User login
Using “design thinking” to improve health care
Health care workers creating innovations by applying “design thinking” – “a human-centered approach to innovation” that comes from the business world – is a growing trend, according to a recent New York Times article.
“With design thinking, the innovations come from those who actually work there, providing feedback to designers to improve the final product,” wrote author Amitha Kalaichandran, MD, MHS.
“Health providers ... are uniquely positioned to come up with fresh solutions to health care problems,” Dr. Kalaichandran wrote. An example at her own hospital: The leader of the trauma team now wears an orange vest, clearly identifying who’s in charge in a potentially chaotic situation. It was an idea created by a hospital nurse.
“A 2016 report that looked at ways in which a health system can implement design thinking identified three principles behind the approach: empathy for the user, in this case a patient, doctor or other health care provider; the involvement of an interdisciplinary team; and rapid prototyping of the idea,” she wrote. “To develop a truly useful product, a comprehensive understanding of the problem the innovation aims to solve is paramount.”
In design thinking, described as creative, multidisciplinary thinking around a problem, groups naturally coalesce to find such solutions. The article cites examples such as Clinicians for Design, an international group of providers focused on improving hospital layouts, and Health Design by Us, a collaborative group that supports health care innovations such as a mobile system for diabetes management, designed by a patient.
Reference
Kalaichandran A. Design thinking for doctors and nurses. The New York Times. Aug. 3, 2017. Accessed Aug. 7, 2017.
Health care workers creating innovations by applying “design thinking” – “a human-centered approach to innovation” that comes from the business world – is a growing trend, according to a recent New York Times article.
“With design thinking, the innovations come from those who actually work there, providing feedback to designers to improve the final product,” wrote author Amitha Kalaichandran, MD, MHS.
“Health providers ... are uniquely positioned to come up with fresh solutions to health care problems,” Dr. Kalaichandran wrote. An example at her own hospital: The leader of the trauma team now wears an orange vest, clearly identifying who’s in charge in a potentially chaotic situation. It was an idea created by a hospital nurse.
“A 2016 report that looked at ways in which a health system can implement design thinking identified three principles behind the approach: empathy for the user, in this case a patient, doctor or other health care provider; the involvement of an interdisciplinary team; and rapid prototyping of the idea,” she wrote. “To develop a truly useful product, a comprehensive understanding of the problem the innovation aims to solve is paramount.”
In design thinking, described as creative, multidisciplinary thinking around a problem, groups naturally coalesce to find such solutions. The article cites examples such as Clinicians for Design, an international group of providers focused on improving hospital layouts, and Health Design by Us, a collaborative group that supports health care innovations such as a mobile system for diabetes management, designed by a patient.
Reference
Kalaichandran A. Design thinking for doctors and nurses. The New York Times. Aug. 3, 2017. Accessed Aug. 7, 2017.
Health care workers creating innovations by applying “design thinking” – “a human-centered approach to innovation” that comes from the business world – is a growing trend, according to a recent New York Times article.
“With design thinking, the innovations come from those who actually work there, providing feedback to designers to improve the final product,” wrote author Amitha Kalaichandran, MD, MHS.
“Health providers ... are uniquely positioned to come up with fresh solutions to health care problems,” Dr. Kalaichandran wrote. An example at her own hospital: The leader of the trauma team now wears an orange vest, clearly identifying who’s in charge in a potentially chaotic situation. It was an idea created by a hospital nurse.
“A 2016 report that looked at ways in which a health system can implement design thinking identified three principles behind the approach: empathy for the user, in this case a patient, doctor or other health care provider; the involvement of an interdisciplinary team; and rapid prototyping of the idea,” she wrote. “To develop a truly useful product, a comprehensive understanding of the problem the innovation aims to solve is paramount.”
In design thinking, described as creative, multidisciplinary thinking around a problem, groups naturally coalesce to find such solutions. The article cites examples such as Clinicians for Design, an international group of providers focused on improving hospital layouts, and Health Design by Us, a collaborative group that supports health care innovations such as a mobile system for diabetes management, designed by a patient.
Reference
Kalaichandran A. Design thinking for doctors and nurses. The New York Times. Aug. 3, 2017. Accessed Aug. 7, 2017.
Early weight change has no special effect on mortality in RA
Weight loss at the time of rheumatoid arthritis diagnosis had the same impact on mortality in patients with and without RA, according to research trying to solve the so-called obesity paradox in RA, which has been related to prior observations of a protective effect of obesity on mortality in RA patients.
The finding indicates that clinicians can continue to encourage patients with rheumatoid arthritis to follow healthy weight-loss strategies, according to first author of the study, Jeffrey Sparks, MD, of Brigham and Women’s Hospital in Boston.
Dr. Sparks and his colleagues examined the impact of weight change and mortality in RA patients based on data from the Nurses’ Health Study.
“Our study is the first to focus on weight change around RA diagnosis and risk of death, rather than weight change in patients who had RA for many years,” Dr. Sparks noted.
By examining changes in weight near the time of RA diagnosis, Dr. Sparks and his colleagues said that they hoped to extract information about RA-specific processes rather than the underlying pathologies that might cause weight changes near the end of life.
In the study published in Arthritis & Rheumatology, the researchers compared women diagnosed with RA during follow-up to women without RA during the same index time period of 1976-2016. The study population included 121,701 women. Of these, 902 developed incident RA and were matched with 7,884 non-RA controls.
During an average of 18 years of follow-up, 41% of the RA cohort and 29% of the controls died. The risk of death was approximately twice as high (hazard ratio, 2.78; 95% confidence interval, 1.58-4.89) among those with weight loss greater than 30 pounds at the time of RA diagnosis, compared with those whose weight remained stable. However, the risk for mortality was similarly increased (HR, 2.16; 95% CI, 1.61-2.88) among the controls with weight loss greater than 30 pounds, compared with those with stable weight. No association with mortality was noted in either group among women who gained more than 30 pounds at the time of RA diagnosis.
Dr. Sparks said he was somewhat surprised by the findings.
“We expected severe, pathologic weight loss to be associated with increased risk of death among patients with RA and comparators. It was somewhat surprising that the risks in both groups were similar,” he said. “Conversely, prior studies suggested that weight gain might have been associated with increased risk of death. However, we found no association of weight gain with risk of death,” he noted.
In addition, “Our findings argue that there is not an RA-specific mortality risk based on either weight loss or gain,” he said. “While we found that weight loss was associated with increased mortality, this was most pronounced in the severe weight loss group, so was likely due to unintentional weight loss.”
Joshua F. Baker, MD, of the University of Pennsylvania, Philadelphia, and his colleagues identified an association between weight change and risk of death in RA patients in a study first published online in Arthritis & Rheumatology in 2015 (Arthritis Rheumatol. 2015 Jul;67[7]:1711-17). That study addressed the so-called obesity paradox in RA, and Dr. Baker and his colleagues noted that weight loss associated with the development of chronic illness is a significant confounder that may explain the observed protective effect of obesity on mortality.
“It is not clear how best to monitor changes in weight, when exactly to become concerned, and what to do when changes are observed,” Dr. Baker noted. “RA patients may lose weight for a number of reasons, not all related to their arthritis, and it is unlikely that there is a ‘one size fits all’ approach,” he said.
The study was limited in part by the women-only study population, so the results might not be generalizable to men, Dr. Sparks said. “The reason for weight change was unavailable,” he added. Directions for further research include investigation of how factors such as physical activity, diet, and weight loss may affect the risk of death among individuals with and without RA, he said.
Dr. Sparks had no financial conflicts to disclose. The study was supported in part by the National Institutes of Health and the Rheumatology Research Foundation’s Disease-Targeted Innovative Award and Scientist Development Awards.
SOURCE: Sparks J et al. Arthritis Rheumatol. 2017 Nov 30. doi: 10.1002/art.40346.
Weight loss at the time of rheumatoid arthritis diagnosis had the same impact on mortality in patients with and without RA, according to research trying to solve the so-called obesity paradox in RA, which has been related to prior observations of a protective effect of obesity on mortality in RA patients.
The finding indicates that clinicians can continue to encourage patients with rheumatoid arthritis to follow healthy weight-loss strategies, according to first author of the study, Jeffrey Sparks, MD, of Brigham and Women’s Hospital in Boston.
Dr. Sparks and his colleagues examined the impact of weight change and mortality in RA patients based on data from the Nurses’ Health Study.
“Our study is the first to focus on weight change around RA diagnosis and risk of death, rather than weight change in patients who had RA for many years,” Dr. Sparks noted.
By examining changes in weight near the time of RA diagnosis, Dr. Sparks and his colleagues said that they hoped to extract information about RA-specific processes rather than the underlying pathologies that might cause weight changes near the end of life.
In the study published in Arthritis & Rheumatology, the researchers compared women diagnosed with RA during follow-up to women without RA during the same index time period of 1976-2016. The study population included 121,701 women. Of these, 902 developed incident RA and were matched with 7,884 non-RA controls.
During an average of 18 years of follow-up, 41% of the RA cohort and 29% of the controls died. The risk of death was approximately twice as high (hazard ratio, 2.78; 95% confidence interval, 1.58-4.89) among those with weight loss greater than 30 pounds at the time of RA diagnosis, compared with those whose weight remained stable. However, the risk for mortality was similarly increased (HR, 2.16; 95% CI, 1.61-2.88) among the controls with weight loss greater than 30 pounds, compared with those with stable weight. No association with mortality was noted in either group among women who gained more than 30 pounds at the time of RA diagnosis.
Dr. Sparks said he was somewhat surprised by the findings.
“We expected severe, pathologic weight loss to be associated with increased risk of death among patients with RA and comparators. It was somewhat surprising that the risks in both groups were similar,” he said. “Conversely, prior studies suggested that weight gain might have been associated with increased risk of death. However, we found no association of weight gain with risk of death,” he noted.
In addition, “Our findings argue that there is not an RA-specific mortality risk based on either weight loss or gain,” he said. “While we found that weight loss was associated with increased mortality, this was most pronounced in the severe weight loss group, so was likely due to unintentional weight loss.”
Joshua F. Baker, MD, of the University of Pennsylvania, Philadelphia, and his colleagues identified an association between weight change and risk of death in RA patients in a study first published online in Arthritis & Rheumatology in 2015 (Arthritis Rheumatol. 2015 Jul;67[7]:1711-17). That study addressed the so-called obesity paradox in RA, and Dr. Baker and his colleagues noted that weight loss associated with the development of chronic illness is a significant confounder that may explain the observed protective effect of obesity on mortality.
“It is not clear how best to monitor changes in weight, when exactly to become concerned, and what to do when changes are observed,” Dr. Baker noted. “RA patients may lose weight for a number of reasons, not all related to their arthritis, and it is unlikely that there is a ‘one size fits all’ approach,” he said.
The study was limited in part by the women-only study population, so the results might not be generalizable to men, Dr. Sparks said. “The reason for weight change was unavailable,” he added. Directions for further research include investigation of how factors such as physical activity, diet, and weight loss may affect the risk of death among individuals with and without RA, he said.
Dr. Sparks had no financial conflicts to disclose. The study was supported in part by the National Institutes of Health and the Rheumatology Research Foundation’s Disease-Targeted Innovative Award and Scientist Development Awards.
SOURCE: Sparks J et al. Arthritis Rheumatol. 2017 Nov 30. doi: 10.1002/art.40346.
Weight loss at the time of rheumatoid arthritis diagnosis had the same impact on mortality in patients with and without RA, according to research trying to solve the so-called obesity paradox in RA, which has been related to prior observations of a protective effect of obesity on mortality in RA patients.
The finding indicates that clinicians can continue to encourage patients with rheumatoid arthritis to follow healthy weight-loss strategies, according to first author of the study, Jeffrey Sparks, MD, of Brigham and Women’s Hospital in Boston.
Dr. Sparks and his colleagues examined the impact of weight change and mortality in RA patients based on data from the Nurses’ Health Study.
“Our study is the first to focus on weight change around RA diagnosis and risk of death, rather than weight change in patients who had RA for many years,” Dr. Sparks noted.
By examining changes in weight near the time of RA diagnosis, Dr. Sparks and his colleagues said that they hoped to extract information about RA-specific processes rather than the underlying pathologies that might cause weight changes near the end of life.
In the study published in Arthritis & Rheumatology, the researchers compared women diagnosed with RA during follow-up to women without RA during the same index time period of 1976-2016. The study population included 121,701 women. Of these, 902 developed incident RA and were matched with 7,884 non-RA controls.
During an average of 18 years of follow-up, 41% of the RA cohort and 29% of the controls died. The risk of death was approximately twice as high (hazard ratio, 2.78; 95% confidence interval, 1.58-4.89) among those with weight loss greater than 30 pounds at the time of RA diagnosis, compared with those whose weight remained stable. However, the risk for mortality was similarly increased (HR, 2.16; 95% CI, 1.61-2.88) among the controls with weight loss greater than 30 pounds, compared with those with stable weight. No association with mortality was noted in either group among women who gained more than 30 pounds at the time of RA diagnosis.
Dr. Sparks said he was somewhat surprised by the findings.
“We expected severe, pathologic weight loss to be associated with increased risk of death among patients with RA and comparators. It was somewhat surprising that the risks in both groups were similar,” he said. “Conversely, prior studies suggested that weight gain might have been associated with increased risk of death. However, we found no association of weight gain with risk of death,” he noted.
In addition, “Our findings argue that there is not an RA-specific mortality risk based on either weight loss or gain,” he said. “While we found that weight loss was associated with increased mortality, this was most pronounced in the severe weight loss group, so was likely due to unintentional weight loss.”
Joshua F. Baker, MD, of the University of Pennsylvania, Philadelphia, and his colleagues identified an association between weight change and risk of death in RA patients in a study first published online in Arthritis & Rheumatology in 2015 (Arthritis Rheumatol. 2015 Jul;67[7]:1711-17). That study addressed the so-called obesity paradox in RA, and Dr. Baker and his colleagues noted that weight loss associated with the development of chronic illness is a significant confounder that may explain the observed protective effect of obesity on mortality.
“It is not clear how best to monitor changes in weight, when exactly to become concerned, and what to do when changes are observed,” Dr. Baker noted. “RA patients may lose weight for a number of reasons, not all related to their arthritis, and it is unlikely that there is a ‘one size fits all’ approach,” he said.
The study was limited in part by the women-only study population, so the results might not be generalizable to men, Dr. Sparks said. “The reason for weight change was unavailable,” he added. Directions for further research include investigation of how factors such as physical activity, diet, and weight loss may affect the risk of death among individuals with and without RA, he said.
Dr. Sparks had no financial conflicts to disclose. The study was supported in part by the National Institutes of Health and the Rheumatology Research Foundation’s Disease-Targeted Innovative Award and Scientist Development Awards.
SOURCE: Sparks J et al. Arthritis Rheumatol. 2017 Nov 30. doi: 10.1002/art.40346.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: The risk of death was approximately twice as high among women with weight loss greater than 30 pounds both for those diagnosed around the same time with RA (hazard ratio, 2.78) and for controls (HR, 2.16), compared with those whose weight remained stable.
Study details: A case-control study of 8,786 participants in the Nurses’ Health Study during 1976-2016.
Disclosures: Dr. Sparks had no financial conflicts to disclose. The study was supported in part by the National Institutes of Health and the Rheumatology Research Foundation’s Disease-Targeted Innovative Award and Scientist Development Awards.
Source: Sparks J et al. Arthritis Rheumatol. 2017 Nov 30. doi: 10.1002/art.40346.
PCVs reduced CAP hospitalizations in young children but not other age groups
, but there was no clear impact apparent in other age groups, reported Annemarie van Deursen, MD, of the University Medical Centre (the Netherlands) Utrecht, and her associates.
In the Netherlands, the 7-valent pneumococcal conjugate vaccine (PCV7) was added to the national infant immunization program in 2006; in 2011, PCV7 was replaced by the 10-valent vaccine (PCV10). The investigators undertook a population-based retrospective study during 1999-2014 on all-cause CAP hospitalizations in all ages, identifying 155,994 CAP hospitalizations.
In children aged 0-6 months, the CAP hospitalization rate ratio (RR) was significant from 2012 onward, with an overall post-PCV RR of 0.62 and a RR of 0.19 at the end of the study period in December 2014. In children aged 6 months-1 year, the RR was statistically significant directly after the introduction of PCV, with an overall post-PCV RR of 0.67 and a RR of 0.47 in December 2014, the investigators wrote.
In none of the other age groups did the overall post-PCV hospitalization RR reach statistical significance.
The association of reductions in CAP hospitalizations in children up to 2 years with the introduction of PCV7 “supports the interpretation for a direct causal effect of PCV7, in line with IPD [invasive pneumococcal disease] results that showed a sustained overall IPD reduction in children,” the investigators said. “Furthermore, [during] each subsequent year of the post-PCV period, the reduction in CAP hospitalization rates increased in line with progressive vaccine-type–IPD reduction in the population and limited replacement by nonvaccine type in childhood IPD.”
Read more in Vaccine (2017 Nov 13. doi: 10.1016/j.vaccine.2017.10.090).
, but there was no clear impact apparent in other age groups, reported Annemarie van Deursen, MD, of the University Medical Centre (the Netherlands) Utrecht, and her associates.
In the Netherlands, the 7-valent pneumococcal conjugate vaccine (PCV7) was added to the national infant immunization program in 2006; in 2011, PCV7 was replaced by the 10-valent vaccine (PCV10). The investigators undertook a population-based retrospective study during 1999-2014 on all-cause CAP hospitalizations in all ages, identifying 155,994 CAP hospitalizations.
In children aged 0-6 months, the CAP hospitalization rate ratio (RR) was significant from 2012 onward, with an overall post-PCV RR of 0.62 and a RR of 0.19 at the end of the study period in December 2014. In children aged 6 months-1 year, the RR was statistically significant directly after the introduction of PCV, with an overall post-PCV RR of 0.67 and a RR of 0.47 in December 2014, the investigators wrote.
In none of the other age groups did the overall post-PCV hospitalization RR reach statistical significance.
The association of reductions in CAP hospitalizations in children up to 2 years with the introduction of PCV7 “supports the interpretation for a direct causal effect of PCV7, in line with IPD [invasive pneumococcal disease] results that showed a sustained overall IPD reduction in children,” the investigators said. “Furthermore, [during] each subsequent year of the post-PCV period, the reduction in CAP hospitalization rates increased in line with progressive vaccine-type–IPD reduction in the population and limited replacement by nonvaccine type in childhood IPD.”
