User login
The (Friendly) Ghosts of Emergency Medicine Past, Present, and Yet to Come
Past…
Almost 40 years have elapsed since the American Board of Medical Specialties recognized emergency medicine (EM) as the 23rd medical specialty. Though the fundamental principles of patient care, medical education, and certification established by the American Board of Emergency Medicine (ABEM) have stood the test of time, the ED of today is a very different place than the “ER” of 1979. So too, today’s emergency physicians (EPs) are not only better trained and more capable of providing the highest quality of care in the ED, but are also increasingly doing so in venues outside of the traditional hospital-based ED.
Present...
In 1996, The New York Hospital – Cornell University Medical Center recruited me to be their first emergency physician-in-chief and EMS medical director, and to establish a first-rate academic ED and EM residency program. Starting with an “ER” staff of eight full-time and part-time non-EM-boarded attending physicians and a varying number of medical and surgical residents, over the next 20 years I expanded the complement of board-certified attending EPs to over 50, added attending-supervised nurse practitioners (NPs) and physician assistants (PAs), recruited a residency director, and helped him start a 4-year EM residency on both our Cornell and Columbia campuses. I also supported the initiation of 1-year ED nurse, PA, and NP residency programs. In corroboration with the chair of radiology, we added 24-hour dedicated sonography technologists to supplement the bedside emergency sonography that we had just been credentialed to perform, and established one of the very first divisions of emergency radiology, headed by EM board member and columnist, Keith Hentel, MD. Keith staffed his division with 24/7 attending radiologists to interpret all ED radiographic studies and provide imaging advice. More recently, I was able to arrange for dedicated 24/7 ED pharmacists, 24/7 ED social workers, and a patient safety/quality assurance division.
When I arrived at New York – Cornell, I supported the expansion of the ED patient services already in place, headed by an incredibly skilled and compassionate director, Constance Peterson, MA, who always insisted that her small office open directly off of the ED waiting room. Constance recruited and supervised a group of dedicated patient greeters and facilitators to ensure that no patient would get lost or fall through the “cracks” of our ever-expanding ED.
The plans for a new ED located at the front entrance to the hospital had literally been “carved in stone” by the time I arrived, but a decade later a magnificent gift from a donor gave me the opportunity to design a fourth patient-care area that expanded our ED to two full city blocks. I designed the new addition to serve the specific needs of a rapidly aging population and to provide a secure unit capable of managing patients with new or emerging infectious diseases and those with compromised immune systems. I also included in the new unit a large, state-of-the-art gynecologic (GYN) examination suite for conducting sexual assault exams and other GYN exams while providing the patient with a maximum level of comfort.
To coordinate activities throughout the ED and to provide a rapid expansion of staff when needed to manage surges in patient volume, I divided the ED into three acute areas and one urgent care area, each headed by an attending physician 24/7. One of the attendings was designated as the “administrative attending” or “AA.” Among other responsibilities, the AA was required to e-mail me and Associate Director Jeremy Sperling, MD, (now chair of EM at Einstein/Jacobi) a detailed note on patient volume, rate of new registrations, and any problems, at the end of every 8-hour shift—or more frequently when the need arose. Whenever patient volume was in danger of exceeding capacity, Jeremy immediately sent an urgent e-mail to all of our attending EPs, PAs, and NPs, offering double the hourly rate for 4 to 8 hours of patient care, while adhering to all relevant work-hour requirements. To cover the cost of these additional emergency clinical hours, I made a small portion of our fee-for-service revenues available. Two years ago, I initiated a physician scribe program to restore the physician-patient relationship during patient evaluations and treatments.
With the successful establishment of our EM residency program by Wallace Carter, MD, in 2003, I started 1- and 2-year fellowships in new disciplines for a 21st century ED—using a portion of our fee-for-service revenues designated for research and development to supplement the part-time attending base salaries of non-ACGME fellows. Beginning in 2005, I established the nation’s first geriatric EM fellowship, supported our newly established global EM program, recruited one of our attending EPs, Jay Lemery, MD, to start a wilderness medicine program in the Adirondack Mountains with Cornell (University) Outdoor Education, and appointed a director of EM/critical care. The ED expansion in 2009 enabled me to hire five attending EPs who were also board eligible/certified in medical toxicology, creating a “tox” group for bedside guidance and care in the ED and consultations throughout the hospital. The tox group also provided invaluable assistance to our secure psychiatric ED, headed by renowned emergency psychiatrists Lisa Sombrotto, MD, and Sharon Hird, MD. I also supported the activities of the pediatric EM fellowship, which had been established and nurtured by our extremely capable chief of pediatric EM, Shari Platt, MD. Most recently, I began to develop a new program in women’s health emergencies.
To expedite emergently needed care for an increasing number of oncology patients, I created a special “fast-track” to ensure that febrile cancer-treatment patients received needed antibiotics within an hour of arrival. I created a second fast-track to expedite the diagnosis and treatment (ie, transfer to the OR) of patients with surgical abdomens, and a third track to expedite the care of patients with community-acquired pneumonia.
And Yet to Come…
The programs and divisions described were developed over a 20-year period, always mindful of the standards and quality measures first promulgated by ABEM in 1979. New hospital-based ED initiatives will undoubtedly continue to be created in the future by EPs who are challenged to develop new and effective ways of caring for the ever-increasing numbers of patients in the face of continued hospital and ED closings.
At the same time, the increased numbers of patients seeking care in EDs, most recently created by the Affordable Care Act of 2010, is leading many EPs to apply the skills they learned as residents and their hospital-based ED experiences in new venues for emergency care. In recent years, there has been a virtual explosion in the number of urgent care centers, freestanding EDs, “convenient-care” centers, and even remote patient care in the form of “telehealth” or “telemedicine.” In 2014, when the National Hockey League mandated the presence of EPs at all games, I negotiated a contract that also enables our attending EPs to have senior residents accompany them and observe the practice of EM outside hospital walls. Prehospital and “interhospital” care also continues to expand with an increasing need for critical care and long-distance patient transfers to and from hospitals, and with a growing interest in community para-medicine programs.
In an October 2012 editorial (Emerg Med. 2012;44[10]:4), I wrote about French high-wire acrobat Philippe Petit who had rigged a cable between the two towers of the World Trade Center in August 1974, and then “aided only by a long, custom-made balancing pole, crossed, re-crossed, and danced on the wire without a safety net, for 45 minutes.” Most observers that day were certain he would fall to his death, and no one imagined that he would survive and outlast the 110-story towers he had anchored his cable to. So too, with EM: Hospital-based EDs will certainly remain an essential part of EM in the years to come, but EPs will also have increasing opportunities to practice their specialty in other important venues as well. The EP of the future will not be bound to a particular location to practice EM.
As I have this time of year for the past 11 years, I wish all of our readers, and all EPs everywhere, a joyous and safe holiday season and many happy and healthy new years to come.
Past…
Almost 40 years have elapsed since the American Board of Medical Specialties recognized emergency medicine (EM) as the 23rd medical specialty. Though the fundamental principles of patient care, medical education, and certification established by the American Board of Emergency Medicine (ABEM) have stood the test of time, the ED of today is a very different place than the “ER” of 1979. So too, today’s emergency physicians (EPs) are not only better trained and more capable of providing the highest quality of care in the ED, but are also increasingly doing so in venues outside of the traditional hospital-based ED.
Present...
