User login
Treatment response rates for psychotic bipolar depression similar to those without
MIAMI – Response rates for psychotic and nonpsychotic depression in bipolar disorder were statistically similar, regardless of treatment, an ad hoc analysis has shown.
Over a 6 month period, results from the multisite, randomized, controlled Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder) study showed that 482 patients anywhere on the bipolar spectrum, given either lithium or quetiapine, had similar treatment response rates over 6 months.
“When you look at the course of the improvement for those with psychosis, they had more severe disorder at baseline,and presented with these symptoms throughout the study. But, when we compare curves of improvement, those with severe disorder responded to treatment at the same pace [as those without psychosis],” Dr. Caldieraro said.
The overall scores for the Bipolar Inventory of Symptoms Scale (BISS) at baseline were 75.2 plus or minus 17.6 percentage points for those with psychosis, vs. 54.9 plus or minus 16.3 for those without (P less than .001). At 6 months, the scores were more in range with one another: 37.2 plus or minus 19.7 for those with psychosis and 26.3 plus or minus 18.0 for those without (P = .003). The BISS depression scores at baseline for those with psychosis were 29.5 plus or minus 7.0, compared with 24.9 plus or minus 8.0 for those without (P = .002). At study end, the scores were 13.0 plus or minus 8.6, vs. 10.9 plus or minus 9.5 (P = .253).
Overall Clinical Global Impressions (CGI) scores for bipolar disorder at baseline in the group with psychosis were 5.1 plus or minus 0.9, compared with 4.5 plus or minus 0.8 in those without (P less than .001). At 6 months, the scores were 3.4 plus or minus 1.3, vs. 2.8 plus or minus 1.3 (P = .032). The CGI scores for depression in the psychosis group at baseline were 4.9 plus or minus 0.9, compared with 4.4 plus or minus 0.9 in the nonpsychosis group (P = .006). At 6 months, the psychosis groups’ scores were 3.1 plus or minus 1.4, compared with 2.6 plus or minus 1.3 in the nonpsychosis group (P = .07).
In addition to either lithium or quetiapine, patients in the CHOICE study also received adjunctive personalized treatment. Patients who received lithium plus APT were not given second-generation antipsychotics, while those given quetiapine plus APT were not given lithium or any other second-generation antipsychotic.
In the quetiapine group, 21 people had psychotic depression at baseline. In the lithium group, there were 11. The time to remission was numerically, although not statistically, similar between the patients with psychosis in the lithium and the quetiapine groups.
Compared with the CHOICE study participants without psychosis, the subanalysis showed that the 32 people with psychotic features were far more likely to be single or never married (P = .036), employed at half the rate (P = .035), twice as likely to suffer from generalized anxiety disorder (P = .028), and more likely to have social phobias (P = .018). People with psychotic depression in the study also were more likely to suffer from agoraphobia.
One reason for his interest in the study, Dr. Caldieraro said, was that, despite the worse prognosis for people on the bipolar spectrum with psychotic depression, the literature on treatment outcomes for this cohort is scant.
“Ours is a small sample, so you could say that we didn’t have enough power, but we have some interesting results,” he said during his presentation. “The results need replication, but the study suggests that maybe, if we make the patient better, it doesn’t matter which medication we use.”
Dr. Caldieraro had no relevant disclosures. The Agency for Healthcare Research and Quality funded the CHOICE study, NCT01331304.
[email protected]
On Twitter @whitneymcknight
MIAMI – Response rates for psychotic and nonpsychotic depression in bipolar disorder were statistically similar, regardless of treatment, an ad hoc analysis has shown.
Over a 6 month period, results from the multisite, randomized, controlled Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder) study showed that 482 patients anywhere on the bipolar spectrum, given either lithium or quetiapine, had similar treatment response rates over 6 months.
“When you look at the course of the improvement for those with psychosis, they had more severe disorder at baseline,and presented with these symptoms throughout the study. But, when we compare curves of improvement, those with severe disorder responded to treatment at the same pace [as those without psychosis],” Dr. Caldieraro said.
The overall scores for the Bipolar Inventory of Symptoms Scale (BISS) at baseline were 75.2 plus or minus 17.6 percentage points for those with psychosis, vs. 54.9 plus or minus 16.3 for those without (P less than .001). At 6 months, the scores were more in range with one another: 37.2 plus or minus 19.7 for those with psychosis and 26.3 plus or minus 18.0 for those without (P = .003). The BISS depression scores at baseline for those with psychosis were 29.5 plus or minus 7.0, compared with 24.9 plus or minus 8.0 for those without (P = .002). At study end, the scores were 13.0 plus or minus 8.6, vs. 10.9 plus or minus 9.5 (P = .253).
Overall Clinical Global Impressions (CGI) scores for bipolar disorder at baseline in the group with psychosis were 5.1 plus or minus 0.9, compared with 4.5 plus or minus 0.8 in those without (P less than .001). At 6 months, the scores were 3.4 plus or minus 1.3, vs. 2.8 plus or minus 1.3 (P = .032). The CGI scores for depression in the psychosis group at baseline were 4.9 plus or minus 0.9, compared with 4.4 plus or minus 0.9 in the nonpsychosis group (P = .006). At 6 months, the psychosis groups’ scores were 3.1 plus or minus 1.4, compared with 2.6 plus or minus 1.3 in the nonpsychosis group (P = .07).
In addition to either lithium or quetiapine, patients in the CHOICE study also received adjunctive personalized treatment. Patients who received lithium plus APT were not given second-generation antipsychotics, while those given quetiapine plus APT were not given lithium or any other second-generation antipsychotic.
In the quetiapine group, 21 people had psychotic depression at baseline. In the lithium group, there were 11. The time to remission was numerically, although not statistically, similar between the patients with psychosis in the lithium and the quetiapine groups.
Compared with the CHOICE study participants without psychosis, the subanalysis showed that the 32 people with psychotic features were far more likely to be single or never married (P = .036), employed at half the rate (P = .035), twice as likely to suffer from generalized anxiety disorder (P = .028), and more likely to have social phobias (P = .018). People with psychotic depression in the study also were more likely to suffer from agoraphobia.
One reason for his interest in the study, Dr. Caldieraro said, was that, despite the worse prognosis for people on the bipolar spectrum with psychotic depression, the literature on treatment outcomes for this cohort is scant.
“Ours is a small sample, so you could say that we didn’t have enough power, but we have some interesting results,” he said during his presentation. “The results need replication, but the study suggests that maybe, if we make the patient better, it doesn’t matter which medication we use.”
Dr. Caldieraro had no relevant disclosures. The Agency for Healthcare Research and Quality funded the CHOICE study, NCT01331304.
[email protected]
On Twitter @whitneymcknight
MIAMI – Response rates for psychotic and nonpsychotic depression in bipolar disorder were statistically similar, regardless of treatment, an ad hoc analysis has shown.
Over a 6 month period, results from the multisite, randomized, controlled Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder) study showed that 482 patients anywhere on the bipolar spectrum, given either lithium or quetiapine, had similar treatment response rates over 6 months.
“When you look at the course of the improvement for those with psychosis, they had more severe disorder at baseline,and presented with these symptoms throughout the study. But, when we compare curves of improvement, those with severe disorder responded to treatment at the same pace [as those without psychosis],” Dr. Caldieraro said.
The overall scores for the Bipolar Inventory of Symptoms Scale (BISS) at baseline were 75.2 plus or minus 17.6 percentage points for those with psychosis, vs. 54.9 plus or minus 16.3 for those without (P less than .001). At 6 months, the scores were more in range with one another: 37.2 plus or minus 19.7 for those with psychosis and 26.3 plus or minus 18.0 for those without (P = .003). The BISS depression scores at baseline for those with psychosis were 29.5 plus or minus 7.0, compared with 24.9 plus or minus 8.0 for those without (P = .002). At study end, the scores were 13.0 plus or minus 8.6, vs. 10.9 plus or minus 9.5 (P = .253).
Overall Clinical Global Impressions (CGI) scores for bipolar disorder at baseline in the group with psychosis were 5.1 plus or minus 0.9, compared with 4.5 plus or minus 0.8 in those without (P less than .001). At 6 months, the scores were 3.4 plus or minus 1.3, vs. 2.8 plus or minus 1.3 (P = .032). The CGI scores for depression in the psychosis group at baseline were 4.9 plus or minus 0.9, compared with 4.4 plus or minus 0.9 in the nonpsychosis group (P = .006). At 6 months, the psychosis groups’ scores were 3.1 plus or minus 1.4, compared with 2.6 plus or minus 1.3 in the nonpsychosis group (P = .07).
In addition to either lithium or quetiapine, patients in the CHOICE study also received adjunctive personalized treatment. Patients who received lithium plus APT were not given second-generation antipsychotics, while those given quetiapine plus APT were not given lithium or any other second-generation antipsychotic.
In the quetiapine group, 21 people had psychotic depression at baseline. In the lithium group, there were 11. The time to remission was numerically, although not statistically, similar between the patients with psychosis in the lithium and the quetiapine groups.
Compared with the CHOICE study participants without psychosis, the subanalysis showed that the 32 people with psychotic features were far more likely to be single or never married (P = .036), employed at half the rate (P = .035), twice as likely to suffer from generalized anxiety disorder (P = .028), and more likely to have social phobias (P = .018). People with psychotic depression in the study also were more likely to suffer from agoraphobia.
One reason for his interest in the study, Dr. Caldieraro said, was that, despite the worse prognosis for people on the bipolar spectrum with psychotic depression, the literature on treatment outcomes for this cohort is scant.
“Ours is a small sample, so you could say that we didn’t have enough power, but we have some interesting results,” he said during his presentation. “The results need replication, but the study suggests that maybe, if we make the patient better, it doesn’t matter which medication we use.”
Dr. Caldieraro had no relevant disclosures. The Agency for Healthcare Research and Quality funded the CHOICE study, NCT01331304.
[email protected]
On Twitter @whitneymcknight
AT THE ASCP ANNUAL MEETING
Key clinical point:
Major finding: Patients with psychotic or nonpsychotic bipolar depression responded equally well to lithium and quetiapine when compared with response rates in patients without bipolar depression.
Data source: A secondary analysis of 32 patients from a multisite, randomized, controlled trial of 482 patients with bipolar disorder I or bipolar II and psychosis, assigned to receive either lithium or quetiapine for 6 months.
Disclosures: Dr. Caldieraro had no relevant disclosures. The Agency for Healthcare Research and Quality funded the CHOICE study, NCT01331304.
Intra-amniotic sludge: Does its presence rule out cerclage for short cervix?
CASE: Woman with short cervix, intra-amniotic sludge, and prior preterm delivery
An asymptomatic 32-year-old woman with a prior preterm delivery, presently pregnant with a singleton at 17 weeks of gestation, underwent transvaginal ultrasonography and was found to have a cervical length of 22 mm and dense intra-amniotic sludge. She received one dose of 17α-hydroxyprogesterone caproate (17P) at 16 weeks of gestation. What are your next steps in management?
Intra-amniotic sludge is a conundrum
Intra-amniotic sludge is a sonographic finding of free-floating, hyperechoic, particulate matter in the amniotic fluid close to the internal os. The precise nature of this material varies, and it may include blood, meconium, or vernix and may signal inflammation or infection. In a retrospective case-control study, 27% of asymptomatic women with sludge and a short cervix had positive amniotic fluid cultures, and 27% had evidence of inflammation in the amniotic fluid (>50 white blood cells/mm3).1 In a separate report, the authors proposed that "the detection of amniotic fluid 'sludge' represents a sign that microbial invasion of the amniotic cavity and an inflammatory process are in progress."2
Benefit of cerclage in high-risk women. Several systematic reviews have highlighted the benefit of cerclage for women with a singleton pregnancy, short cervix, and previous preterm birth or second-trimester loss (ultrasound-indicated cerclage for high-risk women).3 Cerclage is presumed to work by providing some degree of structural support and by maintaining a barrier to protect the fetal membranes against exposure to ascending pathogens.4
Since dense intra-amniotic sludge may represent chronic intra-amniotic infection, can cerclage still be expected to be beneficial when microbiologic invasion of the amniotic cavity already has occurred? Furthermore, intra-amniotic infection has been cited as a possible complication of ultrasound-indicated cerclage, with a rate of 10%.5 The traditional view is that the presence of subclinical intra-amniotic infection may further increase this risk and therefore should be considered a contraindication to cerclage.6
Evaluating the patient for cerclage placement
The patient history and physical examination should focus on the signs and symptoms of labor, vaginal bleeding, amniotic membrane rupture, and intra-amniotic infection. Particular attention should be paid to maternal temperature, pulse, and the presence of uterine tenderness or foul-smelling vaginal discharge. A sterile speculum examination followed by digital examination would complement the ultrasonography evaluation in assessing cervical dilation and effacement. The ultrasonography evaluation should be completed to confirm a viable pregnancy with accurate dating and the absence of detectable fetal anomalies.
Currently, evidence is insufficient for recommending routine amniocentesis to exclude intra-amniotic infection in an asymptomatic woman prior to ultrasound-indicated cerclage, even in the presence of intra-amniotic sludge, as there are no data demonstrating improved outcomes.4 In addition, intra-amniotic sludge has been associated with intra-amniotic infection and/or inflammation in the form of microbial biofilms, which may prevent detection of infection by routine culture techniques.7
Related Article:
Universal cervical length screening–saving babies lives
Study results offer limited guidance
Data are limited on the clinical implications of intra-amniotic sludge in women with cervical cerclage. In a retrospective cohort of 177 patients with cerclage, 60 had evidence of sludge and 46 of those with sludge underwent ultrasound-indicated cerclage.8 There were no significant differences in the mean gestational age at delivery, neonatal outcomes, rate of preterm delivery, preterm premature rupture of membranes, or intra-amniotic infection between women with or without intra-amniotic sludge. A subanalysis was performed comparing women with sludge detected before or after cerclage and, again, no difference was found in measured outcomes.
Similarly, in a small (N = 20) retrospective review of the Arabin pessary used as a noninvasive intervention for short cervix, the presence of intra-amniotic sludge in 5 cases did not appear to impact outcomes.9
Case patient: How would you manage her care?
Based on her obstetric history and ultrasonography findings, the patient described in the case vignette is at high risk for preterm delivery. The presence of both intra-amniotic sludge and short cervix is associated with an increased risk for spontaneous preterm delivery. After evaluating for clinical intra-amniotic infection and performing a work-up for other contraindications to cerclage placement, cerclage placement may be offered--even in the presence of intra-amniotic sludge.
The next practical question is whether 17P, already started, should be continued after cerclage placement. From the literature on 17P, it is unclear whether progesterone provides additional benefit. One randomized, placebo-controlled study in women with at least 2 preterm deliveries or mid-trimester losses and cerclage in place showed that the 17P-treated women had a significant reduction in preterm delivery compared with the control group, from 37.8% to 16.1%.10
By contrast, in a secondary analysis of a randomized trial evaluating cerclage in high-risk women with short cervix in the current pregnancy, addition of 17P to cerclage was not beneficial.11 Results of 2 retrospective cohort studies showed the same lack of difference on preterm delivery rates with the addition of 17P.12,13
Accepting that the interpretation of these data is challenging, in our practice we would choose to continue the progesterone supplementation, siding with other recently expressed expert opinions.14
The bottom line
While clinical intra-amniotic infection is a contraindication to cerclage, there is no evidence to support withholding cerclage from eligible women due to the presence of intra-amniotic fluid sludge alone.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid "sludge" in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30(5):706-714.
- Romero R, Kusanovic JP, Espinoza J, et al. What is amniotic fluid "sludge"? Ultrasound Obstet Gynecol. 2007;30(5):793-798.
- Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2012;4:CD008991.
- Abbott D, To M, Shennan A. Cervical cerclage: a review of current evidence. Aust N Z J Obstet Gynaecol. 2012;52(3):220-223.
- Drassinower D, Poggi SH, Landy HJ, Gilo N, Benson JE, Ghidini A. Perioperative complications of history-indicated and ultrasound-indicated cervical cerclage. Am J Obstet Gynecol. 2011;205(1):53.e1-e5.
- Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95(5):652-655.
- Vaisbuch E, Romero R, Erez IO, et al. Clinical significance of early (<20 weeks) vs late (20-24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36(4):471-481.
- Gorski LA, Huang WH, Iriye BK, Hancock J. Clinical implication of intra-amniotic sludge on ultrasound in patients with cervical cerclage. Ultrasound Obstet Gynecol. 2010;36(4):482-485.
- Ting YH, Lao TT, Wa Law LW, et al. Arabin cerclage pessary in the management of cervical insufficiency. J Matern Fetal Neonatal Med. 2012;25(12):2693-2695.
- Yemini M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 1985;151(5):574-577.
- Berghella V, Figueroa D, Szychowski JM, et al; Vaginal Ultrasound Trial Consortium. 17-alpha-hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol. 2010;202(4):351.e1-e6.
- Rebarber A, Cleary-Goldman J, Istwan NB, et al. The use of 17 alpha-hydroxyprogesterone caproate (17P) in women with cervical cerclage. Am J Perinatol. 2008;25(5):271-275.
- Stetson B, Hibbard JU, Wilkins I, Leftwich H. Outcomes with cerclage alone compared with cerclage plus 17 α-hydroxyprogesterone caproate. Obstet Gynecol. 2016;128(5):983-988.
- Iams JD. Identification of candidates for progesterone: why, who, how, and when? Obstet Gynecol. 2014;123(6):1317-1326.
CASE: Woman with short cervix, intra-amniotic sludge, and prior preterm delivery
An asymptomatic 32-year-old woman with a prior preterm delivery, presently pregnant with a singleton at 17 weeks of gestation, underwent transvaginal ultrasonography and was found to have a cervical length of 22 mm and dense intra-amniotic sludge. She received one dose of 17α-hydroxyprogesterone caproate (17P) at 16 weeks of gestation. What are your next steps in management?
Intra-amniotic sludge is a conundrum
Intra-amniotic sludge is a sonographic finding of free-floating, hyperechoic, particulate matter in the amniotic fluid close to the internal os. The precise nature of this material varies, and it may include blood, meconium, or vernix and may signal inflammation or infection. In a retrospective case-control study, 27% of asymptomatic women with sludge and a short cervix had positive amniotic fluid cultures, and 27% had evidence of inflammation in the amniotic fluid (>50 white blood cells/mm3).1 In a separate report, the authors proposed that "the detection of amniotic fluid 'sludge' represents a sign that microbial invasion of the amniotic cavity and an inflammatory process are in progress."2
Benefit of cerclage in high-risk women. Several systematic reviews have highlighted the benefit of cerclage for women with a singleton pregnancy, short cervix, and previous preterm birth or second-trimester loss (ultrasound-indicated cerclage for high-risk women).3 Cerclage is presumed to work by providing some degree of structural support and by maintaining a barrier to protect the fetal membranes against exposure to ascending pathogens.4
Since dense intra-amniotic sludge may represent chronic intra-amniotic infection, can cerclage still be expected to be beneficial when microbiologic invasion of the amniotic cavity already has occurred? Furthermore, intra-amniotic infection has been cited as a possible complication of ultrasound-indicated cerclage, with a rate of 10%.5 The traditional view is that the presence of subclinical intra-amniotic infection may further increase this risk and therefore should be considered a contraindication to cerclage.6
Evaluating the patient for cerclage placement
The patient history and physical examination should focus on the signs and symptoms of labor, vaginal bleeding, amniotic membrane rupture, and intra-amniotic infection. Particular attention should be paid to maternal temperature, pulse, and the presence of uterine tenderness or foul-smelling vaginal discharge. A sterile speculum examination followed by digital examination would complement the ultrasonography evaluation in assessing cervical dilation and effacement. The ultrasonography evaluation should be completed to confirm a viable pregnancy with accurate dating and the absence of detectable fetal anomalies.
