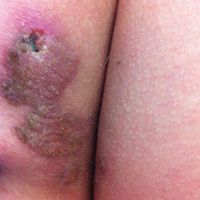
User login
Tubed out
I’m a little concerned about my grandchildren. I worry that when they are in their twenties, no one will want them as trivia teammates. Or when they are hanging out with other 40-something couples, they will fade into the wallpaper when the conversation turns to, “Remember that episode of Big Bang Theory when ... .”
The 5½-year-old and the 8-year-old have grown up in a household that has never had a TV, and the 10- and 12-year-old are surviving with a cable connection so basic that it barely gets more than a few shopping channels and the local school board meetings.
Our children were just too busy doing things to watch much TV. Now as adults they have been paying attention to what they have heard and read about the potential negative influence that TV may have on their own children, and imposed restrictions far more severe than those under which they were raised. It has been interesting to watch how their children are responding to these TV-deprived environments.
For the most part, there has been no whining or begging to turn on the TV. The younger two have no other option and don’t realize what they are missing. The older two, who watched some Sesame Street as toddlers, have been similarly disinterested, although my 10-year-old grandson enjoys watching some sports when the opportunity arises.
So what do my grandchildren do with the 28 hours each week that their peers are spending in front of a TV (“Television and Children,” University of Michigan Medical School/Michigan Medicine website)? The two older girls are voracious readers. One spends hours drawing, and with her younger sister, always has a craft project or two going. The older two are skillful board and card game players, and they play musical instruments. All four are involved in at least one sport per season, and when asked, they would prefer to be playing something outside. And they go to bed at a healthy hour.
In a recent article in AAP News (“How to provide evidence-based pediatric care for the digital age,” May 2017), Michael O. Rich, MD, a member of the American Academy of Pediatrics Council on Communications and Media, writes, “Our traditional advice to limit screen time and restrict content is no longer relevant and often unheard by families.” I agree that for many years that AAP advice had been too focused on content. However, seeing my grandchildren thrive in an environment of what many might consider an extreme screen time restriction has further reinforced my previous observations that the critical issue with screen time is that it replaces health-promoting active alternatives. Even screen time that requires some interaction relegates the child to the role of a sedentary spectator.
Although Dr. Rich is to be commended for suggesting that we look at evidence-based studies as we decide how to counsel parents about screen time, I am always skeptical about the validity of short-term “evidence.” I fear that some of the evidence-based studies are being used to excuse or rationalize an already unhealthy situation. At some point we need to step back and take the longer look. Would you rather see your grandchildren hunched over a screen or couched in front of a television watching other people doing things, or would you prefer that they be physically active doers and creators themselves?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
I’m a little concerned about my grandchildren. I worry that when they are in their twenties, no one will want them as trivia teammates. Or when they are hanging out with other 40-something couples, they will fade into the wallpaper when the conversation turns to, “Remember that episode of Big Bang Theory when ... .”
The 5½-year-old and the 8-year-old have grown up in a household that has never had a TV, and the 10- and 12-year-old are surviving with a cable connection so basic that it barely gets more than a few shopping channels and the local school board meetings.
Our children were just too busy doing things to watch much TV. Now as adults they have been paying attention to what they have heard and read about the potential negative influence that TV may have on their own children, and imposed restrictions far more severe than those under which they were raised. It has been interesting to watch how their children are responding to these TV-deprived environments.
For the most part, there has been no whining or begging to turn on the TV. The younger two have no other option and don’t realize what they are missing. The older two, who watched some Sesame Street as toddlers, have been similarly disinterested, although my 10-year-old grandson enjoys watching some sports when the opportunity arises.
So what do my grandchildren do with the 28 hours each week that their peers are spending in front of a TV (“Television and Children,” University of Michigan Medical School/Michigan Medicine website)? The two older girls are voracious readers. One spends hours drawing, and with her younger sister, always has a craft project or two going. The older two are skillful board and card game players, and they play musical instruments. All four are involved in at least one sport per season, and when asked, they would prefer to be playing something outside. And they go to bed at a healthy hour.
In a recent article in AAP News (“How to provide evidence-based pediatric care for the digital age,” May 2017), Michael O. Rich, MD, a member of the American Academy of Pediatrics Council on Communications and Media, writes, “Our traditional advice to limit screen time and restrict content is no longer relevant and often unheard by families.” I agree that for many years that AAP advice had been too focused on content. However, seeing my grandchildren thrive in an environment of what many might consider an extreme screen time restriction has further reinforced my previous observations that the critical issue with screen time is that it replaces health-promoting active alternatives. Even screen time that requires some interaction relegates the child to the role of a sedentary spectator.
Although Dr. Rich is to be commended for suggesting that we look at evidence-based studies as we decide how to counsel parents about screen time, I am always skeptical about the validity of short-term “evidence.” I fear that some of the evidence-based studies are being used to excuse or rationalize an already unhealthy situation. At some point we need to step back and take the longer look. Would you rather see your grandchildren hunched over a screen or couched in front of a television watching other people doing things, or would you prefer that they be physically active doers and creators themselves?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
I’m a little concerned about my grandchildren. I worry that when they are in their twenties, no one will want them as trivia teammates. Or when they are hanging out with other 40-something couples, they will fade into the wallpaper when the conversation turns to, “Remember that episode of Big Bang Theory when ... .”
The 5½-year-old and the 8-year-old have grown up in a household that has never had a TV, and the 10- and 12-year-old are surviving with a cable connection so basic that it barely gets more than a few shopping channels and the local school board meetings.
Our children were just too busy doing things to watch much TV. Now as adults they have been paying attention to what they have heard and read about the potential negative influence that TV may have on their own children, and imposed restrictions far more severe than those under which they were raised. It has been interesting to watch how their children are responding to these TV-deprived environments.
For the most part, there has been no whining or begging to turn on the TV. The younger two have no other option and don’t realize what they are missing. The older two, who watched some Sesame Street as toddlers, have been similarly disinterested, although my 10-year-old grandson enjoys watching some sports when the opportunity arises.
So what do my grandchildren do with the 28 hours each week that their peers are spending in front of a TV (“Television and Children,” University of Michigan Medical School/Michigan Medicine website)? The two older girls are voracious readers. One spends hours drawing, and with her younger sister, always has a craft project or two going. The older two are skillful board and card game players, and they play musical instruments. All four are involved in at least one sport per season, and when asked, they would prefer to be playing something outside. And they go to bed at a healthy hour.
In a recent article in AAP News (“How to provide evidence-based pediatric care for the digital age,” May 2017), Michael O. Rich, MD, a member of the American Academy of Pediatrics Council on Communications and Media, writes, “Our traditional advice to limit screen time and restrict content is no longer relevant and often unheard by families.” I agree that for many years that AAP advice had been too focused on content. However, seeing my grandchildren thrive in an environment of what many might consider an extreme screen time restriction has further reinforced my previous observations that the critical issue with screen time is that it replaces health-promoting active alternatives. Even screen time that requires some interaction relegates the child to the role of a sedentary spectator.
Although Dr. Rich is to be commended for suggesting that we look at evidence-based studies as we decide how to counsel parents about screen time, I am always skeptical about the validity of short-term “evidence.” I fear that some of the evidence-based studies are being used to excuse or rationalize an already unhealthy situation. At some point we need to step back and take the longer look. Would you rather see your grandchildren hunched over a screen or couched in front of a television watching other people doing things, or would you prefer that they be physically active doers and creators themselves?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Novel evaluation, treatment of NAS decreases medication use
AT PAS 2017
SAN FRANCISCO – A nonpharmacologic approach to neonatal abstinence syndrome (NAS) appears to reduce the use of morphine and may shorten hospital stay, compared with the conventional evaluation that looks at symptoms of opioid withdrawal, a study showed.
“If you focus on the well-being of these infants rather than a list of symptoms, you are much less likely to start medication. Our approach inherently destigmatizes the parents of these infants by allowing them to focus on the same things that any other parent focuses on,” said Matthew Lipshaw, MD, a pediatrician at Yale–New Haven (Conn.) Children’s Hospital.
The novel approach aims instead to avoid drug use. According to Dr. Lipshaw, the nonintrusive approach “assesses infants’ ability to function as infants during their withdrawal.” The approach provides a low-stimulation environment featuring rooming-in by mothers, and frequent feeding of their infants. Dubbed ESC, the approach gauges the ability of an infant to eat 1 ounce or more or breastfeed well, sleep undisturbed for an hour or longer, and be consolable within 10 minutes.
The ESC approach replaced the FNASS at Yale–New Haven Children’s Hospital in 2013. While patient management decisions since then have been based on ESC, FNASS scores have continued to be collected every 2-6 hours. This provided the researchers with the means to conduct a head-to-head comparison of the two systems on the same patients.
The records of 50 consecutive newborns born from March 2014 to August 2015 who had been exposed to opioids for at least 30 days prior to birth were reviewed. The primary outcome was the proportion of infants treated with morphine. Secondary outcomes included disagreements between the two approaches on a daily basis, seizures, 30-day readmissions, and need for more intensive care.
The neonates (56%, female) were mostly white. All were born at greater than 36 weeks’ gestation. Opioid exposure was methadone in 80% of cases and buprenorphine in 14%, with the remaining 6% exposed to hydrocodone, Percocet (acetaminophen/oxycodone), and/or OxyContin (oxycodone).
Morphine was started in 6 (12%) of the 50 patients. If the FNASS protocol had been followed, 31 (62%) of the infants would have been started on morphine (P less than .01). Over a span of 296 hospital days, when the ESC protocol was used, morphine was not used 87% of the time, morphine use was increased 3% of the time, use was decreased 7% of the time, and use was maintained 3% of the time. If decisions had been made based on the FNASS protocol, the frequency of nonuse, increased use, decreased use, and maintained use of morphine would have been 53%, 26%, 12%, and 10%, respectively (all P less than .01).
The use of morphine was less than the FNASS recommendation on 78 days (26% of the total days). Moreover, the FNASS scores on the days following the decreased use of morphine were lower by an average of 0.9 points and were decreased in 69% of cases. The ESC protocol led to greater morphine use than recommended by the FNASS protocol on only 2 days. Both times, the FNASS score was increased the following day.
No adverse events occurred during the study.
“These findings are significant because nearly all institutions use the Finnegan score to guide management, and most research has used Finnegan-based medication thresholds to evaluate new medical therapies. Our point is that if you base your assessment on function, many of these infants may not need medication at all. We have had dramatic reductions in length of stay, which allows these infants to get home and minimize the interruption in this crucial period for maternal-child bonding in these high-risk patients,” Dr. Lipshaw said at the Pediatric Academic Societies meeting.
So far, only the Boston Medical Center has implemented the new system. This does not surprise Dr. Lipshaw: “Most places have been using a symptom-based approach for decades. It requires major buy in from physicians and nurses who have been doing things differently for a long time.”
He said is not deterred, however, and pointed to ongoing efforts by colleagues at Yale–New Haven Hospital and Boston Medical Center that are underway that could led to the ESC’s use in a network of hospitals in New Hampshire and Vermont.
The study was sponsored by Yale–New Haven Children’s Hospital and was not funded. Dr. Lipshaw reported having no relevant financial disclosures.
