User login
It matters how you phrase a child’s flu vaccine recommendation
Vaccine hesitant parents were more likely to change their minds about flu vaccine for their children when pediatricians or pediatric nurse practitioners used a presumptive recommendation that their children get the vaccine, pursued the recommendation if the parent was resistant, and combined their recommendation for the flu vaccine with other childhood vaccines, said Annika M. Hofstetter, MD, PhD, of the University of Washington, Seattle, and her associates.
The researchers recruited 17 pediatricians and pediatric nurse practitioners from eight primary care pediatric practices in the Seattle area to take part in 50 videotaped visits with parents during the 2011-2012 and 2013-2014 flu seasons.
(83% vs. 33%; P less than .01), Dr. Hofstetter and her colleagues said. The various communication patterns did not appear to negatively affect the way parents rated their visit experiences.
Read more at Vaccine. 2017;35:2709-15.
Vaccine hesitant parents were more likely to change their minds about flu vaccine for their children when pediatricians or pediatric nurse practitioners used a presumptive recommendation that their children get the vaccine, pursued the recommendation if the parent was resistant, and combined their recommendation for the flu vaccine with other childhood vaccines, said Annika M. Hofstetter, MD, PhD, of the University of Washington, Seattle, and her associates.
The researchers recruited 17 pediatricians and pediatric nurse practitioners from eight primary care pediatric practices in the Seattle area to take part in 50 videotaped visits with parents during the 2011-2012 and 2013-2014 flu seasons.
(83% vs. 33%; P less than .01), Dr. Hofstetter and her colleagues said. The various communication patterns did not appear to negatively affect the way parents rated their visit experiences.
Read more at Vaccine. 2017;35:2709-15.
Vaccine hesitant parents were more likely to change their minds about flu vaccine for their children when pediatricians or pediatric nurse practitioners used a presumptive recommendation that their children get the vaccine, pursued the recommendation if the parent was resistant, and combined their recommendation for the flu vaccine with other childhood vaccines, said Annika M. Hofstetter, MD, PhD, of the University of Washington, Seattle, and her associates.
The researchers recruited 17 pediatricians and pediatric nurse practitioners from eight primary care pediatric practices in the Seattle area to take part in 50 videotaped visits with parents during the 2011-2012 and 2013-2014 flu seasons.
(83% vs. 33%; P less than .01), Dr. Hofstetter and her colleagues said. The various communication patterns did not appear to negatively affect the way parents rated their visit experiences.
Read more at Vaccine. 2017;35:2709-15.
FROM VACCINE
Former Pharma reps’ new mission: To school docs on high drug costs
As a drug salesman, Mike Courtney worked hard to make health care expensive. He wined and dined doctors, golfed with them and bought lunch for their entire staffs – all to promote pills often costing thousands of dollars a year.
Now he’s on a different mission. When Mr. Courtney calls on doctors these days, he champions generic drugs that frequently cost pennies and work just as well as the kinds of pricey brands he used to push.
Instead of Big Pharma, he works for Capital District Physicians’ Health Plan (CDPHP), an Albany, N.Y., insurer. Instead of maximizing pill profits, his job is to save millions of dollars by educating doctors about expensive prescriptions and the stratagems used to sell them.
“Having come from Big Pharma, I do really feel my soul has been cleansed,” laughs Mr. Courtney, who formerly worked for Pfizer and Johnson & Johnson. “I do feel like I’m more in touch with the physicians” and plan members, he added.
Costs for prescription drugs have been rising faster than those for any other health segment, marked by high-profile cases such as the reported 400% increase for Mylan’s EpiPen and 5,000% spike for Turing Pharmaceuticals’ Daraprim.
Health plans and others paying those costs are fighting back. Many have tried to give doctors academic research on pill effectiveness or simply removed high-cost drugs from coverage lists.
Consumer groups and medical societies have tried to spread the word about expensive drugs. Startup GoodRx lets patients compare retail prices online.
CDPHP is one of the few insurers to have taken the battle against pricey pills a step further. It is recruiting across enemy lines, hiring former pharma representatives and staffing what may be a new job category: a sales force for cost-effective medicine.
“Insurers are taking matters into their own hands,” said Lea Prevel Katsanis, a marketing professor at Canada’s Concordia University who specializes in the pharmaceutical industry. “They’re saying, ‘We can’t really rely on drug companies to talk to doctors about what’s cost-efficient.’ ”
If insurance companies can curb drug costs, premiums paid by employers, taxpayers, and consumers need not rise as fast.
Two years ago, when one company increased the cost of a common diabetes medicine to 20 times what it had been a few years earlier, Mr. Courtney and five other former pharma and medical-device reps working for CDPHP knew what to do.
Valeant Pharmaceuticals had cranked up the price of one common dosage of its Glumetza medicine for lowering blood sugar to an astonishing $81,270 a year, according to Truven Health Analytics, a data firm. Meanwhile a similar, generic version can be bought for as little as a penny a pill.
Because Glumetza was on CDPHP’s list of approved drugs, the insurer and its members had to pay for it when doctors prescribed it, resulting in millions in extra costs and stinging copayments for patients.
Eric Schnakenberg, MD, an upstate New York family medicine doctor, was shocked when patients began complaining about what he assumed was an inexpensive prescription. Doctors are famously unaware about the cost of the care they order, a situation exploited by drug sellers and other vendors.
While physicians’ electronic prescribing programs and even pharmaceutical guides like the Physicians’ Desk Reference contain prescribing information – some are even peppered with ads – they contain no specific information about prices. Drug sales reps who visit their offices don’t highlight high prices as they drop off free samples, and drug makers can quietly, but substantially, hike the price of a drug from one year to the next.
“As physicians, we’re blindsided by that,” Dr. Schnakenberg said. “We get patient complaints saying, ‘Hey, I can’t afford this,’ and we say: ‘It’s cheap!’ ”
After Mr. Courtney and his colleagues alerted doctors to what Valeant was up to, all but a handful of the 60 plan members who were taking Glumetza switched to metformin, the generic alternative. That saved about $5 million in a year.
Following an outcry over its practices, Valeant agreed last year to raise annual prices by no more than single-digit percentages, the company said through a spokesman. But such hikes could still outpace the inflation rate.
Using ‘Those Powers For Good’
Cardiologist John Bennett got the idea to hire pharma reps a few years ago, after he became CDPHP’s chief executive. He knew reps are smart, genial, and motivated. Overhiring by pharma had put many back on the job market.
His sales pitch to them, he says half-jokingly, was: “You know everything they taught you in Big Pharma? How would you like to use those powers for good?”
Pharma companies spend billions on TV ads, doctor blandishments, and expensive salespeople to keep prescriptions flowing.
Pfizer, Johnson & Johnson, and other sellers responded to critics a few years ago by restricting gifts of entertainment, coffee mugs, and some meals. But the industry’s ethics code still allows lavish consulting contracts for doctors and sponsorship of physician conferences as well as meals for doctors and their staffs who listen to an “informational presentation” from sales reps touting expensive pills.
“When those products go generic, nobody’s promoting them anymore,” Mr. Courtney said. Generics makers lack big marketing budgets. CDPHP’s remedy: The insurer promotes generics with its own reps.
“It’s a great idea,” said Alan Sorensen, an economist at the University of Wisconsin who has studied drug prices. “Even a small moving of the needle on their [doctors’] prescribing behavior can have a pretty big impact on costs.”
At first the team concentrated on educating doctors about cheaper alternatives to Lipitor, a widely prescribed cholesterol-lowering medicine, and Nexium, for stomach problems. That saved around $10 million the first year, much in the form of copayments that would have been owed by plan members.
Recently the plan has focused on Seroquel, a branded antipsychotic that costs far more than a similar generic. Switching to the generic saves $600 to $1,000 a month, estimates Eileen Wood, the insurer’s vice president of pharmacy and health quality.
CDPHP’s repurposed reps have helped keep the insurer’s annual drug-cost increases to single-digit percentages, whereas without them and other measures “we would certainly be well into double-digit” increases, she said.
Educating doctors about drug costs is part of a larger push for “transparency” in an industry where Princeton economist Uwe Reinhardt says consumers face the same experience as somebody shopping in Macy’s blindfolded.
Current research by the University of Wisconsin’s Mr. Sorensen finds physicians with access to data about drug prices and insurance coverage are more likely to prescribe generics.
That gives Mr. Courtney and his colleagues a fighting chance, even if, he said, “we don’t have the freewheeling, unlimited green Amex card like I did back in the day.”
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
As a drug salesman, Mike Courtney worked hard to make health care expensive. He wined and dined doctors, golfed with them and bought lunch for their entire staffs – all to promote pills often costing thousands of dollars a year.
Now he’s on a different mission. When Mr. Courtney calls on doctors these days, he champions generic drugs that frequently cost pennies and work just as well as the kinds of pricey brands he used to push.
Instead of Big Pharma, he works for Capital District Physicians’ Health Plan (CDPHP), an Albany, N.Y., insurer. Instead of maximizing pill profits, his job is to save millions of dollars by educating doctors about expensive prescriptions and the stratagems used to sell them.
“Having come from Big Pharma, I do really feel my soul has been cleansed,” laughs Mr. Courtney, who formerly worked for Pfizer and Johnson & Johnson. “I do feel like I’m more in touch with the physicians” and plan members, he added.
Costs for prescription drugs have been rising faster than those for any other health segment, marked by high-profile cases such as the reported 400% increase for Mylan’s EpiPen and 5,000% spike for Turing Pharmaceuticals’ Daraprim.
Health plans and others paying those costs are fighting back. Many have tried to give doctors academic research on pill effectiveness or simply removed high-cost drugs from coverage lists.
Consumer groups and medical societies have tried to spread the word about expensive drugs. Startup GoodRx lets patients compare retail prices online.
CDPHP is one of the few insurers to have taken the battle against pricey pills a step further. It is recruiting across enemy lines, hiring former pharma representatives and staffing what may be a new job category: a sales force for cost-effective medicine.
“Insurers are taking matters into their own hands,” said Lea Prevel Katsanis, a marketing professor at Canada’s Concordia University who specializes in the pharmaceutical industry. “They’re saying, ‘We can’t really rely on drug companies to talk to doctors about what’s cost-efficient.’ ”
If insurance companies can curb drug costs, premiums paid by employers, taxpayers, and consumers need not rise as fast.
Two years ago, when one company increased the cost of a common diabetes medicine to 20 times what it had been a few years earlier, Mr. Courtney and five other former pharma and medical-device reps working for CDPHP knew what to do.
Valeant Pharmaceuticals had cranked up the price of one common dosage of its Glumetza medicine for lowering blood sugar to an astonishing $81,270 a year, according to Truven Health Analytics, a data firm. Meanwhile a similar, generic version can be bought for as little as a penny a pill.
Because Glumetza was on CDPHP’s list of approved drugs, the insurer and its members had to pay for it when doctors prescribed it, resulting in millions in extra costs and stinging copayments for patients.
Eric Schnakenberg, MD, an upstate New York family medicine doctor, was shocked when patients began complaining about what he assumed was an inexpensive prescription. Doctors are famously unaware about the cost of the care they order, a situation exploited by drug sellers and other vendors.
While physicians’ electronic prescribing programs and even pharmaceutical guides like the Physicians’ Desk Reference contain prescribing information – some are even peppered with ads – they contain no specific information about prices. Drug sales reps who visit their offices don’t highlight high prices as they drop off free samples, and drug makers can quietly, but substantially, hike the price of a drug from one year to the next.
“As physicians, we’re blindsided by that,” Dr. Schnakenberg said. “We get patient complaints saying, ‘Hey, I can’t afford this,’ and we say: ‘It’s cheap!’ ”
After Mr. Courtney and his colleagues alerted doctors to what Valeant was up to, all but a handful of the 60 plan members who were taking Glumetza switched to metformin, the generic alternative. That saved about $5 million in a year.
Following an outcry over its practices, Valeant agreed last year to raise annual prices by no more than single-digit percentages, the company said through a spokesman. But such hikes could still outpace the inflation rate.
Using ‘Those Powers For Good’
Cardiologist John Bennett got the idea to hire pharma reps a few years ago, after he became CDPHP’s chief executive. He knew reps are smart, genial, and motivated. Overhiring by pharma had put many back on the job market.
His sales pitch to them, he says half-jokingly, was: “You know everything they taught you in Big Pharma? How would you like to use those powers for good?”
Pharma companies spend billions on TV ads, doctor blandishments, and expensive salespeople to keep prescriptions flowing.
Pfizer, Johnson & Johnson, and other sellers responded to critics a few years ago by restricting gifts of entertainment, coffee mugs, and some meals. But the industry’s ethics code still allows lavish consulting contracts for doctors and sponsorship of physician conferences as well as meals for doctors and their staffs who listen to an “informational presentation” from sales reps touting expensive pills.
“When those products go generic, nobody’s promoting them anymore,” Mr. Courtney said. Generics makers lack big marketing budgets. CDPHP’s remedy: The insurer promotes generics with its own reps.
“It’s a great idea,” said Alan Sorensen, an economist at the University of Wisconsin who has studied drug prices. “Even a small moving of the needle on their [doctors’] prescribing behavior can have a pretty big impact on costs.”