Currently, evidence is insufficient for recommending routine amniocentesis to exclude intra-amniotic infection in an asymptomatic woman prior to ultrasound-indicated cerclage, even in the presence of intra-amniotic sludge, as there are no data demonstrating improved outcomes.4 In addition, intra-amniotic sludge has been associated with intra-amniotic infection and/or inflammation in the form of microbial biofilms, which may prevent detection of infection by routine culture techniques.7
Related Article:
Universal cervical length screening–saving babies lives
Study results offer limited guidance
Data are limited on the clinical implications of intra-amniotic sludge in women with cervical cerclage. In a retrospective cohort of 177 patients with cerclage, 60 had evidence of sludge and 46 of those with sludge underwent ultrasound-indicated cerclage.8 There were no significant differences in the mean gestational age at delivery, neonatal outcomes, rate of preterm delivery, preterm premature rupture of membranes, or intra-amniotic infection between women with or without intra-amniotic sludge. A subanalysis was performed comparing women with sludge detected before or after cerclage and, again, no difference was found in measured outcomes.
Similarly, in a small (N = 20) retrospective review of the Arabin pessary used as a noninvasive intervention for short cervix, the presence of intra-amniotic sludge in 5 cases did not appear to impact outcomes.9
Case patient: How would you manage her care?
Based on her obstetric history and ultrasonography findings, the patient described in the case vignette is at high risk for preterm delivery. The presence of both intra-amniotic sludge and short cervix is associated with an increased risk for spontaneous preterm delivery. After evaluating for clinical intra-amniotic infection and performing a work-up for other contraindications to cerclage placement, cerclage placement may be offered--even in the presence of intra-amniotic sludge.
The next practical question is whether 17P, already started, should be continued after cerclage placement. From the literature on 17P, it is unclear whether progesterone provides additional benefit. One randomized, placebo-controlled study in women with at least 2 preterm deliveries or mid-trimester losses and cerclage in place showed that the 17P-treated women had a significant reduction in preterm delivery compared with the control group, from 37.8% to 16.1%.10
By contrast, in a secondary analysis of a randomized trial evaluating cerclage in high-risk women with short cervix in the current pregnancy, addition of 17P to cerclage was not beneficial.11 Results of 2 retrospective cohort studies showed the same lack of difference on preterm delivery rates with the addition of 17P.12,13
Accepting that the interpretation of these data is challenging, in our practice we would choose to continue the progesterone supplementation, siding with other recently expressed expert opinions.14
The bottom line
While clinical intra-amniotic infection is a contraindication to cerclage, there is no evidence to support withholding cerclage from eligible women due to the presence of intra-amniotic fluid sludge alone.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Woman with short cervix, intra-amniotic sludge, and prior preterm delivery
An asymptomatic 32-year-old woman with a prior preterm delivery, presently pregnant with a singleton at 17 weeks of gestation, underwent transvaginal ultrasonography and was found to have a cervical length of 22 mm and dense intra-amniotic sludge. She received one dose of 17α-hydroxyprogesterone caproate (17P) at 16 weeks of gestation. What are your next steps in management?
Intra-amniotic sludge is a conundrum
Intra-amniotic sludge is a sonographic finding of free-floating, hyperechoic, particulate matter in the amniotic fluid close to the internal os. The precise nature of this material varies, and it may include blood, meconium, or vernix and may signal inflammation or infection. In a retrospective case-control study, 27% of asymptomatic women with sludge and a short cervix had positive amniotic fluid cultures, and 27% had evidence of inflammation in the amniotic fluid (>50 white blood cells/mm3).1 In a separate report, the authors proposed that "the detection of amniotic fluid 'sludge' represents a sign that microbial invasion of the amniotic cavity and an inflammatory process are in progress."2
Benefit of cerclage in high-risk women. Several systematic reviews have highlighted the benefit of cerclage for women with a singleton pregnancy, short cervix, and previous preterm birth or second-trimester loss (ultrasound-indicated cerclage for high-risk women).3 Cerclage is presumed to work by providing some degree of structural support and by maintaining a barrier to protect the fetal membranes against exposure to ascending pathogens.4
Since dense intra-amniotic sludge may represent chronic intra-amniotic infection, can cerclage still be expected to be beneficial when microbiologic invasion of the amniotic cavity already has occurred? Furthermore, intra-amniotic infection has been cited as a possible complication of ultrasound-indicated cerclage, with a rate of 10%.5 The traditional view is that the presence of subclinical intra-amniotic infection may further increase this risk and therefore should be considered a contraindication to cerclage.6
Evaluating the patient for cerclage placement
The patient history and physical examination should focus on the signs and symptoms of labor, vaginal bleeding, amniotic membrane rupture, and intra-amniotic infection. Particular attention should be paid to maternal temperature, pulse, and the presence of uterine tenderness or foul-smelling vaginal discharge. A sterile speculum examination followed by digital examination would complement the ultrasonography evaluation in assessing cervical dilation and effacement. The ultrasonography evaluation should be completed to confirm a viable pregnancy with accurate dating and the absence of detectable fetal anomalies.
Currently, evidence is insufficient for recommending routine amniocentesis to exclude intra-amniotic infection in an asymptomatic woman prior to ultrasound-indicated cerclage, even in the presence of intra-amniotic sludge, as there are no data demonstrating improved outcomes.4 In addition, intra-amniotic sludge has been associated with intra-amniotic infection and/or inflammation in the form of microbial biofilms, which may prevent detection of infection by routine culture techniques.7
Related Article:
Universal cervical length screening–saving babies lives
Study results offer limited guidance
Data are limited on the clinical implications of intra-amniotic sludge in women with cervical cerclage. In a retrospective cohort of 177 patients with cerclage, 60 had evidence of sludge and 46 of those with sludge underwent ultrasound-indicated cerclage.8 There were no significant differences in the mean gestational age at delivery, neonatal outcomes, rate of preterm delivery, preterm premature rupture of membranes, or intra-amniotic infection between women with or without intra-amniotic sludge. A subanalysis was performed comparing women with sludge detected before or after cerclage and, again, no difference was found in measured outcomes.
Similarly, in a small (N = 20) retrospective review of the Arabin pessary used as a noninvasive intervention for short cervix, the presence of intra-amniotic sludge in 5 cases did not appear to impact outcomes.9
Case patient: How would you manage her care?
Based on her obstetric history and ultrasonography findings, the patient described in the case vignette is at high risk for preterm delivery. The presence of both intra-amniotic sludge and short cervix is associated with an increased risk for spontaneous preterm delivery. After evaluating for clinical intra-amniotic infection and performing a work-up for other contraindications to cerclage placement, cerclage placement may be offered--even in the presence of intra-amniotic sludge.
The next practical question is whether 17P, already started, should be continued after cerclage placement. From the literature on 17P, it is unclear whether progesterone provides additional benefit. One randomized, placebo-controlled study in women with at least 2 preterm deliveries or mid-trimester losses and cerclage in place showed that the 17P-treated women had a significant reduction in preterm delivery compared with the control group, from 37.8% to 16.1%.10
By contrast, in a secondary analysis of a randomized trial evaluating cerclage in high-risk women with short cervix in the current pregnancy, addition of 17P to cerclage was not beneficial.11 Results of 2 retrospective cohort studies showed the same lack of difference on preterm delivery rates with the addition of 17P.12,13
Accepting that the interpretation of these data is challenging, in our practice we would choose to continue the progesterone supplementation, siding with other recently expressed expert opinions.14
The bottom line
While clinical intra-amniotic infection is a contraindication to cerclage, there is no evidence to support withholding cerclage from eligible women due to the presence of intra-amniotic fluid sludge alone.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid "sludge" in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30(5):706-714.
- Romero R, Kusanovic JP, Espinoza J, et al. What is amniotic fluid "sludge"? Ultrasound Obstet Gynecol. 2007;30(5):793-798.
- Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2012;4:CD008991.
- Abbott D, To M, Shennan A. Cervical cerclage: a review of current evidence. Aust N Z J Obstet Gynaecol. 2012;52(3):220-223.
- Drassinower D, Poggi SH, Landy HJ, Gilo N, Benson JE, Ghidini A. Perioperative complications of history-indicated and ultrasound-indicated cervical cerclage. Am J Obstet Gynecol. 2011;205(1):53.e1-e5.
- Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95(5):652-655.
- Vaisbuch E, Romero R, Erez IO, et al. Clinical significance of early (<20 weeks) vs late (20-24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36(4):471-481.
- Gorski LA, Huang WH, Iriye BK, Hancock J. Clinical implication of intra-amniotic sludge on ultrasound in patients with cervical cerclage. Ultrasound Obstet Gynecol. 2010;36(4):482-485.
- Ting YH, Lao TT, Wa Law LW, et al. Arabin cerclage pessary in the management of cervical insufficiency. J Matern Fetal Neonatal Med. 2012;25(12):2693-2695.
- Yemini M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 1985;151(5):574-577.
- Berghella V, Figueroa D, Szychowski JM, et al; Vaginal Ultrasound Trial Consortium. 17-alpha-hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol. 2010;202(4):351.e1-e6.
- Rebarber A, Cleary-Goldman J, Istwan NB, et al. The use of 17 alpha-hydroxyprogesterone caproate (17P) in women with cervical cerclage. Am J Perinatol. 2008;25(5):271-275.
- Stetson B, Hibbard JU, Wilkins I, Leftwich H. Outcomes with cerclage alone compared with cerclage plus 17 α-hydroxyprogesterone caproate. Obstet Gynecol. 2016;128(5):983-988.
- Iams JD. Identification of candidates for progesterone: why, who, how, and when? Obstet Gynecol. 2014;123(6):1317-1326.
- Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid "sludge" in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30(5):706-714.
- Romero R, Kusanovic JP, Espinoza J, et al. What is amniotic fluid "sludge"? Ultrasound Obstet Gynecol. 2007;30(5):793-798.
- Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2012;4:CD008991.
- Abbott D, To M, Shennan A. Cervical cerclage: a review of current evidence. Aust N Z J Obstet Gynaecol. 2012;52(3):220-223.
- Drassinower D, Poggi SH, Landy HJ, Gilo N, Benson JE, Ghidini A. Perioperative complications of history-indicated and ultrasound-indicated cervical cerclage. Am J Obstet Gynecol. 2011;205(1):53.e1-e5.
- Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95(5):652-655.
- Vaisbuch E, Romero R, Erez IO, et al. Clinical significance of early (<20 weeks) vs late (20-24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36(4):471-481.
- Gorski LA, Huang WH, Iriye BK, Hancock J. Clinical implication of intra-amniotic sludge on ultrasound in patients with cervical cerclage. Ultrasound Obstet Gynecol. 2010;36(4):482-485.
- Ting YH, Lao TT, Wa Law LW, et al. Arabin cerclage pessary in the management of cervical insufficiency. J Matern Fetal Neonatal Med. 2012;25(12):2693-2695.
- Yemini M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 1985;151(5):574-577.
- Berghella V, Figueroa D, Szychowski JM, et al; Vaginal Ultrasound Trial Consortium. 17-alpha-hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol. 2010;202(4):351.e1-e6.
- Rebarber A, Cleary-Goldman J, Istwan NB, et al. The use of 17 alpha-hydroxyprogesterone caproate (17P) in women with cervical cerclage. Am J Perinatol. 2008;25(5):271-275.
- Stetson B, Hibbard JU, Wilkins I, Leftwich H. Outcomes with cerclage alone compared with cerclage plus 17 α-hydroxyprogesterone caproate. Obstet Gynecol. 2016;128(5):983-988.
- Iams JD. Identification of candidates for progesterone: why, who, how, and when? Obstet Gynecol. 2014;123(6):1317-1326.
DESKTOP III: Secondary surgery for recurrent OC improves PFS, TFST
CHICAGO – Secondary cytoreductive surgery resulted in a clinically meaningful increase in progression-free survival and time to first subsequent therapy in a phase III study of carefully selected women with ovarian cancer who experienced their first relapse after a platin-free interval of 6 months.
These interim findings from the randomized international DESKTOP III trial suggest that until final overall survival data are available to more definitively define the role of secondary cytoreductive surgery in this setting, it should at least be considered as an option in patients who are good candidates based on a positive AGO Study Group score, defined as an ECOG performance status score of 0, ascites of 500 mL or less, and complete resection at initial surgery, Andreas du Bois, MD, reported at the annual meeting of the American Society of Clinical Oncology.
The median progression-free survival (PFS) in 204 women who met this criteria and who were randomized to undergo surgery followed by chemotherapy was 19.6 months, compared with 14 months in 203 women who were randomized to receive only second-line chemotherapy (hazard ratio, 0.66), said Dr. du Bois of AGO and Kliniken Essen-Mitte, Essen, Germany.
“Even more important ... only complete resection makes a difference ... and that adds a median 7.2 months PFS with a hazard ratio of 0.56, which is highly significant. Fortunately that translates into time to first subsequent treatment, which is a more patient-oriented outcome,” he said.
The time to third-line therapy was prolonged by a highly statistically significant median of 7.1 month (hazard ratio, 0.6).
“What was the trade-off for these benefits? The patients did not pay for it with excessive mortality,” he said, explaining that no significant differences were seen between the groups in terms of mortality at 30, 60, 90, or 180 days, and that no excessive toxicity or treatment burden was seen in either group.
Median age of the patients was 60 years; they were enrolled at 80 centers in 12 countries between 2010 and 2015. The platin-free interval exceeded 12 months in 75% and 76% of patient in the surgery and control arms, respectively.
Chemotherapy regimens in both the treatment and control arms were selected according to institutional standards, although platinum-based combination therapy was strongly recommended; 87% and 88% in the groups, respectively, received a platinum-containing second-line therapy.
Macroscopic complete resection was achieved in 72.5% of patients in the surgery arm, which was the rate predicted by the AGO scores.
“We know that the surgery and chemotherapy are the cornerstones of ovarian cancer therapy ... however, surgery in recurrent ovarian cancer has not been based on high-level evidence,” Dr. du Bois said. “So far there are only retrospective series suggesting that there might be a benefit or not.”
The German AGO group and the Gynecologic Oncology Group (GOG) in the United States thus initiated clinical trials to evaluate its role in recurrent ovarian cancer, including the DESKTOP series, he explained, noting that the AGO score was developed through these trials as a way to identify good surgical candidates based on preoperative factors.
It was confirmed in a prospective study that the score, which selects about 50% of all patients with platinum-sensitive recurrent ovarian cancer, could predict successful surgery, he added.
In the current study, the data with respect to overall survival – the primary study endpoint – have not reached maturity, but at 2 years it was 83%.
However, the findings of a meaningful benefit in progression-free survival and time to first subsequent treatment (advantages of 5.6 and 7.1 months, respectively) in secondary cytoreductive surgery patients is at least comparable with all phase III trials in second-line therapy for platinum-sensitive recurrent ovarian cancer so far, he said.
“In fact, it’s the most positive trial ever reported in this population,” he added, noted that he was referring to therapy trials, not maintenance trials.
Further, the fact that the surgery benefit was exclusive to patients with complete resection indicates the importance of selecting both the right center with capability of achieving complete resection in most patients, and the right patients, as identified by the AGO score.
“Hopefully, further follow-up will show that this benefit translates into overall survival,” he concluded, noting that overall survival will be evaluated after extended follow-up when 244 overall survival events are observed.
Dr. Du Bois reported serving as a consultant or adviser for AstraZeneca, Mundipharma, Pfizer, Pharmamar, and Roche/Genentech.
The findings from DESKTOP III complement those from prior retrospective studies, but one key difference is the emphasis on the importance of complete resection, abstract discussant Ritu Salani, MD, said at the meeting.
“I no longer believe that optimal resection is good enough in this patient population,” she said.
In fact, given that 67 patients in the study were not completely resected, it is important to look at whether there are any identifying factors that could help prevent surgery in these patients, and whether there are any minimally invasive or less invasive approaches, such as scoping and scoring these patients, to determine who really is completely resectable, she said.
Other studies of cytoreductive surgery, including GOG 213 and the Dutch SOCceR trial, are ongoing, and the primary endpoint of DESKTOP III is overall survival, she noted.
“We look forward to these data maturing.”
Dr. Salani is with the Ohio State University, Columbus. She has received honoraria from Clovis Oncology and Lynparza, and has served in a consulting or advisory role for Genentech/Roche.
The findings from DESKTOP III complement those from prior retrospective studies, but one key difference is the emphasis on the importance of complete resection, abstract discussant Ritu Salani, MD, said at the meeting.
“I no longer believe that optimal resection is good enough in this patient population,” she said.
In fact, given that 67 patients in the study were not completely resected, it is important to look at whether there are any identifying factors that could help prevent surgery in these patients, and whether there are any minimally invasive or less invasive approaches, such as scoping and scoring these patients, to determine who really is completely resectable, she said.
Other studies of cytoreductive surgery, including GOG 213 and the Dutch SOCceR trial, are ongoing, and the primary endpoint of DESKTOP III is overall survival, she noted.
“We look forward to these data maturing.”
Dr. Salani is with the Ohio State University, Columbus. She has received honoraria from Clovis Oncology and Lynparza, and has served in a consulting or advisory role for Genentech/Roche.
The findings from DESKTOP III complement those from prior retrospective studies, but one key difference is the emphasis on the importance of complete resection, abstract discussant Ritu Salani, MD, said at the meeting.
“I no longer believe that optimal resection is good enough in this patient population,” she said.
In fact, given that 67 patients in the study were not completely resected, it is important to look at whether there are any identifying factors that could help prevent surgery in these patients, and whether there are any minimally invasive or less invasive approaches, such as scoping and scoring these patients, to determine who really is completely resectable, she said.
Other studies of cytoreductive surgery, including GOG 213 and the Dutch SOCceR trial, are ongoing, and the primary endpoint of DESKTOP III is overall survival, she noted.
“We look forward to these data maturing.”
Dr. Salani is with the Ohio State University, Columbus. She has received honoraria from Clovis Oncology and Lynparza, and has served in a consulting or advisory role for Genentech/Roche.
CHICAGO – Secondary cytoreductive surgery resulted in a clinically meaningful increase in progression-free survival and time to first subsequent therapy in a phase III study of carefully selected women with ovarian cancer who experienced their first relapse after a platin-free interval of 6 months.
These interim findings from the randomized international DESKTOP III trial suggest that until final overall survival data are available to more definitively define the role of secondary cytoreductive surgery in this setting, it should at least be considered as an option in patients who are good candidates based on a positive AGO Study Group score, defined as an ECOG performance status score of 0, ascites of 500 mL or less, and complete resection at initial surgery, Andreas du Bois, MD, reported at the annual meeting of the American Society of Clinical Oncology.
