User login
Studies looking at pravastatin for preeclampsia prevention
In the United States, preeclampsia affects 3%-5% of all pregnancies and 10%-20% of pregnancies complicated by diabetes. Up to 20% of maternal deaths in the United States – and a much larger percentage of maternal deaths worldwide – occur in women with the condition, as do numerous maternal and fetal comorbidities. These include severe hypertension, pulmonary edema, stroke, and kidney and liver injury in the mother, and stillbirth, placental abruption, growth restriction, and premature delivery of the fetus.
Longer-term complications for the offspring include chronic lung disease, hearing and vision disorders, cerebral palsy and other neurodevelopmental disorders, and – as shown by more recent research – poor cardiovascular and metabolic outcomes.
Preeclampsia predisposes the mother to at least a twofold increased risk of future heart disease, compared with a woman who does not have the condition. In addition, women with preeclampsia who deliver at term are approximately two times more likely to die prematurely from heart disease than women without a history of preeclampsia, and those who deliver before 34 weeks’ gestation have been shown to have a ninefold greater risk of premature death. The American Heart Association, in fact, now includes preeclampsia in its list of heart disease risk factors.
Much attention now is focused on statins (inhibitors of HMG-CoA reductase), which have been used for more than 30 years for the primary and secondary prevention of heart disease. The properties and mechanisms of this class of drugs – and the similarities in the pathophysiology of cardiovascular disease and preeclampsia – make statins a plausible candidate for preeclampsia prevention. Thus far, data from preclinical work and subsequent pilot studies have been encouraging.
The commonalities
Preeclampsia is unique to pregnancy, but its pathophysiology and risk factors largely overlap with those of adult atherosclerotic cardiovascular disease. The exact pathophysiology of preeclampsia is unknown, but it is generally agreed that angiogenic imbalance and endothelial dysfunction play key roles, as do associated inflammation and oxidative stress.
Women with preeclampsia have been shown, for instance, to have had increased levels of antiangiogenic factors (soluble FMS-like tyrosine kinase 1 and soluble endoglin) and decreased levels of angiogenic factors (vascular endothelial growth factor and placental growth factor) prior to developing the condition clinically. Risk factors common to both preeclampsia and heart disease include chronic hypertension, dyslipidemia, diabetes or insulin resistance, obesity, and a family history of the condition.
Statins, meanwhile, have been shown to prevent or reverse angiogenic imbalance by promoting the release of vascular endothelial growth factor and placental growth factor and by suppressing the production of soluble FMS-like tyrosine kinase 1 and soluble endoglin. The drugs also improve vascular relaxation and exhibit anti-inflammatory and antioxidative effects, thereby broadly improving endovascular health. In the cardiovascular arena, notably, men and women who have elevated inflammatory markers even without hypercholesterolemia have been shown to have improved cardiovascular outcomes with statin treatment.
In various mouse models of preeclampsia studied in the past decade, pravastatin, a hydrophilic statin, has had beneficial effects. Mice with the angiogenic imbalance characteristic of preeclampsia that received this statin have shown a reversal of the imbalance, as well as reduced blood pressure, increased levels of nitric oxide synthase production, decreased oxidative stress, improved vascular reactivity, decreased kidney damage and proteinuria, and other positive effects. These effects occurred without detrimental outcomes to the mice or any increase in the rates of anomalies or resorption in offspring (Clin Obstet Gynecol. 2017 Mar;60:161-8).
Moreover, in addition to ameliorating the preeclampsia phenotype, pravastatin use in these animal models has improved pregnancy outcomes and reduced rates of pregnancy losses.
Safety issues
So, can we use statins in pregnancy? When statins were originally marketed in the 1980s, they were labeled pregnancy category X, which means 1) that there is evidence of fetal abnormalities or risk and 2) that these risks clearly outweigh potential benefits.
This designation for statins was based largely on the second half of the definition (no benefit to outweigh any risk). In addition, there were theoretical concerns about the inhibition of cholesterol synthesis during embryologic development and about a small case series of the original lipophilic statins suggesting an increased risk of malformations. While pregnancy category X does not exist anymore, statins are still labeled as contraindicated in pregnancy.
Pravastatin is one of the safest statins to consider in pregnancy for several reasons: It is one of the most hydrophilic statins and is a substrate of the placental efflux transporters, such as P-glycoprotein; both of those properties limit its ability to cross the placenta. It also has a short-elimination half-life, is cleared through both hepatic and renal routes, and is among the most hepatoselective statins available (one of the weakest inhibitors of HMG-CoA reductase). Indeed, in vitro placental transfer studies suggest that pravastatin transfer is limited and slow and that clearance is significantly higher in the fetal-to-maternal direction than in the maternal-to-fetal direction.
Animal studies have demonstrated that pravastatin is not teratogenic and has no effect on placental weight, pup birth weight, and pup adult weight. Moreover, at least six published cohort studies of women with first-trimester exposure to statins (women who had been prescribed the drugs before becoming pregnant and who received the drugs in the first trimester before realizing they were pregnant) showed no patterns or increased rates of congenital anomalies, compared with women without exposure to known teratogens. Additionally, these cohorts did not show any associations with miscarriage or fetal growth restriction (Obstet Gynecol. 2013 Feb;121:349-53).
A more recent cohort study of close to 900,000 women – of which 1,152 women used a statin (pravastatin or other statins) during their first trimester – similarly found no significant increases in any type of congenital malformation, compared with other completed pregnancies in the larger cohort. Notably, the analysis of this cohort was done using propensity score–based methods to control for potential confounders, including prepregnancy conditions that prompted use of a statin (BMJ. 2015;350:h1035).
A drawback to this body of research is that, with the exception of the BMJ study, the cohorts have been generally small; furthermore, in keeping with current recommendations, most of the statin-exposed patients discontinued use of the drugs upon confirmation of their pregnancies, thereby leaving the effects of long-term use unknown.
A promising pilot
Daily pravastatin use in pregnancy, starting in the second trimester, got its first major test of safety and pharmacokinetics in a pilot randomized, controlled trial undertaken by the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD’s) Obstetric-Fetal Pharmacology Research Units Network. Women with singleton pregnancies and a history of severe preeclampsia requiring delivery prior to 34 weeks’ gestation were randomized between 12 and 16 weeks’ gestation to receive pravastatin or placebo until delivery.
This pilot is the first of three cohorts of women who were or will be randomized in separate pilot trials to escalating doses of pravastatin: 10 mg, 20 mg, and 40 mg (the last of which is the usual dose for lipid lowering in adults). Results of the first cohort, in which 20 patients were randomized to 10 mg pravastatin or placebo, were reported in 2016, and those from the second cohort will be reported soon. The third pilot is currently enrolling women.
In this first pilot we found no differences in rates of congenital anomalies or other identifiable maternal or fetal/neonatal safety risks, no differences in adverse events, and no maternal, fetal, or neonatal deaths. There were also no reports of myopathy/rhabdomyolysis or liver injury; the most common adverse events were heartburn (reported by four patients in the pravastatin group and three in the placebo group) and musculoskeletal pain (reported by four patients and one patient, respectively).
Although not statistically significant, a 10-mg dose of pravastatin was associated with favorable outcomes. None of the women receiving pravastatin developed preeclampsia, while four in the placebo group developed the disorder (with three of these four having severe preeclampsia).
Women in the pravastatin group also were less likely to have an indicated preterm delivery (one vs. five in the placebo group), and their neonates were less likely to be admitted to intermediate nurseries or the neonatal ICU. In addition, their angiogenic profiles were improved (higher placental growth factor and lower FMS-like tyrosine kinase 1 and soluble endoglin).
Importantly, while pravastatin reduced maternal cholesterol concentrations, there were no differences in birth weight or umbilical cord cholesterol concentrations (total cholesterol or LDL) between the two groups (Am J Obstet Gynecol. 2016;214[6]:720.e1-17).
That cholesterol concentrations were not reduced in fetuses exposed to pravastatin is reassuring and aligns with findings from other studies showing that fetal cholesterol concentrations are largely independent from maternal cholesterol concentrations or diet. For instance, we know from studies of Smith-Lemli-Opitz syndrome, a multiple congenital anomaly/intellectual disability syndrome caused by a defect in cholesterol synthesis, that there is not any significant interaction between cholesterol concentrations of the mother and fetus. Only about 10% of the fetal absolute cholesterol requirement comes from the mother, research has demonstrated.
The future
Infant follow-up in the NICHD study is planned, and a large, randomized clinical trial powered to look at the efficacy of pravastatin for preventing preeclampsia in high-risk women has been approved by the NICHD. Once funded, the study is expected to enroll approximately 1,700 pregnant women who have a history of severe preeclampsia and delivery before 36 weeks’ gestation and who are between 10 and 16 weeks’ gestation.
With continued research, we face the question of whether pravastatin may potentiate the benefit of aspirin in pregnant women. In cardiovascular medicine, there is evidence for additive or synergistic effects of the combined use of aspirin and statins.
Interestingly, a recent prospective cohort study of women with antiphospholipid syndrome and poor outcomes in prior pregnancies showed dramatic improvement in both maternal and fetal/neonatal outcomes when pravastatin was administered after the onset of preeclampsia or intrauterine growth restriction. All 21 women in the cohort were treated with low-dose aspirin and low-molecular-weight heparin; after the development of preeclampsia or intrauterine growth restriction, 10 patients were maintained on aspirin and LMWH, and 11 were started on 20 mg daily pravastatin along with the aspirin and LMWH (J Clin Invest. 2016 Aug;126[8]:2933-40; and J Clin Invest. 2016 Aug;126[8]:2792-4).
Those who received the statin had improved uterine artery Doppler velocimetry, lower systemic blood pressure, and delivered infants with higher birth weights and at a more advanced gestational age (median, 36 weeks’ vs. 26.5 weeks’ gestation). The study did not randomly allocate the women and did not include a placebo arm. Still, it is another impressive proof-of-concept study.
Dr. Costantine is an associate professor of obstetrics and gynecology at the University of Texas Medical Branch in Galveston. He reported that he has no financial disclosures.
In the United States, preeclampsia affects 3%-5% of all pregnancies and 10%-20% of pregnancies complicated by diabetes. Up to 20% of maternal deaths in the United States – and a much larger percentage of maternal deaths worldwide – occur in women with the condition, as do numerous maternal and fetal comorbidities. These include severe hypertension, pulmonary edema, stroke, and kidney and liver injury in the mother, and stillbirth, placental abruption, growth restriction, and premature delivery of the fetus.
Longer-term complications for the offspring include chronic lung disease, hearing and vision disorders, cerebral palsy and other neurodevelopmental disorders, and – as shown by more recent research – poor cardiovascular and metabolic outcomes.
Preeclampsia predisposes the mother to at least a twofold increased risk of future heart disease, compared with a woman who does not have the condition. In addition, women with preeclampsia who deliver at term are approximately two times more likely to die prematurely from heart disease than women without a history of preeclampsia, and those who deliver before 34 weeks’ gestation have been shown to have a ninefold greater risk of premature death. The American Heart Association, in fact, now includes preeclampsia in its list of heart disease risk factors.
Much attention now is focused on statins (inhibitors of HMG-CoA reductase), which have been used for more than 30 years for the primary and secondary prevention of heart disease. The properties and mechanisms of this class of drugs – and the similarities in the pathophysiology of cardiovascular disease and preeclampsia – make statins a plausible candidate for preeclampsia prevention. Thus far, data from preclinical work and subsequent pilot studies have been encouraging.
The commonalities
Preeclampsia is unique to pregnancy, but its pathophysiology and risk factors largely overlap with those of adult atherosclerotic cardiovascular disease. The exact pathophysiology of preeclampsia is unknown, but it is generally agreed that angiogenic imbalance and endothelial dysfunction play key roles, as do associated inflammation and oxidative stress.
Women with preeclampsia have been shown, for instance, to have had increased levels of antiangiogenic factors (soluble FMS-like tyrosine kinase 1 and soluble endoglin) and decreased levels of angiogenic factors (vascular endothelial growth factor and placental growth factor) prior to developing the condition clinically. Risk factors common to both preeclampsia and heart disease include chronic hypertension, dyslipidemia, diabetes or insulin resistance, obesity, and a family history of the condition.
Statins, meanwhile, have been shown to prevent or reverse angiogenic imbalance by promoting the release of vascular endothelial growth factor and placental growth factor and by suppressing the production of soluble FMS-like tyrosine kinase 1 and soluble endoglin. The drugs also improve vascular relaxation and exhibit anti-inflammatory and antioxidative effects, thereby broadly improving endovascular health. In the cardiovascular arena, notably, men and women who have elevated inflammatory markers even without hypercholesterolemia have been shown to have improved cardiovascular outcomes with statin treatment.
In various mouse models of preeclampsia studied in the past decade, pravastatin, a hydrophilic statin, has had beneficial effects. Mice with the angiogenic imbalance characteristic of preeclampsia that received this statin have shown a reversal of the imbalance, as well as reduced blood pressure, increased levels of nitric oxide synthase production, decreased oxidative stress, improved vascular reactivity, decreased kidney damage and proteinuria, and other positive effects. These effects occurred without detrimental outcomes to the mice or any increase in the rates of anomalies or resorption in offspring (Clin Obstet Gynecol. 2017 Mar;60:161-8).
Moreover, in addition to ameliorating the preeclampsia phenotype, pravastatin use in these animal models has improved pregnancy outcomes and reduced rates of pregnancy losses.
Safety issues
So, can we use statins in pregnancy? When statins were originally marketed in the 1980s, they were labeled pregnancy category X, which means 1) that there is evidence of fetal abnormalities or risk and 2) that these risks clearly outweigh potential benefits.
This designation for statins was based largely on the second half of the definition (no benefit to outweigh any risk). In addition, there were theoretical concerns about the inhibition of cholesterol synthesis during embryologic development and about a small case series of the original lipophilic statins suggesting an increased risk of malformations. While pregnancy category X does not exist anymore, statins are still labeled as contraindicated in pregnancy.
Pravastatin is one of the safest statins to consider in pregnancy for several reasons: It is one of the most hydrophilic statins and is a substrate of the placental efflux transporters, such as P-glycoprotein; both of those properties limit its ability to cross the placenta. It also has a short-elimination half-life, is cleared through both hepatic and renal routes, and is among the most hepatoselective statins available (one of the weakest inhibitors of HMG-CoA reductase). Indeed, in vitro placental transfer studies suggest that pravastatin transfer is limited and slow and that clearance is significantly higher in the fetal-to-maternal direction than in the maternal-to-fetal direction.
Animal studies have demonstrated that pravastatin is not teratogenic and has no effect on placental weight, pup birth weight, and pup adult weight. Moreover, at least six published cohort studies of women with first-trimester exposure to statins (women who had been prescribed the drugs before becoming pregnant and who received the drugs in the first trimester before realizing they were pregnant) showed no patterns or increased rates of congenital anomalies, compared with women without exposure to known teratogens. Additionally, these cohorts did not show any associations with miscarriage or fetal growth restriction (Obstet Gynecol. 2013 Feb;121:349-53).
A more recent cohort study of close to 900,000 women – of which 1,152 women used a statin (pravastatin or other statins) during their first trimester – similarly found no significant increases in any type of congenital malformation, compared with other completed pregnancies in the larger cohort. Notably, the analysis of this cohort was done using propensity score–based methods to control for potential confounders, including prepregnancy conditions that prompted use of a statin (BMJ. 2015;350:h1035).
A drawback to this body of research is that, with the exception of the BMJ study, the cohorts have been generally small; furthermore, in keeping with current recommendations, most of the statin-exposed patients discontinued use of the drugs upon confirmation of their pregnancies, thereby leaving the effects of long-term use unknown.
A promising pilot
Daily pravastatin use in pregnancy, starting in the second trimester, got its first major test of safety and pharmacokinetics in a pilot randomized, controlled trial undertaken by the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD’s) Obstetric-Fetal Pharmacology Research Units Network. Women with singleton pregnancies and a history of severe preeclampsia requiring delivery prior to 34 weeks’ gestation were randomized between 12 and 16 weeks’ gestation to receive pravastatin or placebo until delivery.
