User login
VIDEO: Immunotherapy ups disease control rate in relapsed mesothelioma
CHICAGO – Early data from a phase II trial of immune checkpoint inhibitors to treat relapsed mesothelioma give hope that immunotherapy may be an effective therapeutic option for the rapidly progressive, currently incurable cancer.
Reporting on 12 weeks of data from the randomized multicenter trial, Arnaud Scherpereel, MD, the study’s first author, said in a video interview, “We were very pleased to see that we were able to increase ... the disease control rate to 44% with nivolumab, and 50% with nivolumab plus ipilimumab. This was translated into a overall survival gain for these patients.” The best previous disease control rate seen with other therapies was less than 30%, said Dr. Scherpereel at the annual meeting of the American Society of Clinical Oncology.
Discussing the early results in a video interview, Dr. Scherpereel, head of the pulmonary and thoracic oncology department at the University Hospital of Lille, France noted that the median overall survival for the nivolumab patients was 10.4 months, and has not yet been reached for the nivolumab plus ipilimumab patients. Further, he said in a press briefing, “Tumors shrunk in 18% of patients treated with nivolumab and 26% of those treated with nivolumab plus ipilimumab.”
The French MAPS-2 study has enrolled 125 adult patients with malignant pleural mesothelioma who had measurable disease progression after one or two prior lines of chemotherapy, including pemetrexed/platinum doublet. Patients were randomized 1:1 to receive either nivolumab or nivolumab plus ipilimumab, until disease control or unacceptable toxicity was reached, for a maximum of 2 years. Patients were mostly (80%) male, with a median age of 71.8 years, and most had the epithelioid malignant pleural mesothelioma subtype.
In commentary at the press briefing announcing the findings, ASCO expert Michael Sabel, MD, said, “I need to emphasize that this is amazing, in that we are seeing [the use of] checkpoint inhibitors expanding beyond melanoma, to other cancers that we thought were not amenable to immunotherapy approaches.”
“This is a great example of how basic cancer research in one field can expand across others,” said Dr. Sabel of the departments of surgery and surgical oncology at the University of Michigan, Ann Arbor.
Most side effects were not severe, but there were three potentially drug-related deaths in the nivolumab-ipilimumab combo arm: one patient died of fulminant hepatitis, one from metabolic encephalitis, and one from acute renal failure. “There is no identified factor that is predictive” in terms of which patients will have the more significant known adverse effects of checkpoint inhibitors, said Dr. Scherpereel. Patients, caregivers, and health care professionals all need to be alert to the possibility of adverse events and act promptly if concerning symptoms crop up, he said.
Dr. Scherpereel said that though his study group has not yet reported the quality of life findings from MAPS-2, he sees that his patients who are study participants are doing better. “In my patients, they have a very good tolerance to this treatment compared to chemotherapy. They have less dyspnea, less chest pain. Clearly, we hope to get these drugs into the routine very quickly for them.”
Bristol-Myers Squibb manufactures both nivolumab and ipilimumab and provided the study drugs. Dr. Sabel disclosed a financial relationship with Merck. Dr. Scherpereel has no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
CHICAGO – Early data from a phase II trial of immune checkpoint inhibitors to treat relapsed mesothelioma give hope that immunotherapy may be an effective therapeutic option for the rapidly progressive, currently incurable cancer.
Reporting on 12 weeks of data from the randomized multicenter trial, Arnaud Scherpereel, MD, the study’s first author, said in a video interview, “We were very pleased to see that we were able to increase ... the disease control rate to 44% with nivolumab, and 50% with nivolumab plus ipilimumab. This was translated into a overall survival gain for these patients.” The best previous disease control rate seen with other therapies was less than 30%, said Dr. Scherpereel at the annual meeting of the American Society of Clinical Oncology.
Discussing the early results in a video interview, Dr. Scherpereel, head of the pulmonary and thoracic oncology department at the University Hospital of Lille, France noted that the median overall survival for the nivolumab patients was 10.4 months, and has not yet been reached for the nivolumab plus ipilimumab patients. Further, he said in a press briefing, “Tumors shrunk in 18% of patients treated with nivolumab and 26% of those treated with nivolumab plus ipilimumab.”
The French MAPS-2 study has enrolled 125 adult patients with malignant pleural mesothelioma who had measurable disease progression after one or two prior lines of chemotherapy, including pemetrexed/platinum doublet. Patients were randomized 1:1 to receive either nivolumab or nivolumab plus ipilimumab, until disease control or unacceptable toxicity was reached, for a maximum of 2 years. Patients were mostly (80%) male, with a median age of 71.8 years, and most had the epithelioid malignant pleural mesothelioma subtype.
In commentary at the press briefing announcing the findings, ASCO expert Michael Sabel, MD, said, “I need to emphasize that this is amazing, in that we are seeing [the use of] checkpoint inhibitors expanding beyond melanoma, to other cancers that we thought were not amenable to immunotherapy approaches.”
“This is a great example of how basic cancer research in one field can expand across others,” said Dr. Sabel of the departments of surgery and surgical oncology at the University of Michigan, Ann Arbor.
Most side effects were not severe, but there were three potentially drug-related deaths in the nivolumab-ipilimumab combo arm: one patient died of fulminant hepatitis, one from metabolic encephalitis, and one from acute renal failure. “There is no identified factor that is predictive” in terms of which patients will have the more significant known adverse effects of checkpoint inhibitors, said Dr. Scherpereel. Patients, caregivers, and health care professionals all need to be alert to the possibility of adverse events and act promptly if concerning symptoms crop up, he said.
Dr. Scherpereel said that though his study group has not yet reported the quality of life findings from MAPS-2, he sees that his patients who are study participants are doing better. “In my patients, they have a very good tolerance to this treatment compared to chemotherapy. They have less dyspnea, less chest pain. Clearly, we hope to get these drugs into the routine very quickly for them.”
Bristol-Myers Squibb manufactures both nivolumab and ipilimumab and provided the study drugs. Dr. Sabel disclosed a financial relationship with Merck. Dr. Scherpereel has no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
CHICAGO – Early data from a phase II trial of immune checkpoint inhibitors to treat relapsed mesothelioma give hope that immunotherapy may be an effective therapeutic option for the rapidly progressive, currently incurable cancer.
Reporting on 12 weeks of data from the randomized multicenter trial, Arnaud Scherpereel, MD, the study’s first author, said in a video interview, “We were very pleased to see that we were able to increase ... the disease control rate to 44% with nivolumab, and 50% with nivolumab plus ipilimumab. This was translated into a overall survival gain for these patients.” The best previous disease control rate seen with other therapies was less than 30%, said Dr. Scherpereel at the annual meeting of the American Society of Clinical Oncology.
Discussing the early results in a video interview, Dr. Scherpereel, head of the pulmonary and thoracic oncology department at the University Hospital of Lille, France noted that the median overall survival for the nivolumab patients was 10.4 months, and has not yet been reached for the nivolumab plus ipilimumab patients. Further, he said in a press briefing, “Tumors shrunk in 18% of patients treated with nivolumab and 26% of those treated with nivolumab plus ipilimumab.”
The French MAPS-2 study has enrolled 125 adult patients with malignant pleural mesothelioma who had measurable disease progression after one or two prior lines of chemotherapy, including pemetrexed/platinum doublet. Patients were randomized 1:1 to receive either nivolumab or nivolumab plus ipilimumab, until disease control or unacceptable toxicity was reached, for a maximum of 2 years. Patients were mostly (80%) male, with a median age of 71.8 years, and most had the epithelioid malignant pleural mesothelioma subtype.
In commentary at the press briefing announcing the findings, ASCO expert Michael Sabel, MD, said, “I need to emphasize that this is amazing, in that we are seeing [the use of] checkpoint inhibitors expanding beyond melanoma, to other cancers that we thought were not amenable to immunotherapy approaches.”
“This is a great example of how basic cancer research in one field can expand across others,” said Dr. Sabel of the departments of surgery and surgical oncology at the University of Michigan, Ann Arbor.
Most side effects were not severe, but there were three potentially drug-related deaths in the nivolumab-ipilimumab combo arm: one patient died of fulminant hepatitis, one from metabolic encephalitis, and one from acute renal failure. “There is no identified factor that is predictive” in terms of which patients will have the more significant known adverse effects of checkpoint inhibitors, said Dr. Scherpereel. Patients, caregivers, and health care professionals all need to be alert to the possibility of adverse events and act promptly if concerning symptoms crop up, he said.
Dr. Scherpereel said that though his study group has not yet reported the quality of life findings from MAPS-2, he sees that his patients who are study participants are doing better. “In my patients, they have a very good tolerance to this treatment compared to chemotherapy. They have less dyspnea, less chest pain. Clearly, we hope to get these drugs into the routine very quickly for them.”
Bristol-Myers Squibb manufactures both nivolumab and ipilimumab and provided the study drugs. Dr. Sabel disclosed a financial relationship with Merck. Dr. Scherpereel has no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
AT ASCO 2017
Depressed, acutely ill elders benefit from short-term CBT
Short-term cognitive-behavioral therapy is an effective intervention for geriatric patients with comorbid acute illness and depression, judging from the findings of a trial of 155 patients.
“The results presented here confirm the interaction of depression with cognitive and functional performance in this vulnerable study population,” wrote Jana Hummel, MD. “However, the extent of the effect on almost all levels of health was unexpected.”
CBT previously had not been used as a treatment for older patients with acute physical illness and comorbid depression, according to Dr. Hummel and her associates (JAMDA. 2017;18:341-9).
The authors recruited patients aged 82 years, plus or minus 6 years, from a 170-bed hospital that served as the Center for Geriatric Medicine at the University of Heidelberg, Germany. Patients who had been admitted to the hospital had a Hospital Anxiety and Depression Scale (HADS) score of greater than 7. Those with dementia or a life expectancy of less than 1 year were excluded from the study, reported Dr. Hummel of the Geriatric and Gerontopsychotherapeutic Practice in Mannheim, Germany, and her associates.
After the patients were discharged, the clinicians began the active intervention. The investigators randomized 56 people into the CBT intervention, a 15-session, manualized program designed for elderly patients who were based at home. The program included several group sessions held at the hospital’s day clinic and two individual sessions conducted by psychotherapists with expertise in gerontology.
The control group was made up of 99 people. Patients in both groups received antidepressants and other medication, the authors reported in the Journal of the American Medical Directors Association.
Four months after discharge, the patients’ severity of depression as measured by HADS scores was significantly lower among the patients in the psychotherapy group than in the usual care group (1.56 plus or minus 1.4, compared with 3.13 plus or minus 1; P less than .001). Likewise, patients in the psychotherapy group also scored lower on the Hamilton Rating Scale for Depression than did those in the control group (10.57 plus or minus 6.4, compared with 21.47 plus or minus 6.9; P less than .001).
In order for CBT to be effective for this population, arrangements might need to be made to transport patients to intervention sites, according to Dr. Hummel and her associates. Alternatively, the treatment could be administered in nursing homes. Ultimately, the authors said, “the interventions have to be tailored to the individual needs, severity of depression, and health situation.”
One limitation of the study is that the cognitive function of some elderly patients could prevent them from benefiting from CBT. However, Dr. Hummel and her associates said they are optimistic about such interventions for other geriatric patients.
“CBT provides psychological benefit to older patients with depressive symptoms, and it also can reverse some of the functional and cognitive decline associated with depression, they wrote. “It may prove to be an important tool in the treatment of depression in old age and multimorbidity.”
The study was funded with grants from the Robert Bosch Foundation and the Dietmar Hopp Foundation. Dr, Hummel and her associates declared no conflicts of interest.
[email protected]
On Twitter @ginalhenderson
Short-term cognitive-behavioral therapy is an effective intervention for geriatric patients with comorbid acute illness and depression, judging from the findings of a trial of 155 patients.
“The results presented here confirm the interaction of depression with cognitive and functional performance in this vulnerable study population,” wrote Jana Hummel, MD. “However, the extent of the effect on almost all levels of health was unexpected.”
CBT previously had not been used as a treatment for older patients with acute physical illness and comorbid depression, according to Dr. Hummel and her associates (JAMDA. 2017;18:341-9).
The authors recruited patients aged 82 years, plus or minus 6 years, from a 170-bed hospital that served as the Center for Geriatric Medicine at the University of Heidelberg, Germany. Patients who had been admitted to the hospital had a Hospital Anxiety and Depression Scale (HADS) score of greater than 7. Those with dementia or a life expectancy of less than 1 year were excluded from the study, reported Dr. Hummel of the Geriatric and Gerontopsychotherapeutic Practice in Mannheim, Germany, and her associates.
After the patients were discharged, the clinicians began the active intervention. The investigators randomized 56 people into the CBT intervention, a 15-session, manualized program designed for elderly patients who were based at home. The program included several group sessions held at the hospital’s day clinic and two individual sessions conducted by psychotherapists with expertise in gerontology.
The control group was made up of 99 people. Patients in both groups received antidepressants and other medication, the authors reported in the Journal of the American Medical Directors Association.
Four months after discharge, the patients’ severity of depression as measured by HADS scores was significantly lower among the patients in the psychotherapy group than in the usual care group (1.56 plus or minus 1.4, compared with 3.13 plus or minus 1; P less than .001). Likewise, patients in the psychotherapy group also scored lower on the Hamilton Rating Scale for Depression than did those in the control group (10.57 plus or minus 6.4, compared with 21.47 plus or minus 6.9; P less than .001).
In order for CBT to be effective for this population, arrangements might need to be made to transport patients to intervention sites, according to Dr. Hummel and her associates. Alternatively, the treatment could be administered in nursing homes. Ultimately, the authors said, “the interventions have to be tailored to the individual needs, severity of depression, and health situation.”
One limitation of the study is that the cognitive function of some elderly patients could prevent them from benefiting from CBT. However, Dr. Hummel and her associates said they are optimistic about such interventions for other geriatric patients.
“CBT provides psychological benefit to older patients with depressive symptoms, and it also can reverse some of the functional and cognitive decline associated with depression, they wrote. “It may prove to be an important tool in the treatment of depression in old age and multimorbidity.”
The study was funded with grants from the Robert Bosch Foundation and the Dietmar Hopp Foundation. Dr, Hummel and her associates declared no conflicts of interest.
[email protected]
On Twitter @ginalhenderson
Short-term cognitive-behavioral therapy is an effective intervention for geriatric patients with comorbid acute illness and depression, judging from the findings of a trial of 155 patients.
“The results presented here confirm the interaction of depression with cognitive and functional performance in this vulnerable study population,” wrote Jana Hummel, MD. “However, the extent of the effect on almost all levels of health was unexpected.”
CBT previously had not been used as a treatment for older patients with acute physical illness and comorbid depression, according to Dr. Hummel and her associates (JAMDA. 2017;18:341-9).
The authors recruited patients aged 82 years, plus or minus 6 years, from a 170-bed hospital that served as the Center for Geriatric Medicine at the University of Heidelberg, Germany. Patients who had been admitted to the hospital had a Hospital Anxiety and Depression Scale (HADS) score of greater than 7. Those with dementia or a life expectancy of less than 1 year were excluded from the study, reported Dr. Hummel of the Geriatric and Gerontopsychotherapeutic Practice in Mannheim, Germany, and her associates.
After the patients were discharged, the clinicians began the active intervention. The investigators randomized 56 people into the CBT intervention, a 15-session, manualized program designed for elderly patients who were based at home. The program included several group sessions held at the hospital’s day clinic and two individual sessions conducted by psychotherapists with expertise in gerontology.
The control group was made up of 99 people. Patients in both groups received antidepressants and other medication, the authors reported in the Journal of the American Medical Directors Association.
Four months after discharge, the patients’ severity of depression as measured by HADS scores was significantly lower among the patients in the psychotherapy group than in the usual care group (1.56 plus or minus 1.4, compared with 3.13 plus or minus 1; P less than .001). Likewise, patients in the psychotherapy group also scored lower on the Hamilton Rating Scale for Depression than did those in the control group (10.57 plus or minus 6.4, compared with 21.47 plus or minus 6.9; P less than .001).
In order for CBT to be effective for this population, arrangements might need to be made to transport patients to intervention sites, according to Dr. Hummel and her associates. Alternatively, the treatment could be administered in nursing homes. Ultimately, the authors said, “the interventions have to be tailored to the individual needs, severity of depression, and health situation.”
One limitation of the study is that the cognitive function of some elderly patients could prevent them from benefiting from CBT. However, Dr. Hummel and her associates said they are optimistic about such interventions for other geriatric patients.
“CBT provides psychological benefit to older patients with depressive symptoms, and it also can reverse some of the functional and cognitive decline associated with depression, they wrote. “It may prove to be an important tool in the treatment of depression in old age and multimorbidity.”
The study was funded with grants from the Robert Bosch Foundation and the Dietmar Hopp Foundation. Dr, Hummel and her associates declared no conflicts of interest.
[email protected]
On Twitter @ginalhenderson
FROM JAMDA
Managing patients who are somatizing
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Weight gain in pregnancy: Too much and too little can be harmful
Both high and low gestational weight gain, compared with the recommended weight gain, are associated with increased risks of adverse perinatal outcomes, according to a systematic review and meta-analysis.
Researchers reviewed 302 studies that categorized women by their prepregnancy body mass index and followed them for gestational weight gain, as well as maternal and neonatal outcomes. They included in their meta-analysis 23 cohort studies involving 1,309,136 pregnancies in 10 countries. Only studies assessing singleton pregnancies in women aged 18 years and older were included.
Compared with the recommended gestational weight gain, low weight gain was associated with a 5% higher risk of a small-for-gestational-age (SGA) neonate and a 5% higher risk of preterm birth. However, low weight gain reduced the risks of a large-for-gestational-age (LGA) neonate and macrosomia.
Compared with the recommended gestational weight gain, high weight gain was associated with a 4% higher risk of cesarean delivery, a 4% higher risk of a LGA neonate, and a 6% higher risk of macrosomia. However, high weight gain reduced the risk of an SGA neonate by 3% and that of preterm birth by 2%, the researchers reported (JAMA. 2017;317[21]:2207-25).
These increases and decreases in risks remained consistent regardless of the mother’s prepregnancy BMI, they noted.
The effect of either high or low gestational weight gain on the risk of gestational diabetes could not be determined because of inconsistencies across the 23 cohort studies concerning definitions and treatments.
The Australian Department of Education and Training and the Australian National Health and Medical Research Council supported the study. Dr. Goldstein reported having no relevant financial disclosures; one of her associates reported serving on the Women’s Health Global Advisory Board for Pfizer.
The findings by Goldstein et al. raise the question: Can clinicians change the amount of weight women gain in pregnancy?
Behavioral economics has demonstrated that loss avoidance is a stronger motivator than the promise of a gain. So rather than positive incentives for adherent behavior, emphasizing how a pregnant woman’s nonadherent behavior will lead to greater harm to her developing baby may be more effective in changing behavior. Sending a message that gaining too much weight could potentially lead to an increased risk of obesity in her child and that gaining too little weight could lead to growth restriction in the child may be better than a simple positive message that eating well leads to greater health for both the mother and infant.
Given the overwhelming environment of constant advertising of high-caloric foods that pregnant women are exposed to, such reminders need to be delivered persistently and frequently – perhaps with the enhanced messaging capacity of social media and public health campaigns.
Aaron B. Caughey, MD, PhD, is in the department of ob.gyn. at Oregon Health & Science University, Portland. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (JAMA 2017;317[21]:2175-6).
The findings by Goldstein et al. raise the question: Can clinicians change the amount of weight women gain in pregnancy?
Behavioral economics has demonstrated that loss avoidance is a stronger motivator than the promise of a gain. So rather than positive incentives for adherent behavior, emphasizing how a pregnant woman’s nonadherent behavior will lead to greater harm to her developing baby may be more effective in changing behavior. Sending a message that gaining too much weight could potentially lead to an increased risk of obesity in her child and that gaining too little weight could lead to growth restriction in the child may be better than a simple positive message that eating well leads to greater health for both the mother and infant.
Given the overwhelming environment of constant advertising of high-caloric foods that pregnant women are exposed to, such reminders need to be delivered persistently and frequently – perhaps with the enhanced messaging capacity of social media and public health campaigns.
Aaron B. Caughey, MD, PhD, is in the department of ob.gyn. at Oregon Health & Science University, Portland. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (JAMA 2017;317[21]:2175-6).
