User login
FDA approves daratumumab-POM-Dex combo for MM
The US Food and Drug Administration (FDA) has approved daratumumab (Darzalex®) in combination with pomalidomide (POM) and dexamethasone (dex) for the treatment of multiple myeloma (MM) in patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor (PI).
The FDA previously approved daratumumab in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with MM who had at least 1 prior therapy.
Daratumumab is also approved by the FDA as monotherapy in MM patients who had at least 3 prior lines of therapy, including a PI and an immunomodulatory (IMiD) agent, or who are double refractory to a PI and an IMiD.
The latest indication is based on the results of the phase 1b MMY1001 EQUULEUS study, which demonstrated that daratumumab produced an overall response (OR) rate of 59% in combination with pomalidomide and dexamethasone, and a very good partial response (VGPR) in 28% of patients.
EQUULEUS study
The daratumumab-POM-Dex arm of the phase 1 open-label EQUULEUS study included 103 MM patients who had received prior treatment with a PI and an immunomodulatory agent.
Patients were a median age of 64 years, and 8% were older than 75.
They had received a median of 4 prior lines of therapy, and 74% had received prior autologous stem cell transplant.
Most (89%) were refractory to lenalidomide and 71% were refractory to bortezomib. Almost two thirds (64%) were refractory to bortezomib and lenalidomide.
Patients were treated with 16 mg/kg of daratumumab in combination with POM and Dex, and 6% achieved a complete response (CR) and 8% achieved a stringent CR.
The median time to response was 1 month (range, 0.9 to 2.8), and the median duration of response was 13.6 months (range, 0.9+ to 14.6+ months).
The most frequent adverse events (AEs) reported in more than 20% of patients were infusion reactions, fatigue, and upper respiratory tract infections (50% each), cough (43%), diarrhea (38%), dyspnea (33%), nausea (30%), muscle spasms (26%), pyrexia (25%), and vomiting (21%).
The overall incidence of serious adverse reactions was 49%.
Grade 3/4 serious AEs reported in 5% of patients or more included pneumonia (7%).
The most common treatment-emergent hematologic laboratory abnormalities included lymphopenia (94%), neutropenia (95%), thrombocytopenia (75%), and anemia (57%).
And the most common grade 3/4 treatment-emergent hematology laboratory abnormalities were neutropenia (82%), lymphopenia (71%), anemia (30%), and thrombocytopenia (20%).
Daratumumab is being developed by Janssen Biotech, Inc., under an exclusive worldwide license to develop, manufacture, and commercialize daratumumab from Genmab.
See the package insert for full prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved daratumumab (Darzalex®) in combination with pomalidomide (POM) and dexamethasone (dex) for the treatment of multiple myeloma (MM) in patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor (PI).
The FDA previously approved daratumumab in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with MM who had at least 1 prior therapy.
Daratumumab is also approved by the FDA as monotherapy in MM patients who had at least 3 prior lines of therapy, including a PI and an immunomodulatory (IMiD) agent, or who are double refractory to a PI and an IMiD.
The latest indication is based on the results of the phase 1b MMY1001 EQUULEUS study, which demonstrated that daratumumab produced an overall response (OR) rate of 59% in combination with pomalidomide and dexamethasone, and a very good partial response (VGPR) in 28% of patients.
EQUULEUS study
The daratumumab-POM-Dex arm of the phase 1 open-label EQUULEUS study included 103 MM patients who had received prior treatment with a PI and an immunomodulatory agent.
Patients were a median age of 64 years, and 8% were older than 75.
They had received a median of 4 prior lines of therapy, and 74% had received prior autologous stem cell transplant.
Most (89%) were refractory to lenalidomide and 71% were refractory to bortezomib. Almost two thirds (64%) were refractory to bortezomib and lenalidomide.
Patients were treated with 16 mg/kg of daratumumab in combination with POM and Dex, and 6% achieved a complete response (CR) and 8% achieved a stringent CR.
The median time to response was 1 month (range, 0.9 to 2.8), and the median duration of response was 13.6 months (range, 0.9+ to 14.6+ months).
The most frequent adverse events (AEs) reported in more than 20% of patients were infusion reactions, fatigue, and upper respiratory tract infections (50% each), cough (43%), diarrhea (38%), dyspnea (33%), nausea (30%), muscle spasms (26%), pyrexia (25%), and vomiting (21%).
The overall incidence of serious adverse reactions was 49%.
Grade 3/4 serious AEs reported in 5% of patients or more included pneumonia (7%).
The most common treatment-emergent hematologic laboratory abnormalities included lymphopenia (94%), neutropenia (95%), thrombocytopenia (75%), and anemia (57%).
And the most common grade 3/4 treatment-emergent hematology laboratory abnormalities were neutropenia (82%), lymphopenia (71%), anemia (30%), and thrombocytopenia (20%).
Daratumumab is being developed by Janssen Biotech, Inc., under an exclusive worldwide license to develop, manufacture, and commercialize daratumumab from Genmab.
See the package insert for full prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved daratumumab (Darzalex®) in combination with pomalidomide (POM) and dexamethasone (dex) for the treatment of multiple myeloma (MM) in patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor (PI).
The FDA previously approved daratumumab in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with MM who had at least 1 prior therapy.
Daratumumab is also approved by the FDA as monotherapy in MM patients who had at least 3 prior lines of therapy, including a PI and an immunomodulatory (IMiD) agent, or who are double refractory to a PI and an IMiD.
The latest indication is based on the results of the phase 1b MMY1001 EQUULEUS study, which demonstrated that daratumumab produced an overall response (OR) rate of 59% in combination with pomalidomide and dexamethasone, and a very good partial response (VGPR) in 28% of patients.
EQUULEUS study
The daratumumab-POM-Dex arm of the phase 1 open-label EQUULEUS study included 103 MM patients who had received prior treatment with a PI and an immunomodulatory agent.
Patients were a median age of 64 years, and 8% were older than 75.
They had received a median of 4 prior lines of therapy, and 74% had received prior autologous stem cell transplant.
Most (89%) were refractory to lenalidomide and 71% were refractory to bortezomib. Almost two thirds (64%) were refractory to bortezomib and lenalidomide.
Patients were treated with 16 mg/kg of daratumumab in combination with POM and Dex, and 6% achieved a complete response (CR) and 8% achieved a stringent CR.
The median time to response was 1 month (range, 0.9 to 2.8), and the median duration of response was 13.6 months (range, 0.9+ to 14.6+ months).
The most frequent adverse events (AEs) reported in more than 20% of patients were infusion reactions, fatigue, and upper respiratory tract infections (50% each), cough (43%), diarrhea (38%), dyspnea (33%), nausea (30%), muscle spasms (26%), pyrexia (25%), and vomiting (21%).
The overall incidence of serious adverse reactions was 49%.
Grade 3/4 serious AEs reported in 5% of patients or more included pneumonia (7%).
The most common treatment-emergent hematologic laboratory abnormalities included lymphopenia (94%), neutropenia (95%), thrombocytopenia (75%), and anemia (57%).
And the most common grade 3/4 treatment-emergent hematology laboratory abnormalities were neutropenia (82%), lymphopenia (71%), anemia (30%), and thrombocytopenia (20%).
Daratumumab is being developed by Janssen Biotech, Inc., under an exclusive worldwide license to develop, manufacture, and commercialize daratumumab from Genmab.
See the package insert for full prescribing information. ![]()
Large MM trial finds denosumab non-inferior to ZA for SRE
CHICAGO—The largest international multiple myeloma (MM) trial ever conducted, according to the trial sponsor, met its primary endpoint, demonstrating that denosumab is non-inferior to zoledronic acid (ZA) in delaying the time to first on-study skeletal-related event (SRE) in patients with MM.
In addition to bone-specific benefits, denosumab-treated patients had significantly fewer renal adverse events and possible prolongation of progression-free survival.
Denosumab “may in fact be a new standard of care for multiple myeloma-related bone disease,” according to one of the investigators.
“The other important thing to note,” Noopur S. Raje, MD, said during her presentation at the ASCO 2017 Annual Meeting, “is denosumab can be administered despite renal function in patients with myeloma.” It does not need to be dose-adjusted, unlike bisphosphonates.
Dr Raje, of the Massachusetts General Hospital Cancer Center in Boston, Massachusetts, presented the study results as abstract 8005.
Study design
The international, phase 3, randomized, double-blind study is evaluating the safety of denosumab compared with ZA in newly diagnosed MM patients.
Investigators enrolled 1718 patients from 259 sites and 29 countries.
They randomized 859 patients to receive denosumab 120 mg subcutaneously every 4 weeks plus intravenous placebo every 4 weeks, and 859 patients to the standard ZA dose of 4 mg intravenously plus subcutaneous placebo every 4 weeks.
Patients were stratified by whether they were on novel-based anti-myeloma therapy, whether they planned to have an autologous peripheral blood stem cell (PBSC) transplant, disease stage, and previous SRE.
“We were looking for 676 on-study SREs, and if we saw a benefit, patients would be offered open-label denosumab for up to 2 years after this,” Dr Raje said.
“Patients had to have radiographic evidence of bone disease, and this is different from some of the other bone disease studies that you’ve seen in the recent past,” she added.
In addition to documented evidence of MM, patients had to be 18 years or older, be ECOG status of 2 or better, have adequate organ function, and plan to receive or be receiving primary frontline anti-myeloma therapy.
Patients were excluded if they had nonsecretory MM, more than 30 days of previous treatment with anti-myeloma therapy prior to screening, prior use of denosumab, use of oral bisphosphonates with a cumulative dose of more than 1 year, more than 1 previous dose of intravenous bisphosphonate, or prior history or current evidence of osteonecrosis/osteomyelitis of the jaw.
The primary endpoint was time to first on-study SRE, “and the idea here was to look for non-inferiority,” Dr Raje explained.
Secondary endpoints included time to the first on-study SRE (superiority), time to the first-and-subsequent on-study SRE (superiority), and overall survival.
Investigators also included the exploratory objective of progression-free survival (PFS).
Patient demographics
Patients were well balanced across the 2 arms, Dr Raje noted, and the breakdown of myeloma disease stage at diagnosis was comparable between the ZA and denosumab arms.
About 32% of patients were stage I, 37% stage II, and 29% stage III. Stage was not available for 49 patients.
A little more than half (54%) were male, mean age was 63 years, and 82% were white.
Two thirds had prior SRE history, and 54% of patients intended to undergo autologous PBSC transplant.
Enrollment began May 2012 and continued through the end of March 2016. The primary analysis cutoff was July 19, 2016.
Results
The primary endpoint for non-inferiority for time to first on-study SRE was met by denosumab (HR=0.98, 95%CI: 0.85, 1.14; P=0.01).
“When we looked at the secondary endpoints for superiority, we were not able to confirm superiority in this analysis, either for time to first SRE or time to first-and subsequent SRE on this study,” Dr Raje said.
The investigators also did not observe a survival difference between denosumab and ZA, with a hazard ratio (HR) (95% CI) of 0.90 (0.70, 1.16), P=0.41.
“Importantly, we had an exploratory endpoint where we looked at progression-free survival in this newly diagnosed patient population,” she added, “and we saw an interestingly increased or prolonged progression-free survival in patients getting denosumab.”
“And that survival difference was more than 10 months between denosumab and zoledronic acid, favoring the denosumab arm,” she affirmed. The HR was 0.82, 95% CI: 0.68, 0.99, P=0.036 (descriptive).
Safety
“[I]f you look at all treatment-emergent adverse events between denosumab and zoledronic acid, we really could not find a big difference in either of these 2 groups of patients,” Dr Raje said.
“We saw that in general both denosumab and zoledronic acid were extremely well tolerated between the 2 groups of patients.”
The investigators “drilled down” on certain toxicity issues of interest and examined events such as atypical stress fractures, hypersensitivity reactions, musculoskeletal pain, infections and infestations, new primary malignancies, and acute phase reactions.
They observed no atypical femur fractures on the study, nor did they see any big differences with respect to hypersensitivity or acute phase reactions.
The investigators examined closely any renal issues because dosing of ZA specifically is impacted by renal function.
The data showed that treatment-emergent adverse event (TEAE) renal toxicity was significantly higher in the ZA group compared to the denosumab group, 17% and 10%, respectively (P<0.001).
“When you look at patients who had a creatinine clearance less than 60 mL per minute,” Dr Raje emphasized, “we saw an almost doubling of renal toxicity in the zoledronic acid arm (26.4%) compared to the denosumab arm (12.9%).”
Patients with a creatinine level greater than 2 mg/dL had a significant increase in creatinine in the ZA arm (P=0.010), which was also significantly increased if their creatinine clearance was less than 60 mL/minute (P=0.054).
“There was a doubling of creatinine from baseline, more so in the zoledronic acid arm compared to the patients with denosumab,” Dr Raje said. “And this was again more pronounced if you had a creatinine clearance of less than 60.”
Hypocalcemia was “not surprisingly” more common in the denosumab arm than the ZA arm (P=0.009) for all patients, and osteonecrosis of the jaw was equal in both arms (P=0.147), although numerically slightly higher with denosumab treatment.
Dr Raje summarized that there was no difference in overall survival at the time of this analysis, “but I will say that the follow-up for a newly diagnosed patient population is fairly short right now.”
“Progression-free survival, which we saw [cut] off 10.7 months, was actually quite striking when denosumab was compared to zoledronic acid, and this was statistically highly significant.”
“The bone-specific benefits in combination with significantly fewer renal adverse events and possible prolongation of PFS with denosumab therapy we do think is very promising,” she said, “and may in fact be a new standard of care for multiple myeloma-related bone disease.”
The study was funded by Amgen Inc.
Denosumab (XGEVA®) is indicated by the US Food and Drug Administration for the prevention of fractures and other SREs in patients with bone metastases from solid tumors. It is currently not indicated for the prevention of SREs in patients with MM. ![]()
CHICAGO—The largest international multiple myeloma (MM) trial ever conducted, according to the trial sponsor, met its primary endpoint, demonstrating that denosumab is non-inferior to zoledronic acid (ZA) in delaying the time to first on-study skeletal-related event (SRE) in patients with MM.
