User login
Case Studies in Toxicology: Start Low and Go Slow
Case
A woman in her third decade with no known medical history was dropped off at the waiting area of the ED for evaluation of depressed mental status. Upon arrival, the patient was unresponsive and cyanotic, with a pulse oximetry of 65% on room air. Bag-valve mask (BVM) ventilation rapidly improved oxygen saturation to 90%. The patient’s other vital signs were: heart rate, 141 beats/min; blood pressure (BP), 117/65 mm Hg; and temperature, afebrile.
Upon examination, the patient’s pupils were pinpoint and her ventilatory effort was shallow, leading the emergency physician (EP) to suspect the patient’s depressed mental status was due to an opioid overdose.
The patient was given 2 mg of intravenous (IV) naloxone, after which she became more alert and responsive, with improved respiratory effort. After receiving naloxone, the patient vomited copiously. Pulmonary examination revealed diffuse rales, most prominently at the right lung base, and a cough productive of thick sputum.
During the patient’s course in the ED, she became increasingly hypotensive with systolic BP readings around 70 mm Hg; tachycardia, fluctuating at around 120 beats/min; and persistent hypoxia of 90% saturation on a nonrebreather mask. A chest X-ray demonstrated pulmonary edema with a continuous diaphragm sign suggesting pneumomediastinum. A computed tomography (CT) scan of the chest confirmed pulmonary edema with extensive pneumomediastinum, and the patient was admitted to the intensive care unit (ICU).
What is naloxone and why is it used?
Naloxone is a nonselective, short-acting, pure opioid antagonist that works at the mu, kappa, and sigma receptors, with the highest affinity for the mu receptor. It is a competitive opioid receptor antagonist that has an elimination half-life of approximately 30 minutes. Though naloxone was originally developed to reverse the effects of anesthesia postoperatively,1 today it is more commonly used to treat ventilatory depression in patients whose clinical findings are most likely due to an opioid overdose.
Opioid-dependent individuals who abstain from use for more than a few hours generally develop opioid withdrawal syndrome (OWS). The effects of OWS include mild-to-moderate tachycardia and hypertension, nausea, vomiting, piloerection, rhinorrhea, and agitated behavior. However, when opioid-dependent patients receive naloxone, OWS develops at a much faster rate (ie, seconds after naloxone administration) and is often more severe.
Findings of naloxone-precipitated OWS include pronounced vital sign abnormalities, seizures,pulmonary edema, and cardiac arrhythmias such as ventricular tachycardia.2 These latter findings are primarily due to the sudden release of catecholamines.3 In addition, patients suffer the psychological pangs of withdrawal, including dysphoria and drug craving, which often leads to poor decision-making as they search for additional opioids to alleviate these troubling effects.
What determines response to naloxone and development of OWS?
The severity of precipitated OWS following naloxone administration is determined by both the degree of the patient’s opioid dependency and the dosage and rate at which naloxone is given. The depth of opioid dependence is determined to a large extent by the quantity of opioid regularly used and the frequency of exposure. For example, a patient who takes 30 mg of oxycodone daily will likely demonstrate mild OWS, while one who uses 300 mg daily will demonstrate more severe OWS—whether due to abstinence or naloxone.
In addition, longer exposure time of the patient’s brain to opioids increases the dependency level. Continuous use of extended-release opioids or methadone, which are both of long duration, essentially “bathe” the brain receptors in opioid around the clock, whereas short-acting opioids, such as fentanyl or heroin, cause peaks and troughs in brain concentrations throughout the day. These trough periods reduce dependency, but increase the abuse liability of the opioid. Patients who only use opioids on the weekend, for example, will have minimal or no OWS following naloxone administration, nor will the toddler with an exploratory ingestion of an opioid medication found in the home. It is therefore important to gauge the extent of a patient’s opioid use to improve the safe use of naloxone in the ED.
What is the optimal dosing of naloxone and proper patient management?
It is essential for clinicians to remember that the ultimate goal of naloxone administration in the ED is to reverse ventilatory depression—not to restore a patient to a normal mental status.4 In fact, full awakening, in addition to precipitating OWS, may lead to difficult interpersonal situations in the ED, since such patients often insist on leaving the ED before the effects of naloxone wear off. This situation places the EP in the undesirable position of discharging a patient who may predictably relapse—though unlikely to die—after release.5
Management in the Hospital Setting. Given the advanced medical care environment in a hospital, the approach to opioid overdose patients can be metered. This means providing temporary noninvasive mechanical ventilatory support through BVM or laryngeal mask airways, which allow both oxygenation and ventilation (reducing the patient’s partial pressure of carbon dioxide), prior to giving naloxone.6 Studies on animal models have shown that lowering the partial pressure of carbon dioxide reduces the catecholamine response to naloxone.7
Although recent literature and textbook recommendations regarding naloxone dosages vary,1 the safest initial dose of naloxone in the hospital setting is 0.04 mg (40 mcg) IV, or 0.08 mg (80 mcg) intramuscularly (IM).8 Whether given by IV or IM route, frequent reassessment of the adequacy of spontaneous ventilatory effort and oxygenation are required.
While the rate of opioid reversal is slower when giving lower doses of naloxone, this approach reduces the severity of precipitated OWS. In fact, in most patients who receive low-dose naloxone administration will not awaken but will develop life-sustaining spontaneous ventilation.8
By monitoring of the patient’s ventilatory rate and depth, along with capnometry and pulse oximetry (without providing exogenous oxygen), the EP can identify the need for additional naloxone. Since the half-life of naloxone is shorter than that of many opioids, proper ventilatory monitoring is essential to assess for the waning of naloxone’s effects and return of respiratory depression.
Treatment in the Nonhospital Setting. Emergency medical service (EMS) workers typically, and often by situational necessity, approach opioid overdose patients more aggressively than do EPs in the ED. Although some EMS systems utilize the IV route, most EMS workers, like laypersons, administer an initial naloxone dose of 0.4 mg IM or 2 or 4 mg intranasally (IN). Due to the slower rate of absorption and lower bioavailability (with IN administration), both IM and IN naloxone equate to roughly 0.08 mg IV.
For patients in whom there is no risk for opioid dependence, the initial dose of naloxone is relatively inconsequential, and higher doses can be safely administered. However, for most patients, including those in the ED setting, in whom one cannot be certain of their depth of dependence, the safest approach is to “start low and go slow” with naloxone administration, while providing supportive care.
Case Conclusion
The patient was not opioid-naïve, explaining the catecholamine surge and related cardiovascular dysfunction and pulmonary edema. The pneumomediastinum and pulmonary aspiration were due to the violent retching and vomiting. After being admitted to the ICU, the patient was started on vancomycin and piperacillin/tazobactam for empiric coverage for mediastinal emphysema. She was kept NPO, assessed by cardiothoracic surgery, and treated with gentle fluid hydration.
A repeat CT showed a stable pneumomediastinum. Her hypoxia, tachycardia, and hypotension gradually improved over about 6 hours. The following day, the patient’s mental status normalized, and she discharged herself from the hospital against medical advice.
1. Connors NJ, Nelson LS. The evolution of recommended naloxone dosing for opioid overdose by medical specialty. J Med Toxicol. 2016;12(3):276-281. doi:10.1007/s13181-016-0559-3.
2. Lameijer, H, Azizi N, Ligtenberg JJ, Ter Maaten JC. Ventricular tachycardia after naloxone administration: a drug related complication? Case report and literature review. Drug Saf Case Rep. 2014;1(1):2. doi:10.1007/s40800-014-0002-0.
3. Kienbaum P, Thürauf N, Michel MC, Scherbaum N, Gastpar M, Peters J. Profound increase in epinephrine concentration in plasma and cardiovascular stimulation after mu-opioid receptor blockade in opioid-addicted patients during barbiturate-induced anesthesia for acute detoxification. Anesthesiology. 1998;88(5):1154-1161.
4. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
5. Willman MW, Liss DB, Schwarz ES, Mullins ME. Do heroin overdose patients require observation after receiving naloxone? Clin Toxicol (Phila). 2017;55(2):81-87. doi:10.1080/15563650.2016.1253846.
6. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155. doi:10.1056/NEJMra1202561.
7. Mills CA, Flacke JW, Miller JD, Davis LJ, Bloor BC, Flacke WE. Cardiovascular effects of fentanyl reversal by naloxone at varying arterial carbon dioxide tensions in dogs. Anesth Analg. 1988;67(8):730-736.
8. Kim HK, Nelson LS. Reversal of opioid-induced ventilatory depression using low-dose naloxone (0.04 mg): a case series. J Med Toxicol. 2015;12(1):107-110. doi:10.1007/s13181-015-0499-3.
Case
A woman in her third decade with no known medical history was dropped off at the waiting area of the ED for evaluation of depressed mental status. Upon arrival, the patient was unresponsive and cyanotic, with a pulse oximetry of 65% on room air. Bag-valve mask (BVM) ventilation rapidly improved oxygen saturation to 90%. The patient’s other vital signs were: heart rate, 141 beats/min; blood pressure (BP), 117/65 mm Hg; and temperature, afebrile.
Upon examination, the patient’s pupils were pinpoint and her ventilatory effort was shallow, leading the emergency physician (EP) to suspect the patient’s depressed mental status was due to an opioid overdose.
The patient was given 2 mg of intravenous (IV) naloxone, after which she became more alert and responsive, with improved respiratory effort. After receiving naloxone, the patient vomited copiously. Pulmonary examination revealed diffuse rales, most prominently at the right lung base, and a cough productive of thick sputum.
During the patient’s course in the ED, she became increasingly hypotensive with systolic BP readings around 70 mm Hg; tachycardia, fluctuating at around 120 beats/min; and persistent hypoxia of 90% saturation on a nonrebreather mask. A chest X-ray demonstrated pulmonary edema with a continuous diaphragm sign suggesting pneumomediastinum. A computed tomography (CT) scan of the chest confirmed pulmonary edema with extensive pneumomediastinum, and the patient was admitted to the intensive care unit (ICU).
What is naloxone and why is it used?
Naloxone is a nonselective, short-acting, pure opioid antagonist that works at the mu, kappa, and sigma receptors, with the highest affinity for the mu receptor. It is a competitive opioid receptor antagonist that has an elimination half-life of approximately 30 minutes. Though naloxone was originally developed to reverse the effects of anesthesia postoperatively,1 today it is more commonly used to treat ventilatory depression in patients whose clinical findings are most likely due to an opioid overdose.
Opioid-dependent individuals who abstain from use for more than a few hours generally develop opioid withdrawal syndrome (OWS). The effects of OWS include mild-to-moderate tachycardia and hypertension, nausea, vomiting, piloerection, rhinorrhea, and agitated behavior. However, when opioid-dependent patients receive naloxone, OWS develops at a much faster rate (ie, seconds after naloxone administration) and is often more severe.
Findings of naloxone-precipitated OWS include pronounced vital sign abnormalities, seizures,pulmonary edema, and cardiac arrhythmias such as ventricular tachycardia.2 These latter findings are primarily due to the sudden release of catecholamines.3 In addition, patients suffer the psychological pangs of withdrawal, including dysphoria and drug craving, which often leads to poor decision-making as they search for additional opioids to alleviate these troubling effects.
What determines response to naloxone and development of OWS?
The severity of precipitated OWS following naloxone administration is determined by both the degree of the patient’s opioid dependency and the dosage and rate at which naloxone is given. The depth of opioid dependence is determined to a large extent by the quantity of opioid regularly used and the frequency of exposure. For example, a patient who takes 30 mg of oxycodone daily will likely demonstrate mild OWS, while one who uses 300 mg daily will demonstrate more severe OWS—whether due to abstinence or naloxone.
In addition, longer exposure time of the patient’s brain to opioids increases the dependency level. Continuous use of extended-release opioids or methadone, which are both of long duration, essentially “bathe” the brain receptors in opioid around the clock, whereas short-acting opioids, such as fentanyl or heroin, cause peaks and troughs in brain concentrations throughout the day. These trough periods reduce dependency, but increase the abuse liability of the opioid. Patients who only use opioids on the weekend, for example, will have minimal or no OWS following naloxone administration, nor will the toddler with an exploratory ingestion of an opioid medication found in the home. It is therefore important to gauge the extent of a patient’s opioid use to improve the safe use of naloxone in the ED.
What is the optimal dosing of naloxone and proper patient management?
It is essential for clinicians to remember that the ultimate goal of naloxone administration in the ED is to reverse ventilatory depression—not to restore a patient to a normal mental status.4 In fact, full awakening, in addition to precipitating OWS, may lead to difficult interpersonal situations in the ED, since such patients often insist on leaving the ED before the effects of naloxone wear off. This situation places the EP in the undesirable position of discharging a patient who may predictably relapse—though unlikely to die—after release.5
Management in the Hospital Setting. Given the advanced medical care environment in a hospital, the approach to opioid overdose patients can be metered. This means providing temporary noninvasive mechanical ventilatory support through BVM or laryngeal mask airways, which allow both oxygenation and ventilation (reducing the patient’s partial pressure of carbon dioxide), prior to giving naloxone.6 Studies on animal models have shown that lowering the partial pressure of carbon dioxide reduces the catecholamine response to naloxone.7
Although recent literature and textbook recommendations regarding naloxone dosages vary,1 the safest initial dose of naloxone in the hospital setting is 0.04 mg (40 mcg) IV, or 0.08 mg (80 mcg) intramuscularly (IM).8 Whether given by IV or IM route, frequent reassessment of the adequacy of spontaneous ventilatory effort and oxygenation are required.
While the rate of opioid reversal is slower when giving lower doses of naloxone, this approach reduces the severity of precipitated OWS. In fact, in most patients who receive low-dose naloxone administration will not awaken but will develop life-sustaining spontaneous ventilation.8
By monitoring of the patient’s ventilatory rate and depth, along with capnometry and pulse oximetry (without providing exogenous oxygen), the EP can identify the need for additional naloxone. Since the half-life of naloxone is shorter than that of many opioids, proper ventilatory monitoring is essential to assess for the waning of naloxone’s effects and return of respiratory depression.
Treatment in the Nonhospital Setting. Emergency medical service (EMS) workers typically, and often by situational necessity, approach opioid overdose patients more aggressively than do EPs in the ED. Although some EMS systems utilize the IV route, most EMS workers, like laypersons, administer an initial naloxone dose of 0.4 mg IM or 2 or 4 mg intranasally (IN). Due to the slower rate of absorption and lower bioavailability (with IN administration), both IM and IN naloxone equate to roughly 0.08 mg IV.
For patients in whom there is no risk for opioid dependence, the initial dose of naloxone is relatively inconsequential, and higher doses can be safely administered. However, for most patients, including those in the ED setting, in whom one cannot be certain of their depth of dependence, the safest approach is to “start low and go slow” with naloxone administration, while providing supportive care.
Case Conclusion
The patient was not opioid-naïve, explaining the catecholamine surge and related cardiovascular dysfunction and pulmonary edema. The pneumomediastinum and pulmonary aspiration were due to the violent retching and vomiting. After being admitted to the ICU, the patient was started on vancomycin and piperacillin/tazobactam for empiric coverage for mediastinal emphysema. She was kept NPO, assessed by cardiothoracic surgery, and treated with gentle fluid hydration.
A repeat CT showed a stable pneumomediastinum. Her hypoxia, tachycardia, and hypotension gradually improved over about 6 hours. The following day, the patient’s mental status normalized, and she discharged herself from the hospital against medical advice.
Case
A woman in her third decade with no known medical history was dropped off at the waiting area of the ED for evaluation of depressed mental status. Upon arrival, the patient was unresponsive and cyanotic, with a pulse oximetry of 65% on room air. Bag-valve mask (BVM) ventilation rapidly improved oxygen saturation to 90%. The patient’s other vital signs were: heart rate, 141 beats/min; blood pressure (BP), 117/65 mm Hg; and temperature, afebrile.
Upon examination, the patient’s pupils were pinpoint and her ventilatory effort was shallow, leading the emergency physician (EP) to suspect the patient’s depressed mental status was due to an opioid overdose.
The patient was given 2 mg of intravenous (IV) naloxone, after which she became more alert and responsive, with improved respiratory effort. After receiving naloxone, the patient vomited copiously. Pulmonary examination revealed diffuse rales, most prominently at the right lung base, and a cough productive of thick sputum.
During the patient’s course in the ED, she became increasingly hypotensive with systolic BP readings around 70 mm Hg; tachycardia, fluctuating at around 120 beats/min; and persistent hypoxia of 90% saturation on a nonrebreather mask. A chest X-ray demonstrated pulmonary edema with a continuous diaphragm sign suggesting pneumomediastinum. A computed tomography (CT) scan of the chest confirmed pulmonary edema with extensive pneumomediastinum, and the patient was admitted to the intensive care unit (ICU).
What is naloxone and why is it used?
Naloxone is a nonselective, short-acting, pure opioid antagonist that works at the mu, kappa, and sigma receptors, with the highest affinity for the mu receptor. It is a competitive opioid receptor antagonist that has an elimination half-life of approximately 30 minutes. Though naloxone was originally developed to reverse the effects of anesthesia postoperatively,1 today it is more commonly used to treat ventilatory depression in patients whose clinical findings are most likely due to an opioid overdose.
Opioid-dependent individuals who abstain from use for more than a few hours generally develop opioid withdrawal syndrome (OWS). The effects of OWS include mild-to-moderate tachycardia and hypertension, nausea, vomiting, piloerection, rhinorrhea, and agitated behavior. However, when opioid-dependent patients receive naloxone, OWS develops at a much faster rate (ie, seconds after naloxone administration) and is often more severe.
Findings of naloxone-precipitated OWS include pronounced vital sign abnormalities, seizures,pulmonary edema, and cardiac arrhythmias such as ventricular tachycardia.2 These latter findings are primarily due to the sudden release of catecholamines.3 In addition, patients suffer the psychological pangs of withdrawal, including dysphoria and drug craving, which often leads to poor decision-making as they search for additional opioids to alleviate these troubling effects.
What determines response to naloxone and development of OWS?
The severity of precipitated OWS following naloxone administration is determined by both the degree of the patient’s opioid dependency and the dosage and rate at which naloxone is given. The depth of opioid dependence is determined to a large extent by the quantity of opioid regularly used and the frequency of exposure. For example, a patient who takes 30 mg of oxycodone daily will likely demonstrate mild OWS, while one who uses 300 mg daily will demonstrate more severe OWS—whether due to abstinence or naloxone.
