User login
Mandar Jog, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
DEVOTE: Degludec and glargine had similar risk with less severe hypoglycemia
SAN DIEGO – For patients with type 2 diabetes at high risk of cardiovascular disease, the ultra–long-acting, once-daily basal insulin degludec produced a similar risk of major adverse cardiovascular events as glargine with a significantly lower risk of severe hypoglycemia, new data show.
Nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death occurred in 325 (8.5%) patients on degludec and 356 (9.3%) patients on glargine (hazard ratio, 0.91; 95% confidence interval, 0.78 to 1.06; P = .21) in DEVOTE (the Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events). Rates of severe hypoglycemia were 4.9% and 6.6%, respectively (P less than .001), investigators reported at the annual scientific sessions of the American Diabetes Association and simultaneously in the New England Journal of Medicine (2017 Jun 12. doi: 10.1056/NEJMoa1615692).
Insulin degludec injection (Tresiba®, Novo Nordisk) is a basal insulin analog, the long, soluble hexamer chains of which are metabolized only at the ends, yielding at least a 42-hour duration of action, Todd Hobbs, MD, chief medical officer of Novo Nordisk, Princeton, N.J., explained in an interview. In contrast, glargine has about a 12-hour half-life. Previous trials of degludec did not adjudicate cardiovascular endpoints, which the U.S. Food and Drug Administration only recently began requiring for insulins, Dr. Hobbs said. In response to an FDA request, the phase III, international, randomized, double-blind DEVOTE trial compared the cardiovascular safety of daily basal insulin degludec (100 U per mL) with that of glargine U100 in more than 7,600 adults with type 2 diabetes.
Fully 98% of patients completed the 2-year trial. The overall risk of major adverse cardiac events resembled that of each individual component, including cardiovascular death (HR, 0.96; 95% CI, 0.76-1.21; P = .71), nonfatal myocardial infarction (HR, 0.85; 95% CI, 0.68-1.06; P = .15), and nonfatal stroke (HR, 0.90; 95% CI, 0.65-1.23; P =. 50). Degludec remained noninferior to glargine when researchers added unstable angina to the primary endpoint and accounted for patient location, treatment duration, length of follow-up, and age, sex, body mass index, and renal function.
Both insulins produced similar HbA1c levels of about 7.5%, but degludec cut fasting plasma glucose by about 5 mg per mL more, compared with glargine (P less than .001), the investigators reported. Furthermore, the odds ratio for severe hypoglycemia significantly favored degludec (HR, 0.73; 95% CI, 0.60-0.89; P less than .001). In other words, 40 patients would need to receive degludec rather than glargine to prevent one event of severe hypoglycemia. Previous studies have shown similar results, Dr. Buse said. In a pooled analysis of all five trials of type 2 diabetes in the degludec clinical development program, degludec was associated with a significantly lower risk of hypoglycemia, particularly nocturnal episodes, than was glargine (Diabetes Obes Metab. 2013;15:175-84).
Findings were similar in the double-blind SWITCH 2 trial (Diabetologia. 2016;59[Suppl 1]:1-581).
DEVOTE identified no safety issues for degludec, compared with glargine. Each arm had similar rates of serious or severe adverse events, leading to treatment discontinuation, and neoplasms. Nonetheless, DEVOTE had several limitations, said Elizabeth R. Seaquist, MD, of the University of Minnesota, Minneapolis, who was not involved in the study. “Investigators could modify the titration protocol based on clinical judgment, and it isn’t clear whether this modification was applied equally in both arms,” she said at ADA. “Another weakness is that there were no data collected about moderate symptomatic hypoglycemia, the most common type of hypoglycemia that patients experience.” DEVOTE also did not examine how often blood glucose dropped below 54 mg per dL, the point at which patients often do not know they are hypoglycemic. Investigators also should examine whether degludec cuts health care costs or improves sleep or quality of life, whether its glycemic benefits extends to patients who are insulin-naive or have severe kidney disease, and how it compares with glargine U300, she added.
Insulin degludec received FDA approval in September 2015, based on interim results of DEVOTE. Novo Nordisk makes insulin degludec and sponsored the trial. Dr. Buse disclosed consulting fees from Novo Nordisk and ties to many other pharmaceutical companies. Dr. Seaquist disclosed ties to Novo Nordisk, Eli Lilly, Locemia, and Lucera. Dr. Hobbs is chief medical officer for Novo Nordisk.
SAN DIEGO – For patients with type 2 diabetes at high risk of cardiovascular disease, the ultra–long-acting, once-daily basal insulin degludec produced a similar risk of major adverse cardiovascular events as glargine with a significantly lower risk of severe hypoglycemia, new data show.
Nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death occurred in 325 (8.5%) patients on degludec and 356 (9.3%) patients on glargine (hazard ratio, 0.91; 95% confidence interval, 0.78 to 1.06; P = .21) in DEVOTE (the Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events). Rates of severe hypoglycemia were 4.9% and 6.6%, respectively (P less than .001), investigators reported at the annual scientific sessions of the American Diabetes Association and simultaneously in the New England Journal of Medicine (2017 Jun 12. doi: 10.1056/NEJMoa1615692).
Insulin degludec injection (Tresiba®, Novo Nordisk) is a basal insulin analog, the long, soluble hexamer chains of which are metabolized only at the ends, yielding at least a 42-hour duration of action, Todd Hobbs, MD, chief medical officer of Novo Nordisk, Princeton, N.J., explained in an interview. In contrast, glargine has about a 12-hour half-life. Previous trials of degludec did not adjudicate cardiovascular endpoints, which the U.S. Food and Drug Administration only recently began requiring for insulins, Dr. Hobbs said. In response to an FDA request, the phase III, international, randomized, double-blind DEVOTE trial compared the cardiovascular safety of daily basal insulin degludec (100 U per mL) with that of glargine U100 in more than 7,600 adults with type 2 diabetes.
Fully 98% of patients completed the 2-year trial. The overall risk of major adverse cardiac events resembled that of each individual component, including cardiovascular death (HR, 0.96; 95% CI, 0.76-1.21; P = .71), nonfatal myocardial infarction (HR, 0.85; 95% CI, 0.68-1.06; P = .15), and nonfatal stroke (HR, 0.90; 95% CI, 0.65-1.23; P =. 50). Degludec remained noninferior to glargine when researchers added unstable angina to the primary endpoint and accounted for patient location, treatment duration, length of follow-up, and age, sex, body mass index, and renal function.
Both insulins produced similar HbA1c levels of about 7.5%, but degludec cut fasting plasma glucose by about 5 mg per mL more, compared with glargine (P less than .001), the investigators reported. Furthermore, the odds ratio for severe hypoglycemia significantly favored degludec (HR, 0.73; 95% CI, 0.60-0.89; P less than .001). In other words, 40 patients would need to receive degludec rather than glargine to prevent one event of severe hypoglycemia. Previous studies have shown similar results, Dr. Buse said. In a pooled analysis of all five trials of type 2 diabetes in the degludec clinical development program, degludec was associated with a significantly lower risk of hypoglycemia, particularly nocturnal episodes, than was glargine (Diabetes Obes Metab. 2013;15:175-84).
Findings were similar in the double-blind SWITCH 2 trial (Diabetologia. 2016;59[Suppl 1]:1-581).
DEVOTE identified no safety issues for degludec, compared with glargine. Each arm had similar rates of serious or severe adverse events, leading to treatment discontinuation, and neoplasms. Nonetheless, DEVOTE had several limitations, said Elizabeth R. Seaquist, MD, of the University of Minnesota, Minneapolis, who was not involved in the study. “Investigators could modify the titration protocol based on clinical judgment, and it isn’t clear whether this modification was applied equally in both arms,” she said at ADA. “Another weakness is that there were no data collected about moderate symptomatic hypoglycemia, the most common type of hypoglycemia that patients experience.” DEVOTE also did not examine how often blood glucose dropped below 54 mg per dL, the point at which patients often do not know they are hypoglycemic. Investigators also should examine whether degludec cuts health care costs or improves sleep or quality of life, whether its glycemic benefits extends to patients who are insulin-naive or have severe kidney disease, and how it compares with glargine U300, she added.
Insulin degludec received FDA approval in September 2015, based on interim results of DEVOTE. Novo Nordisk makes insulin degludec and sponsored the trial. Dr. Buse disclosed consulting fees from Novo Nordisk and ties to many other pharmaceutical companies. Dr. Seaquist disclosed ties to Novo Nordisk, Eli Lilly, Locemia, and Lucera. Dr. Hobbs is chief medical officer for Novo Nordisk.
SAN DIEGO – For patients with type 2 diabetes at high risk of cardiovascular disease, the ultra–long-acting, once-daily basal insulin degludec produced a similar risk of major adverse cardiovascular events as glargine with a significantly lower risk of severe hypoglycemia, new data show.
Nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death occurred in 325 (8.5%) patients on degludec and 356 (9.3%) patients on glargine (hazard ratio, 0.91; 95% confidence interval, 0.78 to 1.06; P = .21) in DEVOTE (the Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events). Rates of severe hypoglycemia were 4.9% and 6.6%, respectively (P less than .001), investigators reported at the annual scientific sessions of the American Diabetes Association and simultaneously in the New England Journal of Medicine (2017 Jun 12. doi: 10.1056/NEJMoa1615692).