In 1996, The New York Hospital – Cornell University Medical Center recruited me to be their first emergency physician-in-chief and EMS medical director, and to establish a first-rate academic ED and EM residency program. Starting with an “ER” staff of eight full-time and part-time non-EM-boarded attending physicians and a varying number of medical and surgical residents, over the next 20 years I expanded the complement of board-certified attending EPs to over 50, added attending-supervised nurse practitioners (NPs) and physician assistants (PAs), recruited a residency director, and helped him start a 4-year EM residency on both our Cornell and Columbia campuses. I also supported the initiation of 1-year ED nurse, PA, and NP residency programs. In corroboration with the chair of radiology, we added 24-hour dedicated sonography technologists to supplement the bedside emergency sonography that we had just been credentialed to perform, and established one of the very first divisions of emergency radiology, headed by EM board member and columnist, Keith Hentel, MD. Keith staffed his division with 24/7 attending radiologists to interpret all ED radiographic studies and provide imaging advice. More recently, I was able to arrange for dedicated 24/7 ED pharmacists, 24/7 ED social workers, and a patient safety/quality assurance division.
When I arrived at New York – Cornell, I supported the expansion of the ED patient services already in place, headed by an incredibly skilled and compassionate director, Constance Peterson, MA, who always insisted that her small office open directly off of the ED waiting room. Constance recruited and supervised a group of dedicated patient greeters and facilitators to ensure that no patient would get lost or fall through the “cracks” of our ever-expanding ED.
The plans for a new ED located at the front entrance to the hospital had literally been “carved in stone” by the time I arrived, but a decade later a magnificent gift from a donor gave me the opportunity to design a fourth patient-care area that expanded our ED to two full city blocks. I designed the new addition to serve the specific needs of a rapidly aging population and to provide a secure unit capable of managing patients with new or emerging infectious diseases and those with compromised immune systems. I also included in the new unit a large, state-of-the-art gynecologic (GYN) examination suite for conducting sexual assault exams and other GYN exams while providing the patient with a maximum level of comfort.
To coordinate activities throughout the ED and to provide a rapid expansion of staff when needed to manage surges in patient volume, I divided the ED into three acute areas and one urgent care area, each headed by an attending physician 24/7. One of the attendings was designated as the “administrative attending” or “AA.” Among other responsibilities, the AA was required to e-mail me and Associate Director Jeremy Sperling, MD, (now chair of EM at Einstein/Jacobi) a detailed note on patient volume, rate of new registrations, and any problems, at the end of every 8-hour shift—or more frequently when the need arose. Whenever patient volume was in danger of exceeding capacity, Jeremy immediately sent an urgent e-mail to all of our attending EPs, PAs, and NPs, offering double the hourly rate for 4 to 8 hours of patient care, while adhering to all relevant work-hour requirements. To cover the cost of these additional emergency clinical hours, I made a small portion of our fee-for-service revenues available. Two years ago, I initiated a physician scribe program to restore the physician-patient relationship during patient evaluations and treatments.
With the successful establishment of our EM residency program by Wallace Carter, MD, in 2003, I started 1- and 2-year fellowships in new disciplines for a 21st century ED—using a portion of our fee-for-service revenues designated for research and development to supplement the part-time attending base salaries of non-ACGME fellows. Beginning in 2005, I established the nation’s first geriatric EM fellowship, supported our newly established global EM program, recruited one of our attending EPs, Jay Lemery, MD, to start a wilderness medicine program in the Adirondack Mountains with Cornell (University) Outdoor Education, and appointed a director of EM/critical care. The ED expansion in 2009 enabled me to hire five attending EPs who were also board eligible/certified in medical toxicology, creating a “tox” group for bedside guidance and care in the ED and consultations throughout the hospital. The tox group also provided invaluable assistance to our secure psychiatric ED, headed by renowned emergency psychiatrists Lisa Sombrotto, MD, and Sharon Hird, MD. I also supported the activities of the pediatric EM fellowship, which had been established and nurtured by our extremely capable chief of pediatric EM, Shari Platt, MD. Most recently, I began to develop a new program in women’s health emergencies.
To expedite emergently needed care for an increasing number of oncology patients, I created a special “fast-track” to ensure that febrile cancer-treatment patients received needed antibiotics within an hour of arrival. I created a second fast-track to expedite the diagnosis and treatment (ie, transfer to the OR) of patients with surgical abdomens, and a third track to expedite the care of patients with community-acquired pneumonia.
And Yet to Come…
The programs and divisions described were developed over a 20-year period, always mindful of the standards and quality measures first promulgated by ABEM in 1979. New hospital-based ED initiatives will undoubtedly continue to be created in the future by EPs who are challenged to develop new and effective ways of caring for the ever-increasing numbers of patients in the face of continued hospital and ED closings.
At the same time, the increased numbers of patients seeking care in EDs, most recently created by the Affordable Care Act of 2010, is leading many EPs to apply the skills they learned as residents and their hospital-based ED experiences in new venues for emergency care. In recent years, there has been a virtual explosion in the number of urgent care centers, freestanding EDs, “convenient-care” centers, and even remote patient care in the form of “telehealth” or “telemedicine.” In 2014, when the National Hockey League mandated the presence of EPs at all games, I negotiated a contract that also enables our attending EPs to have senior residents accompany them and observe the practice of EM outside hospital walls. Prehospital and “interhospital” care also continues to expand with an increasing need for critical care and long-distance patient transfers to and from hospitals, and with a growing interest in community para-medicine programs.
In an October 2012 editorial (Emerg Med. 2012;44[10]:4), I wrote about French high-wire acrobat Philippe Petit who had rigged a cable between the two towers of the World Trade Center in August 1974, and then “aided only by a long, custom-made balancing pole, crossed, re-crossed, and danced on the wire without a safety net, for 45 minutes.” Most observers that day were certain he would fall to his death, and no one imagined that he would survive and outlast the 110-story towers he had anchored his cable to. So too, with EM: Hospital-based EDs will certainly remain an essential part of EM in the years to come, but EPs will also have increasing opportunities to practice their specialty in other important venues as well. The EP of the future will not be bound to a particular location to practice EM.
As I have this time of year for the past 11 years, I wish all of our readers, and all EPs everywhere, a joyous and safe holiday season and many happy and healthy new years to come.
Past…
Almost 40 years have elapsed since the American Board of Medical Specialties recognized emergency medicine (EM) as the 23rd medical specialty. Though the fundamental principles of patient care, medical education, and certification established by the American Board of Emergency Medicine (ABEM) have stood the test of time, the ED of today is a very different place than the “ER” of 1979. So too, today’s emergency physicians (EPs) are not only better trained and more capable of providing the highest quality of care in the ED, but are also increasingly doing so in venues outside of the traditional hospital-based ED.
Present...
In 1996, The New York Hospital – Cornell University Medical Center recruited me to be their first emergency physician-in-chief and EMS medical director, and to establish a first-rate academic ED and EM residency program. Starting with an “ER” staff of eight full-time and part-time non-EM-boarded attending physicians and a varying number of medical and surgical residents, over the next 20 years I expanded the complement of board-certified attending EPs to over 50, added attending-supervised nurse practitioners (NPs) and physician assistants (PAs), recruited a residency director, and helped him start a 4-year EM residency on both our Cornell and Columbia campuses. I also supported the initiation of 1-year ED nurse, PA, and NP residency programs. In corroboration with the chair of radiology, we added 24-hour dedicated sonography technologists to supplement the bedside emergency sonography that we had just been credentialed to perform, and established one of the very first divisions of emergency radiology, headed by EM board member and columnist, Keith Hentel, MD. Keith staffed his division with 24/7 attending radiologists to interpret all ED radiographic studies and provide imaging advice. More recently, I was able to arrange for dedicated 24/7 ED pharmacists, 24/7 ED social workers, and a patient safety/quality assurance division.
