User login
Therapeutic patient education is a new way to approach atopic dermatitis
(AD) resulting from poor adherence to treatment.
The aim of TPE, a multidisciplinary approach to caring for and managing AD, is to improve patient and caregiver adherence to physician-directed treatments through education and to improve quality of life, according to several presenters who spoke at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and University of California, San Diego. They reviewed the approach to using TPE in chronic disease states, demonstrating this state-of-the-art approach to educating patients, families, and health care providers about AD management, and allowing for the exchange of ideas for best practices on AD therapeutic education programs and use in the U.S. health care environment.
Other successful meetings on TPE in Europe, Asia, and South America inspired this meeting, the first in the United States aimed to train health care professionals in TPE principles for AD. The meeting was sponsored by the Fondation Dermatite Atopique (Atopic Dermatitis Foundation for Research and Education), Rady’s Eczema and Inflammatory Skin Disease Center, and UCSD.
TPE is defined by the World Health Organization as an approach to help patients with chronic illness acquire or maintain the skills necessary to manage their life and illness in the best way possible. TPE involves patient preferences, shared decision-making, organized activities, psychosocial support, hospital organization and procedures, and health- and disease-related behaviors.
Sébastien Barbarot, MD, of the departments of dermatology and pediatric dermatology, Nantes (France) University Hospital, said that there are four main components to the TPE process:
• Assessing and understanding the patient’s knowledge and values.
• Developing age-appropriate personalized educational objectives.
• Transferring the necessary skills to the patient or caregiver.
• Assessing the effectiveness of the educational program.
Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at UCSD, discussed how to conduct the initial visit with potential candidates for TPE. At the new patient visit, the pediatric patient and their caregivers should first be presented with the concept of TPE, which includes proposing a personalized education approach. Next, explain the goals and benefits of TPE (with a time and location for the sessions), provide educational materials, allow time for questions, and establish the patient’s consent to participate.
The certain skills that patients should acquire fall under three main categories: knowledge of the disease, practical skills, and relational skills.
Alain Golay, MD, professor and chief of the department of therapeutic education in chronic diseases at the Geneva University Hospital, said that patients and caregivers should be familiar with the pathophysiology and natural history of the disease, as well as with aggravating factors and the rationale behind elements of the treatment plan – and they should understand a reasonable timeline for treatment responses.
Learning how to properly apply the treatment is among the practical skills that the patient needs to acquire. In terms of relational skills, patients should know enough about their disease to be able to explain it to others. Educational methods can include interactive presentations, case studies, roundtable meetings, workshops, and role play. Other tools that can be used as a resource include written action plans, posters, informational videos, reminders, and booklets. Nurse-led educational sessions that increase teaching time is another modality. Multidisciplinary clinics should include an allergist, dermatologist, psychologist, dietitian, and nurse. These clinics also can form workshops or teaching groups. This allows for smaller groups where ideas can be exchanged, and can be targeted specifically based on the audiences’ needs, he said.
Dr. Barbarot outlined the fourth step of TPE, which involves assessment of effectiveness. Several outcome measures can be used, including clinical outcomes, quality of life, patient global assessment, and knowledge questionnaires.
(AD) resulting from poor adherence to treatment.
The aim of TPE, a multidisciplinary approach to caring for and managing AD, is to improve patient and caregiver adherence to physician-directed treatments through education and to improve quality of life, according to several presenters who spoke at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and University of California, San Diego. They reviewed the approach to using TPE in chronic disease states, demonstrating this state-of-the-art approach to educating patients, families, and health care providers about AD management, and allowing for the exchange of ideas for best practices on AD therapeutic education programs and use in the U.S. health care environment.
Other successful meetings on TPE in Europe, Asia, and South America inspired this meeting, the first in the United States aimed to train health care professionals in TPE principles for AD. The meeting was sponsored by the Fondation Dermatite Atopique (Atopic Dermatitis Foundation for Research and Education), Rady’s Eczema and Inflammatory Skin Disease Center, and UCSD.
TPE is defined by the World Health Organization as an approach to help patients with chronic illness acquire or maintain the skills necessary to manage their life and illness in the best way possible. TPE involves patient preferences, shared decision-making, organized activities, psychosocial support, hospital organization and procedures, and health- and disease-related behaviors.
Sébastien Barbarot, MD, of the departments of dermatology and pediatric dermatology, Nantes (France) University Hospital, said that there are four main components to the TPE process:
• Assessing and understanding the patient’s knowledge and values.
• Developing age-appropriate personalized educational objectives.
• Transferring the necessary skills to the patient or caregiver.
• Assessing the effectiveness of the educational program.
Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at UCSD, discussed how to conduct the initial visit with potential candidates for TPE. At the new patient visit, the pediatric patient and their caregivers should first be presented with the concept of TPE, which includes proposing a personalized education approach. Next, explain the goals and benefits of TPE (with a time and location for the sessions), provide educational materials, allow time for questions, and establish the patient’s consent to participate.
The certain skills that patients should acquire fall under three main categories: knowledge of the disease, practical skills, and relational skills.
Alain Golay, MD, professor and chief of the department of therapeutic education in chronic diseases at the Geneva University Hospital, said that patients and caregivers should be familiar with the pathophysiology and natural history of the disease, as well as with aggravating factors and the rationale behind elements of the treatment plan – and they should understand a reasonable timeline for treatment responses.
Learning how to properly apply the treatment is among the practical skills that the patient needs to acquire. In terms of relational skills, patients should know enough about their disease to be able to explain it to others. Educational methods can include interactive presentations, case studies, roundtable meetings, workshops, and role play. Other tools that can be used as a resource include written action plans, posters, informational videos, reminders, and booklets. Nurse-led educational sessions that increase teaching time is another modality. Multidisciplinary clinics should include an allergist, dermatologist, psychologist, dietitian, and nurse. These clinics also can form workshops or teaching groups. This allows for smaller groups where ideas can be exchanged, and can be targeted specifically based on the audiences’ needs, he said.
Dr. Barbarot outlined the fourth step of TPE, which involves assessment of effectiveness. Several outcome measures can be used, including clinical outcomes, quality of life, patient global assessment, and knowledge questionnaires.
(AD) resulting from poor adherence to treatment.
The aim of TPE, a multidisciplinary approach to caring for and managing AD, is to improve patient and caregiver adherence to physician-directed treatments through education and to improve quality of life, according to several presenters who spoke at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and University of California, San Diego. They reviewed the approach to using TPE in chronic disease states, demonstrating this state-of-the-art approach to educating patients, families, and health care providers about AD management, and allowing for the exchange of ideas for best practices on AD therapeutic education programs and use in the U.S. health care environment.
Other successful meetings on TPE in Europe, Asia, and South America inspired this meeting, the first in the United States aimed to train health care professionals in TPE principles for AD. The meeting was sponsored by the Fondation Dermatite Atopique (Atopic Dermatitis Foundation for Research and Education), Rady’s Eczema and Inflammatory Skin Disease Center, and UCSD.
TPE is defined by the World Health Organization as an approach to help patients with chronic illness acquire or maintain the skills necessary to manage their life and illness in the best way possible. TPE involves patient preferences, shared decision-making, organized activities, psychosocial support, hospital organization and procedures, and health- and disease-related behaviors.
Sébastien Barbarot, MD, of the departments of dermatology and pediatric dermatology, Nantes (France) University Hospital, said that there are four main components to the TPE process:
• Assessing and understanding the patient’s knowledge and values.
• Developing age-appropriate personalized educational objectives.
• Transferring the necessary skills to the patient or caregiver.
• Assessing the effectiveness of the educational program.
Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at UCSD, discussed how to conduct the initial visit with potential candidates for TPE. At the new patient visit, the pediatric patient and their caregivers should first be presented with the concept of TPE, which includes proposing a personalized education approach. Next, explain the goals and benefits of TPE (with a time and location for the sessions), provide educational materials, allow time for questions, and establish the patient’s consent to participate.
The certain skills that patients should acquire fall under three main categories: knowledge of the disease, practical skills, and relational skills.
Alain Golay, MD, professor and chief of the department of therapeutic education in chronic diseases at the Geneva University Hospital, said that patients and caregivers should be familiar with the pathophysiology and natural history of the disease, as well as with aggravating factors and the rationale behind elements of the treatment plan – and they should understand a reasonable timeline for treatment responses.
Learning how to properly apply the treatment is among the practical skills that the patient needs to acquire. In terms of relational skills, patients should know enough about their disease to be able to explain it to others. Educational methods can include interactive presentations, case studies, roundtable meetings, workshops, and role play. Other tools that can be used as a resource include written action plans, posters, informational videos, reminders, and booklets. Nurse-led educational sessions that increase teaching time is another modality. Multidisciplinary clinics should include an allergist, dermatologist, psychologist, dietitian, and nurse. These clinics also can form workshops or teaching groups. This allows for smaller groups where ideas can be exchanged, and can be targeted specifically based on the audiences’ needs, he said.
Dr. Barbarot outlined the fourth step of TPE, which involves assessment of effectiveness. Several outcome measures can be used, including clinical outcomes, quality of life, patient global assessment, and knowledge questionnaires.
VIDEO: Cancer immunotherapies activate rheumatologic adverse effects
MADRID – The introduction of immune checkpoint inhibitor drugs has “been great for cancer but bad for rheumatology.”
That’s the gist of the immunologic adverse effect fallout from the immunomodulatory revolution that’s recently swept oncology, Leonard Calabrese, DO, said in a video interview during the European Congress of Rheumatology.
Results from a recent survey of U.S. rheumatologists run by Dr. Calabrese and his associates showed that “more than a quarter” now have seen at least one patient who experienced activation of a rheumatologic disease after starting treatment with an immune checkpoint inhibitor, said Dr. Calabrese, head of the section of clinical immunology at the Cleveland Clinic in Ohio.
Unlike most other immunological adverse effects caused by immune checkpoint inhibitors, the rheumatologic complications usually don’t resolve when treatment stops, he added.
These adverse effects represent a new wrinkle for the practice of rheumatology and are now something that clinicians must familiarize themselves with, Dr. Calabrese advised.
Dr. Calabrese reported that he is a consultant to Bristol-Myers Squibb.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
MADRID – The introduction of immune checkpoint inhibitor drugs has “been great for cancer but bad for rheumatology.”
That’s the gist of the immunologic adverse effect fallout from the immunomodulatory revolution that’s recently swept oncology, Leonard Calabrese, DO, said in a video interview during the European Congress of Rheumatology.
Results from a recent survey of U.S. rheumatologists run by Dr. Calabrese and his associates showed that “more than a quarter” now have seen at least one patient who experienced activation of a rheumatologic disease after starting treatment with an immune checkpoint inhibitor, said Dr. Calabrese, head of the section of clinical immunology at the Cleveland Clinic in Ohio.
Unlike most other immunological adverse effects caused by immune checkpoint inhibitors, the rheumatologic complications usually don’t resolve when treatment stops, he added.
These adverse effects represent a new wrinkle for the practice of rheumatology and are now something that clinicians must familiarize themselves with, Dr. Calabrese advised.
Dr. Calabrese reported that he is a consultant to Bristol-Myers Squibb.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
MADRID – The introduction of immune checkpoint inhibitor drugs has “been great for cancer but bad for rheumatology.”
That’s the gist of the immunologic adverse effect fallout from the immunomodulatory revolution that’s recently swept oncology, Leonard Calabrese, DO, said in a video interview during the European Congress of Rheumatology.
Results from a recent survey of U.S. rheumatologists run by Dr. Calabrese and his associates showed that “more than a quarter” now have seen at least one patient who experienced activation of a rheumatologic disease after starting treatment with an immune checkpoint inhibitor, said Dr. Calabrese, head of the section of clinical immunology at the Cleveland Clinic in Ohio.
Unlike most other immunological adverse effects caused by immune checkpoint inhibitors, the rheumatologic complications usually don’t resolve when treatment stops, he added.
These adverse effects represent a new wrinkle for the practice of rheumatology and are now something that clinicians must familiarize themselves with, Dr. Calabrese advised.
Dr. Calabrese reported that he is a consultant to Bristol-Myers Squibb.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE EULAR 2017 CONGRESS
SUNSHINE: High-dose vitamin D boosts PFS in metastatic CRC
CHICAGO – High-dose vitamin D supplementation is a simple, safe approach for improving on the efficacy of first-line chemotherapy for metastatic colorectal cancer, suggest findings of the SUNSHINE trial reported at the annual meeting of the American Society of Clinical Oncology.
“Vitamin D has shown anti-neoplastic properties in the laboratory, including inhibition of cell proliferation and angiogenesis, induction of cell differentiation and apoptosis, as well as anti-inflammatory and immunomodulatory effects,” said lead author Kimmie Ng, MD, director of clinical research and a Gastrointestinal Cancer Center physician at the Dana-Farber Cancer Institute in Boston.
“The vitamin D hypothesis is also supported by a large body of epidemiologic evidence, both from our group as well as from others, that have shown that higher plasma 25-hydroxyvitamin D levels are associated with improved survival in patients with colorectal cancer,” she added.
Notably, in the CALGB/SWOG 80405 trial of first-line therapy for colorectal cancer, patients’ median 25-hydroxy vitamin D level at baseline fell below the cutoff for deficiency (ASCO 2015 meeting, Abstract 3503). Moreover, those having higher levels ultimately had better overall survival even after other factors were taken into account.