Insulin degludec injection (Tresiba®, Novo Nordisk) is a basal insulin analog, the long, soluble hexamer chains of which are metabolized only at the ends, yielding at least a 42-hour duration of action, Todd Hobbs, MD, chief medical officer of Novo Nordisk, Princeton, N.J., explained in an interview. In contrast, glargine has about a 12-hour half-life. Previous trials of degludec did not adjudicate cardiovascular endpoints, which the U.S. Food and Drug Administration only recently began requiring for insulins, Dr. Hobbs said. In response to an FDA request, the phase III, international, randomized, double-blind DEVOTE trial compared the cardiovascular safety of daily basal insulin degludec (100 U per mL) with that of glargine U100 in more than 7,600 adults with type 2 diabetes.
Fully 98% of patients completed the 2-year trial. The overall risk of major adverse cardiac events resembled that of each individual component, including cardiovascular death (HR, 0.96; 95% CI, 0.76-1.21; P = .71), nonfatal myocardial infarction (HR, 0.85; 95% CI, 0.68-1.06; P = .15), and nonfatal stroke (HR, 0.90; 95% CI, 0.65-1.23; P =. 50). Degludec remained noninferior to glargine when researchers added unstable angina to the primary endpoint and accounted for patient location, treatment duration, length of follow-up, and age, sex, body mass index, and renal function.
Both insulins produced similar HbA1c levels of about 7.5%, but degludec cut fasting plasma glucose by about 5 mg per mL more, compared with glargine (P less than .001), the investigators reported. Furthermore, the odds ratio for severe hypoglycemia significantly favored degludec (HR, 0.73; 95% CI, 0.60-0.89; P less than .001). In other words, 40 patients would need to receive degludec rather than glargine to prevent one event of severe hypoglycemia. Previous studies have shown similar results, Dr. Buse said. In a pooled analysis of all five trials of type 2 diabetes in the degludec clinical development program, degludec was associated with a significantly lower risk of hypoglycemia, particularly nocturnal episodes, than was glargine (Diabetes Obes Metab. 2013;15:175-84).
Findings were similar in the double-blind SWITCH 2 trial (Diabetologia. 2016;59[Suppl 1]:1-581).
DEVOTE identified no safety issues for degludec, compared with glargine. Each arm had similar rates of serious or severe adverse events, leading to treatment discontinuation, and neoplasms. Nonetheless, DEVOTE had several limitations, said Elizabeth R. Seaquist, MD, of the University of Minnesota, Minneapolis, who was not involved in the study. “Investigators could modify the titration protocol based on clinical judgment, and it isn’t clear whether this modification was applied equally in both arms,” she said at ADA. “Another weakness is that there were no data collected about moderate symptomatic hypoglycemia, the most common type of hypoglycemia that patients experience.” DEVOTE also did not examine how often blood glucose dropped below 54 mg per dL, the point at which patients often do not know they are hypoglycemic. Investigators also should examine whether degludec cuts health care costs or improves sleep or quality of life, whether its glycemic benefits extends to patients who are insulin-naive or have severe kidney disease, and how it compares with glargine U300, she added.
Insulin degludec received FDA approval in September 2015, based on interim results of DEVOTE. Novo Nordisk makes insulin degludec and sponsored the trial. Dr. Buse disclosed consulting fees from Novo Nordisk and ties to many other pharmaceutical companies. Dr. Seaquist disclosed ties to Novo Nordisk, Eli Lilly, Locemia, and Lucera. Dr. Hobbs is chief medical officer for Novo Nordisk.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: The ultra–long-acting basal insulin degludec was noninferior to glargine in terms of cardiovascular risk and was superior in terms of severe hypoglycemia.
Major finding: Nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death occurred in 325 (8.5%) patients on degludec and 356 (9.3%) patients on glargine (HR, 0.91; 95% CI, 0.78-1.06; P = .21). Rates of severe hypoglycemia were 4.9% and 6.6%, respectively (P less than .001).
Data source: A randomized, double-blind, multicenter trial of 7,637 adults with type 2 diabetes at high risk of cardiovascular disease.
Disclosures: Novo Nordisk makes insulin degludec and sponsored the trial. Dr. Buse disclosed consulting fees from Novo Nordisk and ties to many other pharmaceutical companies. Dr. Seaquist disclosed ties to Novo Nordisk, Eli Lilly, Locemia, and Lucera. Dr. Hobbs is chief medical officer for Novo Nordisk.
Angiotensin II may improve vasopressors’ efficacy
WASHINGTON – Adding angiotensin II to available vasopressor therapies correlated with significantly improved arterial pressure in patients with catecholamine-resistant vasodilatory shock and less adverse effects, according to a study presented at the recent international conference of the American Thoracic Society.
In a double blind, controlled, phase III study, 70% of 163 patients given angiotensin II reached arterial pressure of at least 75 mm HG or improved by at least 10 mm Hg three hours later, compared with 23.4% of the 158 patients given a placebo (P less than .001).
Those in the angiotensin II group also saw a mean pressure increase of 12.5 mm Hg in the first 3 hours after initiating treatment, compared with 2.9 mm Hg in the placebo group (P less than .001), according to Ashish Khanna, MD, FCCP, of the Cleveland Clinic, and his fellow researchers (N Engl J Med. 2017 May 21. doi: 10.1056/NEJMoa1704154).
Current vasopressor therapies for vasodilatory patients are associated with dangerous side effects and a 30-day mortality rate of more than 50%, which is a major concern for patients who do not have many options to begin with, the researchers noted.
“Treatment options for patients with catecholamine-resistant vasodilatory shock are limited, and the treatments that are available are often associated with side effects,” said Dr. Khanna and his colleagues.
The researchers added the naturally occurring peptide hormone angiotensin II to vasodilatory patients’ treatment regimen in order to “more closely [mimic] natural physiologic responses to shock, which include increased secretion of catecholamines, vasopressin, and RAAS hormones.”
To test the efficacy of angiotensin II, researchers gathered patients with a median age of 64 years and a mean arterial pressure of 66.3 mm Hg.
Sepsis was the predominant cause of shock for 80.7% of the study’s participants.
Patients were injected with either 20 ng/kg of body weight per minute of angiotensin II or an equivalent dose of a placebo until mean arterial pressure reached 75 mm Hg. After 3 hours and 15 minutes of treatment, the dosages were adjusted to keep pressure between 65 and 75 mm Hg for the next 48 hours.
Among patients in the angiotensin II group, 67% of patients were able to decrease angiotensin II and vasopressor doses within 30 minutes of injection, according to researchers.
When researchers measured improvement using the cardiovascular Sequential Organ Failure Assessment, patients in the angiotensin II group saw an average decrease of 1.75 points, compared with 1.28 points in patients in the placebo group (P = .01) 48 hours after treatment.
The Sequential Organ Failure Assessment is scaled from 0-4, with higher scores indicating more severe organ failure.
When measuring for adverse affects, serious effects occurred in 60.7% of the angiotensin II patients, compared with in 67.1% of those in the placebo group.
At the 28-day mark, 75 angiotensin II patients (46.0%) died, compared with 85 patients (53.8%) of the placebo group.
This study was limited by the small sample size, “so the possibility of clinically important side effects attributable to angiotensin II therapy cannot be exuded,” the researches warned.
Also, the follow-up timeline of 28 days, may not have given researchers enough time to uncover the full extent of positive and negative long-term effects associated with angiotensin II.
This study was supported by La Jolla Pharmaceutical, from which multiple researchers reported receiving financial support in the form of personal fees and grants. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
[email protected]
On Twitter @eaztweets
WASHINGTON – Adding angiotensin II to available vasopressor therapies correlated with significantly improved arterial pressure in patients with catecholamine-resistant vasodilatory shock and less adverse effects, according to a study presented at the recent international conference of the American Thoracic Society.
In a double blind, controlled, phase III study, 70% of 163 patients given angiotensin II reached arterial pressure of at least 75 mm HG or improved by at least 10 mm Hg three hours later, compared with 23.4% of the 158 patients given a placebo (P less than .001).
Those in the angiotensin II group also saw a mean pressure increase of 12.5 mm Hg in the first 3 hours after initiating treatment, compared with 2.9 mm Hg in the placebo group (P less than .001), according to Ashish Khanna, MD, FCCP, of the Cleveland Clinic, and his fellow researchers (N Engl J Med. 2017 May 21. doi: 10.1056/NEJMoa1704154).
Current vasopressor therapies for vasodilatory patients are associated with dangerous side effects and a 30-day mortality rate of more than 50%, which is a major concern for patients who do not have many options to begin with, the researchers noted.
“Treatment options for patients with catecholamine-resistant vasodilatory shock are limited, and the treatments that are available are often associated with side effects,” said Dr. Khanna and his colleagues.
The researchers added the naturally occurring peptide hormone angiotensin II to vasodilatory patients’ treatment regimen in order to “more closely [mimic] natural physiologic responses to shock, which include increased secretion of catecholamines, vasopressin, and RAAS hormones.”
To test the efficacy of angiotensin II, researchers gathered patients with a median age of 64 years and a mean arterial pressure of 66.3 mm Hg.
Sepsis was the predominant cause of shock for 80.7% of the study’s participants.
