User login
Dengue vaccine appears safe, immunogenic in children aged 2-17 years
whether or not they previously have been exposed to the virus, said Xavier Sáez-Llorens, MD, of Hospital del Niño Dr José Renán Esquivel, Panama City, Republic of Panama, and his associates.
Studies show dengue to be the most common mosquito-borne viral disease affecting humans, occurring in 125 countries and causing approximately 100 million symptomatic infections annually. Vector control has not halted the global spread of dengue, necessitating development of vaccines. A vaccine for people aged 9 years and older has been licensed, but there remains the need for a safe and effective vaccine against all four dengue virus serotypes for all ages, said Dr. Sáez-Llorens and his associates.
Takeda’s live tetravalent dengue vaccine, TDV, has an “attenuated [dengue virus serotype] DENV-2 virus strain (TDV-2) and three chimeric (dengue–dengue) viruses containing the premembrane and envelope protein genes of DENV-1, DENV-3, and DENV-4 genetically engineered into the attenuated TDV-2 genome backbone (TDV-1, TDV-3, and TDV-4),” the investigators explained.
In an ongoing, randomized, double-blind, placebo-controlled study in dengue-endemic Panama, the Dominican Republic, and the Philippines, healthy children aged 2-17 years were randomly assigned to receive either one TDV dose at 0 months and one dose at 3 months (group 1); one dose at 0 months (group 2); one dose at 0 months and a booster at 12 months (group 3); or a placebo (group 4). At 6 months, 68% of children were seropositive for all four serotypes after one dose, and 85% were seropositive for all four serotypes after two doses; this occurred regardless of whether the children had previously been exposed to dengue. Group 3 has not yet received its 3-month booster.
The vaccine was well tolerated and safe. In children younger than 6 years, fever and irritability were the only systemic adverse events reported more often with the vaccine than with placebo. In children older than 6 years, only headache and myalgia were more common with the vaccine than with placebo, Dr. Sáez-Llorens and his associates noted.
“These data support phase III evaluation of the efficacy and safety of TDV given in a two-dose schedule 3 months apart, with analyses that take into account baseline age and dengue serostatus,” they concluded.
Read more in The Lancet Infectious Diseases (2017 June; 17[6]:615-25).
whether or not they previously have been exposed to the virus, said Xavier Sáez-Llorens, MD, of Hospital del Niño Dr José Renán Esquivel, Panama City, Republic of Panama, and his associates.
Studies show dengue to be the most common mosquito-borne viral disease affecting humans, occurring in 125 countries and causing approximately 100 million symptomatic infections annually. Vector control has not halted the global spread of dengue, necessitating development of vaccines. A vaccine for people aged 9 years and older has been licensed, but there remains the need for a safe and effective vaccine against all four dengue virus serotypes for all ages, said Dr. Sáez-Llorens and his associates.
Takeda’s live tetravalent dengue vaccine, TDV, has an “attenuated [dengue virus serotype] DENV-2 virus strain (TDV-2) and three chimeric (dengue–dengue) viruses containing the premembrane and envelope protein genes of DENV-1, DENV-3, and DENV-4 genetically engineered into the attenuated TDV-2 genome backbone (TDV-1, TDV-3, and TDV-4),” the investigators explained.
In an ongoing, randomized, double-blind, placebo-controlled study in dengue-endemic Panama, the Dominican Republic, and the Philippines, healthy children aged 2-17 years were randomly assigned to receive either one TDV dose at 0 months and one dose at 3 months (group 1); one dose at 0 months (group 2); one dose at 0 months and a booster at 12 months (group 3); or a placebo (group 4). At 6 months, 68% of children were seropositive for all four serotypes after one dose, and 85% were seropositive for all four serotypes after two doses; this occurred regardless of whether the children had previously been exposed to dengue. Group 3 has not yet received its 3-month booster.
The vaccine was well tolerated and safe. In children younger than 6 years, fever and irritability were the only systemic adverse events reported more often with the vaccine than with placebo. In children older than 6 years, only headache and myalgia were more common with the vaccine than with placebo, Dr. Sáez-Llorens and his associates noted.
“These data support phase III evaluation of the efficacy and safety of TDV given in a two-dose schedule 3 months apart, with analyses that take into account baseline age and dengue serostatus,” they concluded.
Read more in The Lancet Infectious Diseases (2017 June; 17[6]:615-25).
whether or not they previously have been exposed to the virus, said Xavier Sáez-Llorens, MD, of Hospital del Niño Dr José Renán Esquivel, Panama City, Republic of Panama, and his associates.
Studies show dengue to be the most common mosquito-borne viral disease affecting humans, occurring in 125 countries and causing approximately 100 million symptomatic infections annually. Vector control has not halted the global spread of dengue, necessitating development of vaccines. A vaccine for people aged 9 years and older has been licensed, but there remains the need for a safe and effective vaccine against all four dengue virus serotypes for all ages, said Dr. Sáez-Llorens and his associates.
Takeda’s live tetravalent dengue vaccine, TDV, has an “attenuated [dengue virus serotype] DENV-2 virus strain (TDV-2) and three chimeric (dengue–dengue) viruses containing the premembrane and envelope protein genes of DENV-1, DENV-3, and DENV-4 genetically engineered into the attenuated TDV-2 genome backbone (TDV-1, TDV-3, and TDV-4),” the investigators explained.
In an ongoing, randomized, double-blind, placebo-controlled study in dengue-endemic Panama, the Dominican Republic, and the Philippines, healthy children aged 2-17 years were randomly assigned to receive either one TDV dose at 0 months and one dose at 3 months (group 1); one dose at 0 months (group 2); one dose at 0 months and a booster at 12 months (group 3); or a placebo (group 4). At 6 months, 68% of children were seropositive for all four serotypes after one dose, and 85% were seropositive for all four serotypes after two doses; this occurred regardless of whether the children had previously been exposed to dengue. Group 3 has not yet received its 3-month booster.
The vaccine was well tolerated and safe. In children younger than 6 years, fever and irritability were the only systemic adverse events reported more often with the vaccine than with placebo. In children older than 6 years, only headache and myalgia were more common with the vaccine than with placebo, Dr. Sáez-Llorens and his associates noted.
“These data support phase III evaluation of the efficacy and safety of TDV given in a two-dose schedule 3 months apart, with analyses that take into account baseline age and dengue serostatus,” they concluded.
Read more in The Lancet Infectious Diseases (2017 June; 17[6]:615-25).
FROM LANCET INFECTIOUS DISEASES
Cutaneous Myoepithelial Carcinoma With Disseminated Metastases
Cutaneous myoepithelial tumors are rare neoplasms but are being increasingly recognized and reported in the literature.1-7 Myoepithelial tumors are related to benign mixed tumors of the skin but lack the epithelial ductules that are present in mixed tumors. Cutaneous myoepithelial tumors may show a variety of architectural, cytological, and stromal features. Their immunophenotype usually is characterized by coexpression of an epithelial marker (eg, keratin, epithelial membrane antigen [EMA]) and S-100 protein; they also may express a variety of other myoepithelial markers, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin.7 EWS RNA binding protein 1 (EWSR1) and pleomorphic adenoma gene 1 (PLAG1) gene rearrangement has been detected in subsets of these tumors on in situ hybridization.8-10
Malignant myoepithelial tumors of the skin, also referred to as cutaneous myoepithelial carcinomas, are exceedingly rare. Including the current case, a search of PubMed articles indexed for MEDLINE and Google Scholar using the terms myoepithelial carcinoma and cutaneous revealed 12 cases that have been reported in the literature (Table).1-7,11-13 These tumors often occur in the head and neck areas and the lower extremities and display a bimodal age distribution, generally occurring in patients younger than 21 years and older than 50 years of age; they also show a slight female predominance. Available follow-up data from the literature have shown local recurrence or metastasis in 3 cases3,4,6; however, in one case the metastatic focus was not histologically identified.4 Cutaneous myoepithelial carcinoma presenting with metastatic disease further limits treatment options. Here, we describe a case of metastatic cutaneous myoepithelial carcinoma in a 47-year-old man, a rare example of cutaneous myoepithelial carcinoma with histologically documented metastatic disease at the initial presentation.
Case Report
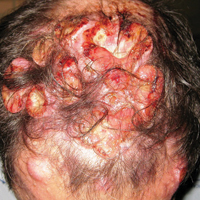
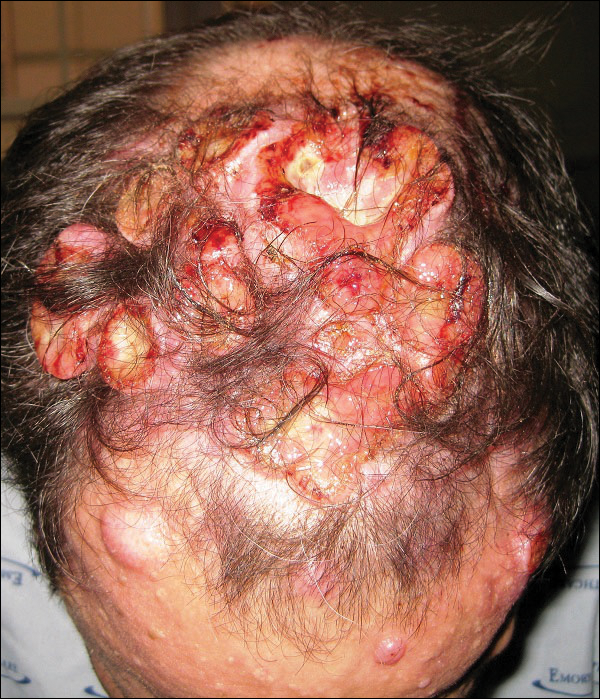
A 47-year-old man who underwent a renal transplant 19 years prior presented with a weeping, ulcerated, mildly tender lesion on the scalp of 4 months’ duration with neck and back pain of 3 months’ duration. Physical examination demonstrated a 6-cm area of ulceration on the anterior crown of the scalp with adjacent enlarged keratoacanthomalike craters and satellite nodules (Figure 1). He was previously diagnosed with basal cell carcinoma (BCC) of the scalp at an outside institution 4 years prior and was treated with radiation therapy. The prior scalp biopsy for BCC diagnosis was unavailable for review. The patient had a history of chronic eczematous dermatitis in the waistband area that had been present for 19 years and another BCC with nodular and infiltrative patterns on the left helix. Of note, he also had been taking long-term immunosuppressant medications (ie, cyclosporine, azathioprine) for maintenance following the renal transplant.
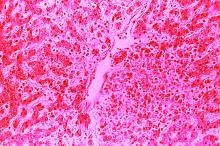
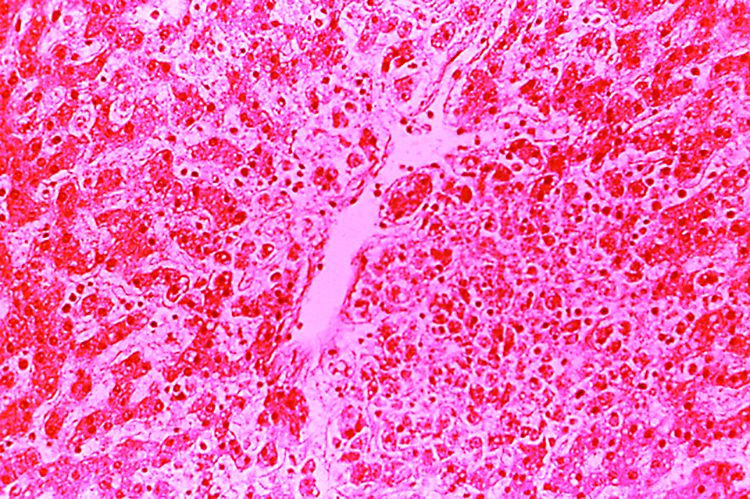
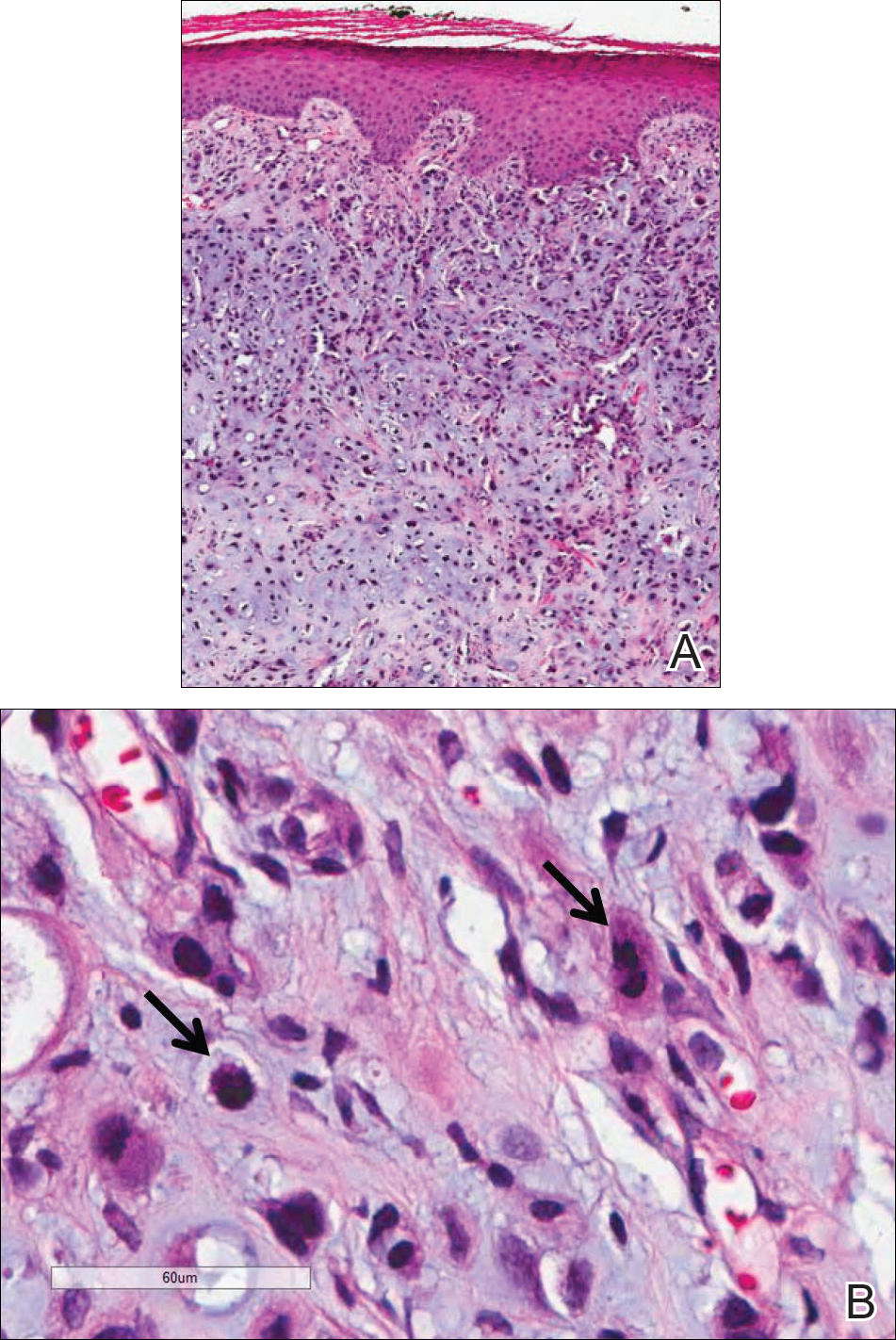
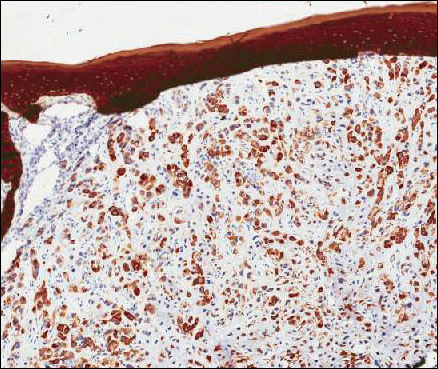
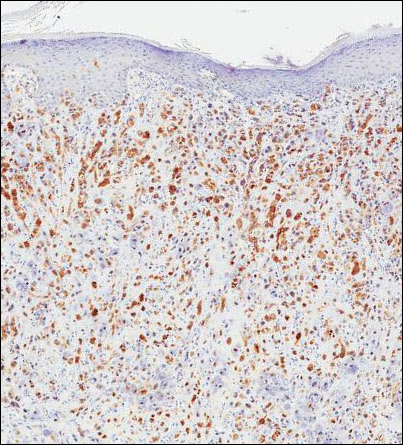
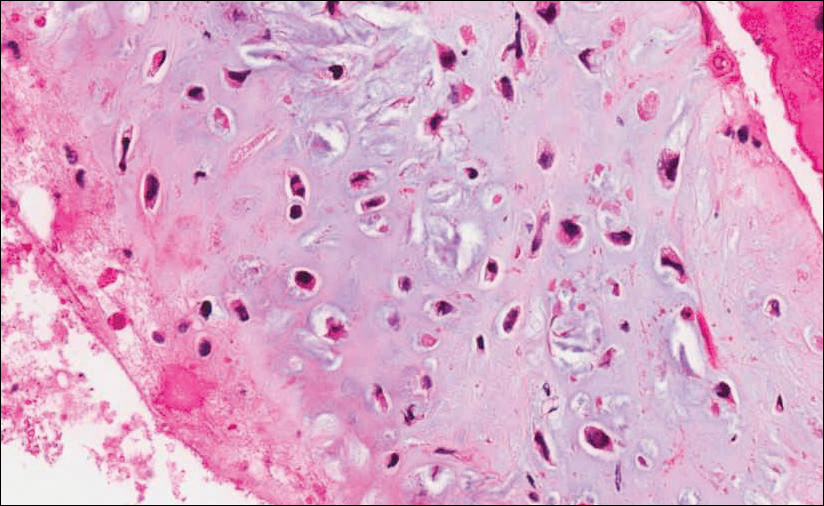
Because of the extensive ulceration of the primary lesion, a shave biopsy of the scalp was performed on an adjacent satellite nodule. Histopathologic findings showed an intradermal neoplasm characterized by poorly cohesive cells exhibiting epithelioid to plasmacytoid morphologic features surrounded by abundant chondromyxoid stroma. Ductular differentiation was not identified (Figure 2A). The neoplastic cells displayed hyperchromatic nuclei with marked nuclear pleomorphism and atypical mitotic figures (Figure 2B). On immunohistochemistry the tumor cells stained positive for cytokeratin AE1/AE3 (Figure 3), S-100 protein (Figure 4), and p63, and were negative for calponin, desmin, melan-A, cytokeratin 7, and brachyury (Figure 5).
