User login
Weekly buprenorphine depot effective for opioid use disorder
A weekly subcutaneous buprenorphine depot could improve adherence and reduce the potential for misuse, according to a study presented at the annual meeting of the College on Problems of Drug Dependence and published simultaneously June 22 in JAMA Psychiatry.
In a phase II, double-blind, randomized study, 47 adults with moderate to severe opioid use disorder were randomized to a weekly dose of the subcutaneous buprenorphine depot CAM2038 – either 24 mg or 32 mg – for 2 weeks. They also underwent five 3-day test sessions to evaluate their response to 6 mg and 18 mg doses of intramuscular hydromorphone and a 0 mg control. One qualification session was held before randomization and the remaining four sessions after.
The primary outcome of the study was drug liking, and both doses of CAM2038 were associated with immediate, sustained, and dose-related suppression of participants’ response to hydromorphone (JAMA Psychiatry. 2017 June 22. doi:10.1001/jamapsychiatry.2017.1874).
The depot also appeared to block the dose-dependent increase in drowsiness that was seen in response to hydromorphone in the qualification session.
However, there was a reversal of the dose-dependent response in participants’ desire to use opioids, with greater suppression seen at the lower depot dose.
Buprenorphine is known to be safer than methadone for the treatment of opioid use disorders, but Sharon L. Walsh, PhD, of the Center on Drug and Alcohol Research at the University of Kentucky, Lexington, and her coauthors said sublingual buprenorphine itself has become an abuse liability in some countries.
“Sublingual formulations of buprenorphine can be injected or snorted to enhance euphoric effects,” they wrote. “Unintentional overdose with buprenorphine has been reported, leading to toxicity and fatality in children and those who coingest buprenorphine with benzodiazepines or alcohol.”
The results mean the formulation meets the U.S. Food and Drug Administration criteria for complete opioid blockade.
CAM2038 achieved complete suppression of opioid withdrawal at both doses, and Clinical Opiate Withdrawal Scale (COWS) remained suppressed for the entire duration of the study.
“During treatment initiation, it is important that withdrawal symptoms are well-controlled,” the authors wrote. “The COWS and [Objective Opioid Withdrawal Scale] scores were reduced to near zero on the first dosing day with suppression thereafter.”
The mean COWS preinjection score was 11, which did not include five participants who accidentally were inducted with CAM2038 before they had achieved the prespecified criteria of a COWS score of 8 or above.
They also noted that the pharmacokinetic profile of CAM2038 showed gradually increasing buprenorphine concentrations, reaching maximum at around 24 hours after the dose was given. Perhaps because of this effect – which they likened to a de factor induction procedure mimicking the recommended induction with sublingual buprenorphine – the authors suggested that patients using CAM2038 could likely be inducted directly using the depot.
Researchers also looked at some key physiological outcomes before and after treatment with the depot. They found that, while patients showed significant dose-dependent reductions in oxygen saturation with hydromorphone before receiving depot treatment, these were significantly attenuated after treatment with CAM2038.
One subject withdrew from the study because of ventricular extrasystoles, and one showed abnormal liver function at discharge that was later attributed a hepatitis C diagnosis. Neither event was linked to the study drug.
However, 81% of patients experienced at least one adverse event, with significantly more events reported in the higher dose group. The most common reported events that were possibly related to the study drug were constipation, injection site pain and erythema, and headache. Most were rated as mild.
The College on Problems of Drug Dependence, a nonprofit corporation, is holding its annual meeting in Montreal. The study was supported by research contracts from Braeburn Pharmaceuticals, with additional support from the National Institute on Drug Abuse, the National Center for Research Resources, and the National Center for Advancing of Translational Sciences. Two authors are employees of study sponsor Braeburn Pharmaceuticals, and one is an employee of Camurus – a partner to the sponsor. Three authors also received research contract support from Braeburn, four research consultant fees from Braeburn, and two received consulting fees or partial salary support from Camurus, and other pharmaceutical companies.
A weekly subcutaneous buprenorphine depot could improve adherence and reduce the potential for misuse, according to a study presented at the annual meeting of the College on Problems of Drug Dependence and published simultaneously June 22 in JAMA Psychiatry.
In a phase II, double-blind, randomized study, 47 adults with moderate to severe opioid use disorder were randomized to a weekly dose of the subcutaneous buprenorphine depot CAM2038 – either 24 mg or 32 mg – for 2 weeks. They also underwent five 3-day test sessions to evaluate their response to 6 mg and 18 mg doses of intramuscular hydromorphone and a 0 mg control. One qualification session was held before randomization and the remaining four sessions after.
The primary outcome of the study was drug liking, and both doses of CAM2038 were associated with immediate, sustained, and dose-related suppression of participants’ response to hydromorphone (JAMA Psychiatry. 2017 June 22. doi:10.1001/jamapsychiatry.2017.1874).
The depot also appeared to block the dose-dependent increase in drowsiness that was seen in response to hydromorphone in the qualification session.
However, there was a reversal of the dose-dependent response in participants’ desire to use opioids, with greater suppression seen at the lower depot dose.
Buprenorphine is known to be safer than methadone for the treatment of opioid use disorders, but Sharon L. Walsh, PhD, of the Center on Drug and Alcohol Research at the University of Kentucky, Lexington, and her coauthors said sublingual buprenorphine itself has become an abuse liability in some countries.
“Sublingual formulations of buprenorphine can be injected or snorted to enhance euphoric effects,” they wrote. “Unintentional overdose with buprenorphine has been reported, leading to toxicity and fatality in children and those who coingest buprenorphine with benzodiazepines or alcohol.”
The results mean the formulation meets the U.S. Food and Drug Administration criteria for complete opioid blockade.
CAM2038 achieved complete suppression of opioid withdrawal at both doses, and Clinical Opiate Withdrawal Scale (COWS) remained suppressed for the entire duration of the study.
“During treatment initiation, it is important that withdrawal symptoms are well-controlled,” the authors wrote. “The COWS and [Objective Opioid Withdrawal Scale] scores were reduced to near zero on the first dosing day with suppression thereafter.”
The mean COWS preinjection score was 11, which did not include five participants who accidentally were inducted with CAM2038 before they had achieved the prespecified criteria of a COWS score of 8 or above.
They also noted that the pharmacokinetic profile of CAM2038 showed gradually increasing buprenorphine concentrations, reaching maximum at around 24 hours after the dose was given. Perhaps because of this effect – which they likened to a de factor induction procedure mimicking the recommended induction with sublingual buprenorphine – the authors suggested that patients using CAM2038 could likely be inducted directly using the depot.
Researchers also looked at some key physiological outcomes before and after treatment with the depot. They found that, while patients showed significant dose-dependent reductions in oxygen saturation with hydromorphone before receiving depot treatment, these were significantly attenuated after treatment with CAM2038.
One subject withdrew from the study because of ventricular extrasystoles, and one showed abnormal liver function at discharge that was later attributed a hepatitis C diagnosis. Neither event was linked to the study drug.
However, 81% of patients experienced at least one adverse event, with significantly more events reported in the higher dose group. The most common reported events that were possibly related to the study drug were constipation, injection site pain and erythema, and headache. Most were rated as mild.
The College on Problems of Drug Dependence, a nonprofit corporation, is holding its annual meeting in Montreal. The study was supported by research contracts from Braeburn Pharmaceuticals, with additional support from the National Institute on Drug Abuse, the National Center for Research Resources, and the National Center for Advancing of Translational Sciences. Two authors are employees of study sponsor Braeburn Pharmaceuticals, and one is an employee of Camurus – a partner to the sponsor. Three authors also received research contract support from Braeburn, four research consultant fees from Braeburn, and two received consulting fees or partial salary support from Camurus, and other pharmaceutical companies.
A weekly subcutaneous buprenorphine depot could improve adherence and reduce the potential for misuse, according to a study presented at the annual meeting of the College on Problems of Drug Dependence and published simultaneously June 22 in JAMA Psychiatry.
In a phase II, double-blind, randomized study, 47 adults with moderate to severe opioid use disorder were randomized to a weekly dose of the subcutaneous buprenorphine depot CAM2038 – either 24 mg or 32 mg – for 2 weeks. They also underwent five 3-day test sessions to evaluate their response to 6 mg and 18 mg doses of intramuscular hydromorphone and a 0 mg control. One qualification session was held before randomization and the remaining four sessions after.
The primary outcome of the study was drug liking, and both doses of CAM2038 were associated with immediate, sustained, and dose-related suppression of participants’ response to hydromorphone (JAMA Psychiatry. 2017 June 22. doi:10.1001/jamapsychiatry.2017.1874).
The depot also appeared to block the dose-dependent increase in drowsiness that was seen in response to hydromorphone in the qualification session.
However, there was a reversal of the dose-dependent response in participants’ desire to use opioids, with greater suppression seen at the lower depot dose.
Buprenorphine is known to be safer than methadone for the treatment of opioid use disorders, but Sharon L. Walsh, PhD, of the Center on Drug and Alcohol Research at the University of Kentucky, Lexington, and her coauthors said sublingual buprenorphine itself has become an abuse liability in some countries.
“Sublingual formulations of buprenorphine can be injected or snorted to enhance euphoric effects,” they wrote. “Unintentional overdose with buprenorphine has been reported, leading to toxicity and fatality in children and those who coingest buprenorphine with benzodiazepines or alcohol.”
The results mean the formulation meets the U.S. Food and Drug Administration criteria for complete opioid blockade.
CAM2038 achieved complete suppression of opioid withdrawal at both doses, and Clinical Opiate Withdrawal Scale (COWS) remained suppressed for the entire duration of the study.
“During treatment initiation, it is important that withdrawal symptoms are well-controlled,” the authors wrote. “The COWS and [Objective Opioid Withdrawal Scale] scores were reduced to near zero on the first dosing day with suppression thereafter.”
The mean COWS preinjection score was 11, which did not include five participants who accidentally were inducted with CAM2038 before they had achieved the prespecified criteria of a COWS score of 8 or above.
They also noted that the pharmacokinetic profile of CAM2038 showed gradually increasing buprenorphine concentrations, reaching maximum at around 24 hours after the dose was given. Perhaps because of this effect – which they likened to a de factor induction procedure mimicking the recommended induction with sublingual buprenorphine – the authors suggested that patients using CAM2038 could likely be inducted directly using the depot.
Researchers also looked at some key physiological outcomes before and after treatment with the depot. They found that, while patients showed significant dose-dependent reductions in oxygen saturation with hydromorphone before receiving depot treatment, these were significantly attenuated after treatment with CAM2038.
One subject withdrew from the study because of ventricular extrasystoles, and one showed abnormal liver function at discharge that was later attributed a hepatitis C diagnosis. Neither event was linked to the study drug.
However, 81% of patients experienced at least one adverse event, with significantly more events reported in the higher dose group. The most common reported events that were possibly related to the study drug were constipation, injection site pain and erythema, and headache. Most were rated as mild.
The College on Problems of Drug Dependence, a nonprofit corporation, is holding its annual meeting in Montreal. The study was supported by research contracts from Braeburn Pharmaceuticals, with additional support from the National Institute on Drug Abuse, the National Center for Research Resources, and the National Center for Advancing of Translational Sciences. Two authors are employees of study sponsor Braeburn Pharmaceuticals, and one is an employee of Camurus – a partner to the sponsor. Three authors also received research contract support from Braeburn, four research consultant fees from Braeburn, and two received consulting fees or partial salary support from Camurus, and other pharmaceutical companies.
FROM JAMA Psychiatry
Key clinical point: A weekly subcutaneous buprenorphine depot is effective and could reduce the potential for misuse of buprenorphine in patients with opioid use disorder.
Major finding: Buprenorphine depot CAM2038 achieves immediate and sustained opioid blockade and suppression of opioid withdrawal.
Data source: A double-blind, randomized phase II study in 47 patients with opioid use disorder.
Disclosures: The study was supported by research contracts from Braeburn Pharmaceuticals, with additional support from the National Institute on Drug Abuse, the National Center for Research Resources, and the National Center for Advancing of Translational Sciences. Two authors are employees of study sponsor Braeburn Pharmaceuticals, and one is an employee of Camurus – a partner to the sponsor. Three authors also received research contract support from Braeburn, four recieved research consultant fees from Braeburn, and two received consulting fees or partial salary support from Camurus and other pharmaceutical companies.
If doctors recommend it, teens are more likely to get HPV vaccine
, Brandon Brown, PhD, at the University of California, Riverside, and his associates said.
In a year-long survey of 200 parents’ reasons for agreeing or refusing initial HPV vaccination following practitioner recommendation in a California pediatric practice of six pediatricians, 82% of parents accepted initiation of the HPV series. A significantly higher percentage of parents of male teens did so, compared with parents of female teens (89% vs. 71%; P less than .01), but there were more male children (61.5%) among offspring of the study participants.
Among parents who refused initiation of the HPV vaccine for their adolescents, the most common reason for refusing (53%) and most influential reason (49%) was “I want to learn more about this vaccine,” while 25% said their child was too young to get the vaccine. Some in this latter group said they would vaccinate their child when they were older.
Of the 195 parents who answered a question regarding whether they had a friend or family member diagnosed with cervical cancer, 13% said yes. Of these parents, 92% agreed to have their child get the HPV vaccine.
Read more at Papillomavirus Research (2017 Jan 17. doi: 10.1016/j.pvr.2017.01.002).
, Brandon Brown, PhD, at the University of California, Riverside, and his associates said.
In a year-long survey of 200 parents’ reasons for agreeing or refusing initial HPV vaccination following practitioner recommendation in a California pediatric practice of six pediatricians, 82% of parents accepted initiation of the HPV series. A significantly higher percentage of parents of male teens did so, compared with parents of female teens (89% vs. 71%; P less than .01), but there were more male children (61.5%) among offspring of the study participants.
Among parents who refused initiation of the HPV vaccine for their adolescents, the most common reason for refusing (53%) and most influential reason (49%) was “I want to learn more about this vaccine,” while 25% said their child was too young to get the vaccine. Some in this latter group said they would vaccinate their child when they were older.
Of the 195 parents who answered a question regarding whether they had a friend or family member diagnosed with cervical cancer, 13% said yes. Of these parents, 92% agreed to have their child get the HPV vaccine.
Read more at Papillomavirus Research (2017 Jan 17. doi: 10.1016/j.pvr.2017.01.002).
, Brandon Brown, PhD, at the University of California, Riverside, and his associates said.
In a year-long survey of 200 parents’ reasons for agreeing or refusing initial HPV vaccination following practitioner recommendation in a California pediatric practice of six pediatricians, 82% of parents accepted initiation of the HPV series. A significantly higher percentage of parents of male teens did so, compared with parents of female teens (89% vs. 71%; P less than .01), but there were more male children (61.5%) among offspring of the study participants.
Among parents who refused initiation of the HPV vaccine for their adolescents, the most common reason for refusing (53%) and most influential reason (49%) was “I want to learn more about this vaccine,” while 25% said their child was too young to get the vaccine. Some in this latter group said they would vaccinate their child when they were older.