Patients were injected with either 20 ng/kg of body weight per minute of angiotensin II or an equivalent dose of a placebo until mean arterial pressure reached 75 mm Hg. After 3 hours and 15 minutes of treatment, the dosages were adjusted to keep pressure between 65 and 75 mm Hg for the next 48 hours.
Among patients in the angiotensin II group, 67% of patients were able to decrease angiotensin II and vasopressor doses within 30 minutes of injection, according to researchers.
When researchers measured improvement using the cardiovascular Sequential Organ Failure Assessment, patients in the angiotensin II group saw an average decrease of 1.75 points, compared with 1.28 points in patients in the placebo group (P = .01) 48 hours after treatment.
The Sequential Organ Failure Assessment is scaled from 0-4, with higher scores indicating more severe organ failure.
When measuring for adverse affects, serious effects occurred in 60.7% of the angiotensin II patients, compared with in 67.1% of those in the placebo group.
At the 28-day mark, 75 angiotensin II patients (46.0%) died, compared with 85 patients (53.8%) of the placebo group.
This study was limited by the small sample size, “so the possibility of clinically important side effects attributable to angiotensin II therapy cannot be exuded,” the researches warned.
Also, the follow-up timeline of 28 days, may not have given researchers enough time to uncover the full extent of positive and negative long-term effects associated with angiotensin II.
This study was supported by La Jolla Pharmaceutical, from which multiple researchers reported receiving financial support in the form of personal fees and grants. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
[email protected]
On Twitter @eaztweets
WASHINGTON – Adding angiotensin II to available vasopressor therapies correlated with significantly improved arterial pressure in patients with catecholamine-resistant vasodilatory shock and less adverse effects, according to a study presented at the recent international conference of the American Thoracic Society.
In a double blind, controlled, phase III study, 70% of 163 patients given angiotensin II reached arterial pressure of at least 75 mm HG or improved by at least 10 mm Hg three hours later, compared with 23.4% of the 158 patients given a placebo (P less than .001).
Those in the angiotensin II group also saw a mean pressure increase of 12.5 mm Hg in the first 3 hours after initiating treatment, compared with 2.9 mm Hg in the placebo group (P less than .001), according to Ashish Khanna, MD, FCCP, of the Cleveland Clinic, and his fellow researchers (N Engl J Med. 2017 May 21. doi: 10.1056/NEJMoa1704154).
Current vasopressor therapies for vasodilatory patients are associated with dangerous side effects and a 30-day mortality rate of more than 50%, which is a major concern for patients who do not have many options to begin with, the researchers noted.
“Treatment options for patients with catecholamine-resistant vasodilatory shock are limited, and the treatments that are available are often associated with side effects,” said Dr. Khanna and his colleagues.
The researchers added the naturally occurring peptide hormone angiotensin II to vasodilatory patients’ treatment regimen in order to “more closely [mimic] natural physiologic responses to shock, which include increased secretion of catecholamines, vasopressin, and RAAS hormones.”
To test the efficacy of angiotensin II, researchers gathered patients with a median age of 64 years and a mean arterial pressure of 66.3 mm Hg.
Sepsis was the predominant cause of shock for 80.7% of the study’s participants.
Patients were injected with either 20 ng/kg of body weight per minute of angiotensin II or an equivalent dose of a placebo until mean arterial pressure reached 75 mm Hg. After 3 hours and 15 minutes of treatment, the dosages were adjusted to keep pressure between 65 and 75 mm Hg for the next 48 hours.
Among patients in the angiotensin II group, 67% of patients were able to decrease angiotensin II and vasopressor doses within 30 minutes of injection, according to researchers.
When researchers measured improvement using the cardiovascular Sequential Organ Failure Assessment, patients in the angiotensin II group saw an average decrease of 1.75 points, compared with 1.28 points in patients in the placebo group (P = .01) 48 hours after treatment.
The Sequential Organ Failure Assessment is scaled from 0-4, with higher scores indicating more severe organ failure.
When measuring for adverse affects, serious effects occurred in 60.7% of the angiotensin II patients, compared with in 67.1% of those in the placebo group.
At the 28-day mark, 75 angiotensin II patients (46.0%) died, compared with 85 patients (53.8%) of the placebo group.
This study was limited by the small sample size, “so the possibility of clinically important side effects attributable to angiotensin II therapy cannot be exuded,” the researches warned.
Also, the follow-up timeline of 28 days, may not have given researchers enough time to uncover the full extent of positive and negative long-term effects associated with angiotensin II.
This study was supported by La Jolla Pharmaceutical, from which multiple researchers reported receiving financial support in the form of personal fees and grants. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
[email protected]
On Twitter @eaztweets
At ATS 2017
Key clinical point:
Major finding: In the first 3 hours, patients taking angiotensin II improved arterial pressure by an average of 12.5 mm Hg, compared with 2.9 mm Hg in patients taking the placebo (P less than .001)
Data source: Double blind, randomized, control trial of 321 patients with catecholamine-resistant vasodilatory shock collected from 75 intensive care units globally during May 2015-January 2017.
Disclosures: Multiple investigators reported receiving support from La Jolla Pharmaceutical and similar companies in the form of grants and/or personal fees. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
VA and DoE to Use Supercomputing for Transformative Science
The VA and the Department of Energy (DoE) have formed a new partnership focused on secure analysis of “big data.” The VA-DoE Big Data Science Initiative will use digital health and genomic data from the Million Veteran Program (MVP), the VA’s electronic health records system, DoD, Centers for Medicare and Medicaid Services, and the CDC’s National Death Index.
The partnership is based in DoE’s National Laboratory system, one of the world’s top resources for supercomputing, where machines are capable of millions of billions of calculations per second. The partnership will allow thousands of researchers access to this unprecedented data resource over time in a secure environment, said VA Secretary David J. Shulkin, MD.
An initial suite of specific studies is already being planned, the VA says. One group of researchers will build algorithms to generate “highly tailored” risk scores for suicide, which could help VA clinicians and researchers predict which patients are at highest risk and evaluate prevention strategies.
Other projects include one to find new ways to distinguish lethal from nonlethal prostate cancer and another to determine which risk factors best predict certain forms of cardiovascular disease.
“The transformative science that will be developed through this partnership,” Shulkin says, “will improve health care for veterans and all Americans.”
The VA and the Department of Energy (DoE) have formed a new partnership focused on secure analysis of “big data.” The VA-DoE Big Data Science Initiative will use digital health and genomic data from the Million Veteran Program (MVP), the VA’s electronic health records system, DoD, Centers for Medicare and Medicaid Services, and the CDC’s National Death Index.
The partnership is based in DoE’s National Laboratory system, one of the world’s top resources for supercomputing, where machines are capable of millions of billions of calculations per second. The partnership will allow thousands of researchers access to this unprecedented data resource over time in a secure environment, said VA Secretary David J. Shulkin, MD.
An initial suite of specific studies is already being planned, the VA says. One group of researchers will build algorithms to generate “highly tailored” risk scores for suicide, which could help VA clinicians and researchers predict which patients are at highest risk and evaluate prevention strategies.
Other projects include one to find new ways to distinguish lethal from nonlethal prostate cancer and another to determine which risk factors best predict certain forms of cardiovascular disease.
“The transformative science that will be developed through this partnership,” Shulkin says, “will improve health care for veterans and all Americans.”
The VA and the Department of Energy (DoE) have formed a new partnership focused on secure analysis of “big data.” The VA-DoE Big Data Science Initiative will use digital health and genomic data from the Million Veteran Program (MVP), the VA’s electronic health records system, DoD, Centers for Medicare and Medicaid Services, and the CDC’s National Death Index.
The partnership is based in DoE’s National Laboratory system, one of the world’s top resources for supercomputing, where machines are capable of millions of billions of calculations per second. The partnership will allow thousands of researchers access to this unprecedented data resource over time in a secure environment, said VA Secretary David J. Shulkin, MD.
An initial suite of specific studies is already being planned, the VA says. One group of researchers will build algorithms to generate “highly tailored” risk scores for suicide, which could help VA clinicians and researchers predict which patients are at highest risk and evaluate prevention strategies.
Other projects include one to find new ways to distinguish lethal from nonlethal prostate cancer and another to determine which risk factors best predict certain forms of cardiovascular disease.
“The transformative science that will be developed through this partnership,” Shulkin says, “will improve health care for veterans and all Americans.”
Company pauses enrollment on 2 trials of pembrolizumab in MM
Merck announced that it is pausing enrollment onto 2 phase 3 trials of pembrolizumab (Keytruda®) in combination with other agents to treat multiple myeloma (MM).
An external Data Monitoring Committee recommended the trial be interrupted “to allow for additional information be collected to better understand more reports of death” in the pembrolizumab groups in the KEYNOTE-183 and KEYNOTE-185 trials.
Patients currently enrolled on the trials can continue to receive treatment. Other pembrolizumab trials are continuing without changes.
Merck in its statement did not disclose the number of deaths nor provide any other details on the deaths.
Pembrolizumab is a humanized monoclonal antibody that blocks interaction between the programmed cell death protein 1 (PD-1) and its receptor ligands, PD-L1 and PD-L2.
The US Food & Drug Administration approved pembrolizumab to treat unresectable or metastatic melanoma after ipilimumab treatment.