Read more in Vaccine (2017 Nov 13. doi: 10.1016/j.vaccine.2017.10.090).
, but there was no clear impact apparent in other age groups, reported Annemarie van Deursen, MD, of the University Medical Centre (the Netherlands) Utrecht, and her associates.
In the Netherlands, the 7-valent pneumococcal conjugate vaccine (PCV7) was added to the national infant immunization program in 2006; in 2011, PCV7 was replaced by the 10-valent vaccine (PCV10). The investigators undertook a population-based retrospective study during 1999-2014 on all-cause CAP hospitalizations in all ages, identifying 155,994 CAP hospitalizations.
In children aged 0-6 months, the CAP hospitalization rate ratio (RR) was significant from 2012 onward, with an overall post-PCV RR of 0.62 and a RR of 0.19 at the end of the study period in December 2014. In children aged 6 months-1 year, the RR was statistically significant directly after the introduction of PCV, with an overall post-PCV RR of 0.67 and a RR of 0.47 in December 2014, the investigators wrote.
In none of the other age groups did the overall post-PCV hospitalization RR reach statistical significance.
The association of reductions in CAP hospitalizations in children up to 2 years with the introduction of PCV7 “supports the interpretation for a direct causal effect of PCV7, in line with IPD [invasive pneumococcal disease] results that showed a sustained overall IPD reduction in children,” the investigators said. “Furthermore, [during] each subsequent year of the post-PCV period, the reduction in CAP hospitalization rates increased in line with progressive vaccine-type–IPD reduction in the population and limited replacement by nonvaccine type in childhood IPD.”
Read more in Vaccine (2017 Nov 13. doi: 10.1016/j.vaccine.2017.10.090).
FROM VACCINE
VIDEO: Daratumumab gives kick to standard first-line myeloma therapy
ATLANTA – The VMP regimen, consisting of bortezomib, melphalan, and prednisone, is a standard of care in Europe for frontline therapy for patients with multiple myeloma who, for reasons of age or infirmity, are not good candidates for autologous stem cell transplant.
In this video interview at the annual meeting of the American Society of Hematology, Jesus San-Miguel, MD, of the Clinical University of Navarra in Pamplona, Spain, discusses the results of the phase 3 international ALCYONE trial, comparing VMP with the same regimen plus the addition of the anti-CD38 monoclonal antibody daratumumab (Darzalex).
Adding daratumumab to VMP regimen as first-line therapy for 706 patients with multiple myeloma cut in half the risk of disease progression or death and substantially improved the rate of minimal residual disease negativity, Dr. San-Miguel reported. There were no new safety signals from adding the monoclonal antibody to VMP.
The ALCYONE study was supported by Janssen Research & Development. Dr. San-Miguel reported serving as an adviser to the company and several others.
ATLANTA – The VMP regimen, consisting of bortezomib, melphalan, and prednisone, is a standard of care in Europe for frontline therapy for patients with multiple myeloma who, for reasons of age or infirmity, are not good candidates for autologous stem cell transplant.
In this video interview at the annual meeting of the American Society of Hematology, Jesus San-Miguel, MD, of the Clinical University of Navarra in Pamplona, Spain, discusses the results of the phase 3 international ALCYONE trial, comparing VMP with the same regimen plus the addition of the anti-CD38 monoclonal antibody daratumumab (Darzalex).
Adding daratumumab to VMP regimen as first-line therapy for 706 patients with multiple myeloma cut in half the risk of disease progression or death and substantially improved the rate of minimal residual disease negativity, Dr. San-Miguel reported. There were no new safety signals from adding the monoclonal antibody to VMP.
The ALCYONE study was supported by Janssen Research & Development. Dr. San-Miguel reported serving as an adviser to the company and several others.
ATLANTA – The VMP regimen, consisting of bortezomib, melphalan, and prednisone, is a standard of care in Europe for frontline therapy for patients with multiple myeloma who, for reasons of age or infirmity, are not good candidates for autologous stem cell transplant.
In this video interview at the annual meeting of the American Society of Hematology, Jesus San-Miguel, MD, of the Clinical University of Navarra in Pamplona, Spain, discusses the results of the phase 3 international ALCYONE trial, comparing VMP with the same regimen plus the addition of the anti-CD38 monoclonal antibody daratumumab (Darzalex).
Adding daratumumab to VMP regimen as first-line therapy for 706 patients with multiple myeloma cut in half the risk of disease progression or death and substantially improved the rate of minimal residual disease negativity, Dr. San-Miguel reported. There were no new safety signals from adding the monoclonal antibody to VMP.
The ALCYONE study was supported by Janssen Research & Development. Dr. San-Miguel reported serving as an adviser to the company and several others.
REPORTING FROM ASH 2017
Daratumumab plus VMP boosts PFS, MRD-negativity in de novo myeloma
ATLANTA – Adding the anti-CD38 monoclonal antibody daratumumab (Darzalex) to the standard VMP regimen as first-line therapy for patients with multiple myeloma cut in half the risk of disease progression or death and substantially improved the rate of minimal residual disease (MRD) negativity, investigators in the ALCYONE trial reported.
This difference translated into a hazard ratio for progression or death with D-VMP of 0.50 (P less than .0001), said Jesus San-Miguel, MD, from the Clinical University of Navarra in Pamplona, Spain.
“This result clearly indicated for the first time that, in a phase 3 randomized study conducted with a monoclonal antibody in newly diagnosed myeloma patients, the addition of daratumumab to the standard of care reduced the risk of progression or death by 50%, and this is associated with significantly deeper responses, including a threefold higher MRD negativity rate,” he said at a media briefing prior to presentation of the data in a late-breaking abstract session at the annual meeting of the American Society of Hematology.
The VMP regimen is used more commonly in Europe than the United States as first-line therapy for patients with previously untreated multiple myeloma who are aged 65 years or older or are otherwise not suitable candidates for autologous stem cell transplants (ASCT).
In the ALCYONE trial, patients who met this definition were enrolled and stratified by International Staging System scores, region, and age (younger or older than 75 years) and were then randomized to 6-week cycles of VMP, with or without daratumumab. In the experimental arm, daratumumab was given at 16 mg/kg IV weekly for cycle 1, every 3 weeks for cycles 2-9, and every 4 weeks for cycles 10 and beyond (post VMP-treatment phase) until disease progression.
As noted before, the primary endpoint of investigator-assessed PFS significantly favored the addition of daratumumab. Dr. San-Miguel attributed this difference to the overall response rates, which were 91%, including 43% complete responses with daratumumab, vs. 74% ORR with 24% CR, without the monoclonal antibody.
The rate of MRD negativity, measured with a threshold sensitivity of 10–5, was also significantly higher with daratumumab at 22% vs. 6% (P less than .0001).
Among all patients who achieved MRD negativity, regardless of treatment, there was a lower risk of progression or death, Dr. San-Miguel said.
The rate of treatment discontinuation because of infection was higher with VMP (1.4%) than with D-VMP (0.9%). One patient in each trial arm discontinued therapy because of pneumonia. Rates of any serious adverse event were higher with D-VMP (42%, compared with 33%). Infusion-related reactions occurred in 27.7% of patients assigned to daratumumab.
Rates of grade 3 or 4 hematologic and nonhematologic toxicities were generally similar between the treatment arms, and there were no new safety signals with daratumumab, Dr. San-Miguel said.
The ALCYONE trial is one of several ongoing studies looking at the addition of daratumumab to standard therapies in the frontline, including the phase 3 MAIA trial (with daratumumab added to lenalidomide and dexamethasone), the phase 3 CASSIOPEIA trial (with the antibody added to bortezomib, thalidomide, and dexamethasone), the phase 2 GRIFFIN trial (with daratumumab plus lenalidomide, bortezomib, and dexamethasone), and the phase 2 LYRA trial (with the antibody added to cyclophosphamide, bortezomib, and dexamethasone).
The ALCYONE study was supported by Janssen Research & Development. Dr. San-Miguel reported serving as an adviser to the company and several others. Multiple coauthors disclosed similar relationships.
SOURCE: Mateos MV et al. ASH Abstract LBA-4.
ATLANTA – Adding the anti-CD38 monoclonal antibody daratumumab (Darzalex) to the standard VMP regimen as first-line therapy for patients with multiple myeloma cut in half the risk of disease progression or death and substantially improved the rate of minimal residual disease (MRD) negativity, investigators in the ALCYONE trial reported.
This difference translated into a hazard ratio for progression or death with D-VMP of 0.50 (P less than .0001), said Jesus San-Miguel, MD, from the Clinical University of Navarra in Pamplona, Spain.
“This result clearly indicated for the first time that, in a phase 3 randomized study conducted with a monoclonal antibody in newly diagnosed myeloma patients, the addition of daratumumab to the standard of care reduced the risk of progression or death by 50%, and this is associated with significantly deeper responses, including a threefold higher MRD negativity rate,” he said at a media briefing prior to presentation of the data in a late-breaking abstract session at the annual meeting of the American Society of Hematology.
The VMP regimen is used more commonly in Europe than the United States as first-line therapy for patients with previously untreated multiple myeloma who are aged 65 years or older or are otherwise not suitable candidates for autologous stem cell transplants (ASCT).
In the ALCYONE trial, patients who met this definition were enrolled and stratified by International Staging System scores, region, and age (younger or older than 75 years) and were then randomized to 6-week cycles of VMP, with or without daratumumab. In the experimental arm, daratumumab was given at 16 mg/kg IV weekly for cycle 1, every 3 weeks for cycles 2-9, and every 4 weeks for cycles 10 and beyond (post VMP-treatment phase) until disease progression.
As noted before, the primary endpoint of investigator-assessed PFS significantly favored the addition of daratumumab. Dr. San-Miguel attributed this difference to the overall response rates, which were 91%, including 43% complete responses with daratumumab, vs. 74% ORR with 24% CR, without the monoclonal antibody.
The rate of MRD negativity, measured with a threshold sensitivity of 10–5, was also significantly higher with daratumumab at 22% vs. 6% (P less than .0001).
Among all patients who achieved MRD negativity, regardless of treatment, there was a lower risk of progression or death, Dr. San-Miguel said.
The rate of treatment discontinuation because of infection was higher with VMP (1.4%) than with D-VMP (0.9%). One patient in each trial arm discontinued therapy because of pneumonia. Rates of any serious adverse event were higher with D-VMP (42%, compared with 33%). Infusion-related reactions occurred in 27.7% of patients assigned to daratumumab.
Rates of grade 3 or 4 hematologic and nonhematologic toxicities were generally similar between the treatment arms, and there were no new safety signals with daratumumab, Dr. San-Miguel said.
The ALCYONE trial is one of several ongoing studies looking at the addition of daratumumab to standard therapies in the frontline, including the phase 3 MAIA trial (with daratumumab added to lenalidomide and dexamethasone), the phase 3 CASSIOPEIA trial (with the antibody added to bortezomib, thalidomide, and dexamethasone), the phase 2 GRIFFIN trial (with daratumumab plus lenalidomide, bortezomib, and dexamethasone), and the phase 2 LYRA trial (with the antibody added to cyclophosphamide, bortezomib, and dexamethasone).
The ALCYONE study was supported by Janssen Research & Development. Dr. San-Miguel reported serving as an adviser to the company and several others. Multiple coauthors disclosed similar relationships.
SOURCE: Mateos MV et al. ASH Abstract LBA-4.
ATLANTA – Adding the anti-CD38 monoclonal antibody daratumumab (Darzalex) to the standard VMP regimen as first-line therapy for patients with multiple myeloma cut in half the risk of disease progression or death and substantially improved the rate of minimal residual disease (MRD) negativity, investigators in the ALCYONE trial reported.
This difference translated into a hazard ratio for progression or death with D-VMP of 0.50 (P less than .0001), said Jesus San-Miguel, MD, from the Clinical University of Navarra in Pamplona, Spain.
“This result clearly indicated for the first time that, in a phase 3 randomized study conducted with a monoclonal antibody in newly diagnosed myeloma patients, the addition of daratumumab to the standard of care reduced the risk of progression or death by 50%, and this is associated with significantly deeper responses, including a threefold higher MRD negativity rate,” he said at a media briefing prior to presentation of the data in a late-breaking abstract session at the annual meeting of the American Society of Hematology.
The VMP regimen is used more commonly in Europe than the United States as first-line therapy for patients with previously untreated multiple myeloma who are aged 65 years or older or are otherwise not suitable candidates for autologous stem cell transplants (ASCT).
In the ALCYONE trial, patients who met this definition were enrolled and stratified by International Staging System scores, region, and age (younger or older than 75 years) and were then randomized to 6-week cycles of VMP, with or without daratumumab. In the experimental arm, daratumumab was given at 16 mg/kg IV weekly for cycle 1, every 3 weeks for cycles 2-9, and every 4 weeks for cycles 10 and beyond (post VMP-treatment phase) until disease progression.
As noted before, the primary endpoint of investigator-assessed PFS significantly favored the addition of daratumumab. Dr. San-Miguel attributed this difference to the overall response rates, which were 91%, including 43% complete responses with daratumumab, vs. 74% ORR with 24% CR, without the monoclonal antibody.
The rate of MRD negativity, measured with a threshold sensitivity of 10–5, was also significantly higher with daratumumab at 22% vs. 6% (P less than .0001).
Among all patients who achieved MRD negativity, regardless of treatment, there was a lower risk of progression or death, Dr. San-Miguel said.
The rate of treatment discontinuation because of infection was higher with VMP (1.4%) than with D-VMP (0.9%). One patient in each trial arm discontinued therapy because of pneumonia. Rates of any serious adverse event were higher with D-VMP (42%, compared with 33%). Infusion-related reactions occurred in 27.7% of patients assigned to daratumumab.
Rates of grade 3 or 4 hematologic and nonhematologic toxicities were generally similar between the treatment arms, and there were no new safety signals with daratumumab, Dr. San-Miguel said.
The ALCYONE trial is one of several ongoing studies looking at the addition of daratumumab to standard therapies in the frontline, including the phase 3 MAIA trial (with daratumumab added to lenalidomide and dexamethasone), the phase 3 CASSIOPEIA trial (with the antibody added to bortezomib, thalidomide, and dexamethasone), the phase 2 GRIFFIN trial (with daratumumab plus lenalidomide, bortezomib, and dexamethasone), and the phase 2 LYRA trial (with the antibody added to cyclophosphamide, bortezomib, and dexamethasone).
The ALCYONE study was supported by Janssen Research & Development. Dr. San-Miguel reported serving as an adviser to the company and several others. Multiple coauthors disclosed similar relationships.
SOURCE: Mateos MV et al. ASH Abstract LBA-4.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: The hazard ratio for progression or death with daratumumab plus VMP was 0.50 (P less than .0001).
Study details: Randomized phase 3 trial in 706 patients with multiple myeloma who were ineligible for transplant.
Disclosures: The ALCYONE study was supported by Janssen Research & Development. Dr. San-Miguel reported serving as an adviser to the company and several others. Multiple coauthors disclosed similar relationships.
Source: Mateos MV et al. ASH Abstract LBA-4.
JAK inhibitors for atopic dermatitis might hit JAK-pot
GENEVA – at the annual congress of the European Academy of Dermatology and Venereology, with three positive phase 2 randomized trials featuring one topical and two oral agents presented to enthusiastic audiences.
The way has already been paved for dermatologic researchers by veterinarians, who developed oclacitinib (Apoquel), a relatively selective Janus kinase 1 (JAK1) inhibitor, for canine AD. The medication was approved by the Food and Drug Administration in 2013 for treating AD and for controlling pruritus associated with allergic dermatitis in dogs.
PF-04965842
“Get out your pencils, everyone. This is why you’re all here at 8 o’clock on a Sunday morning,” Melinda Gooderham, MD, said, standing before a packed house at the main arena of the Geneva Convention Center, as she launched into the results of a phase II randomized, double-blind, placebo-controlled, 12-week trial of a JAK inhibitor known for now as PF-04965842. This is a JAK1-selective agent with a good effect on interleukin-4 and -13, key mediators of the Th2 cytokines implicated in the pathogenesis of AD.
The dose-ranging study included 250 adults with AD and an inadequate response to or intolerance of topical therapy. Their mean baseline Eczema Area and Severity Index (EASI) score was 25 with a 60/40 ratio of moderate to severe AD. The five-arm trial randomized patients to PF-04965842 at 10 mg, 30 mg, 100 mg, or 200 mg once daily or placebo.
The primary endpoint was the proportion of patients achieving an Investigator Global Assessment (IGA) score of 0 or 1 – clear or almost clear – along with at least a 2-grade improvement from baseline at week 12. A clear dose-response effect was evident, with the 100- and 200-mg doses achieving response rates of 28% and 45%, respectively, compared with 6% in placebo-treated controls, reported Dr. Gooderham, medical director of the Skin Center for Dermatology in Peterborough, Ont., and a dermatologist at Queen’s University in Kingston, Ont.
Onset of action was speedy: patients in the 200-mg group reached their full improvement in IGA score by week 4 and maintained that response through week 12. Maximum improvement in EASI score – a mean 80% reduction – was achieved by week 6 and sustained thereafter. The proportion of patients in the 200-mg group achieving at least a 4-point improvement on the Pruritus Numeric Rating Scale significantly exceeded that in the placebo group as early as day 2 of the trial. At week 12, 64% of patients in the 200-mg group had achieved this level of improvement in itch, compared with 26% of controls.
A dose-dependent drop in platelet count occurred in the study, reaching a 30% decline at the 4-week nadir in the 200-mg group, followed by gradual on-treatment recovery. Both LDL and HDL cholesterol rose on active therapy – a class effect of JAK inhibitors – but the ratio between the two lipid levels remained unchanged. The two serious adverse events deemed treatment related were a case of eczema herpeticum in a patient on the 100-mg dose and pneumonia in a patient on the 200-mg dose.
Baricitinib
This once-daily oral JAK1/2 inhibitor is approved for treatment of rheumatoid arthritis in Europe and Japan. Emma Guttman-Yassky, MD, PhD, presented a phase 2 study of baricitinib in 124 adults with moderate to severe AD. Notably, prior to enrollment, all participants had to have failed to respond to a 4-week run-in period of supervised treatment with 0.1% triamcinolone cream, a midpotency topical steroid. They were then randomized to 2 mg or 4 mg of once-daily baricitinib or placebo, in all cases supplemented as needed with the topical steroid. Their median baseline EASI score was 21.