AT PAS 2017
SAN FRANCISCO – A nonpharmacologic approach to neonatal abstinence syndrome (NAS) appears to reduce the use of morphine and may shorten hospital stay, compared with the conventional evaluation that looks at symptoms of opioid withdrawal, a study showed.
“If you focus on the well-being of these infants rather than a list of symptoms, you are much less likely to start medication. Our approach inherently destigmatizes the parents of these infants by allowing them to focus on the same things that any other parent focuses on,” said Matthew Lipshaw, MD, a pediatrician at Yale–New Haven (Conn.) Children’s Hospital.
The novel approach aims instead to avoid drug use. According to Dr. Lipshaw, the nonintrusive approach “assesses infants’ ability to function as infants during their withdrawal.” The approach provides a low-stimulation environment featuring rooming-in by mothers, and frequent feeding of their infants. Dubbed ESC, the approach gauges the ability of an infant to eat 1 ounce or more or breastfeed well, sleep undisturbed for an hour or longer, and be consolable within 10 minutes.
The ESC approach replaced the FNASS at Yale–New Haven Children’s Hospital in 2013. While patient management decisions since then have been based on ESC, FNASS scores have continued to be collected every 2-6 hours. This provided the researchers with the means to conduct a head-to-head comparison of the two systems on the same patients.
The records of 50 consecutive newborns born from March 2014 to August 2015 who had been exposed to opioids for at least 30 days prior to birth were reviewed. The primary outcome was the proportion of infants treated with morphine. Secondary outcomes included disagreements between the two approaches on a daily basis, seizures, 30-day readmissions, and need for more intensive care.
The neonates (56%, female) were mostly white. All were born at greater than 36 weeks’ gestation. Opioid exposure was methadone in 80% of cases and buprenorphine in 14%, with the remaining 6% exposed to hydrocodone, Percocet (acetaminophen/oxycodone), and/or OxyContin (oxycodone).
Morphine was started in 6 (12%) of the 50 patients. If the FNASS protocol had been followed, 31 (62%) of the infants would have been started on morphine (P less than .01). Over a span of 296 hospital days, when the ESC protocol was used, morphine was not used 87% of the time, morphine use was increased 3% of the time, use was decreased 7% of the time, and use was maintained 3% of the time. If decisions had been made based on the FNASS protocol, the frequency of nonuse, increased use, decreased use, and maintained use of morphine would have been 53%, 26%, 12%, and 10%, respectively (all P less than .01).
The use of morphine was less than the FNASS recommendation on 78 days (26% of the total days). Moreover, the FNASS scores on the days following the decreased use of morphine were lower by an average of 0.9 points and were decreased in 69% of cases. The ESC protocol led to greater morphine use than recommended by the FNASS protocol on only 2 days. Both times, the FNASS score was increased the following day.
No adverse events occurred during the study.
“These findings are significant because nearly all institutions use the Finnegan score to guide management, and most research has used Finnegan-based medication thresholds to evaluate new medical therapies. Our point is that if you base your assessment on function, many of these infants may not need medication at all. We have had dramatic reductions in length of stay, which allows these infants to get home and minimize the interruption in this crucial period for maternal-child bonding in these high-risk patients,” Dr. Lipshaw said at the Pediatric Academic Societies meeting.
So far, only the Boston Medical Center has implemented the new system. This does not surprise Dr. Lipshaw: “Most places have been using a symptom-based approach for decades. It requires major buy in from physicians and nurses who have been doing things differently for a long time.”
He said is not deterred, however, and pointed to ongoing efforts by colleagues at Yale–New Haven Hospital and Boston Medical Center that are underway that could led to the ESC’s use in a network of hospitals in New Hampshire and Vermont.
The study was sponsored by Yale–New Haven Children’s Hospital and was not funded. Dr. Lipshaw reported having no relevant financial disclosures.
AT PAS 2017
SAN FRANCISCO – A nonpharmacologic approach to neonatal abstinence syndrome (NAS) appears to reduce the use of morphine and may shorten hospital stay, compared with the conventional evaluation that looks at symptoms of opioid withdrawal, a study showed.
“If you focus on the well-being of these infants rather than a list of symptoms, you are much less likely to start medication. Our approach inherently destigmatizes the parents of these infants by allowing them to focus on the same things that any other parent focuses on,” said Matthew Lipshaw, MD, a pediatrician at Yale–New Haven (Conn.) Children’s Hospital.
The novel approach aims instead to avoid drug use. According to Dr. Lipshaw, the nonintrusive approach “assesses infants’ ability to function as infants during their withdrawal.” The approach provides a low-stimulation environment featuring rooming-in by mothers, and frequent feeding of their infants. Dubbed ESC, the approach gauges the ability of an infant to eat 1 ounce or more or breastfeed well, sleep undisturbed for an hour or longer, and be consolable within 10 minutes.
The ESC approach replaced the FNASS at Yale–New Haven Children’s Hospital in 2013. While patient management decisions since then have been based on ESC, FNASS scores have continued to be collected every 2-6 hours. This provided the researchers with the means to conduct a head-to-head comparison of the two systems on the same patients.
The records of 50 consecutive newborns born from March 2014 to August 2015 who had been exposed to opioids for at least 30 days prior to birth were reviewed. The primary outcome was the proportion of infants treated with morphine. Secondary outcomes included disagreements between the two approaches on a daily basis, seizures, 30-day readmissions, and need for more intensive care.
The neonates (56%, female) were mostly white. All were born at greater than 36 weeks’ gestation. Opioid exposure was methadone in 80% of cases and buprenorphine in 14%, with the remaining 6% exposed to hydrocodone, Percocet (acetaminophen/oxycodone), and/or OxyContin (oxycodone).
Morphine was started in 6 (12%) of the 50 patients. If the FNASS protocol had been followed, 31 (62%) of the infants would have been started on morphine (P less than .01). Over a span of 296 hospital days, when the ESC protocol was used, morphine was not used 87% of the time, morphine use was increased 3% of the time, use was decreased 7% of the time, and use was maintained 3% of the time. If decisions had been made based on the FNASS protocol, the frequency of nonuse, increased use, decreased use, and maintained use of morphine would have been 53%, 26%, 12%, and 10%, respectively (all P less than .01).
The use of morphine was less than the FNASS recommendation on 78 days (26% of the total days). Moreover, the FNASS scores on the days following the decreased use of morphine were lower by an average of 0.9 points and were decreased in 69% of cases. The ESC protocol led to greater morphine use than recommended by the FNASS protocol on only 2 days. Both times, the FNASS score was increased the following day.
No adverse events occurred during the study.
“These findings are significant because nearly all institutions use the Finnegan score to guide management, and most research has used Finnegan-based medication thresholds to evaluate new medical therapies. Our point is that if you base your assessment on function, many of these infants may not need medication at all. We have had dramatic reductions in length of stay, which allows these infants to get home and minimize the interruption in this crucial period for maternal-child bonding in these high-risk patients,” Dr. Lipshaw said at the Pediatric Academic Societies meeting.
So far, only the Boston Medical Center has implemented the new system. This does not surprise Dr. Lipshaw: “Most places have been using a symptom-based approach for decades. It requires major buy in from physicians and nurses who have been doing things differently for a long time.”
He said is not deterred, however, and pointed to ongoing efforts by colleagues at Yale–New Haven Hospital and Boston Medical Center that are underway that could led to the ESC’s use in a network of hospitals in New Hampshire and Vermont.
The study was sponsored by Yale–New Haven Children’s Hospital and was not funded. Dr. Lipshaw reported having no relevant financial disclosures.
Key clinical point: Evaluation and treatment of neonatal abstinence syndrome that focuses on feeding, quality of sleep, and ability to be consoled significantly reduces morphine use, compared with the established system.
Major finding: The novel ESC approach decreased morphine use, compared with the established FNASS approach (3% vs. 26%, P less than .01).
Data source: A retrospective examination of patient medical records.
Disclosures: The study was sponsored by Yale–New Haven Children’s Hospital and was not funded. Dr. Lipshaw reported having no relevant financial disclosures.
Dining alone
I have a repertoire of about a dozen soups that I enjoy preparing, but I certainly don’t consider myself a gourmet chef. However, I can legitimately claim to be a master of the microwave. Hand me a potentially edible substance, and I will nuke it to a palatable temperature in one step. This skill comes from 30 years of practice and requires a sixth sense that includes factoring in the object’s water content, shape, and density, and knowing whether I am starting from the frozen state, refrigerator cool, or room temperature.
Sadly, our 30-some-year-old microwave nuked its last leftover in a shower of sparks a few weeks ago, and I have been forced to recalibrate my technique with a new machine. Not to worry, I am just one or two more rewarmed meals away from returning to my old “nukelear” mastery.
Unfortunately, as with any new technology, the ubiquity of countertop microwave ovens has come with some downsides. While they do offer the cooking challenged among us a broad choice of foods we can prepare in minutes or seconds, the choices we make are not always nutritiously sound.
The microwave oven and single-serving prepared frozen meals have been a great boon to people who live alone or live or work on schedules out of sync with their families’ meal schedule. However, there is a point when this technologically-enabled nutritional independence begins to take precedence over communal dining. The family meal slips on to the endangered species list. Although there is some debate about whether family meals are any more valuable as character-building exercises than other shared family experiences, there is no question that children in families who dine together on a regular basis enjoy substantial health benefits, such as less depressive symptoms, more healthy foods, fewer weight problems, and less delinquency.
The forces that have driven the family meal into decline are numerous and powerful. However, we should not underestimate the role that the microwave oven has had in greasing this path toward extinction. Even if the family has one member with the time, skills, and commitment to create nutritious and complete meals, the microwave oven offers even the youngest member an easy way to opt out of sharing it with the rest of his family. A parent who must work late can rewarm his serving at 9:00 p.m. when he or she gets home. The high school thespian can nuke her own prepared frozen dinner at 5 p.m. so she can get to a rehearsal at 6 p.m. And, the 4-year-old picky eater who won’t touch anything green can have his treasured mac ‘n cheese warmed to his taste while everyone else is enjoying fish tacos. And, there you have it. Poof! With the touch of a couple buttons, the opportunity for a family to enjoy a meal together and share their experiences of the day has vanished into thin air along with a valuable lesson in cooperation and compromise.
But, we needn’t worry about those family members who are dining separately getting lonely because more than likely they each have their own electronic companion to keep them company while they eat their microwaved meal.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
I have a repertoire of about a dozen soups that I enjoy preparing, but I certainly don’t consider myself a gourmet chef. However, I can legitimately claim to be a master of the microwave. Hand me a potentially edible substance, and I will nuke it to a palatable temperature in one step. This skill comes from 30 years of practice and requires a sixth sense that includes factoring in the object’s water content, shape, and density, and knowing whether I am starting from the frozen state, refrigerator cool, or room temperature.
Sadly, our 30-some-year-old microwave nuked its last leftover in a shower of sparks a few weeks ago, and I have been forced to recalibrate my technique with a new machine. Not to worry, I am just one or two more rewarmed meals away from returning to my old “nukelear” mastery.
Unfortunately, as with any new technology, the ubiquity of countertop microwave ovens has come with some downsides. While they do offer the cooking challenged among us a broad choice of foods we can prepare in minutes or seconds, the choices we make are not always nutritiously sound.
