User login
Tweaking CBT to boost outcomes in GAD
SAN FRANCISCO – The search is on for ways to refine cognitive-behavioral therapy for generalized anxiety disorder in order to improve upon current relatively modest success rates.
Cognitive-behavioral therapy (CBT) is less effective for generalized anxiety disorder (GAD) than for the other anxiety disorders. Only about one-half of patients are improved post-treatment, and less than one-third reach recovery, noted Richard E. Zinbarg, PhD, at the annual conference of the Anxiety and Depression Association of America.
At a session on advances in treatment of GAD, investigators presented randomized clinical trials assessing a variety of specific strategies aimed at enhancing the effectiveness of CBT in evidence-based fashion. The trials included a study of the impact of having patients keep a worry outcome journal, an exploration of the potential deleterious effects of a phenomenon known as relaxation-induced anxiety, and a study of the effectiveness of emotion regulation therapy, a relatively recent form of psychotherapy that’s part of the so-called “third wave” of CBT.
Worry outcome journal
Lucas LaFreniere observed that while CBT has been broadly shown to be effective for GAD, the various forms of CBT are packages of components that often include psychoeducation, stimulus control, behavioral experiments, exposure, cognitive reframing, relaxation training, and other elements in various combinations and sequences. Almost none of these specific components has been evaluated formally to learn whether they are pulling their weight therapeutically and making a positive contribution to outcomes.
Mr. LaFreniere, a doctoral student in clinical psychology at Pennsylvania State University in Hershey, presented a randomized trial of one such component, worry outcome monitoring, which currently is incorporated in some but not all CBT programs for GAD. Mr. LaFreniere and his coinvestigators developed a version of worry outcome monitoring they dubbed the worry outcome journal, or WOJ, which he characterized as “a brief ecological momentary intervention for worry.”
The WOJ works like this: At four random times per day, WOJ users receive a phone message to drop what they’re doing and record on a chart what they’re currently worrying about. They briefly note the date and time, the content of their worry, the distress it’s causing on a 1-7 scale, how much time they’re spending thinking about it, and their prediction as to the likelihood that this negative event actually will come to pass, which by the nature of their illness generally is unrealistically sky high early on in treatment. Later, they return to record whether the worrisome outcome occurred. The WOJ data are often reviewed in session.
The hypothesis was that the WOJ would reduce worry by aiding GAD patients in attending to their worries more thoroughly and objectively, recognizing in the moment the high cost of their worrying in terms of distress and cognitive interference, forming more realistic predictions about the future, and changing their conviction that excessive worrying is a worthwhile use of their time.
“One thing that particularly motivates me as a treatment researcher is the idea that those with GAD could be making themselves chronically miserable in an effort to protect themselves from future catastrophes that likely are not even going to happen. That’s a lot of human suffering that isn’t necessary. What we can do to help with that, we should do,” Mr. LaFreniere said.
On the other hand, this was a matter that cried out for a controlled trial because of the possibility that attempts to reduce worry might have unintended harmful consequences.
“Those with GAD have positive beliefs about worry. They believe it’s useful: it motivates, buffers emotional shifts, facilitates problem solving, and marks you as caring and conscientious – good personality traits,” he explained.
His study included 51 GAD patients randomized to 10 days using the WOJ or to a thought log control condition in which prompted by their cell phone four times daily, they recorded whatever everyday thought was on their mind at the moment. An example drawn from personal experience, Mr. LaFreniere said, might be “I love enchiladas!”
Outcome measures evaluated at baseline, again at 10 days upon conclusion of the intervention, and finally at 30 days of follow-up were the Penn State Worry Questionnaire, the GAD Questionnaire for DSM-IV, and the Meta-Cognitions Questionnaire subscales for positive beliefs about worry, uncontrollability of one’s thoughts, and negative beliefs about worry.
“The big reveal was that 91% of their worries did not come true,” he reported.
The primary outcome was reduction in worries as measured by the Penn State Questionnaire. The WOJ group showed a significant reduction, compared with controls, immediately post-treatment – which remained significant, albeit attenuated to a moderate effect size, at 30 days.
At day 10, 18 of 29 WOJ users no longer met diagnostic criteria for GAD, compared with 6 of 22 controls. By day 30, however, there was no significant between-group difference on this secondary endpoint.
The WOJ group showed a significantly greater reduction than controls on the secondary endpoint of uncontrollability of beliefs at both days 10 and 30.
“The WOJ may be a viable ecological momentary intervention for reducing worry in GAD. Therapist-free use of WOJ led to decreased worrying after only 10 days. It’s quite possible that longer practice may yield even stronger results. After all, for a normal CBT protocol, we’re looking at 8-20 weeks of treatment,” Mr. LaFreniere observed.
“I’d like to underscore that there was no harm done: The WOJ didn’t increase detrimental beliefs about worry,” he added. “We had a worry ourselves as researchers – disconfirmed by the trial – that patients may take the non-occurrence of their worries as some kind of proof that worry prevented those bad things from happening.”
Mr. LaFreniere is interested in studying the WOJ for worry reduction in non-GAD populations.
“Worry can be very high in other anxiety disorders, major depressive disorder, bipolar disorders, and in insomnia. The WOJ is highly cost-effective and easy to disseminate. It could very easily be made into a smartphone app,” he said.
Relaxation-induced anxiety
Relaxation training often is incorporated in treatment packages for GAD. Yet, it’s possible that one reason CBT is only modestly effective for GAD is because of relaxation-induced anxiety (RIA), an understudied phenomenon defined as a paradoxical increase in the physiological, behavioral, and cognitive aspects of anxiety when a person tries to relax.
“It has been theorized that individuals who are especially concerned with maintaining control over physical and psychological processes find relaxation vulnerable, unpleasant, and activating. Thus, discomfort with perceived lack of control during relaxed moments – an inability to let go – may result in unsought increase in anxiety during therapeutic attempts at relaxation,” according to Michelle G. Newman, PhD, professor of psychology at Penn State.
She presented a secondary analysis of a published randomized clinical trial she coauthored (J Consult Clin Psychol. 2002 Apr;70[2]:288-98) in which 41 participants with GAD were assigned to CBT with relaxation therapy using standard progressive muscle relaxation techniques or to self-control desensitization. Relaxation therapy and relaxation-induced anxiety ratings were recorded at each session. Outcomes were assessed post-treatment and at 6, 12, and 24 months of follow-up using the Penn State Worry Questionnaire, the State-Trait Anxiety Inventory, the Hamilton Anxiety Rating Scale, and the Clinician Severity Rating for GAD symptoms. In addition, immediately after each in-session relaxation practice, patients were asked to rate on a 9-point scale how much they noticed an increase in anxiety during the relaxation session.
All subjects improved significantly, but those with a lower peak RIA – defined as the highest level of RIA experienced in any of the 14 treatment sessions – had significantly fewer GAD symptoms at the end of therapy as well as at 2-year follow-up. Peak RIA was unrelated to baseline GAD symptom severity or change over time in anxiety symptoms. However, patients whose peak RIA occurred during the last several treatment sessions showed less improvement in GAD symptoms at the conclusion of treatment than those whose peak came earlier.
The clinical implications of these findings are that therapists who use progressive muscle relaxation in the treatment of GAD should assess RIA at the conclusion of every session, and if a patient reports moderate or higher RIA, the duration of the relaxation training portion of therapy should not be shortened until after several consecutive sessions of lower RIA have been reported, according to Dr. Newman.
Emotion regulation therapy
Megan E. Renna brought attendees up to speed on emotion regulation therapy (ERT), a third wave variant of CBT that incorporates principles from more traditional CBT, such as skills training and exposure, supplemented by teaching emotion regulation skills. Those skills include the development of present moment awareness and cultivation of compassion. Both are grounded in research on motivational and regulatory learning mechanisms related to threat vs. safety and reward versus loss.
As detailed in a recent review article for which she was first author (Front Psychol. 2017 Feb 6;8:98), ERT is a manualized, mechanism-targeted treatment for what she termed “distress disorders”; namely, GAD and major depressive disorder, which are highly comorbid, share key underlying temperamental features, and for whom adequate therapeutic success is all too often elusive. ERT appears to be particularly useful during the emerging adulthood years and across a broad range of ethnic and racial patients, according to Ms. Renna, a PhD student in clinical psychology at Hunter College in New York.
The efficacy of the original 20-session, individual therapy version of ERT was established in a study of 20 GAD patients, half of whom also had major depression (Depress Anxiety. 2015 Aug;32[8]:614-23). But Ms. Renna said ERT’s developers – Douglas S. Mennin, PhD, of Hunter College, and David M. Fresco, PhD, of Kent State (Ohio) University, are interested in determining the minimum effective therapeutic dose of ERT. They have conducted an open randomized trial of a 16-session version of ERT in which the results proved similar to those seen with 20 sessions. Now they’re carrying out a study of 8 vs. 16 sessions. The study is ongoing, but at first look, the results with 8 sessions of ERT appear similar to 16, Ms. Renna said.
None of the speakers reported having any financial conflicts of interest.
SAN FRANCISCO – The search is on for ways to refine cognitive-behavioral therapy for generalized anxiety disorder in order to improve upon current relatively modest success rates.
Cognitive-behavioral therapy (CBT) is less effective for generalized anxiety disorder (GAD) than for the other anxiety disorders. Only about one-half of patients are improved post-treatment, and less than one-third reach recovery, noted Richard E. Zinbarg, PhD, at the annual conference of the Anxiety and Depression Association of America.
At a session on advances in treatment of GAD, investigators presented randomized clinical trials assessing a variety of specific strategies aimed at enhancing the effectiveness of CBT in evidence-based fashion. The trials included a study of the impact of having patients keep a worry outcome journal, an exploration of the potential deleterious effects of a phenomenon known as relaxation-induced anxiety, and a study of the effectiveness of emotion regulation therapy, a relatively recent form of psychotherapy that’s part of the so-called “third wave” of CBT.
Worry outcome journal
Lucas LaFreniere observed that while CBT has been broadly shown to be effective for GAD, the various forms of CBT are packages of components that often include psychoeducation, stimulus control, behavioral experiments, exposure, cognitive reframing, relaxation training, and other elements in various combinations and sequences. Almost none of these specific components has been evaluated formally to learn whether they are pulling their weight therapeutically and making a positive contribution to outcomes.
Mr. LaFreniere, a doctoral student in clinical psychology at Pennsylvania State University in Hershey, presented a randomized trial of one such component, worry outcome monitoring, which currently is incorporated in some but not all CBT programs for GAD. Mr. LaFreniere and his coinvestigators developed a version of worry outcome monitoring they dubbed the worry outcome journal, or WOJ, which he characterized as “a brief ecological momentary intervention for worry.”
The WOJ works like this: At four random times per day, WOJ users receive a phone message to drop what they’re doing and record on a chart what they’re currently worrying about. They briefly note the date and time, the content of their worry, the distress it’s causing on a 1-7 scale, how much time they’re spending thinking about it, and their prediction as to the likelihood that this negative event actually will come to pass, which by the nature of their illness generally is unrealistically sky high early on in treatment. Later, they return to record whether the worrisome outcome occurred. The WOJ data are often reviewed in session.
The hypothesis was that the WOJ would reduce worry by aiding GAD patients in attending to their worries more thoroughly and objectively, recognizing in the moment the high cost of their worrying in terms of distress and cognitive interference, forming more realistic predictions about the future, and changing their conviction that excessive worrying is a worthwhile use of their time.
“One thing that particularly motivates me as a treatment researcher is the idea that those with GAD could be making themselves chronically miserable in an effort to protect themselves from future catastrophes that likely are not even going to happen. That’s a lot of human suffering that isn’t necessary. What we can do to help with that, we should do,” Mr. LaFreniere said.
On the other hand, this was a matter that cried out for a controlled trial because of the possibility that attempts to reduce worry might have unintended harmful consequences.
“Those with GAD have positive beliefs about worry. They believe it’s useful: it motivates, buffers emotional shifts, facilitates problem solving, and marks you as caring and conscientious – good personality traits,” he explained.
His study included 51 GAD patients randomized to 10 days using the WOJ or to a thought log control condition in which prompted by their cell phone four times daily, they recorded whatever everyday thought was on their mind at the moment. An example drawn from personal experience, Mr. LaFreniere said, might be “I love enchiladas!”
Outcome measures evaluated at baseline, again at 10 days upon conclusion of the intervention, and finally at 30 days of follow-up were the Penn State Worry Questionnaire, the GAD Questionnaire for DSM-IV, and the Meta-Cognitions Questionnaire subscales for positive beliefs about worry, uncontrollability of one’s thoughts, and negative beliefs about worry.
“The big reveal was that 91% of their worries did not come true,” he reported.
The primary outcome was reduction in worries as measured by the Penn State Questionnaire. The WOJ group showed a significant reduction, compared with controls, immediately post-treatment – which remained significant, albeit attenuated to a moderate effect size, at 30 days.
At day 10, 18 of 29 WOJ users no longer met diagnostic criteria for GAD, compared with 6 of 22 controls. By day 30, however, there was no significant between-group difference on this secondary endpoint.
The WOJ group showed a significantly greater reduction than controls on the secondary endpoint of uncontrollability of beliefs at both days 10 and 30.
“The WOJ may be a viable ecological momentary intervention for reducing worry in GAD. Therapist-free use of WOJ led to decreased worrying after only 10 days. It’s quite possible that longer practice may yield even stronger results. After all, for a normal CBT protocol, we’re looking at 8-20 weeks of treatment,” Mr. LaFreniere observed.
“I’d like to underscore that there was no harm done: The WOJ didn’t increase detrimental beliefs about worry,” he added. “We had a worry ourselves as researchers – disconfirmed by the trial – that patients may take the non-occurrence of their worries as some kind of proof that worry prevented those bad things from happening.”
Mr. LaFreniere is interested in studying the WOJ for worry reduction in non-GAD populations.
“Worry can be very high in other anxiety disorders, major depressive disorder, bipolar disorders, and in insomnia. The WOJ is highly cost-effective and easy to disseminate. It could very easily be made into a smartphone app,” he said.
Relaxation-induced anxiety
Relaxation training often is incorporated in treatment packages for GAD. Yet, it’s possible that one reason CBT is only modestly effective for GAD is because of relaxation-induced anxiety (RIA), an understudied phenomenon defined as a paradoxical increase in the physiological, behavioral, and cognitive aspects of anxiety when a person tries to relax.
“It has been theorized that individuals who are especially concerned with maintaining control over physical and psychological processes find relaxation vulnerable, unpleasant, and activating. Thus, discomfort with perceived lack of control during relaxed moments – an inability to let go – may result in unsought increase in anxiety during therapeutic attempts at relaxation,” according to Michelle G. Newman, PhD, professor of psychology at Penn State.
She presented a secondary analysis of a published randomized clinical trial she coauthored (J Consult Clin Psychol. 2002 Apr;70[2]:288-98) in which 41 participants with GAD were assigned to CBT with relaxation therapy using standard progressive muscle relaxation techniques or to self-control desensitization. Relaxation therapy and relaxation-induced anxiety ratings were recorded at each session. Outcomes were assessed post-treatment and at 6, 12, and 24 months of follow-up using the Penn State Worry Questionnaire, the State-Trait Anxiety Inventory, the Hamilton Anxiety Rating Scale, and the Clinician Severity Rating for GAD symptoms. In addition, immediately after each in-session relaxation practice, patients were asked to rate on a 9-point scale how much they noticed an increase in anxiety during the relaxation session.
All subjects improved significantly, but those with a lower peak RIA – defined as the highest level of RIA experienced in any of the 14 treatment sessions – had significantly fewer GAD symptoms at the end of therapy as well as at 2-year follow-up. Peak RIA was unrelated to baseline GAD symptom severity or change over time in anxiety symptoms. However, patients whose peak RIA occurred during the last several treatment sessions showed less improvement in GAD symptoms at the conclusion of treatment than those whose peak came earlier.
The clinical implications of these findings are that therapists who use progressive muscle relaxation in the treatment of GAD should assess RIA at the conclusion of every session, and if a patient reports moderate or higher RIA, the duration of the relaxation training portion of therapy should not be shortened until after several consecutive sessions of lower RIA have been reported, according to Dr. Newman.
Emotion regulation therapy
Megan E. Renna brought attendees up to speed on emotion regulation therapy (ERT), a third wave variant of CBT that incorporates principles from more traditional CBT, such as skills training and exposure, supplemented by teaching emotion regulation skills. Those skills include the development of present moment awareness and cultivation of compassion. Both are grounded in research on motivational and regulatory learning mechanisms related to threat vs. safety and reward versus loss.