At first the team concentrated on educating doctors about cheaper alternatives to Lipitor, a widely prescribed cholesterol-lowering medicine, and Nexium, for stomach problems. That saved around $10 million the first year, much in the form of copayments that would have been owed by plan members.
Recently the plan has focused on Seroquel, a branded antipsychotic that costs far more than a similar generic. Switching to the generic saves $600 to $1,000 a month, estimates Eileen Wood, the insurer’s vice president of pharmacy and health quality.
CDPHP’s repurposed reps have helped keep the insurer’s annual drug-cost increases to single-digit percentages, whereas without them and other measures “we would certainly be well into double-digit” increases, she said.
Educating doctors about drug costs is part of a larger push for “transparency” in an industry where Princeton economist Uwe Reinhardt says consumers face the same experience as somebody shopping in Macy’s blindfolded.
Current research by the University of Wisconsin’s Mr. Sorensen finds physicians with access to data about drug prices and insurance coverage are more likely to prescribe generics.
That gives Mr. Courtney and his colleagues a fighting chance, even if, he said, “we don’t have the freewheeling, unlimited green Amex card like I did back in the day.”
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
As a drug salesman, Mike Courtney worked hard to make health care expensive. He wined and dined doctors, golfed with them and bought lunch for their entire staffs – all to promote pills often costing thousands of dollars a year.
Now he’s on a different mission. When Mr. Courtney calls on doctors these days, he champions generic drugs that frequently cost pennies and work just as well as the kinds of pricey brands he used to push.
Instead of Big Pharma, he works for Capital District Physicians’ Health Plan (CDPHP), an Albany, N.Y., insurer. Instead of maximizing pill profits, his job is to save millions of dollars by educating doctors about expensive prescriptions and the stratagems used to sell them.
“Having come from Big Pharma, I do really feel my soul has been cleansed,” laughs Mr. Courtney, who formerly worked for Pfizer and Johnson & Johnson. “I do feel like I’m more in touch with the physicians” and plan members, he added.
Costs for prescription drugs have been rising faster than those for any other health segment, marked by high-profile cases such as the reported 400% increase for Mylan’s EpiPen and 5,000% spike for Turing Pharmaceuticals’ Daraprim.
Health plans and others paying those costs are fighting back. Many have tried to give doctors academic research on pill effectiveness or simply removed high-cost drugs from coverage lists.
Consumer groups and medical societies have tried to spread the word about expensive drugs. Startup GoodRx lets patients compare retail prices online.
CDPHP is one of the few insurers to have taken the battle against pricey pills a step further. It is recruiting across enemy lines, hiring former pharma representatives and staffing what may be a new job category: a sales force for cost-effective medicine.
“Insurers are taking matters into their own hands,” said Lea Prevel Katsanis, a marketing professor at Canada’s Concordia University who specializes in the pharmaceutical industry. “They’re saying, ‘We can’t really rely on drug companies to talk to doctors about what’s cost-efficient.’ ”
If insurance companies can curb drug costs, premiums paid by employers, taxpayers, and consumers need not rise as fast.
Two years ago, when one company increased the cost of a common diabetes medicine to 20 times what it had been a few years earlier, Mr. Courtney and five other former pharma and medical-device reps working for CDPHP knew what to do.
Valeant Pharmaceuticals had cranked up the price of one common dosage of its Glumetza medicine for lowering blood sugar to an astonishing $81,270 a year, according to Truven Health Analytics, a data firm. Meanwhile a similar, generic version can be bought for as little as a penny a pill.
Because Glumetza was on CDPHP’s list of approved drugs, the insurer and its members had to pay for it when doctors prescribed it, resulting in millions in extra costs and stinging copayments for patients.
Eric Schnakenberg, MD, an upstate New York family medicine doctor, was shocked when patients began complaining about what he assumed was an inexpensive prescription. Doctors are famously unaware about the cost of the care they order, a situation exploited by drug sellers and other vendors.
While physicians’ electronic prescribing programs and even pharmaceutical guides like the Physicians’ Desk Reference contain prescribing information – some are even peppered with ads – they contain no specific information about prices. Drug sales reps who visit their offices don’t highlight high prices as they drop off free samples, and drug makers can quietly, but substantially, hike the price of a drug from one year to the next.
“As physicians, we’re blindsided by that,” Dr. Schnakenberg said. “We get patient complaints saying, ‘Hey, I can’t afford this,’ and we say: ‘It’s cheap!’ ”
After Mr. Courtney and his colleagues alerted doctors to what Valeant was up to, all but a handful of the 60 plan members who were taking Glumetza switched to metformin, the generic alternative. That saved about $5 million in a year.
Following an outcry over its practices, Valeant agreed last year to raise annual prices by no more than single-digit percentages, the company said through a spokesman. But such hikes could still outpace the inflation rate.
Using ‘Those Powers For Good’
Cardiologist John Bennett got the idea to hire pharma reps a few years ago, after he became CDPHP’s chief executive. He knew reps are smart, genial, and motivated. Overhiring by pharma had put many back on the job market.
His sales pitch to them, he says half-jokingly, was: “You know everything they taught you in Big Pharma? How would you like to use those powers for good?”
Pharma companies spend billions on TV ads, doctor blandishments, and expensive salespeople to keep prescriptions flowing.
Pfizer, Johnson & Johnson, and other sellers responded to critics a few years ago by restricting gifts of entertainment, coffee mugs, and some meals. But the industry’s ethics code still allows lavish consulting contracts for doctors and sponsorship of physician conferences as well as meals for doctors and their staffs who listen to an “informational presentation” from sales reps touting expensive pills.
“When those products go generic, nobody’s promoting them anymore,” Mr. Courtney said. Generics makers lack big marketing budgets. CDPHP’s remedy: The insurer promotes generics with its own reps.
“It’s a great idea,” said Alan Sorensen, an economist at the University of Wisconsin who has studied drug prices. “Even a small moving of the needle on their [doctors’] prescribing behavior can have a pretty big impact on costs.”
At first the team concentrated on educating doctors about cheaper alternatives to Lipitor, a widely prescribed cholesterol-lowering medicine, and Nexium, for stomach problems. That saved around $10 million the first year, much in the form of copayments that would have been owed by plan members.
Recently the plan has focused on Seroquel, a branded antipsychotic that costs far more than a similar generic. Switching to the generic saves $600 to $1,000 a month, estimates Eileen Wood, the insurer’s vice president of pharmacy and health quality.
CDPHP’s repurposed reps have helped keep the insurer’s annual drug-cost increases to single-digit percentages, whereas without them and other measures “we would certainly be well into double-digit” increases, she said.
Educating doctors about drug costs is part of a larger push for “transparency” in an industry where Princeton economist Uwe Reinhardt says consumers face the same experience as somebody shopping in Macy’s blindfolded.
Current research by the University of Wisconsin’s Mr. Sorensen finds physicians with access to data about drug prices and insurance coverage are more likely to prescribe generics.
That gives Mr. Courtney and his colleagues a fighting chance, even if, he said, “we don’t have the freewheeling, unlimited green Amex card like I did back in the day.”
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Medicare Advantage enrollment up again in 2017
Enrollment in Medicare Advantage plans increased for the 13th consecutive year in 2017 and now represents one-third of all Medicare beneficiaries, according to the Kaiser Family Foundation.
Currently, 19 million beneficiaries are enrolled in Medicare Advantage, more than triple the number who were enrolled when the program hit its low point of 5.3 million (13% of all beneficiaries) in 2003 and 2004 and 71% higher since the Affordable Care Act was passed in 2010, Kaiser said in a recent report.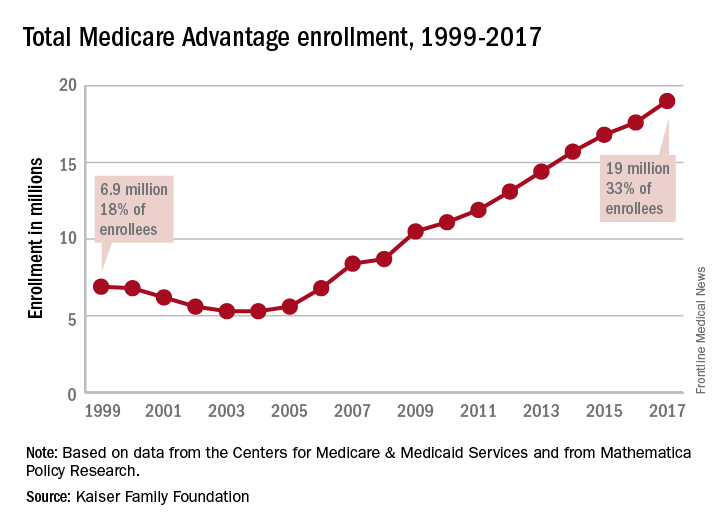
As growth continues, however, so does concentration among the private firms administering the plans. In 2017, UnitedHealthcare and Humana together account for 41% of enrollment in Medicare Advantage, “and, in 17 states, one company has more than half of all Medicare Advantage enrollment – an indicator that these markets may not be very competitive,” they noted.
Enrollment in Medicare Advantage plans increased for the 13th consecutive year in 2017 and now represents one-third of all Medicare beneficiaries, according to the Kaiser Family Foundation.
Currently, 19 million beneficiaries are enrolled in Medicare Advantage, more than triple the number who were enrolled when the program hit its low point of 5.3 million (13% of all beneficiaries) in 2003 and 2004 and 71% higher since the Affordable Care Act was passed in 2010, Kaiser said in a recent report.
As growth continues, however, so does concentration among the private firms administering the plans. In 2017, UnitedHealthcare and Humana together account for 41% of enrollment in Medicare Advantage, “and, in 17 states, one company has more than half of all Medicare Advantage enrollment – an indicator that these markets may not be very competitive,” they noted.
Enrollment in Medicare Advantage plans increased for the 13th consecutive year in 2017 and now represents one-third of all Medicare beneficiaries, according to the Kaiser Family Foundation.
Currently, 19 million beneficiaries are enrolled in Medicare Advantage, more than triple the number who were enrolled when the program hit its low point of 5.3 million (13% of all beneficiaries) in 2003 and 2004 and 71% higher since the Affordable Care Act was passed in 2010, Kaiser said in a recent report.
As growth continues, however, so does concentration among the private firms administering the plans. In 2017, UnitedHealthcare and Humana together account for 41% of enrollment in Medicare Advantage, “and, in 17 states, one company has more than half of all Medicare Advantage enrollment – an indicator that these markets may not be very competitive,” they noted.
Low payment for pulmonary rehab explained
A new review of 2015 Medicare data clearly points fingers at hospitals for the historically low payment rates for pulmonary rehabilitation.
To fully understand these data, everyone involved in the delivery of pulmonary rehabilitation services needs to know some of the specifics regarding Medicare’s rate setting process for hospital outpatient services. Those services are paid on the basis of a prospective payment methodology, similar to the DRG system for inpatient services. Under the outpatient system, APCs (ambulatory payment classifications) are computed with two key data sources, both provided by hospitals.
The second key component used by CMS for rate setting is the hospital cost report, submitted annually to CMS tied to the individual hospital’s fiscal year. This flow of data to CMS is ongoing because of differing fiscal years and is somewhat attributable to changes in Medicare proposed rates for the following year, published in July, compared with final rates, published in early November.
The other key historical fact that needs emphasis is what happened in 2010 when CMS began reimbursing for pulmonary rehab under new HCPCS code G0424. Clearly, there were no charge data to examine, so the Agency had to do a bit of guesswork, estimating what would be a reasonable payment. CMS turned to payment information tied to codes G0237 and G0238, codes that had been used by many institutions for the previous decade for billing pulmonary rehab. But one critical difference existed. The new code, G0424, was a 1-hour code, while G0237-38 were 15-minute codes. Over the next 2 years, even CMS cited the failure of hospitals to adjust their charges to reflect all the component services included in this new, bundled 1-hour code, compared with the unbundled 15-minute code.
The new review of CMS data bears out this problem. With approximately 1,350 institutions billing for hospital outpatient pulmonary rehab via code G0424, there is incredibly wide variance in charge data. The range is from a high of $1,981 to a low of $44, with 1,350 institutions in-between. The average charge was $247, but the difference between the lowest charge and the highest charge is approximately 44-fold.
For cost report data, the spread is from $1,265 to $4 (yes, $4, based on data provided to CMS). Approximately 750 hospitals, more than half, submit data to CMS reflecting costs associated with the delivery of pulmonary rehab, per hour, at $50 or less.
There are probably several reasons why hospitals behave this way. First, there is the historical phenomenon cited by CMS that it often takes years for hospitals to adjust charges appropriately when any new HCPCS code is adopted by CMS. And, in fact, CMS cited pulmonary rehab as a glaring example of that failure by hospitals. Second, there is the cost report data, and we believe it, too, falls victim to hospital neglect. We can understand that a service such as pulmonary rehab falls so far below the radar by chargemasters, hospital administrators and others associated with information submitted to CMS that little attention is paid to accuracy of charges or administrative costs culled from the hospital cost report. And then, there is the matter of community relations. The hospitals at the very high end of the spectrum in terms of charges ($1,100 and up) are unlikely to build good community relations if they let people know of those charges. Ironically, it is fair to presume that hospitals do pay very close attention to their charges and cost report data for very high-end hospital outpatient services, micro-examining that information to ensure desirable payment rates.
