User login
How would you handle predictions of Alzheimer’s disease?
We love to try and predict the future. Some of it is scientific, like checking the weather forecast to see what we’re in for. (Here in Phoenix, it’s always hot, hotter, or melting.)
On the other hand, some of it is just for entertainment, like checking a horoscope or seeing what a fortune cookie says.
The breakthroughs in biomarkers for Alzheimer’s disease are accelerating. Although still experimental, we’re getting pretty close to predicting the disease many years before it develops. At the same time, we aren’t nearly as close to a treatment that will have a meaningful impact on the course of the disease.
In 1993, the genetic marker for Huntington’s disease was identified, quickly leading to a blood test with high accuracy to know if you were – or were not – going to develop the fatal disorder down the road.
Some wanted to know and used the information to decide if they wanted to have families. Others, understandably fearful, decided not to and let their lives play out as they will. Sadly, either way we have nothing close to a cure for the disease.
Now, we come to Alzheimer’s disease, many times more common than Huntington’s. Close to predicting its coming and not really close to a cure.
What would you do?
[polldaddy:9778279]
In “Back to the Future,” Doc Brown said “no man should know too much about their own destiny” (though later changed his mind). But, for Doc Brown, a bulletproof vest was all he needed. In Alzheimer’s disease, it’s not that simple.
I’m sure some would see it as a way to have their affairs in order long in advance, to spare themselves and their loved ones the frantic scramble that often comes after a diagnosis. Others would be afraid to know what the future holds, with every misplaced set of keys or iPhone becoming a reason to panic.
Obviously, if we had a true cure for the disorder, the decision would be easy. Then, it becomes a preventive measure in the same category as mammograms and colonoscopies. Early detection saves lives.
What would you do? And how will you guide the patients who ask your opinion?
For better or worse, these questions are coming. All of us need to think about how we’ll handle them.
Dr. Block has a solo neurology private practice in Scottsdale, Ariz.
We love to try and predict the future. Some of it is scientific, like checking the weather forecast to see what we’re in for. (Here in Phoenix, it’s always hot, hotter, or melting.)
On the other hand, some of it is just for entertainment, like checking a horoscope or seeing what a fortune cookie says.
The breakthroughs in biomarkers for Alzheimer’s disease are accelerating. Although still experimental, we’re getting pretty close to predicting the disease many years before it develops. At the same time, we aren’t nearly as close to a treatment that will have a meaningful impact on the course of the disease.
In 1993, the genetic marker for Huntington’s disease was identified, quickly leading to a blood test with high accuracy to know if you were – or were not – going to develop the fatal disorder down the road.
Some wanted to know and used the information to decide if they wanted to have families. Others, understandably fearful, decided not to and let their lives play out as they will. Sadly, either way we have nothing close to a cure for the disease.
Now, we come to Alzheimer’s disease, many times more common than Huntington’s. Close to predicting its coming and not really close to a cure.
What would you do?
[polldaddy:9778279]
In “Back to the Future,” Doc Brown said “no man should know too much about their own destiny” (though later changed his mind). But, for Doc Brown, a bulletproof vest was all he needed. In Alzheimer’s disease, it’s not that simple.
I’m sure some would see it as a way to have their affairs in order long in advance, to spare themselves and their loved ones the frantic scramble that often comes after a diagnosis. Others would be afraid to know what the future holds, with every misplaced set of keys or iPhone becoming a reason to panic.
Obviously, if we had a true cure for the disorder, the decision would be easy. Then, it becomes a preventive measure in the same category as mammograms and colonoscopies. Early detection saves lives.
What would you do? And how will you guide the patients who ask your opinion?
For better or worse, these questions are coming. All of us need to think about how we’ll handle them.
Dr. Block has a solo neurology private practice in Scottsdale, Ariz.
We love to try and predict the future. Some of it is scientific, like checking the weather forecast to see what we’re in for. (Here in Phoenix, it’s always hot, hotter, or melting.)
On the other hand, some of it is just for entertainment, like checking a horoscope or seeing what a fortune cookie says.
The breakthroughs in biomarkers for Alzheimer’s disease are accelerating. Although still experimental, we’re getting pretty close to predicting the disease many years before it develops. At the same time, we aren’t nearly as close to a treatment that will have a meaningful impact on the course of the disease.
In 1993, the genetic marker for Huntington’s disease was identified, quickly leading to a blood test with high accuracy to know if you were – or were not – going to develop the fatal disorder down the road.
Some wanted to know and used the information to decide if they wanted to have families. Others, understandably fearful, decided not to and let their lives play out as they will. Sadly, either way we have nothing close to a cure for the disease.
Now, we come to Alzheimer’s disease, many times more common than Huntington’s. Close to predicting its coming and not really close to a cure.
What would you do?
[polldaddy:9778279]
In “Back to the Future,” Doc Brown said “no man should know too much about their own destiny” (though later changed his mind). But, for Doc Brown, a bulletproof vest was all he needed. In Alzheimer’s disease, it’s not that simple.
I’m sure some would see it as a way to have their affairs in order long in advance, to spare themselves and their loved ones the frantic scramble that often comes after a diagnosis. Others would be afraid to know what the future holds, with every misplaced set of keys or iPhone becoming a reason to panic.
Obviously, if we had a true cure for the disorder, the decision would be easy. Then, it becomes a preventive measure in the same category as mammograms and colonoscopies. Early detection saves lives.
What would you do? And how will you guide the patients who ask your opinion?
For better or worse, these questions are coming. All of us need to think about how we’ll handle them.
Dr. Block has a solo neurology private practice in Scottsdale, Ariz.
Nocturia and sleep apnea
Author’s note: I have been writing “Myth of the Month” columns for the last several years. I will try to continue to write about myths when possible, but I would like to introduce a new column, “Pearl of the Month.” I want to share with you pearls that I have found really helpful in medical practice. Some of these will be new news, while some may be old news that may not be well known.
A 65-year-old man comes to a clinic concerned about frequent nocturia. He is getting up four times a night to urinate, and he has been urinating about every 5 hours during the day. He has been seen twice for this problem and was diagnosed with benign prostatic hyperplasia and started on tamsulosin.
He found a slight improvement when he started on 0.4 mg qhs, reducing his nocturia episodes from four to three. His dose was increased to 0.8 mg qhs, with no improvement in nocturia.
Exam today: BP, 140/94; pulse, 70. Rectal exam: Prostate is twice normal size without nodules. Labs: Na, 140; K, 4.0; glucose, 80; Ca, 9.6.
He is frustrated because he feels tired and sleepy from having to get up so often to urinate every night.
What is the best treatment/advice at this point?
A. Check hemoglobin A1C.
B. Start finasteride.
C. Switch tamsulosin to terazosin.
D. Evaluate for sleep apnea.
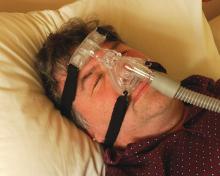
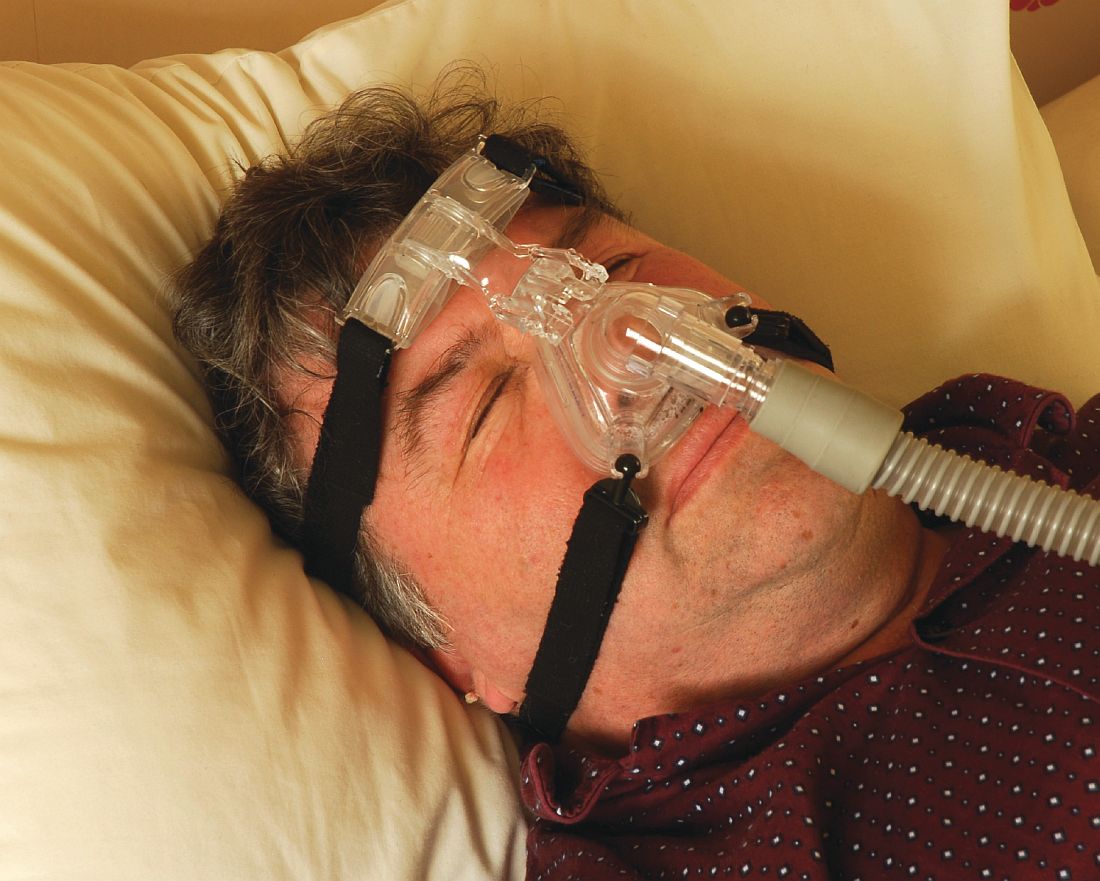
Umpei Yamamoto, MD, of Kyushu University Hospital, Japan, and colleagues studied the prevalence of sleep-disordered breathing among patients who presented to a urology clinic with nocturia and in those who visited a sleep apnea clinic with symptoms of excessive daytime sleepiness.1 Sleep-disordered breathing was found in 91% of the patients from the sleep apnea clinic and 70% of the patients from the urology clinic. The frequency of nocturia was reduced with continuous positive airway pressure (CPAP) in both groups in the patients who had not responded to conventional therapy or nocturia.
The symptom of nocturia as a symptom of sleep apnea might be even more common in women.2 Ozen K. Basoglu, MD, and Mehmet Sezai Tasbakan, MD, of Ege University, Izmir, Turkey, described clinical similarities and differences based on gender in a large group of patients with sleep apnea. Both men and women with sleep apnea had similar rates of excessive daytime sleepiness, snoring, and impaired concentration. Women had more frequent nocturia.
Nocturia especially should be considered a possible clue for the presence of sleep apnea in younger patients who have fewer other reasons to have nocturia. Takahiro Maeda, MD, of Keio University, Tokyo, and colleagues found that men younger than 50 years had more nocturnal urinations the worse their apnea-hypopnea index was.3 Overall in the study, 85% of the patients had a reduction in nighttime urination after CPAP therapy.
Treatment of sleep apnea has been shown in several studies to improve the nocturia that occurs in patients with sleep apnea. Hyoung Keun Park, MD, of Konkuk University, Seoul, and colleagues studied whether surgical intervention with uvulopalatopharyngoplasty (UPPP) reduced nocturia in patients with sleep apnea.4 In the study, there was a 73% success rate in treatment for sleep apnea with the UPPP surgery, and, among those who had successful surgeries, nocturia episodes decreased from 1.9 preoperatively to 0.7 postoperatively (P less than .001).
Minoru Miyazato, MD, PhD, of University of the Ryukyus, Okinawa, Japan, and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.5 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P less than .01).
Pearl: Sleep apnea should be considered in the differential diagnosis of patients with nocturia, and treatment of sleep apnea may decrease nocturia.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Intern Med. 2016;55(8):901-5.
2. Sleep Breath. 2017 Feb 14. doi: 10.1007/s11325-017-1482-9.
3. Can Urol Assoc J. 2016 Jul-Aug;10(7-8):E241-5.
4. Int Neurourol J. 2016 Dec;20(4):329-34.
5. Neurourol Urodyn. 2017 Feb;36(2):376-9.
Author’s note: I have been writing “Myth of the Month” columns for the last several years. I will try to continue to write about myths when possible, but I would like to introduce a new column, “Pearl of the Month.” I want to share with you pearls that I have found really helpful in medical practice. Some of these will be new news, while some may be old news that may not be well known.