As detailed in a recent review article for which she was first author (Front Psychol. 2017 Feb 6;8:98), ERT is a manualized, mechanism-targeted treatment for what she termed “distress disorders”; namely, GAD and major depressive disorder, which are highly comorbid, share key underlying temperamental features, and for whom adequate therapeutic success is all too often elusive. ERT appears to be particularly useful during the emerging adulthood years and across a broad range of ethnic and racial patients, according to Ms. Renna, a PhD student in clinical psychology at Hunter College in New York.
The efficacy of the original 20-session, individual therapy version of ERT was established in a study of 20 GAD patients, half of whom also had major depression (Depress Anxiety. 2015 Aug;32[8]:614-23). But Ms. Renna said ERT’s developers – Douglas S. Mennin, PhD, of Hunter College, and David M. Fresco, PhD, of Kent State (Ohio) University, are interested in determining the minimum effective therapeutic dose of ERT. They have conducted an open randomized trial of a 16-session version of ERT in which the results proved similar to those seen with 20 sessions. Now they’re carrying out a study of 8 vs. 16 sessions. The study is ongoing, but at first look, the results with 8 sessions of ERT appear similar to 16, Ms. Renna said.
None of the speakers reported having any financial conflicts of interest.
SAN FRANCISCO – The search is on for ways to refine cognitive-behavioral therapy for generalized anxiety disorder in order to improve upon current relatively modest success rates.
Cognitive-behavioral therapy (CBT) is less effective for generalized anxiety disorder (GAD) than for the other anxiety disorders. Only about one-half of patients are improved post-treatment, and less than one-third reach recovery, noted Richard E. Zinbarg, PhD, at the annual conference of the Anxiety and Depression Association of America.
At a session on advances in treatment of GAD, investigators presented randomized clinical trials assessing a variety of specific strategies aimed at enhancing the effectiveness of CBT in evidence-based fashion. The trials included a study of the impact of having patients keep a worry outcome journal, an exploration of the potential deleterious effects of a phenomenon known as relaxation-induced anxiety, and a study of the effectiveness of emotion regulation therapy, a relatively recent form of psychotherapy that’s part of the so-called “third wave” of CBT.
Worry outcome journal
Lucas LaFreniere observed that while CBT has been broadly shown to be effective for GAD, the various forms of CBT are packages of components that often include psychoeducation, stimulus control, behavioral experiments, exposure, cognitive reframing, relaxation training, and other elements in various combinations and sequences. Almost none of these specific components has been evaluated formally to learn whether they are pulling their weight therapeutically and making a positive contribution to outcomes.
Mr. LaFreniere, a doctoral student in clinical psychology at Pennsylvania State University in Hershey, presented a randomized trial of one such component, worry outcome monitoring, which currently is incorporated in some but not all CBT programs for GAD. Mr. LaFreniere and his coinvestigators developed a version of worry outcome monitoring they dubbed the worry outcome journal, or WOJ, which he characterized as “a brief ecological momentary intervention for worry.”
The WOJ works like this: At four random times per day, WOJ users receive a phone message to drop what they’re doing and record on a chart what they’re currently worrying about. They briefly note the date and time, the content of their worry, the distress it’s causing on a 1-7 scale, how much time they’re spending thinking about it, and their prediction as to the likelihood that this negative event actually will come to pass, which by the nature of their illness generally is unrealistically sky high early on in treatment. Later, they return to record whether the worrisome outcome occurred. The WOJ data are often reviewed in session.
The hypothesis was that the WOJ would reduce worry by aiding GAD patients in attending to their worries more thoroughly and objectively, recognizing in the moment the high cost of their worrying in terms of distress and cognitive interference, forming more realistic predictions about the future, and changing their conviction that excessive worrying is a worthwhile use of their time.
“One thing that particularly motivates me as a treatment researcher is the idea that those with GAD could be making themselves chronically miserable in an effort to protect themselves from future catastrophes that likely are not even going to happen. That’s a lot of human suffering that isn’t necessary. What we can do to help with that, we should do,” Mr. LaFreniere said.
On the other hand, this was a matter that cried out for a controlled trial because of the possibility that attempts to reduce worry might have unintended harmful consequences.
“Those with GAD have positive beliefs about worry. They believe it’s useful: it motivates, buffers emotional shifts, facilitates problem solving, and marks you as caring and conscientious – good personality traits,” he explained.
His study included 51 GAD patients randomized to 10 days using the WOJ or to a thought log control condition in which prompted by their cell phone four times daily, they recorded whatever everyday thought was on their mind at the moment. An example drawn from personal experience, Mr. LaFreniere said, might be “I love enchiladas!”
Outcome measures evaluated at baseline, again at 10 days upon conclusion of the intervention, and finally at 30 days of follow-up were the Penn State Worry Questionnaire, the GAD Questionnaire for DSM-IV, and the Meta-Cognitions Questionnaire subscales for positive beliefs about worry, uncontrollability of one’s thoughts, and negative beliefs about worry.
“The big reveal was that 91% of their worries did not come true,” he reported.
The primary outcome was reduction in worries as measured by the Penn State Questionnaire. The WOJ group showed a significant reduction, compared with controls, immediately post-treatment – which remained significant, albeit attenuated to a moderate effect size, at 30 days.
At day 10, 18 of 29 WOJ users no longer met diagnostic criteria for GAD, compared with 6 of 22 controls. By day 30, however, there was no significant between-group difference on this secondary endpoint.
The WOJ group showed a significantly greater reduction than controls on the secondary endpoint of uncontrollability of beliefs at both days 10 and 30.
“The WOJ may be a viable ecological momentary intervention for reducing worry in GAD. Therapist-free use of WOJ led to decreased worrying after only 10 days. It’s quite possible that longer practice may yield even stronger results. After all, for a normal CBT protocol, we’re looking at 8-20 weeks of treatment,” Mr. LaFreniere observed.
“I’d like to underscore that there was no harm done: The WOJ didn’t increase detrimental beliefs about worry,” he added. “We had a worry ourselves as researchers – disconfirmed by the trial – that patients may take the non-occurrence of their worries as some kind of proof that worry prevented those bad things from happening.”
Mr. LaFreniere is interested in studying the WOJ for worry reduction in non-GAD populations.
“Worry can be very high in other anxiety disorders, major depressive disorder, bipolar disorders, and in insomnia. The WOJ is highly cost-effective and easy to disseminate. It could very easily be made into a smartphone app,” he said.
Relaxation-induced anxiety
Relaxation training often is incorporated in treatment packages for GAD. Yet, it’s possible that one reason CBT is only modestly effective for GAD is because of relaxation-induced anxiety (RIA), an understudied phenomenon defined as a paradoxical increase in the physiological, behavioral, and cognitive aspects of anxiety when a person tries to relax.
“It has been theorized that individuals who are especially concerned with maintaining control over physical and psychological processes find relaxation vulnerable, unpleasant, and activating. Thus, discomfort with perceived lack of control during relaxed moments – an inability to let go – may result in unsought increase in anxiety during therapeutic attempts at relaxation,” according to Michelle G. Newman, PhD, professor of psychology at Penn State.
She presented a secondary analysis of a published randomized clinical trial she coauthored (J Consult Clin Psychol. 2002 Apr;70[2]:288-98) in which 41 participants with GAD were assigned to CBT with relaxation therapy using standard progressive muscle relaxation techniques or to self-control desensitization. Relaxation therapy and relaxation-induced anxiety ratings were recorded at each session. Outcomes were assessed post-treatment and at 6, 12, and 24 months of follow-up using the Penn State Worry Questionnaire, the State-Trait Anxiety Inventory, the Hamilton Anxiety Rating Scale, and the Clinician Severity Rating for GAD symptoms. In addition, immediately after each in-session relaxation practice, patients were asked to rate on a 9-point scale how much they noticed an increase in anxiety during the relaxation session.
All subjects improved significantly, but those with a lower peak RIA – defined as the highest level of RIA experienced in any of the 14 treatment sessions – had significantly fewer GAD symptoms at the end of therapy as well as at 2-year follow-up. Peak RIA was unrelated to baseline GAD symptom severity or change over time in anxiety symptoms. However, patients whose peak RIA occurred during the last several treatment sessions showed less improvement in GAD symptoms at the conclusion of treatment than those whose peak came earlier.
The clinical implications of these findings are that therapists who use progressive muscle relaxation in the treatment of GAD should assess RIA at the conclusion of every session, and if a patient reports moderate or higher RIA, the duration of the relaxation training portion of therapy should not be shortened until after several consecutive sessions of lower RIA have been reported, according to Dr. Newman.
Emotion regulation therapy
Megan E. Renna brought attendees up to speed on emotion regulation therapy (ERT), a third wave variant of CBT that incorporates principles from more traditional CBT, such as skills training and exposure, supplemented by teaching emotion regulation skills. Those skills include the development of present moment awareness and cultivation of compassion. Both are grounded in research on motivational and regulatory learning mechanisms related to threat vs. safety and reward versus loss.
As detailed in a recent review article for which she was first author (Front Psychol. 2017 Feb 6;8:98), ERT is a manualized, mechanism-targeted treatment for what she termed “distress disorders”; namely, GAD and major depressive disorder, which are highly comorbid, share key underlying temperamental features, and for whom adequate therapeutic success is all too often elusive. ERT appears to be particularly useful during the emerging adulthood years and across a broad range of ethnic and racial patients, according to Ms. Renna, a PhD student in clinical psychology at Hunter College in New York.
The efficacy of the original 20-session, individual therapy version of ERT was established in a study of 20 GAD patients, half of whom also had major depression (Depress Anxiety. 2015 Aug;32[8]:614-23). But Ms. Renna said ERT’s developers – Douglas S. Mennin, PhD, of Hunter College, and David M. Fresco, PhD, of Kent State (Ohio) University, are interested in determining the minimum effective therapeutic dose of ERT. They have conducted an open randomized trial of a 16-session version of ERT in which the results proved similar to those seen with 20 sessions. Now they’re carrying out a study of 8 vs. 16 sessions. The study is ongoing, but at first look, the results with 8 sessions of ERT appear similar to 16, Ms. Renna said.
None of the speakers reported having any financial conflicts of interest.
EXPERT ANALYSIS FROM THE ANXIETY AND DEPRESSION CONFERENCE 2017
Docs to Senate: Abandon your secret efforts on ACA repeal/replace
Six major organizations representing physicians have written to Senate leaders asking them to step back and take a different tack on health reform.
Senators have been meeting behind closed doors to craft health reform legislation as an alternative to the House-passed American Health Care Act (AHCA). To date, no draft legislation has surfaced publicly and no hearings have been held to discuss health reform provisions under consideration.
The letter was signed by the American Academy of Family Physicians, the American Academy of Pediatrics, the American College of Physicians, the American Congress of Obstetricians and Gynecologists, the American Osteopathic Association, and the American Psychiatric Association.
The groups raised their specific objections to two provisions of the AHCA that senators are said to be considering: caps on Medicaid funding and repeal of the Affordable Care Act’s essential benefits package.
Throughout the current health reform efforts “our organizations continually offered constructive ideas on achieving agreement on legislation consistent with our shared principles,” according to the letter. “Regrettably, both the House and Senate seem to be heading to a vote on legislation that would violate those principles by rolling back coverage and patient protections for tens of millions of patients.”
[email protected]
On Twitter @denisefulton
Six major organizations representing physicians have written to Senate leaders asking them to step back and take a different tack on health reform.
Senators have been meeting behind closed doors to craft health reform legislation as an alternative to the House-passed American Health Care Act (AHCA). To date, no draft legislation has surfaced publicly and no hearings have been held to discuss health reform provisions under consideration.
The letter was signed by the American Academy of Family Physicians, the American Academy of Pediatrics, the American College of Physicians, the American Congress of Obstetricians and Gynecologists, the American Osteopathic Association, and the American Psychiatric Association.
The groups raised their specific objections to two provisions of the AHCA that senators are said to be considering: caps on Medicaid funding and repeal of the Affordable Care Act’s essential benefits package.
Throughout the current health reform efforts “our organizations continually offered constructive ideas on achieving agreement on legislation consistent with our shared principles,” according to the letter. “Regrettably, both the House and Senate seem to be heading to a vote on legislation that would violate those principles by rolling back coverage and patient protections for tens of millions of patients.”
[email protected]
On Twitter @denisefulton
Six major organizations representing physicians have written to Senate leaders asking them to step back and take a different tack on health reform.
Senators have been meeting behind closed doors to craft health reform legislation as an alternative to the House-passed American Health Care Act (AHCA). To date, no draft legislation has surfaced publicly and no hearings have been held to discuss health reform provisions under consideration.
The letter was signed by the American Academy of Family Physicians, the American Academy of Pediatrics, the American College of Physicians, the American Congress of Obstetricians and Gynecologists, the American Osteopathic Association, and the American Psychiatric Association.
The groups raised their specific objections to two provisions of the AHCA that senators are said to be considering: caps on Medicaid funding and repeal of the Affordable Care Act’s essential benefits package.
Throughout the current health reform efforts “our organizations continually offered constructive ideas on achieving agreement on legislation consistent with our shared principles,” according to the letter. “Regrettably, both the House and Senate seem to be heading to a vote on legislation that would violate those principles by rolling back coverage and patient protections for tens of millions of patients.”
[email protected]
On Twitter @denisefulton
Vaccine delivery costs challenge physicians
, reported Mandy A. Allison, MD, of the University of Colorado at Denver, Aurora, and her associates.
Using the American Academy of Pediatrics’ recommended level of payment for first vaccine administration at that time – $25 – more than three-quarters of the practices surveyed said that all payer types paid less than the cost of vaccine administration, the researchers noted.
Those reporting that payment for vaccine administration was $11 or more was 74% for FFS, 74% for PPOs, 57% for MCOs/HMOs, 37% for CHIP, and 34% for Medicaid, the investigators reported.
In terms of how profit margins for vaccine delivery had changed in the last 3 years, an increase was reported by 25% of pediatric practices (Ped) and 15% of family physician practices (FP), no change was reported by 38% of Ped and 49% of FP, and a decrease by 37% of Ped and 36% of FP practices.
Of those practices that used strategies to reduce vaccine purchase cost or increase payment, 81% Ped and 36% FP used online purchasing discounts, 78% Ped and 49% FP used prompt pay discounts, 65% Ped and 49% FP used bulk order discounts, 69% Ped and 42% FP used group purchasing, and 69% Ped and 33% FP used promotional pricing.
Fewer than half of the practices said that they negotiated with private insurers regarding payment for vaccines (44% Ped, 33% FP) and administration fees (44% Ped, 35% FP).
When asked if they had stopped purchasing one or more pediatric vaccines for financial reasons, the answer was “yes” for 12% of Ped and 23% of FP, “no, but have seriously considered” for 24% of Ped and 26% of FP, and “no” for 64% of Ped and 51% of FP, reported Dr. Allison and her colleagues.
Read more in Academic Pediatrics (2017. doi: 10.1016/j.acap.2017.06.001).
, reported Mandy A. Allison, MD, of the University of Colorado at Denver, Aurora, and her associates.
Using the American Academy of Pediatrics’ recommended level of payment for first vaccine administration at that time – $25 – more than three-quarters of the practices surveyed said that all payer types paid less than the cost of vaccine administration, the researchers noted.
Those reporting that payment for vaccine administration was $11 or more was 74% for FFS, 74% for PPOs, 57% for MCOs/HMOs, 37% for CHIP, and 34% for Medicaid, the investigators reported.
In terms of how profit margins for vaccine delivery had changed in the last 3 years, an increase was reported by 25% of pediatric practices (Ped) and 15% of family physician practices (FP), no change was reported by 38% of Ped and 49% of FP, and a decrease by 37% of Ped and 36% of FP practices.
Of those practices that used strategies to reduce vaccine purchase cost or increase payment, 81% Ped and 36% FP used online purchasing discounts, 78% Ped and 49% FP used prompt pay discounts, 65% Ped and 49% FP used bulk order discounts, 69% Ped and 42% FP used group purchasing, and 69% Ped and 33% FP used promotional pricing.
Fewer than half of the practices said that they negotiated with private insurers regarding payment for vaccines (44% Ped, 33% FP) and administration fees (44% Ped, 35% FP).
When asked if they had stopped purchasing one or more pediatric vaccines for financial reasons, the answer was “yes” for 12% of Ped and 23% of FP, “no, but have seriously considered” for 24% of Ped and 26% of FP, and “no” for 64% of Ped and 51% of FP, reported Dr. Allison and her colleagues.
Read more in Academic Pediatrics (2017. doi: 10.1016/j.acap.2017.06.001).
