User login
Avapritinib yields high response rate in patients with systemic mastocytosis
ATLANTA – An oral investigational drug with specific activity against a mutation frequently found in advanced systemic mastocytosis (ASM) produced clinical responses in the majority treated patients, according to preliminary data presented at the annual meeting of the American Society of Hematology.
that included a 56% rate of complete or partial response, according to lead study author Daniel J. DeAngelo, MD, PhD, director of clinical and translational research at Dana-Farber Cancer Institute, Boston.
Currently, midostaurin, a multikinase inhibitor, is the only Food and Drug Administration–approved drug for the treatment of systemic mastocytosis. That approval, announced in April 2017, was based in part on a 17% rate of complete or partial response, Dr. DeAngelo noted at a press briefing.
The primary goal of the phase 1 trial was to evaluate the safety profile and establish a maximum-tolerated dose for once-daily oral avapritinib administration. Treatment-emergent side effects were primarily grade 1-2, according to Dr. DeAngelo. Most hematologic toxicities were mild to moderate, and the most common grade 3 nonhematologic toxicities were periorbital edema and fatigue.
This part of the phase 1 trial enrolled 18 patients with ASM, systemic mastocytosis with associated hematologic neoplasm (SM-AHN), and mantle cell lymphoma (MCL). Efficacy of avapritinib was assessed on International Working Group criteria for response rate in myelodysplasia.
The overall response rate was 72% (13 of 18 patients saw complete response, partial response, or clinical improvement), and a 56% rate of complete and partial response (10 of 18 patients), Dr. DeAngelo said.
Avapritinib was active in all ASM subtypes evaluated, including in patients who had previously been treated with midostaurin or chemotherapy, according to the investigators.
The data on avapritinib suggests the drug “has a potent and clinically important activity in systemic mastocytosis,” he said. “It has been a wonderful success in terms of getting the majority of patients into complete and partial remissions, and so as this evolves, having better targeted agents, I think, can improve the outcome for these patients.”
More patients are being enrolled as the phase 1 study continues into the dose-expansion phase at 300 mg once daily, and 30 of 32 patients remain on treatment with median duration of 9 months, Dr. DeAngelo said.
A phase 2 study in advanced systemic mastocytosis is planned for 2018, as well as phase 1 and phase 2 studies that will include patients with indolent or smoldering disease, he added.
Avapritinib is manufactured by Blueprint Medicines, which also supported the study. Dr. DeAngelo reported disclosures from Blueprint and several other companies in the hematologic space.
SOURCE: DeAngelo D et al. ASH 2017 Abstract 2.
ATLANTA – An oral investigational drug with specific activity against a mutation frequently found in advanced systemic mastocytosis (ASM) produced clinical responses in the majority treated patients, according to preliminary data presented at the annual meeting of the American Society of Hematology.
that included a 56% rate of complete or partial response, according to lead study author Daniel J. DeAngelo, MD, PhD, director of clinical and translational research at Dana-Farber Cancer Institute, Boston.
Currently, midostaurin, a multikinase inhibitor, is the only Food and Drug Administration–approved drug for the treatment of systemic mastocytosis. That approval, announced in April 2017, was based in part on a 17% rate of complete or partial response, Dr. DeAngelo noted at a press briefing.
The primary goal of the phase 1 trial was to evaluate the safety profile and establish a maximum-tolerated dose for once-daily oral avapritinib administration. Treatment-emergent side effects were primarily grade 1-2, according to Dr. DeAngelo. Most hematologic toxicities were mild to moderate, and the most common grade 3 nonhematologic toxicities were periorbital edema and fatigue.
This part of the phase 1 trial enrolled 18 patients with ASM, systemic mastocytosis with associated hematologic neoplasm (SM-AHN), and mantle cell lymphoma (MCL). Efficacy of avapritinib was assessed on International Working Group criteria for response rate in myelodysplasia.
The overall response rate was 72% (13 of 18 patients saw complete response, partial response, or clinical improvement), and a 56% rate of complete and partial response (10 of 18 patients), Dr. DeAngelo said.
Avapritinib was active in all ASM subtypes evaluated, including in patients who had previously been treated with midostaurin or chemotherapy, according to the investigators.
The data on avapritinib suggests the drug “has a potent and clinically important activity in systemic mastocytosis,” he said. “It has been a wonderful success in terms of getting the majority of patients into complete and partial remissions, and so as this evolves, having better targeted agents, I think, can improve the outcome for these patients.”
More patients are being enrolled as the phase 1 study continues into the dose-expansion phase at 300 mg once daily, and 30 of 32 patients remain on treatment with median duration of 9 months, Dr. DeAngelo said.
A phase 2 study in advanced systemic mastocytosis is planned for 2018, as well as phase 1 and phase 2 studies that will include patients with indolent or smoldering disease, he added.
Avapritinib is manufactured by Blueprint Medicines, which also supported the study. Dr. DeAngelo reported disclosures from Blueprint and several other companies in the hematologic space.
SOURCE: DeAngelo D et al. ASH 2017 Abstract 2.
ATLANTA – An oral investigational drug with specific activity against a mutation frequently found in advanced systemic mastocytosis (ASM) produced clinical responses in the majority treated patients, according to preliminary data presented at the annual meeting of the American Society of Hematology.
that included a 56% rate of complete or partial response, according to lead study author Daniel J. DeAngelo, MD, PhD, director of clinical and translational research at Dana-Farber Cancer Institute, Boston.
Currently, midostaurin, a multikinase inhibitor, is the only Food and Drug Administration–approved drug for the treatment of systemic mastocytosis. That approval, announced in April 2017, was based in part on a 17% rate of complete or partial response, Dr. DeAngelo noted at a press briefing.
The primary goal of the phase 1 trial was to evaluate the safety profile and establish a maximum-tolerated dose for once-daily oral avapritinib administration. Treatment-emergent side effects were primarily grade 1-2, according to Dr. DeAngelo. Most hematologic toxicities were mild to moderate, and the most common grade 3 nonhematologic toxicities were periorbital edema and fatigue.
This part of the phase 1 trial enrolled 18 patients with ASM, systemic mastocytosis with associated hematologic neoplasm (SM-AHN), and mantle cell lymphoma (MCL). Efficacy of avapritinib was assessed on International Working Group criteria for response rate in myelodysplasia.
The overall response rate was 72% (13 of 18 patients saw complete response, partial response, or clinical improvement), and a 56% rate of complete and partial response (10 of 18 patients), Dr. DeAngelo said.
Avapritinib was active in all ASM subtypes evaluated, including in patients who had previously been treated with midostaurin or chemotherapy, according to the investigators.
The data on avapritinib suggests the drug “has a potent and clinically important activity in systemic mastocytosis,” he said. “It has been a wonderful success in terms of getting the majority of patients into complete and partial remissions, and so as this evolves, having better targeted agents, I think, can improve the outcome for these patients.”
More patients are being enrolled as the phase 1 study continues into the dose-expansion phase at 300 mg once daily, and 30 of 32 patients remain on treatment with median duration of 9 months, Dr. DeAngelo said.
A phase 2 study in advanced systemic mastocytosis is planned for 2018, as well as phase 1 and phase 2 studies that will include patients with indolent or smoldering disease, he added.
Avapritinib is manufactured by Blueprint Medicines, which also supported the study. Dr. DeAngelo reported disclosures from Blueprint and several other companies in the hematologic space.
SOURCE: DeAngelo D et al. ASH 2017 Abstract 2.
REPORTING FROM ASH 2017
Key clinical point: Avapritinib produced complete or partial responses in the majority of patients with advanced systemic mastocytosis.
Major finding: The overall response rate was 72%, including a 56% rate of complete or partial response.
Data source: Phase 1 dose-escalation study of 18 patients with advanced systemic mastocytosis.
Disclosures: The study was supported by Blueprint Medicines. Dr. DeAngelo reported disclosures from Blueprint and several other companies in the hematologic space.
Source: DeAngelo D et al. ASH 2017 Abstract 2
Intrabone gene therapy shows promise in beta-thalassemia
ATLANTA – Intrabone gene therapy could offer long-term hope for patients with beta thalassemia who cannot be treated by allogeneic hematopoietic stem cell transplant (HSCT), suggest the results of a phase 1/2 trial.