Radiographic imaging was performed due to the patient’s history of neck and back pain. Magnetic resonance imaging showed innumerable slightly expansile, T1-hypointense, T2-hyperintense, and robustly enhancing lesions involving the cervical, thoracic, lumbar, and sacral spine, as well as the thoracic ribs and bilateral iliac bones. There was no evidence of soft tissue tumor around the bone lesions. Ventral cervical spinal cord compression was detected at the C4 vertebra, causing a symptomatic radiculopathy; however, due to widely metastatic disease, the patient was not considered appropriate for neurosurgical intervention of the compression. Computerized tomography of the chest, abdomen, and pelvis did not identify any visceral source of malignancy, though multiple bilaterally enlarged cervical lymph nodes were identified on magnetic resonance imaging.

Fine needle aspiration of a left iliac bone lesion demonstrated neoplastic cells and chondromyxoid stroma essentially identical to the features shown in the skin biopsy (Figure 6). Given the morphologic features of the tumor and coexpression of cytokeratin and S-100 protein, the findings were interpreted as primary cutaneous myoepithelial carcinoma with disseminated metastatic lesions. The patient began treatment with carboplatin and paclitaxel chemotherapy. To combat the symptomatic bone pain and upper extremity radiculopathy, palliative radiation was administered to the cervical spine, lumbar spine, and right sacrum (30 Gy to each site in 10 fractions at 3 Gy per fraction). Despite the attempted chemotherapy and radiation, the patient continued to decline, and after 2 months, he elected to pursue palliative care. The patient died after 3 months in palliative care (5 months after the initial presentation).
Comment
Myoepithelial cells normally surround ducts in secretory organs, such as the breasts, salivary glands, and cutaneous sweat glands. Myoepithelial neoplasms are well recognized in the salivary glands14,15; however, myoepithelial neoplasms also can arise in other sites, including the soft tissue4,5,16-18 and skin.1-3,7,11,19,20 Myoepithelioma of soft tissue was first described by Burke et al21 in 1995 and later described in the skin by Fernandez-Figueras et al22 in 1998. Since then, diagnostic criteria for cutaneous myoepithelial neoplasms have evolved, suggesting a spectrum of disease rather than a single distinct entity.11 Most often, cutaneous myoepithelial carcinomas arise as soft nodular lesions in the head and neck areas or extremities of adults. The nodules typically are nontender and range in size from 0.5 to 18.0 cm. Our review of the literature revealed 11 additional cases of cutaneous myoepithelial carcinomas have been reported, ranging in size from 0.7 to 7.0 cm (Table). In our case, the main lesion was 6 cm, mildly tender, ulcerated, and accompanied by satellite nodules.
Histologically, cutaneous myoepithelial tumors typically are well-defined, dermal-based nodules with no connection to the overlying epidermis. Similar to myoepithelial tumors of other sites, they can be diagnostically challenging due to the heterogeneity of both their architectural and cytological features. The presence of a chondromyxoid or hyalinized stroma is common but not always present. Neoplastic myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show growth patterns in clusters, cords, glands, or sheets. Focal epithelial cells can be present. Although benign myoepithelial neoplasms with overt ductal differentiation are consistent with cutaneous mixed tumors (chondroid syringomas), those without ducts are characterized as myoepitheliomas. It is uncertain if cases with only focal ductal differentiation should be classified as mixed tumors or as myoepitheliomas. Malignant myoepithelial tumors show infiltrative borders, nuclear pleomorphism, coarse nuclear chromatin, prominent nucleoli, and increased mitotic activity. A 2003 study by Hornick and Fletcher16 found that cytologic atypia was the primary predictor of malignant behavior for myoepithelial neoplasms of the soft tissue.
Despite a wide variety of expression patterns, immunohistochemistry is critical in demonstrating myoepithelial differentiation and establishing a diagnosis of a myoepithelial neoplasm. Most cases display coexpression of epithelial markers, including keratins and/or EMA as well as S-100 protein. Myogenic markers also may be variably expressed; however, the absence of myogenic markers does not exclude the diagnosis of a myoepithelial tumor. Commonly expressed epithelial markers are cytokeratin AE1/AE3, cytokeratin 8/18, and EMA, while commonly expressed myogenic markers include muscle specific actin and smooth muscle actin.5,7,11,19 Myoepithelial tumors also may express calponin, p63, and glial fibrillary acidic protein.16
Molecular studies also can aid in the diagnosis of myoepithelial tumors. A study by Antonescu et al8 demonstrated EWSR1 gene rearrangement in 45% (30/66) of extrasalivary myoepithelial tumors and the absence of EWSR1 gene rearrangement in salivary gland myoepithelial tumors. The authors also showed that EWSR1-negative tumors were more likely to be superficially located, display ductal differentiation, and possess a benign clinical course.8 In another study, Bahrami et al23 suggested that a subset of mixed tumors, specifically those with tubuloductal differentiation, are genetically linked to salivary gland pleomorphic adenomas, which was achieved by the coexpression of the PLAG1 protein and PLAG1 gene rearrangement on immunohistochemistry and fluorescence in situ hybridization (FISH), respectively. Of the 19 cases evaluated, 11 (58%) expressed nuclear staining for PLAG1 immunohistochemistry; 8 of those 11 showed positive gene rearrangement for PLAG1 using FISH. These findings raise the possibility that cutaneous mixed tumors may be more closely related to those of the salivary glands, while deep myoepithelial tumors that lack ductal differentiation may represent a distinct group. Similar to the study by Antonescu et al,8 Flucke et al10 investigated EWSR1 gene rearrangement but limited their sample to cutaneous tumors, including myoepitheliomas, mixed tumors, and myoepithelial carcinoma. The authors found that 44% of cases (7/16) expressed EWSR1; this expression suggests that cutaneous myoepithelial tumors may have a genetic relationship to their soft tissue, bone, and visceral counterparts.10
Myoepithelial tumors display a broad spectrum of morphologic features; however, one of the most common growth patterns is that of oval to round cells forming cords and chains in a chondromyxoid stroma. As such, the histopathologic differential diagnosis for myoepithelial tumors includes other epithelioid or round-cell neoplasms with similar growth patterns including extraskeletal myxoid chondrosarcoma (EMC), ossifying fibromyxoid tumor of soft parts, and extra-axial soft tissue chordoma. Extraskeletal myxoid chondrosarcoma bears the closest similarity to myoepithelial tumors both histologically and by ancillary studies. It typically possesses cords or chains of small round tumor cells set in a chondromyxoid or myxoid background. In contrast to myoepithelial tumors, which typically have more abundant cytoplasm and can show at least focal areas of spindle cell growth, the cells of EMC are more uniform, small, round cells with relatively scant cytoplasm. Extraskeletal myxoid chondrosarcomas lack the typical myoepithelial coexpression of cytokeratin and S-100 protein, with a minority of EMCs expressing S-100 protein but rarely cytokeratin. Most cases of EMC possess a balanced t(9;22) translocation involving the EWSR1 gene,24 a finding that could lead to confusion with soft tissue myoepithelial tumors, which also may show EWSR1 rearrangement on FISH. Ossifying fibromyxoid tumor of soft parts is also composed of round cells arranged in cords in a myxoid or fibrous stroma; the majority of cases also display a peripheral rim of mature bone, a feature that is not typically seen in myoepithelial tumors. Similar to myoepithelial tumors, ossifying fibromyxoid tumor of soft parts often is positive for S-100 protein; however, it rarely is positive for cytokeratins. Ossifying fibromyxoid tumor of soft parts has been shown to have a rearrangement of the PHD finger protein 1 (PHF1) gene in approximately half of cases, a molecular finding that has not been reported for myoepithelial tumors.25 Finally, extra-axial soft tissue chordomas, though quite rare, may possess striking similarities to myoepithelial tumors both histopathologically and immunohistochemically. Chordomas are composed of epithelioid cells arranged in nests, nodules, and chains with a variably myxoid background. A variable amount of cells with bubbly cytoplasm (known as physaliphorous cells) can be seen. High mitotic activity is not a characteristic feature in chordomas. They classically coexpress cytokeratins and S-100 protein, similar to myoepithelial tumors.
Because cutaneous myoepithelial tumors are relatively rare, a well-defined standard of care for treatment is lacking. Surgical excision is the primary treatment method in most reported cases in the literature.17,19 Miller et al29 reported the successful treatment of recurrent cutaneous myoepitheliomas with Mohs micrographic surgery. Chemotherapy may be useful in the setting of metastatic myoepithelial carcinomas in adults, but reported results are inconsistent.30,31 Radiation treatment of recurrent or metastatic disease has not been shown to be effective. A study of children treated with surgical resection and chemotherapy using ifosfamide, cisplatin, and etoposide followed by radiation therapy showed positive results.32
Our case highlights several challenges that may arise in establishing a diagnosis of cutaneous myoepithelial carcinoma with disseminated metastases. The diagnostic difficulty in our case was compounded by the advanced nature of the lesion at the time of presentation. Given the rarity of metastatic cutaneous myoepithelial carcinomas and the lack of a prior primary diagnosis of a malignant myoepithelioma, the index of suspicion for this entity was not high. A report of myoepithelial carcinoma of the parotid gland metastatic to the skin has been reported,33 but in the absence of salivary gland involvement or other visceral lesions, metastasis from any source other than our patient’s cutaneous scalp lesion is unlikely. The histopathologic features in combination with the characteristic immunophenotype, unique clinical setting, and radiographic findings were essential to arriving at the correct diagnosis. Unlike previously reported metastatic lesions, our case is unique in that metastatic lesions were identified at the time of initial clinical presentation.
Conclusion
Cutaneous myoepithelial carcinomas are exceedingly rare tumors with a wide range of histopathologic and immunohistochemical findings. In challenging cases, studies for EWSR1 or PLAG1 gene rearrangement can be helpful. Furthermore, this case illustrates the potential for widespread dissemination of myoepithelial carcinomas requiring clinical evaluation and imaging studies to exclude metastatic lesions.
- Frost MW, Steiniche T, Damsgaard TE, et al. Primary cutaneous myoepithelial carcinoma: a case report and review of the literature. APMIS. 2014;122:369-379.
- Stojsic Z, Brasanac D, Boricic I, et al. Clear cell myoepithelial carcinoma of the skin. a case report. J Cutan Pathol. 2009;36:680-683.
- Tanahashi J, Kashima K, Daa T, et al. A case of cutaneous myoepithelial carcinoma. J Cutan Pathol. 2007;34:648-653.
- Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. report of 12 cases. Virchows Arch. 1999;434:393-400.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Law RM, Viglione MP, Barrett TL. Metastatic myoepithelial carcinoma in a child. J Cutan Pathol. 2008;35:779-781.
- Hornick JL, Fletcher CD. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004;35:14-24.
- Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. a molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114-1124.
- Antonescu CR, Zhang L, Shao SY, et al. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer. 2013;52:675-682.
- Flucke U, Palmedo G, Blankenhorn N, et al. EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: a study of 18 cases. Mod Pathol. 2011;24:1444-1450.
- Mentzel T, Requena L, Kaddu S, et al. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol. 2003;30:294-302.
- Garcia-Sanchez S, Elices M, Nieto S. Cutaneous myoepithelial carcinoma (malignant myoepithelial tumor of skin). Virchows Archiv. 2009;455(suppl 1):1-482.
- Bajoghli A, Limpert J. Treatment of cutaneous malignant myoepithelioma on the nasal ala using Mohs micrographic surgery in a two and a half year old child. J Invest Dermatol. 2009;129:S44.
- Prasad AR, Savera AT, Gown AM, et al. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801-806.
- Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands. a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761-774.
- Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-1196.
- Kilpatrick SE, Hitchcock MG, Kraus MD, et al. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13-22.
- Neto AG, Pineda-Daboin K, Luna MA. Myoepithelioma of the soft tissue of the head and neck: a case report and review of the literature. Head Neck. 2004;26:470-473.
- Kutzner H, Mentzel T, Kaddu S, et al. Cutaneous myoepithelioma: an under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol. 2001;25:348-355.
- Dix BT, Hentges MJ, Saltrick KR, et al. Cutaneous myoepithelioma in the foot: case report. Foot Ankle Spec. 2013;6:239-241.
- Burke T, Sahin A, Johnson DE, et al. Myoepithelioma of the retroperitoneum. Ultrastruct Pathol. 1995;19:269-274.
- Fernandez-Figueras MT, Puig L, Trias I, et al. Benign myoepithelioma of the skin. Am J Dermatopathol. 1998;20:208-212.
- Bahrami A, Dalton JD, Krane JF, et al. A subset of cutaneous and soft tissue mixed tumors are genetically linked to their salivary gland counterpart. Genes Chromosomes Cancer. 2012;51:140-148.
- Panagopoulos I, Mertens F, Isaksson M, et al. Molecular genetic characterization of the EWS/CHN and RBP56/CHN fusion genes in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2002;35:340-352.
- Graham RP, Weiss SW, Sukov WR, et al. PHF1 rearrangements in ossifying fibromyxoid tumors of soft parts: a fluorescence in situ hybridization study of 41 cases with emphasis on the malignant variant. Am J Surg Pathol. 2013;37:1751-1755.
- Dabska M. Parachordoma: a new clinicopathologic entity. Cancer. 1977;40:1586-1592.
- Fletcher CDM, Mertens F, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Lauer SR, Edgar MA, Gardner JM, et al. Soft tissue chordomas: a clinicopathologic analysis of 11 cases. Am J Surg Pathol. 2013;37:719-726.
- Miller TD, McCalmont T, Tope WD. Recurrent cutaneous myoepithelioma treated using Mohs micrographic surgery: case report and review of the literature. Dermatol Surg. 2009;35:139-143.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Noronha V, Cooper DL, Higgins SA, et al. Metastatic myoepithelial carcinoma of the vulva treated with carboplatin and paclitaxel. Lancet Oncol. 2006;7:270-271.
- Bisogno G, Tagarelli A, Schiavetti A, et al. Myoepithelial carcinoma treatment in children: a report from the TREP project. Pediatr Blood Cancer. 2014;61:643-646.
- He DQ, Hua CG, Tang XF, et al. Cutaneous metastasis from a parotid myoepithelial carcinoma: a case report and review of the literature. J Cutan Pathol. 2008;35:1138-1143.
Cutaneous myoepithelial tumors are rare neoplasms but are being increasingly recognized and reported in the literature.1-7 Myoepithelial tumors are related to benign mixed tumors of the skin but lack the epithelial ductules that are present in mixed tumors. Cutaneous myoepithelial tumors may show a variety of architectural, cytological, and stromal features. Their immunophenotype usually is characterized by coexpression of an epithelial marker (eg, keratin, epithelial membrane antigen [EMA]) and S-100 protein; they also may express a variety of other myoepithelial markers, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin.7 EWS RNA binding protein 1 (EWSR1) and pleomorphic adenoma gene 1 (PLAG1) gene rearrangement has been detected in subsets of these tumors on in situ hybridization.8-10
Malignant myoepithelial tumors of the skin, also referred to as cutaneous myoepithelial carcinomas, are exceedingly rare. Including the current case, a search of PubMed articles indexed for MEDLINE and Google Scholar using the terms myoepithelial carcinoma and cutaneous revealed 12 cases that have been reported in the literature (Table).1-7,11-13 These tumors often occur in the head and neck areas and the lower extremities and display a bimodal age distribution, generally occurring in patients younger than 21 years and older than 50 years of age; they also show a slight female predominance. Available follow-up data from the literature have shown local recurrence or metastasis in 3 cases3,4,6; however, in one case the metastatic focus was not histologically identified.4 Cutaneous myoepithelial carcinoma presenting with metastatic disease further limits treatment options. Here, we describe a case of metastatic cutaneous myoepithelial carcinoma in a 47-year-old man, a rare example of cutaneous myoepithelial carcinoma with histologically documented metastatic disease at the initial presentation.

Case Report
A 47-year-old man who underwent a renal transplant 19 years prior presented with a weeping, ulcerated, mildly tender lesion on the scalp of 4 months’ duration with neck and back pain of 3 months’ duration. Physical examination demonstrated a 6-cm area of ulceration on the anterior crown of the scalp with adjacent enlarged keratoacanthomalike craters and satellite nodules (Figure 1). He was previously diagnosed with basal cell carcinoma (BCC) of the scalp at an outside institution 4 years prior and was treated with radiation therapy. The prior scalp biopsy for BCC diagnosis was unavailable for review. The patient had a history of chronic eczematous dermatitis in the waistband area that had been present for 19 years and another BCC with nodular and infiltrative patterns on the left helix. Of note, he also had been taking long-term immunosuppressant medications (ie, cyclosporine, azathioprine) for maintenance following the renal transplant.

Because of the extensive ulceration of the primary lesion, a shave biopsy of the scalp was performed on an adjacent satellite nodule. Histopathologic findings showed an intradermal neoplasm characterized by poorly cohesive cells exhibiting epithelioid to plasmacytoid morphologic features surrounded by abundant chondromyxoid stroma. Ductular differentiation was not identified (Figure 2A). The neoplastic cells displayed hyperchromatic nuclei with marked nuclear pleomorphism and atypical mitotic figures (Figure 2B). On immunohistochemistry the tumor cells stained positive for cytokeratin AE1/AE3 (Figure 3), S-100 protein (Figure 4), and p63, and were negative for calponin, desmin, melan-A, cytokeratin 7, and brachyury (Figure 5).
Radiographic imaging was performed due to the patient’s history of neck and back pain. Magnetic resonance imaging showed innumerable slightly expansile, T1-hypointense, T2-hyperintense, and robustly enhancing lesions involving the cervical, thoracic, lumbar, and sacral spine, as well as the thoracic ribs and bilateral iliac bones. There was no evidence of soft tissue tumor around the bone lesions. Ventral cervical spinal cord compression was detected at the C4 vertebra, causing a symptomatic radiculopathy; however, due to widely metastatic disease, the patient was not considered appropriate for neurosurgical intervention of the compression. Computerized tomography of the chest, abdomen, and pelvis did not identify any visceral source of malignancy, though multiple bilaterally enlarged cervical lymph nodes were identified on magnetic resonance imaging.