A 65-year-old man comes to a clinic concerned about frequent nocturia. He is getting up four times a night to urinate, and he has been urinating about every 5 hours during the day. He has been seen twice for this problem and was diagnosed with benign prostatic hyperplasia and started on tamsulosin.
He found a slight improvement when he started on 0.4 mg qhs, reducing his nocturia episodes from four to three. His dose was increased to 0.8 mg qhs, with no improvement in nocturia.
Exam today: BP, 140/94; pulse, 70. Rectal exam: Prostate is twice normal size without nodules. Labs: Na, 140; K, 4.0; glucose, 80; Ca, 9.6.
He is frustrated because he feels tired and sleepy from having to get up so often to urinate every night.
What is the best treatment/advice at this point?
A. Check hemoglobin A1C.
B. Start finasteride.
C. Switch tamsulosin to terazosin.
D. Evaluate for sleep apnea.
Umpei Yamamoto, MD, of Kyushu University Hospital, Japan, and colleagues studied the prevalence of sleep-disordered breathing among patients who presented to a urology clinic with nocturia and in those who visited a sleep apnea clinic with symptoms of excessive daytime sleepiness.1 Sleep-disordered breathing was found in 91% of the patients from the sleep apnea clinic and 70% of the patients from the urology clinic. The frequency of nocturia was reduced with continuous positive airway pressure (CPAP) in both groups in the patients who had not responded to conventional therapy or nocturia.
The symptom of nocturia as a symptom of sleep apnea might be even more common in women.2 Ozen K. Basoglu, MD, and Mehmet Sezai Tasbakan, MD, of Ege University, Izmir, Turkey, described clinical similarities and differences based on gender in a large group of patients with sleep apnea. Both men and women with sleep apnea had similar rates of excessive daytime sleepiness, snoring, and impaired concentration. Women had more frequent nocturia.
Nocturia especially should be considered a possible clue for the presence of sleep apnea in younger patients who have fewer other reasons to have nocturia. Takahiro Maeda, MD, of Keio University, Tokyo, and colleagues found that men younger than 50 years had more nocturnal urinations the worse their apnea-hypopnea index was.3 Overall in the study, 85% of the patients had a reduction in nighttime urination after CPAP therapy.
Treatment of sleep apnea has been shown in several studies to improve the nocturia that occurs in patients with sleep apnea. Hyoung Keun Park, MD, of Konkuk University, Seoul, and colleagues studied whether surgical intervention with uvulopalatopharyngoplasty (UPPP) reduced nocturia in patients with sleep apnea.4 In the study, there was a 73% success rate in treatment for sleep apnea with the UPPP surgery, and, among those who had successful surgeries, nocturia episodes decreased from 1.9 preoperatively to 0.7 postoperatively (P less than .001).
Minoru Miyazato, MD, PhD, of University of the Ryukyus, Okinawa, Japan, and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.5 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P less than .01).
Pearl: Sleep apnea should be considered in the differential diagnosis of patients with nocturia, and treatment of sleep apnea may decrease nocturia.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Intern Med. 2016;55(8):901-5.
2. Sleep Breath. 2017 Feb 14. doi: 10.1007/s11325-017-1482-9.
3. Can Urol Assoc J. 2016 Jul-Aug;10(7-8):E241-5.
4. Int Neurourol J. 2016 Dec;20(4):329-34.
5. Neurourol Urodyn. 2017 Feb;36(2):376-9.
Author’s note: I have been writing “Myth of the Month” columns for the last several years. I will try to continue to write about myths when possible, but I would like to introduce a new column, “Pearl of the Month.” I want to share with you pearls that I have found really helpful in medical practice. Some of these will be new news, while some may be old news that may not be well known.
A 65-year-old man comes to a clinic concerned about frequent nocturia. He is getting up four times a night to urinate, and he has been urinating about every 5 hours during the day. He has been seen twice for this problem and was diagnosed with benign prostatic hyperplasia and started on tamsulosin.
He found a slight improvement when he started on 0.4 mg qhs, reducing his nocturia episodes from four to three. His dose was increased to 0.8 mg qhs, with no improvement in nocturia.
Exam today: BP, 140/94; pulse, 70. Rectal exam: Prostate is twice normal size without nodules. Labs: Na, 140; K, 4.0; glucose, 80; Ca, 9.6.
He is frustrated because he feels tired and sleepy from having to get up so often to urinate every night.
What is the best treatment/advice at this point?
A. Check hemoglobin A1C.
B. Start finasteride.
C. Switch tamsulosin to terazosin.
D. Evaluate for sleep apnea.
Umpei Yamamoto, MD, of Kyushu University Hospital, Japan, and colleagues studied the prevalence of sleep-disordered breathing among patients who presented to a urology clinic with nocturia and in those who visited a sleep apnea clinic with symptoms of excessive daytime sleepiness.1 Sleep-disordered breathing was found in 91% of the patients from the sleep apnea clinic and 70% of the patients from the urology clinic. The frequency of nocturia was reduced with continuous positive airway pressure (CPAP) in both groups in the patients who had not responded to conventional therapy or nocturia.
The symptom of nocturia as a symptom of sleep apnea might be even more common in women.2 Ozen K. Basoglu, MD, and Mehmet Sezai Tasbakan, MD, of Ege University, Izmir, Turkey, described clinical similarities and differences based on gender in a large group of patients with sleep apnea. Both men and women with sleep apnea had similar rates of excessive daytime sleepiness, snoring, and impaired concentration. Women had more frequent nocturia.
Nocturia especially should be considered a possible clue for the presence of sleep apnea in younger patients who have fewer other reasons to have nocturia. Takahiro Maeda, MD, of Keio University, Tokyo, and colleagues found that men younger than 50 years had more nocturnal urinations the worse their apnea-hypopnea index was.3 Overall in the study, 85% of the patients had a reduction in nighttime urination after CPAP therapy.
Treatment of sleep apnea has been shown in several studies to improve the nocturia that occurs in patients with sleep apnea. Hyoung Keun Park, MD, of Konkuk University, Seoul, and colleagues studied whether surgical intervention with uvulopalatopharyngoplasty (UPPP) reduced nocturia in patients with sleep apnea.4 In the study, there was a 73% success rate in treatment for sleep apnea with the UPPP surgery, and, among those who had successful surgeries, nocturia episodes decreased from 1.9 preoperatively to 0.7 postoperatively (P less than .001).
Minoru Miyazato, MD, PhD, of University of the Ryukyus, Okinawa, Japan, and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.5 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P less than .01).
Pearl: Sleep apnea should be considered in the differential diagnosis of patients with nocturia, and treatment of sleep apnea may decrease nocturia.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Intern Med. 2016;55(8):901-5.
2. Sleep Breath. 2017 Feb 14. doi: 10.1007/s11325-017-1482-9.
3. Can Urol Assoc J. 2016 Jul-Aug;10(7-8):E241-5.
4. Int Neurourol J. 2016 Dec;20(4):329-34.
5. Neurourol Urodyn. 2017 Feb;36(2):376-9.
Preliminary results promising from ublituximab phase II study for MS
NEW ORLEANS – The investigative agent ublituximab was well tolerated and demonstrated rapid B-cell depletion in patients with relapsing multiple sclerosis, results from an ongoing phase II trial showed.
“Ublituximab is a novel, chimeric monoclonal antibody targeting a unique epitope on the CD20 antigen, and glycoengineered to enhance affinity for all variants of FcyRIlla receptors, thereby demonstrating greater antibody-dependent cellular toxicity activity than rituximab and ofatumumab,” Amy Lovett-Racke, PhD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In a trial supported by TG Therapeutics, which is developing the agent, Dr. Lovett-Racke of the department of microbial infection and immunity at the Ohio State University Medical Center, Columbus, and her associates reported data from 24 patients who received ublituximab at doses markedly less than those used in ongoing phase III oncology studies, and at a range of infusion times, with a goal of rapid infusions.
The study ran for 48 weeks, and the primary endpoint was responders rate, defined as a percent of patients with at least a 95% reduction in peripheral CD19-positive B-cells within 2 weeks after the second infusion. All patients received the same total dose of 600 mg; only infusion times differed. (Ublituximab was administered on day 1, day 15, and week 24. Placebo IV infusion dose was administered on day 1 and day 15 only.)
The mean age of the study participants was 40 years, and the distribution of time from diagnosis was less than 5 years in 11 patients, 5-10 years in 7 patients, and greater than 10 years in 6 patients.
To date, ublituximab has been well tolerated, with only mild infusion reactions being observed, even with infusion times reduced to 1 hour. The researchers also found that ublituximab resulted in 99% B-cell depletion, meeting the study endpoint of greater than 95% depletion within 2 weeks of the second dose, which is comparable to ocrelizumab.
“Every patient has met the endpoint so far,” Dr. Lovett-Racke said. “Although there is a transient decrease in T cells after the initial dose of ublituximab, T-cell numbers are fairly stable over time. Memory B cells seem slightly more resistant to depletion, but are efficiently depleted in all patients. A comprehensive analysis of B- and T-cell profiles is being performed to understand how B-cell depletion influences T-cell profiles, and to characterize the B-cell repletion.”
TG Therapeutics funded the study. Dr. Lovett-Racke disclosed having received research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Strategic Pharmaceutical Academic Research Consortium.
NEW ORLEANS – The investigative agent ublituximab was well tolerated and demonstrated rapid B-cell depletion in patients with relapsing multiple sclerosis, results from an ongoing phase II trial showed.
“Ublituximab is a novel, chimeric monoclonal antibody targeting a unique epitope on the CD20 antigen, and glycoengineered to enhance affinity for all variants of FcyRIlla receptors, thereby demonstrating greater antibody-dependent cellular toxicity activity than rituximab and ofatumumab,” Amy Lovett-Racke, PhD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In a trial supported by TG Therapeutics, which is developing the agent, Dr. Lovett-Racke of the department of microbial infection and immunity at the Ohio State University Medical Center, Columbus, and her associates reported data from 24 patients who received ublituximab at doses markedly less than those used in ongoing phase III oncology studies, and at a range of infusion times, with a goal of rapid infusions.
The study ran for 48 weeks, and the primary endpoint was responders rate, defined as a percent of patients with at least a 95% reduction in peripheral CD19-positive B-cells within 2 weeks after the second infusion. All patients received the same total dose of 600 mg; only infusion times differed. (Ublituximab was administered on day 1, day 15, and week 24. Placebo IV infusion dose was administered on day 1 and day 15 only.)
The mean age of the study participants was 40 years, and the distribution of time from diagnosis was less than 5 years in 11 patients, 5-10 years in 7 patients, and greater than 10 years in 6 patients.
To date, ublituximab has been well tolerated, with only mild infusion reactions being observed, even with infusion times reduced to 1 hour. The researchers also found that ublituximab resulted in 99% B-cell depletion, meeting the study endpoint of greater than 95% depletion within 2 weeks of the second dose, which is comparable to ocrelizumab.
“Every patient has met the endpoint so far,” Dr. Lovett-Racke said. “Although there is a transient decrease in T cells after the initial dose of ublituximab, T-cell numbers are fairly stable over time. Memory B cells seem slightly more resistant to depletion, but are efficiently depleted in all patients. A comprehensive analysis of B- and T-cell profiles is being performed to understand how B-cell depletion influences T-cell profiles, and to characterize the B-cell repletion.”
TG Therapeutics funded the study. Dr. Lovett-Racke disclosed having received research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Strategic Pharmaceutical Academic Research Consortium.
NEW ORLEANS – The investigative agent ublituximab was well tolerated and demonstrated rapid B-cell depletion in patients with relapsing multiple sclerosis, results from an ongoing phase II trial showed.
“Ublituximab is a novel, chimeric monoclonal antibody targeting a unique epitope on the CD20 antigen, and glycoengineered to enhance affinity for all variants of FcyRIlla receptors, thereby demonstrating greater antibody-dependent cellular toxicity activity than rituximab and ofatumumab,” Amy Lovett-Racke, PhD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In a trial supported by TG Therapeutics, which is developing the agent, Dr. Lovett-Racke of the department of microbial infection and immunity at the Ohio State University Medical Center, Columbus, and her associates reported data from 24 patients who received ublituximab at doses markedly less than those used in ongoing phase III oncology studies, and at a range of infusion times, with a goal of rapid infusions.
The study ran for 48 weeks, and the primary endpoint was responders rate, defined as a percent of patients with at least a 95% reduction in peripheral CD19-positive B-cells within 2 weeks after the second infusion. All patients received the same total dose of 600 mg; only infusion times differed. (Ublituximab was administered on day 1, day 15, and week 24. Placebo IV infusion dose was administered on day 1 and day 15 only.)
The mean age of the study participants was 40 years, and the distribution of time from diagnosis was less than 5 years in 11 patients, 5-10 years in 7 patients, and greater than 10 years in 6 patients.