This pilot is the first of three cohorts of women who were or will be randomized in separate pilot trials to escalating doses of pravastatin: 10 mg, 20 mg, and 40 mg (the last of which is the usual dose for lipid lowering in adults). Results of the first cohort, in which 20 patients were randomized to 10 mg pravastatin or placebo, were reported in 2016, and those from the second cohort will be reported soon. The third pilot is currently enrolling women.
In this first pilot we found no differences in rates of congenital anomalies or other identifiable maternal or fetal/neonatal safety risks, no differences in adverse events, and no maternal, fetal, or neonatal deaths. There were also no reports of myopathy/rhabdomyolysis or liver injury; the most common adverse events were heartburn (reported by four patients in the pravastatin group and three in the placebo group) and musculoskeletal pain (reported by four patients and one patient, respectively).
Although not statistically significant, a 10-mg dose of pravastatin was associated with favorable outcomes. None of the women receiving pravastatin developed preeclampsia, while four in the placebo group developed the disorder (with three of these four having severe preeclampsia).
Women in the pravastatin group also were less likely to have an indicated preterm delivery (one vs. five in the placebo group), and their neonates were less likely to be admitted to intermediate nurseries or the neonatal ICU. In addition, their angiogenic profiles were improved (higher placental growth factor and lower FMS-like tyrosine kinase 1 and soluble endoglin).
Importantly, while pravastatin reduced maternal cholesterol concentrations, there were no differences in birth weight or umbilical cord cholesterol concentrations (total cholesterol or LDL) between the two groups (Am J Obstet Gynecol. 2016;214[6]:720.e1-17).
That cholesterol concentrations were not reduced in fetuses exposed to pravastatin is reassuring and aligns with findings from other studies showing that fetal cholesterol concentrations are largely independent from maternal cholesterol concentrations or diet. For instance, we know from studies of Smith-Lemli-Opitz syndrome, a multiple congenital anomaly/intellectual disability syndrome caused by a defect in cholesterol synthesis, that there is not any significant interaction between cholesterol concentrations of the mother and fetus. Only about 10% of the fetal absolute cholesterol requirement comes from the mother, research has demonstrated.
The future
Infant follow-up in the NICHD study is planned, and a large, randomized clinical trial powered to look at the efficacy of pravastatin for preventing preeclampsia in high-risk women has been approved by the NICHD. Once funded, the study is expected to enroll approximately 1,700 pregnant women who have a history of severe preeclampsia and delivery before 36 weeks’ gestation and who are between 10 and 16 weeks’ gestation.
With continued research, we face the question of whether pravastatin may potentiate the benefit of aspirin in pregnant women. In cardiovascular medicine, there is evidence for additive or synergistic effects of the combined use of aspirin and statins.
Interestingly, a recent prospective cohort study of women with antiphospholipid syndrome and poor outcomes in prior pregnancies showed dramatic improvement in both maternal and fetal/neonatal outcomes when pravastatin was administered after the onset of preeclampsia or intrauterine growth restriction. All 21 women in the cohort were treated with low-dose aspirin and low-molecular-weight heparin; after the development of preeclampsia or intrauterine growth restriction, 10 patients were maintained on aspirin and LMWH, and 11 were started on 20 mg daily pravastatin along with the aspirin and LMWH (J Clin Invest. 2016 Aug;126[8]:2933-40; and J Clin Invest. 2016 Aug;126[8]:2792-4).
Those who received the statin had improved uterine artery Doppler velocimetry, lower systemic blood pressure, and delivered infants with higher birth weights and at a more advanced gestational age (median, 36 weeks’ vs. 26.5 weeks’ gestation). The study did not randomly allocate the women and did not include a placebo arm. Still, it is another impressive proof-of-concept study.
Dr. Costantine is an associate professor of obstetrics and gynecology at the University of Texas Medical Branch in Galveston. He reported that he has no financial disclosures.
In the United States, preeclampsia affects 3%-5% of all pregnancies and 10%-20% of pregnancies complicated by diabetes. Up to 20% of maternal deaths in the United States – and a much larger percentage of maternal deaths worldwide – occur in women with the condition, as do numerous maternal and fetal comorbidities. These include severe hypertension, pulmonary edema, stroke, and kidney and liver injury in the mother, and stillbirth, placental abruption, growth restriction, and premature delivery of the fetus.
Longer-term complications for the offspring include chronic lung disease, hearing and vision disorders, cerebral palsy and other neurodevelopmental disorders, and – as shown by more recent research – poor cardiovascular and metabolic outcomes.
Preeclampsia predisposes the mother to at least a twofold increased risk of future heart disease, compared with a woman who does not have the condition. In addition, women with preeclampsia who deliver at term are approximately two times more likely to die prematurely from heart disease than women without a history of preeclampsia, and those who deliver before 34 weeks’ gestation have been shown to have a ninefold greater risk of premature death. The American Heart Association, in fact, now includes preeclampsia in its list of heart disease risk factors.
Much attention now is focused on statins (inhibitors of HMG-CoA reductase), which have been used for more than 30 years for the primary and secondary prevention of heart disease. The properties and mechanisms of this class of drugs – and the similarities in the pathophysiology of cardiovascular disease and preeclampsia – make statins a plausible candidate for preeclampsia prevention. Thus far, data from preclinical work and subsequent pilot studies have been encouraging.
The commonalities
Preeclampsia is unique to pregnancy, but its pathophysiology and risk factors largely overlap with those of adult atherosclerotic cardiovascular disease. The exact pathophysiology of preeclampsia is unknown, but it is generally agreed that angiogenic imbalance and endothelial dysfunction play key roles, as do associated inflammation and oxidative stress.
Women with preeclampsia have been shown, for instance, to have had increased levels of antiangiogenic factors (soluble FMS-like tyrosine kinase 1 and soluble endoglin) and decreased levels of angiogenic factors (vascular endothelial growth factor and placental growth factor) prior to developing the condition clinically. Risk factors common to both preeclampsia and heart disease include chronic hypertension, dyslipidemia, diabetes or insulin resistance, obesity, and a family history of the condition.
Statins, meanwhile, have been shown to prevent or reverse angiogenic imbalance by promoting the release of vascular endothelial growth factor and placental growth factor and by suppressing the production of soluble FMS-like tyrosine kinase 1 and soluble endoglin. The drugs also improve vascular relaxation and exhibit anti-inflammatory and antioxidative effects, thereby broadly improving endovascular health. In the cardiovascular arena, notably, men and women who have elevated inflammatory markers even without hypercholesterolemia have been shown to have improved cardiovascular outcomes with statin treatment.
In various mouse models of preeclampsia studied in the past decade, pravastatin, a hydrophilic statin, has had beneficial effects. Mice with the angiogenic imbalance characteristic of preeclampsia that received this statin have shown a reversal of the imbalance, as well as reduced blood pressure, increased levels of nitric oxide synthase production, decreased oxidative stress, improved vascular reactivity, decreased kidney damage and proteinuria, and other positive effects. These effects occurred without detrimental outcomes to the mice or any increase in the rates of anomalies or resorption in offspring (Clin Obstet Gynecol. 2017 Mar;60:161-8).
Moreover, in addition to ameliorating the preeclampsia phenotype, pravastatin use in these animal models has improved pregnancy outcomes and reduced rates of pregnancy losses.
Safety issues
So, can we use statins in pregnancy? When statins were originally marketed in the 1980s, they were labeled pregnancy category X, which means 1) that there is evidence of fetal abnormalities or risk and 2) that these risks clearly outweigh potential benefits.
This designation for statins was based largely on the second half of the definition (no benefit to outweigh any risk). In addition, there were theoretical concerns about the inhibition of cholesterol synthesis during embryologic development and about a small case series of the original lipophilic statins suggesting an increased risk of malformations. While pregnancy category X does not exist anymore, statins are still labeled as contraindicated in pregnancy.
Pravastatin is one of the safest statins to consider in pregnancy for several reasons: It is one of the most hydrophilic statins and is a substrate of the placental efflux transporters, such as P-glycoprotein; both of those properties limit its ability to cross the placenta. It also has a short-elimination half-life, is cleared through both hepatic and renal routes, and is among the most hepatoselective statins available (one of the weakest inhibitors of HMG-CoA reductase). Indeed, in vitro placental transfer studies suggest that pravastatin transfer is limited and slow and that clearance is significantly higher in the fetal-to-maternal direction than in the maternal-to-fetal direction.
Animal studies have demonstrated that pravastatin is not teratogenic and has no effect on placental weight, pup birth weight, and pup adult weight. Moreover, at least six published cohort studies of women with first-trimester exposure to statins (women who had been prescribed the drugs before becoming pregnant and who received the drugs in the first trimester before realizing they were pregnant) showed no patterns or increased rates of congenital anomalies, compared with women without exposure to known teratogens. Additionally, these cohorts did not show any associations with miscarriage or fetal growth restriction (Obstet Gynecol. 2013 Feb;121:349-53).
A more recent cohort study of close to 900,000 women – of which 1,152 women used a statin (pravastatin or other statins) during their first trimester – similarly found no significant increases in any type of congenital malformation, compared with other completed pregnancies in the larger cohort. Notably, the analysis of this cohort was done using propensity score–based methods to control for potential confounders, including prepregnancy conditions that prompted use of a statin (BMJ. 2015;350:h1035).
A drawback to this body of research is that, with the exception of the BMJ study, the cohorts have been generally small; furthermore, in keeping with current recommendations, most of the statin-exposed patients discontinued use of the drugs upon confirmation of their pregnancies, thereby leaving the effects of long-term use unknown.
A promising pilot
Daily pravastatin use in pregnancy, starting in the second trimester, got its first major test of safety and pharmacokinetics in a pilot randomized, controlled trial undertaken by the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD’s) Obstetric-Fetal Pharmacology Research Units Network. Women with singleton pregnancies and a history of severe preeclampsia requiring delivery prior to 34 weeks’ gestation were randomized between 12 and 16 weeks’ gestation to receive pravastatin or placebo until delivery.
This pilot is the first of three cohorts of women who were or will be randomized in separate pilot trials to escalating doses of pravastatin: 10 mg, 20 mg, and 40 mg (the last of which is the usual dose for lipid lowering in adults). Results of the first cohort, in which 20 patients were randomized to 10 mg pravastatin or placebo, were reported in 2016, and those from the second cohort will be reported soon. The third pilot is currently enrolling women.
In this first pilot we found no differences in rates of congenital anomalies or other identifiable maternal or fetal/neonatal safety risks, no differences in adverse events, and no maternal, fetal, or neonatal deaths. There were also no reports of myopathy/rhabdomyolysis or liver injury; the most common adverse events were heartburn (reported by four patients in the pravastatin group and three in the placebo group) and musculoskeletal pain (reported by four patients and one patient, respectively).
Although not statistically significant, a 10-mg dose of pravastatin was associated with favorable outcomes. None of the women receiving pravastatin developed preeclampsia, while four in the placebo group developed the disorder (with three of these four having severe preeclampsia).
Women in the pravastatin group also were less likely to have an indicated preterm delivery (one vs. five in the placebo group), and their neonates were less likely to be admitted to intermediate nurseries or the neonatal ICU. In addition, their angiogenic profiles were improved (higher placental growth factor and lower FMS-like tyrosine kinase 1 and soluble endoglin).
Importantly, while pravastatin reduced maternal cholesterol concentrations, there were no differences in birth weight or umbilical cord cholesterol concentrations (total cholesterol or LDL) between the two groups (Am J Obstet Gynecol. 2016;214[6]:720.e1-17).
That cholesterol concentrations were not reduced in fetuses exposed to pravastatin is reassuring and aligns with findings from other studies showing that fetal cholesterol concentrations are largely independent from maternal cholesterol concentrations or diet. For instance, we know from studies of Smith-Lemli-Opitz syndrome, a multiple congenital anomaly/intellectual disability syndrome caused by a defect in cholesterol synthesis, that there is not any significant interaction between cholesterol concentrations of the mother and fetus. Only about 10% of the fetal absolute cholesterol requirement comes from the mother, research has demonstrated.
The future
Infant follow-up in the NICHD study is planned, and a large, randomized clinical trial powered to look at the efficacy of pravastatin for preventing preeclampsia in high-risk women has been approved by the NICHD. Once funded, the study is expected to enroll approximately 1,700 pregnant women who have a history of severe preeclampsia and delivery before 36 weeks’ gestation and who are between 10 and 16 weeks’ gestation.
With continued research, we face the question of whether pravastatin may potentiate the benefit of aspirin in pregnant women. In cardiovascular medicine, there is evidence for additive or synergistic effects of the combined use of aspirin and statins.
Interestingly, a recent prospective cohort study of women with antiphospholipid syndrome and poor outcomes in prior pregnancies showed dramatic improvement in both maternal and fetal/neonatal outcomes when pravastatin was administered after the onset of preeclampsia or intrauterine growth restriction. All 21 women in the cohort were treated with low-dose aspirin and low-molecular-weight heparin; after the development of preeclampsia or intrauterine growth restriction, 10 patients were maintained on aspirin and LMWH, and 11 were started on 20 mg daily pravastatin along with the aspirin and LMWH (J Clin Invest. 2016 Aug;126[8]:2933-40; and J Clin Invest. 2016 Aug;126[8]:2792-4).
Those who received the statin had improved uterine artery Doppler velocimetry, lower systemic blood pressure, and delivered infants with higher birth weights and at a more advanced gestational age (median, 36 weeks’ vs. 26.5 weeks’ gestation). The study did not randomly allocate the women and did not include a placebo arm. Still, it is another impressive proof-of-concept study.
Dr. Costantine is an associate professor of obstetrics and gynecology at the University of Texas Medical Branch in Galveston. He reported that he has no financial disclosures.
Hope for targeted management of preeclampsia
In medicine, there are many diseases and conditions that pose significant challenges to health care practitioners. For example, within brain science, there are patients with debilitating neurodegenerative diseases; within emergency medicine, there are patients who have suffered severe and acute trauma; within pediatrics, there are patients with terminal illnesses, such as cancer. In ob.gyn., one of the great obstetrical syndromes is preeclampsia.
Humans have known about preeclampsia for thousands of years, dating back to the 4th and 5th centuries B.C., since the time of Hippocrates. Ancient writings on medical conditions of women reflect a recognition of preeclampsia and eclampsia, although formal classification of the condition as a hypertensive disorder associated specifically with pregnancy did not occur until the late 1800s. Despite this, the pathology of preeclampsia is significantly underdefined, and because the underlying causes of preeclampsia are largely unknown, prevention and management continue to be hindered.
However, recent research indicating an association between statin use and prevention of preeclampsia has given us some hope that targeted management is possible. As ob.gyns. continue to grapple with the intricacies of managing patients with preeclampsia, a condition we increasingly see because of concurrent rises in being overweight, being obese, and having diabetes during pregnancy, so we are eager to see whether the research might confirm an effective role for statins in patient care. Although statins are still quite far from becoming a new standard of care, we are, as a specialty, hungry for a solution to this millennia-old problem.
Therefore, this month we have invited Maged Costantine, MD, an associate professor in the department of obstetrics and gynecology and division of maternal fetal medicine at the University of Texas Medical Branch in Galveston, to discuss this novel and exciting new area of research.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
In medicine, there are many diseases and conditions that pose significant challenges to health care practitioners. For example, within brain science, there are patients with debilitating neurodegenerative diseases; within emergency medicine, there are patients who have suffered severe and acute trauma; within pediatrics, there are patients with terminal illnesses, such as cancer. In ob.gyn., one of the great obstetrical syndromes is preeclampsia.
Humans have known about preeclampsia for thousands of years, dating back to the 4th and 5th centuries B.C., since the time of Hippocrates. Ancient writings on medical conditions of women reflect a recognition of preeclampsia and eclampsia, although formal classification of the condition as a hypertensive disorder associated specifically with pregnancy did not occur until the late 1800s. Despite this, the pathology of preeclampsia is significantly underdefined, and because the underlying causes of preeclampsia are largely unknown, prevention and management continue to be hindered.
However, recent research indicating an association between statin use and prevention of preeclampsia has given us some hope that targeted management is possible. As ob.gyns. continue to grapple with the intricacies of managing patients with preeclampsia, a condition we increasingly see because of concurrent rises in being overweight, being obese, and having diabetes during pregnancy, so we are eager to see whether the research might confirm an effective role for statins in patient care. Although statins are still quite far from becoming a new standard of care, we are, as a specialty, hungry for a solution to this millennia-old problem.