, reported Mandy A. Allison, MD, of the University of Colorado at Denver, Aurora, and her associates.
Using the American Academy of Pediatrics’ recommended level of payment for first vaccine administration at that time – $25 – more than three-quarters of the practices surveyed said that all payer types paid less than the cost of vaccine administration, the researchers noted.
Those reporting that payment for vaccine administration was $11 or more was 74% for FFS, 74% for PPOs, 57% for MCOs/HMOs, 37% for CHIP, and 34% for Medicaid, the investigators reported.
In terms of how profit margins for vaccine delivery had changed in the last 3 years, an increase was reported by 25% of pediatric practices (Ped) and 15% of family physician practices (FP), no change was reported by 38% of Ped and 49% of FP, and a decrease by 37% of Ped and 36% of FP practices.
Of those practices that used strategies to reduce vaccine purchase cost or increase payment, 81% Ped and 36% FP used online purchasing discounts, 78% Ped and 49% FP used prompt pay discounts, 65% Ped and 49% FP used bulk order discounts, 69% Ped and 42% FP used group purchasing, and 69% Ped and 33% FP used promotional pricing.
Fewer than half of the practices said that they negotiated with private insurers regarding payment for vaccines (44% Ped, 33% FP) and administration fees (44% Ped, 35% FP).
When asked if they had stopped purchasing one or more pediatric vaccines for financial reasons, the answer was “yes” for 12% of Ped and 23% of FP, “no, but have seriously considered” for 24% of Ped and 26% of FP, and “no” for 64% of Ped and 51% of FP, reported Dr. Allison and her colleagues.
Read more in Academic Pediatrics (2017. doi: 10.1016/j.acap.2017.06.001).
FROM ACADEMIC PEDIATRICS
Painful Necrotic Ulcer on the Vulva
The Diagnosis: Mucormycosis
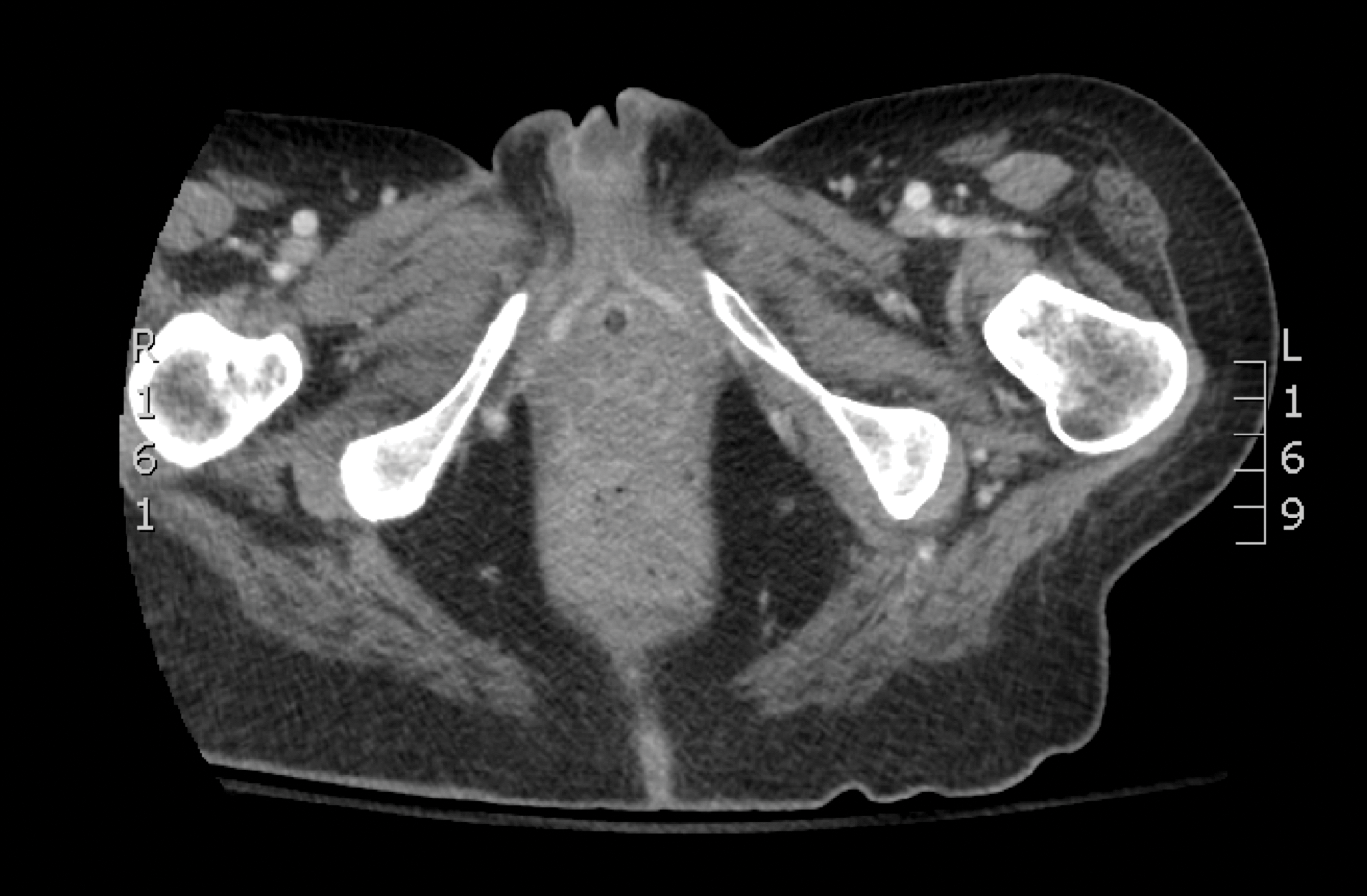
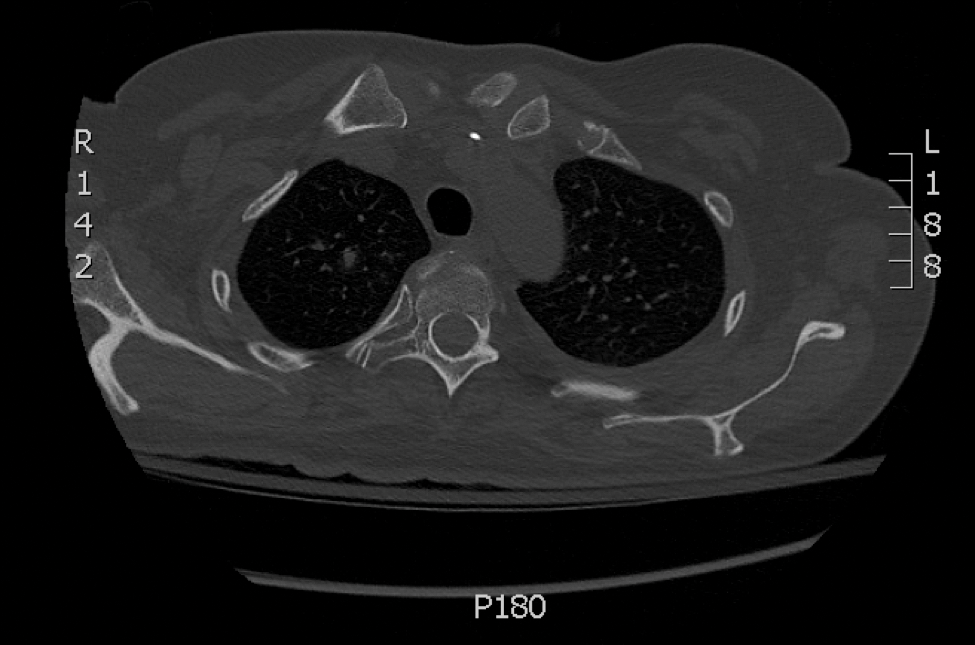
Skin biopsy and histology revealed broad, wide-angle, branched, nonseptate hyphae suggestive of mucormycosis infection (Figure 1). Computed tomography of the abdomen and pelvis revealed marked stranding in the vulvar region and urothelial thickening and enhancement suggestive of infection (Figure 2). Computed tomography of the chest demonstrated multiple irregular nodules in the bilateral upper lobes consistent with disseminated mucormycosis (Figure 3). The patient was started on intravenous amphotericin B and posaconazole. Surgery was not pursued given the poor prognosis of her refractory acute lymphoblastic leukemia, pancytopenia, and disseminated fungal infection. The patient was discharged home with hospice care.
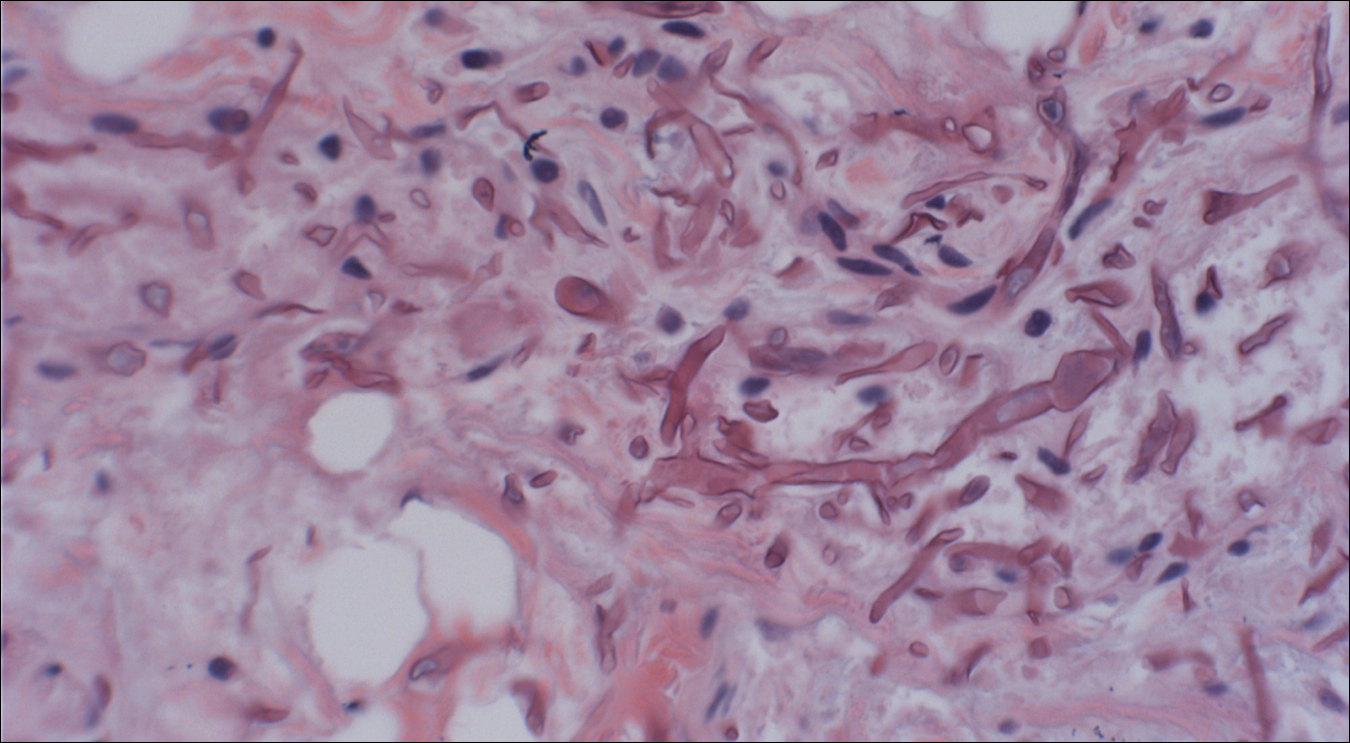
Mucormycosis is an infection caused by fungi that belong to the order Mucorales. The most common genera responsible for human disease are Rhizopus, Mucor, and Rhizomucor, which are organisms ubiquitous in nature and found in soil.1 Mucorales hyphae are widely branched and primarily nonseptate, which distinguishes them from hyphae of ascomycetous molds such as Aspergillus, which are narrowly branched and septate.
Mucormycosis primarily affects immunocompromised individuals. The overall incidence of mucormycosis is difficult to estimate, and the risk for infection varies based on the patient population. For example, the incidence of mucormycosis in hematologic malignancy ranges from 1% to 8% and from 0.4% to 16.0% in solid organ transplant recipients.2 One large series of 929 cases noted that the most common risk factors were associated with impaired immune function including diabetes mellitus and diabetic ketoacidosis (36% of cases), hematologic malignancy (17%), and solid organ (7%) or bone marrow transplantation (5%). Other risk factors include neutropenia, steroid therapy, and other immunocompromising conditions.3 Healthy individuals have a strong natural immunity to mucormycosis and rarely are affected by the disease.2
The host response to Mucorales is primarily driven by phagocyte-mediated killing via oxidative metabolites and cationic peptides called defensins.1 Thus, severely neutropenic patients are at high risk for developing mucormycosis.1 In contrast, it appears as though AIDS patients are not at increased risk for mucormycosis, supporting the theory that T lymphocytes are not involved in the host response.1 The conditions of diabetic ketoacidosis leave patients susceptible to mucormycosis for several reasons. First, hyperglycemia and low pH induce phagocyte dysfunction and thus inhibit the host response to Mucorales.4 Second, these organisms have an active ketone reductase system that may allow them to grow more readily in high glucose, acidic conditions.1 Third, diabetic ketoacidosis conditions increase serum free iron, and Mucorales utilizes host iron for cell growth and development.1 Individuals such as hemodialysis patients receiving the iron chelator deferoxamine also are at risk for mucormycosis, as Rhizopus can bind to this molecule and transport the bound iron intracellularly for growth utilization.1
Mucormycosis infection is characterized by infarction and rapid necrosis of host tissues resulting from vascular infiltration by fungal hyphae. The most common site of infection is rhino-orbital-cerebral (39%), followed by lungs (24%) and skin (19%).3 Dissemination occurs in 23% of cases.3 Inoculation most commonly occurs via inhalation of airborne fungal spores by an immunocompromised host with resultant fungal proliferation in the paranasal sinuses, bronchioles, or alveoli. Gastrointestinal tract infection is presumed to occur via ingestion of spores.5
Cutaneous infection, as in our patient, occurs via the inoculation of spores into the dermis through breaks in the skin such as from intravenous lines, urinary catheters, injection sites, surgical sites, and traumatic wounds. Cutaneous infections typically present as a single erythematous, painful, indurated papule that rapidly progresses to a necrotic ulcer with overlying black eschar. In some cases, the progression may be more indolent over the course of several weeks.2 There are few reported cases of primary vulvar mucormycosis, as in our patient.6,7 The previously reported cases involved severely immunocompromised patients who developed large necrotic lesions over the vulva that demonstrated widely branching, nonseptate hyphae on histologic examination. Each patient required extensive surgical debridement with systemic antifungal treatment.6,7
A timely diagnosis of mucormycosis often hinges on a high index of suspicion on behalf of the clinician. A fungal etiology always should be considered for an infection in an immunocompromised patient. Furthermore, nonresponse to antibiotic treatment should be an important diagnostic clue that the infection could be fungal in origin. The definitive diagnosis of mucormycosis is confirmed by tissue biopsy and the presence of broad, widely branching, nonseptate hyphae seen on histopathologic examination.
Treatment involves aggressive surgical debridement of all necrotic tissues and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and immunosuppressive medications. Early initiation of antifungal therapy with the lipid formulation of amphotericin B is recommended. Oral posaconazole or isavuconazole typically are used as step-down therapy after a favorable clinical response with initial amphotericin B treatment. Deferasirox, in contrast to deferoxamine, is an iron chelator that may reduce the pathogenicity of Mucorales and may help as an adjunctive therapy.8 In addition, hyperbaric oxygen therapy may have limited benefit in some cases.9 In spite of these treatments, the overall mortality of mucormycosis is 50% or higher and approaches nearly 100% in cases of disseminated disease, such as in our patient.1,3
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-1129.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus [published online July 29, 2009]. J Clin Microbiol. 2009;47:2834-2843.
- Colon M, Romaguera J, Mendez K, et al. Mucormycosis of the vulva in an immunocompromised pediatric patient. Bol Asoc Med P R. 2013;105:65-67.
- Nomura J, Ruskin J, Sahebi F, et al. Mucormycosis of the vulva following bone marrow transplantation. Bone Marrow Transplant. 1997;19:859-860.
- Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53:3122-3125.
- Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
The Diagnosis: Mucormycosis
Skin biopsy and histology revealed broad, wide-angle, branched, nonseptate hyphae suggestive of mucormycosis infection (Figure 1). Computed tomography of the abdomen and pelvis revealed marked stranding in the vulvar region and urothelial thickening and enhancement suggestive of infection (Figure 2). Computed tomography of the chest demonstrated multiple irregular nodules in the bilateral upper lobes consistent with disseminated mucormycosis (Figure 3). The patient was started on intravenous amphotericin B and posaconazole. Surgery was not pursued given the poor prognosis of her refractory acute lymphoblastic leukemia, pancytopenia, and disseminated fungal infection. The patient was discharged home with hospice care.



Mucormycosis is an infection caused by fungi that belong to the order Mucorales. The most common genera responsible for human disease are Rhizopus, Mucor, and Rhizomucor, which are organisms ubiquitous in nature and found in soil.1 Mucorales hyphae are widely branched and primarily nonseptate, which distinguishes them from hyphae of ascomycetous molds such as Aspergillus, which are narrowly branched and septate.
Mucormycosis primarily affects immunocompromised individuals. The overall incidence of mucormycosis is difficult to estimate, and the risk for infection varies based on the patient population. For example, the incidence of mucormycosis in hematologic malignancy ranges from 1% to 8% and from 0.4% to 16.0% in solid organ transplant recipients.2 One large series of 929 cases noted that the most common risk factors were associated with impaired immune function including diabetes mellitus and diabetic ketoacidosis (36% of cases), hematologic malignancy (17%), and solid organ (7%) or bone marrow transplantation (5%). Other risk factors include neutropenia, steroid therapy, and other immunocompromising conditions.3 Healthy individuals have a strong natural immunity to mucormycosis and rarely are affected by the disease.2
The host response to Mucorales is primarily driven by phagocyte-mediated killing via oxidative metabolites and cationic peptides called defensins.1 Thus, severely neutropenic patients are at high risk for developing mucormycosis.1 In contrast, it appears as though AIDS patients are not at increased risk for mucormycosis, supporting the theory that T lymphocytes are not involved in the host response.1 The conditions of diabetic ketoacidosis leave patients susceptible to mucormycosis for several reasons. First, hyperglycemia and low pH induce phagocyte dysfunction and thus inhibit the host response to Mucorales.4 Second, these organisms have an active ketone reductase system that may allow them to grow more readily in high glucose, acidic conditions.1 Third, diabetic ketoacidosis conditions increase serum free iron, and Mucorales utilizes host iron for cell growth and development.1 Individuals such as hemodialysis patients receiving the iron chelator deferoxamine also are at risk for mucormycosis, as Rhizopus can bind to this molecule and transport the bound iron intracellularly for growth utilization.1
Mucormycosis infection is characterized by infarction and rapid necrosis of host tissues resulting from vascular infiltration by fungal hyphae. The most common site of infection is rhino-orbital-cerebral (39%), followed by lungs (24%) and skin (19%).3 Dissemination occurs in 23% of cases.3 Inoculation most commonly occurs via inhalation of airborne fungal spores by an immunocompromised host with resultant fungal proliferation in the paranasal sinuses, bronchioles, or alveoli. Gastrointestinal tract infection is presumed to occur via ingestion of spores.5
Cutaneous infection, as in our patient, occurs via the inoculation of spores into the dermis through breaks in the skin such as from intravenous lines, urinary catheters, injection sites, surgical sites, and traumatic wounds. Cutaneous infections typically present as a single erythematous, painful, indurated papule that rapidly progresses to a necrotic ulcer with overlying black eschar. In some cases, the progression may be more indolent over the course of several weeks.2 There are few reported cases of primary vulvar mucormycosis, as in our patient.6,7 The previously reported cases involved severely immunocompromised patients who developed large necrotic lesions over the vulva that demonstrated widely branching, nonseptate hyphae on histologic examination. Each patient required extensive surgical debridement with systemic antifungal treatment.6,7
A timely diagnosis of mucormycosis often hinges on a high index of suspicion on behalf of the clinician. A fungal etiology always should be considered for an infection in an immunocompromised patient. Furthermore, nonresponse to antibiotic treatment should be an important diagnostic clue that the infection could be fungal in origin. The definitive diagnosis of mucormycosis is confirmed by tissue biopsy and the presence of broad, widely branching, nonseptate hyphae seen on histopathologic examination.
Treatment involves aggressive surgical debridement of all necrotic tissues and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and immunosuppressive medications. Early initiation of antifungal therapy with the lipid formulation of amphotericin B is recommended. Oral posaconazole or isavuconazole typically are used as step-down therapy after a favorable clinical response with initial amphotericin B treatment. Deferasirox, in contrast to deferoxamine, is an iron chelator that may reduce the pathogenicity of Mucorales and may help as an adjunctive therapy.8 In addition, hyperbaric oxygen therapy may have limited benefit in some cases.9 In spite of these treatments, the overall mortality of mucormycosis is 50% or higher and approaches nearly 100% in cases of disseminated disease, such as in our patient.1,3
The Diagnosis: Mucormycosis
Skin biopsy and histology revealed broad, wide-angle, branched, nonseptate hyphae suggestive of mucormycosis infection (Figure 1). Computed tomography of the abdomen and pelvis revealed marked stranding in the vulvar region and urothelial thickening and enhancement suggestive of infection (Figure 2). Computed tomography of the chest demonstrated multiple irregular nodules in the bilateral upper lobes consistent with disseminated mucormycosis (Figure 3). The patient was started on intravenous amphotericin B and posaconazole. Surgery was not pursued given the poor prognosis of her refractory acute lymphoblastic leukemia, pancytopenia, and disseminated fungal infection. The patient was discharged home with hospice care.



Mucormycosis is an infection caused by fungi that belong to the order Mucorales. The most common genera responsible for human disease are Rhizopus, Mucor, and Rhizomucor, which are organisms ubiquitous in nature and found in soil.1 Mucorales hyphae are widely branched and primarily nonseptate, which distinguishes them from hyphae of ascomycetous molds such as Aspergillus, which are narrowly branched and septate.
Mucormycosis primarily affects immunocompromised individuals. The overall incidence of mucormycosis is difficult to estimate, and the risk for infection varies based on the patient population. For example, the incidence of mucormycosis in hematologic malignancy ranges from 1% to 8% and from 0.4% to 16.0% in solid organ transplant recipients.2 One large series of 929 cases noted that the most common risk factors were associated with impaired immune function including diabetes mellitus and diabetic ketoacidosis (36% of cases), hematologic malignancy (17%), and solid organ (7%) or bone marrow transplantation (5%). Other risk factors include neutropenia, steroid therapy, and other immunocompromising conditions.3 Healthy individuals have a strong natural immunity to mucormycosis and rarely are affected by the disease.2
The host response to Mucorales is primarily driven by phagocyte-mediated killing via oxidative metabolites and cationic peptides called defensins.1 Thus, severely neutropenic patients are at high risk for developing mucormycosis.1 In contrast, it appears as though AIDS patients are not at increased risk for mucormycosis, supporting the theory that T lymphocytes are not involved in the host response.1 The conditions of diabetic ketoacidosis leave patients susceptible to mucormycosis for several reasons. First, hyperglycemia and low pH induce phagocyte dysfunction and thus inhibit the host response to Mucorales.4 Second, these organisms have an active ketone reductase system that may allow them to grow more readily in high glucose, acidic conditions.1 Third, diabetic ketoacidosis conditions increase serum free iron, and Mucorales utilizes host iron for cell growth and development.1 Individuals such as hemodialysis patients receiving the iron chelator deferoxamine also are at risk for mucormycosis, as Rhizopus can bind to this molecule and transport the bound iron intracellularly for growth utilization.1
Mucormycosis infection is characterized by infarction and rapid necrosis of host tissues resulting from vascular infiltration by fungal hyphae. The most common site of infection is rhino-orbital-cerebral (39%), followed by lungs (24%) and skin (19%).3 Dissemination occurs in 23% of cases.3 Inoculation most commonly occurs via inhalation of airborne fungal spores by an immunocompromised host with resultant fungal proliferation in the paranasal sinuses, bronchioles, or alveoli. Gastrointestinal tract infection is presumed to occur via ingestion of spores.5
Cutaneous infection, as in our patient, occurs via the inoculation of spores into the dermis through breaks in the skin such as from intravenous lines, urinary catheters, injection sites, surgical sites, and traumatic wounds. Cutaneous infections typically present as a single erythematous, painful, indurated papule that rapidly progresses to a necrotic ulcer with overlying black eschar. In some cases, the progression may be more indolent over the course of several weeks.2 There are few reported cases of primary vulvar mucormycosis, as in our patient.6,7 The previously reported cases involved severely immunocompromised patients who developed large necrotic lesions over the vulva that demonstrated widely branching, nonseptate hyphae on histologic examination. Each patient required extensive surgical debridement with systemic antifungal treatment.6,7
A timely diagnosis of mucormycosis often hinges on a high index of suspicion on behalf of the clinician. A fungal etiology always should be considered for an infection in an immunocompromised patient. Furthermore, nonresponse to antibiotic treatment should be an important diagnostic clue that the infection could be fungal in origin. The definitive diagnosis of mucormycosis is confirmed by tissue biopsy and the presence of broad, widely branching, nonseptate hyphae seen on histopathologic examination.
Treatment involves aggressive surgical debridement of all necrotic tissues and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and immunosuppressive medications. Early initiation of antifungal therapy with the lipid formulation of amphotericin B is recommended. Oral posaconazole or isavuconazole typically are used as step-down therapy after a favorable clinical response with initial amphotericin B treatment. Deferasirox, in contrast to deferoxamine, is an iron chelator that may reduce the pathogenicity of Mucorales and may help as an adjunctive therapy.8 In addition, hyperbaric oxygen therapy may have limited benefit in some cases.9 In spite of these treatments, the overall mortality of mucormycosis is 50% or higher and approaches nearly 100% in cases of disseminated disease, such as in our patient.1,3
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-1129.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus [published online July 29, 2009]. J Clin Microbiol. 2009;47:2834-2843.
- Colon M, Romaguera J, Mendez K, et al. Mucormycosis of the vulva in an immunocompromised pediatric patient. Bol Asoc Med P R. 2013;105:65-67.
- Nomura J, Ruskin J, Sahebi F, et al. Mucormycosis of the vulva following bone marrow transplantation. Bone Marrow Transplant. 1997;19:859-860.
- Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53:3122-3125.
- Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-1129.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus [published online July 29, 2009]. J Clin Microbiol. 2009;47:2834-2843.
- Colon M, Romaguera J, Mendez K, et al. Mucormycosis of the vulva in an immunocompromised pediatric patient. Bol Asoc Med P R. 2013;105:65-67.
- Nomura J, Ruskin J, Sahebi F, et al. Mucormycosis of the vulva following bone marrow transplantation. Bone Marrow Transplant. 1997;19:859-860.
- Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53:3122-3125.
- Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
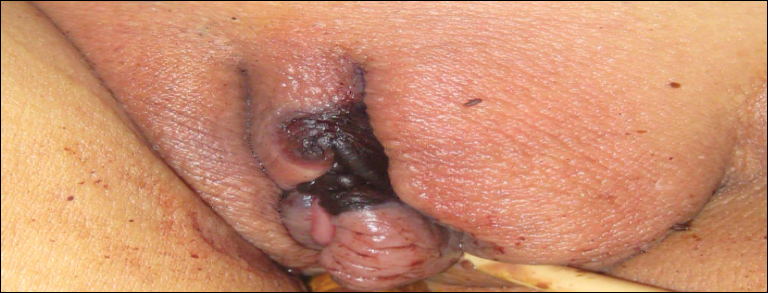
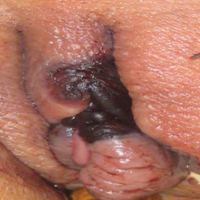
A 48-year-old woman with relapsed T-cell acute lymphoblastic leukemia was admitted to the oncology service for salvage chemotherapy and allogeneic stem cell transplant. Her admission was complicated by extended-spectrum β-lactamase-producing Escherichia coli sepsis and persistent pancytopenia, which required transfer to the intensive care unit. After 2 weeks and while still in the intensive care unit, she developed a painful necrotic vulvar ulcer over the right labia and clitoris that progressed and formed an overlying black eschar.
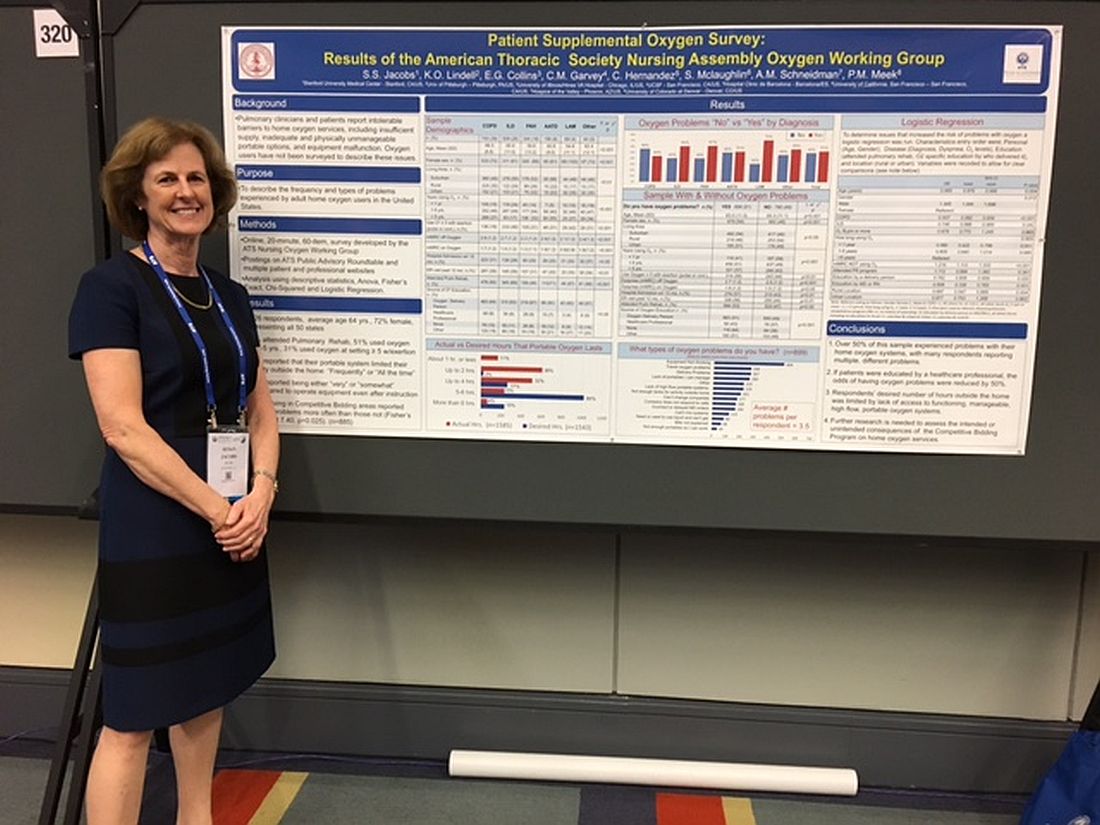
Patients report issues with home O2
WASHINGTON – Patient education in the use of home oxygen halves the number of system use issues reported by patients, based on results of a survey of nearly 2,000 patients.
Pulmonary clinicians and patients report “intolerable barriers to home oxygen services,” lead researcher Susan S. Jacobs, RN, MS, said in a poster session at an international conference of the American Thoracic Society. These barriers include insufficient oxygen supply, inadequate and physically unmanageable portable options, and equipment malfunction.
“We’ve demonstrated that, if the patients are educated by a health care professional, the problems with oxygen go down, Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University, said in an interview. “While physicians can provide oxygen for their patients, the patient oxygen education will most likely lie with the nurses and respiratory therapists.”
Of patients who responded to the survey question "Do you have oxygen problems?" 51% (899) said yes*. On average, these patients said they had experienced 3.5 types of problems with their systems.
Patients who were educated by a health care professional reported fewer problems and were more likely to report having no problems with their oxygen system. Of the patients who received oxygen therapy instruction from a health care professional, 76 (57%) did not report having any issues with their system. In contrast, of the patients who received no instruction, 116 (64%) said they had problems with their oxygen.
Most survey participants (1,113 patients) received oxygen therapy instruction from an oxygen delivery person instead of a health care professional. This group’s opinions about their oxygen systems were split, with 51% (563 patients) experiencing issues with their systems. The other 49% reported no problems.
Survey participants most frequently complained that their equipment was not working; 499 selected this response to the question, “What types of oxygen problems do you have?”