In addition to bone-specific benefits, denosumab-treated patients had significantly fewer renal adverse events and possible prolongation of progression-free survival.
Denosumab “may in fact be a new standard of care for multiple myeloma-related bone disease,” according to one of the investigators.
“The other important thing to note,” Noopur S. Raje, MD, said during her presentation at the ASCO 2017 Annual Meeting, “is denosumab can be administered despite renal function in patients with myeloma.” It does not need to be dose-adjusted, unlike bisphosphonates.
Dr Raje, of the Massachusetts General Hospital Cancer Center in Boston, Massachusetts, presented the study results as abstract 8005.
Study design
The international, phase 3, randomized, double-blind study is evaluating the safety of denosumab compared with ZA in newly diagnosed MM patients.
Investigators enrolled 1718 patients from 259 sites and 29 countries.
They randomized 859 patients to receive denosumab 120 mg subcutaneously every 4 weeks plus intravenous placebo every 4 weeks, and 859 patients to the standard ZA dose of 4 mg intravenously plus subcutaneous placebo every 4 weeks.
Patients were stratified by whether they were on novel-based anti-myeloma therapy, whether they planned to have an autologous peripheral blood stem cell (PBSC) transplant, disease stage, and previous SRE.
“We were looking for 676 on-study SREs, and if we saw a benefit, patients would be offered open-label denosumab for up to 2 years after this,” Dr Raje said.
“Patients had to have radiographic evidence of bone disease, and this is different from some of the other bone disease studies that you’ve seen in the recent past,” she added.
In addition to documented evidence of MM, patients had to be 18 years or older, be ECOG status of 2 or better, have adequate organ function, and plan to receive or be receiving primary frontline anti-myeloma therapy.
Patients were excluded if they had nonsecretory MM, more than 30 days of previous treatment with anti-myeloma therapy prior to screening, prior use of denosumab, use of oral bisphosphonates with a cumulative dose of more than 1 year, more than 1 previous dose of intravenous bisphosphonate, or prior history or current evidence of osteonecrosis/osteomyelitis of the jaw.
The primary endpoint was time to first on-study SRE, “and the idea here was to look for non-inferiority,” Dr Raje explained.
Secondary endpoints included time to the first on-study SRE (superiority), time to the first-and-subsequent on-study SRE (superiority), and overall survival.
Investigators also included the exploratory objective of progression-free survival (PFS).
Patient demographics
Patients were well balanced across the 2 arms, Dr Raje noted, and the breakdown of myeloma disease stage at diagnosis was comparable between the ZA and denosumab arms.
About 32% of patients were stage I, 37% stage II, and 29% stage III. Stage was not available for 49 patients.
A little more than half (54%) were male, mean age was 63 years, and 82% were white.
Two thirds had prior SRE history, and 54% of patients intended to undergo autologous PBSC transplant.
Enrollment began May 2012 and continued through the end of March 2016. The primary analysis cutoff was July 19, 2016.
Results
The primary endpoint for non-inferiority for time to first on-study SRE was met by denosumab (HR=0.98, 95%CI: 0.85, 1.14; P=0.01).
“When we looked at the secondary endpoints for superiority, we were not able to confirm superiority in this analysis, either for time to first SRE or time to first-and subsequent SRE on this study,” Dr Raje said.
The investigators also did not observe a survival difference between denosumab and ZA, with a hazard ratio (HR) (95% CI) of 0.90 (0.70, 1.16), P=0.41.
“Importantly, we had an exploratory endpoint where we looked at progression-free survival in this newly diagnosed patient population,” she added, “and we saw an interestingly increased or prolonged progression-free survival in patients getting denosumab.”
“And that survival difference was more than 10 months between denosumab and zoledronic acid, favoring the denosumab arm,” she affirmed. The HR was 0.82, 95% CI: 0.68, 0.99, P=0.036 (descriptive).
Safety
“[I]f you look at all treatment-emergent adverse events between denosumab and zoledronic acid, we really could not find a big difference in either of these 2 groups of patients,” Dr Raje said.
“We saw that in general both denosumab and zoledronic acid were extremely well tolerated between the 2 groups of patients.”
The investigators “drilled down” on certain toxicity issues of interest and examined events such as atypical stress fractures, hypersensitivity reactions, musculoskeletal pain, infections and infestations, new primary malignancies, and acute phase reactions.
They observed no atypical femur fractures on the study, nor did they see any big differences with respect to hypersensitivity or acute phase reactions.
The investigators examined closely any renal issues because dosing of ZA specifically is impacted by renal function.
The data showed that treatment-emergent adverse event (TEAE) renal toxicity was significantly higher in the ZA group compared to the denosumab group, 17% and 10%, respectively (P<0.001).
“When you look at patients who had a creatinine clearance less than 60 mL per minute,” Dr Raje emphasized, “we saw an almost doubling of renal toxicity in the zoledronic acid arm (26.4%) compared to the denosumab arm (12.9%).”
Patients with a creatinine level greater than 2 mg/dL had a significant increase in creatinine in the ZA arm (P=0.010), which was also significantly increased if their creatinine clearance was less than 60 mL/minute (P=0.054).
“There was a doubling of creatinine from baseline, more so in the zoledronic acid arm compared to the patients with denosumab,” Dr Raje said. “And this was again more pronounced if you had a creatinine clearance of less than 60.”
Hypocalcemia was “not surprisingly” more common in the denosumab arm than the ZA arm (P=0.009) for all patients, and osteonecrosis of the jaw was equal in both arms (P=0.147), although numerically slightly higher with denosumab treatment.
Dr Raje summarized that there was no difference in overall survival at the time of this analysis, “but I will say that the follow-up for a newly diagnosed patient population is fairly short right now.”
“Progression-free survival, which we saw [cut] off 10.7 months, was actually quite striking when denosumab was compared to zoledronic acid, and this was statistically highly significant.”
“The bone-specific benefits in combination with significantly fewer renal adverse events and possible prolongation of PFS with denosumab therapy we do think is very promising,” she said, “and may in fact be a new standard of care for multiple myeloma-related bone disease.”
The study was funded by Amgen Inc.
Denosumab (XGEVA®) is indicated by the US Food and Drug Administration for the prevention of fractures and other SREs in patients with bone metastases from solid tumors. It is currently not indicated for the prevention of SREs in patients with MM. ![]()
CHICAGO—The largest international multiple myeloma (MM) trial ever conducted, according to the trial sponsor, met its primary endpoint, demonstrating that denosumab is non-inferior to zoledronic acid (ZA) in delaying the time to first on-study skeletal-related event (SRE) in patients with MM.
In addition to bone-specific benefits, denosumab-treated patients had significantly fewer renal adverse events and possible prolongation of progression-free survival.
Denosumab “may in fact be a new standard of care for multiple myeloma-related bone disease,” according to one of the investigators.
“The other important thing to note,” Noopur S. Raje, MD, said during her presentation at the ASCO 2017 Annual Meeting, “is denosumab can be administered despite renal function in patients with myeloma.” It does not need to be dose-adjusted, unlike bisphosphonates.
Dr Raje, of the Massachusetts General Hospital Cancer Center in Boston, Massachusetts, presented the study results as abstract 8005.
Study design
The international, phase 3, randomized, double-blind study is evaluating the safety of denosumab compared with ZA in newly diagnosed MM patients.
Investigators enrolled 1718 patients from 259 sites and 29 countries.
They randomized 859 patients to receive denosumab 120 mg subcutaneously every 4 weeks plus intravenous placebo every 4 weeks, and 859 patients to the standard ZA dose of 4 mg intravenously plus subcutaneous placebo every 4 weeks.
Patients were stratified by whether they were on novel-based anti-myeloma therapy, whether they planned to have an autologous peripheral blood stem cell (PBSC) transplant, disease stage, and previous SRE.
“We were looking for 676 on-study SREs, and if we saw a benefit, patients would be offered open-label denosumab for up to 2 years after this,” Dr Raje said.
“Patients had to have radiographic evidence of bone disease, and this is different from some of the other bone disease studies that you’ve seen in the recent past,” she added.
In addition to documented evidence of MM, patients had to be 18 years or older, be ECOG status of 2 or better, have adequate organ function, and plan to receive or be receiving primary frontline anti-myeloma therapy.
Patients were excluded if they had nonsecretory MM, more than 30 days of previous treatment with anti-myeloma therapy prior to screening, prior use of denosumab, use of oral bisphosphonates with a cumulative dose of more than 1 year, more than 1 previous dose of intravenous bisphosphonate, or prior history or current evidence of osteonecrosis/osteomyelitis of the jaw.
The primary endpoint was time to first on-study SRE, “and the idea here was to look for non-inferiority,” Dr Raje explained.
Secondary endpoints included time to the first on-study SRE (superiority), time to the first-and-subsequent on-study SRE (superiority), and overall survival.
Investigators also included the exploratory objective of progression-free survival (PFS).
Patient demographics
Patients were well balanced across the 2 arms, Dr Raje noted, and the breakdown of myeloma disease stage at diagnosis was comparable between the ZA and denosumab arms.
About 32% of patients were stage I, 37% stage II, and 29% stage III. Stage was not available for 49 patients.
A little more than half (54%) were male, mean age was 63 years, and 82% were white.
Two thirds had prior SRE history, and 54% of patients intended to undergo autologous PBSC transplant.
Enrollment began May 2012 and continued through the end of March 2016. The primary analysis cutoff was July 19, 2016.
Results
The primary endpoint for non-inferiority for time to first on-study SRE was met by denosumab (HR=0.98, 95%CI: 0.85, 1.14; P=0.01).
“When we looked at the secondary endpoints for superiority, we were not able to confirm superiority in this analysis, either for time to first SRE or time to first-and subsequent SRE on this study,” Dr Raje said.
The investigators also did not observe a survival difference between denosumab and ZA, with a hazard ratio (HR) (95% CI) of 0.90 (0.70, 1.16), P=0.41.
“Importantly, we had an exploratory endpoint where we looked at progression-free survival in this newly diagnosed patient population,” she added, “and we saw an interestingly increased or prolonged progression-free survival in patients getting denosumab.”
“And that survival difference was more than 10 months between denosumab and zoledronic acid, favoring the denosumab arm,” she affirmed. The HR was 0.82, 95% CI: 0.68, 0.99, P=0.036 (descriptive).
Safety
“[I]f you look at all treatment-emergent adverse events between denosumab and zoledronic acid, we really could not find a big difference in either of these 2 groups of patients,” Dr Raje said.
“We saw that in general both denosumab and zoledronic acid were extremely well tolerated between the 2 groups of patients.”
The investigators “drilled down” on certain toxicity issues of interest and examined events such as atypical stress fractures, hypersensitivity reactions, musculoskeletal pain, infections and infestations, new primary malignancies, and acute phase reactions.
They observed no atypical femur fractures on the study, nor did they see any big differences with respect to hypersensitivity or acute phase reactions.
The investigators examined closely any renal issues because dosing of ZA specifically is impacted by renal function.
The data showed that treatment-emergent adverse event (TEAE) renal toxicity was significantly higher in the ZA group compared to the denosumab group, 17% and 10%, respectively (P<0.001).
“When you look at patients who had a creatinine clearance less than 60 mL per minute,” Dr Raje emphasized, “we saw an almost doubling of renal toxicity in the zoledronic acid arm (26.4%) compared to the denosumab arm (12.9%).”
Patients with a creatinine level greater than 2 mg/dL had a significant increase in creatinine in the ZA arm (P=0.010), which was also significantly increased if their creatinine clearance was less than 60 mL/minute (P=0.054).
“There was a doubling of creatinine from baseline, more so in the zoledronic acid arm compared to the patients with denosumab,” Dr Raje said. “And this was again more pronounced if you had a creatinine clearance of less than 60.”
Hypocalcemia was “not surprisingly” more common in the denosumab arm than the ZA arm (P=0.009) for all patients, and osteonecrosis of the jaw was equal in both arms (P=0.147), although numerically slightly higher with denosumab treatment.
Dr Raje summarized that there was no difference in overall survival at the time of this analysis, “but I will say that the follow-up for a newly diagnosed patient population is fairly short right now.”
“Progression-free survival, which we saw [cut] off 10.7 months, was actually quite striking when denosumab was compared to zoledronic acid, and this was statistically highly significant.”
“The bone-specific benefits in combination with significantly fewer renal adverse events and possible prolongation of PFS with denosumab therapy we do think is very promising,” she said, “and may in fact be a new standard of care for multiple myeloma-related bone disease.”
The study was funded by Amgen Inc.
Denosumab (XGEVA®) is indicated by the US Food and Drug Administration for the prevention of fractures and other SREs in patients with bone metastases from solid tumors. It is currently not indicated for the prevention of SREs in patients with MM. ![]()
Child firearm suicide at highest rate in more than a decade
Boys, older children, and minorities are disproportionately affected when it comes to firearm injuries and deaths in U.S. children and adolescents, and child firearm suicide rates are at the highest they have been in more than a decade, new study results revealed.
Around 19 children are either medically treated for a gunshot wound or killed by one every day in the United States. “The majority of these children are boys 13-17 years old, African American in the case of firearm homicide, and white and American Indian in the case of firearm suicide. Pediatric firearm injuries and deaths are an important public health problem in the United States, contributing substantially each year to premature death, illness, and disability of children,” said Katherine A. Fowler, PhD, of the National Center for Injury Prevention and Control, Atlanta, and her associates. “Finding ways to prevent such injuries and ensure that all children have safe, stable, nurturing relationships and environments remains one of our most important priorities” (Pediatrics. 2017;140(1):e20163486).
“From 2012 to 2014, the average annual case fatality rate was 74% for firearm-related self-harm, 14% for firearm-related assaults, and 6% for unintentional firearm injuries,” the investigators reported.
