User login
VIDEO: Hip, knee replacements fall in Danish RA patients
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
[email protected]
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
[email protected]
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: RA patient hip replacements fell from nearly 9/1,000 person-years in 1996 to about 4.5/1,000 person-years in 2011.
Data source: Records from more than 300,000 people in the Danish National Patient Register.
Disclosures: Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
Obesity blunts TNFi response in axial spondyloarthritis
MADRID – Obese patients with axial spondyloarthritis were substantially less responsive to treatment with a tumor necrosis factor inhibitor than were healthy-weight patients in a multicenter Swiss study with 531 patients.
In a multivariate analysis that controlled for several demographic and clinical factors, including baseline disease severity, obese patients with axial spondyloarthritis (SpA) were 70% less likely to achieve a 40% or better improvement in their Assessment in SpondyloArthritis International Society improvement criteria (ASAS 40) when compared with patients with a healthy body mass index (BMI), Raphael Micheroli, MD, reported in a poster at the European Congress of Rheumatology.
The finding supplies a third reason why patients with newly diagnosed axial SpA should try to lose weight if they are obese (or overweight) – to potentially improve their responsiveness to a TNFi. The other two reasons are to reduce cardiovascular disease risk in patients who are already at risk for these complications because of their disease, and to also help improve their ability to perform physical activities, he explained in an interview.
Dr. Micheroli proposed three possible reasons why obese patients with axial SpA might be less responsive to a TNFi than healthy-weight patients: They receive an inadequate TNFi dosage, their increased adipose tissue produces excess proinflammatory cytokines that exacerbate their axial SpA, or it is possible that obese patients are more likely to be misdiagnosed with axial SpA and because they don’t really have this disease their symptoms cannot improve with TNFi treatment. They may instead have, for example, degenerative back pain, a condition that can be challenging to distinguish from axial SpA, he said.
A role for obesity in blunting the beneficial effects of TNFi treatment has been well described for psoriatic arthritis, for example, in an Italian study with 138 patients (Ann Rheum Dis. 2014 June;73[6]:1157-62), and in a Danish study with more than 1,200 patients (Rheumatology [Oxford]. 2016 Dec;55[12]:2191-9).
Dr. Micheroli’s study included 624 patients with axial SpA enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases axial spondyloarthritis cohort who met the ASAS classification criteria for axial SpA and started treatment with their first TNFi after they entered the cohort. Follow-up data after 1 year on treatment were available for 531 of these patients. The entry group included 332 patients (53%) with a healthy BMI, 204 (33%) with an overweight BMI (25-30 kg/m2), and 88 (14%) obese patients (BMI more than 30 kg/m2). The patients averaged about 40 years old and had been symptomatic for an average of about 13 years. About one-third of patients started on adalimumab (Humira) treatment, about one-quarter started etanercept (Enbrel), more than one-fifth began infliximab (Remicade), and some patients started treatment with either golimumab (Simponi) or certolizumab pegol (Cimzia).
After 1 year on TNFi treatment, ASAS 40 improvement occurred in 44% of 282 healthy-BMI patients, 34% of 178 overweight patients, and in 29% of 71 obese patients, Dr. Micheroli reported. In a baseline-adjusted multivariate model, this difference translated into an odds ratio of 0.30 for obese patients achieving an ASAS 40 response, compared with the healthy-BMI patients after 1 year, a statistically significant difference. Further analysis showed no statistically significant differences in TNFi discontinuation rates among the three BMI subgroups.
Dr. Micheroli had no disclosures.
[email protected]
On Twitter @mitchelzoler
MADRID – Obese patients with axial spondyloarthritis were substantially less responsive to treatment with a tumor necrosis factor inhibitor than were healthy-weight patients in a multicenter Swiss study with 531 patients.
In a multivariate analysis that controlled for several demographic and clinical factors, including baseline disease severity, obese patients with axial spondyloarthritis (SpA) were 70% less likely to achieve a 40% or better improvement in their Assessment in SpondyloArthritis International Society improvement criteria (ASAS 40) when compared with patients with a healthy body mass index (BMI), Raphael Micheroli, MD, reported in a poster at the European Congress of Rheumatology.
The finding supplies a third reason why patients with newly diagnosed axial SpA should try to lose weight if they are obese (or overweight) – to potentially improve their responsiveness to a TNFi. The other two reasons are to reduce cardiovascular disease risk in patients who are already at risk for these complications because of their disease, and to also help improve their ability to perform physical activities, he explained in an interview.
Dr. Micheroli proposed three possible reasons why obese patients with axial SpA might be less responsive to a TNFi than healthy-weight patients: They receive an inadequate TNFi dosage, their increased adipose tissue produces excess proinflammatory cytokines that exacerbate their axial SpA, or it is possible that obese patients are more likely to be misdiagnosed with axial SpA and because they don’t really have this disease their symptoms cannot improve with TNFi treatment. They may instead have, for example, degenerative back pain, a condition that can be challenging to distinguish from axial SpA, he said.
A role for obesity in blunting the beneficial effects of TNFi treatment has been well described for psoriatic arthritis, for example, in an Italian study with 138 patients (Ann Rheum Dis. 2014 June;73[6]:1157-62), and in a Danish study with more than 1,200 patients (Rheumatology [Oxford]. 2016 Dec;55[12]:2191-9).
Dr. Micheroli’s study included 624 patients with axial SpA enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases axial spondyloarthritis cohort who met the ASAS classification criteria for axial SpA and started treatment with their first TNFi after they entered the cohort. Follow-up data after 1 year on treatment were available for 531 of these patients. The entry group included 332 patients (53%) with a healthy BMI, 204 (33%) with an overweight BMI (25-30 kg/m2), and 88 (14%) obese patients (BMI more than 30 kg/m2). The patients averaged about 40 years old and had been symptomatic for an average of about 13 years. About one-third of patients started on adalimumab (Humira) treatment, about one-quarter started etanercept (Enbrel), more than one-fifth began infliximab (Remicade), and some patients started treatment with either golimumab (Simponi) or certolizumab pegol (Cimzia).
After 1 year on TNFi treatment, ASAS 40 improvement occurred in 44% of 282 healthy-BMI patients, 34% of 178 overweight patients, and in 29% of 71 obese patients, Dr. Micheroli reported. In a baseline-adjusted multivariate model, this difference translated into an odds ratio of 0.30 for obese patients achieving an ASAS 40 response, compared with the healthy-BMI patients after 1 year, a statistically significant difference. Further analysis showed no statistically significant differences in TNFi discontinuation rates among the three BMI subgroups.
Dr. Micheroli had no disclosures.
[email protected]
On Twitter @mitchelzoler
MADRID – Obese patients with axial spondyloarthritis were substantially less responsive to treatment with a tumor necrosis factor inhibitor than were healthy-weight patients in a multicenter Swiss study with 531 patients.
In a multivariate analysis that controlled for several demographic and clinical factors, including baseline disease severity, obese patients with axial spondyloarthritis (SpA) were 70% less likely to achieve a 40% or better improvement in their Assessment in SpondyloArthritis International Society improvement criteria (ASAS 40) when compared with patients with a healthy body mass index (BMI), Raphael Micheroli, MD, reported in a poster at the European Congress of Rheumatology.
The finding supplies a third reason why patients with newly diagnosed axial SpA should try to lose weight if they are obese (or overweight) – to potentially improve their responsiveness to a TNFi. The other two reasons are to reduce cardiovascular disease risk in patients who are already at risk for these complications because of their disease, and to also help improve their ability to perform physical activities, he explained in an interview.
Dr. Micheroli proposed three possible reasons why obese patients with axial SpA might be less responsive to a TNFi than healthy-weight patients: They receive an inadequate TNFi dosage, their increased adipose tissue produces excess proinflammatory cytokines that exacerbate their axial SpA, or it is possible that obese patients are more likely to be misdiagnosed with axial SpA and because they don’t really have this disease their symptoms cannot improve with TNFi treatment. They may instead have, for example, degenerative back pain, a condition that can be challenging to distinguish from axial SpA, he said.
A role for obesity in blunting the beneficial effects of TNFi treatment has been well described for psoriatic arthritis, for example, in an Italian study with 138 patients (Ann Rheum Dis. 2014 June;73[6]:1157-62), and in a Danish study with more than 1,200 patients (Rheumatology [Oxford]. 2016 Dec;55[12]:2191-9).
Dr. Micheroli’s study included 624 patients with axial SpA enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases axial spondyloarthritis cohort who met the ASAS classification criteria for axial SpA and started treatment with their first TNFi after they entered the cohort. Follow-up data after 1 year on treatment were available for 531 of these patients. The entry group included 332 patients (53%) with a healthy BMI, 204 (33%) with an overweight BMI (25-30 kg/m2), and 88 (14%) obese patients (BMI more than 30 kg/m2). The patients averaged about 40 years old and had been symptomatic for an average of about 13 years. About one-third of patients started on adalimumab (Humira) treatment, about one-quarter started etanercept (Enbrel), more than one-fifth began infliximab (Remicade), and some patients started treatment with either golimumab (Simponi) or certolizumab pegol (Cimzia).
After 1 year on TNFi treatment, ASAS 40 improvement occurred in 44% of 282 healthy-BMI patients, 34% of 178 overweight patients, and in 29% of 71 obese patients, Dr. Micheroli reported. In a baseline-adjusted multivariate model, this difference translated into an odds ratio of 0.30 for obese patients achieving an ASAS 40 response, compared with the healthy-BMI patients after 1 year, a statistically significant difference. Further analysis showed no statistically significant differences in TNFi discontinuation rates among the three BMI subgroups.
Dr. Micheroli had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Obese patients had a 70% lower response rate to a tumor necrosis factor inhibitor, compared with healthy-weight patients.
Data source: A cohort of 531 axial spondyloarthritis patients enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases program.
Disclosures: Dr. Micheroli had no disclosures.
Transcranial direct-current stimulation does not show noninferiority to escitalopram
Transcranial direct-current stimulation did not show noninferiority to escitalopram for major depressive disorder in a single-center trial. The results were published online June 29.