Many patients also reported being unable to spend as much time out of their homes as they wanted. This limitation resulted from their lack of access to functioning, manageable, high flow, portable oxygen systems, according to the researchers. Further, 43% of patients reported that their portable system limited their activity outside the home frequently or all of the time.
“Most of the reported problems were related to respondents not having portable systems that let them be out of their house for more than 2 to 4 hours or [to systems that] were too heavy for the patients to lift up and down their stairs and out of their cars, and they had problems operating them,” said Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University.
The survey respondents also reported experiencing delivery problems, not being able to change the company providing them with oxygen, receiving incorrect or delayed orders from a physician, or being unable to get liquid oxygen. These responses were provided by 267, 177, 166, and 68 patients, respectively.
“There is a lot of confusion for the physicians as well as the nurses about what types of systems the patients can use [and] the pros and cons of each system. There’s lots of confusion and time spent about getting the initial orders right, getting them set up with a supplier, and ensuring the patient gets the equipment that was ordered. There is a lot of back and forth, which results in a delay to the patient, and the patients are upset because they are waiting for their oxygen supply,” she explained. “So, I think that physicians are very much wanting clarification to streamline the process and identify what patient systems are appropriate, which are high flow, [and] what their patients’ needs are to help physicians spend less time on this and help the patients get their oxygen set up in a timely manner.”
The study participants came from all 50 states and were 64 years of age on average and mostly women. A high percentage (39%) of the sample had chronic obstructive pulmonary disease, while 26% had interstitial lung diseases, 18% had pulmonary arterial hypertension, 8% had alpha-1 antitrypsin deficiency, and 4% had lymphangioleiomyomatosis.
Ms. Jacobs noted that she thought patients would benefit from greater physician knowledge of their prescribing options.
“A physician can dictate exactly what system they want. ... You can try to give [patients] a lighter system, a backpack, a smaller tank, more tanks per week, depending on their lifestyle and their needs. But physicians, a lot of times, like all of us and our patients, [are] not aware of all these choices,” she said, during the interview.
An online resource providing all of the pros and cons of the different types of portable oxygen systems that would be appropriate for physicians, nurses, and patients, as well as an examination of the quality standards of the oxygen suppliers, are needed, she noted
Ms. Jacobs reported no financial disclosures.
*This article was corrected June 16, 2017
WASHINGTON – Patient education in the use of home oxygen halves the number of system use issues reported by patients, based on results of a survey of nearly 2,000 patients.
Pulmonary clinicians and patients report “intolerable barriers to home oxygen services,” lead researcher Susan S. Jacobs, RN, MS, said in a poster session at an international conference of the American Thoracic Society. These barriers include insufficient oxygen supply, inadequate and physically unmanageable portable options, and equipment malfunction.
“We’ve demonstrated that, if the patients are educated by a health care professional, the problems with oxygen go down, Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University, said in an interview. “While physicians can provide oxygen for their patients, the patient oxygen education will most likely lie with the nurses and respiratory therapists.”
Of patients who responded to the survey question "Do you have oxygen problems?" 51% (899) said yes*. On average, these patients said they had experienced 3.5 types of problems with their systems.
Patients who were educated by a health care professional reported fewer problems and were more likely to report having no problems with their oxygen system. Of the patients who received oxygen therapy instruction from a health care professional, 76 (57%) did not report having any issues with their system. In contrast, of the patients who received no instruction, 116 (64%) said they had problems with their oxygen.
Most survey participants (1,113 patients) received oxygen therapy instruction from an oxygen delivery person instead of a health care professional. This group’s opinions about their oxygen systems were split, with 51% (563 patients) experiencing issues with their systems. The other 49% reported no problems.
Survey participants most frequently complained that their equipment was not working; 499 selected this response to the question, “What types of oxygen problems do you have?”
Many patients also reported being unable to spend as much time out of their homes as they wanted. This limitation resulted from their lack of access to functioning, manageable, high flow, portable oxygen systems, according to the researchers. Further, 43% of patients reported that their portable system limited their activity outside the home frequently or all of the time.
“Most of the reported problems were related to respondents not having portable systems that let them be out of their house for more than 2 to 4 hours or [to systems that] were too heavy for the patients to lift up and down their stairs and out of their cars, and they had problems operating them,” said Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University.
The survey respondents also reported experiencing delivery problems, not being able to change the company providing them with oxygen, receiving incorrect or delayed orders from a physician, or being unable to get liquid oxygen. These responses were provided by 267, 177, 166, and 68 patients, respectively.
“There is a lot of confusion for the physicians as well as the nurses about what types of systems the patients can use [and] the pros and cons of each system. There’s lots of confusion and time spent about getting the initial orders right, getting them set up with a supplier, and ensuring the patient gets the equipment that was ordered. There is a lot of back and forth, which results in a delay to the patient, and the patients are upset because they are waiting for their oxygen supply,” she explained. “So, I think that physicians are very much wanting clarification to streamline the process and identify what patient systems are appropriate, which are high flow, [and] what their patients’ needs are to help physicians spend less time on this and help the patients get their oxygen set up in a timely manner.”
The study participants came from all 50 states and were 64 years of age on average and mostly women. A high percentage (39%) of the sample had chronic obstructive pulmonary disease, while 26% had interstitial lung diseases, 18% had pulmonary arterial hypertension, 8% had alpha-1 antitrypsin deficiency, and 4% had lymphangioleiomyomatosis.
Ms. Jacobs noted that she thought patients would benefit from greater physician knowledge of their prescribing options.
“A physician can dictate exactly what system they want. ... You can try to give [patients] a lighter system, a backpack, a smaller tank, more tanks per week, depending on their lifestyle and their needs. But physicians, a lot of times, like all of us and our patients, [are] not aware of all these choices,” she said, during the interview.
An online resource providing all of the pros and cons of the different types of portable oxygen systems that would be appropriate for physicians, nurses, and patients, as well as an examination of the quality standards of the oxygen suppliers, are needed, she noted
Ms. Jacobs reported no financial disclosures.
*This article was corrected June 16, 2017
WASHINGTON – Patient education in the use of home oxygen halves the number of system use issues reported by patients, based on results of a survey of nearly 2,000 patients.
Pulmonary clinicians and patients report “intolerable barriers to home oxygen services,” lead researcher Susan S. Jacobs, RN, MS, said in a poster session at an international conference of the American Thoracic Society. These barriers include insufficient oxygen supply, inadequate and physically unmanageable portable options, and equipment malfunction.
“We’ve demonstrated that, if the patients are educated by a health care professional, the problems with oxygen go down, Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University, said in an interview. “While physicians can provide oxygen for their patients, the patient oxygen education will most likely lie with the nurses and respiratory therapists.”
Of patients who responded to the survey question "Do you have oxygen problems?" 51% (899) said yes*. On average, these patients said they had experienced 3.5 types of problems with their systems.
Patients who were educated by a health care professional reported fewer problems and were more likely to report having no problems with their oxygen system. Of the patients who received oxygen therapy instruction from a health care professional, 76 (57%) did not report having any issues with their system. In contrast, of the patients who received no instruction, 116 (64%) said they had problems with their oxygen.
Most survey participants (1,113 patients) received oxygen therapy instruction from an oxygen delivery person instead of a health care professional. This group’s opinions about their oxygen systems were split, with 51% (563 patients) experiencing issues with their systems. The other 49% reported no problems.
Survey participants most frequently complained that their equipment was not working; 499 selected this response to the question, “What types of oxygen problems do you have?”
Many patients also reported being unable to spend as much time out of their homes as they wanted. This limitation resulted from their lack of access to functioning, manageable, high flow, portable oxygen systems, according to the researchers. Further, 43% of patients reported that their portable system limited their activity outside the home frequently or all of the time.
“Most of the reported problems were related to respondents not having portable systems that let them be out of their house for more than 2 to 4 hours or [to systems that] were too heavy for the patients to lift up and down their stairs and out of their cars, and they had problems operating them,” said Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University.
The survey respondents also reported experiencing delivery problems, not being able to change the company providing them with oxygen, receiving incorrect or delayed orders from a physician, or being unable to get liquid oxygen. These responses were provided by 267, 177, 166, and 68 patients, respectively.
“There is a lot of confusion for the physicians as well as the nurses about what types of systems the patients can use [and] the pros and cons of each system. There’s lots of confusion and time spent about getting the initial orders right, getting them set up with a supplier, and ensuring the patient gets the equipment that was ordered. There is a lot of back and forth, which results in a delay to the patient, and the patients are upset because they are waiting for their oxygen supply,” she explained. “So, I think that physicians are very much wanting clarification to streamline the process and identify what patient systems are appropriate, which are high flow, [and] what their patients’ needs are to help physicians spend less time on this and help the patients get their oxygen set up in a timely manner.”
The study participants came from all 50 states and were 64 years of age on average and mostly women. A high percentage (39%) of the sample had chronic obstructive pulmonary disease, while 26% had interstitial lung diseases, 18% had pulmonary arterial hypertension, 8% had alpha-1 antitrypsin deficiency, and 4% had lymphangioleiomyomatosis.
Ms. Jacobs noted that she thought patients would benefit from greater physician knowledge of their prescribing options.
“A physician can dictate exactly what system they want. ... You can try to give [patients] a lighter system, a backpack, a smaller tank, more tanks per week, depending on their lifestyle and their needs. But physicians, a lot of times, like all of us and our patients, [are] not aware of all these choices,” she said, during the interview.
An online resource providing all of the pros and cons of the different types of portable oxygen systems that would be appropriate for physicians, nurses, and patients, as well as an examination of the quality standards of the oxygen suppliers, are needed, she noted
Ms. Jacobs reported no financial disclosures.
*This article was corrected June 16, 2017
AT ATS 2017
Key clinical point:
Major finding: Patients reported experiencing an average of 3.5 types of problems with their home oxygen systems.
Data source: An analysis of 1,926 home-oxygen users’ responses to an online, 60-question survey.
Disclosures: Ms. Jacobs reported no financial disclosures.
Pregnancy and MS: How do they affect each other?
NEW ORLEANS – Multiple sclerosis has little to no impact on the ability to conceive, on pregnancy, or on fetal status, according to Patricia K. Coyle, MD.
“That’s very reassuring,” Dr. Coyle said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “We don’t see an increase in birth defects just because the mother has MS. There is no consistent increase in abortions, ectopic pregnancies, or assisted vaginal/cesarean deliveries.”
Dr. Coyle, director of the MS Comprehensive Care Center at Stony Brook (N.Y.) University Medical Center, said that the most dramatic changes for pregnant patients with MS occur in the final trimester and mainly involve rising levels of multiple hormones: estrogens, cortisol, progesterone, norepinephrine, and 1,25-dihydroxyvitamin D, which increase late in pregnancy, then rapidly drop off postpartum. This has led to the evaluation of sex hormone therapy for MS.
The impact of other pregnancy factors on MS disease activity remains unknown. One is microchimerism, a maternal-fetal exchange of cells and DNA. “These cells can last for a long time; you can find them in the blood, as well as in the [central nervous system],” Dr. Coyle said. “It’s been reported that fetal microchimerism may be increased in immune-mediated diseases like MS, but we really don’t have a lot of good data.”
Researchers also are studying the impact of changes in the gut microbiota that occur during pregnancy. “Could this be a potential target for MS therapy?” Dr. Coyle asked. “This is in its infancy.”
Counseling tips
She went on to share counseling tips for MS patients of childbearing age, including the fact that some studies report slightly smaller babies born to mothers with MS, while others have not found that association. “This is a question mark, but it doesn’t seem to be a major issue,” she said. One thing you can tell patients for certain is that MS is not inherited. “There are well over 230 genes linked to MS, so there’s a genetic enrichment that can make somebody vulnerable to MS, but there’s no gene that passes on MS,” Dr. Coyle said. “The risk is slightly higher for a first-degree relative, so when a parent has MS, the risk for the child is in the range of 2% to 2.5%, compared with the expected 0.13% in the general population. But there’s a slightly higher risk when you’re a sibling than when you’re a parent – 2.7% – which is speaking to environmental factors having an important impact on genes.”
Controversial data exist as to whether a maternal deficiency in vitamin D poses a risk of MS in the offspring. Dr. Coyle makes it a point to “normalize” vitamin D levels in pregnant MS patients, particularly in white patients. “You’d want to have them on prenatal vitamins and folic acid and tell them not to smoke, to limit their alcohol use, and advise them to have good sleep hygiene.”
Dr. Coyle, vice chair of clinical affairs at Stony Brook University Medical Center, said that up until the 1950s, physicians advised women with MS against having children. “They were told not to get pregnant or to have an abortion, because it was thought to make MS worse,” she said. “It turns out that was fiction. That was completely wrong. Pregnancy has no negative effect on long-term MS prognosis. It may have long-term benefits for relapsing MS, but there are not enough data to comment on its impact on progressive MS. Pregnancy makes it less likely that someone will develop a clinically isolated syndrome, but it may increase the radiologically isolated syndrome risk for clinical attack. That’s based on 7 pregnant patients out of a cohort of 60, so we need further data to explain that.”
Disease-modifying therapies
When it comes to washouts of disease-modifying therapies (DMTs), no one-size-fits-all approach exists. Interferon betas and glatiramer acetate have more than 1,000 pregnancy exposures that yield no evidence for teratogenicity or negative fetal impact. No washout is needed prior to pregnancy. “These agents can be used during pregnancy and breastfeeding,” she said.
The other DMTs paint a somewhat different picture. “There is insufficient pregnancy exposure to the three available oral DMTs to comment definitively on their safety, but there is no clear human teratogenicity to date,” Dr. Coyle said. The conventional washout for fingolimod is 8 weeks. In Dr. Coyle’s opinion, no washout is required with dimethyl fumarate. “The half-life is 40 minutes. There are no good signs of issues. For teriflunomide, it can hang around in individuals for 18-24 months. You should go through an accelerated elimination procedure with oral cholestyramine 8 mg three times a day for 11 days until blood level of the agent is less than 0.02 mcg/mL. Avoid all the orals with breastfeeding.”
Monoclonal antibodies – another form of DMTs – lack sufficient pregnancy exposures to merit comment on safety, but they should not be used during breastfeeding. Natalizumab is a humanized IgG4 antibody that crosses the placenta. “This has been used in several dozen pregnancies because the patients got so bad when they were taken off that it required reinstituting natalizumab even though they were pregnant,” Dr. Coyle said. “Human pregnancy exposures have been associated with transient hematologic issues in the newborn, including anemia, thrombocytopenia, and pancytopenia.” Data indicate that the rate of spontaneous abortion among pregnant women treated with natalizumab was 9%, the rate of major birth defects was 5.05%, and no malformation pattern was observed. The drug is detected in human breast milk and has a half-life of 11 days.
Alemtuzumab is a humanized IgG1 monoclonal antibody that crosses the placenta. The half-life elimination is about 14 days. In transgenic mice, giving alemtuzumab during organogenesis was found to be embryolethal. In human pregnancy, hypothyroidism is a concern. “The recommendation has been to wait 4 months after the last treatment before you try to become pregnant. Alemtuzumab is considered a two-cycle treatment. You don’t get the maximum benefit after the first cycle of 5 days. The complete treatment is the second cycle 3 days.”
Daclizumab, another humanized IgG1 monoclonal antibody, also crosses the placenta. Monkey exposure during gestation led to embryofetal death and decreased fetal growth, “but this was at greater than 30 times the human dose,” she said. “It was found to be excreted in monkey breast milk and the half-life is 21 days.” In humans, there have been 36 exposed women who had 38 pregnancies and 20 live births. The rate of spontaneous abortions/miscarriages was 11%, there were eight elective terminations, two ectopic pregnancies, and one congenital heart defect. “This is very limited data, but nothing that would raise the level of concern,” Dr. Coyle said.
Ocrelizumab, another humanized IgG1 monoclonal antibody, was approved by the Food and Drug Administration in March 2017. Prior studies of anti-CD20 antibodies in human pregnancy noted transient lymphocytopenia and peripheral B cell depletion in the newborns. In studies of pregnant monkeys that used 2 and 10 times human doses during organogenesis, it was associated with B cell depletion in spleen/lymph nodes, Dr. Coyle said. “During organogenesis and throughout the neonatal period, treatment could be associated with perinatal death, some associated with bacterial infection; glomerulonephropathy with inflammation; a decrease in circulating B cells, a decrease in testicular weight, and bone marrow lymphoid follicle formation.” Ocrelizumab is excreted in monkey breast milk and the prescription label suggests a 6-month delay in pregnancy. The drug’s half-life is 26 days.