Fine needle aspiration of a left iliac bone lesion demonstrated neoplastic cells and chondromyxoid stroma essentially identical to the features shown in the skin biopsy (Figure 6). Given the morphologic features of the tumor and coexpression of cytokeratin and S-100 protein, the findings were interpreted as primary cutaneous myoepithelial carcinoma with disseminated metastatic lesions. The patient began treatment with carboplatin and paclitaxel chemotherapy. To combat the symptomatic bone pain and upper extremity radiculopathy, palliative radiation was administered to the cervical spine, lumbar spine, and right sacrum (30 Gy to each site in 10 fractions at 3 Gy per fraction). Despite the attempted chemotherapy and radiation, the patient continued to decline, and after 2 months, he elected to pursue palliative care. The patient died after 3 months in palliative care (5 months after the initial presentation).

Comment
Myoepithelial cells normally surround ducts in secretory organs, such as the breasts, salivary glands, and cutaneous sweat glands. Myoepithelial neoplasms are well recognized in the salivary glands14,15; however, myoepithelial neoplasms also can arise in other sites, including the soft tissue4,5,16-18 and skin.1-3,7,11,19,20 Myoepithelioma of soft tissue was first described by Burke et al21 in 1995 and later described in the skin by Fernandez-Figueras et al22 in 1998. Since then, diagnostic criteria for cutaneous myoepithelial neoplasms have evolved, suggesting a spectrum of disease rather than a single distinct entity.11 Most often, cutaneous myoepithelial carcinomas arise as soft nodular lesions in the head and neck areas or extremities of adults. The nodules typically are nontender and range in size from 0.5 to 18.0 cm. Our review of the literature revealed 11 additional cases of cutaneous myoepithelial carcinomas have been reported, ranging in size from 0.7 to 7.0 cm (Table). In our case, the main lesion was 6 cm, mildly tender, ulcerated, and accompanied by satellite nodules.
Histologically, cutaneous myoepithelial tumors typically are well-defined, dermal-based nodules with no connection to the overlying epidermis. Similar to myoepithelial tumors of other sites, they can be diagnostically challenging due to the heterogeneity of both their architectural and cytological features. The presence of a chondromyxoid or hyalinized stroma is common but not always present. Neoplastic myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show growth patterns in clusters, cords, glands, or sheets. Focal epithelial cells can be present. Although benign myoepithelial neoplasms with overt ductal differentiation are consistent with cutaneous mixed tumors (chondroid syringomas), those without ducts are characterized as myoepitheliomas. It is uncertain if cases with only focal ductal differentiation should be classified as mixed tumors or as myoepitheliomas. Malignant myoepithelial tumors show infiltrative borders, nuclear pleomorphism, coarse nuclear chromatin, prominent nucleoli, and increased mitotic activity. A 2003 study by Hornick and Fletcher16 found that cytologic atypia was the primary predictor of malignant behavior for myoepithelial neoplasms of the soft tissue.
Despite a wide variety of expression patterns, immunohistochemistry is critical in demonstrating myoepithelial differentiation and establishing a diagnosis of a myoepithelial neoplasm. Most cases display coexpression of epithelial markers, including keratins and/or EMA as well as S-100 protein. Myogenic markers also may be variably expressed; however, the absence of myogenic markers does not exclude the diagnosis of a myoepithelial tumor. Commonly expressed epithelial markers are cytokeratin AE1/AE3, cytokeratin 8/18, and EMA, while commonly expressed myogenic markers include muscle specific actin and smooth muscle actin.5,7,11,19 Myoepithelial tumors also may express calponin, p63, and glial fibrillary acidic protein.16
Molecular studies also can aid in the diagnosis of myoepithelial tumors. A study by Antonescu et al8 demonstrated EWSR1 gene rearrangement in 45% (30/66) of extrasalivary myoepithelial tumors and the absence of EWSR1 gene rearrangement in salivary gland myoepithelial tumors. The authors also showed that EWSR1-negative tumors were more likely to be superficially located, display ductal differentiation, and possess a benign clinical course.8 In another study, Bahrami et al23 suggested that a subset of mixed tumors, specifically those with tubuloductal differentiation, are genetically linked to salivary gland pleomorphic adenomas, which was achieved by the coexpression of the PLAG1 protein and PLAG1 gene rearrangement on immunohistochemistry and fluorescence in situ hybridization (FISH), respectively. Of the 19 cases evaluated, 11 (58%) expressed nuclear staining for PLAG1 immunohistochemistry; 8 of those 11 showed positive gene rearrangement for PLAG1 using FISH. These findings raise the possibility that cutaneous mixed tumors may be more closely related to those of the salivary glands, while deep myoepithelial tumors that lack ductal differentiation may represent a distinct group. Similar to the study by Antonescu et al,8 Flucke et al10 investigated EWSR1 gene rearrangement but limited their sample to cutaneous tumors, including myoepitheliomas, mixed tumors, and myoepithelial carcinoma. The authors found that 44% of cases (7/16) expressed EWSR1; this expression suggests that cutaneous myoepithelial tumors may have a genetic relationship to their soft tissue, bone, and visceral counterparts.10
Myoepithelial tumors display a broad spectrum of morphologic features; however, one of the most common growth patterns is that of oval to round cells forming cords and chains in a chondromyxoid stroma. As such, the histopathologic differential diagnosis for myoepithelial tumors includes other epithelioid or round-cell neoplasms with similar growth patterns including extraskeletal myxoid chondrosarcoma (EMC), ossifying fibromyxoid tumor of soft parts, and extra-axial soft tissue chordoma. Extraskeletal myxoid chondrosarcoma bears the closest similarity to myoepithelial tumors both histologically and by ancillary studies. It typically possesses cords or chains of small round tumor cells set in a chondromyxoid or myxoid background. In contrast to myoepithelial tumors, which typically have more abundant cytoplasm and can show at least focal areas of spindle cell growth, the cells of EMC are more uniform, small, round cells with relatively scant cytoplasm. Extraskeletal myxoid chondrosarcomas lack the typical myoepithelial coexpression of cytokeratin and S-100 protein, with a minority of EMCs expressing S-100 protein but rarely cytokeratin. Most cases of EMC possess a balanced t(9;22) translocation involving the EWSR1 gene,24 a finding that could lead to confusion with soft tissue myoepithelial tumors, which also may show EWSR1 rearrangement on FISH. Ossifying fibromyxoid tumor of soft parts is also composed of round cells arranged in cords in a myxoid or fibrous stroma; the majority of cases also display a peripheral rim of mature bone, a feature that is not typically seen in myoepithelial tumors. Similar to myoepithelial tumors, ossifying fibromyxoid tumor of soft parts often is positive for S-100 protein; however, it rarely is positive for cytokeratins. Ossifying fibromyxoid tumor of soft parts has been shown to have a rearrangement of the PHD finger protein 1 (PHF1) gene in approximately half of cases, a molecular finding that has not been reported for myoepithelial tumors.25 Finally, extra-axial soft tissue chordomas, though quite rare, may possess striking similarities to myoepithelial tumors both histopathologically and immunohistochemically. Chordomas are composed of epithelioid cells arranged in nests, nodules, and chains with a variably myxoid background. A variable amount of cells with bubbly cytoplasm (known as physaliphorous cells) can be seen. High mitotic activity is not a characteristic feature in chordomas. They classically coexpress cytokeratins and S-100 protein, similar to myoepithelial tumors.
Because cutaneous myoepithelial tumors are relatively rare, a well-defined standard of care for treatment is lacking. Surgical excision is the primary treatment method in most reported cases in the literature.17,19 Miller et al29 reported the successful treatment of recurrent cutaneous myoepitheliomas with Mohs micrographic surgery. Chemotherapy may be useful in the setting of metastatic myoepithelial carcinomas in adults, but reported results are inconsistent.30,31 Radiation treatment of recurrent or metastatic disease has not been shown to be effective. A study of children treated with surgical resection and chemotherapy using ifosfamide, cisplatin, and etoposide followed by radiation therapy showed positive results.32
Our case highlights several challenges that may arise in establishing a diagnosis of cutaneous myoepithelial carcinoma with disseminated metastases. The diagnostic difficulty in our case was compounded by the advanced nature of the lesion at the time of presentation. Given the rarity of metastatic cutaneous myoepithelial carcinomas and the lack of a prior primary diagnosis of a malignant myoepithelioma, the index of suspicion for this entity was not high. A report of myoepithelial carcinoma of the parotid gland metastatic to the skin has been reported,33 but in the absence of salivary gland involvement or other visceral lesions, metastasis from any source other than our patient’s cutaneous scalp lesion is unlikely. The histopathologic features in combination with the characteristic immunophenotype, unique clinical setting, and radiographic findings were essential to arriving at the correct diagnosis. Unlike previously reported metastatic lesions, our case is unique in that metastatic lesions were identified at the time of initial clinical presentation.
Conclusion
Cutaneous myoepithelial carcinomas are exceedingly rare tumors with a wide range of histopathologic and immunohistochemical findings. In challenging cases, studies for EWSR1 or PLAG1 gene rearrangement can be helpful. Furthermore, this case illustrates the potential for widespread dissemination of myoepithelial carcinomas requiring clinical evaluation and imaging studies to exclude metastatic lesions.
Cutaneous myoepithelial tumors are rare neoplasms but are being increasingly recognized and reported in the literature.1-7 Myoepithelial tumors are related to benign mixed tumors of the skin but lack the epithelial ductules that are present in mixed tumors. Cutaneous myoepithelial tumors may show a variety of architectural, cytological, and stromal features. Their immunophenotype usually is characterized by coexpression of an epithelial marker (eg, keratin, epithelial membrane antigen [EMA]) and S-100 protein; they also may express a variety of other myoepithelial markers, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin.7 EWS RNA binding protein 1 (EWSR1) and pleomorphic adenoma gene 1 (PLAG1) gene rearrangement has been detected in subsets of these tumors on in situ hybridization.8-10
Malignant myoepithelial tumors of the skin, also referred to as cutaneous myoepithelial carcinomas, are exceedingly rare. Including the current case, a search of PubMed articles indexed for MEDLINE and Google Scholar using the terms myoepithelial carcinoma and cutaneous revealed 12 cases that have been reported in the literature (Table).1-7,11-13 These tumors often occur in the head and neck areas and the lower extremities and display a bimodal age distribution, generally occurring in patients younger than 21 years and older than 50 years of age; they also show a slight female predominance. Available follow-up data from the literature have shown local recurrence or metastasis in 3 cases3,4,6; however, in one case the metastatic focus was not histologically identified.4 Cutaneous myoepithelial carcinoma presenting with metastatic disease further limits treatment options. Here, we describe a case of metastatic cutaneous myoepithelial carcinoma in a 47-year-old man, a rare example of cutaneous myoepithelial carcinoma with histologically documented metastatic disease at the initial presentation.

Case Report
A 47-year-old man who underwent a renal transplant 19 years prior presented with a weeping, ulcerated, mildly tender lesion on the scalp of 4 months’ duration with neck and back pain of 3 months’ duration. Physical examination demonstrated a 6-cm area of ulceration on the anterior crown of the scalp with adjacent enlarged keratoacanthomalike craters and satellite nodules (Figure 1). He was previously diagnosed with basal cell carcinoma (BCC) of the scalp at an outside institution 4 years prior and was treated with radiation therapy. The prior scalp biopsy for BCC diagnosis was unavailable for review. The patient had a history of chronic eczematous dermatitis in the waistband area that had been present for 19 years and another BCC with nodular and infiltrative patterns on the left helix. Of note, he also had been taking long-term immunosuppressant medications (ie, cyclosporine, azathioprine) for maintenance following the renal transplant.

Because of the extensive ulceration of the primary lesion, a shave biopsy of the scalp was performed on an adjacent satellite nodule. Histopathologic findings showed an intradermal neoplasm characterized by poorly cohesive cells exhibiting epithelioid to plasmacytoid morphologic features surrounded by abundant chondromyxoid stroma. Ductular differentiation was not identified (Figure 2A). The neoplastic cells displayed hyperchromatic nuclei with marked nuclear pleomorphism and atypical mitotic figures (Figure 2B). On immunohistochemistry the tumor cells stained positive for cytokeratin AE1/AE3 (Figure 3), S-100 protein (Figure 4), and p63, and were negative for calponin, desmin, melan-A, cytokeratin 7, and brachyury (Figure 5).
Radiographic imaging was performed due to the patient’s history of neck and back pain. Magnetic resonance imaging showed innumerable slightly expansile, T1-hypointense, T2-hyperintense, and robustly enhancing lesions involving the cervical, thoracic, lumbar, and sacral spine, as well as the thoracic ribs and bilateral iliac bones. There was no evidence of soft tissue tumor around the bone lesions. Ventral cervical spinal cord compression was detected at the C4 vertebra, causing a symptomatic radiculopathy; however, due to widely metastatic disease, the patient was not considered appropriate for neurosurgical intervention of the compression. Computerized tomography of the chest, abdomen, and pelvis did not identify any visceral source of malignancy, though multiple bilaterally enlarged cervical lymph nodes were identified on magnetic resonance imaging.




Fine needle aspiration of a left iliac bone lesion demonstrated neoplastic cells and chondromyxoid stroma essentially identical to the features shown in the skin biopsy (Figure 6). Given the morphologic features of the tumor and coexpression of cytokeratin and S-100 protein, the findings were interpreted as primary cutaneous myoepithelial carcinoma with disseminated metastatic lesions. The patient began treatment with carboplatin and paclitaxel chemotherapy. To combat the symptomatic bone pain and upper extremity radiculopathy, palliative radiation was administered to the cervical spine, lumbar spine, and right sacrum (30 Gy to each site in 10 fractions at 3 Gy per fraction). Despite the attempted chemotherapy and radiation, the patient continued to decline, and after 2 months, he elected to pursue palliative care. The patient died after 3 months in palliative care (5 months after the initial presentation).

Comment
Myoepithelial cells normally surround ducts in secretory organs, such as the breasts, salivary glands, and cutaneous sweat glands. Myoepithelial neoplasms are well recognized in the salivary glands14,15; however, myoepithelial neoplasms also can arise in other sites, including the soft tissue4,5,16-18 and skin.1-3,7,11,19,20 Myoepithelioma of soft tissue was first described by Burke et al21 in 1995 and later described in the skin by Fernandez-Figueras et al22 in 1998. Since then, diagnostic criteria for cutaneous myoepithelial neoplasms have evolved, suggesting a spectrum of disease rather than a single distinct entity.11 Most often, cutaneous myoepithelial carcinomas arise as soft nodular lesions in the head and neck areas or extremities of adults. The nodules typically are nontender and range in size from 0.5 to 18.0 cm. Our review of the literature revealed 11 additional cases of cutaneous myoepithelial carcinomas have been reported, ranging in size from 0.7 to 7.0 cm (Table). In our case, the main lesion was 6 cm, mildly tender, ulcerated, and accompanied by satellite nodules.
Histologically, cutaneous myoepithelial tumors typically are well-defined, dermal-based nodules with no connection to the overlying epidermis. Similar to myoepithelial tumors of other sites, they can be diagnostically challenging due to the heterogeneity of both their architectural and cytological features. The presence of a chondromyxoid or hyalinized stroma is common but not always present. Neoplastic myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show growth patterns in clusters, cords, glands, or sheets. Focal epithelial cells can be present. Although benign myoepithelial neoplasms with overt ductal differentiation are consistent with cutaneous mixed tumors (chondroid syringomas), those without ducts are characterized as myoepitheliomas. It is uncertain if cases with only focal ductal differentiation should be classified as mixed tumors or as myoepitheliomas. Malignant myoepithelial tumors show infiltrative borders, nuclear pleomorphism, coarse nuclear chromatin, prominent nucleoli, and increased mitotic activity. A 2003 study by Hornick and Fletcher16 found that cytologic atypia was the primary predictor of malignant behavior for myoepithelial neoplasms of the soft tissue.
Despite a wide variety of expression patterns, immunohistochemistry is critical in demonstrating myoepithelial differentiation and establishing a diagnosis of a myoepithelial neoplasm. Most cases display coexpression of epithelial markers, including keratins and/or EMA as well as S-100 protein. Myogenic markers also may be variably expressed; however, the absence of myogenic markers does not exclude the diagnosis of a myoepithelial tumor. Commonly expressed epithelial markers are cytokeratin AE1/AE3, cytokeratin 8/18, and EMA, while commonly expressed myogenic markers include muscle specific actin and smooth muscle actin.5,7,11,19 Myoepithelial tumors also may express calponin, p63, and glial fibrillary acidic protein.16
Molecular studies also can aid in the diagnosis of myoepithelial tumors. A study by Antonescu et al8 demonstrated EWSR1 gene rearrangement in 45% (30/66) of extrasalivary myoepithelial tumors and the absence of EWSR1 gene rearrangement in salivary gland myoepithelial tumors. The authors also showed that EWSR1-negative tumors were more likely to be superficially located, display ductal differentiation, and possess a benign clinical course.8 In another study, Bahrami et al23 suggested that a subset of mixed tumors, specifically those with tubuloductal differentiation, are genetically linked to salivary gland pleomorphic adenomas, which was achieved by the coexpression of the PLAG1 protein and PLAG1 gene rearrangement on immunohistochemistry and fluorescence in situ hybridization (FISH), respectively. Of the 19 cases evaluated, 11 (58%) expressed nuclear staining for PLAG1 immunohistochemistry; 8 of those 11 showed positive gene rearrangement for PLAG1 using FISH. These findings raise the possibility that cutaneous mixed tumors may be more closely related to those of the salivary glands, while deep myoepithelial tumors that lack ductal differentiation may represent a distinct group. Similar to the study by Antonescu et al,8 Flucke et al10 investigated EWSR1 gene rearrangement but limited their sample to cutaneous tumors, including myoepitheliomas, mixed tumors, and myoepithelial carcinoma. The authors found that 44% of cases (7/16) expressed EWSR1; this expression suggests that cutaneous myoepithelial tumors may have a genetic relationship to their soft tissue, bone, and visceral counterparts.10
Myoepithelial tumors display a broad spectrum of morphologic features; however, one of the most common growth patterns is that of oval to round cells forming cords and chains in a chondromyxoid stroma. As such, the histopathologic differential diagnosis for myoepithelial tumors includes other epithelioid or round-cell neoplasms with similar growth patterns including extraskeletal myxoid chondrosarcoma (EMC), ossifying fibromyxoid tumor of soft parts, and extra-axial soft tissue chordoma. Extraskeletal myxoid chondrosarcoma bears the closest similarity to myoepithelial tumors both histologically and by ancillary studies. It typically possesses cords or chains of small round tumor cells set in a chondromyxoid or myxoid background. In contrast to myoepithelial tumors, which typically have more abundant cytoplasm and can show at least focal areas of spindle cell growth, the cells of EMC are more uniform, small, round cells with relatively scant cytoplasm. Extraskeletal myxoid chondrosarcomas lack the typical myoepithelial coexpression of cytokeratin and S-100 protein, with a minority of EMCs expressing S-100 protein but rarely cytokeratin. Most cases of EMC possess a balanced t(9;22) translocation involving the EWSR1 gene,24 a finding that could lead to confusion with soft tissue myoepithelial tumors, which also may show EWSR1 rearrangement on FISH. Ossifying fibromyxoid tumor of soft parts is also composed of round cells arranged in cords in a myxoid or fibrous stroma; the majority of cases also display a peripheral rim of mature bone, a feature that is not typically seen in myoepithelial tumors. Similar to myoepithelial tumors, ossifying fibromyxoid tumor of soft parts often is positive for S-100 protein; however, it rarely is positive for cytokeratins. Ossifying fibromyxoid tumor of soft parts has been shown to have a rearrangement of the PHD finger protein 1 (PHF1) gene in approximately half of cases, a molecular finding that has not been reported for myoepithelial tumors.25 Finally, extra-axial soft tissue chordomas, though quite rare, may possess striking similarities to myoepithelial tumors both histopathologically and immunohistochemically. Chordomas are composed of epithelioid cells arranged in nests, nodules, and chains with a variably myxoid background. A variable amount of cells with bubbly cytoplasm (known as physaliphorous cells) can be seen. High mitotic activity is not a characteristic feature in chordomas. They classically coexpress cytokeratins and S-100 protein, similar to myoepithelial tumors.