The primary endpoint was the proportion of patients achieving at least a 50% improvement in EASI score, or EASI 50 response, by week 16 from baseline in a nonresponder imputation analysis. This was achieved in 65% of patients on the 4-mg dose of baricitinib, 64% on the 2-mg dose, and 46% of controls on placebo plus the topical steroid. A statistically significant difference in EASI 50 response between the baricitinib groups and controls was seen at 1 week, with nearly the maximum effect achieved at week 4. Patients with a baseline EASI score above the median had a much more impressive treatment response because the placebo effect was smaller in participants with more severe AD.
“I think this drug can be an exciting new addition to the field,” declared Dr. Guttman-Yassky, professor and vice chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
From a baseline total SCORAD (Scoring Atopic Dermatitis) score of 55, major improvements were seen in the JAK inhibitor–treated patients by week 4. At week 16, the average reduction from baseline was 47% in the 4-mg group, 41% in the 2-mg group, and 21% in the placebo group. Both the SCORAD pruritus and sleep loss subscores showed significantly more robust improvement in the baricitinib groups than in controls. Indeed, a significant drop in pruritus scores was noted within the first week.
The 4-mg dose was associated with greater improvement and faster onset of action than the 2-mg dose on some but not all disease measures.
Dr. Guttman-Yassky described baricitinib as having “an overall acceptable safety profile,” with no serious treatment-related adverse events noted. Headache, nasopharyngitis, and asymptomatic increases in serum creatinine phosphokinase were more common in baricitinib-treated patients than with placebo.
JTE-052
Hidemi Nakagawa, MD, presented a phase 2 study of topical JTE-052 ointment in 327 Japanese adults with moderate to severe AD. The drug inhibits JAK1/2/3 as well as the tyrosine kinase pathway. It also promotes keratinocyte production of filaggrin in the skin barrier. Participants were randomized to twice-daily application of JTE-052 ointment (at 0.25%, 0.5%, 1%, or 3%), vehicle ointment, or 0.1% tacrolimus ointment twice a day for 4 weeks. The primary outcome was the change from baseline in modified EASI score in the active treatment groups compared with placebo. All doses of JTE-052 proved significantly more effective than vehicle. A dose-response effect was noted, with a 42% reduction from baseline in modified EASI score in the 0.25% JTE-052 group, a 57% reduction with 0.5%, a 55% reduction with 1% ointment, and a 73% reduction with 3%, compared with a 12% reduction decrease in patients who received vehicle. The topical tacrolimus group showed a 62% reduction from baseline, reported Dr. Nakagawa, professor and head of the division of dermatology at Jikei University, Tokyo.
All doses of JTE-052 were also significantly more effective than placebo on all secondary endpoints, which included IGA, percent body surface area affected, and Pruritus Numeric Rating Scale score.
At all but the weakest concentration, JTE-052 resulted in significant reduction in pruritus starting with the second dose on day 1 of the trial, he added.
Mild nasopharyngitis occurred in 3.4% of JTE-052–treated patients. There were no serious adverse events and no changes in laboratory parameters in the study. One patient discontinued JTE-052 because of application-site contact dermatitis, another because of application-site irritation. The results of this study were recently published in the British Journal of Dermatology (Br J Dermatol. 2017 Sep 28. doi: 10.1111/bjd.16014).
Dr. Nakagawa reported receiving research grants from and serving as a consultant to Japan Tobacco, which is developing JTE-052. Dr. Guttman-Yassky reported having financial relationships with Eli Lilly and Incyte, which sponsored the baricitinib study, as well as most other pharmaceutical companies developing therapies for AD. Dr. Gooderham reported receiving research funding from and serving as a consultant to Pfizer, which sponsored the PF-04965842 study, as well as numerous other pharmaceutical companies.
GENEVA – at the annual congress of the European Academy of Dermatology and Venereology, with three positive phase 2 randomized trials featuring one topical and two oral agents presented to enthusiastic audiences.
The way has already been paved for dermatologic researchers by veterinarians, who developed oclacitinib (Apoquel), a relatively selective Janus kinase 1 (JAK1) inhibitor, for canine AD. The medication was approved by the Food and Drug Administration in 2013 for treating AD and for controlling pruritus associated with allergic dermatitis in dogs.
PF-04965842
“Get out your pencils, everyone. This is why you’re all here at 8 o’clock on a Sunday morning,” Melinda Gooderham, MD, said, standing before a packed house at the main arena of the Geneva Convention Center, as she launched into the results of a phase II randomized, double-blind, placebo-controlled, 12-week trial of a JAK inhibitor known for now as PF-04965842. This is a JAK1-selective agent with a good effect on interleukin-4 and -13, key mediators of the Th2 cytokines implicated in the pathogenesis of AD.
The dose-ranging study included 250 adults with AD and an inadequate response to or intolerance of topical therapy. Their mean baseline Eczema Area and Severity Index (EASI) score was 25 with a 60/40 ratio of moderate to severe AD. The five-arm trial randomized patients to PF-04965842 at 10 mg, 30 mg, 100 mg, or 200 mg once daily or placebo.
The primary endpoint was the proportion of patients achieving an Investigator Global Assessment (IGA) score of 0 or 1 – clear or almost clear – along with at least a 2-grade improvement from baseline at week 12. A clear dose-response effect was evident, with the 100- and 200-mg doses achieving response rates of 28% and 45%, respectively, compared with 6% in placebo-treated controls, reported Dr. Gooderham, medical director of the Skin Center for Dermatology in Peterborough, Ont., and a dermatologist at Queen’s University in Kingston, Ont.
Onset of action was speedy: patients in the 200-mg group reached their full improvement in IGA score by week 4 and maintained that response through week 12. Maximum improvement in EASI score – a mean 80% reduction – was achieved by week 6 and sustained thereafter. The proportion of patients in the 200-mg group achieving at least a 4-point improvement on the Pruritus Numeric Rating Scale significantly exceeded that in the placebo group as early as day 2 of the trial. At week 12, 64% of patients in the 200-mg group had achieved this level of improvement in itch, compared with 26% of controls.
A dose-dependent drop in platelet count occurred in the study, reaching a 30% decline at the 4-week nadir in the 200-mg group, followed by gradual on-treatment recovery. Both LDL and HDL cholesterol rose on active therapy – a class effect of JAK inhibitors – but the ratio between the two lipid levels remained unchanged. The two serious adverse events deemed treatment related were a case of eczema herpeticum in a patient on the 100-mg dose and pneumonia in a patient on the 200-mg dose.
Baricitinib
This once-daily oral JAK1/2 inhibitor is approved for treatment of rheumatoid arthritis in Europe and Japan. Emma Guttman-Yassky, MD, PhD, presented a phase 2 study of baricitinib in 124 adults with moderate to severe AD. Notably, prior to enrollment, all participants had to have failed to respond to a 4-week run-in period of supervised treatment with 0.1% triamcinolone cream, a midpotency topical steroid. They were then randomized to 2 mg or 4 mg of once-daily baricitinib or placebo, in all cases supplemented as needed with the topical steroid. Their median baseline EASI score was 21.
The primary endpoint was the proportion of patients achieving at least a 50% improvement in EASI score, or EASI 50 response, by week 16 from baseline in a nonresponder imputation analysis. This was achieved in 65% of patients on the 4-mg dose of baricitinib, 64% on the 2-mg dose, and 46% of controls on placebo plus the topical steroid. A statistically significant difference in EASI 50 response between the baricitinib groups and controls was seen at 1 week, with nearly the maximum effect achieved at week 4. Patients with a baseline EASI score above the median had a much more impressive treatment response because the placebo effect was smaller in participants with more severe AD.
“I think this drug can be an exciting new addition to the field,” declared Dr. Guttman-Yassky, professor and vice chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
From a baseline total SCORAD (Scoring Atopic Dermatitis) score of 55, major improvements were seen in the JAK inhibitor–treated patients by week 4. At week 16, the average reduction from baseline was 47% in the 4-mg group, 41% in the 2-mg group, and 21% in the placebo group. Both the SCORAD pruritus and sleep loss subscores showed significantly more robust improvement in the baricitinib groups than in controls. Indeed, a significant drop in pruritus scores was noted within the first week.
The 4-mg dose was associated with greater improvement and faster onset of action than the 2-mg dose on some but not all disease measures.
Dr. Guttman-Yassky described baricitinib as having “an overall acceptable safety profile,” with no serious treatment-related adverse events noted. Headache, nasopharyngitis, and asymptomatic increases in serum creatinine phosphokinase were more common in baricitinib-treated patients than with placebo.
JTE-052
Hidemi Nakagawa, MD, presented a phase 2 study of topical JTE-052 ointment in 327 Japanese adults with moderate to severe AD. The drug inhibits JAK1/2/3 as well as the tyrosine kinase pathway. It also promotes keratinocyte production of filaggrin in the skin barrier. Participants were randomized to twice-daily application of JTE-052 ointment (at 0.25%, 0.5%, 1%, or 3%), vehicle ointment, or 0.1% tacrolimus ointment twice a day for 4 weeks. The primary outcome was the change from baseline in modified EASI score in the active treatment groups compared with placebo. All doses of JTE-052 proved significantly more effective than vehicle. A dose-response effect was noted, with a 42% reduction from baseline in modified EASI score in the 0.25% JTE-052 group, a 57% reduction with 0.5%, a 55% reduction with 1% ointment, and a 73% reduction with 3%, compared with a 12% reduction decrease in patients who received vehicle. The topical tacrolimus group showed a 62% reduction from baseline, reported Dr. Nakagawa, professor and head of the division of dermatology at Jikei University, Tokyo.
All doses of JTE-052 were also significantly more effective than placebo on all secondary endpoints, which included IGA, percent body surface area affected, and Pruritus Numeric Rating Scale score.
At all but the weakest concentration, JTE-052 resulted in significant reduction in pruritus starting with the second dose on day 1 of the trial, he added.
Mild nasopharyngitis occurred in 3.4% of JTE-052–treated patients. There were no serious adverse events and no changes in laboratory parameters in the study. One patient discontinued JTE-052 because of application-site contact dermatitis, another because of application-site irritation. The results of this study were recently published in the British Journal of Dermatology (Br J Dermatol. 2017 Sep 28. doi: 10.1111/bjd.16014).
Dr. Nakagawa reported receiving research grants from and serving as a consultant to Japan Tobacco, which is developing JTE-052. Dr. Guttman-Yassky reported having financial relationships with Eli Lilly and Incyte, which sponsored the baricitinib study, as well as most other pharmaceutical companies developing therapies for AD. Dr. Gooderham reported receiving research funding from and serving as a consultant to Pfizer, which sponsored the PF-04965842 study, as well as numerous other pharmaceutical companies.
GENEVA – at the annual congress of the European Academy of Dermatology and Venereology, with three positive phase 2 randomized trials featuring one topical and two oral agents presented to enthusiastic audiences.
The way has already been paved for dermatologic researchers by veterinarians, who developed oclacitinib (Apoquel), a relatively selective Janus kinase 1 (JAK1) inhibitor, for canine AD. The medication was approved by the Food and Drug Administration in 2013 for treating AD and for controlling pruritus associated with allergic dermatitis in dogs.
PF-04965842
“Get out your pencils, everyone. This is why you’re all here at 8 o’clock on a Sunday morning,” Melinda Gooderham, MD, said, standing before a packed house at the main arena of the Geneva Convention Center, as she launched into the results of a phase II randomized, double-blind, placebo-controlled, 12-week trial of a JAK inhibitor known for now as PF-04965842. This is a JAK1-selective agent with a good effect on interleukin-4 and -13, key mediators of the Th2 cytokines implicated in the pathogenesis of AD.
The dose-ranging study included 250 adults with AD and an inadequate response to or intolerance of topical therapy. Their mean baseline Eczema Area and Severity Index (EASI) score was 25 with a 60/40 ratio of moderate to severe AD. The five-arm trial randomized patients to PF-04965842 at 10 mg, 30 mg, 100 mg, or 200 mg once daily or placebo.
The primary endpoint was the proportion of patients achieving an Investigator Global Assessment (IGA) score of 0 or 1 – clear or almost clear – along with at least a 2-grade improvement from baseline at week 12. A clear dose-response effect was evident, with the 100- and 200-mg doses achieving response rates of 28% and 45%, respectively, compared with 6% in placebo-treated controls, reported Dr. Gooderham, medical director of the Skin Center for Dermatology in Peterborough, Ont., and a dermatologist at Queen’s University in Kingston, Ont.
Onset of action was speedy: patients in the 200-mg group reached their full improvement in IGA score by week 4 and maintained that response through week 12. Maximum improvement in EASI score – a mean 80% reduction – was achieved by week 6 and sustained thereafter. The proportion of patients in the 200-mg group achieving at least a 4-point improvement on the Pruritus Numeric Rating Scale significantly exceeded that in the placebo group as early as day 2 of the trial. At week 12, 64% of patients in the 200-mg group had achieved this level of improvement in itch, compared with 26% of controls.
A dose-dependent drop in platelet count occurred in the study, reaching a 30% decline at the 4-week nadir in the 200-mg group, followed by gradual on-treatment recovery. Both LDL and HDL cholesterol rose on active therapy – a class effect of JAK inhibitors – but the ratio between the two lipid levels remained unchanged. The two serious adverse events deemed treatment related were a case of eczema herpeticum in a patient on the 100-mg dose and pneumonia in a patient on the 200-mg dose.
Baricitinib
This once-daily oral JAK1/2 inhibitor is approved for treatment of rheumatoid arthritis in Europe and Japan. Emma Guttman-Yassky, MD, PhD, presented a phase 2 study of baricitinib in 124 adults with moderate to severe AD. Notably, prior to enrollment, all participants had to have failed to respond to a 4-week run-in period of supervised treatment with 0.1% triamcinolone cream, a midpotency topical steroid. They were then randomized to 2 mg or 4 mg of once-daily baricitinib or placebo, in all cases supplemented as needed with the topical steroid. Their median baseline EASI score was 21.
The primary endpoint was the proportion of patients achieving at least a 50% improvement in EASI score, or EASI 50 response, by week 16 from baseline in a nonresponder imputation analysis. This was achieved in 65% of patients on the 4-mg dose of baricitinib, 64% on the 2-mg dose, and 46% of controls on placebo plus the topical steroid. A statistically significant difference in EASI 50 response between the baricitinib groups and controls was seen at 1 week, with nearly the maximum effect achieved at week 4. Patients with a baseline EASI score above the median had a much more impressive treatment response because the placebo effect was smaller in participants with more severe AD.
“I think this drug can be an exciting new addition to the field,” declared Dr. Guttman-Yassky, professor and vice chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
From a baseline total SCORAD (Scoring Atopic Dermatitis) score of 55, major improvements were seen in the JAK inhibitor–treated patients by week 4. At week 16, the average reduction from baseline was 47% in the 4-mg group, 41% in the 2-mg group, and 21% in the placebo group. Both the SCORAD pruritus and sleep loss subscores showed significantly more robust improvement in the baricitinib groups than in controls. Indeed, a significant drop in pruritus scores was noted within the first week.
The 4-mg dose was associated with greater improvement and faster onset of action than the 2-mg dose on some but not all disease measures.
Dr. Guttman-Yassky described baricitinib as having “an overall acceptable safety profile,” with no serious treatment-related adverse events noted. Headache, nasopharyngitis, and asymptomatic increases in serum creatinine phosphokinase were more common in baricitinib-treated patients than with placebo.
JTE-052
Hidemi Nakagawa, MD, presented a phase 2 study of topical JTE-052 ointment in 327 Japanese adults with moderate to severe AD. The drug inhibits JAK1/2/3 as well as the tyrosine kinase pathway. It also promotes keratinocyte production of filaggrin in the skin barrier. Participants were randomized to twice-daily application of JTE-052 ointment (at 0.25%, 0.5%, 1%, or 3%), vehicle ointment, or 0.1% tacrolimus ointment twice a day for 4 weeks. The primary outcome was the change from baseline in modified EASI score in the active treatment groups compared with placebo. All doses of JTE-052 proved significantly more effective than vehicle. A dose-response effect was noted, with a 42% reduction from baseline in modified EASI score in the 0.25% JTE-052 group, a 57% reduction with 0.5%, a 55% reduction with 1% ointment, and a 73% reduction with 3%, compared with a 12% reduction decrease in patients who received vehicle. The topical tacrolimus group showed a 62% reduction from baseline, reported Dr. Nakagawa, professor and head of the division of dermatology at Jikei University, Tokyo.
All doses of JTE-052 were also significantly more effective than placebo on all secondary endpoints, which included IGA, percent body surface area affected, and Pruritus Numeric Rating Scale score.
At all but the weakest concentration, JTE-052 resulted in significant reduction in pruritus starting with the second dose on day 1 of the trial, he added.
Mild nasopharyngitis occurred in 3.4% of JTE-052–treated patients. There were no serious adverse events and no changes in laboratory parameters in the study. One patient discontinued JTE-052 because of application-site contact dermatitis, another because of application-site irritation. The results of this study were recently published in the British Journal of Dermatology (Br J Dermatol. 2017 Sep 28. doi: 10.1111/bjd.16014).
Dr. Nakagawa reported receiving research grants from and serving as a consultant to Japan Tobacco, which is developing JTE-052. Dr. Guttman-Yassky reported having financial relationships with Eli Lilly and Incyte, which sponsored the baricitinib study, as well as most other pharmaceutical companies developing therapies for AD. Dr. Gooderham reported receiving research funding from and serving as a consultant to Pfizer, which sponsored the PF-04965842 study, as well as numerous other pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
Innovative Therapies for Severe Asthma
More than 39.5 million people in the U.S. have been diagnosed with asthma, and about 3,400 deaths occur annually due to asthma complications.1 Although the prevalence of atopy and asthma have increased over the past few decades in western countries, control and outcomes are improving.2 Use of asthma protocols and early recognition by the primary care provider (PCP) are among the main reasons for trends toward decreased hospitalization and fewer asthma-related deaths.3,4
In addition to the mainstay of treatments, including trigger avoidance, inhaled corticosteroids (ICS), and rescue bronchodilators, new therapies have been developed to supplement the treatment of severe persistent asthma, which constitutes about 5% to 10% of asthma cases. Severe asthma is defined as asthma that is unresponsive to baseline therapy.5
Three sets of guidelines and recommendations exist to provide structure to asthma treatment decision making. The Expert Panel Report-3 (EPR-3) was created by the National Education and Prevention Program (NAEPP) and was last published in 2007. The NAEPP favors a stepwise approach, based on asthma severity and age group.3 The International European Respiratory Society (ERS) and American Thoracic Society (ATS) task force report was updated in 2014.5 The Global Initiative for Asthma (GINA) report, updated in 2016, now includes several of the advances in asthma care for those patients refractory to standard treatments.