Therefore, this month we have invited Maged Costantine, MD, an associate professor in the department of obstetrics and gynecology and division of maternal fetal medicine at the University of Texas Medical Branch in Galveston, to discuss this novel and exciting new area of research.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
In medicine, there are many diseases and conditions that pose significant challenges to health care practitioners. For example, within brain science, there are patients with debilitating neurodegenerative diseases; within emergency medicine, there are patients who have suffered severe and acute trauma; within pediatrics, there are patients with terminal illnesses, such as cancer. In ob.gyn., one of the great obstetrical syndromes is preeclampsia.
Humans have known about preeclampsia for thousands of years, dating back to the 4th and 5th centuries B.C., since the time of Hippocrates. Ancient writings on medical conditions of women reflect a recognition of preeclampsia and eclampsia, although formal classification of the condition as a hypertensive disorder associated specifically with pregnancy did not occur until the late 1800s. Despite this, the pathology of preeclampsia is significantly underdefined, and because the underlying causes of preeclampsia are largely unknown, prevention and management continue to be hindered.
However, recent research indicating an association between statin use and prevention of preeclampsia has given us some hope that targeted management is possible. As ob.gyns. continue to grapple with the intricacies of managing patients with preeclampsia, a condition we increasingly see because of concurrent rises in being overweight, being obese, and having diabetes during pregnancy, so we are eager to see whether the research might confirm an effective role for statins in patient care. Although statins are still quite far from becoming a new standard of care, we are, as a specialty, hungry for a solution to this millennia-old problem.
Therefore, this month we have invited Maged Costantine, MD, an associate professor in the department of obstetrics and gynecology and division of maternal fetal medicine at the University of Texas Medical Branch in Galveston, to discuss this novel and exciting new area of research.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Reducing outpatient medication costs
For many patients, paying for medication presents a serious challenge. Studies show that up to 45% of Americans do not fill prescriptions secondary to cost, and medication nonadherence leads to morbidity and mortality, with costs from $100 billion to $300 billion annually.
One way to address the problem is by empowering clinicians to reduce patient outpatient medication costs – the goal described in a recent abstract.
Initial testing was promising. One patient, admitted for the fourth time in 14 months for hypertriglyceridemia-induced pancreatitis secondary to medication nonadherence, was able to reduce 90-day outpatient medication cost by 95%, from $1,287.00 to $61.79. By reducing his readmissions, the institution saved more than $20,000 a year.
The researchers secured internal grant funding to develop an automated version of the tool. “We currently have technology that can dramatically reduce the cost of many medications with early promising results for patient outcomes, readmissions rates and overall systemic cost,” Dr. Kubey said. “We are working rapidly to further develop and study our tool and, if prospective results confirm our initial findings, we will seek to provide this tool to clinicians broadly.”
Such tools are a true win-win. Hospitalists using them help ensure that discharged patients are able to afford the often life-saving medications that will keep them healthy and out of the hospital, improve readmission rates, patient satisfaction metrics, total system cost, and, most important, do right by our patients in need for whom we are charged to care, Dr. Kubey said.
“Hospitalists first must be aware that savings of 90% or more are possible for many medications and that medication nonadherence because of cost is a serious issue affecting nearly half the patients we see,” he said. “The first step is simply asking patients if medication cost is proving troublesome – we cannot address what we do not see. The second step is to use current discount tools such as GoodRx, NeedyMeds, and the like – and, we hope, in the not too distant future, our tool, which we plan to integrate into EHR prescribing to make it easy and nearly instantaneous for hospitalists to prescribe the most high-value, low-cost medication regimen for each individual patient at discharge.”
Reference
Kubey A et al. Expensive free hospitalizations – A novel approach to reducing outpatient medication cost [abstract]. J Hosp Med. 2017; 12 (suppl 2). Accessed Aug. 7, 2017.
For many patients, paying for medication presents a serious challenge. Studies show that up to 45% of Americans do not fill prescriptions secondary to cost, and medication nonadherence leads to morbidity and mortality, with costs from $100 billion to $300 billion annually.
One way to address the problem is by empowering clinicians to reduce patient outpatient medication costs – the goal described in a recent abstract.
Initial testing was promising. One patient, admitted for the fourth time in 14 months for hypertriglyceridemia-induced pancreatitis secondary to medication nonadherence, was able to reduce 90-day outpatient medication cost by 95%, from $1,287.00 to $61.79. By reducing his readmissions, the institution saved more than $20,000 a year.
The researchers secured internal grant funding to develop an automated version of the tool. “We currently have technology that can dramatically reduce the cost of many medications with early promising results for patient outcomes, readmissions rates and overall systemic cost,” Dr. Kubey said. “We are working rapidly to further develop and study our tool and, if prospective results confirm our initial findings, we will seek to provide this tool to clinicians broadly.”
Such tools are a true win-win. Hospitalists using them help ensure that discharged patients are able to afford the often life-saving medications that will keep them healthy and out of the hospital, improve readmission rates, patient satisfaction metrics, total system cost, and, most important, do right by our patients in need for whom we are charged to care, Dr. Kubey said.
“Hospitalists first must be aware that savings of 90% or more are possible for many medications and that medication nonadherence because of cost is a serious issue affecting nearly half the patients we see,” he said. “The first step is simply asking patients if medication cost is proving troublesome – we cannot address what we do not see. The second step is to use current discount tools such as GoodRx, NeedyMeds, and the like – and, we hope, in the not too distant future, our tool, which we plan to integrate into EHR prescribing to make it easy and nearly instantaneous for hospitalists to prescribe the most high-value, low-cost medication regimen for each individual patient at discharge.”
Reference
Kubey A et al. Expensive free hospitalizations – A novel approach to reducing outpatient medication cost [abstract]. J Hosp Med. 2017; 12 (suppl 2). Accessed Aug. 7, 2017.
For many patients, paying for medication presents a serious challenge. Studies show that up to 45% of Americans do not fill prescriptions secondary to cost, and medication nonadherence leads to morbidity and mortality, with costs from $100 billion to $300 billion annually.
One way to address the problem is by empowering clinicians to reduce patient outpatient medication costs – the goal described in a recent abstract.
Initial testing was promising. One patient, admitted for the fourth time in 14 months for hypertriglyceridemia-induced pancreatitis secondary to medication nonadherence, was able to reduce 90-day outpatient medication cost by 95%, from $1,287.00 to $61.79. By reducing his readmissions, the institution saved more than $20,000 a year.
The researchers secured internal grant funding to develop an automated version of the tool. “We currently have technology that can dramatically reduce the cost of many medications with early promising results for patient outcomes, readmissions rates and overall systemic cost,” Dr. Kubey said. “We are working rapidly to further develop and study our tool and, if prospective results confirm our initial findings, we will seek to provide this tool to clinicians broadly.”
Such tools are a true win-win. Hospitalists using them help ensure that discharged patients are able to afford the often life-saving medications that will keep them healthy and out of the hospital, improve readmission rates, patient satisfaction metrics, total system cost, and, most important, do right by our patients in need for whom we are charged to care, Dr. Kubey said.
“Hospitalists first must be aware that savings of 90% or more are possible for many medications and that medication nonadherence because of cost is a serious issue affecting nearly half the patients we see,” he said. “The first step is simply asking patients if medication cost is proving troublesome – we cannot address what we do not see. The second step is to use current discount tools such as GoodRx, NeedyMeds, and the like – and, we hope, in the not too distant future, our tool, which we plan to integrate into EHR prescribing to make it easy and nearly instantaneous for hospitalists to prescribe the most high-value, low-cost medication regimen for each individual patient at discharge.”
Reference
Kubey A et al. Expensive free hospitalizations – A novel approach to reducing outpatient medication cost [abstract]. J Hosp Med. 2017; 12 (suppl 2). Accessed Aug. 7, 2017.
Up to 47 million Americans may have “preclinical” Alzheimer’s disease, study estimates
A treatment that would cut the risk of developing amyloid plaques in the brain by 50% could save more than 4 million U.S. residents from mild cognitive impairment and 2.5 million from Alzheimer’s disease by 2060.
The conclusion that even modestly effective preventive therapy could vastly improve the Alzheimer’s outlook is especially important given another startling finding in a new mathematical modeling study by Ron Brookmeyer, PhD, and his colleagues: Right now, they assert, .
“This is the first major attempt to forecast these proposed preclinical Alzheimer’s disease and [mild cognitive impairment] due to Alzheimer’s disease numbers. If confirmed, these data provide essential information for public health planning, and for informing and guiding the public and private investment in Alzheimer’s and dementia research. We need more research to confirm the findings from this model, and more Alzheimer’s and dementia research that includes diverse populations.”
In an interview, Dr. Brookmeyer, a biostatistician at the University of California, Los Angeles, attempted to put those numbers into perspective.
“I want to emphasize that of the 47 million with these Alzheimer’s brain changes, but without clinical symptoms, most will not progress to clinical disease during their lifetimes. In fact, perhaps only 1 in 7 will progress to full-blown dementia.”
Nevertheless, the numbers are disturbing and represent a reality that must be confronted and managed proactively if at all possible, Dr. Brookmeyer said.
“The numbers are what they are,” he said. “They may sound alarmist, but I have every confidence in them. And they’re important because they allow us to understand how many people could potentially benefit from treatment, at what point on the disease continuum it would be useful to implement treatment, and how those treatments could impact public health.”
To carry out the modeling, Dr. Brookmeyer extrapolated from data in two prospective longitudinal cohort studies: the Mayo Clinic Study of Aging, and one by Stephanie J. Vos, PhD, of Maastricht (the Netherlands) University.
The Mayo study followed 1,541 cognitively normal older adults and provided data on the rate of transition from normal cognition to mild cognitive impairment (MCI). The study by Dr. Vos and her associates followed 353 subjects with MCI and brain amyloid, and 222 with late MCI as they progressed. It’s the largest prospective study of progression from MCI to AD that also contains data on baseline neurodegeneration and amyloid burden (Brain. 2015;138[5]:1327-38).
“These studies gave us the rates of transition from one state to another,” Dr. Brookmeyer said. “For example; the Mayo study gave us rates of transition from normal to amyloidosis: 3% of normal 60-year-olds will convert to this state every year.”
The Vos study, Dr. Brookmeyer said, determined rates of progression from MCI to Alzheimer’s dementia, given two preclinical states: asymptomatic amyloid brain plaques alone, or plaques with evidence of neurodegeneration and cognitive signs. Both of these transitional states were first defined in 2011 in a joint paper by the Alzheimer’s Association and the National Institute on Aging (Alzheimers Dement. 2011;7[3]:280-92). While acknowledging that the root causes of Alzheimer’s are unknown, and probably multifactorial, the paper hypothesized a pathophysiologic time line beginning with a three-stage preclinical phase:
• Stage 1: Asymptomatic cerebral amyloidosis: amyloid-positive PET brain imaging with an amyloid-binding ligand and/or a cerebrospinal fluid assay with low amyloid-beta 42 in the presence of normal cognition.
• Stage 2: Amyloid positivity and evidence of synaptic dysfunction and/or early neurodegeneration in the presence of normal cognition.
• Stage 3: Amyloid positivity with evidence of neurodegeneration in the presence of subtle cognitive decline.
“Using those definitions, and piecing together the numbers from these studies, we constructed a computer model based on U.S. census population projections to simulate how many people might be in these different states of disease,” Dr. Brookmeyer said.
In 2017, 6 million Americans were in one of the clinical disease states (MCI due to AD, early clinical AD, or late clinical AD). Dr. Brookmeyer and his colleagues predicted that number will grow to 15 million by 2060. Similarly, in 2017, about 47 million Americans were in one of the preclinical AD states: 22 million with amyloidosis, 8.3 million with only neurodegeneration, and 16.2 million with both. He projects that number will increase to 75.7 million by 2060.
The team then remodeled those numbers in three hypothetical intervention scenarios. In general, Alzheimer’s researchers say a treatment that slows decline by at least 30% would have a meaningful clinical, financial, and societal impact. However, Dr. Brookmeyer modeled treatment scenarios with a greater effect than that.
A primary prevention that reduced the annual risk of new amyloidosis by 50% could decrease the prevalence of MCI by about 700,000 in 2060. A secondary prevention strategy that reduced the annual risk progression to MCI by 50% would decrease the prevalence of MCI by more than 2 million and the prevalence of AD by about 3.8 million.
The results were more complicated with a secondary prevention strategy that would reduce annual risk of MCI-AD conversion by 50%. In this scenario, the prevalence of MCI in 2060 would actually increase by 2.8 million, but the prevalence of AD would decrease by 2.5 million.
These scenarios also developed over different time courses, the researchers wrote.
“We find that the highly effective primary prevention strategy resulted in the lowest AD prevalence by the year 2060. However, [it] was associated with the largest AD prevalence in the 15 years immediately after its introduction ... The explanation for this finding is that the full benefits of delaying amyloidosis in terms of reduced AD prevalence are not realized for many years because of the long lag time between amyloidosis and clinical AD. A take-home message is that the full impact on disease burden of primary prevention that targets the early stages of the pathogenesis of AD on clinical disease burden may not be realized for decades.”
Decreasing preclinical conversion to MCI with a secondary prevention strategy would result in the highest AD prevalence reduction for most of the period. But by 2054, the primary prevention strategy would surpass it.
The intervention targeting MCI-AD conversion would reduce AD prevalence the quickest, with a slight decrease in the first 3 years after introduction. “The explanation for this finding is that MCI is proximate to clinical AD diagnosis, and thus, the impact of delaying progression of MCI will be seen relatively quickly on AD prevalence compared to interventions that delay onset of amyloidosis or MCI.”
The study sharply illustrates two futures: one with an unimpeded tsunami of Alzheimer’s cases, and one in which prevention strategies, while not a floodgate, at least stem the tide somewhat. And while researchers hold out hope for the primary and secondary treatments currently in clinical trials, the AD community is nowhere close to finding even a modestly effective therapy.
Seen in this light, Dr. Brookmeyer’s projections are a cry for action, the Alzheimer’s Association statement proclaimed.
“By focusing attention on a concerning reality – that tens of millions of American adults may face the possibility of dementia due to Alzheimer’s disease – the results reported in this new article, if confirmed, illustrate and greatly amplify the need for more research to develop effective treatments and proven prevention strategies for Alzheimer’s disease. This is especially true as we get better at early detection, and are able to more accurately identify people who have the early brain changes associated with Alzheimer’s disease and other dementias.”
Dr. Brookmeyer reported receiving fees from Takeda for serving as a member of a data safety monitoring board.
SOURCE: Brookemeyer R et al. Alzheimers Dement. 2017 Dec 6. doi: 10.1016/j.jalz.2017.10.009
The study by Brookmeyer and colleagues is a logical and thoughtful attempt to “size” the potential impact of Alzheimer’s disease now and in the future, updating “old technology” estimates based on actual diagnoses with new technologically derived diagnoses of preclinical neurodegenerative states. They acknowledge that the uncertainty in the actual disease burden we will face is centered on the question of conversion rates, which vary between studies and are far less certain in the preclinical stages than the symptomatic ones.
The attention paid by the Alzheimer’s Association is understandable given its mission of increasing awareness and supporting more funding, but it omits mention of another important article that is showing that dementia rates are actually declining (JAMA Neurol. 2017;74[11]:1345-51) when adjusted for our aging population (observed vs. expected).
In my opinion, we need some rational balance between maintaining public awareness without creating unnecessary panic. There is no question that AD is a major public health issue that warrants all the funding we can provide to researchers seeking a cure. How to balance that need with the need to give our population hope that all is not lost when they misplace their keys is the challenge this article raises in my mind.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale. He is also associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He has no relevant disclosures.
The study by Brookmeyer and colleagues is a logical and thoughtful attempt to “size” the potential impact of Alzheimer’s disease now and in the future, updating “old technology” estimates based on actual diagnoses with new technologically derived diagnoses of preclinical neurodegenerative states. They acknowledge that the uncertainty in the actual disease burden we will face is centered on the question of conversion rates, which vary between studies and are far less certain in the preclinical stages than the symptomatic ones.
