User login
ODYSSEY: Alirocumab improves lipids but not glycemic targets in 2DM
SAN DIEGO – The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab helped correct dyslipidemia but did not improve glucose control in patients with type 2 diabetes mellitus, investigators reported at the annual scientific sessions of the American Diabetes Association.
In the international, double-blind ODYSSEY DM-Insulin trial, 24 weeks of alirocumab (Praluent, Sanofi and Regeneron) therapy cut low-density lipoprotein (LDL) cholesterol levels by an average of 49% more than placebo (P less than .0001), said Lawrence A. Leiter, MD, professor of medicine and nutritional sciences at the University of Toronto. Alirocumab also significantly reduced non–high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a) levels. However, hemoglobin A1c, fasting plasma glucose, total insulin dose, and number of antihyperglycemic drugs remained nearly identical between the trial arms throughout follow-up.
About 94% of patients in each arm completed the trial. Most were in their mid-60s, white, obese, and already on a moderate or high-intensity statin, with baseline fasting plasma glucose levels of about 150 mg per dL and HbA1c levels of 7.5%. The most common treatment-associated adverse events were myalgia (4%) and arthralgia (3%). Rates of local and systemic allergic drug reactions, neurologic or neurocognitive events, and elevated transaminases were low and similar between groups, according to Dr. Leiter, who is also director of the lipid clinic at the Li Ka Shing Knowledge Institute at St. Michael’s Hospital.
Robert R. Henry, MD, who is professor of medicine at the University of California, San Diego, discussed the ODYSSEY DM-Dyslipidemia trial which compared alirocumab with usual care in patients with type 2 diabetes whose mixed dyslipidemia was inadequately controlled with maximum tolerable statin therapy. In all, 413 patients received open-label alirocumab (75 mg–150 mg) or placebo plus optional ezetimibe, fenofibrate, omega-3 fatty acids, or nicotinic acid every 2 weeks for 24 weeks. At the end of treatment, non-HDL cholesterol dropped about 33% more with alirocumab than usual care (P less than .0001). Alirocumab also produced significant declines in LDL cholesterol, apolipoprotein B, total cholesterol, and lipoprotein(a), and a 6% increase in HDL cholesterol as compared with usual care. Once again, alirocumab induced no changes in HbA1c or fasting plasma glucose levels. The most common treatment-related adverse events were urinary tract infections, diarrhea, and nasopharyngitis.
Sanofi US and Regeneron Pharmaceuticals make alirocumab and funded the trials. Dr. Leiter disclosed research grants and consulting relationships with Regeneron, Sanofi, Eli Lilly and Company, and several other pharmaceutical companies. Dr. Henry disclosed consulting and advisory relationships with Sanofi and many other pharmaceutical companies.
SAN DIEGO – The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab helped correct dyslipidemia but did not improve glucose control in patients with type 2 diabetes mellitus, investigators reported at the annual scientific sessions of the American Diabetes Association.
In the international, double-blind ODYSSEY DM-Insulin trial, 24 weeks of alirocumab (Praluent, Sanofi and Regeneron) therapy cut low-density lipoprotein (LDL) cholesterol levels by an average of 49% more than placebo (P less than .0001), said Lawrence A. Leiter, MD, professor of medicine and nutritional sciences at the University of Toronto. Alirocumab also significantly reduced non–high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a) levels. However, hemoglobin A1c, fasting plasma glucose, total insulin dose, and number of antihyperglycemic drugs remained nearly identical between the trial arms throughout follow-up.
About 94% of patients in each arm completed the trial. Most were in their mid-60s, white, obese, and already on a moderate or high-intensity statin, with baseline fasting plasma glucose levels of about 150 mg per dL and HbA1c levels of 7.5%. The most common treatment-associated adverse events were myalgia (4%) and arthralgia (3%). Rates of local and systemic allergic drug reactions, neurologic or neurocognitive events, and elevated transaminases were low and similar between groups, according to Dr. Leiter, who is also director of the lipid clinic at the Li Ka Shing Knowledge Institute at St. Michael’s Hospital.
Robert R. Henry, MD, who is professor of medicine at the University of California, San Diego, discussed the ODYSSEY DM-Dyslipidemia trial which compared alirocumab with usual care in patients with type 2 diabetes whose mixed dyslipidemia was inadequately controlled with maximum tolerable statin therapy. In all, 413 patients received open-label alirocumab (75 mg–150 mg) or placebo plus optional ezetimibe, fenofibrate, omega-3 fatty acids, or nicotinic acid every 2 weeks for 24 weeks. At the end of treatment, non-HDL cholesterol dropped about 33% more with alirocumab than usual care (P less than .0001). Alirocumab also produced significant declines in LDL cholesterol, apolipoprotein B, total cholesterol, and lipoprotein(a), and a 6% increase in HDL cholesterol as compared with usual care. Once again, alirocumab induced no changes in HbA1c or fasting plasma glucose levels. The most common treatment-related adverse events were urinary tract infections, diarrhea, and nasopharyngitis.
Sanofi US and Regeneron Pharmaceuticals make alirocumab and funded the trials. Dr. Leiter disclosed research grants and consulting relationships with Regeneron, Sanofi, Eli Lilly and Company, and several other pharmaceutical companies. Dr. Henry disclosed consulting and advisory relationships with Sanofi and many other pharmaceutical companies.
SAN DIEGO – The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab helped correct dyslipidemia but did not improve glucose control in patients with type 2 diabetes mellitus, investigators reported at the annual scientific sessions of the American Diabetes Association.
In the international, double-blind ODYSSEY DM-Insulin trial, 24 weeks of alirocumab (Praluent, Sanofi and Regeneron) therapy cut low-density lipoprotein (LDL) cholesterol levels by an average of 49% more than placebo (P less than .0001), said Lawrence A. Leiter, MD, professor of medicine and nutritional sciences at the University of Toronto. Alirocumab also significantly reduced non–high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a) levels. However, hemoglobin A1c, fasting plasma glucose, total insulin dose, and number of antihyperglycemic drugs remained nearly identical between the trial arms throughout follow-up.
About 94% of patients in each arm completed the trial. Most were in their mid-60s, white, obese, and already on a moderate or high-intensity statin, with baseline fasting plasma glucose levels of about 150 mg per dL and HbA1c levels of 7.5%. The most common treatment-associated adverse events were myalgia (4%) and arthralgia (3%). Rates of local and systemic allergic drug reactions, neurologic or neurocognitive events, and elevated transaminases were low and similar between groups, according to Dr. Leiter, who is also director of the lipid clinic at the Li Ka Shing Knowledge Institute at St. Michael’s Hospital.
Robert R. Henry, MD, who is professor of medicine at the University of California, San Diego, discussed the ODYSSEY DM-Dyslipidemia trial which compared alirocumab with usual care in patients with type 2 diabetes whose mixed dyslipidemia was inadequately controlled with maximum tolerable statin therapy. In all, 413 patients received open-label alirocumab (75 mg–150 mg) or placebo plus optional ezetimibe, fenofibrate, omega-3 fatty acids, or nicotinic acid every 2 weeks for 24 weeks. At the end of treatment, non-HDL cholesterol dropped about 33% more with alirocumab than usual care (P less than .0001). Alirocumab also produced significant declines in LDL cholesterol, apolipoprotein B, total cholesterol, and lipoprotein(a), and a 6% increase in HDL cholesterol as compared with usual care. Once again, alirocumab induced no changes in HbA1c or fasting plasma glucose levels. The most common treatment-related adverse events were urinary tract infections, diarrhea, and nasopharyngitis.
Sanofi US and Regeneron Pharmaceuticals make alirocumab and funded the trials. Dr. Leiter disclosed research grants and consulting relationships with Regeneron, Sanofi, Eli Lilly and Company, and several other pharmaceutical companies. Dr. Henry disclosed consulting and advisory relationships with Sanofi and many other pharmaceutical companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point:
Major finding: After 24 weeks of treatment, average low-density lipoprotein levels fell by about 43%-49% (P less than .0001, compared with placebo, in each trial).
Data source: ODYSSEY DM-Insulin included 441 patients on insulin for type 2 diabetes who had elevated LDL levels and other cardiovascular risk factors. ODYSSEY DM-Dyslipidemia included 413 patients with type 2 diabetes and mixed dyslipidemia despite maximally tolerated statins.
Disclosures: Sanofi and Regeneron Pharmaceuticals funded the trial. Dr. Leiter disclosed research grants and consulting relationships with Regeneron, Sanofi, Eli Lilly, and several other pharmaceutical companies.
Aspirin triples major bleeding risk after age 75 years
Older adults who take aspirin daily are at greater risk for serious bleeding than previously thought, based on data from roughly 3,000 patients.
“The risk of upper gastrointestinal bleeding on antiplatelet treatment increases with age, but it is uncertain whether older age alone is a sufficient indicator of high risk to justify routine coprescription of PPIs [proton pump inhibitors],” wrote Linxin Li, DPhil, of the University of Oxford (England) and her colleagues.