Asthma Therapies
In this review, the authors cover therapies for severe asthma that are becoming more important for PCPs to consider, including exhaled nitric oxide (NO) levels, the use of tiotropium for asthma, the applicability of biologic agents, the use of allergen immunotherapy, and the usefulness of roflumilast. This review also covers antileukotriene therapy, bronchial thermoplasty, and a discussion of long-acting beta-agonist (LABA) therapy.
Fractional Exhaled Nitric Oxide
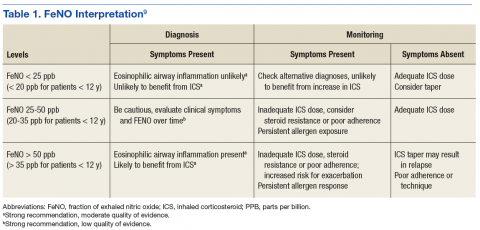
Nitric oxide is present in the exhaled breath and is elevated in those with eosinophilic asthma.6 The role of NO in asthma pathology is complex, involving proinflammatory qualities that contribute to airway hyperresponsiveness (AHR) and as a weak mediator of smooth muscle relaxation. In exhaled air, NO correlates with up-regulation of NO synthase (NOS), which occurs with inflammation, therefore, quantifying airway inflammation.6-8
There has been some variability in the evidence supporting the use of fractional exhaled NO (FeNO) levels as a diagnostic tool. Some studies have suggested that FeNO is also elevated in other nonasthma conditions, such as eosinophilic bronchitis, atopy, and allergic rhinitis. Also, FeNO levels have been shown to be variably influenced by smoking, bronchoconstriction, and viral respiratory infections.9 However, FeNO levels > 50 ppb correlated most strongly with eosinophilic asthma and steroid responsiveness.9
Fractional exhaled NO tests now can be performed in the PCP office with NIOX VERO (Chicago, IL), a small, relatively inexpensive device. Although the 2016 GINA guidelines and the 2015 ERS/ATS guidelines do not offer specific recommendations for use and do not support withholding ICS based on FeNO test results, guidelines for FeNO use do exist. In 2011, ATS published a specific set of FeNO interpretive guidelines for office-based use.9 When performed in conjunction with standard testing, FeNO levels can provide valuable clinically relevant information, such as (1) detection of eosinophilic airway inflammation; (2) determining the likelihood of corticosteroid responsiveness; (3) monitoring of airway inflammation to determine the need for steroids; and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy (Table 1).
Tiotropium as an Adjunct Treatment
Tiotropium is a long-acting inhaled anticholinergic. A sentinel 1984 study by Gross and Skorodin demonstrated that parasympathetic activity is the dominant reversible component in patients with chronic obstructive pulmonary disease (COPD), including emphysema.10 In addition, all achievable bronchodilation was obtained with an inhaled anticholinergic compared with that of separate or simultaneous administration of adrenergics. Sympathetic neural pathways are sparse in human lungs and have their endings on the cells of the cholinergic postganglionic fibers, because sympathetic terminals on airway smooth muscle cells are rare or nonexistent.11 Therefore, sympathetic modulation or activation of beta cells could change the parasympathetic tone.11
The FDA approved the addition of tiotropium for treating asthma in September 2015 for patients aged ≥ 12 years. The use of tiotropium is supported by both the ERS/ASTS and GINA 2016 guidelines. The recommended and approved dose of tiotropium for asthma is 2.5 µg daily (the recommended dose for COPD treatment is 5 µg).12 A recent phase 3 study compared 2.5 µg vs 5 µg dosing with ICS but no LABA in adolescents, noting significant improvement with the 2.5 µg dose.13 Adding tiotropium to ICS + LABA in patients with severe symptomatic asthma has been associated with positive results in initial studies by Kersjens and colleagues.14 Even as early as 2010, the use of tiotropium was shown to produce statistically significant improvement in morning peak expiratory flow (PEF), with a mean difference of 25.8 L/min (n = 210, P < .001).15
Tiotropium also has been shown to provide a sustained reduction in lung hyperinflation for those with COPD, thus providing an improvement in exertional dyspnea and exercise tolerance. On day 42 of a randomized, double-blinded, placebo-controlled, parallel-group study of 187 patients, vital capacity and inspiratory capacity were noted to be increased with decreases in residual volume and functional residual capacity. Exercise endurance times increased by 105 ± 40 sec (21%).16 This effect has not been studied yet in a population of patients with asthma; however, the same principles may hold true.
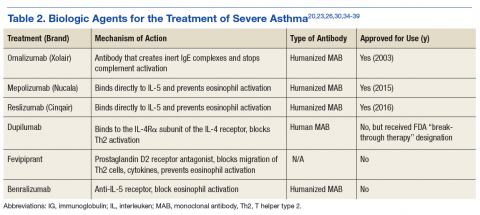
Biologic Agents
Recent asthma research has been focused on disrupting the inflammatory cascade. Both GINA and ERS/ATS divide asthma into allergic vs nonallergic endotypes. Allergic asthma usually is manifested by sputum eosinophilia and high serum eosinophil counts, whereas other endotypes include aspirin-sensitive and exercise-induced asthmas that present with a neutrophilic predominance. Nonallergic asthma is more severe typically and has been linked to steroid resistance.17 Many differentphenotypes have been identified, but the main categories include eosinophilic, neutrophilic, mixed, and paucigranulocytic.18
Mast cells, bronchial epithelium, and macrophages are involved in asthma progression. Targeting the cytokines produced by these pathways can be achieved through direct and indirect modulation. Interleukin (IL)-13 is central to development of AHR, and its effect is mediated through binding to its receptor and IL-4 receptor α complexes.19 Patients with severe asthma with an eosinophilic phenotype can benefit with the use of the new biologics, which decrease the amount of eosinophilia in lung tissue by blocking specific receptors for IL-5.
Omalizumab
Omalizumab, an anti-immunoglobulin E (IgE) antibody, has been shown to be helpful in treating patients with allergic asthma. Omalizumab is a 95% humanized IgE monoclonal antibody (MAB) that binds to the IgE molecule at the fc region and prevents IgE from binding to cell-surface receptors. In a humanized MAB, only the hypervariable regions are from mouse origin vs the newer completely human MABs. Omalizumab forms small, biologically inert IgE+ anti-IgE complexes that cannot activate the complement cascade. The serum free IgE level is decreased.20 Approved in 2003 for those aged ≤ 12 years, its use is restricted to patients with severe asthma, allergic sensitization (positive allergen skin testing), and an elevated serum IgE level (30-700 IU/mL). It is administered subcutaneously every 2 to 4 weeks, based on body weight and serum IgE levels.
For those with baseline eosinophil counts > 300 µL, addition of omalizumab most likely has been shown to improve quality of life (QOL) and reduce exacerbations, the use of rescue medications, ICS dosages, and ED visits.21-26 The most dangerous adverse effect (AE) was found to be an anaphylaxis rate of 0.09%, most frequently occurring in the first 2 hours after the first dose. Therefore, the patient must be monitored for 2 hours after the first dose and 30 minutes after subsequent doses. Epinephrine injection also should be prescribed. Although a 5-year prospective cohort study and retrospective pooled analysis of more than 10,000 patients did not support any relationship with malignancy.27,28 A higher incidence of cardiovascular and cerebrovascular AEs has been observed, and the FDA issued a safety announcement regarding this finding.29
Both ERS/ATS and GINA 2016 recommended that a therapeutic trial of omalizumab should be performed in all patients with severe confirmed IgE dependent allergic asthma.4,5 If there is no response in 4 months, it is unlikely that further administration would be beneficial.
Mepolizumab
Interleukin-5 is a key cytokine in the eosinophil life pathway. There are receptors for IL-5 on eosinophils, basophils, and β cells.30 Mepolizumab is an anti-IL-5 antibody for those with refractory eosinophilic asthma and a history of continued exacerbations. It has beneficial effects in the management of persistent airways eosinophilia among corticosteroid-resistant subjects. In a 2009 study, rates of exacerbations at 50 weeks were significantly lower than with placebo (2.0 vs 3.4 mean exacerbations per subject, 95% confidence interval [CI], 0.32-0.92; P = .002) as were eosinophil counts in blood and sputum (P < .001 and P = .002 respectively.31 A 2014 randomized, double-blind trial by Ortega and colleagues demonstrated reduction in rate of asthma exacerbations (primary outcome) to 47% (95% CI, 29-61) among patients receiving IV dosing and 53% (95% CI, 37-65) in the oral mepolizumab group (P < .001 for both groups, n = 576).32
In addition, there is significant data to show that even if the patient did not respond to omalizumab, he or she might still respond to mepolizumab. Data were collected from 2 randomized, double-blind, placebo-controlled studies with rate of exacerbation and percentage reduction in oral corticosteroid dose as the primary outcomes. In one of the studies (n = 576), the subjects were noted to have prior omalizumab use but still decreased exacerbation rate by 57%.33
Reslizumab
Reslizumab also is an FDA-approved anti-IL-5 antibody. It binds directly to IL-5 and prevents it from binding to eosinophils.34 For adults with severe eosinophilic asthma and refractory exacerbations, the goal of reslizumab therapy is to reduce eosinophil maturation, recruitment, and activation. Reslizumab is delivered in a weight-based IV dose (3 mg/kg) every 4 weeks. The FDA has issued a boxed warning for a 0.3% anaphylaxis rate.35 The most common AEs are elevated creatinine kinase, musculoskeletal pain, and oropharyngeal pain. Use of reslizumab resulted in greater reduction in sputum eosinophils, improvements in airway function, and a trend toward greater asthma control compared with that of placebo.34
Other Biologic Therapies
Many biologics are being developed as medical researchers continue to understand more of the mechanisms and pathways that contribute to allergic disease (Table 2). Dupilumab is an IL-4 inhibitor designated as a “breakthrough therapy” in 2014 by the FDA. This biologic blocks the downstream signaling events induced by IL-4 and IL-13 by binding to a subunit of the IL-4 receptor in the complexes. It has been found beneficial for those with high blood eosinophil counts and moderate-to-severe asthma and decreased asthma exacerbations when LABA and ICS were withdrawn.36,37
Fevipiprant is a prostaglandin D2 inhibitor that blocks T-helper type 2 (Th2) cell migration and subsequent bronchoconstriction and cytokine effects with decreased IL-4, IL-5, and IL-13. Although sputum eosinophil percentage was noted to be decreased in a study involving 61 patients randomized to treatment for 12 weeks, asthma QOL questionnaires and prebronchodilator spirometry did not change.38,39
Benralizumab is an anti-IL-5 receptor antibody that has been more effective in reduction of airway and blood eosinophils levels compared with that of mepolizumab (undetectable vs 52% reduction), within 24 hours of IV dosing. In contrast, the anti-IL-5 antibodies take about 4 weeks to decrease eosinophil levels in blood and sputum.34 There have been no documented AEs aside from nasopharyngitis and injection site reactions and no safety concerns to date. It is currently undergoing phase 3 trials.40
Immunotherapies
Allergen immunotherapy is recommended for mild-to-moderate asthma. A 2010 Cochrane Review found that subcutaneous immunotherapy compared with placebo demonstrated improvements in bronchial hyperresponsiveness and decreased medication use.41 Expert Panel Report-3 guidelines recommend consideration of immunotherapy for mild-to-moderate asthma.5 While ERS/ATS guidelines for severe asthma do not address allergen immunotherapy, GINA guidelines incorporate it as Evidence A for treating modifiable risk factors to reduce exacerbations, although the efficacy is limited.6
Roflumilast
Roflumilast is a selective PDE4 inhibitor that has shown an anti-inflammatory effect in COPD. Studies evaluating the reversibility and prevention of airway remodeling showed good promise in mouse models.42 Data from 8 placebo-controlled, double-blind, phase 1, 2, and 3 studies conducted at 14 sites in Europe, North America, and South Africa from 1997 to 2005 showed reduced sputum eosinophil and neutrophil counts, consistent with findings during COPD treatment. However, forced expiratory volume in one second (FEV1) and PEF values were unchanged, suggesting that there was no acute bronchodilatory effect with roflumilast therapy.43 Roflumilast is not addressed in the 2016 GINA guidelines and at this time does not have a role in the treatment of severe asthma.
Antileukotrienes
After the activation of mast cells and eosinophils, leukotrienes are generated by 5-lipoxygenase from arachidonic acid and create bronchoconstriction, vasodilation, increased mucus production, increased recruitment of eosinophils, and decreased ciliary motility. Some studies have encouraged addingleukotriene receptor blockers (both montelukast and zafirlukast) to ICS therapy44,45 and to therapy for patients with aspirin-intolerant asthma or allergic asthma.46,47 However, other studies have shown them to be of limited benefit.48,49 A recent Cochrane Reviewof 18 randomized-controlled trials with 7,208 adults and children compared ICS + leukotriene receptor antagonist (LTRA) vs ICS + LABA.50 The ICS + LABA resulted in greater improvements in lung function, symptoms score, and rates of exacerbations.50
Most recommendations recognize the limitations of antileukotriene medications and agree that they are an adjunct rather than primary therapy. The GINA 2016 guidelines support the use of LTRAs in mild asthma, stating that although LTRAs are less effective than ICS (Evidence A), they may be appropriate for initial controller treatment for some patients who are unable or unwilling to use ICS or for patients with concurrent allergic rhinitis (Evidence B).51,52
Zileuton is a different type of antileukotriene. It inhibits leukotrienes B4, C4, D4, and E4 by inhibition of 5-lipoxygenase, interfering with leukotriene formation. It is approved for patients aged ≥ 12 years and is more expensive than montelukast or zafirlukast. Most studies supporting its use were conducted in patients with mild-to-moderate asthma on β-agonist therapy only. A 1988 study showed that zileuton therapy improved FEV1, reduced nasal symptoms, and decreased bronchial responsiveness to inhaled aspirin and histamine.53 All but 1 study patient were on ICS or oral corticosteroids. Zileuton was noted to be effective for patients with aspirin-intolerant asthma.
Some earlier studies reported that a small number of subjects had an increase in transaminases that resolved when they discontinued the medication. Therefore, it is recommended to check baseline laboratory results every 2 to 3 months.54,55 Neither GINA nor ERS/ATS guidelines address the use of zileuton.
Bronchial Thermoplasty
With asthma there is marked hypertrophy and hyperplasia that occurs in the airway smooth muscle. The airway of the patient with asthma also is lined with cells that promote inflammation. Thermal energy is used to perform controlled destruction of the inflammatory lining and pathologic hyperplasia. Three sequential bronchoscopies are performed 3 weeks apart to treat the right lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is not treated due to its smaller diameter. Each bronchoscopy takes about 30 to 60 minutes. Patients are given perioperative steroids.56
Three large phase 3 clinical trials have evaluated the efficacy of bronchial thermoplasty (BT). The AIR (Asthma Intervention Research) trial in 2007 was a randomized controlled study of 112 patients with moderate or severe asthma that showed improved exacerbation rates, symptom-free days and QOL scores (1.3 ± 1.0 vs 0.6 ± 1.1; P = .003), but no difference in prebronchodilator FEV1 or AHR.57 There was a significant reduction in the rate of mild exacerbations and increase in morning PEF rates.57 Findings at 5 years showed improved AHR but no difference in frequency of need for oral corticosteroids and frequency of hospital or emergency department (ED) visits.58
The Research in Severe Asthma (RISA) clinical trial was a randomized controlled study (n = 32, 15 randomly assigned to BT) that showed improved prebronchodilator FEV1 in patients with severe, symptomatic asthma and baseline FEV1 of 62% to 66% with half the patients requiring oral corticosteroids (percentage predicted; 14.9 ± 17.4 vs -0.9 ± 22.3; P =.04).57 Quality of life scores were also significantly improved. At 5 years (14 BT patients were followed), the frequency of hospitalizations and ED visits decreased.59
The 2010 AIR2 study was a randomized, double blind, sham-controlled study (n = 288) developed to address the limitations of the 2 previous studies. It excluded the severest asthma cases, and its blinded nature was created with sham bronchoscopy to eliminate possible placebo effect. The study questionnaires showed improved QOL overall (79% vs 64%); however, there was a definite placebo effect noted.60 Decreased frequency of severe exacerbations as well as ED visits and days lost from work or school also were documented as secondary endpoints. At 5 years, decreased frequency of severe exacerbations and ED visits continued in the control group (85% consented to follow-up).61 Importantly, despite the placebo effect in QOL scores, there were no improvements in exacerbation rates or hospitalizations in those receiving sham bronchoscopy at the 1-year mark.61
Although more longitudinal studies need to be planned, including evaluation of those with the most severe asthma, there seems to be a sustained improvement in patients. Those who have received BT generally are found to have reduced airway smooth muscle with lower concentration of key inflammatory cytokines on follow-up bronchoscopy. However, variability in response has been documented.56 There has been no documented deterioration in pulmonary function with BT, and no significant structural abnormalities have been seen on high-resolution computed tomography.56,58 Both GINA 2016 and ERS/ATS support the use of BT in the context of adults with severe asthma, calling for more long-term studies to address delayed benefits and safety.