When I arrived at New York – Cornell, I supported the expansion of the ED patient services already in place, headed by an incredibly skilled and compassionate director, Constance Peterson, MA, who always insisted that her small office open directly off of the ED waiting room. Constance recruited and supervised a group of dedicated patient greeters and facilitators to ensure that no patient would get lost or fall through the “cracks” of our ever-expanding ED.
The plans for a new ED located at the front entrance to the hospital had literally been “carved in stone” by the time I arrived, but a decade later a magnificent gift from a donor gave me the opportunity to design a fourth patient-care area that expanded our ED to two full city blocks. I designed the new addition to serve the specific needs of a rapidly aging population and to provide a secure unit capable of managing patients with new or emerging infectious diseases and those with compromised immune systems. I also included in the new unit a large, state-of-the-art gynecologic (GYN) examination suite for conducting sexual assault exams and other GYN exams while providing the patient with a maximum level of comfort.
To coordinate activities throughout the ED and to provide a rapid expansion of staff when needed to manage surges in patient volume, I divided the ED into three acute areas and one urgent care area, each headed by an attending physician 24/7. One of the attendings was designated as the “administrative attending” or “AA.” Among other responsibilities, the AA was required to e-mail me and Associate Director Jeremy Sperling, MD, (now chair of EM at Einstein/Jacobi) a detailed note on patient volume, rate of new registrations, and any problems, at the end of every 8-hour shift—or more frequently when the need arose. Whenever patient volume was in danger of exceeding capacity, Jeremy immediately sent an urgent e-mail to all of our attending EPs, PAs, and NPs, offering double the hourly rate for 4 to 8 hours of patient care, while adhering to all relevant work-hour requirements. To cover the cost of these additional emergency clinical hours, I made a small portion of our fee-for-service revenues available. Two years ago, I initiated a physician scribe program to restore the physician-patient relationship during patient evaluations and treatments.
With the successful establishment of our EM residency program by Wallace Carter, MD, in 2003, I started 1- and 2-year fellowships in new disciplines for a 21st century ED—using a portion of our fee-for-service revenues designated for research and development to supplement the part-time attending base salaries of non-ACGME fellows. Beginning in 2005, I established the nation’s first geriatric EM fellowship, supported our newly established global EM program, recruited one of our attending EPs, Jay Lemery, MD, to start a wilderness medicine program in the Adirondack Mountains with Cornell (University) Outdoor Education, and appointed a director of EM/critical care. The ED expansion in 2009 enabled me to hire five attending EPs who were also board eligible/certified in medical toxicology, creating a “tox” group for bedside guidance and care in the ED and consultations throughout the hospital. The tox group also provided invaluable assistance to our secure psychiatric ED, headed by renowned emergency psychiatrists Lisa Sombrotto, MD, and Sharon Hird, MD. I also supported the activities of the pediatric EM fellowship, which had been established and nurtured by our extremely capable chief of pediatric EM, Shari Platt, MD. Most recently, I began to develop a new program in women’s health emergencies.
To expedite emergently needed care for an increasing number of oncology patients, I created a special “fast-track” to ensure that febrile cancer-treatment patients received needed antibiotics within an hour of arrival. I created a second fast-track to expedite the diagnosis and treatment (ie, transfer to the OR) of patients with surgical abdomens, and a third track to expedite the care of patients with community-acquired pneumonia.
And Yet to Come…
The programs and divisions described were developed over a 20-year period, always mindful of the standards and quality measures first promulgated by ABEM in 1979. New hospital-based ED initiatives will undoubtedly continue to be created in the future by EPs who are challenged to develop new and effective ways of caring for the ever-increasing numbers of patients in the face of continued hospital and ED closings.
At the same time, the increased numbers of patients seeking care in EDs, most recently created by the Affordable Care Act of 2010, is leading many EPs to apply the skills they learned as residents and their hospital-based ED experiences in new venues for emergency care. In recent years, there has been a virtual explosion in the number of urgent care centers, freestanding EDs, “convenient-care” centers, and even remote patient care in the form of “telehealth” or “telemedicine.” In 2014, when the National Hockey League mandated the presence of EPs at all games, I negotiated a contract that also enables our attending EPs to have senior residents accompany them and observe the practice of EM outside hospital walls. Prehospital and “interhospital” care also continues to expand with an increasing need for critical care and long-distance patient transfers to and from hospitals, and with a growing interest in community para-medicine programs.
In an October 2012 editorial (Emerg Med. 2012;44[10]:4), I wrote about French high-wire acrobat Philippe Petit who had rigged a cable between the two towers of the World Trade Center in August 1974, and then “aided only by a long, custom-made balancing pole, crossed, re-crossed, and danced on the wire without a safety net, for 45 minutes.” Most observers that day were certain he would fall to his death, and no one imagined that he would survive and outlast the 110-story towers he had anchored his cable to. So too, with EM: Hospital-based EDs will certainly remain an essential part of EM in the years to come, but EPs will also have increasing opportunities to practice their specialty in other important venues as well. The EP of the future will not be bound to a particular location to practice EM.
As I have this time of year for the past 11 years, I wish all of our readers, and all EPs everywhere, a joyous and safe holiday season and many happy and healthy new years to come.
A+AVD improves modified PFS in advanced HL
ATLANTA—Phase 3 trial results suggest one 4-drug combination may be more effective than another as frontline treatment for advanced Hodgkin lymphoma (HL).
In the ECHELON-1 trial, treatment with brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) staved off progression, death, and the need for subsequent therapy more effectively than treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).
However, there was no significant difference between the treatment arms when it came to response rates or overall survival.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
These data were presented at the 2017 ASH Annual Meeting (abstract 6) and simultaneously published in The New England Journal of Medicine. The trial was funded by Millennium Pharmaceuticals and Seattle Genetics, Inc.
“The standard of care in the treatment of Hodgkin lymphoma has not changed over the last several decades, and there remains an unmet need for additional regimens in frontline treatment,” said Joseph M. Connors, MD, of BC Cancer in Vancouver, British Columbia, Canada.
With this in mind, he and his colleagues conducted ECHELON-1. The study enrolled 1334 patients who had stage III or IV HL and had not previously received systemic chemotherapy or radiotherapy.
Fifty-eight percent of patients were male, and the median age was 36 (range, 18-83). Sixty-four percent of patients had stage IV disease, 62% had extranodal involvement at diagnosis, and 58% had B symptoms.
The patients were randomized to receive A+AVD (n=664) or ABVD (n=670) on days 1 and 15 of each 28-day cycle for up to 6 cycles. Baseline characteristics were well-balanced between the treatment arms.
The median follow-up was 24.9 months (range, 0-49.3).
Primary endpoint
The study’s primary endpoint is modified progression-free survival (PFS), which is defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review facility, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
“Reducing the risk of relapse is an important concern for patients and their physicians,” Dr Connors noted. “In the trial, 33% fewer patients [in the A+AVD arm] required subsequent salvage chemotherapy or high-dose chemotherapy and transplant compared to the patients treated with ABVD.”
According to the independent review facility, the 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
Certain pre-specified subgroups of patients appeared to benefit more with A+AVD than with ABVD, including:
- Males
- Patients treated in North America
- Patients with involvement of more than 1 extranodal site
- Patients with International Prognostic Scores of 4 to 7
- Patients with stage IV disease
- Patients younger than 60.
Secondary endpoints
Secondary endpoints trended in favor of the A+AVD arm, although there were no significant differences between the treatment arms.
The objective response rate at the end of the randomized regimen was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The proportion of patients with a Deauville score ≤2 after the completion of frontline therapy was 85% in the A+AVD arm and 80% in the ABVD arm (P=0.03).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
Safety
“[T]he safety profile [of A+AVD] was generally consistent with that known for the single-agent components of the regimen,” Dr Connors said.