The randomized double-blind placebo-controlled noninferiority study involved 245 adults with moderate to severe depression, many of whom had coexisting anxiety disorder. This “reflects a typical clinical population in which treatment for depression is indicated,” said André R. Brunoni, MD, PhD, of the Service of Interdisciplinary Neuromodulation, Institute of Psychiatry and Laboratory of Neurosciences, University of São Paulo, Brazil, and his associates.
A total of 94 patients were assigned to receive active tDCS plus oral placebo (tDCS group), 91 to receive sham tDCS plus escitalopram (escitalopram group), and 60 to receive sham tDCS plus oral placebo (placebo group) for 10 weeks. The primary outcome – decrease in mean depression score on the 17-item Hamilton Depression Rating Scale – was 9.0 points with tDCS, 11.3 points with escitalopram, and 5.8 points with placebo. Thus, tDCS did not achieve noninferiority to escitalopram, the investigators said (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMoa1612999).
Although tDCS was superior to placebo in some secondary outcomes, including rate of clinical response (defined as a reduction of 50% or more in the Hamilton or the Montgomery-Åsberg Depression Rating Scale), it was associated with significantly more adverse events. The tDCS group reported more tinnitus, more nervousness, and more itching, tingling, burning, and skin redness at the electrode sites than did the other study groups. The escitalopram group reported higher rates of sleepiness and obstipation than did the other study groups.
One concerning finding was that two patients in the tDCS group developed new-onset mania during treatment, Dr. Brunoni and his associates noted.
“Future studies of tDCS could investigate different total doses of electrical stimulation in patients with major depressive disorder,” they wrote.
This trial was supported by the Fundacão de Amparo à Pesquisa do Estado de São Paulo, the Brain and Behavior Research Foundation, the Sao Paulo State Foundation, the National Council for Scientific and Technological Development, the Associacao Beneficente Alzira Denise Hertzog de Silva, and the Brazilian Coordination for the Improvement of Higher Education Personnel. Soterix Medical supplied the tDCS devices, and Libbs Laboratory supplied the escitalopram used in this study free of charge. Dr. Brunoni reported ties to Soterix Medical, Libbs Laboratory, and Delta Medical. His associates reported ties to numerous industry sources.
Even though the antidepressant efficacy of tDCS remains uncertain, this study shows key knowledge gaps, particularly regarding dosing, that must be addressed in this and other forms of noninvasive brain stimulation before an effective therapy can be developed, Sarah H. Lisanby, MD, wrote in an accompanying editorial (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMe1702492).
“Ultimately, the more we know about the ways in which noninvasive brain stimulation influences brain activity at a mechanistic level,” she wrote, “ the closer we come to determining the clinical usefulness of these new therapies.”
However, she said one important limitation in this trial was that most of the patients in the medication group became aware of their assigned therapy, presumably because they experienced side effects. This might have inflated the efficacy of escitalopram, which in turn may have invalidated the noninferiority comparison.
Dr. Lisanby is affiliated with the National Institute of Mental Health, Bethesda, Md. She reported ties to Oxford University Press, the Stanley Medical Research Foundation, Neosync, Brainsway, and the Brain Behavior Research Foundation. She also holds a patent for magnetic stimulation methods, apparatus, and systems.
Even though the antidepressant efficacy of tDCS remains uncertain, this study shows key knowledge gaps, particularly regarding dosing, that must be addressed in this and other forms of noninvasive brain stimulation before an effective therapy can be developed, Sarah H. Lisanby, MD, wrote in an accompanying editorial (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMe1702492).
“Ultimately, the more we know about the ways in which noninvasive brain stimulation influences brain activity at a mechanistic level,” she wrote, “ the closer we come to determining the clinical usefulness of these new therapies.”
However, she said one important limitation in this trial was that most of the patients in the medication group became aware of their assigned therapy, presumably because they experienced side effects. This might have inflated the efficacy of escitalopram, which in turn may have invalidated the noninferiority comparison.
Dr. Lisanby is affiliated with the National Institute of Mental Health, Bethesda, Md. She reported ties to Oxford University Press, the Stanley Medical Research Foundation, Neosync, Brainsway, and the Brain Behavior Research Foundation. She also holds a patent for magnetic stimulation methods, apparatus, and systems.
Even though the antidepressant efficacy of tDCS remains uncertain, this study shows key knowledge gaps, particularly regarding dosing, that must be addressed in this and other forms of noninvasive brain stimulation before an effective therapy can be developed, Sarah H. Lisanby, MD, wrote in an accompanying editorial (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMe1702492).
“Ultimately, the more we know about the ways in which noninvasive brain stimulation influences brain activity at a mechanistic level,” she wrote, “ the closer we come to determining the clinical usefulness of these new therapies.”
However, she said one important limitation in this trial was that most of the patients in the medication group became aware of their assigned therapy, presumably because they experienced side effects. This might have inflated the efficacy of escitalopram, which in turn may have invalidated the noninferiority comparison.
Dr. Lisanby is affiliated with the National Institute of Mental Health, Bethesda, Md. She reported ties to Oxford University Press, the Stanley Medical Research Foundation, Neosync, Brainsway, and the Brain Behavior Research Foundation. She also holds a patent for magnetic stimulation methods, apparatus, and systems.
Transcranial direct-current stimulation did not show noninferiority to escitalopram for major depressive disorder in a single-center trial. The results were published online June 29.
The randomized double-blind placebo-controlled noninferiority study involved 245 adults with moderate to severe depression, many of whom had coexisting anxiety disorder. This “reflects a typical clinical population in which treatment for depression is indicated,” said André R. Brunoni, MD, PhD, of the Service of Interdisciplinary Neuromodulation, Institute of Psychiatry and Laboratory of Neurosciences, University of São Paulo, Brazil, and his associates.
A total of 94 patients were assigned to receive active tDCS plus oral placebo (tDCS group), 91 to receive sham tDCS plus escitalopram (escitalopram group), and 60 to receive sham tDCS plus oral placebo (placebo group) for 10 weeks. The primary outcome – decrease in mean depression score on the 17-item Hamilton Depression Rating Scale – was 9.0 points with tDCS, 11.3 points with escitalopram, and 5.8 points with placebo. Thus, tDCS did not achieve noninferiority to escitalopram, the investigators said (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMoa1612999).
Although tDCS was superior to placebo in some secondary outcomes, including rate of clinical response (defined as a reduction of 50% or more in the Hamilton or the Montgomery-Åsberg Depression Rating Scale), it was associated with significantly more adverse events. The tDCS group reported more tinnitus, more nervousness, and more itching, tingling, burning, and skin redness at the electrode sites than did the other study groups. The escitalopram group reported higher rates of sleepiness and obstipation than did the other study groups.
One concerning finding was that two patients in the tDCS group developed new-onset mania during treatment, Dr. Brunoni and his associates noted.
“Future studies of tDCS could investigate different total doses of electrical stimulation in patients with major depressive disorder,” they wrote.
This trial was supported by the Fundacão de Amparo à Pesquisa do Estado de São Paulo, the Brain and Behavior Research Foundation, the Sao Paulo State Foundation, the National Council for Scientific and Technological Development, the Associacao Beneficente Alzira Denise Hertzog de Silva, and the Brazilian Coordination for the Improvement of Higher Education Personnel. Soterix Medical supplied the tDCS devices, and Libbs Laboratory supplied the escitalopram used in this study free of charge. Dr. Brunoni reported ties to Soterix Medical, Libbs Laboratory, and Delta Medical. His associates reported ties to numerous industry sources.
Transcranial direct-current stimulation did not show noninferiority to escitalopram for major depressive disorder in a single-center trial. The results were published online June 29.
The randomized double-blind placebo-controlled noninferiority study involved 245 adults with moderate to severe depression, many of whom had coexisting anxiety disorder. This “reflects a typical clinical population in which treatment for depression is indicated,” said André R. Brunoni, MD, PhD, of the Service of Interdisciplinary Neuromodulation, Institute of Psychiatry and Laboratory of Neurosciences, University of São Paulo, Brazil, and his associates.
A total of 94 patients were assigned to receive active tDCS plus oral placebo (tDCS group), 91 to receive sham tDCS plus escitalopram (escitalopram group), and 60 to receive sham tDCS plus oral placebo (placebo group) for 10 weeks. The primary outcome – decrease in mean depression score on the 17-item Hamilton Depression Rating Scale – was 9.0 points with tDCS, 11.3 points with escitalopram, and 5.8 points with placebo. Thus, tDCS did not achieve noninferiority to escitalopram, the investigators said (N Engl J Med. 2017 June 29. doi: 10.1056/NEJMoa1612999).
Although tDCS was superior to placebo in some secondary outcomes, including rate of clinical response (defined as a reduction of 50% or more in the Hamilton or the Montgomery-Åsberg Depression Rating Scale), it was associated with significantly more adverse events. The tDCS group reported more tinnitus, more nervousness, and more itching, tingling, burning, and skin redness at the electrode sites than did the other study groups. The escitalopram group reported higher rates of sleepiness and obstipation than did the other study groups.
One concerning finding was that two patients in the tDCS group developed new-onset mania during treatment, Dr. Brunoni and his associates noted.
“Future studies of tDCS could investigate different total doses of electrical stimulation in patients with major depressive disorder,” they wrote.
This trial was supported by the Fundacão de Amparo à Pesquisa do Estado de São Paulo, the Brain and Behavior Research Foundation, the Sao Paulo State Foundation, the National Council for Scientific and Technological Development, the Associacao Beneficente Alzira Denise Hertzog de Silva, and the Brazilian Coordination for the Improvement of Higher Education Personnel. Soterix Medical supplied the tDCS devices, and Libbs Laboratory supplied the escitalopram used in this study free of charge. Dr. Brunoni reported ties to Soterix Medical, Libbs Laboratory, and Delta Medical. His associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Transcranial direct-current stimulation did not show noninferiority to escitalopram for major depressive disorder in a single-center trial.