After a median of 16 months of follow-up, five of seven patients who received this novel gene therapy needed markedly fewer blood transfusions than at baseline, lead investigator Sarah Marktel, MD, reported at the annual meeting of the American Society of Hematology.
Even more strikingly, (indicating severe disease), said Dr. Marktel of San Raffaele Scientific Institute and San Raffaele Telethon Institute for Gene Therapy (SR-Tiget), Milan.
All patients met the trial’s primary safety endpoint and experienced no treatment-related adverse effects except those caused by conditioning chemotherapy, such as infections, Dr. Marktel said. She and her coinvestigators are expanding the study by administering intrabone gene therapy to three more children.
Beta-thalassemia is a genetic anemia linked to multiple mutations of the beta-globin gene. Patients who can’t undergo allogeneic HSCT face a lifetime of blood transfusions and iron chelation. This is the reality for most because they lack a compatible donor, have exclusionary risk factors for allogeneic transplant, or cannot access treatment, Dr. Marktel said during a press briefing.
Although this is not the first human study of gene therapy in beta-thalassemia, it is the first to infuse treatment directly into bone marrow instead of peripheral blood.
“Compared to previous trials, patients showed evidence of successful engraftment [proliferation in bone marrow] sooner after receiving the therapy,” Dr. Marktel said. Researchers saw evidence of engraftment as soon as 10 days after treatment – noticeably faster than in prior gene therapy studies of beta-thalassemia, she added.
To develop this treatment, investigators created a self-inactivating lentiviral vector (dubbed GLOBE) that carries a normal beta-globin gene. The vector posted encouraging safety and efficacy signals in studies of human thalassemic cells and in a mouse model, Dr. Marktel said.
For the phase 1/2 trial, the researchers extracted circulating CD34+ stem cells from the peripheral blood from three adults and four children with transfusion-dependent beta-thalassemia. For each patient, they transduced these stem cells with GLOBE. Next, patients underwent a 3-day conditioning regimen of treosulfan and thiotepa, after which their individual cell-gene product was infused into their own bone marrow.
This is a small study, but if results hold up in more patients, gene therapy “could represent an alternative to bone marrow transplantation that does not require a matched donor or immunosuppression and that carries no risk of graft-versus-host disease or transplant rejection,” Dr. Marktel said. Children in this study might have had better results because their younger stem cells are more amenable to gene transduction and engraftment, she hypothesized.
Both beta-0/beta-0 patients in the study are children. One continues to need blood transfusions because he experiences a drop in genetically modified cells and vector copy numbers soon after each infusion of gene therapy. The other was treated more than a year ago and remains transfusion free.
“The beta-0/beta-0 genotype is toughest to treat with gene therapy,” Dr. Marktel noted. “In comparison, beta-0/beta+ or beta+/beta+ patients have the highest chances of becoming transfusion independent because they can contribute their own hemoglobin to the total hemoglobin output.”
Telethon Foundation provided funding. Dr. Marktel disclosed research funding from GlaxoSmithKline, which has licensed the therapy.
SOURCE: Marktel S et al. ASH 2017 Abstract 355.
ATLANTA – Intrabone gene therapy could offer long-term hope for patients with beta thalassemia who cannot be treated by allogeneic hematopoietic stem cell transplant (HSCT), suggest the results of a phase 1/2 trial.
After a median of 16 months of follow-up, five of seven patients who received this novel gene therapy needed markedly fewer blood transfusions than at baseline, lead investigator Sarah Marktel, MD, reported at the annual meeting of the American Society of Hematology.
Even more strikingly, (indicating severe disease), said Dr. Marktel of San Raffaele Scientific Institute and San Raffaele Telethon Institute for Gene Therapy (SR-Tiget), Milan.
All patients met the trial’s primary safety endpoint and experienced no treatment-related adverse effects except those caused by conditioning chemotherapy, such as infections, Dr. Marktel said. She and her coinvestigators are expanding the study by administering intrabone gene therapy to three more children.
Beta-thalassemia is a genetic anemia linked to multiple mutations of the beta-globin gene. Patients who can’t undergo allogeneic HSCT face a lifetime of blood transfusions and iron chelation. This is the reality for most because they lack a compatible donor, have exclusionary risk factors for allogeneic transplant, or cannot access treatment, Dr. Marktel said during a press briefing.
Although this is not the first human study of gene therapy in beta-thalassemia, it is the first to infuse treatment directly into bone marrow instead of peripheral blood.
“Compared to previous trials, patients showed evidence of successful engraftment [proliferation in bone marrow] sooner after receiving the therapy,” Dr. Marktel said. Researchers saw evidence of engraftment as soon as 10 days after treatment – noticeably faster than in prior gene therapy studies of beta-thalassemia, she added.
To develop this treatment, investigators created a self-inactivating lentiviral vector (dubbed GLOBE) that carries a normal beta-globin gene. The vector posted encouraging safety and efficacy signals in studies of human thalassemic cells and in a mouse model, Dr. Marktel said.
For the phase 1/2 trial, the researchers extracted circulating CD34+ stem cells from the peripheral blood from three adults and four children with transfusion-dependent beta-thalassemia. For each patient, they transduced these stem cells with GLOBE. Next, patients underwent a 3-day conditioning regimen of treosulfan and thiotepa, after which their individual cell-gene product was infused into their own bone marrow.
This is a small study, but if results hold up in more patients, gene therapy “could represent an alternative to bone marrow transplantation that does not require a matched donor or immunosuppression and that carries no risk of graft-versus-host disease or transplant rejection,” Dr. Marktel said. Children in this study might have had better results because their younger stem cells are more amenable to gene transduction and engraftment, she hypothesized.
Both beta-0/beta-0 patients in the study are children. One continues to need blood transfusions because he experiences a drop in genetically modified cells and vector copy numbers soon after each infusion of gene therapy. The other was treated more than a year ago and remains transfusion free.
“The beta-0/beta-0 genotype is toughest to treat with gene therapy,” Dr. Marktel noted. “In comparison, beta-0/beta+ or beta+/beta+ patients have the highest chances of becoming transfusion independent because they can contribute their own hemoglobin to the total hemoglobin output.”
Telethon Foundation provided funding. Dr. Marktel disclosed research funding from GlaxoSmithKline, which has licensed the therapy.
SOURCE: Marktel S et al. ASH 2017 Abstract 355.
ATLANTA – Intrabone gene therapy could offer long-term hope for patients with beta thalassemia who cannot be treated by allogeneic hematopoietic stem cell transplant (HSCT), suggest the results of a phase 1/2 trial.
After a median of 16 months of follow-up, five of seven patients who received this novel gene therapy needed markedly fewer blood transfusions than at baseline, lead investigator Sarah Marktel, MD, reported at the annual meeting of the American Society of Hematology.
Even more strikingly, (indicating severe disease), said Dr. Marktel of San Raffaele Scientific Institute and San Raffaele Telethon Institute for Gene Therapy (SR-Tiget), Milan.
All patients met the trial’s primary safety endpoint and experienced no treatment-related adverse effects except those caused by conditioning chemotherapy, such as infections, Dr. Marktel said. She and her coinvestigators are expanding the study by administering intrabone gene therapy to three more children.
Beta-thalassemia is a genetic anemia linked to multiple mutations of the beta-globin gene. Patients who can’t undergo allogeneic HSCT face a lifetime of blood transfusions and iron chelation. This is the reality for most because they lack a compatible donor, have exclusionary risk factors for allogeneic transplant, or cannot access treatment, Dr. Marktel said during a press briefing.
Although this is not the first human study of gene therapy in beta-thalassemia, it is the first to infuse treatment directly into bone marrow instead of peripheral blood.
“Compared to previous trials, patients showed evidence of successful engraftment [proliferation in bone marrow] sooner after receiving the therapy,” Dr. Marktel said. Researchers saw evidence of engraftment as soon as 10 days after treatment – noticeably faster than in prior gene therapy studies of beta-thalassemia, she added.
To develop this treatment, investigators created a self-inactivating lentiviral vector (dubbed GLOBE) that carries a normal beta-globin gene. The vector posted encouraging safety and efficacy signals in studies of human thalassemic cells and in a mouse model, Dr. Marktel said.
For the phase 1/2 trial, the researchers extracted circulating CD34+ stem cells from the peripheral blood from three adults and four children with transfusion-dependent beta-thalassemia. For each patient, they transduced these stem cells with GLOBE. Next, patients underwent a 3-day conditioning regimen of treosulfan and thiotepa, after which their individual cell-gene product was infused into their own bone marrow.
