User login
Obesity an independent risk factor for rosacea in cohort study of U.S. women
Obesity was associated with a significantly higher risk of rosacea, compared with a healthy weight, particularly when the weight was gained after age 18 years, according to an analysis of data from the Nurses Health Study II.
Investigators evaluated data on 89,886 women in the U.S. study from 1991 through 2005 – 5,249 diagnosed with rosacea – over 14 years to determine what, if any, relationship existed between body mass index (BMI) and rosacea risk.
As BMI increased, the risk of rosacea increased: Compared with participants with a BMI of 21.0 to 22.9 kg/m2 (considered a healthy weight), the risk of rosacea for those with a BMI of 25.0-29.9 (overweight) was 11% greater (95% confidence interval, 1.02-1.21), and among those with a BMI of 30.0-34.9, was 21% greater (95% CI, 1.09-1.34). The risk was 48% greater among those with a BMI of 35 or above (95% CI, 1.33-1.64). (A BMI over 30 is considered obese). The results were published in the December issue of the Journal of the American Academy of Dermatology.
The association between weight and rosacea risk was only significant after age 18 years, when the risk of rosacea increased 4% for every 10 pounds of weight gain, even after adjusting for BMI.
The investigators also found a significant relationship between waist circumference and rosacea risk that was independent of BMI, with the highest quintile of waist circumference associated with a 32% greater risk of rosacea, compared with the lowest quintile. Similarly, the highest quintile of hip circumference was associated with a 38% higher risk of rosacea, also independent of BMI. However there was no association between waist-to-hip ratio and rosacea risk.
While there were associations between the risk of rosacea and smoking, alcohol intake, and physical activity, the relationship between BMI and rosacea risk was not modified by these other risk factors.
Suyun Li, PhD, of the Guangzhou (China) Medical University School of Public Health and coauthors from the department of dermatology, Brown University, Providence, R.I.; and Brigham and Women’s Hospital, Boston, wrote that previous epidemiologic studies examining the interaction between obesity and rosacea have shown inconsistent results. Longitudinal studies on the issue had focused only on BMI and ignored other measures of central obesity.
“To our knowledge, this is the first cohort study on the association between obesity and risk for incident rosacea,” they wrote. “The study contributes to the understanding of rosacea etiology and informs clinical practice related to rosacea prevention and patient care.”
They suggested a number of different mechanisms that might explain how obesity increases the risk of rosacea, including the chronic, low-grade inflammatory state associated with obesity. “Adiposity can augment proinflammatory cytokine expression, such as interleukin 6 and tumor necrosis factor–alpha, both relevant to rosacea pathophysiology,” they noted. “Vascular changes associated with obesity might be another mechanism, considering obesity can lead to abnormalities of vascular function and structure, which might lead to the vasodilatation in rosacea.”
In the NHS II study, data are collected every 2 years, which the authors said ensured they had the most up-to-date information. While rosacea diagnoses relied on self-report, the authors said the study participants – nurses – were likely to have a high validity of self-reporting of rosacea. They acknowledged, however, that the lack of information on rosacea subtypes was a limitation of the study.
The study was supported by the department of dermatology, Brown University and a Nurses’ Health Study II grant. Dr. Li was supported by a research grant from the National Rosacea Society and the Dermatology Foundation. Another author declared having served as an investigator and receiving research funds from Sanofi and Regeneron; serving as a consultant for Sanofi and RTI Health Solutions, and having received honoraria from Astellas Canada, Prime, and Spire Learning. The remaining three authors had no disclosures.
SOURCE: Li S et al. J Am Acad Dermatol. 2017 Dec;77(6):1083-7.E5.
Obesity was associated with a significantly higher risk of rosacea, compared with a healthy weight, particularly when the weight was gained after age 18 years, according to an analysis of data from the Nurses Health Study II.
Investigators evaluated data on 89,886 women in the U.S. study from 1991 through 2005 – 5,249 diagnosed with rosacea – over 14 years to determine what, if any, relationship existed between body mass index (BMI) and rosacea risk.
As BMI increased, the risk of rosacea increased: Compared with participants with a BMI of 21.0 to 22.9 kg/m2 (considered a healthy weight), the risk of rosacea for those with a BMI of 25.0-29.9 (overweight) was 11% greater (95% confidence interval, 1.02-1.21), and among those with a BMI of 30.0-34.9, was 21% greater (95% CI, 1.09-1.34). The risk was 48% greater among those with a BMI of 35 or above (95% CI, 1.33-1.64). (A BMI over 30 is considered obese). The results were published in the December issue of the Journal of the American Academy of Dermatology.
The association between weight and rosacea risk was only significant after age 18 years, when the risk of rosacea increased 4% for every 10 pounds of weight gain, even after adjusting for BMI.
The investigators also found a significant relationship between waist circumference and rosacea risk that was independent of BMI, with the highest quintile of waist circumference associated with a 32% greater risk of rosacea, compared with the lowest quintile. Similarly, the highest quintile of hip circumference was associated with a 38% higher risk of rosacea, also independent of BMI. However there was no association between waist-to-hip ratio and rosacea risk.
While there were associations between the risk of rosacea and smoking, alcohol intake, and physical activity, the relationship between BMI and rosacea risk was not modified by these other risk factors.
Suyun Li, PhD, of the Guangzhou (China) Medical University School of Public Health and coauthors from the department of dermatology, Brown University, Providence, R.I.; and Brigham and Women’s Hospital, Boston, wrote that previous epidemiologic studies examining the interaction between obesity and rosacea have shown inconsistent results. Longitudinal studies on the issue had focused only on BMI and ignored other measures of central obesity.
“To our knowledge, this is the first cohort study on the association between obesity and risk for incident rosacea,” they wrote. “The study contributes to the understanding of rosacea etiology and informs clinical practice related to rosacea prevention and patient care.”
They suggested a number of different mechanisms that might explain how obesity increases the risk of rosacea, including the chronic, low-grade inflammatory state associated with obesity. “Adiposity can augment proinflammatory cytokine expression, such as interleukin 6 and tumor necrosis factor–alpha, both relevant to rosacea pathophysiology,” they noted. “Vascular changes associated with obesity might be another mechanism, considering obesity can lead to abnormalities of vascular function and structure, which might lead to the vasodilatation in rosacea.”
In the NHS II study, data are collected every 2 years, which the authors said ensured they had the most up-to-date information. While rosacea diagnoses relied on self-report, the authors said the study participants – nurses – were likely to have a high validity of self-reporting of rosacea. They acknowledged, however, that the lack of information on rosacea subtypes was a limitation of the study.
The study was supported by the department of dermatology, Brown University and a Nurses’ Health Study II grant. Dr. Li was supported by a research grant from the National Rosacea Society and the Dermatology Foundation. Another author declared having served as an investigator and receiving research funds from Sanofi and Regeneron; serving as a consultant for Sanofi and RTI Health Solutions, and having received honoraria from Astellas Canada, Prime, and Spire Learning. The remaining three authors had no disclosures.
SOURCE: Li S et al. J Am Acad Dermatol. 2017 Dec;77(6):1083-7.E5.
Obesity was associated with a significantly higher risk of rosacea, compared with a healthy weight, particularly when the weight was gained after age 18 years, according to an analysis of data from the Nurses Health Study II.
Investigators evaluated data on 89,886 women in the U.S. study from 1991 through 2005 – 5,249 diagnosed with rosacea – over 14 years to determine what, if any, relationship existed between body mass index (BMI) and rosacea risk.
As BMI increased, the risk of rosacea increased: Compared with participants with a BMI of 21.0 to 22.9 kg/m2 (considered a healthy weight), the risk of rosacea for those with a BMI of 25.0-29.9 (overweight) was 11% greater (95% confidence interval, 1.02-1.21), and among those with a BMI of 30.0-34.9, was 21% greater (95% CI, 1.09-1.34). The risk was 48% greater among those with a BMI of 35 or above (95% CI, 1.33-1.64). (A BMI over 30 is considered obese). The results were published in the December issue of the Journal of the American Academy of Dermatology.
The association between weight and rosacea risk was only significant after age 18 years, when the risk of rosacea increased 4% for every 10 pounds of weight gain, even after adjusting for BMI.
The investigators also found a significant relationship between waist circumference and rosacea risk that was independent of BMI, with the highest quintile of waist circumference associated with a 32% greater risk of rosacea, compared with the lowest quintile. Similarly, the highest quintile of hip circumference was associated with a 38% higher risk of rosacea, also independent of BMI. However there was no association between waist-to-hip ratio and rosacea risk.
While there were associations between the risk of rosacea and smoking, alcohol intake, and physical activity, the relationship between BMI and rosacea risk was not modified by these other risk factors.
Suyun Li, PhD, of the Guangzhou (China) Medical University School of Public Health and coauthors from the department of dermatology, Brown University, Providence, R.I.; and Brigham and Women’s Hospital, Boston, wrote that previous epidemiologic studies examining the interaction between obesity and rosacea have shown inconsistent results. Longitudinal studies on the issue had focused only on BMI and ignored other measures of central obesity.
“To our knowledge, this is the first cohort study on the association between obesity and risk for incident rosacea,” they wrote. “The study contributes to the understanding of rosacea etiology and informs clinical practice related to rosacea prevention and patient care.”
They suggested a number of different mechanisms that might explain how obesity increases the risk of rosacea, including the chronic, low-grade inflammatory state associated with obesity. “Adiposity can augment proinflammatory cytokine expression, such as interleukin 6 and tumor necrosis factor–alpha, both relevant to rosacea pathophysiology,” they noted. “Vascular changes associated with obesity might be another mechanism, considering obesity can lead to abnormalities of vascular function and structure, which might lead to the vasodilatation in rosacea.”
In the NHS II study, data are collected every 2 years, which the authors said ensured they had the most up-to-date information. While rosacea diagnoses relied on self-report, the authors said the study participants – nurses – were likely to have a high validity of self-reporting of rosacea. They acknowledged, however, that the lack of information on rosacea subtypes was a limitation of the study.
The study was supported by the department of dermatology, Brown University and a Nurses’ Health Study II grant. Dr. Li was supported by a research grant from the National Rosacea Society and the Dermatology Foundation. Another author declared having served as an investigator and receiving research funds from Sanofi and Regeneron; serving as a consultant for Sanofi and RTI Health Solutions, and having received honoraria from Astellas Canada, Prime, and Spire Learning. The remaining three authors had no disclosures.
SOURCE: Li S et al. J Am Acad Dermatol. 2017 Dec;77(6):1083-7.E5.
FROM JAAD
Key clinical point: independent of other risk factors such as smoking and alcohol intake.
Major finding: A BMI greater than 35 is associated with a 48% higher risk of rosacea, compared with a BMI of 21.
Data source: Information on rosacea diagnoses among 89,886 Nurses’ Health Study II participants.
Disclosures: The study was supported by the Warren Alpert Medical School of Brown University and a Nurses’ Health Study II grant. One author was supported by a research grant from the National Rosacea Society and the Dermatology Foundation; another author declared research funding, consultancies, and honoraria from the pharmaceutical industry.
Source: Li S et al. J Am Acad Dermatol. 2017 Dec;77(6):1083-7.E5.
VIDEO: Weight loss cut risk of breast cancer
SAN ANTONIO – A new analysis from the Women’s Health Initiative Observational Study gives postmenopausal women yet another reason to mind their weight. Results showed that women losing at least 5% of their body weight had a significant 12% reduction in adjusted breast cancer risk relative to peers who maintained a stable weight, reported lead author Rowan Chlebowski, MD, PhD, research professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif. Findings were much the same regardless of whether women were of normal weight, overweight, or obese at baseline. Dr. Chlebowski discussed the implications for patient counseling and insurance coverage of weight loss interventions, as well as planned research that will assess the physiologic mechanisms at play in a video interview.
SAN ANTONIO – A new analysis from the Women’s Health Initiative Observational Study gives postmenopausal women yet another reason to mind their weight. Results showed that women losing at least 5% of their body weight had a significant 12% reduction in adjusted breast cancer risk relative to peers who maintained a stable weight, reported lead author Rowan Chlebowski, MD, PhD, research professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif. Findings were much the same regardless of whether women were of normal weight, overweight, or obese at baseline. Dr. Chlebowski discussed the implications for patient counseling and insurance coverage of weight loss interventions, as well as planned research that will assess the physiologic mechanisms at play in a video interview.
SAN ANTONIO – A new analysis from the Women’s Health Initiative Observational Study gives postmenopausal women yet another reason to mind their weight. Results showed that women losing at least 5% of their body weight had a significant 12% reduction in adjusted breast cancer risk relative to peers who maintained a stable weight, reported lead author Rowan Chlebowski, MD, PhD, research professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif. Findings were much the same regardless of whether women were of normal weight, overweight, or obese at baseline. Dr. Chlebowski discussed the implications for patient counseling and insurance coverage of weight loss interventions, as well as planned research that will assess the physiologic mechanisms at play in a video interview.
