User login
Meet the two newest SHM board members
SHM’s two newest board members – pediatric hospitalist Kris Rehm, MD, SFHM, and perioperative specialist Rachel Thompson, MD, MPH, SFHM – will bring their expertise to bear on the society’s top panel.
However, neither woman sees her role as shaping the board. In fact, they see themselves as lucky to be joining the team.
“I really want to hear everyone’s voice, and I hope to see how we can all move to better places together,” added Dr. Rehm, associate professor of clinical pediatrics and director of the division of hospital medicine at Vanderbilt University in Nashville.
Both board members were officially seated for three-year terms at HM17 in Las Vegas. They replace former SHM president Robert Harrington, MD, SFHM, and veteran pediatric hospitalist Erin Stucky Fisher, MD, MHM.
Each of the new board members brings a strong perspective to the panel.
For Dr. Thompson, that viewpoint is based in engagement. She is the former chair of SHM’s Pacific Northwest chapter and has spent the past few years leading the perioperative issues for the society’s work group.
“We get to a certain point of our career as hospitalists, and if we’re just plugging in and working, doing our shifts, somewhere in that 8- to 10-year range, we might get a little bored, tired, worn out,” Dr. Thompson said. “I believe, if we have the community and professional home to keep us engaged, that helps us see the value in what we’re doing every day. It helps us continue to grow, so we don’t hit that wall.”
Given Dr. Thompson’s involvement both with her chapter and the society’s chapter support committee, she will likely continue that effort to make sure SHM’s board sees the value of encouraging and partnering with local chapters. She will also work with SHM president Ron Greeno, MD, FCCP, MHM, on policy issues, as her background in public health has aligned her interests on health care reform and other headwinds facing the specialty.
“I went in to do my masters in public health with the idea that I wanted to build the skill set so that I could be more analytical in how I approach our problem solving, our discovery in the hospital setting,” she said. “It really speaks to a part of me that has always been interested in finding ways to prevent illness and moving beyond that reactivity that we have in medicine into a prevention-based [approach].”
Dr. Thompson noted that her background in perioperative medicine helps her work as part of a team because it “entirely relies on collaboration and coordination of care, which is pretty much the basis of what we do in the hospital any day.”
Dr. Rehm, who serves as a pediatric hospitalist at the Monroe Carell Jr. Children’s Hospital at Vanderbilt, said she will also bring a teamwork-focused perspective to the SHM board.
She could be expected to view everything through the lens of inpatient pediatrics, but that’s not her style.
“I think we have so many similarities and so many things that [pediatric and adult hospitalists] can partner to do together,” she said. “We all are involved in, for example, medication reconciliation or discharge-management planning or postacute care follow-up. There’s a lot of synergy, and I think we can learn so much from each other.”
Dr. Rehm, who chairs SHM’s Pediatrics Committee and the 2017 Pediatric Hospital Medicine meeting, pointed out that working well with others is a natural skill set for hospitalists.
“Collaboration is probably my biggest skill set and that of many hospital medicine providers,” she said. “I think I do that in my job here at Vanderbilt in thinking about complicated patients that requirement multiple subspecialists and in bringing together people to figure out the question at hand. That is definitely my leadership style.”
If Dr. Rehm has one goal on the board, it is to become a little bit more like Dr. Thompson and focus on chapter development for pediatric hospitalists.
“I’m really interested in engaging with members to better understand the struggles on the front line so that we can make sure that, as an organization, we’re offering a brand of things that our membership needs,” she said. “So, I’m really looking forward to becoming more involved in the chapter engagement and development. The Nashville chapter is getting re-engaged now and I’m excited to be involved.”
To prepare for her debut board meeting in Las Vegas, Dr. Rehm attended SHM board meetings at the group’s Philadelphia headquarters over the past two years.
“I’ve been lucky enough to attend the fall board meeting in Philadelphia and observe the board in action, and I think that has helped me get to know some of the current board members and to have a little bit of a vision of what the meetings will be like,” she said.
SHM’s two newest board members – pediatric hospitalist Kris Rehm, MD, SFHM, and perioperative specialist Rachel Thompson, MD, MPH, SFHM – will bring their expertise to bear on the society’s top panel.
However, neither woman sees her role as shaping the board. In fact, they see themselves as lucky to be joining the team.
“I really want to hear everyone’s voice, and I hope to see how we can all move to better places together,” added Dr. Rehm, associate professor of clinical pediatrics and director of the division of hospital medicine at Vanderbilt University in Nashville.
Both board members were officially seated for three-year terms at HM17 in Las Vegas. They replace former SHM president Robert Harrington, MD, SFHM, and veteran pediatric hospitalist Erin Stucky Fisher, MD, MHM.
Each of the new board members brings a strong perspective to the panel.
For Dr. Thompson, that viewpoint is based in engagement. She is the former chair of SHM’s Pacific Northwest chapter and has spent the past few years leading the perioperative issues for the society’s work group.
“We get to a certain point of our career as hospitalists, and if we’re just plugging in and working, doing our shifts, somewhere in that 8- to 10-year range, we might get a little bored, tired, worn out,” Dr. Thompson said. “I believe, if we have the community and professional home to keep us engaged, that helps us see the value in what we’re doing every day. It helps us continue to grow, so we don’t hit that wall.”
Given Dr. Thompson’s involvement both with her chapter and the society’s chapter support committee, she will likely continue that effort to make sure SHM’s board sees the value of encouraging and partnering with local chapters. She will also work with SHM president Ron Greeno, MD, FCCP, MHM, on policy issues, as her background in public health has aligned her interests on health care reform and other headwinds facing the specialty.
“I went in to do my masters in public health with the idea that I wanted to build the skill set so that I could be more analytical in how I approach our problem solving, our discovery in the hospital setting,” she said. “It really speaks to a part of me that has always been interested in finding ways to prevent illness and moving beyond that reactivity that we have in medicine into a prevention-based [approach].”
Dr. Thompson noted that her background in perioperative medicine helps her work as part of a team because it “entirely relies on collaboration and coordination of care, which is pretty much the basis of what we do in the hospital any day.”
Dr. Rehm, who serves as a pediatric hospitalist at the Monroe Carell Jr. Children’s Hospital at Vanderbilt, said she will also bring a teamwork-focused perspective to the SHM board.
She could be expected to view everything through the lens of inpatient pediatrics, but that’s not her style.
“I think we have so many similarities and so many things that [pediatric and adult hospitalists] can partner to do together,” she said. “We all are involved in, for example, medication reconciliation or discharge-management planning or postacute care follow-up. There’s a lot of synergy, and I think we can learn so much from each other.”
Dr. Rehm, who chairs SHM’s Pediatrics Committee and the 2017 Pediatric Hospital Medicine meeting, pointed out that working well with others is a natural skill set for hospitalists.
“Collaboration is probably my biggest skill set and that of many hospital medicine providers,” she said. “I think I do that in my job here at Vanderbilt in thinking about complicated patients that requirement multiple subspecialists and in bringing together people to figure out the question at hand. That is definitely my leadership style.”
If Dr. Rehm has one goal on the board, it is to become a little bit more like Dr. Thompson and focus on chapter development for pediatric hospitalists.
“I’m really interested in engaging with members to better understand the struggles on the front line so that we can make sure that, as an organization, we’re offering a brand of things that our membership needs,” she said. “So, I’m really looking forward to becoming more involved in the chapter engagement and development. The Nashville chapter is getting re-engaged now and I’m excited to be involved.”
To prepare for her debut board meeting in Las Vegas, Dr. Rehm attended SHM board meetings at the group’s Philadelphia headquarters over the past two years.
“I’ve been lucky enough to attend the fall board meeting in Philadelphia and observe the board in action, and I think that has helped me get to know some of the current board members and to have a little bit of a vision of what the meetings will be like,” she said.
SHM’s two newest board members – pediatric hospitalist Kris Rehm, MD, SFHM, and perioperative specialist Rachel Thompson, MD, MPH, SFHM – will bring their expertise to bear on the society’s top panel.
However, neither woman sees her role as shaping the board. In fact, they see themselves as lucky to be joining the team.
“I really want to hear everyone’s voice, and I hope to see how we can all move to better places together,” added Dr. Rehm, associate professor of clinical pediatrics and director of the division of hospital medicine at Vanderbilt University in Nashville.
Both board members were officially seated for three-year terms at HM17 in Las Vegas. They replace former SHM president Robert Harrington, MD, SFHM, and veteran pediatric hospitalist Erin Stucky Fisher, MD, MHM.
Each of the new board members brings a strong perspective to the panel.
For Dr. Thompson, that viewpoint is based in engagement. She is the former chair of SHM’s Pacific Northwest chapter and has spent the past few years leading the perioperative issues for the society’s work group.
“We get to a certain point of our career as hospitalists, and if we’re just plugging in and working, doing our shifts, somewhere in that 8- to 10-year range, we might get a little bored, tired, worn out,” Dr. Thompson said. “I believe, if we have the community and professional home to keep us engaged, that helps us see the value in what we’re doing every day. It helps us continue to grow, so we don’t hit that wall.”
Given Dr. Thompson’s involvement both with her chapter and the society’s chapter support committee, she will likely continue that effort to make sure SHM’s board sees the value of encouraging and partnering with local chapters. She will also work with SHM president Ron Greeno, MD, FCCP, MHM, on policy issues, as her background in public health has aligned her interests on health care reform and other headwinds facing the specialty.
“I went in to do my masters in public health with the idea that I wanted to build the skill set so that I could be more analytical in how I approach our problem solving, our discovery in the hospital setting,” she said. “It really speaks to a part of me that has always been interested in finding ways to prevent illness and moving beyond that reactivity that we have in medicine into a prevention-based [approach].”
Dr. Thompson noted that her background in perioperative medicine helps her work as part of a team because it “entirely relies on collaboration and coordination of care, which is pretty much the basis of what we do in the hospital any day.”
Dr. Rehm, who serves as a pediatric hospitalist at the Monroe Carell Jr. Children’s Hospital at Vanderbilt, said she will also bring a teamwork-focused perspective to the SHM board.
She could be expected to view everything through the lens of inpatient pediatrics, but that’s not her style.
“I think we have so many similarities and so many things that [pediatric and adult hospitalists] can partner to do together,” she said. “We all are involved in, for example, medication reconciliation or discharge-management planning or postacute care follow-up. There’s a lot of synergy, and I think we can learn so much from each other.”
Dr. Rehm, who chairs SHM’s Pediatrics Committee and the 2017 Pediatric Hospital Medicine meeting, pointed out that working well with others is a natural skill set for hospitalists.
“Collaboration is probably my biggest skill set and that of many hospital medicine providers,” she said. “I think I do that in my job here at Vanderbilt in thinking about complicated patients that requirement multiple subspecialists and in bringing together people to figure out the question at hand. That is definitely my leadership style.”
If Dr. Rehm has one goal on the board, it is to become a little bit more like Dr. Thompson and focus on chapter development for pediatric hospitalists.
“I’m really interested in engaging with members to better understand the struggles on the front line so that we can make sure that, as an organization, we’re offering a brand of things that our membership needs,” she said. “So, I’m really looking forward to becoming more involved in the chapter engagement and development. The Nashville chapter is getting re-engaged now and I’m excited to be involved.”
To prepare for her debut board meeting in Las Vegas, Dr. Rehm attended SHM board meetings at the group’s Philadelphia headquarters over the past two years.
“I’ve been lucky enough to attend the fall board meeting in Philadelphia and observe the board in action, and I think that has helped me get to know some of the current board members and to have a little bit of a vision of what the meetings will be like,” she said.
What Does the Accountability Act Mean for VA Employees?
On Friday June 23, President Trump signed into law the VA Accountability and Whistleblower Protection Act, which established a revised disciplinary system for VA employees who are accused of poor performance or misconduct and protects those who report them. The bill amends Title 38 of the U.S. Code, adding VA employees to the list of those that the VA Secretary can remove if necessary. The act speeds up the termination and suspension processes for civil servants. The VA also claims that the bill will shorten the process of hiring new administrators. Still unclear is what this bill means for VA health care providers.
VA employees are protected under both Title 5 and Title 38 of the U.S. code. Title 5 governs the employment of all federal civil servants. Title 38 specifically governs the employment of VA employees in certain health care fields. Both titles specify processes for termination, demotion, or suspension and an appeals process.
Critics, including VA Secretary David J. Shulkin, MD, have complained that the previous civil service protections were time consuming and made it difficult to penalize or remove problem employees. Supporters insist that Title 5 and Title 38 protected the rights of good employees and whistleblowers from unfair workplace retaliation.
Under the new law, the VA Secretary can more easily remove, demote, or suspend any employee who warrants a penalty, including executives and supervisors. Although many of the Title 5 and Title 38 protections still cover VA employees, there are several notable changes.
What’s changing for VA employees?
- Time line: The act shortens time between the initial report, employee notification, Secretary’s decision, and appeal request/decision.
- Standard of proof: Previously, the standard of proof for misconduct was “preponderance of evidence.” The new bill lowers this standard to “supported by substantial evidence.” Changing the standard of proof effectively means that the Secretary will need to present less evidence of wrongdoing/negligence for the employee to be removed, demoted, or suspended.
- Appeals: Employees may still appeal removal, demotion, or suspension decisions, but rather than going through the Disciplinary Appeals Board, the Merit Systems Protection Board will hear appeals. Appeals must be filed within 10 days of the Secretary’s final decision. Additionally, administrative judges are instructed to uphold the decision of the Secretary if the evidence meets the “substantial evidence” standard. Administrative judges are prohibited from reducing the penalty.
Analysis
Although these changes are meant to increase the accountability of health care providers in the VA, there are several other legal implications for VA employees. Ultimately, the bill gives the VA Secretary more discretion. Whether this is a good or bad change is open to debate. Even though the bill easily passed in both the House of Representatives and the Senate, some lawmakers remain skeptical because the act increases the power of the Secretary and limits due process for VA employees. Under the new law, VA employees will have less time to prepare their defense and be held to a lower standard of proof, which will make it easier to remove VA employees from their jobs.
The Fifth Amendment of the U.S. Constitution guarantees that all Americans shall not be “deprived of life, liberty, or property, without due process of law.” Furthermore, due process rights increase when the limitations on ones’ rights are greater; this is why the burden of proof falls on the prosecution and why the standard of proof is “beyond a reasonable doubt” in criminal cases, because the rights that are at risk are liberty and, in some cases, life.
Due process is particularly important for civil servants because they are employed by the federal government. The rights to property are at risk of being limited if they are to be removed, demoted, or suspended from their jobs. The VA Accountability and Whistleblower Protection Act limits the due process of VA civil servants. However, it also is important to highlight that under this law, the VA Secretary has the right to remove, demote, or suspend any person who threatens the function and service of the VA or endangers veterans. In other words, while the VA employee’s due process may have decreased, it has increased for those whose lives depend on their service.
On Friday June 23, President Trump signed into law the VA Accountability and Whistleblower Protection Act, which established a revised disciplinary system for VA employees who are accused of poor performance or misconduct and protects those who report them. The bill amends Title 38 of the U.S. Code, adding VA employees to the list of those that the VA Secretary can remove if necessary. The act speeds up the termination and suspension processes for civil servants. The VA also claims that the bill will shorten the process of hiring new administrators. Still unclear is what this bill means for VA health care providers.
VA employees are protected under both Title 5 and Title 38 of the U.S. code. Title 5 governs the employment of all federal civil servants. Title 38 specifically governs the employment of VA employees in certain health care fields. Both titles specify processes for termination, demotion, or suspension and an appeals process.
Critics, including VA Secretary David J. Shulkin, MD, have complained that the previous civil service protections were time consuming and made it difficult to penalize or remove problem employees. Supporters insist that Title 5 and Title 38 protected the rights of good employees and whistleblowers from unfair workplace retaliation.
Under the new law, the VA Secretary can more easily remove, demote, or suspend any employee who warrants a penalty, including executives and supervisors. Although many of the Title 5 and Title 38 protections still cover VA employees, there are several notable changes.
What’s changing for VA employees?
- Time line: The act shortens time between the initial report, employee notification, Secretary’s decision, and appeal request/decision.
- Standard of proof: Previously, the standard of proof for misconduct was “preponderance of evidence.” The new bill lowers this standard to “supported by substantial evidence.” Changing the standard of proof effectively means that the Secretary will need to present less evidence of wrongdoing/negligence for the employee to be removed, demoted, or suspended.
- Appeals: Employees may still appeal removal, demotion, or suspension decisions, but rather than going through the Disciplinary Appeals Board, the Merit Systems Protection Board will hear appeals. Appeals must be filed within 10 days of the Secretary’s final decision. Additionally, administrative judges are instructed to uphold the decision of the Secretary if the evidence meets the “substantial evidence” standard. Administrative judges are prohibited from reducing the penalty.
Analysis
Although these changes are meant to increase the accountability of health care providers in the VA, there are several other legal implications for VA employees. Ultimately, the bill gives the VA Secretary more discretion. Whether this is a good or bad change is open to debate. Even though the bill easily passed in both the House of Representatives and the Senate, some lawmakers remain skeptical because the act increases the power of the Secretary and limits due process for VA employees. Under the new law, VA employees will have less time to prepare their defense and be held to a lower standard of proof, which will make it easier to remove VA employees from their jobs.