Major finding: The primary outcome – decrease in mean depression score on the 17-item Hamilton Depression Rating Scale – was 9.0 points with tDCS, 11.3 points with escitalopram, and 5.8 points with placebo.
Data source: A single-center randomized double-blind placebo-controlled noninferiority trial involving 245 adults with moderate to severe major depression.
Disclosures: This trial was funded by the Fundacão de Amparo à Pesquisa do Estado de São Paulo, the Brain and Behavior Research Foundation, the São Paulo State Foundation, the National Council for Scientific and Technological Development, the Associacao Beneficente Alzira Denise Hertzog de Silva, and the Brazilian Coordination for the Improvement of Higher Education Personnel. Soterix Medical supplied the tDCS devices, and Libbs Laboratory supplied the escitalopram used in this study free of charge. Dr. Brunoni reported ties to Soterix Medical, Libbs Laboratory, and Delta Medical. His associates reported ties to numerous industry sources.
Small skin abscesses: Add antibiotics to drainage
For patients who have a single, small skin abscess, the addition of oral antibiotics to standard incision and drainage of the lesion improves cure rates and decreases recurrence rates, according to a study published online June 28 in the New England Journal of Medicine.
The results of this multicenter prospective randomized double-blind placebo-controlled trial, taken together with those of another recent large study, “call into question the perception – largely based on expert opinion or smaller, underpowered, and lower-quality noninferiority trials – that cure rates do not improve with the addition of systemic antibiotic treatment after incision and drainage,” said Robert S. Daum, MD, professor of pediatrics at the University of Chicago, and his associates.
The trial involved 786 patients of all ages (64% were adults and 36% were children; mean age was 25.5 years) who had a single, uncomplicated skin abscess of 5 cm or smaller and were treated at the University of Chicago; San Francisco General Hospital; Harbor-UCLA Medical Center; Vanderbilt University Medical Center, Nashville; Washington University, St. Louis; or Emory University, Atlanta. A total of 266 patients were assigned to receive oral clindamycin, 263 to receive oral trimethoprim–sulfamethoxazole (TMP-SMX), and 257 to receive matching placebo for 10 days after the lesions were incised and drained.
At follow-up 7-10 days following the conclusion of treatment, the rates of clinical cure were 83.1% with clindamycin and 81.7% with TMP-SMX, both significantly greater than the 68.9% cure rate with placebo (P less than .001 for both comparisons). Similarly, at 1-month follow-up, 78.6% of the clindamycin group and 73.0% of the TMP-SMX group “remained cured,” compared with 62.6% of the placebo group (N Engl J Med. 2017 June 28. doi: 10.1056/NEJMoa1607033).
Among those with cultures positive for Staphylococcus aureus, cure rates 7-10 days after treatment ended were 83.5% and 83.2% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (63.8%). Among those positive for methicillin-resistant S. aureus, cure rates were 81.7% and 84.6% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (62.9%).
The rate of treatment-associated adverse events was higher with clindamycin (21.9%) than with TMP-SMX (11.1%) or with placebo (12.5%). The most common adverse events were diarrhea and nausea, which were mild to moderate in severity and resolved with sequelae. There were no cases of Clostridium difficile–associated diarrhea or severe allergic reactions. One patient had a hypersensitivity reaction that was considered to be related to TMP-SMX, which involved fever, rash, thrombocytopenia, and hepatitis and which resolved without sequelae.
The National Institute of Allergy and Infectious Diseases and the National Center for Research Resources supported the study. Dr. Daum reported ties to Pfizer, Dynavax, Theravance, and Merck, and his associates reported ties to numerous industry sources.
For patients who have a single, small skin abscess, the addition of oral antibiotics to standard incision and drainage of the lesion improves cure rates and decreases recurrence rates, according to a study published online June 28 in the New England Journal of Medicine.
The results of this multicenter prospective randomized double-blind placebo-controlled trial, taken together with those of another recent large study, “call into question the perception – largely based on expert opinion or smaller, underpowered, and lower-quality noninferiority trials – that cure rates do not improve with the addition of systemic antibiotic treatment after incision and drainage,” said Robert S. Daum, MD, professor of pediatrics at the University of Chicago, and his associates.
The trial involved 786 patients of all ages (64% were adults and 36% were children; mean age was 25.5 years) who had a single, uncomplicated skin abscess of 5 cm or smaller and were treated at the University of Chicago; San Francisco General Hospital; Harbor-UCLA Medical Center; Vanderbilt University Medical Center, Nashville; Washington University, St. Louis; or Emory University, Atlanta. A total of 266 patients were assigned to receive oral clindamycin, 263 to receive oral trimethoprim–sulfamethoxazole (TMP-SMX), and 257 to receive matching placebo for 10 days after the lesions were incised and drained.
At follow-up 7-10 days following the conclusion of treatment, the rates of clinical cure were 83.1% with clindamycin and 81.7% with TMP-SMX, both significantly greater than the 68.9% cure rate with placebo (P less than .001 for both comparisons). Similarly, at 1-month follow-up, 78.6% of the clindamycin group and 73.0% of the TMP-SMX group “remained cured,” compared with 62.6% of the placebo group (N Engl J Med. 2017 June 28. doi: 10.1056/NEJMoa1607033).
Among those with cultures positive for Staphylococcus aureus, cure rates 7-10 days after treatment ended were 83.5% and 83.2% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (63.8%). Among those positive for methicillin-resistant S. aureus, cure rates were 81.7% and 84.6% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (62.9%).
The rate of treatment-associated adverse events was higher with clindamycin (21.9%) than with TMP-SMX (11.1%) or with placebo (12.5%). The most common adverse events were diarrhea and nausea, which were mild to moderate in severity and resolved with sequelae. There were no cases of Clostridium difficile–associated diarrhea or severe allergic reactions. One patient had a hypersensitivity reaction that was considered to be related to TMP-SMX, which involved fever, rash, thrombocytopenia, and hepatitis and which resolved without sequelae.
The National Institute of Allergy and Infectious Diseases and the National Center for Research Resources supported the study. Dr. Daum reported ties to Pfizer, Dynavax, Theravance, and Merck, and his associates reported ties to numerous industry sources.
For patients who have a single, small skin abscess, the addition of oral antibiotics to standard incision and drainage of the lesion improves cure rates and decreases recurrence rates, according to a study published online June 28 in the New England Journal of Medicine.
The results of this multicenter prospective randomized double-blind placebo-controlled trial, taken together with those of another recent large study, “call into question the perception – largely based on expert opinion or smaller, underpowered, and lower-quality noninferiority trials – that cure rates do not improve with the addition of systemic antibiotic treatment after incision and drainage,” said Robert S. Daum, MD, professor of pediatrics at the University of Chicago, and his associates.
The trial involved 786 patients of all ages (64% were adults and 36% were children; mean age was 25.5 years) who had a single, uncomplicated skin abscess of 5 cm or smaller and were treated at the University of Chicago; San Francisco General Hospital; Harbor-UCLA Medical Center; Vanderbilt University Medical Center, Nashville; Washington University, St. Louis; or Emory University, Atlanta. A total of 266 patients were assigned to receive oral clindamycin, 263 to receive oral trimethoprim–sulfamethoxazole (TMP-SMX), and 257 to receive matching placebo for 10 days after the lesions were incised and drained.
At follow-up 7-10 days following the conclusion of treatment, the rates of clinical cure were 83.1% with clindamycin and 81.7% with TMP-SMX, both significantly greater than the 68.9% cure rate with placebo (P less than .001 for both comparisons). Similarly, at 1-month follow-up, 78.6% of the clindamycin group and 73.0% of the TMP-SMX group “remained cured,” compared with 62.6% of the placebo group (N Engl J Med. 2017 June 28. doi: 10.1056/NEJMoa1607033).
Among those with cultures positive for Staphylococcus aureus, cure rates 7-10 days after treatment ended were 83.5% and 83.2% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (63.8%). Among those positive for methicillin-resistant S. aureus, cure rates were 81.7% and 84.6% in the clindamycin and TMP-SMX groups, respectively, significantly higher than in the placebo group (62.9%).
The rate of treatment-associated adverse events was higher with clindamycin (21.9%) than with TMP-SMX (11.1%) or with placebo (12.5%). The most common adverse events were diarrhea and nausea, which were mild to moderate in severity and resolved with sequelae. There were no cases of Clostridium difficile–associated diarrhea or severe allergic reactions. One patient had a hypersensitivity reaction that was considered to be related to TMP-SMX, which involved fever, rash, thrombocytopenia, and hepatitis and which resolved without sequelae.
The National Institute of Allergy and Infectious Diseases and the National Center for Research Resources supported the study. Dr. Daum reported ties to Pfizer, Dynavax, Theravance, and Merck, and his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: For patients who have a single, small skin abscess, the addition of oral antibiotics to standard incision and drainage of the lesion improves cure rates and decreases recurrence rates.
Major finding: At follow-up 7-10 days following the completion of treatment, clinical cure rates were 83.1% with clindamycin and 81.7% with TMP-SMX, both significantly greater than the 68.9% cure rate with placebo.
Data source: A multicenter prospective randomized double-blind placebo-controlled trial involving 786 adults and children who had single, small skin abscesses.
Disclosures: The National Institute of Allergy and Infectious Diseases and the National Center for Research Resources supported the study. Dr. Daum reported ties to Pfizer, Dynavax, Theravance, and Merck, and his associates reported ties to numerous industry sources.
Blood vessels injured during trocar insertion: $8.7M verdict
Blood vessels injured during trocar insertion: $8.7M verdict
PATIENT’S CLAIM:
The resident was negligent in performing trocar insertion during laparoscopic surgery by inserting the trocar too far into the abdomen. The attending ObGyn did not supervise the resident properly. There is nothing in the patient's medical records to indicate that she had abnormal anatomy. The woman's life is in turmoil after what was supposed to be a routine procedure.
DEFENDANTS' DEFENSE:
There was no negligence. The patient's anatomy was abnormal, making the risk of surgery higher. The injury is a known complication of laparoscopic surgery.