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Common AEs (in the A+AVD and ABVD arms, respectively) included neutropenia (58% and 45%), constipation (42% and 37%), vomiting (33% and 28%), fatigue (both 32%), diarrhea (27% and 18%), pyrexia (27% and 22%), abdominal pain (21% and 10%), and stomatitis (21% and 16%).
Peripheral neuropathy events were observed in 67% of patients in the A+AVD arm and 43% in the ABVD arm. Grade 3 or higher peripheral neuropathy was reported in 11% and 2%, respectively.
Febrile neutropenia occurred in 19% of patients in the A+AVD arm and 8% of those in the ABVD arm. However, prophylaxis with granulocyte colony-stimulating factor (G-CSF) was able to reduce the incidence of febrile neutropenia. In the A+AVD arm, the rate of febrile neutropenia was 11% among patients who received G-CSF and 21% among patients who did not.
Pulmonary toxicity occurred in 2% of patients in the A+AVD arm and 7% of those in the ABVD arm. Grade 3 or higher pulmonary toxicity was reported in 0.76% and 3%, respectively.
There were 9 deaths during treatment in the A+AVD arm. Seven were due to neutropenia or associated complications, and 2 were due to myocardial infarction. One of the patients who died of neutropenia had the condition prior to trial enrollment. The remaining 6 patients did not receive G-CSF prophylaxis.
In the ABVD arm, there were 13 deaths during treatment. Eleven were due to or associated with pulmonary-related toxicity, 1 was due to cardiopulmonary failure, and 1 death had an unknown cause. ![]()
ATLANTA—Phase 3 trial results suggest one 4-drug combination may be more effective than another as frontline treatment for advanced Hodgkin lymphoma (HL).
In the ECHELON-1 trial, treatment with brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) staved off progression, death, and the need for subsequent therapy more effectively than treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).
However, there was no significant difference between the treatment arms when it came to response rates or overall survival.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
These data were presented at the 2017 ASH Annual Meeting (abstract 6) and simultaneously published in The New England Journal of Medicine. The trial was funded by Millennium Pharmaceuticals and Seattle Genetics, Inc.
“The standard of care in the treatment of Hodgkin lymphoma has not changed over the last several decades, and there remains an unmet need for additional regimens in frontline treatment,” said Joseph M. Connors, MD, of BC Cancer in Vancouver, British Columbia, Canada.
With this in mind, he and his colleagues conducted ECHELON-1. The study enrolled 1334 patients who had stage III or IV HL and had not previously received systemic chemotherapy or radiotherapy.
Fifty-eight percent of patients were male, and the median age was 36 (range, 18-83). Sixty-four percent of patients had stage IV disease, 62% had extranodal involvement at diagnosis, and 58% had B symptoms.
The patients were randomized to receive A+AVD (n=664) or ABVD (n=670) on days 1 and 15 of each 28-day cycle for up to 6 cycles. Baseline characteristics were well-balanced between the treatment arms.
The median follow-up was 24.9 months (range, 0-49.3).
Primary endpoint
The study’s primary endpoint is modified progression-free survival (PFS), which is defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review facility, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
“Reducing the risk of relapse is an important concern for patients and their physicians,” Dr Connors noted. “In the trial, 33% fewer patients [in the A+AVD arm] required subsequent salvage chemotherapy or high-dose chemotherapy and transplant compared to the patients treated with ABVD.”
According to the independent review facility, the 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
Certain pre-specified subgroups of patients appeared to benefit more with A+AVD than with ABVD, including:
- Males
- Patients treated in North America
- Patients with involvement of more than 1 extranodal site
- Patients with International Prognostic Scores of 4 to 7
- Patients with stage IV disease
- Patients younger than 60.
Secondary endpoints
Secondary endpoints trended in favor of the A+AVD arm, although there were no significant differences between the treatment arms.
The objective response rate at the end of the randomized regimen was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The proportion of patients with a Deauville score ≤2 after the completion of frontline therapy was 85% in the A+AVD arm and 80% in the ABVD arm (P=0.03).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
Safety
“[T]he safety profile [of A+AVD] was generally consistent with that known for the single-agent components of the regimen,” Dr Connors said.
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Common AEs (in the A+AVD and ABVD arms, respectively) included neutropenia (58% and 45%), constipation (42% and 37%), vomiting (33% and 28%), fatigue (both 32%), diarrhea (27% and 18%), pyrexia (27% and 22%), abdominal pain (21% and 10%), and stomatitis (21% and 16%).
Peripheral neuropathy events were observed in 67% of patients in the A+AVD arm and 43% in the ABVD arm. Grade 3 or higher peripheral neuropathy was reported in 11% and 2%, respectively.
Febrile neutropenia occurred in 19% of patients in the A+AVD arm and 8% of those in the ABVD arm. However, prophylaxis with granulocyte colony-stimulating factor (G-CSF) was able to reduce the incidence of febrile neutropenia. In the A+AVD arm, the rate of febrile neutropenia was 11% among patients who received G-CSF and 21% among patients who did not.
Pulmonary toxicity occurred in 2% of patients in the A+AVD arm and 7% of those in the ABVD arm. Grade 3 or higher pulmonary toxicity was reported in 0.76% and 3%, respectively.
There were 9 deaths during treatment in the A+AVD arm. Seven were due to neutropenia or associated complications, and 2 were due to myocardial infarction. One of the patients who died of neutropenia had the condition prior to trial enrollment. The remaining 6 patients did not receive G-CSF prophylaxis.
In the ABVD arm, there were 13 deaths during treatment. Eleven were due to or associated with pulmonary-related toxicity, 1 was due to cardiopulmonary failure, and 1 death had an unknown cause. ![]()
ATLANTA—Phase 3 trial results suggest one 4-drug combination may be more effective than another as frontline treatment for advanced Hodgkin lymphoma (HL).
In the ECHELON-1 trial, treatment with brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) staved off progression, death, and the need for subsequent therapy more effectively than treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).
However, there was no significant difference between the treatment arms when it came to response rates or overall survival.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
These data were presented at the 2017 ASH Annual Meeting (abstract 6) and simultaneously published in The New England Journal of Medicine. The trial was funded by Millennium Pharmaceuticals and Seattle Genetics, Inc.
“The standard of care in the treatment of Hodgkin lymphoma has not changed over the last several decades, and there remains an unmet need for additional regimens in frontline treatment,” said Joseph M. Connors, MD, of BC Cancer in Vancouver, British Columbia, Canada.
With this in mind, he and his colleagues conducted ECHELON-1. The study enrolled 1334 patients who had stage III or IV HL and had not previously received systemic chemotherapy or radiotherapy.
Fifty-eight percent of patients were male, and the median age was 36 (range, 18-83). Sixty-four percent of patients had stage IV disease, 62% had extranodal involvement at diagnosis, and 58% had B symptoms.
The patients were randomized to receive A+AVD (n=664) or ABVD (n=670) on days 1 and 15 of each 28-day cycle for up to 6 cycles. Baseline characteristics were well-balanced between the treatment arms.
The median follow-up was 24.9 months (range, 0-49.3).
Primary endpoint
The study’s primary endpoint is modified progression-free survival (PFS), which is defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review facility, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
“Reducing the risk of relapse is an important concern for patients and their physicians,” Dr Connors noted. “In the trial, 33% fewer patients [in the A+AVD arm] required subsequent salvage chemotherapy or high-dose chemotherapy and transplant compared to the patients treated with ABVD.”
According to the independent review facility, the 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
Certain pre-specified subgroups of patients appeared to benefit more with A+AVD than with ABVD, including:
- Males
- Patients treated in North America
- Patients with involvement of more than 1 extranodal site
- Patients with International Prognostic Scores of 4 to 7
- Patients with stage IV disease
- Patients younger than 60.