To date, ublituximab has been well tolerated, with only mild infusion reactions being observed, even with infusion times reduced to 1 hour. The researchers also found that ublituximab resulted in 99% B-cell depletion, meeting the study endpoint of greater than 95% depletion within 2 weeks of the second dose, which is comparable to ocrelizumab.
“Every patient has met the endpoint so far,” Dr. Lovett-Racke said. “Although there is a transient decrease in T cells after the initial dose of ublituximab, T-cell numbers are fairly stable over time. Memory B cells seem slightly more resistant to depletion, but are efficiently depleted in all patients. A comprehensive analysis of B- and T-cell profiles is being performed to understand how B-cell depletion influences T-cell profiles, and to characterize the B-cell repletion.”
TG Therapeutics funded the study. Dr. Lovett-Racke disclosed having received research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Strategic Pharmaceutical Academic Research Consortium.
AT THE CMSC ANNUAL MEETING
Key clinical point:
Major finding: Use of ublituximab resulted in 99% B-cell depletion among patients with relapsing MS.
Data source: Preliminary results from a phase II placebo-controlled study of 24 MS patients who received ublituximab.
Disclosures: TG Therapeutics funded the study. Dr. Lovett-Racke disclosed having received research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Strategic Pharmaceutical Academic Research Consortium.
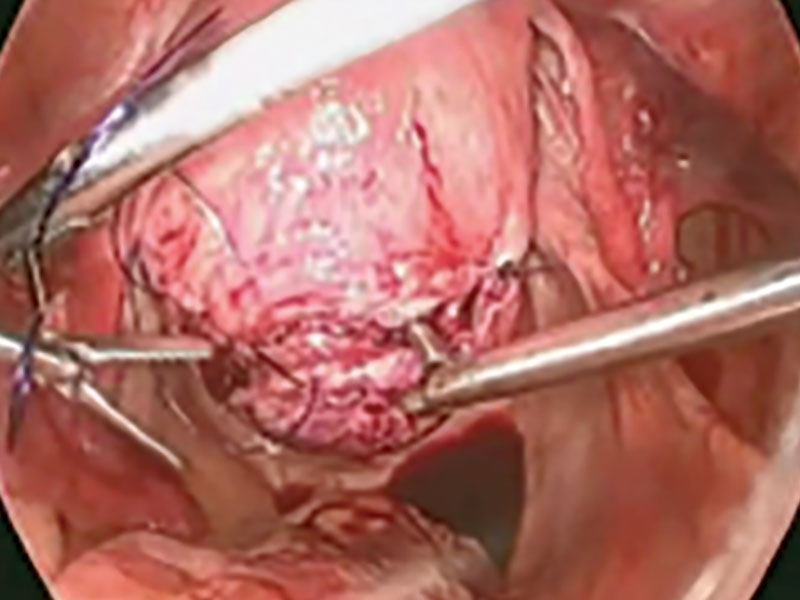
Laparoscopic myomectomy technique
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
App allows monitoring of drug effects in Parkinson’s
BOSTON – The developers of a free app that tests mental and physical symptoms in patients with Parkinson’s disease (PD) report that their software allows the monitoring of responses to treatment.
“We can track markers of medication,” Larsson Omberg, PhD, vice president of systems biology at the nonprofit Sage Bionetworks, said in an interview. “We can statistically predict whether someone has taken their medication or not, and we can even subdivide populations into strong responders to l-dopa and individuals who don’t necessarily have strong responses to medication but have strong fluctuations based on the time of day.”
A total of 10,326 participants (86%) served as a control group of people who do not have PD. Their average age was 32 (interquartile range, 23-38). The other 14% of participants (n = 2,373) disclosed that they have PD. Their average age was 60 (IQR, 54-68), and they reported having the disease for an average of 8 years (IQR, 4-10).
Overall, 80% of the control participants were male, as were 64% of the PD participants.
Researchers found that 96% of the PD participants were taking PD medications, and 10% reported having had deep brain stimulation.
Participants were asked to use the app’s surveys and tests to measure things such as tremor, sleep quality, frequency of gait, tapping ability, and voice jitter.
“We ask them to perform these active tasks, things they might do in the clinic,” Dr. Omberg said. “The difference is that we are capturing measurements using the sensors in the phone. It’s relatively easy so we can track changes over a day and from day to day.”
For example, one of the tests measures how quickly users can tap the screen over a 20-second interval. “It is a two-finger tap between two fingers and two buttons on the screen,” Dr. Omberg said. “Finger tapping is associated with the severity of the disease.”
The researchers found that they could track patient variability throughout the day and connect the data to the times when patients took medication. In half of the PD patients, the data showed a correlation between medication use and performance of at least one of five types of activities – gait, balance, voice, memory, and tapping.
The app and the information it has provided have limitations, he said. While one participant has used the app at least three times a day for 2 years, many users tried it out for a short time and became inactive. And while users can access some of their own data, it’s not very helpful yet: “It’s very raw and probably not the most useful to a clinician,” Dr. Omberg said.
Regarding whether the app could be used to reveal early signs of PD, Dr. Omberg cautioned that it wasn’t designed for that purpose. Still, “I’m pretty sure applications like mPower would be technically able to do this at some point in the future,” he said. “But there is a lot of validation work that will have to be done first.”
For now, the app remains available for free for iOS devices, and researchers are working on a new version. Meanwhile, Dr. Omberg said the app is serving as a research tool to collect baseline and trial data in the Safety of Urate Elevation in Parkinson’s Disease (SURE-PD) study, which is examining the use of oral inosine to boost serum urate in PD patients.
The study was funded by the Robert Wood Johnson Foundation.
BOSTON – The developers of a free app that tests mental and physical symptoms in patients with Parkinson’s disease (PD) report that their software allows the monitoring of responses to treatment.
“We can track markers of medication,” Larsson Omberg, PhD, vice president of systems biology at the nonprofit Sage Bionetworks, said in an interview. “We can statistically predict whether someone has taken their medication or not, and we can even subdivide populations into strong responders to l-dopa and individuals who don’t necessarily have strong responses to medication but have strong fluctuations based on the time of day.”
A total of 10,326 participants (86%) served as a control group of people who do not have PD. Their average age was 32 (interquartile range, 23-38). The other 14% of participants (n = 2,373) disclosed that they have PD. Their average age was 60 (IQR, 54-68), and they reported having the disease for an average of 8 years (IQR, 4-10).
Overall, 80% of the control participants were male, as were 64% of the PD participants.
Researchers found that 96% of the PD participants were taking PD medications, and 10% reported having had deep brain stimulation.
Participants were asked to use the app’s surveys and tests to measure things such as tremor, sleep quality, frequency of gait, tapping ability, and voice jitter.
“We ask them to perform these active tasks, things they might do in the clinic,” Dr. Omberg said. “The difference is that we are capturing measurements using the sensors in the phone. It’s relatively easy so we can track changes over a day and from day to day.”
For example, one of the tests measures how quickly users can tap the screen over a 20-second interval. “It is a two-finger tap between two fingers and two buttons on the screen,” Dr. Omberg said. “Finger tapping is associated with the severity of the disease.”
The researchers found that they could track patient variability throughout the day and connect the data to the times when patients took medication. In half of the PD patients, the data showed a correlation between medication use and performance of at least one of five types of activities – gait, balance, voice, memory, and tapping.
The app and the information it has provided have limitations, he said. While one participant has used the app at least three times a day for 2 years, many users tried it out for a short time and became inactive. And while users can access some of their own data, it’s not very helpful yet: “It’s very raw and probably not the most useful to a clinician,” Dr. Omberg said.
Regarding whether the app could be used to reveal early signs of PD, Dr. Omberg cautioned that it wasn’t designed for that purpose. Still, “I’m pretty sure applications like mPower would be technically able to do this at some point in the future,” he said. “But there is a lot of validation work that will have to be done first.”
For now, the app remains available for free for iOS devices, and researchers are working on a new version. Meanwhile, Dr. Omberg said the app is serving as a research tool to collect baseline and trial data in the Safety of Urate Elevation in Parkinson’s Disease (SURE-PD) study, which is examining the use of oral inosine to boost serum urate in PD patients.
The study was funded by the Robert Wood Johnson Foundation.
BOSTON – The developers of a free app that tests mental and physical symptoms in patients with Parkinson’s disease (PD) report that their software allows the monitoring of responses to treatment.
“We can track markers of medication,” Larsson Omberg, PhD, vice president of systems biology at the nonprofit Sage Bionetworks, said in an interview. “We can statistically predict whether someone has taken their medication or not, and we can even subdivide populations into strong responders to l-dopa and individuals who don’t necessarily have strong responses to medication but have strong fluctuations based on the time of day.”
A total of 10,326 participants (86%) served as a control group of people who do not have PD. Their average age was 32 (interquartile range, 23-38). The other 14% of participants (n = 2,373) disclosed that they have PD. Their average age was 60 (IQR, 54-68), and they reported having the disease for an average of 8 years (IQR, 4-10).
Overall, 80% of the control participants were male, as were 64% of the PD participants.
Researchers found that 96% of the PD participants were taking PD medications, and 10% reported having had deep brain stimulation.
Participants were asked to use the app’s surveys and tests to measure things such as tremor, sleep quality, frequency of gait, tapping ability, and voice jitter.
“We ask them to perform these active tasks, things they might do in the clinic,” Dr. Omberg said. “The difference is that we are capturing measurements using the sensors in the phone. It’s relatively easy so we can track changes over a day and from day to day.”
For example, one of the tests measures how quickly users can tap the screen over a 20-second interval. “It is a two-finger tap between two fingers and two buttons on the screen,” Dr. Omberg said. “Finger tapping is associated with the severity of the disease.”
The researchers found that they could track patient variability throughout the day and connect the data to the times when patients took medication. In half of the PD patients, the data showed a correlation between medication use and performance of at least one of five types of activities – gait, balance, voice, memory, and tapping.
The app and the information it has provided have limitations, he said. While one participant has used the app at least three times a day for 2 years, many users tried it out for a short time and became inactive. And while users can access some of their own data, it’s not very helpful yet: “It’s very raw and probably not the most useful to a clinician,” Dr. Omberg said.
Regarding whether the app could be used to reveal early signs of PD, Dr. Omberg cautioned that it wasn’t designed for that purpose. Still, “I’m pretty sure applications like mPower would be technically able to do this at some point in the future,” he said. “But there is a lot of validation work that will have to be done first.”
For now, the app remains available for free for iOS devices, and researchers are working on a new version. Meanwhile, Dr. Omberg said the app is serving as a research tool to collect baseline and trial data in the Safety of Urate Elevation in Parkinson’s Disease (SURE-PD) study, which is examining the use of oral inosine to boost serum urate in PD patients.
The study was funded by the Robert Wood Johnson Foundation.
AT AAN 2017
Solving stool refusal
When parents bring in their delightful, verbal 3-year-old for refusing to poop on the potty, it may seem laughable. But with impending preschool and costs of diapers, stool refusal can be a major aggravation for families! Fortunately,
Commonly a healthy, typically developing boy stands and urinates in the toilet just fine, but sneaks off behind the sofa to poop. Parent gyrations have gone from cajoling, to punishing, to offering trips to Disney! Flaring tempers can set the stage for stool refusal to be a power play.
There are a number of reasons stool refusal may give clues to child and family tendencies and relevant intervention. We always should be alert to rare medical problems such as Hirschsprung disease or traumas (from slammed toilet lids to sexual abuse). But while learning to use the toilet for urination and defecation generally occur around the same time, there are pitfalls making pooping in the potty different. An impending stool provides stronger sensations and more advance warning than urine and tends to come at regular times, making it logical to start toilet learning with sitting on the potty after meals.
But once seated on the potty, stools can require some waiting – not a typical toddler forte! While running to sit has novelty at first and may be reinforced by celebration, this quickly becomes routine and boring. Very active or very intense children especially hate having their play interrupted by a trip to the bathroom. Oppositional children just won’t perform if they think the parent cares! And unlike for urination, everyone can inhibit defecation long enough for the urge to pass. Repeated stool retention from ignoring the urge makes stools dessicated and harder, with resulting pain when finally passed. One painful stool makes many a young child decide “Never again!” and simply refuse the toilet. A rectal fissure can both start
During unclogging and establishing a new stool pattern, the toddler should be matter-of-factly put back in diapers (not pull ups) saying “Oh well, you are just not ready for pants yet.” Dramatically placing the treasured Superhero underwear on the top shelf increases motivation (or promised if none have been acquired). Returning to diapers without shaming the child is key, and all caregivers need to buy in. They need to be good “actors,” conveying that they don’t really care about toileting to reduce the power struggle. If controlling poop is a battle, only the child can win!
