User login
Hypogonadism after testicular cancer treatment can have lifelong impact
CHICAGO – Hypogonadism may compromise the long-term health outlook for many younger men who have been successfully treated for testicular cancer, according to an analysis conducted by the Platinum Study Group.
“Today, 95% of all testicular cancer patients are cured of their disease thanks to cisplatin-based chemotherapy. Nowadays, testicular cancer survivors can expect to live for over 40 years from the time of their diagnosis,” lead investigator Mohammad Issam Abu Zaid, MBBS, said in a press briefing at the annual meeting of the American Society of Clinical Oncology. “However, they are at risk of other health problems that may be related to their cancer treatment, including late complications from chemotherapy.”
“Testicular cancer survivors, especially those treated with chemotherapy, are at increased risk for hypogonadism, a problem that can be associated with predisposing factors for heart disease,” summarized Dr. Abu Zaid, of Indiana University in Indianapolis. “Mitigating approaches are the usual weight control, exercise, and monitoring of blood pressure and cholesterol levels.”
Expert perspective
“This is an important study, and it sends a loud message to those of us who take care of testis cancer patients, my area of expertise. ... We need to watch for hypogonadism, and we need to ask survivors about it. We need to examine them thinking about it, and, in patients who we are worried [they] might have hypogonadism, we need to do blood tests for testosterone and other hormone levels,” commented ASCO Expert Timothy D. Gilligan, MD, MSc, of the Cleveland Clinic in Ohio. “These are young patients, they have many years of life, so it’s many decades of suffering from consequences of this if it’s undetected.”
The findings were not surprising based on his personal experience and on evidence from the prostate cancer field showing the adverse metabolic effects of withdrawing testosterone, he said. Although the prevalence of hypogonadism found in the study was higher than that found in other studies, given the large size of the cohort, it should be taken seriously. Additionally, even if the true prevalence is somewhat lower, the absolute number of survivors affected would be substantial.
“We need to be cautious though and make sure people don’t misunderstand and think that this means we should test testosterone levels in all patients, which is a risky thing to do because the definition of normal testosterone is very fuzzy,” Dr. Gilligan stressed. “There is a wide range of normal, and what’s normal for me may not be the same as what’s normal for another man. So, looking for symptoms is really what guides this work, and, when there are symptoms, then testing is important.”
Study details
The Platinum Study is a large, ongoing, multicenter North American–based cohort study of testicular cancer survivors who received cisplatin as part of their chemotherapy.
Dr. Abu Zaid and his colleagues analyzed data from 491 participants who were aged 50 years or younger at diagnosis. All completed questionnaires addressing comorbidities, medications, and health behaviors; underwent physical examination; had measurement of testosterone levels; and had a genetic analysis.
The men were by and large young at the time of evaluation, with a mean age of just 38 years, he reported in the press briefing and a poster session at the meeting.
Overall, 38.5% had hypogonadism, defined in the study as a serum testosterone level of 3 ng/mL or lower or as use of testosterone replacement therapy.
In a multivariate analysis, survivors’ odds of hypogonadism increased with age (odds ratio, 1.41 per 10-year increment; P = .007) and were higher for those having a body mass index of 25 kg/m2 or greater, vs. lower (OR, 2.22; P = .003). On the other hand, there was a trend whereby survivors who engaged in any vigorous-intensity physical activity, vs. none, were less likely to have hypogonadism (OR, 0.64; P = .06).
The odds rose with number of risk alleles in the sex hormone–binding globulin gene, but findings were not significant, Dr. Abu Zaid said. They were also statistically indistinguishable across groups differing with respect to the chemotherapy regimen received and socioeconomic factors.
Compared with counterparts having normal testosterone levels, survivors having hypogonadism were up to four times more likely to be taking medication to treat dyslipidemia (20.1% vs. 6.0%; P less than .001), hypertension (18.5% vs. 10.6%; P = .013), erectile dysfunction (19.6% vs. 11.9%; P = .018), diabetes (5.8% vs. 2.6%; P = .067), and anxiety or depression (14.8% vs. 9.3%; P = .060).
Testicular cancer survivors should not universally have testosterone testing, agreed Dr. Abu Zaid. Such a practice might lead to overtreatment, and, in older men at least, use of testosterone itself has been linked to elevated cardiovascular risk.
“You really want to treat somebody who has symptoms related to hypogonadism: constant fatigue, night sweats, and depressed mood and some other symptoms that are well known to the clinician,” he maintained. “At the moment, we have no studies done looking at testosterone replacement in young men. I would hypothesize that we actually help them by giving them testosterone, and that’s what I tell my patients.”
CHICAGO – Hypogonadism may compromise the long-term health outlook for many younger men who have been successfully treated for testicular cancer, according to an analysis conducted by the Platinum Study Group.
“Today, 95% of all testicular cancer patients are cured of their disease thanks to cisplatin-based chemotherapy. Nowadays, testicular cancer survivors can expect to live for over 40 years from the time of their diagnosis,” lead investigator Mohammad Issam Abu Zaid, MBBS, said in a press briefing at the annual meeting of the American Society of Clinical Oncology. “However, they are at risk of other health problems that may be related to their cancer treatment, including late complications from chemotherapy.”
“Testicular cancer survivors, especially those treated with chemotherapy, are at increased risk for hypogonadism, a problem that can be associated with predisposing factors for heart disease,” summarized Dr. Abu Zaid, of Indiana University in Indianapolis. “Mitigating approaches are the usual weight control, exercise, and monitoring of blood pressure and cholesterol levels.”
Expert perspective
“This is an important study, and it sends a loud message to those of us who take care of testis cancer patients, my area of expertise. ... We need to watch for hypogonadism, and we need to ask survivors about it. We need to examine them thinking about it, and, in patients who we are worried [they] might have hypogonadism, we need to do blood tests for testosterone and other hormone levels,” commented ASCO Expert Timothy D. Gilligan, MD, MSc, of the Cleveland Clinic in Ohio. “These are young patients, they have many years of life, so it’s many decades of suffering from consequences of this if it’s undetected.”
The findings were not surprising based on his personal experience and on evidence from the prostate cancer field showing the adverse metabolic effects of withdrawing testosterone, he said. Although the prevalence of hypogonadism found in the study was higher than that found in other studies, given the large size of the cohort, it should be taken seriously. Additionally, even if the true prevalence is somewhat lower, the absolute number of survivors affected would be substantial.
“We need to be cautious though and make sure people don’t misunderstand and think that this means we should test testosterone levels in all patients, which is a risky thing to do because the definition of normal testosterone is very fuzzy,” Dr. Gilligan stressed. “There is a wide range of normal, and what’s normal for me may not be the same as what’s normal for another man. So, looking for symptoms is really what guides this work, and, when there are symptoms, then testing is important.”
Study details
The Platinum Study is a large, ongoing, multicenter North American–based cohort study of testicular cancer survivors who received cisplatin as part of their chemotherapy.
Dr. Abu Zaid and his colleagues analyzed data from 491 participants who were aged 50 years or younger at diagnosis. All completed questionnaires addressing comorbidities, medications, and health behaviors; underwent physical examination; had measurement of testosterone levels; and had a genetic analysis.
The men were by and large young at the time of evaluation, with a mean age of just 38 years, he reported in the press briefing and a poster session at the meeting.
Overall, 38.5% had hypogonadism, defined in the study as a serum testosterone level of 3 ng/mL or lower or as use of testosterone replacement therapy.
In a multivariate analysis, survivors’ odds of hypogonadism increased with age (odds ratio, 1.41 per 10-year increment; P = .007) and were higher for those having a body mass index of 25 kg/m2 or greater, vs. lower (OR, 2.22; P = .003). On the other hand, there was a trend whereby survivors who engaged in any vigorous-intensity physical activity, vs. none, were less likely to have hypogonadism (OR, 0.64; P = .06).
The odds rose with number of risk alleles in the sex hormone–binding globulin gene, but findings were not significant, Dr. Abu Zaid said. They were also statistically indistinguishable across groups differing with respect to the chemotherapy regimen received and socioeconomic factors.
Compared with counterparts having normal testosterone levels, survivors having hypogonadism were up to four times more likely to be taking medication to treat dyslipidemia (20.1% vs. 6.0%; P less than .001), hypertension (18.5% vs. 10.6%; P = .013), erectile dysfunction (19.6% vs. 11.9%; P = .018), diabetes (5.8% vs. 2.6%; P = .067), and anxiety or depression (14.8% vs. 9.3%; P = .060).
Testicular cancer survivors should not universally have testosterone testing, agreed Dr. Abu Zaid. Such a practice might lead to overtreatment, and, in older men at least, use of testosterone itself has been linked to elevated cardiovascular risk.
“You really want to treat somebody who has symptoms related to hypogonadism: constant fatigue, night sweats, and depressed mood and some other symptoms that are well known to the clinician,” he maintained. “At the moment, we have no studies done looking at testosterone replacement in young men. I would hypothesize that we actually help them by giving them testosterone, and that’s what I tell my patients.”
CHICAGO – Hypogonadism may compromise the long-term health outlook for many younger men who have been successfully treated for testicular cancer, according to an analysis conducted by the Platinum Study Group.
“Today, 95% of all testicular cancer patients are cured of their disease thanks to cisplatin-based chemotherapy. Nowadays, testicular cancer survivors can expect to live for over 40 years from the time of their diagnosis,” lead investigator Mohammad Issam Abu Zaid, MBBS, said in a press briefing at the annual meeting of the American Society of Clinical Oncology. “However, they are at risk of other health problems that may be related to their cancer treatment, including late complications from chemotherapy.”
“Testicular cancer survivors, especially those treated with chemotherapy, are at increased risk for hypogonadism, a problem that can be associated with predisposing factors for heart disease,” summarized Dr. Abu Zaid, of Indiana University in Indianapolis. “Mitigating approaches are the usual weight control, exercise, and monitoring of blood pressure and cholesterol levels.”
Expert perspective
“This is an important study, and it sends a loud message to those of us who take care of testis cancer patients, my area of expertise. ... We need to watch for hypogonadism, and we need to ask survivors about it. We need to examine them thinking about it, and, in patients who we are worried [they] might have hypogonadism, we need to do blood tests for testosterone and other hormone levels,” commented ASCO Expert Timothy D. Gilligan, MD, MSc, of the Cleveland Clinic in Ohio. “These are young patients, they have many years of life, so it’s many decades of suffering from consequences of this if it’s undetected.”
The findings were not surprising based on his personal experience and on evidence from the prostate cancer field showing the adverse metabolic effects of withdrawing testosterone, he said. Although the prevalence of hypogonadism found in the study was higher than that found in other studies, given the large size of the cohort, it should be taken seriously. Additionally, even if the true prevalence is somewhat lower, the absolute number of survivors affected would be substantial.
“We need to be cautious though and make sure people don’t misunderstand and think that this means we should test testosterone levels in all patients, which is a risky thing to do because the definition of normal testosterone is very fuzzy,” Dr. Gilligan stressed. “There is a wide range of normal, and what’s normal for me may not be the same as what’s normal for another man. So, looking for symptoms is really what guides this work, and, when there are symptoms, then testing is important.”
Study details
The Platinum Study is a large, ongoing, multicenter North American–based cohort study of testicular cancer survivors who received cisplatin as part of their chemotherapy.
