User login
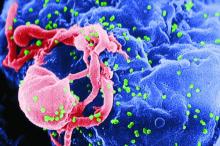
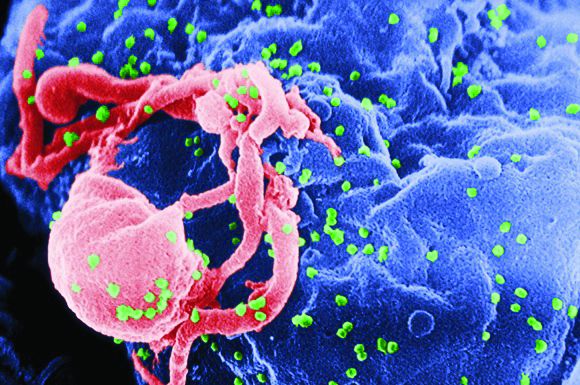
Lung cancer linked to suicide
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: During 1973-2013, suicide among U.S. lung cancer patients was four times higher than the general adult U.S. population.
Data source: Statistics on more than 3.6 million U.S. cancer patients in the SEER Program.
Disclosures: Dr. Rahouma had no disclosures.
Transradial PCI in acute coronary syndrome causes less kidney damage
PARIS – Transradial-access percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS) results in a significantly lower risk of acute kidney injury (AKI), compared with the transfemoral approach, according to a new analysis from the large randomized MATRIX trial.
The results of this prespecified secondary subgroup analysis of MATRIX suggest it’s time to update the classic “five golden rules” for reduction of contrast medium–induced AKI by adding a sixth. “Use a transradial approach,” Bernardo Cortese, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He reported on 8,210 participants in the MATRIX trial (Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) who were randomized to transradial- or transfemoral-access PCI for non–ST-elevation MI or ST-elevation MI.
The primary results of the 78-site, four-country European study, previously published, showed that transradial PCI reduced the composite risk of death, MI, stroke, or major bleeding by 17%, compared with transfemoral PCI, a benefit mainly driven by a marked reduction in clinically important bleeding (Lancet. 2015 Jun 20;385[9986]:2465-76).
Left unanswered by the primary analysis was the question of whether transradial PCI in ACS patients also reduced AKI risk, as had previously been suggested by a meta-analysis of observational studies (Int J Cardiol. 2015 Jan 20;179:309-11). In designing the MATRIX trial, Dr. Cortese and the other investigators decided to address that issue separately in a prespecified secondary analysis known as AKI-MATRIX. For this purpose, AKI was defined as either a post-PCI in-hospital increase in serum creatinine level of more than 25%, compared with the preangiography baseline, or an absolute increase in serum creatinine of greater than 0.5 mg/dL.
AKI occurred in 15.4% of ACS patients who underwent PCI with transradial access and 17.3% of those randomized to transfemoral access, for a significant 13% relative risk reduction. This was accomplished without any increase in the volume of contrast media required. The average was 200 mL in both study groups.
The reduction in AKI achieved with transradial-access PCI was seen in all patient subgroups, including those at increased AKI risk because of an estimated glomerular filtration rate below 60 mL/min, age 75 or older, Killup class III or IV, or a Mehran score greater than 10.
Dr. Cortese proposed several possible mechanisms for the observed reduction in AKI seen with transradial-access PCI. The major factor in his view is that the transradial approach entails less bleeding, as earlier demonstrated in the primary analysis – and bleeding has been associated with impaired renal perfusion in several prior studies. Also, it’s plausible that the passage of the catheter across the renal arteries during the transfemoral approach dislodges atherosclerotic debris, which then travels down the renal vessels.
The five golden rules for preventing contrast media–induced AKI, he noted, are
1. Discontinue nephrotoxic drugs before the procedure.
2. Identify high-risk patients.
3. Hydrate them.
4. Choose an ideal contrast medium.
5. Adapt the dose of contrast medium to the patient’s specific situation.
Discussant Jacek Legutko, MD, PhD, of Jagiellonian University in Krakow, Poland, said the primary results of the MATRIX trial published in 2015 have had a major impact on Polish interventional cardiology, where transradial PCI is now used in 80% of PCIs. The AKI study results will reinforce this trend, he added.
“You have shown something opposite to what we’ve thought in the past, that maybe, with a radial approach, we would use more contrast medium, which is a risk factor for AKI. In your study – at least in ACS with very experienced transradial operators – there was no increase in contrast volume, and the risk of AKI decreased,” Dr. Legutko said.
Asked about the possibility that transradial PCI might be associated with an increased risk of embolization to the brain, much as the transfemoral approach might cause embolization to the kidneys, Dr. Cortese said there was no significant difference between the two AKI-MATRIX study arms in rates of transient ischemic attack or stroke.
“I did my first transradial PCI in 2003, and I haven’t seen any increase in these events or later dementia,” he added.
The prespecified secondary analysis of the MATRIX trial was conducted without commercial support. The presenter reported serving as a consultant to Abbott, AstraZeneca, Daiichi Sankyo, Eli Lilly, and Stentys.
Simultaneous with his presentation in Paris, the AKI-MATRIX study was published online at www.sciencedirect.com/science/article/pii/S0735109717368973.
PARIS – Transradial-access percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS) results in a significantly lower risk of acute kidney injury (AKI), compared with the transfemoral approach, according to a new analysis from the large randomized MATRIX trial.
The results of this prespecified secondary subgroup analysis of MATRIX suggest it’s time to update the classic “five golden rules” for reduction of contrast medium–induced AKI by adding a sixth. “Use a transradial approach,” Bernardo Cortese, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He reported on 8,210 participants in the MATRIX trial (Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) who were randomized to transradial- or transfemoral-access PCI for non–ST-elevation MI or ST-elevation MI.
The primary results of the 78-site, four-country European study, previously published, showed that transradial PCI reduced the composite risk of death, MI, stroke, or major bleeding by 17%, compared with transfemoral PCI, a benefit mainly driven by a marked reduction in clinically important bleeding (Lancet. 2015 Jun 20;385[9986]:2465-76).
Left unanswered by the primary analysis was the question of whether transradial PCI in ACS patients also reduced AKI risk, as had previously been suggested by a meta-analysis of observational studies (Int J Cardiol. 2015 Jan 20;179:309-11). In designing the MATRIX trial, Dr. Cortese and the other investigators decided to address that issue separately in a prespecified secondary analysis known as AKI-MATRIX. For this purpose, AKI was defined as either a post-PCI in-hospital increase in serum creatinine level of more than 25%, compared with the preangiography baseline, or an absolute increase in serum creatinine of greater than 0.5 mg/dL.
AKI occurred in 15.4% of ACS patients who underwent PCI with transradial access and 17.3% of those randomized to transfemoral access, for a significant 13% relative risk reduction. This was accomplished without any increase in the volume of contrast media required. The average was 200 mL in both study groups.
The reduction in AKI achieved with transradial-access PCI was seen in all patient subgroups, including those at increased AKI risk because of an estimated glomerular filtration rate below 60 mL/min, age 75 or older, Killup class III or IV, or a Mehran score greater than 10.
Dr. Cortese proposed several possible mechanisms for the observed reduction in AKI seen with transradial-access PCI. The major factor in his view is that the transradial approach entails less bleeding, as earlier demonstrated in the primary analysis – and bleeding has been associated with impaired renal perfusion in several prior studies. Also, it’s plausible that the passage of the catheter across the renal arteries during the transfemoral approach dislodges atherosclerotic debris, which then travels down the renal vessels.
The five golden rules for preventing contrast media–induced AKI, he noted, are
1. Discontinue nephrotoxic drugs before the procedure.
2. Identify high-risk patients.
3. Hydrate them.
4. Choose an ideal contrast medium.
5. Adapt the dose of contrast medium to the patient’s specific situation.
Discussant Jacek Legutko, MD, PhD, of Jagiellonian University in Krakow, Poland, said the primary results of the MATRIX trial published in 2015 have had a major impact on Polish interventional cardiology, where transradial PCI is now used in 80% of PCIs. The AKI study results will reinforce this trend, he added.
“You have shown something opposite to what we’ve thought in the past, that maybe, with a radial approach, we would use more contrast medium, which is a risk factor for AKI. In your study – at least in ACS with very experienced transradial operators – there was no increase in contrast volume, and the risk of AKI decreased,” Dr. Legutko said.
Asked about the possibility that transradial PCI might be associated with an increased risk of embolization to the brain, much as the transfemoral approach might cause embolization to the kidneys, Dr. Cortese said there was no significant difference between the two AKI-MATRIX study arms in rates of transient ischemic attack or stroke.
“I did my first transradial PCI in 2003, and I haven’t seen any increase in these events or later dementia,” he added.
The prespecified secondary analysis of the MATRIX trial was conducted without commercial support. The presenter reported serving as a consultant to Abbott, AstraZeneca, Daiichi Sankyo, Eli Lilly, and Stentys.
Simultaneous with his presentation in Paris, the AKI-MATRIX study was published online at www.sciencedirect.com/science/article/pii/S0735109717368973.
PARIS – Transradial-access percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS) results in a significantly lower risk of acute kidney injury (AKI), compared with the transfemoral approach, according to a new analysis from the large randomized MATRIX trial.
The results of this prespecified secondary subgroup analysis of MATRIX suggest it’s time to update the classic “five golden rules” for reduction of contrast medium–induced AKI by adding a sixth. “Use a transradial approach,” Bernardo Cortese, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He reported on 8,210 participants in the MATRIX trial (Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) who were randomized to transradial- or transfemoral-access PCI for non–ST-elevation MI or ST-elevation MI.
The primary results of the 78-site, four-country European study, previously published, showed that transradial PCI reduced the composite risk of death, MI, stroke, or major bleeding by 17%, compared with transfemoral PCI, a benefit mainly driven by a marked reduction in clinically important bleeding (Lancet. 2015 Jun 20;385[9986]:2465-76).
Left unanswered by the primary analysis was the question of whether transradial PCI in ACS patients also reduced AKI risk, as had previously been suggested by a meta-analysis of observational studies (Int J Cardiol. 2015 Jan 20;179:309-11). In designing the MATRIX trial, Dr. Cortese and the other investigators decided to address that issue separately in a prespecified secondary analysis known as AKI-MATRIX. For this purpose, AKI was defined as either a post-PCI in-hospital increase in serum creatinine level of more than 25%, compared with the preangiography baseline, or an absolute increase in serum creatinine of greater than 0.5 mg/dL.
AKI occurred in 15.4% of ACS patients who underwent PCI with transradial access and 17.3% of those randomized to transfemoral access, for a significant 13% relative risk reduction. This was accomplished without any increase in the volume of contrast media required. The average was 200 mL in both study groups.
The reduction in AKI achieved with transradial-access PCI was seen in all patient subgroups, including those at increased AKI risk because of an estimated glomerular filtration rate below 60 mL/min, age 75 or older, Killup class III or IV, or a Mehran score greater than 10.
Dr. Cortese proposed several possible mechanisms for the observed reduction in AKI seen with transradial-access PCI. The major factor in his view is that the transradial approach entails less bleeding, as earlier demonstrated in the primary analysis – and bleeding has been associated with impaired renal perfusion in several prior studies. Also, it’s plausible that the passage of the catheter across the renal arteries during the transfemoral approach dislodges atherosclerotic debris, which then travels down the renal vessels.
The five golden rules for preventing contrast media–induced AKI, he noted, are
1. Discontinue nephrotoxic drugs before the procedure.
2. Identify high-risk patients.
3. Hydrate them.
4. Choose an ideal contrast medium.
5. Adapt the dose of contrast medium to the patient’s specific situation.
Discussant Jacek Legutko, MD, PhD, of Jagiellonian University in Krakow, Poland, said the primary results of the MATRIX trial published in 2015 have had a major impact on Polish interventional cardiology, where transradial PCI is now used in 80% of PCIs. The AKI study results will reinforce this trend, he added.
“You have shown something opposite to what we’ve thought in the past, that maybe, with a radial approach, we would use more contrast medium, which is a risk factor for AKI. In your study – at least in ACS with very experienced transradial operators – there was no increase in contrast volume, and the risk of AKI decreased,” Dr. Legutko said.
Asked about the possibility that transradial PCI might be associated with an increased risk of embolization to the brain, much as the transfemoral approach might cause embolization to the kidneys, Dr. Cortese said there was no significant difference between the two AKI-MATRIX study arms in rates of transient ischemic attack or stroke.
“I did my first transradial PCI in 2003, and I haven’t seen any increase in these events or later dementia,” he added.
The prespecified secondary analysis of the MATRIX trial was conducted without commercial support. The presenter reported serving as a consultant to Abbott, AstraZeneca, Daiichi Sankyo, Eli Lilly, and Stentys.
Simultaneous with his presentation in Paris, the AKI-MATRIX study was published online at www.sciencedirect.com/science/article/pii/S0735109717368973.
AT EUROPCR
Key clinical point:
Major finding: Transradial-access PCI for ACS resulted in a 13% lower risk of acute kidney injury than the transfemoral approach.
Data source: A four-country European randomized trial of transradial- vs. transfemoral-access PCI in more than 8,200 patients with ACS.
Disclosures: This prespecified secondary analysis of the MATRIX trial was conducted without commercial support. The presenter reported serving as a consultant to Abbott, AstraZeneca, Daiichi Sankyo, Eli Lilly, and Stentys.
Netherton Syndrome in Association With Vitamin D Deficiency
To the Editor:
Netherton syndrome (NS) is a rare genodermatosis that presents with erythroderma accompanied with failure to thrive in the neonatal period. Ichthyosis linearis circumflexa, or double-edged scale, is a typical skin finding. Chronic severe atopic dermatitis with diffuse generalized xerosis usually develops and often is associated with elevated IgE levels; however, a feature most associated with and crucial for the diagnosis of NS is trichorrhexis invaginata, or bamboo hair, that causes patchy hair thinning. The triad of ichthyosis linearis circumflexa, atopic dermatitis, and trichorrhexis invaginata is diagnostic of NS. Several other clinical features, including delayed growth, skeletal age delay, and short stature also can develop during its clinical course.1
Netherton syndrome is an autosomal-recessive disorder resulting from a mutation in the SPINK5 gene, which encodes a serine protease inhibitor important in skin barrier formation and immunity.2 Thus, frequent infections are common in these patients. Current treatment options include emollients and topical anti-inflammatory agents to minimize and control the classic manifestations of NS.
A 10-year-old girl with a history of allergic rhinitis and multiple food allergies presented to the dermatology clinic with a long history of diffuse generalized xerosis and erythema with areas of lichenification and scaly patches on the face, trunk, and extremities. She was born prematurely at 34 weeks and developed scaling and erythema involving most of the body shortly after birth. She exhibited severe failure to thrive that necessitated placement of a gastrostomy feeding tube at 8 months of age, resulting in satisfactory weight gain and the tube was later removed. A liver biopsy obtained at that time revealed early intrahepatic duct obstruction and early cirrhosis. She continued to have severe atopic dermatitis, poor growth, milk intolerance, and frequent infections. She had a history of dysfunctional voiding, necessitating the use of oxybutynin. The patient also was taking desmopressin to help with insensible water losses. She had no family history of dermatologic disorders.
At presentation she had diffuse scaling and erythema around the nasal vestibule and bilateral oral commissures. She also was noted to have coarse, brittle, and sparse scalp hair and eyebrows. Her current medications included hydrocortisone cream 2.5%, loratadine 10 mg daily, desmopressin 0.1 mg twice daily, and oxybutynin. Laboratory DNA analysis revealed 2 deletion mutations involving the SPINK5 gene that combined with physical findings led to the diagnosis of NS. Due to her severe growth retardation (approximately 6 SDs below the mean), she was referred to the pediatric endocrinology department. Our patient’s skeletal age was markedly delayed (6.5 years), and she was vitamin D deficient with a total vitamin D level of 16 ng/mL (reference range, 30–80 ng/mL). She is now under the care of a dietitian and taking a vitamin D supplement of 2000 IU of vitamin D3 daily. Growth hormone therapy trials have not been helpful.
An important feature of NS is growth retardation, which is multifactorial, resulting from increased caloric requirements, percutaneous fluid loss, and food allergies. Komatsu et al3 proposed that the SPINK5 inhibitory domain in addition to its role in skin barrier function is involved in regulating proteolytic processing of growth hormone in the pituitary gland. Its dysfunction may lead to a decrease in human growth hormone levels, resulting in short stature.3 This association suggested that our patient would be a good candidate for growth hormone therapy.
Furthermore, our patient was found to be vitamin D deficient, which was not surprising, as cholecalciferol (vitamin D3) is synthesized in the epidermis with UV exposure. This finding suggests that vitamin D deficiency should be suspected in patients with an impaired skin barrier. In addition to calcium regulation and bone mineralization, vitamin D plays a preventative role in cardiovascular disease, autoimmune diseases such as Crohn disease and multiple sclerosis, type 2 diabetes mellitus, infectious diseases such as tuberculosis and influenza, and many cancers.4
Vitamin D has 2 primary derivatives: (1) vitamin D3 from the skin and dietary animal sources, and (2) ergocalciferol (vitamin D2), which is obtained primarily from dietary plant sources and fortified foods. The most common test for vitamin D sufficiency is an assay for serum 25-hydroxyvitamin D (25[OH]D) concentration; 25(OH)D is derived primarily from vitamin D3, which is 3 times more potent than vitamin D2 in the production of 25(OH)D.5 The American Academy of Pediatrics recommends vitamin D replacement therapy for children with 25(OH)D levels less than 20 ng/mL (50 nmol/L) or in children who are clinically symptomatic.6 The Endocrine Society Clinical Practice Guidelines suggest screening for vitamin D deficiency only in individuals at risk.7 We suggest that serum vitamin D testing should be routine in children with NS and other atopic dermatitis conditions in which UV absorption may be impaired.
- Sun J, Linden K. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45:693-697.
- Bitoun E, Chavanas S, Irvine AD, et al. Netherton syndrome: disease expression and spectrum of SPINK5 mutations in 21 families. J Invest Dermatol. 2002;118:352-361.
