User login
VIDEO - New lymphoma drug approvals: Clinical use, future directions
ATLANTA – 2017 was a banner year for the approval of new drugs to treat hematologic disorders.
At a special interest session at the annual meeting of American Society of Hematology, representatives from the Food and Drug Administration joined forces with clinicians to discuss the use of the newly approved treatments in the real-world setting.
In this video interview,
“This is extremely exciting,” she said regarding the pace of new approvals for hematologic malignancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Axicabtagene ciloleucel, a CAR T-cell product approved in October for the treatment of relapsed/refractory large B-cell lymphoma in adults, is particularly interesting, she said.
“The data shows that if you look at a population of diffuse large B-cell lymphoma patients, that historically have a very poor outcome, there is definitely an impressive response rate and improved survival, compared to the natural history cohort,” said Dr. Heslop of Baylor College of Medicine, Houston.
However, while the findings are encouraging, only 30%-40% are having a durable response, she added.
“So I think there’ll be lots of efforts to try and improve the response rate by combination with other agents such as checkpoint inhibitors or other immunomodulators,” she said.
With respect to the second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib, which was approved in October for adults with mantle cell lymphoma who have been treated with at least one prior therapy, she discussed the potential for improved outcomes and the importance of looking further into its use in patients who have failed ibrutinib therapy, as well as its use in combination with other agents, such as bendamustine and rituximab early in the course of disease.
Copanlisib, a PI3 kinase inhibitor approved in September, is an addition to the armamentarium for adult patients with relapsed follicular lymphoma after two lines of previous therapy.
“It still does have some side effects, as do other drugs in this class, so I think it’s place will still need to be defined,” Dr. Heslop said.
She reported having no relevant financial disclosures.
ATLANTA – 2017 was a banner year for the approval of new drugs to treat hematologic disorders.
At a special interest session at the annual meeting of American Society of Hematology, representatives from the Food and Drug Administration joined forces with clinicians to discuss the use of the newly approved treatments in the real-world setting.
In this video interview,
“This is extremely exciting,” she said regarding the pace of new approvals for hematologic malignancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Axicabtagene ciloleucel, a CAR T-cell product approved in October for the treatment of relapsed/refractory large B-cell lymphoma in adults, is particularly interesting, she said.
“The data shows that if you look at a population of diffuse large B-cell lymphoma patients, that historically have a very poor outcome, there is definitely an impressive response rate and improved survival, compared to the natural history cohort,” said Dr. Heslop of Baylor College of Medicine, Houston.
However, while the findings are encouraging, only 30%-40% are having a durable response, she added.
“So I think there’ll be lots of efforts to try and improve the response rate by combination with other agents such as checkpoint inhibitors or other immunomodulators,” she said.
With respect to the second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib, which was approved in October for adults with mantle cell lymphoma who have been treated with at least one prior therapy, she discussed the potential for improved outcomes and the importance of looking further into its use in patients who have failed ibrutinib therapy, as well as its use in combination with other agents, such as bendamustine and rituximab early in the course of disease.
Copanlisib, a PI3 kinase inhibitor approved in September, is an addition to the armamentarium for adult patients with relapsed follicular lymphoma after two lines of previous therapy.
“It still does have some side effects, as do other drugs in this class, so I think it’s place will still need to be defined,” Dr. Heslop said.
She reported having no relevant financial disclosures.
ATLANTA – 2017 was a banner year for the approval of new drugs to treat hematologic disorders.
At a special interest session at the annual meeting of American Society of Hematology, representatives from the Food and Drug Administration joined forces with clinicians to discuss the use of the newly approved treatments in the real-world setting.
In this video interview,
“This is extremely exciting,” she said regarding the pace of new approvals for hematologic malignancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Axicabtagene ciloleucel, a CAR T-cell product approved in October for the treatment of relapsed/refractory large B-cell lymphoma in adults, is particularly interesting, she said.
“The data shows that if you look at a population of diffuse large B-cell lymphoma patients, that historically have a very poor outcome, there is definitely an impressive response rate and improved survival, compared to the natural history cohort,” said Dr. Heslop of Baylor College of Medicine, Houston.
However, while the findings are encouraging, only 30%-40% are having a durable response, she added.
“So I think there’ll be lots of efforts to try and improve the response rate by combination with other agents such as checkpoint inhibitors or other immunomodulators,” she said.
With respect to the second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib, which was approved in October for adults with mantle cell lymphoma who have been treated with at least one prior therapy, she discussed the potential for improved outcomes and the importance of looking further into its use in patients who have failed ibrutinib therapy, as well as its use in combination with other agents, such as bendamustine and rituximab early in the course of disease.
Copanlisib, a PI3 kinase inhibitor approved in September, is an addition to the armamentarium for adult patients with relapsed follicular lymphoma after two lines of previous therapy.
“It still does have some side effects, as do other drugs in this class, so I think it’s place will still need to be defined,” Dr. Heslop said.
She reported having no relevant financial disclosures.
REPORTING FROM ASH 2017
Peroneus Quartus Muscle
Take-Home Points
- PQ is easily mistaken for a PB tear.
- PQ is best identified on MRI, but commonly missed.
- For symptomatic cases, excision is the best treatment.
- Consider PQ in patients with chronic ankle pain, swelling, and/or instability.
The peroneus quartus (PQ) is an accessory muscle arising from the leg’s lateral compartment, which typically contains the peroneus longus (PL) and the peroneus brevis (PB). The many cadaveric studies that have been conducted indicate a general population prevalence ranging from 6.6% to 23%.1 Radiographic studies, including magnetic resonance imaging (MRI) and ultrasonography, have shown a similar prevalence.2 Although the PQ is asymptomatic in most cases, it may compromise the space of the superior peroneal tunnel and cause problems, including ankle pain, PB tear, subluxation of peroneal tendons, tendinous calcification, painful hypertrophy of retrotrochlear eminence, and recurrent hematomas.1,3-5 Given its differing anatomy, the PQ variously has been referred to as peroneocalcaneus externum, peroneocuboideus, long peroneal tendon, and peroneoperoneolongus.1
Although the PQ’s origin and insertion differ between subjects, the most common origin is the muscle fibers of the PB, and the most common insertion is the retrotrochlear eminence of the calcaneum.3
We report a case of peroneal tendon pathology that was initially thought to be caused primarily by impingement of a large osteochondroma on the tendons, but was later thought to be caused in part by a PQ and a split PB tendon seen only at the time of the second operation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
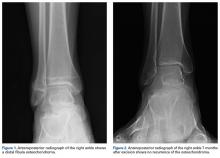
A 16-year-old boy with an osteochondroma of the right distal fibula presented to clinic with the chief complaint of lateral right ankle pain. A “sharp” pain accompanied by audible “popping” occurred with ankle motion. Medical history was significant only for non-Hodgkin lymphoma treated with bone marrow transplantation and whole body radiation at a young age. Physical examination revealed a palpable exostosis of the distal right fibula and associated ankle swelling.
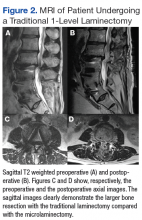
One year after surgery, the patient returned with recurring right ankle pain and audible popping during ankle movement. There was no appreciable peroneal tendon subluxation on physical examination. Repeat imaging of the ankle showed no recurrence of the osteochondroma (Figure 2).
Discussion
Absence of a PQ muscle in simian and prosimian species suggests that the PQ represents an evolutionary adaptation to evert the lateral foot and improve bipedal gait. Although the 3 peroneal (PL, PB, peroneus tertius [PT]) muscles evert the middle part of the lateral border of the foot, the PQ inserts on the retrotrochlear eminence, which everts the posterior part of the lateral border of the foot.1,6 The PQ has often been described as a variation of the PB. The PQ may also stabilize the ankle and reduce the energy required for walking. A similar functional adaptation has been proposed for the PT, which dorsiflexes at the ankle. Although presence of a PT also varies in the population, its occurrence does not correlate with presence of a PQ. In people with PQ muscles, there is an 83% to 95% incidence of also having PT muscles.7
PQ prevalence has ranged from 6.6% to 23% in cadaver studies2 and from 7% to 17% in radiologic studies.1 To better evaluate prevalence, Yammine2 performed a meta-analysis of data from 46 studies (cadaveric dissection, MRI, ultrasonography) and 3928 legs and found an overall incidence of 10.2% and a higher incidence in the Indian population than in other races. Another study found no correlation between PQ presence and sex.7
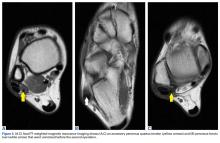
MRI is the best imaging modality for assessing for PQ but must be performed specifically for this anatomical variation. Axial images may show a fat pad separating the PQ muscle from the PB muscle.8 On imaging, a PQ muscle can be mistaken for a peroneal tendon tear. A feature that helps in distinguishing the 2 is location; the PQ typically is found posterior and medial to the PL and PB tendons, whereas PB tears are anterior to the retromalleolar groove.2 Presence of a PQ muscle may be missed on initial MRI, as occurred in our patient’s case. Zammit and Singh3 reviewed 80 leg MRIs and found 6 PQs. Only 1 of the 6 reports described the PQ as an “atypical appearance of peroneus brevis [that] appears to be made up of more than one tendon.”
Surgical excision is often adequate treatment for a symptomatic PQ. If the PQ muscle is small and symptomatic from pressure to the muscle mass, a short fasciotomy may be performed.9 More commonly, complete excision of the accessory muscle is required. Although the PQ muscle is usually asymptomatic, it should be considered in cases of chronic ankle pain, swelling, or instability; recurrent hematomas; and peroneal tendon subluxation or tears.5,7
Our patient’s diagnosis was initially overlooked because of an osteochondroma in the region of interest. It remains unclear whether his pain was caused by the PQ itself or, more likely, from the PB tear. It is thought that the accessory muscle adds bulk to the peroneal tunnel, predisposing to peroneal pathology, such as muscle tears and tendon subluxation. Regardless, advanced imaging performed before the index procedure, along with a general understanding of the PQ and its classic MRI findings, may have prevented the repeat operation in this case.
The PQ muscle is a rare but sometimes missed potential etiology of ankle pain and tendon subluxation. In our patient’s case, the most obvious abnormality, an osteochondroma, may have masked the true cause.
1. Athavale SA, Gupta V, Kotgirwar S, Singh V. The peroneus quartus muscle: clinical correlation with evolutionary importance. Anat Sci Int. 2012;87(2):106-110.
2. Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat. 2015;37(6):617-627.
3. Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br. 2003;85(8):1134-1137.
4. Kulshreshtha R, Kadri S, Rajan DT. A case of unusual combination of injuries around the lateral malleolus. Foot. 2006;16(1):51-53.
5. Donley BG, Leyes M. Peroneus quartus muscle. A rare cause of chronic lateral ankle pain. Am J Sports Med. 2001;29(3):373-375.
6. Hecker P. Study on the peroneus of the tarsus. Anat Rec. 1923;26(1):79-82.
7. Rios Nascimento SR, Watanabe Costa R, Ruiz CR, Wafae N. Analysis on the incidence of the fibularis quartus muscle using magnetic resonance imaging. Anat Res Int. 2012;(2012):485149.
8. Wang XT, Rosenberg ZS, Mechlin MB, Schweitzer ME. Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. Radiographics. 2005;25(3):587-602.
9. Martinelli B, Bernobi S. Peroneus quartus muscle and ankle pain. Foot Ankle Surg. 2002;8(3):223-225.
Take-Home Points
- PQ is easily mistaken for a PB tear.
- PQ is best identified on MRI, but commonly missed.
- For symptomatic cases, excision is the best treatment.
- Consider PQ in patients with chronic ankle pain, swelling, and/or instability.
The peroneus quartus (PQ) is an accessory muscle arising from the leg’s lateral compartment, which typically contains the peroneus longus (PL) and the peroneus brevis (PB). The many cadaveric studies that have been conducted indicate a general population prevalence ranging from 6.6% to 23%.1 Radiographic studies, including magnetic resonance imaging (MRI) and ultrasonography, have shown a similar prevalence.2 Although the PQ is asymptomatic in most cases, it may compromise the space of the superior peroneal tunnel and cause problems, including ankle pain, PB tear, subluxation of peroneal tendons, tendinous calcification, painful hypertrophy of retrotrochlear eminence, and recurrent hematomas.1,3-5 Given its differing anatomy, the PQ variously has been referred to as peroneocalcaneus externum, peroneocuboideus, long peroneal tendon, and peroneoperoneolongus.1
Although the PQ’s origin and insertion differ between subjects, the most common origin is the muscle fibers of the PB, and the most common insertion is the retrotrochlear eminence of the calcaneum.3
We report a case of peroneal tendon pathology that was initially thought to be caused primarily by impingement of a large osteochondroma on the tendons, but was later thought to be caused in part by a PQ and a split PB tendon seen only at the time of the second operation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 16-year-old boy with an osteochondroma of the right distal fibula presented to clinic with the chief complaint of lateral right ankle pain. A “sharp” pain accompanied by audible “popping” occurred with ankle motion. Medical history was significant only for non-Hodgkin lymphoma treated with bone marrow transplantation and whole body radiation at a young age. Physical examination revealed a palpable exostosis of the distal right fibula and associated ankle swelling.
One year after surgery, the patient returned with recurring right ankle pain and audible popping during ankle movement. There was no appreciable peroneal tendon subluxation on physical examination. Repeat imaging of the ankle showed no recurrence of the osteochondroma (Figure 2).
Discussion
Absence of a PQ muscle in simian and prosimian species suggests that the PQ represents an evolutionary adaptation to evert the lateral foot and improve bipedal gait. Although the 3 peroneal (PL, PB, peroneus tertius [PT]) muscles evert the middle part of the lateral border of the foot, the PQ inserts on the retrotrochlear eminence, which everts the posterior part of the lateral border of the foot.1,6 The PQ has often been described as a variation of the PB. The PQ may also stabilize the ankle and reduce the energy required for walking. A similar functional adaptation has been proposed for the PT, which dorsiflexes at the ankle. Although presence of a PT also varies in the population, its occurrence does not correlate with presence of a PQ. In people with PQ muscles, there is an 83% to 95% incidence of also having PT muscles.7
PQ prevalence has ranged from 6.6% to 23% in cadaver studies2 and from 7% to 17% in radiologic studies.1 To better evaluate prevalence, Yammine2 performed a meta-analysis of data from 46 studies (cadaveric dissection, MRI, ultrasonography) and 3928 legs and found an overall incidence of 10.2% and a higher incidence in the Indian population than in other races. Another study found no correlation between PQ presence and sex.7
MRI is the best imaging modality for assessing for PQ but must be performed specifically for this anatomical variation. Axial images may show a fat pad separating the PQ muscle from the PB muscle.8 On imaging, a PQ muscle can be mistaken for a peroneal tendon tear. A feature that helps in distinguishing the 2 is location; the PQ typically is found posterior and medial to the PL and PB tendons, whereas PB tears are anterior to the retromalleolar groove.2 Presence of a PQ muscle may be missed on initial MRI, as occurred in our patient’s case. Zammit and Singh3 reviewed 80 leg MRIs and found 6 PQs. Only 1 of the 6 reports described the PQ as an “atypical appearance of peroneus brevis [that] appears to be made up of more than one tendon.”
Surgical excision is often adequate treatment for a symptomatic PQ. If the PQ muscle is small and symptomatic from pressure to the muscle mass, a short fasciotomy may be performed.9 More commonly, complete excision of the accessory muscle is required. Although the PQ muscle is usually asymptomatic, it should be considered in cases of chronic ankle pain, swelling, or instability; recurrent hematomas; and peroneal tendon subluxation or tears.5,7
Our patient’s diagnosis was initially overlooked because of an osteochondroma in the region of interest. It remains unclear whether his pain was caused by the PQ itself or, more likely, from the PB tear. It is thought that the accessory muscle adds bulk to the peroneal tunnel, predisposing to peroneal pathology, such as muscle tears and tendon subluxation. Regardless, advanced imaging performed before the index procedure, along with a general understanding of the PQ and its classic MRI findings, may have prevented the repeat operation in this case.
The PQ muscle is a rare but sometimes missed potential etiology of ankle pain and tendon subluxation. In our patient’s case, the most obvious abnormality, an osteochondroma, may have masked the true cause.
Take-Home Points
- PQ is easily mistaken for a PB tear.
- PQ is best identified on MRI, but commonly missed.
- For symptomatic cases, excision is the best treatment.
- Consider PQ in patients with chronic ankle pain, swelling, and/or instability.
The peroneus quartus (PQ) is an accessory muscle arising from the leg’s lateral compartment, which typically contains the peroneus longus (PL) and the peroneus brevis (PB). The many cadaveric studies that have been conducted indicate a general population prevalence ranging from 6.6% to 23%.1 Radiographic studies, including magnetic resonance imaging (MRI) and ultrasonography, have shown a similar prevalence.2 Although the PQ is asymptomatic in most cases, it may compromise the space of the superior peroneal tunnel and cause problems, including ankle pain, PB tear, subluxation of peroneal tendons, tendinous calcification, painful hypertrophy of retrotrochlear eminence, and recurrent hematomas.1,3-5 Given its differing anatomy, the PQ variously has been referred to as peroneocalcaneus externum, peroneocuboideus, long peroneal tendon, and peroneoperoneolongus.1
Although the PQ’s origin and insertion differ between subjects, the most common origin is the muscle fibers of the PB, and the most common insertion is the retrotrochlear eminence of the calcaneum.3
We report a case of peroneal tendon pathology that was initially thought to be caused primarily by impingement of a large osteochondroma on the tendons, but was later thought to be caused in part by a PQ and a split PB tendon seen only at the time of the second operation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 16-year-old boy with an osteochondroma of the right distal fibula presented to clinic with the chief complaint of lateral right ankle pain. A “sharp” pain accompanied by audible “popping” occurred with ankle motion. Medical history was significant only for non-Hodgkin lymphoma treated with bone marrow transplantation and whole body radiation at a young age. Physical examination revealed a palpable exostosis of the distal right fibula and associated ankle swelling.
One year after surgery, the patient returned with recurring right ankle pain and audible popping during ankle movement. There was no appreciable peroneal tendon subluxation on physical examination. Repeat imaging of the ankle showed no recurrence of the osteochondroma (Figure 2).
Discussion
Absence of a PQ muscle in simian and prosimian species suggests that the PQ represents an evolutionary adaptation to evert the lateral foot and improve bipedal gait. Although the 3 peroneal (PL, PB, peroneus tertius [PT]) muscles evert the middle part of the lateral border of the foot, the PQ inserts on the retrotrochlear eminence, which everts the posterior part of the lateral border of the foot.1,6 The PQ has often been described as a variation of the PB. The PQ may also stabilize the ankle and reduce the energy required for walking. A similar functional adaptation has been proposed for the PT, which dorsiflexes at the ankle. Although presence of a PT also varies in the population, its occurrence does not correlate with presence of a PQ. In people with PQ muscles, there is an 83% to 95% incidence of also having PT muscles.7
PQ prevalence has ranged from 6.6% to 23% in cadaver studies2 and from 7% to 17% in radiologic studies.1 To better evaluate prevalence, Yammine2 performed a meta-analysis of data from 46 studies (cadaveric dissection, MRI, ultrasonography) and 3928 legs and found an overall incidence of 10.2% and a higher incidence in the Indian population than in other races. Another study found no correlation between PQ presence and sex.7
MRI is the best imaging modality for assessing for PQ but must be performed specifically for this anatomical variation. Axial images may show a fat pad separating the PQ muscle from the PB muscle.8 On imaging, a PQ muscle can be mistaken for a peroneal tendon tear. A feature that helps in distinguishing the 2 is location; the PQ typically is found posterior and medial to the PL and PB tendons, whereas PB tears are anterior to the retromalleolar groove.2 Presence of a PQ muscle may be missed on initial MRI, as occurred in our patient’s case. Zammit and Singh3 reviewed 80 leg MRIs and found 6 PQs. Only 1 of the 6 reports described the PQ as an “atypical appearance of peroneus brevis [that] appears to be made up of more than one tendon.”
Surgical excision is often adequate treatment for a symptomatic PQ. If the PQ muscle is small and symptomatic from pressure to the muscle mass, a short fasciotomy may be performed.9 More commonly, complete excision of the accessory muscle is required. Although the PQ muscle is usually asymptomatic, it should be considered in cases of chronic ankle pain, swelling, or instability; recurrent hematomas; and peroneal tendon subluxation or tears.5,7
Our patient’s diagnosis was initially overlooked because of an osteochondroma in the region of interest. It remains unclear whether his pain was caused by the PQ itself or, more likely, from the PB tear. It is thought that the accessory muscle adds bulk to the peroneal tunnel, predisposing to peroneal pathology, such as muscle tears and tendon subluxation. Regardless, advanced imaging performed before the index procedure, along with a general understanding of the PQ and its classic MRI findings, may have prevented the repeat operation in this case.
The PQ muscle is a rare but sometimes missed potential etiology of ankle pain and tendon subluxation. In our patient’s case, the most obvious abnormality, an osteochondroma, may have masked the true cause.
1. Athavale SA, Gupta V, Kotgirwar S, Singh V. The peroneus quartus muscle: clinical correlation with evolutionary importance. Anat Sci Int. 2012;87(2):106-110.
2. Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat. 2015;37(6):617-627.
3. Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br. 2003;85(8):1134-1137.
4. Kulshreshtha R, Kadri S, Rajan DT. A case of unusual combination of injuries around the lateral malleolus. Foot. 2006;16(1):51-53.
5. Donley BG, Leyes M. Peroneus quartus muscle. A rare cause of chronic lateral ankle pain. Am J Sports Med. 2001;29(3):373-375.
6. Hecker P. Study on the peroneus of the tarsus. Anat Rec. 1923;26(1):79-82.
7. Rios Nascimento SR, Watanabe Costa R, Ruiz CR, Wafae N. Analysis on the incidence of the fibularis quartus muscle using magnetic resonance imaging. Anat Res Int. 2012;(2012):485149.
8. Wang XT, Rosenberg ZS, Mechlin MB, Schweitzer ME. Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. Radiographics. 2005;25(3):587-602.
9. Martinelli B, Bernobi S. Peroneus quartus muscle and ankle pain. Foot Ankle Surg. 2002;8(3):223-225.
1. Athavale SA, Gupta V, Kotgirwar S, Singh V. The peroneus quartus muscle: clinical correlation with evolutionary importance. Anat Sci Int. 2012;87(2):106-110.
2. Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat. 2015;37(6):617-627.
3. Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br. 2003;85(8):1134-1137.
4. Kulshreshtha R, Kadri S, Rajan DT. A case of unusual combination of injuries around the lateral malleolus. Foot. 2006;16(1):51-53.
5. Donley BG, Leyes M. Peroneus quartus muscle. A rare cause of chronic lateral ankle pain. Am J Sports Med. 2001;29(3):373-375.
6. Hecker P. Study on the peroneus of the tarsus. Anat Rec. 1923;26(1):79-82.
7. Rios Nascimento SR, Watanabe Costa R, Ruiz CR, Wafae N. Analysis on the incidence of the fibularis quartus muscle using magnetic resonance imaging. Anat Res Int. 2012;(2012):485149.
8. Wang XT, Rosenberg ZS, Mechlin MB, Schweitzer ME. Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. Radiographics. 2005;25(3):587-602.
9. Martinelli B, Bernobi S. Peroneus quartus muscle and ankle pain. Foot Ankle Surg. 2002;8(3):223-225.
Here’s what’s trending at SHM – Dec. 2017
State of Hospital Medicine Survey opens next month!
The 2018 State of Hospital Medicine Survey will begin in January and last through March with the release of the report in September 2018. Whether you are in a hospital medicine group in an academic or community setting, employed by a hospital or health system, a management company, a private group, or you serve adult or pediatric patients (or both), we need your participation.
Help us help you have the most comprehensive, up-to-date landscape of hospital medicine at your fingertips by participating. As a thank-you for your participation, your group will receive a free copy of the report. Sign up at hospitalmedicine.org/survey.
Apply for SHM’s MARQUIS Med Rec Collaborative kicking off in February 2018
SHM’s MARQUIS Med Rec Collaborative is designed to help hospitals across the United States implement evidence-based best practice medication reconciliation process change and improvement. The collaborative is a 14-month program, spanning from prelaunch to completion.