Secondary endpoints
Secondary endpoints trended in favor of the A+AVD arm, although there were no significant differences between the treatment arms.
The objective response rate at the end of the randomized regimen was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The proportion of patients with a Deauville score ≤2 after the completion of frontline therapy was 85% in the A+AVD arm and 80% in the ABVD arm (P=0.03).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
Safety
“[T]he safety profile [of A+AVD] was generally consistent with that known for the single-agent components of the regimen,” Dr Connors said.
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Common AEs (in the A+AVD and ABVD arms, respectively) included neutropenia (58% and 45%), constipation (42% and 37%), vomiting (33% and 28%), fatigue (both 32%), diarrhea (27% and 18%), pyrexia (27% and 22%), abdominal pain (21% and 10%), and stomatitis (21% and 16%).
Peripheral neuropathy events were observed in 67% of patients in the A+AVD arm and 43% in the ABVD arm. Grade 3 or higher peripheral neuropathy was reported in 11% and 2%, respectively.
Febrile neutropenia occurred in 19% of patients in the A+AVD arm and 8% of those in the ABVD arm. However, prophylaxis with granulocyte colony-stimulating factor (G-CSF) was able to reduce the incidence of febrile neutropenia. In the A+AVD arm, the rate of febrile neutropenia was 11% among patients who received G-CSF and 21% among patients who did not.
Pulmonary toxicity occurred in 2% of patients in the A+AVD arm and 7% of those in the ABVD arm. Grade 3 or higher pulmonary toxicity was reported in 0.76% and 3%, respectively.
There were 9 deaths during treatment in the A+AVD arm. Seven were due to neutropenia or associated complications, and 2 were due to myocardial infarction. One of the patients who died of neutropenia had the condition prior to trial enrollment. The remaining 6 patients did not receive G-CSF prophylaxis.
In the ABVD arm, there were 13 deaths during treatment. Eleven were due to or associated with pulmonary-related toxicity, 1 was due to cardiopulmonary failure, and 1 death had an unknown cause. ![]()
EC approves new formulation of pegaspargase
The European Commission (EC) has granted marketing authorization for a lyophilized formulation of pegaspargase (ONCASPAR).
The product is intended for use as a component of antineoplastic combination therapy in acute lymphoblastic leukemia patients of all ages.
The EC’s approval authorizes Shire to market lyophilized pegaspargase in the 28 member states of the European Union as well as Iceland, Liechtenstein, and Norway.
Lyophilized pegaspargase works the same way as the liquid formulation. By depleting serum L-asparagine levels and thereby interfering with protein synthesis, pegaspargase deprives lymphoblasts of L-asparagine, resulting in cell death.
The lyophilized formulation offers the same dosing regimen as liquid pegaspargase but also provides a shelf life of up to 24 months—3 times longer than that of the liquid formulation.
Shire expects lyophilized pegaspargase to be available in European markets beginning in the first half of 2018.
“With this lyophilized formulation, we aim to make pegylated asparaginase, part of the pediatric standard therapy in acute lymphoblastic leukemia, available to patients in countries where liquid ONCASPAR is not currently offered,” said Howard B. Mayer, MD, senior vice-president and ad-interim head of global research and development at Shire.
“Additionally, with extended shelf life up to 24 months, treatment centers will have flexibility in inventory management to help ensure continuous treatment supply for patients.” ![]()
The European Commission (EC) has granted marketing authorization for a lyophilized formulation of pegaspargase (ONCASPAR).
The product is intended for use as a component of antineoplastic combination therapy in acute lymphoblastic leukemia patients of all ages.
The EC’s approval authorizes Shire to market lyophilized pegaspargase in the 28 member states of the European Union as well as Iceland, Liechtenstein, and Norway.
Lyophilized pegaspargase works the same way as the liquid formulation. By depleting serum L-asparagine levels and thereby interfering with protein synthesis, pegaspargase deprives lymphoblasts of L-asparagine, resulting in cell death.
The lyophilized formulation offers the same dosing regimen as liquid pegaspargase but also provides a shelf life of up to 24 months—3 times longer than that of the liquid formulation.
Shire expects lyophilized pegaspargase to be available in European markets beginning in the first half of 2018.
“With this lyophilized formulation, we aim to make pegylated asparaginase, part of the pediatric standard therapy in acute lymphoblastic leukemia, available to patients in countries where liquid ONCASPAR is not currently offered,” said Howard B. Mayer, MD, senior vice-president and ad-interim head of global research and development at Shire.
“Additionally, with extended shelf life up to 24 months, treatment centers will have flexibility in inventory management to help ensure continuous treatment supply for patients.” ![]()
The European Commission (EC) has granted marketing authorization for a lyophilized formulation of pegaspargase (ONCASPAR).
The product is intended for use as a component of antineoplastic combination therapy in acute lymphoblastic leukemia patients of all ages.
The EC’s approval authorizes Shire to market lyophilized pegaspargase in the 28 member states of the European Union as well as Iceland, Liechtenstein, and Norway.
Lyophilized pegaspargase works the same way as the liquid formulation. By depleting serum L-asparagine levels and thereby interfering with protein synthesis, pegaspargase deprives lymphoblasts of L-asparagine, resulting in cell death.
The lyophilized formulation offers the same dosing regimen as liquid pegaspargase but also provides a shelf life of up to 24 months—3 times longer than that of the liquid formulation.
Shire expects lyophilized pegaspargase to be available in European markets beginning in the first half of 2018.
“With this lyophilized formulation, we aim to make pegylated asparaginase, part of the pediatric standard therapy in acute lymphoblastic leukemia, available to patients in countries where liquid ONCASPAR is not currently offered,” said Howard B. Mayer, MD, senior vice-president and ad-interim head of global research and development at Shire.
“Additionally, with extended shelf life up to 24 months, treatment centers will have flexibility in inventory management to help ensure continuous treatment supply for patients.” ![]()
Technique may be effective for diagnosing NHL
Preclinical research suggests infrared spectroscopy could be used to diagnose non-Hodgkin lymphoma (NHL).
Researchers used mid-infrared spectroscopy to analyze blood serum derived from mice and differentiate mice with NHL and subcutaneous melanoma from each other and from healthy control mice.
The findings suggest infrared spectroscopy can detect biochemical changes induced by NHL and melanoma and therefore has diagnostic potential as a screening technique for these cancers.
A.G. Unil Perera, PhD, of Georgia State University in Atlanta, and his colleagues detailed these findings in Scientific Reports.
The researchers said that Fourier Transform Infrared (FTIR) spectroscopy in Attenuated Total Reflection (ATR) sampling mode provides high-quality results with better reproducibility compared to other vibrational spectroscopy.
With previous work, Dr Perera and his colleagues discovered that a blood test for ulcerative colitis using ATR-FTIR spectroscopy could provide a cheaper, less invasive alternative for screening compared to colonoscopy.
In the current study, the researchers tested ATR-FTIR spectroscopy in mouse models of malignancy—EL4 NHL and B16 subcutaneous melanoma.
The team extracted blood serum from these mice and control mice. Droplets of serum were placed on an ATR crystal of the FTIR instrument.
Incident infrared beams were absorbed and reflected by the serum, creating a wave that was recorded and used to produce an absorbance curve with peaks that identified the presence of certain biomarkers in the sample.
The researchers compared the absorbance curves from the control and cancer mice and assessed biochemical changes induced by NHL and melanoma.
The team found “remarkable” differences between the ATR-FTIR spectra of serum samples from tumor-bearing mice and control mice.
Dr Perera said these findings are applicable to humans because mice and humans have some biomarkers and chemicals in common.