Of the 195 parents who answered a question regarding whether they had a friend or family member diagnosed with cervical cancer, 13% said yes. Of these parents, 92% agreed to have their child get the HPV vaccine.
Read more at Papillomavirus Research (2017 Jan 17. doi: 10.1016/j.pvr.2017.01.002).
FROM PAPILLOMAVIRUS RESEARCH
Proper catheter removal promotes colorectal surgery recovery
Adherence to guidelines for urinary catheter removal significantly reduced rates of urinary tract infection and length of hospital stay, based on data from almost 3,000 patients.
UTIs occurred in 0.8% of colonic surgery patients who were guideline compliant, compared with 4.1% of noncompliant patients. Similarly, UTIs were significantly less likely in rectal surgery patients who complied with guidelines, compared with those who did not (3.5% vs. 9.6%).
The Implementation of the Enhanced Recovery After Surgery (iERAS) program involved 15 academic hospitals in Ontario, Canada. The guidelines include prompt removal of catheters from colonic and rectal surgery patients. Recommended removal times ranged from 24 hours to 72 hours, depending on the procedure.
“Although numerous reports have documented the overall effectiveness of bundled ERAS interventions improving outcome, the contribution of individual components of the pathway in the context of an ERAS implementation program is uncertain,” wrote Allan Okrainec, MD, of the University of Toronto, and his colleagues.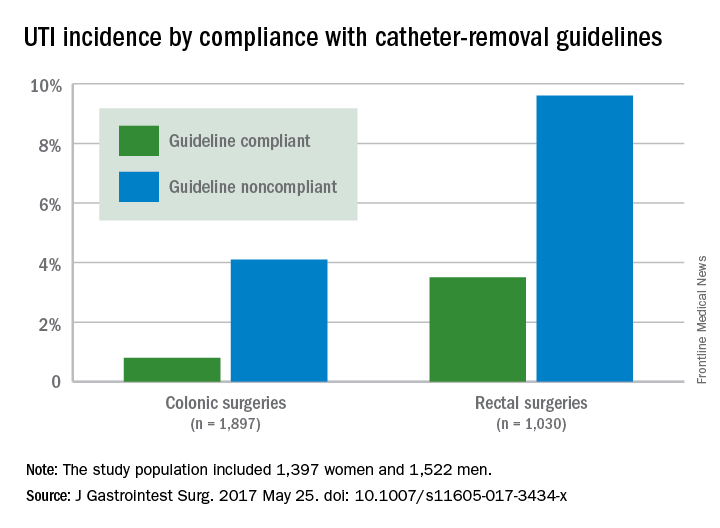
Overall, 53% of patients had catheters removed in compliance with the guidelines, including 47.2% of those with colonic resections and 69.5% of those with rectal resections.
The average length of hospital stay for colonic surgery patients who complied with the urinary catheter guideline was 4 days, compared with 5 days for noncompliant patients, a statistically significant difference. Similarly, the average length of stay was significantly shorter for compliant rectal surgery patients, compared with noncompliant patients (5 days vs. 8 days, respectively).
Reinsertion of the urinary catheter was needed for 6% of the patients, including 122 patients with suspected or confirmed urinary retention and 36 patients who had other indications for reinsertion.
“One of the concerns about early removal of catheters is that it might lead to an increased need for reinsertion,” the researchers noted. “In our study, guideline compliance was associated with an increased rate of catheter reinsertion, but [it] only increased the risk by 2%-3% in patients having rectal or colonic procedures,” the investigators said.
The study results were limited by a lack of information about reasons for noncompliance with the guidelines, the researchers added. However, the data support the value of early catheter removal for surgical patients. “It is noteworthy that, in our study, as well as others, urinary retention rates are relatively low,” they said.
The Council of Academic Hospitals of Ontario funded the study. The researchers had no financial conflicts to disclose.
Adherence to guidelines for urinary catheter removal significantly reduced rates of urinary tract infection and length of hospital stay, based on data from almost 3,000 patients.
UTIs occurred in 0.8% of colonic surgery patients who were guideline compliant, compared with 4.1% of noncompliant patients. Similarly, UTIs were significantly less likely in rectal surgery patients who complied with guidelines, compared with those who did not (3.5% vs. 9.6%).
The Implementation of the Enhanced Recovery After Surgery (iERAS) program involved 15 academic hospitals in Ontario, Canada. The guidelines include prompt removal of catheters from colonic and rectal surgery patients. Recommended removal times ranged from 24 hours to 72 hours, depending on the procedure.
“Although numerous reports have documented the overall effectiveness of bundled ERAS interventions improving outcome, the contribution of individual components of the pathway in the context of an ERAS implementation program is uncertain,” wrote Allan Okrainec, MD, of the University of Toronto, and his colleagues.
Overall, 53% of patients had catheters removed in compliance with the guidelines, including 47.2% of those with colonic resections and 69.5% of those with rectal resections.
The average length of hospital stay for colonic surgery patients who complied with the urinary catheter guideline was 4 days, compared with 5 days for noncompliant patients, a statistically significant difference. Similarly, the average length of stay was significantly shorter for compliant rectal surgery patients, compared with noncompliant patients (5 days vs. 8 days, respectively).
Reinsertion of the urinary catheter was needed for 6% of the patients, including 122 patients with suspected or confirmed urinary retention and 36 patients who had other indications for reinsertion.
“One of the concerns about early removal of catheters is that it might lead to an increased need for reinsertion,” the researchers noted. “In our study, guideline compliance was associated with an increased rate of catheter reinsertion, but [it] only increased the risk by 2%-3% in patients having rectal or colonic procedures,” the investigators said.
The study results were limited by a lack of information about reasons for noncompliance with the guidelines, the researchers added. However, the data support the value of early catheter removal for surgical patients. “It is noteworthy that, in our study, as well as others, urinary retention rates are relatively low,” they said.
The Council of Academic Hospitals of Ontario funded the study. The researchers had no financial conflicts to disclose.
Adherence to guidelines for urinary catheter removal significantly reduced rates of urinary tract infection and length of hospital stay, based on data from almost 3,000 patients.
UTIs occurred in 0.8% of colonic surgery patients who were guideline compliant, compared with 4.1% of noncompliant patients. Similarly, UTIs were significantly less likely in rectal surgery patients who complied with guidelines, compared with those who did not (3.5% vs. 9.6%).
The Implementation of the Enhanced Recovery After Surgery (iERAS) program involved 15 academic hospitals in Ontario, Canada. The guidelines include prompt removal of catheters from colonic and rectal surgery patients. Recommended removal times ranged from 24 hours to 72 hours, depending on the procedure.
“Although numerous reports have documented the overall effectiveness of bundled ERAS interventions improving outcome, the contribution of individual components of the pathway in the context of an ERAS implementation program is uncertain,” wrote Allan Okrainec, MD, of the University of Toronto, and his colleagues.
Overall, 53% of patients had catheters removed in compliance with the guidelines, including 47.2% of those with colonic resections and 69.5% of those with rectal resections.
The average length of hospital stay for colonic surgery patients who complied with the urinary catheter guideline was 4 days, compared with 5 days for noncompliant patients, a statistically significant difference. Similarly, the average length of stay was significantly shorter for compliant rectal surgery patients, compared with noncompliant patients (5 days vs. 8 days, respectively).
Reinsertion of the urinary catheter was needed for 6% of the patients, including 122 patients with suspected or confirmed urinary retention and 36 patients who had other indications for reinsertion.
“One of the concerns about early removal of catheters is that it might lead to an increased need for reinsertion,” the researchers noted. “In our study, guideline compliance was associated with an increased rate of catheter reinsertion, but [it] only increased the risk by 2%-3% in patients having rectal or colonic procedures,” the investigators said.
The study results were limited by a lack of information about reasons for noncompliance with the guidelines, the researchers added. However, the data support the value of early catheter removal for surgical patients. “It is noteworthy that, in our study, as well as others, urinary retention rates are relatively low,” they said.
The Council of Academic Hospitals of Ontario funded the study. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF GASTROINTESTINAL SURGERY
Key clinical point: Compliance with guidelines for urinary catheter removal significantly reduced hospital stay and UTI rates in colon and rectal surgery patients.
Major finding: UTI rates in colonic surgery patients were 0.8% and 4.1%, respectively, for those who were compliant and noncompliant with the guidelines. Rates were 3.5% and 9.6%, respectively, for compliant and noncompliant rectal surgery patients.
Data source: A prospective study of 2,927 adults who underwent colonic or rectal surgery between September 2012 and April 2015.
Disclosures: The Council of Academic Hospitals of Ontario funded the study. The researchers had no financial conflicts to disclose.
ACIP approves new hepatitis A vaccine draft recommendations
, including a focus on catch-up vaccines for adolescents and those over age 40 years.
While hepatitis A cases have dropped significantly since the vaccine’s debut – with the number of reported cases in 2015 dropping to 1,390, compared with 9,606 in 1971 – previous recommendations regarding catch-up vaccinations suggested patients should consider treatment, as opposed to catch-up vaccination.
Adult catch-up vaccines now are recommended to be considered in areas with increasing disease risks, an addition that was not part of the current recommendations but has been changed because of evidence that patients over 40 years old are more vulnerable to the virus and more likely to be hospitalized if infected, said Noele Nelson, MD, PhD, of the Division of Viral Hepatitis at the CDC.
“Increasing proportions of adults in the United States are susceptible to hepatitis A ... due to reduced exposure to virus early in life and significant serum prevalence in older adults greater and equal to 40 years,” said Dr. Nelson. “In addition, there is low two-dose vaccination coverage among adults, including high risk adults, and morbidity and mortality increases with age.”
Recommendations for pregnant women also have been updated with a more definitive message. Previous recommendations advised pregnant women to weigh the options of acquiring hepatitis A against possible adverse effects of the vaccine. But, new evidence was presented at the meeting: in a study of 139 pregnant women vaccinated between 1996 and 2015 who experienced adverse effects, only seven of the effects were considered serious, and no maternal or infant deaths were apparent. In light of this, the ACIP approved the recommendation change to advise all pregnant women to be vaccinated, if they have not already been so before pregnancy.
Updates also included recommendations for patients with chronic liver disease, who are considered to be members of a high-risk population. Newly approved recommendations include a section on epidemiology, which states that, while those with chronic liver disease are not at increased risk for hepatitis A virus infection unless they experience fecal-oral exposure to the virus, those with acute hepatitis A may be more at risk to develop more severe liver disease. Recommendations for those with chronic liver disease also include a statement advising patients to seek immunoglobulin, as well as a hepatitis A, vaccination as soon as possible after exposure.
The ACIP also approved a change in recommendations to advise all residents and caretakers of those living in a group home, specifically those caring for developmentally disabled patients, to be vaccinated because of the historically high endemic nature of such institutions.
Committee members hope these new recommendations will help the United States reach its goal of a national hepatitis A case ratio of 0.3/100,000 people and a hepatitis A vaccination rate of 85%.
If these recommendations are approved by the director of the CDC and the U.S. Health Department, as they usually are, they will be published in the CDC’s Weekly Morbidity and Mortality Report.
Members of the committee reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
, including a focus on catch-up vaccines for adolescents and those over age 40 years.
While hepatitis A cases have dropped significantly since the vaccine’s debut – with the number of reported cases in 2015 dropping to 1,390, compared with 9,606 in 1971 – previous recommendations regarding catch-up vaccinations suggested patients should consider treatment, as opposed to catch-up vaccination.
Adult catch-up vaccines now are recommended to be considered in areas with increasing disease risks, an addition that was not part of the current recommendations but has been changed because of evidence that patients over 40 years old are more vulnerable to the virus and more likely to be hospitalized if infected, said Noele Nelson, MD, PhD, of the Division of Viral Hepatitis at the CDC.
“Increasing proportions of adults in the United States are susceptible to hepatitis A ... due to reduced exposure to virus early in life and significant serum prevalence in older adults greater and equal to 40 years,” said Dr. Nelson. “In addition, there is low two-dose vaccination coverage among adults, including high risk adults, and morbidity and mortality increases with age.”
Recommendations for pregnant women also have been updated with a more definitive message. Previous recommendations advised pregnant women to weigh the options of acquiring hepatitis A against possible adverse effects of the vaccine. But, new evidence was presented at the meeting: in a study of 139 pregnant women vaccinated between 1996 and 2015 who experienced adverse effects, only seven of the effects were considered serious, and no maternal or infant deaths were apparent. In light of this, the ACIP approved the recommendation change to advise all pregnant women to be vaccinated, if they have not already been so before pregnancy.
Updates also included recommendations for patients with chronic liver disease, who are considered to be members of a high-risk population. Newly approved recommendations include a section on epidemiology, which states that, while those with chronic liver disease are not at increased risk for hepatitis A virus infection unless they experience fecal-oral exposure to the virus, those with acute hepatitis A may be more at risk to develop more severe liver disease. Recommendations for those with chronic liver disease also include a statement advising patients to seek immunoglobulin, as well as a hepatitis A, vaccination as soon as possible after exposure.
The ACIP also approved a change in recommendations to advise all residents and caretakers of those living in a group home, specifically those caring for developmentally disabled patients, to be vaccinated because of the historically high endemic nature of such institutions.
Committee members hope these new recommendations will help the United States reach its goal of a national hepatitis A case ratio of 0.3/100,000 people and a hepatitis A vaccination rate of 85%.
If these recommendations are approved by the director of the CDC and the U.S. Health Department, as they usually are, they will be published in the CDC’s Weekly Morbidity and Mortality Report.
Members of the committee reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
, including a focus on catch-up vaccines for adolescents and those over age 40 years.
While hepatitis A cases have dropped significantly since the vaccine’s debut – with the number of reported cases in 2015 dropping to 1,390, compared with 9,606 in 1971 – previous recommendations regarding catch-up vaccinations suggested patients should consider treatment, as opposed to catch-up vaccination.
Adult catch-up vaccines now are recommended to be considered in areas with increasing disease risks, an addition that was not part of the current recommendations but has been changed because of evidence that patients over 40 years old are more vulnerable to the virus and more likely to be hospitalized if infected, said Noele Nelson, MD, PhD, of the Division of Viral Hepatitis at the CDC.
“Increasing proportions of adults in the United States are susceptible to hepatitis A ... due to reduced exposure to virus early in life and significant serum prevalence in older adults greater and equal to 40 years,” said Dr. Nelson. “In addition, there is low two-dose vaccination coverage among adults, including high risk adults, and morbidity and mortality increases with age.”
Recommendations for pregnant women also have been updated with a more definitive message. Previous recommendations advised pregnant women to weigh the options of acquiring hepatitis A against possible adverse effects of the vaccine. But, new evidence was presented at the meeting: in a study of 139 pregnant women vaccinated between 1996 and 2015 who experienced adverse effects, only seven of the effects were considered serious, and no maternal or infant deaths were apparent. In light of this, the ACIP approved the recommendation change to advise all pregnant women to be vaccinated, if they have not already been so before pregnancy.