LABA Inhalers
A multicenter, double-blind, 26-week study of 11,693 patients randomized to ICS + LABA (budesonide/formoterol) vs ICS (budesonide) alone has shown no increased AEs in either arm. The study found that treatment with budesonide/formoterol was associated with lower risk of asthma exacerbations than using budesonide alone (16.5%; P = .002).62
The safety of adding a LABA to fluticasone also has been evaluated recently. A 2016 study of almost 12,000 patients (aged > 12 years) compared fluticasone proprionate alone vs fluticasone with salmeterol.63 There were no asthma-related deaths, but 2 patients in the fluticasone-only group were intubated with asthma complications. The risk of a severe asthma exacerbation seemed to be lower in the combination group (8% vs 10%; P < .001).63
A 2014 Cochrane Review supported the view that LABAs in adults seem to be safe when used concurrently with an ICS with a A-level recommendation, based on consistent good-quality, patient-oriented evidence.64 Multiple organizations have issued guidelines to this effect in the past, but previous results of studies showed that asthma deaths and a small increase in nonfatal serious AEs were noted in those using LABA monotherapy alone.64
NAEPP (EPR-3) and ERS/ATS recommend stepwise increases in the dose of ICS in combination with a LABA. The GINA guidelines recommend controller therapy to include combination IHS and LABA but with the consideration of higher doses of ICS than are routinely recommended for general use.
Inhaler and Inhaler Combinations
Many different inhalers of ICS alone and ICS/LABA combinations exist on the market today. There are differences in delivery that affect patient preference but these differences have not been found to improve delivery. Small particle ICS therapy could possibly correlate with improved delivery to the small airways.65 There are 3 preparations of inhaled steroids that fit in to this group, including beclomethasone, ciclesonide, and flunisolide. Other inhaled steroid formulations include budesonide, fluticasone propionate, fluticasone furoate, and mometasone.
Combination therapy (ICS + LABA) inhalers also are widely available. They include budesonide/formoterol, fluticasone proprionate/salmeterol, mometasone/formoterol, and the newer fluticasone furoate/vilanterol, a once-daily combination approved for those aged ≥ 18 years.
Conclusion
The treatment of severe asthma has progressed from simple manipulation of avoidance, bronchodilators, and corticosteroids to include many other treatments that have improved QOL for patients with refractory asthma. Although many of these options are delivered in coordination with an allergy and pulmonary specialist, it is important for the PCP to have a good knowledge base and awareness of additional treatments that are currently available.
1. Centers for Disease Control and Prevention. Asthma facts: CDC’s national asthma control program grantees. https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Published July 2013. Accessed November 9, 2017.
2. Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990-2003. Med J Aust. 2006;184(5):226-229.
3. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
4. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 update. http://ginasthma.org/wp-content/up loads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed November 9, 2017.
5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
6. Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology. 2003;8(4):479-486.
7. De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621-1630.
8. Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182.
9. Dweik RA, Boggs PB, Erzurum SC, et al; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
10. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421-425.
11. Gelb AF, Nadel JA. Affirmation of the adoration of the vagi and role of tiotropium in asthmatic patients. J Allergy Clin Immunol. 2016;138(4):1011-1013.
12. Chin SJ, Durmowicz AG, Chowdhury BA. Tiotropium respimat is effective for the treatment of asthma at a dose lower than that for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):173-179.
13. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441-450.e8.
14. Kerstjens HA, Casale TB, Bleeker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367-376.
15. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715-1726.
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnea, and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
17. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355-360.
18. Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170(6):579-580.
19. Wang E, Hoyte FC. Traditional therapies for severe asthma. Immunol Allergy Clin North Am. 2016;36(3):581-608.
20. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689-2695.
21. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378-1386.
22. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309-316.
23. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573-582.
24. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408-416.
25. Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111(2):278-284.
26. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184-190.
27. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4.
28. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs /DrugSafety/ucm414911.htm. Updated February 10, 2016. Accessed November 9, 2017.
30. Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16(10):70.
31. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973-984.
32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
33. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335-1344.
34. Castro M, Mathur S, Hargreave F, et al; Res-5-0010 Study Group. Resilizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125-1132.
35. Cinqair [package insert]. Frazier, PA: Teva Respiratory; 2016.
36. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455-2466.
37. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3-4.
38. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699-707.
39. Erpenbeck VJ, Popov TA, Miller D, et al. The oral CRTh2 antagonist QAWO39 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54-63.
40. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71-81.
41. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
42. Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodeling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754-763.
43. Bardin P, Kanniess F, Gauvreau G, Bredenbröker D, Rabe KF. Roflumilast for asthma: efficacy findings in mechanism of action studies. Pulm Pharmacol Ther. 2015;(suppl 35):S4-S10.
44. Virchow JC Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Resp Crit Care Med. 2000;162(2, pt 1):558-585.
45. Price DB, Hernandez D, Magyar P, et al; Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211-216.
46. Dahlén SE, Malmström K, Nizankowska E, et al. Improvement of aspirin intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(1):9-14.
47. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006; 61(6):737-742.
48. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357(9273):2007-2011.
49. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1):CD003137.
50. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
51. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549-1558.
52. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31(4):616-624.
53. Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4, pt 1):1187-1194.
54. Israel E, Cohn J, Dubé L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zilueton Clinical Trial Group. JAMA. 1996;275(12):931-936.
55. Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99(2):178-184.
56. Laxmanan B, Egressy K, Murgu SD, White SR, Hogarth DK. Advances in bronchial thermoplasty. Chest. 2016;150(3):694-704.
57. Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327-1337.
58. Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011:11:8.
59. Pavord, ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185-1191.
60. Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116-124.
61. Wechsler ME, Laviolette M, Rubin AS, et al; Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295-1302.
62. Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860.
63. Stempel DA, Raphiou IH, Kral KM, et al; AUSTRI Investigators. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830.
64. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
65. Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am. 2016;36(3):473-482.
More than 39.5 million people in the U.S. have been diagnosed with asthma, and about 3,400 deaths occur annually due to asthma complications.1 Although the prevalence of atopy and asthma have increased over the past few decades in western countries, control and outcomes are improving.2 Use of asthma protocols and early recognition by the primary care provider (PCP) are among the main reasons for trends toward decreased hospitalization and fewer asthma-related deaths.3,4
In addition to the mainstay of treatments, including trigger avoidance, inhaled corticosteroids (ICS), and rescue bronchodilators, new therapies have been developed to supplement the treatment of severe persistent asthma, which constitutes about 5% to 10% of asthma cases. Severe asthma is defined as asthma that is unresponsive to baseline therapy.5
Three sets of guidelines and recommendations exist to provide structure to asthma treatment decision making. The Expert Panel Report-3 (EPR-3) was created by the National Education and Prevention Program (NAEPP) and was last published in 2007. The NAEPP favors a stepwise approach, based on asthma severity and age group.3 The International European Respiratory Society (ERS) and American Thoracic Society (ATS) task force report was updated in 2014.5 The Global Initiative for Asthma (GINA) report, updated in 2016, now includes several of the advances in asthma care for those patients refractory to standard treatments.
Asthma Therapies
In this review, the authors cover therapies for severe asthma that are becoming more important for PCPs to consider, including exhaled nitric oxide (NO) levels, the use of tiotropium for asthma, the applicability of biologic agents, the use of allergen immunotherapy, and the usefulness of roflumilast. This review also covers antileukotriene therapy, bronchial thermoplasty, and a discussion of long-acting beta-agonist (LABA) therapy.
Fractional Exhaled Nitric Oxide
Nitric oxide is present in the exhaled breath and is elevated in those with eosinophilic asthma.6 The role of NO in asthma pathology is complex, involving proinflammatory qualities that contribute to airway hyperresponsiveness (AHR) and as a weak mediator of smooth muscle relaxation. In exhaled air, NO correlates with up-regulation of NO synthase (NOS), which occurs with inflammation, therefore, quantifying airway inflammation.6-8
There has been some variability in the evidence supporting the use of fractional exhaled NO (FeNO) levels as a diagnostic tool. Some studies have suggested that FeNO is also elevated in other nonasthma conditions, such as eosinophilic bronchitis, atopy, and allergic rhinitis. Also, FeNO levels have been shown to be variably influenced by smoking, bronchoconstriction, and viral respiratory infections.9 However, FeNO levels > 50 ppb correlated most strongly with eosinophilic asthma and steroid responsiveness.9
Fractional exhaled NO tests now can be performed in the PCP office with NIOX VERO (Chicago, IL), a small, relatively inexpensive device. Although the 2016 GINA guidelines and the 2015 ERS/ATS guidelines do not offer specific recommendations for use and do not support withholding ICS based on FeNO test results, guidelines for FeNO use do exist. In 2011, ATS published a specific set of FeNO interpretive guidelines for office-based use.9 When performed in conjunction with standard testing, FeNO levels can provide valuable clinically relevant information, such as (1) detection of eosinophilic airway inflammation; (2) determining the likelihood of corticosteroid responsiveness; (3) monitoring of airway inflammation to determine the need for steroids; and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy (Table 1).
Tiotropium as an Adjunct Treatment
Tiotropium is a long-acting inhaled anticholinergic. A sentinel 1984 study by Gross and Skorodin demonstrated that parasympathetic activity is the dominant reversible component in patients with chronic obstructive pulmonary disease (COPD), including emphysema.10 In addition, all achievable bronchodilation was obtained with an inhaled anticholinergic compared with that of separate or simultaneous administration of adrenergics. Sympathetic neural pathways are sparse in human lungs and have their endings on the cells of the cholinergic postganglionic fibers, because sympathetic terminals on airway smooth muscle cells are rare or nonexistent.11 Therefore, sympathetic modulation or activation of beta cells could change the parasympathetic tone.11
The FDA approved the addition of tiotropium for treating asthma in September 2015 for patients aged ≥ 12 years. The use of tiotropium is supported by both the ERS/ASTS and GINA 2016 guidelines. The recommended and approved dose of tiotropium for asthma is 2.5 µg daily (the recommended dose for COPD treatment is 5 µg).12 A recent phase 3 study compared 2.5 µg vs 5 µg dosing with ICS but no LABA in adolescents, noting significant improvement with the 2.5 µg dose.13 Adding tiotropium to ICS + LABA in patients with severe symptomatic asthma has been associated with positive results in initial studies by Kersjens and colleagues.14 Even as early as 2010, the use of tiotropium was shown to produce statistically significant improvement in morning peak expiratory flow (PEF), with a mean difference of 25.8 L/min (n = 210, P < .001).15
Tiotropium also has been shown to provide a sustained reduction in lung hyperinflation for those with COPD, thus providing an improvement in exertional dyspnea and exercise tolerance. On day 42 of a randomized, double-blinded, placebo-controlled, parallel-group study of 187 patients, vital capacity and inspiratory capacity were noted to be increased with decreases in residual volume and functional residual capacity. Exercise endurance times increased by 105 ± 40 sec (21%).16 This effect has not been studied yet in a population of patients with asthma; however, the same principles may hold true.
Biologic Agents
Recent asthma research has been focused on disrupting the inflammatory cascade. Both GINA and ERS/ATS divide asthma into allergic vs nonallergic endotypes. Allergic asthma usually is manifested by sputum eosinophilia and high serum eosinophil counts, whereas other endotypes include aspirin-sensitive and exercise-induced asthmas that present with a neutrophilic predominance. Nonallergic asthma is more severe typically and has been linked to steroid resistance.17 Many differentphenotypes have been identified, but the main categories include eosinophilic, neutrophilic, mixed, and paucigranulocytic.18
Mast cells, bronchial epithelium, and macrophages are involved in asthma progression. Targeting the cytokines produced by these pathways can be achieved through direct and indirect modulation. Interleukin (IL)-13 is central to development of AHR, and its effect is mediated through binding to its receptor and IL-4 receptor α complexes.19 Patients with severe asthma with an eosinophilic phenotype can benefit with the use of the new biologics, which decrease the amount of eosinophilia in lung tissue by blocking specific receptors for IL-5.
Omalizumab
Omalizumab, an anti-immunoglobulin E (IgE) antibody, has been shown to be helpful in treating patients with allergic asthma. Omalizumab is a 95% humanized IgE monoclonal antibody (MAB) that binds to the IgE molecule at the fc region and prevents IgE from binding to cell-surface receptors. In a humanized MAB, only the hypervariable regions are from mouse origin vs the newer completely human MABs. Omalizumab forms small, biologically inert IgE+ anti-IgE complexes that cannot activate the complement cascade. The serum free IgE level is decreased.20 Approved in 2003 for those aged ≤ 12 years, its use is restricted to patients with severe asthma, allergic sensitization (positive allergen skin testing), and an elevated serum IgE level (30-700 IU/mL). It is administered subcutaneously every 2 to 4 weeks, based on body weight and serum IgE levels.
For those with baseline eosinophil counts > 300 µL, addition of omalizumab most likely has been shown to improve quality of life (QOL) and reduce exacerbations, the use of rescue medications, ICS dosages, and ED visits.21-26 The most dangerous adverse effect (AE) was found to be an anaphylaxis rate of 0.09%, most frequently occurring in the first 2 hours after the first dose. Therefore, the patient must be monitored for 2 hours after the first dose and 30 minutes after subsequent doses. Epinephrine injection also should be prescribed. Although a 5-year prospective cohort study and retrospective pooled analysis of more than 10,000 patients did not support any relationship with malignancy.27,28 A higher incidence of cardiovascular and cerebrovascular AEs has been observed, and the FDA issued a safety announcement regarding this finding.29
Both ERS/ATS and GINA 2016 recommended that a therapeutic trial of omalizumab should be performed in all patients with severe confirmed IgE dependent allergic asthma.4,5 If there is no response in 4 months, it is unlikely that further administration would be beneficial.
Mepolizumab
Interleukin-5 is a key cytokine in the eosinophil life pathway. There are receptors for IL-5 on eosinophils, basophils, and β cells.30 Mepolizumab is an anti-IL-5 antibody for those with refractory eosinophilic asthma and a history of continued exacerbations. It has beneficial effects in the management of persistent airways eosinophilia among corticosteroid-resistant subjects. In a 2009 study, rates of exacerbations at 50 weeks were significantly lower than with placebo (2.0 vs 3.4 mean exacerbations per subject, 95% confidence interval [CI], 0.32-0.92; P = .002) as were eosinophil counts in blood and sputum (P < .001 and P = .002 respectively.31 A 2014 randomized, double-blind trial by Ortega and colleagues demonstrated reduction in rate of asthma exacerbations (primary outcome) to 47% (95% CI, 29-61) among patients receiving IV dosing and 53% (95% CI, 37-65) in the oral mepolizumab group (P < .001 for both groups, n = 576).32
In addition, there is significant data to show that even if the patient did not respond to omalizumab, he or she might still respond to mepolizumab. Data were collected from 2 randomized, double-blind, placebo-controlled studies with rate of exacerbation and percentage reduction in oral corticosteroid dose as the primary outcomes. In one of the studies (n = 576), the subjects were noted to have prior omalizumab use but still decreased exacerbation rate by 57%.33
Reslizumab
Reslizumab also is an FDA-approved anti-IL-5 antibody. It binds directly to IL-5 and prevents it from binding to eosinophils.34 For adults with severe eosinophilic asthma and refractory exacerbations, the goal of reslizumab therapy is to reduce eosinophil maturation, recruitment, and activation. Reslizumab is delivered in a weight-based IV dose (3 mg/kg) every 4 weeks. The FDA has issued a boxed warning for a 0.3% anaphylaxis rate.35 The most common AEs are elevated creatinine kinase, musculoskeletal pain, and oropharyngeal pain. Use of reslizumab resulted in greater reduction in sputum eosinophils, improvements in airway function, and a trend toward greater asthma control compared with that of placebo.34
Other Biologic Therapies
Many biologics are being developed as medical researchers continue to understand more of the mechanisms and pathways that contribute to allergic disease (Table 2). Dupilumab is an IL-4 inhibitor designated as a “breakthrough therapy” in 2014 by the FDA. This biologic blocks the downstream signaling events induced by IL-4 and IL-13 by binding to a subunit of the IL-4 receptor in the complexes. It has been found beneficial for those with high blood eosinophil counts and moderate-to-severe asthma and decreased asthma exacerbations when LABA and ICS were withdrawn.36,37
Fevipiprant is a prostaglandin D2 inhibitor that blocks T-helper type 2 (Th2) cell migration and subsequent bronchoconstriction and cytokine effects with decreased IL-4, IL-5, and IL-13. Although sputum eosinophil percentage was noted to be decreased in a study involving 61 patients randomized to treatment for 12 weeks, asthma QOL questionnaires and prebronchodilator spirometry did not change.38,39
Benralizumab is an anti-IL-5 receptor antibody that has been more effective in reduction of airway and blood eosinophils levels compared with that of mepolizumab (undetectable vs 52% reduction), within 24 hours of IV dosing. In contrast, the anti-IL-5 antibodies take about 4 weeks to decrease eosinophil levels in blood and sputum.34 There have been no documented AEs aside from nasopharyngitis and injection site reactions and no safety concerns to date. It is currently undergoing phase 3 trials.40
Immunotherapies
Allergen immunotherapy is recommended for mild-to-moderate asthma. A 2010 Cochrane Review found that subcutaneous immunotherapy compared with placebo demonstrated improvements in bronchial hyperresponsiveness and decreased medication use.41 Expert Panel Report-3 guidelines recommend consideration of immunotherapy for mild-to-moderate asthma.5 While ERS/ATS guidelines for severe asthma do not address allergen immunotherapy, GINA guidelines incorporate it as Evidence A for treating modifiable risk factors to reduce exacerbations, although the efficacy is limited.6
Roflumilast
Roflumilast is a selective PDE4 inhibitor that has shown an anti-inflammatory effect in COPD. Studies evaluating the reversibility and prevention of airway remodeling showed good promise in mouse models.42 Data from 8 placebo-controlled, double-blind, phase 1, 2, and 3 studies conducted at 14 sites in Europe, North America, and South Africa from 1997 to 2005 showed reduced sputum eosinophil and neutrophil counts, consistent with findings during COPD treatment. However, forced expiratory volume in one second (FEV1) and PEF values were unchanged, suggesting that there was no acute bronchodilatory effect with roflumilast therapy.43 Roflumilast is not addressed in the 2016 GINA guidelines and at this time does not have a role in the treatment of severe asthma.