So, the critical challenge to the pulmonary community is to focus on those two very specific bits of data submitted by hospitals to CMS: what a hospital identifies as the “charge” for code G0424 and is then entered on every claim submitted to G0424; and second, information correlated to the administrative aspects of pulmonary rehab that hospitals submit to CMS annually in their cost report to CMS. Until those adjustments are made, pulmonary rehab will live with unacceptable payment rates.
A new review of 2015 Medicare data clearly points fingers at hospitals for the historically low payment rates for pulmonary rehabilitation.
To fully understand these data, everyone involved in the delivery of pulmonary rehabilitation services needs to know some of the specifics regarding Medicare’s rate setting process for hospital outpatient services. Those services are paid on the basis of a prospective payment methodology, similar to the DRG system for inpatient services. Under the outpatient system, APCs (ambulatory payment classifications) are computed with two key data sources, both provided by hospitals.
The second key component used by CMS for rate setting is the hospital cost report, submitted annually to CMS tied to the individual hospital’s fiscal year. This flow of data to CMS is ongoing because of differing fiscal years and is somewhat attributable to changes in Medicare proposed rates for the following year, published in July, compared with final rates, published in early November.
The other key historical fact that needs emphasis is what happened in 2010 when CMS began reimbursing for pulmonary rehab under new HCPCS code G0424. Clearly, there were no charge data to examine, so the Agency had to do a bit of guesswork, estimating what would be a reasonable payment. CMS turned to payment information tied to codes G0237 and G0238, codes that had been used by many institutions for the previous decade for billing pulmonary rehab. But one critical difference existed. The new code, G0424, was a 1-hour code, while G0237-38 were 15-minute codes. Over the next 2 years, even CMS cited the failure of hospitals to adjust their charges to reflect all the component services included in this new, bundled 1-hour code, compared with the unbundled 15-minute code.
The new review of CMS data bears out this problem. With approximately 1,350 institutions billing for hospital outpatient pulmonary rehab via code G0424, there is incredibly wide variance in charge data. The range is from a high of $1,981 to a low of $44, with 1,350 institutions in-between. The average charge was $247, but the difference between the lowest charge and the highest charge is approximately 44-fold.
For cost report data, the spread is from $1,265 to $4 (yes, $4, based on data provided to CMS). Approximately 750 hospitals, more than half, submit data to CMS reflecting costs associated with the delivery of pulmonary rehab, per hour, at $50 or less.
There are probably several reasons why hospitals behave this way. First, there is the historical phenomenon cited by CMS that it often takes years for hospitals to adjust charges appropriately when any new HCPCS code is adopted by CMS. And, in fact, CMS cited pulmonary rehab as a glaring example of that failure by hospitals. Second, there is the cost report data, and we believe it, too, falls victim to hospital neglect. We can understand that a service such as pulmonary rehab falls so far below the radar by chargemasters, hospital administrators and others associated with information submitted to CMS that little attention is paid to accuracy of charges or administrative costs culled from the hospital cost report. And then, there is the matter of community relations. The hospitals at the very high end of the spectrum in terms of charges ($1,100 and up) are unlikely to build good community relations if they let people know of those charges. Ironically, it is fair to presume that hospitals do pay very close attention to their charges and cost report data for very high-end hospital outpatient services, micro-examining that information to ensure desirable payment rates.
So, the critical challenge to the pulmonary community is to focus on those two very specific bits of data submitted by hospitals to CMS: what a hospital identifies as the “charge” for code G0424 and is then entered on every claim submitted to G0424; and second, information correlated to the administrative aspects of pulmonary rehab that hospitals submit to CMS annually in their cost report to CMS. Until those adjustments are made, pulmonary rehab will live with unacceptable payment rates.
A new review of 2015 Medicare data clearly points fingers at hospitals for the historically low payment rates for pulmonary rehabilitation.
To fully understand these data, everyone involved in the delivery of pulmonary rehabilitation services needs to know some of the specifics regarding Medicare’s rate setting process for hospital outpatient services. Those services are paid on the basis of a prospective payment methodology, similar to the DRG system for inpatient services. Under the outpatient system, APCs (ambulatory payment classifications) are computed with two key data sources, both provided by hospitals.
The second key component used by CMS for rate setting is the hospital cost report, submitted annually to CMS tied to the individual hospital’s fiscal year. This flow of data to CMS is ongoing because of differing fiscal years and is somewhat attributable to changes in Medicare proposed rates for the following year, published in July, compared with final rates, published in early November.
The other key historical fact that needs emphasis is what happened in 2010 when CMS began reimbursing for pulmonary rehab under new HCPCS code G0424. Clearly, there were no charge data to examine, so the Agency had to do a bit of guesswork, estimating what would be a reasonable payment. CMS turned to payment information tied to codes G0237 and G0238, codes that had been used by many institutions for the previous decade for billing pulmonary rehab. But one critical difference existed. The new code, G0424, was a 1-hour code, while G0237-38 were 15-minute codes. Over the next 2 years, even CMS cited the failure of hospitals to adjust their charges to reflect all the component services included in this new, bundled 1-hour code, compared with the unbundled 15-minute code.
The new review of CMS data bears out this problem. With approximately 1,350 institutions billing for hospital outpatient pulmonary rehab via code G0424, there is incredibly wide variance in charge data. The range is from a high of $1,981 to a low of $44, with 1,350 institutions in-between. The average charge was $247, but the difference between the lowest charge and the highest charge is approximately 44-fold.
For cost report data, the spread is from $1,265 to $4 (yes, $4, based on data provided to CMS). Approximately 750 hospitals, more than half, submit data to CMS reflecting costs associated with the delivery of pulmonary rehab, per hour, at $50 or less.
There are probably several reasons why hospitals behave this way. First, there is the historical phenomenon cited by CMS that it often takes years for hospitals to adjust charges appropriately when any new HCPCS code is adopted by CMS. And, in fact, CMS cited pulmonary rehab as a glaring example of that failure by hospitals. Second, there is the cost report data, and we believe it, too, falls victim to hospital neglect. We can understand that a service such as pulmonary rehab falls so far below the radar by chargemasters, hospital administrators and others associated with information submitted to CMS that little attention is paid to accuracy of charges or administrative costs culled from the hospital cost report. And then, there is the matter of community relations. The hospitals at the very high end of the spectrum in terms of charges ($1,100 and up) are unlikely to build good community relations if they let people know of those charges. Ironically, it is fair to presume that hospitals do pay very close attention to their charges and cost report data for very high-end hospital outpatient services, micro-examining that information to ensure desirable payment rates.
So, the critical challenge to the pulmonary community is to focus on those two very specific bits of data submitted by hospitals to CMS: what a hospital identifies as the “charge” for code G0424 and is then entered on every claim submitted to G0424; and second, information correlated to the administrative aspects of pulmonary rehab that hospitals submit to CMS annually in their cost report to CMS. Until those adjustments are made, pulmonary rehab will live with unacceptable payment rates.
From the EVP/ CEO
It is an incredible honor to be recently confirmed as the EVP/CEO for the CHEST organization. As a 23-year veteran of CHEST, I have had the privilege of working with and for many of our leaders, volunteers, and members. Being only the fifth person to lead the organization in an executive leadership role is both humbling and invigorating. CHEST is a dynamic and innovative organization, with a mission to “champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.” That mission resonates deeply with me on a personal level, because my mother had COPD. Toward the end of her life, I saw firsthand how it impacted her quality of life and her ability to be a mother and grandmother, and I also saw the important role her pulmonologist and re
I am both fortunate and grateful to have such a phenomenal professional staff to work with here at CHEST and to have the outstanding leadership of our Presidents, Past Presidents, Boards, Committees, and NetWorks – all of which have been tremendously supportive during the past 9 months as I filled the Interim EVP role. I am also deeply grateful to those of you who choose to be members and Fellows of CHEST and to be engaged as volunteer leadership, faculty, content experts, authors, and more. It is your time, energy, involvement, and vision that make this organization what it is. The fact that you choose to give some of your valuable time toward helping CHEST achieve its mission and vision is so greatly appreciated by all of us in this organization. Thank you for all that you do for CHEST.
In recent years, the College has continued to realize the following significant achievements:
1. Growth of our educational programs in simulation, skills training, and procedures;
2. The building and of our new global HQ and Innovation, Simulation, and Training Center;
3. An increasingly global footprint as we deliver education to our physician and advance practice provider members and nonmembers in the US and around the world;
4. Increasing development of digital publications and essential content, such as our journal; CHEST®, CHEST-SEEK ™ products, e-learning modules, evidence-based guidelines, and more that can be served up to anyone on any device;
5. Growth and maturation of our CHEST Foundation and its research and service awards;
6. Expansion of patient education initiatives and materials;
7. Development of a data warehouse that will allow us to serve our members and partners more effectively; and
8. Far too many more achievements to list here.
Since taking on the EVP/CEO role, I’ve been asked what do I consider my primary responsibilities to be. I think this is best summed up by Rick Moyers, in The Nonprofit Chief Executive’s Ten Basic Responsibilities (BoardSource, 2006). In it, he outlines the executive’s responsibilities as follows:
1. Commit to the mission.
2. Lead the staff and manage the organization.
3. Exercise responsible financial stewardship.
4. Lead and manage fundraising.
5. Follow the highest ethical standards, ensure accountability, and comply with the law.
6. Engage the board in planning and lead the implementation.
7. Develop future leadership.
8. Build external relationships and serve as an advocate.
9. Ensure the quality and effectiveness of programs.
10. Support the board.
These 10 basic responsibilities provide the framework and foundation for how I plan to serve as EVP/CEO of CHEST. In many cases, I’ve been doing much of this as a senior executive at CHEST for the past 23 years, and I look forward to continuing to build on that foundation.
I am also often asked what my vision for the organization is, as its new EVP/CEO. And my answer is simple: to ensure that the American College of Chest Physicians stays relevant in this environment of change and disruption, that it continues to fulfill its mission, and that members, leadership, volunteers, and staff work together, make a positive impact on patient care, and, ultimately, have fun doing the good work of CHEST. This organization has an outstanding reputation, legacy, and brand. I will do everything I can to maintain and improve upon those key attributes.
It is my ultimate responsibility to ensure that we operationalize the educational programs and activities that align with the strategic plan and achieve the organizational goals of CHEST, which have been set by your Boards and Committees. I look forward to proudly and humbly serving as the CHEST evangelist and advocate to our members, patients, partners, and sister societies. I look forward to hearing from you, our members, about how CHEST is doing, and how we can continue to meet – and exceed – your educational and professional needs.
It is an incredible honor to be recently confirmed as the EVP/CEO for the CHEST organization. As a 23-year veteran of CHEST, I have had the privilege of working with and for many of our leaders, volunteers, and members. Being only the fifth person to lead the organization in an executive leadership role is both humbling and invigorating. CHEST is a dynamic and innovative organization, with a mission to “champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.” That mission resonates deeply with me on a personal level, because my mother had COPD. Toward the end of her life, I saw firsthand how it impacted her quality of life and her ability to be a mother and grandmother, and I also saw the important role her pulmonologist and re
I am both fortunate and grateful to have such a phenomenal professional staff to work with here at CHEST and to have the outstanding leadership of our Presidents, Past Presidents, Boards, Committees, and NetWorks – all of which have been tremendously supportive during the past 9 months as I filled the Interim EVP role. I am also deeply grateful to those of you who choose to be members and Fellows of CHEST and to be engaged as volunteer leadership, faculty, content experts, authors, and more. It is your time, energy, involvement, and vision that make this organization what it is. The fact that you choose to give some of your valuable time toward helping CHEST achieve its mission and vision is so greatly appreciated by all of us in this organization. Thank you for all that you do for CHEST.
In recent years, the College has continued to realize the following significant achievements:
1. Growth of our educational programs in simulation, skills training, and procedures;
2. The building and of our new global HQ and Innovation, Simulation, and Training Center;
3. An increasingly global footprint as we deliver education to our physician and advance practice provider members and nonmembers in the US and around the world;
4. Increasing development of digital publications and essential content, such as our journal; CHEST®, CHEST-SEEK ™ products, e-learning modules, evidence-based guidelines, and more that can be served up to anyone on any device;
5. Growth and maturation of our CHEST Foundation and its research and service awards;
6. Expansion of patient education initiatives and materials;
7. Development of a data warehouse that will allow us to serve our members and partners more effectively; and
8. Far too many more achievements to list here.
Since taking on the EVP/CEO role, I’ve been asked what do I consider my primary responsibilities to be. I think this is best summed up by Rick Moyers, in The Nonprofit Chief Executive’s Ten Basic Responsibilities (BoardSource, 2006). In it, he outlines the executive’s responsibilities as follows:
1. Commit to the mission.
2. Lead the staff and manage the organization.
3. Exercise responsible financial stewardship.
4. Lead and manage fundraising.
5. Follow the highest ethical standards, ensure accountability, and comply with the law.
6. Engage the board in planning and lead the implementation.
7. Develop future leadership.
8. Build external relationships and serve as an advocate.
9. Ensure the quality and effectiveness of programs.
10. Support the board.
These 10 basic responsibilities provide the framework and foundation for how I plan to serve as EVP/CEO of CHEST. In many cases, I’ve been doing much of this as a senior executive at CHEST for the past 23 years, and I look forward to continuing to build on that foundation.