VERDICT:
An $8,718,848 Illinois verdict was returned.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Wrong fallopian tube transected: $1.8M award
A 28-year-old woman underwent an appendectomy. During the operation, the surgeon saw an abscess on the patient's right fallopian tube and called in an ObGyn to remove the abscess. While doing so, the ObGyn transected the left fallopian tube. Both fallopian tubes were removed.
PATIENT’S CLAIM:
The surgeon did not tell the ObGyn which fallopian tube was abscessed and therefore the ObGyn operated on the wrong tube. In addition, the surgeon failed to obtain informed consent for bilateral salpingectomy. The patient is now unable to conceive without assisted reproductive treatment.
PHYSICIAN’S DEFENSE:
The surgeon admitted his mistakes but disputed the informed consent claim. The patient probably would not have been able to conceive naturally due to the infection.
VERDICT:
A $1.8 million Connecticut verdict was returned.
Related article:
Elective laparoscopic appendectomy in gynecologic surgery: When, why, and how
Complications after vaginal hysterectomy
A woman underwent laparoscopic vaginal hysterectomy and bilateral salpingo-oophorectomy with anterior and posterior repair using mesh in August 2010. Shortly after surgery, the patient reported vaginal discharge with pain and bleeding. She was treated with antibiotics. Results of a CT scan identified the cause of her symptoms as vaginal cuff granulations.
Her pain continued and in June 2011, she underwent vaginal tissue biopsy. After testing revealed the presence of fecal matter, a small-bowel vaginal fistula was identified. She underwent laparoscopic enterectomy, urethral lysis, an omental pedicle flap, and cystoscopy. The mesh had perforated several loops of the small bowel.
In August 2011, the patient reported spinal pain. Magnetic resonance imaging (MRI) revealed a new fluid abscess in a disc extending through the tract anterior to the soft tissue of the pelvis. She underwent intensive antibiotic therapy.
PATIENT’S CLAIM:
The gynecologic surgeon fell below the standard of care in his treatment of her conditions.
PHYSICIAN’S DEFENSE:
The surgeon denied allegations.
VERDICT:
A Nevada defense verdict was returned.
Related article:
Vaginal hysterectomy with basic instrumentation
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Blood vessels injured during trocar insertion: $8.7M verdict
PATIENT’S CLAIM:
The resident was negligent in performing trocar insertion during laparoscopic surgery by inserting the trocar too far into the abdomen. The attending ObGyn did not supervise the resident properly. There is nothing in the patient's medical records to indicate that she had abnormal anatomy. The woman's life is in turmoil after what was supposed to be a routine procedure.
DEFENDANTS' DEFENSE:
There was no negligence. The patient's anatomy was abnormal, making the risk of surgery higher. The injury is a known complication of laparoscopic surgery.
VERDICT:
An $8,718,848 Illinois verdict was returned.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Wrong fallopian tube transected: $1.8M award
A 28-year-old woman underwent an appendectomy. During the operation, the surgeon saw an abscess on the patient's right fallopian tube and called in an ObGyn to remove the abscess. While doing so, the ObGyn transected the left fallopian tube. Both fallopian tubes were removed.
PATIENT’S CLAIM:
The surgeon did not tell the ObGyn which fallopian tube was abscessed and therefore the ObGyn operated on the wrong tube. In addition, the surgeon failed to obtain informed consent for bilateral salpingectomy. The patient is now unable to conceive without assisted reproductive treatment.
PHYSICIAN’S DEFENSE:
The surgeon admitted his mistakes but disputed the informed consent claim. The patient probably would not have been able to conceive naturally due to the infection.
VERDICT:
A $1.8 million Connecticut verdict was returned.
Related article:
Elective laparoscopic appendectomy in gynecologic surgery: When, why, and how
Complications after vaginal hysterectomy
A woman underwent laparoscopic vaginal hysterectomy and bilateral salpingo-oophorectomy with anterior and posterior repair using mesh in August 2010. Shortly after surgery, the patient reported vaginal discharge with pain and bleeding. She was treated with antibiotics. Results of a CT scan identified the cause of her symptoms as vaginal cuff granulations.
Her pain continued and in June 2011, she underwent vaginal tissue biopsy. After testing revealed the presence of fecal matter, a small-bowel vaginal fistula was identified. She underwent laparoscopic enterectomy, urethral lysis, an omental pedicle flap, and cystoscopy. The mesh had perforated several loops of the small bowel.
In August 2011, the patient reported spinal pain. Magnetic resonance imaging (MRI) revealed a new fluid abscess in a disc extending through the tract anterior to the soft tissue of the pelvis. She underwent intensive antibiotic therapy.
PATIENT’S CLAIM:
The gynecologic surgeon fell below the standard of care in his treatment of her conditions.
PHYSICIAN’S DEFENSE:
The surgeon denied allegations.
VERDICT:
A Nevada defense verdict was returned.
Related article:
Vaginal hysterectomy with basic instrumentation
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Blood vessels injured during trocar insertion: $8.7M verdict
PATIENT’S CLAIM:
The resident was negligent in performing trocar insertion during laparoscopic surgery by inserting the trocar too far into the abdomen. The attending ObGyn did not supervise the resident properly. There is nothing in the patient's medical records to indicate that she had abnormal anatomy. The woman's life is in turmoil after what was supposed to be a routine procedure.
DEFENDANTS' DEFENSE:
There was no negligence. The patient's anatomy was abnormal, making the risk of surgery higher. The injury is a known complication of laparoscopic surgery.
VERDICT:
An $8,718,848 Illinois verdict was returned.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Wrong fallopian tube transected: $1.8M award
A 28-year-old woman underwent an appendectomy. During the operation, the surgeon saw an abscess on the patient's right fallopian tube and called in an ObGyn to remove the abscess. While doing so, the ObGyn transected the left fallopian tube. Both fallopian tubes were removed.
PATIENT’S CLAIM:
The surgeon did not tell the ObGyn which fallopian tube was abscessed and therefore the ObGyn operated on the wrong tube. In addition, the surgeon failed to obtain informed consent for bilateral salpingectomy. The patient is now unable to conceive without assisted reproductive treatment.
PHYSICIAN’S DEFENSE:
The surgeon admitted his mistakes but disputed the informed consent claim. The patient probably would not have been able to conceive naturally due to the infection.
VERDICT:
A $1.8 million Connecticut verdict was returned.
Related article:
Elective laparoscopic appendectomy in gynecologic surgery: When, why, and how
Complications after vaginal hysterectomy
A woman underwent laparoscopic vaginal hysterectomy and bilateral salpingo-oophorectomy with anterior and posterior repair using mesh in August 2010. Shortly after surgery, the patient reported vaginal discharge with pain and bleeding. She was treated with antibiotics. Results of a CT scan identified the cause of her symptoms as vaginal cuff granulations.
Her pain continued and in June 2011, she underwent vaginal tissue biopsy. After testing revealed the presence of fecal matter, a small-bowel vaginal fistula was identified. She underwent laparoscopic enterectomy, urethral lysis, an omental pedicle flap, and cystoscopy. The mesh had perforated several loops of the small bowel.
In August 2011, the patient reported spinal pain. Magnetic resonance imaging (MRI) revealed a new fluid abscess in a disc extending through the tract anterior to the soft tissue of the pelvis. She underwent intensive antibiotic therapy.
PATIENT’S CLAIM:
The gynecologic surgeon fell below the standard of care in his treatment of her conditions.
PHYSICIAN’S DEFENSE:
The surgeon denied allegations.
VERDICT:
A Nevada defense verdict was returned.
Related article:
Vaginal hysterectomy with basic instrumentation
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Medicaid expansion produced opposing cost effects
States that expanded Medicaid in 2014 both increased and decreased their Medicaid spending that year, compared with the nonexpansion states, according to an analysis from the Centers for Medicare & Medicaid Services.
The 26 states, along with the District of Columbia, that expanded Medicaid eligibility by the end of 2014 had an increase in total Medicaid spending of 12.3% over 2013, compared with an increase of 6.2% in nonexpansion states.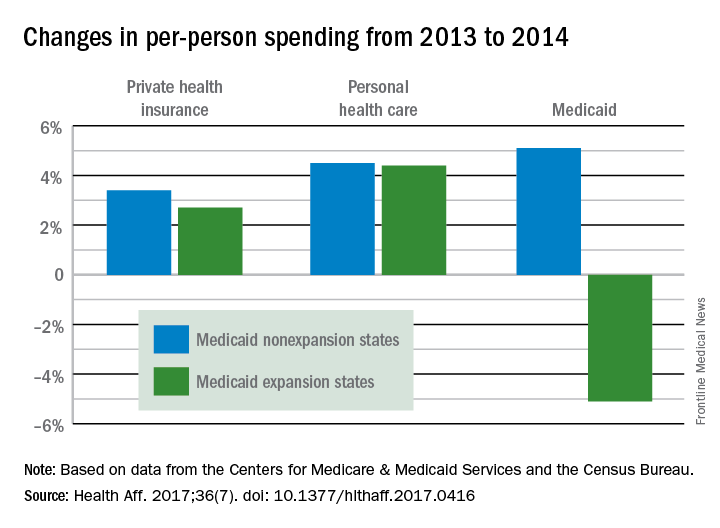
The expansion of coverage “increased the share of ... less expensive enrollees relative to the previous Medicaid beneficiary population mix,” the investigators said. Medicaid expansion brought in more relatively inexpensive adults – 43% of total enrollment in 2014, compared with 32% in 2013 – and reduced the proportion of disabled and aged enrollees, whose cost per person is much higher, they explained.
Private health insurance spending showed a different pattern: Nonexpansion states had larger increases in both higher per-person and overall costs than did expansion states. Per-person costs were up 3.4% for nonexpanders and 2.7% for expanders, and total costs rose 6.8% in nonexpansion states and 4.6% in the Medicaid expanders, Mr. Lassman and his associates said. Higher per-person spending growth for enrollees in the state and federal marketplaces, compared with nonmarketplace individual coverage, was partially responsible for this trend, they pointed out.