The Fifth Amendment of the U.S. Constitution guarantees that all Americans shall not be “deprived of life, liberty, or property, without due process of law.” Furthermore, due process rights increase when the limitations on ones’ rights are greater; this is why the burden of proof falls on the prosecution and why the standard of proof is “beyond a reasonable doubt” in criminal cases, because the rights that are at risk are liberty and, in some cases, life.
Due process is particularly important for civil servants because they are employed by the federal government. The rights to property are at risk of being limited if they are to be removed, demoted, or suspended from their jobs. The VA Accountability and Whistleblower Protection Act limits the due process of VA civil servants. However, it also is important to highlight that under this law, the VA Secretary has the right to remove, demote, or suspend any person who threatens the function and service of the VA or endangers veterans. In other words, while the VA employee’s due process may have decreased, it has increased for those whose lives depend on their service.
On Friday June 23, President Trump signed into law the VA Accountability and Whistleblower Protection Act, which established a revised disciplinary system for VA employees who are accused of poor performance or misconduct and protects those who report them. The bill amends Title 38 of the U.S. Code, adding VA employees to the list of those that the VA Secretary can remove if necessary. The act speeds up the termination and suspension processes for civil servants. The VA also claims that the bill will shorten the process of hiring new administrators. Still unclear is what this bill means for VA health care providers.
VA employees are protected under both Title 5 and Title 38 of the U.S. code. Title 5 governs the employment of all federal civil servants. Title 38 specifically governs the employment of VA employees in certain health care fields. Both titles specify processes for termination, demotion, or suspension and an appeals process.
Critics, including VA Secretary David J. Shulkin, MD, have complained that the previous civil service protections were time consuming and made it difficult to penalize or remove problem employees. Supporters insist that Title 5 and Title 38 protected the rights of good employees and whistleblowers from unfair workplace retaliation.
Under the new law, the VA Secretary can more easily remove, demote, or suspend any employee who warrants a penalty, including executives and supervisors. Although many of the Title 5 and Title 38 protections still cover VA employees, there are several notable changes.
What’s changing for VA employees?
- Time line: The act shortens time between the initial report, employee notification, Secretary’s decision, and appeal request/decision.
- Standard of proof: Previously, the standard of proof for misconduct was “preponderance of evidence.” The new bill lowers this standard to “supported by substantial evidence.” Changing the standard of proof effectively means that the Secretary will need to present less evidence of wrongdoing/negligence for the employee to be removed, demoted, or suspended.
- Appeals: Employees may still appeal removal, demotion, or suspension decisions, but rather than going through the Disciplinary Appeals Board, the Merit Systems Protection Board will hear appeals. Appeals must be filed within 10 days of the Secretary’s final decision. Additionally, administrative judges are instructed to uphold the decision of the Secretary if the evidence meets the “substantial evidence” standard. Administrative judges are prohibited from reducing the penalty.
Analysis
Although these changes are meant to increase the accountability of health care providers in the VA, there are several other legal implications for VA employees. Ultimately, the bill gives the VA Secretary more discretion. Whether this is a good or bad change is open to debate. Even though the bill easily passed in both the House of Representatives and the Senate, some lawmakers remain skeptical because the act increases the power of the Secretary and limits due process for VA employees. Under the new law, VA employees will have less time to prepare their defense and be held to a lower standard of proof, which will make it easier to remove VA employees from their jobs.
The Fifth Amendment of the U.S. Constitution guarantees that all Americans shall not be “deprived of life, liberty, or property, without due process of law.” Furthermore, due process rights increase when the limitations on ones’ rights are greater; this is why the burden of proof falls on the prosecution and why the standard of proof is “beyond a reasonable doubt” in criminal cases, because the rights that are at risk are liberty and, in some cases, life.
Due process is particularly important for civil servants because they are employed by the federal government. The rights to property are at risk of being limited if they are to be removed, demoted, or suspended from their jobs. The VA Accountability and Whistleblower Protection Act limits the due process of VA civil servants. However, it also is important to highlight that under this law, the VA Secretary has the right to remove, demote, or suspend any person who threatens the function and service of the VA or endangers veterans. In other words, while the VA employee’s due process may have decreased, it has increased for those whose lives depend on their service.
Quality of Chronic Obstructive Pulmonary Disease-Related Health Care in Rural and Urban Veterans Affairs Clinics
Chronic obstructive pulmonary disease (COPD) affects between 11 and 24 million people in the U.S. and is the third leading cause of death in this country.1,2 Airflow obstruction on spirometry in addition to respiratory symptoms is required to establish a diagnosis of COPD.3,4 As many as 40% of patients with a clinical diagnosis of COPD have not had spirometry or have spirometry results inconsistent with the diagnosis of COPD.5,6 In addition to recommended spirometry, many patients with COPD do not receive other evidence-based therapies.7,8
About 50% of patients in the Minneapolis VA Health Care System (MVAHCS) receive care in its rural community-based outreach clinics (CBOCs). Data regarding the quality of general medical care between rural and urban populations are sparse; however, studies suggest that the quality of care delivered in rural clinics may be lower than the care provided in an urban setting.9-12 Care for patients with COPD in an urban setting is suboptimal with only 58% of patients receiving guideline-based care, and there are no comparative data for penetrance in the rural setting.8 Most published studies on patients with COPD treated in rural vs urban locations are outcomes studies that queried statewide or national registry data evaluating the frequency of emergency department (ED) visits or hospital admissions for COPD exacerbations, all-cause mortality, or COPD exacerbation-related mortality.13-18 There are no studies examining potential differences in the quality of health care received by patients with COPD in rural vs urban locations or whether these potential differences are associated with changes in health care utilization.
The authors sought to determine whether patients with the diagnosis of COPD treated in the MVAHCS and its 13 CBOCs receive similar quality of disease-related health care in rural vs urban primary care clinic locations. The authors hypothesized that patients who receive their primary care in rural clinics would be less likely to have had spirometry or to receive respiratory immunizations and short- or long-acting inhalers and that discrepancies would be associated with increased health care utilization in rural areas as measured by prescriptions for systemic corticosteroids, antibiotics, ED visits, or hospital admissions for COPD exacerbations.
Methods
The MVAHCS has 14 primary care locations; these locations were designated as rural or urban based on the Rural-Urban Commuting Area codes.19,20 There were 4 urban locations and 10 rural clinics; all rural clinics were farther than 40 miles from the main Minneapolis VAMC.
Patient Selection
The authors performed a retrospective chart review after receiving an institutional review board waiver for this quality assessment study. All patients who had a prior ICD-9 encounter diagnosis of COPD (codes: 491.0, 491.1, 491.2, 491.20, 491.21, 491.22, 491.8, 491.9, 492.0, 492.8, 494, 494.0, 494.1, 496) and who were seen in primary care during March 2015 were identified. Each subject’s first visit during that month was used as the start of the retrospective 1-year look-back period. All eligible subjects were sorted based on their rural or urban location and a randomly assigned number. Patients were then selected according to ascending numbers from each rural and urban clinic in proportion to the clinic’s representation among all eligible patients.
Outcomes
The primary outcomes—possible discrepancies in quality of health care for patients with COPD in rural vs urban primary care clinics—were assessed by (1) prior spirometry; (2) any prior pneumonia vaccination; (3) an influenza vaccination within the past year; (4) prescriptions within the past year for a short-acting beta agonist (SABA) metered-dose inhaler; and (5) prescriptions for a long-acting inhalers, including long-acting beta agonists (LABAs), long-acting muscarinic antagonists (LAMAs), or inhaled corticosteroids (ICSs).
Secondary outcomes included (1) an active prescription for home oxygen within the past calendar year; (2) health care utilization assessed via prescriptions for intermittent courses of oral corticosteroids; (3) prescriptions for respiratory antibiotics (macrolides, tetracyclines, fluoroquinolones) within the past year for COPD exacerbations; (4) ED visits; (5) hospital admissions (and need for mechanical ventilation) for COPD exacerbations within the past year; and (6) whether patients were seen by either VA or Non-VA pulmonology providers.
Data Collection
Patients’ demographic data and comorbidities were collected via chart review. A 1-year prescription medication list was obtained by an electronic database search of the MVAHCS electronic medical record (EMR). Additional antibiotics and corticosteroid prescriptions for COPD exacerbations paid for by the VA but filled at a local pharmacy were manually searched from a separate database to supplement the electronic prescription list. Comparison of the electronic prescription list and pharmacy records in 25% of patients found 100% concordance in the prescription lists. The investigator manually reviewed and extracted the following data from the EMR, scanned-in records, and a Midwest VA COPD registry database: most recent spirometry results; immunization status for influenza in the past year; prior pneumonia vaccination; home oxygen prescription; whether the patient received respiratory antibiotic or intermittent oral corticosteroid treatment for COPD exacerbations; whether the patient had a ED visit or hospital admission for COPD exacerbation with or without need for mechanical ventilation; and whether the patient had been seen by a pulmonology provider. The investigator reviewed all primary care provider notes in the past year for documentation of non-VA ED visits or hospitalizations that were not present in the EMR, Midwest VA COPD registry database, or scanned patient records.
Data Analysis
Results are described as mean ± standard deviation, median (interquartile range) or proportion, expressed as a percentage as appropriate for the level of measurement and distribution. The proportions meeting the COPD quality of health care outcomes in the urban and rural groups were compared using a chi-square test of proportions, and 95% confidence intervals (CI) on the differences were estimated. Samples of 400 patients each from the rural and urban groups were estimated to provide a 95% 2-sided CI on the differences of about ± 0.05 (5%), assuming the proportion meeting the quality of care outcomes in the urban group would be at least 0.8 (80%).
Results
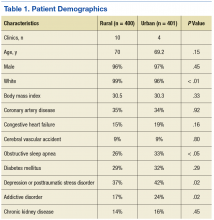
The authors identified 1,538 patients with a previous encounter diagnosis of COPD who were seen in a primary care clinic in the MVAHCS in March of 2015. The authors reviewed the medical records of 801 randomly selected patients: 400 rural clinic patients and 401 urban clinic patients. Demographic characteristics and major comorbidities of rural and urban patients were similar except more rural patients were white, and fewer had a record of obstructive sleep apnea, alcoholism, or addictive disorders (Table 1). Prescriptions for common chronic medical conditions were similar for rural and urban groups, including medications for depression (31% vs 33%) or diabetes mellitus (25% vs 28%). In patients who had spirometry, the severity of COPD, as assessed by mean forced expiratory volume (FEV1), was similar between rural and urban patients (2.06 L vs 2.10 L).
Quality of COPD Care
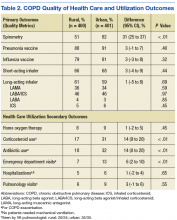
Spirometry was documented in fewer rural clinic patients than in urban clinic patients (51% vs 82%; difference 31%, 95% CI: 25% to 37%) (Table 2).
COPD Outcomes
Home oxygen prescription rates were similar for rural and urban clinic patients (8% vs 9%; difference 1%, 95% CI: -2% to 5%). Rural patients received fewer prescriptions for intermittent oral corticosteroids (17% vs 31%; difference 14%, 95% CI: 9% to 20%) and antibiotics for COPD exacerbations (18% vs 32%; difference 14%, 95% CI: 8% to 20%). Rural patients had fewer ED visits for COPD exacerbations (7% vs 13%; difference 6%, 95% CI: 2% to 10%), and similar admission rates for COPD exacerbations (5% vs 6%; difference 1%, 95% CI: -2% to 4%). Of the few patients hospitalized for COPD exacerbations, none required mechanical ventilation. There was no significant difference in the number of rural vs urban patients seen by a pulmonologist in the calendar year of the study (6% vs 9%; difference 3%, 95% CI:-1% to 6%), with the majority seen by VA providers: 20/24 rural patients and 35/35 urban patients.
Discussion
Fewer rural patients had prior spirometry; otherwise, the COPD-related quality metrics were similar between rural and urban patient groups in the MVAHCS, including immunizations for pneumonia and influenza, and prescribing rates for short- and long-acting inhaler therapy. Despite the similarity in these COPD quality measures, rural clinic patients seemed to have less health care utilization related to COPD exacerbations.
Spirometry with airflow obstruction in the presence of respiratory symptoms is required for accurate diagnosis of COPD.3,4 Spirometry has been available at the MVAHCS hospital-based clinic for years. Efforts to address this disparity led to implementation of on-site spirometry at all rural and urban clinics about 2 years prior to the patient enrollment visit date for the study. Fewer rural patients had spirometry, which is possibly from prior disparity in resources; yet rates of spirometry in all patients with a clinical diagnosis of COPD in the MVAHCS are higher (rural 51%, urban 82%) than previously reported. A nationwide study of 94,000 veterans with recent clinical diagnosis of COPD found only 37% had spirometry within 2.5 years of diagnosis,21 and another non-VA study (n = 553) showed only 31% of patients discharged from a hospital with a diagnosis of COPD exacerbation had spirometry performed within a 8-year period prior to hospitalization.22
Annual influenza vaccines are recommended for everyone aged > 6 months, and the pneumonia vaccine is recommended for all patients with COPD in order to reduce the risk of COPD exacerbations and pneumonias.23,24 The rates of vaccination at MVAHCS rural and urban clinics for both influenza (79% vs 81%) and pneumococcus (88% vs 91%) are higher than previously published studies of patients with COPD for influenza vaccination (30%-51%) and pneumonia vaccination (21%-51%) and did not differ between rural and urban clinics.7,25-28 The observed high vaccination rates may be due to EMR prompts and requirements to document vaccination status and offer recommended vaccinations.
Long-acting inhalers have been shown to reduce rates of COPD exacerbations and improve patients’ quality of life.29 The authors found no disparity in the prescription rate of short- or long-acting inhalers between rural and urban patients, and no difference in the severity of COPD, as indicated by FEV1, that might influence prescription rates.
The authors attempted to evaluate health care resource utilization as an indicator of health care quality and outcomes. Based on previous reports, the authors expected to find lower quality of care and increased utilization in rural patients. Previous studies have shown rural patients can be more symptomatic with a higher body mass index, airflow obstruction, dyspnea, and exercise capacity index (BODE index) than are patients in urban settings.16,30 Statewide and national registry data have shown rural patients have higher rates of primary care visits, ER visits, and hospitalizations for COPD exacerbations.
Rural patients also have been shown to have higher mortality rates and were more likely to be in a long-term care center and less likely to have home care or palliative care than were their urban counterparts.13-18,31 If the severity of illness is similar in rural and urban areas, higher health care utilization related to COPD would suggest that patients in rural settings may be receiving inferior quality of health care. The authors could not find any previous reports of the quality of COPD care delivered in rural vs urban settings.
In this study the only difference in quality of care was the lower proportion of rural patients with a record of spirometry that is needed to confirm the diagnosis. The observed differences in the quality of care measures wouldn’t be expected to lead to large differences in the outcome measures. Contrary to the literature and the observed similarity in quality of care, rural patients had better COPD outcomes perhaps due to unmeasured differences in risk or failure to capture medical visits outside of the VA system. The severity of COPD based on FEV1 and concurrent diagnoses, such as heart failure, did not suggest that rural patients in this comparison had a higher burden of illness or risk of poor COPD outcomes.
More than 70,000 patients spanning a large geographic region receive primary care at MVAHCS, which provides comparable care to all COPD patients, regardless of location, by using the same EMR system, providing evidence-based order sets for disease management, proactively offering on-site and remote COPD case managers for high-risk patients, and more recently, implementing on-site spirometry testing in all clinics. This approach as opposed to the traditional outreach clinic model may in part explain the similarity in quality of care in urban and rural clinics that was not reported in previous studies.
Limitations
This study was performed retrospectively, increasing the potential of missing data, especially from outside the VA health care system. Patients were not randomly assigned to rural or urban clinics, so differences in patient characteristics could exist. Alcohol and addictive disorders were more common in urban patients, which might affect adherence to prescribed medications. In addition, lower rate of obstructive sleep apnea was found in the rural population, which has been linked to increased airway inflammation and COPD exacerbations resulting in hospitalization.32,33 Mortality was not assessed as all patients were alive and seen in clinic at time of enrollment.
The authors were not able to record a patients’ residence in a long-term care facility or institutionalization, use of home care, or palliative care services due to limitations in the EMR system. Whether patients received comanaged primary care or underwent pulmonary rehabilitation could not be obtained from the EMR. Most patients never had lung volumes or diffusion capacity and thus were not included. The authors could not report whether inhaler therapy was appropriate compared with the Global Initiative for Chronic Obstructive Lund Disease(GOLD)severity score because most of the spirometry was done at a discordant time to when inhaler therapy was assessed, and new GOLD guidelines include patient symptoms that were not reliably recorded in the EMR. Last, the authors had hoped to include smoking status and cessation practices as part of the quality measures, but due to significant variability in patients’ documented smoking status in the same period, the data were deemed unreliable.
Conclusion
No disparities were found between rural and urban clinics in the quality of health care for patients with COPD in the MVAHCS except that fewer rural patients had prior spirometry; a difference that is likely due to the fact that only recently has spirometry been implemented in the MVAHCS rural clinics. Overall the quality of COPD care was high and above the previously reported rates. Further larger studies of rural and urban quality of health care for patients with COPD are needed in other VA and non-VA systems to determine whether disparities exist and whether they are associated with clinical outcomes, including ED visits, hospitalizations, and mortality.
1. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014(260):1-161.
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256-1276.
5. Ghattas C, Dai A, Gemmel DJ, Awad MH. Over diagnosis of chronic obstructive pulmonary disease in an underserved patient population. Int J Chron Obstruct Pulmon Dis. 2013;8:545-549.
6. Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust. 2011;195(4):168-171.
7. Lopez-Campos JL, Abad Arranz M, Calero-Acuña C, et al. Guideline adherence in outpatient clinics for chronic obstructive pulmonary disease: results from a clinical audit. PLoS One. 2016;11(3):e0151896.
8. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645.
9. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: U.S. Department of Veteran Affairs; 2011.
10. Weeks WB, Wallace AE, Wang S, Lee A, Kazis LE. Rural-urban disparities in health-related quality of life within disease categories of Veterans. J Rural Health. 2006;22(3):204-211.
11. Wallace AE, Weeks WB, Wang S, Lee AF, Kazis LE. Rural and urban disparities in health-related quality of life among veterans with psychiatric disorders. Psychiatr Serv. 2006;57(6):851-856.
12. Meit M, Knudson A, Gilbert T, et al; Rural Health Reform Policy Research Center. The 2014 update of the rural-urban chartbook. https://ruralhealth.und .edu/projects/health-reform-policy-research-center/pdf/2014-rural-urban-chartbook-update.pdf. Published October 2014. Accessed April 18, 2017.
13. Jackson BE, Suzuki S, Coultas D, et al. Safety-net facilities and hospitalization rates of chronic obstructive pulmonary disease: a cross-sectional analysis of the 2007 Texas Health Care Information Council inpatient data. Int J Chron Obstruct Pulmon Dis. 2011;6:563-571.
14. Jackson BE, Suzuki S, Lo K, et al. Geographic disparity in COPD hospitalization rates among the Texas population. Respir Med. 2011;105(5):734-739.15. Skinner HG, Blanchard J, Elixhauser A. Trends in emergency department visits, 2006-2011: statistical brief #179. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb179-Emergency-Department-Trends.pdf. Published September 2014. Accessed May 9, 2017.
16. Jackson BE, Coultas DB, Suzuki S, Singh KP, Bae S. Rural-urban disparities in quality of life among patients with COPD. J Rural Health. 2013;29(suppl 1):62S-69S.
17. Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am J Prev Med. 2014;46(2):e19-e29.
18. Abrams TE, Vaughan-Sarrazin M, Fan VS, Kaboli PJ. Geographic isolation and the risk for chronic obstructive pulmonary disease-related mortality: a cohort study. Ann Intern Med. 2011;155(2):80-86.
19. Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149-1155.
20. U.S. Department of Agriculture, Economic Research Service. Rural-urban commuting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx#U20K1F50H0A. Updated October 12, 2016. Accessed May 9, 2017.
21. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134(1):38-45.
22. Damarla M, Celli BR, Mullerova HX, Pinto-Plata VM. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51(10):1120-1124.
23. Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015-16 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(30):818-825.
24. Kim DK, Bridges CB, Harriman KH; Advisory Committee on Immunization Practices. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older: United States, 2016. Ann Intern Med. 2016;164(3):184-194.
25. Cimen P, Unlu M, Kirakli C, et al. Should patients with COPD be vaccinated? Respir Care. 2015;60(2):239-243.
26. Mowls DS, Cheruvu VK, Zullo MD. Influenza vaccination in adults with chronic obstructive pulmonary disease: the impact of a diagnostic breathing test on vaccination rates PLoS One. 2013;8(6):e67600.
27. Shoup JA, Madrid C, Koehler C, et al. Effectiveness and cost of influenza vaccine reminders for adults with asthma or chronic obstructive pulmonary disease. Am J Manag Care. 2015;21(7):e405-e413.
28. Arinez-Fernandez MC, Carrasco-Garrido P, Garcia-Carballo M, Hernandez-Barrera V, de Miguel AG, Jimenez-Garcia R. Determinants of pneumococcal vaccination among patients with chronic obstructive pulmonary disease in Spain. Hum Vaccin. 2006;2(3):99-104.29. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
30. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012.
31. Goodridge D, Lawson J, Rennie D, Marciniuk D. Rural/urban differences in health care utilization and place of death for persons with respiratory illness in the last year of life. Rural Remote Health. 2010;10(2):1349.
32. Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123-1130.
33. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325-331
Chronic obstructive pulmonary disease (COPD) affects between 11 and 24 million people in the U.S. and is the third leading cause of death in this country.1,2 Airflow obstruction on spirometry in addition to respiratory symptoms is required to establish a diagnosis of COPD.3,4 As many as 40% of patients with a clinical diagnosis of COPD have not had spirometry or have spirometry results inconsistent with the diagnosis of COPD.5,6 In addition to recommended spirometry, many patients with COPD do not receive other evidence-based therapies.7,8
About 50% of patients in the Minneapolis VA Health Care System (MVAHCS) receive care in its rural community-based outreach clinics (CBOCs). Data regarding the quality of general medical care between rural and urban populations are sparse; however, studies suggest that the quality of care delivered in rural clinics may be lower than the care provided in an urban setting.9-12 Care for patients with COPD in an urban setting is suboptimal with only 58% of patients receiving guideline-based care, and there are no comparative data for penetrance in the rural setting.8 Most published studies on patients with COPD treated in rural vs urban locations are outcomes studies that queried statewide or national registry data evaluating the frequency of emergency department (ED) visits or hospital admissions for COPD exacerbations, all-cause mortality, or COPD exacerbation-related mortality.13-18 There are no studies examining potential differences in the quality of health care received by patients with COPD in rural vs urban locations or whether these potential differences are associated with changes in health care utilization.
The authors sought to determine whether patients with the diagnosis of COPD treated in the MVAHCS and its 13 CBOCs receive similar quality of disease-related health care in rural vs urban primary care clinic locations. The authors hypothesized that patients who receive their primary care in rural clinics would be less likely to have had spirometry or to receive respiratory immunizations and short- or long-acting inhalers and that discrepancies would be associated with increased health care utilization in rural areas as measured by prescriptions for systemic corticosteroids, antibiotics, ED visits, or hospital admissions for COPD exacerbations.
Methods
The MVAHCS has 14 primary care locations; these locations were designated as rural or urban based on the Rural-Urban Commuting Area codes.19,20 There were 4 urban locations and 10 rural clinics; all rural clinics were farther than 40 miles from the main Minneapolis VAMC.
Patient Selection
The authors performed a retrospective chart review after receiving an institutional review board waiver for this quality assessment study. All patients who had a prior ICD-9 encounter diagnosis of COPD (codes: 491.0, 491.1, 491.2, 491.20, 491.21, 491.22, 491.8, 491.9, 492.0, 492.8, 494, 494.0, 494.1, 496) and who were seen in primary care during March 2015 were identified. Each subject’s first visit during that month was used as the start of the retrospective 1-year look-back period. All eligible subjects were sorted based on their rural or urban location and a randomly assigned number. Patients were then selected according to ascending numbers from each rural and urban clinic in proportion to the clinic’s representation among all eligible patients.
Outcomes
The primary outcomes—possible discrepancies in quality of health care for patients with COPD in rural vs urban primary care clinics—were assessed by (1) prior spirometry; (2) any prior pneumonia vaccination; (3) an influenza vaccination within the past year; (4) prescriptions within the past year for a short-acting beta agonist (SABA) metered-dose inhaler; and (5) prescriptions for a long-acting inhalers, including long-acting beta agonists (LABAs), long-acting muscarinic antagonists (LAMAs), or inhaled corticosteroids (ICSs).
Secondary outcomes included (1) an active prescription for home oxygen within the past calendar year; (2) health care utilization assessed via prescriptions for intermittent courses of oral corticosteroids; (3) prescriptions for respiratory antibiotics (macrolides, tetracyclines, fluoroquinolones) within the past year for COPD exacerbations; (4) ED visits; (5) hospital admissions (and need for mechanical ventilation) for COPD exacerbations within the past year; and (6) whether patients were seen by either VA or Non-VA pulmonology providers.
Data Collection
Patients’ demographic data and comorbidities were collected via chart review. A 1-year prescription medication list was obtained by an electronic database search of the MVAHCS electronic medical record (EMR). Additional antibiotics and corticosteroid prescriptions for COPD exacerbations paid for by the VA but filled at a local pharmacy were manually searched from a separate database to supplement the electronic prescription list. Comparison of the electronic prescription list and pharmacy records in 25% of patients found 100% concordance in the prescription lists. The investigator manually reviewed and extracted the following data from the EMR, scanned-in records, and a Midwest VA COPD registry database: most recent spirometry results; immunization status for influenza in the past year; prior pneumonia vaccination; home oxygen prescription; whether the patient received respiratory antibiotic or intermittent oral corticosteroid treatment for COPD exacerbations; whether the patient had a ED visit or hospital admission for COPD exacerbation with or without need for mechanical ventilation; and whether the patient had been seen by a pulmonology provider. The investigator reviewed all primary care provider notes in the past year for documentation of non-VA ED visits or hospitalizations that were not present in the EMR, Midwest VA COPD registry database, or scanned patient records.
Data Analysis
Results are described as mean ± standard deviation, median (interquartile range) or proportion, expressed as a percentage as appropriate for the level of measurement and distribution. The proportions meeting the COPD quality of health care outcomes in the urban and rural groups were compared using a chi-square test of proportions, and 95% confidence intervals (CI) on the differences were estimated. Samples of 400 patients each from the rural and urban groups were estimated to provide a 95% 2-sided CI on the differences of about ± 0.05 (5%), assuming the proportion meeting the quality of care outcomes in the urban group would be at least 0.8 (80%).
Results
The authors identified 1,538 patients with a previous encounter diagnosis of COPD who were seen in a primary care clinic in the MVAHCS in March of 2015. The authors reviewed the medical records of 801 randomly selected patients: 400 rural clinic patients and 401 urban clinic patients. Demographic characteristics and major comorbidities of rural and urban patients were similar except more rural patients were white, and fewer had a record of obstructive sleep apnea, alcoholism, or addictive disorders (Table 1). Prescriptions for common chronic medical conditions were similar for rural and urban groups, including medications for depression (31% vs 33%) or diabetes mellitus (25% vs 28%). In patients who had spirometry, the severity of COPD, as assessed by mean forced expiratory volume (FEV1), was similar between rural and urban patients (2.06 L vs 2.10 L).
Quality of COPD Care
Spirometry was documented in fewer rural clinic patients than in urban clinic patients (51% vs 82%; difference 31%, 95% CI: 25% to 37%) (Table 2).
COPD Outcomes
Home oxygen prescription rates were similar for rural and urban clinic patients (8% vs 9%; difference 1%, 95% CI: -2% to 5%). Rural patients received fewer prescriptions for intermittent oral corticosteroids (17% vs 31%; difference 14%, 95% CI: 9% to 20%) and antibiotics for COPD exacerbations (18% vs 32%; difference 14%, 95% CI: 8% to 20%). Rural patients had fewer ED visits for COPD exacerbations (7% vs 13%; difference 6%, 95% CI: 2% to 10%), and similar admission rates for COPD exacerbations (5% vs 6%; difference 1%, 95% CI: -2% to 4%). Of the few patients hospitalized for COPD exacerbations, none required mechanical ventilation. There was no significant difference in the number of rural vs urban patients seen by a pulmonologist in the calendar year of the study (6% vs 9%; difference 3%, 95% CI:-1% to 6%), with the majority seen by VA providers: 20/24 rural patients and 35/35 urban patients.
Discussion
Fewer rural patients had prior spirometry; otherwise, the COPD-related quality metrics were similar between rural and urban patient groups in the MVAHCS, including immunizations for pneumonia and influenza, and prescribing rates for short- and long-acting inhaler therapy. Despite the similarity in these COPD quality measures, rural clinic patients seemed to have less health care utilization related to COPD exacerbations.
Spirometry with airflow obstruction in the presence of respiratory symptoms is required for accurate diagnosis of COPD.3,4 Spirometry has been available at the MVAHCS hospital-based clinic for years. Efforts to address this disparity led to implementation of on-site spirometry at all rural and urban clinics about 2 years prior to the patient enrollment visit date for the study. Fewer rural patients had spirometry, which is possibly from prior disparity in resources; yet rates of spirometry in all patients with a clinical diagnosis of COPD in the MVAHCS are higher (rural 51%, urban 82%) than previously reported. A nationwide study of 94,000 veterans with recent clinical diagnosis of COPD found only 37% had spirometry within 2.5 years of diagnosis,21 and another non-VA study (n = 553) showed only 31% of patients discharged from a hospital with a diagnosis of COPD exacerbation had spirometry performed within a 8-year period prior to hospitalization.22
Annual influenza vaccines are recommended for everyone aged > 6 months, and the pneumonia vaccine is recommended for all patients with COPD in order to reduce the risk of COPD exacerbations and pneumonias.23,24 The rates of vaccination at MVAHCS rural and urban clinics for both influenza (79% vs 81%) and pneumococcus (88% vs 91%) are higher than previously published studies of patients with COPD for influenza vaccination (30%-51%) and pneumonia vaccination (21%-51%) and did not differ between rural and urban clinics.7,25-28 The observed high vaccination rates may be due to EMR prompts and requirements to document vaccination status and offer recommended vaccinations.
Long-acting inhalers have been shown to reduce rates of COPD exacerbations and improve patients’ quality of life.29 The authors found no disparity in the prescription rate of short- or long-acting inhalers between rural and urban patients, and no difference in the severity of COPD, as indicated by FEV1, that might influence prescription rates.
The authors attempted to evaluate health care resource utilization as an indicator of health care quality and outcomes. Based on previous reports, the authors expected to find lower quality of care and increased utilization in rural patients. Previous studies have shown rural patients can be more symptomatic with a higher body mass index, airflow obstruction, dyspnea, and exercise capacity index (BODE index) than are patients in urban settings.16,30 Statewide and national registry data have shown rural patients have higher rates of primary care visits, ER visits, and hospitalizations for COPD exacerbations.
Rural patients also have been shown to have higher mortality rates and were more likely to be in a long-term care center and less likely to have home care or palliative care than were their urban counterparts.13-18,31 If the severity of illness is similar in rural and urban areas, higher health care utilization related to COPD would suggest that patients in rural settings may be receiving inferior quality of health care. The authors could not find any previous reports of the quality of COPD care delivered in rural vs urban settings.
In this study the only difference in quality of care was the lower proportion of rural patients with a record of spirometry that is needed to confirm the diagnosis. The observed differences in the quality of care measures wouldn’t be expected to lead to large differences in the outcome measures. Contrary to the literature and the observed similarity in quality of care, rural patients had better COPD outcomes perhaps due to unmeasured differences in risk or failure to capture medical visits outside of the VA system. The severity of COPD based on FEV1 and concurrent diagnoses, such as heart failure, did not suggest that rural patients in this comparison had a higher burden of illness or risk of poor COPD outcomes.
More than 70,000 patients spanning a large geographic region receive primary care at MVAHCS, which provides comparable care to all COPD patients, regardless of location, by using the same EMR system, providing evidence-based order sets for disease management, proactively offering on-site and remote COPD case managers for high-risk patients, and more recently, implementing on-site spirometry testing in all clinics. This approach as opposed to the traditional outreach clinic model may in part explain the similarity in quality of care in urban and rural clinics that was not reported in previous studies.
Limitations
This study was performed retrospectively, increasing the potential of missing data, especially from outside the VA health care system. Patients were not randomly assigned to rural or urban clinics, so differences in patient characteristics could exist. Alcohol and addictive disorders were more common in urban patients, which might affect adherence to prescribed medications. In addition, lower rate of obstructive sleep apnea was found in the rural population, which has been linked to increased airway inflammation and COPD exacerbations resulting in hospitalization.32,33 Mortality was not assessed as all patients were alive and seen in clinic at time of enrollment.
The authors were not able to record a patients’ residence in a long-term care facility or institutionalization, use of home care, or palliative care services due to limitations in the EMR system. Whether patients received comanaged primary care or underwent pulmonary rehabilitation could not be obtained from the EMR. Most patients never had lung volumes or diffusion capacity and thus were not included. The authors could not report whether inhaler therapy was appropriate compared with the Global Initiative for Chronic Obstructive Lund Disease(GOLD)severity score because most of the spirometry was done at a discordant time to when inhaler therapy was assessed, and new GOLD guidelines include patient symptoms that were not reliably recorded in the EMR. Last, the authors had hoped to include smoking status and cessation practices as part of the quality measures, but due to significant variability in patients’ documented smoking status in the same period, the data were deemed unreliable.
Conclusion
No disparities were found between rural and urban clinics in the quality of health care for patients with COPD in the MVAHCS except that fewer rural patients had prior spirometry; a difference that is likely due to the fact that only recently has spirometry been implemented in the MVAHCS rural clinics. Overall the quality of COPD care was high and above the previously reported rates. Further larger studies of rural and urban quality of health care for patients with COPD are needed in other VA and non-VA systems to determine whether disparities exist and whether they are associated with clinical outcomes, including ED visits, hospitalizations, and mortality.
