User login
Accelerated aging in schizophrenia
Excessive daytime sleepiness
Nutraceuticals for traumatic brain injury: Should you recommend their use?
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
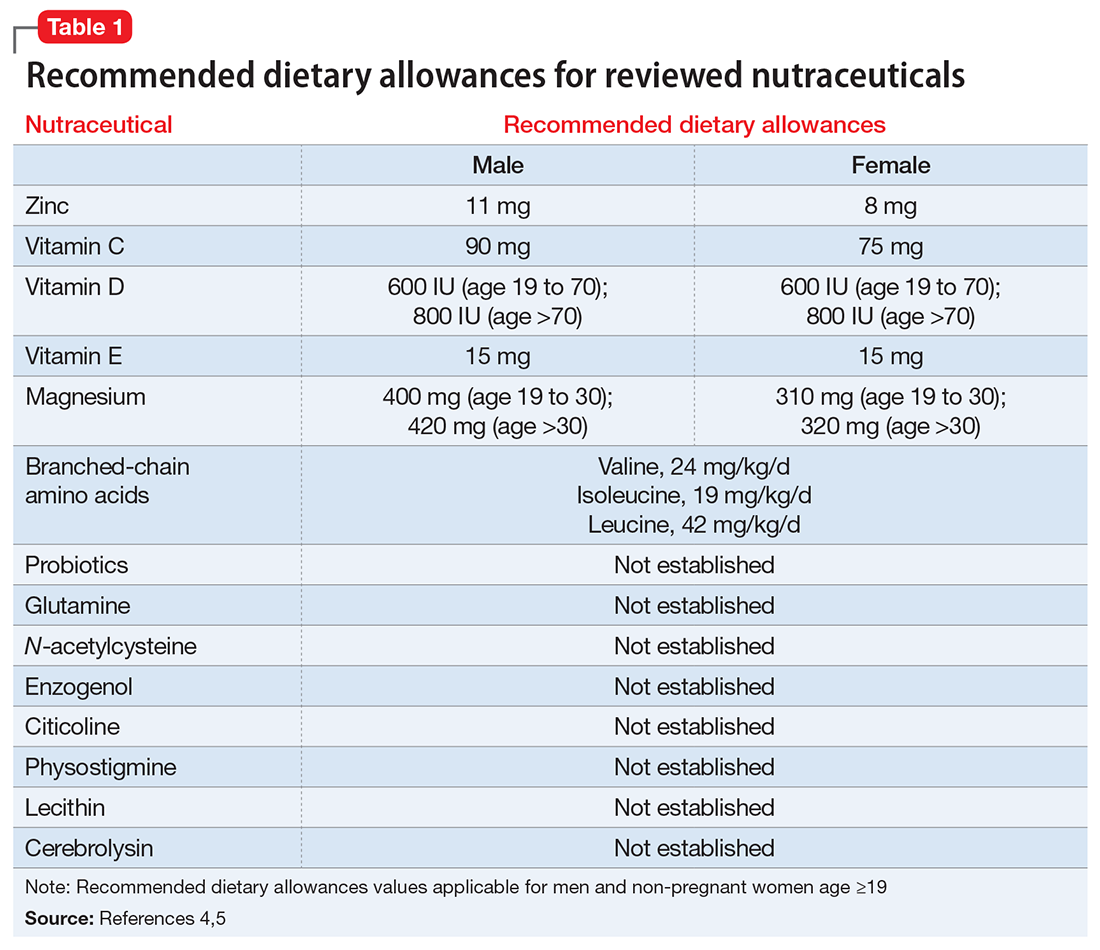
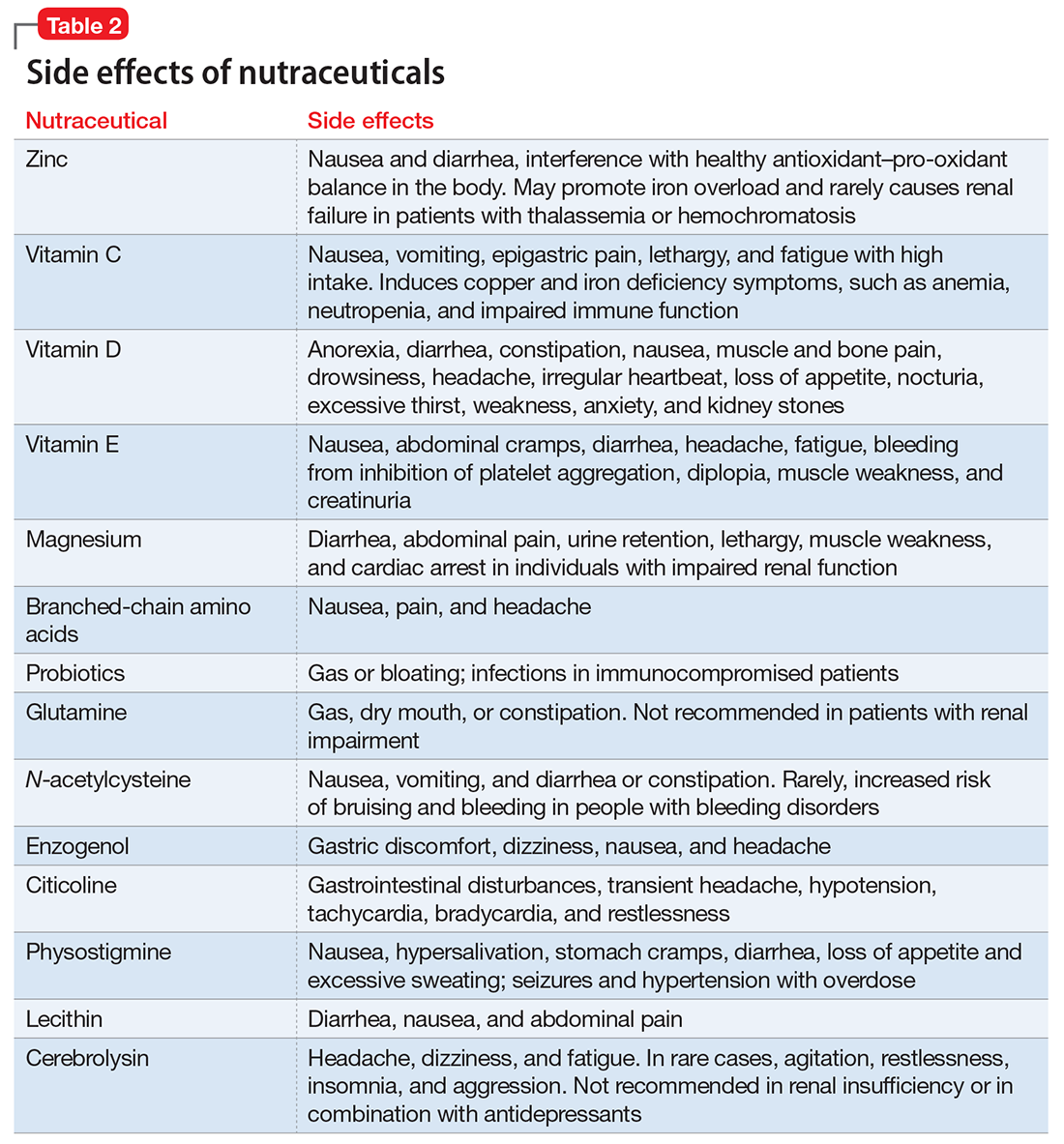
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.
Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10
What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
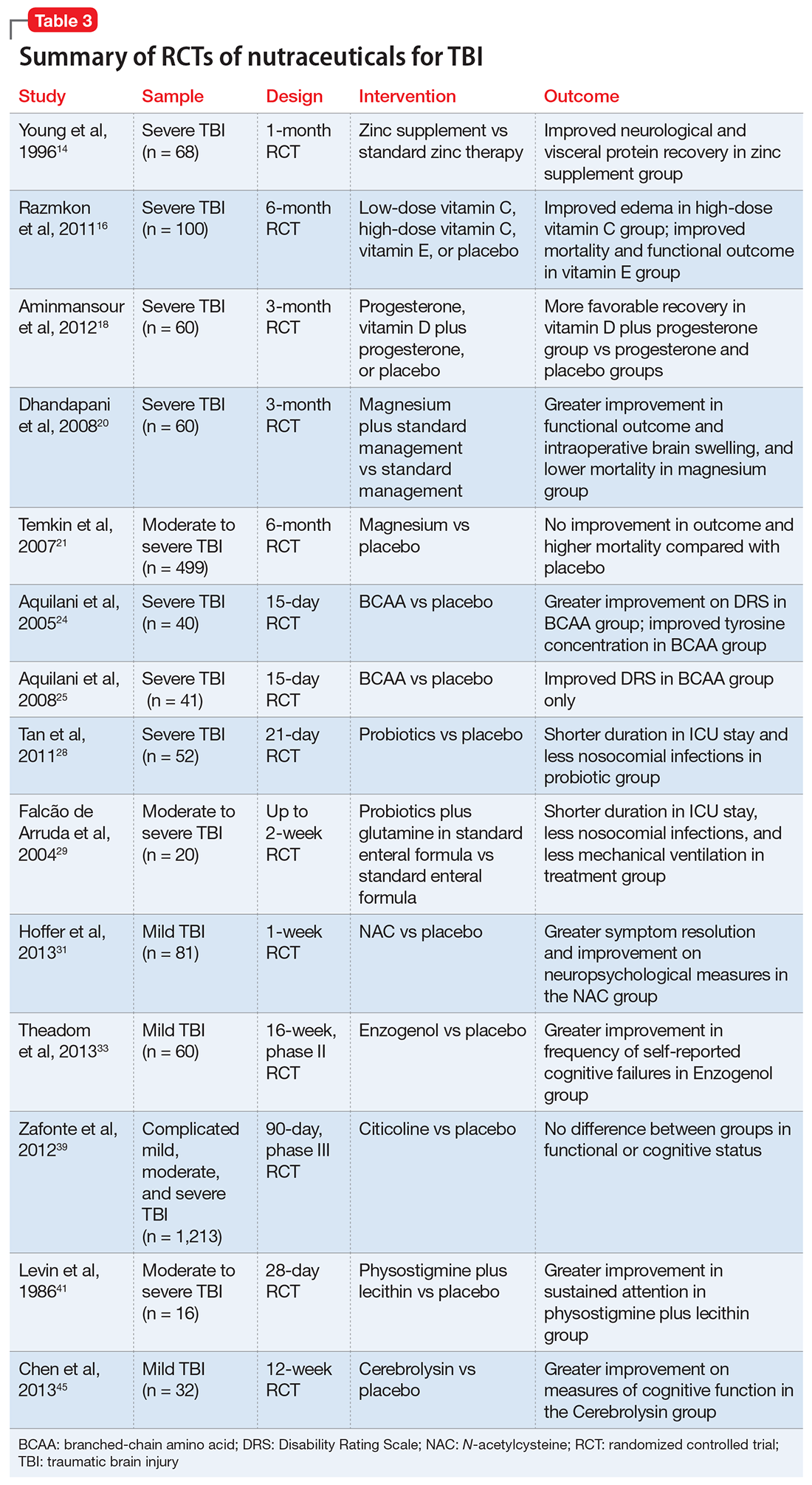
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.
Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.

Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10

What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.

Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.

Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10

What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.

Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
Glutamate’s exciting roles in body, brain, and mind: A fertile future pharmacotherapy target
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.
GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
GLU is now recognized as the most abundant neurotransmitter in the brain, and its excitatory properties are vital for brain structure and function. Importantly, it also is the precursor of γ-aminobutyric acid, the ubiquitous inhibitory neurotransmitter in the brain. GLU is one of the first molecules produced during fetal life and plays a critical role in brain development and in organ development because it is a building block for protein synthesis and for manufacturing muscle and other body tissue. Therefore, aberrations in GLU activity can have a major impact on neurodevelopment—the underpinning of most psychiatric disorders due to genetic and environmental factors—and the general health of the brain and body.