Boys accounted for 82% of all child firearm deaths from 2012 to 2014. In this time period, the annual rate of firearm death for boys was 4.5 times higher than the annual rate for girls (2.8 vs. 0.6 per 100,000). This difference was even more pronounced by age, with the rate for 13- to 17-year-old boys being six times higher than the rate for same-aged girls. Similarly, boys suffer the majority of nonfatal firearm injuries treated in U.S. emergency departments, accounting for 84% of all nonfatal firearm injuries medically treated each year from 2012 to 2014. The average annual rate of nonfatal firearm injuries for boys was five times the rate for girls at 13 vs. 3 per 100,000.
The annual rate of firearm homicide was 10 times higher among 13- to 17-year-olds versus 0- to 12-year-olds (3 vs. 0.3 per 100,000). Unintentional firearm death rates were approximately twice as high when comparing these two groups (0.2 vs. 0.1 per 100,000).
Dr. Fowler and her associates wrote, “Our findings indicate that most children who died of unintentional firearm injuries were shot by another child in their own age range and most often in the context of playing with a gun or showing it to others. More than one-third of the deaths of older children occurred in incidents in which the shooter thought that the gun was unloaded or thought that the safety was engaged.”
“Child firearm suicide rates showed a significant upward trend between 2007 and 2014, increasing 60% from 1.0 to 1.6 (P less than .05) to the highest rate seen over the period examined,” Dr. Fowler and her associates said.
Firearm suicide rates were 11 times higher among 13- to 17-year-olds vs. 10- to 12-year-olds (2 vs. 0.2 per 100,000). Older children also accounted for 88% of all nonfatal firearm injuries treated in an ED. The overall average annual rate of nonfatal firearm injuries for older children was 19 times that of younger children (24 vs. 1 per 100,000).
The annual firearm homicide rate for African American children was nearly 10 times higher than the rate for white children (4 vs. 0.4 per 100,000). However, the annual rate of firearm suicide among white children was nearly four times higher than the rate for African American children (2. vs. 0.6 per 100,000).
Awareness of the availability of firearms during times of crisis is crucial because suicides are often impulsive in young people, Dr. Fowler and her associates said, “with previous findings indicating that many who attempt suicide spend 10 minutes or less deliberating. Safe storage practices (i.e., unloading and locking all firearms and ammunition) can potentially be lifesaving in these instances,” as the results of previous studies in this age group attest.
Firearm deaths are the third leading cause of death overall among children in the United States aged 1-17 years, beating pediatric congenital anomalies, heart disease, influenza/pneumonia, chronic lower respiratory disease, and cerebrovascular causes. Understanding the nature, scale, and impact of firearm violence against children is an important first step, concluded Dr. Fowler and her associates.
Dr. Fowler and her associates said they had no relevant financial disclosures.
Dr. Fowler’s study brings a lot of uncomfortable statistics to light. Subsequently we are left wondering, What can we do about it? With firearm injury mortality rates in children increasing over the past 2 years according to the CDC’s Web-based Injury Statistics Query and Reporting System, it is crucial that we continue to demand the appropriate support for this public health crisis.
Recognizing the prevalence of guns in homes and the potential dangers of easy access to them makes it essential to ask about firearms as part of our injury prevention guidance and offer counseling to parents and children about safe gun storage, gun locks, or support for legislation to protect children from gun violence.
It is important to engage gun owners about gun safety and “point out that parents may underestimate kids’ propensity to handle guns unsafely, even when they’ve been taught,” as well as the “impulsivity, risk-taking, and unpredictability of adolescence. ... We pediatric clinicians tend not to discuss firearm injury prevention much,” but a recent review of the problem offers some excellent guidance that “may help us to do more,” according to Dr. Nelson (Adolesc Med State Art Rev. 2016;27[2]:323-40).
However problematic it may be to confront the problem of firearm injuries in children and adolescents, we cannot ignore the scale of this current epidemic.
Eliot W. Nelson, MD, is a pediatrician at the University of Vermont Children’s Hospital. He reported having no relevant financial disclosures. These remarks are adapted from an editorial accompanying the report by Fowler et al. (Pediatrics. 2017. doi: 10.1542/peds.2017-1300).
Dr. Fowler’s study brings a lot of uncomfortable statistics to light. Subsequently we are left wondering, What can we do about it? With firearm injury mortality rates in children increasing over the past 2 years according to the CDC’s Web-based Injury Statistics Query and Reporting System, it is crucial that we continue to demand the appropriate support for this public health crisis.
Recognizing the prevalence of guns in homes and the potential dangers of easy access to them makes it essential to ask about firearms as part of our injury prevention guidance and offer counseling to parents and children about safe gun storage, gun locks, or support for legislation to protect children from gun violence.
It is important to engage gun owners about gun safety and “point out that parents may underestimate kids’ propensity to handle guns unsafely, even when they’ve been taught,” as well as the “impulsivity, risk-taking, and unpredictability of adolescence. ... We pediatric clinicians tend not to discuss firearm injury prevention much,” but a recent review of the problem offers some excellent guidance that “may help us to do more,” according to Dr. Nelson (Adolesc Med State Art Rev. 2016;27[2]:323-40).
However problematic it may be to confront the problem of firearm injuries in children and adolescents, we cannot ignore the scale of this current epidemic.
Eliot W. Nelson, MD, is a pediatrician at the University of Vermont Children’s Hospital. He reported having no relevant financial disclosures. These remarks are adapted from an editorial accompanying the report by Fowler et al. (Pediatrics. 2017. doi: 10.1542/peds.2017-1300).
Dr. Fowler’s study brings a lot of uncomfortable statistics to light. Subsequently we are left wondering, What can we do about it? With firearm injury mortality rates in children increasing over the past 2 years according to the CDC’s Web-based Injury Statistics Query and Reporting System, it is crucial that we continue to demand the appropriate support for this public health crisis.
Recognizing the prevalence of guns in homes and the potential dangers of easy access to them makes it essential to ask about firearms as part of our injury prevention guidance and offer counseling to parents and children about safe gun storage, gun locks, or support for legislation to protect children from gun violence.
It is important to engage gun owners about gun safety and “point out that parents may underestimate kids’ propensity to handle guns unsafely, even when they’ve been taught,” as well as the “impulsivity, risk-taking, and unpredictability of adolescence. ... We pediatric clinicians tend not to discuss firearm injury prevention much,” but a recent review of the problem offers some excellent guidance that “may help us to do more,” according to Dr. Nelson (Adolesc Med State Art Rev. 2016;27[2]:323-40).
However problematic it may be to confront the problem of firearm injuries in children and adolescents, we cannot ignore the scale of this current epidemic.
Eliot W. Nelson, MD, is a pediatrician at the University of Vermont Children’s Hospital. He reported having no relevant financial disclosures. These remarks are adapted from an editorial accompanying the report by Fowler et al. (Pediatrics. 2017. doi: 10.1542/peds.2017-1300).
Boys, older children, and minorities are disproportionately affected when it comes to firearm injuries and deaths in U.S. children and adolescents, and child firearm suicide rates are at the highest they have been in more than a decade, new study results revealed.
Around 19 children are either medically treated for a gunshot wound or killed by one every day in the United States. “The majority of these children are boys 13-17 years old, African American in the case of firearm homicide, and white and American Indian in the case of firearm suicide. Pediatric firearm injuries and deaths are an important public health problem in the United States, contributing substantially each year to premature death, illness, and disability of children,” said Katherine A. Fowler, PhD, of the National Center for Injury Prevention and Control, Atlanta, and her associates. “Finding ways to prevent such injuries and ensure that all children have safe, stable, nurturing relationships and environments remains one of our most important priorities” (Pediatrics. 2017;140(1):e20163486).
“From 2012 to 2014, the average annual case fatality rate was 74% for firearm-related self-harm, 14% for firearm-related assaults, and 6% for unintentional firearm injuries,” the investigators reported.
Boys accounted for 82% of all child firearm deaths from 2012 to 2014. In this time period, the annual rate of firearm death for boys was 4.5 times higher than the annual rate for girls (2.8 vs. 0.6 per 100,000). This difference was even more pronounced by age, with the rate for 13- to 17-year-old boys being six times higher than the rate for same-aged girls. Similarly, boys suffer the majority of nonfatal firearm injuries treated in U.S. emergency departments, accounting for 84% of all nonfatal firearm injuries medically treated each year from 2012 to 2014. The average annual rate of nonfatal firearm injuries for boys was five times the rate for girls at 13 vs. 3 per 100,000.
The annual rate of firearm homicide was 10 times higher among 13- to 17-year-olds versus 0- to 12-year-olds (3 vs. 0.3 per 100,000). Unintentional firearm death rates were approximately twice as high when comparing these two groups (0.2 vs. 0.1 per 100,000).
Dr. Fowler and her associates wrote, “Our findings indicate that most children who died of unintentional firearm injuries were shot by another child in their own age range and most often in the context of playing with a gun or showing it to others. More than one-third of the deaths of older children occurred in incidents in which the shooter thought that the gun was unloaded or thought that the safety was engaged.”
“Child firearm suicide rates showed a significant upward trend between 2007 and 2014, increasing 60% from 1.0 to 1.6 (P less than .05) to the highest rate seen over the period examined,” Dr. Fowler and her associates said.
Firearm suicide rates were 11 times higher among 13- to 17-year-olds vs. 10- to 12-year-olds (2 vs. 0.2 per 100,000). Older children also accounted for 88% of all nonfatal firearm injuries treated in an ED. The overall average annual rate of nonfatal firearm injuries for older children was 19 times that of younger children (24 vs. 1 per 100,000).
The annual firearm homicide rate for African American children was nearly 10 times higher than the rate for white children (4 vs. 0.4 per 100,000). However, the annual rate of firearm suicide among white children was nearly four times higher than the rate for African American children (2. vs. 0.6 per 100,000).
Awareness of the availability of firearms during times of crisis is crucial because suicides are often impulsive in young people, Dr. Fowler and her associates said, “with previous findings indicating that many who attempt suicide spend 10 minutes or less deliberating. Safe storage practices (i.e., unloading and locking all firearms and ammunition) can potentially be lifesaving in these instances,” as the results of previous studies in this age group attest.
Firearm deaths are the third leading cause of death overall among children in the United States aged 1-17 years, beating pediatric congenital anomalies, heart disease, influenza/pneumonia, chronic lower respiratory disease, and cerebrovascular causes. Understanding the nature, scale, and impact of firearm violence against children is an important first step, concluded Dr. Fowler and her associates.
Dr. Fowler and her associates said they had no relevant financial disclosures.
Boys, older children, and minorities are disproportionately affected when it comes to firearm injuries and deaths in U.S. children and adolescents, and child firearm suicide rates are at the highest they have been in more than a decade, new study results revealed.
Around 19 children are either medically treated for a gunshot wound or killed by one every day in the United States. “The majority of these children are boys 13-17 years old, African American in the case of firearm homicide, and white and American Indian in the case of firearm suicide. Pediatric firearm injuries and deaths are an important public health problem in the United States, contributing substantially each year to premature death, illness, and disability of children,” said Katherine A. Fowler, PhD, of the National Center for Injury Prevention and Control, Atlanta, and her associates. “Finding ways to prevent such injuries and ensure that all children have safe, stable, nurturing relationships and environments remains one of our most important priorities” (Pediatrics. 2017;140(1):e20163486).
“From 2012 to 2014, the average annual case fatality rate was 74% for firearm-related self-harm, 14% for firearm-related assaults, and 6% for unintentional firearm injuries,” the investigators reported.
Boys accounted for 82% of all child firearm deaths from 2012 to 2014. In this time period, the annual rate of firearm death for boys was 4.5 times higher than the annual rate for girls (2.8 vs. 0.6 per 100,000). This difference was even more pronounced by age, with the rate for 13- to 17-year-old boys being six times higher than the rate for same-aged girls. Similarly, boys suffer the majority of nonfatal firearm injuries treated in U.S. emergency departments, accounting for 84% of all nonfatal firearm injuries medically treated each year from 2012 to 2014. The average annual rate of nonfatal firearm injuries for boys was five times the rate for girls at 13 vs. 3 per 100,000.
The annual rate of firearm homicide was 10 times higher among 13- to 17-year-olds versus 0- to 12-year-olds (3 vs. 0.3 per 100,000). Unintentional firearm death rates were approximately twice as high when comparing these two groups (0.2 vs. 0.1 per 100,000).
Dr. Fowler and her associates wrote, “Our findings indicate that most children who died of unintentional firearm injuries were shot by another child in their own age range and most often in the context of playing with a gun or showing it to others. More than one-third of the deaths of older children occurred in incidents in which the shooter thought that the gun was unloaded or thought that the safety was engaged.”
“Child firearm suicide rates showed a significant upward trend between 2007 and 2014, increasing 60% from 1.0 to 1.6 (P less than .05) to the highest rate seen over the period examined,” Dr. Fowler and her associates said.
Firearm suicide rates were 11 times higher among 13- to 17-year-olds vs. 10- to 12-year-olds (2 vs. 0.2 per 100,000). Older children also accounted for 88% of all nonfatal firearm injuries treated in an ED. The overall average annual rate of nonfatal firearm injuries for older children was 19 times that of younger children (24 vs. 1 per 100,000).
The annual firearm homicide rate for African American children was nearly 10 times higher than the rate for white children (4 vs. 0.4 per 100,000). However, the annual rate of firearm suicide among white children was nearly four times higher than the rate for African American children (2. vs. 0.6 per 100,000).
Awareness of the availability of firearms during times of crisis is crucial because suicides are often impulsive in young people, Dr. Fowler and her associates said, “with previous findings indicating that many who attempt suicide spend 10 minutes or less deliberating. Safe storage practices (i.e., unloading and locking all firearms and ammunition) can potentially be lifesaving in these instances,” as the results of previous studies in this age group attest.
Firearm deaths are the third leading cause of death overall among children in the United States aged 1-17 years, beating pediatric congenital anomalies, heart disease, influenza/pneumonia, chronic lower respiratory disease, and cerebrovascular causes. Understanding the nature, scale, and impact of firearm violence against children is an important first step, concluded Dr. Fowler and her associates.
Dr. Fowler and her associates said they had no relevant financial disclosures.