Because cutaneous myoepithelial tumors are relatively rare, a well-defined standard of care for treatment is lacking. Surgical excision is the primary treatment method in most reported cases in the literature.17,19 Miller et al29 reported the successful treatment of recurrent cutaneous myoepitheliomas with Mohs micrographic surgery. Chemotherapy may be useful in the setting of metastatic myoepithelial carcinomas in adults, but reported results are inconsistent.30,31 Radiation treatment of recurrent or metastatic disease has not been shown to be effective. A study of children treated with surgical resection and chemotherapy using ifosfamide, cisplatin, and etoposide followed by radiation therapy showed positive results.32
Our case highlights several challenges that may arise in establishing a diagnosis of cutaneous myoepithelial carcinoma with disseminated metastases. The diagnostic difficulty in our case was compounded by the advanced nature of the lesion at the time of presentation. Given the rarity of metastatic cutaneous myoepithelial carcinomas and the lack of a prior primary diagnosis of a malignant myoepithelioma, the index of suspicion for this entity was not high. A report of myoepithelial carcinoma of the parotid gland metastatic to the skin has been reported,33 but in the absence of salivary gland involvement or other visceral lesions, metastasis from any source other than our patient’s cutaneous scalp lesion is unlikely. The histopathologic features in combination with the characteristic immunophenotype, unique clinical setting, and radiographic findings were essential to arriving at the correct diagnosis. Unlike previously reported metastatic lesions, our case is unique in that metastatic lesions were identified at the time of initial clinical presentation.
Conclusion
Cutaneous myoepithelial carcinomas are exceedingly rare tumors with a wide range of histopathologic and immunohistochemical findings. In challenging cases, studies for EWSR1 or PLAG1 gene rearrangement can be helpful. Furthermore, this case illustrates the potential for widespread dissemination of myoepithelial carcinomas requiring clinical evaluation and imaging studies to exclude metastatic lesions.
- Frost MW, Steiniche T, Damsgaard TE, et al. Primary cutaneous myoepithelial carcinoma: a case report and review of the literature. APMIS. 2014;122:369-379.
- Stojsic Z, Brasanac D, Boricic I, et al. Clear cell myoepithelial carcinoma of the skin. a case report. J Cutan Pathol. 2009;36:680-683.
- Tanahashi J, Kashima K, Daa T, et al. A case of cutaneous myoepithelial carcinoma. J Cutan Pathol. 2007;34:648-653.
- Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. report of 12 cases. Virchows Arch. 1999;434:393-400.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Law RM, Viglione MP, Barrett TL. Metastatic myoepithelial carcinoma in a child. J Cutan Pathol. 2008;35:779-781.
- Hornick JL, Fletcher CD. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004;35:14-24.
- Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. a molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114-1124.
- Antonescu CR, Zhang L, Shao SY, et al. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer. 2013;52:675-682.
- Flucke U, Palmedo G, Blankenhorn N, et al. EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: a study of 18 cases. Mod Pathol. 2011;24:1444-1450.
- Mentzel T, Requena L, Kaddu S, et al. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol. 2003;30:294-302.
- Garcia-Sanchez S, Elices M, Nieto S. Cutaneous myoepithelial carcinoma (malignant myoepithelial tumor of skin). Virchows Archiv. 2009;455(suppl 1):1-482.
- Bajoghli A, Limpert J. Treatment of cutaneous malignant myoepithelioma on the nasal ala using Mohs micrographic surgery in a two and a half year old child. J Invest Dermatol. 2009;129:S44.
- Prasad AR, Savera AT, Gown AM, et al. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801-806.
- Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands. a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761-774.
- Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-1196.
- Kilpatrick SE, Hitchcock MG, Kraus MD, et al. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13-22.
- Neto AG, Pineda-Daboin K, Luna MA. Myoepithelioma of the soft tissue of the head and neck: a case report and review of the literature. Head Neck. 2004;26:470-473.
- Kutzner H, Mentzel T, Kaddu S, et al. Cutaneous myoepithelioma: an under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol. 2001;25:348-355.
- Dix BT, Hentges MJ, Saltrick KR, et al. Cutaneous myoepithelioma in the foot: case report. Foot Ankle Spec. 2013;6:239-241.
- Burke T, Sahin A, Johnson DE, et al. Myoepithelioma of the retroperitoneum. Ultrastruct Pathol. 1995;19:269-274.
- Fernandez-Figueras MT, Puig L, Trias I, et al. Benign myoepithelioma of the skin. Am J Dermatopathol. 1998;20:208-212.
- Bahrami A, Dalton JD, Krane JF, et al. A subset of cutaneous and soft tissue mixed tumors are genetically linked to their salivary gland counterpart. Genes Chromosomes Cancer. 2012;51:140-148.
- Panagopoulos I, Mertens F, Isaksson M, et al. Molecular genetic characterization of the EWS/CHN and RBP56/CHN fusion genes in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2002;35:340-352.
- Graham RP, Weiss SW, Sukov WR, et al. PHF1 rearrangements in ossifying fibromyxoid tumors of soft parts: a fluorescence in situ hybridization study of 41 cases with emphasis on the malignant variant. Am J Surg Pathol. 2013;37:1751-1755.
- Dabska M. Parachordoma: a new clinicopathologic entity. Cancer. 1977;40:1586-1592.
- Fletcher CDM, Mertens F, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Lauer SR, Edgar MA, Gardner JM, et al. Soft tissue chordomas: a clinicopathologic analysis of 11 cases. Am J Surg Pathol. 2013;37:719-726.
- Miller TD, McCalmont T, Tope WD. Recurrent cutaneous myoepithelioma treated using Mohs micrographic surgery: case report and review of the literature. Dermatol Surg. 2009;35:139-143.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Noronha V, Cooper DL, Higgins SA, et al. Metastatic myoepithelial carcinoma of the vulva treated with carboplatin and paclitaxel. Lancet Oncol. 2006;7:270-271.
- Bisogno G, Tagarelli A, Schiavetti A, et al. Myoepithelial carcinoma treatment in children: a report from the TREP project. Pediatr Blood Cancer. 2014;61:643-646.
- He DQ, Hua CG, Tang XF, et al. Cutaneous metastasis from a parotid myoepithelial carcinoma: a case report and review of the literature. J Cutan Pathol. 2008;35:1138-1143.
- Frost MW, Steiniche T, Damsgaard TE, et al. Primary cutaneous myoepithelial carcinoma: a case report and review of the literature. APMIS. 2014;122:369-379.
- Stojsic Z, Brasanac D, Boricic I, et al. Clear cell myoepithelial carcinoma of the skin. a case report. J Cutan Pathol. 2009;36:680-683.
- Tanahashi J, Kashima K, Daa T, et al. A case of cutaneous myoepithelial carcinoma. J Cutan Pathol. 2007;34:648-653.
- Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. report of 12 cases. Virchows Arch. 1999;434:393-400.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Law RM, Viglione MP, Barrett TL. Metastatic myoepithelial carcinoma in a child. J Cutan Pathol. 2008;35:779-781.
- Hornick JL, Fletcher CD. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004;35:14-24.
- Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. a molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114-1124.
- Antonescu CR, Zhang L, Shao SY, et al. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer. 2013;52:675-682.
- Flucke U, Palmedo G, Blankenhorn N, et al. EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: a study of 18 cases. Mod Pathol. 2011;24:1444-1450.
- Mentzel T, Requena L, Kaddu S, et al. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol. 2003;30:294-302.
- Garcia-Sanchez S, Elices M, Nieto S. Cutaneous myoepithelial carcinoma (malignant myoepithelial tumor of skin). Virchows Archiv. 2009;455(suppl 1):1-482.
- Bajoghli A, Limpert J. Treatment of cutaneous malignant myoepithelioma on the nasal ala using Mohs micrographic surgery in a two and a half year old child. J Invest Dermatol. 2009;129:S44.
- Prasad AR, Savera AT, Gown AM, et al. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801-806.
- Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands. a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761-774.
- Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-1196.
- Kilpatrick SE, Hitchcock MG, Kraus MD, et al. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13-22.
- Neto AG, Pineda-Daboin K, Luna MA. Myoepithelioma of the soft tissue of the head and neck: a case report and review of the literature. Head Neck. 2004;26:470-473.
- Kutzner H, Mentzel T, Kaddu S, et al. Cutaneous myoepithelioma: an under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol. 2001;25:348-355.
- Dix BT, Hentges MJ, Saltrick KR, et al. Cutaneous myoepithelioma in the foot: case report. Foot Ankle Spec. 2013;6:239-241.
- Burke T, Sahin A, Johnson DE, et al. Myoepithelioma of the retroperitoneum. Ultrastruct Pathol. 1995;19:269-274.
- Fernandez-Figueras MT, Puig L, Trias I, et al. Benign myoepithelioma of the skin. Am J Dermatopathol. 1998;20:208-212.
- Bahrami A, Dalton JD, Krane JF, et al. A subset of cutaneous and soft tissue mixed tumors are genetically linked to their salivary gland counterpart. Genes Chromosomes Cancer. 2012;51:140-148.
- Panagopoulos I, Mertens F, Isaksson M, et al. Molecular genetic characterization of the EWS/CHN and RBP56/CHN fusion genes in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2002;35:340-352.
- Graham RP, Weiss SW, Sukov WR, et al. PHF1 rearrangements in ossifying fibromyxoid tumors of soft parts: a fluorescence in situ hybridization study of 41 cases with emphasis on the malignant variant. Am J Surg Pathol. 2013;37:1751-1755.
- Dabska M. Parachordoma: a new clinicopathologic entity. Cancer. 1977;40:1586-1592.
- Fletcher CDM, Mertens F, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Lauer SR, Edgar MA, Gardner JM, et al. Soft tissue chordomas: a clinicopathologic analysis of 11 cases. Am J Surg Pathol. 2013;37:719-726.
- Miller TD, McCalmont T, Tope WD. Recurrent cutaneous myoepithelioma treated using Mohs micrographic surgery: case report and review of the literature. Dermatol Surg. 2009;35:139-143.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Noronha V, Cooper DL, Higgins SA, et al. Metastatic myoepithelial carcinoma of the vulva treated with carboplatin and paclitaxel. Lancet Oncol. 2006;7:270-271.
- Bisogno G, Tagarelli A, Schiavetti A, et al. Myoepithelial carcinoma treatment in children: a report from the TREP project. Pediatr Blood Cancer. 2014;61:643-646.
- He DQ, Hua CG, Tang XF, et al. Cutaneous metastasis from a parotid myoepithelial carcinoma: a case report and review of the literature. J Cutan Pathol. 2008;35:1138-1143.
Practice Points
- Cutaneous myoepithelial carcinoma is a rare malignant adnexal neoplasm with metastatic potential that can present in the skin.
- Cutaneous myoepithelial carcinoma is a tumor that can occasionally show EWSR1 gene rearrangement.
- Excision with negative margins and close follow-up is recommended for cutaneous myoepithelial carcinoma.
Sooner is better than later for acute UC surgery
AT ASCRS 2017
SEATTLE – Postponing surgery for acute ulcerative colitis more than a day increases postoperative complications, lengths of stay, and hospital costs, according to a review by Johns Hopkins University, Baltimore, of almost 2,000 patients.
It’s not uncommon to wait 5 or even 10 days to give biologics a chance to work when patients are admitted for acute ulcerative colitis (UC). Based on the review, however, “we believe that the need for prolonged medical therapy and resuscitation in this patient population prior to colectomy may be overstated,” and that “the lasting effects of persistent inflammation cascade are underestimated.”
There has to be “a conversation with the gastroenterologist to strike the right balance between medical and surgical therapy. Early surgical intervention” should be considered, lead author and general surgery resident Ira Leeds, MD, said at the American Society of Colon and Rectal Surgeons annual meeting.
The team reviewed 1,953 index UC admissions with emergent non-elective abdominal surgery in the National Inpatient Sample (NIS) database from 2008-13; 546 patients (28%) had early operations - within 24 hours of admission – and the other 1,407 had operations after that time.
Although it’s impossible to say for sure given the limits of administrative data in the NIS, patients who had surgery soon after admission were probably sicker. Even so, they were less likely to have complications than patients in the delayed surgery group (55% versus 43%), and they had shorter hospital stays, with just 8% in the hospital past 21 days, versus 29% of patients who had delayed operations. The findings were similar for both overall length of stay and post-op length of stay.
Renal complications (8% versus 14%), pulmonary complications (20% versus 25%), and thromboembolic events (4% versus 6%) were also less common in the early surgery group. On multivariable analysis, delayed surgery increased the complication rate by 64%.
With fewer complications and shorter hospital stays, early operations were also less expensive, with a mean total hospitalization cost of $19,985 versus $34,258. The findings were all statistically significant.
Dr. Leeds noted the limits of the study; medical management regimes and the reasons for variations in surgical timing are unknown, among other things. “This is not the final answer on what to do with patients like this, but it opens the door to prospective studies that could control” for such variables, he said.
Early surgery patients were more likely to be male (57% versus 51%) and from households with incomes higher than the national median. There were no difference in age, race, comorbidities, region, or hospital type between the two groups.
Dr. Leeds said he had no disclosures.
AGA Resource
Visit www.gastro.org/ibd for patient education guides that you can share with your patients to help them understand and manage their ulcerative colitis and IBD.
AT ASCRS 2017
SEATTLE – Postponing surgery for acute ulcerative colitis more than a day increases postoperative complications, lengths of stay, and hospital costs, according to a review by Johns Hopkins University, Baltimore, of almost 2,000 patients.
It’s not uncommon to wait 5 or even 10 days to give biologics a chance to work when patients are admitted for acute ulcerative colitis (UC). Based on the review, however, “we believe that the need for prolonged medical therapy and resuscitation in this patient population prior to colectomy may be overstated,” and that “the lasting effects of persistent inflammation cascade are underestimated.”
There has to be “a conversation with the gastroenterologist to strike the right balance between medical and surgical therapy. Early surgical intervention” should be considered, lead author and general surgery resident Ira Leeds, MD, said at the American Society of Colon and Rectal Surgeons annual meeting.
The team reviewed 1,953 index UC admissions with emergent non-elective abdominal surgery in the National Inpatient Sample (NIS) database from 2008-13; 546 patients (28%) had early operations - within 24 hours of admission – and the other 1,407 had operations after that time.
Although it’s impossible to say for sure given the limits of administrative data in the NIS, patients who had surgery soon after admission were probably sicker. Even so, they were less likely to have complications than patients in the delayed surgery group (55% versus 43%), and they had shorter hospital stays, with just 8% in the hospital past 21 days, versus 29% of patients who had delayed operations. The findings were similar for both overall length of stay and post-op length of stay.
Renal complications (8% versus 14%), pulmonary complications (20% versus 25%), and thromboembolic events (4% versus 6%) were also less common in the early surgery group. On multivariable analysis, delayed surgery increased the complication rate by 64%.
With fewer complications and shorter hospital stays, early operations were also less expensive, with a mean total hospitalization cost of $19,985 versus $34,258. The findings were all statistically significant.
Dr. Leeds noted the limits of the study; medical management regimes and the reasons for variations in surgical timing are unknown, among other things. “This is not the final answer on what to do with patients like this, but it opens the door to prospective studies that could control” for such variables, he said.
Early surgery patients were more likely to be male (57% versus 51%) and from households with incomes higher than the national median. There were no difference in age, race, comorbidities, region, or hospital type between the two groups.
Dr. Leeds said he had no disclosures.
AGA Resource
Visit www.gastro.org/ibd for patient education guides that you can share with your patients to help them understand and manage their ulcerative colitis and IBD.
AT ASCRS 2017
SEATTLE – Postponing surgery for acute ulcerative colitis more than a day increases postoperative complications, lengths of stay, and hospital costs, according to a review by Johns Hopkins University, Baltimore, of almost 2,000 patients.
It’s not uncommon to wait 5 or even 10 days to give biologics a chance to work when patients are admitted for acute ulcerative colitis (UC). Based on the review, however, “we believe that the need for prolonged medical therapy and resuscitation in this patient population prior to colectomy may be overstated,” and that “the lasting effects of persistent inflammation cascade are underestimated.”