Pembrolizumab has also been approved to treat non-small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, and microsatellite instability-high solid tumors.
KEYNOTE-183 (NCT02576977), which has an estimated enrollment of 300 patients, is comparing the combination of pembrolizumab, pomalidomide, and low-dose dexamethasone to pomalidomide and low-dose dexamethasone alone in patients with relapsed or refractory MM who have undergone at least 2 lines of prior therapy.
KEYNOTE-185 (NCT02579863), which has an estimated enrollment of 640 patients, is comparing the combination of pembrolizumab, lenalidomide, and low-dose dexamethasone to lenalidomide and low-dose desamethasone alone in patients with newly diagnosed and treatment-native MM who are ineligible for autologous stem cell transplant.
The comparator agents pomalidomide (Pomalyst®) and lenalidomide (Revlimid®) are products of Celgene Corporation. ![]()
Merck announced that it is pausing enrollment onto 2 phase 3 trials of pembrolizumab (Keytruda®) in combination with other agents to treat multiple myeloma (MM).
An external Data Monitoring Committee recommended the trial be interrupted “to allow for additional information be collected to better understand more reports of death” in the pembrolizumab groups in the KEYNOTE-183 and KEYNOTE-185 trials.
Patients currently enrolled on the trials can continue to receive treatment. Other pembrolizumab trials are continuing without changes.
Merck in its statement did not disclose the number of deaths nor provide any other details on the deaths.
Pembrolizumab is a humanized monoclonal antibody that blocks interaction between the programmed cell death protein 1 (PD-1) and its receptor ligands, PD-L1 and PD-L2.
The US Food & Drug Administration approved pembrolizumab to treat unresectable or metastatic melanoma after ipilimumab treatment.
Pembrolizumab has also been approved to treat non-small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, and microsatellite instability-high solid tumors.
KEYNOTE-183 (NCT02576977), which has an estimated enrollment of 300 patients, is comparing the combination of pembrolizumab, pomalidomide, and low-dose dexamethasone to pomalidomide and low-dose dexamethasone alone in patients with relapsed or refractory MM who have undergone at least 2 lines of prior therapy.
KEYNOTE-185 (NCT02579863), which has an estimated enrollment of 640 patients, is comparing the combination of pembrolizumab, lenalidomide, and low-dose dexamethasone to lenalidomide and low-dose desamethasone alone in patients with newly diagnosed and treatment-native MM who are ineligible for autologous stem cell transplant.
The comparator agents pomalidomide (Pomalyst®) and lenalidomide (Revlimid®) are products of Celgene Corporation. ![]()
Merck announced that it is pausing enrollment onto 2 phase 3 trials of pembrolizumab (Keytruda®) in combination with other agents to treat multiple myeloma (MM).
An external Data Monitoring Committee recommended the trial be interrupted “to allow for additional information be collected to better understand more reports of death” in the pembrolizumab groups in the KEYNOTE-183 and KEYNOTE-185 trials.
Patients currently enrolled on the trials can continue to receive treatment. Other pembrolizumab trials are continuing without changes.
Merck in its statement did not disclose the number of deaths nor provide any other details on the deaths.
Pembrolizumab is a humanized monoclonal antibody that blocks interaction between the programmed cell death protein 1 (PD-1) and its receptor ligands, PD-L1 and PD-L2.
The US Food & Drug Administration approved pembrolizumab to treat unresectable or metastatic melanoma after ipilimumab treatment.
Pembrolizumab has also been approved to treat non-small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, and microsatellite instability-high solid tumors.
KEYNOTE-183 (NCT02576977), which has an estimated enrollment of 300 patients, is comparing the combination of pembrolizumab, pomalidomide, and low-dose dexamethasone to pomalidomide and low-dose dexamethasone alone in patients with relapsed or refractory MM who have undergone at least 2 lines of prior therapy.
KEYNOTE-185 (NCT02579863), which has an estimated enrollment of 640 patients, is comparing the combination of pembrolizumab, lenalidomide, and low-dose dexamethasone to lenalidomide and low-dose desamethasone alone in patients with newly diagnosed and treatment-native MM who are ineligible for autologous stem cell transplant.
The comparator agents pomalidomide (Pomalyst®) and lenalidomide (Revlimid®) are products of Celgene Corporation. ![]()
BM-MSCs may be an option for AA patients refractory to IST
Researchers report that infusions of bone marrow-derived mesenchymal stromal cells (BM-MSCs) may be a treatment option for patients with aplastic anemia (AA) who are refractory to immunosuppressive therapy (IST).
They conducted a phase 2, non-comparative multicenter study to assess the safety and efficacy of this approach and found that after 12 months, 28.4% of patients responded, with 6.8% achieving a complete response (CR) and 21.6% a partial response (PR).
The trial involved 74 patients at 7 centers in China. The research team reported its findings in Stem Cells Translational Medicine.
About 30% to 40% of patients with severe AA (sAA) don’t respond well to IST and continue to have abnormally low levels of red blood cells, white blood cells, and platelets.
The benefit of treatment with BM-MSCs is they support hematopoiesis, express low levels of major histocompatibility (MHC)-I, and lack expression of MHC-II surface molecules.
And BM-MSCs have been reported to cure diseases, including graft-versus-host disease, arthritis, lupus, and other immune and non-immune disorders.
The current study, led by Yan Pang, MD, and Yang Xiao, MD, PhD, of Guangzhou General Hospital of Guangzhou Military Command in China, is based on their previous data evaluating intravenous administration of MSCs from a related donor in 18 patients with refractory AA.
This earlier data showed that 33% of patients with AA refractory to IST achieved a CR or PR to BM-MSC treatment.
So the team undertook to further investigate the use of BM-MSCs in AA.
Study design
Each of the 74 patients received 4 doses of BM-MSCs over a period of 4 weeks. If patients responded after the first month, they continued to receive 4 doses.
Investigators obtained the BM-MSCs from 74 healthy donors—48 males and 26 females. Forty were related donors, 27 haploidentical donors, and 7 unrelated donors.
Patients with AA by standard criteria had to be 16 years or older, had an incomplete response to antithymocyte globulin (ATG) and cyclosporine for at leas 6 months or cyclosporine alone for at least 12 months, did not have a donor available for bone marrow transplantation, and had at least one of the following: hemoglobin <70 g/L, neutrophilic granulocytes <1 × 109/L, or platelet count <30 × 109/L.
Almost half the patients (47%) were between 20 and 40 years, 54% were male, and 32% had severe aplastic anemia.
Patients’ previous therapy for aplastic anemia included cyclosporine and andriol (65%) cyclosporine and ATG (19%), and cyclosporine (16%).
Patients could continue cyclosporine, but no immunosuppressive agents were permitted.
Fifty-three patients (71.6%) completed 1 course of therapy, and 21 patients (18.4%) completed 2 courses.
Response
At 1 year, the overall response rate was 28.4% (n=21) and the PR rate was 21.6% (n=16).
The median time to a leukocyte response was 19 days (range, 11 – 29), to an erythrocyte response 17 days (range, 12 – 25), and to a megakaryocyte response 31 days (range, 26 – 84).
Ten of the patients with hematologic response had normalization of cellularity for more than 1 year.
The median follow-up among survivors was 17 months (range, 3 – 24). The 2-year overall survival was 87.8%.
Three patients progressed to myelodysplasia, 1 with refractory anemia with excess blasts (RAEB)-I and 2 with RAEB-II.
The median time to progression was 11 months (range, 8 – 12).
Nine patients died, all of whom had severe AA. One patient with RAEB-II died of disease progression, two patients died of intracranial hemorrhage, and six patients died of serious infection.
Safety
Adverse events included grade 1 (n=5) and grade 2 (n=2) fever. Two of these patients also had grade 1 headache.
The investigators observed no other adverse events.
Response predictors
The investigators determined that 2 factors predicted response in patients: prior treatment with antithymocyte globulin (ATG) and absence of infection throughout the treatment.
The odds ratio for patients treated with ATG was 1.41 (95% CI: -0.50, 3.31) and for patients without infection, 2.19 (95% CI: 0.50, 3.87).
“Our study strongly indicates that MSC infusion is a promising therapy for severe AA," Dr Pang said, “but improved MSC cultures in vitro and the MSC doses need further study to maximize their therapeutic potential." ![]()
Researchers report that infusions of bone marrow-derived mesenchymal stromal cells (BM-MSCs) may be a treatment option for patients with aplastic anemia (AA) who are refractory to immunosuppressive therapy (IST).
They conducted a phase 2, non-comparative multicenter study to assess the safety and efficacy of this approach and found that after 12 months, 28.4% of patients responded, with 6.8% achieving a complete response (CR) and 21.6% a partial response (PR).
The trial involved 74 patients at 7 centers in China. The research team reported its findings in Stem Cells Translational Medicine.
About 30% to 40% of patients with severe AA (sAA) don’t respond well to IST and continue to have abnormally low levels of red blood cells, white blood cells, and platelets.
The benefit of treatment with BM-MSCs is they support hematopoiesis, express low levels of major histocompatibility (MHC)-I, and lack expression of MHC-II surface molecules.
And BM-MSCs have been reported to cure diseases, including graft-versus-host disease, arthritis, lupus, and other immune and non-immune disorders.