Dr. Abu Zaid and his colleagues analyzed data from 491 participants who were aged 50 years or younger at diagnosis. All completed questionnaires addressing comorbidities, medications, and health behaviors; underwent physical examination; had measurement of testosterone levels; and had a genetic analysis.
The men were by and large young at the time of evaluation, with a mean age of just 38 years, he reported in the press briefing and a poster session at the meeting.
Overall, 38.5% had hypogonadism, defined in the study as a serum testosterone level of 3 ng/mL or lower or as use of testosterone replacement therapy.
In a multivariate analysis, survivors’ odds of hypogonadism increased with age (odds ratio, 1.41 per 10-year increment; P = .007) and were higher for those having a body mass index of 25 kg/m2 or greater, vs. lower (OR, 2.22; P = .003). On the other hand, there was a trend whereby survivors who engaged in any vigorous-intensity physical activity, vs. none, were less likely to have hypogonadism (OR, 0.64; P = .06).
The odds rose with number of risk alleles in the sex hormone–binding globulin gene, but findings were not significant, Dr. Abu Zaid said. They were also statistically indistinguishable across groups differing with respect to the chemotherapy regimen received and socioeconomic factors.
Compared with counterparts having normal testosterone levels, survivors having hypogonadism were up to four times more likely to be taking medication to treat dyslipidemia (20.1% vs. 6.0%; P less than .001), hypertension (18.5% vs. 10.6%; P = .013), erectile dysfunction (19.6% vs. 11.9%; P = .018), diabetes (5.8% vs. 2.6%; P = .067), and anxiety or depression (14.8% vs. 9.3%; P = .060).
Testicular cancer survivors should not universally have testosterone testing, agreed Dr. Abu Zaid. Such a practice might lead to overtreatment, and, in older men at least, use of testosterone itself has been linked to elevated cardiovascular risk.
“You really want to treat somebody who has symptoms related to hypogonadism: constant fatigue, night sweats, and depressed mood and some other symptoms that are well known to the clinician,” he maintained. “At the moment, we have no studies done looking at testosterone replacement in young men. I would hypothesize that we actually help them by giving them testosterone, and that’s what I tell my patients.”
AT ASCO 2017
Key clinical point:
Major finding: Fully 38.5% of survivors had low testosterone levels, and men in this group were more likely to be taking medications for dyslipidemia, hypertension, diabetes, erectile dysfunction, and anxiety or depression.
Data source: A cohort study of 491 testicular cancer survivors who had been treated with cisplatin-based chemotherapy (Platinum Study).
Disclosures: Dr. Abu Zaid reported that he had no relevant disclosures.
Consistent weight benefits seen in empagliflozin use
SAN DIEGO – In a follow-up to the blockbuster trial results linking the type 2 diabetes drug empagliflozin (Jardiance) to a dramatically lower risk of cardiac death, researchers report that the drug improved weight-related measures in multiple groups.
Two daily doses of empagliflozin, 10 mg and 25 mg, “had consistent and robust effects on lowering weight, waist circumference, and other markers of body fat across most patients regardless of their age, sex, or degree of abdominal obesity,” study lead author Ian J. Neeland, MD, of the department of medicine at UT Southwestern Medical Center, Dallas, said in an interview. “Our next step is to determine if these effects may contribute to the improvement in cardiovascular risk seen with the drug.”
“In the EMPA-REG OUTCOME study, empagliflozin treatment significantly reduced the risk of cardiovascular death by 38%,” Dr. Neeland said. “We also observed that patients treated with empagliflozin had improvements in markers of body fatness such as weight, waist circumference, and estimated total body fat. Since we know that obesity is a major risk factor for cardiovascular disease, we were interested in finding out if the improvements in weight and other markers of body fatness may have contributed to the observed cardiovascular benefits of empagliflozin in the study. One part of this was to examine whether the drug had consistent effects on body fat according to other important cardiovascular risk factors.”
The researchers analyzed changes in body weight, waist circumference, index of central obesity, and estimated total body fat from baseline to week 164 in a study that randomly assigned participants with type 2 diabetes and cardiovascular disease to placebo or 10 mg or 25 mg of empagliflozin. The number of patients in the groups were 2,333, 2,345 and 2,342, respectively, and their mean baseline weight was around 86.0 kg.
In general, researchers found that across groups, weight measures improved more in drug-treated patients than those treated with placebo. The higher dose (25 mg) often had a greater effect; the two available doses of the drug are 10 mg and 20 mg.
For example, the placebo-adjusted mean reduction in weight was –1.70 kg in men (95% confidence interval, –2.14 to –1.27) in the 10-mg group and 2.18 kg (95% CI, –2.61 to –1.75) in the 25-mg group. For women, the reduction was –1.32 kg (95% CI, –2.02 to –0.62) in the 10-mg group and –1.44 kg (95% CI, –2.15 to –0.73) in the 25-mg group.
“Patients lost on average 1.5-2 kg of weight – about 4 pounds – with empagliflozin, compared with placebo,” Dr. Neeland said. “Although quality of life and other metrics of better health were not systematically collected, we do know that people who lose weight and waist circumference tend to feel better, have fewer health problems, and live longer, compared with people who remain obese.”
Dr. Neeland said researchers still need to understand whether the improvements in obesity markers contribute to the drug’s positive cardiac effects.
Study funding was not reported. The original EMPA-REG OUTCOME trial was funded by Boehringer Ingelheim and Eli Lilly. Dr. Neeland disclosed consultant/speakers bureau support from Boehringer Ingelheim. He is a scientific advisory board member for Advanced MR Analytics AB.
SAN DIEGO – In a follow-up to the blockbuster trial results linking the type 2 diabetes drug empagliflozin (Jardiance) to a dramatically lower risk of cardiac death, researchers report that the drug improved weight-related measures in multiple groups.
Two daily doses of empagliflozin, 10 mg and 25 mg, “had consistent and robust effects on lowering weight, waist circumference, and other markers of body fat across most patients regardless of their age, sex, or degree of abdominal obesity,” study lead author Ian J. Neeland, MD, of the department of medicine at UT Southwestern Medical Center, Dallas, said in an interview. “Our next step is to determine if these effects may contribute to the improvement in cardiovascular risk seen with the drug.”
“In the EMPA-REG OUTCOME study, empagliflozin treatment significantly reduced the risk of cardiovascular death by 38%,” Dr. Neeland said. “We also observed that patients treated with empagliflozin had improvements in markers of body fatness such as weight, waist circumference, and estimated total body fat. Since we know that obesity is a major risk factor for cardiovascular disease, we were interested in finding out if the improvements in weight and other markers of body fatness may have contributed to the observed cardiovascular benefits of empagliflozin in the study. One part of this was to examine whether the drug had consistent effects on body fat according to other important cardiovascular risk factors.”
The researchers analyzed changes in body weight, waist circumference, index of central obesity, and estimated total body fat from baseline to week 164 in a study that randomly assigned participants with type 2 diabetes and cardiovascular disease to placebo or 10 mg or 25 mg of empagliflozin. The number of patients in the groups were 2,333, 2,345 and 2,342, respectively, and their mean baseline weight was around 86.0 kg.
In general, researchers found that across groups, weight measures improved more in drug-treated patients than those treated with placebo. The higher dose (25 mg) often had a greater effect; the two available doses of the drug are 10 mg and 20 mg.
For example, the placebo-adjusted mean reduction in weight was –1.70 kg in men (95% confidence interval, –2.14 to –1.27) in the 10-mg group and 2.18 kg (95% CI, –2.61 to –1.75) in the 25-mg group. For women, the reduction was –1.32 kg (95% CI, –2.02 to –0.62) in the 10-mg group and –1.44 kg (95% CI, –2.15 to –0.73) in the 25-mg group.
“Patients lost on average 1.5-2 kg of weight – about 4 pounds – with empagliflozin, compared with placebo,” Dr. Neeland said. “Although quality of life and other metrics of better health were not systematically collected, we do know that people who lose weight and waist circumference tend to feel better, have fewer health problems, and live longer, compared with people who remain obese.”
Dr. Neeland said researchers still need to understand whether the improvements in obesity markers contribute to the drug’s positive cardiac effects.
Study funding was not reported. The original EMPA-REG OUTCOME trial was funded by Boehringer Ingelheim and Eli Lilly. Dr. Neeland disclosed consultant/speakers bureau support from Boehringer Ingelheim. He is a scientific advisory board member for Advanced MR Analytics AB.
SAN DIEGO – In a follow-up to the blockbuster trial results linking the type 2 diabetes drug empagliflozin (Jardiance) to a dramatically lower risk of cardiac death, researchers report that the drug improved weight-related measures in multiple groups.
Two daily doses of empagliflozin, 10 mg and 25 mg, “had consistent and robust effects on lowering weight, waist circumference, and other markers of body fat across most patients regardless of their age, sex, or degree of abdominal obesity,” study lead author Ian J. Neeland, MD, of the department of medicine at UT Southwestern Medical Center, Dallas, said in an interview. “Our next step is to determine if these effects may contribute to the improvement in cardiovascular risk seen with the drug.”
“In the EMPA-REG OUTCOME study, empagliflozin treatment significantly reduced the risk of cardiovascular death by 38%,” Dr. Neeland said. “We also observed that patients treated with empagliflozin had improvements in markers of body fatness such as weight, waist circumference, and estimated total body fat. Since we know that obesity is a major risk factor for cardiovascular disease, we were interested in finding out if the improvements in weight and other markers of body fatness may have contributed to the observed cardiovascular benefits of empagliflozin in the study. One part of this was to examine whether the drug had consistent effects on body fat according to other important cardiovascular risk factors.”
The researchers analyzed changes in body weight, waist circumference, index of central obesity, and estimated total body fat from baseline to week 164 in a study that randomly assigned participants with type 2 diabetes and cardiovascular disease to placebo or 10 mg or 25 mg of empagliflozin. The number of patients in the groups were 2,333, 2,345 and 2,342, respectively, and their mean baseline weight was around 86.0 kg.
In general, researchers found that across groups, weight measures improved more in drug-treated patients than those treated with placebo. The higher dose (25 mg) often had a greater effect; the two available doses of the drug are 10 mg and 20 mg.
For example, the placebo-adjusted mean reduction in weight was –1.70 kg in men (95% confidence interval, –2.14 to –1.27) in the 10-mg group and 2.18 kg (95% CI, –2.61 to –1.75) in the 25-mg group. For women, the reduction was –1.32 kg (95% CI, –2.02 to –0.62) in the 10-mg group and –1.44 kg (95% CI, –2.15 to –0.73) in the 25-mg group.
“Patients lost on average 1.5-2 kg of weight – about 4 pounds – with empagliflozin, compared with placebo,” Dr. Neeland said. “Although quality of life and other metrics of better health were not systematically collected, we do know that people who lose weight and waist circumference tend to feel better, have fewer health problems, and live longer, compared with people who remain obese.”
Dr. Neeland said researchers still need to understand whether the improvements in obesity markers contribute to the drug’s positive cardiac effects.
Study funding was not reported. The original EMPA-REG OUTCOME trial was funded by Boehringer Ingelheim and Eli Lilly. Dr. Neeland disclosed consultant/speakers bureau support from Boehringer Ingelheim. He is a scientific advisory board member for Advanced MR Analytics AB.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Placebo-adjusted mean reduction in weight was –1.70 kg in men for daily 10-mg dose group and –2.18 kg in daily 25-mg group. For women, the losses were –1.32 kg in the 10-mg group and –1.44 kg in the 25-mg group.