There has to be “a conversation with the gastroenterologist to strike the right balance between medical and surgical therapy. Early surgical intervention” should be considered, lead author and general surgery resident Ira Leeds, MD, said at the American Society of Colon and Rectal Surgeons annual meeting.
The team reviewed 1,953 index UC admissions with emergent non-elective abdominal surgery in the National Inpatient Sample (NIS) database from 2008-13; 546 patients (28%) had early operations - within 24 hours of admission – and the other 1,407 had operations after that time.
Although it’s impossible to say for sure given the limits of administrative data in the NIS, patients who had surgery soon after admission were probably sicker. Even so, they were less likely to have complications than patients in the delayed surgery group (55% versus 43%), and they had shorter hospital stays, with just 8% in the hospital past 21 days, versus 29% of patients who had delayed operations. The findings were similar for both overall length of stay and post-op length of stay.
Renal complications (8% versus 14%), pulmonary complications (20% versus 25%), and thromboembolic events (4% versus 6%) were also less common in the early surgery group. On multivariable analysis, delayed surgery increased the complication rate by 64%.
With fewer complications and shorter hospital stays, early operations were also less expensive, with a mean total hospitalization cost of $19,985 versus $34,258. The findings were all statistically significant.
Dr. Leeds noted the limits of the study; medical management regimes and the reasons for variations in surgical timing are unknown, among other things. “This is not the final answer on what to do with patients like this, but it opens the door to prospective studies that could control” for such variables, he said.
Early surgery patients were more likely to be male (57% versus 51%) and from households with incomes higher than the national median. There were no difference in age, race, comorbidities, region, or hospital type between the two groups.
Dr. Leeds said he had no disclosures.
AGA Resource
Visit www.gastro.org/ibd for patient education guides that you can share with your patients to help them understand and manage their ulcerative colitis and IBD.
Key clinical point:
Major finding: Patients who had surgery soon after admission were probably sicker. Even so, they were less likely to have complications than patients in the delayed surgery group (55% versus 43%), and they had shorter hospital stays, with just 8% in the hospital past 21 days, versus 29% of patients who had delayed operations.
Data source: Review of almost 2,000 patients in the National Inpatient Sample
Disclosures: The lead investigator had no disclosures.
SEATTLE – Postponing surgery for acute ulcerative colitis more than a day increases postoperative complications, lengths of stay, and hospital costs, according to a review by Johns Hopkins University, Baltimore, of almost 2,000 patients.
It’s not uncommon to wait 5 or even 10 days to give biologics a chance to work when patients are admitted for acute ulcerative colitis (UC). Based on the review, however, “we believe that the need for prolonged medical therapy and resuscitation in this patient population prior to colectomy may be overstated,” and that “the lasting effects of persistent inflammation cascade are underestimated.”
[[{"attributes":{},"fields":{}}]]
There has to be “a conversation with the gastroenterologist to strike the right balance between medical and surgical therapy. Early surgical intervention” should be considered, lead author and general surgery resident Ira Leeds, MD, said at the American Society of Colon and Rectal Surgeons annual meeting.
The team reviewed 1,953 index UC admissions with emergent non-elective abdominal surgery in the National Inpatient Sample (NIS) database from 2008-13; 546 patients (28%) had early operations - within 24 hours of admission – and the other 1,407 had operations after that time.
Although it’s impossible to say for sure given the limits of administrative data in the NIS, patients who had surgery soon after admission were probably sicker. Even so, they were less likely to have complications than patients in the delayed surgery group (55% versus 43%), and they had shorter hospital stays, with just 8% in the hospital past 21 days, versus 29% of patients who had delayed operations. The findings were similar for both overall length of stay and post-op length of stay.
Renal complications (8% versus 14%), pulmonary complications (20% versus 25%), and thromboembolic events (4% versus 6%) were also less common in the early surgery group. On multivariable analysis, delayed surgery increased the complication rate by 64%.
With fewer complications and shorter hospital stays, early operations were also less expensive, with a mean total hospitalization cost of $19,985 versus $34,258. The findings were all statistically significant.
Dr. Leeds noted the limits of the study; medical management regimes and the reasons for variations in surgical timing are unknown, among other things. “This is not the final answer on what to do with patients like this, but it opens the door to prospective studies that could control” for such variables, he said.
Early surgery patients were more likely to be male (57% versus 51%) and from households with incomes higher than the national median. There were no difference in age, race, comorbidities, region, or hospital type between the two groups.
Dr. Leeds said he had no disclosures.
Patient Satisfaction With Prophylactic Migraine Medications
BOSTON—Effectiveness and tolerability were the key factors influencing patients’ satisfaction with prophylactic migraine medications, according to a study presented at the 59th Annual Scientific Meeting of the American Headache Society. “There is an unmet need for treatments that have better efficacy and tolerability profiles than existing prophylactic migraine medications,” said lead author Marci Clark, PharmD, Director of Patient-Centered Outcomes Assessment at RTI Health Solutions in Ann Arbor, Michigan, on behalf of her study collaborators.
To gain insight into reasons for patient satisfaction or dissatisfaction with prophylactic migraine medications and identify medication characteristics that would increase patient satisfaction with future prophylactic migraine medications, the researchers recruited study participants from research facilities in Charlotte, North Carolina; St. Louis, Missouri; Southfield, Michigan; and Tampa, Florida. Individuals were eligible to participate if they met the following self-reported criteria: age 18 to 55, physician diagnosis of migraine for 12 or more months, current or prior experience taking one or more prescription prophylactic migraine medication in the past six months, experiencing four or more migraine headache days per month, and further classification as having episodic or chronic migraine (based on the headache/migraine days reported). Individual discussions with all participants were conducted by two experienced interviewers.
Forty participants were recruited and interviewed; 36 met all eligibility criteria. Twenty-one participants met the criteria for episodic migraine, 15 met the criteria for chronic migraine, and four participants, who were current migraineurs, were unable to be classified as episodic or chronic migraine. Participants provided feedback on 20 prescription prophylactic migraine medications. The most commonly used current or prior migraine prophylactics were topiramate (n = 27), amitriptyline (n = 6), propranolol (n = 6), onabotulinumtoxinA (n = 5), and zonisamide (n = 3).
In general, medication effectiveness and tolerability were the most influential factors on patient satisfaction or dissatisfaction with their prophylactic migraine medications. Lack of efficacy, cited by 56% of respondents, was the most frequently reported reason for participants’ dissatisfaction with their medications. Regarding tolerability, 51% of participants reported that a particular side effect was the most dissatisfying characteristic of their medication. The most bothersome side effects reported by participants were memory loss (n = 6), dizziness or light-headedness (n = 5), tiredness/drowsiness/grogginess/sluggishness (n = 5), nausea (n = 3), and dry mouth (n = 2).
Among those who were satisfied with their prophylactic migraine medications, decreased migraine frequency was the most common reason for their satisfaction (65%), followed by decreased migraine severity or intensity, which was reported by 28% of participants.
The most frequently reported desirable characteristics of a new prophylactic migraine medication that would increase participants’ satisfaction were related to the medication’s ability to decrease the frequency and severity or intensity of migraines. Nausea (36%), weight gain (26%), dizziness/light-headedness (23%), drowsiness/grogginess (15%), and memory loss, including the ability to remember words and speak clearly (13%), are side effects that patients reported that they do not want to see in a new or future prophylactic migraine medication.
BOSTON—Effectiveness and tolerability were the key factors influencing patients’ satisfaction with prophylactic migraine medications, according to a study presented at the 59th Annual Scientific Meeting of the American Headache Society. “There is an unmet need for treatments that have better efficacy and tolerability profiles than existing prophylactic migraine medications,” said lead author Marci Clark, PharmD, Director of Patient-Centered Outcomes Assessment at RTI Health Solutions in Ann Arbor, Michigan, on behalf of her study collaborators.
To gain insight into reasons for patient satisfaction or dissatisfaction with prophylactic migraine medications and identify medication characteristics that would increase patient satisfaction with future prophylactic migraine medications, the researchers recruited study participants from research facilities in Charlotte, North Carolina; St. Louis, Missouri; Southfield, Michigan; and Tampa, Florida. Individuals were eligible to participate if they met the following self-reported criteria: age 18 to 55, physician diagnosis of migraine for 12 or more months, current or prior experience taking one or more prescription prophylactic migraine medication in the past six months, experiencing four or more migraine headache days per month, and further classification as having episodic or chronic migraine (based on the headache/migraine days reported). Individual discussions with all participants were conducted by two experienced interviewers.
Forty participants were recruited and interviewed; 36 met all eligibility criteria. Twenty-one participants met the criteria for episodic migraine, 15 met the criteria for chronic migraine, and four participants, who were current migraineurs, were unable to be classified as episodic or chronic migraine. Participants provided feedback on 20 prescription prophylactic migraine medications. The most commonly used current or prior migraine prophylactics were topiramate (n = 27), amitriptyline (n = 6), propranolol (n = 6), onabotulinumtoxinA (n = 5), and zonisamide (n = 3).
In general, medication effectiveness and tolerability were the most influential factors on patient satisfaction or dissatisfaction with their prophylactic migraine medications. Lack of efficacy, cited by 56% of respondents, was the most frequently reported reason for participants’ dissatisfaction with their medications. Regarding tolerability, 51% of participants reported that a particular side effect was the most dissatisfying characteristic of their medication. The most bothersome side effects reported by participants were memory loss (n = 6), dizziness or light-headedness (n = 5), tiredness/drowsiness/grogginess/sluggishness (n = 5), nausea (n = 3), and dry mouth (n = 2).
Among those who were satisfied with their prophylactic migraine medications, decreased migraine frequency was the most common reason for their satisfaction (65%), followed by decreased migraine severity or intensity, which was reported by 28% of participants.
The most frequently reported desirable characteristics of a new prophylactic migraine medication that would increase participants’ satisfaction were related to the medication’s ability to decrease the frequency and severity or intensity of migraines. Nausea (36%), weight gain (26%), dizziness/light-headedness (23%), drowsiness/grogginess (15%), and memory loss, including the ability to remember words and speak clearly (13%), are side effects that patients reported that they do not want to see in a new or future prophylactic migraine medication.
BOSTON—Effectiveness and tolerability were the key factors influencing patients’ satisfaction with prophylactic migraine medications, according to a study presented at the 59th Annual Scientific Meeting of the American Headache Society. “There is an unmet need for treatments that have better efficacy and tolerability profiles than existing prophylactic migraine medications,” said lead author Marci Clark, PharmD, Director of Patient-Centered Outcomes Assessment at RTI Health Solutions in Ann Arbor, Michigan, on behalf of her study collaborators.
To gain insight into reasons for patient satisfaction or dissatisfaction with prophylactic migraine medications and identify medication characteristics that would increase patient satisfaction with future prophylactic migraine medications, the researchers recruited study participants from research facilities in Charlotte, North Carolina; St. Louis, Missouri; Southfield, Michigan; and Tampa, Florida. Individuals were eligible to participate if they met the following self-reported criteria: age 18 to 55, physician diagnosis of migraine for 12 or more months, current or prior experience taking one or more prescription prophylactic migraine medication in the past six months, experiencing four or more migraine headache days per month, and further classification as having episodic or chronic migraine (based on the headache/migraine days reported). Individual discussions with all participants were conducted by two experienced interviewers.
Forty participants were recruited and interviewed; 36 met all eligibility criteria. Twenty-one participants met the criteria for episodic migraine, 15 met the criteria for chronic migraine, and four participants, who were current migraineurs, were unable to be classified as episodic or chronic migraine. Participants provided feedback on 20 prescription prophylactic migraine medications. The most commonly used current or prior migraine prophylactics were topiramate (n = 27), amitriptyline (n = 6), propranolol (n = 6), onabotulinumtoxinA (n = 5), and zonisamide (n = 3).
In general, medication effectiveness and tolerability were the most influential factors on patient satisfaction or dissatisfaction with their prophylactic migraine medications. Lack of efficacy, cited by 56% of respondents, was the most frequently reported reason for participants’ dissatisfaction with their medications. Regarding tolerability, 51% of participants reported that a particular side effect was the most dissatisfying characteristic of their medication. The most bothersome side effects reported by participants were memory loss (n = 6), dizziness or light-headedness (n = 5), tiredness/drowsiness/grogginess/sluggishness (n = 5), nausea (n = 3), and dry mouth (n = 2).
Among those who were satisfied with their prophylactic migraine medications, decreased migraine frequency was the most common reason for their satisfaction (65%), followed by decreased migraine severity or intensity, which was reported by 28% of participants.
The most frequently reported desirable characteristics of a new prophylactic migraine medication that would increase participants’ satisfaction were related to the medication’s ability to decrease the frequency and severity or intensity of migraines. Nausea (36%), weight gain (26%), dizziness/light-headedness (23%), drowsiness/grogginess (15%), and memory loss, including the ability to remember words and speak clearly (13%), are side effects that patients reported that they do not want to see in a new or future prophylactic migraine medication.
Lenalidomide-rituximab induces high CR rate in untreated follicular lymphoma
LUGANO, SWITZERLAND – The chemotherapy-free combination of lenalidomide (Revlimid) and rituximab was highly active as frontline therapy for patients with low- and intermediate-risk follicular lymphoma in a multicenter phase II trial.
Among 66 patients with previously untreated follicular lymphoma, the overall response rate to the combination was 95%, including complete responses in 72% of patients. The 5-year progression-free survival (PFS) rate was 70%, reported John P. Leonard, MD, of Weill Cornell Medicine, New York.
“I think this overall is a useful validation – confirmation of the single-center data that showed that this [combination] in a multicenter setting can be a highly effective and reasonably well-tolerated treatment approach for patients with untreated follicular lymphoma,” he said at the International Conference on Malignant Lymphoma, on behalf of colleagues in the National Cancer Institute Alliance for Clinical Trials in Oncology and Cancer and Leukemia Group B (CALGB) 50803 trial.
In the 50803 study, the investigators enrolled 66 treatment-naive patients. The median age was 53 years (range 32-79). Patients were eligible if they had grade 1-3a, stage 3-4 or bulky stage 2 untreated follicular lymphoma, with Follicular Lymphoma International Prognostic Index (FLIPI) scores of 0-2.
They received lenalidomide 20 mg/day on days 1 through 21 of each 28-day cycle for 12 cycles, plus rituximab administered once weekly for each week of cycle 1, and on the first day of cycles 4, 6, 8 and 10.
The investigators also evaluated polymorphisms in the Fc fragment of immunoglobulin G receptor IIa and IIIa (FcGR2A/FcGR3A).
One of the 66 patients never started treatment, leaving 65 for the response analysis.
As noted, the overall response rate was 95%, including 94% of 21 patients with FLIPI 0 or 1 disease, and 96% of 44 patients with FLIPI 2 or 3. There were no associations between FLIPI score and likelihood of achieving a complete response, and no associations between FcR polymorphism or change in angiogenic markers and either complete responses or PFS, Dr. Leonard said.
Complete responses, the primary endpoint, were seen in 15 of the 21 (71%) of patients with FLIPI 0-1, and in 32 of the 44 (73%) with FLIPI 2-3, for an overall complete response rate of 72%.
Partial responses occurred in 5 patients (23%) with FLIPI 0-1 and 10 patients (23%) with FLIPI 2-3, for an overall PR rate of 23%.
Respective rates of stable disease were 0, 2%, and 2%. One patient in each FLIPI group was not evaluated because of adverse events.
After a median follow-up of 5 years, the progression free survival rate was 70%.
The most common grade 3 or 4 adverse events were neutropenia, seen in 21% of patients, and infections, seen in 40% (including one grade 3 febrile neutropenia).
Grade 1-2 fatigue was reported by 51 patients, and grade 3 fatigue was reported by 4.
Other grade 3 or 4 events seen in more than 5% of patients included rash in 9%, and hyperglycemia, hypophosphatemia, or hypertension, each in 6% of patients.
Celgene and Genentech supported the study. Dr. Leonard has served as an adviser/consultant to Celgene and other companies.
LUGANO, SWITZERLAND – The chemotherapy-free combination of lenalidomide (Revlimid) and rituximab was highly active as frontline therapy for patients with low- and intermediate-risk follicular lymphoma in a multicenter phase II trial.
Among 66 patients with previously untreated follicular lymphoma, the overall response rate to the combination was 95%, including complete responses in 72% of patients. The 5-year progression-free survival (PFS) rate was 70%, reported John P. Leonard, MD, of Weill Cornell Medicine, New York.
“I think this overall is a useful validation – confirmation of the single-center data that showed that this [combination] in a multicenter setting can be a highly effective and reasonably well-tolerated treatment approach for patients with untreated follicular lymphoma,” he said at the International Conference on Malignant Lymphoma, on behalf of colleagues in the National Cancer Institute Alliance for Clinical Trials in Oncology and Cancer and Leukemia Group B (CALGB) 50803 trial.
In the 50803 study, the investigators enrolled 66 treatment-naive patients. The median age was 53 years (range 32-79). Patients were eligible if they had grade 1-3a, stage 3-4 or bulky stage 2 untreated follicular lymphoma, with Follicular Lymphoma International Prognostic Index (FLIPI) scores of 0-2.
They received lenalidomide 20 mg/day on days 1 through 21 of each 28-day cycle for 12 cycles, plus rituximab administered once weekly for each week of cycle 1, and on the first day of cycles 4, 6, 8 and 10.
The investigators also evaluated polymorphisms in the Fc fragment of immunoglobulin G receptor IIa and IIIa (FcGR2A/FcGR3A).
One of the 66 patients never started treatment, leaving 65 for the response analysis.
As noted, the overall response rate was 95%, including 94% of 21 patients with FLIPI 0 or 1 disease, and 96% of 44 patients with FLIPI 2 or 3. There were no associations between FLIPI score and likelihood of achieving a complete response, and no associations between FcR polymorphism or change in angiogenic markers and either complete responses or PFS, Dr. Leonard said.
Complete responses, the primary endpoint, were seen in 15 of the 21 (71%) of patients with FLIPI 0-1, and in 32 of the 44 (73%) with FLIPI 2-3, for an overall complete response rate of 72%.
Partial responses occurred in 5 patients (23%) with FLIPI 0-1 and 10 patients (23%) with FLIPI 2-3, for an overall PR rate of 23%.
Respective rates of stable disease were 0, 2%, and 2%. One patient in each FLIPI group was not evaluated because of adverse events.
After a median follow-up of 5 years, the progression free survival rate was 70%.