Updates also included recommendations for patients with chronic liver disease, who are considered to be members of a high-risk population. Newly approved recommendations include a section on epidemiology, which states that, while those with chronic liver disease are not at increased risk for hepatitis A virus infection unless they experience fecal-oral exposure to the virus, those with acute hepatitis A may be more at risk to develop more severe liver disease. Recommendations for those with chronic liver disease also include a statement advising patients to seek immunoglobulin, as well as a hepatitis A, vaccination as soon as possible after exposure.
The ACIP also approved a change in recommendations to advise all residents and caretakers of those living in a group home, specifically those caring for developmentally disabled patients, to be vaccinated because of the historically high endemic nature of such institutions.
Committee members hope these new recommendations will help the United States reach its goal of a national hepatitis A case ratio of 0.3/100,000 people and a hepatitis A vaccination rate of 85%.
If these recommendations are approved by the director of the CDC and the U.S. Health Department, as they usually are, they will be published in the CDC’s Weekly Morbidity and Mortality Report.
Members of the committee reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
FROM ACIP MEETING
Bendamustine plus rituximab may have edge for treating indolent NHL, MCL
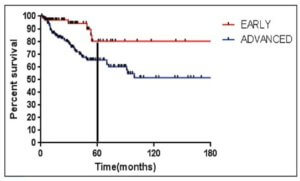
CHICAGO – Overall survival was comparable at 5 years of follow up for three regimens in treatment-naive patients with indolent non-Hodgkin lymphoma (NHL) or mantle cell lymphoma (MCL), based on long-term results from the BRIGHT study.
While progression-free survival, event-free survival, and duration of response were significantly better with bendamustine plus rituximab (BR), overall survival at 5 years did not significantly differ in patients given this regimen and compared to patients given rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine and prednisone (R-CVP), Ian Flinn, MD, of Tennessee Oncology, Nashville, reported at the annual meeting of the American Society of Clinical Oncology.
Quality of life was somewhat better for the patients given BR, but those patients were also at higher risk for secondary malignancies (42 vs. 24), most of which were squamous cell carcinomas, observed Dr. Kahl, professor of medicine at Washington University, St. Louis.
In BRIGHT, 224 treatment-naive patients with indolent NHL or MCL were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients). At least six cycles of therapy were completed by 203 patients in the BR group and by 196 in the R-CHOP/R-CVP group. Rituximab maintenance therapy was given to 43% of the BR group and to 45% of the R-CHOP/R-CVP group.
For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively. The overall survival rate for the entire patient group was 81.7% (75.7-86.3) and 85% (79.3-89.3) respectively. Comparing BR and R-CHOP/R-CVP, the hazard ratio (95% CI) for progression-free survival was 0.61 (0.45-0.85; P = .0025), the HR for event-free survival was 0.63 (0.46-0.84; P = .0020), the HR for duration of response was 0.66 (0.47-0.92; P = .0134), and the HR for overall survival was 1.15 (0.72-1.84; P = .5461).
Similar results were found in indolent NHL (progression-free survival 0.70 [0.49-1.01; P = .0582]) and MCL (progression-free survival 0.40 [0.21-0.75; P = .0035]), with the strongest effect in MCL, Dr. Flinn said.
Dr. Kahl noted that the advantages for the BR regimen include that it is not associated with alopecia, neuropathy, or steroid issues, and that it may extend progression-free survival and time to next treatment. On the other hand, R-CHOP is associated with less GI toxicity, rash, opportunistic infections, and prolonged cytopenia. Also, the BR regimen was associated with a higher risk of secondary cancers, primarily squamous cell carcinomas.
There were 42 secondary malignancies in the BR group and 24 in the R-CHOP/R-CVP group, Dr. Flinn reported.
It is theoretically possible that BR equals R-CHOP plus maintenance therapy from an efficacy perspective, Dr. Kahl said.
As virtually all excess adverse event fatalities occurred during maintenance therapy, it is possible that maintenance therapy after BR “does more harm than good.” This high priority issue “should be evaluated in the BRIGHT data set,” Dr. Kahl recommended.
Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
[email protected]
On Twitter @maryjodales
CHICAGO – Overall survival was comparable at 5 years of follow up for three regimens in treatment-naive patients with indolent non-Hodgkin lymphoma (NHL) or mantle cell lymphoma (MCL), based on long-term results from the BRIGHT study.
While progression-free survival, event-free survival, and duration of response were significantly better with bendamustine plus rituximab (BR), overall survival at 5 years did not significantly differ in patients given this regimen and compared to patients given rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine and prednisone (R-CVP), Ian Flinn, MD, of Tennessee Oncology, Nashville, reported at the annual meeting of the American Society of Clinical Oncology.
Quality of life was somewhat better for the patients given BR, but those patients were also at higher risk for secondary malignancies (42 vs. 24), most of which were squamous cell carcinomas, observed Dr. Kahl, professor of medicine at Washington University, St. Louis.
In BRIGHT, 224 treatment-naive patients with indolent NHL or MCL were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients). At least six cycles of therapy were completed by 203 patients in the BR group and by 196 in the R-CHOP/R-CVP group. Rituximab maintenance therapy was given to 43% of the BR group and to 45% of the R-CHOP/R-CVP group.
For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively. The overall survival rate for the entire patient group was 81.7% (75.7-86.3) and 85% (79.3-89.3) respectively. Comparing BR and R-CHOP/R-CVP, the hazard ratio (95% CI) for progression-free survival was 0.61 (0.45-0.85; P = .0025), the HR for event-free survival was 0.63 (0.46-0.84; P = .0020), the HR for duration of response was 0.66 (0.47-0.92; P = .0134), and the HR for overall survival was 1.15 (0.72-1.84; P = .5461).
Similar results were found in indolent NHL (progression-free survival 0.70 [0.49-1.01; P = .0582]) and MCL (progression-free survival 0.40 [0.21-0.75; P = .0035]), with the strongest effect in MCL, Dr. Flinn said.
Dr. Kahl noted that the advantages for the BR regimen include that it is not associated with alopecia, neuropathy, or steroid issues, and that it may extend progression-free survival and time to next treatment. On the other hand, R-CHOP is associated with less GI toxicity, rash, opportunistic infections, and prolonged cytopenia. Also, the BR regimen was associated with a higher risk of secondary cancers, primarily squamous cell carcinomas.
There were 42 secondary malignancies in the BR group and 24 in the R-CHOP/R-CVP group, Dr. Flinn reported.
It is theoretically possible that BR equals R-CHOP plus maintenance therapy from an efficacy perspective, Dr. Kahl said.
As virtually all excess adverse event fatalities occurred during maintenance therapy, it is possible that maintenance therapy after BR “does more harm than good.” This high priority issue “should be evaluated in the BRIGHT data set,” Dr. Kahl recommended.
Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
[email protected]
On Twitter @maryjodales
CHICAGO – Overall survival was comparable at 5 years of follow up for three regimens in treatment-naive patients with indolent non-Hodgkin lymphoma (NHL) or mantle cell lymphoma (MCL), based on long-term results from the BRIGHT study.
While progression-free survival, event-free survival, and duration of response were significantly better with bendamustine plus rituximab (BR), overall survival at 5 years did not significantly differ in patients given this regimen and compared to patients given rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine and prednisone (R-CVP), Ian Flinn, MD, of Tennessee Oncology, Nashville, reported at the annual meeting of the American Society of Clinical Oncology.
Quality of life was somewhat better for the patients given BR, but those patients were also at higher risk for secondary malignancies (42 vs. 24), most of which were squamous cell carcinomas, observed Dr. Kahl, professor of medicine at Washington University, St. Louis.
In BRIGHT, 224 treatment-naive patients with indolent NHL or MCL were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients). At least six cycles of therapy were completed by 203 patients in the BR group and by 196 in the R-CHOP/R-CVP group. Rituximab maintenance therapy was given to 43% of the BR group and to 45% of the R-CHOP/R-CVP group.
For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively. The overall survival rate for the entire patient group was 81.7% (75.7-86.3) and 85% (79.3-89.3) respectively. Comparing BR and R-CHOP/R-CVP, the hazard ratio (95% CI) for progression-free survival was 0.61 (0.45-0.85; P = .0025), the HR for event-free survival was 0.63 (0.46-0.84; P = .0020), the HR for duration of response was 0.66 (0.47-0.92; P = .0134), and the HR for overall survival was 1.15 (0.72-1.84; P = .5461).
Similar results were found in indolent NHL (progression-free survival 0.70 [0.49-1.01; P = .0582]) and MCL (progression-free survival 0.40 [0.21-0.75; P = .0035]), with the strongest effect in MCL, Dr. Flinn said.
Dr. Kahl noted that the advantages for the BR regimen include that it is not associated with alopecia, neuropathy, or steroid issues, and that it may extend progression-free survival and time to next treatment. On the other hand, R-CHOP is associated with less GI toxicity, rash, opportunistic infections, and prolonged cytopenia. Also, the BR regimen was associated with a higher risk of secondary cancers, primarily squamous cell carcinomas.
There were 42 secondary malignancies in the BR group and 24 in the R-CHOP/R-CVP group, Dr. Flinn reported.
It is theoretically possible that BR equals R-CHOP plus maintenance therapy from an efficacy perspective, Dr. Kahl said.
As virtually all excess adverse event fatalities occurred during maintenance therapy, it is possible that maintenance therapy after BR “does more harm than good.” This high priority issue “should be evaluated in the BRIGHT data set,” Dr. Kahl recommended.
Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
[email protected]
On Twitter @maryjodales
AT ASCO 2017
Key clinical point:
Major finding: For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively.
Data source: In BRIGHT, 224 treatment-naive patients with indolent non-Hodgkin lymphoma or mantle cell lymphoma were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients).
Disclosures: Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
Hospitalist meta-leader: Your new mission has arrived
If you are a hospitalist and leader in your health care organization, the ongoing controversies surrounding the Affordable Care Act repeal and replace campaign are unsettling. No matter your politics, Washington’s political drama and gamesmanship pose a genuine threat to the solvency of your hospital’s budget, services, workforce, and patients.
Health care has devolved into a political football, tossed from skirmish to skirmish. Political leaders warn of the implosion of the health care system as a political tactic, not an outcome that could cost and ruin lives. Both Democrats and Republicans hope that if or when that happens, it does so in ways that allow them to blame the other side. For them, this is a game of partisan advantage that wagers the well-being of your health care system.
For you, the situation remains predictably unpredictable. The future directives from Washington are unknowable. This makes your strategic planning – and health care leadership itself – a complex and puzzling task. Your job now is not simply leading your organization for today. Your more important mission is preparing your organization to perform in this unpredictable and perplexing future.
Forecasting is the life blood of leadership: Craft a vision and the work to achieve it; be mindful of the range of obstacles and opportunities; and know and coalesce your followers. The problem is that today’s prospects are loaded with puzzling twists and turns. The viability of both the private insurance market and public dollars are – maybe! – in future jeopardy. Patients and the workforce are understandably jittery. What is a hospitalist leader to do?
It is time to refresh your thinking, to take a big picture view of what is happening and to assess what can be done about it. There is a tendency for leaders to look at problems and then wonder how to fit solutions into their established organizational framework. In other words, solutions are cast into the mold of retaining what you have, ignoring larger options and innovative possibilities. Solutions are expected to adapt to the organization rather than the organization adapting to the solutions.
The hospitalist movement grew as early leaders – true innovators – recognized the problems of costly, inefficient and uncoordinated care. Rather than tinkering with what was, hospitalist leaders introduced a new and proactive model to provide care. It had to first prove itself and once it did, a once revolutionary idea evolved into an institutionalized solution.
No matter what emerges from the current policy debate, the national pressures on the health care system persist: rising expectations for access; decreasing patience for spending; increasing appetite for breakthrough technology; shifting workforce requirements; all combined with a population that is aging and more in need of care. These are meta-trends that will redefine how the health system operates and what it will achieve. What is a health care leader to do?
Think and act like a “meta-leader.” This framework, developed at the Harvard T.H. Chan School of Public Health, guides leaders facing complex and transformational problem solving. The prefix “meta-” encourages expansive analysis directed toward a wide range of options and opportunities. In keeping with the strategies employed by hospitalist pioneers, rather than building solutions around “what already is,” meta-leaders pursue “what could be.” In this way, solutions are designed and constructed to fit the problems they are intended to overcome.
There are three critical dimensions to the thinking and practices of meta-leadership.
The first is the Person of the meta-leader. This is who you are, your priorities and values. This is how other people regard your leadership, translated into the respect, trust, and “followership” you garner. Be a role model. This involves building your own confidence for the task at hand so that you gain and then foster the confidence of those you lead. As a meta-leader, you shape your mindset and that of others for innovation, sharpening the curiosity necessary for fostering discovery and exploration of new ideas. Be ready to take appropriate risks.
The second dimension of meta-leadership practice is the Situation. This is what is happening and what can be done about it. You did not create the complex circumstances that derive from the political showdown in Washington. However, it is your job to understand them and to develop effective strategies and operations in response. This is where the “think big” of meta-leadership comes into play. You distinguish the chasm between the adversarial policy confrontation in Washington and the collaborative solution building needed in your home institution. You want to set the stage to meaningfully coalesce the thinking, resources, and people in your organization. The invigorated shared mission is a health care system that leads into the future.
The third dimension of meta-leadership practice is about building the Connectivity needed to make that happen. This involves developing the communication, coordination, and cooperation necessary for constructing something new. Many of your answers lie within the walls of your organization, even the most innovative among them. This is where you sow adaptability and flexibility. It translates into necessary change and transformation. This is reorienting what you and others do and how you go about doing it, from shifts and adjustments to, when necessary, disruptive innovation.
A recent Harvard Business School and Harvard Medical School forum on health care innovation identified five imperatives for meeting innovation challenges in health care: 1) Creating value is the key aim for innovation and it requires a combination of care coordination along with communication; 2) Seek opportunities for process improvement that allows new ideas to be tested, accepting that failure is a step on the road to discovery; 3) Adopt a consumerism strategy for service organization that engages and involves active patients; 4) Decentralize problem solving to encourage field innovation and collaboration; and 5) Integrate new models into established institutions, introducing fresh thinking to replace outdated practices.
Meta-leadership is not a formula for an easy fix. While much remains unpredictable, an impending economic squeeze is a likely scenario. There is nothing easy about a shortage of dollars to serve more and more people in need of clinical care. This may very well be the prompt – today – that encourages the sort of innovative thinking and disruptive solution development that the future requires. Will you and your organization get ahead of this curve?
Your mission as a hospitalist meta-leader is in forging this process of discovery. Perceive what is going on through a wide lens. Orient yourself to emerging trends. Predict what is likely to emerge from this unpredictable policy environment. Take decisions and operationalize them in ways responsive to the circumstances at hand. And then communicate with your constituencies, not only to inform them of direction but also to learn from them what is working and what not. And then you start the process again, trying on ideas and practices, learning from them and through this continuous process, finding solutions that fit your situation at hand.