Data source: Secondary analysis of 164-week randomized, double-blind, placebo-controlled study of patients with type 2 diabetes and cardiovascular disease assigned to placebo or 10-mg or 25-mg doses of empagliflozin.
Disclosures: Study funding was not reported. The original EMPA-REG OUTCOME trial was funded by Boehringer Ingelheim and Eli Lilly.
HFrEF mortality halved when treatment matches guidelines
PARIS – Heart failure patients who received guideline-directed pharmacotherapy, at dosages that approached guideline-directed levels, had roughly half the 6-month mortality as did similar patients who did not receive this level of treatment in a real-world, observational study with more than 6,000 patients.
Adherence to pharmacologic treatment guidelines for patients with heart failure with reduced ejection fraction (HFrEF) “was strongly associated with clinical outcomes during 6-month follow-up,” Michel Komajda, MD, said at a meeting held by the Heart Failure Association of the European Society of Cardiology. The findings highlight the importance of closely following guideline recommendations in routine practice.
[polldaddy:9772629]
The analysis used data collected in the QUALIFY (Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure: an International Survey) registry, which enrolled 7,127 HFrEF patients during September 2013–December 2014 at 547 centers in 36 countries, mostly in Europe, Asia, and Africa but also in Canada, Ecuador, and Australia. All enrolled patients had to have been hospitalized for worsening heart failure at least once during the 1-15 months before they entered QUALIFY.
Dr. Komajda and his associates assessed each enrolled patient at baseline by their treatment with each of four guideline-recommended drug classes: an ACE inhibitor or angiotensin receptor blocker; a beta-blocker; an aldosterone receptor antagonist (ARA) if the patient’s functional status was rated as New York Heart Association class II, III, or IV; and ivabradine (Corlanor) if the patient was in NYHA class II, III, or IV, in sinus rhythm, had a heart rate of at least 70 beats per minute, and if the patient was in a country where ivabradine was available. Because patient enrollment occurred in 2013 and 2014, the study couldn’t include the new formulation of sacubitril plus valsartan (Entresto) in its analysis.
For each eligible drug class a patient received 1 point if their daily prescribed dosage was at least 50% of the recommended dosage (or 100% for an ARA), 0.5 points if the patient received the recommended drug but at a lower dosage, and no points if the drug wasn’t given. A patient also received 1 point if they were appropriately not given a drug because of a documented contraindication or intolerance. The researchers then calculated each patient’s “adherence score” by dividing their point total by the potentially maximum number of points that each patient could have received (a number that ranged from 2 to 4). They defined a score of 1 (which meant the patients received at least half the recommended dosage of all recommended drugs) as good adherence, a score of 0.51-0.99 as moderate adherence, and a score of 0.5 or less as poor adherence.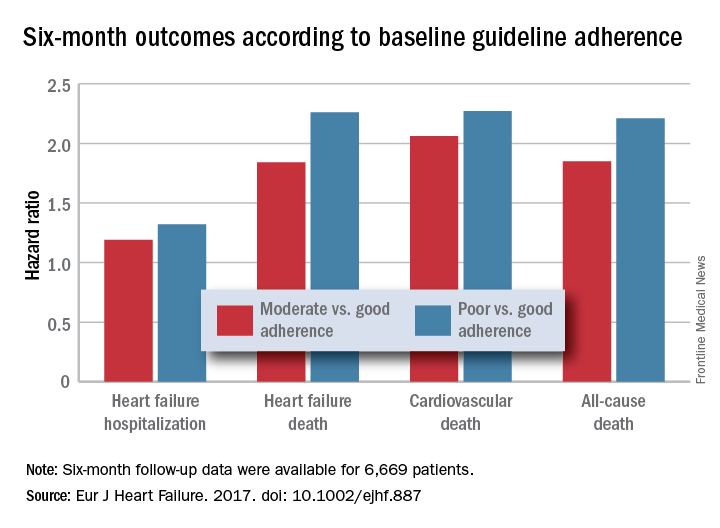
Because patient enrollment occurred during 2013 and 2014, the benchmark heart failure treatment guidelines were those issued by the European Society of Cardiology in 2012 (Eur Heart J. 2012 July;33[14]:1787-1847).
Concurrently with Dr. Komajda’s report at the meeting the results appeared in an article online (Eur J Heart Failure. 2017. doi: 10.1002/ejhf.887).
QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
[email protected]
On Twitter @mitchelzoler
This was a wonderful and useful study. It was large, prospective, covered a wide geographic area, and showed that drug dosage matters when treating heart failure with reduced ejection fraction.
The geographic diversity was a strength, but also a potential weakness because of the resulting differences among the enrolled patients in financial resources, ethnic and genetic makeup, and their tolerance to various drugs. I hope that further research can dissect the role that each of these factors played in the results.
Another limitation is the relatively simplistic approach used for assessing drug dosages to calculate the adherence scores. While it is a very useful step forward to classify patients by the drug dosages they received, there could be some very legitimate variations in dosages based on parameters such as blood pressure. An important question to address in the future is whether it is better to use all the recommended drugs at reduced dosages if necessary, or to use fewer agents at higher dosages.
But these are just quibbles about what is a very important study.
Andrew J.S. Coats, MD , is a cardiologist, professor of medicine, and academic vice-president for the Monash Warwick Alliance of Warwick University in Coventry, England, and Monash University in Melbourne. He has received honoraria from Impulse Dynamics, Menarini, PsiOxus, ResMed, Respicardia, and Servier. He made these comments as designated discussant for Dr. Komajda’s report.
This was a wonderful and useful study. It was large, prospective, covered a wide geographic area, and showed that drug dosage matters when treating heart failure with reduced ejection fraction.
The geographic diversity was a strength, but also a potential weakness because of the resulting differences among the enrolled patients in financial resources, ethnic and genetic makeup, and their tolerance to various drugs. I hope that further research can dissect the role that each of these factors played in the results.
Another limitation is the relatively simplistic approach used for assessing drug dosages to calculate the adherence scores. While it is a very useful step forward to classify patients by the drug dosages they received, there could be some very legitimate variations in dosages based on parameters such as blood pressure. An important question to address in the future is whether it is better to use all the recommended drugs at reduced dosages if necessary, or to use fewer agents at higher dosages.
But these are just quibbles about what is a very important study.
Andrew J.S. Coats, MD , is a cardiologist, professor of medicine, and academic vice-president for the Monash Warwick Alliance of Warwick University in Coventry, England, and Monash University in Melbourne. He has received honoraria from Impulse Dynamics, Menarini, PsiOxus, ResMed, Respicardia, and Servier. He made these comments as designated discussant for Dr. Komajda’s report.
This was a wonderful and useful study. It was large, prospective, covered a wide geographic area, and showed that drug dosage matters when treating heart failure with reduced ejection fraction.
The geographic diversity was a strength, but also a potential weakness because of the resulting differences among the enrolled patients in financial resources, ethnic and genetic makeup, and their tolerance to various drugs. I hope that further research can dissect the role that each of these factors played in the results.
Another limitation is the relatively simplistic approach used for assessing drug dosages to calculate the adherence scores. While it is a very useful step forward to classify patients by the drug dosages they received, there could be some very legitimate variations in dosages based on parameters such as blood pressure. An important question to address in the future is whether it is better to use all the recommended drugs at reduced dosages if necessary, or to use fewer agents at higher dosages.
But these are just quibbles about what is a very important study.
Andrew J.S. Coats, MD , is a cardiologist, professor of medicine, and academic vice-president for the Monash Warwick Alliance of Warwick University in Coventry, England, and Monash University in Melbourne. He has received honoraria from Impulse Dynamics, Menarini, PsiOxus, ResMed, Respicardia, and Servier. He made these comments as designated discussant for Dr. Komajda’s report.
PARIS – Heart failure patients who received guideline-directed pharmacotherapy, at dosages that approached guideline-directed levels, had roughly half the 6-month mortality as did similar patients who did not receive this level of treatment in a real-world, observational study with more than 6,000 patients.
Adherence to pharmacologic treatment guidelines for patients with heart failure with reduced ejection fraction (HFrEF) “was strongly associated with clinical outcomes during 6-month follow-up,” Michel Komajda, MD, said at a meeting held by the Heart Failure Association of the European Society of Cardiology. The findings highlight the importance of closely following guideline recommendations in routine practice.
[polldaddy:9772629]
The analysis used data collected in the QUALIFY (Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure: an International Survey) registry, which enrolled 7,127 HFrEF patients during September 2013–December 2014 at 547 centers in 36 countries, mostly in Europe, Asia, and Africa but also in Canada, Ecuador, and Australia. All enrolled patients had to have been hospitalized for worsening heart failure at least once during the 1-15 months before they entered QUALIFY.
Dr. Komajda and his associates assessed each enrolled patient at baseline by their treatment with each of four guideline-recommended drug classes: an ACE inhibitor or angiotensin receptor blocker; a beta-blocker; an aldosterone receptor antagonist (ARA) if the patient’s functional status was rated as New York Heart Association class II, III, or IV; and ivabradine (Corlanor) if the patient was in NYHA class II, III, or IV, in sinus rhythm, had a heart rate of at least 70 beats per minute, and if the patient was in a country where ivabradine was available. Because patient enrollment occurred in 2013 and 2014, the study couldn’t include the new formulation of sacubitril plus valsartan (Entresto) in its analysis.
For each eligible drug class a patient received 1 point if their daily prescribed dosage was at least 50% of the recommended dosage (or 100% for an ARA), 0.5 points if the patient received the recommended drug but at a lower dosage, and no points if the drug wasn’t given. A patient also received 1 point if they were appropriately not given a drug because of a documented contraindication or intolerance. The researchers then calculated each patient’s “adherence score” by dividing their point total by the potentially maximum number of points that each patient could have received (a number that ranged from 2 to 4). They defined a score of 1 (which meant the patients received at least half the recommended dosage of all recommended drugs) as good adherence, a score of 0.51-0.99 as moderate adherence, and a score of 0.5 or less as poor adherence.
Because patient enrollment occurred during 2013 and 2014, the benchmark heart failure treatment guidelines were those issued by the European Society of Cardiology in 2012 (Eur Heart J. 2012 July;33[14]:1787-1847).
Concurrently with Dr. Komajda’s report at the meeting the results appeared in an article online (Eur J Heart Failure. 2017. doi: 10.1002/ejhf.887).
QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
[email protected]
On Twitter @mitchelzoler
PARIS – Heart failure patients who received guideline-directed pharmacotherapy, at dosages that approached guideline-directed levels, had roughly half the 6-month mortality as did similar patients who did not receive this level of treatment in a real-world, observational study with more than 6,000 patients.
Adherence to pharmacologic treatment guidelines for patients with heart failure with reduced ejection fraction (HFrEF) “was strongly associated with clinical outcomes during 6-month follow-up,” Michel Komajda, MD, said at a meeting held by the Heart Failure Association of the European Society of Cardiology. The findings highlight the importance of closely following guideline recommendations in routine practice.
[polldaddy:9772629]
The analysis used data collected in the QUALIFY (Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure: an International Survey) registry, which enrolled 7,127 HFrEF patients during September 2013–December 2014 at 547 centers in 36 countries, mostly in Europe, Asia, and Africa but also in Canada, Ecuador, and Australia. All enrolled patients had to have been hospitalized for worsening heart failure at least once during the 1-15 months before they entered QUALIFY.