The microwave oven and single-serving prepared frozen meals have been a great boon to people who live alone or live or work on schedules out of sync with their families’ meal schedule. However, there is a point when this technologically-enabled nutritional independence begins to take precedence over communal dining. The family meal slips on to the endangered species list. Although there is some debate about whether family meals are any more valuable as character-building exercises than other shared family experiences, there is no question that children in families who dine together on a regular basis enjoy substantial health benefits, such as less depressive symptoms, more healthy foods, fewer weight problems, and less delinquency.
The forces that have driven the family meal into decline are numerous and powerful. However, we should not underestimate the role that the microwave oven has had in greasing this path toward extinction. Even if the family has one member with the time, skills, and commitment to create nutritious and complete meals, the microwave oven offers even the youngest member an easy way to opt out of sharing it with the rest of his family. A parent who must work late can rewarm his serving at 9:00 p.m. when he or she gets home. The high school thespian can nuke her own prepared frozen dinner at 5 p.m. so she can get to a rehearsal at 6 p.m. And, the 4-year-old picky eater who won’t touch anything green can have his treasured mac ‘n cheese warmed to his taste while everyone else is enjoying fish tacos. And, there you have it. Poof! With the touch of a couple buttons, the opportunity for a family to enjoy a meal together and share their experiences of the day has vanished into thin air along with a valuable lesson in cooperation and compromise.
But, we needn’t worry about those family members who are dining separately getting lonely because more than likely they each have their own electronic companion to keep them company while they eat their microwaved meal.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
I have a repertoire of about a dozen soups that I enjoy preparing, but I certainly don’t consider myself a gourmet chef. However, I can legitimately claim to be a master of the microwave. Hand me a potentially edible substance, and I will nuke it to a palatable temperature in one step. This skill comes from 30 years of practice and requires a sixth sense that includes factoring in the object’s water content, shape, and density, and knowing whether I am starting from the frozen state, refrigerator cool, or room temperature.
Sadly, our 30-some-year-old microwave nuked its last leftover in a shower of sparks a few weeks ago, and I have been forced to recalibrate my technique with a new machine. Not to worry, I am just one or two more rewarmed meals away from returning to my old “nukelear” mastery.
Unfortunately, as with any new technology, the ubiquity of countertop microwave ovens has come with some downsides. While they do offer the cooking challenged among us a broad choice of foods we can prepare in minutes or seconds, the choices we make are not always nutritiously sound.
The microwave oven and single-serving prepared frozen meals have been a great boon to people who live alone or live or work on schedules out of sync with their families’ meal schedule. However, there is a point when this technologically-enabled nutritional independence begins to take precedence over communal dining. The family meal slips on to the endangered species list. Although there is some debate about whether family meals are any more valuable as character-building exercises than other shared family experiences, there is no question that children in families who dine together on a regular basis enjoy substantial health benefits, such as less depressive symptoms, more healthy foods, fewer weight problems, and less delinquency.
The forces that have driven the family meal into decline are numerous and powerful. However, we should not underestimate the role that the microwave oven has had in greasing this path toward extinction. Even if the family has one member with the time, skills, and commitment to create nutritious and complete meals, the microwave oven offers even the youngest member an easy way to opt out of sharing it with the rest of his family. A parent who must work late can rewarm his serving at 9:00 p.m. when he or she gets home. The high school thespian can nuke her own prepared frozen dinner at 5 p.m. so she can get to a rehearsal at 6 p.m. And, the 4-year-old picky eater who won’t touch anything green can have his treasured mac ‘n cheese warmed to his taste while everyone else is enjoying fish tacos. And, there you have it. Poof! With the touch of a couple buttons, the opportunity for a family to enjoy a meal together and share their experiences of the day has vanished into thin air along with a valuable lesson in cooperation and compromise.
But, we needn’t worry about those family members who are dining separately getting lonely because more than likely they each have their own electronic companion to keep them company while they eat their microwaved meal.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Three developmental screens differ in outcomes in comparative study
SAN FRANCISCO – The use of three different screening instruments to gauge behavioral development in children up to 5 years of age has yielded results that vary within a single practice and between different practices. This heterogeneity complicates the accurate and early identification of developmental disorders children in the primary care setting.
“The burden of diagnostic services that go along with developmental screening depends on the number of positive screens and the referral completion rate. These rates may vary markedly across practices that from the outside seem relatively homogeneous. This differential burden may help explain the variation between practices that has been observed,” said Radley Sheldrick, PhD, of Boston University School of Public Health.
The American Academy of Pediatrics has recommended the use of developmental screening instruments that have a track record in prior studies of a sensitivity and specificity of at least 70% each. Children who score positive can receive further services. The aim is laudable, Dr. Sheldrick said, but little is known of how different screens compare to one another in the results obtained, and the consistency of their performance in different practice settings.
A few years ago, Dr. Sheldrick and his colleagues at Tufts Medical Center, Boston, initiated the Screen Early, Screen Accurately for Child Well-Being (SESAW) head-to-head comparison of the effectiveness of three sets of developmental behavioral screening instruments used in the pediatric primary care setting: the Ages and Stages Questionnaire, 2nd edition (ASQ-2), Parent’s Evaluation of Developmental Status (PEDS), and the Survey of Well-Being of Young Children (SWYC).
The ASQ-2 and PEDS instruments have been in use for some time. Differences in their sensitivity and specificity of developmental concerns have been noted, although both can be used at the discretion of the physician. SWYC is a more recent instrument, which was developed at Tufts Medical Center. It was designed to be easy to read and quickly completed.
In the study, 1,000 parents of children aged 9 months to 5.5 years were enrolled at six pediatric practices in Massachusetts. About 50% of the children were boys, 10% were Hispanic, and 10% were African American. About one-quarter of the parents were receiving some form of public assistance. The parents completed the three screens. Children scoring positive on any screen were assessed further.
The researchers were especially interested in the agreement between the three screens and the variance across the six practices in the performance of the screens and the proportion of children who tested positive and actually received referral care.
Overall, about 44% of the children scored positive on at least one screen. Of these, 72% were assessed more comprehensively. A closer look at those who were assessed revealed agreement between all three screens in only 16% of the children.
The performance of the three screens was not consistent from practice to practice. Variations were evident with each screen in the different practices, and between the three screens in individual practices. The differences in the performance of the screens in the individual practices were not significantly different. However, considerable difference was noted between practices, the extreme being a 70% higher difference in one practice compared to another.
Referral completion rates also displayed variation between practices, although no significant difference was evident. Still, the extreme case was a 30% higher rate of completion of one of the practices, compared with another.
“As I’ve gotten further into this research, I’ve become struck by the number of things we don’t know about developmental screens [compared to] what we do know. Whether, for example, the sensitivity and specificity of a screen in one population carries over to other populations is an assumption we have made, but which we don’t really know,” said Dr. Sheldrick.
Another unknown is whether a developmental disorder identified by a screen at one age can be identified at a later age in someone who has not received specialized care.
Finally, the issue of false positive results is vexing. While a false positive might be suspected, not to do anything sends the wrong message.
“What to do when there is a problem between a clinical result and a screening result is one of the most important clinical questions we have right now. Clinicians have to make up their minds on this issue every day, and there is not a lot of research on it. The results need to be evaluated while recognizing that there are still some uncertainties with screening results, and recognizing other forms of information, such as parent reporting and observations of the child, that can be informative,” explained Dr. Sheldrick.
Tufts Medical Center sponsored the study, which was funded by the National Institutes of Health. Dr. Sheldrick reported having no relevant financial disclosures.
SAN FRANCISCO – The use of three different screening instruments to gauge behavioral development in children up to 5 years of age has yielded results that vary within a single practice and between different practices. This heterogeneity complicates the accurate and early identification of developmental disorders children in the primary care setting.
“The burden of diagnostic services that go along with developmental screening depends on the number of positive screens and the referral completion rate. These rates may vary markedly across practices that from the outside seem relatively homogeneous. This differential burden may help explain the variation between practices that has been observed,” said Radley Sheldrick, PhD, of Boston University School of Public Health.
The American Academy of Pediatrics has recommended the use of developmental screening instruments that have a track record in prior studies of a sensitivity and specificity of at least 70% each. Children who score positive can receive further services. The aim is laudable, Dr. Sheldrick said, but little is known of how different screens compare to one another in the results obtained, and the consistency of their performance in different practice settings.
A few years ago, Dr. Sheldrick and his colleagues at Tufts Medical Center, Boston, initiated the Screen Early, Screen Accurately for Child Well-Being (SESAW) head-to-head comparison of the effectiveness of three sets of developmental behavioral screening instruments used in the pediatric primary care setting: the Ages and Stages Questionnaire, 2nd edition (ASQ-2), Parent’s Evaluation of Developmental Status (PEDS), and the Survey of Well-Being of Young Children (SWYC).
The ASQ-2 and PEDS instruments have been in use for some time. Differences in their sensitivity and specificity of developmental concerns have been noted, although both can be used at the discretion of the physician. SWYC is a more recent instrument, which was developed at Tufts Medical Center. It was designed to be easy to read and quickly completed.
In the study, 1,000 parents of children aged 9 months to 5.5 years were enrolled at six pediatric practices in Massachusetts. About 50% of the children were boys, 10% were Hispanic, and 10% were African American. About one-quarter of the parents were receiving some form of public assistance. The parents completed the three screens. Children scoring positive on any screen were assessed further.
The researchers were especially interested in the agreement between the three screens and the variance across the six practices in the performance of the screens and the proportion of children who tested positive and actually received referral care.
Overall, about 44% of the children scored positive on at least one screen. Of these, 72% were assessed more comprehensively. A closer look at those who were assessed revealed agreement between all three screens in only 16% of the children.
The performance of the three screens was not consistent from practice to practice. Variations were evident with each screen in the different practices, and between the three screens in individual practices. The differences in the performance of the screens in the individual practices were not significantly different. However, considerable difference was noted between practices, the extreme being a 70% higher difference in one practice compared to another.
Referral completion rates also displayed variation between practices, although no significant difference was evident. Still, the extreme case was a 30% higher rate of completion of one of the practices, compared with another.
“As I’ve gotten further into this research, I’ve become struck by the number of things we don’t know about developmental screens [compared to] what we do know. Whether, for example, the sensitivity and specificity of a screen in one population carries over to other populations is an assumption we have made, but which we don’t really know,” said Dr. Sheldrick.
Another unknown is whether a developmental disorder identified by a screen at one age can be identified at a later age in someone who has not received specialized care.
Finally, the issue of false positive results is vexing. While a false positive might be suspected, not to do anything sends the wrong message.
“What to do when there is a problem between a clinical result and a screening result is one of the most important clinical questions we have right now. Clinicians have to make up their minds on this issue every day, and there is not a lot of research on it. The results need to be evaluated while recognizing that there are still some uncertainties with screening results, and recognizing other forms of information, such as parent reporting and observations of the child, that can be informative,” explained Dr. Sheldrick.
Tufts Medical Center sponsored the study, which was funded by the National Institutes of Health. Dr. Sheldrick reported having no relevant financial disclosures.
SAN FRANCISCO – The use of three different screening instruments to gauge behavioral development in children up to 5 years of age has yielded results that vary within a single practice and between different practices. This heterogeneity complicates the accurate and early identification of developmental disorders children in the primary care setting.