Antileukotrienes
After the activation of mast cells and eosinophils, leukotrienes are generated by 5-lipoxygenase from arachidonic acid and create bronchoconstriction, vasodilation, increased mucus production, increased recruitment of eosinophils, and decreased ciliary motility. Some studies have encouraged addingleukotriene receptor blockers (both montelukast and zafirlukast) to ICS therapy44,45 and to therapy for patients with aspirin-intolerant asthma or allergic asthma.46,47 However, other studies have shown them to be of limited benefit.48,49 A recent Cochrane Reviewof 18 randomized-controlled trials with 7,208 adults and children compared ICS + leukotriene receptor antagonist (LTRA) vs ICS + LABA.50 The ICS + LABA resulted in greater improvements in lung function, symptoms score, and rates of exacerbations.50
Most recommendations recognize the limitations of antileukotriene medications and agree that they are an adjunct rather than primary therapy. The GINA 2016 guidelines support the use of LTRAs in mild asthma, stating that although LTRAs are less effective than ICS (Evidence A), they may be appropriate for initial controller treatment for some patients who are unable or unwilling to use ICS or for patients with concurrent allergic rhinitis (Evidence B).51,52
Zileuton is a different type of antileukotriene. It inhibits leukotrienes B4, C4, D4, and E4 by inhibition of 5-lipoxygenase, interfering with leukotriene formation. It is approved for patients aged ≥ 12 years and is more expensive than montelukast or zafirlukast. Most studies supporting its use were conducted in patients with mild-to-moderate asthma on β-agonist therapy only. A 1988 study showed that zileuton therapy improved FEV1, reduced nasal symptoms, and decreased bronchial responsiveness to inhaled aspirin and histamine.53 All but 1 study patient were on ICS or oral corticosteroids. Zileuton was noted to be effective for patients with aspirin-intolerant asthma.
Some earlier studies reported that a small number of subjects had an increase in transaminases that resolved when they discontinued the medication. Therefore, it is recommended to check baseline laboratory results every 2 to 3 months.54,55 Neither GINA nor ERS/ATS guidelines address the use of zileuton.
Bronchial Thermoplasty
With asthma there is marked hypertrophy and hyperplasia that occurs in the airway smooth muscle. The airway of the patient with asthma also is lined with cells that promote inflammation. Thermal energy is used to perform controlled destruction of the inflammatory lining and pathologic hyperplasia. Three sequential bronchoscopies are performed 3 weeks apart to treat the right lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is not treated due to its smaller diameter. Each bronchoscopy takes about 30 to 60 minutes. Patients are given perioperative steroids.56
Three large phase 3 clinical trials have evaluated the efficacy of bronchial thermoplasty (BT). The AIR (Asthma Intervention Research) trial in 2007 was a randomized controlled study of 112 patients with moderate or severe asthma that showed improved exacerbation rates, symptom-free days and QOL scores (1.3 ± 1.0 vs 0.6 ± 1.1; P = .003), but no difference in prebronchodilator FEV1 or AHR.57 There was a significant reduction in the rate of mild exacerbations and increase in morning PEF rates.57 Findings at 5 years showed improved AHR but no difference in frequency of need for oral corticosteroids and frequency of hospital or emergency department (ED) visits.58
The Research in Severe Asthma (RISA) clinical trial was a randomized controlled study (n = 32, 15 randomly assigned to BT) that showed improved prebronchodilator FEV1 in patients with severe, symptomatic asthma and baseline FEV1 of 62% to 66% with half the patients requiring oral corticosteroids (percentage predicted; 14.9 ± 17.4 vs -0.9 ± 22.3; P =.04).57 Quality of life scores were also significantly improved. At 5 years (14 BT patients were followed), the frequency of hospitalizations and ED visits decreased.59
The 2010 AIR2 study was a randomized, double blind, sham-controlled study (n = 288) developed to address the limitations of the 2 previous studies. It excluded the severest asthma cases, and its blinded nature was created with sham bronchoscopy to eliminate possible placebo effect. The study questionnaires showed improved QOL overall (79% vs 64%); however, there was a definite placebo effect noted.60 Decreased frequency of severe exacerbations as well as ED visits and days lost from work or school also were documented as secondary endpoints. At 5 years, decreased frequency of severe exacerbations and ED visits continued in the control group (85% consented to follow-up).61 Importantly, despite the placebo effect in QOL scores, there were no improvements in exacerbation rates or hospitalizations in those receiving sham bronchoscopy at the 1-year mark.61
Although more longitudinal studies need to be planned, including evaluation of those with the most severe asthma, there seems to be a sustained improvement in patients. Those who have received BT generally are found to have reduced airway smooth muscle with lower concentration of key inflammatory cytokines on follow-up bronchoscopy. However, variability in response has been documented.56 There has been no documented deterioration in pulmonary function with BT, and no significant structural abnormalities have been seen on high-resolution computed tomography.56,58 Both GINA 2016 and ERS/ATS support the use of BT in the context of adults with severe asthma, calling for more long-term studies to address delayed benefits and safety.
LABA Inhalers
A multicenter, double-blind, 26-week study of 11,693 patients randomized to ICS + LABA (budesonide/formoterol) vs ICS (budesonide) alone has shown no increased AEs in either arm. The study found that treatment with budesonide/formoterol was associated with lower risk of asthma exacerbations than using budesonide alone (16.5%; P = .002).62
The safety of adding a LABA to fluticasone also has been evaluated recently. A 2016 study of almost 12,000 patients (aged > 12 years) compared fluticasone proprionate alone vs fluticasone with salmeterol.63 There were no asthma-related deaths, but 2 patients in the fluticasone-only group were intubated with asthma complications. The risk of a severe asthma exacerbation seemed to be lower in the combination group (8% vs 10%; P < .001).63
A 2014 Cochrane Review supported the view that LABAs in adults seem to be safe when used concurrently with an ICS with a A-level recommendation, based on consistent good-quality, patient-oriented evidence.64 Multiple organizations have issued guidelines to this effect in the past, but previous results of studies showed that asthma deaths and a small increase in nonfatal serious AEs were noted in those using LABA monotherapy alone.64
NAEPP (EPR-3) and ERS/ATS recommend stepwise increases in the dose of ICS in combination with a LABA. The GINA guidelines recommend controller therapy to include combination IHS and LABA but with the consideration of higher doses of ICS than are routinely recommended for general use.
Inhaler and Inhaler Combinations
Many different inhalers of ICS alone and ICS/LABA combinations exist on the market today. There are differences in delivery that affect patient preference but these differences have not been found to improve delivery. Small particle ICS therapy could possibly correlate with improved delivery to the small airways.65 There are 3 preparations of inhaled steroids that fit in to this group, including beclomethasone, ciclesonide, and flunisolide. Other inhaled steroid formulations include budesonide, fluticasone propionate, fluticasone furoate, and mometasone.
Combination therapy (ICS + LABA) inhalers also are widely available. They include budesonide/formoterol, fluticasone proprionate/salmeterol, mometasone/formoterol, and the newer fluticasone furoate/vilanterol, a once-daily combination approved for those aged ≥ 18 years.
Conclusion
The treatment of severe asthma has progressed from simple manipulation of avoidance, bronchodilators, and corticosteroids to include many other treatments that have improved QOL for patients with refractory asthma. Although many of these options are delivered in coordination with an allergy and pulmonary specialist, it is important for the PCP to have a good knowledge base and awareness of additional treatments that are currently available.
More than 39.5 million people in the U.S. have been diagnosed with asthma, and about 3,400 deaths occur annually due to asthma complications.1 Although the prevalence of atopy and asthma have increased over the past few decades in western countries, control and outcomes are improving.2 Use of asthma protocols and early recognition by the primary care provider (PCP) are among the main reasons for trends toward decreased hospitalization and fewer asthma-related deaths.3,4
In addition to the mainstay of treatments, including trigger avoidance, inhaled corticosteroids (ICS), and rescue bronchodilators, new therapies have been developed to supplement the treatment of severe persistent asthma, which constitutes about 5% to 10% of asthma cases. Severe asthma is defined as asthma that is unresponsive to baseline therapy.5
Three sets of guidelines and recommendations exist to provide structure to asthma treatment decision making. The Expert Panel Report-3 (EPR-3) was created by the National Education and Prevention Program (NAEPP) and was last published in 2007. The NAEPP favors a stepwise approach, based on asthma severity and age group.3 The International European Respiratory Society (ERS) and American Thoracic Society (ATS) task force report was updated in 2014.5 The Global Initiative for Asthma (GINA) report, updated in 2016, now includes several of the advances in asthma care for those patients refractory to standard treatments.
Asthma Therapies
In this review, the authors cover therapies for severe asthma that are becoming more important for PCPs to consider, including exhaled nitric oxide (NO) levels, the use of tiotropium for asthma, the applicability of biologic agents, the use of allergen immunotherapy, and the usefulness of roflumilast. This review also covers antileukotriene therapy, bronchial thermoplasty, and a discussion of long-acting beta-agonist (LABA) therapy.
Fractional Exhaled Nitric Oxide
Nitric oxide is present in the exhaled breath and is elevated in those with eosinophilic asthma.6 The role of NO in asthma pathology is complex, involving proinflammatory qualities that contribute to airway hyperresponsiveness (AHR) and as a weak mediator of smooth muscle relaxation. In exhaled air, NO correlates with up-regulation of NO synthase (NOS), which occurs with inflammation, therefore, quantifying airway inflammation.6-8
There has been some variability in the evidence supporting the use of fractional exhaled NO (FeNO) levels as a diagnostic tool. Some studies have suggested that FeNO is also elevated in other nonasthma conditions, such as eosinophilic bronchitis, atopy, and allergic rhinitis. Also, FeNO levels have been shown to be variably influenced by smoking, bronchoconstriction, and viral respiratory infections.9 However, FeNO levels > 50 ppb correlated most strongly with eosinophilic asthma and steroid responsiveness.9
Fractional exhaled NO tests now can be performed in the PCP office with NIOX VERO (Chicago, IL), a small, relatively inexpensive device. Although the 2016 GINA guidelines and the 2015 ERS/ATS guidelines do not offer specific recommendations for use and do not support withholding ICS based on FeNO test results, guidelines for FeNO use do exist. In 2011, ATS published a specific set of FeNO interpretive guidelines for office-based use.9 When performed in conjunction with standard testing, FeNO levels can provide valuable clinically relevant information, such as (1) detection of eosinophilic airway inflammation; (2) determining the likelihood of corticosteroid responsiveness; (3) monitoring of airway inflammation to determine the need for steroids; and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy (Table 1).
Tiotropium as an Adjunct Treatment
Tiotropium is a long-acting inhaled anticholinergic. A sentinel 1984 study by Gross and Skorodin demonstrated that parasympathetic activity is the dominant reversible component in patients with chronic obstructive pulmonary disease (COPD), including emphysema.10 In addition, all achievable bronchodilation was obtained with an inhaled anticholinergic compared with that of separate or simultaneous administration of adrenergics. Sympathetic neural pathways are sparse in human lungs and have their endings on the cells of the cholinergic postganglionic fibers, because sympathetic terminals on airway smooth muscle cells are rare or nonexistent.11 Therefore, sympathetic modulation or activation of beta cells could change the parasympathetic tone.11
The FDA approved the addition of tiotropium for treating asthma in September 2015 for patients aged ≥ 12 years. The use of tiotropium is supported by both the ERS/ASTS and GINA 2016 guidelines. The recommended and approved dose of tiotropium for asthma is 2.5 µg daily (the recommended dose for COPD treatment is 5 µg).12 A recent phase 3 study compared 2.5 µg vs 5 µg dosing with ICS but no LABA in adolescents, noting significant improvement with the 2.5 µg dose.13 Adding tiotropium to ICS + LABA in patients with severe symptomatic asthma has been associated with positive results in initial studies by Kersjens and colleagues.14 Even as early as 2010, the use of tiotropium was shown to produce statistically significant improvement in morning peak expiratory flow (PEF), with a mean difference of 25.8 L/min (n = 210, P < .001).15
Tiotropium also has been shown to provide a sustained reduction in lung hyperinflation for those with COPD, thus providing an improvement in exertional dyspnea and exercise tolerance. On day 42 of a randomized, double-blinded, placebo-controlled, parallel-group study of 187 patients, vital capacity and inspiratory capacity were noted to be increased with decreases in residual volume and functional residual capacity. Exercise endurance times increased by 105 ± 40 sec (21%).16 This effect has not been studied yet in a population of patients with asthma; however, the same principles may hold true.
Biologic Agents
Recent asthma research has been focused on disrupting the inflammatory cascade. Both GINA and ERS/ATS divide asthma into allergic vs nonallergic endotypes. Allergic asthma usually is manifested by sputum eosinophilia and high serum eosinophil counts, whereas other endotypes include aspirin-sensitive and exercise-induced asthmas that present with a neutrophilic predominance. Nonallergic asthma is more severe typically and has been linked to steroid resistance.17 Many differentphenotypes have been identified, but the main categories include eosinophilic, neutrophilic, mixed, and paucigranulocytic.18
Mast cells, bronchial epithelium, and macrophages are involved in asthma progression. Targeting the cytokines produced by these pathways can be achieved through direct and indirect modulation. Interleukin (IL)-13 is central to development of AHR, and its effect is mediated through binding to its receptor and IL-4 receptor α complexes.19 Patients with severe asthma with an eosinophilic phenotype can benefit with the use of the new biologics, which decrease the amount of eosinophilia in lung tissue by blocking specific receptors for IL-5.
Omalizumab
Omalizumab, an anti-immunoglobulin E (IgE) antibody, has been shown to be helpful in treating patients with allergic asthma. Omalizumab is a 95% humanized IgE monoclonal antibody (MAB) that binds to the IgE molecule at the fc region and prevents IgE from binding to cell-surface receptors. In a humanized MAB, only the hypervariable regions are from mouse origin vs the newer completely human MABs. Omalizumab forms small, biologically inert IgE+ anti-IgE complexes that cannot activate the complement cascade. The serum free IgE level is decreased.20 Approved in 2003 for those aged ≤ 12 years, its use is restricted to patients with severe asthma, allergic sensitization (positive allergen skin testing), and an elevated serum IgE level (30-700 IU/mL). It is administered subcutaneously every 2 to 4 weeks, based on body weight and serum IgE levels.
For those with baseline eosinophil counts > 300 µL, addition of omalizumab most likely has been shown to improve quality of life (QOL) and reduce exacerbations, the use of rescue medications, ICS dosages, and ED visits.21-26 The most dangerous adverse effect (AE) was found to be an anaphylaxis rate of 0.09%, most frequently occurring in the first 2 hours after the first dose. Therefore, the patient must be monitored for 2 hours after the first dose and 30 minutes after subsequent doses. Epinephrine injection also should be prescribed. Although a 5-year prospective cohort study and retrospective pooled analysis of more than 10,000 patients did not support any relationship with malignancy.27,28 A higher incidence of cardiovascular and cerebrovascular AEs has been observed, and the FDA issued a safety announcement regarding this finding.29
Both ERS/ATS and GINA 2016 recommended that a therapeutic trial of omalizumab should be performed in all patients with severe confirmed IgE dependent allergic asthma.4,5 If there is no response in 4 months, it is unlikely that further administration would be beneficial.
Mepolizumab
Interleukin-5 is a key cytokine in the eosinophil life pathway. There are receptors for IL-5 on eosinophils, basophils, and β cells.30 Mepolizumab is an anti-IL-5 antibody for those with refractory eosinophilic asthma and a history of continued exacerbations. It has beneficial effects in the management of persistent airways eosinophilia among corticosteroid-resistant subjects. In a 2009 study, rates of exacerbations at 50 weeks were significantly lower than with placebo (2.0 vs 3.4 mean exacerbations per subject, 95% confidence interval [CI], 0.32-0.92; P = .002) as were eosinophil counts in blood and sputum (P < .001 and P = .002 respectively.31 A 2014 randomized, double-blind trial by Ortega and colleagues demonstrated reduction in rate of asthma exacerbations (primary outcome) to 47% (95% CI, 29-61) among patients receiving IV dosing and 53% (95% CI, 37-65) in the oral mepolizumab group (P < .001 for both groups, n = 576).32
In addition, there is significant data to show that even if the patient did not respond to omalizumab, he or she might still respond to mepolizumab. Data were collected from 2 randomized, double-blind, placebo-controlled studies with rate of exacerbation and percentage reduction in oral corticosteroid dose as the primary outcomes. In one of the studies (n = 576), the subjects were noted to have prior omalizumab use but still decreased exacerbation rate by 57%.33
Reslizumab
Reslizumab also is an FDA-approved anti-IL-5 antibody. It binds directly to IL-5 and prevents it from binding to eosinophils.34 For adults with severe eosinophilic asthma and refractory exacerbations, the goal of reslizumab therapy is to reduce eosinophil maturation, recruitment, and activation. Reslizumab is delivered in a weight-based IV dose (3 mg/kg) every 4 weeks. The FDA has issued a boxed warning for a 0.3% anaphylaxis rate.35 The most common AEs are elevated creatinine kinase, musculoskeletal pain, and oropharyngeal pain. Use of reslizumab resulted in greater reduction in sputum eosinophils, improvements in airway function, and a trend toward greater asthma control compared with that of placebo.34
Other Biologic Therapies
Many biologics are being developed as medical researchers continue to understand more of the mechanisms and pathways that contribute to allergic disease (Table 2). Dupilumab is an IL-4 inhibitor designated as a “breakthrough therapy” in 2014 by the FDA. This biologic blocks the downstream signaling events induced by IL-4 and IL-13 by binding to a subunit of the IL-4 receptor in the complexes. It has been found beneficial for those with high blood eosinophil counts and moderate-to-severe asthma and decreased asthma exacerbations when LABA and ICS were withdrawn.36,37
Fevipiprant is a prostaglandin D2 inhibitor that blocks T-helper type 2 (Th2) cell migration and subsequent bronchoconstriction and cytokine effects with decreased IL-4, IL-5, and IL-13. Although sputum eosinophil percentage was noted to be decreased in a study involving 61 patients randomized to treatment for 12 weeks, asthma QOL questionnaires and prebronchodilator spirometry did not change.38,39
Benralizumab is an anti-IL-5 receptor antibody that has been more effective in reduction of airway and blood eosinophils levels compared with that of mepolizumab (undetectable vs 52% reduction), within 24 hours of IV dosing. In contrast, the anti-IL-5 antibodies take about 4 weeks to decrease eosinophil levels in blood and sputum.34 There have been no documented AEs aside from nasopharyngitis and injection site reactions and no safety concerns to date. It is currently undergoing phase 3 trials.40
Immunotherapies
Allergen immunotherapy is recommended for mild-to-moderate asthma. A 2010 Cochrane Review found that subcutaneous immunotherapy compared with placebo demonstrated improvements in bronchial hyperresponsiveness and decreased medication use.41 Expert Panel Report-3 guidelines recommend consideration of immunotherapy for mild-to-moderate asthma.5 While ERS/ATS guidelines for severe asthma do not address allergen immunotherapy, GINA guidelines incorporate it as Evidence A for treating modifiable risk factors to reduce exacerbations, although the efficacy is limited.6
Roflumilast
Roflumilast is a selective PDE4 inhibitor that has shown an anti-inflammatory effect in COPD. Studies evaluating the reversibility and prevention of airway remodeling showed good promise in mouse models.42 Data from 8 placebo-controlled, double-blind, phase 1, 2, and 3 studies conducted at 14 sites in Europe, North America, and South Africa from 1997 to 2005 showed reduced sputum eosinophil and neutrophil counts, consistent with findings during COPD treatment. However, forced expiratory volume in one second (FEV1) and PEF values were unchanged, suggesting that there was no acute bronchodilatory effect with roflumilast therapy.43 Roflumilast is not addressed in the 2016 GINA guidelines and at this time does not have a role in the treatment of severe asthma.