Dr. Komajda and his associates assessed each enrolled patient at baseline by their treatment with each of four guideline-recommended drug classes: an ACE inhibitor or angiotensin receptor blocker; a beta-blocker; an aldosterone receptor antagonist (ARA) if the patient’s functional status was rated as New York Heart Association class II, III, or IV; and ivabradine (Corlanor) if the patient was in NYHA class II, III, or IV, in sinus rhythm, had a heart rate of at least 70 beats per minute, and if the patient was in a country where ivabradine was available. Because patient enrollment occurred in 2013 and 2014, the study couldn’t include the new formulation of sacubitril plus valsartan (Entresto) in its analysis.
For each eligible drug class a patient received 1 point if their daily prescribed dosage was at least 50% of the recommended dosage (or 100% for an ARA), 0.5 points if the patient received the recommended drug but at a lower dosage, and no points if the drug wasn’t given. A patient also received 1 point if they were appropriately not given a drug because of a documented contraindication or intolerance. The researchers then calculated each patient’s “adherence score” by dividing their point total by the potentially maximum number of points that each patient could have received (a number that ranged from 2 to 4). They defined a score of 1 (which meant the patients received at least half the recommended dosage of all recommended drugs) as good adherence, a score of 0.51-0.99 as moderate adherence, and a score of 0.5 or less as poor adherence.
Because patient enrollment occurred during 2013 and 2014, the benchmark heart failure treatment guidelines were those issued by the European Society of Cardiology in 2012 (Eur Heart J. 2012 July;33[14]:1787-1847).
Concurrently with Dr. Komajda’s report at the meeting the results appeared in an article online (Eur J Heart Failure. 2017. doi: 10.1002/ejhf.887).
QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
[email protected]
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: Six-month all-cause, cardiovascular, and heart failure mortalities were doubled in patients not on guideline-adherent therapy and dosages.
Data source: QUANTIFY, an international registry with 6,669 HFrEF patients followed for 6 months.
Disclosures: QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
Rural patients less likely to have bariatric surgery
Obese patients living in rural areas of West Virginia were substantially less likely than were their urban and suburban counterparts to have bariatric surgery, according to findings from a study comparing outcomes in two patient groups.
This discrepancy is attributed to a difference between rural and nonrural patients in type of insurance coverage. In this 2-year study, rural patients were nearly five times more likely to be covered by West Virginia Medicaid than were patients living in nonrural areas of the state, said Kristie L. Bergmann, PhD, of the department of behavioral medicine and psychiatry, West Virginia University, Morgantown, and her associates. The findings were published in Surgery for Obesity and Related Diseases (2017;13[4]:632-6), the journal of the American Society for Metabolic and Bariatric Surgery.
Previous research has identified rural patients’ lack of insurance as a barrier to health care. “Our results suggest that insurance denial represents a successive barrier. Despite being insured, rural individuals may be barred from surgery if insurance carriers do not offer it as a covered benefit, deny approval, or require indomitable prerequisites for surgery,” Dr. Bergmann and her associates said.
They examined the associations among rural status, access to bariatric surgery, and surgical outcomes in West Virginia in part because the state’s residents “have been consistently ranked as the most obese population in the United States, with approximately 35.1% of residents meeting criteria for obesity.” West Virginia also has the highest rates of diabetes (13%) and hypertension (41%) in the United States.
At the same time, rural populations are known to have decreased access to all forms of health care and specifically to bariatric surgery. This makes rural West Virginians “a particularly vulnerable population of interest,” the investigators said.
They performed a retrospective cohort study involving 122 obese patients seeking bariatric surgery at their university’s medical center during 2012-2014. A total of 97% of these patients were white, 83% were women, and the mean age was 47 years. Only 82 of the 122 study participants underwent bariatric surgery: 77 had Roux-en-Y gastric bypass and 5 had sleeve gastrectomy.
Rural residents were significantly less likely to undergo bariatric surgery than were nonrural patients, but coverage by West Virginia Medicaid explained 83.6% of this difference. When Medicaid patients were excluded from the analysis, nonrural status no longer predicted the use of bariatric surgery.
Moreover, when Medicaid coverage was controlled for, rural status had no effect on the effectiveness of bariatric surgery. Patients residing in rural areas had the same attendance at follow-up visits and the same reduction in body mass index at 6 months and at 12 months as did nonrural patients.
In addition, patients who had higher levels of education and who worked full-time were more likely to undergo bariatric surgery, but overall, rural patients were more likely to have comorbidities, disability, and lower rates of full-time work. “An argument can be made that rural individuals may have a greater medical need for bariatric surgery, as obesity and associated health conditions may contribute to lower rates of employment. Unfortunately, barring these patients from receiving care may reinforce a cycle of disability and declining health status,” Dr. Bergmann and her associates noted.
This study was limited in that it had a relatively small sample size, particularly in analyses that excluded Medicaid recipients. It also had a follow-up of only 1 year, so longer-term outcomes of bariatric surgery could not be assessed. “Our sample is also predominantly Caucasian and may have unique culturally-based characteristics” that limit the generalizability of the study findings, they added.
No specific sponsor was cited for this study. Dr. Bergmann and her associates reported having no relevant financial disclosures.
Obese patients living in rural areas of West Virginia were substantially less likely than were their urban and suburban counterparts to have bariatric surgery, according to findings from a study comparing outcomes in two patient groups.
This discrepancy is attributed to a difference between rural and nonrural patients in type of insurance coverage. In this 2-year study, rural patients were nearly five times more likely to be covered by West Virginia Medicaid than were patients living in nonrural areas of the state, said Kristie L. Bergmann, PhD, of the department of behavioral medicine and psychiatry, West Virginia University, Morgantown, and her associates. The findings were published in Surgery for Obesity and Related Diseases (2017;13[4]:632-6), the journal of the American Society for Metabolic and Bariatric Surgery.
Previous research has identified rural patients’ lack of insurance as a barrier to health care. “Our results suggest that insurance denial represents a successive barrier. Despite being insured, rural individuals may be barred from surgery if insurance carriers do not offer it as a covered benefit, deny approval, or require indomitable prerequisites for surgery,” Dr. Bergmann and her associates said.
They examined the associations among rural status, access to bariatric surgery, and surgical outcomes in West Virginia in part because the state’s residents “have been consistently ranked as the most obese population in the United States, with approximately 35.1% of residents meeting criteria for obesity.” West Virginia also has the highest rates of diabetes (13%) and hypertension (41%) in the United States.
At the same time, rural populations are known to have decreased access to all forms of health care and specifically to bariatric surgery. This makes rural West Virginians “a particularly vulnerable population of interest,” the investigators said.
They performed a retrospective cohort study involving 122 obese patients seeking bariatric surgery at their university’s medical center during 2012-2014. A total of 97% of these patients were white, 83% were women, and the mean age was 47 years. Only 82 of the 122 study participants underwent bariatric surgery: 77 had Roux-en-Y gastric bypass and 5 had sleeve gastrectomy.
Rural residents were significantly less likely to undergo bariatric surgery than were nonrural patients, but coverage by West Virginia Medicaid explained 83.6% of this difference. When Medicaid patients were excluded from the analysis, nonrural status no longer predicted the use of bariatric surgery.
Moreover, when Medicaid coverage was controlled for, rural status had no effect on the effectiveness of bariatric surgery. Patients residing in rural areas had the same attendance at follow-up visits and the same reduction in body mass index at 6 months and at 12 months as did nonrural patients.
In addition, patients who had higher levels of education and who worked full-time were more likely to undergo bariatric surgery, but overall, rural patients were more likely to have comorbidities, disability, and lower rates of full-time work. “An argument can be made that rural individuals may have a greater medical need for bariatric surgery, as obesity and associated health conditions may contribute to lower rates of employment. Unfortunately, barring these patients from receiving care may reinforce a cycle of disability and declining health status,” Dr. Bergmann and her associates noted.
This study was limited in that it had a relatively small sample size, particularly in analyses that excluded Medicaid recipients. It also had a follow-up of only 1 year, so longer-term outcomes of bariatric surgery could not be assessed. “Our sample is also predominantly Caucasian and may have unique culturally-based characteristics” that limit the generalizability of the study findings, they added.
No specific sponsor was cited for this study. Dr. Bergmann and her associates reported having no relevant financial disclosures.
Obese patients living in rural areas of West Virginia were substantially less likely than were their urban and suburban counterparts to have bariatric surgery, according to findings from a study comparing outcomes in two patient groups.
This discrepancy is attributed to a difference between rural and nonrural patients in type of insurance coverage. In this 2-year study, rural patients were nearly five times more likely to be covered by West Virginia Medicaid than were patients living in nonrural areas of the state, said Kristie L. Bergmann, PhD, of the department of behavioral medicine and psychiatry, West Virginia University, Morgantown, and her associates. The findings were published in Surgery for Obesity and Related Diseases (2017;13[4]:632-6), the journal of the American Society for Metabolic and Bariatric Surgery.
Previous research has identified rural patients’ lack of insurance as a barrier to health care. “Our results suggest that insurance denial represents a successive barrier. Despite being insured, rural individuals may be barred from surgery if insurance carriers do not offer it as a covered benefit, deny approval, or require indomitable prerequisites for surgery,” Dr. Bergmann and her associates said.
They examined the associations among rural status, access to bariatric surgery, and surgical outcomes in West Virginia in part because the state’s residents “have been consistently ranked as the most obese population in the United States, with approximately 35.1% of residents meeting criteria for obesity.” West Virginia also has the highest rates of diabetes (13%) and hypertension (41%) in the United States.
At the same time, rural populations are known to have decreased access to all forms of health care and specifically to bariatric surgery. This makes rural West Virginians “a particularly vulnerable population of interest,” the investigators said.
They performed a retrospective cohort study involving 122 obese patients seeking bariatric surgery at their university’s medical center during 2012-2014. A total of 97% of these patients were white, 83% were women, and the mean age was 47 years. Only 82 of the 122 study participants underwent bariatric surgery: 77 had Roux-en-Y gastric bypass and 5 had sleeve gastrectomy.
Rural residents were significantly less likely to undergo bariatric surgery than were nonrural patients, but coverage by West Virginia Medicaid explained 83.6% of this difference. When Medicaid patients were excluded from the analysis, nonrural status no longer predicted the use of bariatric surgery.
Moreover, when Medicaid coverage was controlled for, rural status had no effect on the effectiveness of bariatric surgery. Patients residing in rural areas had the same attendance at follow-up visits and the same reduction in body mass index at 6 months and at 12 months as did nonrural patients.
In addition, patients who had higher levels of education and who worked full-time were more likely to undergo bariatric surgery, but overall, rural patients were more likely to have comorbidities, disability, and lower rates of full-time work. “An argument can be made that rural individuals may have a greater medical need for bariatric surgery, as obesity and associated health conditions may contribute to lower rates of employment. Unfortunately, barring these patients from receiving care may reinforce a cycle of disability and declining health status,” Dr. Bergmann and her associates noted.
This study was limited in that it had a relatively small sample size, particularly in analyses that excluded Medicaid recipients. It also had a follow-up of only 1 year, so longer-term outcomes of bariatric surgery could not be assessed. “Our sample is also predominantly Caucasian and may have unique culturally-based characteristics” that limit the generalizability of the study findings, they added.