The findings by Goldstein et al. raise the question: Can clinicians change the amount of weight women gain in pregnancy?
Behavioral economics has demonstrated that loss avoidance is a stronger motivator than the promise of a gain. So rather than positive incentives for adherent behavior, emphasizing how a pregnant woman’s nonadherent behavior will lead to greater harm to her developing baby may be more effective in changing behavior. Sending a message that gaining too much weight could potentially lead to an increased risk of obesity in her child and that gaining too little weight could lead to growth restriction in the child may be better than a simple positive message that eating well leads to greater health for both the mother and infant.
Given the overwhelming environment of constant advertising of high-caloric foods that pregnant women are exposed to, such reminders need to be delivered persistently and frequently – perhaps with the enhanced messaging capacity of social media and public health campaigns.
Aaron B. Caughey, MD, PhD, is in the department of ob.gyn. at Oregon Health & Science University, Portland. He reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (JAMA 2017;317[21]:2175-6).
Both high and low gestational weight gain, compared with the recommended weight gain, are associated with increased risks of adverse perinatal outcomes, according to a systematic review and meta-analysis.
Researchers reviewed 302 studies that categorized women by their prepregnancy body mass index and followed them for gestational weight gain, as well as maternal and neonatal outcomes. They included in their meta-analysis 23 cohort studies involving 1,309,136 pregnancies in 10 countries. Only studies assessing singleton pregnancies in women aged 18 years and older were included.
Compared with the recommended gestational weight gain, low weight gain was associated with a 5% higher risk of a small-for-gestational-age (SGA) neonate and a 5% higher risk of preterm birth. However, low weight gain reduced the risks of a large-for-gestational-age (LGA) neonate and macrosomia.
Compared with the recommended gestational weight gain, high weight gain was associated with a 4% higher risk of cesarean delivery, a 4% higher risk of a LGA neonate, and a 6% higher risk of macrosomia. However, high weight gain reduced the risk of an SGA neonate by 3% and that of preterm birth by 2%, the researchers reported (JAMA. 2017;317[21]:2207-25).
These increases and decreases in risks remained consistent regardless of the mother’s prepregnancy BMI, they noted.
The effect of either high or low gestational weight gain on the risk of gestational diabetes could not be determined because of inconsistencies across the 23 cohort studies concerning definitions and treatments.
The Australian Department of Education and Training and the Australian National Health and Medical Research Council supported the study. Dr. Goldstein reported having no relevant financial disclosures; one of her associates reported serving on the Women’s Health Global Advisory Board for Pfizer.
Both high and low gestational weight gain, compared with the recommended weight gain, are associated with increased risks of adverse perinatal outcomes, according to a systematic review and meta-analysis.
Researchers reviewed 302 studies that categorized women by their prepregnancy body mass index and followed them for gestational weight gain, as well as maternal and neonatal outcomes. They included in their meta-analysis 23 cohort studies involving 1,309,136 pregnancies in 10 countries. Only studies assessing singleton pregnancies in women aged 18 years and older were included.
Compared with the recommended gestational weight gain, low weight gain was associated with a 5% higher risk of a small-for-gestational-age (SGA) neonate and a 5% higher risk of preterm birth. However, low weight gain reduced the risks of a large-for-gestational-age (LGA) neonate and macrosomia.
Compared with the recommended gestational weight gain, high weight gain was associated with a 4% higher risk of cesarean delivery, a 4% higher risk of a LGA neonate, and a 6% higher risk of macrosomia. However, high weight gain reduced the risk of an SGA neonate by 3% and that of preterm birth by 2%, the researchers reported (JAMA. 2017;317[21]:2207-25).
These increases and decreases in risks remained consistent regardless of the mother’s prepregnancy BMI, they noted.
The effect of either high or low gestational weight gain on the risk of gestational diabetes could not be determined because of inconsistencies across the 23 cohort studies concerning definitions and treatments.
The Australian Department of Education and Training and the Australian National Health and Medical Research Council supported the study. Dr. Goldstein reported having no relevant financial disclosures; one of her associates reported serving on the Women’s Health Global Advisory Board for Pfizer.
FROM JAMA
Key clinical point: Both high and low gestational weight gain are associated with increased risks of adverse perinatal outcomes.
Major finding: Low gestational weight gain was associated with a 5% higher risk of an SGA neonate and a 5% higher risk of preterm birth, while high weight gain was associated with a 4% higher risk of cesarean delivery, a 4% higher risk of an LGA neonate, and a 6% higher risk of macrosomia.
Data source: A systematic review and meta-analysis of 23 studies involving 1,309,136 pregnancies from diverse international cohorts.
Disclosures: The Australian Department of Education and Training and the Australian National Health and Medical Research Council supported the study. Dr. Goldstein reported having no relevant financial disclosures; one of her associates reported serving on the Women’s Health Global Advisory Board for Pfizer.
Does Preoperative Pneumonia Affect Complications of Geriatric Hip Fracture Surgery?
Take-Home Points
- The prevalence of preoperative pneumonia is 1.2% among hip fracture patients aged >65 years.
- Preoperative pneumonia is an independent risk factor for mortality and adverse events including renal failure, prolonged ventilator dependence, and prolonged altered mental status after geriatric hip fracture surgery.
- Underweight BMI (<18.5 kg/m2) was associated with higher mortality within 30 days among hip fracture patients admitted with pneumonia.
- The mortality rate normalized to that of patients without pneumonia within 2 weeks of hip fracture surgery.
- Time from admission to surgery was not associated with adverse events or mortality among hip fracture patients admitted with pneumonia.
Preoperative pneumonia remains relatively unexplored as a risk factor for adverse outcomes in geriatric hip fracture surgery. Dated studies report a 0.3% to 3.2% prevalence of “recent pneumonia” in patients presenting with hip fracture but provide neither a definition of pneumonia based on clinical criteria nor a subset analysis of outcomes in the pneumonia group.1-3 Although active pneumonia has been identified as a preoperative optimization target in the management guidelines for geriatric hip fracture,4 we are unaware of any studies that have reported on differences in demographics, comorbidities, delay to surgery, or adverse outcomes between hip fracture patients with and without preoperative pneumonia.
This paucity of information on the effect of preoperative pneumonia in the hip fracture population may be related to low prevalence of preoperative pneumonia and a cadre of variable definitions, which limit identification of a cohort of patients with preoperative pneumonia large enough from which to draw meaningful results. Database studies, especially those using surgical registries rather than administrative or reimbursement data, offer particular advantages for investigation of such rare clinical entities.5Medical care of patients with pneumonia alone is known to be facilitated by assessments of mortality risk from clinical and laboratory data. The modified British Thoracic Society rule/CURB-65 (confusion, urea, respiratory rate, blood pressure) score is strongly predictive of mortality in hospitalized adults with pneumonia (odds ratio [OR], 4.59; 95% confidence interval [CI], 1.42-14.85; P = .011) and may guide antibiotic therapy, laboratory investigations, and the decision to intubate in a patient with pneumonia.6-8 This score is predictive of adverse events (AEs), hospital length of stay, and use of intensive care services.6,7,9-13 We hypothesized that preoperative clinical indicators assessed by pneumonia severity scores as well as patient demographics and baseline comorbidities may also have prognostic value for risk of AEs in a cohort of geriatric hip fracture surgery patients with preoperative pneumonia.
In this article, we first describe the prevalence of preoperative pneumonia in geriatric hip fracture surgery patients as well as demographic and operative differences between patients with and without the disease. We then ask 3 questions: Is preoperative pneumonia an independent risk factor for mortality and adverse outcomes in geriatric hip fracture surgery? Is there a postoperative interval during which the unadjusted mortality rate is higher among patients with preoperative pneumonia? In patients with preoperative pneumonia, what are the predictors of morbidity and mortality?
Methods
Yale University’s Human Investigations Committee approved this retrospective cohort study, which used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for the period 2005 to 2012. ACS-NSQIP is a prospective, multi-institutional outcomes program that collects data on preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes for patients undergoing surgical procedures in inpatient and outpatient settings.14
Unlike administrative databases, which are based on reimbursement data, ACS-NSQIP data are collected by trained surgical clinical reviewers for the purposes of quality improvement and clinical research, and data quality is ensured with routine auditing.15 The program has gained a high degree of respect as a powerful and valid data source in both general16 and orthopedic17 surgery literature. The database offers a particular advantage with respect to the study of preoperative pneumonia: Only patients with new or recently diagnosed pneumonia on antibiotic therapy who meet strict criteria for characteristic findings on chest radiography, clinical signs and symptoms of respiratory illness, and positive cultures are coded as having actively treated pneumonia at time of surgery.15
To identify hip fracture patients over the age of 65 years who underwent operative fixation of a hip fracture, we used Current Procedural Terminology (CPT) hip fracture codes, including 27235 (percutaneous screw fixation), 27236 or 27244 (plate-and-screw fixation), and 27245 (intramedullary device), as well as 27125 (hemiarthroplasty) and 27130 (arthroplasty) for patients with a postoperative International Classification of Disease, Ninth Revision (ICD-9) diagnosis code (820.x, 820.2x, or 820.8) consistent with acute hip fracture.18,19 Procedure type, anesthesia type, and delay from admission to surgery were captured for all procedures.
Preoperative demographics included age, sex, transfer origin, functional status, and body mass index (BMI) category. Binary comorbidities were classified as preoperative anemia (hematocrit, <0.41 for men, <0.36 for women), confusion, dyspnea at rest, uremia (blood urea nitrogen, >6.8 mmol/L), history of cardiovascular disease (congestive heart failure, myocardial infarction, percutaneous coronary intervention, angina pectoris, medically treated hypertension, peripheral vascular disease, or resting claudication), chronic obstructive pulmonary disease, diabetes, renal disease (renal failure or dialysis), and cigarette use in preceding 12 months.20,21 Although preoperative hypotension and respiratory rate are often considered in patients with pneumonia, these variables were not available from the ACS-NSQIP data.6,22Pearson χ2 test for categorical variables was used to compare baseline demographics and operative characteristics between patients with and without pneumonia, and Student t test was used to compare intervals from hospital admission to hip fracture surgery, surgery start to surgery stop, and surgery to discharge between patients with and without preoperative pneumonia.
Binary outcome measures were compared between patients with and without preoperative pneumonia. “Any AE” included any serious AE (SAE) or any minor AE. SAEs included death, acute renal failure, ventilator use >48 hours, unplanned intubation, septic shock, sepsis, return to operating room, coma >24 hours, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, thromboembolic event (deep vein thrombosis or pulmonary embolism), and stroke/cerebrovascular accident. Minor AEs included progressive renal insufficiency, urinary tract infection, organ/space infection, superficial surgical-site infection, deep surgical-site infection, and wound dehiscence. Other binary outcome measures included discharge destination and unplanned readmission within 30 days after hip fracture surgery.23Poisson regression with robust error variance as described by Zou24 was used to compare the rates of any, minor, and individual AEs, and any SAEs, between patients with and without pneumonia. Multivariate analysis accounted for the baseline variables in Table 1. AEs that occurred more than once in each group were included in the analyses.
Kaplan-Meier survival analysis was performed for postoperative mortality within 30 days. Within the preoperative pneumonia group, covariates from Table 1 were identified as predictors of any AE, SAE, or death within 30 days after hip fracture surgery by stepwise multivariate Poisson regression with robust error variance. When interval from admission to surgery was longer than 24, 48, 72, or 96 hours, it was also included as a covariate. Variables that did not show an association with AEs at the P < .20 level were not included in the final regression model. All analyses were performed with Stata/SE Version 12.0 statistical software (StataCorp).
Results
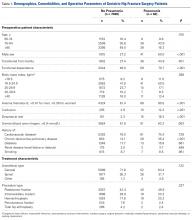
Of the 7128 geriatric hip fracture patients in this study, 82 (1.2%) had active pneumonia at time of surgery (Table 1). Age, BMI, preoperative uremia, history of cardiovascular disease, diabetes, renal disease, and smoking were similar between groups. In addition, there was no difference in anesthesia type or fixation procedure between the pneumonia and no-pneumonia groups. Patients with preoperative pneumonia differed significantly with respect to sex, transfer from facility, preoperative functional dependence, anemia, confusion, dyspnea at rest, and history of chronic obstructive pulmonary disease (Table 1).
Interval from admission to surgery was longer (P < .001) for geriatric hip fracture patients with preoperative pneumonia (mean, 6.8 days; 95% CI, 2.5-11.1 days) than for those without pneumonia (mean, 1.5 days; CI, 1.4-1.5 days). There was no difference (P = .124) in operative time between the pneumonia group (mean, 72.8 min; CI, 64.0-81.5 min) and the no-pneumonia group (mean, 66.1 min; CI, 61.2-67.0 min). Interval from surgery to discharge was longer (P < .001) for patients with preoperative pneumonia (mean, 10.1 days; CI, 6.9-13.4 days) than for those without pneumonia (mean, 6.3 days; CI, 6.1-6.4 days).
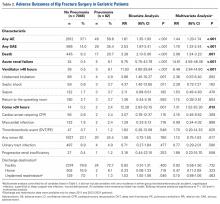
Adverse outcomes of geriatric hip fracture surgery are listed in Table 2. In the multivariate analysis, preoperative pneumonia was significantly associated with any AE (relative risk [RR]) = 1.44) and any SAE (RR = 1.79).
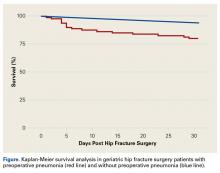
Survival patterns diverged between patients with and without preoperative pneumonia (Figure). The unadjusted mortality rate was qualitatively higher in patients with preoperative pneumonia than in patients without pneumonia during the first days after hip fracture (slopes of unadjusted mortality curves in Figure). Of note, no patient under age 75 years with pneumonia at time of surgery died within the 30-day study period.
Among geriatric hip fracture patients with preoperative pneumonia, multivariate analyses revealed no significant association of any preoperative comorbidity with any AE or any SAE. Given the gravity of the death complication, however, death within 30 days after surgery was analyzed separately, and was found to be significantly associated (RR = 4.67) with being underweight (BMI, <18.5 kg/m2) (Table 3). Admission-to-surgery interval longer than 24, 48, 72, or 96 hours did not reach significance at the P < 0.2 level in the stepwise regressions and therefore was not associated with a higher or lower risk of any AE, SAE, or death.
Discussion
In the general US population, pneumonia accounts for 1.4% of deaths in people 65 years to 74 years old, 2.1% in people 75 years to 84 years, and 3.1% in people 85 years or older. In total, 3.4% of hospital inpatient deaths are attributed to pneumonia.25 In hospitalized general orthopedic surgical patients as well as hip fracture patients, pneumonia is strongly associated with increased mortality.26,27
We identified a preoperative pneumonia prevalence of 1.2%, which is comparable to the rates reported in the literature (0.3%-3.2%).1-3 To our knowledge, our study represents the largest series of patients with preoperative pneumonia at time of hip fracture repair, and the first to independently associate preoperative pneumonia with increased incidence of AEs, including death.
This study had its limitations. First, the ACS-NSQIP morbidity and mortality data, which are limited to the first 30 postoperative days, may be skewed because AEs that occurred after that interval are not captured. Second, coding of pneumonia in ACS-NSQIP does not convey specific information about the disease and its severity—infectious organism(s) responsible; acquisition setting (healthcare or community); treatment given, including antibiotic(s) selection, steroid use, dosing, and duration; and measures of treatment efficacy—limiting interpretation of the difference in delay to surgery. We cannot say whether the longer interval in patients with pneumonia reflects medical optimization, or whether the delay itself or any interventions during that time positively or negatively affected outcomes. In addition, despite using a large national database, we obtained a relatively small sample of patients (82) who had pneumonia before surgical hip fracture repair.
Multivariate analysis controlling for baseline demographics and comorbidities revealed that multiple SAEs were independently associated with preoperative pneumonia (overall SAE, RR = 1.79). Postoperative use of ventilator support for longer than 48 hours (RR = 6.48) and coma longer than 24 hours (RR = 7.31) are expected given the severity of pulmonary compromise in the study cohort.28,29 Acute renal failure (RR = 14.61) can occur in both hip fracture patients and community-acquired pneumonia patients and may be a multifactorial complication of the pulmonary infection, of the anesthesia, or of the surgical intervention in this cohort.30-32Unadjusted mortality in hip fracture takes months to a year to normalize to that of age-matched controls.32-34 In our series, the unadjusted death rate in the pneumonia cohort (Figure) was transiently elevated during the first weeks after surgery but then drew nearer the rate in the nondiseased hip fracture cohort by the end of the first month. Early death in the pneumonia group likely was multifactorial, potentially influenced by the increased burden of comorbidities in the pneumonia group at baseline, and the longer delay to surgery,35-38 as well as by the natural history of treated pneumonia in hospital patients, who, compared with age-matched hospitalized controls, also exhibit higher mortality during only the first 2 to 4 months of hospitalization for pneumonia.39 We regret that quality improvement strategies in the treatment of geriatric hip fracture surgery with pneumonia cannot be extrapolated from these results.
Similarly, the utility of BMI <18.5 kg/m2 as an actionable preoperative finding cannot be assessed from these results. However, we propose that underweight geriatric hip fracture patients with pneumonia may benefit from more aggressive preoperative optimization that does not delay surgery. Higher acuity of postoperative care, including more intensive nursing care and early coordination of care with respiratory therapists and medical comanagement teams, may also be beneficial.
Anesthesia type did not differ between patients with and without preoperative pneumonia and was not associated with AEs in patients with preoperative pneumonia. Consistent with our findings, multiple studies have reported no significant differences in short-term outcomes of hip fracture repair between general and spinal anesthesia, though no other study has compared the benefits of general and spinal anesthesia for patients with preoperative pneumonia.40-44 Although spinal anesthesia (relative to general anesthesia) has been reported to have benefits in hip and knee arthroplasty, these benefits appear not to translate to hip fracture repair.45-50 The results of the present study suggest that general and spinal anesthesia may be equivalent in terms of risk for the geriatric hip fracture patient with preoperative pneumonia.43,44Our attempt to evaluate the CURB-65 pneumonia severity score as a prognosticator of AEs was thwarted by the absence of required variables in the ACS-NSQIP dataset (confusion, uremia, dyspnea, and age were available; hypotension and blood pressure were not). In our analysis, we did include, individually, variables previously found to predict AEs in the medical pneumonia population (confusion, uremia, dyspnea at rest, anemia).9-11,32 However, these clinical findings are nonspecific in hip fracture patients, who may become anemic, confused, dyspneic, or uremic from a multitude of factors related to their injury and unrelated to pneumonia, including but not limited to hemorrhage, muscle damage, renal injury, and pulmonary embolism. It is not surprising that confusion, uremia, dyspnea at rest, and anemia were not individually predictive of AEs or death within 30 days after surgery in the cohort of geriatric hip fracture patients with pneumonia.
There is no literature that argues for or against delaying hip fracture surgery in geriatric hip fracture patients with pneumonia. The surgical delay observed in this population is ostensibly related to medical optimization of the pneumonia and/or underlying comorbidities. However, we did not find a morbidity or mortality detriment or benefit in delaying surgery by 1 to 4 days in this population. Delay of surgery is a poor covariate, given extensive confounding by medical management and preoperative optimizing of comorbid conditions (reflected in our independent variable and covariates) as well as institutional and surgeon variations in policy and behavior and other unaccounted influences. Although some authors have found no difference in mortality or major AEs between hip fracture patients who had a surgical delay and those who did not,31,51-53 other series and meta-analyses have suggested a mortality detriment in a surgical delay of more than 2 days36,54 or 4 days55 from admission. Given our data, we cannot recommend against immediate hip fracture repair in the subpopulation of geriatric hip fracture patients with pneumonia.
Our study findings suggest that preoperative pneumonia is a rare independent risk factor for AEs after hip fracture surgery in geriatric patients. Underweight BMI is predictive of death in geriatric hip fracture surgery patients who present with pneumonia, whereas early surgical repair appears not to be associated with adverse outcomes. Further investigation is warranted to determine if such patients benefit from specific preoperative and postoperative strategies for optimizing medical and surgical care based on these findings.
Am J Orthop. 2017;46(3):E177-E185. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sexson SB, Lehner JT. Factors affecting hip fracture mortality. J Orthop Trauma. 1987;1(4):298-305.
2. Mullen JO, Mullen NL. Hip fracture mortality: a prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;(280):214-222.
3. Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;(186):45-56.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24(4):701-719.
5. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382.
7. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BDW. The CURB (confusion, urea, respiratory rate and blood pressure) criteria in community-acquired pneumonia (CAP) in hospitalised elderly patients aged 65 years and over: a prospective observational cohort study. Age Ageing. 2005;34(1):75-77.
8. Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care. 2004;10(1):59-64.
9. Buising K, Thursky K, Black J, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419-424.
10. Ewig S, De Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421-427.
11. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572-1579.
12. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223.
13. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med. 2006;260(1):93-101.
14. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837-843.
15. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed October 8, 2014.
16. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267.
17. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889.
18. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42.
19. Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620-625.
20. Fisher MA, Matthei JD, Obirieze A, et al. Open reduction internal fixation versus hemiarthroplasty versus total hip arthroplasty in the elderly: a review of the National Surgical Quality Improvement Program database. J Surg Res. 2013;181(2):193-198.
21. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63-69.
22. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134-141.
23. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
24. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004:159(7):702-706.
25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 20138;61(4):1-117.
26. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84(4):562-572.
27. Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
28. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
29. Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21(1):24-31.
30. Urwin S, Parker M, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455.
31. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
32. Niederman MS, Mandell LA, Anzueto A, et al; American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754.
33. Koval KJ, Skovron ML, Aharonoff GB, Zuckerman JD. Predictors of functional recovery after hip fracture in the elderly. Clin Orthop Relat Res. 1998;(348):22-28.
34. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
35. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology. 2000;39(4):346-349.
36. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947-951.
37. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709.
38. Simunovic N, Devereaux P, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616.
39. Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317-323.
40. Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521.
41. Chakladar A, White SM. Cost estimates of spinal versus general anaesthesia for fractured neck of femur surgery. Anaesthesia. 2010;65(8):810-814.
42. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
43. Gilbert TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general anesthesia for hip fracture repair: a longitudinal observation of 741 elderly patients during 2-year follow-up. Am J Orthop. 2000;29(1):25-35.
44. O’Hara DA, Duff A, Berlin JA, et al. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92(4):947-957.
45. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279-287.
46. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006;49(6):391-396.
47. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279-284.
48. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103(4):1018-1025.
49. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-942.
50. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
51. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697.
52. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559.
53. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489.
54. Shiga T, Wajima Zi, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154.
55. Streubel P, Ricci W, Wong A, Gardner M. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
Take-Home Points
- The prevalence of preoperative pneumonia is 1.2% among hip fracture patients aged >65 years.
- Preoperative pneumonia is an independent risk factor for mortality and adverse events including renal failure, prolonged ventilator dependence, and prolonged altered mental status after geriatric hip fracture surgery.
- Underweight BMI (<18.5 kg/m2) was associated with higher mortality within 30 days among hip fracture patients admitted with pneumonia.
- The mortality rate normalized to that of patients without pneumonia within 2 weeks of hip fracture surgery.
- Time from admission to surgery was not associated with adverse events or mortality among hip fracture patients admitted with pneumonia.
Preoperative pneumonia remains relatively unexplored as a risk factor for adverse outcomes in geriatric hip fracture surgery. Dated studies report a 0.3% to 3.2% prevalence of “recent pneumonia” in patients presenting with hip fracture but provide neither a definition of pneumonia based on clinical criteria nor a subset analysis of outcomes in the pneumonia group.1-3 Although active pneumonia has been identified as a preoperative optimization target in the management guidelines for geriatric hip fracture,4 we are unaware of any studies that have reported on differences in demographics, comorbidities, delay to surgery, or adverse outcomes between hip fracture patients with and without preoperative pneumonia.
This paucity of information on the effect of preoperative pneumonia in the hip fracture population may be related to low prevalence of preoperative pneumonia and a cadre of variable definitions, which limit identification of a cohort of patients with preoperative pneumonia large enough from which to draw meaningful results. Database studies, especially those using surgical registries rather than administrative or reimbursement data, offer particular advantages for investigation of such rare clinical entities.5Medical care of patients with pneumonia alone is known to be facilitated by assessments of mortality risk from clinical and laboratory data. The modified British Thoracic Society rule/CURB-65 (confusion, urea, respiratory rate, blood pressure) score is strongly predictive of mortality in hospitalized adults with pneumonia (odds ratio [OR], 4.59; 95% confidence interval [CI], 1.42-14.85; P = .011) and may guide antibiotic therapy, laboratory investigations, and the decision to intubate in a patient with pneumonia.6-8 This score is predictive of adverse events (AEs), hospital length of stay, and use of intensive care services.6,7,9-13 We hypothesized that preoperative clinical indicators assessed by pneumonia severity scores as well as patient demographics and baseline comorbidities may also have prognostic value for risk of AEs in a cohort of geriatric hip fracture surgery patients with preoperative pneumonia.
In this article, we first describe the prevalence of preoperative pneumonia in geriatric hip fracture surgery patients as well as demographic and operative differences between patients with and without the disease. We then ask 3 questions: Is preoperative pneumonia an independent risk factor for mortality and adverse outcomes in geriatric hip fracture surgery? Is there a postoperative interval during which the unadjusted mortality rate is higher among patients with preoperative pneumonia? In patients with preoperative pneumonia, what are the predictors of morbidity and mortality?
Methods
Yale University’s Human Investigations Committee approved this retrospective cohort study, which used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for the period 2005 to 2012. ACS-NSQIP is a prospective, multi-institutional outcomes program that collects data on preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes for patients undergoing surgical procedures in inpatient and outpatient settings.14
Unlike administrative databases, which are based on reimbursement data, ACS-NSQIP data are collected by trained surgical clinical reviewers for the purposes of quality improvement and clinical research, and data quality is ensured with routine auditing.15 The program has gained a high degree of respect as a powerful and valid data source in both general16 and orthopedic17 surgery literature. The database offers a particular advantage with respect to the study of preoperative pneumonia: Only patients with new or recently diagnosed pneumonia on antibiotic therapy who meet strict criteria for characteristic findings on chest radiography, clinical signs and symptoms of respiratory illness, and positive cultures are coded as having actively treated pneumonia at time of surgery.15
To identify hip fracture patients over the age of 65 years who underwent operative fixation of a hip fracture, we used Current Procedural Terminology (CPT) hip fracture codes, including 27235 (percutaneous screw fixation), 27236 or 27244 (plate-and-screw fixation), and 27245 (intramedullary device), as well as 27125 (hemiarthroplasty) and 27130 (arthroplasty) for patients with a postoperative International Classification of Disease, Ninth Revision (ICD-9) diagnosis code (820.x, 820.2x, or 820.8) consistent with acute hip fracture.18,19 Procedure type, anesthesia type, and delay from admission to surgery were captured for all procedures.
Preoperative demographics included age, sex, transfer origin, functional status, and body mass index (BMI) category. Binary comorbidities were classified as preoperative anemia (hematocrit, <0.41 for men, <0.36 for women), confusion, dyspnea at rest, uremia (blood urea nitrogen, >6.8 mmol/L), history of cardiovascular disease (congestive heart failure, myocardial infarction, percutaneous coronary intervention, angina pectoris, medically treated hypertension, peripheral vascular disease, or resting claudication), chronic obstructive pulmonary disease, diabetes, renal disease (renal failure or dialysis), and cigarette use in preceding 12 months.20,21 Although preoperative hypotension and respiratory rate are often considered in patients with pneumonia, these variables were not available from the ACS-NSQIP data.6,22Pearson χ2 test for categorical variables was used to compare baseline demographics and operative characteristics between patients with and without pneumonia, and Student t test was used to compare intervals from hospital admission to hip fracture surgery, surgery start to surgery stop, and surgery to discharge between patients with and without preoperative pneumonia.
Binary outcome measures were compared between patients with and without preoperative pneumonia. “Any AE” included any serious AE (SAE) or any minor AE. SAEs included death, acute renal failure, ventilator use >48 hours, unplanned intubation, septic shock, sepsis, return to operating room, coma >24 hours, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, thromboembolic event (deep vein thrombosis or pulmonary embolism), and stroke/cerebrovascular accident. Minor AEs included progressive renal insufficiency, urinary tract infection, organ/space infection, superficial surgical-site infection, deep surgical-site infection, and wound dehiscence. Other binary outcome measures included discharge destination and unplanned readmission within 30 days after hip fracture surgery.23Poisson regression with robust error variance as described by Zou24 was used to compare the rates of any, minor, and individual AEs, and any SAEs, between patients with and without pneumonia. Multivariate analysis accounted for the baseline variables in Table 1. AEs that occurred more than once in each group were included in the analyses.
Kaplan-Meier survival analysis was performed for postoperative mortality within 30 days. Within the preoperative pneumonia group, covariates from Table 1 were identified as predictors of any AE, SAE, or death within 30 days after hip fracture surgery by stepwise multivariate Poisson regression with robust error variance. When interval from admission to surgery was longer than 24, 48, 72, or 96 hours, it was also included as a covariate. Variables that did not show an association with AEs at the P < .20 level were not included in the final regression model. All analyses were performed with Stata/SE Version 12.0 statistical software (StataCorp).
Results
Of the 7128 geriatric hip fracture patients in this study, 82 (1.2%) had active pneumonia at time of surgery (Table 1). Age, BMI, preoperative uremia, history of cardiovascular disease, diabetes, renal disease, and smoking were similar between groups. In addition, there was no difference in anesthesia type or fixation procedure between the pneumonia and no-pneumonia groups. Patients with preoperative pneumonia differed significantly with respect to sex, transfer from facility, preoperative functional dependence, anemia, confusion, dyspnea at rest, and history of chronic obstructive pulmonary disease (Table 1).
Interval from admission to surgery was longer (P < .001) for geriatric hip fracture patients with preoperative pneumonia (mean, 6.8 days; 95% CI, 2.5-11.1 days) than for those without pneumonia (mean, 1.5 days; CI, 1.4-1.5 days). There was no difference (P = .124) in operative time between the pneumonia group (mean, 72.8 min; CI, 64.0-81.5 min) and the no-pneumonia group (mean, 66.1 min; CI, 61.2-67.0 min). Interval from surgery to discharge was longer (P < .001) for patients with preoperative pneumonia (mean, 10.1 days; CI, 6.9-13.4 days) than for those without pneumonia (mean, 6.3 days; CI, 6.1-6.4 days).
Adverse outcomes of geriatric hip fracture surgery are listed in Table 2. In the multivariate analysis, preoperative pneumonia was significantly associated with any AE (relative risk [RR]) = 1.44) and any SAE (RR = 1.79).
Survival patterns diverged between patients with and without preoperative pneumonia (Figure). The unadjusted mortality rate was qualitatively higher in patients with preoperative pneumonia than in patients without pneumonia during the first days after hip fracture (slopes of unadjusted mortality curves in Figure). Of note, no patient under age 75 years with pneumonia at time of surgery died within the 30-day study period.
Among geriatric hip fracture patients with preoperative pneumonia, multivariate analyses revealed no significant association of any preoperative comorbidity with any AE or any SAE. Given the gravity of the death complication, however, death within 30 days after surgery was analyzed separately, and was found to be significantly associated (RR = 4.67) with being underweight (BMI, <18.5 kg/m2) (Table 3). Admission-to-surgery interval longer than 24, 48, 72, or 96 hours did not reach significance at the P < 0.2 level in the stepwise regressions and therefore was not associated with a higher or lower risk of any AE, SAE, or death.
Discussion
In the general US population, pneumonia accounts for 1.4% of deaths in people 65 years to 74 years old, 2.1% in people 75 years to 84 years, and 3.1% in people 85 years or older. In total, 3.4% of hospital inpatient deaths are attributed to pneumonia.25 In hospitalized general orthopedic surgical patients as well as hip fracture patients, pneumonia is strongly associated with increased mortality.26,27
We identified a preoperative pneumonia prevalence of 1.2%, which is comparable to the rates reported in the literature (0.3%-3.2%).1-3 To our knowledge, our study represents the largest series of patients with preoperative pneumonia at time of hip fracture repair, and the first to independently associate preoperative pneumonia with increased incidence of AEs, including death.
This study had its limitations. First, the ACS-NSQIP morbidity and mortality data, which are limited to the first 30 postoperative days, may be skewed because AEs that occurred after that interval are not captured. Second, coding of pneumonia in ACS-NSQIP does not convey specific information about the disease and its severity—infectious organism(s) responsible; acquisition setting (healthcare or community); treatment given, including antibiotic(s) selection, steroid use, dosing, and duration; and measures of treatment efficacy—limiting interpretation of the difference in delay to surgery. We cannot say whether the longer interval in patients with pneumonia reflects medical optimization, or whether the delay itself or any interventions during that time positively or negatively affected outcomes. In addition, despite using a large national database, we obtained a relatively small sample of patients (82) who had pneumonia before surgical hip fracture repair.
Multivariate analysis controlling for baseline demographics and comorbidities revealed that multiple SAEs were independently associated with preoperative pneumonia (overall SAE, RR = 1.79). Postoperative use of ventilator support for longer than 48 hours (RR = 6.48) and coma longer than 24 hours (RR = 7.31) are expected given the severity of pulmonary compromise in the study cohort.28,29 Acute renal failure (RR = 14.61) can occur in both hip fracture patients and community-acquired pneumonia patients and may be a multifactorial complication of the pulmonary infection, of the anesthesia, or of the surgical intervention in this cohort.30-32Unadjusted mortality in hip fracture takes months to a year to normalize to that of age-matched controls.32-34 In our series, the unadjusted death rate in the pneumonia cohort (Figure) was transiently elevated during the first weeks after surgery but then drew nearer the rate in the nondiseased hip fracture cohort by the end of the first month. Early death in the pneumonia group likely was multifactorial, potentially influenced by the increased burden of comorbidities in the pneumonia group at baseline, and the longer delay to surgery,35-38 as well as by the natural history of treated pneumonia in hospital patients, who, compared with age-matched hospitalized controls, also exhibit higher mortality during only the first 2 to 4 months of hospitalization for pneumonia.39 We regret that quality improvement strategies in the treatment of geriatric hip fracture surgery with pneumonia cannot be extrapolated from these results.
Similarly, the utility of BMI <18.5 kg/m2 as an actionable preoperative finding cannot be assessed from these results. However, we propose that underweight geriatric hip fracture patients with pneumonia may benefit from more aggressive preoperative optimization that does not delay surgery. Higher acuity of postoperative care, including more intensive nursing care and early coordination of care with respiratory therapists and medical comanagement teams, may also be beneficial.
Anesthesia type did not differ between patients with and without preoperative pneumonia and was not associated with AEs in patients with preoperative pneumonia. Consistent with our findings, multiple studies have reported no significant differences in short-term outcomes of hip fracture repair between general and spinal anesthesia, though no other study has compared the benefits of general and spinal anesthesia for patients with preoperative pneumonia.40-44 Although spinal anesthesia (relative to general anesthesia) has been reported to have benefits in hip and knee arthroplasty, these benefits appear not to translate to hip fracture repair.45-50 The results of the present study suggest that general and spinal anesthesia may be equivalent in terms of risk for the geriatric hip fracture patient with preoperative pneumonia.43,44Our attempt to evaluate the CURB-65 pneumonia severity score as a prognosticator of AEs was thwarted by the absence of required variables in the ACS-NSQIP dataset (confusion, uremia, dyspnea, and age were available; hypotension and blood pressure were not). In our analysis, we did include, individually, variables previously found to predict AEs in the medical pneumonia population (confusion, uremia, dyspnea at rest, anemia).9-11,32 However, these clinical findings are nonspecific in hip fracture patients, who may become anemic, confused, dyspneic, or uremic from a multitude of factors related to their injury and unrelated to pneumonia, including but not limited to hemorrhage, muscle damage, renal injury, and pulmonary embolism. It is not surprising that confusion, uremia, dyspnea at rest, and anemia were not individually predictive of AEs or death within 30 days after surgery in the cohort of geriatric hip fracture patients with pneumonia.
There is no literature that argues for or against delaying hip fracture surgery in geriatric hip fracture patients with pneumonia. The surgical delay observed in this population is ostensibly related to medical optimization of the pneumonia and/or underlying comorbidities. However, we did not find a morbidity or mortality detriment or benefit in delaying surgery by 1 to 4 days in this population. Delay of surgery is a poor covariate, given extensive confounding by medical management and preoperative optimizing of comorbid conditions (reflected in our independent variable and covariates) as well as institutional and surgeon variations in policy and behavior and other unaccounted influences. Although some authors have found no difference in mortality or major AEs between hip fracture patients who had a surgical delay and those who did not,31,51-53 other series and meta-analyses have suggested a mortality detriment in a surgical delay of more than 2 days36,54 or 4 days55 from admission. Given our data, we cannot recommend against immediate hip fracture repair in the subpopulation of geriatric hip fracture patients with pneumonia.
Our study findings suggest that preoperative pneumonia is a rare independent risk factor for AEs after hip fracture surgery in geriatric patients. Underweight BMI is predictive of death in geriatric hip fracture surgery patients who present with pneumonia, whereas early surgical repair appears not to be associated with adverse outcomes. Further investigation is warranted to determine if such patients benefit from specific preoperative and postoperative strategies for optimizing medical and surgical care based on these findings.
Am J Orthop. 2017;46(3):E177-E185. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- The prevalence of preoperative pneumonia is 1.2% among hip fracture patients aged >65 years.
- Preoperative pneumonia is an independent risk factor for mortality and adverse events including renal failure, prolonged ventilator dependence, and prolonged altered mental status after geriatric hip fracture surgery.
- Underweight BMI (<18.5 kg/m2) was associated with higher mortality within 30 days among hip fracture patients admitted with pneumonia.
- The mortality rate normalized to that of patients without pneumonia within 2 weeks of hip fracture surgery.
- Time from admission to surgery was not associated with adverse events or mortality among hip fracture patients admitted with pneumonia.
Preoperative pneumonia remains relatively unexplored as a risk factor for adverse outcomes in geriatric hip fracture surgery. Dated studies report a 0.3% to 3.2% prevalence of “recent pneumonia” in patients presenting with hip fracture but provide neither a definition of pneumonia based on clinical criteria nor a subset analysis of outcomes in the pneumonia group.1-3 Although active pneumonia has been identified as a preoperative optimization target in the management guidelines for geriatric hip fracture,4 we are unaware of any studies that have reported on differences in demographics, comorbidities, delay to surgery, or adverse outcomes between hip fracture patients with and without preoperative pneumonia.
This paucity of information on the effect of preoperative pneumonia in the hip fracture population may be related to low prevalence of preoperative pneumonia and a cadre of variable definitions, which limit identification of a cohort of patients with preoperative pneumonia large enough from which to draw meaningful results. Database studies, especially those using surgical registries rather than administrative or reimbursement data, offer particular advantages for investigation of such rare clinical entities.5Medical care of patients with pneumonia alone is known to be facilitated by assessments of mortality risk from clinical and laboratory data. The modified British Thoracic Society rule/CURB-65 (confusion, urea, respiratory rate, blood pressure) score is strongly predictive of mortality in hospitalized adults with pneumonia (odds ratio [OR], 4.59; 95% confidence interval [CI], 1.42-14.85; P = .011) and may guide antibiotic therapy, laboratory investigations, and the decision to intubate in a patient with pneumonia.6-8 This score is predictive of adverse events (AEs), hospital length of stay, and use of intensive care services.6,7,9-13 We hypothesized that preoperative clinical indicators assessed by pneumonia severity scores as well as patient demographics and baseline comorbidities may also have prognostic value for risk of AEs in a cohort of geriatric hip fracture surgery patients with preoperative pneumonia.
In this article, we first describe the prevalence of preoperative pneumonia in geriatric hip fracture surgery patients as well as demographic and operative differences between patients with and without the disease. We then ask 3 questions: Is preoperative pneumonia an independent risk factor for mortality and adverse outcomes in geriatric hip fracture surgery? Is there a postoperative interval during which the unadjusted mortality rate is higher among patients with preoperative pneumonia? In patients with preoperative pneumonia, what are the predictors of morbidity and mortality?