Health care meta-leaders today must keep both eyes firmly on their feet, to know that current operations are achieving necessary success. At the same time, they must also keep both eyes focused on the horizon, to ensure that when conditions change, their organizations are ready to adaptively innovate and transform.
Leonard J. Marcus, Ph.D. is coauthor of Renegotiating Health Care: Resolving Conflict to Build Collaboration, Second Edition (San Francisco: Jossey-Bass Publishers, 2011) and is director of the program for health care negotiation and conflict resolution, Harvard T.H. Chan School of Public Health. Dr. Marcus teaches regularly in the SHM Leadership Academy. He can be reached at [email protected]
If you are a hospitalist and leader in your health care organization, the ongoing controversies surrounding the Affordable Care Act repeal and replace campaign are unsettling. No matter your politics, Washington’s political drama and gamesmanship pose a genuine threat to the solvency of your hospital’s budget, services, workforce, and patients.
Health care has devolved into a political football, tossed from skirmish to skirmish. Political leaders warn of the implosion of the health care system as a political tactic, not an outcome that could cost and ruin lives. Both Democrats and Republicans hope that if or when that happens, it does so in ways that allow them to blame the other side. For them, this is a game of partisan advantage that wagers the well-being of your health care system.
For you, the situation remains predictably unpredictable. The future directives from Washington are unknowable. This makes your strategic planning – and health care leadership itself – a complex and puzzling task. Your job now is not simply leading your organization for today. Your more important mission is preparing your organization to perform in this unpredictable and perplexing future.
Forecasting is the life blood of leadership: Craft a vision and the work to achieve it; be mindful of the range of obstacles and opportunities; and know and coalesce your followers. The problem is that today’s prospects are loaded with puzzling twists and turns. The viability of both the private insurance market and public dollars are – maybe! – in future jeopardy. Patients and the workforce are understandably jittery. What is a hospitalist leader to do?
It is time to refresh your thinking, to take a big picture view of what is happening and to assess what can be done about it. There is a tendency for leaders to look at problems and then wonder how to fit solutions into their established organizational framework. In other words, solutions are cast into the mold of retaining what you have, ignoring larger options and innovative possibilities. Solutions are expected to adapt to the organization rather than the organization adapting to the solutions.
The hospitalist movement grew as early leaders – true innovators – recognized the problems of costly, inefficient and uncoordinated care. Rather than tinkering with what was, hospitalist leaders introduced a new and proactive model to provide care. It had to first prove itself and once it did, a once revolutionary idea evolved into an institutionalized solution.
No matter what emerges from the current policy debate, the national pressures on the health care system persist: rising expectations for access; decreasing patience for spending; increasing appetite for breakthrough technology; shifting workforce requirements; all combined with a population that is aging and more in need of care. These are meta-trends that will redefine how the health system operates and what it will achieve. What is a health care leader to do?
Think and act like a “meta-leader.” This framework, developed at the Harvard T.H. Chan School of Public Health, guides leaders facing complex and transformational problem solving. The prefix “meta-” encourages expansive analysis directed toward a wide range of options and opportunities. In keeping with the strategies employed by hospitalist pioneers, rather than building solutions around “what already is,” meta-leaders pursue “what could be.” In this way, solutions are designed and constructed to fit the problems they are intended to overcome.
There are three critical dimensions to the thinking and practices of meta-leadership.
The first is the Person of the meta-leader. This is who you are, your priorities and values. This is how other people regard your leadership, translated into the respect, trust, and “followership” you garner. Be a role model. This involves building your own confidence for the task at hand so that you gain and then foster the confidence of those you lead. As a meta-leader, you shape your mindset and that of others for innovation, sharpening the curiosity necessary for fostering discovery and exploration of new ideas. Be ready to take appropriate risks.
The second dimension of meta-leadership practice is the Situation. This is what is happening and what can be done about it. You did not create the complex circumstances that derive from the political showdown in Washington. However, it is your job to understand them and to develop effective strategies and operations in response. This is where the “think big” of meta-leadership comes into play. You distinguish the chasm between the adversarial policy confrontation in Washington and the collaborative solution building needed in your home institution. You want to set the stage to meaningfully coalesce the thinking, resources, and people in your organization. The invigorated shared mission is a health care system that leads into the future.
The third dimension of meta-leadership practice is about building the Connectivity needed to make that happen. This involves developing the communication, coordination, and cooperation necessary for constructing something new. Many of your answers lie within the walls of your organization, even the most innovative among them. This is where you sow adaptability and flexibility. It translates into necessary change and transformation. This is reorienting what you and others do and how you go about doing it, from shifts and adjustments to, when necessary, disruptive innovation.
A recent Harvard Business School and Harvard Medical School forum on health care innovation identified five imperatives for meeting innovation challenges in health care: 1) Creating value is the key aim for innovation and it requires a combination of care coordination along with communication; 2) Seek opportunities for process improvement that allows new ideas to be tested, accepting that failure is a step on the road to discovery; 3) Adopt a consumerism strategy for service organization that engages and involves active patients; 4) Decentralize problem solving to encourage field innovation and collaboration; and 5) Integrate new models into established institutions, introducing fresh thinking to replace outdated practices.
Meta-leadership is not a formula for an easy fix. While much remains unpredictable, an impending economic squeeze is a likely scenario. There is nothing easy about a shortage of dollars to serve more and more people in need of clinical care. This may very well be the prompt – today – that encourages the sort of innovative thinking and disruptive solution development that the future requires. Will you and your organization get ahead of this curve?
Your mission as a hospitalist meta-leader is in forging this process of discovery. Perceive what is going on through a wide lens. Orient yourself to emerging trends. Predict what is likely to emerge from this unpredictable policy environment. Take decisions and operationalize them in ways responsive to the circumstances at hand. And then communicate with your constituencies, not only to inform them of direction but also to learn from them what is working and what not. And then you start the process again, trying on ideas and practices, learning from them and through this continuous process, finding solutions that fit your situation at hand.
Health care meta-leaders today must keep both eyes firmly on their feet, to know that current operations are achieving necessary success. At the same time, they must also keep both eyes focused on the horizon, to ensure that when conditions change, their organizations are ready to adaptively innovate and transform.
Leonard J. Marcus, Ph.D. is coauthor of Renegotiating Health Care: Resolving Conflict to Build Collaboration, Second Edition (San Francisco: Jossey-Bass Publishers, 2011) and is director of the program for health care negotiation and conflict resolution, Harvard T.H. Chan School of Public Health. Dr. Marcus teaches regularly in the SHM Leadership Academy. He can be reached at [email protected]
If you are a hospitalist and leader in your health care organization, the ongoing controversies surrounding the Affordable Care Act repeal and replace campaign are unsettling. No matter your politics, Washington’s political drama and gamesmanship pose a genuine threat to the solvency of your hospital’s budget, services, workforce, and patients.
Health care has devolved into a political football, tossed from skirmish to skirmish. Political leaders warn of the implosion of the health care system as a political tactic, not an outcome that could cost and ruin lives. Both Democrats and Republicans hope that if or when that happens, it does so in ways that allow them to blame the other side. For them, this is a game of partisan advantage that wagers the well-being of your health care system.
For you, the situation remains predictably unpredictable. The future directives from Washington are unknowable. This makes your strategic planning – and health care leadership itself – a complex and puzzling task. Your job now is not simply leading your organization for today. Your more important mission is preparing your organization to perform in this unpredictable and perplexing future.
Forecasting is the life blood of leadership: Craft a vision and the work to achieve it; be mindful of the range of obstacles and opportunities; and know and coalesce your followers. The problem is that today’s prospects are loaded with puzzling twists and turns. The viability of both the private insurance market and public dollars are – maybe! – in future jeopardy. Patients and the workforce are understandably jittery. What is a hospitalist leader to do?
It is time to refresh your thinking, to take a big picture view of what is happening and to assess what can be done about it. There is a tendency for leaders to look at problems and then wonder how to fit solutions into their established organizational framework. In other words, solutions are cast into the mold of retaining what you have, ignoring larger options and innovative possibilities. Solutions are expected to adapt to the organization rather than the organization adapting to the solutions.
The hospitalist movement grew as early leaders – true innovators – recognized the problems of costly, inefficient and uncoordinated care. Rather than tinkering with what was, hospitalist leaders introduced a new and proactive model to provide care. It had to first prove itself and once it did, a once revolutionary idea evolved into an institutionalized solution.
No matter what emerges from the current policy debate, the national pressures on the health care system persist: rising expectations for access; decreasing patience for spending; increasing appetite for breakthrough technology; shifting workforce requirements; all combined with a population that is aging and more in need of care. These are meta-trends that will redefine how the health system operates and what it will achieve. What is a health care leader to do?
Think and act like a “meta-leader.” This framework, developed at the Harvard T.H. Chan School of Public Health, guides leaders facing complex and transformational problem solving. The prefix “meta-” encourages expansive analysis directed toward a wide range of options and opportunities. In keeping with the strategies employed by hospitalist pioneers, rather than building solutions around “what already is,” meta-leaders pursue “what could be.” In this way, solutions are designed and constructed to fit the problems they are intended to overcome.
There are three critical dimensions to the thinking and practices of meta-leadership.
The first is the Person of the meta-leader. This is who you are, your priorities and values. This is how other people regard your leadership, translated into the respect, trust, and “followership” you garner. Be a role model. This involves building your own confidence for the task at hand so that you gain and then foster the confidence of those you lead. As a meta-leader, you shape your mindset and that of others for innovation, sharpening the curiosity necessary for fostering discovery and exploration of new ideas. Be ready to take appropriate risks.
The second dimension of meta-leadership practice is the Situation. This is what is happening and what can be done about it. You did not create the complex circumstances that derive from the political showdown in Washington. However, it is your job to understand them and to develop effective strategies and operations in response. This is where the “think big” of meta-leadership comes into play. You distinguish the chasm between the adversarial policy confrontation in Washington and the collaborative solution building needed in your home institution. You want to set the stage to meaningfully coalesce the thinking, resources, and people in your organization. The invigorated shared mission is a health care system that leads into the future.
The third dimension of meta-leadership practice is about building the Connectivity needed to make that happen. This involves developing the communication, coordination, and cooperation necessary for constructing something new. Many of your answers lie within the walls of your organization, even the most innovative among them. This is where you sow adaptability and flexibility. It translates into necessary change and transformation. This is reorienting what you and others do and how you go about doing it, from shifts and adjustments to, when necessary, disruptive innovation.
A recent Harvard Business School and Harvard Medical School forum on health care innovation identified five imperatives for meeting innovation challenges in health care: 1) Creating value is the key aim for innovation and it requires a combination of care coordination along with communication; 2) Seek opportunities for process improvement that allows new ideas to be tested, accepting that failure is a step on the road to discovery; 3) Adopt a consumerism strategy for service organization that engages and involves active patients; 4) Decentralize problem solving to encourage field innovation and collaboration; and 5) Integrate new models into established institutions, introducing fresh thinking to replace outdated practices.
Meta-leadership is not a formula for an easy fix. While much remains unpredictable, an impending economic squeeze is a likely scenario. There is nothing easy about a shortage of dollars to serve more and more people in need of clinical care. This may very well be the prompt – today – that encourages the sort of innovative thinking and disruptive solution development that the future requires. Will you and your organization get ahead of this curve?
Your mission as a hospitalist meta-leader is in forging this process of discovery. Perceive what is going on through a wide lens. Orient yourself to emerging trends. Predict what is likely to emerge from this unpredictable policy environment. Take decisions and operationalize them in ways responsive to the circumstances at hand. And then communicate with your constituencies, not only to inform them of direction but also to learn from them what is working and what not. And then you start the process again, trying on ideas and practices, learning from them and through this continuous process, finding solutions that fit your situation at hand.
Health care meta-leaders today must keep both eyes firmly on their feet, to know that current operations are achieving necessary success. At the same time, they must also keep both eyes focused on the horizon, to ensure that when conditions change, their organizations are ready to adaptively innovate and transform.
Leonard J. Marcus, Ph.D. is coauthor of Renegotiating Health Care: Resolving Conflict to Build Collaboration, Second Edition (San Francisco: Jossey-Bass Publishers, 2011) and is director of the program for health care negotiation and conflict resolution, Harvard T.H. Chan School of Public Health. Dr. Marcus teaches regularly in the SHM Leadership Academy. He can be reached at [email protected]
Orthorexia Nervosa: An Obsession With Healthy Eating
First named by Steven Bratman in 1997, orthorexia nervosa (ON) from the Greek ortho, meaning correct, and orexi, meaning appetite, is classified as an unspecified feeding and eating disorder in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5).1,2
Hypothetical Case
Mr. P is a 30-year-old male who presented to the mental health clinic with his wife. The patient recounted that he had wanted to “be healthy” since childhood and has focused on exercise and proper diet, but anxiety about diet and food intake have steadily increased. Two years ago, he adopted a vegetarian diet by progressively eliminating several foods and food groups from his diet. He now feels “proud” to eat certain organically grown fruits, vegetables, nuts, beans, and drink only fruit or vegetable juice.
His wife stated that he spent between 3 and 5 hours daily preparing food or talking to friends and family about “correct foods to eat.” He also believed that errors in dietary habits caused physical or mental illnesses. He reported significant guilt and shame whenever he “slips up” on his dietary regimen and eats anything containing seafood, beef, or pork products, which he corrects by a day of fasting. His wife was frustrated because he refused to go to restaurants and started declining offers from friends to eat dinner at their homes unless he could bring his prepared food. He describes feeling “annoyed” when he sees other people eating fast food or meat.
Mr. P reported no significant medical or surgical history. His family history was significant for anxiety in his mother. He used to drink alcohol socially but ceased a few years ago due to its carbohydrate content. He never smoked or used illicit drugs.
A mental status exam revealed a thin male who appeared his stated age. He was cooperative, casually dressed, and made fair eye contact. He spoke clearly with an anxious tone and appropriate rate and volume. His affect was congruent with stated anxious mood. He was alert, awake, and oriented to person, place, and time. He reported no paranoia, auditory or visual hallucinations, and suicidal or homicidal ideation.
A physical exam revealed a thin male in no distress who measured 5 feet 10 inches tall and weighed 145 pounds, which yielded a body mass index of 20.8. His vitals included temperature of 98° F, blood pressure 115/76, pulse 74, and oxygen saturation 98% on room air. The remaining physical examination revealed no abnormalities. A complete blood count, thyroid function, urinalysis, and urine drug screens were within normal limits. Comprehensive metabolic profile revealed decreased sodium of 130 meq/L. Electrocardiogram revealed bradycardia.
An ON diagnosis is made primarily through a clinical interview. Collateral information from individuals familiar with the patient can be helpful. Experts have proposed and recently revised criteria for ON (Table). Although the ORTO-15 assessment tool may assist with diagnosis, the tool does not substitute for the clinical interview.