The attention paid by the Alzheimer’s Association is understandable given its mission of increasing awareness and supporting more funding, but it omits mention of another important article that is showing that dementia rates are actually declining (JAMA Neurol. 2017;74[11]:1345-51) when adjusted for our aging population (observed vs. expected).
In my opinion, we need some rational balance between maintaining public awareness without creating unnecessary panic. There is no question that AD is a major public health issue that warrants all the funding we can provide to researchers seeking a cure. How to balance that need with the need to give our population hope that all is not lost when they misplace their keys is the challenge this article raises in my mind.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale. He is also associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He has no relevant disclosures.
The study by Brookmeyer and colleagues is a logical and thoughtful attempt to “size” the potential impact of Alzheimer’s disease now and in the future, updating “old technology” estimates based on actual diagnoses with new technologically derived diagnoses of preclinical neurodegenerative states. They acknowledge that the uncertainty in the actual disease burden we will face is centered on the question of conversion rates, which vary between studies and are far less certain in the preclinical stages than the symptomatic ones.
The attention paid by the Alzheimer’s Association is understandable given its mission of increasing awareness and supporting more funding, but it omits mention of another important article that is showing that dementia rates are actually declining (JAMA Neurol. 2017;74[11]:1345-51) when adjusted for our aging population (observed vs. expected).
In my opinion, we need some rational balance between maintaining public awareness without creating unnecessary panic. There is no question that AD is a major public health issue that warrants all the funding we can provide to researchers seeking a cure. How to balance that need with the need to give our population hope that all is not lost when they misplace their keys is the challenge this article raises in my mind.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale. He is also associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He has no relevant disclosures.
A treatment that would cut the risk of developing amyloid plaques in the brain by 50% could save more than 4 million U.S. residents from mild cognitive impairment and 2.5 million from Alzheimer’s disease by 2060.
The conclusion that even modestly effective preventive therapy could vastly improve the Alzheimer’s outlook is especially important given another startling finding in a new mathematical modeling study by Ron Brookmeyer, PhD, and his colleagues: Right now, they assert, .
“This is the first major attempt to forecast these proposed preclinical Alzheimer’s disease and [mild cognitive impairment] due to Alzheimer’s disease numbers. If confirmed, these data provide essential information for public health planning, and for informing and guiding the public and private investment in Alzheimer’s and dementia research. We need more research to confirm the findings from this model, and more Alzheimer’s and dementia research that includes diverse populations.”
In an interview, Dr. Brookmeyer, a biostatistician at the University of California, Los Angeles, attempted to put those numbers into perspective.
“I want to emphasize that of the 47 million with these Alzheimer’s brain changes, but without clinical symptoms, most will not progress to clinical disease during their lifetimes. In fact, perhaps only 1 in 7 will progress to full-blown dementia.”
Nevertheless, the numbers are disturbing and represent a reality that must be confronted and managed proactively if at all possible, Dr. Brookmeyer said.
“The numbers are what they are,” he said. “They may sound alarmist, but I have every confidence in them. And they’re important because they allow us to understand how many people could potentially benefit from treatment, at what point on the disease continuum it would be useful to implement treatment, and how those treatments could impact public health.”
To carry out the modeling, Dr. Brookmeyer extrapolated from data in two prospective longitudinal cohort studies: the Mayo Clinic Study of Aging, and one by Stephanie J. Vos, PhD, of Maastricht (the Netherlands) University.
The Mayo study followed 1,541 cognitively normal older adults and provided data on the rate of transition from normal cognition to mild cognitive impairment (MCI). The study by Dr. Vos and her associates followed 353 subjects with MCI and brain amyloid, and 222 with late MCI as they progressed. It’s the largest prospective study of progression from MCI to AD that also contains data on baseline neurodegeneration and amyloid burden (Brain. 2015;138[5]:1327-38).
“These studies gave us the rates of transition from one state to another,” Dr. Brookmeyer said. “For example; the Mayo study gave us rates of transition from normal to amyloidosis: 3% of normal 60-year-olds will convert to this state every year.”
The Vos study, Dr. Brookmeyer said, determined rates of progression from MCI to Alzheimer’s dementia, given two preclinical states: asymptomatic amyloid brain plaques alone, or plaques with evidence of neurodegeneration and cognitive signs. Both of these transitional states were first defined in 2011 in a joint paper by the Alzheimer’s Association and the National Institute on Aging (Alzheimers Dement. 2011;7[3]:280-92). While acknowledging that the root causes of Alzheimer’s are unknown, and probably multifactorial, the paper hypothesized a pathophysiologic time line beginning with a three-stage preclinical phase:
• Stage 1: Asymptomatic cerebral amyloidosis: amyloid-positive PET brain imaging with an amyloid-binding ligand and/or a cerebrospinal fluid assay with low amyloid-beta 42 in the presence of normal cognition.
• Stage 2: Amyloid positivity and evidence of synaptic dysfunction and/or early neurodegeneration in the presence of normal cognition.
• Stage 3: Amyloid positivity with evidence of neurodegeneration in the presence of subtle cognitive decline.
“Using those definitions, and piecing together the numbers from these studies, we constructed a computer model based on U.S. census population projections to simulate how many people might be in these different states of disease,” Dr. Brookmeyer said.
In 2017, 6 million Americans were in one of the clinical disease states (MCI due to AD, early clinical AD, or late clinical AD). Dr. Brookmeyer and his colleagues predicted that number will grow to 15 million by 2060. Similarly, in 2017, about 47 million Americans were in one of the preclinical AD states: 22 million with amyloidosis, 8.3 million with only neurodegeneration, and 16.2 million with both. He projects that number will increase to 75.7 million by 2060.
The team then remodeled those numbers in three hypothetical intervention scenarios. In general, Alzheimer’s researchers say a treatment that slows decline by at least 30% would have a meaningful clinical, financial, and societal impact. However, Dr. Brookmeyer modeled treatment scenarios with a greater effect than that.
A primary prevention that reduced the annual risk of new amyloidosis by 50% could decrease the prevalence of MCI by about 700,000 in 2060. A secondary prevention strategy that reduced the annual risk progression to MCI by 50% would decrease the prevalence of MCI by more than 2 million and the prevalence of AD by about 3.8 million.
The results were more complicated with a secondary prevention strategy that would reduce annual risk of MCI-AD conversion by 50%. In this scenario, the prevalence of MCI in 2060 would actually increase by 2.8 million, but the prevalence of AD would decrease by 2.5 million.
These scenarios also developed over different time courses, the researchers wrote.
“We find that the highly effective primary prevention strategy resulted in the lowest AD prevalence by the year 2060. However, [it] was associated with the largest AD prevalence in the 15 years immediately after its introduction ... The explanation for this finding is that the full benefits of delaying amyloidosis in terms of reduced AD prevalence are not realized for many years because of the long lag time between amyloidosis and clinical AD. A take-home message is that the full impact on disease burden of primary prevention that targets the early stages of the pathogenesis of AD on clinical disease burden may not be realized for decades.”
Decreasing preclinical conversion to MCI with a secondary prevention strategy would result in the highest AD prevalence reduction for most of the period. But by 2054, the primary prevention strategy would surpass it.
The intervention targeting MCI-AD conversion would reduce AD prevalence the quickest, with a slight decrease in the first 3 years after introduction. “The explanation for this finding is that MCI is proximate to clinical AD diagnosis, and thus, the impact of delaying progression of MCI will be seen relatively quickly on AD prevalence compared to interventions that delay onset of amyloidosis or MCI.”
The study sharply illustrates two futures: one with an unimpeded tsunami of Alzheimer’s cases, and one in which prevention strategies, while not a floodgate, at least stem the tide somewhat. And while researchers hold out hope for the primary and secondary treatments currently in clinical trials, the AD community is nowhere close to finding even a modestly effective therapy.
Seen in this light, Dr. Brookmeyer’s projections are a cry for action, the Alzheimer’s Association statement proclaimed.
“By focusing attention on a concerning reality – that tens of millions of American adults may face the possibility of dementia due to Alzheimer’s disease – the results reported in this new article, if confirmed, illustrate and greatly amplify the need for more research to develop effective treatments and proven prevention strategies for Alzheimer’s disease. This is especially true as we get better at early detection, and are able to more accurately identify people who have the early brain changes associated with Alzheimer’s disease and other dementias.”
Dr. Brookmeyer reported receiving fees from Takeda for serving as a member of a data safety monitoring board.
SOURCE: Brookemeyer R et al. Alzheimers Dement. 2017 Dec 6. doi: 10.1016/j.jalz.2017.10.009
A treatment that would cut the risk of developing amyloid plaques in the brain by 50% could save more than 4 million U.S. residents from mild cognitive impairment and 2.5 million from Alzheimer’s disease by 2060.
The conclusion that even modestly effective preventive therapy could vastly improve the Alzheimer’s outlook is especially important given another startling finding in a new mathematical modeling study by Ron Brookmeyer, PhD, and his colleagues: Right now, they assert, .
“This is the first major attempt to forecast these proposed preclinical Alzheimer’s disease and [mild cognitive impairment] due to Alzheimer’s disease numbers. If confirmed, these data provide essential information for public health planning, and for informing and guiding the public and private investment in Alzheimer’s and dementia research. We need more research to confirm the findings from this model, and more Alzheimer’s and dementia research that includes diverse populations.”
In an interview, Dr. Brookmeyer, a biostatistician at the University of California, Los Angeles, attempted to put those numbers into perspective.
“I want to emphasize that of the 47 million with these Alzheimer’s brain changes, but without clinical symptoms, most will not progress to clinical disease during their lifetimes. In fact, perhaps only 1 in 7 will progress to full-blown dementia.”
Nevertheless, the numbers are disturbing and represent a reality that must be confronted and managed proactively if at all possible, Dr. Brookmeyer said.
“The numbers are what they are,” he said. “They may sound alarmist, but I have every confidence in them. And they’re important because they allow us to understand how many people could potentially benefit from treatment, at what point on the disease continuum it would be useful to implement treatment, and how those treatments could impact public health.”
To carry out the modeling, Dr. Brookmeyer extrapolated from data in two prospective longitudinal cohort studies: the Mayo Clinic Study of Aging, and one by Stephanie J. Vos, PhD, of Maastricht (the Netherlands) University.
The Mayo study followed 1,541 cognitively normal older adults and provided data on the rate of transition from normal cognition to mild cognitive impairment (MCI). The study by Dr. Vos and her associates followed 353 subjects with MCI and brain amyloid, and 222 with late MCI as they progressed. It’s the largest prospective study of progression from MCI to AD that also contains data on baseline neurodegeneration and amyloid burden (Brain. 2015;138[5]:1327-38).
“These studies gave us the rates of transition from one state to another,” Dr. Brookmeyer said. “For example; the Mayo study gave us rates of transition from normal to amyloidosis: 3% of normal 60-year-olds will convert to this state every year.”
The Vos study, Dr. Brookmeyer said, determined rates of progression from MCI to Alzheimer’s dementia, given two preclinical states: asymptomatic amyloid brain plaques alone, or plaques with evidence of neurodegeneration and cognitive signs. Both of these transitional states were first defined in 2011 in a joint paper by the Alzheimer’s Association and the National Institute on Aging (Alzheimers Dement. 2011;7[3]:280-92). While acknowledging that the root causes of Alzheimer’s are unknown, and probably multifactorial, the paper hypothesized a pathophysiologic time line beginning with a three-stage preclinical phase:
• Stage 1: Asymptomatic cerebral amyloidosis: amyloid-positive PET brain imaging with an amyloid-binding ligand and/or a cerebrospinal fluid assay with low amyloid-beta 42 in the presence of normal cognition.
• Stage 2: Amyloid positivity and evidence of synaptic dysfunction and/or early neurodegeneration in the presence of normal cognition.
• Stage 3: Amyloid positivity with evidence of neurodegeneration in the presence of subtle cognitive decline.
“Using those definitions, and piecing together the numbers from these studies, we constructed a computer model based on U.S. census population projections to simulate how many people might be in these different states of disease,” Dr. Brookmeyer said.
In 2017, 6 million Americans were in one of the clinical disease states (MCI due to AD, early clinical AD, or late clinical AD). Dr. Brookmeyer and his colleagues predicted that number will grow to 15 million by 2060. Similarly, in 2017, about 47 million Americans were in one of the preclinical AD states: 22 million with amyloidosis, 8.3 million with only neurodegeneration, and 16.2 million with both. He projects that number will increase to 75.7 million by 2060.
The team then remodeled those numbers in three hypothetical intervention scenarios. In general, Alzheimer’s researchers say a treatment that slows decline by at least 30% would have a meaningful clinical, financial, and societal impact. However, Dr. Brookmeyer modeled treatment scenarios with a greater effect than that.
A primary prevention that reduced the annual risk of new amyloidosis by 50% could decrease the prevalence of MCI by about 700,000 in 2060. A secondary prevention strategy that reduced the annual risk progression to MCI by 50% would decrease the prevalence of MCI by more than 2 million and the prevalence of AD by about 3.8 million.
The results were more complicated with a secondary prevention strategy that would reduce annual risk of MCI-AD conversion by 50%. In this scenario, the prevalence of MCI in 2060 would actually increase by 2.8 million, but the prevalence of AD would decrease by 2.5 million.
These scenarios also developed over different time courses, the researchers wrote.
“We find that the highly effective primary prevention strategy resulted in the lowest AD prevalence by the year 2060. However, [it] was associated with the largest AD prevalence in the 15 years immediately after its introduction ... The explanation for this finding is that the full benefits of delaying amyloidosis in terms of reduced AD prevalence are not realized for many years because of the long lag time between amyloidosis and clinical AD. A take-home message is that the full impact on disease burden of primary prevention that targets the early stages of the pathogenesis of AD on clinical disease burden may not be realized for decades.”
Decreasing preclinical conversion to MCI with a secondary prevention strategy would result in the highest AD prevalence reduction for most of the period. But by 2054, the primary prevention strategy would surpass it.
The intervention targeting MCI-AD conversion would reduce AD prevalence the quickest, with a slight decrease in the first 3 years after introduction. “The explanation for this finding is that MCI is proximate to clinical AD diagnosis, and thus, the impact of delaying progression of MCI will be seen relatively quickly on AD prevalence compared to interventions that delay onset of amyloidosis or MCI.”
The study sharply illustrates two futures: one with an unimpeded tsunami of Alzheimer’s cases, and one in which prevention strategies, while not a floodgate, at least stem the tide somewhat. And while researchers hold out hope for the primary and secondary treatments currently in clinical trials, the AD community is nowhere close to finding even a modestly effective therapy.
Seen in this light, Dr. Brookmeyer’s projections are a cry for action, the Alzheimer’s Association statement proclaimed.
“By focusing attention on a concerning reality – that tens of millions of American adults may face the possibility of dementia due to Alzheimer’s disease – the results reported in this new article, if confirmed, illustrate and greatly amplify the need for more research to develop effective treatments and proven prevention strategies for Alzheimer’s disease. This is especially true as we get better at early detection, and are able to more accurately identify people who have the early brain changes associated with Alzheimer’s disease and other dementias.”
Dr. Brookmeyer reported receiving fees from Takeda for serving as a member of a data safety monitoring board.
SOURCE: Brookemeyer R et al. Alzheimers Dement. 2017 Dec 6. doi: 10.1016/j.jalz.2017.10.009
FROM ALZHEIMER’S & DEMENTIA
Key clinical point: Preventing amyloid accumulation would save millions from MCI and later AD.
Major finding: A treatment that decreased the risk of amyloidosis by 50% would prevent 4 million MCI cases and 2 million AD cases in the United States by 2060.
Study details: A computer modeling study based on two prospective longitudinal studies of aging.
Disclosures: This research was funded by a grant from the National Institutes of Health. Dr. Brookmeyer reported remuneration from Takeda as part of a data safety monitoring board.
Source: Brookemeyer R et al. Alzheimers Dement. 2017 Dec 6. doi: 10.1016/j.jalz.2017.10.009
Best of Acne: 2017
The Best of Acne collection consists of our top-accessed content online this year on acne in one convenient file.
Topics include:
- Hormonal therapies such as oral contraceptives and spironolactone
- Oral therapies
- Alternative therapies for acne scarring
- Patient management in populations such as children
Gary Goldenberg, MD, Digital Editor, Cutis, provides commentary on the top 10 articles.