In addition, longer exposure time of the patient’s brain to opioids increases the dependency level. Continuous use of extended-release opioids or methadone, which are both of long duration, essentially “bathe” the brain receptors in opioid around the clock, whereas short-acting opioids, such as fentanyl or heroin, cause peaks and troughs in brain concentrations throughout the day. These trough periods reduce dependency, but increase the abuse liability of the opioid. Patients who only use opioids on the weekend, for example, will have minimal or no OWS following naloxone administration, nor will the toddler with an exploratory ingestion of an opioid medication found in the home. It is therefore important to gauge the extent of a patient’s opioid use to improve the safe use of naloxone in the ED.
What is the optimal dosing of naloxone and proper patient management?
It is essential for clinicians to remember that the ultimate goal of naloxone administration in the ED is to reverse ventilatory depression—not to restore a patient to a normal mental status.4 In fact, full awakening, in addition to precipitating OWS, may lead to difficult interpersonal situations in the ED, since such patients often insist on leaving the ED before the effects of naloxone wear off. This situation places the EP in the undesirable position of discharging a patient who may predictably relapse—though unlikely to die—after release.5
Management in the Hospital Setting. Given the advanced medical care environment in a hospital, the approach to opioid overdose patients can be metered. This means providing temporary noninvasive mechanical ventilatory support through BVM or laryngeal mask airways, which allow both oxygenation and ventilation (reducing the patient’s partial pressure of carbon dioxide), prior to giving naloxone.6 Studies on animal models have shown that lowering the partial pressure of carbon dioxide reduces the catecholamine response to naloxone.7
Although recent literature and textbook recommendations regarding naloxone dosages vary,1 the safest initial dose of naloxone in the hospital setting is 0.04 mg (40 mcg) IV, or 0.08 mg (80 mcg) intramuscularly (IM).8 Whether given by IV or IM route, frequent reassessment of the adequacy of spontaneous ventilatory effort and oxygenation are required.
While the rate of opioid reversal is slower when giving lower doses of naloxone, this approach reduces the severity of precipitated OWS. In fact, in most patients who receive low-dose naloxone administration will not awaken but will develop life-sustaining spontaneous ventilation.8
By monitoring of the patient’s ventilatory rate and depth, along with capnometry and pulse oximetry (without providing exogenous oxygen), the EP can identify the need for additional naloxone. Since the half-life of naloxone is shorter than that of many opioids, proper ventilatory monitoring is essential to assess for the waning of naloxone’s effects and return of respiratory depression.
Treatment in the Nonhospital Setting. Emergency medical service (EMS) workers typically, and often by situational necessity, approach opioid overdose patients more aggressively than do EPs in the ED. Although some EMS systems utilize the IV route, most EMS workers, like laypersons, administer an initial naloxone dose of 0.4 mg IM or 2 or 4 mg intranasally (IN). Due to the slower rate of absorption and lower bioavailability (with IN administration), both IM and IN naloxone equate to roughly 0.08 mg IV.
For patients in whom there is no risk for opioid dependence, the initial dose of naloxone is relatively inconsequential, and higher doses can be safely administered. However, for most patients, including those in the ED setting, in whom one cannot be certain of their depth of dependence, the safest approach is to “start low and go slow” with naloxone administration, while providing supportive care.
Case Conclusion
The patient was not opioid-naïve, explaining the catecholamine surge and related cardiovascular dysfunction and pulmonary edema. The pneumomediastinum and pulmonary aspiration were due to the violent retching and vomiting. After being admitted to the ICU, the patient was started on vancomycin and piperacillin/tazobactam for empiric coverage for mediastinal emphysema. She was kept NPO, assessed by cardiothoracic surgery, and treated with gentle fluid hydration.
A repeat CT showed a stable pneumomediastinum. Her hypoxia, tachycardia, and hypotension gradually improved over about 6 hours. The following day, the patient’s mental status normalized, and she discharged herself from the hospital against medical advice.
1. Connors NJ, Nelson LS. The evolution of recommended naloxone dosing for opioid overdose by medical specialty. J Med Toxicol. 2016;12(3):276-281. doi:10.1007/s13181-016-0559-3.
2. Lameijer, H, Azizi N, Ligtenberg JJ, Ter Maaten JC. Ventricular tachycardia after naloxone administration: a drug related complication? Case report and literature review. Drug Saf Case Rep. 2014;1(1):2. doi:10.1007/s40800-014-0002-0.
3. Kienbaum P, Thürauf N, Michel MC, Scherbaum N, Gastpar M, Peters J. Profound increase in epinephrine concentration in plasma and cardiovascular stimulation after mu-opioid receptor blockade in opioid-addicted patients during barbiturate-induced anesthesia for acute detoxification. Anesthesiology. 1998;88(5):1154-1161.
4. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
5. Willman MW, Liss DB, Schwarz ES, Mullins ME. Do heroin overdose patients require observation after receiving naloxone? Clin Toxicol (Phila). 2017;55(2):81-87. doi:10.1080/15563650.2016.1253846.
6. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155. doi:10.1056/NEJMra1202561.
7. Mills CA, Flacke JW, Miller JD, Davis LJ, Bloor BC, Flacke WE. Cardiovascular effects of fentanyl reversal by naloxone at varying arterial carbon dioxide tensions in dogs. Anesth Analg. 1988;67(8):730-736.
8. Kim HK, Nelson LS. Reversal of opioid-induced ventilatory depression using low-dose naloxone (0.04 mg): a case series. J Med Toxicol. 2015;12(1):107-110. doi:10.1007/s13181-015-0499-3.
1. Connors NJ, Nelson LS. The evolution of recommended naloxone dosing for opioid overdose by medical specialty. J Med Toxicol. 2016;12(3):276-281. doi:10.1007/s13181-016-0559-3.
2. Lameijer, H, Azizi N, Ligtenberg JJ, Ter Maaten JC. Ventricular tachycardia after naloxone administration: a drug related complication? Case report and literature review. Drug Saf Case Rep. 2014;1(1):2. doi:10.1007/s40800-014-0002-0.
3. Kienbaum P, Thürauf N, Michel MC, Scherbaum N, Gastpar M, Peters J. Profound increase in epinephrine concentration in plasma and cardiovascular stimulation after mu-opioid receptor blockade in opioid-addicted patients during barbiturate-induced anesthesia for acute detoxification. Anesthesiology. 1998;88(5):1154-1161.
4. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
5. Willman MW, Liss DB, Schwarz ES, Mullins ME. Do heroin overdose patients require observation after receiving naloxone? Clin Toxicol (Phila). 2017;55(2):81-87. doi:10.1080/15563650.2016.1253846.
6. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155. doi:10.1056/NEJMra1202561.
7. Mills CA, Flacke JW, Miller JD, Davis LJ, Bloor BC, Flacke WE. Cardiovascular effects of fentanyl reversal by naloxone at varying arterial carbon dioxide tensions in dogs. Anesth Analg. 1988;67(8):730-736.
8. Kim HK, Nelson LS. Reversal of opioid-induced ventilatory depression using low-dose naloxone (0.04 mg): a case series. J Med Toxicol. 2015;12(1):107-110. doi:10.1007/s13181-015-0499-3.
Playing by the Rules: Using Decision Rules Wisely Part 1, Trauma
Emergency physicians (EPs) rely on rapid diagnostic testing to help screen patients for illnesses. While the decision to order a test for a patient should be driven by an objective assessment of pretest probability, other factors such as fear of litigation, clinical inexperience, or desire for increased patient satisfaction can prompt testing even when the likelihood of disease is low. This in turn leads to practice variability, increased cost, and decreased ED throughput, as well as other risks attendant to overtesting and overtreatment. Conversely, practitioners may fail to order necessary tests despite the presence of high-risk clinical features, which in turn may lead to misdiagnoses and delay in initiating life-saving treatments.
Development of Decision Rules
Clinical decision rules seek to decrease resource utilization in instances of low probability of disease and to identify high-risk features that should prompt further investigation. The formation of clinical decision rules entails at least three steps, which Ian Stiell, MD, emergency medicine’s (EM’s) most prolific author of these instruments, describes as follows:
- Creation of the rule or derivation;
- Prospective assessment of the reliability, accuracy, and impact of the rule in a validation study; and
- Gauging the effect of the rule on patient care through an implementation study.1
In addition to these three steps, many clinicians argue that there should also be an important fourth step included in this process: the external validation or assessment of the rule outside of the original study site(s), to assure reliability of the rule across a variety of populations for which its use was intended.2
Critiques and Caveats
A common critique of clinical decision rules is that they may not necessarily outperform subjective physician judgment, and that those who create these rules often do not explicitly compare their instruments against independent unassisted decision-making by clinicians.2,3
Another drawback is that the misapplication of these rules can lead to increased testing, something particularly problematic for one-way rules, which only guide the provider in a single clinical direction. An example of a one-way rule is the Pulmonary Embolism Rule-Out Criteria (PERC), which advises that a low-risk patient who does not have any of the PERC factors will not require any further testing. This, however, does not necessarily mean that further testing is indicated in patients who have one of the PERC factors present.2 Thus, applying PERC and other one-way decision-making rules in a two-way fashion can prompt testing that would not be ordered based on clinician gestalt. Rules that are designed to help determine when testing is necessary and when it is unnecessary are referred to as two-way rules, an example of which is the Ottawa Ankle Rule.
Controversies aside, the incorporation of clinical decision rules in the electronic medical record of many institutions and the proliferation of smartphone applications utilizing these instruments have further cemented their place in EM. This article describes the more commonly used ED clinical decision rules, as well as pearls and pitfalls pertaining to their use. Part 1 that follows covers important validated rules related to trauma patients in the ED. Part 2, which will appear in an upcoming 2018 issue, will cover nontrauma medical-diagnosis decision rules, including pulmonary embolism, and pneumonia.
Head Trauma
The increased utilization of computed tomography (CT) studies to assess for minor blunt head trauma spurred the development of clinical decision rules. In adult patients, the most popular and well-studied instruments are the New Orleans Criteria (NOC), the Canadian CT Head Rule (CCHR), and the National Emergency X-Radiography Utilization Study (NEXUS) CT Head Rule.
New Orleans Criteria
The NOC validation cohort examined over 900 cases at a single trauma center, enrolling all patients 3 years of age and older who had suffered minor head trauma (defined as loss of consciousness (LOC) in a patient with grossly normal neurological examination and a Glasgow Coma Scale [GCS] score of 15), in the preceding 24 hours.4 Patients who experienced no LOC, had focal neurological deficit (except isolated short-term memory deficits), or who did not have any CT study performed, were excluded.
The NOC describes seven factors for consideration:
- Short-term memory deficits;
- Intoxication with drugs or alcohol;
- Physical evidence of trauma above the clavicles;
- Patients older than age 60 years;
- Seizure;
- Headache; and
- Vomiting.
The presence of at least one of these factors was found to be 100% sensitive and 25% specific for the presence of any traumatic intracranial abnormality on CT, though only 6.5% of patients in the derivation and validation cohorts had positive CT scans, and ultimately less than 1% had lesions that required surgery.4
Canadian CT Head Rule
The CCHR validation study assessed over 2,700 patients at nine Canadian EDs, enrolling all patients aged 16 years and older who sustained a blunt head trauma less than 24 hours prior to presentation and who had a GCS score of 13 or higher. The investigators of the CCHR study specified that included patients should have suffered a witnessed LOC, definite amnesia, or a witnessed disorientation.5 Patients who did not have any of these factors were deemed to have minimal head trauma and were excluded from the study. Also excluded from this study were patients who had seizure prior to ED arrival, had a coagulopathy or used oral anticoagulants, had acute focal neurological deficit or obvious depressed skull fracture, had unstable vitals associated with a major trauma, or were pregnant.5 The CCHR was not designed to be applied to these excluded populations. Of note, patients with drug and alcohol intoxication were included in their validation.6
The CCHR describes five high-risk factors that increase the likelihood of requiring acute neurosurgical intervention:
- A GCS score of less than 15 at 2 hours after injury;
- Suspected open or depressed skull fracture;
- Any sign of basal skull fracture (eg, hemotympanum, raccoon eyes, cerebrospinal fluid otorrhea/rhinorrhea, Battle’s sign;
- Two or more episodes of vomiting; or
- Patients aged 65 years or older.
In addition to these five high-risk factors, the CCHR also describes two medium risk criteria for finding any traumatic lesion on CT that would not necessitate acute neurosurgical intervention: amnesia of greater than 30 minutes before impact; and injury resulting from a dangerous mechanism such as a pedestrian struck by motor vehicle, occupant ejected from a motor vehicle, or a fall from a height greater than 3 feet or from over 5 stair-steps.
The presence of any one or more of the five high-risk factors was 100% sensitive for predicting the need for neurosurgical intervention, and taken together, having one or more of the seven factors was 100% sensitive for predicting clinically important brain injury.5
NEXUS CT Head Rule
The NEXUS CT Head Rule validation cohort included over 11,000 pediatric and adult blunt head trauma patients undergoing CT imaging at four hospital EDs, and excluded patients with penetrating injuries or presentation greater than 24 hours after injury.7 Patients were considered to be low risk if none of the following criteria were present:
- Age 65 years or older;
- Evidence of significant skull fracture;
- Scalp hematoma;
- Neurological deficit;
- Altered level of alertness;
- Abnormal behavior;
- Coagulopathy; and
- Recurrent vomiting.
Patients who have one or more of these factors were considered as high risk. The presence of one of these factors was 100% sensitive for detecting patients with lesions requiring neurosurgery and 99% sensitive for detecting any significant intracranial injury, with specificity for either condition at 25%.7
Decision-Rule Sensitivity Comparison
Though the CCHR, NOC, and NEXUS rules differ in their inclusion/exclusion criteria, they have been compared to each other in different populations in the medical literature. A subgroup of the CCHR validation cohort and an accompanying external validation study of over 3,000 patients both found that the CCHR and the NOC were at least as sensitive (100%) in detecting lesions requiring neurosurgical intervention, but the CCHR was more specific and had greater potential to reduce imaging rates.5,8 Another study examined the performance of NEXUS, CCHR, NOC, and other decision instruments in a database of nearly 8,000 adolescent and adult head trauma patients. The authors of this study found the three rules to have similarly high sensitivities (97% to 99%) for detecting clinically important findings, but felt NEXUS to have the best combination of sensitivity and specificity compared to CCHR and NOC.9
Comment: A decision rule to decrease CT utilization in intoxicated head trauma patients is particularly useful, but only the CCHR can help potentially avoid imaging a drug or alcohol-intoxicated patient with abnormal behavior or altered level of alertness. Similarly, distinguishing superficial scalp trauma from more worrisome signs of intracranial injury is important, but the NOC and NEXUS rules recommend imaging for any trauma above the clavicles and
Though the rules have similar sensitivities, the CCHR appears to have the greatest potential for aiding clinical decision-making. Of note, all of the adult decision rules consider patients older than age 60 or 65 years to be a high-risk feature. An instrument with better specificity for geriatric patients would be immensely helpful as our population continues to age.
The Pediatric Population: The PECARN Rule
The CCHR, NEXUS, and NOC have been variously applied to pediatric populations, and the NEXUS and NOC included children (patients aged 3 years and older in the NOC) in their validation cohorts. Perhaps the most widely utilized validated rule for pediatric head trauma in the United States is the Pediatric Emergency Care Applied Research Network (PECARN) rule.
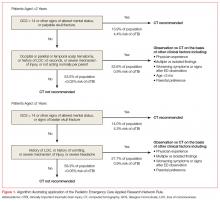
The PECARN rule is an age-specific instrument for assessing pediatric blunt head trauma patients for clinically important traumatic brain injury (TBI)—ie, brain injuries resulting in death, neurosurgery, intubation longer than 24 hours, or hospital admission length of more than two nights.10 The PECARN algorithm as described in its validation study is reproduced in Figure 1.
Patients with trivial injury mechanisms such as ground level falls or running into stationary objects and no signs or symptoms of head trauma other than scalp abrasions or lacerations were excluded from the PECARN analysis, as were patients with pre-existing neurological disorders, including ventricular shunts and brain tumors, patients with penetrating trauma, and those with bleeding disorders. The validation study of over 8,000 children found the presence of any of the criteria to be 100% and 96.8% sensitive in detecting clinically important TBI in children aged less than 2 years and children aged 2 years or older, respectively.
The PECARN rule was recently assessed in a large prospective cohort against two less extensively studied clinical decision rules: the Children’s Head Injury Algorithm for the Prediction of Important Clinical Events (CHALICE) and the Canadian Assessment of Tomography for Childhood Head Injury (CATCH). Though the sample size was over 20,000 patients, only less than 1% required neurosurgery or died. The PECARN rule was determined to have the highest validation sensitivities (100% for <2 years, 99% for ≥2 years) of all of the rules; however, it has been noted by some clinicians that the strict application of the PECARN rule to the study population would have increased the rate of CT scanning 5-fold without providing any clear benefit over clinical judgment in detecting injury.11,12
Comment: The PECARN tool is by far the most robust instrument for pediatric head trauma, but striking a balance between finding otherwise clinically occult injury and reducing unnecessary testing/irradiation remains difficult in this vulnerable population.
Cervical Spine Trauma
Though the incidence of serious cervical spine injury in blunt trauma is low, the potentially devastating consequences of a missed lesion is a potent driver of radiographic testing.13 The Nexus Criteria and Canadian C-Spine Rule were developed to assist the clinician in determining when radiographic imaging is indicated in patients presenting with a blunt-trauma-related injury. The NEXUS criteria were developed to decrease cervical spine X-ray use based on five low-risk factors:
- No posterior midline tenderness;
- No focal neurological deficit;
- Normal level of alertness;
- No evidence of intoxication; and
- No clinically apparent pain that could distract the patient from the pain of a cervical spine injury.13
Patients meeting these low-risk criteria were considered to not have a clinically significant cervical spine injury. Of note, the NEXUS investigators deemed several isolated radiographic lesions to be clinically insignificant in their analysis, including spinous process fractures, simple wedge-compression fractures without loss of 25% or more of vertebral body height, isolated avulsion fractures without ligamentous injury, type 1 odontoid fractures, transverse process fractures, end-plate fractures, trabecular bony injuries, and osteophyte fractures, except corner or teardrop fractures.13
The NEXUS validation study included 34,000 blunt trauma patients, including 3,000 patients aged 1 to 17 years and nearly 3,000 elderly patients, all of whom received at least 3-view X-rays (cross-table lateral, anteroposterior view, and open-mouth odontoid view) unless CT or magnetic resonance imaging was performed because X-rays were deemed impractical or impossible.13-15
The five low-risk features of NEXUS had a sensitivity of 99% in excluding clinically significant cervical spine trauma, and the authors determined that X-rays could have been avoided in more than 12% of the study population.