Methods
Yale University’s Human Investigations Committee approved this retrospective cohort study, which used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for the period 2005 to 2012. ACS-NSQIP is a prospective, multi-institutional outcomes program that collects data on preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes for patients undergoing surgical procedures in inpatient and outpatient settings.14
Unlike administrative databases, which are based on reimbursement data, ACS-NSQIP data are collected by trained surgical clinical reviewers for the purposes of quality improvement and clinical research, and data quality is ensured with routine auditing.15 The program has gained a high degree of respect as a powerful and valid data source in both general16 and orthopedic17 surgery literature. The database offers a particular advantage with respect to the study of preoperative pneumonia: Only patients with new or recently diagnosed pneumonia on antibiotic therapy who meet strict criteria for characteristic findings on chest radiography, clinical signs and symptoms of respiratory illness, and positive cultures are coded as having actively treated pneumonia at time of surgery.15
To identify hip fracture patients over the age of 65 years who underwent operative fixation of a hip fracture, we used Current Procedural Terminology (CPT) hip fracture codes, including 27235 (percutaneous screw fixation), 27236 or 27244 (plate-and-screw fixation), and 27245 (intramedullary device), as well as 27125 (hemiarthroplasty) and 27130 (arthroplasty) for patients with a postoperative International Classification of Disease, Ninth Revision (ICD-9) diagnosis code (820.x, 820.2x, or 820.8) consistent with acute hip fracture.18,19 Procedure type, anesthesia type, and delay from admission to surgery were captured for all procedures.
Preoperative demographics included age, sex, transfer origin, functional status, and body mass index (BMI) category. Binary comorbidities were classified as preoperative anemia (hematocrit, <0.41 for men, <0.36 for women), confusion, dyspnea at rest, uremia (blood urea nitrogen, >6.8 mmol/L), history of cardiovascular disease (congestive heart failure, myocardial infarction, percutaneous coronary intervention, angina pectoris, medically treated hypertension, peripheral vascular disease, or resting claudication), chronic obstructive pulmonary disease, diabetes, renal disease (renal failure or dialysis), and cigarette use in preceding 12 months.20,21 Although preoperative hypotension and respiratory rate are often considered in patients with pneumonia, these variables were not available from the ACS-NSQIP data.6,22Pearson χ2 test for categorical variables was used to compare baseline demographics and operative characteristics between patients with and without pneumonia, and Student t test was used to compare intervals from hospital admission to hip fracture surgery, surgery start to surgery stop, and surgery to discharge between patients with and without preoperative pneumonia.
Binary outcome measures were compared between patients with and without preoperative pneumonia. “Any AE” included any serious AE (SAE) or any minor AE. SAEs included death, acute renal failure, ventilator use >48 hours, unplanned intubation, septic shock, sepsis, return to operating room, coma >24 hours, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, thromboembolic event (deep vein thrombosis or pulmonary embolism), and stroke/cerebrovascular accident. Minor AEs included progressive renal insufficiency, urinary tract infection, organ/space infection, superficial surgical-site infection, deep surgical-site infection, and wound dehiscence. Other binary outcome measures included discharge destination and unplanned readmission within 30 days after hip fracture surgery.23Poisson regression with robust error variance as described by Zou24 was used to compare the rates of any, minor, and individual AEs, and any SAEs, between patients with and without pneumonia. Multivariate analysis accounted for the baseline variables in Table 1. AEs that occurred more than once in each group were included in the analyses.
Kaplan-Meier survival analysis was performed for postoperative mortality within 30 days. Within the preoperative pneumonia group, covariates from Table 1 were identified as predictors of any AE, SAE, or death within 30 days after hip fracture surgery by stepwise multivariate Poisson regression with robust error variance. When interval from admission to surgery was longer than 24, 48, 72, or 96 hours, it was also included as a covariate. Variables that did not show an association with AEs at the P < .20 level were not included in the final regression model. All analyses were performed with Stata/SE Version 12.0 statistical software (StataCorp).
Results
Of the 7128 geriatric hip fracture patients in this study, 82 (1.2%) had active pneumonia at time of surgery (Table 1). Age, BMI, preoperative uremia, history of cardiovascular disease, diabetes, renal disease, and smoking were similar between groups. In addition, there was no difference in anesthesia type or fixation procedure between the pneumonia and no-pneumonia groups. Patients with preoperative pneumonia differed significantly with respect to sex, transfer from facility, preoperative functional dependence, anemia, confusion, dyspnea at rest, and history of chronic obstructive pulmonary disease (Table 1).
Interval from admission to surgery was longer (P < .001) for geriatric hip fracture patients with preoperative pneumonia (mean, 6.8 days; 95% CI, 2.5-11.1 days) than for those without pneumonia (mean, 1.5 days; CI, 1.4-1.5 days). There was no difference (P = .124) in operative time between the pneumonia group (mean, 72.8 min; CI, 64.0-81.5 min) and the no-pneumonia group (mean, 66.1 min; CI, 61.2-67.0 min). Interval from surgery to discharge was longer (P < .001) for patients with preoperative pneumonia (mean, 10.1 days; CI, 6.9-13.4 days) than for those without pneumonia (mean, 6.3 days; CI, 6.1-6.4 days).
Adverse outcomes of geriatric hip fracture surgery are listed in Table 2. In the multivariate analysis, preoperative pneumonia was significantly associated with any AE (relative risk [RR]) = 1.44) and any SAE (RR = 1.79).
Survival patterns diverged between patients with and without preoperative pneumonia (Figure). The unadjusted mortality rate was qualitatively higher in patients with preoperative pneumonia than in patients without pneumonia during the first days after hip fracture (slopes of unadjusted mortality curves in Figure). Of note, no patient under age 75 years with pneumonia at time of surgery died within the 30-day study period.
Among geriatric hip fracture patients with preoperative pneumonia, multivariate analyses revealed no significant association of any preoperative comorbidity with any AE or any SAE. Given the gravity of the death complication, however, death within 30 days after surgery was analyzed separately, and was found to be significantly associated (RR = 4.67) with being underweight (BMI, <18.5 kg/m2) (Table 3). Admission-to-surgery interval longer than 24, 48, 72, or 96 hours did not reach significance at the P < 0.2 level in the stepwise regressions and therefore was not associated with a higher or lower risk of any AE, SAE, or death.
Discussion
In the general US population, pneumonia accounts for 1.4% of deaths in people 65 years to 74 years old, 2.1% in people 75 years to 84 years, and 3.1% in people 85 years or older. In total, 3.4% of hospital inpatient deaths are attributed to pneumonia.25 In hospitalized general orthopedic surgical patients as well as hip fracture patients, pneumonia is strongly associated with increased mortality.26,27
We identified a preoperative pneumonia prevalence of 1.2%, which is comparable to the rates reported in the literature (0.3%-3.2%).1-3 To our knowledge, our study represents the largest series of patients with preoperative pneumonia at time of hip fracture repair, and the first to independently associate preoperative pneumonia with increased incidence of AEs, including death.
This study had its limitations. First, the ACS-NSQIP morbidity and mortality data, which are limited to the first 30 postoperative days, may be skewed because AEs that occurred after that interval are not captured. Second, coding of pneumonia in ACS-NSQIP does not convey specific information about the disease and its severity—infectious organism(s) responsible; acquisition setting (healthcare or community); treatment given, including antibiotic(s) selection, steroid use, dosing, and duration; and measures of treatment efficacy—limiting interpretation of the difference in delay to surgery. We cannot say whether the longer interval in patients with pneumonia reflects medical optimization, or whether the delay itself or any interventions during that time positively or negatively affected outcomes. In addition, despite using a large national database, we obtained a relatively small sample of patients (82) who had pneumonia before surgical hip fracture repair.
Multivariate analysis controlling for baseline demographics and comorbidities revealed that multiple SAEs were independently associated with preoperative pneumonia (overall SAE, RR = 1.79). Postoperative use of ventilator support for longer than 48 hours (RR = 6.48) and coma longer than 24 hours (RR = 7.31) are expected given the severity of pulmonary compromise in the study cohort.28,29 Acute renal failure (RR = 14.61) can occur in both hip fracture patients and community-acquired pneumonia patients and may be a multifactorial complication of the pulmonary infection, of the anesthesia, or of the surgical intervention in this cohort.30-32Unadjusted mortality in hip fracture takes months to a year to normalize to that of age-matched controls.32-34 In our series, the unadjusted death rate in the pneumonia cohort (Figure) was transiently elevated during the first weeks after surgery but then drew nearer the rate in the nondiseased hip fracture cohort by the end of the first month. Early death in the pneumonia group likely was multifactorial, potentially influenced by the increased burden of comorbidities in the pneumonia group at baseline, and the longer delay to surgery,35-38 as well as by the natural history of treated pneumonia in hospital patients, who, compared with age-matched hospitalized controls, also exhibit higher mortality during only the first 2 to 4 months of hospitalization for pneumonia.39 We regret that quality improvement strategies in the treatment of geriatric hip fracture surgery with pneumonia cannot be extrapolated from these results.
Similarly, the utility of BMI <18.5 kg/m2 as an actionable preoperative finding cannot be assessed from these results. However, we propose that underweight geriatric hip fracture patients with pneumonia may benefit from more aggressive preoperative optimization that does not delay surgery. Higher acuity of postoperative care, including more intensive nursing care and early coordination of care with respiratory therapists and medical comanagement teams, may also be beneficial.
Anesthesia type did not differ between patients with and without preoperative pneumonia and was not associated with AEs in patients with preoperative pneumonia. Consistent with our findings, multiple studies have reported no significant differences in short-term outcomes of hip fracture repair between general and spinal anesthesia, though no other study has compared the benefits of general and spinal anesthesia for patients with preoperative pneumonia.40-44 Although spinal anesthesia (relative to general anesthesia) has been reported to have benefits in hip and knee arthroplasty, these benefits appear not to translate to hip fracture repair.45-50 The results of the present study suggest that general and spinal anesthesia may be equivalent in terms of risk for the geriatric hip fracture patient with preoperative pneumonia.43,44Our attempt to evaluate the CURB-65 pneumonia severity score as a prognosticator of AEs was thwarted by the absence of required variables in the ACS-NSQIP dataset (confusion, uremia, dyspnea, and age were available; hypotension and blood pressure were not). In our analysis, we did include, individually, variables previously found to predict AEs in the medical pneumonia population (confusion, uremia, dyspnea at rest, anemia).9-11,32 However, these clinical findings are nonspecific in hip fracture patients, who may become anemic, confused, dyspneic, or uremic from a multitude of factors related to their injury and unrelated to pneumonia, including but not limited to hemorrhage, muscle damage, renal injury, and pulmonary embolism. It is not surprising that confusion, uremia, dyspnea at rest, and anemia were not individually predictive of AEs or death within 30 days after surgery in the cohort of geriatric hip fracture patients with pneumonia.
There is no literature that argues for or against delaying hip fracture surgery in geriatric hip fracture patients with pneumonia. The surgical delay observed in this population is ostensibly related to medical optimization of the pneumonia and/or underlying comorbidities. However, we did not find a morbidity or mortality detriment or benefit in delaying surgery by 1 to 4 days in this population. Delay of surgery is a poor covariate, given extensive confounding by medical management and preoperative optimizing of comorbid conditions (reflected in our independent variable and covariates) as well as institutional and surgeon variations in policy and behavior and other unaccounted influences. Although some authors have found no difference in mortality or major AEs between hip fracture patients who had a surgical delay and those who did not,31,51-53 other series and meta-analyses have suggested a mortality detriment in a surgical delay of more than 2 days36,54 or 4 days55 from admission. Given our data, we cannot recommend against immediate hip fracture repair in the subpopulation of geriatric hip fracture patients with pneumonia.
Our study findings suggest that preoperative pneumonia is a rare independent risk factor for AEs after hip fracture surgery in geriatric patients. Underweight BMI is predictive of death in geriatric hip fracture surgery patients who present with pneumonia, whereas early surgical repair appears not to be associated with adverse outcomes. Further investigation is warranted to determine if such patients benefit from specific preoperative and postoperative strategies for optimizing medical and surgical care based on these findings.
Am J Orthop. 2017;46(3):E177-E185. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sexson SB, Lehner JT. Factors affecting hip fracture mortality. J Orthop Trauma. 1987;1(4):298-305.
2. Mullen JO, Mullen NL. Hip fracture mortality: a prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;(280):214-222.
3. Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;(186):45-56.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24(4):701-719.
5. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382.
7. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BDW. The CURB (confusion, urea, respiratory rate and blood pressure) criteria in community-acquired pneumonia (CAP) in hospitalised elderly patients aged 65 years and over: a prospective observational cohort study. Age Ageing. 2005;34(1):75-77.
8. Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care. 2004;10(1):59-64.
9. Buising K, Thursky K, Black J, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419-424.
10. Ewig S, De Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421-427.
11. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572-1579.
12. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223.
13. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med. 2006;260(1):93-101.
14. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837-843.
15. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed October 8, 2014.
16. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267.
17. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889.
18. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42.
19. Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620-625.
20. Fisher MA, Matthei JD, Obirieze A, et al. Open reduction internal fixation versus hemiarthroplasty versus total hip arthroplasty in the elderly: a review of the National Surgical Quality Improvement Program database. J Surg Res. 2013;181(2):193-198.
21. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63-69.
22. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134-141.
23. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
24. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004:159(7):702-706.
25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 20138;61(4):1-117.
26. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84(4):562-572.
27. Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
28. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
29. Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21(1):24-31.
30. Urwin S, Parker M, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455.
31. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
32. Niederman MS, Mandell LA, Anzueto A, et al; American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754.
33. Koval KJ, Skovron ML, Aharonoff GB, Zuckerman JD. Predictors of functional recovery after hip fracture in the elderly. Clin Orthop Relat Res. 1998;(348):22-28.
34. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
35. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology. 2000;39(4):346-349.
36. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947-951.
37. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709.
38. Simunovic N, Devereaux P, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616.
39. Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317-323.
40. Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521.
41. Chakladar A, White SM. Cost estimates of spinal versus general anaesthesia for fractured neck of femur surgery. Anaesthesia. 2010;65(8):810-814.
42. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
43. Gilbert TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general anesthesia for hip fracture repair: a longitudinal observation of 741 elderly patients during 2-year follow-up. Am J Orthop. 2000;29(1):25-35.
44. O’Hara DA, Duff A, Berlin JA, et al. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92(4):947-957.
45. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279-287.
46. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006;49(6):391-396.
47. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279-284.
48. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103(4):1018-1025.
49. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-942.
50. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
51. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697.
52. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559.
53. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489.
54. Shiga T, Wajima Zi, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154.
55. Streubel P, Ricci W, Wong A, Gardner M. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
1. Sexson SB, Lehner JT. Factors affecting hip fracture mortality. J Orthop Trauma. 1987;1(4):298-305.
2. Mullen JO, Mullen NL. Hip fracture mortality: a prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;(280):214-222.
3. Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;(186):45-56.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24(4):701-719.
5. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382.
7. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BDW. The CURB (confusion, urea, respiratory rate and blood pressure) criteria in community-acquired pneumonia (CAP) in hospitalised elderly patients aged 65 years and over: a prospective observational cohort study. Age Ageing. 2005;34(1):75-77.
8. Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care. 2004;10(1):59-64.
9. Buising K, Thursky K, Black J, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419-424.
10. Ewig S, De Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421-427.
11. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572-1579.
12. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223.
13. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med. 2006;260(1):93-101.
14. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837-843.
15. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed October 8, 2014.
16. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267.
17. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889.
18. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42.
19. Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620-625.
20. Fisher MA, Matthei JD, Obirieze A, et al. Open reduction internal fixation versus hemiarthroplasty versus total hip arthroplasty in the elderly: a review of the National Surgical Quality Improvement Program database. J Surg Res. 2013;181(2):193-198.
21. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63-69.
22. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134-141.
23. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
24. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004:159(7):702-706.
25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 20138;61(4):1-117.
26. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84(4):562-572.
27. Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
28. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
29. Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21(1):24-31.
30. Urwin S, Parker M, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455.
31. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
32. Niederman MS, Mandell LA, Anzueto A, et al; American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754.
33. Koval KJ, Skovron ML, Aharonoff GB, Zuckerman JD. Predictors of functional recovery after hip fracture in the elderly. Clin Orthop Relat Res. 1998;(348):22-28.
34. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
35. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology. 2000;39(4):346-349.
36. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947-951.
37. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709.
38. Simunovic N, Devereaux P, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616.
39. Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317-323.
40. Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521.
41. Chakladar A, White SM. Cost estimates of spinal versus general anaesthesia for fractured neck of femur surgery. Anaesthesia. 2010;65(8):810-814.
42. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
43. Gilbert TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general anesthesia for hip fracture repair: a longitudinal observation of 741 elderly patients during 2-year follow-up. Am J Orthop. 2000;29(1):25-35.
44. O’Hara DA, Duff A, Berlin JA, et al. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92(4):947-957.
45. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279-287.
46. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006;49(6):391-396.
47. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279-284.
48. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103(4):1018-1025.
49. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-942.
50. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
51. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697.
52. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559.
53. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489.
54. Shiga T, Wajima Zi, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154.
55. Streubel P, Ricci W, Wong A, Gardner M. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
VIDEO: Alectinib doubles PFS and then some over crizotinib in ALK+ NSCLC
CHICAGO – The standard of care for patients with non–small cell lung cancer positive for the anaplastic lymphoma kinase (ALK) is the ALK inhibitor crizotinib (Xalkori). However, many patients on crizotinib will have disease progression within the first year of therapy, and many will go on to have central nervous system (CNS) metastases.
The multicenter international ALEX trial compared crizotinib with the second-generation ALK inhibitor alectinib (Alecensa). The investigators found that alectinib reduced the risk of progression by 53% and the time to CNS progression by 84%.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Alice T. Shaw, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, outlines the ALEX trial results, which are being hailed as “practice changing.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The standard of care for patients with non–small cell lung cancer positive for the anaplastic lymphoma kinase (ALK) is the ALK inhibitor crizotinib (Xalkori). However, many patients on crizotinib will have disease progression within the first year of therapy, and many will go on to have central nervous system (CNS) metastases.
The multicenter international ALEX trial compared crizotinib with the second-generation ALK inhibitor alectinib (Alecensa). The investigators found that alectinib reduced the risk of progression by 53% and the time to CNS progression by 84%.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Alice T. Shaw, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, outlines the ALEX trial results, which are being hailed as “practice changing.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The standard of care for patients with non–small cell lung cancer positive for the anaplastic lymphoma kinase (ALK) is the ALK inhibitor crizotinib (Xalkori). However, many patients on crizotinib will have disease progression within the first year of therapy, and many will go on to have central nervous system (CNS) metastases.
The multicenter international ALEX trial compared crizotinib with the second-generation ALK inhibitor alectinib (Alecensa). The investigators found that alectinib reduced the risk of progression by 53% and the time to CNS progression by 84%.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Alice T. Shaw, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, outlines the ALEX trial results, which are being hailed as “practice changing.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASCO 2017
VIDEO: Combined immunotherapy strategy shows promise in advanced solid tumors
CHICAGO – Adding an experimental immune-enhancing agent to a checkpoint inhibitor was safe and showed early promise of activity against advanced solid tumors in a phase I/IIa clinical trial.
BMS-986156 is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucorticoid-induced tumor necrosis factor receptor-related gene. The drug acts synergistically with the programmed-death 1 inhibitor (PD-1) nivolumab (Opdivo) by increasing survival of T effector cells, promoting regulatory T-cell depletion and reduction, and reducing regulatory T cell suppression of T effector cells to produce a more robust antitumor immune response.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Lillian Siu, MD, from the Princess Margaret Hospital, Toronto, describes how the combination has induced durable partial responses in patients with tumors thought to be insensitive to immunotherapy, as well as patients who had disease progression while on a PD-1 inhibitor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Adding an experimental immune-enhancing agent to a checkpoint inhibitor was safe and showed early promise of activity against advanced solid tumors in a phase I/IIa clinical trial.
BMS-986156 is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucorticoid-induced tumor necrosis factor receptor-related gene. The drug acts synergistically with the programmed-death 1 inhibitor (PD-1) nivolumab (Opdivo) by increasing survival of T effector cells, promoting regulatory T-cell depletion and reduction, and reducing regulatory T cell suppression of T effector cells to produce a more robust antitumor immune response.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Lillian Siu, MD, from the Princess Margaret Hospital, Toronto, describes how the combination has induced durable partial responses in patients with tumors thought to be insensitive to immunotherapy, as well as patients who had disease progression while on a PD-1 inhibitor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Adding an experimental immune-enhancing agent to a checkpoint inhibitor was safe and showed early promise of activity against advanced solid tumors in a phase I/IIa clinical trial.