FROM PEDIATRICS
Key clinical point:
Major finding: Firearm suicide rates were 11 times higher among 13- to 17-year-olds versus 10- to 12-year-olds (2 vs. 0.2 per 100,000).
Data source: National 2012-2014 data from CDC’s National Vital Statistics System and the National Electronic Injury Surveillance System.
Disclosures: Dr. Fowler and her associates said they had no relevant financial disclosures.
Common Conditions in Persons with Skin of Color
1. This patient is concerned about the numerous long-standing “moles” on her face. Only occasionally pruritic, they are otherwise asymptomatic. Nevertheless, the patient wants them investigated and removed.
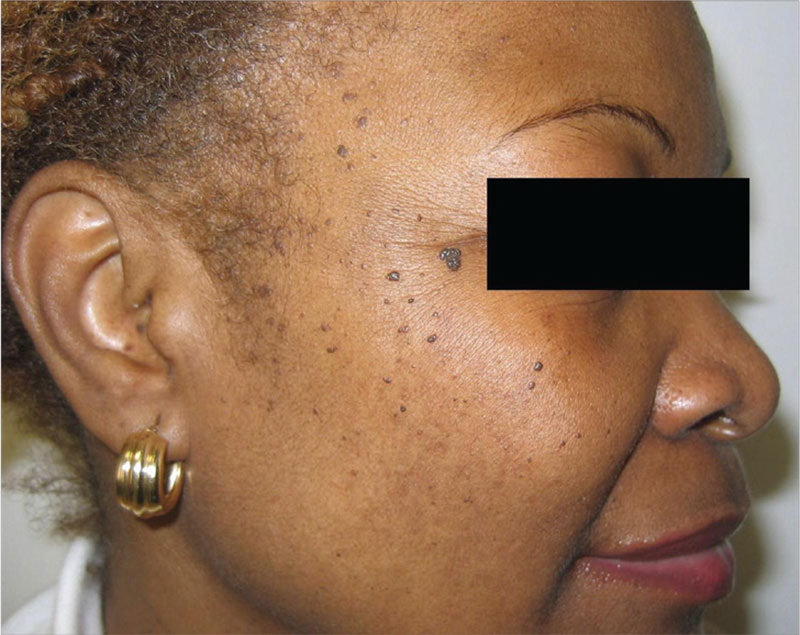
Diagnosis: Dermatosis papulosa nigra (DPN) is a benign condition that presents as multiple brown to dark-brown 1- to 5-mm papules on the face, neck, and trunk. The lesions are a sign of aging in darker skinned patients and do not require treatment. They can, however, be safely, easily, and effectively removed. Both electrodesiccation and KTP (532 nm) laser have comparable efficacy in the removal of DPN, according to a 2009 study published in the American Journal of Dermatologic Surgery. Without use of anesthetics, the KTP laser is preferred for patient comfort.
For more information, see “Skin of Color: Dermatosis Papulosa Nigra Removal.” Dermatology News. February 1, 2012.
2. A 17-year-old African-American boy likes to keep his face clean-shaven, but his skin becomes irritated every time he shaves. He states that his father also has this problem, but doesn’t have a solution.
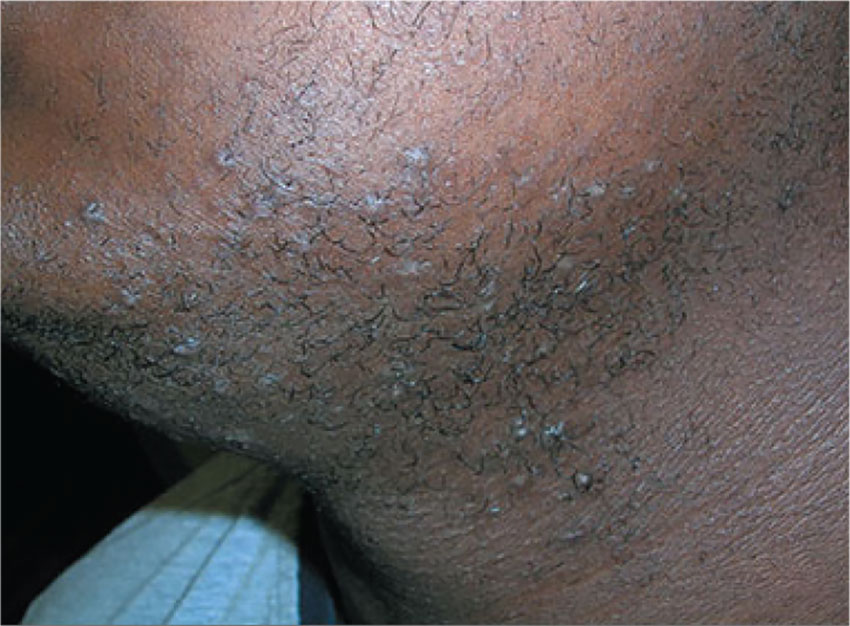
Diagnosis: Pseudofolliculitis is a common skin condition affecting hair-bearing areas of the body that are shaved. It develops when the free end of a tightly coiled hair reenters the skin after shaving, causing a foreign-body–like inflammatory reaction. Tightly curled hair has a greater tendency to pierce the follicle and the surface of the skin, explaining the relative predominance of pseudofolliculitis in patients of African descent; at least half of black men who shave develop the condition.
For more information, see “Bumps in beard area.” J Fam Pract. 2015 November;64(11).
3. A 15-year-old African-American boy presents with a two-month history of hair loss and pruritic papules on the back of his head that developed after a barber shaved the area. There are two dozen 1- to 2-mm papules on his posterior neck and occipital scalp with areas of focal crusting. The patient had a similar episode a year ago after shaving the same area, but those papules cleared after one month of applying rubbing alcohol.

Diagnosis: This patient’s diagnosis is acne keloidalis nuchae (AKN), a chronic folliculitis characterized by smooth, dome-shaped papules on the posterior scalp and neck that become confluent and form firm papules and hairless, keloid-like plaques. Seen almost exclusively in young, postpubescent African-American males, the condition is often asymptomatic, although some patients complain of itching in the affected area.
For more information, see “Scalp papules in a teenage boy.” J Fam Pract. 2017 March;66(3).
4. Several years after a motorcycle accident and resulting road rash, this 23-year-old Hispanic woman is left with complex scars. She experiences pruritus, pain, and hypersensitivity and is seeking relief.
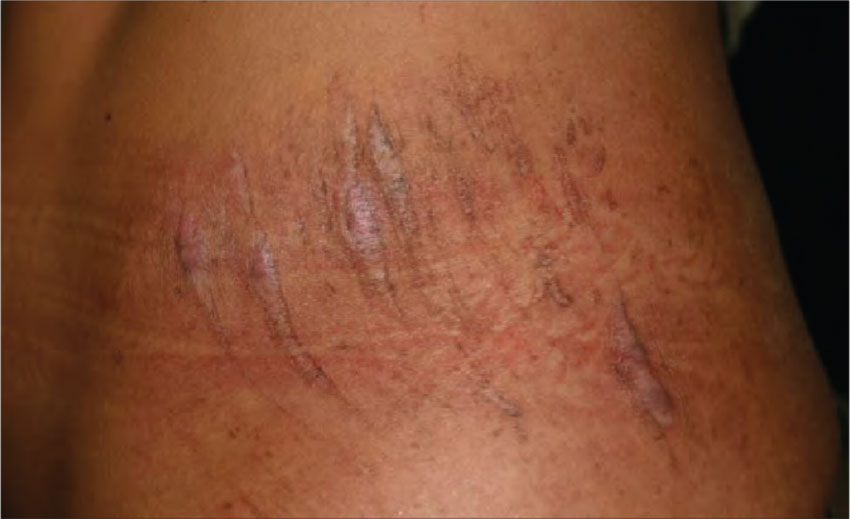
Diagnosis: Often confused with hypertrophic scars, keloids are benign overgrowths of scar tissue that can occur months to years after skin trauma. They are most common in those ages 10 to 30, with a higher incidence among blacks, Hispanics, and Asians. Although the exact etiology of keloids is unknown, they are related to aberrant collagen production and breakdown in wound healing.
For more information, see “Laser treatment of scars and keloids.” Cosmet Dermatol. 2012;25:318-325.
1. This patient is concerned about the numerous long-standing “moles” on her face. Only occasionally pruritic, they are otherwise asymptomatic. Nevertheless, the patient wants them investigated and removed.

Diagnosis: Dermatosis papulosa nigra (DPN) is a benign condition that presents as multiple brown to dark-brown 1- to 5-mm papules on the face, neck, and trunk. The lesions are a sign of aging in darker skinned patients and do not require treatment. They can, however, be safely, easily, and effectively removed. Both electrodesiccation and KTP (532 nm) laser have comparable efficacy in the removal of DPN, according to a 2009 study published in the American Journal of Dermatologic Surgery. Without use of anesthetics, the KTP laser is preferred for patient comfort.
For more information, see “Skin of Color: Dermatosis Papulosa Nigra Removal.” Dermatology News. February 1, 2012.
2. A 17-year-old African-American boy likes to keep his face clean-shaven, but his skin becomes irritated every time he shaves. He states that his father also has this problem, but doesn’t have a solution.

Diagnosis: Pseudofolliculitis is a common skin condition affecting hair-bearing areas of the body that are shaved. It develops when the free end of a tightly coiled hair reenters the skin after shaving, causing a foreign-body–like inflammatory reaction. Tightly curled hair has a greater tendency to pierce the follicle and the surface of the skin, explaining the relative predominance of pseudofolliculitis in patients of African descent; at least half of black men who shave develop the condition.
For more information, see “Bumps in beard area.” J Fam Pract. 2015 November;64(11).
3. A 15-year-old African-American boy presents with a two-month history of hair loss and pruritic papules on the back of his head that developed after a barber shaved the area. There are two dozen 1- to 2-mm papules on his posterior neck and occipital scalp with areas of focal crusting. The patient had a similar episode a year ago after shaving the same area, but those papules cleared after one month of applying rubbing alcohol.

Diagnosis: This patient’s diagnosis is acne keloidalis nuchae (AKN), a chronic folliculitis characterized by smooth, dome-shaped papules on the posterior scalp and neck that become confluent and form firm papules and hairless, keloid-like plaques. Seen almost exclusively in young, postpubescent African-American males, the condition is often asymptomatic, although some patients complain of itching in the affected area.
For more information, see “Scalp papules in a teenage boy.” J Fam Pract. 2017 March;66(3).
4. Several years after a motorcycle accident and resulting road rash, this 23-year-old Hispanic woman is left with complex scars. She experiences pruritus, pain, and hypersensitivity and is seeking relief.

Diagnosis: Often confused with hypertrophic scars, keloids are benign overgrowths of scar tissue that can occur months to years after skin trauma. They are most common in those ages 10 to 30, with a higher incidence among blacks, Hispanics, and Asians. Although the exact etiology of keloids is unknown, they are related to aberrant collagen production and breakdown in wound healing.
For more information, see “Laser treatment of scars and keloids.” Cosmet Dermatol. 2012;25:318-325.
1. This patient is concerned about the numerous long-standing “moles” on her face. Only occasionally pruritic, they are otherwise asymptomatic. Nevertheless, the patient wants them investigated and removed.

Diagnosis: Dermatosis papulosa nigra (DPN) is a benign condition that presents as multiple brown to dark-brown 1- to 5-mm papules on the face, neck, and trunk. The lesions are a sign of aging in darker skinned patients and do not require treatment. They can, however, be safely, easily, and effectively removed. Both electrodesiccation and KTP (532 nm) laser have comparable efficacy in the removal of DPN, according to a 2009 study published in the American Journal of Dermatologic Surgery. Without use of anesthetics, the KTP laser is preferred for patient comfort.
For more information, see “Skin of Color: Dermatosis Papulosa Nigra Removal.” Dermatology News. February 1, 2012.
2. A 17-year-old African-American boy likes to keep his face clean-shaven, but his skin becomes irritated every time he shaves. He states that his father also has this problem, but doesn’t have a solution.

Diagnosis: Pseudofolliculitis is a common skin condition affecting hair-bearing areas of the body that are shaved. It develops when the free end of a tightly coiled hair reenters the skin after shaving, causing a foreign-body–like inflammatory reaction. Tightly curled hair has a greater tendency to pierce the follicle and the surface of the skin, explaining the relative predominance of pseudofolliculitis in patients of African descent; at least half of black men who shave develop the condition.
For more information, see “Bumps in beard area.” J Fam Pract. 2015 November;64(11).
3. A 15-year-old African-American boy presents with a two-month history of hair loss and pruritic papules on the back of his head that developed after a barber shaved the area. There are two dozen 1- to 2-mm papules on his posterior neck and occipital scalp with areas of focal crusting. The patient had a similar episode a year ago after shaving the same area, but those papules cleared after one month of applying rubbing alcohol.

Diagnosis: This patient’s diagnosis is acne keloidalis nuchae (AKN), a chronic folliculitis characterized by smooth, dome-shaped papules on the posterior scalp and neck that become confluent and form firm papules and hairless, keloid-like plaques. Seen almost exclusively in young, postpubescent African-American males, the condition is often asymptomatic, although some patients complain of itching in the affected area.
For more information, see “Scalp papules in a teenage boy.” J Fam Pract. 2017 March;66(3).
4. Several years after a motorcycle accident and resulting road rash, this 23-year-old Hispanic woman is left with complex scars. She experiences pruritus, pain, and hypersensitivity and is seeking relief.

Diagnosis: Often confused with hypertrophic scars, keloids are benign overgrowths of scar tissue that can occur months to years after skin trauma. They are most common in those ages 10 to 30, with a higher incidence among blacks, Hispanics, and Asians. Although the exact etiology of keloids is unknown, they are related to aberrant collagen production and breakdown in wound healing.
For more information, see “Laser treatment of scars and keloids.” Cosmet Dermatol. 2012;25:318-325.
Shift to minimally invasive MV surgery picks up
NEW YORK – An analysis of a Society of Thoracic Surgeons database has identified a significant increase in volumes for isolated mitral valve surgery and leaflet prolapse this decade, with a shift toward minimally invasive approaches, according to a study of trends in mitral valve surgery in the United States presented here at the American Association for Thoracic Surgery Mitral Conclave 2017.