This is a small study, but if results hold up in more patients, gene therapy “could represent an alternative to bone marrow transplantation that does not require a matched donor or immunosuppression and that carries no risk of graft-versus-host disease or transplant rejection,” Dr. Marktel said. Children in this study might have had better results because their younger stem cells are more amenable to gene transduction and engraftment, she hypothesized.
Both beta-0/beta-0 patients in the study are children. One continues to need blood transfusions because he experiences a drop in genetically modified cells and vector copy numbers soon after each infusion of gene therapy. The other was treated more than a year ago and remains transfusion free.
“The beta-0/beta-0 genotype is toughest to treat with gene therapy,” Dr. Marktel noted. “In comparison, beta-0/beta+ or beta+/beta+ patients have the highest chances of becoming transfusion independent because they can contribute their own hemoglobin to the total hemoglobin output.”
Telethon Foundation provided funding. Dr. Marktel disclosed research funding from GlaxoSmithKline, which has licensed the therapy.
SOURCE: Marktel S et al. ASH 2017 Abstract 355.
REPORTING FROM ASH 2017
Key clinical point: Gene therapy engraftment was achieved sooner using intrabone delivery.
Major finding: After a median of 16 months of follow-up, five of seven patients who received this novel gene therapy needed markedly fewer blood transfusions than at baseline.
Data source: An interventional phase 1/2 trial of seven patients with transfusion-dependent beta thalassemia.
Disclosures: Telethon Foundation provided funding. Dr. Marktel disclosed research funding from GlaxoSmithKline, which has licensed the therapy.
Source: Marktel S et al. ASH 2017 Abstract 355.
Cancer Care Facilities Receive CoC Outstanding Achievement Award
The Commission on Cancer (CoC) of the American College of Surgeons (ACS) has granted its mid-year 2017 Outstanding Achievement Award to a select group of 16 accredited cancer programs throughout the U.S. Award criteria are based on qualitative and quantitative surveys conducted in the first half of 2017. Visit the ACS website for a list of these award-winning cancer programs at facs.org/quality-programs/cancer/coc/info/outstanding/2017-part-1.
The purpose of the award is to raise the bar on quality cancer care, with the ultimate goal of increasing awareness about quality care choices among cancer patients and their loved ones. In addition, the award is intended to fulfill the following goals:
• Recognize those cancer programs that achieve excellence in providing quality care to cancer patients
• Motivate other cancer programs to work toward improving their level of care
• Facilitate dialogue between award recipients and health care professionals at other cancer facilities for the purpose of sharing best practices
• Encourage honorees to serve as quality care resources to other cancer programs
“More and more, we’re finding that patients and their families want to know how the health care institutions in their communities compare with one another,” said Lawrence N. Shulman, MD, FACP, Chair of the CoC. “They want access to information in terms of who’s providing the best quality of care, and they want to know about overall patient outcomes. Through this recognition program, I’d like to think we’re playing a small, but vital, role in helping them make informed decisions on their cancer care. ”The 16 award-winning cancer care programs represent approximately six percent of programs surveyed by the CoC January 1–June 30, 2017. “These cancer programs currently represent the best of the best when it comes to cancer care,” Dr. Shulman added.
The Commission on Cancer (CoC) of the American College of Surgeons (ACS) has granted its mid-year 2017 Outstanding Achievement Award to a select group of 16 accredited cancer programs throughout the U.S. Award criteria are based on qualitative and quantitative surveys conducted in the first half of 2017. Visit the ACS website for a list of these award-winning cancer programs at facs.org/quality-programs/cancer/coc/info/outstanding/2017-part-1.
The purpose of the award is to raise the bar on quality cancer care, with the ultimate goal of increasing awareness about quality care choices among cancer patients and their loved ones. In addition, the award is intended to fulfill the following goals:
• Recognize those cancer programs that achieve excellence in providing quality care to cancer patients
• Motivate other cancer programs to work toward improving their level of care
• Facilitate dialogue between award recipients and health care professionals at other cancer facilities for the purpose of sharing best practices
• Encourage honorees to serve as quality care resources to other cancer programs
“More and more, we’re finding that patients and their families want to know how the health care institutions in their communities compare with one another,” said Lawrence N. Shulman, MD, FACP, Chair of the CoC. “They want access to information in terms of who’s providing the best quality of care, and they want to know about overall patient outcomes. Through this recognition program, I’d like to think we’re playing a small, but vital, role in helping them make informed decisions on their cancer care. ”The 16 award-winning cancer care programs represent approximately six percent of programs surveyed by the CoC January 1–June 30, 2017. “These cancer programs currently represent the best of the best when it comes to cancer care,” Dr. Shulman added.
The Commission on Cancer (CoC) of the American College of Surgeons (ACS) has granted its mid-year 2017 Outstanding Achievement Award to a select group of 16 accredited cancer programs throughout the U.S. Award criteria are based on qualitative and quantitative surveys conducted in the first half of 2017. Visit the ACS website for a list of these award-winning cancer programs at facs.org/quality-programs/cancer/coc/info/outstanding/2017-part-1.
The purpose of the award is to raise the bar on quality cancer care, with the ultimate goal of increasing awareness about quality care choices among cancer patients and their loved ones. In addition, the award is intended to fulfill the following goals:
• Recognize those cancer programs that achieve excellence in providing quality care to cancer patients
• Motivate other cancer programs to work toward improving their level of care
• Facilitate dialogue between award recipients and health care professionals at other cancer facilities for the purpose of sharing best practices
• Encourage honorees to serve as quality care resources to other cancer programs
“More and more, we’re finding that patients and their families want to know how the health care institutions in their communities compare with one another,” said Lawrence N. Shulman, MD, FACP, Chair of the CoC. “They want access to information in terms of who’s providing the best quality of care, and they want to know about overall patient outcomes. Through this recognition program, I’d like to think we’re playing a small, but vital, role in helping them make informed decisions on their cancer care. ”The 16 award-winning cancer care programs represent approximately six percent of programs surveyed by the CoC January 1–June 30, 2017. “These cancer programs currently represent the best of the best when it comes to cancer care,” Dr. Shulman added.
Dr. Frank Opelka Testifies before U.S. House Energy and Commerce Health Subcommittee teaser
Frank G. Opelka, MD, FACS, Medical Director, Quality and Health Policy, American College of Surgeons (ACS) Division of Advocacy and Health Policy, testified November 8 before the U.S. House Committee on Energy and Commerce Health Subcommittee. The subcommittee conducted the hearing—MACRA (Medicare Access and CHIP [Children’s Health Insurance Program] Reauthorization Act) and Alternative Payment Models: Developing Options for Value-based Care—to explore how Medicare payment reforms are shaping the way physicians treat patients.
Dr. Opelka described for lawmakers how the Advanced Alternative Payment Model (A-APM) developed by the ACS and Brandeis University, Waltham, MA, the ACS-Brandeis A-APM—proceeded through the Payment Model Technical Advisory Committee (PTAC) review and approval process. He shared how the ACS-Brandeis A-APM can revolutionize physician payment, as well as encourage and incentivize a team-based approach to patient care. Dr. Opelka expressed the College’s ongoing willingness to work with Congress on ways to improve and enhance patient care and Medicare physician payment.
A replay of the hearing is available on the Energy and Commerce Committee website at https://goo.gl/X2xjqJ.
For more information, contact Matt Coffron, ACS Manager of Policy Development, at [email protected].
Frank G. Opelka, MD, FACS, Medical Director, Quality and Health Policy, American College of Surgeons (ACS) Division of Advocacy and Health Policy, testified November 8 before the U.S. House Committee on Energy and Commerce Health Subcommittee. The subcommittee conducted the hearing—MACRA (Medicare Access and CHIP [Children’s Health Insurance Program] Reauthorization Act) and Alternative Payment Models: Developing Options for Value-based Care—to explore how Medicare payment reforms are shaping the way physicians treat patients.
Dr. Opelka described for lawmakers how the Advanced Alternative Payment Model (A-APM) developed by the ACS and Brandeis University, Waltham, MA, the ACS-Brandeis A-APM—proceeded through the Payment Model Technical Advisory Committee (PTAC) review and approval process. He shared how the ACS-Brandeis A-APM can revolutionize physician payment, as well as encourage and incentivize a team-based approach to patient care. Dr. Opelka expressed the College’s ongoing willingness to work with Congress on ways to improve and enhance patient care and Medicare physician payment.