GLU is derived from glutamic acid, which is not considered an essential amino acid because it is synthesized in the body via the citric acid cycle. It is readily available from many food items, including cheese, soy, and tomatoes. Monosodium GLU2 is used as a food additive to enhance flavor (Chinese food, anyone?). Incidentally, GLU represents >50% of all amino acids in breast milk, which underscores its importance for a baby’s brain and body development.
GLU’s many brain receptors
Amazingly, although it has been long known that GLU is present in all body tissues, the role of GLU in the CNS and brain was not recognized until the 1980s. This was several decades after the discovery of other neurotransmitters, such as acetylcholine, norepinephrine, and serotonin, which are less widely distributed in the CNS. Over the past 30 years, advances in psychiatric research have elucidated the numerous effects of GLU and its receptors on neuropsychiatric disorders. Multiple receptors of GLU have been discovered, including 16 ion channel receptors (7 for N-methyl-
GLU and neurodegeneration
An excess of GLU activity can be neurotoxic and can lead to brain damage.3 Therefore, it is not surprising that excess GLU activity has been found in many neurodegenerative disorders (Table). Similar to other neurologic disorders that are considered neurodegenerative, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease, major psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, also are neurodegenerative if left untreated or if multiple relapses recur because of treatment discontinuation (Table). Several neuroimaging studies have documented brain tissue loss in psychotic and mood disorders after repeated episodes. Therefore, targeting GLU in psychotic and mood disorders is legitimately a “hot” research area in psychiatry.

GLU models of psychiatric neurobiology
Advances in biological psychiatry have moved GLU to the forefront of the neurobiology and pathophysiology of the most serious psychiatric disorders. Overactivity or underactivity of the GLU NMDA receptor has emerged as scientifically plausible mechanisms underlying psychotic and mood disorders. The GLU hypothesis of schizophrenia4 grew out of the observation that phencyclidine, a drug of abuse that is a potent NMDA antagonist (50-fold stronger than ketamine), can trigger in healthy individuals a severe psychosis indistinguishable from schizophrenia, with positive and negative symptoms, cognitive impairment, thought disorder, catatonia, and agitation. Similarly, the recently discovered paraneoplastic encephalitis caused by an ovarian teratoma that secretes antibodies to the NMDA receptor produces acute psychosis, seizures, delirium, dyskinesia, headache, bizarre behavior, confusion, paranoia, auditory and visual hallucinations, and cognitive deficits.5 This demonstrates how the GLU NMDA receptor and its 7 subunits are intimately associated with various psychotic symptoms when genetic or non-genetic factors (antagonists or antibodies) drastically reduce its activity.
On the other hand, there is an impressive body of evidence that, unlike the hypofunction of NMDA receptors in schizophrenia, there appears to be increased activity of NMDA receptors in both unipolar and bipolar depression.6 Several NMDA antagonists have been shown in controlled clinical trials to be highly effective in rapidly reversing severe, chronic depression that did not respond to standard antidepressants.7 A number of NMDA antagonists have been reported to rapidly reverse—within a few hours—severe and chronic depression when administered intravenously (ketamine, rapastinel, scopolamine), intranasally (S-ketamine), or via inhalation (nitrous oxide). NMDA antagonists also show promise in other serious psychiatric disorders such as obsessive-compulsive disorder.8 Riluzole and memantine reduce GLU activity and both are FDA-approved for treating neurodegenerative disorders, such as ALS and AD, respectively.9,10 Therefore, antagonism of GLU is considered neuroprotective and can be therapeutically beneficial in managing neurodegenerative brain disorders.
GLU and the future of psychopharmacology
Based on the wealth of data generated over the past 2 decades regarding the central role of GLU receptors (NMDA, AMPA, kainate, and others) in brain health and disease, modulating GLU pathways is rapidly emerging as a key target for drug development for neuropsychiatric disorders. This approach could help with some medical comorbidities, such as diabetes11 and pain,12 that co-occur frequently with schizophrenia and depression. GLU has been implicated in diabetes via toxicity that destroys pancreatic beta cells.11 It is possible that novel drug development in the future could exploit GLU signaling and pathways to concurrently repair disorders of the brain and body, such as schizophrenia with comorbid diabetes or depression with comorbid pain. It is worth noting that glucose dysregulation has been shown to exist at the onset of schizophrenia before treatment is started.13 This might be related to GLU toxicity occurring simultaneously in the body and the brain. Also worth noting is that ketamine, an NMDA antagonist which has emerged as an ultra-rapid acting antidepressant, is an anesthetic, suggesting that perhaps it may help mitigate the pain symptoms that often accompany major depression.
It is logical to conclude that GLU pathways show exciting prospects for therapeutic advances for the brain, body, and mind. This merits intensive scientific effort for novel drug development in neuropsychiatric disorder that may parsimoniously rectify co-occurring GLU-related diseases of the brain, body, and mind.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S suppl):1007S-1015S.
2. Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract. 2005;18(10):482-486.
3. Novelli A, Pérez-Basterrechea M, Fernández-Sánchez MT. Glutamate and neurodegeneration. In: Schmidt WJ, Reith MEA, eds. Dopamine and glutamate in psychiatric disorders. Totowa, NJ: Humana Press; 2005:447-474.
4. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69-108.
5. Dalmau E, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
6. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97-114.
7. Wohleb ES, Gerhard D, Thomas A, et al. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15(1):11-20.
8. Pittenger C. Glutamate modulators in the treatment of obsessive-compulsive disorder. Psychiatr Ann. 2015;45(6):308-315.
9. Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-000917.
10. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591.
11. Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol. 2012;49(3):167-183.
12. Wozniak KM, Rojas C, Wu Y, et al. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19(9):1323-1334.
13. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
Suicidal and paranoid thoughts after starting hepatitis C virus treatment
CASE Suicidal and paranoid
Ms. B, age 53, has a 30-year history of bipolar disorder, a 1-year history of hepatitis C virus (HCV), and previous inpatient psychiatric hospitalizations secondary to acute mania. She presents to our hospital describing her symptoms as the “worst depression ever” and reports suicidal ideation and paranoid thoughts of people watching and following her. Ms. B describes significant neurovegetative symptoms of depression, including poor sleep, poor appetite, low energy and concentration, and chronic feelings of hopelessness with thoughts of “ending it all.” Ms. B reports that her symptoms started 3 weeks ago, a few days after she started taking sofosbuvir and ribavirin for refractory HCV.
Ms. B’s medication regimen consisted of quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, for bipolar disorder, when she started taking sofosbuvir and ribavirin. Ms. B admits she stopped taking her psychotropic and antiviral medications after she noticed progressively worsening depression with intrusive suicidal thoughts, including ruminative thoughts of overdosing on them.
At evaluation, Ms. B is casually dressed, pleasant, with fair hygiene and poor eye contact. Her speech is decreased in rate, volume, and tone; mood is “devastated and depressed”; affect is labile and tearful. Her thought process reveals occasional thought blocking and her thought content includes suicidal ideations and paranoid thoughts. Her cognition is intact; insight and judgment are poor. During evaluation, Ms. B reveals a history of alcohol and marijuana use, but reports that she has not used either for the past 15 years. She further states that she had agreed to a trial of medication first for her liver disease and had deferred any discussion of liver transplant at the time of her diagnosis with HCV.
Laboratory tests reveal a normal complete blood count, creatinine, and electrolytes. However, liver functions were elevated, including aspartate aminotransferase (AST) of 107 U/L (reference range, 8 to 48 U/L) and alanine aminotransferase of 117 U/L (reference range, 7 to 55 U/L). Although increased, the levels of AST and ALT were slightly less than her levels pre-sofosbuvir–ribavirin trial, indicating some response to the medication.
[polldaddy:9777325]
The authors’ observations
Approximately 170 million people worldwide suffer from chronic HCV infection, affecting 2.7 to 5.2 million people in the United States, with 350,000 deaths attributed to liver disease caused by HCV.1
The standard treatment of HCV genotype 1, which represents 70% of all cases of chronic HCV in the United States, is 12 to 32 weeks of an oral protease inhibitor combined with 24 to 48 weeks of peg-interferon (IFN)–alpha-2a plus ribavirin, with the duration of therapy guided by the on-treatment response and the stage of hepatic fibrosis.1
In 2013, the FDA approved sofosbuvir, a direct-acting antiviral drug for chronic HCV. It is a nucleotide analogue HCV NS5B polymerase inhibitor with similar in vitro activity against all HCV genotypes.1 This medication is efficient when used with an antiviral regimen in adults with HCV with liver disease, cirrhosis, HIV coinfection, and hepatocellular carcinoma awaiting liver transplant.2
TREATMENT Medication restarted
Ms. B is admitted to the psychiatric unit for management of severe depression and suicidal thoughts, and quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, are restarted. The hepatology team is consulted for further evaluation and management of her liver disease.
She receives supportive psychotherapy, art therapy, and group therapy to develop better coping skills for her depression and suicidal thoughts and psychoeducation about her medical and psychiatric illness to understand the importance of treatment adherence for symptom improvement. Over the course of her hospital stay, Ms. B has subjective and objective improvements of her depressive symptoms.
The authors’ observations
Psychiatric adverse effects associated with IFN-α therapy in chronic HCV patients are the main cause of antiviral treatment discontinuation, resulting in a decreased rate of sustained viral response.3 Chronic HCV is a major health burden; therefore there is a need for treatment options that are more efficient, safer, simpler, more convenient, and preferably IFN-free.
Sofosbuvir has met many of these criteria and has been found to be safe and well tolerated when administered alone or with ribavirin. Sofosbuvir represents a major breakthrough in HCV care to achieve cures and prevent IFN-associated morbidity and mortality.4,5
Our case report highlights, however, that significant depressive symptoms may be associated with sofosbuvir. Hepatologists should be cautious when prescribing sofosbuvir in patients with comorbid psychiatric illness to avoid exacerbating depressive symptoms and increasing the risk of suicidality.
[polldaddy:9777328]
OUTCOME Refuses treatment
Ms. B is seen by the hepatology team who discuss the best treatment options for HCV, including ledipasvir/sofosbuvir, daclatasvir and ribavirin, and ombitasvir/paritaprevir/ritonavir plus dasabuvir. However, she refuses treatment for HCV stating, “I would rather have no depression with hepatitis C than feel depressed and suicidal while getting treatment for hepatitis C.”
Ms. B is discharged with referral to the outpatient psychiatry clinic and hepatology clinic for monitoring her liver function and restarting sofosbuvir and ribavirin for HCV once her mood symptoms improved.
The authors’ observations
A robust psychiatric evaluation is required before initiating the previously mentioned antiviral therapy to identify high-risk patients to prevent emergence or exacerbation of new psychiatric symptoms, including depression and mania, when treating with IFN-free or IFN-containing regimens. Collaborative care involving a hepatologist and psychiatrist is necessary for comprehensive monitoring of a patient’s psychiatric symptoms and management with medication and psychotherapy. This will limit psychiatric morbidity in patients receiving antiviral treatment with sofosbuvir and ribavirin.
It’s imperative to improve medication adherence for patients by adopting strategies, such as:
- identifying factors leading to noncompliance
- establishing a strong rapport with the patients
- providing psychoeducation about the illness, discussing the benefits and risks of medications and the importance of maintenance treatment
- simplifying medication regimen.6
More research on medication management of HCV in patients with comorbid psychiatric illness should be encouraged and focused on initiating and monitoring non-IFN treatment regimens for patients with HCV and preexisting bipolar disorder or other mood disorders.
1. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
2. Centers for Disease Control and Prevention. Hepatitis C FAQ for health professionals. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section4. Updated January 27, 2017. Accessed June 2, 2017.
3. Lucaciu LA, Dumitrascu DL. Depression and suicide ideation in chronic hepatitis C patients untreated and treated with interferon: prevalence, prevention, and treatment. Ann Gastroenterol. 2015;28(4):440-447.
4. Lam B, Henry L, Younossi Z. Sofosbuvir (Sovaldi) for the treatment of hepatitis C. Expert Rev Clin Pharmacol. 2014;7(5):555-566.
5. Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomized, phase 2 trial. Lancet 2014;383(9916):515-523.
6. Balon R. Managing compliance. Psychiatric Times. www.psychiatrictimes.com/articles/managing-compliance. Published May 1, 2002. Accessed June 14, 2017.
CASE Suicidal and paranoid
Ms. B, age 53, has a 30-year history of bipolar disorder, a 1-year history of hepatitis C virus (HCV), and previous inpatient psychiatric hospitalizations secondary to acute mania. She presents to our hospital describing her symptoms as the “worst depression ever” and reports suicidal ideation and paranoid thoughts of people watching and following her. Ms. B describes significant neurovegetative symptoms of depression, including poor sleep, poor appetite, low energy and concentration, and chronic feelings of hopelessness with thoughts of “ending it all.” Ms. B reports that her symptoms started 3 weeks ago, a few days after she started taking sofosbuvir and ribavirin for refractory HCV.
Ms. B’s medication regimen consisted of quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, for bipolar disorder, when she started taking sofosbuvir and ribavirin. Ms. B admits she stopped taking her psychotropic and antiviral medications after she noticed progressively worsening depression with intrusive suicidal thoughts, including ruminative thoughts of overdosing on them.
At evaluation, Ms. B is casually dressed, pleasant, with fair hygiene and poor eye contact. Her speech is decreased in rate, volume, and tone; mood is “devastated and depressed”; affect is labile and tearful. Her thought process reveals occasional thought blocking and her thought content includes suicidal ideations and paranoid thoughts. Her cognition is intact; insight and judgment are poor. During evaluation, Ms. B reveals a history of alcohol and marijuana use, but reports that she has not used either for the past 15 years. She further states that she had agreed to a trial of medication first for her liver disease and had deferred any discussion of liver transplant at the time of her diagnosis with HCV.
Laboratory tests reveal a normal complete blood count, creatinine, and electrolytes. However, liver functions were elevated, including aspartate aminotransferase (AST) of 107 U/L (reference range, 8 to 48 U/L) and alanine aminotransferase of 117 U/L (reference range, 7 to 55 U/L). Although increased, the levels of AST and ALT were slightly less than her levels pre-sofosbuvir–ribavirin trial, indicating some response to the medication.
[polldaddy:9777325]
The authors’ observations
Approximately 170 million people worldwide suffer from chronic HCV infection, affecting 2.7 to 5.2 million people in the United States, with 350,000 deaths attributed to liver disease caused by HCV.1
The standard treatment of HCV genotype 1, which represents 70% of all cases of chronic HCV in the United States, is 12 to 32 weeks of an oral protease inhibitor combined with 24 to 48 weeks of peg-interferon (IFN)–alpha-2a plus ribavirin, with the duration of therapy guided by the on-treatment response and the stage of hepatic fibrosis.1
In 2013, the FDA approved sofosbuvir, a direct-acting antiviral drug for chronic HCV. It is a nucleotide analogue HCV NS5B polymerase inhibitor with similar in vitro activity against all HCV genotypes.1 This medication is efficient when used with an antiviral regimen in adults with HCV with liver disease, cirrhosis, HIV coinfection, and hepatocellular carcinoma awaiting liver transplant.2
TREATMENT Medication restarted
Ms. B is admitted to the psychiatric unit for management of severe depression and suicidal thoughts, and quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, are restarted. The hepatology team is consulted for further evaluation and management of her liver disease.
She receives supportive psychotherapy, art therapy, and group therapy to develop better coping skills for her depression and suicidal thoughts and psychoeducation about her medical and psychiatric illness to understand the importance of treatment adherence for symptom improvement. Over the course of her hospital stay, Ms. B has subjective and objective improvements of her depressive symptoms.
The authors’ observations
Psychiatric adverse effects associated with IFN-α therapy in chronic HCV patients are the main cause of antiviral treatment discontinuation, resulting in a decreased rate of sustained viral response.3 Chronic HCV is a major health burden; therefore there is a need for treatment options that are more efficient, safer, simpler, more convenient, and preferably IFN-free.
Sofosbuvir has met many of these criteria and has been found to be safe and well tolerated when administered alone or with ribavirin. Sofosbuvir represents a major breakthrough in HCV care to achieve cures and prevent IFN-associated morbidity and mortality.4,5
Our case report highlights, however, that significant depressive symptoms may be associated with sofosbuvir. Hepatologists should be cautious when prescribing sofosbuvir in patients with comorbid psychiatric illness to avoid exacerbating depressive symptoms and increasing the risk of suicidality.
[polldaddy:9777328]
OUTCOME Refuses treatment
Ms. B is seen by the hepatology team who discuss the best treatment options for HCV, including ledipasvir/sofosbuvir, daclatasvir and ribavirin, and ombitasvir/paritaprevir/ritonavir plus dasabuvir. However, she refuses treatment for HCV stating, “I would rather have no depression with hepatitis C than feel depressed and suicidal while getting treatment for hepatitis C.”
Ms. B is discharged with referral to the outpatient psychiatry clinic and hepatology clinic for monitoring her liver function and restarting sofosbuvir and ribavirin for HCV once her mood symptoms improved.
The authors’ observations
A robust psychiatric evaluation is required before initiating the previously mentioned antiviral therapy to identify high-risk patients to prevent emergence or exacerbation of new psychiatric symptoms, including depression and mania, when treating with IFN-free or IFN-containing regimens. Collaborative care involving a hepatologist and psychiatrist is necessary for comprehensive monitoring of a patient’s psychiatric symptoms and management with medication and psychotherapy. This will limit psychiatric morbidity in patients receiving antiviral treatment with sofosbuvir and ribavirin.
It’s imperative to improve medication adherence for patients by adopting strategies, such as:
- identifying factors leading to noncompliance
- establishing a strong rapport with the patients
- providing psychoeducation about the illness, discussing the benefits and risks of medications and the importance of maintenance treatment
- simplifying medication regimen.6
More research on medication management of HCV in patients with comorbid psychiatric illness should be encouraged and focused on initiating and monitoring non-IFN treatment regimens for patients with HCV and preexisting bipolar disorder or other mood disorders.
CASE Suicidal and paranoid
Ms. B, age 53, has a 30-year history of bipolar disorder, a 1-year history of hepatitis C virus (HCV), and previous inpatient psychiatric hospitalizations secondary to acute mania. She presents to our hospital describing her symptoms as the “worst depression ever” and reports suicidal ideation and paranoid thoughts of people watching and following her. Ms. B describes significant neurovegetative symptoms of depression, including poor sleep, poor appetite, low energy and concentration, and chronic feelings of hopelessness with thoughts of “ending it all.” Ms. B reports that her symptoms started 3 weeks ago, a few days after she started taking sofosbuvir and ribavirin for refractory HCV.
Ms. B’s medication regimen consisted of quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, for bipolar disorder, when she started taking sofosbuvir and ribavirin. Ms. B admits she stopped taking her psychotropic and antiviral medications after she noticed progressively worsening depression with intrusive suicidal thoughts, including ruminative thoughts of overdosing on them.
At evaluation, Ms. B is casually dressed, pleasant, with fair hygiene and poor eye contact. Her speech is decreased in rate, volume, and tone; mood is “devastated and depressed”; affect is labile and tearful. Her thought process reveals occasional thought blocking and her thought content includes suicidal ideations and paranoid thoughts. Her cognition is intact; insight and judgment are poor. During evaluation, Ms. B reveals a history of alcohol and marijuana use, but reports that she has not used either for the past 15 years. She further states that she had agreed to a trial of medication first for her liver disease and had deferred any discussion of liver transplant at the time of her diagnosis with HCV.
Laboratory tests reveal a normal complete blood count, creatinine, and electrolytes. However, liver functions were elevated, including aspartate aminotransferase (AST) of 107 U/L (reference range, 8 to 48 U/L) and alanine aminotransferase of 117 U/L (reference range, 7 to 55 U/L). Although increased, the levels of AST and ALT were slightly less than her levels pre-sofosbuvir–ribavirin trial, indicating some response to the medication.
[polldaddy:9777325]
The authors’ observations
Approximately 170 million people worldwide suffer from chronic HCV infection, affecting 2.7 to 5.2 million people in the United States, with 350,000 deaths attributed to liver disease caused by HCV.1
The standard treatment of HCV genotype 1, which represents 70% of all cases of chronic HCV in the United States, is 12 to 32 weeks of an oral protease inhibitor combined with 24 to 48 weeks of peg-interferon (IFN)–alpha-2a plus ribavirin, with the duration of therapy guided by the on-treatment response and the stage of hepatic fibrosis.1
In 2013, the FDA approved sofosbuvir, a direct-acting antiviral drug for chronic HCV. It is a nucleotide analogue HCV NS5B polymerase inhibitor with similar in vitro activity against all HCV genotypes.1 This medication is efficient when used with an antiviral regimen in adults with HCV with liver disease, cirrhosis, HIV coinfection, and hepatocellular carcinoma awaiting liver transplant.2
TREATMENT Medication restarted
Ms. B is admitted to the psychiatric unit for management of severe depression and suicidal thoughts, and quetiapine, 400 mg at bedtime, fluoxetine, 40 mg/d, and lamotrigine, 150 mg/d, are restarted. The hepatology team is consulted for further evaluation and management of her liver disease.
She receives supportive psychotherapy, art therapy, and group therapy to develop better coping skills for her depression and suicidal thoughts and psychoeducation about her medical and psychiatric illness to understand the importance of treatment adherence for symptom improvement. Over the course of her hospital stay, Ms. B has subjective and objective improvements of her depressive symptoms.
The authors’ observations
Psychiatric adverse effects associated with IFN-α therapy in chronic HCV patients are the main cause of antiviral treatment discontinuation, resulting in a decreased rate of sustained viral response.3 Chronic HCV is a major health burden; therefore there is a need for treatment options that are more efficient, safer, simpler, more convenient, and preferably IFN-free.
Sofosbuvir has met many of these criteria and has been found to be safe and well tolerated when administered alone or with ribavirin. Sofosbuvir represents a major breakthrough in HCV care to achieve cures and prevent IFN-associated morbidity and mortality.4,5
Our case report highlights, however, that significant depressive symptoms may be associated with sofosbuvir. Hepatologists should be cautious when prescribing sofosbuvir in patients with comorbid psychiatric illness to avoid exacerbating depressive symptoms and increasing the risk of suicidality.
[polldaddy:9777328]
OUTCOME Refuses treatment
Ms. B is seen by the hepatology team who discuss the best treatment options for HCV, including ledipasvir/sofosbuvir, daclatasvir and ribavirin, and ombitasvir/paritaprevir/ritonavir plus dasabuvir. However, she refuses treatment for HCV stating, “I would rather have no depression with hepatitis C than feel depressed and suicidal while getting treatment for hepatitis C.”
Ms. B is discharged with referral to the outpatient psychiatry clinic and hepatology clinic for monitoring her liver function and restarting sofosbuvir and ribavirin for HCV once her mood symptoms improved.