Discussion
There is no reliable measure of prevalence of ON, though Varga and colleagues initially estimated ON to occur in 6.9% of the general population, and ON may occur more frequently in health care professionals and performance artists.3 However, these may be overestimates, as the assessment tool used in the study does not adequately separate people with healthy eating habits from those with ON.4,5
Most prevalence studies were conducted in Europe and Turkey, and prevalence of ON may differ in the U.S. population. A recent assessment determined a prevalence of about 1%, similar to that of other eating disorders.5 No study has reported a correlation between ON and gender, but a survey of 448 college students in the U.S. (mean age 22 years) reported highest ON tendencies in Hispanic/Latino and overweight/obese students.6
Relationship to Other Illnesses
There is significant debate whether ON is a single syndrome, a variance of other syndromes, or a behavioral and culturally influenced attitude.7,8 Although ON may lead to or be comorbid with anorexia nervosa (AN) or obsessive-compulsive disorder (OCD), subtle differences exist between ON and these conditions.
To meet DSM-5 diagnostic criteria for AN, patients must weigh below minimally normal weight for their height and age, have an intense fear of gaining weight or becoming fat, and have a disturbed experience of their weight or body shape or cannot recognize the severity of the low weight.2 In contrast, an individual with ON may possess normal or low-normal weight. Patients with AN focus on food quantity, while patients with ON tend to focus on food quality. As summarized by Bratman, “People are ashamed of their anorexia, but they actively evangelize their orthorexia. People with anorexia skip meals; people with orthorexia do not (unless they are fasting). Those with anorexia focus only on avoiding foods, while those with orthorexia both avoid foods they think are bad and embrace foods they think are super-healthy.”9
Similarities between ON and OCD include anxiety, a need to exert control, and perfectionism. However, patients with OCD tend to report distress from compulsive behavior and a desire to change, thus exhibiting insight into their illness.8,10 Similarities between obsessive-compulsive personality disorder (OCPD) and ON include perfectionism, rigid thinking, excessive devotion, hypermorality, and a preoccupation with details and perceived rules.11
While no studies have yet described ON as a feature of somatoform disorders, some experts have hypothesized that preoccupation with illness in a patient with somatization disorder may engender a preoccupation with food and diet as a way to combat either real or perceived illness.11 Finally, there is a report of ON associated with the prodromal phase of schizophrenia, and the development of ON may increase risk for future psychotic disorders.11,12
Pathophysiology
The exact cause of ON is unknown, though it is likely multifactorial. Individuals with ON have neurocognitive deficits similar to those seen in patients with AN and OCD, including impairments in set-shifting (flexible problem solving), external attention, and working memory.11,13 Given these cognitive deficits as well as similar symptomatology, there may be analogous brain dysfunction in patients with ON and AN or OCD. Neuroimaging studies of patients with AN have revealed dysregulation of dopamine transmission in the reward circuitry of the ventral striatum and the food regulatory mechanism in the hypothalamus.14
Dysmorphology of and dysfunction in neural circuitry, particularly the cortico-striato-thalamo-cortical pathway, have been implicated in OCD.15 Neuroimaging studies have revealed increased volume and activation of the orbitofrontal cortex, which may be associated with obsessions and difficulty with extinction recall.14,15 In contrast, decreased volume and activity of the thalamus may impair its ability to inhibit the orbitofrontal cortex.15,16 Decreased volume and activity of the cingulate gyrus may be associated with difficulty in error monitoring and fear conditioning, while overactivation of the parietal lobe and cerebellum may be associated with compulsive behaviors.15,16
Risk Factors
Factors that contribute to the development of AN and possibly ON include development of food preferences, inherited differences in taste perception, food neophobia or pickiness, being premorbidly overweight or obese, parental feeding practices, and a history of parental eating disorders.14 One survey associated orthorexic tendencies with perfectionism, appearance orientation, overweight preoccupation, self-classified weight, and fearful and dismissing attachment styles.17 Significant predictors of ON included overweight preoccupation, appearance orientation, and a history of an eating disorder.17
Treatment
In contrast to patients with AN, patients with ON may be easily amenable to treatment, given their pursuit of and emphasis on wellness.18 Experts recommend a multidisciplinary team approach that includes physicians, psychotherapists, and dieticians.11 Treatment may be undertaken in an outpatient setting, but hospitalization for refeeding is recommended in cases with significant weight loss or malnourishment.11 Physical examination and laboratory studies are warranted, as excessive dietary restrictions can lead to weight loss and medical complications similar to those seen in AN, including osteopenia, anemia, hyponatremia, pancytopenia, bradycardia, and even pneumothorax and pneumomediastinum.19-21
There are no reported studies exploring the efficacy of psychotherapy or psychotropic medications for patients with ON. However, several treatments have been proposed given the symptom overlap with AN. Serotonin reuptake inhibitors may be beneficial for anxiety and obsessive-compulsive traits.18 However, patients with ON may refuse medications as unnatural substances.18
Cognitive behavioral therapy may be beneficial to address perfectionism and cognitive distortions, and exposure and response prevention may reduce obsessive-compulsive behaviors.11 Relaxation therapy may reduce mealtime anxiety. Psychoeducation may correct inaccurate beliefs about food groups, purity, and preparation, but it may induce emotional stress for the patient with ON.11
Conclusion
Orthorexia nervosa is perhaps best summarized as an obsession with healthy eating with associated restrictive behaviors. However, the attempt to attain optimum health through attention to diet may lead to malnourishment, loss of relationships, and poor quality of life.11 It is a little-understood disorder with uncertain etiology, imprecise assessment tools, and no formal diagnostic criteria or classification. Orthorexic characteristics vary from normal to pathologic in degree, and making a diagnosis remains a clinical judgment.22 Further research is needed to develop valid diagnostic tools and determining whether ON should be classified as a unique illness or a variation of other eating or anxiety disorders. Further research also may identify the etiology of ON, thus enabling targeted multidisciplinary treatment.
1. Bratman S. Health food junkie. Yoga J. 1997;136:42-50.
2. American Psychiatric Association. Feeding and eating disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013:329-354.
3. Varga M, Dukay-Szabó S, Túry F, van Furth EF. Evidence and gaps in the literature on orthorexia nervosa. Eat Weight Disord. 2013;18(2):103-111.
4. Donini LM, Marsili D, Graziani MP, Imbriale M, Cannella C. Orthorexia nervosa: validation of a diagnosis questionnaire. Eat Weight Disord. 2005;10(2):e28-e32.
5. Dunn TM, Gibbs J, Whitney N, Starosta A. Prevalence of orthorexia nervosa is less than 1 %: data from a US sample. Eat Weight Disord. 2016;22(1):185-192.
6. Bundros J, Clifford D, Silliman K, Neyman Morris M. Prevalence of orthorexia nervosa among college students based on Bratman’s test and associated tendencies. Appetite. 2016;101:86-94.
7. Vandereycken W. Media hype, diagnostic fad or genuine disorder? Professionals’ opinions about night eating syndrome, orthorexia, muscle dysmorphia, and emetophobia. Eat Disord. 2011;19(2):145-155.
8. Dell’Osso L, Abelli M, Carpita B, et al. Historical evolution of the concept of anorexia nervosa and relationships with orthorexia nervosa, autism, and obsessive-compulsive spectrum. Neuropsychiatr Dis Treat. 2016;12:1651-1660.
9. Bratman S. Orthorexia: an update. http://www.orthorexia.com/orthorexia-an-update. Updated October 5, 2015. Accessed April 18, 2017.
10. Dunn TM, Bratman S. On orthorexia nervosa: a review of the literature and proposed diagnostic criteria. Eat Behav. 2016;21:11-17.
11. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
12. Saddichha S, Babu GN, Chandra P. Orthorexia nervosa presenting as prodrome of schizophrenia. Schizophr Res. 2012;134(1):110.
13. Koven NS, Senbonmatsu R. A neuropsychological evaluation of orthorexia nervosa. Open J Psychiatry. 2013;3(2):214-222.
14. Gorwood P, Blanchet-Collet C, Chartrel N, et al. New insights in anorexia nervosa. Front Neurosci. 2016;10:256.
15. Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43-51.
16. Tang W, Zhu Q, Gong X, Zhu C, Wang Y, Chen S. Cortico-striato-thalamo-cortical circuit abnormalities in obsessive-compulsive disorder: A voxel-based morphometric and fMRI study of the whole brain. Behav Brain Res. 2016;313:17-22.
17. Barnes MA, Caltabiano ML. The interrelationship between orthorexia nervosa, perfectionism, body image and attachment style. Eat Weight Disord. 2017;22(1):177-184.
18. Mathieu J. What is orthorexia? J Am Diet Assoc. 2005;105(10):1510-1512.
19. Catalina Zamora ML, Bote Bonaechea B, García Sánchez F, Ríos Rial B. Orthorexia nervosa. A new eating behavior disorder? [in Spanish]. Actas Esp Psiquiatr. 2005;33(1):66-68.
20. Moroze RM, Dunn TM, Craig Holland J, Yager J, Weintraub P. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
21. Park SW, Kim JY, Go GJ, Jeon ES, Pyo HJ, Kwon YJ. Orthorexia nervosa with hyponatremia, subcutaneous emphysema, pneumomediastimum, pneumothorax, and pancytopenia. Electrolyte Blood Press. 2011;9(1):32-37.
22. Mogallapu RNG, Aynampudi AR, Scarff JR, Lippmann S. Orthorexia nervosa. The Kentucky Psychiatrist. 2012;22(3):3-6.
First named by Steven Bratman in 1997, orthorexia nervosa (ON) from the Greek ortho, meaning correct, and orexi, meaning appetite, is classified as an unspecified feeding and eating disorder in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5).1,2
Hypothetical Case
Mr. P is a 30-year-old male who presented to the mental health clinic with his wife. The patient recounted that he had wanted to “be healthy” since childhood and has focused on exercise and proper diet, but anxiety about diet and food intake have steadily increased. Two years ago, he adopted a vegetarian diet by progressively eliminating several foods and food groups from his diet. He now feels “proud” to eat certain organically grown fruits, vegetables, nuts, beans, and drink only fruit or vegetable juice.
His wife stated that he spent between 3 and 5 hours daily preparing food or talking to friends and family about “correct foods to eat.” He also believed that errors in dietary habits caused physical or mental illnesses. He reported significant guilt and shame whenever he “slips up” on his dietary regimen and eats anything containing seafood, beef, or pork products, which he corrects by a day of fasting. His wife was frustrated because he refused to go to restaurants and started declining offers from friends to eat dinner at their homes unless he could bring his prepared food. He describes feeling “annoyed” when he sees other people eating fast food or meat.
Mr. P reported no significant medical or surgical history. His family history was significant for anxiety in his mother. He used to drink alcohol socially but ceased a few years ago due to its carbohydrate content. He never smoked or used illicit drugs.
A mental status exam revealed a thin male who appeared his stated age. He was cooperative, casually dressed, and made fair eye contact. He spoke clearly with an anxious tone and appropriate rate and volume. His affect was congruent with stated anxious mood. He was alert, awake, and oriented to person, place, and time. He reported no paranoia, auditory or visual hallucinations, and suicidal or homicidal ideation.
A physical exam revealed a thin male in no distress who measured 5 feet 10 inches tall and weighed 145 pounds, which yielded a body mass index of 20.8. His vitals included temperature of 98° F, blood pressure 115/76, pulse 74, and oxygen saturation 98% on room air. The remaining physical examination revealed no abnormalities. A complete blood count, thyroid function, urinalysis, and urine drug screens were within normal limits. Comprehensive metabolic profile revealed decreased sodium of 130 meq/L. Electrocardiogram revealed bradycardia.
An ON diagnosis is made primarily through a clinical interview. Collateral information from individuals familiar with the patient can be helpful. Experts have proposed and recently revised criteria for ON (Table). Although the ORTO-15 assessment tool may assist with diagnosis, the tool does not substitute for the clinical interview.
Discussion
There is no reliable measure of prevalence of ON, though Varga and colleagues initially estimated ON to occur in 6.9% of the general population, and ON may occur more frequently in health care professionals and performance artists.3 However, these may be overestimates, as the assessment tool used in the study does not adequately separate people with healthy eating habits from those with ON.4,5
Most prevalence studies were conducted in Europe and Turkey, and prevalence of ON may differ in the U.S. population. A recent assessment determined a prevalence of about 1%, similar to that of other eating disorders.5 No study has reported a correlation between ON and gender, but a survey of 448 college students in the U.S. (mean age 22 years) reported highest ON tendencies in Hispanic/Latino and overweight/obese students.6
Relationship to Other Illnesses
There is significant debate whether ON is a single syndrome, a variance of other syndromes, or a behavioral and culturally influenced attitude.7,8 Although ON may lead to or be comorbid with anorexia nervosa (AN) or obsessive-compulsive disorder (OCD), subtle differences exist between ON and these conditions.