The NEXUS criteria have been criticized as being less reliable at the extremes of age, and the study authors have specifically urged caution in the use of this rule for infants and toddlers given the small number of these patients in the validation cohort.14
There have been multiple case reports and studies suggesting that the NEXUS rule does not perform well in elderly patients.15,16 Recent research has suggested that substituting “deviation from baseline mental status” for “normal level of alertness” and signs of head and neck trauma for distracting injury may improve specificity for detecting clinically significant injuries in geriatric fall patients, though the sample size does not approach the numbers from the original validation cohort.15,17
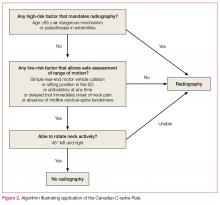
Canadian C-Spine Rule
The Canadian C-spine Rule (CCR), described in Figure 2, is more complex than the NEXUS criteria, but there are data to suggest it performs better in head-to-head evaluations.18
Patients were assessed primarily on the basis of the three-view X-rays used in the NEXUS validation, however, only 71.7% of patients received radiographs and the remainder of patients were assessed using a telephone survey the authors had created during the derivation phase.18 Some lesions were deemed to be clinically unimportant, including isolated osteophyte avulsion, isolated fracture of a transverse process not involving a facet joint, isolated fracture of a spinous process not involving the lamina, or simple compression fracture involving less than 25% of the vertebral body height.18
Unfortunately, the CCR validation study was limited by incomplete evaluation in 10% of cases, though multiple subgroup analyses consistently demonstrated the CCR was 95% sensitive or higher for detecting clinically important cervical spine injury, outperforming NEXUS. Subsequently, a large systematic review of 15 studies shows sensitivity of the CCR to be 90% to 100% and NEXUS to be 83% to 100%.19
Comment: The NEXUS criteria were validated in a larger and more heterogeneous cohort than CCR, and also have the advantage of being easier to remember. Using either rule, clinicians must consider that “clinically unimportant” cervical spine fractures are not excluded. Ultimately, the landscape of cervical spine assessment has shifted to performing CT over plain radiographs, and these rules should be re-evaluated in this context.20
Blunt Chest Trauma
NEXUS Chest Guidelines
The NEXUS chest guidelines are more recent developments to help assess the need for chest imaging in the patient presenting with a blunt trauma. The first rule derived and validated by the investigators examined the utility of seven clinical criteria in predicting the need for chest imaging—either X-ray or CT:
- Patients older than age 60 years;
- Rapid deceleration mechanism defined as fall greater than 20 feet or motor vehicle crash greater than 40 mph;
- Chest pain;
- Intoxication;
- Abnormal alertness/mental status;
- Painful distracting injury; and
- Chest wall tenderness to palpation, with the exception of isolated clavicular tenderness to palpation.21
Of note, pericardial tamponade and cardiac contusion were not studied as they “are not primarily diagnosed by chest X-ray (CXR) or chest CT.”21 The NEXUS chest validation cohort included over 9,000 blunt trauma patients older than age 14 years receiving a variety of imaging modalities, but 43% received only a single CXR. The presence of one or more of the criteria had a sensitivity of 98.8% for detecting any traumatic injury on chest imaging, as well as a sensitivity of 99.7% for any major injury.
CT-All Rule and CT-Major Rule
Subsequently, the investigators of the NEXUS chest rule focused on creating a decision rule to decrease chest CT utilization in blunt trauma, as they found chest CT after a normal CXR to be low-yield.22 After classifying injuries as major or minor based on the necessity of procedural intervention, the authors derived two rules: CT-All, designed to not miss any injuries; and CT-Major, to identify major injuries requiring procedural intervention.
Hemothorax, pneumothorax, pneumomediastinum, or pulmonary contusion that were found on CT but did not require inpatient observation, intervention, or mechanical ventilation were considered clinically insignificant.23
The CT-Major includes six factors:
- Abnormal CXR showing any thoracic injury including clavicle fracture or widened mediastinum;
- Distracting injury;
- Any chest wall tenderness;
- Sternum tenderness;
- Thoracic spine tenderness; or
- Scapular tenderness.
The CT-All includes all of the CT-Major criteria and the additional criteria of rapid deceleration mechanism as defined previously. The validation cohort included over 5,000 patients aged 14 years and older. Having one or more of the major criteria was found to be 99.2% sensitive for major injury, and meeting one or more of the CT-All criteria were found to be 95.4% sensitive for any clinically significant injury. Both CT-Major and CT-All rules identified all 21 aortic/great vessel injuries in their study; the authors assert that vigilance for these types of injuries is often the primary justification for ordering CT imaging.23
Comment: Given the ubiquity and utility of the CXR, it seems unlikely that one would forgo a simple film on the basis of these rules. The presence of an abnormal CXR was by far the best screening criterion for major injury seen on subsequent chest CT, with sensitivity and specificity of 74.7% and 83.9%, respectively.24 Of note, focused assessment with sonography for trauma (FAST) or eFAST (extended FAST) examinations were reported in only 63% or fewer patients in the derivation phase, so the rules did not incorporate point of care ultrasound findings as a criterion despite the growing influence of this modality on trauma decision-making.23
Knee Trauma
Ottawa Knee Rules
The Ottawa Knee Rules state that X-rays are only indicated if patients present with the following:
- Age older than 55 years;
- Isolated tenderness of the patella or fibular head;
- Inability to flex the knee to 90°; or
- Inability to bear weight (limping is allowed) for four steps both immediately following injury and at the ED.
In a prospective validation cohort of 1,096 adult patients, the rule was found to be 100% sensitive for detecting clinically important fractures—defined as any bone fragment at least 5 mm or avulsion if associated with complete disruption as tendons or ligaments—with a potential reduction in radiography use by 28%.25 Though the rule was designed for adult patients, it was validated in 750 children aged 2 to 16 years and found to be 100% sensitive with a 31% potential reduction in radiographs.26
Pittsburgh Knee Rules
The Pittsburgh Knee Rules only apply to patients with blunt trauma or fall to the knee—not twisting injury—and state X-rays are indicated if patients are younger than age 12 years or older than age 50 years, and are unable to bear weight fully on toe pads and heels for four steps (limping is not allowed).
An initial validation of 133 patients demonstrating 100% sensitivity was followed by a larger external validation study.27 That study prospectively applied the Pittsburgh and the Ottawa rules in over 700 patients and found the sensitivity and specificity of the rules to be 99% and 60% for Pittsburgh and 97% and 27% for Ottawa.28
Comment: The ability to apply a rule to “all-comers” is important to EPs. The Ottawa instrument is more broadly applicable and has even been assessed in pediatric populations.
Foot and Ankle Trauma
Ottawa Foot and Ankle Rule
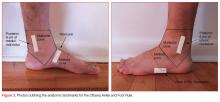
The most well-studied clinical decision rule in this category is the Ottawa Foot and Ankle Rule.29 This rule states that an ankle X-ray is only required if there is pain in the malleolar zone and bony tenderness to palpation at the posterior edge or tip of either the lateral or medial malleolus, or the inability to bear weight both immediately after injury and in the ED. The anatomic landmarks are shown in Figure 3.
- Pain in the midfoot zone and either tenderness at the base of the fifth metatarsal;
- Tenderness over the navicular; or
- Inability to bear weight both immediately after the injury and at the ED.
The prospective validation study included over 1,400 adult patients with blunt trauma or twisting injury within 10 days of presentation. Clinically significant fractures were defined as malleolar or midfoot fractures with bone fragments greater than 3 mm. The rule was found to be 100% sensitive for detecting both ankle and midfoot fractures, and would lead to a reduction of radiographs by 34% for the ankle and 30% for the foot.29 These results have borne out in multiple studies including a recent meta-analysis comparing six different decision instruments where the Ottawa rule was found to be superior.30
Though the initial validation cohort of the Ottawa rule did not include pediatric patients, it has subsequently been applied to this population. The initial validation study in the pediatric population included 670 patients aged 2 to 16 years. In addition to the criteria for clinically significant injuries in the original validation study, Salter Harris type I fractures, though treated with immobilization, were not deemed to be clinically significant injuries in this cohort.
Although the Ottawa rules were determined to be 100% sensitive for detecting significant ankle and midfoot fractures in the pediatric cohort, one of the study sites experienced an increase rather than a decrease in X-rays when this rule was applied.31
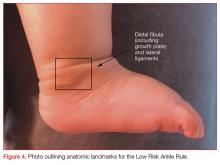
Low Risk Ankle Rule
Investigators for the pediatric specific Low Risk Ankle Rule posited that many pediatric patients with mild ankle injury refuse to bear weight on the extremity. Also, the most common fracture among preadolescent patients is a Salter-Harris type I fracture of the distal fibular epiphysis, which the investigators felt was commonly a clinical diagnosis with little to be gained from X-ray.32 These factors would prompt clinicians using the Ottawa rule to order imaging that may not be necessary in pediatric patients.
The Low Risk Ankle Rule states that if a patient has a low-risk examination, which is defined as tenderness and swelling isolated to the distal fibula and/or adjacent lateral ligaments distal to the tibial anterior joint line, then X-rays may not be necessary to exclude a high-risk ankle injury.
High-risk injuries were defined as any injury leading to an unstable ankle, and do not include avulsion, buckle, and nondisplaced Salter-Harris types I and II fractures of the distal fibula. In the validation study of nearly 600 children, the sensitivity for detecting a high risk fracture was 100% and X-rays could have been reduced in 62.8% of children with low-risk examinations compared to only 12% with the Ottawa Ankle Rule.
Comment: Although the Low Risk Ankle Rule was shown to reduce radiographic imaging by almost 63%, it omits many patients who would require splinting or subspecialty follow-up. Even the Salter-Harris type I fractures in the pediatric validation of the Ottawa rule were treated with splinting, though they were not regarded as “significant” injuries. Clinicians applying these rules, especially to a pediatric population, should have a good sense of what type of injuries these rules are designed to detect.
Conclusion
In the midst of a busy shift, clinical decision rules can help save time and expense. However, few of the rules described are meant to be applied to “all-comers,” and practitioners should be careful to not apply these rules to populations that were excluded in the validation cohorts. While clinical decision rules can help identify high-risk features, they are not a substitute for performing a thorough history and physical examination. Further studies should focus on whether these rules truly outperform unaided clinical decision-making.
Part 2 of “Playing by the Rules” will examine the use of clinician decision rules for nontraumatic conditions.
1. Stiell IG, Bennett C. Implementation of clinical decision rules in the emergency department. Acad Emerg Med. 2007;14(11):955-959. doi:10.1197/j.aem.2007.06.039.
2. Green SM. When do clinical decision rules improve patient care? Ann Emerg Med. 2013;62(2):132-135. doi:10.1016/j.annemergmed.2013.02.006.
3. Schriger DL, Elder JW, Cooper RJ. Structured clinical decision aids are seldom compared with subjective physician judgment, and are seldom superior. Ann Emerg Med. 2017;70(3):338-344.e3. doi:10.1016/j.annemergmed.2016.12.004.
4. Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343(2):100-105. doi:10.1056/NEJM200007133430204.
5. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT head rule and the New Orleans criteria in patients with minor head injury. JAMA. 2005;294(12):1511-1518. doi:10.1001/jama.294.12.1511
6. Stiell IG, Perry JJ. Traumatic intracranial injury in intoxicate patients with minor head trauma. Acad Emerg Med. 2014;21(2):221. doi:10.1111/acem.12306.
7. Mower WR, Gupta M, Rodriguez R, Hendey GW. Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) head computed tomographic (CT) decision instrument for selective imaging of blunt head injury patients: An observational study. PLoS Med. 2017;14(7):e1002313. doi:10.1371/journal.pmed.1002313.
8. Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT head rule and the New Orleans criteria for CT scanning in patients with minor head injury. JAMA. 2005;294(12):1519-1525. doi:10.1001/jama.294.12.1519.
9. Stein SC, Fabbri A, Servadei F, Glick HA. A critical comparison of clinical decision instruments for computed tomographic scanning in mild closed traumatic brain injury in adolescents and adults. Ann Emerg Med. 2009;53(2):180-188. doi:10.1016/j.annemergmed.2008.01.002.
10. Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160-1170. doi:10.1016/S0140-6736(09)61558-0.
11. Babi FE, Borland ML, Phillips N, et al. Accuracy of PECARN, CATCH, and CHALICE head injury decision rules in children: a prospective cohort study. Lancet. 2017;389(10087):2393-2402. doi:10.1016/S0140-6736(17)30555-X.
12. Mower WR. Paediatric head imaging decisions are not child’s play. Lancet. 2017;389(10087):2354-2355. doi:10.1016/S0140-6736(17)30932-7.
13. Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343(2):94-99. doi:10.1056/NEJM200007133430203.
14. Viccellio P, Simon H, Pressman BD, Shah NM, Mower WR, Hoffman JR; NEXUS Group. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001;108(2):20.
15. Tran J, Jeanmonod D, Agresti D, Hamden K, Jeanmonod RK. Prospective validation of modified NEXUS cervical spine injury criteria in low-risk elderly fall patients. West J Emerg Med. 2016;17(3):252-257. doi:10.5811/westjem.2016.3.29702.
16. Paykin G, O’Reilly G, Ackland HM, Mitra B. The NEXUS criteria are insufficient to exclude cervical spine fractures in older blunt trauma patients. Injury. 2017;48(5):1020-1024. doi:10.1016/j.injury.2017.02.013.
17. Evans D, Vera L, Jeanmonod D, Pester J, Jeanmonod R. Application of National Emergency X-Ray Utilizations Study low-risk c-spine criteria in high-risk geriatric falls. Am J Emerg Med. 2015;33(9):1184-1187. doi:10.1016/j.ajem.2015.05.031.
18. Stiell IG, Clement CM, McKnight RD, et al. The Canadian C-spine rule versus the NEXUS low-risk criteria in patients with trauma. N Engl J Med. 2003;349(26):2510-2518.
19. Michaleff ZA, Maher CG, Verhagen AP, Rebbeck T, Lin CW. Accuracy of the Canadian C-spine rule and NEXUS to screen for clinically important cervical spine injury in patients following blunt trauma: A systematic review. CMAJ. 2012;184(16):E867-E876. doi:10.1503/cmaj.120675.
20. Gale SC, Gracias VH, Reilly PM, Schwab CW. The inefficiency of plain radiography to evaluate the cervical spine after blunt trauma. J Trauma. 2005;59(5):1121-1125.
21. Rodriguez RM, Anglin D, Langdorf MI, et al. NEXUS chest: validation of a decision instrument for selective chest imaging in blunt trauma. JAMA Surg. 2013;148(10):940-946. doi:10.1001/jamasurg.2013.2757.
22. Rodriguez RM, Baumann BM, Raja AS, et al. Diagnostic yields, charges, and radiation dose of chest imaging in blunt trauma evaluations. Acad Emerg Med. 2014;21(6):644-650. doi:10.1111/acem.12396.
23. Rodriguez RM, Langdorf MI, Nishijima D, et al. Derivation and validation of two decision instruments for selective chest CT in blunt trauma: a multicenter prospective observational study (NEXUS Chest CT). PLoS Med. 2015;12(10):e1001883. doi:10.1371/journal.pmed.1001883.
24. Rodriguez RM, Hendey GW, Mower WR, et al. Selective chest imaging for blunt trauma patients: The national emergency X-ray utilization studies (NEXUS-chest algorithm). Am J Emerg Med. 2017;35(1):164-170. doi:10.1016/j.ajem.2016.10.066.
25. Stiell IG, Greenberg GH, Wells GA, et al. Prospective validation of a decision rule for the use of radiography in acute knee injuries. JAMA. 1996;275(8):611-615.
26. Bulloch B, Neto G, Plint A, et al. Validation of the Ottawa Knee Rule in children: a multicenter study. Ann Emerg Med. 2003;42(1):48-55. doi:10.1067/mem.2003.196.
27. Seaberg DC, Jackson R. Clinical decision rule for knee radiographs. Am J Emerg Med. 1994;12(5):541-543.
28. Seaberg DC, Yealy DM, Lukens T, Auble T, Mathias S. Multicenter comparison of two clinical decision rules for the use of radiography in acute, high-risk knee injuries. Ann Emerg Med. 1998;32(1):8-13.
29. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269(9):1127-1132.
30. Barelds I, Krijnen WP, van de Leur JP, van der Schans CP, Goddard RJ. Diagnostic accuracy of clinical decision rules to exclude fractures in acute ankle injuries: Systematic review and meta-analysis. J Emerg Med. 2017;53(3):353-368. doi:10.1016/j.jemermed.2017.04.035.
31. Plint AC, Bulloch B, Osmond MH, et al. Validation of the Ottawa Ankle Rules in children with ankle injuries. Acad Emerg Med. 1999;6(10):1005-1009.
32. Boutis K, Komar L, Jaramillo D, et al. Sensitivity of a clinical examination to predict need for radiography in children with ankle injuries: a prospective study. Lancet. 2001;358(9299):2118-2121. doi:10.1016/S0140-6736(01)07218-X.
Emergency physicians (EPs) rely on rapid diagnostic testing to help screen patients for illnesses. While the decision to order a test for a patient should be driven by an objective assessment of pretest probability, other factors such as fear of litigation, clinical inexperience, or desire for increased patient satisfaction can prompt testing even when the likelihood of disease is low. This in turn leads to practice variability, increased cost, and decreased ED throughput, as well as other risks attendant to overtesting and overtreatment. Conversely, practitioners may fail to order necessary tests despite the presence of high-risk clinical features, which in turn may lead to misdiagnoses and delay in initiating life-saving treatments.