Antileukotrienes
After the activation of mast cells and eosinophils, leukotrienes are generated by 5-lipoxygenase from arachidonic acid and create bronchoconstriction, vasodilation, increased mucus production, increased recruitment of eosinophils, and decreased ciliary motility. Some studies have encouraged addingleukotriene receptor blockers (both montelukast and zafirlukast) to ICS therapy44,45 and to therapy for patients with aspirin-intolerant asthma or allergic asthma.46,47 However, other studies have shown them to be of limited benefit.48,49 A recent Cochrane Reviewof 18 randomized-controlled trials with 7,208 adults and children compared ICS + leukotriene receptor antagonist (LTRA) vs ICS + LABA.50 The ICS + LABA resulted in greater improvements in lung function, symptoms score, and rates of exacerbations.50
Most recommendations recognize the limitations of antileukotriene medications and agree that they are an adjunct rather than primary therapy. The GINA 2016 guidelines support the use of LTRAs in mild asthma, stating that although LTRAs are less effective than ICS (Evidence A), they may be appropriate for initial controller treatment for some patients who are unable or unwilling to use ICS or for patients with concurrent allergic rhinitis (Evidence B).51,52
Zileuton is a different type of antileukotriene. It inhibits leukotrienes B4, C4, D4, and E4 by inhibition of 5-lipoxygenase, interfering with leukotriene formation. It is approved for patients aged ≥ 12 years and is more expensive than montelukast or zafirlukast. Most studies supporting its use were conducted in patients with mild-to-moderate asthma on β-agonist therapy only. A 1988 study showed that zileuton therapy improved FEV1, reduced nasal symptoms, and decreased bronchial responsiveness to inhaled aspirin and histamine.53 All but 1 study patient were on ICS or oral corticosteroids. Zileuton was noted to be effective for patients with aspirin-intolerant asthma.
Some earlier studies reported that a small number of subjects had an increase in transaminases that resolved when they discontinued the medication. Therefore, it is recommended to check baseline laboratory results every 2 to 3 months.54,55 Neither GINA nor ERS/ATS guidelines address the use of zileuton.
Bronchial Thermoplasty
With asthma there is marked hypertrophy and hyperplasia that occurs in the airway smooth muscle. The airway of the patient with asthma also is lined with cells that promote inflammation. Thermal energy is used to perform controlled destruction of the inflammatory lining and pathologic hyperplasia. Three sequential bronchoscopies are performed 3 weeks apart to treat the right lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is not treated due to its smaller diameter. Each bronchoscopy takes about 30 to 60 minutes. Patients are given perioperative steroids.56
Three large phase 3 clinical trials have evaluated the efficacy of bronchial thermoplasty (BT). The AIR (Asthma Intervention Research) trial in 2007 was a randomized controlled study of 112 patients with moderate or severe asthma that showed improved exacerbation rates, symptom-free days and QOL scores (1.3 ± 1.0 vs 0.6 ± 1.1; P = .003), but no difference in prebronchodilator FEV1 or AHR.57 There was a significant reduction in the rate of mild exacerbations and increase in morning PEF rates.57 Findings at 5 years showed improved AHR but no difference in frequency of need for oral corticosteroids and frequency of hospital or emergency department (ED) visits.58
The Research in Severe Asthma (RISA) clinical trial was a randomized controlled study (n = 32, 15 randomly assigned to BT) that showed improved prebronchodilator FEV1 in patients with severe, symptomatic asthma and baseline FEV1 of 62% to 66% with half the patients requiring oral corticosteroids (percentage predicted; 14.9 ± 17.4 vs -0.9 ± 22.3; P =.04).57 Quality of life scores were also significantly improved. At 5 years (14 BT patients were followed), the frequency of hospitalizations and ED visits decreased.59
The 2010 AIR2 study was a randomized, double blind, sham-controlled study (n = 288) developed to address the limitations of the 2 previous studies. It excluded the severest asthma cases, and its blinded nature was created with sham bronchoscopy to eliminate possible placebo effect. The study questionnaires showed improved QOL overall (79% vs 64%); however, there was a definite placebo effect noted.60 Decreased frequency of severe exacerbations as well as ED visits and days lost from work or school also were documented as secondary endpoints. At 5 years, decreased frequency of severe exacerbations and ED visits continued in the control group (85% consented to follow-up).61 Importantly, despite the placebo effect in QOL scores, there were no improvements in exacerbation rates or hospitalizations in those receiving sham bronchoscopy at the 1-year mark.61
Although more longitudinal studies need to be planned, including evaluation of those with the most severe asthma, there seems to be a sustained improvement in patients. Those who have received BT generally are found to have reduced airway smooth muscle with lower concentration of key inflammatory cytokines on follow-up bronchoscopy. However, variability in response has been documented.56 There has been no documented deterioration in pulmonary function with BT, and no significant structural abnormalities have been seen on high-resolution computed tomography.56,58 Both GINA 2016 and ERS/ATS support the use of BT in the context of adults with severe asthma, calling for more long-term studies to address delayed benefits and safety.
LABA Inhalers
A multicenter, double-blind, 26-week study of 11,693 patients randomized to ICS + LABA (budesonide/formoterol) vs ICS (budesonide) alone has shown no increased AEs in either arm. The study found that treatment with budesonide/formoterol was associated with lower risk of asthma exacerbations than using budesonide alone (16.5%; P = .002).62
The safety of adding a LABA to fluticasone also has been evaluated recently. A 2016 study of almost 12,000 patients (aged > 12 years) compared fluticasone proprionate alone vs fluticasone with salmeterol.63 There were no asthma-related deaths, but 2 patients in the fluticasone-only group were intubated with asthma complications. The risk of a severe asthma exacerbation seemed to be lower in the combination group (8% vs 10%; P < .001).63
A 2014 Cochrane Review supported the view that LABAs in adults seem to be safe when used concurrently with an ICS with a A-level recommendation, based on consistent good-quality, patient-oriented evidence.64 Multiple organizations have issued guidelines to this effect in the past, but previous results of studies showed that asthma deaths and a small increase in nonfatal serious AEs were noted in those using LABA monotherapy alone.64
NAEPP (EPR-3) and ERS/ATS recommend stepwise increases in the dose of ICS in combination with a LABA. The GINA guidelines recommend controller therapy to include combination IHS and LABA but with the consideration of higher doses of ICS than are routinely recommended for general use.
Inhaler and Inhaler Combinations
Many different inhalers of ICS alone and ICS/LABA combinations exist on the market today. There are differences in delivery that affect patient preference but these differences have not been found to improve delivery. Small particle ICS therapy could possibly correlate with improved delivery to the small airways.65 There are 3 preparations of inhaled steroids that fit in to this group, including beclomethasone, ciclesonide, and flunisolide. Other inhaled steroid formulations include budesonide, fluticasone propionate, fluticasone furoate, and mometasone.
Combination therapy (ICS + LABA) inhalers also are widely available. They include budesonide/formoterol, fluticasone proprionate/salmeterol, mometasone/formoterol, and the newer fluticasone furoate/vilanterol, a once-daily combination approved for those aged ≥ 18 years.
Conclusion
The treatment of severe asthma has progressed from simple manipulation of avoidance, bronchodilators, and corticosteroids to include many other treatments that have improved QOL for patients with refractory asthma. Although many of these options are delivered in coordination with an allergy and pulmonary specialist, it is important for the PCP to have a good knowledge base and awareness of additional treatments that are currently available.
1. Centers for Disease Control and Prevention. Asthma facts: CDC’s national asthma control program grantees. https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Published July 2013. Accessed November 9, 2017.
2. Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990-2003. Med J Aust. 2006;184(5):226-229.
3. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
4. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 update. http://ginasthma.org/wp-content/up loads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed November 9, 2017.
5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
6. Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology. 2003;8(4):479-486.
7. De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621-1630.
8. Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182.
9. Dweik RA, Boggs PB, Erzurum SC, et al; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
10. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421-425.
11. Gelb AF, Nadel JA. Affirmation of the adoration of the vagi and role of tiotropium in asthmatic patients. J Allergy Clin Immunol. 2016;138(4):1011-1013.
12. Chin SJ, Durmowicz AG, Chowdhury BA. Tiotropium respimat is effective for the treatment of asthma at a dose lower than that for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):173-179.
13. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441-450.e8.
14. Kerstjens HA, Casale TB, Bleeker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367-376.
15. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715-1726.
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnea, and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
17. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355-360.
18. Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170(6):579-580.
19. Wang E, Hoyte FC. Traditional therapies for severe asthma. Immunol Allergy Clin North Am. 2016;36(3):581-608.
20. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689-2695.
21. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378-1386.
22. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309-316.
23. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573-582.
24. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408-416.
25. Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111(2):278-284.
26. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184-190.
27. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4.
28. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs /DrugSafety/ucm414911.htm. Updated February 10, 2016. Accessed November 9, 2017.
30. Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16(10):70.
31. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973-984.
32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
33. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335-1344.
34. Castro M, Mathur S, Hargreave F, et al; Res-5-0010 Study Group. Resilizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125-1132.
35. Cinqair [package insert]. Frazier, PA: Teva Respiratory; 2016.
36. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455-2466.
37. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3-4.
38. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699-707.
39. Erpenbeck VJ, Popov TA, Miller D, et al. The oral CRTh2 antagonist QAWO39 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54-63.
40. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71-81.
41. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
42. Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodeling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754-763.
43. Bardin P, Kanniess F, Gauvreau G, Bredenbröker D, Rabe KF. Roflumilast for asthma: efficacy findings in mechanism of action studies. Pulm Pharmacol Ther. 2015;(suppl 35):S4-S10.
44. Virchow JC Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Resp Crit Care Med. 2000;162(2, pt 1):558-585.
45. Price DB, Hernandez D, Magyar P, et al; Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211-216.
46. Dahlén SE, Malmström K, Nizankowska E, et al. Improvement of aspirin intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(1):9-14.
47. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006; 61(6):737-742.
48. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357(9273):2007-2011.
49. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1):CD003137.
50. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
51. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549-1558.
52. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31(4):616-624.
53. Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4, pt 1):1187-1194.
54. Israel E, Cohn J, Dubé L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zilueton Clinical Trial Group. JAMA. 1996;275(12):931-936.
55. Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99(2):178-184.
56. Laxmanan B, Egressy K, Murgu SD, White SR, Hogarth DK. Advances in bronchial thermoplasty. Chest. 2016;150(3):694-704.
57. Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327-1337.
58. Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011:11:8.
59. Pavord, ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185-1191.
60. Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116-124.
61. Wechsler ME, Laviolette M, Rubin AS, et al; Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295-1302.
62. Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860.
63. Stempel DA, Raphiou IH, Kral KM, et al; AUSTRI Investigators. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830.
64. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
65. Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am. 2016;36(3):473-482.
1. Centers for Disease Control and Prevention. Asthma facts: CDC’s national asthma control program grantees. https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Published July 2013. Accessed November 9, 2017.
2. Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990-2003. Med J Aust. 2006;184(5):226-229.
3. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
4. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 update. http://ginasthma.org/wp-content/up loads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed November 9, 2017.
5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
6. Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology. 2003;8(4):479-486.
7. De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621-1630.
8. Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182.
9. Dweik RA, Boggs PB, Erzurum SC, et al; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
10. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421-425.
11. Gelb AF, Nadel JA. Affirmation of the adoration of the vagi and role of tiotropium in asthmatic patients. J Allergy Clin Immunol. 2016;138(4):1011-1013.
12. Chin SJ, Durmowicz AG, Chowdhury BA. Tiotropium respimat is effective for the treatment of asthma at a dose lower than that for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):173-179.
13. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441-450.e8.
14. Kerstjens HA, Casale TB, Bleeker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367-376.
15. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715-1726.
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnea, and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
17. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355-360.
18. Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170(6):579-580.
19. Wang E, Hoyte FC. Traditional therapies for severe asthma. Immunol Allergy Clin North Am. 2016;36(3):581-608.
20. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689-2695.
21. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378-1386.
22. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309-316.
23. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573-582.
24. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408-416.
25. Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111(2):278-284.
26. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184-190.
27. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4.
28. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs /DrugSafety/ucm414911.htm. Updated February 10, 2016. Accessed November 9, 2017.
30. Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16(10):70.
31. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973-984.
32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
33. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335-1344.
34. Castro M, Mathur S, Hargreave F, et al; Res-5-0010 Study Group. Resilizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125-1132.
35. Cinqair [package insert]. Frazier, PA: Teva Respiratory; 2016.
36. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455-2466.
37. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3-4.
38. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699-707.
39. Erpenbeck VJ, Popov TA, Miller D, et al. The oral CRTh2 antagonist QAWO39 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54-63.
40. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71-81.
41. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
42. Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodeling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754-763.
43. Bardin P, Kanniess F, Gauvreau G, Bredenbröker D, Rabe KF. Roflumilast for asthma: efficacy findings in mechanism of action studies. Pulm Pharmacol Ther. 2015;(suppl 35):S4-S10.
44. Virchow JC Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Resp Crit Care Med. 2000;162(2, pt 1):558-585.
45. Price DB, Hernandez D, Magyar P, et al; Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211-216.
46. Dahlén SE, Malmström K, Nizankowska E, et al. Improvement of aspirin intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(1):9-14.
47. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006; 61(6):737-742.
48. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357(9273):2007-2011.
49. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1):CD003137.
50. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
51. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549-1558.
52. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31(4):616-624.
53. Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4, pt 1):1187-1194.
54. Israel E, Cohn J, Dubé L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zilueton Clinical Trial Group. JAMA. 1996;275(12):931-936.
55. Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99(2):178-184.
56. Laxmanan B, Egressy K, Murgu SD, White SR, Hogarth DK. Advances in bronchial thermoplasty. Chest. 2016;150(3):694-704.
57. Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327-1337.
58. Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011:11:8.
59. Pavord, ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185-1191.
60. Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116-124.
61. Wechsler ME, Laviolette M, Rubin AS, et al; Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295-1302.
62. Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860.
63. Stempel DA, Raphiou IH, Kral KM, et al; AUSTRI Investigators. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830.
64. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
65. Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am. 2016;36(3):473-482.
Antibiotics Before Dental Surgery—or Not?
Does antibiotic prophylaxis protect patients with cardiac conditions against endocarditis from invasive dental procedures? Studies have long suggested both “yes” and “no.” Researchers from Université Paris Diderot and others note that clinical trials and cohort studies have not proved efficacy. Only 2 case-control studies done in the past 30 years have established an association between dental procedures and streptococcal infective endocarditis; neither was sufficiently powered to establish the efficacy of antibiotic prophylaxis.
Current U.S. and European guidelines vary in who must be covered: all patients, certain patients, or no patients at all. But a rise in the incidence of infective endocarditis among patients with prosthetic heart valves, implicating invasive dental procedures, “raised the question of whether the indications for antibiotic prophylaxis may be broadened again,” the researchers say. They cite 2016 NICE guidelines that “clearly specify” that it may be appropriate in individual cases.
Given that endocarditis can be fatal or expensive to treat—hospitals stays are long and valve surgery may be needed—the researchers decided to evaluate the association between invasive dental procedures and oral streptococcal infective endocarditis in a population-based cohort and a case crossover study.
In the first study of 138,876 patients with prosthetic heart valves, 69,303 underwent at least 1 dental procedure. Of 396,615 dental procedures, 26% were invasive. Patients received prophylactic antibiotics before half of the procedures.
Over a median 1.7 years of follow-up, 267 people developed infective endocarditis associated with oral streptococci. However, the rate of oral streptococcal infective endocarditis did not rise significantly in the 3 months after an invasive dental procedure, with or without antibiotic prophylaxis.
The case crossover study of patients with endocarditis indicated “the same direction of effect”: Although invasive dental procedures may be associated with oral streptococcal infective endocarditis, the magnitude of the association “remains uncertain.”
Source:
Tubiana S, Blotière PO, Hoen B, et al. BMJ. 2017;358: j3776.
doi: 10.1136/bmj.j3776.
Does antibiotic prophylaxis protect patients with cardiac conditions against endocarditis from invasive dental procedures? Studies have long suggested both “yes” and “no.” Researchers from Université Paris Diderot and others note that clinical trials and cohort studies have not proved efficacy. Only 2 case-control studies done in the past 30 years have established an association between dental procedures and streptococcal infective endocarditis; neither was sufficiently powered to establish the efficacy of antibiotic prophylaxis.
Current U.S. and European guidelines vary in who must be covered: all patients, certain patients, or no patients at all. But a rise in the incidence of infective endocarditis among patients with prosthetic heart valves, implicating invasive dental procedures, “raised the question of whether the indications for antibiotic prophylaxis may be broadened again,” the researchers say. They cite 2016 NICE guidelines that “clearly specify” that it may be appropriate in individual cases.
Given that endocarditis can be fatal or expensive to treat—hospitals stays are long and valve surgery may be needed—the researchers decided to evaluate the association between invasive dental procedures and oral streptococcal infective endocarditis in a population-based cohort and a case crossover study.
In the first study of 138,876 patients with prosthetic heart valves, 69,303 underwent at least 1 dental procedure. Of 396,615 dental procedures, 26% were invasive. Patients received prophylactic antibiotics before half of the procedures.