REPORTING FROM SABCS 2017
Inside the ‘mad rush’ for ketamine treatment
Ketamine, once best known as a pet anesthetic and party drug, is taking the United States by storm. Dozens of ketamine treatment centers are operating from coast to coast.
Big cities like Baltimore, Boston, and Phoenix have them. So do Charleston, S.C., and Boise, Idaho. Two such clinics are in sparsely populated New Mexico. And one national chain went from a pair of clinics to 10 in fewer than 2 years.
Never mind that these expensive treatments for conditions like depression are not covered by insurers or approved for this use by the Food and Drug Administration. Other questions also persist. “There is a considerable body of evidence that proves it really does work,” Dr. Lieberman said. “But we don’t know the extent of the range of conditions for which it might be effective, what the optimal frequency and concentration for dosing is, and what the long-term consequences are.”
To make matters more complicated, it’s anesthesiologists – not psychiatrists – who are leading the way toward a ketamine-infused future.
For now, however, hundreds and perhaps even thousands of patients are serving as ketamine test cases with psychiatrists only assisting remotely, if at all.
A stunningly rapid rise
Sara M. Markey, MD, is one of the rare psychiatrists in the United States who’s fully embraced ketamine treatment for mental illness.
She recalled first hearing about ketamine as an anesthetic in medical school. Best known as an anesthetic in animals, it’s also occasionally given to children and adults, although the drug’s dissociative properties have prevented widespread use.
In 2006, word spread about ketamine’s use as a painkiller. “I also began hearing and reading about its potential use/efficacy in treatment-resistant depression,” said Dr. Markey, who practices in Denver. “It was difficult to find information about ketamine, and many of my colleagues were hostile to the idea of using ketamine in clinical practice.”
She persisted, however, and prescribed intranasal and oral ketamine to depressed patients with “mild success.” She also saw patients whose psychiatrists refused to consider ketamine.
In early 2016, with Steven P. Levine, MD, a New Jersey psychiatrist who pioneered ketamine use for depression, Dr. Markey opened a ketamine infusion clinic in the Mile High City.
At at that time, it was only the second in a national chain called Ketamine Treatment Centers. Now, not even 2 years later, the chain has a new name – Actify Neurotherapies – and a total of 10 clinics from San Francisco and Beverly Hills, Calif., to Palm Beach, Fla.; Raleigh, N.C.; and New York City.
“It is wonderful,” she said, “to have an opportunity to provide a medication to people that does not cause weight gain, has very few medication interactions, and which is well tolerated and generic.”
Big short-term benefits
Treatment outcomes with ketamine – which is thought to act on glutamate and N-methyl-D-aspartate receptors – can be dramatic. “Some patients describe the ketamine treatments as life saving,” said Allison F. Wells, MD, an anesthesiologist who runs a clinic in Houston.
One depressed young man who tried ketamine at a Phoenix clinic in 2013 reportedly told the news site vice.com that he “felt good for a week” after his first treatment: “Not the kind of bipolar ‘good’ where I’d be manic. I just felt pleasant, and not crazy or compulsive. I felt normal for the first time in a long time.” Another depressed patient told National Public Radio that ketamine transformed his life: “I remember I was in my bathroom, and I literally fell to my knees crying because I had no anxiety; I had no depression.”
Enrique A. Abreu, DO, an anesthesiologist who offers ketamine therapy in Seattle and Portland, Ore., said he’s seen anxiety relief and a decrease in rumination in these patients. “They’re able to go back to work; a lot haven’t been able to work for a long time. And motivation is a big thing. They’re able to do things they haven’t been able to do.”
In addition, ketamine can reduce suicidal thinking, Dr. Markey said. “I am continually astounded to hear patients who come in with acute or chronic suicidal thinking report that those ideas and/or intrusive thoughts have disappeared. When they are absent, people need to be reminded of when they had them. They seem to have forgotten about them.”
‘Mystical experiences’
Dr. Markey said a retrospective analysis of about 740 patients at her chain’s clinics showed a response rate of about 75%. Other research has shown similarly high response levels.
“Multiple clinical trials suggest that a single low dose (0.5mg/kg) of IV ketamine results in a 50%-70% response rate in patients with treatment-resistant depression,” reported a 2016 clinical review. “Additional research has shown that depressed patients can experience symptom relief as early as 2 [hours], and lasting up to 2 weeks after a single administration of IV ketamine,” according to the review in Evidence Based Mental Health (2016 May;19[2]:35-8).
Adverse effects can include nausea and headache in patients with a history of migraine, he said. Over the long term, ketamine use can lead to incontinence and urinary urgency, he said.
As for ketamine addiction, Dr. Simelgor calls it unlikely at the lower doses that are used. However, he said, “I can’t say 100% that it won’t cause addiction.”
Who benefits? The jury’s still out
Considering its positive effects, why shouldn’t the mental health community embrace ketamine? Because, two prominent researchers say, best practices are still absent in a whole range of areas.
For example, there’s no agreement about who should undergo ketamine treatments beyond patients with treatment-resistant depression, especially those who have failed or cannot undergo electroconvulsive therapy. Ketamine therapy also is being touted by some as a treatment for a long list of other conditions from obsessive-compulsive disorder and anxiety to fibromyalgia and chronic pain disorders.
There are also limited data about dosing, making it “not possible to clarify the relative benefits and risks of doses other than 0.5 mg/kg delivered intravenously over 40 minutes,” cautioned Dr. Sanacora and Samuel T. Wilkinson, MD, also at Yale, in a 2017 commentary in JAMA (2017;318[9]:793-4).
In fact, they write, “Most published data supporting the use of ketamine as a treatment for mood disorders are based on trials that have followed up patients for just 1 week after a single administration of the drug.”
Unchartered waters
There’s also no accepted protocol beyond a typical six treatments over 2 or more weeks. This is relevant because the benefits of a series of treatments often fade away after a few weeks.
“Some patients describe the results lasting indefinitely, while most patients who respond to the treatments get to the point where they are going roughly 4-12 weeks with sustained results,” Dr. Wells said.
“When the effects start to wear off, they don’t crash,” said Dr. Abreu. Instead, he said, symptoms slowly reappear.
It’s typical for patients at Dr. Abreu’s clinic and others to return within a couple of months to go through another round of ketamine treatments. In some cases, “they continue to see us indefinitely to get them back up to where they need to be with a booster type of session,” he said.
Ketamine treatment costs vary widely, and insurers don’t cover this off-label treatment. The clinic operators quoted in this article reported a range of per-infusion costs from $350 (Dr. Markey’s clinic in Denver) to $675 (Dr. Abreu’s clinics in the Northwest).
“We have to have a talk with them: Can you afford this? This is going to take a significant amount of money every month to keep you well,” Dr. Abreu said. On the other hand, he said, the need for other medications goes away, eliminating that cost. (“They’re on [selective serotonin reputake inhibitors] usually,” he said, “but those drugs don’t work.”)
Nonpsychiatrists in forefront
At Dr. Markey’s clinic in Denver, all patients are required to see either her or a psychiatrist colleague. Some other ketamine clinics are run by psychiatrists, but that’s far from common.
Clinics often have no mental health professionals on staff and are run by anesthesiologists or other kinds of physicians.
Some clinic owners, Dr. Wells said, require patients to be under the care of a psychiatrist, neurologist, pain doctor, or other appropriate professional. “I do not intend, nor do I act, to displace psychiatrists, or the relationships our patients have with their psychiatrists, or the care they receive from their psychiatrists,” she said.
In the Northwest, Dr. Abreu said his patients take mood questionnaires, and he’s experimenting with a text-based mood monitoring system. In the Minneapolis area, anesthesiologist Dr. Simelgor is looking for a psychiatrist or psychiatrist partner for his ketamine clinic. “My thinking,” he said, “is that we need to work together.”
Still, there do not appear to be any requirements that ketamine clinic practitioners have connections to mental health professionals. Yet, as Dr. Sanacora put it: “Delivering the drug is the easiest part of the treatment. The hard part is managing the depression.”
Dr. Lieberman, Dr. Wells, Dr. Abreu, Dr. Simelgor, and Dr. Markey reported no relevant disclosures. Dr. Sanacora reported consulting fees and research contracts for multiple drug makers over the past 24 months. He holds shares in Biohaven Pharmaceuticals and is a coinventor on a U.S. patent (No. 8778979) on using glutamate agents to treat mental disorders held by Yale University.
Ketamine, once best known as a pet anesthetic and party drug, is taking the United States by storm. Dozens of ketamine treatment centers are operating from coast to coast.
Big cities like Baltimore, Boston, and Phoenix have them. So do Charleston, S.C., and Boise, Idaho. Two such clinics are in sparsely populated New Mexico. And one national chain went from a pair of clinics to 10 in fewer than 2 years.
Never mind that these expensive treatments for conditions like depression are not covered by insurers or approved for this use by the Food and Drug Administration. Other questions also persist. “There is a considerable body of evidence that proves it really does work,” Dr. Lieberman said. “But we don’t know the extent of the range of conditions for which it might be effective, what the optimal frequency and concentration for dosing is, and what the long-term consequences are.”
To make matters more complicated, it’s anesthesiologists – not psychiatrists – who are leading the way toward a ketamine-infused future.
For now, however, hundreds and perhaps even thousands of patients are serving as ketamine test cases with psychiatrists only assisting remotely, if at all.
A stunningly rapid rise
Sara M. Markey, MD, is one of the rare psychiatrists in the United States who’s fully embraced ketamine treatment for mental illness.
She recalled first hearing about ketamine as an anesthetic in medical school. Best known as an anesthetic in animals, it’s also occasionally given to children and adults, although the drug’s dissociative properties have prevented widespread use.
In 2006, word spread about ketamine’s use as a painkiller. “I also began hearing and reading about its potential use/efficacy in treatment-resistant depression,” said Dr. Markey, who practices in Denver. “It was difficult to find information about ketamine, and many of my colleagues were hostile to the idea of using ketamine in clinical practice.”
She persisted, however, and prescribed intranasal and oral ketamine to depressed patients with “mild success.” She also saw patients whose psychiatrists refused to consider ketamine.
In early 2016, with Steven P. Levine, MD, a New Jersey psychiatrist who pioneered ketamine use for depression, Dr. Markey opened a ketamine infusion clinic in the Mile High City.
At at that time, it was only the second in a national chain called Ketamine Treatment Centers. Now, not even 2 years later, the chain has a new name – Actify Neurotherapies – and a total of 10 clinics from San Francisco and Beverly Hills, Calif., to Palm Beach, Fla.; Raleigh, N.C.; and New York City.
“It is wonderful,” she said, “to have an opportunity to provide a medication to people that does not cause weight gain, has very few medication interactions, and which is well tolerated and generic.”
Big short-term benefits
Treatment outcomes with ketamine – which is thought to act on glutamate and N-methyl-D-aspartate receptors – can be dramatic. “Some patients describe the ketamine treatments as life saving,” said Allison F. Wells, MD, an anesthesiologist who runs a clinic in Houston.
One depressed young man who tried ketamine at a Phoenix clinic in 2013 reportedly told the news site vice.com that he “felt good for a week” after his first treatment: “Not the kind of bipolar ‘good’ where I’d be manic. I just felt pleasant, and not crazy or compulsive. I felt normal for the first time in a long time.” Another depressed patient told National Public Radio that ketamine transformed his life: “I remember I was in my bathroom, and I literally fell to my knees crying because I had no anxiety; I had no depression.”
Enrique A. Abreu, DO, an anesthesiologist who offers ketamine therapy in Seattle and Portland, Ore., said he’s seen anxiety relief and a decrease in rumination in these patients. “They’re able to go back to work; a lot haven’t been able to work for a long time. And motivation is a big thing. They’re able to do things they haven’t been able to do.”
In addition, ketamine can reduce suicidal thinking, Dr. Markey said. “I am continually astounded to hear patients who come in with acute or chronic suicidal thinking report that those ideas and/or intrusive thoughts have disappeared. When they are absent, people need to be reminded of when they had them. They seem to have forgotten about them.”
‘Mystical experiences’
Dr. Markey said a retrospective analysis of about 740 patients at her chain’s clinics showed a response rate of about 75%. Other research has shown similarly high response levels.
“Multiple clinical trials suggest that a single low dose (0.5mg/kg) of IV ketamine results in a 50%-70% response rate in patients with treatment-resistant depression,” reported a 2016 clinical review. “Additional research has shown that depressed patients can experience symptom relief as early as 2 [hours], and lasting up to 2 weeks after a single administration of IV ketamine,” according to the review in Evidence Based Mental Health (2016 May;19[2]:35-8).