The authors’ observations
A robust psychiatric evaluation is required before initiating the previously mentioned antiviral therapy to identify high-risk patients to prevent emergence or exacerbation of new psychiatric symptoms, including depression and mania, when treating with IFN-free or IFN-containing regimens. Collaborative care involving a hepatologist and psychiatrist is necessary for comprehensive monitoring of a patient’s psychiatric symptoms and management with medication and psychotherapy. This will limit psychiatric morbidity in patients receiving antiviral treatment with sofosbuvir and ribavirin.
It’s imperative to improve medication adherence for patients by adopting strategies, such as:
- identifying factors leading to noncompliance
- establishing a strong rapport with the patients
- providing psychoeducation about the illness, discussing the benefits and risks of medications and the importance of maintenance treatment
- simplifying medication regimen.6
More research on medication management of HCV in patients with comorbid psychiatric illness should be encouraged and focused on initiating and monitoring non-IFN treatment regimens for patients with HCV and preexisting bipolar disorder or other mood disorders.
1. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
2. Centers for Disease Control and Prevention. Hepatitis C FAQ for health professionals. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section4. Updated January 27, 2017. Accessed June 2, 2017.
3. Lucaciu LA, Dumitrascu DL. Depression and suicide ideation in chronic hepatitis C patients untreated and treated with interferon: prevalence, prevention, and treatment. Ann Gastroenterol. 2015;28(4):440-447.
4. Lam B, Henry L, Younossi Z. Sofosbuvir (Sovaldi) for the treatment of hepatitis C. Expert Rev Clin Pharmacol. 2014;7(5):555-566.
5. Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomized, phase 2 trial. Lancet 2014;383(9916):515-523.
6. Balon R. Managing compliance. Psychiatric Times. www.psychiatrictimes.com/articles/managing-compliance. Published May 1, 2002. Accessed June 14, 2017.
1. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
2. Centers for Disease Control and Prevention. Hepatitis C FAQ for health professionals. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section4. Updated January 27, 2017. Accessed June 2, 2017.
3. Lucaciu LA, Dumitrascu DL. Depression and suicide ideation in chronic hepatitis C patients untreated and treated with interferon: prevalence, prevention, and treatment. Ann Gastroenterol. 2015;28(4):440-447.
4. Lam B, Henry L, Younossi Z. Sofosbuvir (Sovaldi) for the treatment of hepatitis C. Expert Rev Clin Pharmacol. 2014;7(5):555-566.
5. Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomized, phase 2 trial. Lancet 2014;383(9916):515-523.
6. Balon R. Managing compliance. Psychiatric Times. www.psychiatrictimes.com/articles/managing-compliance. Published May 1, 2002. Accessed June 14, 2017.
Eating disorders: Are they age-restricted?
Eating disorders are thought to affect only the young. Although the mean age of presentation is 17 years for anorexia nervosa and 18 to 25 years for bulimia nervosa, many women >65 years suffer from these disorders.1 Often, geriatric patients with a history of eating disorders during their youth that partially remitted have the same disorders re-emerge during their golden years. Because many practitioners think of eating disorders as a younger person’s illness, we could miss an opportunity to help these individuals when screening our geriatric patients.
DSM-52 categorizes feeding and eating disorders as:
- binge eating disorder
- anorexia nervosa
- bulimia nervosa
- other specified feeding and eating disorders
- pica
- avoidant/restrictive food intake disorder.
Binge eating disorder’s main feature is recurrent binge eating, which is the sense that one has lost control when consuming a larger amount of food within a discrete time period than what most people might eat in the same time period. Binge eating may include eating rapidly, feeling uncomfortably
Anorexia nervosa is defined as the restriction of energy intake relative to necessary energy requirements, leading to significantly low body weight in the context of age, sex, developmental trajectory, and physical health, as well as an intense fear of gaining weight or persistent behaviors interfering with weight gain.
Bulimia nervosa is repetitive loss of control when eating large amounts of food (more than most would eat in a period), with compensatory behaviors to prevent weight gain. It is possible that the value attached to youthful slenderness leads to dissatisfaction among older women as their bodies change; binging might provide a sense of control during a time of uncertainty.
Body mass index typically is highest at middle age and slowly declines. In part, this decline is caused by a reduction in energy intake because of modifications in eating habits and lowered appetite often seen during aging. Older women eat 30% fewer calories than younger women.3,4 Social isolation, chronic disease, and depression also contribute to diminished food intake. It is important to remember that distorted body image can occur in older individuals as well. Anorexia nervosa has the highest fatality rate among psychiatric conditions,5 and geriatric patients could be at particularly high risk.
Assessment
Assess for eating disorders in a geriatric patient by exploring the patient’s perception of body image and ruling out underlying causes of weight loss and medical comorbidities. Take a detailed history, including:
- body image and disordered thinking about food
- abnormal behaviors or rituals surrounding food
- history of eating disorders, psychiatric illness, or hospitalization
- medical history
- current and past medications
- illicit drug use or addiction to prescription medications.
Collateral informants, such as partners and adult children of the patient, may yield important information. Because geriatric patients often take several medications, contacting the primary care physician is important in the integrated care of the patient.
A thorough physical and mental status examination will provide information about the patient’s physical appearance. For example, if the patient appears emaciated or weak, the content and process of thoughts related to food will help rule out other etiologies, such as psychosis, depressive disorders, or anxiety. Vital signs and a full physical examination are needed when caring for patients with an eating disorder, regardless of age, but particularly in medically fragile geriatric patients. Because osteoporosis and osteopenia are concerns for many older patients, it’s important to collaborate with the primary care physician early to help minimize bone loss.
Treatment
While ensuring medical stability of the patient, psychotherapy is the treatment of choice for eating disorders in geriatric patients. Moderate to severe binge eating disorder can be treated with lisdexamfetamine. For bulimia nervosa, consider a combination of SSRI and psychotherapy. There is no FDA-approved medication for treating anorexia nervosa; therefore identifying and treating underlying medical causes and/or psychiatric comorbidities can help improve prognosis. Despite this, 1 study showed 20% of geriatric patients with an eating disorder die of complications from eating disorders.6
1. Currin L, Schmidt U, Treasure J, et al. Time trends in eating disorder incidence. Br J Psychiatry. 2005;186(2):132-135.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
4. Morley JE. Peptides and aging: their role in anorexia and memory. Peptides. 2015;72(10):112-118.
5. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. Arch Gen Psychiatry. 2011;68(7):724-731.
6. Lapid MI, Prom MC, Burton MC, et al. Eating disorders in the elderly. Int Psychogeriatr. 2010;22(4):523-536.
Eating disorders are thought to affect only the young. Although the mean age of presentation is 17 years for anorexia nervosa and 18 to 25 years for bulimia nervosa, many women >65 years suffer from these disorders.1 Often, geriatric patients with a history of eating disorders during their youth that partially remitted have the same disorders re-emerge during their golden years. Because many practitioners think of eating disorders as a younger person’s illness, we could miss an opportunity to help these individuals when screening our geriatric patients.
DSM-52 categorizes feeding and eating disorders as:
- binge eating disorder
- anorexia nervosa
- bulimia nervosa
- other specified feeding and eating disorders
- pica
- avoidant/restrictive food intake disorder.
Binge eating disorder’s main feature is recurrent binge eating, which is the sense that one has lost control when consuming a larger amount of food within a discrete time period than what most people might eat in the same time period. Binge eating may include eating rapidly, feeling uncomfortably
Anorexia nervosa is defined as the restriction of energy intake relative to necessary energy requirements, leading to significantly low body weight in the context of age, sex, developmental trajectory, and physical health, as well as an intense fear of gaining weight or persistent behaviors interfering with weight gain.
Bulimia nervosa is repetitive loss of control when eating large amounts of food (more than most would eat in a period), with compensatory behaviors to prevent weight gain. It is possible that the value attached to youthful slenderness leads to dissatisfaction among older women as their bodies change; binging might provide a sense of control during a time of uncertainty.
Body mass index typically is highest at middle age and slowly declines. In part, this decline is caused by a reduction in energy intake because of modifications in eating habits and lowered appetite often seen during aging. Older women eat 30% fewer calories than younger women.3,4 Social isolation, chronic disease, and depression also contribute to diminished food intake. It is important to remember that distorted body image can occur in older individuals as well. Anorexia nervosa has the highest fatality rate among psychiatric conditions,5 and geriatric patients could be at particularly high risk.
Assessment
Assess for eating disorders in a geriatric patient by exploring the patient’s perception of body image and ruling out underlying causes of weight loss and medical comorbidities. Take a detailed history, including:
- body image and disordered thinking about food
- abnormal behaviors or rituals surrounding food
- history of eating disorders, psychiatric illness, or hospitalization
- medical history
- current and past medications
- illicit drug use or addiction to prescription medications.
Collateral informants, such as partners and adult children of the patient, may yield important information. Because geriatric patients often take several medications, contacting the primary care physician is important in the integrated care of the patient.
A thorough physical and mental status examination will provide information about the patient’s physical appearance. For example, if the patient appears emaciated or weak, the content and process of thoughts related to food will help rule out other etiologies, such as psychosis, depressive disorders, or anxiety. Vital signs and a full physical examination are needed when caring for patients with an eating disorder, regardless of age, but particularly in medically fragile geriatric patients. Because osteoporosis and osteopenia are concerns for many older patients, it’s important to collaborate with the primary care physician early to help minimize bone loss.
Treatment
While ensuring medical stability of the patient, psychotherapy is the treatment of choice for eating disorders in geriatric patients. Moderate to severe binge eating disorder can be treated with lisdexamfetamine. For bulimia nervosa, consider a combination of SSRI and psychotherapy. There is no FDA-approved medication for treating anorexia nervosa; therefore identifying and treating underlying medical causes and/or psychiatric comorbidities can help improve prognosis. Despite this, 1 study showed 20% of geriatric patients with an eating disorder die of complications from eating disorders.6
Eating disorders are thought to affect only the young. Although the mean age of presentation is 17 years for anorexia nervosa and 18 to 25 years for bulimia nervosa, many women >65 years suffer from these disorders.1 Often, geriatric patients with a history of eating disorders during their youth that partially remitted have the same disorders re-emerge during their golden years. Because many practitioners think of eating disorders as a younger person’s illness, we could miss an opportunity to help these individuals when screening our geriatric patients.
DSM-52 categorizes feeding and eating disorders as:
- binge eating disorder
- anorexia nervosa
- bulimia nervosa
- other specified feeding and eating disorders
- pica
- avoidant/restrictive food intake disorder.
Binge eating disorder’s main feature is recurrent binge eating, which is the sense that one has lost control when consuming a larger amount of food within a discrete time period than what most people might eat in the same time period. Binge eating may include eating rapidly, feeling uncomfortably
Anorexia nervosa is defined as the restriction of energy intake relative to necessary energy requirements, leading to significantly low body weight in the context of age, sex, developmental trajectory, and physical health, as well as an intense fear of gaining weight or persistent behaviors interfering with weight gain.
Bulimia nervosa is repetitive loss of control when eating large amounts of food (more than most would eat in a period), with compensatory behaviors to prevent weight gain. It is possible that the value attached to youthful slenderness leads to dissatisfaction among older women as their bodies change; binging might provide a sense of control during a time of uncertainty.
Body mass index typically is highest at middle age and slowly declines. In part, this decline is caused by a reduction in energy intake because of modifications in eating habits and lowered appetite often seen during aging. Older women eat 30% fewer calories than younger women.3,4 Social isolation, chronic disease, and depression also contribute to diminished food intake. It is important to remember that distorted body image can occur in older individuals as well. Anorexia nervosa has the highest fatality rate among psychiatric conditions,5 and geriatric patients could be at particularly high risk.