Chronic obstructive pulmonary disease (COPD) affects between 11 and 24 million people in the U.S. and is the third leading cause of death in this country.1,2 Airflow obstruction on spirometry in addition to respiratory symptoms is required to establish a diagnosis of COPD.3,4 As many as 40% of patients with a clinical diagnosis of COPD have not had spirometry or have spirometry results inconsistent with the diagnosis of COPD.5,6 In addition to recommended spirometry, many patients with COPD do not receive other evidence-based therapies.7,8
About 50% of patients in the Minneapolis VA Health Care System (MVAHCS) receive care in its rural community-based outreach clinics (CBOCs). Data regarding the quality of general medical care between rural and urban populations are sparse; however, studies suggest that the quality of care delivered in rural clinics may be lower than the care provided in an urban setting.9-12 Care for patients with COPD in an urban setting is suboptimal with only 58% of patients receiving guideline-based care, and there are no comparative data for penetrance in the rural setting.8 Most published studies on patients with COPD treated in rural vs urban locations are outcomes studies that queried statewide or national registry data evaluating the frequency of emergency department (ED) visits or hospital admissions for COPD exacerbations, all-cause mortality, or COPD exacerbation-related mortality.13-18 There are no studies examining potential differences in the quality of health care received by patients with COPD in rural vs urban locations or whether these potential differences are associated with changes in health care utilization.
The authors sought to determine whether patients with the diagnosis of COPD treated in the MVAHCS and its 13 CBOCs receive similar quality of disease-related health care in rural vs urban primary care clinic locations. The authors hypothesized that patients who receive their primary care in rural clinics would be less likely to have had spirometry or to receive respiratory immunizations and short- or long-acting inhalers and that discrepancies would be associated with increased health care utilization in rural areas as measured by prescriptions for systemic corticosteroids, antibiotics, ED visits, or hospital admissions for COPD exacerbations.
Methods
The MVAHCS has 14 primary care locations; these locations were designated as rural or urban based on the Rural-Urban Commuting Area codes.19,20 There were 4 urban locations and 10 rural clinics; all rural clinics were farther than 40 miles from the main Minneapolis VAMC.
Patient Selection
The authors performed a retrospective chart review after receiving an institutional review board waiver for this quality assessment study. All patients who had a prior ICD-9 encounter diagnosis of COPD (codes: 491.0, 491.1, 491.2, 491.20, 491.21, 491.22, 491.8, 491.9, 492.0, 492.8, 494, 494.0, 494.1, 496) and who were seen in primary care during March 2015 were identified. Each subject’s first visit during that month was used as the start of the retrospective 1-year look-back period. All eligible subjects were sorted based on their rural or urban location and a randomly assigned number. Patients were then selected according to ascending numbers from each rural and urban clinic in proportion to the clinic’s representation among all eligible patients.
Outcomes
The primary outcomes—possible discrepancies in quality of health care for patients with COPD in rural vs urban primary care clinics—were assessed by (1) prior spirometry; (2) any prior pneumonia vaccination; (3) an influenza vaccination within the past year; (4) prescriptions within the past year for a short-acting beta agonist (SABA) metered-dose inhaler; and (5) prescriptions for a long-acting inhalers, including long-acting beta agonists (LABAs), long-acting muscarinic antagonists (LAMAs), or inhaled corticosteroids (ICSs).
Secondary outcomes included (1) an active prescription for home oxygen within the past calendar year; (2) health care utilization assessed via prescriptions for intermittent courses of oral corticosteroids; (3) prescriptions for respiratory antibiotics (macrolides, tetracyclines, fluoroquinolones) within the past year for COPD exacerbations; (4) ED visits; (5) hospital admissions (and need for mechanical ventilation) for COPD exacerbations within the past year; and (6) whether patients were seen by either VA or Non-VA pulmonology providers.
Data Collection
Patients’ demographic data and comorbidities were collected via chart review. A 1-year prescription medication list was obtained by an electronic database search of the MVAHCS electronic medical record (EMR). Additional antibiotics and corticosteroid prescriptions for COPD exacerbations paid for by the VA but filled at a local pharmacy were manually searched from a separate database to supplement the electronic prescription list. Comparison of the electronic prescription list and pharmacy records in 25% of patients found 100% concordance in the prescription lists. The investigator manually reviewed and extracted the following data from the EMR, scanned-in records, and a Midwest VA COPD registry database: most recent spirometry results; immunization status for influenza in the past year; prior pneumonia vaccination; home oxygen prescription; whether the patient received respiratory antibiotic or intermittent oral corticosteroid treatment for COPD exacerbations; whether the patient had a ED visit or hospital admission for COPD exacerbation with or without need for mechanical ventilation; and whether the patient had been seen by a pulmonology provider. The investigator reviewed all primary care provider notes in the past year for documentation of non-VA ED visits or hospitalizations that were not present in the EMR, Midwest VA COPD registry database, or scanned patient records.
Data Analysis
Results are described as mean ± standard deviation, median (interquartile range) or proportion, expressed as a percentage as appropriate for the level of measurement and distribution. The proportions meeting the COPD quality of health care outcomes in the urban and rural groups were compared using a chi-square test of proportions, and 95% confidence intervals (CI) on the differences were estimated. Samples of 400 patients each from the rural and urban groups were estimated to provide a 95% 2-sided CI on the differences of about ± 0.05 (5%), assuming the proportion meeting the quality of care outcomes in the urban group would be at least 0.8 (80%).
Results
The authors identified 1,538 patients with a previous encounter diagnosis of COPD who were seen in a primary care clinic in the MVAHCS in March of 2015. The authors reviewed the medical records of 801 randomly selected patients: 400 rural clinic patients and 401 urban clinic patients. Demographic characteristics and major comorbidities of rural and urban patients were similar except more rural patients were white, and fewer had a record of obstructive sleep apnea, alcoholism, or addictive disorders (Table 1). Prescriptions for common chronic medical conditions were similar for rural and urban groups, including medications for depression (31% vs 33%) or diabetes mellitus (25% vs 28%). In patients who had spirometry, the severity of COPD, as assessed by mean forced expiratory volume (FEV1), was similar between rural and urban patients (2.06 L vs 2.10 L).
Quality of COPD Care
Spirometry was documented in fewer rural clinic patients than in urban clinic patients (51% vs 82%; difference 31%, 95% CI: 25% to 37%) (Table 2).
COPD Outcomes
Home oxygen prescription rates were similar for rural and urban clinic patients (8% vs 9%; difference 1%, 95% CI: -2% to 5%). Rural patients received fewer prescriptions for intermittent oral corticosteroids (17% vs 31%; difference 14%, 95% CI: 9% to 20%) and antibiotics for COPD exacerbations (18% vs 32%; difference 14%, 95% CI: 8% to 20%). Rural patients had fewer ED visits for COPD exacerbations (7% vs 13%; difference 6%, 95% CI: 2% to 10%), and similar admission rates for COPD exacerbations (5% vs 6%; difference 1%, 95% CI: -2% to 4%). Of the few patients hospitalized for COPD exacerbations, none required mechanical ventilation. There was no significant difference in the number of rural vs urban patients seen by a pulmonologist in the calendar year of the study (6% vs 9%; difference 3%, 95% CI:-1% to 6%), with the majority seen by VA providers: 20/24 rural patients and 35/35 urban patients.
Discussion
Fewer rural patients had prior spirometry; otherwise, the COPD-related quality metrics were similar between rural and urban patient groups in the MVAHCS, including immunizations for pneumonia and influenza, and prescribing rates for short- and long-acting inhaler therapy. Despite the similarity in these COPD quality measures, rural clinic patients seemed to have less health care utilization related to COPD exacerbations.
Spirometry with airflow obstruction in the presence of respiratory symptoms is required for accurate diagnosis of COPD.3,4 Spirometry has been available at the MVAHCS hospital-based clinic for years. Efforts to address this disparity led to implementation of on-site spirometry at all rural and urban clinics about 2 years prior to the patient enrollment visit date for the study. Fewer rural patients had spirometry, which is possibly from prior disparity in resources; yet rates of spirometry in all patients with a clinical diagnosis of COPD in the MVAHCS are higher (rural 51%, urban 82%) than previously reported. A nationwide study of 94,000 veterans with recent clinical diagnosis of COPD found only 37% had spirometry within 2.5 years of diagnosis,21 and another non-VA study (n = 553) showed only 31% of patients discharged from a hospital with a diagnosis of COPD exacerbation had spirometry performed within a 8-year period prior to hospitalization.22
Annual influenza vaccines are recommended for everyone aged > 6 months, and the pneumonia vaccine is recommended for all patients with COPD in order to reduce the risk of COPD exacerbations and pneumonias.23,24 The rates of vaccination at MVAHCS rural and urban clinics for both influenza (79% vs 81%) and pneumococcus (88% vs 91%) are higher than previously published studies of patients with COPD for influenza vaccination (30%-51%) and pneumonia vaccination (21%-51%) and did not differ between rural and urban clinics.7,25-28 The observed high vaccination rates may be due to EMR prompts and requirements to document vaccination status and offer recommended vaccinations.
Long-acting inhalers have been shown to reduce rates of COPD exacerbations and improve patients’ quality of life.29 The authors found no disparity in the prescription rate of short- or long-acting inhalers between rural and urban patients, and no difference in the severity of COPD, as indicated by FEV1, that might influence prescription rates.
The authors attempted to evaluate health care resource utilization as an indicator of health care quality and outcomes. Based on previous reports, the authors expected to find lower quality of care and increased utilization in rural patients. Previous studies have shown rural patients can be more symptomatic with a higher body mass index, airflow obstruction, dyspnea, and exercise capacity index (BODE index) than are patients in urban settings.16,30 Statewide and national registry data have shown rural patients have higher rates of primary care visits, ER visits, and hospitalizations for COPD exacerbations.
Rural patients also have been shown to have higher mortality rates and were more likely to be in a long-term care center and less likely to have home care or palliative care than were their urban counterparts.13-18,31 If the severity of illness is similar in rural and urban areas, higher health care utilization related to COPD would suggest that patients in rural settings may be receiving inferior quality of health care. The authors could not find any previous reports of the quality of COPD care delivered in rural vs urban settings.
In this study the only difference in quality of care was the lower proportion of rural patients with a record of spirometry that is needed to confirm the diagnosis. The observed differences in the quality of care measures wouldn’t be expected to lead to large differences in the outcome measures. Contrary to the literature and the observed similarity in quality of care, rural patients had better COPD outcomes perhaps due to unmeasured differences in risk or failure to capture medical visits outside of the VA system. The severity of COPD based on FEV1 and concurrent diagnoses, such as heart failure, did not suggest that rural patients in this comparison had a higher burden of illness or risk of poor COPD outcomes.
More than 70,000 patients spanning a large geographic region receive primary care at MVAHCS, which provides comparable care to all COPD patients, regardless of location, by using the same EMR system, providing evidence-based order sets for disease management, proactively offering on-site and remote COPD case managers for high-risk patients, and more recently, implementing on-site spirometry testing in all clinics. This approach as opposed to the traditional outreach clinic model may in part explain the similarity in quality of care in urban and rural clinics that was not reported in previous studies.
Limitations
This study was performed retrospectively, increasing the potential of missing data, especially from outside the VA health care system. Patients were not randomly assigned to rural or urban clinics, so differences in patient characteristics could exist. Alcohol and addictive disorders were more common in urban patients, which might affect adherence to prescribed medications. In addition, lower rate of obstructive sleep apnea was found in the rural population, which has been linked to increased airway inflammation and COPD exacerbations resulting in hospitalization.32,33 Mortality was not assessed as all patients were alive and seen in clinic at time of enrollment.
The authors were not able to record a patients’ residence in a long-term care facility or institutionalization, use of home care, or palliative care services due to limitations in the EMR system. Whether patients received comanaged primary care or underwent pulmonary rehabilitation could not be obtained from the EMR. Most patients never had lung volumes or diffusion capacity and thus were not included. The authors could not report whether inhaler therapy was appropriate compared with the Global Initiative for Chronic Obstructive Lund Disease(GOLD)severity score because most of the spirometry was done at a discordant time to when inhaler therapy was assessed, and new GOLD guidelines include patient symptoms that were not reliably recorded in the EMR. Last, the authors had hoped to include smoking status and cessation practices as part of the quality measures, but due to significant variability in patients’ documented smoking status in the same period, the data were deemed unreliable.
Conclusion
No disparities were found between rural and urban clinics in the quality of health care for patients with COPD in the MVAHCS except that fewer rural patients had prior spirometry; a difference that is likely due to the fact that only recently has spirometry been implemented in the MVAHCS rural clinics. Overall the quality of COPD care was high and above the previously reported rates. Further larger studies of rural and urban quality of health care for patients with COPD are needed in other VA and non-VA systems to determine whether disparities exist and whether they are associated with clinical outcomes, including ED visits, hospitalizations, and mortality.
1. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014(260):1-161.
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256-1276.
5. Ghattas C, Dai A, Gemmel DJ, Awad MH. Over diagnosis of chronic obstructive pulmonary disease in an underserved patient population. Int J Chron Obstruct Pulmon Dis. 2013;8:545-549.
6. Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust. 2011;195(4):168-171.
7. Lopez-Campos JL, Abad Arranz M, Calero-Acuña C, et al. Guideline adherence in outpatient clinics for chronic obstructive pulmonary disease: results from a clinical audit. PLoS One. 2016;11(3):e0151896.
8. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645.
9. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: U.S. Department of Veteran Affairs; 2011.
10. Weeks WB, Wallace AE, Wang S, Lee A, Kazis LE. Rural-urban disparities in health-related quality of life within disease categories of Veterans. J Rural Health. 2006;22(3):204-211.
11. Wallace AE, Weeks WB, Wang S, Lee AF, Kazis LE. Rural and urban disparities in health-related quality of life among veterans with psychiatric disorders. Psychiatr Serv. 2006;57(6):851-856.
12. Meit M, Knudson A, Gilbert T, et al; Rural Health Reform Policy Research Center. The 2014 update of the rural-urban chartbook. https://ruralhealth.und .edu/projects/health-reform-policy-research-center/pdf/2014-rural-urban-chartbook-update.pdf. Published October 2014. Accessed April 18, 2017.
13. Jackson BE, Suzuki S, Coultas D, et al. Safety-net facilities and hospitalization rates of chronic obstructive pulmonary disease: a cross-sectional analysis of the 2007 Texas Health Care Information Council inpatient data. Int J Chron Obstruct Pulmon Dis. 2011;6:563-571.
14. Jackson BE, Suzuki S, Lo K, et al. Geographic disparity in COPD hospitalization rates among the Texas population. Respir Med. 2011;105(5):734-739.15. Skinner HG, Blanchard J, Elixhauser A. Trends in emergency department visits, 2006-2011: statistical brief #179. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb179-Emergency-Department-Trends.pdf. Published September 2014. Accessed May 9, 2017.
16. Jackson BE, Coultas DB, Suzuki S, Singh KP, Bae S. Rural-urban disparities in quality of life among patients with COPD. J Rural Health. 2013;29(suppl 1):62S-69S.
17. Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am J Prev Med. 2014;46(2):e19-e29.
18. Abrams TE, Vaughan-Sarrazin M, Fan VS, Kaboli PJ. Geographic isolation and the risk for chronic obstructive pulmonary disease-related mortality: a cohort study. Ann Intern Med. 2011;155(2):80-86.
19. Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149-1155.
20. U.S. Department of Agriculture, Economic Research Service. Rural-urban commuting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx#U20K1F50H0A. Updated October 12, 2016. Accessed May 9, 2017.
21. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134(1):38-45.
22. Damarla M, Celli BR, Mullerova HX, Pinto-Plata VM. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51(10):1120-1124.
23. Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015-16 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(30):818-825.
24. Kim DK, Bridges CB, Harriman KH; Advisory Committee on Immunization Practices. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older: United States, 2016. Ann Intern Med. 2016;164(3):184-194.
25. Cimen P, Unlu M, Kirakli C, et al. Should patients with COPD be vaccinated? Respir Care. 2015;60(2):239-243.
26. Mowls DS, Cheruvu VK, Zullo MD. Influenza vaccination in adults with chronic obstructive pulmonary disease: the impact of a diagnostic breathing test on vaccination rates PLoS One. 2013;8(6):e67600.
27. Shoup JA, Madrid C, Koehler C, et al. Effectiveness and cost of influenza vaccine reminders for adults with asthma or chronic obstructive pulmonary disease. Am J Manag Care. 2015;21(7):e405-e413.
28. Arinez-Fernandez MC, Carrasco-Garrido P, Garcia-Carballo M, Hernandez-Barrera V, de Miguel AG, Jimenez-Garcia R. Determinants of pneumococcal vaccination among patients with chronic obstructive pulmonary disease in Spain. Hum Vaccin. 2006;2(3):99-104.29. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
30. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012.
31. Goodridge D, Lawson J, Rennie D, Marciniuk D. Rural/urban differences in health care utilization and place of death for persons with respiratory illness in the last year of life. Rural Remote Health. 2010;10(2):1349.
32. Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123-1130.
33. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325-331
1. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014(260):1-161.
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256-1276.
5. Ghattas C, Dai A, Gemmel DJ, Awad MH. Over diagnosis of chronic obstructive pulmonary disease in an underserved patient population. Int J Chron Obstruct Pulmon Dis. 2013;8:545-549.
6. Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust. 2011;195(4):168-171.
7. Lopez-Campos JL, Abad Arranz M, Calero-Acuña C, et al. Guideline adherence in outpatient clinics for chronic obstructive pulmonary disease: results from a clinical audit. PLoS One. 2016;11(3):e0151896.
8. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645.
9. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: U.S. Department of Veteran Affairs; 2011.
10. Weeks WB, Wallace AE, Wang S, Lee A, Kazis LE. Rural-urban disparities in health-related quality of life within disease categories of Veterans. J Rural Health. 2006;22(3):204-211.
11. Wallace AE, Weeks WB, Wang S, Lee AF, Kazis LE. Rural and urban disparities in health-related quality of life among veterans with psychiatric disorders. Psychiatr Serv. 2006;57(6):851-856.
12. Meit M, Knudson A, Gilbert T, et al; Rural Health Reform Policy Research Center. The 2014 update of the rural-urban chartbook. https://ruralhealth.und .edu/projects/health-reform-policy-research-center/pdf/2014-rural-urban-chartbook-update.pdf. Published October 2014. Accessed April 18, 2017.
13. Jackson BE, Suzuki S, Coultas D, et al. Safety-net facilities and hospitalization rates of chronic obstructive pulmonary disease: a cross-sectional analysis of the 2007 Texas Health Care Information Council inpatient data. Int J Chron Obstruct Pulmon Dis. 2011;6:563-571.
14. Jackson BE, Suzuki S, Lo K, et al. Geographic disparity in COPD hospitalization rates among the Texas population. Respir Med. 2011;105(5):734-739.15. Skinner HG, Blanchard J, Elixhauser A. Trends in emergency department visits, 2006-2011: statistical brief #179. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb179-Emergency-Department-Trends.pdf. Published September 2014. Accessed May 9, 2017.
16. Jackson BE, Coultas DB, Suzuki S, Singh KP, Bae S. Rural-urban disparities in quality of life among patients with COPD. J Rural Health. 2013;29(suppl 1):62S-69S.
17. Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am J Prev Med. 2014;46(2):e19-e29.
18. Abrams TE, Vaughan-Sarrazin M, Fan VS, Kaboli PJ. Geographic isolation and the risk for chronic obstructive pulmonary disease-related mortality: a cohort study. Ann Intern Med. 2011;155(2):80-86.
19. Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149-1155.
20. U.S. Department of Agriculture, Economic Research Service. Rural-urban commuting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx#U20K1F50H0A. Updated October 12, 2016. Accessed May 9, 2017.
21. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134(1):38-45.
22. Damarla M, Celli BR, Mullerova HX, Pinto-Plata VM. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51(10):1120-1124.
23. Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015-16 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(30):818-825.
24. Kim DK, Bridges CB, Harriman KH; Advisory Committee on Immunization Practices. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older: United States, 2016. Ann Intern Med. 2016;164(3):184-194.
25. Cimen P, Unlu M, Kirakli C, et al. Should patients with COPD be vaccinated? Respir Care. 2015;60(2):239-243.
26. Mowls DS, Cheruvu VK, Zullo MD. Influenza vaccination in adults with chronic obstructive pulmonary disease: the impact of a diagnostic breathing test on vaccination rates PLoS One. 2013;8(6):e67600.
27. Shoup JA, Madrid C, Koehler C, et al. Effectiveness and cost of influenza vaccine reminders for adults with asthma or chronic obstructive pulmonary disease. Am J Manag Care. 2015;21(7):e405-e413.
28. Arinez-Fernandez MC, Carrasco-Garrido P, Garcia-Carballo M, Hernandez-Barrera V, de Miguel AG, Jimenez-Garcia R. Determinants of pneumococcal vaccination among patients with chronic obstructive pulmonary disease in Spain. Hum Vaccin. 2006;2(3):99-104.29. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191.
30. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012.
31. Goodridge D, Lawson J, Rennie D, Marciniuk D. Rural/urban differences in health care utilization and place of death for persons with respiratory illness in the last year of life. Rural Remote Health. 2010;10(2):1349.
32. Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16(9):1123-1130.
33. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325-331
Betrixaban receives FDA approval for VTE prophylaxis in adults
The oral, once-daily factor Xa inhibitor betrixaban (Bevyxxa®) was granted approval by the US Food and Drug Administration (FDA) under priority review.
Betrixaban is the first and only anticoagulant for hospital and extended duration prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness at risk for thromboembolic complications due to restricted mobility and other risk factors for VTE, according to Portola Pharmaceuticals, Inc, the drug’s developer.
Betrixaban had also received fast track designation from the FDA.
Betrixaban is Portola’s first commercial product.
The FDA approved betrixaban based on data from the phase 3 APEX trial, a randomized, double-blind multinational clinical trial comparing extended duration betrixaban to short duration of enoxaparin.
The trial enrolled 7513 patients at more than 450 clinical sites worldwide. The patients were acutely ill with medical conditions, hospitalized, and had risk factors for VTE.
Patients who received betrixaban had an initial dose of 160 mg orally on day 1, 80 mg once daily for 35 to 42 days, and a placebo injection once daily for 6 to 14 days.
Patients who received enoxaparin had a 40 mg injection subcutaneously once daily for 6 to 14 days and a placebo pill orally once daily for 35 to 42 days.
Efficacy data was analyzed in 7441 patients and assessed by a composite outcome score comprising either the occurrence of asymptomatic proximal deep vein thrombosis (DVT) or symptomatic DVT, non-fatal pulmonary embolism (PE), or VTE-related death.
Investigators observed fewer events in patients receiving betrixaban (4.4%) compared with those taking enoxaparin (6%) (relative risk 0.75, 95% CI: 0.61, 0.91) with no significant increase in major bleeding (0.67% vs 0.57%, respectively).
The most common adverse reactions with betrixaban occurring in 5% or more of patients were related to bleeding.
Overall, 54% of patients receiving betrixaban experienced at least one adverse reaction compared with 52% taking enoxaparin.
The frequency of patients reporting serious adverse reactions was similar between betrixaban (18%) and enoxaparin (17%).
The most frequent reason for treatment discontinuation was bleeding, with an incidence rate for all bleeding episodes of 2.4% and 1.2% for betrixaban and enoxaparin, respectively.
The incidence rate for major bleeding episodes was 0.67% and 0.57% for betrixaban and enoxaparin, respectively.
Portola expects to launch betrixaban between August and November 2017.
The European Medicines Agency’s Committee for Human Medicinal Products (CHMP) is reviewing betrixaban for marketing authorization under its standard review period.
Portola is also advancing clinical development of andexanet alfa (AndexXa®) and cerdulatinib.
Andexanet alfa is a recombinant protein designed to reverse the anticoagulant effect in patients treated with an oral or injectable factor Xa inhibitor.
Cerdulatinib is a Syk/JAK inhibitor to treat hematologic cancers.
See the full prescribing information for more details on betrixaban. ![]()
The oral, once-daily factor Xa inhibitor betrixaban (Bevyxxa®) was granted approval by the US Food and Drug Administration (FDA) under priority review.
Betrixaban is the first and only anticoagulant for hospital and extended duration prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness at risk for thromboembolic complications due to restricted mobility and other risk factors for VTE, according to Portola Pharmaceuticals, Inc, the drug’s developer.
Betrixaban had also received fast track designation from the FDA.
Betrixaban is Portola’s first commercial product.
The FDA approved betrixaban based on data from the phase 3 APEX trial, a randomized, double-blind multinational clinical trial comparing extended duration betrixaban to short duration of enoxaparin.
The trial enrolled 7513 patients at more than 450 clinical sites worldwide. The patients were acutely ill with medical conditions, hospitalized, and had risk factors for VTE.
Patients who received betrixaban had an initial dose of 160 mg orally on day 1, 80 mg once daily for 35 to 42 days, and a placebo injection once daily for 6 to 14 days.
Patients who received enoxaparin had a 40 mg injection subcutaneously once daily for 6 to 14 days and a placebo pill orally once daily for 35 to 42 days.
Efficacy data was analyzed in 7441 patients and assessed by a composite outcome score comprising either the occurrence of asymptomatic proximal deep vein thrombosis (DVT) or symptomatic DVT, non-fatal pulmonary embolism (PE), or VTE-related death.
Investigators observed fewer events in patients receiving betrixaban (4.4%) compared with those taking enoxaparin (6%) (relative risk 0.75, 95% CI: 0.61, 0.91) with no significant increase in major bleeding (0.67% vs 0.57%, respectively).
The most common adverse reactions with betrixaban occurring in 5% or more of patients were related to bleeding.
Overall, 54% of patients receiving betrixaban experienced at least one adverse reaction compared with 52% taking enoxaparin.
The frequency of patients reporting serious adverse reactions was similar between betrixaban (18%) and enoxaparin (17%).
The most frequent reason for treatment discontinuation was bleeding, with an incidence rate for all bleeding episodes of 2.4% and 1.2% for betrixaban and enoxaparin, respectively.
The incidence rate for major bleeding episodes was 0.67% and 0.57% for betrixaban and enoxaparin, respectively.
Portola expects to launch betrixaban between August and November 2017.
The European Medicines Agency’s Committee for Human Medicinal Products (CHMP) is reviewing betrixaban for marketing authorization under its standard review period.
Portola is also advancing clinical development of andexanet alfa (AndexXa®) and cerdulatinib.
Andexanet alfa is a recombinant protein designed to reverse the anticoagulant effect in patients treated with an oral or injectable factor Xa inhibitor.
Cerdulatinib is a Syk/JAK inhibitor to treat hematologic cancers.
See the full prescribing information for more details on betrixaban. ![]()
The oral, once-daily factor Xa inhibitor betrixaban (Bevyxxa®) was granted approval by the US Food and Drug Administration (FDA) under priority review.
Betrixaban is the first and only anticoagulant for hospital and extended duration prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness at risk for thromboembolic complications due to restricted mobility and other risk factors for VTE, according to Portola Pharmaceuticals, Inc, the drug’s developer.
Betrixaban had also received fast track designation from the FDA.
Betrixaban is Portola’s first commercial product.
The FDA approved betrixaban based on data from the phase 3 APEX trial, a randomized, double-blind multinational clinical trial comparing extended duration betrixaban to short duration of enoxaparin.
The trial enrolled 7513 patients at more than 450 clinical sites worldwide. The patients were acutely ill with medical conditions, hospitalized, and had risk factors for VTE.
Patients who received betrixaban had an initial dose of 160 mg orally on day 1, 80 mg once daily for 35 to 42 days, and a placebo injection once daily for 6 to 14 days.
Patients who received enoxaparin had a 40 mg injection subcutaneously once daily for 6 to 14 days and a placebo pill orally once daily for 35 to 42 days.
Efficacy data was analyzed in 7441 patients and assessed by a composite outcome score comprising either the occurrence of asymptomatic proximal deep vein thrombosis (DVT) or symptomatic DVT, non-fatal pulmonary embolism (PE), or VTE-related death.
Investigators observed fewer events in patients receiving betrixaban (4.4%) compared with those taking enoxaparin (6%) (relative risk 0.75, 95% CI: 0.61, 0.91) with no significant increase in major bleeding (0.67% vs 0.57%, respectively).
The most common adverse reactions with betrixaban occurring in 5% or more of patients were related to bleeding.
Overall, 54% of patients receiving betrixaban experienced at least one adverse reaction compared with 52% taking enoxaparin.
The frequency of patients reporting serious adverse reactions was similar between betrixaban (18%) and enoxaparin (17%).
The most frequent reason for treatment discontinuation was bleeding, with an incidence rate for all bleeding episodes of 2.4% and 1.2% for betrixaban and enoxaparin, respectively.
The incidence rate for major bleeding episodes was 0.67% and 0.57% for betrixaban and enoxaparin, respectively.
Portola expects to launch betrixaban between August and November 2017.
The European Medicines Agency’s Committee for Human Medicinal Products (CHMP) is reviewing betrixaban for marketing authorization under its standard review period.
Portola is also advancing clinical development of andexanet alfa (AndexXa®) and cerdulatinib.
Andexanet alfa is a recombinant protein designed to reverse the anticoagulant effect in patients treated with an oral or injectable factor Xa inhibitor.
Cerdulatinib is a Syk/JAK inhibitor to treat hematologic cancers.
See the full prescribing information for more details on betrixaban. ![]()
Guidelines on classification, diagnosis, and treatment of acne fulminans
The incidence of acne fulminans may be decreasing but the isotretinoin-induced form is on the rise, say the authors of new guidelines on this severe variant of inflammatory acne.
Writing in the July issue of the Journal of the American Academy of Dermatology, Tanya Greywal, MD, from the University of California, San Diego, and coauthors, presented guidelines that aimed to better characterize this relatively rare condition and provide guidance on classification, treatment, and prevention.
Acne fulminans presents with rapid onset of painful erosions and hemorrhagic crusts – usually on the trunk – that can lead to severe, disfiguring scars. A more extreme form of the disorder also features systemic inflammation including fever, arthralgia, osteolytic bony lesions, and can even require hospitalization.
It is most commonly seen in white adolescent males, many of whom have a prior history of acne (J Am Acad Dermatol. 2017;77:109-17).
While alterations in innate immunity, autoimmunity, adaptive immunity, and autoinflammation have all been suggested as playing a role in the pathogenesis of acne fulminans, it can also be associated with isotretinoin use.
The overall incidence of the disorder has been decreasing over the last decade, which some suggest may be because the disease is being recognized and treated earlier. However, isotretinoin-induced acne fulminans is on the rise, perhaps because of more widespread use of the drug.
Acne fulminans previously has been described with a range of terms, including acne maligna, acute febrile ulcerative acne conglobota, and pseudo-acne fulminans, which the authors said has led to some confusion.
However, the expert panel behind the guidelines proposed that acne fulminans should be classified as being either with or without systemic symptoms, and either isotretinoin-induced or not.
They also recognized a range of associated disorders, including SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), and PASH (pyoderma gangrenosum, acne, and hidradenitis suppurativa).
In the absence of large-scale randomized controlled trials of treatments for acne fulminans, the authors said case series and individual reports supported the use of systemic corticosteroids in combination with isotretinoin when treating all forms of the disorder.
The expert group recommended starting patients on prednisone 0.5 to 1 mg/kg per day as monotherapy for at least 4 weeks for acne fulminans with systemic symptoms, and for at least 2 weeks in the absence of systemic symptoms.
They proposed a typical isotretinoin cumulative goal dose of 120-150 mg/kg, starting at a lower dose and gradually increasing, and overlapping with prednisone.
Case studies suggest that tetracyclines are minimally effective against acne fulminans, but the authors said there was a need for studies to examine whether the use of antibiotics overlapping with isotretinoin might reduce the development of isotretinoin-induced acne fulminans.
No funding or conflicts of interest were declared.
The incidence of acne fulminans may be decreasing but the isotretinoin-induced form is on the rise, say the authors of new guidelines on this severe variant of inflammatory acne.
Writing in the July issue of the Journal of the American Academy of Dermatology, Tanya Greywal, MD, from the University of California, San Diego, and coauthors, presented guidelines that aimed to better characterize this relatively rare condition and provide guidance on classification, treatment, and prevention.
Acne fulminans presents with rapid onset of painful erosions and hemorrhagic crusts – usually on the trunk – that can lead to severe, disfiguring scars. A more extreme form of the disorder also features systemic inflammation including fever, arthralgia, osteolytic bony lesions, and can even require hospitalization.
It is most commonly seen in white adolescent males, many of whom have a prior history of acne (J Am Acad Dermatol. 2017;77:109-17).
While alterations in innate immunity, autoimmunity, adaptive immunity, and autoinflammation have all been suggested as playing a role in the pathogenesis of acne fulminans, it can also be associated with isotretinoin use.
The overall incidence of the disorder has been decreasing over the last decade, which some suggest may be because the disease is being recognized and treated earlier. However, isotretinoin-induced acne fulminans is on the rise, perhaps because of more widespread use of the drug.
Acne fulminans previously has been described with a range of terms, including acne maligna, acute febrile ulcerative acne conglobota, and pseudo-acne fulminans, which the authors said has led to some confusion.
However, the expert panel behind the guidelines proposed that acne fulminans should be classified as being either with or without systemic symptoms, and either isotretinoin-induced or not.
They also recognized a range of associated disorders, including SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), and PASH (pyoderma gangrenosum, acne, and hidradenitis suppurativa).
In the absence of large-scale randomized controlled trials of treatments for acne fulminans, the authors said case series and individual reports supported the use of systemic corticosteroids in combination with isotretinoin when treating all forms of the disorder.
The expert group recommended starting patients on prednisone 0.5 to 1 mg/kg per day as monotherapy for at least 4 weeks for acne fulminans with systemic symptoms, and for at least 2 weeks in the absence of systemic symptoms.
They proposed a typical isotretinoin cumulative goal dose of 120-150 mg/kg, starting at a lower dose and gradually increasing, and overlapping with prednisone.
Case studies suggest that tetracyclines are minimally effective against acne fulminans, but the authors said there was a need for studies to examine whether the use of antibiotics overlapping with isotretinoin might reduce the development of isotretinoin-induced acne fulminans.
No funding or conflicts of interest were declared.
The incidence of acne fulminans may be decreasing but the isotretinoin-induced form is on the rise, say the authors of new guidelines on this severe variant of inflammatory acne.