Dr. Coyle reported that she has served as a consultant for Accordant, Acorda, Bayer, Biogen, Celgene, Genentech/Roche, Genzyme/Sanofi, Novartis, Serono, and Teva. She has also received research support from Actelion, Alkermes, Genentech/Roche, MedDay, the National Institute of Neurological Disorders and Stroke, and Novartis.
NEW ORLEANS – Multiple sclerosis has little to no impact on the ability to conceive, on pregnancy, or on fetal status, according to Patricia K. Coyle, MD.
“That’s very reassuring,” Dr. Coyle said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “We don’t see an increase in birth defects just because the mother has MS. There is no consistent increase in abortions, ectopic pregnancies, or assisted vaginal/cesarean deliveries.”
Dr. Coyle, director of the MS Comprehensive Care Center at Stony Brook (N.Y.) University Medical Center, said that the most dramatic changes for pregnant patients with MS occur in the final trimester and mainly involve rising levels of multiple hormones: estrogens, cortisol, progesterone, norepinephrine, and 1,25-dihydroxyvitamin D, which increase late in pregnancy, then rapidly drop off postpartum. This has led to the evaluation of sex hormone therapy for MS.
The impact of other pregnancy factors on MS disease activity remains unknown. One is microchimerism, a maternal-fetal exchange of cells and DNA. “These cells can last for a long time; you can find them in the blood, as well as in the [central nervous system],” Dr. Coyle said. “It’s been reported that fetal microchimerism may be increased in immune-mediated diseases like MS, but we really don’t have a lot of good data.”
Researchers also are studying the impact of changes in the gut microbiota that occur during pregnancy. “Could this be a potential target for MS therapy?” Dr. Coyle asked. “This is in its infancy.”
Counseling tips
She went on to share counseling tips for MS patients of childbearing age, including the fact that some studies report slightly smaller babies born to mothers with MS, while others have not found that association. “This is a question mark, but it doesn’t seem to be a major issue,” she said. One thing you can tell patients for certain is that MS is not inherited. “There are well over 230 genes linked to MS, so there’s a genetic enrichment that can make somebody vulnerable to MS, but there’s no gene that passes on MS,” Dr. Coyle said. “The risk is slightly higher for a first-degree relative, so when a parent has MS, the risk for the child is in the range of 2% to 2.5%, compared with the expected 0.13% in the general population. But there’s a slightly higher risk when you’re a sibling than when you’re a parent – 2.7% – which is speaking to environmental factors having an important impact on genes.”
Controversial data exist as to whether a maternal deficiency in vitamin D poses a risk of MS in the offspring. Dr. Coyle makes it a point to “normalize” vitamin D levels in pregnant MS patients, particularly in white patients. “You’d want to have them on prenatal vitamins and folic acid and tell them not to smoke, to limit their alcohol use, and advise them to have good sleep hygiene.”
Dr. Coyle, vice chair of clinical affairs at Stony Brook University Medical Center, said that up until the 1950s, physicians advised women with MS against having children. “They were told not to get pregnant or to have an abortion, because it was thought to make MS worse,” she said. “It turns out that was fiction. That was completely wrong. Pregnancy has no negative effect on long-term MS prognosis. It may have long-term benefits for relapsing MS, but there are not enough data to comment on its impact on progressive MS. Pregnancy makes it less likely that someone will develop a clinically isolated syndrome, but it may increase the radiologically isolated syndrome risk for clinical attack. That’s based on 7 pregnant patients out of a cohort of 60, so we need further data to explain that.”
Disease-modifying therapies
When it comes to washouts of disease-modifying therapies (DMTs), no one-size-fits-all approach exists. Interferon betas and glatiramer acetate have more than 1,000 pregnancy exposures that yield no evidence for teratogenicity or negative fetal impact. No washout is needed prior to pregnancy. “These agents can be used during pregnancy and breastfeeding,” she said.
The other DMTs paint a somewhat different picture. “There is insufficient pregnancy exposure to the three available oral DMTs to comment definitively on their safety, but there is no clear human teratogenicity to date,” Dr. Coyle said. The conventional washout for fingolimod is 8 weeks. In Dr. Coyle’s opinion, no washout is required with dimethyl fumarate. “The half-life is 40 minutes. There are no good signs of issues. For teriflunomide, it can hang around in individuals for 18-24 months. You should go through an accelerated elimination procedure with oral cholestyramine 8 mg three times a day for 11 days until blood level of the agent is less than 0.02 mcg/mL. Avoid all the orals with breastfeeding.”
Monoclonal antibodies – another form of DMTs – lack sufficient pregnancy exposures to merit comment on safety, but they should not be used during breastfeeding. Natalizumab is a humanized IgG4 antibody that crosses the placenta. “This has been used in several dozen pregnancies because the patients got so bad when they were taken off that it required reinstituting natalizumab even though they were pregnant,” Dr. Coyle said. “Human pregnancy exposures have been associated with transient hematologic issues in the newborn, including anemia, thrombocytopenia, and pancytopenia.” Data indicate that the rate of spontaneous abortion among pregnant women treated with natalizumab was 9%, the rate of major birth defects was 5.05%, and no malformation pattern was observed. The drug is detected in human breast milk and has a half-life of 11 days.
Alemtuzumab is a humanized IgG1 monoclonal antibody that crosses the placenta. The half-life elimination is about 14 days. In transgenic mice, giving alemtuzumab during organogenesis was found to be embryolethal. In human pregnancy, hypothyroidism is a concern. “The recommendation has been to wait 4 months after the last treatment before you try to become pregnant. Alemtuzumab is considered a two-cycle treatment. You don’t get the maximum benefit after the first cycle of 5 days. The complete treatment is the second cycle 3 days.”
Daclizumab, another humanized IgG1 monoclonal antibody, also crosses the placenta. Monkey exposure during gestation led to embryofetal death and decreased fetal growth, “but this was at greater than 30 times the human dose,” she said. “It was found to be excreted in monkey breast milk and the half-life is 21 days.” In humans, there have been 36 exposed women who had 38 pregnancies and 20 live births. The rate of spontaneous abortions/miscarriages was 11%, there were eight elective terminations, two ectopic pregnancies, and one congenital heart defect. “This is very limited data, but nothing that would raise the level of concern,” Dr. Coyle said.
Ocrelizumab, another humanized IgG1 monoclonal antibody, was approved by the Food and Drug Administration in March 2017. Prior studies of anti-CD20 antibodies in human pregnancy noted transient lymphocytopenia and peripheral B cell depletion in the newborns. In studies of pregnant monkeys that used 2 and 10 times human doses during organogenesis, it was associated with B cell depletion in spleen/lymph nodes, Dr. Coyle said. “During organogenesis and throughout the neonatal period, treatment could be associated with perinatal death, some associated with bacterial infection; glomerulonephropathy with inflammation; a decrease in circulating B cells, a decrease in testicular weight, and bone marrow lymphoid follicle formation.” Ocrelizumab is excreted in monkey breast milk and the prescription label suggests a 6-month delay in pregnancy. The drug’s half-life is 26 days.
Dr. Coyle reported that she has served as a consultant for Accordant, Acorda, Bayer, Biogen, Celgene, Genentech/Roche, Genzyme/Sanofi, Novartis, Serono, and Teva. She has also received research support from Actelion, Alkermes, Genentech/Roche, MedDay, the National Institute of Neurological Disorders and Stroke, and Novartis.
NEW ORLEANS – Multiple sclerosis has little to no impact on the ability to conceive, on pregnancy, or on fetal status, according to Patricia K. Coyle, MD.
“That’s very reassuring,” Dr. Coyle said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “We don’t see an increase in birth defects just because the mother has MS. There is no consistent increase in abortions, ectopic pregnancies, or assisted vaginal/cesarean deliveries.”
Dr. Coyle, director of the MS Comprehensive Care Center at Stony Brook (N.Y.) University Medical Center, said that the most dramatic changes for pregnant patients with MS occur in the final trimester and mainly involve rising levels of multiple hormones: estrogens, cortisol, progesterone, norepinephrine, and 1,25-dihydroxyvitamin D, which increase late in pregnancy, then rapidly drop off postpartum. This has led to the evaluation of sex hormone therapy for MS.
The impact of other pregnancy factors on MS disease activity remains unknown. One is microchimerism, a maternal-fetal exchange of cells and DNA. “These cells can last for a long time; you can find them in the blood, as well as in the [central nervous system],” Dr. Coyle said. “It’s been reported that fetal microchimerism may be increased in immune-mediated diseases like MS, but we really don’t have a lot of good data.”
Researchers also are studying the impact of changes in the gut microbiota that occur during pregnancy. “Could this be a potential target for MS therapy?” Dr. Coyle asked. “This is in its infancy.”
Counseling tips
She went on to share counseling tips for MS patients of childbearing age, including the fact that some studies report slightly smaller babies born to mothers with MS, while others have not found that association. “This is a question mark, but it doesn’t seem to be a major issue,” she said. One thing you can tell patients for certain is that MS is not inherited. “There are well over 230 genes linked to MS, so there’s a genetic enrichment that can make somebody vulnerable to MS, but there’s no gene that passes on MS,” Dr. Coyle said. “The risk is slightly higher for a first-degree relative, so when a parent has MS, the risk for the child is in the range of 2% to 2.5%, compared with the expected 0.13% in the general population. But there’s a slightly higher risk when you’re a sibling than when you’re a parent – 2.7% – which is speaking to environmental factors having an important impact on genes.”
Controversial data exist as to whether a maternal deficiency in vitamin D poses a risk of MS in the offspring. Dr. Coyle makes it a point to “normalize” vitamin D levels in pregnant MS patients, particularly in white patients. “You’d want to have them on prenatal vitamins and folic acid and tell them not to smoke, to limit their alcohol use, and advise them to have good sleep hygiene.”
Dr. Coyle, vice chair of clinical affairs at Stony Brook University Medical Center, said that up until the 1950s, physicians advised women with MS against having children. “They were told not to get pregnant or to have an abortion, because it was thought to make MS worse,” she said. “It turns out that was fiction. That was completely wrong. Pregnancy has no negative effect on long-term MS prognosis. It may have long-term benefits for relapsing MS, but there are not enough data to comment on its impact on progressive MS. Pregnancy makes it less likely that someone will develop a clinically isolated syndrome, but it may increase the radiologically isolated syndrome risk for clinical attack. That’s based on 7 pregnant patients out of a cohort of 60, so we need further data to explain that.”
Disease-modifying therapies
When it comes to washouts of disease-modifying therapies (DMTs), no one-size-fits-all approach exists. Interferon betas and glatiramer acetate have more than 1,000 pregnancy exposures that yield no evidence for teratogenicity or negative fetal impact. No washout is needed prior to pregnancy. “These agents can be used during pregnancy and breastfeeding,” she said.
The other DMTs paint a somewhat different picture. “There is insufficient pregnancy exposure to the three available oral DMTs to comment definitively on their safety, but there is no clear human teratogenicity to date,” Dr. Coyle said. The conventional washout for fingolimod is 8 weeks. In Dr. Coyle’s opinion, no washout is required with dimethyl fumarate. “The half-life is 40 minutes. There are no good signs of issues. For teriflunomide, it can hang around in individuals for 18-24 months. You should go through an accelerated elimination procedure with oral cholestyramine 8 mg three times a day for 11 days until blood level of the agent is less than 0.02 mcg/mL. Avoid all the orals with breastfeeding.”
Monoclonal antibodies – another form of DMTs – lack sufficient pregnancy exposures to merit comment on safety, but they should not be used during breastfeeding. Natalizumab is a humanized IgG4 antibody that crosses the placenta. “This has been used in several dozen pregnancies because the patients got so bad when they were taken off that it required reinstituting natalizumab even though they were pregnant,” Dr. Coyle said. “Human pregnancy exposures have been associated with transient hematologic issues in the newborn, including anemia, thrombocytopenia, and pancytopenia.” Data indicate that the rate of spontaneous abortion among pregnant women treated with natalizumab was 9%, the rate of major birth defects was 5.05%, and no malformation pattern was observed. The drug is detected in human breast milk and has a half-life of 11 days.
Alemtuzumab is a humanized IgG1 monoclonal antibody that crosses the placenta. The half-life elimination is about 14 days. In transgenic mice, giving alemtuzumab during organogenesis was found to be embryolethal. In human pregnancy, hypothyroidism is a concern. “The recommendation has been to wait 4 months after the last treatment before you try to become pregnant. Alemtuzumab is considered a two-cycle treatment. You don’t get the maximum benefit after the first cycle of 5 days. The complete treatment is the second cycle 3 days.”
Daclizumab, another humanized IgG1 monoclonal antibody, also crosses the placenta. Monkey exposure during gestation led to embryofetal death and decreased fetal growth, “but this was at greater than 30 times the human dose,” she said. “It was found to be excreted in monkey breast milk and the half-life is 21 days.” In humans, there have been 36 exposed women who had 38 pregnancies and 20 live births. The rate of spontaneous abortions/miscarriages was 11%, there were eight elective terminations, two ectopic pregnancies, and one congenital heart defect. “This is very limited data, but nothing that would raise the level of concern,” Dr. Coyle said.
Ocrelizumab, another humanized IgG1 monoclonal antibody, was approved by the Food and Drug Administration in March 2017. Prior studies of anti-CD20 antibodies in human pregnancy noted transient lymphocytopenia and peripheral B cell depletion in the newborns. In studies of pregnant monkeys that used 2 and 10 times human doses during organogenesis, it was associated with B cell depletion in spleen/lymph nodes, Dr. Coyle said. “During organogenesis and throughout the neonatal period, treatment could be associated with perinatal death, some associated with bacterial infection; glomerulonephropathy with inflammation; a decrease in circulating B cells, a decrease in testicular weight, and bone marrow lymphoid follicle formation.” Ocrelizumab is excreted in monkey breast milk and the prescription label suggests a 6-month delay in pregnancy. The drug’s half-life is 26 days.
Dr. Coyle reported that she has served as a consultant for Accordant, Acorda, Bayer, Biogen, Celgene, Genentech/Roche, Genzyme/Sanofi, Novartis, Serono, and Teva. She has also received research support from Actelion, Alkermes, Genentech/Roche, MedDay, the National Institute of Neurological Disorders and Stroke, and Novartis.
EXPERT ANALYSIS AT THE CMSC ANNUAL MEETING
Mindfulness May Alleviate Chronic Migraine Associated With Medication Overuse
Mindfulness training is as effective as prophylactic medications for treating chronic migraine associated with medication overuse (CM-MO), according to research published online ahead of print February 4 in the Journal of Headache and Pain.
“Our results further suggest that a mindfulness-based treatment may be comparable to standard pharmacologic prophylaxis with regard to relevant primary outcomes such as headache frequency reduction and reduction in the consumption of acute medications,” said Licia Grazzi, MD, a neurologist at Istituto Neurologico Carlo Besta in Milan.
Research has suggested that mindfulness may be beneficial for headache. Previous studies, however, have been limited by inadequate consideration of several significant end points in chronic headache, such as frequency of headache and consumption of medications for acute headache management, said the authors.
To address these limitations, Dr. Grazzi and colleagues conducted an exploratory clinical trial that compared conventional prophylactic pharmacologic treatment with a mindfulness-based treatment for patients diagnosed with CM-MO. Researchers hypothesized that the mindfulness-based approach would be as effective as conventional prophylactic treatment.
Eligible participants were between ages 18 and 65 and had been diagnosed with CM-MO according to the International Classification of Headache Disorders, third edition (beta version), and had presented for treatment at the Headache Center of the Istituto Neurologico Carlo Besta between February 2014 and June 2015. In addition, participants had a history of chronic migraine for at least 10 years that was associated with overuse of triptans and nonsteroidal anti-inflammatory drugs for a minimum of the past five years.
All patients completed a five-day medication withdrawal program and were encouraged to exercise at least 45 minutes twice a week, to stay properly hydrated, and to consume three meals every day.
Participants were separated into two groups. In one group, patients were treated with prophylactic medications. In the second group, patients participated in a mindfulness-based training that consisted of six weekly sessions of guided mindfulness. Patients were invited to practice mindfulness training for seven to 10 minutes per day. At each follow-up visit, the Headache Impact Test, the Migraine Disability Assessment, the State and Trait Anxiety Inventory, and the Beck Depression Inventory were administered. Patients also kept headache diaries.
A total of 44 patients participated in the study. The average age was 44.5, the average headache frequency per month was 20.5, and the average monthly medication intake was 18.4 pills. Overall, data indicated a similar improvement over time in the mindfulness group and pharmacologic prophylaxis group for headache frequency, use of medication, Migraine Disability Assessment, Headache Impact Test, and Beck Depression Inventory. No changes on State and Trait Anxiety Inventory were reported. Both groups had significant and equivalent proportions of participants who achieved at least 50% reduction of headaches, compared with baseline. The majority of patients in each group no longer satisfied criteria for chronic migraine.