No specific sponsor was cited for this study. Dr. Bergmann and her associates reported having no relevant financial disclosures.
FROM SURGERY FOR OBESITY AND RELATED DISEASES
Key clinical point: Obese patients living in rural areas of West Virginia are substantially less likely than are their urban and suburban counterparts to have bariatric surgery.
Major finding: Rural residents were significantly less likely to undergo bariatric surgery than were nonrural patients, but coverage by West Virginia Medicaid explained 83.6% of this difference.
Data source: A retrospective single-center cohort study involving 122 obese West Virginians seeking bariatric surgery in 2012-2014.
Disclosures: No specific sponsor was cited for this study. Dr. Bergmann and her associates reported having no relevant financial disclosures.
VIDEO: Metastatic Trial Search links MBC patients to relevant trials
CHICAGO – Metastatic Trial Search was launched in 2015 by ClinicalTrials.org to make it easier for patients with metastatic breast cancer to consider clinical trials as a routine option as they are making treatment decisions with their physicians.
In a video interview, Shirley A. Mertz, JD, president of the Metastatic Breast Cancer Network, describes the tool, the mixed response from physicians, the barriers to trial participation still faced by patients, and the tweaked version 2.0 of the search tool, expected to launch by the end of this year.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @nikolaideslaura
CHICAGO – Metastatic Trial Search was launched in 2015 by ClinicalTrials.org to make it easier for patients with metastatic breast cancer to consider clinical trials as a routine option as they are making treatment decisions with their physicians.
In a video interview, Shirley A. Mertz, JD, president of the Metastatic Breast Cancer Network, describes the tool, the mixed response from physicians, the barriers to trial participation still faced by patients, and the tweaked version 2.0 of the search tool, expected to launch by the end of this year.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @nikolaideslaura
CHICAGO – Metastatic Trial Search was launched in 2015 by ClinicalTrials.org to make it easier for patients with metastatic breast cancer to consider clinical trials as a routine option as they are making treatment decisions with their physicians.
In a video interview, Shirley A. Mertz, JD, president of the Metastatic Breast Cancer Network, describes the tool, the mixed response from physicians, the barriers to trial participation still faced by patients, and the tweaked version 2.0 of the search tool, expected to launch by the end of this year.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @nikolaideslaura
AT ASCO 2017
Waiving screening copayments could cut colorectal cancer deaths
CHICAGO – Out-of-pocket costs may present a barrier to colorectal screening, and removing those costs could reduce colorectal cancer deaths, according to new data presented at the annual Digestive Disease Week®.
These data imply that removing copayments could result in a 16% decrease in colorectal cancer–related deaths among Medicare beneficiaries, explained lead author Elisabeth Peterse, PhD, of the department of public health, Erasmus Medical Center, Rotterdam, the Netherlands.
The research also demonstrated that waiving copayments is cost effective, she added.
Despite the effectiveness of colorectal cancer screening, only 58% of eligible individuals adhere to current screening recommendations, Dr. Peterse noted. Financial barriers may play a role in the lack of adherence, as studies have found that removing out-of-pocket costs is one of the most effective interventions for increasing screening.
“But despite the fact that the Affordable Care Act has been successful in partially eliminating cost sharing for colorectal screening, Medicare beneficiaries may still face unexpected out-of-pocket liabilities,” said Dr. Peterse.
Out-of-pocket costs can be complicated, given that they can depend largely on how a procedure is coded. A screening colonoscopy or fecal immunochemical test (FIT) is completely covered if it is coded as a screening test, but follow-up colonoscopies come with 20% copayments.
A screening colonoscopy with polypectomy and a follow-up colonoscopy that is done after a positive fecal immunochemical test are coded as diagnostic rather than screening, so the patient has out-of-pocket costs, she explained.
To explore how waiving the cost of screening could impact colorectal cancer–related mortality and cost effectiveness, the researchers conducted an analysis using a microsimulation model for a cohort composed of 65-year-old individuals.
In the simulation, they estimated colorectal cancer–related mortality, quality-adjusted life-years, and total cost of screening and treatment using the current Medicare copayment schedule. These were then compared with outcomes for alternative situations.
The study was conducted in two parts, explained Dr. Peterse. In the first part, the researchers looked at five scenarios: one in which the 20% copayment was intact. In the second, the copayment was waived without having any impact on adherence. In the third, the investigators looked at a 5% increase in adherence but only at diagnostic follow-up.
In the fourth and fifth scenarios, the investigators looked at 5% and 10% increases in adherence, in both first screening and diagnostic follow-up, she added.
In the study’s second part, the researchers also estimated the threshold increase in participation at which copayment removal would be cost effective, using a $50,000 willingness-to-pay threshold.
They found that without screening, the expected mortality would be 25 colorectal deaths per 1,000 people in a population of 65-year-old individuals. With screening, the number was reduced to 12.8 deaths per 1,000 65-year-olds for colonoscopy, and 14.9 deaths per 1,000 for FIT screening. The total associated costs for screening and treatment for the two modalities were $3.02 million and $2.87 million.
If waiving the copayments had no impact in increasing screening levels, the cost of screening was estimated to increase to $3.1 million (2.8% increase) for colonoscopy and $2.9 million (1.6% increase) for FIT.
But if copayments were removed and there were a 5% increase in adherence, colorectal cancer deaths were estimated to decline to 11.7 (–8.3%) and 13.9 (–6.3%) per 1,000 for colonoscopy and FIT, respectively. That would result in cost-effectiveness ratios of $19,288 and $7,894 for no copayment versus having a copayment. Increasing adherence to 10% would result in an even lower ratio, noted Dr. Peterse.
The threshold increase for participating in screening programs – the point where removing a copayment becomes cost effective – was a 1.8% increase in colonoscopy screening and a 0.8% increase for FIT.
The conclusion is that waiving copayments is cost effective, Dr. Peterse said.
Dr. Peterse added that a limitation to the analysis is that the study authors don’t know to what extent patients are even aware of the copayments. “So, we don’t know if it is a barrier, and we didn’t take other insurance scenarios into account,” she said.
Dr. Peterse declared no relevant disclosures.
AGA Resource
Screening colonoscopy is the most cost-effective test for prevention of colorectal cancer. Patients should be incentivized, through the elimination of cost sharing, to use colonoscopy as a colorectal cancer screening mechanism. Additionally, the preventive screening benefit has contributed to the decline in colorectal cancer rates in our country, and AGA believes that this benefit should be preserved in any health care reform legislation. Read more at http://www.gastro.org/take-action/top-issues/patient-cost-sharing-for-screening-colonoscopy.
CHICAGO – Out-of-pocket costs may present a barrier to colorectal screening, and removing those costs could reduce colorectal cancer deaths, according to new data presented at the annual Digestive Disease Week®.
These data imply that removing copayments could result in a 16% decrease in colorectal cancer–related deaths among Medicare beneficiaries, explained lead author Elisabeth Peterse, PhD, of the department of public health, Erasmus Medical Center, Rotterdam, the Netherlands.
The research also demonstrated that waiving copayments is cost effective, she added.
Despite the effectiveness of colorectal cancer screening, only 58% of eligible individuals adhere to current screening recommendations, Dr. Peterse noted. Financial barriers may play a role in the lack of adherence, as studies have found that removing out-of-pocket costs is one of the most effective interventions for increasing screening.
“But despite the fact that the Affordable Care Act has been successful in partially eliminating cost sharing for colorectal screening, Medicare beneficiaries may still face unexpected out-of-pocket liabilities,” said Dr. Peterse.
Out-of-pocket costs can be complicated, given that they can depend largely on how a procedure is coded. A screening colonoscopy or fecal immunochemical test (FIT) is completely covered if it is coded as a screening test, but follow-up colonoscopies come with 20% copayments.
A screening colonoscopy with polypectomy and a follow-up colonoscopy that is done after a positive fecal immunochemical test are coded as diagnostic rather than screening, so the patient has out-of-pocket costs, she explained.
To explore how waiving the cost of screening could impact colorectal cancer–related mortality and cost effectiveness, the researchers conducted an analysis using a microsimulation model for a cohort composed of 65-year-old individuals.
In the simulation, they estimated colorectal cancer–related mortality, quality-adjusted life-years, and total cost of screening and treatment using the current Medicare copayment schedule. These were then compared with outcomes for alternative situations.
The study was conducted in two parts, explained Dr. Peterse. In the first part, the researchers looked at five scenarios: one in which the 20% copayment was intact. In the second, the copayment was waived without having any impact on adherence. In the third, the investigators looked at a 5% increase in adherence but only at diagnostic follow-up.
In the fourth and fifth scenarios, the investigators looked at 5% and 10% increases in adherence, in both first screening and diagnostic follow-up, she added.
In the study’s second part, the researchers also estimated the threshold increase in participation at which copayment removal would be cost effective, using a $50,000 willingness-to-pay threshold.
They found that without screening, the expected mortality would be 25 colorectal deaths per 1,000 people in a population of 65-year-old individuals. With screening, the number was reduced to 12.8 deaths per 1,000 65-year-olds for colonoscopy, and 14.9 deaths per 1,000 for FIT screening. The total associated costs for screening and treatment for the two modalities were $3.02 million and $2.87 million.
If waiving the copayments had no impact in increasing screening levels, the cost of screening was estimated to increase to $3.1 million (2.8% increase) for colonoscopy and $2.9 million (1.6% increase) for FIT.
But if copayments were removed and there were a 5% increase in adherence, colorectal cancer deaths were estimated to decline to 11.7 (–8.3%) and 13.9 (–6.3%) per 1,000 for colonoscopy and FIT, respectively. That would result in cost-effectiveness ratios of $19,288 and $7,894 for no copayment versus having a copayment. Increasing adherence to 10% would result in an even lower ratio, noted Dr. Peterse.
The threshold increase for participating in screening programs – the point where removing a copayment becomes cost effective – was a 1.8% increase in colonoscopy screening and a 0.8% increase for FIT.
The conclusion is that waiving copayments is cost effective, Dr. Peterse said.
Dr. Peterse added that a limitation to the analysis is that the study authors don’t know to what extent patients are even aware of the copayments. “So, we don’t know if it is a barrier, and we didn’t take other insurance scenarios into account,” she said.
Dr. Peterse declared no relevant disclosures.
AGA Resource
Screening colonoscopy is the most cost-effective test for prevention of colorectal cancer. Patients should be incentivized, through the elimination of cost sharing, to use colonoscopy as a colorectal cancer screening mechanism. Additionally, the preventive screening benefit has contributed to the decline in colorectal cancer rates in our country, and AGA believes that this benefit should be preserved in any health care reform legislation. Read more at http://www.gastro.org/take-action/top-issues/patient-cost-sharing-for-screening-colonoscopy.
CHICAGO – Out-of-pocket costs may present a barrier to colorectal screening, and removing those costs could reduce colorectal cancer deaths, according to new data presented at the annual Digestive Disease Week®.
These data imply that removing copayments could result in a 16% decrease in colorectal cancer–related deaths among Medicare beneficiaries, explained lead author Elisabeth Peterse, PhD, of the department of public health, Erasmus Medical Center, Rotterdam, the Netherlands.
The research also demonstrated that waiving copayments is cost effective, she added.