BMS-986156 is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucorticoid-induced tumor necrosis factor receptor-related gene. The drug acts synergistically with the programmed-death 1 inhibitor (PD-1) nivolumab (Opdivo) by increasing survival of T effector cells, promoting regulatory T-cell depletion and reduction, and reducing regulatory T cell suppression of T effector cells to produce a more robust antitumor immune response.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Lillian Siu, MD, from the Princess Margaret Hospital, Toronto, describes how the combination has induced durable partial responses in patients with tumors thought to be insensitive to immunotherapy, as well as patients who had disease progression while on a PD-1 inhibitor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASCO 2017
Scheduling patterns: Time for a change?
Bob Wachter, MD, created buzz in March 2016 when, at the SHM annual meeting in San Diego, he displayed a slide titled “What did we get wrong?” The slide contained the copy, “Hospitalist shifts run 7 a.m.-7 p.m.; 10 a.m.-10 p.m. 7on/7off” circled in bold red.
Over the last several years, thought leaders in the hospital medicine field have expressed concern that this one-size-fits-all schedule model is a threat to the well-being of many physicians and, by extension, the sustainability of their hospital medicine groups. Despite this, the 2016 State of Hospital Medicine Report reveals relatively little change in the way hospital medicine groups schedule their physicians.
Night shifts echo this trend. There is an even greater number of groups utilizing the 12- to 13.9-hour shift length (79%), which has also varied less at just approximately 5% in either direction over the last two surveys. It is likely very hard to be creative with the shift length for your night physicians when the group is structured predominately around a 12-hour day position.
The 12-hour shift scheduled in long blocks is straightforward to employ for the scheduler, limits hand offs of care, and maximizes number of days off. So, why are Wachter et al. calling for change? Seven day stretches off may seem attractive when you are just starting out, but, as physicians mature, the very long day competes with family time that cannot be made up on weekday mornings when others are at school and work. Furthermore, the very long hours for 7 days straight lead to burn out and eventually retention issues as well. Some argue that this design promotes disengagement. It sets the expectation that, during “off” weeks, physicians might be unavailable for email responses, committee meetings, or participation in quality improvement initiatives, which disrupts integration into the larger hospital community and perhaps even our own career advancement.
Some groups are trying to address these concerns with innovative approaches to block scheduling. While the hallmark hospital medicine schedule of 7on/7off blocks remains the predominant model – 38.1% of all groups – this represents a drop of approximately 15%, compared with the prior survey. A new large contingent of groups entering the survey this year utilize a Monday-Friday model with rotating moonlighter/weekend coverage. This lifestyle and family-friendly model predominates in the Midwest. It is also found more in smaller groups, which may employ this model to keep the most system-knowledgeable worker around during high volume times, as well as to preserve the well-being and retention of their limited physician work force.
Of note, reconfiguring the 7on/7off model does not necessarily translate into more time off. The median number of shifts per year is also relatively stable at 182 which is the exact number of shifts per year in a strict 7on/7off schedule. This number does not vary by region of the country, group size, or teaching status. Some might argue that working 182 annual shifts is ideal, giving hospitalists a “vacation” every other week. However, this line of thought does not take into account the very long workdays, nor the 52 weekend days spent in the hospital – far more than most specialty peers who serve fewer weekend calls often with more limited in-house hours. In addition, one might argue that defining ourselves as available only during our 182 clinical “on” days is not in our own best interest, as it is the important nonclinical quality and committee service activities that are likely to lead to professional recognition and advancement.
Our hospital medicine group has deviated from this scheduling mainstay and requires only 160 shifts per year. We have set this number based on removal of the number of shifts equivalent to the vacation hours received by our medical group peers. The model poses a challenge in terms of matching our productivity up to benchmarks when talking to system leaders. This challenge pales in comparison to the increased buy-in from our physicians, as they feel equitable vacation time signifies respect from the medical group leadership.
In addition, our group has had success in being flexible around the number of days worked in a continuity stretch. We utilize everything from a 3-day block over holidays to a 7-day block. In general, we allow physicians to select their desired block length. The scheduler then works to accommodate that stretch as much as is feasible. The upfront work in this system is significant, but the downstream effect is decreased turnover costs. Even our own entrenched standard of 7on/7off schedules for house staff services (designed to protect continuity for the learner) have been the target of change. A pilot of alternating 4 and 5 day runs in a 4-week stretch has been implemented over the last few months. The number of days the residents are exposed to a given attending is the same in this model, but there is one additional switch day. The additional switch day puts the residents at risk of managing a change in care plan related to change in attending, but this was mitigated by paring attendings with very similar teaching and patient management styles. For our group, the extra administrative effort needed to work around the 7on/7off model has always paid off in terms of provider satisfaction and retention.
On the other hand, although I lead a large academic group, we have not yet developed flexibility around the shift length. Only one of the 29 roles our providers fill each 24-hour period is not a 12-hour shift. Over the years, I have tried to offer alternate models with shorter shifts to improve flow, reduce burn out, and increase family time. No matter how eloquent the reasoning, the response from the group was always the same: a resounding “no.” Most providers felt that they would wind up with a very similar work load and not actually leave the hospital earlier. Other reasons included not wanting to come in more days per month and concerns about increased handoffs/cross coverage.
There is some reason to think change may actually come. For one, burnout is high and may lead physicians to try a new model even with fear of the unknown. Our practice may be reconsidering this one-size-fits-all shift length in the very near future as an increasing percentage of candidates seeking to join our group express a strong interest in finding more accommodating hours.
Overall, I am hopeful that, in the coming years, my hospital medicine group, as well as many others, will heed the thoughts expressed by Dr. Wachter. Finding the flexibility to break out of these rigid scheduling models will be a first step in promoting both physician and system well being.
Dr. Eisenstock, MD, FHM, is clinical chief, division of hospital medicine, at the University of Massachusetts Memorial Health Care, Worcester.
Bob Wachter, MD, created buzz in March 2016 when, at the SHM annual meeting in San Diego, he displayed a slide titled “What did we get wrong?” The slide contained the copy, “Hospitalist shifts run 7 a.m.-7 p.m.; 10 a.m.-10 p.m. 7on/7off” circled in bold red.
Over the last several years, thought leaders in the hospital medicine field have expressed concern that this one-size-fits-all schedule model is a threat to the well-being of many physicians and, by extension, the sustainability of their hospital medicine groups. Despite this, the 2016 State of Hospital Medicine Report reveals relatively little change in the way hospital medicine groups schedule their physicians.
Night shifts echo this trend. There is an even greater number of groups utilizing the 12- to 13.9-hour shift length (79%), which has also varied less at just approximately 5% in either direction over the last two surveys. It is likely very hard to be creative with the shift length for your night physicians when the group is structured predominately around a 12-hour day position.
The 12-hour shift scheduled in long blocks is straightforward to employ for the scheduler, limits hand offs of care, and maximizes number of days off. So, why are Wachter et al. calling for change? Seven day stretches off may seem attractive when you are just starting out, but, as physicians mature, the very long day competes with family time that cannot be made up on weekday mornings when others are at school and work. Furthermore, the very long hours for 7 days straight lead to burn out and eventually retention issues as well. Some argue that this design promotes disengagement. It sets the expectation that, during “off” weeks, physicians might be unavailable for email responses, committee meetings, or participation in quality improvement initiatives, which disrupts integration into the larger hospital community and perhaps even our own career advancement.
Some groups are trying to address these concerns with innovative approaches to block scheduling. While the hallmark hospital medicine schedule of 7on/7off blocks remains the predominant model – 38.1% of all groups – this represents a drop of approximately 15%, compared with the prior survey. A new large contingent of groups entering the survey this year utilize a Monday-Friday model with rotating moonlighter/weekend coverage. This lifestyle and family-friendly model predominates in the Midwest. It is also found more in smaller groups, which may employ this model to keep the most system-knowledgeable worker around during high volume times, as well as to preserve the well-being and retention of their limited physician work force.
Of note, reconfiguring the 7on/7off model does not necessarily translate into more time off. The median number of shifts per year is also relatively stable at 182 which is the exact number of shifts per year in a strict 7on/7off schedule. This number does not vary by region of the country, group size, or teaching status. Some might argue that working 182 annual shifts is ideal, giving hospitalists a “vacation” every other week. However, this line of thought does not take into account the very long workdays, nor the 52 weekend days spent in the hospital – far more than most specialty peers who serve fewer weekend calls often with more limited in-house hours. In addition, one might argue that defining ourselves as available only during our 182 clinical “on” days is not in our own best interest, as it is the important nonclinical quality and committee service activities that are likely to lead to professional recognition and advancement.
Our hospital medicine group has deviated from this scheduling mainstay and requires only 160 shifts per year. We have set this number based on removal of the number of shifts equivalent to the vacation hours received by our medical group peers. The model poses a challenge in terms of matching our productivity up to benchmarks when talking to system leaders. This challenge pales in comparison to the increased buy-in from our physicians, as they feel equitable vacation time signifies respect from the medical group leadership.
In addition, our group has had success in being flexible around the number of days worked in a continuity stretch. We utilize everything from a 3-day block over holidays to a 7-day block. In general, we allow physicians to select their desired block length. The scheduler then works to accommodate that stretch as much as is feasible. The upfront work in this system is significant, but the downstream effect is decreased turnover costs. Even our own entrenched standard of 7on/7off schedules for house staff services (designed to protect continuity for the learner) have been the target of change. A pilot of alternating 4 and 5 day runs in a 4-week stretch has been implemented over the last few months. The number of days the residents are exposed to a given attending is the same in this model, but there is one additional switch day. The additional switch day puts the residents at risk of managing a change in care plan related to change in attending, but this was mitigated by paring attendings with very similar teaching and patient management styles. For our group, the extra administrative effort needed to work around the 7on/7off model has always paid off in terms of provider satisfaction and retention.
On the other hand, although I lead a large academic group, we have not yet developed flexibility around the shift length. Only one of the 29 roles our providers fill each 24-hour period is not a 12-hour shift. Over the years, I have tried to offer alternate models with shorter shifts to improve flow, reduce burn out, and increase family time. No matter how eloquent the reasoning, the response from the group was always the same: a resounding “no.” Most providers felt that they would wind up with a very similar work load and not actually leave the hospital earlier. Other reasons included not wanting to come in more days per month and concerns about increased handoffs/cross coverage.
There is some reason to think change may actually come. For one, burnout is high and may lead physicians to try a new model even with fear of the unknown. Our practice may be reconsidering this one-size-fits-all shift length in the very near future as an increasing percentage of candidates seeking to join our group express a strong interest in finding more accommodating hours.
Overall, I am hopeful that, in the coming years, my hospital medicine group, as well as many others, will heed the thoughts expressed by Dr. Wachter. Finding the flexibility to break out of these rigid scheduling models will be a first step in promoting both physician and system well being.
Dr. Eisenstock, MD, FHM, is clinical chief, division of hospital medicine, at the University of Massachusetts Memorial Health Care, Worcester.
Bob Wachter, MD, created buzz in March 2016 when, at the SHM annual meeting in San Diego, he displayed a slide titled “What did we get wrong?” The slide contained the copy, “Hospitalist shifts run 7 a.m.-7 p.m.; 10 a.m.-10 p.m. 7on/7off” circled in bold red.
Over the last several years, thought leaders in the hospital medicine field have expressed concern that this one-size-fits-all schedule model is a threat to the well-being of many physicians and, by extension, the sustainability of their hospital medicine groups. Despite this, the 2016 State of Hospital Medicine Report reveals relatively little change in the way hospital medicine groups schedule their physicians.
Night shifts echo this trend. There is an even greater number of groups utilizing the 12- to 13.9-hour shift length (79%), which has also varied less at just approximately 5% in either direction over the last two surveys. It is likely very hard to be creative with the shift length for your night physicians when the group is structured predominately around a 12-hour day position.
The 12-hour shift scheduled in long blocks is straightforward to employ for the scheduler, limits hand offs of care, and maximizes number of days off. So, why are Wachter et al. calling for change? Seven day stretches off may seem attractive when you are just starting out, but, as physicians mature, the very long day competes with family time that cannot be made up on weekday mornings when others are at school and work. Furthermore, the very long hours for 7 days straight lead to burn out and eventually retention issues as well. Some argue that this design promotes disengagement. It sets the expectation that, during “off” weeks, physicians might be unavailable for email responses, committee meetings, or participation in quality improvement initiatives, which disrupts integration into the larger hospital community and perhaps even our own career advancement.
Some groups are trying to address these concerns with innovative approaches to block scheduling. While the hallmark hospital medicine schedule of 7on/7off blocks remains the predominant model – 38.1% of all groups – this represents a drop of approximately 15%, compared with the prior survey. A new large contingent of groups entering the survey this year utilize a Monday-Friday model with rotating moonlighter/weekend coverage. This lifestyle and family-friendly model predominates in the Midwest. It is also found more in smaller groups, which may employ this model to keep the most system-knowledgeable worker around during high volume times, as well as to preserve the well-being and retention of their limited physician work force.
Of note, reconfiguring the 7on/7off model does not necessarily translate into more time off. The median number of shifts per year is also relatively stable at 182 which is the exact number of shifts per year in a strict 7on/7off schedule. This number does not vary by region of the country, group size, or teaching status. Some might argue that working 182 annual shifts is ideal, giving hospitalists a “vacation” every other week. However, this line of thought does not take into account the very long workdays, nor the 52 weekend days spent in the hospital – far more than most specialty peers who serve fewer weekend calls often with more limited in-house hours. In addition, one might argue that defining ourselves as available only during our 182 clinical “on” days is not in our own best interest, as it is the important nonclinical quality and committee service activities that are likely to lead to professional recognition and advancement.
Our hospital medicine group has deviated from this scheduling mainstay and requires only 160 shifts per year. We have set this number based on removal of the number of shifts equivalent to the vacation hours received by our medical group peers. The model poses a challenge in terms of matching our productivity up to benchmarks when talking to system leaders. This challenge pales in comparison to the increased buy-in from our physicians, as they feel equitable vacation time signifies respect from the medical group leadership.
In addition, our group has had success in being flexible around the number of days worked in a continuity stretch. We utilize everything from a 3-day block over holidays to a 7-day block. In general, we allow physicians to select their desired block length. The scheduler then works to accommodate that stretch as much as is feasible. The upfront work in this system is significant, but the downstream effect is decreased turnover costs. Even our own entrenched standard of 7on/7off schedules for house staff services (designed to protect continuity for the learner) have been the target of change. A pilot of alternating 4 and 5 day runs in a 4-week stretch has been implemented over the last few months. The number of days the residents are exposed to a given attending is the same in this model, but there is one additional switch day. The additional switch day puts the residents at risk of managing a change in care plan related to change in attending, but this was mitigated by paring attendings with very similar teaching and patient management styles. For our group, the extra administrative effort needed to work around the 7on/7off model has always paid off in terms of provider satisfaction and retention.
On the other hand, although I lead a large academic group, we have not yet developed flexibility around the shift length. Only one of the 29 roles our providers fill each 24-hour period is not a 12-hour shift. Over the years, I have tried to offer alternate models with shorter shifts to improve flow, reduce burn out, and increase family time. No matter how eloquent the reasoning, the response from the group was always the same: a resounding “no.” Most providers felt that they would wind up with a very similar work load and not actually leave the hospital earlier. Other reasons included not wanting to come in more days per month and concerns about increased handoffs/cross coverage.
There is some reason to think change may actually come. For one, burnout is high and may lead physicians to try a new model even with fear of the unknown. Our practice may be reconsidering this one-size-fits-all shift length in the very near future as an increasing percentage of candidates seeking to join our group express a strong interest in finding more accommodating hours.
Overall, I am hopeful that, in the coming years, my hospital medicine group, as well as many others, will heed the thoughts expressed by Dr. Wachter. Finding the flexibility to break out of these rigid scheduling models will be a first step in promoting both physician and system well being.
Dr. Eisenstock, MD, FHM, is clinical chief, division of hospital medicine, at the University of Massachusetts Memorial Health Care, Worcester.
BIO-RESORT: A mandate to prescreen PCI patients for silent diabetes
PARIS – Undetected diabetes and prediabetes are pervasive in patients undergoing percutaneous coronary intervention, and they’re associated with a sharply increased risk of major adverse cardiovascular events, according to the results of the potentially practice-changing BIO-RESORT Silent Diabetes Study, Clemens von Birgelen, MD, PhD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Our data support screening PCI all-comers for silent diabetes, which may help identify patients with an increased event risk and improve their therapy,” said Dr. von Birgelen, professor of cardiology at the Thoraxcentrum of Twente, a high-volume center for cardiac interventions in Enschede, the Netherlands.
A substantial one-third of subjects turned out to have abnormal glucose tolerance according to World Health Organization criteria and an International Expert Committee Report (Diabetes Care. 2009 Jul;32[7]:1327-34). In a multivariate analysis, their 1-year rate of the primary study endpoint – target vessel failure, a composite of cardiac death, target vessel-related MI, or target vessel revascularization – was an adjusted 2.2 times greater than in the 788 normoglycemic patients.
Moreover, among the 7% of study participants who met diagnostic criteria for silent diabetes, the risk of target vessel failure was more than 4.4 times greater than in the normoglycemic group.
“To a very great extent, periprocedural MI is the driving force behind this difference that we saw. From a biological point of view, I think that the vulnerability of the vessel in the diabetic or prediabetic patient features more brittle plaque with a higher risk of cholesterol embolization, and with more plaque mass that can be pushed to the side so that side branch vessels can become occluded, leading to periprocedural MI,” he observed.
Glucose metabolism was assessed in all participants by two methods using the conventional cutoffs: a 2-hour oral glucose tolerance test (OGTT), and the combination of fasting plasma glucose and hemoglobin A1c. By OGTT, 7% of patients had silent, previously unrecognized diabetes and another 13% had prediabetes. Using the combination of fasting plasma glucose and HbA1c, a total of 25% of subjects had silent diabetes or prediabetes. Fully 33% of participants had abnormal glucose metabolism by one yardstick or the other.
“What we have seen is there is a group of patients that are missed with either. With the OGTT you don’t see all the diabetics, and with HbA1c and fasting blood glucose you also miss some patients,” said Dr. von Birgelen.
The 1-year cumulative incidence of target vessel failure was 13.2% in patients with silent diabetes as identified by the OGTT and 12.1% in those detected by the alternative method, compared with rates of 2.8% and 3.1%, respectively, in normoglycemic PCI patients. The event rate was 6.1% in patients with prediabetes by OGTT and similar at 5.5% in those found to be prediabetic based on fasting blood glucose and HbA1c, versus rates of 2.8% and 3.1%, respectively, in normoglycemic patients.
“The findings of this study suggest that post-PCI event risk associated with hyperglycemia is a continuum without a clear threshold effect, extending well beyond the threshold that currently defines diabetes,” Dr. von Birgelen said.
Once again, it’s worth emphasizing that the elevated target vessel failure rates seen in patients with abnormal glucose metabolism were due mostly to increased rates of acute MI within the first 24 hours after PCI. The target vessel–related MI rate was 10.3% in patients with silent diabetes, compared with just 1.8% in normoglycemic controls.
Asked what the take-home message for clinicians is from this study, he noted that the Netherlands has a relatively low prevalence of diabetes, and a highly developed primary care medicine system.
“We have a very good one-to-one relationship between the patient and the GP. So if we find 7% silent diabetes and up to one-third of patients with undetected abnormal glucose tolerance in a country with a relatively low prevalence of diabetes, you may expect that in other countries with a higher prevalence and perhaps a less developed primary care system the rate may be much, much higher,” Dr. von Birgelen cautioned.
The implications for the daily clinical practice of interventional cardiology are clear, he continued: “We’ve seen in several trials that the new stents are doing a fantastic job. So if we want to further improve the outcomes in our patients we have to do something else. We should look for subgroups of our PCI patients who have a particularly high risk. And we all realize that diabetics are such a problem, but I think we have shown that the prediabetic patients are also important. So we should identify and pretreat these patients, perhaps with aggressive lipid-lowering therapy during the weeks before a scheduled elective PCI.”
“There are data showing that with aggressive lipid-lowering you might reduce the risk of periprocedural MI,” the cardiologist noted.