James Gammie, MD, of the University of Maryland School of Medicine, Baltimore, reported on the analysis of the STS Adult Cardiac Surgery Database in which more than 90% of the adult cardiac surgery centers in North America participate.
“Degenerative disease remains the most common reason patients are referred for surgery,” Dr. Gammie said, noting that 60.7% of patients with an identified etiology had degenerative leaflet prolapse (etiology was unknown in 31% of the patients in the dataset).
“The operative approach has changed and continues to shift toward a less invasive approach,” Dr. Gammie said. Overall, 74.1% of operations involved sternotomy, but only 67.5% of those in the leaflet prolapse subgroup, with less invasive operations comprising 23% of all operations and 29.1% of those in the leaflet prolapse subgroup. From 2011 to 2016, total mitral surgical volume grew at a rate of 1.1% annually, but the volume for isolated MV operations grew 4.4% annually while leaflet prolapse procedures increased 7.6% annually, Dr. Gammie said.
Dr. Gammie described surgeons’ decision to perform ablation for preoperative AF during MV surgery a “coin toss.” One-third (34%) had AF, but only 51.2% of patients with AF in the total cohort and 54.4% of those in the leaflet prolapse subgroup got ablation. The overall MV repair rate was 65% for the total cohort but 83% for those with leaflet prolapse. For those who had MV replacement, the share of bioprosthetic devices increased steadily through the study period, from around 65% to 75.8%, Dr. Gammie said.
In the leaflet prolapse subgroup, 96.1% had annuloplasty and 29.2% had artificial chords implanted, with an average of two chords per operation. “There’s an increasing use of artificial ePTFE chords,” Dr. Gammie said. He noted the leaflet prolapse subgroup was composed of low-risk healthy patients. The mean ejection fraction (EF) for the cohort was 57%, and 47% of patients had EF of less than 60%. The overwhelming majority of patients (88%) had Class I indications for surgery with the remainder having Class IIa indications.
Dr. Gammie noted a few other emerging trends of MV surgery during the study period. “Patients with functional mitral regurgitation are rarely referred for operation: these patients made up fewer than 5% of patients undergoing mitral valve operations during the study period,” he said.;
With regard to outcomes, Dr. Gammie said, “There remain substantial differences between repair and replacement.” Replacement had almost twice the rate of reoperation for bleeding than repair (4.1% vs. 2.1%) and renal failure (3.4% vs. 1.4%).
“We observed that a substantial number of patients have an unsuccessful attempt at mitral valve repair before undergoing replacement – 16 % of the overall replacement group and 27% of patients having replacement for degenerative leaflet prolapse,” he said. “This does not appear to penalize patients in terms of outcome.”
The rate of permanent pacemaker was also significantly higher in the replacement cohort, 9.8% vs. 3.8% for repair operations, as was operative mortality, 3.7% vs. 1.1%. Said Dr. Gammie.
“This is something to think about as we move to less invasive approaches and overall operative mortality remains over threefold higher for replacement than repair.”
The leaflet prolapse subgroup showed similar disparities between replacement and repair groups. “The increased application of repair when feasible will be of value to improve outcomes, as will referral of patients earlier in the disease process ,” Dr. Gammie said.
“In our own STS database, about one-third of patients who were coded as having AF had no evidence of it, and that’s why in our institution they don’t get treated.” He added, “I’m not that surprised at the low repair rate when the average surgeon in the United States does five mitral repairs a year.”
Dr. Gammie disclosed that he is a consultant to Edwards Lifesciences and has an ownership interest in Harpoon Medical. Dr. Damiano disclosed that he is a speaker for LivaNova and a consultant to and a research grant recipient of Atricure.
NEW YORK – An analysis of a Society of Thoracic Surgeons database has identified a significant increase in volumes for isolated mitral valve surgery and leaflet prolapse this decade, with a shift toward minimally invasive approaches, according to a study of trends in mitral valve surgery in the United States presented here at the American Association for Thoracic Surgery Mitral Conclave 2017.
James Gammie, MD, of the University of Maryland School of Medicine, Baltimore, reported on the analysis of the STS Adult Cardiac Surgery Database in which more than 90% of the adult cardiac surgery centers in North America participate.
“Degenerative disease remains the most common reason patients are referred for surgery,” Dr. Gammie said, noting that 60.7% of patients with an identified etiology had degenerative leaflet prolapse (etiology was unknown in 31% of the patients in the dataset).
“The operative approach has changed and continues to shift toward a less invasive approach,” Dr. Gammie said. Overall, 74.1% of operations involved sternotomy, but only 67.5% of those in the leaflet prolapse subgroup, with less invasive operations comprising 23% of all operations and 29.1% of those in the leaflet prolapse subgroup. From 2011 to 2016, total mitral surgical volume grew at a rate of 1.1% annually, but the volume for isolated MV operations grew 4.4% annually while leaflet prolapse procedures increased 7.6% annually, Dr. Gammie said.
Dr. Gammie described surgeons’ decision to perform ablation for preoperative AF during MV surgery a “coin toss.” One-third (34%) had AF, but only 51.2% of patients with AF in the total cohort and 54.4% of those in the leaflet prolapse subgroup got ablation. The overall MV repair rate was 65% for the total cohort but 83% for those with leaflet prolapse. For those who had MV replacement, the share of bioprosthetic devices increased steadily through the study period, from around 65% to 75.8%, Dr. Gammie said.
In the leaflet prolapse subgroup, 96.1% had annuloplasty and 29.2% had artificial chords implanted, with an average of two chords per operation. “There’s an increasing use of artificial ePTFE chords,” Dr. Gammie said. He noted the leaflet prolapse subgroup was composed of low-risk healthy patients. The mean ejection fraction (EF) for the cohort was 57%, and 47% of patients had EF of less than 60%. The overwhelming majority of patients (88%) had Class I indications for surgery with the remainder having Class IIa indications.
Dr. Gammie noted a few other emerging trends of MV surgery during the study period. “Patients with functional mitral regurgitation are rarely referred for operation: these patients made up fewer than 5% of patients undergoing mitral valve operations during the study period,” he said.;
With regard to outcomes, Dr. Gammie said, “There remain substantial differences between repair and replacement.” Replacement had almost twice the rate of reoperation for bleeding than repair (4.1% vs. 2.1%) and renal failure (3.4% vs. 1.4%).
“We observed that a substantial number of patients have an unsuccessful attempt at mitral valve repair before undergoing replacement – 16 % of the overall replacement group and 27% of patients having replacement for degenerative leaflet prolapse,” he said. “This does not appear to penalize patients in terms of outcome.”
The rate of permanent pacemaker was also significantly higher in the replacement cohort, 9.8% vs. 3.8% for repair operations, as was operative mortality, 3.7% vs. 1.1%. Said Dr. Gammie.
“This is something to think about as we move to less invasive approaches and overall operative mortality remains over threefold higher for replacement than repair.”
The leaflet prolapse subgroup showed similar disparities between replacement and repair groups. “The increased application of repair when feasible will be of value to improve outcomes, as will referral of patients earlier in the disease process ,” Dr. Gammie said.
“In our own STS database, about one-third of patients who were coded as having AF had no evidence of it, and that’s why in our institution they don’t get treated.” He added, “I’m not that surprised at the low repair rate when the average surgeon in the United States does five mitral repairs a year.”
Dr. Gammie disclosed that he is a consultant to Edwards Lifesciences and has an ownership interest in Harpoon Medical. Dr. Damiano disclosed that he is a speaker for LivaNova and a consultant to and a research grant recipient of Atricure.
NEW YORK – An analysis of a Society of Thoracic Surgeons database has identified a significant increase in volumes for isolated mitral valve surgery and leaflet prolapse this decade, with a shift toward minimally invasive approaches, according to a study of trends in mitral valve surgery in the United States presented here at the American Association for Thoracic Surgery Mitral Conclave 2017.
James Gammie, MD, of the University of Maryland School of Medicine, Baltimore, reported on the analysis of the STS Adult Cardiac Surgery Database in which more than 90% of the adult cardiac surgery centers in North America participate.
“Degenerative disease remains the most common reason patients are referred for surgery,” Dr. Gammie said, noting that 60.7% of patients with an identified etiology had degenerative leaflet prolapse (etiology was unknown in 31% of the patients in the dataset).
“The operative approach has changed and continues to shift toward a less invasive approach,” Dr. Gammie said. Overall, 74.1% of operations involved sternotomy, but only 67.5% of those in the leaflet prolapse subgroup, with less invasive operations comprising 23% of all operations and 29.1% of those in the leaflet prolapse subgroup. From 2011 to 2016, total mitral surgical volume grew at a rate of 1.1% annually, but the volume for isolated MV operations grew 4.4% annually while leaflet prolapse procedures increased 7.6% annually, Dr. Gammie said.
Dr. Gammie described surgeons’ decision to perform ablation for preoperative AF during MV surgery a “coin toss.” One-third (34%) had AF, but only 51.2% of patients with AF in the total cohort and 54.4% of those in the leaflet prolapse subgroup got ablation. The overall MV repair rate was 65% for the total cohort but 83% for those with leaflet prolapse. For those who had MV replacement, the share of bioprosthetic devices increased steadily through the study period, from around 65% to 75.8%, Dr. Gammie said.
In the leaflet prolapse subgroup, 96.1% had annuloplasty and 29.2% had artificial chords implanted, with an average of two chords per operation. “There’s an increasing use of artificial ePTFE chords,” Dr. Gammie said. He noted the leaflet prolapse subgroup was composed of low-risk healthy patients. The mean ejection fraction (EF) for the cohort was 57%, and 47% of patients had EF of less than 60%. The overwhelming majority of patients (88%) had Class I indications for surgery with the remainder having Class IIa indications.
Dr. Gammie noted a few other emerging trends of MV surgery during the study period. “Patients with functional mitral regurgitation are rarely referred for operation: these patients made up fewer than 5% of patients undergoing mitral valve operations during the study period,” he said.;
With regard to outcomes, Dr. Gammie said, “There remain substantial differences between repair and replacement.” Replacement had almost twice the rate of reoperation for bleeding than repair (4.1% vs. 2.1%) and renal failure (3.4% vs. 1.4%).
“We observed that a substantial number of patients have an unsuccessful attempt at mitral valve repair before undergoing replacement – 16 % of the overall replacement group and 27% of patients having replacement for degenerative leaflet prolapse,” he said. “This does not appear to penalize patients in terms of outcome.”
The rate of permanent pacemaker was also significantly higher in the replacement cohort, 9.8% vs. 3.8% for repair operations, as was operative mortality, 3.7% vs. 1.1%. Said Dr. Gammie.
“This is something to think about as we move to less invasive approaches and overall operative mortality remains over threefold higher for replacement than repair.”
The leaflet prolapse subgroup showed similar disparities between replacement and repair groups. “The increased application of repair when feasible will be of value to improve outcomes, as will referral of patients earlier in the disease process ,” Dr. Gammie said.
“In our own STS database, about one-third of patients who were coded as having AF had no evidence of it, and that’s why in our institution they don’t get treated.” He added, “I’m not that surprised at the low repair rate when the average surgeon in the United States does five mitral repairs a year.”
Dr. Gammie disclosed that he is a consultant to Edwards Lifesciences and has an ownership interest in Harpoon Medical. Dr. Damiano disclosed that he is a speaker for LivaNova and a consultant to and a research grant recipient of Atricure.
AT THE AATS MITRAL CONCLAVE 2017
Key clinical point: The volume of mitral valve surgery has increased substantially, according to an analysis of the Society for Thoracic Surgery database, and an increasing percentage of procedures are minimally invasive in nature.
Major finding: Sternotomy continues to be the most widely used approach for mitral valve surgery, but less invasive options most recently comprised 23% of the overall group and 29.1% of those with isolated leaflet prolapse.
Data source: Retrospective study of 15,360 isolated mitral valve operations performed from July 2011 to September 2016 in the Society of Thoracic Surgeons database.
Disclosures: Dr. Gammie reported being a consultant to Edwards Lifesciences and having an ownership interest in Harpoon Medical.
Urgent surgery said to deserve separate classification
Urgent surgery deserves a separate classification from elective surgery and emergency surgery for assessments of healthcare quality and performance, because the three types of surgery have distinct morbidity and mortality profiles, according to a report published in JAMA Surgery.
Current methods of assessing pay-for-performance reimbursement, surgical outcomes, and value-based care programs all classify surgeries as either elective or emergent procedures. They do not account for the many surgeries that are instead urgent – performed after a trial of nonoperative conservative management or after patients with acute disease processes undergo a brief period of medical optimization.
Common examples of surgeries that occupy this middle ‘urgent’ ground between elective and emergent procedures are those done for cholecystitis, adhesive small-bowel obstruction, and acute diverticulitis, said Matthew G. Mullen, MD, and his associates at the University of Virginia Health System, Charlottesville.
Such urgent surgeries should not be lumped together with elective surgeries, as they usually are at present, because they carry substantially higher complication rates and mortality. “At a time when reimbursement is contingent on value-based outcomes reporting and performance, it is imperative to ensure that appropriate risk adjustment is performed,” the researchers stated.
“Surgeons who commonly operate on an urgent basis, including many acute-care and emergency general surgeons, are at risk of being penalized” in Medicare’s value-based reimbursement for their services.
“These surgeons may even unfairly be labeled as poor performers by current outcome reporting guidelines,” the investigators noted.
Morbidity and mortality rates associated with the “substantial” population of patients undergoing urgent surgery have not been well-studied until now. Dr. Mullen and his associates examined the issue using information from a national database, the American College of Surgeons’ National Quality Improvement Program Participant Use File. They focused on 173,643 general surgeries performed at 435 hospitals during a 1-year period: 130,235 (75%) were categorized as elective, 20,816 (12%) as urgent (nonelective and nonemergency), and 22,592 (13%) as emergency procedures.
Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality. These rates are much greater than those of elective surgeries (6.7% and 0.4%, respectively), even though urgent surgeries typically fall into the category of “elective.” In fact, the morbidity and mortality rates for urgent surgeries closely approached those of emergency surgeries (13.8% and 3.7%, respectively), the investigators said (JAMA Surg. 2017 May 10 [doi:10.1001/jamasurg.2017.0918]).
In this cohort, patients in the “urgent” surgery category had the highest preoperative rates of congestive heart failure, chronic obstructive pulmonary disease, diabetes, hemodialysis, corticosteroid use, and disseminated cancer – all factors that markedly elevate mortality and morbidity risks.
“We have identified operative urgency as a key consideration for patient risk stratification. If this issue is not recognized, quality outcome reporting and value-based reimbursement will continue to incentivize operating on an elective basis and will make surgeons more reluctant to operate on patients who urgently require care,” Dr. Mullen and his associates said. And such delays in surgical intervention could further increase patient morbidity and mortality.
This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
Urgent surgery deserves a separate classification from elective surgery and emergency surgery for assessments of healthcare quality and performance, because the three types of surgery have distinct morbidity and mortality profiles, according to a report published in JAMA Surgery.
Current methods of assessing pay-for-performance reimbursement, surgical outcomes, and value-based care programs all classify surgeries as either elective or emergent procedures. They do not account for the many surgeries that are instead urgent – performed after a trial of nonoperative conservative management or after patients with acute disease processes undergo a brief period of medical optimization.
Common examples of surgeries that occupy this middle ‘urgent’ ground between elective and emergent procedures are those done for cholecystitis, adhesive small-bowel obstruction, and acute diverticulitis, said Matthew G. Mullen, MD, and his associates at the University of Virginia Health System, Charlottesville.
Such urgent surgeries should not be lumped together with elective surgeries, as they usually are at present, because they carry substantially higher complication rates and mortality. “At a time when reimbursement is contingent on value-based outcomes reporting and performance, it is imperative to ensure that appropriate risk adjustment is performed,” the researchers stated.
“Surgeons who commonly operate on an urgent basis, including many acute-care and emergency general surgeons, are at risk of being penalized” in Medicare’s value-based reimbursement for their services.
“These surgeons may even unfairly be labeled as poor performers by current outcome reporting guidelines,” the investigators noted.
Morbidity and mortality rates associated with the “substantial” population of patients undergoing urgent surgery have not been well-studied until now. Dr. Mullen and his associates examined the issue using information from a national database, the American College of Surgeons’ National Quality Improvement Program Participant Use File. They focused on 173,643 general surgeries performed at 435 hospitals during a 1-year period: 130,235 (75%) were categorized as elective, 20,816 (12%) as urgent (nonelective and nonemergency), and 22,592 (13%) as emergency procedures.
Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality. These rates are much greater than those of elective surgeries (6.7% and 0.4%, respectively), even though urgent surgeries typically fall into the category of “elective.” In fact, the morbidity and mortality rates for urgent surgeries closely approached those of emergency surgeries (13.8% and 3.7%, respectively), the investigators said (JAMA Surg. 2017 May 10 [doi:10.1001/jamasurg.2017.0918]).
In this cohort, patients in the “urgent” surgery category had the highest preoperative rates of congestive heart failure, chronic obstructive pulmonary disease, diabetes, hemodialysis, corticosteroid use, and disseminated cancer – all factors that markedly elevate mortality and morbidity risks.
“We have identified operative urgency as a key consideration for patient risk stratification. If this issue is not recognized, quality outcome reporting and value-based reimbursement will continue to incentivize operating on an elective basis and will make surgeons more reluctant to operate on patients who urgently require care,” Dr. Mullen and his associates said. And such delays in surgical intervention could further increase patient morbidity and mortality.
This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
Urgent surgery deserves a separate classification from elective surgery and emergency surgery for assessments of healthcare quality and performance, because the three types of surgery have distinct morbidity and mortality profiles, according to a report published in JAMA Surgery.
Current methods of assessing pay-for-performance reimbursement, surgical outcomes, and value-based care programs all classify surgeries as either elective or emergent procedures. They do not account for the many surgeries that are instead urgent – performed after a trial of nonoperative conservative management or after patients with acute disease processes undergo a brief period of medical optimization.
Common examples of surgeries that occupy this middle ‘urgent’ ground between elective and emergent procedures are those done for cholecystitis, adhesive small-bowel obstruction, and acute diverticulitis, said Matthew G. Mullen, MD, and his associates at the University of Virginia Health System, Charlottesville.
Such urgent surgeries should not be lumped together with elective surgeries, as they usually are at present, because they carry substantially higher complication rates and mortality. “At a time when reimbursement is contingent on value-based outcomes reporting and performance, it is imperative to ensure that appropriate risk adjustment is performed,” the researchers stated.
“Surgeons who commonly operate on an urgent basis, including many acute-care and emergency general surgeons, are at risk of being penalized” in Medicare’s value-based reimbursement for their services.
“These surgeons may even unfairly be labeled as poor performers by current outcome reporting guidelines,” the investigators noted.
Morbidity and mortality rates associated with the “substantial” population of patients undergoing urgent surgery have not been well-studied until now. Dr. Mullen and his associates examined the issue using information from a national database, the American College of Surgeons’ National Quality Improvement Program Participant Use File. They focused on 173,643 general surgeries performed at 435 hospitals during a 1-year period: 130,235 (75%) were categorized as elective, 20,816 (12%) as urgent (nonelective and nonemergency), and 22,592 (13%) as emergency procedures.
Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality. These rates are much greater than those of elective surgeries (6.7% and 0.4%, respectively), even though urgent surgeries typically fall into the category of “elective.” In fact, the morbidity and mortality rates for urgent surgeries closely approached those of emergency surgeries (13.8% and 3.7%, respectively), the investigators said (JAMA Surg. 2017 May 10 [doi:10.1001/jamasurg.2017.0918]).
In this cohort, patients in the “urgent” surgery category had the highest preoperative rates of congestive heart failure, chronic obstructive pulmonary disease, diabetes, hemodialysis, corticosteroid use, and disseminated cancer – all factors that markedly elevate mortality and morbidity risks.
“We have identified operative urgency as a key consideration for patient risk stratification. If this issue is not recognized, quality outcome reporting and value-based reimbursement will continue to incentivize operating on an elective basis and will make surgeons more reluctant to operate on patients who urgently require care,” Dr. Mullen and his associates said. And such delays in surgical intervention could further increase patient morbidity and mortality.
This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point: Urgent surgery deserves a separate classification from elective and emergency surgeries for assessments of healthcare quality and performance.
Major finding: Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality, approximately double the morbidity rate and 6 times the mortality rate of elective surgeries (6.7% and 0.4%, respectively).
Data source: A retrospective analysis of outcomes for 173,643 general surgeries performed during a 1-year period at 435 hospitals across U.S.
Disclosures: This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
Sooner is better than later for acute UC surgery
SEATTLE – Postponing surgery for acute ulcerative colitis more than a day increases postoperative complications, lengths of stay, and hospital costs, according to a review by Johns Hopkins University, Baltimore, of almost 2,000 patients.
It’s not uncommon to wait 5 or even 10 days to give biologics a chance to work when patients are admitted for acute ulcerative colitis (UC). Based on the review, however, “we believe that the need for prolonged medical therapy and resuscitation in this patient population prior to colectomy may be overstated,” and that “the lasting effects of persistent inflammation cascade are underestimated.”
The team reviewed 1,953 index UC admissions with emergent non-elective abdominal surgery in the National Inpatient Sample (NIS) database from 2008-13; 546 patients (28%) had early operations - within 24 hours of admission – and the other 1,407 had operations after that time.
Although it’s impossible to say for sure given the limits of administrative data in the NIS, patients who had surgery soon after admission were probably sicker. Even so, they were less likely to have complications than patients in the delayed surgery group (55% versus 43%), and they had shorter hospital stays, with just 8% in the hospital past 21 days, versus 29% of patients who had delayed operations. The findings were similar for both overall length of stay and post-op length of stay.
Renal complications (8% versus 14%), pulmonary complications (20% versus 25%), and thromboembolic events (4% versus 6%) were also less common in the early surgery group. On multivariable analysis, delayed surgery increased the complication rate by 64%.
With fewer complications and shorter hospital stays, early operations were also less expensive, with a mean total hospitalization cost of $19,985 versus $34,258. The findings were all statistically significant.
Dr. Leeds noted the limits of the study; medical management regimes and the reasons for variations in surgical timing are unknown, among other things. “This is not the final answer on what to do with patients like this, but it opens the door to prospective studies that could control” for such variables, he said.
Early surgery patients were more likely to be male (57% versus 51%) and from households with incomes higher than the national median. There were no difference in age, race, comorbidities, region, or hospital type between the two groups.
Dr. Leeds said he had no disclosures.
SEATTLE – Postponing surgery for acute ulcerative colitis more than a day increases postoperative complications, lengths of stay, and hospital costs, according to a review by Johns Hopkins University, Baltimore, of almost 2,000 patients.
It’s not uncommon to wait 5 or even 10 days to give biologics a chance to work when patients are admitted for acute ulcerative colitis (UC). Based on the review, however, “we believe that the need for prolonged medical therapy and resuscitation in this patient population prior to colectomy may be overstated,” and that “the lasting effects of persistent inflammation cascade are underestimated.”
The team reviewed 1,953 index UC admissions with emergent non-elective abdominal surgery in the National Inpatient Sample (NIS) database from 2008-13; 546 patients (28%) had early operations - within 24 hours of admission – and the other 1,407 had operations after that time.
Although it’s impossible to say for sure given the limits of administrative data in the NIS, patients who had surgery soon after admission were probably sicker. Even so, they were less likely to have complications than patients in the delayed surgery group (55% versus 43%), and they had shorter hospital stays, with just 8% in the hospital past 21 days, versus 29% of patients who had delayed operations. The findings were similar for both overall length of stay and post-op length of stay.
Renal complications (8% versus 14%), pulmonary complications (20% versus 25%), and thromboembolic events (4% versus 6%) were also less common in the early surgery group. On multivariable analysis, delayed surgery increased the complication rate by 64%.
With fewer complications and shorter hospital stays, early operations were also less expensive, with a mean total hospitalization cost of $19,985 versus $34,258. The findings were all statistically significant.
Dr. Leeds noted the limits of the study; medical management regimes and the reasons for variations in surgical timing are unknown, among other things. “This is not the final answer on what to do with patients like this, but it opens the door to prospective studies that could control” for such variables, he said.
Early surgery patients were more likely to be male (57% versus 51%) and from households with incomes higher than the national median. There were no difference in age, race, comorbidities, region, or hospital type between the two groups.
Dr. Leeds said he had no disclosures.
SEATTLE – Postponing surgery for acute ulcerative colitis more than a day increases postoperative complications, lengths of stay, and hospital costs, according to a review by Johns Hopkins University, Baltimore, of almost 2,000 patients.
It’s not uncommon to wait 5 or even 10 days to give biologics a chance to work when patients are admitted for acute ulcerative colitis (UC). Based on the review, however, “we believe that the need for prolonged medical therapy and resuscitation in this patient population prior to colectomy may be overstated,” and that “the lasting effects of persistent inflammation cascade are underestimated.”
The team reviewed 1,953 index UC admissions with emergent non-elective abdominal surgery in the National Inpatient Sample (NIS) database from 2008-13; 546 patients (28%) had early operations - within 24 hours of admission – and the other 1,407 had operations after that time.
Although it’s impossible to say for sure given the limits of administrative data in the NIS, patients who had surgery soon after admission were probably sicker. Even so, they were less likely to have complications than patients in the delayed surgery group (55% versus 43%), and they had shorter hospital stays, with just 8% in the hospital past 21 days, versus 29% of patients who had delayed operations. The findings were similar for both overall length of stay and post-op length of stay.
Renal complications (8% versus 14%), pulmonary complications (20% versus 25%), and thromboembolic events (4% versus 6%) were also less common in the early surgery group. On multivariable analysis, delayed surgery increased the complication rate by 64%.
With fewer complications and shorter hospital stays, early operations were also less expensive, with a mean total hospitalization cost of $19,985 versus $34,258. The findings were all statistically significant.
Dr. Leeds noted the limits of the study; medical management regimes and the reasons for variations in surgical timing are unknown, among other things. “This is not the final answer on what to do with patients like this, but it opens the door to prospective studies that could control” for such variables, he said.
Early surgery patients were more likely to be male (57% versus 51%) and from households with incomes higher than the national median. There were no difference in age, race, comorbidities, region, or hospital type between the two groups.
Dr. Leeds said he had no disclosures.
AT ASCRS 2017
Key clinical point:
Major finding: Patients who had surgery soon after admission were probably sicker. Even so, they were less likely to have complications than patients in the delayed surgery group (55% versus 43%), and they had shorter hospital stays, with just 8% in the hospital past 21 days, versus 29% of patients who had delayed operations.
Data source: Review of almost 2,000 patients in the National Inpatient Sample
Disclosures: The lead investigator had no disclosures.
Anal dysplasia surveillance called into question
SEATTLE – Aggressive screening and treatment of HPV anal dysplasia probably doesn’t decrease the incidence of anal cancer, even in high-risk groups, according to investigators from Kaiser Permanente of Southern California.
The increase in anal squamous cell carcinoma (SCC) over the past 20 years has led to surveillance programs for anal dysplasia in many institutions, based on the assumption that finding and destroying the lesions will prevent anal cancer, similar to the reason why polyps are removed during colonoscopy to prevent colon cancer, said lead investigator Marco Tomassi, MD, a general surgeon with Kaiser in San Diego.
Kaiser in Southern California doesn’t have an intensive surveillance program for anal HPV. Instead, high risk patients – those with HIV, past cervical cancer, anogenital warts, among others – are screened with digital rectal exams and anoscopy every 3-12 months, and only anal warts and other grossly abnormal growths are removed.
Physicians there do not routinely use high resolution anoscopy (HRA), Pap smears, and other techniques to identify and destroy microscopic foci of dysplasia, as in more intensive programs.