To meet DSM-5 diagnostic criteria for AN, patients must weigh below minimally normal weight for their height and age, have an intense fear of gaining weight or becoming fat, and have a disturbed experience of their weight or body shape or cannot recognize the severity of the low weight.2 In contrast, an individual with ON may possess normal or low-normal weight. Patients with AN focus on food quantity, while patients with ON tend to focus on food quality. As summarized by Bratman, “People are ashamed of their anorexia, but they actively evangelize their orthorexia. People with anorexia skip meals; people with orthorexia do not (unless they are fasting). Those with anorexia focus only on avoiding foods, while those with orthorexia both avoid foods they think are bad and embrace foods they think are super-healthy.”9
Similarities between ON and OCD include anxiety, a need to exert control, and perfectionism. However, patients with OCD tend to report distress from compulsive behavior and a desire to change, thus exhibiting insight into their illness.8,10 Similarities between obsessive-compulsive personality disorder (OCPD) and ON include perfectionism, rigid thinking, excessive devotion, hypermorality, and a preoccupation with details and perceived rules.11
While no studies have yet described ON as a feature of somatoform disorders, some experts have hypothesized that preoccupation with illness in a patient with somatization disorder may engender a preoccupation with food and diet as a way to combat either real or perceived illness.11 Finally, there is a report of ON associated with the prodromal phase of schizophrenia, and the development of ON may increase risk for future psychotic disorders.11,12
Pathophysiology
The exact cause of ON is unknown, though it is likely multifactorial. Individuals with ON have neurocognitive deficits similar to those seen in patients with AN and OCD, including impairments in set-shifting (flexible problem solving), external attention, and working memory.11,13 Given these cognitive deficits as well as similar symptomatology, there may be analogous brain dysfunction in patients with ON and AN or OCD. Neuroimaging studies of patients with AN have revealed dysregulation of dopamine transmission in the reward circuitry of the ventral striatum and the food regulatory mechanism in the hypothalamus.14
Dysmorphology of and dysfunction in neural circuitry, particularly the cortico-striato-thalamo-cortical pathway, have been implicated in OCD.15 Neuroimaging studies have revealed increased volume and activation of the orbitofrontal cortex, which may be associated with obsessions and difficulty with extinction recall.14,15 In contrast, decreased volume and activity of the thalamus may impair its ability to inhibit the orbitofrontal cortex.15,16 Decreased volume and activity of the cingulate gyrus may be associated with difficulty in error monitoring and fear conditioning, while overactivation of the parietal lobe and cerebellum may be associated with compulsive behaviors.15,16
Risk Factors
Factors that contribute to the development of AN and possibly ON include development of food preferences, inherited differences in taste perception, food neophobia or pickiness, being premorbidly overweight or obese, parental feeding practices, and a history of parental eating disorders.14 One survey associated orthorexic tendencies with perfectionism, appearance orientation, overweight preoccupation, self-classified weight, and fearful and dismissing attachment styles.17 Significant predictors of ON included overweight preoccupation, appearance orientation, and a history of an eating disorder.17
Treatment
In contrast to patients with AN, patients with ON may be easily amenable to treatment, given their pursuit of and emphasis on wellness.18 Experts recommend a multidisciplinary team approach that includes physicians, psychotherapists, and dieticians.11 Treatment may be undertaken in an outpatient setting, but hospitalization for refeeding is recommended in cases with significant weight loss or malnourishment.11 Physical examination and laboratory studies are warranted, as excessive dietary restrictions can lead to weight loss and medical complications similar to those seen in AN, including osteopenia, anemia, hyponatremia, pancytopenia, bradycardia, and even pneumothorax and pneumomediastinum.19-21
There are no reported studies exploring the efficacy of psychotherapy or psychotropic medications for patients with ON. However, several treatments have been proposed given the symptom overlap with AN. Serotonin reuptake inhibitors may be beneficial for anxiety and obsessive-compulsive traits.18 However, patients with ON may refuse medications as unnatural substances.18
Cognitive behavioral therapy may be beneficial to address perfectionism and cognitive distortions, and exposure and response prevention may reduce obsessive-compulsive behaviors.11 Relaxation therapy may reduce mealtime anxiety. Psychoeducation may correct inaccurate beliefs about food groups, purity, and preparation, but it may induce emotional stress for the patient with ON.11
Conclusion
Orthorexia nervosa is perhaps best summarized as an obsession with healthy eating with associated restrictive behaviors. However, the attempt to attain optimum health through attention to diet may lead to malnourishment, loss of relationships, and poor quality of life.11 It is a little-understood disorder with uncertain etiology, imprecise assessment tools, and no formal diagnostic criteria or classification. Orthorexic characteristics vary from normal to pathologic in degree, and making a diagnosis remains a clinical judgment.22 Further research is needed to develop valid diagnostic tools and determining whether ON should be classified as a unique illness or a variation of other eating or anxiety disorders. Further research also may identify the etiology of ON, thus enabling targeted multidisciplinary treatment.
First named by Steven Bratman in 1997, orthorexia nervosa (ON) from the Greek ortho, meaning correct, and orexi, meaning appetite, is classified as an unspecified feeding and eating disorder in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5).1,2
Hypothetical Case
Mr. P is a 30-year-old male who presented to the mental health clinic with his wife. The patient recounted that he had wanted to “be healthy” since childhood and has focused on exercise and proper diet, but anxiety about diet and food intake have steadily increased. Two years ago, he adopted a vegetarian diet by progressively eliminating several foods and food groups from his diet. He now feels “proud” to eat certain organically grown fruits, vegetables, nuts, beans, and drink only fruit or vegetable juice.
His wife stated that he spent between 3 and 5 hours daily preparing food or talking to friends and family about “correct foods to eat.” He also believed that errors in dietary habits caused physical or mental illnesses. He reported significant guilt and shame whenever he “slips up” on his dietary regimen and eats anything containing seafood, beef, or pork products, which he corrects by a day of fasting. His wife was frustrated because he refused to go to restaurants and started declining offers from friends to eat dinner at their homes unless he could bring his prepared food. He describes feeling “annoyed” when he sees other people eating fast food or meat.
Mr. P reported no significant medical or surgical history. His family history was significant for anxiety in his mother. He used to drink alcohol socially but ceased a few years ago due to its carbohydrate content. He never smoked or used illicit drugs.
A mental status exam revealed a thin male who appeared his stated age. He was cooperative, casually dressed, and made fair eye contact. He spoke clearly with an anxious tone and appropriate rate and volume. His affect was congruent with stated anxious mood. He was alert, awake, and oriented to person, place, and time. He reported no paranoia, auditory or visual hallucinations, and suicidal or homicidal ideation.
A physical exam revealed a thin male in no distress who measured 5 feet 10 inches tall and weighed 145 pounds, which yielded a body mass index of 20.8. His vitals included temperature of 98° F, blood pressure 115/76, pulse 74, and oxygen saturation 98% on room air. The remaining physical examination revealed no abnormalities. A complete blood count, thyroid function, urinalysis, and urine drug screens were within normal limits. Comprehensive metabolic profile revealed decreased sodium of 130 meq/L. Electrocardiogram revealed bradycardia.
An ON diagnosis is made primarily through a clinical interview. Collateral information from individuals familiar with the patient can be helpful. Experts have proposed and recently revised criteria for ON (Table). Although the ORTO-15 assessment tool may assist with diagnosis, the tool does not substitute for the clinical interview.
Discussion
There is no reliable measure of prevalence of ON, though Varga and colleagues initially estimated ON to occur in 6.9% of the general population, and ON may occur more frequently in health care professionals and performance artists.3 However, these may be overestimates, as the assessment tool used in the study does not adequately separate people with healthy eating habits from those with ON.4,5
Most prevalence studies were conducted in Europe and Turkey, and prevalence of ON may differ in the U.S. population. A recent assessment determined a prevalence of about 1%, similar to that of other eating disorders.5 No study has reported a correlation between ON and gender, but a survey of 448 college students in the U.S. (mean age 22 years) reported highest ON tendencies in Hispanic/Latino and overweight/obese students.6
Relationship to Other Illnesses
There is significant debate whether ON is a single syndrome, a variance of other syndromes, or a behavioral and culturally influenced attitude.7,8 Although ON may lead to or be comorbid with anorexia nervosa (AN) or obsessive-compulsive disorder (OCD), subtle differences exist between ON and these conditions.
To meet DSM-5 diagnostic criteria for AN, patients must weigh below minimally normal weight for their height and age, have an intense fear of gaining weight or becoming fat, and have a disturbed experience of their weight or body shape or cannot recognize the severity of the low weight.2 In contrast, an individual with ON may possess normal or low-normal weight. Patients with AN focus on food quantity, while patients with ON tend to focus on food quality. As summarized by Bratman, “People are ashamed of their anorexia, but they actively evangelize their orthorexia. People with anorexia skip meals; people with orthorexia do not (unless they are fasting). Those with anorexia focus only on avoiding foods, while those with orthorexia both avoid foods they think are bad and embrace foods they think are super-healthy.”9
Similarities between ON and OCD include anxiety, a need to exert control, and perfectionism. However, patients with OCD tend to report distress from compulsive behavior and a desire to change, thus exhibiting insight into their illness.8,10 Similarities between obsessive-compulsive personality disorder (OCPD) and ON include perfectionism, rigid thinking, excessive devotion, hypermorality, and a preoccupation with details and perceived rules.11
While no studies have yet described ON as a feature of somatoform disorders, some experts have hypothesized that preoccupation with illness in a patient with somatization disorder may engender a preoccupation with food and diet as a way to combat either real or perceived illness.11 Finally, there is a report of ON associated with the prodromal phase of schizophrenia, and the development of ON may increase risk for future psychotic disorders.11,12
Pathophysiology
The exact cause of ON is unknown, though it is likely multifactorial. Individuals with ON have neurocognitive deficits similar to those seen in patients with AN and OCD, including impairments in set-shifting (flexible problem solving), external attention, and working memory.11,13 Given these cognitive deficits as well as similar symptomatology, there may be analogous brain dysfunction in patients with ON and AN or OCD. Neuroimaging studies of patients with AN have revealed dysregulation of dopamine transmission in the reward circuitry of the ventral striatum and the food regulatory mechanism in the hypothalamus.14
Dysmorphology of and dysfunction in neural circuitry, particularly the cortico-striato-thalamo-cortical pathway, have been implicated in OCD.15 Neuroimaging studies have revealed increased volume and activation of the orbitofrontal cortex, which may be associated with obsessions and difficulty with extinction recall.14,15 In contrast, decreased volume and activity of the thalamus may impair its ability to inhibit the orbitofrontal cortex.15,16 Decreased volume and activity of the cingulate gyrus may be associated with difficulty in error monitoring and fear conditioning, while overactivation of the parietal lobe and cerebellum may be associated with compulsive behaviors.15,16
Risk Factors
Factors that contribute to the development of AN and possibly ON include development of food preferences, inherited differences in taste perception, food neophobia or pickiness, being premorbidly overweight or obese, parental feeding practices, and a history of parental eating disorders.14 One survey associated orthorexic tendencies with perfectionism, appearance orientation, overweight preoccupation, self-classified weight, and fearful and dismissing attachment styles.17 Significant predictors of ON included overweight preoccupation, appearance orientation, and a history of an eating disorder.17
Treatment
In contrast to patients with AN, patients with ON may be easily amenable to treatment, given their pursuit of and emphasis on wellness.18 Experts recommend a multidisciplinary team approach that includes physicians, psychotherapists, and dieticians.11 Treatment may be undertaken in an outpatient setting, but hospitalization for refeeding is recommended in cases with significant weight loss or malnourishment.11 Physical examination and laboratory studies are warranted, as excessive dietary restrictions can lead to weight loss and medical complications similar to those seen in AN, including osteopenia, anemia, hyponatremia, pancytopenia, bradycardia, and even pneumothorax and pneumomediastinum.19-21
There are no reported studies exploring the efficacy of psychotherapy or psychotropic medications for patients with ON. However, several treatments have been proposed given the symptom overlap with AN. Serotonin reuptake inhibitors may be beneficial for anxiety and obsessive-compulsive traits.18 However, patients with ON may refuse medications as unnatural substances.18
Cognitive behavioral therapy may be beneficial to address perfectionism and cognitive distortions, and exposure and response prevention may reduce obsessive-compulsive behaviors.11 Relaxation therapy may reduce mealtime anxiety. Psychoeducation may correct inaccurate beliefs about food groups, purity, and preparation, but it may induce emotional stress for the patient with ON.11
Conclusion
Orthorexia nervosa is perhaps best summarized as an obsession with healthy eating with associated restrictive behaviors. However, the attempt to attain optimum health through attention to diet may lead to malnourishment, loss of relationships, and poor quality of life.11 It is a little-understood disorder with uncertain etiology, imprecise assessment tools, and no formal diagnostic criteria or classification. Orthorexic characteristics vary from normal to pathologic in degree, and making a diagnosis remains a clinical judgment.22 Further research is needed to develop valid diagnostic tools and determining whether ON should be classified as a unique illness or a variation of other eating or anxiety disorders. Further research also may identify the etiology of ON, thus enabling targeted multidisciplinary treatment.
1. Bratman S. Health food junkie. Yoga J. 1997;136:42-50.
2. American Psychiatric Association. Feeding and eating disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013:329-354.
3. Varga M, Dukay-Szabó S, Túry F, van Furth EF. Evidence and gaps in the literature on orthorexia nervosa. Eat Weight Disord. 2013;18(2):103-111.
4. Donini LM, Marsili D, Graziani MP, Imbriale M, Cannella C. Orthorexia nervosa: validation of a diagnosis questionnaire. Eat Weight Disord. 2005;10(2):e28-e32.
5. Dunn TM, Gibbs J, Whitney N, Starosta A. Prevalence of orthorexia nervosa is less than 1 %: data from a US sample. Eat Weight Disord. 2016;22(1):185-192.
6. Bundros J, Clifford D, Silliman K, Neyman Morris M. Prevalence of orthorexia nervosa among college students based on Bratman’s test and associated tendencies. Appetite. 2016;101:86-94.
7. Vandereycken W. Media hype, diagnostic fad or genuine disorder? Professionals’ opinions about night eating syndrome, orthorexia, muscle dysmorphia, and emetophobia. Eat Disord. 2011;19(2):145-155.
8. Dell’Osso L, Abelli M, Carpita B, et al. Historical evolution of the concept of anorexia nervosa and relationships with orthorexia nervosa, autism, and obsessive-compulsive spectrum. Neuropsychiatr Dis Treat. 2016;12:1651-1660.
9. Bratman S. Orthorexia: an update. http://www.orthorexia.com/orthorexia-an-update. Updated October 5, 2015. Accessed April 18, 2017.
10. Dunn TM, Bratman S. On orthorexia nervosa: a review of the literature and proposed diagnostic criteria. Eat Behav. 2016;21:11-17.
11. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
12. Saddichha S, Babu GN, Chandra P. Orthorexia nervosa presenting as prodrome of schizophrenia. Schizophr Res. 2012;134(1):110.
13. Koven NS, Senbonmatsu R. A neuropsychological evaluation of orthorexia nervosa. Open J Psychiatry. 2013;3(2):214-222.
14. Gorwood P, Blanchet-Collet C, Chartrel N, et al. New insights in anorexia nervosa. Front Neurosci. 2016;10:256.
15. Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43-51.
16. Tang W, Zhu Q, Gong X, Zhu C, Wang Y, Chen S. Cortico-striato-thalamo-cortical circuit abnormalities in obsessive-compulsive disorder: A voxel-based morphometric and fMRI study of the whole brain. Behav Brain Res. 2016;313:17-22.
17. Barnes MA, Caltabiano ML. The interrelationship between orthorexia nervosa, perfectionism, body image and attachment style. Eat Weight Disord. 2017;22(1):177-184.
18. Mathieu J. What is orthorexia? J Am Diet Assoc. 2005;105(10):1510-1512.
19. Catalina Zamora ML, Bote Bonaechea B, García Sánchez F, Ríos Rial B. Orthorexia nervosa. A new eating behavior disorder? [in Spanish]. Actas Esp Psiquiatr. 2005;33(1):66-68.
20. Moroze RM, Dunn TM, Craig Holland J, Yager J, Weintraub P. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
21. Park SW, Kim JY, Go GJ, Jeon ES, Pyo HJ, Kwon YJ. Orthorexia nervosa with hyponatremia, subcutaneous emphysema, pneumomediastimum, pneumothorax, and pancytopenia. Electrolyte Blood Press. 2011;9(1):32-37.
22. Mogallapu RNG, Aynampudi AR, Scarff JR, Lippmann S. Orthorexia nervosa. The Kentucky Psychiatrist. 2012;22(3):3-6.
1. Bratman S. Health food junkie. Yoga J. 1997;136:42-50.
2. American Psychiatric Association. Feeding and eating disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013:329-354.
3. Varga M, Dukay-Szabó S, Túry F, van Furth EF. Evidence and gaps in the literature on orthorexia nervosa. Eat Weight Disord. 2013;18(2):103-111.