Despite the effectiveness of colorectal cancer screening, only 58% of eligible individuals adhere to current screening recommendations, Dr. Peterse noted. Financial barriers may play a role in the lack of adherence, as studies have found that removing out-of-pocket costs is one of the most effective interventions for increasing screening.
“But despite the fact that the Affordable Care Act has been successful in partially eliminating cost sharing for colorectal screening, Medicare beneficiaries may still face unexpected out-of-pocket liabilities,” said Dr. Peterse.
Out-of-pocket costs can be complicated, given that they can depend largely on how a procedure is coded. A screening colonoscopy or fecal immunochemical test (FIT) is completely covered if it is coded as a screening test, but follow-up colonoscopies come with 20% copayments.
A screening colonoscopy with polypectomy and a follow-up colonoscopy that is done after a positive fecal immunochemical test are coded as diagnostic rather than screening, so the patient has out-of-pocket costs, she explained.
To explore how waiving the cost of screening could impact colorectal cancer–related mortality and cost effectiveness, the researchers conducted an analysis using a microsimulation model for a cohort composed of 65-year-old individuals.
In the simulation, they estimated colorectal cancer–related mortality, quality-adjusted life-years, and total cost of screening and treatment using the current Medicare copayment schedule. These were then compared with outcomes for alternative situations.
The study was conducted in two parts, explained Dr. Peterse. In the first part, the researchers looked at five scenarios: one in which the 20% copayment was intact. In the second, the copayment was waived without having any impact on adherence. In the third, the investigators looked at a 5% increase in adherence but only at diagnostic follow-up.
In the fourth and fifth scenarios, the investigators looked at 5% and 10% increases in adherence, in both first screening and diagnostic follow-up, she added.
In the study’s second part, the researchers also estimated the threshold increase in participation at which copayment removal would be cost effective, using a $50,000 willingness-to-pay threshold.
They found that without screening, the expected mortality would be 25 colorectal deaths per 1,000 people in a population of 65-year-old individuals. With screening, the number was reduced to 12.8 deaths per 1,000 65-year-olds for colonoscopy, and 14.9 deaths per 1,000 for FIT screening. The total associated costs for screening and treatment for the two modalities were $3.02 million and $2.87 million.
If waiving the copayments had no impact in increasing screening levels, the cost of screening was estimated to increase to $3.1 million (2.8% increase) for colonoscopy and $2.9 million (1.6% increase) for FIT.
But if copayments were removed and there were a 5% increase in adherence, colorectal cancer deaths were estimated to decline to 11.7 (–8.3%) and 13.9 (–6.3%) per 1,000 for colonoscopy and FIT, respectively. That would result in cost-effectiveness ratios of $19,288 and $7,894 for no copayment versus having a copayment. Increasing adherence to 10% would result in an even lower ratio, noted Dr. Peterse.
The threshold increase for participating in screening programs – the point where removing a copayment becomes cost effective – was a 1.8% increase in colonoscopy screening and a 0.8% increase for FIT.
The conclusion is that waiving copayments is cost effective, Dr. Peterse said.
Dr. Peterse added that a limitation to the analysis is that the study authors don’t know to what extent patients are even aware of the copayments. “So, we don’t know if it is a barrier, and we didn’t take other insurance scenarios into account,” she said.
Dr. Peterse declared no relevant disclosures.
AGA Resource
Screening colonoscopy is the most cost-effective test for prevention of colorectal cancer. Patients should be incentivized, through the elimination of cost sharing, to use colonoscopy as a colorectal cancer screening mechanism. Additionally, the preventive screening benefit has contributed to the decline in colorectal cancer rates in our country, and AGA believes that this benefit should be preserved in any health care reform legislation. Read more at http://www.gastro.org/take-action/top-issues/patient-cost-sharing-for-screening-colonoscopy.
AT DDW
The perils of the National Practitioner Data Bank
Question: With reference to the National Practitioner Data Bank (NPDB), which of the following statements is incorrect?
A. Both court judgments and out-of-court settlements are reportable to the NPDB.
B. Adverse actions by a hospital against a physician are reportable within 15 days.
C. In states with “Disclosure, Apology, and Offer” laws, a prompt settlement through mediation need not be reported.
D. Hospitals, state licensing boards, medical organizations, and the physician himself/herself can access the NPDB.
E. A plaintiff’s attorney cannot access the NPDB for information regarding a defendant.
Answer: C. Congress implemented the NPDB to collect information about an individual doctor’s malpractice and disciplinary histories, with the objective of restricting errant doctors from moving from one state to another.1
Federal law requires medical liability payments stemming from either a court judgment or an out-of-court settlement be reported to the NPDB. An institution’s disciplinary actions against a medical staff member must also be reported. In turn, the NPDB is obligated to make its information available to hospitals, state licensure boards, and legitimate medical organizations charged with granting privileges or membership. A physician also can ask to see his or her own records, but a plaintiff’s attorney cannot access the NPDB unless there is evidence that a hospital failed to query the NPDB as part of its credentialing process.
Some observers have claimed that the NPDB’s existence has hindered settlement negotiations, because many doctors fear being listed in the NPDB, thus significantly diminishing the likelihood of payments to satisfy a claim. It has been stated that within 6 years of NPDB’s inception, the probability that an injured patient’s claim would receive payment had fallen to 59% of the pre-NPDB level.
Many states have enacted so-called “apology laws” that promote full disclosure of medical errors and prompt out-of-court settlements, if warranted. However, the federal Department of Health and Human Services has ruled that all written demands for payment must be reported, even if the cases are resolved under state programs designed for early out-of-court resolution.
For example, a provision in the Oregon law asserts that a payment under the measure’s mediation mechanism “is not a payment resulting from a written claim or demand for payment.” The HHS has rejected this as “explicitly designed to avoid medical malpractice reporting to the NPDB for any claims that are part of the new process that do not proceed to litigation.”
Massachusetts’ 2012 apology law had proposed reporting only those cases where it was determined that a practitioner failed to meet the standard of care. The HHS responded by indicating that all cases had to be reported, regardless of whether care was determined to be up to standards, and that the state’s prelitigation notice to initiate the meditation process qualified as a reportable “written claim.”
Physicians can be impacted greatly by the NPDB. How much of an impact depends in large part on the underlying events and the wording of the report.
An NPDB account of a medical malpractice payment doesn’t necessarily affect a physician’s ability to practice, while those – especially when “severely-worded” – involving denial or restriction of privileges are taken more seriously by state licensing boards and employers. Physicians should therefore play an active role whenever a report to the NPDB appears likely.
The dispute review process is highly technical and requires the knowledge and skill of an experienced health law attorney. To start out, consider making a request to the reporting entity to correct or vacate the report due to error. If the reporting entity declines, the physician may request a review by the HHS and file an accompanying statement seeking to explain the report.
Yet, out of more than 800,000 total reports for all practitioner types captured in the system, apparently only 44,273 included accompanying clarifying statements by the physician. Risk managers have urged vigilance.
For example, it may be that multiple reports involved a single incident, leading to a “piling on” effect. If an adverse decision at one hospital caused a physician’s clinical privileges to be terminated, this might lead the state medical board to restrict the physician’s license. It is necessary to explain that both of these NPDB-reportable events involved the same incident, and that the state board did not have any independent knowledge of anything that was wrong.
Others have advised that one should always clarify one’s involvement, e.g., “I was not the main doctor in the case.” And if dismissed in a malpractice lawsuit, be sure your name or identifying information isn’t included in the judgment or settlement agreement.
Hospital disciplinary actions being far more serious, physicians would do well to familiarize themselves with medical staff bylaws dealing with peer review and investigations. To avoid being reported to the NPDB, physicians must resist adverse actions that would be in effect for more than 30 days and fight attempts to place restrictions or sanctions on their licenses by the hospital or professional societies. Don’t withdraw applications for privileges during an investigation.
The 2015 NPDB Guidebook, the first update in more than 10 years, contains important changes pertaining to hospital adverse actions.2 The regulations now require any “surrender” of privileges while the physician is a subject of an investigation to be a reportable event. Previously, physicians sought to avoid being reported by simply giving up their privileges when an adverse decision appeared imminent.
Surrender includes not renewing one’s hospital privileges or the taking of a leave of absence, and “investigation” is widely defined to include any formal inquiry into a physician’s competence or conduct. And there need not be any “nexus,” i.e., connection, between what is being investigated and the privileges surrendered, in order to be reportable.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Health Care Quality Improvement Act of 1986, 42 U.S.C. 11101 et seq.
2. 2015 NPDB e-Guidebook, available at www.npdb.hrsa.gov/resources/aboutGuidebooks.jsp.
Question: With reference to the National Practitioner Data Bank (NPDB), which of the following statements is incorrect?
A. Both court judgments and out-of-court settlements are reportable to the NPDB.
B. Adverse actions by a hospital against a physician are reportable within 15 days.
C. In states with “Disclosure, Apology, and Offer” laws, a prompt settlement through mediation need not be reported.
D. Hospitals, state licensing boards, medical organizations, and the physician himself/herself can access the NPDB.
E. A plaintiff’s attorney cannot access the NPDB for information regarding a defendant.
Answer: C. Congress implemented the NPDB to collect information about an individual doctor’s malpractice and disciplinary histories, with the objective of restricting errant doctors from moving from one state to another.1
Federal law requires medical liability payments stemming from either a court judgment or an out-of-court settlement be reported to the NPDB. An institution’s disciplinary actions against a medical staff member must also be reported. In turn, the NPDB is obligated to make its information available to hospitals, state licensure boards, and legitimate medical organizations charged with granting privileges or membership. A physician also can ask to see his or her own records, but a plaintiff’s attorney cannot access the NPDB unless there is evidence that a hospital failed to query the NPDB as part of its credentialing process.
Some observers have claimed that the NPDB’s existence has hindered settlement negotiations, because many doctors fear being listed in the NPDB, thus significantly diminishing the likelihood of payments to satisfy a claim. It has been stated that within 6 years of NPDB’s inception, the probability that an injured patient’s claim would receive payment had fallen to 59% of the pre-NPDB level.
Many states have enacted so-called “apology laws” that promote full disclosure of medical errors and prompt out-of-court settlements, if warranted. However, the federal Department of Health and Human Services has ruled that all written demands for payment must be reported, even if the cases are resolved under state programs designed for early out-of-court resolution.
For example, a provision in the Oregon law asserts that a payment under the measure’s mediation mechanism “is not a payment resulting from a written claim or demand for payment.” The HHS has rejected this as “explicitly designed to avoid medical malpractice reporting to the NPDB for any claims that are part of the new process that do not proceed to litigation.”
Massachusetts’ 2012 apology law had proposed reporting only those cases where it was determined that a practitioner failed to meet the standard of care. The HHS responded by indicating that all cases had to be reported, regardless of whether care was determined to be up to standards, and that the state’s prelitigation notice to initiate the meditation process qualified as a reportable “written claim.”
Physicians can be impacted greatly by the NPDB. How much of an impact depends in large part on the underlying events and the wording of the report.
An NPDB account of a medical malpractice payment doesn’t necessarily affect a physician’s ability to practice, while those – especially when “severely-worded” – involving denial or restriction of privileges are taken more seriously by state licensing boards and employers. Physicians should therefore play an active role whenever a report to the NPDB appears likely.