States that expanded Medicaid in 2014 both increased and decreased their Medicaid spending that year, compared with the nonexpansion states, according to an analysis from the Centers for Medicare & Medicaid Services.
The 26 states, along with the District of Columbia, that expanded Medicaid eligibility by the end of 2014 had an increase in total Medicaid spending of 12.3% over 2013, compared with an increase of 6.2% in nonexpansion states.
The expansion of coverage “increased the share of ... less expensive enrollees relative to the previous Medicaid beneficiary population mix,” the investigators said. Medicaid expansion brought in more relatively inexpensive adults – 43% of total enrollment in 2014, compared with 32% in 2013 – and reduced the proportion of disabled and aged enrollees, whose cost per person is much higher, they explained.
Private health insurance spending showed a different pattern: Nonexpansion states had larger increases in both higher per-person and overall costs than did expansion states. Per-person costs were up 3.4% for nonexpanders and 2.7% for expanders, and total costs rose 6.8% in nonexpansion states and 4.6% in the Medicaid expanders, Mr. Lassman and his associates said. Higher per-person spending growth for enrollees in the state and federal marketplaces, compared with nonmarketplace individual coverage, was partially responsible for this trend, they pointed out.
States that expanded Medicaid in 2014 both increased and decreased their Medicaid spending that year, compared with the nonexpansion states, according to an analysis from the Centers for Medicare & Medicaid Services.
The 26 states, along with the District of Columbia, that expanded Medicaid eligibility by the end of 2014 had an increase in total Medicaid spending of 12.3% over 2013, compared with an increase of 6.2% in nonexpansion states.
The expansion of coverage “increased the share of ... less expensive enrollees relative to the previous Medicaid beneficiary population mix,” the investigators said. Medicaid expansion brought in more relatively inexpensive adults – 43% of total enrollment in 2014, compared with 32% in 2013 – and reduced the proportion of disabled and aged enrollees, whose cost per person is much higher, they explained.
Private health insurance spending showed a different pattern: Nonexpansion states had larger increases in both higher per-person and overall costs than did expansion states. Per-person costs were up 3.4% for nonexpanders and 2.7% for expanders, and total costs rose 6.8% in nonexpansion states and 4.6% in the Medicaid expanders, Mr. Lassman and his associates said. Higher per-person spending growth for enrollees in the state and federal marketplaces, compared with nonmarketplace individual coverage, was partially responsible for this trend, they pointed out.
FROM HEALTH AFFAIRS
Patient Handout: Finding the Right Epilepsy Resources
TWEAKing inflammation: Studies reflect potential treatment target for psoriasis, atopic dermatitis
An immunomodulatory pathway that has been linked to cancer, kidney disease, and other disease processes is becoming a focus of dermatologic research.
New evidence suggests that TNF-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis family (TNF) superfamily, may be involved in both atopic dermatitis (AD) and psoriasis (Nat Commun. 2017 May 22;8:15395. doi: 10.1038/ncomms15395). The research showed that mice engineered to have low TWEAK levels had less severe disease when both AD and psoriasis were induced.
The TWEAK receptor, Fn14, was upregulated in keratinocytes and dermal fibroblasts in mouse disease models of AD and psoriasis, and TWEAK induced production of a range of cytokines associated with both AD and psoriasis. Subcutaneous injection of recombinant TWEAK led to cutaneous inflammation, as well as histological and molecular signals of the two diseases.
The pathophysiology of both AD and psoriasis is nebulously complex, sharing a similar theme of immune dysregulation, but historically polar opposites based on the different branches of the immune response implicated.
The study is not the only recent work tying TWEAK/Fn14 to dermatologic diseases. Other recent papers have shown evidence of their involvement in chronic cutaneous lupus (J Invest Dermatol. 2015;135[8]:1986-95), UVB irradiation-induced cutaneous lupus (Exp Dermatol. 2016 Dec;25[12]:969-76), and bullous pemphigoid (J Invest Dermatol. 2017 Jul;137[7]:1512-22).
The spate of findings hint that TWEAK/Fn14 could be a novel therapeutic pathway to attack inflammatory disease. Many therapies for autoimmune disease focus on immunosuppressive agents, which are associated with an increased risk of infection. But mice engineered to lack either TWEAK or Fn14 appear normal, and a phase I trial of an anti-TWEAK antibody in patients with rheumatoid arthritis did not reveal any worrisome safety concerns. “It doesn’t seem to have the broad immunosuppressive effects which characterize the therapies we currently use,” said Chaim Putterman, MD, chief of the division of rheumatology and professor of medicine and microbiology & immunology at the Albert Einstein College of Medicine, New York.
Instead, TWEAK seems to be regulating inflammation in target organs. It almost certainly plays a role in healthy functions like wound healing and cell survival, but Dr. Putterman believes there are redundant mechanisms that can pick up the slack, as the healthy knockout mice attest. The evidence suggests that the TWEAK pathway may become overactive in some diseases and, if so, a therapeutic antibody might be able to reset it to a more normal balance. “The utopian vision is that you would block this cytokine and bring its downstream effects back to normal levels, rather than totally abrogating its homeostatic functions,” Dr. Putterman noted.
Because blocking TWEAK has no apparent immunosuppressive effects, it might be a candidate for combination therapy with existing cytotoxic drugs. “If you have a disease like psoriasis where some standard of care medications are immunosuppressive, such as methotrexate, you might not get more risk by adding an antibody targeting TWEAK, as opposed to using immunosuppressives in combination. That, I think, has potential,” he said.
Work remains, however. A proof-of-concept study in lupus nephritis, sponsored by Biogen, failed to show a benefit when an anti-TWEAK antibody was combined with the standard of care.
But the potential impact of this approach holds much promise, and the fact that TWEAK has been linked to multiple diseases should make it a more attractive drug target for drug companies. “Now we have a target, that if you knock it out, or its receptor, you can potentially affect both diseases. This may the start of a whole new direction for biologics to treat inflammatory disease, and cancer as well,” Dr. Friedman said.
Dr. Putterman and Dr. Friedman were among the authors of the 2015 JID study on TWEAK/Fn14 signaling in spontaneous lupus and the Experimental Dermatology study. Dr. Putterman has research funding from Biogen Idec. Dr. Friedman had no related disclosures. The authors of the Nature Communications study were from the La Jolla Institute for Allergy and Immunology, and Biogen.
An immunomodulatory pathway that has been linked to cancer, kidney disease, and other disease processes is becoming a focus of dermatologic research.
New evidence suggests that TNF-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis family (TNF) superfamily, may be involved in both atopic dermatitis (AD) and psoriasis (Nat Commun. 2017 May 22;8:15395. doi: 10.1038/ncomms15395). The research showed that mice engineered to have low TWEAK levels had less severe disease when both AD and psoriasis were induced.
The TWEAK receptor, Fn14, was upregulated in keratinocytes and dermal fibroblasts in mouse disease models of AD and psoriasis, and TWEAK induced production of a range of cytokines associated with both AD and psoriasis. Subcutaneous injection of recombinant TWEAK led to cutaneous inflammation, as well as histological and molecular signals of the two diseases.
The pathophysiology of both AD and psoriasis is nebulously complex, sharing a similar theme of immune dysregulation, but historically polar opposites based on the different branches of the immune response implicated.
The study is not the only recent work tying TWEAK/Fn14 to dermatologic diseases. Other recent papers have shown evidence of their involvement in chronic cutaneous lupus (J Invest Dermatol. 2015;135[8]:1986-95), UVB irradiation-induced cutaneous lupus (Exp Dermatol. 2016 Dec;25[12]:969-76), and bullous pemphigoid (J Invest Dermatol. 2017 Jul;137[7]:1512-22).
The spate of findings hint that TWEAK/Fn14 could be a novel therapeutic pathway to attack inflammatory disease. Many therapies for autoimmune disease focus on immunosuppressive agents, which are associated with an increased risk of infection. But mice engineered to lack either TWEAK or Fn14 appear normal, and a phase I trial of an anti-TWEAK antibody in patients with rheumatoid arthritis did not reveal any worrisome safety concerns. “It doesn’t seem to have the broad immunosuppressive effects which characterize the therapies we currently use,” said Chaim Putterman, MD, chief of the division of rheumatology and professor of medicine and microbiology & immunology at the Albert Einstein College of Medicine, New York.
Instead, TWEAK seems to be regulating inflammation in target organs. It almost certainly plays a role in healthy functions like wound healing and cell survival, but Dr. Putterman believes there are redundant mechanisms that can pick up the slack, as the healthy knockout mice attest. The evidence suggests that the TWEAK pathway may become overactive in some diseases and, if so, a therapeutic antibody might be able to reset it to a more normal balance. “The utopian vision is that you would block this cytokine and bring its downstream effects back to normal levels, rather than totally abrogating its homeostatic functions,” Dr. Putterman noted.
Because blocking TWEAK has no apparent immunosuppressive effects, it might be a candidate for combination therapy with existing cytotoxic drugs. “If you have a disease like psoriasis where some standard of care medications are immunosuppressive, such as methotrexate, you might not get more risk by adding an antibody targeting TWEAK, as opposed to using immunosuppressives in combination. That, I think, has potential,” he said.
Work remains, however. A proof-of-concept study in lupus nephritis, sponsored by Biogen, failed to show a benefit when an anti-TWEAK antibody was combined with the standard of care.
But the potential impact of this approach holds much promise, and the fact that TWEAK has been linked to multiple diseases should make it a more attractive drug target for drug companies. “Now we have a target, that if you knock it out, or its receptor, you can potentially affect both diseases. This may the start of a whole new direction for biologics to treat inflammatory disease, and cancer as well,” Dr. Friedman said.
Dr. Putterman and Dr. Friedman were among the authors of the 2015 JID study on TWEAK/Fn14 signaling in spontaneous lupus and the Experimental Dermatology study. Dr. Putterman has research funding from Biogen Idec. Dr. Friedman had no related disclosures. The authors of the Nature Communications study were from the La Jolla Institute for Allergy and Immunology, and Biogen.