A replay of the hearing is available on the Energy and Commerce Committee website at https://goo.gl/X2xjqJ.
For more information, contact Matt Coffron, ACS Manager of Policy Development, at [email protected].
Frank G. Opelka, MD, FACS, Medical Director, Quality and Health Policy, American College of Surgeons (ACS) Division of Advocacy and Health Policy, testified November 8 before the U.S. House Committee on Energy and Commerce Health Subcommittee. The subcommittee conducted the hearing—MACRA (Medicare Access and CHIP [Children’s Health Insurance Program] Reauthorization Act) and Alternative Payment Models: Developing Options for Value-based Care—to explore how Medicare payment reforms are shaping the way physicians treat patients.
Dr. Opelka described for lawmakers how the Advanced Alternative Payment Model (A-APM) developed by the ACS and Brandeis University, Waltham, MA, the ACS-Brandeis A-APM—proceeded through the Payment Model Technical Advisory Committee (PTAC) review and approval process. He shared how the ACS-Brandeis A-APM can revolutionize physician payment, as well as encourage and incentivize a team-based approach to patient care. Dr. Opelka expressed the College’s ongoing willingness to work with Congress on ways to improve and enhance patient care and Medicare physician payment.
A replay of the hearing is available on the Energy and Commerce Committee website at https://goo.gl/X2xjqJ.
For more information, contact Matt Coffron, ACS Manager of Policy Development, at [email protected].
Call for nominations for the ACS Board of Regents and ACS Officers-Elect
The American College of Surgeons (ACS) 2018 Nominating Committee of the Fellows (NCF) and the Nominating Committee of the Board of Governors (NCBG) will be selecting nominees for leadership positions in the College as follows.
Call for nominations for Officers-Elect
The 2018 NCF will select nominees for the three Officer-Elect positions of the ACS: President-Elect, First Vice-President-Elect, and Second Vice-President-Elect. The deadline for submitting nominations is February 23, 2018.
Criteria for consideration
The NCF will use the following guidelines when considering potential candidates:
• Nominees must be loyal members of the College who have demonstrated outstanding integrity and an unquestioned devotion to the highest principles of surgical practice.
• Nominees must have demonstrated leadership qualities, such as service and active participation on ACS committees or in other components of the College.
• The ACS encourages consideration of women and underrepresented minorities for all leadership positions.
All nominations must include the following:
• A letter/letters of nomination
• A personal statement from the candidate detailing his or her ACS service and interest in the position (for President-Elect position only)
• A current curriculum vitae (CV)
• The name of one individual who can serve as a reference
Further details
Entities such as surgical specialty societies, ACS Advisory Councils, ACS committees, and ACS chapters that would like to provide a letter of nomination must provide a description of their selection process and the total list of applicants reviewed.
Any attempt to contact members of the NCF by a candidate or on behalf of a candidate will be viewed negatively, and may result in disqualification. Applications submitted without the requested information will not be considered.
Nominations must be submitted to o[email protected]. If you have any questions, contact Emily Kalata at 312-202-5360 or e[email protected].
Call for Nominations for Board of Regents
The 2018 NCBG will select nominees for pending vacancies on the Board of Regents to be filled at Clinical Congress 2018. The deadline for submitting nominations is February 23, 2018.
Criteria
The NCBG will use the following guidelines when considering potential candidates:
• Nominees must be loyal members of the College who have demonstrated outstanding integrity along with an unquestioned devotion to the highest principles of surgical practice.
• Nominees must have demonstrated leadership qualities, such as service and active participation on ACS committees or in other components of the College.
• The ACS encourages consideration of women and underrepresented minorities for all leadership positions.
• The NCBG recognizes the importance of the Board of Regents representing all who practice surgery in both academic and community practice, regardless of practice location or configuration.
• Nominations are open to surgeons of all specialties, but particular consideration will be given this nomination cycle to those in the following specialties:
o Burn and critical care surgery
o Gastrointestinal surgery
o General surgery
o Surgical oncology
o Transplantation
o Trauma
o Vascular surgery
Only individuals who are currently and expected to remain in active surgical practice for their entire term may be nominated for election or reelection to the Board of Regents.
All nominations must include the following:
• A letter of nomination
• A personal statement from the candidate detailing his or her ACS service and interest in the position
• A current curriculum vitae
• The name of one individual who can serve as a reference
Further details
Entities such as surgical specialty societies, ACS Advisory Councils, ACS Committees, and ACS chapters that would like to provide a letter of nomination must provide at least two nominees and a description of their selection process along with the total list of applicants reviewed.
Any attempt to contact members of the NCBG by a candidate or on behalf of a candidate will be viewed negatively, and may possibly result in disqualification. Applications submitted without the requested information will not be considered.
Nominations may be submitted to [email protected]. If you have any questions, contact Emily Kalata at 312-202-5360 or e[email protected].
For information only, the current members of the Board of Regents who will be considered for re-election are (all MD, FACS): John L. D. Atkinson, James C. Denneny III, Timothy J. Eberlein, Henri R. Ford, Enrique Hernandez, L. Scott Levin, Linda Phillips, Anton A. Sidawy, Beth H. Sutton, and Steven D. Wexner.
The American College of Surgeons (ACS) 2018 Nominating Committee of the Fellows (NCF) and the Nominating Committee of the Board of Governors (NCBG) will be selecting nominees for leadership positions in the College as follows.
Call for nominations for Officers-Elect
The 2018 NCF will select nominees for the three Officer-Elect positions of the ACS: President-Elect, First Vice-President-Elect, and Second Vice-President-Elect. The deadline for submitting nominations is February 23, 2018.
Criteria for consideration
The NCF will use the following guidelines when considering potential candidates:
• Nominees must be loyal members of the College who have demonstrated outstanding integrity and an unquestioned devotion to the highest principles of surgical practice.
• Nominees must have demonstrated leadership qualities, such as service and active participation on ACS committees or in other components of the College.
• The ACS encourages consideration of women and underrepresented minorities for all leadership positions.
All nominations must include the following:
• A letter/letters of nomination
• A personal statement from the candidate detailing his or her ACS service and interest in the position (for President-Elect position only)
• A current curriculum vitae (CV)
• The name of one individual who can serve as a reference
Further details
Entities such as surgical specialty societies, ACS Advisory Councils, ACS committees, and ACS chapters that would like to provide a letter of nomination must provide a description of their selection process and the total list of applicants reviewed.
Any attempt to contact members of the NCF by a candidate or on behalf of a candidate will be viewed negatively, and may result in disqualification. Applications submitted without the requested information will not be considered.
Nominations must be submitted to o[email protected]. If you have any questions, contact Emily Kalata at 312-202-5360 or e[email protected].
Call for Nominations for Board of Regents
The 2018 NCBG will select nominees for pending vacancies on the Board of Regents to be filled at Clinical Congress 2018. The deadline for submitting nominations is February 23, 2018.
Criteria
The NCBG will use the following guidelines when considering potential candidates:
• Nominees must be loyal members of the College who have demonstrated outstanding integrity along with an unquestioned devotion to the highest principles of surgical practice.
• Nominees must have demonstrated leadership qualities, such as service and active participation on ACS committees or in other components of the College.
• The ACS encourages consideration of women and underrepresented minorities for all leadership positions.
• The NCBG recognizes the importance of the Board of Regents representing all who practice surgery in both academic and community practice, regardless of practice location or configuration.
• Nominations are open to surgeons of all specialties, but particular consideration will be given this nomination cycle to those in the following specialties:
o Burn and critical care surgery
o Gastrointestinal surgery
o General surgery
o Surgical oncology
o Transplantation
o Trauma
o Vascular surgery
Only individuals who are currently and expected to remain in active surgical practice for their entire term may be nominated for election or reelection to the Board of Regents.
All nominations must include the following:
• A letter of nomination
• A personal statement from the candidate detailing his or her ACS service and interest in the position
• A current curriculum vitae
• The name of one individual who can serve as a reference
Further details
Entities such as surgical specialty societies, ACS Advisory Councils, ACS Committees, and ACS chapters that would like to provide a letter of nomination must provide at least two nominees and a description of their selection process along with the total list of applicants reviewed.