The current study, led by Yan Pang, MD, and Yang Xiao, MD, PhD, of Guangzhou General Hospital of Guangzhou Military Command in China, is based on their previous data evaluating intravenous administration of MSCs from a related donor in 18 patients with refractory AA.
This earlier data showed that 33% of patients with AA refractory to IST achieved a CR or PR to BM-MSC treatment.
So the team undertook to further investigate the use of BM-MSCs in AA.
Study design
Each of the 74 patients received 4 doses of BM-MSCs over a period of 4 weeks. If patients responded after the first month, they continued to receive 4 doses.
Investigators obtained the BM-MSCs from 74 healthy donors—48 males and 26 females. Forty were related donors, 27 haploidentical donors, and 7 unrelated donors.
Patients with AA by standard criteria had to be 16 years or older, had an incomplete response to antithymocyte globulin (ATG) and cyclosporine for at leas 6 months or cyclosporine alone for at least 12 months, did not have a donor available for bone marrow transplantation, and had at least one of the following: hemoglobin <70 g/L, neutrophilic granulocytes <1 × 109/L, or platelet count <30 × 109/L.
Almost half the patients (47%) were between 20 and 40 years, 54% were male, and 32% had severe aplastic anemia.
Patients’ previous therapy for aplastic anemia included cyclosporine and andriol (65%) cyclosporine and ATG (19%), and cyclosporine (16%).
Patients could continue cyclosporine, but no immunosuppressive agents were permitted.
Fifty-three patients (71.6%) completed 1 course of therapy, and 21 patients (18.4%) completed 2 courses.
Response
At 1 year, the overall response rate was 28.4% (n=21) and the PR rate was 21.6% (n=16).
The median time to a leukocyte response was 19 days (range, 11 – 29), to an erythrocyte response 17 days (range, 12 – 25), and to a megakaryocyte response 31 days (range, 26 – 84).
Ten of the patients with hematologic response had normalization of cellularity for more than 1 year.
The median follow-up among survivors was 17 months (range, 3 – 24). The 2-year overall survival was 87.8%.
Three patients progressed to myelodysplasia, 1 with refractory anemia with excess blasts (RAEB)-I and 2 with RAEB-II.
The median time to progression was 11 months (range, 8 – 12).
Nine patients died, all of whom had severe AA. One patient with RAEB-II died of disease progression, two patients died of intracranial hemorrhage, and six patients died of serious infection.
Safety
Adverse events included grade 1 (n=5) and grade 2 (n=2) fever. Two of these patients also had grade 1 headache.
The investigators observed no other adverse events.
Response predictors
The investigators determined that 2 factors predicted response in patients: prior treatment with antithymocyte globulin (ATG) and absence of infection throughout the treatment.
The odds ratio for patients treated with ATG was 1.41 (95% CI: -0.50, 3.31) and for patients without infection, 2.19 (95% CI: 0.50, 3.87).
“Our study strongly indicates that MSC infusion is a promising therapy for severe AA," Dr Pang said, “but improved MSC cultures in vitro and the MSC doses need further study to maximize their therapeutic potential." ![]()
Researchers report that infusions of bone marrow-derived mesenchymal stromal cells (BM-MSCs) may be a treatment option for patients with aplastic anemia (AA) who are refractory to immunosuppressive therapy (IST).
They conducted a phase 2, non-comparative multicenter study to assess the safety and efficacy of this approach and found that after 12 months, 28.4% of patients responded, with 6.8% achieving a complete response (CR) and 21.6% a partial response (PR).
The trial involved 74 patients at 7 centers in China. The research team reported its findings in Stem Cells Translational Medicine.
About 30% to 40% of patients with severe AA (sAA) don’t respond well to IST and continue to have abnormally low levels of red blood cells, white blood cells, and platelets.
The benefit of treatment with BM-MSCs is they support hematopoiesis, express low levels of major histocompatibility (MHC)-I, and lack expression of MHC-II surface molecules.
And BM-MSCs have been reported to cure diseases, including graft-versus-host disease, arthritis, lupus, and other immune and non-immune disorders.
The current study, led by Yan Pang, MD, and Yang Xiao, MD, PhD, of Guangzhou General Hospital of Guangzhou Military Command in China, is based on their previous data evaluating intravenous administration of MSCs from a related donor in 18 patients with refractory AA.
This earlier data showed that 33% of patients with AA refractory to IST achieved a CR or PR to BM-MSC treatment.
So the team undertook to further investigate the use of BM-MSCs in AA.
Study design
Each of the 74 patients received 4 doses of BM-MSCs over a period of 4 weeks. If patients responded after the first month, they continued to receive 4 doses.
Investigators obtained the BM-MSCs from 74 healthy donors—48 males and 26 females. Forty were related donors, 27 haploidentical donors, and 7 unrelated donors.
Patients with AA by standard criteria had to be 16 years or older, had an incomplete response to antithymocyte globulin (ATG) and cyclosporine for at leas 6 months or cyclosporine alone for at least 12 months, did not have a donor available for bone marrow transplantation, and had at least one of the following: hemoglobin <70 g/L, neutrophilic granulocytes <1 × 109/L, or platelet count <30 × 109/L.
Almost half the patients (47%) were between 20 and 40 years, 54% were male, and 32% had severe aplastic anemia.
Patients’ previous therapy for aplastic anemia included cyclosporine and andriol (65%) cyclosporine and ATG (19%), and cyclosporine (16%).
Patients could continue cyclosporine, but no immunosuppressive agents were permitted.
Fifty-three patients (71.6%) completed 1 course of therapy, and 21 patients (18.4%) completed 2 courses.
Response
At 1 year, the overall response rate was 28.4% (n=21) and the PR rate was 21.6% (n=16).
The median time to a leukocyte response was 19 days (range, 11 – 29), to an erythrocyte response 17 days (range, 12 – 25), and to a megakaryocyte response 31 days (range, 26 – 84).
Ten of the patients with hematologic response had normalization of cellularity for more than 1 year.
The median follow-up among survivors was 17 months (range, 3 – 24). The 2-year overall survival was 87.8%.
Three patients progressed to myelodysplasia, 1 with refractory anemia with excess blasts (RAEB)-I and 2 with RAEB-II.
The median time to progression was 11 months (range, 8 – 12).
Nine patients died, all of whom had severe AA. One patient with RAEB-II died of disease progression, two patients died of intracranial hemorrhage, and six patients died of serious infection.
Safety
Adverse events included grade 1 (n=5) and grade 2 (n=2) fever. Two of these patients also had grade 1 headache.
The investigators observed no other adverse events.
Response predictors
The investigators determined that 2 factors predicted response in patients: prior treatment with antithymocyte globulin (ATG) and absence of infection throughout the treatment.
The odds ratio for patients treated with ATG was 1.41 (95% CI: -0.50, 3.31) and for patients without infection, 2.19 (95% CI: 0.50, 3.87).
“Our study strongly indicates that MSC infusion is a promising therapy for severe AA," Dr Pang said, “but improved MSC cultures in vitro and the MSC doses need further study to maximize their therapeutic potential." ![]()
Supportive parenting program reduced African American teen smoking
Particularly in high-risk populations, participation in a family-centered parenting intervention can reduce adolescent and early adult smoking, according to results of a randomized controlled trial of the Strong African American Families (SAAF) program published in Pediatrics June 14.
African Americans have the “highest mortality rates for coronary heart disease and stroke,” compared with other racial groups, and the high rate of smoking among young adult African Americans is of concern, wrote Yi-fu Chen, PhD, of National Taipei University, New Taipei City, Taiwan, and his coinvestigators.
In a study of 424 African American adolescents from small towns in nine rural counties of southern Georgia, the 257 who were randomized to SAAF at age 11 years overall displayed significantly lower cotinine scores a
The SAAF families each attended seven meetings that consisted of both a separate session for the youth and the parent, and a joint session in which they practiced the skills they learned separately. The parents focused on “consistent provision of instrumental and emotional support, along with high levels of monitoring, consistent discipline that is not harsh, predictable family routines, and the establishment of clear norms and expectations for the use of drugs,” while the youth focused on “the importance of having and abiding by household rules, adaptive behaviors to use when encountering racism, and the importance of forming goals for the future and making plans to attain them,” the researchers explained.
These results “may provide a strategy for reducing health vulnerabilities associated with smoking among African American youth who grow up in challenging rural contexts,” the authors wrote.
Read more in Pediatrics (doi: 10.1542/peds.2016-4162).
Particularly in high-risk populations, participation in a family-centered parenting intervention can reduce adolescent and early adult smoking, according to results of a randomized controlled trial of the Strong African American Families (SAAF) program published in Pediatrics June 14.
African Americans have the “highest mortality rates for coronary heart disease and stroke,” compared with other racial groups, and the high rate of smoking among young adult African Americans is of concern, wrote Yi-fu Chen, PhD, of National Taipei University, New Taipei City, Taiwan, and his coinvestigators.