“The burden of diagnostic services that go along with developmental screening depends on the number of positive screens and the referral completion rate. These rates may vary markedly across practices that from the outside seem relatively homogeneous. This differential burden may help explain the variation between practices that has been observed,” said Radley Sheldrick, PhD, of Boston University School of Public Health.
The American Academy of Pediatrics has recommended the use of developmental screening instruments that have a track record in prior studies of a sensitivity and specificity of at least 70% each. Children who score positive can receive further services. The aim is laudable, Dr. Sheldrick said, but little is known of how different screens compare to one another in the results obtained, and the consistency of their performance in different practice settings.
A few years ago, Dr. Sheldrick and his colleagues at Tufts Medical Center, Boston, initiated the Screen Early, Screen Accurately for Child Well-Being (SESAW) head-to-head comparison of the effectiveness of three sets of developmental behavioral screening instruments used in the pediatric primary care setting: the Ages and Stages Questionnaire, 2nd edition (ASQ-2), Parent’s Evaluation of Developmental Status (PEDS), and the Survey of Well-Being of Young Children (SWYC).
The ASQ-2 and PEDS instruments have been in use for some time. Differences in their sensitivity and specificity of developmental concerns have been noted, although both can be used at the discretion of the physician. SWYC is a more recent instrument, which was developed at Tufts Medical Center. It was designed to be easy to read and quickly completed.
In the study, 1,000 parents of children aged 9 months to 5.5 years were enrolled at six pediatric practices in Massachusetts. About 50% of the children were boys, 10% were Hispanic, and 10% were African American. About one-quarter of the parents were receiving some form of public assistance. The parents completed the three screens. Children scoring positive on any screen were assessed further.
The researchers were especially interested in the agreement between the three screens and the variance across the six practices in the performance of the screens and the proportion of children who tested positive and actually received referral care.
Overall, about 44% of the children scored positive on at least one screen. Of these, 72% were assessed more comprehensively. A closer look at those who were assessed revealed agreement between all three screens in only 16% of the children.
The performance of the three screens was not consistent from practice to practice. Variations were evident with each screen in the different practices, and between the three screens in individual practices. The differences in the performance of the screens in the individual practices were not significantly different. However, considerable difference was noted between practices, the extreme being a 70% higher difference in one practice compared to another.
Referral completion rates also displayed variation between practices, although no significant difference was evident. Still, the extreme case was a 30% higher rate of completion of one of the practices, compared with another.
“As I’ve gotten further into this research, I’ve become struck by the number of things we don’t know about developmental screens [compared to] what we do know. Whether, for example, the sensitivity and specificity of a screen in one population carries over to other populations is an assumption we have made, but which we don’t really know,” said Dr. Sheldrick.
Another unknown is whether a developmental disorder identified by a screen at one age can be identified at a later age in someone who has not received specialized care.
Finally, the issue of false positive results is vexing. While a false positive might be suspected, not to do anything sends the wrong message.
“What to do when there is a problem between a clinical result and a screening result is one of the most important clinical questions we have right now. Clinicians have to make up their minds on this issue every day, and there is not a lot of research on it. The results need to be evaluated while recognizing that there are still some uncertainties with screening results, and recognizing other forms of information, such as parent reporting and observations of the child, that can be informative,” explained Dr. Sheldrick.
Tufts Medical Center sponsored the study, which was funded by the National Institutes of Health. Dr. Sheldrick reported having no relevant financial disclosures.
AT PAS 17
Key clinical point: There were significant differences in the results of different developmental screening instruments within and between practices in a comparative study.
Major finding: The PEDS, ASQ-2, and SWYC developmental screens coidentified only 16% of 317 children aged 9 months to 5.5 years, with significant differences in the results of each developmental screen between practices.
Data source: The Screen Early, Screen Accurately for Child Well-Being (SESAW) head-to-head comparative effectiveness trial of three sets of developmental behavioral screening instruments used in pediatric primary care.
Disclosures: Tufts Medical Center sponsored the study, which was funded by the National Institutes of Health. Dr. Sheldrick reported having no relevant financial disclosures.
Endometrial cancer rates increased following WHI
SAN DIEGO – Endometrial cancer (EC) rates increased after 2002, coinciding with the release of results from the Women’s Health Initiative and including a 10% spike between 2006 and 2014, according to a large analysis of national data.
“Be aware of an increase of endometrial cancer and, whenever possible, look to minimize possible inciting causes,” Ginger Constantine, MD, lead study author, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Be certain that the hormonal products that patients are taking are delivering adequate progesterone to the endometrium in those patients at an increased risk of endometrial cancer, such as those with unopposed estrogen.”
“We sought to look at possible causes for the increase of the known risk factors for EC,” she said. “What has changed in the years leading up to 2006?” To find out, she and her associates obtained EC incidence from the Surveillance, Epidemiology, and End Result Program database from 1975 through 2014. They evaluated the incidence of risk factors thought to be associated with EC, including age, obesity, race, number of menstrual cycles, gravidity and parity, metabolic syndromes, diet and exercise, and medications, including various types of hormone therapy, tamoxifen, and hormonal contraceptives.
Shelli Graham, PhD, vice president of medical affairs for Boca Raton, Florida–based TherapeuticsMD, presented the study findings on behalf of Dr. Constantine, who was unable to attend the meeting. The rates of EC were relatively constant from 1992 to 2002 (at about 76/100,000 cases per year) but have increased 2.5% annually, with a 10% increase from 2006 to 2014, especially in women aged 55-64 years.
Use of estrogen and progestin combinations have decreased while risk factors remained constant or decreased during the same time period. However, the researchers observed a “huge increase (of 1-2.5 million U.S. women) using non-FDA approved compounded estrogen and estrogen and progesterone, which may not provide adequate endometrial protection from estrogen, a known cause of EC,” Dr. Constantine said. “Additionally, there is less progestin use subsequent to the [Women’s Health Initiative] and it is known that progestin is protective on the endometrium.”
The researchers also examined the incidence of obesity – another known risk factor for EC – and found that, although obesity has continued to increase in incidence, “it does not appear to be increasing at the same rate as EC, so [it] does not appear to be enough to explain the increase in EC from 2006,” Dr. Constantine said. “I was surprised at the rate of increase of EC from 2006 to 2014 and have also been surprised by the amount of non-FDA compounded hormone use.”
Dr. Graham characterized the increase in EC incidence as “a public health concern that is most likely multifactorial in etiology.” Contributors, she said, include the combination of an increase in obesity with an inherent increase in endogenous estrogen, decreasing progesterone use from a decrease in the use of FDA-approved hormone therapy products, and an increase in compounded hormone therapy that may not deliver adequate endometrial protection.
Dr. Constantine acknowledged certain limitations of the study, including the fact that it is “an ecological analysis, not a randomized clinical trial. It is hypothesis-generating.”
Dr. Constantine reported that she is a consultant/advisory member for TherapeuticsMD and other pharmaceutical companies. She owns stock in TherapeuticsMD. Coauthor Steven R. Goldstein, MD, reported having numerous financial relationships with pharmaceutical companies including TherapeuticsMD. Dr. Graham is an employee of TherapeuticsMD.
SAN DIEGO – Endometrial cancer (EC) rates increased after 2002, coinciding with the release of results from the Women’s Health Initiative and including a 10% spike between 2006 and 2014, according to a large analysis of national data.
“Be aware of an increase of endometrial cancer and, whenever possible, look to minimize possible inciting causes,” Ginger Constantine, MD, lead study author, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Be certain that the hormonal products that patients are taking are delivering adequate progesterone to the endometrium in those patients at an increased risk of endometrial cancer, such as those with unopposed estrogen.”
“We sought to look at possible causes for the increase of the known risk factors for EC,” she said. “What has changed in the years leading up to 2006?” To find out, she and her associates obtained EC incidence from the Surveillance, Epidemiology, and End Result Program database from 1975 through 2014. They evaluated the incidence of risk factors thought to be associated with EC, including age, obesity, race, number of menstrual cycles, gravidity and parity, metabolic syndromes, diet and exercise, and medications, including various types of hormone therapy, tamoxifen, and hormonal contraceptives.
Shelli Graham, PhD, vice president of medical affairs for Boca Raton, Florida–based TherapeuticsMD, presented the study findings on behalf of Dr. Constantine, who was unable to attend the meeting. The rates of EC were relatively constant from 1992 to 2002 (at about 76/100,000 cases per year) but have increased 2.5% annually, with a 10% increase from 2006 to 2014, especially in women aged 55-64 years.
Use of estrogen and progestin combinations have decreased while risk factors remained constant or decreased during the same time period. However, the researchers observed a “huge increase (of 1-2.5 million U.S. women) using non-FDA approved compounded estrogen and estrogen and progesterone, which may not provide adequate endometrial protection from estrogen, a known cause of EC,” Dr. Constantine said. “Additionally, there is less progestin use subsequent to the [Women’s Health Initiative] and it is known that progestin is protective on the endometrium.”
The researchers also examined the incidence of obesity – another known risk factor for EC – and found that, although obesity has continued to increase in incidence, “it does not appear to be increasing at the same rate as EC, so [it] does not appear to be enough to explain the increase in EC from 2006,” Dr. Constantine said. “I was surprised at the rate of increase of EC from 2006 to 2014 and have also been surprised by the amount of non-FDA compounded hormone use.”
Dr. Graham characterized the increase in EC incidence as “a public health concern that is most likely multifactorial in etiology.” Contributors, she said, include the combination of an increase in obesity with an inherent increase in endogenous estrogen, decreasing progesterone use from a decrease in the use of FDA-approved hormone therapy products, and an increase in compounded hormone therapy that may not deliver adequate endometrial protection.
Dr. Constantine acknowledged certain limitations of the study, including the fact that it is “an ecological analysis, not a randomized clinical trial. It is hypothesis-generating.”
Dr. Constantine reported that she is a consultant/advisory member for TherapeuticsMD and other pharmaceutical companies. She owns stock in TherapeuticsMD. Coauthor Steven R. Goldstein, MD, reported having numerous financial relationships with pharmaceutical companies including TherapeuticsMD. Dr. Graham is an employee of TherapeuticsMD.
SAN DIEGO – Endometrial cancer (EC) rates increased after 2002, coinciding with the release of results from the Women’s Health Initiative and including a 10% spike between 2006 and 2014, according to a large analysis of national data.
“Be aware of an increase of endometrial cancer and, whenever possible, look to minimize possible inciting causes,” Ginger Constantine, MD, lead study author, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Be certain that the hormonal products that patients are taking are delivering adequate progesterone to the endometrium in those patients at an increased risk of endometrial cancer, such as those with unopposed estrogen.”
“We sought to look at possible causes for the increase of the known risk factors for EC,” she said. “What has changed in the years leading up to 2006?” To find out, she and her associates obtained EC incidence from the Surveillance, Epidemiology, and End Result Program database from 1975 through 2014. They evaluated the incidence of risk factors thought to be associated with EC, including age, obesity, race, number of menstrual cycles, gravidity and parity, metabolic syndromes, diet and exercise, and medications, including various types of hormone therapy, tamoxifen, and hormonal contraceptives.