To see how the approach has worked, Dr. Tomassi and his colleagues reviewed the incidence of anal SCC among almost 6 million Kaiser patients from 2005 through 2015.
The cumulative incidence in all groups of patients, even those at risk for the disease, was less than 1%; 425 of the 460 anal cancers (92%) were in the general population, among patients who would not have been part of a high risk surveillance program.
Even without an aggressive surveillance program, high grade anal dysplasia was identified in 377 patients; their incidence of anal SCC was 0.8%, the highest found in the study.
There were no incident cases of SCC among the 133 HIV patients with anal dysplasia. Among over 5,000 HIV patients in the study, the anal SCC incidence was 0.09%. In the general population of 5.86 million, it was 0.01%.
“The cumulative incidence in our group, despite not performing ablative techniques for dysplasia, was comparable to those institutions that routinely perform high resolution anoscopy and destruction of dysplasia. The low rate of malignant conversion suggests that aggressive surveillance regimens, such as HRA, may lead to unnecessary procedures even in high risk patients,” Dr. Tomassi said.
Intensive surveillance doesn’t seem to make much difference because “the incidence of anal cancer is very low even in very high risk populations,” and “most SCC cases develop in the general population,” so “regimens dedicated to identifying cancer in high risk groups will ultimate miss the majority of patients who will succumb to this disease,” he said.
“It’s rare that I come to a meeting and hear a paper that’s going to change my practice. Thank you for that,” an audience member said after Dr. Tomassi presented his findings at the American Society of Colon and Rectal Surgeons annual meeting.
Dr. Tomassi did not have any disclosures.
SEATTLE – Aggressive screening and treatment of HPV anal dysplasia probably doesn’t decrease the incidence of anal cancer, even in high-risk groups, according to investigators from Kaiser Permanente of Southern California.
The increase in anal squamous cell carcinoma (SCC) over the past 20 years has led to surveillance programs for anal dysplasia in many institutions, based on the assumption that finding and destroying the lesions will prevent anal cancer, similar to the reason why polyps are removed during colonoscopy to prevent colon cancer, said lead investigator Marco Tomassi, MD, a general surgeon with Kaiser in San Diego.
Kaiser in Southern California doesn’t have an intensive surveillance program for anal HPV. Instead, high risk patients – those with HIV, past cervical cancer, anogenital warts, among others – are screened with digital rectal exams and anoscopy every 3-12 months, and only anal warts and other grossly abnormal growths are removed.
Physicians there do not routinely use high resolution anoscopy (HRA), Pap smears, and other techniques to identify and destroy microscopic foci of dysplasia, as in more intensive programs.
To see how the approach has worked, Dr. Tomassi and his colleagues reviewed the incidence of anal SCC among almost 6 million Kaiser patients from 2005 through 2015.
The cumulative incidence in all groups of patients, even those at risk for the disease, was less than 1%; 425 of the 460 anal cancers (92%) were in the general population, among patients who would not have been part of a high risk surveillance program.
Even without an aggressive surveillance program, high grade anal dysplasia was identified in 377 patients; their incidence of anal SCC was 0.8%, the highest found in the study.
There were no incident cases of SCC among the 133 HIV patients with anal dysplasia. Among over 5,000 HIV patients in the study, the anal SCC incidence was 0.09%. In the general population of 5.86 million, it was 0.01%.
“The cumulative incidence in our group, despite not performing ablative techniques for dysplasia, was comparable to those institutions that routinely perform high resolution anoscopy and destruction of dysplasia. The low rate of malignant conversion suggests that aggressive surveillance regimens, such as HRA, may lead to unnecessary procedures even in high risk patients,” Dr. Tomassi said.
Intensive surveillance doesn’t seem to make much difference because “the incidence of anal cancer is very low even in very high risk populations,” and “most SCC cases develop in the general population,” so “regimens dedicated to identifying cancer in high risk groups will ultimate miss the majority of patients who will succumb to this disease,” he said.
“It’s rare that I come to a meeting and hear a paper that’s going to change my practice. Thank you for that,” an audience member said after Dr. Tomassi presented his findings at the American Society of Colon and Rectal Surgeons annual meeting.
Dr. Tomassi did not have any disclosures.
SEATTLE – Aggressive screening and treatment of HPV anal dysplasia probably doesn’t decrease the incidence of anal cancer, even in high-risk groups, according to investigators from Kaiser Permanente of Southern California.
The increase in anal squamous cell carcinoma (SCC) over the past 20 years has led to surveillance programs for anal dysplasia in many institutions, based on the assumption that finding and destroying the lesions will prevent anal cancer, similar to the reason why polyps are removed during colonoscopy to prevent colon cancer, said lead investigator Marco Tomassi, MD, a general surgeon with Kaiser in San Diego.
Kaiser in Southern California doesn’t have an intensive surveillance program for anal HPV. Instead, high risk patients – those with HIV, past cervical cancer, anogenital warts, among others – are screened with digital rectal exams and anoscopy every 3-12 months, and only anal warts and other grossly abnormal growths are removed.
Physicians there do not routinely use high resolution anoscopy (HRA), Pap smears, and other techniques to identify and destroy microscopic foci of dysplasia, as in more intensive programs.
To see how the approach has worked, Dr. Tomassi and his colleagues reviewed the incidence of anal SCC among almost 6 million Kaiser patients from 2005 through 2015.
The cumulative incidence in all groups of patients, even those at risk for the disease, was less than 1%; 425 of the 460 anal cancers (92%) were in the general population, among patients who would not have been part of a high risk surveillance program.
Even without an aggressive surveillance program, high grade anal dysplasia was identified in 377 patients; their incidence of anal SCC was 0.8%, the highest found in the study.
There were no incident cases of SCC among the 133 HIV patients with anal dysplasia. Among over 5,000 HIV patients in the study, the anal SCC incidence was 0.09%. In the general population of 5.86 million, it was 0.01%.
“The cumulative incidence in our group, despite not performing ablative techniques for dysplasia, was comparable to those institutions that routinely perform high resolution anoscopy and destruction of dysplasia. The low rate of malignant conversion suggests that aggressive surveillance regimens, such as HRA, may lead to unnecessary procedures even in high risk patients,” Dr. Tomassi said.
Intensive surveillance doesn’t seem to make much difference because “the incidence of anal cancer is very low even in very high risk populations,” and “most SCC cases develop in the general population,” so “regimens dedicated to identifying cancer in high risk groups will ultimate miss the majority of patients who will succumb to this disease,” he said.
“It’s rare that I come to a meeting and hear a paper that’s going to change my practice. Thank you for that,” an audience member said after Dr. Tomassi presented his findings at the American Society of Colon and Rectal Surgeons annual meeting.
Dr. Tomassi did not have any disclosures.
AT ASCRS 2017
Key clinical point:
Major finding: When high risk patients were not screened and treated for anal dysplasia, the cumulative incidence of anal squamous cell carcinoma was 0.8% or less, similar to programs that target and treat the lesions.
Data source: Review of almost 6 million patients at Kaiser Permanente of Southern California
Disclosures: The lead investigator had no disclosures.
Fewer early neurologic complications with TAVR than SAVR
PARIS – Patients with severe aortic stenosis at intermediate operative risk have a significantly lower 30-day risk of stroke and other neurologic complications with transcatheter aortic valve replacement than with surgical replacement, according to new results from the landmark SURTAVI trial.
“This is the first time the stroke rate has been shown to be lower with TAVR than with surgery,” A. Pieter Kappetein, MD, noted in presenting the results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
It’s a finding that adds to the momentum for studying TAVR in low-surgical-risk patients, he added.
“As we move toward lower-risk patients it will become even more important to see whether there is a difference in stroke. Suppose the stroke rate in SURTAVI had been a little higher with TAVR than SAVR? It would really make us more cautious about moving toward lower-risk patients. Now that we see that in intermediate-risk patients the stroke rate is actually a little bit lower than with surgery, I think we’ll feel more comfortable moving toward lower-risk patients,” according to Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University Medical Center in Rotterdam.
SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) involved randomization of 1,660 patients with severe symptomatic aortic stenosis to TAVR or SAVR. All participants were deemed to be at intermediate operative risk based upon a predicted surgical mortality of 3%-15%. The primary outcome -- a composite of all-cause mortality and disabling stroke at 2 years -- was presented at the 2017 meeting of the American College of Cardiology and simultaneously published (N Engl J Med. 2017 Apr 6;376(14):1321-1331). The rate was 12.6% with TAVR using the self-expanding CoreValve or Evolut R bioprosthesis and noninferior at 14% with SAVR.
Dr. Kappetein presented a prespecified secondary analysis of the 30-day rate of all neurologic complications, including nondisabling strokes and encephalopathy. He and the other SURTAVI organizers felt this was an important outcome because these early neurologic events have a major impact upon quality of life, including whether a patient will be discharged home or to a rehabilitation clinic or skilled nursing facility following aortic valve replacement.
The 30-day incidence of all stroke was 3.3% in the TAVR patients, significantly lower than the 5.4% rate in the SAVR group. By the 2-year mark, however, the difference was no longer statistically significant, with a rate of 6.3% in the TAVR group compared with 8.0% with SAVR.
Ninety-five percent of the early strokes were ischemic.
The 30-day incidence of disabling stroke was 1.2% with TAVR and 2.4% with SAVR, a difference that was not significant (P=0.057). The 2-year rate was 2.4% in the TAVR arm and 4.5% with SAVR, again not significantly different.
Half of the strokes in the TAVR group had a modified Rankin score of 0-1 at 30 days, meaning no or only minimal signs of stroke. In contrast, most of the strokes in the SAVR group were disabling, with a modified Rankin score of 2-6.
Only 36% of patients who had an early stroke were discharged home, compared with 87% of patients without a stroke. Not surprisingly, quality of life as assessed using the SF-36 physical summary was significantly worse in the early-stroke group. However, with or without stroke, TAVR patients recovered quality of life faster than SAVR patients.
He noted that the timing of the early strokes differed between the two groups. The great majority of both disabling and nondisabling strokes in the TAVR patients were periprocedural, occurring on the day of TAVR or the next day. Strokes in the SAVR group occurred then as well, but also on days 2-6.
One reason why SURTAVI is the first study to show a lower stroke risk with TAVR is that it was the first TAVR-versus-SAVR study to feature comprehensive neurologic testing pre- and post-procedure, along with evaluation of all suspected events by a neurologist or stroke specialist, according to Dr. Kappetein.
“As surgeons we all have said the stroke rate after SAVR is 1%-1.5%, but only when the patient wasn’t waving to us the next morning would we say, ‘Oh, that patient may have a stroke.’ Then we would call a neurologist. So there were many more subtle strokes that we never actually detected. If you do a proper examination of the patient before and after the procedure you’ll find many more strokes,” he said.
He and his coinvestigators systematically searched in vain for predictors of increased stroke risk among the TAVR and SAVR patients.
“Actually, the stroke risk is present for every patient we treat with TAVR or SAVR,” the surgeon continued.
However, discussant Adnan Kastrati, MD, chief physician at the German Heart Center in Munich, thought he spied in the SURTAVI data a potential opportunity to reduce early strokes in SAVR patients. He noted that new-onset atrial fibrillation is consistently more common in SAVR than TAVR patients, and that many of the strokes in the SAVR group occurred on days 2-6. When do heart surgeons typically start oral anticoagulation in their patients with postsurgical atrial fibrillation? he asked.
Not until after 48 hours, Dr. Kappetein replied.
“Those strokes on days 4, 5, and 6 might have to do with atrial fibrillation, and we may need to be more aggressive as surgeons in anticoagulating patients with atrial fibrillation after surgery,” he said.
Dr. Kappetein reported receiving research grant support from Medtronic, sponsor of SURTAVI.
This article was updated July 28, 2107.
PARIS – Patients with severe aortic stenosis at intermediate operative risk have a significantly lower 30-day risk of stroke and other neurologic complications with transcatheter aortic valve replacement than with surgical replacement, according to new results from the landmark SURTAVI trial.
“This is the first time the stroke rate has been shown to be lower with TAVR than with surgery,” A. Pieter Kappetein, MD, noted in presenting the results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
It’s a finding that adds to the momentum for studying TAVR in low-surgical-risk patients, he added.
“As we move toward lower-risk patients it will become even more important to see whether there is a difference in stroke. Suppose the stroke rate in SURTAVI had been a little higher with TAVR than SAVR? It would really make us more cautious about moving toward lower-risk patients. Now that we see that in intermediate-risk patients the stroke rate is actually a little bit lower than with surgery, I think we’ll feel more comfortable moving toward lower-risk patients,” according to Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University Medical Center in Rotterdam.
SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) involved randomization of 1,660 patients with severe symptomatic aortic stenosis to TAVR or SAVR. All participants were deemed to be at intermediate operative risk based upon a predicted surgical mortality of 3%-15%. The primary outcome -- a composite of all-cause mortality and disabling stroke at 2 years -- was presented at the 2017 meeting of the American College of Cardiology and simultaneously published (N Engl J Med. 2017 Apr 6;376(14):1321-1331). The rate was 12.6% with TAVR using the self-expanding CoreValve or Evolut R bioprosthesis and noninferior at 14% with SAVR.
Dr. Kappetein presented a prespecified secondary analysis of the 30-day rate of all neurologic complications, including nondisabling strokes and encephalopathy. He and the other SURTAVI organizers felt this was an important outcome because these early neurologic events have a major impact upon quality of life, including whether a patient will be discharged home or to a rehabilitation clinic or skilled nursing facility following aortic valve replacement.
The 30-day incidence of all stroke was 3.3% in the TAVR patients, significantly lower than the 5.4% rate in the SAVR group. By the 2-year mark, however, the difference was no longer statistically significant, with a rate of 6.3% in the TAVR group compared with 8.0% with SAVR.
Ninety-five percent of the early strokes were ischemic.
The 30-day incidence of disabling stroke was 1.2% with TAVR and 2.4% with SAVR, a difference that was not significant (P=0.057). The 2-year rate was 2.4% in the TAVR arm and 4.5% with SAVR, again not significantly different.