In a study of 424 African American adolescents from small towns in nine rural counties of southern Georgia, the 257 who were randomized to SAAF at age 11 years overall displayed significantly lower cotinine scores a
The SAAF families each attended seven meetings that consisted of both a separate session for the youth and the parent, and a joint session in which they practiced the skills they learned separately. The parents focused on “consistent provision of instrumental and emotional support, along with high levels of monitoring, consistent discipline that is not harsh, predictable family routines, and the establishment of clear norms and expectations for the use of drugs,” while the youth focused on “the importance of having and abiding by household rules, adaptive behaviors to use when encountering racism, and the importance of forming goals for the future and making plans to attain them,” the researchers explained.
These results “may provide a strategy for reducing health vulnerabilities associated with smoking among African American youth who grow up in challenging rural contexts,” the authors wrote.
Read more in Pediatrics (doi: 10.1542/peds.2016-4162).
Particularly in high-risk populations, participation in a family-centered parenting intervention can reduce adolescent and early adult smoking, according to results of a randomized controlled trial of the Strong African American Families (SAAF) program published in Pediatrics June 14.
African Americans have the “highest mortality rates for coronary heart disease and stroke,” compared with other racial groups, and the high rate of smoking among young adult African Americans is of concern, wrote Yi-fu Chen, PhD, of National Taipei University, New Taipei City, Taiwan, and his coinvestigators.
In a study of 424 African American adolescents from small towns in nine rural counties of southern Georgia, the 257 who were randomized to SAAF at age 11 years overall displayed significantly lower cotinine scores a
The SAAF families each attended seven meetings that consisted of both a separate session for the youth and the parent, and a joint session in which they practiced the skills they learned separately. The parents focused on “consistent provision of instrumental and emotional support, along with high levels of monitoring, consistent discipline that is not harsh, predictable family routines, and the establishment of clear norms and expectations for the use of drugs,” while the youth focused on “the importance of having and abiding by household rules, adaptive behaviors to use when encountering racism, and the importance of forming goals for the future and making plans to attain them,” the researchers explained.
These results “may provide a strategy for reducing health vulnerabilities associated with smoking among African American youth who grow up in challenging rural contexts,” the authors wrote.
Read more in Pediatrics (doi: 10.1542/peds.2016-4162).
FROM PEDIATRICS
The Man With No Medical History
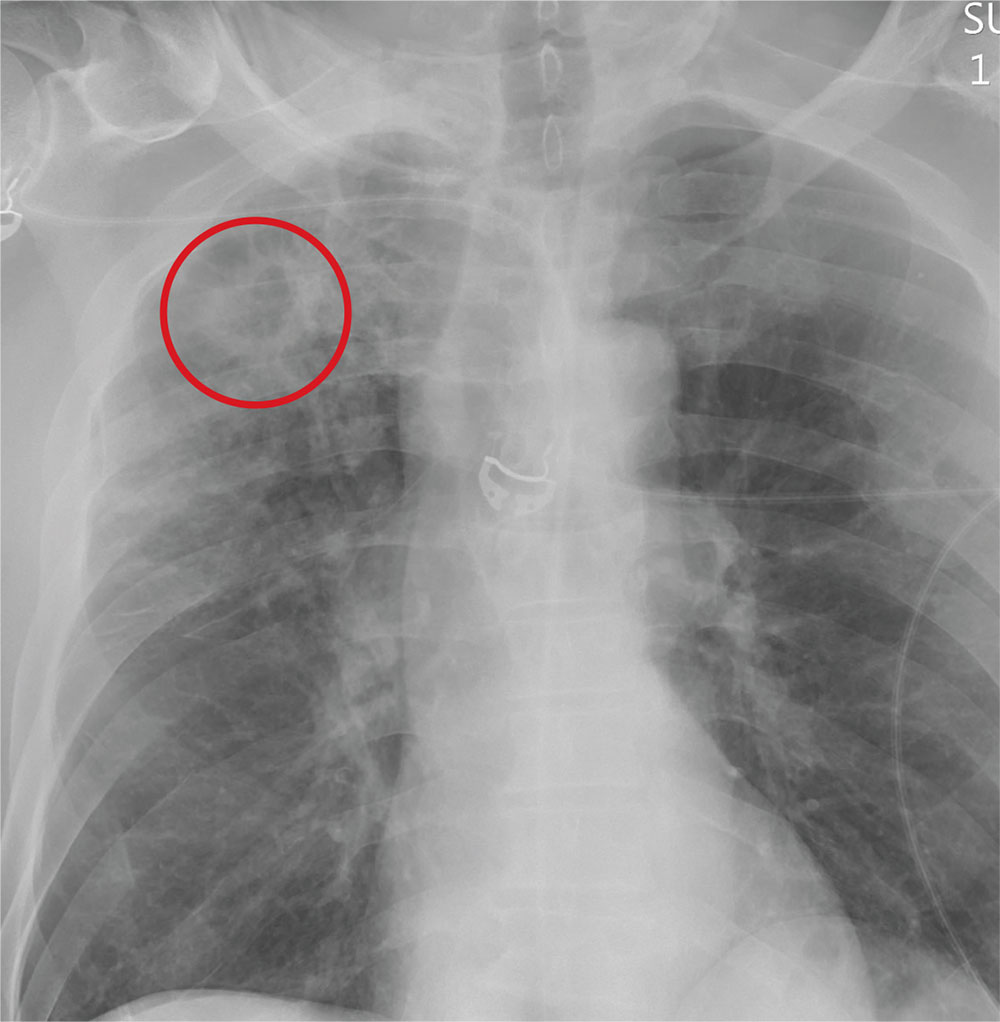
ANSWER
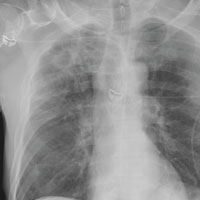
The radiograph demonstrates no acute fractures or pneumothorax. Of note is a right upper lobe infiltrate, which is a rounded cavitary lesion measuring approximately 4 cm. The differential includes pulmonary malignancy, active or previous pulmonary infection (eg, tuberculosis), or pneumatocele. Further evaluation with CT and a pulmonary consultation was coordinated.

ANSWER
The radiograph demonstrates no acute fractures or pneumothorax. Of note is a right upper lobe infiltrate, which is a rounded cavitary lesion measuring approximately 4 cm. The differential includes pulmonary malignancy, active or previous pulmonary infection (eg, tuberculosis), or pneumatocele. Further evaluation with CT and a pulmonary consultation was coordinated.

ANSWER
The radiograph demonstrates no acute fractures or pneumothorax. Of note is a right upper lobe infiltrate, which is a rounded cavitary lesion measuring approximately 4 cm. The differential includes pulmonary malignancy, active or previous pulmonary infection (eg, tuberculosis), or pneumatocele. Further evaluation with CT and a pulmonary consultation was coordinated.
Following a motor vehicle collision, a 60-year-old man is brought to the emergency department via ambulance. He was an unrestrained front-seat passenger in a vehicle that lost control on the roadway and went into a ditch. The patient complains of headache, chest wall pain, and left arm pain. He does not believe he lost consciousness.
He denies any medical history and adds that he does not seek regular medical treatment. He admits to tobacco use and frequent alcohol use.
On physical exam, you note an elderly-appearing male in no obvious distress with a Glasgow Coma Scale score of 15. His vital signs are all within normal limits. Other than slight swelling on the left side of his head, tenderness in the anterior chest wall, and pain in his left humerus, his exam is normal.
You order trauma lab tests and appropriate radiographic studies; a portable chest radiograph is completed (shown). What is your impression?
Endometriosis after menopause: Weigh the treatment risks
VANCOUVER – Endometriosis, while generally considered a premenopausal condition, can also occur in women following surgical or natural menopause, and can undergo malignant transformation, although this risk is likely very small.
That was the main message from a new meta-analysis presented at the World Congress on Endometriosis. “We wanted to synthesize the case reports out there to show some common factors so physicians can be aware of them,” said Laura Gemmell, a second-year medical student at Case Western Reserve University, Cleveland, who presented the research.
The researchers surveyed the literature for studies in postmenopausal women with a confirmed or clinically suspected history of endometriosis, and who discussed the management of their menopausal symptoms. They included 33 case reports and case series (42 patients, 36 surgical menopause, 4 natural, 2 presumed natural with later oophorectomy), as well as 6 observational studies and clinical trials.
In the case reports, patients were on HT for a mean of 7.8 years, and 17 of 42 women experienced a recurrence of endometriosis. Also, 25 women had a malignant transformation and there was some overlap with the recurrence group.
Among 17 patients with recurrence, 6 had “severe” or “extensive” endometriosis, and 14 had surgical menopause, with a mean of 7.1 years between surgical menopause and presentation. Twelve of 17 received unopposed estrogen. Following surgical excision (16 of 17 cases), 10 had symptom regression without relapses.
When the researchers looked at the 25 cases of malignant transformation, they found that 13 women had endometriosis at more than one site, 22 had undergone surgical menopause, 19 were on unopposed estrogen, and the mean duration of HT was 6.7 years. Seven women presented with vaginal bleeding and nine with masses. Three died from the disease. These three women had severe endometriosis complicating factors, including older age and multiple malignancies.
The analysis also included six observational studies and clinical trials that explored recurrence of endometriosis, and whether HT should be given to women with a history of endometriosis, whether it should be given immediately after surgical menopause, and the most appropriate menopause treatments.
Predictably, the evidence could not be summed up neatly, but Ms. Gemmell emphasized the need to individually weigh the risks and benefits of HT in each patient, with consideration of characteristics such as age, previous disease severity, family history, comorbidities, and body mass index.