Development of Decision Rules
Clinical decision rules seek to decrease resource utilization in instances of low probability of disease and to identify high-risk features that should prompt further investigation. The formation of clinical decision rules entails at least three steps, which Ian Stiell, MD, emergency medicine’s (EM’s) most prolific author of these instruments, describes as follows:
- Creation of the rule or derivation;
- Prospective assessment of the reliability, accuracy, and impact of the rule in a validation study; and
- Gauging the effect of the rule on patient care through an implementation study.1
In addition to these three steps, many clinicians argue that there should also be an important fourth step included in this process: the external validation or assessment of the rule outside of the original study site(s), to assure reliability of the rule across a variety of populations for which its use was intended.2
Critiques and Caveats
A common critique of clinical decision rules is that they may not necessarily outperform subjective physician judgment, and that those who create these rules often do not explicitly compare their instruments against independent unassisted decision-making by clinicians.2,3
Another drawback is that the misapplication of these rules can lead to increased testing, something particularly problematic for one-way rules, which only guide the provider in a single clinical direction. An example of a one-way rule is the Pulmonary Embolism Rule-Out Criteria (PERC), which advises that a low-risk patient who does not have any of the PERC factors will not require any further testing. This, however, does not necessarily mean that further testing is indicated in patients who have one of the PERC factors present.2 Thus, applying PERC and other one-way decision-making rules in a two-way fashion can prompt testing that would not be ordered based on clinician gestalt. Rules that are designed to help determine when testing is necessary and when it is unnecessary are referred to as two-way rules, an example of which is the Ottawa Ankle Rule.
Controversies aside, the incorporation of clinical decision rules in the electronic medical record of many institutions and the proliferation of smartphone applications utilizing these instruments have further cemented their place in EM. This article describes the more commonly used ED clinical decision rules, as well as pearls and pitfalls pertaining to their use. Part 1 that follows covers important validated rules related to trauma patients in the ED. Part 2, which will appear in an upcoming 2018 issue, will cover nontrauma medical-diagnosis decision rules, including pulmonary embolism, and pneumonia.
Head Trauma
The increased utilization of computed tomography (CT) studies to assess for minor blunt head trauma spurred the development of clinical decision rules. In adult patients, the most popular and well-studied instruments are the New Orleans Criteria (NOC), the Canadian CT Head Rule (CCHR), and the National Emergency X-Radiography Utilization Study (NEXUS) CT Head Rule.
New Orleans Criteria
The NOC validation cohort examined over 900 cases at a single trauma center, enrolling all patients 3 years of age and older who had suffered minor head trauma (defined as loss of consciousness (LOC) in a patient with grossly normal neurological examination and a Glasgow Coma Scale [GCS] score of 15), in the preceding 24 hours.4 Patients who experienced no LOC, had focal neurological deficit (except isolated short-term memory deficits), or who did not have any CT study performed, were excluded.
The NOC describes seven factors for consideration:
- Short-term memory deficits;
- Intoxication with drugs or alcohol;
- Physical evidence of trauma above the clavicles;
- Patients older than age 60 years;
- Seizure;
- Headache; and
- Vomiting.
The presence of at least one of these factors was found to be 100% sensitive and 25% specific for the presence of any traumatic intracranial abnormality on CT, though only 6.5% of patients in the derivation and validation cohorts had positive CT scans, and ultimately less than 1% had lesions that required surgery.4
Canadian CT Head Rule
The CCHR validation study assessed over 2,700 patients at nine Canadian EDs, enrolling all patients aged 16 years and older who sustained a blunt head trauma less than 24 hours prior to presentation and who had a GCS score of 13 or higher. The investigators of the CCHR study specified that included patients should have suffered a witnessed LOC, definite amnesia, or a witnessed disorientation.5 Patients who did not have any of these factors were deemed to have minimal head trauma and were excluded from the study. Also excluded from this study were patients who had seizure prior to ED arrival, had a coagulopathy or used oral anticoagulants, had acute focal neurological deficit or obvious depressed skull fracture, had unstable vitals associated with a major trauma, or were pregnant.5 The CCHR was not designed to be applied to these excluded populations. Of note, patients with drug and alcohol intoxication were included in their validation.6
The CCHR describes five high-risk factors that increase the likelihood of requiring acute neurosurgical intervention:
- A GCS score of less than 15 at 2 hours after injury;
- Suspected open or depressed skull fracture;
- Any sign of basal skull fracture (eg, hemotympanum, raccoon eyes, cerebrospinal fluid otorrhea/rhinorrhea, Battle’s sign;
- Two or more episodes of vomiting; or
- Patients aged 65 years or older.
In addition to these five high-risk factors, the CCHR also describes two medium risk criteria for finding any traumatic lesion on CT that would not necessitate acute neurosurgical intervention: amnesia of greater than 30 minutes before impact; and injury resulting from a dangerous mechanism such as a pedestrian struck by motor vehicle, occupant ejected from a motor vehicle, or a fall from a height greater than 3 feet or from over 5 stair-steps.
The presence of any one or more of the five high-risk factors was 100% sensitive for predicting the need for neurosurgical intervention, and taken together, having one or more of the seven factors was 100% sensitive for predicting clinically important brain injury.5
NEXUS CT Head Rule
The NEXUS CT Head Rule validation cohort included over 11,000 pediatric and adult blunt head trauma patients undergoing CT imaging at four hospital EDs, and excluded patients with penetrating injuries or presentation greater than 24 hours after injury.7 Patients were considered to be low risk if none of the following criteria were present:
- Age 65 years or older;
- Evidence of significant skull fracture;
- Scalp hematoma;
- Neurological deficit;
- Altered level of alertness;
- Abnormal behavior;
- Coagulopathy; and
- Recurrent vomiting.
Patients who have one or more of these factors were considered as high risk. The presence of one of these factors was 100% sensitive for detecting patients with lesions requiring neurosurgery and 99% sensitive for detecting any significant intracranial injury, with specificity for either condition at 25%.7
Decision-Rule Sensitivity Comparison
Though the CCHR, NOC, and NEXUS rules differ in their inclusion/exclusion criteria, they have been compared to each other in different populations in the medical literature. A subgroup of the CCHR validation cohort and an accompanying external validation study of over 3,000 patients both found that the CCHR and the NOC were at least as sensitive (100%) in detecting lesions requiring neurosurgical intervention, but the CCHR was more specific and had greater potential to reduce imaging rates.5,8 Another study examined the performance of NEXUS, CCHR, NOC, and other decision instruments in a database of nearly 8,000 adolescent and adult head trauma patients. The authors of this study found the three rules to have similarly high sensitivities (97% to 99%) for detecting clinically important findings, but felt NEXUS to have the best combination of sensitivity and specificity compared to CCHR and NOC.9
Comment: A decision rule to decrease CT utilization in intoxicated head trauma patients is particularly useful, but only the CCHR can help potentially avoid imaging a drug or alcohol-intoxicated patient with abnormal behavior or altered level of alertness. Similarly, distinguishing superficial scalp trauma from more worrisome signs of intracranial injury is important, but the NOC and NEXUS rules recommend imaging for any trauma above the clavicles and
Though the rules have similar sensitivities, the CCHR appears to have the greatest potential for aiding clinical decision-making. Of note, all of the adult decision rules consider patients older than age 60 or 65 years to be a high-risk feature. An instrument with better specificity for geriatric patients would be immensely helpful as our population continues to age.
The Pediatric Population: The PECARN Rule
The CCHR, NEXUS, and NOC have been variously applied to pediatric populations, and the NEXUS and NOC included children (patients aged 3 years and older in the NOC) in their validation cohorts. Perhaps the most widely utilized validated rule for pediatric head trauma in the United States is the Pediatric Emergency Care Applied Research Network (PECARN) rule.
The PECARN rule is an age-specific instrument for assessing pediatric blunt head trauma patients for clinically important traumatic brain injury (TBI)—ie, brain injuries resulting in death, neurosurgery, intubation longer than 24 hours, or hospital admission length of more than two nights.10 The PECARN algorithm as described in its validation study is reproduced in Figure 1.
Patients with trivial injury mechanisms such as ground level falls or running into stationary objects and no signs or symptoms of head trauma other than scalp abrasions or lacerations were excluded from the PECARN analysis, as were patients with pre-existing neurological disorders, including ventricular shunts and brain tumors, patients with penetrating trauma, and those with bleeding disorders. The validation study of over 8,000 children found the presence of any of the criteria to be 100% and 96.8% sensitive in detecting clinically important TBI in children aged less than 2 years and children aged 2 years or older, respectively.
The PECARN rule was recently assessed in a large prospective cohort against two less extensively studied clinical decision rules: the Children’s Head Injury Algorithm for the Prediction of Important Clinical Events (CHALICE) and the Canadian Assessment of Tomography for Childhood Head Injury (CATCH). Though the sample size was over 20,000 patients, only less than 1% required neurosurgery or died. The PECARN rule was determined to have the highest validation sensitivities (100% for <2 years, 99% for ≥2 years) of all of the rules; however, it has been noted by some clinicians that the strict application of the PECARN rule to the study population would have increased the rate of CT scanning 5-fold without providing any clear benefit over clinical judgment in detecting injury.11,12
Comment: The PECARN tool is by far the most robust instrument for pediatric head trauma, but striking a balance between finding otherwise clinically occult injury and reducing unnecessary testing/irradiation remains difficult in this vulnerable population.
Cervical Spine Trauma
Though the incidence of serious cervical spine injury in blunt trauma is low, the potentially devastating consequences of a missed lesion is a potent driver of radiographic testing.13 The Nexus Criteria and Canadian C-Spine Rule were developed to assist the clinician in determining when radiographic imaging is indicated in patients presenting with a blunt-trauma-related injury. The NEXUS criteria were developed to decrease cervical spine X-ray use based on five low-risk factors:
- No posterior midline tenderness;
- No focal neurological deficit;
- Normal level of alertness;
- No evidence of intoxication; and
- No clinically apparent pain that could distract the patient from the pain of a cervical spine injury.13
Patients meeting these low-risk criteria were considered to not have a clinically significant cervical spine injury. Of note, the NEXUS investigators deemed several isolated radiographic lesions to be clinically insignificant in their analysis, including spinous process fractures, simple wedge-compression fractures without loss of 25% or more of vertebral body height, isolated avulsion fractures without ligamentous injury, type 1 odontoid fractures, transverse process fractures, end-plate fractures, trabecular bony injuries, and osteophyte fractures, except corner or teardrop fractures.13
The NEXUS validation study included 34,000 blunt trauma patients, including 3,000 patients aged 1 to 17 years and nearly 3,000 elderly patients, all of whom received at least 3-view X-rays (cross-table lateral, anteroposterior view, and open-mouth odontoid view) unless CT or magnetic resonance imaging was performed because X-rays were deemed impractical or impossible.13-15
The five low-risk features of NEXUS had a sensitivity of 99% in excluding clinically significant cervical spine trauma, and the authors determined that X-rays could have been avoided in more than 12% of the study population.
The NEXUS criteria have been criticized as being less reliable at the extremes of age, and the study authors have specifically urged caution in the use of this rule for infants and toddlers given the small number of these patients in the validation cohort.14
There have been multiple case reports and studies suggesting that the NEXUS rule does not perform well in elderly patients.15,16 Recent research has suggested that substituting “deviation from baseline mental status” for “normal level of alertness” and signs of head and neck trauma for distracting injury may improve specificity for detecting clinically significant injuries in geriatric fall patients, though the sample size does not approach the numbers from the original validation cohort.15,17
Canadian C-Spine Rule
The Canadian C-spine Rule (CCR), described in Figure 2, is more complex than the NEXUS criteria, but there are data to suggest it performs better in head-to-head evaluations.18
Patients were assessed primarily on the basis of the three-view X-rays used in the NEXUS validation, however, only 71.7% of patients received radiographs and the remainder of patients were assessed using a telephone survey the authors had created during the derivation phase.18 Some lesions were deemed to be clinically unimportant, including isolated osteophyte avulsion, isolated fracture of a transverse process not involving a facet joint, isolated fracture of a spinous process not involving the lamina, or simple compression fracture involving less than 25% of the vertebral body height.18
Unfortunately, the CCR validation study was limited by incomplete evaluation in 10% of cases, though multiple subgroup analyses consistently demonstrated the CCR was 95% sensitive or higher for detecting clinically important cervical spine injury, outperforming NEXUS. Subsequently, a large systematic review of 15 studies shows sensitivity of the CCR to be 90% to 100% and NEXUS to be 83% to 100%.19
Comment: The NEXUS criteria were validated in a larger and more heterogeneous cohort than CCR, and also have the advantage of being easier to remember. Using either rule, clinicians must consider that “clinically unimportant” cervical spine fractures are not excluded. Ultimately, the landscape of cervical spine assessment has shifted to performing CT over plain radiographs, and these rules should be re-evaluated in this context.20
Blunt Chest Trauma
NEXUS Chest Guidelines
The NEXUS chest guidelines are more recent developments to help assess the need for chest imaging in the patient presenting with a blunt trauma. The first rule derived and validated by the investigators examined the utility of seven clinical criteria in predicting the need for chest imaging—either X-ray or CT:
- Patients older than age 60 years;
- Rapid deceleration mechanism defined as fall greater than 20 feet or motor vehicle crash greater than 40 mph;
- Chest pain;
- Intoxication;
- Abnormal alertness/mental status;
- Painful distracting injury; and
- Chest wall tenderness to palpation, with the exception of isolated clavicular tenderness to palpation.21
Of note, pericardial tamponade and cardiac contusion were not studied as they “are not primarily diagnosed by chest X-ray (CXR) or chest CT.”21 The NEXUS chest validation cohort included over 9,000 blunt trauma patients older than age 14 years receiving a variety of imaging modalities, but 43% received only a single CXR. The presence of one or more of the criteria had a sensitivity of 98.8% for detecting any traumatic injury on chest imaging, as well as a sensitivity of 99.7% for any major injury.
CT-All Rule and CT-Major Rule
Subsequently, the investigators of the NEXUS chest rule focused on creating a decision rule to decrease chest CT utilization in blunt trauma, as they found chest CT after a normal CXR to be low-yield.22 After classifying injuries as major or minor based on the necessity of procedural intervention, the authors derived two rules: CT-All, designed to not miss any injuries; and CT-Major, to identify major injuries requiring procedural intervention.
Hemothorax, pneumothorax, pneumomediastinum, or pulmonary contusion that were found on CT but did not require inpatient observation, intervention, or mechanical ventilation were considered clinically insignificant.23
The CT-Major includes six factors:
- Abnormal CXR showing any thoracic injury including clavicle fracture or widened mediastinum;
- Distracting injury;
- Any chest wall tenderness;
- Sternum tenderness;
- Thoracic spine tenderness; or
- Scapular tenderness.
The CT-All includes all of the CT-Major criteria and the additional criteria of rapid deceleration mechanism as defined previously. The validation cohort included over 5,000 patients aged 14 years and older. Having one or more of the major criteria was found to be 99.2% sensitive for major injury, and meeting one or more of the CT-All criteria were found to be 95.4% sensitive for any clinically significant injury. Both CT-Major and CT-All rules identified all 21 aortic/great vessel injuries in their study; the authors assert that vigilance for these types of injuries is often the primary justification for ordering CT imaging.23
Comment: Given the ubiquity and utility of the CXR, it seems unlikely that one would forgo a simple film on the basis of these rules. The presence of an abnormal CXR was by far the best screening criterion for major injury seen on subsequent chest CT, with sensitivity and specificity of 74.7% and 83.9%, respectively.24 Of note, focused assessment with sonography for trauma (FAST) or eFAST (extended FAST) examinations were reported in only 63% or fewer patients in the derivation phase, so the rules did not incorporate point of care ultrasound findings as a criterion despite the growing influence of this modality on trauma decision-making.23
Knee Trauma
Ottawa Knee Rules
The Ottawa Knee Rules state that X-rays are only indicated if patients present with the following:
- Age older than 55 years;
- Isolated tenderness of the patella or fibular head;
- Inability to flex the knee to 90°; or
- Inability to bear weight (limping is allowed) for four steps both immediately following injury and at the ED.
In a prospective validation cohort of 1,096 adult patients, the rule was found to be 100% sensitive for detecting clinically important fractures—defined as any bone fragment at least 5 mm or avulsion if associated with complete disruption as tendons or ligaments—with a potential reduction in radiography use by 28%.25 Though the rule was designed for adult patients, it was validated in 750 children aged 2 to 16 years and found to be 100% sensitive with a 31% potential reduction in radiographs.26
Pittsburgh Knee Rules
The Pittsburgh Knee Rules only apply to patients with blunt trauma or fall to the knee—not twisting injury—and state X-rays are indicated if patients are younger than age 12 years or older than age 50 years, and are unable to bear weight fully on toe pads and heels for four steps (limping is not allowed).
An initial validation of 133 patients demonstrating 100% sensitivity was followed by a larger external validation study.27 That study prospectively applied the Pittsburgh and the Ottawa rules in over 700 patients and found the sensitivity and specificity of the rules to be 99% and 60% for Pittsburgh and 97% and 27% for Ottawa.28
Comment: The ability to apply a rule to “all-comers” is important to EPs. The Ottawa instrument is more broadly applicable and has even been assessed in pediatric populations.
Foot and Ankle Trauma
Ottawa Foot and Ankle Rule
The most well-studied clinical decision rule in this category is the Ottawa Foot and Ankle Rule.29 This rule states that an ankle X-ray is only required if there is pain in the malleolar zone and bony tenderness to palpation at the posterior edge or tip of either the lateral or medial malleolus, or the inability to bear weight both immediately after injury and in the ED. The anatomic landmarks are shown in Figure 3.
- Pain in the midfoot zone and either tenderness at the base of the fifth metatarsal;
- Tenderness over the navicular; or
- Inability to bear weight both immediately after the injury and at the ED.