In the SUNSHINE trial, the investigators studied 139 patients with untreated metastatic colorectal cancer. Results showed that those given FOLFOX chemotherapy and the antiangiogenic agent bevacizumab (Avastin) plus high-dose vitamin D had a one-third lower risk of progression or death compared with counterparts given the same regimen plus low-dose vitamin D.
High-dose supplementation was not associated with greater toxicity. In fact, patients in that group had a much lower incidence of grade 3 or 4 diarrhea.
“After a decade of observational data linking higher vitamin D status with improved outcomes in colorectal cancer patients, SUNSHINE is the first completed randomized double-blind controlled clinical trial of vitamin D supplementation for treatment of colorectal cancer,” Dr. Ng said. “The trial met its primary endpoint. Given this data, a larger, confirmatory phase III trial is warranted.”
The investigators are performing subgroup analyses and analyzing overall survival, and will measure patients’ 25-hydroxyvitamin D levels in plasma samples collected serially throughout the study to determine whether they correlate with outcomes.
In addition, “to help understand and elucidate underlying mechanisms and biology, we have planned several correlative studies looking at tumoral and plasma biomarkers related to the vitamin D pathway, inflammation, and tumor immunity, among other pathways,” she further noted. “We will also be conducting next-generation sequencing and gene expression analyses.”
Expert perspective
“It will be interesting to know [patients’ vitamin D levels] as the majority of patients were enrolled in New England, where I think there is a little less sunshine than in other parts of the United States, so perhaps the vitamin D levels will reflect that,” said invited discussant Andrea Cercek, MD, of Memorial Sloan Kettering Cancer Center in New York.
Data on vitamin D pertaining to chemoprevention and to outcomes in other malignancies have been mixed, she cautioned. For example, supplementation did not reduce the risk of recurrent colorectal adenomas in one study (N Engl J Med. 2015;373:1519-30), and benefit or harm in terms of developing advanced colorectal adenomas in another study hinged on vitamin D receptor genotype (JAMA Oncol. 2017;3:628-35). Among patients with prostate cancer, addition of vitamin D to chemotherapy was actually associated with poorer overall survival (J Clin Oncol. 2011;29:2191-8).
“SUNSHINE was a positive study. It was a very well carried out phase II trial with a significant progression-free survival benefit. Correlative analyses are ongoing, and these will be critical, looking at biomarkers, perhaps helping us identify those patients who will benefit,” Dr. Cercek summarized. “I agree 100% with the investigators that a phase III study is warranted, and I look forward to it.”
Study details
Patients in SUNSHINE were randomized to receive first-line modified FOLFOX chemotherapy and bevacizumab plus either high-dose vitamin D (oral vitamin D3 8,000 IU/day for 2 weeks as a loading dose, followed by 4,000 IU/day) or low-dose vitamin D (oral vitamin D3 400 IU/day) on a double-blind basis. The latter “is an amount you would find in a multivitamin and only increases plasma levels by about 3 ng/mL, thus serving as a useful active control” Dr. Ng noted.
Receipt of chemotherapy and bevacizumab was generally similar between groups. “It is interesting to note that more patients receiving high-dose vitamin D discontinued treatment to undergo potentially curative surgery compared to those in the control arm, though this was not statistically different,” she noted.
In intent-to-treat analysis conducted at a median follow-up of 17-18 months, progression-free survival, the trial’s primary endpoint, was a median of 13.1 months in the high-dose group and 11.2 months in the low-dose group (P = .04). The difference in favor of high-dose vitamin D remained significant in multivariate analysis (hazard ratio, 0.67; P = .02).
The groups were statistically indistinguishable with respect to overall response rate, but the disease control rate was marginally better with high-dose versus low-dose vitamin D (96% vs. 84%, P = .05).
Rates of most grade 3 or 4 adverse events did not differ significantly between groups. However, diarrhea of these grades was less common in the high-dose vitamin D group (1% vs. 12%; P = .02). With respect to grade 3 or 4 events possibly related to the vitamin therapy, there was one case of hyperphosphatemia in the high-dose group and one case of kidney stones in the low-dose group.
Dr. Ng disclosed that she receives honoraria from Prime Oncology and Sage Publications; has a consulting or advisory role with Defined Health and Genentech/Roche; and receives research funding from Celgene, Genentech/Roche (institutional), Gilead Sciences, Pharmavite (institutional), and Trovagene.
CHICAGO – High-dose vitamin D supplementation is a simple, safe approach for improving on the efficacy of first-line chemotherapy for metastatic colorectal cancer, suggest findings of the SUNSHINE trial reported at the annual meeting of the American Society of Clinical Oncology.
“Vitamin D has shown anti-neoplastic properties in the laboratory, including inhibition of cell proliferation and angiogenesis, induction of cell differentiation and apoptosis, as well as anti-inflammatory and immunomodulatory effects,” said lead author Kimmie Ng, MD, director of clinical research and a Gastrointestinal Cancer Center physician at the Dana-Farber Cancer Institute in Boston.
“The vitamin D hypothesis is also supported by a large body of epidemiologic evidence, both from our group as well as from others, that have shown that higher plasma 25-hydroxyvitamin D levels are associated with improved survival in patients with colorectal cancer,” she added.
Notably, in the CALGB/SWOG 80405 trial of first-line therapy for colorectal cancer, patients’ median 25-hydroxy vitamin D level at baseline fell below the cutoff for deficiency (ASCO 2015 meeting, Abstract 3503). Moreover, those having higher levels ultimately had better overall survival even after other factors were taken into account.
In the SUNSHINE trial, the investigators studied 139 patients with untreated metastatic colorectal cancer. Results showed that those given FOLFOX chemotherapy and the antiangiogenic agent bevacizumab (Avastin) plus high-dose vitamin D had a one-third lower risk of progression or death compared with counterparts given the same regimen plus low-dose vitamin D.
High-dose supplementation was not associated with greater toxicity. In fact, patients in that group had a much lower incidence of grade 3 or 4 diarrhea.
“After a decade of observational data linking higher vitamin D status with improved outcomes in colorectal cancer patients, SUNSHINE is the first completed randomized double-blind controlled clinical trial of vitamin D supplementation for treatment of colorectal cancer,” Dr. Ng said. “The trial met its primary endpoint. Given this data, a larger, confirmatory phase III trial is warranted.”
The investigators are performing subgroup analyses and analyzing overall survival, and will measure patients’ 25-hydroxyvitamin D levels in plasma samples collected serially throughout the study to determine whether they correlate with outcomes.
In addition, “to help understand and elucidate underlying mechanisms and biology, we have planned several correlative studies looking at tumoral and plasma biomarkers related to the vitamin D pathway, inflammation, and tumor immunity, among other pathways,” she further noted. “We will also be conducting next-generation sequencing and gene expression analyses.”
Expert perspective
“It will be interesting to know [patients’ vitamin D levels] as the majority of patients were enrolled in New England, where I think there is a little less sunshine than in other parts of the United States, so perhaps the vitamin D levels will reflect that,” said invited discussant Andrea Cercek, MD, of Memorial Sloan Kettering Cancer Center in New York.
Data on vitamin D pertaining to chemoprevention and to outcomes in other malignancies have been mixed, she cautioned. For example, supplementation did not reduce the risk of recurrent colorectal adenomas in one study (N Engl J Med. 2015;373:1519-30), and benefit or harm in terms of developing advanced colorectal adenomas in another study hinged on vitamin D receptor genotype (JAMA Oncol. 2017;3:628-35). Among patients with prostate cancer, addition of vitamin D to chemotherapy was actually associated with poorer overall survival (J Clin Oncol. 2011;29:2191-8).
“SUNSHINE was a positive study. It was a very well carried out phase II trial with a significant progression-free survival benefit. Correlative analyses are ongoing, and these will be critical, looking at biomarkers, perhaps helping us identify those patients who will benefit,” Dr. Cercek summarized. “I agree 100% with the investigators that a phase III study is warranted, and I look forward to it.”
Study details
Patients in SUNSHINE were randomized to receive first-line modified FOLFOX chemotherapy and bevacizumab plus either high-dose vitamin D (oral vitamin D3 8,000 IU/day for 2 weeks as a loading dose, followed by 4,000 IU/day) or low-dose vitamin D (oral vitamin D3 400 IU/day) on a double-blind basis. The latter “is an amount you would find in a multivitamin and only increases plasma levels by about 3 ng/mL, thus serving as a useful active control” Dr. Ng noted.
Receipt of chemotherapy and bevacizumab was generally similar between groups. “It is interesting to note that more patients receiving high-dose vitamin D discontinued treatment to undergo potentially curative surgery compared to those in the control arm, though this was not statistically different,” she noted.
In intent-to-treat analysis conducted at a median follow-up of 17-18 months, progression-free survival, the trial’s primary endpoint, was a median of 13.1 months in the high-dose group and 11.2 months in the low-dose group (P = .04). The difference in favor of high-dose vitamin D remained significant in multivariate analysis (hazard ratio, 0.67; P = .02).
The groups were statistically indistinguishable with respect to overall response rate, but the disease control rate was marginally better with high-dose versus low-dose vitamin D (96% vs. 84%, P = .05).
Rates of most grade 3 or 4 adverse events did not differ significantly between groups. However, diarrhea of these grades was less common in the high-dose vitamin D group (1% vs. 12%; P = .02). With respect to grade 3 or 4 events possibly related to the vitamin therapy, there was one case of hyperphosphatemia in the high-dose group and one case of kidney stones in the low-dose group.
Dr. Ng disclosed that she receives honoraria from Prime Oncology and Sage Publications; has a consulting or advisory role with Defined Health and Genentech/Roche; and receives research funding from Celgene, Genentech/Roche (institutional), Gilead Sciences, Pharmavite (institutional), and Trovagene.
CHICAGO – High-dose vitamin D supplementation is a simple, safe approach for improving on the efficacy of first-line chemotherapy for metastatic colorectal cancer, suggest findings of the SUNSHINE trial reported at the annual meeting of the American Society of Clinical Oncology.
“Vitamin D has shown anti-neoplastic properties in the laboratory, including inhibition of cell proliferation and angiogenesis, induction of cell differentiation and apoptosis, as well as anti-inflammatory and immunomodulatory effects,” said lead author Kimmie Ng, MD, director of clinical research and a Gastrointestinal Cancer Center physician at the Dana-Farber Cancer Institute in Boston.
“The vitamin D hypothesis is also supported by a large body of epidemiologic evidence, both from our group as well as from others, that have shown that higher plasma 25-hydroxyvitamin D levels are associated with improved survival in patients with colorectal cancer,” she added.
Notably, in the CALGB/SWOG 80405 trial of first-line therapy for colorectal cancer, patients’ median 25-hydroxy vitamin D level at baseline fell below the cutoff for deficiency (ASCO 2015 meeting, Abstract 3503). Moreover, those having higher levels ultimately had better overall survival even after other factors were taken into account.
In the SUNSHINE trial, the investigators studied 139 patients with untreated metastatic colorectal cancer. Results showed that those given FOLFOX chemotherapy and the antiangiogenic agent bevacizumab (Avastin) plus high-dose vitamin D had a one-third lower risk of progression or death compared with counterparts given the same regimen plus low-dose vitamin D.
High-dose supplementation was not associated with greater toxicity. In fact, patients in that group had a much lower incidence of grade 3 or 4 diarrhea.
“After a decade of observational data linking higher vitamin D status with improved outcomes in colorectal cancer patients, SUNSHINE is the first completed randomized double-blind controlled clinical trial of vitamin D supplementation for treatment of colorectal cancer,” Dr. Ng said. “The trial met its primary endpoint. Given this data, a larger, confirmatory phase III trial is warranted.”
The investigators are performing subgroup analyses and analyzing overall survival, and will measure patients’ 25-hydroxyvitamin D levels in plasma samples collected serially throughout the study to determine whether they correlate with outcomes.
In addition, “to help understand and elucidate underlying mechanisms and biology, we have planned several correlative studies looking at tumoral and plasma biomarkers related to the vitamin D pathway, inflammation, and tumor immunity, among other pathways,” she further noted. “We will also be conducting next-generation sequencing and gene expression analyses.”
Expert perspective
“It will be interesting to know [patients’ vitamin D levels] as the majority of patients were enrolled in New England, where I think there is a little less sunshine than in other parts of the United States, so perhaps the vitamin D levels will reflect that,” said invited discussant Andrea Cercek, MD, of Memorial Sloan Kettering Cancer Center in New York.
Data on vitamin D pertaining to chemoprevention and to outcomes in other malignancies have been mixed, she cautioned. For example, supplementation did not reduce the risk of recurrent colorectal adenomas in one study (N Engl J Med. 2015;373:1519-30), and benefit or harm in terms of developing advanced colorectal adenomas in another study hinged on vitamin D receptor genotype (JAMA Oncol. 2017;3:628-35). Among patients with prostate cancer, addition of vitamin D to chemotherapy was actually associated with poorer overall survival (J Clin Oncol. 2011;29:2191-8).
“SUNSHINE was a positive study. It was a very well carried out phase II trial with a significant progression-free survival benefit. Correlative analyses are ongoing, and these will be critical, looking at biomarkers, perhaps helping us identify those patients who will benefit,” Dr. Cercek summarized. “I agree 100% with the investigators that a phase III study is warranted, and I look forward to it.”
Study details
Patients in SUNSHINE were randomized to receive first-line modified FOLFOX chemotherapy and bevacizumab plus either high-dose vitamin D (oral vitamin D3 8,000 IU/day for 2 weeks as a loading dose, followed by 4,000 IU/day) or low-dose vitamin D (oral vitamin D3 400 IU/day) on a double-blind basis. The latter “is an amount you would find in a multivitamin and only increases plasma levels by about 3 ng/mL, thus serving as a useful active control” Dr. Ng noted.