Shelli Graham, PhD, vice president of medical affairs for Boca Raton, Florida–based TherapeuticsMD, presented the study findings on behalf of Dr. Constantine, who was unable to attend the meeting. The rates of EC were relatively constant from 1992 to 2002 (at about 76/100,000 cases per year) but have increased 2.5% annually, with a 10% increase from 2006 to 2014, especially in women aged 55-64 years.
Use of estrogen and progestin combinations have decreased while risk factors remained constant or decreased during the same time period. However, the researchers observed a “huge increase (of 1-2.5 million U.S. women) using non-FDA approved compounded estrogen and estrogen and progesterone, which may not provide adequate endometrial protection from estrogen, a known cause of EC,” Dr. Constantine said. “Additionally, there is less progestin use subsequent to the [Women’s Health Initiative] and it is known that progestin is protective on the endometrium.”
The researchers also examined the incidence of obesity – another known risk factor for EC – and found that, although obesity has continued to increase in incidence, “it does not appear to be increasing at the same rate as EC, so [it] does not appear to be enough to explain the increase in EC from 2006,” Dr. Constantine said. “I was surprised at the rate of increase of EC from 2006 to 2014 and have also been surprised by the amount of non-FDA compounded hormone use.”
Dr. Graham characterized the increase in EC incidence as “a public health concern that is most likely multifactorial in etiology.” Contributors, she said, include the combination of an increase in obesity with an inherent increase in endogenous estrogen, decreasing progesterone use from a decrease in the use of FDA-approved hormone therapy products, and an increase in compounded hormone therapy that may not deliver adequate endometrial protection.
Dr. Constantine acknowledged certain limitations of the study, including the fact that it is “an ecological analysis, not a randomized clinical trial. It is hypothesis-generating.”
Dr. Constantine reported that she is a consultant/advisory member for TherapeuticsMD and other pharmaceutical companies. She owns stock in TherapeuticsMD. Coauthor Steven R. Goldstein, MD, reported having numerous financial relationships with pharmaceutical companies including TherapeuticsMD. Dr. Graham is an employee of TherapeuticsMD.
Key clinical point:
Major finding: Rates of endometrial cancer increased 10% between 2006 and 2014.
Data source: An analysis of the Surveillance, Epidemiology, and End Result Program database.
Disclosures: Dr. Constantine reported that she is a consultant/advisory board member for TherapeuticsMD as well as other pharmaceutical companies. She also owns stock in TherapeuticsMD. Coauthor Steven R. Goldstein, MD, reported having numerous financial relationships with pharmaceutical companies including TherapeuticsMD. Dr. Graham is an employee of TherapeuticsMD.
Hospitalists share strategies to secure, excel at jobs
LAS VEGAS – In the view of academic hospitalist Alfred Burger, MD, SFHM, portability was long a dirty word in HM circles. But not anymore.
“My good friends in law and business do this all the time,” said Dr. Burger, associate program director of the internal medicine residency program at Mount Sinai Beth Israel in New York. “You’re not going to make partner in city X, but they’ve got an opening to be partner in city Y if you go there and perform for a year. People up and leave coasts, people up and leave states, people have up and left the country. ... Doctors are starting to view it the same way.”
The lessons of career development were a focal point of HM17, particularly for younger physicians who could take advantage of the Early-Career Hospitalists mini-track. But Dr. Burger said that those strategies of upward mobility can apply whether someone is chasing their first job or their fifth.
First, identify one’s strengths and play to those. Then identify the skills you don’t have or don’t excel at, and address those deficiencies.
“How can you acquire the skills to put yourself in the best position to move up, if you wish to develop your career as a leader?” Dr. Burger said. “If you wish to be the best clinician, you still need to stay on top of the game. Things like coming to SHM, staying on top of the content. That’s important.”
Another skill set is self-advocacy.
“Be your own champion,” said Brian Markoff, MD, SFHM, chief of hospital medicine at Mount Sinai St. Luke’s in New York. “Many of us are very good at this and many of us are terrible at this. You may fall somewhere in between, but you do have to be your own champion.”
Dr. Burger said that he understands that there is a fine line between too much self-promotion and too little. But he urged hospitalists at all career points to take responsibility for marketing themselves.
“Nobody is going to invest in your career unless you yourself invest in it,” he added. “You have to put it as a priority, and not in a selfish way, but in a way [that,] if you wish to move forward and move up, you’ve got to put the time in. It’s not a natural assumption anymore that, if you are the best and brightest of a group of doctors, you will just be chosen to lead.”
In a similar vein, networking is a major boon to career development that can be a double-edged scalpel.
“Having a great ‘social game’ is important, but if all you bring to the table is a social game, you’ll find yourself out of a job just as quickly as you found that job,” Dr. Burger said. “Meaning, you might be able to get it based on that, but you’re not going to be able to sustain it. At the same time, being highly accomplished and having no social graces is also a killer. So, you need to be sort of strong in both areas.”
Many of the meeting’s opportunities for tips on professional development are personal, but HM group leaders have to consider developing the careers of their employees. One of the main planks of that is physician engagement, said Flora Kisuule, MD, MPH, SFHM, of Johns Hopkins University, Baltimore.
“I don’t believe your institution or your organization can go anywhere if your employees are not engaged or if the people you work with are not engaged,” she said, adding that disengaged employees “are actively working against you. You don’t want that. You can’t go in any direction when there are people rowing in the opposite direction. At best, you stay in one place. At worst, you can end up losing ground.”
Hospitalist Christie Masters, MD, MBA, MHA, who practices at UCLA, disclosed during a session that she also runs a wellness coaching firm. She added that a focus on personal wellness and well-being is its own form of career development. It works in tandem with engagement, morale, and professional growth.
“If you’re only focusing on wellness and you don’t have hospitalists or a group that’s engaged or with high morale, they’re going to burnout or they’re going to leave,” Dr. Masters said. “And nobody wants that for their group. So, if we surround ourselves with people who feel well and feel whole, that’s going to have intangible benefits ... that affect the bottom line.”
LAS VEGAS – In the view of academic hospitalist Alfred Burger, MD, SFHM, portability was long a dirty word in HM circles. But not anymore.
“My good friends in law and business do this all the time,” said Dr. Burger, associate program director of the internal medicine residency program at Mount Sinai Beth Israel in New York. “You’re not going to make partner in city X, but they’ve got an opening to be partner in city Y if you go there and perform for a year. People up and leave coasts, people up and leave states, people have up and left the country. ... Doctors are starting to view it the same way.”
The lessons of career development were a focal point of HM17, particularly for younger physicians who could take advantage of the Early-Career Hospitalists mini-track. But Dr. Burger said that those strategies of upward mobility can apply whether someone is chasing their first job or their fifth.
First, identify one’s strengths and play to those. Then identify the skills you don’t have or don’t excel at, and address those deficiencies.
“How can you acquire the skills to put yourself in the best position to move up, if you wish to develop your career as a leader?” Dr. Burger said. “If you wish to be the best clinician, you still need to stay on top of the game. Things like coming to SHM, staying on top of the content. That’s important.”
Another skill set is self-advocacy.
“Be your own champion,” said Brian Markoff, MD, SFHM, chief of hospital medicine at Mount Sinai St. Luke’s in New York. “Many of us are very good at this and many of us are terrible at this. You may fall somewhere in between, but you do have to be your own champion.”
Dr. Burger said that he understands that there is a fine line between too much self-promotion and too little. But he urged hospitalists at all career points to take responsibility for marketing themselves.
“Nobody is going to invest in your career unless you yourself invest in it,” he added. “You have to put it as a priority, and not in a selfish way, but in a way [that,] if you wish to move forward and move up, you’ve got to put the time in. It’s not a natural assumption anymore that, if you are the best and brightest of a group of doctors, you will just be chosen to lead.”
In a similar vein, networking is a major boon to career development that can be a double-edged scalpel.
“Having a great ‘social game’ is important, but if all you bring to the table is a social game, you’ll find yourself out of a job just as quickly as you found that job,” Dr. Burger said. “Meaning, you might be able to get it based on that, but you’re not going to be able to sustain it. At the same time, being highly accomplished and having no social graces is also a killer. So, you need to be sort of strong in both areas.”
Many of the meeting’s opportunities for tips on professional development are personal, but HM group leaders have to consider developing the careers of their employees. One of the main planks of that is physician engagement, said Flora Kisuule, MD, MPH, SFHM, of Johns Hopkins University, Baltimore.
“I don’t believe your institution or your organization can go anywhere if your employees are not engaged or if the people you work with are not engaged,” she said, adding that disengaged employees “are actively working against you. You don’t want that. You can’t go in any direction when there are people rowing in the opposite direction. At best, you stay in one place. At worst, you can end up losing ground.”
Hospitalist Christie Masters, MD, MBA, MHA, who practices at UCLA, disclosed during a session that she also runs a wellness coaching firm. She added that a focus on personal wellness and well-being is its own form of career development. It works in tandem with engagement, morale, and professional growth.
“If you’re only focusing on wellness and you don’t have hospitalists or a group that’s engaged or with high morale, they’re going to burnout or they’re going to leave,” Dr. Masters said. “And nobody wants that for their group. So, if we surround ourselves with people who feel well and feel whole, that’s going to have intangible benefits ... that affect the bottom line.”
LAS VEGAS – In the view of academic hospitalist Alfred Burger, MD, SFHM, portability was long a dirty word in HM circles. But not anymore.
“My good friends in law and business do this all the time,” said Dr. Burger, associate program director of the internal medicine residency program at Mount Sinai Beth Israel in New York. “You’re not going to make partner in city X, but they’ve got an opening to be partner in city Y if you go there and perform for a year. People up and leave coasts, people up and leave states, people have up and left the country. ... Doctors are starting to view it the same way.”
The lessons of career development were a focal point of HM17, particularly for younger physicians who could take advantage of the Early-Career Hospitalists mini-track. But Dr. Burger said that those strategies of upward mobility can apply whether someone is chasing their first job or their fifth.
First, identify one’s strengths and play to those. Then identify the skills you don’t have or don’t excel at, and address those deficiencies.
“How can you acquire the skills to put yourself in the best position to move up, if you wish to develop your career as a leader?” Dr. Burger said. “If you wish to be the best clinician, you still need to stay on top of the game. Things like coming to SHM, staying on top of the content. That’s important.”
Another skill set is self-advocacy.
“Be your own champion,” said Brian Markoff, MD, SFHM, chief of hospital medicine at Mount Sinai St. Luke’s in New York. “Many of us are very good at this and many of us are terrible at this. You may fall somewhere in between, but you do have to be your own champion.”
Dr. Burger said that he understands that there is a fine line between too much self-promotion and too little. But he urged hospitalists at all career points to take responsibility for marketing themselves.
“Nobody is going to invest in your career unless you yourself invest in it,” he added. “You have to put it as a priority, and not in a selfish way, but in a way [that,] if you wish to move forward and move up, you’ve got to put the time in. It’s not a natural assumption anymore that, if you are the best and brightest of a group of doctors, you will just be chosen to lead.”
In a similar vein, networking is a major boon to career development that can be a double-edged scalpel.
“Having a great ‘social game’ is important, but if all you bring to the table is a social game, you’ll find yourself out of a job just as quickly as you found that job,” Dr. Burger said. “Meaning, you might be able to get it based on that, but you’re not going to be able to sustain it. At the same time, being highly accomplished and having no social graces is also a killer. So, you need to be sort of strong in both areas.”