- Komatsu N, Saijoh K, Otsuki N, et al. Proteolytic processing of human growth hormone by multiple tissue kallikreins and regulation by the serine protease inhibitor Kazal-Type5 (SPINK5) protein. Clin Chim Acta. 2007;377:228-236.
- Wacker M, Holick MF. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111-148.
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387-5391.
- Madhusmita M, Pacaud D, Collett-Solberg PF, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
- Holick MF, Binkley NC, Bisckoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
To the Editor:
Netherton syndrome (NS) is a rare genodermatosis that presents with erythroderma accompanied with failure to thrive in the neonatal period. Ichthyosis linearis circumflexa, or double-edged scale, is a typical skin finding. Chronic severe atopic dermatitis with diffuse generalized xerosis usually develops and often is associated with elevated IgE levels; however, a feature most associated with and crucial for the diagnosis of NS is trichorrhexis invaginata, or bamboo hair, that causes patchy hair thinning. The triad of ichthyosis linearis circumflexa, atopic dermatitis, and trichorrhexis invaginata is diagnostic of NS. Several other clinical features, including delayed growth, skeletal age delay, and short stature also can develop during its clinical course.1
Netherton syndrome is an autosomal-recessive disorder resulting from a mutation in the SPINK5 gene, which encodes a serine protease inhibitor important in skin barrier formation and immunity.2 Thus, frequent infections are common in these patients. Current treatment options include emollients and topical anti-inflammatory agents to minimize and control the classic manifestations of NS.
A 10-year-old girl with a history of allergic rhinitis and multiple food allergies presented to the dermatology clinic with a long history of diffuse generalized xerosis and erythema with areas of lichenification and scaly patches on the face, trunk, and extremities. She was born prematurely at 34 weeks and developed scaling and erythema involving most of the body shortly after birth. She exhibited severe failure to thrive that necessitated placement of a gastrostomy feeding tube at 8 months of age, resulting in satisfactory weight gain and the tube was later removed. A liver biopsy obtained at that time revealed early intrahepatic duct obstruction and early cirrhosis. She continued to have severe atopic dermatitis, poor growth, milk intolerance, and frequent infections. She had a history of dysfunctional voiding, necessitating the use of oxybutynin. The patient also was taking desmopressin to help with insensible water losses. She had no family history of dermatologic disorders.
At presentation she had diffuse scaling and erythema around the nasal vestibule and bilateral oral commissures. She also was noted to have coarse, brittle, and sparse scalp hair and eyebrows. Her current medications included hydrocortisone cream 2.5%, loratadine 10 mg daily, desmopressin 0.1 mg twice daily, and oxybutynin. Laboratory DNA analysis revealed 2 deletion mutations involving the SPINK5 gene that combined with physical findings led to the diagnosis of NS. Due to her severe growth retardation (approximately 6 SDs below the mean), she was referred to the pediatric endocrinology department. Our patient’s skeletal age was markedly delayed (6.5 years), and she was vitamin D deficient with a total vitamin D level of 16 ng/mL (reference range, 30–80 ng/mL). She is now under the care of a dietitian and taking a vitamin D supplement of 2000 IU of vitamin D3 daily. Growth hormone therapy trials have not been helpful.
An important feature of NS is growth retardation, which is multifactorial, resulting from increased caloric requirements, percutaneous fluid loss, and food allergies. Komatsu et al3 proposed that the SPINK5 inhibitory domain in addition to its role in skin barrier function is involved in regulating proteolytic processing of growth hormone in the pituitary gland. Its dysfunction may lead to a decrease in human growth hormone levels, resulting in short stature.3 This association suggested that our patient would be a good candidate for growth hormone therapy.
Furthermore, our patient was found to be vitamin D deficient, which was not surprising, as cholecalciferol (vitamin D3) is synthesized in the epidermis with UV exposure. This finding suggests that vitamin D deficiency should be suspected in patients with an impaired skin barrier. In addition to calcium regulation and bone mineralization, vitamin D plays a preventative role in cardiovascular disease, autoimmune diseases such as Crohn disease and multiple sclerosis, type 2 diabetes mellitus, infectious diseases such as tuberculosis and influenza, and many cancers.4
Vitamin D has 2 primary derivatives: (1) vitamin D3 from the skin and dietary animal sources, and (2) ergocalciferol (vitamin D2), which is obtained primarily from dietary plant sources and fortified foods. The most common test for vitamin D sufficiency is an assay for serum 25-hydroxyvitamin D (25[OH]D) concentration; 25(OH)D is derived primarily from vitamin D3, which is 3 times more potent than vitamin D2 in the production of 25(OH)D.5 The American Academy of Pediatrics recommends vitamin D replacement therapy for children with 25(OH)D levels less than 20 ng/mL (50 nmol/L) or in children who are clinically symptomatic.6 The Endocrine Society Clinical Practice Guidelines suggest screening for vitamin D deficiency only in individuals at risk.7 We suggest that serum vitamin D testing should be routine in children with NS and other atopic dermatitis conditions in which UV absorption may be impaired.
To the Editor:
Netherton syndrome (NS) is a rare genodermatosis that presents with erythroderma accompanied with failure to thrive in the neonatal period. Ichthyosis linearis circumflexa, or double-edged scale, is a typical skin finding. Chronic severe atopic dermatitis with diffuse generalized xerosis usually develops and often is associated with elevated IgE levels; however, a feature most associated with and crucial for the diagnosis of NS is trichorrhexis invaginata, or bamboo hair, that causes patchy hair thinning. The triad of ichthyosis linearis circumflexa, atopic dermatitis, and trichorrhexis invaginata is diagnostic of NS. Several other clinical features, including delayed growth, skeletal age delay, and short stature also can develop during its clinical course.1
Netherton syndrome is an autosomal-recessive disorder resulting from a mutation in the SPINK5 gene, which encodes a serine protease inhibitor important in skin barrier formation and immunity.2 Thus, frequent infections are common in these patients. Current treatment options include emollients and topical anti-inflammatory agents to minimize and control the classic manifestations of NS.
A 10-year-old girl with a history of allergic rhinitis and multiple food allergies presented to the dermatology clinic with a long history of diffuse generalized xerosis and erythema with areas of lichenification and scaly patches on the face, trunk, and extremities. She was born prematurely at 34 weeks and developed scaling and erythema involving most of the body shortly after birth. She exhibited severe failure to thrive that necessitated placement of a gastrostomy feeding tube at 8 months of age, resulting in satisfactory weight gain and the tube was later removed. A liver biopsy obtained at that time revealed early intrahepatic duct obstruction and early cirrhosis. She continued to have severe atopic dermatitis, poor growth, milk intolerance, and frequent infections. She had a history of dysfunctional voiding, necessitating the use of oxybutynin. The patient also was taking desmopressin to help with insensible water losses. She had no family history of dermatologic disorders.
At presentation she had diffuse scaling and erythema around the nasal vestibule and bilateral oral commissures. She also was noted to have coarse, brittle, and sparse scalp hair and eyebrows. Her current medications included hydrocortisone cream 2.5%, loratadine 10 mg daily, desmopressin 0.1 mg twice daily, and oxybutynin. Laboratory DNA analysis revealed 2 deletion mutations involving the SPINK5 gene that combined with physical findings led to the diagnosis of NS. Due to her severe growth retardation (approximately 6 SDs below the mean), she was referred to the pediatric endocrinology department. Our patient’s skeletal age was markedly delayed (6.5 years), and she was vitamin D deficient with a total vitamin D level of 16 ng/mL (reference range, 30–80 ng/mL). She is now under the care of a dietitian and taking a vitamin D supplement of 2000 IU of vitamin D3 daily. Growth hormone therapy trials have not been helpful.
An important feature of NS is growth retardation, which is multifactorial, resulting from increased caloric requirements, percutaneous fluid loss, and food allergies. Komatsu et al3 proposed that the SPINK5 inhibitory domain in addition to its role in skin barrier function is involved in regulating proteolytic processing of growth hormone in the pituitary gland. Its dysfunction may lead to a decrease in human growth hormone levels, resulting in short stature.3 This association suggested that our patient would be a good candidate for growth hormone therapy.
Furthermore, our patient was found to be vitamin D deficient, which was not surprising, as cholecalciferol (vitamin D3) is synthesized in the epidermis with UV exposure. This finding suggests that vitamin D deficiency should be suspected in patients with an impaired skin barrier. In addition to calcium regulation and bone mineralization, vitamin D plays a preventative role in cardiovascular disease, autoimmune diseases such as Crohn disease and multiple sclerosis, type 2 diabetes mellitus, infectious diseases such as tuberculosis and influenza, and many cancers.4
Vitamin D has 2 primary derivatives: (1) vitamin D3 from the skin and dietary animal sources, and (2) ergocalciferol (vitamin D2), which is obtained primarily from dietary plant sources and fortified foods. The most common test for vitamin D sufficiency is an assay for serum 25-hydroxyvitamin D (25[OH]D) concentration; 25(OH)D is derived primarily from vitamin D3, which is 3 times more potent than vitamin D2 in the production of 25(OH)D.5 The American Academy of Pediatrics recommends vitamin D replacement therapy for children with 25(OH)D levels less than 20 ng/mL (50 nmol/L) or in children who are clinically symptomatic.6 The Endocrine Society Clinical Practice Guidelines suggest screening for vitamin D deficiency only in individuals at risk.7 We suggest that serum vitamin D testing should be routine in children with NS and other atopic dermatitis conditions in which UV absorption may be impaired.
- Sun J, Linden K. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45:693-697.
- Bitoun E, Chavanas S, Irvine AD, et al. Netherton syndrome: disease expression and spectrum of SPINK5 mutations in 21 families. J Invest Dermatol. 2002;118:352-361.
- Komatsu N, Saijoh K, Otsuki N, et al. Proteolytic processing of human growth hormone by multiple tissue kallikreins and regulation by the serine protease inhibitor Kazal-Type5 (SPINK5) protein. Clin Chim Acta. 2007;377:228-236.
- Wacker M, Holick MF. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111-148.
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387-5391.
- Madhusmita M, Pacaud D, Collett-Solberg PF, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
- Holick MF, Binkley NC, Bisckoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Sun J, Linden K. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45:693-697.
- Bitoun E, Chavanas S, Irvine AD, et al. Netherton syndrome: disease expression and spectrum of SPINK5 mutations in 21 families. J Invest Dermatol. 2002;118:352-361.
- Komatsu N, Saijoh K, Otsuki N, et al. Proteolytic processing of human growth hormone by multiple tissue kallikreins and regulation by the serine protease inhibitor Kazal-Type5 (SPINK5) protein. Clin Chim Acta. 2007;377:228-236.
- Wacker M, Holick MF. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111-148.
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387-5391.
- Madhusmita M, Pacaud D, Collett-Solberg PF, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
- Holick MF, Binkley NC, Bisckoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
Practice Points
- Netherton syndrome (NS) is characterized by severe atopic dermatitis, ichthyosis linearis circumflexa, and trichorrhexis invaginata.
- Children with NS are at increased risk for vitamin D deficiency.
- Consider screening patients with chronic severe dermatitis for vitamin D deficiency.
New SHM Members – February/March 2017
The Society of Hospital Medicine welcomes its newest members:
Kwie-Hoa Siem, MD, Alaska
Frank Abene, Alabama
Kayla Maldonado, Alabama
Kenny Murray, MD, Alabama
Shanthan Ramidi, MD, Alabama
Lauren Hancock, APRN, Arkansas
William Hawkins, MD, Arkansas
Matthew Law, Arkansas
Emily Smith, MD, Arkansas
Firas Abbas, MBchB, Arizona
Shahid Ahmad, MD, MBBS, Arizona
Praveen Bheemanathini, Arizona
Atoosa Hosseini, Arizona
William McGrade, DO, Arizona
Konstantin Mazursky, DO, Arizona
Ibrahim Taweel, MD, Arizona
Kevin Virk, MD, FACP, Arizona
Kevin Virk, MD, FACP, Arizona
Mohemmedd Khalid Abbas, Arizona
Hasan Chaudhry, MD, Arizona
Kelly Kelleher, FAAP, Arizona
Priyanka Sultania Dudani, MBBS, Arizona
Krishna Kasireddy, MD, Arizona
Melanie Meguro, Arizona
Puneet Tuli, MD, Arizona
Jonathan Byrdy, DO, Arizona
Sarah Corral, DO, Arizona
Edward Maharam, MD, Arizona
Arvind Satyanarayan, DO, Arizona
Mayank Aggarwal, MD, Arizona
Syed Jafri, Arizona
Bujji Ainapurapu, MD, Arizona
Aaron Fernandes, MD, Arizona
Sonal Gandhi, Arizona
Sudhir Tutiki, Arizona
Navaneeth Kumar, MD, Arizona
Brian T. Courtney, MD, California
Won Jin Jeon, California
Veena Panduranga, MD, California
Jennifer Tinloy, DO, California
Debra Buckland Coffey, MCUSN, MD, California
Kathleen Teves, MD, California
Paul Goebel, MD, ACMPE, California
Shainy Hegde, California
Summaiya Muhammad, California
Desmond Wah, California
Chonn Khristin Ng, California
Almira Yang, DO, California
Salimah Boghani, MD, California
Stella Abhyankar, California
Cherie Ginwalla, MD, California
Armond Esmaili, California
Sarah Schaeffer, MD, MPH, California