The median progression-free survival (PFS) in 204 women who met this criteria and who were randomized to undergo surgery followed by chemotherapy was 19.6 months, compared with 14 months in 203 women who were randomized to receive only second-line chemotherapy (hazard ratio, 0.66), said Dr. du Bois of AGO and Kliniken Essen-Mitte, Essen, Germany.
“Even more important ... only complete resection makes a difference ... and that adds a median 7.2 months PFS with a hazard ratio of 0.56, which is highly significant. Fortunately that translates into time to first subsequent treatment, which is a more patient-oriented outcome,” he said.
The time to third-line therapy was prolonged by a highly statistically significant median of 7.1 month (hazard ratio, 0.6).
“What was the trade-off for these benefits? The patients did not pay for it with excessive mortality,” he said, explaining that no significant differences were seen between the groups in terms of mortality at 30, 60, 90, or 180 days, and that no excessive toxicity or treatment burden was seen in either group.
Median age of the patients was 60 years; they were enrolled at 80 centers in 12 countries between 2010 and 2015. The platin-free interval exceeded 12 months in 75% and 76% of patient in the surgery and control arms, respectively.
Chemotherapy regimens in both the treatment and control arms were selected according to institutional standards, although platinum-based combination therapy was strongly recommended; 87% and 88% in the groups, respectively, received a platinum-containing second-line therapy.
Macroscopic complete resection was achieved in 72.5% of patients in the surgery arm, which was the rate predicted by the AGO scores.
“We know that the surgery and chemotherapy are the cornerstones of ovarian cancer therapy ... however, surgery in recurrent ovarian cancer has not been based on high-level evidence,” Dr. du Bois said. “So far there are only retrospective series suggesting that there might be a benefit or not.”
The German AGO group and the Gynecologic Oncology Group (GOG) in the United States thus initiated clinical trials to evaluate its role in recurrent ovarian cancer, including the DESKTOP series, he explained, noting that the AGO score was developed through these trials as a way to identify good surgical candidates based on preoperative factors.
It was confirmed in a prospective study that the score, which selects about 50% of all patients with platinum-sensitive recurrent ovarian cancer, could predict successful surgery, he added.
In the current study, the data with respect to overall survival – the primary study endpoint – have not reached maturity, but at 2 years it was 83%.
However, the findings of a meaningful benefit in progression-free survival and time to first subsequent treatment (advantages of 5.6 and 7.1 months, respectively) in secondary cytoreductive surgery patients is at least comparable with all phase III trials in second-line therapy for platinum-sensitive recurrent ovarian cancer so far, he said.
“In fact, it’s the most positive trial ever reported in this population,” he added, noted that he was referring to therapy trials, not maintenance trials.
Further, the fact that the surgery benefit was exclusive to patients with complete resection indicates the importance of selecting both the right center with capability of achieving complete resection in most patients, and the right patients, as identified by the AGO score.
“Hopefully, further follow-up will show that this benefit translates into overall survival,” he concluded, noting that overall survival will be evaluated after extended follow-up when 244 overall survival events are observed.
Dr. Du Bois reported serving as a consultant or adviser for AstraZeneca, Mundipharma, Pfizer, Pharmamar, and Roche/Genentech.
CHICAGO – Secondary cytoreductive surgery resulted in a clinically meaningful increase in progression-free survival and time to first subsequent therapy in a phase III study of carefully selected women with ovarian cancer who experienced their first relapse after a platin-free interval of 6 months.
These interim findings from the randomized international DESKTOP III trial suggest that until final overall survival data are available to more definitively define the role of secondary cytoreductive surgery in this setting, it should at least be considered as an option in patients who are good candidates based on a positive AGO Study Group score, defined as an ECOG performance status score of 0, ascites of 500 mL or less, and complete resection at initial surgery, Andreas du Bois, MD, reported at the annual meeting of the American Society of Clinical Oncology.
The median progression-free survival (PFS) in 204 women who met this criteria and who were randomized to undergo surgery followed by chemotherapy was 19.6 months, compared with 14 months in 203 women who were randomized to receive only second-line chemotherapy (hazard ratio, 0.66), said Dr. du Bois of AGO and Kliniken Essen-Mitte, Essen, Germany.
“Even more important ... only complete resection makes a difference ... and that adds a median 7.2 months PFS with a hazard ratio of 0.56, which is highly significant. Fortunately that translates into time to first subsequent treatment, which is a more patient-oriented outcome,” he said.
The time to third-line therapy was prolonged by a highly statistically significant median of 7.1 month (hazard ratio, 0.6).
“What was the trade-off for these benefits? The patients did not pay for it with excessive mortality,” he said, explaining that no significant differences were seen between the groups in terms of mortality at 30, 60, 90, or 180 days, and that no excessive toxicity or treatment burden was seen in either group.
Median age of the patients was 60 years; they were enrolled at 80 centers in 12 countries between 2010 and 2015. The platin-free interval exceeded 12 months in 75% and 76% of patient in the surgery and control arms, respectively.
Chemotherapy regimens in both the treatment and control arms were selected according to institutional standards, although platinum-based combination therapy was strongly recommended; 87% and 88% in the groups, respectively, received a platinum-containing second-line therapy.
Macroscopic complete resection was achieved in 72.5% of patients in the surgery arm, which was the rate predicted by the AGO scores.
“We know that the surgery and chemotherapy are the cornerstones of ovarian cancer therapy ... however, surgery in recurrent ovarian cancer has not been based on high-level evidence,” Dr. du Bois said. “So far there are only retrospective series suggesting that there might be a benefit or not.”
The German AGO group and the Gynecologic Oncology Group (GOG) in the United States thus initiated clinical trials to evaluate its role in recurrent ovarian cancer, including the DESKTOP series, he explained, noting that the AGO score was developed through these trials as a way to identify good surgical candidates based on preoperative factors.
It was confirmed in a prospective study that the score, which selects about 50% of all patients with platinum-sensitive recurrent ovarian cancer, could predict successful surgery, he added.
In the current study, the data with respect to overall survival – the primary study endpoint – have not reached maturity, but at 2 years it was 83%.
However, the findings of a meaningful benefit in progression-free survival and time to first subsequent treatment (advantages of 5.6 and 7.1 months, respectively) in secondary cytoreductive surgery patients is at least comparable with all phase III trials in second-line therapy for platinum-sensitive recurrent ovarian cancer so far, he said.
“In fact, it’s the most positive trial ever reported in this population,” he added, noted that he was referring to therapy trials, not maintenance trials.
Further, the fact that the surgery benefit was exclusive to patients with complete resection indicates the importance of selecting both the right center with capability of achieving complete resection in most patients, and the right patients, as identified by the AGO score.
“Hopefully, further follow-up will show that this benefit translates into overall survival,” he concluded, noting that overall survival will be evaluated after extended follow-up when 244 overall survival events are observed.
Dr. Du Bois reported serving as a consultant or adviser for AstraZeneca, Mundipharma, Pfizer, Pharmamar, and Roche/Genentech.
At THE 2017 ASCO ANNUAL MEETING
Key clinical point:
Major finding: Median progression-free survival was 19.6 vs. 14 months in the surgery vs. second-line chemotherapy-only arm.
Data source: The randomized phase III DESKTOP III study of 407 patients.
Disclosures: Dr. du Bois reported serving as a consultant or adviser for AstraZeneca, Mundipharma, Pfizer, Pharmamar, and Roche/Genentech.
Consensus on isotretinoin monitoring elusive
For dermatologists searching for consensus on isotretinoin monitoring – keep looking. There is no consensus to be found from a new survey of U.S. dermatologists.
There were some trends among the 2,322 responses to the national email survey of American Academy of Dermatology members, but the researchers at the University of Vermont in Burlington said that no real agreement on monitoring emerged.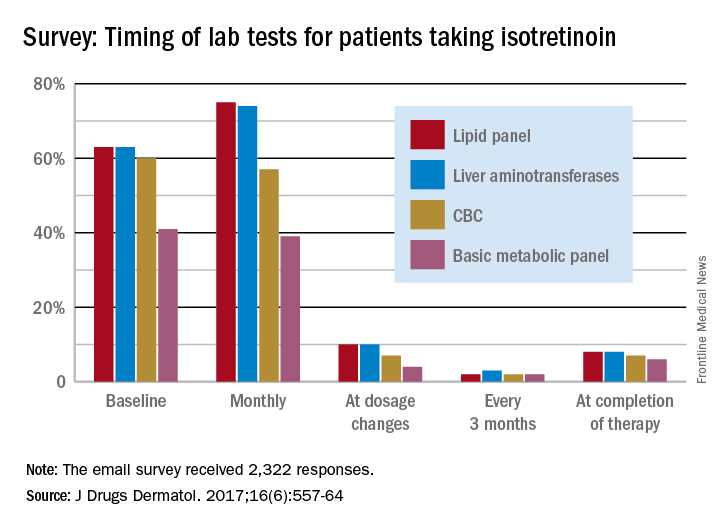
Very few dermatologists do any testing when the dosage is changed – a lipid panel and liver enzymes being the most popular response at 10% – or when therapy is completed – a lipid panel (8%) and liver enzyme check (8%) were the most common, they said.
In response to abnormal test results, 75% of respondents would take patients off isotretinoin if their liver enzyme levels were three times normal, and 89% would stop the medication if the enzyme level were four times normal. Between 30% and 40% of dermatologists would “order further testing” if liver enzyme levels were even slightly elevated, the investigators said.
Most respondents (70%) would not make a change if triglyceride levels were twice normal or less, and 72% would stop isotretinoin if triglyceride levels were four times normal, but “only 26% would add a lipid-lowering agent when triglyceride levels reach three times normal,” Dr. Hobson and her associates wrote.
“Based on the available literature and low incidence of adverse events, less monitoring is probably required,” they said, adding that “over-monitoring may no longer be justified or considered a good use of resources.”
The investigators said that they had no conflicts of interest to report.
For dermatologists searching for consensus on isotretinoin monitoring – keep looking. There is no consensus to be found from a new survey of U.S. dermatologists.
There were some trends among the 2,322 responses to the national email survey of American Academy of Dermatology members, but the researchers at the University of Vermont in Burlington said that no real agreement on monitoring emerged.
Very few dermatologists do any testing when the dosage is changed – a lipid panel and liver enzymes being the most popular response at 10% – or when therapy is completed – a lipid panel (8%) and liver enzyme check (8%) were the most common, they said.
In response to abnormal test results, 75% of respondents would take patients off isotretinoin if their liver enzyme levels were three times normal, and 89% would stop the medication if the enzyme level were four times normal. Between 30% and 40% of dermatologists would “order further testing” if liver enzyme levels were even slightly elevated, the investigators said.
Most respondents (70%) would not make a change if triglyceride levels were twice normal or less, and 72% would stop isotretinoin if triglyceride levels were four times normal, but “only 26% would add a lipid-lowering agent when triglyceride levels reach three times normal,” Dr. Hobson and her associates wrote.
“Based on the available literature and low incidence of adverse events, less monitoring is probably required,” they said, adding that “over-monitoring may no longer be justified or considered a good use of resources.”
The investigators said that they had no conflicts of interest to report.
For dermatologists searching for consensus on isotretinoin monitoring – keep looking. There is no consensus to be found from a new survey of U.S. dermatologists.
There were some trends among the 2,322 responses to the national email survey of American Academy of Dermatology members, but the researchers at the University of Vermont in Burlington said that no real agreement on monitoring emerged.
Very few dermatologists do any testing when the dosage is changed – a lipid panel and liver enzymes being the most popular response at 10% – or when therapy is completed – a lipid panel (8%) and liver enzyme check (8%) were the most common, they said.
In response to abnormal test results, 75% of respondents would take patients off isotretinoin if their liver enzyme levels were three times normal, and 89% would stop the medication if the enzyme level were four times normal. Between 30% and 40% of dermatologists would “order further testing” if liver enzyme levels were even slightly elevated, the investigators said.
Most respondents (70%) would not make a change if triglyceride levels were twice normal or less, and 72% would stop isotretinoin if triglyceride levels were four times normal, but “only 26% would add a lipid-lowering agent when triglyceride levels reach three times normal,” Dr. Hobson and her associates wrote.
“Based on the available literature and low incidence of adverse events, less monitoring is probably required,” they said, adding that “over-monitoring may no longer be justified or considered a good use of resources.”
The investigators said that they had no conflicts of interest to report.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Prescribing Patterns Shift After Detailing-Policy Change
In the past decade, medical institutions have begun to check the practice of detailing—pharmaceutical reps promoting medications during sales visits to physicians. This NIH study is one of the first to document the effect of these restrictions. The researchers compared prescribing at 19 academic medical centers (AMCs) that, between 2006 and 2012 instituted policies restricting detailing.
The study compared prescribing by 2,126 physicians at AMCs with that by 24,593 physicians from a pharmacy benefits database. The analysis covered 16.1 million prescriptions in 8 major drug classes: lipid lowering, gastroesophageal reflux disease, diabetes, hypertension, sleep, attention deficit hyperactivity disorder, depression, and antipsychosis.
At the centers with restrictions, physicians prescribed fewer of the promoted drugs and more nonpromoted drugs in the same drug classes. The mean market share of detailed drugs (across all the drug classes) in AMCs before the policy changes was 19.3%. Over the study period, the market share of detailed drugs prescribed by AMC physicians declined by 1.67 percentage point, an 8.7% decrease relative to the prechange level. The comparison group of physicians saw a slight decline over the same period. Although the drop was “modest,” NIH notes, proportionally small changes can represent thousands of prescriptions. The market share of nondetailed drugs increased by a relative 5.6%.
The changes were statistically significant for 6 of the 8 drug classes and for all drugs in the aggregate. The magnitude of changes differed across AMCs, the researchers found. The decline was greatest at centers with the most stringent policies, such as bans on salespeople in patient care areas. In 8 of 11 AMCs with more stringent policies, the changes in prescribing were significant, compared with only 1 of 8 AMCs with more limited measures.
In the past decade, medical institutions have begun to check the practice of detailing—pharmaceutical reps promoting medications during sales visits to physicians. This NIH study is one of the first to document the effect of these restrictions. The researchers compared prescribing at 19 academic medical centers (AMCs) that, between 2006 and 2012 instituted policies restricting detailing.
The study compared prescribing by 2,126 physicians at AMCs with that by 24,593 physicians from a pharmacy benefits database. The analysis covered 16.1 million prescriptions in 8 major drug classes: lipid lowering, gastroesophageal reflux disease, diabetes, hypertension, sleep, attention deficit hyperactivity disorder, depression, and antipsychosis.
At the centers with restrictions, physicians prescribed fewer of the promoted drugs and more nonpromoted drugs in the same drug classes. The mean market share of detailed drugs (across all the drug classes) in AMCs before the policy changes was 19.3%. Over the study period, the market share of detailed drugs prescribed by AMC physicians declined by 1.67 percentage point, an 8.7% decrease relative to the prechange level. The comparison group of physicians saw a slight decline over the same period. Although the drop was “modest,” NIH notes, proportionally small changes can represent thousands of prescriptions. The market share of nondetailed drugs increased by a relative 5.6%.
The changes were statistically significant for 6 of the 8 drug classes and for all drugs in the aggregate. The magnitude of changes differed across AMCs, the researchers found. The decline was greatest at centers with the most stringent policies, such as bans on salespeople in patient care areas. In 8 of 11 AMCs with more stringent policies, the changes in prescribing were significant, compared with only 1 of 8 AMCs with more limited measures.
In the past decade, medical institutions have begun to check the practice of detailing—pharmaceutical reps promoting medications during sales visits to physicians. This NIH study is one of the first to document the effect of these restrictions. The researchers compared prescribing at 19 academic medical centers (AMCs) that, between 2006 and 2012 instituted policies restricting detailing.
The study compared prescribing by 2,126 physicians at AMCs with that by 24,593 physicians from a pharmacy benefits database. The analysis covered 16.1 million prescriptions in 8 major drug classes: lipid lowering, gastroesophageal reflux disease, diabetes, hypertension, sleep, attention deficit hyperactivity disorder, depression, and antipsychosis.
At the centers with restrictions, physicians prescribed fewer of the promoted drugs and more nonpromoted drugs in the same drug classes. The mean market share of detailed drugs (across all the drug classes) in AMCs before the policy changes was 19.3%. Over the study period, the market share of detailed drugs prescribed by AMC physicians declined by 1.67 percentage point, an 8.7% decrease relative to the prechange level. The comparison group of physicians saw a slight decline over the same period. Although the drop was “modest,” NIH notes, proportionally small changes can represent thousands of prescriptions. The market share of nondetailed drugs increased by a relative 5.6%.
The changes were statistically significant for 6 of the 8 drug classes and for all drugs in the aggregate. The magnitude of changes differed across AMCs, the researchers found. The decline was greatest at centers with the most stringent policies, such as bans on salespeople in patient care areas. In 8 of 11 AMCs with more stringent policies, the changes in prescribing were significant, compared with only 1 of 8 AMCs with more limited measures.
Interprofessional Education in Patient Aligned Care Team Primary Care-Mental Health Integration
Over the past 10 years, the VHA has been a national leader in primary care-mental health integration (PC-MHI) within patient aligned care teams (PACTs).1,2 Studies of the PC-MHI collaborative care model consistently have shown increased access to MH services, higher levels of MH treatment engagement, improved MH treatment outcomes, and high patient and provider satisfaction.3-7 Primary care-mental health integration relies heavily on interprofessional team-based practice with providers from diverse educational and clinical backgrounds who work together to deliver integrated mental and behavioral health services within PACTs. This model requires a unique blending of professional cultures and communication and practice styles.
To sustain PC-MHI in PACT, health care professionals (HCPs) must be well trained to work effectively in interprofessional teams. Across health care organizations, training in collaborative interprofessional team-based practice has been identified as an important and challenging task.8-11
Integrating educational experiences among different HCP learners is an approach to developing competency in interprofessional collaboration early in training. The World Health Organization defined interprofessional education (IPE) as occurring “when students from two or more professions learn about, from, and with each other to enable effective collaboration and improve health outcomes.”9 Fundamental to this definition is the belief that interaction among learners from different disciplines during their training develops competency in subsequent effective collaborative practice. Studies of IPE in MH professional training have found that prelicensure IPE contributes to increased knowledge of roles and responsibilities of different disciplines, improved interprofessional communication and attitudes, and increased willingness to work in teams.12-17
Interprofessional education is a valuable training model, but developing interprofessional learning experiences in a system of diverse and often siloed training programs is difficult. More information about design and implementation of IPE training experiences is needed, particularly in outpatient settings in which integration of traditionally separate discipline-specific care is central to the health care mission. The VA PACT PC-MHI is a strong team-based care model that represents a unique opportunity for training across disciplines in interprofessional collaborative care.
To find innovative approaches to meeting the need for IPE in PACT PC-MHI, the authors developed a new IPE program in PC-MHI at the William S. Middleton Memorial Veterans Hospital (WSMMVH) in Madison, Wisconsin. This article reviews the development, implementation, and first-year evaluation of the training program and discusses the challenges and the IPE areas in need of improvement in PACT PC-MHI.