Receipt of chemotherapy and bevacizumab was generally similar between groups. “It is interesting to note that more patients receiving high-dose vitamin D discontinued treatment to undergo potentially curative surgery compared to those in the control arm, though this was not statistically different,” she noted.
In intent-to-treat analysis conducted at a median follow-up of 17-18 months, progression-free survival, the trial’s primary endpoint, was a median of 13.1 months in the high-dose group and 11.2 months in the low-dose group (P = .04). The difference in favor of high-dose vitamin D remained significant in multivariate analysis (hazard ratio, 0.67; P = .02).
The groups were statistically indistinguishable with respect to overall response rate, but the disease control rate was marginally better with high-dose versus low-dose vitamin D (96% vs. 84%, P = .05).
Rates of most grade 3 or 4 adverse events did not differ significantly between groups. However, diarrhea of these grades was less common in the high-dose vitamin D group (1% vs. 12%; P = .02). With respect to grade 3 or 4 events possibly related to the vitamin therapy, there was one case of hyperphosphatemia in the high-dose group and one case of kidney stones in the low-dose group.
Dr. Ng disclosed that she receives honoraria from Prime Oncology and Sage Publications; has a consulting or advisory role with Defined Health and Genentech/Roche; and receives research funding from Celgene, Genentech/Roche (institutional), Gilead Sciences, Pharmavite (institutional), and Trovagene.
AT ASCO 2017
Key clinical point:
Major finding: Compared with chemotherapy plus low-dose vitamin D, chemotherapy plus high-dose vitamin D was associated with a reduced risk of progression or death (hazard ratio, 0.67).
Data source: A phase II randomized trial (SUNSHINE) among 139 patients with untreated metastatic colorectal cancer.
Disclosures: Dr. Ng disclosed that she receives honoraria from Prime Oncology and Sage Publications; has a consulting or advisory role with Defined Health and Genentech/Roche; and receives research funding from Celgene, Genentech/Roche (institutional), Gilead Sciences, Pharmavite (institutional), and Trovagene.
New drug choices emerging to battle antibiotic resistance
SAN FRANCISCO – When the Infectious Diseases Society of America released the “Bad Bugs, No Drugs” report in 2004, its authors warned that effective antibiotics may not be available to treat seriously ill patients in the near future.
It also proposed legislative, regulatory, and funding solutions with a goal of developing and licensing 10 new antibiotics by the year 2020.
One such advancement was the Generating Antibiotics Incentives Now Act, which was signed into law in 2012 and created a designation for new antibiotics that are used to treat serious and/or life-threatening diseases due to certain pathogens. It also extends the patent life of these antibiotics and allows for fast-track Food and Drug Administration approval.
According to Dr. Erlich, chief of staff and medical director of infection control and antibiotic stewardship at Mills Peninsula Medical Center, Burlingame, Calif., increasingly common antibiotic-resistant pathogens besides MRSA and VRE include penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase–producing gram-negative rods, carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Mycobacterium tuberculosis, Salmonella enterica serotype Typhimurium DT 104, and drug-resistant Candida species.
Since 2010, several new antibiotics have been introduced to the market, including three second-generation lipoglycopeptide antibiotics with gram-positive coverage that are approved primarily for skin and soft tissue infections: dalbavancin (Dalvance), telavancin (Vibativ), and oritavancin (Orbactiv).
Compared with vancomycin, these new agents have more convenient dosing and a longer half life, “but they’re also more expensive,” said Dr. Erlich. Dalbavancin can be dosed once a week intravenously, telavancin can be dosed once daily intravenously, and oritavancin requires just one dose.
Another new agent is tedizolid phosphate (Sivextro), a second-generation oxazolidinone that is in the same drug class as linezolid (Zyvox). Tedizolid phosphate has gram-positive coverage including MRSA, but it is not approved for VRE. “It’s FDA approved for skin and soft-tissue infections (SSTI) but can be used for other locations as well,” Dr. Erlich said. “It features once-daily dosing IV or PO.”
Ceftaroline fosamil (Teflaro), ceftolozane/tazobactam (Zerbaxa), and ceftazidime/avibactam (Avycaz) are broad-spectrum cephalosporins with or without beta-lactamase inhibitors resulting in extended gram-negative coverage. FDA-approved indications include complicated urinary tract infections, complicated abdominal infections, SSTI, and pneumonia.
The primary advantage of these drugs, compared with other agents, is for multidrug-resistant gram-negative bacteria such as extended-spectrum beta-lactamase producers and CRE. “We’re not using a lot of these drugs in clinical practice, but they are available for patients with multidrug-resistant gram-negative rods who have no other options,” Dr. Erlich said.
Practical ways that clinicians can prevent antibiotic resistance include prescribing antibiotics only when necessary. “Be aware of local resistance patterns, avoid antibiotics for probable viral infections, use narrow-spectrum choices when possible, use shorter durations when appropriate, and consult published guidelines for optimal empiric antibiotic therapy,” Dr. Erlich advised.
In addition, “advocate infection control measures to keep patients from developing infections, including proper wound care, hand washing, respiratory etiquette, vaccinations, and social isolation for symptomatic individuals,” he noted.
Dr. Erlich reported having no relevant financial disclosures.
SAN FRANCISCO – When the Infectious Diseases Society of America released the “Bad Bugs, No Drugs” report in 2004, its authors warned that effective antibiotics may not be available to treat seriously ill patients in the near future.
It also proposed legislative, regulatory, and funding solutions with a goal of developing and licensing 10 new antibiotics by the year 2020.
One such advancement was the Generating Antibiotics Incentives Now Act, which was signed into law in 2012 and created a designation for new antibiotics that are used to treat serious and/or life-threatening diseases due to certain pathogens. It also extends the patent life of these antibiotics and allows for fast-track Food and Drug Administration approval.
According to Dr. Erlich, chief of staff and medical director of infection control and antibiotic stewardship at Mills Peninsula Medical Center, Burlingame, Calif., increasingly common antibiotic-resistant pathogens besides MRSA and VRE include penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase–producing gram-negative rods, carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Mycobacterium tuberculosis, Salmonella enterica serotype Typhimurium DT 104, and drug-resistant Candida species.
Since 2010, several new antibiotics have been introduced to the market, including three second-generation lipoglycopeptide antibiotics with gram-positive coverage that are approved primarily for skin and soft tissue infections: dalbavancin (Dalvance), telavancin (Vibativ), and oritavancin (Orbactiv).
Compared with vancomycin, these new agents have more convenient dosing and a longer half life, “but they’re also more expensive,” said Dr. Erlich. Dalbavancin can be dosed once a week intravenously, telavancin can be dosed once daily intravenously, and oritavancin requires just one dose.
Another new agent is tedizolid phosphate (Sivextro), a second-generation oxazolidinone that is in the same drug class as linezolid (Zyvox). Tedizolid phosphate has gram-positive coverage including MRSA, but it is not approved for VRE. “It’s FDA approved for skin and soft-tissue infections (SSTI) but can be used for other locations as well,” Dr. Erlich said. “It features once-daily dosing IV or PO.”
Ceftaroline fosamil (Teflaro), ceftolozane/tazobactam (Zerbaxa), and ceftazidime/avibactam (Avycaz) are broad-spectrum cephalosporins with or without beta-lactamase inhibitors resulting in extended gram-negative coverage. FDA-approved indications include complicated urinary tract infections, complicated abdominal infections, SSTI, and pneumonia.
The primary advantage of these drugs, compared with other agents, is for multidrug-resistant gram-negative bacteria such as extended-spectrum beta-lactamase producers and CRE. “We’re not using a lot of these drugs in clinical practice, but they are available for patients with multidrug-resistant gram-negative rods who have no other options,” Dr. Erlich said.
Practical ways that clinicians can prevent antibiotic resistance include prescribing antibiotics only when necessary. “Be aware of local resistance patterns, avoid antibiotics for probable viral infections, use narrow-spectrum choices when possible, use shorter durations when appropriate, and consult published guidelines for optimal empiric antibiotic therapy,” Dr. Erlich advised.
In addition, “advocate infection control measures to keep patients from developing infections, including proper wound care, hand washing, respiratory etiquette, vaccinations, and social isolation for symptomatic individuals,” he noted.
Dr. Erlich reported having no relevant financial disclosures.
SAN FRANCISCO – When the Infectious Diseases Society of America released the “Bad Bugs, No Drugs” report in 2004, its authors warned that effective antibiotics may not be available to treat seriously ill patients in the near future.
It also proposed legislative, regulatory, and funding solutions with a goal of developing and licensing 10 new antibiotics by the year 2020.
One such advancement was the Generating Antibiotics Incentives Now Act, which was signed into law in 2012 and created a designation for new antibiotics that are used to treat serious and/or life-threatening diseases due to certain pathogens. It also extends the patent life of these antibiotics and allows for fast-track Food and Drug Administration approval.
According to Dr. Erlich, chief of staff and medical director of infection control and antibiotic stewardship at Mills Peninsula Medical Center, Burlingame, Calif., increasingly common antibiotic-resistant pathogens besides MRSA and VRE include penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase–producing gram-negative rods, carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Mycobacterium tuberculosis, Salmonella enterica serotype Typhimurium DT 104, and drug-resistant Candida species.
Since 2010, several new antibiotics have been introduced to the market, including three second-generation lipoglycopeptide antibiotics with gram-positive coverage that are approved primarily for skin and soft tissue infections: dalbavancin (Dalvance), telavancin (Vibativ), and oritavancin (Orbactiv).
Compared with vancomycin, these new agents have more convenient dosing and a longer half life, “but they’re also more expensive,” said Dr. Erlich. Dalbavancin can be dosed once a week intravenously, telavancin can be dosed once daily intravenously, and oritavancin requires just one dose.
Another new agent is tedizolid phosphate (Sivextro), a second-generation oxazolidinone that is in the same drug class as linezolid (Zyvox). Tedizolid phosphate has gram-positive coverage including MRSA, but it is not approved for VRE. “It’s FDA approved for skin and soft-tissue infections (SSTI) but can be used for other locations as well,” Dr. Erlich said. “It features once-daily dosing IV or PO.”
Ceftaroline fosamil (Teflaro), ceftolozane/tazobactam (Zerbaxa), and ceftazidime/avibactam (Avycaz) are broad-spectrum cephalosporins with or without beta-lactamase inhibitors resulting in extended gram-negative coverage. FDA-approved indications include complicated urinary tract infections, complicated abdominal infections, SSTI, and pneumonia.
The primary advantage of these drugs, compared with other agents, is for multidrug-resistant gram-negative bacteria such as extended-spectrum beta-lactamase producers and CRE. “We’re not using a lot of these drugs in clinical practice, but they are available for patients with multidrug-resistant gram-negative rods who have no other options,” Dr. Erlich said.
Practical ways that clinicians can prevent antibiotic resistance include prescribing antibiotics only when necessary. “Be aware of local resistance patterns, avoid antibiotics for probable viral infections, use narrow-spectrum choices when possible, use shorter durations when appropriate, and consult published guidelines for optimal empiric antibiotic therapy,” Dr. Erlich advised.
In addition, “advocate infection control measures to keep patients from developing infections, including proper wound care, hand washing, respiratory etiquette, vaccinations, and social isolation for symptomatic individuals,” he noted.
Dr. Erlich reported having no relevant financial disclosures.
AT THE ANNUAL ADVANCES IN INTERNAL MEDICINE
Ibrutinib/buparlisib looks good for relapsed mantle cell lymphoma
LUGANO, SWITZERLAND – A combination of the Bruton’s tyrosine kinase (BTK) ibrutinib (Imbruvica) and the pan-phosphoinositide 3-kinase (PI3K) inhibitor buparlisib showed clinical activity superior to that of single-agent ibrutinib in patients with relapsed/refractory mantle cell lymphoma (MCL).
In a phase I/IB dose escalation study and expansion cohort testing the combination in patients with diffuse large B cell lymphoma (DLBCL), follicular lymphoma, and MCL, the overall response rate (ORR) among patients with MCL was 100%, consisting of complete responses (CR) in 8 of 11 patients, and partial responses (PR) in 3 patients, reported Connie Lee Batlevi, MD, of Memorial Sloan Kettering Cancer Center in New York.
In contrast, the response rate to ibrutinib monotherapy among patients with relapsed MCL is around 20%, she said.
Preclinical studies have demonstrated synergism between BTK inhibitors and PI3K inhibitors in B-cell non-Hodgkin lymphoma (NHL), prompting the investigators to look into the combination in patients with relapsed or refractory DLBCL, follicular lymphoma (FL), and MCL.
They enrolled 25 patients (9 with DLBCL, 5 with FL, and 11 with MCL). The patients received escalating doses of once daily ibrutinib and buparlisib in three dose levels (ibrutinib 420-560 mg; buparlisib 80-100 mg). Dose level 3, consisting of ibrutinib 560 mg and buparlisib 100 mg, was selected for dose expansion based on one of six patients developing a dose-limiting toxicity.
Using the Lugano Response Criteria, the overall ORR (all histologies) was 52%. Among nine patients with DLBCL, the ORR was 11%, with one CR and no PR. Among five patients with FL, the ORR was 20%, consisting of one CR and no PR.
Among 11 patients with MCL, however, the ORR was far more impressive, at 100%, including eight CR and three PR. No patients with MCL had either stable or progressive disease.