4. Donini LM, Marsili D, Graziani MP, Imbriale M, Cannella C. Orthorexia nervosa: validation of a diagnosis questionnaire. Eat Weight Disord. 2005;10(2):e28-e32.
5. Dunn TM, Gibbs J, Whitney N, Starosta A. Prevalence of orthorexia nervosa is less than 1 %: data from a US sample. Eat Weight Disord. 2016;22(1):185-192.
6. Bundros J, Clifford D, Silliman K, Neyman Morris M. Prevalence of orthorexia nervosa among college students based on Bratman’s test and associated tendencies. Appetite. 2016;101:86-94.
7. Vandereycken W. Media hype, diagnostic fad or genuine disorder? Professionals’ opinions about night eating syndrome, orthorexia, muscle dysmorphia, and emetophobia. Eat Disord. 2011;19(2):145-155.
8. Dell’Osso L, Abelli M, Carpita B, et al. Historical evolution of the concept of anorexia nervosa and relationships with orthorexia nervosa, autism, and obsessive-compulsive spectrum. Neuropsychiatr Dis Treat. 2016;12:1651-1660.
9. Bratman S. Orthorexia: an update. http://www.orthorexia.com/orthorexia-an-update. Updated October 5, 2015. Accessed April 18, 2017.
10. Dunn TM, Bratman S. On orthorexia nervosa: a review of the literature and proposed diagnostic criteria. Eat Behav. 2016;21:11-17.
11. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
12. Saddichha S, Babu GN, Chandra P. Orthorexia nervosa presenting as prodrome of schizophrenia. Schizophr Res. 2012;134(1):110.
13. Koven NS, Senbonmatsu R. A neuropsychological evaluation of orthorexia nervosa. Open J Psychiatry. 2013;3(2):214-222.
14. Gorwood P, Blanchet-Collet C, Chartrel N, et al. New insights in anorexia nervosa. Front Neurosci. 2016;10:256.
15. Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43-51.
16. Tang W, Zhu Q, Gong X, Zhu C, Wang Y, Chen S. Cortico-striato-thalamo-cortical circuit abnormalities in obsessive-compulsive disorder: A voxel-based morphometric and fMRI study of the whole brain. Behav Brain Res. 2016;313:17-22.
17. Barnes MA, Caltabiano ML. The interrelationship between orthorexia nervosa, perfectionism, body image and attachment style. Eat Weight Disord. 2017;22(1):177-184.
18. Mathieu J. What is orthorexia? J Am Diet Assoc. 2005;105(10):1510-1512.
19. Catalina Zamora ML, Bote Bonaechea B, García Sánchez F, Ríos Rial B. Orthorexia nervosa. A new eating behavior disorder? [in Spanish]. Actas Esp Psiquiatr. 2005;33(1):66-68.
20. Moroze RM, Dunn TM, Craig Holland J, Yager J, Weintraub P. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
21. Park SW, Kim JY, Go GJ, Jeon ES, Pyo HJ, Kwon YJ. Orthorexia nervosa with hyponatremia, subcutaneous emphysema, pneumomediastimum, pneumothorax, and pancytopenia. Electrolyte Blood Press. 2011;9(1):32-37.
22. Mogallapu RNG, Aynampudi AR, Scarff JR, Lippmann S. Orthorexia nervosa. The Kentucky Psychiatrist. 2012;22(3):3-6.
Inhibitor elicits responses in heavily pretreated FL, DLBCL
LUGANO, SWITZERLAND—Interim results of a phase 2 trial suggest tazemetostat can be effective in patients with heavily pretreated, relapsed or refractory non-Hodgkin lymphoma.
The EZH2 inhibitor produced the highest overall response rate in patients with EZH2-mutated follicular lymphoma (FL), followed by EZH2-mutated diffuse large B-cell lymphoma (DLBCL).
However, the drug also produced complete responses in FL and DLBCL patients with wild-type EZH2.
“If we had focused [only] on patients with EZH2 mutations, we would have missed those other complete responders in the wild-type setting,” said study investigator Franck Morschhauser, MD, PhD, of Centre Hospitalier Régional Universitaire de Lille in France.
He presented results of the trial* during the plenary session of the 14th International Conference on Malignant Lymphoma (ICML). The research was sponsored by Epizyme, the company developing tazemetostat.
The trial enrolled patients with relapsed or refractory DLBCL or FL who had received at least 2 prior therapies. The patients received tazemetostat at 800 mg twice daily until disease progression or study withdrawal.
Efficacy in FL
Dr Morschhauser presented efficacy data on 67 patients with FL. Thirteen had EZH2 mutations, and 54 had wild-type EZH2. The median age was 62 in the mutated group and 61 in the wild-type group.
Both groups had a median of 4 prior lines of therapy. Fifty-four percent of EZH2-mutated patients were refractory to their last treatment, as were 48% of wild-type patients.
The median time from diagnosis was 7.4 years in mutated patients and 4.9 years in wild-type patients. The median time from last therapy was 13 weeks and 41.3 weeks, respectively.
The overall response rate was 92% (12/13) in EZH2-mutated patients and 26% (14/54) in wild-type patients. The complete response rates were 8% (n=1) and 6% (n=3), respectively.
The median time to first response was 11.9 weeks and 15.2 weeks, respectively.
None of the EZH2-mutated patients have progressed, but 13 (24%) wild-type patients have.
Forty-eight percent of all FL patients remain on study. One EZH2-mutated patient with stable disease is still on study, as are 23 wild-type patients with stable disease.
Efficacy in DLBCL
Dr Morschhauser presented data on 137 patients with DLBCL, 17 with EZH2 mutations and 120 with wild-type EZH2. The median age was 61 in the mutated group and 69 in the wild-type group.
Both groups had a median of 3 prior lines of therapy. Eighty-two percent of EZH2-mutated patients were refractory to their last treatment, as were 63% of wild-type patients.
The median time from diagnosis was 1 year in mutated patients and 2 years in wild-type patients. The median time from last therapy was 8.6 weeks and 11.6 weeks, respectively.
The overall response rate was 29% (5/17) in EZH2-mutated patients and 15% (18/119) in wild-type patients. The complete response rates were 0% (n=0) and 8% (n=10), respectively.
The median time to first response was 8.3 weeks and 8.5 weeks, respectively.
Six (35%) of the EZH2-mutated patients have progressed, as have 60 (50%) wild-type patients.
Twelve percent of all DLBCL patients remain on study. One EZH2-mutated patient with stable disease is still on therapy, as are 4 wild-type patients with stable disease.
Predictors of response
Dr Morschhauser and his colleagues performed next-generation sequencing of samples from 92 patients in an attempt to identify predictors of response to tazemetostat.
The data suggested that EZH2 and MYD88 activating mutations are positive predictors of response, and negative predictors include MYC, TP53, and HIST1H1E.
Safety
Safety data were available for 210 patients. The overall rate of treatment-related adverse events (AEs) was 59%, the rate of grade 3 or higher treatment-related AEs was 18%, and the rate of serious treatment-related AEs was 10%.
There were treatment-related AEs leading to dose interruption (15%), dose reduction (3%), and discontinuation of tazemetostat (2%).
The most common treatment-related AEs were nausea (14%), thrombocytopenia (13%), anemia (10%), neutropenia (9%), diarrhea (8%), asthenia (8%), and fatigue (7%).
Dr Morschhauser said these results “confirm that tazemetostat is quite safe” in this patient population, and enrollment in this trial is ongoing. ![]()
*Data in the abstract differ from the presentation.
LUGANO, SWITZERLAND—Interim results of a phase 2 trial suggest tazemetostat can be effective in patients with heavily pretreated, relapsed or refractory non-Hodgkin lymphoma.
The EZH2 inhibitor produced the highest overall response rate in patients with EZH2-mutated follicular lymphoma (FL), followed by EZH2-mutated diffuse large B-cell lymphoma (DLBCL).
However, the drug also produced complete responses in FL and DLBCL patients with wild-type EZH2.
“If we had focused [only] on patients with EZH2 mutations, we would have missed those other complete responders in the wild-type setting,” said study investigator Franck Morschhauser, MD, PhD, of Centre Hospitalier Régional Universitaire de Lille in France.
He presented results of the trial* during the plenary session of the 14th International Conference on Malignant Lymphoma (ICML). The research was sponsored by Epizyme, the company developing tazemetostat.
The trial enrolled patients with relapsed or refractory DLBCL or FL who had received at least 2 prior therapies. The patients received tazemetostat at 800 mg twice daily until disease progression or study withdrawal.
Efficacy in FL
Dr Morschhauser presented efficacy data on 67 patients with FL. Thirteen had EZH2 mutations, and 54 had wild-type EZH2. The median age was 62 in the mutated group and 61 in the wild-type group.
Both groups had a median of 4 prior lines of therapy. Fifty-four percent of EZH2-mutated patients were refractory to their last treatment, as were 48% of wild-type patients.
The median time from diagnosis was 7.4 years in mutated patients and 4.9 years in wild-type patients. The median time from last therapy was 13 weeks and 41.3 weeks, respectively.
The overall response rate was 92% (12/13) in EZH2-mutated patients and 26% (14/54) in wild-type patients. The complete response rates were 8% (n=1) and 6% (n=3), respectively.
The median time to first response was 11.9 weeks and 15.2 weeks, respectively.
None of the EZH2-mutated patients have progressed, but 13 (24%) wild-type patients have.
Forty-eight percent of all FL patients remain on study. One EZH2-mutated patient with stable disease is still on study, as are 23 wild-type patients with stable disease.
Efficacy in DLBCL
Dr Morschhauser presented data on 137 patients with DLBCL, 17 with EZH2 mutations and 120 with wild-type EZH2. The median age was 61 in the mutated group and 69 in the wild-type group.
Both groups had a median of 3 prior lines of therapy. Eighty-two percent of EZH2-mutated patients were refractory to their last treatment, as were 63% of wild-type patients.
The median time from diagnosis was 1 year in mutated patients and 2 years in wild-type patients. The median time from last therapy was 8.6 weeks and 11.6 weeks, respectively.
The overall response rate was 29% (5/17) in EZH2-mutated patients and 15% (18/119) in wild-type patients. The complete response rates were 0% (n=0) and 8% (n=10), respectively.
The median time to first response was 8.3 weeks and 8.5 weeks, respectively.
Six (35%) of the EZH2-mutated patients have progressed, as have 60 (50%) wild-type patients.
Twelve percent of all DLBCL patients remain on study. One EZH2-mutated patient with stable disease is still on therapy, as are 4 wild-type patients with stable disease.
Predictors of response
Dr Morschhauser and his colleagues performed next-generation sequencing of samples from 92 patients in an attempt to identify predictors of response to tazemetostat.
The data suggested that EZH2 and MYD88 activating mutations are positive predictors of response, and negative predictors include MYC, TP53, and HIST1H1E.
Safety
Safety data were available for 210 patients. The overall rate of treatment-related adverse events (AEs) was 59%, the rate of grade 3 or higher treatment-related AEs was 18%, and the rate of serious treatment-related AEs was 10%.
There were treatment-related AEs leading to dose interruption (15%), dose reduction (3%), and discontinuation of tazemetostat (2%).
The most common treatment-related AEs were nausea (14%), thrombocytopenia (13%), anemia (10%), neutropenia (9%), diarrhea (8%), asthenia (8%), and fatigue (7%).
Dr Morschhauser said these results “confirm that tazemetostat is quite safe” in this patient population, and enrollment in this trial is ongoing. ![]()
*Data in the abstract differ from the presentation.
LUGANO, SWITZERLAND—Interim results of a phase 2 trial suggest tazemetostat can be effective in patients with heavily pretreated, relapsed or refractory non-Hodgkin lymphoma.
The EZH2 inhibitor produced the highest overall response rate in patients with EZH2-mutated follicular lymphoma (FL), followed by EZH2-mutated diffuse large B-cell lymphoma (DLBCL).
However, the drug also produced complete responses in FL and DLBCL patients with wild-type EZH2.
“If we had focused [only] on patients with EZH2 mutations, we would have missed those other complete responders in the wild-type setting,” said study investigator Franck Morschhauser, MD, PhD, of Centre Hospitalier Régional Universitaire de Lille in France.
He presented results of the trial* during the plenary session of the 14th International Conference on Malignant Lymphoma (ICML). The research was sponsored by Epizyme, the company developing tazemetostat.
The trial enrolled patients with relapsed or refractory DLBCL or FL who had received at least 2 prior therapies. The patients received tazemetostat at 800 mg twice daily until disease progression or study withdrawal.
Efficacy in FL
Dr Morschhauser presented efficacy data on 67 patients with FL. Thirteen had EZH2 mutations, and 54 had wild-type EZH2. The median age was 62 in the mutated group and 61 in the wild-type group.
Both groups had a median of 4 prior lines of therapy. Fifty-four percent of EZH2-mutated patients were refractory to their last treatment, as were 48% of wild-type patients.
The median time from diagnosis was 7.4 years in mutated patients and 4.9 years in wild-type patients. The median time from last therapy was 13 weeks and 41.3 weeks, respectively.
The overall response rate was 92% (12/13) in EZH2-mutated patients and 26% (14/54) in wild-type patients. The complete response rates were 8% (n=1) and 6% (n=3), respectively.
The median time to first response was 11.9 weeks and 15.2 weeks, respectively.
None of the EZH2-mutated patients have progressed, but 13 (24%) wild-type patients have.
Forty-eight percent of all FL patients remain on study. One EZH2-mutated patient with stable disease is still on study, as are 23 wild-type patients with stable disease.
Efficacy in DLBCL
Dr Morschhauser presented data on 137 patients with DLBCL, 17 with EZH2 mutations and 120 with wild-type EZH2. The median age was 61 in the mutated group and 69 in the wild-type group.
Both groups had a median of 3 prior lines of therapy. Eighty-two percent of EZH2-mutated patients were refractory to their last treatment, as were 63% of wild-type patients.
The median time from diagnosis was 1 year in mutated patients and 2 years in wild-type patients. The median time from last therapy was 8.6 weeks and 11.6 weeks, respectively.
The overall response rate was 29% (5/17) in EZH2-mutated patients and 15% (18/119) in wild-type patients. The complete response rates were 0% (n=0) and 8% (n=10), respectively.
The median time to first response was 8.3 weeks and 8.5 weeks, respectively.
Six (35%) of the EZH2-mutated patients have progressed, as have 60 (50%) wild-type patients.
Twelve percent of all DLBCL patients remain on study. One EZH2-mutated patient with stable disease is still on therapy, as are 4 wild-type patients with stable disease.
Predictors of response
Dr Morschhauser and his colleagues performed next-generation sequencing of samples from 92 patients in an attempt to identify predictors of response to tazemetostat.
The data suggested that EZH2 and MYD88 activating mutations are positive predictors of response, and negative predictors include MYC, TP53, and HIST1H1E.