Any attempt to contact members of the NCBG by a candidate or on behalf of a candidate will be viewed negatively, and may possibly result in disqualification. Applications submitted without the requested information will not be considered.
Nominations may be submitted to [email protected]. If you have any questions, contact Emily Kalata at 312-202-5360 or e[email protected].
For information only, the current members of the Board of Regents who will be considered for re-election are (all MD, FACS): John L. D. Atkinson, James C. Denneny III, Timothy J. Eberlein, Henri R. Ford, Enrique Hernandez, L. Scott Levin, Linda Phillips, Anton A. Sidawy, Beth H. Sutton, and Steven D. Wexner.
The American College of Surgeons (ACS) 2018 Nominating Committee of the Fellows (NCF) and the Nominating Committee of the Board of Governors (NCBG) will be selecting nominees for leadership positions in the College as follows.
Call for nominations for Officers-Elect
The 2018 NCF will select nominees for the three Officer-Elect positions of the ACS: President-Elect, First Vice-President-Elect, and Second Vice-President-Elect. The deadline for submitting nominations is February 23, 2018.
Criteria for consideration
The NCF will use the following guidelines when considering potential candidates:
• Nominees must be loyal members of the College who have demonstrated outstanding integrity and an unquestioned devotion to the highest principles of surgical practice.
• Nominees must have demonstrated leadership qualities, such as service and active participation on ACS committees or in other components of the College.
• The ACS encourages consideration of women and underrepresented minorities for all leadership positions.
All nominations must include the following:
• A letter/letters of nomination
• A personal statement from the candidate detailing his or her ACS service and interest in the position (for President-Elect position only)
• A current curriculum vitae (CV)
• The name of one individual who can serve as a reference
Further details
Entities such as surgical specialty societies, ACS Advisory Councils, ACS committees, and ACS chapters that would like to provide a letter of nomination must provide a description of their selection process and the total list of applicants reviewed.
Any attempt to contact members of the NCF by a candidate or on behalf of a candidate will be viewed negatively, and may result in disqualification. Applications submitted without the requested information will not be considered.
Nominations must be submitted to o[email protected]. If you have any questions, contact Emily Kalata at 312-202-5360 or e[email protected].
Call for Nominations for Board of Regents
The 2018 NCBG will select nominees for pending vacancies on the Board of Regents to be filled at Clinical Congress 2018. The deadline for submitting nominations is February 23, 2018.
Criteria
The NCBG will use the following guidelines when considering potential candidates:
• Nominees must be loyal members of the College who have demonstrated outstanding integrity along with an unquestioned devotion to the highest principles of surgical practice.
• Nominees must have demonstrated leadership qualities, such as service and active participation on ACS committees or in other components of the College.
• The ACS encourages consideration of women and underrepresented minorities for all leadership positions.
• The NCBG recognizes the importance of the Board of Regents representing all who practice surgery in both academic and community practice, regardless of practice location or configuration.
• Nominations are open to surgeons of all specialties, but particular consideration will be given this nomination cycle to those in the following specialties:
o Burn and critical care surgery
o Gastrointestinal surgery
o General surgery
o Surgical oncology
o Transplantation
o Trauma
o Vascular surgery
Only individuals who are currently and expected to remain in active surgical practice for their entire term may be nominated for election or reelection to the Board of Regents.
All nominations must include the following:
• A letter of nomination
• A personal statement from the candidate detailing his or her ACS service and interest in the position
• A current curriculum vitae
• The name of one individual who can serve as a reference
Further details
Entities such as surgical specialty societies, ACS Advisory Councils, ACS Committees, and ACS chapters that would like to provide a letter of nomination must provide at least two nominees and a description of their selection process along with the total list of applicants reviewed.
Any attempt to contact members of the NCBG by a candidate or on behalf of a candidate will be viewed negatively, and may possibly result in disqualification. Applications submitted without the requested information will not be considered.
Nominations may be submitted to [email protected]. If you have any questions, contact Emily Kalata at 312-202-5360 or e[email protected].
For information only, the current members of the Board of Regents who will be considered for re-election are (all MD, FACS): John L. D. Atkinson, James C. Denneny III, Timothy J. Eberlein, Henri R. Ford, Enrique Hernandez, L. Scott Levin, Linda Phillips, Anton A. Sidawy, Beth H. Sutton, and Steven D. Wexner.
Ronald V. Maier, MD, FACS, Elected ACS President-Elect
Ronald V. Maier, MD, FACS, the Jane and Donald D. Trunkey Endowed Chair in Trauma Surgery, vice-chairman, department of surgery, and professor of surgery, University of Washington School of Medicine, Seattle, was elected President-Elect of the American College of Surgeons (ACS) at the October 25 Annual Business Meeting of the Members.
Read more about President-Elect and Vice-Presidents-Elect in the December Bulletin at bulletin.facs.org.
Ronald V. Maier, MD, FACS, the Jane and Donald D. Trunkey Endowed Chair in Trauma Surgery, vice-chairman, department of surgery, and professor of surgery, University of Washington School of Medicine, Seattle, was elected President-Elect of the American College of Surgeons (ACS) at the October 25 Annual Business Meeting of the Members.
Read more about President-Elect and Vice-Presidents-Elect in the December Bulletin at bulletin.facs.org.
Ronald V. Maier, MD, FACS, the Jane and Donald D. Trunkey Endowed Chair in Trauma Surgery, vice-chairman, department of surgery, and professor of surgery, University of Washington School of Medicine, Seattle, was elected President-Elect of the American College of Surgeons (ACS) at the October 25 Annual Business Meeting of the Members.
Read more about President-Elect and Vice-Presidents-Elect in the December Bulletin at bulletin.facs.org.
Read the December Bulletin: The joy and privilege of a surgical career
The December issue of the Bulletin of the American College of Surgeons is now available online at bulletin.facs.org. This month’s Bulletin includes the following features, columns, and news stories, among other:
Features
-Presidential Address: The joy and privilege of a surgical career
-ACS leaders visit Cuba, discover opportunities for collaboration
-Blockchain technology in health care: A primer for surgeons
Columns
-Looking forward: Highlights of College activities in 2017
-What surgeons should know about…The 2018 Inpatient Prospective Payment System final rule
-From residency to retirement: ACS Health Policy Scholar reports on the value of small acts
News
-ACSPA-SurgeonsPAC 2017–2018 election cycle update
-Medicare participation: Know your options
-Making quality stick: Optimal Resources for Surgical Quality and Safety: The SQSC and credentialing and privileging processes ensure sustainability of standards
The Bulletin is available in a variety of digital formats to satisfy every reader’s preference, including an interactive version and a smartphone app. Go to the Bulletin website at bulletin.facs.org to connect to any of these versions or to read the articles directly online.
The December issue of the Bulletin of the American College of Surgeons is now available online at bulletin.facs.org. This month’s Bulletin includes the following features, columns, and news stories, among other:
Features
-Presidential Address: The joy and privilege of a surgical career
-ACS leaders visit Cuba, discover opportunities for collaboration
-Blockchain technology in health care: A primer for surgeons
Columns
-Looking forward: Highlights of College activities in 2017
-What surgeons should know about…The 2018 Inpatient Prospective Payment System final rule
-From residency to retirement: ACS Health Policy Scholar reports on the value of small acts
News
-ACSPA-SurgeonsPAC 2017–2018 election cycle update
-Medicare participation: Know your options
-Making quality stick: Optimal Resources for Surgical Quality and Safety: The SQSC and credentialing and privileging processes ensure sustainability of standards
The Bulletin is available in a variety of digital formats to satisfy every reader’s preference, including an interactive version and a smartphone app. Go to the Bulletin website at bulletin.facs.org to connect to any of these versions or to read the articles directly online.
The December issue of the Bulletin of the American College of Surgeons is now available online at bulletin.facs.org. This month’s Bulletin includes the following features, columns, and news stories, among other:
Features
-Presidential Address: The joy and privilege of a surgical career
-ACS leaders visit Cuba, discover opportunities for collaboration
-Blockchain technology in health care: A primer for surgeons
Columns
-Looking forward: Highlights of College activities in 2017
-What surgeons should know about…The 2018 Inpatient Prospective Payment System final rule
-From residency to retirement: ACS Health Policy Scholar reports on the value of small acts
News
-ACSPA-SurgeonsPAC 2017–2018 election cycle update
-Medicare participation: Know your options
-Making quality stick: Optimal Resources for Surgical Quality and Safety: The SQSC and credentialing and privileging processes ensure sustainability of standards
The Bulletin is available in a variety of digital formats to satisfy every reader’s preference, including an interactive version and a smartphone app. Go to the Bulletin website at bulletin.facs.org to connect to any of these versions or to read the articles directly online.