Writing in the July issue of the Journal of the American Academy of Dermatology, Tanya Greywal, MD, from the University of California, San Diego, and coauthors, presented guidelines that aimed to better characterize this relatively rare condition and provide guidance on classification, treatment, and prevention.
Acne fulminans presents with rapid onset of painful erosions and hemorrhagic crusts – usually on the trunk – that can lead to severe, disfiguring scars. A more extreme form of the disorder also features systemic inflammation including fever, arthralgia, osteolytic bony lesions, and can even require hospitalization.
It is most commonly seen in white adolescent males, many of whom have a prior history of acne (J Am Acad Dermatol. 2017;77:109-17).
While alterations in innate immunity, autoimmunity, adaptive immunity, and autoinflammation have all been suggested as playing a role in the pathogenesis of acne fulminans, it can also be associated with isotretinoin use.
The overall incidence of the disorder has been decreasing over the last decade, which some suggest may be because the disease is being recognized and treated earlier. However, isotretinoin-induced acne fulminans is on the rise, perhaps because of more widespread use of the drug.
Acne fulminans previously has been described with a range of terms, including acne maligna, acute febrile ulcerative acne conglobota, and pseudo-acne fulminans, which the authors said has led to some confusion.
However, the expert panel behind the guidelines proposed that acne fulminans should be classified as being either with or without systemic symptoms, and either isotretinoin-induced or not.
They also recognized a range of associated disorders, including SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), and PASH (pyoderma gangrenosum, acne, and hidradenitis suppurativa).
In the absence of large-scale randomized controlled trials of treatments for acne fulminans, the authors said case series and individual reports supported the use of systemic corticosteroids in combination with isotretinoin when treating all forms of the disorder.
The expert group recommended starting patients on prednisone 0.5 to 1 mg/kg per day as monotherapy for at least 4 weeks for acne fulminans with systemic symptoms, and for at least 2 weeks in the absence of systemic symptoms.
They proposed a typical isotretinoin cumulative goal dose of 120-150 mg/kg, starting at a lower dose and gradually increasing, and overlapping with prednisone.
Case studies suggest that tetracyclines are minimally effective against acne fulminans, but the authors said there was a need for studies to examine whether the use of antibiotics overlapping with isotretinoin might reduce the development of isotretinoin-induced acne fulminans.
No funding or conflicts of interest were declared.
FROM THE JOURNAL OF AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Experts have presented evidence-based guidelines on the diagnosis, classification, and treatment of acne fulminans.
Major finding: Acne fulminans is classified as either presenting with or without systemic symptoms, and being either isotretinoin-induced or not.
Data source: Review.
Disclosures: No conflicts of interest were declared.
IL-6 nanobody vobarilizumab advances despite equivocal phase II data
MADRID – Despite failing its phase II primary endpoint, the single-domain antibody vobarilizumab, being developed for rheumatoid arthritis, will advance to phase III studies.
A robust placebo response obscured the strength of clinical response to vobarilizumab, a nanobody that blocks the soluble interleukin-6 receptor, Thomas Dorner, MD, said at the European Congress of Rheumatology. But by 24 weeks of treatment, vobarilizumab effected a deep, sustained response in up to half of patients with rheumatoid arthritis who responded inadequately to methotrexate.
The safety profile was encouraging, Dr. Dorner said, with just one case of gastrointestinal perforation. These events have been a problem in some IL-6 blockade studies; the protein stimulates repair of the intestinal epithelium and blocking it leaves the tissue vulnerable to injury. The single case in this trial occurred 17 days after the patient withdrew from the study for other reasons, so it was not considered to be related to the study medication, Dr. Dorner said.
Vobarilizumab is composed of two heavy chains, lacking the two light-chain arms of a full antibody. It is minuscule – just 10% of the molecular weight of a conventional antibody. One chain targets human serum albumin and is designed to prolong the molecule’s half-life by up to 14 days; the other chain targets the soluble IL-6 receptor.
The phase IIb study comprised 345 patients with active RA despite treatment with methotrexate (mean dose 16 mg/week). While staying on their methotrexate, they were equally assigned to five treatment groups: placebo or vobarilizumab at 75 mg every 4 weeks; 150 mg every 4 weeks; 150 mg every 2 weeks; or 225 mg every 2 weeks.
The primary endpoint was the difference in ACR20 response at week 12 between placebo and the four dosing groups. While the majority of patients taking vobarilizumab met that endpoint, the percentages did not significantly differ from each other, nor from placebo. ACR20 was met by 62% of the placebo group and 81%-72% of the vobarilizumab groups. ACR50 was met by 28% of the placebo group and 45%-29% of the vobarilizumab groups and ACR70 by 9% of the placebo group and 21%-14% of the vobarilizumab groups.
However, Dr. Dorner said, response in the placebo group tended to tail off as the study progressed, while staying significantly stronger in the higher-dose vobarilizumab groups. By week 24, 16% of the placebo group still had an ACR50 – significantly less than in the vobarilizumab 150 mg/2 weeks and 225 mg/2 weeks groups (31%, 39%, respectively).
On a measure of remission that combined C-reactive protein of less than 2.6 mg/L and low disease activity, vobarilizumab looked much better at week 24. At that point, 16% of placebo patients met the endpoint, compared to 39% of the 150 mg/4 weeks and 150 mg/2 weeks vobarilizumab groups, and 51% of the 225 mg/2 weeks group.
Adverse events occurred in 36% of placebo patients and about 44% of vobarilizumab patients and did not show any dose-response relationship. About 38% of these were considered treatment related; 7% of the cohort withdrew due to an adverse event. There were seven serious infections among those taking vobarilizumab (three pneumonia, and one each herpes zoster, ear infection, staphylococcus sepsis, and bacterial arthritis). One patient died of a myocardial infarction, but she had experienced a previous heart attack and undergone percutaneous coronary intervention. The single gastrointestinal perforation occurred in a woman taking the highest dose of vobarilizumab, more than 2 weeks after she stopped taking it.
Vobarilizumab will continue into a phase III study, according to the Ablynx website. The company is seeking an industry partner. AbbVie had an option to license vobarilizumab, but declined last fall on the mixed results of Ablynx’s phase II studies.
[email protected]
On Twitter @Alz_gal
MADRID – Despite failing its phase II primary endpoint, the single-domain antibody vobarilizumab, being developed for rheumatoid arthritis, will advance to phase III studies.
A robust placebo response obscured the strength of clinical response to vobarilizumab, a nanobody that blocks the soluble interleukin-6 receptor, Thomas Dorner, MD, said at the European Congress of Rheumatology. But by 24 weeks of treatment, vobarilizumab effected a deep, sustained response in up to half of patients with rheumatoid arthritis who responded inadequately to methotrexate.
The safety profile was encouraging, Dr. Dorner said, with just one case of gastrointestinal perforation. These events have been a problem in some IL-6 blockade studies; the protein stimulates repair of the intestinal epithelium and blocking it leaves the tissue vulnerable to injury. The single case in this trial occurred 17 days after the patient withdrew from the study for other reasons, so it was not considered to be related to the study medication, Dr. Dorner said.
Vobarilizumab is composed of two heavy chains, lacking the two light-chain arms of a full antibody. It is minuscule – just 10% of the molecular weight of a conventional antibody. One chain targets human serum albumin and is designed to prolong the molecule’s half-life by up to 14 days; the other chain targets the soluble IL-6 receptor.
The phase IIb study comprised 345 patients with active RA despite treatment with methotrexate (mean dose 16 mg/week). While staying on their methotrexate, they were equally assigned to five treatment groups: placebo or vobarilizumab at 75 mg every 4 weeks; 150 mg every 4 weeks; 150 mg every 2 weeks; or 225 mg every 2 weeks.
The primary endpoint was the difference in ACR20 response at week 12 between placebo and the four dosing groups. While the majority of patients taking vobarilizumab met that endpoint, the percentages did not significantly differ from each other, nor from placebo. ACR20 was met by 62% of the placebo group and 81%-72% of the vobarilizumab groups. ACR50 was met by 28% of the placebo group and 45%-29% of the vobarilizumab groups and ACR70 by 9% of the placebo group and 21%-14% of the vobarilizumab groups.
However, Dr. Dorner said, response in the placebo group tended to tail off as the study progressed, while staying significantly stronger in the higher-dose vobarilizumab groups. By week 24, 16% of the placebo group still had an ACR50 – significantly less than in the vobarilizumab 150 mg/2 weeks and 225 mg/2 weeks groups (31%, 39%, respectively).
On a measure of remission that combined C-reactive protein of less than 2.6 mg/L and low disease activity, vobarilizumab looked much better at week 24. At that point, 16% of placebo patients met the endpoint, compared to 39% of the 150 mg/4 weeks and 150 mg/2 weeks vobarilizumab groups, and 51% of the 225 mg/2 weeks group.
Adverse events occurred in 36% of placebo patients and about 44% of vobarilizumab patients and did not show any dose-response relationship. About 38% of these were considered treatment related; 7% of the cohort withdrew due to an adverse event. There were seven serious infections among those taking vobarilizumab (three pneumonia, and one each herpes zoster, ear infection, staphylococcus sepsis, and bacterial arthritis). One patient died of a myocardial infarction, but she had experienced a previous heart attack and undergone percutaneous coronary intervention. The single gastrointestinal perforation occurred in a woman taking the highest dose of vobarilizumab, more than 2 weeks after she stopped taking it.
Vobarilizumab will continue into a phase III study, according to the Ablynx website. The company is seeking an industry partner. AbbVie had an option to license vobarilizumab, but declined last fall on the mixed results of Ablynx’s phase II studies.
[email protected]
On Twitter @Alz_gal
MADRID – Despite failing its phase II primary endpoint, the single-domain antibody vobarilizumab, being developed for rheumatoid arthritis, will advance to phase III studies.
A robust placebo response obscured the strength of clinical response to vobarilizumab, a nanobody that blocks the soluble interleukin-6 receptor, Thomas Dorner, MD, said at the European Congress of Rheumatology. But by 24 weeks of treatment, vobarilizumab effected a deep, sustained response in up to half of patients with rheumatoid arthritis who responded inadequately to methotrexate.
The safety profile was encouraging, Dr. Dorner said, with just one case of gastrointestinal perforation. These events have been a problem in some IL-6 blockade studies; the protein stimulates repair of the intestinal epithelium and blocking it leaves the tissue vulnerable to injury. The single case in this trial occurred 17 days after the patient withdrew from the study for other reasons, so it was not considered to be related to the study medication, Dr. Dorner said.
Vobarilizumab is composed of two heavy chains, lacking the two light-chain arms of a full antibody. It is minuscule – just 10% of the molecular weight of a conventional antibody. One chain targets human serum albumin and is designed to prolong the molecule’s half-life by up to 14 days; the other chain targets the soluble IL-6 receptor.
The phase IIb study comprised 345 patients with active RA despite treatment with methotrexate (mean dose 16 mg/week). While staying on their methotrexate, they were equally assigned to five treatment groups: placebo or vobarilizumab at 75 mg every 4 weeks; 150 mg every 4 weeks; 150 mg every 2 weeks; or 225 mg every 2 weeks.
The primary endpoint was the difference in ACR20 response at week 12 between placebo and the four dosing groups. While the majority of patients taking vobarilizumab met that endpoint, the percentages did not significantly differ from each other, nor from placebo. ACR20 was met by 62% of the placebo group and 81%-72% of the vobarilizumab groups. ACR50 was met by 28% of the placebo group and 45%-29% of the vobarilizumab groups and ACR70 by 9% of the placebo group and 21%-14% of the vobarilizumab groups.
However, Dr. Dorner said, response in the placebo group tended to tail off as the study progressed, while staying significantly stronger in the higher-dose vobarilizumab groups. By week 24, 16% of the placebo group still had an ACR50 – significantly less than in the vobarilizumab 150 mg/2 weeks and 225 mg/2 weeks groups (31%, 39%, respectively).
On a measure of remission that combined C-reactive protein of less than 2.6 mg/L and low disease activity, vobarilizumab looked much better at week 24. At that point, 16% of placebo patients met the endpoint, compared to 39% of the 150 mg/4 weeks and 150 mg/2 weeks vobarilizumab groups, and 51% of the 225 mg/2 weeks group.
Adverse events occurred in 36% of placebo patients and about 44% of vobarilizumab patients and did not show any dose-response relationship. About 38% of these were considered treatment related; 7% of the cohort withdrew due to an adverse event. There were seven serious infections among those taking vobarilizumab (three pneumonia, and one each herpes zoster, ear infection, staphylococcus sepsis, and bacterial arthritis). One patient died of a myocardial infarction, but she had experienced a previous heart attack and undergone percutaneous coronary intervention. The single gastrointestinal perforation occurred in a woman taking the highest dose of vobarilizumab, more than 2 weeks after she stopped taking it.
Vobarilizumab will continue into a phase III study, according to the Ablynx website. The company is seeking an industry partner. AbbVie had an option to license vobarilizumab, but declined last fall on the mixed results of Ablynx’s phase II studies.
[email protected]
On Twitter @Alz_gal
AT EULAR 2017
CARs race for supremacy against aggressive non-Hodgkin lymphoma
MADRID – Two chimeric antigen receptor T cell (CAR-T) constructs are showing promising activity against treatment-refractory, aggressive forms of non-Hodgkin lymphoma in multicenter clinical trials.
In the ZUMA-1 trial, axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 CAR-T product, was associated with an 82% objective response rate (ORR), including 54% complete responses, in patients with refractory diffuse large B cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL), reported Yi Lin, MD, PhD, from the Mayo Clinic in Rochester Minnesota.
In an interim analysis from the JULIET study, a different anti-CD19 CAR-T construct labeled CTL019 was associated with a 59% ORR, consisting of 43% complete responses and 16% partial responses (PR) in patients with relapsed or refractory DLBCL, reported Gilles Salles, MD, PhD, from the University of Lyon, France.
The analysis “confirms the high response rates and durable responses observed in the previous single-center trial,” Dr. Salles said.
Although the CAR-T cell constructs in the study have different costimulatory molecules, each is created in a centralized facility, which allows for consistent manufacturing of cells sufficient for harvesting, transfecting, expanding, and reinfusing into heavily pretreated patients.
The construct used in ZUMA-1, also called KTE-C19 (Kite Pharma), has CD28 and CD3-zeta signaling domains. CTL019 (Novarits, U. Pennsylvania, and Oxford Biomedica) has CD3-zeta and 4-1BB costimulatory domains.
ZUMA-1
Dr. Lin reported phase II results from ZUMA-1, investigating axi-cel at a target dose of 2 x 106 cells per kilogram in 72 patients with refractory DLBCL (cohort 1), and 20 patients with refractory PMBCL or TFL (cohort 2).
The median patient age was 58 years. Patients had stage III or IV disease, 47% had International Prognostic Index (IPI) scores of 3-4, 77% had disease that was refractory to second-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
The axi-cel construct was successfully manufactured in 99% of patients, with an average turnaround time from apheresis to the clinical site of 17 days.
As noted before, the trial met its primary endpoint with an 82% ORR, consisting of 54% complete responses and 28% partial responses.
The median duration of response was 8.2 months, and for patients with complete responses the median duration has not been reached.
Median overall survival has also not been reached.
The treatment was generally safe, with only 13% of patients experiencing grade 3 or greater cytokine release syndrome (CRS), and 28% having grade 3 or greater neurologic events. The events were generally reversible, and the rates of each declined over time. The use of tociluzumab or steroids to control adverse events did not have a negative effect on responses, Dr. Lin said.
JULIET
In the ongoing JULIET study, patients with relapsed/refractory DLBCL after at least two prior lines of therapy and who are not candidates for stem cell transplants are enrolled.
In a safety analysis including 85 patients, the CRS was seen in 57% of all patients, including grade 3 in 17% and grade 4 in 9%.
Other common adverse events occurring within 8 weeks of CTL019 infusion were infections in 26% of patients, cytopenias lasting longer than 28 days in 26%, neurologic events in 21%, febrile neutropenia in 14%, and tumor lysis syndrome in 1%.
There were no cases of cerebral edema, and no deaths attributable to the CAR-T cell construct, Dr. Salles said.
Peter Borchmann, MD, from the University of Cologne, Germany, who attended the briefing but was not involved with either study, commented that investigators in ZUMA-1 need to monitor patients carefully, because previous clinical trials using other CAR-T cells with CD28 costimuatory domains have been associated with several cases of fatal cerebral edema.
“I think you can use CD28 in lymphoma, and it’s highly active as we have seen, but my personal impression is that you have to be aware that this might happen,” he said in an interview.
The ZUMA-1 study is funded by Kite Pharma. Dr. Lin disclosed research funding from Janssen. The JULIET study is supported by Novartis. Dr. Salles disclosed serving on an advisory board for the company. Dr. Borchmann had no disclosures.
MADRID – Two chimeric antigen receptor T cell (CAR-T) constructs are showing promising activity against treatment-refractory, aggressive forms of non-Hodgkin lymphoma in multicenter clinical trials.
In the ZUMA-1 trial, axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 CAR-T product, was associated with an 82% objective response rate (ORR), including 54% complete responses, in patients with refractory diffuse large B cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL), reported Yi Lin, MD, PhD, from the Mayo Clinic in Rochester Minnesota.