Under the RECIL (International Working Group) criteria, the ORR was 48% including one CR each for DLBCL and FL, and eight CR and two PR for patients with MCL.
In the safety analysis, there were two dose-limiting toxicities in the lowest and highest dose groups, but none at dose level 2 (ibrutinib 560 mg and buparlisib 80 mg).
Grade 3 or greater adverse events occurred in 63% of patients. The most common events were hyperglycemia and rash in 19% each, and diarrhea, anorexia, and neurologic changes in 11% each,
The grade 3 neurologic changes included depression, agitation, mood swings, confusion and memory impairment, all of which resolved after buparlisib was withdrawn.
Dr. Batlevi showed scans of two patients with representative clinical responses in MCL. One 55-year-old man with blastoid MCL who had relapsed 18 months after frontline therapy with ofatumumab and bendamustine had near total clearance of lesions after two cycles of ibrutinib and buparlisib. He remains in CR after 12 months on the combination.
A second patient, a 77-year-old man with MCL that relapsed 10 years after R-CHOP and rituximab maintenance followed by autologous stem cell transplant, showed a complete response upon restaging after two cycles of ibrutinib/buparlisib.
The investigators are currently enrolling patients for phase IB expansions, with the goal of better estimating the safety and efficacy of the combination.
Memorial Sloan Kettering Cancer Center sponsored the study, with support from Janssen and Novartis. Dr. Batlevi reported no conflicts of interest.
LUGANO, SWITZERLAND – A combination of the Bruton’s tyrosine kinase (BTK) ibrutinib (Imbruvica) and the pan-phosphoinositide 3-kinase (PI3K) inhibitor buparlisib showed clinical activity superior to that of single-agent ibrutinib in patients with relapsed/refractory mantle cell lymphoma (MCL).
In a phase I/IB dose escalation study and expansion cohort testing the combination in patients with diffuse large B cell lymphoma (DLBCL), follicular lymphoma, and MCL, the overall response rate (ORR) among patients with MCL was 100%, consisting of complete responses (CR) in 8 of 11 patients, and partial responses (PR) in 3 patients, reported Connie Lee Batlevi, MD, of Memorial Sloan Kettering Cancer Center in New York.
In contrast, the response rate to ibrutinib monotherapy among patients with relapsed MCL is around 20%, she said.
Preclinical studies have demonstrated synergism between BTK inhibitors and PI3K inhibitors in B-cell non-Hodgkin lymphoma (NHL), prompting the investigators to look into the combination in patients with relapsed or refractory DLBCL, follicular lymphoma (FL), and MCL.
They enrolled 25 patients (9 with DLBCL, 5 with FL, and 11 with MCL). The patients received escalating doses of once daily ibrutinib and buparlisib in three dose levels (ibrutinib 420-560 mg; buparlisib 80-100 mg). Dose level 3, consisting of ibrutinib 560 mg and buparlisib 100 mg, was selected for dose expansion based on one of six patients developing a dose-limiting toxicity.
Using the Lugano Response Criteria, the overall ORR (all histologies) was 52%. Among nine patients with DLBCL, the ORR was 11%, with one CR and no PR. Among five patients with FL, the ORR was 20%, consisting of one CR and no PR.
Among 11 patients with MCL, however, the ORR was far more impressive, at 100%, including eight CR and three PR. No patients with MCL had either stable or progressive disease.
Under the RECIL (International Working Group) criteria, the ORR was 48% including one CR each for DLBCL and FL, and eight CR and two PR for patients with MCL.
In the safety analysis, there were two dose-limiting toxicities in the lowest and highest dose groups, but none at dose level 2 (ibrutinib 560 mg and buparlisib 80 mg).
Grade 3 or greater adverse events occurred in 63% of patients. The most common events were hyperglycemia and rash in 19% each, and diarrhea, anorexia, and neurologic changes in 11% each,
The grade 3 neurologic changes included depression, agitation, mood swings, confusion and memory impairment, all of which resolved after buparlisib was withdrawn.
Dr. Batlevi showed scans of two patients with representative clinical responses in MCL. One 55-year-old man with blastoid MCL who had relapsed 18 months after frontline therapy with ofatumumab and bendamustine had near total clearance of lesions after two cycles of ibrutinib and buparlisib. He remains in CR after 12 months on the combination.
A second patient, a 77-year-old man with MCL that relapsed 10 years after R-CHOP and rituximab maintenance followed by autologous stem cell transplant, showed a complete response upon restaging after two cycles of ibrutinib/buparlisib.
The investigators are currently enrolling patients for phase IB expansions, with the goal of better estimating the safety and efficacy of the combination.
Memorial Sloan Kettering Cancer Center sponsored the study, with support from Janssen and Novartis. Dr. Batlevi reported no conflicts of interest.
LUGANO, SWITZERLAND – A combination of the Bruton’s tyrosine kinase (BTK) ibrutinib (Imbruvica) and the pan-phosphoinositide 3-kinase (PI3K) inhibitor buparlisib showed clinical activity superior to that of single-agent ibrutinib in patients with relapsed/refractory mantle cell lymphoma (MCL).
In a phase I/IB dose escalation study and expansion cohort testing the combination in patients with diffuse large B cell lymphoma (DLBCL), follicular lymphoma, and MCL, the overall response rate (ORR) among patients with MCL was 100%, consisting of complete responses (CR) in 8 of 11 patients, and partial responses (PR) in 3 patients, reported Connie Lee Batlevi, MD, of Memorial Sloan Kettering Cancer Center in New York.
In contrast, the response rate to ibrutinib monotherapy among patients with relapsed MCL is around 20%, she said.
Preclinical studies have demonstrated synergism between BTK inhibitors and PI3K inhibitors in B-cell non-Hodgkin lymphoma (NHL), prompting the investigators to look into the combination in patients with relapsed or refractory DLBCL, follicular lymphoma (FL), and MCL.
They enrolled 25 patients (9 with DLBCL, 5 with FL, and 11 with MCL). The patients received escalating doses of once daily ibrutinib and buparlisib in three dose levels (ibrutinib 420-560 mg; buparlisib 80-100 mg). Dose level 3, consisting of ibrutinib 560 mg and buparlisib 100 mg, was selected for dose expansion based on one of six patients developing a dose-limiting toxicity.
Using the Lugano Response Criteria, the overall ORR (all histologies) was 52%. Among nine patients with DLBCL, the ORR was 11%, with one CR and no PR. Among five patients with FL, the ORR was 20%, consisting of one CR and no PR.
Among 11 patients with MCL, however, the ORR was far more impressive, at 100%, including eight CR and three PR. No patients with MCL had either stable or progressive disease.
Under the RECIL (International Working Group) criteria, the ORR was 48% including one CR each for DLBCL and FL, and eight CR and two PR for patients with MCL.
In the safety analysis, there were two dose-limiting toxicities in the lowest and highest dose groups, but none at dose level 2 (ibrutinib 560 mg and buparlisib 80 mg).
Grade 3 or greater adverse events occurred in 63% of patients. The most common events were hyperglycemia and rash in 19% each, and diarrhea, anorexia, and neurologic changes in 11% each,
The grade 3 neurologic changes included depression, agitation, mood swings, confusion and memory impairment, all of which resolved after buparlisib was withdrawn.
Dr. Batlevi showed scans of two patients with representative clinical responses in MCL. One 55-year-old man with blastoid MCL who had relapsed 18 months after frontline therapy with ofatumumab and bendamustine had near total clearance of lesions after two cycles of ibrutinib and buparlisib. He remains in CR after 12 months on the combination.
A second patient, a 77-year-old man with MCL that relapsed 10 years after R-CHOP and rituximab maintenance followed by autologous stem cell transplant, showed a complete response upon restaging after two cycles of ibrutinib/buparlisib.
The investigators are currently enrolling patients for phase IB expansions, with the goal of better estimating the safety and efficacy of the combination.
Memorial Sloan Kettering Cancer Center sponsored the study, with support from Janssen and Novartis. Dr. Batlevi reported no conflicts of interest.
AT 14-ICML
Key clinical point: The combination of ibrutinib and buparlisib showed efficacy against mantle cell lymphoma in a dose-escalation and safety study,
Major finding: The overall response rate to the combination among 11 patients with relapsed MCL was 100%.
Data source: Open label phase I/IB study of 25 patients with B-cell lymphomas.
Disclosures: Memorial Sloan Kettering Cancer Center sponsored the study, with support from Janssen and Novartis. Dr. Batlevi reported no conflicts of interest.
Knee bone density improved in osteoarthritis with load-reducing shoe
LAS VEGAS – Patients with medial compartment knee osteoarthritis who wore a patented flexible mobility shoe experienced a favorable reduction in medial tibial bone mineral density that directly correlated with their improved gait biomechanics and reduced peak knee adduction moment, Najia Shakoor, MD, reported at the World Congress on Osteoarthritis.
“Our results suggest that bone can be modified with sustained load reduction and that evaluation of tibial bone density may be an inexpensive tool for evaluating the consequences of load-reducing interventions,” said Dr. Shakoor, a rheumatologist at Rush University in Chicago.
Indeed, measuring changes in medial tibial bone density over time via serial dual x-ray absorptiometry is an attractive surrogate anatomic marker of a patient’s response to a biomechanical load-reducing intervention such as a special shoe or knee brace, Dr. Shakoor noted at the meeting sponsored by the Osteoarthritis Research Society International.
After all, she added, bone density measurement is simpler than sending a patient to a motion analysis laboratory for multicamera gait analysis using a force plate to evaluate changes in the peak external knee adduction moment (a validated marker of load distribution across the tibial plateau).
Studies suggest that bone, not cartilage, bears the bulk of the load burden across the knee joint. That’s why patients with knee osteoarthritis have increased proximal tibial bone mineral density. Dr. Shakoor presented evidence that sustained reduction in dynamic knee loading results in a proportionate reduction in medial tibial bone density over the course of 6 months.
She reported on 51 patients with mild to moderate radiographic and symptomatic medial compartment knee osteoarthritis who were randomized to wear a commercially available flexible mobility shoe or a similar-looking but nonflexible control shoe for 6 hours per day for at least 6 days per week for 6 months. At baseline and again at 6 months, the participants underwent knee bone density measurement and formal gait analysis.
Peak knee adduction moment decreased by 14% over the course of 6 months in the flexible shoe group, significantly greater than the 6% reduction in the controls. Moreover, Dr. Shakoor and her coinvestigators documented a significant reduction in medial tibial bone density in the flexible shoe group. The greater the improvement in knee adduction moment, the larger the reduction in bone density.
In contrast, medial tibial bone density didn’t change significantly in the controls.
Dr. Shakoor said that she had also expected to see a reduction in the ratio of medial to lateral tibial bone density in the flexible shoe group. However, there was no statistically significant change, although there was a trend in that direction.
Asked if reduction in knee adduction moment and/or medial tibial bone density correlated with improved knee pain scores, Dr. Shakoor replied that almost everyone in the study reported improvement in pain, suggesting a placebo effect for that endpoint. In any event, the relatively small study wasn’t powered to evaluate change in pain over time.
The Arthritis Foundation funded the study. Dr. Shakoor is coinventor of the flexible shoe used in the study. The patent, owned by Rush University, has been licensed to Dr. Comfort, which markets the shoe as the Dr. Comfort Flex-OA Mobility Shoe. A percentage of the proceeds from shoe sales is distributed to the university and the coinventors.
LAS VEGAS – Patients with medial compartment knee osteoarthritis who wore a patented flexible mobility shoe experienced a favorable reduction in medial tibial bone mineral density that directly correlated with their improved gait biomechanics and reduced peak knee adduction moment, Najia Shakoor, MD, reported at the World Congress on Osteoarthritis.
“Our results suggest that bone can be modified with sustained load reduction and that evaluation of tibial bone density may be an inexpensive tool for evaluating the consequences of load-reducing interventions,” said Dr. Shakoor, a rheumatologist at Rush University in Chicago.
Indeed, measuring changes in medial tibial bone density over time via serial dual x-ray absorptiometry is an attractive surrogate anatomic marker of a patient’s response to a biomechanical load-reducing intervention such as a special shoe or knee brace, Dr. Shakoor noted at the meeting sponsored by the Osteoarthritis Research Society International.
After all, she added, bone density measurement is simpler than sending a patient to a motion analysis laboratory for multicamera gait analysis using a force plate to evaluate changes in the peak external knee adduction moment (a validated marker of load distribution across the tibial plateau).
Studies suggest that bone, not cartilage, bears the bulk of the load burden across the knee joint. That’s why patients with knee osteoarthritis have increased proximal tibial bone mineral density. Dr. Shakoor presented evidence that sustained reduction in dynamic knee loading results in a proportionate reduction in medial tibial bone density over the course of 6 months.
She reported on 51 patients with mild to moderate radiographic and symptomatic medial compartment knee osteoarthritis who were randomized to wear a commercially available flexible mobility shoe or a similar-looking but nonflexible control shoe for 6 hours per day for at least 6 days per week for 6 months. At baseline and again at 6 months, the participants underwent knee bone density measurement and formal gait analysis.
Peak knee adduction moment decreased by 14% over the course of 6 months in the flexible shoe group, significantly greater than the 6% reduction in the controls. Moreover, Dr. Shakoor and her coinvestigators documented a significant reduction in medial tibial bone density in the flexible shoe group. The greater the improvement in knee adduction moment, the larger the reduction in bone density.
In contrast, medial tibial bone density didn’t change significantly in the controls.