Assessment
Assess for eating disorders in a geriatric patient by exploring the patient’s perception of body image and ruling out underlying causes of weight loss and medical comorbidities. Take a detailed history, including:
- body image and disordered thinking about food
- abnormal behaviors or rituals surrounding food
- history of eating disorders, psychiatric illness, or hospitalization
- medical history
- current and past medications
- illicit drug use or addiction to prescription medications.
Collateral informants, such as partners and adult children of the patient, may yield important information. Because geriatric patients often take several medications, contacting the primary care physician is important in the integrated care of the patient.
A thorough physical and mental status examination will provide information about the patient’s physical appearance. For example, if the patient appears emaciated or weak, the content and process of thoughts related to food will help rule out other etiologies, such as psychosis, depressive disorders, or anxiety. Vital signs and a full physical examination are needed when caring for patients with an eating disorder, regardless of age, but particularly in medically fragile geriatric patients. Because osteoporosis and osteopenia are concerns for many older patients, it’s important to collaborate with the primary care physician early to help minimize bone loss.
Treatment
While ensuring medical stability of the patient, psychotherapy is the treatment of choice for eating disorders in geriatric patients. Moderate to severe binge eating disorder can be treated with lisdexamfetamine. For bulimia nervosa, consider a combination of SSRI and psychotherapy. There is no FDA-approved medication for treating anorexia nervosa; therefore identifying and treating underlying medical causes and/or psychiatric comorbidities can help improve prognosis. Despite this, 1 study showed 20% of geriatric patients with an eating disorder die of complications from eating disorders.6
1. Currin L, Schmidt U, Treasure J, et al. Time trends in eating disorder incidence. Br J Psychiatry. 2005;186(2):132-135.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
4. Morley JE. Peptides and aging: their role in anorexia and memory. Peptides. 2015;72(10):112-118.
5. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. Arch Gen Psychiatry. 2011;68(7):724-731.
6. Lapid MI, Prom MC, Burton MC, et al. Eating disorders in the elderly. Int Psychogeriatr. 2010;22(4):523-536.
1. Currin L, Schmidt U, Treasure J, et al. Time trends in eating disorder incidence. Br J Psychiatry. 2005;186(2):132-135.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Morley JE, Thomas DR. Anorexia and aging: pathophysiology. Nutrition. 1999;15(6):499-503.
4. Morley JE. Peptides and aging: their role in anorexia and memory. Peptides. 2015;72(10):112-118.
5. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. Arch Gen Psychiatry. 2011;68(7):724-731.
6. Lapid MI, Prom MC, Burton MC, et al. Eating disorders in the elderly. Int Psychogeriatr. 2010;22(4):523-536.
A practical approach to interviewing a somatizing patient
Somatization is the experience of psychological distress in the form of bodil
Collecting a detailed history of physical symptoms can help the patient feel that you are listening to him (her) and that the chief concern is important. A detailed review of psychiatric symptoms (eg, hallucinations, paranoia, suicidality, etc.) should be deferred until later in the examination. Asking questions relating to psychiatric symptoms early could lead to further resistance by reinforcing negative preconceptions that the patient might have regarding mental illness.
Explicitly express empathy regarding physical symptoms throughout the interview to acknowledge any real suffering the patient is experiencing and to contradict any notion that psychiatric evaluation implies that the suffering could be imaginary.
Ask, “How has this illness affected your life?” This question helps make the connection between the patient’s physical state and social milieu. If somatization is confirmed, then the provider should assist the patient in reversing the arrow of causation. Although the ultimate goal is for the patient to understand how his (her) life has affected the symptoms, simply understanding that there are connections between the two is a start toward this goal.1
Explore the response to the previous question. Expand upon it to elicit a detailed social history, listening for any social stressors.
Obtain family and personal histories of allergies, substance abuse, and medical or psychiatric illness.
Review psychiatric symptoms. Make questions less jarring2 by adapting them to the patient’s situation, such as “Has your illness become so painful that at times you don’t even want to live?”
Perform cognitive and physical examinations. Conducting a physical examination could further reassure the patient that you are not ignoring physical complaints.
Educate the patient that the mind and body are connected and emotions affect how one feels physically. Use examples, such as “When I feel anxious, my heart beats faster” or “A headache might hurt more at work than at the beach.”
Elicit feedback and questions from the patient.
Discuss your treatment plan with the patient. Resistant patients with confirmed somatization disorders might accept psychiatric care as a means of dealing with the stress or pain of their physical symptoms.
Consider asking:
- What would you be doing if you weren’t in the hospital right now?
- Aside from your health, what’s the biggest challenge in your life?
- Everything has a good side and a bad side. Is there anything positive about dealing with your illness? Providing the patient with an example of negative aspects of a good thing (such as the calories in ice cream, the high cost of gold, etc.) can help make this point.
- What would your life look like if you didn’t have these symptoms?
1. Creed F, Guthrie E. Techniques for interviewing the somatising patient. Br J Psychiatry. 1993;162:467-471.
2. Carlat DJ. The psychiatric interview: a practical guide. 2nd ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2005.
Somatization is the experience of psychological distress in the form of bodil
Collecting a detailed history of physical symptoms can help the patient feel that you are listening to him (her) and that the chief concern is important. A detailed review of psychiatric symptoms (eg, hallucinations, paranoia, suicidality, etc.) should be deferred until later in the examination. Asking questions relating to psychiatric symptoms early could lead to further resistance by reinforcing negative preconceptions that the patient might have regarding mental illness.
Explicitly express empathy regarding physical symptoms throughout the interview to acknowledge any real suffering the patient is experiencing and to contradict any notion that psychiatric evaluation implies that the suffering could be imaginary.
Ask, “How has this illness affected your life?” This question helps make the connection between the patient’s physical state and social milieu. If somatization is confirmed, then the provider should assist the patient in reversing the arrow of causation. Although the ultimate goal is for the patient to understand how his (her) life has affected the symptoms, simply understanding that there are connections between the two is a start toward this goal.1
Explore the response to the previous question. Expand upon it to elicit a detailed social history, listening for any social stressors.
Obtain family and personal histories of allergies, substance abuse, and medical or psychiatric illness.
Review psychiatric symptoms. Make questions less jarring2 by adapting them to the patient’s situation, such as “Has your illness become so painful that at times you don’t even want to live?”
Perform cognitive and physical examinations. Conducting a physical examination could further reassure the patient that you are not ignoring physical complaints.
Educate the patient that the mind and body are connected and emotions affect how one feels physically. Use examples, such as “When I feel anxious, my heart beats faster” or “A headache might hurt more at work than at the beach.”
Elicit feedback and questions from the patient.
Discuss your treatment plan with the patient. Resistant patients with confirmed somatization disorders might accept psychiatric care as a means of dealing with the stress or pain of their physical symptoms.
Consider asking:
- What would you be doing if you weren’t in the hospital right now?
- Aside from your health, what’s the biggest challenge in your life?
- Everything has a good side and a bad side. Is there anything positive about dealing with your illness? Providing the patient with an example of negative aspects of a good thing (such as the calories in ice cream, the high cost of gold, etc.) can help make this point.
- What would your life look like if you didn’t have these symptoms?
Somatization is the experience of psychological distress in the form of bodil
Collecting a detailed history of physical symptoms can help the patient feel that you are listening to him (her) and that the chief concern is important. A detailed review of psychiatric symptoms (eg, hallucinations, paranoia, suicidality, etc.) should be deferred until later in the examination. Asking questions relating to psychiatric symptoms early could lead to further resistance by reinforcing negative preconceptions that the patient might have regarding mental illness.
Explicitly express empathy regarding physical symptoms throughout the interview to acknowledge any real suffering the patient is experiencing and to contradict any notion that psychiatric evaluation implies that the suffering could be imaginary.
Ask, “How has this illness affected your life?” This question helps make the connection between the patient’s physical state and social milieu. If somatization is confirmed, then the provider should assist the patient in reversing the arrow of causation. Although the ultimate goal is for the patient to understand how his (her) life has affected the symptoms, simply understanding that there are connections between the two is a start toward this goal.1
Explore the response to the previous question. Expand upon it to elicit a detailed social history, listening for any social stressors.
Obtain family and personal histories of allergies, substance abuse, and medical or psychiatric illness.
Review psychiatric symptoms. Make questions less jarring2 by adapting them to the patient’s situation, such as “Has your illness become so painful that at times you don’t even want to live?”
Perform cognitive and physical examinations. Conducting a physical examination could further reassure the patient that you are not ignoring physical complaints.
Educate the patient that the mind and body are connected and emotions affect how one feels physically. Use examples, such as “When I feel anxious, my heart beats faster” or “A headache might hurt more at work than at the beach.”
Elicit feedback and questions from the patient.
Discuss your treatment plan with the patient. Resistant patients with confirmed somatization disorders might accept psychiatric care as a means of dealing with the stress or pain of their physical symptoms.
Consider asking:
- What would you be doing if you weren’t in the hospital right now?
- Aside from your health, what’s the biggest challenge in your life?
- Everything has a good side and a bad side. Is there anything positive about dealing with your illness? Providing the patient with an example of negative aspects of a good thing (such as the calories in ice cream, the high cost of gold, etc.) can help make this point.
- What would your life look like if you didn’t have these symptoms?
1. Creed F, Guthrie E. Techniques for interviewing the somatising patient. Br J Psychiatry. 1993;162:467-471.
2. Carlat DJ. The psychiatric interview: a practical guide. 2nd ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2005.
1. Creed F, Guthrie E. Techniques for interviewing the somatising patient. Br J Psychiatry. 1993;162:467-471.
2. Carlat DJ. The psychiatric interview: a practical guide. 2nd ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2005.
Should you recommend acupuncture to patients with substance use disorders?
Acupuncture is an ancient therapeutic tool known to be the core of traditional Chinese medicine. Two theories suggest positive outcomes in patients treated with acupuncture:
- The oxidative stress reduction theory states that a “large body of evidences demonstrated that acupuncture has [an] antioxidative effect in various diseases, but the exact mechanism remains unclear.”1
- The neurophysiological theory states that “acupuncture stimulation can facilitate the release of certain neuropeptides in the CNS, eliciting profound physiological effects and even activating self-healing mechanisms.”2
For decades, acupuncture has been used for addiction management. Here we provide information on its utility for patients with substance use disorders.
Opioid use disorder. Multiple studies have looked at withdrawal, comorbid mood disorders, and its management with acupuncture alone or in combination with psychotherapy and/or opioid agonists. Studies from Asia reported good treatment outcomes but had low-method quality.3 Western studies had superior method quality but found that acupuncture was no better than placebo as monotherapy. When acupuncture is combined with psychotherapy and an opioid agonist, treatment results are promising, showing faster taper of medications (methadone and buprenorphine/naloxone) with fewer adverse effects.
Cocaine use disorder. Most studies had poor treatment outcomes of acupuncture over placebo and were of low quality. A number of small studies were promising and found that patients treated with acupuncture were most likely to have a negative urine drug screen.3 Although acupuncture is widely used in the United States to treat cocaine dependence, evidence does not confirm its efficacy.
Tobacco use disorder. A small group of studies favored acupuncture for smoking cessation.3 Other studies reported no benefit compared with placebo or neutral results. Some studies agreed that any intervention (acupuncture or sham acupuncture) with good results is better than no intervention at all.
Alcohol use disorder. Almost no advantage over placebo was found. Studies with significant findings were in small populations.3
Amphetamine, Cannabis, and other hallucinogen use disorders. Available data on stimulants were too limited to be relevant. No studies were found on Cannabis and hallucinogens.
Further studies are needed
There is a lack of conclusive, good quality studies supporting acupuncture’s benefits in treating substance abuse. Acupuncture has been known to lack adverse effects other than those related to needle manipulation, which is dependent on the methods (depth of needle insertion, accurate anatomical location, angle, etc.). Because this treatment option is virtually side-effect free, inexpensive, with positive synergistic results, more high-method quality studies are needed to consider it for our patients.