The most common grade 3 or 4 adverse events were neutropenia, seen in 21% of patients, and infections, seen in 40% (including one grade 3 febrile neutropenia).
Grade 1-2 fatigue was reported by 51 patients, and grade 3 fatigue was reported by 4.
Other grade 3 or 4 events seen in more than 5% of patients included rash in 9%, and hyperglycemia, hypophosphatemia, or hypertension, each in 6% of patients.
Celgene and Genentech supported the study. Dr. Leonard has served as an adviser/consultant to Celgene and other companies.
LUGANO, SWITZERLAND – The chemotherapy-free combination of lenalidomide (Revlimid) and rituximab was highly active as frontline therapy for patients with low- and intermediate-risk follicular lymphoma in a multicenter phase II trial.
Among 66 patients with previously untreated follicular lymphoma, the overall response rate to the combination was 95%, including complete responses in 72% of patients. The 5-year progression-free survival (PFS) rate was 70%, reported John P. Leonard, MD, of Weill Cornell Medicine, New York.
“I think this overall is a useful validation – confirmation of the single-center data that showed that this [combination] in a multicenter setting can be a highly effective and reasonably well-tolerated treatment approach for patients with untreated follicular lymphoma,” he said at the International Conference on Malignant Lymphoma, on behalf of colleagues in the National Cancer Institute Alliance for Clinical Trials in Oncology and Cancer and Leukemia Group B (CALGB) 50803 trial.
In the 50803 study, the investigators enrolled 66 treatment-naive patients. The median age was 53 years (range 32-79). Patients were eligible if they had grade 1-3a, stage 3-4 or bulky stage 2 untreated follicular lymphoma, with Follicular Lymphoma International Prognostic Index (FLIPI) scores of 0-2.
They received lenalidomide 20 mg/day on days 1 through 21 of each 28-day cycle for 12 cycles, plus rituximab administered once weekly for each week of cycle 1, and on the first day of cycles 4, 6, 8 and 10.
The investigators also evaluated polymorphisms in the Fc fragment of immunoglobulin G receptor IIa and IIIa (FcGR2A/FcGR3A).
One of the 66 patients never started treatment, leaving 65 for the response analysis.
As noted, the overall response rate was 95%, including 94% of 21 patients with FLIPI 0 or 1 disease, and 96% of 44 patients with FLIPI 2 or 3. There were no associations between FLIPI score and likelihood of achieving a complete response, and no associations between FcR polymorphism or change in angiogenic markers and either complete responses or PFS, Dr. Leonard said.
Complete responses, the primary endpoint, were seen in 15 of the 21 (71%) of patients with FLIPI 0-1, and in 32 of the 44 (73%) with FLIPI 2-3, for an overall complete response rate of 72%.
Partial responses occurred in 5 patients (23%) with FLIPI 0-1 and 10 patients (23%) with FLIPI 2-3, for an overall PR rate of 23%.
Respective rates of stable disease were 0, 2%, and 2%. One patient in each FLIPI group was not evaluated because of adverse events.
After a median follow-up of 5 years, the progression free survival rate was 70%.
The most common grade 3 or 4 adverse events were neutropenia, seen in 21% of patients, and infections, seen in 40% (including one grade 3 febrile neutropenia).
Grade 1-2 fatigue was reported by 51 patients, and grade 3 fatigue was reported by 4.
Other grade 3 or 4 events seen in more than 5% of patients included rash in 9%, and hyperglycemia, hypophosphatemia, or hypertension, each in 6% of patients.
Celgene and Genentech supported the study. Dr. Leonard has served as an adviser/consultant to Celgene and other companies.
AT 14-ICML
Key clinical point: The combination of lenalidomide and rituximab was highly active against previously untreated follicular lymphoma.
Major finding: The overall response rate was 95%; 5-year progression-free survival was 70%.
Data source: Open-label prospective study of lenalidomide-rituximab in 66 patients with previously untreated follicular lymphoma.
Disclosures: Celgene and Genentech supported the study. Dr. Leonard has served as an adviser/consultant to Celgene and other companies.
Sequential triple therapy produces high response rates in CLL
Lugano, Switzerland– It’s in the BAG: For patients with treatment-naive or relapsed/refractory chronic lymphocytic leukemia, a regimen consisting of debulking with bendamustine (Treanda) followed sequentially by obinutuzumab (Gazyva) and venetoclax (Venclexta) was associated with a high overall response rate and a large majority of patients being negative for minimal residual disease (MRD) in peripheral blood.
In the open-label, phase II CLL2-BAG study in 66 patients, the overall response rate at the end of induction was 95%, and 87% of patients were negative for MRD, reported Paula Cramer, MD, of the University of Cologne (Germany).
“This sequential treatment of bendamustine followed by obinutuzumab and venetoclax does not lead to any cumulative or unexpected toxicity. As compared to other trials, it’s important to point out that only one laboratory TLS [tumor lysis syndrome] occurred with venetoclax. Maybe that’s explained by the sequential start of all three drugs,” she said at the International Conference on Malignant Lymphoma.
The trial is based on a concept the investigators call “sequential triple-T” (tailored and targeted treatment) aimed at complete eradication of MRD.
The 66 patients (median age, 59 years) had either previously untreated or relapsed/refractory CLL; 34 of the treatment-naive and 29 of the relapsed/refractory patients were evaluable for response.
At enrollment, patients who had an absolute lymphocyte count of 25,000/mcL or greater and/or lymph nodes 5 cm or larger received two cycles of debulking with bendamustine 70 mg/m2 on days 1 and 2 every 28 days, unless this was contraindicated.
The induction phase consisted of obinutuzumab 1,000 mg administered three times in cycle 1, then every 4 weeks for cycles 2 through 6. Venetoclax was started during cycle 2 with a dose escalation to 400 mg daily over a period of 5 weeks.
Maintenance therapy consisted of daily venetoclax and obinutuzumab every 3 months until MRD negativity or up to 24 months.
A total of 45 patients (31 who were treatment naive and 14 with relapsed/refractory disease) underwent debulking. Of this group 36 patients received both cycles, and 9 discontinued early because of either adverse events (5 patients), disease progression (1), or unknown reasons (3).
Sixty of the 66 patients received the full six induction cycles. Three patients who had fewer than two cycles were excluded according to protocol. Of these three, two heavily pretreated patients died of sepsis and one of myocardial infarction in cycle 1.
Responses
In all, 24 patients (53%) who had debulking had a response during that phase based on International Workshop on CLL (IWCLL) criteria, for an overall response rate (ORR) of 53%. Thirteen patients had stable disease after debulking, and four had disease progression.
Responses after induction according to IWCLL criteria, the primary endpoint, included five patients with a complete response (three treatment-naive and two relapsed/refractory patients), and 20 patients (14 treatment-naive and six relapsed/refractory patients) with an unconfirmed or clinical complete response or a complete response with incomplete recovery of counts.
In addition, 35 patients (17 who were treatment naive and 18 with relapsed/refractory disease) had a partial response. No patients had stable disease, and three patients, all with relapsed/refractory disease, had disease progression.
Of the 63 patients who had at least two cycles of therapy, 87% were MRD negative. Eight patients had MRD assessments by bone marrow, and all eight were MRD negative.
The maintenance phase of the trial is ongoing and the data are not mature, but 56 patients (30 who were treatment naive and 26 with relapsed/refractory disease) have started on maintenance therapy. Of this group, 28 have stopped maintenance, including 21 patients who stopped because they achieved MRD negativity, 5 who discontinued because of adverse events, and 2 with disease progression or Richter’s transformation.
At 15 months of follow-up, progression-free survival was 100% in patients who received the combination as the frontline therapy, and 83% in the patients with relapsed/refractory disease.
Adverse events
In the debulking phase, 34 patients (72%) experienced adverse events of any grade, including 16 patients with grade 3 or 4 events
Adverse events associated with debulking included neutropenia and anemia, each seen in five patients, thrombocytopenia and infection each seen in three patients, and coronary artery disorders, rash, tumor lysis syndrome, vomiting, and pyrexia in one patient each.
Sixty of the 66 patients received the full six induction cycles. Three patients who had fewer than two cycles were excluded according to protocol. Of these three, two heavily pretreated patients died of sepsis and one of myocardial infarction in cycle 1.
All but 3 of the 63 patients had an adverse event, and 44 patients had at least one grade 3 or 4 adverse event.
Cytopenias, infections, infusion-related reactions, and neoplasms were the most common adverse events.
There were three grade 3 laboratory-confirmed cases of tumor lysis syndrome, one each occurring during bendamustine debulking, obinutuzumab administration, and venetoclax administration.
Something other than venetoclax?
Davide Rossi, MD, PhD, of the Oncology Institute of Southern Switzerland in Bellinzona, the invited discussant, questioned whether venetoclax is the best agent to use in sequential triple-T therapy.
“We know that venetoclax is more active in blood and bone marrow than in lymph nodes and, indeed, a proportion of cases achieving all the criteria for a CR [complete response] in the bone marrow and the peripheral blood, including MRD negativity, may still have some residual [disease in] lymph nodes,” he said.
Drugs that inhibit the B-cell receptor pathway may be more effective at eradicating MRD in the lymph node compartment, Dr. Cramer said.
The study was sponsored by the German CLL study group. Dr. Cramer disclosed financial ties to multiple entities. Dr. Rossi did not report disclosures.
Lugano, Switzerland– It’s in the BAG: For patients with treatment-naive or relapsed/refractory chronic lymphocytic leukemia, a regimen consisting of debulking with bendamustine (Treanda) followed sequentially by obinutuzumab (Gazyva) and venetoclax (Venclexta) was associated with a high overall response rate and a large majority of patients being negative for minimal residual disease (MRD) in peripheral blood.
In the open-label, phase II CLL2-BAG study in 66 patients, the overall response rate at the end of induction was 95%, and 87% of patients were negative for MRD, reported Paula Cramer, MD, of the University of Cologne (Germany).
“This sequential treatment of bendamustine followed by obinutuzumab and venetoclax does not lead to any cumulative or unexpected toxicity. As compared to other trials, it’s important to point out that only one laboratory TLS [tumor lysis syndrome] occurred with venetoclax. Maybe that’s explained by the sequential start of all three drugs,” she said at the International Conference on Malignant Lymphoma.
The trial is based on a concept the investigators call “sequential triple-T” (tailored and targeted treatment) aimed at complete eradication of MRD.
The 66 patients (median age, 59 years) had either previously untreated or relapsed/refractory CLL; 34 of the treatment-naive and 29 of the relapsed/refractory patients were evaluable for response.
At enrollment, patients who had an absolute lymphocyte count of 25,000/mcL or greater and/or lymph nodes 5 cm or larger received two cycles of debulking with bendamustine 70 mg/m2 on days 1 and 2 every 28 days, unless this was contraindicated.
The induction phase consisted of obinutuzumab 1,000 mg administered three times in cycle 1, then every 4 weeks for cycles 2 through 6. Venetoclax was started during cycle 2 with a dose escalation to 400 mg daily over a period of 5 weeks.
Maintenance therapy consisted of daily venetoclax and obinutuzumab every 3 months until MRD negativity or up to 24 months.
A total of 45 patients (31 who were treatment naive and 14 with relapsed/refractory disease) underwent debulking. Of this group 36 patients received both cycles, and 9 discontinued early because of either adverse events (5 patients), disease progression (1), or unknown reasons (3).
Sixty of the 66 patients received the full six induction cycles. Three patients who had fewer than two cycles were excluded according to protocol. Of these three, two heavily pretreated patients died of sepsis and one of myocardial infarction in cycle 1.
Responses
In all, 24 patients (53%) who had debulking had a response during that phase based on International Workshop on CLL (IWCLL) criteria, for an overall response rate (ORR) of 53%. Thirteen patients had stable disease after debulking, and four had disease progression.
Responses after induction according to IWCLL criteria, the primary endpoint, included five patients with a complete response (three treatment-naive and two relapsed/refractory patients), and 20 patients (14 treatment-naive and six relapsed/refractory patients) with an unconfirmed or clinical complete response or a complete response with incomplete recovery of counts.
In addition, 35 patients (17 who were treatment naive and 18 with relapsed/refractory disease) had a partial response. No patients had stable disease, and three patients, all with relapsed/refractory disease, had disease progression.
Of the 63 patients who had at least two cycles of therapy, 87% were MRD negative. Eight patients had MRD assessments by bone marrow, and all eight were MRD negative.
The maintenance phase of the trial is ongoing and the data are not mature, but 56 patients (30 who were treatment naive and 26 with relapsed/refractory disease) have started on maintenance therapy. Of this group, 28 have stopped maintenance, including 21 patients who stopped because they achieved MRD negativity, 5 who discontinued because of adverse events, and 2 with disease progression or Richter’s transformation.
At 15 months of follow-up, progression-free survival was 100% in patients who received the combination as the frontline therapy, and 83% in the patients with relapsed/refractory disease.
Adverse events
In the debulking phase, 34 patients (72%) experienced adverse events of any grade, including 16 patients with grade 3 or 4 events
Adverse events associated with debulking included neutropenia and anemia, each seen in five patients, thrombocytopenia and infection each seen in three patients, and coronary artery disorders, rash, tumor lysis syndrome, vomiting, and pyrexia in one patient each.
Sixty of the 66 patients received the full six induction cycles. Three patients who had fewer than two cycles were excluded according to protocol. Of these three, two heavily pretreated patients died of sepsis and one of myocardial infarction in cycle 1.
All but 3 of the 63 patients had an adverse event, and 44 patients had at least one grade 3 or 4 adverse event.
Cytopenias, infections, infusion-related reactions, and neoplasms were the most common adverse events.
There were three grade 3 laboratory-confirmed cases of tumor lysis syndrome, one each occurring during bendamustine debulking, obinutuzumab administration, and venetoclax administration.
Something other than venetoclax?
Davide Rossi, MD, PhD, of the Oncology Institute of Southern Switzerland in Bellinzona, the invited discussant, questioned whether venetoclax is the best agent to use in sequential triple-T therapy.
“We know that venetoclax is more active in blood and bone marrow than in lymph nodes and, indeed, a proportion of cases achieving all the criteria for a CR [complete response] in the bone marrow and the peripheral blood, including MRD negativity, may still have some residual [disease in] lymph nodes,” he said.
Drugs that inhibit the B-cell receptor pathway may be more effective at eradicating MRD in the lymph node compartment, Dr. Cramer said.
The study was sponsored by the German CLL study group. Dr. Cramer disclosed financial ties to multiple entities. Dr. Rossi did not report disclosures.
Lugano, Switzerland– It’s in the BAG: For patients with treatment-naive or relapsed/refractory chronic lymphocytic leukemia, a regimen consisting of debulking with bendamustine (Treanda) followed sequentially by obinutuzumab (Gazyva) and venetoclax (Venclexta) was associated with a high overall response rate and a large majority of patients being negative for minimal residual disease (MRD) in peripheral blood.
In the open-label, phase II CLL2-BAG study in 66 patients, the overall response rate at the end of induction was 95%, and 87% of patients were negative for MRD, reported Paula Cramer, MD, of the University of Cologne (Germany).
“This sequential treatment of bendamustine followed by obinutuzumab and venetoclax does not lead to any cumulative or unexpected toxicity. As compared to other trials, it’s important to point out that only one laboratory TLS [tumor lysis syndrome] occurred with venetoclax. Maybe that’s explained by the sequential start of all three drugs,” she said at the International Conference on Malignant Lymphoma.
The trial is based on a concept the investigators call “sequential triple-T” (tailored and targeted treatment) aimed at complete eradication of MRD.
The 66 patients (median age, 59 years) had either previously untreated or relapsed/refractory CLL; 34 of the treatment-naive and 29 of the relapsed/refractory patients were evaluable for response.
At enrollment, patients who had an absolute lymphocyte count of 25,000/mcL or greater and/or lymph nodes 5 cm or larger received two cycles of debulking with bendamustine 70 mg/m2 on days 1 and 2 every 28 days, unless this was contraindicated.
The induction phase consisted of obinutuzumab 1,000 mg administered three times in cycle 1, then every 4 weeks for cycles 2 through 6. Venetoclax was started during cycle 2 with a dose escalation to 400 mg daily over a period of 5 weeks.
Maintenance therapy consisted of daily venetoclax and obinutuzumab every 3 months until MRD negativity or up to 24 months.
A total of 45 patients (31 who were treatment naive and 14 with relapsed/refractory disease) underwent debulking. Of this group 36 patients received both cycles, and 9 discontinued early because of either adverse events (5 patients), disease progression (1), or unknown reasons (3).
Sixty of the 66 patients received the full six induction cycles. Three patients who had fewer than two cycles were excluded according to protocol. Of these three, two heavily pretreated patients died of sepsis and one of myocardial infarction in cycle 1.
Responses
In all, 24 patients (53%) who had debulking had a response during that phase based on International Workshop on CLL (IWCLL) criteria, for an overall response rate (ORR) of 53%. Thirteen patients had stable disease after debulking, and four had disease progression.
Responses after induction according to IWCLL criteria, the primary endpoint, included five patients with a complete response (three treatment-naive and two relapsed/refractory patients), and 20 patients (14 treatment-naive and six relapsed/refractory patients) with an unconfirmed or clinical complete response or a complete response with incomplete recovery of counts.
In addition, 35 patients (17 who were treatment naive and 18 with relapsed/refractory disease) had a partial response. No patients had stable disease, and three patients, all with relapsed/refractory disease, had disease progression.
Of the 63 patients who had at least two cycles of therapy, 87% were MRD negative. Eight patients had MRD assessments by bone marrow, and all eight were MRD negative.
The maintenance phase of the trial is ongoing and the data are not mature, but 56 patients (30 who were treatment naive and 26 with relapsed/refractory disease) have started on maintenance therapy. Of this group, 28 have stopped maintenance, including 21 patients who stopped because they achieved MRD negativity, 5 who discontinued because of adverse events, and 2 with disease progression or Richter’s transformation.
At 15 months of follow-up, progression-free survival was 100% in patients who received the combination as the frontline therapy, and 83% in the patients with relapsed/refractory disease.
Adverse events
In the debulking phase, 34 patients (72%) experienced adverse events of any grade, including 16 patients with grade 3 or 4 events
Adverse events associated with debulking included neutropenia and anemia, each seen in five patients, thrombocytopenia and infection each seen in three patients, and coronary artery disorders, rash, tumor lysis syndrome, vomiting, and pyrexia in one patient each.