I am also often asked what my vision for the organization is, as its new EVP/CEO. And my answer is simple: to ensure that the American College of Chest Physicians stays relevant in this environment of change and disruption, that it continues to fulfill its mission, and that members, leadership, volunteers, and staff work together, make a positive impact on patient care, and, ultimately, have fun doing the good work of CHEST. This organization has an outstanding reputation, legacy, and brand. I will do everything I can to maintain and improve upon those key attributes.
It is my ultimate responsibility to ensure that we operationalize the educational programs and activities that align with the strategic plan and achieve the organizational goals of CHEST, which have been set by your Boards and Committees. I look forward to proudly and humbly serving as the CHEST evangelist and advocate to our members, patients, partners, and sister societies. I look forward to hearing from you, our members, about how CHEST is doing, and how we can continue to meet – and exceed – your educational and professional needs.
It is an incredible honor to be recently confirmed as the EVP/CEO for the CHEST organization. As a 23-year veteran of CHEST, I have had the privilege of working with and for many of our leaders, volunteers, and members. Being only the fifth person to lead the organization in an executive leadership role is both humbling and invigorating. CHEST is a dynamic and innovative organization, with a mission to “champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.” That mission resonates deeply with me on a personal level, because my mother had COPD. Toward the end of her life, I saw firsthand how it impacted her quality of life and her ability to be a mother and grandmother, and I also saw the important role her pulmonologist and re
I am both fortunate and grateful to have such a phenomenal professional staff to work with here at CHEST and to have the outstanding leadership of our Presidents, Past Presidents, Boards, Committees, and NetWorks – all of which have been tremendously supportive during the past 9 months as I filled the Interim EVP role. I am also deeply grateful to those of you who choose to be members and Fellows of CHEST and to be engaged as volunteer leadership, faculty, content experts, authors, and more. It is your time, energy, involvement, and vision that make this organization what it is. The fact that you choose to give some of your valuable time toward helping CHEST achieve its mission and vision is so greatly appreciated by all of us in this organization. Thank you for all that you do for CHEST.
In recent years, the College has continued to realize the following significant achievements:
1. Growth of our educational programs in simulation, skills training, and procedures;
2. The building and of our new global HQ and Innovation, Simulation, and Training Center;
3. An increasingly global footprint as we deliver education to our physician and advance practice provider members and nonmembers in the US and around the world;
4. Increasing development of digital publications and essential content, such as our journal; CHEST®, CHEST-SEEK ™ products, e-learning modules, evidence-based guidelines, and more that can be served up to anyone on any device;
5. Growth and maturation of our CHEST Foundation and its research and service awards;
6. Expansion of patient education initiatives and materials;
7. Development of a data warehouse that will allow us to serve our members and partners more effectively; and
8. Far too many more achievements to list here.
Since taking on the EVP/CEO role, I’ve been asked what do I consider my primary responsibilities to be. I think this is best summed up by Rick Moyers, in The Nonprofit Chief Executive’s Ten Basic Responsibilities (BoardSource, 2006). In it, he outlines the executive’s responsibilities as follows:
1. Commit to the mission.
2. Lead the staff and manage the organization.
3. Exercise responsible financial stewardship.
4. Lead and manage fundraising.
5. Follow the highest ethical standards, ensure accountability, and comply with the law.
6. Engage the board in planning and lead the implementation.
7. Develop future leadership.
8. Build external relationships and serve as an advocate.
9. Ensure the quality and effectiveness of programs.
10. Support the board.
These 10 basic responsibilities provide the framework and foundation for how I plan to serve as EVP/CEO of CHEST. In many cases, I’ve been doing much of this as a senior executive at CHEST for the past 23 years, and I look forward to continuing to build on that foundation.
I am also often asked what my vision for the organization is, as its new EVP/CEO. And my answer is simple: to ensure that the American College of Chest Physicians stays relevant in this environment of change and disruption, that it continues to fulfill its mission, and that members, leadership, volunteers, and staff work together, make a positive impact on patient care, and, ultimately, have fun doing the good work of CHEST. This organization has an outstanding reputation, legacy, and brand. I will do everything I can to maintain and improve upon those key attributes.
It is my ultimate responsibility to ensure that we operationalize the educational programs and activities that align with the strategic plan and achieve the organizational goals of CHEST, which have been set by your Boards and Committees. I look forward to proudly and humbly serving as the CHEST evangelist and advocate to our members, patients, partners, and sister societies. I look forward to hearing from you, our members, about how CHEST is doing, and how we can continue to meet – and exceed – your educational and professional needs.
Pulmonary Perspectives® China’s Pulmonary Crisis
Over the past 2 years, we had the opportunity to participate in an annual cross-cultural exchange that has broadened our horizons. Xi’an, the ancient capital of China and home of the Terracotta warriors, is a sprawling megapolis similar to Los Angeles. In the southern suburb of Huxian, US trained pulmonary, neurosurgical, and critical care physicians from Cooper University Hospital and Morehouse School of Medicine partnered with physicians of Ji-Ren Teaching Hospital to deliver a Chinese Medical Association accredited continuing medical education conference. The conference agenda included a variety of pulmonary and critical care topics highlighting sepsis, neurovascular disease, and lung cancer screening and diagnosis. We also provided a hands-on workshop for point of care ultrasound, and, in return, received education about Chinese medicine.
We found our hosts appreciative and hospitable, and they treated us with the highest level of respect (the cornerstone of Chinese culture). The audience was receptive and very interested in learning. However, while we were impressed with their rapid growth and interest in incorporating western medicine into their daily practice, it was impossible to overlook the major pulmonary health-care concerns threatening their communities. Tobacco use was omnipresent, and the haze of air pollution made the sky a constant shade of grey. In both public and private spaces, powerful echoes of a once familiar America resonated, and they served to underscore the obstacles the Chinese medical community now faces in caring for their country’s pulmonary health.
An Old, Familiar Foe
The China National Tobacco Corporation (CNTC) is the largest tobacco company in the world, as well as China’s most profitable state-owned enterprise (Pratt, A, et al. WHO Report. 2017. ISBN 9789290617907 [http://www.wpro.who.int/china/publications/2017_china_tobacco_control_report_en_web_final.pdf?ua=1]). As such, the CNTC controls every aspect of its production and supply chain with the force of the federal government and also exerts heavy influence over regulatory policy. It controls about 98% of domestic crop production and manages to price cigarettes just short of one American dollar per pack, yet contributes about $170 billion annually to the government (Rich, et al. Nicotine Tob Res. 2012;14[3]:258). This accounted for nearly 7% of total governmental revenue in 2015 (Pratt, 2017).
To date, nearly 44% of the world’s cigarettes are manufactured and consumed in China (Pratt 2017, Rich 2012). In 2015, more than 315 million Chinese adults were daily smokers, or about 28% of the adult population and nearly half of all men (Pratt, 2017). This is about double the proportion of US smokers (about 15.1%) and more than eight times the 36.5 million daily smokers in the United States (CDC Online Tobacco Use Report, 2016 [https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/]). However, to visit China is not only to know a love for tobacco, but also an overwhelming guest and gift culture. Gift giving and hospitality is central to the Chinese identity, from business meetings to afternoon tea. Given their economy and such rich supply, people gift cigarettes to one another at all times for nearly any occasion. Unfortunately, tobacco smoke in China is as inescapable as its health consequences.
The direct effects of smoking on China’s pulmonary health have been catastrophic. Cancers of the lung and bronchus constitute their most common malignancy across both sexes, accounting for the majority of the annual 4.3 million new cancer diagnoses (Chen et al. CA Cancer J Clin. 2016;66[2]:115). In Chinese men, lung cancer is the second most common cancer before the age of 60, and over the age of 75, it is the most common malignancy and also accounts for the majority of that group’s cancer mortality. Women fare only slightly better, with breast cancer being their most common malignancy, but with lung cancer remaining the most pervasive across all age groups, and, by far, the most deadly (Chen, 2016). All told, of the projected 2.8 million cancer deaths occurring in 2015 in China, 21% were directly a result of lung cancer.
Likewise, COPD also threatens China. The Global Burden of Disease study conducted in 2004 demonstrated that nearly 3 million people die of COPD each year. Chinese adults over the age of 40 had an overall prevalence of COPD of 9% for the last decade, though this may be higher given the high rate of underdiagnosis in rural China (Fang X, et al. Chest. 2011;139[4]:920). After 2004, the Chinese Ministry of Health affirmed that COPD was the fourth leading cause of mortality in urban areas, but third in rural ones (Fang, 2011). When investigators analyzed deaths secondary to cor pulmonale coexisting with COPD, they found COPD-related mortality increased to 179.9 for men and 141.3 for women per 100,000 persons, which is about double the COPD mortality for other countries in the Asian-Pacific region (Reilly K, et al. Am J Epidemiol. 2008;167[8]:998).
Both cancer and COPD in China disproportionately affect those in rural areas and with lower socioeconomic status, with smoking being the most potent causative exposure. On average, the annual direct and indirect per-patient cost of treating COPD amounted to about $2,000, comprising about 40% of a family’s total annual income (Fang, 2011). The cost of treating malignancy is even more expensive, but the higher likelihood of death results in an additional 10% to 20% reduction of family income when a working family member dies (Pratt, 2016). Taken together, and especially since rural Chinese citizens spend close to 20% of their income on tobacco products, the pulmonary health consequences of smoking are a significant driver of both health and economic inequality.
The Air We Breathe
Air pollution comprises a second pulmonary insult to China’s health. The International Agency for Research on Cancer designated particulate matter (PM) as a class I carcinogen (Kurt O, et al. Curr Opin Pulm Med. 2016;22[2]:138). PM forms from combustion of bio-mass fuel, as well as from dust storms or construction. Once particulates are smaller than 2.5 microns (PM2.5), they cause substantial harm to the pulmonary microenvironment. Guo and colleagues demonstrated markedly increased lung cancer risks associated with spatial mapping of ozone and PM2.5 concentrations (Guo Y, et al. Environ Res. 2016;144;60). PM2.5 also doubles the odds of contracting COPD in nonsmoking adults, conferring as much as a three-fold risk of contracting the disease in nonsmoking women (Fang, 2011).
Apart from causing pulmonary disease, studies also implicate air pollution as frequently causing exacerbations of existing disease. One study found an incremental increase in ED visits for respiratory illnesses for every 10 µg/m3 above the median PM2.5 level (Xu, et al. PLoS One. 2016;11(4): e0153099). In 2013, 83% of Chinese lived in places where PM2.5 levels exceeded China’s own ambient air standard. In this cohort, elevated PM2.5 levels contributed directly to 300,000 premature deaths from lung cancer and COPD, with PM2.5 causing 1.2 million premature deaths overall (Liu J, et al. Sci Total Environ. 2016;568;1253).
Moving Forward
The Chinese have few illusions about these pulmonary concerns, and they are making progress. The government recently introduced stricter smoking controls in Beijing and Shanghai and continues to explore ways to decrease emissions. President Xi has put forward strong initiatives to improve the health of the Chinese. However, the nation is trying to balance its national priorities in the context of a fluid, and, at times, perilous geopolitical climate. In some ways, their position is not too dissimilar from the US geopolitical and health-care situation of the 1970s. While challenging, the issue of Chinese health care should not overshadow the remarkable resources or the truly remarkable culture of their people. Friendship, cooperation, the reduction of suffering: these are ideals where all clinicians find common ground, regardless of nationality.
Dr. Mackay is Chief Fellow of Critical Care Medicine, Cooper University Hospital, Cooper Medical School of Rowan University, Camden, New Jersey; Dr. Flenaugh is Associate Professor of Medicine, Division Chief of Pulmonary and Critical Care Medicine, Director of Advance Diagnostic and Interventional Pulmonary, Morehouse School of Medicine, Atlanta, Georgia.
Editor’s Note
This excellent, up-close Pulmonary Perspective details observations of Drs. Mackay and Flenaugh as they have participated in cross-cultural exchanges in
The American College of Chest Physicians, likewise concerned about pulmonary health in China, has approached the problem on a different front, working closely with partners, such as the Chinese Thoracic Society, the Chinese Association of Chest Physicians, and the Chinese Medical Doctor Association, to implement China’s first ever fellowship program offering standardized training in PCCM for Chinese physicians. Read more at http://www.mdedge.com/chestphysician/article/131179/society-news/pccm-endorsed-pilot-subspecialty-chinese-national-health.
Nitin Puri, MD, FCCP, is the section editor of Pulmonary Perspectives.
Over the past 2 years, we had the opportunity to participate in an annual cross-cultural exchange that has broadened our horizons. Xi’an, the ancient capital of China and home of the Terracotta warriors, is a sprawling megapolis similar to Los Angeles. In the southern suburb of Huxian, US trained pulmonary, neurosurgical, and critical care physicians from Cooper University Hospital and Morehouse School of Medicine partnered with physicians of Ji-Ren Teaching Hospital to deliver a Chinese Medical Association accredited continuing medical education conference. The conference agenda included a variety of pulmonary and critical care topics highlighting sepsis, neurovascular disease, and lung cancer screening and diagnosis. We also provided a hands-on workshop for point of care ultrasound, and, in return, received education about Chinese medicine.