Sophia Virani, MD, California
Dipti Munshi, MD, California
Judy Nguyen, DO, California
Daniel Owyang, DO, California
Christian Chiavetta, DO, California
David Reinert, DO, California
Joseph Pawlowski, MD, California
Eleanor Yang, California
Adrian Campo, MD, California
Emerson De Jesus, MD, California
Zachary Edmonds, MD, California
Trit Garg, California
Alexandra G. Ianculescu, MD, PhD, California
Felix Karp, MD, California
Cara Lai, California
Kristen Lew, MD, California
John Mogannam, California
Ameer Moussa, California
Neil Parikh, MD, MBA, California
Priya Reddy, California
Adam Simons, California
Sanjay Vadgama, MD, California
Kristofer Wills, DO, California
Michael Yang, MD, MS, California
Victor Ekuta, California,
Donna Colobong, PA-C, Colorado
Janna B. Dreason, FNP-C, Colorado
Cheryl English, NP-C, Colorado
Melanie Gerrior, MD, Colorado
Marciann Harris, NP, Colorado
Marsha Henke, MD, Colorado
Brett Hesse, Colorado
Naomi J Hipp, MD, Colorado
Aurell Horing, Colorado
Rachel Koch, DO, Colorado
Ed Marino, PA-C, Colorado
Marcus Reinhardt, MD, Colorado
Carol Runge, Colorado
Harshal Shah, Colorado
Leo Soehnlen, DO, Colorado
Anna Villalobos, MD, Colorado
Kathryn Whitfield, PA-C, Colorado
Jonathan Bei-Shing Young, MD, Colorado
Leah Damiani, MD, Colorado
Kathy Lynch, MD, Colorado
Micah Friedman, Colorado
Rachael Hilton, MD, Colorado
Madeline Koerner, Colorado
Chi Zheng, MD, Colorado
Chin-Kun Baw, MD, Connecticut
Alexandra Hawkins, NP, Connecticut
Vasundhara Singh, MD, MBBS, Connecticut
Ryan Quarles, MD, Connecticut
Debra Hernandez, APRN, BC, Connecticut
Karine Karapetyan, MD, Delaware
Choosak Burr, ARNP, Florida
Nelsi Mora, Florida
Mary Quillinan, Florida
Thuntanat Rachanakul, Florida
Samual W. Sauer, MD, MPH, Florida
Jennifer Tibangin, Florida
Keith Williams, MD, Florida
Eric Penedo, MD, Florida
Margaret Webb, Florida
Mark Bender, Florida
Brett Waress, MD, MHA, Florida
Giselle Racho, Florida
Bryan Thiel, Florida
Juan Loor Tuarez, MD, Florida
Christine Stopyra, Florida
Betsy Screws, ARNP, Florida
Jaimie Weber, MD, Florida
Priti Amin, MHA, Georgia
Naga Doddapaneni, Georgia
Stephanie Fletcher, Georgia
Disha Spath, MD, Georgia
Rafaela Wesley, DO, Georgia
Nikky Keer, DO, Georgia
James Kim, Georgia
Todd Martin, Georgia
Eli Mlaver, Georgia
Andrew Ritter, Georgia
Ali Al-Zubaidi, MBchB, Georgia
Deann Bing, MD, Georgia
Tushar Shah, Georgia
Cameron Straughn, DO, Georgia
Nobuhiro Ariyoshi, MEd, Hawaii
Prerna Kumar, Iowa
Jonathan Sebolt, MD, Iowa
Amy Tesar, DO, Iowa
Houng Chea, NP, Idaho
Finnegan Greer, PA-C, Idaho
Thao Nelson, PA, Idaho
Malatesha Gangappa, Idaho
Gloria Alumona, ACNP, Illinois
Ram Sanjeev Alur, Illinois
James Antoon, MD, FAAP, PhD, Illinois
Stefania Bailuc, MD, Illinois
Richard Huh, Illinois
Bhakti Patel, MD, Illinois
Frances Uy, ACNP, Illinois
Fernando Velazquez Vazquez, MD, Illinois
Tiffany White, MD, Illinois
Bryan P. Tully, MD, Illinois
Swati Gobhil, MBBS, Illinois
Lianghe Gao, Illinois
Gopi Astik, MD, Illinois
Marina Kovacevic, MD, Illinois
Abbie Raymond, DO, Illinois
Timothy Yung, Illinois
Ahmed Zahid, MD, Illinois
Cristina Corsini, MEd, Illinois
Faisal Rashid, MD, FACP, Illinois
Mansoor Ahmad, MD, Illinois
Matthew A. Strauch, DO, Illinois
Purshotham Reddy Grinne, Illinois
Nadia Nasreen, MD, Illinois
Maham Ashraf, MD, Indiana
Jennifer Gross, Indiana
Debasmita Mohapatra, MBBS, Indiana
Eric Scheper, Indiana
Katherine Gray, APRNBC, FNP, Indiana
Venkata Kureti, Indiana
Omer Al-Buoshkor, MD, Indiana
David Johnson, FNP, MSN, Indiana
Jonathan Salisbury, MD, Indiana
Debra Shapert, MSN, RN, Iowa
Lisa Carter, ARNP, Iowa
Matthew Woodham, Iowa
Tomoharu Suzuki, MD, Pharm, Japan
Khaldoun Haj, Kansas
Will Rogers, ACMPE, MA, MBA, Kansas
Karen Shumate, Kansas
Lisa Unruh, MD, Kansas
Matthew George, Kansas
Katie Washburn, DO, Kansas
Edwin Avallone, DO, Kentucky
Matthew Morris, Kentucky
Samantha Cappetto, MD, Kentucky
Jaison John, Kentucky
Ammar Al Jajeh, Kentucky
Joseph Bolger, MD, PhD, Louisiana
Clairissa Mulloy, Louisiana
Harish Talla, MD, Louisiana
John Amadon, Louisiana
Karthik Krishnareddy, Louisiana
Cheryl DeGrandpre, PA-C, Maine
Katherine Liu, MD, Maine
Sarah Sedney, MD, Maine
Aksana Afanasenka, MD, Maryland
Syed Nazeer Mahmood, MBBS, Maryland
Joseph Apata, MD, Maryland
Russom Ghebrai, MD, Maryland
Musa Momoh, MD, Maryland
Antanina Voit, Maryland
Dejene Kassaye, MD, MSC, Maryland
Shams Quazi, MD, FACP, MS, Maryland
Dawn Roelofs, FNP, MSN, Maryland
Kirsten Austad, MD, Massachusetts
Yoel Carrasquillo Vega, MD, Massachusetts
Michele Gaudet, NP, Massachusetts
Karina Mejias, Massachusetts
Peter Rohloff, MD, PhD, Massachusetts
Jennifer Schaeffer, Massachusetts
James Shaw, MD, Massachusetts
Renee Wheeler, Massachusetts
Angela Freeman, PA, PA-C, Massachusetts
Supriya Parvatini, MD, Massachusetts
Karen Jiang, MD, Massachusetts
Roula E. Abou-Nader, MD, Massachusetts
Shreekant Vasudhev, MD, Massachusetts
Nivedita Adabala, MD, MBBS, Michigan
Robert Behrendt, RN, BSN, Michigan
Molly Belisle, Michigan
Christine Dugan, MD, Michigan
Baljinder Gill, Michigan
Kellie Herringa, PA-C, Michigan
Christine Klingert, Michigan
Kathy Mitchell, Michigan
Aimee Vos, Michigan
Alyssa Churchill, DO, Michigan
Mailvaganam Sridharan, MD, Michigan
Atul Kapoor, MD, MBBS, Michigan
Anitha Kompally, MD, MBBS, Michigan
Nicole Webb, PA-C, Michigan
Abdulqadir Ahmad, MD, Minnesota
John Patrick Eikens, Minnesota
Bobbi Jo Jensen, PA-C, Minnesota
Rachel Keuseman, Minnesota
Stephen Palmquist, Minnesota
Manit Singla, MD, Minnesota
Douglas Berg, Minnesota
Nathan Palmolea, Minnesota
Molly Tureson, PAC, Minnesota
Mehdi Dastrange, MD, MHA, Minnesota
Kent Svee, Minnesota
Ashley Viere, PA-C, Minnesota
Molly Yang, MD, Minnesota
Paige Sams, DO, Minnesota
Amit Reddy, MBBS, Mississippi
Jacqueline Brooke Banks, FNP-C, Mississippi
Lori Foxworth, CFNP, Mississippi
Nicki Lawson, FNP-C, Mississippi
Bikash Acharya, Missouri
Zafar Ahmad, PA-C, Missouri
Harleen Chela, MD, Missouri
Jeffrey Chung, MD, Missouri
Daniel Kornfeld, Missouri
Erika Leung, MD, MSc, Missouri
Lisa Moser, PA, Missouri
Mark Stiffler, Missouri
Tushar Tarun, MBBS, Missouri
Nicole McLaughlin, Missouri
Katy Lohmann, PA-C, Missouri
Jayasree Bodagala, MD, Missouri
Ravi Kiran Morumuru, ACMPE, Missouri
Matthew Brown, MD, FAAFP, Missouri
Ravikanth Tadi, Missouri
Bazgha Ahmad, DO, Missouri
Monica Hawkins, RN, Missouri
Karri Vesey, BSN, Montana
Madison Vertin, PA-C, Montana
Urmila Mukherjee, MD, Nebraska
Noah Wiedel, MD, Nebraska
Sidrah Sheikh, MD, MBBS, Nebraska
Mohammad Esmadi, MBBS, Nebraska
Jill Zabih, MD, Nebraska
Jody Frey-Burns, RN, Nevada
Adnan Akbar, MD, Nevada
Peter Gayed, MRCP, New Hampshire
Jonathan T. Huntington, MD, New Hampshire
Meghan Meehan, ACNP, New Hampshire
Saurabh Mehta, MD, New Jersey
Hanaa Benchekroun Belabbes, MD, MHA, New Jersey
Hwan Kim, MD, New Jersey
Mary Tobiasson, USA, New Jersey
Muhammad Khakwani, MD, New Jersey
Amita Maibam, MD, MPH, New Jersey
Kumar Rohit, MBBS, New Jersey
Crystal Benjamin, MD, New Jersey
Rafael Garabis, New Mexico
Sam MacBride, MD, New Mexico
Indra Peram, MD, New Mexico
Sarah Vertrees, DO, New Mexico
Aswani Kumar Alavala, MD, New Mexico
Christopher Anstine, New Mexico
Prathima Guruguri, MD, New Mexico
Diedre Hofinger, MD, FACP, New Mexico
Katharine Juarez, New Mexico
Amtul Mahavesh, MD, New Mexico
Francisco Marquez, New Mexico
Payal Sen, MD, New Mexico
Morgan Wong, DO, New Mexico
Kelly Berchou, New York
Ronald Cho, New York
Nishil Dalsania, New York
Carolyn Drake, MD, MPH, New York
Leanne Forman, New York
Valerie Gausman, New York
Laurie Jacobs, New York
Janice Jang, MD, New York
Sonia Kohli, MD, New York
Nancy Lee, PA, New York
Allen Lee, MD, New York
Matthew McCarthy, FACP, New York
Akram Mohammed, MD, New York
Jennifer Nead, New York
Kristal Persaud, PA, New York
Mariya Rozenblit, MD, New York
Christian Torres, MD, New York
Sasha De Jesus, MD, New York
Gabriella Polyak, New York
Nataliya Yuklyaeva, MD, New York
Riyaz Kamadoli, MD, New York
Ramanuj Chakravarty, New York
Adil Zaidi, MD, New York
Allison Walker, MD, New York
Himali Gandhi, New York
Alexey Yanilshtein, MD, New York
Ramsey Al-Khalil, New York
Latoya Codougan, MD, New York
Khan Najmi, MD, New York
Sara Stream, MD, New York
Bhuwan Poudyal, MD, New York
Khalil Anchouche, New York
Sarah Azarchi, New York
Susana Bejar, New York
Brian Chang, New York
Jonathan Chen, New York
Hailey Gupta, MD, New York
Medhavi Gupta, New York
Ali Khan, New York
Benjamin Kwok, MD, New York
Billy Lin, New York
Katherine Ni, New York
Jina Park, New York
Gabriel Perreault, New York
Luis Alberto Romero, New York
Payal Shah, New York
Punita Shroff, New York
Scott Statman, New York
Maria Sunseri, New York
Benjamin Verplanke, New York
Audrey Zhang, New York
Gaby Razzouk, MD, New York
Pranitha Mantrala, MD, New York
Marsha Antoine, New York
Kanica Yashi, New York
Navid Ahmed, New York
Tasha Richards, PA, New York
Connor Tryon, MD, New York
Naveen Yarlagadda, MD, New York
Alex Hogan, New York
Andrew Donohoe, CCM, MD, North Carolina
Brittany Forshay, MD, North Carolina
Kelly Hammerbeck, FNP, North Carolina
Jennifer Hausman, North Carolina
Babajide Obisesan, North Carolina
Kwadwo Ofori, MD, North Carolina
Eric Ofosu, MD, North Carolina
Kale Roth, North Carolina
Robert Soma, PA-C, North Carolina
Sommany Weber, North Carolina
Ronnie Jacobs, North Carolina
Muhammad Ghani, MD, MACP, MBBS, North Carolina
Madeline Treasure, North Carolina
Andrew McWilliams, MD, North Carolina
Karen Payne, ACNP, MPH, North Carolina
Rafal Poplawski, MD, North Carolina
James Seal, PA-C, North Carolina
Farheen Qureshi, DO, North Carolina
Basavatti Sowmya, MD, MBBS, North Carolina
Eshwar Lal, MD, North Carolina
Catherine Hathaway, MD, North Carolina
Sherif Naguib, FAAFP, North Carolina
Sara Skavroneck, North Carolina
Charles Ofosu, North Carolina
Alex Alburquerque, MD, Ohio
Isha Butler, DO, Ohio
Anne Carrol, MD, Ohio
Scott Childers, MD, Ohio
Philip Jonas, MD, Ohio
Ahmadreza Karimianpour, Ohio
Rahul Kumar, MD, Ohio
George Maidaa, MD, Ohio
Kevin McAninch, Ohio
Jill Mccourt, FNP, Ohio
Roxanne Oliver, Ohio
Farah Hussain, Ohio
Natasha Axton, PA-C, Ohio
Brooke Harris, ACNP, Ohio
Vidhya Murukesan, MD, Ohio
Sara Dong, Ohio
Christie Astor, FNP, Ohio
Sunita Mall, MD, Ohio
Sunita Mall, MD, Ohio
Fouzia Tariq, MD, Ohio
Kaveri Sivaruban, MD, Ohio
Eunice Quicho, Ohio
Smitha Achuthankutty, MD, Ohio
Harmanpreet Shinh, MD, Ohio
Maereg Tesfaye, Ohio
Kalyn Jolivette, MD, Ohio
Richelle Voth, PA-C, Oklahoma
Samuel J. Ratermann, MD, FAAFP, Oklahoma
Richelle Voth, PA-C, Oklahoma
Alden Forrester, MD, Oregon
Nicholas Brown, DO, Oregon
Ian Pennell-Walklin, MD, Oregon
Bruce Ramsey, Oregon
Kyle Brekke, DO, Oregon
Sarah Webber, MD, Oregon
Brian Beaudoin, MD, Pennsylvania
Glenn Bedell, MHSA, Pennsylvania
Cristina Green, AGACNP-DNP, Pennsylvania
Andrew Groff, Pennsylvania
Sulman Masood Hashmi, MBBS, Pennsylvania
Eric Kasprowicz, MD, MPH, Pennsylvania
Laura Leuenberger, Pennsylvania
James Liszewski, MD, Pennsylvania
Caitlyn Moss, Pennsylvania
Paul Seunghyun Nho, Pennsylvania
Rishan Patel, MD, Pennsylvania
Dilli R. Poudel, MBBS, Pennsylvania
Naveen Yellappa, MBBS, Pennsylvania
Usman Zulfiqar, Pennsylvania
Nina Jain, Pennsylvania
Bhumika Patel, DO, Pennsylvania
Jenna M. Diasio, PA-C, Pennsylvania
Malachi Courtney, MD, Pennsylvania
Sonia Arneja, MD, Pennsylvania
Ross Ellis, MD, Pennsylvania
Samreen Siddiqui, Pennsylvania
Jillian Zavodnick, Pennsylvania
Kinan Kassar, MD, Pennsylvania
Maritsa M. Scoulos-Hanson, Pennsylvania
Jennifer Taylor, PA-C, Pennsylvania
Steven Delaveris, DO, Pennsylvania
Danica Buzniak, DO, Rhode Island
Paul Browning, MD, South Carolina
Matt Coones, MD, South Carolina
Cedric Fisher, MD, South Carolina
Aloysius Jackson, MD, South Carolina
Katharine DuPont, MD, South Carolina
Michael Jenkins, MD, South Carolina
Jessica Hamilton, APRN, BC, FNP, South Carolina
Pamela Pyle, DO, South Carolina
Shakeel Ahmed, MBBS, MD, South Dakota
D. Bruce Eaton, MD, South Dakota
Drew Jorgensen, MD, South Dakota
Shelly Turbak, MSN, RN, South Dakota
Tamera Sturm, DO, South Dakota
Peggy Brooks, Tennessee
Joseph Garrido, MD, Tennessee
Lisa Grimes, FNP, Tennessee
Chennakesava Kummathi, MBBS, Tennessee
Victoria Okafor, Tennessee
Ashley Smith, Tennessee
Monisha Bhatia, Tennessee
Belinda Jenkins, APRN-BC, Tennessee
Kim Zahnke, MD, Tennessee
Robert Arias, Texas
Nicolas Batterton, MD, Texas
Scott DePaul, MD, Texas
Nancy Foster, Texas
Larry Hughes, Texas
Erin Koval, Texas
Femi Layiwola, MD, Texas
Krysta Lin, Texas
James J. Onorato, MD, PhD, Texas
Allison Stephenson, PA-C, Texas
Brandon Stormes, Texas
Rubin Simon, MD, Texas
Brian Anderson, DO, Texas
Hatim Chhatriwala, MD, Texas
Aziz Hammoud, Texas
Haru Yamamoto, MD, Texas
Lauren Schiegg, Texas
Victoria Grasso, DO, Texas
Victor Salcedo, MD, Texas
Rajiv Bhattarai, Texas
Iram Qureshi, DO, Texas
Lisa Hafemeister, FACHE, MHA, Texas
Helena Kurian, MD, Texas
Jessica Lin, Texas
Nathan Nowalk, MD, Texas
Keely Smith, MD, Texas
Jonathan Weiser, MD, Texas
Roland Prezas, DO, FAAFP, Texas
Allan Recto, AHIP, Texas
Regina Dimbo, Texas
Venkata Ghanta, Texas
Richmond Hunt, Texas
Vishal Patel, MD, Texas
Zain Sharif, MD, Texas
Rommel Del Rosario, MD, Texas
Khawer Khadimally, DO, Texas
Diogenes Valderrama, MD, Texas
Charles Ewoh, MD, Texas
Deepika Kilaru, Texas
Tilahun Belay, MD, Texas
Chandra S Reddy Navuluri, MD, Texas
Bradley Goad, DO, FACP, Virginia
Patrick Higdon, MD, Virginia
Gabriella Miller, MD, HMDC, Virginia
Miklos Szentirmai, MD, Virginia
Hyder Tamton, Virginia
Andra Mirescu, MD, Virginia
Olukayode Ojo, Virginia
Robert Szeles, MD, Virginia
Anya Cope, DO, Virginia
OsCiriah Press, MD, Virginia
Rikin Kadakia, MD, Virginia
Bryant Self, DO, Virginia
Sarah Sabo, ACNP, Virginia
Pedro A. Gonzales Alvarez, MD, Virginia
William Best, Virginia
Pushpanjali Basnyat, MD, Washington
Nikki Hartley-Jonason, Washington
Helen Johnsonwall, MD, Washington
Eric LaMotte, MD, Washington
Maher Muraywid, Washington
Evan Neal Paul, MD, Washington
Sarah Rogers, MD, Washington
Lindee Strizich, Washington
Maryam Tariq, MBBS, Washington
Meghaan Walsh, MD, Washington
Oleg Zbirun, MD, Washington
Meeta Sabnis, MD, Washington
James Kuo, MD, Washington
Liang Du, Washington
Syed Farhan Tabraiz Hashmi, MD, Washington
Jessica Jung, MD, Washington
Joshua Pelley, MD, Washington
Alex Yu, MD, Washington
Alfred Curnow, MD, Washington
Duhwan Kang, Washington
Gilbert Daniel, MD, Washington, D.C.
Eleanor Fitall, Washington, D.C.
Vinay Srinivasan, Washington, D.C.