Methods
In 2012, the VHA launched phase 1 of the Mental Health Education Expansion Initiative (MHEEI), a collaboration of the Office of Academic Affiliations (OAA), VHA Mental Health Services (VHA-MHS), and the Office of Mental Health Operations (OMHO).18 The MHEEI was intended to “increase expertise in critical areas of need, expand the recruitment pipeline of well-trained, highly qualified health care providers in behavioral and mental health disciplines, and promote the utilization of interprofessional team-based care.”18 In response, WSMMVH organized a planning committee and submitted a funding request through the section of MHEEI called PACT With Integrated Behavioral Health Providers. The planning committee included training program directors and staff from psychiatry, pharmacy, social work, psychology, and primary care. The authors received funding for trainees in psychiatry (postgraduate year 4 [PGY-4]), pharmacy/MH residency (PGY-2), pharmacy/ambulatory care (PGY-1), and social work (interns).
Curriculum Development
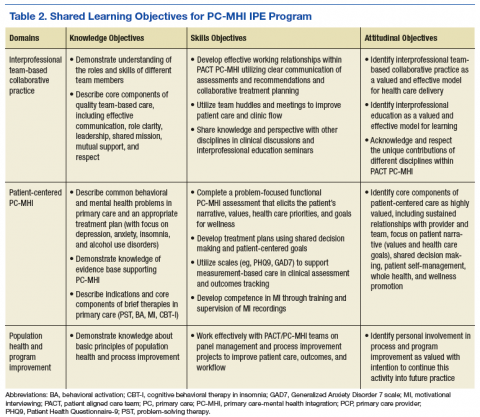
The planning committee met regularly for 6 months to develop the organization, learning objectives, educational strategies, and implementation plan for the IPE program. The program was organized as a 4- to 12-month clinical rotation with the PC-MHI team in PACT, combined with 12 months of protected weekly IPE time (Table 1).
Learning Objectives
To better understand the educational needs and foci for learning objectives, the interprofessional planning committee reviewed guidelines on training in integrated care and collaborative team-based practice.2,9,10,19-21 These guidelines were compared with existing training opportunities for each discipline to identify training gaps and needs.
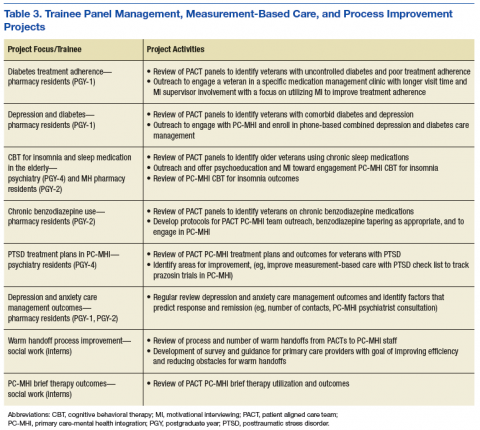
Learning objectives were organized into 3 domains: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Table 2 outlines the shared learning objectives linked to each domain that were common to the psychiatry, pharmacy, and social work disciplines. Although many of the learning objectives were shared among all disciplines, each trainee also had discipline-specific clinical activities and learning objectives. Psychiatry and pharmacy residents focused on primary care psychiatric medication consultation and care management for antidepressant medication starts. Social work interns focused on psychosocial and functional assessment and brief problem-focused psychotherapies. Learning objectives were met through direct veteran care in the primary care clinic as part of the PACT PC-MHI team and through interprofessional learning activities during protected weekly education time.
Implementation
Critical stakeholders in implementing the IPE program involved themselves early and throughout the planning process. Stakeholders included VAMC leadership, primary care and MH service line chiefs and clinic managers, training program directors, and PACT staff. Planning committee members gave presentations on the IPE program at MH service line and PACT meetings in the 2 months before program initiation in order to orient staff to learning objectives, program structure, and impact on PACT PC-MHI operations. Throughout the first year, the planning committee continued to meet every 2 weeks to review progress, solve implementation problems, and revise learning objectives and activities.
Trainee Clinical Activities
A wide range of educational strategies were planned to meet learning objectives across the 3 domains. There was strong emphasis on experiential learning through daily PACT and PC-MHI clinical work, team huddles and meetings, and trainee-led program improvement projects.
Psychiatry and PGY-2 pharmacy/MH residents focused on direct and indirect medication consultation and problem-focused assessments. Their clinical activities included PC-MHI medication evaluation and follow-up visits; chart reviews and e-consults for medication recommendations to PACT providers; reviews of care management data and consultations on veterans enrolled in depression and anxiety care management; “curbside consultations” for providers in PACT huddles and meetings and throughout the clinic day; and “warm handoffs,” same-day initial PC-MHI problem-focused assessments performed on PACT provider request. The residents were part of a pool of staff and trainees who performed these assessments.
PGY-1 pharmacy residents made care management phone calls for antidepressant trials for depression and anxiety. These residents were trained in motivational interviewing (MI). They applied their MI skills during care management calls focused on medication adherence and behavioral interventions for depression (eg, exercise, planning pleasurable activity) and during other clinical rotations, including tobacco cessation and medication management for diabetes and hypertension. Particularly challenging veteran cases from these clinics were cosupervised with medication management and PC-MHIstaff for added consultation on engagement, behavior change, and treatment plan adherence.
Social work interns completed initial PC-MHI psychosocial and functional assessments by phone and directly by same-day warm handoffs from PACT staff. The PC-MHI therapies they provided included problem-solving therapy, behavioral activation, stress management based on cognitive behavioral therapy, and brief alcohol interventions.
Group IPE Activities
All trainees had a weekly protected block of 3 hours during which they came together for group IPE that was designed to elicit active participation; facilitate interprofessional communication; and develop an understanding of and respect for the knowledge, culture, and practice style of the different disciplines.
Trainees participated in a Herrmann Brain Dominance Instrument (HBDI) workshop focused on developing a better understanding of individual differences in thinking and problem solving, with the goal of improving communication and learning within teams.22 In a seminar series on professionalism and boundaries in health care, trainees from each discipline gave a presentation on the traditional structure and content of their discipline’s training and discussed similarities and differences in their disciplines’ professional oaths, codes of ethics, and boundary guidelines.
Motivational interviewing training was conducted early in the year so trainees would be prepared to apply MI skills in their daily PACT PC-MHI clinical work. Motivational inteviewing is a patient-centered approach to engaging patients in health promoting behavior change. It is defined as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence.”23
Trainees recorded MI sessions with at least 2 live-patient visits and at least 2 simulated-patient interviews (with staff serving as patient actors). The structure of MI training and supervision was deliberately designed to facilitate interprofessional communication and learning. In accord with a group supervision model for MI recorded reviews, the trainees presented their tapes to the entire learning group in the presence of a facilitating supervisor. Trainees had the opportunity to observe different interview styles and exchange feedback within a peer group of interprofessional learners.
Seminars were focused on core PC-MHI clinical content (eg, depression, anxiety, alcohol use disorders) and organized around case-based learning. Trainees divided into small teams in which representatives of each discipline offered their perspective on how to approach planning patient assessment and treatment. During the seminars, the authors engaged trainees as teachers and leaders whenever possible. All trainees presented on a topic in which they had some discipline expertise. For example, social work interns led a seminar on support and social services for victims of domestic violence, and PGY-1 pharmacy/ambulatory care residents led seminars and a panel management project focused on diabetes and depression.
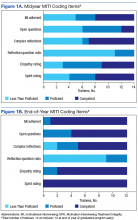
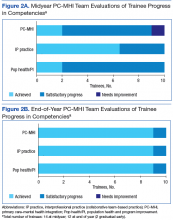
Trainees participated in several PACT PC-MHI projects focused on population- and measurement-based care, panel management, and program improvement (Table 3). Protected IPE time was used to teach trainees about population health principles and different tools for process improvement (eg, Vision-Analysis Team-Aim-Map-Measure-Change-Sustain) and provide a forum in which trainees could share their work with one another.
Evaluations
Several tools were used for trainee and program evaluations. Clinical skills were evaluated during daily supervision. Trainees began the year with PC-MHI staff directly observing all their clinical contacts with veterans. Staff evaluated and offered feedback on trainee clinical interviewing and on assessment and treatment planning skills. Once they were assessed to be ready to see veterans on their own, trainees were advanced by staff to “drop-in” direct supervision: Toward the end of a veteran’s visit, a staff preceptor entered the room to review relevant clinical findings, assessment and finalized treatment planning with the trainee and veteran. When appropriate for trainee competence level, clinical contacts were indirectly supervised: Trainees discussed their assessment and treatment plan with a staff supervisor at the end of the day.
Motivational interviewing recordings were reviewed during group supervision. To objectively evaluate MI skills, supervisors who were VA-certified in MI used the Motivational Interviewing Treatment Integrity (MITI) coding tool to review and code both the live- and simulated-patient recordings.24 The MITI coding involves quantitative and qualitative analysis using standardized coding items.
Quantitative items included percentage of open-ended questions (Proficiency: 50%; competency: 70%); percentage of reflections considered complex reflections, or reflective statements adding substantial meaning or emphasis and conveying a deeper or more complex picture of what patients say (Proficiency: 40%; competency: 50%); reflection-to-question ratio (Proficiency: 1:1; competency: 2:1); and percentage of MI-adherent provider statements (Proficiency: 90%; competency: 100%).
Qualitative coding items were a global rating of therapist “empathy,” which evaluated the extent to which the trainee understood or made an effort to grasp the patient’s perspective, and “MI spirit.” This coding intended to capture the overall competence of the trainee in emphasizing collaboration, patient autonomy, and evocation of the patient experience (Proficiency score: 5; competency score: 6).
The PC-MHI teaching staff met midyear and end of year as a team to complete trainee evaluations focused on the 3 areas of learning objectives: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Patient-centered PC-MHI involves direct observation and supervision of trainee clinical contacts with veterans, including assessment and treatment planning, clinical documentation, and review of live- and simulated-patient MI recordings. Collaborative team-based practice involves review of trainee participation in day-to-day teamwork, huddles, team meetings, and IPE activities. Population health and program improvement involves review of trainee work on a panel measurement-based care management or program improvement project. In each learning objectives area, trainees were rated on a 3-point scale: needs improvement (1); satisfactory (2); achieved (3). Core knowledge about PC-MHI evidence base, structure, and clinical topics was assessed with case-based written examination at midyear and end of year.
Surveys and qualitative interviews were used to assess trainee perceptions about the IPE program. A midyear and end-of-year survey assessed trainee satisfaction and perceived efficacy of the IPE training program in meeting core learning objectives. A midyear survey designed by pharmacy residents as part of their program improvement project evaluated attitudes around interprofessional learning and team practice. Trainees met individually with the PC-MHI IPE director at midyear and end of year to gather qualitative feedback on the IPE program.
Outcomes
All trainees advanced to the expected level of supervision for clinical contacts (drop-in or indirect clinical supervision). Over the year, there was significant improvement in trainees’ MI skills as measured by MITI coding of at least 2 live-patient or 2 simulated-patient recordings (Figures 1A and 1B). By end of year, most trainees had reached proficiency or competency in several MITI coded items: percentage of open-ended questions (4/12 proficient, 8/12 competent), percentage of complex reflections (2/12 less than proficient, 3/12 proficient, 7/12 competent), MI adherence (1/12 proficient, 11/12 competent), global empathy rating (2/12 proficient, 10/12 competent), and global MI spirit rating (12/12 competent). Average reflection-to-question ratio for the trainee group increased from 0.63 to 0.96 from midyear to end of year, but only 3 of 12 trainees reached the proficiency level of a 1:1 ratio, and no trainee reached the competency level of a 2:1 ratio.
According to the PC-MHI team’s midyear evaluation, most trainees were already making satisfactory progress in the 3 domains of learning objectives for the training program. At end of year, 13 of 14 trainees were assessed as competent in all 3 domains (Figures 2A and B). All trainees passed the midyear and end-of-year written examinations with overall high scores (average score, 82%) demonstrating acquisition of core PC-MHI clinical knowledge.
Trainee evaluations of the IPE program were overall highly favorable at both midyear and at end of year. Trainees rated the program effective or extremely effective in developing their skills in patient-centered care, interprofessional communication, and collaborative team-based practice. They also rated the program highly effective in preparing them to use team-based practice skills in other settings. On a midyear survey, trainees moderately to strongly agreed with several positive beliefs and attitudes about team-based care.
In qualitative interviews at program completion, trainees across disciplines rated the MI training with group supervision as one of their most valuable interprofessional learning experiences. Other highly valued training experiences were PACT PC-MHI panel management projects, team-based clinical case reviews, and cross-disciplinary supervision.
Discussion
This article describes the successful development and implementation of a VA-based IPE program in PACT PC-MHI. Interprofessional clinical training and educational experiences were highly valued, and trainees identified positive attitudes and improved skills related to team-based care. These findings support previous findings that IPE is associated with high satisfaction and positive attitudes toward team-based collaborative practice.12-17 Program implementation presented several challenges: nonsynchronous academic calendars and rotation schedules, cross-disciplinary supervision regulations, variations in clinical and supervisory requirements for accreditation standards, the traditional health care hierarchy, and measurement of the impact of IPE experiences.11,25,26
Rotation schedules and academic calendars varied across the psychiatry, pharmacy, and social work home programs. Organizing different trainee rotation schedules was a significant challenge. Collaboration with training directors and support staff was crucial in planning rotations that offered a longitudinal training experience in PACT PC-MHI. Given the participants’ different starting dates, protected IPE time early in the calendar year was focused on developing clinical skills specific to the pharmacy and psychiatry trainees who would be starting in July, and IPE activities that required the presence of all trainee disciplines (eg, MI training, HBDI) were planned for after the September start of the social work interns.
Cross-disciplinary supervision was highly valued by trainees because it offered exposure to the disciplines’ different communication styles and approaches to clinical assessment and decision making. Throughout the year, however, trainees encountered several obstacles to cross-disciplinary supervision, with respect to coding/billing and home program supervisory policies. The authors worked closely with the VA administration and each training program to develop and revise supervising policies and procedures to meet the necessary administrative and program supervisory requirements for accreditation standards.
In some cases, this work resulted in dual supervision by a preceptor of the same discipline (to meet requirements for coding/billing and home program supervision) and clinical supervision by a preceptor of a different discipline (eg, in team meetings or in clinical case reviews during protected IPE time). Preceptors from each discipline met regularly to discuss challenges in cross-disciplinary supervision, review scope-of-practice issues, share information on discipline-specific training, and revise supervisory procedures.
Interprofessional education activities during weekly protected time were designed to improve collaboration and to challenge trainees to examine traditional hierarchical roles and patterns of communication in health care. An emphasis on case-based learning in small groups encouraged trainees to share perspectives on their discipline-specific approach to assessment and treatment planning. Motivational interviewing training, one of the IPE experiences most valued by trainees and staff, created the opportunity for a truly shared learning environment, as trainees largely started at about the same skill level despite having different educational backgrounds. Group supervision of MI recordings offered trainees the opportunity to learn from each other and develop comfort in offering and receiving interprofessional constructive feedback.
Limitations
There were considerable methodologic limitations in the authors’ efforts to evaluate the impact of this training program on trainee attitudes and skills in collaborative team-based practice. Although trainee surveys revealed highly positive attitudes and beliefs about team-based care as well as perceived competence in collaborative practice, these were not validated surveys, and changes could not be accurately measured over time (trainees were not assessed at baseline). Trainees also were involved in other clinical teams within the VA during the year, and it is difficult to assess the specific impact of their PC-MHI IPE experiences. The PC-MHI staff evaluations of trainee competence in collaborative team-based practice were largely observational and potentially vulnerable to biases, as the staff evaluating trainee competence also were part of the IPE program planning process and invested in its success.
To address these limitations in the future, the authors will use better assessment tools at baseline and end of year to more effectively evaluate the impact of the training program on teamwork skills as well changes in attitudes and beliefs about interprofessional learning and teamwork. The Attitudes Toward Interprofessional Health Care Teams Scale and the Perceptions of Effective Interpersonal Teams Scale should be considered as they have published reliability and validity.27,28 The authors could improve the reliability and depth of trainee evaluations with use of a “360-degree evaluation” model for trainee evaluations that includes other PACT members (beyond PC-MHI staff) as well as veterans.
Assessment of MI skills and competency was limited by use of both live- and simulated-patient recordings. Simulation is a valuable learning tool but often does not accurately represent actual clinical situations and challenges. To appropriately assess MI competence, future MI training should emphasize live-patient recordings over simulated-patient visits. Furthermore, whereas trainees reached competency in several MI coding items, none reached competency in the reflection-to-question ratio, and only about half reached competency in percentage of complex reflections. Future MI training will need to focus on further development of reflection skills.
Trainee intentions to remain involved in program improvement and collaborative team-based care in future professional work were core attitudinal learning objectives, but neither was adequately assessed in end-of-year surveys. Ideally, future evaluations will assess subjective trainee intentions and goals around team-based work and objectively measure future professional choices and activities. For example, it would be interesting to determine whether trainees who participated in this program will choose to practice in an interprofessional team-based model or participate in program improvement activities.
Last, the absence of psychology interns was considered a weakness in the learning environment, resulting in a relatively “prescriber-heavy” balance in discipline perspectives for IPE-focused case discussions and other training. Furthermore, the discipline representation in the IPE program did not exemplify the typical PC-MHI team in most VA clinic settings and community practices in which psychology has a strong presence. Adding psychology trainees was an important goal for the IPE program leadership. In 2015, WSMMVH was awarded additional funding for a PACT PC-MHI predoctoral psychology intern through the phase 4 MHEEI. The PC-MHI track psychology intern joined the IPE program in the 2016-2017 training year.
Conclusion
There is broad consensus that interprofessional team-based practice is crucial for providers in the VA and all health care systems. Primary care-mental health integration is an area of VA care in which interprofessional collaboration is uniquely important to implementation and sustainability of the model. Interprofessional education is an effective approach for preparing HCPs for team-based practice, but implementation is challenging. Several factors crucially contributed to the successful implementation of this program: collaboration of the interprofessional planning team with representation from key stakeholders in different departments and training programs; a well-established PACT PC-MHI team with experience in collaborative team-based care; a curriculum structure that emphasized experiential educational strategies designed to promote interprofessional learning and communication; VA leadership support at the national level (MHEEI funding) and local level; and PACT PC-MHI clinical staff committed to teaching and to the IPE mission.
1. Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105-116.
2. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
3. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of loner-term outcomes. Arch Intern Med. 2006;166(21):2314-2321.
4. Unützer J, Katon W, Callahan CM, et al; IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) Investigators. Collaborative care management in late-life depression in the primary care setting: a randomized control trial. JAMA. 2002;288(22):2836-2845.
5. Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;3(4):305-310.
6. Bruce ML, Tenhave TR, Reynolds CF III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091.
7. Alexopoulos GS, Reynolds CF III , Bruce ML, et al; PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166(8):882-890.
8. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
9. World Health Organization, Study Group on Interprofessional Education and Collaborative Practice. Framework for Action on Interprofessional Education and Collaborative Practice. Geneva, Switzerland: World Health Organization; 2010.
10. Interprofessional Education Collaborative Expert Panel. Core Competencies for Interprofessional Collaborative Practice: Report of an Expert Panel. Washington, DC: Interprofessional Education Collaborative; 2011.
