User login
What stops physicians from getting mental health care?
Physician burnout is rampant, and every seat was taken at a workshop on physician burnout and depression at this year’s APA annual meeting in San Diego.
In his book, “Why Physicians Die By Suicide” (Amazon, 2017), Michael F. Myers, MD, describes “burnout.”
Myers notes that there is no stigma to having burnout, as there is to having major depression – a condition that may have remarkably similar symptoms.
What stops physicians from getting help? It’s a complex question – especially in a group that has the means to access medical services – but one factor is that most state licensing boards specifically ask about mental illnesses and substance abuse in intrusive and stigmatizing ways. States vary both with their questions and with their responses to a box checked “yes.”
Katherine Gold, MD, MSW, MS, a family physician at the University of Michigan, Ann Arbor, has studied the topic extensively. Her review of licensing questions from all 50 states revealed that most states ask for information about mental health, and there is tremendous variation as to what is asked (Fam Med. 2017 Jun;49[6]:464-7).
“Some states are very specific and very intrusive,” Gold noted. “They may ask if a physician has a specific diagnosis, a history of treatment or hospitalization. The questions may ask about current impairment, or they may ask about mental health conditions back to age 18 years. There may be very specific questions about diagnosis that are not asked about medical conditions, such as whether the applicant has kleptomania, pyromania, or seasonal affective disorder.”
Gold conducted an online survey of physician-mothers. Nearly half believed they had met criteria for an episode of mental illness at some point during their lives. Of those who did have a diagnosis, only 6% of physicians reported this on licensing forms, though she was quick to say that not all states ask for this information, and some may just ask about current impairment. “The people who are self-disclosing are probably not the physicians we need to be worrying about,” she said.
There is no research that supports the idea that asking physicians about mental illness improves patient safety. Not every state licensing board asks about psychiatric history, but many do ask these questions in a way that violates the Americans with Disabilities Act (Acad Med. 2009;84[6]:776-81). This is not a new issue: In 1993, The State Medical Society of New Jersey filed an injunction against the New Jersey medical board (Medical Society vs. Jacobs et al.) and questions asked on the licensing forms were changed.
Dr. Gold noted that if a physician checks yes to a question about a mental health history, the board response also varies. The doctor can be asked to provide a letter from his physician stating he is fit to work, or can be required to release all of his psychiatric record, or even to appear before the board to justify his fitness to practice.
Chae Kwak, LCSW-C, is the director of the Maryland Physician Health Program for Maryland MedChi. In the fall 2016 Board of Physicians newsletter, Kwak wrote, “An applicant has to affirmatively answer this question only if a current condition affects their ability to practice medicine. Diagnosis and/or treatment of mental health issues such as depression or anxiety is not the same as ‘impairment’ in the practice of medicine.”
Kwak was pleased that the board published his letter. “We want physicians to get the help they need. But this is not just about licensing boards, it’s an issue with hospital credentialing and applications for malpractice insurance as well.”
“We need to advocate on the level of the Federation of State Medical Boards on this subject, and there is a sense of increasing awareness that this is a problem, said Richard Summers, MD, who cochaired the American Psychiatric Association workshop on physician burnout and depression. “The increased salience and awareness of physician burnout, and its relationship to stigma might help this organization and the various state boards become more sympathetic and open to questioning the stigmatizing element of their questions. So, we’ve got to work on this situation both nationally and at the level of the state boards. Hopefully, some successes will stimulate others and will begin to help to change the culture of secrecy and shame.”
Nathaniel Morris, MD, is doing his psychiatry residency at Stanford (Calif.) University. He wrote about this issue in a Washington Post article, “Why doctors are leery about seeking mental health care for themselves” (Jan. 7, 2017). Morris wrote, “When I was a medical student, I suffered an episode of depression and refused to seek treatment for weeks. My fears about licensing applications were a major reason I kept quiet. I didn’t want a mark on my record. I didn’t want to check “yes” on those forms.”
Questions about mental health on licensing board applications were recently addressed by the American Medical Association’s House of Delegates meeting as part of Resolution 301. The AMA concluded with a suggestion that state medical boards inquire about mental health and physical health in a similar way and went on to suggest that boards not request psychotherapy records if the psychotherapy were a requirement of training. This is a profoundly disappointing and inadequate response from the AMA, and my hope is that the APA will move ahead with both words and actions that condemn stigmatizing inquiries.
Questions that differentiate other medical disabilities from psychiatric disabilities need to be stricken from licensing and credentialing forms. Our treatments work, and the cost of not getting care can be catastrophic for both physicians and for their patients. Why ask intrusive and detailed questions about mental illness or substance abuse, and not about diabetes control, seizures, hypotension, atrial fibrillation, or any illness that may cause impairment? It would seem enough to simply ask if the applicant suffers from any condition that impairs ability to function as a physician. Furthermore, it is unreasonable to ask for a full release of psychiatric records following an affirmative statement if detailed records of other illnesses are not required to confirm competency to practice and may prevent psychiatrists from being honest with their therapists. Self-report has limited value on applications, and questions about past sanctions, employment history, and criminal records are more likely to identify physicians who are impaired for any reason.
Dr. Miller, who practices in Baltimore, is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care,” (Baltimore: Johns Hopkins University Press, 2016).
Physician burnout is rampant, and every seat was taken at a workshop on physician burnout and depression at this year’s APA annual meeting in San Diego.
In his book, “Why Physicians Die By Suicide” (Amazon, 2017), Michael F. Myers, MD, describes “burnout.”
Myers notes that there is no stigma to having burnout, as there is to having major depression – a condition that may have remarkably similar symptoms.
What stops physicians from getting help? It’s a complex question – especially in a group that has the means to access medical services – but one factor is that most state licensing boards specifically ask about mental illnesses and substance abuse in intrusive and stigmatizing ways. States vary both with their questions and with their responses to a box checked “yes.”
Katherine Gold, MD, MSW, MS, a family physician at the University of Michigan, Ann Arbor, has studied the topic extensively. Her review of licensing questions from all 50 states revealed that most states ask for information about mental health, and there is tremendous variation as to what is asked (Fam Med. 2017 Jun;49[6]:464-7).
“Some states are very specific and very intrusive,” Gold noted. “They may ask if a physician has a specific diagnosis, a history of treatment or hospitalization. The questions may ask about current impairment, or they may ask about mental health conditions back to age 18 years. There may be very specific questions about diagnosis that are not asked about medical conditions, such as whether the applicant has kleptomania, pyromania, or seasonal affective disorder.”
Gold conducted an online survey of physician-mothers. Nearly half believed they had met criteria for an episode of mental illness at some point during their lives. Of those who did have a diagnosis, only 6% of physicians reported this on licensing forms, though she was quick to say that not all states ask for this information, and some may just ask about current impairment. “The people who are self-disclosing are probably not the physicians we need to be worrying about,” she said.
There is no research that supports the idea that asking physicians about mental illness improves patient safety. Not every state licensing board asks about psychiatric history, but many do ask these questions in a way that violates the Americans with Disabilities Act (Acad Med. 2009;84[6]:776-81). This is not a new issue: In 1993, The State Medical Society of New Jersey filed an injunction against the New Jersey medical board (Medical Society vs. Jacobs et al.) and questions asked on the licensing forms were changed.
Dr. Gold noted that if a physician checks yes to a question about a mental health history, the board response also varies. The doctor can be asked to provide a letter from his physician stating he is fit to work, or can be required to release all of his psychiatric record, or even to appear before the board to justify his fitness to practice.
Chae Kwak, LCSW-C, is the director of the Maryland Physician Health Program for Maryland MedChi. In the fall 2016 Board of Physicians newsletter, Kwak wrote, “An applicant has to affirmatively answer this question only if a current condition affects their ability to practice medicine. Diagnosis and/or treatment of mental health issues such as depression or anxiety is not the same as ‘impairment’ in the practice of medicine.”
Kwak was pleased that the board published his letter. “We want physicians to get the help they need. But this is not just about licensing boards, it’s an issue with hospital credentialing and applications for malpractice insurance as well.”
“We need to advocate on the level of the Federation of State Medical Boards on this subject, and there is a sense of increasing awareness that this is a problem, said Richard Summers, MD, who cochaired the American Psychiatric Association workshop on physician burnout and depression. “The increased salience and awareness of physician burnout, and its relationship to stigma might help this organization and the various state boards become more sympathetic and open to questioning the stigmatizing element of their questions. So, we’ve got to work on this situation both nationally and at the level of the state boards. Hopefully, some successes will stimulate others and will begin to help to change the culture of secrecy and shame.”
Nathaniel Morris, MD, is doing his psychiatry residency at Stanford (Calif.) University. He wrote about this issue in a Washington Post article, “Why doctors are leery about seeking mental health care for themselves” (Jan. 7, 2017). Morris wrote, “When I was a medical student, I suffered an episode of depression and refused to seek treatment for weeks. My fears about licensing applications were a major reason I kept quiet. I didn’t want a mark on my record. I didn’t want to check “yes” on those forms.”
Questions about mental health on licensing board applications were recently addressed by the American Medical Association’s House of Delegates meeting as part of Resolution 301. The AMA concluded with a suggestion that state medical boards inquire about mental health and physical health in a similar way and went on to suggest that boards not request psychotherapy records if the psychotherapy were a requirement of training. This is a profoundly disappointing and inadequate response from the AMA, and my hope is that the APA will move ahead with both words and actions that condemn stigmatizing inquiries.
Questions that differentiate other medical disabilities from psychiatric disabilities need to be stricken from licensing and credentialing forms. Our treatments work, and the cost of not getting care can be catastrophic for both physicians and for their patients. Why ask intrusive and detailed questions about mental illness or substance abuse, and not about diabetes control, seizures, hypotension, atrial fibrillation, or any illness that may cause impairment? It would seem enough to simply ask if the applicant suffers from any condition that impairs ability to function as a physician. Furthermore, it is unreasonable to ask for a full release of psychiatric records following an affirmative statement if detailed records of other illnesses are not required to confirm competency to practice and may prevent psychiatrists from being honest with their therapists. Self-report has limited value on applications, and questions about past sanctions, employment history, and criminal records are more likely to identify physicians who are impaired for any reason.
Dr. Miller, who practices in Baltimore, is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care,” (Baltimore: Johns Hopkins University Press, 2016).
Physician burnout is rampant, and every seat was taken at a workshop on physician burnout and depression at this year’s APA annual meeting in San Diego.
In his book, “Why Physicians Die By Suicide” (Amazon, 2017), Michael F. Myers, MD, describes “burnout.”
Myers notes that there is no stigma to having burnout, as there is to having major depression – a condition that may have remarkably similar symptoms.
What stops physicians from getting help? It’s a complex question – especially in a group that has the means to access medical services – but one factor is that most state licensing boards specifically ask about mental illnesses and substance abuse in intrusive and stigmatizing ways. States vary both with their questions and with their responses to a box checked “yes.”
Katherine Gold, MD, MSW, MS, a family physician at the University of Michigan, Ann Arbor, has studied the topic extensively. Her review of licensing questions from all 50 states revealed that most states ask for information about mental health, and there is tremendous variation as to what is asked (Fam Med. 2017 Jun;49[6]:464-7).
“Some states are very specific and very intrusive,” Gold noted. “They may ask if a physician has a specific diagnosis, a history of treatment or hospitalization. The questions may ask about current impairment, or they may ask about mental health conditions back to age 18 years. There may be very specific questions about diagnosis that are not asked about medical conditions, such as whether the applicant has kleptomania, pyromania, or seasonal affective disorder.”
Gold conducted an online survey of physician-mothers. Nearly half believed they had met criteria for an episode of mental illness at some point during their lives. Of those who did have a diagnosis, only 6% of physicians reported this on licensing forms, though she was quick to say that not all states ask for this information, and some may just ask about current impairment. “The people who are self-disclosing are probably not the physicians we need to be worrying about,” she said.
There is no research that supports the idea that asking physicians about mental illness improves patient safety. Not every state licensing board asks about psychiatric history, but many do ask these questions in a way that violates the Americans with Disabilities Act (Acad Med. 2009;84[6]:776-81). This is not a new issue: In 1993, The State Medical Society of New Jersey filed an injunction against the New Jersey medical board (Medical Society vs. Jacobs et al.) and questions asked on the licensing forms were changed.
Dr. Gold noted that if a physician checks yes to a question about a mental health history, the board response also varies. The doctor can be asked to provide a letter from his physician stating he is fit to work, or can be required to release all of his psychiatric record, or even to appear before the board to justify his fitness to practice.
Chae Kwak, LCSW-C, is the director of the Maryland Physician Health Program for Maryland MedChi. In the fall 2016 Board of Physicians newsletter, Kwak wrote, “An applicant has to affirmatively answer this question only if a current condition affects their ability to practice medicine. Diagnosis and/or treatment of mental health issues such as depression or anxiety is not the same as ‘impairment’ in the practice of medicine.”
Kwak was pleased that the board published his letter. “We want physicians to get the help they need. But this is not just about licensing boards, it’s an issue with hospital credentialing and applications for malpractice insurance as well.”
“We need to advocate on the level of the Federation of State Medical Boards on this subject, and there is a sense of increasing awareness that this is a problem, said Richard Summers, MD, who cochaired the American Psychiatric Association workshop on physician burnout and depression. “The increased salience and awareness of physician burnout, and its relationship to stigma might help this organization and the various state boards become more sympathetic and open to questioning the stigmatizing element of their questions. So, we’ve got to work on this situation both nationally and at the level of the state boards. Hopefully, some successes will stimulate others and will begin to help to change the culture of secrecy and shame.”
Nathaniel Morris, MD, is doing his psychiatry residency at Stanford (Calif.) University. He wrote about this issue in a Washington Post article, “Why doctors are leery about seeking mental health care for themselves” (Jan. 7, 2017). Morris wrote, “When I was a medical student, I suffered an episode of depression and refused to seek treatment for weeks. My fears about licensing applications were a major reason I kept quiet. I didn’t want a mark on my record. I didn’t want to check “yes” on those forms.”
Questions about mental health on licensing board applications were recently addressed by the American Medical Association’s House of Delegates meeting as part of Resolution 301. The AMA concluded with a suggestion that state medical boards inquire about mental health and physical health in a similar way and went on to suggest that boards not request psychotherapy records if the psychotherapy were a requirement of training. This is a profoundly disappointing and inadequate response from the AMA, and my hope is that the APA will move ahead with both words and actions that condemn stigmatizing inquiries.
Questions that differentiate other medical disabilities from psychiatric disabilities need to be stricken from licensing and credentialing forms. Our treatments work, and the cost of not getting care can be catastrophic for both physicians and for their patients. Why ask intrusive and detailed questions about mental illness or substance abuse, and not about diabetes control, seizures, hypotension, atrial fibrillation, or any illness that may cause impairment? It would seem enough to simply ask if the applicant suffers from any condition that impairs ability to function as a physician. Furthermore, it is unreasonable to ask for a full release of psychiatric records following an affirmative statement if detailed records of other illnesses are not required to confirm competency to practice and may prevent psychiatrists from being honest with their therapists. Self-report has limited value on applications, and questions about past sanctions, employment history, and criminal records are more likely to identify physicians who are impaired for any reason.
Dr. Miller, who practices in Baltimore, is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care,” (Baltimore: Johns Hopkins University Press, 2016).
Is female genital cosmetic surgery going mainstream?
Experts describe the field of female genital cosmetic surgery as the “Wild West,” but the lack of regulation and consensus has not kept it from exploding in recent years.
More than 10,000 labiaplasties were performed in 2016, a 23% jump over the previous year, and the procedures are offered by more than 35% of all plastic surgeons, according to data from the American Society for Aesthetic Plastic Surgery. As another indicator of increasing attention to the appearance of female genitalia, a 2013 survey of U.S. women revealed that more than 80% performed some sort of pubic hair grooming (JAMA Dermatol. 2016;152[10]:1106-13).
Dr. Iglesia recounted being contacted by a National Gallery of Art staff member, who, when confronted with Gustave Courbet’s L’Origine du Monde, an 1866 below-the-waist portrait of a nude woman, asked, “Is this normal? Do women have this much hair?” Dr. Iglesia said she reassured the staff member that the woman in the portrait did indeed have a normal female Tanner stage IV or V escutcheon. However, she said, social media and other images in the popular press have essentially erased female pubic hair from the public eye, even in explicit imagery that involves female nudity.
“This is an ideal that men and women are seeing in social media, in pornography, and even in the lay press.” And now, she said, “We’re in a new era of sex surgeries, with these ‘nips and tucks’ below the belt.”
Labiaplasty
The combination of a newly-hairless genital region, together with portrayals of adult women with a “Barbie doll” appearance, may contribute to women feeling self-conscious about labia minora protruding beyond the labia majora. Dr. Iglesia, who is section director for female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, Washington, D.C., said this is true even though the normal length of labia minora can range from 7 mm to 5 cm.
That’s where labiaplasty comes in. The procedure, which can be performed with conventional surgical techniques or with a laser, is sometimes done for functional reasons.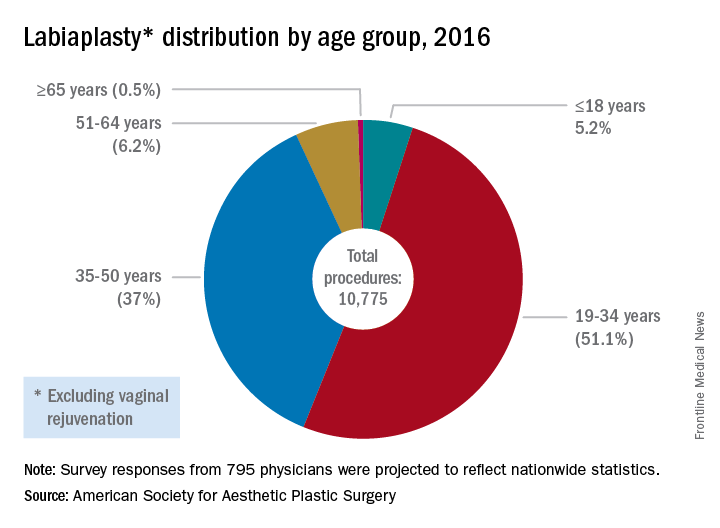
The waters are murkier when labiaplasty is performed for cosmetic reasons, to get that “Barbie doll” look, with some offices advertising the procedure as “designer lips,” Dr. Iglesia said.
In 2007, ACOG issued a committee opinion expressing concern about the lack of data and sometimes deceptive marketing practices surrounding a number of cosmetic vaginal surgeries (Obstet Gynecol 2007;110:737–8). The policy was reaffirmed in 2017.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/is-it-appropriate-to-perform-gynecologic-procedures-such-as-labiaplasty-for-cosmetic-reasons?iframe=1"}]Similarly, the Society of Obstetricians and Gynaecologists of Canada issued a 2013 statement about labiaplasty and other female genital cosmetic surgeries saying that “there is little evidence to support any of the female genital cosmetic surgeries in terms of improvement to sexual satisfaction or self-images. Physicians choosing to proceed with these cosmetic procedures should not promote these surgeries for the enhancement of sexual function and advertising of female genital cosmetic surgical procedures should be avoided.”
However, Mickey Karram, MD, who is director of the urogynecology program at Christ Hospital, Cincinnatti, said that informed consent is the key to dealing appropriately with these procedures.
“If a patient is physically bothered from a cosmetic standpoint that her labia are larger than she thinks they should be, and they are bothering her, is it appropriate or inappropriate to potentially discuss with her a labiaplasty?” Dr. Karram said at the ACOG meeting. For the patient who understands the risk and is also clear that the procedure is not medically necessary, he said he “feels strongly” that labiaplasty should be an option.
Fractional laser
The introduction of the fractional laser to gynecology is also adding to the debate about the appropriate integration of gynecologic procedures that may have nonmedical uses, such as vaginal “tightening.” Used primarily intravaginally, these devices have shallow penetration and are meant to stimulate collagen, proteoglycan, and hyaluronic acid synthesis with minimal tissue damage and downtime. One such device, the MonaLisa Touch, is marketed in the United States by Cynosure.
These energy sources hold great promise for the genitourinary syndrome of menopause (GSM) and other conditions, Dr. Karram said. “Many of these energy sources are being promoted for actual disease states, like vulvovaginal atrophy and lichen sclerosus,” he said.
Dr. Iglesia is not so sure: “This is not the fountain of youth.” She pointed out that the vasculature and innervation of the vagina and vulva are complex, with the outer one-eighth of the vagina being much more highly innervated. Laser treatment with a shallow penetration depth may not get at all of the issues that contribute to GSM.
“Is marketing ahead of the science? I would say yes,” she said. “There’s too much hype about this curing vaginal dryness and making your sex life better.”
Dr. Zahn also urged caution with the use of this technology. “The data are very limited, but, despite this, it’s become a very popular and highly-advertised approach. We need larger studies and more longitudinal data. This is especially true since one of the proposed ways this device works is by stimulating fibrosis. In every other body system, fibrosis stimulation may result in scarring. We have no idea if this is the case with this device. If it is, its application could result in worsening of bodily function, especially in regard to dyspareunia,” he said. “We clearly need more data.”
In 2016, ACOG issued a position statement about the fractional carbon dioxide and yttrium-aluminum-garnet laser systems that had received clearance from the Food and Drug Administration. The statement advised both ob.gyns. and patients that “this technology is, in fact, neither approved nor cleared by the FDA for the specific indication of treating vulvovaginal atrophy.”
Both Dr. Karram and Dr. Iglesia are investigators in an ongoing randomized, placebo- and sham-controlled trial comparing vaginal estrogen and laser therapy used both in conjunction and singly.
‘No-go’ procedures
Though Dr. Karram and Dr. Iglesia disagree on whether cosmetic labiaplasty is appropriate, they were in agreement that certain procedures are so untested, or have such potential risk with no proven benefit, that they should not be performed at all. The procedures on both physicians’ “no-go” lists included clitoral unhooding, G-spot amplification, “revirginification” in any form, vulval recontouring with autologous fat, and the so-called “O-shot,” injections of platelet-rich plasma that are touted as augmenting the sexual experience.
What’s to be done?
There is also agreement that a lack of common terminology is a significant problem. Step one, Dr. Karram said, is doing away with the term vaginal rejuvenation. “This is a terrible term. … There’s no real definition for this term.” He called for a multidisciplinary working group that would bring together gynecologists, plastic surgeons, and dermatologists to begin the work of terminology standardization.
From there, he proposed that the group develop a classification system that clarifies whether procedures are being done for cosmetic reasons, to enhance the sexual experience, or to address a specific disease state. Finally, he said, the group should recommend standardized outcome metrics that can be used to study the various interventions.
Dr. Zahn applauded this notion. “It’s a great point. I agree that multiple disciplines should be involved in examining outcomes, statistics, and criteria for evaluating procedures.”
And gynecologists should be leading this effort, Dr. Karram suggested. “Who knows this anatomy the best? We do.” He added, “If it’s going to be addressed, it should be addressed by us.”
But, Dr. Iglesia said she worries about vulnerable populations, such as adolescents and cancer survivors, who may undergo surgeries, for which the benefits may not outweigh the potential risks. For labiaplasty and laser resurfacing techniques, there have been a small number of studies on outcomes and patient satisfaction that have generally been conducted at single centers with no comparison arms and limited follow-up, she said.
“I also am concerned about pain, scarring, altered sensation, painful sex that could develop, wound complications, and what happens over time,” especially when these procedures may be performed on adolescents or women in their 20s or 30s who may later go on to have children, Dr. Iglesia said.
The question, she said, is not just whether gynecologists are better equipped than plastic surgeons or dermatologists to be performing female genital cosmetic surgery, “but should we be doing this at all?”
Dr. Zahn emphasized the need for evidence to guide decision making. “There has to be data that there is benefit and that the benefit outweighs the potential harm. There is no data on most cosmetic gynecologic procedures. If there are no data, they shouldn’t be done because we would not have the information necessary to appropriately counsel patients,” he said.
Dr. Karram has a financial relationship with Cynosure, which markets the MonaLisa Touch system in the United States. Dr. Iglesia reported that she had no relevant financial disclosures. Dr. Zahn is employed by ACOG.
[email protected]
On Twitter @karioakes
Experts describe the field of female genital cosmetic surgery as the “Wild West,” but the lack of regulation and consensus has not kept it from exploding in recent years.
More than 10,000 labiaplasties were performed in 2016, a 23% jump over the previous year, and the procedures are offered by more than 35% of all plastic surgeons, according to data from the American Society for Aesthetic Plastic Surgery. As another indicator of increasing attention to the appearance of female genitalia, a 2013 survey of U.S. women revealed that more than 80% performed some sort of pubic hair grooming (JAMA Dermatol. 2016;152[10]:1106-13).
Dr. Iglesia recounted being contacted by a National Gallery of Art staff member, who, when confronted with Gustave Courbet’s L’Origine du Monde, an 1866 below-the-waist portrait of a nude woman, asked, “Is this normal? Do women have this much hair?” Dr. Iglesia said she reassured the staff member that the woman in the portrait did indeed have a normal female Tanner stage IV or V escutcheon. However, she said, social media and other images in the popular press have essentially erased female pubic hair from the public eye, even in explicit imagery that involves female nudity.
“This is an ideal that men and women are seeing in social media, in pornography, and even in the lay press.” And now, she said, “We’re in a new era of sex surgeries, with these ‘nips and tucks’ below the belt.”
Labiaplasty
The combination of a newly-hairless genital region, together with portrayals of adult women with a “Barbie doll” appearance, may contribute to women feeling self-conscious about labia minora protruding beyond the labia majora. Dr. Iglesia, who is section director for female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, Washington, D.C., said this is true even though the normal length of labia minora can range from 7 mm to 5 cm.
That’s where labiaplasty comes in. The procedure, which can be performed with conventional surgical techniques or with a laser, is sometimes done for functional reasons.
The waters are murkier when labiaplasty is performed for cosmetic reasons, to get that “Barbie doll” look, with some offices advertising the procedure as “designer lips,” Dr. Iglesia said.
In 2007, ACOG issued a committee opinion expressing concern about the lack of data and sometimes deceptive marketing practices surrounding a number of cosmetic vaginal surgeries (Obstet Gynecol 2007;110:737–8). The policy was reaffirmed in 2017.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/is-it-appropriate-to-perform-gynecologic-procedures-such-as-labiaplasty-for-cosmetic-reasons?iframe=1"}]Similarly, the Society of Obstetricians and Gynaecologists of Canada issued a 2013 statement about labiaplasty and other female genital cosmetic surgeries saying that “there is little evidence to support any of the female genital cosmetic surgeries in terms of improvement to sexual satisfaction or self-images. Physicians choosing to proceed with these cosmetic procedures should not promote these surgeries for the enhancement of sexual function and advertising of female genital cosmetic surgical procedures should be avoided.”
However, Mickey Karram, MD, who is director of the urogynecology program at Christ Hospital, Cincinnatti, said that informed consent is the key to dealing appropriately with these procedures.
“If a patient is physically bothered from a cosmetic standpoint that her labia are larger than she thinks they should be, and they are bothering her, is it appropriate or inappropriate to potentially discuss with her a labiaplasty?” Dr. Karram said at the ACOG meeting. For the patient who understands the risk and is also clear that the procedure is not medically necessary, he said he “feels strongly” that labiaplasty should be an option.
Fractional laser
The introduction of the fractional laser to gynecology is also adding to the debate about the appropriate integration of gynecologic procedures that may have nonmedical uses, such as vaginal “tightening.” Used primarily intravaginally, these devices have shallow penetration and are meant to stimulate collagen, proteoglycan, and hyaluronic acid synthesis with minimal tissue damage and downtime. One such device, the MonaLisa Touch, is marketed in the United States by Cynosure.
These energy sources hold great promise for the genitourinary syndrome of menopause (GSM) and other conditions, Dr. Karram said. “Many of these energy sources are being promoted for actual disease states, like vulvovaginal atrophy and lichen sclerosus,” he said.
Dr. Iglesia is not so sure: “This is not the fountain of youth.” She pointed out that the vasculature and innervation of the vagina and vulva are complex, with the outer one-eighth of the vagina being much more highly innervated. Laser treatment with a shallow penetration depth may not get at all of the issues that contribute to GSM.
“Is marketing ahead of the science? I would say yes,” she said. “There’s too much hype about this curing vaginal dryness and making your sex life better.”
Dr. Zahn also urged caution with the use of this technology. “The data are very limited, but, despite this, it’s become a very popular and highly-advertised approach. We need larger studies and more longitudinal data. This is especially true since one of the proposed ways this device works is by stimulating fibrosis. In every other body system, fibrosis stimulation may result in scarring. We have no idea if this is the case with this device. If it is, its application could result in worsening of bodily function, especially in regard to dyspareunia,” he said. “We clearly need more data.”
In 2016, ACOG issued a position statement about the fractional carbon dioxide and yttrium-aluminum-garnet laser systems that had received clearance from the Food and Drug Administration. The statement advised both ob.gyns. and patients that “this technology is, in fact, neither approved nor cleared by the FDA for the specific indication of treating vulvovaginal atrophy.”
Both Dr. Karram and Dr. Iglesia are investigators in an ongoing randomized, placebo- and sham-controlled trial comparing vaginal estrogen and laser therapy used both in conjunction and singly.
‘No-go’ procedures
Though Dr. Karram and Dr. Iglesia disagree on whether cosmetic labiaplasty is appropriate, they were in agreement that certain procedures are so untested, or have such potential risk with no proven benefit, that they should not be performed at all. The procedures on both physicians’ “no-go” lists included clitoral unhooding, G-spot amplification, “revirginification” in any form, vulval recontouring with autologous fat, and the so-called “O-shot,” injections of platelet-rich plasma that are touted as augmenting the sexual experience.
What’s to be done?
There is also agreement that a lack of common terminology is a significant problem. Step one, Dr. Karram said, is doing away with the term vaginal rejuvenation. “This is a terrible term. … There’s no real definition for this term.” He called for a multidisciplinary working group that would bring together gynecologists, plastic surgeons, and dermatologists to begin the work of terminology standardization.
From there, he proposed that the group develop a classification system that clarifies whether procedures are being done for cosmetic reasons, to enhance the sexual experience, or to address a specific disease state. Finally, he said, the group should recommend standardized outcome metrics that can be used to study the various interventions.
Dr. Zahn applauded this notion. “It’s a great point. I agree that multiple disciplines should be involved in examining outcomes, statistics, and criteria for evaluating procedures.”
And gynecologists should be leading this effort, Dr. Karram suggested. “Who knows this anatomy the best? We do.” He added, “If it’s going to be addressed, it should be addressed by us.”
But, Dr. Iglesia said she worries about vulnerable populations, such as adolescents and cancer survivors, who may undergo surgeries, for which the benefits may not outweigh the potential risks. For labiaplasty and laser resurfacing techniques, there have been a small number of studies on outcomes and patient satisfaction that have generally been conducted at single centers with no comparison arms and limited follow-up, she said.
“I also am concerned about pain, scarring, altered sensation, painful sex that could develop, wound complications, and what happens over time,” especially when these procedures may be performed on adolescents or women in their 20s or 30s who may later go on to have children, Dr. Iglesia said.
The question, she said, is not just whether gynecologists are better equipped than plastic surgeons or dermatologists to be performing female genital cosmetic surgery, “but should we be doing this at all?”
Dr. Zahn emphasized the need for evidence to guide decision making. “There has to be data that there is benefit and that the benefit outweighs the potential harm. There is no data on most cosmetic gynecologic procedures. If there are no data, they shouldn’t be done because we would not have the information necessary to appropriately counsel patients,” he said.
Dr. Karram has a financial relationship with Cynosure, which markets the MonaLisa Touch system in the United States. Dr. Iglesia reported that she had no relevant financial disclosures. Dr. Zahn is employed by ACOG.
[email protected]
On Twitter @karioakes
Experts describe the field of female genital cosmetic surgery as the “Wild West,” but the lack of regulation and consensus has not kept it from exploding in recent years.
More than 10,000 labiaplasties were performed in 2016, a 23% jump over the previous year, and the procedures are offered by more than 35% of all plastic surgeons, according to data from the American Society for Aesthetic Plastic Surgery. As another indicator of increasing attention to the appearance of female genitalia, a 2013 survey of U.S. women revealed that more than 80% performed some sort of pubic hair grooming (JAMA Dermatol. 2016;152[10]:1106-13).
Dr. Iglesia recounted being contacted by a National Gallery of Art staff member, who, when confronted with Gustave Courbet’s L’Origine du Monde, an 1866 below-the-waist portrait of a nude woman, asked, “Is this normal? Do women have this much hair?” Dr. Iglesia said she reassured the staff member that the woman in the portrait did indeed have a normal female Tanner stage IV or V escutcheon. However, she said, social media and other images in the popular press have essentially erased female pubic hair from the public eye, even in explicit imagery that involves female nudity.
“This is an ideal that men and women are seeing in social media, in pornography, and even in the lay press.” And now, she said, “We’re in a new era of sex surgeries, with these ‘nips and tucks’ below the belt.”
Labiaplasty
The combination of a newly-hairless genital region, together with portrayals of adult women with a “Barbie doll” appearance, may contribute to women feeling self-conscious about labia minora protruding beyond the labia majora. Dr. Iglesia, who is section director for female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, Washington, D.C., said this is true even though the normal length of labia minora can range from 7 mm to 5 cm.
That’s where labiaplasty comes in. The procedure, which can be performed with conventional surgical techniques or with a laser, is sometimes done for functional reasons.
The waters are murkier when labiaplasty is performed for cosmetic reasons, to get that “Barbie doll” look, with some offices advertising the procedure as “designer lips,” Dr. Iglesia said.
In 2007, ACOG issued a committee opinion expressing concern about the lack of data and sometimes deceptive marketing practices surrounding a number of cosmetic vaginal surgeries (Obstet Gynecol 2007;110:737–8). The policy was reaffirmed in 2017.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/is-it-appropriate-to-perform-gynecologic-procedures-such-as-labiaplasty-for-cosmetic-reasons?iframe=1"}]Similarly, the Society of Obstetricians and Gynaecologists of Canada issued a 2013 statement about labiaplasty and other female genital cosmetic surgeries saying that “there is little evidence to support any of the female genital cosmetic surgeries in terms of improvement to sexual satisfaction or self-images. Physicians choosing to proceed with these cosmetic procedures should not promote these surgeries for the enhancement of sexual function and advertising of female genital cosmetic surgical procedures should be avoided.”
However, Mickey Karram, MD, who is director of the urogynecology program at Christ Hospital, Cincinnatti, said that informed consent is the key to dealing appropriately with these procedures.
“If a patient is physically bothered from a cosmetic standpoint that her labia are larger than she thinks they should be, and they are bothering her, is it appropriate or inappropriate to potentially discuss with her a labiaplasty?” Dr. Karram said at the ACOG meeting. For the patient who understands the risk and is also clear that the procedure is not medically necessary, he said he “feels strongly” that labiaplasty should be an option.
Fractional laser
The introduction of the fractional laser to gynecology is also adding to the debate about the appropriate integration of gynecologic procedures that may have nonmedical uses, such as vaginal “tightening.” Used primarily intravaginally, these devices have shallow penetration and are meant to stimulate collagen, proteoglycan, and hyaluronic acid synthesis with minimal tissue damage and downtime. One such device, the MonaLisa Touch, is marketed in the United States by Cynosure.
These energy sources hold great promise for the genitourinary syndrome of menopause (GSM) and other conditions, Dr. Karram said. “Many of these energy sources are being promoted for actual disease states, like vulvovaginal atrophy and lichen sclerosus,” he said.
Dr. Iglesia is not so sure: “This is not the fountain of youth.” She pointed out that the vasculature and innervation of the vagina and vulva are complex, with the outer one-eighth of the vagina being much more highly innervated. Laser treatment with a shallow penetration depth may not get at all of the issues that contribute to GSM.
“Is marketing ahead of the science? I would say yes,” she said. “There’s too much hype about this curing vaginal dryness and making your sex life better.”
Dr. Zahn also urged caution with the use of this technology. “The data are very limited, but, despite this, it’s become a very popular and highly-advertised approach. We need larger studies and more longitudinal data. This is especially true since one of the proposed ways this device works is by stimulating fibrosis. In every other body system, fibrosis stimulation may result in scarring. We have no idea if this is the case with this device. If it is, its application could result in worsening of bodily function, especially in regard to dyspareunia,” he said. “We clearly need more data.”
In 2016, ACOG issued a position statement about the fractional carbon dioxide and yttrium-aluminum-garnet laser systems that had received clearance from the Food and Drug Administration. The statement advised both ob.gyns. and patients that “this technology is, in fact, neither approved nor cleared by the FDA for the specific indication of treating vulvovaginal atrophy.”
Both Dr. Karram and Dr. Iglesia are investigators in an ongoing randomized, placebo- and sham-controlled trial comparing vaginal estrogen and laser therapy used both in conjunction and singly.
‘No-go’ procedures
Though Dr. Karram and Dr. Iglesia disagree on whether cosmetic labiaplasty is appropriate, they were in agreement that certain procedures are so untested, or have such potential risk with no proven benefit, that they should not be performed at all. The procedures on both physicians’ “no-go” lists included clitoral unhooding, G-spot amplification, “revirginification” in any form, vulval recontouring with autologous fat, and the so-called “O-shot,” injections of platelet-rich plasma that are touted as augmenting the sexual experience.
What’s to be done?
There is also agreement that a lack of common terminology is a significant problem. Step one, Dr. Karram said, is doing away with the term vaginal rejuvenation. “This is a terrible term. … There’s no real definition for this term.” He called for a multidisciplinary working group that would bring together gynecologists, plastic surgeons, and dermatologists to begin the work of terminology standardization.
From there, he proposed that the group develop a classification system that clarifies whether procedures are being done for cosmetic reasons, to enhance the sexual experience, or to address a specific disease state. Finally, he said, the group should recommend standardized outcome metrics that can be used to study the various interventions.
Dr. Zahn applauded this notion. “It’s a great point. I agree that multiple disciplines should be involved in examining outcomes, statistics, and criteria for evaluating procedures.”
And gynecologists should be leading this effort, Dr. Karram suggested. “Who knows this anatomy the best? We do.” He added, “If it’s going to be addressed, it should be addressed by us.”
But, Dr. Iglesia said she worries about vulnerable populations, such as adolescents and cancer survivors, who may undergo surgeries, for which the benefits may not outweigh the potential risks. For labiaplasty and laser resurfacing techniques, there have been a small number of studies on outcomes and patient satisfaction that have generally been conducted at single centers with no comparison arms and limited follow-up, she said.
“I also am concerned about pain, scarring, altered sensation, painful sex that could develop, wound complications, and what happens over time,” especially when these procedures may be performed on adolescents or women in their 20s or 30s who may later go on to have children, Dr. Iglesia said.
The question, she said, is not just whether gynecologists are better equipped than plastic surgeons or dermatologists to be performing female genital cosmetic surgery, “but should we be doing this at all?”
Dr. Zahn emphasized the need for evidence to guide decision making. “There has to be data that there is benefit and that the benefit outweighs the potential harm. There is no data on most cosmetic gynecologic procedures. If there are no data, they shouldn’t be done because we would not have the information necessary to appropriately counsel patients,” he said.
Dr. Karram has a financial relationship with Cynosure, which markets the MonaLisa Touch system in the United States. Dr. Iglesia reported that she had no relevant financial disclosures. Dr. Zahn is employed by ACOG.
[email protected]
On Twitter @karioakes
Metastatic Crohn Disease: A Review of Dermatologic Manifestations and Treatment
Almost half of Crohn disease (CD) patients experience a dermatologic manifestation of the disease. A rare entity, metastatic CD (MCD) presents a diagnostic challenge without a high index of suspicion. Its etiology is not well defined; however, it appears to be an autoimmune response to gut antigens. Herein, we review the etiology/epidemiology, diagnostic criteria, and treatment for this uncommon condition.
Epidemiology and Clinical Characteristics of MCD
Metastatic CD was first described by Parks et al1 in 1965 and refers to a diverse collection of macroscopic dermatologic manifestations in tissue not contiguous with the gastrointestinal (GI) tract. To be classified as MCD, the tissue must demonstrate characteristic histopathologic findings, which invariably include noncaseating granulomas.
Crohn disease may affect any part of the GI tract from the mouth to anus, with a multitude of associated cutaneous manifestations having been described. The terminal ileum is the most commonly affected portion of the GI tract in CD, but the large intestine also may be involved in 55% to 80% of cases.2 The incidence of non-MCD-associated anal lesions seems to correlate with intestinal involvement in that as few as 25% of patients with ileal-localized CD have anal lesions compared to nearly 80% of patients with large intestinal involvement.3
It has been estimated that 18% to 44% of patients with CD have some form of cutaneous manifestation,4 with MCD being a rare subcategory. As few as 100 cases have been described from 1965 to the present.5 The presence of MCD does not correlate well with severity of intestinal CD, and although a majority of MCD cases present after at least 6 months of GI symptoms,6 there are instances in which MCD presents without prior or existing evidence of intestinal CD.7
With regard to MCD, the term metastatic is sometimes supplanted in the literature by cutaneous to avoid any implication of cancer; however, due to a myriad of dermatologic manifestations, both terms can cause confusion. The categorization of the various types of cutaneous findings in CD is well summarized in a review by Palamaras et al8 with the following classifications: (1) granulomatous by direct extension (oral or perianal), (2) MCD lesions (genital and nongenital), (3) immune-related lesions, and (4) lesions from nutritional deficiencies. Of the cutaneous manifestations relating to CD, MCD is the least common cutaneous categorical manifestation and is further divided into subcategories of genital and nongenital lesions.8
The nongenital distribution of MCD is the more common variety in adults and particularly seems to affect the legs and plantar surfaces (38%), the trunk and abdomen (24%), and the face (15%).5,9 These nongenital MCD manifestations are most commonly described as nodules, ulcerations, or erythematous to purple plaques, and less commonly described as abscesses, pustules, or papules.