Adverse effects can include nausea and headache in patients with a history of migraine, he said. Over the long term, ketamine use can lead to incontinence and urinary urgency, he said.
As for ketamine addiction, Dr. Simelgor calls it unlikely at the lower doses that are used. However, he said, “I can’t say 100% that it won’t cause addiction.”
Who benefits? The jury’s still out
Considering its positive effects, why shouldn’t the mental health community embrace ketamine? Because, two prominent researchers say, best practices are still absent in a whole range of areas.
For example, there’s no agreement about who should undergo ketamine treatments beyond patients with treatment-resistant depression, especially those who have failed or cannot undergo electroconvulsive therapy. Ketamine therapy also is being touted by some as a treatment for a long list of other conditions from obsessive-compulsive disorder and anxiety to fibromyalgia and chronic pain disorders.
There are also limited data about dosing, making it “not possible to clarify the relative benefits and risks of doses other than 0.5 mg/kg delivered intravenously over 40 minutes,” cautioned Dr. Sanacora and Samuel T. Wilkinson, MD, also at Yale, in a 2017 commentary in JAMA (2017;318[9]:793-4).
In fact, they write, “Most published data supporting the use of ketamine as a treatment for mood disorders are based on trials that have followed up patients for just 1 week after a single administration of the drug.”
Unchartered waters
There’s also no accepted protocol beyond a typical six treatments over 2 or more weeks. This is relevant because the benefits of a series of treatments often fade away after a few weeks.
“Some patients describe the results lasting indefinitely, while most patients who respond to the treatments get to the point where they are going roughly 4-12 weeks with sustained results,” Dr. Wells said.
“When the effects start to wear off, they don’t crash,” said Dr. Abreu. Instead, he said, symptoms slowly reappear.
It’s typical for patients at Dr. Abreu’s clinic and others to return within a couple of months to go through another round of ketamine treatments. In some cases, “they continue to see us indefinitely to get them back up to where they need to be with a booster type of session,” he said.
Ketamine treatment costs vary widely, and insurers don’t cover this off-label treatment. The clinic operators quoted in this article reported a range of per-infusion costs from $350 (Dr. Markey’s clinic in Denver) to $675 (Dr. Abreu’s clinics in the Northwest).
“We have to have a talk with them: Can you afford this? This is going to take a significant amount of money every month to keep you well,” Dr. Abreu said. On the other hand, he said, the need for other medications goes away, eliminating that cost. (“They’re on [selective serotonin reputake inhibitors] usually,” he said, “but those drugs don’t work.”)
Nonpsychiatrists in forefront
At Dr. Markey’s clinic in Denver, all patients are required to see either her or a psychiatrist colleague. Some other ketamine clinics are run by psychiatrists, but that’s far from common.
Clinics often have no mental health professionals on staff and are run by anesthesiologists or other kinds of physicians.
Some clinic owners, Dr. Wells said, require patients to be under the care of a psychiatrist, neurologist, pain doctor, or other appropriate professional. “I do not intend, nor do I act, to displace psychiatrists, or the relationships our patients have with their psychiatrists, or the care they receive from their psychiatrists,” she said.
In the Northwest, Dr. Abreu said his patients take mood questionnaires, and he’s experimenting with a text-based mood monitoring system. In the Minneapolis area, anesthesiologist Dr. Simelgor is looking for a psychiatrist or psychiatrist partner for his ketamine clinic. “My thinking,” he said, “is that we need to work together.”
Still, there do not appear to be any requirements that ketamine clinic practitioners have connections to mental health professionals. Yet, as Dr. Sanacora put it: “Delivering the drug is the easiest part of the treatment. The hard part is managing the depression.”
Dr. Lieberman, Dr. Wells, Dr. Abreu, Dr. Simelgor, and Dr. Markey reported no relevant disclosures. Dr. Sanacora reported consulting fees and research contracts for multiple drug makers over the past 24 months. He holds shares in Biohaven Pharmaceuticals and is a coinventor on a U.S. patent (No. 8778979) on using glutamate agents to treat mental disorders held by Yale University.
Ketamine, once best known as a pet anesthetic and party drug, is taking the United States by storm. Dozens of ketamine treatment centers are operating from coast to coast.
Big cities like Baltimore, Boston, and Phoenix have them. So do Charleston, S.C., and Boise, Idaho. Two such clinics are in sparsely populated New Mexico. And one national chain went from a pair of clinics to 10 in fewer than 2 years.
Never mind that these expensive treatments for conditions like depression are not covered by insurers or approved for this use by the Food and Drug Administration. Other questions also persist. “There is a considerable body of evidence that proves it really does work,” Dr. Lieberman said. “But we don’t know the extent of the range of conditions for which it might be effective, what the optimal frequency and concentration for dosing is, and what the long-term consequences are.”
To make matters more complicated, it’s anesthesiologists – not psychiatrists – who are leading the way toward a ketamine-infused future.
For now, however, hundreds and perhaps even thousands of patients are serving as ketamine test cases with psychiatrists only assisting remotely, if at all.
A stunningly rapid rise
Sara M. Markey, MD, is one of the rare psychiatrists in the United States who’s fully embraced ketamine treatment for mental illness.
She recalled first hearing about ketamine as an anesthetic in medical school. Best known as an anesthetic in animals, it’s also occasionally given to children and adults, although the drug’s dissociative properties have prevented widespread use.
In 2006, word spread about ketamine’s use as a painkiller. “I also began hearing and reading about its potential use/efficacy in treatment-resistant depression,” said Dr. Markey, who practices in Denver. “It was difficult to find information about ketamine, and many of my colleagues were hostile to the idea of using ketamine in clinical practice.”
She persisted, however, and prescribed intranasal and oral ketamine to depressed patients with “mild success.” She also saw patients whose psychiatrists refused to consider ketamine.
In early 2016, with Steven P. Levine, MD, a New Jersey psychiatrist who pioneered ketamine use for depression, Dr. Markey opened a ketamine infusion clinic in the Mile High City.
At at that time, it was only the second in a national chain called Ketamine Treatment Centers. Now, not even 2 years later, the chain has a new name – Actify Neurotherapies – and a total of 10 clinics from San Francisco and Beverly Hills, Calif., to Palm Beach, Fla.; Raleigh, N.C.; and New York City.
“It is wonderful,” she said, “to have an opportunity to provide a medication to people that does not cause weight gain, has very few medication interactions, and which is well tolerated and generic.”
Big short-term benefits
Treatment outcomes with ketamine – which is thought to act on glutamate and N-methyl-D-aspartate receptors – can be dramatic. “Some patients describe the ketamine treatments as life saving,” said Allison F. Wells, MD, an anesthesiologist who runs a clinic in Houston.
One depressed young man who tried ketamine at a Phoenix clinic in 2013 reportedly told the news site vice.com that he “felt good for a week” after his first treatment: “Not the kind of bipolar ‘good’ where I’d be manic. I just felt pleasant, and not crazy or compulsive. I felt normal for the first time in a long time.” Another depressed patient told National Public Radio that ketamine transformed his life: “I remember I was in my bathroom, and I literally fell to my knees crying because I had no anxiety; I had no depression.”
Enrique A. Abreu, DO, an anesthesiologist who offers ketamine therapy in Seattle and Portland, Ore., said he’s seen anxiety relief and a decrease in rumination in these patients. “They’re able to go back to work; a lot haven’t been able to work for a long time. And motivation is a big thing. They’re able to do things they haven’t been able to do.”
In addition, ketamine can reduce suicidal thinking, Dr. Markey said. “I am continually astounded to hear patients who come in with acute or chronic suicidal thinking report that those ideas and/or intrusive thoughts have disappeared. When they are absent, people need to be reminded of when they had them. They seem to have forgotten about them.”
‘Mystical experiences’
Dr. Markey said a retrospective analysis of about 740 patients at her chain’s clinics showed a response rate of about 75%. Other research has shown similarly high response levels.
“Multiple clinical trials suggest that a single low dose (0.5mg/kg) of IV ketamine results in a 50%-70% response rate in patients with treatment-resistant depression,” reported a 2016 clinical review. “Additional research has shown that depressed patients can experience symptom relief as early as 2 [hours], and lasting up to 2 weeks after a single administration of IV ketamine,” according to the review in Evidence Based Mental Health (2016 May;19[2]:35-8).
Adverse effects can include nausea and headache in patients with a history of migraine, he said. Over the long term, ketamine use can lead to incontinence and urinary urgency, he said.
As for ketamine addiction, Dr. Simelgor calls it unlikely at the lower doses that are used. However, he said, “I can’t say 100% that it won’t cause addiction.”
Who benefits? The jury’s still out
Considering its positive effects, why shouldn’t the mental health community embrace ketamine? Because, two prominent researchers say, best practices are still absent in a whole range of areas.
For example, there’s no agreement about who should undergo ketamine treatments beyond patients with treatment-resistant depression, especially those who have failed or cannot undergo electroconvulsive therapy. Ketamine therapy also is being touted by some as a treatment for a long list of other conditions from obsessive-compulsive disorder and anxiety to fibromyalgia and chronic pain disorders.
There are also limited data about dosing, making it “not possible to clarify the relative benefits and risks of doses other than 0.5 mg/kg delivered intravenously over 40 minutes,” cautioned Dr. Sanacora and Samuel T. Wilkinson, MD, also at Yale, in a 2017 commentary in JAMA (2017;318[9]:793-4).
In fact, they write, “Most published data supporting the use of ketamine as a treatment for mood disorders are based on trials that have followed up patients for just 1 week after a single administration of the drug.”
Unchartered waters
There’s also no accepted protocol beyond a typical six treatments over 2 or more weeks. This is relevant because the benefits of a series of treatments often fade away after a few weeks.
“Some patients describe the results lasting indefinitely, while most patients who respond to the treatments get to the point where they are going roughly 4-12 weeks with sustained results,” Dr. Wells said.
“When the effects start to wear off, they don’t crash,” said Dr. Abreu. Instead, he said, symptoms slowly reappear.
It’s typical for patients at Dr. Abreu’s clinic and others to return within a couple of months to go through another round of ketamine treatments. In some cases, “they continue to see us indefinitely to get them back up to where they need to be with a booster type of session,” he said.
Ketamine treatment costs vary widely, and insurers don’t cover this off-label treatment. The clinic operators quoted in this article reported a range of per-infusion costs from $350 (Dr. Markey’s clinic in Denver) to $675 (Dr. Abreu’s clinics in the Northwest).
“We have to have a talk with them: Can you afford this? This is going to take a significant amount of money every month to keep you well,” Dr. Abreu said. On the other hand, he said, the need for other medications goes away, eliminating that cost. (“They’re on [selective serotonin reputake inhibitors] usually,” he said, “but those drugs don’t work.”)
Nonpsychiatrists in forefront
At Dr. Markey’s clinic in Denver, all patients are required to see either her or a psychiatrist colleague. Some other ketamine clinics are run by psychiatrists, but that’s far from common.
Clinics often have no mental health professionals on staff and are run by anesthesiologists or other kinds of physicians.
Some clinic owners, Dr. Wells said, require patients to be under the care of a psychiatrist, neurologist, pain doctor, or other appropriate professional. “I do not intend, nor do I act, to displace psychiatrists, or the relationships our patients have with their psychiatrists, or the care they receive from their psychiatrists,” she said.
In the Northwest, Dr. Abreu said his patients take mood questionnaires, and he’s experimenting with a text-based mood monitoring system. In the Minneapolis area, anesthesiologist Dr. Simelgor is looking for a psychiatrist or psychiatrist partner for his ketamine clinic. “My thinking,” he said, “is that we need to work together.”
Still, there do not appear to be any requirements that ketamine clinic practitioners have connections to mental health professionals. Yet, as Dr. Sanacora put it: “Delivering the drug is the easiest part of the treatment. The hard part is managing the depression.”
Dr. Lieberman, Dr. Wells, Dr. Abreu, Dr. Simelgor, and Dr. Markey reported no relevant disclosures. Dr. Sanacora reported consulting fees and research contracts for multiple drug makers over the past 24 months. He holds shares in Biohaven Pharmaceuticals and is a coinventor on a U.S. patent (No. 8778979) on using glutamate agents to treat mental disorders held by Yale University.
Tc-325 effective for immediate GI tumor bleeding
The powder Tc-325 is effective for immediate hemostasis in patients with malignant gastrointestinal bleeding, according to results published in Gastrointestinal Endoscopy.
The compound achieved immediate hemostasis in 97.7% of patients with GI tumor bleeding, reported Alan Barkun, MD, of the division of gastroenterology at McGill University Health Centre, Montreal, and his coauthors. Conventional endoscopic hemostatic methods, by contrast, have shown highly variable hemostasis rates in prior studies, ranging from 31% to 93%, the authors said.
“Tc-325 seems to be more predictably effective in providing initial hemostasis in upper GI tumor bleeding compared with conventional methods,” they added.