To assess the rate of bleeding among older adults on long-term aspirin therapy, Dr. Li and her colleagues reviewed data from the Oxford Vascular Study, a prospective population-based study of 3,166 patients. Of those, 1,584 were younger than 75 years, with an average age of 61 years, and 1,582 were at least 75 years old, with average age of 83 years. Patients were followed at 30 days, 6 months, and 1, 5, and 10 years to determine bleeding, recurrent ischemic events, and disability (Lancet. 2017. doi: 10.1016/S0140-6736[17]30770-5).
In addition, more than twice the major upper GI bleeds were disabling or fatal in adults aged 75 years and older than in the younger patients (62% vs. 25%).
Only a third of the patients in the study were taking proton pump inhibitors (PPIs), partly because current clinical guidelines don’t specifically recommend their use and partly in the absence of an accepted definition of which patients are at high risk for upper GI bleeding, the researchers said. They estimated that the number needed to treat with PPIs to prevent a major GI bleed after 5 years decreased with age: “80 for patients younger than 65 years, 75 for patients aged 65-74 years, 23 for patients aged 75-84 years, and 21 for patients aged 85 years or older.” In addition, the number needed to treat with PPIs to prevent a disabling or fatal upper GI bleed after 5 years was 338 for patients younger than 65 years but dropped to 25 for patients aged 85 years and older.
The findings were limited by the observational nature of the study and inability to show that increased risk of bleeding was caused by aspirin alone, the researchers said. However, based on the data, “age 75 years would be an appropriate threshold to start a PPI both in patients newly initiated on antiplatelet drugs and in patients on established treatment,” they wrote.
The study data were taken from the Oxford Vascular Study, which was funded by the National Institute of Health Research and several other research institutions. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
In patients with stroke with a cardiac source of embolism who qualify for oral anticoagulation, we obsess about the association between benefit and bleeding risk. Specific risk scores were developed to assess the bleeding risk for patients with atrial fibrillation who qualified for anticoagulation. However, similar risk scores are not applied for patients who undergo long-term prevention with antiplatelet therapy.
On the basis of this study’s results, the benefit-risk association in long-term antiplatelet therapy should be evaluated every 3-5 years in patients older than 75 years, and PPIs should be used in patients on antiplatelet therapy who are at least 75 years old or in patients with a history of gastrointestinal bleeds.
Hans-Christoph Diener, MD, of the department of neurology at the University Duisburg-Essen in Essen, Germany, made these remarks in an accompanying editorial (Lancet. 2017 Jun 13. doi: 10.1016/S0140-6736[17]31507-6). He disclosed relationships with multiple companies, including AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Novartis.
In patients with stroke with a cardiac source of embolism who qualify for oral anticoagulation, we obsess about the association between benefit and bleeding risk. Specific risk scores were developed to assess the bleeding risk for patients with atrial fibrillation who qualified for anticoagulation. However, similar risk scores are not applied for patients who undergo long-term prevention with antiplatelet therapy.
On the basis of this study’s results, the benefit-risk association in long-term antiplatelet therapy should be evaluated every 3-5 years in patients older than 75 years, and PPIs should be used in patients on antiplatelet therapy who are at least 75 years old or in patients with a history of gastrointestinal bleeds.
Hans-Christoph Diener, MD, of the department of neurology at the University Duisburg-Essen in Essen, Germany, made these remarks in an accompanying editorial (Lancet. 2017 Jun 13. doi: 10.1016/S0140-6736[17]31507-6). He disclosed relationships with multiple companies, including AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Novartis.
In patients with stroke with a cardiac source of embolism who qualify for oral anticoagulation, we obsess about the association between benefit and bleeding risk. Specific risk scores were developed to assess the bleeding risk for patients with atrial fibrillation who qualified for anticoagulation. However, similar risk scores are not applied for patients who undergo long-term prevention with antiplatelet therapy.
On the basis of this study’s results, the benefit-risk association in long-term antiplatelet therapy should be evaluated every 3-5 years in patients older than 75 years, and PPIs should be used in patients on antiplatelet therapy who are at least 75 years old or in patients with a history of gastrointestinal bleeds.
Hans-Christoph Diener, MD, of the department of neurology at the University Duisburg-Essen in Essen, Germany, made these remarks in an accompanying editorial (Lancet. 2017 Jun 13. doi: 10.1016/S0140-6736[17]31507-6). He disclosed relationships with multiple companies, including AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Novartis.
Older adults who take aspirin daily are at greater risk for serious bleeding than previously thought, based on data from roughly 3,000 patients.
“The risk of upper gastrointestinal bleeding on antiplatelet treatment increases with age, but it is uncertain whether older age alone is a sufficient indicator of high risk to justify routine coprescription of PPIs [proton pump inhibitors],” wrote Linxin Li, DPhil, of the University of Oxford (England) and her colleagues.
To assess the rate of bleeding among older adults on long-term aspirin therapy, Dr. Li and her colleagues reviewed data from the Oxford Vascular Study, a prospective population-based study of 3,166 patients. Of those, 1,584 were younger than 75 years, with an average age of 61 years, and 1,582 were at least 75 years old, with average age of 83 years. Patients were followed at 30 days, 6 months, and 1, 5, and 10 years to determine bleeding, recurrent ischemic events, and disability (Lancet. 2017. doi: 10.1016/S0140-6736[17]30770-5).
In addition, more than twice the major upper GI bleeds were disabling or fatal in adults aged 75 years and older than in the younger patients (62% vs. 25%).
Only a third of the patients in the study were taking proton pump inhibitors (PPIs), partly because current clinical guidelines don’t specifically recommend their use and partly in the absence of an accepted definition of which patients are at high risk for upper GI bleeding, the researchers said. They estimated that the number needed to treat with PPIs to prevent a major GI bleed after 5 years decreased with age: “80 for patients younger than 65 years, 75 for patients aged 65-74 years, 23 for patients aged 75-84 years, and 21 for patients aged 85 years or older.” In addition, the number needed to treat with PPIs to prevent a disabling or fatal upper GI bleed after 5 years was 338 for patients younger than 65 years but dropped to 25 for patients aged 85 years and older.
The findings were limited by the observational nature of the study and inability to show that increased risk of bleeding was caused by aspirin alone, the researchers said. However, based on the data, “age 75 years would be an appropriate threshold to start a PPI both in patients newly initiated on antiplatelet drugs and in patients on established treatment,” they wrote.
The study data were taken from the Oxford Vascular Study, which was funded by the National Institute of Health Research and several other research institutions. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
Older adults who take aspirin daily are at greater risk for serious bleeding than previously thought, based on data from roughly 3,000 patients.
“The risk of upper gastrointestinal bleeding on antiplatelet treatment increases with age, but it is uncertain whether older age alone is a sufficient indicator of high risk to justify routine coprescription of PPIs [proton pump inhibitors],” wrote Linxin Li, DPhil, of the University of Oxford (England) and her colleagues.
To assess the rate of bleeding among older adults on long-term aspirin therapy, Dr. Li and her colleagues reviewed data from the Oxford Vascular Study, a prospective population-based study of 3,166 patients. Of those, 1,584 were younger than 75 years, with an average age of 61 years, and 1,582 were at least 75 years old, with average age of 83 years. Patients were followed at 30 days, 6 months, and 1, 5, and 10 years to determine bleeding, recurrent ischemic events, and disability (Lancet. 2017. doi: 10.1016/S0140-6736[17]30770-5).
In addition, more than twice the major upper GI bleeds were disabling or fatal in adults aged 75 years and older than in the younger patients (62% vs. 25%).
Only a third of the patients in the study were taking proton pump inhibitors (PPIs), partly because current clinical guidelines don’t specifically recommend their use and partly in the absence of an accepted definition of which patients are at high risk for upper GI bleeding, the researchers said. They estimated that the number needed to treat with PPIs to prevent a major GI bleed after 5 years decreased with age: “80 for patients younger than 65 years, 75 for patients aged 65-74 years, 23 for patients aged 75-84 years, and 21 for patients aged 85 years or older.” In addition, the number needed to treat with PPIs to prevent a disabling or fatal upper GI bleed after 5 years was 338 for patients younger than 65 years but dropped to 25 for patients aged 85 years and older.
The findings were limited by the observational nature of the study and inability to show that increased risk of bleeding was caused by aspirin alone, the researchers said. However, based on the data, “age 75 years would be an appropriate threshold to start a PPI both in patients newly initiated on antiplatelet drugs and in patients on established treatment,” they wrote.
The study data were taken from the Oxford Vascular Study, which was funded by the National Institute of Health Research and several other research institutions. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
FROM THE LANCET
Key clinical point:
Major finding: The annual rate of life-threatening or fatal bleeding episodes was less than 0.5% for patients younger than 65 years but rose to 1.5% in those aged 75-84 years and 2.5% in those aged 85 years and older.
Data source: A prospective, population-based cohort study of 3,166 adults who had one transient ischemic attack, ischemic stroke, or MI and who were treated with antiplatelet drugs.
Disclosures: The study data were taken from the Oxford Vascular Study, which was funded by the Wellcome Trust, Wolfson Foundation, British Heart Foundation, Dunhill Medical Trust, the National Institute of Health Research (NIHR), and the NIHR Oxford Biomedical Research Centre. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
USPSTF recommends screening children, adolescents for obesity
Children and adolescents aged 6 years and older should be screened for obesity and referred to comprehensive, intensive behavioral interventions with at least 26 hours of intervention contact, according to a U.S. Preventive Services Task Force Recommendation Statement that was published online June 20 in JAMA.