11. Blue AV, Mitcham M, Smith T, Raymond J, Greenburg R. Changing the future of health professions: embedding interprofessional education within an academic health center. Acad Med. 2010;85(8):1290-1295.
12. Priest HM, Roberts P, Dent H, Blincoe C, Lawton D, Armstrong C. Interprofessional education and working in mental health: in search of the evidence base. J Nurs Manag. 2008;16(4):474-485.
13. Reeves S. A systematic review of the effects of interprofessional education on staff involved in the care of adults with mental health problems. J Psychiatr Ment Health Nurs. 2001;8(6):533-542.
14. Carpenter J, Barnes D, Dickinson C, Wooff D. Outcomes of interprofessional education for community mental health services in England: the longitudinal evaluation of a postgraduate programme. J Interprof Care. 2006;20(2):145-161.
15. Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: BEME guide no. 9. Med Teach. 2007;29(8):735-751.
16. Pauzé E, Reeves S. Examining the effects of interprofessional education on mental health providers: findings from an updated systematic review. J Ment Health. 2010;19(3):258-271.
17. Curran V, Heath O, Adey T, et al. An approach to integrating interprofessional education in collaborative mental health care. Acad Psychiatry. 2012;36(2):91-95.
18. Veterans Health Administration. VA Interprofessional Mental Health Education Expansion Initiative, Phase I. Washington, DC; Department of Veterans Affairs; 2012.
19. Dundon M, Dollar K, Schohn M, Lantinga LJ. Primary care–mental health integration: co-located, collaborative care: an operations manual. https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/MH-IPC_CCC_Operations_Manual_Version_2_1.pdf. Published February 2011. Accessed May 19, 2017.
20. McDaniel SH, Grus CL, Cubic BA, et al. Competencies for psychology practice in primary care. Am Psychol. 2014;69(4):409-429.
21. Cowley D, Dunaway K, Forstein M, et al. Teaching psychiatry residents to work at the interface of mental health and primary care. Acad Psychiatry. 2014;38(4):398-404.
22. Herrmann N. The creative brain. J Creat Behav. 1991;25:275-295.
23. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
24. Pierson HM, Hayes SC, Gifford EV, et al. An examination of the Motivational Interviewing Treatment Integrity code. J Subst Abuse Treat. 2007;32(1):11-17.
25. Shaw D, Blue A. Should psychiatry champion interprofessional education? Acad Psychiatry. 2012;36(3):163-166.
26. Gilbert JH. Interprofessional learning and higher education structural barriers. J Interprof Care. 2005;19(suppl 1):87-106.
27. Heinemann GD, Schmitt MH, Farrell PP, Brallier SA. Development of an Attitudes Toward Health Care Teams Scale. Eval Health Prof. 1999;22(1):123-142.
28. Hepburn K, Tsukuda RA, Fasser C. Team Skills Scale. In: Heinemann GD, Zeiss AM, eds. Team Performance in Healthcare: Assessment and Development. New York, NY: Kluwer Academic/Plenum; 2002:159-163.
Over the past 10 years, the VHA has been a national leader in primary care-mental health integration (PC-MHI) within patient aligned care teams (PACTs).1,2 Studies of the PC-MHI collaborative care model consistently have shown increased access to MH services, higher levels of MH treatment engagement, improved MH treatment outcomes, and high patient and provider satisfaction.3-7 Primary care-mental health integration relies heavily on interprofessional team-based practice with providers from diverse educational and clinical backgrounds who work together to deliver integrated mental and behavioral health services within PACTs. This model requires a unique blending of professional cultures and communication and practice styles.
To sustain PC-MHI in PACT, health care professionals (HCPs) must be well trained to work effectively in interprofessional teams. Across health care organizations, training in collaborative interprofessional team-based practice has been identified as an important and challenging task.8-11
Integrating educational experiences among different HCP learners is an approach to developing competency in interprofessional collaboration early in training. The World Health Organization defined interprofessional education (IPE) as occurring “when students from two or more professions learn about, from, and with each other to enable effective collaboration and improve health outcomes.”9 Fundamental to this definition is the belief that interaction among learners from different disciplines during their training develops competency in subsequent effective collaborative practice. Studies of IPE in MH professional training have found that prelicensure IPE contributes to increased knowledge of roles and responsibilities of different disciplines, improved interprofessional communication and attitudes, and increased willingness to work in teams.12-17
Interprofessional education is a valuable training model, but developing interprofessional learning experiences in a system of diverse and often siloed training programs is difficult. More information about design and implementation of IPE training experiences is needed, particularly in outpatient settings in which integration of traditionally separate discipline-specific care is central to the health care mission. The VA PACT PC-MHI is a strong team-based care model that represents a unique opportunity for training across disciplines in interprofessional collaborative care.
To find innovative approaches to meeting the need for IPE in PACT PC-MHI, the authors developed a new IPE program in PC-MHI at the William S. Middleton Memorial Veterans Hospital (WSMMVH) in Madison, Wisconsin. This article reviews the development, implementation, and first-year evaluation of the training program and discusses the challenges and the IPE areas in need of improvement in PACT PC-MHI.
Methods
In 2012, the VHA launched phase 1 of the Mental Health Education Expansion Initiative (MHEEI), a collaboration of the Office of Academic Affiliations (OAA), VHA Mental Health Services (VHA-MHS), and the Office of Mental Health Operations (OMHO).18 The MHEEI was intended to “increase expertise in critical areas of need, expand the recruitment pipeline of well-trained, highly qualified health care providers in behavioral and mental health disciplines, and promote the utilization of interprofessional team-based care.”18 In response, WSMMVH organized a planning committee and submitted a funding request through the section of MHEEI called PACT With Integrated Behavioral Health Providers. The planning committee included training program directors and staff from psychiatry, pharmacy, social work, psychology, and primary care. The authors received funding for trainees in psychiatry (postgraduate year 4 [PGY-4]), pharmacy/MH residency (PGY-2), pharmacy/ambulatory care (PGY-1), and social work (interns).
Curriculum Development
The planning committee met regularly for 6 months to develop the organization, learning objectives, educational strategies, and implementation plan for the IPE program. The program was organized as a 4- to 12-month clinical rotation with the PC-MHI team in PACT, combined with 12 months of protected weekly IPE time (Table 1).
Learning Objectives
To better understand the educational needs and foci for learning objectives, the interprofessional planning committee reviewed guidelines on training in integrated care and collaborative team-based practice.2,9,10,19-21 These guidelines were compared with existing training opportunities for each discipline to identify training gaps and needs.
Learning objectives were organized into 3 domains: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Table 2 outlines the shared learning objectives linked to each domain that were common to the psychiatry, pharmacy, and social work disciplines. Although many of the learning objectives were shared among all disciplines, each trainee also had discipline-specific clinical activities and learning objectives. Psychiatry and pharmacy residents focused on primary care psychiatric medication consultation and care management for antidepressant medication starts. Social work interns focused on psychosocial and functional assessment and brief problem-focused psychotherapies. Learning objectives were met through direct veteran care in the primary care clinic as part of the PACT PC-MHI team and through interprofessional learning activities during protected weekly education time.
Implementation
Critical stakeholders in implementing the IPE program involved themselves early and throughout the planning process. Stakeholders included VAMC leadership, primary care and MH service line chiefs and clinic managers, training program directors, and PACT staff. Planning committee members gave presentations on the IPE program at MH service line and PACT meetings in the 2 months before program initiation in order to orient staff to learning objectives, program structure, and impact on PACT PC-MHI operations. Throughout the first year, the planning committee continued to meet every 2 weeks to review progress, solve implementation problems, and revise learning objectives and activities.
Trainee Clinical Activities
A wide range of educational strategies were planned to meet learning objectives across the 3 domains. There was strong emphasis on experiential learning through daily PACT and PC-MHI clinical work, team huddles and meetings, and trainee-led program improvement projects.
Psychiatry and PGY-2 pharmacy/MH residents focused on direct and indirect medication consultation and problem-focused assessments. Their clinical activities included PC-MHI medication evaluation and follow-up visits; chart reviews and e-consults for medication recommendations to PACT providers; reviews of care management data and consultations on veterans enrolled in depression and anxiety care management; “curbside consultations” for providers in PACT huddles and meetings and throughout the clinic day; and “warm handoffs,” same-day initial PC-MHI problem-focused assessments performed on PACT provider request. The residents were part of a pool of staff and trainees who performed these assessments.
PGY-1 pharmacy residents made care management phone calls for antidepressant trials for depression and anxiety. These residents were trained in motivational interviewing (MI). They applied their MI skills during care management calls focused on medication adherence and behavioral interventions for depression (eg, exercise, planning pleasurable activity) and during other clinical rotations, including tobacco cessation and medication management for diabetes and hypertension. Particularly challenging veteran cases from these clinics were cosupervised with medication management and PC-MHIstaff for added consultation on engagement, behavior change, and treatment plan adherence.
Social work interns completed initial PC-MHI psychosocial and functional assessments by phone and directly by same-day warm handoffs from PACT staff. The PC-MHI therapies they provided included problem-solving therapy, behavioral activation, stress management based on cognitive behavioral therapy, and brief alcohol interventions.
Group IPE Activities
All trainees had a weekly protected block of 3 hours during which they came together for group IPE that was designed to elicit active participation; facilitate interprofessional communication; and develop an understanding of and respect for the knowledge, culture, and practice style of the different disciplines.
Trainees participated in a Herrmann Brain Dominance Instrument (HBDI) workshop focused on developing a better understanding of individual differences in thinking and problem solving, with the goal of improving communication and learning within teams.22 In a seminar series on professionalism and boundaries in health care, trainees from each discipline gave a presentation on the traditional structure and content of their discipline’s training and discussed similarities and differences in their disciplines’ professional oaths, codes of ethics, and boundary guidelines.
Motivational interviewing training was conducted early in the year so trainees would be prepared to apply MI skills in their daily PACT PC-MHI clinical work. Motivational inteviewing is a patient-centered approach to engaging patients in health promoting behavior change. It is defined as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence.”23
Trainees recorded MI sessions with at least 2 live-patient visits and at least 2 simulated-patient interviews (with staff serving as patient actors). The structure of MI training and supervision was deliberately designed to facilitate interprofessional communication and learning. In accord with a group supervision model for MI recorded reviews, the trainees presented their tapes to the entire learning group in the presence of a facilitating supervisor. Trainees had the opportunity to observe different interview styles and exchange feedback within a peer group of interprofessional learners.
Seminars were focused on core PC-MHI clinical content (eg, depression, anxiety, alcohol use disorders) and organized around case-based learning. Trainees divided into small teams in which representatives of each discipline offered their perspective on how to approach planning patient assessment and treatment. During the seminars, the authors engaged trainees as teachers and leaders whenever possible. All trainees presented on a topic in which they had some discipline expertise. For example, social work interns led a seminar on support and social services for victims of domestic violence, and PGY-1 pharmacy/ambulatory care residents led seminars and a panel management project focused on diabetes and depression.
Trainees participated in several PACT PC-MHI projects focused on population- and measurement-based care, panel management, and program improvement (Table 3). Protected IPE time was used to teach trainees about population health principles and different tools for process improvement (eg, Vision-Analysis Team-Aim-Map-Measure-Change-Sustain) and provide a forum in which trainees could share their work with one another.
Evaluations
Several tools were used for trainee and program evaluations. Clinical skills were evaluated during daily supervision. Trainees began the year with PC-MHI staff directly observing all their clinical contacts with veterans. Staff evaluated and offered feedback on trainee clinical interviewing and on assessment and treatment planning skills. Once they were assessed to be ready to see veterans on their own, trainees were advanced by staff to “drop-in” direct supervision: Toward the end of a veteran’s visit, a staff preceptor entered the room to review relevant clinical findings, assessment and finalized treatment planning with the trainee and veteran. When appropriate for trainee competence level, clinical contacts were indirectly supervised: Trainees discussed their assessment and treatment plan with a staff supervisor at the end of the day.
Motivational interviewing recordings were reviewed during group supervision. To objectively evaluate MI skills, supervisors who were VA-certified in MI used the Motivational Interviewing Treatment Integrity (MITI) coding tool to review and code both the live- and simulated-patient recordings.24 The MITI coding involves quantitative and qualitative analysis using standardized coding items.
Quantitative items included percentage of open-ended questions (Proficiency: 50%; competency: 70%); percentage of reflections considered complex reflections, or reflective statements adding substantial meaning or emphasis and conveying a deeper or more complex picture of what patients say (Proficiency: 40%; competency: 50%); reflection-to-question ratio (Proficiency: 1:1; competency: 2:1); and percentage of MI-adherent provider statements (Proficiency: 90%; competency: 100%).
Qualitative coding items were a global rating of therapist “empathy,” which evaluated the extent to which the trainee understood or made an effort to grasp the patient’s perspective, and “MI spirit.” This coding intended to capture the overall competence of the trainee in emphasizing collaboration, patient autonomy, and evocation of the patient experience (Proficiency score: 5; competency score: 6).
The PC-MHI teaching staff met midyear and end of year as a team to complete trainee evaluations focused on the 3 areas of learning objectives: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Patient-centered PC-MHI involves direct observation and supervision of trainee clinical contacts with veterans, including assessment and treatment planning, clinical documentation, and review of live- and simulated-patient MI recordings. Collaborative team-based practice involves review of trainee participation in day-to-day teamwork, huddles, team meetings, and IPE activities. Population health and program improvement involves review of trainee work on a panel measurement-based care management or program improvement project. In each learning objectives area, trainees were rated on a 3-point scale: needs improvement (1); satisfactory (2); achieved (3). Core knowledge about PC-MHI evidence base, structure, and clinical topics was assessed with case-based written examination at midyear and end of year.
Surveys and qualitative interviews were used to assess trainee perceptions about the IPE program. A midyear and end-of-year survey assessed trainee satisfaction and perceived efficacy of the IPE training program in meeting core learning objectives. A midyear survey designed by pharmacy residents as part of their program improvement project evaluated attitudes around interprofessional learning and team practice. Trainees met individually with the PC-MHI IPE director at midyear and end of year to gather qualitative feedback on the IPE program.
Outcomes
All trainees advanced to the expected level of supervision for clinical contacts (drop-in or indirect clinical supervision). Over the year, there was significant improvement in trainees’ MI skills as measured by MITI coding of at least 2 live-patient or 2 simulated-patient recordings (Figures 1A and 1B). By end of year, most trainees had reached proficiency or competency in several MITI coded items: percentage of open-ended questions (4/12 proficient, 8/12 competent), percentage of complex reflections (2/12 less than proficient, 3/12 proficient, 7/12 competent), MI adherence (1/12 proficient, 11/12 competent), global empathy rating (2/12 proficient, 10/12 competent), and global MI spirit rating (12/12 competent). Average reflection-to-question ratio for the trainee group increased from 0.63 to 0.96 from midyear to end of year, but only 3 of 12 trainees reached the proficiency level of a 1:1 ratio, and no trainee reached the competency level of a 2:1 ratio.
According to the PC-MHI team’s midyear evaluation, most trainees were already making satisfactory progress in the 3 domains of learning objectives for the training program. At end of year, 13 of 14 trainees were assessed as competent in all 3 domains (Figures 2A and B). All trainees passed the midyear and end-of-year written examinations with overall high scores (average score, 82%) demonstrating acquisition of core PC-MHI clinical knowledge.
Trainee evaluations of the IPE program were overall highly favorable at both midyear and at end of year. Trainees rated the program effective or extremely effective in developing their skills in patient-centered care, interprofessional communication, and collaborative team-based practice. They also rated the program highly effective in preparing them to use team-based practice skills in other settings. On a midyear survey, trainees moderately to strongly agreed with several positive beliefs and attitudes about team-based care.
In qualitative interviews at program completion, trainees across disciplines rated the MI training with group supervision as one of their most valuable interprofessional learning experiences. Other highly valued training experiences were PACT PC-MHI panel management projects, team-based clinical case reviews, and cross-disciplinary supervision.
Discussion
This article describes the successful development and implementation of a VA-based IPE program in PACT PC-MHI. Interprofessional clinical training and educational experiences were highly valued, and trainees identified positive attitudes and improved skills related to team-based care. These findings support previous findings that IPE is associated with high satisfaction and positive attitudes toward team-based collaborative practice.12-17 Program implementation presented several challenges: nonsynchronous academic calendars and rotation schedules, cross-disciplinary supervision regulations, variations in clinical and supervisory requirements for accreditation standards, the traditional health care hierarchy, and measurement of the impact of IPE experiences.11,25,26
Rotation schedules and academic calendars varied across the psychiatry, pharmacy, and social work home programs. Organizing different trainee rotation schedules was a significant challenge. Collaboration with training directors and support staff was crucial in planning rotations that offered a longitudinal training experience in PACT PC-MHI. Given the participants’ different starting dates, protected IPE time early in the calendar year was focused on developing clinical skills specific to the pharmacy and psychiatry trainees who would be starting in July, and IPE activities that required the presence of all trainee disciplines (eg, MI training, HBDI) were planned for after the September start of the social work interns.
Cross-disciplinary supervision was highly valued by trainees because it offered exposure to the disciplines’ different communication styles and approaches to clinical assessment and decision making. Throughout the year, however, trainees encountered several obstacles to cross-disciplinary supervision, with respect to coding/billing and home program supervisory policies. The authors worked closely with the VA administration and each training program to develop and revise supervising policies and procedures to meet the necessary administrative and program supervisory requirements for accreditation standards.
In some cases, this work resulted in dual supervision by a preceptor of the same discipline (to meet requirements for coding/billing and home program supervision) and clinical supervision by a preceptor of a different discipline (eg, in team meetings or in clinical case reviews during protected IPE time). Preceptors from each discipline met regularly to discuss challenges in cross-disciplinary supervision, review scope-of-practice issues, share information on discipline-specific training, and revise supervisory procedures.
Interprofessional education activities during weekly protected time were designed to improve collaboration and to challenge trainees to examine traditional hierarchical roles and patterns of communication in health care. An emphasis on case-based learning in small groups encouraged trainees to share perspectives on their discipline-specific approach to assessment and treatment planning. Motivational interviewing training, one of the IPE experiences most valued by trainees and staff, created the opportunity for a truly shared learning environment, as trainees largely started at about the same skill level despite having different educational backgrounds. Group supervision of MI recordings offered trainees the opportunity to learn from each other and develop comfort in offering and receiving interprofessional constructive feedback.
Limitations
There were considerable methodologic limitations in the authors’ efforts to evaluate the impact of this training program on trainee attitudes and skills in collaborative team-based practice. Although trainee surveys revealed highly positive attitudes and beliefs about team-based care as well as perceived competence in collaborative practice, these were not validated surveys, and changes could not be accurately measured over time (trainees were not assessed at baseline). Trainees also were involved in other clinical teams within the VA during the year, and it is difficult to assess the specific impact of their PC-MHI IPE experiences. The PC-MHI staff evaluations of trainee competence in collaborative team-based practice were largely observational and potentially vulnerable to biases, as the staff evaluating trainee competence also were part of the IPE program planning process and invested in its success.