We found our hosts appreciative and hospitable, and they treated us with the highest level of respect (the cornerstone of Chinese culture). The audience was receptive and very interested in learning. However, while we were impressed with their rapid growth and interest in incorporating western medicine into their daily practice, it was impossible to overlook the major pulmonary health-care concerns threatening their communities. Tobacco use was omnipresent, and the haze of air pollution made the sky a constant shade of grey. In both public and private spaces, powerful echoes of a once familiar America resonated, and they served to underscore the obstacles the Chinese medical community now faces in caring for their country’s pulmonary health.
An Old, Familiar Foe
The China National Tobacco Corporation (CNTC) is the largest tobacco company in the world, as well as China’s most profitable state-owned enterprise (Pratt, A, et al. WHO Report. 2017. ISBN 9789290617907 [http://www.wpro.who.int/china/publications/2017_china_tobacco_control_report_en_web_final.pdf?ua=1]). As such, the CNTC controls every aspect of its production and supply chain with the force of the federal government and also exerts heavy influence over regulatory policy. It controls about 98% of domestic crop production and manages to price cigarettes just short of one American dollar per pack, yet contributes about $170 billion annually to the government (Rich, et al. Nicotine Tob Res. 2012;14[3]:258). This accounted for nearly 7% of total governmental revenue in 2015 (Pratt, 2017).
To date, nearly 44% of the world’s cigarettes are manufactured and consumed in China (Pratt 2017, Rich 2012). In 2015, more than 315 million Chinese adults were daily smokers, or about 28% of the adult population and nearly half of all men (Pratt, 2017). This is about double the proportion of US smokers (about 15.1%) and more than eight times the 36.5 million daily smokers in the United States (CDC Online Tobacco Use Report, 2016 [https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/]). However, to visit China is not only to know a love for tobacco, but also an overwhelming guest and gift culture. Gift giving and hospitality is central to the Chinese identity, from business meetings to afternoon tea. Given their economy and such rich supply, people gift cigarettes to one another at all times for nearly any occasion. Unfortunately, tobacco smoke in China is as inescapable as its health consequences.
The direct effects of smoking on China’s pulmonary health have been catastrophic. Cancers of the lung and bronchus constitute their most common malignancy across both sexes, accounting for the majority of the annual 4.3 million new cancer diagnoses (Chen et al. CA Cancer J Clin. 2016;66[2]:115). In Chinese men, lung cancer is the second most common cancer before the age of 60, and over the age of 75, it is the most common malignancy and also accounts for the majority of that group’s cancer mortality. Women fare only slightly better, with breast cancer being their most common malignancy, but with lung cancer remaining the most pervasive across all age groups, and, by far, the most deadly (Chen, 2016). All told, of the projected 2.8 million cancer deaths occurring in 2015 in China, 21% were directly a result of lung cancer.
Likewise, COPD also threatens China. The Global Burden of Disease study conducted in 2004 demonstrated that nearly 3 million people die of COPD each year. Chinese adults over the age of 40 had an overall prevalence of COPD of 9% for the last decade, though this may be higher given the high rate of underdiagnosis in rural China (Fang X, et al. Chest. 2011;139[4]:920). After 2004, the Chinese Ministry of Health affirmed that COPD was the fourth leading cause of mortality in urban areas, but third in rural ones (Fang, 2011). When investigators analyzed deaths secondary to cor pulmonale coexisting with COPD, they found COPD-related mortality increased to 179.9 for men and 141.3 for women per 100,000 persons, which is about double the COPD mortality for other countries in the Asian-Pacific region (Reilly K, et al. Am J Epidemiol. 2008;167[8]:998).
Both cancer and COPD in China disproportionately affect those in rural areas and with lower socioeconomic status, with smoking being the most potent causative exposure. On average, the annual direct and indirect per-patient cost of treating COPD amounted to about $2,000, comprising about 40% of a family’s total annual income (Fang, 2011). The cost of treating malignancy is even more expensive, but the higher likelihood of death results in an additional 10% to 20% reduction of family income when a working family member dies (Pratt, 2016). Taken together, and especially since rural Chinese citizens spend close to 20% of their income on tobacco products, the pulmonary health consequences of smoking are a significant driver of both health and economic inequality.
The Air We Breathe
Air pollution comprises a second pulmonary insult to China’s health. The International Agency for Research on Cancer designated particulate matter (PM) as a class I carcinogen (Kurt O, et al. Curr Opin Pulm Med. 2016;22[2]:138). PM forms from combustion of bio-mass fuel, as well as from dust storms or construction. Once particulates are smaller than 2.5 microns (PM2.5), they cause substantial harm to the pulmonary microenvironment. Guo and colleagues demonstrated markedly increased lung cancer risks associated with spatial mapping of ozone and PM2.5 concentrations (Guo Y, et al. Environ Res. 2016;144;60). PM2.5 also doubles the odds of contracting COPD in nonsmoking adults, conferring as much as a three-fold risk of contracting the disease in nonsmoking women (Fang, 2011).
Apart from causing pulmonary disease, studies also implicate air pollution as frequently causing exacerbations of existing disease. One study found an incremental increase in ED visits for respiratory illnesses for every 10 µg/m3 above the median PM2.5 level (Xu, et al. PLoS One. 2016;11(4): e0153099). In 2013, 83% of Chinese lived in places where PM2.5 levels exceeded China’s own ambient air standard. In this cohort, elevated PM2.5 levels contributed directly to 300,000 premature deaths from lung cancer and COPD, with PM2.5 causing 1.2 million premature deaths overall (Liu J, et al. Sci Total Environ. 2016;568;1253).
Moving Forward
The Chinese have few illusions about these pulmonary concerns, and they are making progress. The government recently introduced stricter smoking controls in Beijing and Shanghai and continues to explore ways to decrease emissions. President Xi has put forward strong initiatives to improve the health of the Chinese. However, the nation is trying to balance its national priorities in the context of a fluid, and, at times, perilous geopolitical climate. In some ways, their position is not too dissimilar from the US geopolitical and health-care situation of the 1970s. While challenging, the issue of Chinese health care should not overshadow the remarkable resources or the truly remarkable culture of their people. Friendship, cooperation, the reduction of suffering: these are ideals where all clinicians find common ground, regardless of nationality.
Dr. Mackay is Chief Fellow of Critical Care Medicine, Cooper University Hospital, Cooper Medical School of Rowan University, Camden, New Jersey; Dr. Flenaugh is Associate Professor of Medicine, Division Chief of Pulmonary and Critical Care Medicine, Director of Advance Diagnostic and Interventional Pulmonary, Morehouse School of Medicine, Atlanta, Georgia.
Editor’s Note
This excellent, up-close Pulmonary Perspective details observations of Drs. Mackay and Flenaugh as they have participated in cross-cultural exchanges in
The American College of Chest Physicians, likewise concerned about pulmonary health in China, has approached the problem on a different front, working closely with partners, such as the Chinese Thoracic Society, the Chinese Association of Chest Physicians, and the Chinese Medical Doctor Association, to implement China’s first ever fellowship program offering standardized training in PCCM for Chinese physicians. Read more at http://www.mdedge.com/chestphysician/article/131179/society-news/pccm-endorsed-pilot-subspecialty-chinese-national-health.
Nitin Puri, MD, FCCP, is the section editor of Pulmonary Perspectives.
Over the past 2 years, we had the opportunity to participate in an annual cross-cultural exchange that has broadened our horizons. Xi’an, the ancient capital of China and home of the Terracotta warriors, is a sprawling megapolis similar to Los Angeles. In the southern suburb of Huxian, US trained pulmonary, neurosurgical, and critical care physicians from Cooper University Hospital and Morehouse School of Medicine partnered with physicians of Ji-Ren Teaching Hospital to deliver a Chinese Medical Association accredited continuing medical education conference. The conference agenda included a variety of pulmonary and critical care topics highlighting sepsis, neurovascular disease, and lung cancer screening and diagnosis. We also provided a hands-on workshop for point of care ultrasound, and, in return, received education about Chinese medicine.
We found our hosts appreciative and hospitable, and they treated us with the highest level of respect (the cornerstone of Chinese culture). The audience was receptive and very interested in learning. However, while we were impressed with their rapid growth and interest in incorporating western medicine into their daily practice, it was impossible to overlook the major pulmonary health-care concerns threatening their communities. Tobacco use was omnipresent, and the haze of air pollution made the sky a constant shade of grey. In both public and private spaces, powerful echoes of a once familiar America resonated, and they served to underscore the obstacles the Chinese medical community now faces in caring for their country’s pulmonary health.
An Old, Familiar Foe
The China National Tobacco Corporation (CNTC) is the largest tobacco company in the world, as well as China’s most profitable state-owned enterprise (Pratt, A, et al. WHO Report. 2017. ISBN 9789290617907 [http://www.wpro.who.int/china/publications/2017_china_tobacco_control_report_en_web_final.pdf?ua=1]). As such, the CNTC controls every aspect of its production and supply chain with the force of the federal government and also exerts heavy influence over regulatory policy. It controls about 98% of domestic crop production and manages to price cigarettes just short of one American dollar per pack, yet contributes about $170 billion annually to the government (Rich, et al. Nicotine Tob Res. 2012;14[3]:258). This accounted for nearly 7% of total governmental revenue in 2015 (Pratt, 2017).
To date, nearly 44% of the world’s cigarettes are manufactured and consumed in China (Pratt 2017, Rich 2012). In 2015, more than 315 million Chinese adults were daily smokers, or about 28% of the adult population and nearly half of all men (Pratt, 2017). This is about double the proportion of US smokers (about 15.1%) and more than eight times the 36.5 million daily smokers in the United States (CDC Online Tobacco Use Report, 2016 [https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/]). However, to visit China is not only to know a love for tobacco, but also an overwhelming guest and gift culture. Gift giving and hospitality is central to the Chinese identity, from business meetings to afternoon tea. Given their economy and such rich supply, people gift cigarettes to one another at all times for nearly any occasion. Unfortunately, tobacco smoke in China is as inescapable as its health consequences.
The direct effects of smoking on China’s pulmonary health have been catastrophic. Cancers of the lung and bronchus constitute their most common malignancy across both sexes, accounting for the majority of the annual 4.3 million new cancer diagnoses (Chen et al. CA Cancer J Clin. 2016;66[2]:115). In Chinese men, lung cancer is the second most common cancer before the age of 60, and over the age of 75, it is the most common malignancy and also accounts for the majority of that group’s cancer mortality. Women fare only slightly better, with breast cancer being their most common malignancy, but with lung cancer remaining the most pervasive across all age groups, and, by far, the most deadly (Chen, 2016). All told, of the projected 2.8 million cancer deaths occurring in 2015 in China, 21% were directly a result of lung cancer.
Likewise, COPD also threatens China. The Global Burden of Disease study conducted in 2004 demonstrated that nearly 3 million people die of COPD each year. Chinese adults over the age of 40 had an overall prevalence of COPD of 9% for the last decade, though this may be higher given the high rate of underdiagnosis in rural China (Fang X, et al. Chest. 2011;139[4]:920). After 2004, the Chinese Ministry of Health affirmed that COPD was the fourth leading cause of mortality in urban areas, but third in rural ones (Fang, 2011). When investigators analyzed deaths secondary to cor pulmonale coexisting with COPD, they found COPD-related mortality increased to 179.9 for men and 141.3 for women per 100,000 persons, which is about double the COPD mortality for other countries in the Asian-Pacific region (Reilly K, et al. Am J Epidemiol. 2008;167[8]:998).
Both cancer and COPD in China disproportionately affect those in rural areas and with lower socioeconomic status, with smoking being the most potent causative exposure. On average, the annual direct and indirect per-patient cost of treating COPD amounted to about $2,000, comprising about 40% of a family’s total annual income (Fang, 2011). The cost of treating malignancy is even more expensive, but the higher likelihood of death results in an additional 10% to 20% reduction of family income when a working family member dies (Pratt, 2016). Taken together, and especially since rural Chinese citizens spend close to 20% of their income on tobacco products, the pulmonary health consequences of smoking are a significant driver of both health and economic inequality.
The Air We Breathe
Air pollution comprises a second pulmonary insult to China’s health. The International Agency for Research on Cancer designated particulate matter (PM) as a class I carcinogen (Kurt O, et al. Curr Opin Pulm Med. 2016;22[2]:138). PM forms from combustion of bio-mass fuel, as well as from dust storms or construction. Once particulates are smaller than 2.5 microns (PM2.5), they cause substantial harm to the pulmonary microenvironment. Guo and colleagues demonstrated markedly increased lung cancer risks associated with spatial mapping of ozone and PM2.5 concentrations (Guo Y, et al. Environ Res. 2016;144;60). PM2.5 also doubles the odds of contracting COPD in nonsmoking adults, conferring as much as a three-fold risk of contracting the disease in nonsmoking women (Fang, 2011).