1. Zeng XH, Li QQ, Xu Q, et al. Acupuncture mechanism and redox equilibrium. Evid Based Complement and Alternat Med. 2014;2014:483294. doi: 10.1155/2014/483294
2. Bai L, Lao L. Neurobiological foundations of acupuncture: the relevance and future prospect based on neuroimaging evidence. Evid Based Complement and Alternat Med. 2013;2013:812568. doi: 10.1155/2013/812568.
3. Boyuan Z, Yang C, Ke C, et al. Efficacy of acupuncture for psychological symptoms associated with opioid addiction: a systematic review and meta-analysis. Evid Based Complement and Alternat Med. 2014;2014:313549. doi: 10.1155/2014/313549.
Acupuncture is an ancient therapeutic tool known to be the core of traditional Chinese medicine. Two theories suggest positive outcomes in patients treated with acupuncture:
- The oxidative stress reduction theory states that a “large body of evidences demonstrated that acupuncture has [an] antioxidative effect in various diseases, but the exact mechanism remains unclear.”1
- The neurophysiological theory states that “acupuncture stimulation can facilitate the release of certain neuropeptides in the CNS, eliciting profound physiological effects and even activating self-healing mechanisms.”2
For decades, acupuncture has been used for addiction management. Here we provide information on its utility for patients with substance use disorders.
Opioid use disorder. Multiple studies have looked at withdrawal, comorbid mood disorders, and its management with acupuncture alone or in combination with psychotherapy and/or opioid agonists. Studies from Asia reported good treatment outcomes but had low-method quality.3 Western studies had superior method quality but found that acupuncture was no better than placebo as monotherapy. When acupuncture is combined with psychotherapy and an opioid agonist, treatment results are promising, showing faster taper of medications (methadone and buprenorphine/naloxone) with fewer adverse effects.
Cocaine use disorder. Most studies had poor treatment outcomes of acupuncture over placebo and were of low quality. A number of small studies were promising and found that patients treated with acupuncture were most likely to have a negative urine drug screen.3 Although acupuncture is widely used in the United States to treat cocaine dependence, evidence does not confirm its efficacy.
Tobacco use disorder. A small group of studies favored acupuncture for smoking cessation.3 Other studies reported no benefit compared with placebo or neutral results. Some studies agreed that any intervention (acupuncture or sham acupuncture) with good results is better than no intervention at all.
Alcohol use disorder. Almost no advantage over placebo was found. Studies with significant findings were in small populations.3
Amphetamine, Cannabis, and other hallucinogen use disorders. Available data on stimulants were too limited to be relevant. No studies were found on Cannabis and hallucinogens.
Further studies are needed
There is a lack of conclusive, good quality studies supporting acupuncture’s benefits in treating substance abuse. Acupuncture has been known to lack adverse effects other than those related to needle manipulation, which is dependent on the methods (depth of needle insertion, accurate anatomical location, angle, etc.). Because this treatment option is virtually side-effect free, inexpensive, with positive synergistic results, more high-method quality studies are needed to consider it for our patients.
Acupuncture is an ancient therapeutic tool known to be the core of traditional Chinese medicine. Two theories suggest positive outcomes in patients treated with acupuncture:
- The oxidative stress reduction theory states that a “large body of evidences demonstrated that acupuncture has [an] antioxidative effect in various diseases, but the exact mechanism remains unclear.”1
- The neurophysiological theory states that “acupuncture stimulation can facilitate the release of certain neuropeptides in the CNS, eliciting profound physiological effects and even activating self-healing mechanisms.”2
For decades, acupuncture has been used for addiction management. Here we provide information on its utility for patients with substance use disorders.
Opioid use disorder. Multiple studies have looked at withdrawal, comorbid mood disorders, and its management with acupuncture alone or in combination with psychotherapy and/or opioid agonists. Studies from Asia reported good treatment outcomes but had low-method quality.3 Western studies had superior method quality but found that acupuncture was no better than placebo as monotherapy. When acupuncture is combined with psychotherapy and an opioid agonist, treatment results are promising, showing faster taper of medications (methadone and buprenorphine/naloxone) with fewer adverse effects.
Cocaine use disorder. Most studies had poor treatment outcomes of acupuncture over placebo and were of low quality. A number of small studies were promising and found that patients treated with acupuncture were most likely to have a negative urine drug screen.3 Although acupuncture is widely used in the United States to treat cocaine dependence, evidence does not confirm its efficacy.
Tobacco use disorder. A small group of studies favored acupuncture for smoking cessation.3 Other studies reported no benefit compared with placebo or neutral results. Some studies agreed that any intervention (acupuncture or sham acupuncture) with good results is better than no intervention at all.
Alcohol use disorder. Almost no advantage over placebo was found. Studies with significant findings were in small populations.3
Amphetamine, Cannabis, and other hallucinogen use disorders. Available data on stimulants were too limited to be relevant. No studies were found on Cannabis and hallucinogens.
Further studies are needed
There is a lack of conclusive, good quality studies supporting acupuncture’s benefits in treating substance abuse. Acupuncture has been known to lack adverse effects other than those related to needle manipulation, which is dependent on the methods (depth of needle insertion, accurate anatomical location, angle, etc.). Because this treatment option is virtually side-effect free, inexpensive, with positive synergistic results, more high-method quality studies are needed to consider it for our patients.
1. Zeng XH, Li QQ, Xu Q, et al. Acupuncture mechanism and redox equilibrium. Evid Based Complement and Alternat Med. 2014;2014:483294. doi: 10.1155/2014/483294
2. Bai L, Lao L. Neurobiological foundations of acupuncture: the relevance and future prospect based on neuroimaging evidence. Evid Based Complement and Alternat Med. 2013;2013:812568. doi: 10.1155/2013/812568.
3. Boyuan Z, Yang C, Ke C, et al. Efficacy of acupuncture for psychological symptoms associated with opioid addiction: a systematic review and meta-analysis. Evid Based Complement and Alternat Med. 2014;2014:313549. doi: 10.1155/2014/313549.
1. Zeng XH, Li QQ, Xu Q, et al. Acupuncture mechanism and redox equilibrium. Evid Based Complement and Alternat Med. 2014;2014:483294. doi: 10.1155/2014/483294
2. Bai L, Lao L. Neurobiological foundations of acupuncture: the relevance and future prospect based on neuroimaging evidence. Evid Based Complement and Alternat Med. 2013;2013:812568. doi: 10.1155/2013/812568.
3. Boyuan Z, Yang C, Ke C, et al. Efficacy of acupuncture for psychological symptoms associated with opioid addiction: a systematic review and meta-analysis. Evid Based Complement and Alternat Med. 2014;2014:313549. doi: 10.1155/2014/313549.
FDA unveils plan to eliminate orphan designation backlog
The US Food and Drug Administration (FDA) has unveiled a plan to eliminate the agency’s existing backlog of orphan designation requests and ensure timely responses to all new requests with firm deadlines.
The agency’s Orphan Drug Modernization Plan comes a week after FDA Commissioner Scott Gottlieb committed to eliminating the backlog within 90 days and responding to all new requests for designation within 90 days of receipt during his testimony before a Senate subcommittee.
As authorized under the Orphan Drug Act, the Orphan Drug Designation Program provides orphan status to drugs and biologics intended for the treatment, diagnosis, or prevention of rare diseases, which are generally defined as diseases that affect fewer than 200,000 people in the US.
Orphan designation qualifies a product’s developer for various development incentives, including tax credits for clinical trial costs, relief from prescription drug user fee if the indication is for a rare disease or condition, and eligibility for 7 years of marketing exclusivity if the product is approved.
A request for orphan designation is one step that can be taken in the development process and is different than the filing of a marketing application with the FDA.
Currently, the FDA has about 200 orphan drug designation requests that are pending review. The number of orphan drug designation requests has steadily increased over the past 5 years.
In 2016, the FDA’s Office of Orphan Products Development received 568 new requests for designation – more than double the number of requests received in 2012.
The FDA says this increased interest in the program is a positive development for patients with rare diseases, and, with the Orphan Drug Modernization Plan, the FDA is committing to advancing the program to ensure it can efficiently and adequately review these requests.
“People who suffer with rare diseases are too often faced with no or limited treatment options, and what treatment options they have may be quite expensive due, in part, to significant costs of developing therapies for smaller populations,” said FDA Commissioner Scott Gottlieb, MD.
“Congress gave us tools to incentivize the development of novel therapies for rare diseases, and we intend to use these resources to their fullest extent in order to ensure Americans get the safe and effective medicines they need, and that the process for developing these innovations is as modern and efficient as possible.”
Among the elements of the Orphan Drug Modernization Plan, the FDA will deploy a Backlog SWAT team comprised of senior, experienced reviewers with significant expertise in orphan drug designation. The team will focus solely on the backlogged applications, starting with the oldest requests.
The agency will also employ a streamlined Designation Review Template to increase consistency and efficiency of its reviews.
In addition, the Orphan Drug Designation Program will look to collaborate within the agency’s medical product centers to create greater efficiency, including conducting joint reviews with the Office of Pediatric Therapeutics to review rare pediatric disease designation requests.
To ensure all future requests receive a response within 90 days of receipt, the FDA will take a multifaceted approach.
These efforts include, among other new steps:
- Reorganizing the review staff to maximize expertise and improve workload efficiencies
- Better leveraging the expertise across the FDA’s medical product centers
- Establishing a new FDA Orphan Products Council that will help address scientific and regulatory issues to ensure the agency is applying a consistent approach to regulating orphan drug products and reviewing designation requests.
The FDA is planning for the backlog to be eliminated by mid-September.
The Orphan Drug Modernization Plan is the first element of several efforts the FDA plans to undertake under its new “Medical Innovation Development Plan,” which is aimed at ensuring the FDA’s regulatory tools and policies are modern, risk-based, and efficient.
The goal of the plan is to seek ways the FDA can help facilitate the development of safe, effective, and transformative medical innovations that have the potential to significantly impact disease and reduce overall healthcare costs. ![]()
The US Food and Drug Administration (FDA) has unveiled a plan to eliminate the agency’s existing backlog of orphan designation requests and ensure timely responses to all new requests with firm deadlines.
The agency’s Orphan Drug Modernization Plan comes a week after FDA Commissioner Scott Gottlieb committed to eliminating the backlog within 90 days and responding to all new requests for designation within 90 days of receipt during his testimony before a Senate subcommittee.
As authorized under the Orphan Drug Act, the Orphan Drug Designation Program provides orphan status to drugs and biologics intended for the treatment, diagnosis, or prevention of rare diseases, which are generally defined as diseases that affect fewer than 200,000 people in the US.
Orphan designation qualifies a product’s developer for various development incentives, including tax credits for clinical trial costs, relief from prescription drug user fee if the indication is for a rare disease or condition, and eligibility for 7 years of marketing exclusivity if the product is approved.
A request for orphan designation is one step that can be taken in the development process and is different than the filing of a marketing application with the FDA.
Currently, the FDA has about 200 orphan drug designation requests that are pending review. The number of orphan drug designation requests has steadily increased over the past 5 years.
In 2016, the FDA’s Office of Orphan Products Development received 568 new requests for designation – more than double the number of requests received in 2012.
The FDA says this increased interest in the program is a positive development for patients with rare diseases, and, with the Orphan Drug Modernization Plan, the FDA is committing to advancing the program to ensure it can efficiently and adequately review these requests.
“People who suffer with rare diseases are too often faced with no or limited treatment options, and what treatment options they have may be quite expensive due, in part, to significant costs of developing therapies for smaller populations,” said FDA Commissioner Scott Gottlieb, MD.