As a practical matter, screening via fasting blood glucose and HbA1c is probably the way to go in clinical practice, according to Dr. von Birgelen.
“In this study, we performed the OGTT because it is still considered by many the gold standard. But there is increasing evidence favoring HbA1c data and fasting blood glucose,” he said.
Other possible pre-PCI interventions worthy of consideration in patients found to have previously unsuspected abnormal glucose tolerance might include medical therapy aimed at normalizing glucose metabolism, as well as perhaps resorting to the most potent forms of dual-antiplatelet therapy in patients with stable angina who have impaired glucose tolerance. However, these are possibilities that should be tested in randomized controlled trials before widespread adoption, he added.
The BIO-RESORT Silent Diabetes Study, which will continue for 5 years of post-PCI follow-up, is a prespecified substudy of the previously reported BIO-RESORT trial, which addressed another issue entirely. It was a three-arm, patient-blinded clinical trial comparing 1-year safety and efficacy outcomes in nearly 3,500 PCI patients randomized to PCI with very thin strut biodegradable polymer everolimus- or sirolimus-eluting stents or a durable polymer zotarolimus-eluting stent. Outcomes proved noninferior across the three treatment groups (Lancet. 2016 Nov 26;388[10060]:2607-17).
Dr. von Birgelen observed that the silent diabetes study broke new ground. Prior studies of PCI outcomes in patients with unrecognized diabetes were limited to recipients of plain old balloon angioplasty, bare metal, or first-generation drug-eluting stents. And studies of PCI in patients with unrecognized prediabetes are virtually nonexistent.
As the principal investigator for both the parent BIO-RESORT trial and the silent diabetes substudy, Dr. von Birgelen received research grants from Biotronik, Boston Scientific, and Medtronic, the cosponsors. He applauded the three companies for funding the silent diabetes substudy in the interest of science even though it had no commercial relevance to their stent businesses.
PARIS – Undetected diabetes and prediabetes are pervasive in patients undergoing percutaneous coronary intervention, and they’re associated with a sharply increased risk of major adverse cardiovascular events, according to the results of the potentially practice-changing BIO-RESORT Silent Diabetes Study, Clemens von Birgelen, MD, PhD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Our data support screening PCI all-comers for silent diabetes, which may help identify patients with an increased event risk and improve their therapy,” said Dr. von Birgelen, professor of cardiology at the Thoraxcentrum of Twente, a high-volume center for cardiac interventions in Enschede, the Netherlands.
A substantial one-third of subjects turned out to have abnormal glucose tolerance according to World Health Organization criteria and an International Expert Committee Report (Diabetes Care. 2009 Jul;32[7]:1327-34). In a multivariate analysis, their 1-year rate of the primary study endpoint – target vessel failure, a composite of cardiac death, target vessel-related MI, or target vessel revascularization – was an adjusted 2.2 times greater than in the 788 normoglycemic patients.
Moreover, among the 7% of study participants who met diagnostic criteria for silent diabetes, the risk of target vessel failure was more than 4.4 times greater than in the normoglycemic group.
“To a very great extent, periprocedural MI is the driving force behind this difference that we saw. From a biological point of view, I think that the vulnerability of the vessel in the diabetic or prediabetic patient features more brittle plaque with a higher risk of cholesterol embolization, and with more plaque mass that can be pushed to the side so that side branch vessels can become occluded, leading to periprocedural MI,” he observed.
Glucose metabolism was assessed in all participants by two methods using the conventional cutoffs: a 2-hour oral glucose tolerance test (OGTT), and the combination of fasting plasma glucose and hemoglobin A1c. By OGTT, 7% of patients had silent, previously unrecognized diabetes and another 13% had prediabetes. Using the combination of fasting plasma glucose and HbA1c, a total of 25% of subjects had silent diabetes or prediabetes. Fully 33% of participants had abnormal glucose metabolism by one yardstick or the other.
“What we have seen is there is a group of patients that are missed with either. With the OGTT you don’t see all the diabetics, and with HbA1c and fasting blood glucose you also miss some patients,” said Dr. von Birgelen.
The 1-year cumulative incidence of target vessel failure was 13.2% in patients with silent diabetes as identified by the OGTT and 12.1% in those detected by the alternative method, compared with rates of 2.8% and 3.1%, respectively, in normoglycemic PCI patients. The event rate was 6.1% in patients with prediabetes by OGTT and similar at 5.5% in those found to be prediabetic based on fasting blood glucose and HbA1c, versus rates of 2.8% and 3.1%, respectively, in normoglycemic patients.
“The findings of this study suggest that post-PCI event risk associated with hyperglycemia is a continuum without a clear threshold effect, extending well beyond the threshold that currently defines diabetes,” Dr. von Birgelen said.
Once again, it’s worth emphasizing that the elevated target vessel failure rates seen in patients with abnormal glucose metabolism were due mostly to increased rates of acute MI within the first 24 hours after PCI. The target vessel–related MI rate was 10.3% in patients with silent diabetes, compared with just 1.8% in normoglycemic controls.
Asked what the take-home message for clinicians is from this study, he noted that the Netherlands has a relatively low prevalence of diabetes, and a highly developed primary care medicine system.
“We have a very good one-to-one relationship between the patient and the GP. So if we find 7% silent diabetes and up to one-third of patients with undetected abnormal glucose tolerance in a country with a relatively low prevalence of diabetes, you may expect that in other countries with a higher prevalence and perhaps a less developed primary care system the rate may be much, much higher,” Dr. von Birgelen cautioned.
The implications for the daily clinical practice of interventional cardiology are clear, he continued: “We’ve seen in several trials that the new stents are doing a fantastic job. So if we want to further improve the outcomes in our patients we have to do something else. We should look for subgroups of our PCI patients who have a particularly high risk. And we all realize that diabetics are such a problem, but I think we have shown that the prediabetic patients are also important. So we should identify and pretreat these patients, perhaps with aggressive lipid-lowering therapy during the weeks before a scheduled elective PCI.”
“There are data showing that with aggressive lipid-lowering you might reduce the risk of periprocedural MI,” the cardiologist noted.
As a practical matter, screening via fasting blood glucose and HbA1c is probably the way to go in clinical practice, according to Dr. von Birgelen.
“In this study, we performed the OGTT because it is still considered by many the gold standard. But there is increasing evidence favoring HbA1c data and fasting blood glucose,” he said.
Other possible pre-PCI interventions worthy of consideration in patients found to have previously unsuspected abnormal glucose tolerance might include medical therapy aimed at normalizing glucose metabolism, as well as perhaps resorting to the most potent forms of dual-antiplatelet therapy in patients with stable angina who have impaired glucose tolerance. However, these are possibilities that should be tested in randomized controlled trials before widespread adoption, he added.
The BIO-RESORT Silent Diabetes Study, which will continue for 5 years of post-PCI follow-up, is a prespecified substudy of the previously reported BIO-RESORT trial, which addressed another issue entirely. It was a three-arm, patient-blinded clinical trial comparing 1-year safety and efficacy outcomes in nearly 3,500 PCI patients randomized to PCI with very thin strut biodegradable polymer everolimus- or sirolimus-eluting stents or a durable polymer zotarolimus-eluting stent. Outcomes proved noninferior across the three treatment groups (Lancet. 2016 Nov 26;388[10060]:2607-17).
Dr. von Birgelen observed that the silent diabetes study broke new ground. Prior studies of PCI outcomes in patients with unrecognized diabetes were limited to recipients of plain old balloon angioplasty, bare metal, or first-generation drug-eluting stents. And studies of PCI in patients with unrecognized prediabetes are virtually nonexistent.
As the principal investigator for both the parent BIO-RESORT trial and the silent diabetes substudy, Dr. von Birgelen received research grants from Biotronik, Boston Scientific, and Medtronic, the cosponsors. He applauded the three companies for funding the silent diabetes substudy in the interest of science even though it had no commercial relevance to their stent businesses.
PARIS – Undetected diabetes and prediabetes are pervasive in patients undergoing percutaneous coronary intervention, and they’re associated with a sharply increased risk of major adverse cardiovascular events, according to the results of the potentially practice-changing BIO-RESORT Silent Diabetes Study, Clemens von Birgelen, MD, PhD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Our data support screening PCI all-comers for silent diabetes, which may help identify patients with an increased event risk and improve their therapy,” said Dr. von Birgelen, professor of cardiology at the Thoraxcentrum of Twente, a high-volume center for cardiac interventions in Enschede, the Netherlands.
A substantial one-third of subjects turned out to have abnormal glucose tolerance according to World Health Organization criteria and an International Expert Committee Report (Diabetes Care. 2009 Jul;32[7]:1327-34). In a multivariate analysis, their 1-year rate of the primary study endpoint – target vessel failure, a composite of cardiac death, target vessel-related MI, or target vessel revascularization – was an adjusted 2.2 times greater than in the 788 normoglycemic patients.
Moreover, among the 7% of study participants who met diagnostic criteria for silent diabetes, the risk of target vessel failure was more than 4.4 times greater than in the normoglycemic group.
“To a very great extent, periprocedural MI is the driving force behind this difference that we saw. From a biological point of view, I think that the vulnerability of the vessel in the diabetic or prediabetic patient features more brittle plaque with a higher risk of cholesterol embolization, and with more plaque mass that can be pushed to the side so that side branch vessels can become occluded, leading to periprocedural MI,” he observed.
Glucose metabolism was assessed in all participants by two methods using the conventional cutoffs: a 2-hour oral glucose tolerance test (OGTT), and the combination of fasting plasma glucose and hemoglobin A1c. By OGTT, 7% of patients had silent, previously unrecognized diabetes and another 13% had prediabetes. Using the combination of fasting plasma glucose and HbA1c, a total of 25% of subjects had silent diabetes or prediabetes. Fully 33% of participants had abnormal glucose metabolism by one yardstick or the other.
“What we have seen is there is a group of patients that are missed with either. With the OGTT you don’t see all the diabetics, and with HbA1c and fasting blood glucose you also miss some patients,” said Dr. von Birgelen.
The 1-year cumulative incidence of target vessel failure was 13.2% in patients with silent diabetes as identified by the OGTT and 12.1% in those detected by the alternative method, compared with rates of 2.8% and 3.1%, respectively, in normoglycemic PCI patients. The event rate was 6.1% in patients with prediabetes by OGTT and similar at 5.5% in those found to be prediabetic based on fasting blood glucose and HbA1c, versus rates of 2.8% and 3.1%, respectively, in normoglycemic patients.
“The findings of this study suggest that post-PCI event risk associated with hyperglycemia is a continuum without a clear threshold effect, extending well beyond the threshold that currently defines diabetes,” Dr. von Birgelen said.
Once again, it’s worth emphasizing that the elevated target vessel failure rates seen in patients with abnormal glucose metabolism were due mostly to increased rates of acute MI within the first 24 hours after PCI. The target vessel–related MI rate was 10.3% in patients with silent diabetes, compared with just 1.8% in normoglycemic controls.
Asked what the take-home message for clinicians is from this study, he noted that the Netherlands has a relatively low prevalence of diabetes, and a highly developed primary care medicine system.
“We have a very good one-to-one relationship between the patient and the GP. So if we find 7% silent diabetes and up to one-third of patients with undetected abnormal glucose tolerance in a country with a relatively low prevalence of diabetes, you may expect that in other countries with a higher prevalence and perhaps a less developed primary care system the rate may be much, much higher,” Dr. von Birgelen cautioned.
The implications for the daily clinical practice of interventional cardiology are clear, he continued: “We’ve seen in several trials that the new stents are doing a fantastic job. So if we want to further improve the outcomes in our patients we have to do something else. We should look for subgroups of our PCI patients who have a particularly high risk. And we all realize that diabetics are such a problem, but I think we have shown that the prediabetic patients are also important. So we should identify and pretreat these patients, perhaps with aggressive lipid-lowering therapy during the weeks before a scheduled elective PCI.”
“There are data showing that with aggressive lipid-lowering you might reduce the risk of periprocedural MI,” the cardiologist noted.
As a practical matter, screening via fasting blood glucose and HbA1c is probably the way to go in clinical practice, according to Dr. von Birgelen.
“In this study, we performed the OGTT because it is still considered by many the gold standard. But there is increasing evidence favoring HbA1c data and fasting blood glucose,” he said.
Other possible pre-PCI interventions worthy of consideration in patients found to have previously unsuspected abnormal glucose tolerance might include medical therapy aimed at normalizing glucose metabolism, as well as perhaps resorting to the most potent forms of dual-antiplatelet therapy in patients with stable angina who have impaired glucose tolerance. However, these are possibilities that should be tested in randomized controlled trials before widespread adoption, he added.
The BIO-RESORT Silent Diabetes Study, which will continue for 5 years of post-PCI follow-up, is a prespecified substudy of the previously reported BIO-RESORT trial, which addressed another issue entirely. It was a three-arm, patient-blinded clinical trial comparing 1-year safety and efficacy outcomes in nearly 3,500 PCI patients randomized to PCI with very thin strut biodegradable polymer everolimus- or sirolimus-eluting stents or a durable polymer zotarolimus-eluting stent. Outcomes proved noninferior across the three treatment groups (Lancet. 2016 Nov 26;388[10060]:2607-17).
Dr. von Birgelen observed that the silent diabetes study broke new ground. Prior studies of PCI outcomes in patients with unrecognized diabetes were limited to recipients of plain old balloon angioplasty, bare metal, or first-generation drug-eluting stents. And studies of PCI in patients with unrecognized prediabetes are virtually nonexistent.
As the principal investigator for both the parent BIO-RESORT trial and the silent diabetes substudy, Dr. von Birgelen received research grants from Biotronik, Boston Scientific, and Medtronic, the cosponsors. He applauded the three companies for funding the silent diabetes substudy in the interest of science even though it had no commercial relevance to their stent businesses.
AT EUROPCR
Key clinical point:
Major finding: One-third of patients undergoing PCI have unsuspected silent diabetes or prediabetes, placing them at increased risk for major adverse cardiac events.
Data source: This prospective observational study included 988 patients not known to have diabetes who underwent screening for abnormal glucose tolerance 6 weeks after PCI with stenting.
Disclosures: The study was cosponsored by Biotronik, Boston Scientific, and Medtronic.
Outcomes Associated With a Multidisciplinary Pain Oversight Committee to Facilitate Appropriate Management of Chronic Opioid Therapy
The use of opioids to treat chronic noncancer pain (CNCP) has become increasingly common over the previous 2 decades. The Office of National Drug Control Policy (ONDCP) reported that from 1997 to 2007, there was a 4-fold increase in the mg per person per year sale of prescription opioids, from 74 mg to 369 mg.1 The number of opioid prescriptions dispensed by pharmacies also has increased by 48% from 2000 to 2009.2 Within the VA population, about half of the 1.44 million patients with a diagnosis of pain (excluding cancer pain) received opioids during 2011, and 57% of these patients received chronic opioid therapy (COT), which is at least 90 days of opioid use in a year.3
Despite this increased use of opioids, data regarding the efficacy of long-term opioid use for noncancer pain remain limited.1,4-8 Instead, there is a growing body of evidence describing potential adverse effects (AEs) of long-term opioid use at even relatively modest doses, including sexual dysfunction, hyperalgesia, and altered brain structure.9-11 Additionally, increases in the misuse and abuse of opioids as well as mortality associated with opioid toxicity have been observed.12-14 Opioid pain relievers were involved in nearly 17,000 deaths in the U.S. in 2010, which represents a 3-fold increase since 1999. This number also represents 75% of all deaths that were attributed to prescription drug poisoning in 2010.13 Unfortunately, this alarming trend parallels the aforementioned increases in the utilization of prescription opioids for CNCP.
Given this accumulating data regarding the profound risks and limited benefit of COT, many organizations have advocated a reassessment of the upward trajectory of opioid utilization. In 2009, the American Pain Society (APS) in partnership with the American Academy of Pain Medicine (AAPM) released clinical guidelines for the use of COT in CNCP.6 In this guideline, the authors advocate a balanced approach to opioid use: Clinicians consider both the legitimate medical need for opioids in some patients with CNCP as well as the serious public health problem of abuse, addiction, and diversion.6 In 2011, the FDA, Drug Enforcement Agency (DEA), and ONDCP enacted the Prescription Drug Abuse Prevention Plan, which focused on 4 major areas: education, prescription monitoring, proper medication disposal, and law enforcement.4
In March 2016, the CDC released a new guideline for prescribing opioids for chronic pain that included 12 recommendations based on 3 key principles. First, nonopioids are preferred for chronic pain in all settings except for active cancer, palliative, and end-of-life care. Next, when opioids are used for chronic pain, they always should be prescribed at the lowest possible effective dose to reduce the risk of opioid use disorder and overdose. Finally, clinicians should exercise caution when prescribing opioids and monitor all patients closely for opioid-related risk.15
Recently, an August 2016 FDA review found that the combined use of opioids and benzodiazepines (BZDs) resulted in serious AEs, including respiratory depression and death. Based on these findings, the FDA requires that updated boxed warnings be added to the labeling of prescription opioid and BZDs.16
The VHA also has been at the forefront of this national movement to promote the appropriate use of opioids. In 2009, the VHA released a pain management directive that highlighted the risks of COT and required adoption of a stepped-care approach to opioid prescribing that focused on quality of life as the primary determinant of treatment quality.17 In 2010, the VHA released its guideline on opioid therapy for chronic pain, which also included tools for providers, such as a sample opioid therapy agreement, equivalent potency tables, and a urine drug screening guide.18 In 2014, the VHA released the Opioid Safety Initiative (OSI), which advocates for a team-based approach to reduce the use of opioids for veterans through a focus on alternate methods to alleviate pain.
At the Ralph H. Johnson VAMC (RHJVAMC) in Charleston, South Carolina, a multidisciplinary pain oversight committee (POC) was tasked with assisting in achieving the goals set forth in the VHA OSI. To reach these goals, the POC sought to develop and implement a population-based initiative targeting modifiable factors that are known to increase the risk of opioid-related toxicity and overdose. These factors included patient utilization of multiple prescribers or multiple pharmacies, high-dose COT (defined in the APS/AAPM guidelines as a morphine equivalent daily dose [MEDD] > 200 mg6), and use of concomitant central nervous system-active medications, chiefly BZDs.19-23 The POC consisted of the RHJVAMC chiefs of mental health, primary care, and pharmacy; a physician specializing in pain and addiction medicine; a pharmacist specializing in pain and palliative care; quality management personnel; a patient advocate; and multiple physicians from the mental health and primary care departments.
Previous studies have described the successful implementation of opioid management initiatives in a variety of health care settings.2,21,24-27 However, most of this work focused only on strategies to decrease prescribing of high-dose and long-acting opioid formulations. The study presented here sought to add to the existing body of knowledge through evaluation of an initiative aimed at increasing appropriate monitoring as a tool to decrease opioid-related patient risk. The primary aim of this study was to describe the types of interventions implemented by the POC during the study period. The secondary aim was to evaluate the effect of these interventions on the appropriate monitoring of COT as well as the appropriate management of high-risk COT > 200 mg MEDD.
Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.
Informatics Tool
In September 2014, an e-consult tool was created to enable PCPs to efficiently consult the RHJVAMC pain clinic for advice on opioid-related issues in patients who require specialized attention. On activation of this EMR-based tool, the following patient data autopopulated in the consult: recent and active opioid prescription(s), UDS data from the previous 365 days, and PDMP review data from the previous 365 days. The consulting provider was then required to enter data on concomitant mental health disorders that were deemed pertinent to opioid safety as well as obstructive sleep apnea (OSA) status (OSA diagnosis and continuous positive airway pressure machine receipt and adherence).
The consulting provider was required to indicate whether the patient had an active BZD prescription. If yes, a text field allowed the provider to enter the specific agent(s) prescribed and dose(s). Data were required in all fields for the e-consult to be considered ready for pain clinic review. Common pain clinic recommendations included orders for additional laboratory tests to assess adherence and potential toxicity, drug tapers, and consideration for complementary and alternative medicine (CAM). If a drug taper was recommended (either opioid or BZD), specific taper schedules would be provided by a pharmacist specializing in pain management.