Scott Wine, West Virginia
Trevor Miller, MBA, PA-C, West Virginia
Audrey Hiltunen, Wisconsin
Elina Litinskaya, Wisconsin
John M. Murphy, MD, Wisconsin
Tanya Pedretti, PA, Wisconsin
Adine Rodemeyer, MD, Wisconsin
Oghomwen Sule, MBBS, Wisconsin
Terrence Witt, MD, Wisconsin
Mayank Arora, Wisconsin
John D. MacDonald, MD, Wisconsin
Abigail Cook, Wisconsin
Mohamed Ibrahim, MD, Wisconsin
Aymen Khogali, MD, Wisconsin
Nicholas Haun, Wisconsin
Sandra Brown, Victoria, Australia
Alessandra Gessner, Alberta, Canada
Courtney Carlucci, British Columbia, Canada
Muhanad Y. Al Habash, Canada
Karen Tong, MD, Canada
Taku Yabuki, Japan
Liza van Loon, the Netherlands
Edward Gebuis, MD, the Netherlands
Abdisalan Afrah, MD, Qatar
Akhnuwkh Jones, Qatar
Mashuk Uddin, MBBS, MRCP, FRCP, Qatar
Ibrahim Yusuf Abubeker, MRCP, Qatar
Chih-Wei Tseng, Taiwan
Sawsan Abdel-Razig, MD, FACP, United Arab Emirates
The Society of Hospital Medicine welcomes its newest members:
Kwie-Hoa Siem, MD, Alaska
Frank Abene, Alabama
Kayla Maldonado, Alabama
Kenny Murray, MD, Alabama
Shanthan Ramidi, MD, Alabama
Lauren Hancock, APRN, Arkansas
William Hawkins, MD, Arkansas
Matthew Law, Arkansas
Emily Smith, MD, Arkansas
Firas Abbas, MBchB, Arizona
Shahid Ahmad, MD, MBBS, Arizona
Praveen Bheemanathini, Arizona
Atoosa Hosseini, Arizona
William McGrade, DO, Arizona
Konstantin Mazursky, DO, Arizona
Ibrahim Taweel, MD, Arizona
Kevin Virk, MD, FACP, Arizona
Kevin Virk, MD, FACP, Arizona
Mohemmedd Khalid Abbas, Arizona
Hasan Chaudhry, MD, Arizona
Kelly Kelleher, FAAP, Arizona
Priyanka Sultania Dudani, MBBS, Arizona
Krishna Kasireddy, MD, Arizona
Melanie Meguro, Arizona
Puneet Tuli, MD, Arizona
Jonathan Byrdy, DO, Arizona
Sarah Corral, DO, Arizona
Edward Maharam, MD, Arizona
Arvind Satyanarayan, DO, Arizona
Mayank Aggarwal, MD, Arizona
Syed Jafri, Arizona
Bujji Ainapurapu, MD, Arizona
Aaron Fernandes, MD, Arizona
Sonal Gandhi, Arizona
Sudhir Tutiki, Arizona
Navaneeth Kumar, MD, Arizona
Brian T. Courtney, MD, California
Won Jin Jeon, California
Veena Panduranga, MD, California
Jennifer Tinloy, DO, California
Debra Buckland Coffey, MCUSN, MD, California
Kathleen Teves, MD, California
Paul Goebel, MD, ACMPE, California
Shainy Hegde, California
Summaiya Muhammad, California
Desmond Wah, California
Chonn Khristin Ng, California
Almira Yang, DO, California
Salimah Boghani, MD, California
Stella Abhyankar, California
Cherie Ginwalla, MD, California
Armond Esmaili, California
Sarah Schaeffer, MD, MPH, California
Sophia Virani, MD, California
Dipti Munshi, MD, California
Judy Nguyen, DO, California
Daniel Owyang, DO, California
Christian Chiavetta, DO, California
David Reinert, DO, California
Joseph Pawlowski, MD, California
Eleanor Yang, California
Adrian Campo, MD, California
Emerson De Jesus, MD, California
Zachary Edmonds, MD, California
Trit Garg, California
Alexandra G. Ianculescu, MD, PhD, California
Felix Karp, MD, California
Cara Lai, California
Kristen Lew, MD, California
John Mogannam, California
Ameer Moussa, California
Neil Parikh, MD, MBA, California
Priya Reddy, California
Adam Simons, California
Sanjay Vadgama, MD, California
Kristofer Wills, DO, California
Michael Yang, MD, MS, California
Victor Ekuta, California,
Donna Colobong, PA-C, Colorado
Janna B. Dreason, FNP-C, Colorado
Cheryl English, NP-C, Colorado
Melanie Gerrior, MD, Colorado
Marciann Harris, NP, Colorado
Marsha Henke, MD, Colorado
Brett Hesse, Colorado
Naomi J Hipp, MD, Colorado
Aurell Horing, Colorado
Rachel Koch, DO, Colorado
Ed Marino, PA-C, Colorado
Marcus Reinhardt, MD, Colorado
Carol Runge, Colorado
Harshal Shah, Colorado
Leo Soehnlen, DO, Colorado
Anna Villalobos, MD, Colorado
Kathryn Whitfield, PA-C, Colorado
Jonathan Bei-Shing Young, MD, Colorado
Leah Damiani, MD, Colorado
Kathy Lynch, MD, Colorado
Micah Friedman, Colorado
Rachael Hilton, MD, Colorado
Madeline Koerner, Colorado
Chi Zheng, MD, Colorado
Chin-Kun Baw, MD, Connecticut
Alexandra Hawkins, NP, Connecticut
Vasundhara Singh, MD, MBBS, Connecticut
Ryan Quarles, MD, Connecticut
Debra Hernandez, APRN, BC, Connecticut
Karine Karapetyan, MD, Delaware
Choosak Burr, ARNP, Florida
Nelsi Mora, Florida
Mary Quillinan, Florida
Thuntanat Rachanakul, Florida
Samual W. Sauer, MD, MPH, Florida
Jennifer Tibangin, Florida
Keith Williams, MD, Florida
Eric Penedo, MD, Florida
Margaret Webb, Florida
Mark Bender, Florida
Brett Waress, MD, MHA, Florida
Giselle Racho, Florida
Bryan Thiel, Florida
Juan Loor Tuarez, MD, Florida
Christine Stopyra, Florida
Betsy Screws, ARNP, Florida
Jaimie Weber, MD, Florida
Priti Amin, MHA, Georgia
Naga Doddapaneni, Georgia
Stephanie Fletcher, Georgia
Disha Spath, MD, Georgia
Rafaela Wesley, DO, Georgia
Nikky Keer, DO, Georgia
James Kim, Georgia
Todd Martin, Georgia
Eli Mlaver, Georgia
Andrew Ritter, Georgia
Ali Al-Zubaidi, MBchB, Georgia
Deann Bing, MD, Georgia
Tushar Shah, Georgia
Cameron Straughn, DO, Georgia
Nobuhiro Ariyoshi, MEd, Hawaii
Prerna Kumar, Iowa
Jonathan Sebolt, MD, Iowa
Amy Tesar, DO, Iowa
Houng Chea, NP, Idaho
Finnegan Greer, PA-C, Idaho
Thao Nelson, PA, Idaho
Malatesha Gangappa, Idaho
Gloria Alumona, ACNP, Illinois
Ram Sanjeev Alur, Illinois
James Antoon, MD, FAAP, PhD, Illinois
Stefania Bailuc, MD, Illinois
Richard Huh, Illinois
Bhakti Patel, MD, Illinois
Frances Uy, ACNP, Illinois
Fernando Velazquez Vazquez, MD, Illinois
Tiffany White, MD, Illinois
Bryan P. Tully, MD, Illinois
Swati Gobhil, MBBS, Illinois
Lianghe Gao, Illinois
Gopi Astik, MD, Illinois
Marina Kovacevic, MD, Illinois
Abbie Raymond, DO, Illinois
Timothy Yung, Illinois
Ahmed Zahid, MD, Illinois
Cristina Corsini, MEd, Illinois
Faisal Rashid, MD, FACP, Illinois
Mansoor Ahmad, MD, Illinois
Matthew A. Strauch, DO, Illinois
Purshotham Reddy Grinne, Illinois
Nadia Nasreen, MD, Illinois
Maham Ashraf, MD, Indiana
Jennifer Gross, Indiana
Debasmita Mohapatra, MBBS, Indiana
Eric Scheper, Indiana
Katherine Gray, APRNBC, FNP, Indiana
Venkata Kureti, Indiana
Omer Al-Buoshkor, MD, Indiana
David Johnson, FNP, MSN, Indiana
Jonathan Salisbury, MD, Indiana
Debra Shapert, MSN, RN, Iowa
Lisa Carter, ARNP, Iowa
Matthew Woodham, Iowa
Tomoharu Suzuki, MD, Pharm, Japan
Khaldoun Haj, Kansas
Will Rogers, ACMPE, MA, MBA, Kansas
Karen Shumate, Kansas
Lisa Unruh, MD, Kansas
Matthew George, Kansas
Katie Washburn, DO, Kansas
Edwin Avallone, DO, Kentucky
Matthew Morris, Kentucky
Samantha Cappetto, MD, Kentucky
Jaison John, Kentucky
Ammar Al Jajeh, Kentucky
Joseph Bolger, MD, PhD, Louisiana
Clairissa Mulloy, Louisiana
Harish Talla, MD, Louisiana
John Amadon, Louisiana
Karthik Krishnareddy, Louisiana
Cheryl DeGrandpre, PA-C, Maine
Katherine Liu, MD, Maine
Sarah Sedney, MD, Maine
Aksana Afanasenka, MD, Maryland
Syed Nazeer Mahmood, MBBS, Maryland
Joseph Apata, MD, Maryland
Russom Ghebrai, MD, Maryland
Musa Momoh, MD, Maryland
Antanina Voit, Maryland
Dejene Kassaye, MD, MSC, Maryland
Shams Quazi, MD, FACP, MS, Maryland
Dawn Roelofs, FNP, MSN, Maryland
Kirsten Austad, MD, Massachusetts
Yoel Carrasquillo Vega, MD, Massachusetts
Michele Gaudet, NP, Massachusetts
Karina Mejias, Massachusetts
Peter Rohloff, MD, PhD, Massachusetts
Jennifer Schaeffer, Massachusetts
James Shaw, MD, Massachusetts
Renee Wheeler, Massachusetts
Angela Freeman, PA, PA-C, Massachusetts
Supriya Parvatini, MD, Massachusetts
Karen Jiang, MD, Massachusetts
Roula E. Abou-Nader, MD, Massachusetts
Shreekant Vasudhev, MD, Massachusetts
Nivedita Adabala, MD, MBBS, Michigan
Robert Behrendt, RN, BSN, Michigan
Molly Belisle, Michigan
Christine Dugan, MD, Michigan
Baljinder Gill, Michigan
Kellie Herringa, PA-C, Michigan
Christine Klingert, Michigan
Kathy Mitchell, Michigan
Aimee Vos, Michigan
Alyssa Churchill, DO, Michigan
Mailvaganam Sridharan, MD, Michigan
Atul Kapoor, MD, MBBS, Michigan
Anitha Kompally, MD, MBBS, Michigan
Nicole Webb, PA-C, Michigan
Abdulqadir Ahmad, MD, Minnesota
John Patrick Eikens, Minnesota
Bobbi Jo Jensen, PA-C, Minnesota
Rachel Keuseman, Minnesota
Stephen Palmquist, Minnesota
Manit Singla, MD, Minnesota
Douglas Berg, Minnesota
Nathan Palmolea, Minnesota
Molly Tureson, PAC, Minnesota
Mehdi Dastrange, MD, MHA, Minnesota
Kent Svee, Minnesota
Ashley Viere, PA-C, Minnesota
Molly Yang, MD, Minnesota
Paige Sams, DO, Minnesota
Amit Reddy, MBBS, Mississippi
Jacqueline Brooke Banks, FNP-C, Mississippi
Lori Foxworth, CFNP, Mississippi
Nicki Lawson, FNP-C, Mississippi
Bikash Acharya, Missouri
Zafar Ahmad, PA-C, Missouri
Harleen Chela, MD, Missouri
Jeffrey Chung, MD, Missouri
Daniel Kornfeld, Missouri
Erika Leung, MD, MSc, Missouri
Lisa Moser, PA, Missouri
Mark Stiffler, Missouri
Tushar Tarun, MBBS, Missouri
Nicole McLaughlin, Missouri
Katy Lohmann, PA-C, Missouri
Jayasree Bodagala, MD, Missouri
Ravi Kiran Morumuru, ACMPE, Missouri
Matthew Brown, MD, FAAFP, Missouri
Ravikanth Tadi, Missouri
Bazgha Ahmad, DO, Missouri
Monica Hawkins, RN, Missouri
Karri Vesey, BSN, Montana
Madison Vertin, PA-C, Montana
Urmila Mukherjee, MD, Nebraska
Noah Wiedel, MD, Nebraska
Sidrah Sheikh, MD, MBBS, Nebraska
Mohammad Esmadi, MBBS, Nebraska
Jill Zabih, MD, Nebraska
Jody Frey-Burns, RN, Nevada
Adnan Akbar, MD, Nevada
Peter Gayed, MRCP, New Hampshire
Jonathan T. Huntington, MD, New Hampshire
Meghan Meehan, ACNP, New Hampshire
Saurabh Mehta, MD, New Jersey
Hanaa Benchekroun Belabbes, MD, MHA, New Jersey
Hwan Kim, MD, New Jersey
Mary Tobiasson, USA, New Jersey
Muhammad Khakwani, MD, New Jersey
Amita Maibam, MD, MPH, New Jersey
Kumar Rohit, MBBS, New Jersey
Crystal Benjamin, MD, New Jersey
Rafael Garabis, New Mexico
Sam MacBride, MD, New Mexico
Indra Peram, MD, New Mexico
Sarah Vertrees, DO, New Mexico
Aswani Kumar Alavala, MD, New Mexico
Christopher Anstine, New Mexico
Prathima Guruguri, MD, New Mexico
Diedre Hofinger, MD, FACP, New Mexico
Katharine Juarez, New Mexico
Amtul Mahavesh, MD, New Mexico
Francisco Marquez, New Mexico
Payal Sen, MD, New Mexico
Morgan Wong, DO, New Mexico
Kelly Berchou, New York
Ronald Cho, New York
Nishil Dalsania, New York
Carolyn Drake, MD, MPH, New York
Leanne Forman, New York
Valerie Gausman, New York
Laurie Jacobs, New York
Janice Jang, MD, New York
Sonia Kohli, MD, New York
Nancy Lee, PA, New York
Allen Lee, MD, New York
Matthew McCarthy, FACP, New York
Akram Mohammed, MD, New York
Jennifer Nead, New York
Kristal Persaud, PA, New York
Mariya Rozenblit, MD, New York
Christian Torres, MD, New York
Sasha De Jesus, MD, New York
Gabriella Polyak, New York
Nataliya Yuklyaeva, MD, New York
Riyaz Kamadoli, MD, New York
Ramanuj Chakravarty, New York
Adil Zaidi, MD, New York
Allison Walker, MD, New York
Himali Gandhi, New York
Alexey Yanilshtein, MD, New York
Ramsey Al-Khalil, New York
Latoya Codougan, MD, New York
Khan Najmi, MD, New York
Sara Stream, MD, New York
Bhuwan Poudyal, MD, New York
Khalil Anchouche, New York
Sarah Azarchi, New York
Susana Bejar, New York
Brian Chang, New York
Jonathan Chen, New York
Hailey Gupta, MD, New York
Medhavi Gupta, New York
Ali Khan, New York
Benjamin Kwok, MD, New York
Billy Lin, New York
Katherine Ni, New York
Jina Park, New York
Gabriel Perreault, New York
Luis Alberto Romero, New York
Payal Shah, New York
Punita Shroff, New York
Scott Statman, New York
Maria Sunseri, New York
Benjamin Verplanke, New York
Audrey Zhang, New York
Gaby Razzouk, MD, New York
Pranitha Mantrala, MD, New York
Marsha Antoine, New York
Kanica Yashi, New York
Navid Ahmed, New York
Tasha Richards, PA, New York
Connor Tryon, MD, New York
Naveen Yarlagadda, MD, New York
Alex Hogan, New York
Andrew Donohoe, CCM, MD, North Carolina
Brittany Forshay, MD, North Carolina
Kelly Hammerbeck, FNP, North Carolina
Jennifer Hausman, North Carolina
Babajide Obisesan, North Carolina
Kwadwo Ofori, MD, North Carolina
Eric Ofosu, MD, North Carolina
Kale Roth, North Carolina
Robert Soma, PA-C, North Carolina
Sommany Weber, North Carolina
Ronnie Jacobs, North Carolina
Muhammad Ghani, MD, MACP, MBBS, North Carolina
Madeline Treasure, North Carolina
Andrew McWilliams, MD, North Carolina
Karen Payne, ACNP, MPH, North Carolina
Rafal Poplawski, MD, North Carolina
James Seal, PA-C, North Carolina
Farheen Qureshi, DO, North Carolina
Basavatti Sowmya, MD, MBBS, North Carolina
Eshwar Lal, MD, North Carolina
Catherine Hathaway, MD, North Carolina
Sherif Naguib, FAAFP, North Carolina
Sara Skavroneck, North Carolina
Charles Ofosu, North Carolina
Alex Alburquerque, MD, Ohio
Isha Butler, DO, Ohio
Anne Carrol, MD, Ohio
Scott Childers, MD, Ohio
Philip Jonas, MD, Ohio
Ahmadreza Karimianpour, Ohio
Rahul Kumar, MD, Ohio
George Maidaa, MD, Ohio
Kevin McAninch, Ohio
Jill Mccourt, FNP, Ohio
Roxanne Oliver, Ohio
Farah Hussain, Ohio
Natasha Axton, PA-C, Ohio
Brooke Harris, ACNP, Ohio
Vidhya Murukesan, MD, Ohio
Sara Dong, Ohio
Christie Astor, FNP, Ohio
Sunita Mall, MD, Ohio
Sunita Mall, MD, Ohio
Fouzia Tariq, MD, Ohio
Kaveri Sivaruban, MD, Ohio
Eunice Quicho, Ohio
Smitha Achuthankutty, MD, Ohio
Harmanpreet Shinh, MD, Ohio
Maereg Tesfaye, Ohio
Kalyn Jolivette, MD, Ohio
Richelle Voth, PA-C, Oklahoma
Samuel J. Ratermann, MD, FAAFP, Oklahoma
Richelle Voth, PA-C, Oklahoma
Alden Forrester, MD, Oregon
Nicholas Brown, DO, Oregon
Ian Pennell-Walklin, MD, Oregon
Bruce Ramsey, Oregon
Kyle Brekke, DO, Oregon
Sarah Webber, MD, Oregon
Brian Beaudoin, MD, Pennsylvania
Glenn Bedell, MHSA, Pennsylvania
Cristina Green, AGACNP-DNP, Pennsylvania
Andrew Groff, Pennsylvania
Sulman Masood Hashmi, MBBS, Pennsylvania
Eric Kasprowicz, MD, MPH, Pennsylvania
Laura Leuenberger, Pennsylvania
James Liszewski, MD, Pennsylvania
Caitlyn Moss, Pennsylvania
Paul Seunghyun Nho, Pennsylvania
Rishan Patel, MD, Pennsylvania
Dilli R. Poudel, MBBS, Pennsylvania
Naveen Yellappa, MBBS, Pennsylvania
Usman Zulfiqar, Pennsylvania
Nina Jain, Pennsylvania
Bhumika Patel, DO, Pennsylvania
Jenna M. Diasio, PA-C, Pennsylvania
Malachi Courtney, MD, Pennsylvania
Sonia Arneja, MD, Pennsylvania
Ross Ellis, MD, Pennsylvania
Samreen Siddiqui, Pennsylvania
Jillian Zavodnick, Pennsylvania
Kinan Kassar, MD, Pennsylvania
Maritsa M. Scoulos-Hanson, Pennsylvania
Jennifer Taylor, PA-C, Pennsylvania
Steven Delaveris, DO, Pennsylvania
Danica Buzniak, DO, Rhode Island
Paul Browning, MD, South Carolina
Matt Coones, MD, South Carolina
Cedric Fisher, MD, South Carolina
Aloysius Jackson, MD, South Carolina
Katharine DuPont, MD, South Carolina
Michael Jenkins, MD, South Carolina
Jessica Hamilton, APRN, BC, FNP, South Carolina
Pamela Pyle, DO, South Carolina
Shakeel Ahmed, MBBS, MD, South Dakota
D. Bruce Eaton, MD, South Dakota
Drew Jorgensen, MD, South Dakota
Shelly Turbak, MSN, RN, South Dakota
Tamera Sturm, DO, South Dakota
Peggy Brooks, Tennessee
Joseph Garrido, MD, Tennessee
Lisa Grimes, FNP, Tennessee
Chennakesava Kummathi, MBBS, Tennessee
Victoria Okafor, Tennessee
Ashley Smith, Tennessee
Monisha Bhatia, Tennessee
Belinda Jenkins, APRN-BC, Tennessee
Kim Zahnke, MD, Tennessee
Robert Arias, Texas
Nicolas Batterton, MD, Texas
Scott DePaul, MD, Texas
Nancy Foster, Texas
Larry Hughes, Texas
Erin Koval, Texas
Femi Layiwola, MD, Texas
Krysta Lin, Texas
James J. Onorato, MD, PhD, Texas
Allison Stephenson, PA-C, Texas
Brandon Stormes, Texas
Rubin Simon, MD, Texas
Brian Anderson, DO, Texas
Hatim Chhatriwala, MD, Texas
Aziz Hammoud, Texas
Haru Yamamoto, MD, Texas
Lauren Schiegg, Texas
Victoria Grasso, DO, Texas
Victor Salcedo, MD, Texas
Rajiv Bhattarai, Texas
Iram Qureshi, DO, Texas
Lisa Hafemeister, FACHE, MHA, Texas
Helena Kurian, MD, Texas
Jessica Lin, Texas
Nathan Nowalk, MD, Texas
Keely Smith, MD, Texas
Jonathan Weiser, MD, Texas
Roland Prezas, DO, FAAFP, Texas
Allan Recto, AHIP, Texas
Regina Dimbo, Texas
Venkata Ghanta, Texas
Richmond Hunt, Texas
Vishal Patel, MD, Texas
Zain Sharif, MD, Texas
Rommel Del Rosario, MD, Texas
Khawer Khadimally, DO, Texas
Diogenes Valderrama, MD, Texas
Charles Ewoh, MD, Texas
Deepika Kilaru, Texas
Tilahun Belay, MD, Texas
Chandra S Reddy Navuluri, MD, Texas
Bradley Goad, DO, FACP, Virginia
Patrick Higdon, MD, Virginia
Gabriella Miller, MD, HMDC, Virginia
Miklos Szentirmai, MD, Virginia
Hyder Tamton, Virginia
Andra Mirescu, MD, Virginia
Olukayode Ojo, Virginia
Robert Szeles, MD, Virginia
Anya Cope, DO, Virginia
OsCiriah Press, MD, Virginia
Rikin Kadakia, MD, Virginia
Bryant Self, DO, Virginia
Sarah Sabo, ACNP, Virginia
Pedro A. Gonzales Alvarez, MD, Virginia
William Best, Virginia
Pushpanjali Basnyat, MD, Washington
Nikki Hartley-Jonason, Washington
Helen Johnsonwall, MD, Washington
Eric LaMotte, MD, Washington
Maher Muraywid, Washington
Evan Neal Paul, MD, Washington
Sarah Rogers, MD, Washington
Lindee Strizich, Washington
Maryam Tariq, MBBS, Washington
Meghaan Walsh, MD, Washington
Oleg Zbirun, MD, Washington
Meeta Sabnis, MD, Washington
James Kuo, MD, Washington
Liang Du, Washington
Syed Farhan Tabraiz Hashmi, MD, Washington
Jessica Jung, MD, Washington
Joshua Pelley, MD, Washington
Alex Yu, MD, Washington
Alfred Curnow, MD, Washington
Duhwan Kang, Washington
Gilbert Daniel, MD, Washington, D.C.