Gene therapy normalized or near-normalized factor VIII in hemophilia A
ATLANTA – A single infusion of valoctocogene roxaparvovec normalized or nearly normalized factor VIII levels in 11 of 13 adults with severe hemophilia A, eliminated spontaneous bleeds and the need for factor VIII infusions, showed durable effects for up to 72 weeks of follow-up, K. John Pasi, MD, said at the annual meeting of the American Society of Hematology.
Nicknamed valrox and designated as a breakthrough therapy by the Food and Drug Administration in October 2017, valoctocogene roxaparvovec uses an adenoviral vector to deliver a functional copy of the factor VIII gene to patients with hemophilia A, said Dr. Pasi of Barts and The London School of Medicine and Dentistry.
Gene therapy has long been the “holy grail” for managing hemophilia because it is a single-gene disorder with a clear relationship between clotting factor level and bleeding severity, Dr. Pasi said. In a mouse model of hemophilia A, valrox restored factor VIII plasma concentrations to levels thought to be adequate to support normal clotting in humans.
Accordingly, the phase 2/3 enrolled 13 patients with severe hemophilia A whose baseline factor VIII levels were less than 1 IU/dL. Patients started at the lowest dose of gene therapy (4 x 1013 vector genomes/kg) and then received a higher dose ( 6 x 1013 VG/kg) if their factor VIII level remained under 5 IU/dL at week 3. Six patients received the lower dose and seven received the higher dose.
At 78 weeks, median factor VIII level in the higher-dose cohort was 90 IU/mL, as Dr. Pasi and his associates reported simultaneously in the New England Journal of Medicine (2017 Dec 9. doi: 10. 1056/NEJMoa1708483).
Before undergoing gene therapy, study participants had endured up to 41 breakthrough bleeds per year despite often receiving more than 150 infusions of factor VIII annually. Median annualized bleeding rates, which at baseline were 16.5 in the higher dose group and 8 in the lower dose group, zeroed out in both groups after factor VIII activity rose above 5%. Quality of life was evaluated in five patients, who reported substantial improvements across all domains.
All patients began producing factor VIII several weeks after infusion. Median levels plateaued within normal range by 20 weeks in the higher-dose group. At the lower dose, median levels rose steadily to a median of 34 IU/dL by 20 weeks. Additionally, three recipients of the lower dose who were followed for 32 weeks achieved factor VIII levels within normal range (median 51 IU/dL). Levels of factor VIII remained within normal range for up to 78 weeks of posttreatment follow-up, Dr. Pasi said.
No patients developed inhibitors or signs of immune-related adverse effects, nor were adverse events qualitatively different between dose groups, Dr. Pasi said. The most common adverse effects were transient increases in alanine transaminase (ALT), which peaked between 44 IU/L and 141 IU/L and lasted anywhere from several days to 15 weeks. Patients whose ALT rose 1.5-fold above baseline received short-term corticosteroids with no adverse effects. All but one was tapered off. There were two serious adverse events – one elective knee surgery and one case of transient fever, headache, and myalgia at time of infusion.
So far, valrox appears to be long lasting, but “durability is a huge question for any gene therapy approach,” Dr. Pasi said. “The only way to answer it is to follow patients through.”
In hemophilia B, studies indicate that some patients continue expressing factor IX years after a single infusion of gene therapy (N Engl J Med. 2017 Dec 7;377:2215-27).
Two phase 3 trials will further evaluate safety and optimal dosing of valrox, Dr. Pasi said. The GENEr8-1 trial will use the 6 x 1013 VG/kg dose and the GENEr8-2 trial will use the 4 x 1013 VG/kg dose. Like the pilot study, these trials will exclude patients with inhibitors, but they may include patients with comorbidities such as liver disease, he said.
Valrox was previously known as BMN 270.
The study was sponsored by BioMarin. Dr. Pasi disclosed research funding, consultancy fees, and speaker and advisory relationships with BioMarin. He disclosed ties to many other companies that develop hemophilia therapies.
SOURCE: Pasi KJ et al. ASH 2017 Abstract 603
ATLANTA – A single infusion of valoctocogene roxaparvovec normalized or nearly normalized factor VIII levels in 11 of 13 adults with severe hemophilia A, eliminated spontaneous bleeds and the need for factor VIII infusions, showed durable effects for up to 72 weeks of follow-up, K. John Pasi, MD, said at the annual meeting of the American Society of Hematology.
Nicknamed valrox and designated as a breakthrough therapy by the Food and Drug Administration in October 2017, valoctocogene roxaparvovec uses an adenoviral vector to deliver a functional copy of the factor VIII gene to patients with hemophilia A, said Dr. Pasi of Barts and The London School of Medicine and Dentistry.
Gene therapy has long been the “holy grail” for managing hemophilia because it is a single-gene disorder with a clear relationship between clotting factor level and bleeding severity, Dr. Pasi said. In a mouse model of hemophilia A, valrox restored factor VIII plasma concentrations to levels thought to be adequate to support normal clotting in humans.
Accordingly, the phase 2/3 enrolled 13 patients with severe hemophilia A whose baseline factor VIII levels were less than 1 IU/dL. Patients started at the lowest dose of gene therapy (4 x 1013 vector genomes/kg) and then received a higher dose ( 6 x 1013 VG/kg) if their factor VIII level remained under 5 IU/dL at week 3. Six patients received the lower dose and seven received the higher dose.
At 78 weeks, median factor VIII level in the higher-dose cohort was 90 IU/mL, as Dr. Pasi and his associates reported simultaneously in the New England Journal of Medicine (2017 Dec 9. doi: 10. 1056/NEJMoa1708483).
Before undergoing gene therapy, study participants had endured up to 41 breakthrough bleeds per year despite often receiving more than 150 infusions of factor VIII annually. Median annualized bleeding rates, which at baseline were 16.5 in the higher dose group and 8 in the lower dose group, zeroed out in both groups after factor VIII activity rose above 5%. Quality of life was evaluated in five patients, who reported substantial improvements across all domains.
All patients began producing factor VIII several weeks after infusion. Median levels plateaued within normal range by 20 weeks in the higher-dose group. At the lower dose, median levels rose steadily to a median of 34 IU/dL by 20 weeks. Additionally, three recipients of the lower dose who were followed for 32 weeks achieved factor VIII levels within normal range (median 51 IU/dL). Levels of factor VIII remained within normal range for up to 78 weeks of posttreatment follow-up, Dr. Pasi said.
No patients developed inhibitors or signs of immune-related adverse effects, nor were adverse events qualitatively different between dose groups, Dr. Pasi said. The most common adverse effects were transient increases in alanine transaminase (ALT), which peaked between 44 IU/L and 141 IU/L and lasted anywhere from several days to 15 weeks. Patients whose ALT rose 1.5-fold above baseline received short-term corticosteroids with no adverse effects. All but one was tapered off. There were two serious adverse events – one elective knee surgery and one case of transient fever, headache, and myalgia at time of infusion.
So far, valrox appears to be long lasting, but “durability is a huge question for any gene therapy approach,” Dr. Pasi said. “The only way to answer it is to follow patients through.”
In hemophilia B, studies indicate that some patients continue expressing factor IX years after a single infusion of gene therapy (N Engl J Med. 2017 Dec 7;377:2215-27).
Two phase 3 trials will further evaluate safety and optimal dosing of valrox, Dr. Pasi said. The GENEr8-1 trial will use the 6 x 1013 VG/kg dose and the GENEr8-2 trial will use the 4 x 1013 VG/kg dose. Like the pilot study, these trials will exclude patients with inhibitors, but they may include patients with comorbidities such as liver disease, he said.
Valrox was previously known as BMN 270.
The study was sponsored by BioMarin. Dr. Pasi disclosed research funding, consultancy fees, and speaker and advisory relationships with BioMarin. He disclosed ties to many other companies that develop hemophilia therapies.