The prospective validation study included over 1,400 adult patients with blunt trauma or twisting injury within 10 days of presentation. Clinically significant fractures were defined as malleolar or midfoot fractures with bone fragments greater than 3 mm. The rule was found to be 100% sensitive for detecting both ankle and midfoot fractures, and would lead to a reduction of radiographs by 34% for the ankle and 30% for the foot.29 These results have borne out in multiple studies including a recent meta-analysis comparing six different decision instruments where the Ottawa rule was found to be superior.30
Though the initial validation cohort of the Ottawa rule did not include pediatric patients, it has subsequently been applied to this population. The initial validation study in the pediatric population included 670 patients aged 2 to 16 years. In addition to the criteria for clinically significant injuries in the original validation study, Salter Harris type I fractures, though treated with immobilization, were not deemed to be clinically significant injuries in this cohort.
Although the Ottawa rules were determined to be 100% sensitive for detecting significant ankle and midfoot fractures in the pediatric cohort, one of the study sites experienced an increase rather than a decrease in X-rays when this rule was applied.31
Low Risk Ankle Rule
Investigators for the pediatric specific Low Risk Ankle Rule posited that many pediatric patients with mild ankle injury refuse to bear weight on the extremity. Also, the most common fracture among preadolescent patients is a Salter-Harris type I fracture of the distal fibular epiphysis, which the investigators felt was commonly a clinical diagnosis with little to be gained from X-ray.32 These factors would prompt clinicians using the Ottawa rule to order imaging that may not be necessary in pediatric patients.
The Low Risk Ankle Rule states that if a patient has a low-risk examination, which is defined as tenderness and swelling isolated to the distal fibula and/or adjacent lateral ligaments distal to the tibial anterior joint line, then X-rays may not be necessary to exclude a high-risk ankle injury.
High-risk injuries were defined as any injury leading to an unstable ankle, and do not include avulsion, buckle, and nondisplaced Salter-Harris types I and II fractures of the distal fibula. In the validation study of nearly 600 children, the sensitivity for detecting a high risk fracture was 100% and X-rays could have been reduced in 62.8% of children with low-risk examinations compared to only 12% with the Ottawa Ankle Rule.
Comment: Although the Low Risk Ankle Rule was shown to reduce radiographic imaging by almost 63%, it omits many patients who would require splinting or subspecialty follow-up. Even the Salter-Harris type I fractures in the pediatric validation of the Ottawa rule were treated with splinting, though they were not regarded as “significant” injuries. Clinicians applying these rules, especially to a pediatric population, should have a good sense of what type of injuries these rules are designed to detect.
Conclusion
In the midst of a busy shift, clinical decision rules can help save time and expense. However, few of the rules described are meant to be applied to “all-comers,” and practitioners should be careful to not apply these rules to populations that were excluded in the validation cohorts. While clinical decision rules can help identify high-risk features, they are not a substitute for performing a thorough history and physical examination. Further studies should focus on whether these rules truly outperform unaided clinical decision-making.
Part 2 of “Playing by the Rules” will examine the use of clinician decision rules for nontraumatic conditions.
Emergency physicians (EPs) rely on rapid diagnostic testing to help screen patients for illnesses. While the decision to order a test for a patient should be driven by an objective assessment of pretest probability, other factors such as fear of litigation, clinical inexperience, or desire for increased patient satisfaction can prompt testing even when the likelihood of disease is low. This in turn leads to practice variability, increased cost, and decreased ED throughput, as well as other risks attendant to overtesting and overtreatment. Conversely, practitioners may fail to order necessary tests despite the presence of high-risk clinical features, which in turn may lead to misdiagnoses and delay in initiating life-saving treatments.
Development of Decision Rules
Clinical decision rules seek to decrease resource utilization in instances of low probability of disease and to identify high-risk features that should prompt further investigation. The formation of clinical decision rules entails at least three steps, which Ian Stiell, MD, emergency medicine’s (EM’s) most prolific author of these instruments, describes as follows:
- Creation of the rule or derivation;
- Prospective assessment of the reliability, accuracy, and impact of the rule in a validation study; and
- Gauging the effect of the rule on patient care through an implementation study.1
In addition to these three steps, many clinicians argue that there should also be an important fourth step included in this process: the external validation or assessment of the rule outside of the original study site(s), to assure reliability of the rule across a variety of populations for which its use was intended.2
Critiques and Caveats
A common critique of clinical decision rules is that they may not necessarily outperform subjective physician judgment, and that those who create these rules often do not explicitly compare their instruments against independent unassisted decision-making by clinicians.2,3
Another drawback is that the misapplication of these rules can lead to increased testing, something particularly problematic for one-way rules, which only guide the provider in a single clinical direction. An example of a one-way rule is the Pulmonary Embolism Rule-Out Criteria (PERC), which advises that a low-risk patient who does not have any of the PERC factors will not require any further testing. This, however, does not necessarily mean that further testing is indicated in patients who have one of the PERC factors present.2 Thus, applying PERC and other one-way decision-making rules in a two-way fashion can prompt testing that would not be ordered based on clinician gestalt. Rules that are designed to help determine when testing is necessary and when it is unnecessary are referred to as two-way rules, an example of which is the Ottawa Ankle Rule.
Controversies aside, the incorporation of clinical decision rules in the electronic medical record of many institutions and the proliferation of smartphone applications utilizing these instruments have further cemented their place in EM. This article describes the more commonly used ED clinical decision rules, as well as pearls and pitfalls pertaining to their use. Part 1 that follows covers important validated rules related to trauma patients in the ED. Part 2, which will appear in an upcoming 2018 issue, will cover nontrauma medical-diagnosis decision rules, including pulmonary embolism, and pneumonia.
Head Trauma
The increased utilization of computed tomography (CT) studies to assess for minor blunt head trauma spurred the development of clinical decision rules. In adult patients, the most popular and well-studied instruments are the New Orleans Criteria (NOC), the Canadian CT Head Rule (CCHR), and the National Emergency X-Radiography Utilization Study (NEXUS) CT Head Rule.
New Orleans Criteria
The NOC validation cohort examined over 900 cases at a single trauma center, enrolling all patients 3 years of age and older who had suffered minor head trauma (defined as loss of consciousness (LOC) in a patient with grossly normal neurological examination and a Glasgow Coma Scale [GCS] score of 15), in the preceding 24 hours.4 Patients who experienced no LOC, had focal neurological deficit (except isolated short-term memory deficits), or who did not have any CT study performed, were excluded.
The NOC describes seven factors for consideration:
- Short-term memory deficits;
- Intoxication with drugs or alcohol;
- Physical evidence of trauma above the clavicles;
- Patients older than age 60 years;
- Seizure;
- Headache; and
- Vomiting.
The presence of at least one of these factors was found to be 100% sensitive and 25% specific for the presence of any traumatic intracranial abnormality on CT, though only 6.5% of patients in the derivation and validation cohorts had positive CT scans, and ultimately less than 1% had lesions that required surgery.4
Canadian CT Head Rule
The CCHR validation study assessed over 2,700 patients at nine Canadian EDs, enrolling all patients aged 16 years and older who sustained a blunt head trauma less than 24 hours prior to presentation and who had a GCS score of 13 or higher. The investigators of the CCHR study specified that included patients should have suffered a witnessed LOC, definite amnesia, or a witnessed disorientation.5 Patients who did not have any of these factors were deemed to have minimal head trauma and were excluded from the study. Also excluded from this study were patients who had seizure prior to ED arrival, had a coagulopathy or used oral anticoagulants, had acute focal neurological deficit or obvious depressed skull fracture, had unstable vitals associated with a major trauma, or were pregnant.5 The CCHR was not designed to be applied to these excluded populations. Of note, patients with drug and alcohol intoxication were included in their validation.6
The CCHR describes five high-risk factors that increase the likelihood of requiring acute neurosurgical intervention:
- A GCS score of less than 15 at 2 hours after injury;
- Suspected open or depressed skull fracture;
- Any sign of basal skull fracture (eg, hemotympanum, raccoon eyes, cerebrospinal fluid otorrhea/rhinorrhea, Battle’s sign;
- Two or more episodes of vomiting; or
- Patients aged 65 years or older.
In addition to these five high-risk factors, the CCHR also describes two medium risk criteria for finding any traumatic lesion on CT that would not necessitate acute neurosurgical intervention: amnesia of greater than 30 minutes before impact; and injury resulting from a dangerous mechanism such as a pedestrian struck by motor vehicle, occupant ejected from a motor vehicle, or a fall from a height greater than 3 feet or from over 5 stair-steps.
The presence of any one or more of the five high-risk factors was 100% sensitive for predicting the need for neurosurgical intervention, and taken together, having one or more of the seven factors was 100% sensitive for predicting clinically important brain injury.5
NEXUS CT Head Rule
The NEXUS CT Head Rule validation cohort included over 11,000 pediatric and adult blunt head trauma patients undergoing CT imaging at four hospital EDs, and excluded patients with penetrating injuries or presentation greater than 24 hours after injury.7 Patients were considered to be low risk if none of the following criteria were present:
- Age 65 years or older;
- Evidence of significant skull fracture;
- Scalp hematoma;
- Neurological deficit;
- Altered level of alertness;
- Abnormal behavior;
- Coagulopathy; and
- Recurrent vomiting.
Patients who have one or more of these factors were considered as high risk. The presence of one of these factors was 100% sensitive for detecting patients with lesions requiring neurosurgery and 99% sensitive for detecting any significant intracranial injury, with specificity for either condition at 25%.7
Decision-Rule Sensitivity Comparison
Though the CCHR, NOC, and NEXUS rules differ in their inclusion/exclusion criteria, they have been compared to each other in different populations in the medical literature. A subgroup of the CCHR validation cohort and an accompanying external validation study of over 3,000 patients both found that the CCHR and the NOC were at least as sensitive (100%) in detecting lesions requiring neurosurgical intervention, but the CCHR was more specific and had greater potential to reduce imaging rates.5,8 Another study examined the performance of NEXUS, CCHR, NOC, and other decision instruments in a database of nearly 8,000 adolescent and adult head trauma patients. The authors of this study found the three rules to have similarly high sensitivities (97% to 99%) for detecting clinically important findings, but felt NEXUS to have the best combination of sensitivity and specificity compared to CCHR and NOC.9
Comment: A decision rule to decrease CT utilization in intoxicated head trauma patients is particularly useful, but only the CCHR can help potentially avoid imaging a drug or alcohol-intoxicated patient with abnormal behavior or altered level of alertness. Similarly, distinguishing superficial scalp trauma from more worrisome signs of intracranial injury is important, but the NOC and NEXUS rules recommend imaging for any trauma above the clavicles and
Though the rules have similar sensitivities, the CCHR appears to have the greatest potential for aiding clinical decision-making. Of note, all of the adult decision rules consider patients older than age 60 or 65 years to be a high-risk feature. An instrument with better specificity for geriatric patients would be immensely helpful as our population continues to age.
The Pediatric Population: The PECARN Rule
The CCHR, NEXUS, and NOC have been variously applied to pediatric populations, and the NEXUS and NOC included children (patients aged 3 years and older in the NOC) in their validation cohorts. Perhaps the most widely utilized validated rule for pediatric head trauma in the United States is the Pediatric Emergency Care Applied Research Network (PECARN) rule.
The PECARN rule is an age-specific instrument for assessing pediatric blunt head trauma patients for clinically important traumatic brain injury (TBI)—ie, brain injuries resulting in death, neurosurgery, intubation longer than 24 hours, or hospital admission length of more than two nights.10 The PECARN algorithm as described in its validation study is reproduced in Figure 1.
Patients with trivial injury mechanisms such as ground level falls or running into stationary objects and no signs or symptoms of head trauma other than scalp abrasions or lacerations were excluded from the PECARN analysis, as were patients with pre-existing neurological disorders, including ventricular shunts and brain tumors, patients with penetrating trauma, and those with bleeding disorders. The validation study of over 8,000 children found the presence of any of the criteria to be 100% and 96.8% sensitive in detecting clinically important TBI in children aged less than 2 years and children aged 2 years or older, respectively.
The PECARN rule was recently assessed in a large prospective cohort against two less extensively studied clinical decision rules: the Children’s Head Injury Algorithm for the Prediction of Important Clinical Events (CHALICE) and the Canadian Assessment of Tomography for Childhood Head Injury (CATCH). Though the sample size was over 20,000 patients, only less than 1% required neurosurgery or died. The PECARN rule was determined to have the highest validation sensitivities (100% for <2 years, 99% for ≥2 years) of all of the rules; however, it has been noted by some clinicians that the strict application of the PECARN rule to the study population would have increased the rate of CT scanning 5-fold without providing any clear benefit over clinical judgment in detecting injury.11,12
Comment: The PECARN tool is by far the most robust instrument for pediatric head trauma, but striking a balance between finding otherwise clinically occult injury and reducing unnecessary testing/irradiation remains difficult in this vulnerable population.
Cervical Spine Trauma
Though the incidence of serious cervical spine injury in blunt trauma is low, the potentially devastating consequences of a missed lesion is a potent driver of radiographic testing.13 The Nexus Criteria and Canadian C-Spine Rule were developed to assist the clinician in determining when radiographic imaging is indicated in patients presenting with a blunt-trauma-related injury. The NEXUS criteria were developed to decrease cervical spine X-ray use based on five low-risk factors:
- No posterior midline tenderness;
- No focal neurological deficit;
- Normal level of alertness;
- No evidence of intoxication; and
- No clinically apparent pain that could distract the patient from the pain of a cervical spine injury.13
Patients meeting these low-risk criteria were considered to not have a clinically significant cervical spine injury. Of note, the NEXUS investigators deemed several isolated radiographic lesions to be clinically insignificant in their analysis, including spinous process fractures, simple wedge-compression fractures without loss of 25% or more of vertebral body height, isolated avulsion fractures without ligamentous injury, type 1 odontoid fractures, transverse process fractures, end-plate fractures, trabecular bony injuries, and osteophyte fractures, except corner or teardrop fractures.13
The NEXUS validation study included 34,000 blunt trauma patients, including 3,000 patients aged 1 to 17 years and nearly 3,000 elderly patients, all of whom received at least 3-view X-rays (cross-table lateral, anteroposterior view, and open-mouth odontoid view) unless CT or magnetic resonance imaging was performed because X-rays were deemed impractical or impossible.13-15
The five low-risk features of NEXUS had a sensitivity of 99% in excluding clinically significant cervical spine trauma, and the authors determined that X-rays could have been avoided in more than 12% of the study population.
The NEXUS criteria have been criticized as being less reliable at the extremes of age, and the study authors have specifically urged caution in the use of this rule for infants and toddlers given the small number of these patients in the validation cohort.14
There have been multiple case reports and studies suggesting that the NEXUS rule does not perform well in elderly patients.15,16 Recent research has suggested that substituting “deviation from baseline mental status” for “normal level of alertness” and signs of head and neck trauma for distracting injury may improve specificity for detecting clinically significant injuries in geriatric fall patients, though the sample size does not approach the numbers from the original validation cohort.15,17
Canadian C-Spine Rule
The Canadian C-spine Rule (CCR), described in Figure 2, is more complex than the NEXUS criteria, but there are data to suggest it performs better in head-to-head evaluations.18
Patients were assessed primarily on the basis of the three-view X-rays used in the NEXUS validation, however, only 71.7% of patients received radiographs and the remainder of patients were assessed using a telephone survey the authors had created during the derivation phase.18 Some lesions were deemed to be clinically unimportant, including isolated osteophyte avulsion, isolated fracture of a transverse process not involving a facet joint, isolated fracture of a spinous process not involving the lamina, or simple compression fracture involving less than 25% of the vertebral body height.18
Unfortunately, the CCR validation study was limited by incomplete evaluation in 10% of cases, though multiple subgroup analyses consistently demonstrated the CCR was 95% sensitive or higher for detecting clinically important cervical spine injury, outperforming NEXUS. Subsequently, a large systematic review of 15 studies shows sensitivity of the CCR to be 90% to 100% and NEXUS to be 83% to 100%.19
Comment: The NEXUS criteria were validated in a larger and more heterogeneous cohort than CCR, and also have the advantage of being easier to remember. Using either rule, clinicians must consider that “clinically unimportant” cervical spine fractures are not excluded. Ultimately, the landscape of cervical spine assessment has shifted to performing CT over plain radiographs, and these rules should be re-evaluated in this context.20
Blunt Chest Trauma
NEXUS Chest Guidelines
The NEXUS chest guidelines are more recent developments to help assess the need for chest imaging in the patient presenting with a blunt trauma. The first rule derived and validated by the investigators examined the utility of seven clinical criteria in predicting the need for chest imaging—either X-ray or CT:
- Patients older than age 60 years;
- Rapid deceleration mechanism defined as fall greater than 20 feet or motor vehicle crash greater than 40 mph;
- Chest pain;
- Intoxication;
- Abnormal alertness/mental status;
- Painful distracting injury; and
- Chest wall tenderness to palpation, with the exception of isolated clavicular tenderness to palpation.21
Of note, pericardial tamponade and cardiac contusion were not studied as they “are not primarily diagnosed by chest X-ray (CXR) or chest CT.”21 The NEXUS chest validation cohort included over 9,000 blunt trauma patients older than age 14 years receiving a variety of imaging modalities, but 43% received only a single CXR. The presence of one or more of the criteria had a sensitivity of 98.8% for detecting any traumatic injury on chest imaging, as well as a sensitivity of 99.7% for any major injury.
CT-All Rule and CT-Major Rule
Subsequently, the investigators of the NEXUS chest rule focused on creating a decision rule to decrease chest CT utilization in blunt trauma, as they found chest CT after a normal CXR to be low-yield.22 After classifying injuries as major or minor based on the necessity of procedural intervention, the authors derived two rules: CT-All, designed to not miss any injuries; and CT-Major, to identify major injuries requiring procedural intervention.
Hemothorax, pneumothorax, pneumomediastinum, or pulmonary contusion that were found on CT but did not require inpatient observation, intervention, or mechanical ventilation were considered clinically insignificant.23
The CT-Major includes six factors:
- Abnormal CXR showing any thoracic injury including clavicle fracture or widened mediastinum;
- Distracting injury;
- Any chest wall tenderness;
- Sternum tenderness;
- Thoracic spine tenderness; or
- Scapular tenderness.