Dr. Shakoor said that she had also expected to see a reduction in the ratio of medial to lateral tibial bone density in the flexible shoe group. However, there was no statistically significant change, although there was a trend in that direction.
Asked if reduction in knee adduction moment and/or medial tibial bone density correlated with improved knee pain scores, Dr. Shakoor replied that almost everyone in the study reported improvement in pain, suggesting a placebo effect for that endpoint. In any event, the relatively small study wasn’t powered to evaluate change in pain over time.
The Arthritis Foundation funded the study. Dr. Shakoor is coinventor of the flexible shoe used in the study. The patent, owned by Rush University, has been licensed to Dr. Comfort, which markets the shoe as the Dr. Comfort Flex-OA Mobility Shoe. A percentage of the proceeds from shoe sales is distributed to the university and the coinventors.
LAS VEGAS – Patients with medial compartment knee osteoarthritis who wore a patented flexible mobility shoe experienced a favorable reduction in medial tibial bone mineral density that directly correlated with their improved gait biomechanics and reduced peak knee adduction moment, Najia Shakoor, MD, reported at the World Congress on Osteoarthritis.
“Our results suggest that bone can be modified with sustained load reduction and that evaluation of tibial bone density may be an inexpensive tool for evaluating the consequences of load-reducing interventions,” said Dr. Shakoor, a rheumatologist at Rush University in Chicago.
Indeed, measuring changes in medial tibial bone density over time via serial dual x-ray absorptiometry is an attractive surrogate anatomic marker of a patient’s response to a biomechanical load-reducing intervention such as a special shoe or knee brace, Dr. Shakoor noted at the meeting sponsored by the Osteoarthritis Research Society International.
After all, she added, bone density measurement is simpler than sending a patient to a motion analysis laboratory for multicamera gait analysis using a force plate to evaluate changes in the peak external knee adduction moment (a validated marker of load distribution across the tibial plateau).
Studies suggest that bone, not cartilage, bears the bulk of the load burden across the knee joint. That’s why patients with knee osteoarthritis have increased proximal tibial bone mineral density. Dr. Shakoor presented evidence that sustained reduction in dynamic knee loading results in a proportionate reduction in medial tibial bone density over the course of 6 months.
She reported on 51 patients with mild to moderate radiographic and symptomatic medial compartment knee osteoarthritis who were randomized to wear a commercially available flexible mobility shoe or a similar-looking but nonflexible control shoe for 6 hours per day for at least 6 days per week for 6 months. At baseline and again at 6 months, the participants underwent knee bone density measurement and formal gait analysis.
Peak knee adduction moment decreased by 14% over the course of 6 months in the flexible shoe group, significantly greater than the 6% reduction in the controls. Moreover, Dr. Shakoor and her coinvestigators documented a significant reduction in medial tibial bone density in the flexible shoe group. The greater the improvement in knee adduction moment, the larger the reduction in bone density.
In contrast, medial tibial bone density didn’t change significantly in the controls.
Dr. Shakoor said that she had also expected to see a reduction in the ratio of medial to lateral tibial bone density in the flexible shoe group. However, there was no statistically significant change, although there was a trend in that direction.
Asked if reduction in knee adduction moment and/or medial tibial bone density correlated with improved knee pain scores, Dr. Shakoor replied that almost everyone in the study reported improvement in pain, suggesting a placebo effect for that endpoint. In any event, the relatively small study wasn’t powered to evaluate change in pain over time.
The Arthritis Foundation funded the study. Dr. Shakoor is coinventor of the flexible shoe used in the study. The patent, owned by Rush University, has been licensed to Dr. Comfort, which markets the shoe as the Dr. Comfort Flex-OA Mobility Shoe. A percentage of the proceeds from shoe sales is distributed to the university and the coinventors.
AT OARSI 2017
Key clinical point:
Major finding: Knee osteoarthritis patients who wore a flexible mobility shoe designed to reduce dynamic loading of the joint had a 14% reduction in peak external knee adduction moment over a 6-month period, with a parallel decrease in medial tibial bone density.
Data source: A 6-month randomized trial involving 51 patients with symptomatic radiographic medial compartment knee osteoarthritis, who were assigned to wear a shoe designed to reduce dynamic knee loading or a similar-looking control shoe.
Disclosures: The Arthritis Foundation funded the study. Dr. Shakoor is coinventor of the flexible shoe used in the study. The patent, owned by Rush University, has been licensed to Dr. Comfort, which markets the shoe as the Dr. Comfort Flex-OA Mobility Shoe. A percentage of the proceeds from shoe sales is distributed to the university and the coinventors.
Female genital mutilation is seen by most ob.gyns.
SAN DIEGO – Nearly 60% of ob.gyns. have seen patients who have experienced female genital mutilation (FGM), according to the results of a survey of 288 fellows of the American College of Obstetricians and Gynecologists.
Additionally, the survey found that there are few guidelines for care of these patients. Among fellows who had seen patients with FGM, 80.1% said their institutions had no policies or guidelines for management of these patients. Additionally, just 56.7% of fellows who had treated these women were aware that federal laws prohibit FGM.
The findings came as a surprise to the study’s senior author, Alireza Shamshirsaz, MD, a maternal-fetal medicine specialist at Baylor College of Medicine, Houston. “I really see the gap of knowledge of [ob.gyns.] in the States, and the emergent need for guidelines and regulation.”
Ob.gyns. who cared for women who had experienced FGM reported that they used a combination of approaches in treating these patients. Nearly half of physicians (45.8%) said they generally treated FGM patients the same as their other patients, but nearly three-quarters said they also discuss potential complications of FGM procedures. About 28% of physicians reported providing information about surgical repair options, while less than 5% provided mental health referrals.
For minors who had experienced FGM, low numbers of survey respondents made law enforcement referrals (7.7%) or social service referrals (7.7%). There were a few respondents (5.4%) who said they didn’t feel comfortable managing patients who had experienced FGM.
In another surprise to the researchers, genital mutilation procedures were not all performed abroad. Three ob.gyns. reported caring for patients who had previously undergone FGM in the United States.
The findings highlight the need for structured education in ob.gyn. residency training programs regarding how to approach at-risk patients and those who have experienced FGM, Dr. Shamshirsaz said in an interview. He also called for specific guidelines from ACOG regarding virginity testing and hymenoplasty and for clearer statutes to guide physicians when patients request FGM or virginity testing procedures.
“All [ob.gyns.] should be aware that their at-risk patients may have a history of honor-related practices and should obtain a full history in a culturally sensitive and professionally responsible manner so that they may respond to and address the unique needs of these patients,” the researchers wrote.
The researchers reported having no relevant financial disclosures. The study was supported by a grant from ACOG.
[email protected]
On Twitter @karioakes
SAN DIEGO – Nearly 60% of ob.gyns. have seen patients who have experienced female genital mutilation (FGM), according to the results of a survey of 288 fellows of the American College of Obstetricians and Gynecologists.
Additionally, the survey found that there are few guidelines for care of these patients. Among fellows who had seen patients with FGM, 80.1% said their institutions had no policies or guidelines for management of these patients. Additionally, just 56.7% of fellows who had treated these women were aware that federal laws prohibit FGM.
The findings came as a surprise to the study’s senior author, Alireza Shamshirsaz, MD, a maternal-fetal medicine specialist at Baylor College of Medicine, Houston. “I really see the gap of knowledge of [ob.gyns.] in the States, and the emergent need for guidelines and regulation.”
Ob.gyns. who cared for women who had experienced FGM reported that they used a combination of approaches in treating these patients. Nearly half of physicians (45.8%) said they generally treated FGM patients the same as their other patients, but nearly three-quarters said they also discuss potential complications of FGM procedures. About 28% of physicians reported providing information about surgical repair options, while less than 5% provided mental health referrals.
For minors who had experienced FGM, low numbers of survey respondents made law enforcement referrals (7.7%) or social service referrals (7.7%). There were a few respondents (5.4%) who said they didn’t feel comfortable managing patients who had experienced FGM.
In another surprise to the researchers, genital mutilation procedures were not all performed abroad. Three ob.gyns. reported caring for patients who had previously undergone FGM in the United States.
The findings highlight the need for structured education in ob.gyn. residency training programs regarding how to approach at-risk patients and those who have experienced FGM, Dr. Shamshirsaz said in an interview. He also called for specific guidelines from ACOG regarding virginity testing and hymenoplasty and for clearer statutes to guide physicians when patients request FGM or virginity testing procedures.
“All [ob.gyns.] should be aware that their at-risk patients may have a history of honor-related practices and should obtain a full history in a culturally sensitive and professionally responsible manner so that they may respond to and address the unique needs of these patients,” the researchers wrote.
The researchers reported having no relevant financial disclosures. The study was supported by a grant from ACOG.
[email protected]
On Twitter @karioakes
SAN DIEGO – Nearly 60% of ob.gyns. have seen patients who have experienced female genital mutilation (FGM), according to the results of a survey of 288 fellows of the American College of Obstetricians and Gynecologists.
Additionally, the survey found that there are few guidelines for care of these patients. Among fellows who had seen patients with FGM, 80.1% said their institutions had no policies or guidelines for management of these patients. Additionally, just 56.7% of fellows who had treated these women were aware that federal laws prohibit FGM.
The findings came as a surprise to the study’s senior author, Alireza Shamshirsaz, MD, a maternal-fetal medicine specialist at Baylor College of Medicine, Houston. “I really see the gap of knowledge of [ob.gyns.] in the States, and the emergent need for guidelines and regulation.”
Ob.gyns. who cared for women who had experienced FGM reported that they used a combination of approaches in treating these patients. Nearly half of physicians (45.8%) said they generally treated FGM patients the same as their other patients, but nearly three-quarters said they also discuss potential complications of FGM procedures. About 28% of physicians reported providing information about surgical repair options, while less than 5% provided mental health referrals.
For minors who had experienced FGM, low numbers of survey respondents made law enforcement referrals (7.7%) or social service referrals (7.7%). There were a few respondents (5.4%) who said they didn’t feel comfortable managing patients who had experienced FGM.
In another surprise to the researchers, genital mutilation procedures were not all performed abroad. Three ob.gyns. reported caring for patients who had previously undergone FGM in the United States.
The findings highlight the need for structured education in ob.gyn. residency training programs regarding how to approach at-risk patients and those who have experienced FGM, Dr. Shamshirsaz said in an interview. He also called for specific guidelines from ACOG regarding virginity testing and hymenoplasty and for clearer statutes to guide physicians when patients request FGM or virginity testing procedures.
“All [ob.gyns.] should be aware that their at-risk patients may have a history of honor-related practices and should obtain a full history in a culturally sensitive and professionally responsible manner so that they may respond to and address the unique needs of these patients,” the researchers wrote.
The researchers reported having no relevant financial disclosures. The study was supported by a grant from ACOG.
[email protected]
On Twitter @karioakes
AT ACOG 2017
Key clinical point:
Major finding: Of the 288 ACOG fellows surveyed, 58.6% have seen patients who have experienced female genital mutilation.
Data source: An anonymous online survey of 288 randomly-selected and stratified ACOG fellows.
Disclosures: The study was supported by a grant from ACOG. The researchers reported having no relevant financial disclosures.
Hepatitis C is a pediatric disease now
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Mesenchymal precursor cells simmer down rheumatoid arthritis
MADRID – A single injection of allogeneic mesenchymal precursor cells was apparently both safe and effective for relieving some symptoms of rheumatoid arthritis in biological-refractory patients in a phase II randomized trial with 48 patients.
Most of the efficacy measures ran out to 12 weeks, but researchers followed one measure, the American College of Rheumatology N (ACR-N) index of improvement, out to 39 weeks after a single infusion, and this measure showed a statistically significant, durable benefit from the higher tested dose of mesenchymal precursor cells (MPC) when compared with placebo, Suzanne Kafaja, MD, said while presenting a poster at the European Congress of Rheumatology.
“The trial met its primary endpoints, and so far the data look pretty encouraging, but it’s still pretty early,” she said.
“I was surprised by the durability” of the anti-inflammatory effect of the MPC at 39 weeks, Dr. Kafaja added in an interview.
The study, run at 16 U.S. centers and 2 sites in Australia, enrolled 48 patients with rheumatoid arthritis and on a stable methotrexate regimen for at least 4 months who had a history of failing to adequately respond to at least one biological disease-modifying drug, most commonly a tumor necrosis factor inhibitor. The average age of the enrolled patients was 55 years; nearly three-quarters were women. The patients had been diagnosed with rheumatoid arthritis for an average of 13 years, and more than one-third of the patients had a history of inadequate response to three or more biological agents.
Thirty-six of the patients received a single infusion of a preparation of MPC taken from the bone marrow of healthy donors and isolated and expanded ex vivo. Half the patients in the active-treatment group received 1 million MPC per kg, and the other half received 2 million MPC per kg. An additional 16 patients received placebo. The researchers obtained the MPC from Mesoblast Limited, an Australia-based company that sponsored the study and uses a proprietary method for MPC processing to produce an “off-the-shelf” product.
The allogeneic MPC used in the study is a homogeneous population of cells that respond to pro-inflammatory cytokines by releasing cytokines of their own that induce monocytes and T-cells into an anti-inflammatory state.
The treatment groups showed no difference, compared with the placebo group, for the ACR20, ACR50, and ACR70 measurements, but for two other measures, the Health Assessment Questionnaire and the 28-joint Disease Activity Score, the results showed trends toward dose-related improvements following MPC treatment. The results showed statistically significant improvements after 12 and 39 weeks in average ACR-N in the patients who received 2 million MPC per kg. After 39 weeks, the average ACR-N improvement rate was about 35% among patients who had received 2 million MPC per kg, about 10% among those who received 1 million cells per kg, and about 8% among placebo patients, Dr. Kafaja reported.