The sequence of cutaneous symptoms of MCD relative to intestinal disease depends to some degree on patient age. In adults diagnosed with MCD, it has been noted that a GI flare is expected 2 months to 4 years after diagnosis; however, in children the subsequent GI flare has been noted to vary more widely from 9 months to 14 years following presentation of MCD.8 Furthermore, roughly 50% of children diagnosed with MCD present concomitantly with their first symptoms of a GI flare, whereas 70% of adults with MCD had been previously diagnosed with intestinal CD.8 In one review of 80 reported cases of MCD, 20% (16/80) had no symptoms of intestinal disease at the time of MCD diagnosis, and the majority of the asymptomatic cases were in children; interestingly, the majority of these same children were diagnosed with CD months to years later.9
Both the location and characteristics of cutaneous findings in MCD correlate with age.9 Metastatic CD has been identified in all age groups; however, lymphedema is more common in children/young adults, while nodules, ulceration, and fistulating disease are more often seen in adults.10 Affected children and adolescents with MCD range from 5 to 17 years of age, with a mean age at disease onset of 11.1 years and equal incidence in males and females.8 Adults with MCD range from 18 to 78 years of age, with a mean age at presentation of 38.4 years.8,11
Concerning anatomic location of disease, adults with MCD most commonly have nodules with or without plaques on the arms and legs and less commonly in the genital area.8 In contrast, children with MCD are more prone to genital lesions, with up to 85% of cases including some degree of genital erythematous or nonerythematous swelling with or without induration.8 Genitourinary complications of CD as a broad category, however, are estimated to occur in only 5% to 20% of intestinal CD cases in both children and adults.12
There have been conflicting reports regarding gender predilection in MCD. Based on a review by Samitz et al13 of 200 cases of CD over an 18-year period, 22% of patients with CD were found to have cutaneous manifestations--presumably not MCD but rather perianal, perineal, vulvar fistulae, fissures, or abscesses--with a male to female preponderance of almost 2 to 1. A more recent review of the literature by Palamaras et al8 in 2008 reported that contiguous non-MCD affects adult females and children more often than adult males, with 63% adult cases being female. This review seems to be more congruent with other reports in the literature implicating that females are twice as commonly affected by MCD than males.9,14
Pathophysiology
The etiology of MCD has not been well defined. One proposed mechanism of the distal tissue involvement of MCD is through passage of antigens to the skin with subsequent granulomatous response at the level of the dermis.10 Another proposed mechanism suggests antibody sensitization to gut antigens, possibly bacterial antigens, that then coincidentally cross-react with analogous skin antigens.8,14 Burgdorf11 supported this notion in a 1981 report in which it was suggested that the granulomatous reaction was related to deposition of immune complexes in the skin. Slater et al15 and Tatnall et al16 offered a variation of Burgdorf's notion, suggesting that it was sensitized T cells to circulating antigens that were the initiators of granuloma formation in the periphery.
An examination of MCD tissue in 1990 by Shum and Guenther17 under electron microscopy and immunofluorescence provided evidence against prior studies that purported to have identified immune complexes as the causative agents of MCD. In this study, the authors found no evidence of immune complexes in the dermis of MCD lesions. In addition, an attempt to react serum antibodies of a patient with MCD, which were postulated to have IgG, IgM, and IgA antibodies to specific gut antigens, yielded no response when reacted with the tongue, ileum, and colon tissue from a rat. As a culminant finding, the authors also noted MCD dermis tissue with granulomas without vasculitis, suggesting a T-cell mediated type IV hypersensitivity response with a secondary vasculitis from T-cell origin lymphokines and T-cell mediated monocyte activation.17
Research implicating other immunologic entities involved in the pathophysiology of CD such as β-2 integrin,18 CD14+ monocytes,19 and the role of the DNA repair gene MLH1 (mutL homolog 1)20 have been considered but without a clearly definitive role in the manifestations of MCD.
The utility of metronidazole in the treatment of MCD has been suggested as evidence that certain bacteria in the gut may either serve as the causative antigen or may induce its formation21; however, the causative antigen has yet to be identified, and whether it travels distally to the skin or merely resembles a similar antigen normally present in the dermis has not yet been determined. Some research has used in situ polymerase chain reaction techniques to attempt to detect similar microbial pathogens in both the vasculature of active bowel lesions and in the skin, but to date, bacterial RNA noted to be present in the gut vasculature adjacent to CD lesions has not been detected in skin lesions.22
Diagnosis
Physical Findings
Overall, it is estimated that roughly 56% of all MCD cases affect the external genitalia.23 The classic appearance of MCD includes well-demarcated ulcerations in the areas of intertriginous skin folds with or without diffuse edema and tenderness to palpation.23 Although MCD has been historically noted as having a predilection for moist skin folds, there are numerous case reports of MCD all over the body, including the face,7,24-29 retroauricular areas,30 arms and legs,16,17,31-34 lower abdomen,3,5 under the breasts,1 perineum,35 external genitalia,1,9,36-40 and even the lungs41 and bladder.42
As a dermatologic disease, MCD has been referred to as yet another great imitator, both on the macroscopic and microscopic levels.8 As such, more common causes of genital edema should be considered first and investigated based on the patient's history, physical examination, skin biopsy, lymphangiogram, ultrasound, and cystogram.43 Ultrasonography and color Doppler sonography have been shown to be helpful in patients with genital involvement. This modality can evaluate not only the presence of normal testes but also intratesticular and scrotal wall fluid, especially when the physical examination reveals swelling that makes testicle palpation more difficult.6 Clinically, the correct diagnosis of MCD often is made through suspicion of inflammatory bowel disease based on classic symptoms and/or physical findings including abdominal pain, weight loss, bloody stool, diarrhea, perianal skin tags, and anal fissures or fistulas. Any of these GI findings should prompt an intestinal biopsy to rule out any histologic evidence of CD.
Metastatic CD affecting the vulva often presents with vulvar pain and pruritus and may clinically mimic a more benign disease such as balanitis plasmacellularis, also referred to as Zoon vulvitis.23 Similar to MCD on any given body surface, there is dramatic variation in the macroscopic presentation of vulvar MCD, with physical examination findings ranging from bilateral diffuse, edematous, deeply macerated, red, ulcerated lesions over the vulva with lymphadenopathy to findings of bilateral vulvar pain with yellow drainage from the labia majora.23 There have been cases of vulvar MCD that include exquisite vulvar pain but without structural abnormalities including normal uterus, cervix, adnexa, rectovaginal septum, and rectum. In these more nebulous cases of vulvar MCD, the diagnosis often is discovered incidentally when nonspecific diagnostic imaging suggests underlying CD.23
Beyond the case-by-case variations on physical examination, the great difficulty in diagnosis, particularly in children, occurs in the absence of any GI symptoms and therefore no logical consideration of underlying CD. Consequently, there have been cases of children presenting with irritation of the vulva who were eventually diagnosed with MCD only after erroneous treatment of contact dermatitis, candidiasis, and even consideration of sexual abuse.37 Because it is so rare and obscure among practicing clinicians, the diagnosis of MCD often is considered only after irritation or swelling of the external genitalia has not responded to standard therapies. If and when the diagnosis of MCD is considered in children, it has been suggested to screen patients for anorectal stricture, as case studies have found the condition to be relatively common in this subpopulation.44
In the less common case of adults with genitourinary symptoms that suggest possible MCD, the differential diagnosis for penile or vaginal ulcers should include contact and irritant dermatitis, chronic infectious lesions (eg, hidradenitis suppurativa, actinomycosis, tuberculosis),45 sexually transmitted ulcerative diseases (eg, chancroid, lymphogranuloma venereum, herpes genitalia, granuloma inguinale),46 drug reactions, and even extramammary Paget disease.47
Histologic Findings
Because MCD has so much macroscopic variation and can present anywhere on the surface of the body, formal diagnosis relies on microscopy. As an added measure of difficulty in diagnosis, one random biopsy of a suspicious segment of tissue may not contain the expected histologic findings; therefore, clinical suspicion may warrant a second biopsy.10 There have been reported cases of an adult patient without history of CD presenting with a lesion that resembled a more common pathology, such as a genital wart, and the correct diagnosis of MCD with pseudocondylomatous morphology was made only after intestinal manifestations prompted the clinician to consider such an unusual diagnosis.48
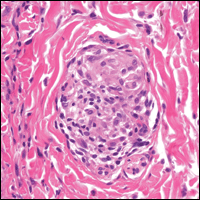
From a histopathologic perspective, MCD is characterized by discrete, noncaseating, sarcoidlike granulomas with abundant multinucleated giant cells (Langhans giant cells) in the superficial dermis (papillary), deep dermis (reticular), and adipose tissue (Figure).8,17 In the presence of concomitant intestinal disease, the granulomas of both the intestinal and dermal tissues should share the same microscopic characteristics.8 In addition, copious neutrophils and granulomas surrounding the microvasculature have been described,34 as well as general lymphocyte and plasma cell infiltrate.45 Some histologic samples have included collagen degeneration termed necrobiosis in the middle dermal layer as another variable finding in MCD.14,34
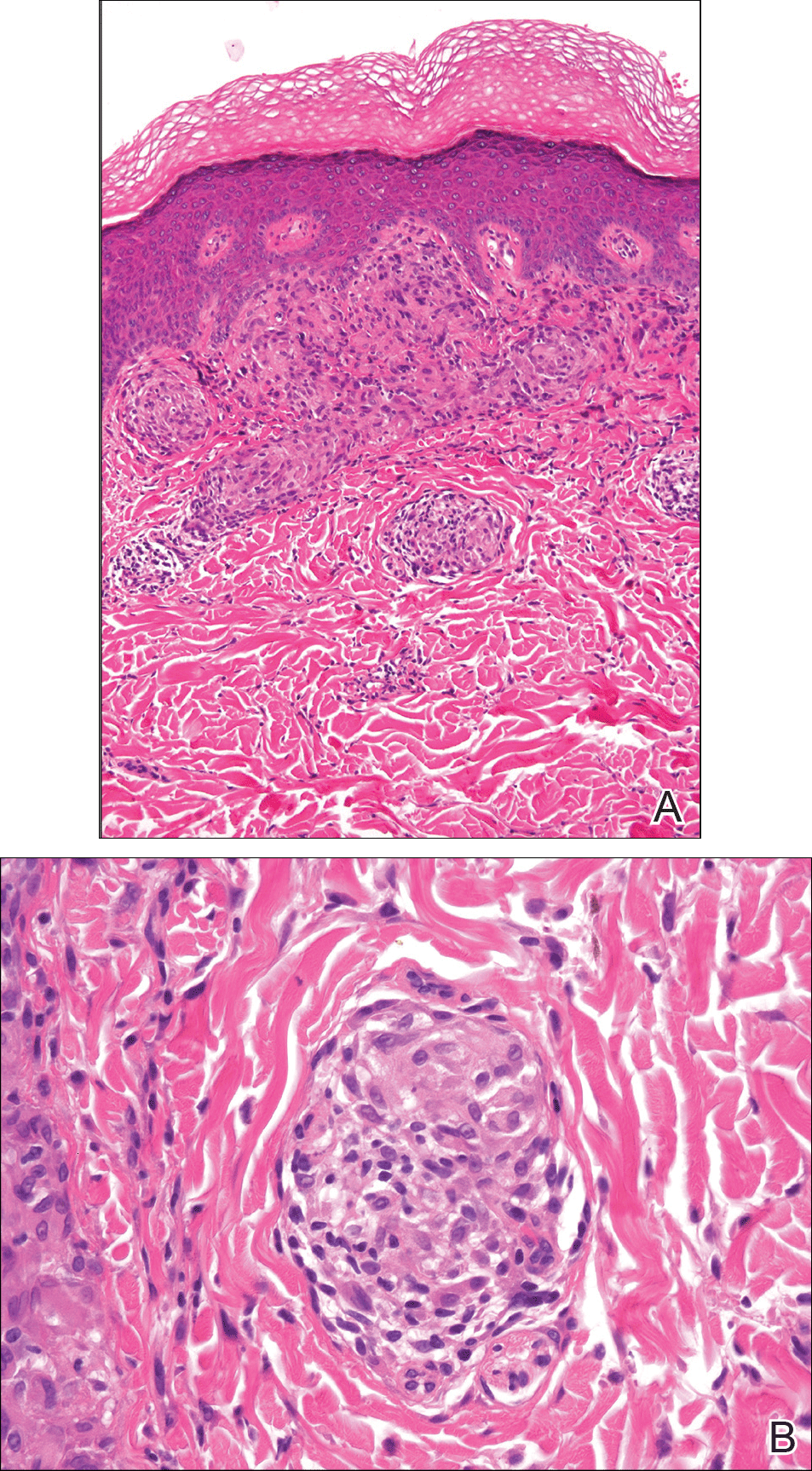
On microscopy, it has been reported that use of Verhoeff-van Gieson staining may be helpful to highlight the presence of neutrophil obstruction within the dermal vasculature, particularly the arterial lumen, as well as to aid in highlighting swelling of the endothelium with fragmentation of the internal elastic lamina.17 Although not part of the routine diagnosis, electron microscopy of MCD tissue samples have confirmed hypertrophy of the endothelial cells composing the capillaries with resulting extravasation of fibrin, red blood cells, lymphocytes, and epithelioid histiocytes.17 Observation of tissue under direct immunofluorescence has been less helpful, as it has shown only nonspecific fibrinogen deposition within the dermis and dermal vessels.17
In an article on treatment of MCD, Escher et al43 reinforced that the macroscopic findings of MCD are diverse, and the microscopic findings characteristic of MCD also can be mimicked by other etiologies such as sarcoidosis, tuberculosis, fungal infections, lymphogranuloma venereum, leishmaniasis, and connective tissue disorders.43 As such, the workup to rule out infectious, anatomic, and autoimmune etiologies should be diverse. Often, the workup for MCD will include special stains such as Ziehl-Neelsen stain to rule out Mycobacterium tuberculosis and acid-fast bacilli and Fite stain to consider atypical mycobacteria. Other tests such as tissue culture, chest radiograph, tuberculin skin test (Mantoux test), IFN-γ release assay, or polarized light microscopy may rule out infectious etiologies.9,49 Serologic testing might include VDRL test, Treponema pallidum hemagglutination assay, hepatitis B, hepatitis C, and human immunodeficiency virus.5
Crohn disease is characterized histologically by sarcoidlike noncaseating granulomas, and as such, it is important to differentiate MCD from sarcoidosis prior to histologic analysis. Sarcoidosis also can be considered much less likely with a normal chest radiograph and in the absence of increased serum calcium and angiotensin-converting enzyme levels.7 The differentiation of sarcoidosis from MCD on the microscopic scale is subtle but is sometimes facilitated in the presence of an ulcerated epidermis or lymphocytic/eosinophilic infiltrate and edema within the dermis, all suggestive of MCD.14
Metastatic CD also should be differentiated from erythema nodosum and pyoderma gangrenosum, which are among the most common cutaneous findings associated with CD.14 Pyoderma gangrenosum can be distinguished histologically by identifying copious neutrophilic infiltrate with pseudoepitheliomatous hyperplasia.50
Treatment
Because MCD is relatively rare, there are no known randomized trials suggesting a particular medical or surgical treatment. In a review of perineal MCD from 2007, the 40-year-old recommendation by Moutain3 opting for surgical debridement versus medical management still resonates, particularly for perineal disease, as an effective measure in all but the mildest of presentations.51 However, recent case reports also suggest that the tumor necrosis factor α (TNF-α) inhibitors such as infliximab and adalimumab should be considered prior to surgery even with severe perineal MCD.51 Moreover, even if medical management with TNF-α inhibitors or some combination of immunosuppressants and antibiotics does not eradicate the disease, it often helps reduce the size of the ulcers prior to surgery.52 With a limited understanding of MCD, one might think that removal of the affected bowel would eliminate cutaneous disease, but it has been shown that this strategy is not effective.53,54
The composition and location of the particular lesion affects the trajectory of treatment. For example, MCD manifesting as local ulcers and plaques has been described as responding well to topical and intralesional steroids.10,55,56 In the case of penile swelling and/or phimosis, circumcision has been helpful to improve the patient's ability to void as well as to attain and maintain erection.10 In the case of scrotal swelling secondary to MCD, early treatment (ie, within 4 to 6 months) with oral steroids and/or metronidazole is likely beneficial to prevent refractory edematous organization of the tissue.57
As a general rule, an effective treatment will include a combination of an immunosuppressant, antibiotic therapy, and sometimes surgery. The most commonly used immunosuppressant agents include topical or intralesional steroids, infliximab,43,58 cyclosporine A,59,60 dapsone, minocycline, thalidomide, methotrexate, mycophenolate mofetil, sulfasalazine, azathioprine, tacrolimus, and 6-mercaptopurine.4 Steroids have been the conventional treatment of extraintestinal manifestations of CD61; however, perineal CD has been poorly controlled with systemic steroids.62 If steroids are found not to be effective, sometimes agents such as dapsone or thalidomide are considered. One case report noted stabilization of MCD penile ulcers with oral thalidomide 300 mg once daily, oral minocycline 100 mg once daily, and topical tacrolimus 0.3% with benzocaine twice daily with continuation of prednisolone and methotrexate as parts of previously unsuccessful regimen.52
Metronidazole is perhaps the most commonly used antibiotic, having been a component of many successful regimens.4,63 For example, a 27-year-old patient with MCD presenting as a nonhealing ulcerative lesion in the subcoronal area of the penis and scrotum was treated successfully with a 6-month course of mesalamine, prednisone, and metronidazole.45 Another case report of vulvar MCD reported initial success with intravenous methylprednisolone, ciprofloxacin, and metronidazole.23 The primary limitation of metronidazole is that subsequent tapering of the dose seems to result in recurrence of disease.64 Consequently, patients must remain on the antibiotic for an indeterminate course, with dosages ranging from 5 mg/kg daily in adolescents65 to 1000 to 1500 mg daily in adults.66
Of the various immunosuppressants available, infliximab has been listed in numerous reports as a successful agent in both the induction and maintenance of extraintestinal manifestations of CD including MCD.67-71 Infliximab has been reported to be effective in the treatment of penile and scrotal edema secondary to MCD that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine.43 Infliximab may be a good option to help heal draining fistulas, particularly in combination with an antibiotic such as metronidazole and ciprofloxacin, which helps to prevent abscess formation during healing.72 The response to infliximab has been dramatic, with resolution of cutaneous lesions after just 6 weeks in some cases.73 The dosing regimen of infliximab has been suggested at 5 mg/kg administered at 0, 2, and 6 weeks, with subsequent maintenance infusions every 10 weeks,70 or at 0, 4, and 12 weeks, with subsequent infusions every 8 weeks.43
Adalimumab may be considered as an alternative to infliximab and is potentially less allergenic as a fully humanized monoclonal antibody to TNF-α, which also has been used successfully to both induce and maintain remission of moderate to severe CD.42,74,75 Proposed dosing of adalimumab includes a loading dose of 160 mg subcutaneously on day 1, followed by an 80-mg dose 2 weeks later and a 40-mg maintenance dose every other week indefinitely.48 Of note, adalimumab has been noted in the literature to have many potential side effects, including one particular case in which severe headaches were attributed to its use.59 As a consequence of the headaches, the patient was switched from adalimumab to cyclosporine and responded well with no subsequent flare-ups on follow-up.
In summary, treatment of MCD depends on cutaneous location, severity, physician experience with certain antibiotics or immunosuppressants, availability of medication, and patient disposition. It seems reasonable to attempt medical management with one or more medical regimens before committing to surgical intervention. Furthermore, even with debridement, curettage, skin graft, or other surgical strategy, the patient is likely to require some period of immunosuppression to provide long-lasting remission.
Conclusion
Patients with inflammatory bowel disease often develop dermatologic sequelae, with MCD being a rare but serious process. Patients may present with a wide array of physical concerns and symptoms, many resembling other disease processes. As such, education and a high index of suspicion are needed for proper diagnosis and treatment.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Friedman S, Blumber RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005:1778-1784.
- Moutain JC. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18-26.
- Lester LU, Rapini RP. Dermatologic manifestations of colonic disorders. Curr Opin Gastroenterol. 2008;25:66-73.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Simoneaux SF, Ball TI, Atkinson GO Jr. Scrotal swelling: unusual first presentation of Crohn's disease. Pediatr Radiol. 1995;25:375-376.
- Albuquerque A, Magro F, Rodrigues S, et al. Metastatic cutaneous Crohn's disease of the face: a case report and review of literature. Eur J Gastroenterol Hepatol. 2011;23:954-956.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Vint R, Husain E, Hassain F, et al. Metastatic Crohn's disease of the penis: two cases. Int Urol Nephrol. 2012;44:45-49.
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol. 1981;5:689-695.
- Resnick MI, Kursh ED. Extrinsic obstruction of the ureter. In: Walsh PC, Retik AB, Stamey TA, et al, eds. Campbell's Urology. 7th ed. Philadelphia, PA: WB Saunders; 1998:400-402.
- Samitz MH, Dana AS Jr, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease--review of statistics of skin complications. Cutis. 1970;6:51-56.
- Emanuel PO, Phelps RG. Metastatic Crohn's disease: a histo-pathologic study of 12 cases. J Cutan Pathol. 2008;35:457-461.
- Slater DN, Waller PC, Reilly G. Cutaneous granulomatous vasculitis: presenting features of Crohn's disease. J R Soc Med. 1985;78:589-590.
- Tatnall FM, Dodd HJ, Sarkany I. Crohn's disease with metastatic cutaneous involvement and granulomatous cheilitis. J R Soc Med. 1987;80:49-51.
- Shum DT, Guenther L. Metastatic Crohn's disease: case report and review of literature. Arch Dermatol. 1990;126:645-648.
- Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn's disease. Clin Immunol Immunopathol. 1998;86:147-160.
- Grimm MC, Pavli P, Van de Pol E, et al. Evidence for a CD-14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297.
- Pokorny RM, Hofmeister A, Galandiuk S, et al. Crohn's disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725.
- Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn's disease, its spectrum, and pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn's disease. Hum Pathol. 2003;34:1185.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn's disease. Dig Dis Sci. 2009;54:1565-1571.
- Chen W, Blume-Peytavi U, Goerdt S, et al. Metastatic Crohn's disease of the face. J Am Acad Dermatol. 1996;35:986-988.
- Ogram AE, Sobanko JF, Nigra TP. Metastatic cutaneous Crohn disease of the face: a case report and review of the literature. Cutis. 2010;85:25-27.
- Graham D, Jager D, Borum M. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062-2063.
- Biancone L, Geboes K, Spagnoli LG, et al. Metastatic Crohn's disease of the forehead. Inflamm Bowel Dis. 2002;8:101-105.
- Kolansky G, Green CK, Dubin H. Metastatic Crohn's disease of the face: an uncommon presentation. Arch Dermatol. 1993;129:1348-1349.
- Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38-42.
- McCallum DI, Gray WM. Metastatic Crohn's disease. Br J Dermatol. 1976;95:551-554.
- Lieberman TR, Greene JF Jr. Transient subcutaneous granulomatosis of the upper extremities in Crohn's disease. Am J Gastroenterol. 1979;72:89-91.
- Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn's disease: a rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300-303.
- Marotta PJ, Reynolds RP. Metastatic Crohn's disease. Am J Gastroenterol. 1996;91:373-375.
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn's disease. report of 3 cases with special reference to histopathologic findings. Arch Dermatol. 1996;132:928-932.
- van Dulleman HM, de Jong E, Slors F, et al. Treatment of therapy resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: report of two cases. Dis Colon Rectum. 1998;41:98-102.
- Lavery HA, Pinkerton JH, Sloan J. Crohn's disease of the vulva--two further cases. Br J Dermatol. 1985;113:359-363.
- Lally MR, Orenstein SR, Cohen BA. Crohn's disease of the vulva in an 8-year-old girl. Pediatr Dermatol. 1988;5:103-106.
- Tuffnell D, Buchan PC. Crohn's disease of the vulva in childhood. Br J Clin Pract. 1991;45:159-160.
- Schrodt BJ, Callen JP. Metastatic Crohn's disease presenting as chronic perivulvar and perirectal ulcerations in an adolescent patient. Pediatrics. 1999;103:500-502.
- Slaney G, Muller S, Clay J, et al. Crohn's disease involving the penis. Gut. 1986;27:329-333.
- Calder CJ, Lacy D, Raafat F, et al. Crohn's disease with pulmonary involvement in a 3 year old boy. Gut. 1993;34:1636-1638.
- Saha S, Fichera A, Bales G, et al. Metastatic Crohn's disease of the bladder. Inflamm Bowel Dis. 2008;14:140-142.
- Escher JC, Stoof TJ, van Deventer SJ, et al. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420-423.
- Saadah OI, Oliver MR, Bines JE, et al. Anorectal strictures and genital Crohn's disease: an unusual clinical association. J Pediatr Gastroenterol Nutr. 2003;36:403-406.
- Martinez-Salamanca JI, Jara J, Miralles P, et al. Metastatic Crohn's disease: penile and scrotal involvement. Scand J Urol Nephrol. 2004;38:436-437.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429.
- Acker SM, Sahn EE, Rogers HC, et al. Genital cutaneous Crohn disease. Am J Dermatopathol. 2000;22:443-446.
- Lestre S, Ramos J, Joao A, et al. Cutaneous Crohn's disease presenting as genital warts: successful treatment with adalimumab. Eur J Dermatol. 2010;20:504-505.
- Yu JT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467-469.
- Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol. 1988;18:511-521.
- Moyes LH, Glen P, Pickford IR. Perineal metastatic Crohn's disease: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W1-W3.
- Rajpara SM, Siddha SK, Ormerod AD, et al. Cutaneous penile and perianal Crohn's disease treated with a combination of medical and surgical interventions. Australas J Dermatol. 2008;49:21-24.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764-1766.
- Sangueza OP, Davis LS, Gourdin FW. Metastatic Crohn disease. South Med J. 1997;90:897-900.
- Chiba M, Iizuka M, Horie Y, et al. Metastatic Crohn's disease involving the penis. J Gastroenterol. 1997;32:817-821.
- Poon KS, Gilks CB, Masterson JS. Metastatic Crohn's disease involving the genitalia. J Urol. 2002;167:2541-2542.
- Shanahan F. Anti-TNF therapy for Crohn's disease: a perspective (infliximab is not the drug we have been waiting for). Inflamm Bowel Dis. 2000;6:137-139.
- Carranza DC, Young L. Successful treatment of metastatic Crohn's disease with cyclosporine. J Drugs Dermatol. 2008;7:789-791.
- Bardazzi F, Guidetti MS, Passarini B, et al. Cyclosporine A in metastatic Crohn's disease. Acta Derm Venereol. 1995;75:324-325.
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260.
- Gelbmann CM, Rogler G, Gross V, et al. Prior bowel resections, perianal disease, and a high initial Crohn's disease activity index are associated with corticosteroid resistance in active Crohn's disease. Am J Gastroenterol. 2002;97:1438-1445.
- Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223-228.
- Brandt LJ, Berstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology. 1982;83:383-387.
- Lehrnbecher T, Kontny HU, Jeschke R. Metastatic Crohn's disease in a 9-year-old boy. J Pediatr Gastroenterol Nutr. 1999;28:321-323.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387-391.
- Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406-410.
- Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351-356.
- Miller AM, Elliott PR, Fink R, et al. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940-942.
- Chuah JH, Kim DS, Allen C, et al. Metastatic Crohn's disease of the ear. Int J Otolaryngol. 2009;2009:871567.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405.
- Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous metastatic Crohn's disease responsive to infliximab. Am J Gastroenterol. 2009;104:1058.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333.
- Cury DB, Moss A, Elias G, et al. Adalimumab for cutaneous metastatic Crohn's disease. Inflamm Bowel Dis. 2010;16:723-724.
Almost half of Crohn disease (CD) patients experience a dermatologic manifestation of the disease. A rare entity, metastatic CD (MCD) presents a diagnostic challenge without a high index of suspicion. Its etiology is not well defined; however, it appears to be an autoimmune response to gut antigens. Herein, we review the etiology/epidemiology, diagnostic criteria, and treatment for this uncommon condition.
Epidemiology and Clinical Characteristics of MCD
Metastatic CD was first described by Parks et al1 in 1965 and refers to a diverse collection of macroscopic dermatologic manifestations in tissue not contiguous with the gastrointestinal (GI) tract. To be classified as MCD, the tissue must demonstrate characteristic histopathologic findings, which invariably include noncaseating granulomas.
Crohn disease may affect any part of the GI tract from the mouth to anus, with a multitude of associated cutaneous manifestations having been described. The terminal ileum is the most commonly affected portion of the GI tract in CD, but the large intestine also may be involved in 55% to 80% of cases.2 The incidence of non-MCD-associated anal lesions seems to correlate with intestinal involvement in that as few as 25% of patients with ileal-localized CD have anal lesions compared to nearly 80% of patients with large intestinal involvement.3
It has been estimated that 18% to 44% of patients with CD have some form of cutaneous manifestation,4 with MCD being a rare subcategory. As few as 100 cases have been described from 1965 to the present.5 The presence of MCD does not correlate well with severity of intestinal CD, and although a majority of MCD cases present after at least 6 months of GI symptoms,6 there are instances in which MCD presents without prior or existing evidence of intestinal CD.7
With regard to MCD, the term metastatic is sometimes supplanted in the literature by cutaneous to avoid any implication of cancer; however, due to a myriad of dermatologic manifestations, both terms can cause confusion. The categorization of the various types of cutaneous findings in CD is well summarized in a review by Palamaras et al8 with the following classifications: (1) granulomatous by direct extension (oral or perianal), (2) MCD lesions (genital and nongenital), (3) immune-related lesions, and (4) lesions from nutritional deficiencies. Of the cutaneous manifestations relating to CD, MCD is the least common cutaneous categorical manifestation and is further divided into subcategories of genital and nongenital lesions.8
The nongenital distribution of MCD is the more common variety in adults and particularly seems to affect the legs and plantar surfaces (38%), the trunk and abdomen (24%), and the face (15%).5,9 These nongenital MCD manifestations are most commonly described as nodules, ulcerations, or erythematous to purple plaques, and less commonly described as abscesses, pustules, or papules.
The sequence of cutaneous symptoms of MCD relative to intestinal disease depends to some degree on patient age. In adults diagnosed with MCD, it has been noted that a GI flare is expected 2 months to 4 years after diagnosis; however, in children the subsequent GI flare has been noted to vary more widely from 9 months to 14 years following presentation of MCD.8 Furthermore, roughly 50% of children diagnosed with MCD present concomitantly with their first symptoms of a GI flare, whereas 70% of adults with MCD had been previously diagnosed with intestinal CD.8 In one review of 80 reported cases of MCD, 20% (16/80) had no symptoms of intestinal disease at the time of MCD diagnosis, and the majority of the asymptomatic cases were in children; interestingly, the majority of these same children were diagnosed with CD months to years later.9
Both the location and characteristics of cutaneous findings in MCD correlate with age.9 Metastatic CD has been identified in all age groups; however, lymphedema is more common in children/young adults, while nodules, ulceration, and fistulating disease are more often seen in adults.10 Affected children and adolescents with MCD range from 5 to 17 years of age, with a mean age at disease onset of 11.1 years and equal incidence in males and females.8 Adults with MCD range from 18 to 78 years of age, with a mean age at presentation of 38.4 years.8,11
Concerning anatomic location of disease, adults with MCD most commonly have nodules with or without plaques on the arms and legs and less commonly in the genital area.8 In contrast, children with MCD are more prone to genital lesions, with up to 85% of cases including some degree of genital erythematous or nonerythematous swelling with or without induration.8 Genitourinary complications of CD as a broad category, however, are estimated to occur in only 5% to 20% of intestinal CD cases in both children and adults.12
There have been conflicting reports regarding gender predilection in MCD. Based on a review by Samitz et al13 of 200 cases of CD over an 18-year period, 22% of patients with CD were found to have cutaneous manifestations--presumably not MCD but rather perianal, perineal, vulvar fistulae, fissures, or abscesses--with a male to female preponderance of almost 2 to 1. A more recent review of the literature by Palamaras et al8 in 2008 reported that contiguous non-MCD affects adult females and children more often than adult males, with 63% adult cases being female. This review seems to be more congruent with other reports in the literature implicating that females are twice as commonly affected by MCD than males.9,14
Pathophysiology
The etiology of MCD has not been well defined. One proposed mechanism of the distal tissue involvement of MCD is through passage of antigens to the skin with subsequent granulomatous response at the level of the dermis.10 Another proposed mechanism suggests antibody sensitization to gut antigens, possibly bacterial antigens, that then coincidentally cross-react with analogous skin antigens.8,14 Burgdorf11 supported this notion in a 1981 report in which it was suggested that the granulomatous reaction was related to deposition of immune complexes in the skin. Slater et al15 and Tatnall et al16 offered a variation of Burgdorf's notion, suggesting that it was sensitized T cells to circulating antigens that were the initiators of granuloma formation in the periphery.
An examination of MCD tissue in 1990 by Shum and Guenther17 under electron microscopy and immunofluorescence provided evidence against prior studies that purported to have identified immune complexes as the causative agents of MCD. In this study, the authors found no evidence of immune complexes in the dermis of MCD lesions. In addition, an attempt to react serum antibodies of a patient with MCD, which were postulated to have IgG, IgM, and IgA antibodies to specific gut antigens, yielded no response when reacted with the tongue, ileum, and colon tissue from a rat. As a culminant finding, the authors also noted MCD dermis tissue with granulomas without vasculitis, suggesting a T-cell mediated type IV hypersensitivity response with a secondary vasculitis from T-cell origin lymphokines and T-cell mediated monocyte activation.17
Research implicating other immunologic entities involved in the pathophysiology of CD such as β-2 integrin,18 CD14+ monocytes,19 and the role of the DNA repair gene MLH1 (mutL homolog 1)20 have been considered but without a clearly definitive role in the manifestations of MCD.
The utility of metronidazole in the treatment of MCD has been suggested as evidence that certain bacteria in the gut may either serve as the causative antigen or may induce its formation21; however, the causative antigen has yet to be identified, and whether it travels distally to the skin or merely resembles a similar antigen normally present in the dermis has not yet been determined. Some research has used in situ polymerase chain reaction techniques to attempt to detect similar microbial pathogens in both the vasculature of active bowel lesions and in the skin, but to date, bacterial RNA noted to be present in the gut vasculature adjacent to CD lesions has not been detected in skin lesions.22
Diagnosis
Physical Findings
Overall, it is estimated that roughly 56% of all MCD cases affect the external genitalia.23 The classic appearance of MCD includes well-demarcated ulcerations in the areas of intertriginous skin folds with or without diffuse edema and tenderness to palpation.23 Although MCD has been historically noted as having a predilection for moist skin folds, there are numerous case reports of MCD all over the body, including the face,7,24-29 retroauricular areas,30 arms and legs,16,17,31-34 lower abdomen,3,5 under the breasts,1 perineum,35 external genitalia,1,9,36-40 and even the lungs41 and bladder.42
As a dermatologic disease, MCD has been referred to as yet another great imitator, both on the macroscopic and microscopic levels.8 As such, more common causes of genital edema should be considered first and investigated based on the patient's history, physical examination, skin biopsy, lymphangiogram, ultrasound, and cystogram.43 Ultrasonography and color Doppler sonography have been shown to be helpful in patients with genital involvement. This modality can evaluate not only the presence of normal testes but also intratesticular and scrotal wall fluid, especially when the physical examination reveals swelling that makes testicle palpation more difficult.6 Clinically, the correct diagnosis of MCD often is made through suspicion of inflammatory bowel disease based on classic symptoms and/or physical findings including abdominal pain, weight loss, bloody stool, diarrhea, perianal skin tags, and anal fissures or fistulas. Any of these GI findings should prompt an intestinal biopsy to rule out any histologic evidence of CD.