The study included 88 eligible patients who initially presented with bleeding either as a result of a primary GI tumor, or metastases to the upper or lower GI tract. Almost 60% had an upper GI cancer site. Twenty-five patients died before the end of the 30-day observation period.
The recurrent bleeding rate at 72 hours was 15%. Bleeding rates at 7, 14, and 30 days’ follow-up were 7%, 7.8%, and 1.9%, respectively.
Overall, 27.3% of patients experienced repeat bleeding within 30 days of Tc-325 treatment, all from upper GI sites. No recurrent bleeding occurred from lower GI lesions. Recurrent bleeding occurred in 38% patients who did not receive definite hemostasis within 30 days.
An international normalized ratio value greater than 1.3 was significantly associated with early recurrent bleeding in univariable analysis (P = .02; odds ratio, 5.08; 95% confidence interval, 1.33-19.33), as was an Eastern Cooperative Oncology Group (ECOG) score of at least 3 (P = .049; OR, 3.94; 95% CI, 1.01-15.38). Definite hemostatic treatment was associated with less recurrent bleeding (P = .009; OR, 0.15; 95% CI, 0.04-0.62).
Factors significantly associated with 6-month survival in multivariable analysis were an ECOG score of 0-2 (P = .001; hazard ratio, 0.14; 95% CI, 0.04-0.47); cancer stage 1-3 (P = .042; HR, 0.31; 95% CI, 0.10-0.96), and receiving definite hemostastic treatment (P = .002; HR, 0.24; 95% CI, 0.09-0.59), Dr. Barkun and his colleagues reported.
Although the results show promise for Tc-325 as an immediate treatment in the case of failed standard endoscopic hemostatic techniques or when definite hemostasis via radiation, surgery, and chemotherapy are unavailable, the long-term effects are comparable with conventional methods, at least in the upper GI tract, the authors said. Better results in the lower GI tract may be attributed to the presence of gastric juice in the upper GI tract, the investigators noted.
Limitations of the study include “its retrospective design with the possibility of missing information and selective data collection,” as well as the possibility of decreased generalizability of results, Dr. Barkun and coauthors wrote. Nevertheless, its immediate effectiveness at achieving hemostasis and prevention of early bleeding indicate that the results may still be “used with confidence as guidance for any physician managing such patients,” the authors said.
The investigators did not disclose any conflicts of interest. The study was funded by the Grant for International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund.
SOURCE: Pittayanon R et al. Gastrointest Endosc. 2017 Nov 17. doi: 10.1016/j.gie.2017.11.013.
The powder Tc-325 is effective for immediate hemostasis in patients with malignant gastrointestinal bleeding, according to results published in Gastrointestinal Endoscopy.
The compound achieved immediate hemostasis in 97.7% of patients with GI tumor bleeding, reported Alan Barkun, MD, of the division of gastroenterology at McGill University Health Centre, Montreal, and his coauthors. Conventional endoscopic hemostatic methods, by contrast, have shown highly variable hemostasis rates in prior studies, ranging from 31% to 93%, the authors said.
“Tc-325 seems to be more predictably effective in providing initial hemostasis in upper GI tumor bleeding compared with conventional methods,” they added.
The study included 88 eligible patients who initially presented with bleeding either as a result of a primary GI tumor, or metastases to the upper or lower GI tract. Almost 60% had an upper GI cancer site. Twenty-five patients died before the end of the 30-day observation period.
The recurrent bleeding rate at 72 hours was 15%. Bleeding rates at 7, 14, and 30 days’ follow-up were 7%, 7.8%, and 1.9%, respectively.
Overall, 27.3% of patients experienced repeat bleeding within 30 days of Tc-325 treatment, all from upper GI sites. No recurrent bleeding occurred from lower GI lesions. Recurrent bleeding occurred in 38% patients who did not receive definite hemostasis within 30 days.
An international normalized ratio value greater than 1.3 was significantly associated with early recurrent bleeding in univariable analysis (P = .02; odds ratio, 5.08; 95% confidence interval, 1.33-19.33), as was an Eastern Cooperative Oncology Group (ECOG) score of at least 3 (P = .049; OR, 3.94; 95% CI, 1.01-15.38). Definite hemostatic treatment was associated with less recurrent bleeding (P = .009; OR, 0.15; 95% CI, 0.04-0.62).
Factors significantly associated with 6-month survival in multivariable analysis were an ECOG score of 0-2 (P = .001; hazard ratio, 0.14; 95% CI, 0.04-0.47); cancer stage 1-3 (P = .042; HR, 0.31; 95% CI, 0.10-0.96), and receiving definite hemostastic treatment (P = .002; HR, 0.24; 95% CI, 0.09-0.59), Dr. Barkun and his colleagues reported.
Although the results show promise for Tc-325 as an immediate treatment in the case of failed standard endoscopic hemostatic techniques or when definite hemostasis via radiation, surgery, and chemotherapy are unavailable, the long-term effects are comparable with conventional methods, at least in the upper GI tract, the authors said. Better results in the lower GI tract may be attributed to the presence of gastric juice in the upper GI tract, the investigators noted.
Limitations of the study include “its retrospective design with the possibility of missing information and selective data collection,” as well as the possibility of decreased generalizability of results, Dr. Barkun and coauthors wrote. Nevertheless, its immediate effectiveness at achieving hemostasis and prevention of early bleeding indicate that the results may still be “used with confidence as guidance for any physician managing such patients,” the authors said.
The investigators did not disclose any conflicts of interest. The study was funded by the Grant for International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund.
SOURCE: Pittayanon R et al. Gastrointest Endosc. 2017 Nov 17. doi: 10.1016/j.gie.2017.11.013.
The powder Tc-325 is effective for immediate hemostasis in patients with malignant gastrointestinal bleeding, according to results published in Gastrointestinal Endoscopy.
The compound achieved immediate hemostasis in 97.7% of patients with GI tumor bleeding, reported Alan Barkun, MD, of the division of gastroenterology at McGill University Health Centre, Montreal, and his coauthors. Conventional endoscopic hemostatic methods, by contrast, have shown highly variable hemostasis rates in prior studies, ranging from 31% to 93%, the authors said.
“Tc-325 seems to be more predictably effective in providing initial hemostasis in upper GI tumor bleeding compared with conventional methods,” they added.
The study included 88 eligible patients who initially presented with bleeding either as a result of a primary GI tumor, or metastases to the upper or lower GI tract. Almost 60% had an upper GI cancer site. Twenty-five patients died before the end of the 30-day observation period.
The recurrent bleeding rate at 72 hours was 15%. Bleeding rates at 7, 14, and 30 days’ follow-up were 7%, 7.8%, and 1.9%, respectively.
Overall, 27.3% of patients experienced repeat bleeding within 30 days of Tc-325 treatment, all from upper GI sites. No recurrent bleeding occurred from lower GI lesions. Recurrent bleeding occurred in 38% patients who did not receive definite hemostasis within 30 days.
An international normalized ratio value greater than 1.3 was significantly associated with early recurrent bleeding in univariable analysis (P = .02; odds ratio, 5.08; 95% confidence interval, 1.33-19.33), as was an Eastern Cooperative Oncology Group (ECOG) score of at least 3 (P = .049; OR, 3.94; 95% CI, 1.01-15.38). Definite hemostatic treatment was associated with less recurrent bleeding (P = .009; OR, 0.15; 95% CI, 0.04-0.62).
Factors significantly associated with 6-month survival in multivariable analysis were an ECOG score of 0-2 (P = .001; hazard ratio, 0.14; 95% CI, 0.04-0.47); cancer stage 1-3 (P = .042; HR, 0.31; 95% CI, 0.10-0.96), and receiving definite hemostastic treatment (P = .002; HR, 0.24; 95% CI, 0.09-0.59), Dr. Barkun and his colleagues reported.
Although the results show promise for Tc-325 as an immediate treatment in the case of failed standard endoscopic hemostatic techniques or when definite hemostasis via radiation, surgery, and chemotherapy are unavailable, the long-term effects are comparable with conventional methods, at least in the upper GI tract, the authors said. Better results in the lower GI tract may be attributed to the presence of gastric juice in the upper GI tract, the investigators noted.
Limitations of the study include “its retrospective design with the possibility of missing information and selective data collection,” as well as the possibility of decreased generalizability of results, Dr. Barkun and coauthors wrote. Nevertheless, its immediate effectiveness at achieving hemostasis and prevention of early bleeding indicate that the results may still be “used with confidence as guidance for any physician managing such patients,” the authors said.
The investigators did not disclose any conflicts of interest. The study was funded by the Grant for International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund.
SOURCE: Pittayanon R et al. Gastrointest Endosc. 2017 Nov 17. doi: 10.1016/j.gie.2017.11.013.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: Tc-325 is promising for initial hemostasis in patients with gastrointestinal tumor bleeding.
Major finding: Tc-325 achieved immediate hemostasis in 97.7% of patients with bleeding from GI tumors.
Data source: A multicenter retrospective study of 88 eligible patients with GI tumor-related hemorrhage from 2011 to 2016.
Disclosures: The authors did not disclose any conflicts of interest. The study was funded by the Grant for International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund.
Source: Pittayanon R et al. Gastrointest Endosc. 2017 Nov 17. doi: 10.1016/j.gie.2017.11.013.
Hyperbaric oxygen may cut CO deaths
In patients with carbon monoxide poisoning, hyperbaric oxygen therapy was associated with a lower rate of mortality, according to results of a recent retrospective study.
The mortality reduction was particularly evident among patients under 20 years of age and in patients with acute respiratory failure, authors of the study said in a report published in Chest (2017 Nov. doi: 10.1016/j.chest.2017.03.049).
“The results provide important references for decision making in the treatment of carbon monoxide poisoning,” Chien-Cheng Huang, MD, department of emergency medicine, Chi-Mei Medical Center, Tainan, Taiwan, and colleagues said in their report.
While hyperbaric oxygen has been suggested for severe carbon monoxide poisoning, 100% normobaric oxygen is considered standard treatment, according to Dr. Huang and colleagues.
“There has been no consensus about whether hyperbaric oxygen therapy is better than 100% normobaric oxygen alone, or the number of sessions of hyperbaric oxygen therapy that are necessary regarding mortality and morbidity,” they wrote.
In a Taiwanese nationwide poisoning database, Dr. Huang and colleagues identified 25,737 patients diagnosed with carbon monoxide poisoning between 1999 and 2012. Of those patients, 7,278 had hyperbaric oxygen therapy.
After researchers adjusted for variables including age, sex, and underlying comorbidities, the mortality rate was lower in patients who underwent hyperbaric oxygen therapy, compared with those who did not (adjusted hazard ratio, 0.74; 95% confidence interval, 0.67-0.81), data show.
The reduction in mortality was especially notable in patients younger than age 20 years (adjusted HR, 0.45; 95% CI, 0.26-0.80), according to the researchers.
A similarly greater magnitude of mortality benefit also was found for patients who had acute respiratory failure, “which supports acute respiratory failure being an indication for hyperbaric oxygen therapy,” investigators wrote. “Further studies are warranted to clarify this issue.”
The number of hyperbaric oxygen therapy sessions appeared to make a difference in mortality. Patients who received two or more sessions had a lower rate of mortality than did those who had only one session, according to the report.
Predictors of mortality, described in more detail in the published report, included older age, diabetes, alcoholism, and suicide attempts, among other factors.
“In addition to considering hyperbaric oxygen therapy for reducing mortality, control of other concomitant mortality predictors is necessary,” the authors concluded based on their results.
Accidental deaths from carbon monoxide poisoning also are a major issue in the United States, where each year, there are an estimated 1,000-2,000 cases, according to the authors. Additionally, accidental carbon monoxide poisoning has “increased greatly in the past 10 years,” they said in the report.
Other studies have shown that, compared with normobaric oxygen, hyperbaric oxygen therapy did not reduce neurologic complications, the authors noted. Even so, that fact “does not suggest that hyperbaric oxygen therapy is not beneficial regarding mortality,” they wrote. “In fact, it is possible that reducing mortality may increase morbidities such as neurologic sequelae.”
Dr. Huang and coauthors reported no conflicts of interest related to the study, which was supported by Chi-Mei Medical Center in Taiwan.
Use of hyperbaric oxygen therapy to treat carbon monoxide poisioning has been “controversial since its inception” since early promoters “tended to place hyperbaric treatment ahead of strong supporting data,” wrote Clayton T. Cowl, MD, FCCP, in an editorial regarding the study by Dr. Huang and colleagues.
These data are compelling because they come from what is believed to be the first large-scale study that specifically examines mortality as an endpoint in an entire nation, as opposed to smaller cohorts in single centers or even multiple institutions, he said in his editorial.