The staff has the expertise and experience of having completed two previous mentored implementation studies, involving 23 sites and thousands of patients. The collaborative also offers numerous resources, including training materials, project management and process improvement tools for hospitals to use to adapt for their needs to improve the medication reconciliation process. Visit hospitalmedicine.org/MARQUISrecruit to learn more.
Get engaged with public policy
Health care legislation is constantly evolving, and hospitalists play an important role in advocating for hospitalized patients and the hospital medicine movement. SHM is an active voice in many conversations on policy development and reform. Visit hospitalmedicine.org and sign up for the Grassroots Network to stay updated on developments in health care policy, share your experiences with health care programs and participate in policy forums.
Develop your career at Hospital Medicine 2018
Don’t miss SHM’s Annual Conference, Hospital Medicine 2018, to be held April 8-11, 2018. in Orlando. This year, the program was created to help you develop your hospital medicine career, no matter what stage you are in. Two new tracks include Seasoning Your Career and the Career Development Workshops.
Seasoning Your Career focuses on didactics designed to augment those committed to a career in hospital medicine, including topics such as career growth and development, resiliency, work-life balance, and how practical work matters such as schedules affect your career.
The new Career Development Workshops track includes six sessions that aim to help you use skills that will advance your career, such as: Leadership Essentials for Success in Hospital Medicine; Being Female in Hospital Medicine: Overcoming Individual and Institutional Barriers in the Workplace; Do You Have a Minute to Talk? Peer-to-Peer Feedback, and more.
Just starting out in hospital medicine? Back by popular demand, The Early-Career Hospitalists track has been designed for new hospitalists, resident physicians, and medical students interested in pursuing a career in hospital medicine. Designed by SHM’s Physicians-in-Training Committee, which includes nationally recognized hospitalists with expertise in scholarship, career development and medical education, this track aims to inspire future hospitalist leaders.
Visit shmannualconference.org/schedule to learn more.
Two new modules debut on SHM’s Learning Portal
SHM members have access to free continuing medical education (CME) and Maintenance of Certification (MOC) points with the SHM Learning Portal. Don’t miss two new modules: Role of the Medical Consultant and Anesthesia for Internists.
Medical consultation is an important clinical component for most hospitalists. Today, hospitalists also are asked to provide both “curbside” advice and more comprehensive comanagement of medical problems. Hospitalists who are effective consultants communicate skillfully and act professionally. The Role of the Medical Consultant module describes the different roles that hospitalists can perform as medical consultants and provides strategies for improving communications and referring physician satisfaction.
Looking for up-to-date information about surgical anesthesia? The Anesthesia for Internists module discusses the basic forms of surgical anesthesia and contraindications to each, as well as the most commonly used anesthetic drugs, their mechanisms of actions, and side effects.
Both modules are free for SHM members and $45.00 per module for nonmembers. Earn 2 AMA PRA Category 1 Credits™ and 2 MOC points per each module. Visit shmlearningportal.org to get started today.
Not a member? Join the movement today
More than 15,000 members have joined SHM to show their commitment to revolutionizing patient care. As a member, you will be connected with a wealth of opportunities designed to help you grow professionally, network with colleagues nationwide, and shape the practice of hospital medicine. See a full list of member benefits or become a member today at hospitalmedicine.org/join.
Join a chapter and connect to your local hospital medicine community
SHM hosts more than 50 local chapters nationwide to encourage networking, collaboration, and innovation within the hospital medicine community. Getting involved with your local chapter allows you to share knowledge, engage with colleagues, and stay current on the latest developments in hospital medicine.
Visit hospitalmedicine.org/chapters to find a chapter in your area.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
State of Hospital Medicine Survey opens next month!
The 2018 State of Hospital Medicine Survey will begin in January and last through March with the release of the report in September 2018. Whether you are in a hospital medicine group in an academic or community setting, employed by a hospital or health system, a management company, a private group, or you serve adult or pediatric patients (or both), we need your participation.
Help us help you have the most comprehensive, up-to-date landscape of hospital medicine at your fingertips by participating. As a thank-you for your participation, your group will receive a free copy of the report. Sign up at hospitalmedicine.org/survey.
Apply for SHM’s MARQUIS Med Rec Collaborative kicking off in February 2018
SHM’s MARQUIS Med Rec Collaborative is designed to help hospitals across the United States implement evidence-based best practice medication reconciliation process change and improvement. The collaborative is a 14-month program, spanning from prelaunch to completion.
The staff has the expertise and experience of having completed two previous mentored implementation studies, involving 23 sites and thousands of patients. The collaborative also offers numerous resources, including training materials, project management and process improvement tools for hospitals to use to adapt for their needs to improve the medication reconciliation process. Visit hospitalmedicine.org/MARQUISrecruit to learn more.
Get engaged with public policy
Health care legislation is constantly evolving, and hospitalists play an important role in advocating for hospitalized patients and the hospital medicine movement. SHM is an active voice in many conversations on policy development and reform. Visit hospitalmedicine.org and sign up for the Grassroots Network to stay updated on developments in health care policy, share your experiences with health care programs and participate in policy forums.
Develop your career at Hospital Medicine 2018
Don’t miss SHM’s Annual Conference, Hospital Medicine 2018, to be held April 8-11, 2018. in Orlando. This year, the program was created to help you develop your hospital medicine career, no matter what stage you are in. Two new tracks include Seasoning Your Career and the Career Development Workshops.
Seasoning Your Career focuses on didactics designed to augment those committed to a career in hospital medicine, including topics such as career growth and development, resiliency, work-life balance, and how practical work matters such as schedules affect your career.
The new Career Development Workshops track includes six sessions that aim to help you use skills that will advance your career, such as: Leadership Essentials for Success in Hospital Medicine; Being Female in Hospital Medicine: Overcoming Individual and Institutional Barriers in the Workplace; Do You Have a Minute to Talk? Peer-to-Peer Feedback, and more.
Just starting out in hospital medicine? Back by popular demand, The Early-Career Hospitalists track has been designed for new hospitalists, resident physicians, and medical students interested in pursuing a career in hospital medicine. Designed by SHM’s Physicians-in-Training Committee, which includes nationally recognized hospitalists with expertise in scholarship, career development and medical education, this track aims to inspire future hospitalist leaders.
Visit shmannualconference.org/schedule to learn more.
Two new modules debut on SHM’s Learning Portal
SHM members have access to free continuing medical education (CME) and Maintenance of Certification (MOC) points with the SHM Learning Portal. Don’t miss two new modules: Role of the Medical Consultant and Anesthesia for Internists.
Medical consultation is an important clinical component for most hospitalists. Today, hospitalists also are asked to provide both “curbside” advice and more comprehensive comanagement of medical problems. Hospitalists who are effective consultants communicate skillfully and act professionally. The Role of the Medical Consultant module describes the different roles that hospitalists can perform as medical consultants and provides strategies for improving communications and referring physician satisfaction.
Looking for up-to-date information about surgical anesthesia? The Anesthesia for Internists module discusses the basic forms of surgical anesthesia and contraindications to each, as well as the most commonly used anesthetic drugs, their mechanisms of actions, and side effects.
Both modules are free for SHM members and $45.00 per module for nonmembers. Earn 2 AMA PRA Category 1 Credits™ and 2 MOC points per each module. Visit shmlearningportal.org to get started today.
Not a member? Join the movement today
More than 15,000 members have joined SHM to show their commitment to revolutionizing patient care. As a member, you will be connected with a wealth of opportunities designed to help you grow professionally, network with colleagues nationwide, and shape the practice of hospital medicine. See a full list of member benefits or become a member today at hospitalmedicine.org/join.
Join a chapter and connect to your local hospital medicine community
SHM hosts more than 50 local chapters nationwide to encourage networking, collaboration, and innovation within the hospital medicine community. Getting involved with your local chapter allows you to share knowledge, engage with colleagues, and stay current on the latest developments in hospital medicine.
Visit hospitalmedicine.org/chapters to find a chapter in your area.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
State of Hospital Medicine Survey opens next month!
The 2018 State of Hospital Medicine Survey will begin in January and last through March with the release of the report in September 2018. Whether you are in a hospital medicine group in an academic or community setting, employed by a hospital or health system, a management company, a private group, or you serve adult or pediatric patients (or both), we need your participation.
Help us help you have the most comprehensive, up-to-date landscape of hospital medicine at your fingertips by participating. As a thank-you for your participation, your group will receive a free copy of the report. Sign up at hospitalmedicine.org/survey.
Apply for SHM’s MARQUIS Med Rec Collaborative kicking off in February 2018
SHM’s MARQUIS Med Rec Collaborative is designed to help hospitals across the United States implement evidence-based best practice medication reconciliation process change and improvement. The collaborative is a 14-month program, spanning from prelaunch to completion.
The staff has the expertise and experience of having completed two previous mentored implementation studies, involving 23 sites and thousands of patients. The collaborative also offers numerous resources, including training materials, project management and process improvement tools for hospitals to use to adapt for their needs to improve the medication reconciliation process. Visit hospitalmedicine.org/MARQUISrecruit to learn more.
Get engaged with public policy
Health care legislation is constantly evolving, and hospitalists play an important role in advocating for hospitalized patients and the hospital medicine movement. SHM is an active voice in many conversations on policy development and reform. Visit hospitalmedicine.org and sign up for the Grassroots Network to stay updated on developments in health care policy, share your experiences with health care programs and participate in policy forums.
Develop your career at Hospital Medicine 2018
Don’t miss SHM’s Annual Conference, Hospital Medicine 2018, to be held April 8-11, 2018. in Orlando. This year, the program was created to help you develop your hospital medicine career, no matter what stage you are in. Two new tracks include Seasoning Your Career and the Career Development Workshops.
Seasoning Your Career focuses on didactics designed to augment those committed to a career in hospital medicine, including topics such as career growth and development, resiliency, work-life balance, and how practical work matters such as schedules affect your career.
The new Career Development Workshops track includes six sessions that aim to help you use skills that will advance your career, such as: Leadership Essentials for Success in Hospital Medicine; Being Female in Hospital Medicine: Overcoming Individual and Institutional Barriers in the Workplace; Do You Have a Minute to Talk? Peer-to-Peer Feedback, and more.
Just starting out in hospital medicine? Back by popular demand, The Early-Career Hospitalists track has been designed for new hospitalists, resident physicians, and medical students interested in pursuing a career in hospital medicine. Designed by SHM’s Physicians-in-Training Committee, which includes nationally recognized hospitalists with expertise in scholarship, career development and medical education, this track aims to inspire future hospitalist leaders.
Visit shmannualconference.org/schedule to learn more.
Two new modules debut on SHM’s Learning Portal
SHM members have access to free continuing medical education (CME) and Maintenance of Certification (MOC) points with the SHM Learning Portal. Don’t miss two new modules: Role of the Medical Consultant and Anesthesia for Internists.
Medical consultation is an important clinical component for most hospitalists. Today, hospitalists also are asked to provide both “curbside” advice and more comprehensive comanagement of medical problems. Hospitalists who are effective consultants communicate skillfully and act professionally. The Role of the Medical Consultant module describes the different roles that hospitalists can perform as medical consultants and provides strategies for improving communications and referring physician satisfaction.
Looking for up-to-date information about surgical anesthesia? The Anesthesia for Internists module discusses the basic forms of surgical anesthesia and contraindications to each, as well as the most commonly used anesthetic drugs, their mechanisms of actions, and side effects.
Both modules are free for SHM members and $45.00 per module for nonmembers. Earn 2 AMA PRA Category 1 Credits™ and 2 MOC points per each module. Visit shmlearningportal.org to get started today.
Not a member? Join the movement today
More than 15,000 members have joined SHM to show their commitment to revolutionizing patient care. As a member, you will be connected with a wealth of opportunities designed to help you grow professionally, network with colleagues nationwide, and shape the practice of hospital medicine. See a full list of member benefits or become a member today at hospitalmedicine.org/join.
Join a chapter and connect to your local hospital medicine community
SHM hosts more than 50 local chapters nationwide to encourage networking, collaboration, and innovation within the hospital medicine community. Getting involved with your local chapter allows you to share knowledge, engage with colleagues, and stay current on the latest developments in hospital medicine.
Visit hospitalmedicine.org/chapters to find a chapter in your area.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Characterization and Surgical Management of Metastatic Disease of the Tibia
Take-Home Points
- Metastatic disease of the tibia is a rare but significant event in a subset of patients.
- Cancer histologies with historically “acral” spread may not apply to tibial disease.
- Patients with leg pain and any cancer diagnosis should be worked up for tibial metastases.
- Tibial disease is probably a late manifestation, and early detection may indicate late diagnosis of malignancy.
- The ultimate surgical plan for these patients should be a patient-centered multidisciplinary decision making process.
Metastatic dissemination to bones is common in advanced cancer stages and affects the axial and appendicular skeleton.1-4 The appendicular skeleton bones most often involved are the proximal femur and the proximal humerus.5,6 The tibia is involved third most often but is comparatively rarely affected.4-6 Metastatic involvement distal to the knee or elbow is more typical of advanced disease.1,3 Distal appendicular lesions are called acral metastases, but the term is inconsistently used and may refer to lesions either distal to the knee and elbow or distal to the ankle and wrist. Regardless of terminology, tibia lesions are uncommon and not well described.1,4,7,8
The tibia is the primary weight-bearing leg bone. Metastatic tibia lesions may cause pain and instability and impair mobility. Although distal skeletal dissemination often presents late in advanced disease in patients with relatively poor prognoses, in some cases early surgical intervention is indicated for pain relief, increased mobility, and improved quality of life.4,8-10
Materials and Methods
Our Institutional Review Board approved this single-institution retrospective study. We used proprietary research software (Clinical Looking Glass) to identify eligible patients treated between 2000 and 2013. The software was used to search all radiology and pathology reports for the term tibia or any variation (eg, tibial) and metastasis or any variation (eg, metastatic). The software was then used to search by Current Procedural Terminology code for any patients treated with intramedullary nail (IMN) or another tibial fixation method. This list was cross-referenced with the list of patients originally identified to help ensure that all eligible patients were identified.
Inclusion criteria were known malignancy and imaging or biopsy evidence of a metastatic tibia lesion. Treatment strategies for patients with metastatic disease and patients with multiple myeloma are sometimes considered together because of similar goals and methodologies. We specifically excluded patients with multiple myeloma in order to more accurately characterize the natural history of metastatic disease and the timing of metastatic development and to report on a more homogeneous population. Patients were excluded if their electronic medical records were inadequate in establishing a diagnosis.
Demographic and pathology data were collected directly from the institutional electronic medical records system. Dr. Geller and Dr. Greenbaum used Centricity software (General Electric Healthcare) to review all imaging on medical diagnostic display monitors. If their interpretation differed from that in the radiology report, or if clarification was needed, the study was sent to Dr. Thornhill, the institution’s director of musculoskeletal radiology, for review and interpretation. Investigated radiographic characteristics included location, cortical breakthrough, presence of fracture, and size (if advanced imaging was available). Surgical interventions were recorded from reviews of operative reports and postoperative imaging studies.
Time to metastasis was defined as number of days from diagnosis of malignancy to diagnosis of tibial osseous spread. Date of diagnosis of malignancy was the date that a biopsy or other confirmatory test was performed. In cases in which that date was unavailable, an imaging study consistent with disease or a clinical note documenting the known diagnosis date was used instead. When only month and year (ie, not an exact date) of diagnosis were available, the 15th of the month was used as an estimate. Of the 36 patients, 4 had records insufficient for establishing date of diagnosis. The first date of any imaging study confirming (or suggestive of) a metastatic lesion of the tibia was used as the date of tibial metastasis.
Many patients had osseous lesions at sites other than the tibiae. These lesions were noted on review of imaging studies, screening examinations, and physicians’ clinical notes. Widespread disease was defined as including both axial and appendicular lesions, and lesions of the tibiae.
Tibia lesion presentation was recorded as either symptomatic or incidental. If the tibiae were imaged for pain, including posttraumatic pain, the presentation was symptomatic. If a lesion was identified on staging examination (eg, bone or positron emission tomography scan), or if the tibiae were imaged for another reason, the presentation was incidental.
Results
Demographics
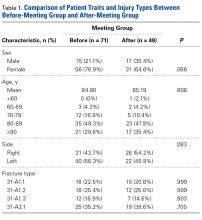
Thirty-six patients had 43 affected tibiae. Sixteen male patients (44.4% of the total) had 19 (44.2%) of the affected tibiae, and 20 female patients (55.6%) had the other 24 affected tibiae (55.8%). Mean age was 63.5 years for all patients (range, 6-95 years), 68.1 years for males, and 59.8 years for females. Of the 36 patients, 32 (88.9%) were over age 40 years (Table). All patients had radiographic evidence of ≥1 tibia lesion, and 6 (16.7%) also had biopsy-proven metastatic disease of the tibia.
Tumor Characteristics
There were 12 different primary neoplasms (Table). The most common were prostate cancer (7 patients, 19.4%; 10 tibiae, 23.3%), breast cancer (7 patients, 19.4%; 9 tibiae, 20.9%), and lung cancer (7 patients, 19.4%; 7 tibiae, 16.3%). For males, the most common diagnoses were prostate cancer (7 cases, 43.8% of males) and diffuse large B-cell lymphoma and lung cancer (3 cases and 18.8% of males each). For females, the most common diagnoses were breast cancer (7 cases, 35.0% of females) and lung cancer (4 cases, 20.0% of females).
Most of the lesions were proximal (31 tibiae, 72.1%), followed by diaphyseal (7, 16.3%) and distal (2, 4.7%) (Table). Three tibiae (7.0%) were entirely involved, but 1 of these was more affected at the distal end. One tibia had 2 lesions, 1 proximal and 1 distal.
Time to Metastasis, Other Osseous Disease
Mean time from diagnosis of malignancy to diagnosis of osseous disease of the tibia was 1282 days (range, 0-3708 days) (Table). Of the 36 patients, 32 (88.9%) had other metastatic lesions, 3 (8.3%) had isolated tibia lesions, and 1 (2.8%) had a medical record insufficient for establishing lesion status (isolated or not). Of the 32 patients with known other osseous metastases, 14 (43.8%) had widespread (axial and additional appendicular) disease, and 3 (9.4%) had additional lesions only distal to the identified tibial metastases.
Clinical Presentation
Of the 36 lesions, 18 (50%) were asymptomatic and were found on screening examinations, 17 (47.2%) presented with pain, and 1 (2.8%) had a presentation that could not be determined from the medical record (Table). Of the 17 painful lesions, 3 (17.6%) were found after a trauma brought attention to the site, and the other 14 (82.4%) were atraumatic in origin.
Of the 10 patients with cortical breakthrough, 8 (80%) had painful lesions, 1 (10%) had a lesion that was found on screening examination, and 1 (10%) had a medical record insufficient for establishing clinical presentation. Of the 8 patients who underwent surgical stabilization, 6 (75%) had painful lesions. Only 1 patient with an asymptomatic tibia lesion underwent surgical intervention (total knee arthroplasty).
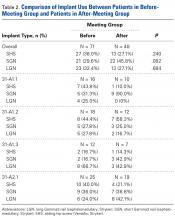
Surgical Intervention
Two patients (5.6%) with affected tibiae (4.8%) had pathologic fractures. One fracture (non-small cell lung cancer) was treated with open reduction and internal fixation (periarticular locking plate with cement augmentation), and the other (urothelial cancer) was treated with IMN fixation.
Ten patients (27.8%) with affected tibiae (23.8%) had radiographs that showed cortical breakthrough (Table). Two of the 10 cases were managed nonoperatively, and the patients died before surgical stabilization could be attempted. Of the 8 surgically managed cases, 3 were prophylactically stabilized with IMN (2 of these were augmented with cement, and the third with a screw-plate construct), 2 were treated with periarticular resection and reconstruction (total knee megaprosthesis), 1 was treated with an approach undertaken to address a concomitant distal femoral pathologic fracture, and 1 was treated with total knee arthroplasty undertaken to address lesions at the proximal end of the tibia and the distal end of the femur.
Discussion
We have described a retrospective descriptive study conducted to characterize tibial metastases, their histologies, and the circumstances surrounding diagnosis and surgical management. In all cases, general findings confirmed advanced metastatic disease. In only 3 cases, the tibia lesion was an isolated metastatic lesion.
Sex predilection of tibial metastases remains controversial. One study found males had up to twice as many hand and foot metastases as women,11 but this contrasts with the relatively equal sex ratio found in other studies8,10 and in the present study. We found metastatic disease of the tibia was unsurprisingly concentrated in patients over age 40 years, in whom the vast majority of all cancers develop.12,13 Our study agrees with those that have found most tibia lesions develop in patients in the 6th decade of life on average.8,10 Mean age was 8.3 years higher in our male patients than in our female patients.
Tumor Characteristics
The most common primary neoplasms in our cohort were prostate, breast, and lung cancers, which are among the most common cancers in the United States12,13 and which have a predilection for osseous spread.2,6,9,14 Renal cell carcinoma has been reported to spread to distal (or “acral”) skeletal sites,2-4,9,11,14 but the present study did not identify any patients with this diagnosis. Of our patients with a primary lung cancer for whom a histologic description was available (5/7), all had non-small cell lung cancer. Three patients had a primary malignancy of colorectal cancer, which occasionally metastasizes to the distal skeleton.3,8,11 We identified 3 patients with diffuse large B-cell lymphoma, a histology not widely reported to metastasize to distal skeletal sites.
Metastatic disease of the tibia is most common at the proximal end of the bone.1,10,11,14 Other studies8,10 have found the proximal tibia is affected much more commonly than the tibial diaphysis, and even fewer cases develop at the distal end. Our findings agree with theirs: Proximal lesions outnumber all other lesions combined (Table).
Time to Metastasis
Distal metastases are typical of late-stage metastatic disease,1,3 but quantification of the time from diagnosis of malignancy to presentation of a tibia lesion is not well defined. In our study, time to metastasis was <100 days for some patients (Table). As osseous involvement, especially acral disease, was considered a late-stage manifestation of malignancy, this result was unexpected and most likely represents undiagnosed and untreated malignancy. Six patients in this group were diagnosed with tibial metastases within 30 days, essentially at the same time the primary neoplasm was diagnosed. These findings suggest that a tibia lesion found at time of patient presentation should raise concern for late-stage undiagnosed metastatic cancer.
Other Osseous Disease
The patients identified in this study had advanced malignancy, and most had widespread bony dissemination. Those with the lowest disease burden had isolated tibia lesions or additional metastases only distal to the tibia lesion in the ipsilateral lower extremity. Most of these patients had undergone surgery or were scheduled for it (Table). Most of the patients with appendicular metastases proximal to the tibia lesion had disease of the femora, the most common long bones affected by osseous metastatic disease.5,6 In accordance with orthopedic oncology principles, all other osseous disease should be thoroughly identified and staged before any surgical planning for identified tibia lesions. Ipsilateral distal femoral lesions are of particular importance for patients with proximal tibia lesions, as reconstruction with total knee endoprosthesis can potentially provide a functional reconstructive option after resection of both lesions.
Clinical Presentation
Most of the patients who had cortical breakthrough or required surgical stabilization had painful lesions. Although tibial metastasis is rare, its potential occurrence should raise concerns and be investigated in the patient with tibial pain.
Surgical Intervention
General surgical management of metastatic disease of other long bones has been extensively studied,6,7,9,14 but there are fewer published recommendations regarding specific treatments for metastatic lesions of the tibia. In 2003, Kelly and colleagues8 described an algorithm based on the anatomical location of the lesion, with either internal fixation or IMN fixation representing the preferred management for lesions in the metaphyseal or diaphyseal regions. For epiphyseal or extensive proximal metaphyseal lesions, modular oncology endoprostheses are described as the procedure of choice. Piccioli and colleagues10 in 2013 and Beauchamp and Sim1 in 1988 described a similar operative approach.
It is unknown if the algorithm of Kelly and colleagues8 was referenced during clinical decision-making, but it appears operative management mirrored these principles. Deviations from this general approach in the operative management of the patients in the present study included modifications such as the addition of a screw-plate construct to an IMN for better stability.