The dispute review process is highly technical and requires the knowledge and skill of an experienced health law attorney. To start out, consider making a request to the reporting entity to correct or vacate the report due to error. If the reporting entity declines, the physician may request a review by the HHS and file an accompanying statement seeking to explain the report.
Yet, out of more than 800,000 total reports for all practitioner types captured in the system, apparently only 44,273 included accompanying clarifying statements by the physician. Risk managers have urged vigilance.
For example, it may be that multiple reports involved a single incident, leading to a “piling on” effect. If an adverse decision at one hospital caused a physician’s clinical privileges to be terminated, this might lead the state medical board to restrict the physician’s license. It is necessary to explain that both of these NPDB-reportable events involved the same incident, and that the state board did not have any independent knowledge of anything that was wrong.
Others have advised that one should always clarify one’s involvement, e.g., “I was not the main doctor in the case.” And if dismissed in a malpractice lawsuit, be sure your name or identifying information isn’t included in the judgment or settlement agreement.
Hospital disciplinary actions being far more serious, physicians would do well to familiarize themselves with medical staff bylaws dealing with peer review and investigations. To avoid being reported to the NPDB, physicians must resist adverse actions that would be in effect for more than 30 days and fight attempts to place restrictions or sanctions on their licenses by the hospital or professional societies. Don’t withdraw applications for privileges during an investigation.
The 2015 NPDB Guidebook, the first update in more than 10 years, contains important changes pertaining to hospital adverse actions.2 The regulations now require any “surrender” of privileges while the physician is a subject of an investigation to be a reportable event. Previously, physicians sought to avoid being reported by simply giving up their privileges when an adverse decision appeared imminent.
Surrender includes not renewing one’s hospital privileges or the taking of a leave of absence, and “investigation” is widely defined to include any formal inquiry into a physician’s competence or conduct. And there need not be any “nexus,” i.e., connection, between what is being investigated and the privileges surrendered, in order to be reportable.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Health Care Quality Improvement Act of 1986, 42 U.S.C. 11101 et seq.
2. 2015 NPDB e-Guidebook, available at www.npdb.hrsa.gov/resources/aboutGuidebooks.jsp.
Question: With reference to the National Practitioner Data Bank (NPDB), which of the following statements is incorrect?
A. Both court judgments and out-of-court settlements are reportable to the NPDB.
B. Adverse actions by a hospital against a physician are reportable within 15 days.
C. In states with “Disclosure, Apology, and Offer” laws, a prompt settlement through mediation need not be reported.
D. Hospitals, state licensing boards, medical organizations, and the physician himself/herself can access the NPDB.
E. A plaintiff’s attorney cannot access the NPDB for information regarding a defendant.
Answer: C. Congress implemented the NPDB to collect information about an individual doctor’s malpractice and disciplinary histories, with the objective of restricting errant doctors from moving from one state to another.1
Federal law requires medical liability payments stemming from either a court judgment or an out-of-court settlement be reported to the NPDB. An institution’s disciplinary actions against a medical staff member must also be reported. In turn, the NPDB is obligated to make its information available to hospitals, state licensure boards, and legitimate medical organizations charged with granting privileges or membership. A physician also can ask to see his or her own records, but a plaintiff’s attorney cannot access the NPDB unless there is evidence that a hospital failed to query the NPDB as part of its credentialing process.
Some observers have claimed that the NPDB’s existence has hindered settlement negotiations, because many doctors fear being listed in the NPDB, thus significantly diminishing the likelihood of payments to satisfy a claim. It has been stated that within 6 years of NPDB’s inception, the probability that an injured patient’s claim would receive payment had fallen to 59% of the pre-NPDB level.
Many states have enacted so-called “apology laws” that promote full disclosure of medical errors and prompt out-of-court settlements, if warranted. However, the federal Department of Health and Human Services has ruled that all written demands for payment must be reported, even if the cases are resolved under state programs designed for early out-of-court resolution.
For example, a provision in the Oregon law asserts that a payment under the measure’s mediation mechanism “is not a payment resulting from a written claim or demand for payment.” The HHS has rejected this as “explicitly designed to avoid medical malpractice reporting to the NPDB for any claims that are part of the new process that do not proceed to litigation.”
Massachusetts’ 2012 apology law had proposed reporting only those cases where it was determined that a practitioner failed to meet the standard of care. The HHS responded by indicating that all cases had to be reported, regardless of whether care was determined to be up to standards, and that the state’s prelitigation notice to initiate the meditation process qualified as a reportable “written claim.”
Physicians can be impacted greatly by the NPDB. How much of an impact depends in large part on the underlying events and the wording of the report.
An NPDB account of a medical malpractice payment doesn’t necessarily affect a physician’s ability to practice, while those – especially when “severely-worded” – involving denial or restriction of privileges are taken more seriously by state licensing boards and employers. Physicians should therefore play an active role whenever a report to the NPDB appears likely.
The dispute review process is highly technical and requires the knowledge and skill of an experienced health law attorney. To start out, consider making a request to the reporting entity to correct or vacate the report due to error. If the reporting entity declines, the physician may request a review by the HHS and file an accompanying statement seeking to explain the report.
Yet, out of more than 800,000 total reports for all practitioner types captured in the system, apparently only 44,273 included accompanying clarifying statements by the physician. Risk managers have urged vigilance.
For example, it may be that multiple reports involved a single incident, leading to a “piling on” effect. If an adverse decision at one hospital caused a physician’s clinical privileges to be terminated, this might lead the state medical board to restrict the physician’s license. It is necessary to explain that both of these NPDB-reportable events involved the same incident, and that the state board did not have any independent knowledge of anything that was wrong.
Others have advised that one should always clarify one’s involvement, e.g., “I was not the main doctor in the case.” And if dismissed in a malpractice lawsuit, be sure your name or identifying information isn’t included in the judgment or settlement agreement.
Hospital disciplinary actions being far more serious, physicians would do well to familiarize themselves with medical staff bylaws dealing with peer review and investigations. To avoid being reported to the NPDB, physicians must resist adverse actions that would be in effect for more than 30 days and fight attempts to place restrictions or sanctions on their licenses by the hospital or professional societies. Don’t withdraw applications for privileges during an investigation.
The 2015 NPDB Guidebook, the first update in more than 10 years, contains important changes pertaining to hospital adverse actions.2 The regulations now require any “surrender” of privileges while the physician is a subject of an investigation to be a reportable event. Previously, physicians sought to avoid being reported by simply giving up their privileges when an adverse decision appeared imminent.
Surrender includes not renewing one’s hospital privileges or the taking of a leave of absence, and “investigation” is widely defined to include any formal inquiry into a physician’s competence or conduct. And there need not be any “nexus,” i.e., connection, between what is being investigated and the privileges surrendered, in order to be reportable.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Health Care Quality Improvement Act of 1986, 42 U.S.C. 11101 et seq.
2. 2015 NPDB e-Guidebook, available at www.npdb.hrsa.gov/resources/aboutGuidebooks.jsp.
Study sheds light on pregnancy outcomes following ocrelizumab treatment
NEW ORLEANS – Data from the ocrelizumab clinical development program gives clinicians a first look at pregnancy outcomes after exposure to the drug, but the small size limits the ability to draw firm conclusions.
In the United States, prescribing information for ocrelizumab states that women of childbearing potential should use contraception while receiving ocrelizumab and for 6 months after the last infusion. At the annual meeting of the Consortium of Multiple Sclerosis Centers, researchers led by Sibyl Wray, MD, set out to assess the pregnancy, fetal, neonatal and infant outcomes in patients who became pregnant during ocrelizumab trials in MS, rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) through Sept. 15, 2015.
The analysis included ocrelizumab-exposed women in primarily European-based clinical trials in patients with MS, RA, or SLE, in whom doses ranged from 20 mg to 2,000 mg. These included three randomized trials of its use in MS, totaling 1,876 patients with a mean age of 40 years; four trials of its use in RA, totaling 2,759 patients with a mean age of 53 years; and one trial of its use in SLE, totaling 381 patients with a mean age of 31 years. Between 2008 and Sept. 14, 2015, a total of 48 women who were enrolled in the trials reported pregnancies.
MS data
Of the 15 pregnancies in the MS trials, three involved the delivery of full term, healthy newborns. In one case, the last ocrelizumab infusion was given 28 months before conception. In the second case, an infusion was given 20 weeks before conception, and a further infusion was given 17 days after conception. In the third case, the last ocrelizumab infusion was given 26.5 weeks before conception.
One live term birth occurred with an abnormal finding. In this case, the last infusion of ocrelizumab was 23 weeks prior to the last menstrual period or about 6 months prior to conception. The embryo/fetus was not exposed to the drug in utero. The researchers also found that seven elective terminations occurred among MS patients and that four pregnancies were ongoing at the time of this report.
“We have to be cautious because we don’t have enough data yet to know, but it’s encouraging to see that, if you follow the guidelines, the patient population and the newborns seem to be healthy in these exposed individuals,” Dr. Wray said.
RA data
Data from the RA clinical trials revealed 22 pregnancies in 21 patients exposed to ocrelizumab. Of these, eight pregnancies resulted in healthy term babies; four resulted in live births with abnormal findings (structural malformation, growth abnormality) or preterm birth; and eight pregnancies in seven women resulted in spontaneous abortion (one patient experienced a spontaneous abortion on two occasions), missed abortion, or an embryonic pregnancy. One pregnancy was lost to follow-up and another resulted in elective termination.
SLE data
During the SLE trials, 11 pregnancies occurred in 10 patients. Three pregnancies in two women resulted in healthy term babies. Three other pregnancies resulted in live births with an abnormal finding (structural malformation, functional deficit, growth abnormality) and/or preterm birth. Two pregnancies resulted in spontaneous/missed abortion. One pregnancy resulted in fetal death at 7.5 months’ gestation secondary to fatal pulmonary embolism in the mother; one pregnancy resulted in elective termination; and one pregnancy resulted in a healthy baby born at an unknown gestational week.
Dr. Wray emphasized that the small numbers of patients studied make it difficult to draw conclusions about pregnancy outcomes following ocrelizumab in patients with MS and other autoimmune diseases. “We need to pay attention to the half-life of this drug, the time it takes to clear, and how to plan pregnancies around that,” she said. She noted that pregnancy outcomes in ongoing ocrelizumab studies and postmarketing experiences will continue to be collected and assessed.
The study was funded by Roche, Basel, Switzerland. Dr. Wray reported that she has received honoraria and/or research funding from Actelion, Alkermes, Biogen, Celgene, EMD Serono, Genentech/Roche, Genzyme/Sanofi, Novartis, and TG Therapeutics.
NEW ORLEANS – Data from the ocrelizumab clinical development program gives clinicians a first look at pregnancy outcomes after exposure to the drug, but the small size limits the ability to draw firm conclusions.
In the United States, prescribing information for ocrelizumab states that women of childbearing potential should use contraception while receiving ocrelizumab and for 6 months after the last infusion. At the annual meeting of the Consortium of Multiple Sclerosis Centers, researchers led by Sibyl Wray, MD, set out to assess the pregnancy, fetal, neonatal and infant outcomes in patients who became pregnant during ocrelizumab trials in MS, rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) through Sept. 15, 2015.