“Congress gave us tools to incentivize the development of novel therapies for rare diseases, and we intend to use these resources to their fullest extent in order to ensure Americans get the safe and effective medicines they need, and that the process for developing these innovations is as modern and efficient as possible.”
Among the elements of the Orphan Drug Modernization Plan, the FDA will deploy a Backlog SWAT team comprised of senior, experienced reviewers with significant expertise in orphan drug designation. The team will focus solely on the backlogged applications, starting with the oldest requests.
The agency will also employ a streamlined Designation Review Template to increase consistency and efficiency of its reviews.
In addition, the Orphan Drug Designation Program will look to collaborate within the agency’s medical product centers to create greater efficiency, including conducting joint reviews with the Office of Pediatric Therapeutics to review rare pediatric disease designation requests.
To ensure all future requests receive a response within 90 days of receipt, the FDA will take a multifaceted approach.
These efforts include, among other new steps:
- Reorganizing the review staff to maximize expertise and improve workload efficiencies
- Better leveraging the expertise across the FDA’s medical product centers
- Establishing a new FDA Orphan Products Council that will help address scientific and regulatory issues to ensure the agency is applying a consistent approach to regulating orphan drug products and reviewing designation requests.
The FDA is planning for the backlog to be eliminated by mid-September.
The Orphan Drug Modernization Plan is the first element of several efforts the FDA plans to undertake under its new “Medical Innovation Development Plan,” which is aimed at ensuring the FDA’s regulatory tools and policies are modern, risk-based, and efficient.
The goal of the plan is to seek ways the FDA can help facilitate the development of safe, effective, and transformative medical innovations that have the potential to significantly impact disease and reduce overall healthcare costs. ![]()
The US Food and Drug Administration (FDA) has unveiled a plan to eliminate the agency’s existing backlog of orphan designation requests and ensure timely responses to all new requests with firm deadlines.
The agency’s Orphan Drug Modernization Plan comes a week after FDA Commissioner Scott Gottlieb committed to eliminating the backlog within 90 days and responding to all new requests for designation within 90 days of receipt during his testimony before a Senate subcommittee.
As authorized under the Orphan Drug Act, the Orphan Drug Designation Program provides orphan status to drugs and biologics intended for the treatment, diagnosis, or prevention of rare diseases, which are generally defined as diseases that affect fewer than 200,000 people in the US.
Orphan designation qualifies a product’s developer for various development incentives, including tax credits for clinical trial costs, relief from prescription drug user fee if the indication is for a rare disease or condition, and eligibility for 7 years of marketing exclusivity if the product is approved.
A request for orphan designation is one step that can be taken in the development process and is different than the filing of a marketing application with the FDA.
Currently, the FDA has about 200 orphan drug designation requests that are pending review. The number of orphan drug designation requests has steadily increased over the past 5 years.
In 2016, the FDA’s Office of Orphan Products Development received 568 new requests for designation – more than double the number of requests received in 2012.
The FDA says this increased interest in the program is a positive development for patients with rare diseases, and, with the Orphan Drug Modernization Plan, the FDA is committing to advancing the program to ensure it can efficiently and adequately review these requests.
“People who suffer with rare diseases are too often faced with no or limited treatment options, and what treatment options they have may be quite expensive due, in part, to significant costs of developing therapies for smaller populations,” said FDA Commissioner Scott Gottlieb, MD.
“Congress gave us tools to incentivize the development of novel therapies for rare diseases, and we intend to use these resources to their fullest extent in order to ensure Americans get the safe and effective medicines they need, and that the process for developing these innovations is as modern and efficient as possible.”
Among the elements of the Orphan Drug Modernization Plan, the FDA will deploy a Backlog SWAT team comprised of senior, experienced reviewers with significant expertise in orphan drug designation. The team will focus solely on the backlogged applications, starting with the oldest requests.
The agency will also employ a streamlined Designation Review Template to increase consistency and efficiency of its reviews.
In addition, the Orphan Drug Designation Program will look to collaborate within the agency’s medical product centers to create greater efficiency, including conducting joint reviews with the Office of Pediatric Therapeutics to review rare pediatric disease designation requests.
To ensure all future requests receive a response within 90 days of receipt, the FDA will take a multifaceted approach.
These efforts include, among other new steps:
- Reorganizing the review staff to maximize expertise and improve workload efficiencies
- Better leveraging the expertise across the FDA’s medical product centers
- Establishing a new FDA Orphan Products Council that will help address scientific and regulatory issues to ensure the agency is applying a consistent approach to regulating orphan drug products and reviewing designation requests.
The FDA is planning for the backlog to be eliminated by mid-September.
The Orphan Drug Modernization Plan is the first element of several efforts the FDA plans to undertake under its new “Medical Innovation Development Plan,” which is aimed at ensuring the FDA’s regulatory tools and policies are modern, risk-based, and efficient.
The goal of the plan is to seek ways the FDA can help facilitate the development of safe, effective, and transformative medical innovations that have the potential to significantly impact disease and reduce overall healthcare costs. ![]()
Oral Agent Offers Relief From Generalized Hyperhidrosis

A 34-year-old woman presents to your office for unbearable sweating on her hands, face, and axillary regions. It occurs nearly daily, causing social embarrassment. She has tried multiple antiperspirants to no avail. What can she can take to reduce the sweating?
Hyperhidrosis is a common, self-limiting problem that affects 2% to 3% of the United States population.2 Patients may complain of localized sweating of the hands, feet, face, or underarms, or more systemic, generalized sweating in multiple locations. Either way, patients note a significant impact on their quality of life.
Treatment of hyperhidrosis has traditionally focused on topical therapies to the affected areas. Research by both subjective report and objective measurements has shown that antiperspirants containing aluminum salt are effective at reducing sweating, particularly in the axilla, hands, and feet.3,4 Additionally, a systematic review of observational and experimental studies found topical glycopyrrolate to be efficacious for craniofacial hyperhidrosis, with minimal adverse effects.5 The availability of low-cost prescription and OTC aluminum-based antiperspirant agents makes topicals the firstline choice.
More invasive treatments are available for hyperhidrosis refractory to topicals. In a double-blind RCT, researchers injected either botulinum toxin type A (BTX-A) 50 U or placebo in patients with bilateral primary axillary hyperhidrosis.6 Of the 207 patients who received treatment injections, 96.1% had at least a 50% reduction of axillary sweating four weeks after initial injection, as measured by gravimetric assessment. The BTX-A injections also produced a prolonged effect; mean duration between injections was 30.6 weeks.
Other invasive treatments include iontophoresis, surgery, and laser therapy; however, these methods are not suitable for body-wide application and are thus not appropriate for patients with generalized hyperhidrosis.
Oxybutynin is the first oral agent to emerge as a treatment option for hyperhidrosis. This cholinergic antagonist has historically been used to treat overactive bladder. But oxybutynin not only reduces urinary frequency, it also decreases secretions in various locations and can therefore reduce perspiration and cause dry mouth.
In one prospective placebo-controlled trial, 50 patients with generalized hyperhidrosis were randomly assigned to either oxybutynin (titrated from 2.5 mg orally once daily to 5 mg orally twice daily) or placebo for six weeks.7 Seventeen patients (73.9%) receiving oxybutynin for palmar or axillary hyperhidrosis reported moderate to “great” resolution of their symptoms, compared with six patients (27.3%) in the placebo group. Dry mouth was reported in 34.8% of patients receiving oxybutynin versus 9.1% of those who received placebo; however, no patients dropped out of the study due to this adverse effect.7
STUDY SUMMARY
This multicenter RCT compared oxybutynin to placebo in 62 adults with localized or generalized hyperhidrosis from 12 outpatient dermatology practices in France. It is the first study to include patients with both localized and generalized forms of the condition.
Patients were included if they were older than 18, enrolled in the National Health Insurance system in France, and reported a Hyperhidrosis Disease Severity Scale (HDSS) score ≥ 2. The HDSS is a validated, one-question tool (“How would you rate the severity of your sweating?”). Patients provide a score of 1 (no perceptible sweating and no interference with everyday life) to 4 (intolerable sweating with constant interference with everyday life).8 Patients were excluded if they had any contraindications to the use of an anticholinergic medication.
Patients randomly assigned to oxybutynin took 2.5 mg/d by mouth initially and increased gradually over eight days until reaching an effective dose that was no more than 7.5 mg/d. They then continued at that dose for six weeks.
The primary outcome was improvement on the HDSS by one or more points, measured at the beginning of the trial and again at six weeks. Secondary outcomes included change in quality of life, as measured by the Dermatology Life Quality Index (DLQI) and reported adverse effects. The DLQI is a dermatology-specific quality-of-life measure consisting of 10 questions. Scores range from 0 (where disease has no impact on quality of life) to 30 (maximum impact of disease on quality of life).9
Improved HDSS and DLQI scores. Most patients (83%) in the study had generalized hyperhidrosis. Patients were in their mid-30s. Sixty percent of patients in the oxybutynin group had an improvement of one point or more on the 4-point HDSS, compared to 27% in the placebo group. DLQI scores improved by 6.9 points in the oxybutynin group and 2.3 points in the placebo group.
The most common adverse effect was dry mouth, which occurred in 13 patients (43%) in the oxybutynin group and in three patients (11%) in the placebo group; it did not cause any patients to drop out of the study. The second most common adverse effect was blurred vision, which only occurred in the oxybutynin group (four patients; 13%).
WHAT’S NEW
This is the first RCT to demonstrate the efficacy of an oral agent for generalized primary hyperhidrosis. This trial used a relatively low dose of oxybutynin, which produced significant benefit while minimizing anticholinergic adverse effects.
CAVEATS
There are many situations for which anticholinergic medications are inappropriate, including use by geriatric patients and those with gastrointestinal disorders, urinary retention, or glaucoma.
CHALLENGES TO IMPLEMENTATION
Few, if any, challenges exist to the utilization of oxybutynin; inexpensive generic versions are widely available.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[6]:392-394).
1. Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis: a randomized, placebo-controlled trial. Br J Dermatol. 2015; 173:1163-1168.
2. Grabell DA, Hebert AA. Current and emerging medical therapies for primary hyperhidrosis. Dermatol Ther (Heidelb). 2017;7:25-36.
3. Innocenzi D, Lupi F, Bruni F, et al. Efficacy of a new aluminium salt thermophobic foam in the treatment of axillary and palmar primary hyperhidrosis: a pilot exploratory trial. Curr Med Res Opin. 2005;21:1949-1953.
4. Goh CL. Aluminum chloride hexahydrate versus palmar hyperhidrosis. Evaporimeter assessment. Int J Dermatol. 1990;29:368-370.
5. Nicholas R, Quddus A, Baker DM. Treatment of primary craniofacial hyperhidrosis: a systematic review. Am J Clin Dermatol. 2015;16:361-370.
6. Naumann M, Lowe NJ, Kumar CR, et al. Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003; 139:731-736.
7. Wolosker N, de Campos JR, Kauffman P, et al. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012; 55:1696-1700.
8. Varella AY, Fukuda JM, Teivelis MP, et al. Translation and validation of Hyperhidrosis Disease Severity Scale. Rev Assoc Med Bras. 2016;62:843-847.
9. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.

A 34-year-old woman presents to your office for unbearable sweating on her hands, face, and axillary regions. It occurs nearly daily, causing social embarrassment. She has tried multiple antiperspirants to no avail. What can she can take to reduce the sweating?
Hyperhidrosis is a common, self-limiting problem that affects 2% to 3% of the United States population.2 Patients may complain of localized sweating of the hands, feet, face, or underarms, or more systemic, generalized sweating in multiple locations. Either way, patients note a significant impact on their quality of life.