Using the data collected on the biomarkers for NHL and melanoma, the researchers could develop detectors for these particular absorbance peaks, which doctors could use to test patients’ blood samples for these cancers.
“Our final goal is to say we can use this infrared technique to identify various diseases,” Dr Perera said. “This study shows infrared spectroscopy can identify cancer. Right now, when you go to the doctor, they do blood tests for sugar and several other things but not for serious diseases like cancer and colitis.”
“One day, we hope that even these serious diseases can be rapidly screened. Your primary doctor could keep a record of your number and check that every time you come back. Then, if there is some indication of cancer or colitis, they can do biopsies, colonoscopies, etc.” ![]()
Preclinical research suggests infrared spectroscopy could be used to diagnose non-Hodgkin lymphoma (NHL).
Researchers used mid-infrared spectroscopy to analyze blood serum derived from mice and differentiate mice with NHL and subcutaneous melanoma from each other and from healthy control mice.
The findings suggest infrared spectroscopy can detect biochemical changes induced by NHL and melanoma and therefore has diagnostic potential as a screening technique for these cancers.
A.G. Unil Perera, PhD, of Georgia State University in Atlanta, and his colleagues detailed these findings in Scientific Reports.
The researchers said that Fourier Transform Infrared (FTIR) spectroscopy in Attenuated Total Reflection (ATR) sampling mode provides high-quality results with better reproducibility compared to other vibrational spectroscopy.
With previous work, Dr Perera and his colleagues discovered that a blood test for ulcerative colitis using ATR-FTIR spectroscopy could provide a cheaper, less invasive alternative for screening compared to colonoscopy.
In the current study, the researchers tested ATR-FTIR spectroscopy in mouse models of malignancy—EL4 NHL and B16 subcutaneous melanoma.
The team extracted blood serum from these mice and control mice. Droplets of serum were placed on an ATR crystal of the FTIR instrument.
Incident infrared beams were absorbed and reflected by the serum, creating a wave that was recorded and used to produce an absorbance curve with peaks that identified the presence of certain biomarkers in the sample.
The researchers compared the absorbance curves from the control and cancer mice and assessed biochemical changes induced by NHL and melanoma.
The team found “remarkable” differences between the ATR-FTIR spectra of serum samples from tumor-bearing mice and control mice.
Dr Perera said these findings are applicable to humans because mice and humans have some biomarkers and chemicals in common.
Using the data collected on the biomarkers for NHL and melanoma, the researchers could develop detectors for these particular absorbance peaks, which doctors could use to test patients’ blood samples for these cancers.
“Our final goal is to say we can use this infrared technique to identify various diseases,” Dr Perera said. “This study shows infrared spectroscopy can identify cancer. Right now, when you go to the doctor, they do blood tests for sugar and several other things but not for serious diseases like cancer and colitis.”
“One day, we hope that even these serious diseases can be rapidly screened. Your primary doctor could keep a record of your number and check that every time you come back. Then, if there is some indication of cancer or colitis, they can do biopsies, colonoscopies, etc.” ![]()
Preclinical research suggests infrared spectroscopy could be used to diagnose non-Hodgkin lymphoma (NHL).
Researchers used mid-infrared spectroscopy to analyze blood serum derived from mice and differentiate mice with NHL and subcutaneous melanoma from each other and from healthy control mice.
The findings suggest infrared spectroscopy can detect biochemical changes induced by NHL and melanoma and therefore has diagnostic potential as a screening technique for these cancers.
A.G. Unil Perera, PhD, of Georgia State University in Atlanta, and his colleagues detailed these findings in Scientific Reports.
The researchers said that Fourier Transform Infrared (FTIR) spectroscopy in Attenuated Total Reflection (ATR) sampling mode provides high-quality results with better reproducibility compared to other vibrational spectroscopy.
With previous work, Dr Perera and his colleagues discovered that a blood test for ulcerative colitis using ATR-FTIR spectroscopy could provide a cheaper, less invasive alternative for screening compared to colonoscopy.
In the current study, the researchers tested ATR-FTIR spectroscopy in mouse models of malignancy—EL4 NHL and B16 subcutaneous melanoma.
The team extracted blood serum from these mice and control mice. Droplets of serum were placed on an ATR crystal of the FTIR instrument.
Incident infrared beams were absorbed and reflected by the serum, creating a wave that was recorded and used to produce an absorbance curve with peaks that identified the presence of certain biomarkers in the sample.
The researchers compared the absorbance curves from the control and cancer mice and assessed biochemical changes induced by NHL and melanoma.
The team found “remarkable” differences between the ATR-FTIR spectra of serum samples from tumor-bearing mice and control mice.
Dr Perera said these findings are applicable to humans because mice and humans have some biomarkers and chemicals in common.
Using the data collected on the biomarkers for NHL and melanoma, the researchers could develop detectors for these particular absorbance peaks, which doctors could use to test patients’ blood samples for these cancers.
“Our final goal is to say we can use this infrared technique to identify various diseases,” Dr Perera said. “This study shows infrared spectroscopy can identify cancer. Right now, when you go to the doctor, they do blood tests for sugar and several other things but not for serious diseases like cancer and colitis.”
“One day, we hope that even these serious diseases can be rapidly screened. Your primary doctor could keep a record of your number and check that every time you come back. Then, if there is some indication of cancer or colitis, they can do biopsies, colonoscopies, etc.” ![]()
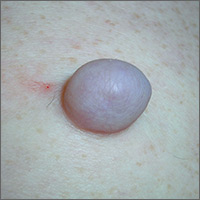
Growing mass on trunk
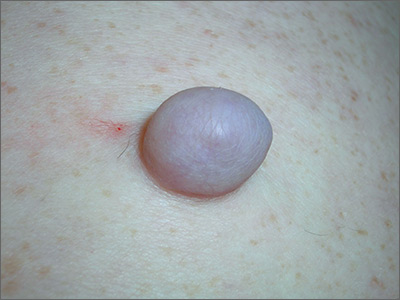
The FP recognized that the patient had a large skin tag. He offered to excise it for the patient and explained that it could be removed with a deep elliptical excision and then repaired with sutures, or that it could be shaved off and allowed to heal by secondary intention. The patient opted for the shave excision because he preferred not to have sutures.
The FP numbed the area with an injection of 1% lidocaine and epinephrine, then shaved off the growth using a DermaBlade and sent it to pathology. (See a video on how to perform a shave biopsy here.) The FP used aluminum chloride to stop the bleeding. The Pathology report came back and indicated the lesion was a fibroepithelial polyp, which is essentially a large skin tag. At a 2-week follow-up, the biopsied area was healing well.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith AM. Skin tags. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 922-925.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP recognized that the patient had a large skin tag. He offered to excise it for the patient and explained that it could be removed with a deep elliptical excision and then repaired with sutures, or that it could be shaved off and allowed to heal by secondary intention. The patient opted for the shave excision because he preferred not to have sutures.
The FP numbed the area with an injection of 1% lidocaine and epinephrine, then shaved off the growth using a DermaBlade and sent it to pathology. (See a video on how to perform a shave biopsy here.) The FP used aluminum chloride to stop the bleeding. The Pathology report came back and indicated the lesion was a fibroepithelial polyp, which is essentially a large skin tag. At a 2-week follow-up, the biopsied area was healing well.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith AM. Skin tags. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 922-925.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP recognized that the patient had a large skin tag. He offered to excise it for the patient and explained that it could be removed with a deep elliptical excision and then repaired with sutures, or that it could be shaved off and allowed to heal by secondary intention. The patient opted for the shave excision because he preferred not to have sutures.