Save this collection, print it, and/or share it with your colleagues. We hope this comprehensive collection will positively impact how you manage acne patients.

The Best of Acne collection consists of our top-accessed content online this year on acne in one convenient file.
Topics include:
- Hormonal therapies such as oral contraceptives and spironolactone
- Oral therapies
- Alternative therapies for acne scarring
- Patient management in populations such as children
Gary Goldenberg, MD, Digital Editor, Cutis, provides commentary on the top 10 articles.
Save this collection, print it, and/or share it with your colleagues. We hope this comprehensive collection will positively impact how you manage acne patients.

The Best of Acne collection consists of our top-accessed content online this year on acne in one convenient file.
Topics include:
- Hormonal therapies such as oral contraceptives and spironolactone
- Oral therapies
- Alternative therapies for acne scarring
- Patient management in populations such as children
Gary Goldenberg, MD, Digital Editor, Cutis, provides commentary on the top 10 articles.
Save this collection, print it, and/or share it with your colleagues. We hope this comprehensive collection will positively impact how you manage acne patients.


My choices for best books of 2017
In this month’s column, I am providing my recommendations of what I consider were the best books published in 2017, starting with “Attending: Medicine, Mindfulness and Humanity” by Ronald Epstein, MD, (New York: Simon & Schuster, 2017). These days, it’s difficult to have a conversation about medicine without mention of mindfulness. Most mindfulness and medicine articles are nothing more than a list of bromides written by people who’ve never seen a patient. Ronald Epstein is not one of them. A practicing family physician and professor of medicine, he is “The Attending,” and uses a play on words to encourage us to attend, or be present. Like a good instructor, he uses stories supported by studies in this book (there are nearly 25 pages of references) to argue why being mindful is essential and how you can strengthen your mindfulness muscles in your practice. Mastering these skills will help you become a better diagnostician and reduce the chance you’ll become a burnout statistic. We physicians “miss more by not seeing than by not knowing,” said physician William Osler, and Dr. Epstein helps us to see.
“Astrophysics for People in a Hurry,” by Neil de Grasse Tyson, (New York: W.W. Norton & Company, 2017). For no other reason than you’d like to be the compelling conversationalist at your next party, get this book. It is the lightest take I’ve read on the heaviest of subject matters. One can’t help but be fascinated by the universe we call home (there may be others, but you’ll have to get the book to find out). If ever you find yourself the victim of a bad outcome or serious error, reread his last chapter on the cosmic perspective. We all share a mere speck of dust as our home.
“The Power of Moments: Why Certain Experiences Have Extraordinary Impact,” by Chip Heath and Dan Heath, (New York: Simon & Schuster, 2017). The Heath brothers are back at it. This book, which focuses on experiences, is closest to our daily lives in medicine. Patient experience surveys are ubiquitous, and more often than not, public, fairly or not. Fortunately, service experiences have key factors that are common and modifiable. In their usual engaging prose, they make those key factors easy to understand and hard to forget. They end each chapter fittingly with a clinic to get you practicing. You and your patients will benefit from what you learn here.
“The Butchering Art: Joseph Lister’s Quest to Transform the Grisly World of Victorian Medicine,” by Lindsey Fitzharris, PhD, (New York: Farrar, Straus, and Giroux, 2017). This book is a real treat: a good old-fashioned medical history spiced with a bit of gore. The story of Dr. Joseph Lister’s discovery of antisepsis is a compelling and critical milestone in our history. (Yes, the germ-killing mouthwash, Listerine, was named in his honor.) Dr. Fitzharris, an Oxford scholar on the history of science and medicine, vividly re-creates the world of Victorian medicine with its gritty and sometimes messy pursuit of the truth. Lister was mocked and ostracized for his controversial ideas on the role of microbes in surgical infection before he was lionized. In a year dominated by “fake news,” it’s refreshing to read a story about how truth not only wins in the end but also saves lives.
“Autumn,” by Ali Smith, (New York: Pantheon Books, 2017). At the heart of this eloquent novel is the deeply felt, platonic 25-year-long relationship between Elisabeth Demand, a 32-year-old art history lecturer and 101-year-old Daniel Gluck, who is living out his final days in a nursing home. Set in post-Brexit Britain, the book jumps back and forth in time touching on many relevant issues including xenophobia and neo-nationalism, art and beauty, and the ever-evolving definitions of love of family. Some of the most touching moments occur in scenes when Elizabeth and Daniel discuss art and literature and the profound impact it has on their lives. The novel has got me thinking ... perhaps by better understanding art, we can better understand our patients and our roles in their lives.
“Salt, Fat, Acid, Heat: Mastering the Elements of Good Cooking,” by Samin Nosrat, (New York: Simon & Schuster, 2017). If like me, you prefer good old-fashioned cooking to meal replacements and food delivery apps, get this book. Though I don’t plan to dethrone my wife as our family’s chef, I am a much better sous chef after having perused this enlightening and charmingly illustrated cookbook. Ms. Nosrat, who learned how to cook at the famed Chez Panisse in Berkeley, Calif., demystifies cooking by breaking it down to four essential elements: salt, fat, acid and heat. Master these fundamentals, and you’ll be able to cook good food just about every time.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
In this month’s column, I am providing my recommendations of what I consider were the best books published in 2017, starting with “Attending: Medicine, Mindfulness and Humanity” by Ronald Epstein, MD, (New York: Simon & Schuster, 2017). These days, it’s difficult to have a conversation about medicine without mention of mindfulness. Most mindfulness and medicine articles are nothing more than a list of bromides written by people who’ve never seen a patient. Ronald Epstein is not one of them. A practicing family physician and professor of medicine, he is “The Attending,” and uses a play on words to encourage us to attend, or be present. Like a good instructor, he uses stories supported by studies in this book (there are nearly 25 pages of references) to argue why being mindful is essential and how you can strengthen your mindfulness muscles in your practice. Mastering these skills will help you become a better diagnostician and reduce the chance you’ll become a burnout statistic. We physicians “miss more by not seeing than by not knowing,” said physician William Osler, and Dr. Epstein helps us to see.
“Astrophysics for People in a Hurry,” by Neil de Grasse Tyson, (New York: W.W. Norton & Company, 2017). For no other reason than you’d like to be the compelling conversationalist at your next party, get this book. It is the lightest take I’ve read on the heaviest of subject matters. One can’t help but be fascinated by the universe we call home (there may be others, but you’ll have to get the book to find out). If ever you find yourself the victim of a bad outcome or serious error, reread his last chapter on the cosmic perspective. We all share a mere speck of dust as our home.
“The Power of Moments: Why Certain Experiences Have Extraordinary Impact,” by Chip Heath and Dan Heath, (New York: Simon & Schuster, 2017). The Heath brothers are back at it. This book, which focuses on experiences, is closest to our daily lives in medicine. Patient experience surveys are ubiquitous, and more often than not, public, fairly or not. Fortunately, service experiences have key factors that are common and modifiable. In their usual engaging prose, they make those key factors easy to understand and hard to forget. They end each chapter fittingly with a clinic to get you practicing. You and your patients will benefit from what you learn here.
“The Butchering Art: Joseph Lister’s Quest to Transform the Grisly World of Victorian Medicine,” by Lindsey Fitzharris, PhD, (New York: Farrar, Straus, and Giroux, 2017). This book is a real treat: a good old-fashioned medical history spiced with a bit of gore. The story of Dr. Joseph Lister’s discovery of antisepsis is a compelling and critical milestone in our history. (Yes, the germ-killing mouthwash, Listerine, was named in his honor.) Dr. Fitzharris, an Oxford scholar on the history of science and medicine, vividly re-creates the world of Victorian medicine with its gritty and sometimes messy pursuit of the truth. Lister was mocked and ostracized for his controversial ideas on the role of microbes in surgical infection before he was lionized. In a year dominated by “fake news,” it’s refreshing to read a story about how truth not only wins in the end but also saves lives.
“Autumn,” by Ali Smith, (New York: Pantheon Books, 2017). At the heart of this eloquent novel is the deeply felt, platonic 25-year-long relationship between Elisabeth Demand, a 32-year-old art history lecturer and 101-year-old Daniel Gluck, who is living out his final days in a nursing home. Set in post-Brexit Britain, the book jumps back and forth in time touching on many relevant issues including xenophobia and neo-nationalism, art and beauty, and the ever-evolving definitions of love of family. Some of the most touching moments occur in scenes when Elizabeth and Daniel discuss art and literature and the profound impact it has on their lives. The novel has got me thinking ... perhaps by better understanding art, we can better understand our patients and our roles in their lives.
“Salt, Fat, Acid, Heat: Mastering the Elements of Good Cooking,” by Samin Nosrat, (New York: Simon & Schuster, 2017). If like me, you prefer good old-fashioned cooking to meal replacements and food delivery apps, get this book. Though I don’t plan to dethrone my wife as our family’s chef, I am a much better sous chef after having perused this enlightening and charmingly illustrated cookbook. Ms. Nosrat, who learned how to cook at the famed Chez Panisse in Berkeley, Calif., demystifies cooking by breaking it down to four essential elements: salt, fat, acid and heat. Master these fundamentals, and you’ll be able to cook good food just about every time.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
In this month’s column, I am providing my recommendations of what I consider were the best books published in 2017, starting with “Attending: Medicine, Mindfulness and Humanity” by Ronald Epstein, MD, (New York: Simon & Schuster, 2017). These days, it’s difficult to have a conversation about medicine without mention of mindfulness. Most mindfulness and medicine articles are nothing more than a list of bromides written by people who’ve never seen a patient. Ronald Epstein is not one of them. A practicing family physician and professor of medicine, he is “The Attending,” and uses a play on words to encourage us to attend, or be present. Like a good instructor, he uses stories supported by studies in this book (there are nearly 25 pages of references) to argue why being mindful is essential and how you can strengthen your mindfulness muscles in your practice. Mastering these skills will help you become a better diagnostician and reduce the chance you’ll become a burnout statistic. We physicians “miss more by not seeing than by not knowing,” said physician William Osler, and Dr. Epstein helps us to see.
“Astrophysics for People in a Hurry,” by Neil de Grasse Tyson, (New York: W.W. Norton & Company, 2017). For no other reason than you’d like to be the compelling conversationalist at your next party, get this book. It is the lightest take I’ve read on the heaviest of subject matters. One can’t help but be fascinated by the universe we call home (there may be others, but you’ll have to get the book to find out). If ever you find yourself the victim of a bad outcome or serious error, reread his last chapter on the cosmic perspective. We all share a mere speck of dust as our home.
“The Power of Moments: Why Certain Experiences Have Extraordinary Impact,” by Chip Heath and Dan Heath, (New York: Simon & Schuster, 2017). The Heath brothers are back at it. This book, which focuses on experiences, is closest to our daily lives in medicine. Patient experience surveys are ubiquitous, and more often than not, public, fairly or not. Fortunately, service experiences have key factors that are common and modifiable. In their usual engaging prose, they make those key factors easy to understand and hard to forget. They end each chapter fittingly with a clinic to get you practicing. You and your patients will benefit from what you learn here.
“The Butchering Art: Joseph Lister’s Quest to Transform the Grisly World of Victorian Medicine,” by Lindsey Fitzharris, PhD, (New York: Farrar, Straus, and Giroux, 2017). This book is a real treat: a good old-fashioned medical history spiced with a bit of gore. The story of Dr. Joseph Lister’s discovery of antisepsis is a compelling and critical milestone in our history. (Yes, the germ-killing mouthwash, Listerine, was named in his honor.) Dr. Fitzharris, an Oxford scholar on the history of science and medicine, vividly re-creates the world of Victorian medicine with its gritty and sometimes messy pursuit of the truth. Lister was mocked and ostracized for his controversial ideas on the role of microbes in surgical infection before he was lionized. In a year dominated by “fake news,” it’s refreshing to read a story about how truth not only wins in the end but also saves lives.
“Autumn,” by Ali Smith, (New York: Pantheon Books, 2017). At the heart of this eloquent novel is the deeply felt, platonic 25-year-long relationship between Elisabeth Demand, a 32-year-old art history lecturer and 101-year-old Daniel Gluck, who is living out his final days in a nursing home. Set in post-Brexit Britain, the book jumps back and forth in time touching on many relevant issues including xenophobia and neo-nationalism, art and beauty, and the ever-evolving definitions of love of family. Some of the most touching moments occur in scenes when Elizabeth and Daniel discuss art and literature and the profound impact it has on their lives. The novel has got me thinking ... perhaps by better understanding art, we can better understand our patients and our roles in their lives.
“Salt, Fat, Acid, Heat: Mastering the Elements of Good Cooking,” by Samin Nosrat, (New York: Simon & Schuster, 2017). If like me, you prefer good old-fashioned cooking to meal replacements and food delivery apps, get this book. Though I don’t plan to dethrone my wife as our family’s chef, I am a much better sous chef after having perused this enlightening and charmingly illustrated cookbook. Ms. Nosrat, who learned how to cook at the famed Chez Panisse in Berkeley, Calif., demystifies cooking by breaking it down to four essential elements: salt, fat, acid and heat. Master these fundamentals, and you’ll be able to cook good food just about every time.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
A Nondrug Way to Relieve Withdrawal Symptoms
The FDA has given the nod to a new indication for the NSS-2 Bridge, an electronic device that was cleared for use in acupuncture in 2014. Now it is approved for reducing the symptoms of opioid withdrawal.
The NSS-2 Bridge is a small electrical nerve stimulator placed behind a patient’s ear. A battery-powered chip sends electrical pulses to stimulate branches of certain cranial nerves. Patients can use the device for up to 5 days during the acute phase of physical withdrawal.
The approval was based on results from a clinical study of 73 patients in withdrawal, evaluating their symptoms on the Clinical Opiate Withdrawal Scale (COWS), which measures signs and symptoms such as resting pulse rate, sweating, pupil size, gastrointestinal issues, bone and joint aches, tremors, and anxiety. The COWS scores range from 0 to > 36. The higher the score, the more severe the symptoms.
Before patients used the device, their average COWS score was 20.1. Within 30 minutes, all patient scores dropped by at least 31%. Nearly all (88%) the patients transitioned to medication-assisted therapy after 5 days of using the device.
The FDA has given the nod to a new indication for the NSS-2 Bridge, an electronic device that was cleared for use in acupuncture in 2014. Now it is approved for reducing the symptoms of opioid withdrawal.
The NSS-2 Bridge is a small electrical nerve stimulator placed behind a patient’s ear. A battery-powered chip sends electrical pulses to stimulate branches of certain cranial nerves. Patients can use the device for up to 5 days during the acute phase of physical withdrawal.
The approval was based on results from a clinical study of 73 patients in withdrawal, evaluating their symptoms on the Clinical Opiate Withdrawal Scale (COWS), which measures signs and symptoms such as resting pulse rate, sweating, pupil size, gastrointestinal issues, bone and joint aches, tremors, and anxiety. The COWS scores range from 0 to > 36. The higher the score, the more severe the symptoms.
Before patients used the device, their average COWS score was 20.1. Within 30 minutes, all patient scores dropped by at least 31%. Nearly all (88%) the patients transitioned to medication-assisted therapy after 5 days of using the device.
The FDA has given the nod to a new indication for the NSS-2 Bridge, an electronic device that was cleared for use in acupuncture in 2014. Now it is approved for reducing the symptoms of opioid withdrawal.
The NSS-2 Bridge is a small electrical nerve stimulator placed behind a patient’s ear. A battery-powered chip sends electrical pulses to stimulate branches of certain cranial nerves. Patients can use the device for up to 5 days during the acute phase of physical withdrawal.
The approval was based on results from a clinical study of 73 patients in withdrawal, evaluating their symptoms on the Clinical Opiate Withdrawal Scale (COWS), which measures signs and symptoms such as resting pulse rate, sweating, pupil size, gastrointestinal issues, bone and joint aches, tremors, and anxiety. The COWS scores range from 0 to > 36. The higher the score, the more severe the symptoms.
Before patients used the device, their average COWS score was 20.1. Within 30 minutes, all patient scores dropped by at least 31%. Nearly all (88%) the patients transitioned to medication-assisted therapy after 5 days of using the device.