Eleanor Fitall, Washington, D.C.
Vinay Srinivasan, Washington, D.C.
Scott Wine, West Virginia
Trevor Miller, MBA, PA-C, West Virginia
Audrey Hiltunen, Wisconsin
Elina Litinskaya, Wisconsin
John M. Murphy, MD, Wisconsin
Tanya Pedretti, PA, Wisconsin
Adine Rodemeyer, MD, Wisconsin
Oghomwen Sule, MBBS, Wisconsin
Terrence Witt, MD, Wisconsin
Mayank Arora, Wisconsin
John D. MacDonald, MD, Wisconsin
Abigail Cook, Wisconsin
Mohamed Ibrahim, MD, Wisconsin
Aymen Khogali, MD, Wisconsin
Nicholas Haun, Wisconsin
Sandra Brown, Victoria, Australia
Alessandra Gessner, Alberta, Canada
Courtney Carlucci, British Columbia, Canada
Muhanad Y. Al Habash, Canada
Karen Tong, MD, Canada
Taku Yabuki, Japan
Liza van Loon, the Netherlands
Edward Gebuis, MD, the Netherlands
Abdisalan Afrah, MD, Qatar
Akhnuwkh Jones, Qatar
Mashuk Uddin, MBBS, MRCP, FRCP, Qatar
Ibrahim Yusuf Abubeker, MRCP, Qatar
Chih-Wei Tseng, Taiwan
Sawsan Abdel-Razig, MD, FACP, United Arab Emirates
The Society of Hospital Medicine welcomes its newest members:
Kwie-Hoa Siem, MD, Alaska
Frank Abene, Alabama
Kayla Maldonado, Alabama
Kenny Murray, MD, Alabama
Shanthan Ramidi, MD, Alabama
Lauren Hancock, APRN, Arkansas
William Hawkins, MD, Arkansas
Matthew Law, Arkansas
Emily Smith, MD, Arkansas
Firas Abbas, MBchB, Arizona
Shahid Ahmad, MD, MBBS, Arizona
Praveen Bheemanathini, Arizona
Atoosa Hosseini, Arizona
William McGrade, DO, Arizona
Konstantin Mazursky, DO, Arizona
Ibrahim Taweel, MD, Arizona
Kevin Virk, MD, FACP, Arizona
Kevin Virk, MD, FACP, Arizona
Mohemmedd Khalid Abbas, Arizona
Hasan Chaudhry, MD, Arizona
Kelly Kelleher, FAAP, Arizona
Priyanka Sultania Dudani, MBBS, Arizona
Krishna Kasireddy, MD, Arizona
Melanie Meguro, Arizona
Puneet Tuli, MD, Arizona
Jonathan Byrdy, DO, Arizona
Sarah Corral, DO, Arizona
Edward Maharam, MD, Arizona
Arvind Satyanarayan, DO, Arizona
Mayank Aggarwal, MD, Arizona
Syed Jafri, Arizona
Bujji Ainapurapu, MD, Arizona
Aaron Fernandes, MD, Arizona
Sonal Gandhi, Arizona
Sudhir Tutiki, Arizona
Navaneeth Kumar, MD, Arizona
Brian T. Courtney, MD, California
Won Jin Jeon, California
Veena Panduranga, MD, California
Jennifer Tinloy, DO, California
Debra Buckland Coffey, MCUSN, MD, California
Kathleen Teves, MD, California
Paul Goebel, MD, ACMPE, California
Shainy Hegde, California
Summaiya Muhammad, California
Desmond Wah, California
Chonn Khristin Ng, California
Almira Yang, DO, California
Salimah Boghani, MD, California
Stella Abhyankar, California
Cherie Ginwalla, MD, California
Armond Esmaili, California
Sarah Schaeffer, MD, MPH, California
Sophia Virani, MD, California
Dipti Munshi, MD, California
Judy Nguyen, DO, California
Daniel Owyang, DO, California
Christian Chiavetta, DO, California
David Reinert, DO, California
Joseph Pawlowski, MD, California
Eleanor Yang, California
Adrian Campo, MD, California
Emerson De Jesus, MD, California
Zachary Edmonds, MD, California
Trit Garg, California
Alexandra G. Ianculescu, MD, PhD, California
Felix Karp, MD, California
Cara Lai, California
Kristen Lew, MD, California
John Mogannam, California
Ameer Moussa, California
Neil Parikh, MD, MBA, California
Priya Reddy, California
Adam Simons, California
Sanjay Vadgama, MD, California
Kristofer Wills, DO, California
Michael Yang, MD, MS, California
Victor Ekuta, California,
Donna Colobong, PA-C, Colorado
Janna B. Dreason, FNP-C, Colorado
Cheryl English, NP-C, Colorado
Melanie Gerrior, MD, Colorado
Marciann Harris, NP, Colorado
Marsha Henke, MD, Colorado
Brett Hesse, Colorado
Naomi J Hipp, MD, Colorado
Aurell Horing, Colorado
Rachel Koch, DO, Colorado
Ed Marino, PA-C, Colorado
Marcus Reinhardt, MD, Colorado
Carol Runge, Colorado
Harshal Shah, Colorado
Leo Soehnlen, DO, Colorado
Anna Villalobos, MD, Colorado
Kathryn Whitfield, PA-C, Colorado
Jonathan Bei-Shing Young, MD, Colorado
Leah Damiani, MD, Colorado
Kathy Lynch, MD, Colorado
Micah Friedman, Colorado
Rachael Hilton, MD, Colorado
Madeline Koerner, Colorado
Chi Zheng, MD, Colorado
Chin-Kun Baw, MD, Connecticut
Alexandra Hawkins, NP, Connecticut
Vasundhara Singh, MD, MBBS, Connecticut
Ryan Quarles, MD, Connecticut
Debra Hernandez, APRN, BC, Connecticut
Karine Karapetyan, MD, Delaware
Choosak Burr, ARNP, Florida
Nelsi Mora, Florida
Mary Quillinan, Florida
Thuntanat Rachanakul, Florida
Samual W. Sauer, MD, MPH, Florida
Jennifer Tibangin, Florida
Keith Williams, MD, Florida
Eric Penedo, MD, Florida
Margaret Webb, Florida
Mark Bender, Florida
Brett Waress, MD, MHA, Florida
Giselle Racho, Florida
Bryan Thiel, Florida
Juan Loor Tuarez, MD, Florida
Christine Stopyra, Florida
Betsy Screws, ARNP, Florida
Jaimie Weber, MD, Florida
Priti Amin, MHA, Georgia
Naga Doddapaneni, Georgia
Stephanie Fletcher, Georgia
Disha Spath, MD, Georgia
Rafaela Wesley, DO, Georgia
Nikky Keer, DO, Georgia
James Kim, Georgia
Todd Martin, Georgia
Eli Mlaver, Georgia
Andrew Ritter, Georgia
Ali Al-Zubaidi, MBchB, Georgia
Deann Bing, MD, Georgia
Tushar Shah, Georgia
Cameron Straughn, DO, Georgia
Nobuhiro Ariyoshi, MEd, Hawaii
Prerna Kumar, Iowa
Jonathan Sebolt, MD, Iowa
Amy Tesar, DO, Iowa
Houng Chea, NP, Idaho
Finnegan Greer, PA-C, Idaho
Thao Nelson, PA, Idaho
Malatesha Gangappa, Idaho
Gloria Alumona, ACNP, Illinois
Ram Sanjeev Alur, Illinois
James Antoon, MD, FAAP, PhD, Illinois
Stefania Bailuc, MD, Illinois
Richard Huh, Illinois
Bhakti Patel, MD, Illinois
Frances Uy, ACNP, Illinois
Fernando Velazquez Vazquez, MD, Illinois
Tiffany White, MD, Illinois
Bryan P. Tully, MD, Illinois
Swati Gobhil, MBBS, Illinois
Lianghe Gao, Illinois
Gopi Astik, MD, Illinois
Marina Kovacevic, MD, Illinois
Abbie Raymond, DO, Illinois
Timothy Yung, Illinois
Ahmed Zahid, MD, Illinois
Cristina Corsini, MEd, Illinois
Faisal Rashid, MD, FACP, Illinois
Mansoor Ahmad, MD, Illinois
Matthew A. Strauch, DO, Illinois
Purshotham Reddy Grinne, Illinois
Nadia Nasreen, MD, Illinois
Maham Ashraf, MD, Indiana
Jennifer Gross, Indiana
Debasmita Mohapatra, MBBS, Indiana
Eric Scheper, Indiana
Katherine Gray, APRNBC, FNP, Indiana
Venkata Kureti, Indiana
Omer Al-Buoshkor, MD, Indiana
David Johnson, FNP, MSN, Indiana
Jonathan Salisbury, MD, Indiana
Debra Shapert, MSN, RN, Iowa
Lisa Carter, ARNP, Iowa
Matthew Woodham, Iowa
Tomoharu Suzuki, MD, Pharm, Japan
Khaldoun Haj, Kansas
Will Rogers, ACMPE, MA, MBA, Kansas
Karen Shumate, Kansas
Lisa Unruh, MD, Kansas
Matthew George, Kansas
Katie Washburn, DO, Kansas
Edwin Avallone, DO, Kentucky
Matthew Morris, Kentucky
Samantha Cappetto, MD, Kentucky
Jaison John, Kentucky
Ammar Al Jajeh, Kentucky
Joseph Bolger, MD, PhD, Louisiana
Clairissa Mulloy, Louisiana
Harish Talla, MD, Louisiana
John Amadon, Louisiana
Karthik Krishnareddy, Louisiana
Cheryl DeGrandpre, PA-C, Maine
Katherine Liu, MD, Maine
Sarah Sedney, MD, Maine
Aksana Afanasenka, MD, Maryland
Syed Nazeer Mahmood, MBBS, Maryland
Joseph Apata, MD, Maryland
Russom Ghebrai, MD, Maryland
Musa Momoh, MD, Maryland
Antanina Voit, Maryland
Dejene Kassaye, MD, MSC, Maryland
Shams Quazi, MD, FACP, MS, Maryland
Dawn Roelofs, FNP, MSN, Maryland
Kirsten Austad, MD, Massachusetts
Yoel Carrasquillo Vega, MD, Massachusetts
Michele Gaudet, NP, Massachusetts
Karina Mejias, Massachusetts
Peter Rohloff, MD, PhD, Massachusetts
Jennifer Schaeffer, Massachusetts
James Shaw, MD, Massachusetts
Renee Wheeler, Massachusetts
Angela Freeman, PA, PA-C, Massachusetts
Supriya Parvatini, MD, Massachusetts
Karen Jiang, MD, Massachusetts
Roula E. Abou-Nader, MD, Massachusetts
Shreekant Vasudhev, MD, Massachusetts
Nivedita Adabala, MD, MBBS, Michigan
Robert Behrendt, RN, BSN, Michigan
Molly Belisle, Michigan
Christine Dugan, MD, Michigan
Baljinder Gill, Michigan
Kellie Herringa, PA-C, Michigan
Christine Klingert, Michigan
Kathy Mitchell, Michigan
Aimee Vos, Michigan
Alyssa Churchill, DO, Michigan
Mailvaganam Sridharan, MD, Michigan
Atul Kapoor, MD, MBBS, Michigan
Anitha Kompally, MD, MBBS, Michigan
Nicole Webb, PA-C, Michigan
Abdulqadir Ahmad, MD, Minnesota
John Patrick Eikens, Minnesota
Bobbi Jo Jensen, PA-C, Minnesota
Rachel Keuseman, Minnesota
Stephen Palmquist, Minnesota
Manit Singla, MD, Minnesota
Douglas Berg, Minnesota
Nathan Palmolea, Minnesota
Molly Tureson, PAC, Minnesota
Mehdi Dastrange, MD, MHA, Minnesota
Kent Svee, Minnesota
Ashley Viere, PA-C, Minnesota
Molly Yang, MD, Minnesota
Paige Sams, DO, Minnesota
Amit Reddy, MBBS, Mississippi
Jacqueline Brooke Banks, FNP-C, Mississippi
Lori Foxworth, CFNP, Mississippi
Nicki Lawson, FNP-C, Mississippi
Bikash Acharya, Missouri
Zafar Ahmad, PA-C, Missouri
Harleen Chela, MD, Missouri
Jeffrey Chung, MD, Missouri
Daniel Kornfeld, Missouri
Erika Leung, MD, MSc, Missouri
Lisa Moser, PA, Missouri
Mark Stiffler, Missouri
Tushar Tarun, MBBS, Missouri
Nicole McLaughlin, Missouri
Katy Lohmann, PA-C, Missouri
Jayasree Bodagala, MD, Missouri
Ravi Kiran Morumuru, ACMPE, Missouri
Matthew Brown, MD, FAAFP, Missouri
Ravikanth Tadi, Missouri
Bazgha Ahmad, DO, Missouri
Monica Hawkins, RN, Missouri
Karri Vesey, BSN, Montana
Madison Vertin, PA-C, Montana
Urmila Mukherjee, MD, Nebraska
Noah Wiedel, MD, Nebraska
Sidrah Sheikh, MD, MBBS, Nebraska
Mohammad Esmadi, MBBS, Nebraska
Jill Zabih, MD, Nebraska
Jody Frey-Burns, RN, Nevada
Adnan Akbar, MD, Nevada
Peter Gayed, MRCP, New Hampshire
Jonathan T. Huntington, MD, New Hampshire
Meghan Meehan, ACNP, New Hampshire
Saurabh Mehta, MD, New Jersey
Hanaa Benchekroun Belabbes, MD, MHA, New Jersey
Hwan Kim, MD, New Jersey
Mary Tobiasson, USA, New Jersey
Muhammad Khakwani, MD, New Jersey
Amita Maibam, MD, MPH, New Jersey
Kumar Rohit, MBBS, New Jersey
Crystal Benjamin, MD, New Jersey
Rafael Garabis, New Mexico
Sam MacBride, MD, New Mexico
Indra Peram, MD, New Mexico
Sarah Vertrees, DO, New Mexico
Aswani Kumar Alavala, MD, New Mexico
Christopher Anstine, New Mexico
Prathima Guruguri, MD, New Mexico
Diedre Hofinger, MD, FACP, New Mexico
Katharine Juarez, New Mexico
Amtul Mahavesh, MD, New Mexico
Francisco Marquez, New Mexico
Payal Sen, MD, New Mexico
Morgan Wong, DO, New Mexico
Kelly Berchou, New York
Ronald Cho, New York
Nishil Dalsania, New York
Carolyn Drake, MD, MPH, New York
Leanne Forman, New York
Valerie Gausman, New York
Laurie Jacobs, New York
Janice Jang, MD, New York
Sonia Kohli, MD, New York
Nancy Lee, PA, New York
Allen Lee, MD, New York
Matthew McCarthy, FACP, New York
Akram Mohammed, MD, New York
Jennifer Nead, New York
Kristal Persaud, PA, New York
Mariya Rozenblit, MD, New York
Christian Torres, MD, New York
Sasha De Jesus, MD, New York
Gabriella Polyak, New York
Nataliya Yuklyaeva, MD, New York
Riyaz Kamadoli, MD, New York
Ramanuj Chakravarty, New York
Adil Zaidi, MD, New York
Allison Walker, MD, New York
Himali Gandhi, New York
Alexey Yanilshtein, MD, New York
Ramsey Al-Khalil, New York
Latoya Codougan, MD, New York
Khan Najmi, MD, New York
Sara Stream, MD, New York
Bhuwan Poudyal, MD, New York
Khalil Anchouche, New York
Sarah Azarchi, New York
Susana Bejar, New York
Brian Chang, New York
Jonathan Chen, New York
Hailey Gupta, MD, New York
Medhavi Gupta, New York
Ali Khan, New York
Benjamin Kwok, MD, New York
Billy Lin, New York
Katherine Ni, New York
Jina Park, New York
Gabriel Perreault, New York
Luis Alberto Romero, New York
Payal Shah, New York
Punita Shroff, New York
Scott Statman, New York
Maria Sunseri, New York
Benjamin Verplanke, New York
Audrey Zhang, New York
Gaby Razzouk, MD, New York
Pranitha Mantrala, MD, New York
Marsha Antoine, New York
Kanica Yashi, New York
Navid Ahmed, New York
Tasha Richards, PA, New York
Connor Tryon, MD, New York
Naveen Yarlagadda, MD, New York
Alex Hogan, New York
Andrew Donohoe, CCM, MD, North Carolina
Brittany Forshay, MD, North Carolina
Kelly Hammerbeck, FNP, North Carolina
Jennifer Hausman, North Carolina
Babajide Obisesan, North Carolina
Kwadwo Ofori, MD, North Carolina
Eric Ofosu, MD, North Carolina
Kale Roth, North Carolina
Robert Soma, PA-C, North Carolina
Sommany Weber, North Carolina
Ronnie Jacobs, North Carolina
Muhammad Ghani, MD, MACP, MBBS, North Carolina
Madeline Treasure, North Carolina
Andrew McWilliams, MD, North Carolina
Karen Payne, ACNP, MPH, North Carolina
Rafal Poplawski, MD, North Carolina
James Seal, PA-C, North Carolina
Farheen Qureshi, DO, North Carolina
Basavatti Sowmya, MD, MBBS, North Carolina
Eshwar Lal, MD, North Carolina
Catherine Hathaway, MD, North Carolina
Sherif Naguib, FAAFP, North Carolina
Sara Skavroneck, North Carolina
Charles Ofosu, North Carolina
Alex Alburquerque, MD, Ohio
Isha Butler, DO, Ohio
Anne Carrol, MD, Ohio
Scott Childers, MD, Ohio
Philip Jonas, MD, Ohio
Ahmadreza Karimianpour, Ohio
Rahul Kumar, MD, Ohio
George Maidaa, MD, Ohio
Kevin McAninch, Ohio
Jill Mccourt, FNP, Ohio
Roxanne Oliver, Ohio
Farah Hussain, Ohio
Natasha Axton, PA-C, Ohio
Brooke Harris, ACNP, Ohio
Vidhya Murukesan, MD, Ohio
Sara Dong, Ohio
Christie Astor, FNP, Ohio
Sunita Mall, MD, Ohio
Sunita Mall, MD, Ohio
Fouzia Tariq, MD, Ohio
Kaveri Sivaruban, MD, Ohio
Eunice Quicho, Ohio
Smitha Achuthankutty, MD, Ohio
Harmanpreet Shinh, MD, Ohio
Maereg Tesfaye, Ohio
Kalyn Jolivette, MD, Ohio
Richelle Voth, PA-C, Oklahoma
Samuel J. Ratermann, MD, FAAFP, Oklahoma
Richelle Voth, PA-C, Oklahoma
Alden Forrester, MD, Oregon