This updated recommendation is largely consistent with the previous 2010 recommendation “but includes the word ‘adolescents’ to further clarify the population to which this recommendation applies,” according to a press release accompanying the Recommendation Statement and the Evidence Report on which it is based.
The behavioral interventions that proved most beneficial included at least 26 hours of contact over a period of 2-12 months. Those that included 52 or more hours of contact achieved even greater weight loss, as well as some improvements in cardiovascular and metabolic risk factors (JAMA. 2017 Jun 20. doi: 10.1001/jama.2017.6803).
In general, children and adolescents who received intensive behavioral intervention showed absolute reductions in BMI z scores of 0.20 and maintained their baseline weight within approximately 5 pounds, while control subjects showed small or no reductions in BMI z scores and typically gained a mean of 5-17 pounds.
The components of these comprehensive interventions varied, but the most successful ones included sessions involving both the child and the parent (separately, together, or both); offered both family and group sessions; provided education regarding healthy eating, exercising, and reading food labels; encouraged stimulus-control measures such as limiting access to unhealthy foods and limiting screen time (that is, physical inactivity); and included supervised physical activity. Additional beneficial components are assisting patients to identify and accomplish goals, self-monitor, and problem-solve, as well as teaching them coping skills and addressing their body image.
In contrast to behavioral interventions, pharmacotherapy was not endorsed by the USPSTF. The current evidence was deemed inadequate to determine whether the slight weight loss achieved with pharmacotherapy is clinically significant and whether it outweighs the harms of the medications.
The two agents currently used in this regard are metformin, which is not Food and Drug Administration–approved for this purpose, and orlistat, which is approved for patients aged 12 years and older. Orlistat in particular frequently causes adverse events including fatty or oily stools, abdominal pain or cramping, flatus with stool discharge, and fecal incontinence, Dr. Grossman and his associates said.
The USPSTF is an independent voluntary group supported by the U.S. Agency for Healthcare Research and Quality as mandated by Congress. The authors’ conflicts of interest are available at https://www.uspreventiveservicestaskforce.org/Page/Name/conflict-of-interest-disclosures.
Letter to the Editor
It is not surprising that there are so many overweight and obese children, despite the fact that in order to prevent bad feelings, children have to be much more overweight than adults to be classified as obese or overweight.
If one is waiting until age 6 years to screen for obesity, that horse will be long out of the barn. The problem begins when the rapid weight gain of infants does not slow down in the 2nd and 3rd years of life. This is also when bad food choices and eating habits often begin.
The other problem is that body mass index is a terrible tool and so is any indicator that tries to define obesity using only height and weight. None of these distinguish between the muscular child and the slender child with a large belly. Waist to height or body volume measurements are far better indicators.
Lastly, parents have come to see the mildly overweight child as the norm because so many children are. Until parents see pictures of children from a few decades ago and are educated as to what normal looks like, we will have great difficulty making a dent in this problem.
Richard H. Feuille Jr., MD
This USPSTF recommendation simply confirms what pediatric clinicians always do in the everyday care for children and adolescents: monitor growth, counsel on healthy lifestyles, and refer for specialized care when appropriate.
Intensive behavioral interventions are impractical for many families and frequently aren’t covered by insurance. At best, implementing this recommendation will have only a modest effect on obesity in the United States. At worst, it could divert attention and resources away from population-health approaches to prevention and toward weight management programs that are not well equipped to meet the demand and very often don’t exist within local communities.
Improving neighborhood walkability, increasing the availability of healthy foods, and providing safe physical spaces would be more effective at reducing childhood obesity, as would improving school nutrition and curtailing the marketing of sugar-sweetened drinks and other unhealthy foods to children.
Rachel L. J. Thornton, MD, PhD, Raquel G. Hernandez, MD, MPH; Tina L. Cheng, MD, MPH, are in the department of pediatrics at Johns Hopkins University, Baltimore. They reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the USPSTF report (JAMA. 2017;317:2378-80).
This USPSTF recommendation simply confirms what pediatric clinicians always do in the everyday care for children and adolescents: monitor growth, counsel on healthy lifestyles, and refer for specialized care when appropriate.
Intensive behavioral interventions are impractical for many families and frequently aren’t covered by insurance. At best, implementing this recommendation will have only a modest effect on obesity in the United States. At worst, it could divert attention and resources away from population-health approaches to prevention and toward weight management programs that are not well equipped to meet the demand and very often don’t exist within local communities.
Improving neighborhood walkability, increasing the availability of healthy foods, and providing safe physical spaces would be more effective at reducing childhood obesity, as would improving school nutrition and curtailing the marketing of sugar-sweetened drinks and other unhealthy foods to children.
Rachel L. J. Thornton, MD, PhD, Raquel G. Hernandez, MD, MPH; Tina L. Cheng, MD, MPH, are in the department of pediatrics at Johns Hopkins University, Baltimore. They reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the USPSTF report (JAMA. 2017;317:2378-80).
This USPSTF recommendation simply confirms what pediatric clinicians always do in the everyday care for children and adolescents: monitor growth, counsel on healthy lifestyles, and refer for specialized care when appropriate.
Intensive behavioral interventions are impractical for many families and frequently aren’t covered by insurance. At best, implementing this recommendation will have only a modest effect on obesity in the United States. At worst, it could divert attention and resources away from population-health approaches to prevention and toward weight management programs that are not well equipped to meet the demand and very often don’t exist within local communities.
Improving neighborhood walkability, increasing the availability of healthy foods, and providing safe physical spaces would be more effective at reducing childhood obesity, as would improving school nutrition and curtailing the marketing of sugar-sweetened drinks and other unhealthy foods to children.
Rachel L. J. Thornton, MD, PhD, Raquel G. Hernandez, MD, MPH; Tina L. Cheng, MD, MPH, are in the department of pediatrics at Johns Hopkins University, Baltimore. They reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the USPSTF report (JAMA. 2017;317:2378-80).
Children and adolescents aged 6 years and older should be screened for obesity and referred to comprehensive, intensive behavioral interventions with at least 26 hours of intervention contact, according to a U.S. Preventive Services Task Force Recommendation Statement that was published online June 20 in JAMA.
This updated recommendation is largely consistent with the previous 2010 recommendation “but includes the word ‘adolescents’ to further clarify the population to which this recommendation applies,” according to a press release accompanying the Recommendation Statement and the Evidence Report on which it is based.
The behavioral interventions that proved most beneficial included at least 26 hours of contact over a period of 2-12 months. Those that included 52 or more hours of contact achieved even greater weight loss, as well as some improvements in cardiovascular and metabolic risk factors (JAMA. 2017 Jun 20. doi: 10.1001/jama.2017.6803).
In general, children and adolescents who received intensive behavioral intervention showed absolute reductions in BMI z scores of 0.20 and maintained their baseline weight within approximately 5 pounds, while control subjects showed small or no reductions in BMI z scores and typically gained a mean of 5-17 pounds.
The components of these comprehensive interventions varied, but the most successful ones included sessions involving both the child and the parent (separately, together, or both); offered both family and group sessions; provided education regarding healthy eating, exercising, and reading food labels; encouraged stimulus-control measures such as limiting access to unhealthy foods and limiting screen time (that is, physical inactivity); and included supervised physical activity. Additional beneficial components are assisting patients to identify and accomplish goals, self-monitor, and problem-solve, as well as teaching them coping skills and addressing their body image.
In contrast to behavioral interventions, pharmacotherapy was not endorsed by the USPSTF. The current evidence was deemed inadequate to determine whether the slight weight loss achieved with pharmacotherapy is clinically significant and whether it outweighs the harms of the medications.
The two agents currently used in this regard are metformin, which is not Food and Drug Administration–approved for this purpose, and orlistat, which is approved for patients aged 12 years and older. Orlistat in particular frequently causes adverse events including fatty or oily stools, abdominal pain or cramping, flatus with stool discharge, and fecal incontinence, Dr. Grossman and his associates said.
The USPSTF is an independent voluntary group supported by the U.S. Agency for Healthcare Research and Quality as mandated by Congress. The authors’ conflicts of interest are available at https://www.uspreventiveservicestaskforce.org/Page/Name/conflict-of-interest-disclosures.
Letter to the Editor
It is not surprising that there are so many overweight and obese children, despite the fact that in order to prevent bad feelings, children have to be much more overweight than adults to be classified as obese or overweight.
If one is waiting until age 6 years to screen for obesity, that horse will be long out of the barn. The problem begins when the rapid weight gain of infants does not slow down in the 2nd and 3rd years of life. This is also when bad food choices and eating habits often begin.
The other problem is that body mass index is a terrible tool and so is any indicator that tries to define obesity using only height and weight. None of these distinguish between the muscular child and the slender child with a large belly. Waist to height or body volume measurements are far better indicators.
Lastly, parents have come to see the mildly overweight child as the norm because so many children are. Until parents see pictures of children from a few decades ago and are educated as to what normal looks like, we will have great difficulty making a dent in this problem.