Job Satisfaction
You know that good feeling you get when you think about what you do for a living? That’s job satisfaction. So, what contributes to that feeling? Why does it matter?
Job satisfaction among both NPs and PAs remains high since last year’s survey.
The major determinants of job satisfaction include autonomy, appropriate pay, having adequate time to interact with patients, collegial support, and opportunities for professional growth.1-3
Dissatisfaction—due, for example, to work-life imbalance, adverse working conditions, or threat of malpractice lawsuits—may motivate experienced clinicians to leave their jobs. Clearly, keeping NPs and PAs engaged and satisfied is key to creating and retaining effective health care teams, resulting in better patient care and lower health care costs.1,4
So, are you interested in discovering ways to increase your career satisfaction? Actively seeking a new position? Looking to hire or retain staff? Check out the PDF for information on pay, benefits, and reasons your peers leave their jobs—broken out by profession and by region.
You know that good feeling you get when you think about what you do for a living? That’s job satisfaction. So, what contributes to that feeling? Why does it matter?
Job satisfaction among both NPs and PAs remains high since last year’s survey.
The major determinants of job satisfaction include autonomy, appropriate pay, having adequate time to interact with patients, collegial support, and opportunities for professional growth.1-3
Dissatisfaction—due, for example, to work-life imbalance, adverse working conditions, or threat of malpractice lawsuits—may motivate experienced clinicians to leave their jobs. Clearly, keeping NPs and PAs engaged and satisfied is key to creating and retaining effective health care teams, resulting in better patient care and lower health care costs.1,4
So, are you interested in discovering ways to increase your career satisfaction? Actively seeking a new position? Looking to hire or retain staff? Check out the PDF for information on pay, benefits, and reasons your peers leave their jobs—broken out by profession and by region.
You know that good feeling you get when you think about what you do for a living? That’s job satisfaction. So, what contributes to that feeling? Why does it matter?
Job satisfaction among both NPs and PAs remains high since last year’s survey.
The major determinants of job satisfaction include autonomy, appropriate pay, having adequate time to interact with patients, collegial support, and opportunities for professional growth.1-3
Dissatisfaction—due, for example, to work-life imbalance, adverse working conditions, or threat of malpractice lawsuits—may motivate experienced clinicians to leave their jobs. Clearly, keeping NPs and PAs engaged and satisfied is key to creating and retaining effective health care teams, resulting in better patient care and lower health care costs.1,4
So, are you interested in discovering ways to increase your career satisfaction? Actively seeking a new position? Looking to hire or retain staff? Check out the PDF for information on pay, benefits, and reasons your peers leave their jobs—broken out by profession and by region.
Combo should be standard in MM, doc says
ATLANTA—Study results “strongly support” a new standard of care for transplant-ineligible patients with newly diagnosed multiple myeloma (MM), according to a speaker at the 2017 ASH Annual Meeting.
The study, ALCYONE, suggests treatment with bortezomib, melphalan, and prednisone (VMP) can be improved by the addition of daratumumab (D).
D-VMP produced deeper responses and prolonged progression-free survival (PFS) when compared to VMP.
“In this first phase 3, randomized study with a monoclonal antibody in newly diagnosed multiple myeloma, daratumumab reduced the risk of progression or death by 50%,” said Maria-Victoria Mateos, MD, PhD, of University Hospital of Salamanca in Spain.
“No new safety signals were observed [with D-VMP], except for higher infectious events that resolved. I would say the results of this study strongly support daratumumab in combination with VMP as a standard of care in transplant-ineligible, newly diagnosed multiple myeloma.”
Dr Mateos presented results from ALCYONE as a late-breaking abstract (LBA-4) at the ASH Annual Meeting. The study was simultaneously published in NEJM. The research was supported by Janssen Research and Development.
Patients and treatment
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant.
Patients were randomized to receive VMP or D-VMP. They were stratified by International Staging System (I, II, III), region (Europe vs other), and age (<75 vs ≥75 years).
All patients received up to 9 cycles of VMP:
- Bortezomib at 1.3 mg/m2 twice weekly on weeks 1, 2, 4, and 5 of cycle 1 and once weekly on weeks 1, 2, 4, and 5 of cycles 2 through 9
- Melphalan at 9 mg/m2 once daily on days 1 to 4 of each cycle
- Prednisone at 60 mg/m2 once daily on days 1 to 4 of each cycle.
Patients in the daratumumab arm received the drug at 16 mg/kg once-weekly for the first cycle, every 3 weeks for cycles 2 to 9, and every 4 weeks thereafter, until disease progression. These patients also received dexamethasone (to manage infusion reactions) at 20 mg on the same schedule.
Baseline characteristics were similar between the VMP (n=356) and D-VMP (n=350) arms. The median age was 71 in both arms (range, 50-91 in the VMP arm and 40-93 in the D-VMP arm). Males made up 47% of the VMP arm and 46% of the D-VMP arm.
Forty-nine percent of patients in the VMP arm and 52% in the D-VMP arm had an ECOG performance status of 1. Twenty-eight percent and 22%, respectively, had a status of 0.
The median follow-up was 16.5 months (range, 0.1-28.1). At the clinical cutoff date (June 12, 2017), 5% of patients in the VMP arm were still on study treatment, as were 71% of patients in the D-VMP arm.
Response and survival
“I would like to note that the benefit of the addition of daratumumab was observed since the beginning of the treatment,” Dr Mateos said.
The overall response rate was 74% in the VMP arm and 91% in the D-VMP arm (P<0.0001). The median duration of response was 21.3 months in the VMP arm and was not reached in the D-VMP arm.
The rate of complete response was 24% in the VMP arm and 43% in the D-VMP arm (P<0.0001). Six percent of patients in the VMP arm and 22% in the D-VMP arm were negative for minimal residual disease (P<0.0001).
The hazard ratio for disease progression or death in the D-VMP arm versus the VMP arm was 0.50 (P<0.0001).
The median PFS was 18.1 months in the VMP arm and was not reached in the D-VMP arm. The 12-month PFS was 76% and 87%, respectively. And the 18-month PFS was 50% and 72%, respectively.
D-VMP prolonged PFS regardless of patient sex, age, cytogenetic risk, ECOG performance status, baseline renal function, and other factors.
The median overall survival was not reached in either treatment arm. There were 48 deaths in the VMP arm and 45 in the D-VMP arm.
Adverse events
The most common treatment-emergent adverse events (TEAEs; in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
The most common grade 3/4 TEAEs (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
There were 6 deaths due to TEAEs in the D-VMP arm and 5 such deaths in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. The most common of these was pneumonia, with rates of 11% and 4%, respectively.
Infections resolved in 88% of cases in the D-VMP arm and 87% of cases in the VMP arm. Rates of treatment discontinuation due to infection were 0.9% and 1.4%, respectively. One patient in each group stopped treatment due to pneumonia.
Twenty-eight percent of patients in the D-VMP arm had infusion-related reactions (15% grade 3 and 2% grade 4). Most of these reactions occurred during the first infusion. Five patients (1.4%) discontinued daratumumab due to infusion-related reactions. ![]()
ATLANTA—Study results “strongly support” a new standard of care for transplant-ineligible patients with newly diagnosed multiple myeloma (MM), according to a speaker at the 2017 ASH Annual Meeting.
The study, ALCYONE, suggests treatment with bortezomib, melphalan, and prednisone (VMP) can be improved by the addition of daratumumab (D).
D-VMP produced deeper responses and prolonged progression-free survival (PFS) when compared to VMP.
“In this first phase 3, randomized study with a monoclonal antibody in newly diagnosed multiple myeloma, daratumumab reduced the risk of progression or death by 50%,” said Maria-Victoria Mateos, MD, PhD, of University Hospital of Salamanca in Spain.
“No new safety signals were observed [with D-VMP], except for higher infectious events that resolved. I would say the results of this study strongly support daratumumab in combination with VMP as a standard of care in transplant-ineligible, newly diagnosed multiple myeloma.”
Dr Mateos presented results from ALCYONE as a late-breaking abstract (LBA-4) at the ASH Annual Meeting. The study was simultaneously published in NEJM. The research was supported by Janssen Research and Development.
Patients and treatment
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant.
Patients were randomized to receive VMP or D-VMP. They were stratified by International Staging System (I, II, III), region (Europe vs other), and age (<75 vs ≥75 years).
All patients received up to 9 cycles of VMP:
- Bortezomib at 1.3 mg/m2 twice weekly on weeks 1, 2, 4, and 5 of cycle 1 and once weekly on weeks 1, 2, 4, and 5 of cycles 2 through 9
- Melphalan at 9 mg/m2 once daily on days 1 to 4 of each cycle
- Prednisone at 60 mg/m2 once daily on days 1 to 4 of each cycle.
Patients in the daratumumab arm received the drug at 16 mg/kg once-weekly for the first cycle, every 3 weeks for cycles 2 to 9, and every 4 weeks thereafter, until disease progression. These patients also received dexamethasone (to manage infusion reactions) at 20 mg on the same schedule.
Baseline characteristics were similar between the VMP (n=356) and D-VMP (n=350) arms. The median age was 71 in both arms (range, 50-91 in the VMP arm and 40-93 in the D-VMP arm). Males made up 47% of the VMP arm and 46% of the D-VMP arm.
Forty-nine percent of patients in the VMP arm and 52% in the D-VMP arm had an ECOG performance status of 1. Twenty-eight percent and 22%, respectively, had a status of 0.
The median follow-up was 16.5 months (range, 0.1-28.1). At the clinical cutoff date (June 12, 2017), 5% of patients in the VMP arm were still on study treatment, as were 71% of patients in the D-VMP arm.
Response and survival
“I would like to note that the benefit of the addition of daratumumab was observed since the beginning of the treatment,” Dr Mateos said.
The overall response rate was 74% in the VMP arm and 91% in the D-VMP arm (P<0.0001). The median duration of response was 21.3 months in the VMP arm and was not reached in the D-VMP arm.
The rate of complete response was 24% in the VMP arm and 43% in the D-VMP arm (P<0.0001). Six percent of patients in the VMP arm and 22% in the D-VMP arm were negative for minimal residual disease (P<0.0001).
The hazard ratio for disease progression or death in the D-VMP arm versus the VMP arm was 0.50 (P<0.0001).
The median PFS was 18.1 months in the VMP arm and was not reached in the D-VMP arm. The 12-month PFS was 76% and 87%, respectively. And the 18-month PFS was 50% and 72%, respectively.
D-VMP prolonged PFS regardless of patient sex, age, cytogenetic risk, ECOG performance status, baseline renal function, and other factors.
The median overall survival was not reached in either treatment arm. There were 48 deaths in the VMP arm and 45 in the D-VMP arm.
Adverse events
The most common treatment-emergent adverse events (TEAEs; in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
The most common grade 3/4 TEAEs (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
There were 6 deaths due to TEAEs in the D-VMP arm and 5 such deaths in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. The most common of these was pneumonia, with rates of 11% and 4%, respectively.
Infections resolved in 88% of cases in the D-VMP arm and 87% of cases in the VMP arm. Rates of treatment discontinuation due to infection were 0.9% and 1.4%, respectively. One patient in each group stopped treatment due to pneumonia.
Twenty-eight percent of patients in the D-VMP arm had infusion-related reactions (15% grade 3 and 2% grade 4). Most of these reactions occurred during the first infusion. Five patients (1.4%) discontinued daratumumab due to infusion-related reactions. ![]()
ATLANTA—Study results “strongly support” a new standard of care for transplant-ineligible patients with newly diagnosed multiple myeloma (MM), according to a speaker at the 2017 ASH Annual Meeting.
The study, ALCYONE, suggests treatment with bortezomib, melphalan, and prednisone (VMP) can be improved by the addition of daratumumab (D).
D-VMP produced deeper responses and prolonged progression-free survival (PFS) when compared to VMP.
“In this first phase 3, randomized study with a monoclonal antibody in newly diagnosed multiple myeloma, daratumumab reduced the risk of progression or death by 50%,” said Maria-Victoria Mateos, MD, PhD, of University Hospital of Salamanca in Spain.
“No new safety signals were observed [with D-VMP], except for higher infectious events that resolved. I would say the results of this study strongly support daratumumab in combination with VMP as a standard of care in transplant-ineligible, newly diagnosed multiple myeloma.”
Dr Mateos presented results from ALCYONE as a late-breaking abstract (LBA-4) at the ASH Annual Meeting. The study was simultaneously published in NEJM. The research was supported by Janssen Research and Development.
Patients and treatment
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant.
Patients were randomized to receive VMP or D-VMP. They were stratified by International Staging System (I, II, III), region (Europe vs other), and age (<75 vs ≥75 years).
All patients received up to 9 cycles of VMP:
- Bortezomib at 1.3 mg/m2 twice weekly on weeks 1, 2, 4, and 5 of cycle 1 and once weekly on weeks 1, 2, 4, and 5 of cycles 2 through 9
- Melphalan at 9 mg/m2 once daily on days 1 to 4 of each cycle
- Prednisone at 60 mg/m2 once daily on days 1 to 4 of each cycle.
Patients in the daratumumab arm received the drug at 16 mg/kg once-weekly for the first cycle, every 3 weeks for cycles 2 to 9, and every 4 weeks thereafter, until disease progression. These patients also received dexamethasone (to manage infusion reactions) at 20 mg on the same schedule.
Baseline characteristics were similar between the VMP (n=356) and D-VMP (n=350) arms. The median age was 71 in both arms (range, 50-91 in the VMP arm and 40-93 in the D-VMP arm). Males made up 47% of the VMP arm and 46% of the D-VMP arm.
Forty-nine percent of patients in the VMP arm and 52% in the D-VMP arm had an ECOG performance status of 1. Twenty-eight percent and 22%, respectively, had a status of 0.
The median follow-up was 16.5 months (range, 0.1-28.1). At the clinical cutoff date (June 12, 2017), 5% of patients in the VMP arm were still on study treatment, as were 71% of patients in the D-VMP arm.
Response and survival
“I would like to note that the benefit of the addition of daratumumab was observed since the beginning of the treatment,” Dr Mateos said.
The overall response rate was 74% in the VMP arm and 91% in the D-VMP arm (P<0.0001). The median duration of response was 21.3 months in the VMP arm and was not reached in the D-VMP arm.
The rate of complete response was 24% in the VMP arm and 43% in the D-VMP arm (P<0.0001). Six percent of patients in the VMP arm and 22% in the D-VMP arm were negative for minimal residual disease (P<0.0001).
The hazard ratio for disease progression or death in the D-VMP arm versus the VMP arm was 0.50 (P<0.0001).
The median PFS was 18.1 months in the VMP arm and was not reached in the D-VMP arm. The 12-month PFS was 76% and 87%, respectively. And the 18-month PFS was 50% and 72%, respectively.
D-VMP prolonged PFS regardless of patient sex, age, cytogenetic risk, ECOG performance status, baseline renal function, and other factors.
The median overall survival was not reached in either treatment arm. There were 48 deaths in the VMP arm and 45 in the D-VMP arm.
Adverse events
The most common treatment-emergent adverse events (TEAEs; in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
The most common grade 3/4 TEAEs (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
There were 6 deaths due to TEAEs in the D-VMP arm and 5 such deaths in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. The most common of these was pneumonia, with rates of 11% and 4%, respectively.
Infections resolved in 88% of cases in the D-VMP arm and 87% of cases in the VMP arm. Rates of treatment discontinuation due to infection were 0.9% and 1.4%, respectively. One patient in each group stopped treatment due to pneumonia.
Twenty-eight percent of patients in the D-VMP arm had infusion-related reactions (15% grade 3 and 2% grade 4). Most of these reactions occurred during the first infusion. Five patients (1.4%) discontinued daratumumab due to infusion-related reactions. ![]()
Lichen Planus and Lichenoid Drug Eruption After Vaccination
Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.