To address these limitations in the future, the authors will use better assessment tools at baseline and end of year to more effectively evaluate the impact of the training program on teamwork skills as well changes in attitudes and beliefs about interprofessional learning and teamwork. The Attitudes Toward Interprofessional Health Care Teams Scale and the Perceptions of Effective Interpersonal Teams Scale should be considered as they have published reliability and validity.27,28 The authors could improve the reliability and depth of trainee evaluations with use of a “360-degree evaluation” model for trainee evaluations that includes other PACT members (beyond PC-MHI staff) as well as veterans.
Assessment of MI skills and competency was limited by use of both live- and simulated-patient recordings. Simulation is a valuable learning tool but often does not accurately represent actual clinical situations and challenges. To appropriately assess MI competence, future MI training should emphasize live-patient recordings over simulated-patient visits. Furthermore, whereas trainees reached competency in several MI coding items, none reached competency in the reflection-to-question ratio, and only about half reached competency in percentage of complex reflections. Future MI training will need to focus on further development of reflection skills.
Trainee intentions to remain involved in program improvement and collaborative team-based care in future professional work were core attitudinal learning objectives, but neither was adequately assessed in end-of-year surveys. Ideally, future evaluations will assess subjective trainee intentions and goals around team-based work and objectively measure future professional choices and activities. For example, it would be interesting to determine whether trainees who participated in this program will choose to practice in an interprofessional team-based model or participate in program improvement activities.
Last, the absence of psychology interns was considered a weakness in the learning environment, resulting in a relatively “prescriber-heavy” balance in discipline perspectives for IPE-focused case discussions and other training. Furthermore, the discipline representation in the IPE program did not exemplify the typical PC-MHI team in most VA clinic settings and community practices in which psychology has a strong presence. Adding psychology trainees was an important goal for the IPE program leadership. In 2015, WSMMVH was awarded additional funding for a PACT PC-MHI predoctoral psychology intern through the phase 4 MHEEI. The PC-MHI track psychology intern joined the IPE program in the 2016-2017 training year.
Conclusion
There is broad consensus that interprofessional team-based practice is crucial for providers in the VA and all health care systems. Primary care-mental health integration is an area of VA care in which interprofessional collaboration is uniquely important to implementation and sustainability of the model. Interprofessional education is an effective approach for preparing HCPs for team-based practice, but implementation is challenging. Several factors crucially contributed to the successful implementation of this program: collaboration of the interprofessional planning team with representation from key stakeholders in different departments and training programs; a well-established PACT PC-MHI team with experience in collaborative team-based care; a curriculum structure that emphasized experiential educational strategies designed to promote interprofessional learning and communication; VA leadership support at the national level (MHEEI funding) and local level; and PACT PC-MHI clinical staff committed to teaching and to the IPE mission.
Over the past 10 years, the VHA has been a national leader in primary care-mental health integration (PC-MHI) within patient aligned care teams (PACTs).1,2 Studies of the PC-MHI collaborative care model consistently have shown increased access to MH services, higher levels of MH treatment engagement, improved MH treatment outcomes, and high patient and provider satisfaction.3-7 Primary care-mental health integration relies heavily on interprofessional team-based practice with providers from diverse educational and clinical backgrounds who work together to deliver integrated mental and behavioral health services within PACTs. This model requires a unique blending of professional cultures and communication and practice styles.
To sustain PC-MHI in PACT, health care professionals (HCPs) must be well trained to work effectively in interprofessional teams. Across health care organizations, training in collaborative interprofessional team-based practice has been identified as an important and challenging task.8-11
Integrating educational experiences among different HCP learners is an approach to developing competency in interprofessional collaboration early in training. The World Health Organization defined interprofessional education (IPE) as occurring “when students from two or more professions learn about, from, and with each other to enable effective collaboration and improve health outcomes.”9 Fundamental to this definition is the belief that interaction among learners from different disciplines during their training develops competency in subsequent effective collaborative practice. Studies of IPE in MH professional training have found that prelicensure IPE contributes to increased knowledge of roles and responsibilities of different disciplines, improved interprofessional communication and attitudes, and increased willingness to work in teams.12-17
Interprofessional education is a valuable training model, but developing interprofessional learning experiences in a system of diverse and often siloed training programs is difficult. More information about design and implementation of IPE training experiences is needed, particularly in outpatient settings in which integration of traditionally separate discipline-specific care is central to the health care mission. The VA PACT PC-MHI is a strong team-based care model that represents a unique opportunity for training across disciplines in interprofessional collaborative care.
To find innovative approaches to meeting the need for IPE in PACT PC-MHI, the authors developed a new IPE program in PC-MHI at the William S. Middleton Memorial Veterans Hospital (WSMMVH) in Madison, Wisconsin. This article reviews the development, implementation, and first-year evaluation of the training program and discusses the challenges and the IPE areas in need of improvement in PACT PC-MHI.
Methods
In 2012, the VHA launched phase 1 of the Mental Health Education Expansion Initiative (MHEEI), a collaboration of the Office of Academic Affiliations (OAA), VHA Mental Health Services (VHA-MHS), and the Office of Mental Health Operations (OMHO).18 The MHEEI was intended to “increase expertise in critical areas of need, expand the recruitment pipeline of well-trained, highly qualified health care providers in behavioral and mental health disciplines, and promote the utilization of interprofessional team-based care.”18 In response, WSMMVH organized a planning committee and submitted a funding request through the section of MHEEI called PACT With Integrated Behavioral Health Providers. The planning committee included training program directors and staff from psychiatry, pharmacy, social work, psychology, and primary care. The authors received funding for trainees in psychiatry (postgraduate year 4 [PGY-4]), pharmacy/MH residency (PGY-2), pharmacy/ambulatory care (PGY-1), and social work (interns).
Curriculum Development
The planning committee met regularly for 6 months to develop the organization, learning objectives, educational strategies, and implementation plan for the IPE program. The program was organized as a 4- to 12-month clinical rotation with the PC-MHI team in PACT, combined with 12 months of protected weekly IPE time (Table 1).
Learning Objectives
To better understand the educational needs and foci for learning objectives, the interprofessional planning committee reviewed guidelines on training in integrated care and collaborative team-based practice.2,9,10,19-21 These guidelines were compared with existing training opportunities for each discipline to identify training gaps and needs.
Learning objectives were organized into 3 domains: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Table 2 outlines the shared learning objectives linked to each domain that were common to the psychiatry, pharmacy, and social work disciplines. Although many of the learning objectives were shared among all disciplines, each trainee also had discipline-specific clinical activities and learning objectives. Psychiatry and pharmacy residents focused on primary care psychiatric medication consultation and care management for antidepressant medication starts. Social work interns focused on psychosocial and functional assessment and brief problem-focused psychotherapies. Learning objectives were met through direct veteran care in the primary care clinic as part of the PACT PC-MHI team and through interprofessional learning activities during protected weekly education time.
Implementation
Critical stakeholders in implementing the IPE program involved themselves early and throughout the planning process. Stakeholders included VAMC leadership, primary care and MH service line chiefs and clinic managers, training program directors, and PACT staff. Planning committee members gave presentations on the IPE program at MH service line and PACT meetings in the 2 months before program initiation in order to orient staff to learning objectives, program structure, and impact on PACT PC-MHI operations. Throughout the first year, the planning committee continued to meet every 2 weeks to review progress, solve implementation problems, and revise learning objectives and activities.
Trainee Clinical Activities
A wide range of educational strategies were planned to meet learning objectives across the 3 domains. There was strong emphasis on experiential learning through daily PACT and PC-MHI clinical work, team huddles and meetings, and trainee-led program improvement projects.
Psychiatry and PGY-2 pharmacy/MH residents focused on direct and indirect medication consultation and problem-focused assessments. Their clinical activities included PC-MHI medication evaluation and follow-up visits; chart reviews and e-consults for medication recommendations to PACT providers; reviews of care management data and consultations on veterans enrolled in depression and anxiety care management; “curbside consultations” for providers in PACT huddles and meetings and throughout the clinic day; and “warm handoffs,” same-day initial PC-MHI problem-focused assessments performed on PACT provider request. The residents were part of a pool of staff and trainees who performed these assessments.
PGY-1 pharmacy residents made care management phone calls for antidepressant trials for depression and anxiety. These residents were trained in motivational interviewing (MI). They applied their MI skills during care management calls focused on medication adherence and behavioral interventions for depression (eg, exercise, planning pleasurable activity) and during other clinical rotations, including tobacco cessation and medication management for diabetes and hypertension. Particularly challenging veteran cases from these clinics were cosupervised with medication management and PC-MHIstaff for added consultation on engagement, behavior change, and treatment plan adherence.
Social work interns completed initial PC-MHI psychosocial and functional assessments by phone and directly by same-day warm handoffs from PACT staff. The PC-MHI therapies they provided included problem-solving therapy, behavioral activation, stress management based on cognitive behavioral therapy, and brief alcohol interventions.
Group IPE Activities
All trainees had a weekly protected block of 3 hours during which they came together for group IPE that was designed to elicit active participation; facilitate interprofessional communication; and develop an understanding of and respect for the knowledge, culture, and practice style of the different disciplines.
Trainees participated in a Herrmann Brain Dominance Instrument (HBDI) workshop focused on developing a better understanding of individual differences in thinking and problem solving, with the goal of improving communication and learning within teams.22 In a seminar series on professionalism and boundaries in health care, trainees from each discipline gave a presentation on the traditional structure and content of their discipline’s training and discussed similarities and differences in their disciplines’ professional oaths, codes of ethics, and boundary guidelines.
Motivational interviewing training was conducted early in the year so trainees would be prepared to apply MI skills in their daily PACT PC-MHI clinical work. Motivational inteviewing is a patient-centered approach to engaging patients in health promoting behavior change. It is defined as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence.”23
Trainees recorded MI sessions with at least 2 live-patient visits and at least 2 simulated-patient interviews (with staff serving as patient actors). The structure of MI training and supervision was deliberately designed to facilitate interprofessional communication and learning. In accord with a group supervision model for MI recorded reviews, the trainees presented their tapes to the entire learning group in the presence of a facilitating supervisor. Trainees had the opportunity to observe different interview styles and exchange feedback within a peer group of interprofessional learners.
Seminars were focused on core PC-MHI clinical content (eg, depression, anxiety, alcohol use disorders) and organized around case-based learning. Trainees divided into small teams in which representatives of each discipline offered their perspective on how to approach planning patient assessment and treatment. During the seminars, the authors engaged trainees as teachers and leaders whenever possible. All trainees presented on a topic in which they had some discipline expertise. For example, social work interns led a seminar on support and social services for victims of domestic violence, and PGY-1 pharmacy/ambulatory care residents led seminars and a panel management project focused on diabetes and depression.
Trainees participated in several PACT PC-MHI projects focused on population- and measurement-based care, panel management, and program improvement (Table 3). Protected IPE time was used to teach trainees about population health principles and different tools for process improvement (eg, Vision-Analysis Team-Aim-Map-Measure-Change-Sustain) and provide a forum in which trainees could share their work with one another.
Evaluations
Several tools were used for trainee and program evaluations. Clinical skills were evaluated during daily supervision. Trainees began the year with PC-MHI staff directly observing all their clinical contacts with veterans. Staff evaluated and offered feedback on trainee clinical interviewing and on assessment and treatment planning skills. Once they were assessed to be ready to see veterans on their own, trainees were advanced by staff to “drop-in” direct supervision: Toward the end of a veteran’s visit, a staff preceptor entered the room to review relevant clinical findings, assessment and finalized treatment planning with the trainee and veteran. When appropriate for trainee competence level, clinical contacts were indirectly supervised: Trainees discussed their assessment and treatment plan with a staff supervisor at the end of the day.
Motivational interviewing recordings were reviewed during group supervision. To objectively evaluate MI skills, supervisors who were VA-certified in MI used the Motivational Interviewing Treatment Integrity (MITI) coding tool to review and code both the live- and simulated-patient recordings.24 The MITI coding involves quantitative and qualitative analysis using standardized coding items.
Quantitative items included percentage of open-ended questions (Proficiency: 50%; competency: 70%); percentage of reflections considered complex reflections, or reflective statements adding substantial meaning or emphasis and conveying a deeper or more complex picture of what patients say (Proficiency: 40%; competency: 50%); reflection-to-question ratio (Proficiency: 1:1; competency: 2:1); and percentage of MI-adherent provider statements (Proficiency: 90%; competency: 100%).
Qualitative coding items were a global rating of therapist “empathy,” which evaluated the extent to which the trainee understood or made an effort to grasp the patient’s perspective, and “MI spirit.” This coding intended to capture the overall competence of the trainee in emphasizing collaboration, patient autonomy, and evocation of the patient experience (Proficiency score: 5; competency score: 6).
The PC-MHI teaching staff met midyear and end of year as a team to complete trainee evaluations focused on the 3 areas of learning objectives: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Patient-centered PC-MHI involves direct observation and supervision of trainee clinical contacts with veterans, including assessment and treatment planning, clinical documentation, and review of live- and simulated-patient MI recordings. Collaborative team-based practice involves review of trainee participation in day-to-day teamwork, huddles, team meetings, and IPE activities. Population health and program improvement involves review of trainee work on a panel measurement-based care management or program improvement project. In each learning objectives area, trainees were rated on a 3-point scale: needs improvement (1); satisfactory (2); achieved (3). Core knowledge about PC-MHI evidence base, structure, and clinical topics was assessed with case-based written examination at midyear and end of year.
Surveys and qualitative interviews were used to assess trainee perceptions about the IPE program. A midyear and end-of-year survey assessed trainee satisfaction and perceived efficacy of the IPE training program in meeting core learning objectives. A midyear survey designed by pharmacy residents as part of their program improvement project evaluated attitudes around interprofessional learning and team practice. Trainees met individually with the PC-MHI IPE director at midyear and end of year to gather qualitative feedback on the IPE program.
Outcomes
All trainees advanced to the expected level of supervision for clinical contacts (drop-in or indirect clinical supervision). Over the year, there was significant improvement in trainees’ MI skills as measured by MITI coding of at least 2 live-patient or 2 simulated-patient recordings (Figures 1A and 1B). By end of year, most trainees had reached proficiency or competency in several MITI coded items: percentage of open-ended questions (4/12 proficient, 8/12 competent), percentage of complex reflections (2/12 less than proficient, 3/12 proficient, 7/12 competent), MI adherence (1/12 proficient, 11/12 competent), global empathy rating (2/12 proficient, 10/12 competent), and global MI spirit rating (12/12 competent). Average reflection-to-question ratio for the trainee group increased from 0.63 to 0.96 from midyear to end of year, but only 3 of 12 trainees reached the proficiency level of a 1:1 ratio, and no trainee reached the competency level of a 2:1 ratio.
According to the PC-MHI team’s midyear evaluation, most trainees were already making satisfactory progress in the 3 domains of learning objectives for the training program. At end of year, 13 of 14 trainees were assessed as competent in all 3 domains (Figures 2A and B). All trainees passed the midyear and end-of-year written examinations with overall high scores (average score, 82%) demonstrating acquisition of core PC-MHI clinical knowledge.
Trainee evaluations of the IPE program were overall highly favorable at both midyear and at end of year. Trainees rated the program effective or extremely effective in developing their skills in patient-centered care, interprofessional communication, and collaborative team-based practice. They also rated the program highly effective in preparing them to use team-based practice skills in other settings. On a midyear survey, trainees moderately to strongly agreed with several positive beliefs and attitudes about team-based care.
In qualitative interviews at program completion, trainees across disciplines rated the MI training with group supervision as one of their most valuable interprofessional learning experiences. Other highly valued training experiences were PACT PC-MHI panel management projects, team-based clinical case reviews, and cross-disciplinary supervision.
Discussion
This article describes the successful development and implementation of a VA-based IPE program in PACT PC-MHI. Interprofessional clinical training and educational experiences were highly valued, and trainees identified positive attitudes and improved skills related to team-based care. These findings support previous findings that IPE is associated with high satisfaction and positive attitudes toward team-based collaborative practice.12-17 Program implementation presented several challenges: nonsynchronous academic calendars and rotation schedules, cross-disciplinary supervision regulations, variations in clinical and supervisory requirements for accreditation standards, the traditional health care hierarchy, and measurement of the impact of IPE experiences.11,25,26
Rotation schedules and academic calendars varied across the psychiatry, pharmacy, and social work home programs. Organizing different trainee rotation schedules was a significant challenge. Collaboration with training directors and support staff was crucial in planning rotations that offered a longitudinal training experience in PACT PC-MHI. Given the participants’ different starting dates, protected IPE time early in the calendar year was focused on developing clinical skills specific to the pharmacy and psychiatry trainees who would be starting in July, and IPE activities that required the presence of all trainee disciplines (eg, MI training, HBDI) were planned for after the September start of the social work interns.
Cross-disciplinary supervision was highly valued by trainees because it offered exposure to the disciplines’ different communication styles and approaches to clinical assessment and decision making. Throughout the year, however, trainees encountered several obstacles to cross-disciplinary supervision, with respect to coding/billing and home program supervisory policies. The authors worked closely with the VA administration and each training program to develop and revise supervising policies and procedures to meet the necessary administrative and program supervisory requirements for accreditation standards.
In some cases, this work resulted in dual supervision by a preceptor of the same discipline (to meet requirements for coding/billing and home program supervision) and clinical supervision by a preceptor of a different discipline (eg, in team meetings or in clinical case reviews during protected IPE time). Preceptors from each discipline met regularly to discuss challenges in cross-disciplinary supervision, review scope-of-practice issues, share information on discipline-specific training, and revise supervisory procedures.
Interprofessional education activities during weekly protected time were designed to improve collaboration and to challenge trainees to examine traditional hierarchical roles and patterns of communication in health care. An emphasis on case-based learning in small groups encouraged trainees to share perspectives on their discipline-specific approach to assessment and treatment planning. Motivational interviewing training, one of the IPE experiences most valued by trainees and staff, created the opportunity for a truly shared learning environment, as trainees largely started at about the same skill level despite having different educational backgrounds. Group supervision of MI recordings offered trainees the opportunity to learn from each other and develop comfort in offering and receiving interprofessional constructive feedback.
Limitations
There were considerable methodologic limitations in the authors’ efforts to evaluate the impact of this training program on trainee attitudes and skills in collaborative team-based practice. Although trainee surveys revealed highly positive attitudes and beliefs about team-based care as well as perceived competence in collaborative practice, these were not validated surveys, and changes could not be accurately measured over time (trainees were not assessed at baseline). Trainees also were involved in other clinical teams within the VA during the year, and it is difficult to assess the specific impact of their PC-MHI IPE experiences. The PC-MHI staff evaluations of trainee competence in collaborative team-based practice were largely observational and potentially vulnerable to biases, as the staff evaluating trainee competence also were part of the IPE program planning process and invested in its success.
To address these limitations in the future, the authors will use better assessment tools at baseline and end of year to more effectively evaluate the impact of the training program on teamwork skills as well changes in attitudes and beliefs about interprofessional learning and teamwork. The Attitudes Toward Interprofessional Health Care Teams Scale and the Perceptions of Effective Interpersonal Teams Scale should be considered as they have published reliability and validity.27,28 The authors could improve the reliability and depth of trainee evaluations with use of a “360-degree evaluation” model for trainee evaluations that includes other PACT members (beyond PC-MHI staff) as well as veterans.