Richard H. Feuille Jr., MD
Children and adolescents aged 6 years and older should be screened for obesity and referred to comprehensive, intensive behavioral interventions with at least 26 hours of intervention contact, according to a U.S. Preventive Services Task Force Recommendation Statement that was published online June 20 in JAMA.
This updated recommendation is largely consistent with the previous 2010 recommendation “but includes the word ‘adolescents’ to further clarify the population to which this recommendation applies,” according to a press release accompanying the Recommendation Statement and the Evidence Report on which it is based.
The behavioral interventions that proved most beneficial included at least 26 hours of contact over a period of 2-12 months. Those that included 52 or more hours of contact achieved even greater weight loss, as well as some improvements in cardiovascular and metabolic risk factors (JAMA. 2017 Jun 20. doi: 10.1001/jama.2017.6803).
In general, children and adolescents who received intensive behavioral intervention showed absolute reductions in BMI z scores of 0.20 and maintained their baseline weight within approximately 5 pounds, while control subjects showed small or no reductions in BMI z scores and typically gained a mean of 5-17 pounds.
The components of these comprehensive interventions varied, but the most successful ones included sessions involving both the child and the parent (separately, together, or both); offered both family and group sessions; provided education regarding healthy eating, exercising, and reading food labels; encouraged stimulus-control measures such as limiting access to unhealthy foods and limiting screen time (that is, physical inactivity); and included supervised physical activity. Additional beneficial components are assisting patients to identify and accomplish goals, self-monitor, and problem-solve, as well as teaching them coping skills and addressing their body image.
In contrast to behavioral interventions, pharmacotherapy was not endorsed by the USPSTF. The current evidence was deemed inadequate to determine whether the slight weight loss achieved with pharmacotherapy is clinically significant and whether it outweighs the harms of the medications.
The two agents currently used in this regard are metformin, which is not Food and Drug Administration–approved for this purpose, and orlistat, which is approved for patients aged 12 years and older. Orlistat in particular frequently causes adverse events including fatty or oily stools, abdominal pain or cramping, flatus with stool discharge, and fecal incontinence, Dr. Grossman and his associates said.
The USPSTF is an independent voluntary group supported by the U.S. Agency for Healthcare Research and Quality as mandated by Congress. The authors’ conflicts of interest are available at https://www.uspreventiveservicestaskforce.org/Page/Name/conflict-of-interest-disclosures.
Letter to the Editor
It is not surprising that there are so many overweight and obese children, despite the fact that in order to prevent bad feelings, children have to be much more overweight than adults to be classified as obese or overweight.
If one is waiting until age 6 years to screen for obesity, that horse will be long out of the barn. The problem begins when the rapid weight gain of infants does not slow down in the 2nd and 3rd years of life. This is also when bad food choices and eating habits often begin.
The other problem is that body mass index is a terrible tool and so is any indicator that tries to define obesity using only height and weight. None of these distinguish between the muscular child and the slender child with a large belly. Waist to height or body volume measurements are far better indicators.
Lastly, parents have come to see the mildly overweight child as the norm because so many children are. Until parents see pictures of children from a few decades ago and are educated as to what normal looks like, we will have great difficulty making a dent in this problem.
Richard H. Feuille Jr., MD
FROM JAMA
Key clinical point: with at least 26 hours of contact.
Major finding: Children and adolescents who received intensive behavioral intervention showed absolute reductions in BMI z scores of 0.20 and maintained their baseline weight within approximately 5 pounds, while control subjects showed small or no reductions in BMI z scores and typically gained a mean of 5-17 pounds.
Data source: A review of the literature since the previous USPSTF recommendation statement in 2010, including 45 studies of lifestyle-based interventions involving 7,099 overweight and obese children.
Disclosures: The USPSTF is an independent voluntary group supported by the U.S. Agency for Healthcare Research and Quality as mandated by Congress. The authors’ conflicts of interest are available at www.uspreventiveservicestaskforce.org.
MMR vaccine cut hospitalizations for unrelated respiratory infections
, said Giuseppe La Torre of the Sapienza University of Rome and his associates.
The 2-year retrospective database study included 11,004 children, 21% of whom did not receive the MMR vaccine; 49% received one dose, and 30% received two doses. There were 12 hospitalizations for measles (9 in unvaccinated children, 3 in those who received one dose, and none in those who received two doses), 2 hospitalizations for mumps (1 among vaccinated and 1 among unvaccinated children), and no hospitalizations for rubella (P less than .001).
There were 414 hospitalizations for all infectious diseases, 11% in unvaccinated children, 1.5% in those who had received one dose of vaccine, and 1% in those who had received two doses (P less than .001). MMR vaccine also was highly protective against hospitalizations for all infectious diseases (HR, 0.29).
Of 809 hospitalizations for respiratory diseases, 18% involved children who had not been vaccinated, 4% involved children who had received one dose, and 5.5% involved children vaccinated with two doses (P less than .001). MMR likewise was highly protective against hospitalizations for respiratory diseases (HR, 0.18).
Read more in the journal Human Vaccines & Immunotherapeutics (2017 Jun 12. doi: 10.1080/21645515.2017.1330733).
, said Giuseppe La Torre of the Sapienza University of Rome and his associates.
The 2-year retrospective database study included 11,004 children, 21% of whom did not receive the MMR vaccine; 49% received one dose, and 30% received two doses. There were 12 hospitalizations for measles (9 in unvaccinated children, 3 in those who received one dose, and none in those who received two doses), 2 hospitalizations for mumps (1 among vaccinated and 1 among unvaccinated children), and no hospitalizations for rubella (P less than .001).
There were 414 hospitalizations for all infectious diseases, 11% in unvaccinated children, 1.5% in those who had received one dose of vaccine, and 1% in those who had received two doses (P less than .001). MMR vaccine also was highly protective against hospitalizations for all infectious diseases (HR, 0.29).
Of 809 hospitalizations for respiratory diseases, 18% involved children who had not been vaccinated, 4% involved children who had received one dose, and 5.5% involved children vaccinated with two doses (P less than .001). MMR likewise was highly protective against hospitalizations for respiratory diseases (HR, 0.18).
Read more in the journal Human Vaccines & Immunotherapeutics (2017 Jun 12. doi: 10.1080/21645515.2017.1330733).
, said Giuseppe La Torre of the Sapienza University of Rome and his associates.
The 2-year retrospective database study included 11,004 children, 21% of whom did not receive the MMR vaccine; 49% received one dose, and 30% received two doses. There were 12 hospitalizations for measles (9 in unvaccinated children, 3 in those who received one dose, and none in those who received two doses), 2 hospitalizations for mumps (1 among vaccinated and 1 among unvaccinated children), and no hospitalizations for rubella (P less than .001).
There were 414 hospitalizations for all infectious diseases, 11% in unvaccinated children, 1.5% in those who had received one dose of vaccine, and 1% in those who had received two doses (P less than .001). MMR vaccine also was highly protective against hospitalizations for all infectious diseases (HR, 0.29).
Of 809 hospitalizations for respiratory diseases, 18% involved children who had not been vaccinated, 4% involved children who had received one dose, and 5.5% involved children vaccinated with two doses (P less than .001). MMR likewise was highly protective against hospitalizations for respiratory diseases (HR, 0.18).
Read more in the journal Human Vaccines & Immunotherapeutics (2017 Jun 12. doi: 10.1080/21645515.2017.1330733).
FROM HUMAN VACCINES & IMMUNOTHERAPEUTICS
Massive blood transfusions increase risk with CRS/HIPEC
Massive allogenic blood transfusion during cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) increased the risk of major complications and reduced overall survival in a review of 936 cases at St. George Hospital near Sydney, Australia.
CRS/HIPEC is a long, complex procedure for peritoneal carcinomatosis, pseudomyxoma peritonei, peritoneal mesothelioma, and other abdominal cancers. The abdomen is opened, the cancer is debulked as much as possible, and the cavity is filled with heated chemotherapy drugs. Because CRS/HIPEC often requires multivisceral resection and dissection in multiple abdominal regions, up to 77% of patients require intraoperative transfusions, and up to 37% require massive allogenic blood transfusions (MABT) with five or more units.
Blood transfusions are known to be associated with poorer cancer surgery outcomes, but their effect in CRS/HIPEC hasn’t been much studied, which is “surprising given the extent to which blood products are used in” the procedure, said investigators led by Akshat Saxena, MD, a surgeon at St. George Hospital (J Gastrointest Surg. 2017 May 30. doi: 10.1007/s11605-017-3444-8).
Based on their findings, the researchers concluded that “there is a real need to evaluate new strategies to reduce the rate of MABT during CRS/HIPEC.”
The procedures in the study were performed from 1996 to 2016. The in-hospital mortality rate was 0.3% in patients who did not have MABT but 4.4% among the 337 patients (36%) who did. Even after adjusting for confounders on multivariate analysis, including the fact that MABT patients had more extensive disease and longer surgeries, MABT significantly increased the risk of in-hospital mortality (relative risk, 7.72; P = .021). In patients requiring MABT had a 5-year survival of 5%. In patients not requiring MABT, 5-year survival was at 36%. The difference remained significant on multivariate analysis.