Sixty of the 66 patients received the full six induction cycles. Three patients who had fewer than two cycles were excluded according to protocol. Of these three, two heavily pretreated patients died of sepsis and one of myocardial infarction in cycle 1.
All but 3 of the 63 patients had an adverse event, and 44 patients had at least one grade 3 or 4 adverse event.
Cytopenias, infections, infusion-related reactions, and neoplasms were the most common adverse events.
There were three grade 3 laboratory-confirmed cases of tumor lysis syndrome, one each occurring during bendamustine debulking, obinutuzumab administration, and venetoclax administration.
Something other than venetoclax?
Davide Rossi, MD, PhD, of the Oncology Institute of Southern Switzerland in Bellinzona, the invited discussant, questioned whether venetoclax is the best agent to use in sequential triple-T therapy.
“We know that venetoclax is more active in blood and bone marrow than in lymph nodes and, indeed, a proportion of cases achieving all the criteria for a CR [complete response] in the bone marrow and the peripheral blood, including MRD negativity, may still have some residual [disease in] lymph nodes,” he said.
Drugs that inhibit the B-cell receptor pathway may be more effective at eradicating MRD in the lymph node compartment, Dr. Cramer said.
The study was sponsored by the German CLL study group. Dr. Cramer disclosed financial ties to multiple entities. Dr. Rossi did not report disclosures.
AT 14-ICML
Key clinical point: Sequential therapy with bendamustine, obinutuzumab, and venetoclax was associated with high response rates in patients with both treatment-naive and relapsed/refractory chronic lymphocytic leukemia.
Major finding: The overall response rate at the end of induction was 95%.
Data source: An open-label prospective study in 66 patients with CLL.
Disclosures: The study was sponsored by the German CLL study group. Dr. Cramer and her coauthors disclosed financial ties to multiple entities. Dr. Rossi did not report disclosures.
In Sweden, very few osteoporotic fractures prompt treatment within 12 months
MADRID – In Sweden, less than 7% of those who experience an osteoporotic fracture receive osteoporosis treatment within the first year, when the risk of a second fracture is the highest.
Women are more likely than are men to get a prescription, but people older than 80 years, and those with dementia or low socioeconomic status, are less likely to receive one, Anna Spångeus, MD, PhD, said at the European Congress of Rheumatology.
She expressed particular concern about the age differential observed in the study. “These drugs are equally as effective in the aged as in younger subjects. … It’s quite reasonable for a women aged 80-85 years to have treatment if she has a lower biological age and a good survival time that would allow her to benefit from the treatment.”
The investigators took advantage of Sweden’s multiple, inked national health registries to examine the rates of new osteoporosis prescriptions in treatment-naive patients who experienced a first osteoporotic bone fracture. Prescribing information came from the Prescribed Drug Register, which covers 100% of outpatient pharmacies. This database doesn’t include medications dispensed in hospitals, though, or intravenous osteoporotic treatments – a weakness of the study, Dr. Spångeus noted. The 6-year study period covered 2006-2012.
In all, almost 260,000 Swedish citizens naive to osteoporosis treatment had an osteoporotic bone fracture during that period. Most of these (68%) were women; the mean age was 73 years. Of these, 16,970 (6.6%) received therapy for osteoporosis within the first year after their fracture.
Vertebral fractures were the most likely to prompt treatment (21%). Only about 5% of all other fracture types prompted an osteoporosis treatment prescription, despite a clear clinical link to the disorder, Dr. Spångeus said. “Almost four times as many patients with clinical vertebral fractures initiated osteoporosis treatment in the year after the fracture. In contrast, treatment after hip fracture was significantly lower, despite the high subsequent risk.”
A good bit of news emerged when she parsed the group by comorbidities: 18% of patients with rheumatoid arthritis got an osteoporosis prescription, suggesting that clinicians were aware of the risk to bone posed by glucocorticoid therapy. Of all fracture patients on glucocorticoids, 17% got a prescription after their break. Only 6% of those not taking glucocorticoids got an osteoporosis medication.
Patients with chronic pulmonary diseases also tended to be treated more often, with about 10% getting a prescription. But those with dementia were much less likely to receive a prescription (1.5%), as were patients with any kind of social dependency – a marker of lower socioeconomic status (2%).
In an age analysis, patients aged 70-80 years were most often treated (19%). The youngest patients (50-60 years) and the oldest (90 and older) were the least likely to be treated (4% and 1.6%). About 6% of those aged 80-90 years were treated.
Women were almost four times more likely to get a prescription than were men (8.5% vs. 2.3%; odds ratio, 3.7). “This was concerning, because about a third of our cohort consisted of men,” Dr. Spångeus said. “Men do develop osteoporotic bone fractures, but this is not being recognized.”
The study was funded by an unrestricted grant from UCB Pharma. Dr. Spångeus disclosed that she has received research funding from Amgen and Eli Lilly.
[email protected]
On Twitter @alz_gal
MADRID – In Sweden, less than 7% of those who experience an osteoporotic fracture receive osteoporosis treatment within the first year, when the risk of a second fracture is the highest.
Women are more likely than are men to get a prescription, but people older than 80 years, and those with dementia or low socioeconomic status, are less likely to receive one, Anna Spångeus, MD, PhD, said at the European Congress of Rheumatology.
She expressed particular concern about the age differential observed in the study. “These drugs are equally as effective in the aged as in younger subjects. … It’s quite reasonable for a women aged 80-85 years to have treatment if she has a lower biological age and a good survival time that would allow her to benefit from the treatment.”
The investigators took advantage of Sweden’s multiple, inked national health registries to examine the rates of new osteoporosis prescriptions in treatment-naive patients who experienced a first osteoporotic bone fracture. Prescribing information came from the Prescribed Drug Register, which covers 100% of outpatient pharmacies. This database doesn’t include medications dispensed in hospitals, though, or intravenous osteoporotic treatments – a weakness of the study, Dr. Spångeus noted. The 6-year study period covered 2006-2012.
In all, almost 260,000 Swedish citizens naive to osteoporosis treatment had an osteoporotic bone fracture during that period. Most of these (68%) were women; the mean age was 73 years. Of these, 16,970 (6.6%) received therapy for osteoporosis within the first year after their fracture.
Vertebral fractures were the most likely to prompt treatment (21%). Only about 5% of all other fracture types prompted an osteoporosis treatment prescription, despite a clear clinical link to the disorder, Dr. Spångeus said. “Almost four times as many patients with clinical vertebral fractures initiated osteoporosis treatment in the year after the fracture. In contrast, treatment after hip fracture was significantly lower, despite the high subsequent risk.”
A good bit of news emerged when she parsed the group by comorbidities: 18% of patients with rheumatoid arthritis got an osteoporosis prescription, suggesting that clinicians were aware of the risk to bone posed by glucocorticoid therapy. Of all fracture patients on glucocorticoids, 17% got a prescription after their break. Only 6% of those not taking glucocorticoids got an osteoporosis medication.
Patients with chronic pulmonary diseases also tended to be treated more often, with about 10% getting a prescription. But those with dementia were much less likely to receive a prescription (1.5%), as were patients with any kind of social dependency – a marker of lower socioeconomic status (2%).
In an age analysis, patients aged 70-80 years were most often treated (19%). The youngest patients (50-60 years) and the oldest (90 and older) were the least likely to be treated (4% and 1.6%). About 6% of those aged 80-90 years were treated.
Women were almost four times more likely to get a prescription than were men (8.5% vs. 2.3%; odds ratio, 3.7). “This was concerning, because about a third of our cohort consisted of men,” Dr. Spångeus said. “Men do develop osteoporotic bone fractures, but this is not being recognized.”
The study was funded by an unrestricted grant from UCB Pharma. Dr. Spångeus disclosed that she has received research funding from Amgen and Eli Lilly.
[email protected]
On Twitter @alz_gal
MADRID – In Sweden, less than 7% of those who experience an osteoporotic fracture receive osteoporosis treatment within the first year, when the risk of a second fracture is the highest.
Women are more likely than are men to get a prescription, but people older than 80 years, and those with dementia or low socioeconomic status, are less likely to receive one, Anna Spångeus, MD, PhD, said at the European Congress of Rheumatology.
She expressed particular concern about the age differential observed in the study. “These drugs are equally as effective in the aged as in younger subjects. … It’s quite reasonable for a women aged 80-85 years to have treatment if she has a lower biological age and a good survival time that would allow her to benefit from the treatment.”
The investigators took advantage of Sweden’s multiple, inked national health registries to examine the rates of new osteoporosis prescriptions in treatment-naive patients who experienced a first osteoporotic bone fracture. Prescribing information came from the Prescribed Drug Register, which covers 100% of outpatient pharmacies. This database doesn’t include medications dispensed in hospitals, though, or intravenous osteoporotic treatments – a weakness of the study, Dr. Spångeus noted. The 6-year study period covered 2006-2012.
In all, almost 260,000 Swedish citizens naive to osteoporosis treatment had an osteoporotic bone fracture during that period. Most of these (68%) were women; the mean age was 73 years. Of these, 16,970 (6.6%) received therapy for osteoporosis within the first year after their fracture.
Vertebral fractures were the most likely to prompt treatment (21%). Only about 5% of all other fracture types prompted an osteoporosis treatment prescription, despite a clear clinical link to the disorder, Dr. Spångeus said. “Almost four times as many patients with clinical vertebral fractures initiated osteoporosis treatment in the year after the fracture. In contrast, treatment after hip fracture was significantly lower, despite the high subsequent risk.”
A good bit of news emerged when she parsed the group by comorbidities: 18% of patients with rheumatoid arthritis got an osteoporosis prescription, suggesting that clinicians were aware of the risk to bone posed by glucocorticoid therapy. Of all fracture patients on glucocorticoids, 17% got a prescription after their break. Only 6% of those not taking glucocorticoids got an osteoporosis medication.
Patients with chronic pulmonary diseases also tended to be treated more often, with about 10% getting a prescription. But those with dementia were much less likely to receive a prescription (1.5%), as were patients with any kind of social dependency – a marker of lower socioeconomic status (2%).
In an age analysis, patients aged 70-80 years were most often treated (19%). The youngest patients (50-60 years) and the oldest (90 and older) were the least likely to be treated (4% and 1.6%). About 6% of those aged 80-90 years were treated.
Women were almost four times more likely to get a prescription than were men (8.5% vs. 2.3%; odds ratio, 3.7). “This was concerning, because about a third of our cohort consisted of men,” Dr. Spångeus said. “Men do develop osteoporotic bone fractures, but this is not being recognized.”
The study was funded by an unrestricted grant from UCB Pharma. Dr. Spångeus disclosed that she has received research funding from Amgen and Eli Lilly.
[email protected]
On Twitter @alz_gal
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Only 6.6% of patients who were naive to osteoporotic therapy and experienced a break received a prescription.
Data source: The national registry study, comprising about 290,000 patients.
Disclosures: UCB Pharma sponsored the study with an unrestricted grant. Dr. Spångeus has received research funding from Amgen and Eli Lilly.
Knee OA structural defects predict pain trajectories
The presence of bone marrow lesions and cartilage defects on baseline MRI scans of knee osteoarthritis (OA) patients may help to predict moderate to severe pain trajectories, based on data from a population-based study presented here at the European Congress of Rheumatology.
Three pain trajectories were identified: 52% of the patients reported stable mild pain over time, 33% reported moderate pain over time, and 15% reported fluctuating or severe pain over time.
“Knee pain is the most prominent symptom of knee osteoarthritis,” Dr. Pan said in an interview. “It is also a main reason for people to seek joint replacement. Despite this, the causes of knee pain are poorly understood. Therapy will only improve if we understand this better.”
Dr. Pan and his colleagues studied 1,099 adults with knee OA who participated in a population-based study. The mean age was 63 years (ranging from 51 to 81 years). A total of 875 participants were assessed using the knee pain components of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 2.6 years’ follow-up, 768 at 5.1 years’ follow-up, and 563 at 10.7 years’ follow-up.
Knee OA was assessed at baseline by x-ray, and T1-weighted or T2-weighted MRI scans of the right knee were used to assess knee structural pathology. The researchers applied group-based trajectory modeling to identify pain trajectories.
“We identified three distinct trajectories over time,” Dr. Pan said. “Remarkably, structural, environmental, sociodemographic, and psychological factors are associated with a more ‘severe pain’ trajectory, suggesting that pain course is determined by an integrated mix of all these factors.”
Dr. Pan added: “We already know that structural damage is a major driver in the development and maintenance of pain severity, but less is known about the other factors.”
The researchers found a significant dose-response relationship between the number of knee structural abnormalities, including cartilage defects and bone marrow lesions, and the ‘moderate pain’ and ‘severe pain’ trajectories (P less than .001 comparing both categories to the ‘mild pain’ trajectory).
Other factors that were significantly associated with both moderate and severe pain, compared with mild pain, included higher body mass index (overweight but not obese), emotional problems, and musculoskeletal diseases.
The findings provide support to the concept that “pain experience is both complex and individual in nature, suggesting the clinician should target treatments according to which of these factors predominate in the individual,” Dr. Pan said.
Future research may aim to develop “a predictive model that could be utilized in clinical practice and target therapies based on these trajectories.”
Dr. Pan had no financial conflicts to disclose.
The presence of bone marrow lesions and cartilage defects on baseline MRI scans of knee osteoarthritis (OA) patients may help to predict moderate to severe pain trajectories, based on data from a population-based study presented here at the European Congress of Rheumatology.
Three pain trajectories were identified: 52% of the patients reported stable mild pain over time, 33% reported moderate pain over time, and 15% reported fluctuating or severe pain over time.
“Knee pain is the most prominent symptom of knee osteoarthritis,” Dr. Pan said in an interview. “It is also a main reason for people to seek joint replacement. Despite this, the causes of knee pain are poorly understood. Therapy will only improve if we understand this better.”
Dr. Pan and his colleagues studied 1,099 adults with knee OA who participated in a population-based study. The mean age was 63 years (ranging from 51 to 81 years). A total of 875 participants were assessed using the knee pain components of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 2.6 years’ follow-up, 768 at 5.1 years’ follow-up, and 563 at 10.7 years’ follow-up.
Knee OA was assessed at baseline by x-ray, and T1-weighted or T2-weighted MRI scans of the right knee were used to assess knee structural pathology. The researchers applied group-based trajectory modeling to identify pain trajectories.
“We identified three distinct trajectories over time,” Dr. Pan said. “Remarkably, structural, environmental, sociodemographic, and psychological factors are associated with a more ‘severe pain’ trajectory, suggesting that pain course is determined by an integrated mix of all these factors.”
Dr. Pan added: “We already know that structural damage is a major driver in the development and maintenance of pain severity, but less is known about the other factors.”
The researchers found a significant dose-response relationship between the number of knee structural abnormalities, including cartilage defects and bone marrow lesions, and the ‘moderate pain’ and ‘severe pain’ trajectories (P less than .001 comparing both categories to the ‘mild pain’ trajectory).
Other factors that were significantly associated with both moderate and severe pain, compared with mild pain, included higher body mass index (overweight but not obese), emotional problems, and musculoskeletal diseases.
The findings provide support to the concept that “pain experience is both complex and individual in nature, suggesting the clinician should target treatments according to which of these factors predominate in the individual,” Dr. Pan said.
Future research may aim to develop “a predictive model that could be utilized in clinical practice and target therapies based on these trajectories.”
Dr. Pan had no financial conflicts to disclose.
The presence of bone marrow lesions and cartilage defects on baseline MRI scans of knee osteoarthritis (OA) patients may help to predict moderate to severe pain trajectories, based on data from a population-based study presented here at the European Congress of Rheumatology.
Three pain trajectories were identified: 52% of the patients reported stable mild pain over time, 33% reported moderate pain over time, and 15% reported fluctuating or severe pain over time.
“Knee pain is the most prominent symptom of knee osteoarthritis,” Dr. Pan said in an interview. “It is also a main reason for people to seek joint replacement. Despite this, the causes of knee pain are poorly understood. Therapy will only improve if we understand this better.”
Dr. Pan and his colleagues studied 1,099 adults with knee OA who participated in a population-based study. The mean age was 63 years (ranging from 51 to 81 years). A total of 875 participants were assessed using the knee pain components of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 2.6 years’ follow-up, 768 at 5.1 years’ follow-up, and 563 at 10.7 years’ follow-up.
Knee OA was assessed at baseline by x-ray, and T1-weighted or T2-weighted MRI scans of the right knee were used to assess knee structural pathology. The researchers applied group-based trajectory modeling to identify pain trajectories.
“We identified three distinct trajectories over time,” Dr. Pan said. “Remarkably, structural, environmental, sociodemographic, and psychological factors are associated with a more ‘severe pain’ trajectory, suggesting that pain course is determined by an integrated mix of all these factors.”
Dr. Pan added: “We already know that structural damage is a major driver in the development and maintenance of pain severity, but less is known about the other factors.”
The researchers found a significant dose-response relationship between the number of knee structural abnormalities, including cartilage defects and bone marrow lesions, and the ‘moderate pain’ and ‘severe pain’ trajectories (P less than .001 comparing both categories to the ‘mild pain’ trajectory).
Other factors that were significantly associated with both moderate and severe pain, compared with mild pain, included higher body mass index (overweight but not obese), emotional problems, and musculoskeletal diseases.
The findings provide support to the concept that “pain experience is both complex and individual in nature, suggesting the clinician should target treatments according to which of these factors predominate in the individual,” Dr. Pan said.
Future research may aim to develop “a predictive model that could be utilized in clinical practice and target therapies based on these trajectories.”
Dr. Pan had no financial conflicts to disclose.
FROM THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Three pain trajectories were identified: stable mild pain (52% of patients), moderate pain (33%), and fluctuating or severe pain (15%).
Data source: Population-based cohort study of 1,099 patients with knee OA.
Disclosures: The presenter had no financial conflicts to disclose.
Physician-created APMs: Dermatology aims to learn from early recommendations
Dermatologists looking for a way to participate in an advanced alternative payment model under Medicare’s new Quality Payment Program will likely need to develop their own and get it approved.
Advanced alternative payment models (APMs) involve physicians taking on two-sided risk along with Medicare in exchange for the potential for higher bonus payments for delivering higher-value care to patients. Officials at the Centers for Medicare & Medicaid Services have created seven APMs (some primary care and some specialty focused), but they do not appeal to everyone.