“Our findings support the value of conducting further … well-controlled studies (incorporating random assignment, larger sample sizes, and checks on integrity of treatment),” said Dr. Grazzi. “[Such studies] are warranted to more fully explore the benefits, boundaries, and mechanisms of action for mindfulness in treating chronic migraine by itself and when it is complicated by medication overuse and medical or psychological comorbidities.”
—Erica Tricarico
Suggested Reading
Grazzi L, Sansone E, Raggi A, et al. Mindfulness and pharmacological prophylaxis after withdrawal from medication overuse in patients with chronic migraine: an effectiveness trial with a one-year follow-up. J Headache Pain. 2017;18(1):15.
Mindfulness training is as effective as prophylactic medications for treating chronic migraine associated with medication overuse (CM-MO), according to research published online ahead of print February 4 in the Journal of Headache and Pain.
“Our results further suggest that a mindfulness-based treatment may be comparable to standard pharmacologic prophylaxis with regard to relevant primary outcomes such as headache frequency reduction and reduction in the consumption of acute medications,” said Licia Grazzi, MD, a neurologist at Istituto Neurologico Carlo Besta in Milan.
Research has suggested that mindfulness may be beneficial for headache. Previous studies, however, have been limited by inadequate consideration of several significant end points in chronic headache, such as frequency of headache and consumption of medications for acute headache management, said the authors.
To address these limitations, Dr. Grazzi and colleagues conducted an exploratory clinical trial that compared conventional prophylactic pharmacologic treatment with a mindfulness-based treatment for patients diagnosed with CM-MO. Researchers hypothesized that the mindfulness-based approach would be as effective as conventional prophylactic treatment.
Eligible participants were between ages 18 and 65 and had been diagnosed with CM-MO according to the International Classification of Headache Disorders, third edition (beta version), and had presented for treatment at the Headache Center of the Istituto Neurologico Carlo Besta between February 2014 and June 2015. In addition, participants had a history of chronic migraine for at least 10 years that was associated with overuse of triptans and nonsteroidal anti-inflammatory drugs for a minimum of the past five years.
All patients completed a five-day medication withdrawal program and were encouraged to exercise at least 45 minutes twice a week, to stay properly hydrated, and to consume three meals every day.
Participants were separated into two groups. In one group, patients were treated with prophylactic medications. In the second group, patients participated in a mindfulness-based training that consisted of six weekly sessions of guided mindfulness. Patients were invited to practice mindfulness training for seven to 10 minutes per day. At each follow-up visit, the Headache Impact Test, the Migraine Disability Assessment, the State and Trait Anxiety Inventory, and the Beck Depression Inventory were administered. Patients also kept headache diaries.
A total of 44 patients participated in the study. The average age was 44.5, the average headache frequency per month was 20.5, and the average monthly medication intake was 18.4 pills. Overall, data indicated a similar improvement over time in the mindfulness group and pharmacologic prophylaxis group for headache frequency, use of medication, Migraine Disability Assessment, Headache Impact Test, and Beck Depression Inventory. No changes on State and Trait Anxiety Inventory were reported. Both groups had significant and equivalent proportions of participants who achieved at least 50% reduction of headaches, compared with baseline. The majority of patients in each group no longer satisfied criteria for chronic migraine.
“Our findings support the value of conducting further … well-controlled studies (incorporating random assignment, larger sample sizes, and checks on integrity of treatment),” said Dr. Grazzi. “[Such studies] are warranted to more fully explore the benefits, boundaries, and mechanisms of action for mindfulness in treating chronic migraine by itself and when it is complicated by medication overuse and medical or psychological comorbidities.”
—Erica Tricarico
Suggested Reading
Grazzi L, Sansone E, Raggi A, et al. Mindfulness and pharmacological prophylaxis after withdrawal from medication overuse in patients with chronic migraine: an effectiveness trial with a one-year follow-up. J Headache Pain. 2017;18(1):15.
Mindfulness training is as effective as prophylactic medications for treating chronic migraine associated with medication overuse (CM-MO), according to research published online ahead of print February 4 in the Journal of Headache and Pain.
“Our results further suggest that a mindfulness-based treatment may be comparable to standard pharmacologic prophylaxis with regard to relevant primary outcomes such as headache frequency reduction and reduction in the consumption of acute medications,” said Licia Grazzi, MD, a neurologist at Istituto Neurologico Carlo Besta in Milan.
Research has suggested that mindfulness may be beneficial for headache. Previous studies, however, have been limited by inadequate consideration of several significant end points in chronic headache, such as frequency of headache and consumption of medications for acute headache management, said the authors.
To address these limitations, Dr. Grazzi and colleagues conducted an exploratory clinical trial that compared conventional prophylactic pharmacologic treatment with a mindfulness-based treatment for patients diagnosed with CM-MO. Researchers hypothesized that the mindfulness-based approach would be as effective as conventional prophylactic treatment.
Eligible participants were between ages 18 and 65 and had been diagnosed with CM-MO according to the International Classification of Headache Disorders, third edition (beta version), and had presented for treatment at the Headache Center of the Istituto Neurologico Carlo Besta between February 2014 and June 2015. In addition, participants had a history of chronic migraine for at least 10 years that was associated with overuse of triptans and nonsteroidal anti-inflammatory drugs for a minimum of the past five years.
All patients completed a five-day medication withdrawal program and were encouraged to exercise at least 45 minutes twice a week, to stay properly hydrated, and to consume three meals every day.
Participants were separated into two groups. In one group, patients were treated with prophylactic medications. In the second group, patients participated in a mindfulness-based training that consisted of six weekly sessions of guided mindfulness. Patients were invited to practice mindfulness training for seven to 10 minutes per day. At each follow-up visit, the Headache Impact Test, the Migraine Disability Assessment, the State and Trait Anxiety Inventory, and the Beck Depression Inventory were administered. Patients also kept headache diaries.
A total of 44 patients participated in the study. The average age was 44.5, the average headache frequency per month was 20.5, and the average monthly medication intake was 18.4 pills. Overall, data indicated a similar improvement over time in the mindfulness group and pharmacologic prophylaxis group for headache frequency, use of medication, Migraine Disability Assessment, Headache Impact Test, and Beck Depression Inventory. No changes on State and Trait Anxiety Inventory were reported. Both groups had significant and equivalent proportions of participants who achieved at least 50% reduction of headaches, compared with baseline. The majority of patients in each group no longer satisfied criteria for chronic migraine.
“Our findings support the value of conducting further … well-controlled studies (incorporating random assignment, larger sample sizes, and checks on integrity of treatment),” said Dr. Grazzi. “[Such studies] are warranted to more fully explore the benefits, boundaries, and mechanisms of action for mindfulness in treating chronic migraine by itself and when it is complicated by medication overuse and medical or psychological comorbidities.”
—Erica Tricarico
Suggested Reading
Grazzi L, Sansone E, Raggi A, et al. Mindfulness and pharmacological prophylaxis after withdrawal from medication overuse in patients with chronic migraine: an effectiveness trial with a one-year follow-up. J Headache Pain. 2017;18(1):15.
ABIM finds 69% concordance between MOC questions and common practice
, according to new research conducted and funded by the board.
“In this study comparing the percentages of 186 categories of medical conditions seen by general internists in office visits and hospital stays with the percentages of 3,461 questions on IM-MOC examinations from 2010 to 2013, 69% of examination questions were concordant with conditions seen,” Bradley Gray, PhD, ABIM senior health services researcher, Philadelphia, and his colleagues said in a report published in JAMA (2017 Jun 13;317[22]:2317-24).
Questions’ discordance with conditions seen specifically in the office outpatient setting and in the hospital was greater. Comparing questions and office-based practice, 58% of questions (2,010) involving 145 conditions were categorized as concordant. Comparing questions and hospital stays only, 42% of questions (1,456) involving 122 conditions were categorized as concordant.
The study did not evaluate the importance of the conditions that were found in questions that were in discordance with what physicians see in practice.
“Most of the discordant conditions where the frequency of questions on the exam was greater than the frequency of conditions seen were conditions that may have been uncommon but were rated as extremely important to patient care by internists responding to a survey involving review of exam blueprints,” Marianne Green, MD, study coauthor and ABIM board member, said in a statement. “An example of this is diagnosis of vasculitis, a rare but painful condition that can slow vital blood supply to tissues and organs. Based on physicians’ input, these conditions continue to be included on the exam.”
Conversely, there are questions that appear infrequently despite the fact that they cover conditions commonly seen in practice.
“For example, the question percentage for hypertension was judged to be concordant because it was similar to hospital stay percentage (1.91% vs. 1.84%), even though the question percentage was much lower than office visit percentage for this condition (1.91% vs. 13.87%),” the authors stated in the report.
ABIM researchers added that certain common conditions do not require more questions “because care guidelines are widely disseminated and more questions in these areas may be repetitive in terms of content and, therefore, do not contribute significantly to the assessment of a physician’s clinical judgment, especially when limited testing time is available.”
The overall percentage was a reasonable range, Andrea Paul, MD, chief medical officer of BoardVitals, a New York-based company that helps doctors prepare for MOC exams, said in an interview.
“Aiming for somewhere between 65% and 75% on commonly experienced patient conditions is reasonable,” Dr. Paul said. “The reason for that is that those common things that physicians see regularly in practice are just that – they are regularly practiced. So, they don’t necessarily need as much review and testing on topics that they are maintaining their own knowledge of on a daily basis.”
It is important for maintenance of certification exams to cover the rarer conditions, compared with what doctors see regularly, she added.
“I think the exams are aiming to cover those in greater detail because, if a rare condition were to walk into someone’s practice, there would be a great lapse in reviewing those obscure or rare conditions, and it might lead to a delay in diagnosing it or a delay in treating one of those conditions,” Dr. Paul said. “While people find it a nuisance to review something they don’t see regularly, that’s the reason that it’s important.”
The study’s findings have helped inform the review of the MOC testing blueprint that began in 2015, the ABIM noted, so the results may not match the current rate of concordance between questions asked and conditions that physicians are seeing. The report’s authors added that not enough new exams have been administered and not enough information on office visits has been made available to determine the latest concordance rates.
Dr. Paul suggested that, even in internal medicine, it might be better to offer examinations that are more specialized.
“Of people taking that exam, a great proportion of them don’t practice in general outpatient internal medicine,” she said. “They are either specialized in oncology or nephrology, or they practice in academia or are researchers. So, although you would think that everyone would have what they define as a general internal medicine practice, a great proportion doesn’t practice general internal medicine, especially outpatient internal medicine.
“The way to get that [concordance] number up would be to create different maintenance of certification exams based on people’s practice type,” Dr. Paul added. “To have an exam that tests what that person’s specific area is ... That would be a way that I could see to improve it.”
All of the researchers are either employed by or affiliated with ABIM. No additional conflicts of interest were disclosed.
In recent years, ABIM examinations have placed greater emphasis on evidence-based medicine. Yet, the ABIM has been too slow to develop rigorous evidence to support the validity of its recertification examination. There will never be a perfect examination, nor will there be a perfect way to assess physician knowledge, understanding, decision making, and clinical skills.
However, the study by Gray et al. represents an important effort to provide evidence on the validity of the recertification examination and its relevance to practicing internists.
The ABIM must strive for continuous improvement and is most relevant and useful when it asks for help and listens to its members, a process that it has been involved with for the past several years. Open, ongoing evaluation of the recertification examination will be essential, along with frank and productive discussion about how to ensure the continuous improvement, excellence, and relevance of the recertification process.
Adam B. Schwartz, MD, of New York University and J. Sanford Schwartz, MD, of the University of Pennsylvania, Philadelphia, made these comments in an editorial (JAMA. 2017 Jun 13;317[22]:2288-9).
In recent years, ABIM examinations have placed greater emphasis on evidence-based medicine. Yet, the ABIM has been too slow to develop rigorous evidence to support the validity of its recertification examination. There will never be a perfect examination, nor will there be a perfect way to assess physician knowledge, understanding, decision making, and clinical skills.
However, the study by Gray et al. represents an important effort to provide evidence on the validity of the recertification examination and its relevance to practicing internists.
The ABIM must strive for continuous improvement and is most relevant and useful when it asks for help and listens to its members, a process that it has been involved with for the past several years. Open, ongoing evaluation of the recertification examination will be essential, along with frank and productive discussion about how to ensure the continuous improvement, excellence, and relevance of the recertification process.
Adam B. Schwartz, MD, of New York University and J. Sanford Schwartz, MD, of the University of Pennsylvania, Philadelphia, made these comments in an editorial (JAMA. 2017 Jun 13;317[22]:2288-9).
In recent years, ABIM examinations have placed greater emphasis on evidence-based medicine. Yet, the ABIM has been too slow to develop rigorous evidence to support the validity of its recertification examination. There will never be a perfect examination, nor will there be a perfect way to assess physician knowledge, understanding, decision making, and clinical skills.
However, the study by Gray et al. represents an important effort to provide evidence on the validity of the recertification examination and its relevance to practicing internists.
The ABIM must strive for continuous improvement and is most relevant and useful when it asks for help and listens to its members, a process that it has been involved with for the past several years. Open, ongoing evaluation of the recertification examination will be essential, along with frank and productive discussion about how to ensure the continuous improvement, excellence, and relevance of the recertification process.
Adam B. Schwartz, MD, of New York University and J. Sanford Schwartz, MD, of the University of Pennsylvania, Philadelphia, made these comments in an editorial (JAMA. 2017 Jun 13;317[22]:2288-9).
, according to new research conducted and funded by the board.
“In this study comparing the percentages of 186 categories of medical conditions seen by general internists in office visits and hospital stays with the percentages of 3,461 questions on IM-MOC examinations from 2010 to 2013, 69% of examination questions were concordant with conditions seen,” Bradley Gray, PhD, ABIM senior health services researcher, Philadelphia, and his colleagues said in a report published in JAMA (2017 Jun 13;317[22]:2317-24).
Questions’ discordance with conditions seen specifically in the office outpatient setting and in the hospital was greater. Comparing questions and office-based practice, 58% of questions (2,010) involving 145 conditions were categorized as concordant. Comparing questions and hospital stays only, 42% of questions (1,456) involving 122 conditions were categorized as concordant.
The study did not evaluate the importance of the conditions that were found in questions that were in discordance with what physicians see in practice.
“Most of the discordant conditions where the frequency of questions on the exam was greater than the frequency of conditions seen were conditions that may have been uncommon but were rated as extremely important to patient care by internists responding to a survey involving review of exam blueprints,” Marianne Green, MD, study coauthor and ABIM board member, said in a statement. “An example of this is diagnosis of vasculitis, a rare but painful condition that can slow vital blood supply to tissues and organs. Based on physicians’ input, these conditions continue to be included on the exam.”
Conversely, there are questions that appear infrequently despite the fact that they cover conditions commonly seen in practice.
“For example, the question percentage for hypertension was judged to be concordant because it was similar to hospital stay percentage (1.91% vs. 1.84%), even though the question percentage was much lower than office visit percentage for this condition (1.91% vs. 13.87%),” the authors stated in the report.
ABIM researchers added that certain common conditions do not require more questions “because care guidelines are widely disseminated and more questions in these areas may be repetitive in terms of content and, therefore, do not contribute significantly to the assessment of a physician’s clinical judgment, especially when limited testing time is available.”
The overall percentage was a reasonable range, Andrea Paul, MD, chief medical officer of BoardVitals, a New York-based company that helps doctors prepare for MOC exams, said in an interview.
“Aiming for somewhere between 65% and 75% on commonly experienced patient conditions is reasonable,” Dr. Paul said. “The reason for that is that those common things that physicians see regularly in practice are just that – they are regularly practiced. So, they don’t necessarily need as much review and testing on topics that they are maintaining their own knowledge of on a daily basis.”
It is important for maintenance of certification exams to cover the rarer conditions, compared with what doctors see regularly, she added.
“I think the exams are aiming to cover those in greater detail because, if a rare condition were to walk into someone’s practice, there would be a great lapse in reviewing those obscure or rare conditions, and it might lead to a delay in diagnosing it or a delay in treating one of those conditions,” Dr. Paul said. “While people find it a nuisance to review something they don’t see regularly, that’s the reason that it’s important.”
The study’s findings have helped inform the review of the MOC testing blueprint that began in 2015, the ABIM noted, so the results may not match the current rate of concordance between questions asked and conditions that physicians are seeing. The report’s authors added that not enough new exams have been administered and not enough information on office visits has been made available to determine the latest concordance rates.