SOURCE: Pasi KJ et al. ASH 2017 Abstract 603
ATLANTA – A single infusion of valoctocogene roxaparvovec normalized or nearly normalized factor VIII levels in 11 of 13 adults with severe hemophilia A, eliminated spontaneous bleeds and the need for factor VIII infusions, showed durable effects for up to 72 weeks of follow-up, K. John Pasi, MD, said at the annual meeting of the American Society of Hematology.
Nicknamed valrox and designated as a breakthrough therapy by the Food and Drug Administration in October 2017, valoctocogene roxaparvovec uses an adenoviral vector to deliver a functional copy of the factor VIII gene to patients with hemophilia A, said Dr. Pasi of Barts and The London School of Medicine and Dentistry.
Gene therapy has long been the “holy grail” for managing hemophilia because it is a single-gene disorder with a clear relationship between clotting factor level and bleeding severity, Dr. Pasi said. In a mouse model of hemophilia A, valrox restored factor VIII plasma concentrations to levels thought to be adequate to support normal clotting in humans.
Accordingly, the phase 2/3 enrolled 13 patients with severe hemophilia A whose baseline factor VIII levels were less than 1 IU/dL. Patients started at the lowest dose of gene therapy (4 x 1013 vector genomes/kg) and then received a higher dose ( 6 x 1013 VG/kg) if their factor VIII level remained under 5 IU/dL at week 3. Six patients received the lower dose and seven received the higher dose.
At 78 weeks, median factor VIII level in the higher-dose cohort was 90 IU/mL, as Dr. Pasi and his associates reported simultaneously in the New England Journal of Medicine (2017 Dec 9. doi: 10. 1056/NEJMoa1708483).
Before undergoing gene therapy, study participants had endured up to 41 breakthrough bleeds per year despite often receiving more than 150 infusions of factor VIII annually. Median annualized bleeding rates, which at baseline were 16.5 in the higher dose group and 8 in the lower dose group, zeroed out in both groups after factor VIII activity rose above 5%. Quality of life was evaluated in five patients, who reported substantial improvements across all domains.
All patients began producing factor VIII several weeks after infusion. Median levels plateaued within normal range by 20 weeks in the higher-dose group. At the lower dose, median levels rose steadily to a median of 34 IU/dL by 20 weeks. Additionally, three recipients of the lower dose who were followed for 32 weeks achieved factor VIII levels within normal range (median 51 IU/dL). Levels of factor VIII remained within normal range for up to 78 weeks of posttreatment follow-up, Dr. Pasi said.
No patients developed inhibitors or signs of immune-related adverse effects, nor were adverse events qualitatively different between dose groups, Dr. Pasi said. The most common adverse effects were transient increases in alanine transaminase (ALT), which peaked between 44 IU/L and 141 IU/L and lasted anywhere from several days to 15 weeks. Patients whose ALT rose 1.5-fold above baseline received short-term corticosteroids with no adverse effects. All but one was tapered off. There were two serious adverse events – one elective knee surgery and one case of transient fever, headache, and myalgia at time of infusion.
So far, valrox appears to be long lasting, but “durability is a huge question for any gene therapy approach,” Dr. Pasi said. “The only way to answer it is to follow patients through.”
In hemophilia B, studies indicate that some patients continue expressing factor IX years after a single infusion of gene therapy (N Engl J Med. 2017 Dec 7;377:2215-27).
Two phase 3 trials will further evaluate safety and optimal dosing of valrox, Dr. Pasi said. The GENEr8-1 trial will use the 6 x 1013 VG/kg dose and the GENEr8-2 trial will use the 4 x 1013 VG/kg dose. Like the pilot study, these trials will exclude patients with inhibitors, but they may include patients with comorbidities such as liver disease, he said.
Valrox was previously known as BMN 270.
The study was sponsored by BioMarin. Dr. Pasi disclosed research funding, consultancy fees, and speaker and advisory relationships with BioMarin. He disclosed ties to many other companies that develop hemophilia therapies.
SOURCE: Pasi KJ et al. ASH 2017 Abstract 603
REPORTING FROM ASH 2017
Key clinical point: The investigational gene therapy BMN 270 (valoctocogene roxaparvovec; valrox) eliminated spontaneous bleeds and the need for factor VIII infusions in patients with hemophilia A, and the effects persisted for up to 78 weeks.
Major finding: Median FVIII level was 90 IU/dL at 78 weeks in the higher (6 x 1013 VG/kg) dose cohort.
Data source: A phase I/II, first-in-human study of adenoassociated viral factor VIII gene transfer in 15 patients with severe hemophilia A without inhibitors.
Disclosures: The study was sponsored by BioMarin. Dr. Pasi disclosed research funding, consultancy fees, and speaker and advisory relationships with BioMarin. He disclosed ties to many other companies that develop hemophilia therapies.
Source: Pasi KJ et al. ASH 2017 Abstract 603
Hallmark tumor metabolism becomes a validated therapeutic target
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
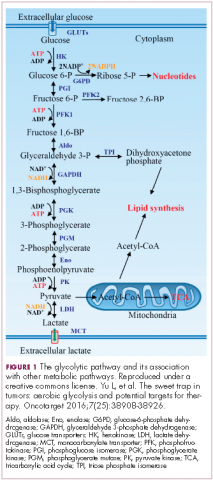
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
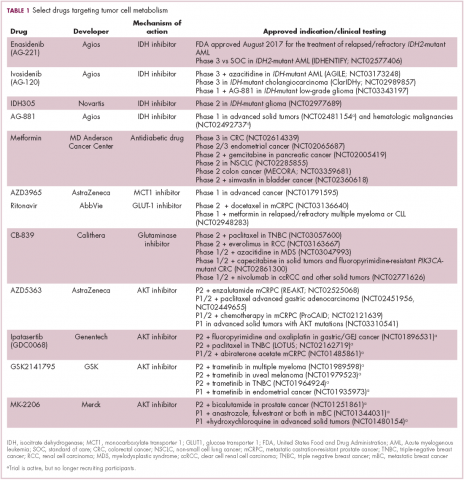
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
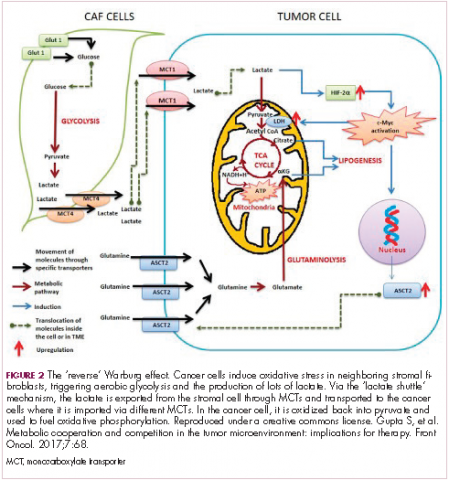
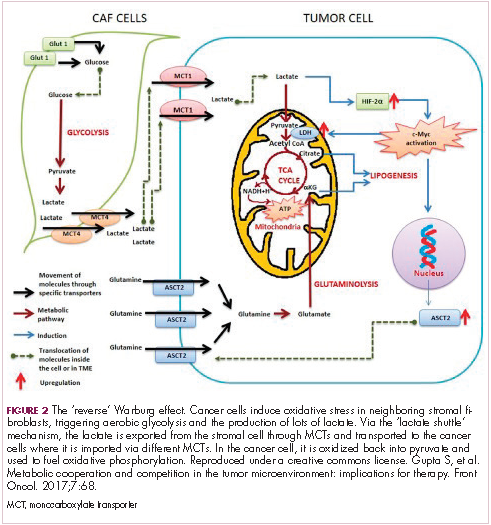
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
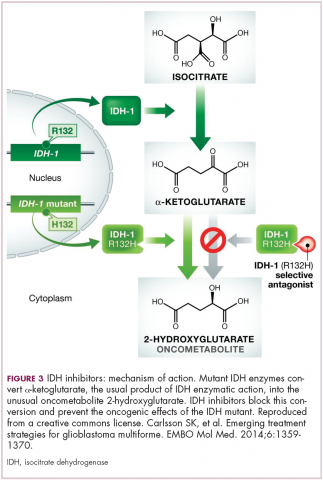
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
Gene therapy regimen for XSCID shows rapid results in newly diagnosed infants
ATLANTA – A new approach to gene therapy produced rapid T cell reconstruction and normal markers of B-cell and natural killer (NK)-cell function in newly diagnosed infants with X-linked Severe Combined Immunodeficiency (XSCID), according to initial results from a phase 1/2 trial.