Surgical management depends largely on the anatomical location within the bone and on remaining bone stock. Generally, extensive proximal disease is managed with total knee endoprosthesis reconstruction, diaphyseal disease with IMN, and distal disease with internal fixation. Construct augmentation, such as the addition of cement or use of additional hardware, is decided case by case on the basis of desired stability and surrounding bone stock.
Study Limitations
Despite being a larger series, this single-institution study had a relatively small sample size, and its patient demographics and primary malignancies may reflect institutional recruitment bias. In addition, the study was limited by its retrospective design and some incomplete medical records. Eleven patients had only a bone or positron emission tomography scan depicting metastatic disease, limiting characterization of these lesions. One patient lacked radiologic images, and characterizations were based on written reports. As multiple physicians were involved in diagnosis and treatment, there were many inconsistencies in clinical decision-making across the group.
Conclusion
Metastasis to the tibia is a rare but significant event in a subset of patients over the course of their treatment and surveillance. Patients may present with pain secondary to either pathologic or impending pathologic fractures, and in such instances surgical intervention is often needed. Despite the historical reports of “acral” histologies, tibia lesions are not indicative of histology, and biopsy should be considered, especially if management will depend on histology. Patients with lower leg pain and known malignancy should be evaluated to rule out tibial metastasis, but screening examinations may be prudent for asymptomatic patients as well. Increased vigilance may be indicated for those with prostate, breast, or lung cancer. These lesions should be surgically managed case by case using fundamental tenets of both orthopedic fracture care and orthopedic oncology. Ideally, patients should be treated by a multidisciplinary team using a patient-centered approach.
1. Beauchamp CP, Sim FH. Lesions of the tibia. In: Sim FH, ed. Diagnosis and Management of Metastatic Bone Disease: A Multidisciplinary Approach. New York, NY: Raven; 1988:201-212.
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s-6249s.
3. Healy JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
4. Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;(206):94-99.
5. De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67(1):54-59.
6. Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55(2):95-98.
7. Jasmin C. Textbook of Bone Metastases. Chichester, England: Wiley; 2005.
8. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;(415 suppl):S219-S229.
9. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509-524.
10. Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44(8):1092-1096.
11. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
12. American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
14. Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83(4):471-481.
Take-Home Points
- Metastatic disease of the tibia is a rare but significant event in a subset of patients.
- Cancer histologies with historically “acral” spread may not apply to tibial disease.
- Patients with leg pain and any cancer diagnosis should be worked up for tibial metastases.
- Tibial disease is probably a late manifestation, and early detection may indicate late diagnosis of malignancy.
- The ultimate surgical plan for these patients should be a patient-centered multidisciplinary decision making process.
Metastatic dissemination to bones is common in advanced cancer stages and affects the axial and appendicular skeleton.1-4 The appendicular skeleton bones most often involved are the proximal femur and the proximal humerus.5,6 The tibia is involved third most often but is comparatively rarely affected.4-6 Metastatic involvement distal to the knee or elbow is more typical of advanced disease.1,3 Distal appendicular lesions are called acral metastases, but the term is inconsistently used and may refer to lesions either distal to the knee and elbow or distal to the ankle and wrist. Regardless of terminology, tibia lesions are uncommon and not well described.1,4,7,8
The tibia is the primary weight-bearing leg bone. Metastatic tibia lesions may cause pain and instability and impair mobility. Although distal skeletal dissemination often presents late in advanced disease in patients with relatively poor prognoses, in some cases early surgical intervention is indicated for pain relief, increased mobility, and improved quality of life.4,8-10
Materials and Methods
Our Institutional Review Board approved this single-institution retrospective study. We used proprietary research software (Clinical Looking Glass) to identify eligible patients treated between 2000 and 2013. The software was used to search all radiology and pathology reports for the term tibia or any variation (eg, tibial) and metastasis or any variation (eg, metastatic). The software was then used to search by Current Procedural Terminology code for any patients treated with intramedullary nail (IMN) or another tibial fixation method. This list was cross-referenced with the list of patients originally identified to help ensure that all eligible patients were identified.
Inclusion criteria were known malignancy and imaging or biopsy evidence of a metastatic tibia lesion. Treatment strategies for patients with metastatic disease and patients with multiple myeloma are sometimes considered together because of similar goals and methodologies. We specifically excluded patients with multiple myeloma in order to more accurately characterize the natural history of metastatic disease and the timing of metastatic development and to report on a more homogeneous population. Patients were excluded if their electronic medical records were inadequate in establishing a diagnosis.
Demographic and pathology data were collected directly from the institutional electronic medical records system. Dr. Geller and Dr. Greenbaum used Centricity software (General Electric Healthcare) to review all imaging on medical diagnostic display monitors. If their interpretation differed from that in the radiology report, or if clarification was needed, the study was sent to Dr. Thornhill, the institution’s director of musculoskeletal radiology, for review and interpretation. Investigated radiographic characteristics included location, cortical breakthrough, presence of fracture, and size (if advanced imaging was available). Surgical interventions were recorded from reviews of operative reports and postoperative imaging studies.
Time to metastasis was defined as number of days from diagnosis of malignancy to diagnosis of tibial osseous spread. Date of diagnosis of malignancy was the date that a biopsy or other confirmatory test was performed. In cases in which that date was unavailable, an imaging study consistent with disease or a clinical note documenting the known diagnosis date was used instead. When only month and year (ie, not an exact date) of diagnosis were available, the 15th of the month was used as an estimate. Of the 36 patients, 4 had records insufficient for establishing date of diagnosis. The first date of any imaging study confirming (or suggestive of) a metastatic lesion of the tibia was used as the date of tibial metastasis.
Many patients had osseous lesions at sites other than the tibiae. These lesions were noted on review of imaging studies, screening examinations, and physicians’ clinical notes. Widespread disease was defined as including both axial and appendicular lesions, and lesions of the tibiae.
Tibia lesion presentation was recorded as either symptomatic or incidental. If the tibiae were imaged for pain, including posttraumatic pain, the presentation was symptomatic. If a lesion was identified on staging examination (eg, bone or positron emission tomography scan), or if the tibiae were imaged for another reason, the presentation was incidental.
Results
Demographics
Thirty-six patients had 43 affected tibiae. Sixteen male patients (44.4% of the total) had 19 (44.2%) of the affected tibiae, and 20 female patients (55.6%) had the other 24 affected tibiae (55.8%). Mean age was 63.5 years for all patients (range, 6-95 years), 68.1 years for males, and 59.8 years for females. Of the 36 patients, 32 (88.9%) were over age 40 years (Table). All patients had radiographic evidence of ≥1 tibia lesion, and 6 (16.7%) also had biopsy-proven metastatic disease of the tibia.
Tumor Characteristics
There were 12 different primary neoplasms (Table). The most common were prostate cancer (7 patients, 19.4%; 10 tibiae, 23.3%), breast cancer (7 patients, 19.4%; 9 tibiae, 20.9%), and lung cancer (7 patients, 19.4%; 7 tibiae, 16.3%). For males, the most common diagnoses were prostate cancer (7 cases, 43.8% of males) and diffuse large B-cell lymphoma and lung cancer (3 cases and 18.8% of males each). For females, the most common diagnoses were breast cancer (7 cases, 35.0% of females) and lung cancer (4 cases, 20.0% of females).
Most of the lesions were proximal (31 tibiae, 72.1%), followed by diaphyseal (7, 16.3%) and distal (2, 4.7%) (Table). Three tibiae (7.0%) were entirely involved, but 1 of these was more affected at the distal end. One tibia had 2 lesions, 1 proximal and 1 distal.
Time to Metastasis, Other Osseous Disease
Mean time from diagnosis of malignancy to diagnosis of osseous disease of the tibia was 1282 days (range, 0-3708 days) (Table). Of the 36 patients, 32 (88.9%) had other metastatic lesions, 3 (8.3%) had isolated tibia lesions, and 1 (2.8%) had a medical record insufficient for establishing lesion status (isolated or not). Of the 32 patients with known other osseous metastases, 14 (43.8%) had widespread (axial and additional appendicular) disease, and 3 (9.4%) had additional lesions only distal to the identified tibial metastases.
Clinical Presentation
Of the 36 lesions, 18 (50%) were asymptomatic and were found on screening examinations, 17 (47.2%) presented with pain, and 1 (2.8%) had a presentation that could not be determined from the medical record (Table). Of the 17 painful lesions, 3 (17.6%) were found after a trauma brought attention to the site, and the other 14 (82.4%) were atraumatic in origin.
Of the 10 patients with cortical breakthrough, 8 (80%) had painful lesions, 1 (10%) had a lesion that was found on screening examination, and 1 (10%) had a medical record insufficient for establishing clinical presentation. Of the 8 patients who underwent surgical stabilization, 6 (75%) had painful lesions. Only 1 patient with an asymptomatic tibia lesion underwent surgical intervention (total knee arthroplasty).
Surgical Intervention
Two patients (5.6%) with affected tibiae (4.8%) had pathologic fractures. One fracture (non-small cell lung cancer) was treated with open reduction and internal fixation (periarticular locking plate with cement augmentation), and the other (urothelial cancer) was treated with IMN fixation.
Ten patients (27.8%) with affected tibiae (23.8%) had radiographs that showed cortical breakthrough (Table). Two of the 10 cases were managed nonoperatively, and the patients died before surgical stabilization could be attempted. Of the 8 surgically managed cases, 3 were prophylactically stabilized with IMN (2 of these were augmented with cement, and the third with a screw-plate construct), 2 were treated with periarticular resection and reconstruction (total knee megaprosthesis), 1 was treated with an approach undertaken to address a concomitant distal femoral pathologic fracture, and 1 was treated with total knee arthroplasty undertaken to address lesions at the proximal end of the tibia and the distal end of the femur.
Discussion
We have described a retrospective descriptive study conducted to characterize tibial metastases, their histologies, and the circumstances surrounding diagnosis and surgical management. In all cases, general findings confirmed advanced metastatic disease. In only 3 cases, the tibia lesion was an isolated metastatic lesion.
Sex predilection of tibial metastases remains controversial. One study found males had up to twice as many hand and foot metastases as women,11 but this contrasts with the relatively equal sex ratio found in other studies8,10 and in the present study. We found metastatic disease of the tibia was unsurprisingly concentrated in patients over age 40 years, in whom the vast majority of all cancers develop.12,13 Our study agrees with those that have found most tibia lesions develop in patients in the 6th decade of life on average.8,10 Mean age was 8.3 years higher in our male patients than in our female patients.
Tumor Characteristics
The most common primary neoplasms in our cohort were prostate, breast, and lung cancers, which are among the most common cancers in the United States12,13 and which have a predilection for osseous spread.2,6,9,14 Renal cell carcinoma has been reported to spread to distal (or “acral”) skeletal sites,2-4,9,11,14 but the present study did not identify any patients with this diagnosis. Of our patients with a primary lung cancer for whom a histologic description was available (5/7), all had non-small cell lung cancer. Three patients had a primary malignancy of colorectal cancer, which occasionally metastasizes to the distal skeleton.3,8,11 We identified 3 patients with diffuse large B-cell lymphoma, a histology not widely reported to metastasize to distal skeletal sites.
Metastatic disease of the tibia is most common at the proximal end of the bone.1,10,11,14 Other studies8,10 have found the proximal tibia is affected much more commonly than the tibial diaphysis, and even fewer cases develop at the distal end. Our findings agree with theirs: Proximal lesions outnumber all other lesions combined (Table).
Time to Metastasis
Distal metastases are typical of late-stage metastatic disease,1,3 but quantification of the time from diagnosis of malignancy to presentation of a tibia lesion is not well defined. In our study, time to metastasis was <100 days for some patients (Table). As osseous involvement, especially acral disease, was considered a late-stage manifestation of malignancy, this result was unexpected and most likely represents undiagnosed and untreated malignancy. Six patients in this group were diagnosed with tibial metastases within 30 days, essentially at the same time the primary neoplasm was diagnosed. These findings suggest that a tibia lesion found at time of patient presentation should raise concern for late-stage undiagnosed metastatic cancer.
Other Osseous Disease
The patients identified in this study had advanced malignancy, and most had widespread bony dissemination. Those with the lowest disease burden had isolated tibia lesions or additional metastases only distal to the tibia lesion in the ipsilateral lower extremity. Most of these patients had undergone surgery or were scheduled for it (Table). Most of the patients with appendicular metastases proximal to the tibia lesion had disease of the femora, the most common long bones affected by osseous metastatic disease.5,6 In accordance with orthopedic oncology principles, all other osseous disease should be thoroughly identified and staged before any surgical planning for identified tibia lesions. Ipsilateral distal femoral lesions are of particular importance for patients with proximal tibia lesions, as reconstruction with total knee endoprosthesis can potentially provide a functional reconstructive option after resection of both lesions.
Clinical Presentation
Most of the patients who had cortical breakthrough or required surgical stabilization had painful lesions. Although tibial metastasis is rare, its potential occurrence should raise concerns and be investigated in the patient with tibial pain.
Surgical Intervention
General surgical management of metastatic disease of other long bones has been extensively studied,6,7,9,14 but there are fewer published recommendations regarding specific treatments for metastatic lesions of the tibia. In 2003, Kelly and colleagues8 described an algorithm based on the anatomical location of the lesion, with either internal fixation or IMN fixation representing the preferred management for lesions in the metaphyseal or diaphyseal regions. For epiphyseal or extensive proximal metaphyseal lesions, modular oncology endoprostheses are described as the procedure of choice. Piccioli and colleagues10 in 2013 and Beauchamp and Sim1 in 1988 described a similar operative approach.
It is unknown if the algorithm of Kelly and colleagues8 was referenced during clinical decision-making, but it appears operative management mirrored these principles. Deviations from this general approach in the operative management of the patients in the present study included modifications such as the addition of a screw-plate construct to an IMN for better stability.
Surgical management depends largely on the anatomical location within the bone and on remaining bone stock. Generally, extensive proximal disease is managed with total knee endoprosthesis reconstruction, diaphyseal disease with IMN, and distal disease with internal fixation. Construct augmentation, such as the addition of cement or use of additional hardware, is decided case by case on the basis of desired stability and surrounding bone stock.
Study Limitations
Despite being a larger series, this single-institution study had a relatively small sample size, and its patient demographics and primary malignancies may reflect institutional recruitment bias. In addition, the study was limited by its retrospective design and some incomplete medical records. Eleven patients had only a bone or positron emission tomography scan depicting metastatic disease, limiting characterization of these lesions. One patient lacked radiologic images, and characterizations were based on written reports. As multiple physicians were involved in diagnosis and treatment, there were many inconsistencies in clinical decision-making across the group.
Conclusion
Metastasis to the tibia is a rare but significant event in a subset of patients over the course of their treatment and surveillance. Patients may present with pain secondary to either pathologic or impending pathologic fractures, and in such instances surgical intervention is often needed. Despite the historical reports of “acral” histologies, tibia lesions are not indicative of histology, and biopsy should be considered, especially if management will depend on histology. Patients with lower leg pain and known malignancy should be evaluated to rule out tibial metastasis, but screening examinations may be prudent for asymptomatic patients as well. Increased vigilance may be indicated for those with prostate, breast, or lung cancer. These lesions should be surgically managed case by case using fundamental tenets of both orthopedic fracture care and orthopedic oncology. Ideally, patients should be treated by a multidisciplinary team using a patient-centered approach.
Take-Home Points
- Metastatic disease of the tibia is a rare but significant event in a subset of patients.
- Cancer histologies with historically “acral” spread may not apply to tibial disease.
- Patients with leg pain and any cancer diagnosis should be worked up for tibial metastases.
- Tibial disease is probably a late manifestation, and early detection may indicate late diagnosis of malignancy.
- The ultimate surgical plan for these patients should be a patient-centered multidisciplinary decision making process.
Metastatic dissemination to bones is common in advanced cancer stages and affects the axial and appendicular skeleton.1-4 The appendicular skeleton bones most often involved are the proximal femur and the proximal humerus.5,6 The tibia is involved third most often but is comparatively rarely affected.4-6 Metastatic involvement distal to the knee or elbow is more typical of advanced disease.1,3 Distal appendicular lesions are called acral metastases, but the term is inconsistently used and may refer to lesions either distal to the knee and elbow or distal to the ankle and wrist. Regardless of terminology, tibia lesions are uncommon and not well described.1,4,7,8
The tibia is the primary weight-bearing leg bone. Metastatic tibia lesions may cause pain and instability and impair mobility. Although distal skeletal dissemination often presents late in advanced disease in patients with relatively poor prognoses, in some cases early surgical intervention is indicated for pain relief, increased mobility, and improved quality of life.4,8-10
Materials and Methods
Our Institutional Review Board approved this single-institution retrospective study. We used proprietary research software (Clinical Looking Glass) to identify eligible patients treated between 2000 and 2013. The software was used to search all radiology and pathology reports for the term tibia or any variation (eg, tibial) and metastasis or any variation (eg, metastatic). The software was then used to search by Current Procedural Terminology code for any patients treated with intramedullary nail (IMN) or another tibial fixation method. This list was cross-referenced with the list of patients originally identified to help ensure that all eligible patients were identified.
Inclusion criteria were known malignancy and imaging or biopsy evidence of a metastatic tibia lesion. Treatment strategies for patients with metastatic disease and patients with multiple myeloma are sometimes considered together because of similar goals and methodologies. We specifically excluded patients with multiple myeloma in order to more accurately characterize the natural history of metastatic disease and the timing of metastatic development and to report on a more homogeneous population. Patients were excluded if their electronic medical records were inadequate in establishing a diagnosis.
Demographic and pathology data were collected directly from the institutional electronic medical records system. Dr. Geller and Dr. Greenbaum used Centricity software (General Electric Healthcare) to review all imaging on medical diagnostic display monitors. If their interpretation differed from that in the radiology report, or if clarification was needed, the study was sent to Dr. Thornhill, the institution’s director of musculoskeletal radiology, for review and interpretation. Investigated radiographic characteristics included location, cortical breakthrough, presence of fracture, and size (if advanced imaging was available). Surgical interventions were recorded from reviews of operative reports and postoperative imaging studies.
Time to metastasis was defined as number of days from diagnosis of malignancy to diagnosis of tibial osseous spread. Date of diagnosis of malignancy was the date that a biopsy or other confirmatory test was performed. In cases in which that date was unavailable, an imaging study consistent with disease or a clinical note documenting the known diagnosis date was used instead. When only month and year (ie, not an exact date) of diagnosis were available, the 15th of the month was used as an estimate. Of the 36 patients, 4 had records insufficient for establishing date of diagnosis. The first date of any imaging study confirming (or suggestive of) a metastatic lesion of the tibia was used as the date of tibial metastasis.
Many patients had osseous lesions at sites other than the tibiae. These lesions were noted on review of imaging studies, screening examinations, and physicians’ clinical notes. Widespread disease was defined as including both axial and appendicular lesions, and lesions of the tibiae.
Tibia lesion presentation was recorded as either symptomatic or incidental. If the tibiae were imaged for pain, including posttraumatic pain, the presentation was symptomatic. If a lesion was identified on staging examination (eg, bone or positron emission tomography scan), or if the tibiae were imaged for another reason, the presentation was incidental.
Results
Demographics
Thirty-six patients had 43 affected tibiae. Sixteen male patients (44.4% of the total) had 19 (44.2%) of the affected tibiae, and 20 female patients (55.6%) had the other 24 affected tibiae (55.8%). Mean age was 63.5 years for all patients (range, 6-95 years), 68.1 years for males, and 59.8 years for females. Of the 36 patients, 32 (88.9%) were over age 40 years (Table). All patients had radiographic evidence of ≥1 tibia lesion, and 6 (16.7%) also had biopsy-proven metastatic disease of the tibia.
Tumor Characteristics
There were 12 different primary neoplasms (Table). The most common were prostate cancer (7 patients, 19.4%; 10 tibiae, 23.3%), breast cancer (7 patients, 19.4%; 9 tibiae, 20.9%), and lung cancer (7 patients, 19.4%; 7 tibiae, 16.3%). For males, the most common diagnoses were prostate cancer (7 cases, 43.8% of males) and diffuse large B-cell lymphoma and lung cancer (3 cases and 18.8% of males each). For females, the most common diagnoses were breast cancer (7 cases, 35.0% of females) and lung cancer (4 cases, 20.0% of females).
Most of the lesions were proximal (31 tibiae, 72.1%), followed by diaphyseal (7, 16.3%) and distal (2, 4.7%) (Table). Three tibiae (7.0%) were entirely involved, but 1 of these was more affected at the distal end. One tibia had 2 lesions, 1 proximal and 1 distal.
Time to Metastasis, Other Osseous Disease
Mean time from diagnosis of malignancy to diagnosis of osseous disease of the tibia was 1282 days (range, 0-3708 days) (Table). Of the 36 patients, 32 (88.9%) had other metastatic lesions, 3 (8.3%) had isolated tibia lesions, and 1 (2.8%) had a medical record insufficient for establishing lesion status (isolated or not). Of the 32 patients with known other osseous metastases, 14 (43.8%) had widespread (axial and additional appendicular) disease, and 3 (9.4%) had additional lesions only distal to the identified tibial metastases.
Clinical Presentation
Of the 36 lesions, 18 (50%) were asymptomatic and were found on screening examinations, 17 (47.2%) presented with pain, and 1 (2.8%) had a presentation that could not be determined from the medical record (Table). Of the 17 painful lesions, 3 (17.6%) were found after a trauma brought attention to the site, and the other 14 (82.4%) were atraumatic in origin.
Of the 10 patients with cortical breakthrough, 8 (80%) had painful lesions, 1 (10%) had a lesion that was found on screening examination, and 1 (10%) had a medical record insufficient for establishing clinical presentation. Of the 8 patients who underwent surgical stabilization, 6 (75%) had painful lesions. Only 1 patient with an asymptomatic tibia lesion underwent surgical intervention (total knee arthroplasty).
Surgical Intervention
Two patients (5.6%) with affected tibiae (4.8%) had pathologic fractures. One fracture (non-small cell lung cancer) was treated with open reduction and internal fixation (periarticular locking plate with cement augmentation), and the other (urothelial cancer) was treated with IMN fixation.
Ten patients (27.8%) with affected tibiae (23.8%) had radiographs that showed cortical breakthrough (Table). Two of the 10 cases were managed nonoperatively, and the patients died before surgical stabilization could be attempted. Of the 8 surgically managed cases, 3 were prophylactically stabilized with IMN (2 of these were augmented with cement, and the third with a screw-plate construct), 2 were treated with periarticular resection and reconstruction (total knee megaprosthesis), 1 was treated with an approach undertaken to address a concomitant distal femoral pathologic fracture, and 1 was treated with total knee arthroplasty undertaken to address lesions at the proximal end of the tibia and the distal end of the femur.
Discussion
We have described a retrospective descriptive study conducted to characterize tibial metastases, their histologies, and the circumstances surrounding diagnosis and surgical management. In all cases, general findings confirmed advanced metastatic disease. In only 3 cases, the tibia lesion was an isolated metastatic lesion.
Sex predilection of tibial metastases remains controversial. One study found males had up to twice as many hand and foot metastases as women,11 but this contrasts with the relatively equal sex ratio found in other studies8,10 and in the present study. We found metastatic disease of the tibia was unsurprisingly concentrated in patients over age 40 years, in whom the vast majority of all cancers develop.12,13 Our study agrees with those that have found most tibia lesions develop in patients in the 6th decade of life on average.8,10 Mean age was 8.3 years higher in our male patients than in our female patients.
Tumor Characteristics
The most common primary neoplasms in our cohort were prostate, breast, and lung cancers, which are among the most common cancers in the United States12,13 and which have a predilection for osseous spread.2,6,9,14 Renal cell carcinoma has been reported to spread to distal (or “acral”) skeletal sites,2-4,9,11,14 but the present study did not identify any patients with this diagnosis. Of our patients with a primary lung cancer for whom a histologic description was available (5/7), all had non-small cell lung cancer. Three patients had a primary malignancy of colorectal cancer, which occasionally metastasizes to the distal skeleton.3,8,11 We identified 3 patients with diffuse large B-cell lymphoma, a histology not widely reported to metastasize to distal skeletal sites.