When the soft stools are occurring several times per day, I suggest “M&M treatment”: 1 for sitting, 2 for peeing, and 3 for pooping = 6 potential M&Ms per episode. The “1 for sitting” (the easiest part), is not painful and restores the habit of complying. Remember, M&Ms are no match for a game on an iPad! By charting the times of stools, the parent can remove electronics ½ hour before the expected poop and restrict the child to one room of the house with a potty nearby. Parents can interact, but should avoid making this a rewarding playtime. When the child uses the potty rather than their pants, the room restriction is removed until the next window for pooping. If they poop outside the toilet, they remain restricted (and no electronics) until the next window (even the next day).
Some parents are especially sensitive to the smell and mess of stools and pass that attitude along to their child by saying “Ugh, you stink!” or “I can’t stand this mess!” or even handing the child to another caregiver in a gesture of rejection. These messages are not missed by the child, who may then not want to deal with the mess, either. I coach parents to stay at least neutral about stools, reminding them that, “Your child is going to have to poop her whole life!”
Demanding a diaper and then getting the special intimacy of bottom cleaning can be reinforcing. If there is a younger sibling, diaper changes may be a desired opportunity for the toddler to regress and retain some “baby privileges.” Other clues to this dynamic include thumb sucking, baby talk, clinginess, or being rough on the sibling. One part of addressing this issue is to prescribe “babying” the toddler by holding in arms, rocking, talking baby talk, offering a pacifier, and feeding him during daily parent-child one-on-one Special Time. This sounds crazy to parents aiming for grown up toileting, but I promise them the child will not go backwards! It addresses the child’s deep fear that the nurturing of infancy is no longer available.
You may have noticed that boys are much more likely to refuse stools than girls. Some of this difference may be that high activity, but learning to urinate standing up also is fun, a Big Boy feat, and a source of pride to fathers. If regular sitting to poop has not been well established before the fun of standing to pee is offered, the little guys are not so interested in sitting again to poop. Plus the wiping and hand washing after poops are further aggravations delaying return to the Legos. But more! By around age 3 years, both genders make the horrifying discovery that boys have a penis and girls don’t. At this age of confusion about potential transformations, the obvious conclusion is that the girl’s penis was lost! And that turd disappearing down the toilet looks a lot like a dismembered body part! Reassurance and education is in order. I address this with my “Penis Talk”: “Boys are made with a penis and girls are made with a vagina. (For boys:) When you get big like your Dad, your penis will be big, too. No one can ever take your penis away. (For girls, a less common concern.) You have always had a vagina. You did not lose a penis.” I recommend that you practice this in front of a mirror before first use!
Another cognitive milestone concerns what sorts of things can disappear dow
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. Email her at [email protected].
When parents bring in their delightful, verbal 3-year-old for refusing to poop on the potty, it may seem laughable. But with impending preschool and costs of diapers, stool refusal can be a major aggravation for families! Fortunately,
Commonly a healthy, typically developing boy stands and urinates in the toilet just fine, but sneaks off behind the sofa to poop. Parent gyrations have gone from cajoling, to punishing, to offering trips to Disney! Flaring tempers can set the stage for stool refusal to be a power play.
There are a number of reasons stool refusal may give clues to child and family tendencies and relevant intervention. We always should be alert to rare medical problems such as Hirschsprung disease or traumas (from slammed toilet lids to sexual abuse). But while learning to use the toilet for urination and defecation generally occur around the same time, there are pitfalls making pooping in the potty different. An impending stool provides stronger sensations and more advance warning than urine and tends to come at regular times, making it logical to start toilet learning with sitting on the potty after meals.
But once seated on the potty, stools can require some waiting – not a typical toddler forte! While running to sit has novelty at first and may be reinforced by celebration, this quickly becomes routine and boring. Very active or very intense children especially hate having their play interrupted by a trip to the bathroom. Oppositional children just won’t perform if they think the parent cares! And unlike for urination, everyone can inhibit defecation long enough for the urge to pass. Repeated stool retention from ignoring the urge makes stools dessicated and harder, with resulting pain when finally passed. One painful stool makes many a young child decide “Never again!” and simply refuse the toilet. A rectal fissure can both start
During unclogging and establishing a new stool pattern, the toddler should be matter-of-factly put back in diapers (not pull ups) saying “Oh well, you are just not ready for pants yet.” Dramatically placing the treasured Superhero underwear on the top shelf increases motivation (or promised if none have been acquired). Returning to diapers without shaming the child is key, and all caregivers need to buy in. They need to be good “actors,” conveying that they don’t really care about toileting to reduce the power struggle. If controlling poop is a battle, only the child can win!
When the soft stools are occurring several times per day, I suggest “M&M treatment”: 1 for sitting, 2 for peeing, and 3 for pooping = 6 potential M&Ms per episode. The “1 for sitting” (the easiest part), is not painful and restores the habit of complying. Remember, M&Ms are no match for a game on an iPad! By charting the times of stools, the parent can remove electronics ½ hour before the expected poop and restrict the child to one room of the house with a potty nearby. Parents can interact, but should avoid making this a rewarding playtime. When the child uses the potty rather than their pants, the room restriction is removed until the next window for pooping. If they poop outside the toilet, they remain restricted (and no electronics) until the next window (even the next day).
Some parents are especially sensitive to the smell and mess of stools and pass that attitude along to their child by saying “Ugh, you stink!” or “I can’t stand this mess!” or even handing the child to another caregiver in a gesture of rejection. These messages are not missed by the child, who may then not want to deal with the mess, either. I coach parents to stay at least neutral about stools, reminding them that, “Your child is going to have to poop her whole life!”
Demanding a diaper and then getting the special intimacy of bottom cleaning can be reinforcing. If there is a younger sibling, diaper changes may be a desired opportunity for the toddler to regress and retain some “baby privileges.” Other clues to this dynamic include thumb sucking, baby talk, clinginess, or being rough on the sibling. One part of addressing this issue is to prescribe “babying” the toddler by holding in arms, rocking, talking baby talk, offering a pacifier, and feeding him during daily parent-child one-on-one Special Time. This sounds crazy to parents aiming for grown up toileting, but I promise them the child will not go backwards! It addresses the child’s deep fear that the nurturing of infancy is no longer available.
You may have noticed that boys are much more likely to refuse stools than girls. Some of this difference may be that high activity, but learning to urinate standing up also is fun, a Big Boy feat, and a source of pride to fathers. If regular sitting to poop has not been well established before the fun of standing to pee is offered, the little guys are not so interested in sitting again to poop. Plus the wiping and hand washing after poops are further aggravations delaying return to the Legos. But more! By around age 3 years, both genders make the horrifying discovery that boys have a penis and girls don’t. At this age of confusion about potential transformations, the obvious conclusion is that the girl’s penis was lost! And that turd disappearing down the toilet looks a lot like a dismembered body part! Reassurance and education is in order. I address this with my “Penis Talk”: “Boys are made with a penis and girls are made with a vagina. (For boys:) When you get big like your Dad, your penis will be big, too. No one can ever take your penis away. (For girls, a less common concern.) You have always had a vagina. You did not lose a penis.” I recommend that you practice this in front of a mirror before first use!
Another cognitive milestone concerns what sorts of things can disappear dow
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. Email her at [email protected].
When parents bring in their delightful, verbal 3-year-old for refusing to poop on the potty, it may seem laughable. But with impending preschool and costs of diapers, stool refusal can be a major aggravation for families! Fortunately,
Commonly a healthy, typically developing boy stands and urinates in the toilet just fine, but sneaks off behind the sofa to poop. Parent gyrations have gone from cajoling, to punishing, to offering trips to Disney! Flaring tempers can set the stage for stool refusal to be a power play.
There are a number of reasons stool refusal may give clues to child and family tendencies and relevant intervention. We always should be alert to rare medical problems such as Hirschsprung disease or traumas (from slammed toilet lids to sexual abuse). But while learning to use the toilet for urination and defecation generally occur around the same time, there are pitfalls making pooping in the potty different. An impending stool provides stronger sensations and more advance warning than urine and tends to come at regular times, making it logical to start toilet learning with sitting on the potty after meals.
But once seated on the potty, stools can require some waiting – not a typical toddler forte! While running to sit has novelty at first and may be reinforced by celebration, this quickly becomes routine and boring. Very active or very intense children especially hate having their play interrupted by a trip to the bathroom. Oppositional children just won’t perform if they think the parent cares! And unlike for urination, everyone can inhibit defecation long enough for the urge to pass. Repeated stool retention from ignoring the urge makes stools dessicated and harder, with resulting pain when finally passed. One painful stool makes many a young child decide “Never again!” and simply refuse the toilet. A rectal fissure can both start
During unclogging and establishing a new stool pattern, the toddler should be matter-of-factly put back in diapers (not pull ups) saying “Oh well, you are just not ready for pants yet.” Dramatically placing the treasured Superhero underwear on the top shelf increases motivation (or promised if none have been acquired). Returning to diapers without shaming the child is key, and all caregivers need to buy in. They need to be good “actors,” conveying that they don’t really care about toileting to reduce the power struggle. If controlling poop is a battle, only the child can win!
When the soft stools are occurring several times per day, I suggest “M&M treatment”: 1 for sitting, 2 for peeing, and 3 for pooping = 6 potential M&Ms per episode. The “1 for sitting” (the easiest part), is not painful and restores the habit of complying. Remember, M&Ms are no match for a game on an iPad! By charting the times of stools, the parent can remove electronics ½ hour before the expected poop and restrict the child to one room of the house with a potty nearby. Parents can interact, but should avoid making this a rewarding playtime. When the child uses the potty rather than their pants, the room restriction is removed until the next window for pooping. If they poop outside the toilet, they remain restricted (and no electronics) until the next window (even the next day).
Some parents are especially sensitive to the smell and mess of stools and pass that attitude along to their child by saying “Ugh, you stink!” or “I can’t stand this mess!” or even handing the child to another caregiver in a gesture of rejection. These messages are not missed by the child, who may then not want to deal with the mess, either. I coach parents to stay at least neutral about stools, reminding them that, “Your child is going to have to poop her whole life!”
Demanding a diaper and then getting the special intimacy of bottom cleaning can be reinforcing. If there is a younger sibling, diaper changes may be a desired opportunity for the toddler to regress and retain some “baby privileges.” Other clues to this dynamic include thumb sucking, baby talk, clinginess, or being rough on the sibling. One part of addressing this issue is to prescribe “babying” the toddler by holding in arms, rocking, talking baby talk, offering a pacifier, and feeding him during daily parent-child one-on-one Special Time. This sounds crazy to parents aiming for grown up toileting, but I promise them the child will not go backwards! It addresses the child’s deep fear that the nurturing of infancy is no longer available.
You may have noticed that boys are much more likely to refuse stools than girls. Some of this difference may be that high activity, but learning to urinate standing up also is fun, a Big Boy feat, and a source of pride to fathers. If regular sitting to poop has not been well established before the fun of standing to pee is offered, the little guys are not so interested in sitting again to poop. Plus the wiping and hand washing after poops are further aggravations delaying return to the Legos. But more! By around age 3 years, both genders make the horrifying discovery that boys have a penis and girls don’t. At this age of confusion about potential transformations, the obvious conclusion is that the girl’s penis was lost! And that turd disappearing down the toilet looks a lot like a dismembered body part! Reassurance and education is in order. I address this with my “Penis Talk”: “Boys are made with a penis and girls are made with a vagina. (For boys:) When you get big like your Dad, your penis will be big, too. No one can ever take your penis away. (For girls, a less common concern.) You have always had a vagina. You did not lose a penis.” I recommend that you practice this in front of a mirror before first use!
Another cognitive milestone concerns what sorts of things can disappear dow
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. Email her at [email protected].
Labor and delivery mismanaged, child has CP: $30.5M award
Labor and delivery mismanaged, child has CP: $30.5M award
Two days later, the mother reported decreased fetal movement; she was admitted to the hospital for continuous fetal heart-rate (FHR) monitoring, and a maternal-fetal medicine (MFM) specialist was consulted. The mother was not placed on FHR monitoring until 2 hours after admission. Three hours after admission, the MFM, by phone, recommended further testing and later, cesarean delivery.
The child was found to have spastic quadriplegic cerebral palsy, profound developmental delays, a seizure disorder, and cortical blindness.
PARENTS' CLAIM:
The child's injuries were due to mismanagement of labor and delivery. The MFM prescribed ultrasonographic biophysical profiles, but they were not performed until 2 hours after ordered. There were 3 ultrasonography (US) technicians at the hospital when the mother was admitted: 1 was on break, another was performing other tests, and the third was not notified because the hospital's computer system was down. When test results were unfavorable, the MFM recommended emergency cesarean delivery. An earlier delivery could have prevented the child's injuries.