Researchers used a regimen of safety-modified lentiviral vector plus reduced-exposure busulfan conditioning in seven newly diagnosed infants and found that it was well tolerated in all patients and quickly improved T-cell immunity in five of seven patients.
Initial gene therapy trials used a mouse vector without busulfan conditioning. These trials produced T-cell correction but were unable to restore B-cell function, NK-cell function, or myeloid-cell function. As a result, patients continued to experience viral infections and required monthly, life-long intravenous immunoglobulin infusions, Dr. Mamcarz said.
The phase 1/2 multicenter safety and efficacy study tested the gene therapy regimen for the first time in seven newly diagnosed infants. The regimen involved transducing purified bone marrow CD34+ cells with the lentiviral vector, which was generated by a stable producer cell line and then cryopreserved. The busulfan was given as two single daily doses, which were tailored based on patient age and weight.
In total, seven patients have been treated and all tolerated the low-dose busulfan chemotherapy well. Five patients had normal numbers of previously defective cells (T cells, B cells, and NK cells) and were taken off protective isolation and prophylactic medications. Those infants appear to have functioning immune systems, Dr. Mamcarz said. One infant has stopped monthly intravenous immunoglobulin infusions and has received normal pediatric vaccines, but responses have not yet been tested.
For the two infants who did not show responses, one is early in the trial and there is not yet adequate follow-up data to assess the immune system reconstitution, Dr. Mamcarz said.
The other infant had high levels of maternal T cell engraftment, severe neutropenia, and ongoing cytomegalovirus infection, resulting in delayed and partial T-cell reconstitution. The researchers sought to boost the patient’s immunity through an infusion of corrected cells – without busulfan – at 1 year after receiving the initial therapy. Three months after the second treatment, the infant has normal functioning of T cells and NK cells, but he is not yet engrafting B cells, Dr. Mamcarz said.
Overall, there was no evidence of vector-mediated effects on blood formation, Dr. Mamcarz reported.
The ultimate evaluation of the efficacy of the trial would be to assess vaccine responses in the infants treated with gene therapy, she said.
The study was supported by the Assisi Foundation of Memphis and the California Institute of Regenerative Medicine. Dr. Mamcarz reported having no relevant financial disclosures. Her coauthors reported financial relationships with InsightRX, UpToDate, Invitae, and Homology Medicines.
[email protected]
SOURCE: Mamcarz E et al. Abstract 523.
ATLANTA – A new approach to gene therapy produced rapid T cell reconstruction and normal markers of B-cell and natural killer (NK)-cell function in newly diagnosed infants with X-linked Severe Combined Immunodeficiency (XSCID), according to initial results from a phase 1/2 trial.
Researchers used a regimen of safety-modified lentiviral vector plus reduced-exposure busulfan conditioning in seven newly diagnosed infants and found that it was well tolerated in all patients and quickly improved T-cell immunity in five of seven patients.
Initial gene therapy trials used a mouse vector without busulfan conditioning. These trials produced T-cell correction but were unable to restore B-cell function, NK-cell function, or myeloid-cell function. As a result, patients continued to experience viral infections and required monthly, life-long intravenous immunoglobulin infusions, Dr. Mamcarz said.
The phase 1/2 multicenter safety and efficacy study tested the gene therapy regimen for the first time in seven newly diagnosed infants. The regimen involved transducing purified bone marrow CD34+ cells with the lentiviral vector, which was generated by a stable producer cell line and then cryopreserved. The busulfan was given as two single daily doses, which were tailored based on patient age and weight.
In total, seven patients have been treated and all tolerated the low-dose busulfan chemotherapy well. Five patients had normal numbers of previously defective cells (T cells, B cells, and NK cells) and were taken off protective isolation and prophylactic medications. Those infants appear to have functioning immune systems, Dr. Mamcarz said. One infant has stopped monthly intravenous immunoglobulin infusions and has received normal pediatric vaccines, but responses have not yet been tested.
For the two infants who did not show responses, one is early in the trial and there is not yet adequate follow-up data to assess the immune system reconstitution, Dr. Mamcarz said.
The other infant had high levels of maternal T cell engraftment, severe neutropenia, and ongoing cytomegalovirus infection, resulting in delayed and partial T-cell reconstitution. The researchers sought to boost the patient’s immunity through an infusion of corrected cells – without busulfan – at 1 year after receiving the initial therapy. Three months after the second treatment, the infant has normal functioning of T cells and NK cells, but he is not yet engrafting B cells, Dr. Mamcarz said.
Overall, there was no evidence of vector-mediated effects on blood formation, Dr. Mamcarz reported.
The ultimate evaluation of the efficacy of the trial would be to assess vaccine responses in the infants treated with gene therapy, she said.
The study was supported by the Assisi Foundation of Memphis and the California Institute of Regenerative Medicine. Dr. Mamcarz reported having no relevant financial disclosures. Her coauthors reported financial relationships with InsightRX, UpToDate, Invitae, and Homology Medicines.
[email protected]
SOURCE: Mamcarz E et al. Abstract 523.
ATLANTA – A new approach to gene therapy produced rapid T cell reconstruction and normal markers of B-cell and natural killer (NK)-cell function in newly diagnosed infants with X-linked Severe Combined Immunodeficiency (XSCID), according to initial results from a phase 1/2 trial.
Researchers used a regimen of safety-modified lentiviral vector plus reduced-exposure busulfan conditioning in seven newly diagnosed infants and found that it was well tolerated in all patients and quickly improved T-cell immunity in five of seven patients.
Initial gene therapy trials used a mouse vector without busulfan conditioning. These trials produced T-cell correction but were unable to restore B-cell function, NK-cell function, or myeloid-cell function. As a result, patients continued to experience viral infections and required monthly, life-long intravenous immunoglobulin infusions, Dr. Mamcarz said.
The phase 1/2 multicenter safety and efficacy study tested the gene therapy regimen for the first time in seven newly diagnosed infants. The regimen involved transducing purified bone marrow CD34+ cells with the lentiviral vector, which was generated by a stable producer cell line and then cryopreserved. The busulfan was given as two single daily doses, which were tailored based on patient age and weight.
In total, seven patients have been treated and all tolerated the low-dose busulfan chemotherapy well. Five patients had normal numbers of previously defective cells (T cells, B cells, and NK cells) and were taken off protective isolation and prophylactic medications. Those infants appear to have functioning immune systems, Dr. Mamcarz said. One infant has stopped monthly intravenous immunoglobulin infusions and has received normal pediatric vaccines, but responses have not yet been tested.
For the two infants who did not show responses, one is early in the trial and there is not yet adequate follow-up data to assess the immune system reconstitution, Dr. Mamcarz said.
The other infant had high levels of maternal T cell engraftment, severe neutropenia, and ongoing cytomegalovirus infection, resulting in delayed and partial T-cell reconstitution. The researchers sought to boost the patient’s immunity through an infusion of corrected cells – without busulfan – at 1 year after receiving the initial therapy. Three months after the second treatment, the infant has normal functioning of T cells and NK cells, but he is not yet engrafting B cells, Dr. Mamcarz said.
Overall, there was no evidence of vector-mediated effects on blood formation, Dr. Mamcarz reported.
The ultimate evaluation of the efficacy of the trial would be to assess vaccine responses in the infants treated with gene therapy, she said.
The study was supported by the Assisi Foundation of Memphis and the California Institute of Regenerative Medicine. Dr. Mamcarz reported having no relevant financial disclosures. Her coauthors reported financial relationships with InsightRX, UpToDate, Invitae, and Homology Medicines.
[email protected]
SOURCE: Mamcarz E et al. Abstract 523.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: In total, five of seven infants with XSCID had normal functioning of T cells, B cells, and NK cells.
Study details: A phase 1/2 multicenter study of seven infants with newly diagnosed XSCID.
Disclosures: The study was supported by the Assisi Foundation of Memphis and the California Institute of Regenerative Medicine. Dr. Mamcarz reported having no relevant financial disclosures. Her coauthors reported financial relationships with InsightRX, UpToDate, Invitae, and Homology Medicines.
Source: Mamcarz E et al. Abstract 523.