MABT patients also had twice the risk of life-threatening complications and complications requiring surgical, endoscopic, or radiological intervention (62% versus 30%; RR, 2.05; P less than .001). MABT patients were more likely to stay in the ICU for 4 or more days and in the hospital for 28 or more days.
Worse overall survival with MABT was driven at least in part by patients who had CRS/HIPEC for colorectal cancer peritoneal carcinomatosis and pseudomyxoma peritonei. MABT did not seem to contribute to lower survival in patients who had the procedure for appendiceal or ovarian cancer. “It seems that the impact of long-term immunomodulation induced by blood transfusion” – the suspected mechanism through which transfusions cause problems – “varies according to the disease subtype. This warrants further investigation,” the investigators said.
Several strategies have been tried to reduce the need for transfusions during CRS/HIPEC. The study team previously reported that preemptive clotting factor replacement helps. Others have had success with preemptive tranexamic acid and cryoprecipitate to address low serum fibrinogen levels during CRS/HIPEC. “Further evaluation of both these strategies is warranted,” the researchers said.
Funding source and disclosure information were not included in the study report.
Massive allogenic blood transfusion during cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) increased the risk of major complications and reduced overall survival in a review of 936 cases at St. George Hospital near Sydney, Australia.
CRS/HIPEC is a long, complex procedure for peritoneal carcinomatosis, pseudomyxoma peritonei, peritoneal mesothelioma, and other abdominal cancers. The abdomen is opened, the cancer is debulked as much as possible, and the cavity is filled with heated chemotherapy drugs. Because CRS/HIPEC often requires multivisceral resection and dissection in multiple abdominal regions, up to 77% of patients require intraoperative transfusions, and up to 37% require massive allogenic blood transfusions (MABT) with five or more units.
Blood transfusions are known to be associated with poorer cancer surgery outcomes, but their effect in CRS/HIPEC hasn’t been much studied, which is “surprising given the extent to which blood products are used in” the procedure, said investigators led by Akshat Saxena, MD, a surgeon at St. George Hospital (J Gastrointest Surg. 2017 May 30. doi: 10.1007/s11605-017-3444-8).
Based on their findings, the researchers concluded that “there is a real need to evaluate new strategies to reduce the rate of MABT during CRS/HIPEC.”
The procedures in the study were performed from 1996 to 2016. The in-hospital mortality rate was 0.3% in patients who did not have MABT but 4.4% among the 337 patients (36%) who did. Even after adjusting for confounders on multivariate analysis, including the fact that MABT patients had more extensive disease and longer surgeries, MABT significantly increased the risk of in-hospital mortality (relative risk, 7.72; P = .021). In patients requiring MABT had a 5-year survival of 5%. In patients not requiring MABT, 5-year survival was at 36%. The difference remained significant on multivariate analysis.
MABT patients also had twice the risk of life-threatening complications and complications requiring surgical, endoscopic, or radiological intervention (62% versus 30%; RR, 2.05; P less than .001). MABT patients were more likely to stay in the ICU for 4 or more days and in the hospital for 28 or more days.
Worse overall survival with MABT was driven at least in part by patients who had CRS/HIPEC for colorectal cancer peritoneal carcinomatosis and pseudomyxoma peritonei. MABT did not seem to contribute to lower survival in patients who had the procedure for appendiceal or ovarian cancer. “It seems that the impact of long-term immunomodulation induced by blood transfusion” – the suspected mechanism through which transfusions cause problems – “varies according to the disease subtype. This warrants further investigation,” the investigators said.
Several strategies have been tried to reduce the need for transfusions during CRS/HIPEC. The study team previously reported that preemptive clotting factor replacement helps. Others have had success with preemptive tranexamic acid and cryoprecipitate to address low serum fibrinogen levels during CRS/HIPEC. “Further evaluation of both these strategies is warranted,” the researchers said.
Funding source and disclosure information were not included in the study report.
Massive allogenic blood transfusion during cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) increased the risk of major complications and reduced overall survival in a review of 936 cases at St. George Hospital near Sydney, Australia.
CRS/HIPEC is a long, complex procedure for peritoneal carcinomatosis, pseudomyxoma peritonei, peritoneal mesothelioma, and other abdominal cancers. The abdomen is opened, the cancer is debulked as much as possible, and the cavity is filled with heated chemotherapy drugs. Because CRS/HIPEC often requires multivisceral resection and dissection in multiple abdominal regions, up to 77% of patients require intraoperative transfusions, and up to 37% require massive allogenic blood transfusions (MABT) with five or more units.
Blood transfusions are known to be associated with poorer cancer surgery outcomes, but their effect in CRS/HIPEC hasn’t been much studied, which is “surprising given the extent to which blood products are used in” the procedure, said investigators led by Akshat Saxena, MD, a surgeon at St. George Hospital (J Gastrointest Surg. 2017 May 30. doi: 10.1007/s11605-017-3444-8).
Based on their findings, the researchers concluded that “there is a real need to evaluate new strategies to reduce the rate of MABT during CRS/HIPEC.”
The procedures in the study were performed from 1996 to 2016. The in-hospital mortality rate was 0.3% in patients who did not have MABT but 4.4% among the 337 patients (36%) who did. Even after adjusting for confounders on multivariate analysis, including the fact that MABT patients had more extensive disease and longer surgeries, MABT significantly increased the risk of in-hospital mortality (relative risk, 7.72; P = .021). In patients requiring MABT had a 5-year survival of 5%. In patients not requiring MABT, 5-year survival was at 36%. The difference remained significant on multivariate analysis.
MABT patients also had twice the risk of life-threatening complications and complications requiring surgical, endoscopic, or radiological intervention (62% versus 30%; RR, 2.05; P less than .001). MABT patients were more likely to stay in the ICU for 4 or more days and in the hospital for 28 or more days.
Worse overall survival with MABT was driven at least in part by patients who had CRS/HIPEC for colorectal cancer peritoneal carcinomatosis and pseudomyxoma peritonei. MABT did not seem to contribute to lower survival in patients who had the procedure for appendiceal or ovarian cancer. “It seems that the impact of long-term immunomodulation induced by blood transfusion” – the suspected mechanism through which transfusions cause problems – “varies according to the disease subtype. This warrants further investigation,” the investigators said.
Several strategies have been tried to reduce the need for transfusions during CRS/HIPEC. The study team previously reported that preemptive clotting factor replacement helps. Others have had success with preemptive tranexamic acid and cryoprecipitate to address low serum fibrinogen levels during CRS/HIPEC. “Further evaluation of both these strategies is warranted,” the researchers said.
Funding source and disclosure information were not included in the study report.
FROM THE JOURNAL OF GASTROINTESTINAL SURGERY
Key clinical point:
Major finding: Even after adjusting for confounders, MABT significantly increased the risk of in-hospital mortality (RR, 7.72; P = .021).
Data source: A single institution review of 936 cases.
Disclosures: Funding source and disclosure information were not included in the study report.
Sneak Peek: Journal of Hospital Medicine – July 2017
BACKGROUND: Medicare patients account for approximately 50% of hospital days. Hospitalization in older adults often results in poor outcomes.
OBJECTIVE: To test the feasibility and impact of using Assessing Care of Vulnerable Elders (ACOVE) quality indicators (QIs) as a therapeutic intervention to improve care of hospitalized older adults.
SETTING: Large tertiary hospital in the greater New York Metropolitan area.
PATIENTS: Hospitalized patients, 75 and over, admitted to medical units.
INTERVENTION: A checklist, comprised of four ACOVE QIs, administered during daily interdisciplinary rounds: venous thrombosis prophylaxis (VTE) (QI 1), indwelling bladder catheters (QI 2), mobilization (QI 3), and delirium evaluation (QI 4).
MEASUREMENTS: Variables were extracted from electronic medical records with QI compliance as the primary outcome, and length of stay (LOS), discharge disposition, and readmissions as secondary outcomes. Generalized linear mixed models for binary clustered data were used to estimate compliance rates for each group (intervention group or control group) in the postintervention period, along with their corresponding 95% confidence intervals.
RESULTS: Of the 2,396 patients, 530 were on an intervention unit. In those patients not already compliant with VTE, the compliance rate was 57% in intervention vs. 39% in control (P less than .0056). For indwelling catheters, mobilization, and delirium evaluation, overall compliance was significantly higher in the intervention group 72.2% vs. 54.4% (P = .1061), 62.9% vs. 48.2% (P less than .0001), and 27.9% vs. 21.7% (P = .0027), respectively.
CONCLUSIONS: The study demonstrates the feasibility and effectiveness of integrating ACOVE QIs to improve the quality of care in hospitalized older adults.