Treatment of hyperhidrosis has traditionally focused on topical therapies to the affected areas. Research by both subjective report and objective measurements has shown that antiperspirants containing aluminum salt are effective at reducing sweating, particularly in the axilla, hands, and feet.3,4 Additionally, a systematic review of observational and experimental studies found topical glycopyrrolate to be efficacious for craniofacial hyperhidrosis, with minimal adverse effects.5 The availability of low-cost prescription and OTC aluminum-based antiperspirant agents makes topicals the firstline choice.
More invasive treatments are available for hyperhidrosis refractory to topicals. In a double-blind RCT, researchers injected either botulinum toxin type A (BTX-A) 50 U or placebo in patients with bilateral primary axillary hyperhidrosis.6 Of the 207 patients who received treatment injections, 96.1% had at least a 50% reduction of axillary sweating four weeks after initial injection, as measured by gravimetric assessment. The BTX-A injections also produced a prolonged effect; mean duration between injections was 30.6 weeks.
Other invasive treatments include iontophoresis, surgery, and laser therapy; however, these methods are not suitable for body-wide application and are thus not appropriate for patients with generalized hyperhidrosis.
Oxybutynin is the first oral agent to emerge as a treatment option for hyperhidrosis. This cholinergic antagonist has historically been used to treat overactive bladder. But oxybutynin not only reduces urinary frequency, it also decreases secretions in various locations and can therefore reduce perspiration and cause dry mouth.
In one prospective placebo-controlled trial, 50 patients with generalized hyperhidrosis were randomly assigned to either oxybutynin (titrated from 2.5 mg orally once daily to 5 mg orally twice daily) or placebo for six weeks.7 Seventeen patients (73.9%) receiving oxybutynin for palmar or axillary hyperhidrosis reported moderate to “great” resolution of their symptoms, compared with six patients (27.3%) in the placebo group. Dry mouth was reported in 34.8% of patients receiving oxybutynin versus 9.1% of those who received placebo; however, no patients dropped out of the study due to this adverse effect.7
STUDY SUMMARY
This multicenter RCT compared oxybutynin to placebo in 62 adults with localized or generalized hyperhidrosis from 12 outpatient dermatology practices in France. It is the first study to include patients with both localized and generalized forms of the condition.
Patients were included if they were older than 18, enrolled in the National Health Insurance system in France, and reported a Hyperhidrosis Disease Severity Scale (HDSS) score ≥ 2. The HDSS is a validated, one-question tool (“How would you rate the severity of your sweating?”). Patients provide a score of 1 (no perceptible sweating and no interference with everyday life) to 4 (intolerable sweating with constant interference with everyday life).8 Patients were excluded if they had any contraindications to the use of an anticholinergic medication.
Patients randomly assigned to oxybutynin took 2.5 mg/d by mouth initially and increased gradually over eight days until reaching an effective dose that was no more than 7.5 mg/d. They then continued at that dose for six weeks.
The primary outcome was improvement on the HDSS by one or more points, measured at the beginning of the trial and again at six weeks. Secondary outcomes included change in quality of life, as measured by the Dermatology Life Quality Index (DLQI) and reported adverse effects. The DLQI is a dermatology-specific quality-of-life measure consisting of 10 questions. Scores range from 0 (where disease has no impact on quality of life) to 30 (maximum impact of disease on quality of life).9
Improved HDSS and DLQI scores. Most patients (83%) in the study had generalized hyperhidrosis. Patients were in their mid-30s. Sixty percent of patients in the oxybutynin group had an improvement of one point or more on the 4-point HDSS, compared to 27% in the placebo group. DLQI scores improved by 6.9 points in the oxybutynin group and 2.3 points in the placebo group.
The most common adverse effect was dry mouth, which occurred in 13 patients (43%) in the oxybutynin group and in three patients (11%) in the placebo group; it did not cause any patients to drop out of the study. The second most common adverse effect was blurred vision, which only occurred in the oxybutynin group (four patients; 13%).
WHAT’S NEW
This is the first RCT to demonstrate the efficacy of an oral agent for generalized primary hyperhidrosis. This trial used a relatively low dose of oxybutynin, which produced significant benefit while minimizing anticholinergic adverse effects.
CAVEATS
There are many situations for which anticholinergic medications are inappropriate, including use by geriatric patients and those with gastrointestinal disorders, urinary retention, or glaucoma.
CHALLENGES TO IMPLEMENTATION
Few, if any, challenges exist to the utilization of oxybutynin; inexpensive generic versions are widely available.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[6]:392-394).

A 34-year-old woman presents to your office for unbearable sweating on her hands, face, and axillary regions. It occurs nearly daily, causing social embarrassment. She has tried multiple antiperspirants to no avail. What can she can take to reduce the sweating?
Hyperhidrosis is a common, self-limiting problem that affects 2% to 3% of the United States population.2 Patients may complain of localized sweating of the hands, feet, face, or underarms, or more systemic, generalized sweating in multiple locations. Either way, patients note a significant impact on their quality of life.
Treatment of hyperhidrosis has traditionally focused on topical therapies to the affected areas. Research by both subjective report and objective measurements has shown that antiperspirants containing aluminum salt are effective at reducing sweating, particularly in the axilla, hands, and feet.3,4 Additionally, a systematic review of observational and experimental studies found topical glycopyrrolate to be efficacious for craniofacial hyperhidrosis, with minimal adverse effects.5 The availability of low-cost prescription and OTC aluminum-based antiperspirant agents makes topicals the firstline choice.
More invasive treatments are available for hyperhidrosis refractory to topicals. In a double-blind RCT, researchers injected either botulinum toxin type A (BTX-A) 50 U or placebo in patients with bilateral primary axillary hyperhidrosis.6 Of the 207 patients who received treatment injections, 96.1% had at least a 50% reduction of axillary sweating four weeks after initial injection, as measured by gravimetric assessment. The BTX-A injections also produced a prolonged effect; mean duration between injections was 30.6 weeks.
Other invasive treatments include iontophoresis, surgery, and laser therapy; however, these methods are not suitable for body-wide application and are thus not appropriate for patients with generalized hyperhidrosis.
Oxybutynin is the first oral agent to emerge as a treatment option for hyperhidrosis. This cholinergic antagonist has historically been used to treat overactive bladder. But oxybutynin not only reduces urinary frequency, it also decreases secretions in various locations and can therefore reduce perspiration and cause dry mouth.
In one prospective placebo-controlled trial, 50 patients with generalized hyperhidrosis were randomly assigned to either oxybutynin (titrated from 2.5 mg orally once daily to 5 mg orally twice daily) or placebo for six weeks.7 Seventeen patients (73.9%) receiving oxybutynin for palmar or axillary hyperhidrosis reported moderate to “great” resolution of their symptoms, compared with six patients (27.3%) in the placebo group. Dry mouth was reported in 34.8% of patients receiving oxybutynin versus 9.1% of those who received placebo; however, no patients dropped out of the study due to this adverse effect.7
STUDY SUMMARY
This multicenter RCT compared oxybutynin to placebo in 62 adults with localized or generalized hyperhidrosis from 12 outpatient dermatology practices in France. It is the first study to include patients with both localized and generalized forms of the condition.
Patients were included if they were older than 18, enrolled in the National Health Insurance system in France, and reported a Hyperhidrosis Disease Severity Scale (HDSS) score ≥ 2. The HDSS is a validated, one-question tool (“How would you rate the severity of your sweating?”). Patients provide a score of 1 (no perceptible sweating and no interference with everyday life) to 4 (intolerable sweating with constant interference with everyday life).8 Patients were excluded if they had any contraindications to the use of an anticholinergic medication.
Patients randomly assigned to oxybutynin took 2.5 mg/d by mouth initially and increased gradually over eight days until reaching an effective dose that was no more than 7.5 mg/d. They then continued at that dose for six weeks.
The primary outcome was improvement on the HDSS by one or more points, measured at the beginning of the trial and again at six weeks. Secondary outcomes included change in quality of life, as measured by the Dermatology Life Quality Index (DLQI) and reported adverse effects. The DLQI is a dermatology-specific quality-of-life measure consisting of 10 questions. Scores range from 0 (where disease has no impact on quality of life) to 30 (maximum impact of disease on quality of life).9
Improved HDSS and DLQI scores. Most patients (83%) in the study had generalized hyperhidrosis. Patients were in their mid-30s. Sixty percent of patients in the oxybutynin group had an improvement of one point or more on the 4-point HDSS, compared to 27% in the placebo group. DLQI scores improved by 6.9 points in the oxybutynin group and 2.3 points in the placebo group.
The most common adverse effect was dry mouth, which occurred in 13 patients (43%) in the oxybutynin group and in three patients (11%) in the placebo group; it did not cause any patients to drop out of the study. The second most common adverse effect was blurred vision, which only occurred in the oxybutynin group (four patients; 13%).
WHAT’S NEW
This is the first RCT to demonstrate the efficacy of an oral agent for generalized primary hyperhidrosis. This trial used a relatively low dose of oxybutynin, which produced significant benefit while minimizing anticholinergic adverse effects.
CAVEATS
There are many situations for which anticholinergic medications are inappropriate, including use by geriatric patients and those with gastrointestinal disorders, urinary retention, or glaucoma.
CHALLENGES TO IMPLEMENTATION
Few, if any, challenges exist to the utilization of oxybutynin; inexpensive generic versions are widely available.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[6]:392-394).
1. Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis: a randomized, placebo-controlled trial. Br J Dermatol. 2015; 173:1163-1168.
2. Grabell DA, Hebert AA. Current and emerging medical therapies for primary hyperhidrosis. Dermatol Ther (Heidelb). 2017;7:25-36.
3. Innocenzi D, Lupi F, Bruni F, et al. Efficacy of a new aluminium salt thermophobic foam in the treatment of axillary and palmar primary hyperhidrosis: a pilot exploratory trial. Curr Med Res Opin. 2005;21:1949-1953.
4. Goh CL. Aluminum chloride hexahydrate versus palmar hyperhidrosis. Evaporimeter assessment. Int J Dermatol. 1990;29:368-370.
5. Nicholas R, Quddus A, Baker DM. Treatment of primary craniofacial hyperhidrosis: a systematic review. Am J Clin Dermatol. 2015;16:361-370.
6. Naumann M, Lowe NJ, Kumar CR, et al. Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003; 139:731-736.
7. Wolosker N, de Campos JR, Kauffman P, et al. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012; 55:1696-1700.
8. Varella AY, Fukuda JM, Teivelis MP, et al. Translation and validation of Hyperhidrosis Disease Severity Scale. Rev Assoc Med Bras. 2016;62:843-847.
9. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
1. Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis: a randomized, placebo-controlled trial. Br J Dermatol. 2015; 173:1163-1168.
2. Grabell DA, Hebert AA. Current and emerging medical therapies for primary hyperhidrosis. Dermatol Ther (Heidelb). 2017;7:25-36.
3. Innocenzi D, Lupi F, Bruni F, et al. Efficacy of a new aluminium salt thermophobic foam in the treatment of axillary and palmar primary hyperhidrosis: a pilot exploratory trial. Curr Med Res Opin. 2005;21:1949-1953.
4. Goh CL. Aluminum chloride hexahydrate versus palmar hyperhidrosis. Evaporimeter assessment. Int J Dermatol. 1990;29:368-370.
5. Nicholas R, Quddus A, Baker DM. Treatment of primary craniofacial hyperhidrosis: a systematic review. Am J Clin Dermatol. 2015;16:361-370.
6. Naumann M, Lowe NJ, Kumar CR, et al. Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003; 139:731-736.
7. Wolosker N, de Campos JR, Kauffman P, et al. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012; 55:1696-1700.
8. Varella AY, Fukuda JM, Teivelis MP, et al. Translation and validation of Hyperhidrosis Disease Severity Scale. Rev Assoc Med Bras. 2016;62:843-847.
9. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.