Targeted Patient Intervention
In April 2014, the POC and Mental Health service began a targeted review of all outpatients receiving combination opioid and BZD therapy. First, the POC distributed to each mental health provider a list of patients who were receiving combination opioid and BZD therapy. An opioid/BZD combination risk assessment tool (Table 2) was developed by the POC and made available to assist with these patient reviews. This tool prompted a provider to assess a patient’s stability on the current regimen as well as the presence of any absolute or relative contraindications to concomitant BZD and opioid use. Providers documented whether a discussion regarding the risks and benefits of opioids and BZDs had occurred with the patient. The tool encouraged providers to document a continued indication for combined BZD and opioid therapy use and whether the lowest effective BZD dose was being prescribed. A standardized BZD taper protocol also was developed by the POC to assist providers if a BZD taper was indicated. A total of 222 patients were reviewed over 7 months from April 2014 to October 2015.
Following completion of this targeted review in October 2015, the POC required that starting any patients on opioid and BZD combination therapy would require a specialist consult. For existing COT patients, a mental health consult would be required to initiate BZD therapy. For stable patients on BZD therapy, a pain clinic consult was required before initiating an opioid prescription. The Pharmacy service acted as a gatekeeper for these agents and refused to dispense either new agent until the proper consults had been submitted unless clinical necessity of an agent was apparent (ie, opioid prescription following invasive surgery).
The final targeted patient intervention occurred following deployment of the opioid safety review e-consult tool in September 2015. To review the highest risk COT patients, each PCP was given a list of their patients who were taking ≥ 200 mg MEDD. With support from the primary care service chief, PCPs were required to submit an e-consult for every patient who did not meet the e-consult exclusion criteria. In the fourth quarter of fiscal year (FY) 2014, and first quarter of FY 2015, 116 RHJVAMC patients received ≥ 200 mg MEDD with 49 meeting the exclusion criteria. Of the 67 patients eligible for pain clinic review, e-consults were placed for 58 patients over a 7-month period. The remaining 9 patients did not receive an e-consult because taper was initiated by the patient’s PCP without pain clinic assistance (6), aberrant patient behavior was identified during data collection (2), and patient was transitioned to palliative care (1).
Provider Education
A primary goal of the POC was to educate PCPs on opioid safety, to ensure that each provider was able to use evidence-based medicine and identify potential high-risk situations during patient encounters. Provider education was delivered by physician and pharmacist pain specialists and took place from September 2013 to January 2015 at existing primary care meetings. Topics included UDS interpretation, opioid/BZD combination risks, the goals and requirements of the VA OSI, and legal requirements of the South Carolina Reporting and Identification Prescription Tracking System (SCRIPTS) PDMP.
Patient Education
Patient education was delivered through informational brochures either mailed or given out during clinic visits. The first brochure was mailed to patients and described the VA OSI goals and its potential impact on patients. A second handout described the risks associated with opioid/BZD combination therapy and encouraged patients to discuss these risks and alternate options with their providers. It was made available to primary care and mental health teams for distribution to patients.
Results
Interventions spanned 19 months, with an average of 1 intervention per month. The highest number of POC interventions in a single month was observed in October 2014, with 3 individual interventions from 3 separate categories.
Impact of POC Interventions on COT Monitoring
During the study period, patients meeting COT criteria who received an annual UDS increased from 47.8% to 75.5%, a 56.7% increase from baseline (Figure 1). During the same period, patients with an annual PDMP review note in their medical record also increased from 4.1% to 19.6%, a 324% increase from baseline (Figure 2). Although the study period began in FY 2012 third quarter, FY 2014 first quarter was the baseline for PDMP review note data collection because VA providers were not legally allowed to access the SCRIPTS database prior to FY 2014.
Impact of Interventions on High-Risk Opioid Prescribing
Patients who received an opioid prescription and a BZD derivative in the same fiscal quarter decreased 41.7% during the study period (Figure 3). A significant improvement was observed in each clinical variable at 6 months postintervention among high-dose COT patients who received an opioid safety review e-consult (Table 3). The median opioid dose per patient decreased 20% from baseline, from 300 mg MEDD to 240 mg MEDD. The number of patients with an annual UDS increased 31.7% from 41 to 54 patients. The number of patients with an annual PDMP review also increased 345%, from 11 patients to 49 patients. Finally, the number of patients with an active BZD order decreased > 75% from 17 patients at baseline to 4 patients at 6-month follow-up.
In the FY 2014 third quarter, prior to activation of the opioid safety review e-consult tool, 100 patients received high-dose COT. Follow-up at the conclusion of fourth quarter FY 2015 revealed 64 such patients, which represented a 36% decrease from baseline.
Discussion
During the study period, the POC used a variety of interventions from 4 distinct categories. Overall, these interventions successfully increased measures of appropriate COT monitoring (ie, UDS and PDMP utilization) and management of high-risk COT. Substantial improvements also were seen in the subgroup of patients receiving high-dose COT following creation and use of the opioid safety review e-consult tool.
Other VHA opioid management improvement initiatives were successful at reducing high-dose opioid prescribing through interventions similar to those described in this study. However, these initiatives did not address opioid monitoring practices or opioid/BZD combination therapy.25,26 To the authors’ knowledge, no previous opioid management improvement initiatives have reported improvements in provider use of a state PDMP database.
There are a number of factors that also may have helped lead to the successful outcomes observed during the study period. First, the creation of an informatics tool allowed for sustained interventions over time. While targeted interventions and patient/provider education were certainly beneficial, the impact of these efforts wanes as time moves forward. Inevitably, a patient’s and a provider’s focus move to the next important issue, and new patients meet the criteria of the original targeted intervention.
Group Health Cooperative implemented an opioid risk reduction initiative that successfully increased UDS use over a 2-year postimplementation period.21,26 While this initiative used a number of similar interventions to those implemented in this study (patient and provider education, targeted patient intervention), an informatics tool was not used. The annual UDS rate at the conclusion of the Group Health initiative was 50%, which contrasts with a final rate of 75.5% in this study. Although it is difficult to draw comparisons between the studies given differences in populations studied, periods of evaluation, and varying baseline annual UDS rates, the current study results demonstrate the potential effectiveness of informatics tools to help drive enduring changes in practice.
An additional factor that had a positive impact on outcomes the continued support and advocacy from RHJVAMC clinical and administrative leadership. A targeted review of all patients receiving concomitant BZDs and opioids would not be possible without mental health department leaders who believed in the value of the time consuming undertaking. Furthermore, an e-consult tool is effective only if actually submitted for patients and if a specialist’s recommendations are then followed by a PCP.
Finally, the interdisciplinary nature of the POC contributed to the success of each intervention described in this study. Patients receiving COT often have many complex physical, psychological, and social issues that must be considered in order to make a positive impact on patient care. To appropriately and effectively address these issues requires close collaboration between specialists from multiple disciplines.
Limitations
This study has several important limitations. First, its retrospective nature presents obvious documentation challenges. A second limitation is the brief period of evaluation following a number of POC interventions. For instance, 3 interventions took place in January 2015, leaving only 3 FY quarters of effectiveness data. Furthermore, increased awareness of the risks associated with opioid therapy in the VHA and the health care industry across the study period may have independently impacted the improvements observed in this study.
The lack of an assessment of both patient-centered and clinical outcomes is an additional limitation of this study. Rates of annual UDS and PDMP database reviews and the number of patients receiving high-risk COT are only surrogate metrics that may indicate appropriate prescribing and monitoring of these. Obtaining a UDS or PDMP review is meant to provide a practitioner with additional information to interpret when caring for a patient. These data are only meant to complement—not replace—skilled patient assessment by a provider. Although the authors observed no major patient or clinical adverse events during the study period, the possibility exists that a patient may have been negatively impacted by a population-level initiative to improve surrogate measures of appropriate drug use.
Future studies should assess changes in measures, such as pain scores, legitimate adverse events, and overdose occurrences in order to evaluate whether such opioid improvement initiatives truly benefit the patients who are ultimately affected by each intervention.
Conclusion
This study demonstrates the successful implementation of a VHA-based opioid management initiative to increase appropriate COT monitoring and appropriate management of high-risk patients. It is the authors’ hope that the findings may add to the growing body of literature describing successful opioid improvement initiatives and serve as a tool for other health systems that are confronted with these same issues.
1. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401-435.
2. Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447-454.
3. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337-2343.
4. Office of National Drug Control Policy. Prescription drug abuse. https://obamawhitehouse.archives.gov /ondcp/prescription-drug-abuse1. Accessed April 18, 2017.
5. Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147-159.
6. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
7. The American Pain Society, The American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic non-cancer pain: evidence review. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy -cncp.pdf. Accessed April 18, 2017.
8. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207-209.
9. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570-587.
10. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222.
11. Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803-1810.
12. U.S Department of Health and Human Services, Substance Abuse and Mental Health Service Administration Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. http://medicalmarijuana.procon .org/sourcefiles/2k4results.pdf. Updated September 8, 2005. Accessed April 18, 2017.
13. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
14. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705-709.
15. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
16. U.S. Federal Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety /ucm518473.htm. Published August 31, 2016. Accessed April 18, 2017.
17. U.S. Department of Veterans Affairs. Pain management, VHA directive 2009-053. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed April 18, 2017.
18. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed May 8, 2017.
19. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
20. Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
21. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929.
22. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
23. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501.
24. Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573-580.
25. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019-1026.
26. Kryskalla J, Kern S, Gray D, Hauser P. Using dashboard technology to monitor overdose risk. Fed Pract. 2014;31(9):8-14.
27. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
28. U.S. Department of Veterans Affairs. Patient aligned care team (PACT). https://www.patientcare.va.gov /primarycare/PACT.asp. Updated September 22, 2016. Accessed April 18, 2017.
29. Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-665.
30. Reisfield GM, Webster LR. Benzodiazepines in long-term opioid therapy. Pain Med. 2013;14(10):1441-1446.
The use of opioids to treat chronic noncancer pain (CNCP) has become increasingly common over the previous 2 decades. The Office of National Drug Control Policy (ONDCP) reported that from 1997 to 2007, there was a 4-fold increase in the mg per person per year sale of prescription opioids, from 74 mg to 369 mg.1 The number of opioid prescriptions dispensed by pharmacies also has increased by 48% from 2000 to 2009.2 Within the VA population, about half of the 1.44 million patients with a diagnosis of pain (excluding cancer pain) received opioids during 2011, and 57% of these patients received chronic opioid therapy (COT), which is at least 90 days of opioid use in a year.3
Despite this increased use of opioids, data regarding the efficacy of long-term opioid use for noncancer pain remain limited.1,4-8 Instead, there is a growing body of evidence describing potential adverse effects (AEs) of long-term opioid use at even relatively modest doses, including sexual dysfunction, hyperalgesia, and altered brain structure.9-11 Additionally, increases in the misuse and abuse of opioids as well as mortality associated with opioid toxicity have been observed.12-14 Opioid pain relievers were involved in nearly 17,000 deaths in the U.S. in 2010, which represents a 3-fold increase since 1999. This number also represents 75% of all deaths that were attributed to prescription drug poisoning in 2010.13 Unfortunately, this alarming trend parallels the aforementioned increases in the utilization of prescription opioids for CNCP.
Given this accumulating data regarding the profound risks and limited benefit of COT, many organizations have advocated a reassessment of the upward trajectory of opioid utilization. In 2009, the American Pain Society (APS) in partnership with the American Academy of Pain Medicine (AAPM) released clinical guidelines for the use of COT in CNCP.6 In this guideline, the authors advocate a balanced approach to opioid use: Clinicians consider both the legitimate medical need for opioids in some patients with CNCP as well as the serious public health problem of abuse, addiction, and diversion.6 In 2011, the FDA, Drug Enforcement Agency (DEA), and ONDCP enacted the Prescription Drug Abuse Prevention Plan, which focused on 4 major areas: education, prescription monitoring, proper medication disposal, and law enforcement.4
In March 2016, the CDC released a new guideline for prescribing opioids for chronic pain that included 12 recommendations based on 3 key principles. First, nonopioids are preferred for chronic pain in all settings except for active cancer, palliative, and end-of-life care. Next, when opioids are used for chronic pain, they always should be prescribed at the lowest possible effective dose to reduce the risk of opioid use disorder and overdose. Finally, clinicians should exercise caution when prescribing opioids and monitor all patients closely for opioid-related risk.15
Recently, an August 2016 FDA review found that the combined use of opioids and benzodiazepines (BZDs) resulted in serious AEs, including respiratory depression and death. Based on these findings, the FDA requires that updated boxed warnings be added to the labeling of prescription opioid and BZDs.16
The VHA also has been at the forefront of this national movement to promote the appropriate use of opioids. In 2009, the VHA released a pain management directive that highlighted the risks of COT and required adoption of a stepped-care approach to opioid prescribing that focused on quality of life as the primary determinant of treatment quality.17 In 2010, the VHA released its guideline on opioid therapy for chronic pain, which also included tools for providers, such as a sample opioid therapy agreement, equivalent potency tables, and a urine drug screening guide.18 In 2014, the VHA released the Opioid Safety Initiative (OSI), which advocates for a team-based approach to reduce the use of opioids for veterans through a focus on alternate methods to alleviate pain.
At the Ralph H. Johnson VAMC (RHJVAMC) in Charleston, South Carolina, a multidisciplinary pain oversight committee (POC) was tasked with assisting in achieving the goals set forth in the VHA OSI. To reach these goals, the POC sought to develop and implement a population-based initiative targeting modifiable factors that are known to increase the risk of opioid-related toxicity and overdose. These factors included patient utilization of multiple prescribers or multiple pharmacies, high-dose COT (defined in the APS/AAPM guidelines as a morphine equivalent daily dose [MEDD] > 200 mg6), and use of concomitant central nervous system-active medications, chiefly BZDs.19-23 The POC consisted of the RHJVAMC chiefs of mental health, primary care, and pharmacy; a physician specializing in pain and addiction medicine; a pharmacist specializing in pain and palliative care; quality management personnel; a patient advocate; and multiple physicians from the mental health and primary care departments.
Previous studies have described the successful implementation of opioid management initiatives in a variety of health care settings.2,21,24-27 However, most of this work focused only on strategies to decrease prescribing of high-dose and long-acting opioid formulations. The study presented here sought to add to the existing body of knowledge through evaluation of an initiative aimed at increasing appropriate monitoring as a tool to decrease opioid-related patient risk. The primary aim of this study was to describe the types of interventions implemented by the POC during the study period. The secondary aim was to evaluate the effect of these interventions on the appropriate monitoring of COT as well as the appropriate management of high-risk COT > 200 mg MEDD.
Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.
Informatics Tool
In September 2014, an e-consult tool was created to enable PCPs to efficiently consult the RHJVAMC pain clinic for advice on opioid-related issues in patients who require specialized attention. On activation of this EMR-based tool, the following patient data autopopulated in the consult: recent and active opioid prescription(s), UDS data from the previous 365 days, and PDMP review data from the previous 365 days. The consulting provider was then required to enter data on concomitant mental health disorders that were deemed pertinent to opioid safety as well as obstructive sleep apnea (OSA) status (OSA diagnosis and continuous positive airway pressure machine receipt and adherence).
The consulting provider was required to indicate whether the patient had an active BZD prescription. If yes, a text field allowed the provider to enter the specific agent(s) prescribed and dose(s). Data were required in all fields for the e-consult to be considered ready for pain clinic review. Common pain clinic recommendations included orders for additional laboratory tests to assess adherence and potential toxicity, drug tapers, and consideration for complementary and alternative medicine (CAM). If a drug taper was recommended (either opioid or BZD), specific taper schedules would be provided by a pharmacist specializing in pain management.
Targeted Patient Intervention
In April 2014, the POC and Mental Health service began a targeted review of all outpatients receiving combination opioid and BZD therapy. First, the POC distributed to each mental health provider a list of patients who were receiving combination opioid and BZD therapy. An opioid/BZD combination risk assessment tool (Table 2) was developed by the POC and made available to assist with these patient reviews. This tool prompted a provider to assess a patient’s stability on the current regimen as well as the presence of any absolute or relative contraindications to concomitant BZD and opioid use. Providers documented whether a discussion regarding the risks and benefits of opioids and BZDs had occurred with the patient. The tool encouraged providers to document a continued indication for combined BZD and opioid therapy use and whether the lowest effective BZD dose was being prescribed. A standardized BZD taper protocol also was developed by the POC to assist providers if a BZD taper was indicated. A total of 222 patients were reviewed over 7 months from April 2014 to October 2015.
Following completion of this targeted review in October 2015, the POC required that starting any patients on opioid and BZD combination therapy would require a specialist consult. For existing COT patients, a mental health consult would be required to initiate BZD therapy. For stable patients on BZD therapy, a pain clinic consult was required before initiating an opioid prescription. The Pharmacy service acted as a gatekeeper for these agents and refused to dispense either new agent until the proper consults had been submitted unless clinical necessity of an agent was apparent (ie, opioid prescription following invasive surgery).
The final targeted patient intervention occurred following deployment of the opioid safety review e-consult tool in September 2015. To review the highest risk COT patients, each PCP was given a list of their patients who were taking ≥ 200 mg MEDD. With support from the primary care service chief, PCPs were required to submit an e-consult for every patient who did not meet the e-consult exclusion criteria. In the fourth quarter of fiscal year (FY) 2014, and first quarter of FY 2015, 116 RHJVAMC patients received ≥ 200 mg MEDD with 49 meeting the exclusion criteria. Of the 67 patients eligible for pain clinic review, e-consults were placed for 58 patients over a 7-month period. The remaining 9 patients did not receive an e-consult because taper was initiated by the patient’s PCP without pain clinic assistance (6), aberrant patient behavior was identified during data collection (2), and patient was transitioned to palliative care (1).
Provider Education
A primary goal of the POC was to educate PCPs on opioid safety, to ensure that each provider was able to use evidence-based medicine and identify potential high-risk situations during patient encounters. Provider education was delivered by physician and pharmacist pain specialists and took place from September 2013 to January 2015 at existing primary care meetings. Topics included UDS interpretation, opioid/BZD combination risks, the goals and requirements of the VA OSI, and legal requirements of the South Carolina Reporting and Identification Prescription Tracking System (SCRIPTS) PDMP.
Patient Education
Patient education was delivered through informational brochures either mailed or given out during clinic visits. The first brochure was mailed to patients and described the VA OSI goals and its potential impact on patients. A second handout described the risks associated with opioid/BZD combination therapy and encouraged patients to discuss these risks and alternate options with their providers. It was made available to primary care and mental health teams for distribution to patients.
Results
Interventions spanned 19 months, with an average of 1 intervention per month. The highest number of POC interventions in a single month was observed in October 2014, with 3 individual interventions from 3 separate categories.
Impact of POC Interventions on COT Monitoring
During the study period, patients meeting COT criteria who received an annual UDS increased from 47.8% to 75.5%, a 56.7% increase from baseline (Figure 1). During the same period, patients with an annual PDMP review note in their medical record also increased from 4.1% to 19.6%, a 324% increase from baseline (Figure 2). Although the study period began in FY 2012 third quarter, FY 2014 first quarter was the baseline for PDMP review note data collection because VA providers were not legally allowed to access the SCRIPTS database prior to FY 2014.
Impact of Interventions on High-Risk Opioid Prescribing
Patients who received an opioid prescription and a BZD derivative in the same fiscal quarter decreased 41.7% during the study period (Figure 3). A significant improvement was observed in each clinical variable at 6 months postintervention among high-dose COT patients who received an opioid safety review e-consult (Table 3). The median opioid dose per patient decreased 20% from baseline, from 300 mg MEDD to 240 mg MEDD. The number of patients with an annual UDS increased 31.7% from 41 to 54 patients. The number of patients with an annual PDMP review also increased 345%, from 11 patients to 49 patients. Finally, the number of patients with an active BZD order decreased > 75% from 17 patients at baseline to 4 patients at 6-month follow-up.
In the FY 2014 third quarter, prior to activation of the opioid safety review e-consult tool, 100 patients received high-dose COT. Follow-up at the conclusion of fourth quarter FY 2015 revealed 64 such patients, which represented a 36% decrease from baseline.
Discussion
During the study period, the POC used a variety of interventions from 4 distinct categories. Overall, these interventions successfully increased measures of appropriate COT monitoring (ie, UDS and PDMP utilization) and management of high-risk COT. Substantial improvements also were seen in the subgroup of patients receiving high-dose COT following creation and use of the opioid safety review e-consult tool.
Other VHA opioid management improvement initiatives were successful at reducing high-dose opioid prescribing through interventions similar to those described in this study. However, these initiatives did not address opioid monitoring practices or opioid/BZD combination therapy.25,26 To the authors’ knowledge, no previous opioid management improvement initiatives have reported improvements in provider use of a state PDMP database.