Many of the meeting’s opportunities for tips on professional development are personal, but HM group leaders have to consider developing the careers of their employees. One of the main planks of that is physician engagement, said Flora Kisuule, MD, MPH, SFHM, of Johns Hopkins University, Baltimore.
“I don’t believe your institution or your organization can go anywhere if your employees are not engaged or if the people you work with are not engaged,” she said, adding that disengaged employees “are actively working against you. You don’t want that. You can’t go in any direction when there are people rowing in the opposite direction. At best, you stay in one place. At worst, you can end up losing ground.”
Hospitalist Christie Masters, MD, MBA, MHA, who practices at UCLA, disclosed during a session that she also runs a wellness coaching firm. She added that a focus on personal wellness and well-being is its own form of career development. It works in tandem with engagement, morale, and professional growth.
“If you’re only focusing on wellness and you don’t have hospitalists or a group that’s engaged or with high morale, they’re going to burnout or they’re going to leave,” Dr. Masters said. “And nobody wants that for their group. So, if we surround ourselves with people who feel well and feel whole, that’s going to have intangible benefits ... that affect the bottom line.”
Hospitalists’ EMR frustrations continue: SHM report
LAS VEGAS – Ronald Schaefer, MD, a hospitalist with Hawaii Pacific Health who also works on creating digital templates for his hospital, can’t input hemoglobin A1c levels from three different labs into his electronic medical records (EMR) system the same way.
Hospitalist George Dimitriou, MD, FHM, who splits his time at Allegheny Health Network in Pittsburgh between clinical work and medical informatics, worries there are so many fields in his EMR that physicians can get distracted.
Yevgeniy “Eugene” Gitelman, MD, a clinical informatics manager at the Perelman School of Medicine at University of Pennsylvania Health in Philadelphia, wonders how good any systems can be with the privacy concerns related to HIPAA.
This was the nexus of IT and HM17, a time when hospitalists said they are stymied and frustrated by continuing issues of interoperability, functionality, and access. The meeting highlighted new smartphone and tablet applications, as well as medical devices available to hospitalists, but tech-focused physicians say the biggest issue remains the day-to-day workings of EMR.
“If you build something really good, people will use it. If you build something that makes their documentation process a lot easier and a lot faster and a lot better, they’ll use it,” said Dr. Schaefer. “The tools aren’t there yet. I don’t think the technology is mature enough.”
If the tech hasn’t yet come of full age, the concerns surely have. SHM unveiled a white paper at HM17 that codified hospitalists’ worries about the current state of IT. The report, “Hospitalist Perspectives on Electronic Medical Records,” found that “a staggering” 85% of providers said they spend more time interacting with their inpatient EMR than their actual inpatients.
Rupesh Prasad, MD, MPH, SFHM, chair of SHM’s Health IT Committee, says the report is meant to foster discussion about the issues surrounding EMRs. The data points, generated from 462 respondents, are stark. Just 40% said they were happy with their EMR. Some 52% would change vendors if they could. One-quarter of respondents would revert to using paper if given the option.
“By sharing these results, we hope to raise awareness of the unacceptable performance of existing systems,” the report states. “This continues to contribute to our slower than desired improvement in quality and safety, as well as increasing provider frustration. We strongly believe that we need a renewed focus on initial goals of technology adoption in health care.”
Dr. Prasad said that he hopes hospitalists heed that call to action and use the report in discussions with various stakeholders, including vendors, public policy officials, and their own bosses.
“We want to give hospitalists ammunition to go back to their systems and talk to their administrators to see if they can influence [it],” he said.
Dr. Prasad is pleased that the society is sensitive to the issues surrounding technology. He encourages hospitalists to actively participate in HMX, SHM’s online portal to discuss health IT issues and crowd-source potential solutions. Patrick Vulgamore, MPH, SHM’s director of governance and practice management, said the society is formulating a potential special-interest working group to further seek to solve problems.
Hospitalists were also urged to apply for American Board of Medical Specialties (ABMS) certification in clinical informatics. Physicians can grandfather into eligibility via the “practice pathway” through the end of the year, if they’ve been working in informatics professionally for at least 25% of their time during any three of the previous five years. Next year, only graduates of two-year Accreditation Council for Graduate Medical Education–accredited fellowships will be board eligible.
“As end users of technology, we understand the problems better than anybody else,” Dr. Prasad said. “Obviously, the next step would be try to solve the problems. And what better way then to get involved and become experts in what you do?”
While much of the meeting’s tech talk was frustration, both former National Coordinator for Health IT Karen DeSalvo, MD, MPH, MSc, and HM Dean Robert Wachter, MD, MHM, forecast a future when artificial intelligence and intuitive computers work alongside physicians. Imagine the user-friendliness of Apple’s Siri or Google’s Alexa married to the existing functionalities provided by firms such as Epic or Cerner.
But that’s years away, and hospitalists like Dr. Dimitriou want help now.
“The speed of medicine, the speed of what’s happening in real time, is still faster than what our electronic tools seem to be able to keep up with,” he said. “There are encouraging signs that we’ve definitely moved in the right direction. We’ve come a long way ... but again, the speed at which things are moving? We aren’t keeping up. We’ve got to do more.”
LAS VEGAS – Ronald Schaefer, MD, a hospitalist with Hawaii Pacific Health who also works on creating digital templates for his hospital, can’t input hemoglobin A1c levels from three different labs into his electronic medical records (EMR) system the same way.
Hospitalist George Dimitriou, MD, FHM, who splits his time at Allegheny Health Network in Pittsburgh between clinical work and medical informatics, worries there are so many fields in his EMR that physicians can get distracted.
Yevgeniy “Eugene” Gitelman, MD, a clinical informatics manager at the Perelman School of Medicine at University of Pennsylvania Health in Philadelphia, wonders how good any systems can be with the privacy concerns related to HIPAA.
This was the nexus of IT and HM17, a time when hospitalists said they are stymied and frustrated by continuing issues of interoperability, functionality, and access. The meeting highlighted new smartphone and tablet applications, as well as medical devices available to hospitalists, but tech-focused physicians say the biggest issue remains the day-to-day workings of EMR.
“If you build something really good, people will use it. If you build something that makes their documentation process a lot easier and a lot faster and a lot better, they’ll use it,” said Dr. Schaefer. “The tools aren’t there yet. I don’t think the technology is mature enough.”
If the tech hasn’t yet come of full age, the concerns surely have. SHM unveiled a white paper at HM17 that codified hospitalists’ worries about the current state of IT. The report, “Hospitalist Perspectives on Electronic Medical Records,” found that “a staggering” 85% of providers said they spend more time interacting with their inpatient EMR than their actual inpatients.
Rupesh Prasad, MD, MPH, SFHM, chair of SHM’s Health IT Committee, says the report is meant to foster discussion about the issues surrounding EMRs. The data points, generated from 462 respondents, are stark. Just 40% said they were happy with their EMR. Some 52% would change vendors if they could. One-quarter of respondents would revert to using paper if given the option.
“By sharing these results, we hope to raise awareness of the unacceptable performance of existing systems,” the report states. “This continues to contribute to our slower than desired improvement in quality and safety, as well as increasing provider frustration. We strongly believe that we need a renewed focus on initial goals of technology adoption in health care.”
Dr. Prasad said that he hopes hospitalists heed that call to action and use the report in discussions with various stakeholders, including vendors, public policy officials, and their own bosses.
“We want to give hospitalists ammunition to go back to their systems and talk to their administrators to see if they can influence [it],” he said.
Dr. Prasad is pleased that the society is sensitive to the issues surrounding technology. He encourages hospitalists to actively participate in HMX, SHM’s online portal to discuss health IT issues and crowd-source potential solutions. Patrick Vulgamore, MPH, SHM’s director of governance and practice management, said the society is formulating a potential special-interest working group to further seek to solve problems.
Hospitalists were also urged to apply for American Board of Medical Specialties (ABMS) certification in clinical informatics. Physicians can grandfather into eligibility via the “practice pathway” through the end of the year, if they’ve been working in informatics professionally for at least 25% of their time during any three of the previous five years. Next year, only graduates of two-year Accreditation Council for Graduate Medical Education–accredited fellowships will be board eligible.
“As end users of technology, we understand the problems better than anybody else,” Dr. Prasad said. “Obviously, the next step would be try to solve the problems. And what better way then to get involved and become experts in what you do?”
While much of the meeting’s tech talk was frustration, both former National Coordinator for Health IT Karen DeSalvo, MD, MPH, MSc, and HM Dean Robert Wachter, MD, MHM, forecast a future when artificial intelligence and intuitive computers work alongside physicians. Imagine the user-friendliness of Apple’s Siri or Google’s Alexa married to the existing functionalities provided by firms such as Epic or Cerner.
But that’s years away, and hospitalists like Dr. Dimitriou want help now.
“The speed of medicine, the speed of what’s happening in real time, is still faster than what our electronic tools seem to be able to keep up with,” he said. “There are encouraging signs that we’ve definitely moved in the right direction. We’ve come a long way ... but again, the speed at which things are moving? We aren’t keeping up. We’ve got to do more.”
LAS VEGAS – Ronald Schaefer, MD, a hospitalist with Hawaii Pacific Health who also works on creating digital templates for his hospital, can’t input hemoglobin A1c levels from three different labs into his electronic medical records (EMR) system the same way.
Hospitalist George Dimitriou, MD, FHM, who splits his time at Allegheny Health Network in Pittsburgh between clinical work and medical informatics, worries there are so many fields in his EMR that physicians can get distracted.
Yevgeniy “Eugene” Gitelman, MD, a clinical informatics manager at the Perelman School of Medicine at University of Pennsylvania Health in Philadelphia, wonders how good any systems can be with the privacy concerns related to HIPAA.
This was the nexus of IT and HM17, a time when hospitalists said they are stymied and frustrated by continuing issues of interoperability, functionality, and access. The meeting highlighted new smartphone and tablet applications, as well as medical devices available to hospitalists, but tech-focused physicians say the biggest issue remains the day-to-day workings of EMR.
“If you build something really good, people will use it. If you build something that makes their documentation process a lot easier and a lot faster and a lot better, they’ll use it,” said Dr. Schaefer. “The tools aren’t there yet. I don’t think the technology is mature enough.”
If the tech hasn’t yet come of full age, the concerns surely have. SHM unveiled a white paper at HM17 that codified hospitalists’ worries about the current state of IT. The report, “Hospitalist Perspectives on Electronic Medical Records,” found that “a staggering” 85% of providers said they spend more time interacting with their inpatient EMR than their actual inpatients.
Rupesh Prasad, MD, MPH, SFHM, chair of SHM’s Health IT Committee, says the report is meant to foster discussion about the issues surrounding EMRs. The data points, generated from 462 respondents, are stark. Just 40% said they were happy with their EMR. Some 52% would change vendors if they could. One-quarter of respondents would revert to using paper if given the option.
“By sharing these results, we hope to raise awareness of the unacceptable performance of existing systems,” the report states. “This continues to contribute to our slower than desired improvement in quality and safety, as well as increasing provider frustration. We strongly believe that we need a renewed focus on initial goals of technology adoption in health care.”
Dr. Prasad said that he hopes hospitalists heed that call to action and use the report in discussions with various stakeholders, including vendors, public policy officials, and their own bosses.