Metastatic disease of the tibia is most common at the proximal end of the bone.1,10,11,14 Other studies8,10 have found the proximal tibia is affected much more commonly than the tibial diaphysis, and even fewer cases develop at the distal end. Our findings agree with theirs: Proximal lesions outnumber all other lesions combined (Table).
Time to Metastasis
Distal metastases are typical of late-stage metastatic disease,1,3 but quantification of the time from diagnosis of malignancy to presentation of a tibia lesion is not well defined. In our study, time to metastasis was <100 days for some patients (Table). As osseous involvement, especially acral disease, was considered a late-stage manifestation of malignancy, this result was unexpected and most likely represents undiagnosed and untreated malignancy. Six patients in this group were diagnosed with tibial metastases within 30 days, essentially at the same time the primary neoplasm was diagnosed. These findings suggest that a tibia lesion found at time of patient presentation should raise concern for late-stage undiagnosed metastatic cancer.
Other Osseous Disease
The patients identified in this study had advanced malignancy, and most had widespread bony dissemination. Those with the lowest disease burden had isolated tibia lesions or additional metastases only distal to the tibia lesion in the ipsilateral lower extremity. Most of these patients had undergone surgery or were scheduled for it (Table). Most of the patients with appendicular metastases proximal to the tibia lesion had disease of the femora, the most common long bones affected by osseous metastatic disease.5,6 In accordance with orthopedic oncology principles, all other osseous disease should be thoroughly identified and staged before any surgical planning for identified tibia lesions. Ipsilateral distal femoral lesions are of particular importance for patients with proximal tibia lesions, as reconstruction with total knee endoprosthesis can potentially provide a functional reconstructive option after resection of both lesions.
Clinical Presentation
Most of the patients who had cortical breakthrough or required surgical stabilization had painful lesions. Although tibial metastasis is rare, its potential occurrence should raise concerns and be investigated in the patient with tibial pain.
Surgical Intervention
General surgical management of metastatic disease of other long bones has been extensively studied,6,7,9,14 but there are fewer published recommendations regarding specific treatments for metastatic lesions of the tibia. In 2003, Kelly and colleagues8 described an algorithm based on the anatomical location of the lesion, with either internal fixation or IMN fixation representing the preferred management for lesions in the metaphyseal or diaphyseal regions. For epiphyseal or extensive proximal metaphyseal lesions, modular oncology endoprostheses are described as the procedure of choice. Piccioli and colleagues10 in 2013 and Beauchamp and Sim1 in 1988 described a similar operative approach.
It is unknown if the algorithm of Kelly and colleagues8 was referenced during clinical decision-making, but it appears operative management mirrored these principles. Deviations from this general approach in the operative management of the patients in the present study included modifications such as the addition of a screw-plate construct to an IMN for better stability.
Surgical management depends largely on the anatomical location within the bone and on remaining bone stock. Generally, extensive proximal disease is managed with total knee endoprosthesis reconstruction, diaphyseal disease with IMN, and distal disease with internal fixation. Construct augmentation, such as the addition of cement or use of additional hardware, is decided case by case on the basis of desired stability and surrounding bone stock.
Study Limitations
Despite being a larger series, this single-institution study had a relatively small sample size, and its patient demographics and primary malignancies may reflect institutional recruitment bias. In addition, the study was limited by its retrospective design and some incomplete medical records. Eleven patients had only a bone or positron emission tomography scan depicting metastatic disease, limiting characterization of these lesions. One patient lacked radiologic images, and characterizations were based on written reports. As multiple physicians were involved in diagnosis and treatment, there were many inconsistencies in clinical decision-making across the group.
Conclusion
Metastasis to the tibia is a rare but significant event in a subset of patients over the course of their treatment and surveillance. Patients may present with pain secondary to either pathologic or impending pathologic fractures, and in such instances surgical intervention is often needed. Despite the historical reports of “acral” histologies, tibia lesions are not indicative of histology, and biopsy should be considered, especially if management will depend on histology. Patients with lower leg pain and known malignancy should be evaluated to rule out tibial metastasis, but screening examinations may be prudent for asymptomatic patients as well. Increased vigilance may be indicated for those with prostate, breast, or lung cancer. These lesions should be surgically managed case by case using fundamental tenets of both orthopedic fracture care and orthopedic oncology. Ideally, patients should be treated by a multidisciplinary team using a patient-centered approach.
1. Beauchamp CP, Sim FH. Lesions of the tibia. In: Sim FH, ed. Diagnosis and Management of Metastatic Bone Disease: A Multidisciplinary Approach. New York, NY: Raven; 1988:201-212.
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s-6249s.
3. Healy JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
4. Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;(206):94-99.
5. De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67(1):54-59.
6. Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55(2):95-98.
7. Jasmin C. Textbook of Bone Metastases. Chichester, England: Wiley; 2005.
8. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;(415 suppl):S219-S229.
9. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509-524.
10. Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44(8):1092-1096.
11. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
12. American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
14. Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83(4):471-481.
1. Beauchamp CP, Sim FH. Lesions of the tibia. In: Sim FH, ed. Diagnosis and Management of Metastatic Bone Disease: A Multidisciplinary Approach. New York, NY: Raven; 1988:201-212.
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s-6249s.
3. Healy JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
4. Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;(206):94-99.
5. De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67(1):54-59.
6. Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55(2):95-98.
7. Jasmin C. Textbook of Bone Metastases. Chichester, England: Wiley; 2005.
8. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;(415 suppl):S219-S229.
9. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509-524.
10. Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44(8):1092-1096.
11. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
12. American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
14. Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83(4):471-481.
VIDEO: Joint FDA-ASH session highlights new AML drugs
ATLANTA – The past year brought a flurry of new drug approvals for the treatment of acute myeloid leukemia (AML), including CPX-351, midostaurin, gemtuzumab ozogamicin, and enasidenib.
During a special interest session at the annual meeting of the American Society of Hematology, Food and Drug Administration representatives discussed the available data and approval process for these drugs, and clinicians discussed their use in the real-world setting.
In this video interview, – a liposome-encapsulated combination of daunorubicin and cytarabine approved in August for patients with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes, and midostaurin (Rydapt), which was approved in April for the treatment of newly diagnosed AML patients who are FLT3 mutation-positive. She also discussed future directions for these agents.
“So what clinicians are faced with is, all of a sudden, a number of new agents, and no particularly vetted or data-based algorithm by which to assign patients from one to the other,” said Dr. Michaelis, of the Medical College of Wisconsin, Milwaukee, adding that none of the drugs have been compared against one another.
In her own practice, when it comes to CPX-351, she said she first discusses the pros and cons with patients.
“This drug is used for older individuals ... with very adverse risk disease, and so the first question is do you fit the trial entry criteria, do you want to go through induction, do you understand what that’s going to mean, and am I going to take you to transplant after we go through this.”
As for midostaurin, she said she tries to use it on anyone who fits the trial criteria and is FLT-3 positive.
“The trick with that is that we don’t know the FLT-3 status at the time we have to start induction, so it’s hard to determine the exact right doses of your induction regimen knowing that you’re not going to get the test back until day 6, 7, 8, and you’re supposed to start delivering the drug on day 8, so we still have a ways as a care community to catch up with being able to give these drugs in a manner that was the same as what was delivered in the trials that led to approval.”
She also discussed the potential for combining treatments.
“I think there’s really room for studies on combinations of inhibitors plus the CPX, the safety of using a variety of induction regimens alongside midostaurin, and safety of combining things, like with midostaurin for example, with some of our antifungals ... and to make sure that that’s safe. So yeah, we’ve got a lot more to do,” she said.
Dr. Michaelis serves on an advisory board for Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ATLANTA – The past year brought a flurry of new drug approvals for the treatment of acute myeloid leukemia (AML), including CPX-351, midostaurin, gemtuzumab ozogamicin, and enasidenib.
During a special interest session at the annual meeting of the American Society of Hematology, Food and Drug Administration representatives discussed the available data and approval process for these drugs, and clinicians discussed their use in the real-world setting.
In this video interview, – a liposome-encapsulated combination of daunorubicin and cytarabine approved in August for patients with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes, and midostaurin (Rydapt), which was approved in April for the treatment of newly diagnosed AML patients who are FLT3 mutation-positive. She also discussed future directions for these agents.
“So what clinicians are faced with is, all of a sudden, a number of new agents, and no particularly vetted or data-based algorithm by which to assign patients from one to the other,” said Dr. Michaelis, of the Medical College of Wisconsin, Milwaukee, adding that none of the drugs have been compared against one another.
In her own practice, when it comes to CPX-351, she said she first discusses the pros and cons with patients.
“This drug is used for older individuals ... with very adverse risk disease, and so the first question is do you fit the trial entry criteria, do you want to go through induction, do you understand what that’s going to mean, and am I going to take you to transplant after we go through this.”
As for midostaurin, she said she tries to use it on anyone who fits the trial criteria and is FLT-3 positive.
“The trick with that is that we don’t know the FLT-3 status at the time we have to start induction, so it’s hard to determine the exact right doses of your induction regimen knowing that you’re not going to get the test back until day 6, 7, 8, and you’re supposed to start delivering the drug on day 8, so we still have a ways as a care community to catch up with being able to give these drugs in a manner that was the same as what was delivered in the trials that led to approval.”
She also discussed the potential for combining treatments.
“I think there’s really room for studies on combinations of inhibitors plus the CPX, the safety of using a variety of induction regimens alongside midostaurin, and safety of combining things, like with midostaurin for example, with some of our antifungals ... and to make sure that that’s safe. So yeah, we’ve got a lot more to do,” she said.
Dr. Michaelis serves on an advisory board for Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ATLANTA – The past year brought a flurry of new drug approvals for the treatment of acute myeloid leukemia (AML), including CPX-351, midostaurin, gemtuzumab ozogamicin, and enasidenib.
During a special interest session at the annual meeting of the American Society of Hematology, Food and Drug Administration representatives discussed the available data and approval process for these drugs, and clinicians discussed their use in the real-world setting.
In this video interview, – a liposome-encapsulated combination of daunorubicin and cytarabine approved in August for patients with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes, and midostaurin (Rydapt), which was approved in April for the treatment of newly diagnosed AML patients who are FLT3 mutation-positive. She also discussed future directions for these agents.
“So what clinicians are faced with is, all of a sudden, a number of new agents, and no particularly vetted or data-based algorithm by which to assign patients from one to the other,” said Dr. Michaelis, of the Medical College of Wisconsin, Milwaukee, adding that none of the drugs have been compared against one another.
In her own practice, when it comes to CPX-351, she said she first discusses the pros and cons with patients.
“This drug is used for older individuals ... with very adverse risk disease, and so the first question is do you fit the trial entry criteria, do you want to go through induction, do you understand what that’s going to mean, and am I going to take you to transplant after we go through this.”
As for midostaurin, she said she tries to use it on anyone who fits the trial criteria and is FLT-3 positive.
“The trick with that is that we don’t know the FLT-3 status at the time we have to start induction, so it’s hard to determine the exact right doses of your induction regimen knowing that you’re not going to get the test back until day 6, 7, 8, and you’re supposed to start delivering the drug on day 8, so we still have a ways as a care community to catch up with being able to give these drugs in a manner that was the same as what was delivered in the trials that led to approval.”
She also discussed the potential for combining treatments.
“I think there’s really room for studies on combinations of inhibitors plus the CPX, the safety of using a variety of induction regimens alongside midostaurin, and safety of combining things, like with midostaurin for example, with some of our antifungals ... and to make sure that that’s safe. So yeah, we’ve got a lot more to do,” she said.
Dr. Michaelis serves on an advisory board for Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM ASH 2017
Physician-assisted suicide – an update
Question: Choose the best answer regarding physician-assisted suicide in the United States:
A. It is now legal in most states.
B. Under California law, assisting or causing one to commit suicide, including physician-assisted suicide, still remains a felony.
C. Both the U.S. Supreme Court and the New York Court of Appeals have held there is no constitutional right to physician-assisted suicide.
D. The American Medical Association is neutral on the issue.
E. Pain relief is the overriding reason for patients who request physician-assisted suicide.
Answer: C. We reviewed this topic in one of our regular columns in 2013.1 At that time, efforts to legalize physician-assisted suicide (PAS) appeared to be gathering momentum across the country, with four jurisdictions having legalized the practice, beginning with Oregon in 1994. The other states were Washington, Vermont, and Montana, whose Supreme Court held that there was no public interest reason against the practice.2
Since that time, California, Colorado, and the District of Columbia have joined the group. Currently, PAS – but not euthanasia – is legally available in these jurisdictions and in Switzerland, but both can be legally practiced in Belgium, Canada, Colombia, Luxembourg, and the Netherlands.
All state statutes permitting PAS provide similar provisions and safeguards. Only competent individuals who are terminally ill, i.e., death expected within 6 months, can make a request for a lethal dose of medication to carry out the suicidal act. The request to the doctor is first made verbally, then in writing, and a second opinion must be obtained to confirm the patient’s intent, understanding, and free choice. There is also a waiting period.
Public support for euthanasia and PAS in the United States is said to have plateaued since the 1990s. But a significant number of Americans, 67%, still favor PAS, up from 56% a decade ago.3 However, not many patients resort to PAS – usually those with terminal cancers or neuromuscular conditions – and only a minority of physicians are participants.
For example, 61 physicians in Oregon wrote a total of 115 prescriptions in 2012; there were 77 known Death With Dignity Act deaths in Oregon that year.4 In Oregon and Washington State, less than 1% of licensed physicians write prescriptions for physician-assisted suicide each year. In contrast, about half or more of physicians in the Netherlands and Belgium reported ever having received a request, and 60% of Dutch physicians have granted such requests.
The California Department of Public Health reported that 111 terminally ill patients availed themselves of California’s End of Life Option Act in the 7 months after it became effective on June 9, 2016.
In a recent review on euthanasia and PAS for the period 1947-2016, Ezekiel Emanuel, MD, and colleagues noted that typical patients were older, white, and well educated, and pain was mostly not reported as the primary motivation.5 A large portion of patients receiving PAS in Oregon and Washington were enrolled in hospice or palliative care. Abuses have not been apparent.
In the vast majority of jurisdictions, assisting or causing one to commit suicide, including PAS, still remains a crime; for example, it is considered manslaughter under Hawaii state law §707-702.
In distinguishing between assisting suicide and withdrawing life-sustaining treatment, the U.S. Supreme Court’s landmark 1997 Vacco v. Quill decision emphasized issues of causation and intent.6 On causation, the court reasoned that when a patient refuses life-sustaining treatment, he dies from an underlying fatal disease; but if a patient ingests a lethal medication, he is killed by that medication. As to intent, a physician who honors a patient’s refusal of treatment purposefully intends only to respect his patient’s wishes and to cease doing futile or degrading things. On the other hand, a doctor who assists a suicide “must, necessarily and indubitably, intend primarily that the patient be made dead.”
In its companion case Washington v. Glucksberg, the Supreme Court held that the asserted “right” to assistance in committing suicide is not a fundamental liberty interest protected by the due process clause.7
State supreme courts in Florida, New Mexico, and elsewhere have likewise rebuffed claims of any constitutional right to PAS. The latest court to so rule is in New York, which has a long history of criminalizing assisted suicide.8 The New York Court of Appeals recently addressed claims brought by three terminally ill individuals, several medical providers, and a nonprofit entity seeking a declaration that New York’s “assisted suicide” statutes exclude physicians from prescribing a lethal dose of drugs to terminally ill, competent patients.
The court unequivocally rejected such claims and affirmed that a physician who assists a suicide by prescribing lethal doses of drugs is subject to criminal prosecution for second-degree manslaughter. It refused to regard PAS as being different from assisted suicide in general, and it rejected the constitutional claim to assisted suicide by a terminally ill person. The state appeals court reiterated the U.S. Supreme Court’s distinction between refusing life-sustaining treatment and assisted suicide, the former being “at least partially rooted in notions of bodily integrity, as the right to refuse treatment is a consequence of a person’s right to resist unwanted bodily invasions.” The New York Court of Appeals also noted that the state has a legitimate purpose and a rational basis for guarding against the risks of mistake and abuse.
These developments may signal a shift away from the legalization of PAS, as recently suggested in a Washington Post article.9 According to the end-of-life advocacy organization Compassion and Choices, none of the 27 states where such measures were introduced in 2017 passed them into law, including states such as Connecticut, Hawaii, and Rhode Island. In Central and Eastern Europe, support is decreasing, whereas the opposite is true in Western Europe.
U.S. federal lawmakers also appear to be pushing back. On July 13, 2017, the U.S. House Committee on Appropriations voted to block implementation of a “death with dignity” statute passed by the District of Columbia. Further, 11 House members – including 6 Democrats – have introduced a resolution asserting that PAS undermines a key safeguard that protects our nation’s most vulnerable citizens, including the elderly, people with disabilities, and people experiencing psychiatric diagnoses.10
The American Medical Association is steadfast in its opposition to PAS and euthanasia. In its latest Code of Ethics, the AMA reaffirmed its long-held position that “allowing physicians to engage in assisted suicide would cause more harm than good. Physician-assisted suicide is fundamentally incompatible with the physician’s role as healer, would be difficult or impossible to control, and would pose serious societal risks. … Instead of participating in assisted suicide, physicians must aggressively respond to the needs of patients at the end of life.”11
References
1. “Physician-assisted suicide,” Internal Medicine News, Oct. 14, 2013.
2. Baxter v. State of Montana, 224 P. 3d 1211 (2010).
3. “Majority of Americans Remain Supportive of Euthanasia,” Gallup News, June 12, 2017.
4. Statistics available at public.health.oregon.gov under Oregon Death with Dignity Act.
5. JAMA. 2016 Jul 5;316(1):79-90.
6. Vacco v. Quill, 117 S. Ct. 2293 (1997).
7. Washington v. Glucksberg, 521 U.S. 702 (1997).
8. Myers v. Schneiderman, New York Court of Appeals, 2017.
9. “Legalizing assisted suicide has stalled at every level,” Washington Post, Oct. 24, 2017.
10. H. Con. Res. 80, 115th Congress (2017-2018).
11. AMA Code of Medical Ethics §5.7 (2017).
Question: Choose the best answer regarding physician-assisted suicide in the United States:
A. It is now legal in most states.
B. Under California law, assisting or causing one to commit suicide, including physician-assisted suicide, still remains a felony.
C. Both the U.S. Supreme Court and the New York Court of Appeals have held there is no constitutional right to physician-assisted suicide.
D. The American Medical Association is neutral on the issue.
E. Pain relief is the overriding reason for patients who request physician-assisted suicide.
Answer: C. We reviewed this topic in one of our regular columns in 2013.1 At that time, efforts to legalize physician-assisted suicide (PAS) appeared to be gathering momentum across the country, with four jurisdictions having legalized the practice, beginning with Oregon in 1994. The other states were Washington, Vermont, and Montana, whose Supreme Court held that there was no public interest reason against the practice.2
Since that time, California, Colorado, and the District of Columbia have joined the group. Currently, PAS – but not euthanasia – is legally available in these jurisdictions and in Switzerland, but both can be legally practiced in Belgium, Canada, Colombia, Luxembourg, and the Netherlands.
All state statutes permitting PAS provide similar provisions and safeguards. Only competent individuals who are terminally ill, i.e., death expected within 6 months, can make a request for a lethal dose of medication to carry out the suicidal act. The request to the doctor is first made verbally, then in writing, and a second opinion must be obtained to confirm the patient’s intent, understanding, and free choice. There is also a waiting period.
Public support for euthanasia and PAS in the United States is said to have plateaued since the 1990s. But a significant number of Americans, 67%, still favor PAS, up from 56% a decade ago.3 However, not many patients resort to PAS – usually those with terminal cancers or neuromuscular conditions – and only a minority of physicians are participants.
For example, 61 physicians in Oregon wrote a total of 115 prescriptions in 2012; there were 77 known Death With Dignity Act deaths in Oregon that year.4 In Oregon and Washington State, less than 1% of licensed physicians write prescriptions for physician-assisted suicide each year. In contrast, about half or more of physicians in the Netherlands and Belgium reported ever having received a request, and 60% of Dutch physicians have granted such requests.
The California Department of Public Health reported that 111 terminally ill patients availed themselves of California’s End of Life Option Act in the 7 months after it became effective on June 9, 2016.
In a recent review on euthanasia and PAS for the period 1947-2016, Ezekiel Emanuel, MD, and colleagues noted that typical patients were older, white, and well educated, and pain was mostly not reported as the primary motivation.5 A large portion of patients receiving PAS in Oregon and Washington were enrolled in hospice or palliative care. Abuses have not been apparent.
In the vast majority of jurisdictions, assisting or causing one to commit suicide, including PAS, still remains a crime; for example, it is considered manslaughter under Hawaii state law §707-702.
In distinguishing between assisting suicide and withdrawing life-sustaining treatment, the U.S. Supreme Court’s landmark 1997 Vacco v. Quill decision emphasized issues of causation and intent.6 On causation, the court reasoned that when a patient refuses life-sustaining treatment, he dies from an underlying fatal disease; but if a patient ingests a lethal medication, he is killed by that medication. As to intent, a physician who honors a patient’s refusal of treatment purposefully intends only to respect his patient’s wishes and to cease doing futile or degrading things. On the other hand, a doctor who assists a suicide “must, necessarily and indubitably, intend primarily that the patient be made dead.”
In its companion case Washington v. Glucksberg, the Supreme Court held that the asserted “right” to assistance in committing suicide is not a fundamental liberty interest protected by the due process clause.7
State supreme courts in Florida, New Mexico, and elsewhere have likewise rebuffed claims of any constitutional right to PAS. The latest court to so rule is in New York, which has a long history of criminalizing assisted suicide.8 The New York Court of Appeals recently addressed claims brought by three terminally ill individuals, several medical providers, and a nonprofit entity seeking a declaration that New York’s “assisted suicide” statutes exclude physicians from prescribing a lethal dose of drugs to terminally ill, competent patients.
The court unequivocally rejected such claims and affirmed that a physician who assists a suicide by prescribing lethal doses of drugs is subject to criminal prosecution for second-degree manslaughter. It refused to regard PAS as being different from assisted suicide in general, and it rejected the constitutional claim to assisted suicide by a terminally ill person. The state appeals court reiterated the U.S. Supreme Court’s distinction between refusing life-sustaining treatment and assisted suicide, the former being “at least partially rooted in notions of bodily integrity, as the right to refuse treatment is a consequence of a person’s right to resist unwanted bodily invasions.” The New York Court of Appeals also noted that the state has a legitimate purpose and a rational basis for guarding against the risks of mistake and abuse.
These developments may signal a shift away from the legalization of PAS, as recently suggested in a Washington Post article.9 According to the end-of-life advocacy organization Compassion and Choices, none of the 27 states where such measures were introduced in 2017 passed them into law, including states such as Connecticut, Hawaii, and Rhode Island. In Central and Eastern Europe, support is decreasing, whereas the opposite is true in Western Europe.
U.S. federal lawmakers also appear to be pushing back. On July 13, 2017, the U.S. House Committee on Appropriations voted to block implementation of a “death with dignity” statute passed by the District of Columbia. Further, 11 House members – including 6 Democrats – have introduced a resolution asserting that PAS undermines a key safeguard that protects our nation’s most vulnerable citizens, including the elderly, people with disabilities, and people experiencing psychiatric diagnoses.10
The American Medical Association is steadfast in its opposition to PAS and euthanasia. In its latest Code of Ethics, the AMA reaffirmed its long-held position that “allowing physicians to engage in assisted suicide would cause more harm than good. Physician-assisted suicide is fundamentally incompatible with the physician’s role as healer, would be difficult or impossible to control, and would pose serious societal risks. … Instead of participating in assisted suicide, physicians must aggressively respond to the needs of patients at the end of life.”11
References
1. “Physician-assisted suicide,” Internal Medicine News, Oct. 14, 2013.
2. Baxter v. State of Montana, 224 P. 3d 1211 (2010).
3. “Majority of Americans Remain Supportive of Euthanasia,” Gallup News, June 12, 2017.