DEFENDANTS' DEFENSE:
The infant's injuries were a result of her mother's failure to keep her GDM under control.
VERDICT:
A $30,545,655 Georgia verdict was returned.
Related article:
10 tips for overcoming common challenges of intrapartum fetal monitoring
Did oxytocin cause child's spastic CP? $14.4M verdict
When a woman went to the hospital in labor, her ObGyn ordered oxytocin to enhance delivery. The FHR monitor showed repetitive decelerations for the next hour, dropping to 60 bpm by 8:00 pm, when the ObGyn expedited delivery but did not stop the oxytocin. By 8:20 pm, the baby's head was crowning, but the ObGyn waited another 10 minutes before performing an episiotomy and delivering the baby.
The child, intubated 5 minutes after birth, was found to have spastic tetraparesis cerebral palsy (CP) with impaired cognition, seizures, and global aphasia.
PARENTS' CLAIM:
The ObGyn and nurses failed to properly monitor labor and delivery. The ObGyn should not have started oxytocin because the patient's labor was progressing normally. He should have taken the mother off oxytocin at 8:00 pm when the FHR dropped to 60 bpm. He should have performed an operative delivery at 8:20 pm when the baby's head crowned. An earlier delivery would have prevented injury.
DEFENDANTS' DEFENSE:
The ObGyn's treatment was within the standard of care. He properly determined that vaginal delivery would be the quickest. It is his practice to stop oxytocin when the FHR slows, though he had no memory of halting oxytocin administration in this case. The baby's CP stemmed from insufficiencies in the placenta, seizures, and meconium aspiration syndrome.
VERDICT:
A $14.4 million Pennsylvania verdict was returned. The ObGyn was found 60% liable for the baby's injuries and the hospital 40% responsible.
Related article:
Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding?
Woman with preeclampsia dies: $6M verdict
A 34-year-old woman had been a patient of her family practitioner (FP) for many years. Her blood pressure (BP) averaged 105/63 mm Hg over that time. At a regular prenatal visit on February 26, the patient reported a headache and cough. Her BP was 130/90 mm Hg and she had gained 8.6 lb since her last visit 4 weeks earlier. She was told to return in 2 weeks.
She contacted her FP 2 days later to report acute vaginal bleeding and a severe headache. The FP sent her to the hospital, where potential placental abruption was considered. Two US studies demonstrated oligohydramnios, intrauterine growth restriction, and a grade II placenta. She continued to have repeated high BP readings, headaches, variable and late decelerations, and a dropping platelet count.
She was discharged on the morning of March 3 and sent to another hospital for a specialized US. The FP spoke to the physician who was to perform the US, advising him by phone and in writing to evaluate the oligohydramnios and intrauterine growth restriction. No other information was provided.
At 6:00 pm on March 3, the patient's husband called the FP to report that his wife was vomiting, reporting abdominal pain and intense headache. He was advised to call back in 1 hour, and when he did, he was told to take his wife to the hospital. At the hospital at 8:50 pm on March 3, her BP was 128/103 mm Hg. She reported throbbing headache, vomiting, and facial edema. She was admitted for observation.
At 9:30 pm, when the patient's BP was 155/100 mm Hg, a nurse contacted the FP to report the patient's continued throbbing headache and elevated, labile BP. The FP neither requested a consultation with an attending ObGyn nor went to the hospital until 4:31 am on March 4.
At 3:15 am on March 4, a nurse found the patient with her head hanging over the side of the bed in an obtunded state, having vomited. The rapid response team and an attending ObGyn were called. The ObGyn diagnosed eclampsia, ordered magnesium sulfate and hydralazine and immediately transported her to the operating room for an emergency cesarean delivery. Although the baby was healthy, the mother remained unresponsive. A computed tomography (CT) scan confirmed a massive intracranial hemorrhage. She was pronounced dead at 5:10 pm.
ESTATE'S CLAIM:
The FP negligently deviated from the standard of care, leading to the mother's death. The FP fraudulently misrepresented her experience and training for obstetric conditions. She was negligent for failing to adequately diagnose and react to the patient's condition or refer her to an ObGyn, per hospital policy.
DEFENDANTS' DEFENSE:
The patient's treatment met the standard of care. The FP was credentialed to practice obstetrics at the hospital. The patient's BP never reached or sustained a level that would require the FP to consult an ObGyn until 3:15 am on March 4. When the patient first presented with a headache, the FP had consulted a board-certified ObGyn and an MFM, who suggested continued antepartum testing and induction at 39 weeks. The patient's death was unforeseeable because her BP values were inconsistent; the FP had no knowledge of a family history of stroke. The autopsy reported that a ruptured aneurysm was the cause of death.
VERDICT:
A $6,067,830 Ohio verdict was returned. The award was reduced to $900,000 due to a high/low agreement.
Related article:
Start offering aspirin to pregnant women at high risk for preeclampsia
Fetal abnormalities not diagnosed: Baby has Down syndrome
On September 6, at 10 weeks' gestation, a woman began prenatal care at a clinic with Dr. A, an ObGyn. The mother participated in the California Prenatal Screening Program and received test results on October 23 that showed normal risk for birth defects. On November 1, she saw Dr. B, another ObGyn, who confirmed the negative prenatal screening and ordered an US. A radiologist reported to Dr. Bthat the fetal anatomy was not well visualized. When the mother was at 23 2/7 weeks' gestation (December 6), Dr. B told the parents that the US results were normal.
On January 2, the parents saw Dr. A, who disclosed that the US radiology report indicated an incomplete fetal anatomy scan and ordered a repeat US. The US performed on January 17 showed a cardiac defect. Further testing confirmed that the fetus had Down syndrome. The parents scheduled but did not appear for a late-term abortion because they feared that the procedure was illegal.
PARENTS' CLAIM:
The parents told both ObGyns that they wanted extensive prenatal testing because of a family history of birth defects and that they would terminate the pregnancy if birth defects were discovered. Because Dr. B did not discuss prenatal testing, the parents did not know their child had Down syndrome until it was too late to legally terminate the pregnancy. The mother testified that she had never heard of amniocentesis until mid-January, when a perinatologist confirmed that the baby had Down syndrome.
DEFENDANTS' DEFENSE:
The ObGyns denied having any discussions with the parents about their request for extensive prenatal tests or desire for termination. Difficulty in visualizing the fetus is common in second trimester US, and therefore Dr. B routinely performs another US later in the pregnancy. He also denied responsibility for discussing prenatal testing with the parents, stating that such discussions should happen in the first trimester. Since the parents saw Dr. A during that time, Dr. B believed that those conversations had already taken place. The prenatal screening pamphlet that the mother signed on September 6 discussed amniocentesis. The child's grandmother testified that she had discussed amniocentesis with the parents early in the pregnancy. A clinic employee testified that in January she asked the mother why she had not chosen amniocentesis earlier in the pregnancy; the mother replied that she had decided against it because her prenatal screening test was normal.
VERDICT:
A California defense verdict was returned.
Related article:
When is cell-free DNA best used as a primary screen?
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Labor and delivery mismanaged, child has CP: $30.5M award
Two days later, the mother reported decreased fetal movement; she was admitted to the hospital for continuous fetal heart-rate (FHR) monitoring, and a maternal-fetal medicine (MFM) specialist was consulted. The mother was not placed on FHR monitoring until 2 hours after admission. Three hours after admission, the MFM, by phone, recommended further testing and later, cesarean delivery.
The child was found to have spastic quadriplegic cerebral palsy, profound developmental delays, a seizure disorder, and cortical blindness.
PARENTS' CLAIM:
The child's injuries were due to mismanagement of labor and delivery. The MFM prescribed ultrasonographic biophysical profiles, but they were not performed until 2 hours after ordered. There were 3 ultrasonography (US) technicians at the hospital when the mother was admitted: 1 was on break, another was performing other tests, and the third was not notified because the hospital's computer system was down. When test results were unfavorable, the MFM recommended emergency cesarean delivery. An earlier delivery could have prevented the child's injuries.
DEFENDANTS' DEFENSE:
The infant's injuries were a result of her mother's failure to keep her GDM under control.
VERDICT:
A $30,545,655 Georgia verdict was returned.
Related article:
10 tips for overcoming common challenges of intrapartum fetal monitoring
Did oxytocin cause child's spastic CP? $14.4M verdict
When a woman went to the hospital in labor, her ObGyn ordered oxytocin to enhance delivery. The FHR monitor showed repetitive decelerations for the next hour, dropping to 60 bpm by 8:00 pm, when the ObGyn expedited delivery but did not stop the oxytocin. By 8:20 pm, the baby's head was crowning, but the ObGyn waited another 10 minutes before performing an episiotomy and delivering the baby.
The child, intubated 5 minutes after birth, was found to have spastic tetraparesis cerebral palsy (CP) with impaired cognition, seizures, and global aphasia.
PARENTS' CLAIM:
The ObGyn and nurses failed to properly monitor labor and delivery. The ObGyn should not have started oxytocin because the patient's labor was progressing normally. He should have taken the mother off oxytocin at 8:00 pm when the FHR dropped to 60 bpm. He should have performed an operative delivery at 8:20 pm when the baby's head crowned. An earlier delivery would have prevented injury.
DEFENDANTS' DEFENSE:
The ObGyn's treatment was within the standard of care. He properly determined that vaginal delivery would be the quickest. It is his practice to stop oxytocin when the FHR slows, though he had no memory of halting oxytocin administration in this case. The baby's CP stemmed from insufficiencies in the placenta, seizures, and meconium aspiration syndrome.
VERDICT:
A $14.4 million Pennsylvania verdict was returned. The ObGyn was found 60% liable for the baby's injuries and the hospital 40% responsible.
Related article:
Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding?
Woman with preeclampsia dies: $6M verdict
A 34-year-old woman had been a patient of her family practitioner (FP) for many years. Her blood pressure (BP) averaged 105/63 mm Hg over that time. At a regular prenatal visit on February 26, the patient reported a headache and cough. Her BP was 130/90 mm Hg and she had gained 8.6 lb since her last visit 4 weeks earlier. She was told to return in 2 weeks.
She contacted her FP 2 days later to report acute vaginal bleeding and a severe headache. The FP sent her to the hospital, where potential placental abruption was considered. Two US studies demonstrated oligohydramnios, intrauterine growth restriction, and a grade II placenta. She continued to have repeated high BP readings, headaches, variable and late decelerations, and a dropping platelet count.
She was discharged on the morning of March 3 and sent to another hospital for a specialized US. The FP spoke to the physician who was to perform the US, advising him by phone and in writing to evaluate the oligohydramnios and intrauterine growth restriction. No other information was provided.
At 6:00 pm on March 3, the patient's husband called the FP to report that his wife was vomiting, reporting abdominal pain and intense headache. He was advised to call back in 1 hour, and when he did, he was told to take his wife to the hospital. At the hospital at 8:50 pm on March 3, her BP was 128/103 mm Hg. She reported throbbing headache, vomiting, and facial edema. She was admitted for observation.
At 9:30 pm, when the patient's BP was 155/100 mm Hg, a nurse contacted the FP to report the patient's continued throbbing headache and elevated, labile BP. The FP neither requested a consultation with an attending ObGyn nor went to the hospital until 4:31 am on March 4.
At 3:15 am on March 4, a nurse found the patient with her head hanging over the side of the bed in an obtunded state, having vomited. The rapid response team and an attending ObGyn were called. The ObGyn diagnosed eclampsia, ordered magnesium sulfate and hydralazine and immediately transported her to the operating room for an emergency cesarean delivery. Although the baby was healthy, the mother remained unresponsive. A computed tomography (CT) scan confirmed a massive intracranial hemorrhage. She was pronounced dead at 5:10 pm.
ESTATE'S CLAIM:
The FP negligently deviated from the standard of care, leading to the mother's death. The FP fraudulently misrepresented her experience and training for obstetric conditions. She was negligent for failing to adequately diagnose and react to the patient's condition or refer her to an ObGyn, per hospital policy.
DEFENDANTS' DEFENSE:
The patient's treatment met the standard of care. The FP was credentialed to practice obstetrics at the hospital. The patient's BP never reached or sustained a level that would require the FP to consult an ObGyn until 3:15 am on March 4. When the patient first presented with a headache, the FP had consulted a board-certified ObGyn and an MFM, who suggested continued antepartum testing and induction at 39 weeks. The patient's death was unforeseeable because her BP values were inconsistent; the FP had no knowledge of a family history of stroke. The autopsy reported that a ruptured aneurysm was the cause of death.
VERDICT:
A $6,067,830 Ohio verdict was returned. The award was reduced to $900,000 due to a high/low agreement.