Metastatic CD affecting the vulva often presents with vulvar pain and pruritus and may clinically mimic a more benign disease such as balanitis plasmacellularis, also referred to as Zoon vulvitis.23 Similar to MCD on any given body surface, there is dramatic variation in the macroscopic presentation of vulvar MCD, with physical examination findings ranging from bilateral diffuse, edematous, deeply macerated, red, ulcerated lesions over the vulva with lymphadenopathy to findings of bilateral vulvar pain with yellow drainage from the labia majora.23 There have been cases of vulvar MCD that include exquisite vulvar pain but without structural abnormalities including normal uterus, cervix, adnexa, rectovaginal septum, and rectum. In these more nebulous cases of vulvar MCD, the diagnosis often is discovered incidentally when nonspecific diagnostic imaging suggests underlying CD.23
Beyond the case-by-case variations on physical examination, the great difficulty in diagnosis, particularly in children, occurs in the absence of any GI symptoms and therefore no logical consideration of underlying CD. Consequently, there have been cases of children presenting with irritation of the vulva who were eventually diagnosed with MCD only after erroneous treatment of contact dermatitis, candidiasis, and even consideration of sexual abuse.37 Because it is so rare and obscure among practicing clinicians, the diagnosis of MCD often is considered only after irritation or swelling of the external genitalia has not responded to standard therapies. If and when the diagnosis of MCD is considered in children, it has been suggested to screen patients for anorectal stricture, as case studies have found the condition to be relatively common in this subpopulation.44
In the less common case of adults with genitourinary symptoms that suggest possible MCD, the differential diagnosis for penile or vaginal ulcers should include contact and irritant dermatitis, chronic infectious lesions (eg, hidradenitis suppurativa, actinomycosis, tuberculosis),45 sexually transmitted ulcerative diseases (eg, chancroid, lymphogranuloma venereum, herpes genitalia, granuloma inguinale),46 drug reactions, and even extramammary Paget disease.47
Histologic Findings
Because MCD has so much macroscopic variation and can present anywhere on the surface of the body, formal diagnosis relies on microscopy. As an added measure of difficulty in diagnosis, one random biopsy of a suspicious segment of tissue may not contain the expected histologic findings; therefore, clinical suspicion may warrant a second biopsy.10 There have been reported cases of an adult patient without history of CD presenting with a lesion that resembled a more common pathology, such as a genital wart, and the correct diagnosis of MCD with pseudocondylomatous morphology was made only after intestinal manifestations prompted the clinician to consider such an unusual diagnosis.48
From a histopathologic perspective, MCD is characterized by discrete, noncaseating, sarcoidlike granulomas with abundant multinucleated giant cells (Langhans giant cells) in the superficial dermis (papillary), deep dermis (reticular), and adipose tissue (Figure).8,17 In the presence of concomitant intestinal disease, the granulomas of both the intestinal and dermal tissues should share the same microscopic characteristics.8 In addition, copious neutrophils and granulomas surrounding the microvasculature have been described,34 as well as general lymphocyte and plasma cell infiltrate.45 Some histologic samples have included collagen degeneration termed necrobiosis in the middle dermal layer as another variable finding in MCD.14,34

On microscopy, it has been reported that use of Verhoeff-van Gieson staining may be helpful to highlight the presence of neutrophil obstruction within the dermal vasculature, particularly the arterial lumen, as well as to aid in highlighting swelling of the endothelium with fragmentation of the internal elastic lamina.17 Although not part of the routine diagnosis, electron microscopy of MCD tissue samples have confirmed hypertrophy of the endothelial cells composing the capillaries with resulting extravasation of fibrin, red blood cells, lymphocytes, and epithelioid histiocytes.17 Observation of tissue under direct immunofluorescence has been less helpful, as it has shown only nonspecific fibrinogen deposition within the dermis and dermal vessels.17
In an article on treatment of MCD, Escher et al43 reinforced that the macroscopic findings of MCD are diverse, and the microscopic findings characteristic of MCD also can be mimicked by other etiologies such as sarcoidosis, tuberculosis, fungal infections, lymphogranuloma venereum, leishmaniasis, and connective tissue disorders.43 As such, the workup to rule out infectious, anatomic, and autoimmune etiologies should be diverse. Often, the workup for MCD will include special stains such as Ziehl-Neelsen stain to rule out Mycobacterium tuberculosis and acid-fast bacilli and Fite stain to consider atypical mycobacteria. Other tests such as tissue culture, chest radiograph, tuberculin skin test (Mantoux test), IFN-γ release assay, or polarized light microscopy may rule out infectious etiologies.9,49 Serologic testing might include VDRL test, Treponema pallidum hemagglutination assay, hepatitis B, hepatitis C, and human immunodeficiency virus.5
Crohn disease is characterized histologically by sarcoidlike noncaseating granulomas, and as such, it is important to differentiate MCD from sarcoidosis prior to histologic analysis. Sarcoidosis also can be considered much less likely with a normal chest radiograph and in the absence of increased serum calcium and angiotensin-converting enzyme levels.7 The differentiation of sarcoidosis from MCD on the microscopic scale is subtle but is sometimes facilitated in the presence of an ulcerated epidermis or lymphocytic/eosinophilic infiltrate and edema within the dermis, all suggestive of MCD.14
Metastatic CD also should be differentiated from erythema nodosum and pyoderma gangrenosum, which are among the most common cutaneous findings associated with CD.14 Pyoderma gangrenosum can be distinguished histologically by identifying copious neutrophilic infiltrate with pseudoepitheliomatous hyperplasia.50
Treatment
Because MCD is relatively rare, there are no known randomized trials suggesting a particular medical or surgical treatment. In a review of perineal MCD from 2007, the 40-year-old recommendation by Moutain3 opting for surgical debridement versus medical management still resonates, particularly for perineal disease, as an effective measure in all but the mildest of presentations.51 However, recent case reports also suggest that the tumor necrosis factor α (TNF-α) inhibitors such as infliximab and adalimumab should be considered prior to surgery even with severe perineal MCD.51 Moreover, even if medical management with TNF-α inhibitors or some combination of immunosuppressants and antibiotics does not eradicate the disease, it often helps reduce the size of the ulcers prior to surgery.52 With a limited understanding of MCD, one might think that removal of the affected bowel would eliminate cutaneous disease, but it has been shown that this strategy is not effective.53,54
The composition and location of the particular lesion affects the trajectory of treatment. For example, MCD manifesting as local ulcers and plaques has been described as responding well to topical and intralesional steroids.10,55,56 In the case of penile swelling and/or phimosis, circumcision has been helpful to improve the patient's ability to void as well as to attain and maintain erection.10 In the case of scrotal swelling secondary to MCD, early treatment (ie, within 4 to 6 months) with oral steroids and/or metronidazole is likely beneficial to prevent refractory edematous organization of the tissue.57
As a general rule, an effective treatment will include a combination of an immunosuppressant, antibiotic therapy, and sometimes surgery. The most commonly used immunosuppressant agents include topical or intralesional steroids, infliximab,43,58 cyclosporine A,59,60 dapsone, minocycline, thalidomide, methotrexate, mycophenolate mofetil, sulfasalazine, azathioprine, tacrolimus, and 6-mercaptopurine.4 Steroids have been the conventional treatment of extraintestinal manifestations of CD61; however, perineal CD has been poorly controlled with systemic steroids.62 If steroids are found not to be effective, sometimes agents such as dapsone or thalidomide are considered. One case report noted stabilization of MCD penile ulcers with oral thalidomide 300 mg once daily, oral minocycline 100 mg once daily, and topical tacrolimus 0.3% with benzocaine twice daily with continuation of prednisolone and methotrexate as parts of previously unsuccessful regimen.52
Metronidazole is perhaps the most commonly used antibiotic, having been a component of many successful regimens.4,63 For example, a 27-year-old patient with MCD presenting as a nonhealing ulcerative lesion in the subcoronal area of the penis and scrotum was treated successfully with a 6-month course of mesalamine, prednisone, and metronidazole.45 Another case report of vulvar MCD reported initial success with intravenous methylprednisolone, ciprofloxacin, and metronidazole.23 The primary limitation of metronidazole is that subsequent tapering of the dose seems to result in recurrence of disease.64 Consequently, patients must remain on the antibiotic for an indeterminate course, with dosages ranging from 5 mg/kg daily in adolescents65 to 1000 to 1500 mg daily in adults.66
Of the various immunosuppressants available, infliximab has been listed in numerous reports as a successful agent in both the induction and maintenance of extraintestinal manifestations of CD including MCD.67-71 Infliximab has been reported to be effective in the treatment of penile and scrotal edema secondary to MCD that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine.43 Infliximab may be a good option to help heal draining fistulas, particularly in combination with an antibiotic such as metronidazole and ciprofloxacin, which helps to prevent abscess formation during healing.72 The response to infliximab has been dramatic, with resolution of cutaneous lesions after just 6 weeks in some cases.73 The dosing regimen of infliximab has been suggested at 5 mg/kg administered at 0, 2, and 6 weeks, with subsequent maintenance infusions every 10 weeks,70 or at 0, 4, and 12 weeks, with subsequent infusions every 8 weeks.43
Adalimumab may be considered as an alternative to infliximab and is potentially less allergenic as a fully humanized monoclonal antibody to TNF-α, which also has been used successfully to both induce and maintain remission of moderate to severe CD.42,74,75 Proposed dosing of adalimumab includes a loading dose of 160 mg subcutaneously on day 1, followed by an 80-mg dose 2 weeks later and a 40-mg maintenance dose every other week indefinitely.48 Of note, adalimumab has been noted in the literature to have many potential side effects, including one particular case in which severe headaches were attributed to its use.59 As a consequence of the headaches, the patient was switched from adalimumab to cyclosporine and responded well with no subsequent flare-ups on follow-up.
In summary, treatment of MCD depends on cutaneous location, severity, physician experience with certain antibiotics or immunosuppressants, availability of medication, and patient disposition. It seems reasonable to attempt medical management with one or more medical regimens before committing to surgical intervention. Furthermore, even with debridement, curettage, skin graft, or other surgical strategy, the patient is likely to require some period of immunosuppression to provide long-lasting remission.
Conclusion
Patients with inflammatory bowel disease often develop dermatologic sequelae, with MCD being a rare but serious process. Patients may present with a wide array of physical concerns and symptoms, many resembling other disease processes. As such, education and a high index of suspicion are needed for proper diagnosis and treatment.
Almost half of Crohn disease (CD) patients experience a dermatologic manifestation of the disease. A rare entity, metastatic CD (MCD) presents a diagnostic challenge without a high index of suspicion. Its etiology is not well defined; however, it appears to be an autoimmune response to gut antigens. Herein, we review the etiology/epidemiology, diagnostic criteria, and treatment for this uncommon condition.
Epidemiology and Clinical Characteristics of MCD
Metastatic CD was first described by Parks et al1 in 1965 and refers to a diverse collection of macroscopic dermatologic manifestations in tissue not contiguous with the gastrointestinal (GI) tract. To be classified as MCD, the tissue must demonstrate characteristic histopathologic findings, which invariably include noncaseating granulomas.
Crohn disease may affect any part of the GI tract from the mouth to anus, with a multitude of associated cutaneous manifestations having been described. The terminal ileum is the most commonly affected portion of the GI tract in CD, but the large intestine also may be involved in 55% to 80% of cases.2 The incidence of non-MCD-associated anal lesions seems to correlate with intestinal involvement in that as few as 25% of patients with ileal-localized CD have anal lesions compared to nearly 80% of patients with large intestinal involvement.3
It has been estimated that 18% to 44% of patients with CD have some form of cutaneous manifestation,4 with MCD being a rare subcategory. As few as 100 cases have been described from 1965 to the present.5 The presence of MCD does not correlate well with severity of intestinal CD, and although a majority of MCD cases present after at least 6 months of GI symptoms,6 there are instances in which MCD presents without prior or existing evidence of intestinal CD.7
With regard to MCD, the term metastatic is sometimes supplanted in the literature by cutaneous to avoid any implication of cancer; however, due to a myriad of dermatologic manifestations, both terms can cause confusion. The categorization of the various types of cutaneous findings in CD is well summarized in a review by Palamaras et al8 with the following classifications: (1) granulomatous by direct extension (oral or perianal), (2) MCD lesions (genital and nongenital), (3) immune-related lesions, and (4) lesions from nutritional deficiencies. Of the cutaneous manifestations relating to CD, MCD is the least common cutaneous categorical manifestation and is further divided into subcategories of genital and nongenital lesions.8
The nongenital distribution of MCD is the more common variety in adults and particularly seems to affect the legs and plantar surfaces (38%), the trunk and abdomen (24%), and the face (15%).5,9 These nongenital MCD manifestations are most commonly described as nodules, ulcerations, or erythematous to purple plaques, and less commonly described as abscesses, pustules, or papules.
The sequence of cutaneous symptoms of MCD relative to intestinal disease depends to some degree on patient age. In adults diagnosed with MCD, it has been noted that a GI flare is expected 2 months to 4 years after diagnosis; however, in children the subsequent GI flare has been noted to vary more widely from 9 months to 14 years following presentation of MCD.8 Furthermore, roughly 50% of children diagnosed with MCD present concomitantly with their first symptoms of a GI flare, whereas 70% of adults with MCD had been previously diagnosed with intestinal CD.8 In one review of 80 reported cases of MCD, 20% (16/80) had no symptoms of intestinal disease at the time of MCD diagnosis, and the majority of the asymptomatic cases were in children; interestingly, the majority of these same children were diagnosed with CD months to years later.9
Both the location and characteristics of cutaneous findings in MCD correlate with age.9 Metastatic CD has been identified in all age groups; however, lymphedema is more common in children/young adults, while nodules, ulceration, and fistulating disease are more often seen in adults.10 Affected children and adolescents with MCD range from 5 to 17 years of age, with a mean age at disease onset of 11.1 years and equal incidence in males and females.8 Adults with MCD range from 18 to 78 years of age, with a mean age at presentation of 38.4 years.8,11
Concerning anatomic location of disease, adults with MCD most commonly have nodules with or without plaques on the arms and legs and less commonly in the genital area.8 In contrast, children with MCD are more prone to genital lesions, with up to 85% of cases including some degree of genital erythematous or nonerythematous swelling with or without induration.8 Genitourinary complications of CD as a broad category, however, are estimated to occur in only 5% to 20% of intestinal CD cases in both children and adults.12
There have been conflicting reports regarding gender predilection in MCD. Based on a review by Samitz et al13 of 200 cases of CD over an 18-year period, 22% of patients with CD were found to have cutaneous manifestations--presumably not MCD but rather perianal, perineal, vulvar fistulae, fissures, or abscesses--with a male to female preponderance of almost 2 to 1. A more recent review of the literature by Palamaras et al8 in 2008 reported that contiguous non-MCD affects adult females and children more often than adult males, with 63% adult cases being female. This review seems to be more congruent with other reports in the literature implicating that females are twice as commonly affected by MCD than males.9,14
Pathophysiology
The etiology of MCD has not been well defined. One proposed mechanism of the distal tissue involvement of MCD is through passage of antigens to the skin with subsequent granulomatous response at the level of the dermis.10 Another proposed mechanism suggests antibody sensitization to gut antigens, possibly bacterial antigens, that then coincidentally cross-react with analogous skin antigens.8,14 Burgdorf11 supported this notion in a 1981 report in which it was suggested that the granulomatous reaction was related to deposition of immune complexes in the skin. Slater et al15 and Tatnall et al16 offered a variation of Burgdorf's notion, suggesting that it was sensitized T cells to circulating antigens that were the initiators of granuloma formation in the periphery.
An examination of MCD tissue in 1990 by Shum and Guenther17 under electron microscopy and immunofluorescence provided evidence against prior studies that purported to have identified immune complexes as the causative agents of MCD. In this study, the authors found no evidence of immune complexes in the dermis of MCD lesions. In addition, an attempt to react serum antibodies of a patient with MCD, which were postulated to have IgG, IgM, and IgA antibodies to specific gut antigens, yielded no response when reacted with the tongue, ileum, and colon tissue from a rat. As a culminant finding, the authors also noted MCD dermis tissue with granulomas without vasculitis, suggesting a T-cell mediated type IV hypersensitivity response with a secondary vasculitis from T-cell origin lymphokines and T-cell mediated monocyte activation.17
Research implicating other immunologic entities involved in the pathophysiology of CD such as β-2 integrin,18 CD14+ monocytes,19 and the role of the DNA repair gene MLH1 (mutL homolog 1)20 have been considered but without a clearly definitive role in the manifestations of MCD.
The utility of metronidazole in the treatment of MCD has been suggested as evidence that certain bacteria in the gut may either serve as the causative antigen or may induce its formation21; however, the causative antigen has yet to be identified, and whether it travels distally to the skin or merely resembles a similar antigen normally present in the dermis has not yet been determined. Some research has used in situ polymerase chain reaction techniques to attempt to detect similar microbial pathogens in both the vasculature of active bowel lesions and in the skin, but to date, bacterial RNA noted to be present in the gut vasculature adjacent to CD lesions has not been detected in skin lesions.22
Diagnosis
Physical Findings
Overall, it is estimated that roughly 56% of all MCD cases affect the external genitalia.23 The classic appearance of MCD includes well-demarcated ulcerations in the areas of intertriginous skin folds with or without diffuse edema and tenderness to palpation.23 Although MCD has been historically noted as having a predilection for moist skin folds, there are numerous case reports of MCD all over the body, including the face,7,24-29 retroauricular areas,30 arms and legs,16,17,31-34 lower abdomen,3,5 under the breasts,1 perineum,35 external genitalia,1,9,36-40 and even the lungs41 and bladder.42
As a dermatologic disease, MCD has been referred to as yet another great imitator, both on the macroscopic and microscopic levels.8 As such, more common causes of genital edema should be considered first and investigated based on the patient's history, physical examination, skin biopsy, lymphangiogram, ultrasound, and cystogram.43 Ultrasonography and color Doppler sonography have been shown to be helpful in patients with genital involvement. This modality can evaluate not only the presence of normal testes but also intratesticular and scrotal wall fluid, especially when the physical examination reveals swelling that makes testicle palpation more difficult.6 Clinically, the correct diagnosis of MCD often is made through suspicion of inflammatory bowel disease based on classic symptoms and/or physical findings including abdominal pain, weight loss, bloody stool, diarrhea, perianal skin tags, and anal fissures or fistulas. Any of these GI findings should prompt an intestinal biopsy to rule out any histologic evidence of CD.
Metastatic CD affecting the vulva often presents with vulvar pain and pruritus and may clinically mimic a more benign disease such as balanitis plasmacellularis, also referred to as Zoon vulvitis.23 Similar to MCD on any given body surface, there is dramatic variation in the macroscopic presentation of vulvar MCD, with physical examination findings ranging from bilateral diffuse, edematous, deeply macerated, red, ulcerated lesions over the vulva with lymphadenopathy to findings of bilateral vulvar pain with yellow drainage from the labia majora.23 There have been cases of vulvar MCD that include exquisite vulvar pain but without structural abnormalities including normal uterus, cervix, adnexa, rectovaginal septum, and rectum. In these more nebulous cases of vulvar MCD, the diagnosis often is discovered incidentally when nonspecific diagnostic imaging suggests underlying CD.23
Beyond the case-by-case variations on physical examination, the great difficulty in diagnosis, particularly in children, occurs in the absence of any GI symptoms and therefore no logical consideration of underlying CD. Consequently, there have been cases of children presenting with irritation of the vulva who were eventually diagnosed with MCD only after erroneous treatment of contact dermatitis, candidiasis, and even consideration of sexual abuse.37 Because it is so rare and obscure among practicing clinicians, the diagnosis of MCD often is considered only after irritation or swelling of the external genitalia has not responded to standard therapies. If and when the diagnosis of MCD is considered in children, it has been suggested to screen patients for anorectal stricture, as case studies have found the condition to be relatively common in this subpopulation.44
In the less common case of adults with genitourinary symptoms that suggest possible MCD, the differential diagnosis for penile or vaginal ulcers should include contact and irritant dermatitis, chronic infectious lesions (eg, hidradenitis suppurativa, actinomycosis, tuberculosis),45 sexually transmitted ulcerative diseases (eg, chancroid, lymphogranuloma venereum, herpes genitalia, granuloma inguinale),46 drug reactions, and even extramammary Paget disease.47
Histologic Findings
Because MCD has so much macroscopic variation and can present anywhere on the surface of the body, formal diagnosis relies on microscopy. As an added measure of difficulty in diagnosis, one random biopsy of a suspicious segment of tissue may not contain the expected histologic findings; therefore, clinical suspicion may warrant a second biopsy.10 There have been reported cases of an adult patient without history of CD presenting with a lesion that resembled a more common pathology, such as a genital wart, and the correct diagnosis of MCD with pseudocondylomatous morphology was made only after intestinal manifestations prompted the clinician to consider such an unusual diagnosis.48
From a histopathologic perspective, MCD is characterized by discrete, noncaseating, sarcoidlike granulomas with abundant multinucleated giant cells (Langhans giant cells) in the superficial dermis (papillary), deep dermis (reticular), and adipose tissue (Figure).8,17 In the presence of concomitant intestinal disease, the granulomas of both the intestinal and dermal tissues should share the same microscopic characteristics.8 In addition, copious neutrophils and granulomas surrounding the microvasculature have been described,34 as well as general lymphocyte and plasma cell infiltrate.45 Some histologic samples have included collagen degeneration termed necrobiosis in the middle dermal layer as another variable finding in MCD.14,34

On microscopy, it has been reported that use of Verhoeff-van Gieson staining may be helpful to highlight the presence of neutrophil obstruction within the dermal vasculature, particularly the arterial lumen, as well as to aid in highlighting swelling of the endothelium with fragmentation of the internal elastic lamina.17 Although not part of the routine diagnosis, electron microscopy of MCD tissue samples have confirmed hypertrophy of the endothelial cells composing the capillaries with resulting extravasation of fibrin, red blood cells, lymphocytes, and epithelioid histiocytes.17 Observation of tissue under direct immunofluorescence has been less helpful, as it has shown only nonspecific fibrinogen deposition within the dermis and dermal vessels.17
In an article on treatment of MCD, Escher et al43 reinforced that the macroscopic findings of MCD are diverse, and the microscopic findings characteristic of MCD also can be mimicked by other etiologies such as sarcoidosis, tuberculosis, fungal infections, lymphogranuloma venereum, leishmaniasis, and connective tissue disorders.43 As such, the workup to rule out infectious, anatomic, and autoimmune etiologies should be diverse. Often, the workup for MCD will include special stains such as Ziehl-Neelsen stain to rule out Mycobacterium tuberculosis and acid-fast bacilli and Fite stain to consider atypical mycobacteria. Other tests such as tissue culture, chest radiograph, tuberculin skin test (Mantoux test), IFN-γ release assay, or polarized light microscopy may rule out infectious etiologies.9,49 Serologic testing might include VDRL test, Treponema pallidum hemagglutination assay, hepatitis B, hepatitis C, and human immunodeficiency virus.5
Crohn disease is characterized histologically by sarcoidlike noncaseating granulomas, and as such, it is important to differentiate MCD from sarcoidosis prior to histologic analysis. Sarcoidosis also can be considered much less likely with a normal chest radiograph and in the absence of increased serum calcium and angiotensin-converting enzyme levels.7 The differentiation of sarcoidosis from MCD on the microscopic scale is subtle but is sometimes facilitated in the presence of an ulcerated epidermis or lymphocytic/eosinophilic infiltrate and edema within the dermis, all suggestive of MCD.14
Metastatic CD also should be differentiated from erythema nodosum and pyoderma gangrenosum, which are among the most common cutaneous findings associated with CD.14 Pyoderma gangrenosum can be distinguished histologically by identifying copious neutrophilic infiltrate with pseudoepitheliomatous hyperplasia.50
Treatment
Because MCD is relatively rare, there are no known randomized trials suggesting a particular medical or surgical treatment. In a review of perineal MCD from 2007, the 40-year-old recommendation by Moutain3 opting for surgical debridement versus medical management still resonates, particularly for perineal disease, as an effective measure in all but the mildest of presentations.51 However, recent case reports also suggest that the tumor necrosis factor α (TNF-α) inhibitors such as infliximab and adalimumab should be considered prior to surgery even with severe perineal MCD.51 Moreover, even if medical management with TNF-α inhibitors or some combination of immunosuppressants and antibiotics does not eradicate the disease, it often helps reduce the size of the ulcers prior to surgery.52 With a limited understanding of MCD, one might think that removal of the affected bowel would eliminate cutaneous disease, but it has been shown that this strategy is not effective.53,54
The composition and location of the particular lesion affects the trajectory of treatment. For example, MCD manifesting as local ulcers and plaques has been described as responding well to topical and intralesional steroids.10,55,56 In the case of penile swelling and/or phimosis, circumcision has been helpful to improve the patient's ability to void as well as to attain and maintain erection.10 In the case of scrotal swelling secondary to MCD, early treatment (ie, within 4 to 6 months) with oral steroids and/or metronidazole is likely beneficial to prevent refractory edematous organization of the tissue.57
As a general rule, an effective treatment will include a combination of an immunosuppressant, antibiotic therapy, and sometimes surgery. The most commonly used immunosuppressant agents include topical or intralesional steroids, infliximab,43,58 cyclosporine A,59,60 dapsone, minocycline, thalidomide, methotrexate, mycophenolate mofetil, sulfasalazine, azathioprine, tacrolimus, and 6-mercaptopurine.4 Steroids have been the conventional treatment of extraintestinal manifestations of CD61; however, perineal CD has been poorly controlled with systemic steroids.62 If steroids are found not to be effective, sometimes agents such as dapsone or thalidomide are considered. One case report noted stabilization of MCD penile ulcers with oral thalidomide 300 mg once daily, oral minocycline 100 mg once daily, and topical tacrolimus 0.3% with benzocaine twice daily with continuation of prednisolone and methotrexate as parts of previously unsuccessful regimen.52
Metronidazole is perhaps the most commonly used antibiotic, having been a component of many successful regimens.4,63 For example, a 27-year-old patient with MCD presenting as a nonhealing ulcerative lesion in the subcoronal area of the penis and scrotum was treated successfully with a 6-month course of mesalamine, prednisone, and metronidazole.45 Another case report of vulvar MCD reported initial success with intravenous methylprednisolone, ciprofloxacin, and metronidazole.23 The primary limitation of metronidazole is that subsequent tapering of the dose seems to result in recurrence of disease.64 Consequently, patients must remain on the antibiotic for an indeterminate course, with dosages ranging from 5 mg/kg daily in adolescents65 to 1000 to 1500 mg daily in adults.66
Of the various immunosuppressants available, infliximab has been listed in numerous reports as a successful agent in both the induction and maintenance of extraintestinal manifestations of CD including MCD.67-71 Infliximab has been reported to be effective in the treatment of penile and scrotal edema secondary to MCD that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine.43 Infliximab may be a good option to help heal draining fistulas, particularly in combination with an antibiotic such as metronidazole and ciprofloxacin, which helps to prevent abscess formation during healing.72 The response to infliximab has been dramatic, with resolution of cutaneous lesions after just 6 weeks in some cases.73 The dosing regimen of infliximab has been suggested at 5 mg/kg administered at 0, 2, and 6 weeks, with subsequent maintenance infusions every 10 weeks,70 or at 0, 4, and 12 weeks, with subsequent infusions every 8 weeks.43
Adalimumab may be considered as an alternative to infliximab and is potentially less allergenic as a fully humanized monoclonal antibody to TNF-α, which also has been used successfully to both induce and maintain remission of moderate to severe CD.42,74,75 Proposed dosing of adalimumab includes a loading dose of 160 mg subcutaneously on day 1, followed by an 80-mg dose 2 weeks later and a 40-mg maintenance dose every other week indefinitely.48 Of note, adalimumab has been noted in the literature to have many potential side effects, including one particular case in which severe headaches were attributed to its use.59 As a consequence of the headaches, the patient was switched from adalimumab to cyclosporine and responded well with no subsequent flare-ups on follow-up.
In summary, treatment of MCD depends on cutaneous location, severity, physician experience with certain antibiotics or immunosuppressants, availability of medication, and patient disposition. It seems reasonable to attempt medical management with one or more medical regimens before committing to surgical intervention. Furthermore, even with debridement, curettage, skin graft, or other surgical strategy, the patient is likely to require some period of immunosuppression to provide long-lasting remission.
Conclusion
Patients with inflammatory bowel disease often develop dermatologic sequelae, with MCD being a rare but serious process. Patients may present with a wide array of physical concerns and symptoms, many resembling other disease processes. As such, education and a high index of suspicion are needed for proper diagnosis and treatment.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Friedman S, Blumber RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005:1778-1784.
- Moutain JC. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18-26.
- Lester LU, Rapini RP. Dermatologic manifestations of colonic disorders. Curr Opin Gastroenterol. 2008;25:66-73.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Simoneaux SF, Ball TI, Atkinson GO Jr. Scrotal swelling: unusual first presentation of Crohn's disease. Pediatr Radiol. 1995;25:375-376.
- Albuquerque A, Magro F, Rodrigues S, et al. Metastatic cutaneous Crohn's disease of the face: a case report and review of literature. Eur J Gastroenterol Hepatol. 2011;23:954-956.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Vint R, Husain E, Hassain F, et al. Metastatic Crohn's disease of the penis: two cases. Int Urol Nephrol. 2012;44:45-49.
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol. 1981;5:689-695.
- Resnick MI, Kursh ED. Extrinsic obstruction of the ureter. In: Walsh PC, Retik AB, Stamey TA, et al, eds. Campbell's Urology. 7th ed. Philadelphia, PA: WB Saunders; 1998:400-402.
- Samitz MH, Dana AS Jr, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease--review of statistics of skin complications. Cutis. 1970;6:51-56.
- Emanuel PO, Phelps RG. Metastatic Crohn's disease: a histo-pathologic study of 12 cases. J Cutan Pathol. 2008;35:457-461.
- Slater DN, Waller PC, Reilly G. Cutaneous granulomatous vasculitis: presenting features of Crohn's disease. J R Soc Med. 1985;78:589-590.
- Tatnall FM, Dodd HJ, Sarkany I. Crohn's disease with metastatic cutaneous involvement and granulomatous cheilitis. J R Soc Med. 1987;80:49-51.
- Shum DT, Guenther L. Metastatic Crohn's disease: case report and review of literature. Arch Dermatol. 1990;126:645-648.
- Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn's disease. Clin Immunol Immunopathol. 1998;86:147-160.
- Grimm MC, Pavli P, Van de Pol E, et al. Evidence for a CD-14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297.
- Pokorny RM, Hofmeister A, Galandiuk S, et al. Crohn's disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725.
- Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn's disease, its spectrum, and pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn's disease. Hum Pathol. 2003;34:1185.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn's disease. Dig Dis Sci. 2009;54:1565-1571.
- Chen W, Blume-Peytavi U, Goerdt S, et al. Metastatic Crohn's disease of the face. J Am Acad Dermatol. 1996;35:986-988.
- Ogram AE, Sobanko JF, Nigra TP. Metastatic cutaneous Crohn disease of the face: a case report and review of the literature. Cutis. 2010;85:25-27.
- Graham D, Jager D, Borum M. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062-2063.
- Biancone L, Geboes K, Spagnoli LG, et al. Metastatic Crohn's disease of the forehead. Inflamm Bowel Dis. 2002;8:101-105.
- Kolansky G, Green CK, Dubin H. Metastatic Crohn's disease of the face: an uncommon presentation. Arch Dermatol. 1993;129:1348-1349.
- Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38-42.
- McCallum DI, Gray WM. Metastatic Crohn's disease. Br J Dermatol. 1976;95:551-554.
- Lieberman TR, Greene JF Jr. Transient subcutaneous granulomatosis of the upper extremities in Crohn's disease. Am J Gastroenterol. 1979;72:89-91.
- Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn's disease: a rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300-303.
- Marotta PJ, Reynolds RP. Metastatic Crohn's disease. Am J Gastroenterol. 1996;91:373-375.
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn's disease. report of 3 cases with special reference to histopathologic findings. Arch Dermatol. 1996;132:928-932.
- van Dulleman HM, de Jong E, Slors F, et al. Treatment of therapy resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: report of two cases. Dis Colon Rectum. 1998;41:98-102.
- Lavery HA, Pinkerton JH, Sloan J. Crohn's disease of the vulva--two further cases. Br J Dermatol. 1985;113:359-363.
- Lally MR, Orenstein SR, Cohen BA. Crohn's disease of the vulva in an 8-year-old girl. Pediatr Dermatol. 1988;5:103-106.
- Tuffnell D, Buchan PC. Crohn's disease of the vulva in childhood. Br J Clin Pract. 1991;45:159-160.
- Schrodt BJ, Callen JP. Metastatic Crohn's disease presenting as chronic perivulvar and perirectal ulcerations in an adolescent patient. Pediatrics. 1999;103:500-502.
- Slaney G, Muller S, Clay J, et al. Crohn's disease involving the penis. Gut. 1986;27:329-333.
- Calder CJ, Lacy D, Raafat F, et al. Crohn's disease with pulmonary involvement in a 3 year old boy. Gut. 1993;34:1636-1638.
- Saha S, Fichera A, Bales G, et al. Metastatic Crohn's disease of the bladder. Inflamm Bowel Dis. 2008;14:140-142.
- Escher JC, Stoof TJ, van Deventer SJ, et al. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420-423.
- Saadah OI, Oliver MR, Bines JE, et al. Anorectal strictures and genital Crohn's disease: an unusual clinical association. J Pediatr Gastroenterol Nutr. 2003;36:403-406.
- Martinez-Salamanca JI, Jara J, Miralles P, et al. Metastatic Crohn's disease: penile and scrotal involvement. Scand J Urol Nephrol. 2004;38:436-437.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429.
- Acker SM, Sahn EE, Rogers HC, et al. Genital cutaneous Crohn disease. Am J Dermatopathol. 2000;22:443-446.
- Lestre S, Ramos J, Joao A, et al. Cutaneous Crohn's disease presenting as genital warts: successful treatment with adalimumab. Eur J Dermatol. 2010;20:504-505.
- Yu JT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467-469.
- Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol. 1988;18:511-521.
- Moyes LH, Glen P, Pickford IR. Perineal metastatic Crohn's disease: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W1-W3.
- Rajpara SM, Siddha SK, Ormerod AD, et al. Cutaneous penile and perianal Crohn's disease treated with a combination of medical and surgical interventions. Australas J Dermatol. 2008;49:21-24.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764-1766.
- Sangueza OP, Davis LS, Gourdin FW. Metastatic Crohn disease. South Med J. 1997;90:897-900.
- Chiba M, Iizuka M, Horie Y, et al. Metastatic Crohn's disease involving the penis. J Gastroenterol. 1997;32:817-821.
- Poon KS, Gilks CB, Masterson JS. Metastatic Crohn's disease involving the genitalia. J Urol. 2002;167:2541-2542.
- Shanahan F. Anti-TNF therapy for Crohn's disease: a perspective (infliximab is not the drug we have been waiting for). Inflamm Bowel Dis. 2000;6:137-139.
- Carranza DC, Young L. Successful treatment of metastatic Crohn's disease with cyclosporine. J Drugs Dermatol. 2008;7:789-791.
- Bardazzi F, Guidetti MS, Passarini B, et al. Cyclosporine A in metastatic Crohn's disease. Acta Derm Venereol. 1995;75:324-325.
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260.
- Gelbmann CM, Rogler G, Gross V, et al. Prior bowel resections, perianal disease, and a high initial Crohn's disease activity index are associated with corticosteroid resistance in active Crohn's disease. Am J Gastroenterol. 2002;97:1438-1445.
- Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223-228.
- Brandt LJ, Berstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology. 1982;83:383-387.
- Lehrnbecher T, Kontny HU, Jeschke R. Metastatic Crohn's disease in a 9-year-old boy. J Pediatr Gastroenterol Nutr. 1999;28:321-323.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387-391.
- Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406-410.
- Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351-356.
- Miller AM, Elliott PR, Fink R, et al. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940-942.
- Chuah JH, Kim DS, Allen C, et al. Metastatic Crohn's disease of the ear. Int J Otolaryngol. 2009;2009:871567.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405.
- Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous metastatic Crohn's disease responsive to infliximab. Am J Gastroenterol. 2009;104:1058.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333.
- Cury DB, Moss A, Elias G, et al. Adalimumab for cutaneous metastatic Crohn's disease. Inflamm Bowel Dis. 2010;16:723-724.
- Parks AG, Morson BC, Pegum JS. Crohn's disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Friedman S, Blumber RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2005:1778-1784.
- Moutain JC. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18-26.
- Lester LU, Rapini RP. Dermatologic manifestations of colonic disorders. Curr Opin Gastroenterol. 2008;25:66-73.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Simoneaux SF, Ball TI, Atkinson GO Jr. Scrotal swelling: unusual first presentation of Crohn's disease. Pediatr Radiol. 1995;25:375-376.
- Albuquerque A, Magro F, Rodrigues S, et al. Metastatic cutaneous Crohn's disease of the face: a case report and review of literature. Eur J Gastroenterol Hepatol. 2011;23:954-956.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Vint R, Husain E, Hassain F, et al. Metastatic Crohn's disease of the penis: two cases. Int Urol Nephrol. 2012;44:45-49.
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol. 1981;5:689-695.
- Resnick MI, Kursh ED. Extrinsic obstruction of the ureter. In: Walsh PC, Retik AB, Stamey TA, et al, eds. Campbell's Urology. 7th ed. Philadelphia, PA: WB Saunders; 1998:400-402.
- Samitz MH, Dana AS Jr, Rosenberg P. Cutaneous vasculitis in association with Crohn's disease--review of statistics of skin complications. Cutis. 1970;6:51-56.
- Emanuel PO, Phelps RG. Metastatic Crohn's disease: a histo-pathologic study of 12 cases. J Cutan Pathol. 2008;35:457-461.
- Slater DN, Waller PC, Reilly G. Cutaneous granulomatous vasculitis: presenting features of Crohn's disease. J R Soc Med. 1985;78:589-590.
- Tatnall FM, Dodd HJ, Sarkany I. Crohn's disease with metastatic cutaneous involvement and granulomatous cheilitis. J R Soc Med. 1987;80:49-51.
- Shum DT, Guenther L. Metastatic Crohn's disease: case report and review of literature. Arch Dermatol. 1990;126:645-648.
- Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn's disease. Clin Immunol Immunopathol. 1998;86:147-160.
- Grimm MC, Pavli P, Van de Pol E, et al. Evidence for a CD-14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297.
- Pokorny RM, Hofmeister A, Galandiuk S, et al. Crohn's disease and ulcerative colitis are associated with the DNA repair gene MLH1. Ann Surg. 1997;225:718-723; discussion 723-725.
- Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn's disease, its spectrum, and pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn's disease. Hum Pathol. 2003;34:1185.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulva metastatic Crohn's disease. Dig Dis Sci. 2009;54:1565-1571.
- Chen W, Blume-Peytavi U, Goerdt S, et al. Metastatic Crohn's disease of the face. J Am Acad Dermatol. 1996;35:986-988.
- Ogram AE, Sobanko JF, Nigra TP. Metastatic cutaneous Crohn disease of the face: a case report and review of the literature. Cutis. 2010;85:25-27.
- Graham D, Jager D, Borum M. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062-2063.
- Biancone L, Geboes K, Spagnoli LG, et al. Metastatic Crohn's disease of the forehead. Inflamm Bowel Dis. 2002;8:101-105.
- Kolansky G, Green CK, Dubin H. Metastatic Crohn's disease of the face: an uncommon presentation. Arch Dermatol. 1993;129:1348-1349.
- Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38-42.
- McCallum DI, Gray WM. Metastatic Crohn's disease. Br J Dermatol. 1976;95:551-554.
- Lieberman TR, Greene JF Jr. Transient subcutaneous granulomatosis of the upper extremities in Crohn's disease. Am J Gastroenterol. 1979;72:89-91.
- Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn's disease: a rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300-303.
- Marotta PJ, Reynolds RP. Metastatic Crohn's disease. Am J Gastroenterol. 1996;91:373-375.
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn's disease. report of 3 cases with special reference to histopathologic findings. Arch Dermatol. 1996;132:928-932.
- van Dulleman HM, de Jong E, Slors F, et al. Treatment of therapy resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: report of two cases. Dis Colon Rectum. 1998;41:98-102.
- Lavery HA, Pinkerton JH, Sloan J. Crohn's disease of the vulva--two further cases. Br J Dermatol. 1985;113:359-363.
- Lally MR, Orenstein SR, Cohen BA. Crohn's disease of the vulva in an 8-year-old girl. Pediatr Dermatol. 1988;5:103-106.
- Tuffnell D, Buchan PC. Crohn's disease of the vulva in childhood. Br J Clin Pract. 1991;45:159-160.
- Schrodt BJ, Callen JP. Metastatic Crohn's disease presenting as chronic perivulvar and perirectal ulcerations in an adolescent patient. Pediatrics. 1999;103:500-502.
- Slaney G, Muller S, Clay J, et al. Crohn's disease involving the penis. Gut. 1986;27:329-333.
- Calder CJ, Lacy D, Raafat F, et al. Crohn's disease with pulmonary involvement in a 3 year old boy. Gut. 1993;34:1636-1638.
- Saha S, Fichera A, Bales G, et al. Metastatic Crohn's disease of the bladder. Inflamm Bowel Dis. 2008;14:140-142.
- Escher JC, Stoof TJ, van Deventer SJ, et al. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420-423.
- Saadah OI, Oliver MR, Bines JE, et al. Anorectal strictures and genital Crohn's disease: an unusual clinical association. J Pediatr Gastroenterol Nutr. 2003;36:403-406.
- Martinez-Salamanca JI, Jara J, Miralles P, et al. Metastatic Crohn's disease: penile and scrotal involvement. Scand J Urol Nephrol. 2004;38:436-437.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429.
- Acker SM, Sahn EE, Rogers HC, et al. Genital cutaneous Crohn disease. Am J Dermatopathol. 2000;22:443-446.
- Lestre S, Ramos J, Joao A, et al. Cutaneous Crohn's disease presenting as genital warts: successful treatment with adalimumab. Eur J Dermatol. 2010;20:504-505.
- Yu JT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467-469.
- Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol. 1988;18:511-521.
- Moyes LH, Glen P, Pickford IR. Perineal metastatic Crohn's disease: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W1-W3.
- Rajpara SM, Siddha SK, Ormerod AD, et al. Cutaneous penile and perianal Crohn's disease treated with a combination of medical and surgical interventions. Australas J Dermatol. 2008;49:21-24.
- Cockburn AG, Krolikowski J, Balogh K, et al. Crohn disease of penile and scrotal skin. Urology. 1980;15:596-598.
- Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764-1766.
- Sangueza OP, Davis LS, Gourdin FW. Metastatic Crohn disease. South Med J. 1997;90:897-900.
- Chiba M, Iizuka M, Horie Y, et al. Metastatic Crohn's disease involving the penis. J Gastroenterol. 1997;32:817-821.
- Poon KS, Gilks CB, Masterson JS. Metastatic Crohn's disease involving the genitalia. J Urol. 2002;167:2541-2542.
- Shanahan F. Anti-TNF therapy for Crohn's disease: a perspective (infliximab is not the drug we have been waiting for). Inflamm Bowel Dis. 2000;6:137-139.
- Carranza DC, Young L. Successful treatment of metastatic Crohn's disease with cyclosporine. J Drugs Dermatol. 2008;7:789-791.
- Bardazzi F, Guidetti MS, Passarini B, et al. Cyclosporine A in metastatic Crohn's disease. Acta Derm Venereol. 1995;75:324-325.
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260.
- Gelbmann CM, Rogler G, Gross V, et al. Prior bowel resections, perianal disease, and a high initial Crohn's disease activity index are associated with corticosteroid resistance in active Crohn's disease. Am J Gastroenterol. 2002;97:1438-1445.
- Thukral C, Travassos WJ, Peppercorn MA. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:223-228.
- Brandt LJ, Berstein LH, Boley SJ, et al. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology. 1982;83:383-387.
- Lehrnbecher T, Kontny HU, Jeschke R. Metastatic Crohn's disease in a 9-year-old boy. J Pediatr Gastroenterol Nutr. 1999;28:321-323.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol. 2005;34:387-391.
- Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406-410.
- Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351-356.
- Miller AM, Elliott PR, Fink R, et al. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940-942.
- Chuah JH, Kim DS, Allen C, et al. Metastatic Crohn's disease of the ear. Int J Otolaryngol. 2009;2009:871567.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405.
- Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous metastatic Crohn's disease responsive to infliximab. Am J Gastroenterol. 2009;104:1058.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333.
- Cury DB, Moss A, Elias G, et al. Adalimumab for cutaneous metastatic Crohn's disease. Inflamm Bowel Dis. 2010;16:723-724.
Practice Points
- Almost half of patients with Crohn disease develop a dermatologic manifestation of the disease.
- The etiology of metastatic Crohn disease is unknown and diagnosis requires a high index of suspicion with exclusion of other processes.
Should genetic counselors be involved in genetic test ordering for ObGyn patients?
With more than 1,000 diseases for which genetic testing is available, and with the completion of the Human Genome Project, more patients are requesting genetic testing and more clinicians are utilizing such testing; it has become mainstream.1 This increased utilization has resulted in increased cost as well, say Ruzzo and colleagues, who presented research on genetic testing costs and compliance with clinical best practices at the 2017 Annual Clinical and Scientific Meeting of the American College of Obstetricians and Gynecologists (ACOG).2 But, according to previous research say Ruzzo and colleagues, these costs can be quelled by involving genetic counselors in the test-ordering process.
Just how much cost savings can be achieved? In their quality improvement project, the investigators found that 38.6% of 44 genetic tests reviewed were inappropriately ordered—either they were not indicated (21%), misordered for false reassurance (7%), or inadequately ordered (10.5%). If the tests were ordered as appropriately recommended, a cost savings of $20,912.58 would have been realized, according to the researchers.