An immunomodulatory pathway that has been linked to cancer, kidney disease, and other disease processes is becoming a focus of dermatologic research.
New evidence suggests that TNF-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis family (TNF) superfamily, may be involved in both atopic dermatitis (AD) and psoriasis (Nat Commun. 2017 May 22;8:15395. doi: 10.1038/ncomms15395). The research showed that mice engineered to have low TWEAK levels had less severe disease when both AD and psoriasis were induced.
The TWEAK receptor, Fn14, was upregulated in keratinocytes and dermal fibroblasts in mouse disease models of AD and psoriasis, and TWEAK induced production of a range of cytokines associated with both AD and psoriasis. Subcutaneous injection of recombinant TWEAK led to cutaneous inflammation, as well as histological and molecular signals of the two diseases.
The pathophysiology of both AD and psoriasis is nebulously complex, sharing a similar theme of immune dysregulation, but historically polar opposites based on the different branches of the immune response implicated.
The study is not the only recent work tying TWEAK/Fn14 to dermatologic diseases. Other recent papers have shown evidence of their involvement in chronic cutaneous lupus (J Invest Dermatol. 2015;135[8]:1986-95), UVB irradiation-induced cutaneous lupus (Exp Dermatol. 2016 Dec;25[12]:969-76), and bullous pemphigoid (J Invest Dermatol. 2017 Jul;137[7]:1512-22).
The spate of findings hint that TWEAK/Fn14 could be a novel therapeutic pathway to attack inflammatory disease. Many therapies for autoimmune disease focus on immunosuppressive agents, which are associated with an increased risk of infection. But mice engineered to lack either TWEAK or Fn14 appear normal, and a phase I trial of an anti-TWEAK antibody in patients with rheumatoid arthritis did not reveal any worrisome safety concerns. “It doesn’t seem to have the broad immunosuppressive effects which characterize the therapies we currently use,” said Chaim Putterman, MD, chief of the division of rheumatology and professor of medicine and microbiology & immunology at the Albert Einstein College of Medicine, New York.
Instead, TWEAK seems to be regulating inflammation in target organs. It almost certainly plays a role in healthy functions like wound healing and cell survival, but Dr. Putterman believes there are redundant mechanisms that can pick up the slack, as the healthy knockout mice attest. The evidence suggests that the TWEAK pathway may become overactive in some diseases and, if so, a therapeutic antibody might be able to reset it to a more normal balance. “The utopian vision is that you would block this cytokine and bring its downstream effects back to normal levels, rather than totally abrogating its homeostatic functions,” Dr. Putterman noted.
Because blocking TWEAK has no apparent immunosuppressive effects, it might be a candidate for combination therapy with existing cytotoxic drugs. “If you have a disease like psoriasis where some standard of care medications are immunosuppressive, such as methotrexate, you might not get more risk by adding an antibody targeting TWEAK, as opposed to using immunosuppressives in combination. That, I think, has potential,” he said.
Work remains, however. A proof-of-concept study in lupus nephritis, sponsored by Biogen, failed to show a benefit when an anti-TWEAK antibody was combined with the standard of care.
But the potential impact of this approach holds much promise, and the fact that TWEAK has been linked to multiple diseases should make it a more attractive drug target for drug companies. “Now we have a target, that if you knock it out, or its receptor, you can potentially affect both diseases. This may the start of a whole new direction for biologics to treat inflammatory disease, and cancer as well,” Dr. Friedman said.
Dr. Putterman and Dr. Friedman were among the authors of the 2015 JID study on TWEAK/Fn14 signaling in spontaneous lupus and the Experimental Dermatology study. Dr. Putterman has research funding from Biogen Idec. Dr. Friedman had no related disclosures. The authors of the Nature Communications study were from the La Jolla Institute for Allergy and Immunology, and Biogen.
CBO: Senate health care proposal marginally better than House-passed bill
The Senate health care proposal is only marginally better in terms of the number of uninsured Americans, compared with the House-passed bill it aims to replace, but it still would leave 22 million more Americans without insurance coverage, according to a June 26 analysis by the Congressional Budget Office.
The analysis raised voices of opposition from the medical community.
BCRA would lower the federal deficit by $321 billion between 2017-2026, driven by the dramatic cuts in spending on Medicaid (estimated to be $772 billion), as well as $408 billion saved from reduced tax credits and other subsidies to help people afford health insurance.
The CBO’s estimate also addresses how the bill could impact access to health care.
Initially, patients can expect another short-term spike in insurance premiums, with average premiums in 2018 increasing by 20%, compared with current law, “mainly because the penalty for not having insurance would be eliminated, inducing fewer comparatively healthy people to sign up.” In 2019, premiums are predicted to be about 10% higher than under current law; however, by 2020, premiums for benchmark plans would be 30% lower than with current law.
However, as premiums come down, deductibles would continue to rise for plans that would offer lower levels of coverage, according to the CBO report. Additionally, “starting in 2020, the premium for a silver plan would typically be a relatively high percentage of income for low income people. The deductible for a plan ... would be a significantly higher percentage of income – also making such a plan unattractive but for a different reason. As a result, despite being eligible for premium tax credits, few low-income people would purchase any plan.”
The report also notes that the Senate proposal would not necessarily reverse current concerns regarding consumer choice in the individual markets, stating that “a small fraction of the population resides in areas which – because of this legislation, for at least some of the years after 2019 – no insurers will participate in the nongroup market or insurance would be offered only with very high premiums.” Additionally, removing the employer mandate could result in employers forgoing offering health insurance to their employees.
The bill faces an uphill battle in the Senate as there seemingly are not enough votes to pass the bill at this time. The measure is using the budget reconciliation process, meaning it will need 50 of the 52 Senate Republicans to pass it (all 48 Democrats are expected to vote against it). At least six GOP senators have said they are not ready to start debate. Senate Majority Leader Mitch McConnell (R-Ky) will not present the bill to the chamber for consideration until after the July 4 recess in an effort to tweak the language to garner the 50 votes needed to pass.
Medical societies are pushing back against the bill as well.
The American Medical Association, in a letter to Senate leaders, notes that the first principal that medical professionals operate under is to do no harm. “The draft legislation violates that standard on many levels,” according to the AMA letter.
In a statement from the American Gastroenterological Association, it was pointed out that both the House and Senate bills allow states to opt out of the Affordable Care Act’s essential health benefits package, which includes coverage of colorectal cancer screenings. “A core mission of AGA is to ensure that patients have access to high-quality medical care,“ said Timothy Wang, MD, AGAF, AGA chair. “We have made great strides in the increase in screening and prevention of colorectal cancer, which reduces deaths and downstream health care costs. Erecting barriers to screening will only reverse this process.” The budget cuts and restrictions on patient access under the congressional proposals will create tremendous burdens for the health care system as patients will increasingly rely on practices and academic medical centers to provide uncompensated care, according to the AGA.
The American Osteopathic Association reiterated its objections to BCRA in a statement, citing the CBO’s determination that 22 million would lose coverage.
“As patient advocates, we cannot accept that under [BCRA] patients in need will no longer have the coverage they require to access health care services,” the association said in a statement. “The BCRA does nothing to control health costs but instead focuses on reducing federal health care expenditures by cutting coverage of our nation’s most vulnerable individuals and eliminating policies that promote access to preventive care services that can actually drive down expenses while improving patient outcomes.”
The American College of Cardiology noted that CBO analysis “makes it clear that the [BCRA] would lead to loss of coverage for millions of Americans and limit access to care for our most vulnerable populations. ... The ACC opposes the BCRA as it does not align with our Principles for Health Reform, which stress the need for patient access to meaningful insurance coverage and high-quality care.”
The Senate health care proposal is only marginally better in terms of the number of uninsured Americans, compared with the House-passed bill it aims to replace, but it still would leave 22 million more Americans without insurance coverage, according to a June 26 analysis by the Congressional Budget Office.
The analysis raised voices of opposition from the medical community.
BCRA would lower the federal deficit by $321 billion between 2017-2026, driven by the dramatic cuts in spending on Medicaid (estimated to be $772 billion), as well as $408 billion saved from reduced tax credits and other subsidies to help people afford health insurance.
The CBO’s estimate also addresses how the bill could impact access to health care.
Initially, patients can expect another short-term spike in insurance premiums, with average premiums in 2018 increasing by 20%, compared with current law, “mainly because the penalty for not having insurance would be eliminated, inducing fewer comparatively healthy people to sign up.” In 2019, premiums are predicted to be about 10% higher than under current law; however, by 2020, premiums for benchmark plans would be 30% lower than with current law.
However, as premiums come down, deductibles would continue to rise for plans that would offer lower levels of coverage, according to the CBO report. Additionally, “starting in 2020, the premium for a silver plan would typically be a relatively high percentage of income for low income people. The deductible for a plan ... would be a significantly higher percentage of income – also making such a plan unattractive but for a different reason. As a result, despite being eligible for premium tax credits, few low-income people would purchase any plan.”
The report also notes that the Senate proposal would not necessarily reverse current concerns regarding consumer choice in the individual markets, stating that “a small fraction of the population resides in areas which – because of this legislation, for at least some of the years after 2019 – no insurers will participate in the nongroup market or insurance would be offered only with very high premiums.” Additionally, removing the employer mandate could result in employers forgoing offering health insurance to their employees.
The bill faces an uphill battle in the Senate as there seemingly are not enough votes to pass the bill at this time. The measure is using the budget reconciliation process, meaning it will need 50 of the 52 Senate Republicans to pass it (all 48 Democrats are expected to vote against it). At least six GOP senators have said they are not ready to start debate. Senate Majority Leader Mitch McConnell (R-Ky) will not present the bill to the chamber for consideration until after the July 4 recess in an effort to tweak the language to garner the 50 votes needed to pass.
Medical societies are pushing back against the bill as well.
The American Medical Association, in a letter to Senate leaders, notes that the first principal that medical professionals operate under is to do no harm. “The draft legislation violates that standard on many levels,” according to the AMA letter.