4. Statistics available at public.health.oregon.gov under Oregon Death with Dignity Act.
5. JAMA. 2016 Jul 5;316(1):79-90.
6. Vacco v. Quill, 117 S. Ct. 2293 (1997).
7. Washington v. Glucksberg, 521 U.S. 702 (1997).
8. Myers v. Schneiderman, New York Court of Appeals, 2017.
9. “Legalizing assisted suicide has stalled at every level,” Washington Post, Oct. 24, 2017.
10. H. Con. Res. 80, 115th Congress (2017-2018).
11. AMA Code of Medical Ethics §5.7 (2017).
Question: Choose the best answer regarding physician-assisted suicide in the United States:
A. It is now legal in most states.
B. Under California law, assisting or causing one to commit suicide, including physician-assisted suicide, still remains a felony.
C. Both the U.S. Supreme Court and the New York Court of Appeals have held there is no constitutional right to physician-assisted suicide.
D. The American Medical Association is neutral on the issue.
E. Pain relief is the overriding reason for patients who request physician-assisted suicide.
Answer: C. We reviewed this topic in one of our regular columns in 2013.1 At that time, efforts to legalize physician-assisted suicide (PAS) appeared to be gathering momentum across the country, with four jurisdictions having legalized the practice, beginning with Oregon in 1994. The other states were Washington, Vermont, and Montana, whose Supreme Court held that there was no public interest reason against the practice.2
Since that time, California, Colorado, and the District of Columbia have joined the group. Currently, PAS – but not euthanasia – is legally available in these jurisdictions and in Switzerland, but both can be legally practiced in Belgium, Canada, Colombia, Luxembourg, and the Netherlands.
All state statutes permitting PAS provide similar provisions and safeguards. Only competent individuals who are terminally ill, i.e., death expected within 6 months, can make a request for a lethal dose of medication to carry out the suicidal act. The request to the doctor is first made verbally, then in writing, and a second opinion must be obtained to confirm the patient’s intent, understanding, and free choice. There is also a waiting period.
Public support for euthanasia and PAS in the United States is said to have plateaued since the 1990s. But a significant number of Americans, 67%, still favor PAS, up from 56% a decade ago.3 However, not many patients resort to PAS – usually those with terminal cancers or neuromuscular conditions – and only a minority of physicians are participants.
For example, 61 physicians in Oregon wrote a total of 115 prescriptions in 2012; there were 77 known Death With Dignity Act deaths in Oregon that year.4 In Oregon and Washington State, less than 1% of licensed physicians write prescriptions for physician-assisted suicide each year. In contrast, about half or more of physicians in the Netherlands and Belgium reported ever having received a request, and 60% of Dutch physicians have granted such requests.
The California Department of Public Health reported that 111 terminally ill patients availed themselves of California’s End of Life Option Act in the 7 months after it became effective on June 9, 2016.
In a recent review on euthanasia and PAS for the period 1947-2016, Ezekiel Emanuel, MD, and colleagues noted that typical patients were older, white, and well educated, and pain was mostly not reported as the primary motivation.5 A large portion of patients receiving PAS in Oregon and Washington were enrolled in hospice or palliative care. Abuses have not been apparent.
In the vast majority of jurisdictions, assisting or causing one to commit suicide, including PAS, still remains a crime; for example, it is considered manslaughter under Hawaii state law §707-702.
In distinguishing between assisting suicide and withdrawing life-sustaining treatment, the U.S. Supreme Court’s landmark 1997 Vacco v. Quill decision emphasized issues of causation and intent.6 On causation, the court reasoned that when a patient refuses life-sustaining treatment, he dies from an underlying fatal disease; but if a patient ingests a lethal medication, he is killed by that medication. As to intent, a physician who honors a patient’s refusal of treatment purposefully intends only to respect his patient’s wishes and to cease doing futile or degrading things. On the other hand, a doctor who assists a suicide “must, necessarily and indubitably, intend primarily that the patient be made dead.”
In its companion case Washington v. Glucksberg, the Supreme Court held that the asserted “right” to assistance in committing suicide is not a fundamental liberty interest protected by the due process clause.7
State supreme courts in Florida, New Mexico, and elsewhere have likewise rebuffed claims of any constitutional right to PAS. The latest court to so rule is in New York, which has a long history of criminalizing assisted suicide.8 The New York Court of Appeals recently addressed claims brought by three terminally ill individuals, several medical providers, and a nonprofit entity seeking a declaration that New York’s “assisted suicide” statutes exclude physicians from prescribing a lethal dose of drugs to terminally ill, competent patients.
The court unequivocally rejected such claims and affirmed that a physician who assists a suicide by prescribing lethal doses of drugs is subject to criminal prosecution for second-degree manslaughter. It refused to regard PAS as being different from assisted suicide in general, and it rejected the constitutional claim to assisted suicide by a terminally ill person. The state appeals court reiterated the U.S. Supreme Court’s distinction between refusing life-sustaining treatment and assisted suicide, the former being “at least partially rooted in notions of bodily integrity, as the right to refuse treatment is a consequence of a person’s right to resist unwanted bodily invasions.” The New York Court of Appeals also noted that the state has a legitimate purpose and a rational basis for guarding against the risks of mistake and abuse.
These developments may signal a shift away from the legalization of PAS, as recently suggested in a Washington Post article.9 According to the end-of-life advocacy organization Compassion and Choices, none of the 27 states where such measures were introduced in 2017 passed them into law, including states such as Connecticut, Hawaii, and Rhode Island. In Central and Eastern Europe, support is decreasing, whereas the opposite is true in Western Europe.
U.S. federal lawmakers also appear to be pushing back. On July 13, 2017, the U.S. House Committee on Appropriations voted to block implementation of a “death with dignity” statute passed by the District of Columbia. Further, 11 House members – including 6 Democrats – have introduced a resolution asserting that PAS undermines a key safeguard that protects our nation’s most vulnerable citizens, including the elderly, people with disabilities, and people experiencing psychiatric diagnoses.10
The American Medical Association is steadfast in its opposition to PAS and euthanasia. In its latest Code of Ethics, the AMA reaffirmed its long-held position that “allowing physicians to engage in assisted suicide would cause more harm than good. Physician-assisted suicide is fundamentally incompatible with the physician’s role as healer, would be difficult or impossible to control, and would pose serious societal risks. … Instead of participating in assisted suicide, physicians must aggressively respond to the needs of patients at the end of life.”11
References
1. “Physician-assisted suicide,” Internal Medicine News, Oct. 14, 2013.
2. Baxter v. State of Montana, 224 P. 3d 1211 (2010).
3. “Majority of Americans Remain Supportive of Euthanasia,” Gallup News, June 12, 2017.
4. Statistics available at public.health.oregon.gov under Oregon Death with Dignity Act.
5. JAMA. 2016 Jul 5;316(1):79-90.
6. Vacco v. Quill, 117 S. Ct. 2293 (1997).
7. Washington v. Glucksberg, 521 U.S. 702 (1997).
8. Myers v. Schneiderman, New York Court of Appeals, 2017.
9. “Legalizing assisted suicide has stalled at every level,” Washington Post, Oct. 24, 2017.
10. H. Con. Res. 80, 115th Congress (2017-2018).
11. AMA Code of Medical Ethics §5.7 (2017).
Clinic eases pediatric-adult transition in sickle cell disease
CONCORD, N.C. – Teenage sickle cell disease patients transitioning to adulthood can often find the move to adult providers challenging, causing some patients to lose interest in self-care at a critical point in life, but a transitional program can help them develop the skills they need to manage their disease and avoid risky behaviors, according to psychologist Anya Griffin, PhD.
Managing pain in teenagers and young adults with sickle cell disease (SCD) is fraught with challenges, said Dr. Griffin, who led the SCD transition program at Children’s Healthcare of Atlanta and is now the clinical director of the Stanford (Calif.) Children’s Health Pediatric Rehabilitation Program, an intensive pain management program for pediatric chronic pain.
“Think about who you are when you’re a teenager, when you’re a young adult, what’s going on: dating, sex, parties, college, all-night study sessions,” she said at a Sickle Cell Disease Symposium held by Carolinas Health Care System. “But in the world of sickle cell, these are critical choices that have dire consequences.” Those consequences include dehydration from drinking, fatigue from lack of sleep, and pain crises.
Compounding these challenges is the prevalence of depression and other psychological complications in this age group. And among SCD patients, there can be a sense of grief, Dr. Griffin said.
“Grief is something we tend not to talk too much about,” she said. That grief can manifest in excessive absences from school or work. “Sudden academic declines are something we really have to pay attention to,” Dr. Griffin said.
Silent strokes are also of concern in this age group. “I don’t know if we fully understand the impact on each individual unless we do neuropsychological testing,” she said. The intervals for neuropsychological testing should be in childhood to determine a baseline, then again in adolescence and adulthood. For college-bound students, testing may be a requirement for them to receive medical and physical accommodations, Dr. Griffin said.
While in their late teens and early twenties, SCD patients often rely on pediatric care and can get caught between pediatric and adult providers, she said. That prompted Children’s Healthcare of Atlanta to start a program that essentially hands off those patients from pediatric to adult providers and works with patients to reduce their risks.
As teens approach age 18, they come to the clinic to meet with adult providers and tour the facility. The program involves social workers, vocational and school counselors, and mentors and peer support. “It takes an entire village to address the concerns of transition,” Dr. Griffin said.
Support groups and home visits by providers can also play a key role in the transition protocol, as can telemedicine. “The technology is now there; now we have to figure out how we’re going to start using it,” she said.
This full transitional process can involve multiple appointments with a variety of providers. It’s also important that patients – not parents – interact with providers, Dr. Griffin said.
From January 2007 to September 2012, 74 patients participated in the SCD transition at Children’s Healthcare of Atlanta. Participants who attended more than one transition clinic visit in Atlanta (n = 9) had an average baseline score of 60% on an SCD knowledge questionnaire. But 6 months later, those scores improved to 80%, on average. “We found that teenagers who came to that type of clinic more than once improved pretty well,” Dr. Griffin said.
Dr. Griffin reported having no relevant financial disclosures.
CONCORD, N.C. – Teenage sickle cell disease patients transitioning to adulthood can often find the move to adult providers challenging, causing some patients to lose interest in self-care at a critical point in life, but a transitional program can help them develop the skills they need to manage their disease and avoid risky behaviors, according to psychologist Anya Griffin, PhD.
Managing pain in teenagers and young adults with sickle cell disease (SCD) is fraught with challenges, said Dr. Griffin, who led the SCD transition program at Children’s Healthcare of Atlanta and is now the clinical director of the Stanford (Calif.) Children’s Health Pediatric Rehabilitation Program, an intensive pain management program for pediatric chronic pain.
“Think about who you are when you’re a teenager, when you’re a young adult, what’s going on: dating, sex, parties, college, all-night study sessions,” she said at a Sickle Cell Disease Symposium held by Carolinas Health Care System. “But in the world of sickle cell, these are critical choices that have dire consequences.” Those consequences include dehydration from drinking, fatigue from lack of sleep, and pain crises.
Compounding these challenges is the prevalence of depression and other psychological complications in this age group. And among SCD patients, there can be a sense of grief, Dr. Griffin said.
“Grief is something we tend not to talk too much about,” she said. That grief can manifest in excessive absences from school or work. “Sudden academic declines are something we really have to pay attention to,” Dr. Griffin said.
Silent strokes are also of concern in this age group. “I don’t know if we fully understand the impact on each individual unless we do neuropsychological testing,” she said. The intervals for neuropsychological testing should be in childhood to determine a baseline, then again in adolescence and adulthood. For college-bound students, testing may be a requirement for them to receive medical and physical accommodations, Dr. Griffin said.
While in their late teens and early twenties, SCD patients often rely on pediatric care and can get caught between pediatric and adult providers, she said. That prompted Children’s Healthcare of Atlanta to start a program that essentially hands off those patients from pediatric to adult providers and works with patients to reduce their risks.
As teens approach age 18, they come to the clinic to meet with adult providers and tour the facility. The program involves social workers, vocational and school counselors, and mentors and peer support. “It takes an entire village to address the concerns of transition,” Dr. Griffin said.
Support groups and home visits by providers can also play a key role in the transition protocol, as can telemedicine. “The technology is now there; now we have to figure out how we’re going to start using it,” she said.
This full transitional process can involve multiple appointments with a variety of providers. It’s also important that patients – not parents – interact with providers, Dr. Griffin said.
From January 2007 to September 2012, 74 patients participated in the SCD transition at Children’s Healthcare of Atlanta. Participants who attended more than one transition clinic visit in Atlanta (n = 9) had an average baseline score of 60% on an SCD knowledge questionnaire. But 6 months later, those scores improved to 80%, on average. “We found that teenagers who came to that type of clinic more than once improved pretty well,” Dr. Griffin said.
Dr. Griffin reported having no relevant financial disclosures.
CONCORD, N.C. – Teenage sickle cell disease patients transitioning to adulthood can often find the move to adult providers challenging, causing some patients to lose interest in self-care at a critical point in life, but a transitional program can help them develop the skills they need to manage their disease and avoid risky behaviors, according to psychologist Anya Griffin, PhD.
Managing pain in teenagers and young adults with sickle cell disease (SCD) is fraught with challenges, said Dr. Griffin, who led the SCD transition program at Children’s Healthcare of Atlanta and is now the clinical director of the Stanford (Calif.) Children’s Health Pediatric Rehabilitation Program, an intensive pain management program for pediatric chronic pain.
“Think about who you are when you’re a teenager, when you’re a young adult, what’s going on: dating, sex, parties, college, all-night study sessions,” she said at a Sickle Cell Disease Symposium held by Carolinas Health Care System. “But in the world of sickle cell, these are critical choices that have dire consequences.” Those consequences include dehydration from drinking, fatigue from lack of sleep, and pain crises.
Compounding these challenges is the prevalence of depression and other psychological complications in this age group. And among SCD patients, there can be a sense of grief, Dr. Griffin said.
“Grief is something we tend not to talk too much about,” she said. That grief can manifest in excessive absences from school or work. “Sudden academic declines are something we really have to pay attention to,” Dr. Griffin said.
Silent strokes are also of concern in this age group. “I don’t know if we fully understand the impact on each individual unless we do neuropsychological testing,” she said. The intervals for neuropsychological testing should be in childhood to determine a baseline, then again in adolescence and adulthood. For college-bound students, testing may be a requirement for them to receive medical and physical accommodations, Dr. Griffin said.
While in their late teens and early twenties, SCD patients often rely on pediatric care and can get caught between pediatric and adult providers, she said. That prompted Children’s Healthcare of Atlanta to start a program that essentially hands off those patients from pediatric to adult providers and works with patients to reduce their risks.
As teens approach age 18, they come to the clinic to meet with adult providers and tour the facility. The program involves social workers, vocational and school counselors, and mentors and peer support. “It takes an entire village to address the concerns of transition,” Dr. Griffin said.
Support groups and home visits by providers can also play a key role in the transition protocol, as can telemedicine. “The technology is now there; now we have to figure out how we’re going to start using it,” she said.
This full transitional process can involve multiple appointments with a variety of providers. It’s also important that patients – not parents – interact with providers, Dr. Griffin said.
From January 2007 to September 2012, 74 patients participated in the SCD transition at Children’s Healthcare of Atlanta. Participants who attended more than one transition clinic visit in Atlanta (n = 9) had an average baseline score of 60% on an SCD knowledge questionnaire. But 6 months later, those scores improved to 80%, on average. “We found that teenagers who came to that type of clinic more than once improved pretty well,” Dr. Griffin said.
Dr. Griffin reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM A MEETING ON SICKLE CELL DISEASE
Lumbar Microlaminectomy vs Traditional Laminectomy
Lumbar spinal stenosis (LSS) is a common debilitating issue in older patients. Open laminectomies traditionally are the standard treatment for LSS; however, minimally invasive surgery (MIS) has recently become a popular option to facilitate recovery and improve efficiency of care regarding spine procedures.
Guiot and colleagues described the technique for an MIS decompressive lumbar laminectomy procedure.1 The surgery may represent an important strategy to improve the efficiency of care for patients with severe LSS. Several authors have reported clinical benefits with the MIS lumbar laminectomy, leading to a significant improvement in the Oswetry Disability Index (ODI) 25 in the degenerative stenosis group in cases of LSS.2-5 In a recent reviewof 13 studies Wong and colleagues concluded that the MIS laminectomy was efficacious in terms of symptomatic relief and patient satisfaction for patients with LSS.6 Further, Rosen and colleaguesfound a significant improvement in the ODI scores and in the Short Form-36 body pain and physical functions scores in patients aged ≥ 75 years.7
Perioperative measures, including blood loss and narcotic consumption, have been shown to significantly decrease with MIS surgery compared with open decompression.8,9 Decreased narcotic use is of particular interest for the geriatric population because it is expected to allow those patients to remain more physically active and mentally agile.10
Also, long-term success is important when assessing the efficacy of new MIS procedures. Oertel and colleagues found that 85% of patients reported long-term success after unilateral laminotomy of bilateral decompression (ULBD).11 These results indicate that a MIS laminectomy is effective in older patients with LSS and neurogenic claudication.
Although there are numerous MIS approaches to alleviating LSS, more research is needed to determine whether it is superior to the open laminectomy.9,12,13 Skovrliand and colleagues reviewed publications comparing ULBD and open laminectomies and determined that currently insufficient evidence exists to define which technique leads to more positive outcomes.14 Thus, the purpose of this study is 2-fold. First, this study adds to the current research by comparing estimated blood loss and length of stay (LOS) for microscopic MIS laminectomy vs traditional laminectomy. Second, this study aims to address the difference in health care costs between the 2 types of surgery in the VHA.
The U.S. health care system is facing several challenges and in particular pressure for cost reduction.15 VA hospitals are not exempt from those challenges, and their operating budgets are influenced by political and economic factors.16 Because of those challenges, cost-effectiveness is gaining importance.7 Future decisions for procedure coverage and reimbursement rates are likely to consider ratios like the cost to quality-adjusted life-years (QALY). Improving this ratio requires a reduction of cost and/or an improvement in outcome.
Minimally invasive spine surgery (MISS) may lower the cost of spine procedures. Wang and colleagues reported that minimally invasive posterior lumbar interbody fusion (PLIF) led to shorter stay and lower blood loss compared with traditional PLIF.17 These improvements led to about $8,000 in savings for a single-level PLIF.17
Lumbar degenerative disease is a frequently encountered condition, and lumbar laminectomy is one of the most frequently performed spine procedures at VA hospitals. Consequently, MISS may be an important strategy for the VA to face systematic challenges. At the Southern Arizona VA Health Care System (SAVAHCS) in Tucson, the authors converted lumbar laminectomies from traditional open surgery to a MIS procedure using a tubular retractor system and a paramedian approach. To the authors’ knowledge, no studies have evaluated outcomes and cost efficiency of MIS surgery at the VA. The results of such a study may be instrumental in choosing which surgery is appropriate in a patient-centered health care model.
Material and Methods
Fifty veterans with severe lumbar stenosis and neurogenic claudication underwent a 1- or 2-level laminectomy at SAVAHCS (Table). A traditional laminectomy was performed for all patients until conversion to the MIS procedure, then all subsequent patients underwent the microlaminectomy. There was 1 female patient in each group. The preoperative magnetic resonance imaging (MRI) of the patients showed severe spinal canal stenosis defined radiographically by the absence of cerebrospinal fluid signal at the affected level on MRI (Figures 1A and 2A) and clinically by the presence of neurogenic claudication.
Procedure
The open laminectomies were performed in a traditional midline approach with removal of the spinous process along with the lamina bilaterally to provide spinal canal decompression (Figure 2).
The patients were given the choice of going home or being admitted. Overall admission costs were determined by the VA hospital following described models.18 The LOS in rehabilitation were determined from the records of the SAVAHCS rehabilitation center.
Results
There was not a significant difference in age between the 2 groups; mean age was 69.7 ± 9.8 years for the traditional laminectomy group and 64.4 ± 8.3 years for the MIS group. Operating room time was just over 2 hours on average in both groups. Blood loss was estimated and reported by the surgeon and the anesthesiologist, based on values from the surgical suction system. Patients in the MIS group lost on average 46 cc ± 70 cc compared with 135 cc ± 78 cc in the traditional group. The average number of operated levels was higher in the traditional group (1.7 ± 0.5) compared with the MIS group (1.4 ± 0.5), but this difference did not reach significance (P > .05).
Length of Stay and Cost
The LOS was lower for the MIS group, and 76% chose to be discharged from the recovery room. After a traditional laminectomy, the average patient’s stay was 3 days in the hospital and 5 days in the rehabilitation center. The average MIS group patient stayed < 1 day in the hospital. There were no readmissions within 30 days and no severe morbidity (including no new neurologic deficits or death) in the MIS cohort.
Only 1 MIS patient needed transfer to the rehabilitation center. The estimated cost of care (hospital and rehabilitation) for the traditional group was $10,846 compared with $1,961 for the MIS group.
Discussion
In the authors’ experience, the use of MISS microlaminectomy for the treatment of LSS seems to have led to shorter hospital stays and faster recoveries. Some of the possible reasons for faster patient mobilization included a reduction in postoperative pain and the absence of a wound drain. Larger dissections with a traditional laminectomy often lead to the placement of a wound drain, which requires an inpatient stay until the wound output reaches a certain threshold. The absence of a drain and the reduction in pain with the MISS approach allowed the providers to focus on early ambulation and discharge planning. The microlaminectomy technique allowed for a proper surgical decompression with less tissue dissection than is required for a traditional laminectomy. Previous studies have shown that the microlaminectomy technique provides significant symptomatic relief.5-7,17
In most cases, the microlaminectomy can be performed on an outpatient basis. The improvement in bed availability is particularly important as surgical procedures may be delayed when hospitals operate at full capacity. Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves availability, allowing for better patient access to health care.19
Other authors have studied opportunities to transform inpatient neurosurgical care into outpatient procedures. For instance, Purzner and colleagues presented a large series of successful outpatient neurosurgical cases, including craniotomies, cervical fusions, and lumbar microdiscectomies.20 The MISS techniques offer a critical option to facilitate postoperative recovery and improve efficiency of care in regards to spine procedures.5,17
Cost-Effectiveness Within the VHA
The VA has been described as one of the best health care systems in the U.S.9 The arguments in favor of the VA system include its integrated computerized system and its resistance to health care cost inflation over the years.21 The $186.5 billion 2018 fiscal year VA budget is surpassed only by the total DoD budget, and it is expected to rise substantially in the near future.22
Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves bed availability and reduces cost.19 The authors have demonstrated that a minimally invasive unilateral paramedian approach for the treatment of lumbar stenosis leads to shorter hospital stay, improved bed availability, and lower cost while allowing for a proper surgical decompression. These clinical results are in accord with previous MIS surgery studies.5,17 The improvement in bed availability is particularly important within the VA system. Elective surgeries occasionally are delayed or cancelled because hospitals operate at full capacity. However, the authors’ outpatient microlaminectomy patients avoid delays or cancellations.
Given that both laminectomy procedures use similar operating room resources (time and material), the lower LOS associated with the microlaminectomy translates in cost saving. At SAVAHCS, acute care hospitalization is estimated at $3,000 per day when accounting for various costs, including nursing, pharmacy, ancillary services, and maintenance. The MIS procedure costs about $9,000 less than the open surgery. Over a 2-year period with 37 MIS patients, SAVAHCS saved about $300,000.
Patient Satisfaction
Patient satisfaction was assessed 1 day after the lumbar microdecompression outpatient surgery. Patients were asked to rate their overall surgical experience on a scale of 1 (worst) to 10 (best). All 24 patients who were contacted following outpatient lumbar microdecompression surgery rated the experience 10. These results indicate that patients do not expect or desire an admission following lumbar surgery, and they may recover comfortably at home. Studies are needed to compare outpatient and inpatient satisfaction ratings.
Conclusion
In this small sample, lumbar microlaminectomy significantly reduced LOS, successfully decompressed the spinal canal, and achieved symptomatic relief. Also, the procedure is associated with a lower blood loss than a traditional laminectomy and may reduce the rate of perioperative morbidity over time. In addition to faster recovery, the reduction in LOS can improve access to care by increasing the availability to inpatient admission.
1. Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila PA 1976). 2002;27(4):432-438.
2. Rahman M, Summers LE, Richter B, Mimran RI, Jacob RP. Comparison of techniques for decompressive lumbar laminectomy: the minimally invasive versus the “classic” open approach. Minim Invasive Neurosurg. 2008;51(2)100-105.
3. Sasai K, Umeda M, Maruyama T, Wakabayashi E, Iida H. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008;9(6):554-559.
4. Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009;18(5):672-678.
5. Yagi M, Okada, E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10(4):293-299.
6. Wong AP, Smith ZA, Lall RR, Bresnahan LE, Fessler RG. The microendoscopic decompression of lumbar stenosis: a review of the current literature and clinical results. Minim Invasive Surg. 2012;2012:325095.
7. Rosen DS, O’Toole JE, Eichholz KM, et al. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60(3):503-509.
8. Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51(suppl 5):S146-S154.
9. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21(2):179-186.
10. Avila MJ, Walter CM, Baaj AA. Outcomes and complications of minimally invasive surgery of the lumbar spine in the elderly. Cureus. 2016;8(3):e519.
11. Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59(6):1264-1269.
12. Haddadi K, Ganjeh Qazvini HR. Outcome after surgery of lumbar spinal stenosis: a randomized comparison of bilateral laminotomy, trumpet laminectomy, and conventional laminectomy. Front Surg. 2016;3:199.
13. Watanabe K, Matsumoto M, Ikegami T, et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine. 2011;14(1):51-58.
14. Skovrlj B, Belton P, Zarzour H, Qureshi SA. Perioperative outcomes in minimally invasive lumbar spine surgery: a systematic review. World J. Orthop. 2015;6(11):996-1005.
15. Hellander I. The deepening crisis in U.S. health care: a review of data. Int J Health Serv. 2011;41(3):575-586.
16. Chokshi DA. Improving health care for veterans—a watershed moment for the VA. N Engl J Med. 2014;371(4):297-299.
17. Wang MY, Cummock MD, Yu Y, Trivedi RA. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg Spine. 2010;12(6):694-699.
18. Barnett PG. Determination of VA health care costs. Med Care Res Rev. 2003;60(suppl 3):S124-S141.
19. Congressional Budget Office. The health care system for veterans: interim report. https://www.cbo.gov/sites/default/files/110th-congress-2007-2008/reports/12-21-va_healthcare.pdf. Published December 2007. Accessed October 13, 2017.
20. Purzner T, Purzner J, Massicotte EM, Bernstein M. Outpatient brain tumor surgery and spinal decompression: a prospective study of 1003 patients. Neurosurgery. 2011;69(1):119-126.
21. Waller D. How veterans’ hospitals became the best in health care. Time Magazine. http://content.time.com/time/magazine/article/0,9171,1376238,00.html. Published August 27, 2006. Accessed October 13, 2017.
22. U.S. Department of Veterans Affairs, Office of Budget. Annual budget submission—office of budget. https://www.va.gov/budget/products.asp. Updated July 12, 2017. Published October 13, 2017. Accessed October 27, 2017.
Lumbar spinal stenosis (LSS) is a common debilitating issue in older patients. Open laminectomies traditionally are the standard treatment for LSS; however, minimally invasive surgery (MIS) has recently become a popular option to facilitate recovery and improve efficiency of care regarding spine procedures.
Guiot and colleagues described the technique for an MIS decompressive lumbar laminectomy procedure.1 The surgery may represent an important strategy to improve the efficiency of care for patients with severe LSS. Several authors have reported clinical benefits with the MIS lumbar laminectomy, leading to a significant improvement in the Oswetry Disability Index (ODI) 25 in the degenerative stenosis group in cases of LSS.2-5 In a recent reviewof 13 studies Wong and colleagues concluded that the MIS laminectomy was efficacious in terms of symptomatic relief and patient satisfaction for patients with LSS.6 Further, Rosen and colleaguesfound a significant improvement in the ODI scores and in the Short Form-36 body pain and physical functions scores in patients aged ≥ 75 years.7
Perioperative measures, including blood loss and narcotic consumption, have been shown to significantly decrease with MIS surgery compared with open decompression.8,9 Decreased narcotic use is of particular interest for the geriatric population because it is expected to allow those patients to remain more physically active and mentally agile.10
Also, long-term success is important when assessing the efficacy of new MIS procedures. Oertel and colleagues found that 85% of patients reported long-term success after unilateral laminotomy of bilateral decompression (ULBD).11 These results indicate that a MIS laminectomy is effective in older patients with LSS and neurogenic claudication.
Although there are numerous MIS approaches to alleviating LSS, more research is needed to determine whether it is superior to the open laminectomy.9,12,13 Skovrliand and colleagues reviewed publications comparing ULBD and open laminectomies and determined that currently insufficient evidence exists to define which technique leads to more positive outcomes.14 Thus, the purpose of this study is 2-fold. First, this study adds to the current research by comparing estimated blood loss and length of stay (LOS) for microscopic MIS laminectomy vs traditional laminectomy. Second, this study aims to address the difference in health care costs between the 2 types of surgery in the VHA.
The U.S. health care system is facing several challenges and in particular pressure for cost reduction.15 VA hospitals are not exempt from those challenges, and their operating budgets are influenced by political and economic factors.16 Because of those challenges, cost-effectiveness is gaining importance.7 Future decisions for procedure coverage and reimbursement rates are likely to consider ratios like the cost to quality-adjusted life-years (QALY). Improving this ratio requires a reduction of cost and/or an improvement in outcome.
Minimally invasive spine surgery (MISS) may lower the cost of spine procedures. Wang and colleagues reported that minimally invasive posterior lumbar interbody fusion (PLIF) led to shorter stay and lower blood loss compared with traditional PLIF.17 These improvements led to about $8,000 in savings for a single-level PLIF.17
Lumbar degenerative disease is a frequently encountered condition, and lumbar laminectomy is one of the most frequently performed spine procedures at VA hospitals. Consequently, MISS may be an important strategy for the VA to face systematic challenges. At the Southern Arizona VA Health Care System (SAVAHCS) in Tucson, the authors converted lumbar laminectomies from traditional open surgery to a MIS procedure using a tubular retractor system and a paramedian approach. To the authors’ knowledge, no studies have evaluated outcomes and cost efficiency of MIS surgery at the VA. The results of such a study may be instrumental in choosing which surgery is appropriate in a patient-centered health care model.
Material and Methods
Fifty veterans with severe lumbar stenosis and neurogenic claudication underwent a 1- or 2-level laminectomy at SAVAHCS (Table). A traditional laminectomy was performed for all patients until conversion to the MIS procedure, then all subsequent patients underwent the microlaminectomy. There was 1 female patient in each group. The preoperative magnetic resonance imaging (MRI) of the patients showed severe spinal canal stenosis defined radiographically by the absence of cerebrospinal fluid signal at the affected level on MRI (Figures 1A and 2A) and clinically by the presence of neurogenic claudication.
Procedure
The open laminectomies were performed in a traditional midline approach with removal of the spinous process along with the lamina bilaterally to provide spinal canal decompression (Figure 2).
The patients were given the choice of going home or being admitted. Overall admission costs were determined by the VA hospital following described models.18 The LOS in rehabilitation were determined from the records of the SAVAHCS rehabilitation center.
Results
There was not a significant difference in age between the 2 groups; mean age was 69.7 ± 9.8 years for the traditional laminectomy group and 64.4 ± 8.3 years for the MIS group. Operating room time was just over 2 hours on average in both groups. Blood loss was estimated and reported by the surgeon and the anesthesiologist, based on values from the surgical suction system. Patients in the MIS group lost on average 46 cc ± 70 cc compared with 135 cc ± 78 cc in the traditional group. The average number of operated levels was higher in the traditional group (1.7 ± 0.5) compared with the MIS group (1.4 ± 0.5), but this difference did not reach significance (P > .05).
Length of Stay and Cost
The LOS was lower for the MIS group, and 76% chose to be discharged from the recovery room. After a traditional laminectomy, the average patient’s stay was 3 days in the hospital and 5 days in the rehabilitation center. The average MIS group patient stayed < 1 day in the hospital. There were no readmissions within 30 days and no severe morbidity (including no new neurologic deficits or death) in the MIS cohort.
Only 1 MIS patient needed transfer to the rehabilitation center. The estimated cost of care (hospital and rehabilitation) for the traditional group was $10,846 compared with $1,961 for the MIS group.
Discussion
In the authors’ experience, the use of MISS microlaminectomy for the treatment of LSS seems to have led to shorter hospital stays and faster recoveries. Some of the possible reasons for faster patient mobilization included a reduction in postoperative pain and the absence of a wound drain. Larger dissections with a traditional laminectomy often lead to the placement of a wound drain, which requires an inpatient stay until the wound output reaches a certain threshold. The absence of a drain and the reduction in pain with the MISS approach allowed the providers to focus on early ambulation and discharge planning. The microlaminectomy technique allowed for a proper surgical decompression with less tissue dissection than is required for a traditional laminectomy. Previous studies have shown that the microlaminectomy technique provides significant symptomatic relief.5-7,17
In most cases, the microlaminectomy can be performed on an outpatient basis. The improvement in bed availability is particularly important as surgical procedures may be delayed when hospitals operate at full capacity. Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves availability, allowing for better patient access to health care.19
Other authors have studied opportunities to transform inpatient neurosurgical care into outpatient procedures. For instance, Purzner and colleagues presented a large series of successful outpatient neurosurgical cases, including craniotomies, cervical fusions, and lumbar microdiscectomies.20 The MISS techniques offer a critical option to facilitate postoperative recovery and improve efficiency of care in regards to spine procedures.5,17
Cost-Effectiveness Within the VHA
The VA has been described as one of the best health care systems in the U.S.9 The arguments in favor of the VA system include its integrated computerized system and its resistance to health care cost inflation over the years.21 The $186.5 billion 2018 fiscal year VA budget is surpassed only by the total DoD budget, and it is expected to rise substantially in the near future.22
Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves bed availability and reduces cost.19 The authors have demonstrated that a minimally invasive unilateral paramedian approach for the treatment of lumbar stenosis leads to shorter hospital stay, improved bed availability, and lower cost while allowing for a proper surgical decompression. These clinical results are in accord with previous MIS surgery studies.5,17 The improvement in bed availability is particularly important within the VA system. Elective surgeries occasionally are delayed or cancelled because hospitals operate at full capacity. However, the authors’ outpatient microlaminectomy patients avoid delays or cancellations.
Given that both laminectomy procedures use similar operating room resources (time and material), the lower LOS associated with the microlaminectomy translates in cost saving. At SAVAHCS, acute care hospitalization is estimated at $3,000 per day when accounting for various costs, including nursing, pharmacy, ancillary services, and maintenance. The MIS procedure costs about $9,000 less than the open surgery. Over a 2-year period with 37 MIS patients, SAVAHCS saved about $300,000.
Patient Satisfaction
Patient satisfaction was assessed 1 day after the lumbar microdecompression outpatient surgery. Patients were asked to rate their overall surgical experience on a scale of 1 (worst) to 10 (best). All 24 patients who were contacted following outpatient lumbar microdecompression surgery rated the experience 10. These results indicate that patients do not expect or desire an admission following lumbar surgery, and they may recover comfortably at home. Studies are needed to compare outpatient and inpatient satisfaction ratings.
Conclusion
In this small sample, lumbar microlaminectomy significantly reduced LOS, successfully decompressed the spinal canal, and achieved symptomatic relief. Also, the procedure is associated with a lower blood loss than a traditional laminectomy and may reduce the rate of perioperative morbidity over time. In addition to faster recovery, the reduction in LOS can improve access to care by increasing the availability to inpatient admission.
Lumbar spinal stenosis (LSS) is a common debilitating issue in older patients. Open laminectomies traditionally are the standard treatment for LSS; however, minimally invasive surgery (MIS) has recently become a popular option to facilitate recovery and improve efficiency of care regarding spine procedures.
Guiot and colleagues described the technique for an MIS decompressive lumbar laminectomy procedure.1 The surgery may represent an important strategy to improve the efficiency of care for patients with severe LSS. Several authors have reported clinical benefits with the MIS lumbar laminectomy, leading to a significant improvement in the Oswetry Disability Index (ODI) 25 in the degenerative stenosis group in cases of LSS.2-5 In a recent reviewof 13 studies Wong and colleagues concluded that the MIS laminectomy was efficacious in terms of symptomatic relief and patient satisfaction for patients with LSS.6 Further, Rosen and colleaguesfound a significant improvement in the ODI scores and in the Short Form-36 body pain and physical functions scores in patients aged ≥ 75 years.7
Perioperative measures, including blood loss and narcotic consumption, have been shown to significantly decrease with MIS surgery compared with open decompression.8,9 Decreased narcotic use is of particular interest for the geriatric population because it is expected to allow those patients to remain more physically active and mentally agile.10
Also, long-term success is important when assessing the efficacy of new MIS procedures. Oertel and colleagues found that 85% of patients reported long-term success after unilateral laminotomy of bilateral decompression (ULBD).11 These results indicate that a MIS laminectomy is effective in older patients with LSS and neurogenic claudication.
Although there are numerous MIS approaches to alleviating LSS, more research is needed to determine whether it is superior to the open laminectomy.9,12,13 Skovrliand and colleagues reviewed publications comparing ULBD and open laminectomies and determined that currently insufficient evidence exists to define which technique leads to more positive outcomes.14 Thus, the purpose of this study is 2-fold. First, this study adds to the current research by comparing estimated blood loss and length of stay (LOS) for microscopic MIS laminectomy vs traditional laminectomy. Second, this study aims to address the difference in health care costs between the 2 types of surgery in the VHA.
The U.S. health care system is facing several challenges and in particular pressure for cost reduction.15 VA hospitals are not exempt from those challenges, and their operating budgets are influenced by political and economic factors.16 Because of those challenges, cost-effectiveness is gaining importance.7 Future decisions for procedure coverage and reimbursement rates are likely to consider ratios like the cost to quality-adjusted life-years (QALY). Improving this ratio requires a reduction of cost and/or an improvement in outcome.
Minimally invasive spine surgery (MISS) may lower the cost of spine procedures. Wang and colleagues reported that minimally invasive posterior lumbar interbody fusion (PLIF) led to shorter stay and lower blood loss compared with traditional PLIF.17 These improvements led to about $8,000 in savings for a single-level PLIF.17
Lumbar degenerative disease is a frequently encountered condition, and lumbar laminectomy is one of the most frequently performed spine procedures at VA hospitals. Consequently, MISS may be an important strategy for the VA to face systematic challenges. At the Southern Arizona VA Health Care System (SAVAHCS) in Tucson, the authors converted lumbar laminectomies from traditional open surgery to a MIS procedure using a tubular retractor system and a paramedian approach. To the authors’ knowledge, no studies have evaluated outcomes and cost efficiency of MIS surgery at the VA. The results of such a study may be instrumental in choosing which surgery is appropriate in a patient-centered health care model.
Material and Methods
Fifty veterans with severe lumbar stenosis and neurogenic claudication underwent a 1- or 2-level laminectomy at SAVAHCS (Table). A traditional laminectomy was performed for all patients until conversion to the MIS procedure, then all subsequent patients underwent the microlaminectomy. There was 1 female patient in each group. The preoperative magnetic resonance imaging (MRI) of the patients showed severe spinal canal stenosis defined radiographically by the absence of cerebrospinal fluid signal at the affected level on MRI (Figures 1A and 2A) and clinically by the presence of neurogenic claudication.
Procedure
The open laminectomies were performed in a traditional midline approach with removal of the spinous process along with the lamina bilaterally to provide spinal canal decompression (Figure 2).
The patients were given the choice of going home or being admitted. Overall admission costs were determined by the VA hospital following described models.18 The LOS in rehabilitation were determined from the records of the SAVAHCS rehabilitation center.
Results
There was not a significant difference in age between the 2 groups; mean age was 69.7 ± 9.8 years for the traditional laminectomy group and 64.4 ± 8.3 years for the MIS group. Operating room time was just over 2 hours on average in both groups. Blood loss was estimated and reported by the surgeon and the anesthesiologist, based on values from the surgical suction system. Patients in the MIS group lost on average 46 cc ± 70 cc compared with 135 cc ± 78 cc in the traditional group. The average number of operated levels was higher in the traditional group (1.7 ± 0.5) compared with the MIS group (1.4 ± 0.5), but this difference did not reach significance (P > .05).
Length of Stay and Cost
The LOS was lower for the MIS group, and 76% chose to be discharged from the recovery room. After a traditional laminectomy, the average patient’s stay was 3 days in the hospital and 5 days in the rehabilitation center. The average MIS group patient stayed < 1 day in the hospital. There were no readmissions within 30 days and no severe morbidity (including no new neurologic deficits or death) in the MIS cohort.
Only 1 MIS patient needed transfer to the rehabilitation center. The estimated cost of care (hospital and rehabilitation) for the traditional group was $10,846 compared with $1,961 for the MIS group.
Discussion
In the authors’ experience, the use of MISS microlaminectomy for the treatment of LSS seems to have led to shorter hospital stays and faster recoveries. Some of the possible reasons for faster patient mobilization included a reduction in postoperative pain and the absence of a wound drain. Larger dissections with a traditional laminectomy often lead to the placement of a wound drain, which requires an inpatient stay until the wound output reaches a certain threshold. The absence of a drain and the reduction in pain with the MISS approach allowed the providers to focus on early ambulation and discharge planning. The microlaminectomy technique allowed for a proper surgical decompression with less tissue dissection than is required for a traditional laminectomy. Previous studies have shown that the microlaminectomy technique provides significant symptomatic relief.5-7,17
In most cases, the microlaminectomy can be performed on an outpatient basis. The improvement in bed availability is particularly important as surgical procedures may be delayed when hospitals operate at full capacity. Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves availability, allowing for better patient access to health care.19
Other authors have studied opportunities to transform inpatient neurosurgical care into outpatient procedures. For instance, Purzner and colleagues presented a large series of successful outpatient neurosurgical cases, including craniotomies, cervical fusions, and lumbar microdiscectomies.20 The MISS techniques offer a critical option to facilitate postoperative recovery and improve efficiency of care in regards to spine procedures.5,17
Cost-Effectiveness Within the VHA
The VA has been described as one of the best health care systems in the U.S.9 The arguments in favor of the VA system include its integrated computerized system and its resistance to health care cost inflation over the years.21 The $186.5 billion 2018 fiscal year VA budget is surpassed only by the total DoD budget, and it is expected to rise substantially in the near future.22
Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves bed availability and reduces cost.19 The authors have demonstrated that a minimally invasive unilateral paramedian approach for the treatment of lumbar stenosis leads to shorter hospital stay, improved bed availability, and lower cost while allowing for a proper surgical decompression. These clinical results are in accord with previous MIS surgery studies.5,17 The improvement in bed availability is particularly important within the VA system. Elective surgeries occasionally are delayed or cancelled because hospitals operate at full capacity. However, the authors’ outpatient microlaminectomy patients avoid delays or cancellations.
Given that both laminectomy procedures use similar operating room resources (time and material), the lower LOS associated with the microlaminectomy translates in cost saving. At SAVAHCS, acute care hospitalization is estimated at $3,000 per day when accounting for various costs, including nursing, pharmacy, ancillary services, and maintenance. The MIS procedure costs about $9,000 less than the open surgery. Over a 2-year period with 37 MIS patients, SAVAHCS saved about $300,000.
Patient Satisfaction
Patient satisfaction was assessed 1 day after the lumbar microdecompression outpatient surgery. Patients were asked to rate their overall surgical experience on a scale of 1 (worst) to 10 (best). All 24 patients who were contacted following outpatient lumbar microdecompression surgery rated the experience 10. These results indicate that patients do not expect or desire an admission following lumbar surgery, and they may recover comfortably at home. Studies are needed to compare outpatient and inpatient satisfaction ratings.
Conclusion
In this small sample, lumbar microlaminectomy significantly reduced LOS, successfully decompressed the spinal canal, and achieved symptomatic relief. Also, the procedure is associated with a lower blood loss than a traditional laminectomy and may reduce the rate of perioperative morbidity over time. In addition to faster recovery, the reduction in LOS can improve access to care by increasing the availability to inpatient admission.
1. Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila PA 1976). 2002;27(4):432-438.
2. Rahman M, Summers LE, Richter B, Mimran RI, Jacob RP. Comparison of techniques for decompressive lumbar laminectomy: the minimally invasive versus the “classic” open approach. Minim Invasive Neurosurg. 2008;51(2)100-105.
3. Sasai K, Umeda M, Maruyama T, Wakabayashi E, Iida H. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008;9(6):554-559.
4. Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009;18(5):672-678.
5. Yagi M, Okada, E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10(4):293-299.
6. Wong AP, Smith ZA, Lall RR, Bresnahan LE, Fessler RG. The microendoscopic decompression of lumbar stenosis: a review of the current literature and clinical results. Minim Invasive Surg. 2012;2012:325095.
7. Rosen DS, O’Toole JE, Eichholz KM, et al. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60(3):503-509.
8. Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51(suppl 5):S146-S154.
9. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21(2):179-186.
10. Avila MJ, Walter CM, Baaj AA. Outcomes and complications of minimally invasive surgery of the lumbar spine in the elderly. Cureus. 2016;8(3):e519.
11. Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59(6):1264-1269.
12. Haddadi K, Ganjeh Qazvini HR. Outcome after surgery of lumbar spinal stenosis: a randomized comparison of bilateral laminotomy, trumpet laminectomy, and conventional laminectomy. Front Surg. 2016;3:199.
13. Watanabe K, Matsumoto M, Ikegami T, et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine. 2011;14(1):51-58.
14. Skovrlj B, Belton P, Zarzour H, Qureshi SA. Perioperative outcomes in minimally invasive lumbar spine surgery: a systematic review. World J. Orthop. 2015;6(11):996-1005.
15. Hellander I. The deepening crisis in U.S. health care: a review of data. Int J Health Serv. 2011;41(3):575-586.
16. Chokshi DA. Improving health care for veterans—a watershed moment for the VA. N Engl J Med. 2014;371(4):297-299.
17. Wang MY, Cummock MD, Yu Y, Trivedi RA. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg Spine. 2010;12(6):694-699.
18. Barnett PG. Determination of VA health care costs. Med Care Res Rev. 2003;60(suppl 3):S124-S141.
19. Congressional Budget Office. The health care system for veterans: interim report. https://www.cbo.gov/sites/default/files/110th-congress-2007-2008/reports/12-21-va_healthcare.pdf. Published December 2007. Accessed October 13, 2017.
20. Purzner T, Purzner J, Massicotte EM, Bernstein M. Outpatient brain tumor surgery and spinal decompression: a prospective study of 1003 patients. Neurosurgery. 2011;69(1):119-126.
21. Waller D. How veterans’ hospitals became the best in health care. Time Magazine. http://content.time.com/time/magazine/article/0,9171,1376238,00.html. Published August 27, 2006. Accessed October 13, 2017.
22. U.S. Department of Veterans Affairs, Office of Budget. Annual budget submission—office of budget. https://www.va.gov/budget/products.asp. Updated July 12, 2017. Published October 13, 2017. Accessed October 27, 2017.
1. Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila PA 1976). 2002;27(4):432-438.
2. Rahman M, Summers LE, Richter B, Mimran RI, Jacob RP. Comparison of techniques for decompressive lumbar laminectomy: the minimally invasive versus the “classic” open approach. Minim Invasive Neurosurg. 2008;51(2)100-105.
3. Sasai K, Umeda M, Maruyama T, Wakabayashi E, Iida H. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008;9(6):554-559.
4. Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009;18(5):672-678.
5. Yagi M, Okada, E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10(4):293-299.
6. Wong AP, Smith ZA, Lall RR, Bresnahan LE, Fessler RG. The microendoscopic decompression of lumbar stenosis: a review of the current literature and clinical results. Minim Invasive Surg. 2012;2012:325095.
7. Rosen DS, O’Toole JE, Eichholz KM, et al. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60(3):503-509.
8. Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51(suppl 5):S146-S154.
9. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21(2):179-186.