“We want to give hospitalists ammunition to go back to their systems and talk to their administrators to see if they can influence [it],” he said.
Dr. Prasad is pleased that the society is sensitive to the issues surrounding technology. He encourages hospitalists to actively participate in HMX, SHM’s online portal to discuss health IT issues and crowd-source potential solutions. Patrick Vulgamore, MPH, SHM’s director of governance and practice management, said the society is formulating a potential special-interest working group to further seek to solve problems.
Hospitalists were also urged to apply for American Board of Medical Specialties (ABMS) certification in clinical informatics. Physicians can grandfather into eligibility via the “practice pathway” through the end of the year, if they’ve been working in informatics professionally for at least 25% of their time during any three of the previous five years. Next year, only graduates of two-year Accreditation Council for Graduate Medical Education–accredited fellowships will be board eligible.
“As end users of technology, we understand the problems better than anybody else,” Dr. Prasad said. “Obviously, the next step would be try to solve the problems. And what better way then to get involved and become experts in what you do?”
While much of the meeting’s tech talk was frustration, both former National Coordinator for Health IT Karen DeSalvo, MD, MPH, MSc, and HM Dean Robert Wachter, MD, MHM, forecast a future when artificial intelligence and intuitive computers work alongside physicians. Imagine the user-friendliness of Apple’s Siri or Google’s Alexa married to the existing functionalities provided by firms such as Epic or Cerner.
But that’s years away, and hospitalists like Dr. Dimitriou want help now.
“The speed of medicine, the speed of what’s happening in real time, is still faster than what our electronic tools seem to be able to keep up with,” he said. “There are encouraging signs that we’ve definitely moved in the right direction. We’ve come a long way ... but again, the speed at which things are moving? We aren’t keeping up. We’ve got to do more.”
UTI predictors identified in infants under 3 months of age
SAN FRANCISCO – Serum leukocytosis, pyuria, and urine leukocyte esterase have been identified as predictors of urinary tract infection (UTI) in infants younger than 90 days of age, with males being more likely to develop UTI than females.
The chance of the infection declined in older infants.
“Really young infants have a less well developed immune system, which may increase their susceptibility to UTIs,” said Sarah Berman, DO, a pediatric resident at Winthrop-University Hospital, Mineola, N.Y.
In 2011, the American Academy of Pediatrics issued “Urinary Tract Infection: Clinical Practice Guidelines for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months” (Pediatrics. 2011. doi: 10.1542/peds.2011-1330) concerning the diagnosis and management of the presence of bacteria in urine – a possible warning sign of UTI, but the AAP guidelines did not consider infants in the first 2 months of life.
To address this gap, the Winthrop-University Hospital researchers reviewed the medical records for all infants younger than 90 days of age diagnosed with UTI, fever, or viral illness from the beginning of 2009 to September 2015. Residence in neonatal intensive care, known congenital abnormalities, and prior antibiotic use were grounds for exclusion. Standard growth-based definitions of UTI and bacteriuria were used.
Of the 315 mainly white or Hispanic patients, 73 had a diagnosis of UTI and 261 did not. Both groups had the same mean age of 45 days. Fever within 24 hours of admission was more prevalent in those without UTI than those with (57% vs. 41%; P = .035). Those with UTI were significantly more likely (all P lesser than .001) to display serum leukocytosis (35% vs. 12%), pyuria (71% vs. 12%), and urine detection of leukocyte esterase (87% vs. 14%) and nitrite (19% vs. 0%).
In a univariate analysis, factors that were associated with UTI included serum leukocytosis, white blood cells in the urine, and urine leukocyte esterase, with fever within 24 hours of admission associated with reduced chance of UTI. A multivariate analysis that accounted for age, gender, and gestational age identified serum leukocytosis, pyruria, urine leukocyte esterase, and male sex as predictors of UTI, with increased odds ratio of 6.25, 3.19, 28, and 3.88, respectively.
The reduced likelihood of UTI in those who developed a fever within 24 hours of admission held up in the multivariate analysis (odds ratio 0.34).
Validation of the findings will require a prospective study, which is in the planning stage. If the findings bear out, the result could be improved diagnosis of UTI from birth onward, according to Dr. Berman.
Dr. Berman reported having no relevant financial disclosures.
SAN FRANCISCO – Serum leukocytosis, pyuria, and urine leukocyte esterase have been identified as predictors of urinary tract infection (UTI) in infants younger than 90 days of age, with males being more likely to develop UTI than females.
The chance of the infection declined in older infants.
“Really young infants have a less well developed immune system, which may increase their susceptibility to UTIs,” said Sarah Berman, DO, a pediatric resident at Winthrop-University Hospital, Mineola, N.Y.
In 2011, the American Academy of Pediatrics issued “Urinary Tract Infection: Clinical Practice Guidelines for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months” (Pediatrics. 2011. doi: 10.1542/peds.2011-1330) concerning the diagnosis and management of the presence of bacteria in urine – a possible warning sign of UTI, but the AAP guidelines did not consider infants in the first 2 months of life.
To address this gap, the Winthrop-University Hospital researchers reviewed the medical records for all infants younger than 90 days of age diagnosed with UTI, fever, or viral illness from the beginning of 2009 to September 2015. Residence in neonatal intensive care, known congenital abnormalities, and prior antibiotic use were grounds for exclusion. Standard growth-based definitions of UTI and bacteriuria were used.
Of the 315 mainly white or Hispanic patients, 73 had a diagnosis of UTI and 261 did not. Both groups had the same mean age of 45 days. Fever within 24 hours of admission was more prevalent in those without UTI than those with (57% vs. 41%; P = .035). Those with UTI were significantly more likely (all P lesser than .001) to display serum leukocytosis (35% vs. 12%), pyuria (71% vs. 12%), and urine detection of leukocyte esterase (87% vs. 14%) and nitrite (19% vs. 0%).
In a univariate analysis, factors that were associated with UTI included serum leukocytosis, white blood cells in the urine, and urine leukocyte esterase, with fever within 24 hours of admission associated with reduced chance of UTI. A multivariate analysis that accounted for age, gender, and gestational age identified serum leukocytosis, pyruria, urine leukocyte esterase, and male sex as predictors of UTI, with increased odds ratio of 6.25, 3.19, 28, and 3.88, respectively.
The reduced likelihood of UTI in those who developed a fever within 24 hours of admission held up in the multivariate analysis (odds ratio 0.34).
Validation of the findings will require a prospective study, which is in the planning stage. If the findings bear out, the result could be improved diagnosis of UTI from birth onward, according to Dr. Berman.
Dr. Berman reported having no relevant financial disclosures.
SAN FRANCISCO – Serum leukocytosis, pyuria, and urine leukocyte esterase have been identified as predictors of urinary tract infection (UTI) in infants younger than 90 days of age, with males being more likely to develop UTI than females.
The chance of the infection declined in older infants.
“Really young infants have a less well developed immune system, which may increase their susceptibility to UTIs,” said Sarah Berman, DO, a pediatric resident at Winthrop-University Hospital, Mineola, N.Y.
In 2011, the American Academy of Pediatrics issued “Urinary Tract Infection: Clinical Practice Guidelines for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months” (Pediatrics. 2011. doi: 10.1542/peds.2011-1330) concerning the diagnosis and management of the presence of bacteria in urine – a possible warning sign of UTI, but the AAP guidelines did not consider infants in the first 2 months of life.
To address this gap, the Winthrop-University Hospital researchers reviewed the medical records for all infants younger than 90 days of age diagnosed with UTI, fever, or viral illness from the beginning of 2009 to September 2015. Residence in neonatal intensive care, known congenital abnormalities, and prior antibiotic use were grounds for exclusion. Standard growth-based definitions of UTI and bacteriuria were used.
Of the 315 mainly white or Hispanic patients, 73 had a diagnosis of UTI and 261 did not. Both groups had the same mean age of 45 days. Fever within 24 hours of admission was more prevalent in those without UTI than those with (57% vs. 41%; P = .035). Those with UTI were significantly more likely (all P lesser than .001) to display serum leukocytosis (35% vs. 12%), pyuria (71% vs. 12%), and urine detection of leukocyte esterase (87% vs. 14%) and nitrite (19% vs. 0%).
In a univariate analysis, factors that were associated with UTI included serum leukocytosis, white blood cells in the urine, and urine leukocyte esterase, with fever within 24 hours of admission associated with reduced chance of UTI. A multivariate analysis that accounted for age, gender, and gestational age identified serum leukocytosis, pyruria, urine leukocyte esterase, and male sex as predictors of UTI, with increased odds ratio of 6.25, 3.19, 28, and 3.88, respectively.
The reduced likelihood of UTI in those who developed a fever within 24 hours of admission held up in the multivariate analysis (odds ratio 0.34).
Validation of the findings will require a prospective study, which is in the planning stage. If the findings bear out, the result could be improved diagnosis of UTI from birth onward, according to Dr. Berman.
Dr. Berman reported having no relevant financial disclosures.
AT PAS 17
Key clinical point: Pyuria, urine leukocyte esterase, and serum leukocytosis appear to be predictors of urinary tract infection in infants younger than 90 days of age.
Major finding: A multivariate analysis identified serum leukocytosis, pyruria, and urine leukocyte esterase as predictors of UTI, with increased odds ratio of 6.25, 3.19, and 28, respectively.
Data source: Retrospective analysis of medical records from one hospital.
Disclosures: Dr. Berman reported having no relevant financial disclosures.
Unilateral Verrucous Porokeratosis of the Gluteal Cleft
To the Editor:
Verrucous porokeratosis of the gluteal cleft is a rare skin condition that has distinct clinical and histologic features. A review of 5 cases described a characteristic clinical presentation of a butterfly-shaped bilateral gluteal cleft lesion on most patients.1 We present an unusual case of verrucous porokeratosis presenting as a unilateral single lesion in the gluteal area that emulated seborrheic keratosis with histology consistent with verrucous porokeratosis. This case adds to the variable presentation of this unusual disease.
A 40-year-old man who presented to the dermatology clinic for a follow-up on a basal cell carcinoma of the temple region was concerned about a lesion on the left buttock of 1 year’s duration. Physical examination revealed a unilateral hyperkeratotic plaque that clinically resembled seborrheic keratosis (Figure 1). Biopsy revealed hyperkeratosis with numerous columns of parakeratosis, psoriasiform epidermal hyperplasia (Figures 2A and 2B), dyskeratotic keratinocytes (Figure 2C), pigment incontinence, and mild superficial chronic inflammation consistent with verrucous porokeratosis. The patient was treated with urea lotion but ultimately was lost to follow-up.