There are a number of factors that also may have helped lead to the successful outcomes observed during the study period. First, the creation of an informatics tool allowed for sustained interventions over time. While targeted interventions and patient/provider education were certainly beneficial, the impact of these efforts wanes as time moves forward. Inevitably, a patient’s and a provider’s focus move to the next important issue, and new patients meet the criteria of the original targeted intervention.
Group Health Cooperative implemented an opioid risk reduction initiative that successfully increased UDS use over a 2-year postimplementation period.21,26 While this initiative used a number of similar interventions to those implemented in this study (patient and provider education, targeted patient intervention), an informatics tool was not used. The annual UDS rate at the conclusion of the Group Health initiative was 50%, which contrasts with a final rate of 75.5% in this study. Although it is difficult to draw comparisons between the studies given differences in populations studied, periods of evaluation, and varying baseline annual UDS rates, the current study results demonstrate the potential effectiveness of informatics tools to help drive enduring changes in practice.
An additional factor that had a positive impact on outcomes the continued support and advocacy from RHJVAMC clinical and administrative leadership. A targeted review of all patients receiving concomitant BZDs and opioids would not be possible without mental health department leaders who believed in the value of the time consuming undertaking. Furthermore, an e-consult tool is effective only if actually submitted for patients and if a specialist’s recommendations are then followed by a PCP.
Finally, the interdisciplinary nature of the POC contributed to the success of each intervention described in this study. Patients receiving COT often have many complex physical, psychological, and social issues that must be considered in order to make a positive impact on patient care. To appropriately and effectively address these issues requires close collaboration between specialists from multiple disciplines.
Limitations
This study has several important limitations. First, its retrospective nature presents obvious documentation challenges. A second limitation is the brief period of evaluation following a number of POC interventions. For instance, 3 interventions took place in January 2015, leaving only 3 FY quarters of effectiveness data. Furthermore, increased awareness of the risks associated with opioid therapy in the VHA and the health care industry across the study period may have independently impacted the improvements observed in this study.
The lack of an assessment of both patient-centered and clinical outcomes is an additional limitation of this study. Rates of annual UDS and PDMP database reviews and the number of patients receiving high-risk COT are only surrogate metrics that may indicate appropriate prescribing and monitoring of these. Obtaining a UDS or PDMP review is meant to provide a practitioner with additional information to interpret when caring for a patient. These data are only meant to complement—not replace—skilled patient assessment by a provider. Although the authors observed no major patient or clinical adverse events during the study period, the possibility exists that a patient may have been negatively impacted by a population-level initiative to improve surrogate measures of appropriate drug use.
Future studies should assess changes in measures, such as pain scores, legitimate adverse events, and overdose occurrences in order to evaluate whether such opioid improvement initiatives truly benefit the patients who are ultimately affected by each intervention.
Conclusion
This study demonstrates the successful implementation of a VHA-based opioid management initiative to increase appropriate COT monitoring and appropriate management of high-risk patients. It is the authors’ hope that the findings may add to the growing body of literature describing successful opioid improvement initiatives and serve as a tool for other health systems that are confronted with these same issues.
The use of opioids to treat chronic noncancer pain (CNCP) has become increasingly common over the previous 2 decades. The Office of National Drug Control Policy (ONDCP) reported that from 1997 to 2007, there was a 4-fold increase in the mg per person per year sale of prescription opioids, from 74 mg to 369 mg.1 The number of opioid prescriptions dispensed by pharmacies also has increased by 48% from 2000 to 2009.2 Within the VA population, about half of the 1.44 million patients with a diagnosis of pain (excluding cancer pain) received opioids during 2011, and 57% of these patients received chronic opioid therapy (COT), which is at least 90 days of opioid use in a year.3
Despite this increased use of opioids, data regarding the efficacy of long-term opioid use for noncancer pain remain limited.1,4-8 Instead, there is a growing body of evidence describing potential adverse effects (AEs) of long-term opioid use at even relatively modest doses, including sexual dysfunction, hyperalgesia, and altered brain structure.9-11 Additionally, increases in the misuse and abuse of opioids as well as mortality associated with opioid toxicity have been observed.12-14 Opioid pain relievers were involved in nearly 17,000 deaths in the U.S. in 2010, which represents a 3-fold increase since 1999. This number also represents 75% of all deaths that were attributed to prescription drug poisoning in 2010.13 Unfortunately, this alarming trend parallels the aforementioned increases in the utilization of prescription opioids for CNCP.
Given this accumulating data regarding the profound risks and limited benefit of COT, many organizations have advocated a reassessment of the upward trajectory of opioid utilization. In 2009, the American Pain Society (APS) in partnership with the American Academy of Pain Medicine (AAPM) released clinical guidelines for the use of COT in CNCP.6 In this guideline, the authors advocate a balanced approach to opioid use: Clinicians consider both the legitimate medical need for opioids in some patients with CNCP as well as the serious public health problem of abuse, addiction, and diversion.6 In 2011, the FDA, Drug Enforcement Agency (DEA), and ONDCP enacted the Prescription Drug Abuse Prevention Plan, which focused on 4 major areas: education, prescription monitoring, proper medication disposal, and law enforcement.4
In March 2016, the CDC released a new guideline for prescribing opioids for chronic pain that included 12 recommendations based on 3 key principles. First, nonopioids are preferred for chronic pain in all settings except for active cancer, palliative, and end-of-life care. Next, when opioids are used for chronic pain, they always should be prescribed at the lowest possible effective dose to reduce the risk of opioid use disorder and overdose. Finally, clinicians should exercise caution when prescribing opioids and monitor all patients closely for opioid-related risk.15
Recently, an August 2016 FDA review found that the combined use of opioids and benzodiazepines (BZDs) resulted in serious AEs, including respiratory depression and death. Based on these findings, the FDA requires that updated boxed warnings be added to the labeling of prescription opioid and BZDs.16
The VHA also has been at the forefront of this national movement to promote the appropriate use of opioids. In 2009, the VHA released a pain management directive that highlighted the risks of COT and required adoption of a stepped-care approach to opioid prescribing that focused on quality of life as the primary determinant of treatment quality.17 In 2010, the VHA released its guideline on opioid therapy for chronic pain, which also included tools for providers, such as a sample opioid therapy agreement, equivalent potency tables, and a urine drug screening guide.18 In 2014, the VHA released the Opioid Safety Initiative (OSI), which advocates for a team-based approach to reduce the use of opioids for veterans through a focus on alternate methods to alleviate pain.
At the Ralph H. Johnson VAMC (RHJVAMC) in Charleston, South Carolina, a multidisciplinary pain oversight committee (POC) was tasked with assisting in achieving the goals set forth in the VHA OSI. To reach these goals, the POC sought to develop and implement a population-based initiative targeting modifiable factors that are known to increase the risk of opioid-related toxicity and overdose. These factors included patient utilization of multiple prescribers or multiple pharmacies, high-dose COT (defined in the APS/AAPM guidelines as a morphine equivalent daily dose [MEDD] > 200 mg6), and use of concomitant central nervous system-active medications, chiefly BZDs.19-23 The POC consisted of the RHJVAMC chiefs of mental health, primary care, and pharmacy; a physician specializing in pain and addiction medicine; a pharmacist specializing in pain and palliative care; quality management personnel; a patient advocate; and multiple physicians from the mental health and primary care departments.
Previous studies have described the successful implementation of opioid management initiatives in a variety of health care settings.2,21,24-27 However, most of this work focused only on strategies to decrease prescribing of high-dose and long-acting opioid formulations. The study presented here sought to add to the existing body of knowledge through evaluation of an initiative aimed at increasing appropriate monitoring as a tool to decrease opioid-related patient risk. The primary aim of this study was to describe the types of interventions implemented by the POC during the study period. The secondary aim was to evaluate the effect of these interventions on the appropriate monitoring of COT as well as the appropriate management of high-risk COT > 200 mg MEDD.
Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.
Informatics Tool
In September 2014, an e-consult tool was created to enable PCPs to efficiently consult the RHJVAMC pain clinic for advice on opioid-related issues in patients who require specialized attention. On activation of this EMR-based tool, the following patient data autopopulated in the consult: recent and active opioid prescription(s), UDS data from the previous 365 days, and PDMP review data from the previous 365 days. The consulting provider was then required to enter data on concomitant mental health disorders that were deemed pertinent to opioid safety as well as obstructive sleep apnea (OSA) status (OSA diagnosis and continuous positive airway pressure machine receipt and adherence).
The consulting provider was required to indicate whether the patient had an active BZD prescription. If yes, a text field allowed the provider to enter the specific agent(s) prescribed and dose(s). Data were required in all fields for the e-consult to be considered ready for pain clinic review. Common pain clinic recommendations included orders for additional laboratory tests to assess adherence and potential toxicity, drug tapers, and consideration for complementary and alternative medicine (CAM). If a drug taper was recommended (either opioid or BZD), specific taper schedules would be provided by a pharmacist specializing in pain management.
Targeted Patient Intervention
In April 2014, the POC and Mental Health service began a targeted review of all outpatients receiving combination opioid and BZD therapy. First, the POC distributed to each mental health provider a list of patients who were receiving combination opioid and BZD therapy. An opioid/BZD combination risk assessment tool (Table 2) was developed by the POC and made available to assist with these patient reviews. This tool prompted a provider to assess a patient’s stability on the current regimen as well as the presence of any absolute or relative contraindications to concomitant BZD and opioid use. Providers documented whether a discussion regarding the risks and benefits of opioids and BZDs had occurred with the patient. The tool encouraged providers to document a continued indication for combined BZD and opioid therapy use and whether the lowest effective BZD dose was being prescribed. A standardized BZD taper protocol also was developed by the POC to assist providers if a BZD taper was indicated. A total of 222 patients were reviewed over 7 months from April 2014 to October 2015.
Following completion of this targeted review in October 2015, the POC required that starting any patients on opioid and BZD combination therapy would require a specialist consult. For existing COT patients, a mental health consult would be required to initiate BZD therapy. For stable patients on BZD therapy, a pain clinic consult was required before initiating an opioid prescription. The Pharmacy service acted as a gatekeeper for these agents and refused to dispense either new agent until the proper consults had been submitted unless clinical necessity of an agent was apparent (ie, opioid prescription following invasive surgery).
The final targeted patient intervention occurred following deployment of the opioid safety review e-consult tool in September 2015. To review the highest risk COT patients, each PCP was given a list of their patients who were taking ≥ 200 mg MEDD. With support from the primary care service chief, PCPs were required to submit an e-consult for every patient who did not meet the e-consult exclusion criteria. In the fourth quarter of fiscal year (FY) 2014, and first quarter of FY 2015, 116 RHJVAMC patients received ≥ 200 mg MEDD with 49 meeting the exclusion criteria. Of the 67 patients eligible for pain clinic review, e-consults were placed for 58 patients over a 7-month period. The remaining 9 patients did not receive an e-consult because taper was initiated by the patient’s PCP without pain clinic assistance (6), aberrant patient behavior was identified during data collection (2), and patient was transitioned to palliative care (1).
Provider Education
A primary goal of the POC was to educate PCPs on opioid safety, to ensure that each provider was able to use evidence-based medicine and identify potential high-risk situations during patient encounters. Provider education was delivered by physician and pharmacist pain specialists and took place from September 2013 to January 2015 at existing primary care meetings. Topics included UDS interpretation, opioid/BZD combination risks, the goals and requirements of the VA OSI, and legal requirements of the South Carolina Reporting and Identification Prescription Tracking System (SCRIPTS) PDMP.
Patient Education
Patient education was delivered through informational brochures either mailed or given out during clinic visits. The first brochure was mailed to patients and described the VA OSI goals and its potential impact on patients. A second handout described the risks associated with opioid/BZD combination therapy and encouraged patients to discuss these risks and alternate options with their providers. It was made available to primary care and mental health teams for distribution to patients.
Results
Interventions spanned 19 months, with an average of 1 intervention per month. The highest number of POC interventions in a single month was observed in October 2014, with 3 individual interventions from 3 separate categories.
Impact of POC Interventions on COT Monitoring
During the study period, patients meeting COT criteria who received an annual UDS increased from 47.8% to 75.5%, a 56.7% increase from baseline (Figure 1). During the same period, patients with an annual PDMP review note in their medical record also increased from 4.1% to 19.6%, a 324% increase from baseline (Figure 2). Although the study period began in FY 2012 third quarter, FY 2014 first quarter was the baseline for PDMP review note data collection because VA providers were not legally allowed to access the SCRIPTS database prior to FY 2014.
Impact of Interventions on High-Risk Opioid Prescribing
Patients who received an opioid prescription and a BZD derivative in the same fiscal quarter decreased 41.7% during the study period (Figure 3). A significant improvement was observed in each clinical variable at 6 months postintervention among high-dose COT patients who received an opioid safety review e-consult (Table 3). The median opioid dose per patient decreased 20% from baseline, from 300 mg MEDD to 240 mg MEDD. The number of patients with an annual UDS increased 31.7% from 41 to 54 patients. The number of patients with an annual PDMP review also increased 345%, from 11 patients to 49 patients. Finally, the number of patients with an active BZD order decreased > 75% from 17 patients at baseline to 4 patients at 6-month follow-up.
In the FY 2014 third quarter, prior to activation of the opioid safety review e-consult tool, 100 patients received high-dose COT. Follow-up at the conclusion of fourth quarter FY 2015 revealed 64 such patients, which represented a 36% decrease from baseline.
Discussion
During the study period, the POC used a variety of interventions from 4 distinct categories. Overall, these interventions successfully increased measures of appropriate COT monitoring (ie, UDS and PDMP utilization) and management of high-risk COT. Substantial improvements also were seen in the subgroup of patients receiving high-dose COT following creation and use of the opioid safety review e-consult tool.
Other VHA opioid management improvement initiatives were successful at reducing high-dose opioid prescribing through interventions similar to those described in this study. However, these initiatives did not address opioid monitoring practices or opioid/BZD combination therapy.25,26 To the authors’ knowledge, no previous opioid management improvement initiatives have reported improvements in provider use of a state PDMP database.
There are a number of factors that also may have helped lead to the successful outcomes observed during the study period. First, the creation of an informatics tool allowed for sustained interventions over time. While targeted interventions and patient/provider education were certainly beneficial, the impact of these efforts wanes as time moves forward. Inevitably, a patient’s and a provider’s focus move to the next important issue, and new patients meet the criteria of the original targeted intervention.
Group Health Cooperative implemented an opioid risk reduction initiative that successfully increased UDS use over a 2-year postimplementation period.21,26 While this initiative used a number of similar interventions to those implemented in this study (patient and provider education, targeted patient intervention), an informatics tool was not used. The annual UDS rate at the conclusion of the Group Health initiative was 50%, which contrasts with a final rate of 75.5% in this study. Although it is difficult to draw comparisons between the studies given differences in populations studied, periods of evaluation, and varying baseline annual UDS rates, the current study results demonstrate the potential effectiveness of informatics tools to help drive enduring changes in practice.
An additional factor that had a positive impact on outcomes the continued support and advocacy from RHJVAMC clinical and administrative leadership. A targeted review of all patients receiving concomitant BZDs and opioids would not be possible without mental health department leaders who believed in the value of the time consuming undertaking. Furthermore, an e-consult tool is effective only if actually submitted for patients and if a specialist’s recommendations are then followed by a PCP.
Finally, the interdisciplinary nature of the POC contributed to the success of each intervention described in this study. Patients receiving COT often have many complex physical, psychological, and social issues that must be considered in order to make a positive impact on patient care. To appropriately and effectively address these issues requires close collaboration between specialists from multiple disciplines.
Limitations
This study has several important limitations. First, its retrospective nature presents obvious documentation challenges. A second limitation is the brief period of evaluation following a number of POC interventions. For instance, 3 interventions took place in January 2015, leaving only 3 FY quarters of effectiveness data. Furthermore, increased awareness of the risks associated with opioid therapy in the VHA and the health care industry across the study period may have independently impacted the improvements observed in this study.
The lack of an assessment of both patient-centered and clinical outcomes is an additional limitation of this study. Rates of annual UDS and PDMP database reviews and the number of patients receiving high-risk COT are only surrogate metrics that may indicate appropriate prescribing and monitoring of these. Obtaining a UDS or PDMP review is meant to provide a practitioner with additional information to interpret when caring for a patient. These data are only meant to complement—not replace—skilled patient assessment by a provider. Although the authors observed no major patient or clinical adverse events during the study period, the possibility exists that a patient may have been negatively impacted by a population-level initiative to improve surrogate measures of appropriate drug use.
Future studies should assess changes in measures, such as pain scores, legitimate adverse events, and overdose occurrences in order to evaluate whether such opioid improvement initiatives truly benefit the patients who are ultimately affected by each intervention.
Conclusion
This study demonstrates the successful implementation of a VHA-based opioid management initiative to increase appropriate COT monitoring and appropriate management of high-risk patients. It is the authors’ hope that the findings may add to the growing body of literature describing successful opioid improvement initiatives and serve as a tool for other health systems that are confronted with these same issues.
1. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401-435.
2. Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447-454.
3. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337-2343.
4. Office of National Drug Control Policy. Prescription drug abuse. https://obamawhitehouse.archives.gov /ondcp/prescription-drug-abuse1. Accessed April 18, 2017.
5. Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147-159.
6. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
7. The American Pain Society, The American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic non-cancer pain: evidence review. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy -cncp.pdf. Accessed April 18, 2017.
8. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207-209.
9. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570-587.
10. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222.
11. Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803-1810.
12. U.S Department of Health and Human Services, Substance Abuse and Mental Health Service Administration Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. http://medicalmarijuana.procon .org/sourcefiles/2k4results.pdf. Updated September 8, 2005. Accessed April 18, 2017.
13. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
14. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705-709.
15. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
16. U.S. Federal Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety /ucm518473.htm. Published August 31, 2016. Accessed April 18, 2017.
17. U.S. Department of Veterans Affairs. Pain management, VHA directive 2009-053. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed April 18, 2017.
18. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed May 8, 2017.
19. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
20. Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
21. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929.
22. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
23. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501.
24. Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573-580.
25. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019-1026.
26. Kryskalla J, Kern S, Gray D, Hauser P. Using dashboard technology to monitor overdose risk. Fed Pract. 2014;31(9):8-14.
27. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
28. U.S. Department of Veterans Affairs. Patient aligned care team (PACT). https://www.patientcare.va.gov /primarycare/PACT.asp. Updated September 22, 2016. Accessed April 18, 2017.
29. Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-665.
30. Reisfield GM, Webster LR. Benzodiazepines in long-term opioid therapy. Pain Med. 2013;14(10):1441-1446.
1. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401-435.
2. Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447-454.
3. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337-2343.
4. Office of National Drug Control Policy. Prescription drug abuse. https://obamawhitehouse.archives.gov /ondcp/prescription-drug-abuse1. Accessed April 18, 2017.
5. Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147-159.
6. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
7. The American Pain Society, The American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic non-cancer pain: evidence review. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy -cncp.pdf. Accessed April 18, 2017.
8. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207-209.
9. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570-587.
10. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222.
11. Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803-1810.
12. U.S Department of Health and Human Services, Substance Abuse and Mental Health Service Administration Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. http://medicalmarijuana.procon .org/sourcefiles/2k4results.pdf. Updated September 8, 2005. Accessed April 18, 2017.
13. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
14. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705-709.
15. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
16. U.S. Federal Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety /ucm518473.htm. Published August 31, 2016. Accessed April 18, 2017.
17. U.S. Department of Veterans Affairs. Pain management, VHA directive 2009-053. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed April 18, 2017.
18. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed May 8, 2017.
19. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
20. Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
21. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929.
22. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
23. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501.
24. Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573-580.
25. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019-1026.
26. Kryskalla J, Kern S, Gray D, Hauser P. Using dashboard technology to monitor overdose risk. Fed Pract. 2014;31(9):8-14.
27. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
28. U.S. Department of Veterans Affairs. Patient aligned care team (PACT). https://www.patientcare.va.gov /primarycare/PACT.asp. Updated September 22, 2016. Accessed April 18, 2017.
29. Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-665.
30. Reisfield GM, Webster LR. Benzodiazepines in long-term opioid therapy. Pain Med. 2013;14(10):1441-1446.