Also in JHM
Use of simulation to assess incoming interns’ recognition of opportunities to choose wisely
AUTHORS: Kathleen M. Wiest, Jeanne M. Farnan, MD, MHPE, Ellen Byrne, Lukas Matern, Melissa Cappaert, MA, Kristen Hirsch, Vineet M. Arora, MD, MAPP
Clinician attitudes regarding ICD deactivation in DNR/DNI patients
AUTHORS: Andrew J. Bradley, MD, Adam D. Marks, MD, MPH
Using standardized patients to assess hospitalist communication skills
AUTHORS: Dennis T. Chang, MD, Micah Mann, MD, Terry Sommer, BFA, Robert Fallar, PhD, Alan Weinberg, MS, Erica Friedman, MD
Techniques and behaviors associated with exemplary inpatient general medicine teaching: An exploratory qualitative study
AUTHORS: Nathan Houchens, MD, Molly Harrod, PhD, Stephanie Moody, PhD, Karen E. Fowler, MPH, Sanjay Saint, MD, MPH
A simple algorithm for predicting bacteremia using food consumption and shaking chills: A prospective observational study
AUTHORS: Takayuki Komatsu, MD, PhD, Erika Takahashi, MD, Kentaro Mishima, MD, Takeo Toyoda, MD, Fumihiro Saitoh, MD, Akari Yasuda, RN, Joe Matsuoka, PhD, Manabu Sugita, MD, PhD, Joel Branch, MD, Makoto Aoki, MD, Lawrence M. Tierney Jr., MD, Kenji Inoue, MD, PhD
For more articles and subscription information, visit www.journalofhospitalmedicine.com.
BACKGROUND: Medicare patients account for approximately 50% of hospital days. Hospitalization in older adults often results in poor outcomes.
OBJECTIVE: To test the feasibility and impact of using Assessing Care of Vulnerable Elders (ACOVE) quality indicators (QIs) as a therapeutic intervention to improve care of hospitalized older adults.
SETTING: Large tertiary hospital in the greater New York Metropolitan area.
PATIENTS: Hospitalized patients, 75 and over, admitted to medical units.
INTERVENTION: A checklist, comprised of four ACOVE QIs, administered during daily interdisciplinary rounds: venous thrombosis prophylaxis (VTE) (QI 1), indwelling bladder catheters (QI 2), mobilization (QI 3), and delirium evaluation (QI 4).
MEASUREMENTS: Variables were extracted from electronic medical records with QI compliance as the primary outcome, and length of stay (LOS), discharge disposition, and readmissions as secondary outcomes. Generalized linear mixed models for binary clustered data were used to estimate compliance rates for each group (intervention group or control group) in the postintervention period, along with their corresponding 95% confidence intervals.
RESULTS: Of the 2,396 patients, 530 were on an intervention unit. In those patients not already compliant with VTE, the compliance rate was 57% in intervention vs. 39% in control (P less than .0056). For indwelling catheters, mobilization, and delirium evaluation, overall compliance was significantly higher in the intervention group 72.2% vs. 54.4% (P = .1061), 62.9% vs. 48.2% (P less than .0001), and 27.9% vs. 21.7% (P = .0027), respectively.
CONCLUSIONS: The study demonstrates the feasibility and effectiveness of integrating ACOVE QIs to improve the quality of care in hospitalized older adults.
Also in JHM
Use of simulation to assess incoming interns’ recognition of opportunities to choose wisely
AUTHORS: Kathleen M. Wiest, Jeanne M. Farnan, MD, MHPE, Ellen Byrne, Lukas Matern, Melissa Cappaert, MA, Kristen Hirsch, Vineet M. Arora, MD, MAPP
Clinician attitudes regarding ICD deactivation in DNR/DNI patients
AUTHORS: Andrew J. Bradley, MD, Adam D. Marks, MD, MPH
Using standardized patients to assess hospitalist communication skills
AUTHORS: Dennis T. Chang, MD, Micah Mann, MD, Terry Sommer, BFA, Robert Fallar, PhD, Alan Weinberg, MS, Erica Friedman, MD
Techniques and behaviors associated with exemplary inpatient general medicine teaching: An exploratory qualitative study
AUTHORS: Nathan Houchens, MD, Molly Harrod, PhD, Stephanie Moody, PhD, Karen E. Fowler, MPH, Sanjay Saint, MD, MPH
A simple algorithm for predicting bacteremia using food consumption and shaking chills: A prospective observational study
AUTHORS: Takayuki Komatsu, MD, PhD, Erika Takahashi, MD, Kentaro Mishima, MD, Takeo Toyoda, MD, Fumihiro Saitoh, MD, Akari Yasuda, RN, Joe Matsuoka, PhD, Manabu Sugita, MD, PhD, Joel Branch, MD, Makoto Aoki, MD, Lawrence M. Tierney Jr., MD, Kenji Inoue, MD, PhD
For more articles and subscription information, visit www.journalofhospitalmedicine.com.
BACKGROUND: Medicare patients account for approximately 50% of hospital days. Hospitalization in older adults often results in poor outcomes.
OBJECTIVE: To test the feasibility and impact of using Assessing Care of Vulnerable Elders (ACOVE) quality indicators (QIs) as a therapeutic intervention to improve care of hospitalized older adults.
SETTING: Large tertiary hospital in the greater New York Metropolitan area.
PATIENTS: Hospitalized patients, 75 and over, admitted to medical units.
INTERVENTION: A checklist, comprised of four ACOVE QIs, administered during daily interdisciplinary rounds: venous thrombosis prophylaxis (VTE) (QI 1), indwelling bladder catheters (QI 2), mobilization (QI 3), and delirium evaluation (QI 4).
MEASUREMENTS: Variables were extracted from electronic medical records with QI compliance as the primary outcome, and length of stay (LOS), discharge disposition, and readmissions as secondary outcomes. Generalized linear mixed models for binary clustered data were used to estimate compliance rates for each group (intervention group or control group) in the postintervention period, along with their corresponding 95% confidence intervals.
RESULTS: Of the 2,396 patients, 530 were on an intervention unit. In those patients not already compliant with VTE, the compliance rate was 57% in intervention vs. 39% in control (P less than .0056). For indwelling catheters, mobilization, and delirium evaluation, overall compliance was significantly higher in the intervention group 72.2% vs. 54.4% (P = .1061), 62.9% vs. 48.2% (P less than .0001), and 27.9% vs. 21.7% (P = .0027), respectively.
CONCLUSIONS: The study demonstrates the feasibility and effectiveness of integrating ACOVE QIs to improve the quality of care in hospitalized older adults.
Also in JHM
Use of simulation to assess incoming interns’ recognition of opportunities to choose wisely
AUTHORS: Kathleen M. Wiest, Jeanne M. Farnan, MD, MHPE, Ellen Byrne, Lukas Matern, Melissa Cappaert, MA, Kristen Hirsch, Vineet M. Arora, MD, MAPP
Clinician attitudes regarding ICD deactivation in DNR/DNI patients
AUTHORS: Andrew J. Bradley, MD, Adam D. Marks, MD, MPH
Using standardized patients to assess hospitalist communication skills
AUTHORS: Dennis T. Chang, MD, Micah Mann, MD, Terry Sommer, BFA, Robert Fallar, PhD, Alan Weinberg, MS, Erica Friedman, MD
Techniques and behaviors associated with exemplary inpatient general medicine teaching: An exploratory qualitative study
AUTHORS: Nathan Houchens, MD, Molly Harrod, PhD, Stephanie Moody, PhD, Karen E. Fowler, MPH, Sanjay Saint, MD, MPH
A simple algorithm for predicting bacteremia using food consumption and shaking chills: A prospective observational study
AUTHORS: Takayuki Komatsu, MD, PhD, Erika Takahashi, MD, Kentaro Mishima, MD, Takeo Toyoda, MD, Fumihiro Saitoh, MD, Akari Yasuda, RN, Joe Matsuoka, PhD, Manabu Sugita, MD, PhD, Joel Branch, MD, Makoto Aoki, MD, Lawrence M. Tierney Jr., MD, Kenji Inoue, MD, PhD
For more articles and subscription information, visit www.journalofhospitalmedicine.com.
Multimodality Approach to a Stener Lesion: Radiographic, Ultrasound, Magnetic Resonance Imaging, and Surgical Correlation
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
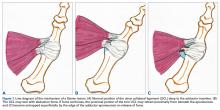
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
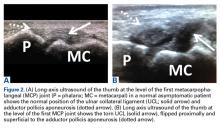
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
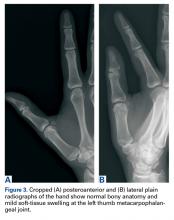
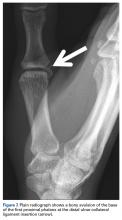
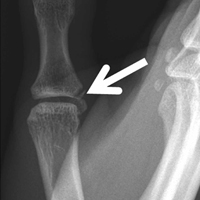
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
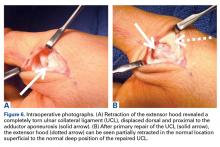
Surgical Findings
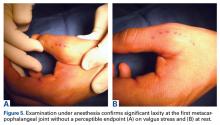
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
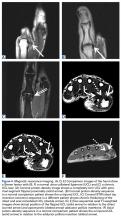
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
Treating Traumatic Injuries and the Issues They Cause
In the Madigan Intrepid Spirit Transitions (MIST) program, holistic treatment for traumatic brain injuries (TBIs) includes traditional and nontraditional therapies as well as a little help from friends.