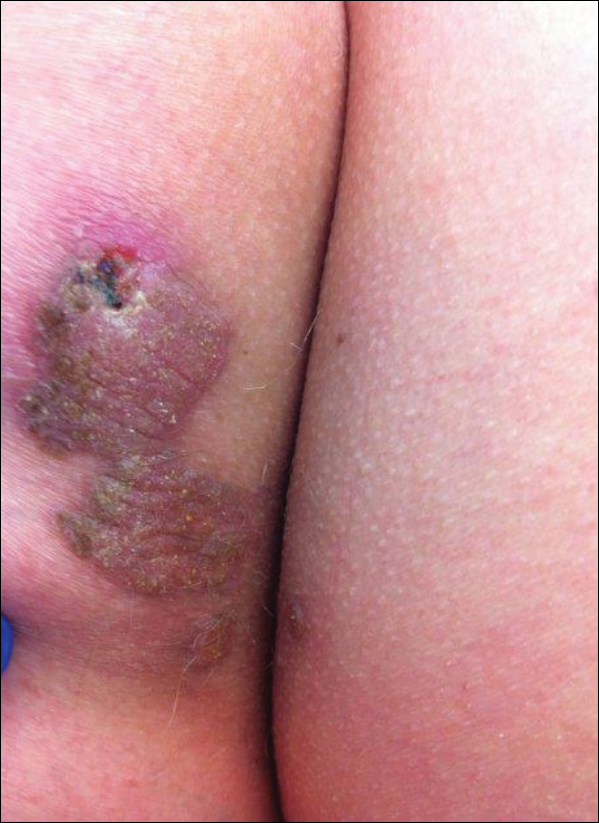
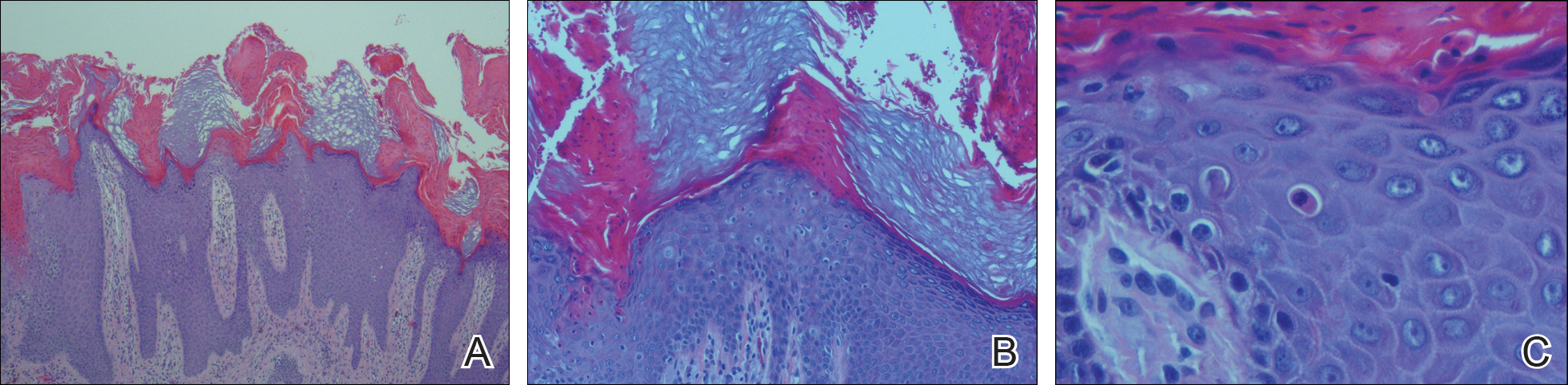
We present a unique case of unilateral verrucous porokeratosis of the gluteal cleft. The clinical differential diagnosis included seborrheic keratosis, condyloma acuminata, and inflammatory linear verrucous epidermal nevus. Histopathology was consistent with verrucous porokeratosis. Porokeratosis is a heterogeneous group of keratinization disorders containing several described variants such as classici porokeratosis of Mibelli, disseminated superficial porokeratosis, porokeratosis palmaris et plantaris disseminata, linear porokeratosis, and punctuate porokeratosis.1,2 Most patients present clinically with plaquelike bilateral (butterfly) lesions with threadlike (ridge) borders, though some patients initially have a unilateral lesion that subsequently develops into a bilateral lesion.1 The clinical course is slow growing, but it can potentially give rise to malignancies such as squamous cell carcinoma.3 Histologically, numerous columns of parakeratosis overlying epidermal cells with attenuated granular layer are observed with the concentric cornoid lamellae considered unique to the verrucous variant.1 Although our patient had only a single unilateral lesion on the gluteal cleft, the histology was consistent with verrucous porokeratosis. Our case adds to the growing clinical presentations of this unusual disease.
- Takiguchi R, White K, Clifton W, et al. Verrucous porokeratosis of the gluteal cleft (porokeratosis stychotropica): a rare disorder easily misdiagnosed. J Cutan Pathol. 2010;37:802-807.
- McGuigan K, Shurman D, Campanelli C, et al. Porokeratosis ptychotropica: a clinically distinct variant of porokeratosis. J Am Acad Dermatol. 2009;60:501-503.
- Malek J, Chedraoui A, Kibbi AG, et al. Genitogluteal porokeratosis: 10 years to make the diagnosis! Am J Dermatopathol. 2009;31:604-606.
To the Editor:
Verrucous porokeratosis of the gluteal cleft is a rare skin condition that has distinct clinical and histologic features. A review of 5 cases described a characteristic clinical presentation of a butterfly-shaped bilateral gluteal cleft lesion on most patients.1 We present an unusual case of verrucous porokeratosis presenting as a unilateral single lesion in the gluteal area that emulated seborrheic keratosis with histology consistent with verrucous porokeratosis. This case adds to the variable presentation of this unusual disease.
A 40-year-old man who presented to the dermatology clinic for a follow-up on a basal cell carcinoma of the temple region was concerned about a lesion on the left buttock of 1 year’s duration. Physical examination revealed a unilateral hyperkeratotic plaque that clinically resembled seborrheic keratosis (Figure 1). Biopsy revealed hyperkeratosis with numerous columns of parakeratosis, psoriasiform epidermal hyperplasia (Figures 2A and 2B), dyskeratotic keratinocytes (Figure 2C), pigment incontinence, and mild superficial chronic inflammation consistent with verrucous porokeratosis. The patient was treated with urea lotion but ultimately was lost to follow-up.


We present a unique case of unilateral verrucous porokeratosis of the gluteal cleft. The clinical differential diagnosis included seborrheic keratosis, condyloma acuminata, and inflammatory linear verrucous epidermal nevus. Histopathology was consistent with verrucous porokeratosis. Porokeratosis is a heterogeneous group of keratinization disorders containing several described variants such as classici porokeratosis of Mibelli, disseminated superficial porokeratosis, porokeratosis palmaris et plantaris disseminata, linear porokeratosis, and punctuate porokeratosis.1,2 Most patients present clinically with plaquelike bilateral (butterfly) lesions with threadlike (ridge) borders, though some patients initially have a unilateral lesion that subsequently develops into a bilateral lesion.1 The clinical course is slow growing, but it can potentially give rise to malignancies such as squamous cell carcinoma.3 Histologically, numerous columns of parakeratosis overlying epidermal cells with attenuated granular layer are observed with the concentric cornoid lamellae considered unique to the verrucous variant.1 Although our patient had only a single unilateral lesion on the gluteal cleft, the histology was consistent with verrucous porokeratosis. Our case adds to the growing clinical presentations of this unusual disease.
To the Editor:
Verrucous porokeratosis of the gluteal cleft is a rare skin condition that has distinct clinical and histologic features. A review of 5 cases described a characteristic clinical presentation of a butterfly-shaped bilateral gluteal cleft lesion on most patients.1 We present an unusual case of verrucous porokeratosis presenting as a unilateral single lesion in the gluteal area that emulated seborrheic keratosis with histology consistent with verrucous porokeratosis. This case adds to the variable presentation of this unusual disease.
A 40-year-old man who presented to the dermatology clinic for a follow-up on a basal cell carcinoma of the temple region was concerned about a lesion on the left buttock of 1 year’s duration. Physical examination revealed a unilateral hyperkeratotic plaque that clinically resembled seborrheic keratosis (Figure 1). Biopsy revealed hyperkeratosis with numerous columns of parakeratosis, psoriasiform epidermal hyperplasia (Figures 2A and 2B), dyskeratotic keratinocytes (Figure 2C), pigment incontinence, and mild superficial chronic inflammation consistent with verrucous porokeratosis. The patient was treated with urea lotion but ultimately was lost to follow-up.


We present a unique case of unilateral verrucous porokeratosis of the gluteal cleft. The clinical differential diagnosis included seborrheic keratosis, condyloma acuminata, and inflammatory linear verrucous epidermal nevus. Histopathology was consistent with verrucous porokeratosis. Porokeratosis is a heterogeneous group of keratinization disorders containing several described variants such as classici porokeratosis of Mibelli, disseminated superficial porokeratosis, porokeratosis palmaris et plantaris disseminata, linear porokeratosis, and punctuate porokeratosis.1,2 Most patients present clinically with plaquelike bilateral (butterfly) lesions with threadlike (ridge) borders, though some patients initially have a unilateral lesion that subsequently develops into a bilateral lesion.1 The clinical course is slow growing, but it can potentially give rise to malignancies such as squamous cell carcinoma.3 Histologically, numerous columns of parakeratosis overlying epidermal cells with attenuated granular layer are observed with the concentric cornoid lamellae considered unique to the verrucous variant.1 Although our patient had only a single unilateral lesion on the gluteal cleft, the histology was consistent with verrucous porokeratosis. Our case adds to the growing clinical presentations of this unusual disease.
- Takiguchi R, White K, Clifton W, et al. Verrucous porokeratosis of the gluteal cleft (porokeratosis stychotropica): a rare disorder easily misdiagnosed. J Cutan Pathol. 2010;37:802-807.
- McGuigan K, Shurman D, Campanelli C, et al. Porokeratosis ptychotropica: a clinically distinct variant of porokeratosis. J Am Acad Dermatol. 2009;60:501-503.
- Malek J, Chedraoui A, Kibbi AG, et al. Genitogluteal porokeratosis: 10 years to make the diagnosis! Am J Dermatopathol. 2009;31:604-606.
- Takiguchi R, White K, Clifton W, et al. Verrucous porokeratosis of the gluteal cleft (porokeratosis stychotropica): a rare disorder easily misdiagnosed. J Cutan Pathol. 2010;37:802-807.
- McGuigan K, Shurman D, Campanelli C, et al. Porokeratosis ptychotropica: a clinically distinct variant of porokeratosis. J Am Acad Dermatol. 2009;60:501-503.
- Malek J, Chedraoui A, Kibbi AG, et al. Genitogluteal porokeratosis: 10 years to make the diagnosis! Am J Dermatopathol. 2009;31:604-606.
Practice Point
- Porokeratosis of the gluteal cleft typically is bilateral but may be unilateral.
Is Your Practice Biosimilar Ready?
Click Here to Read the Full Supplement.
Topics include:
- The Pathway for Biosimilar Approval
- The Difference between Biosimilars and Generics
- Draft Interchangeablity Guidelines
- ACR Position Statement on Biosimilars
- Effect of the Reimbursement Model on Biosimilars
Click Here to Read the Full Supplement.
Topics include:
- The Pathway for Biosimilar Approval
- The Difference between Biosimilars and Generics
- Draft Interchangeablity Guidelines
- ACR Position Statement on Biosimilars
- Effect of the Reimbursement Model on Biosimilars
Click Here to Read the Full Supplement.
Topics include:
- The Pathway for Biosimilar Approval
- The Difference between Biosimilars and Generics
- Draft Interchangeablity Guidelines
- ACR Position Statement on Biosimilars
- Effect of the Reimbursement Model on Biosimilars