Ruzzo and colleagues reviewed 114 charts over a 3-month period for adherence with published clinical practice guidelines. All of the charts were associated with a genetic test billing code for common tests ordered through LabCorp (for cystic fibrosis, BRCA mutation, factor V Leiden, prothrombin, alpha-thalassemia, hemochromatosis, and cell-free DNA).
The researchers concluded that genetic counselor review or involvement in genetic test ordering can decrease inappropriate spending and improve patient care. They pointed out that the 114 charts reviewed represent a fraction of genetic tests ordered at their institution, and further study should broaden the research scope to determine the full extent of the problem.
Ruzzo and colleagues were awarded first prize for their research as presented at ACOG.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Petition requesting a genetic testing specialty and standards for proficiency testing. Public Citizen website. https://www.citizen.org/our-work/health-and-safety/petition-requesting-genetic-testing-specialty-and-standards#_ftn28. Accessed June 9, 2017.
- Ruzzo K, Sale TJ, Willis MJ, Harding AJ, Lutgendorf MA. Genetic testing costs and compliance with clinical best practices. Paper presented at: 2017 Annual Clinical and Scientific Meeting of The American College of Obstetricians and Gynecologists; May 6, 2017; San Diego, CA.
With more than 1,000 diseases for which genetic testing is available, and with the completion of the Human Genome Project, more patients are requesting genetic testing and more clinicians are utilizing such testing; it has become mainstream.1 This increased utilization has resulted in increased cost as well, say Ruzzo and colleagues, who presented research on genetic testing costs and compliance with clinical best practices at the 2017 Annual Clinical and Scientific Meeting of the American College of Obstetricians and Gynecologists (ACOG).2 But, according to previous research say Ruzzo and colleagues, these costs can be quelled by involving genetic counselors in the test-ordering process.
Just how much cost savings can be achieved? In their quality improvement project, the investigators found that 38.6% of 44 genetic tests reviewed were inappropriately ordered—either they were not indicated (21%), misordered for false reassurance (7%), or inadequately ordered (10.5%). If the tests were ordered as appropriately recommended, a cost savings of $20,912.58 would have been realized, according to the researchers.
Ruzzo and colleagues reviewed 114 charts over a 3-month period for adherence with published clinical practice guidelines. All of the charts were associated with a genetic test billing code for common tests ordered through LabCorp (for cystic fibrosis, BRCA mutation, factor V Leiden, prothrombin, alpha-thalassemia, hemochromatosis, and cell-free DNA).
The researchers concluded that genetic counselor review or involvement in genetic test ordering can decrease inappropriate spending and improve patient care. They pointed out that the 114 charts reviewed represent a fraction of genetic tests ordered at their institution, and further study should broaden the research scope to determine the full extent of the problem.
Ruzzo and colleagues were awarded first prize for their research as presented at ACOG.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
With more than 1,000 diseases for which genetic testing is available, and with the completion of the Human Genome Project, more patients are requesting genetic testing and more clinicians are utilizing such testing; it has become mainstream.1 This increased utilization has resulted in increased cost as well, say Ruzzo and colleagues, who presented research on genetic testing costs and compliance with clinical best practices at the 2017 Annual Clinical and Scientific Meeting of the American College of Obstetricians and Gynecologists (ACOG).2 But, according to previous research say Ruzzo and colleagues, these costs can be quelled by involving genetic counselors in the test-ordering process.
Just how much cost savings can be achieved? In their quality improvement project, the investigators found that 38.6% of 44 genetic tests reviewed were inappropriately ordered—either they were not indicated (21%), misordered for false reassurance (7%), or inadequately ordered (10.5%). If the tests were ordered as appropriately recommended, a cost savings of $20,912.58 would have been realized, according to the researchers.
Ruzzo and colleagues reviewed 114 charts over a 3-month period for adherence with published clinical practice guidelines. All of the charts were associated with a genetic test billing code for common tests ordered through LabCorp (for cystic fibrosis, BRCA mutation, factor V Leiden, prothrombin, alpha-thalassemia, hemochromatosis, and cell-free DNA).
The researchers concluded that genetic counselor review or involvement in genetic test ordering can decrease inappropriate spending and improve patient care. They pointed out that the 114 charts reviewed represent a fraction of genetic tests ordered at their institution, and further study should broaden the research scope to determine the full extent of the problem.
Ruzzo and colleagues were awarded first prize for their research as presented at ACOG.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Petition requesting a genetic testing specialty and standards for proficiency testing. Public Citizen website. https://www.citizen.org/our-work/health-and-safety/petition-requesting-genetic-testing-specialty-and-standards#_ftn28. Accessed June 9, 2017.
- Ruzzo K, Sale TJ, Willis MJ, Harding AJ, Lutgendorf MA. Genetic testing costs and compliance with clinical best practices. Paper presented at: 2017 Annual Clinical and Scientific Meeting of The American College of Obstetricians and Gynecologists; May 6, 2017; San Diego, CA.
- Petition requesting a genetic testing specialty and standards for proficiency testing. Public Citizen website. https://www.citizen.org/our-work/health-and-safety/petition-requesting-genetic-testing-specialty-and-standards#_ftn28. Accessed June 9, 2017.
- Ruzzo K, Sale TJ, Willis MJ, Harding AJ, Lutgendorf MA. Genetic testing costs and compliance with clinical best practices. Paper presented at: 2017 Annual Clinical and Scientific Meeting of The American College of Obstetricians and Gynecologists; May 6, 2017; San Diego, CA.
Integrative approach to MS care underused, expert says
NEW ORLEANS – The integration of lifestyle, alternative, and conventional medicine into the care of patients with multiple sclerosis can be transformative for patients and clinicians alike, according to Allen C. Bowling, MD, PhD.
This approach not only emphasizes health and wellness of the whole person, it supports the clinician-patient relationship since neurologists serve as point persons for MS patients, Dr. Bowling said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “It’s critical to not just think about MS the disease, but to think about the patient’s overall health, maintaining health,” he said. “That’s a big mind shift. I think sometimes people hear the term ‘integrative medicine’ and they start walking out of the room or think it’s getting ‘woo-woo,’ but there is a very evidence-based approach to integrative medicine. The core of it still is not familiar to a lot of physicians.”
Harness ‘built-in’ resources
Dr. Bowling, author of “Optimal Health With Multiple Sclerosis: A Guide to Integrating Lifestyle, Alternative, and Conventional Medicine” (New York: Demos Medical Publishing, 2014) and developer of a website devoted to integrative care and MS (www.neurologycare.net), said that some of best treatment approaches may be those that use the “built-in” resources of the human body and do not require any medications, supplements, devices, or technology. “The most effective and long-lasting changes in lifestyle may be those that are small and consistent,” he said. For example, when he first started applying integrative care principles to his practice more than 16 years ago, some patients told him that MS was “one of the best things that happened to them, and that it was a gift in that it helped them clean up their lifestyle.” In the summer of 2001, one such patient came to see Dr. Bowling and said, “Since my last appointment I’ve lost 20 pounds, bought a bike. I exercise four times weekly. My wife and I have changed our diet. This is real; I’m on it.”
“I said. ‘Very impressive. How did that happen? What motivated you?’ ”
“He said, ‘You did.’ This was like a turning point in his life.”
As of May 2017, this patient has maintained his healthy lifestyle changes.
Dr. Bowling told meeting attendees that early in his neurology career he didn’t always consider other ways he could impact MS patients beyond helping them determine the best disease-modifying treatment and/or assisting them in managing symptoms. Thinking to counsel them in areas such as a balanced diet, exercise, and emotional wellness “was a mind shift for me, and made me realize how narrow my focus was,” he said. “It transformed me to go back to thinking more about general medicine. I’m a detail-oriented guy, but I don’t think that’s in the best interests of our patients. I think we need to combine very disease-specific advice with very general advice. We’re at a very important place with our patients where we can potentially have a very significant impact with our recommendations about MS as well as other medical conditions and health maintenance.”
Take baby steps
Dr. Bowling recommended that clinicians take baby steps to incorporate aspects of integrated care, including use of brief, strong supportive statements; focusing on only one issue per visit; referring patients to information resources; and sharing or transferring responsibility/accountability with other providers. Excessive focus on one therapy – including unusual and unproven CAM therapies – may detract from, or be used to avoid, other valuable approaches. For example, some of Dr. Bowling’s patients may come in very enchanted with a particular dietary supplement yet their own diet is unhealthy. “I don’t scold them, but I say, ‘I don’t think this supplement’s going to hurt you but there’s much more evidence that we should shift the focus of your motivation and drive to dietary approaches.’ ”
Use leverage to tackle emotional health
In the area of emotional health, Dr. Bowling said that an MS diagnosis often serves as a springboard to help young patients develop emotional literacy so that they don’t develop high levels of stress and anxiety. “What I find are people on the verge of entering into depression or anxiety and not really knowing how to address those emotional issues or even to do basic identification and processing of emotions, and getting attached to mind-body approaches, which I think have a clear benefit, but for many patients it does not lead to emotional literacy,” he said. “I emphasize ‘I can’t be with you 24/7. That’s your piece of the equation.’ You can also help patients identify things that give them joy, meaning, and fun in life. That’s a motivator for a lot of them – to keep doing what they find joyful, meaningful, and fun.” Dr. Bowling said that this kind of approach brings “plain old common sense” to the clinician-patient relationship. “I think we all have lots of leverage with our patients, but there’s no cookbook or algorithm here; you need to start where patients are,” he said. “Our younger patients in particular are not really listening to their primary care doctor too much. They’re paying very close attention to what we’re talking about: disease-modifying therapies and symptomatic management.”
In Dr. Bowling’s clinical experience, men often struggle with emotional health and emotional literacy issues. “I have seen amazing transformations of men in their early 20s with very low emotional literacy, and working with them, sometimes with psychodynamic psychotherapy and other methods, sometimes just talking with friends and family – really encouraging them before there’s serious anxiety or depression.” One patient told him that MS “forced me to do a year of psychotherapy and some intensive introspection for a few years. This helped me and also my family.” He recommended that clinicians tread carefully when advising men about tobacco and alcohol use, and when advising women about weight management. “Be prepared; it’s a little different every time you bring those potential issues up,” he said.
Dr. Bowling added that MS often affects how patients view the aging process itself. “It can be a crash course in how to understand the body and best use it before people technically get to the aging years,” he said. “There’s also more acceptance of disability in people who are older.” In the setting of patients who experience disease flare-ups, they may ask you if there’s anything they can do from a lifestyle standpoint to make them go away. “Be primed for these teaching moments and opportunities where there’s a seed of motivation,” he advised.
Approach treats, prevents clinician burnout
Dr. Bowling described the integrative approach to treating MS patients as “an ever-changing skill set. There’s all this talk about neurologist burnout, which is very real. If you practice this way, this is a great treatment and preventive approach for clinician burnout because your patients check you out to see if you ‘walk the walk’ on advice you give them. My success with my patients in some of these areas is less than 50%. But when there’s a real change, it’s for the life of that person. That’s the most rewarding part of my career at this point.”
Dr. Bowling disclosed that he has received research, consulting, advising, and speaking fees from Acorda, Biogen, EMD Serono, Genentech, Genzyme, Sanofi-Aventis, Teva, the American Academy of Neurology, the CMSC, the Mandell Center for Multiple Sclerosis, and the National MS Society.
NEW ORLEANS – The integration of lifestyle, alternative, and conventional medicine into the care of patients with multiple sclerosis can be transformative for patients and clinicians alike, according to Allen C. Bowling, MD, PhD.
This approach not only emphasizes health and wellness of the whole person, it supports the clinician-patient relationship since neurologists serve as point persons for MS patients, Dr. Bowling said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “It’s critical to not just think about MS the disease, but to think about the patient’s overall health, maintaining health,” he said. “That’s a big mind shift. I think sometimes people hear the term ‘integrative medicine’ and they start walking out of the room or think it’s getting ‘woo-woo,’ but there is a very evidence-based approach to integrative medicine. The core of it still is not familiar to a lot of physicians.”
Harness ‘built-in’ resources
Dr. Bowling, author of “Optimal Health With Multiple Sclerosis: A Guide to Integrating Lifestyle, Alternative, and Conventional Medicine” (New York: Demos Medical Publishing, 2014) and developer of a website devoted to integrative care and MS (www.neurologycare.net), said that some of best treatment approaches may be those that use the “built-in” resources of the human body and do not require any medications, supplements, devices, or technology. “The most effective and long-lasting changes in lifestyle may be those that are small and consistent,” he said. For example, when he first started applying integrative care principles to his practice more than 16 years ago, some patients told him that MS was “one of the best things that happened to them, and that it was a gift in that it helped them clean up their lifestyle.” In the summer of 2001, one such patient came to see Dr. Bowling and said, “Since my last appointment I’ve lost 20 pounds, bought a bike. I exercise four times weekly. My wife and I have changed our diet. This is real; I’m on it.”
“I said. ‘Very impressive. How did that happen? What motivated you?’ ”
“He said, ‘You did.’ This was like a turning point in his life.”
As of May 2017, this patient has maintained his healthy lifestyle changes.
Dr. Bowling told meeting attendees that early in his neurology career he didn’t always consider other ways he could impact MS patients beyond helping them determine the best disease-modifying treatment and/or assisting them in managing symptoms. Thinking to counsel them in areas such as a balanced diet, exercise, and emotional wellness “was a mind shift for me, and made me realize how narrow my focus was,” he said. “It transformed me to go back to thinking more about general medicine. I’m a detail-oriented guy, but I don’t think that’s in the best interests of our patients. I think we need to combine very disease-specific advice with very general advice. We’re at a very important place with our patients where we can potentially have a very significant impact with our recommendations about MS as well as other medical conditions and health maintenance.”
Take baby steps
Dr. Bowling recommended that clinicians take baby steps to incorporate aspects of integrated care, including use of brief, strong supportive statements; focusing on only one issue per visit; referring patients to information resources; and sharing or transferring responsibility/accountability with other providers. Excessive focus on one therapy – including unusual and unproven CAM therapies – may detract from, or be used to avoid, other valuable approaches. For example, some of Dr. Bowling’s patients may come in very enchanted with a particular dietary supplement yet their own diet is unhealthy. “I don’t scold them, but I say, ‘I don’t think this supplement’s going to hurt you but there’s much more evidence that we should shift the focus of your motivation and drive to dietary approaches.’ ”
Use leverage to tackle emotional health
In the area of emotional health, Dr. Bowling said that an MS diagnosis often serves as a springboard to help young patients develop emotional literacy so that they don’t develop high levels of stress and anxiety. “What I find are people on the verge of entering into depression or anxiety and not really knowing how to address those emotional issues or even to do basic identification and processing of emotions, and getting attached to mind-body approaches, which I think have a clear benefit, but for many patients it does not lead to emotional literacy,” he said. “I emphasize ‘I can’t be with you 24/7. That’s your piece of the equation.’ You can also help patients identify things that give them joy, meaning, and fun in life. That’s a motivator for a lot of them – to keep doing what they find joyful, meaningful, and fun.” Dr. Bowling said that this kind of approach brings “plain old common sense” to the clinician-patient relationship. “I think we all have lots of leverage with our patients, but there’s no cookbook or algorithm here; you need to start where patients are,” he said. “Our younger patients in particular are not really listening to their primary care doctor too much. They’re paying very close attention to what we’re talking about: disease-modifying therapies and symptomatic management.”
In Dr. Bowling’s clinical experience, men often struggle with emotional health and emotional literacy issues. “I have seen amazing transformations of men in their early 20s with very low emotional literacy, and working with them, sometimes with psychodynamic psychotherapy and other methods, sometimes just talking with friends and family – really encouraging them before there’s serious anxiety or depression.” One patient told him that MS “forced me to do a year of psychotherapy and some intensive introspection for a few years. This helped me and also my family.” He recommended that clinicians tread carefully when advising men about tobacco and alcohol use, and when advising women about weight management. “Be prepared; it’s a little different every time you bring those potential issues up,” he said.
Dr. Bowling added that MS often affects how patients view the aging process itself. “It can be a crash course in how to understand the body and best use it before people technically get to the aging years,” he said. “There’s also more acceptance of disability in people who are older.” In the setting of patients who experience disease flare-ups, they may ask you if there’s anything they can do from a lifestyle standpoint to make them go away. “Be primed for these teaching moments and opportunities where there’s a seed of motivation,” he advised.
Approach treats, prevents clinician burnout
Dr. Bowling described the integrative approach to treating MS patients as “an ever-changing skill set. There’s all this talk about neurologist burnout, which is very real. If you practice this way, this is a great treatment and preventive approach for clinician burnout because your patients check you out to see if you ‘walk the walk’ on advice you give them. My success with my patients in some of these areas is less than 50%. But when there’s a real change, it’s for the life of that person. That’s the most rewarding part of my career at this point.”
Dr. Bowling disclosed that he has received research, consulting, advising, and speaking fees from Acorda, Biogen, EMD Serono, Genentech, Genzyme, Sanofi-Aventis, Teva, the American Academy of Neurology, the CMSC, the Mandell Center for Multiple Sclerosis, and the National MS Society.
NEW ORLEANS – The integration of lifestyle, alternative, and conventional medicine into the care of patients with multiple sclerosis can be transformative for patients and clinicians alike, according to Allen C. Bowling, MD, PhD.
This approach not only emphasizes health and wellness of the whole person, it supports the clinician-patient relationship since neurologists serve as point persons for MS patients, Dr. Bowling said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “It’s critical to not just think about MS the disease, but to think about the patient’s overall health, maintaining health,” he said. “That’s a big mind shift. I think sometimes people hear the term ‘integrative medicine’ and they start walking out of the room or think it’s getting ‘woo-woo,’ but there is a very evidence-based approach to integrative medicine. The core of it still is not familiar to a lot of physicians.”
Harness ‘built-in’ resources
Dr. Bowling, author of “Optimal Health With Multiple Sclerosis: A Guide to Integrating Lifestyle, Alternative, and Conventional Medicine” (New York: Demos Medical Publishing, 2014) and developer of a website devoted to integrative care and MS (www.neurologycare.net), said that some of best treatment approaches may be those that use the “built-in” resources of the human body and do not require any medications, supplements, devices, or technology. “The most effective and long-lasting changes in lifestyle may be those that are small and consistent,” he said. For example, when he first started applying integrative care principles to his practice more than 16 years ago, some patients told him that MS was “one of the best things that happened to them, and that it was a gift in that it helped them clean up their lifestyle.” In the summer of 2001, one such patient came to see Dr. Bowling and said, “Since my last appointment I’ve lost 20 pounds, bought a bike. I exercise four times weekly. My wife and I have changed our diet. This is real; I’m on it.”
“I said. ‘Very impressive. How did that happen? What motivated you?’ ”
“He said, ‘You did.’ This was like a turning point in his life.”
As of May 2017, this patient has maintained his healthy lifestyle changes.
Dr. Bowling told meeting attendees that early in his neurology career he didn’t always consider other ways he could impact MS patients beyond helping them determine the best disease-modifying treatment and/or assisting them in managing symptoms. Thinking to counsel them in areas such as a balanced diet, exercise, and emotional wellness “was a mind shift for me, and made me realize how narrow my focus was,” he said. “It transformed me to go back to thinking more about general medicine. I’m a detail-oriented guy, but I don’t think that’s in the best interests of our patients. I think we need to combine very disease-specific advice with very general advice. We’re at a very important place with our patients where we can potentially have a very significant impact with our recommendations about MS as well as other medical conditions and health maintenance.”
Take baby steps
Dr. Bowling recommended that clinicians take baby steps to incorporate aspects of integrated care, including use of brief, strong supportive statements; focusing on only one issue per visit; referring patients to information resources; and sharing or transferring responsibility/accountability with other providers. Excessive focus on one therapy – including unusual and unproven CAM therapies – may detract from, or be used to avoid, other valuable approaches. For example, some of Dr. Bowling’s patients may come in very enchanted with a particular dietary supplement yet their own diet is unhealthy. “I don’t scold them, but I say, ‘I don’t think this supplement’s going to hurt you but there’s much more evidence that we should shift the focus of your motivation and drive to dietary approaches.’ ”
Use leverage to tackle emotional health
In the area of emotional health, Dr. Bowling said that an MS diagnosis often serves as a springboard to help young patients develop emotional literacy so that they don’t develop high levels of stress and anxiety. “What I find are people on the verge of entering into depression or anxiety and not really knowing how to address those emotional issues or even to do basic identification and processing of emotions, and getting attached to mind-body approaches, which I think have a clear benefit, but for many patients it does not lead to emotional literacy,” he said. “I emphasize ‘I can’t be with you 24/7. That’s your piece of the equation.’ You can also help patients identify things that give them joy, meaning, and fun in life. That’s a motivator for a lot of them – to keep doing what they find joyful, meaningful, and fun.” Dr. Bowling said that this kind of approach brings “plain old common sense” to the clinician-patient relationship. “I think we all have lots of leverage with our patients, but there’s no cookbook or algorithm here; you need to start where patients are,” he said. “Our younger patients in particular are not really listening to their primary care doctor too much. They’re paying very close attention to what we’re talking about: disease-modifying therapies and symptomatic management.”
In Dr. Bowling’s clinical experience, men often struggle with emotional health and emotional literacy issues. “I have seen amazing transformations of men in their early 20s with very low emotional literacy, and working with them, sometimes with psychodynamic psychotherapy and other methods, sometimes just talking with friends and family – really encouraging them before there’s serious anxiety or depression.” One patient told him that MS “forced me to do a year of psychotherapy and some intensive introspection for a few years. This helped me and also my family.” He recommended that clinicians tread carefully when advising men about tobacco and alcohol use, and when advising women about weight management. “Be prepared; it’s a little different every time you bring those potential issues up,” he said.
Dr. Bowling added that MS often affects how patients view the aging process itself. “It can be a crash course in how to understand the body and best use it before people technically get to the aging years,” he said. “There’s also more acceptance of disability in people who are older.” In the setting of patients who experience disease flare-ups, they may ask you if there’s anything they can do from a lifestyle standpoint to make them go away. “Be primed for these teaching moments and opportunities where there’s a seed of motivation,” he advised.
Approach treats, prevents clinician burnout
Dr. Bowling described the integrative approach to treating MS patients as “an ever-changing skill set. There’s all this talk about neurologist burnout, which is very real. If you practice this way, this is a great treatment and preventive approach for clinician burnout because your patients check you out to see if you ‘walk the walk’ on advice you give them. My success with my patients in some of these areas is less than 50%. But when there’s a real change, it’s for the life of that person. That’s the most rewarding part of my career at this point.”
Dr. Bowling disclosed that he has received research, consulting, advising, and speaking fees from Acorda, Biogen, EMD Serono, Genentech, Genzyme, Sanofi-Aventis, Teva, the American Academy of Neurology, the CMSC, the Mandell Center for Multiple Sclerosis, and the National MS Society.
EXPERT ANALYSIS FROM THE CMSC ANNUAL MEETING
From hospitalist to health plan CMO
Several times a year, I’m privileged to step away from my role as chief medical officer of a health insurance company and return to a previous role I cherish – teaching.
This isn’t the clinical teaching that I used to do as a hospital medicine attending or palliative medicine consultant. These are mostly 4th-year medical students who have 90 minutes or so set aside during their primary care rotation to learn about “the business of medicine.”
I always begin by telling them that when I went to medical school, “I always intended to become a health insurance executive – NOT!” (If I get a few laughs, I know the time will fly by.) I share the history of my improbable career arc and how I wound up doing something I didn’t even know existed when I was their age. And, I still try to impart some pearls of wisdom in case they remember any of this discussion as they embark on their own personal and professional journeys, knowing that at this stage in their young careers, they will be almost totally immersed in their clinical training.
1. Do what you love
Sounds simple, but too many of us make the expedient choice, or the one expected of us. Work is hard enough without being able to find some joy and meaning every day in what you do. Every job has aspects that must be tolerated, but if you don’t find a greater purpose in practicing medicine, then find a way to get it back – or think about doing something else.
2. When opportunity knocks, be prepared to answer the door
For me, I enjoyed caring for patients one at a time, perhaps 15 or so during any particular day. Being a hospitalist is important and fulfilling work. But my experience as a hospitalist enabled me to recognize the “quality chasms” that existed in my hospital and across the “system,” namely lost opportunities to provide better end-of-life care and to better coordinate care within the hospital and across the care continuum. A new mission evolved for me: to do whatever I could to improve the safety, quality, and efficiency of the care we provided, and to make the hospital a better place to work. I taught myself the clinical skills to practice palliative medicine, and I attended courses that helped me prepare to become a service line medical director in hopes of starting a program at my hospital. I also took on the role of medical director of care management at my hospital, which in a sense allowed me to help take care of several hundred patients at a time – the beginning of my transition to population health.
3. Be a lifelong learner
When these opportunities arose, I was prepared for the challenges thanks to training opportunities I actively sought out, and thanks to the support of my mentors and my medical group to attend leadership training, such as SHM’s Leadership Academy. No matter what your role in your group or at your hospital, gaining these valuable skills outside of the usual medical training will help position you for new opportunities that can only help you create a more sustainable career. And although I never went back to school to earn another advanced degree such as an MBA or MHA, additional formal education is something to consider. You can never have too many tools in your toolkit.
4. Diversify!
It’s good advice from your financial adviser, and it’s good advice for your career. I’m not suggesting you take on a side job as a lawyer or a carpenter, but you might want to think about becoming an expert in a related field like perioperative medicine, primary palliative care, or postacute care. Or consider developing a niche as a sought-after leader for hospital-based committees, such as Quality or P&T. Or maybe consider clinical research, even if you’re not at an academic medical center. The point I’m making – and I know this may seem controversial – is that practicing medicine 100% of the time is probably no longer a sustainable plan for the entire 30- to 40-year span of your postgraduate career. Find an area that you can develop into protected, paid time apart from providing direct clinical care.
5. If you are thinking of changing jobs or even careers, run toward something – not away from something else
When I was first recruited to be a medical director at another health plan, I struggled mightily as to whether leaving full-time practice was an opportunity or a foolish, dead-end career move. Ultimately, I made my decision not to avoid night call or working every other weekend; I did it because I felt I had made a difference in the hospital where I had worked for 15 years and was ready to take on a new challenge by learning the business side of health care. It provided me the opportunity to positively impact the care of not just several hundred patients, but as many as two million! My current position now allows me to have even greater influence in pursuing my personal mission to improve the quality, safety, and affordability of health care.
6. Seek balance in life
Again, sounds trite, but think about it. Most health care professionals, especially physicians, have spent most of their adult lives focused on a single goal – and that often comes at a great cost, both financially and personally. (By the way, I say “seek” because at this point in my career, I doubt any of us ever really find balance.) The best you can hope for is to be wise enough to know that amongst all the balls we are juggling, there are a few that you just can’t let drop without possibly breaking without repair.
As I conclude my talks, I tell those young medical students to practice resiliency; the only constant is change. Remain inquisitive, open yourself to whatever life may bring and enjoy the ride. With a specialty as dynamic and diverse as hospital medicine, you never know where it will take you.
Dr. Epstein is executive vice president & chief medical officer at PreferredOne, and adjunct assistant professor of medicine at the University of Minnesota, Minneapolis. He also serves as Board Secretary of SHM.
Several times a year, I’m privileged to step away from my role as chief medical officer of a health insurance company and return to a previous role I cherish – teaching.
This isn’t the clinical teaching that I used to do as a hospital medicine attending or palliative medicine consultant. These are mostly 4th-year medical students who have 90 minutes or so set aside during their primary care rotation to learn about “the business of medicine.”
I always begin by telling them that when I went to medical school, “I always intended to become a health insurance executive – NOT!” (If I get a few laughs, I know the time will fly by.) I share the history of my improbable career arc and how I wound up doing something I didn’t even know existed when I was their age. And, I still try to impart some pearls of wisdom in case they remember any of this discussion as they embark on their own personal and professional journeys, knowing that at this stage in their young careers, they will be almost totally immersed in their clinical training.
1. Do what you love
Sounds simple, but too many of us make the expedient choice, or the one expected of us. Work is hard enough without being able to find some joy and meaning every day in what you do. Every job has aspects that must be tolerated, but if you don’t find a greater purpose in practicing medicine, then find a way to get it back – or think about doing something else.
2. When opportunity knocks, be prepared to answer the door
For me, I enjoyed caring for patients one at a time, perhaps 15 or so during any particular day. Being a hospitalist is important and fulfilling work. But my experience as a hospitalist enabled me to recognize the “quality chasms” that existed in my hospital and across the “system,” namely lost opportunities to provide better end-of-life care and to better coordinate care within the hospital and across the care continuum. A new mission evolved for me: to do whatever I could to improve the safety, quality, and efficiency of the care we provided, and to make the hospital a better place to work. I taught myself the clinical skills to practice palliative medicine, and I attended courses that helped me prepare to become a service line medical director in hopes of starting a program at my hospital. I also took on the role of medical director of care management at my hospital, which in a sense allowed me to help take care of several hundred patients at a time – the beginning of my transition to population health.
3. Be a lifelong learner
When these opportunities arose, I was prepared for the challenges thanks to training opportunities I actively sought out, and thanks to the support of my mentors and my medical group to attend leadership training, such as SHM’s Leadership Academy. No matter what your role in your group or at your hospital, gaining these valuable skills outside of the usual medical training will help position you for new opportunities that can only help you create a more sustainable career. And although I never went back to school to earn another advanced degree such as an MBA or MHA, additional formal education is something to consider. You can never have too many tools in your toolkit.
4. Diversify!
It’s good advice from your financial adviser, and it’s good advice for your career. I’m not suggesting you take on a side job as a lawyer or a carpenter, but you might want to think about becoming an expert in a related field like perioperative medicine, primary palliative care, or postacute care. Or consider developing a niche as a sought-after leader for hospital-based committees, such as Quality or P&T. Or maybe consider clinical research, even if you’re not at an academic medical center. The point I’m making – and I know this may seem controversial – is that practicing medicine 100% of the time is probably no longer a sustainable plan for the entire 30- to 40-year span of your postgraduate career. Find an area that you can develop into protected, paid time apart from providing direct clinical care.
5. If you are thinking of changing jobs or even careers, run toward something – not away from something else
When I was first recruited to be a medical director at another health plan, I struggled mightily as to whether leaving full-time practice was an opportunity or a foolish, dead-end career move. Ultimately, I made my decision not to avoid night call or working every other weekend; I did it because I felt I had made a difference in the hospital where I had worked for 15 years and was ready to take on a new challenge by learning the business side of health care. It provided me the opportunity to positively impact the care of not just several hundred patients, but as many as two million! My current position now allows me to have even greater influence in pursuing my personal mission to improve the quality, safety, and affordability of health care.
6. Seek balance in life
Again, sounds trite, but think about it. Most health care professionals, especially physicians, have spent most of their adult lives focused on a single goal – and that often comes at a great cost, both financially and personally. (By the way, I say “seek” because at this point in my career, I doubt any of us ever really find balance.) The best you can hope for is to be wise enough to know that amongst all the balls we are juggling, there are a few that you just can’t let drop without possibly breaking without repair.
As I conclude my talks, I tell those young medical students to practice resiliency; the only constant is change. Remain inquisitive, open yourself to whatever life may bring and enjoy the ride. With a specialty as dynamic and diverse as hospital medicine, you never know where it will take you.
Dr. Epstein is executive vice president & chief medical officer at PreferredOne, and adjunct assistant professor of medicine at the University of Minnesota, Minneapolis. He also serves as Board Secretary of SHM.
Several times a year, I’m privileged to step away from my role as chief medical officer of a health insurance company and return to a previous role I cherish – teaching.
This isn’t the clinical teaching that I used to do as a hospital medicine attending or palliative medicine consultant. These are mostly 4th-year medical students who have 90 minutes or so set aside during their primary care rotation to learn about “the business of medicine.”
I always begin by telling them that when I went to medical school, “I always intended to become a health insurance executive – NOT!” (If I get a few laughs, I know the time will fly by.) I share the history of my improbable career arc and how I wound up doing something I didn’t even know existed when I was their age. And, I still try to impart some pearls of wisdom in case they remember any of this discussion as they embark on their own personal and professional journeys, knowing that at this stage in their young careers, they will be almost totally immersed in their clinical training.
1. Do what you love
Sounds simple, but too many of us make the expedient choice, or the one expected of us. Work is hard enough without being able to find some joy and meaning every day in what you do. Every job has aspects that must be tolerated, but if you don’t find a greater purpose in practicing medicine, then find a way to get it back – or think about doing something else.
2. When opportunity knocks, be prepared to answer the door
For me, I enjoyed caring for patients one at a time, perhaps 15 or so during any particular day. Being a hospitalist is important and fulfilling work. But my experience as a hospitalist enabled me to recognize the “quality chasms” that existed in my hospital and across the “system,” namely lost opportunities to provide better end-of-life care and to better coordinate care within the hospital and across the care continuum. A new mission evolved for me: to do whatever I could to improve the safety, quality, and efficiency of the care we provided, and to make the hospital a better place to work. I taught myself the clinical skills to practice palliative medicine, and I attended courses that helped me prepare to become a service line medical director in hopes of starting a program at my hospital. I also took on the role of medical director of care management at my hospital, which in a sense allowed me to help take care of several hundred patients at a time – the beginning of my transition to population health.
3. Be a lifelong learner
When these opportunities arose, I was prepared for the challenges thanks to training opportunities I actively sought out, and thanks to the support of my mentors and my medical group to attend leadership training, such as SHM’s Leadership Academy. No matter what your role in your group or at your hospital, gaining these valuable skills outside of the usual medical training will help position you for new opportunities that can only help you create a more sustainable career. And although I never went back to school to earn another advanced degree such as an MBA or MHA, additional formal education is something to consider. You can never have too many tools in your toolkit.
4. Diversify!
It’s good advice from your financial adviser, and it’s good advice for your career. I’m not suggesting you take on a side job as a lawyer or a carpenter, but you might want to think about becoming an expert in a related field like perioperative medicine, primary palliative care, or postacute care. Or consider developing a niche as a sought-after leader for hospital-based committees, such as Quality or P&T. Or maybe consider clinical research, even if you’re not at an academic medical center. The point I’m making – and I know this may seem controversial – is that practicing medicine 100% of the time is probably no longer a sustainable plan for the entire 30- to 40-year span of your postgraduate career. Find an area that you can develop into protected, paid time apart from providing direct clinical care.
5. If you are thinking of changing jobs or even careers, run toward something – not away from something else
When I was first recruited to be a medical director at another health plan, I struggled mightily as to whether leaving full-time practice was an opportunity or a foolish, dead-end career move. Ultimately, I made my decision not to avoid night call or working every other weekend; I did it because I felt I had made a difference in the hospital where I had worked for 15 years and was ready to take on a new challenge by learning the business side of health care. It provided me the opportunity to positively impact the care of not just several hundred patients, but as many as two million! My current position now allows me to have even greater influence in pursuing my personal mission to improve the quality, safety, and affordability of health care.
6. Seek balance in life
Again, sounds trite, but think about it. Most health care professionals, especially physicians, have spent most of their adult lives focused on a single goal – and that often comes at a great cost, both financially and personally. (By the way, I say “seek” because at this point in my career, I doubt any of us ever really find balance.) The best you can hope for is to be wise enough to know that amongst all the balls we are juggling, there are a few that you just can’t let drop without possibly breaking without repair.
As I conclude my talks, I tell those young medical students to practice resiliency; the only constant is change. Remain inquisitive, open yourself to whatever life may bring and enjoy the ride. With a specialty as dynamic and diverse as hospital medicine, you never know where it will take you.
Dr. Epstein is executive vice president & chief medical officer at PreferredOne, and adjunct assistant professor of medicine at the University of Minnesota, Minneapolis. He also serves as Board Secretary of SHM.
Implementing ACOVE quality indicators as an intervention checklist to improve care for hospitalized older adults
In 2014, the United States spent $3 trillion on healthcare; hospitalization consumed 32% of these expenditures.1 Today, Medicare patients account for over 50% of hospital days and over 30% of all hospital discharges in the United States.2 Despite this staggering financial burden, hospitalization of older adults often results in poor patient outcomes.3-6 The exponential growth of the hospitalist movement, from 350 hospitalists nationwide in 1995 to over 44,000 in 2014, has become the key strategy for providing care to hospitalized geriatric patients.7-10 Most of these hospitalists have not received geriatric training.11-15
There is growing evidence that a geriatric approach, emphasizing multidisciplinary management of the complex needs of older patients, leads to improved outcomes. Geriatric Evaluation and Management Units (GEMUs), such as Acute Care for Elderly (ACE) models, have demonstrated significant decreases in functional decline, institutionalization, and death in randomized controlled trials.16,17 Multidisciplinary, nonunit based efforts, such as the mobile acute care of elderly (MACE), proactive consultation models (Sennour/Counsell), and the Hospital Elder Life Program (HELP), have demonstrated success in preventing adverse events and decreasing length of stay (LOS).17-20
However, these models have not been systematically implemented due to challenges in generalizability and replicability in diverse settings. To address this concern, an alternative approach must be developed to widely “generalize” geriatric expertise throughout hospitals, regardless of their location, size, and resources. This initiative will require systematic integration of evidence-based decision support tools for the standardization of clinical management in hospitalized older adults.21
The 1998 Assessing Care of Vulnerable Elders (ACOVE) project developed a standardized tool to measure and evaluate the quality of care by using a comprehensive set of quality indicators (QIs) to improve the care of “vulnerable elders” (VEs) at a high risk for functional and cognitive decline and death.22-24 The latest systematic review concludes that, although many studies have used ACOVE as an assessment tool of quality, there has been a dearth of studies investigating the ACOVE QIs as an intervention to improve patient care.25
Our study investigated the role of ACOVE as an intervention by using the QIs as a standardized checklist in the acute care setting. We selected the 4 most commonly encountered QIs in the hospital setting, namely venous thrombosis prophylaxis (VTE), indwelling bladder catheter, mobilization, and delirium evaluation, in order to test the feasibility and impact of systematically implementing these ACOVE QIs as a therapeutic intervention for all hospitalized older adults.
METHODS
This study (IRB #13-644B) was conducted using a prospective intervention with a nonequivalent control group design comprised of retrospective chart data from May 1, 2014, to June 30, 2015. Process and outcome variables were extracted from electronic medical records ([EMR], Sunrise Clinical Manager [SCM]) of 2,396 patients, with 530 patients in the intervention unit and 1,866 on the control units, at a large academic tertiary center operating in the greater New York metropolitan area. Our study investigated the role of ACOVE as an intervention to improve patient care by using selected QIs as a standardized checklist tool in the acute care setting. Of the original 30 hospital-specific QIs, our study focused on the care of older adults admitted to the medicine service.26 We selected commonly encountered QIs, with the objective of testing the feasibility and impact of implementing the ACOVE QIs as an intervention to improve care of hospitalized older adults. This intervention consisted of applying the checklist tool, constructed with 4 selected ACOVE QIs and administered daily during interdisciplinary rounds, namely: 2 general “medical” indicators, VTE prophylaxis and indwelling bladder catheters, and 2 “geriatric”-focused indicators, mobilization and delirium evaluation.
Subject matter experts (hospitalists, geriatricians, researchers, administrators, and nurses) reviewed the ACOVE QIs and agreed upon the adaptation of the QIs from a quality measure assessment into a feasible and acceptable intervention checklist tool (Table 1). The checklist was reviewed during daily interdisciplinary rounds for all patients 75 years and older. While ACOVE defined vulnerable elders by using the Vulnerable Elder Screen (VES), we wanted to apply this intervention more broadly to all hospitalized older adults who are most at risk for poor outcomes.27 Patients admitted to the intensive care unit, inpatient psychiatry, inpatient leukemia/lymphoma, and surgical services were excluded.
Daily interdisciplinary rounds are held on every one of the five 40-bed medical units; they last approximately 1 hour, and consist of a lead hospitalist, nurse manager, nurse practitioners, case managers, and the nursing staff. During interdisciplinary rounds, nurses present the case to the team members who then discuss the care plan. These 5 medical units did not differ in terms of patient characteristics or staffing patterns; the intervention unit was chosen simply for logistical reasons, in that the principal investigator (PI) had been assigned to this unit prior to study start-up.
Prior to the intervention, LS held an education session for staff on the intervention unit staff (who participated on interdisciplinary rounds) to explain the concept of the ACOVE QI initiative and describe the four QIs selected for the study. Three subsequent educational sessions were held during the first week of the intervention, with new incoming staff receiving a brief individual educational session. The staff demonstrated significant knowledge improvement after session completion (pre/post mean score 70.6% vs 90.0%; P < .0001).
The Clinical Information System for the Health System EMR, The Eclipsys SCM, has alerts with different levels of severity from “soft” (user must acknowledge a recommendation) to “hard” (requires an action in order to proceed).
To measure compliance of the quality indicators, we collected the following variables:
QI 1: VTE prophylaxis
Through SCM, we collected type of VTE prophylaxis ordered (pharmacologic and/or mechanical) as well as start and stop dates for all agents. International normalized ratio levels were checked for patients receiving warfarin. Days of compliance were calculated.
QI 2: Indwelling Bladder Catheters
SCM data were collected on catheter entry and discontinuation dates, the presence of an indication, and order renewal for bladder catheter at least every 3 days.