“Have we reached the point of clearly establishing that delivery of pure oxygen in a high-pressure environment is more effective in treating patients who have carbon monoxide poisoning than is normobaric supplemental oxygen alone? Probably not,” Dr. Cowl wrote.
“The retrospective database study by Huang et al, despite its large size and interesting findings, remains distant from the ideal of a large blinded multicenter randomized controlled trial using a standardized protocol to compare normobaric supplemental oxygenation with hyperbaric oxygen therapy delivery for this cohort,” he explained. “However, its size, scale, and findings add credibility to the mounting data supporting HBOT for this indication.”
Clayton T. Cowl, MD, FCCP, is with the division of preventive, occupational, and aerospace medicine and the division of pulmonary and critical care medicine, Mayo Clinic. His comments came from an editorial in Chest (doi: 10.1016/j.chest.2017.07.022) He declared no financial or nonfinancial disclosures related to the editorial.
Use of hyperbaric oxygen therapy to treat carbon monoxide poisioning has been “controversial since its inception” since early promoters “tended to place hyperbaric treatment ahead of strong supporting data,” wrote Clayton T. Cowl, MD, FCCP, in an editorial regarding the study by Dr. Huang and colleagues.
These data are compelling because they come from what is believed to be the first large-scale study that specifically examines mortality as an endpoint in an entire nation, as opposed to smaller cohorts in single centers or even multiple institutions, he said in his editorial.
“Have we reached the point of clearly establishing that delivery of pure oxygen in a high-pressure environment is more effective in treating patients who have carbon monoxide poisoning than is normobaric supplemental oxygen alone? Probably not,” Dr. Cowl wrote.
“The retrospective database study by Huang et al, despite its large size and interesting findings, remains distant from the ideal of a large blinded multicenter randomized controlled trial using a standardized protocol to compare normobaric supplemental oxygenation with hyperbaric oxygen therapy delivery for this cohort,” he explained. “However, its size, scale, and findings add credibility to the mounting data supporting HBOT for this indication.”
Clayton T. Cowl, MD, FCCP, is with the division of preventive, occupational, and aerospace medicine and the division of pulmonary and critical care medicine, Mayo Clinic. His comments came from an editorial in Chest (doi: 10.1016/j.chest.2017.07.022) He declared no financial or nonfinancial disclosures related to the editorial.
Use of hyperbaric oxygen therapy to treat carbon monoxide poisioning has been “controversial since its inception” since early promoters “tended to place hyperbaric treatment ahead of strong supporting data,” wrote Clayton T. Cowl, MD, FCCP, in an editorial regarding the study by Dr. Huang and colleagues.
These data are compelling because they come from what is believed to be the first large-scale study that specifically examines mortality as an endpoint in an entire nation, as opposed to smaller cohorts in single centers or even multiple institutions, he said in his editorial.
“Have we reached the point of clearly establishing that delivery of pure oxygen in a high-pressure environment is more effective in treating patients who have carbon monoxide poisoning than is normobaric supplemental oxygen alone? Probably not,” Dr. Cowl wrote.
“The retrospective database study by Huang et al, despite its large size and interesting findings, remains distant from the ideal of a large blinded multicenter randomized controlled trial using a standardized protocol to compare normobaric supplemental oxygenation with hyperbaric oxygen therapy delivery for this cohort,” he explained. “However, its size, scale, and findings add credibility to the mounting data supporting HBOT for this indication.”
Clayton T. Cowl, MD, FCCP, is with the division of preventive, occupational, and aerospace medicine and the division of pulmonary and critical care medicine, Mayo Clinic. His comments came from an editorial in Chest (doi: 10.1016/j.chest.2017.07.022) He declared no financial or nonfinancial disclosures related to the editorial.
In patients with carbon monoxide poisoning, hyperbaric oxygen therapy was associated with a lower rate of mortality, according to results of a recent retrospective study.
The mortality reduction was particularly evident among patients under 20 years of age and in patients with acute respiratory failure, authors of the study said in a report published in Chest (2017 Nov. doi: 10.1016/j.chest.2017.03.049).
“The results provide important references for decision making in the treatment of carbon monoxide poisoning,” Chien-Cheng Huang, MD, department of emergency medicine, Chi-Mei Medical Center, Tainan, Taiwan, and colleagues said in their report.
While hyperbaric oxygen has been suggested for severe carbon monoxide poisoning, 100% normobaric oxygen is considered standard treatment, according to Dr. Huang and colleagues.
“There has been no consensus about whether hyperbaric oxygen therapy is better than 100% normobaric oxygen alone, or the number of sessions of hyperbaric oxygen therapy that are necessary regarding mortality and morbidity,” they wrote.
In a Taiwanese nationwide poisoning database, Dr. Huang and colleagues identified 25,737 patients diagnosed with carbon monoxide poisoning between 1999 and 2012. Of those patients, 7,278 had hyperbaric oxygen therapy.
After researchers adjusted for variables including age, sex, and underlying comorbidities, the mortality rate was lower in patients who underwent hyperbaric oxygen therapy, compared with those who did not (adjusted hazard ratio, 0.74; 95% confidence interval, 0.67-0.81), data show.
The reduction in mortality was especially notable in patients younger than age 20 years (adjusted HR, 0.45; 95% CI, 0.26-0.80), according to the researchers.
A similarly greater magnitude of mortality benefit also was found for patients who had acute respiratory failure, “which supports acute respiratory failure being an indication for hyperbaric oxygen therapy,” investigators wrote. “Further studies are warranted to clarify this issue.”
The number of hyperbaric oxygen therapy sessions appeared to make a difference in mortality. Patients who received two or more sessions had a lower rate of mortality than did those who had only one session, according to the report.
Predictors of mortality, described in more detail in the published report, included older age, diabetes, alcoholism, and suicide attempts, among other factors.
“In addition to considering hyperbaric oxygen therapy for reducing mortality, control of other concomitant mortality predictors is necessary,” the authors concluded based on their results.
Accidental deaths from carbon monoxide poisoning also are a major issue in the United States, where each year, there are an estimated 1,000-2,000 cases, according to the authors. Additionally, accidental carbon monoxide poisoning has “increased greatly in the past 10 years,” they said in the report.
Other studies have shown that, compared with normobaric oxygen, hyperbaric oxygen therapy did not reduce neurologic complications, the authors noted. Even so, that fact “does not suggest that hyperbaric oxygen therapy is not beneficial regarding mortality,” they wrote. “In fact, it is possible that reducing mortality may increase morbidities such as neurologic sequelae.”
Dr. Huang and coauthors reported no conflicts of interest related to the study, which was supported by Chi-Mei Medical Center in Taiwan.
In patients with carbon monoxide poisoning, hyperbaric oxygen therapy was associated with a lower rate of mortality, according to results of a recent retrospective study.
The mortality reduction was particularly evident among patients under 20 years of age and in patients with acute respiratory failure, authors of the study said in a report published in Chest (2017 Nov. doi: 10.1016/j.chest.2017.03.049).
“The results provide important references for decision making in the treatment of carbon monoxide poisoning,” Chien-Cheng Huang, MD, department of emergency medicine, Chi-Mei Medical Center, Tainan, Taiwan, and colleagues said in their report.
While hyperbaric oxygen has been suggested for severe carbon monoxide poisoning, 100% normobaric oxygen is considered standard treatment, according to Dr. Huang and colleagues.
“There has been no consensus about whether hyperbaric oxygen therapy is better than 100% normobaric oxygen alone, or the number of sessions of hyperbaric oxygen therapy that are necessary regarding mortality and morbidity,” they wrote.
In a Taiwanese nationwide poisoning database, Dr. Huang and colleagues identified 25,737 patients diagnosed with carbon monoxide poisoning between 1999 and 2012. Of those patients, 7,278 had hyperbaric oxygen therapy.
After researchers adjusted for variables including age, sex, and underlying comorbidities, the mortality rate was lower in patients who underwent hyperbaric oxygen therapy, compared with those who did not (adjusted hazard ratio, 0.74; 95% confidence interval, 0.67-0.81), data show.
The reduction in mortality was especially notable in patients younger than age 20 years (adjusted HR, 0.45; 95% CI, 0.26-0.80), according to the researchers.
A similarly greater magnitude of mortality benefit also was found for patients who had acute respiratory failure, “which supports acute respiratory failure being an indication for hyperbaric oxygen therapy,” investigators wrote. “Further studies are warranted to clarify this issue.”
The number of hyperbaric oxygen therapy sessions appeared to make a difference in mortality. Patients who received two or more sessions had a lower rate of mortality than did those who had only one session, according to the report.
Predictors of mortality, described in more detail in the published report, included older age, diabetes, alcoholism, and suicide attempts, among other factors.
“In addition to considering hyperbaric oxygen therapy for reducing mortality, control of other concomitant mortality predictors is necessary,” the authors concluded based on their results.
Accidental deaths from carbon monoxide poisoning also are a major issue in the United States, where each year, there are an estimated 1,000-2,000 cases, according to the authors. Additionally, accidental carbon monoxide poisoning has “increased greatly in the past 10 years,” they said in the report.
Other studies have shown that, compared with normobaric oxygen, hyperbaric oxygen therapy did not reduce neurologic complications, the authors noted. Even so, that fact “does not suggest that hyperbaric oxygen therapy is not beneficial regarding mortality,” they wrote. “In fact, it is possible that reducing mortality may increase morbidities such as neurologic sequelae.”
Dr. Huang and coauthors reported no conflicts of interest related to the study, which was supported by Chi-Mei Medical Center in Taiwan.
FROM CHEST
Key clinical point: In patients with carbon monoxide poisoning, hyperbaric oxygen therapy was associated with a lower rate of mortality.
Major finding: The mortality rate was lower in patients who received hyperbaric oxygen therapy, compared with those who did not (adjusted HR, 0.74; 95% CI, 0.67-0.81).
Data source: A retrospective nationwide population-based study from Taiwan including 25,737 individuals diagnosed with carbon monoxide poisoning during 1999-2012.
Disclosures: Chi-Mei Medical Center in Taiwan supported the study. The authors reported no conflicts of interest.
Adherence to Psychotherapy May Improve Outcomes in Patients With Psychogenic Nonepileptic Seizures
WASHINGTON, DC—Adherence to psychotherapy is associated with a 50% or greater reduction in psychogenic nonepileptic seizures (PNES), improved quality of life, and decreased emergency department visits, according to research presented at the 71st Annual Meeting of the American Epilepsy Society.
Twenty-five percent to 40% of patients in epilepsy monitoring units are ultimately diagnosed with PNES. Research has indicated that psychotherapy reduces seizure frequency and improves quality of life in patients with PNES, but 20% to 30% of patients fail to attend their first appointment after diagnosis.
An Obstacle to Treatment
To determine whether nonadherence to psychotherapy may affect patient outcomes, Benjamin Tolchin, MD, Assistant Professor of Neurology at Yale School of Medicine in New Haven, Connecticut, and colleagues followed 105 consecutive patients with video-EEG confirmed PNES at Brigham and Women’s Hospital in Boston from March 2013 to August 2015. At baseline, researchers assessed patients’ psychiatric comorbidities and abuse history in a semistructured neuropsychiatric interview.
Following diagnosis, participants were referred for psychotherapy at Brigham and Women’s Hospital or with a local community therapist. Psychotherapy at Brigham and Women’s Hospital included a manualized regimen of 12 weekly one-hour sessions. In addition, a neuropsychiatrist called all local therapists to review the diagnosis and principles of treatment.
The researchers defined adherence to psychotherapy as attending at least eight sessions over the course of 16 weeks. The primary outcome was the proportion of participants with a 50% or greater reduction in PNES frequency at a 12- to 24-month telephone follow-up. Secondary outcomes included the rate of seizure freedom, change in quality of life, and change in number of monthly emergency department visits, which were assessed during the telephone follow-up and with medical chart review. Researchers used Fischer’s exact test and Student’s t-test to assess the correlation of outcomes with adherence to psychotherapy.
At follow-up, 84% of patients who were adherent with treatment achieved a significant reduction in seizure frequency, compared with 61% of patients who were nonadherent. Patients who were adherent had an average 7.2-point improvement on the Quality of Life in Epilepsy-10, whereas patients who were nonadherent had a mean 2.8-point improvement. Mean change in monthly emergency department visits was significantly reduced among patients who were adherent to treatment, compared with patients who were not adherent. After adjusting for potential confounders, treatment adherence remained a significant predictor of 50% reduction in seizure frequency (odds ratio, 3.81). Overall, 60% of participants were nonadherent. Risk factors for nonadherence included self-identified minority status and history of childhood abuse.
“New interventions, such as motivational interviewing, are needed to improve adherence with psychotherapy among patients with PNES,” Dr. Tolchin said.