In a statement from the American Gastroenterological Association, it was pointed out that both the House and Senate bills allow states to opt out of the Affordable Care Act’s essential health benefits package, which includes coverage of colorectal cancer screenings. “A core mission of AGA is to ensure that patients have access to high-quality medical care,“ said Timothy Wang, MD, AGAF, AGA chair. “We have made great strides in the increase in screening and prevention of colorectal cancer, which reduces deaths and downstream health care costs. Erecting barriers to screening will only reverse this process.” The budget cuts and restrictions on patient access under the congressional proposals will create tremendous burdens for the health care system as patients will increasingly rely on practices and academic medical centers to provide uncompensated care, according to the AGA.
The American Osteopathic Association reiterated its objections to BCRA in a statement, citing the CBO’s determination that 22 million would lose coverage.
“As patient advocates, we cannot accept that under [BCRA] patients in need will no longer have the coverage they require to access health care services,” the association said in a statement. “The BCRA does nothing to control health costs but instead focuses on reducing federal health care expenditures by cutting coverage of our nation’s most vulnerable individuals and eliminating policies that promote access to preventive care services that can actually drive down expenses while improving patient outcomes.”
The American College of Cardiology noted that CBO analysis “makes it clear that the [BCRA] would lead to loss of coverage for millions of Americans and limit access to care for our most vulnerable populations. ... The ACC opposes the BCRA as it does not align with our Principles for Health Reform, which stress the need for patient access to meaningful insurance coverage and high-quality care.”
The Senate health care proposal is only marginally better in terms of the number of uninsured Americans, compared with the House-passed bill it aims to replace, but it still would leave 22 million more Americans without insurance coverage, according to a June 26 analysis by the Congressional Budget Office.
The analysis raised voices of opposition from the medical community.
BCRA would lower the federal deficit by $321 billion between 2017-2026, driven by the dramatic cuts in spending on Medicaid (estimated to be $772 billion), as well as $408 billion saved from reduced tax credits and other subsidies to help people afford health insurance.
The CBO’s estimate also addresses how the bill could impact access to health care.
Initially, patients can expect another short-term spike in insurance premiums, with average premiums in 2018 increasing by 20%, compared with current law, “mainly because the penalty for not having insurance would be eliminated, inducing fewer comparatively healthy people to sign up.” In 2019, premiums are predicted to be about 10% higher than under current law; however, by 2020, premiums for benchmark plans would be 30% lower than with current law.
However, as premiums come down, deductibles would continue to rise for plans that would offer lower levels of coverage, according to the CBO report. Additionally, “starting in 2020, the premium for a silver plan would typically be a relatively high percentage of income for low income people. The deductible for a plan ... would be a significantly higher percentage of income – also making such a plan unattractive but for a different reason. As a result, despite being eligible for premium tax credits, few low-income people would purchase any plan.”
The report also notes that the Senate proposal would not necessarily reverse current concerns regarding consumer choice in the individual markets, stating that “a small fraction of the population resides in areas which – because of this legislation, for at least some of the years after 2019 – no insurers will participate in the nongroup market or insurance would be offered only with very high premiums.” Additionally, removing the employer mandate could result in employers forgoing offering health insurance to their employees.
The bill faces an uphill battle in the Senate as there seemingly are not enough votes to pass the bill at this time. The measure is using the budget reconciliation process, meaning it will need 50 of the 52 Senate Republicans to pass it (all 48 Democrats are expected to vote against it). At least six GOP senators have said they are not ready to start debate. Senate Majority Leader Mitch McConnell (R-Ky) will not present the bill to the chamber for consideration until after the July 4 recess in an effort to tweak the language to garner the 50 votes needed to pass.
Medical societies are pushing back against the bill as well.
The American Medical Association, in a letter to Senate leaders, notes that the first principal that medical professionals operate under is to do no harm. “The draft legislation violates that standard on many levels,” according to the AMA letter.
In a statement from the American Gastroenterological Association, it was pointed out that both the House and Senate bills allow states to opt out of the Affordable Care Act’s essential health benefits package, which includes coverage of colorectal cancer screenings. “A core mission of AGA is to ensure that patients have access to high-quality medical care,“ said Timothy Wang, MD, AGAF, AGA chair. “We have made great strides in the increase in screening and prevention of colorectal cancer, which reduces deaths and downstream health care costs. Erecting barriers to screening will only reverse this process.” The budget cuts and restrictions on patient access under the congressional proposals will create tremendous burdens for the health care system as patients will increasingly rely on practices and academic medical centers to provide uncompensated care, according to the AGA.
The American Osteopathic Association reiterated its objections to BCRA in a statement, citing the CBO’s determination that 22 million would lose coverage.
“As patient advocates, we cannot accept that under [BCRA] patients in need will no longer have the coverage they require to access health care services,” the association said in a statement. “The BCRA does nothing to control health costs but instead focuses on reducing federal health care expenditures by cutting coverage of our nation’s most vulnerable individuals and eliminating policies that promote access to preventive care services that can actually drive down expenses while improving patient outcomes.”
The American College of Cardiology noted that CBO analysis “makes it clear that the [BCRA] would lead to loss of coverage for millions of Americans and limit access to care for our most vulnerable populations. ... The ACC opposes the BCRA as it does not align with our Principles for Health Reform, which stress the need for patient access to meaningful insurance coverage and high-quality care.”
Senate health care proposal already facing uphill battle
Senate Republican leaders are facing pushback from almost every side on their Affordable Care Act repeal/replace proposal – so much so that the current plan is unlikely to gain enough support to pass.
Sen. Rand Paul (R-Ky.), Sen. Ted Cruz (R-Texas), Sen. Ron Johnson (R-Wis.), and Sen. Mike Lee (R-Utah) said in a joint statement issued June 22, the day the plan was published, “There are provisions in this draft that represent an improvement to our current health care system, but it does not appear this draft as written will accomplish the most important promise that we made to Americans to repeal Obamacare and lower their health care costs.”
Like the House-passed American Health Care Act (H.R. 1628), the proposed BCRA would reduce Medicaid spending and would address rising premiums in the individual health insurance marketplace; however, BCRA would take a slightly different path to the same destination.
Like the House bill, BCRA also targets funding for Planned Parenthood, although because of Senate procedural rules, it is a more indirect funding ban.
A key difference between BCRA and the House bill is how insurance premium support is calculated. The AHCA would base tax credits on age, providing a lesser benefit for older, but pre–Medicare-age adults. In contrast, BCRA would base tax credits on income while limiting eligibility to households at 350% of the federal poverty line. Further, credits would cover only 58% of the actuarial value of health insurance under BCRA.
The draft Senate plan would not allow states to request a waiver from the ACA’s so-called community waiver provisions – the portion of the law that requires health insurance premiums to be the same regardless of age or preexisting condition; the House-passed AHCA would allow those waivers.
Medicaid expansion would be rolled back under the Senate plan, but at a slower pace than the AHCA would require – by 2023 under BCRA vs. 2020 under AHCA.
The BCRA would establish a per capita funding mechanism for Medicaid going forward, which would base funding on historic Medicaid expenditures and uses an economic index to track inflation and adjust payments accordingly.
To address the needs of people with greater health care needs, the Senate proposal would provide $57 billion over the first 4 years, then another $57 billion over the next 8. The funds would be available for programs such as premium support or high-risk pools to help individuals who are expected to be high users of health care. States would be required to match funds starting in 2022.
Experts were quick to weigh in on the Senate plan.
The BCRA needs to do three things, according to Grace-Marie Turner, president of the Galen Institute: Provide a safety net for those covered through the ACA so that they do not lose coverage in the transition, modernize Medicaid, and give states more authority and options to reform their own health insurance markets.
“We have learned that the federal government is not able to regulate something as local as health insurance,” Ms. Turner said. “They cannot create policies and legislation that works for people in downtown Manhattan and rural Montana and southern New Mexico and the panhandle of Florida. There are too many different populations. The states need to do that, and this bill also would give the states more authority to begin to oversee their health insurance markets but with new funding to provide extra help for the people who have difficulty buying progress.”
She said it could be much better if the Senate did not use the reconciliation process, “but within the confines of that, both the House and Senate bills do the same thing.”
Ms. Turner also stressed that there are more reforms coming later, as the Senate and House address other portions of ACA repeal/replacement.
“I hope that [senators] would see moving this forward as beneficial so that then they can move additional pieces of legislation, hopefully, with 60 votes to go through the regular process, to have additional follow-up bills. This is not the end of the story. This is just rescuing us from Obamacare,” she said. “Then we need to go forward and think about what do we need to do to make our health sector work better in the future by putting doctors and patients, rather than government, in charge of choices.”
Doctors, however, did not agree.
“This bill significantly decreases patients’ ability to access high-quality health care, and affordable coverage for millions of Americans will be in jeopardy if the legislation is passed,” Boyd Buser, MD, a doctor of osteopathy and president of the American Osteopathic Association, said in a statement.
He noted that the Medicaid cuts will have a “devastating impact, especially in areas of our country hardest hit by the ongoing opioid epidemic. ... The Senate bill should have prioritized prevention and care coordination, two measures proven to reduce overall health costs by eliminating waste and addressing health problems at the most treatable stage. Decreasing the number of Americans with coverage as it intends does will not lower costs.”
In a statement from the American Gastroenterological Association, it was noted that steep cuts to Medicaid funding are a main driver of loss of coverage. Both bills also allow states to opt out of the Affordable Care Act’s essential health benefits package, which includes coverage of colorectal cancer screenings. “A core mission of AGA is to ensure that patients have access to high-quality medical care,” said Timothy Wang, MD, AGAF, AGA chair. “We have made great strides in the increase in screening and prevention of colorectal cancer, which reduces deaths and downstream health-care costs. Erecting barriers to screening will only reverse this progress.” The budget cuts and restrictions on patient access under the congressional proposals will create tremendous burdens for the health-care system as patients will increasingly rely on practices and academic medical centers to provide uncompensated care, according to the AGA.