QI 3: Mobilization
Ambulation status prior to admission was extracted from nursing documentation completed on admission to the medical ward. Patients documented as bedfast were categorized as nonambulatory prior to admission. Nursing documentation of activity level and amount of feet ambulated per nursing shift were collected. In addition, hospital day of physical therapy (PT) order and hospital days with PT performed were charted. Compliance with QI 3 in patients documented as ambulatory prior to hospital admission was recorded as present if there was a PT order within 48 hours of admission.
QI 4: Delirium Evaluation
During daily rounds, the hospitalist (PI) questioned nurses about delirium evaluation, using the first feature of the Confusion Assessment Method (CAM) as well as the “single question in delirium,” namely, “Is there evidence of an acute change in mental status from the patient’s baseline?” and “Do you think [name of patient] has been more confused lately?”28,29 Because EMR does not contain a specified field for delirium screening and documentation, and patients are not routinely included in rounds, documentation with QI 4 was recorded using the “key words” method as described in the work by Puelle et al.30 To extract SCM key words, nursing documentation of the “cognitive/perceptual/neurological exam” section of the EMR on admission and on all subsequent documentation (once per shift) was retrieved to identify acute changes in mental status (eg, “altered mental status, delirium/delirious, alert and oriented X 3, confused/confusion, disoriented, lethargy/lethargic”).30 In addition, nurses were asked to activate an SCM parameter, “Acute Confusion” SCM parameter, in the nursing documentation section, which includes potential risk factors for confusion.
In addition to QI compliance, we collected LOS, discharge disposition, and 30-day readmission data.
Generalized linear mixed models (GLMM) for binary clustered (ie, hierarchical) data were used to estimate compliance rates (ie, nurse adherence) for each group (intervention group or control group) in the postintervention period, along with their corresponding 95% confidence intervals. GLMM was used to account for the hierarchical structure of the data: nursing units within a hospital. In order to calculate the Charlson Comorbidity Index, we extracted past medical history from the EMR.31
Subjects (N = 2,396) were included in the comparison of the intervention group vs control group for each of the following 4 ACOVE QI compliance measures: DVT, mobilization, bladder catheter, and delirium.
RESULTS
Of the 2,396 patient admissions, 530 were in the intervention unit and 1,866 were in the control unit. In the intervention group, the average age was 84.65 years, 75.58% were white and 47.21% were married. There was no difference in patient demographics between groups (Table 2).
QI 1: VTE Prophylaxis
Compliance with VTE prophylaxis was met in 78.3% of the intervention subjects and 76.5% of the controls (P < .4371) (Table 3). Of note, the rate of VTE prophylaxis was 57% in the intervention vs 39% in the control group (P < .0056), in the 554 patients for whom compliance was not met. Mechanical prophylaxis was used in 35.6% of intervention subjects vs 30.6 in the control (P = .048). Patients who received no form of prophylaxis were 0.5% in the intervention and 3% in the control (P = .027).
QI 2: Indwelling Bladder Catheters
Out of 2,396 subjects, 406 had an indwelling bladder catheter (16.9%). Compliance with the catheter was met in 72.2% of the intervention group vs 54.4% in the control group (P = .1061). An indication for indwelling bladder catheters was documented in 100% of the subjects. The average number of catheter days was 5.16 in the intervention vs 5.88 in the control (P < .2284). There was statistical significance in catheter compliance in the longer stay (>15 days) subjects, decreasing to 23.32% in the control group while staying constant in the intervention group 71.5% (P = .0006).
QI 3: Mobilization
Of the 2,396 patients, 1,991 (83.1%) were reported as ambulatory prior to admission. In the intervention vs control group, 74 (14%) vs 297 (15.7%), respectively, were nonambulatory. Overall compliance with Q3 was 62.9% in the intervention vs 48.2% in the control (P < .0001). More specifically, the average time to PT order in the intervention group was 1.83 days vs 2.22 days in the control group (P <
QI 4: Delirium Evaluation
In terms of nursing documentation indicating the presence of an acute confusional state, the intervention group had 148 out of 530 nursing notes (27.9%) vs 405 out of 1,866 in the control group (21.7%; P = .0027). However, utilization of the “acute confusion” parameter with documentation of a risk factor did not differ between the groups (5.8% in the intervention group vs 5.6% in the control group, P < .94).
LOS, Discharge Disposition, and 30-Day Readmissions
LOS did not differ between intervention and control groups (6.37 days vs 6.27 days, respectively), with a median of 5 days (P = .877). Discharge disposition in the 2 groups included the following: home/home with services (71.32% vs 68.7%), skilled nursing facility/assisted living/long-term care (24.34 versus 25.83), inpatient hospice/home hospice (2.64 vs 2.25), and expired (1.13 vs 1.77; P < .3282). In addition, 30-day readmissions did not differ (21% vs 20%, respectively, P = .41).
DISCUSSION
Our goal was to explore an evidence-based, standardized approach to improve the care of hospitalized older adults. This approach leverages existing automated EMR alert functions with an additional level of decision support for VEs, integrated into daily multidisciplinary rounds. The use of a daily checklist-based tool offers a cost-effective and practical pathway to distribute the burden of compliance responsibility amongst team members.
As we anticipated and similar to study findings in hospitalized medicine, geriatric trauma, and primary care, compliance with general care QIs was better than geriatric-focused QIs.27,32 Wenger et al33 demonstrated significant improvements with screening for falls and incontinence; however, screening for cognitive impairment did not improve in the outpatient setting by imbedding ACOVE QIs into routine physician practice.
Increased compliance with VTE prophylaxis and indwelling bladder catheters may be explained by national financial incentives for widespread implementation of EMR alert systems. Conversely, mobilization, delirium assessment, and management in hospitalized older adults don’t benefit from similar incentives.
VTE Prophylaxis
The American College of Chest Physicians (ACCP) supports the use of VTE prophylaxis, especially in hospitalized older adults with decreased mobility.34 While greater adoption of EMR has already increased adherence, our intervention resulted in an even higher rate of compliance with the use of pharmacologic VTE prophylaxis.35 In the future, validated scores for risk of thrombosis and bleeding may be integrated into our QI-based checklist.
Indwelling Bladder Catheters
The potential harms of catheters have been described for over 50 years, yet remain frequently used.36,37 Previous studies have shown success in decreasing catheter days with computer-based and multidisciplinary protocols.36-39
Our health system’s EMR has built-in “soft” and “hard” alerts for indwelling bladder catheters, so we did not expect intervention-associated changes in compliance.
Mobilization
Hospitalization in older adults frequently results in functional decline.4,5,40 In response, the mobilization QI recommends an ambulation plan within 48 hours for those patients who were ambulatory prior to admission; it does not specifically define the components of the plan.26 There are several multicomponent interventions that have demonstrated improvement in functional decline, yet they require skilled providers.41,42 Our intervention implemented specific ambulation plan components: daily ambulation and documentation reminders and early PT evaluation.
While functional status measures have existed for decades, most are primarily geared to assess community-residing individuals and not designed to measure changes in function during hospitalization.43,44 Furthermore, performance-based hospital measures are difficult to integrate into the daily nursing workflow as they are time consuming.45,46 In practice, nurses routinely use free text to document functional status in the hospital setting, rendering comparative analysis problematic. Yet, we demonstrated that nurses were more engaged in reporting mobilization (increased documentation of ambulation distance and a decrease in time to PT). Future research should focus on the development of a standardized tool, integrated into the EMR, to accurately measure function in the acute care setting.
Delirium Evaluation
Delirium evaluation remains one of the most difficult clinical challenges for healthcare providers in hospitalized individuals, and our study reiterated these concerns. Previous research has consistently demonstrated that the diagnosis of delirium is missed by up to 75% of clinicians.47,48 Indeed, our study, which exclusively examined nursing documentation of the delirium evaluation QI, found that both groups showed strikingly low compliance rates. This may have been due to the fact that we only evaluated nursing documentation of suspected or definite diagnosis of delirium and a documented attempt to attribute the altered mental state to a potential etiology.31 By utilizing the concept of “key words,” as developed by Puelle et al.30, we were able to demonstrate a statistically significant improvement in nursing delirium documentation in the intervention group. This result should be interpreted with caution, as this approach is not validated. Furthermore, our operational definition of delirium compliance (ie, nurse documentation of delirium, requiring the launching of a separate parameter) may have been simply too cumbersome to readily integrate into the daily workflow. Future research should study the efficacy of a sensitive EMR-integrated screening tool that facilitates recognition, by all team members, of acute changes in cognition.
Although a number of QI improved for the intervention group, acute care utilization measures such as LOS, discharge disposition, and 30-day readmissions did not differ between groups. It may well be that improving quality for this very frail, vulnerable population may simply not result in decreased utilization. Our ability to further decrease LOS and readmission rates may be limited due to restriction of range in this complex patient population (eg, median LOS value of 5 days).
Limitations
Although our study had a large sample size, data were only collected from a single-center and thus require further exploration in different settings to ensure generalizability. In addition, QI observance was based on the medical record, which was problematic for some indicators, notably delirium identification. While prior literature highlights the difficulty in identifying delirium, especially during clinical practice without specialized training, our compliance was strikingly low.47 While validated measures such as CAM may have been included as part of the assessment, there is currently no EMR documentation of such measures and therefore, these data could not be obtained.
CONCLUSION
In summary, our study demonstrates the successful integration of the established ACOVE QIs as an intervention, rather than as an assessment method, for improving care of hospitalized older patients. By utilizing a checklist-based tool at the bedside allows the multidisciplinary team to implement evidence-based practices with the ultimate goal of standardizing care, not only for VEs, but potentially for other high-risk populations with multimorbidity.49 This innovative approach provides a much-needed direction to healthcare providers in the ever increasing stressful conditions of today’s acute care environment and for the ultimate benefit and safety of our older patients.
Disclosure
The authors declare no conflicts of interest. This study was supported by New York State Empire Clinical Research Investigators Program (ECRIP). The sponsor had no role in the conception, study design, data collection, data analysis, interpretation of data, manuscript preparation, or the decision to submit the manuscript for publication.
1. National Center for Health Statistics (US). Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics (US); 2016. http://www.ncbi.nlm.nih.gov/books/NBK367640/. Accessed November 2, 2016.
2. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief #180. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. http://www.ncbi.nlm.nih.gov/books/NBK259100/. Accessed November 2, 2016.
3. Jencks SF, Cuerdon T, Burwen DR, et al. Quality of medical care delivered to medicare beneficiaries: A profile at state and national levels. JAMA. 2000;284(13):1670-1676. PubMed
4. Covinsky KE, Pierluissi E, Johnston C. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. PubMed
5. Creditor MC. Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
6. Graf C. Functional decline in hospitalized older adults. Am J Nurs. 2006;106(1):58-67, NaN-68. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. PubMed
8. Lindenauer PK, Pantilat SZ, Katz PP, Wachter RM. Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians. Ann Intern Med. 1999;130(4 Pt 2):343-349. PubMed
9. Wachter RM. The hospitalist movement 5 years later. JAMA. 2002;287(4):487. PubMed
10. Shank B. 2016: Celebrating 20 years of hospital medicine and looking toward a bright future. Hosp Natl Assoc Inpatient Physicians. 2016. http://www.the-hospitalist.org/hospitalist/article/121925/2016-celebrating-20-years-hospital-medicine-and-looking-toward-bright. Accessed June 2, 2017.
11. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC.: National Academies Press; 2008. http://www.nap.edu/catalog/12089. Accessed November 2, 2016.
12. Boult C, Counsell SR, Leipzig RM, Berenson RA. The urgency of preparing primary care physicians to care for older people with chronic illnesses. Health Aff Proj Hope. 2010;29(5):811-818. PubMed
13. Warshaw GA, Bragg EJ, Thomas DC, Ho ML, Brewer DE, Association of Directors of Geriatric Academic Programs. Are internal medicine residency programs adequately preparing physicians to care for the baby boomers? A national survey from the Association of Directors of Geriatric Academic Programs Status of Geriatrics Workforce Study. J Am Geriatr Soc. 2006;54(10):1603-1609. PubMed
14. Tanner CE, Eckstrom E, Desai SS, Joseph CL, Ririe MR, Bowen JL. Uncovering frustrations: A qualitative needs assessment of academic general internists as geriatric care providers and teachers. J Gen Intern Med. 2006;21(1):51-55. PubMed
15. Warshaw GA, Bragg EJ, Brewer DE, Meganathan K, Ho M. The development of academic geriatric medicine: progress toward preparing the nation’s physicians to care for an aging population. J Am Geriatr Soc. 2007;55(12):2075-2082. PubMed
16. Fox MT, Sidani S, Persaud M, et al. Acute care for elders components of acute geriatric unit care: Systematic descriptive review. J Am Geriatr Soc. 2013;61(6):939-946. PubMed
17. Palmer RM, Landefeld CS, Kresevic D, Kowal J. A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994;42(5):545-552.
18. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. PubMed
19. Sennour Y, Counsell SR, Jones J, Weiner M. Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139-2145. PubMed
20. Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211. PubMed
21. Mattison MLP, Catic A, Davis RB, et al. A standardized, bundled approach to providing geriatric-focused acute care. J Am Geriatr Soc. 2014;62(5):936-942. doi:10.1111/jgs.12780. PubMed
22. Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135(8 Pt 2):642-646. PubMed
23. Wenger NS, Roth CP, Shekelle P, ACOVE Investigators. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55 Suppl 2:S247-S252. PubMed
24. Reuben DB, Roth C, Kamberg C, Wenger NS. Restructuring primary care practices to manage geriatric syndromes: the ACOVE-2 intervention. J Am Geriatr Soc. 2003;51(12):1787-1793. PubMed
25. Askari M, Wierenga PC, Eslami S, Medlock S, De Rooij SE, Abu-Hanna A. Studies pertaining to the ACOVE quality criteria: a systematic review. Int J Qual Health Care. 2012;24(1):80-87. PubMed
26. Arora VM, McGory ML, Fung CH. Quality indicators for hospitalization and surgery in vulnerable elders. J Am Geriatr Soc. 2007;55 Suppl 2:S347-S358. PubMed
27. Arora VM, Johnson M, Olson J, et al. Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders. J Am Geriatr Soc. 2007;55(11):1705-1711. PubMed
28. Sands M, Dantoc B, Hartshorn A, Ryan C, Lujic S. Single Question in Delirium (SQiD): testing its efficacy against psychiatrist interview, the Confusion Assessment Method and the Memorial Delirium Assessment Scale. Palliat Med. 2010;24(6):561-565. PubMed
29. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. PubMed
30. Puelle MR, Kosar CM, Xu G, et al. The language of delirium: Keywords for identifying delirium from medical records. J Gerontol Nurs. 2015;41(8):34-42. PubMed
31. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed
32. Boult C, Boult L, Morishita L, Smith SL, Kane RL. Outpatient geriatric evaluation and management. J Am Geriatr Soc. 1998;46(3):296-302.33. Wenger NS, Roth CP, Shekelle PG, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc. 2009;57(3):547-555. PubMed
34. Geerts WH. Prevention of Venous Thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest J. 2008;133(6_suppl):381S.
35. Rosenman M, Liu X, Phatak H, et al. Pharmacological prophylaxis for venous thromboembolism among hospitalized patients with acute medical illness: An electronic medical records study. Am J Ther. 2016;23(2):e328-e335. PubMed
36. Ghanem A, Artime C, Moser M, Caceres L, Basconcillo A. Holy moley! Take out that foley! Measuring compliance with a nurse driven protocol for foley catheter removal to decrease utilization. Am J Infect Control. 2015;43(6):S51.
37. Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114(5):404-407. PubMed
38. Huang W-C, Wann S-R, Lin S-L, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
39. Topal J, Conklin S, Camp K, Morris V, Balcezak T, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121-126. PubMed
40. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63(1):55-62. PubMed
41. Inouye SK, Bogardus ST, Baker DI, Leo-Summers L, Cooney LM. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48(12):1697-1706. PubMed
42. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-347. PubMed
43. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61-65. PubMed
44. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. PubMed
45. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119-126. PubMed
46. Smith R. Validation and Reliability of the Elderly Mobility Scale. Physiotherapy. 1994;80(11):744-747.
47. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM. Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467-2473. PubMed
48. Gustafson Y, Brännström B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760-765. PubMed
49. Brenner SK, Kaushal R, Grinspan Z, et al. Effects of health information technology on patient outcomes: a systematic review. J Am Med Inform Assoc. 2016;23(5):1016-1036. PubMed
In 2014, the United States spent $3 trillion on healthcare; hospitalization consumed 32% of these expenditures.1 Today, Medicare patients account for over 50% of hospital days and over 30% of all hospital discharges in the United States.2 Despite this staggering financial burden, hospitalization of older adults often results in poor patient outcomes.3-6 The exponential growth of the hospitalist movement, from 350 hospitalists nationwide in 1995 to over 44,000 in 2014, has become the key strategy for providing care to hospitalized geriatric patients.7-10 Most of these hospitalists have not received geriatric training.11-15
There is growing evidence that a geriatric approach, emphasizing multidisciplinary management of the complex needs of older patients, leads to improved outcomes. Geriatric Evaluation and Management Units (GEMUs), such as Acute Care for Elderly (ACE) models, have demonstrated significant decreases in functional decline, institutionalization, and death in randomized controlled trials.16,17 Multidisciplinary, nonunit based efforts, such as the mobile acute care of elderly (MACE), proactive consultation models (Sennour/Counsell), and the Hospital Elder Life Program (HELP), have demonstrated success in preventing adverse events and decreasing length of stay (LOS).17-20
However, these models have not been systematically implemented due to challenges in generalizability and replicability in diverse settings. To address this concern, an alternative approach must be developed to widely “generalize” geriatric expertise throughout hospitals, regardless of their location, size, and resources. This initiative will require systematic integration of evidence-based decision support tools for the standardization of clinical management in hospitalized older adults.21
The 1998 Assessing Care of Vulnerable Elders (ACOVE) project developed a standardized tool to measure and evaluate the quality of care by using a comprehensive set of quality indicators (QIs) to improve the care of “vulnerable elders” (VEs) at a high risk for functional and cognitive decline and death.22-24 The latest systematic review concludes that, although many studies have used ACOVE as an assessment tool of quality, there has been a dearth of studies investigating the ACOVE QIs as an intervention to improve patient care.25
Our study investigated the role of ACOVE as an intervention by using the QIs as a standardized checklist in the acute care setting. We selected the 4 most commonly encountered QIs in the hospital setting, namely venous thrombosis prophylaxis (VTE), indwelling bladder catheter, mobilization, and delirium evaluation, in order to test the feasibility and impact of systematically implementing these ACOVE QIs as a therapeutic intervention for all hospitalized older adults.
METHODS
This study (IRB #13-644B) was conducted using a prospective intervention with a nonequivalent control group design comprised of retrospective chart data from May 1, 2014, to June 30, 2015. Process and outcome variables were extracted from electronic medical records ([EMR], Sunrise Clinical Manager [SCM]) of 2,396 patients, with 530 patients in the intervention unit and 1,866 on the control units, at a large academic tertiary center operating in the greater New York metropolitan area. Our study investigated the role of ACOVE as an intervention to improve patient care by using selected QIs as a standardized checklist tool in the acute care setting. Of the original 30 hospital-specific QIs, our study focused on the care of older adults admitted to the medicine service.26 We selected commonly encountered QIs, with the objective of testing the feasibility and impact of implementing the ACOVE QIs as an intervention to improve care of hospitalized older adults. This intervention consisted of applying the checklist tool, constructed with 4 selected ACOVE QIs and administered daily during interdisciplinary rounds, namely: 2 general “medical” indicators, VTE prophylaxis and indwelling bladder catheters, and 2 “geriatric”-focused indicators, mobilization and delirium evaluation.
Subject matter experts (hospitalists, geriatricians, researchers, administrators, and nurses) reviewed the ACOVE QIs and agreed upon the adaptation of the QIs from a quality measure assessment into a feasible and acceptable intervention checklist tool (Table 1). The checklist was reviewed during daily interdisciplinary rounds for all patients 75 years and older. While ACOVE defined vulnerable elders by using the Vulnerable Elder Screen (VES), we wanted to apply this intervention more broadly to all hospitalized older adults who are most at risk for poor outcomes.27 Patients admitted to the intensive care unit, inpatient psychiatry, inpatient leukemia/lymphoma, and surgical services were excluded.
Daily interdisciplinary rounds are held on every one of the five 40-bed medical units; they last approximately 1 hour, and consist of a lead hospitalist, nurse manager, nurse practitioners, case managers, and the nursing staff. During interdisciplinary rounds, nurses present the case to the team members who then discuss the care plan. These 5 medical units did not differ in terms of patient characteristics or staffing patterns; the intervention unit was chosen simply for logistical reasons, in that the principal investigator (PI) had been assigned to this unit prior to study start-up.
Prior to the intervention, LS held an education session for staff on the intervention unit staff (who participated on interdisciplinary rounds) to explain the concept of the ACOVE QI initiative and describe the four QIs selected for the study. Three subsequent educational sessions were held during the first week of the intervention, with new incoming staff receiving a brief individual educational session. The staff demonstrated significant knowledge improvement after session completion (pre/post mean score 70.6% vs 90.0%; P < .0001).
The Clinical Information System for the Health System EMR, The Eclipsys SCM, has alerts with different levels of severity from “soft” (user must acknowledge a recommendation) to “hard” (requires an action in order to proceed).
To measure compliance of the quality indicators, we collected the following variables:
QI 1: VTE prophylaxis
Through SCM, we collected type of VTE prophylaxis ordered (pharmacologic and/or mechanical) as well as start and stop dates for all agents. International normalized ratio levels were checked for patients receiving warfarin. Days of compliance were calculated.
QI 2: Indwelling Bladder Catheters
SCM data were collected on catheter entry and discontinuation dates, the presence of an indication, and order renewal for bladder catheter at least every 3 days.
QI 3: Mobilization
Ambulation status prior to admission was extracted from nursing documentation completed on admission to the medical ward. Patients documented as bedfast were categorized as nonambulatory prior to admission. Nursing documentation of activity level and amount of feet ambulated per nursing shift were collected. In addition, hospital day of physical therapy (PT) order and hospital days with PT performed were charted. Compliance with QI 3 in patients documented as ambulatory prior to hospital admission was recorded as present if there was a PT order within 48 hours of admission.
QI 4: Delirium Evaluation
During daily rounds, the hospitalist (PI) questioned nurses about delirium evaluation, using the first feature of the Confusion Assessment Method (CAM) as well as the “single question in delirium,” namely, “Is there evidence of an acute change in mental status from the patient’s baseline?” and “Do you think [name of patient] has been more confused lately?”28,29 Because EMR does not contain a specified field for delirium screening and documentation, and patients are not routinely included in rounds, documentation with QI 4 was recorded using the “key words” method as described in the work by Puelle et al.30 To extract SCM key words, nursing documentation of the “cognitive/perceptual/neurological exam” section of the EMR on admission and on all subsequent documentation (once per shift) was retrieved to identify acute changes in mental status (eg, “altered mental status, delirium/delirious, alert and oriented X 3, confused/confusion, disoriented, lethargy/lethargic”).30 In addition, nurses were asked to activate an SCM parameter, “Acute Confusion” SCM parameter, in the nursing documentation section, which includes potential risk factors for confusion.
In addition to QI compliance, we collected LOS, discharge disposition, and 30-day readmission data.
Generalized linear mixed models (GLMM) for binary clustered (ie, hierarchical) data were used to estimate compliance rates (ie, nurse adherence) for each group (intervention group or control group) in the postintervention period, along with their corresponding 95% confidence intervals. GLMM was used to account for the hierarchical structure of the data: nursing units within a hospital. In order to calculate the Charlson Comorbidity Index, we extracted past medical history from the EMR.31
Subjects (N = 2,396) were included in the comparison of the intervention group vs control group for each of the following 4 ACOVE QI compliance measures: DVT, mobilization, bladder catheter, and delirium.
RESULTS
Of the 2,396 patient admissions, 530 were in the intervention unit and 1,866 were in the control unit. In the intervention group, the average age was 84.65 years, 75.58% were white and 47.21% were married. There was no difference in patient demographics between groups (Table 2).
QI 1: VTE Prophylaxis
Compliance with VTE prophylaxis was met in 78.3% of the intervention subjects and 76.5% of the controls (P < .4371) (Table 3). Of note, the rate of VTE prophylaxis was 57% in the intervention vs 39% in the control group (P < .0056), in the 554 patients for whom compliance was not met. Mechanical prophylaxis was used in 35.6% of intervention subjects vs 30.6 in the control (P = .048). Patients who received no form of prophylaxis were 0.5% in the intervention and 3% in the control (P = .027).
QI 2: Indwelling Bladder Catheters
Out of 2,396 subjects, 406 had an indwelling bladder catheter (16.9%). Compliance with the catheter was met in 72.2% of the intervention group vs 54.4% in the control group (P = .1061). An indication for indwelling bladder catheters was documented in 100% of the subjects. The average number of catheter days was 5.16 in the intervention vs 5.88 in the control (P < .2284). There was statistical significance in catheter compliance in the longer stay (>15 days) subjects, decreasing to 23.32% in the control group while staying constant in the intervention group 71.5% (P = .0006).
QI 3: Mobilization
Of the 2,396 patients, 1,991 (83.1%) were reported as ambulatory prior to admission. In the intervention vs control group, 74 (14%) vs 297 (15.7%), respectively, were nonambulatory. Overall compliance with Q3 was 62.9% in the intervention vs 48.2% in the control (P < .0001). More specifically, the average time to PT order in the intervention group was 1.83 days vs 2.22 days in the control group (P <
QI 4: Delirium Evaluation
In terms of nursing documentation indicating the presence of an acute confusional state, the intervention group had 148 out of 530 nursing notes (27.9%) vs 405 out of 1,866 in the control group (21.7%; P = .0027). However, utilization of the “acute confusion” parameter with documentation of a risk factor did not differ between the groups (5.8% in the intervention group vs 5.6% in the control group, P < .94).
LOS, Discharge Disposition, and 30-Day Readmissions
LOS did not differ between intervention and control groups (6.37 days vs 6.27 days, respectively), with a median of 5 days (P = .877). Discharge disposition in the 2 groups included the following: home/home with services (71.32% vs 68.7%), skilled nursing facility/assisted living/long-term care (24.34 versus 25.83), inpatient hospice/home hospice (2.64 vs 2.25), and expired (1.13 vs 1.77; P < .3282). In addition, 30-day readmissions did not differ (21% vs 20%, respectively, P = .41).
DISCUSSION
Our goal was to explore an evidence-based, standardized approach to improve the care of hospitalized older adults. This approach leverages existing automated EMR alert functions with an additional level of decision support for VEs, integrated into daily multidisciplinary rounds. The use of a daily checklist-based tool offers a cost-effective and practical pathway to distribute the burden of compliance responsibility amongst team members.
As we anticipated and similar to study findings in hospitalized medicine, geriatric trauma, and primary care, compliance with general care QIs was better than geriatric-focused QIs.27,32 Wenger et al33 demonstrated significant improvements with screening for falls and incontinence; however, screening for cognitive impairment did not improve in the outpatient setting by imbedding ACOVE QIs into routine physician practice.
Increased compliance with VTE prophylaxis and indwelling bladder catheters may be explained by national financial incentives for widespread implementation of EMR alert systems. Conversely, mobilization, delirium assessment, and management in hospitalized older adults don’t benefit from similar incentives.
VTE Prophylaxis
The American College of Chest Physicians (ACCP) supports the use of VTE prophylaxis, especially in hospitalized older adults with decreased mobility.34 While greater adoption of EMR has already increased adherence, our intervention resulted in an even higher rate of compliance with the use of pharmacologic VTE prophylaxis.35 In the future, validated scores for risk of thrombosis and bleeding may be integrated into our QI-based checklist.
Indwelling Bladder Catheters
The potential harms of catheters have been described for over 50 years, yet remain frequently used.36,37 Previous studies have shown success in decreasing catheter days with computer-based and multidisciplinary protocols.36-39
Our health system’s EMR has built-in “soft” and “hard” alerts for indwelling bladder catheters, so we did not expect intervention-associated changes in compliance.
Mobilization
Hospitalization in older adults frequently results in functional decline.4,5,40 In response, the mobilization QI recommends an ambulation plan within 48 hours for those patients who were ambulatory prior to admission; it does not specifically define the components of the plan.26 There are several multicomponent interventions that have demonstrated improvement in functional decline, yet they require skilled providers.41,42 Our intervention implemented specific ambulation plan components: daily ambulation and documentation reminders and early PT evaluation.
While functional status measures have existed for decades, most are primarily geared to assess community-residing individuals and not designed to measure changes in function during hospitalization.43,44 Furthermore, performance-based hospital measures are difficult to integrate into the daily nursing workflow as they are time consuming.45,46 In practice, nurses routinely use free text to document functional status in the hospital setting, rendering comparative analysis problematic. Yet, we demonstrated that nurses were more engaged in reporting mobilization (increased documentation of ambulation distance and a decrease in time to PT). Future research should focus on the development of a standardized tool, integrated into the EMR, to accurately measure function in the acute care setting.
Delirium Evaluation
Delirium evaluation remains one of the most difficult clinical challenges for healthcare providers in hospitalized individuals, and our study reiterated these concerns. Previous research has consistently demonstrated that the diagnosis of delirium is missed by up to 75% of clinicians.47,48 Indeed, our study, which exclusively examined nursing documentation of the delirium evaluation QI, found that both groups showed strikingly low compliance rates. This may have been due to the fact that we only evaluated nursing documentation of suspected or definite diagnosis of delirium and a documented attempt to attribute the altered mental state to a potential etiology.31 By utilizing the concept of “key words,” as developed by Puelle et al.30, we were able to demonstrate a statistically significant improvement in nursing delirium documentation in the intervention group. This result should be interpreted with caution, as this approach is not validated. Furthermore, our operational definition of delirium compliance (ie, nurse documentation of delirium, requiring the launching of a separate parameter) may have been simply too cumbersome to readily integrate into the daily workflow. Future research should study the efficacy of a sensitive EMR-integrated screening tool that facilitates recognition, by all team members, of acute changes in cognition.
Although a number of QI improved for the intervention group, acute care utilization measures such as LOS, discharge disposition, and 30-day readmissions did not differ between groups. It may well be that improving quality for this very frail, vulnerable population may simply not result in decreased utilization. Our ability to further decrease LOS and readmission rates may be limited due to restriction of range in this complex patient population (eg, median LOS value of 5 days).
Limitations
Although our study had a large sample size, data were only collected from a single-center and thus require further exploration in different settings to ensure generalizability. In addition, QI observance was based on the medical record, which was problematic for some indicators, notably delirium identification. While prior literature highlights the difficulty in identifying delirium, especially during clinical practice without specialized training, our compliance was strikingly low.47 While validated measures such as CAM may have been included as part of the assessment, there is currently no EMR documentation of such measures and therefore, these data could not be obtained.
CONCLUSION
In summary, our study demonstrates the successful integration of the established ACOVE QIs as an intervention, rather than as an assessment method, for improving care of hospitalized older patients. By utilizing a checklist-based tool at the bedside allows the multidisciplinary team to implement evidence-based practices with the ultimate goal of standardizing care, not only for VEs, but potentially for other high-risk populations with multimorbidity.49 This innovative approach provides a much-needed direction to healthcare providers in the ever increasing stressful conditions of today’s acute care environment and for the ultimate benefit and safety of our older patients.
Disclosure
The authors declare no conflicts of interest. This study was supported by New York State Empire Clinical Research Investigators Program (ECRIP). The sponsor had no role in the conception, study design, data collection, data analysis, interpretation of data, manuscript preparation, or the decision to submit the manuscript for publication.
In 2014, the United States spent $3 trillion on healthcare; hospitalization consumed 32% of these expenditures.1 Today, Medicare patients account for over 50% of hospital days and over 30% of all hospital discharges in the United States.2 Despite this staggering financial burden, hospitalization of older adults often results in poor patient outcomes.3-6 The exponential growth of the hospitalist movement, from 350 hospitalists nationwide in 1995 to over 44,000 in 2014, has become the key strategy for providing care to hospitalized geriatric patients.7-10 Most of these hospitalists have not received geriatric training.11-15
There is growing evidence that a geriatric approach, emphasizing multidisciplinary management of the complex needs of older patients, leads to improved outcomes. Geriatric Evaluation and Management Units (GEMUs), such as Acute Care for Elderly (ACE) models, have demonstrated significant decreases in functional decline, institutionalization, and death in randomized controlled trials.16,17 Multidisciplinary, nonunit based efforts, such as the mobile acute care of elderly (MACE), proactive consultation models (Sennour/Counsell), and the Hospital Elder Life Program (HELP), have demonstrated success in preventing adverse events and decreasing length of stay (LOS).17-20
However, these models have not been systematically implemented due to challenges in generalizability and replicability in diverse settings. To address this concern, an alternative approach must be developed to widely “generalize” geriatric expertise throughout hospitals, regardless of their location, size, and resources. This initiative will require systematic integration of evidence-based decision support tools for the standardization of clinical management in hospitalized older adults.21
The 1998 Assessing Care of Vulnerable Elders (ACOVE) project developed a standardized tool to measure and evaluate the quality of care by using a comprehensive set of quality indicators (QIs) to improve the care of “vulnerable elders” (VEs) at a high risk for functional and cognitive decline and death.22-24 The latest systematic review concludes that, although many studies have used ACOVE as an assessment tool of quality, there has been a dearth of studies investigating the ACOVE QIs as an intervention to improve patient care.25
Our study investigated the role of ACOVE as an intervention by using the QIs as a standardized checklist in the acute care setting. We selected the 4 most commonly encountered QIs in the hospital setting, namely venous thrombosis prophylaxis (VTE), indwelling bladder catheter, mobilization, and delirium evaluation, in order to test the feasibility and impact of systematically implementing these ACOVE QIs as a therapeutic intervention for all hospitalized older adults.
METHODS
This study (IRB #13-644B) was conducted using a prospective intervention with a nonequivalent control group design comprised of retrospective chart data from May 1, 2014, to June 30, 2015. Process and outcome variables were extracted from electronic medical records ([EMR], Sunrise Clinical Manager [SCM]) of 2,396 patients, with 530 patients in the intervention unit and 1,866 on the control units, at a large academic tertiary center operating in the greater New York metropolitan area. Our study investigated the role of ACOVE as an intervention to improve patient care by using selected QIs as a standardized checklist tool in the acute care setting. Of the original 30 hospital-specific QIs, our study focused on the care of older adults admitted to the medicine service.26 We selected commonly encountered QIs, with the objective of testing the feasibility and impact of implementing the ACOVE QIs as an intervention to improve care of hospitalized older adults. This intervention consisted of applying the checklist tool, constructed with 4 selected ACOVE QIs and administered daily during interdisciplinary rounds, namely: 2 general “medical” indicators, VTE prophylaxis and indwelling bladder catheters, and 2 “geriatric”-focused indicators, mobilization and delirium evaluation.
Subject matter experts (hospitalists, geriatricians, researchers, administrators, and nurses) reviewed the ACOVE QIs and agreed upon the adaptation of the QIs from a quality measure assessment into a feasible and acceptable intervention checklist tool (Table 1). The checklist was reviewed during daily interdisciplinary rounds for all patients 75 years and older. While ACOVE defined vulnerable elders by using the Vulnerable Elder Screen (VES), we wanted to apply this intervention more broadly to all hospitalized older adults who are most at risk for poor outcomes.27 Patients admitted to the intensive care unit, inpatient psychiatry, inpatient leukemia/lymphoma, and surgical services were excluded.
Daily interdisciplinary rounds are held on every one of the five 40-bed medical units; they last approximately 1 hour, and consist of a lead hospitalist, nurse manager, nurse practitioners, case managers, and the nursing staff. During interdisciplinary rounds, nurses present the case to the team members who then discuss the care plan. These 5 medical units did not differ in terms of patient characteristics or staffing patterns; the intervention unit was chosen simply for logistical reasons, in that the principal investigator (PI) had been assigned to this unit prior to study start-up.
Prior to the intervention, LS held an education session for staff on the intervention unit staff (who participated on interdisciplinary rounds) to explain the concept of the ACOVE QI initiative and describe the four QIs selected for the study. Three subsequent educational sessions were held during the first week of the intervention, with new incoming staff receiving a brief individual educational session. The staff demonstrated significant knowledge improvement after session completion (pre/post mean score 70.6% vs 90.0%; P < .0001).
The Clinical Information System for the Health System EMR, The Eclipsys SCM, has alerts with different levels of severity from “soft” (user must acknowledge a recommendation) to “hard” (requires an action in order to proceed).
To measure compliance of the quality indicators, we collected the following variables:
QI 1: VTE prophylaxis
Through SCM, we collected type of VTE prophylaxis ordered (pharmacologic and/or mechanical) as well as start and stop dates for all agents. International normalized ratio levels were checked for patients receiving warfarin. Days of compliance were calculated.
QI 2: Indwelling Bladder Catheters
SCM data were collected on catheter entry and discontinuation dates, the presence of an indication, and order renewal for bladder catheter at least every 3 days.
QI 3: Mobilization
Ambulation status prior to admission was extracted from nursing documentation completed on admission to the medical ward. Patients documented as bedfast were categorized as nonambulatory prior to admission. Nursing documentation of activity level and amount of feet ambulated per nursing shift were collected. In addition, hospital day of physical therapy (PT) order and hospital days with PT performed were charted. Compliance with QI 3 in patients documented as ambulatory prior to hospital admission was recorded as present if there was a PT order within 48 hours of admission.
QI 4: Delirium Evaluation
During daily rounds, the hospitalist (PI) questioned nurses about delirium evaluation, using the first feature of the Confusion Assessment Method (CAM) as well as the “single question in delirium,” namely, “Is there evidence of an acute change in mental status from the patient’s baseline?” and “Do you think [name of patient] has been more confused lately?”28,29 Because EMR does not contain a specified field for delirium screening and documentation, and patients are not routinely included in rounds, documentation with QI 4 was recorded using the “key words” method as described in the work by Puelle et al.30 To extract SCM key words, nursing documentation of the “cognitive/perceptual/neurological exam” section of the EMR on admission and on all subsequent documentation (once per shift) was retrieved to identify acute changes in mental status (eg, “altered mental status, delirium/delirious, alert and oriented X 3, confused/confusion, disoriented, lethargy/lethargic”).30 In addition, nurses were asked to activate an SCM parameter, “Acute Confusion” SCM parameter, in the nursing documentation section, which includes potential risk factors for confusion.
In addition to QI compliance, we collected LOS, discharge disposition, and 30-day readmission data.
Generalized linear mixed models (GLMM) for binary clustered (ie, hierarchical) data were used to estimate compliance rates (ie, nurse adherence) for each group (intervention group or control group) in the postintervention period, along with their corresponding 95% confidence intervals. GLMM was used to account for the hierarchical structure of the data: nursing units within a hospital. In order to calculate the Charlson Comorbidity Index, we extracted past medical history from the EMR.31
Subjects (N = 2,396) were included in the comparison of the intervention group vs control group for each of the following 4 ACOVE QI compliance measures: DVT, mobilization, bladder catheter, and delirium.
RESULTS
Of the 2,396 patient admissions, 530 were in the intervention unit and 1,866 were in the control unit. In the intervention group, the average age was 84.65 years, 75.58% were white and 47.21% were married. There was no difference in patient demographics between groups (Table 2).
QI 1: VTE Prophylaxis
Compliance with VTE prophylaxis was met in 78.3% of the intervention subjects and 76.5% of the controls (P < .4371) (Table 3). Of note, the rate of VTE prophylaxis was 57% in the intervention vs 39% in the control group (P < .0056), in the 554 patients for whom compliance was not met. Mechanical prophylaxis was used in 35.6% of intervention subjects vs 30.6 in the control (P = .048). Patients who received no form of prophylaxis were 0.5% in the intervention and 3% in the control (P = .027).
QI 2: Indwelling Bladder Catheters
Out of 2,396 subjects, 406 had an indwelling bladder catheter (16.9%). Compliance with the catheter was met in 72.2% of the intervention group vs 54.4% in the control group (P = .1061). An indication for indwelling bladder catheters was documented in 100% of the subjects. The average number of catheter days was 5.16 in the intervention vs 5.88 in the control (P < .2284). There was statistical significance in catheter compliance in the longer stay (>15 days) subjects, decreasing to 23.32% in the control group while staying constant in the intervention group 71.5% (P = .0006).
QI 3: Mobilization
Of the 2,396 patients, 1,991 (83.1%) were reported as ambulatory prior to admission. In the intervention vs control group, 74 (14%) vs 297 (15.7%), respectively, were nonambulatory. Overall compliance with Q3 was 62.9% in the intervention vs 48.2% in the control (P < .0001). More specifically, the average time to PT order in the intervention group was 1.83 days vs 2.22 days in the control group (P <
QI 4: Delirium Evaluation
In terms of nursing documentation indicating the presence of an acute confusional state, the intervention group had 148 out of 530 nursing notes (27.9%) vs 405 out of 1,866 in the control group (21.7%; P = .0027). However, utilization of the “acute confusion” parameter with documentation of a risk factor did not differ between the groups (5.8% in the intervention group vs 5.6% in the control group, P < .94).