Nicholas Brown, DO, Oregon
Ian Pennell-Walklin, MD, Oregon
Bruce Ramsey, Oregon
Kyle Brekke, DO, Oregon
Sarah Webber, MD, Oregon
Brian Beaudoin, MD, Pennsylvania
Glenn Bedell, MHSA, Pennsylvania
Cristina Green, AGACNP-DNP, Pennsylvania
Andrew Groff, Pennsylvania
Sulman Masood Hashmi, MBBS, Pennsylvania
Eric Kasprowicz, MD, MPH, Pennsylvania
Laura Leuenberger, Pennsylvania
James Liszewski, MD, Pennsylvania
Caitlyn Moss, Pennsylvania
Paul Seunghyun Nho, Pennsylvania
Rishan Patel, MD, Pennsylvania
Dilli R. Poudel, MBBS, Pennsylvania
Naveen Yellappa, MBBS, Pennsylvania
Usman Zulfiqar, Pennsylvania
Nina Jain, Pennsylvania
Bhumika Patel, DO, Pennsylvania
Jenna M. Diasio, PA-C, Pennsylvania
Malachi Courtney, MD, Pennsylvania
Sonia Arneja, MD, Pennsylvania
Ross Ellis, MD, Pennsylvania
Samreen Siddiqui, Pennsylvania
Jillian Zavodnick, Pennsylvania
Kinan Kassar, MD, Pennsylvania
Maritsa M. Scoulos-Hanson, Pennsylvania
Jennifer Taylor, PA-C, Pennsylvania
Steven Delaveris, DO, Pennsylvania
Danica Buzniak, DO, Rhode Island
Paul Browning, MD, South Carolina
Matt Coones, MD, South Carolina
Cedric Fisher, MD, South Carolina
Aloysius Jackson, MD, South Carolina
Katharine DuPont, MD, South Carolina
Michael Jenkins, MD, South Carolina
Jessica Hamilton, APRN, BC, FNP, South Carolina
Pamela Pyle, DO, South Carolina
Shakeel Ahmed, MBBS, MD, South Dakota
D. Bruce Eaton, MD, South Dakota
Drew Jorgensen, MD, South Dakota
Shelly Turbak, MSN, RN, South Dakota
Tamera Sturm, DO, South Dakota
Peggy Brooks, Tennessee
Joseph Garrido, MD, Tennessee
Lisa Grimes, FNP, Tennessee
Chennakesava Kummathi, MBBS, Tennessee
Victoria Okafor, Tennessee
Ashley Smith, Tennessee
Monisha Bhatia, Tennessee
Belinda Jenkins, APRN-BC, Tennessee
Kim Zahnke, MD, Tennessee
Robert Arias, Texas
Nicolas Batterton, MD, Texas
Scott DePaul, MD, Texas
Nancy Foster, Texas
Larry Hughes, Texas
Erin Koval, Texas
Femi Layiwola, MD, Texas
Krysta Lin, Texas
James J. Onorato, MD, PhD, Texas
Allison Stephenson, PA-C, Texas
Brandon Stormes, Texas
Rubin Simon, MD, Texas
Brian Anderson, DO, Texas
Hatim Chhatriwala, MD, Texas
Aziz Hammoud, Texas
Haru Yamamoto, MD, Texas
Lauren Schiegg, Texas
Victoria Grasso, DO, Texas
Victor Salcedo, MD, Texas
Rajiv Bhattarai, Texas
Iram Qureshi, DO, Texas
Lisa Hafemeister, FACHE, MHA, Texas
Helena Kurian, MD, Texas
Jessica Lin, Texas
Nathan Nowalk, MD, Texas
Keely Smith, MD, Texas
Jonathan Weiser, MD, Texas
Roland Prezas, DO, FAAFP, Texas
Allan Recto, AHIP, Texas
Regina Dimbo, Texas
Venkata Ghanta, Texas
Richmond Hunt, Texas
Vishal Patel, MD, Texas
Zain Sharif, MD, Texas
Rommel Del Rosario, MD, Texas
Khawer Khadimally, DO, Texas
Diogenes Valderrama, MD, Texas
Charles Ewoh, MD, Texas
Deepika Kilaru, Texas
Tilahun Belay, MD, Texas
Chandra S Reddy Navuluri, MD, Texas
Bradley Goad, DO, FACP, Virginia
Patrick Higdon, MD, Virginia
Gabriella Miller, MD, HMDC, Virginia
Miklos Szentirmai, MD, Virginia
Hyder Tamton, Virginia
Andra Mirescu, MD, Virginia
Olukayode Ojo, Virginia
Robert Szeles, MD, Virginia
Anya Cope, DO, Virginia
OsCiriah Press, MD, Virginia
Rikin Kadakia, MD, Virginia
Bryant Self, DO, Virginia
Sarah Sabo, ACNP, Virginia
Pedro A. Gonzales Alvarez, MD, Virginia
William Best, Virginia
Pushpanjali Basnyat, MD, Washington
Nikki Hartley-Jonason, Washington
Helen Johnsonwall, MD, Washington
Eric LaMotte, MD, Washington
Maher Muraywid, Washington
Evan Neal Paul, MD, Washington
Sarah Rogers, MD, Washington
Lindee Strizich, Washington
Maryam Tariq, MBBS, Washington
Meghaan Walsh, MD, Washington
Oleg Zbirun, MD, Washington
Meeta Sabnis, MD, Washington
James Kuo, MD, Washington
Liang Du, Washington
Syed Farhan Tabraiz Hashmi, MD, Washington
Jessica Jung, MD, Washington
Joshua Pelley, MD, Washington
Alex Yu, MD, Washington
Alfred Curnow, MD, Washington
Duhwan Kang, Washington
Gilbert Daniel, MD, Washington, D.C.
Eleanor Fitall, Washington, D.C.
Vinay Srinivasan, Washington, D.C.
Scott Wine, West Virginia
Trevor Miller, MBA, PA-C, West Virginia
Audrey Hiltunen, Wisconsin
Elina Litinskaya, Wisconsin
John M. Murphy, MD, Wisconsin
Tanya Pedretti, PA, Wisconsin
Adine Rodemeyer, MD, Wisconsin
Oghomwen Sule, MBBS, Wisconsin
Terrence Witt, MD, Wisconsin
Mayank Arora, Wisconsin
John D. MacDonald, MD, Wisconsin
Abigail Cook, Wisconsin
Mohamed Ibrahim, MD, Wisconsin
Aymen Khogali, MD, Wisconsin
Nicholas Haun, Wisconsin
Sandra Brown, Victoria, Australia
Alessandra Gessner, Alberta, Canada
Courtney Carlucci, British Columbia, Canada
Muhanad Y. Al Habash, Canada
Karen Tong, MD, Canada
Taku Yabuki, Japan
Liza van Loon, the Netherlands
Edward Gebuis, MD, the Netherlands
Abdisalan Afrah, MD, Qatar
Akhnuwkh Jones, Qatar
Mashuk Uddin, MBBS, MRCP, FRCP, Qatar
Ibrahim Yusuf Abubeker, MRCP, Qatar
Chih-Wei Tseng, Taiwan
Sawsan Abdel-Razig, MD, FACP, United Arab Emirates
Oral clozapine, long-acting injectables tied to lower relapse risk in schizophrenia
Clozapine and long-acting injectable antipsychotic medications were most effective at preventing rehospitalization and treatment failure among patients with schizophrenia, according to a large prospective cohort study reported online June 7.
“The risk of rehospitalization is about 20% to 30% lower during long-acting injectable treatments, compared with equivalent oral formulations,” wrote Jari Tiihonen, MD, PhD, of the department of clinical neuroscience at the Karolinska Institutet in Stockholm, together with his associates.
Selection bias is a major problem in clinical trials of antipsychotics because up to 90% of patients are excluded for suicidal or antisocial behavior, substance abuse, refusal to participate, or comorbidities, the researchers noted. Observational studies also suffer from selection bias because of residual confounding. To address this issue, the researchers analyzed linked databases of 29,823 adults in Sweden with schizophrenia by using within-individual Cox proportional hazards regression analysis, in which each patient serves as his or her own control. Patients were diagnosed by 2006 and followed through 2013 (JAMA Psychiatry. 2017 June 7. doi: 10.1001/jamapsychiatry.2017.1322). A total of 13,042 patients (44%) were rehospitalized during follow-up. Rehospitalization was significantly less likely when patients were receiving oral clozapine or long-acting injectable antipsychotics, including paliperidone, zuclopenthixol, perphenazine, and olanzapine, than when they were not on antipsychotics. Hazard ratios for these comparisons were statistically significant, ranging from 0.51 to 0.58, the researchers reported.
In the overall cohort, patients were about 22% less likely to be rehospitalized when they received long-acting injectable antipsychotics instead of equivalent oral formulations (HR, 0.78; 95% confidence interval, 0.72-0.84; P less than .001). For newly diagnosed patients, this protective effect reached 32% (HR, 0.68; 95% CI, 0.53-0.86). The overall rate of treatment failure was 72%, but this outcome was significantly less likely when patients received clozapine (HR, 0.58; 95% CI, 0.53-0.63) or any of the long-acting injectable antipsychotics (HR, 0.65-0.80) instead of the most widely used medication, oral olanzapine. Those trends held up during several sensitivity analyses, the researchers noted.
The major reason long-acting injectables led to better outcomes probably is tied to adherence, “and the rationale for developing long-acting injectable formulations was to improve adherence,” Dr. Tilhonen and his associates wrote.
Dr. Tiihonen and his associates cited several limitations. For example, their results comparing the effectiveness of antipsychotics “can be generalized only to white populations and high-income countries in which all antipsychotic treatments are reimbursed by the state,” they wrote.
Nevertheless, they concluded that their results suggest that substantial differences exist “between specific antipsychotic agents and between routes of administration concerning the risk of rehospitalization and treatment failure among patients with schizophrenia.”
Janssen-Cilag funded the study. Dr. Tiihonen disclosed ties to Janssen-Cilag and several other pharmaceutical companies.
Clozapine and long-acting injectable antipsychotic medications were most effective at preventing rehospitalization and treatment failure among patients with schizophrenia, according to a large prospective cohort study reported online June 7.
“The risk of rehospitalization is about 20% to 30% lower during long-acting injectable treatments, compared with equivalent oral formulations,” wrote Jari Tiihonen, MD, PhD, of the department of clinical neuroscience at the Karolinska Institutet in Stockholm, together with his associates.
Selection bias is a major problem in clinical trials of antipsychotics because up to 90% of patients are excluded for suicidal or antisocial behavior, substance abuse, refusal to participate, or comorbidities, the researchers noted. Observational studies also suffer from selection bias because of residual confounding. To address this issue, the researchers analyzed linked databases of 29,823 adults in Sweden with schizophrenia by using within-individual Cox proportional hazards regression analysis, in which each patient serves as his or her own control. Patients were diagnosed by 2006 and followed through 2013 (JAMA Psychiatry. 2017 June 7. doi: 10.1001/jamapsychiatry.2017.1322). A total of 13,042 patients (44%) were rehospitalized during follow-up. Rehospitalization was significantly less likely when patients were receiving oral clozapine or long-acting injectable antipsychotics, including paliperidone, zuclopenthixol, perphenazine, and olanzapine, than when they were not on antipsychotics. Hazard ratios for these comparisons were statistically significant, ranging from 0.51 to 0.58, the researchers reported.
In the overall cohort, patients were about 22% less likely to be rehospitalized when they received long-acting injectable antipsychotics instead of equivalent oral formulations (HR, 0.78; 95% confidence interval, 0.72-0.84; P less than .001). For newly diagnosed patients, this protective effect reached 32% (HR, 0.68; 95% CI, 0.53-0.86). The overall rate of treatment failure was 72%, but this outcome was significantly less likely when patients received clozapine (HR, 0.58; 95% CI, 0.53-0.63) or any of the long-acting injectable antipsychotics (HR, 0.65-0.80) instead of the most widely used medication, oral olanzapine. Those trends held up during several sensitivity analyses, the researchers noted.
The major reason long-acting injectables led to better outcomes probably is tied to adherence, “and the rationale for developing long-acting injectable formulations was to improve adherence,” Dr. Tilhonen and his associates wrote.
Dr. Tiihonen and his associates cited several limitations. For example, their results comparing the effectiveness of antipsychotics “can be generalized only to white populations and high-income countries in which all antipsychotic treatments are reimbursed by the state,” they wrote.
Nevertheless, they concluded that their results suggest that substantial differences exist “between specific antipsychotic agents and between routes of administration concerning the risk of rehospitalization and treatment failure among patients with schizophrenia.”
Janssen-Cilag funded the study. Dr. Tiihonen disclosed ties to Janssen-Cilag and several other pharmaceutical companies.
Clozapine and long-acting injectable antipsychotic medications were most effective at preventing rehospitalization and treatment failure among patients with schizophrenia, according to a large prospective cohort study reported online June 7.
“The risk of rehospitalization is about 20% to 30% lower during long-acting injectable treatments, compared with equivalent oral formulations,” wrote Jari Tiihonen, MD, PhD, of the department of clinical neuroscience at the Karolinska Institutet in Stockholm, together with his associates.
Selection bias is a major problem in clinical trials of antipsychotics because up to 90% of patients are excluded for suicidal or antisocial behavior, substance abuse, refusal to participate, or comorbidities, the researchers noted. Observational studies also suffer from selection bias because of residual confounding. To address this issue, the researchers analyzed linked databases of 29,823 adults in Sweden with schizophrenia by using within-individual Cox proportional hazards regression analysis, in which each patient serves as his or her own control. Patients were diagnosed by 2006 and followed through 2013 (JAMA Psychiatry. 2017 June 7. doi: 10.1001/jamapsychiatry.2017.1322). A total of 13,042 patients (44%) were rehospitalized during follow-up. Rehospitalization was significantly less likely when patients were receiving oral clozapine or long-acting injectable antipsychotics, including paliperidone, zuclopenthixol, perphenazine, and olanzapine, than when they were not on antipsychotics. Hazard ratios for these comparisons were statistically significant, ranging from 0.51 to 0.58, the researchers reported.