MIST is a 6-week intensive outpatient group for service members who have TBIs and other traumatic injuries, along with coexisting conditions, such as chronic pain or posttraumatic stress. Coexisting conditions can make cases more complex, said U.S. Army Colonel Beverly Scott, medical and program director of Madigan Army Medical Center’s Traumatic Brain Injury Program and Intrepid Spirit Program in an interview with Health.mil News. But she adds, “It’s never too late to help [patients] address a number of issues they may be having following a traumatic brain injury, dealing with pain, dealing with behavior health issues.”
Related: Let’s Dance: A Holistic Approach to Treating Veterans With Posttraumatic Stress Disorder
The MIST program serves only active-duty service members with referral from their primary care managers and other specialty services at Madigan or throughout the Regional Health Command-Pacific. Commanders must sign memoranda of understanding that patients will be off duty rosters for the duration of the program. “They’re making a commitment to help that service member get better,” Scott said.
The MIST program enrolls 8 to 12 service members at a time. The holistic focus allows patients to address chronic pain, insomnia, and cognitive issues through traditional means as well as less traditional means that include mindfulness training; art; such as creating symbolic masks; and yoga. The variety of approaches lets them “cherry pick the methods they believe will help them the most,” the Health.mil News article reports, or what one member called “customizing their own multitool.”
Participants are encouraged to continue individual care within the TBI/Intrepid Spirit program. The MIST program aims to introduce them to the resources they can use going forward. Giving them tools they can use after they complete the program is an acknowledgment that the recovery process is ongoing. “We recognize it is a transition,” Scott said.
Related: Ideas for Helping TBI Patients
The MIST program has graduated 2 groups. Scott says, “We’ve seen incredible success,” both in wellness and in other areas, such as improved interpersonal relationships. Some of the credit goes to the peer support that MIST promotes. The curriculum is evidence based, but Scott says some “significant success is clearly related to soldiers helping soldiers.”
In the Madigan Intrepid Spirit Transitions (MIST) program, holistic treatment for traumatic brain injuries (TBIs) includes traditional and nontraditional therapies as well as a little help from friends.
MIST is a 6-week intensive outpatient group for service members who have TBIs and other traumatic injuries, along with coexisting conditions, such as chronic pain or posttraumatic stress. Coexisting conditions can make cases more complex, said U.S. Army Colonel Beverly Scott, medical and program director of Madigan Army Medical Center’s Traumatic Brain Injury Program and Intrepid Spirit Program in an interview with Health.mil News. But she adds, “It’s never too late to help [patients] address a number of issues they may be having following a traumatic brain injury, dealing with pain, dealing with behavior health issues.”
Related: Let’s Dance: A Holistic Approach to Treating Veterans With Posttraumatic Stress Disorder
The MIST program serves only active-duty service members with referral from their primary care managers and other specialty services at Madigan or throughout the Regional Health Command-Pacific. Commanders must sign memoranda of understanding that patients will be off duty rosters for the duration of the program. “They’re making a commitment to help that service member get better,” Scott said.
The MIST program enrolls 8 to 12 service members at a time. The holistic focus allows patients to address chronic pain, insomnia, and cognitive issues through traditional means as well as less traditional means that include mindfulness training; art; such as creating symbolic masks; and yoga. The variety of approaches lets them “cherry pick the methods they believe will help them the most,” the Health.mil News article reports, or what one member called “customizing their own multitool.”
Participants are encouraged to continue individual care within the TBI/Intrepid Spirit program. The MIST program aims to introduce them to the resources they can use going forward. Giving them tools they can use after they complete the program is an acknowledgment that the recovery process is ongoing. “We recognize it is a transition,” Scott said.
Related: Ideas for Helping TBI Patients
The MIST program has graduated 2 groups. Scott says, “We’ve seen incredible success,” both in wellness and in other areas, such as improved interpersonal relationships. Some of the credit goes to the peer support that MIST promotes. The curriculum is evidence based, but Scott says some “significant success is clearly related to soldiers helping soldiers.”
In the Madigan Intrepid Spirit Transitions (MIST) program, holistic treatment for traumatic brain injuries (TBIs) includes traditional and nontraditional therapies as well as a little help from friends.
MIST is a 6-week intensive outpatient group for service members who have TBIs and other traumatic injuries, along with coexisting conditions, such as chronic pain or posttraumatic stress. Coexisting conditions can make cases more complex, said U.S. Army Colonel Beverly Scott, medical and program director of Madigan Army Medical Center’s Traumatic Brain Injury Program and Intrepid Spirit Program in an interview with Health.mil News. But she adds, “It’s never too late to help [patients] address a number of issues they may be having following a traumatic brain injury, dealing with pain, dealing with behavior health issues.”
Related: Let’s Dance: A Holistic Approach to Treating Veterans With Posttraumatic Stress Disorder
The MIST program serves only active-duty service members with referral from their primary care managers and other specialty services at Madigan or throughout the Regional Health Command-Pacific. Commanders must sign memoranda of understanding that patients will be off duty rosters for the duration of the program. “They’re making a commitment to help that service member get better,” Scott said.
The MIST program enrolls 8 to 12 service members at a time. The holistic focus allows patients to address chronic pain, insomnia, and cognitive issues through traditional means as well as less traditional means that include mindfulness training; art; such as creating symbolic masks; and yoga. The variety of approaches lets them “cherry pick the methods they believe will help them the most,” the Health.mil News article reports, or what one member called “customizing their own multitool.”
Participants are encouraged to continue individual care within the TBI/Intrepid Spirit program. The MIST program aims to introduce them to the resources they can use going forward. Giving them tools they can use after they complete the program is an acknowledgment that the recovery process is ongoing. “We recognize it is a transition,” Scott said.
Related: Ideas for Helping TBI Patients
The MIST program has graduated 2 groups. Scott says, “We’ve seen incredible success,” both in wellness and in other areas, such as improved interpersonal relationships. Some of the credit goes to the peer support that MIST promotes. The curriculum is evidence based, but Scott says some “significant success is clearly related to soldiers helping soldiers.”
Patients With Cancer Have Higher Plasma Brain Natriuretic Peptide Levels
Natriuretic peptides have been shown to be valuable biomarkers for guiding diagnosis and treatment for cardiovascular disease. Recently, they also have been reported to inhibit progression of several cancers. The link is likely to be inflammation—which is usually a precursor to malignant changes, say researchers from Tokushima University and the National Cerebral and Cardiovascular Center Research Institute, both in Japan. To find out how reliable brain natriuretic peptide and C-reactive protein (CRP) levels might be in cancer, the researchers retrospectively studied 2,923 patients at their hospital who had had brain natriuretic peptide (BNP) measured to rule out heart disease.
Related: The Link Between Low-Density Lipoproteins and Chronic Lymphocytic Leukemia
Of 234 patients included in the final analysis, 80 were diagnosed with cancer. No patients with clinically evident heart failure and cardiac disease requiring medical treatment were included in the study. Both the plasma BNP and serum CRP levels were significantly higher in the patients with cancer (66.4 vs 44.0 pg/mL, and 0.99 vs 0.18 mg/dL, respectively). There were no significant differences in the echocardiographic parameters.
In 28 cured patients with solid cancers who underwent a radical surgery, the plasma BNP levels dropped significantly, from 70.7 to 45.0 pg/mL. However, the levels did not change after surgery in 7 relapsed or “insufficiently treated” patients with solid cancers. And although plasma BNP levels “tended to decrease” after chemotherapy in patients with hematologic cancers, in 13 patients plasma BNP levels did not change significantly.
Related: Novel Neuroendocrine Tumor in Multiple Endocrine Neoplasia Type 1
BNP levels were significantly higher in the patients with stage IV cancer, compared with those who had stages I, II, or III. This might be accompanied by systemic inflammation, the researchers say.
As far as the researchers know, no studies have shown that cancer cells generate BNP. Therefore, they think a higher plasma BNP level may reflect the elevated production from cardiomyocytes in association with inflammation in cancer patients.
Their findings suggest that it’s a good idea to consider the effect of cancer on the BNP levels when using BNP as an indicator of heart failure. Asymptomatic cancer patients with higher BNP levels should be diagnosed whether the elevated BNP is due to asymptomatic heart failure or cancer.
Source:
Bando S, Soeki T, Matsuura T, et al. PLoS One. 2017;12(6):e0178607
Natriuretic peptides have been shown to be valuable biomarkers for guiding diagnosis and treatment for cardiovascular disease. Recently, they also have been reported to inhibit progression of several cancers. The link is likely to be inflammation—which is usually a precursor to malignant changes, say researchers from Tokushima University and the National Cerebral and Cardiovascular Center Research Institute, both in Japan. To find out how reliable brain natriuretic peptide and C-reactive protein (CRP) levels might be in cancer, the researchers retrospectively studied 2,923 patients at their hospital who had had brain natriuretic peptide (BNP) measured to rule out heart disease.
Related: The Link Between Low-Density Lipoproteins and Chronic Lymphocytic Leukemia
Of 234 patients included in the final analysis, 80 were diagnosed with cancer. No patients with clinically evident heart failure and cardiac disease requiring medical treatment were included in the study. Both the plasma BNP and serum CRP levels were significantly higher in the patients with cancer (66.4 vs 44.0 pg/mL, and 0.99 vs 0.18 mg/dL, respectively). There were no significant differences in the echocardiographic parameters.