LOS, Discharge Disposition, and 30-Day Readmissions
LOS did not differ between intervention and control groups (6.37 days vs 6.27 days, respectively), with a median of 5 days (P = .877). Discharge disposition in the 2 groups included the following: home/home with services (71.32% vs 68.7%), skilled nursing facility/assisted living/long-term care (24.34 versus 25.83), inpatient hospice/home hospice (2.64 vs 2.25), and expired (1.13 vs 1.77; P < .3282). In addition, 30-day readmissions did not differ (21% vs 20%, respectively, P = .41).
DISCUSSION
Our goal was to explore an evidence-based, standardized approach to improve the care of hospitalized older adults. This approach leverages existing automated EMR alert functions with an additional level of decision support for VEs, integrated into daily multidisciplinary rounds. The use of a daily checklist-based tool offers a cost-effective and practical pathway to distribute the burden of compliance responsibility amongst team members.
As we anticipated and similar to study findings in hospitalized medicine, geriatric trauma, and primary care, compliance with general care QIs was better than geriatric-focused QIs.27,32 Wenger et al33 demonstrated significant improvements with screening for falls and incontinence; however, screening for cognitive impairment did not improve in the outpatient setting by imbedding ACOVE QIs into routine physician practice.
Increased compliance with VTE prophylaxis and indwelling bladder catheters may be explained by national financial incentives for widespread implementation of EMR alert systems. Conversely, mobilization, delirium assessment, and management in hospitalized older adults don’t benefit from similar incentives.
VTE Prophylaxis
The American College of Chest Physicians (ACCP) supports the use of VTE prophylaxis, especially in hospitalized older adults with decreased mobility.34 While greater adoption of EMR has already increased adherence, our intervention resulted in an even higher rate of compliance with the use of pharmacologic VTE prophylaxis.35 In the future, validated scores for risk of thrombosis and bleeding may be integrated into our QI-based checklist.
Indwelling Bladder Catheters
The potential harms of catheters have been described for over 50 years, yet remain frequently used.36,37 Previous studies have shown success in decreasing catheter days with computer-based and multidisciplinary protocols.36-39
Our health system’s EMR has built-in “soft” and “hard” alerts for indwelling bladder catheters, so we did not expect intervention-associated changes in compliance.
Mobilization
Hospitalization in older adults frequently results in functional decline.4,5,40 In response, the mobilization QI recommends an ambulation plan within 48 hours for those patients who were ambulatory prior to admission; it does not specifically define the components of the plan.26 There are several multicomponent interventions that have demonstrated improvement in functional decline, yet they require skilled providers.41,42 Our intervention implemented specific ambulation plan components: daily ambulation and documentation reminders and early PT evaluation.
While functional status measures have existed for decades, most are primarily geared to assess community-residing individuals and not designed to measure changes in function during hospitalization.43,44 Furthermore, performance-based hospital measures are difficult to integrate into the daily nursing workflow as they are time consuming.45,46 In practice, nurses routinely use free text to document functional status in the hospital setting, rendering comparative analysis problematic. Yet, we demonstrated that nurses were more engaged in reporting mobilization (increased documentation of ambulation distance and a decrease in time to PT). Future research should focus on the development of a standardized tool, integrated into the EMR, to accurately measure function in the acute care setting.
Delirium Evaluation
Delirium evaluation remains one of the most difficult clinical challenges for healthcare providers in hospitalized individuals, and our study reiterated these concerns. Previous research has consistently demonstrated that the diagnosis of delirium is missed by up to 75% of clinicians.47,48 Indeed, our study, which exclusively examined nursing documentation of the delirium evaluation QI, found that both groups showed strikingly low compliance rates. This may have been due to the fact that we only evaluated nursing documentation of suspected or definite diagnosis of delirium and a documented attempt to attribute the altered mental state to a potential etiology.31 By utilizing the concept of “key words,” as developed by Puelle et al.30, we were able to demonstrate a statistically significant improvement in nursing delirium documentation in the intervention group. This result should be interpreted with caution, as this approach is not validated. Furthermore, our operational definition of delirium compliance (ie, nurse documentation of delirium, requiring the launching of a separate parameter) may have been simply too cumbersome to readily integrate into the daily workflow. Future research should study the efficacy of a sensitive EMR-integrated screening tool that facilitates recognition, by all team members, of acute changes in cognition.
Although a number of QI improved for the intervention group, acute care utilization measures such as LOS, discharge disposition, and 30-day readmissions did not differ between groups. It may well be that improving quality for this very frail, vulnerable population may simply not result in decreased utilization. Our ability to further decrease LOS and readmission rates may be limited due to restriction of range in this complex patient population (eg, median LOS value of 5 days).
Limitations
Although our study had a large sample size, data were only collected from a single-center and thus require further exploration in different settings to ensure generalizability. In addition, QI observance was based on the medical record, which was problematic for some indicators, notably delirium identification. While prior literature highlights the difficulty in identifying delirium, especially during clinical practice without specialized training, our compliance was strikingly low.47 While validated measures such as CAM may have been included as part of the assessment, there is currently no EMR documentation of such measures and therefore, these data could not be obtained.
CONCLUSION
In summary, our study demonstrates the successful integration of the established ACOVE QIs as an intervention, rather than as an assessment method, for improving care of hospitalized older patients. By utilizing a checklist-based tool at the bedside allows the multidisciplinary team to implement evidence-based practices with the ultimate goal of standardizing care, not only for VEs, but potentially for other high-risk populations with multimorbidity.49 This innovative approach provides a much-needed direction to healthcare providers in the ever increasing stressful conditions of today’s acute care environment and for the ultimate benefit and safety of our older patients.
Disclosure
The authors declare no conflicts of interest. This study was supported by New York State Empire Clinical Research Investigators Program (ECRIP). The sponsor had no role in the conception, study design, data collection, data analysis, interpretation of data, manuscript preparation, or the decision to submit the manuscript for publication.
1. National Center for Health Statistics (US). Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics (US); 2016. http://www.ncbi.nlm.nih.gov/books/NBK367640/. Accessed November 2, 2016.
2. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief #180. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. http://www.ncbi.nlm.nih.gov/books/NBK259100/. Accessed November 2, 2016.
3. Jencks SF, Cuerdon T, Burwen DR, et al. Quality of medical care delivered to medicare beneficiaries: A profile at state and national levels. JAMA. 2000;284(13):1670-1676. PubMed
4. Covinsky KE, Pierluissi E, Johnston C. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. PubMed
5. Creditor MC. Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
6. Graf C. Functional decline in hospitalized older adults. Am J Nurs. 2006;106(1):58-67, NaN-68. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. PubMed
8. Lindenauer PK, Pantilat SZ, Katz PP, Wachter RM. Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians. Ann Intern Med. 1999;130(4 Pt 2):343-349. PubMed
9. Wachter RM. The hospitalist movement 5 years later. JAMA. 2002;287(4):487. PubMed
10. Shank B. 2016: Celebrating 20 years of hospital medicine and looking toward a bright future. Hosp Natl Assoc Inpatient Physicians. 2016. http://www.the-hospitalist.org/hospitalist/article/121925/2016-celebrating-20-years-hospital-medicine-and-looking-toward-bright. Accessed June 2, 2017.
11. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC.: National Academies Press; 2008. http://www.nap.edu/catalog/12089. Accessed November 2, 2016.
12. Boult C, Counsell SR, Leipzig RM, Berenson RA. The urgency of preparing primary care physicians to care for older people with chronic illnesses. Health Aff Proj Hope. 2010;29(5):811-818. PubMed
13. Warshaw GA, Bragg EJ, Thomas DC, Ho ML, Brewer DE, Association of Directors of Geriatric Academic Programs. Are internal medicine residency programs adequately preparing physicians to care for the baby boomers? A national survey from the Association of Directors of Geriatric Academic Programs Status of Geriatrics Workforce Study. J Am Geriatr Soc. 2006;54(10):1603-1609. PubMed
14. Tanner CE, Eckstrom E, Desai SS, Joseph CL, Ririe MR, Bowen JL. Uncovering frustrations: A qualitative needs assessment of academic general internists as geriatric care providers and teachers. J Gen Intern Med. 2006;21(1):51-55. PubMed
15. Warshaw GA, Bragg EJ, Brewer DE, Meganathan K, Ho M. The development of academic geriatric medicine: progress toward preparing the nation’s physicians to care for an aging population. J Am Geriatr Soc. 2007;55(12):2075-2082. PubMed
16. Fox MT, Sidani S, Persaud M, et al. Acute care for elders components of acute geriatric unit care: Systematic descriptive review. J Am Geriatr Soc. 2013;61(6):939-946. PubMed
17. Palmer RM, Landefeld CS, Kresevic D, Kowal J. A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994;42(5):545-552.
18. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. PubMed
19. Sennour Y, Counsell SR, Jones J, Weiner M. Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139-2145. PubMed
20. Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211. PubMed
21. Mattison MLP, Catic A, Davis RB, et al. A standardized, bundled approach to providing geriatric-focused acute care. J Am Geriatr Soc. 2014;62(5):936-942. doi:10.1111/jgs.12780. PubMed
22. Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135(8 Pt 2):642-646. PubMed
23. Wenger NS, Roth CP, Shekelle P, ACOVE Investigators. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55 Suppl 2:S247-S252. PubMed
24. Reuben DB, Roth C, Kamberg C, Wenger NS. Restructuring primary care practices to manage geriatric syndromes: the ACOVE-2 intervention. J Am Geriatr Soc. 2003;51(12):1787-1793. PubMed
25. Askari M, Wierenga PC, Eslami S, Medlock S, De Rooij SE, Abu-Hanna A. Studies pertaining to the ACOVE quality criteria: a systematic review. Int J Qual Health Care. 2012;24(1):80-87. PubMed
26. Arora VM, McGory ML, Fung CH. Quality indicators for hospitalization and surgery in vulnerable elders. J Am Geriatr Soc. 2007;55 Suppl 2:S347-S358. PubMed
27. Arora VM, Johnson M, Olson J, et al. Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders. J Am Geriatr Soc. 2007;55(11):1705-1711. PubMed
28. Sands M, Dantoc B, Hartshorn A, Ryan C, Lujic S. Single Question in Delirium (SQiD): testing its efficacy against psychiatrist interview, the Confusion Assessment Method and the Memorial Delirium Assessment Scale. Palliat Med. 2010;24(6):561-565. PubMed
29. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. PubMed
30. Puelle MR, Kosar CM, Xu G, et al. The language of delirium: Keywords for identifying delirium from medical records. J Gerontol Nurs. 2015;41(8):34-42. PubMed
31. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed
32. Boult C, Boult L, Morishita L, Smith SL, Kane RL. Outpatient geriatric evaluation and management. J Am Geriatr Soc. 1998;46(3):296-302.33. Wenger NS, Roth CP, Shekelle PG, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc. 2009;57(3):547-555. PubMed
34. Geerts WH. Prevention of Venous Thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest J. 2008;133(6_suppl):381S.
35. Rosenman M, Liu X, Phatak H, et al. Pharmacological prophylaxis for venous thromboembolism among hospitalized patients with acute medical illness: An electronic medical records study. Am J Ther. 2016;23(2):e328-e335. PubMed
36. Ghanem A, Artime C, Moser M, Caceres L, Basconcillo A. Holy moley! Take out that foley! Measuring compliance with a nurse driven protocol for foley catheter removal to decrease utilization. Am J Infect Control. 2015;43(6):S51.
37. Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114(5):404-407. PubMed
38. Huang W-C, Wann S-R, Lin S-L, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
39. Topal J, Conklin S, Camp K, Morris V, Balcezak T, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121-126. PubMed
40. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63(1):55-62. PubMed
41. Inouye SK, Bogardus ST, Baker DI, Leo-Summers L, Cooney LM. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48(12):1697-1706. PubMed
42. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-347. PubMed
43. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61-65. PubMed
44. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. PubMed
45. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119-126. PubMed
46. Smith R. Validation and Reliability of the Elderly Mobility Scale. Physiotherapy. 1994;80(11):744-747.
47. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM. Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467-2473. PubMed
48. Gustafson Y, Brännström B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760-765. PubMed
49. Brenner SK, Kaushal R, Grinspan Z, et al. Effects of health information technology on patient outcomes: a systematic review. J Am Med Inform Assoc. 2016;23(5):1016-1036. PubMed
1. National Center for Health Statistics (US). Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics (US); 2016. http://www.ncbi.nlm.nih.gov/books/NBK367640/. Accessed November 2, 2016.
2. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief #180. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. http://www.ncbi.nlm.nih.gov/books/NBK259100/. Accessed November 2, 2016.
3. Jencks SF, Cuerdon T, Burwen DR, et al. Quality of medical care delivered to medicare beneficiaries: A profile at state and national levels. JAMA. 2000;284(13):1670-1676. PubMed
4. Covinsky KE, Pierluissi E, Johnston C. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. PubMed
5. Creditor MC. Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
6. Graf C. Functional decline in hospitalized older adults. Am J Nurs. 2006;106(1):58-67, NaN-68. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. PubMed
8. Lindenauer PK, Pantilat SZ, Katz PP, Wachter RM. Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians. Ann Intern Med. 1999;130(4 Pt 2):343-349. PubMed
9. Wachter RM. The hospitalist movement 5 years later. JAMA. 2002;287(4):487. PubMed
10. Shank B. 2016: Celebrating 20 years of hospital medicine and looking toward a bright future. Hosp Natl Assoc Inpatient Physicians. 2016. http://www.the-hospitalist.org/hospitalist/article/121925/2016-celebrating-20-years-hospital-medicine-and-looking-toward-bright. Accessed June 2, 2017.
11. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC.: National Academies Press; 2008. http://www.nap.edu/catalog/12089. Accessed November 2, 2016.
12. Boult C, Counsell SR, Leipzig RM, Berenson RA. The urgency of preparing primary care physicians to care for older people with chronic illnesses. Health Aff Proj Hope. 2010;29(5):811-818. PubMed
13. Warshaw GA, Bragg EJ, Thomas DC, Ho ML, Brewer DE, Association of Directors of Geriatric Academic Programs. Are internal medicine residency programs adequately preparing physicians to care for the baby boomers? A national survey from the Association of Directors of Geriatric Academic Programs Status of Geriatrics Workforce Study. J Am Geriatr Soc. 2006;54(10):1603-1609. PubMed
14. Tanner CE, Eckstrom E, Desai SS, Joseph CL, Ririe MR, Bowen JL. Uncovering frustrations: A qualitative needs assessment of academic general internists as geriatric care providers and teachers. J Gen Intern Med. 2006;21(1):51-55. PubMed
15. Warshaw GA, Bragg EJ, Brewer DE, Meganathan K, Ho M. The development of academic geriatric medicine: progress toward preparing the nation’s physicians to care for an aging population. J Am Geriatr Soc. 2007;55(12):2075-2082. PubMed
16. Fox MT, Sidani S, Persaud M, et al. Acute care for elders components of acute geriatric unit care: Systematic descriptive review. J Am Geriatr Soc. 2013;61(6):939-946. PubMed
17. Palmer RM, Landefeld CS, Kresevic D, Kowal J. A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994;42(5):545-552.
18. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. PubMed
19. Sennour Y, Counsell SR, Jones J, Weiner M. Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139-2145. PubMed
20. Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211. PubMed
21. Mattison MLP, Catic A, Davis RB, et al. A standardized, bundled approach to providing geriatric-focused acute care. J Am Geriatr Soc. 2014;62(5):936-942. doi:10.1111/jgs.12780. PubMed
22. Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135(8 Pt 2):642-646. PubMed
23. Wenger NS, Roth CP, Shekelle P, ACOVE Investigators. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55 Suppl 2:S247-S252. PubMed
24. Reuben DB, Roth C, Kamberg C, Wenger NS. Restructuring primary care practices to manage geriatric syndromes: the ACOVE-2 intervention. J Am Geriatr Soc. 2003;51(12):1787-1793. PubMed
25. Askari M, Wierenga PC, Eslami S, Medlock S, De Rooij SE, Abu-Hanna A. Studies pertaining to the ACOVE quality criteria: a systematic review. Int J Qual Health Care. 2012;24(1):80-87. PubMed
26. Arora VM, McGory ML, Fung CH. Quality indicators for hospitalization and surgery in vulnerable elders. J Am Geriatr Soc. 2007;55 Suppl 2:S347-S358. PubMed
27. Arora VM, Johnson M, Olson J, et al. Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders. J Am Geriatr Soc. 2007;55(11):1705-1711. PubMed
28. Sands M, Dantoc B, Hartshorn A, Ryan C, Lujic S. Single Question in Delirium (SQiD): testing its efficacy against psychiatrist interview, the Confusion Assessment Method and the Memorial Delirium Assessment Scale. Palliat Med. 2010;24(6):561-565. PubMed
29. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. PubMed
30. Puelle MR, Kosar CM, Xu G, et al. The language of delirium: Keywords for identifying delirium from medical records. J Gerontol Nurs. 2015;41(8):34-42. PubMed
31. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed
32. Boult C, Boult L, Morishita L, Smith SL, Kane RL. Outpatient geriatric evaluation and management. J Am Geriatr Soc. 1998;46(3):296-302.33. Wenger NS, Roth CP, Shekelle PG, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc. 2009;57(3):547-555. PubMed
34. Geerts WH. Prevention of Venous Thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest J. 2008;133(6_suppl):381S.
35. Rosenman M, Liu X, Phatak H, et al. Pharmacological prophylaxis for venous thromboembolism among hospitalized patients with acute medical illness: An electronic medical records study. Am J Ther. 2016;23(2):e328-e335. PubMed
36. Ghanem A, Artime C, Moser M, Caceres L, Basconcillo A. Holy moley! Take out that foley! Measuring compliance with a nurse driven protocol for foley catheter removal to decrease utilization. Am J Infect Control. 2015;43(6):S51.
37. Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114(5):404-407. PubMed
38. Huang W-C, Wann S-R, Lin S-L, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
39. Topal J, Conklin S, Camp K, Morris V, Balcezak T, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121-126. PubMed
40. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63(1):55-62. PubMed
41. Inouye SK, Bogardus ST, Baker DI, Leo-Summers L, Cooney LM. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48(12):1697-1706. PubMed
42. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-347. PubMed
43. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61-65. PubMed
44. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. PubMed
45. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119-126. PubMed
46. Smith R. Validation and Reliability of the Elderly Mobility Scale. Physiotherapy. 1994;80(11):744-747.
47. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM. Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467-2473. PubMed
48. Gustafson Y, Brännström B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760-765. PubMed
49. Brenner SK, Kaushal R, Grinspan Z, et al. Effects of health information technology on patient outcomes: a systematic review. J Am Med Inform Assoc. 2016;23(5):1016-1036. PubMed
© 2017 Society of Hospital Medicine
A simple algorithm for predicting bacteremia using food consumption and shaking chills: a prospective observational study
Fever in hospitalized patients is a nonspecific finding with many potential causes. Blood cultures (BC) are commonly obtained prior to commencing parenteral antibiotics in febrile patients. However, as many as 35% to 50% of positive BCs represent a contamination with organisms inoculated from the skin into culture bottles at the time of sample collection.1-3 Such results represent false-positive BCs that can lead to unnecessary investigations and treatment.
Recently, Coburn et al. reviewed the severity of chills (graded on an ordinal scale) as the most useful predictor of true bacteremia (positive likelihood ratio [LR], 4.7; 95% confidence interval [CI], 3.0–7.2),4-6 and the lack of the systemic inflammatory response syndrome (SIRS) criteria as the best negative indicator of true bacteremia with a negative LR of 0.09 (95% CI, 0.03-0.3).6,7 We have also previously reported normal food consumption as a negative indicator of true bacteremia, with a 98.3% negative predictive value.8 Henderson’s Basic Principles of Nursing Care emphasizes the importance of evaluating whether a patient can eat and drink adequately,9 and the evaluation of a patient’s food consumption is a routine nursing staff practice, which is treated as vital sign in Japan, in contrast to nursing practices in the United States.
However, these data were the result of a single-center retrospective study using the nursing staff’s assessment of food consumption, and they cannot be generalized to larger patient populations. Therefore, the aim of this prospective, multicenter study was to measure the accuracy of food consumption and shaking chills as predictive factors for true bacteremia.
METHODS
Study Design
This was a prospective multicenter observational study (UMIN ID: R000013768) involving 3 hospitals in Tokyo, Japan, that enrolled consecutive patients who had BCs obtained. This study was approved by the ethical committee at Juntendo University Nerima Hospital and each of the participating centers, and the study was conducted in accordance with the Declaration of Helsinki 1971, as revised in 1983. We evaluated 2,792 consecutive hospitalized patients (mean age, 68.9 ± 17.1 years; 55.3% men) who had BCs obtained between April 2013 and August 2014, inclusive. The indication for BC acquisition was at the discretion of the treating physician. The study protocol and the indication for BCs are described in detail elsewhere.8 We excluded patients with anorexia-inducing conditions such as gastrointestinal disease, including gastrointestinal bleeding, enterocolitis, gastric ulceration, peritonitis, appendicitis, cholangitis, pancreatitis, diverticulitis, and ischemic colitis. We also excluded patients receiving chemotherapy for malignancy. In this study, true bacteremia was defined as identical organisms isolated from 2 sets of blood cultures (a set refers to one aerobic bottle and one anaerobic bottle). Moreover, even if only one set of blood cultures was acquired, when the identified pathogen could account for the clinical presentation, we also defined this as true bacteremia. Briefly, contaminants were defined as organisms common to skin flora, including Bacillus species, coagulase-negative Staphylococcus, Corynebacterium species, and Micrococcus species, without isolation of an identical organism with the same antibiotic susceptibilities from another potentially infected site in a patient with incompatible clinical features and no risk factors for infection with the isolated organism. Single BCs that were positive for organisms that were unlikely to explain the patient’s symptoms were also considered as contaminants. Patients with contaminated BCs were excluded from the analyses.
Structure of Reliability Study Procedures
Nurses in the 3 different hospitals performed daily independent food consumption ratings during each patient’s stay. Interrater reliability assessments were conducted in the morning or afternoon, and none of the raters had access to the other nurses’ scores at any time. The study nurses performed simultaneous ratings during these assessments (one interacted with and rated the patient while the other observed and rated the same patient).
Prediction Variables of True Bacteremia
1. Food consumption. Assessment of food consumption has been previously described in detail.8 Briefly, we characterized the patients’ oral intake based on the meal taken immediately prior to the BCs. For example, if a fever developed at 2
2. Chills. As done previously, the physician evaluated the patient for a history of chills at the time of BCs and classified the patients into 1 of 4 grades4,5: “no chills,” the absence of any chills; “mild chills,” feeling cold, equivalent to needing an outer jacket; “moderate chills,” feeling very cold, equivalent to needing a thick blanket; and “shaking chills,” feeling extremely cold with rigors and generalized bodily shaking, even under a thick blanket. To distinguish between those patients who had shaking chills and those who did not, we divided the patients into 2 groups: the “shaking chills group” and the combination of none, mild, and moderate chills, referred to as the “negative shaking chills group.”
3. Other predictive variables. We considered the following additional predictive variables: age, gender, axillary body temperature (BT), heart rate (HR), systolic blood pressure (SBP), respiratory rate (RR), white blood cell count (WBC), and serum C-reactive protein level (CRP). These predictive variables were obtained immediately prior to the BCs. We defined SIRS based on standard criteria (HR >90 beats/m, RR >20/m, BT <36°C or >38°C, and a WBC <4 × 103 WBC/μL or >12 × 103 WBC/μL). Patients were subcategorized by age into 2 groups (≤69 years and >70 years). CRP levels were dichotomized as >10.0 mg/dL or ≤10.0 mg/dL. We reviewed the patients’ charts to determine whether they had received antibiotics. In the case of walk-in patients, we interviewed the patients regarding whether they had visited a clinic; if they had, they were questioned as to whether any antibiotic therapy had been prescribed.
Statistical Analysis
Continuous variables are presented as the mean with the associated standard deviation (SD). All potential variables predictive of true bacteremia are shown in Table 1. The variables were dichotomized by clinically meaningful thresholds and used as potential risk-adjusted variables. We calculated the sensitivity and specificity and positive and negative predictive value for each criterion. Multiple logistic regression analysis was used to select components that were significantly associated with true bacteremia (the level of statistical significance determined with maximum likelihood methods was set at P < .05). To visualize and quantify other aspects in the prediction of true bacteremia, a recursive partitioning analysis (RPA) was used to make a decision tree model for true bacteremia. This nonparametric regression method produces a classification tree following a series of nonsequential top-down binary splits. The tree-building process starts by considering a set of predictive variables and selects the variable that produces 2 subsets of participants with the greatest purity. Two factors are considered when splitting a node into its daughter nodes: the goodness of the split and the amount of impurity in the daughter nodes. The splitting process is repeated until further partitioning is no longer possible and the terminal nodes have been reached. Details on this method are discussed in Monte Carlo Calibration of Distributions of Partition Statistics (www.jmp.com).
Probability was considered significant at a value of P < .05. All statistical tests were 2-tailed. Statistical analyses were conducted by a physician (KI) and an independent statistician (JM) with the use of the SPSS® v.16.0 software package (SPSS Inc., Chicago, IL) and JMP® version 8.0.2 (SAS Institute, Cary, NC).
RESULTS
Patients Characteristics
Two thousand seven hundred and ninety-two patients met the inclusion criteria for our study, from which 849 were excluded (see Figure 1 for flow diagram). Among the remaining 1,943 patients, there were 317 patients with positive BCs, of which 221 patients (69.7%) were considered to have true-positive BCs and 96 (30.3%) were considered to have contaminated BCs. After excluding these 96 patients, 221 patients with true bacteremia (true bacteremic group) were compared with 1,626 nonbacteremic patients (nonbacteremic group; Figure 1). The baseline characteristics of the subjects are shown in Table 1. The mean BT was 38.4 ± 1.2°C in the true bacteremic group and 37.9 ± 1.0°C in the nonbacteremic group. The mean serum CRP level was 11.6 ± 9.6 mg/dL in the true bacteremic group and 7.3 ± 6.9 mg/dL in the nonbacteremic group. In the true bacteremic group, there were 6 afebrile patients, and 27 patients without leukocytosis. The pathogens identified from the true-positive BCs were Escherichia coli (n = 59, 26.7%), including extended-spectrum beta-lactamase producing species, Staphylococcus aureus (n = 36, 16.3%), including methicillin-resistant Staphylococcus aureus, and Klebsiella pneumoniae (n = 22, 10.0%; Supplemental Table 1).
The underlying clinical diagnoses in the true bacteremic group included urinary tract infection (UTI), pneumonia, abscess, catheter-related bloodstream infection (CRBSI), cellulitis, osteomyelitis, infective endocarditis (IE), chorioamnionitis, iatrogenic infection at hemodialysis puncture sites, bacterial meningitis, septic arthritis, and infection of unknown cause (Supplemental Table 2).
Interrater Reliability Testing of Food Consumption
Patients were evaluated during their hospital stays. The interrater reliability of the evaluation of food consumption was very high across all participating hospitals (Supplemental Table 3). To assess the reliability of the evaluations of food consumption, patients (separate from this main study) were selected randomly and evaluated independently by 2 nurses in 3 different hospitals. The kappa scores of agreement between the nurses at the 3 different hospitals were 0.83 (95% CI, 0.63-0.88), 0.90 (95% CI, 0.80-0.99), and 0.80 (95% CI, 0.67-0.99), respectively. The interrater reliability of food consumption evaluation by the nurses was very high at all participating hospitals.
Food Consumption
The low, moderate, and high food consumption groups consisted of 964 (52.1%), 306 (16.6%), and 577 (31.2%) patients, respectively (Table 1). Of these, 174 (18.0%), 33 (10.8%), and 14 (2.4%) patients, respectively, had true bacteremia. The presence of poor food consumption had a sensitivity of 93.7% (95% CI, 89.4%-97.9%), specificity of 34.6% (95% CI, 33.0%-36.2%), and a positive LR of 1.43 (95% CI, 1.37-1.50) for predicting true bacteremia. Conversely, the absence of poor food consumption (ie, normal food consumption) had a negative LR of 0.18 (95% CI, 0.17-0.19).
Chills
The no, mild, moderate, and shaking chills groups consisted of 1,514 (82.0%), 148 (8.0%), 53 (2.9%), and 132 (7.1%) patients, respectively (Table 1). Of these, 136 (9.0%), 25 (16.9%), 8 (15.1%), and 52 (39.4%) patients, respectively, had true bacteremia. The presence of shaking chills had a sensitivity of 23.5% (95% CI, 22.5%-24.6%), a specificity of 95.1% (95% CI, 90.7%-99.4%), and a positive LR of 4.78 (95% CI, 4.56–5.00) for predicting true bacteremia. Conversely, the absence of shaking chills had a negative LR of 0.80 (95% CI, 0.77-0.84).
Prediction Model for True Bacteremia
The components identified as significantly related to true bacteremia by multiple logistic regression analysis are indicated in Table 2. The significant predictors of true bacteremia were shaking chills (odds ratio [OR], 5.6; 95% CI, 3.6-8.6; P < .01), SBP <90 mmHg (OR, 3.1; 95% CI, 1.6-5.7; P < 01), CRP levels >10.0 mg/dL (OR, 2.2; 95% CI, 1.6-3.1; P < .01), BT <36°C or >38°C (OR, 1.8; 95% CI, 1.3-2.6; P < .01), WBC <4 × 103/μL or >12 × 103/μL (OR, 1.6; 95% CI, 1.2-2.3; P = .003), HR >90 bpm (OR, 1.5; 95% CI, 1.1-2.1; P = .021), and female (OR, 1.4; 95% CI, 1.0-1.9; P = .036). An RPA to create an ideal prediction model for patients with true bacteremia or nonbacteremia is shown in Figure 2. The original group consisted of 1,847 patients, including 221 patients with true bacteremia. The pretest probability of true bacteremia was 2.4% (14/577) for those with normal food consumption (Group 1) and 2.4% (13/552) for those with both normal food consumption and the absence of shaking chills (Group 2). Conversely, the pretest probability of true bacteremia was 16.3% (207/1270) for those with poor food consumption and 47.7% (51/107) for those with both poor food consumption and shaking chills. The patients with true bacteremia with normal food consumption and without shaking chills consisted of 4 cases of CRBSI and UTI, 2 cases of osteomyelitis, 1 case of IE, 1 case of chorioamnionitis, and 1 case for which the focus was unknown (Supplemental Table 4).
DISCUSSION
In this observational study, we evaluated if a simple algorithm using food consumption and shaking chills was useful for assessing whether a patient had true bacteremia. A 2-item screening checklist (nursing assessment of food consumption and shaking chills) had excellent statistical properties as a brief screening instrument for true bacteremia.
We have prospectively validated that food consumption, as assessed by nurses, is a reliable predictor of true bacteremia.8 A previous single-center retrospective study showed similar findings, but these could not be generalized across all institutions because of the limited nature of the study. In this multicenter study, we used 2 statistical methods to reduce selection bias. First, we performed a kappa analysis across the hospitals to evaluate the interrater reliability of the evaluation of food consumption. Second, we used an RPA (Figure 2), also known as a decision tree model. RPA is a step-by-step process by which a decision tree is constructed by either splitting or not splitting each node on the tree into 2 daughter nodes.10 By using this method, we successfully generated an ideal approach to predict true bacteremia using food consumption and shaking chills. After adjusting for food consumption and shaking chills, groups 1 to 2 had sequentially decreasing diagnoses of true bacteremia, varying from 221 patients to only 13 patients.
Appetite is influenced by many factors that are integrated by the brain, most importantly within the hypothalamus. Signals that impinge on the hypothalamic center include neural afferents, hormones, cytokines, and metabolites.11 These factors elicit “sickness behavior,” which includes a decrease in food-motivated behavior.12 Furthermore, exposure to pathogenic bacteria increases serotonin, which has been shown to decrease metabolism in
The strengths of this study include its relatively large sample size, multicenter design, uniformity of data collection across sites, and completeness of data collection from study participants. All of these factors allowed for a robust analysis.
However, there are several limitations of this study. First, the physicians or nurses asked the patients about the presence of shaking chills when they obtained the BCs. It may be difficult for patients, especially elderly patients, to provide this information promptly and accurately. Some patients did not call the nurse when they had shaking chills, and the chills were not witnessed by a healthcare provider. However, we used a more specific definition for shaking chills: a feeling of being extremely cold with rigors and generalized bodily shaking, even under a thick blanket. Second, this algorithm is not applicable to patients with immunosuppressed states because none of the hospitals involved in this study perform bone marrow or organ transplantation. Third, although we included patients with dementia in our cohort, we did not specifically evaluate performance of the algorithm in patients with this medical condition. It is possible that the algorithm would not perform well in this subset of patients owing to their unreliable appetite and food intake. Fourth, some medications may affect appetite, leading to reduced food consumption. Although we have not considered the details of medications in this study, we found that the pretest probability of true bacteremia was low for those patients with normal food consumption regardless of whether the medication affected their appetites or not. However, the question of whether medications truly affect patients’ appetites concurrently with bacteremia would need to be specifically addressed in a future study.
CONCLUSION
In conclusion, we have established a simple algorithm to identify patients with suspected true bacteremia who require the acquisition of blood cultures. This extremely simple model can enable physicians to make a rapid bedside estimation of the risk of true bacteremia.
Acknowledgment
The authors thank Drs. H. Honda and S. Saint, and Ms. A. Okada for their helpful discussions with regard to this study; Ms. M. Takigawa for the collection of data; and Ms. T. Oguri for providing infectious disease consultation on the pathogenicity of the identified organisms.
Disclosure
This work was supported by JSPS KAKENHI Grant Number 15K19294 (to TK) and 20590840 (to KI) from the Japan Society for the Promotion of Science. The authors report no potential conflicts of interest relevant to this article.
1. Weinstein MP, Towns ML, Quartey SM et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584-602. PubMed
2. Strand CL, Wajsbort RR, Sturmann K. Effect of iodophor vs iodine tincture skin preparation on blood culture contamination rate. JAMA. 1993;269:1004-1006. PubMed
3. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265:365-369. PubMed
4. Tokuda Y, Miyasato H, Stein GH. A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98:813-820. PubMed
5. Tokuda Y, Miyasato H, Stein GH, Kishaba T. The degree of chills for risk of bacteremia in acute febrile illness. Am J Med. 2005;118:1417. PubMed
6. Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012;308:502-511. PubMed
7. Shapiro NI, Wolfe RE, Wright SB, Moore R, Bates DW. Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med. 2008;35:255-264. PubMed
8. Komatsu T, Onda T, Murayama G, et al. Predicting bacteremia based on nurse-assessed food consumption at the time of blood culture. J Hosp Med. 2012;7:702-705. PubMed
9. Henderson V. Basic Principles of Nursing Care. 2nd ed. Silver Spring, MD: American Nurses Association; 1969.
10. Therneau T, Atkinson, EJ. An Introduction to Recursive Partitioning using the RPART Routines. Mayo Foundation 2017. https://cran.r-project.org/web/packages/rpart/vignettes/longintro.pdf. Accessed May 5, 2017.
11. Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med .2003;9:125-134. PubMed
12. Hansen MK, Nguyen KT, Fleshner M, et al. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2000;278:R331-336. PubMed
13. Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 2005;438:179-84. PubMed
14. Van Dissel JT, Schijf V, Vogtlander N, Hoogendoorn M, van’t Wout J. Implications of chills. Lancet 1998;352:374. PubMed
15. Fukui S, Uehara Y, Fujibayashi K, et al. Bacteraemia predictive factors among general medical inpatients: a retrospective cross-sectional survey in a Japanese university hospital. BMJ Open 2016;6:e010527. PubMed
Fever in hospitalized patients is a nonspecific finding with many potential causes. Blood cultures (BC) are commonly obtained prior to commencing parenteral antibiotics in febrile patients. However, as many as 35% to 50% of positive BCs represent a contamination with organisms inoculated from the skin into culture bottles at the time of sample collection.1-3 Such results represent false-positive BCs that can lead to unnecessary investigations and treatment.
Recently, Coburn et al. reviewed the severity of chills (graded on an ordinal scale) as the most useful predictor of true bacteremia (positive likelihood ratio [LR], 4.7; 95% confidence interval [CI], 3.0–7.2),4-6 and the lack of the systemic inflammatory response syndrome (SIRS) criteria as the best negative indicator of true bacteremia with a negative LR of 0.09 (95% CI, 0.03-0.3).6,7 We have also previously reported normal food consumption as a negative indicator of true bacteremia, with a 98.3% negative predictive value.8 Henderson’s Basic Principles of Nursing Care emphasizes the importance of evaluating whether a patient can eat and drink adequately,9 and the evaluation of a patient’s food consumption is a routine nursing staff practice, which is treated as vital sign in Japan, in contrast to nursing practices in the United States.
However, these data were the result of a single-center retrospective study using the nursing staff’s assessment of food consumption, and they cannot be generalized to larger patient populations. Therefore, the aim of this prospective, multicenter study was to measure the accuracy of food consumption and shaking chills as predictive factors for true bacteremia.
METHODS
Study Design
This was a prospective multicenter observational study (UMIN ID: R000013768) involving 3 hospitals in Tokyo, Japan, that enrolled consecutive patients who had BCs obtained. This study was approved by the ethical committee at Juntendo University Nerima Hospital and each of the participating centers, and the study was conducted in accordance with the Declaration of Helsinki 1971, as revised in 1983. We evaluated 2,792 consecutive hospitalized patients (mean age, 68.9 ± 17.1 years; 55.3% men) who had BCs obtained between April 2013 and August 2014, inclusive. The indication for BC acquisition was at the discretion of the treating physician. The study protocol and the indication for BCs are described in detail elsewhere.8 We excluded patients with anorexia-inducing conditions such as gastrointestinal disease, including gastrointestinal bleeding, enterocolitis, gastric ulceration, peritonitis, appendicitis, cholangitis, pancreatitis, diverticulitis, and ischemic colitis. We also excluded patients receiving chemotherapy for malignancy. In this study, true bacteremia was defined as identical organisms isolated from 2 sets of blood cultures (a set refers to one aerobic bottle and one anaerobic bottle). Moreover, even if only one set of blood cultures was acquired, when the identified pathogen could account for the clinical presentation, we also defined this as true bacteremia. Briefly, contaminants were defined as organisms common to skin flora, including Bacillus species, coagulase-negative Staphylococcus, Corynebacterium species, and Micrococcus species, without isolation of an identical organism with the same antibiotic susceptibilities from another potentially infected site in a patient with incompatible clinical features and no risk factors for infection with the isolated organism. Single BCs that were positive for organisms that were unlikely to explain the patient’s symptoms were also considered as contaminants. Patients with contaminated BCs were excluded from the analyses.
Structure of Reliability Study Procedures
Nurses in the 3 different hospitals performed daily independent food consumption ratings during each patient’s stay. Interrater reliability assessments were conducted in the morning or afternoon, and none of the raters had access to the other nurses’ scores at any time. The study nurses performed simultaneous ratings during these assessments (one interacted with and rated the patient while the other observed and rated the same patient).
Prediction Variables of True Bacteremia
1. Food consumption. Assessment of food consumption has been previously described in detail.8 Briefly, we characterized the patients’ oral intake based on the meal taken immediately prior to the BCs. For example, if a fever developed at 2
2. Chills. As done previously, the physician evaluated the patient for a history of chills at the time of BCs and classified the patients into 1 of 4 grades4,5: “no chills,” the absence of any chills; “mild chills,” feeling cold, equivalent to needing an outer jacket; “moderate chills,” feeling very cold, equivalent to needing a thick blanket; and “shaking chills,” feeling extremely cold with rigors and generalized bodily shaking, even under a thick blanket. To distinguish between those patients who had shaking chills and those who did not, we divided the patients into 2 groups: the “shaking chills group” and the combination of none, mild, and moderate chills, referred to as the “negative shaking chills group.”
3. Other predictive variables. We considered the following additional predictive variables: age, gender, axillary body temperature (BT), heart rate (HR), systolic blood pressure (SBP), respiratory rate (RR), white blood cell count (WBC), and serum C-reactive protein level (CRP). These predictive variables were obtained immediately prior to the BCs. We defined SIRS based on standard criteria (HR >90 beats/m, RR >20/m, BT <36°C or >38°C, and a WBC <4 × 103 WBC/μL or >12 × 103 WBC/μL). Patients were subcategorized by age into 2 groups (≤69 years and >70 years). CRP levels were dichotomized as >10.0 mg/dL or ≤10.0 mg/dL. We reviewed the patients’ charts to determine whether they had received antibiotics. In the case of walk-in patients, we interviewed the patients regarding whether they had visited a clinic; if they had, they were questioned as to whether any antibiotic therapy had been prescribed.
Statistical Analysis
Continuous variables are presented as the mean with the associated standard deviation (SD). All potential variables predictive of true bacteremia are shown in Table 1. The variables were dichotomized by clinically meaningful thresholds and used as potential risk-adjusted variables. We calculated the sensitivity and specificity and positive and negative predictive value for each criterion. Multiple logistic regression analysis was used to select components that were significantly associated with true bacteremia (the level of statistical significance determined with maximum likelihood methods was set at P < .05). To visualize and quantify other aspects in the prediction of true bacteremia, a recursive partitioning analysis (RPA) was used to make a decision tree model for true bacteremia. This nonparametric regression method produces a classification tree following a series of nonsequential top-down binary splits. The tree-building process starts by considering a set of predictive variables and selects the variable that produces 2 subsets of participants with the greatest purity. Two factors are considered when splitting a node into its daughter nodes: the goodness of the split and the amount of impurity in the daughter nodes. The splitting process is repeated until further partitioning is no longer possible and the terminal nodes have been reached. Details on this method are discussed in Monte Carlo Calibration of Distributions of Partition Statistics (www.jmp.com).