Related article:
Start offering aspirin to pregnant women at high risk for preeclampsia
Fetal abnormalities not diagnosed: Baby has Down syndrome
On September 6, at 10 weeks' gestation, a woman began prenatal care at a clinic with Dr. A, an ObGyn. The mother participated in the California Prenatal Screening Program and received test results on October 23 that showed normal risk for birth defects. On November 1, she saw Dr. B, another ObGyn, who confirmed the negative prenatal screening and ordered an US. A radiologist reported to Dr. Bthat the fetal anatomy was not well visualized. When the mother was at 23 2/7 weeks' gestation (December 6), Dr. B told the parents that the US results were normal.
On January 2, the parents saw Dr. A, who disclosed that the US radiology report indicated an incomplete fetal anatomy scan and ordered a repeat US. The US performed on January 17 showed a cardiac defect. Further testing confirmed that the fetus had Down syndrome. The parents scheduled but did not appear for a late-term abortion because they feared that the procedure was illegal.
PARENTS' CLAIM:
The parents told both ObGyns that they wanted extensive prenatal testing because of a family history of birth defects and that they would terminate the pregnancy if birth defects were discovered. Because Dr. B did not discuss prenatal testing, the parents did not know their child had Down syndrome until it was too late to legally terminate the pregnancy. The mother testified that she had never heard of amniocentesis until mid-January, when a perinatologist confirmed that the baby had Down syndrome.
DEFENDANTS' DEFENSE:
The ObGyns denied having any discussions with the parents about their request for extensive prenatal tests or desire for termination. Difficulty in visualizing the fetus is common in second trimester US, and therefore Dr. B routinely performs another US later in the pregnancy. He also denied responsibility for discussing prenatal testing with the parents, stating that such discussions should happen in the first trimester. Since the parents saw Dr. A during that time, Dr. B believed that those conversations had already taken place. The prenatal screening pamphlet that the mother signed on September 6 discussed amniocentesis. The child's grandmother testified that she had discussed amniocentesis with the parents early in the pregnancy. A clinic employee testified that in January she asked the mother why she had not chosen amniocentesis earlier in the pregnancy; the mother replied that she had decided against it because her prenatal screening test was normal.
VERDICT:
A California defense verdict was returned.
Related article:
When is cell-free DNA best used as a primary screen?
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Labor and delivery mismanaged, child has CP: $30.5M award
Two days later, the mother reported decreased fetal movement; she was admitted to the hospital for continuous fetal heart-rate (FHR) monitoring, and a maternal-fetal medicine (MFM) specialist was consulted. The mother was not placed on FHR monitoring until 2 hours after admission. Three hours after admission, the MFM, by phone, recommended further testing and later, cesarean delivery.
The child was found to have spastic quadriplegic cerebral palsy, profound developmental delays, a seizure disorder, and cortical blindness.
PARENTS' CLAIM:
The child's injuries were due to mismanagement of labor and delivery. The MFM prescribed ultrasonographic biophysical profiles, but they were not performed until 2 hours after ordered. There were 3 ultrasonography (US) technicians at the hospital when the mother was admitted: 1 was on break, another was performing other tests, and the third was not notified because the hospital's computer system was down. When test results were unfavorable, the MFM recommended emergency cesarean delivery. An earlier delivery could have prevented the child's injuries.
DEFENDANTS' DEFENSE:
The infant's injuries were a result of her mother's failure to keep her GDM under control.
VERDICT:
A $30,545,655 Georgia verdict was returned.
Related article:
10 tips for overcoming common challenges of intrapartum fetal monitoring
Did oxytocin cause child's spastic CP? $14.4M verdict
When a woman went to the hospital in labor, her ObGyn ordered oxytocin to enhance delivery. The FHR monitor showed repetitive decelerations for the next hour, dropping to 60 bpm by 8:00 pm, when the ObGyn expedited delivery but did not stop the oxytocin. By 8:20 pm, the baby's head was crowning, but the ObGyn waited another 10 minutes before performing an episiotomy and delivering the baby.
The child, intubated 5 minutes after birth, was found to have spastic tetraparesis cerebral palsy (CP) with impaired cognition, seizures, and global aphasia.
PARENTS' CLAIM:
The ObGyn and nurses failed to properly monitor labor and delivery. The ObGyn should not have started oxytocin because the patient's labor was progressing normally. He should have taken the mother off oxytocin at 8:00 pm when the FHR dropped to 60 bpm. He should have performed an operative delivery at 8:20 pm when the baby's head crowned. An earlier delivery would have prevented injury.
DEFENDANTS' DEFENSE:
The ObGyn's treatment was within the standard of care. He properly determined that vaginal delivery would be the quickest. It is his practice to stop oxytocin when the FHR slows, though he had no memory of halting oxytocin administration in this case. The baby's CP stemmed from insufficiencies in the placenta, seizures, and meconium aspiration syndrome.
VERDICT:
A $14.4 million Pennsylvania verdict was returned. The ObGyn was found 60% liable for the baby's injuries and the hospital 40% responsible.
Related article:
Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding?
Woman with preeclampsia dies: $6M verdict
A 34-year-old woman had been a patient of her family practitioner (FP) for many years. Her blood pressure (BP) averaged 105/63 mm Hg over that time. At a regular prenatal visit on February 26, the patient reported a headache and cough. Her BP was 130/90 mm Hg and she had gained 8.6 lb since her last visit 4 weeks earlier. She was told to return in 2 weeks.
She contacted her FP 2 days later to report acute vaginal bleeding and a severe headache. The FP sent her to the hospital, where potential placental abruption was considered. Two US studies demonstrated oligohydramnios, intrauterine growth restriction, and a grade II placenta. She continued to have repeated high BP readings, headaches, variable and late decelerations, and a dropping platelet count.
She was discharged on the morning of March 3 and sent to another hospital for a specialized US. The FP spoke to the physician who was to perform the US, advising him by phone and in writing to evaluate the oligohydramnios and intrauterine growth restriction. No other information was provided.
At 6:00 pm on March 3, the patient's husband called the FP to report that his wife was vomiting, reporting abdominal pain and intense headache. He was advised to call back in 1 hour, and when he did, he was told to take his wife to the hospital. At the hospital at 8:50 pm on March 3, her BP was 128/103 mm Hg. She reported throbbing headache, vomiting, and facial edema. She was admitted for observation.
At 9:30 pm, when the patient's BP was 155/100 mm Hg, a nurse contacted the FP to report the patient's continued throbbing headache and elevated, labile BP. The FP neither requested a consultation with an attending ObGyn nor went to the hospital until 4:31 am on March 4.
At 3:15 am on March 4, a nurse found the patient with her head hanging over the side of the bed in an obtunded state, having vomited. The rapid response team and an attending ObGyn were called. The ObGyn diagnosed eclampsia, ordered magnesium sulfate and hydralazine and immediately transported her to the operating room for an emergency cesarean delivery. Although the baby was healthy, the mother remained unresponsive. A computed tomography (CT) scan confirmed a massive intracranial hemorrhage. She was pronounced dead at 5:10 pm.
ESTATE'S CLAIM:
The FP negligently deviated from the standard of care, leading to the mother's death. The FP fraudulently misrepresented her experience and training for obstetric conditions. She was negligent for failing to adequately diagnose and react to the patient's condition or refer her to an ObGyn, per hospital policy.
DEFENDANTS' DEFENSE:
The patient's treatment met the standard of care. The FP was credentialed to practice obstetrics at the hospital. The patient's BP never reached or sustained a level that would require the FP to consult an ObGyn until 3:15 am on March 4. When the patient first presented with a headache, the FP had consulted a board-certified ObGyn and an MFM, who suggested continued antepartum testing and induction at 39 weeks. The patient's death was unforeseeable because her BP values were inconsistent; the FP had no knowledge of a family history of stroke. The autopsy reported that a ruptured aneurysm was the cause of death.
VERDICT:
A $6,067,830 Ohio verdict was returned. The award was reduced to $900,000 due to a high/low agreement.
Related article:
Start offering aspirin to pregnant women at high risk for preeclampsia
Fetal abnormalities not diagnosed: Baby has Down syndrome
On September 6, at 10 weeks' gestation, a woman began prenatal care at a clinic with Dr. A, an ObGyn. The mother participated in the California Prenatal Screening Program and received test results on October 23 that showed normal risk for birth defects. On November 1, she saw Dr. B, another ObGyn, who confirmed the negative prenatal screening and ordered an US. A radiologist reported to Dr. Bthat the fetal anatomy was not well visualized. When the mother was at 23 2/7 weeks' gestation (December 6), Dr. B told the parents that the US results were normal.
On January 2, the parents saw Dr. A, who disclosed that the US radiology report indicated an incomplete fetal anatomy scan and ordered a repeat US. The US performed on January 17 showed a cardiac defect. Further testing confirmed that the fetus had Down syndrome. The parents scheduled but did not appear for a late-term abortion because they feared that the procedure was illegal.
PARENTS' CLAIM:
The parents told both ObGyns that they wanted extensive prenatal testing because of a family history of birth defects and that they would terminate the pregnancy if birth defects were discovered. Because Dr. B did not discuss prenatal testing, the parents did not know their child had Down syndrome until it was too late to legally terminate the pregnancy. The mother testified that she had never heard of amniocentesis until mid-January, when a perinatologist confirmed that the baby had Down syndrome.
DEFENDANTS' DEFENSE:
The ObGyns denied having any discussions with the parents about their request for extensive prenatal tests or desire for termination. Difficulty in visualizing the fetus is common in second trimester US, and therefore Dr. B routinely performs another US later in the pregnancy. He also denied responsibility for discussing prenatal testing with the parents, stating that such discussions should happen in the first trimester. Since the parents saw Dr. A during that time, Dr. B believed that those conversations had already taken place. The prenatal screening pamphlet that the mother signed on September 6 discussed amniocentesis. The child's grandmother testified that she had discussed amniocentesis with the parents early in the pregnancy. A clinic employee testified that in January she asked the mother why she had not chosen amniocentesis earlier in the pregnancy; the mother replied that she had decided against it because her prenatal screening test was normal.
VERDICT:
A California defense verdict was returned.
Related article:
When is cell-free DNA best used as a primary screen?
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Promising phase II results for Rytary reformulation for Parkinson’s
BOSTON – The results of a small phase II trial confirm that IPX203, an investigational reformulation of the extended-release carbidopa-levodopa combination called Rytary, holds the potential to greatly extend “on” time in Parkinson’s disease patients.
While it’s early in the research process, “this is a promising new formulation,” said Mark Stacy, MD, who led the trial that pitted IPX203 against immediate-release (IR) carbidopa-levodopa (CD-LD) and Rytary.
Dr. Stacy, professor of neurology and vice dean for clinical research at Duke University, Durham, N.C., and his colleagues released the phase II results at the annual meeting of the American Academy of Neurology.
Parkinson’s disease patients often aren’t adherent to their drugs despite the risk that they’ll see symptoms return. “It’s been demonstrated that they miss medicines as often as in any other disease,” Dr. Stacy said in an interview.
Rytary, which was approved by the Food and Drug Administration in 2015, has improved adherence in Parkinson’s disease patients by allowing them to reduce the number of doses they need to separately take every day to four. “If this new formulation is able to last 8 hours, you could essentially take that at bedtime and have medication in your system when you awaken,” Dr. Stacy said. “Then you could take it twice more during the day.”
The new, open-label, crossover trial randomly assigned 26 patients on stable IR regimens (at least 400 mg LD/day, at least 4 doses a day) to single doses of IR, Rytary, and IPX203. For 10 hours after dose, raters evaluated every 30 minutes whether the subjects were on (with troublesome dyskinesia) or off (without troublesome dyskinesia).
All but one subject finished the trial. Over the first hour, the patients fared about the same in terms of converting to on status. Overall, PX203 significantly decreased average off time (4.5 hours), compared with IR (7.2 hours; P less than .0001) and Rytary (5.4 hours; P less than .05).
Researchers also found that IPX203 had the highest duration of a 4-point improvement in the Unified Parkinson’s Disease Rating Scale Part III score at 6.1 hours, compared with IR (3.7 hours; P less than .0001) and Rytary (5.2 hours; P less than .05). In addition, 13-point improvements were higher for IPX203 at 4.8 hours, compared with IR (2.2 hours; P less than .0001) and Rytary (3.6 hours; P less than .05).
No serious adverse events were reported. Nausea, dizziness, and hypertension occurred in two or more patients in each treatment group and were more common with IR (28.0%) and IPX203 (19.2%) than with Rytary (8.0%).
The study was funded by Impax Laboratories. Dr. Stacy reported several disclosures, including consulting work for numerous drug makers.
BOSTON – The results of a small phase II trial confirm that IPX203, an investigational reformulation of the extended-release carbidopa-levodopa combination called Rytary, holds the potential to greatly extend “on” time in Parkinson’s disease patients.