Safety
Safety data were available for 210 patients. The overall rate of treatment-related adverse events (AEs) was 59%, the rate of grade 3 or higher treatment-related AEs was 18%, and the rate of serious treatment-related AEs was 10%.
There were treatment-related AEs leading to dose interruption (15%), dose reduction (3%), and discontinuation of tazemetostat (2%).
The most common treatment-related AEs were nausea (14%), thrombocytopenia (13%), anemia (10%), neutropenia (9%), diarrhea (8%), asthenia (8%), and fatigue (7%).
Dr Morschhauser said these results “confirm that tazemetostat is quite safe” in this patient population, and enrollment in this trial is ongoing. ![]()
*Data in the abstract differ from the presentation.
New frontline treatments needed for Hodgkin lymphoma
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
In this editorial, Anna Sureda, MD, PhD, details the need for new frontline treatments for patients with Hodgkin lymphoma, including those with advanced stage disease.
Dr Sureda is head of the Hematology Department and Hematopoietic Stem Cell Transplant Programme at the Institut Català d'Oncologia, Hospital Duran i Reynals, in Barcelona, Spain. She has received consultancy fees from Takeda/Millennium Pharmaceuticals, Merck Sharp & Dohme, and Bristol-Myers Squibb.
Hodgkin lymphoma has traditionally been known as a cancer with generally favorable outcomes. Yet, as with any cancer treatment, there is always room for improvement. For Hodgkin lymphoma specifically, there remains a significant unmet need in the frontline setting for patients with advanced disease (Stage III or Stage IV).
Hodgkin lymphoma most commonly affects young adults as well as adults over the age of 55.1 Both age at diagnosis and stage of the disease are significant factors that must be considered when determining treatment plans, as they can affect a patient’s success in achieving long-term remission.
Though early stage patients have demonstrated 5-year survival rates of approximately 90%, this number drops to 70% in patients with advanced stage disease,2-4 underlining the challenges of treating later stage Hodgkin lymphoma.
Additionally, only 50% of patients with relapsed or refractory disease will experience long-term remission with high-dose chemotherapy and an autologous stem cell transplant (ASCT)5-6— a historically and frequently used treatment regimen.
These facts support the importance of successful frontline treatment and highlight a gap with current treatment regimens.7-10
With current frontline Hodgkin lymphoma treatments, it can be a challenge for physicians to balance efficacy with safety. While allowing the patient to achieve long-term remission remains the goal, physicians are also considering the impact of treatment-related side effects including endocrine dysfunction, cardiac dysfunction, lung toxicity, infertility, and an increased risk of secondary cancers when determining the best possible treatment.8-15
Advanced stage vs early stage Hodgkin lymphoma
Stage of disease at diagnosis has a large influence on outcomes, with advanced stage patients having poorer outcomes than earlier stage patients.7,15-16 Advanced Hodgkin lymphoma patients are more likely to progress or relapse,7,15-16 with nearly one third remaining uncured following standard frontline therapy.7-10
As seen in Figure 1 below, there is a clear difference in progression-free survival for early versus advanced stage Hodgkin lymphoma.16
The difference between early stage and advanced stage patients treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) demonstrates the heightened importance of successful frontline treatment for those with advanced stage disease.16
Unmet needs with current frontline Hodgkin lymphoma treatment
Though current treatments for frontline Hodgkin lymphoma, including ABVD and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), have improved outcomes for patients, these standard regimens are more than 20 years old.
ABVD is generally regarded as the treatment of choice based on its efficacy, relative ease of administration, and side effect profile.17
Escalated BEACOPP, on the other hand, was developed to improve outcomes for advanced stage patients but is associated with increased toxicity.8-10,13,18
Positron emission tomography (PET) scans have also been identified as a pathway to help guide further treatment, but patients with advanced stage Hodgkin lymphoma may relapse more often, despite a negative interim PET scan, compared to stage II patients.19
Among current treatments, side effects including lung and cardiotoxicity as well as an increased risk of secondary cancers are a concern for both physicians and their patients.8-10,13-15
Similarly, radiation therapy, often used in conjunction with chemotherapy for patients who have a large tumor burden in one part of the body, usually the chest,20 is also associated with an increased risk of secondary cancers and cardiotoxicity.8-10,21
With these complications in mind, stabilizing the effects between improved efficacy and minimizing the toxicities associated with current frontline treatments needs to be a focus as new therapies are developed.
For young patients specifically, minimizing toxicities is crucial, as many will have a lifetime ahead of them after Hodgkin lymphoma and will want to avoid the risks associated with current treatments including lung disease, heart disease and infertility.8-10,12-15,22
Treating elderly patients can also be challenging due to their reduced ability to tolerate aggressive frontline treatment and multi-agent chemotherapy, which causes inferior survival outcomes when compared to younger patients.23-25 These secondary effects can affect a patient’s quality of life8-9,12,14-15,22,26-28 and exacerbate preexisting conditions commonly experienced by those undergoing treatment, including long-term fatigue, chronic medical and psychosocial complications, and general deterioration in physical well-being.22
Studies have shown that most relapses after ASCT typically occur within 2 years.29 After a relapse, the patient may endure a substantial physical and psychological burden due to the need for additional treatment, impacting quality of life for both the patient and their caregiver.22,26,30
Goals of clinical research
Despite its recognition as a highly treatable cancer, newly diagnosed Hodgkin lymphoma remains incurable in up to 30% of patients with advanced disease.7-10 Though current therapies seek to achieve remission and extend the lives of patients, it is often at the cost of treatment-related toxicities and side effects that can significantly reduce quality of life.
Moving forward, it is critical that these gaps in treatment are addressed in new frontline treatments that aim to benefit patients, including those with advanced stage disease, while reducing short-term and long-term toxicities. ![]()
Acknowledgements: The author would like to acknowledge the W2O Group for their writing support, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
______________________________________________________
1American Cancer Society. What Are the Key Statistics About Hodgkin Disease? https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html. Accessed February 16, 2017.
2Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J (editors). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD, 2007.
3American Cancer Society. Survival Rates for Hodgkin Disease by Stage. https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html. Accessed February 16, 2017.
4Fermé C, et al. New Engl J Med, 2007.357:1916–27.
5Sureda A, et al. Ann Oncol, 2005;16: 625–633.
6Majhail NS, et al. Biol Blood Marrow Transplant, 2006;12:1065–1072.
7Gordon LI, et al. J Clin Oncol, 2013;31:684-691.
8Carde P, et al. J Clin Oncol, 2016;34(17):2028-2036.
9Engert A, et al. J Clin Oncol, 2009;27(27):4548-4554
10Viviani S, et al. New Engl J Med, 2011;365(3):203-212.
11Sklar C, et al. J Endocrinology & Metabolism, 2000;85(9):3227-3232
12Behringer K, et al. J Clin Oncol, 2013;31:231-239.
13Borchmann P, et al. J Clin Oncol, 2011;29(32):4234-4242.
14Duggan DB, et al. J Clin Oncol, 2003;21(4):607-614.
15Johnson P, McKenzie H. Blood, 2015;125(11):1717-1723.
16Maddi RN, et al. Indian J Medical and Paediatric Oncology, 2015;36(4):255-260
17Ansell SM. American Journal of Hematology, 2014;89: 771–779.
18Merli F, et al. J Clin Oncol, 34:1175-1181.
19Johnson P, et al. N Engl J Med. 2016;374:2419‑2429
20American Cancer Society. Treating Hodgkin Disease: Radiation Therapy for Hodgkin Disease. https://www.cancer.org/cancer/hodgkin-lymphoma/treating/radiation.html. Accessed January 30, 2017.
21Adams MJ, et al. J Clin Oncol, 2004; 22: 3139–48.
22Khimani N, et al. Ann Oncol, 2013;24(1):226-230.
23Engert A, et al. J Clin Oncol, 2005;23(22):5052-60.
24Evens AM, et al. Br J Haematol, 2013;161: 76–86.
25Janssen-Heijnen ML, et al. Br J Haematol, 2005;129:597-606.
26Ganz PA et al. J Clin Oncol, 2003;21(18):3512-3519.
27Daniels LA, et al. Br J Cancer 2014;110:868-874.
28Loge JH, et al. Ann Oncol. 1999;10:71-77.
29Brusamolino E, Carella AM. Haematologica, 2007;92:6-10
30Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? Personalized Medicine in Oncology, 2016. http://www.personalizedmedonc.com/article/consolidation-therapy-after-asct-in-hodgkin-lymphoma-why-and-who-to-treat/. Accessed February 16, 2017.
Less sex, more contraception use in teens in last decade
, according to a report by Joyce C. Abma, PhD, and Gladys M. Martinez, PhD, of the Division of Vital Statistics at the National Center for Health Statistics, Hyattsville, Md.
The percentage of female teens who had ever had sexual intercourse declined from 51% in 1988 to 42% in 2011-2015. For male teens, the percentage who were sexually experienced declined from 60% in 1988 to 44% in 2011-2015.
Male teens’ condom use during their first sexual experiences increased from 71% in 2002 to 77% in 2011-2015. This plateau in male contraception is in part the result of various types of contraception for women being developed and widely distributed in the course of these years, such as intrauterine devices.
The report features the most recent data from the National Survey of Family Growth (NSFG), resulting in 4 years of interviews spanning 2011 through 2015 and including 2,047 female teens and 2,087 male teens aged 15-19 years (National Health Statistics Reports; no 104. Hyattsville, MD: National Center for Health Statistics. 2017). Data also was abstracted from the 1988 and 1995 National Surveys of Adolescent Males, a national panel survey of male teens.
NSFG is currently the only source of ongoing data on the topics of sexual activity and contraceptive use for the total U.S. population of teenagers, paralleling the teen population whose pregnancy and birth rates are captured by birth certificates as part of the statistics of the National Center for Health Statistics. “Using data from the 2011-2015 NSFG and earlier NSFG surveys, this report provides an update of information on U.S. teenagers’ sexual activity and contraceptive use, thus helping to improve understanding of their risk of pregnancy,” said Dr. Abma and her associates. “This report … provides insights into the ways in which those determinants are changing with time, how they differ by sociodemographic groups, and what circumstances are most associated with them. The information can be used to monitor and understand trends in teen sexual behavior and contraception use.”
The 2011-2015 National Survey of Family Growth was conducted by the National Center for Health Statistics with the support and assistance of a number of other organizations and individuals. Interviewing and other tasks were carried out by the University of Michigan’s Survey Research Center and the Institute for Social Research under a contract with NCHS.
, according to a report by Joyce C. Abma, PhD, and Gladys M. Martinez, PhD, of the Division of Vital Statistics at the National Center for Health Statistics, Hyattsville, Md.
The percentage of female teens who had ever had sexual intercourse declined from 51% in 1988 to 42% in 2011-2015. For male teens, the percentage who were sexually experienced declined from 60% in 1988 to 44% in 2011-2015.
Male teens’ condom use during their first sexual experiences increased from 71% in 2002 to 77% in 2011-2015. This plateau in male contraception is in part the result of various types of contraception for women being developed and widely distributed in the course of these years, such as intrauterine devices.
The report features the most recent data from the National Survey of Family Growth (NSFG), resulting in 4 years of interviews spanning 2011 through 2015 and including 2,047 female teens and 2,087 male teens aged 15-19 years (National Health Statistics Reports; no 104. Hyattsville, MD: National Center for Health Statistics. 2017). Data also was abstracted from the 1988 and 1995 National Surveys of Adolescent Males, a national panel survey of male teens.
NSFG is currently the only source of ongoing data on the topics of sexual activity and contraceptive use for the total U.S. population of teenagers, paralleling the teen population whose pregnancy and birth rates are captured by birth certificates as part of the statistics of the National Center for Health Statistics. “Using data from the 2011-2015 NSFG and earlier NSFG surveys, this report provides an update of information on U.S. teenagers’ sexual activity and contraceptive use, thus helping to improve understanding of their risk of pregnancy,” said Dr. Abma and her associates. “This report … provides insights into the ways in which those determinants are changing with time, how they differ by sociodemographic groups, and what circumstances are most associated with them. The information can be used to monitor and understand trends in teen sexual behavior and contraception use.”
The 2011-2015 National Survey of Family Growth was conducted by the National Center for Health Statistics with the support and assistance of a number of other organizations and individuals. Interviewing and other tasks were carried out by the University of Michigan’s Survey Research Center and the Institute for Social Research under a contract with NCHS.
, according to a report by Joyce C. Abma, PhD, and Gladys M. Martinez, PhD, of the Division of Vital Statistics at the National Center for Health Statistics, Hyattsville, Md.
The percentage of female teens who had ever had sexual intercourse declined from 51% in 1988 to 42% in 2011-2015. For male teens, the percentage who were sexually experienced declined from 60% in 1988 to 44% in 2011-2015.
Male teens’ condom use during their first sexual experiences increased from 71% in 2002 to 77% in 2011-2015. This plateau in male contraception is in part the result of various types of contraception for women being developed and widely distributed in the course of these years, such as intrauterine devices.
The report features the most recent data from the National Survey of Family Growth (NSFG), resulting in 4 years of interviews spanning 2011 through 2015 and including 2,047 female teens and 2,087 male teens aged 15-19 years (National Health Statistics Reports; no 104. Hyattsville, MD: National Center for Health Statistics. 2017). Data also was abstracted from the 1988 and 1995 National Surveys of Adolescent Males, a national panel survey of male teens.
NSFG is currently the only source of ongoing data on the topics of sexual activity and contraceptive use for the total U.S. population of teenagers, paralleling the teen population whose pregnancy and birth rates are captured by birth certificates as part of the statistics of the National Center for Health Statistics. “Using data from the 2011-2015 NSFG and earlier NSFG surveys, this report provides an update of information on U.S. teenagers’ sexual activity and contraceptive use, thus helping to improve understanding of their risk of pregnancy,” said Dr. Abma and her associates. “This report … provides insights into the ways in which those determinants are changing with time, how they differ by sociodemographic groups, and what circumstances are most associated with them. The information can be used to monitor and understand trends in teen sexual behavior and contraception use.”
The 2011-2015 National Survey of Family Growth was conducted by the National Center for Health Statistics with the support and assistance of a number of other organizations and individuals. Interviewing and other tasks were carried out by the University of Michigan’s Survey Research Center and the Institute for Social Research under a contract with NCHS.
FROM NATIONAL HEALTH STATISTICS REPORTS
Key clinical point: Contraception use has gone up in the last decade
Major finding: Contraception use has increased to 99% in girls and 77% in boys in the last decade.
Data source: This report presents U.S. data on the sexual activity and contraceptive use of males and females aged 15-19 years, using data from the 2011-2015 and earlier National Surveys of Family Growth and the 1988 and 1995 National Surveys of Adolescent Males.
Disclosures: The 2011-2015 National Survey of Family Growth was conducted by the National Center for Health Statistics with the support and assistance of a number of other organizations and individuals. Interviewing and other tasks were carried out by the University of Michigan’s Survey Research Center and the Institute for Social Research under a contract with NCHS.