The analysis included ocrelizumab-exposed women in primarily European-based clinical trials in patients with MS, RA, or SLE, in whom doses ranged from 20 mg to 2,000 mg. These included three randomized trials of its use in MS, totaling 1,876 patients with a mean age of 40 years; four trials of its use in RA, totaling 2,759 patients with a mean age of 53 years; and one trial of its use in SLE, totaling 381 patients with a mean age of 31 years. Between 2008 and Sept. 14, 2015, a total of 48 women who were enrolled in the trials reported pregnancies.
MS data
Of the 15 pregnancies in the MS trials, three involved the delivery of full term, healthy newborns. In one case, the last ocrelizumab infusion was given 28 months before conception. In the second case, an infusion was given 20 weeks before conception, and a further infusion was given 17 days after conception. In the third case, the last ocrelizumab infusion was given 26.5 weeks before conception.
One live term birth occurred with an abnormal finding. In this case, the last infusion of ocrelizumab was 23 weeks prior to the last menstrual period or about 6 months prior to conception. The embryo/fetus was not exposed to the drug in utero. The researchers also found that seven elective terminations occurred among MS patients and that four pregnancies were ongoing at the time of this report.
“We have to be cautious because we don’t have enough data yet to know, but it’s encouraging to see that, if you follow the guidelines, the patient population and the newborns seem to be healthy in these exposed individuals,” Dr. Wray said.
RA data
Data from the RA clinical trials revealed 22 pregnancies in 21 patients exposed to ocrelizumab. Of these, eight pregnancies resulted in healthy term babies; four resulted in live births with abnormal findings (structural malformation, growth abnormality) or preterm birth; and eight pregnancies in seven women resulted in spontaneous abortion (one patient experienced a spontaneous abortion on two occasions), missed abortion, or an embryonic pregnancy. One pregnancy was lost to follow-up and another resulted in elective termination.
SLE data
During the SLE trials, 11 pregnancies occurred in 10 patients. Three pregnancies in two women resulted in healthy term babies. Three other pregnancies resulted in live births with an abnormal finding (structural malformation, functional deficit, growth abnormality) and/or preterm birth. Two pregnancies resulted in spontaneous/missed abortion. One pregnancy resulted in fetal death at 7.5 months’ gestation secondary to fatal pulmonary embolism in the mother; one pregnancy resulted in elective termination; and one pregnancy resulted in a healthy baby born at an unknown gestational week.
Dr. Wray emphasized that the small numbers of patients studied make it difficult to draw conclusions about pregnancy outcomes following ocrelizumab in patients with MS and other autoimmune diseases. “We need to pay attention to the half-life of this drug, the time it takes to clear, and how to plan pregnancies around that,” she said. She noted that pregnancy outcomes in ongoing ocrelizumab studies and postmarketing experiences will continue to be collected and assessed.
The study was funded by Roche, Basel, Switzerland. Dr. Wray reported that she has received honoraria and/or research funding from Actelion, Alkermes, Biogen, Celgene, EMD Serono, Genentech/Roche, Genzyme/Sanofi, Novartis, and TG Therapeutics.
NEW ORLEANS – Data from the ocrelizumab clinical development program gives clinicians a first look at pregnancy outcomes after exposure to the drug, but the small size limits the ability to draw firm conclusions.
In the United States, prescribing information for ocrelizumab states that women of childbearing potential should use contraception while receiving ocrelizumab and for 6 months after the last infusion. At the annual meeting of the Consortium of Multiple Sclerosis Centers, researchers led by Sibyl Wray, MD, set out to assess the pregnancy, fetal, neonatal and infant outcomes in patients who became pregnant during ocrelizumab trials in MS, rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) through Sept. 15, 2015.
The analysis included ocrelizumab-exposed women in primarily European-based clinical trials in patients with MS, RA, or SLE, in whom doses ranged from 20 mg to 2,000 mg. These included three randomized trials of its use in MS, totaling 1,876 patients with a mean age of 40 years; four trials of its use in RA, totaling 2,759 patients with a mean age of 53 years; and one trial of its use in SLE, totaling 381 patients with a mean age of 31 years. Between 2008 and Sept. 14, 2015, a total of 48 women who were enrolled in the trials reported pregnancies.
MS data
Of the 15 pregnancies in the MS trials, three involved the delivery of full term, healthy newborns. In one case, the last ocrelizumab infusion was given 28 months before conception. In the second case, an infusion was given 20 weeks before conception, and a further infusion was given 17 days after conception. In the third case, the last ocrelizumab infusion was given 26.5 weeks before conception.
One live term birth occurred with an abnormal finding. In this case, the last infusion of ocrelizumab was 23 weeks prior to the last menstrual period or about 6 months prior to conception. The embryo/fetus was not exposed to the drug in utero. The researchers also found that seven elective terminations occurred among MS patients and that four pregnancies were ongoing at the time of this report.
“We have to be cautious because we don’t have enough data yet to know, but it’s encouraging to see that, if you follow the guidelines, the patient population and the newborns seem to be healthy in these exposed individuals,” Dr. Wray said.
RA data
Data from the RA clinical trials revealed 22 pregnancies in 21 patients exposed to ocrelizumab. Of these, eight pregnancies resulted in healthy term babies; four resulted in live births with abnormal findings (structural malformation, growth abnormality) or preterm birth; and eight pregnancies in seven women resulted in spontaneous abortion (one patient experienced a spontaneous abortion on two occasions), missed abortion, or an embryonic pregnancy. One pregnancy was lost to follow-up and another resulted in elective termination.
SLE data
During the SLE trials, 11 pregnancies occurred in 10 patients. Three pregnancies in two women resulted in healthy term babies. Three other pregnancies resulted in live births with an abnormal finding (structural malformation, functional deficit, growth abnormality) and/or preterm birth. Two pregnancies resulted in spontaneous/missed abortion. One pregnancy resulted in fetal death at 7.5 months’ gestation secondary to fatal pulmonary embolism in the mother; one pregnancy resulted in elective termination; and one pregnancy resulted in a healthy baby born at an unknown gestational week.
Dr. Wray emphasized that the small numbers of patients studied make it difficult to draw conclusions about pregnancy outcomes following ocrelizumab in patients with MS and other autoimmune diseases. “We need to pay attention to the half-life of this drug, the time it takes to clear, and how to plan pregnancies around that,” she said. She noted that pregnancy outcomes in ongoing ocrelizumab studies and postmarketing experiences will continue to be collected and assessed.
The study was funded by Roche, Basel, Switzerland. Dr. Wray reported that she has received honoraria and/or research funding from Actelion, Alkermes, Biogen, Celgene, EMD Serono, Genentech/Roche, Genzyme/Sanofi, Novartis, and TG Therapeutics.
AT THE CMSC ANNUAL MEETING
Key clinical point:
Major finding: Of 15 pregnancies in the MS trials, three involved the delivery of three full term, healthy newborns; one live term birth occurred with an abnormal finding; seven elective terminations occurred; and four pregnancies were ongoing.
Data source: A review of 48 pregnancies among women enrolled in clinical trials for ocrelizumab in MS, rheumatoid arthritis, and systemic lupus erythematosus.
Disclosures: The study was funded by Roche, Basel, Switzerland. Dr. Wray reported that she has received honoraria and/or research funding from Actelion, Alkermes, Biogen, Celgene, EMD Serono, Genentech/Roche, Genzyme/Sanofi, Novartis, and TG Therapeutics.
Politics and Planned Parenthood
WHAT LIES AHEAD FOR WOMEN’S HEALTH? CHALLENGES, AND OPPORTUNITIES, AS ACOG AND US LEADERSHIP TRANSITION
LUCIA DIVENERE, MA
Politics and Planned Parenthood
As obstetricians and gynecologists, we are all dedicated to women's health care. Many of us are Pro-Life and resent your unqualified support of Planned Parenthood, the world’s largest provider and supporter of unrestricted abortion. There are many venues to deliver women's health care. We should not be cheerleaders for the Planned Parenthood/Democratic party propaganda. As you stated, we should be truly a-political.
Laurence Burns, DO
Grand Rapids, Michigan
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
WHAT LIES AHEAD FOR WOMEN’S HEALTH? CHALLENGES, AND OPPORTUNITIES, AS ACOG AND US LEADERSHIP TRANSITION
LUCIA DIVENERE, MA
Politics and Planned Parenthood
As obstetricians and gynecologists, we are all dedicated to women's health care. Many of us are Pro-Life and resent your unqualified support of Planned Parenthood, the world’s largest provider and supporter of unrestricted abortion. There are many venues to deliver women's health care. We should not be cheerleaders for the Planned Parenthood/Democratic party propaganda. As you stated, we should be truly a-political.
Laurence Burns, DO
Grand Rapids, Michigan
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
WHAT LIES AHEAD FOR WOMEN’S HEALTH? CHALLENGES, AND OPPORTUNITIES, AS ACOG AND US LEADERSHIP TRANSITION
LUCIA DIVENERE, MA
Politics and Planned Parenthood
As obstetricians and gynecologists, we are all dedicated to women's health care. Many of us are Pro-Life and resent your unqualified support of Planned Parenthood, the world’s largest provider and supporter of unrestricted abortion. There are many venues to deliver women's health care. We should not be cheerleaders for the Planned Parenthood/Democratic party propaganda. As you stated, we should be truly a-political.
Laurence Burns, DO
Grand Rapids, Michigan
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
BMS recalls a lot of Eliquis 5 mg tablets
Bristol-Myers Squibb is recalling one lot of Eliquis (apixaban) tablets, according to a company announcement on the Food and Drug Administration website.
The recall is based on a customer complaint that a bottle labeled as containing 5 mg tablets of Eliquis actually contained 2.5 mg tablets of Eliquis. The lot in question was distributed nationwide to wholesalers and retail pharmacies in February 2017. The recall is voluntary, the company noted.
“There are distinct visible differences between the two tablet strengths, including colors, size, and markings that distinguish the 2.5 mg and 5 mg tablets and decrease the likelihood of an incorrect dose,” Bristol-Myers Squibb noted in the press release.
Find the full Bristol-Myers Squibb press release on the FDA website.
Bristol-Myers Squibb is recalling one lot of Eliquis (apixaban) tablets, according to a company announcement on the Food and Drug Administration website.
The recall is based on a customer complaint that a bottle labeled as containing 5 mg tablets of Eliquis actually contained 2.5 mg tablets of Eliquis. The lot in question was distributed nationwide to wholesalers and retail pharmacies in February 2017. The recall is voluntary, the company noted.
“There are distinct visible differences between the two tablet strengths, including colors, size, and markings that distinguish the 2.5 mg and 5 mg tablets and decrease the likelihood of an incorrect dose,” Bristol-Myers Squibb noted in the press release.
Find the full Bristol-Myers Squibb press release on the FDA website.
Bristol-Myers Squibb is recalling one lot of Eliquis (apixaban) tablets, according to a company announcement on the Food and Drug Administration website.
The recall is based on a customer complaint that a bottle labeled as containing 5 mg tablets of Eliquis actually contained 2.5 mg tablets of Eliquis. The lot in question was distributed nationwide to wholesalers and retail pharmacies in February 2017. The recall is voluntary, the company noted.
“There are distinct visible differences between the two tablet strengths, including colors, size, and markings that distinguish the 2.5 mg and 5 mg tablets and decrease the likelihood of an incorrect dose,” Bristol-Myers Squibb noted in the press release.
Find the full Bristol-Myers Squibb press release on the FDA website.