“The likelihood that a private practice dermatologist is going to participate in a risk-bearing APM is, I think, possible but challenging,” said Kathryn Schwarzenberger, MD, chair of the American Academy of Dermatology’s Workgroup on Innovation in Payment and Delivery. “To have a fully qualified, double-sided risk APM for most private practitioners is going to be difficult. The advanced APMs, unless you are in a group, we have figured most dermatologists wouldn’t be interested.”
A physician-created APM might be a different story, however.
Getting approval for this type of APM – technically known as physician-focused payment models (PFPMs) – is tough. Of the first three PFPMs reviewed by Medicare’s Physician-Focused Payment Model Technical Advisory Committee (PTAC), two were recommended for limited trial periods only. A third proposal was not recommended. None of these early proposals were dermatology focused.
Not jumping into the process early was a prudent move, according to Dr. Schwarzenberger.
“We learned that it is probably good to sit back at this point and watch and see what is happening,” she said. “I think the rules have become much more clear. I think now that PTAC has provided us with some better guidelines for how to develop a successful APM, we have a much better chance of doing so.”
In this earliest review, each proposed APM was assigned to three commissioners, including at least one physician, for review against 10 criteria:
• Scope of proposed PFPM (high priority).
• Quality and cost (high priority).
• Payment methodology (high priority).
• Value over volume.
• Flexibility.
• Ability to be evaluated.
• Integration and care coordination.
• Patient choice.
• Patient safety.
• Health information technology.
While each proposal met a few of the criteria, none met all three high-priority criteria, and none was recommended for approval by its preliminary reviewers; however, after committee deliberation, two received provisional recommendation.
“We recommended the two models for small-scale testing,” PTAC Vice-Chairman Elizabeth Mitchell said in an interview. “Even though we think they are very good ideas, we know that more experience and evidence are required before they may be ready.”
The two models that got the limited recommendation were:
• Project Sonar, submitted by the Illinois Gastroenterology Group and SonarMD, a Web-based platform that queries patients with inflammatory bowel disease monthly to determine which are in need of more hands-on care.
• American College of Surgeons–Brandeis APM, submitted by the American College of Surgeons, an episode-based payment model that uses claims data but expands on existing CMS value-based models by not requiring hospitalizations. It creates an episodic payment using outpatient settings, including acute and chronic care.
The COPD [chronic obstructive pulmonary disease] and Asthma Monitoring Project (CAMP), a smartphone app to remotely monitor and guide treatment of patients with asthma and chronic obstructive pulmonary disease, was not recommended. It was submitted by Pulmonary Medicine, Infectious Disease and Critical Care Consultants Medical Group of Sacramento.
PTAC has received more than 20 letters of intent from physicians and aims to hold another round of public hearings in September to determine their usefulness.
“I think it is very safe to say that our whole committee has been really gratified with the level of interest and engagement,” said Ms. Mitchell, president and CEO of Network for Regional Healthcare Improvement in Portland, Maine. The volume of applications “underscores the level of interest from the field. The entire reason PTAC was established was to get those good ideas from practicing physicians and others who are identifying better ways to deliver care but are facing barriers in the current payment system.”
She offered advice to those who are contemplating submission of a payment model: “Really understand the criteria and review the request for proposals. I think the committee lays out what we are looking for in terms of information, and we are hoping that it is really straightforward.”
She also stressed that successful models need to work broadly. “We are not talking about something that works for a single practice,” she said. “We are talking about models that are ready for inclusion in the whole CMS portfolio. It is helpful if there is experience to draw from that informs our deliberations, but we recognize that, in some cases, there has not been the opportunity to test these models broadly.”
Most of all, the highest priority when it comes to the models is related to quality of care and cost.
“We are not soliciting models that are essentially tweaks to fee for service. We are looking for changes that cannot be made without a new method of payment,” she said, adding that the models “have to either reduce cost while maintaining quality or improve quality without raising cost.”
Meeting transcripts and video are posted online and can help potential applicants see how the committee came to its recommendations.
“The committee does not deliberate on the proposals except in public,” Ms. Mitchell said. “So, those public meetings were the first time we had deliberated on any of the proposals we have considered. The preliminary review teams have discussed it [in depth], but the full committee can only deliberate in public.”
Dr. Schwarzenberger said that while the AAD working group is looking at some dermatology-specific conditions that might be good for a PFPM a broader approach may be better. Perhaps the focus should be “where dermatology’s involvement with other physicians, where we might be able to help them practice medicine better or save money,” she said. “Maybe where we can make our biggest financial and potentially quality impacts.”
Dermatologists looking for a way to participate in an advanced alternative payment model under Medicare’s new Quality Payment Program will likely need to develop their own and get it approved.
Advanced alternative payment models (APMs) involve physicians taking on two-sided risk along with Medicare in exchange for the potential for higher bonus payments for delivering higher-value care to patients. Officials at the Centers for Medicare & Medicaid Services have created seven APMs (some primary care and some specialty focused), but they do not appeal to everyone.
“The likelihood that a private practice dermatologist is going to participate in a risk-bearing APM is, I think, possible but challenging,” said Kathryn Schwarzenberger, MD, chair of the American Academy of Dermatology’s Workgroup on Innovation in Payment and Delivery. “To have a fully qualified, double-sided risk APM for most private practitioners is going to be difficult. The advanced APMs, unless you are in a group, we have figured most dermatologists wouldn’t be interested.”
A physician-created APM might be a different story, however.
Getting approval for this type of APM – technically known as physician-focused payment models (PFPMs) – is tough. Of the first three PFPMs reviewed by Medicare’s Physician-Focused Payment Model Technical Advisory Committee (PTAC), two were recommended for limited trial periods only. A third proposal was not recommended. None of these early proposals were dermatology focused.
Not jumping into the process early was a prudent move, according to Dr. Schwarzenberger.
“We learned that it is probably good to sit back at this point and watch and see what is happening,” she said. “I think the rules have become much more clear. I think now that PTAC has provided us with some better guidelines for how to develop a successful APM, we have a much better chance of doing so.”
In this earliest review, each proposed APM was assigned to three commissioners, including at least one physician, for review against 10 criteria:
• Scope of proposed PFPM (high priority).
• Quality and cost (high priority).
• Payment methodology (high priority).
• Value over volume.
• Flexibility.
• Ability to be evaluated.
• Integration and care coordination.
• Patient choice.
• Patient safety.
• Health information technology.
While each proposal met a few of the criteria, none met all three high-priority criteria, and none was recommended for approval by its preliminary reviewers; however, after committee deliberation, two received provisional recommendation.
“We recommended the two models for small-scale testing,” PTAC Vice-Chairman Elizabeth Mitchell said in an interview. “Even though we think they are very good ideas, we know that more experience and evidence are required before they may be ready.”
The two models that got the limited recommendation were:
• Project Sonar, submitted by the Illinois Gastroenterology Group and SonarMD, a Web-based platform that queries patients with inflammatory bowel disease monthly to determine which are in need of more hands-on care.
• American College of Surgeons–Brandeis APM, submitted by the American College of Surgeons, an episode-based payment model that uses claims data but expands on existing CMS value-based models by not requiring hospitalizations. It creates an episodic payment using outpatient settings, including acute and chronic care.
The COPD [chronic obstructive pulmonary disease] and Asthma Monitoring Project (CAMP), a smartphone app to remotely monitor and guide treatment of patients with asthma and chronic obstructive pulmonary disease, was not recommended. It was submitted by Pulmonary Medicine, Infectious Disease and Critical Care Consultants Medical Group of Sacramento.
PTAC has received more than 20 letters of intent from physicians and aims to hold another round of public hearings in September to determine their usefulness.
“I think it is very safe to say that our whole committee has been really gratified with the level of interest and engagement,” said Ms. Mitchell, president and CEO of Network for Regional Healthcare Improvement in Portland, Maine. The volume of applications “underscores the level of interest from the field. The entire reason PTAC was established was to get those good ideas from practicing physicians and others who are identifying better ways to deliver care but are facing barriers in the current payment system.”
She offered advice to those who are contemplating submission of a payment model: “Really understand the criteria and review the request for proposals. I think the committee lays out what we are looking for in terms of information, and we are hoping that it is really straightforward.”
She also stressed that successful models need to work broadly. “We are not talking about something that works for a single practice,” she said. “We are talking about models that are ready for inclusion in the whole CMS portfolio. It is helpful if there is experience to draw from that informs our deliberations, but we recognize that, in some cases, there has not been the opportunity to test these models broadly.”
Most of all, the highest priority when it comes to the models is related to quality of care and cost.
“We are not soliciting models that are essentially tweaks to fee for service. We are looking for changes that cannot be made without a new method of payment,” she said, adding that the models “have to either reduce cost while maintaining quality or improve quality without raising cost.”
Meeting transcripts and video are posted online and can help potential applicants see how the committee came to its recommendations.
“The committee does not deliberate on the proposals except in public,” Ms. Mitchell said. “So, those public meetings were the first time we had deliberated on any of the proposals we have considered. The preliminary review teams have discussed it [in depth], but the full committee can only deliberate in public.”
Dr. Schwarzenberger said that while the AAD working group is looking at some dermatology-specific conditions that might be good for a PFPM a broader approach may be better. Perhaps the focus should be “where dermatology’s involvement with other physicians, where we might be able to help them practice medicine better or save money,” she said. “Maybe where we can make our biggest financial and potentially quality impacts.”
Dermatologists looking for a way to participate in an advanced alternative payment model under Medicare’s new Quality Payment Program will likely need to develop their own and get it approved.
Advanced alternative payment models (APMs) involve physicians taking on two-sided risk along with Medicare in exchange for the potential for higher bonus payments for delivering higher-value care to patients. Officials at the Centers for Medicare & Medicaid Services have created seven APMs (some primary care and some specialty focused), but they do not appeal to everyone.
“The likelihood that a private practice dermatologist is going to participate in a risk-bearing APM is, I think, possible but challenging,” said Kathryn Schwarzenberger, MD, chair of the American Academy of Dermatology’s Workgroup on Innovation in Payment and Delivery. “To have a fully qualified, double-sided risk APM for most private practitioners is going to be difficult. The advanced APMs, unless you are in a group, we have figured most dermatologists wouldn’t be interested.”
A physician-created APM might be a different story, however.
Getting approval for this type of APM – technically known as physician-focused payment models (PFPMs) – is tough. Of the first three PFPMs reviewed by Medicare’s Physician-Focused Payment Model Technical Advisory Committee (PTAC), two were recommended for limited trial periods only. A third proposal was not recommended. None of these early proposals were dermatology focused.
Not jumping into the process early was a prudent move, according to Dr. Schwarzenberger.
“We learned that it is probably good to sit back at this point and watch and see what is happening,” she said. “I think the rules have become much more clear. I think now that PTAC has provided us with some better guidelines for how to develop a successful APM, we have a much better chance of doing so.”
In this earliest review, each proposed APM was assigned to three commissioners, including at least one physician, for review against 10 criteria:
• Scope of proposed PFPM (high priority).
• Quality and cost (high priority).
• Payment methodology (high priority).
• Value over volume.
• Flexibility.
• Ability to be evaluated.
• Integration and care coordination.
• Patient choice.
• Patient safety.
• Health information technology.
While each proposal met a few of the criteria, none met all three high-priority criteria, and none was recommended for approval by its preliminary reviewers; however, after committee deliberation, two received provisional recommendation.
“We recommended the two models for small-scale testing,” PTAC Vice-Chairman Elizabeth Mitchell said in an interview. “Even though we think they are very good ideas, we know that more experience and evidence are required before they may be ready.”
The two models that got the limited recommendation were:
• Project Sonar, submitted by the Illinois Gastroenterology Group and SonarMD, a Web-based platform that queries patients with inflammatory bowel disease monthly to determine which are in need of more hands-on care.
• American College of Surgeons–Brandeis APM, submitted by the American College of Surgeons, an episode-based payment model that uses claims data but expands on existing CMS value-based models by not requiring hospitalizations. It creates an episodic payment using outpatient settings, including acute and chronic care.
The COPD [chronic obstructive pulmonary disease] and Asthma Monitoring Project (CAMP), a smartphone app to remotely monitor and guide treatment of patients with asthma and chronic obstructive pulmonary disease, was not recommended. It was submitted by Pulmonary Medicine, Infectious Disease and Critical Care Consultants Medical Group of Sacramento.
PTAC has received more than 20 letters of intent from physicians and aims to hold another round of public hearings in September to determine their usefulness.
“I think it is very safe to say that our whole committee has been really gratified with the level of interest and engagement,” said Ms. Mitchell, president and CEO of Network for Regional Healthcare Improvement in Portland, Maine. The volume of applications “underscores the level of interest from the field. The entire reason PTAC was established was to get those good ideas from practicing physicians and others who are identifying better ways to deliver care but are facing barriers in the current payment system.”
She offered advice to those who are contemplating submission of a payment model: “Really understand the criteria and review the request for proposals. I think the committee lays out what we are looking for in terms of information, and we are hoping that it is really straightforward.”
She also stressed that successful models need to work broadly. “We are not talking about something that works for a single practice,” she said. “We are talking about models that are ready for inclusion in the whole CMS portfolio. It is helpful if there is experience to draw from that informs our deliberations, but we recognize that, in some cases, there has not been the opportunity to test these models broadly.”
Most of all, the highest priority when it comes to the models is related to quality of care and cost.
“We are not soliciting models that are essentially tweaks to fee for service. We are looking for changes that cannot be made without a new method of payment,” she said, adding that the models “have to either reduce cost while maintaining quality or improve quality without raising cost.”
Meeting transcripts and video are posted online and can help potential applicants see how the committee came to its recommendations.
“The committee does not deliberate on the proposals except in public,” Ms. Mitchell said. “So, those public meetings were the first time we had deliberated on any of the proposals we have considered. The preliminary review teams have discussed it [in depth], but the full committee can only deliberate in public.”
Dr. Schwarzenberger said that while the AAD working group is looking at some dermatology-specific conditions that might be good for a PFPM a broader approach may be better. Perhaps the focus should be “where dermatology’s involvement with other physicians, where we might be able to help them practice medicine better or save money,” she said. “Maybe where we can make our biggest financial and potentially quality impacts.”
Telemedicine Boosts Number of Native Americans Who Can Obtain Retina Exams
American Indians and Alaska Natives (AI/ANs) have a high risk of diabetes-related vision loss, in part because only half get the annual eye exam needed for timely diagnosis and treatment. But using an innovative telemedicine program, IHS increased the number of AI/ANs who receive an annual retina exam by 20% from 2007 to 2015.
The IHS teleophthalmology program, established in 2000, provides high-quality retinal exams in primary care clinics. More than 70,000 retinal exams have been done for AI/AN patients, IHS says. The exam can be done during a regular office visit without dilation. The retinal photographs are sent electronically to a central reading center where they’re interpreted by trained and certified IHS eye doctors.
The program, available at about 100 sites in 25 states, has not only increased access, IHS says, but also reduced the overall cost of care by preventing complications resulting from delayed or missed care. Screening and treating eye disease in patients with diabetes mellitus costs $3,190 per quality-adjusted life-year saved, IHS says; $840,000 would be saved annually in detecting high-risk diabetic retinopathy (DR). Another $1,090,000 would be saved by reducing the need for additional surgery for complications resulting from delayed identification of patients with high-risk DR.
The program has been validated to American Telemedicine Association Category 3, meaning its clinical outcome is equal to or better than a conventional eye examination for DR. As one of the few and largest programs validated and operating at this level, IHS says the teleophthalmology program has brought high-quality point-of-care specialty service to more than 150,000 AI/AN patients.
American Indians and Alaska Natives (AI/ANs) have a high risk of diabetes-related vision loss, in part because only half get the annual eye exam needed for timely diagnosis and treatment. But using an innovative telemedicine program, IHS increased the number of AI/ANs who receive an annual retina exam by 20% from 2007 to 2015.
The IHS teleophthalmology program, established in 2000, provides high-quality retinal exams in primary care clinics. More than 70,000 retinal exams have been done for AI/AN patients, IHS says. The exam can be done during a regular office visit without dilation. The retinal photographs are sent electronically to a central reading center where they’re interpreted by trained and certified IHS eye doctors.
The program, available at about 100 sites in 25 states, has not only increased access, IHS says, but also reduced the overall cost of care by preventing complications resulting from delayed or missed care. Screening and treating eye disease in patients with diabetes mellitus costs $3,190 per quality-adjusted life-year saved, IHS says; $840,000 would be saved annually in detecting high-risk diabetic retinopathy (DR). Another $1,090,000 would be saved by reducing the need for additional surgery for complications resulting from delayed identification of patients with high-risk DR.
The program has been validated to American Telemedicine Association Category 3, meaning its clinical outcome is equal to or better than a conventional eye examination for DR. As one of the few and largest programs validated and operating at this level, IHS says the teleophthalmology program has brought high-quality point-of-care specialty service to more than 150,000 AI/AN patients.
American Indians and Alaska Natives (AI/ANs) have a high risk of diabetes-related vision loss, in part because only half get the annual eye exam needed for timely diagnosis and treatment. But using an innovative telemedicine program, IHS increased the number of AI/ANs who receive an annual retina exam by 20% from 2007 to 2015.
The IHS teleophthalmology program, established in 2000, provides high-quality retinal exams in primary care clinics. More than 70,000 retinal exams have been done for AI/AN patients, IHS says. The exam can be done during a regular office visit without dilation. The retinal photographs are sent electronically to a central reading center where they’re interpreted by trained and certified IHS eye doctors.
The program, available at about 100 sites in 25 states, has not only increased access, IHS says, but also reduced the overall cost of care by preventing complications resulting from delayed or missed care. Screening and treating eye disease in patients with diabetes mellitus costs $3,190 per quality-adjusted life-year saved, IHS says; $840,000 would be saved annually in detecting high-risk diabetic retinopathy (DR). Another $1,090,000 would be saved by reducing the need for additional surgery for complications resulting from delayed identification of patients with high-risk DR.
The program has been validated to American Telemedicine Association Category 3, meaning its clinical outcome is equal to or better than a conventional eye examination for DR. As one of the few and largest programs validated and operating at this level, IHS says the teleophthalmology program has brought high-quality point-of-care specialty service to more than 150,000 AI/AN patients.