The FP numbed the area with an injection of 1% lidocaine and epinephrine, then shaved off the growth using a DermaBlade and sent it to pathology. (See a video on how to perform a shave biopsy here.) The FP used aluminum chloride to stop the bleeding. The Pathology report came back and indicated the lesion was a fibroepithelial polyp, which is essentially a large skin tag. At a 2-week follow-up, the biopsied area was healing well.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith AM. Skin tags. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 922-925.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Even Grandma Can’t Cure This
ANSWER
The correct answer is nummular eczema (NE; choice “c”).
Although fairly common, NE is not a well-known diagnostic entity. Its round shape and scaly surface are often misdiagnosed as fungal infection (choice “a”). But such an infection would have responded to the prescribed medication. In addition, there was no likely source (a new cat, dog, or ferret; participation in a contact sport, such as wrestling), making this diagnosis unlikely.
The scale of psoriasis (choice “b”) is typically white, tenacious, and much thicker than that seen in this case. Other signs would have been visible on the knees, elbows, scalp, or nails.
Impetigo (choice “d”), a superficial skin infection caused by staph and/or strep, first requires a break in the skin to gain entry (eg, picked-over acne, scratches). It manifests with a honey-colored crust on a red base, which were missing in this case.
DISCUSSION
NE is fairly common (seen in every 1 per 1,000 dermatology visits) and can be found on the arms as well as the legs. Predisposing factors include atopy and dry skin—but truth be told, there are many aspects of this condition that remain unexplained. While histopathologic studies can confirm the diagnosis, they offer little explanation of its origin.
What we do know: NE usually begins with a tiny papule, then a surrounding scaly rash develops as the papule disappears. The lesions, which can appear in multiples, are benign and will eventually clear on their own. This is fortunate, because NE can be very difficult to treat.
In this case, the standard regimen was prescribed: a class I topical steroid (clobetasol) to be used under occlusion. For prevention, patients should avoid scented, colored products; long, hot showers (or hot tubs); and heavy moisturizers.
But the bigger take-home message is that not all round, scaly lesions are of fungal origin. The differential includes several items—NE among them.
ANSWER
The correct answer is nummular eczema (NE; choice “c”).
Although fairly common, NE is not a well-known diagnostic entity. Its round shape and scaly surface are often misdiagnosed as fungal infection (choice “a”). But such an infection would have responded to the prescribed medication. In addition, there was no likely source (a new cat, dog, or ferret; participation in a contact sport, such as wrestling), making this diagnosis unlikely.
The scale of psoriasis (choice “b”) is typically white, tenacious, and much thicker than that seen in this case. Other signs would have been visible on the knees, elbows, scalp, or nails.
Impetigo (choice “d”), a superficial skin infection caused by staph and/or strep, first requires a break in the skin to gain entry (eg, picked-over acne, scratches). It manifests with a honey-colored crust on a red base, which were missing in this case.
DISCUSSION
NE is fairly common (seen in every 1 per 1,000 dermatology visits) and can be found on the arms as well as the legs. Predisposing factors include atopy and dry skin—but truth be told, there are many aspects of this condition that remain unexplained. While histopathologic studies can confirm the diagnosis, they offer little explanation of its origin.
What we do know: NE usually begins with a tiny papule, then a surrounding scaly rash develops as the papule disappears. The lesions, which can appear in multiples, are benign and will eventually clear on their own. This is fortunate, because NE can be very difficult to treat.
In this case, the standard regimen was prescribed: a class I topical steroid (clobetasol) to be used under occlusion. For prevention, patients should avoid scented, colored products; long, hot showers (or hot tubs); and heavy moisturizers.
But the bigger take-home message is that not all round, scaly lesions are of fungal origin. The differential includes several items—NE among them.
ANSWER
The correct answer is nummular eczema (NE; choice “c”).
Although fairly common, NE is not a well-known diagnostic entity. Its round shape and scaly surface are often misdiagnosed as fungal infection (choice “a”). But such an infection would have responded to the prescribed medication. In addition, there was no likely source (a new cat, dog, or ferret; participation in a contact sport, such as wrestling), making this diagnosis unlikely.
The scale of psoriasis (choice “b”) is typically white, tenacious, and much thicker than that seen in this case. Other signs would have been visible on the knees, elbows, scalp, or nails.
Impetigo (choice “d”), a superficial skin infection caused by staph and/or strep, first requires a break in the skin to gain entry (eg, picked-over acne, scratches). It manifests with a honey-colored crust on a red base, which were missing in this case.
DISCUSSION
NE is fairly common (seen in every 1 per 1,000 dermatology visits) and can be found on the arms as well as the legs. Predisposing factors include atopy and dry skin—but truth be told, there are many aspects of this condition that remain unexplained. While histopathologic studies can confirm the diagnosis, they offer little explanation of its origin.
What we do know: NE usually begins with a tiny papule, then a surrounding scaly rash develops as the papule disappears. The lesions, which can appear in multiples, are benign and will eventually clear on their own. This is fortunate, because NE can be very difficult to treat.
In this case, the standard regimen was prescribed: a class I topical steroid (clobetasol) to be used under occlusion. For prevention, patients should avoid scented, colored products; long, hot showers (or hot tubs); and heavy moisturizers.
But the bigger take-home message is that not all round, scaly lesions are of fungal origin. The differential includes several items—NE among them.
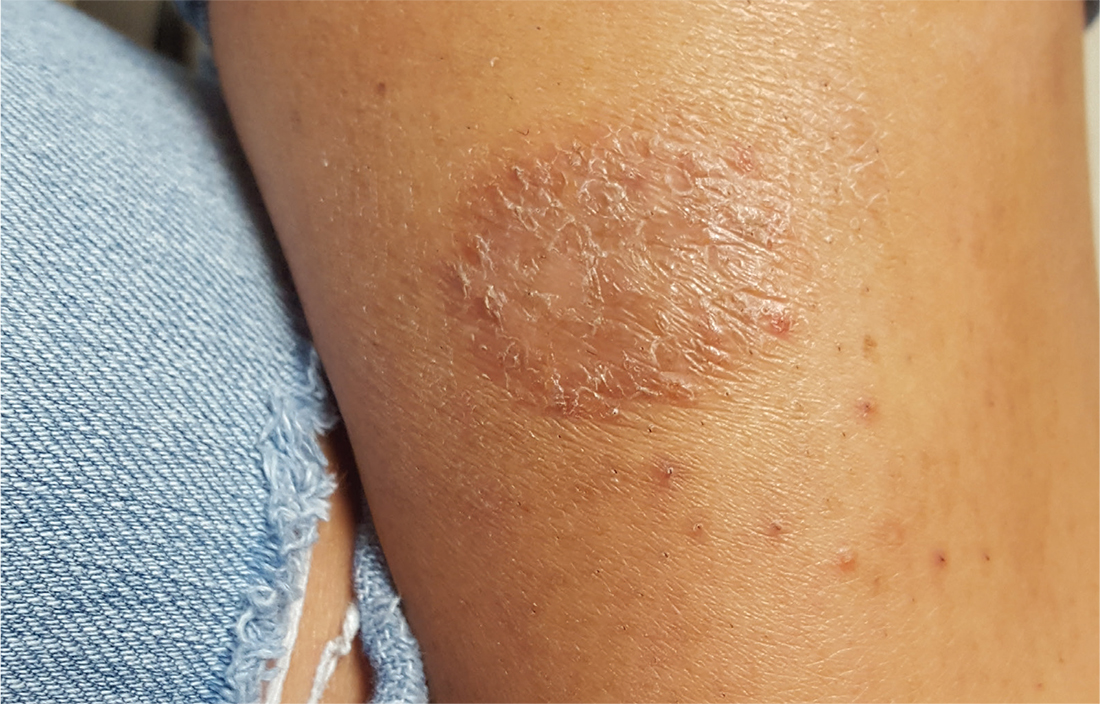
For several weeks, a 16-year-old girl has had an itchy rash on her calf. Her primary care provider, believing it to be fungal, prescribed nystatin cream and oral terbinafine (250 mg/d for a month). This treatment regimen had no effect.