Mesoblast funded the study. Dr. Kafaja had no other disclosures.
[email protected]
On Twitter @mitchelzoler
MADRID – A single injection of allogeneic mesenchymal precursor cells was apparently both safe and effective for relieving some symptoms of rheumatoid arthritis in biological-refractory patients in a phase II randomized trial with 48 patients.
Most of the efficacy measures ran out to 12 weeks, but researchers followed one measure, the American College of Rheumatology N (ACR-N) index of improvement, out to 39 weeks after a single infusion, and this measure showed a statistically significant, durable benefit from the higher tested dose of mesenchymal precursor cells (MPC) when compared with placebo, Suzanne Kafaja, MD, said while presenting a poster at the European Congress of Rheumatology.
“The trial met its primary endpoints, and so far the data look pretty encouraging, but it’s still pretty early,” she said.
“I was surprised by the durability” of the anti-inflammatory effect of the MPC at 39 weeks, Dr. Kafaja added in an interview.
The study, run at 16 U.S. centers and 2 sites in Australia, enrolled 48 patients with rheumatoid arthritis and on a stable methotrexate regimen for at least 4 months who had a history of failing to adequately respond to at least one biological disease-modifying drug, most commonly a tumor necrosis factor inhibitor. The average age of the enrolled patients was 55 years; nearly three-quarters were women. The patients had been diagnosed with rheumatoid arthritis for an average of 13 years, and more than one-third of the patients had a history of inadequate response to three or more biological agents.
Thirty-six of the patients received a single infusion of a preparation of MPC taken from the bone marrow of healthy donors and isolated and expanded ex vivo. Half the patients in the active-treatment group received 1 million MPC per kg, and the other half received 2 million MPC per kg. An additional 16 patients received placebo. The researchers obtained the MPC from Mesoblast Limited, an Australia-based company that sponsored the study and uses a proprietary method for MPC processing to produce an “off-the-shelf” product.
The allogeneic MPC used in the study is a homogeneous population of cells that respond to pro-inflammatory cytokines by releasing cytokines of their own that induce monocytes and T-cells into an anti-inflammatory state.
The treatment groups showed no difference, compared with the placebo group, for the ACR20, ACR50, and ACR70 measurements, but for two other measures, the Health Assessment Questionnaire and the 28-joint Disease Activity Score, the results showed trends toward dose-related improvements following MPC treatment. The results showed statistically significant improvements after 12 and 39 weeks in average ACR-N in the patients who received 2 million MPC per kg. After 39 weeks, the average ACR-N improvement rate was about 35% among patients who had received 2 million MPC per kg, about 10% among those who received 1 million cells per kg, and about 8% among placebo patients, Dr. Kafaja reported.
Mesoblast funded the study. Dr. Kafaja had no other disclosures.
[email protected]
On Twitter @mitchelzoler
MADRID – A single injection of allogeneic mesenchymal precursor cells was apparently both safe and effective for relieving some symptoms of rheumatoid arthritis in biological-refractory patients in a phase II randomized trial with 48 patients.
Most of the efficacy measures ran out to 12 weeks, but researchers followed one measure, the American College of Rheumatology N (ACR-N) index of improvement, out to 39 weeks after a single infusion, and this measure showed a statistically significant, durable benefit from the higher tested dose of mesenchymal precursor cells (MPC) when compared with placebo, Suzanne Kafaja, MD, said while presenting a poster at the European Congress of Rheumatology.
“The trial met its primary endpoints, and so far the data look pretty encouraging, but it’s still pretty early,” she said.
“I was surprised by the durability” of the anti-inflammatory effect of the MPC at 39 weeks, Dr. Kafaja added in an interview.
The study, run at 16 U.S. centers and 2 sites in Australia, enrolled 48 patients with rheumatoid arthritis and on a stable methotrexate regimen for at least 4 months who had a history of failing to adequately respond to at least one biological disease-modifying drug, most commonly a tumor necrosis factor inhibitor. The average age of the enrolled patients was 55 years; nearly three-quarters were women. The patients had been diagnosed with rheumatoid arthritis for an average of 13 years, and more than one-third of the patients had a history of inadequate response to three or more biological agents.
Thirty-six of the patients received a single infusion of a preparation of MPC taken from the bone marrow of healthy donors and isolated and expanded ex vivo. Half the patients in the active-treatment group received 1 million MPC per kg, and the other half received 2 million MPC per kg. An additional 16 patients received placebo. The researchers obtained the MPC from Mesoblast Limited, an Australia-based company that sponsored the study and uses a proprietary method for MPC processing to produce an “off-the-shelf” product.
The allogeneic MPC used in the study is a homogeneous population of cells that respond to pro-inflammatory cytokines by releasing cytokines of their own that induce monocytes and T-cells into an anti-inflammatory state.
The treatment groups showed no difference, compared with the placebo group, for the ACR20, ACR50, and ACR70 measurements, but for two other measures, the Health Assessment Questionnaire and the 28-joint Disease Activity Score, the results showed trends toward dose-related improvements following MPC treatment. The results showed statistically significant improvements after 12 and 39 weeks in average ACR-N in the patients who received 2 million MPC per kg. After 39 weeks, the average ACR-N improvement rate was about 35% among patients who had received 2 million MPC per kg, about 10% among those who received 1 million cells per kg, and about 8% among placebo patients, Dr. Kafaja reported.
Mesoblast funded the study. Dr. Kafaja had no other disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Mesenchymal precursor cells led to an average 35% ACR-N improvement, compared with 8% in placebo-treated patients.
Data source: A multicenter, dose-ranging phase II study with 48 rheumatoid arthritis patients.
Disclosures: Mesoblast funded the study. Dr. Kafaja had no other disclosures.
Evaluation of Patch Test Reactivities in Patients With Chronic Idiopathic Urticaria
Chronic urticaria (CU) is clinically defined as the daily or almost daily presence of wheals on the skin for at least 6 weeks.1 Chronic urticaria severely affects patients’ quality of life and can cause emotional disability and distress.2 In clinical practice, CU is one of the most common and challenging conditions for general practitioners, dermatologists, and allergists. It can be provoked by a wide variety of different causes or may be the clinical presentation of certain systemic diseases3,4; thus, CU often requires a detailed and time-consuming diagnostic procedure that includes screening for allergies, autoimmune diseases, parasites, malignancies, infections, and metabolic disorders.5,6 In many patients (up to 50% in some case series), the cause or pathogenic mechanism cannot be identified, and the disease is then classified as chronic idiopathic urticaria (CIU).7
It has previously been shown that contact sensitization could have some relation with CIU,8 which was further explored in this study. This study sought to evaluate if contact allergy may play a role in disease development in CIU patients in Saudi Arabia and if patch testing should be routinely performed for CIU patients to determine if any allergens can be avoided.
Methods
This prospective study was conducted at the King Khalid University Hospital Allergy Clinic (Riyadh, Saudi Arabia) in patients aged 18 to 60 years who had CU for more than 6 weeks. It was a clinic-based study conducted over a period of 2 years (March 2010 to February 2012). The study protocol was approved by the local ethics committee at King Khalid University Hospital. Valid written consent was obtained from each patient.
Patients were excluded if they had CU caused by physical factors (eg, hot or cold temperature, water, physical contact) or drug reactions that were possible causative factors or if they had taken oral prednisolone or other oral immunosuppressive drugs (eg, azathioprine, cyclosporine) in the last month. However, patients taking antihistamines were not excluded because it was impossible for the patients to discontinue their urticaria treatment. Other exclusion criteria included CU associated with any systemic disease, thyroid disease, diabetes mellitus, autoimmune disorder, or atopic dermatitis. Pregnant and lactating women were not included in this study.
All new adult CU patients (ie, disease duration >6 weeks) were worked up using the routine diagnostic tests that are typically performed for any new CU patient, including complete blood cell count with differential, erythrocyte sedimentation rate, liver function tests, urine analysis, and hepatitis B and C screenings. Further diagnostic tests also were carried out when appropriate according to the patient’s history and physical examination, including levels of urea, electrolytes, thyrotropin, thyroid antibodies (antithyroglobulin and antimicrosomal), and antinuclear antibodies, as well as a Helicobacter pylori test.
All of the patients enrolled in the study were evaluated by skin prick testing to establish the link between CU and its cause. Patch testing was performed in patients who were negative on skin prick testing.
Skin Prick Testing
All patients were advised to temporarily discontinue the use of antihistamines and corticosteroids 5 to 6 days prior to testing.
Patch Testing
Patch tests were carried out using a ready-to-use epicutaneous patch test system for the diagnosis of allergic contact dermatitis (ACD).10 A European standard series was used with the addition of 4 allergens of local relevance: black seed oil, local perfume mix, henna, and myrrh (a topical herbal medicine used to promote healing).
Assessment of Improvement
Assessment of urticaria severity using the Chronic Urticaria Severity Score (CUSS), a simple semiquantitative assessment of disease activity, was calculated as the sum of the number of wheals and the degree of itch severity graded from 0 (none) to 3 (severe), according to the guidelines established by the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization.11 The avoidance group of patients was assessed at baseline and after 1 month to evaluate changes in their CUSS after allergen avoidance for 8 weeks.
Statistical Analysis
All of the statistical analyses were carried out using SPSS software version 16. Results were presented as the median with the range or the mean (SD).
Results
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).
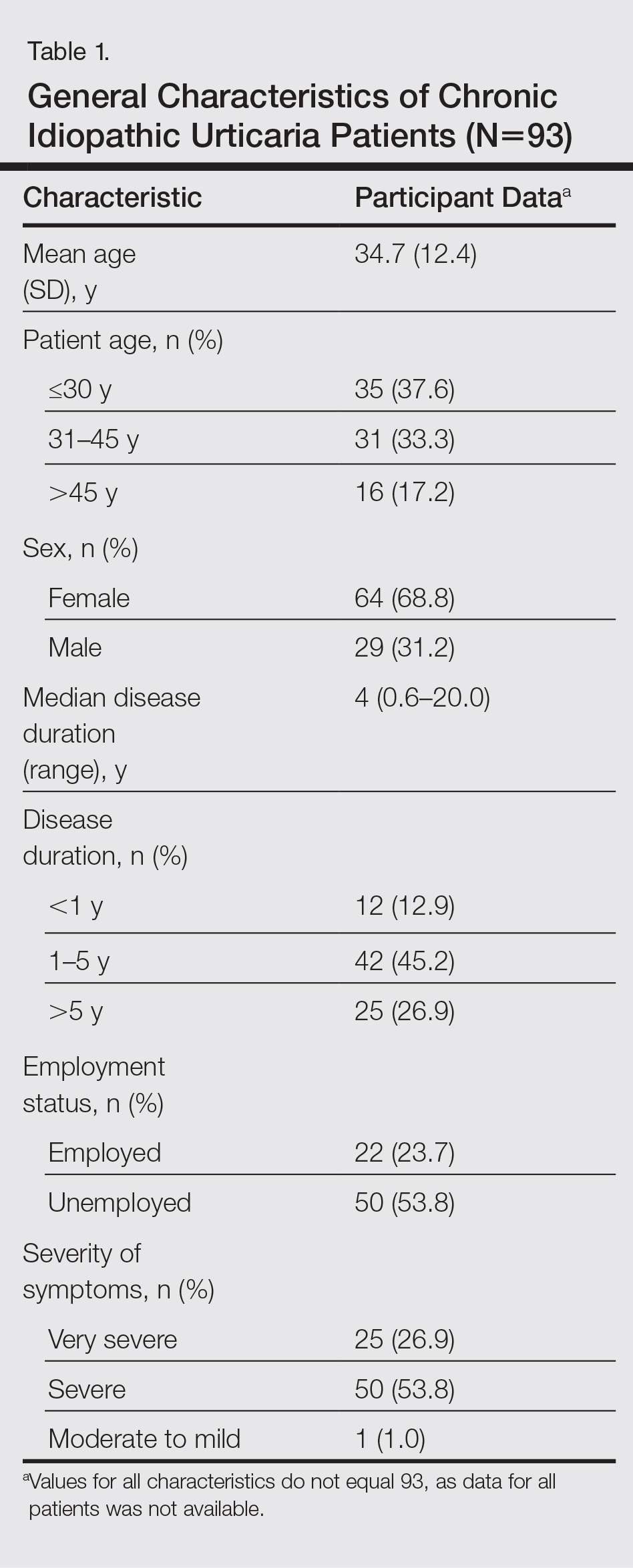
The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).