In an interim analysis from the JULIET study, a different anti-CD19 CAR-T construct labeled CTL019 was associated with a 59% ORR, consisting of 43% complete responses and 16% partial responses (PR) in patients with relapsed or refractory DLBCL, reported Gilles Salles, MD, PhD, from the University of Lyon, France.
The analysis “confirms the high response rates and durable responses observed in the previous single-center trial,” Dr. Salles said.
Although the CAR-T cell constructs in the study have different costimulatory molecules, each is created in a centralized facility, which allows for consistent manufacturing of cells sufficient for harvesting, transfecting, expanding, and reinfusing into heavily pretreated patients.
The construct used in ZUMA-1, also called KTE-C19 (Kite Pharma), has CD28 and CD3-zeta signaling domains. CTL019 (Novarits, U. Pennsylvania, and Oxford Biomedica) has CD3-zeta and 4-1BB costimulatory domains.
ZUMA-1
Dr. Lin reported phase II results from ZUMA-1, investigating axi-cel at a target dose of 2 x 106 cells per kilogram in 72 patients with refractory DLBCL (cohort 1), and 20 patients with refractory PMBCL or TFL (cohort 2).
The median patient age was 58 years. Patients had stage III or IV disease, 47% had International Prognostic Index (IPI) scores of 3-4, 77% had disease that was refractory to second-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
The axi-cel construct was successfully manufactured in 99% of patients, with an average turnaround time from apheresis to the clinical site of 17 days.
As noted before, the trial met its primary endpoint with an 82% ORR, consisting of 54% complete responses and 28% partial responses.
The median duration of response was 8.2 months, and for patients with complete responses the median duration has not been reached.
Median overall survival has also not been reached.
The treatment was generally safe, with only 13% of patients experiencing grade 3 or greater cytokine release syndrome (CRS), and 28% having grade 3 or greater neurologic events. The events were generally reversible, and the rates of each declined over time. The use of tociluzumab or steroids to control adverse events did not have a negative effect on responses, Dr. Lin said.
JULIET
In the ongoing JULIET study, patients with relapsed/refractory DLBCL after at least two prior lines of therapy and who are not candidates for stem cell transplants are enrolled.
In a safety analysis including 85 patients, the CRS was seen in 57% of all patients, including grade 3 in 17% and grade 4 in 9%.
Other common adverse events occurring within 8 weeks of CTL019 infusion were infections in 26% of patients, cytopenias lasting longer than 28 days in 26%, neurologic events in 21%, febrile neutropenia in 14%, and tumor lysis syndrome in 1%.
There were no cases of cerebral edema, and no deaths attributable to the CAR-T cell construct, Dr. Salles said.
Peter Borchmann, MD, from the University of Cologne, Germany, who attended the briefing but was not involved with either study, commented that investigators in ZUMA-1 need to monitor patients carefully, because previous clinical trials using other CAR-T cells with CD28 costimuatory domains have been associated with several cases of fatal cerebral edema.
“I think you can use CD28 in lymphoma, and it’s highly active as we have seen, but my personal impression is that you have to be aware that this might happen,” he said in an interview.
The ZUMA-1 study is funded by Kite Pharma. Dr. Lin disclosed research funding from Janssen. The JULIET study is supported by Novartis. Dr. Salles disclosed serving on an advisory board for the company. Dr. Borchmann had no disclosures.
MADRID – Two chimeric antigen receptor T cell (CAR-T) constructs are showing promising activity against treatment-refractory, aggressive forms of non-Hodgkin lymphoma in multicenter clinical trials.
In the ZUMA-1 trial, axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 CAR-T product, was associated with an 82% objective response rate (ORR), including 54% complete responses, in patients with refractory diffuse large B cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL), reported Yi Lin, MD, PhD, from the Mayo Clinic in Rochester Minnesota.
In an interim analysis from the JULIET study, a different anti-CD19 CAR-T construct labeled CTL019 was associated with a 59% ORR, consisting of 43% complete responses and 16% partial responses (PR) in patients with relapsed or refractory DLBCL, reported Gilles Salles, MD, PhD, from the University of Lyon, France.
The analysis “confirms the high response rates and durable responses observed in the previous single-center trial,” Dr. Salles said.
Although the CAR-T cell constructs in the study have different costimulatory molecules, each is created in a centralized facility, which allows for consistent manufacturing of cells sufficient for harvesting, transfecting, expanding, and reinfusing into heavily pretreated patients.
The construct used in ZUMA-1, also called KTE-C19 (Kite Pharma), has CD28 and CD3-zeta signaling domains. CTL019 (Novarits, U. Pennsylvania, and Oxford Biomedica) has CD3-zeta and 4-1BB costimulatory domains.
ZUMA-1
Dr. Lin reported phase II results from ZUMA-1, investigating axi-cel at a target dose of 2 x 106 cells per kilogram in 72 patients with refractory DLBCL (cohort 1), and 20 patients with refractory PMBCL or TFL (cohort 2).
The median patient age was 58 years. Patients had stage III or IV disease, 47% had International Prognostic Index (IPI) scores of 3-4, 77% had disease that was refractory to second-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
The axi-cel construct was successfully manufactured in 99% of patients, with an average turnaround time from apheresis to the clinical site of 17 days.
As noted before, the trial met its primary endpoint with an 82% ORR, consisting of 54% complete responses and 28% partial responses.
The median duration of response was 8.2 months, and for patients with complete responses the median duration has not been reached.
Median overall survival has also not been reached.
The treatment was generally safe, with only 13% of patients experiencing grade 3 or greater cytokine release syndrome (CRS), and 28% having grade 3 or greater neurologic events. The events were generally reversible, and the rates of each declined over time. The use of tociluzumab or steroids to control adverse events did not have a negative effect on responses, Dr. Lin said.
JULIET
In the ongoing JULIET study, patients with relapsed/refractory DLBCL after at least two prior lines of therapy and who are not candidates for stem cell transplants are enrolled.
In a safety analysis including 85 patients, the CRS was seen in 57% of all patients, including grade 3 in 17% and grade 4 in 9%.
Other common adverse events occurring within 8 weeks of CTL019 infusion were infections in 26% of patients, cytopenias lasting longer than 28 days in 26%, neurologic events in 21%, febrile neutropenia in 14%, and tumor lysis syndrome in 1%.
There were no cases of cerebral edema, and no deaths attributable to the CAR-T cell construct, Dr. Salles said.
Peter Borchmann, MD, from the University of Cologne, Germany, who attended the briefing but was not involved with either study, commented that investigators in ZUMA-1 need to monitor patients carefully, because previous clinical trials using other CAR-T cells with CD28 costimuatory domains have been associated with several cases of fatal cerebral edema.
“I think you can use CD28 in lymphoma, and it’s highly active as we have seen, but my personal impression is that you have to be aware that this might happen,” he said in an interview.
The ZUMA-1 study is funded by Kite Pharma. Dr. Lin disclosed research funding from Janssen. The JULIET study is supported by Novartis. Dr. Salles disclosed serving on an advisory board for the company. Dr. Borchmann had no disclosures.
AT EHA 2017
Key clinical point: CAR-T cell therapies are showing good activity against relapsed/refractory non-Hodgkin lymphomas.
Major finding: In ZUMA-1, the objective response rate was 82%. In JULIET, it was 59%
Data source: Two multicenter trials of CAR-T cells in patients with relapsed/refractory DLBCL, PMBCL, and TFL.
Disclosures: The ZUMA-1 study is funded by Kite Pharma. Dr. Lin disclosed research funding from Janssen. The JULIET study is supported by Novartis. Dr. Salles disclosed serving on an advisory board for the company. Dr. Borchmann had no disclosures.
Biosimilar immunogenicity studies produce no surprises
AT EULAR 2017
MADRID – Two biosimilar tumor necrosis factor inhibitor (TNFi) products are no more immunogenic than their reference biologic counterparts in patients with rheumatoid arthritis, according to the results of two studies presented at the European Congress of Rheumatology.
Results of one study, which involved 544 RA patients, showed 33.1% treated with SB5, a biosimilar candidate for adalimumab (Humira), and 32% of those who were treated with reference adalimumab developed antidrug antibodies (ADAbs) after 24 weeks’ treatment.
In the second study, which involved 596 RA patients, significantly fewer treated with SB4, a biosimilar candidate for etanercept (Enbrel) developed ADAbs versus reference etanercept (0.7% vs. 13.1%, P less than .001).
In both studies the development of ADAbs with the biosimilar or reference products was found to attenuate the clinical responses seen, with trends towards lower American College of Rheumatology (ACR) scores with higher levels of ADAbs.
Dr. Kay observed that for both SB5 and adalimumab, efficacy at week 24 was higher among patients who achieved drug trough levels of 1.274 microg/mL or more than this threshold set for achieving a good EULAR response. At week 24, 32.9% and 43.2% of patients treated with the biosimilar adalimumab and reference adalimumab, respectively, had drug trough levels greater than or equal to the threshold, and achieved a good EULAR response, with 61.7% and 51.8% achieving a moderate EULAR response, and 5.4% and 5% no EULAR response. Dr. Kay also reported that similar percentages of patients treated with the biosimilar and reference adalimumab achieved a low disease activity (LDA) score or were in remission at 24 weeks if the drug trough levels were 1.274 microg/mL or above, at 32.9% and 43.2% for LDA and 20.8% and 25.9% for remission.
“Efficacy tended to be lower in patients with ADAbs,” Dr. Vencovský, who is vice-director of the Institute of Rheumatology, Charles University in Prague, Czech Republic, said. For both the ACR50 and ACR70 responses at week 24, no differences were seen between the patients who received biosimilar or reference etanercept. The impact on the ACR20 response could not be evaluated in the biosimilar group due to the low incidence of ADAbs, Dr. Vencovský said. The effect of injection site reactions on efficacy was also studied, and while no correlation was found between injection site reactions and the development of ADAbs, efficacy did tend to be slightly lower in some parameters measured in the patients who experienced injection site reactions. Dr. Vencovský reported that fewer patients treated with the biosimilar candidate experienced injection site reactions than those with the reference etanercept (3% vs. 16%).
The study presented by Dr. Kay is currently under consideration for publication and has previously been presented at the American College of Rheumatology.
Samsung Biopics sponsored the studies. The company has several biosimilar products in the pipeline, including SB2, which is a biosimilar for infliximab (Remicade) that was approved for use in the United States in April 2017. Although not yet available in the U.S., the company’s biosimilar adalimumab (SB5) is undergoing regulatory review by the European Medicines Agency (EMA) and it’s biosimilar etanercept is available in Europe as Benpali and Canada as Brenzys.
Dr. Kay has received research support paid to his institution from AbbVie, Genentech, Pfizer, Roche Laboratories, and UCB. He has also acted as a consultant to several companies that develop or market biosimilars including Samsung Bioepis who make the two biosimilar products mentioned in this report. Dr. Vencovský disclosed acting as a consultant to Samsung Bioepis and to Biogen, and acting as a speaker for UCB, Pfizer, AbbVie, MSD, Novartis, and Eli Lilly.
AT EULAR 2017
MADRID – Two biosimilar tumor necrosis factor inhibitor (TNFi) products are no more immunogenic than their reference biologic counterparts in patients with rheumatoid arthritis, according to the results of two studies presented at the European Congress of Rheumatology.
Results of one study, which involved 544 RA patients, showed 33.1% treated with SB5, a biosimilar candidate for adalimumab (Humira), and 32% of those who were treated with reference adalimumab developed antidrug antibodies (ADAbs) after 24 weeks’ treatment.
In the second study, which involved 596 RA patients, significantly fewer treated with SB4, a biosimilar candidate for etanercept (Enbrel) developed ADAbs versus reference etanercept (0.7% vs. 13.1%, P less than .001).
In both studies the development of ADAbs with the biosimilar or reference products was found to attenuate the clinical responses seen, with trends towards lower American College of Rheumatology (ACR) scores with higher levels of ADAbs.
Dr. Kay observed that for both SB5 and adalimumab, efficacy at week 24 was higher among patients who achieved drug trough levels of 1.274 microg/mL or more than this threshold set for achieving a good EULAR response. At week 24, 32.9% and 43.2% of patients treated with the biosimilar adalimumab and reference adalimumab, respectively, had drug trough levels greater than or equal to the threshold, and achieved a good EULAR response, with 61.7% and 51.8% achieving a moderate EULAR response, and 5.4% and 5% no EULAR response. Dr. Kay also reported that similar percentages of patients treated with the biosimilar and reference adalimumab achieved a low disease activity (LDA) score or were in remission at 24 weeks if the drug trough levels were 1.274 microg/mL or above, at 32.9% and 43.2% for LDA and 20.8% and 25.9% for remission.
“Efficacy tended to be lower in patients with ADAbs,” Dr. Vencovský, who is vice-director of the Institute of Rheumatology, Charles University in Prague, Czech Republic, said. For both the ACR50 and ACR70 responses at week 24, no differences were seen between the patients who received biosimilar or reference etanercept. The impact on the ACR20 response could not be evaluated in the biosimilar group due to the low incidence of ADAbs, Dr. Vencovský said. The effect of injection site reactions on efficacy was also studied, and while no correlation was found between injection site reactions and the development of ADAbs, efficacy did tend to be slightly lower in some parameters measured in the patients who experienced injection site reactions. Dr. Vencovský reported that fewer patients treated with the biosimilar candidate experienced injection site reactions than those with the reference etanercept (3% vs. 16%).
The study presented by Dr. Kay is currently under consideration for publication and has previously been presented at the American College of Rheumatology.
Samsung Biopics sponsored the studies. The company has several biosimilar products in the pipeline, including SB2, which is a biosimilar for infliximab (Remicade) that was approved for use in the United States in April 2017. Although not yet available in the U.S., the company’s biosimilar adalimumab (SB5) is undergoing regulatory review by the European Medicines Agency (EMA) and it’s biosimilar etanercept is available in Europe as Benpali and Canada as Brenzys.
Dr. Kay has received research support paid to his institution from AbbVie, Genentech, Pfizer, Roche Laboratories, and UCB. He has also acted as a consultant to several companies that develop or market biosimilars including Samsung Bioepis who make the two biosimilar products mentioned in this report. Dr. Vencovský disclosed acting as a consultant to Samsung Bioepis and to Biogen, and acting as a speaker for UCB, Pfizer, AbbVie, MSD, Novartis, and Eli Lilly.
AT EULAR 2017
MADRID – Two biosimilar tumor necrosis factor inhibitor (TNFi) products are no more immunogenic than their reference biologic counterparts in patients with rheumatoid arthritis, according to the results of two studies presented at the European Congress of Rheumatology.
Results of one study, which involved 544 RA patients, showed 33.1% treated with SB5, a biosimilar candidate for adalimumab (Humira), and 32% of those who were treated with reference adalimumab developed antidrug antibodies (ADAbs) after 24 weeks’ treatment.
In the second study, which involved 596 RA patients, significantly fewer treated with SB4, a biosimilar candidate for etanercept (Enbrel) developed ADAbs versus reference etanercept (0.7% vs. 13.1%, P less than .001).
In both studies the development of ADAbs with the biosimilar or reference products was found to attenuate the clinical responses seen, with trends towards lower American College of Rheumatology (ACR) scores with higher levels of ADAbs.
Dr. Kay observed that for both SB5 and adalimumab, efficacy at week 24 was higher among patients who achieved drug trough levels of 1.274 microg/mL or more than this threshold set for achieving a good EULAR response. At week 24, 32.9% and 43.2% of patients treated with the biosimilar adalimumab and reference adalimumab, respectively, had drug trough levels greater than or equal to the threshold, and achieved a good EULAR response, with 61.7% and 51.8% achieving a moderate EULAR response, and 5.4% and 5% no EULAR response. Dr. Kay also reported that similar percentages of patients treated with the biosimilar and reference adalimumab achieved a low disease activity (LDA) score or were in remission at 24 weeks if the drug trough levels were 1.274 microg/mL or above, at 32.9% and 43.2% for LDA and 20.8% and 25.9% for remission.
“Efficacy tended to be lower in patients with ADAbs,” Dr. Vencovský, who is vice-director of the Institute of Rheumatology, Charles University in Prague, Czech Republic, said. For both the ACR50 and ACR70 responses at week 24, no differences were seen between the patients who received biosimilar or reference etanercept. The impact on the ACR20 response could not be evaluated in the biosimilar group due to the low incidence of ADAbs, Dr. Vencovský said. The effect of injection site reactions on efficacy was also studied, and while no correlation was found between injection site reactions and the development of ADAbs, efficacy did tend to be slightly lower in some parameters measured in the patients who experienced injection site reactions. Dr. Vencovský reported that fewer patients treated with the biosimilar candidate experienced injection site reactions than those with the reference etanercept (3% vs. 16%).
The study presented by Dr. Kay is currently under consideration for publication and has previously been presented at the American College of Rheumatology.
Samsung Biopics sponsored the studies. The company has several biosimilar products in the pipeline, including SB2, which is a biosimilar for infliximab (Remicade) that was approved for use in the United States in April 2017. Although not yet available in the U.S., the company’s biosimilar adalimumab (SB5) is undergoing regulatory review by the European Medicines Agency (EMA) and it’s biosimilar etanercept is available in Europe as Benpali and Canada as Brenzys.