Apart from causing pulmonary disease, studies also implicate air pollution as frequently causing exacerbations of existing disease. One study found an incremental increase in ED visits for respiratory illnesses for every 10 µg/m3 above the median PM2.5 level (Xu, et al. PLoS One. 2016;11(4): e0153099). In 2013, 83% of Chinese lived in places where PM2.5 levels exceeded China’s own ambient air standard. In this cohort, elevated PM2.5 levels contributed directly to 300,000 premature deaths from lung cancer and COPD, with PM2.5 causing 1.2 million premature deaths overall (Liu J, et al. Sci Total Environ. 2016;568;1253).
Moving Forward
The Chinese have few illusions about these pulmonary concerns, and they are making progress. The government recently introduced stricter smoking controls in Beijing and Shanghai and continues to explore ways to decrease emissions. President Xi has put forward strong initiatives to improve the health of the Chinese. However, the nation is trying to balance its national priorities in the context of a fluid, and, at times, perilous geopolitical climate. In some ways, their position is not too dissimilar from the US geopolitical and health-care situation of the 1970s. While challenging, the issue of Chinese health care should not overshadow the remarkable resources or the truly remarkable culture of their people. Friendship, cooperation, the reduction of suffering: these are ideals where all clinicians find common ground, regardless of nationality.
Dr. Mackay is Chief Fellow of Critical Care Medicine, Cooper University Hospital, Cooper Medical School of Rowan University, Camden, New Jersey; Dr. Flenaugh is Associate Professor of Medicine, Division Chief of Pulmonary and Critical Care Medicine, Director of Advance Diagnostic and Interventional Pulmonary, Morehouse School of Medicine, Atlanta, Georgia.
Editor’s Note
This excellent, up-close Pulmonary Perspective details observations of Drs. Mackay and Flenaugh as they have participated in cross-cultural exchanges in
The American College of Chest Physicians, likewise concerned about pulmonary health in China, has approached the problem on a different front, working closely with partners, such as the Chinese Thoracic Society, the Chinese Association of Chest Physicians, and the Chinese Medical Doctor Association, to implement China’s first ever fellowship program offering standardized training in PCCM for Chinese physicians. Read more at http://www.mdedge.com/chestphysician/article/131179/society-news/pccm-endorsed-pilot-subspecialty-chinese-national-health.
Nitin Puri, MD, FCCP, is the section editor of Pulmonary Perspectives.
CHEST NetWorks Submassive PE, antibiotic resistance, advanced practice providers
Cardiovascular Medicine and Surgery
Catch 22 of Submassive Pulmonary Emboli
Venous thromboembolism (including deep vein thrombosis (DVT) and pulmonary embolism [PE]) occurs in approximately 1 per 1,000 patients (Piran S, Schulman S. Thromb J. 2016;14[S1]:23) and can be fatal. Pulmonary embolus severity is classified as low risk, intermediate-risk/submassive PE, and massive PE. There is significant controversy about the management of submassive PE, which is defined as PE with right-sided heart strain (elevated troponin or B-type natriuretic peptide, right-axis deviation on ECG, or e
David J. Nagel, MD
Steering Committee Member
Olivier Axler, MD, FCCP
Vice-Chair
Chest Infections
Antibiotic Resistance
One-hundred years ago, infectious diseases caused 5 of the 10 most common causes of deaths in the United States. In 2016, only one infection remained on this list (influenza/pneumonia) (MMWR Morb Mortal Wkly Rep. 2017;66:413).
How medicine has improved with antibiotics. An unfortunate and unintended consequence of widespread antibiotic use has been the progressive resistance to these drugs. It is estimated that, if current trends continue, 10 million lives a year will be at risk from resistant organisms by 2050 (O’Neill, J. (2016). https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf).
Pathogens acquire antibiotic resistance by passing genetic material to one another through plasmids, bacteriophages, or naked DNA. Once acquired, resistance manifests via a number of mechanisms under the stress imposed by antibiotics (Levy SB, et al. Nat Med. 2004;10:S122).
Among the best studied is enzymatic degradation of the antibiotic. This occurs when beta-lactamases degrade penicillin. A second mechanism alters cell transport, thereby blocking cell entry or actively ejecting the antibiotic from the cell. Finally, overexpression or alteration of the antibiotic target may render a drug ineffective at inhibiting any vital cell function.
At the pace with which resistance now develops, the medical community faces a crisis, whereby infections caused by evolving superbugs are no longer effectively controlled by the available menu of antimicrobial agents.
This challenge must be met collectively by the more prudent prescribing of antibiotics, potentially with the help of rapid diagnostics; isolation of patients potentially infected with resistant organisms; and a focus on developing newer drugs that defy known resistant mechanisms.
Marc Feinstein, MD, FCCP
Steering Committee Member
Clinical Pulmonary Medicine
COPD and sleep-disordered breathing; A missing comorbid condition
Subjective, as well as objective, sleep complaints are common in patients with COPD (Krachman S, et al. Proc Am Thorac Soc. 2008;5[4]:536), and sleeping difficulties are ranked the third most frequent complaint (behind dyspnea and fatigue) in patients with COPD (Kinsman RA, et al. Chest. 1983;83[5]:755). Also, sleep quality is poor, and patients with moderate to severe COPD may have higher-than-expected incidence of OSA (Soler X, et al. Ann Am Thorac Soc. 2015;12[8]:1219).
Unfortunately, sleep is usually not assessed during a COPD evaluation. Up to 27% of patients with COPD without hypoxia during wakefulness can experience important desaturation during sleep, so called nocturnal oxygen desaturation (NOD) (Fletcher EC, et al. Chest. 1987;92[4]:604), that may lead to pulmonary hypertension (Chaouat A, et a
Although identification and effective treatment of COPD comorbidities are becoming the cornerstone of COPD management, sleep-disordered breathing has not been identified in current guidelines yet as a true potential contributor in poor outcomes despite emergent clinical evidence. Multidisciplinary programs, such as pulmonary rehabilitation, that improve dyspnea, exercise capacity, and quality of life may also positively impact sleep (Soler X, et al. COPD. 2013;10[2]:156). Because of the background of the staff involved, the comprehensive approach to patient assessment, and access to number of COPD subjects, pulmonary rehabilitation may be an optimal opportunity to assess sleep and identify an important comorbid condition often overlooked in patients with more advanced COPD.
Xavier Soler, MD, PhD
Steering Committee Member
Interprofessional Team
Finding Home
Outside our internal medicine curriculum, there is no formal pulmonary training or post-masters fellowship in pulmonary medicine for Advanced Practice Providers (APPs). Because of this, APPs are left to their own devices to fill educational gaps. To perform at the level expected by the physicians I work for, journal reviews and memorizing guidelines were not going to be enough. Since there is no formal pulmonary APP society, there were no peers to reach out to either. Off to conferences I went.
At first, I found CHEST daunting. After all, it’s run by the American College of Chest “Physicians,” not Nurse Practitioners. I spent most of the first day with my nametag turned around worried I’d be found out as a nonphysician attendee who snuck in. And then the unthinkable happened, I ran into another unicorn—another APP seeking the same information, only her nametag was turned the right way. The best advice she gave was to attend the Interprofessional NetWork meeting. This was ground zero of the conference as far as I was concerned. There I found myself surrounded by RTs, RNs, NPs, PAs, and yes, even physicians.
Over the years, as I’ve gotten further involved with CHEST NetWorks, I have found from top to bottom CHEST striving to incorporate APPs and advance our education. From including us in the FCCP program, reducing conference pricing for APPs, and focusing this year’s conference theme around being team focused, CHEST is creating a home for APPs.
Corinne Preston Young, FNP, FCCP
Steering Committee Member
Cardiovascular Medicine and Surgery
Catch 22 of Submassive Pulmonary Emboli
Venous thromboembolism (including deep vein thrombosis (DVT) and pulmonary embolism [PE]) occurs in approximately 1 per 1,000 patients (Piran S, Schulman S. Thromb J. 2016;14[S1]:23) and can be fatal. Pulmonary embolus severity is classified as low risk, intermediate-risk/submassive PE, and massive PE. There is significant controversy about the management of submassive PE, which is defined as PE with right-sided heart strain (elevated troponin or B-type natriuretic peptide, right-axis deviation on ECG, or e
David J. Nagel, MD
Steering Committee Member
Olivier Axler, MD, FCCP
Vice-Chair
Chest Infections
Antibiotic Resistance
One-hundred years ago, infectious diseases caused 5 of the 10 most common causes of deaths in the United States. In 2016, only one infection remained on this list (influenza/pneumonia) (MMWR Morb Mortal Wkly Rep. 2017;66:413).
How medicine has improved with antibiotics. An unfortunate and unintended consequence of widespread antibiotic use has been the progressive resistance to these drugs. It is estimated that, if current trends continue, 10 million lives a year will be at risk from resistant organisms by 2050 (O’Neill, J. (2016). https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf).
Pathogens acquire antibiotic resistance by passing genetic material to one another through plasmids, bacteriophages, or naked DNA. Once acquired, resistance manifests via a number of mechanisms under the stress imposed by antibiotics (Levy SB, et al. Nat Med. 2004;10:S122).
Among the best studied is enzymatic degradation of the antibiotic. This occurs when beta-lactamases degrade penicillin. A second mechanism alters cell transport, thereby blocking cell entry or actively ejecting the antibiotic from the cell. Finally, overexpression or alteration of the antibiotic target may render a drug ineffective at inhibiting any vital cell function.
At the pace with which resistance now develops, the medical community faces a crisis, whereby infections caused by evolving superbugs are no longer effectively controlled by the available menu of antimicrobial agents.
This challenge must be met collectively by the more prudent prescribing of antibiotics, potentially with the help of rapid diagnostics; isolation of patients potentially infected with resistant organisms; and a focus on developing newer drugs that defy known resistant mechanisms.
Marc Feinstein, MD, FCCP
Steering Committee Member
Clinical Pulmonary Medicine
COPD and sleep-disordered breathing; A missing comorbid condition
Subjective, as well as objective, sleep complaints are common in patients with COPD (Krachman S, et al. Proc Am Thorac Soc. 2008;5[4]:536), and sleeping difficulties are ranked the third most frequent complaint (behind dyspnea and fatigue) in patients with COPD (Kinsman RA, et al. Chest. 1983;83[5]:755). Also, sleep quality is poor, and patients with moderate to severe COPD may have higher-than-expected incidence of OSA (Soler X, et al. Ann Am Thorac Soc. 2015;12[8]:1219).
Unfortunately, sleep is usually not assessed during a COPD evaluation. Up to 27% of patients with COPD without hypoxia during wakefulness can experience important desaturation during sleep, so called nocturnal oxygen desaturation (NOD) (Fletcher EC, et al. Chest. 1987;92[4]:604), that may lead to pulmonary hypertension (Chaouat A, et a
Although identification and effective treatment of COPD comorbidities are becoming the cornerstone of COPD management, sleep-disordered breathing has not been identified in current guidelines yet as a true potential contributor in poor outcomes despite emergent clinical evidence. Multidisciplinary programs, such as pulmonary rehabilitation, that improve dyspnea, exercise capacity, and quality of life may also positively impact sleep (Soler X, et al. COPD. 2013;10[2]:156). Because of the background of the staff involved, the comprehensive approach to patient assessment, and access to number of COPD subjects, pulmonary rehabilitation may be an optimal opportunity to assess sleep and identify an important comorbid condition often overlooked in patients with more advanced COPD.
Xavier Soler, MD, PhD
Steering Committee Member
Interprofessional Team
Finding Home
Outside our internal medicine curriculum, there is no formal pulmonary training or post-masters fellowship in pulmonary medicine for Advanced Practice Providers (APPs). Because of this, APPs are left to their own devices to fill educational gaps. To perform at the level expected by the physicians I work for, journal reviews and memorizing guidelines were not going to be enough. Since there is no formal pulmonary APP society, there were no peers to reach out to either. Off to conferences I went.
At first, I found CHEST daunting. After all, it’s run by the American College of Chest “Physicians,” not Nurse Practitioners. I spent most of the first day with my nametag turned around worried I’d be found out as a nonphysician attendee who snuck in. And then the unthinkable happened, I ran into another unicorn—another APP seeking the same information, only her nametag was turned the right way. The best advice she gave was to attend the Interprofessional NetWork meeting. This was ground zero of the conference as far as I was concerned. There I found myself surrounded by RTs, RNs, NPs, PAs, and yes, even physicians.
Over the years, as I’ve gotten further involved with CHEST NetWorks, I have found from top to bottom CHEST striving to incorporate APPs and advance our education. From including us in the FCCP program, reducing conference pricing for APPs, and focusing this year’s conference theme around being team focused, CHEST is creating a home for APPs.