10. Avila MJ, Walter CM, Baaj AA. Outcomes and complications of minimally invasive surgery of the lumbar spine in the elderly. Cureus. 2016;8(3):e519.
11. Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59(6):1264-1269.
12. Haddadi K, Ganjeh Qazvini HR. Outcome after surgery of lumbar spinal stenosis: a randomized comparison of bilateral laminotomy, trumpet laminectomy, and conventional laminectomy. Front Surg. 2016;3:199.
13. Watanabe K, Matsumoto M, Ikegami T, et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine. 2011;14(1):51-58.
14. Skovrlj B, Belton P, Zarzour H, Qureshi SA. Perioperative outcomes in minimally invasive lumbar spine surgery: a systematic review. World J. Orthop. 2015;6(11):996-1005.
15. Hellander I. The deepening crisis in U.S. health care: a review of data. Int J Health Serv. 2011;41(3):575-586.
16. Chokshi DA. Improving health care for veterans—a watershed moment for the VA. N Engl J Med. 2014;371(4):297-299.
17. Wang MY, Cummock MD, Yu Y, Trivedi RA. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg Spine. 2010;12(6):694-699.
18. Barnett PG. Determination of VA health care costs. Med Care Res Rev. 2003;60(suppl 3):S124-S141.
19. Congressional Budget Office. The health care system for veterans: interim report. https://www.cbo.gov/sites/default/files/110th-congress-2007-2008/reports/12-21-va_healthcare.pdf. Published December 2007. Accessed October 13, 2017.
20. Purzner T, Purzner J, Massicotte EM, Bernstein M. Outpatient brain tumor surgery and spinal decompression: a prospective study of 1003 patients. Neurosurgery. 2011;69(1):119-126.
21. Waller D. How veterans’ hospitals became the best in health care. Time Magazine. http://content.time.com/time/magazine/article/0,9171,1376238,00.html. Published August 27, 2006. Accessed October 13, 2017.
22. U.S. Department of Veterans Affairs, Office of Budget. Annual budget submission—office of budget. https://www.va.gov/budget/products.asp. Updated July 12, 2017. Published October 13, 2017. Accessed October 27, 2017.
Does Knowledge of Implant Cost Affect Fixation Method Choice in the Management of Stable Intertrochanteric Hip Fractures?
Take-Home Points
- The incidence of geriatric hip fractures is rising nationally.
- Costs associated with hip fracture care have risen significantly.
- CMN and SHS are effective for stable, intertrochanteric hip fractures.
- Current trends show increased utilization of CMN compared to SHS for stable introchanteric hip fractures.
- Surgeon awareness of implant cost is a critical factor in delivering cost-effective, evidence-based surgical care.
The continuing increase in the population of patients older than 65 years in the United States is well known. For orthopedic surgeons, this trend highlights the importance of effective geriatric fracture care, particularly hip fracture care. Hip fractures in the elderly are expected to increase 50% by 2025 and to number 500,000 by 2040.1 The growing burden of hip fracture cases is accompanied by increasing costs of care. In 2012, 88% of the healthcare dollars spent on these patients were for direct fracture care, a significant increase from 60% in 2009.2 Although fewer than 1 in 5 fractures in the elderly are hip fractures, these injuries account for 72% of the total cost of geriatric fracture care, more than the total cost of all other osteoporosis-related injuries combined.1 Currently, the direct cost of hip fracture care ranges from $8358 to $32,195 and is expected, in total, to reach $25 billion by 2025.2,3
About 50% of geriatric hip fractures are extracapsular intertrochanteric or pertrochanteric.4 Several studies have compared sliding hip screws (SHSs) with cephalomedullary nails (CMNs) in the effective management of stable intertrochanteric fractures.5-11 Although these implants have shown an increased risk for peri-implant fracture and subsequent reoperation, markers such as mortality, medical complications, and restoration of prefracture function have all been equivocal relative to SHSs.12 Ultimately, one implant cannot be definitively recommended over the other for management of stable intertrochanteric hip fractures.13,14 Nevertheless, the current trend increasingly favors CMNs over SHSs.4,15 Most orthopedic surgeons are unaware of or underestimate the cost difference between these implants—a fact even more pronounced for newer implants.4,16 Considering the ever growing cost burden of hip fractures in the United States, orthopedists must consider not only the efficacy of care but the cost of delivery.
We conducted a study to determine the effect that surgeon knowledge of implant cost had on rates of use of SHSs and CMNs in the management of stable intertrochanteric hip fractures.
Patients and Methods
On May 1, 2012, all 9 attending orthopedic surgeons in a private practice group serving a suburban level II trauma center met to discuss implant prices and implant-related costs for the $850 Versafix SHS, the $1950 short Gamma3 nail (SGN), and the $2900 long Gamma3 nail (LGN), all manufactured by Stryker. All surgeons denied previous knowledge of the costs of these implants. During the discussion, no particular implant was recommended for management of any specific type of fracture. Surgeons were not directly instructed to consider implant cost in subsequent hip fracture surgeries and were not informed of our upcoming study of implant utilization.
After obtaining Institutional Review Board approval, we performed a retrospective chart and radiologic review of all hip fractures (Current Procedural Terminology [CPT] code 27244 or 27245) managed with fixation at our institution between May 1, 2011 and April 30, 2013. Two hundred six patients were identified (Figure 1).
One year later, surgeons were again shown their respective hip fracture radiographs, with patient identifying data removed. They were asked to reclassify their respective cases using the OTA system and then indicate the implant they would use for operative fixation in each of their cases.
Patient age, sex, injury side, fracture types, and utilization rates of the SHS, SGN, and LGN implants were compared between the groups. For each eligible patient, implant cost and other financial data were obtained from the hospital’s finance department. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) Version 20 for Macintosh. Independent 2-sample t test was used for parametric comparisons, and Fisher exact test was used for nonparametric comparisons.
Results
Examination of implant use per fracture classification revealed an interesting change. In the before group, SHS was the implant most commonly used for 31-A1.1 fractures (7/16, 43.8%), 31-A1.2 fractures (8/18, 44.4%), and 31-A2.1 fractures (10/25, 40.0%), and LGN was used in 66.7% (8/12) of 31-A1.3 fractures. By contrast, in the after group, SHS was most commonly used only for 31-A1.2 fractures (7/12, 58.3%), SGN was used in 90% (9/10) of 31-A1.1 fractures, and LGN was used in 42.1% (8/19) of 31-A2.1 fractures. In addition, 85.7% (6/7) of 31-A1.3 fractures were managed with a version of the Gamma nail.
Reclassification resulted in more A2.1 fractures (42.0% vs 37.0%) and fewer A1.3 fractures (10.1% vs 16.0%). About the same numbers of fractures were classified A1.1 (21.0% vs 21.8%) and A1.2 (26.9% vs 25.2%). SHS was favored for A1.1 fractures (92.0%) and A1.2 fractures (65.6%). SGN was favored for A1.3 fractures (75.0%). Gamma nails of both sizes were favored for A2.1 fractures (88.0%).
Discussion
Comparisons of SHS/plate and CMN constructs in the management of stable intertrochanteric hip fractures have long been discussed in the orthopedic literature. The major concern with CMNs (vs SHSs) is a statistically significantly higher rate of revision surgery, most often for peri-implant fracture. Rates of previous revision surgery for peri- implant fracture have ranged from 2.4% to 6% for CMNs and from 0.6% to 4% for SHSs.5-7,9 In a Cochrane review of 22 studies (3749 patients), Parker and Handoll12 compared CMN and SHS outcomes in 23 categories and found a statistically significant difference only in postoperative fracture rate. However, in a meta-analysis of studies conducted between 2000 and 2005, Bhandari and colleagues8 found no statistically significant difference in risk of femoral shaft fracture between CMNs (0.6%) and SHSs (0.1%). In addition, Utrilla and colleagues10 reported no postoperative fractures with use of Gamma3 CMNs. These recent studies may indicate that newer CMN designs can reduce the incidence of postoperative peri-implant fracture.8,10 Other outcome measures, such as 1-year mortality, functional outcome, and medical complication rate, have shown no statistically significant differences between the 2 implants.10-12 Ultimately, the current recommendation for fixation of stable intertrochanteric hip fractures is either SHS or CMN.13,14
Of our study patients, 78.9% (before group) and 64.6% (after group) were female, and 49.3% (before group) and 47.9% (after group) were between 80 and 89 years of age. Similarly, a review of hip fracture Medicare claims made between 1999 and 2002 revealed that >75% of the patients were females and 48% to 49% were octogenarians.4,18 However, our rates of different fracture types differed from those of Adams and colleagues.5 In a 1-year single-institution study, they found that, for both CMNs and SHSs, the most common stable intertrochanteric fractures were 31-A1.1 fractures; in our study’s before and after groups, more than one-third of injuries were 31-A2.1 fractures. Least common were 31-A1.3 fractures, both in the study by Adams and colleagues5 and in our before (16.9%) and after (14.6%) groups. Although our fracture rates differ from those of previous studies, all 4 classification categories fall under the umbrella of stable intertrochanteric hip fracture, which is the sole focus of this study.14
We hypothesized that cost would be a significant driver of implant choice in the management of these injuries. Given that SHS costs $1186.91 less than SGN and $1625.88 less than LGN at our institution, we expected that the before- discussion group’s overall SHS use rate of 38.0% would increase after discussion. Instead, SGN became the favored implant, with use in almost half of all fractures in the after group. Although the change in overall implant use rate was notable, these findings were not statistically significant. Examination of individual fracture patterns revealed 2 areas of interest. First, SHS was assumed to be the implant of choice in the management of the relatively simple 31-A1.1 fractures. Although this assumption was verified in the before group (SHS use in 43.8% of fractures), SGN was used in almost every case (90%) in the after group. However, when surgeons were asked 1 year later to recommend an implant for A1.1 fractures, 92% suggested SHS. The more cost-effective SHS construct may be the most amenable for use in these injury types given all intertrochanteric hip fracture patterns, though this has not been studied.
On the other hand, for 31-A2.1 fractures, perhaps the most complicated of the stable patterns, LGN became the implant of choice (42.1%). Despite surgeons’ awareness of the cost differences, management of these fractures shifted in the after group to the most expensive implant, indicative of surgeon concern about eventual loss of reduction with SHS and surgeon comfort with a particular procedure. This trend held when surgeons were asked to reclassify fractures 1 year later, as CMNs were recommended for 88% of 31-A2.1 fractures. Although both SHS and CMN were acceptable in 97% of the fractures included in this study, SGNs or LGNs were preferred for almost every fracture pattern involving the lesser trochanter. All 9 attending surgeons described involvement of the lesser trochanter as an indicator of posteromedial calcar injury. Surgeons became particularly concerned when this fracture pattern occurred in patients with significant osteopenia; SHS fixation, in their opinion, would be poor in the setting of a combination of greater posteromedial instability and poor bone quality. In a level I prospective, randomized trial, Barton and colleagues7 found no difference in outcomes between LGN and SHS fixation for 31-A2 proximal femur fractures and recommended SHS implants for the cost savings. In the clinical experience of this group, however, A1.3 and A2.1 fractures were at especially high risk for failure with SHS use, which necessitated greater implant stability through CMN fixation. On the other hand, for simpler fracture patterns, most surgeons suggested SHS implants. In their opinion, SGN and LGN implants offered no additional benefit of stability without evidence of posteromedial injury, even in the setting of osteopenia. For A1.2 fractures, posteromedial involvement was judged on the basis of size of the inferomedial spike or the extent of the inferomedial fracture line. Two surgeons preferred CMN for simple fractures, one because of the increased comfort with the implants and the other because of the minimally invasive surgical technique. Overall, our results indicate that knowledge of implant cost is not a strong enough factor to change surgeon behavior in selecting fixation for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients.
The lack of effect could be a consequence of surgeons’ training and comfort with various implants, especially among younger attending surgeons. Most of the attendings in the practice are under age 50 years, which correlates with a preference for CMN fixation.19 Case loads of >80 intertrochanteric hip fractures per calendar year, as in the after group, also correlates with more CMN use.19 However, the before group had more intertrochanteric hip fractures, and yet SHS was the implant of choice. Resident physician experience and comfort with various implants may play a role too, as teaching hospitals with resident assistance also correlate with CMN use.19 However, no major change in resident physician involvement was undertaken during this period. The institution studied is near a major metropolis in the Northeast, a region that has disfavored SHS in recent years.18 The change from before to after fits an overall trend in changing implant use. Anglen and colleagues15 found a significant decrease in SHS use, from 97% in 1999 to 33% in 2006, for intertrochanteric fracture fixation. Simultaneously, CMN use increased from 3% to 67%.
This study had several limitations. First, its overall sample size was small, and therefore any data fluctuations may be exaggerated. Furthermore, changes in utilization rates were compared over 2 years, which may not be long enough to show a changing trend in implant selection. Post hoc analysis of the sample size determined a power of 0.76 for an α of 0.05 and an effect size of 0.50. Second, radiologic classification was performed in a retrospective review, not officially by the operative surgeon. Fractures that we considered stable may have been considered unstable by the operative surgeon, influencing implant selection. Third, patients were selected from only one hospital, and orthopedic surgeons from other institutions may be more sensitive to cost considerations, changing implant selection more quickly. Fourth, initial selection of patients by CPT code might not have captured all those who satisfied the inclusion criteria. Fifth, only a single intervention was used, and follow-up meetings certainly could have increased the effectiveness of the intervention. Last, this and other retrospective studies are inherently weaker because of possible bias.
Conclusion
Our study results showed that implant cost is not a significant factor in implant selection for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients. By itself, knowledge of implant cost may not be a strong enough force to change surgeon behavior or preference secondary to consequences of failure or comfort with particular implants. In an economic climate in which healthcare is scrutinized for both its medical effectiveness and cost-effectiveness, further study of forces that could influence orthopedic surgeons to select a more cost-effective implant is warranted.
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475.
2. Kilgore ML, Curtis JR, Delzell E, et al. A close examination of healthcare expenditures related to fractures. J Bone Miner Res. 2013;28(4):816-820.
3. Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170.
4. Aros B, Tosteson AN, Gottlieb DJ, Koval KJ. Is a sliding hip screw or IM nail the preferred implant for intertrochanteric fracture fixation? Clin Orthop Relat Res. 2008;466(11):2827-2832.
5. Adams CI, Robinson CM, Court-Brown CM, McQueen MM. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 2001;15(6):394-400.
6. Ahrengart L, Törnkvist H, Fornander P, et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 2002;(401):209-222.
7. Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ. A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 2010;92(4):792-798.
8. Bhandari M, Schemitsch E, Jönsson A, Zlowodzki M, Haidukewych GJ. Gamma nails revisited: Gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma. 2009;23(6):460-464.
9. Osnes EK, Lofthus CM, Falch JA, et al. More postoperative femoral fractures with the Gamma nail than the sliding screw plate in the treatment of trochanteric fractures. Acta Orthop Scand. 2001;72(3):252-256.
10. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric Gamma nail and compression hip screw for trochanteric fractures. J Orthop Trauma. 2005;19(4):229-233.
11. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw-plating vs intramedullary nailing. Injury. 2010;41(3):279-284.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Kaplan K, Miyamoto R, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 2008;16(11):665-673.
14. Lindskog DM, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
15. Anglen JO, Weinstein JN; American Board of Orthopaedic Surgery Research Committee. Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700-707.
16. Streit JJ, Youssef A, Coale RM, Carpenter JE, Marcus RE. Orthopaedic surgeons frequently underestimate the cost of orthopaedic implants. Clin Orthop Relat Res. 2013;471(6):1744-1749.
17. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
18. Forte ML, Virnig BA, Kane RL, et al. Geographic variation in device use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2008;90(4):691-699.
19. Forte ML, Virnig BA, Eberly LE, et al. Provider factors associated with intramedullary nail use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2010;92(5):1105-1114.
Take-Home Points
- The incidence of geriatric hip fractures is rising nationally.
- Costs associated with hip fracture care have risen significantly.
- CMN and SHS are effective for stable, intertrochanteric hip fractures.
- Current trends show increased utilization of CMN compared to SHS for stable introchanteric hip fractures.
- Surgeon awareness of implant cost is a critical factor in delivering cost-effective, evidence-based surgical care.
The continuing increase in the population of patients older than 65 years in the United States is well known. For orthopedic surgeons, this trend highlights the importance of effective geriatric fracture care, particularly hip fracture care. Hip fractures in the elderly are expected to increase 50% by 2025 and to number 500,000 by 2040.1 The growing burden of hip fracture cases is accompanied by increasing costs of care. In 2012, 88% of the healthcare dollars spent on these patients were for direct fracture care, a significant increase from 60% in 2009.2 Although fewer than 1 in 5 fractures in the elderly are hip fractures, these injuries account for 72% of the total cost of geriatric fracture care, more than the total cost of all other osteoporosis-related injuries combined.1 Currently, the direct cost of hip fracture care ranges from $8358 to $32,195 and is expected, in total, to reach $25 billion by 2025.2,3
About 50% of geriatric hip fractures are extracapsular intertrochanteric or pertrochanteric.4 Several studies have compared sliding hip screws (SHSs) with cephalomedullary nails (CMNs) in the effective management of stable intertrochanteric fractures.5-11 Although these implants have shown an increased risk for peri-implant fracture and subsequent reoperation, markers such as mortality, medical complications, and restoration of prefracture function have all been equivocal relative to SHSs.12 Ultimately, one implant cannot be definitively recommended over the other for management of stable intertrochanteric hip fractures.13,14 Nevertheless, the current trend increasingly favors CMNs over SHSs.4,15 Most orthopedic surgeons are unaware of or underestimate the cost difference between these implants—a fact even more pronounced for newer implants.4,16 Considering the ever growing cost burden of hip fractures in the United States, orthopedists must consider not only the efficacy of care but the cost of delivery.
We conducted a study to determine the effect that surgeon knowledge of implant cost had on rates of use of SHSs and CMNs in the management of stable intertrochanteric hip fractures.
Patients and Methods
On May 1, 2012, all 9 attending orthopedic surgeons in a private practice group serving a suburban level II trauma center met to discuss implant prices and implant-related costs for the $850 Versafix SHS, the $1950 short Gamma3 nail (SGN), and the $2900 long Gamma3 nail (LGN), all manufactured by Stryker. All surgeons denied previous knowledge of the costs of these implants. During the discussion, no particular implant was recommended for management of any specific type of fracture. Surgeons were not directly instructed to consider implant cost in subsequent hip fracture surgeries and were not informed of our upcoming study of implant utilization.
After obtaining Institutional Review Board approval, we performed a retrospective chart and radiologic review of all hip fractures (Current Procedural Terminology [CPT] code 27244 or 27245) managed with fixation at our institution between May 1, 2011 and April 30, 2013. Two hundred six patients were identified (Figure 1).
One year later, surgeons were again shown their respective hip fracture radiographs, with patient identifying data removed. They were asked to reclassify their respective cases using the OTA system and then indicate the implant they would use for operative fixation in each of their cases.
Patient age, sex, injury side, fracture types, and utilization rates of the SHS, SGN, and LGN implants were compared between the groups. For each eligible patient, implant cost and other financial data were obtained from the hospital’s finance department. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) Version 20 for Macintosh. Independent 2-sample t test was used for parametric comparisons, and Fisher exact test was used for nonparametric comparisons.
Results
Examination of implant use per fracture classification revealed an interesting change. In the before group, SHS was the implant most commonly used for 31-A1.1 fractures (7/16, 43.8%), 31-A1.2 fractures (8/18, 44.4%), and 31-A2.1 fractures (10/25, 40.0%), and LGN was used in 66.7% (8/12) of 31-A1.3 fractures. By contrast, in the after group, SHS was most commonly used only for 31-A1.2 fractures (7/12, 58.3%), SGN was used in 90% (9/10) of 31-A1.1 fractures, and LGN was used in 42.1% (8/19) of 31-A2.1 fractures. In addition, 85.7% (6/7) of 31-A1.3 fractures were managed with a version of the Gamma nail.
Reclassification resulted in more A2.1 fractures (42.0% vs 37.0%) and fewer A1.3 fractures (10.1% vs 16.0%). About the same numbers of fractures were classified A1.1 (21.0% vs 21.8%) and A1.2 (26.9% vs 25.2%). SHS was favored for A1.1 fractures (92.0%) and A1.2 fractures (65.6%). SGN was favored for A1.3 fractures (75.0%). Gamma nails of both sizes were favored for A2.1 fractures (88.0%).
Discussion
Comparisons of SHS/plate and CMN constructs in the management of stable intertrochanteric hip fractures have long been discussed in the orthopedic literature. The major concern with CMNs (vs SHSs) is a statistically significantly higher rate of revision surgery, most often for peri-implant fracture. Rates of previous revision surgery for peri- implant fracture have ranged from 2.4% to 6% for CMNs and from 0.6% to 4% for SHSs.5-7,9 In a Cochrane review of 22 studies (3749 patients), Parker and Handoll12 compared CMN and SHS outcomes in 23 categories and found a statistically significant difference only in postoperative fracture rate. However, in a meta-analysis of studies conducted between 2000 and 2005, Bhandari and colleagues8 found no statistically significant difference in risk of femoral shaft fracture between CMNs (0.6%) and SHSs (0.1%). In addition, Utrilla and colleagues10 reported no postoperative fractures with use of Gamma3 CMNs. These recent studies may indicate that newer CMN designs can reduce the incidence of postoperative peri-implant fracture.8,10 Other outcome measures, such as 1-year mortality, functional outcome, and medical complication rate, have shown no statistically significant differences between the 2 implants.10-12 Ultimately, the current recommendation for fixation of stable intertrochanteric hip fractures is either SHS or CMN.13,14
Of our study patients, 78.9% (before group) and 64.6% (after group) were female, and 49.3% (before group) and 47.9% (after group) were between 80 and 89 years of age. Similarly, a review of hip fracture Medicare claims made between 1999 and 2002 revealed that >75% of the patients were females and 48% to 49% were octogenarians.4,18 However, our rates of different fracture types differed from those of Adams and colleagues.5 In a 1-year single-institution study, they found that, for both CMNs and SHSs, the most common stable intertrochanteric fractures were 31-A1.1 fractures; in our study’s before and after groups, more than one-third of injuries were 31-A2.1 fractures. Least common were 31-A1.3 fractures, both in the study by Adams and colleagues5 and in our before (16.9%) and after (14.6%) groups. Although our fracture rates differ from those of previous studies, all 4 classification categories fall under the umbrella of stable intertrochanteric hip fracture, which is the sole focus of this study.14
We hypothesized that cost would be a significant driver of implant choice in the management of these injuries. Given that SHS costs $1186.91 less than SGN and $1625.88 less than LGN at our institution, we expected that the before- discussion group’s overall SHS use rate of 38.0% would increase after discussion. Instead, SGN became the favored implant, with use in almost half of all fractures in the after group. Although the change in overall implant use rate was notable, these findings were not statistically significant. Examination of individual fracture patterns revealed 2 areas of interest. First, SHS was assumed to be the implant of choice in the management of the relatively simple 31-A1.1 fractures. Although this assumption was verified in the before group (SHS use in 43.8% of fractures), SGN was used in almost every case (90%) in the after group. However, when surgeons were asked 1 year later to recommend an implant for A1.1 fractures, 92% suggested SHS. The more cost-effective SHS construct may be the most amenable for use in these injury types given all intertrochanteric hip fracture patterns, though this has not been studied.