Assessment of MI skills and competency was limited by use of both live- and simulated-patient recordings. Simulation is a valuable learning tool but often does not accurately represent actual clinical situations and challenges. To appropriately assess MI competence, future MI training should emphasize live-patient recordings over simulated-patient visits. Furthermore, whereas trainees reached competency in several MI coding items, none reached competency in the reflection-to-question ratio, and only about half reached competency in percentage of complex reflections. Future MI training will need to focus on further development of reflection skills.
Trainee intentions to remain involved in program improvement and collaborative team-based care in future professional work were core attitudinal learning objectives, but neither was adequately assessed in end-of-year surveys. Ideally, future evaluations will assess subjective trainee intentions and goals around team-based work and objectively measure future professional choices and activities. For example, it would be interesting to determine whether trainees who participated in this program will choose to practice in an interprofessional team-based model or participate in program improvement activities.
Last, the absence of psychology interns was considered a weakness in the learning environment, resulting in a relatively “prescriber-heavy” balance in discipline perspectives for IPE-focused case discussions and other training. Furthermore, the discipline representation in the IPE program did not exemplify the typical PC-MHI team in most VA clinic settings and community practices in which psychology has a strong presence. Adding psychology trainees was an important goal for the IPE program leadership. In 2015, WSMMVH was awarded additional funding for a PACT PC-MHI predoctoral psychology intern through the phase 4 MHEEI. The PC-MHI track psychology intern joined the IPE program in the 2016-2017 training year.
Conclusion
There is broad consensus that interprofessional team-based practice is crucial for providers in the VA and all health care systems. Primary care-mental health integration is an area of VA care in which interprofessional collaboration is uniquely important to implementation and sustainability of the model. Interprofessional education is an effective approach for preparing HCPs for team-based practice, but implementation is challenging. Several factors crucially contributed to the successful implementation of this program: collaboration of the interprofessional planning team with representation from key stakeholders in different departments and training programs; a well-established PACT PC-MHI team with experience in collaborative team-based care; a curriculum structure that emphasized experiential educational strategies designed to promote interprofessional learning and communication; VA leadership support at the national level (MHEEI funding) and local level; and PACT PC-MHI clinical staff committed to teaching and to the IPE mission.
1. Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105-116.
2. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
3. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of loner-term outcomes. Arch Intern Med. 2006;166(21):2314-2321.
4. Unützer J, Katon W, Callahan CM, et al; IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) Investigators. Collaborative care management in late-life depression in the primary care setting: a randomized control trial. JAMA. 2002;288(22):2836-2845.
5. Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;3(4):305-310.
6. Bruce ML, Tenhave TR, Reynolds CF III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091.
7. Alexopoulos GS, Reynolds CF III , Bruce ML, et al; PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166(8):882-890.
8. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
9. World Health Organization, Study Group on Interprofessional Education and Collaborative Practice. Framework for Action on Interprofessional Education and Collaborative Practice. Geneva, Switzerland: World Health Organization; 2010.
10. Interprofessional Education Collaborative Expert Panel. Core Competencies for Interprofessional Collaborative Practice: Report of an Expert Panel. Washington, DC: Interprofessional Education Collaborative; 2011.
11. Blue AV, Mitcham M, Smith T, Raymond J, Greenburg R. Changing the future of health professions: embedding interprofessional education within an academic health center. Acad Med. 2010;85(8):1290-1295.
12. Priest HM, Roberts P, Dent H, Blincoe C, Lawton D, Armstrong C. Interprofessional education and working in mental health: in search of the evidence base. J Nurs Manag. 2008;16(4):474-485.
13. Reeves S. A systematic review of the effects of interprofessional education on staff involved in the care of adults with mental health problems. J Psychiatr Ment Health Nurs. 2001;8(6):533-542.
14. Carpenter J, Barnes D, Dickinson C, Wooff D. Outcomes of interprofessional education for community mental health services in England: the longitudinal evaluation of a postgraduate programme. J Interprof Care. 2006;20(2):145-161.
15. Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: BEME guide no. 9. Med Teach. 2007;29(8):735-751.
16. Pauzé E, Reeves S. Examining the effects of interprofessional education on mental health providers: findings from an updated systematic review. J Ment Health. 2010;19(3):258-271.
17. Curran V, Heath O, Adey T, et al. An approach to integrating interprofessional education in collaborative mental health care. Acad Psychiatry. 2012;36(2):91-95.
18. Veterans Health Administration. VA Interprofessional Mental Health Education Expansion Initiative, Phase I. Washington, DC; Department of Veterans Affairs; 2012.
19. Dundon M, Dollar K, Schohn M, Lantinga LJ. Primary care–mental health integration: co-located, collaborative care: an operations manual. https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/MH-IPC_CCC_Operations_Manual_Version_2_1.pdf. Published February 2011. Accessed May 19, 2017.
20. McDaniel SH, Grus CL, Cubic BA, et al. Competencies for psychology practice in primary care. Am Psychol. 2014;69(4):409-429.
21. Cowley D, Dunaway K, Forstein M, et al. Teaching psychiatry residents to work at the interface of mental health and primary care. Acad Psychiatry. 2014;38(4):398-404.
22. Herrmann N. The creative brain. J Creat Behav. 1991;25:275-295.
23. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
24. Pierson HM, Hayes SC, Gifford EV, et al. An examination of the Motivational Interviewing Treatment Integrity code. J Subst Abuse Treat. 2007;32(1):11-17.
25. Shaw D, Blue A. Should psychiatry champion interprofessional education? Acad Psychiatry. 2012;36(3):163-166.
26. Gilbert JH. Interprofessional learning and higher education structural barriers. J Interprof Care. 2005;19(suppl 1):87-106.
27. Heinemann GD, Schmitt MH, Farrell PP, Brallier SA. Development of an Attitudes Toward Health Care Teams Scale. Eval Health Prof. 1999;22(1):123-142.
28. Hepburn K, Tsukuda RA, Fasser C. Team Skills Scale. In: Heinemann GD, Zeiss AM, eds. Team Performance in Healthcare: Assessment and Development. New York, NY: Kluwer Academic/Plenum; 2002:159-163.
1. Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105-116.
2. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
3. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of loner-term outcomes. Arch Intern Med. 2006;166(21):2314-2321.
4. Unützer J, Katon W, Callahan CM, et al; IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) Investigators. Collaborative care management in late-life depression in the primary care setting: a randomized control trial. JAMA. 2002;288(22):2836-2845.
5. Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;3(4):305-310.
6. Bruce ML, Tenhave TR, Reynolds CF III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091.
7. Alexopoulos GS, Reynolds CF III , Bruce ML, et al; PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166(8):882-890.
8. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
9. World Health Organization, Study Group on Interprofessional Education and Collaborative Practice. Framework for Action on Interprofessional Education and Collaborative Practice. Geneva, Switzerland: World Health Organization; 2010.
10. Interprofessional Education Collaborative Expert Panel. Core Competencies for Interprofessional Collaborative Practice: Report of an Expert Panel. Washington, DC: Interprofessional Education Collaborative; 2011.
11. Blue AV, Mitcham M, Smith T, Raymond J, Greenburg R. Changing the future of health professions: embedding interprofessional education within an academic health center. Acad Med. 2010;85(8):1290-1295.
12. Priest HM, Roberts P, Dent H, Blincoe C, Lawton D, Armstrong C. Interprofessional education and working in mental health: in search of the evidence base. J Nurs Manag. 2008;16(4):474-485.
13. Reeves S. A systematic review of the effects of interprofessional education on staff involved in the care of adults with mental health problems. J Psychiatr Ment Health Nurs. 2001;8(6):533-542.
14. Carpenter J, Barnes D, Dickinson C, Wooff D. Outcomes of interprofessional education for community mental health services in England: the longitudinal evaluation of a postgraduate programme. J Interprof Care. 2006;20(2):145-161.
15. Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: BEME guide no. 9. Med Teach. 2007;29(8):735-751.
16. Pauzé E, Reeves S. Examining the effects of interprofessional education on mental health providers: findings from an updated systematic review. J Ment Health. 2010;19(3):258-271.
17. Curran V, Heath O, Adey T, et al. An approach to integrating interprofessional education in collaborative mental health care. Acad Psychiatry. 2012;36(2):91-95.
18. Veterans Health Administration. VA Interprofessional Mental Health Education Expansion Initiative, Phase I. Washington, DC; Department of Veterans Affairs; 2012.
19. Dundon M, Dollar K, Schohn M, Lantinga LJ. Primary care–mental health integration: co-located, collaborative care: an operations manual. https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/MH-IPC_CCC_Operations_Manual_Version_2_1.pdf. Published February 2011. Accessed May 19, 2017.
20. McDaniel SH, Grus CL, Cubic BA, et al. Competencies for psychology practice in primary care. Am Psychol. 2014;69(4):409-429.
21. Cowley D, Dunaway K, Forstein M, et al. Teaching psychiatry residents to work at the interface of mental health and primary care. Acad Psychiatry. 2014;38(4):398-404.
22. Herrmann N. The creative brain. J Creat Behav. 1991;25:275-295.
23. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
24. Pierson HM, Hayes SC, Gifford EV, et al. An examination of the Motivational Interviewing Treatment Integrity code. J Subst Abuse Treat. 2007;32(1):11-17.
25. Shaw D, Blue A. Should psychiatry champion interprofessional education? Acad Psychiatry. 2012;36(3):163-166.
26. Gilbert JH. Interprofessional learning and higher education structural barriers. J Interprof Care. 2005;19(suppl 1):87-106.
27. Heinemann GD, Schmitt MH, Farrell PP, Brallier SA. Development of an Attitudes Toward Health Care Teams Scale. Eval Health Prof. 1999;22(1):123-142.
28. Hepburn K, Tsukuda RA, Fasser C. Team Skills Scale. In: Heinemann GD, Zeiss AM, eds. Team Performance in Healthcare: Assessment and Development. New York, NY: Kluwer Academic/Plenum; 2002:159-163.
Getting a Candle on Her Condition
A 15-year-old girl is brought in by her mother for evaluation of a rash that developed following a “candling” treatment she underwent two weeks ago at a beauty spa.
The treatment, which was performed to eliminate scaling in her external ear canal, involved dripping hot wax from a burning candle into the external auditory meatus. The cooled wax was peeled away, along with the attached scaling. A new, asymptomatic, scaly rash has since appeared in the same area—far worse than the original.
There is no history of recent infection or joint pain, or family history of skin disease.
EXAMINATION
Heavy, uniform, white scaling on a salmon-colored base covers the external auditory meatus, extending 3 or 4 mm into the concha. Similar, milder changes are observed in the left ear.

There are scattered pits in three fingernails, white scaling in the scalp above and behind both ears, and faint pink scaly patches on both knees and elbows.
What is the diagnosis?
DISCUSSION
Psoriasis is extremely common, affecting about 3% of the white population in this country, and is one of a handful of conditions that manifest with the Koebner phenomenon. This means that any trauma (ie, scrapes, burns, scratches, or cuts) can trigger or extend the condition. In this case, the hot wax was likely the culprit.
The patient’s primary care provider, it turns out, was treating the elbow and knee rashes with antifungal creams (to no avail). He hadn’t made the connection between her various skin problems. It was the acute manifestation in the ear that prompted a broader assessment of the patient’s condition—proving the maxim that to find a diagnosis, you have to look for it.
Given its mild nature, this patient’s condition was easily treated with topical steroids and vitamin D-derived cream (calcipotriene). She was advised of the need to avoid exacerbating factors, such as smoking, obesity, excess alcohol intake, and stress.
There is the possibility of the disease worsening despite treatment. The patient also has about a 25% chance of developing psoriatic arthropathy. For these reasons, she will need to be followed by dermatology.
TAKE-HOME LEARNING POINTS
- Psoriasis, though very common, does not always manifest in its typical form.
- Any trauma (ie, burn, scrape, or cut) can trigger preexisting psoriasis, a response called the Koebner phenomenon.
- Several other conditions—including lichen planus, warts, and molluscum—can exhibit this same phenomenon.
- When psoriasis is suspected, potential corroboratory sites of involvement (eg, knees, elbows, nails, and scalp) should be examined. Biopsy is often needed to confirm the diagnosis.
A 15-year-old girl is brought in by her mother for evaluation of a rash that developed following a “candling” treatment she underwent two weeks ago at a beauty spa.
The treatment, which was performed to eliminate scaling in her external ear canal, involved dripping hot wax from a burning candle into the external auditory meatus. The cooled wax was peeled away, along with the attached scaling. A new, asymptomatic, scaly rash has since appeared in the same area—far worse than the original.
There is no history of recent infection or joint pain, or family history of skin disease.
EXAMINATION
Heavy, uniform, white scaling on a salmon-colored base covers the external auditory meatus, extending 3 or 4 mm into the concha. Similar, milder changes are observed in the left ear.

There are scattered pits in three fingernails, white scaling in the scalp above and behind both ears, and faint pink scaly patches on both knees and elbows.
What is the diagnosis?
DISCUSSION
Psoriasis is extremely common, affecting about 3% of the white population in this country, and is one of a handful of conditions that manifest with the Koebner phenomenon. This means that any trauma (ie, scrapes, burns, scratches, or cuts) can trigger or extend the condition. In this case, the hot wax was likely the culprit.
The patient’s primary care provider, it turns out, was treating the elbow and knee rashes with antifungal creams (to no avail). He hadn’t made the connection between her various skin problems. It was the acute manifestation in the ear that prompted a broader assessment of the patient’s condition—proving the maxim that to find a diagnosis, you have to look for it.
Given its mild nature, this patient’s condition was easily treated with topical steroids and vitamin D-derived cream (calcipotriene). She was advised of the need to avoid exacerbating factors, such as smoking, obesity, excess alcohol intake, and stress.
There is the possibility of the disease worsening despite treatment. The patient also has about a 25% chance of developing psoriatic arthropathy. For these reasons, she will need to be followed by dermatology.
TAKE-HOME LEARNING POINTS
- Psoriasis, though very common, does not always manifest in its typical form.
- Any trauma (ie, burn, scrape, or cut) can trigger preexisting psoriasis, a response called the Koebner phenomenon.
- Several other conditions—including lichen planus, warts, and molluscum—can exhibit this same phenomenon.
- When psoriasis is suspected, potential corroboratory sites of involvement (eg, knees, elbows, nails, and scalp) should be examined. Biopsy is often needed to confirm the diagnosis.
A 15-year-old girl is brought in by her mother for evaluation of a rash that developed following a “candling” treatment she underwent two weeks ago at a beauty spa.
The treatment, which was performed to eliminate scaling in her external ear canal, involved dripping hot wax from a burning candle into the external auditory meatus. The cooled wax was peeled away, along with the attached scaling. A new, asymptomatic, scaly rash has since appeared in the same area—far worse than the original.
There is no history of recent infection or joint pain, or family history of skin disease.
EXAMINATION
Heavy, uniform, white scaling on a salmon-colored base covers the external auditory meatus, extending 3 or 4 mm into the concha. Similar, milder changes are observed in the left ear.

There are scattered pits in three fingernails, white scaling in the scalp above and behind both ears, and faint pink scaly patches on both knees and elbows.
What is the diagnosis?
DISCUSSION
Psoriasis is extremely common, affecting about 3% of the white population in this country, and is one of a handful of conditions that manifest with the Koebner phenomenon. This means that any trauma (ie, scrapes, burns, scratches, or cuts) can trigger or extend the condition. In this case, the hot wax was likely the culprit.
The patient’s primary care provider, it turns out, was treating the elbow and knee rashes with antifungal creams (to no avail). He hadn’t made the connection between her various skin problems. It was the acute manifestation in the ear that prompted a broader assessment of the patient’s condition—proving the maxim that to find a diagnosis, you have to look for it.
Given its mild nature, this patient’s condition was easily treated with topical steroids and vitamin D-derived cream (calcipotriene). She was advised of the need to avoid exacerbating factors, such as smoking, obesity, excess alcohol intake, and stress.
There is the possibility of the disease worsening despite treatment. The patient also has about a 25% chance of developing psoriatic arthropathy. For these reasons, she will need to be followed by dermatology.
TAKE-HOME LEARNING POINTS
- Psoriasis, though very common, does not always manifest in its typical form.
- Any trauma (ie, burn, scrape, or cut) can trigger preexisting psoriasis, a response called the Koebner phenomenon.
- Several other conditions—including lichen planus, warts, and molluscum—can exhibit this same phenomenon.
- When psoriasis is suspected, potential corroboratory sites of involvement (eg, knees, elbows, nails, and scalp) should be examined. Biopsy is often needed to confirm the diagnosis.
Ferrous sulfate bests iron complex in treating IDA in infants, young kids
A trial comparing ferrous sulfate with iron polysaccharide complex to treat infants and young children with nutritional iron-deficiency anemia (IDA) has shown ferrous sulfate to be more effective at raising hemoglobin levels in this population, according to researchers.
Dozens of oral iron supplements exist for IDA treatment, ferrous sulfate being the most commonly used. Iron polysaccharide complex, however, may be better tolerated.
Investigators undertook the BESTIRON study (NCT01904864) to determine whether the iron complex was more efficacious than ferrous sulfate in increasing hemoglobin concentrations in infants and young children aged 9 to 48 months.
Up to 3% of children aged 1 to 2 years in the United States have IDA, as do millions worldwide. IDA is associated with impaired neurodevelopment in the young.
Inadequate dietary iron intake in this group is the most common cause of IDA. It most often results from excessive cow milk consumption and/or prolonged breastfeeding without appropriate iron supplementation.
For this study, investigators randomized 80 infants and young children with nutritional IDA to receive 3 mg/kg of ferrous sulfate (n=40) or iron complex (n=40) drops once daily for 12 weeks.
Patients had to have hemoglobin concentrations of 10 g/dL or less, mean corpuscular volumes of 70 fL or less, reticulocyte hemoglobin equivalents of 25 pg or less, and either serum ferritin level of 15 ng/mL or less or total iron-binding capacity of 425 μg/dL or greater.
And they could have no clinical or laboratory evidence of other causes of anemia.
All 80 patients were included in the primary analysis evaluating change in hemoglobin concentration during the 12 weeks after starting oral iron therapy.
Patient characteristics
Patient characteristics were similar between the groups. The mean age was 23 months and 55% were male.
Most patients (61%) were Hispanic white, 9% were non-Hispanic white, and 11% were black.
Ten patients in the ferrous sulfate group and 8 in the iron complex group had received a packed red blood cell transfusion prior to study enrollment.
Results
Fifty-nine patients completed all study visits, 28 in the ferrous sulfate group and 31 in the iron complex group.
Patients’ mean hemoglobin level in the ferrous sulfate group increased from 7.9 g/dL to 11.9 g/dL over the 12 weeks. In the iron complex group, the patients’ hemoglobin level increased from 7.7 g/dL to 11.1 g/dL.
Using a linear mixed model, the primary outcome demonstrated a significant difference in the change in hemoglobin concentration of 1.0 g/dL (95% CI, 0.4-1.6; P < .001) between the groups, favoring ferrous sulfate.
IDA completely resolved in 8 of 28 (29%) patients in the ferrous sulfate group and 2 of 31 (6%) in the iron complex group (P=0.04).