Motivational Interviewing
In a separate study, Dr. Tolchin and colleagues evaluated whether motivational interviewing improved treatment adherence and outcomes among 60 patients with video-EEG confirmed PNES. Patients who were randomized to receive a 30-minute motivational interviewing session before psychotherapy were more adherent to psychotherapy and had greater improvements in PNES frequency and quality of life, compared with patients randomized to psychotherapy alone. “Motivational interviewing-based interventions can improve the common problem of nonadherence with psychotherapy among patients diagnosed with PNES,” the researchers said. They added that this technique potentially could be delivered remotely to improve outcomes.
—Erica Tricarico
WASHINGTON, DC—Adherence to psychotherapy is associated with a 50% or greater reduction in psychogenic nonepileptic seizures (PNES), improved quality of life, and decreased emergency department visits, according to research presented at the 71st Annual Meeting of the American Epilepsy Society.
Twenty-five percent to 40% of patients in epilepsy monitoring units are ultimately diagnosed with PNES. Research has indicated that psychotherapy reduces seizure frequency and improves quality of life in patients with PNES, but 20% to 30% of patients fail to attend their first appointment after diagnosis.
An Obstacle to Treatment
To determine whether nonadherence to psychotherapy may affect patient outcomes, Benjamin Tolchin, MD, Assistant Professor of Neurology at Yale School of Medicine in New Haven, Connecticut, and colleagues followed 105 consecutive patients with video-EEG confirmed PNES at Brigham and Women’s Hospital in Boston from March 2013 to August 2015. At baseline, researchers assessed patients’ psychiatric comorbidities and abuse history in a semistructured neuropsychiatric interview.
Following diagnosis, participants were referred for psychotherapy at Brigham and Women’s Hospital or with a local community therapist. Psychotherapy at Brigham and Women’s Hospital included a manualized regimen of 12 weekly one-hour sessions. In addition, a neuropsychiatrist called all local therapists to review the diagnosis and principles of treatment.
The researchers defined adherence to psychotherapy as attending at least eight sessions over the course of 16 weeks. The primary outcome was the proportion of participants with a 50% or greater reduction in PNES frequency at a 12- to 24-month telephone follow-up. Secondary outcomes included the rate of seizure freedom, change in quality of life, and change in number of monthly emergency department visits, which were assessed during the telephone follow-up and with medical chart review. Researchers used Fischer’s exact test and Student’s t-test to assess the correlation of outcomes with adherence to psychotherapy.
At follow-up, 84% of patients who were adherent with treatment achieved a significant reduction in seizure frequency, compared with 61% of patients who were nonadherent. Patients who were adherent had an average 7.2-point improvement on the Quality of Life in Epilepsy-10, whereas patients who were nonadherent had a mean 2.8-point improvement. Mean change in monthly emergency department visits was significantly reduced among patients who were adherent to treatment, compared with patients who were not adherent. After adjusting for potential confounders, treatment adherence remained a significant predictor of 50% reduction in seizure frequency (odds ratio, 3.81). Overall, 60% of participants were nonadherent. Risk factors for nonadherence included self-identified minority status and history of childhood abuse.
“New interventions, such as motivational interviewing, are needed to improve adherence with psychotherapy among patients with PNES,” Dr. Tolchin said.
Motivational Interviewing
In a separate study, Dr. Tolchin and colleagues evaluated whether motivational interviewing improved treatment adherence and outcomes among 60 patients with video-EEG confirmed PNES. Patients who were randomized to receive a 30-minute motivational interviewing session before psychotherapy were more adherent to psychotherapy and had greater improvements in PNES frequency and quality of life, compared with patients randomized to psychotherapy alone. “Motivational interviewing-based interventions can improve the common problem of nonadherence with psychotherapy among patients diagnosed with PNES,” the researchers said. They added that this technique potentially could be delivered remotely to improve outcomes.
—Erica Tricarico
WASHINGTON, DC—Adherence to psychotherapy is associated with a 50% or greater reduction in psychogenic nonepileptic seizures (PNES), improved quality of life, and decreased emergency department visits, according to research presented at the 71st Annual Meeting of the American Epilepsy Society.
Twenty-five percent to 40% of patients in epilepsy monitoring units are ultimately diagnosed with PNES. Research has indicated that psychotherapy reduces seizure frequency and improves quality of life in patients with PNES, but 20% to 30% of patients fail to attend their first appointment after diagnosis.
An Obstacle to Treatment
To determine whether nonadherence to psychotherapy may affect patient outcomes, Benjamin Tolchin, MD, Assistant Professor of Neurology at Yale School of Medicine in New Haven, Connecticut, and colleagues followed 105 consecutive patients with video-EEG confirmed PNES at Brigham and Women’s Hospital in Boston from March 2013 to August 2015. At baseline, researchers assessed patients’ psychiatric comorbidities and abuse history in a semistructured neuropsychiatric interview.
Following diagnosis, participants were referred for psychotherapy at Brigham and Women’s Hospital or with a local community therapist. Psychotherapy at Brigham and Women’s Hospital included a manualized regimen of 12 weekly one-hour sessions. In addition, a neuropsychiatrist called all local therapists to review the diagnosis and principles of treatment.
The researchers defined adherence to psychotherapy as attending at least eight sessions over the course of 16 weeks. The primary outcome was the proportion of participants with a 50% or greater reduction in PNES frequency at a 12- to 24-month telephone follow-up. Secondary outcomes included the rate of seizure freedom, change in quality of life, and change in number of monthly emergency department visits, which were assessed during the telephone follow-up and with medical chart review. Researchers used Fischer’s exact test and Student’s t-test to assess the correlation of outcomes with adherence to psychotherapy.
At follow-up, 84% of patients who were adherent with treatment achieved a significant reduction in seizure frequency, compared with 61% of patients who were nonadherent. Patients who were adherent had an average 7.2-point improvement on the Quality of Life in Epilepsy-10, whereas patients who were nonadherent had a mean 2.8-point improvement. Mean change in monthly emergency department visits was significantly reduced among patients who were adherent to treatment, compared with patients who were not adherent. After adjusting for potential confounders, treatment adherence remained a significant predictor of 50% reduction in seizure frequency (odds ratio, 3.81). Overall, 60% of participants were nonadherent. Risk factors for nonadherence included self-identified minority status and history of childhood abuse.
“New interventions, such as motivational interviewing, are needed to improve adherence with psychotherapy among patients with PNES,” Dr. Tolchin said.
Motivational Interviewing
In a separate study, Dr. Tolchin and colleagues evaluated whether motivational interviewing improved treatment adherence and outcomes among 60 patients with video-EEG confirmed PNES. Patients who were randomized to receive a 30-minute motivational interviewing session before psychotherapy were more adherent to psychotherapy and had greater improvements in PNES frequency and quality of life, compared with patients randomized to psychotherapy alone. “Motivational interviewing-based interventions can improve the common problem of nonadherence with psychotherapy among patients diagnosed with PNES,” the researchers said. They added that this technique potentially could be delivered remotely to improve outcomes.
—Erica Tricarico
Dermatologists paying for errors during procedures
according to a review of over 90,000 lawsuits filed from 2006 to 2015.
Data from the Physician Insurers Association of America Data Sharing Project registry show that dermatologists paid 102 claims for errors that occurred during a procedure over that period. Misdiagnosis, with 62 payments, was the next most common reason, followed by medication errors (23 payments). Failure to supervise or monitor case, failure to instruct or communicate with patients, and failure to recognize a complication of treatment each had 15 payments, Heather Kornmehl and her associates reported (JAMA Dermatol. 2017 Dec 6. doi: 10.1001/jamadermatol.2017.3713).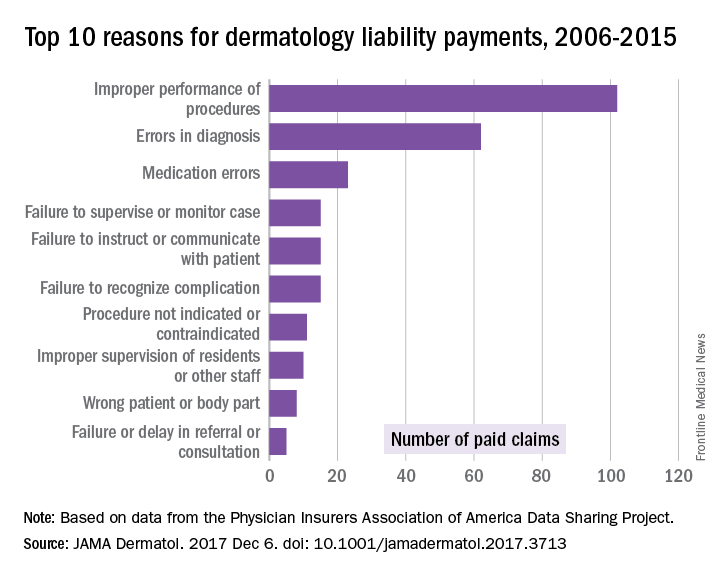
From 2006 to 2015, the total cost of the 281 claims paid by dermatologists was $71.8 million, and dermatologists were involved in 1,084 (1.2%) of the 90,743 closed claims against physicians in all specialties, said Ms. Kornwehl, who is a medical student at Drexel University, Philadelphia, and her associates.
Closed claims include any lawsuit that is subsequently abandoned, withdrawn, or dismissed; is settled; or goes to trial and results in a verdict.
One of Ms. Kornmehl’s coinvestigators has served as an investigator and/or advisor to a number of companies. No other disclosures were reported.
SOURCE: Kornmehl H et al. JAMA Dermatol. 2017 Dec 6. doi: 10.1001/jamadermatol.2017.3713
according to a review of over 90,000 lawsuits filed from 2006 to 2015.
Data from the Physician Insurers Association of America Data Sharing Project registry show that dermatologists paid 102 claims for errors that occurred during a procedure over that period. Misdiagnosis, with 62 payments, was the next most common reason, followed by medication errors (23 payments). Failure to supervise or monitor case, failure to instruct or communicate with patients, and failure to recognize a complication of treatment each had 15 payments, Heather Kornmehl and her associates reported (JAMA Dermatol. 2017 Dec 6. doi: 10.1001/jamadermatol.2017.3713).
From 2006 to 2015, the total cost of the 281 claims paid by dermatologists was $71.8 million, and dermatologists were involved in 1,084 (1.2%) of the 90,743 closed claims against physicians in all specialties, said Ms. Kornwehl, who is a medical student at Drexel University, Philadelphia, and her associates.
Closed claims include any lawsuit that is subsequently abandoned, withdrawn, or dismissed; is settled; or goes to trial and results in a verdict.
One of Ms. Kornmehl’s coinvestigators has served as an investigator and/or advisor to a number of companies. No other disclosures were reported.
SOURCE: Kornmehl H et al. JAMA Dermatol. 2017 Dec 6. doi: 10.1001/jamadermatol.2017.3713
according to a review of over 90,000 lawsuits filed from 2006 to 2015.
Data from the Physician Insurers Association of America Data Sharing Project registry show that dermatologists paid 102 claims for errors that occurred during a procedure over that period. Misdiagnosis, with 62 payments, was the next most common reason, followed by medication errors (23 payments). Failure to supervise or monitor case, failure to instruct or communicate with patients, and failure to recognize a complication of treatment each had 15 payments, Heather Kornmehl and her associates reported (JAMA Dermatol. 2017 Dec 6. doi: 10.1001/jamadermatol.2017.3713).
From 2006 to 2015, the total cost of the 281 claims paid by dermatologists was $71.8 million, and dermatologists were involved in 1,084 (1.2%) of the 90,743 closed claims against physicians in all specialties, said Ms. Kornwehl, who is a medical student at Drexel University, Philadelphia, and her associates.
Closed claims include any lawsuit that is subsequently abandoned, withdrawn, or dismissed; is settled; or goes to trial and results in a verdict.
One of Ms. Kornmehl’s coinvestigators has served as an investigator and/or advisor to a number of companies. No other disclosures were reported.
SOURCE: Kornmehl H et al. JAMA Dermatol. 2017 Dec 6. doi: 10.1001/jamadermatol.2017.3713
FROM JAMA DERMATOLOGY
Vaccine exemptions more common in western states
For the 2016-2017 school year, 2% of American kindergarten students had an exemption from one or more vaccines, said Ranee Seither and associates at the National Center of Immunization and Respiratory Disease, Centers for Disease Control and Prevention, Atlanta.