Corinne Preston Young, FNP, FCCP
Steering Committee Member
Cardiovascular Medicine and Surgery
Catch 22 of Submassive Pulmonary Emboli
Venous thromboembolism (including deep vein thrombosis (DVT) and pulmonary embolism [PE]) occurs in approximately 1 per 1,000 patients (Piran S, Schulman S. Thromb J. 2016;14[S1]:23) and can be fatal. Pulmonary embolus severity is classified as low risk, intermediate-risk/submassive PE, and massive PE. There is significant controversy about the management of submassive PE, which is defined as PE with right-sided heart strain (elevated troponin or B-type natriuretic peptide, right-axis deviation on ECG, or e
David J. Nagel, MD
Steering Committee Member
Olivier Axler, MD, FCCP
Vice-Chair
Chest Infections
Antibiotic Resistance
One-hundred years ago, infectious diseases caused 5 of the 10 most common causes of deaths in the United States. In 2016, only one infection remained on this list (influenza/pneumonia) (MMWR Morb Mortal Wkly Rep. 2017;66:413).
How medicine has improved with antibiotics. An unfortunate and unintended consequence of widespread antibiotic use has been the progressive resistance to these drugs. It is estimated that, if current trends continue, 10 million lives a year will be at risk from resistant organisms by 2050 (O’Neill, J. (2016). https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf).
Pathogens acquire antibiotic resistance by passing genetic material to one another through plasmids, bacteriophages, or naked DNA. Once acquired, resistance manifests via a number of mechanisms under the stress imposed by antibiotics (Levy SB, et al. Nat Med. 2004;10:S122).
Among the best studied is enzymatic degradation of the antibiotic. This occurs when beta-lactamases degrade penicillin. A second mechanism alters cell transport, thereby blocking cell entry or actively ejecting the antibiotic from the cell. Finally, overexpression or alteration of the antibiotic target may render a drug ineffective at inhibiting any vital cell function.
At the pace with which resistance now develops, the medical community faces a crisis, whereby infections caused by evolving superbugs are no longer effectively controlled by the available menu of antimicrobial agents.
This challenge must be met collectively by the more prudent prescribing of antibiotics, potentially with the help of rapid diagnostics; isolation of patients potentially infected with resistant organisms; and a focus on developing newer drugs that defy known resistant mechanisms.
Marc Feinstein, MD, FCCP
Steering Committee Member
Clinical Pulmonary Medicine
COPD and sleep-disordered breathing; A missing comorbid condition
Subjective, as well as objective, sleep complaints are common in patients with COPD (Krachman S, et al. Proc Am Thorac Soc. 2008;5[4]:536), and sleeping difficulties are ranked the third most frequent complaint (behind dyspnea and fatigue) in patients with COPD (Kinsman RA, et al. Chest. 1983;83[5]:755). Also, sleep quality is poor, and patients with moderate to severe COPD may have higher-than-expected incidence of OSA (Soler X, et al. Ann Am Thorac Soc. 2015;12[8]:1219).
Unfortunately, sleep is usually not assessed during a COPD evaluation. Up to 27% of patients with COPD without hypoxia during wakefulness can experience important desaturation during sleep, so called nocturnal oxygen desaturation (NOD) (Fletcher EC, et al. Chest. 1987;92[4]:604), that may lead to pulmonary hypertension (Chaouat A, et a
Although identification and effective treatment of COPD comorbidities are becoming the cornerstone of COPD management, sleep-disordered breathing has not been identified in current guidelines yet as a true potential contributor in poor outcomes despite emergent clinical evidence. Multidisciplinary programs, such as pulmonary rehabilitation, that improve dyspnea, exercise capacity, and quality of life may also positively impact sleep (Soler X, et al. COPD. 2013;10[2]:156). Because of the background of the staff involved, the comprehensive approach to patient assessment, and access to number of COPD subjects, pulmonary rehabilitation may be an optimal opportunity to assess sleep and identify an important comorbid condition often overlooked in patients with more advanced COPD.
Xavier Soler, MD, PhD
Steering Committee Member
Interprofessional Team
Finding Home
Outside our internal medicine curriculum, there is no formal pulmonary training or post-masters fellowship in pulmonary medicine for Advanced Practice Providers (APPs). Because of this, APPs are left to their own devices to fill educational gaps. To perform at the level expected by the physicians I work for, journal reviews and memorizing guidelines were not going to be enough. Since there is no formal pulmonary APP society, there were no peers to reach out to either. Off to conferences I went.
At first, I found CHEST daunting. After all, it’s run by the American College of Chest “Physicians,” not Nurse Practitioners. I spent most of the first day with my nametag turned around worried I’d be found out as a nonphysician attendee who snuck in. And then the unthinkable happened, I ran into another unicorn—another APP seeking the same information, only her nametag was turned the right way. The best advice she gave was to attend the Interprofessional NetWork meeting. This was ground zero of the conference as far as I was concerned. There I found myself surrounded by RTs, RNs, NPs, PAs, and yes, even physicians.
Over the years, as I’ve gotten further involved with CHEST NetWorks, I have found from top to bottom CHEST striving to incorporate APPs and advance our education. From including us in the FCCP program, reducing conference pricing for APPs, and focusing this year’s conference theme around being team focused, CHEST is creating a home for APPs.
Corinne Preston Young, FNP, FCCP
Steering Committee Member
Building bridges: CHEST Foundation collaborations
Partnering with like-minded advocates and organizations strengthens our collective voice to improve patient outcomes. We choose to partner with others who share our values in creating sustainable, long-lasting change by engaging clinicians, patients, caregivers, and the public on the importance of understanding lung health.
Pulmonary Fibrosis Foundation
Allergy & Asthma Network
Over the past 2 years, our relationship with the Allergy & Asthma Network (AAN) has grown to include collaborative disease awareness campaigns, co-branded and co-created patient education materials in asthma and COPD, and an exciting expansion of the platforms we utilize to reach patients. Partnering with the AAN has allowed us to reach new audiences and bring asthma and COPD education to local communities with opportunities, including:
- A Lifetime television segment on Access Health that focuses on asthma education;
- Co-hosted asthma Twitter chats reaching thousands of clinicians and patients; and
- “The Air We Breathe,” an Atlantic Live Summit in Chicago which focused on the relationship between air quality and respiratory health.
COPD Foundation
The COPD Foundation, along with Allergy & Asthma Network, have partnered with us to support our Lung Health Experience, a lung health expo touring Oklahoma City, Nashville, Chicago, and Toronto in 2017. The Lung Health Experience focuses on bringing lung health experts to the public in a comfortable, relaxed, and fun setting. The COPD Foundation and AAN have attended these events to provide the public with educational materials on lung diseases, which support the spirometry screenings performed by local respiratory therapists. We thank the Allergy & Asthma Network and the COPD Foundation for their outstanding support.
It is with these and many other partnerships that the CHEST Foundation is able to elevate its mission to champion lung health and provide local communities with an opportunity to interact with clinicians and physicians outside of a hospital setting. These experiences and collaborations are the key to strengthening the patient and clinician conversation and bridging the gap to improve patient care and outcomes.
Partnering with like-minded advocates and organizations strengthens our collective voice to improve patient outcomes. We choose to partner with others who share our values in creating sustainable, long-lasting change by engaging clinicians, patients, caregivers, and the public on the importance of understanding lung health.
Pulmonary Fibrosis Foundation
Allergy & Asthma Network
Over the past 2 years, our relationship with the Allergy & Asthma Network (AAN) has grown to include collaborative disease awareness campaigns, co-branded and co-created patient education materials in asthma and COPD, and an exciting expansion of the platforms we utilize to reach patients. Partnering with the AAN has allowed us to reach new audiences and bring asthma and COPD education to local communities with opportunities, including:
- A Lifetime television segment on Access Health that focuses on asthma education;
- Co-hosted asthma Twitter chats reaching thousands of clinicians and patients; and
- “The Air We Breathe,” an Atlantic Live Summit in Chicago which focused on the relationship between air quality and respiratory health.
COPD Foundation
The COPD Foundation, along with Allergy & Asthma Network, have partnered with us to support our Lung Health Experience, a lung health expo touring Oklahoma City, Nashville, Chicago, and Toronto in 2017. The Lung Health Experience focuses on bringing lung health experts to the public in a comfortable, relaxed, and fun setting. The COPD Foundation and AAN have attended these events to provide the public with educational materials on lung diseases, which support the spirometry screenings performed by local respiratory therapists. We thank the Allergy & Asthma Network and the COPD Foundation for their outstanding support.
It is with these and many other partnerships that the CHEST Foundation is able to elevate its mission to champion lung health and provide local communities with an opportunity to interact with clinicians and physicians outside of a hospital setting. These experiences and collaborations are the key to strengthening the patient and clinician conversation and bridging the gap to improve patient care and outcomes.
Partnering with like-minded advocates and organizations strengthens our collective voice to improve patient outcomes. We choose to partner with others who share our values in creating sustainable, long-lasting change by engaging clinicians, patients, caregivers, and the public on the importance of understanding lung health.
Pulmonary Fibrosis Foundation
Allergy & Asthma Network
Over the past 2 years, our relationship with the Allergy & Asthma Network (AAN) has grown to include collaborative disease awareness campaigns, co-branded and co-created patient education materials in asthma and COPD, and an exciting expansion of the platforms we utilize to reach patients. Partnering with the AAN has allowed us to reach new audiences and bring asthma and COPD education to local communities with opportunities, including:
- A Lifetime television segment on Access Health that focuses on asthma education;
- Co-hosted asthma Twitter chats reaching thousands of clinicians and patients; and
- “The Air We Breathe,” an Atlantic Live Summit in Chicago which focused on the relationship between air quality and respiratory health.
COPD Foundation
The COPD Foundation, along with Allergy & Asthma Network, have partnered with us to support our Lung Health Experience, a lung health expo touring Oklahoma City, Nashville, Chicago, and Toronto in 2017. The Lung Health Experience focuses on bringing lung health experts to the public in a comfortable, relaxed, and fun setting. The COPD Foundation and AAN have attended these events to provide the public with educational materials on lung diseases, which support the spirometry screenings performed by local respiratory therapists. We thank the Allergy & Asthma Network and the COPD Foundation for their outstanding support.
It is with these and many other partnerships that the CHEST Foundation is able to elevate its mission to champion lung health and provide local communities with an opportunity to interact with clinicians and physicians outside of a hospital setting. These experiences and collaborations are the key to strengthening the patient and clinician conversation and bridging the gap to improve patient care and outcomes.
The Global Impact of Respiratory Disease – Second Edition
The Global Impact of Respiratory Disease – Second Edition was released by the Forum of International Respiratory Societies (FIRS) at the World Health Assembly May 25, 2017, in Geneva, Switzerland, calling attention to the global burden of lung disease and the benefits of prevention and clean air.
We often take our breathing and our respiratory health for granted, but respiratory diseases are a leading cause of death and disability in the world. Sixty-five million people suffer from COPD, and 3 million die of it each year, now making it the third leading cause of death worldwide.1,2 Asthma affects 334 million people in the world and is the most common chronic disease of childhood.3 Pneumonia kills millions of people annually and is a leading cause of death among children under 5 years old.4 Over 10 million people develop TB, and 1.4 million die of it each year, making it the most common deadly infectious disease.5 Lung cancer kills 1.6 million people each year and is the most deadly cancer.6 Globally, at least 2 billion people are exposed to indoor toxic smoke, 1 billion inhale outdoor pollutant air, and 1 billion are exposed to tobacco smoke. Many of us, and the world, are naïve to these staggering realities.
The American College of Chest Physicians® (CHEST), together with FIRS, is working hard to change these realities. CHEST, and our more than 19,000 members around the world, want a better future, one that has less suffering. We want a future that enables and allows everyone to breathe freely.
The 2017 Global Impact of Respiratory Disease report objectively speaks to these issues and outlines an eight-step action plan to impact these serious concerns. It highlights the importance of prevention, control, and cure of these diseases and announces that promotion of respiratory health must be a top priority for health-care systems and decision-makers. In emphasizing that these goals are achievable, it also highlights the reality that the prevention and cure of respiratory diseases are among the most cost-effective health interventions available – a “best-buy” in the view of the World Health Organization (WHO). In addition to reducing so much suffering, investment in respiratory health will pay manifold dividends in longevity, healthy living days, and national economies.
Darcy Marciniuk, MD, FCCP, FRCPC, and Co-Chair of the Report notes, “The Global Impact of Respiratory Disease” report calls attention to the importance of respiratory health in the world. The report and these efforts are required to ensure respiratory health becomes a top priority in global decision-making.”
In addition to focusing attention to the importance of respiratory health in the world and ensuring it becomes a global priority, the 2017 Global Impact of Respiratory Disease report also includes practical information for our members. The report summarizes the current state of our understanding with the “Big 5”: COPD, asthma, pneumonia, lung cancer, and TB, as well as with the environment and clean air, sleep-disordered breathing, pulmonary hypertension, and pulmonary embolism. It highlights key controllable factors, such as a reduction in tobacco smoking and improvement in air quality, which includes reduction in second-hand tobacco smoke, smoke from indoor fire, and unhealthy public and workplace air. The report underlines the value of trained health-care professionals and the need for health-care systems and policies to support those trained professionals. Finally, it emphasizes the reality that investment in respiratory research is more than the hope for today – it is the promise and a genuine commitment for tomorrow. CHEST’s involvement in this important project is only one component of our global engagement and impact. We support and help to educate lung specialists and health-care teams, no matter where they live and work. Our journal CHEST®, and other education offerings, are used every day and in every part of the world. The American College of Chest Physicians® focuses on the prevention, diagnosis, and treatment of chest diseases by providing innovative education and advancing best patient outcomes around the globe.