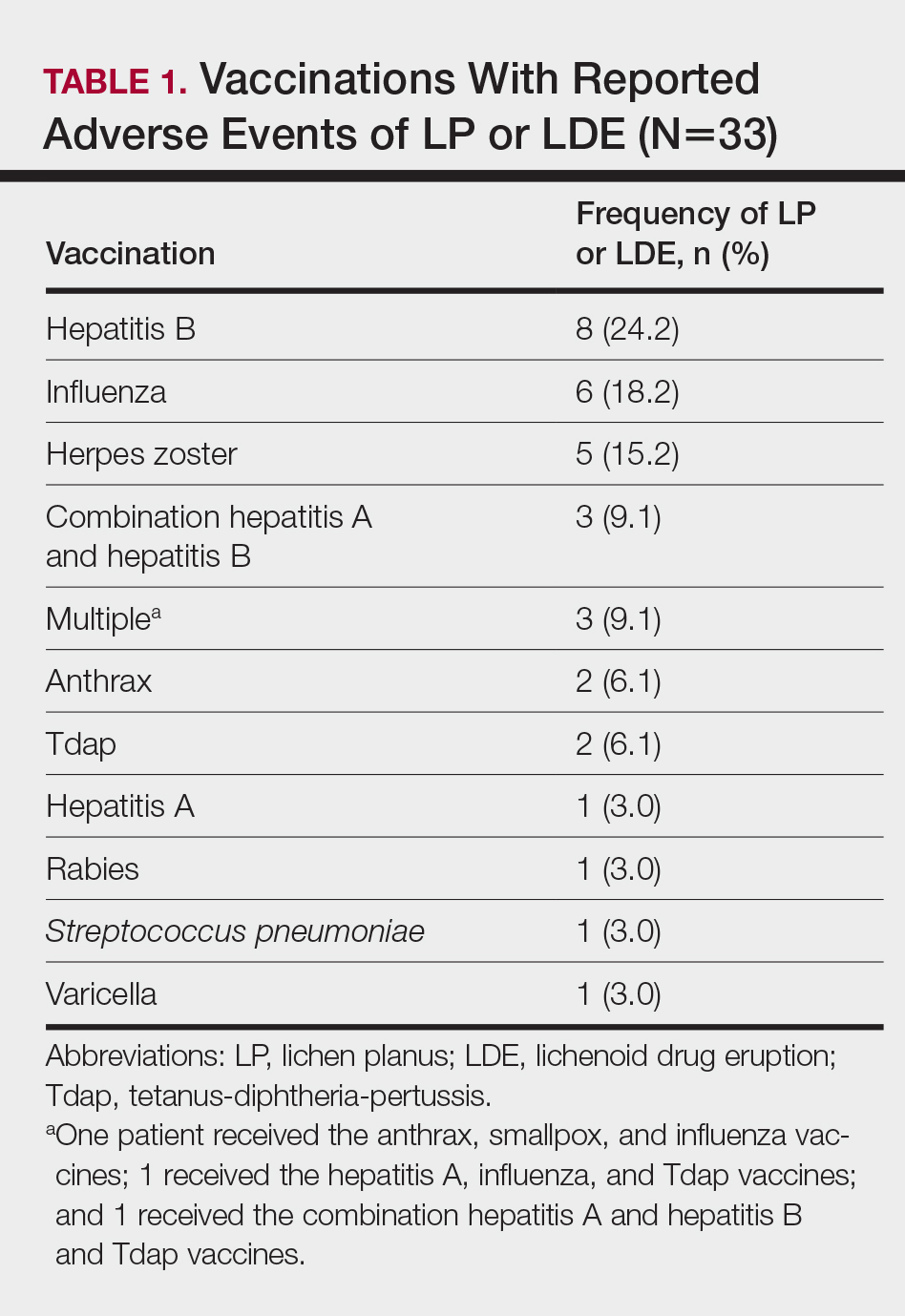
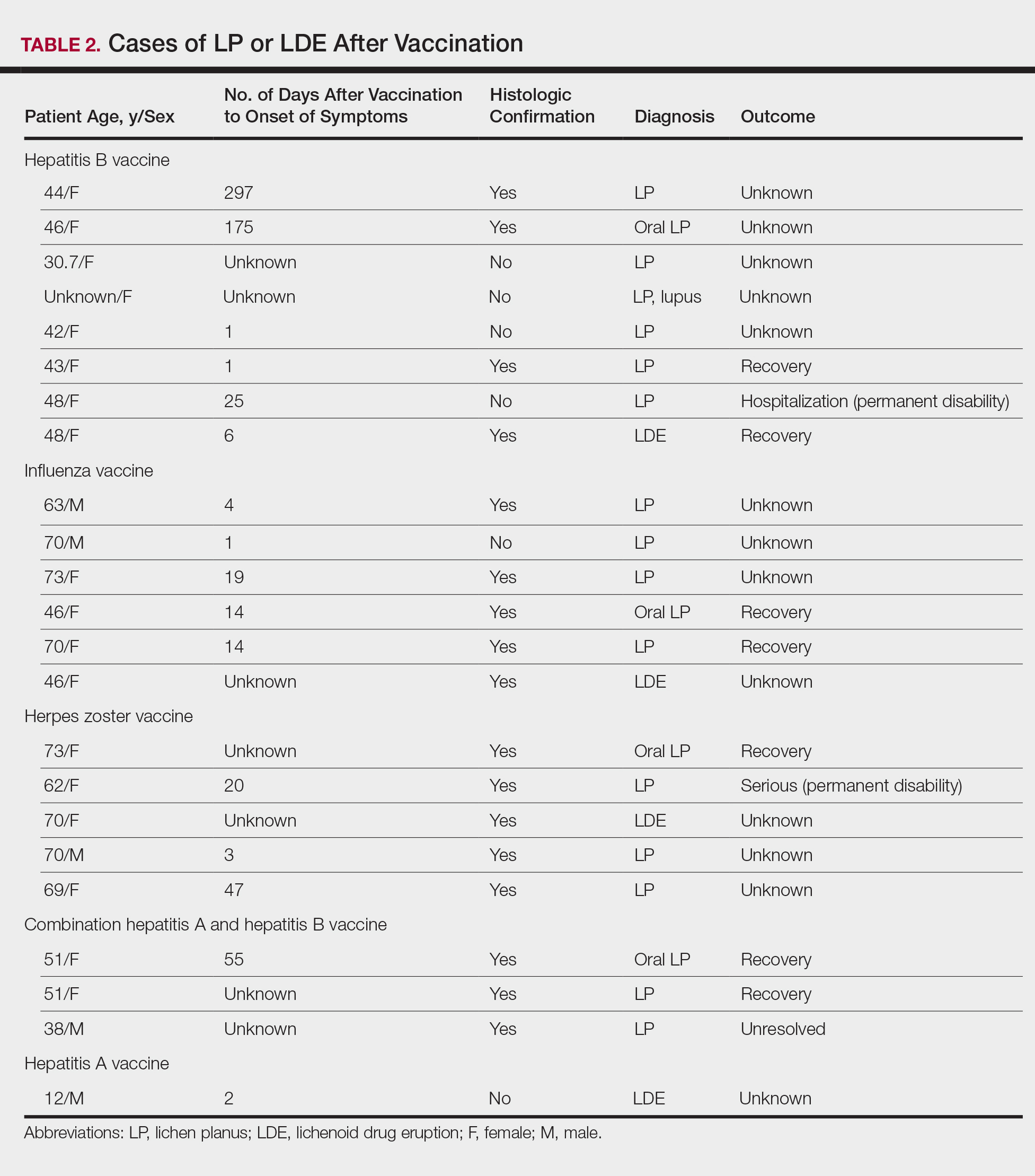
Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. O
Comment
The estimated prevalence of LP ranges from 0.22% to 5% worldwide,11-15 with an incidence of 0.032% to 0.037%.16 Although rare, LP and LDE can occur from certain medications or vaccines. Cases of LP have been reported after hepatitis B and influenza vaccinations. The first case of LP following hepatitis B vaccination was described by Ciaccio and Rebora17 in 1990. Since then, a total of 50 similar cases have been reported worldwide.2 There also have been reports of LP following influenza, tetanus-diphtheria-pertussis, measles-mumps-rubella, and inactivated polio vaccines.3,4,9,10 Table 3 summarizes cases of LP following various vaccinations.
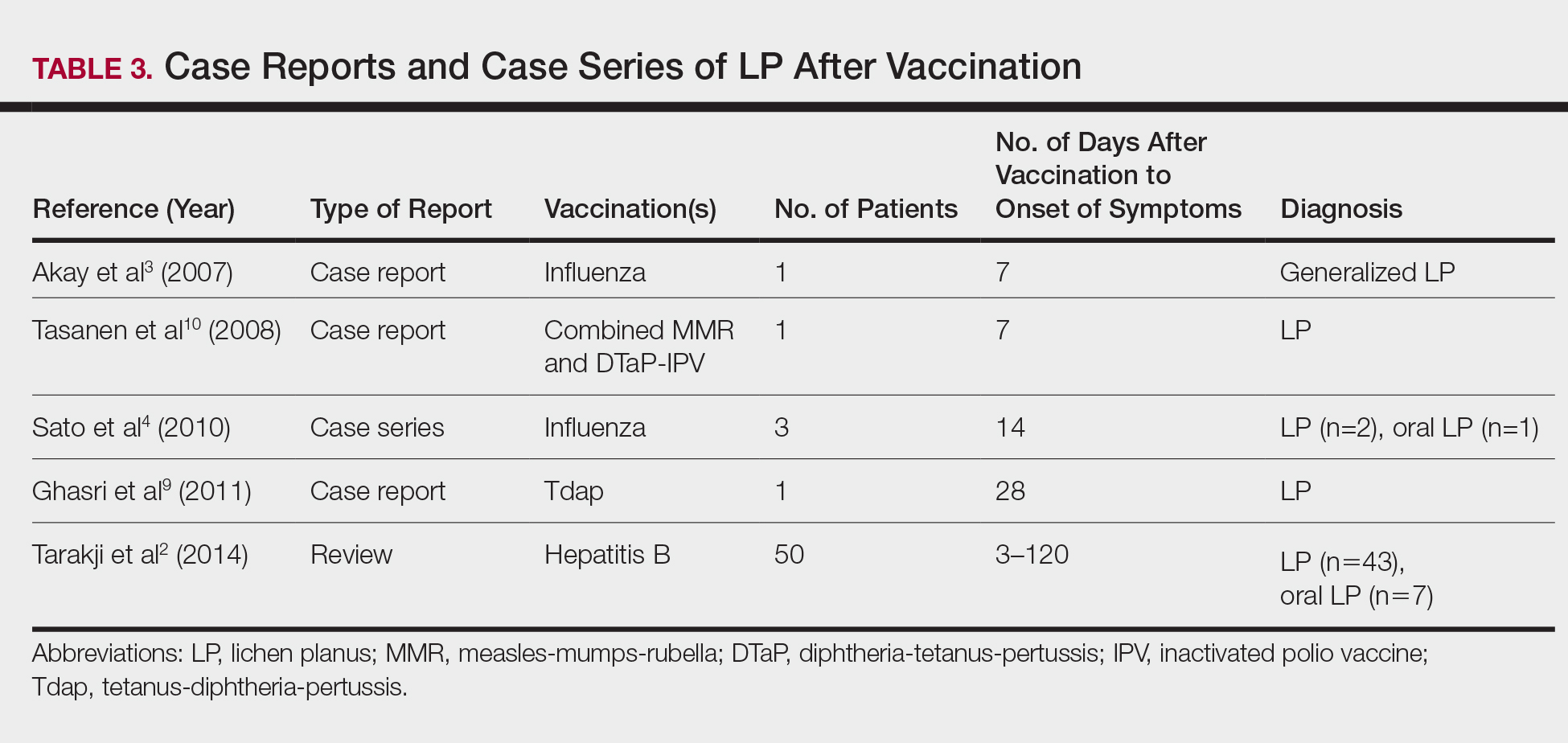
The key initiating event of the pathogenesis for both LP and LDE is not completely understood. Both conditions share similar immunologic mechanisms of persistently activated CD8 autocytotoxic T lymphocytes against epidermal cells.18 These cells can induce apoptosis of basal epidermal keratinocytes and generate various cytokines (eg, IFN-γ, IL-5) to enhance expression of class II MHC molecules and antigen presentation to CD4 T cells.19-22 It is conceivable that one of the initiating factors may be related to components in vaccines.
Hepatitis B, influenza, and herpes zoster vaccines were the 3 most common vaccines implicated in postimmunization LP or LDEs in our study. The excipients of these vaccines were compared based on the product inserts to identify any common components. It was found that all 3 vaccines contain either yeast protein or egg protein with various forms of phosphate buffers, while the hepatitis A and herpes zoster vaccines share Medical Research Council cell strain 5 (human diploid) cells as well as other cellular components.23 Sato et al4 suggested that specific vaccine components, such as the vaccine itself or egg proteins, could have contributed to the development of LP following vaccination. It has been postulated that the protein S fraction of hepatitis B surface antigen plays a crucial role in the pathogenesis of both LP and LDE after hepatitis B vaccination.2,24 It is likely that protein S shares common epitopes on keratinocytes that are recognized by the immune system, thus activating cytotoxic T lymphocytes and inducing apoptosis.2,24
In this study, the median time to onset of vaccine-related LP was 14 days, which is consistent with a case series by Sato et al,4 suggesting that adverse reactions mainly occurred within 2 weeks after influenza vaccination. Onset of symptoms within 2 weeks of vaccination would therefore be a crucial clue for diagnosing possible vaccine-related LP or LDE. On the other hand, at least 4 patients in our study had onset of LP and LDE more than 1 month after vaccination; 2 of 4 cases even reported symptom onset at 175 and 297 days after hepatitis B vaccination, which were much longer than the 120 days reported by Tarakji et al.2 It is not known if these cases constitute true vaccine-associated LP or LDE or if unmeasured confounding factors such as concurrent medications or comorbidities may have contributed to the development of these AEs.
It also is interesting to note that LP and LDE affected mainly middle-aged women. An increased risk of autoimmunity in female adults partly explains this observation.25 Some vaccines, such as herpes zoster and influenza vaccines, generally are recommended for older adults who also are more likely to have multiple comorbidities or take multiple medications/supplements, which can potentially skew the prevalence of AEs toward an older age group. It should be noted, however, that LP and LDE were relatively uncommon AEs following vaccination in the current study. In this study, LP and LDE consisted of only 0.01% (N=42,230) of all AEs after hepatitis B vaccination, while the more common AEs such as pyrexia, nonspecific rashes, nonspecific gastrointestinal symptoms, and headache contributed to approximately 66.5% of all reported events.
One of the strengths of our study is that up to two-thirds of cases were confirmed histologically and all patients were seen and followed up by dermatologists or physicians. The VAERS is an easily accessible, up-to-date, and live reporting system that collects all AEs associated with vaccines in the United States. Important clinical and laboratory information usually is available in the database; however, the main limitation is that this study can only demonstrate a possible association but not a causal relationship between vaccination and LP or LDE. There can be various sources of biases such as underreporting, overreporting, or inaccurate reporting.26,27 Pertinent clinical information (eg, new medications, new dental fillings/implants) that could potentially misrepresent the actual relationship between vaccination and development of AEs also was not available in the VAERS database. A cohort study with long-term follow-up or a large-scale case-control study would be useful in evaluating such associations.
Conclusion
Lichen planus and LDE can occur, albeit rarely, after vaccination, especially following hepatitis B vaccination. When middle-aged adults present to the clinic with LP or LDE, it is important to inquire about recent vaccination history in addition to a detailed medication history.
- Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111.
- Tarakji B, Ashok N, Alakeel R, et al. Hepatitis B vaccination and associated oral manifestations: a non-systemic review of literature and case reports. Ann Med Health Sci Res. 2014;4:829-836.
- Akay BN, Arslan A, Cekirge S, et al. The first reported case of lichen planus following inactivated influenza vaccination. J Drugs Dermatol. 2007;6:536-538.
- Sato NA, Kano Y, Shiohara T. Lichen planus occurring after influenza vaccination: report of three cases and review of the literature. Dermatology. 2010;221:296-299.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm. Updated May 23, 2016. Accessed April 4, 2017.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2013-2014. MMWR Recomm Rep. 2013;62:1-43.
- Rebora A. Hepatitis viruses and lichen planus. Arch Dermatol. 1994;130:1328-1329.
- Black MM. Lichen planus and lichenoid disorders. In: Rook A, Wilkinson DS, Ebling FJG, eds. Textbook of Dermatology. 6th ed. London, England: Blackwell Science Inc; 1998:1899-1890.