In the overall cohort, patients were about 22% less likely to be rehospitalized when they received long-acting injectable antipsychotics instead of equivalent oral formulations (HR, 0.78; 95% confidence interval, 0.72-0.84; P less than .001). For newly diagnosed patients, this protective effect reached 32% (HR, 0.68; 95% CI, 0.53-0.86). The overall rate of treatment failure was 72%, but this outcome was significantly less likely when patients received clozapine (HR, 0.58; 95% CI, 0.53-0.63) or any of the long-acting injectable antipsychotics (HR, 0.65-0.80) instead of the most widely used medication, oral olanzapine. Those trends held up during several sensitivity analyses, the researchers noted.
The major reason long-acting injectables led to better outcomes probably is tied to adherence, “and the rationale for developing long-acting injectable formulations was to improve adherence,” Dr. Tilhonen and his associates wrote.
Dr. Tiihonen and his associates cited several limitations. For example, their results comparing the effectiveness of antipsychotics “can be generalized only to white populations and high-income countries in which all antipsychotic treatments are reimbursed by the state,” they wrote.
Nevertheless, they concluded that their results suggest that substantial differences exist “between specific antipsychotic agents and between routes of administration concerning the risk of rehospitalization and treatment failure among patients with schizophrenia.”
Janssen-Cilag funded the study. Dr. Tiihonen disclosed ties to Janssen-Cilag and several other pharmaceutical companies.
FROM JAMA PSYCHIATRY
Key clinical point: Clozapine and long-acting injectable antipsychotic medications were associated with the lowest rates of relapse in patients with schizophrenia.
Major finding: Patients were about 22% less likely to be rehospitalized when receiving long-acting injectable antipsychotics instead of equivalent oral formulations (HR, 0.78; 95% CI, 0.72-0.84).
Data source: A prospective observational study of 29,823 adults diagnosed with schizophrenia living in Sweden who served as their own controls.
Disclosures: Janssen-Cilag funded the study. Dr. Tiihonen disclosed ties to Janssen-Cilag and several other pharmaceutical companies.
Late-to-bed adolescents may be early-to-diabetes
BOSTON – Late chronotype may have a deleterious impact on glucose homeostasis independent of obesity in preadolescents aged 10-13 years, according to a small study.
Late chronotype – or an individual’s preference for later sleep and food intake – is associated with higher body mass index (BMI), risk incidence of type 2 diabetes mellitus, hemoglobin A1c, and risk of hypertension in adults. It is also associated with higher BMI, lower dietary restraint, and lower HDL cholesterol in adolescents. This study was the first to focus on chronotype and glycemic control in children on the cusp of or in early puberty.
In participants tested during the summer months, the actigraphy-derived mid-sleep time on free days – an objective measure of chronotype – was associated positively with fasting plasma glucose (P = 0.048). “This is an important point because it highlights the objective measure of chronotype, whereas questionnaires can be more subjective,” said Magdalena Dumin, MD, at the annual meeting of Associated Professional Sleep Societies.
The researcher also noted that higher sleep fragmentation and lower sleep efficiency were both associated with hyperglycemia and hyperinsulinemia, independent of obesity.
“Our preliminary findings suggest that having a late chronotype and less efficient and more fragmented sleep in pre- and early adolescence may have a deleterious glycemic impact independent of obesity and pubertal stage,” she said.
“Advancing bedtimes may reduce glucose levels in preadolescents and adolescents,” Dr. Dumin surmised.
This study included 24 adolescents, 13 of whom were normal weight and 11 of whom were obese. They all underwent anthropometric measurements with fasting glucose, insulin, C-peptide, HbA1c, and lipid levels. Exclusion criteria included a clinical diagnosis of sleep, behavioral, or dietary problems. The obese subjects also underwent a 180-minute glucose tolerance test.
Chronotype was assessed via actigraphy over 1 week to provide mid-sleep time on free days, and secondarily through administration to chronotype questionnaires – the Children’s ChronoType Questionnaire and the Morningness-Eveningness Scale for Children.
Among the normal-weight participants, 31% were morning and 23% evening chronotype (the rest being neutral chronotype), and, in the obese subjects, 27% were morning and 36% were evening chronotype.
The obese subjects were more likely to be in puberty, and had higher systolic blood pressures, higher fasting plasma insulin and levels of insulin resistance, as measured by Homeostatic Model Assessment of Insulin Resistance. Hemoglobin A1c tended to be higher in the obese subjects (P = .06), but fasting plasma glucose did not differ significantly between the obese and normal-weight participants (P = .68).
“We chose this population due to the shift from morningness to eveningness that occurs during adolescence followed by a progressive return to an intermediate or early chronotype at the end of adolescence,” Dr. Dumin said.
Bedtime, wake time, and total sleep time were nearly identical between the two groups (7.84 hours and 7.62 hours; P = .49), although sleep efficiency was numerically lower in the obese subjects (87.0 vs. 89.8; P = .14), while sleep fragmentation was somewhat greater (20.16 vs. 17.38; P = .18).
Dr. Dumin suggested that a continuation of her research with more patients would be useful.
This study is supported by the Endocrine Fellows Foundation and the University of Chicago Institute of Translational Medicine. Dr. Dumin reported having no financial disclosures.
BOSTON – Late chronotype may have a deleterious impact on glucose homeostasis independent of obesity in preadolescents aged 10-13 years, according to a small study.
Late chronotype – or an individual’s preference for later sleep and food intake – is associated with higher body mass index (BMI), risk incidence of type 2 diabetes mellitus, hemoglobin A1c, and risk of hypertension in adults. It is also associated with higher BMI, lower dietary restraint, and lower HDL cholesterol in adolescents. This study was the first to focus on chronotype and glycemic control in children on the cusp of or in early puberty.
In participants tested during the summer months, the actigraphy-derived mid-sleep time on free days – an objective measure of chronotype – was associated positively with fasting plasma glucose (P = 0.048). “This is an important point because it highlights the objective measure of chronotype, whereas questionnaires can be more subjective,” said Magdalena Dumin, MD, at the annual meeting of Associated Professional Sleep Societies.
The researcher also noted that higher sleep fragmentation and lower sleep efficiency were both associated with hyperglycemia and hyperinsulinemia, independent of obesity.
“Our preliminary findings suggest that having a late chronotype and less efficient and more fragmented sleep in pre- and early adolescence may have a deleterious glycemic impact independent of obesity and pubertal stage,” she said.
“Advancing bedtimes may reduce glucose levels in preadolescents and adolescents,” Dr. Dumin surmised.
This study included 24 adolescents, 13 of whom were normal weight and 11 of whom were obese. They all underwent anthropometric measurements with fasting glucose, insulin, C-peptide, HbA1c, and lipid levels. Exclusion criteria included a clinical diagnosis of sleep, behavioral, or dietary problems. The obese subjects also underwent a 180-minute glucose tolerance test.
Chronotype was assessed via actigraphy over 1 week to provide mid-sleep time on free days, and secondarily through administration to chronotype questionnaires – the Children’s ChronoType Questionnaire and the Morningness-Eveningness Scale for Children.
Among the normal-weight participants, 31% were morning and 23% evening chronotype (the rest being neutral chronotype), and, in the obese subjects, 27% were morning and 36% were evening chronotype.
The obese subjects were more likely to be in puberty, and had higher systolic blood pressures, higher fasting plasma insulin and levels of insulin resistance, as measured by Homeostatic Model Assessment of Insulin Resistance. Hemoglobin A1c tended to be higher in the obese subjects (P = .06), but fasting plasma glucose did not differ significantly between the obese and normal-weight participants (P = .68).
“We chose this population due to the shift from morningness to eveningness that occurs during adolescence followed by a progressive return to an intermediate or early chronotype at the end of adolescence,” Dr. Dumin said.
Bedtime, wake time, and total sleep time were nearly identical between the two groups (7.84 hours and 7.62 hours; P = .49), although sleep efficiency was numerically lower in the obese subjects (87.0 vs. 89.8; P = .14), while sleep fragmentation was somewhat greater (20.16 vs. 17.38; P = .18).
Dr. Dumin suggested that a continuation of her research with more patients would be useful.
This study is supported by the Endocrine Fellows Foundation and the University of Chicago Institute of Translational Medicine. Dr. Dumin reported having no financial disclosures.
BOSTON – Late chronotype may have a deleterious impact on glucose homeostasis independent of obesity in preadolescents aged 10-13 years, according to a small study.
Late chronotype – or an individual’s preference for later sleep and food intake – is associated with higher body mass index (BMI), risk incidence of type 2 diabetes mellitus, hemoglobin A1c, and risk of hypertension in adults. It is also associated with higher BMI, lower dietary restraint, and lower HDL cholesterol in adolescents. This study was the first to focus on chronotype and glycemic control in children on the cusp of or in early puberty.
In participants tested during the summer months, the actigraphy-derived mid-sleep time on free days – an objective measure of chronotype – was associated positively with fasting plasma glucose (P = 0.048). “This is an important point because it highlights the objective measure of chronotype, whereas questionnaires can be more subjective,” said Magdalena Dumin, MD, at the annual meeting of Associated Professional Sleep Societies.
The researcher also noted that higher sleep fragmentation and lower sleep efficiency were both associated with hyperglycemia and hyperinsulinemia, independent of obesity.
“Our preliminary findings suggest that having a late chronotype and less efficient and more fragmented sleep in pre- and early adolescence may have a deleterious glycemic impact independent of obesity and pubertal stage,” she said.
“Advancing bedtimes may reduce glucose levels in preadolescents and adolescents,” Dr. Dumin surmised.
This study included 24 adolescents, 13 of whom were normal weight and 11 of whom were obese. They all underwent anthropometric measurements with fasting glucose, insulin, C-peptide, HbA1c, and lipid levels. Exclusion criteria included a clinical diagnosis of sleep, behavioral, or dietary problems. The obese subjects also underwent a 180-minute glucose tolerance test.
Chronotype was assessed via actigraphy over 1 week to provide mid-sleep time on free days, and secondarily through administration to chronotype questionnaires – the Children’s ChronoType Questionnaire and the Morningness-Eveningness Scale for Children.
Among the normal-weight participants, 31% were morning and 23% evening chronotype (the rest being neutral chronotype), and, in the obese subjects, 27% were morning and 36% were evening chronotype.
The obese subjects were more likely to be in puberty, and had higher systolic blood pressures, higher fasting plasma insulin and levels of insulin resistance, as measured by Homeostatic Model Assessment of Insulin Resistance. Hemoglobin A1c tended to be higher in the obese subjects (P = .06), but fasting plasma glucose did not differ significantly between the obese and normal-weight participants (P = .68).
“We chose this population due to the shift from morningness to eveningness that occurs during adolescence followed by a progressive return to an intermediate or early chronotype at the end of adolescence,” Dr. Dumin said.
Bedtime, wake time, and total sleep time were nearly identical between the two groups (7.84 hours and 7.62 hours; P = .49), although sleep efficiency was numerically lower in the obese subjects (87.0 vs. 89.8; P = .14), while sleep fragmentation was somewhat greater (20.16 vs. 17.38; P = .18).
Dr. Dumin suggested that a continuation of her research with more patients would be useful.
This study is supported by the Endocrine Fellows Foundation and the University of Chicago Institute of Translational Medicine. Dr. Dumin reported having no financial disclosures.
AT SLEEP 2017
Key clinical point: Preadolescents with a late chronotype show impaired glucose homeostasis independent of obesity.
Major finding: In participants tested during the summer months, the actigraphy-derived mid-sleep time on free days was positively associated with fasting plasma glucose (P = .048).
Data source: Observational study including 24 adolescents between 10 and 13 years of age. Thirteen were normal weight and 11 were obese.
Disclosures: Dr. Dumin reported having no financial disclosures. This study is supported by the Endocrine Fellows Foundation and the University of Chicago Institute of Translational Medicine.
VIDEO: Shorter oxaliplatin-based therapy suffices for some patients with stage III CRC
CHICAGO – When it comes to oxaliplatin-based therapy for stage III colon cancer patients with low recurrence risk, 3 is preferable to 6 months and may also be preferable in those with higher recurrence risk – particularly if they are receiving oxaliplatin with capecitabine (CAPOX), according to findings from a prospective pooled analysis of data from six phase III trials.
The findings have immediate practice-changing implications, according to Axel Grothey, MD, of the Mayo Clinic Cancer Center, Rochester, Minn.
The preplanned analysis of the concurrently conducted trials (the International Duration Evaluation of Adjuvant chemotherapy [IDEA] collaboration), which included 12,834 patients receiving either oxaliplatin with fluorouracil and folinic acid (FOLFOX) or CAPOX, showed that, at a median follow-up of 39 months, the overall 3-year disease-free survival (DFS) rates differed by only 0.9% between those receiving 3 vs. 6 months of therapy (DFS, 74.6% and 75.5%, respectively). The difference between the groups was not statistically significant (estimated disease-free hazard ratio, 1.07), Dr. Grothey reported during a press briefing at the annual meeting of the American Society of Clinical Oncology.
However, grade 2 or greater neurotoxicity was greatly reduced, occurring in 17% vs. 48% of FOLFOX patients treated for 3 vs. 6 months, respectively, and in 15% vs. 45% of CAPOX patients treated for 3 vs. 6 months, respectively.
When outcomes were analyzed based on risk of recurrence, low-risk patients (those with T1-3 N1 disease) had 3-year disease-free survival rates of 83.1% and 83.3% with 3 vs. 6 months of therapy, and high-risk patients (T4 or N2 disease) had 3-year disease-free survival of 62.7% vs. 64.4% with 3 vs. 6 months of therapy.
When outcomes were analyzed by regimen, the FOLFOX patients (about 60% of the patient population) had 3-year DFS of 73.6% and 76.0% with 3 vs. 6 months of therapy, and the CAPOX patients had 3-year DFS of 75.9% and 74.8%, respectively.
In this video interview, Dr. Grothey discusses the findings, as well as the importance of federal funding for cancer research, which is underscored by the findings.
He also discusses the IDEA Collaboration consensus based on the results, which calls for 3 months of therapy in low-risk patients and for an individualized approach based on tolerability of therapy, patient preference, assessment of recurrence risk, and treatment regimen (CAPOX vs. FOLFOX) in higher-risk patients.
“We now have very important, very solid data to engage patients in discussion [about] how to individualize the duration of therapy,” he said.
Dr. Grothey reported having no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – When it comes to oxaliplatin-based therapy for stage III colon cancer patients with low recurrence risk, 3 is preferable to 6 months and may also be preferable in those with higher recurrence risk – particularly if they are receiving oxaliplatin with capecitabine (CAPOX), according to findings from a prospective pooled analysis of data from six phase III trials.
The findings have immediate practice-changing implications, according to Axel Grothey, MD, of the Mayo Clinic Cancer Center, Rochester, Minn.
The preplanned analysis of the concurrently conducted trials (the International Duration Evaluation of Adjuvant chemotherapy [IDEA] collaboration), which included 12,834 patients receiving either oxaliplatin with fluorouracil and folinic acid (FOLFOX) or CAPOX, showed that, at a median follow-up of 39 months, the overall 3-year disease-free survival (DFS) rates differed by only 0.9% between those receiving 3 vs. 6 months of therapy (DFS, 74.6% and 75.5%, respectively). The difference between the groups was not statistically significant (estimated disease-free hazard ratio, 1.07), Dr. Grothey reported during a press briefing at the annual meeting of the American Society of Clinical Oncology.
However, grade 2 or greater neurotoxicity was greatly reduced, occurring in 17% vs. 48% of FOLFOX patients treated for 3 vs. 6 months, respectively, and in 15% vs. 45% of CAPOX patients treated for 3 vs. 6 months, respectively.
When outcomes were analyzed based on risk of recurrence, low-risk patients (those with T1-3 N1 disease) had 3-year disease-free survival rates of 83.1% and 83.3% with 3 vs. 6 months of therapy, and high-risk patients (T4 or N2 disease) had 3-year disease-free survival of 62.7% vs. 64.4% with 3 vs. 6 months of therapy.
When outcomes were analyzed by regimen, the FOLFOX patients (about 60% of the patient population) had 3-year DFS of 73.6% and 76.0% with 3 vs. 6 months of therapy, and the CAPOX patients had 3-year DFS of 75.9% and 74.8%, respectively.
In this video interview, Dr. Grothey discusses the findings, as well as the importance of federal funding for cancer research, which is underscored by the findings.
He also discusses the IDEA Collaboration consensus based on the results, which calls for 3 months of therapy in low-risk patients and for an individualized approach based on tolerability of therapy, patient preference, assessment of recurrence risk, and treatment regimen (CAPOX vs. FOLFOX) in higher-risk patients.
“We now have very important, very solid data to engage patients in discussion [about] how to individualize the duration of therapy,” he said.
Dr. Grothey reported having no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – When it comes to oxaliplatin-based therapy for stage III colon cancer patients with low recurrence risk, 3 is preferable to 6 months and may also be preferable in those with higher recurrence risk – particularly if they are receiving oxaliplatin with capecitabine (CAPOX), according to findings from a prospective pooled analysis of data from six phase III trials.
The findings have immediate practice-changing implications, according to Axel Grothey, MD, of the Mayo Clinic Cancer Center, Rochester, Minn.
The preplanned analysis of the concurrently conducted trials (the International Duration Evaluation of Adjuvant chemotherapy [IDEA] collaboration), which included 12,834 patients receiving either oxaliplatin with fluorouracil and folinic acid (FOLFOX) or CAPOX, showed that, at a median follow-up of 39 months, the overall 3-year disease-free survival (DFS) rates differed by only 0.9% between those receiving 3 vs. 6 months of therapy (DFS, 74.6% and 75.5%, respectively). The difference between the groups was not statistically significant (estimated disease-free hazard ratio, 1.07), Dr. Grothey reported during a press briefing at the annual meeting of the American Society of Clinical Oncology.
However, grade 2 or greater neurotoxicity was greatly reduced, occurring in 17% vs. 48% of FOLFOX patients treated for 3 vs. 6 months, respectively, and in 15% vs. 45% of CAPOX patients treated for 3 vs. 6 months, respectively.