About the Forum of International Respiratory Societies (FIRS) Formed in 2001, the Forum of International Respiratory Societies (FIRS) is composed of the leading international respiratory societies, with more than 70,000 members who devote their working lives to respiratory health and disease. The goal of FIRS is to speak with one voice in promoting respiratory health worldwide and to call for action to reduce, prevent, cure, and control the terrible burden of respiratory disease.
References
1. World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases, a comprehensive approach. 2007.
2. Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J. 2015;45(5):1239-47.
3. International Study of Asthma and Allergies in Childhood (ISAAC). Global Asthma Report. 2014.
4. World Health Organization. Pneumonia: the forgotten killer of children. Geneva: World Health Organization; 2006.
5. World Health Organization. Global Tuberculosis Report 2016. Geneva: World Health Organization; 2016.
6. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
The Global Impact of Respiratory Disease – Second Edition was released by the Forum of International Respiratory Societies (FIRS) at the World Health Assembly May 25, 2017, in Geneva, Switzerland, calling attention to the global burden of lung disease and the benefits of prevention and clean air.
We often take our breathing and our respiratory health for granted, but respiratory diseases are a leading cause of death and disability in the world. Sixty-five million people suffer from COPD, and 3 million die of it each year, now making it the third leading cause of death worldwide.1,2 Asthma affects 334 million people in the world and is the most common chronic disease of childhood.3 Pneumonia kills millions of people annually and is a leading cause of death among children under 5 years old.4 Over 10 million people develop TB, and 1.4 million die of it each year, making it the most common deadly infectious disease.5 Lung cancer kills 1.6 million people each year and is the most deadly cancer.6 Globally, at least 2 billion people are exposed to indoor toxic smoke, 1 billion inhale outdoor pollutant air, and 1 billion are exposed to tobacco smoke. Many of us, and the world, are naïve to these staggering realities.
The American College of Chest Physicians® (CHEST), together with FIRS, is working hard to change these realities. CHEST, and our more than 19,000 members around the world, want a better future, one that has less suffering. We want a future that enables and allows everyone to breathe freely.
The 2017 Global Impact of Respiratory Disease report objectively speaks to these issues and outlines an eight-step action plan to impact these serious concerns. It highlights the importance of prevention, control, and cure of these diseases and announces that promotion of respiratory health must be a top priority for health-care systems and decision-makers. In emphasizing that these goals are achievable, it also highlights the reality that the prevention and cure of respiratory diseases are among the most cost-effective health interventions available – a “best-buy” in the view of the World Health Organization (WHO). In addition to reducing so much suffering, investment in respiratory health will pay manifold dividends in longevity, healthy living days, and national economies.
Darcy Marciniuk, MD, FCCP, FRCPC, and Co-Chair of the Report notes, “The Global Impact of Respiratory Disease” report calls attention to the importance of respiratory health in the world. The report and these efforts are required to ensure respiratory health becomes a top priority in global decision-making.”
In addition to focusing attention to the importance of respiratory health in the world and ensuring it becomes a global priority, the 2017 Global Impact of Respiratory Disease report also includes practical information for our members. The report summarizes the current state of our understanding with the “Big 5”: COPD, asthma, pneumonia, lung cancer, and TB, as well as with the environment and clean air, sleep-disordered breathing, pulmonary hypertension, and pulmonary embolism. It highlights key controllable factors, such as a reduction in tobacco smoking and improvement in air quality, which includes reduction in second-hand tobacco smoke, smoke from indoor fire, and unhealthy public and workplace air. The report underlines the value of trained health-care professionals and the need for health-care systems and policies to support those trained professionals. Finally, it emphasizes the reality that investment in respiratory research is more than the hope for today – it is the promise and a genuine commitment for tomorrow. CHEST’s involvement in this important project is only one component of our global engagement and impact. We support and help to educate lung specialists and health-care teams, no matter where they live and work. Our journal CHEST®, and other education offerings, are used every day and in every part of the world. The American College of Chest Physicians® focuses on the prevention, diagnosis, and treatment of chest diseases by providing innovative education and advancing best patient outcomes around the globe.
About the Forum of International Respiratory Societies (FIRS) Formed in 2001, the Forum of International Respiratory Societies (FIRS) is composed of the leading international respiratory societies, with more than 70,000 members who devote their working lives to respiratory health and disease. The goal of FIRS is to speak with one voice in promoting respiratory health worldwide and to call for action to reduce, prevent, cure, and control the terrible burden of respiratory disease.
References
1. World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases, a comprehensive approach. 2007.
2. Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J. 2015;45(5):1239-47.
3. International Study of Asthma and Allergies in Childhood (ISAAC). Global Asthma Report. 2014.
4. World Health Organization. Pneumonia: the forgotten killer of children. Geneva: World Health Organization; 2006.
5. World Health Organization. Global Tuberculosis Report 2016. Geneva: World Health Organization; 2016.
6. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
The Global Impact of Respiratory Disease – Second Edition was released by the Forum of International Respiratory Societies (FIRS) at the World Health Assembly May 25, 2017, in Geneva, Switzerland, calling attention to the global burden of lung disease and the benefits of prevention and clean air.
We often take our breathing and our respiratory health for granted, but respiratory diseases are a leading cause of death and disability in the world. Sixty-five million people suffer from COPD, and 3 million die of it each year, now making it the third leading cause of death worldwide.1,2 Asthma affects 334 million people in the world and is the most common chronic disease of childhood.3 Pneumonia kills millions of people annually and is a leading cause of death among children under 5 years old.4 Over 10 million people develop TB, and 1.4 million die of it each year, making it the most common deadly infectious disease.5 Lung cancer kills 1.6 million people each year and is the most deadly cancer.6 Globally, at least 2 billion people are exposed to indoor toxic smoke, 1 billion inhale outdoor pollutant air, and 1 billion are exposed to tobacco smoke. Many of us, and the world, are naïve to these staggering realities.
The American College of Chest Physicians® (CHEST), together with FIRS, is working hard to change these realities. CHEST, and our more than 19,000 members around the world, want a better future, one that has less suffering. We want a future that enables and allows everyone to breathe freely.
The 2017 Global Impact of Respiratory Disease report objectively speaks to these issues and outlines an eight-step action plan to impact these serious concerns. It highlights the importance of prevention, control, and cure of these diseases and announces that promotion of respiratory health must be a top priority for health-care systems and decision-makers. In emphasizing that these goals are achievable, it also highlights the reality that the prevention and cure of respiratory diseases are among the most cost-effective health interventions available – a “best-buy” in the view of the World Health Organization (WHO). In addition to reducing so much suffering, investment in respiratory health will pay manifold dividends in longevity, healthy living days, and national economies.
Darcy Marciniuk, MD, FCCP, FRCPC, and Co-Chair of the Report notes, “The Global Impact of Respiratory Disease” report calls attention to the importance of respiratory health in the world. The report and these efforts are required to ensure respiratory health becomes a top priority in global decision-making.”
In addition to focusing attention to the importance of respiratory health in the world and ensuring it becomes a global priority, the 2017 Global Impact of Respiratory Disease report also includes practical information for our members. The report summarizes the current state of our understanding with the “Big 5”: COPD, asthma, pneumonia, lung cancer, and TB, as well as with the environment and clean air, sleep-disordered breathing, pulmonary hypertension, and pulmonary embolism. It highlights key controllable factors, such as a reduction in tobacco smoking and improvement in air quality, which includes reduction in second-hand tobacco smoke, smoke from indoor fire, and unhealthy public and workplace air. The report underlines the value of trained health-care professionals and the need for health-care systems and policies to support those trained professionals. Finally, it emphasizes the reality that investment in respiratory research is more than the hope for today – it is the promise and a genuine commitment for tomorrow. CHEST’s involvement in this important project is only one component of our global engagement and impact. We support and help to educate lung specialists and health-care teams, no matter where they live and work. Our journal CHEST®, and other education offerings, are used every day and in every part of the world. The American College of Chest Physicians® focuses on the prevention, diagnosis, and treatment of chest diseases by providing innovative education and advancing best patient outcomes around the globe.
About the Forum of International Respiratory Societies (FIRS) Formed in 2001, the Forum of International Respiratory Societies (FIRS) is composed of the leading international respiratory societies, with more than 70,000 members who devote their working lives to respiratory health and disease. The goal of FIRS is to speak with one voice in promoting respiratory health worldwide and to call for action to reduce, prevent, cure, and control the terrible burden of respiratory disease.
References
1. World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases, a comprehensive approach. 2007.
2. Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J. 2015;45(5):1239-47.
3. International Study of Asthma and Allergies in Childhood (ISAAC). Global Asthma Report. 2014.
4. World Health Organization. Pneumonia: the forgotten killer of children. Geneva: World Health Organization; 2006.
5. World Health Organization. Global Tuberculosis Report 2016. Geneva: World Health Organization; 2016.
6. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
Patients’ surrogate decision-makers face stress
WASHINGTON – Making health care–related decisions for relatives in the ICU heightens an individual’s risk for psychological stress, a new study showed.
While 90% of patients admitted to the ICU lack decision capacity, only 20%-29% of the U.S. population has written advanced directives. Therefore, most often, family members are called upon to make decisions for ICU patients’ care.
Sean Cohen, MD, of the University of Washington presented the study’s results in a mini symposium at an international conference of the American Thoracic Society in May.
In this cohort study, 265 patients with acute respiratory distress syndrome and 162 of these patients’ family members were surveyed 3 months after each patient’s discharge. Associations between the family members’ roles and psychological symptoms were examined with regression models. These models were adjusted for family characteristics, which included sex, age, education, and legal next-of-kin status. Results showed that the patients’ family members were on average 52 years of age and that 94% were white, 73% were female, and 64% were legal next-of-kin, mostly spouses or children of the patients.
“I think looking more at identification of the decision-making role and attempting to go toward the surrogates’ preferred role actually mitigates some of these psychological symptoms,” concluded Dr. Cohen.
This study was sponsored by the Cambia Palliative Care Center of Excellence at the University of Washington in Seattle. Funding was provided by the National Institutes of Health and the American Lung Association. The authors reported no other disclosures.
WASHINGTON – Making health care–related decisions for relatives in the ICU heightens an individual’s risk for psychological stress, a new study showed.
While 90% of patients admitted to the ICU lack decision capacity, only 20%-29% of the U.S. population has written advanced directives. Therefore, most often, family members are called upon to make decisions for ICU patients’ care.
Sean Cohen, MD, of the University of Washington presented the study’s results in a mini symposium at an international conference of the American Thoracic Society in May.
In this cohort study, 265 patients with acute respiratory distress syndrome and 162 of these patients’ family members were surveyed 3 months after each patient’s discharge. Associations between the family members’ roles and psychological symptoms were examined with regression models. These models were adjusted for family characteristics, which included sex, age, education, and legal next-of-kin status. Results showed that the patients’ family members were on average 52 years of age and that 94% were white, 73% were female, and 64% were legal next-of-kin, mostly spouses or children of the patients.
“I think looking more at identification of the decision-making role and attempting to go toward the surrogates’ preferred role actually mitigates some of these psychological symptoms,” concluded Dr. Cohen.
This study was sponsored by the Cambia Palliative Care Center of Excellence at the University of Washington in Seattle. Funding was provided by the National Institutes of Health and the American Lung Association. The authors reported no other disclosures.
WASHINGTON – Making health care–related decisions for relatives in the ICU heightens an individual’s risk for psychological stress, a new study showed.
While 90% of patients admitted to the ICU lack decision capacity, only 20%-29% of the U.S. population has written advanced directives. Therefore, most often, family members are called upon to make decisions for ICU patients’ care.
Sean Cohen, MD, of the University of Washington presented the study’s results in a mini symposium at an international conference of the American Thoracic Society in May.
In this cohort study, 265 patients with acute respiratory distress syndrome and 162 of these patients’ family members were surveyed 3 months after each patient’s discharge. Associations between the family members’ roles and psychological symptoms were examined with regression models. These models were adjusted for family characteristics, which included sex, age, education, and legal next-of-kin status. Results showed that the patients’ family members were on average 52 years of age and that 94% were white, 73% were female, and 64% were legal next-of-kin, mostly spouses or children of the patients.
“I think looking more at identification of the decision-making role and attempting to go toward the surrogates’ preferred role actually mitigates some of these psychological symptoms,” concluded Dr. Cohen.
This study was sponsored by the Cambia Palliative Care Center of Excellence at the University of Washington in Seattle. Funding was provided by the National Institutes of Health and the American Lung Association. The authors reported no other disclosures.
AT ATS 2017
Key clinical point:
Major finding: Patients’ family members’ active involvement in the health care decision-making process are more likely to experience symptoms of PTSD (OR, 4.43), depression (OR, 1.80), and anxiety (OR, 1.11; P less .05 for all).
Data source: Cohort study of 265 patients with ARDS and 162 family members of the patients.
Disclosures: This study was sponsored by the Cambia Palliative Care Center of Excellence at the University of Washington in Seattle. Funding was provided by the National Institutes of Health and the American Lung Association. The authors reported no other financial disclosures.