Dr. Kay has received research support paid to his institution from AbbVie, Genentech, Pfizer, Roche Laboratories, and UCB. He has also acted as a consultant to several companies that develop or market biosimilars including Samsung Bioepis who make the two biosimilar products mentioned in this report. Dr. Vencovský disclosed acting as a consultant to Samsung Bioepis and to Biogen, and acting as a speaker for UCB, Pfizer, AbbVie, MSD, Novartis, and Eli Lilly.
Key clinical point:
Major finding: Around one-third of biosimilar and reference adalimumab-treated patients developed anti-drug antibodies, while significantly fewer patients treated with biosimilar etanercept versus reference etanercept did so (0.7% vs. 13/1%, P less than .001).
Data source: Two studies in patients with rheumatoid arthritis treated with biosimilar adalimumab or biosimilar etanercept or the reference products.
Disclosures: Samsung Bioepis sponsored the studies. Dr. Kay has received research support paid to his institution from AbbVie, Pfizer, Genentech, Roche Laboratories, and UCB. He has also acted as a consultant to several companies that develop or market biosimilars including Samsung Bioepis who make the two biosimilar products mentioned in this report. Dr. Vencovský disclosed acting as a consultant to Samsung Bioepis and to Biogen, and acting as a speaker for UCB, Pfizer, AbbVie, MSD, Novartis, and Eli Lilly.
Biologics, TNF-inhibitors confer no excess cancer risks upon RA patients
MADRID – Biologics and tumor necrosis factor–inhibitors confer very little – if any – risk of malignancy upon those who take them for rheumatoid arthritis, according to a large Swedish registry study.
A doubling in the risk of squamous cell carcinoma among those who took abatacept was the only significant finding in the 8-year study of almost 70,000 subjects, Hjalmar Wadstrom, said at the European Congress of Rheumatology. And while he said the finding could be spurious, he stressed that it can’t be ignored.
“We think this finding should be interpreted cautiously,” said Mr. Wadstrom, a doctoral student at the Karolinska Institute, Stockholm. “These patients are seen frequently, and this increase in sqaumous cell carcinoma could be related to this multiple screening, or due to study bias. But of course, we cannot disregard it. It should be validated and further examined.”
The study plumbed several national patient registries and administrative databases for its cohort. It comprised 22,500 patients who, from 2006 to 2014, took tocilizumab, abatacept, or rituximab; or a TNF-inhibitor as a first or second disease-modifying antirheumatic drug. These were compared to 46,600 patients who took synthetic DMARDS during the same period and to a matched general population cohort (107,500). The mean age of all groups was about 59 years.
The primary outcome was a first-ever solid or hematologic malignancy excluding nonmelanoma skin cancer. The fully adjusted risk model controlled for age, sex, educational level, comorbidities, seropositivity, number of hospitalizations and days spent in inpatient care, use of prednisone at baseline, use of nonsteroidal anti-inflammatory drugs at baseline, number of prescription drugs at baseline, and sick leave and disability.
A first invasive solid tumor or hematologic malignancy occurred in 50 patients taking tocilizumab; 61 taking abatacept; 141 taking rituximab; 478 taking a TNF-inhibitor as the first biologic DMARD; and 169 taking a TNF-inhibitor as the second DMARD. There was no statistically significant difference between any of these groups, when they were compared either to each other, to those taking a conventional synthetic DMARD, or to the general population.
The doubled risk of squamous cell carcinoma associated with abatacept (HR 2.2) was the only significant positive finding the secondary analysis, Dr. Wadstrom said.
He had no financial disclosures.
[email protected]
On Twitter @Alz_gal
MADRID – Biologics and tumor necrosis factor–inhibitors confer very little – if any – risk of malignancy upon those who take them for rheumatoid arthritis, according to a large Swedish registry study.
A doubling in the risk of squamous cell carcinoma among those who took abatacept was the only significant finding in the 8-year study of almost 70,000 subjects, Hjalmar Wadstrom, said at the European Congress of Rheumatology. And while he said the finding could be spurious, he stressed that it can’t be ignored.
“We think this finding should be interpreted cautiously,” said Mr. Wadstrom, a doctoral student at the Karolinska Institute, Stockholm. “These patients are seen frequently, and this increase in sqaumous cell carcinoma could be related to this multiple screening, or due to study bias. But of course, we cannot disregard it. It should be validated and further examined.”
The study plumbed several national patient registries and administrative databases for its cohort. It comprised 22,500 patients who, from 2006 to 2014, took tocilizumab, abatacept, or rituximab; or a TNF-inhibitor as a first or second disease-modifying antirheumatic drug. These were compared to 46,600 patients who took synthetic DMARDS during the same period and to a matched general population cohort (107,500). The mean age of all groups was about 59 years.
The primary outcome was a first-ever solid or hematologic malignancy excluding nonmelanoma skin cancer. The fully adjusted risk model controlled for age, sex, educational level, comorbidities, seropositivity, number of hospitalizations and days spent in inpatient care, use of prednisone at baseline, use of nonsteroidal anti-inflammatory drugs at baseline, number of prescription drugs at baseline, and sick leave and disability.
A first invasive solid tumor or hematologic malignancy occurred in 50 patients taking tocilizumab; 61 taking abatacept; 141 taking rituximab; 478 taking a TNF-inhibitor as the first biologic DMARD; and 169 taking a TNF-inhibitor as the second DMARD. There was no statistically significant difference between any of these groups, when they were compared either to each other, to those taking a conventional synthetic DMARD, or to the general population.
The doubled risk of squamous cell carcinoma associated with abatacept (HR 2.2) was the only significant positive finding the secondary analysis, Dr. Wadstrom said.
He had no financial disclosures.
[email protected]
On Twitter @Alz_gal
MADRID – Biologics and tumor necrosis factor–inhibitors confer very little – if any – risk of malignancy upon those who take them for rheumatoid arthritis, according to a large Swedish registry study.
A doubling in the risk of squamous cell carcinoma among those who took abatacept was the only significant finding in the 8-year study of almost 70,000 subjects, Hjalmar Wadstrom, said at the European Congress of Rheumatology. And while he said the finding could be spurious, he stressed that it can’t be ignored.
“We think this finding should be interpreted cautiously,” said Mr. Wadstrom, a doctoral student at the Karolinska Institute, Stockholm. “These patients are seen frequently, and this increase in sqaumous cell carcinoma could be related to this multiple screening, or due to study bias. But of course, we cannot disregard it. It should be validated and further examined.”
The study plumbed several national patient registries and administrative databases for its cohort. It comprised 22,500 patients who, from 2006 to 2014, took tocilizumab, abatacept, or rituximab; or a TNF-inhibitor as a first or second disease-modifying antirheumatic drug. These were compared to 46,600 patients who took synthetic DMARDS during the same period and to a matched general population cohort (107,500). The mean age of all groups was about 59 years.
The primary outcome was a first-ever solid or hematologic malignancy excluding nonmelanoma skin cancer. The fully adjusted risk model controlled for age, sex, educational level, comorbidities, seropositivity, number of hospitalizations and days spent in inpatient care, use of prednisone at baseline, use of nonsteroidal anti-inflammatory drugs at baseline, number of prescription drugs at baseline, and sick leave and disability.
A first invasive solid tumor or hematologic malignancy occurred in 50 patients taking tocilizumab; 61 taking abatacept; 141 taking rituximab; 478 taking a TNF-inhibitor as the first biologic DMARD; and 169 taking a TNF-inhibitor as the second DMARD. There was no statistically significant difference between any of these groups, when they were compared either to each other, to those taking a conventional synthetic DMARD, or to the general population.
The doubled risk of squamous cell carcinoma associated with abatacept (HR 2.2) was the only significant positive finding the secondary analysis, Dr. Wadstrom said.
He had no financial disclosures.
[email protected]
On Twitter @Alz_gal
AT EULAR 2017
Key clinical point:
Major finding: A twofold increased risk of squamous cell carcinoma was associated with abatacept.
Data source: The Swedish study examined almost 70,000 people in various national registries and health care databases.
Disclosures: Mr. Wadstrom had no financial disclosures.
Obeticholic acid fails to prevent liver damage in an animal model of short-bowel syndrome
Obeticholic acid failed to prevent the development of short-bowel syndrome–associated liver disease in a preliminary study using piglet models. The findings were published in the July issue of Cellular and Molecular Gastroenterology and Hepatology (doi: 10.1016/j.jcmgh.2017.02.008).
Current treatment options for short-bowel syndrome-associated liver disease are limited, wrote Prue M. Pereira-Fantini, PhD, of Murdoch Childrens Research Institute, Victoria, Australia, and colleagues. However, the farnesoid X receptor, which regulates genes involved in bile acid synthesis, absorption, and transport in the intestine and liver, has shown promise as a pharmaceutical target.
“Recently, we described SBS-ALD-associated alterations in bile acid composition associated with disrupted farnesoid X receptor (FXR) signaling mechanisms,” the researchers said. Obeticholic acid (OCA) has been shown to prevent liver disease in mouse models and human disease, and the researchers explored whether it would be effective in the context of short-bowel syndrome associated liver disease (SBS-ALD).
The researchers randomized piglets into four groups to receive small-bowel resection or sham surgery, and either a daily dose of 2.4 mg/kg per day of OCA or no treatment. The pigs were euthanized 2 weeks after their surgeries, and the researchers collected portal plasma samples, bile samples, and liver samples.
OCA treatment in piglets in the SBS surgery group was associated with decreased stool fat that suggested improved fat absorption, but impacted liver morphology, the researchers noted. “Untreated, sham-operated piglets showed normal liver histology when compared with SBS piglets who showed decreased hepatic lobule area and small clusters of inflammatory cells together with mild-to-moderate vesicular zone 2 lipidosis,” they wrote.
Overall, OCA treatment prevented the depletion of taurine; taurine concentration was approximately 8 ng/mL for piglets with SBS treated with OCA compared with 8 ng/mL in the sham group, 9 ng/mL in the sham plus OCA group, and 3 ng/mL in the SBS-only group. However, bile acid dysmetabolism occurred as shown by HDCA levels, which increased with OCA treatment compared to sham controls but were significantly reduced in SBS piglets treated with OCA vs. untreated SBS piglets.
In addition, the researchers found that small-bowel resection did not impact gene expression levels of FXR targets in the intestine or liver. However, “intestinal FXR gene expression was 11-fold higher in untreated SBS piglets when compared with untreated sham piglets,” they wrote. OCA treatment had no significant impact on FXR gene expression in the OCA-treated group vs. the untreated group and in the OCA-treated SBS group.
Although the findings were limited by use of an animal model, the results suggest that OCA treatment may have clinical benefits for SBS patients by reducing fat malabsorption, which remains a challenge, the researchers wrote. However, OCA “did not prevent the development of SBS-ALD, thereby limiting the potential therapeutic benefit in patients with SBS,” they concluded.
The researchers had no financial conflicts to disclose. The study was supported in part by the National Health and Medical Research Council of Australia and by a research grant from the Science Foundation Ireland.
Obeticholic acid failed to prevent the development of short-bowel syndrome–associated liver disease in a preliminary study using piglet models. The findings were published in the July issue of Cellular and Molecular Gastroenterology and Hepatology (doi: 10.1016/j.jcmgh.2017.02.008).
Current treatment options for short-bowel syndrome-associated liver disease are limited, wrote Prue M. Pereira-Fantini, PhD, of Murdoch Childrens Research Institute, Victoria, Australia, and colleagues. However, the farnesoid X receptor, which regulates genes involved in bile acid synthesis, absorption, and transport in the intestine and liver, has shown promise as a pharmaceutical target.
“Recently, we described SBS-ALD-associated alterations in bile acid composition associated with disrupted farnesoid X receptor (FXR) signaling mechanisms,” the researchers said. Obeticholic acid (OCA) has been shown to prevent liver disease in mouse models and human disease, and the researchers explored whether it would be effective in the context of short-bowel syndrome associated liver disease (SBS-ALD).
The researchers randomized piglets into four groups to receive small-bowel resection or sham surgery, and either a daily dose of 2.4 mg/kg per day of OCA or no treatment. The pigs were euthanized 2 weeks after their surgeries, and the researchers collected portal plasma samples, bile samples, and liver samples.
OCA treatment in piglets in the SBS surgery group was associated with decreased stool fat that suggested improved fat absorption, but impacted liver morphology, the researchers noted. “Untreated, sham-operated piglets showed normal liver histology when compared with SBS piglets who showed decreased hepatic lobule area and small clusters of inflammatory cells together with mild-to-moderate vesicular zone 2 lipidosis,” they wrote.
Overall, OCA treatment prevented the depletion of taurine; taurine concentration was approximately 8 ng/mL for piglets with SBS treated with OCA compared with 8 ng/mL in the sham group, 9 ng/mL in the sham plus OCA group, and 3 ng/mL in the SBS-only group. However, bile acid dysmetabolism occurred as shown by HDCA levels, which increased with OCA treatment compared to sham controls but were significantly reduced in SBS piglets treated with OCA vs. untreated SBS piglets.
In addition, the researchers found that small-bowel resection did not impact gene expression levels of FXR targets in the intestine or liver. However, “intestinal FXR gene expression was 11-fold higher in untreated SBS piglets when compared with untreated sham piglets,” they wrote. OCA treatment had no significant impact on FXR gene expression in the OCA-treated group vs. the untreated group and in the OCA-treated SBS group.
Although the findings were limited by use of an animal model, the results suggest that OCA treatment may have clinical benefits for SBS patients by reducing fat malabsorption, which remains a challenge, the researchers wrote. However, OCA “did not prevent the development of SBS-ALD, thereby limiting the potential therapeutic benefit in patients with SBS,” they concluded.
The researchers had no financial conflicts to disclose. The study was supported in part by the National Health and Medical Research Council of Australia and by a research grant from the Science Foundation Ireland.
Obeticholic acid failed to prevent the development of short-bowel syndrome–associated liver disease in a preliminary study using piglet models. The findings were published in the July issue of Cellular and Molecular Gastroenterology and Hepatology (doi: 10.1016/j.jcmgh.2017.02.008).
Current treatment options for short-bowel syndrome-associated liver disease are limited, wrote Prue M. Pereira-Fantini, PhD, of Murdoch Childrens Research Institute, Victoria, Australia, and colleagues. However, the farnesoid X receptor, which regulates genes involved in bile acid synthesis, absorption, and transport in the intestine and liver, has shown promise as a pharmaceutical target.
“Recently, we described SBS-ALD-associated alterations in bile acid composition associated with disrupted farnesoid X receptor (FXR) signaling mechanisms,” the researchers said. Obeticholic acid (OCA) has been shown to prevent liver disease in mouse models and human disease, and the researchers explored whether it would be effective in the context of short-bowel syndrome associated liver disease (SBS-ALD).
The researchers randomized piglets into four groups to receive small-bowel resection or sham surgery, and either a daily dose of 2.4 mg/kg per day of OCA or no treatment. The pigs were euthanized 2 weeks after their surgeries, and the researchers collected portal plasma samples, bile samples, and liver samples.
OCA treatment in piglets in the SBS surgery group was associated with decreased stool fat that suggested improved fat absorption, but impacted liver morphology, the researchers noted. “Untreated, sham-operated piglets showed normal liver histology when compared with SBS piglets who showed decreased hepatic lobule area and small clusters of inflammatory cells together with mild-to-moderate vesicular zone 2 lipidosis,” they wrote.
Overall, OCA treatment prevented the depletion of taurine; taurine concentration was approximately 8 ng/mL for piglets with SBS treated with OCA compared with 8 ng/mL in the sham group, 9 ng/mL in the sham plus OCA group, and 3 ng/mL in the SBS-only group. However, bile acid dysmetabolism occurred as shown by HDCA levels, which increased with OCA treatment compared to sham controls but were significantly reduced in SBS piglets treated with OCA vs. untreated SBS piglets.
In addition, the researchers found that small-bowel resection did not impact gene expression levels of FXR targets in the intestine or liver. However, “intestinal FXR gene expression was 11-fold higher in untreated SBS piglets when compared with untreated sham piglets,” they wrote. OCA treatment had no significant impact on FXR gene expression in the OCA-treated group vs. the untreated group and in the OCA-treated SBS group.
Although the findings were limited by use of an animal model, the results suggest that OCA treatment may have clinical benefits for SBS patients by reducing fat malabsorption, which remains a challenge, the researchers wrote. However, OCA “did not prevent the development of SBS-ALD, thereby limiting the potential therapeutic benefit in patients with SBS,” they concluded.
The researchers had no financial conflicts to disclose. The study was supported in part by the National Health and Medical Research Council of Australia and by a research grant from the Science Foundation Ireland.
FROM CMGH
Key clinical point: Treatment with obeticholic acid improved absorption and altered bile acid, but did not prevent liver damage in a piglet model of short-bowel syndrome.
Major finding: Overall, taurine concentration was approximately 8 ng/mL for piglets with SBS treated with OCA compared with 8 ng/mL in the sham surgery group, 9 ng/mL in the sham treated with OCA group, and 3 ng/mL in the SBS-only group.
Data source: The data come from piglets treated with obeticholic acid or untreated, and randomized to a small-bowel resection or a sham surgery.
Disclosures: The researchers had no financial conflicts to disclose,