She also suggested that patients should be active participants in decision making.
Finally, if the decision is to go forward or continue with HT, she suggested that clinicians consider a combined treatment rather than estrogen-only, though she pointed out the increased risk for breast cancer that this presents.
Tommaso Falcone, MD, chairman of obstetrics and gynecology at the Cleveland Clinic, sounded a note of caution about the use of progestins during the question-and-answer session. “The data are not strong that it actually prevents the development of cancer in the residual disease, if there is any. Even if you take the hypothesis that progestins are going to prevent cancer of residual disease, which is a low-level risk, the main worry that women have is breast cancer, and progestin is strongly associated with breast cancer,” Dr. Falcone said in an interview.
Ms. Gemmell and Dr. Falcone reported having no financial disclosures.
VANCOUVER – Endometriosis, while generally considered a premenopausal condition, can also occur in women following surgical or natural menopause, and can undergo malignant transformation, although this risk is likely very small.
That was the main message from a new meta-analysis presented at the World Congress on Endometriosis. “We wanted to synthesize the case reports out there to show some common factors so physicians can be aware of them,” said Laura Gemmell, a second-year medical student at Case Western Reserve University, Cleveland, who presented the research.
The researchers surveyed the literature for studies in postmenopausal women with a confirmed or clinically suspected history of endometriosis, and who discussed the management of their menopausal symptoms. They included 33 case reports and case series (42 patients, 36 surgical menopause, 4 natural, 2 presumed natural with later oophorectomy), as well as 6 observational studies and clinical trials.
In the case reports, patients were on HT for a mean of 7.8 years, and 17 of 42 women experienced a recurrence of endometriosis. Also, 25 women had a malignant transformation and there was some overlap with the recurrence group.
Among 17 patients with recurrence, 6 had “severe” or “extensive” endometriosis, and 14 had surgical menopause, with a mean of 7.1 years between surgical menopause and presentation. Twelve of 17 received unopposed estrogen. Following surgical excision (16 of 17 cases), 10 had symptom regression without relapses.
When the researchers looked at the 25 cases of malignant transformation, they found that 13 women had endometriosis at more than one site, 22 had undergone surgical menopause, 19 were on unopposed estrogen, and the mean duration of HT was 6.7 years. Seven women presented with vaginal bleeding and nine with masses. Three died from the disease. These three women had severe endometriosis complicating factors, including older age and multiple malignancies.
The analysis also included six observational studies and clinical trials that explored recurrence of endometriosis, and whether HT should be given to women with a history of endometriosis, whether it should be given immediately after surgical menopause, and the most appropriate menopause treatments.
Predictably, the evidence could not be summed up neatly, but Ms. Gemmell emphasized the need to individually weigh the risks and benefits of HT in each patient, with consideration of characteristics such as age, previous disease severity, family history, comorbidities, and body mass index.
She also suggested that patients should be active participants in decision making.
Finally, if the decision is to go forward or continue with HT, she suggested that clinicians consider a combined treatment rather than estrogen-only, though she pointed out the increased risk for breast cancer that this presents.
Tommaso Falcone, MD, chairman of obstetrics and gynecology at the Cleveland Clinic, sounded a note of caution about the use of progestins during the question-and-answer session. “The data are not strong that it actually prevents the development of cancer in the residual disease, if there is any. Even if you take the hypothesis that progestins are going to prevent cancer of residual disease, which is a low-level risk, the main worry that women have is breast cancer, and progestin is strongly associated with breast cancer,” Dr. Falcone said in an interview.
Ms. Gemmell and Dr. Falcone reported having no financial disclosures.
VANCOUVER – Endometriosis, while generally considered a premenopausal condition, can also occur in women following surgical or natural menopause, and can undergo malignant transformation, although this risk is likely very small.
That was the main message from a new meta-analysis presented at the World Congress on Endometriosis. “We wanted to synthesize the case reports out there to show some common factors so physicians can be aware of them,” said Laura Gemmell, a second-year medical student at Case Western Reserve University, Cleveland, who presented the research.
The researchers surveyed the literature for studies in postmenopausal women with a confirmed or clinically suspected history of endometriosis, and who discussed the management of their menopausal symptoms. They included 33 case reports and case series (42 patients, 36 surgical menopause, 4 natural, 2 presumed natural with later oophorectomy), as well as 6 observational studies and clinical trials.
In the case reports, patients were on HT for a mean of 7.8 years, and 17 of 42 women experienced a recurrence of endometriosis. Also, 25 women had a malignant transformation and there was some overlap with the recurrence group.
Among 17 patients with recurrence, 6 had “severe” or “extensive” endometriosis, and 14 had surgical menopause, with a mean of 7.1 years between surgical menopause and presentation. Twelve of 17 received unopposed estrogen. Following surgical excision (16 of 17 cases), 10 had symptom regression without relapses.
When the researchers looked at the 25 cases of malignant transformation, they found that 13 women had endometriosis at more than one site, 22 had undergone surgical menopause, 19 were on unopposed estrogen, and the mean duration of HT was 6.7 years. Seven women presented with vaginal bleeding and nine with masses. Three died from the disease. These three women had severe endometriosis complicating factors, including older age and multiple malignancies.
The analysis also included six observational studies and clinical trials that explored recurrence of endometriosis, and whether HT should be given to women with a history of endometriosis, whether it should be given immediately after surgical menopause, and the most appropriate menopause treatments.
Predictably, the evidence could not be summed up neatly, but Ms. Gemmell emphasized the need to individually weigh the risks and benefits of HT in each patient, with consideration of characteristics such as age, previous disease severity, family history, comorbidities, and body mass index.
She also suggested that patients should be active participants in decision making.
Finally, if the decision is to go forward or continue with HT, she suggested that clinicians consider a combined treatment rather than estrogen-only, though she pointed out the increased risk for breast cancer that this presents.
Tommaso Falcone, MD, chairman of obstetrics and gynecology at the Cleveland Clinic, sounded a note of caution about the use of progestins during the question-and-answer session. “The data are not strong that it actually prevents the development of cancer in the residual disease, if there is any. Even if you take the hypothesis that progestins are going to prevent cancer of residual disease, which is a low-level risk, the main worry that women have is breast cancer, and progestin is strongly associated with breast cancer,” Dr. Falcone said in an interview.
Ms. Gemmell and Dr. Falcone reported having no financial disclosures.
EXPERT ANALYSIS AT WCE 2017
Maryland passes generic drug anti–price gouging law
Maryland has become the first state to prohibit unreasonable price increases on essential off-patent or generic drugs.
The anti–price gouging law authorizes Maryland’s Attorney General to take legal action against manufacturers or distributors that raise drug prices in noncompetitive drug markets if the firms can’t prove the legitimacy of their price increases. Companies could face a civil penalty of up to $10,000 for each violation, according to the law, which goes into effect in October.
Stephen Rockower, MD, president of MedChi, the Maryland State Medical Society, said the medical society has long supported the bill and hailed its passage as beneficial for doctors and their patients.
“It’s been a long time coming,” Dr. Rockower said in an interview. “There have been lots of problems with the huge rising costs of drugs. Manufacturers and pharmacy benefit managers are completely uncontrolled in terms of their pricing. [With this law], we’ll be able to take care of our patients better. It’s hard to prescribe things if it’s going to cost patients $700 a day [for] a drug that used to cost $5 a day. Patients have to be able to afford their medicine, or they don’t take it.”
Chester “Chip” Davis, Jr. president and CEO of the Association for Accessible Medicines, a trade association for manufacturers and distributors of generic drugs, criticized the law.
“By giving the Attorney General unbounded and unprecedented authority to control pricing in a competitive free market, generic companies will be exposed to a level of risk in Maryland that will require them to evaluate whether they want to continue to market affordable medicines within the state,” Mr. Davis said in a statement. “One day, in the not too distant future, [Maryland lawmakers] should be prepared to defend why Maryland was the first state to lead the nation in creating less market-based competition, higher overall prescription drug costs, while also simultaneously increasing the risk of future drug shortages for Maryland’s patients.”
The law has two provisions. It prevents a generic drug manufacturer or wholesale distributor from engaging in price gouging in the sale of an essential off-patent or generic drug, defined as a prescription drug that no longer has market exclusivity and has been designated as essential for treating a life-threatening or chronic health condition by the Maryland Secretary of Health and Mental Hygiene, is actively marketed in the United States by three or fewer manufacturers, and is available for sale in Maryland.
The law also authorizes the Maryland Medical Assistance Program to notify the Attorney General of a price increase when the wholesale acquisition cost of a prescription drug increases by at least 50% in a given year for medications that cost more than $80 per 30-day course.
This first-of-its-kind law is a way to deter manufacturers from exploiting noncompetitive drug markets for short-term profit through unconscionable behavior that “imperils public health and individual welfare,” according to Jeremy A. Greene, MD, PhD, and William V. Padula, PhD.
“Perhaps, [the law will] help reestablish public trust in U.S. policy’s balancing of innovation and access, by reaffirming that older drugs of proven value should be accessible and subject to competition so that they are priced as the commodities they’ve become,” the authors wrote in a perspective published in the New England Journal of Medicine (doi: 10.1056/NEJMp1704907).