Probability was considered significant at a value of P < .05. All statistical tests were 2-tailed. Statistical analyses were conducted by a physician (KI) and an independent statistician (JM) with the use of the SPSS® v.16.0 software package (SPSS Inc., Chicago, IL) and JMP® version 8.0.2 (SAS Institute, Cary, NC).
RESULTS
Patients Characteristics
Two thousand seven hundred and ninety-two patients met the inclusion criteria for our study, from which 849 were excluded (see Figure 1 for flow diagram). Among the remaining 1,943 patients, there were 317 patients with positive BCs, of which 221 patients (69.7%) were considered to have true-positive BCs and 96 (30.3%) were considered to have contaminated BCs. After excluding these 96 patients, 221 patients with true bacteremia (true bacteremic group) were compared with 1,626 nonbacteremic patients (nonbacteremic group; Figure 1). The baseline characteristics of the subjects are shown in Table 1. The mean BT was 38.4 ± 1.2°C in the true bacteremic group and 37.9 ± 1.0°C in the nonbacteremic group. The mean serum CRP level was 11.6 ± 9.6 mg/dL in the true bacteremic group and 7.3 ± 6.9 mg/dL in the nonbacteremic group. In the true bacteremic group, there were 6 afebrile patients, and 27 patients without leukocytosis. The pathogens identified from the true-positive BCs were Escherichia coli (n = 59, 26.7%), including extended-spectrum beta-lactamase producing species, Staphylococcus aureus (n = 36, 16.3%), including methicillin-resistant Staphylococcus aureus, and Klebsiella pneumoniae (n = 22, 10.0%; Supplemental Table 1).
The underlying clinical diagnoses in the true bacteremic group included urinary tract infection (UTI), pneumonia, abscess, catheter-related bloodstream infection (CRBSI), cellulitis, osteomyelitis, infective endocarditis (IE), chorioamnionitis, iatrogenic infection at hemodialysis puncture sites, bacterial meningitis, septic arthritis, and infection of unknown cause (Supplemental Table 2).
Interrater Reliability Testing of Food Consumption
Patients were evaluated during their hospital stays. The interrater reliability of the evaluation of food consumption was very high across all participating hospitals (Supplemental Table 3). To assess the reliability of the evaluations of food consumption, patients (separate from this main study) were selected randomly and evaluated independently by 2 nurses in 3 different hospitals. The kappa scores of agreement between the nurses at the 3 different hospitals were 0.83 (95% CI, 0.63-0.88), 0.90 (95% CI, 0.80-0.99), and 0.80 (95% CI, 0.67-0.99), respectively. The interrater reliability of food consumption evaluation by the nurses was very high at all participating hospitals.
Food Consumption
The low, moderate, and high food consumption groups consisted of 964 (52.1%), 306 (16.6%), and 577 (31.2%) patients, respectively (Table 1). Of these, 174 (18.0%), 33 (10.8%), and 14 (2.4%) patients, respectively, had true bacteremia. The presence of poor food consumption had a sensitivity of 93.7% (95% CI, 89.4%-97.9%), specificity of 34.6% (95% CI, 33.0%-36.2%), and a positive LR of 1.43 (95% CI, 1.37-1.50) for predicting true bacteremia. Conversely, the absence of poor food consumption (ie, normal food consumption) had a negative LR of 0.18 (95% CI, 0.17-0.19).
Chills
The no, mild, moderate, and shaking chills groups consisted of 1,514 (82.0%), 148 (8.0%), 53 (2.9%), and 132 (7.1%) patients, respectively (Table 1). Of these, 136 (9.0%), 25 (16.9%), 8 (15.1%), and 52 (39.4%) patients, respectively, had true bacteremia. The presence of shaking chills had a sensitivity of 23.5% (95% CI, 22.5%-24.6%), a specificity of 95.1% (95% CI, 90.7%-99.4%), and a positive LR of 4.78 (95% CI, 4.56–5.00) for predicting true bacteremia. Conversely, the absence of shaking chills had a negative LR of 0.80 (95% CI, 0.77-0.84).
Prediction Model for True Bacteremia
The components identified as significantly related to true bacteremia by multiple logistic regression analysis are indicated in Table 2. The significant predictors of true bacteremia were shaking chills (odds ratio [OR], 5.6; 95% CI, 3.6-8.6; P < .01), SBP <90 mmHg (OR, 3.1; 95% CI, 1.6-5.7; P < 01), CRP levels >10.0 mg/dL (OR, 2.2; 95% CI, 1.6-3.1; P < .01), BT <36°C or >38°C (OR, 1.8; 95% CI, 1.3-2.6; P < .01), WBC <4 × 103/μL or >12 × 103/μL (OR, 1.6; 95% CI, 1.2-2.3; P = .003), HR >90 bpm (OR, 1.5; 95% CI, 1.1-2.1; P = .021), and female (OR, 1.4; 95% CI, 1.0-1.9; P = .036). An RPA to create an ideal prediction model for patients with true bacteremia or nonbacteremia is shown in Figure 2. The original group consisted of 1,847 patients, including 221 patients with true bacteremia. The pretest probability of true bacteremia was 2.4% (14/577) for those with normal food consumption (Group 1) and 2.4% (13/552) for those with both normal food consumption and the absence of shaking chills (Group 2). Conversely, the pretest probability of true bacteremia was 16.3% (207/1270) for those with poor food consumption and 47.7% (51/107) for those with both poor food consumption and shaking chills. The patients with true bacteremia with normal food consumption and without shaking chills consisted of 4 cases of CRBSI and UTI, 2 cases of osteomyelitis, 1 case of IE, 1 case of chorioamnionitis, and 1 case for which the focus was unknown (Supplemental Table 4).
DISCUSSION
In this observational study, we evaluated if a simple algorithm using food consumption and shaking chills was useful for assessing whether a patient had true bacteremia. A 2-item screening checklist (nursing assessment of food consumption and shaking chills) had excellent statistical properties as a brief screening instrument for true bacteremia.
We have prospectively validated that food consumption, as assessed by nurses, is a reliable predictor of true bacteremia.8 A previous single-center retrospective study showed similar findings, but these could not be generalized across all institutions because of the limited nature of the study. In this multicenter study, we used 2 statistical methods to reduce selection bias. First, we performed a kappa analysis across the hospitals to evaluate the interrater reliability of the evaluation of food consumption. Second, we used an RPA (Figure 2), also known as a decision tree model. RPA is a step-by-step process by which a decision tree is constructed by either splitting or not splitting each node on the tree into 2 daughter nodes.10 By using this method, we successfully generated an ideal approach to predict true bacteremia using food consumption and shaking chills. After adjusting for food consumption and shaking chills, groups 1 to 2 had sequentially decreasing diagnoses of true bacteremia, varying from 221 patients to only 13 patients.
Appetite is influenced by many factors that are integrated by the brain, most importantly within the hypothalamus. Signals that impinge on the hypothalamic center include neural afferents, hormones, cytokines, and metabolites.11 These factors elicit “sickness behavior,” which includes a decrease in food-motivated behavior.12 Furthermore, exposure to pathogenic bacteria increases serotonin, which has been shown to decrease metabolism in
The strengths of this study include its relatively large sample size, multicenter design, uniformity of data collection across sites, and completeness of data collection from study participants. All of these factors allowed for a robust analysis.
However, there are several limitations of this study. First, the physicians or nurses asked the patients about the presence of shaking chills when they obtained the BCs. It may be difficult for patients, especially elderly patients, to provide this information promptly and accurately. Some patients did not call the nurse when they had shaking chills, and the chills were not witnessed by a healthcare provider. However, we used a more specific definition for shaking chills: a feeling of being extremely cold with rigors and generalized bodily shaking, even under a thick blanket. Second, this algorithm is not applicable to patients with immunosuppressed states because none of the hospitals involved in this study perform bone marrow or organ transplantation. Third, although we included patients with dementia in our cohort, we did not specifically evaluate performance of the algorithm in patients with this medical condition. It is possible that the algorithm would not perform well in this subset of patients owing to their unreliable appetite and food intake. Fourth, some medications may affect appetite, leading to reduced food consumption. Although we have not considered the details of medications in this study, we found that the pretest probability of true bacteremia was low for those patients with normal food consumption regardless of whether the medication affected their appetites or not. However, the question of whether medications truly affect patients’ appetites concurrently with bacteremia would need to be specifically addressed in a future study.
CONCLUSION
In conclusion, we have established a simple algorithm to identify patients with suspected true bacteremia who require the acquisition of blood cultures. This extremely simple model can enable physicians to make a rapid bedside estimation of the risk of true bacteremia.
Acknowledgment
The authors thank Drs. H. Honda and S. Saint, and Ms. A. Okada for their helpful discussions with regard to this study; Ms. M. Takigawa for the collection of data; and Ms. T. Oguri for providing infectious disease consultation on the pathogenicity of the identified organisms.
Disclosure
This work was supported by JSPS KAKENHI Grant Number 15K19294 (to TK) and 20590840 (to KI) from the Japan Society for the Promotion of Science. The authors report no potential conflicts of interest relevant to this article.
Fever in hospitalized patients is a nonspecific finding with many potential causes. Blood cultures (BC) are commonly obtained prior to commencing parenteral antibiotics in febrile patients. However, as many as 35% to 50% of positive BCs represent a contamination with organisms inoculated from the skin into culture bottles at the time of sample collection.1-3 Such results represent false-positive BCs that can lead to unnecessary investigations and treatment.
Recently, Coburn et al. reviewed the severity of chills (graded on an ordinal scale) as the most useful predictor of true bacteremia (positive likelihood ratio [LR], 4.7; 95% confidence interval [CI], 3.0–7.2),4-6 and the lack of the systemic inflammatory response syndrome (SIRS) criteria as the best negative indicator of true bacteremia with a negative LR of 0.09 (95% CI, 0.03-0.3).6,7 We have also previously reported normal food consumption as a negative indicator of true bacteremia, with a 98.3% negative predictive value.8 Henderson’s Basic Principles of Nursing Care emphasizes the importance of evaluating whether a patient can eat and drink adequately,9 and the evaluation of a patient’s food consumption is a routine nursing staff practice, which is treated as vital sign in Japan, in contrast to nursing practices in the United States.
However, these data were the result of a single-center retrospective study using the nursing staff’s assessment of food consumption, and they cannot be generalized to larger patient populations. Therefore, the aim of this prospective, multicenter study was to measure the accuracy of food consumption and shaking chills as predictive factors for true bacteremia.
METHODS
Study Design
This was a prospective multicenter observational study (UMIN ID: R000013768) involving 3 hospitals in Tokyo, Japan, that enrolled consecutive patients who had BCs obtained. This study was approved by the ethical committee at Juntendo University Nerima Hospital and each of the participating centers, and the study was conducted in accordance with the Declaration of Helsinki 1971, as revised in 1983. We evaluated 2,792 consecutive hospitalized patients (mean age, 68.9 ± 17.1 years; 55.3% men) who had BCs obtained between April 2013 and August 2014, inclusive. The indication for BC acquisition was at the discretion of the treating physician. The study protocol and the indication for BCs are described in detail elsewhere.8 We excluded patients with anorexia-inducing conditions such as gastrointestinal disease, including gastrointestinal bleeding, enterocolitis, gastric ulceration, peritonitis, appendicitis, cholangitis, pancreatitis, diverticulitis, and ischemic colitis. We also excluded patients receiving chemotherapy for malignancy. In this study, true bacteremia was defined as identical organisms isolated from 2 sets of blood cultures (a set refers to one aerobic bottle and one anaerobic bottle). Moreover, even if only one set of blood cultures was acquired, when the identified pathogen could account for the clinical presentation, we also defined this as true bacteremia. Briefly, contaminants were defined as organisms common to skin flora, including Bacillus species, coagulase-negative Staphylococcus, Corynebacterium species, and Micrococcus species, without isolation of an identical organism with the same antibiotic susceptibilities from another potentially infected site in a patient with incompatible clinical features and no risk factors for infection with the isolated organism. Single BCs that were positive for organisms that were unlikely to explain the patient’s symptoms were also considered as contaminants. Patients with contaminated BCs were excluded from the analyses.
Structure of Reliability Study Procedures
Nurses in the 3 different hospitals performed daily independent food consumption ratings during each patient’s stay. Interrater reliability assessments were conducted in the morning or afternoon, and none of the raters had access to the other nurses’ scores at any time. The study nurses performed simultaneous ratings during these assessments (one interacted with and rated the patient while the other observed and rated the same patient).
Prediction Variables of True Bacteremia
1. Food consumption. Assessment of food consumption has been previously described in detail.8 Briefly, we characterized the patients’ oral intake based on the meal taken immediately prior to the BCs. For example, if a fever developed at 2
2. Chills. As done previously, the physician evaluated the patient for a history of chills at the time of BCs and classified the patients into 1 of 4 grades4,5: “no chills,” the absence of any chills; “mild chills,” feeling cold, equivalent to needing an outer jacket; “moderate chills,” feeling very cold, equivalent to needing a thick blanket; and “shaking chills,” feeling extremely cold with rigors and generalized bodily shaking, even under a thick blanket. To distinguish between those patients who had shaking chills and those who did not, we divided the patients into 2 groups: the “shaking chills group” and the combination of none, mild, and moderate chills, referred to as the “negative shaking chills group.”
3. Other predictive variables. We considered the following additional predictive variables: age, gender, axillary body temperature (BT), heart rate (HR), systolic blood pressure (SBP), respiratory rate (RR), white blood cell count (WBC), and serum C-reactive protein level (CRP). These predictive variables were obtained immediately prior to the BCs. We defined SIRS based on standard criteria (HR >90 beats/m, RR >20/m, BT <36°C or >38°C, and a WBC <4 × 103 WBC/μL or >12 × 103 WBC/μL). Patients were subcategorized by age into 2 groups (≤69 years and >70 years). CRP levels were dichotomized as >10.0 mg/dL or ≤10.0 mg/dL. We reviewed the patients’ charts to determine whether they had received antibiotics. In the case of walk-in patients, we interviewed the patients regarding whether they had visited a clinic; if they had, they were questioned as to whether any antibiotic therapy had been prescribed.
Statistical Analysis
Continuous variables are presented as the mean with the associated standard deviation (SD). All potential variables predictive of true bacteremia are shown in Table 1. The variables were dichotomized by clinically meaningful thresholds and used as potential risk-adjusted variables. We calculated the sensitivity and specificity and positive and negative predictive value for each criterion. Multiple logistic regression analysis was used to select components that were significantly associated with true bacteremia (the level of statistical significance determined with maximum likelihood methods was set at P < .05). To visualize and quantify other aspects in the prediction of true bacteremia, a recursive partitioning analysis (RPA) was used to make a decision tree model for true bacteremia. This nonparametric regression method produces a classification tree following a series of nonsequential top-down binary splits. The tree-building process starts by considering a set of predictive variables and selects the variable that produces 2 subsets of participants with the greatest purity. Two factors are considered when splitting a node into its daughter nodes: the goodness of the split and the amount of impurity in the daughter nodes. The splitting process is repeated until further partitioning is no longer possible and the terminal nodes have been reached. Details on this method are discussed in Monte Carlo Calibration of Distributions of Partition Statistics (www.jmp.com).
Probability was considered significant at a value of P < .05. All statistical tests were 2-tailed. Statistical analyses were conducted by a physician (KI) and an independent statistician (JM) with the use of the SPSS® v.16.0 software package (SPSS Inc., Chicago, IL) and JMP® version 8.0.2 (SAS Institute, Cary, NC).
RESULTS
Patients Characteristics
Two thousand seven hundred and ninety-two patients met the inclusion criteria for our study, from which 849 were excluded (see Figure 1 for flow diagram). Among the remaining 1,943 patients, there were 317 patients with positive BCs, of which 221 patients (69.7%) were considered to have true-positive BCs and 96 (30.3%) were considered to have contaminated BCs. After excluding these 96 patients, 221 patients with true bacteremia (true bacteremic group) were compared with 1,626 nonbacteremic patients (nonbacteremic group; Figure 1). The baseline characteristics of the subjects are shown in Table 1. The mean BT was 38.4 ± 1.2°C in the true bacteremic group and 37.9 ± 1.0°C in the nonbacteremic group. The mean serum CRP level was 11.6 ± 9.6 mg/dL in the true bacteremic group and 7.3 ± 6.9 mg/dL in the nonbacteremic group. In the true bacteremic group, there were 6 afebrile patients, and 27 patients without leukocytosis. The pathogens identified from the true-positive BCs were Escherichia coli (n = 59, 26.7%), including extended-spectrum beta-lactamase producing species, Staphylococcus aureus (n = 36, 16.3%), including methicillin-resistant Staphylococcus aureus, and Klebsiella pneumoniae (n = 22, 10.0%; Supplemental Table 1).
The underlying clinical diagnoses in the true bacteremic group included urinary tract infection (UTI), pneumonia, abscess, catheter-related bloodstream infection (CRBSI), cellulitis, osteomyelitis, infective endocarditis (IE), chorioamnionitis, iatrogenic infection at hemodialysis puncture sites, bacterial meningitis, septic arthritis, and infection of unknown cause (Supplemental Table 2).
Interrater Reliability Testing of Food Consumption
Patients were evaluated during their hospital stays. The interrater reliability of the evaluation of food consumption was very high across all participating hospitals (Supplemental Table 3). To assess the reliability of the evaluations of food consumption, patients (separate from this main study) were selected randomly and evaluated independently by 2 nurses in 3 different hospitals. The kappa scores of agreement between the nurses at the 3 different hospitals were 0.83 (95% CI, 0.63-0.88), 0.90 (95% CI, 0.80-0.99), and 0.80 (95% CI, 0.67-0.99), respectively. The interrater reliability of food consumption evaluation by the nurses was very high at all participating hospitals.
Food Consumption
The low, moderate, and high food consumption groups consisted of 964 (52.1%), 306 (16.6%), and 577 (31.2%) patients, respectively (Table 1). Of these, 174 (18.0%), 33 (10.8%), and 14 (2.4%) patients, respectively, had true bacteremia. The presence of poor food consumption had a sensitivity of 93.7% (95% CI, 89.4%-97.9%), specificity of 34.6% (95% CI, 33.0%-36.2%), and a positive LR of 1.43 (95% CI, 1.37-1.50) for predicting true bacteremia. Conversely, the absence of poor food consumption (ie, normal food consumption) had a negative LR of 0.18 (95% CI, 0.17-0.19).
Chills
The no, mild, moderate, and shaking chills groups consisted of 1,514 (82.0%), 148 (8.0%), 53 (2.9%), and 132 (7.1%) patients, respectively (Table 1). Of these, 136 (9.0%), 25 (16.9%), 8 (15.1%), and 52 (39.4%) patients, respectively, had true bacteremia. The presence of shaking chills had a sensitivity of 23.5% (95% CI, 22.5%-24.6%), a specificity of 95.1% (95% CI, 90.7%-99.4%), and a positive LR of 4.78 (95% CI, 4.56–5.00) for predicting true bacteremia. Conversely, the absence of shaking chills had a negative LR of 0.80 (95% CI, 0.77-0.84).
Prediction Model for True Bacteremia
The components identified as significantly related to true bacteremia by multiple logistic regression analysis are indicated in Table 2. The significant predictors of true bacteremia were shaking chills (odds ratio [OR], 5.6; 95% CI, 3.6-8.6; P < .01), SBP <90 mmHg (OR, 3.1; 95% CI, 1.6-5.7; P < 01), CRP levels >10.0 mg/dL (OR, 2.2; 95% CI, 1.6-3.1; P < .01), BT <36°C or >38°C (OR, 1.8; 95% CI, 1.3-2.6; P < .01), WBC <4 × 103/μL or >12 × 103/μL (OR, 1.6; 95% CI, 1.2-2.3; P = .003), HR >90 bpm (OR, 1.5; 95% CI, 1.1-2.1; P = .021), and female (OR, 1.4; 95% CI, 1.0-1.9; P = .036). An RPA to create an ideal prediction model for patients with true bacteremia or nonbacteremia is shown in Figure 2. The original group consisted of 1,847 patients, including 221 patients with true bacteremia. The pretest probability of true bacteremia was 2.4% (14/577) for those with normal food consumption (Group 1) and 2.4% (13/552) for those with both normal food consumption and the absence of shaking chills (Group 2). Conversely, the pretest probability of true bacteremia was 16.3% (207/1270) for those with poor food consumption and 47.7% (51/107) for those with both poor food consumption and shaking chills. The patients with true bacteremia with normal food consumption and without shaking chills consisted of 4 cases of CRBSI and UTI, 2 cases of osteomyelitis, 1 case of IE, 1 case of chorioamnionitis, and 1 case for which the focus was unknown (Supplemental Table 4).
DISCUSSION
In this observational study, we evaluated if a simple algorithm using food consumption and shaking chills was useful for assessing whether a patient had true bacteremia. A 2-item screening checklist (nursing assessment of food consumption and shaking chills) had excellent statistical properties as a brief screening instrument for true bacteremia.
We have prospectively validated that food consumption, as assessed by nurses, is a reliable predictor of true bacteremia.8 A previous single-center retrospective study showed similar findings, but these could not be generalized across all institutions because of the limited nature of the study. In this multicenter study, we used 2 statistical methods to reduce selection bias. First, we performed a kappa analysis across the hospitals to evaluate the interrater reliability of the evaluation of food consumption. Second, we used an RPA (Figure 2), also known as a decision tree model. RPA is a step-by-step process by which a decision tree is constructed by either splitting or not splitting each node on the tree into 2 daughter nodes.10 By using this method, we successfully generated an ideal approach to predict true bacteremia using food consumption and shaking chills. After adjusting for food consumption and shaking chills, groups 1 to 2 had sequentially decreasing diagnoses of true bacteremia, varying from 221 patients to only 13 patients.
Appetite is influenced by many factors that are integrated by the brain, most importantly within the hypothalamus. Signals that impinge on the hypothalamic center include neural afferents, hormones, cytokines, and metabolites.11 These factors elicit “sickness behavior,” which includes a decrease in food-motivated behavior.12 Furthermore, exposure to pathogenic bacteria increases serotonin, which has been shown to decrease metabolism in
The strengths of this study include its relatively large sample size, multicenter design, uniformity of data collection across sites, and completeness of data collection from study participants. All of these factors allowed for a robust analysis.
However, there are several limitations of this study. First, the physicians or nurses asked the patients about the presence of shaking chills when they obtained the BCs. It may be difficult for patients, especially elderly patients, to provide this information promptly and accurately. Some patients did not call the nurse when they had shaking chills, and the chills were not witnessed by a healthcare provider. However, we used a more specific definition for shaking chills: a feeling of being extremely cold with rigors and generalized bodily shaking, even under a thick blanket. Second, this algorithm is not applicable to patients with immunosuppressed states because none of the hospitals involved in this study perform bone marrow or organ transplantation. Third, although we included patients with dementia in our cohort, we did not specifically evaluate performance of the algorithm in patients with this medical condition. It is possible that the algorithm would not perform well in this subset of patients owing to their unreliable appetite and food intake. Fourth, some medications may affect appetite, leading to reduced food consumption. Although we have not considered the details of medications in this study, we found that the pretest probability of true bacteremia was low for those patients with normal food consumption regardless of whether the medication affected their appetites or not. However, the question of whether medications truly affect patients’ appetites concurrently with bacteremia would need to be specifically addressed in a future study.
CONCLUSION
In conclusion, we have established a simple algorithm to identify patients with suspected true bacteremia who require the acquisition of blood cultures. This extremely simple model can enable physicians to make a rapid bedside estimation of the risk of true bacteremia.
Acknowledgment
The authors thank Drs. H. Honda and S. Saint, and Ms. A. Okada for their helpful discussions with regard to this study; Ms. M. Takigawa for the collection of data; and Ms. T. Oguri for providing infectious disease consultation on the pathogenicity of the identified organisms.
Disclosure
This work was supported by JSPS KAKENHI Grant Number 15K19294 (to TK) and 20590840 (to KI) from the Japan Society for the Promotion of Science. The authors report no potential conflicts of interest relevant to this article.
1. Weinstein MP, Towns ML, Quartey SM et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584-602. PubMed
2. Strand CL, Wajsbort RR, Sturmann K. Effect of iodophor vs iodine tincture skin preparation on blood culture contamination rate. JAMA. 1993;269:1004-1006. PubMed
3. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265:365-369. PubMed
4. Tokuda Y, Miyasato H, Stein GH. A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98:813-820. PubMed
5. Tokuda Y, Miyasato H, Stein GH, Kishaba T. The degree of chills for risk of bacteremia in acute febrile illness. Am J Med. 2005;118:1417. PubMed
6. Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012;308:502-511. PubMed
7. Shapiro NI, Wolfe RE, Wright SB, Moore R, Bates DW. Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med. 2008;35:255-264. PubMed
8. Komatsu T, Onda T, Murayama G, et al. Predicting bacteremia based on nurse-assessed food consumption at the time of blood culture. J Hosp Med. 2012;7:702-705. PubMed
9. Henderson V. Basic Principles of Nursing Care. 2nd ed. Silver Spring, MD: American Nurses Association; 1969.
10. Therneau T, Atkinson, EJ. An Introduction to Recursive Partitioning using the RPART Routines. Mayo Foundation 2017. https://cran.r-project.org/web/packages/rpart/vignettes/longintro.pdf. Accessed May 5, 2017.
11. Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med .2003;9:125-134. PubMed
12. Hansen MK, Nguyen KT, Fleshner M, et al. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2000;278:R331-336. PubMed
13. Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 2005;438:179-84. PubMed
14. Van Dissel JT, Schijf V, Vogtlander N, Hoogendoorn M, van’t Wout J. Implications of chills. Lancet 1998;352:374. PubMed
15. Fukui S, Uehara Y, Fujibayashi K, et al. Bacteraemia predictive factors among general medical inpatients: a retrospective cross-sectional survey in a Japanese university hospital. BMJ Open 2016;6:e010527. PubMed
1. Weinstein MP, Towns ML, Quartey SM et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584-602. PubMed
2. Strand CL, Wajsbort RR, Sturmann K. Effect of iodophor vs iodine tincture skin preparation on blood culture contamination rate. JAMA. 1993;269:1004-1006. PubMed
3. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265:365-369. PubMed
4. Tokuda Y, Miyasato H, Stein GH. A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98:813-820. PubMed
5. Tokuda Y, Miyasato H, Stein GH, Kishaba T. The degree of chills for risk of bacteremia in acute febrile illness. Am J Med. 2005;118:1417. PubMed
6. Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012;308:502-511. PubMed
7. Shapiro NI, Wolfe RE, Wright SB, Moore R, Bates DW. Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med. 2008;35:255-264. PubMed
8. Komatsu T, Onda T, Murayama G, et al. Predicting bacteremia based on nurse-assessed food consumption at the time of blood culture. J Hosp Med. 2012;7:702-705. PubMed
9. Henderson V. Basic Principles of Nursing Care. 2nd ed. Silver Spring, MD: American Nurses Association; 1969.
10. Therneau T, Atkinson, EJ. An Introduction to Recursive Partitioning using the RPART Routines. Mayo Foundation 2017. https://cran.r-project.org/web/packages/rpart/vignettes/longintro.pdf. Accessed May 5, 2017.
11. Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med .2003;9:125-134. PubMed
12. Hansen MK, Nguyen KT, Fleshner M, et al. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2000;278:R331-336. PubMed
13. Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 2005;438:179-84. PubMed
14. Van Dissel JT, Schijf V, Vogtlander N, Hoogendoorn M, van’t Wout J. Implications of chills. Lancet 1998;352:374. PubMed
15. Fukui S, Uehara Y, Fujibayashi K, et al. Bacteraemia predictive factors among general medical inpatients: a retrospective cross-sectional survey in a Japanese university hospital. BMJ Open 2016;6:e010527. PubMed
© 2017 Society of Hospital Medicine
Clinician attitudes regarding ICD deactivation in DNR/DNI patients
Implantable cardioverter-defibrillators (ICDs) offer lifesaving therapies to many patients and have been implanted in hundreds of thousands of patients.1 The population of patients with ICDs is growing rapidly, and the national ICD Registry reports over 12,000 devices are implanted monthly.2 This population includes patients with congenital heart disease, ischemic cardiomyopathy, and idiopathic arrhythmias. If these patients experience ventricular tachycardia or fibrillation, an ICD attempts to restore sinus rhythm and prevent death. While a shock from an ICD may be lifesaving, it can be a traumatic and startling experience for the patient and perhaps distressful for families to witness.3,4
Although ICDs are intended to save lives, they do not slow the progress of the patient’s underlying cardiac and noncardiac comorbidities. All these patients will eventually die, whether from their cardiac disease or another condition. The literature includes many anecdotes about patients shocked multiple times by their defibrillator while actively dying.4 These situations could be prevented with preemptive ICD deactivation. (ICDs can function not only as cardioverters and defibrillators, as implied by their name, but also as pacemakers. “Deactivation” as used in this paper refers only to disabling the tachycardia therapies. No distinction was made between defibrillation with a shock and anti-tachycardia pacing.) Therefore, research on ICD deactivation has emphasized patients who are acutely terminally ill, while less emphasis has been placed on patients who are not actively dying.4–8
Patients may, for a variety of reasons, request a do-not-resuscitate/do-not-intubate (DNR/DNI) order as their code status. However, it is not necessarily clear what a DNR/DNI order implies for ICD management. One survey of attending physicians found that 19% of respondents felt a DNR/DNI order was equivalent to requesting ICD deactivation.9 On the other hand, patients are split on whether they would want their device deactivated while in hospice or even at the very end of life.6 Heart Rhythm Society (HRS) guidelines favor a nuanced approach to ICD deactivation in DNR/DNI patients that emphasizes the individual patient’s comorbidities and goals.10 A patient’s individual circumstances might justify a choice to be DNR/DNI without deactivating the ICD. Decision-making in these circumstances requires a careful conversation between the patient and clinician. It is important to identify barriers that might prevent optimal shared decision-making.
Clinicians have been surveyed on ICD management at the end of life, but these studies have generally focused on attending physicians.5,9,11 However, physician trainees (ie, residents and fellows) as well as advanced practice providers (ie, physician assistants and nurse practitioners) are responsible for much of the clinical care provided to hospitalized patients. In particular, they are often the clinicians to discuss code status with patients. Different specialties (eg, cardiology, general medicine, and geriatrics) manage different sets of patients, which might affect clinicians’ opinions on ICD management. We therefore designed a survey to assess clinician attitudes and beliefs regarding ICD deactivation, including in non-terminally ill patients, and to evaluate for differences according to training level and specialty.
METHODS
Case-based and Likert-scale questions were considered for this survey, with the latter being chosen for ease of completion by respondents. An online survey tool (SurveyMonkey; San Mateo, CA) was used for data collection; no identifying data were collected. E-mail invitations to participate were sent to a combination of mailing lists and individual addresses for residents, fellows, advanced practice providers, and attending physicians in general internal medicine, cardiology, electrophysiology, and geriatrics. The survey remained open for 2.5 weeks. It was conducted 5 months into the academic year, thus trainees were well-established in their current roles. Two $25 gift cards were offered to respondents who entered their e-mail into a drawing; responses were not tied to e-mail addresses. Approval for the study was obtained from the University of Michigan Institutional Review Board.
The survey posed 12 questions assessing general attitudes about ICDs as well as individual beliefs and behaviors relating to ICD deactivation. Answers were on a Likert scale of 1 to 5 with 1 representing “strongly disagree” and 5 representing “strongly agree.” A score of 3 indicated “unsure or neutral.” The first 3 questions appeared together on the first page and were prefaced with “Please respond to the following statements about ICD shocks.” The next 9 were likewise grouped on the next page and were prefaced with “Please respond to the following statements about ICD deactivation.” All 12 questions are shown in Figures 1 and 2. Respondents could easily return to previous questions and change their answers. The survey ended with a third page showing 3 multiple choice demographic questions. The demographic questions were about clinical role (first-, second-, third-, or fourth-year resident, fellow, advanced practice provider, and attending), specialty, and number of ICD deactivations the respondent had been directly involved in (0, 1 to 5, 5 to 10, and more than 10). Specialty options were internal medicine resident, inpatient general medicine, outpatient general medicine, cardiology, electrophysiology, and geriatrics.
Likert scale answers of “agree” or “strongly agree” were grouped together as an affirmative response, while all other answers were grouped together as a nonaffirmative response. For analysis, residents were grouped together and their responses compared with attending physicians as a group. Additional analysis was done comparing attending physicians stratified by clinical specialty. Given the small number of responses from attending electrophysiologists, they were grouped with attending cardiologists for analysis. Due to the limited number of fellows and advanced practice providers who responded, further evaluation of these groups was not performed. Finally, the number of ICD deactivations respondents had been involved in was stratified by training level. All comparisons were performed using the two-tailed Pearson’s chi-squared test.
RESULTS
A total of 170 responses were collected from 508 individuals on the e-mail lists. Two responses were from registered nurses who were not part of the intended study sample and 7 responses were incomplete, having only answered the first 3 questions. These 9 responses were excluded from further analysis, yielding an overall response rate of 32%. The demographics of the remaining 161 respondents are shown in Table 1. Figure 1 shows overall responses to each question.
When comparing residents to attending physicians, there were no statistically significant between-differences except on questions 5 and 6. Specifically, residents were less comfortable than attending physicians discussing ICD deactivation and did so with less regularity (P < .001 and P = .018, respectively; Figure 2). Comfort levels improved markedly with experience: 29.2% of interns expressed comfort asking about ICD deactivation as compared with 60.7% of third- and-fourth year residents and 78.8% of attending physicians. Furthermore, comfort level seemed to parallel the regularity with which respondents asked about ICD deactivation: 4.2% of interns routinely asked about ICD deactivation as compared with 21.4% of third- and fourth-year residents and 34.8% of attending physicians.
The only statistically significant difference when comparing attending physicians by specialty was on question 6 of the survey with the groups being unequal in their reliability at asking about ICD deactivation during code status discussions (P < .001; Figure 2). Of cardiologists and electrophysiologists, 73.3% said they routinely ask about ICD deactivation, as well as 83.3% of geriatricians. By contrast, only 19.2% of outpatient general internists and 10.5% of inpatient general internists (ie, hospitalists) said they routinely ask about ICD deactivation.
There were no differences between groups when asked whether ICD deactivation was part of a DNR/DNI order (question 8), or if an ICD should be deactivated in DNR/DNI patients (questions 9 and 10). As shown in Figure 1, 21.1% of respondents felt that a DNR/DNI order is equivalent to requesting ICD deactivation, 60.2% felt that terminally ill DNR/DNI patients should have their device deactivated, and 28% felt that non-terminally ill DNR/DNI patients should have their device deactivated.
Groups were unequal with respect to the number of ICD deactivations in which they had been directly involved (Figure 3; P < .001). Over half of interns had not been involved in any ICD deactivations as compared with only 10.7% of third- or fourth-year residents. Of the 20 geriatricians, cardiologists, and electrophysiologists, 45% had been involved in at least 5 ICD deactivations. Of note, although 77.8% of fellows reported being involved in more than 10 ICD deactivations, these 9 respondents were all in cardiology or electrophysiology.
DISCUSSION
Overall, our major findings were (1) residents, who provide much of the clinical care in a teaching hospital, are remarkably uncomfortable discussing ICD deactivation, (2) general internists and residents ask about ICD deactivation infrequently compared to geriatricians and cardiologists, and (3) about one fifth of our respondents believe ICD deactivation is automatically part of a DNR/DNI order.
Although the majority of respondents did not routinely address ICD deactivation in conjunction with code status, there was significant variability among subgroups. For example, 83.3% of geriatricians routinely discussed ICD deactivation as part of code status compared with only 4% of first-year residents and 10.5% of inpatient general internists. This finding is interesting because 90.7% of all respondents believed that discussions of code status should address preferences on ICD deactivation. This apparent discrepancy could be explained by the relatively small number of patients admitted to the hospital who have both an ICD and a request to be DNR/DNI. Residents and inpatient general internists see a very broad spectrum of patients; ICD deactivation is frequently irrelevant in the cases these physicians manage. The subset of patients seen in consultation by cardiologists and geriatricians, by contrast, is expected to include a larger proportion of patients with ICDs. Therefore, discussing ICD deactivation will be more relevant to their daily practice. Fear of alienating patients was not a reason for our findings, as our respondents did not express concern that recommending ICD deactivation would harm the patient-clinician relationship.
There are several possible reasons that residents, particularly interns, are uncomfortable discussing ICD deactivation. A lack of exposure to ICD deactivation is probably a key contributor. Over half of interns had never been involved in any ICD deactivations. Residents and hospitalists may also feel as if they are overstepping their boundaries to discuss deactivating ICD therapies. Their feelings may not be misplaced, as one survey of ICD patients found that over 75% thought responsibility for discussing ICD deactivation, at least at the end of life, rests with cardiologists or electrophysiologists.6
The HRS guidelines call for individualized decisions regarding ICD deactivation, even if a patient is DNR/DNI. However, our respondents frequently felt a standardized approach was indicated, with 21% believing that a DNR/DNI order included ICD deactivation. Additionally, 28% agreed that even non-terminally ill DNR/DNI patients should have their device deactivated. This is relevant because it is the role of clinicians to engage in shared decision-making with their patients. If the clinician holds the fixed belief that a DNR/DNI order, regardless of the precise clinical scenario, should include ICD deactivation, they may pressure a patient to have their device deactivated even if it could still benefit them.
In 2009, Kelley et al published results of a survey on ICD deactivation at the end of life.9 They contacted 4,876 attending physicians in cardiology, electrophysiology, geriatrics, and general medicine, receiving 558 responses. The survey included Likert-scale questions assessing attitudes and knowledge about ICD functionality. Demographic information was also collected, including how many patients in their practice had ICDs and how often they had previously discussed ICD deactivation.
There are some interesting comparisons between Kelley et al’s findings and ours, although we included trainees and the precise wording of questions was different. The specific questions used by Kelley et al to ask whether ICD shocks were painful or distressing and to ask if ICD deactivation is part of a DNR order were: “The shock from an ICD is very painful for most patients.” “The shock of an ICD at the end of life is distressing to a patient and their loved ones.” “A DNR order is equivalent to deactivation of an ICD.”
Only 47% of general internists in the Kelley et al survey thought that ICD shocks were painful, compared with 83% of electrophysiologists. In addition, 65% of general internists and 85% of electrophysiologists viewed shocks at the end of life to be distressing to patients and families. By contrast, our respondents were nearly unanimous in believing shocks to be painful and distressing. This discrepancy may be due to the growing prevalence of ICDs over the past several years as well as the growing body of literature on unnecessary shocks at the end of life. In line with our study, 19% of their respondents believed a DNR order was equivalent to ICD deactivation.9
Taken together, our findings indicate that additional education for clinicians of all levels could be helpful. Didactic lessons cannot replace experience, and it is important for residents to be exposed to discussions of ICD deactivation. However, lessons about ICD therapies and practical examples of how to broach the topic of deactivation could be beneficial, especially for interns whose responsibility includes discussions of code status. Within the context of an internal medicine residency, the fundamentals of ICD functionality could be covered during rotations on cardiology or palliative care services. Additionally, the recommendations of the HRS for device management can be covered in didactic sessions. Similar opportunities could be built into continuing medical education for practicing physicians and the training of advanced practice providers.
There are limitations to this survey, most notably the fact that it was restricted to a single academic medical center, the patient population and practices of which may not be generalizable to medical practice at large. Selection bias is also a distinct possibility given the 32% overall response rate; those who responded may feel more strongly about the survey topic. Our study subgroups may have interpreted questions differently because of their particular area of clinical practice. The small sample size also precluded an effective analysis of fellows and advanced practice practitioners due to lack of power. A major strength of this survey was the inclusion of a large number of residents upon whom the majority of inpatient contact rests. Future work could include expanding the survey to multiple medical centers, which would enhance generalizability and improve the ability to recruit sufficient fellows and advanced practice providers.
CONCLUSION
In summary, we conducted a single-center survey of residents, fellows, advanced practice providers, and attending physicians on their attitudes and beliefs about ICD deactivation in DNR/DNI patients. Residents are particularly uncomfortable discussing ICD deactivation with patients, which is an important finding because of their crucial role in providing patient care. Additionally, residents and hospitalists do not broach the topic of deactivation regularly, especially when compared to geriatricians and cardiologists. Despite HRS guidelines to the contrary, a fifth of our respondents believed that DNR/DNI orders include ICD deactivation. Overall, ICD deactivation in DNR/DNI patients is a topic that needs further attention in clinical education so that patients receive care that respects their individual wishes.
Disclosure
Nothing to report.