In 28 cured patients with solid cancers who underwent a radical surgery, the plasma BNP levels dropped significantly, from 70.7 to 45.0 pg/mL. However, the levels did not change after surgery in 7 relapsed or “insufficiently treated” patients with solid cancers. And although plasma BNP levels “tended to decrease” after chemotherapy in patients with hematologic cancers, in 13 patients plasma BNP levels did not change significantly.
Related: Novel Neuroendocrine Tumor in Multiple Endocrine Neoplasia Type 1
BNP levels were significantly higher in the patients with stage IV cancer, compared with those who had stages I, II, or III. This might be accompanied by systemic inflammation, the researchers say.
As far as the researchers know, no studies have shown that cancer cells generate BNP. Therefore, they think a higher plasma BNP level may reflect the elevated production from cardiomyocytes in association with inflammation in cancer patients.
Their findings suggest that it’s a good idea to consider the effect of cancer on the BNP levels when using BNP as an indicator of heart failure. Asymptomatic cancer patients with higher BNP levels should be diagnosed whether the elevated BNP is due to asymptomatic heart failure or cancer.
Source:
Bando S, Soeki T, Matsuura T, et al. PLoS One. 2017;12(6):e0178607
Natriuretic peptides have been shown to be valuable biomarkers for guiding diagnosis and treatment for cardiovascular disease. Recently, they also have been reported to inhibit progression of several cancers. The link is likely to be inflammation—which is usually a precursor to malignant changes, say researchers from Tokushima University and the National Cerebral and Cardiovascular Center Research Institute, both in Japan. To find out how reliable brain natriuretic peptide and C-reactive protein (CRP) levels might be in cancer, the researchers retrospectively studied 2,923 patients at their hospital who had had brain natriuretic peptide (BNP) measured to rule out heart disease.
Related: The Link Between Low-Density Lipoproteins and Chronic Lymphocytic Leukemia
Of 234 patients included in the final analysis, 80 were diagnosed with cancer. No patients with clinically evident heart failure and cardiac disease requiring medical treatment were included in the study. Both the plasma BNP and serum CRP levels were significantly higher in the patients with cancer (66.4 vs 44.0 pg/mL, and 0.99 vs 0.18 mg/dL, respectively). There were no significant differences in the echocardiographic parameters.
In 28 cured patients with solid cancers who underwent a radical surgery, the plasma BNP levels dropped significantly, from 70.7 to 45.0 pg/mL. However, the levels did not change after surgery in 7 relapsed or “insufficiently treated” patients with solid cancers. And although plasma BNP levels “tended to decrease” after chemotherapy in patients with hematologic cancers, in 13 patients plasma BNP levels did not change significantly.
Related: Novel Neuroendocrine Tumor in Multiple Endocrine Neoplasia Type 1
BNP levels were significantly higher in the patients with stage IV cancer, compared with those who had stages I, II, or III. This might be accompanied by systemic inflammation, the researchers say.
As far as the researchers know, no studies have shown that cancer cells generate BNP. Therefore, they think a higher plasma BNP level may reflect the elevated production from cardiomyocytes in association with inflammation in cancer patients.
Their findings suggest that it’s a good idea to consider the effect of cancer on the BNP levels when using BNP as an indicator of heart failure. Asymptomatic cancer patients with higher BNP levels should be diagnosed whether the elevated BNP is due to asymptomatic heart failure or cancer.
Source:
Bando S, Soeki T, Matsuura T, et al. PLoS One. 2017;12(6):e0178607
Company discontinues phase 3 trial of vadastuximab talirine in AML
Update as of June 21: The US Food and Drug Administration (FDA) has placed the Investigational New Drug (IND) application for vadastuximab talirine on hold. No clinical trial may resume under the IND until the FDA lifts the clinical hold.
On the advice of the Independent Data Monitoring Committee, Seattle Genetics is discontinuing the phase 3 CASCADE clinical trial of vadastuximab talirine as frontline treatment in older patients with acute myeloid leukemia (AML).
The company is also suspending patient enrollment and treatment in all its vadastuximab trials, including the ongoing phase 1/2 trial in frontline high-risk myelodysplastic syndromes (MDS).
In December last year, the US Food and Drug Administration (FDA) had placed the trials of vadastuximab on full and partial clinical holds due to the potential risk of hepatotoxicity.
The FDA lifted the hold in March of this year. However, concerns regarding a higher rate of deaths, including fatal infections but not liver toxicity, in the vadastuximab arm compared to control prompted the company to discontinue the phase 3 trial.
Vadastuximab talirene is an antibody-drug conjugate (ADC) targeted to CD33, which is expressed on most AML and MDS blasts. The ADC technology links anti-cancer compounds with targeting antibodies to precisely kill cancer cells and spare healthy ones.
Seattle Genetics’ ADC for Hodgkin lymphoma, brentuximab vedotin, was granted accelerated approval by the FDA in 2011.
The CASCADE trial was evaluating vadastuximab in combination with the hypomethylating agents (HMAs) azacytidine or decitabine compared to an HMA alone in older patients with newly diagnosed AML.
In addition to the MDS trial, the company is stopping enrollment onto the trial of vadastuximab in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients and vadastuximab given prior to or after allogeneic hematopoietic stem cell transplant in AML patients.
Calling the decision “disappointing and unexpected,” Clay Siegall, PhD, president and CEO of Seattle Genetics, said, “Patient safety is our highest priority, and we will closely review the data and evaluate next steps.” ![]()
Update as of June 21: The US Food and Drug Administration (FDA) has placed the Investigational New Drug (IND) application for vadastuximab talirine on hold. No clinical trial may resume under the IND until the FDA lifts the clinical hold.
On the advice of the Independent Data Monitoring Committee, Seattle Genetics is discontinuing the phase 3 CASCADE clinical trial of vadastuximab talirine as frontline treatment in older patients with acute myeloid leukemia (AML).
The company is also suspending patient enrollment and treatment in all its vadastuximab trials, including the ongoing phase 1/2 trial in frontline high-risk myelodysplastic syndromes (MDS).
In December last year, the US Food and Drug Administration (FDA) had placed the trials of vadastuximab on full and partial clinical holds due to the potential risk of hepatotoxicity.
The FDA lifted the hold in March of this year. However, concerns regarding a higher rate of deaths, including fatal infections but not liver toxicity, in the vadastuximab arm compared to control prompted the company to discontinue the phase 3 trial.
Vadastuximab talirene is an antibody-drug conjugate (ADC) targeted to CD33, which is expressed on most AML and MDS blasts. The ADC technology links anti-cancer compounds with targeting antibodies to precisely kill cancer cells and spare healthy ones.
Seattle Genetics’ ADC for Hodgkin lymphoma, brentuximab vedotin, was granted accelerated approval by the FDA in 2011.
The CASCADE trial was evaluating vadastuximab in combination with the hypomethylating agents (HMAs) azacytidine or decitabine compared to an HMA alone in older patients with newly diagnosed AML.
In addition to the MDS trial, the company is stopping enrollment onto the trial of vadastuximab in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients and vadastuximab given prior to or after allogeneic hematopoietic stem cell transplant in AML patients.
Calling the decision “disappointing and unexpected,” Clay Siegall, PhD, president and CEO of Seattle Genetics, said, “Patient safety is our highest priority, and we will closely review the data and evaluate next steps.” ![]()
Update as of June 21: The US Food and Drug Administration (FDA) has placed the Investigational New Drug (IND) application for vadastuximab talirine on hold. No clinical trial may resume under the IND until the FDA lifts the clinical hold.
On the advice of the Independent Data Monitoring Committee, Seattle Genetics is discontinuing the phase 3 CASCADE clinical trial of vadastuximab talirine as frontline treatment in older patients with acute myeloid leukemia (AML).
The company is also suspending patient enrollment and treatment in all its vadastuximab trials, including the ongoing phase 1/2 trial in frontline high-risk myelodysplastic syndromes (MDS).
In December last year, the US Food and Drug Administration (FDA) had placed the trials of vadastuximab on full and partial clinical holds due to the potential risk of hepatotoxicity.
The FDA lifted the hold in March of this year. However, concerns regarding a higher rate of deaths, including fatal infections but not liver toxicity, in the vadastuximab arm compared to control prompted the company to discontinue the phase 3 trial.
Vadastuximab talirene is an antibody-drug conjugate (ADC) targeted to CD33, which is expressed on most AML and MDS blasts. The ADC technology links anti-cancer compounds with targeting antibodies to precisely kill cancer cells and spare healthy ones.
Seattle Genetics’ ADC for Hodgkin lymphoma, brentuximab vedotin, was granted accelerated approval by the FDA in 2011.
The CASCADE trial was evaluating vadastuximab in combination with the hypomethylating agents (HMAs) azacytidine or decitabine compared to an HMA alone in older patients with newly diagnosed AML.
In addition to the MDS trial, the company is stopping enrollment onto the trial of vadastuximab in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients and vadastuximab given prior to or after allogeneic hematopoietic stem cell transplant in AML patients.
Calling the decision “disappointing and unexpected,” Clay Siegall, PhD, president and CEO of Seattle Genetics, said, “Patient safety is our highest priority, and we will closely review the data and evaluate next steps.” ![]()