- Ghasri P, Roehmholdt BF, Young LC. A case of lichen planus following Tdap vaccination. J Drugs Dermatol. 2011;10:1067-1069.
- Tasanen K, Renko M, Kandelberg P, et al. Childhood lichen planus after simultaneous measles-mumps-rubella and diphtheria-tetanus-pertussis-polio vaccinations. Br J Dermatol. 2008;58:646-648.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo J, Rapini RP, eds. Dermatology. 2nd ed. New York, NY: Mosby Elsevier; 2008:159-180.
- Miller CS, Epstein JB, Hall EH, et al. Changing oral care needs in the United States: the continuing need for oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:34-44.
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373-381.
- Axéll T, Rundquist L. Oral lichen planus—a demographic study. Community Dent Oral Epidemiol. 1987;15:52-56.
- Alabi GO, Akinsanya JB. Lichen planus in tropical Africa. Trop Geogr Med. 1981;33:143-147.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133:985-991.
- Ciaccio M, Rebora A. Lichen planus following HBV vaccination: a coincidence? Br J Dermatol. 1990;122:424.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449-456.
- Yawalkar N, Pichler WJ. Mechanisms of cutaneous drug reactions [in German]. J Dtsch Dermatol Ges. 2004;2:1013-1023; quiz 1024-1026.
- Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299-303.
- Yawalkar N, Egli F, Hari Y, et al. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855.
- Yawalkar N, Shrikhande M, Hari Y, et al. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000;106:1171-1176.
- Grabenstein JD. Immu
noFacts 2013: Vaccines and Immunologic Drugs. St Louis, MO: Wolters Kluwer Health; 2012. - Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780.
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity [published online November 12, 2011]. J Autoimmun. 2012;38:J109-J119.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38:295-301.
- Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225-1231.
Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.


Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. O
Comment
The estimated prevalence of LP ranges from 0.22% to 5% worldwide,11-15 with an incidence of 0.032% to 0.037%.16 Although rare, LP and LDE can occur from certain medications or vaccines. Cases of LP have been reported after hepatitis B and influenza vaccinations. The first case of LP following hepatitis B vaccination was described by Ciaccio and Rebora17 in 1990. Since then, a total of 50 similar cases have been reported worldwide.2 There also have been reports of LP following influenza, tetanus-diphtheria-pertussis, measles-mumps-rubella, and inactivated polio vaccines.3,4,9,10 Table 3 summarizes cases of LP following various vaccinations.

The key initiating event of the pathogenesis for both LP and LDE is not completely understood. Both conditions share similar immunologic mechanisms of persistently activated CD8 autocytotoxic T lymphocytes against epidermal cells.18 These cells can induce apoptosis of basal epidermal keratinocytes and generate various cytokines (eg, IFN-γ, IL-5) to enhance expression of class II MHC molecules and antigen presentation to CD4 T cells.19-22 It is conceivable that one of the initiating factors may be related to components in vaccines.
Hepatitis B, influenza, and herpes zoster vaccines were the 3 most common vaccines implicated in postimmunization LP or LDEs in our study. The excipients of these vaccines were compared based on the product inserts to identify any common components. It was found that all 3 vaccines contain either yeast protein or egg protein with various forms of phosphate buffers, while the hepatitis A and herpes zoster vaccines share Medical Research Council cell strain 5 (human diploid) cells as well as other cellular components.23 Sato et al4 suggested that specific vaccine components, such as the vaccine itself or egg proteins, could have contributed to the development of LP following vaccination. It has been postulated that the protein S fraction of hepatitis B surface antigen plays a crucial role in the pathogenesis of both LP and LDE after hepatitis B vaccination.2,24 It is likely that protein S shares common epitopes on keratinocytes that are recognized by the immune system, thus activating cytotoxic T lymphocytes and inducing apoptosis.2,24
In this study, the median time to onset of vaccine-related LP was 14 days, which is consistent with a case series by Sato et al,4 suggesting that adverse reactions mainly occurred within 2 weeks after influenza vaccination. Onset of symptoms within 2 weeks of vaccination would therefore be a crucial clue for diagnosing possible vaccine-related LP or LDE. On the other hand, at least 4 patients in our study had onset of LP and LDE more than 1 month after vaccination; 2 of 4 cases even reported symptom onset at 175 and 297 days after hepatitis B vaccination, which were much longer than the 120 days reported by Tarakji et al.2 It is not known if these cases constitute true vaccine-associated LP or LDE or if unmeasured confounding factors such as concurrent medications or comorbidities may have contributed to the development of these AEs.
It also is interesting to note that LP and LDE affected mainly middle-aged women. An increased risk of autoimmunity in female adults partly explains this observation.25 Some vaccines, such as herpes zoster and influenza vaccines, generally are recommended for older adults who also are more likely to have multiple comorbidities or take multiple medications/supplements, which can potentially skew the prevalence of AEs toward an older age group. It should be noted, however, that LP and LDE were relatively uncommon AEs following vaccination in the current study. In this study, LP and LDE consisted of only 0.01% (N=42,230) of all AEs after hepatitis B vaccination, while the more common AEs such as pyrexia, nonspecific rashes, nonspecific gastrointestinal symptoms, and headache contributed to approximately 66.5% of all reported events.
One of the strengths of our study is that up to two-thirds of cases were confirmed histologically and all patients were seen and followed up by dermatologists or physicians. The VAERS is an easily accessible, up-to-date, and live reporting system that collects all AEs associated with vaccines in the United States. Important clinical and laboratory information usually is available in the database; however, the main limitation is that this study can only demonstrate a possible association but not a causal relationship between vaccination and LP or LDE. There can be various sources of biases such as underreporting, overreporting, or inaccurate reporting.26,27 Pertinent clinical information (eg, new medications, new dental fillings/implants) that could potentially misrepresent the actual relationship between vaccination and development of AEs also was not available in the VAERS database. A cohort study with long-term follow-up or a large-scale case-control study would be useful in evaluating such associations.
Conclusion
Lichen planus and LDE can occur, albeit rarely, after vaccination, especially following hepatitis B vaccination. When middle-aged adults present to the clinic with LP or LDE, it is important to inquire about recent vaccination history in addition to a detailed medication history.
Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.


Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. O
Comment
The estimated prevalence of LP ranges from 0.22% to 5% worldwide,11-15 with an incidence of 0.032% to 0.037%.16 Although rare, LP and LDE can occur from certain medications or vaccines. Cases of LP have been reported after hepatitis B and influenza vaccinations. The first case of LP following hepatitis B vaccination was described by Ciaccio and Rebora17 in 1990. Since then, a total of 50 similar cases have been reported worldwide.2 There also have been reports of LP following influenza, tetanus-diphtheria-pertussis, measles-mumps-rubella, and inactivated polio vaccines.3,4,9,10 Table 3 summarizes cases of LP following various vaccinations.

The key initiating event of the pathogenesis for both LP and LDE is not completely understood. Both conditions share similar immunologic mechanisms of persistently activated CD8 autocytotoxic T lymphocytes against epidermal cells.18 These cells can induce apoptosis of basal epidermal keratinocytes and generate various cytokines (eg, IFN-γ, IL-5) to enhance expression of class II MHC molecules and antigen presentation to CD4 T cells.19-22 It is conceivable that one of the initiating factors may be related to components in vaccines.
Hepatitis B, influenza, and herpes zoster vaccines were the 3 most common vaccines implicated in postimmunization LP or LDEs in our study. The excipients of these vaccines were compared based on the product inserts to identify any common components. It was found that all 3 vaccines contain either yeast protein or egg protein with various forms of phosphate buffers, while the hepatitis A and herpes zoster vaccines share Medical Research Council cell strain 5 (human diploid) cells as well as other cellular components.23 Sato et al4 suggested that specific vaccine components, such as the vaccine itself or egg proteins, could have contributed to the development of LP following vaccination. It has been postulated that the protein S fraction of hepatitis B surface antigen plays a crucial role in the pathogenesis of both LP and LDE after hepatitis B vaccination.2,24 It is likely that protein S shares common epitopes on keratinocytes that are recognized by the immune system, thus activating cytotoxic T lymphocytes and inducing apoptosis.2,24
In this study, the median time to onset of vaccine-related LP was 14 days, which is consistent with a case series by Sato et al,4 suggesting that adverse reactions mainly occurred within 2 weeks after influenza vaccination. Onset of symptoms within 2 weeks of vaccination would therefore be a crucial clue for diagnosing possible vaccine-related LP or LDE. On the other hand, at least 4 patients in our study had onset of LP and LDE more than 1 month after vaccination; 2 of 4 cases even reported symptom onset at 175 and 297 days after hepatitis B vaccination, which were much longer than the 120 days reported by Tarakji et al.2 It is not known if these cases constitute true vaccine-associated LP or LDE or if unmeasured confounding factors such as concurrent medications or comorbidities may have contributed to the development of these AEs.
It also is interesting to note that LP and LDE affected mainly middle-aged women. An increased risk of autoimmunity in female adults partly explains this observation.25 Some vaccines, such as herpes zoster and influenza vaccines, generally are recommended for older adults who also are more likely to have multiple comorbidities or take multiple medications/supplements, which can potentially skew the prevalence of AEs toward an older age group. It should be noted, however, that LP and LDE were relatively uncommon AEs following vaccination in the current study. In this study, LP and LDE consisted of only 0.01% (N=42,230) of all AEs after hepatitis B vaccination, while the more common AEs such as pyrexia, nonspecific rashes, nonspecific gastrointestinal symptoms, and headache contributed to approximately 66.5% of all reported events.
One of the strengths of our study is that up to two-thirds of cases were confirmed histologically and all patients were seen and followed up by dermatologists or physicians. The VAERS is an easily accessible, up-to-date, and live reporting system that collects all AEs associated with vaccines in the United States. Important clinical and laboratory information usually is available in the database; however, the main limitation is that this study can only demonstrate a possible association but not a causal relationship between vaccination and LP or LDE. There can be various sources of biases such as underreporting, overreporting, or inaccurate reporting.26,27 Pertinent clinical information (eg, new medications, new dental fillings/implants) that could potentially misrepresent the actual relationship between vaccination and development of AEs also was not available in the VAERS database. A cohort study with long-term follow-up or a large-scale case-control study would be useful in evaluating such associations.
Conclusion
Lichen planus and LDE can occur, albeit rarely, after vaccination, especially following hepatitis B vaccination. When middle-aged adults present to the clinic with LP or LDE, it is important to inquire about recent vaccination history in addition to a detailed medication history.
- Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111.
- Tarakji B, Ashok N, Alakeel R, et al. Hepatitis B vaccination and associated oral manifestations: a non-systemic review of literature and case reports. Ann Med Health Sci Res. 2014;4:829-836.
- Akay BN, Arslan A, Cekirge S, et al. The first reported case of lichen planus following inactivated influenza vaccination. J Drugs Dermatol. 2007;6:536-538.
- Sato NA, Kano Y, Shiohara T. Lichen planus occurring after influenza vaccination: report of three cases and review of the literature. Dermatology. 2010;221:296-299.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm. Updated May 23, 2016. Accessed April 4, 2017.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2013-2014. MMWR Recomm Rep. 2013;62:1-43.
- Rebora A. Hepatitis viruses and lichen planus. Arch Dermatol. 1994;130:1328-1329.
- Black MM. Lichen planus and lichenoid disorders. In: Rook A, Wilkinson DS, Ebling FJG, eds. Textbook of Dermatology. 6th ed. London, England: Blackwell Science Inc; 1998:1899-1890.
- Ghasri P, Roehmholdt BF, Young LC. A case of lichen planus following Tdap vaccination. J Drugs Dermatol. 2011;10:1067-1069.
- Tasanen K, Renko M, Kandelberg P, et al. Childhood lichen planus after simultaneous measles-mumps-rubella and diphtheria-tetanus-pertussis-polio vaccinations. Br J Dermatol. 2008;58:646-648.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo J, Rapini RP, eds. Dermatology. 2nd ed. New York, NY: Mosby Elsevier; 2008:159-180.
- Miller CS, Epstein JB, Hall EH, et al. Changing oral care needs in the United States: the continuing need for oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:34-44.
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373-381.
- Axéll T, Rundquist L. Oral lichen planus—a demographic study. Community Dent Oral Epidemiol. 1987;15:52-56.
- Alabi GO, Akinsanya JB. Lichen planus in tropical Africa. Trop Geogr Med. 1981;33:143-147.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133:985-991.
- Ciaccio M, Rebora A. Lichen planus following HBV vaccination: a coincidence? Br J Dermatol. 1990;122:424.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449-456.
- Yawalkar N, Pichler WJ. Mechanisms of cutaneous drug reactions [in German]. J Dtsch Dermatol Ges. 2004;2:1013-1023; quiz 1024-1026.
- Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299-303.
- Yawalkar N, Egli F, Hari Y, et al. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855.
- Yawalkar N, Shrikhande M, Hari Y, et al. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000;106:1171-1176.
- Grabenstein JD. Immu
noFacts 2013: Vaccines and Immunologic Drugs. St Louis, MO: Wolters Kluwer Health; 2012. - Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780.
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity [published online November 12, 2011]. J Autoimmun. 2012;38:J109-J119.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38:295-301.
- Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225-1231.
- Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111.
- Tarakji B, Ashok N, Alakeel R, et al. Hepatitis B vaccination and associated oral manifestations: a non-systemic review of literature and case reports. Ann Med Health Sci Res. 2014;4:829-836.
- Akay BN, Arslan A, Cekirge S, et al. The first reported case of lichen planus following inactivated influenza vaccination. J Drugs Dermatol. 2007;6:536-538.
- Sato NA, Kano Y, Shiohara T. Lichen planus occurring after influenza vaccination: report of three cases and review of the literature. Dermatology. 2010;221:296-299.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm. Updated May 23, 2016. Accessed April 4, 2017.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2013-2014. MMWR Recomm Rep. 2013;62:1-43.
- Rebora A. Hepatitis viruses and lichen planus. Arch Dermatol. 1994;130:1328-1329.
- Black MM. Lichen planus and lichenoid disorders. In: Rook A, Wilkinson DS, Ebling FJG, eds. Textbook of Dermatology. 6th ed. London, England: Blackwell Science Inc; 1998:1899-1890.
- Ghasri P, Roehmholdt BF, Young LC. A case of lichen planus following Tdap vaccination. J Drugs Dermatol. 2011;10:1067-1069.
- Tasanen K, Renko M, Kandelberg P, et al. Childhood lichen planus after simultaneous measles-mumps-rubella and diphtheria-tetanus-pertussis-polio vaccinations. Br J Dermatol. 2008;58:646-648.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo J, Rapini RP, eds. Dermatology. 2nd ed. New York, NY: Mosby Elsevier; 2008:159-180.
- Miller CS, Epstein JB, Hall EH, et al. Changing oral care needs in the United States: the continuing need for oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:34-44.
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373-381.
- Axéll T, Rundquist L. Oral lichen planus—a demographic study. Community Dent Oral Epidemiol. 1987;15:52-56.
- Alabi GO, Akinsanya JB. Lichen planus in tropical Africa. Trop Geogr Med. 1981;33:143-147.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133:985-991.
- Ciaccio M, Rebora A. Lichen planus following HBV vaccination: a coincidence? Br J Dermatol. 1990;122:424.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449-456.
- Yawalkar N, Pichler WJ. Mechanisms of cutaneous drug reactions [in German]. J Dtsch Dermatol Ges. 2004;2:1013-1023; quiz 1024-1026.
- Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299-303.
- Yawalkar N, Egli F, Hari Y, et al. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855.
- Yawalkar N, Shrikhande M, Hari Y, et al. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000;106:1171-1176.
- Grabenstein JD. Immu
noFacts 2013: Vaccines and Immunologic Drugs. St Louis, MO: Wolters Kluwer Health; 2012. - Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780.
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity [published online November 12, 2011]. J Autoimmun. 2012;38:J109-J119.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38:295-301.
- Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225-1231.
Practice Points
- Lichen planus (LP) and lichenoid drug eruptions (LDEs) can uncommonly occur after vaccination.
- Common vaccines associated with LP and LDEs include hepatitis B and influenza vaccinations.
- It is important to be cognizant of such reactions, especially in patients who have recently received these common vaccines.