Over a median 1.7 years of follow-up, 267 people developed infective endocarditis associated with oral streptococci. However, the rate of oral streptococcal infective endocarditis did not rise significantly in the 3 months after an invasive dental procedure, with or without antibiotic prophylaxis.
The case crossover study of patients with endocarditis indicated “the same direction of effect”: Although invasive dental procedures may be associated with oral streptococcal infective endocarditis, the magnitude of the association “remains uncertain.”
Source:
Tubiana S, Blotière PO, Hoen B, et al. BMJ. 2017;358: j3776.
doi: 10.1136/bmj.j3776.
Does antibiotic prophylaxis protect patients with cardiac conditions against endocarditis from invasive dental procedures? Studies have long suggested both “yes” and “no.” Researchers from Université Paris Diderot and others note that clinical trials and cohort studies have not proved efficacy. Only 2 case-control studies done in the past 30 years have established an association between dental procedures and streptococcal infective endocarditis; neither was sufficiently powered to establish the efficacy of antibiotic prophylaxis.
Current U.S. and European guidelines vary in who must be covered: all patients, certain patients, or no patients at all. But a rise in the incidence of infective endocarditis among patients with prosthetic heart valves, implicating invasive dental procedures, “raised the question of whether the indications for antibiotic prophylaxis may be broadened again,” the researchers say. They cite 2016 NICE guidelines that “clearly specify” that it may be appropriate in individual cases.
Given that endocarditis can be fatal or expensive to treat—hospitals stays are long and valve surgery may be needed—the researchers decided to evaluate the association between invasive dental procedures and oral streptococcal infective endocarditis in a population-based cohort and a case crossover study.
In the first study of 138,876 patients with prosthetic heart valves, 69,303 underwent at least 1 dental procedure. Of 396,615 dental procedures, 26% were invasive. Patients received prophylactic antibiotics before half of the procedures.
Over a median 1.7 years of follow-up, 267 people developed infective endocarditis associated with oral streptococci. However, the rate of oral streptococcal infective endocarditis did not rise significantly in the 3 months after an invasive dental procedure, with or without antibiotic prophylaxis.
The case crossover study of patients with endocarditis indicated “the same direction of effect”: Although invasive dental procedures may be associated with oral streptococcal infective endocarditis, the magnitude of the association “remains uncertain.”
Source:
Tubiana S, Blotière PO, Hoen B, et al. BMJ. 2017;358: j3776.
doi: 10.1136/bmj.j3776.
PPIs With Warfarin Regimens: Balancing the Perks and Pitfalls

A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had GERD or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefit of preventing embolization with the risk for serious bleeding. Concurrent use of NSAIDs, aspirin, and other antiplatelet agents further increases the latter risk.2
Clinicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most effective drugs for healing peptic ulcers.3,4 But while previous case-control studies show that PPIs reduce the risk for upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 What’s more, while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk for upper GI bleeding, other guidelines do not address this clinical question.2,7,8
STUDY SUMMARY
Study supports PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding in Medicare and Medicaid patients taking warfarin, with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of severe bleeding or certain illnesses that predispose patients to GI bleeding (eg, esophageal varices). Patients with risk factors for an upper GI bleed (eg, abdominal pain, peptic ulcer disease, anemia) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed more than 75,000 person-years of active warfarin therapy (Medicaid, > 52,000 person-years; Medicare, > 23,000 person-years). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Among all patients taking warfarin (regardless of whether they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR], 0.76), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning that 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk for lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by about half (HR, 0.55). Hospitalizations decreased by 128/10,000 person-years (NNT, 78 person-years). For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly reduce the risk for hospitalization due to upper GI bleeding (HR, 0.86).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk for upper GI bleeding regardless of whether the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to warfarin-only patients
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk for upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Not a randomized controlled trial
This study was observational, not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken OTC medications that influenced or obscured results but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk for upper GI bleeds. We need RCTs to determine whether PPIs cause a risk reduction.
Don’t overlook the risks of PPIs. This study assessed the ability of PPIs to prevent bleeds but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include increased risk for pneumonia, infection with Clostridium difficile, hip and spinal fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and their results are inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another option is to prescribe a direct oral anticoagulant (DOAC; eg, dabigatran, rivaroxaban, or apixaban) instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Compared to warfarin, rivaroxaban has been shown to result in fewer fatal bleeding events due to intracranial bleeds, although it is associated with more GI bleeding.13 Apixaban is associated with fewer GI bleeds and lower bleeding rates overall, compared with warfarin.13 Further research is warranted to determine if PPI therapy is beneficial to patients who are taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When long-term anticoagulation is necessary, providers and patients must attempt to prevent thrombotic events while minimizing the risk for GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[11]:694-696).
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von Holt HK, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.

A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had GERD or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefit of preventing embolization with the risk for serious bleeding. Concurrent use of NSAIDs, aspirin, and other antiplatelet agents further increases the latter risk.2
Clinicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most effective drugs for healing peptic ulcers.3,4 But while previous case-control studies show that PPIs reduce the risk for upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 What’s more, while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk for upper GI bleeding, other guidelines do not address this clinical question.2,7,8
STUDY SUMMARY
Study supports PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding in Medicare and Medicaid patients taking warfarin, with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of severe bleeding or certain illnesses that predispose patients to GI bleeding (eg, esophageal varices). Patients with risk factors for an upper GI bleed (eg, abdominal pain, peptic ulcer disease, anemia) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed more than 75,000 person-years of active warfarin therapy (Medicaid, > 52,000 person-years; Medicare, > 23,000 person-years). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Among all patients taking warfarin (regardless of whether they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR], 0.76), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning that 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk for lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by about half (HR, 0.55). Hospitalizations decreased by 128/10,000 person-years (NNT, 78 person-years). For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly reduce the risk for hospitalization due to upper GI bleeding (HR, 0.86).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk for upper GI bleeding regardless of whether the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to warfarin-only patients
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk for upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Not a randomized controlled trial
This study was observational, not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken OTC medications that influenced or obscured results but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk for upper GI bleeds. We need RCTs to determine whether PPIs cause a risk reduction.
Don’t overlook the risks of PPIs. This study assessed the ability of PPIs to prevent bleeds but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include increased risk for pneumonia, infection with Clostridium difficile, hip and spinal fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and their results are inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another option is to prescribe a direct oral anticoagulant (DOAC; eg, dabigatran, rivaroxaban, or apixaban) instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Compared to warfarin, rivaroxaban has been shown to result in fewer fatal bleeding events due to intracranial bleeds, although it is associated with more GI bleeding.13 Apixaban is associated with fewer GI bleeds and lower bleeding rates overall, compared with warfarin.13 Further research is warranted to determine if PPI therapy is beneficial to patients who are taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When long-term anticoagulation is necessary, providers and patients must attempt to prevent thrombotic events while minimizing the risk for GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[11]:694-696).

A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had GERD or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefit of preventing embolization with the risk for serious bleeding. Concurrent use of NSAIDs, aspirin, and other antiplatelet agents further increases the latter risk.2
Clinicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most effective drugs for healing peptic ulcers.3,4 But while previous case-control studies show that PPIs reduce the risk for upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 What’s more, while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk for upper GI bleeding, other guidelines do not address this clinical question.2,7,8
STUDY SUMMARY
Study supports PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding in Medicare and Medicaid patients taking warfarin, with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of severe bleeding or certain illnesses that predispose patients to GI bleeding (eg, esophageal varices). Patients with risk factors for an upper GI bleed (eg, abdominal pain, peptic ulcer disease, anemia) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed more than 75,000 person-years of active warfarin therapy (Medicaid, > 52,000 person-years; Medicare, > 23,000 person-years). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Among all patients taking warfarin (regardless of whether they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR], 0.76), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning that 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk for lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by about half (HR, 0.55). Hospitalizations decreased by 128/10,000 person-years (NNT, 78 person-years). For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly reduce the risk for hospitalization due to upper GI bleeding (HR, 0.86).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk for upper GI bleeding regardless of whether the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to warfarin-only patients
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk for upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Not a randomized controlled trial
This study was observational, not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken OTC medications that influenced or obscured results but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk for upper GI bleeds. We need RCTs to determine whether PPIs cause a risk reduction.
Don’t overlook the risks of PPIs. This study assessed the ability of PPIs to prevent bleeds but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include increased risk for pneumonia, infection with Clostridium difficile, hip and spinal fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and their results are inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another option is to prescribe a direct oral anticoagulant (DOAC; eg, dabigatran, rivaroxaban, or apixaban) instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Compared to warfarin, rivaroxaban has been shown to result in fewer fatal bleeding events due to intracranial bleeds, although it is associated with more GI bleeding.13 Apixaban is associated with fewer GI bleeds and lower bleeding rates overall, compared with warfarin.13 Further research is warranted to determine if PPI therapy is beneficial to patients who are taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When long-term anticoagulation is necessary, providers and patients must attempt to prevent thrombotic events while minimizing the risk for GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[11]:694-696).
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von Holt HK, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von Holt HK, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
Emicizumab reduces bleeds in kids with hemophilia A and inhibitors
ATLANTA—Updated results from the HAVEN 2 trial have shown that emicizumab prophylaxis can reduce bleeds in children with hemophilia A and factor VIII inhibitors.
Sixty-five percent of all patients enrolled in HAVEN 2 had no bleeds while on emicizumab, and 95% had no treated bleeds.
Among patients who had been on emicizumab for at least 12 weeks, 35% had no bleeds, and 87% had no treated bleeds.
The most common adverse events (AEs) in this trial were viral upper respiratory tract infections and injection site reactions.
Guy Young, MD, of Children’s Hospital Los Angeles in California, presented these results at the 2017 ASH Annual Meeting (abstract 85). The trial was sponsored by Hoffmann-La Roche.
HAVEN 2 enrolled 60 patients, ages 1 to 17, who had hemophilia A and inhibitors. Most patients (95%) had severe hemophilia, 3.3% (n=2) had mild disease, and 1.7% (n=1) had moderate disease.
Nearly a quarter of patients (73.3%) had previously received prophylaxis, and 26.7% had previously received episodic treatment.
The median number of bleeds in the previous 24 weeks was 6.0 (range, 0-155), and 38.3% of patients had target joints.
Patients received emicizumab prophylaxis at 3 mg/kg/week for 4 weeks and 1.5 mg/kg/week thereafter. The median observation time was 9 weeks (range, 1.6 to 41.6 weeks).
Efficacy
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
There were a total of 65 bleeds in 20 patients. Eight were joint bleeds, 2 were muscle bleeds, and the rest were classified as “other.” Of the 55 “other’’ bleeds, 26 (40.0%) were spontaneous, 36 (55.4%) were traumatic, and 3 (4.6%) were due to a procedure/surgery.
A subset of 23 patients received emicizumab for at least 12 weeks. They had a median treatment duration of 38.1 weeks (range, 12.7 to 41.6 weeks).
Of these patients, 34.8% had 0 bleeds, 87.0% had 0 treated bleeds, and 95.7% had 0 treated spontaneous bleeds and 0 treated joint bleeds. There were a total of 41 bleeds in 15 of these patients. Three bleeds (joint, muscle, and hip) were treated.
The median annualized bleeding rate (ABR) for the 23 patients was 1.5 for all bleeds and 0.0 for all types of treated bleeds.
There were 13 patients who had participated in a non-interventional study prior to enrolling in HAVEN 2, so these patients could serve as their own controls. The patients had an overall reduction in ABR of 99% with emicizumab.
Safety
All 60 patients were evaluated for safety. Forty patients had a total of 201 AEs. The most common AEs were viral upper respiratory tract infection (16.7%) and injection site reactions (16.7%)
There were 7 serious AEs in 6 patients—muscle hemorrhage (n=2), eye pain, catheter site injection, device-related infection, mouth hemorrhage, and appendicitis. None of these events were considered treatment-related.
There were no thromboembolic or thrombotic microangiopathy events, and none of the patients tested positive for anti-drug antibodies.
“The safety profile of emicizumab was favorable and well-tolerated,” Dr Young said. “And these updated results from the HAVEN 2 study confirm our prior efficacy results, presented at ISTH, that emicizumab successfully prevents or reduces bleeds.” ![]()
ATLANTA—Updated results from the HAVEN 2 trial have shown that emicizumab prophylaxis can reduce bleeds in children with hemophilia A and factor VIII inhibitors.
Sixty-five percent of all patients enrolled in HAVEN 2 had no bleeds while on emicizumab, and 95% had no treated bleeds.
Among patients who had been on emicizumab for at least 12 weeks, 35% had no bleeds, and 87% had no treated bleeds.
The most common adverse events (AEs) in this trial were viral upper respiratory tract infections and injection site reactions.
Guy Young, MD, of Children’s Hospital Los Angeles in California, presented these results at the 2017 ASH Annual Meeting (abstract 85). The trial was sponsored by Hoffmann-La Roche.
HAVEN 2 enrolled 60 patients, ages 1 to 17, who had hemophilia A and inhibitors. Most patients (95%) had severe hemophilia, 3.3% (n=2) had mild disease, and 1.7% (n=1) had moderate disease.
Nearly a quarter of patients (73.3%) had previously received prophylaxis, and 26.7% had previously received episodic treatment.
The median number of bleeds in the previous 24 weeks was 6.0 (range, 0-155), and 38.3% of patients had target joints.
Patients received emicizumab prophylaxis at 3 mg/kg/week for 4 weeks and 1.5 mg/kg/week thereafter. The median observation time was 9 weeks (range, 1.6 to 41.6 weeks).
Efficacy
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
There were a total of 65 bleeds in 20 patients. Eight were joint bleeds, 2 were muscle bleeds, and the rest were classified as “other.” Of the 55 “other’’ bleeds, 26 (40.0%) were spontaneous, 36 (55.4%) were traumatic, and 3 (4.6%) were due to a procedure/surgery.
A subset of 23 patients received emicizumab for at least 12 weeks. They had a median treatment duration of 38.1 weeks (range, 12.7 to 41.6 weeks).
Of these patients, 34.8% had 0 bleeds, 87.0% had 0 treated bleeds, and 95.7% had 0 treated spontaneous bleeds and 0 treated joint bleeds. There were a total of 41 bleeds in 15 of these patients. Three bleeds (joint, muscle, and hip) were treated.
The median annualized bleeding rate (ABR) for the 23 patients was 1.5 for all bleeds and 0.0 for all types of treated bleeds.
There were 13 patients who had participated in a non-interventional study prior to enrolling in HAVEN 2, so these patients could serve as their own controls. The patients had an overall reduction in ABR of 99% with emicizumab.
Safety
All 60 patients were evaluated for safety. Forty patients had a total of 201 AEs. The most common AEs were viral upper respiratory tract infection (16.7%) and injection site reactions (16.7%)
There were 7 serious AEs in 6 patients—muscle hemorrhage (n=2), eye pain, catheter site injection, device-related infection, mouth hemorrhage, and appendicitis. None of these events were considered treatment-related.
There were no thromboembolic or thrombotic microangiopathy events, and none of the patients tested positive for anti-drug antibodies.
“The safety profile of emicizumab was favorable and well-tolerated,” Dr Young said. “And these updated results from the HAVEN 2 study confirm our prior efficacy results, presented at ISTH, that emicizumab successfully prevents or reduces bleeds.” ![]()
ATLANTA—Updated results from the HAVEN 2 trial have shown that emicizumab prophylaxis can reduce bleeds in children with hemophilia A and factor VIII inhibitors.
Sixty-five percent of all patients enrolled in HAVEN 2 had no bleeds while on emicizumab, and 95% had no treated bleeds.
Among patients who had been on emicizumab for at least 12 weeks, 35% had no bleeds, and 87% had no treated bleeds.
The most common adverse events (AEs) in this trial were viral upper respiratory tract infections and injection site reactions.
Guy Young, MD, of Children’s Hospital Los Angeles in California, presented these results at the 2017 ASH Annual Meeting (abstract 85). The trial was sponsored by Hoffmann-La Roche.
HAVEN 2 enrolled 60 patients, ages 1 to 17, who had hemophilia A and inhibitors. Most patients (95%) had severe hemophilia, 3.3% (n=2) had mild disease, and 1.7% (n=1) had moderate disease.
Nearly a quarter of patients (73.3%) had previously received prophylaxis, and 26.7% had previously received episodic treatment.
The median number of bleeds in the previous 24 weeks was 6.0 (range, 0-155), and 38.3% of patients had target joints.
Patients received emicizumab prophylaxis at 3 mg/kg/week for 4 weeks and 1.5 mg/kg/week thereafter. The median observation time was 9 weeks (range, 1.6 to 41.6 weeks).
Efficacy
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
There were a total of 65 bleeds in 20 patients. Eight were joint bleeds, 2 were muscle bleeds, and the rest were classified as “other.” Of the 55 “other’’ bleeds, 26 (40.0%) were spontaneous, 36 (55.4%) were traumatic, and 3 (4.6%) were due to a procedure/surgery.
A subset of 23 patients received emicizumab for at least 12 weeks. They had a median treatment duration of 38.1 weeks (range, 12.7 to 41.6 weeks).
Of these patients, 34.8% had 0 bleeds, 87.0% had 0 treated bleeds, and 95.7% had 0 treated spontaneous bleeds and 0 treated joint bleeds. There were a total of 41 bleeds in 15 of these patients. Three bleeds (joint, muscle, and hip) were treated.
The median annualized bleeding rate (ABR) for the 23 patients was 1.5 for all bleeds and 0.0 for all types of treated bleeds.
There were 13 patients who had participated in a non-interventional study prior to enrolling in HAVEN 2, so these patients could serve as their own controls. The patients had an overall reduction in ABR of 99% with emicizumab.
Safety
All 60 patients were evaluated for safety. Forty patients had a total of 201 AEs. The most common AEs were viral upper respiratory tract infection (16.7%) and injection site reactions (16.7%)
There were 7 serious AEs in 6 patients—muscle hemorrhage (n=2), eye pain, catheter site injection, device-related infection, mouth hemorrhage, and appendicitis. None of these events were considered treatment-related.
There were no thromboembolic or thrombotic microangiopathy events, and none of the patients tested positive for anti-drug antibodies.
“The safety profile of emicizumab was favorable and well-tolerated,” Dr Young said. “And these updated results from the HAVEN 2 study confirm our prior efficacy results, presented at ISTH, that emicizumab successfully prevents or reduces bleeds.” ![]()