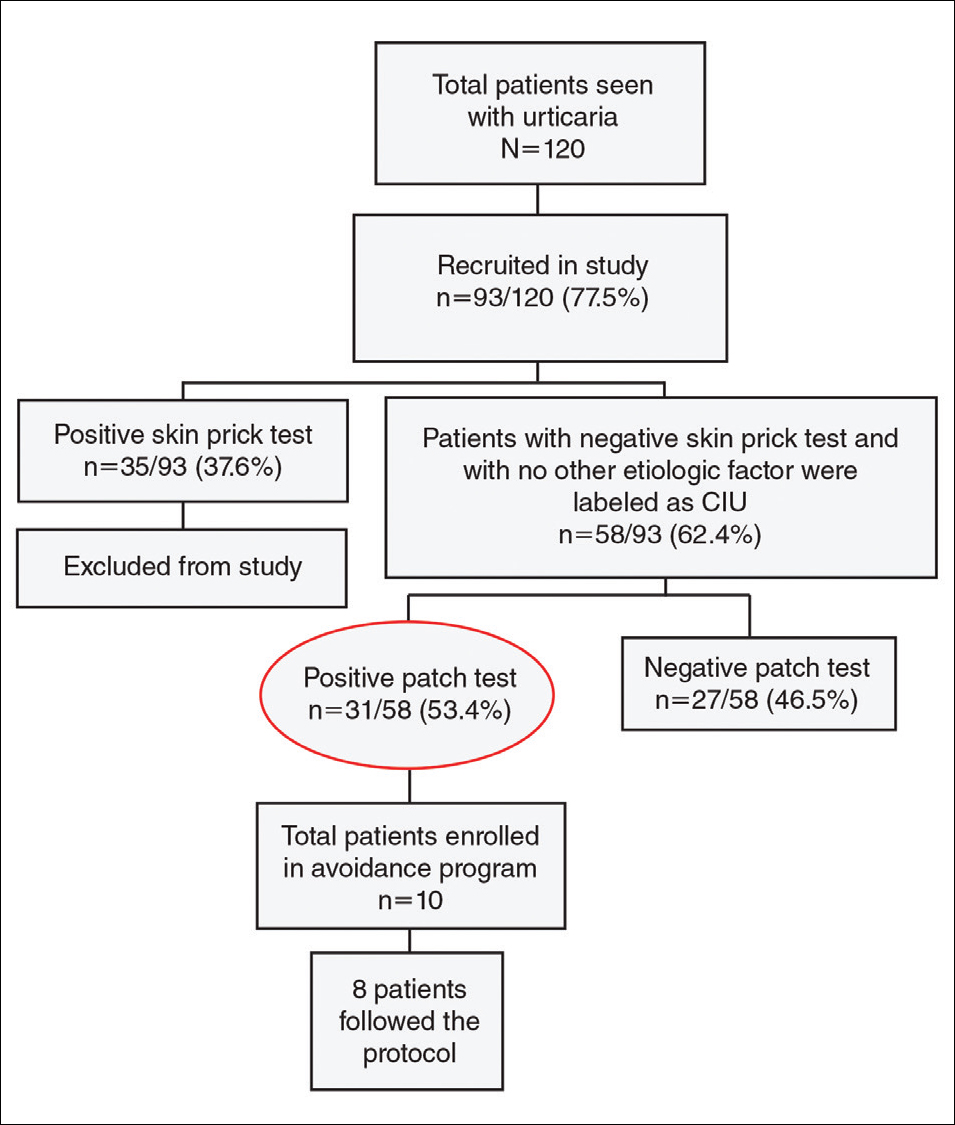
Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). T
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).
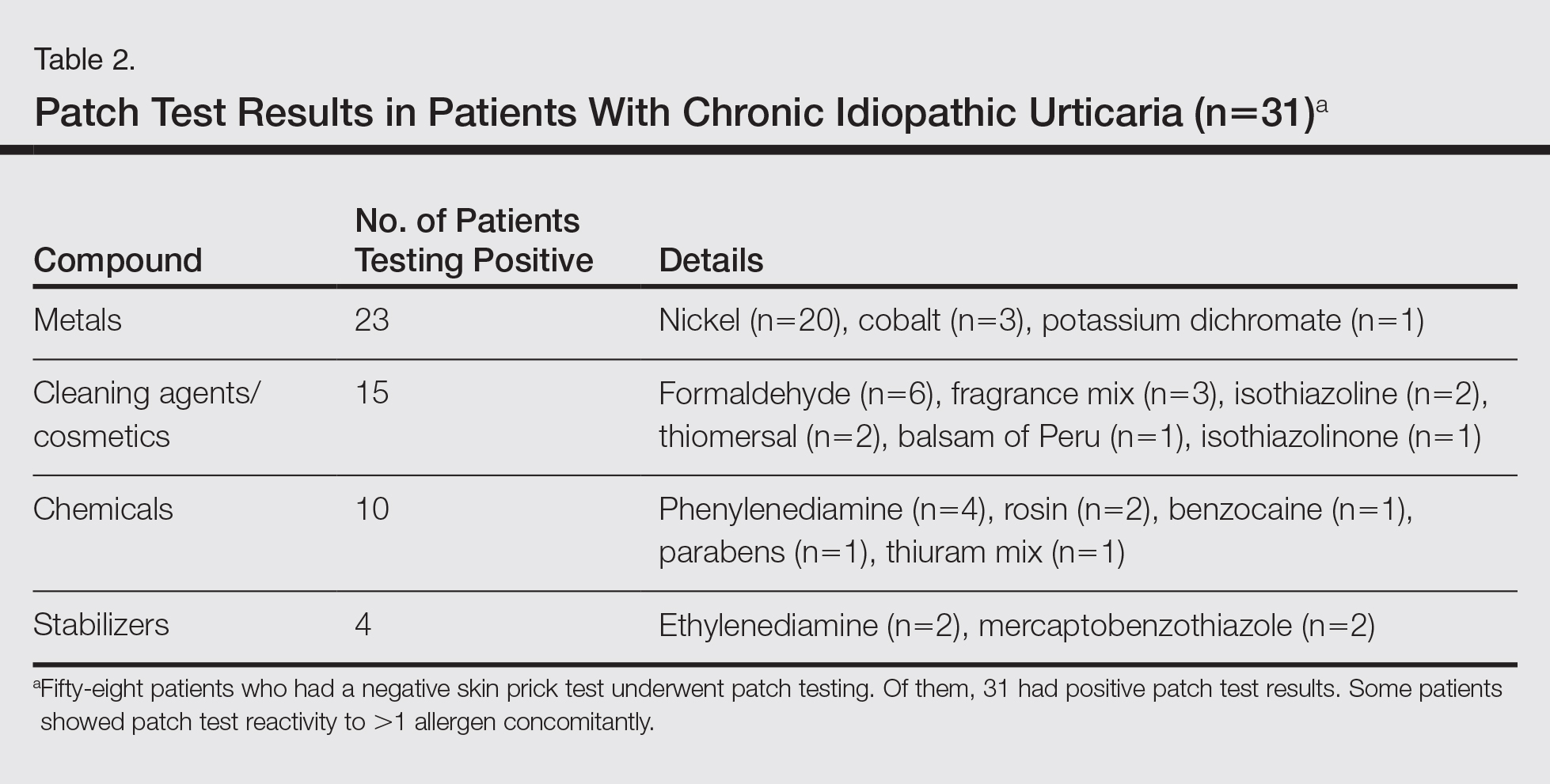
Of
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.
Conclusion
We propose that patch tests should be performed while investigating CU because they give effective diagnostic and therapeutic results in a substantial number of patients. Urticaria, or at least a subgroup of the disease, may have a delayed-type reaction element, which may explain the disease etiology for many CIU patients. Patients with CU without a detectable underlying etiologic factor can have positive patch test results.
- Zuberbier T, Bindslev-Jensen C, Canonica W, et al. Guidelines, definition, classification and diagnosis of urticaria. Allergy. 2006;61:316-331.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427-436.
- Green GA, Koelsche GA, Kierland R. Etiology and pathogenesis of chronic urticaria. Ann Allergy. 1965;23:30-36.
- Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408-412.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Sharma AD. Use of patch testing for identifying allergen causing chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:114-117.
- Li JT, Andrist D, Bamlet WR, et al. Accuracy of patient prediction of allergy skin test results. Ann Allergy Asthma Immunol. 2000;85:382-384.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
- Bindslev-Jensen C, Finzi A, Greaves M, et al. Chronic urticaria: diagnostic recommendations. Eur Acad Dermatol Venereol. 2000;14:175-180.
- Bruze M, Conde-Slazar L, Goossens A, et al. Thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Al-Sheikh OA, Gad El-Rab MO. Allergic contact dermatitis: clinical features and profile of sensitizing allergens in Riyadh, Saudi Arabia. Int J Dermatol. 1996;35:493-497.
- Al-Suwaidan SN, Gad El Rab MO, Al-Fakhiry S, et al. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermatitis. 1998;39:137.
- Henz BM, Zuberbier T. Causes of urticaria. In: Henz B, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer; 1998:19.
- Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol. 2007;56:88-90.
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
- Smart GA, Sherlock JC. Nickel in foods and the diet. Food Addit Contam. 1987;4:61-71.
- Abeck D, Traenckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Derm Venereol. 1993;73:438-439.
- Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria [in French]. Ann Dermatol Venereol. 2003;130(Spec No 1):1S35-1S42.
- Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387-396.
- Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm Venerol. 2002;82:121-123.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003: first results of the European Surveillance System on Contact Allergies. Contact Dermatitis. 2005;53:136-145.
- Magen E, Mishal J, Menachem S. Impact of contact sensitization in chronic spontaneous urticaria. Am J Med Sci. 2011;341:202-206.
- Antico A, Soana R. Chronic allergic-like dermatopathies in nickel sensitive patients: results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
Chronic urticaria (CU) is clinically defined as the daily or almost daily presence of wheals on the skin for at least 6 weeks.1 Chronic urticaria severely affects patients’ quality of life and can cause emotional disability and distress.2 In clinical practice, CU is one of the most common and challenging conditions for general practitioners, dermatologists, and allergists. It can be provoked by a wide variety of different causes or may be the clinical presentation of certain systemic diseases3,4; thus, CU often requires a detailed and time-consuming diagnostic procedure that includes screening for allergies, autoimmune diseases, parasites, malignancies, infections, and metabolic disorders.5,6 In many patients (up to 50% in some case series), the cause or pathogenic mechanism cannot be identified, and the disease is then classified as chronic idiopathic urticaria (CIU).7
It has previously been shown that contact sensitization could have some relation with CIU,8 which was further explored in this study. This study sought to evaluate if contact allergy may play a role in disease development in CIU patients in Saudi Arabia and if patch testing should be routinely performed for CIU patients to determine if any allergens can be avoided.
Methods
This prospective study was conducted at the King Khalid University Hospital Allergy Clinic (Riyadh, Saudi Arabia) in patients aged 18 to 60 years who had CU for more than 6 weeks. It was a clinic-based study conducted over a period of 2 years (March 2010 to February 2012). The study protocol was approved by the local ethics committee at King Khalid University Hospital. Valid written consent was obtained from each patient.
Patients were excluded if they had CU caused by physical factors (eg, hot or cold temperature, water, physical contact) or drug reactions that were possible causative factors or if they had taken oral prednisolone or other oral immunosuppressive drugs (eg, azathioprine, cyclosporine) in the last month. However, patients taking antihistamines were not excluded because it was impossible for the patients to discontinue their urticaria treatment. Other exclusion criteria included CU associated with any systemic disease, thyroid disease, diabetes mellitus, autoimmune disorder, or atopic dermatitis. Pregnant and lactating women were not included in this study.
All new adult CU patients (ie, disease duration >6 weeks) were worked up using the routine diagnostic tests that are typically performed for any new CU patient, including complete blood cell count with differential, erythrocyte sedimentation rate, liver function tests, urine analysis, and hepatitis B and C screenings. Further diagnostic tests also were carried out when appropriate according to the patient’s history and physical examination, including levels of urea, electrolytes, thyrotropin, thyroid antibodies (antithyroglobulin and antimicrosomal), and antinuclear antibodies, as well as a Helicobacter pylori test.
All of the patients enrolled in the study were evaluated by skin prick testing to establish the link between CU and its cause. Patch testing was performed in patients who were negative on skin prick testing.
Skin Prick Testing
All patients were advised to temporarily discontinue the use of antihistamines and corticosteroids 5 to 6 days prior to testing.
Patch Testing
Patch tests were carried out using a ready-to-use epicutaneous patch test system for the diagnosis of allergic contact dermatitis (ACD).10 A European standard series was used with the addition of 4 allergens of local relevance: black seed oil, local perfume mix, henna, and myrrh (a topical herbal medicine used to promote healing).
Assessment of Improvement
Assessment of urticaria severity using the Chronic Urticaria Severity Score (CUSS), a simple semiquantitative assessment of disease activity, was calculated as the sum of the number of wheals and the degree of itch severity graded from 0 (none) to 3 (severe), according to the guidelines established by the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization.11 The avoidance group of patients was assessed at baseline and after 1 month to evaluate changes in their CUSS after allergen avoidance for 8 weeks.
Statistical Analysis
All of the statistical analyses were carried out using SPSS software version 16. Results were presented as the median with the range or the mean (SD).
Results
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).

The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).

Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). T
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).

Of
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.
Conclusion
We propose that patch tests should be performed while investigating CU because they give effective diagnostic and therapeutic results in a substantial number of patients. Urticaria, or at least a subgroup of the disease, may have a delayed-type reaction element, which may explain the disease etiology for many CIU patients. Patients with CU without a detectable underlying etiologic factor can have positive patch test results.
Chronic urticaria (CU) is clinically defined as the daily or almost daily presence of wheals on the skin for at least 6 weeks.1 Chronic urticaria severely affects patients’ quality of life and can cause emotional disability and distress.2 In clinical practice, CU is one of the most common and challenging conditions for general practitioners, dermatologists, and allergists. It can be provoked by a wide variety of different causes or may be the clinical presentation of certain systemic diseases3,4; thus, CU often requires a detailed and time-consuming diagnostic procedure that includes screening for allergies, autoimmune diseases, parasites, malignancies, infections, and metabolic disorders.5,6 In many patients (up to 50% in some case series), the cause or pathogenic mechanism cannot be identified, and the disease is then classified as chronic idiopathic urticaria (CIU).7
It has previously been shown that contact sensitization could have some relation with CIU,8 which was further explored in this study. This study sought to evaluate if contact allergy may play a role in disease development in CIU patients in Saudi Arabia and if patch testing should be routinely performed for CIU patients to determine if any allergens can be avoided.