While it’s early in the research process, “this is a promising new formulation,” said Mark Stacy, MD, who led the trial that pitted IPX203 against immediate-release (IR) carbidopa-levodopa (CD-LD) and Rytary.
Dr. Stacy, professor of neurology and vice dean for clinical research at Duke University, Durham, N.C., and his colleagues released the phase II results at the annual meeting of the American Academy of Neurology.
Parkinson’s disease patients often aren’t adherent to their drugs despite the risk that they’ll see symptoms return. “It’s been demonstrated that they miss medicines as often as in any other disease,” Dr. Stacy said in an interview.
Rytary, which was approved by the Food and Drug Administration in 2015, has improved adherence in Parkinson’s disease patients by allowing them to reduce the number of doses they need to separately take every day to four. “If this new formulation is able to last 8 hours, you could essentially take that at bedtime and have medication in your system when you awaken,” Dr. Stacy said. “Then you could take it twice more during the day.”
The new, open-label, crossover trial randomly assigned 26 patients on stable IR regimens (at least 400 mg LD/day, at least 4 doses a day) to single doses of IR, Rytary, and IPX203. For 10 hours after dose, raters evaluated every 30 minutes whether the subjects were on (with troublesome dyskinesia) or off (without troublesome dyskinesia).
All but one subject finished the trial. Over the first hour, the patients fared about the same in terms of converting to on status. Overall, PX203 significantly decreased average off time (4.5 hours), compared with IR (7.2 hours; P less than .0001) and Rytary (5.4 hours; P less than .05).
Researchers also found that IPX203 had the highest duration of a 4-point improvement in the Unified Parkinson’s Disease Rating Scale Part III score at 6.1 hours, compared with IR (3.7 hours; P less than .0001) and Rytary (5.2 hours; P less than .05). In addition, 13-point improvements were higher for IPX203 at 4.8 hours, compared with IR (2.2 hours; P less than .0001) and Rytary (3.6 hours; P less than .05).
No serious adverse events were reported. Nausea, dizziness, and hypertension occurred in two or more patients in each treatment group and were more common with IR (28.0%) and IPX203 (19.2%) than with Rytary (8.0%).
The study was funded by Impax Laboratories. Dr. Stacy reported several disclosures, including consulting work for numerous drug makers.
BOSTON – The results of a small phase II trial confirm that IPX203, an investigational reformulation of the extended-release carbidopa-levodopa combination called Rytary, holds the potential to greatly extend “on” time in Parkinson’s disease patients.
While it’s early in the research process, “this is a promising new formulation,” said Mark Stacy, MD, who led the trial that pitted IPX203 against immediate-release (IR) carbidopa-levodopa (CD-LD) and Rytary.
Dr. Stacy, professor of neurology and vice dean for clinical research at Duke University, Durham, N.C., and his colleagues released the phase II results at the annual meeting of the American Academy of Neurology.
Parkinson’s disease patients often aren’t adherent to their drugs despite the risk that they’ll see symptoms return. “It’s been demonstrated that they miss medicines as often as in any other disease,” Dr. Stacy said in an interview.
Rytary, which was approved by the Food and Drug Administration in 2015, has improved adherence in Parkinson’s disease patients by allowing them to reduce the number of doses they need to separately take every day to four. “If this new formulation is able to last 8 hours, you could essentially take that at bedtime and have medication in your system when you awaken,” Dr. Stacy said. “Then you could take it twice more during the day.”
The new, open-label, crossover trial randomly assigned 26 patients on stable IR regimens (at least 400 mg LD/day, at least 4 doses a day) to single doses of IR, Rytary, and IPX203. For 10 hours after dose, raters evaluated every 30 minutes whether the subjects were on (with troublesome dyskinesia) or off (without troublesome dyskinesia).
All but one subject finished the trial. Over the first hour, the patients fared about the same in terms of converting to on status. Overall, PX203 significantly decreased average off time (4.5 hours), compared with IR (7.2 hours; P less than .0001) and Rytary (5.4 hours; P less than .05).
Researchers also found that IPX203 had the highest duration of a 4-point improvement in the Unified Parkinson’s Disease Rating Scale Part III score at 6.1 hours, compared with IR (3.7 hours; P less than .0001) and Rytary (5.2 hours; P less than .05). In addition, 13-point improvements were higher for IPX203 at 4.8 hours, compared with IR (2.2 hours; P less than .0001) and Rytary (3.6 hours; P less than .05).
No serious adverse events were reported. Nausea, dizziness, and hypertension occurred in two or more patients in each treatment group and were more common with IR (28.0%) and IPX203 (19.2%) than with Rytary (8.0%).
The study was funded by Impax Laboratories. Dr. Stacy reported several disclosures, including consulting work for numerous drug makers.
AT AAN 2017
Key clinical point: Investigational drug IPX203, a reformulation of Rytary (extended release carbidopa-levodopa), holds promise as an improved extended-release Parkinson’s disease medication.
Major finding: After one dose, PX203 significantly decreased average off time (4.5 hours), compared with immediate-release carbidopa-levodopa (7.2 hours; P less than .0001) and Rytary (5.4 hours; P less than .05).
Data source: Open-label, rater-blinded, randomized, crossover study of 25 Parkinson’s disease patients
Disclosures: The study was funded by Impax Laboratories. The presenter reported several disclosures, including consulting work for numerous drug makers.
Obtaining coverage for transgender and gender-expansive youth
Transgender and gender-expansive youth face many barriers to health care. (Gender-expansive youth are defined as “youth who do not identify with traditional gender roles but are otherwise not confined to one gender narrative or experience.”) Although some of these youth may be fortunate to have a supportive family and access to health care providers proficient in transgender health care, they still face difficulties in having their insurance cover transgender-related services. This is not an impossible task, but it is a constant struggle for many clinicians.
In this column, I will provide some tips and strategies to help clinicians get insurance companies to cover these critical services. However, keep in mind that there is no one-size-fits-all approach to obtaining insurance coverage. In addition, growing uncertainty over the repeal of the Affordable Care Act (ACA) – which was critical in lifting many of the barriers to insurance coverage for transgender individuals – will make this task challenging.
Health insurance is extraordinarily complex. There are multiple private and public plans that vary in the services they cover. This variation is state dependent. And even within states, there is additional variability. Most health insurance plans are purchased by employers, and employers have a choice of what can be covered in their health plans. So even though an insurance company may state that it covers transgender-related services, the patient’s employer may pay for a plan that doesn’t cover such services. The only way to be sure whether a patient’s insurance will cover transgender-related services or not is to contact the insurance provider directly, but with extremely busy schedules and heavy patient loads, this is easier said than done. It would be helpful to have a social worker perform this task, but even having a social worker can be a luxury for some clinics.
The ACA made it easier for transgender individuals to obtain insurance coverage. Three years ago, the U.S. Department of Health and Human Services stated that Medicare’s longstanding exclusion of “transsexual surgical procedures” was no longer valid.1 Although it did not universally ban transgender exclusion policies, it did allow individual states to do so. Thirteen states have explicit policies that ban exclusions of transgender-related services in both private insurance and in Medicaid, and an additional five states have some policies that discourage such practices.2 This allowed some insurance providers and state Medicaid plans to offer coverage of transgender-related services.
Another challenge in obtaining insurance coverage for transgender and gender-expansive youth is claims denial for sex-specific procedures. For example, if a transwoman is designated as “male” in the electronic medical record and requires a breast ultrasound, the insurance company may automatically reject this claim because this procedure is covered for bodies designated as “female.” If the patient’s insurance plan covers transgender-related services, the clinic can notify the insurance company that the patient is transgender; if the patient’s plan does not, then the clinic will need to appeal to the insurance provider. Alternatively, for clinics associated with federally-funded institutions (e.g., most hospitals), the clinician can use Condition Code 45 in the billing to override the sex mismatch, although not all hospitals have implemented this code.3
1. Patient’s identifying information. Usually the patient’s name and date of birth is sufficient. Clinicians should use the patient’s preferred name in the letter, but provide the insurance or legal name of the patient so that the insurance provider can locate the patient’s records.
2. Result of a psychosocial evaluation and diagnosis (if any). Many insurance providers are looking specifically for the gender dysphoria diagnosis.
3. The duration of the referring health professional’s relationship with the patient, which includes the type of evaluation and therapy or counseling (e.g., cognitive behavior therapy or gender coaching).
4. An explanation that the criteria (usually from the World Professional Association for Transgender Health standard of care4 or the Endocrine Society Guidelines titled Endocrine Treatment of Transsexual Persons5) for hormone therapy have been met, and a brief description of the clinical rationale for supporting the client’s request for hormone therapy.
5. A statement that informed consent has been obtained from the patient (or parental permission if the patient is younger than 18 years).
6. A statement that the referring health professional is available for coordination of care.
If the clinician fails to convince the insurance provider of the necessity of covering transgender-related services, the patient still can pay out of pocket. Some hormones can be affordable to certain patients. In the state of Pennsylvania, for example, a 10-mL vial of testosterone can cost anywhere from $60 to $80, and may generally last anywhere from 10 weeks to a year, depending on dosage. Nevertheless, these costs still may be prohibitive for many transgender youth. Many are chronically unemployed or underemployed, or struggle with homelessness.6 Some transgender youth have to the face the excruciatingly difficult choice between having something to eat for the day or living another day with gender dysphoria.
Clinicians should work very hard to make sure that their transgender and gender-expansive patients obtain the care they need. The above strategies may help navigate the complex insurance system. However, insurance policies vary by state, and anti-trans discrimination creates additional barriers to health care. Therefore, clinicians who take care of transgender youth also should advocate for policies that protect these patients from discrimination, and they should advocate for policies that expand medical coverage for this vulnerable population.
Resources
• The Human Rights Campaign keeps a list of insurance plans that cover transgender-related services, but this list is far from comprehensive.
• Healthcare.gov provides some guidance on how to obtain coverage and navigate the insurance system for transgender individuals.
• UCSF Center of Excellence for Transgender Health provides some excellent resources and guidance on obtaining insurance coverage for transgender individuals.
References
1. LGBT Health 2014;1(4):256-8.
2. Map: State Health Insurance Rules: National Center for Transgender Equality, 2016 [Available from: www.transequality.org/issues/resources/map-state-health-insurance-rules].
3. Health insurance coverage issues for transgender people in the United States: University of California, San Fransisco Center of Excellence for Transgender Health, 2017 [Available from: http://transhealth.ucsf.edu/trans?page=guidelines-insurance].
4. International Journal of Transgenderism 2012;13(4):165-232.
5. J Clin Endocrinol Metab 2009;94(9):3132-54.
6. Injustice at Every Turn: A Report of the National Transgender Discrimination Survey. Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011.
Transgender and gender-expansive youth face many barriers to health care. (Gender-expansive youth are defined as “youth who do not identify with traditional gender roles but are otherwise not confined to one gender narrative or experience.”) Although some of these youth may be fortunate to have a supportive family and access to health care providers proficient in transgender health care, they still face difficulties in having their insurance cover transgender-related services. This is not an impossible task, but it is a constant struggle for many clinicians.
In this column, I will provide some tips and strategies to help clinicians get insurance companies to cover these critical services. However, keep in mind that there is no one-size-fits-all approach to obtaining insurance coverage. In addition, growing uncertainty over the repeal of the Affordable Care Act (ACA) – which was critical in lifting many of the barriers to insurance coverage for transgender individuals – will make this task challenging.
Health insurance is extraordinarily complex. There are multiple private and public plans that vary in the services they cover. This variation is state dependent. And even within states, there is additional variability. Most health insurance plans are purchased by employers, and employers have a choice of what can be covered in their health plans. So even though an insurance company may state that it covers transgender-related services, the patient’s employer may pay for a plan that doesn’t cover such services. The only way to be sure whether a patient’s insurance will cover transgender-related services or not is to contact the insurance provider directly, but with extremely busy schedules and heavy patient loads, this is easier said than done. It would be helpful to have a social worker perform this task, but even having a social worker can be a luxury for some clinics.
The ACA made it easier for transgender individuals to obtain insurance coverage. Three years ago, the U.S. Department of Health and Human Services stated that Medicare’s longstanding exclusion of “transsexual surgical procedures” was no longer valid.1 Although it did not universally ban transgender exclusion policies, it did allow individual states to do so. Thirteen states have explicit policies that ban exclusions of transgender-related services in both private insurance and in Medicaid, and an additional five states have some policies that discourage such practices.2 This allowed some insurance providers and state Medicaid plans to offer coverage of transgender-related services.