Republican lawmakers and the Trump administration have vowed to address the ACA in other ways as well, by reviewing and possibly changing all relevant regulations, then using the regular legislative process, which would need 60 votes, to address issues that cannot be handled by the budget reconciliation process.
Senate Republican leaders are facing pushback from almost every side on their Affordable Care Act repeal/replace proposal – so much so that the current plan is unlikely to gain enough support to pass.
Sen. Rand Paul (R-Ky.), Sen. Ted Cruz (R-Texas), Sen. Ron Johnson (R-Wis.), and Sen. Mike Lee (R-Utah) said in a joint statement issued June 22, the day the plan was published, “There are provisions in this draft that represent an improvement to our current health care system, but it does not appear this draft as written will accomplish the most important promise that we made to Americans to repeal Obamacare and lower their health care costs.”
Like the House-passed American Health Care Act (H.R. 1628), the proposed BCRA would reduce Medicaid spending and would address rising premiums in the individual health insurance marketplace; however, BCRA would take a slightly different path to the same destination.
Like the House bill, BCRA also targets funding for Planned Parenthood, although because of Senate procedural rules, it is a more indirect funding ban.
A key difference between BCRA and the House bill is how insurance premium support is calculated. The AHCA would base tax credits on age, providing a lesser benefit for older, but pre–Medicare-age adults. In contrast, BCRA would base tax credits on income while limiting eligibility to households at 350% of the federal poverty line. Further, credits would cover only 58% of the actuarial value of health insurance under BCRA.
The draft Senate plan would not allow states to request a waiver from the ACA’s so-called community waiver provisions – the portion of the law that requires health insurance premiums to be the same regardless of age or preexisting condition; the House-passed AHCA would allow those waivers.
Medicaid expansion would be rolled back under the Senate plan, but at a slower pace than the AHCA would require – by 2023 under BCRA vs. 2020 under AHCA.
The BCRA would establish a per capita funding mechanism for Medicaid going forward, which would base funding on historic Medicaid expenditures and uses an economic index to track inflation and adjust payments accordingly.
To address the needs of people with greater health care needs, the Senate proposal would provide $57 billion over the first 4 years, then another $57 billion over the next 8. The funds would be available for programs such as premium support or high-risk pools to help individuals who are expected to be high users of health care. States would be required to match funds starting in 2022.
Experts were quick to weigh in on the Senate plan.
The BCRA needs to do three things, according to Grace-Marie Turner, president of the Galen Institute: Provide a safety net for those covered through the ACA so that they do not lose coverage in the transition, modernize Medicaid, and give states more authority and options to reform their own health insurance markets.
“We have learned that the federal government is not able to regulate something as local as health insurance,” Ms. Turner said. “They cannot create policies and legislation that works for people in downtown Manhattan and rural Montana and southern New Mexico and the panhandle of Florida. There are too many different populations. The states need to do that, and this bill also would give the states more authority to begin to oversee their health insurance markets but with new funding to provide extra help for the people who have difficulty buying progress.”
She said it could be much better if the Senate did not use the reconciliation process, “but within the confines of that, both the House and Senate bills do the same thing.”
Ms. Turner also stressed that there are more reforms coming later, as the Senate and House address other portions of ACA repeal/replacement.
“I hope that [senators] would see moving this forward as beneficial so that then they can move additional pieces of legislation, hopefully, with 60 votes to go through the regular process, to have additional follow-up bills. This is not the end of the story. This is just rescuing us from Obamacare,” she said. “Then we need to go forward and think about what do we need to do to make our health sector work better in the future by putting doctors and patients, rather than government, in charge of choices.”
Doctors, however, did not agree.
“This bill significantly decreases patients’ ability to access high-quality health care, and affordable coverage for millions of Americans will be in jeopardy if the legislation is passed,” Boyd Buser, MD, a doctor of osteopathy and president of the American Osteopathic Association, said in a statement.
He noted that the Medicaid cuts will have a “devastating impact, especially in areas of our country hardest hit by the ongoing opioid epidemic. ... The Senate bill should have prioritized prevention and care coordination, two measures proven to reduce overall health costs by eliminating waste and addressing health problems at the most treatable stage. Decreasing the number of Americans with coverage as it intends does will not lower costs.”
In a statement from the American Gastroenterological Association, it was noted that steep cuts to Medicaid funding are a main driver of loss of coverage. Both bills also allow states to opt out of the Affordable Care Act’s essential health benefits package, which includes coverage of colorectal cancer screenings. “A core mission of AGA is to ensure that patients have access to high-quality medical care,” said Timothy Wang, MD, AGAF, AGA chair. “We have made great strides in the increase in screening and prevention of colorectal cancer, which reduces deaths and downstream health-care costs. Erecting barriers to screening will only reverse this progress.” The budget cuts and restrictions on patient access under the congressional proposals will create tremendous burdens for the health-care system as patients will increasingly rely on practices and academic medical centers to provide uncompensated care, according to the AGA.
Republican lawmakers and the Trump administration have vowed to address the ACA in other ways as well, by reviewing and possibly changing all relevant regulations, then using the regular legislative process, which would need 60 votes, to address issues that cannot be handled by the budget reconciliation process.
Senate Republican leaders are facing pushback from almost every side on their Affordable Care Act repeal/replace proposal – so much so that the current plan is unlikely to gain enough support to pass.
Sen. Rand Paul (R-Ky.), Sen. Ted Cruz (R-Texas), Sen. Ron Johnson (R-Wis.), and Sen. Mike Lee (R-Utah) said in a joint statement issued June 22, the day the plan was published, “There are provisions in this draft that represent an improvement to our current health care system, but it does not appear this draft as written will accomplish the most important promise that we made to Americans to repeal Obamacare and lower their health care costs.”
Like the House-passed American Health Care Act (H.R. 1628), the proposed BCRA would reduce Medicaid spending and would address rising premiums in the individual health insurance marketplace; however, BCRA would take a slightly different path to the same destination.
Like the House bill, BCRA also targets funding for Planned Parenthood, although because of Senate procedural rules, it is a more indirect funding ban.
A key difference between BCRA and the House bill is how insurance premium support is calculated. The AHCA would base tax credits on age, providing a lesser benefit for older, but pre–Medicare-age adults. In contrast, BCRA would base tax credits on income while limiting eligibility to households at 350% of the federal poverty line. Further, credits would cover only 58% of the actuarial value of health insurance under BCRA.
The draft Senate plan would not allow states to request a waiver from the ACA’s so-called community waiver provisions – the portion of the law that requires health insurance premiums to be the same regardless of age or preexisting condition; the House-passed AHCA would allow those waivers.
Medicaid expansion would be rolled back under the Senate plan, but at a slower pace than the AHCA would require – by 2023 under BCRA vs. 2020 under AHCA.
The BCRA would establish a per capita funding mechanism for Medicaid going forward, which would base funding on historic Medicaid expenditures and uses an economic index to track inflation and adjust payments accordingly.
To address the needs of people with greater health care needs, the Senate proposal would provide $57 billion over the first 4 years, then another $57 billion over the next 8. The funds would be available for programs such as premium support or high-risk pools to help individuals who are expected to be high users of health care. States would be required to match funds starting in 2022.
Experts were quick to weigh in on the Senate plan.
The BCRA needs to do three things, according to Grace-Marie Turner, president of the Galen Institute: Provide a safety net for those covered through the ACA so that they do not lose coverage in the transition, modernize Medicaid, and give states more authority and options to reform their own health insurance markets.
“We have learned that the federal government is not able to regulate something as local as health insurance,” Ms. Turner said. “They cannot create policies and legislation that works for people in downtown Manhattan and rural Montana and southern New Mexico and the panhandle of Florida. There are too many different populations. The states need to do that, and this bill also would give the states more authority to begin to oversee their health insurance markets but with new funding to provide extra help for the people who have difficulty buying progress.”
She said it could be much better if the Senate did not use the reconciliation process, “but within the confines of that, both the House and Senate bills do the same thing.”
Ms. Turner also stressed that there are more reforms coming later, as the Senate and House address other portions of ACA repeal/replacement.
“I hope that [senators] would see moving this forward as beneficial so that then they can move additional pieces of legislation, hopefully, with 60 votes to go through the regular process, to have additional follow-up bills. This is not the end of the story. This is just rescuing us from Obamacare,” she said. “Then we need to go forward and think about what do we need to do to make our health sector work better in the future by putting doctors and patients, rather than government, in charge of choices.”
Doctors, however, did not agree.
“This bill significantly decreases patients’ ability to access high-quality health care, and affordable coverage for millions of Americans will be in jeopardy if the legislation is passed,” Boyd Buser, MD, a doctor of osteopathy and president of the American Osteopathic Association, said in a statement.
He noted that the Medicaid cuts will have a “devastating impact, especially in areas of our country hardest hit by the ongoing opioid epidemic. ... The Senate bill should have prioritized prevention and care coordination, two measures proven to reduce overall health costs by eliminating waste and addressing health problems at the most treatable stage. Decreasing the number of Americans with coverage as it intends does will not lower costs.”
In a statement from the American Gastroenterological Association, it was noted that steep cuts to Medicaid funding are a main driver of loss of coverage. Both bills also allow states to opt out of the Affordable Care Act’s essential health benefits package, which includes coverage of colorectal cancer screenings. “A core mission of AGA is to ensure that patients have access to high-quality medical care,” said Timothy Wang, MD, AGAF, AGA chair. “We have made great strides in the increase in screening and prevention of colorectal cancer, which reduces deaths and downstream health-care costs. Erecting barriers to screening will only reverse this progress.” The budget cuts and restrictions on patient access under the congressional proposals will create tremendous burdens for the health-care system as patients will increasingly rely on practices and academic medical centers to provide uncompensated care, according to the AGA.
Republican lawmakers and the Trump administration have vowed to address the ACA in other ways as well, by reviewing and possibly changing all relevant regulations, then using the regular legislative process, which would need 60 votes, to address issues that cannot be handled by the budget reconciliation process.