Dr. Paul suggested that, even in internal medicine, it might be better to offer examinations that are more specialized.
“Of people taking that exam, a great proportion of them don’t practice in general outpatient internal medicine,” she said. “They are either specialized in oncology or nephrology, or they practice in academia or are researchers. So, although you would think that everyone would have what they define as a general internal medicine practice, a great proportion doesn’t practice general internal medicine, especially outpatient internal medicine.
“The way to get that [concordance] number up would be to create different maintenance of certification exams based on people’s practice type,” Dr. Paul added. “To have an exam that tests what that person’s specific area is ... That would be a way that I could see to improve it.”
All of the researchers are either employed by or affiliated with ABIM. No additional conflicts of interest were disclosed.
, according to new research conducted and funded by the board.
“In this study comparing the percentages of 186 categories of medical conditions seen by general internists in office visits and hospital stays with the percentages of 3,461 questions on IM-MOC examinations from 2010 to 2013, 69% of examination questions were concordant with conditions seen,” Bradley Gray, PhD, ABIM senior health services researcher, Philadelphia, and his colleagues said in a report published in JAMA (2017 Jun 13;317[22]:2317-24).
Questions’ discordance with conditions seen specifically in the office outpatient setting and in the hospital was greater. Comparing questions and office-based practice, 58% of questions (2,010) involving 145 conditions were categorized as concordant. Comparing questions and hospital stays only, 42% of questions (1,456) involving 122 conditions were categorized as concordant.
The study did not evaluate the importance of the conditions that were found in questions that were in discordance with what physicians see in practice.
“Most of the discordant conditions where the frequency of questions on the exam was greater than the frequency of conditions seen were conditions that may have been uncommon but were rated as extremely important to patient care by internists responding to a survey involving review of exam blueprints,” Marianne Green, MD, study coauthor and ABIM board member, said in a statement. “An example of this is diagnosis of vasculitis, a rare but painful condition that can slow vital blood supply to tissues and organs. Based on physicians’ input, these conditions continue to be included on the exam.”
Conversely, there are questions that appear infrequently despite the fact that they cover conditions commonly seen in practice.
“For example, the question percentage for hypertension was judged to be concordant because it was similar to hospital stay percentage (1.91% vs. 1.84%), even though the question percentage was much lower than office visit percentage for this condition (1.91% vs. 13.87%),” the authors stated in the report.
ABIM researchers added that certain common conditions do not require more questions “because care guidelines are widely disseminated and more questions in these areas may be repetitive in terms of content and, therefore, do not contribute significantly to the assessment of a physician’s clinical judgment, especially when limited testing time is available.”
The overall percentage was a reasonable range, Andrea Paul, MD, chief medical officer of BoardVitals, a New York-based company that helps doctors prepare for MOC exams, said in an interview.
“Aiming for somewhere between 65% and 75% on commonly experienced patient conditions is reasonable,” Dr. Paul said. “The reason for that is that those common things that physicians see regularly in practice are just that – they are regularly practiced. So, they don’t necessarily need as much review and testing on topics that they are maintaining their own knowledge of on a daily basis.”
It is important for maintenance of certification exams to cover the rarer conditions, compared with what doctors see regularly, she added.
“I think the exams are aiming to cover those in greater detail because, if a rare condition were to walk into someone’s practice, there would be a great lapse in reviewing those obscure or rare conditions, and it might lead to a delay in diagnosing it or a delay in treating one of those conditions,” Dr. Paul said. “While people find it a nuisance to review something they don’t see regularly, that’s the reason that it’s important.”
The study’s findings have helped inform the review of the MOC testing blueprint that began in 2015, the ABIM noted, so the results may not match the current rate of concordance between questions asked and conditions that physicians are seeing. The report’s authors added that not enough new exams have been administered and not enough information on office visits has been made available to determine the latest concordance rates.
Dr. Paul suggested that, even in internal medicine, it might be better to offer examinations that are more specialized.
“Of people taking that exam, a great proportion of them don’t practice in general outpatient internal medicine,” she said. “They are either specialized in oncology or nephrology, or they practice in academia or are researchers. So, although you would think that everyone would have what they define as a general internal medicine practice, a great proportion doesn’t practice general internal medicine, especially outpatient internal medicine.
“The way to get that [concordance] number up would be to create different maintenance of certification exams based on people’s practice type,” Dr. Paul added. “To have an exam that tests what that person’s specific area is ... That would be a way that I could see to improve it.”
All of the researchers are either employed by or affiliated with ABIM. No additional conflicts of interest were disclosed.
FROM JAMA
EHR Report: Don’t let the electronic health record do the driving
The secret to the care of the patient ... is in caring for the patient.
-Francis W. Peabody, MD1
Last month I received a call from a man who was upset about the way he was treated in our office. He had presented with depression and felt insulted by one of our resident physicians in the way he had interacted with him during his visit. I offered to see him the next day.
When I walked into the exam room, I noticed that his eyes were bloodshot and he was fidgeting in his chair. He explained that it was difficult for him to address this issue, but he had been taken aback at his previous visit to our office when the doctor who saw him, after introducing himself, proceeded to sit down, open his computer, and start typing. The patient went on to describe that the physician – while staring at his computer screen – first acknowledged that he was being seen for depression and then immediately asked him if he had any plans to commit suicide. He did not have any suicidal plans, but he felt strongly that being asked about suicide as the first question in the doctor’s interview missed the point of his visit. He was having trouble concentrating, he felt down, and he was having difficulty sleeping at night, all contributing to trouble both at work and in his personal life. Suicide was not a concern of his. He shook his head. He said he understood that we, as doctors, had to put information into the computer, but he also felt that the doctor’s main goal during that visit appeared to be to get through the forms on the computer rather than taking care of him. He admonished that physicians also need to remember that there is a patient in the room and that we should pay attention to the patient first. The computer should be second. I couldn’t have said it better myself. I told him that I would look into what happened, and then we continued with his visit.
You can already see where this discussion is going. The odd thing about the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Medicare’s quality payment program, is that, unless we are careful, the result of the program may be the opposite of what it’s intended to accomplish. By leading to an over-focus on documentation of the quality of care, we are at risk of diminishing the quality of care itself. In essence, many of the requirements appear to simply be more advanced versions of the meaningful (meaningless?) use provisions with which we have previously grappled. It is clear that we should assess the quality of care that is given and that physician payment should be influenced by that care. It is also clear that the only reasonable way to measure the care provided is by collecting data from the EHR. The problem is that the sophistication of the EHR has not caught up to the sophistication of our goals.
Our challenge as physicians who care for patients therefore occurs at an individual level for each of us. How do we provide the necessary documentation scattered throughout our digital charts to satisfy reporting requirements, yet still meet the very real needs of patients to have their voices heard and their emotions acknowledged? The Physician Charter by the American Board of Internal Medicine discusses “the primacy of patient welfare” as a core tenant of medical practice. It goes on to state that “administrative exigencies must not compromise this principle.”2 Given competing demands, how do we continue to accomplish these goals which are often in conflict with one another?
We cannot provide an answer to this question because unfortunately – or perhaps fortunately – the answer does not come in the form of a clear algorithm of behaviors or a form that we can click on. However that does not mean that it cannot be done. Simply being mindful of how important personal interaction is to our patients will help us stay focused on patient needs. In fact, one of the most exciting aspects of our digital age (and our use of EHRs) is that the need to actually connect with people is more important than ever, and prioritizing this stands to reward those individuals who continue to pay attention to patients. In a future column, we will discuss suggestions and strategies for integrating the EHR into truly patient-centered care. In the early 1920s, Dr. Francis W. Peabody said, “The treatment of a disease may be entirely impersonal: the care of the patient must be completely personal.”1 Medical competency is essential and documentation is required, but neither alone is sufficient for the care of patients.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. Peabody FW. The care of the patient. JAMA. 1927;88:877-82.
2. The Physician Charter. American Board of Internal Medicine Foundation at http://abimfoundation.org/what-we-do/physician-charter.
The secret to the care of the patient ... is in caring for the patient.
-Francis W. Peabody, MD1
Last month I received a call from a man who was upset about the way he was treated in our office. He had presented with depression and felt insulted by one of our resident physicians in the way he had interacted with him during his visit. I offered to see him the next day.
When I walked into the exam room, I noticed that his eyes were bloodshot and he was fidgeting in his chair. He explained that it was difficult for him to address this issue, but he had been taken aback at his previous visit to our office when the doctor who saw him, after introducing himself, proceeded to sit down, open his computer, and start typing. The patient went on to describe that the physician – while staring at his computer screen – first acknowledged that he was being seen for depression and then immediately asked him if he had any plans to commit suicide. He did not have any suicidal plans, but he felt strongly that being asked about suicide as the first question in the doctor’s interview missed the point of his visit. He was having trouble concentrating, he felt down, and he was having difficulty sleeping at night, all contributing to trouble both at work and in his personal life. Suicide was not a concern of his. He shook his head. He said he understood that we, as doctors, had to put information into the computer, but he also felt that the doctor’s main goal during that visit appeared to be to get through the forms on the computer rather than taking care of him. He admonished that physicians also need to remember that there is a patient in the room and that we should pay attention to the patient first. The computer should be second. I couldn’t have said it better myself. I told him that I would look into what happened, and then we continued with his visit.
You can already see where this discussion is going. The odd thing about the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Medicare’s quality payment program, is that, unless we are careful, the result of the program may be the opposite of what it’s intended to accomplish. By leading to an over-focus on documentation of the quality of care, we are at risk of diminishing the quality of care itself. In essence, many of the requirements appear to simply be more advanced versions of the meaningful (meaningless?) use provisions with which we have previously grappled. It is clear that we should assess the quality of care that is given and that physician payment should be influenced by that care. It is also clear that the only reasonable way to measure the care provided is by collecting data from the EHR. The problem is that the sophistication of the EHR has not caught up to the sophistication of our goals.
Our challenge as physicians who care for patients therefore occurs at an individual level for each of us. How do we provide the necessary documentation scattered throughout our digital charts to satisfy reporting requirements, yet still meet the very real needs of patients to have their voices heard and their emotions acknowledged? The Physician Charter by the American Board of Internal Medicine discusses “the primacy of patient welfare” as a core tenant of medical practice. It goes on to state that “administrative exigencies must not compromise this principle.”2 Given competing demands, how do we continue to accomplish these goals which are often in conflict with one another?
We cannot provide an answer to this question because unfortunately – or perhaps fortunately – the answer does not come in the form of a clear algorithm of behaviors or a form that we can click on. However that does not mean that it cannot be done. Simply being mindful of how important personal interaction is to our patients will help us stay focused on patient needs. In fact, one of the most exciting aspects of our digital age (and our use of EHRs) is that the need to actually connect with people is more important than ever, and prioritizing this stands to reward those individuals who continue to pay attention to patients. In a future column, we will discuss suggestions and strategies for integrating the EHR into truly patient-centered care. In the early 1920s, Dr. Francis W. Peabody said, “The treatment of a disease may be entirely impersonal: the care of the patient must be completely personal.”1 Medical competency is essential and documentation is required, but neither alone is sufficient for the care of patients.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. Peabody FW. The care of the patient. JAMA. 1927;88:877-82.
2. The Physician Charter. American Board of Internal Medicine Foundation at http://abimfoundation.org/what-we-do/physician-charter.
The secret to the care of the patient ... is in caring for the patient.
-Francis W. Peabody, MD1
Last month I received a call from a man who was upset about the way he was treated in our office. He had presented with depression and felt insulted by one of our resident physicians in the way he had interacted with him during his visit. I offered to see him the next day.
When I walked into the exam room, I noticed that his eyes were bloodshot and he was fidgeting in his chair. He explained that it was difficult for him to address this issue, but he had been taken aback at his previous visit to our office when the doctor who saw him, after introducing himself, proceeded to sit down, open his computer, and start typing. The patient went on to describe that the physician – while staring at his computer screen – first acknowledged that he was being seen for depression and then immediately asked him if he had any plans to commit suicide. He did not have any suicidal plans, but he felt strongly that being asked about suicide as the first question in the doctor’s interview missed the point of his visit. He was having trouble concentrating, he felt down, and he was having difficulty sleeping at night, all contributing to trouble both at work and in his personal life. Suicide was not a concern of his. He shook his head. He said he understood that we, as doctors, had to put information into the computer, but he also felt that the doctor’s main goal during that visit appeared to be to get through the forms on the computer rather than taking care of him. He admonished that physicians also need to remember that there is a patient in the room and that we should pay attention to the patient first. The computer should be second. I couldn’t have said it better myself. I told him that I would look into what happened, and then we continued with his visit.
You can already see where this discussion is going. The odd thing about the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Medicare’s quality payment program, is that, unless we are careful, the result of the program may be the opposite of what it’s intended to accomplish. By leading to an over-focus on documentation of the quality of care, we are at risk of diminishing the quality of care itself. In essence, many of the requirements appear to simply be more advanced versions of the meaningful (meaningless?) use provisions with which we have previously grappled. It is clear that we should assess the quality of care that is given and that physician payment should be influenced by that care. It is also clear that the only reasonable way to measure the care provided is by collecting data from the EHR. The problem is that the sophistication of the EHR has not caught up to the sophistication of our goals.
Our challenge as physicians who care for patients therefore occurs at an individual level for each of us. How do we provide the necessary documentation scattered throughout our digital charts to satisfy reporting requirements, yet still meet the very real needs of patients to have their voices heard and their emotions acknowledged? The Physician Charter by the American Board of Internal Medicine discusses “the primacy of patient welfare” as a core tenant of medical practice. It goes on to state that “administrative exigencies must not compromise this principle.”2 Given competing demands, how do we continue to accomplish these goals which are often in conflict with one another?
We cannot provide an answer to this question because unfortunately – or perhaps fortunately – the answer does not come in the form of a clear algorithm of behaviors or a form that we can click on. However that does not mean that it cannot be done. Simply being mindful of how important personal interaction is to our patients will help us stay focused on patient needs. In fact, one of the most exciting aspects of our digital age (and our use of EHRs) is that the need to actually connect with people is more important than ever, and prioritizing this stands to reward those individuals who continue to pay attention to patients. In a future column, we will discuss suggestions and strategies for integrating the EHR into truly patient-centered care. In the early 1920s, Dr. Francis W. Peabody said, “The treatment of a disease may be entirely impersonal: the care of the patient must be completely personal.”1 Medical competency is essential and documentation is required, but neither alone is sufficient for the care of patients.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. Peabody FW. The care of the patient. JAMA. 1927;88:877-82.
2. The Physician Charter. American Board of Internal Medicine Foundation at http://abimfoundation.org/what-we-do/physician-charter.
VIDEO: Social networking offers coping help for endometriosis patients
VANCOUVER – Nearly half of the discussion topics on the social networking site www.myendometriosisteam.com are about pain, but other uncontrolled symptoms are popular topics, including fatigue and depression.
The online social support network includes 30,000 women with endometriosis and offers them a chance to connect with other women with the condition, find a provider, and research treatments. Elise-Marie Menke, director of alliance management at MyHealthTeams, which runs the site, presented data at the World Congress on Endometriosis. Among the findings she presented was how symptoms mapped to a woman’s cycle.
In a video interview, Ms. Menke described how this type of patient-generated data can play a role in the management of disease and serve to highlight unmet needs to physicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VANCOUVER – Nearly half of the discussion topics on the social networking site www.myendometriosisteam.com are about pain, but other uncontrolled symptoms are popular topics, including fatigue and depression.
The online social support network includes 30,000 women with endometriosis and offers them a chance to connect with other women with the condition, find a provider, and research treatments. Elise-Marie Menke, director of alliance management at MyHealthTeams, which runs the site, presented data at the World Congress on Endometriosis. Among the findings she presented was how symptoms mapped to a woman’s cycle.
In a video interview, Ms. Menke described how this type of patient-generated data can play a role in the management of disease and serve to highlight unmet needs to physicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VANCOUVER – Nearly half of the discussion topics on the social networking site www.myendometriosisteam.com are about pain, but other uncontrolled symptoms are popular topics, including fatigue and depression.
The online social support network includes 30,000 women with endometriosis and offers them a chance to connect with other women with the condition, find a provider, and research treatments. Elise-Marie Menke, director of alliance management at MyHealthTeams, which runs the site, presented data at the World Congress on Endometriosis. Among the findings she presented was how symptoms mapped to a woman’s cycle.
In a video interview, Ms. Menke described how this type of patient-generated data can play a role in the management of disease and serve to highlight unmet needs to physicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT WCE 2017