When outcomes were analyzed based on risk of recurrence, low-risk patients (those with T1-3 N1 disease) had 3-year disease-free survival rates of 83.1% and 83.3% with 3 vs. 6 months of therapy, and high-risk patients (T4 or N2 disease) had 3-year disease-free survival of 62.7% vs. 64.4% with 3 vs. 6 months of therapy.
When outcomes were analyzed by regimen, the FOLFOX patients (about 60% of the patient population) had 3-year DFS of 73.6% and 76.0% with 3 vs. 6 months of therapy, and the CAPOX patients had 3-year DFS of 75.9% and 74.8%, respectively.
In this video interview, Dr. Grothey discusses the findings, as well as the importance of federal funding for cancer research, which is underscored by the findings.
He also discusses the IDEA Collaboration consensus based on the results, which calls for 3 months of therapy in low-risk patients and for an individualized approach based on tolerability of therapy, patient preference, assessment of recurrence risk, and treatment regimen (CAPOX vs. FOLFOX) in higher-risk patients.
“We now have very important, very solid data to engage patients in discussion [about] how to individualize the duration of therapy,” he said.
Dr. Grothey reported having no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASCO 2017
VIDEO: Pertuzumab prolongs disease-free survival in HER2+ early breast cancer
CHICAGO –
At a median follow-up of 45.4 months, invasive disease-free survival (IDFS) events occurred in 171 of 2,400 patients (7.1%) who received pertuzumab, compared with 210 of 2,405 patients (8.7%) who received placebo (hazard ratio, 0.81). This 19% reduction in risk of an IDFS event was statistically significant, Gunter von Minckwitz, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology.
The estimated IDFS rate at 3 years was 94.1% in the pertuzumab arm, and 93.2% in the placebo arm, said Dr. von Minckwitz of the German Breast Group, Neu-Isenburg.
Study subjects were patients with adequately excised HER2-positive, pT1-3 early breast cancer. Patients were randomized to receive adjuvant chemotherapy plus 1 year of either trastuzumab plus pertuzumab, or trastuzumab plus placebo.
In a video interview, Dr. von Minckwitz discusses the study results, including outcomes in node-positive vs. node-negative patients, early overall survival findings, and safety.
“We are using pertuzumab right now in many countries for the neoadjuvant setting,” he said, explaining that existing approvals were granted conditionally in the absence of evidence regarding long-term benefits. “With the APHINITY study ... use of pertuzumab either in the neoadjuvant setting or in the higher-risk adjuvant setting is something that is supported now with evidence.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO –
At a median follow-up of 45.4 months, invasive disease-free survival (IDFS) events occurred in 171 of 2,400 patients (7.1%) who received pertuzumab, compared with 210 of 2,405 patients (8.7%) who received placebo (hazard ratio, 0.81). This 19% reduction in risk of an IDFS event was statistically significant, Gunter von Minckwitz, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology.
The estimated IDFS rate at 3 years was 94.1% in the pertuzumab arm, and 93.2% in the placebo arm, said Dr. von Minckwitz of the German Breast Group, Neu-Isenburg.
Study subjects were patients with adequately excised HER2-positive, pT1-3 early breast cancer. Patients were randomized to receive adjuvant chemotherapy plus 1 year of either trastuzumab plus pertuzumab, or trastuzumab plus placebo.
In a video interview, Dr. von Minckwitz discusses the study results, including outcomes in node-positive vs. node-negative patients, early overall survival findings, and safety.
“We are using pertuzumab right now in many countries for the neoadjuvant setting,” he said, explaining that existing approvals were granted conditionally in the absence of evidence regarding long-term benefits. “With the APHINITY study ... use of pertuzumab either in the neoadjuvant setting or in the higher-risk adjuvant setting is something that is supported now with evidence.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO –
At a median follow-up of 45.4 months, invasive disease-free survival (IDFS) events occurred in 171 of 2,400 patients (7.1%) who received pertuzumab, compared with 210 of 2,405 patients (8.7%) who received placebo (hazard ratio, 0.81). This 19% reduction in risk of an IDFS event was statistically significant, Gunter von Minckwitz, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology.
The estimated IDFS rate at 3 years was 94.1% in the pertuzumab arm, and 93.2% in the placebo arm, said Dr. von Minckwitz of the German Breast Group, Neu-Isenburg.
Study subjects were patients with adequately excised HER2-positive, pT1-3 early breast cancer. Patients were randomized to receive adjuvant chemotherapy plus 1 year of either trastuzumab plus pertuzumab, or trastuzumab plus placebo.
In a video interview, Dr. von Minckwitz discusses the study results, including outcomes in node-positive vs. node-negative patients, early overall survival findings, and safety.
“We are using pertuzumab right now in many countries for the neoadjuvant setting,” he said, explaining that existing approvals were granted conditionally in the absence of evidence regarding long-term benefits. “With the APHINITY study ... use of pertuzumab either in the neoadjuvant setting or in the higher-risk adjuvant setting is something that is supported now with evidence.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASCO 2017
HIV study: Vaginal bacteria rapidly metabolized topical tenofovir
Certain species of vaginal bacteria rapidly metabolized topical tenofovir, which was three times less effective at preventing HIV in women with predominantly these vaginal microbiota, according to results of a study published in Science.
Key culprits were Gardnerella vaginalis and other anaerobic species associated with bacterial vaginosis, reported Nichole R. Klatt, PhD, of the University of Washington, Seattle, and her associates. The findings could help explain the lower and widely variable efficacy rates of topical microbicides in clinical HIV prevention trials, they added.
Lactobacillus was the primary genus in 423 women (62%), while non-Lactobacillus microbiota predominated in the remaining 256 women. Compared with placebo, tenofovir decreased the incidence of HIV infections by 61% (95% confidence interval, 0.16-0.89; P = .01) in women whose vaginal microbiota consisted mainly of Lactobacillus species, but by only 18% (95% CI, 0.37-1.77; P = .6) when nonlactobacilli predominated. These findings did not change after the investigators controlled for sexually transmitted infections, use of antibiotics, use of depot medroxyprogesterone acetate (DMPA), and sexual behaviors (including frequency of sex, number of partners, and condom use). Furthermore, the two groups were clinically, behaviorally, and demographically similar and had similar rates of adherence to tenofovir gel during the trial.
The non-Lactobacillus group had more bacterial diversity and several distinct subgroups, the largest of which was dominated by Gardnerella vaginalis and other bacteria associated with bacterial vaginosis. This subgroup had lower mucosal levels of tenofovir, compared with women with primarily lactobacilli, the investigators reported. Tenofovir concentrations fell by 51% in the in vivo cultures of G. vaginalis but dropped only slightly in cultures of two common Lactobacillus species (P less than .004 for each comparison). After 24 hours, tenofovir concentrations had decreased by 67% in cultures with G. vaginalis but by only 9%-14% in cultures with lactobacilli.
The researchers also tested three other subtypes of G. vaginalis, all of which metabolized tenofovir. The findings revealed a biologic mechanism that is probably part of “a multifactorial process, including increased vaginal inflammation and adherence, leading to varying levels of HIV prevention efficacy observed across topical microbicide trials,” they concluded. Screening by testing vaginal pH or microbiota might help “guide and enhance prevention of HIV in women,” they wrote.
Funders included the Canadian Institutes of Health Research, the University of Washington, the Public Health Agency of Canada, and the U.S. Agency for International Development. The investigators had no disclosures.
Certain species of vaginal bacteria rapidly metabolized topical tenofovir, which was three times less effective at preventing HIV in women with predominantly these vaginal microbiota, according to results of a study published in Science.
Key culprits were Gardnerella vaginalis and other anaerobic species associated with bacterial vaginosis, reported Nichole R. Klatt, PhD, of the University of Washington, Seattle, and her associates. The findings could help explain the lower and widely variable efficacy rates of topical microbicides in clinical HIV prevention trials, they added.
Lactobacillus was the primary genus in 423 women (62%), while non-Lactobacillus microbiota predominated in the remaining 256 women. Compared with placebo, tenofovir decreased the incidence of HIV infections by 61% (95% confidence interval, 0.16-0.89; P = .01) in women whose vaginal microbiota consisted mainly of Lactobacillus species, but by only 18% (95% CI, 0.37-1.77; P = .6) when nonlactobacilli predominated. These findings did not change after the investigators controlled for sexually transmitted infections, use of antibiotics, use of depot medroxyprogesterone acetate (DMPA), and sexual behaviors (including frequency of sex, number of partners, and condom use). Furthermore, the two groups were clinically, behaviorally, and demographically similar and had similar rates of adherence to tenofovir gel during the trial.
The non-Lactobacillus group had more bacterial diversity and several distinct subgroups, the largest of which was dominated by Gardnerella vaginalis and other bacteria associated with bacterial vaginosis. This subgroup had lower mucosal levels of tenofovir, compared with women with primarily lactobacilli, the investigators reported. Tenofovir concentrations fell by 51% in the in vivo cultures of G. vaginalis but dropped only slightly in cultures of two common Lactobacillus species (P less than .004 for each comparison). After 24 hours, tenofovir concentrations had decreased by 67% in cultures with G. vaginalis but by only 9%-14% in cultures with lactobacilli.
The researchers also tested three other subtypes of G. vaginalis, all of which metabolized tenofovir. The findings revealed a biologic mechanism that is probably part of “a multifactorial process, including increased vaginal inflammation and adherence, leading to varying levels of HIV prevention efficacy observed across topical microbicide trials,” they concluded. Screening by testing vaginal pH or microbiota might help “guide and enhance prevention of HIV in women,” they wrote.
Funders included the Canadian Institutes of Health Research, the University of Washington, the Public Health Agency of Canada, and the U.S. Agency for International Development. The investigators had no disclosures.
Certain species of vaginal bacteria rapidly metabolized topical tenofovir, which was three times less effective at preventing HIV in women with predominantly these vaginal microbiota, according to results of a study published in Science.
Key culprits were Gardnerella vaginalis and other anaerobic species associated with bacterial vaginosis, reported Nichole R. Klatt, PhD, of the University of Washington, Seattle, and her associates. The findings could help explain the lower and widely variable efficacy rates of topical microbicides in clinical HIV prevention trials, they added.
Lactobacillus was the primary genus in 423 women (62%), while non-Lactobacillus microbiota predominated in the remaining 256 women. Compared with placebo, tenofovir decreased the incidence of HIV infections by 61% (95% confidence interval, 0.16-0.89; P = .01) in women whose vaginal microbiota consisted mainly of Lactobacillus species, but by only 18% (95% CI, 0.37-1.77; P = .6) when nonlactobacilli predominated. These findings did not change after the investigators controlled for sexually transmitted infections, use of antibiotics, use of depot medroxyprogesterone acetate (DMPA), and sexual behaviors (including frequency of sex, number of partners, and condom use). Furthermore, the two groups were clinically, behaviorally, and demographically similar and had similar rates of adherence to tenofovir gel during the trial.
The non-Lactobacillus group had more bacterial diversity and several distinct subgroups, the largest of which was dominated by Gardnerella vaginalis and other bacteria associated with bacterial vaginosis. This subgroup had lower mucosal levels of tenofovir, compared with women with primarily lactobacilli, the investigators reported. Tenofovir concentrations fell by 51% in the in vivo cultures of G. vaginalis but dropped only slightly in cultures of two common Lactobacillus species (P less than .004 for each comparison). After 24 hours, tenofovir concentrations had decreased by 67% in cultures with G. vaginalis but by only 9%-14% in cultures with lactobacilli.
The researchers also tested three other subtypes of G. vaginalis, all of which metabolized tenofovir. The findings revealed a biologic mechanism that is probably part of “a multifactorial process, including increased vaginal inflammation and adherence, leading to varying levels of HIV prevention efficacy observed across topical microbicide trials,” they concluded. Screening by testing vaginal pH or microbiota might help “guide and enhance prevention of HIV in women,” they wrote.
Funders included the Canadian Institutes of Health Research, the University of Washington, the Public Health Agency of Canada, and the U.S. Agency for International Development. The investigators had no disclosures.
FROM SCIENCE
Key clinical point: Certain species of vaginal bacteria rapidly metabolized topical tenofovir.
Major finding: After 24 hours in culture, tenofovir concentrations had decreased by 67% in cultures with Gardnerella vaginalis but by only 9%-14% in culture with lactobacilli.
Data source: Mass spectrometry–based proteomics of vaginal specimens from 668 HIV-negative women.
Disclosures: Funders included the Canadian Institutes of Health Research, the University of Washington, the Public Health Agency of Canada, and the U.S. Agency for International Development. The investigators had no disclosures.
CDC specifies risk-reduction strategies for HIV-discordant conception
When HIV-discordant couples wish to conceive, several strategies can effectively prevent men from infecting their female partners, according to an analysis in Morbidity and Mortality Weekly Report.
Using sperm from an HIV-negative donor remains the safest choice, but current methods of sperm washing make in vitro fertilization (IVF) or intrauterine insemination (IUI) a low-risk alternative, especially when used together with highly active antiretroviral therapy (HAART) and preexposure prophylaxis (PrEP), wrote Jennifer F. Kawwass, MD, and her associates in the division of reproductive health at the Centers for Disease Control and Prevention, Atlanta (MMWR Morb Mortal Wkly Rep. 2017;66:554-7).
Previously, the CDC recommended against insemination with semen from HIV-infected men. The new report reverses that position, citing epidemiologic and laboratory evidence that conception can occur safely when HIV-discordant couples use combined strategies including HAART, PrEP, and either condomless intercourse limited to the time around ovulation, or sperm washing prior to IVF or IUI.
Sperm washing has been around for about 2 decades, but current techniques appear to significantly cut the risk of HIV transmission, based on epidemiologic data. Among 11,500 IVF or IUI cycles in which uninfected females used washed sperm from their HIV-infected male partners, there have been no cases of HIV transmission to women or their children, the CDC authors emphasized. The use of HAART and PrEP can add an extra layer of protection, they added.
Serodiscordant couples may also consider or prefer natural conception. An HIV-infected male on HAART whose plasma viral load is undetectable can expect to infect his female partner only about 0.16 times for every 10,000 exposures during condomless intercourse, the report noted. Limiting condomless intercourse to the time of ovulation and using PrEP by the uninfected female can further minimize the risk of sexual transmission, the authors added. Experienced medical providers can help HIV-discordant couples further evaluate the “unique risk profile” of each approach to conception, they concluded.
Dr. Kawwass and her associates had no conflicts of interest.
When HIV-discordant couples wish to conceive, several strategies can effectively prevent men from infecting their female partners, according to an analysis in Morbidity and Mortality Weekly Report.
Using sperm from an HIV-negative donor remains the safest choice, but current methods of sperm washing make in vitro fertilization (IVF) or intrauterine insemination (IUI) a low-risk alternative, especially when used together with highly active antiretroviral therapy (HAART) and preexposure prophylaxis (PrEP), wrote Jennifer F. Kawwass, MD, and her associates in the division of reproductive health at the Centers for Disease Control and Prevention, Atlanta (MMWR Morb Mortal Wkly Rep. 2017;66:554-7).
Previously, the CDC recommended against insemination with semen from HIV-infected men. The new report reverses that position, citing epidemiologic and laboratory evidence that conception can occur safely when HIV-discordant couples use combined strategies including HAART, PrEP, and either condomless intercourse limited to the time around ovulation, or sperm washing prior to IVF or IUI.
Sperm washing has been around for about 2 decades, but current techniques appear to significantly cut the risk of HIV transmission, based on epidemiologic data. Among 11,500 IVF or IUI cycles in which uninfected females used washed sperm from their HIV-infected male partners, there have been no cases of HIV transmission to women or their children, the CDC authors emphasized. The use of HAART and PrEP can add an extra layer of protection, they added.
Serodiscordant couples may also consider or prefer natural conception. An HIV-infected male on HAART whose plasma viral load is undetectable can expect to infect his female partner only about 0.16 times for every 10,000 exposures during condomless intercourse, the report noted. Limiting condomless intercourse to the time of ovulation and using PrEP by the uninfected female can further minimize the risk of sexual transmission, the authors added. Experienced medical providers can help HIV-discordant couples further evaluate the “unique risk profile” of each approach to conception, they concluded.
Dr. Kawwass and her associates had no conflicts of interest.
When HIV-discordant couples wish to conceive, several strategies can effectively prevent men from infecting their female partners, according to an analysis in Morbidity and Mortality Weekly Report.
Using sperm from an HIV-negative donor remains the safest choice, but current methods of sperm washing make in vitro fertilization (IVF) or intrauterine insemination (IUI) a low-risk alternative, especially when used together with highly active antiretroviral therapy (HAART) and preexposure prophylaxis (PrEP), wrote Jennifer F. Kawwass, MD, and her associates in the division of reproductive health at the Centers for Disease Control and Prevention, Atlanta (MMWR Morb Mortal Wkly Rep. 2017;66:554-7).
Previously, the CDC recommended against insemination with semen from HIV-infected men. The new report reverses that position, citing epidemiologic and laboratory evidence that conception can occur safely when HIV-discordant couples use combined strategies including HAART, PrEP, and either condomless intercourse limited to the time around ovulation, or sperm washing prior to IVF or IUI.
Sperm washing has been around for about 2 decades, but current techniques appear to significantly cut the risk of HIV transmission, based on epidemiologic data. Among 11,500 IVF or IUI cycles in which uninfected females used washed sperm from their HIV-infected male partners, there have been no cases of HIV transmission to women or their children, the CDC authors emphasized. The use of HAART and PrEP can add an extra layer of protection, they added.
Serodiscordant couples may also consider or prefer natural conception. An HIV-infected male on HAART whose plasma viral load is undetectable can expect to infect his female partner only about 0.16 times for every 10,000 exposures during condomless intercourse, the report noted. Limiting condomless intercourse to the time of ovulation and using PrEP by the uninfected female can further minimize the risk of sexual transmission, the authors added. Experienced medical providers can help HIV-discordant couples further evaluate the “unique risk profile” of each approach to conception, they concluded.
Dr. Kawwass and her associates had no conflicts of interest.
FROM MMWR