However, successful administration of the supplement—meaning he child did not spit out the medication—was higher in the iron complex group (94%) than the iron sulfate group (82%), P=0.009.
The median serum ferritin level increased from 3.0 ng/mL to 15.6 ng/mL in the ferrous sulfate arm, which was significantly better than in the iron complex arm, which increased from 2.0 ng/mL to 7.5 ng/mL, P<0.001.
And the mean total iron binding capacity significantly increased in the ferrous sulfate group compared with the iron oxide group (P<0.001).
Safety
The investigators reported that patients treated with iron complex had significantly more diahrrea, while patients treated with ferrous sulfate had more vomiting, although the latter was not statistically significant.
A gastrointestinal adverse effect profile created at the end of the study showed no significant differences between the groups.
The investigators noted a few limitations of the study.
First, it was conducted in a single tertiary-care children’s hospital, the Children’s Medical Center in Dallas, Texas.
Second, a disproportionate number of patients were from lower income and minority families and frequently had severe anemia, with approximately 23% requiring blood transfusion prior to study start.
And third, the trial had a high lost-to-follow-up rate of 25% at the final visit.
So the results may not be generalizable to the general pediatric population.
Nevertheless, the investigators concluded, “Once daily, low-dose ferrous sulfate should be considered for children with nutritional iron-deficiency anemia.”
The team reported their findings in JAMA.
The study was an investigator-initiated trial with sponsorship from Gensavis Pharmaceuticals LLC, the manufacturer of the iron polysaccharide complex used in the trial. The company provided funding for both trial drugs.
The study received additional grant support from the National Center for Advancing Translational Sciences and the National Heart, Lung, and Blood Institute. ![]()
A trial comparing ferrous sulfate with iron polysaccharide complex to treat infants and young children with nutritional iron-deficiency anemia (IDA) has shown ferrous sulfate to be more effective at raising hemoglobin levels in this population, according to researchers.
Dozens of oral iron supplements exist for IDA treatment, ferrous sulfate being the most commonly used. Iron polysaccharide complex, however, may be better tolerated.
Investigators undertook the BESTIRON study (NCT01904864) to determine whether the iron complex was more efficacious than ferrous sulfate in increasing hemoglobin concentrations in infants and young children aged 9 to 48 months.
Up to 3% of children aged 1 to 2 years in the United States have IDA, as do millions worldwide. IDA is associated with impaired neurodevelopment in the young.
Inadequate dietary iron intake in this group is the most common cause of IDA. It most often results from excessive cow milk consumption and/or prolonged breastfeeding without appropriate iron supplementation.
For this study, investigators randomized 80 infants and young children with nutritional IDA to receive 3 mg/kg of ferrous sulfate (n=40) or iron complex (n=40) drops once daily for 12 weeks.
Patients had to have hemoglobin concentrations of 10 g/dL or less, mean corpuscular volumes of 70 fL or less, reticulocyte hemoglobin equivalents of 25 pg or less, and either serum ferritin level of 15 ng/mL or less or total iron-binding capacity of 425 μg/dL or greater.
And they could have no clinical or laboratory evidence of other causes of anemia.
All 80 patients were included in the primary analysis evaluating change in hemoglobin concentration during the 12 weeks after starting oral iron therapy.
Patient characteristics
Patient characteristics were similar between the groups. The mean age was 23 months and 55% were male.
Most patients (61%) were Hispanic white, 9% were non-Hispanic white, and 11% were black.
Ten patients in the ferrous sulfate group and 8 in the iron complex group had received a packed red blood cell transfusion prior to study enrollment.
Results
Fifty-nine patients completed all study visits, 28 in the ferrous sulfate group and 31 in the iron complex group.
Patients’ mean hemoglobin level in the ferrous sulfate group increased from 7.9 g/dL to 11.9 g/dL over the 12 weeks. In the iron complex group, the patients’ hemoglobin level increased from 7.7 g/dL to 11.1 g/dL.
Using a linear mixed model, the primary outcome demonstrated a significant difference in the change in hemoglobin concentration of 1.0 g/dL (95% CI, 0.4-1.6; P < .001) between the groups, favoring ferrous sulfate.
IDA completely resolved in 8 of 28 (29%) patients in the ferrous sulfate group and 2 of 31 (6%) in the iron complex group (P=0.04).
However, successful administration of the supplement—meaning he child did not spit out the medication—was higher in the iron complex group (94%) than the iron sulfate group (82%), P=0.009.
The median serum ferritin level increased from 3.0 ng/mL to 15.6 ng/mL in the ferrous sulfate arm, which was significantly better than in the iron complex arm, which increased from 2.0 ng/mL to 7.5 ng/mL, P<0.001.
And the mean total iron binding capacity significantly increased in the ferrous sulfate group compared with the iron oxide group (P<0.001).
Safety
The investigators reported that patients treated with iron complex had significantly more diahrrea, while patients treated with ferrous sulfate had more vomiting, although the latter was not statistically significant.
A gastrointestinal adverse effect profile created at the end of the study showed no significant differences between the groups.
The investigators noted a few limitations of the study.
First, it was conducted in a single tertiary-care children’s hospital, the Children’s Medical Center in Dallas, Texas.
Second, a disproportionate number of patients were from lower income and minority families and frequently had severe anemia, with approximately 23% requiring blood transfusion prior to study start.
And third, the trial had a high lost-to-follow-up rate of 25% at the final visit.
So the results may not be generalizable to the general pediatric population.
Nevertheless, the investigators concluded, “Once daily, low-dose ferrous sulfate should be considered for children with nutritional iron-deficiency anemia.”
The team reported their findings in JAMA.
The study was an investigator-initiated trial with sponsorship from Gensavis Pharmaceuticals LLC, the manufacturer of the iron polysaccharide complex used in the trial. The company provided funding for both trial drugs.
The study received additional grant support from the National Center for Advancing Translational Sciences and the National Heart, Lung, and Blood Institute. ![]()
A trial comparing ferrous sulfate with iron polysaccharide complex to treat infants and young children with nutritional iron-deficiency anemia (IDA) has shown ferrous sulfate to be more effective at raising hemoglobin levels in this population, according to researchers.
Dozens of oral iron supplements exist for IDA treatment, ferrous sulfate being the most commonly used. Iron polysaccharide complex, however, may be better tolerated.
Investigators undertook the BESTIRON study (NCT01904864) to determine whether the iron complex was more efficacious than ferrous sulfate in increasing hemoglobin concentrations in infants and young children aged 9 to 48 months.
Up to 3% of children aged 1 to 2 years in the United States have IDA, as do millions worldwide. IDA is associated with impaired neurodevelopment in the young.
Inadequate dietary iron intake in this group is the most common cause of IDA. It most often results from excessive cow milk consumption and/or prolonged breastfeeding without appropriate iron supplementation.
For this study, investigators randomized 80 infants and young children with nutritional IDA to receive 3 mg/kg of ferrous sulfate (n=40) or iron complex (n=40) drops once daily for 12 weeks.
Patients had to have hemoglobin concentrations of 10 g/dL or less, mean corpuscular volumes of 70 fL or less, reticulocyte hemoglobin equivalents of 25 pg or less, and either serum ferritin level of 15 ng/mL or less or total iron-binding capacity of 425 μg/dL or greater.
And they could have no clinical or laboratory evidence of other causes of anemia.
All 80 patients were included in the primary analysis evaluating change in hemoglobin concentration during the 12 weeks after starting oral iron therapy.
Patient characteristics
Patient characteristics were similar between the groups. The mean age was 23 months and 55% were male.
Most patients (61%) were Hispanic white, 9% were non-Hispanic white, and 11% were black.
Ten patients in the ferrous sulfate group and 8 in the iron complex group had received a packed red blood cell transfusion prior to study enrollment.
Results
Fifty-nine patients completed all study visits, 28 in the ferrous sulfate group and 31 in the iron complex group.
Patients’ mean hemoglobin level in the ferrous sulfate group increased from 7.9 g/dL to 11.9 g/dL over the 12 weeks. In the iron complex group, the patients’ hemoglobin level increased from 7.7 g/dL to 11.1 g/dL.
Using a linear mixed model, the primary outcome demonstrated a significant difference in the change in hemoglobin concentration of 1.0 g/dL (95% CI, 0.4-1.6; P < .001) between the groups, favoring ferrous sulfate.
IDA completely resolved in 8 of 28 (29%) patients in the ferrous sulfate group and 2 of 31 (6%) in the iron complex group (P=0.04).
However, successful administration of the supplement—meaning he child did not spit out the medication—was higher in the iron complex group (94%) than the iron sulfate group (82%), P=0.009.
The median serum ferritin level increased from 3.0 ng/mL to 15.6 ng/mL in the ferrous sulfate arm, which was significantly better than in the iron complex arm, which increased from 2.0 ng/mL to 7.5 ng/mL, P<0.001.
And the mean total iron binding capacity significantly increased in the ferrous sulfate group compared with the iron oxide group (P<0.001).
Safety
The investigators reported that patients treated with iron complex had significantly more diahrrea, while patients treated with ferrous sulfate had more vomiting, although the latter was not statistically significant.
A gastrointestinal adverse effect profile created at the end of the study showed no significant differences between the groups.
The investigators noted a few limitations of the study.
First, it was conducted in a single tertiary-care children’s hospital, the Children’s Medical Center in Dallas, Texas.
Second, a disproportionate number of patients were from lower income and minority families and frequently had severe anemia, with approximately 23% requiring blood transfusion prior to study start.
And third, the trial had a high lost-to-follow-up rate of 25% at the final visit.
So the results may not be generalizable to the general pediatric population.
Nevertheless, the investigators concluded, “Once daily, low-dose ferrous sulfate should be considered for children with nutritional iron-deficiency anemia.”
The team reported their findings in JAMA.
The study was an investigator-initiated trial with sponsorship from Gensavis Pharmaceuticals LLC, the manufacturer of the iron polysaccharide complex used in the trial. The company provided funding for both trial drugs.
The study received additional grant support from the National Center for Advancing Translational Sciences and the National Heart, Lung, and Blood Institute. ![]()
Itchy nodules on legs
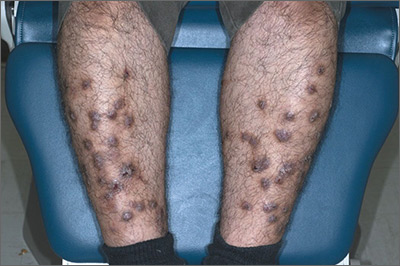
The FP did a 4-mm punch biopsy to confirm that this was a case of prurigo nodularis and prescribed clobetasol cream to be applied twice daily to the pruritic nodules. The FP recommended that the patient apply the cream instead of scratching the area. The FP also said that if the patient couldn’t avoid touching the area, it would be better to lightly rub the area over his clothing instead.
The biopsy results subsequently confirmed a diagnosis of prurigo nodularis. At the patient’s 2-week follow-up, he indicated that his symptoms were 50% better since using the clobetasol. The FP explained to the patient the nature of prurigo nodularis, including the patient’s itch-scratch cycle and how stress was making it worse. The patient acknowledged that his symptoms had become worse when he began having financial trouble. The FP asked the patient if he wanted to see a counselor, but the patient declined, saying that he just needed to get more work.
At a one-month follow-up, 90% of the nodules were resolved, although there were still some stubborn areas that continued to itch. The patient could not control scratching these areas at times. The FP offered intralesional injections with triamcinolone and/or cryotherapy. The patient consented to liquid nitrogen therapy and the remaining nodules were frozen for approximately 10 seconds each using a liquid nitrogen spray.
Four weeks later, the patient had 4 remaining nodules. The FP injected the nodules with 10 mg/mL triamcinolone and refilled the clobetasol cream. At the next appointment, 2 nodules remained. The patient indicated that he would continue using the cream until the nodules went away. In many cases, prurigo nodularis does not respond especially well to treatment, so this patient was fortunate that standard treatments provided a good outcome.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Johnson A. Self-inflicted dermatosis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 856-862.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP did a 4-mm punch biopsy to confirm that this was a case of prurigo nodularis and prescribed clobetasol cream to be applied twice daily to the pruritic nodules. The FP recommended that the patient apply the cream instead of scratching the area. The FP also said that if the patient couldn’t avoid touching the area, it would be better to lightly rub the area over his clothing instead.
The biopsy results subsequently confirmed a diagnosis of prurigo nodularis. At the patient’s 2-week follow-up, he indicated that his symptoms were 50% better since using the clobetasol. The FP explained to the patient the nature of prurigo nodularis, including the patient’s itch-scratch cycle and how stress was making it worse. The patient acknowledged that his symptoms had become worse when he began having financial trouble. The FP asked the patient if he wanted to see a counselor, but the patient declined, saying that he just needed to get more work.
At a one-month follow-up, 90% of the nodules were resolved, although there were still some stubborn areas that continued to itch. The patient could not control scratching these areas at times. The FP offered intralesional injections with triamcinolone and/or cryotherapy. The patient consented to liquid nitrogen therapy and the remaining nodules were frozen for approximately 10 seconds each using a liquid nitrogen spray.
Four weeks later, the patient had 4 remaining nodules. The FP injected the nodules with 10 mg/mL triamcinolone and refilled the clobetasol cream. At the next appointment, 2 nodules remained. The patient indicated that he would continue using the cream until the nodules went away. In many cases, prurigo nodularis does not respond especially well to treatment, so this patient was fortunate that standard treatments provided a good outcome.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Johnson A. Self-inflicted dermatosis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 856-862.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP did a 4-mm punch biopsy to confirm that this was a case of prurigo nodularis and prescribed clobetasol cream to be applied twice daily to the pruritic nodules. The FP recommended that the patient apply the cream instead of scratching the area. The FP also said that if the patient couldn’t avoid touching the area, it would be better to lightly rub the area over his clothing instead.
The biopsy results subsequently confirmed a diagnosis of prurigo nodularis. At the patient’s 2-week follow-up, he indicated that his symptoms were 50% better since using the clobetasol. The FP explained to the patient the nature of prurigo nodularis, including the patient’s itch-scratch cycle and how stress was making it worse. The patient acknowledged that his symptoms had become worse when he began having financial trouble. The FP asked the patient if he wanted to see a counselor, but the patient declined, saying that he just needed to get more work.
At a one-month follow-up, 90% of the nodules were resolved, although there were still some stubborn areas that continued to itch. The patient could not control scratching these areas at times. The FP offered intralesional injections with triamcinolone and/or cryotherapy. The patient consented to liquid nitrogen therapy and the remaining nodules were frozen for approximately 10 seconds each using a liquid nitrogen spray.
Four weeks later, the patient had 4 remaining nodules. The FP injected the nodules with 10 mg/mL triamcinolone and refilled the clobetasol cream. At the next appointment, 2 nodules remained. The patient indicated that he would continue using the cream until the nodules went away. In many cases, prurigo nodularis does not respond especially well to treatment, so this patient was fortunate that standard treatments provided a good outcome.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Johnson A. Self-inflicted dermatosis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 856-862.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
ACS Committee on Diversity Issues seeks two new members
The American College of Surgeons (ACS) Committee on Diversity Issues (CODI) is seeking candidates to fill two vacancies on the committee beginning in October 2017.
The mission of the Committee on Diversity Issues is to study the educational and professional needs of underrepresented surgeons and surgical trainees and the impact that its work may have on the elimination of health care disparities among diverse population groups.
Surgeons interested in developing initiatives to expand diversity within the ACS membership and leadership and to developing resources and programming for surgeons related to diversity and cultural competency should apply. Nominations are open to all, and the committee encourages representation by individuals of diverse cultural, racial, and ethnic backgrounds.
Nominees must meet the following criteria:
- Be an active Fellow of the ACS
- Be able to serve an initial three-year term: 2017–2020
- Attend one in-person meeting at the annual ACS Clinical Congress
- Participate in quarterly conference calls
- Contribute to committee initiatives
To apply, go to www.surveymonkey.com/r/CmteDiversityApp to access the application and submit by June 30.
Applicants will need to do the following:
- Upload a summary of your curriculum vitae (five pages or less)
- Upload a letter of interest highlighting your skills and expertise, along with contributions you would like to make to the committee
Eligible candidates will be selected and notified by the committee in July and will be invited to attend the October 23 meeting of the Committee on Diversity Issues as guests. This meeting is held in conjunction with the 2017 Clinical Congress in San Diego. Travel reimbursement will not be provided.
Direct questions to [email protected].
The American College of Surgeons (ACS) Committee on Diversity Issues (CODI) is seeking candidates to fill two vacancies on the committee beginning in October 2017.
The mission of the Committee on Diversity Issues is to study the educational and professional needs of underrepresented surgeons and surgical trainees and the impact that its work may have on the elimination of health care disparities among diverse population groups.
Surgeons interested in developing initiatives to expand diversity within the ACS membership and leadership and to developing resources and programming for surgeons related to diversity and cultural competency should apply. Nominations are open to all, and the committee encourages representation by individuals of diverse cultural, racial, and ethnic backgrounds.
Nominees must meet the following criteria:
- Be an active Fellow of the ACS
- Be able to serve an initial three-year term: 2017–2020
- Attend one in-person meeting at the annual ACS Clinical Congress
- Participate in quarterly conference calls
- Contribute to committee initiatives
To apply, go to www.surveymonkey.com/r/CmteDiversityApp to access the application and submit by June 30.
Applicants will need to do the following:
- Upload a summary of your curriculum vitae (five pages or less)
- Upload a letter of interest highlighting your skills and expertise, along with contributions you would like to make to the committee
Eligible candidates will be selected and notified by the committee in July and will be invited to attend the October 23 meeting of the Committee on Diversity Issues as guests. This meeting is held in conjunction with the 2017 Clinical Congress in San Diego. Travel reimbursement will not be provided.
Direct questions to [email protected].
The American College of Surgeons (ACS) Committee on Diversity Issues (CODI) is seeking candidates to fill two vacancies on the committee beginning in October 2017.
The mission of the Committee on Diversity Issues is to study the educational and professional needs of underrepresented surgeons and surgical trainees and the impact that its work may have on the elimination of health care disparities among diverse population groups.
Surgeons interested in developing initiatives to expand diversity within the ACS membership and leadership and to developing resources and programming for surgeons related to diversity and cultural competency should apply. Nominations are open to all, and the committee encourages representation by individuals of diverse cultural, racial, and ethnic backgrounds.
Nominees must meet the following criteria:
- Be an active Fellow of the ACS
- Be able to serve an initial three-year term: 2017–2020
- Attend one in-person meeting at the annual ACS Clinical Congress
- Participate in quarterly conference calls
- Contribute to committee initiatives
To apply, go to www.surveymonkey.com/r/CmteDiversityApp to access the application and submit by June 30.
Applicants will need to do the following:
- Upload a summary of your curriculum vitae (five pages or less)
- Upload a letter of interest highlighting your skills and expertise, along with contributions you would like to make to the committee
Eligible candidates will be selected and notified by the committee in July and will be invited to attend the October 23 meeting of the Committee on Diversity Issues as guests. This meeting is held in conjunction with the 2017 Clinical Congress in San Diego. Travel reimbursement will not be provided.
Direct questions to [email protected].