The CT-All includes all of the CT-Major criteria and the additional criteria of rapid deceleration mechanism as defined previously. The validation cohort included over 5,000 patients aged 14 years and older. Having one or more of the major criteria was found to be 99.2% sensitive for major injury, and meeting one or more of the CT-All criteria were found to be 95.4% sensitive for any clinically significant injury. Both CT-Major and CT-All rules identified all 21 aortic/great vessel injuries in their study; the authors assert that vigilance for these types of injuries is often the primary justification for ordering CT imaging.23
Comment: Given the ubiquity and utility of the CXR, it seems unlikely that one would forgo a simple film on the basis of these rules. The presence of an abnormal CXR was by far the best screening criterion for major injury seen on subsequent chest CT, with sensitivity and specificity of 74.7% and 83.9%, respectively.24 Of note, focused assessment with sonography for trauma (FAST) or eFAST (extended FAST) examinations were reported in only 63% or fewer patients in the derivation phase, so the rules did not incorporate point of care ultrasound findings as a criterion despite the growing influence of this modality on trauma decision-making.23
Knee Trauma
Ottawa Knee Rules
The Ottawa Knee Rules state that X-rays are only indicated if patients present with the following:
- Age older than 55 years;
- Isolated tenderness of the patella or fibular head;
- Inability to flex the knee to 90°; or
- Inability to bear weight (limping is allowed) for four steps both immediately following injury and at the ED.
In a prospective validation cohort of 1,096 adult patients, the rule was found to be 100% sensitive for detecting clinically important fractures—defined as any bone fragment at least 5 mm or avulsion if associated with complete disruption as tendons or ligaments—with a potential reduction in radiography use by 28%.25 Though the rule was designed for adult patients, it was validated in 750 children aged 2 to 16 years and found to be 100% sensitive with a 31% potential reduction in radiographs.26
Pittsburgh Knee Rules
The Pittsburgh Knee Rules only apply to patients with blunt trauma or fall to the knee—not twisting injury—and state X-rays are indicated if patients are younger than age 12 years or older than age 50 years, and are unable to bear weight fully on toe pads and heels for four steps (limping is not allowed).
An initial validation of 133 patients demonstrating 100% sensitivity was followed by a larger external validation study.27 That study prospectively applied the Pittsburgh and the Ottawa rules in over 700 patients and found the sensitivity and specificity of the rules to be 99% and 60% for Pittsburgh and 97% and 27% for Ottawa.28
Comment: The ability to apply a rule to “all-comers” is important to EPs. The Ottawa instrument is more broadly applicable and has even been assessed in pediatric populations.
Foot and Ankle Trauma
Ottawa Foot and Ankle Rule
The most well-studied clinical decision rule in this category is the Ottawa Foot and Ankle Rule.29 This rule states that an ankle X-ray is only required if there is pain in the malleolar zone and bony tenderness to palpation at the posterior edge or tip of either the lateral or medial malleolus, or the inability to bear weight both immediately after injury and in the ED. The anatomic landmarks are shown in Figure 3.
- Pain in the midfoot zone and either tenderness at the base of the fifth metatarsal;
- Tenderness over the navicular; or
- Inability to bear weight both immediately after the injury and at the ED.
The prospective validation study included over 1,400 adult patients with blunt trauma or twisting injury within 10 days of presentation. Clinically significant fractures were defined as malleolar or midfoot fractures with bone fragments greater than 3 mm. The rule was found to be 100% sensitive for detecting both ankle and midfoot fractures, and would lead to a reduction of radiographs by 34% for the ankle and 30% for the foot.29 These results have borne out in multiple studies including a recent meta-analysis comparing six different decision instruments where the Ottawa rule was found to be superior.30
Though the initial validation cohort of the Ottawa rule did not include pediatric patients, it has subsequently been applied to this population. The initial validation study in the pediatric population included 670 patients aged 2 to 16 years. In addition to the criteria for clinically significant injuries in the original validation study, Salter Harris type I fractures, though treated with immobilization, were not deemed to be clinically significant injuries in this cohort.
Although the Ottawa rules were determined to be 100% sensitive for detecting significant ankle and midfoot fractures in the pediatric cohort, one of the study sites experienced an increase rather than a decrease in X-rays when this rule was applied.31
Low Risk Ankle Rule
Investigators for the pediatric specific Low Risk Ankle Rule posited that many pediatric patients with mild ankle injury refuse to bear weight on the extremity. Also, the most common fracture among preadolescent patients is a Salter-Harris type I fracture of the distal fibular epiphysis, which the investigators felt was commonly a clinical diagnosis with little to be gained from X-ray.32 These factors would prompt clinicians using the Ottawa rule to order imaging that may not be necessary in pediatric patients.
The Low Risk Ankle Rule states that if a patient has a low-risk examination, which is defined as tenderness and swelling isolated to the distal fibula and/or adjacent lateral ligaments distal to the tibial anterior joint line, then X-rays may not be necessary to exclude a high-risk ankle injury.
High-risk injuries were defined as any injury leading to an unstable ankle, and do not include avulsion, buckle, and nondisplaced Salter-Harris types I and II fractures of the distal fibula. In the validation study of nearly 600 children, the sensitivity for detecting a high risk fracture was 100% and X-rays could have been reduced in 62.8% of children with low-risk examinations compared to only 12% with the Ottawa Ankle Rule.
Comment: Although the Low Risk Ankle Rule was shown to reduce radiographic imaging by almost 63%, it omits many patients who would require splinting or subspecialty follow-up. Even the Salter-Harris type I fractures in the pediatric validation of the Ottawa rule were treated with splinting, though they were not regarded as “significant” injuries. Clinicians applying these rules, especially to a pediatric population, should have a good sense of what type of injuries these rules are designed to detect.
Conclusion
In the midst of a busy shift, clinical decision rules can help save time and expense. However, few of the rules described are meant to be applied to “all-comers,” and practitioners should be careful to not apply these rules to populations that were excluded in the validation cohorts. While clinical decision rules can help identify high-risk features, they are not a substitute for performing a thorough history and physical examination. Further studies should focus on whether these rules truly outperform unaided clinical decision-making.
Part 2 of “Playing by the Rules” will examine the use of clinician decision rules for nontraumatic conditions.
1. Stiell IG, Bennett C. Implementation of clinical decision rules in the emergency department. Acad Emerg Med. 2007;14(11):955-959. doi:10.1197/j.aem.2007.06.039.
2. Green SM. When do clinical decision rules improve patient care? Ann Emerg Med. 2013;62(2):132-135. doi:10.1016/j.annemergmed.2013.02.006.
3. Schriger DL, Elder JW, Cooper RJ. Structured clinical decision aids are seldom compared with subjective physician judgment, and are seldom superior. Ann Emerg Med. 2017;70(3):338-344.e3. doi:10.1016/j.annemergmed.2016.12.004.
4. Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343(2):100-105. doi:10.1056/NEJM200007133430204.
5. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT head rule and the New Orleans criteria in patients with minor head injury. JAMA. 2005;294(12):1511-1518. doi:10.1001/jama.294.12.1511
6. Stiell IG, Perry JJ. Traumatic intracranial injury in intoxicate patients with minor head trauma. Acad Emerg Med. 2014;21(2):221. doi:10.1111/acem.12306.
7. Mower WR, Gupta M, Rodriguez R, Hendey GW. Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) head computed tomographic (CT) decision instrument for selective imaging of blunt head injury patients: An observational study. PLoS Med. 2017;14(7):e1002313. doi:10.1371/journal.pmed.1002313.
8. Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT head rule and the New Orleans criteria for CT scanning in patients with minor head injury. JAMA. 2005;294(12):1519-1525. doi:10.1001/jama.294.12.1519.
9. Stein SC, Fabbri A, Servadei F, Glick HA. A critical comparison of clinical decision instruments for computed tomographic scanning in mild closed traumatic brain injury in adolescents and adults. Ann Emerg Med. 2009;53(2):180-188. doi:10.1016/j.annemergmed.2008.01.002.
10. Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160-1170. doi:10.1016/S0140-6736(09)61558-0.
11. Babi FE, Borland ML, Phillips N, et al. Accuracy of PECARN, CATCH, and CHALICE head injury decision rules in children: a prospective cohort study. Lancet. 2017;389(10087):2393-2402. doi:10.1016/S0140-6736(17)30555-X.
12. Mower WR. Paediatric head imaging decisions are not child’s play. Lancet. 2017;389(10087):2354-2355. doi:10.1016/S0140-6736(17)30932-7.
13. Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343(2):94-99. doi:10.1056/NEJM200007133430203.
14. Viccellio P, Simon H, Pressman BD, Shah NM, Mower WR, Hoffman JR; NEXUS Group. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001;108(2):20.
15. Tran J, Jeanmonod D, Agresti D, Hamden K, Jeanmonod RK. Prospective validation of modified NEXUS cervical spine injury criteria in low-risk elderly fall patients. West J Emerg Med. 2016;17(3):252-257. doi:10.5811/westjem.2016.3.29702.
16. Paykin G, O’Reilly G, Ackland HM, Mitra B. The NEXUS criteria are insufficient to exclude cervical spine fractures in older blunt trauma patients. Injury. 2017;48(5):1020-1024. doi:10.1016/j.injury.2017.02.013.
17. Evans D, Vera L, Jeanmonod D, Pester J, Jeanmonod R. Application of National Emergency X-Ray Utilizations Study low-risk c-spine criteria in high-risk geriatric falls. Am J Emerg Med. 2015;33(9):1184-1187. doi:10.1016/j.ajem.2015.05.031.
18. Stiell IG, Clement CM, McKnight RD, et al. The Canadian C-spine rule versus the NEXUS low-risk criteria in patients with trauma. N Engl J Med. 2003;349(26):2510-2518.
19. Michaleff ZA, Maher CG, Verhagen AP, Rebbeck T, Lin CW. Accuracy of the Canadian C-spine rule and NEXUS to screen for clinically important cervical spine injury in patients following blunt trauma: A systematic review. CMAJ. 2012;184(16):E867-E876. doi:10.1503/cmaj.120675.
20. Gale SC, Gracias VH, Reilly PM, Schwab CW. The inefficiency of plain radiography to evaluate the cervical spine after blunt trauma. J Trauma. 2005;59(5):1121-1125.
21. Rodriguez RM, Anglin D, Langdorf MI, et al. NEXUS chest: validation of a decision instrument for selective chest imaging in blunt trauma. JAMA Surg. 2013;148(10):940-946. doi:10.1001/jamasurg.2013.2757.
22. Rodriguez RM, Baumann BM, Raja AS, et al. Diagnostic yields, charges, and radiation dose of chest imaging in blunt trauma evaluations. Acad Emerg Med. 2014;21(6):644-650. doi:10.1111/acem.12396.
23. Rodriguez RM, Langdorf MI, Nishijima D, et al. Derivation and validation of two decision instruments for selective chest CT in blunt trauma: a multicenter prospective observational study (NEXUS Chest CT). PLoS Med. 2015;12(10):e1001883. doi:10.1371/journal.pmed.1001883.
24. Rodriguez RM, Hendey GW, Mower WR, et al. Selective chest imaging for blunt trauma patients: The national emergency X-ray utilization studies (NEXUS-chest algorithm). Am J Emerg Med. 2017;35(1):164-170. doi:10.1016/j.ajem.2016.10.066.
25. Stiell IG, Greenberg GH, Wells GA, et al. Prospective validation of a decision rule for the use of radiography in acute knee injuries. JAMA. 1996;275(8):611-615.
26. Bulloch B, Neto G, Plint A, et al. Validation of the Ottawa Knee Rule in children: a multicenter study. Ann Emerg Med. 2003;42(1):48-55. doi:10.1067/mem.2003.196.
27. Seaberg DC, Jackson R. Clinical decision rule for knee radiographs. Am J Emerg Med. 1994;12(5):541-543.
28. Seaberg DC, Yealy DM, Lukens T, Auble T, Mathias S. Multicenter comparison of two clinical decision rules for the use of radiography in acute, high-risk knee injuries. Ann Emerg Med. 1998;32(1):8-13.
29. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269(9):1127-1132.
30. Barelds I, Krijnen WP, van de Leur JP, van der Schans CP, Goddard RJ. Diagnostic accuracy of clinical decision rules to exclude fractures in acute ankle injuries: Systematic review and meta-analysis. J Emerg Med. 2017;53(3):353-368. doi:10.1016/j.jemermed.2017.04.035.
31. Plint AC, Bulloch B, Osmond MH, et al. Validation of the Ottawa Ankle Rules in children with ankle injuries. Acad Emerg Med. 1999;6(10):1005-1009.
32. Boutis K, Komar L, Jaramillo D, et al. Sensitivity of a clinical examination to predict need for radiography in children with ankle injuries: a prospective study. Lancet. 2001;358(9299):2118-2121. doi:10.1016/S0140-6736(01)07218-X.
1. Stiell IG, Bennett C. Implementation of clinical decision rules in the emergency department. Acad Emerg Med. 2007;14(11):955-959. doi:10.1197/j.aem.2007.06.039.
2. Green SM. When do clinical decision rules improve patient care? Ann Emerg Med. 2013;62(2):132-135. doi:10.1016/j.annemergmed.2013.02.006.
3. Schriger DL, Elder JW, Cooper RJ. Structured clinical decision aids are seldom compared with subjective physician judgment, and are seldom superior. Ann Emerg Med. 2017;70(3):338-344.e3. doi:10.1016/j.annemergmed.2016.12.004.
4. Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343(2):100-105. doi:10.1056/NEJM200007133430204.
5. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT head rule and the New Orleans criteria in patients with minor head injury. JAMA. 2005;294(12):1511-1518. doi:10.1001/jama.294.12.1511
6. Stiell IG, Perry JJ. Traumatic intracranial injury in intoxicate patients with minor head trauma. Acad Emerg Med. 2014;21(2):221. doi:10.1111/acem.12306.
7. Mower WR, Gupta M, Rodriguez R, Hendey GW. Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) head computed tomographic (CT) decision instrument for selective imaging of blunt head injury patients: An observational study. PLoS Med. 2017;14(7):e1002313. doi:10.1371/journal.pmed.1002313.
8. Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT head rule and the New Orleans criteria for CT scanning in patients with minor head injury. JAMA. 2005;294(12):1519-1525. doi:10.1001/jama.294.12.1519.
9. Stein SC, Fabbri A, Servadei F, Glick HA. A critical comparison of clinical decision instruments for computed tomographic scanning in mild closed traumatic brain injury in adolescents and adults. Ann Emerg Med. 2009;53(2):180-188. doi:10.1016/j.annemergmed.2008.01.002.
10. Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160-1170. doi:10.1016/S0140-6736(09)61558-0.
11. Babi FE, Borland ML, Phillips N, et al. Accuracy of PECARN, CATCH, and CHALICE head injury decision rules in children: a prospective cohort study. Lancet. 2017;389(10087):2393-2402. doi:10.1016/S0140-6736(17)30555-X.
12. Mower WR. Paediatric head imaging decisions are not child’s play. Lancet. 2017;389(10087):2354-2355. doi:10.1016/S0140-6736(17)30932-7.
13. Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343(2):94-99. doi:10.1056/NEJM200007133430203.
14. Viccellio P, Simon H, Pressman BD, Shah NM, Mower WR, Hoffman JR; NEXUS Group. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001;108(2):20.
15. Tran J, Jeanmonod D, Agresti D, Hamden K, Jeanmonod RK. Prospective validation of modified NEXUS cervical spine injury criteria in low-risk elderly fall patients. West J Emerg Med. 2016;17(3):252-257. doi:10.5811/westjem.2016.3.29702.
16. Paykin G, O’Reilly G, Ackland HM, Mitra B. The NEXUS criteria are insufficient to exclude cervical spine fractures in older blunt trauma patients. Injury. 2017;48(5):1020-1024. doi:10.1016/j.injury.2017.02.013.
17. Evans D, Vera L, Jeanmonod D, Pester J, Jeanmonod R. Application of National Emergency X-Ray Utilizations Study low-risk c-spine criteria in high-risk geriatric falls. Am J Emerg Med. 2015;33(9):1184-1187. doi:10.1016/j.ajem.2015.05.031.
18. Stiell IG, Clement CM, McKnight RD, et al. The Canadian C-spine rule versus the NEXUS low-risk criteria in patients with trauma. N Engl J Med. 2003;349(26):2510-2518.
19. Michaleff ZA, Maher CG, Verhagen AP, Rebbeck T, Lin CW. Accuracy of the Canadian C-spine rule and NEXUS to screen for clinically important cervical spine injury in patients following blunt trauma: A systematic review. CMAJ. 2012;184(16):E867-E876. doi:10.1503/cmaj.120675.
20. Gale SC, Gracias VH, Reilly PM, Schwab CW. The inefficiency of plain radiography to evaluate the cervical spine after blunt trauma. J Trauma. 2005;59(5):1121-1125.
21. Rodriguez RM, Anglin D, Langdorf MI, et al. NEXUS chest: validation of a decision instrument for selective chest imaging in blunt trauma. JAMA Surg. 2013;148(10):940-946. doi:10.1001/jamasurg.2013.2757.
22. Rodriguez RM, Baumann BM, Raja AS, et al. Diagnostic yields, charges, and radiation dose of chest imaging in blunt trauma evaluations. Acad Emerg Med. 2014;21(6):644-650. doi:10.1111/acem.12396.
23. Rodriguez RM, Langdorf MI, Nishijima D, et al. Derivation and validation of two decision instruments for selective chest CT in blunt trauma: a multicenter prospective observational study (NEXUS Chest CT). PLoS Med. 2015;12(10):e1001883. doi:10.1371/journal.pmed.1001883.
24. Rodriguez RM, Hendey GW, Mower WR, et al. Selective chest imaging for blunt trauma patients: The national emergency X-ray utilization studies (NEXUS-chest algorithm). Am J Emerg Med. 2017;35(1):164-170. doi:10.1016/j.ajem.2016.10.066.
25. Stiell IG, Greenberg GH, Wells GA, et al. Prospective validation of a decision rule for the use of radiography in acute knee injuries. JAMA. 1996;275(8):611-615.
26. Bulloch B, Neto G, Plint A, et al. Validation of the Ottawa Knee Rule in children: a multicenter study. Ann Emerg Med. 2003;42(1):48-55. doi:10.1067/mem.2003.196.