On the other hand, for 31-A2.1 fractures, perhaps the most complicated of the stable patterns, LGN became the implant of choice (42.1%). Despite surgeons’ awareness of the cost differences, management of these fractures shifted in the after group to the most expensive implant, indicative of surgeon concern about eventual loss of reduction with SHS and surgeon comfort with a particular procedure. This trend held when surgeons were asked to reclassify fractures 1 year later, as CMNs were recommended for 88% of 31-A2.1 fractures. Although both SHS and CMN were acceptable in 97% of the fractures included in this study, SGNs or LGNs were preferred for almost every fracture pattern involving the lesser trochanter. All 9 attending surgeons described involvement of the lesser trochanter as an indicator of posteromedial calcar injury. Surgeons became particularly concerned when this fracture pattern occurred in patients with significant osteopenia; SHS fixation, in their opinion, would be poor in the setting of a combination of greater posteromedial instability and poor bone quality. In a level I prospective, randomized trial, Barton and colleagues7 found no difference in outcomes between LGN and SHS fixation for 31-A2 proximal femur fractures and recommended SHS implants for the cost savings. In the clinical experience of this group, however, A1.3 and A2.1 fractures were at especially high risk for failure with SHS use, which necessitated greater implant stability through CMN fixation. On the other hand, for simpler fracture patterns, most surgeons suggested SHS implants. In their opinion, SGN and LGN implants offered no additional benefit of stability without evidence of posteromedial injury, even in the setting of osteopenia. For A1.2 fractures, posteromedial involvement was judged on the basis of size of the inferomedial spike or the extent of the inferomedial fracture line. Two surgeons preferred CMN for simple fractures, one because of the increased comfort with the implants and the other because of the minimally invasive surgical technique. Overall, our results indicate that knowledge of implant cost is not a strong enough factor to change surgeon behavior in selecting fixation for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients.
The lack of effect could be a consequence of surgeons’ training and comfort with various implants, especially among younger attending surgeons. Most of the attendings in the practice are under age 50 years, which correlates with a preference for CMN fixation.19 Case loads of >80 intertrochanteric hip fractures per calendar year, as in the after group, also correlates with more CMN use.19 However, the before group had more intertrochanteric hip fractures, and yet SHS was the implant of choice. Resident physician experience and comfort with various implants may play a role too, as teaching hospitals with resident assistance also correlate with CMN use.19 However, no major change in resident physician involvement was undertaken during this period. The institution studied is near a major metropolis in the Northeast, a region that has disfavored SHS in recent years.18 The change from before to after fits an overall trend in changing implant use. Anglen and colleagues15 found a significant decrease in SHS use, from 97% in 1999 to 33% in 2006, for intertrochanteric fracture fixation. Simultaneously, CMN use increased from 3% to 67%.
This study had several limitations. First, its overall sample size was small, and therefore any data fluctuations may be exaggerated. Furthermore, changes in utilization rates were compared over 2 years, which may not be long enough to show a changing trend in implant selection. Post hoc analysis of the sample size determined a power of 0.76 for an α of 0.05 and an effect size of 0.50. Second, radiologic classification was performed in a retrospective review, not officially by the operative surgeon. Fractures that we considered stable may have been considered unstable by the operative surgeon, influencing implant selection. Third, patients were selected from only one hospital, and orthopedic surgeons from other institutions may be more sensitive to cost considerations, changing implant selection more quickly. Fourth, initial selection of patients by CPT code might not have captured all those who satisfied the inclusion criteria. Fifth, only a single intervention was used, and follow-up meetings certainly could have increased the effectiveness of the intervention. Last, this and other retrospective studies are inherently weaker because of possible bias.
Conclusion
Our study results showed that implant cost is not a significant factor in implant selection for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients. By itself, knowledge of implant cost may not be a strong enough force to change surgeon behavior or preference secondary to consequences of failure or comfort with particular implants. In an economic climate in which healthcare is scrutinized for both its medical effectiveness and cost-effectiveness, further study of forces that could influence orthopedic surgeons to select a more cost-effective implant is warranted.
Take-Home Points
- The incidence of geriatric hip fractures is rising nationally.
- Costs associated with hip fracture care have risen significantly.
- CMN and SHS are effective for stable, intertrochanteric hip fractures.
- Current trends show increased utilization of CMN compared to SHS for stable introchanteric hip fractures.
- Surgeon awareness of implant cost is a critical factor in delivering cost-effective, evidence-based surgical care.
The continuing increase in the population of patients older than 65 years in the United States is well known. For orthopedic surgeons, this trend highlights the importance of effective geriatric fracture care, particularly hip fracture care. Hip fractures in the elderly are expected to increase 50% by 2025 and to number 500,000 by 2040.1 The growing burden of hip fracture cases is accompanied by increasing costs of care. In 2012, 88% of the healthcare dollars spent on these patients were for direct fracture care, a significant increase from 60% in 2009.2 Although fewer than 1 in 5 fractures in the elderly are hip fractures, these injuries account for 72% of the total cost of geriatric fracture care, more than the total cost of all other osteoporosis-related injuries combined.1 Currently, the direct cost of hip fracture care ranges from $8358 to $32,195 and is expected, in total, to reach $25 billion by 2025.2,3
About 50% of geriatric hip fractures are extracapsular intertrochanteric or pertrochanteric.4 Several studies have compared sliding hip screws (SHSs) with cephalomedullary nails (CMNs) in the effective management of stable intertrochanteric fractures.5-11 Although these implants have shown an increased risk for peri-implant fracture and subsequent reoperation, markers such as mortality, medical complications, and restoration of prefracture function have all been equivocal relative to SHSs.12 Ultimately, one implant cannot be definitively recommended over the other for management of stable intertrochanteric hip fractures.13,14 Nevertheless, the current trend increasingly favors CMNs over SHSs.4,15 Most orthopedic surgeons are unaware of or underestimate the cost difference between these implants—a fact even more pronounced for newer implants.4,16 Considering the ever growing cost burden of hip fractures in the United States, orthopedists must consider not only the efficacy of care but the cost of delivery.
We conducted a study to determine the effect that surgeon knowledge of implant cost had on rates of use of SHSs and CMNs in the management of stable intertrochanteric hip fractures.
Patients and Methods
On May 1, 2012, all 9 attending orthopedic surgeons in a private practice group serving a suburban level II trauma center met to discuss implant prices and implant-related costs for the $850 Versafix SHS, the $1950 short Gamma3 nail (SGN), and the $2900 long Gamma3 nail (LGN), all manufactured by Stryker. All surgeons denied previous knowledge of the costs of these implants. During the discussion, no particular implant was recommended for management of any specific type of fracture. Surgeons were not directly instructed to consider implant cost in subsequent hip fracture surgeries and were not informed of our upcoming study of implant utilization.
After obtaining Institutional Review Board approval, we performed a retrospective chart and radiologic review of all hip fractures (Current Procedural Terminology [CPT] code 27244 or 27245) managed with fixation at our institution between May 1, 2011 and April 30, 2013. Two hundred six patients were identified (Figure 1).
One year later, surgeons were again shown their respective hip fracture radiographs, with patient identifying data removed. They were asked to reclassify their respective cases using the OTA system and then indicate the implant they would use for operative fixation in each of their cases.
Patient age, sex, injury side, fracture types, and utilization rates of the SHS, SGN, and LGN implants were compared between the groups. For each eligible patient, implant cost and other financial data were obtained from the hospital’s finance department. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) Version 20 for Macintosh. Independent 2-sample t test was used for parametric comparisons, and Fisher exact test was used for nonparametric comparisons.
Results
Examination of implant use per fracture classification revealed an interesting change. In the before group, SHS was the implant most commonly used for 31-A1.1 fractures (7/16, 43.8%), 31-A1.2 fractures (8/18, 44.4%), and 31-A2.1 fractures (10/25, 40.0%), and LGN was used in 66.7% (8/12) of 31-A1.3 fractures. By contrast, in the after group, SHS was most commonly used only for 31-A1.2 fractures (7/12, 58.3%), SGN was used in 90% (9/10) of 31-A1.1 fractures, and LGN was used in 42.1% (8/19) of 31-A2.1 fractures. In addition, 85.7% (6/7) of 31-A1.3 fractures were managed with a version of the Gamma nail.
Reclassification resulted in more A2.1 fractures (42.0% vs 37.0%) and fewer A1.3 fractures (10.1% vs 16.0%). About the same numbers of fractures were classified A1.1 (21.0% vs 21.8%) and A1.2 (26.9% vs 25.2%). SHS was favored for A1.1 fractures (92.0%) and A1.2 fractures (65.6%). SGN was favored for A1.3 fractures (75.0%). Gamma nails of both sizes were favored for A2.1 fractures (88.0%).
Discussion
Comparisons of SHS/plate and CMN constructs in the management of stable intertrochanteric hip fractures have long been discussed in the orthopedic literature. The major concern with CMNs (vs SHSs) is a statistically significantly higher rate of revision surgery, most often for peri-implant fracture. Rates of previous revision surgery for peri- implant fracture have ranged from 2.4% to 6% for CMNs and from 0.6% to 4% for SHSs.5-7,9 In a Cochrane review of 22 studies (3749 patients), Parker and Handoll12 compared CMN and SHS outcomes in 23 categories and found a statistically significant difference only in postoperative fracture rate. However, in a meta-analysis of studies conducted between 2000 and 2005, Bhandari and colleagues8 found no statistically significant difference in risk of femoral shaft fracture between CMNs (0.6%) and SHSs (0.1%). In addition, Utrilla and colleagues10 reported no postoperative fractures with use of Gamma3 CMNs. These recent studies may indicate that newer CMN designs can reduce the incidence of postoperative peri-implant fracture.8,10 Other outcome measures, such as 1-year mortality, functional outcome, and medical complication rate, have shown no statistically significant differences between the 2 implants.10-12 Ultimately, the current recommendation for fixation of stable intertrochanteric hip fractures is either SHS or CMN.13,14
Of our study patients, 78.9% (before group) and 64.6% (after group) were female, and 49.3% (before group) and 47.9% (after group) were between 80 and 89 years of age. Similarly, a review of hip fracture Medicare claims made between 1999 and 2002 revealed that >75% of the patients were females and 48% to 49% were octogenarians.4,18 However, our rates of different fracture types differed from those of Adams and colleagues.5 In a 1-year single-institution study, they found that, for both CMNs and SHSs, the most common stable intertrochanteric fractures were 31-A1.1 fractures; in our study’s before and after groups, more than one-third of injuries were 31-A2.1 fractures. Least common were 31-A1.3 fractures, both in the study by Adams and colleagues5 and in our before (16.9%) and after (14.6%) groups. Although our fracture rates differ from those of previous studies, all 4 classification categories fall under the umbrella of stable intertrochanteric hip fracture, which is the sole focus of this study.14
We hypothesized that cost would be a significant driver of implant choice in the management of these injuries. Given that SHS costs $1186.91 less than SGN and $1625.88 less than LGN at our institution, we expected that the before- discussion group’s overall SHS use rate of 38.0% would increase after discussion. Instead, SGN became the favored implant, with use in almost half of all fractures in the after group. Although the change in overall implant use rate was notable, these findings were not statistically significant. Examination of individual fracture patterns revealed 2 areas of interest. First, SHS was assumed to be the implant of choice in the management of the relatively simple 31-A1.1 fractures. Although this assumption was verified in the before group (SHS use in 43.8% of fractures), SGN was used in almost every case (90%) in the after group. However, when surgeons were asked 1 year later to recommend an implant for A1.1 fractures, 92% suggested SHS. The more cost-effective SHS construct may be the most amenable for use in these injury types given all intertrochanteric hip fracture patterns, though this has not been studied.
On the other hand, for 31-A2.1 fractures, perhaps the most complicated of the stable patterns, LGN became the implant of choice (42.1%). Despite surgeons’ awareness of the cost differences, management of these fractures shifted in the after group to the most expensive implant, indicative of surgeon concern about eventual loss of reduction with SHS and surgeon comfort with a particular procedure. This trend held when surgeons were asked to reclassify fractures 1 year later, as CMNs were recommended for 88% of 31-A2.1 fractures. Although both SHS and CMN were acceptable in 97% of the fractures included in this study, SGNs or LGNs were preferred for almost every fracture pattern involving the lesser trochanter. All 9 attending surgeons described involvement of the lesser trochanter as an indicator of posteromedial calcar injury. Surgeons became particularly concerned when this fracture pattern occurred in patients with significant osteopenia; SHS fixation, in their opinion, would be poor in the setting of a combination of greater posteromedial instability and poor bone quality. In a level I prospective, randomized trial, Barton and colleagues7 found no difference in outcomes between LGN and SHS fixation for 31-A2 proximal femur fractures and recommended SHS implants for the cost savings. In the clinical experience of this group, however, A1.3 and A2.1 fractures were at especially high risk for failure with SHS use, which necessitated greater implant stability through CMN fixation. On the other hand, for simpler fracture patterns, most surgeons suggested SHS implants. In their opinion, SGN and LGN implants offered no additional benefit of stability without evidence of posteromedial injury, even in the setting of osteopenia. For A1.2 fractures, posteromedial involvement was judged on the basis of size of the inferomedial spike or the extent of the inferomedial fracture line. Two surgeons preferred CMN for simple fractures, one because of the increased comfort with the implants and the other because of the minimally invasive surgical technique. Overall, our results indicate that knowledge of implant cost is not a strong enough factor to change surgeon behavior in selecting fixation for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients.
The lack of effect could be a consequence of surgeons’ training and comfort with various implants, especially among younger attending surgeons. Most of the attendings in the practice are under age 50 years, which correlates with a preference for CMN fixation.19 Case loads of >80 intertrochanteric hip fractures per calendar year, as in the after group, also correlates with more CMN use.19 However, the before group had more intertrochanteric hip fractures, and yet SHS was the implant of choice. Resident physician experience and comfort with various implants may play a role too, as teaching hospitals with resident assistance also correlate with CMN use.19 However, no major change in resident physician involvement was undertaken during this period. The institution studied is near a major metropolis in the Northeast, a region that has disfavored SHS in recent years.18 The change from before to after fits an overall trend in changing implant use. Anglen and colleagues15 found a significant decrease in SHS use, from 97% in 1999 to 33% in 2006, for intertrochanteric fracture fixation. Simultaneously, CMN use increased from 3% to 67%.
This study had several limitations. First, its overall sample size was small, and therefore any data fluctuations may be exaggerated. Furthermore, changes in utilization rates were compared over 2 years, which may not be long enough to show a changing trend in implant selection. Post hoc analysis of the sample size determined a power of 0.76 for an α of 0.05 and an effect size of 0.50. Second, radiologic classification was performed in a retrospective review, not officially by the operative surgeon. Fractures that we considered stable may have been considered unstable by the operative surgeon, influencing implant selection. Third, patients were selected from only one hospital, and orthopedic surgeons from other institutions may be more sensitive to cost considerations, changing implant selection more quickly. Fourth, initial selection of patients by CPT code might not have captured all those who satisfied the inclusion criteria. Fifth, only a single intervention was used, and follow-up meetings certainly could have increased the effectiveness of the intervention. Last, this and other retrospective studies are inherently weaker because of possible bias.
Conclusion
Our study results showed that implant cost is not a significant factor in implant selection for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients. By itself, knowledge of implant cost may not be a strong enough force to change surgeon behavior or preference secondary to consequences of failure or comfort with particular implants. In an economic climate in which healthcare is scrutinized for both its medical effectiveness and cost-effectiveness, further study of forces that could influence orthopedic surgeons to select a more cost-effective implant is warranted.
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475.
2. Kilgore ML, Curtis JR, Delzell E, et al. A close examination of healthcare expenditures related to fractures. J Bone Miner Res. 2013;28(4):816-820.
3. Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170.
4. Aros B, Tosteson AN, Gottlieb DJ, Koval KJ. Is a sliding hip screw or IM nail the preferred implant for intertrochanteric fracture fixation? Clin Orthop Relat Res. 2008;466(11):2827-2832.
5. Adams CI, Robinson CM, Court-Brown CM, McQueen MM. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 2001;15(6):394-400.
6. Ahrengart L, Törnkvist H, Fornander P, et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 2002;(401):209-222.
7. Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ. A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 2010;92(4):792-798.
8. Bhandari M, Schemitsch E, Jönsson A, Zlowodzki M, Haidukewych GJ. Gamma nails revisited: Gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma. 2009;23(6):460-464.
9. Osnes EK, Lofthus CM, Falch JA, et al. More postoperative femoral fractures with the Gamma nail than the sliding screw plate in the treatment of trochanteric fractures. Acta Orthop Scand. 2001;72(3):252-256.
10. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric Gamma nail and compression hip screw for trochanteric fractures. J Orthop Trauma. 2005;19(4):229-233.
11. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw-plating vs intramedullary nailing. Injury. 2010;41(3):279-284.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Kaplan K, Miyamoto R, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 2008;16(11):665-673.
14. Lindskog DM, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
15. Anglen JO, Weinstein JN; American Board of Orthopaedic Surgery Research Committee. Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700-707.
16. Streit JJ, Youssef A, Coale RM, Carpenter JE, Marcus RE. Orthopaedic surgeons frequently underestimate the cost of orthopaedic implants. Clin Orthop Relat Res. 2013;471(6):1744-1749.
17. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
18. Forte ML, Virnig BA, Kane RL, et al. Geographic variation in device use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2008;90(4):691-699.
19. Forte ML, Virnig BA, Eberly LE, et al. Provider factors associated with intramedullary nail use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2010;92(5):1105-1114.
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475.
2. Kilgore ML, Curtis JR, Delzell E, et al. A close examination of healthcare expenditures related to fractures. J Bone Miner Res. 2013;28(4):816-820.
3. Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170.
4. Aros B, Tosteson AN, Gottlieb DJ, Koval KJ. Is a sliding hip screw or IM nail the preferred implant for intertrochanteric fracture fixation? Clin Orthop Relat Res. 2008;466(11):2827-2832.
5. Adams CI, Robinson CM, Court-Brown CM, McQueen MM. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 2001;15(6):394-400.
6. Ahrengart L, Törnkvist H, Fornander P, et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 2002;(401):209-222.
7. Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ. A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 2010;92(4):792-798.
8. Bhandari M, Schemitsch E, Jönsson A, Zlowodzki M, Haidukewych GJ. Gamma nails revisited: Gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma. 2009;23(6):460-464.
9. Osnes EK, Lofthus CM, Falch JA, et al. More postoperative femoral fractures with the Gamma nail than the sliding screw plate in the treatment of trochanteric fractures. Acta Orthop Scand. 2001;72(3):252-256.
10. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric Gamma nail and compression hip screw for trochanteric fractures. J Orthop Trauma. 2005;19(4):229-233.
11. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw-plating vs intramedullary nailing. Injury. 2010;41(3):279-284.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Kaplan K, Miyamoto R, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 2008;16(11):665-673.
14. Lindskog DM, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
15. Anglen JO, Weinstein JN; American Board of Orthopaedic Surgery Research Committee. Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700-707.
16. Streit JJ, Youssef A, Coale RM, Carpenter JE, Marcus RE. Orthopaedic surgeons frequently underestimate the cost of orthopaedic implants. Clin Orthop Relat Res. 2013;471(6):1744-1749.
17. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
18. Forte ML, Virnig BA, Kane RL, et al. Geographic variation in device use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2008;90(4):691-699.
19. Forte ML, Virnig BA, Eberly LE, et al. Provider factors associated with intramedullary nail use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2010;92(5):1105-1114.
Higher Risk of Secondary Cancers for Patients With Mycosis Fungoides
Adult patients with mycosis fungoides (MF) have a higher risk of secondary malignancies, according to a 20-year population-based cohort study. The researchers, from Bezmialem Vakif University in Istanbul, Turkey, say their findings support earlier research about a higher risk of, for instance, Hodgkin lymphoma, chronic leukemia, and lung cancer.
Between 1998 and 2015, the researchers documented 143 cases of cutaneous T-cell lymphoma (CTCL). The majority of patients had early-stage disease.
The researchers also documented 13 cases (9%) of secondary malignancy diagnosed at least 3 months after the diagnosis of CTCL. The cancers included bladder cancer, nasopharynx cancer, renal cell carcinoma, lung cancer, and superficial spreading malignant melanoma.
Older age, stage IV disease, lymphomatoid papulosis, and having CTCL for > 10 years raised the chances of developing secondary solid tumors. In 60% of patients, the secondary malignancies occurred during the first year of diagnosis.
Research has suggested that antilymphoma drugs, particularly alkylating agents, may lead to leukemia, the researchers note. In this study, 6 patients with secondary cancers were getting systemic treatment with interferon or acitretin; 7 were getting no systemic treatment.
The researchers add that MF and hematologic malignancies may share genetic origin, carcinogens, or viruses that affect lymphocyte precursors. They also note that the first neoplasm may produce cytokines that induce development of the secondary neoplasm. The researchers cite research that found MF is a T helper cell 2–mediated disease associated with human leukocyte antigen 2 alleles. Viruses such as Epstein-Barr and herpes simplex also have been implicated.
“Extensive evaluation” for secondary malignancies in adult patients with MF is wise, the researchers advise, particularly if the patient has lymphomatoid papulosis.
Source:
Cengiz FP, Emiroğlu N, Onsun N. Turk J Haematol. 2017;34(4):378-379.
doi: 10.4274/tjh.2017.0234.
Adult patients with mycosis fungoides (MF) have a higher risk of secondary malignancies, according to a 20-year population-based cohort study. The researchers, from Bezmialem Vakif University in Istanbul, Turkey, say their findings support earlier research about a higher risk of, for instance, Hodgkin lymphoma, chronic leukemia, and lung cancer.
Between 1998 and 2015, the researchers documented 143 cases of cutaneous T-cell lymphoma (CTCL). The majority of patients had early-stage disease.
The researchers also documented 13 cases (9%) of secondary malignancy diagnosed at least 3 months after the diagnosis of CTCL. The cancers included bladder cancer, nasopharynx cancer, renal cell carcinoma, lung cancer, and superficial spreading malignant melanoma.
Older age, stage IV disease, lymphomatoid papulosis, and having CTCL for > 10 years raised the chances of developing secondary solid tumors. In 60% of patients, the secondary malignancies occurred during the first year of diagnosis.
Research has suggested that antilymphoma drugs, particularly alkylating agents, may lead to leukemia, the researchers note. In this study, 6 patients with secondary cancers were getting systemic treatment with interferon or acitretin; 7 were getting no systemic treatment.
The researchers add that MF and hematologic malignancies may share genetic origin, carcinogens, or viruses that affect lymphocyte precursors. They also note that the first neoplasm may produce cytokines that induce development of the secondary neoplasm. The researchers cite research that found MF is a T helper cell 2–mediated disease associated with human leukocyte antigen 2 alleles. Viruses such as Epstein-Barr and herpes simplex also have been implicated.
“Extensive evaluation” for secondary malignancies in adult patients with MF is wise, the researchers advise, particularly if the patient has lymphomatoid papulosis.
Source:
Cengiz FP, Emiroğlu N, Onsun N. Turk J Haematol. 2017;34(4):378-379.
doi: 10.4274/tjh.2017.0234.
Adult patients with mycosis fungoides (MF) have a higher risk of secondary malignancies, according to a 20-year population-based cohort study. The researchers, from Bezmialem Vakif University in Istanbul, Turkey, say their findings support earlier research about a higher risk of, for instance, Hodgkin lymphoma, chronic leukemia, and lung cancer.
Between 1998 and 2015, the researchers documented 143 cases of cutaneous T-cell lymphoma (CTCL). The majority of patients had early-stage disease.
The researchers also documented 13 cases (9%) of secondary malignancy diagnosed at least 3 months after the diagnosis of CTCL. The cancers included bladder cancer, nasopharynx cancer, renal cell carcinoma, lung cancer, and superficial spreading malignant melanoma.
Older age, stage IV disease, lymphomatoid papulosis, and having CTCL for > 10 years raised the chances of developing secondary solid tumors. In 60% of patients, the secondary malignancies occurred during the first year of diagnosis.
Research has suggested that antilymphoma drugs, particularly alkylating agents, may lead to leukemia, the researchers note. In this study, 6 patients with secondary cancers were getting systemic treatment with interferon or acitretin; 7 were getting no systemic treatment.
The researchers add that MF and hematologic malignancies may share genetic origin, carcinogens, or viruses that affect lymphocyte precursors. They also note that the first neoplasm may produce cytokines that induce development of the secondary neoplasm. The researchers cite research that found MF is a T helper cell 2–mediated disease associated with human leukocyte antigen 2 alleles. Viruses such as Epstein-Barr and herpes simplex also have been implicated.
“Extensive evaluation” for secondary malignancies in adult patients with MF is wise, the researchers advise, particularly if the patient has lymphomatoid papulosis.
Source:
Cengiz FP, Emiroğlu N, Onsun N. Turk J Haematol. 2017;34(4):378-379.
doi: 10.4274/tjh.2017.0234.