1. Freeman JV, Wang Y, Curtis JP, Heidenreich PA, Hlatky MA. Physician procedure volume and complications of cardioverter-defibrillator implantation. Circulation. 2012;125(1):57-64. doi:10.1161/CIRCULATIONAHA.111.046995. PubMed
2. Kremers MS, Hammill SC, Berul CI, et al. The National ICD Registry Report: Version 2.1 including leads and pediatrics for years 2010 and 2011. Hear Rhythm. 2013;10(4):e59-e65. doi:10.1016/j.hrthm.2013.01.035. PubMed
3. Goldstein NE, Mehta D, Siddiqui S, et al. “That’s like an act of suicide” patients’ attitudes toward deactivation of implantable defibrillators. J Gen Intern Med. 2008;23 Suppl 1:7-12. PubMed
4. Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med. 2004;141(11):835-838. http://annals.org/article.aspx?articleid=717985&issueno=11. Accessed October 23, 2013.
5. Sherazi S, Daubert JP, Block RC, et al. Physicians’ preferences and attitudes about end-of-life care in patients with an implantable cardioverter-defibrillator. Mayo Clin Proc. 2008;83(10):1139-1141. doi:10.4065/83.10.1139. PubMed
6. Kirkpatrick JN, Gottlieb M, Sehgal P, Patel R, Verdino RJ. Deactivation of implantable cardioverter defibrillators in terminal illness and end of life care. Am J Cardiol. 2012;109(1):91-94. doi:10.1016/j.amjcard.2011.08.011. PubMed
7. Marinskis G, van Erven L. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace. 2010;12(8):1176-1177. doi:10.1093/europace/euq272. PubMed
8. Mueller PS, Jenkins SM, Bramstedt KA, Hayes DL. Deactivating implanted cardiac devices in terminally ill patients: practices and attitudes. Pacing Clin Electrophysiol. 2008;31(5):560-568. doi:10.1111/j.1540-8159.2008.01041.x. PubMed
9. Kelley AS, Reid MC, Miller DH, Fins JJ, Lachs MS. Implantable cardioverter-defibrillator deactivation at the end of life: a physician survey. Am Heart J. 2009;157(4):702-8.e1. doi:10.1016/j.ahj.2008.12.011. PubMed
10. Lampert R, Hayes DL, Annas GJ, et al. HRS Expert Consensus Statement on the Management of Cardiovascular Implantable Electronic Devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Hear Rhythm. 2010;7(7):1008-1026. doi:10.1016/j.hrthm.2010.04.033.PubMed
11. Kelley AS, Mehta SS, Reid MC. Management of patients with ICDs at the end of life (EOL): a qualitative study. Am J Hosp Palliat Care. 2008;25(6):440-446. doi:10.1177/1049909108320885. PubMed
Implantable cardioverter-defibrillators (ICDs) offer lifesaving therapies to many patients and have been implanted in hundreds of thousands of patients.1 The population of patients with ICDs is growing rapidly, and the national ICD Registry reports over 12,000 devices are implanted monthly.2 This population includes patients with congenital heart disease, ischemic cardiomyopathy, and idiopathic arrhythmias. If these patients experience ventricular tachycardia or fibrillation, an ICD attempts to restore sinus rhythm and prevent death. While a shock from an ICD may be lifesaving, it can be a traumatic and startling experience for the patient and perhaps distressful for families to witness.3,4
Although ICDs are intended to save lives, they do not slow the progress of the patient’s underlying cardiac and noncardiac comorbidities. All these patients will eventually die, whether from their cardiac disease or another condition. The literature includes many anecdotes about patients shocked multiple times by their defibrillator while actively dying.4 These situations could be prevented with preemptive ICD deactivation. (ICDs can function not only as cardioverters and defibrillators, as implied by their name, but also as pacemakers. “Deactivation” as used in this paper refers only to disabling the tachycardia therapies. No distinction was made between defibrillation with a shock and anti-tachycardia pacing.) Therefore, research on ICD deactivation has emphasized patients who are acutely terminally ill, while less emphasis has been placed on patients who are not actively dying.4–8
Patients may, for a variety of reasons, request a do-not-resuscitate/do-not-intubate (DNR/DNI) order as their code status. However, it is not necessarily clear what a DNR/DNI order implies for ICD management. One survey of attending physicians found that 19% of respondents felt a DNR/DNI order was equivalent to requesting ICD deactivation.9 On the other hand, patients are split on whether they would want their device deactivated while in hospice or even at the very end of life.6 Heart Rhythm Society (HRS) guidelines favor a nuanced approach to ICD deactivation in DNR/DNI patients that emphasizes the individual patient’s comorbidities and goals.10 A patient’s individual circumstances might justify a choice to be DNR/DNI without deactivating the ICD. Decision-making in these circumstances requires a careful conversation between the patient and clinician. It is important to identify barriers that might prevent optimal shared decision-making.
Clinicians have been surveyed on ICD management at the end of life, but these studies have generally focused on attending physicians.5,9,11 However, physician trainees (ie, residents and fellows) as well as advanced practice providers (ie, physician assistants and nurse practitioners) are responsible for much of the clinical care provided to hospitalized patients. In particular, they are often the clinicians to discuss code status with patients. Different specialties (eg, cardiology, general medicine, and geriatrics) manage different sets of patients, which might affect clinicians’ opinions on ICD management. We therefore designed a survey to assess clinician attitudes and beliefs regarding ICD deactivation, including in non-terminally ill patients, and to evaluate for differences according to training level and specialty.
METHODS
Case-based and Likert-scale questions were considered for this survey, with the latter being chosen for ease of completion by respondents. An online survey tool (SurveyMonkey; San Mateo, CA) was used for data collection; no identifying data were collected. E-mail invitations to participate were sent to a combination of mailing lists and individual addresses for residents, fellows, advanced practice providers, and attending physicians in general internal medicine, cardiology, electrophysiology, and geriatrics. The survey remained open for 2.5 weeks. It was conducted 5 months into the academic year, thus trainees were well-established in their current roles. Two $25 gift cards were offered to respondents who entered their e-mail into a drawing; responses were not tied to e-mail addresses. Approval for the study was obtained from the University of Michigan Institutional Review Board.
The survey posed 12 questions assessing general attitudes about ICDs as well as individual beliefs and behaviors relating to ICD deactivation. Answers were on a Likert scale of 1 to 5 with 1 representing “strongly disagree” and 5 representing “strongly agree.” A score of 3 indicated “unsure or neutral.” The first 3 questions appeared together on the first page and were prefaced with “Please respond to the following statements about ICD shocks.” The next 9 were likewise grouped on the next page and were prefaced with “Please respond to the following statements about ICD deactivation.” All 12 questions are shown in Figures 1 and 2. Respondents could easily return to previous questions and change their answers. The survey ended with a third page showing 3 multiple choice demographic questions. The demographic questions were about clinical role (first-, second-, third-, or fourth-year resident, fellow, advanced practice provider, and attending), specialty, and number of ICD deactivations the respondent had been directly involved in (0, 1 to 5, 5 to 10, and more than 10). Specialty options were internal medicine resident, inpatient general medicine, outpatient general medicine, cardiology, electrophysiology, and geriatrics.
Likert scale answers of “agree” or “strongly agree” were grouped together as an affirmative response, while all other answers were grouped together as a nonaffirmative response. For analysis, residents were grouped together and their responses compared with attending physicians as a group. Additional analysis was done comparing attending physicians stratified by clinical specialty. Given the small number of responses from attending electrophysiologists, they were grouped with attending cardiologists for analysis. Due to the limited number of fellows and advanced practice providers who responded, further evaluation of these groups was not performed. Finally, the number of ICD deactivations respondents had been involved in was stratified by training level. All comparisons were performed using the two-tailed Pearson’s chi-squared test.
RESULTS
A total of 170 responses were collected from 508 individuals on the e-mail lists. Two responses were from registered nurses who were not part of the intended study sample and 7 responses were incomplete, having only answered the first 3 questions. These 9 responses were excluded from further analysis, yielding an overall response rate of 32%. The demographics of the remaining 161 respondents are shown in Table 1. Figure 1 shows overall responses to each question.
When comparing residents to attending physicians, there were no statistically significant between-differences except on questions 5 and 6. Specifically, residents were less comfortable than attending physicians discussing ICD deactivation and did so with less regularity (P < .001 and P = .018, respectively; Figure 2). Comfort levels improved markedly with experience: 29.2% of interns expressed comfort asking about ICD deactivation as compared with 60.7% of third- and-fourth year residents and 78.8% of attending physicians. Furthermore, comfort level seemed to parallel the regularity with which respondents asked about ICD deactivation: 4.2% of interns routinely asked about ICD deactivation as compared with 21.4% of third- and fourth-year residents and 34.8% of attending physicians.
The only statistically significant difference when comparing attending physicians by specialty was on question 6 of the survey with the groups being unequal in their reliability at asking about ICD deactivation during code status discussions (P < .001; Figure 2). Of cardiologists and electrophysiologists, 73.3% said they routinely ask about ICD deactivation, as well as 83.3% of geriatricians. By contrast, only 19.2% of outpatient general internists and 10.5% of inpatient general internists (ie, hospitalists) said they routinely ask about ICD deactivation.
There were no differences between groups when asked whether ICD deactivation was part of a DNR/DNI order (question 8), or if an ICD should be deactivated in DNR/DNI patients (questions 9 and 10). As shown in Figure 1, 21.1% of respondents felt that a DNR/DNI order is equivalent to requesting ICD deactivation, 60.2% felt that terminally ill DNR/DNI patients should have their device deactivated, and 28% felt that non-terminally ill DNR/DNI patients should have their device deactivated.
Groups were unequal with respect to the number of ICD deactivations in which they had been directly involved (Figure 3; P < .001). Over half of interns had not been involved in any ICD deactivations as compared with only 10.7% of third- or fourth-year residents. Of the 20 geriatricians, cardiologists, and electrophysiologists, 45% had been involved in at least 5 ICD deactivations. Of note, although 77.8% of fellows reported being involved in more than 10 ICD deactivations, these 9 respondents were all in cardiology or electrophysiology.
DISCUSSION
Overall, our major findings were (1) residents, who provide much of the clinical care in a teaching hospital, are remarkably uncomfortable discussing ICD deactivation, (2) general internists and residents ask about ICD deactivation infrequently compared to geriatricians and cardiologists, and (3) about one fifth of our respondents believe ICD deactivation is automatically part of a DNR/DNI order.
Although the majority of respondents did not routinely address ICD deactivation in conjunction with code status, there was significant variability among subgroups. For example, 83.3% of geriatricians routinely discussed ICD deactivation as part of code status compared with only 4% of first-year residents and 10.5% of inpatient general internists. This finding is interesting because 90.7% of all respondents believed that discussions of code status should address preferences on ICD deactivation. This apparent discrepancy could be explained by the relatively small number of patients admitted to the hospital who have both an ICD and a request to be DNR/DNI. Residents and inpatient general internists see a very broad spectrum of patients; ICD deactivation is frequently irrelevant in the cases these physicians manage. The subset of patients seen in consultation by cardiologists and geriatricians, by contrast, is expected to include a larger proportion of patients with ICDs. Therefore, discussing ICD deactivation will be more relevant to their daily practice. Fear of alienating patients was not a reason for our findings, as our respondents did not express concern that recommending ICD deactivation would harm the patient-clinician relationship.
There are several possible reasons that residents, particularly interns, are uncomfortable discussing ICD deactivation. A lack of exposure to ICD deactivation is probably a key contributor. Over half of interns had never been involved in any ICD deactivations. Residents and hospitalists may also feel as if they are overstepping their boundaries to discuss deactivating ICD therapies. Their feelings may not be misplaced, as one survey of ICD patients found that over 75% thought responsibility for discussing ICD deactivation, at least at the end of life, rests with cardiologists or electrophysiologists.6
The HRS guidelines call for individualized decisions regarding ICD deactivation, even if a patient is DNR/DNI. However, our respondents frequently felt a standardized approach was indicated, with 21% believing that a DNR/DNI order included ICD deactivation. Additionally, 28% agreed that even non-terminally ill DNR/DNI patients should have their device deactivated. This is relevant because it is the role of clinicians to engage in shared decision-making with their patients. If the clinician holds the fixed belief that a DNR/DNI order, regardless of the precise clinical scenario, should include ICD deactivation, they may pressure a patient to have their device deactivated even if it could still benefit them.
In 2009, Kelley et al published results of a survey on ICD deactivation at the end of life.9 They contacted 4,876 attending physicians in cardiology, electrophysiology, geriatrics, and general medicine, receiving 558 responses. The survey included Likert-scale questions assessing attitudes and knowledge about ICD functionality. Demographic information was also collected, including how many patients in their practice had ICDs and how often they had previously discussed ICD deactivation.
There are some interesting comparisons between Kelley et al’s findings and ours, although we included trainees and the precise wording of questions was different. The specific questions used by Kelley et al to ask whether ICD shocks were painful or distressing and to ask if ICD deactivation is part of a DNR order were: “The shock from an ICD is very painful for most patients.” “The shock of an ICD at the end of life is distressing to a patient and their loved ones.” “A DNR order is equivalent to deactivation of an ICD.”
Only 47% of general internists in the Kelley et al survey thought that ICD shocks were painful, compared with 83% of electrophysiologists. In addition, 65% of general internists and 85% of electrophysiologists viewed shocks at the end of life to be distressing to patients and families. By contrast, our respondents were nearly unanimous in believing shocks to be painful and distressing. This discrepancy may be due to the growing prevalence of ICDs over the past several years as well as the growing body of literature on unnecessary shocks at the end of life. In line with our study, 19% of their respondents believed a DNR order was equivalent to ICD deactivation.9
Taken together, our findings indicate that additional education for clinicians of all levels could be helpful. Didactic lessons cannot replace experience, and it is important for residents to be exposed to discussions of ICD deactivation. However, lessons about ICD therapies and practical examples of how to broach the topic of deactivation could be beneficial, especially for interns whose responsibility includes discussions of code status. Within the context of an internal medicine residency, the fundamentals of ICD functionality could be covered during rotations on cardiology or palliative care services. Additionally, the recommendations of the HRS for device management can be covered in didactic sessions. Similar opportunities could be built into continuing medical education for practicing physicians and the training of advanced practice providers.
There are limitations to this survey, most notably the fact that it was restricted to a single academic medical center, the patient population and practices of which may not be generalizable to medical practice at large. Selection bias is also a distinct possibility given the 32% overall response rate; those who responded may feel more strongly about the survey topic. Our study subgroups may have interpreted questions differently because of their particular area of clinical practice. The small sample size also precluded an effective analysis of fellows and advanced practice practitioners due to lack of power. A major strength of this survey was the inclusion of a large number of residents upon whom the majority of inpatient contact rests. Future work could include expanding the survey to multiple medical centers, which would enhance generalizability and improve the ability to recruit sufficient fellows and advanced practice providers.
CONCLUSION
In summary, we conducted a single-center survey of residents, fellows, advanced practice providers, and attending physicians on their attitudes and beliefs about ICD deactivation in DNR/DNI patients. Residents are particularly uncomfortable discussing ICD deactivation with patients, which is an important finding because of their crucial role in providing patient care. Additionally, residents and hospitalists do not broach the topic of deactivation regularly, especially when compared to geriatricians and cardiologists. Despite HRS guidelines to the contrary, a fifth of our respondents believed that DNR/DNI orders include ICD deactivation. Overall, ICD deactivation in DNR/DNI patients is a topic that needs further attention in clinical education so that patients receive care that respects their individual wishes.
Disclosure
Nothing to report.
Implantable cardioverter-defibrillators (ICDs) offer lifesaving therapies to many patients and have been implanted in hundreds of thousands of patients.1 The population of patients with ICDs is growing rapidly, and the national ICD Registry reports over 12,000 devices are implanted monthly.2 This population includes patients with congenital heart disease, ischemic cardiomyopathy, and idiopathic arrhythmias. If these patients experience ventricular tachycardia or fibrillation, an ICD attempts to restore sinus rhythm and prevent death. While a shock from an ICD may be lifesaving, it can be a traumatic and startling experience for the patient and perhaps distressful for families to witness.3,4
Although ICDs are intended to save lives, they do not slow the progress of the patient’s underlying cardiac and noncardiac comorbidities. All these patients will eventually die, whether from their cardiac disease or another condition. The literature includes many anecdotes about patients shocked multiple times by their defibrillator while actively dying.4 These situations could be prevented with preemptive ICD deactivation. (ICDs can function not only as cardioverters and defibrillators, as implied by their name, but also as pacemakers. “Deactivation” as used in this paper refers only to disabling the tachycardia therapies. No distinction was made between defibrillation with a shock and anti-tachycardia pacing.) Therefore, research on ICD deactivation has emphasized patients who are acutely terminally ill, while less emphasis has been placed on patients who are not actively dying.4–8
Patients may, for a variety of reasons, request a do-not-resuscitate/do-not-intubate (DNR/DNI) order as their code status. However, it is not necessarily clear what a DNR/DNI order implies for ICD management. One survey of attending physicians found that 19% of respondents felt a DNR/DNI order was equivalent to requesting ICD deactivation.9 On the other hand, patients are split on whether they would want their device deactivated while in hospice or even at the very end of life.6 Heart Rhythm Society (HRS) guidelines favor a nuanced approach to ICD deactivation in DNR/DNI patients that emphasizes the individual patient’s comorbidities and goals.10 A patient’s individual circumstances might justify a choice to be DNR/DNI without deactivating the ICD. Decision-making in these circumstances requires a careful conversation between the patient and clinician. It is important to identify barriers that might prevent optimal shared decision-making.
Clinicians have been surveyed on ICD management at the end of life, but these studies have generally focused on attending physicians.5,9,11 However, physician trainees (ie, residents and fellows) as well as advanced practice providers (ie, physician assistants and nurse practitioners) are responsible for much of the clinical care provided to hospitalized patients. In particular, they are often the clinicians to discuss code status with patients. Different specialties (eg, cardiology, general medicine, and geriatrics) manage different sets of patients, which might affect clinicians’ opinions on ICD management. We therefore designed a survey to assess clinician attitudes and beliefs regarding ICD deactivation, including in non-terminally ill patients, and to evaluate for differences according to training level and specialty.
METHODS
Case-based and Likert-scale questions were considered for this survey, with the latter being chosen for ease of completion by respondents. An online survey tool (SurveyMonkey; San Mateo, CA) was used for data collection; no identifying data were collected. E-mail invitations to participate were sent to a combination of mailing lists and individual addresses for residents, fellows, advanced practice providers, and attending physicians in general internal medicine, cardiology, electrophysiology, and geriatrics. The survey remained open for 2.5 weeks. It was conducted 5 months into the academic year, thus trainees were well-established in their current roles. Two $25 gift cards were offered to respondents who entered their e-mail into a drawing; responses were not tied to e-mail addresses. Approval for the study was obtained from the University of Michigan Institutional Review Board.
The survey posed 12 questions assessing general attitudes about ICDs as well as individual beliefs and behaviors relating to ICD deactivation. Answers were on a Likert scale of 1 to 5 with 1 representing “strongly disagree” and 5 representing “strongly agree.” A score of 3 indicated “unsure or neutral.” The first 3 questions appeared together on the first page and were prefaced with “Please respond to the following statements about ICD shocks.” The next 9 were likewise grouped on the next page and were prefaced with “Please respond to the following statements about ICD deactivation.” All 12 questions are shown in Figures 1 and 2. Respondents could easily return to previous questions and change their answers. The survey ended with a third page showing 3 multiple choice demographic questions. The demographic questions were about clinical role (first-, second-, third-, or fourth-year resident, fellow, advanced practice provider, and attending), specialty, and number of ICD deactivations the respondent had been directly involved in (0, 1 to 5, 5 to 10, and more than 10). Specialty options were internal medicine resident, inpatient general medicine, outpatient general medicine, cardiology, electrophysiology, and geriatrics.
Likert scale answers of “agree” or “strongly agree” were grouped together as an affirmative response, while all other answers were grouped together as a nonaffirmative response. For analysis, residents were grouped together and their responses compared with attending physicians as a group. Additional analysis was done comparing attending physicians stratified by clinical specialty. Given the small number of responses from attending electrophysiologists, they were grouped with attending cardiologists for analysis. Due to the limited number of fellows and advanced practice providers who responded, further evaluation of these groups was not performed. Finally, the number of ICD deactivations respondents had been involved in was stratified by training level. All comparisons were performed using the two-tailed Pearson’s chi-squared test.
RESULTS
A total of 170 responses were collected from 508 individuals on the e-mail lists. Two responses were from registered nurses who were not part of the intended study sample and 7 responses were incomplete, having only answered the first 3 questions. These 9 responses were excluded from further analysis, yielding an overall response rate of 32%. The demographics of the remaining 161 respondents are shown in Table 1. Figure 1 shows overall responses to each question.
When comparing residents to attending physicians, there were no statistically significant between-differences except on questions 5 and 6. Specifically, residents were less comfortable than attending physicians discussing ICD deactivation and did so with less regularity (P < .001 and P = .018, respectively; Figure 2). Comfort levels improved markedly with experience: 29.2% of interns expressed comfort asking about ICD deactivation as compared with 60.7% of third- and-fourth year residents and 78.8% of attending physicians. Furthermore, comfort level seemed to parallel the regularity with which respondents asked about ICD deactivation: 4.2% of interns routinely asked about ICD deactivation as compared with 21.4% of third- and fourth-year residents and 34.8% of attending physicians.
The only statistically significant difference when comparing attending physicians by specialty was on question 6 of the survey with the groups being unequal in their reliability at asking about ICD deactivation during code status discussions (P < .001; Figure 2). Of cardiologists and electrophysiologists, 73.3% said they routinely ask about ICD deactivation, as well as 83.3% of geriatricians. By contrast, only 19.2% of outpatient general internists and 10.5% of inpatient general internists (ie, hospitalists) said they routinely ask about ICD deactivation.
There were no differences between groups when asked whether ICD deactivation was part of a DNR/DNI order (question 8), or if an ICD should be deactivated in DNR/DNI patients (questions 9 and 10). As shown in Figure 1, 21.1% of respondents felt that a DNR/DNI order is equivalent to requesting ICD deactivation, 60.2% felt that terminally ill DNR/DNI patients should have their device deactivated, and 28% felt that non-terminally ill DNR/DNI patients should have their device deactivated.
Groups were unequal with respect to the number of ICD deactivations in which they had been directly involved (Figure 3; P < .001). Over half of interns had not been involved in any ICD deactivations as compared with only 10.7% of third- or fourth-year residents. Of the 20 geriatricians, cardiologists, and electrophysiologists, 45% had been involved in at least 5 ICD deactivations. Of note, although 77.8% of fellows reported being involved in more than 10 ICD deactivations, these 9 respondents were all in cardiology or electrophysiology.
DISCUSSION
Overall, our major findings were (1) residents, who provide much of the clinical care in a teaching hospital, are remarkably uncomfortable discussing ICD deactivation, (2) general internists and residents ask about ICD deactivation infrequently compared to geriatricians and cardiologists, and (3) about one fifth of our respondents believe ICD deactivation is automatically part of a DNR/DNI order.
Although the majority of respondents did not routinely address ICD deactivation in conjunction with code status, there was significant variability among subgroups. For example, 83.3% of geriatricians routinely discussed ICD deactivation as part of code status compared with only 4% of first-year residents and 10.5% of inpatient general internists. This finding is interesting because 90.7% of all respondents believed that discussions of code status should address preferences on ICD deactivation. This apparent discrepancy could be explained by the relatively small number of patients admitted to the hospital who have both an ICD and a request to be DNR/DNI. Residents and inpatient general internists see a very broad spectrum of patients; ICD deactivation is frequently irrelevant in the cases these physicians manage. The subset of patients seen in consultation by cardiologists and geriatricians, by contrast, is expected to include a larger proportion of patients with ICDs. Therefore, discussing ICD deactivation will be more relevant to their daily practice. Fear of alienating patients was not a reason for our findings, as our respondents did not express concern that recommending ICD deactivation would harm the patient-clinician relationship.
There are several possible reasons that residents, particularly interns, are uncomfortable discussing ICD deactivation. A lack of exposure to ICD deactivation is probably a key contributor. Over half of interns had never been involved in any ICD deactivations. Residents and hospitalists may also feel as if they are overstepping their boundaries to discuss deactivating ICD therapies. Their feelings may not be misplaced, as one survey of ICD patients found that over 75% thought responsibility for discussing ICD deactivation, at least at the end of life, rests with cardiologists or electrophysiologists.6
The HRS guidelines call for individualized decisions regarding ICD deactivation, even if a patient is DNR/DNI. However, our respondents frequently felt a standardized approach was indicated, with 21% believing that a DNR/DNI order included ICD deactivation. Additionally, 28% agreed that even non-terminally ill DNR/DNI patients should have their device deactivated. This is relevant because it is the role of clinicians to engage in shared decision-making with their patients. If the clinician holds the fixed belief that a DNR/DNI order, regardless of the precise clinical scenario, should include ICD deactivation, they may pressure a patient to have their device deactivated even if it could still benefit them.
In 2009, Kelley et al published results of a survey on ICD deactivation at the end of life.9 They contacted 4,876 attending physicians in cardiology, electrophysiology, geriatrics, and general medicine, receiving 558 responses. The survey included Likert-scale questions assessing attitudes and knowledge about ICD functionality. Demographic information was also collected, including how many patients in their practice had ICDs and how often they had previously discussed ICD deactivation.
There are some interesting comparisons between Kelley et al’s findings and ours, although we included trainees and the precise wording of questions was different. The specific questions used by Kelley et al to ask whether ICD shocks were painful or distressing and to ask if ICD deactivation is part of a DNR order were: “The shock from an ICD is very painful for most patients.” “The shock of an ICD at the end of life is distressing to a patient and their loved ones.” “A DNR order is equivalent to deactivation of an ICD.”
Only 47% of general internists in the Kelley et al survey thought that ICD shocks were painful, compared with 83% of electrophysiologists. In addition, 65% of general internists and 85% of electrophysiologists viewed shocks at the end of life to be distressing to patients and families. By contrast, our respondents were nearly unanimous in believing shocks to be painful and distressing. This discrepancy may be due to the growing prevalence of ICDs over the past several years as well as the growing body of literature on unnecessary shocks at the end of life. In line with our study, 19% of their respondents believed a DNR order was equivalent to ICD deactivation.9
Taken together, our findings indicate that additional education for clinicians of all levels could be helpful. Didactic lessons cannot replace experience, and it is important for residents to be exposed to discussions of ICD deactivation. However, lessons about ICD therapies and practical examples of how to broach the topic of deactivation could be beneficial, especially for interns whose responsibility includes discussions of code status. Within the context of an internal medicine residency, the fundamentals of ICD functionality could be covered during rotations on cardiology or palliative care services. Additionally, the recommendations of the HRS for device management can be covered in didactic sessions. Similar opportunities could be built into continuing medical education for practicing physicians and the training of advanced practice providers.
There are limitations to this survey, most notably the fact that it was restricted to a single academic medical center, the patient population and practices of which may not be generalizable to medical practice at large. Selection bias is also a distinct possibility given the 32% overall response rate; those who responded may feel more strongly about the survey topic. Our study subgroups may have interpreted questions differently because of their particular area of clinical practice. The small sample size also precluded an effective analysis of fellows and advanced practice practitioners due to lack of power. A major strength of this survey was the inclusion of a large number of residents upon whom the majority of inpatient contact rests. Future work could include expanding the survey to multiple medical centers, which would enhance generalizability and improve the ability to recruit sufficient fellows and advanced practice providers.
CONCLUSION
In summary, we conducted a single-center survey of residents, fellows, advanced practice providers, and attending physicians on their attitudes and beliefs about ICD deactivation in DNR/DNI patients. Residents are particularly uncomfortable discussing ICD deactivation with patients, which is an important finding because of their crucial role in providing patient care. Additionally, residents and hospitalists do not broach the topic of deactivation regularly, especially when compared to geriatricians and cardiologists. Despite HRS guidelines to the contrary, a fifth of our respondents believed that DNR/DNI orders include ICD deactivation. Overall, ICD deactivation in DNR/DNI patients is a topic that needs further attention in clinical education so that patients receive care that respects their individual wishes.
Disclosure
Nothing to report.
1. Freeman JV, Wang Y, Curtis JP, Heidenreich PA, Hlatky MA. Physician procedure volume and complications of cardioverter-defibrillator implantation. Circulation. 2012;125(1):57-64. doi:10.1161/CIRCULATIONAHA.111.046995. PubMed
2. Kremers MS, Hammill SC, Berul CI, et al. The National ICD Registry Report: Version 2.1 including leads and pediatrics for years 2010 and 2011. Hear Rhythm. 2013;10(4):e59-e65. doi:10.1016/j.hrthm.2013.01.035. PubMed
3. Goldstein NE, Mehta D, Siddiqui S, et al. “That’s like an act of suicide” patients’ attitudes toward deactivation of implantable defibrillators. J Gen Intern Med. 2008;23 Suppl 1:7-12. PubMed
4. Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med. 2004;141(11):835-838. http://annals.org/article.aspx?articleid=717985&issueno=11. Accessed October 23, 2013.
5. Sherazi S, Daubert JP, Block RC, et al. Physicians’ preferences and attitudes about end-of-life care in patients with an implantable cardioverter-defibrillator. Mayo Clin Proc. 2008;83(10):1139-1141. doi:10.4065/83.10.1139. PubMed
6. Kirkpatrick JN, Gottlieb M, Sehgal P, Patel R, Verdino RJ. Deactivation of implantable cardioverter defibrillators in terminal illness and end of life care. Am J Cardiol. 2012;109(1):91-94. doi:10.1016/j.amjcard.2011.08.011. PubMed
7. Marinskis G, van Erven L. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace. 2010;12(8):1176-1177. doi:10.1093/europace/euq272. PubMed
8. Mueller PS, Jenkins SM, Bramstedt KA, Hayes DL. Deactivating implanted cardiac devices in terminally ill patients: practices and attitudes. Pacing Clin Electrophysiol. 2008;31(5):560-568. doi:10.1111/j.1540-8159.2008.01041.x. PubMed
9. Kelley AS, Reid MC, Miller DH, Fins JJ, Lachs MS. Implantable cardioverter-defibrillator deactivation at the end of life: a physician survey. Am Heart J. 2009;157(4):702-8.e1. doi:10.1016/j.ahj.2008.12.011. PubMed
10. Lampert R, Hayes DL, Annas GJ, et al. HRS Expert Consensus Statement on the Management of Cardiovascular Implantable Electronic Devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Hear Rhythm. 2010;7(7):1008-1026. doi:10.1016/j.hrthm.2010.04.033.PubMed
11. Kelley AS, Mehta SS, Reid MC. Management of patients with ICDs at the end of life (EOL): a qualitative study. Am J Hosp Palliat Care. 2008;25(6):440-446. doi:10.1177/1049909108320885. PubMed
1. Freeman JV, Wang Y, Curtis JP, Heidenreich PA, Hlatky MA. Physician procedure volume and complications of cardioverter-defibrillator implantation. Circulation. 2012;125(1):57-64. doi:10.1161/CIRCULATIONAHA.111.046995. PubMed
2. Kremers MS, Hammill SC, Berul CI, et al. The National ICD Registry Report: Version 2.1 including leads and pediatrics for years 2010 and 2011. Hear Rhythm. 2013;10(4):e59-e65. doi:10.1016/j.hrthm.2013.01.035. PubMed
3. Goldstein NE, Mehta D, Siddiqui S, et al. “That’s like an act of suicide” patients’ attitudes toward deactivation of implantable defibrillators. J Gen Intern Med. 2008;23 Suppl 1:7-12. PubMed
4. Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med. 2004;141(11):835-838. http://annals.org/article.aspx?articleid=717985&issueno=11. Accessed October 23, 2013.
5. Sherazi S, Daubert JP, Block RC, et al. Physicians’ preferences and attitudes about end-of-life care in patients with an implantable cardioverter-defibrillator. Mayo Clin Proc. 2008;83(10):1139-1141. doi:10.4065/83.10.1139. PubMed
6. Kirkpatrick JN, Gottlieb M, Sehgal P, Patel R, Verdino RJ. Deactivation of implantable cardioverter defibrillators in terminal illness and end of life care. Am J Cardiol. 2012;109(1):91-94. doi:10.1016/j.amjcard.2011.08.011. PubMed
7. Marinskis G, van Erven L. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace. 2010;12(8):1176-1177. doi:10.1093/europace/euq272. PubMed
8. Mueller PS, Jenkins SM, Bramstedt KA, Hayes DL. Deactivating implanted cardiac devices in terminally ill patients: practices and attitudes. Pacing Clin Electrophysiol. 2008;31(5):560-568. doi:10.1111/j.1540-8159.2008.01041.x. PubMed
9. Kelley AS, Reid MC, Miller DH, Fins JJ, Lachs MS. Implantable cardioverter-defibrillator deactivation at the end of life: a physician survey. Am Heart J. 2009;157(4):702-8.e1. doi:10.1016/j.ahj.2008.12.011. PubMed
10. Lampert R, Hayes DL, Annas GJ, et al. HRS Expert Consensus Statement on the Management of Cardiovascular Implantable Electronic Devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Hear Rhythm. 2010;7(7):1008-1026. doi:10.1016/j.hrthm.2010.04.033.PubMed
11. Kelley AS, Mehta SS, Reid MC. Management of patients with ICDs at the end of life (EOL): a qualitative study. Am J Hosp Palliat Care. 2008;25(6):440-446. doi:10.1177/1049909108320885. PubMed
© 2017 Society of Hospital Medicine
Imaging after bariatric surgery appears overdone
Nearly 70% of bariatric surgery patients received postoperative imaging, with more than one-third receiving CT imaging. This high level of screening resulted in symptom-related findings in only 23% of cases, and may be excessive, according to researchers who studied nearly 600 adults who underwent bariatric surgery.
As the volume of bariatric surgery has increased, so has the role of postoperative imaging, wrote Dana Haddad, MD, and her colleagues at Harlem Hospital Center, New York.
“However, there is a lack of well-defined postoperative imaging guidelines,” they said. “Detrimental aspects of postoperative imaging include the potential for false-positive findings leading to further and often unnecessary investigations, radiation exposure, and additional cost,” they added.
The primary outcomes were the numbers of initial postimaging studies and whether the findings supported subsequent studies.
The study population included 399 adults who underwent laparoscopic bypass and 144 who underwent sleeve gastrectomy. The average age of the patients was 41 years and 90% were women.
The researchers identified 907 imaging studies performed in 400 patients (69% of the study population). Of these, 38% were ultrasound, 36% were CT, 15% were x-ray, 6.6% were fluoroscopy, 3.3% were MRI, and .6% were nuclear medicine.
On review of the imaging findings, the researchers found that half (50%) were unremarkable, while 13% were either surgery related or symptom related, 6.8% were not related to surgery but might have explained patients’ symptoms, 4.3% were surgery-related but not likely to explain symptoms, and 26% were incidental. “Interestingly, no incidental findings were found to be of major clinical importance; all were benign,” according to the researchers.
However, incidental findings led to a total of 71 additional studies, and to 5 laparoscopic cholecystectomies.
A univariate analysis showed that the factors with a significant impact a patient’s odds of undergoing postoperative abdominal imaging included having a bypass procedure vs. a sleeve procedure, older age, and lower baseline body mass index. In addition, patients with a history of abdominal surgery or dyspepsia or those who had a routine postoperative upper gastrointestinal series were significantly more likely to undergo CT scans. Patients with history of ulcer or reflux were significantly less likely to undergo CT scans.
Although the study was limited by the retrospective design and lack of information about possible imaging of patients at other centers, “results suggest that nonroutine postoperative abdominal imaging in the bariatric population is common and requires streamlined protocols, with almost 70% of patients undergoing imaging and greater than 70% of findings being unrelated to symptoms or negative,” the researchers said.
A clinical algorithm for imaging of bariatric patients should be based on clinical parameters collected during a physical exam. “Once an algorithm is in place, further studies will be needed to validate its accuracy and efficiency,” the researchers stated.
Dr. Haddad and her colleagues had no financial conflicts to disclose.
Nearly 70% of bariatric surgery patients received postoperative imaging, with more than one-third receiving CT imaging. This high level of screening resulted in symptom-related findings in only 23% of cases, and may be excessive, according to researchers who studied nearly 600 adults who underwent bariatric surgery.
As the volume of bariatric surgery has increased, so has the role of postoperative imaging, wrote Dana Haddad, MD, and her colleagues at Harlem Hospital Center, New York.
“However, there is a lack of well-defined postoperative imaging guidelines,” they said. “Detrimental aspects of postoperative imaging include the potential for false-positive findings leading to further and often unnecessary investigations, radiation exposure, and additional cost,” they added.
The primary outcomes were the numbers of initial postimaging studies and whether the findings supported subsequent studies.
The study population included 399 adults who underwent laparoscopic bypass and 144 who underwent sleeve gastrectomy. The average age of the patients was 41 years and 90% were women.
The researchers identified 907 imaging studies performed in 400 patients (69% of the study population). Of these, 38% were ultrasound, 36% were CT, 15% were x-ray, 6.6% were fluoroscopy, 3.3% were MRI, and .6% were nuclear medicine.
On review of the imaging findings, the researchers found that half (50%) were unremarkable, while 13% were either surgery related or symptom related, 6.8% were not related to surgery but might have explained patients’ symptoms, 4.3% were surgery-related but not likely to explain symptoms, and 26% were incidental. “Interestingly, no incidental findings were found to be of major clinical importance; all were benign,” according to the researchers.
However, incidental findings led to a total of 71 additional studies, and to 5 laparoscopic cholecystectomies.
A univariate analysis showed that the factors with a significant impact a patient’s odds of undergoing postoperative abdominal imaging included having a bypass procedure vs. a sleeve procedure, older age, and lower baseline body mass index. In addition, patients with a history of abdominal surgery or dyspepsia or those who had a routine postoperative upper gastrointestinal series were significantly more likely to undergo CT scans. Patients with history of ulcer or reflux were significantly less likely to undergo CT scans.
Although the study was limited by the retrospective design and lack of information about possible imaging of patients at other centers, “results suggest that nonroutine postoperative abdominal imaging in the bariatric population is common and requires streamlined protocols, with almost 70% of patients undergoing imaging and greater than 70% of findings being unrelated to symptoms or negative,” the researchers said.
A clinical algorithm for imaging of bariatric patients should be based on clinical parameters collected during a physical exam. “Once an algorithm is in place, further studies will be needed to validate its accuracy and efficiency,” the researchers stated.
Dr. Haddad and her colleagues had no financial conflicts to disclose.
Nearly 70% of bariatric surgery patients received postoperative imaging, with more than one-third receiving CT imaging. This high level of screening resulted in symptom-related findings in only 23% of cases, and may be excessive, according to researchers who studied nearly 600 adults who underwent bariatric surgery.
As the volume of bariatric surgery has increased, so has the role of postoperative imaging, wrote Dana Haddad, MD, and her colleagues at Harlem Hospital Center, New York.
“However, there is a lack of well-defined postoperative imaging guidelines,” they said. “Detrimental aspects of postoperative imaging include the potential for false-positive findings leading to further and often unnecessary investigations, radiation exposure, and additional cost,” they added.
The primary outcomes were the numbers of initial postimaging studies and whether the findings supported subsequent studies.
The study population included 399 adults who underwent laparoscopic bypass and 144 who underwent sleeve gastrectomy. The average age of the patients was 41 years and 90% were women.
The researchers identified 907 imaging studies performed in 400 patients (69% of the study population). Of these, 38% were ultrasound, 36% were CT, 15% were x-ray, 6.6% were fluoroscopy, 3.3% were MRI, and .6% were nuclear medicine.
On review of the imaging findings, the researchers found that half (50%) were unremarkable, while 13% were either surgery related or symptom related, 6.8% were not related to surgery but might have explained patients’ symptoms, 4.3% were surgery-related but not likely to explain symptoms, and 26% were incidental. “Interestingly, no incidental findings were found to be of major clinical importance; all were benign,” according to the researchers.
However, incidental findings led to a total of 71 additional studies, and to 5 laparoscopic cholecystectomies.
A univariate analysis showed that the factors with a significant impact a patient’s odds of undergoing postoperative abdominal imaging included having a bypass procedure vs. a sleeve procedure, older age, and lower baseline body mass index. In addition, patients with a history of abdominal surgery or dyspepsia or those who had a routine postoperative upper gastrointestinal series were significantly more likely to undergo CT scans. Patients with history of ulcer or reflux were significantly less likely to undergo CT scans.
Although the study was limited by the retrospective design and lack of information about possible imaging of patients at other centers, “results suggest that nonroutine postoperative abdominal imaging in the bariatric population is common and requires streamlined protocols, with almost 70% of patients undergoing imaging and greater than 70% of findings being unrelated to symptoms or negative,” the researchers said.
A clinical algorithm for imaging of bariatric patients should be based on clinical parameters collected during a physical exam. “Once an algorithm is in place, further studies will be needed to validate its accuracy and efficiency,” the researchers stated.
Dr. Haddad and her colleagues had no financial conflicts to disclose.
FROM SURGERY FOR OBESITY AND RELATED DISEASES
Key clinical point: No well-defined guidelines exist for when to use postoperative imaging in bariatric surgery patients.
Major finding: Approximately 70% of postoperative imaging findings were not symptom related, and incidental findings led to 71 additional studies.
Data source: A review of 578 patients who underwent gastric bypass or sleeve gastrectomy.
Disclosures: The researchers had no financial conflicts to disclose.