27. Seaberg DC, Jackson R. Clinical decision rule for knee radiographs. Am J Emerg Med. 1994;12(5):541-543.
28. Seaberg DC, Yealy DM, Lukens T, Auble T, Mathias S. Multicenter comparison of two clinical decision rules for the use of radiography in acute, high-risk knee injuries. Ann Emerg Med. 1998;32(1):8-13.
29. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269(9):1127-1132.
30. Barelds I, Krijnen WP, van de Leur JP, van der Schans CP, Goddard RJ. Diagnostic accuracy of clinical decision rules to exclude fractures in acute ankle injuries: Systematic review and meta-analysis. J Emerg Med. 2017;53(3):353-368. doi:10.1016/j.jemermed.2017.04.035.
31. Plint AC, Bulloch B, Osmond MH, et al. Validation of the Ottawa Ankle Rules in children with ankle injuries. Acad Emerg Med. 1999;6(10):1005-1009.
32. Boutis K, Komar L, Jaramillo D, et al. Sensitivity of a clinical examination to predict need for radiography in children with ankle injuries: a prospective study. Lancet. 2001;358(9299):2118-2121. doi:10.1016/S0140-6736(01)07218-X.
Inpatient antiviral treatment reduces ICU admissions among influenza patients
SAN DIEGO – Administering inpatient antiviral influenza treatment may reduce admissions to the ICU among adults hospitalized with flu, according to a study presented at ID Week 2017, an infectious diseases meeting.
While interventions did not directly affect flu-related deaths, lower ICU admission rates could reduce morbidity as well as ease the financial burden felt during the influenza season.
Investigators retrospectively studied 4,679 influenza patients admitted to Canadian Immunization Research Network Serious Outcomes Surveillance (SOS) Network hospitals during 2011-2014. Of the 54% of patients given inpatient antiviral treatment, the risk of being admitted to the ICU was reduced by 90% (odds ratio, 0.10;95% confidence interval, 0.08-0.13; P less than .001).
Antiviral treatment was not protective against death outcomes in patients with either influenza A or influenza B (OR, 0.9; 95% CI, 0.7-1.2; P =.454).
The median age of patients was 70 years, with a majority older than 75 years(41%); the majority presented with one or more comorbidities (89%), and had influenza A (72%).
Researchers found that, of the 4,679 patients studied, 798 (16%) were admitted to the ICU, 511 (11%) required mechanical ventilation, and the average length of hospital stay was 11 days.
Of those studied, 444 (9%) died within 30 days of discharge.
Researchers also found that only 38% of those studied had received the current seasonal vaccine upon admittance. However, these numbers may be skewed from the general population, because patients who have not taken the vaccine are more likely to be hospitalized.
Along with the results of antivirals on hospitalized patients, researchers wanted to uncover how the effectiveness of inpatient vaccine administration would vary based on treatment timing, said presenter Zach Shaffelburg of the Canadian Center for Vaccinology, Dalhousie University, Halifax, NS.
Even when administered 4.28 days after symptom onset, antiviral treatments in patients proved to be associated with significant reductions in ICU admissions and the need for mechanical ventilation.
The investigators concluded that antivirals show a strong association with positive effects on serious, influenza-related outcomes in hospitalized patients and, while therapy remained effective with later treatment start, patients would benefit the most from initiation as soon as possible.
Currently, the U.S. Centers for Disease Control and Prevention and the Canadian Immunization Research Network (CIRN) have guidelines instructing best practice for inpatient antiviral treatment, however the number of hospitalized patients given treatment has declined in Canada since 2009, according to Mr. Shaffelburg.
The reason more patients were not receiving inpatient antiviral treatment may be related to studies of different populations that failed to show significant impact, Mr. Shaffelburg suggested during a question and answer session following the presentation: “I think a lot of that comes from outpatient studies that involve patients who are younger and quite healthy [who received] antivirals, and it showed a very minimal impact,” Mr. Shaffelburg said. “So a lot of people saw that study and thought, ‘What’s that point of giving it if it’s not going to make an impact?’ ”
Mr. Shaffelburg and his colleagues are planning to continue their study of inpatient antiviral treatment, focusing more on the effectiveness of treatment in relation to time administered after onset.
Mr. Shaffelburg reported having no disclosures. The study was funded by the CIRN SOS network, Canadian Institutes for Health Research, and a partnership with GlaxoSmithKline Biologicals. Some of the investigators were GSK employees or received grant funding from the company.
SOURCE: Shaffelburg Z et al. IDWeek 2017 Abstract 890.
SAN DIEGO – Administering inpatient antiviral influenza treatment may reduce admissions to the ICU among adults hospitalized with flu, according to a study presented at ID Week 2017, an infectious diseases meeting.
While interventions did not directly affect flu-related deaths, lower ICU admission rates could reduce morbidity as well as ease the financial burden felt during the influenza season.
Investigators retrospectively studied 4,679 influenza patients admitted to Canadian Immunization Research Network Serious Outcomes Surveillance (SOS) Network hospitals during 2011-2014. Of the 54% of patients given inpatient antiviral treatment, the risk of being admitted to the ICU was reduced by 90% (odds ratio, 0.10;95% confidence interval, 0.08-0.13; P less than .001).
Antiviral treatment was not protective against death outcomes in patients with either influenza A or influenza B (OR, 0.9; 95% CI, 0.7-1.2; P =.454).
The median age of patients was 70 years, with a majority older than 75 years(41%); the majority presented with one or more comorbidities (89%), and had influenza A (72%).
Researchers found that, of the 4,679 patients studied, 798 (16%) were admitted to the ICU, 511 (11%) required mechanical ventilation, and the average length of hospital stay was 11 days.
Of those studied, 444 (9%) died within 30 days of discharge.
Researchers also found that only 38% of those studied had received the current seasonal vaccine upon admittance. However, these numbers may be skewed from the general population, because patients who have not taken the vaccine are more likely to be hospitalized.
Along with the results of antivirals on hospitalized patients, researchers wanted to uncover how the effectiveness of inpatient vaccine administration would vary based on treatment timing, said presenter Zach Shaffelburg of the Canadian Center for Vaccinology, Dalhousie University, Halifax, NS.
Even when administered 4.28 days after symptom onset, antiviral treatments in patients proved to be associated with significant reductions in ICU admissions and the need for mechanical ventilation.
The investigators concluded that antivirals show a strong association with positive effects on serious, influenza-related outcomes in hospitalized patients and, while therapy remained effective with later treatment start, patients would benefit the most from initiation as soon as possible.
Currently, the U.S. Centers for Disease Control and Prevention and the Canadian Immunization Research Network (CIRN) have guidelines instructing best practice for inpatient antiviral treatment, however the number of hospitalized patients given treatment has declined in Canada since 2009, according to Mr. Shaffelburg.
The reason more patients were not receiving inpatient antiviral treatment may be related to studies of different populations that failed to show significant impact, Mr. Shaffelburg suggested during a question and answer session following the presentation: “I think a lot of that comes from outpatient studies that involve patients who are younger and quite healthy [who received] antivirals, and it showed a very minimal impact,” Mr. Shaffelburg said. “So a lot of people saw that study and thought, ‘What’s that point of giving it if it’s not going to make an impact?’ ”
Mr. Shaffelburg and his colleagues are planning to continue their study of inpatient antiviral treatment, focusing more on the effectiveness of treatment in relation to time administered after onset.
Mr. Shaffelburg reported having no disclosures. The study was funded by the CIRN SOS network, Canadian Institutes for Health Research, and a partnership with GlaxoSmithKline Biologicals. Some of the investigators were GSK employees or received grant funding from the company.
SOURCE: Shaffelburg Z et al. IDWeek 2017 Abstract 890.
SAN DIEGO – Administering inpatient antiviral influenza treatment may reduce admissions to the ICU among adults hospitalized with flu, according to a study presented at ID Week 2017, an infectious diseases meeting.
While interventions did not directly affect flu-related deaths, lower ICU admission rates could reduce morbidity as well as ease the financial burden felt during the influenza season.
Investigators retrospectively studied 4,679 influenza patients admitted to Canadian Immunization Research Network Serious Outcomes Surveillance (SOS) Network hospitals during 2011-2014. Of the 54% of patients given inpatient antiviral treatment, the risk of being admitted to the ICU was reduced by 90% (odds ratio, 0.10;95% confidence interval, 0.08-0.13; P less than .001).
Antiviral treatment was not protective against death outcomes in patients with either influenza A or influenza B (OR, 0.9; 95% CI, 0.7-1.2; P =.454).
The median age of patients was 70 years, with a majority older than 75 years(41%); the majority presented with one or more comorbidities (89%), and had influenza A (72%).
Researchers found that, of the 4,679 patients studied, 798 (16%) were admitted to the ICU, 511 (11%) required mechanical ventilation, and the average length of hospital stay was 11 days.
Of those studied, 444 (9%) died within 30 days of discharge.
Researchers also found that only 38% of those studied had received the current seasonal vaccine upon admittance. However, these numbers may be skewed from the general population, because patients who have not taken the vaccine are more likely to be hospitalized.
Along with the results of antivirals on hospitalized patients, researchers wanted to uncover how the effectiveness of inpatient vaccine administration would vary based on treatment timing, said presenter Zach Shaffelburg of the Canadian Center for Vaccinology, Dalhousie University, Halifax, NS.
Even when administered 4.28 days after symptom onset, antiviral treatments in patients proved to be associated with significant reductions in ICU admissions and the need for mechanical ventilation.
The investigators concluded that antivirals show a strong association with positive effects on serious, influenza-related outcomes in hospitalized patients and, while therapy remained effective with later treatment start, patients would benefit the most from initiation as soon as possible.
Currently, the U.S. Centers for Disease Control and Prevention and the Canadian Immunization Research Network (CIRN) have guidelines instructing best practice for inpatient antiviral treatment, however the number of hospitalized patients given treatment has declined in Canada since 2009, according to Mr. Shaffelburg.
The reason more patients were not receiving inpatient antiviral treatment may be related to studies of different populations that failed to show significant impact, Mr. Shaffelburg suggested during a question and answer session following the presentation: “I think a lot of that comes from outpatient studies that involve patients who are younger and quite healthy [who received] antivirals, and it showed a very minimal impact,” Mr. Shaffelburg said. “So a lot of people saw that study and thought, ‘What’s that point of giving it if it’s not going to make an impact?’ ”
Mr. Shaffelburg and his colleagues are planning to continue their study of inpatient antiviral treatment, focusing more on the effectiveness of treatment in relation to time administered after onset.
Mr. Shaffelburg reported having no disclosures. The study was funded by the CIRN SOS network, Canadian Institutes for Health Research, and a partnership with GlaxoSmithKline Biologicals. Some of the investigators were GSK employees or received grant funding from the company.
SOURCE: Shaffelburg Z et al. IDWeek 2017 Abstract 890.
REPORTING FROM ID WEEK 2017
Key clinical point:
Major finding: Patients who received antiviral treatment were significantly less likely to go to the ICU or need mechanical ventilation (OR, 0.10; 95% CI, 0.08-0.13; P less than .001).
Study details: Study of 4,679 hospitalized influenza patients admitted to the Canadian Immunization Research Network Serious Outcomes Surveillance (CIRN SOS) network hospitals between 2011 to 2014.
Disclosures: Mr. Shaffelburg reported having no disclosures. The study was funded by the CIRN SOS network, Canadian Institutes for Health Research, and a partnership with GlaxoSmithKline Biologicals. Some of the investigators were GSK employees or received grant funding from the company.
Source: Shaffelburg Z et al. IDWeek 2017 Abstract 890.
FDA approves infliximab biosimilar Ixifi for all of Remicade’s indications
The Food and Drug Administration has approved Ixifi (infliximab-qbtx), a biosimilar of Remicade, the original infliximab product. Ixifi is the third infliximab biosimilar to be approved by the FDA, and it is approved for all the same indications as Remicade, according to an announcement from its manufacturer, Pfizer.
Ixifi and Remicade are approved for the treatment of rheumatoid arthritis in combination with methotrexate, Crohn’s disease, pediatric Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis.
The most common adverse events associated with Ixifi are upper respiratory infections, sinusitis, pharyngitis, infusion-related reactions, headache, and abdominal pain.
The Food and Drug Administration has approved Ixifi (infliximab-qbtx), a biosimilar of Remicade, the original infliximab product. Ixifi is the third infliximab biosimilar to be approved by the FDA, and it is approved for all the same indications as Remicade, according to an announcement from its manufacturer, Pfizer.
Ixifi and Remicade are approved for the treatment of rheumatoid arthritis in combination with methotrexate, Crohn’s disease, pediatric Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis.
The most common adverse events associated with Ixifi are upper respiratory infections, sinusitis, pharyngitis, infusion-related reactions, headache, and abdominal pain.
The Food and Drug Administration has approved Ixifi (infliximab-qbtx), a biosimilar of Remicade, the original infliximab product. Ixifi is the third infliximab biosimilar to be approved by the FDA, and it is approved for all the same indications as Remicade, according to an announcement from its manufacturer, Pfizer.
Ixifi and Remicade are approved for the treatment of rheumatoid arthritis in combination with methotrexate, Crohn’s disease, pediatric Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis.
The most common adverse events associated with Ixifi are upper respiratory infections, sinusitis, pharyngitis, infusion-related reactions, headache, and abdominal pain.
Memorial and honorary gifts: a special tribute
Make a tribute gift to honor someone whose life has been touched by GI research or celebrate a special occasion such as a birthday while supporting the AGA Research Awards Program through the AGA Research Foundation. A tribute gift will make your loved one feel special because it honors their passion, and also provides us with needed support in furthering basic digestive disease research.
- Giving a gift to the AGA Research Foundation in memory of a loved one. A memorial gift is a meaningful way to celebrate the legacy of a family member, friend, or colleague.
- Telling your friends and family members to donate to the AGA Research Foundation in YOUR honor.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.gastro.org/contribute or contact Harmony Excellent at 301-272-1602 or [email protected].
Make a tribute gift to honor someone whose life has been touched by GI research or celebrate a special occasion such as a birthday while supporting the AGA Research Awards Program through the AGA Research Foundation. A tribute gift will make your loved one feel special because it honors their passion, and also provides us with needed support in furthering basic digestive disease research.
- Giving a gift to the AGA Research Foundation in memory of a loved one. A memorial gift is a meaningful way to celebrate the legacy of a family member, friend, or colleague.
- Telling your friends and family members to donate to the AGA Research Foundation in YOUR honor.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.gastro.org/contribute or contact Harmony Excellent at 301-272-1602 or [email protected].
Make a tribute gift to honor someone whose life has been touched by GI research or celebrate a special occasion such as a birthday while supporting the AGA Research Awards Program through the AGA Research Foundation. A tribute gift will make your loved one feel special because it honors their passion, and also provides us with needed support in furthering basic digestive disease research.
- Giving a gift to the AGA Research Foundation in memory of a loved one. A memorial gift is a meaningful way to celebrate the legacy of a family member, friend, or colleague.
- Telling your friends and family members to donate to the AGA Research Foundation in YOUR honor.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.gastro.org/contribute or contact Harmony Excellent at 301-272-1602 or [email protected].
The New Gastroenterologist goes digital
Beginning in February 2018, The New Gastroenterologist (TNG) – a supplement to GI & Hepatology News that addresses issues pertinent to trainees and early-career GIs – will switch to a primarily digital format. We are excited about this change and confident that it will allow for a more effective and widespread dissemination of content that is valuable to both AGA members and our readership more broadly.
If you have any questions about these changes, or if there are any topics you’d be interested in writing or reading about in The New Gastroenterologist, please contact Editor in Chief Bryson Katona, MD, PhD ([email protected]) or Managing Editor Ryan Farrell ([email protected]).
Beginning in February 2018, The New Gastroenterologist (TNG) – a supplement to GI & Hepatology News that addresses issues pertinent to trainees and early-career GIs – will switch to a primarily digital format. We are excited about this change and confident that it will allow for a more effective and widespread dissemination of content that is valuable to both AGA members and our readership more broadly.
If you have any questions about these changes, or if there are any topics you’d be interested in writing or reading about in The New Gastroenterologist, please contact Editor in Chief Bryson Katona, MD, PhD ([email protected]) or Managing Editor Ryan Farrell ([email protected]).
Beginning in February 2018, The New Gastroenterologist (TNG) – a supplement to GI & Hepatology News that addresses issues pertinent to trainees and early-career GIs – will switch to a primarily digital format. We are excited about this change and confident that it will allow for a more effective and widespread dissemination of content that is valuable to both AGA members and our readership more broadly.
If you have any questions about these changes, or if there are any topics you’d be interested in writing or reading about in The New Gastroenterologist, please contact Editor in Chief Bryson Katona, MD, PhD ([email protected]) or Managing Editor Ryan Farrell ([email protected]).
Idarucizumab reverses anticoagulation effects of dabigatran
Clinical question: Can idarucizumab reverse anticoagulation effects of dabigatran in a timely manner for urgent surgery or in the event of bleeding?
Background: Reversing the anticoagulant properties of anticoagulants can be important in the event of a life-threatening bleed, or if patients taking these medications need urgent surgery or other interventions. Idarucizumab, a humanized monoclonal antibody fragment, can reverse anticoagulant activity of dabigatran, increasing its acceptance for prescribing physicians as well as patients.
Study design: Multicenter prospective single cohort study.
Setting: 173 sites, 39 countries.
Synopsis: Among 503 patients (median age, 78 years, indication for dabigatran included stroke prophylaxis in setting of atrial fibrillation for most) who had either uncontrolled bleeding (n = 301) or needing emergent surgery (n = 202), a single 5-g dose of idarucizumab was able to reverse anticoagulation rapidly and completely in more than 98% of these patients independent of age, sex, renal function, and dabigatran concentration at baseline. Specifically in 68% of the patients in the bleeding group (excluding intracranial hemorrhage) median time to the cessation of bleeding was 2.5 hours and median time to the initiation of the procedure in the emergent surgery group was 1.6 hours. Study limited by lack of control group.
Bottom line: Idarucizumab can be effective for dabigatran reversal among patients who have uncontrolled bleeding or need to undergo urgent surgery.
Citation: Pollack CV Jr. et al. Idarucizumab for dabigatran reversal: Full cohort analysis. N Engl J Med. 2017 Aug 3;377(5):431-41.
Dr. Kamath is a hospitalist and medical director of quality and patient safety, Duke Regional Hospital, Duke University Health System.