Maryland’s law is part of a growing movement among states to address unreasonable pricing spikes in prescription drugs, noted Dr. Greene and Dr. Padula, both of Johns Hopkins University in Baltimore. In April, Louisiana health officials requested feedback about the possibility of invoking an obscure U.S. patent and copyright law to ensure better affordability of hepatitis drugs. In May, the Nevada Senate passed a bill that would force drug makers to publish the list prices they set and the profits they make on insulin, as well as the amount of insulin discounts they give third parties. Additional pharmaceutical price-transparency laws have been proposed in 16 states and Puerto Rico.
“My first concern relates to the provisions of the legislation which directly regulate interstate commerce and pricing by prohibiting and penalizing manufacturer pricing which may occur outside of Maryland,” Gov. Hogan wrote. “These provisions would likely violate the dormant commerce clause of the Constitution. I am also concerned that the definition of ‘unconscionable increase’ and ‘excessive’ are vague, and would likely not stand a ‘vagueness challenge’ under the procedural due process concepts of the 14th Amendment.”
Gov. Hogan added that he is not convinced that the legislation is a solution to Marylanders having better access to medications and that the law may even have the unintended consequences of harming patients by restricting drug access.
[email protected]
On Twitter @legal_med
Maryland has become the first state to prohibit unreasonable price increases on essential off-patent or generic drugs.
The anti–price gouging law authorizes Maryland’s Attorney General to take legal action against manufacturers or distributors that raise drug prices in noncompetitive drug markets if the firms can’t prove the legitimacy of their price increases. Companies could face a civil penalty of up to $10,000 for each violation, according to the law, which goes into effect in October.
Stephen Rockower, MD, president of MedChi, the Maryland State Medical Society, said the medical society has long supported the bill and hailed its passage as beneficial for doctors and their patients.
“It’s been a long time coming,” Dr. Rockower said in an interview. “There have been lots of problems with the huge rising costs of drugs. Manufacturers and pharmacy benefit managers are completely uncontrolled in terms of their pricing. [With this law], we’ll be able to take care of our patients better. It’s hard to prescribe things if it’s going to cost patients $700 a day [for] a drug that used to cost $5 a day. Patients have to be able to afford their medicine, or they don’t take it.”
Chester “Chip” Davis, Jr. president and CEO of the Association for Accessible Medicines, a trade association for manufacturers and distributors of generic drugs, criticized the law.
“By giving the Attorney General unbounded and unprecedented authority to control pricing in a competitive free market, generic companies will be exposed to a level of risk in Maryland that will require them to evaluate whether they want to continue to market affordable medicines within the state,” Mr. Davis said in a statement. “One day, in the not too distant future, [Maryland lawmakers] should be prepared to defend why Maryland was the first state to lead the nation in creating less market-based competition, higher overall prescription drug costs, while also simultaneously increasing the risk of future drug shortages for Maryland’s patients.”
The law has two provisions. It prevents a generic drug manufacturer or wholesale distributor from engaging in price gouging in the sale of an essential off-patent or generic drug, defined as a prescription drug that no longer has market exclusivity and has been designated as essential for treating a life-threatening or chronic health condition by the Maryland Secretary of Health and Mental Hygiene, is actively marketed in the United States by three or fewer manufacturers, and is available for sale in Maryland.
The law also authorizes the Maryland Medical Assistance Program to notify the Attorney General of a price increase when the wholesale acquisition cost of a prescription drug increases by at least 50% in a given year for medications that cost more than $80 per 30-day course.
This first-of-its-kind law is a way to deter manufacturers from exploiting noncompetitive drug markets for short-term profit through unconscionable behavior that “imperils public health and individual welfare,” according to Jeremy A. Greene, MD, PhD, and William V. Padula, PhD.
“Perhaps, [the law will] help reestablish public trust in U.S. policy’s balancing of innovation and access, by reaffirming that older drugs of proven value should be accessible and subject to competition so that they are priced as the commodities they’ve become,” the authors wrote in a perspective published in the New England Journal of Medicine (doi: 10.1056/NEJMp1704907).
Maryland’s law is part of a growing movement among states to address unreasonable pricing spikes in prescription drugs, noted Dr. Greene and Dr. Padula, both of Johns Hopkins University in Baltimore. In April, Louisiana health officials requested feedback about the possibility of invoking an obscure U.S. patent and copyright law to ensure better affordability of hepatitis drugs. In May, the Nevada Senate passed a bill that would force drug makers to publish the list prices they set and the profits they make on insulin, as well as the amount of insulin discounts they give third parties. Additional pharmaceutical price-transparency laws have been proposed in 16 states and Puerto Rico.
“My first concern relates to the provisions of the legislation which directly regulate interstate commerce and pricing by prohibiting and penalizing manufacturer pricing which may occur outside of Maryland,” Gov. Hogan wrote. “These provisions would likely violate the dormant commerce clause of the Constitution. I am also concerned that the definition of ‘unconscionable increase’ and ‘excessive’ are vague, and would likely not stand a ‘vagueness challenge’ under the procedural due process concepts of the 14th Amendment.”
Gov. Hogan added that he is not convinced that the legislation is a solution to Marylanders having better access to medications and that the law may even have the unintended consequences of harming patients by restricting drug access.
[email protected]
On Twitter @legal_med
Maryland has become the first state to prohibit unreasonable price increases on essential off-patent or generic drugs.
The anti–price gouging law authorizes Maryland’s Attorney General to take legal action against manufacturers or distributors that raise drug prices in noncompetitive drug markets if the firms can’t prove the legitimacy of their price increases. Companies could face a civil penalty of up to $10,000 for each violation, according to the law, which goes into effect in October.
Stephen Rockower, MD, president of MedChi, the Maryland State Medical Society, said the medical society has long supported the bill and hailed its passage as beneficial for doctors and their patients.
“It’s been a long time coming,” Dr. Rockower said in an interview. “There have been lots of problems with the huge rising costs of drugs. Manufacturers and pharmacy benefit managers are completely uncontrolled in terms of their pricing. [With this law], we’ll be able to take care of our patients better. It’s hard to prescribe things if it’s going to cost patients $700 a day [for] a drug that used to cost $5 a day. Patients have to be able to afford their medicine, or they don’t take it.”
Chester “Chip” Davis, Jr. president and CEO of the Association for Accessible Medicines, a trade association for manufacturers and distributors of generic drugs, criticized the law.
“By giving the Attorney General unbounded and unprecedented authority to control pricing in a competitive free market, generic companies will be exposed to a level of risk in Maryland that will require them to evaluate whether they want to continue to market affordable medicines within the state,” Mr. Davis said in a statement. “One day, in the not too distant future, [Maryland lawmakers] should be prepared to defend why Maryland was the first state to lead the nation in creating less market-based competition, higher overall prescription drug costs, while also simultaneously increasing the risk of future drug shortages for Maryland’s patients.”
The law has two provisions. It prevents a generic drug manufacturer or wholesale distributor from engaging in price gouging in the sale of an essential off-patent or generic drug, defined as a prescription drug that no longer has market exclusivity and has been designated as essential for treating a life-threatening or chronic health condition by the Maryland Secretary of Health and Mental Hygiene, is actively marketed in the United States by three or fewer manufacturers, and is available for sale in Maryland.
The law also authorizes the Maryland Medical Assistance Program to notify the Attorney General of a price increase when the wholesale acquisition cost of a prescription drug increases by at least 50% in a given year for medications that cost more than $80 per 30-day course.
This first-of-its-kind law is a way to deter manufacturers from exploiting noncompetitive drug markets for short-term profit through unconscionable behavior that “imperils public health and individual welfare,” according to Jeremy A. Greene, MD, PhD, and William V. Padula, PhD.
“Perhaps, [the law will] help reestablish public trust in U.S. policy’s balancing of innovation and access, by reaffirming that older drugs of proven value should be accessible and subject to competition so that they are priced as the commodities they’ve become,” the authors wrote in a perspective published in the New England Journal of Medicine (doi: 10.1056/NEJMp1704907).
Maryland’s law is part of a growing movement among states to address unreasonable pricing spikes in prescription drugs, noted Dr. Greene and Dr. Padula, both of Johns Hopkins University in Baltimore. In April, Louisiana health officials requested feedback about the possibility of invoking an obscure U.S. patent and copyright law to ensure better affordability of hepatitis drugs. In May, the Nevada Senate passed a bill that would force drug makers to publish the list prices they set and the profits they make on insulin, as well as the amount of insulin discounts they give third parties. Additional pharmaceutical price-transparency laws have been proposed in 16 states and Puerto Rico.
“My first concern relates to the provisions of the legislation which directly regulate interstate commerce and pricing by prohibiting and penalizing manufacturer pricing which may occur outside of Maryland,” Gov. Hogan wrote. “These provisions would likely violate the dormant commerce clause of the Constitution. I am also concerned that the definition of ‘unconscionable increase’ and ‘excessive’ are vague, and would likely not stand a ‘vagueness challenge’ under the procedural due process concepts of the 14th Amendment.”
Gov. Hogan added that he is not convinced that the legislation is a solution to Marylanders having better access to medications and that the law may even have the unintended consequences of harming patients by restricting drug access.
[email protected]
On Twitter @legal_med