Half of the strokes in the TAVR group had a modified Rankin score of 0-1 at 30 days, meaning no or only minimal signs of stroke. In contrast, most of the strokes in the SAVR group were disabling, with a modified Rankin score of 2-6.
Only 36% of patients who had an early stroke were discharged home, compared with 87% of patients without a stroke. Not surprisingly, quality of life as assessed using the SF-36 physical summary was significantly worse in the early-stroke group. However, with or without stroke, TAVR patients recovered quality of life faster than SAVR patients.
He noted that the timing of the early strokes differed between the two groups. The great majority of both disabling and nondisabling strokes in the TAVR patients were periprocedural, occurring on the day of TAVR or the next day. Strokes in the SAVR group occurred then as well, but also on days 2-6.
One reason why SURTAVI is the first study to show a lower stroke risk with TAVR is that it was the first TAVR-versus-SAVR study to feature comprehensive neurologic testing pre- and post-procedure, along with evaluation of all suspected events by a neurologist or stroke specialist, according to Dr. Kappetein.
“As surgeons we all have said the stroke rate after SAVR is 1%-1.5%, but only when the patient wasn’t waving to us the next morning would we say, ‘Oh, that patient may have a stroke.’ Then we would call a neurologist. So there were many more subtle strokes that we never actually detected. If you do a proper examination of the patient before and after the procedure you’ll find many more strokes,” he said.
He and his coinvestigators systematically searched in vain for predictors of increased stroke risk among the TAVR and SAVR patients.
“Actually, the stroke risk is present for every patient we treat with TAVR or SAVR,” the surgeon continued.
However, discussant Adnan Kastrati, MD, chief physician at the German Heart Center in Munich, thought he spied in the SURTAVI data a potential opportunity to reduce early strokes in SAVR patients. He noted that new-onset atrial fibrillation is consistently more common in SAVR than TAVR patients, and that many of the strokes in the SAVR group occurred on days 2-6. When do heart surgeons typically start oral anticoagulation in their patients with postsurgical atrial fibrillation? he asked.
Not until after 48 hours, Dr. Kappetein replied.
“Those strokes on days 4, 5, and 6 might have to do with atrial fibrillation, and we may need to be more aggressive as surgeons in anticoagulating patients with atrial fibrillation after surgery,” he said.
Dr. Kappetein reported receiving research grant support from Medtronic, sponsor of SURTAVI.
This article was updated July 28, 2107.
PARIS – Patients with severe aortic stenosis at intermediate operative risk have a significantly lower 30-day risk of stroke and other neurologic complications with transcatheter aortic valve replacement than with surgical replacement, according to new results from the landmark SURTAVI trial.
“This is the first time the stroke rate has been shown to be lower with TAVR than with surgery,” A. Pieter Kappetein, MD, noted in presenting the results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
It’s a finding that adds to the momentum for studying TAVR in low-surgical-risk patients, he added.
“As we move toward lower-risk patients it will become even more important to see whether there is a difference in stroke. Suppose the stroke rate in SURTAVI had been a little higher with TAVR than SAVR? It would really make us more cautious about moving toward lower-risk patients. Now that we see that in intermediate-risk patients the stroke rate is actually a little bit lower than with surgery, I think we’ll feel more comfortable moving toward lower-risk patients,” according to Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University Medical Center in Rotterdam.
SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) involved randomization of 1,660 patients with severe symptomatic aortic stenosis to TAVR or SAVR. All participants were deemed to be at intermediate operative risk based upon a predicted surgical mortality of 3%-15%. The primary outcome -- a composite of all-cause mortality and disabling stroke at 2 years -- was presented at the 2017 meeting of the American College of Cardiology and simultaneously published (N Engl J Med. 2017 Apr 6;376(14):1321-1331). The rate was 12.6% with TAVR using the self-expanding CoreValve or Evolut R bioprosthesis and noninferior at 14% with SAVR.
Dr. Kappetein presented a prespecified secondary analysis of the 30-day rate of all neurologic complications, including nondisabling strokes and encephalopathy. He and the other SURTAVI organizers felt this was an important outcome because these early neurologic events have a major impact upon quality of life, including whether a patient will be discharged home or to a rehabilitation clinic or skilled nursing facility following aortic valve replacement.
The 30-day incidence of all stroke was 3.3% in the TAVR patients, significantly lower than the 5.4% rate in the SAVR group. By the 2-year mark, however, the difference was no longer statistically significant, with a rate of 6.3% in the TAVR group compared with 8.0% with SAVR.
Ninety-five percent of the early strokes were ischemic.
The 30-day incidence of disabling stroke was 1.2% with TAVR and 2.4% with SAVR, a difference that was not significant (P=0.057). The 2-year rate was 2.4% in the TAVR arm and 4.5% with SAVR, again not significantly different.
Half of the strokes in the TAVR group had a modified Rankin score of 0-1 at 30 days, meaning no or only minimal signs of stroke. In contrast, most of the strokes in the SAVR group were disabling, with a modified Rankin score of 2-6.
Only 36% of patients who had an early stroke were discharged home, compared with 87% of patients without a stroke. Not surprisingly, quality of life as assessed using the SF-36 physical summary was significantly worse in the early-stroke group. However, with or without stroke, TAVR patients recovered quality of life faster than SAVR patients.
He noted that the timing of the early strokes differed between the two groups. The great majority of both disabling and nondisabling strokes in the TAVR patients were periprocedural, occurring on the day of TAVR or the next day. Strokes in the SAVR group occurred then as well, but also on days 2-6.
One reason why SURTAVI is the first study to show a lower stroke risk with TAVR is that it was the first TAVR-versus-SAVR study to feature comprehensive neurologic testing pre- and post-procedure, along with evaluation of all suspected events by a neurologist or stroke specialist, according to Dr. Kappetein.
“As surgeons we all have said the stroke rate after SAVR is 1%-1.5%, but only when the patient wasn’t waving to us the next morning would we say, ‘Oh, that patient may have a stroke.’ Then we would call a neurologist. So there were many more subtle strokes that we never actually detected. If you do a proper examination of the patient before and after the procedure you’ll find many more strokes,” he said.
He and his coinvestigators systematically searched in vain for predictors of increased stroke risk among the TAVR and SAVR patients.
“Actually, the stroke risk is present for every patient we treat with TAVR or SAVR,” the surgeon continued.
However, discussant Adnan Kastrati, MD, chief physician at the German Heart Center in Munich, thought he spied in the SURTAVI data a potential opportunity to reduce early strokes in SAVR patients. He noted that new-onset atrial fibrillation is consistently more common in SAVR than TAVR patients, and that many of the strokes in the SAVR group occurred on days 2-6. When do heart surgeons typically start oral anticoagulation in their patients with postsurgical atrial fibrillation? he asked.
Not until after 48 hours, Dr. Kappetein replied.
“Those strokes on days 4, 5, and 6 might have to do with atrial fibrillation, and we may need to be more aggressive as surgeons in anticoagulating patients with atrial fibrillation after surgery,” he said.
Dr. Kappetein reported receiving research grant support from Medtronic, sponsor of SURTAVI.
This article was updated July 28, 2107.
AT EUROPCR
Key clinical point:
Major finding: The combined incidence of disabling and nondisabling stroke within 30 days of TAVR was 3.3%, significantly better than the 5.4% rate in patients who underwent SAVR.
Data source: SURTAVI was a multicenter trial which included 1,660 patients with severe aortic stenosis who were at intermediate operative risk and were randomized to TAVR or SAVR.
Disclosures: The study presenter reported receiving research grant support from Medtronic, sponsor of the SURTAVI trial.
VIDEO: Childhood second-hand smoke boosts RA incidence
MADRID – Second-hand smoke exposure to children was about as potent a trigger for future rheumatoid arthritis as active smoking by an adult, based on an analysis of data collected from more than 70,000 French women followed for an average of more than 20 years
“This is the first demonstration of a rheumatoid arthritis risk associated with passive smoking,” Raphaèle Seror, MD, said at the European Congress of Rheumatology.
“This is an important finding because we can avoid passive smoke exposure,” Dr. Seror added in a video interview . The imperative to eliminate second-hand smoke exposure to children is particularly acute for those with a genetic risk for developing rheumatoid arthritis (RA), specifically children with a parent diagnosed with RA, suggested Dr. Seror, a professor of rheumatology at the University of Paris–South.
She and her associates used data collected in the E3N, a longitudinal French epidemiological study that enrolled nearly 100,000 women in 1990 when they were 40-65 years old and collected health data by questionnaire every 2-3 years for an average of 21 years. They identified from this cohort women with “confirmed” RA based on a self report of having incident RA during follow-up plus a coincident record of reimbursement for a prescription for an RA-specific treatment, such as methotrexate or a biological disease-modifying drug.
This identified 389 women with confirmed incident RA, including 350 with a complete smoking history that made the current analysis possible. The study also included 70,248 women who did not develop RA and who had provided a complete smoking history.
The analysis showed that women who reported a history of second-hand smoke exposure estimated at more than an hour daily as children but without a history of active smoking had a 43% higher rate of incident RA compared with never smoker women without a history of passive smoke exposure, Dr. Seror reported. This association just missed reaching statistical significance, a limitation that Dr. Seror attributed to a power issue as the analysis included only 30 women who had incident RA and a history of second-hand smoke exposure without adult smoke exposure. By comparison, women in the study with a history of active smoking without childhood exposure linked had a 37% increased incidence of RA, a finding that confirmed the well-known link between smoking and RA incidence.
The study also found that women with both second-hand smoke exposure as children and adult smoking linked with a 73% higher RA incidence, an indication that the contributions from second-hand smoke in children and active smoking by adults were not only similar in magnitude but also worked additively to promote RA development.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
MADRID – Second-hand smoke exposure to children was about as potent a trigger for future rheumatoid arthritis as active smoking by an adult, based on an analysis of data collected from more than 70,000 French women followed for an average of more than 20 years
“This is the first demonstration of a rheumatoid arthritis risk associated with passive smoking,” Raphaèle Seror, MD, said at the European Congress of Rheumatology.
“This is an important finding because we can avoid passive smoke exposure,” Dr. Seror added in a video interview . The imperative to eliminate second-hand smoke exposure to children is particularly acute for those with a genetic risk for developing rheumatoid arthritis (RA), specifically children with a parent diagnosed with RA, suggested Dr. Seror, a professor of rheumatology at the University of Paris–South.
She and her associates used data collected in the E3N, a longitudinal French epidemiological study that enrolled nearly 100,000 women in 1990 when they were 40-65 years old and collected health data by questionnaire every 2-3 years for an average of 21 years. They identified from this cohort women with “confirmed” RA based on a self report of having incident RA during follow-up plus a coincident record of reimbursement for a prescription for an RA-specific treatment, such as methotrexate or a biological disease-modifying drug.
This identified 389 women with confirmed incident RA, including 350 with a complete smoking history that made the current analysis possible. The study also included 70,248 women who did not develop RA and who had provided a complete smoking history.
The analysis showed that women who reported a history of second-hand smoke exposure estimated at more than an hour daily as children but without a history of active smoking had a 43% higher rate of incident RA compared with never smoker women without a history of passive smoke exposure, Dr. Seror reported. This association just missed reaching statistical significance, a limitation that Dr. Seror attributed to a power issue as the analysis included only 30 women who had incident RA and a history of second-hand smoke exposure without adult smoke exposure. By comparison, women in the study with a history of active smoking without childhood exposure linked had a 37% increased incidence of RA, a finding that confirmed the well-known link between smoking and RA incidence.
The study also found that women with both second-hand smoke exposure as children and adult smoking linked with a 73% higher RA incidence, an indication that the contributions from second-hand smoke in children and active smoking by adults were not only similar in magnitude but also worked additively to promote RA development.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
MADRID – Second-hand smoke exposure to children was about as potent a trigger for future rheumatoid arthritis as active smoking by an adult, based on an analysis of data collected from more than 70,000 French women followed for an average of more than 20 years
“This is the first demonstration of a rheumatoid arthritis risk associated with passive smoking,” Raphaèle Seror, MD, said at the European Congress of Rheumatology.
“This is an important finding because we can avoid passive smoke exposure,” Dr. Seror added in a video interview . The imperative to eliminate second-hand smoke exposure to children is particularly acute for those with a genetic risk for developing rheumatoid arthritis (RA), specifically children with a parent diagnosed with RA, suggested Dr. Seror, a professor of rheumatology at the University of Paris–South.
She and her associates used data collected in the E3N, a longitudinal French epidemiological study that enrolled nearly 100,000 women in 1990 when they were 40-65 years old and collected health data by questionnaire every 2-3 years for an average of 21 years. They identified from this cohort women with “confirmed” RA based on a self report of having incident RA during follow-up plus a coincident record of reimbursement for a prescription for an RA-specific treatment, such as methotrexate or a biological disease-modifying drug.
This identified 389 women with confirmed incident RA, including 350 with a complete smoking history that made the current analysis possible. The study also included 70,248 women who did not develop RA and who had provided a complete smoking history.
The analysis showed that women who reported a history of second-hand smoke exposure estimated at more than an hour daily as children but without a history of active smoking had a 43% higher rate of incident RA compared with never smoker women without a history of passive smoke exposure, Dr. Seror reported. This association just missed reaching statistical significance, a limitation that Dr. Seror attributed to a power issue as the analysis included only 30 women who had incident RA and a history of second-hand smoke exposure without adult smoke exposure. By comparison, women in the study with a history of active smoking without childhood exposure linked had a 37% increased incidence of RA, a finding that confirmed the well-known link between smoking and RA incidence.
The study also found that women with both second-hand smoke exposure as children and adult smoking linked with a 73% higher RA incidence, an indication that the contributions from second-hand smoke in children and active smoking by adults were not only similar in magnitude but also worked additively to promote RA development.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Women with significant second-hand smoke exposure as children had a 43% increased rate of incident rheumatoid arthritis.
Data source: E3N, a prospective, longitudinal, observational study of nearly 100,000 French women begun in 1990.
Disclosures: Dr. Seror had no relevant disclosures.