Another challenge in obtaining insurance coverage for transgender and gender-expansive youth is claims denial for sex-specific procedures. For example, if a transwoman is designated as “male” in the electronic medical record and requires a breast ultrasound, the insurance company may automatically reject this claim because this procedure is covered for bodies designated as “female.” If the patient’s insurance plan covers transgender-related services, the clinic can notify the insurance company that the patient is transgender; if the patient’s plan does not, then the clinic will need to appeal to the insurance provider. Alternatively, for clinics associated with federally-funded institutions (e.g., most hospitals), the clinician can use Condition Code 45 in the billing to override the sex mismatch, although not all hospitals have implemented this code.3
1. Patient’s identifying information. Usually the patient’s name and date of birth is sufficient. Clinicians should use the patient’s preferred name in the letter, but provide the insurance or legal name of the patient so that the insurance provider can locate the patient’s records.
2. Result of a psychosocial evaluation and diagnosis (if any). Many insurance providers are looking specifically for the gender dysphoria diagnosis.
3. The duration of the referring health professional’s relationship with the patient, which includes the type of evaluation and therapy or counseling (e.g., cognitive behavior therapy or gender coaching).
4. An explanation that the criteria (usually from the World Professional Association for Transgender Health standard of care4 or the Endocrine Society Guidelines titled Endocrine Treatment of Transsexual Persons5) for hormone therapy have been met, and a brief description of the clinical rationale for supporting the client’s request for hormone therapy.
5. A statement that informed consent has been obtained from the patient (or parental permission if the patient is younger than 18 years).
6. A statement that the referring health professional is available for coordination of care.
If the clinician fails to convince the insurance provider of the necessity of covering transgender-related services, the patient still can pay out of pocket. Some hormones can be affordable to certain patients. In the state of Pennsylvania, for example, a 10-mL vial of testosterone can cost anywhere from $60 to $80, and may generally last anywhere from 10 weeks to a year, depending on dosage. Nevertheless, these costs still may be prohibitive for many transgender youth. Many are chronically unemployed or underemployed, or struggle with homelessness.6 Some transgender youth have to the face the excruciatingly difficult choice between having something to eat for the day or living another day with gender dysphoria.
Clinicians should work very hard to make sure that their transgender and gender-expansive patients obtain the care they need. The above strategies may help navigate the complex insurance system. However, insurance policies vary by state, and anti-trans discrimination creates additional barriers to health care. Therefore, clinicians who take care of transgender youth also should advocate for policies that protect these patients from discrimination, and they should advocate for policies that expand medical coverage for this vulnerable population.
Resources
• The Human Rights Campaign keeps a list of insurance plans that cover transgender-related services, but this list is far from comprehensive.
• Healthcare.gov provides some guidance on how to obtain coverage and navigate the insurance system for transgender individuals.
• UCSF Center of Excellence for Transgender Health provides some excellent resources and guidance on obtaining insurance coverage for transgender individuals.
References
1. LGBT Health 2014;1(4):256-8.
2. Map: State Health Insurance Rules: National Center for Transgender Equality, 2016 [Available from: www.transequality.org/issues/resources/map-state-health-insurance-rules].
3. Health insurance coverage issues for transgender people in the United States: University of California, San Fransisco Center of Excellence for Transgender Health, 2017 [Available from: http://transhealth.ucsf.edu/trans?page=guidelines-insurance].
4. International Journal of Transgenderism 2012;13(4):165-232.
5. J Clin Endocrinol Metab 2009;94(9):3132-54.
6. Injustice at Every Turn: A Report of the National Transgender Discrimination Survey. Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011.
Transgender and gender-expansive youth face many barriers to health care. (Gender-expansive youth are defined as “youth who do not identify with traditional gender roles but are otherwise not confined to one gender narrative or experience.”) Although some of these youth may be fortunate to have a supportive family and access to health care providers proficient in transgender health care, they still face difficulties in having their insurance cover transgender-related services. This is not an impossible task, but it is a constant struggle for many clinicians.
In this column, I will provide some tips and strategies to help clinicians get insurance companies to cover these critical services. However, keep in mind that there is no one-size-fits-all approach to obtaining insurance coverage. In addition, growing uncertainty over the repeal of the Affordable Care Act (ACA) – which was critical in lifting many of the barriers to insurance coverage for transgender individuals – will make this task challenging.
Health insurance is extraordinarily complex. There are multiple private and public plans that vary in the services they cover. This variation is state dependent. And even within states, there is additional variability. Most health insurance plans are purchased by employers, and employers have a choice of what can be covered in their health plans. So even though an insurance company may state that it covers transgender-related services, the patient’s employer may pay for a plan that doesn’t cover such services. The only way to be sure whether a patient’s insurance will cover transgender-related services or not is to contact the insurance provider directly, but with extremely busy schedules and heavy patient loads, this is easier said than done. It would be helpful to have a social worker perform this task, but even having a social worker can be a luxury for some clinics.
The ACA made it easier for transgender individuals to obtain insurance coverage. Three years ago, the U.S. Department of Health and Human Services stated that Medicare’s longstanding exclusion of “transsexual surgical procedures” was no longer valid.1 Although it did not universally ban transgender exclusion policies, it did allow individual states to do so. Thirteen states have explicit policies that ban exclusions of transgender-related services in both private insurance and in Medicaid, and an additional five states have some policies that discourage such practices.2 This allowed some insurance providers and state Medicaid plans to offer coverage of transgender-related services.
Another challenge in obtaining insurance coverage for transgender and gender-expansive youth is claims denial for sex-specific procedures. For example, if a transwoman is designated as “male” in the electronic medical record and requires a breast ultrasound, the insurance company may automatically reject this claim because this procedure is covered for bodies designated as “female.” If the patient’s insurance plan covers transgender-related services, the clinic can notify the insurance company that the patient is transgender; if the patient’s plan does not, then the clinic will need to appeal to the insurance provider. Alternatively, for clinics associated with federally-funded institutions (e.g., most hospitals), the clinician can use Condition Code 45 in the billing to override the sex mismatch, although not all hospitals have implemented this code.3
1. Patient’s identifying information. Usually the patient’s name and date of birth is sufficient. Clinicians should use the patient’s preferred name in the letter, but provide the insurance or legal name of the patient so that the insurance provider can locate the patient’s records.
2. Result of a psychosocial evaluation and diagnosis (if any). Many insurance providers are looking specifically for the gender dysphoria diagnosis.
3. The duration of the referring health professional’s relationship with the patient, which includes the type of evaluation and therapy or counseling (e.g., cognitive behavior therapy or gender coaching).
4. An explanation that the criteria (usually from the World Professional Association for Transgender Health standard of care4 or the Endocrine Society Guidelines titled Endocrine Treatment of Transsexual Persons5) for hormone therapy have been met, and a brief description of the clinical rationale for supporting the client’s request for hormone therapy.
5. A statement that informed consent has been obtained from the patient (or parental permission if the patient is younger than 18 years).
6. A statement that the referring health professional is available for coordination of care.
If the clinician fails to convince the insurance provider of the necessity of covering transgender-related services, the patient still can pay out of pocket. Some hormones can be affordable to certain patients. In the state of Pennsylvania, for example, a 10-mL vial of testosterone can cost anywhere from $60 to $80, and may generally last anywhere from 10 weeks to a year, depending on dosage. Nevertheless, these costs still may be prohibitive for many transgender youth. Many are chronically unemployed or underemployed, or struggle with homelessness.6 Some transgender youth have to the face the excruciatingly difficult choice between having something to eat for the day or living another day with gender dysphoria.
Clinicians should work very hard to make sure that their transgender and gender-expansive patients obtain the care they need. The above strategies may help navigate the complex insurance system. However, insurance policies vary by state, and anti-trans discrimination creates additional barriers to health care. Therefore, clinicians who take care of transgender youth also should advocate for policies that protect these patients from discrimination, and they should advocate for policies that expand medical coverage for this vulnerable population.
Resources
• The Human Rights Campaign keeps a list of insurance plans that cover transgender-related services, but this list is far from comprehensive.
• Healthcare.gov provides some guidance on how to obtain coverage and navigate the insurance system for transgender individuals.
• UCSF Center of Excellence for Transgender Health provides some excellent resources and guidance on obtaining insurance coverage for transgender individuals.
References
1. LGBT Health 2014;1(4):256-8.
2. Map: State Health Insurance Rules: National Center for Transgender Equality, 2016 [Available from: www.transequality.org/issues/resources/map-state-health-insurance-rules].
3. Health insurance coverage issues for transgender people in the United States: University of California, San Fransisco Center of Excellence for Transgender Health, 2017 [Available from: http://transhealth.ucsf.edu/trans?page=guidelines-insurance].
4. International Journal of Transgenderism 2012;13(4):165-232.
5. J Clin Endocrinol Metab 2009;94(9):3132-54.
6. Injustice at Every Turn: A Report of the National Transgender Discrimination Survey. Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011.
Why extended release metformin?
“TREATING POLYCYSTIC OVARY SYNDROME: START USING DUAL MEDICAL THERAPY”
ROBERT L. BARBIERI, MD (EDITORIAL; APRIL 2017)
Why extended release metformin?
I read with interest Dr. Barbieri’s editorial on polycystic ovary syndrome. It left me wondering: Is there a metabolic or pharmacologic reason why you give metformin XR 1,500 mg with dinner instead of 750 mg orally twice per day?
Marcelo Andreoli, MD
Vienna, Virginia
Dr. Barbieri responds
I thank Dr. Andreoli for the important clinical question about one-time or multiple dosing of metformin. To improve patient adherence with metformin treatment, I think once-daily dosing at dinner with an extended-release formulation is more convenient than twice-daily dosing with immediate-release metformin. Following ingestion of immediate- or extended-release metformin, peak metformin blood concentrations are achieved after 2 and 7 hours, respectively.1 There is some evidence that extended-release metformin has fewer gastrointestinal (GI) adverse effects than immediate-release metformin.2 In one study, the reported rates of GI adverse effects were 29% versus 39% with extended-release and immediate-release formulations, respectively.2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ali S, Fonseca V. Overview of metformin: special focus on metformin extended release. Expert Opin Pharmacother. 2012;13(12):1797–1805.
- Fujioka K, Pans M, Joyal S. Glycemic control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended-release formulation. Clin Ther. 2003;25(2):515–529.
“TREATING POLYCYSTIC OVARY SYNDROME: START USING DUAL MEDICAL THERAPY”
ROBERT L. BARBIERI, MD (EDITORIAL; APRIL 2017)
Why extended release metformin?
I read with interest Dr. Barbieri’s editorial on polycystic ovary syndrome. It left me wondering: Is there a metabolic or pharmacologic reason why you give metformin XR 1,500 mg with dinner instead of 750 mg orally twice per day?
Marcelo Andreoli, MD
Vienna, Virginia
Dr. Barbieri responds
I thank Dr. Andreoli for the important clinical question about one-time or multiple dosing of metformin. To improve patient adherence with metformin treatment, I think once-daily dosing at dinner with an extended-release formulation is more convenient than twice-daily dosing with immediate-release metformin. Following ingestion of immediate- or extended-release metformin, peak metformin blood concentrations are achieved after 2 and 7 hours, respectively.1 There is some evidence that extended-release metformin has fewer gastrointestinal (GI) adverse effects than immediate-release metformin.2 In one study, the reported rates of GI adverse effects were 29% versus 39% with extended-release and immediate-release formulations, respectively.2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“TREATING POLYCYSTIC OVARY SYNDROME: START USING DUAL MEDICAL THERAPY”
ROBERT L. BARBIERI, MD (EDITORIAL; APRIL 2017)
Why extended release metformin?
I read with interest Dr. Barbieri’s editorial on polycystic ovary syndrome. It left me wondering: Is there a metabolic or pharmacologic reason why you give metformin XR 1,500 mg with dinner instead of 750 mg orally twice per day?
Marcelo Andreoli, MD
Vienna, Virginia
Dr. Barbieri responds
I thank Dr. Andreoli for the important clinical question about one-time or multiple dosing of metformin. To improve patient adherence with metformin treatment, I think once-daily dosing at dinner with an extended-release formulation is more convenient than twice-daily dosing with immediate-release metformin. Following ingestion of immediate- or extended-release metformin, peak metformin blood concentrations are achieved after 2 and 7 hours, respectively.1 There is some evidence that extended-release metformin has fewer gastrointestinal (GI) adverse effects than immediate-release metformin.2 In one study, the reported rates of GI adverse effects were 29% versus 39% with extended-release and immediate-release formulations, respectively.2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ali S, Fonseca V. Overview of metformin: special focus on metformin extended release. Expert Opin Pharmacother. 2012;13(12):1797–1805.
- Fujioka K, Pans M, Joyal S. Glycemic control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended-release formulation. Clin Ther. 2003;25(2):515–529.
- Ali S, Fonseca V. Overview of metformin: special focus on metformin extended release. Expert Opin Pharmacother. 2012;13(12):1797–1805.
- Fujioka K, Pans M, Joyal S. Glycemic control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended-release formulation. Clin Ther. 2003;25(2):515–529.