Clinical question: Can idarucizumab reverse anticoagulation effects of dabigatran in a timely manner for urgent surgery or in the event of bleeding?
Background: Reversing the anticoagulant properties of anticoagulants can be important in the event of a life-threatening bleed, or if patients taking these medications need urgent surgery or other interventions. Idarucizumab, a humanized monoclonal antibody fragment, can reverse anticoagulant activity of dabigatran, increasing its acceptance for prescribing physicians as well as patients.
Study design: Multicenter prospective single cohort study.
Setting: 173 sites, 39 countries.
Synopsis: Among 503 patients (median age, 78 years, indication for dabigatran included stroke prophylaxis in setting of atrial fibrillation for most) who had either uncontrolled bleeding (n = 301) or needing emergent surgery (n = 202), a single 5-g dose of idarucizumab was able to reverse anticoagulation rapidly and completely in more than 98% of these patients independent of age, sex, renal function, and dabigatran concentration at baseline. Specifically in 68% of the patients in the bleeding group (excluding intracranial hemorrhage) median time to the cessation of bleeding was 2.5 hours and median time to the initiation of the procedure in the emergent surgery group was 1.6 hours. Study limited by lack of control group.
Bottom line: Idarucizumab can be effective for dabigatran reversal among patients who have uncontrolled bleeding or need to undergo urgent surgery.
Citation: Pollack CV Jr. et al. Idarucizumab for dabigatran reversal: Full cohort analysis. N Engl J Med. 2017 Aug 3;377(5):431-41.
Dr. Kamath is a hospitalist and medical director of quality and patient safety, Duke Regional Hospital, Duke University Health System.
Clinical question: Can idarucizumab reverse anticoagulation effects of dabigatran in a timely manner for urgent surgery or in the event of bleeding?
Background: Reversing the anticoagulant properties of anticoagulants can be important in the event of a life-threatening bleed, or if patients taking these medications need urgent surgery or other interventions. Idarucizumab, a humanized monoclonal antibody fragment, can reverse anticoagulant activity of dabigatran, increasing its acceptance for prescribing physicians as well as patients.
Study design: Multicenter prospective single cohort study.
Setting: 173 sites, 39 countries.
Synopsis: Among 503 patients (median age, 78 years, indication for dabigatran included stroke prophylaxis in setting of atrial fibrillation for most) who had either uncontrolled bleeding (n = 301) or needing emergent surgery (n = 202), a single 5-g dose of idarucizumab was able to reverse anticoagulation rapidly and completely in more than 98% of these patients independent of age, sex, renal function, and dabigatran concentration at baseline. Specifically in 68% of the patients in the bleeding group (excluding intracranial hemorrhage) median time to the cessation of bleeding was 2.5 hours and median time to the initiation of the procedure in the emergent surgery group was 1.6 hours. Study limited by lack of control group.
Bottom line: Idarucizumab can be effective for dabigatran reversal among patients who have uncontrolled bleeding or need to undergo urgent surgery.
Citation: Pollack CV Jr. et al. Idarucizumab for dabigatran reversal: Full cohort analysis. N Engl J Med. 2017 Aug 3;377(5):431-41.
Dr. Kamath is a hospitalist and medical director of quality and patient safety, Duke Regional Hospital, Duke University Health System.
More evidence links high-potency marijuana use to first-episode psychosis
SAN DIEGO – High-potency marijuana use appears to be associated with an increased risk of a first psychotic episode, based on a case-control study conducted in Europe.
“Daily users of a strong type of cannabis face a significant increase in the probability of developing a psychotic disorder,” reported Marta Di Forti, MD, PhD, MRC, lead author of a study whose preliminary results were presented at the International Congress on Schizophrenia Research.
Dr. Di Forti spawned a media boomlet in 2015 when she and her colleagues raised the prospect of a possible association between so-called “skunk” marijuana and first psychotic episodes. In their study of subjects in London with first-time psychotic episodes and matched population controls, those who had psychotic episodes were three times (adjusted odds ratio: 2.92; 95% confidence interval, 1.52-3.45; P = .001) as likely as controls to have used “skunk” marijuana (Lancet Psychiatry. 2015 Mar;2[3]:233-8).
In the new study, Dr. Di Forti and her colleagues analyzed 1,200 first-incident cases of psychosis that were captured between the years 2010 and 2013 by the European Network of National Schizophrenia Networks Studying Gene-Environment Interactions project (EU-GEI). The researchers compared the cases to 1,300 population-based controls in five unidentified European countries and found that daily users of high-potency marijuana had the highest adjusted odds ratio (4.5-8, statistical significance not available) of a psychotic episode (Schizophr Bull. 2017 Mar:43:S30. doi: 10.1093/schbul/sbx021.078). “This effect is significant even after controlling for other drugs of abuse such as stimulants, tobacco and alcohol, and main sociodemographic confounders,” the researchers wrote in their abstract.
“The biology of cannabis-associated psychosis is still unclear,” Dr. Di Forti said in an interview. “Nevertheless, we know that THC (tetrahydrocannabinol) binds with two receptors called CB1 and CB2. They’re part of the endocannabinoid system, which from uterus onward protects our central nervous systems from insults. It activates on demand if the brain goes on hypoxia or we experience a brain injury.”
“CB1 activation leads to changes in the transmission of both GABA and glutamate. Downstream, they affect the dopamine system, which is biologically linked to psychosis.”
Dr. Di Forti dismissed the idea that people at risk for psychosis are drawn to high-potency marijuana. “Using genetic data, we’ve showed that cannabis users – both cases and controls – did not have a higher genetic load for schizophrenia than those who never used (marijuana),” she said (Lancet Psychiatry. 2015 May;2[5]:381-2).
The findings point to the importance of asking patients – and students and children – about more than just whether they have ever used marijuana. History-taking for marijuana use needs to be comparable to that performed for alcohol use, she said. “I always ask my patients for details about their past and present use but also try to understand why they use (marijuana). When possible, once I know how frequently and what type (of marijuana) they use, I can negotiate some harm-reduction strategy.”
The study is funded by the U.K.’s Medical Research Council and a European Union grant. Dr. Di Forti reports no relevant disclosures.
SAN DIEGO – High-potency marijuana use appears to be associated with an increased risk of a first psychotic episode, based on a case-control study conducted in Europe.
“Daily users of a strong type of cannabis face a significant increase in the probability of developing a psychotic disorder,” reported Marta Di Forti, MD, PhD, MRC, lead author of a study whose preliminary results were presented at the International Congress on Schizophrenia Research.
Dr. Di Forti spawned a media boomlet in 2015 when she and her colleagues raised the prospect of a possible association between so-called “skunk” marijuana and first psychotic episodes. In their study of subjects in London with first-time psychotic episodes and matched population controls, those who had psychotic episodes were three times (adjusted odds ratio: 2.92; 95% confidence interval, 1.52-3.45; P = .001) as likely as controls to have used “skunk” marijuana (Lancet Psychiatry. 2015 Mar;2[3]:233-8).
In the new study, Dr. Di Forti and her colleagues analyzed 1,200 first-incident cases of psychosis that were captured between the years 2010 and 2013 by the European Network of National Schizophrenia Networks Studying Gene-Environment Interactions project (EU-GEI). The researchers compared the cases to 1,300 population-based controls in five unidentified European countries and found that daily users of high-potency marijuana had the highest adjusted odds ratio (4.5-8, statistical significance not available) of a psychotic episode (Schizophr Bull. 2017 Mar:43:S30. doi: 10.1093/schbul/sbx021.078). “This effect is significant even after controlling for other drugs of abuse such as stimulants, tobacco and alcohol, and main sociodemographic confounders,” the researchers wrote in their abstract.
“The biology of cannabis-associated psychosis is still unclear,” Dr. Di Forti said in an interview. “Nevertheless, we know that THC (tetrahydrocannabinol) binds with two receptors called CB1 and CB2. They’re part of the endocannabinoid system, which from uterus onward protects our central nervous systems from insults. It activates on demand if the brain goes on hypoxia or we experience a brain injury.”
“CB1 activation leads to changes in the transmission of both GABA and glutamate. Downstream, they affect the dopamine system, which is biologically linked to psychosis.”
Dr. Di Forti dismissed the idea that people at risk for psychosis are drawn to high-potency marijuana. “Using genetic data, we’ve showed that cannabis users – both cases and controls – did not have a higher genetic load for schizophrenia than those who never used (marijuana),” she said (Lancet Psychiatry. 2015 May;2[5]:381-2).
The findings point to the importance of asking patients – and students and children – about more than just whether they have ever used marijuana. History-taking for marijuana use needs to be comparable to that performed for alcohol use, she said. “I always ask my patients for details about their past and present use but also try to understand why they use (marijuana). When possible, once I know how frequently and what type (of marijuana) they use, I can negotiate some harm-reduction strategy.”
The study is funded by the U.K.’s Medical Research Council and a European Union grant. Dr. Di Forti reports no relevant disclosures.
SAN DIEGO – High-potency marijuana use appears to be associated with an increased risk of a first psychotic episode, based on a case-control study conducted in Europe.
“Daily users of a strong type of cannabis face a significant increase in the probability of developing a psychotic disorder,” reported Marta Di Forti, MD, PhD, MRC, lead author of a study whose preliminary results were presented at the International Congress on Schizophrenia Research.
Dr. Di Forti spawned a media boomlet in 2015 when she and her colleagues raised the prospect of a possible association between so-called “skunk” marijuana and first psychotic episodes. In their study of subjects in London with first-time psychotic episodes and matched population controls, those who had psychotic episodes were three times (adjusted odds ratio: 2.92; 95% confidence interval, 1.52-3.45; P = .001) as likely as controls to have used “skunk” marijuana (Lancet Psychiatry. 2015 Mar;2[3]:233-8).
In the new study, Dr. Di Forti and her colleagues analyzed 1,200 first-incident cases of psychosis that were captured between the years 2010 and 2013 by the European Network of National Schizophrenia Networks Studying Gene-Environment Interactions project (EU-GEI). The researchers compared the cases to 1,300 population-based controls in five unidentified European countries and found that daily users of high-potency marijuana had the highest adjusted odds ratio (4.5-8, statistical significance not available) of a psychotic episode (Schizophr Bull. 2017 Mar:43:S30. doi: 10.1093/schbul/sbx021.078). “This effect is significant even after controlling for other drugs of abuse such as stimulants, tobacco and alcohol, and main sociodemographic confounders,” the researchers wrote in their abstract.
“The biology of cannabis-associated psychosis is still unclear,” Dr. Di Forti said in an interview. “Nevertheless, we know that THC (tetrahydrocannabinol) binds with two receptors called CB1 and CB2. They’re part of the endocannabinoid system, which from uterus onward protects our central nervous systems from insults. It activates on demand if the brain goes on hypoxia or we experience a brain injury.”
“CB1 activation leads to changes in the transmission of both GABA and glutamate. Downstream, they affect the dopamine system, which is biologically linked to psychosis.”
Dr. Di Forti dismissed the idea that people at risk for psychosis are drawn to high-potency marijuana. “Using genetic data, we’ve showed that cannabis users – both cases and controls – did not have a higher genetic load for schizophrenia than those who never used (marijuana),” she said (Lancet Psychiatry. 2015 May;2[5]:381-2).
The findings point to the importance of asking patients – and students and children – about more than just whether they have ever used marijuana. History-taking for marijuana use needs to be comparable to that performed for alcohol use, she said. “I always ask my patients for details about their past and present use but also try to understand why they use (marijuana). When possible, once I know how frequently and what type (of marijuana) they use, I can negotiate some harm-reduction strategy.”
The study is funded by the U.K.’s Medical Research Council and a European Union grant. Dr. Di Forti reports no relevant disclosures.
REPORTING FROM THE ICSR BIENNIAL MEETING
Clinical Challenges - January 2018 What's your diagnosis?
The diagnosis
Answer: Posttraumatic splenosis
Pathologic examination of the specimen revealed a splenosis showing regular red and white pulp (Figure E). Splenosis is the heterotopic autotransplantation of splenic tissue within the abdominal or pelvic cavity and occurs in 16%–67% of patients with a history of splenic trauma or splenic surgery.1 Nevertheless, hepatic splenosis is rare.2 The literature documents only 16 case reports of hepatic splenosis, although the difficulty of diagnosis could have contributed to underreporting. Although usually harmless, splenosis is a rare cause of bowel obstruction or abdominal pain.
References
1. Fleming, C.R., Dickson, E.R., Harrison, E.G. Splenosis: autotransplantation of splenic tissue. Am J Med. 1976;61:414-9.
2. D’Angelica, M., Fong, Y., Blumgart, L.H. Isolated hepatic splenosis: first reported case. HPB Surg. 1998;11:39-42.
3. Ohmoto, K., Mimura, N., Iguchi, Y. et al. Percutaneous microwave coagulation therapy for superficial hepatocellular carcinoma on the surface of the liver. Hepatogastroenterology. 2003;50:1547-51.
The diagnosis
Answer: Posttraumatic splenosis
Pathologic examination of the specimen revealed a splenosis showing regular red and white pulp (Figure E). Splenosis is the heterotopic autotransplantation of splenic tissue within the abdominal or pelvic cavity and occurs in 16%–67% of patients with a history of splenic trauma or splenic surgery.1 Nevertheless, hepatic splenosis is rare.2 The literature documents only 16 case reports of hepatic splenosis, although the difficulty of diagnosis could have contributed to underreporting. Although usually harmless, splenosis is a rare cause of bowel obstruction or abdominal pain.
References
1. Fleming, C.R., Dickson, E.R., Harrison, E.G. Splenosis: autotransplantation of splenic tissue. Am J Med. 1976;61:414-9.
2. D’Angelica, M., Fong, Y., Blumgart, L.H. Isolated hepatic splenosis: first reported case. HPB Surg. 1998;11:39-42.
3. Ohmoto, K., Mimura, N., Iguchi, Y. et al. Percutaneous microwave coagulation therapy for superficial hepatocellular carcinoma on the surface of the liver. Hepatogastroenterology. 2003;50:1547-51.
The diagnosis
Answer: Posttraumatic splenosis
Pathologic examination of the specimen revealed a splenosis showing regular red and white pulp (Figure E). Splenosis is the heterotopic autotransplantation of splenic tissue within the abdominal or pelvic cavity and occurs in 16%–67% of patients with a history of splenic trauma or splenic surgery.1 Nevertheless, hepatic splenosis is rare.2 The literature documents only 16 case reports of hepatic splenosis, although the difficulty of diagnosis could have contributed to underreporting. Although usually harmless, splenosis is a rare cause of bowel obstruction or abdominal pain.
References
1. Fleming, C.R., Dickson, E.R., Harrison, E.G. Splenosis: autotransplantation of splenic tissue. Am J Med. 1976;61:414-9.
2. D’Angelica, M., Fong, Y., Blumgart, L.H. Isolated hepatic splenosis: first reported case. HPB Surg. 1998;11:39-42.
3. Ohmoto, K., Mimura, N., Iguchi, Y. et al. Percutaneous microwave coagulation therapy for superficial hepatocellular carcinoma on the surface of the liver. Hepatogastroenterology. 2003;50:1547-51.
By Mareike Röther, MD, Jean-Francois Dufour, MD, and Beat Schnüriger, MD. Published previously in Gastroenterology (2013;144[3]:510, 659).
What is your diagnosis and treatment?
DDSEP® 8 Quick Quiz - January 2018 Question 1
Q1. CORRECT ANSWER: A
RATIONALE
In patients with a good response to anti-reflux surgery who develop new dysphagia, an upper endoscopy is the test of choice. This will evaluate the structural integrity of the fundoplication, and evaluate for disruption and paraesophageal herniation. The esophagus can be inspected for esophagitis, and dilation of the fundoplication site can be performed. In the absence of heartburn or the original reflux symptoms, empiric acid suppression is not expected to improve the dysphagia. If the endoscopy is negative, esophageal manometry and barium swallow are the next studies of value. A pH study off PPI therapy is performed if recurrent reflux is suspected that does not respond to anti-reflux medications.
REFERENCE
1. Johnson D.A., Younes Z., Hogan W.J. Endoscopic assessment of hiatal hernia repair. Gastrointest Endosc. 2000;52(5):650-9.
Q1. CORRECT ANSWER: A
RATIONALE
In patients with a good response to anti-reflux surgery who develop new dysphagia, an upper endoscopy is the test of choice. This will evaluate the structural integrity of the fundoplication, and evaluate for disruption and paraesophageal herniation. The esophagus can be inspected for esophagitis, and dilation of the fundoplication site can be performed. In the absence of heartburn or the original reflux symptoms, empiric acid suppression is not expected to improve the dysphagia. If the endoscopy is negative, esophageal manometry and barium swallow are the next studies of value. A pH study off PPI therapy is performed if recurrent reflux is suspected that does not respond to anti-reflux medications.
REFERENCE
1. Johnson D.A., Younes Z., Hogan W.J. Endoscopic assessment of hiatal hernia repair. Gastrointest Endosc. 2000;52(5):650-9.
Q1. CORRECT ANSWER: A
RATIONALE
In patients with a good response to anti-reflux surgery who develop new dysphagia, an upper endoscopy is the test of choice. This will evaluate the structural integrity of the fundoplication, and evaluate for disruption and paraesophageal herniation. The esophagus can be inspected for esophagitis, and dilation of the fundoplication site can be performed. In the absence of heartburn or the original reflux symptoms, empiric acid suppression is not expected to improve the dysphagia. If the endoscopy is negative, esophageal manometry and barium swallow are the next studies of value. A pH study off PPI therapy is performed if recurrent reflux is suspected that does not respond to anti-reflux medications.
REFERENCE
1. Johnson D.A., Younes Z., Hogan W.J. Endoscopic assessment of hiatal hernia repair. Gastrointest Endosc. 2000;52(5):650-9.
Q1. A 45-year-old woman underwent anti-reflux surgery 5 years ago for well-characterized reflux disease and a 5-cm hiatal hernia. After brief initial postoperative dysphagia treated conservatively with dietary adjustment, symptoms completely resolved. However, over the past 3 months, she has developed new dysphagia following solid meals, sometimes associated with epigastric pain. She localizes the dysphagia to the retrosternal region, with infrequent regurgitation but no heartburn.