Among the 46 states – including the District of Columbia – that reported data, nine times as many exemptions were granted on religious or philosophical grounds (1.8%) as were granted for medical reasons (0.2%), they said.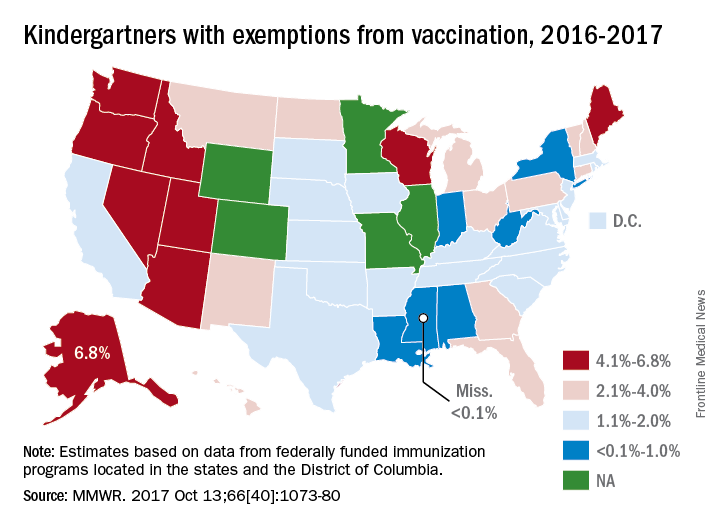
Alaska had the highest rate of medical exemptions at 1.5% and Oregon had the highest rate of religious/philosophical exemptions at 6.5%. Thirty states do not allow philosophical exemptions, Arizona and Mississippi do not allow religious exemptions, and West Virginia does not allow either, they noted.
Exemption data were reported for 3,666,870 kindergartners for the 2016-2017 school year and collected by federally funded immunization programs in the 50 states and D.C.
For the 2016-2017 school year, 2% of American kindergarten students had an exemption from one or more vaccines, said Ranee Seither and associates at the National Center of Immunization and Respiratory Disease, Centers for Disease Control and Prevention, Atlanta.
Among the 46 states – including the District of Columbia – that reported data, nine times as many exemptions were granted on religious or philosophical grounds (1.8%) as were granted for medical reasons (0.2%), they said.
Alaska had the highest rate of medical exemptions at 1.5% and Oregon had the highest rate of religious/philosophical exemptions at 6.5%. Thirty states do not allow philosophical exemptions, Arizona and Mississippi do not allow religious exemptions, and West Virginia does not allow either, they noted.
Exemption data were reported for 3,666,870 kindergartners for the 2016-2017 school year and collected by federally funded immunization programs in the 50 states and D.C.
For the 2016-2017 school year, 2% of American kindergarten students had an exemption from one or more vaccines, said Ranee Seither and associates at the National Center of Immunization and Respiratory Disease, Centers for Disease Control and Prevention, Atlanta.
Among the 46 states – including the District of Columbia – that reported data, nine times as many exemptions were granted on religious or philosophical grounds (1.8%) as were granted for medical reasons (0.2%), they said.
Alaska had the highest rate of medical exemptions at 1.5% and Oregon had the highest rate of religious/philosophical exemptions at 6.5%. Thirty states do not allow philosophical exemptions, Arizona and Mississippi do not allow religious exemptions, and West Virginia does not allow either, they noted.
Exemption data were reported for 3,666,870 kindergartners for the 2016-2017 school year and collected by federally funded immunization programs in the 50 states and D.C.
FROM MMWR
National Academy of Medicine should revisit issue of fetal alcohol exposure
More than 20 years ago the Institute of Medicine (recently renamed the National Academy of Medicine, or NAM) issued its landmark report on fetal alcohol syndrome. Since then, there has been an explosion of research on the issue of fetal alcohol exposure – and NAM needs to revisit the issue and release another report.
Unfortunately, too few physicians and not enough people in the larger society understand public health and that the health status of the unfortunate among us affects the health status of the most fortunate of us. In short, low-income people are the proverbial “canary in the coal mine.”
Accordingly, solving the health care problems of low-income people would solve the health care problems of the middle and upper class. Consider where the United States would be had we paid attention to the opioid epidemic in low-income communities instead of waiting until it spread into everyone’s “safe” communities. We would have tried and tested solutions to the problem as it currently exists.
Another reason NAM needs to revisit FASD – currently proposed to be called neurobehavioral disorders associated with prenatal alcohol exposure in the DSM-5 – are the new findings that link FASD to seizure disorders and other neurodevelopmental disorders of childhood, such as intellectual disability, attention-deficit/hyperactivity disorder, speech and language disorders, motor disorders, specific learning disorders, and autism. In fact, new research is emerging from Robert Freedman, MD, and his team at the University of Colorado Denver, to suggest that preventing choline deficiency in pregnancy (the mechanism producing neurodevelopmental defects in FASD) may not only prevent neurodevelopmental disorders of childhood but also schizophrenia. Further, it has become abundantly clear that choline deficiency (often generated by FASD), is responsible for affect dysregulation, which is a common thread in various forms of violence and suicide.
A recent report highlighted the findings that inmates incarcerated in Mexican prisons have high rates of intellectual disability, and we know that FASD is one of the leading causes of this problem, but we are not screening for it in our juvenile detention centers, jails, or prisons. Consequently, we need to look for the prevalence of FASD in special education as well as in foster care, because these services often feed our correctional institutions. There also is some recent animal evidence suggesting that sufficient prenatal choline during pregnancy may be protective against Alzheimer’s disease – soon to be an even greater public problem in the United States. Lastly, the neuroscience findings regarding FASD also are emerging.
Hence, there is substantial information growing in various, different but overlapping areas of our systems addressing the nation’s public health and well-being. One of the most respected sources of credible science in America is the National Academy Science. The NAM should convene a meeting of the experts to examine the current state of FASD knowledge. If there is sufficient new information, the NAM needs to develop a new report on FASD. It has been 21 years since the first FAS report from the Institute of Medicine and it needs to be revisited. But it appears that the correctional, child protective services, special education, and mental health fields are not aware of the breadth of available research and its importance to the nation’s public health. Determining how fetal alcohol exposure/choline deficiency affects children and adults in special education, foster care, juvenile and adult corrections systems – along with such other social issues as prematurity, disability, unemployment, homelessness, suicide, violence, and mental health – is critical to our nation’s future.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital Family Medicine Clinic in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
More than 20 years ago the Institute of Medicine (recently renamed the National Academy of Medicine, or NAM) issued its landmark report on fetal alcohol syndrome. Since then, there has been an explosion of research on the issue of fetal alcohol exposure – and NAM needs to revisit the issue and release another report.
Unfortunately, too few physicians and not enough people in the larger society understand public health and that the health status of the unfortunate among us affects the health status of the most fortunate of us. In short, low-income people are the proverbial “canary in the coal mine.”
Accordingly, solving the health care problems of low-income people would solve the health care problems of the middle and upper class. Consider where the United States would be had we paid attention to the opioid epidemic in low-income communities instead of waiting until it spread into everyone’s “safe” communities. We would have tried and tested solutions to the problem as it currently exists.
Another reason NAM needs to revisit FASD – currently proposed to be called neurobehavioral disorders associated with prenatal alcohol exposure in the DSM-5 – are the new findings that link FASD to seizure disorders and other neurodevelopmental disorders of childhood, such as intellectual disability, attention-deficit/hyperactivity disorder, speech and language disorders, motor disorders, specific learning disorders, and autism. In fact, new research is emerging from Robert Freedman, MD, and his team at the University of Colorado Denver, to suggest that preventing choline deficiency in pregnancy (the mechanism producing neurodevelopmental defects in FASD) may not only prevent neurodevelopmental disorders of childhood but also schizophrenia. Further, it has become abundantly clear that choline deficiency (often generated by FASD), is responsible for affect dysregulation, which is a common thread in various forms of violence and suicide.
A recent report highlighted the findings that inmates incarcerated in Mexican prisons have high rates of intellectual disability, and we know that FASD is one of the leading causes of this problem, but we are not screening for it in our juvenile detention centers, jails, or prisons. Consequently, we need to look for the prevalence of FASD in special education as well as in foster care, because these services often feed our correctional institutions. There also is some recent animal evidence suggesting that sufficient prenatal choline during pregnancy may be protective against Alzheimer’s disease – soon to be an even greater public problem in the United States. Lastly, the neuroscience findings regarding FASD also are emerging.
Hence, there is substantial information growing in various, different but overlapping areas of our systems addressing the nation’s public health and well-being. One of the most respected sources of credible science in America is the National Academy Science. The NAM should convene a meeting of the experts to examine the current state of FASD knowledge. If there is sufficient new information, the NAM needs to develop a new report on FASD. It has been 21 years since the first FAS report from the Institute of Medicine and it needs to be revisited. But it appears that the correctional, child protective services, special education, and mental health fields are not aware of the breadth of available research and its importance to the nation’s public health. Determining how fetal alcohol exposure/choline deficiency affects children and adults in special education, foster care, juvenile and adult corrections systems – along with such other social issues as prematurity, disability, unemployment, homelessness, suicide, violence, and mental health – is critical to our nation’s future.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital Family Medicine Clinic in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
More than 20 years ago the Institute of Medicine (recently renamed the National Academy of Medicine, or NAM) issued its landmark report on fetal alcohol syndrome. Since then, there has been an explosion of research on the issue of fetal alcohol exposure – and NAM needs to revisit the issue and release another report.
Unfortunately, too few physicians and not enough people in the larger society understand public health and that the health status of the unfortunate among us affects the health status of the most fortunate of us. In short, low-income people are the proverbial “canary in the coal mine.”
Accordingly, solving the health care problems of low-income people would solve the health care problems of the middle and upper class. Consider where the United States would be had we paid attention to the opioid epidemic in low-income communities instead of waiting until it spread into everyone’s “safe” communities. We would have tried and tested solutions to the problem as it currently exists.
Another reason NAM needs to revisit FASD – currently proposed to be called neurobehavioral disorders associated with prenatal alcohol exposure in the DSM-5 – are the new findings that link FASD to seizure disorders and other neurodevelopmental disorders of childhood, such as intellectual disability, attention-deficit/hyperactivity disorder, speech and language disorders, motor disorders, specific learning disorders, and autism. In fact, new research is emerging from Robert Freedman, MD, and his team at the University of Colorado Denver, to suggest that preventing choline deficiency in pregnancy (the mechanism producing neurodevelopmental defects in FASD) may not only prevent neurodevelopmental disorders of childhood but also schizophrenia. Further, it has become abundantly clear that choline deficiency (often generated by FASD), is responsible for affect dysregulation, which is a common thread in various forms of violence and suicide.
A recent report highlighted the findings that inmates incarcerated in Mexican prisons have high rates of intellectual disability, and we know that FASD is one of the leading causes of this problem, but we are not screening for it in our juvenile detention centers, jails, or prisons. Consequently, we need to look for the prevalence of FASD in special education as well as in foster care, because these services often feed our correctional institutions. There also is some recent animal evidence suggesting that sufficient prenatal choline during pregnancy may be protective against Alzheimer’s disease – soon to be an even greater public problem in the United States. Lastly, the neuroscience findings regarding FASD also are emerging.
Hence, there is substantial information growing in various, different but overlapping areas of our systems addressing the nation’s public health and well-being. One of the most respected sources of credible science in America is the National Academy Science. The NAM should convene a meeting of the experts to examine the current state of FASD knowledge. If there is sufficient new information, the NAM needs to develop a new report on FASD. It has been 21 years since the first FAS report from the Institute of Medicine and it needs to be revisited. But it appears that the correctional, child protective services, special education, and mental health fields are not aware of the breadth of available research and its importance to the nation’s public health. Determining how fetal alcohol exposure/choline deficiency affects children and adults in special education, foster care, juvenile and adult corrections systems – along with such other social issues as prematurity, disability, unemployment, homelessness, suicide, violence, and mental health – is critical to our nation’s future.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital Family Medicine Clinic in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
Adjuvant trastuzumab for 1 year remains standard of care for early HER2+ breast cancer
SAN ANTONIO – The phase 3 SOLD trial failed to show a 9-week course of trastuzumab as noninferior to the standard 12-month course when combined with chemotherapy for women with early-stage HER2-positive breast cancer.
Disease-free survival (DFS) with a 9-week course of adjuvant trastuzumab (Herceptin)did not pass muster as noninferior to the standard 12 months, reported Dr. Heikki Joensuu, professor in the department of oncology at the Helsinki University Hospital and University of Helsinki.
“The non-inferiority of 9 week administration of trastuzumab plus docetaxel could not be demonstrated in terms of disease free survival, as compared to chemotherapy and one year duration of adjuvant trastuzumab.”
Based on these findings, he noted that chemotherapy plus one year of anti-HER2 therapy should remain the standard of care.
However, the dose of docetaxel administered along with trastuzumab could influence survival outcomes. Patients who received a higher dose of docetaxel in the 9 week cohort had similar DFS as those who received 12 months of trastuzumab.
Dr. Joensuu explained that the regimen of giving trastuzumab for one year is an arbitrary one and is not based on research data. In the four large randomized trials that established the current standard treatment for this population of breast cancer patients, trastuzumab was administered for one year and that eventually became the standard of care, he said at the San Antonio Breast Cancer Symposium.