Methods
This prospective study was conducted at the King Khalid University Hospital Allergy Clinic (Riyadh, Saudi Arabia) in patients aged 18 to 60 years who had CU for more than 6 weeks. It was a clinic-based study conducted over a period of 2 years (March 2010 to February 2012). The study protocol was approved by the local ethics committee at King Khalid University Hospital. Valid written consent was obtained from each patient.
Patients were excluded if they had CU caused by physical factors (eg, hot or cold temperature, water, physical contact) or drug reactions that were possible causative factors or if they had taken oral prednisolone or other oral immunosuppressive drugs (eg, azathioprine, cyclosporine) in the last month. However, patients taking antihistamines were not excluded because it was impossible for the patients to discontinue their urticaria treatment. Other exclusion criteria included CU associated with any systemic disease, thyroid disease, diabetes mellitus, autoimmune disorder, or atopic dermatitis. Pregnant and lactating women were not included in this study.
All new adult CU patients (ie, disease duration >6 weeks) were worked up using the routine diagnostic tests that are typically performed for any new CU patient, including complete blood cell count with differential, erythrocyte sedimentation rate, liver function tests, urine analysis, and hepatitis B and C screenings. Further diagnostic tests also were carried out when appropriate according to the patient’s history and physical examination, including levels of urea, electrolytes, thyrotropin, thyroid antibodies (antithyroglobulin and antimicrosomal), and antinuclear antibodies, as well as a Helicobacter pylori test.
All of the patients enrolled in the study were evaluated by skin prick testing to establish the link between CU and its cause. Patch testing was performed in patients who were negative on skin prick testing.
Skin Prick Testing
All patients were advised to temporarily discontinue the use of antihistamines and corticosteroids 5 to 6 days prior to testing.
Patch Testing
Patch tests were carried out using a ready-to-use epicutaneous patch test system for the diagnosis of allergic contact dermatitis (ACD).10 A European standard series was used with the addition of 4 allergens of local relevance: black seed oil, local perfume mix, henna, and myrrh (a topical herbal medicine used to promote healing).
Assessment of Improvement
Assessment of urticaria severity using the Chronic Urticaria Severity Score (CUSS), a simple semiquantitative assessment of disease activity, was calculated as the sum of the number of wheals and the degree of itch severity graded from 0 (none) to 3 (severe), according to the guidelines established by the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization.11 The avoidance group of patients was assessed at baseline and after 1 month to evaluate changes in their CUSS after allergen avoidance for 8 weeks.
Statistical Analysis
All of the statistical analyses were carried out using SPSS software version 16. Results were presented as the median with the range or the mean (SD).
Results
During the study period, a total of 120 CU patients were seen at the clinic. Ninety-three patients with CU met our selection criteria (77.5%) and were enrolled in the study. The mean age (SD) of the patients was 34.7 (12.4) years. Women comprised 68.8% (64/93) of the study population (Table 1).

The duration of urticaria ranged from 0.6 to 20 years, with a median duration of 4 years. Approximately half of the patients (50/93) experienced severe symptoms of urticaria, but only 26.9% (25/93) had graded their urticaria as very severe.
Negative results from the skin prick test were reported in 62.4% (58/93) of patients and were subsequently patch tested. These patients also had no other etiologic factors (eg, infection; thyroid, autoimmune, or metabolic disease). Patients who had positive skin prick test results (35/93 [37.6%]) were not considered to be cases of CIU, according to diagnostic recommendations.12 Of the 58 CIU patients who were patch tested, 31 (53.4%) had positive results and 27 (46.5%) had negative results to both skin prick and patch tests (Figure).

Univariate analysis revealed significant associations between age, gender, and duration of urticaria and patch test positivity (χ2 test, P<.05). T
Of the 31 patients with positive patch tests, there were 20 positive reactions to nickel, 6 to formaldehyde, 4 to phenylenediamine, 3 to cobalt, and 3 to a fragrance mix (Table 2). Some patients showed patch test reactivity to more than 1 allergen concomitantly. Overall, these 31 patients had positive reactions to 16 allergens. None of the patients showed actual signs of contact dermatitis (Table 2).

Of
Comment
Chronic idiopathic urticaria is the diagnosis given when urticarial vasculitis, physical urticaria, and all other possible etiologic factors have been excluded in patients with CU. Our study was designed to assess patch test reactivity in patients with CU without any identifiable systemic etiologic factor after detailed laboratory testing and negative skin prick tests.
Chronic idiopathic urticaria can be an extremely disabling and difficult-to-treat condition. Because the cause is unknown, the management of CIU often is frustrating. The
Patch testing is commonly performed to diagnose ACD, and if contact allergens are found via patch testing, patients can often be cured of their dermatitis by avoiding these agents. However, patch testing is not routinely performed in the evaluation of patients with CIU. It is a relatively inexpensive and safe procedure to determine a causal link between sensitization to a specific agent and ACD. In patch test clinics, agents often are tested in standard and screening series. Sensitization that is not suspected from the patient’s history and/or clinical examination can be detected in this manner. Requirements for the inclusion of a chemical in a standard series have been formulated by Bruze et al.13 In addition, ready-to-use materials relevant to the specific leisure activities and working conditions also can be selected for patch testing.
A study conducted in Saudi Arabia showed that the European standard series is suitable for patch testing patients in this community14; however, 3 allergens of local relevance were added in our study: black seed oil, local perfume mix, and henna. Moreover, in our study we added a local allergen known as myrrh, which is a topical herbal medicine used to promote healing that has been reported to cause ACD in some cases.15 We sought to determine if contact allergens can be identified with patch testing in patients with CU and if avoiding these contact allergens would resolve the CU.
Urticaria was once considered an IgE-mediated hypersensitivity reaction, but recent studies have demonstrated the existence of different subgroupsof urticaria, some with an autoimmune mechanism.1-4,11 In CU, skin prick tests are recommended for etiologic workup, while patch testing generally is not recommended.16
It has been observed in clinical practice that a substantial number of patients with CU are positive to patch tests, even without a clear clinical history or signs of contact dermatitis.17 In 2007, Guerra et al17 reported that of 121 patients with CU, 50 (41.3%) tested positive for contact allergens. In all of the patch test–positive patients, avoidance measures led to complete remission within 10 days to 1 month. Therefore, this result suggested that testing for contact sensitization could be helpful in the management of CU. Patients with nickel sensitivity were subsequently allowed to ingest small amounts of nickel-containing foods after 8 weeks of a completely nickel-free diet, and remission persisted.17
Contact dermatitis affects approximately 20% of the general population18; however, there has been little investigation (limited to nickel) into the relationship between contact allergens and CU,19,20 and the underlying mechanisms of the disease are unknown. It has been hypothesized that small amounts of the substances are absorbed through the skin or the digestive tract into the bloodstream over the long-term and are delivered to antigen-presenting cells in the skin, which provide the necessary signals for mast cell activation. Nonetheless, the reasons for a selectively cutaneous localization of the reaction remain largely unclear.
Management of CU is debated among physicians, and several diagnostic flowcharts have been proposed.1,2 In general, patch tests for contact dermatitis are not recommended as a fundamental part of the diagnostic procedure, but Guerra et al17 suggested that contact allergy often plays a role in CU.
There have been inadequate reports of CU found to be caused by common contact sensitizers.21-24 Interestingly, no signs of contact allergy were demonstrated in CU patients before urticarial attack.
Our findings supported our patient selection criteria and also confirmed that contact sensitization may be one of the many possible mechanisms involved in the etiology of CU. Urticaria may have a delayed-type hypersensitivity reaction element, and patients with CU without an obvious causal factor can have positive patch test results.
The
The main findings of our study were that 53.4% of patients with CIU had positive patch test results and that avoidance of the sensitizing substance was effective in 5 of 8 patients who completed an avoidance program. Almost all of the patients showed notable remission of symptoms after limiting their exposure to the offending allergens. This study clearly showed that a cause or pathogenesis for CIU could be identified, thus showing that CIU occurs less frequently than is usually assumed.
Our study had limitations. The first is our lack of a controlled challenge test, which is important to confirm an allergen as a cause of CIU.26 Nonetheless, avoidance of the revealed contact allergen was associated with comparable improvement of CIU severity after 1 month in 5 of 8 patients, though such measures were not tested in all 31 of 58 CIU patients who had positive patch test results.
Conclusion
We propose that patch tests should be performed while investigating CU because they give effective diagnostic and therapeutic results in a substantial number of patients. Urticaria, or at least a subgroup of the disease, may have a delayed-type reaction element, which may explain the disease etiology for many CIU patients. Patients with CU without a detectable underlying etiologic factor can have positive patch test results.
- Zuberbier T, Bindslev-Jensen C, Canonica W, et al. Guidelines, definition, classification and diagnosis of urticaria. Allergy. 2006;61:316-331.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427-436.
- Green GA, Koelsche GA, Kierland R. Etiology and pathogenesis of chronic urticaria. Ann Allergy. 1965;23:30-36.
- Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408-412.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Sharma AD. Use of patch testing for identifying allergen causing chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:114-117.
- Li JT, Andrist D, Bamlet WR, et al. Accuracy of patient prediction of allergy skin test results. Ann Allergy Asthma Immunol. 2000;85:382-384.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
- Bindslev-Jensen C, Finzi A, Greaves M, et al. Chronic urticaria: diagnostic recommendations. Eur Acad Dermatol Venereol. 2000;14:175-180.
- Bruze M, Conde-Slazar L, Goossens A, et al. Thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Al-Sheikh OA, Gad El-Rab MO. Allergic contact dermatitis: clinical features and profile of sensitizing allergens in Riyadh, Saudi Arabia. Int J Dermatol. 1996;35:493-497.
- Al-Suwaidan SN, Gad El Rab MO, Al-Fakhiry S, et al. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermatitis. 1998;39:137.
- Henz BM, Zuberbier T. Causes of urticaria. In: Henz B, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer; 1998:19.
- Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol. 2007;56:88-90.
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
- Smart GA, Sherlock JC. Nickel in foods and the diet. Food Addit Contam. 1987;4:61-71.
- Abeck D, Traenckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Derm Venereol. 1993;73:438-439.
- Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria [in French]. Ann Dermatol Venereol. 2003;130(Spec No 1):1S35-1S42.
- Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387-396.
- Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm Venerol. 2002;82:121-123.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003: first results of the European Surveillance System on Contact Allergies. Contact Dermatitis. 2005;53:136-145.
- Magen E, Mishal J, Menachem S. Impact of contact sensitization in chronic spontaneous urticaria. Am J Med Sci. 2011;341:202-206.
- Antico A, Soana R. Chronic allergic-like dermatopathies in nickel sensitive patients: results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
- Zuberbier T, Bindslev-Jensen C, Canonica W, et al. Guidelines, definition, classification and diagnosis of urticaria. Allergy. 2006;61:316-331.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427-436.
- Green GA, Koelsche GA, Kierland R. Etiology and pathogenesis of chronic urticaria. Ann Allergy. 1965;23:30-36.
- Kaplan AP. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408-412.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Sharma AD. Use of patch testing for identifying allergen causing chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:114-117.
- Li JT, Andrist D, Bamlet WR, et al. Accuracy of patient prediction of allergy skin test results. Ann Allergy Asthma Immunol. 2000;85:382-384.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
- Bindslev-Jensen C, Finzi A, Greaves M, et al. Chronic urticaria: diagnostic recommendations. Eur Acad Dermatol Venereol. 2000;14:175-180.
- Bruze M, Conde-Slazar L, Goossens A, et al. Thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Al-Sheikh OA, Gad El-Rab MO. Allergic contact dermatitis: clinical features and profile of sensitizing allergens in Riyadh, Saudi Arabia. Int J Dermatol. 1996;35:493-497.
- Al-Suwaidan SN, Gad El Rab MO, Al-Fakhiry S, et al. Allergic contact dermatitis from myrrh, a topical herbal medicine used to promote healing. Contact Dermatitis. 1998;39:137.
- Henz BM, Zuberbier T. Causes of urticaria. In: Henz B, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer; 1998:19.
- Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol. 2007;56:88-90.
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
- Smart GA, Sherlock JC. Nickel in foods and the diet. Food Addit Contam. 1987;4:61-71.
- Abeck D, Traenckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Derm Venereol. 1993;73:438-439.
- Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria [in French]. Ann Dermatol Venereol. 2003;130(Spec No 1):1S35-1S42.
- Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387-396.
- Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm Venerol. 2002;82:121-123.
- Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003: first results of the European Surveillance System on Contact Allergies. Contact Dermatitis. 2005;53:136-145.
- Magen E, Mishal J, Menachem S. Impact of contact sensitization in chronic spontaneous urticaria. Am J Med Sci. 2011;341:202-206.
- Antico A, Soana R. Chronic allergic-like dermatopathies in nickel sensitive patients: results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
Practice Points
- Patients with chronic urticaria (CU) without a detectable underlying etiologic factor can have positive patch test results.
- Avoidance of the sensitizing substance can be effective in CU patients and remission of symptoms can be possible after limiting their exposure to the offending allergens.