But in two randomized trials that had relatively limited statistical power, a statistically significant difference in DFS or overall survival was not observed between patients who received only nine weeks of trastuzumab compared with those who received the one year regimen.
In addition, the one year treatment course is costly and has been associated with cardiac-related adverse events, although it is relatively uncommon (less than 3% of patients).
The phase 3 SOLD (Synergism or Long Duration) clinical trial was conducted by Dr. Joensuu and his colleagues to compare DFS between women treated with 9 weeks of concomitant trastuzumab plus docetaxel followed by 5-FU, epirubicin, and cyclophosphamide (FEC) to that of women treated with the same regimen followed by single-agent trastuzumab for a one year duration.
The cohort included 2,176 patients with early-stage HER2-positive breast cancer who were randomized to either the 9-week or the 12-month trastuzumab arm. In addition, cardiac function among women enrolled in the trial had to be normal, Dr. Joensuu said, as determined by left ventricular ejection fraction of 50% or greater.
Patients in both groups received three cycles of docetaxel (80 mg/m2 or 100 mg/m2) and trastuzumab three times a week, followed by three cycles of FEC. The dose of docetaxel was determined by the individual center. Patients in the 12-month arm continued to receive trastuzumab every 3 weeks for 14 cycles and in both groups, those with estrogen receptor–positive cancer received appropriate endocrine treatment per guidelines.
“SOLD was initially designed as a superiority trial,” he said, “But, we redesigned the study and the size during the study because we noticed that the assumptions we made for 5-year disease-free survival were too low.”
“We also realized that the shorter arm cannot be better than the longer arm because less drug is given,” said Dr. Joensuu. “So we changed it from a superiority trial to a noninferiority design, where the shorter arm would be at least equal to the longer arm.”
When looking at results, the DFS favored the longer arm: 90.5% in the 12-month arm vs. 88% in the 9-week arm (hazard ratio, 1.39; 90% confidence interval, 1.12-1.72). However, there were no substantial differences in secondary endpoints. Five-year distant DFS was 93.2% in the 9-week arm vs. 94.2% in the 12-month arm (HR, 1.24; 90% CI, 0.93-1.65), and similarly, 5-year overall survival was 94.7% vs. 95.9% (HR, 1.39; 90% CI, 0.98-1.89).
There was an interaction between the dose of docetaxel given concomitantly with trastuzumab. For patients who received 100 mg/m2 docetaxel, DFS was similar in both groups: 92.2% (long arm) vs. 87.8% (short arm) (HR 0.71, 90% CI 0,44-1.14). But among those who received 80 mg/m2 docetaxel, DFS was superior in the group who received 12 months of trastuzumab, compared with the 9-week group (91.3% vs. 86.8%; HR, 1.66; 90% CI, 1.30-2.11).
Less cardiac toxicity was observed in the 9-week group, for any protocol-defined cardiac adverse event (n = 22 [2%] vs. n = 42 [3.9%]; P = .012) and for heart failure (n = 21 [1.9%] vs. n = 36 [3.3%]; P = .046).
“Docetaxel dosing with trastuzumab requires further study,” Dr. Joensuu said.
SOURCE: Joensuu H et al., Abstract GS3-04
SAN ANTONIO – The phase 3 SOLD trial failed to show a 9-week course of trastuzumab as noninferior to the standard 12-month course when combined with chemotherapy for women with early-stage HER2-positive breast cancer.
Disease-free survival (DFS) with a 9-week course of adjuvant trastuzumab (Herceptin)did not pass muster as noninferior to the standard 12 months, reported Dr. Heikki Joensuu, professor in the department of oncology at the Helsinki University Hospital and University of Helsinki.
“The non-inferiority of 9 week administration of trastuzumab plus docetaxel could not be demonstrated in terms of disease free survival, as compared to chemotherapy and one year duration of adjuvant trastuzumab.”
Based on these findings, he noted that chemotherapy plus one year of anti-HER2 therapy should remain the standard of care.
However, the dose of docetaxel administered along with trastuzumab could influence survival outcomes. Patients who received a higher dose of docetaxel in the 9 week cohort had similar DFS as those who received 12 months of trastuzumab.
Dr. Joensuu explained that the regimen of giving trastuzumab for one year is an arbitrary one and is not based on research data. In the four large randomized trials that established the current standard treatment for this population of breast cancer patients, trastuzumab was administered for one year and that eventually became the standard of care, he said at the San Antonio Breast Cancer Symposium.
But in two randomized trials that had relatively limited statistical power, a statistically significant difference in DFS or overall survival was not observed between patients who received only nine weeks of trastuzumab compared with those who received the one year regimen.
In addition, the one year treatment course is costly and has been associated with cardiac-related adverse events, although it is relatively uncommon (less than 3% of patients).
The phase 3 SOLD (Synergism or Long Duration) clinical trial was conducted by Dr. Joensuu and his colleagues to compare DFS between women treated with 9 weeks of concomitant trastuzumab plus docetaxel followed by 5-FU, epirubicin, and cyclophosphamide (FEC) to that of women treated with the same regimen followed by single-agent trastuzumab for a one year duration.
The cohort included 2,176 patients with early-stage HER2-positive breast cancer who were randomized to either the 9-week or the 12-month trastuzumab arm. In addition, cardiac function among women enrolled in the trial had to be normal, Dr. Joensuu said, as determined by left ventricular ejection fraction of 50% or greater.
Patients in both groups received three cycles of docetaxel (80 mg/m2 or 100 mg/m2) and trastuzumab three times a week, followed by three cycles of FEC. The dose of docetaxel was determined by the individual center. Patients in the 12-month arm continued to receive trastuzumab every 3 weeks for 14 cycles and in both groups, those with estrogen receptor–positive cancer received appropriate endocrine treatment per guidelines.
“SOLD was initially designed as a superiority trial,” he said, “But, we redesigned the study and the size during the study because we noticed that the assumptions we made for 5-year disease-free survival were too low.”
“We also realized that the shorter arm cannot be better than the longer arm because less drug is given,” said Dr. Joensuu. “So we changed it from a superiority trial to a noninferiority design, where the shorter arm would be at least equal to the longer arm.”
When looking at results, the DFS favored the longer arm: 90.5% in the 12-month arm vs. 88% in the 9-week arm (hazard ratio, 1.39; 90% confidence interval, 1.12-1.72). However, there were no substantial differences in secondary endpoints. Five-year distant DFS was 93.2% in the 9-week arm vs. 94.2% in the 12-month arm (HR, 1.24; 90% CI, 0.93-1.65), and similarly, 5-year overall survival was 94.7% vs. 95.9% (HR, 1.39; 90% CI, 0.98-1.89).
There was an interaction between the dose of docetaxel given concomitantly with trastuzumab. For patients who received 100 mg/m2 docetaxel, DFS was similar in both groups: 92.2% (long arm) vs. 87.8% (short arm) (HR 0.71, 90% CI 0,44-1.14). But among those who received 80 mg/m2 docetaxel, DFS was superior in the group who received 12 months of trastuzumab, compared with the 9-week group (91.3% vs. 86.8%; HR, 1.66; 90% CI, 1.30-2.11).
Less cardiac toxicity was observed in the 9-week group, for any protocol-defined cardiac adverse event (n = 22 [2%] vs. n = 42 [3.9%]; P = .012) and for heart failure (n = 21 [1.9%] vs. n = 36 [3.3%]; P = .046).
“Docetaxel dosing with trastuzumab requires further study,” Dr. Joensuu said.
SOURCE: Joensuu H et al., Abstract GS3-04
SAN ANTONIO – The phase 3 SOLD trial failed to show a 9-week course of trastuzumab as noninferior to the standard 12-month course when combined with chemotherapy for women with early-stage HER2-positive breast cancer.
Disease-free survival (DFS) with a 9-week course of adjuvant trastuzumab (Herceptin)did not pass muster as noninferior to the standard 12 months, reported Dr. Heikki Joensuu, professor in the department of oncology at the Helsinki University Hospital and University of Helsinki.
“The non-inferiority of 9 week administration of trastuzumab plus docetaxel could not be demonstrated in terms of disease free survival, as compared to chemotherapy and one year duration of adjuvant trastuzumab.”
Based on these findings, he noted that chemotherapy plus one year of anti-HER2 therapy should remain the standard of care.
However, the dose of docetaxel administered along with trastuzumab could influence survival outcomes. Patients who received a higher dose of docetaxel in the 9 week cohort had similar DFS as those who received 12 months of trastuzumab.
Dr. Joensuu explained that the regimen of giving trastuzumab for one year is an arbitrary one and is not based on research data. In the four large randomized trials that established the current standard treatment for this population of breast cancer patients, trastuzumab was administered for one year and that eventually became the standard of care, he said at the San Antonio Breast Cancer Symposium.
But in two randomized trials that had relatively limited statistical power, a statistically significant difference in DFS or overall survival was not observed between patients who received only nine weeks of trastuzumab compared with those who received the one year regimen.
In addition, the one year treatment course is costly and has been associated with cardiac-related adverse events, although it is relatively uncommon (less than 3% of patients).
The phase 3 SOLD (Synergism or Long Duration) clinical trial was conducted by Dr. Joensuu and his colleagues to compare DFS between women treated with 9 weeks of concomitant trastuzumab plus docetaxel followed by 5-FU, epirubicin, and cyclophosphamide (FEC) to that of women treated with the same regimen followed by single-agent trastuzumab for a one year duration.
The cohort included 2,176 patients with early-stage HER2-positive breast cancer who were randomized to either the 9-week or the 12-month trastuzumab arm. In addition, cardiac function among women enrolled in the trial had to be normal, Dr. Joensuu said, as determined by left ventricular ejection fraction of 50% or greater.
Patients in both groups received three cycles of docetaxel (80 mg/m2 or 100 mg/m2) and trastuzumab three times a week, followed by three cycles of FEC. The dose of docetaxel was determined by the individual center. Patients in the 12-month arm continued to receive trastuzumab every 3 weeks for 14 cycles and in both groups, those with estrogen receptor–positive cancer received appropriate endocrine treatment per guidelines.
“SOLD was initially designed as a superiority trial,” he said, “But, we redesigned the study and the size during the study because we noticed that the assumptions we made for 5-year disease-free survival were too low.”
“We also realized that the shorter arm cannot be better than the longer arm because less drug is given,” said Dr. Joensuu. “So we changed it from a superiority trial to a noninferiority design, where the shorter arm would be at least equal to the longer arm.”
When looking at results, the DFS favored the longer arm: 90.5% in the 12-month arm vs. 88% in the 9-week arm (hazard ratio, 1.39; 90% confidence interval, 1.12-1.72). However, there were no substantial differences in secondary endpoints. Five-year distant DFS was 93.2% in the 9-week arm vs. 94.2% in the 12-month arm (HR, 1.24; 90% CI, 0.93-1.65), and similarly, 5-year overall survival was 94.7% vs. 95.9% (HR, 1.39; 90% CI, 0.98-1.89).
There was an interaction between the dose of docetaxel given concomitantly with trastuzumab. For patients who received 100 mg/m2 docetaxel, DFS was similar in both groups: 92.2% (long arm) vs. 87.8% (short arm) (HR 0.71, 90% CI 0,44-1.14). But among those who received 80 mg/m2 docetaxel, DFS was superior in the group who received 12 months of trastuzumab, compared with the 9-week group (91.3% vs. 86.8%; HR, 1.66; 90% CI, 1.30-2.11).
Less cardiac toxicity was observed in the 9-week group, for any protocol-defined cardiac adverse event (n = 22 [2%] vs. n = 42 [3.9%]; P = .012) and for heart failure (n = 21 [1.9%] vs. n = 36 [3.3%]; P = .046).
“Docetaxel dosing with trastuzumab requires further study,” Dr. Joensuu said.
SOURCE: Joensuu H et al., Abstract GS3-04
REPORTING FROM SABCS 2017
Key clinical point: Disease-free survival with a 9-week course of adjuvant trastuzumab did not pass muster as noninferior to the standard 12 months.
Major finding: DFS favored the longer arm: 90.5% in the 12 month arm vs. 88% in the 9-week arm (HR, 1.39; 90% CI 1.12-1.72), but no substantial differences were observed in secondary endpoints.
Data source: Randomized phase 3 trial that included 2,176 patients with early-stage HER2-positive breast cancer
Disclosures:. The study was funded by Pharmac, Sanofi, Novartis, the Academy of Finland, the Cancer Society of Finland, Helsinki University Hospital research funds, Sigrid Juselius Foundation, and Jane and Aatos Erkko Foundation. Dr. Joensuu is a scientific adviser for Neutron Therapeutics, has received consultation fees from Orion Pharma, and has stock ownership interest in Orion, Faron Pharmaceuticals, and Sartar Therapeutics.
Source: Joensuu H et al., GS3-04