In desperation, her grandmother mixed together a “family recipe” of peroxide, manteca (lard), and alcohol. This concoction was applied to the rash for several days—but alas, even this secret formula failed to bring improvement.
The patient has an extensive history of atopy, including eczema and seasonal allergies. The family has one small dog that stays indoors; there are no new pets in the household.
On the patient’s left medial calf is a round, well-defined, 4-cm patch of scaly skin. The lesion is brownish pink, consistent with her type IV skin. Closer inspection suggests the surface was initially covered with tiny blisters (vesicles); additional questioning confirms this.
Elsewhere, her skin is quite dry. No changes are seen on her knees, elbows, or scalp.
Recommended Reading: Best of 2017
Recommended Reading lists are something of a tradition for ACS Surgery News. This feature has appeared several times over the years and it has always proved among the most popular items in the publication. But the project hinges on input from our Editorial Advisory Board, the members of which are already regularly called upon to help vet the publication’s content and give their advice. They have gone the extra mile and have once again chosen their “Best of 2017” studies in their own specialty areas, along with commentary on why their choices should be of interest to all surgeons. We hope our readers will find the list and the comments of interest.
General surgery
Cogbill TH et al. Rural general surgery: A 38-year experience with a regional network established by an integrated health system in the Midwestern United States. J Am Coll Surg. 2017;225(1):115-24.
This article is of particular interest because it provides details of an innovative, regional system of surgical care at the critical access hospitals and referral centers that cooperate seamlessly to improve quality of care and quality of practice for rural surgeons. It could serve as a model for similar independent hospitals and practices in a region to improve the practice lives of the surgeons in rural communities and preserve access to local care for rural patients.
Dimou FM et al. Outcomes in older patients with grade III cholecystitis and cholecystostomy tube placement: A propensity score analysis. J Am Coll Surg. 2017;224(4):502-14.This study is valuable because it sheds light on the current status of treatment of severe acute cholecystitis in the United States and reports outcomes of patients who get initial tube cholecystostomy. It demonstrates potential drawbacks of following the Tokyo Guidelines: fewer patients receiving definitive treatment (cholecystectomy) and higher mortality rates and readmissions.
Karen E. Deveney, MD, FACS
Palliative Care
Gani F et al. Palliative care utilization among patients admitted for gastrointestinal and thoracic cancers. J Palliat Med. 2017 Nov 3; doi: 10.1089/jpm.2017.0295; epub ahead of print.
Is this a matter of “too little too late”? This retrospective cross-sectional analysis of patients identified in the National Inpatient Sample database admitted with a primary diagnosis of gastrointestinal and/or thoracic cancer determined that only 8.5% of patients admitted received palliative care services. Surgical patients were 79% less likely to have received a palliative care consultation, and then only after a prolonged length of stay or postoperative complication. Is referral to palliative care services hindered by its stigmatization with these outcomes?
Taylor LJ et al. A framework to improve surgeon communication in high-stakes surgical decisions: Best Case/Worst Case. JAMA Surgery. 2017;152(6):531-8.
My chief used to say, “You might not be teachable, but you are trainable!” After surgeons received training in the Best Case/Worst Case framework described in this paper, they demonstrated that it was possible to successfully change the focus of decision-making conversations from an isolated surgical problem – with its menu of technical solutions – instead into a discussion about treatment alternatives and outcomes. This intervention is a useful tool for one of the most invasive procedures of all – an exploration of a patient’s preferences and values that is necessary for shared decision making within the acute setting.
Makhani SS et al. Cognitive impairment and overall survival in frail surgical patients. J Amer Coll Surg. 2017 Nov;225(5):590-600.
In my preoperative discussions with families of frail patients, it is often quite evident that the factor driving their decision is the cognitive state of the patient and the consequences of its further decline, even when they are willing to accept the risks of physical frailty. This study in a large multidisciplinary cohort of patients undergoing major operations determined that a combined frailty (Fried frailty score) and cognitive assessment score (Emory Clock Draw Test) has a more powerful potential to predict adult patients at higher risk of overall survival than does either measurement alone. Dual frailty and cognitive screening appears to be a promising adjunct to the shared decision-making process.
Geoffrey P. Dunn, MD, FACS
Wilson DG et al. Patterns of care in hospitalized vascular patients at end of life. JAMA Surg. 2017;152(2):183-90.
This thoughtful study and the excellent accompanying invited commentary by William Schecter, MD, FACS, address a major, difficult issue that faces all physicians as our patients become older and sicker and our ability to keep them alive expands: How do we speak honestly with patients about their prognosis and likely outcomes and honor their autonomy in decision making?
Karen E. Deveney, MD, FACS
Practice Management
Robinson JR et al. Complexity of medical decision making in care provided by surgeons through patient portals. Surg Res. 2017;214:93-101.
This article describes an analysis of the content of patient portal messages exchanged between surgical providers and patients. The study demonstrates that more than 90% of these exchanges involved the delivery of medical care, and more than two-thirds of the messages contained medical decision making, which might have generated charges if done in a face-to-face outpatient encounter. The articles argues that surgeons are providing substantial medical care to their patients through patient portal message exchanges and suggests that models for compensation of this type of online care should be developed.
Gretchen Purcell Jackson, MD, FACS
Vascular Surgery
Bennett KM et al. Carotid artery stenting is associated with a higher incidence of major adverse clinical events than carotid endarterectomy in female patients. J Vasc Surg. 2017 Sep;66(3):794-801.
This article uses the ACS NSQIP database to assess outcomes of women undergoing intervention for carotid stenosis in a real-world setting and finds that major adverse cardiac events in the first 30 days is higher for carotid artery stenting (12.2%), compared with carotid endarterectomy (5.2%). What we need to keep in mind is that the practice of any intervention for asymptomatic carotid stenosis is being reevaluated in the new CREST study, which will compare current best medical management with carotid stenting and carotid endarterectomy. The indications are likely to change for all, but because women had less relative risk reduction in the early studies, we can expect that the benefits for intervention for women will continue to be less than those for men, calling to question when we should truly intervene, and how best to do so.
Gargiulo M et al. Outcomes of endovascular aneurysm repair performed in abdominal aortic aneurysms with large infrarenal necks. J Vasc Surg. 2017 Oct;66(4);1065-72.
This study found that endovascular aneurysm repair, performed in patients with large necks (greater than 28 mm), was associated with further neck enlargement at 2 years, and a higher risk of proximal type I endoleak, with the need for reintervention. This is one of many recent studies, all with similar findings. The issue becomes how we can best address larger infrarenal necks, whether by use of fenestrated grafts, snorkels/chimneys with extension of the seal zone, aptus, or other technologies. The question of whether all grafts have equal impacts on these more dilated necks has still to be elucidated. Nonetheless, when we stretch the instructions for use, there is an increased likelihood for more interventions.
Zettervall SL et al. Renal complications after EVAR with suprarenal versus infrarenal fixation among all users and routine users. J Vasc Surg. 2017 Oct;66(4):1305.
This study found that endografts with suprarenal fixation were associated with a greater decline in renal function, compared with those with infrarenal fixation, as well as with a longer length of stay. The reasons for the renal function decline are not entirely clear, and there was a slight increase in contrast use for those with suprarenal fixation but were otherwise similar when comparing comorbidities. Clearly, assessment of any impact on long-term renal function is important, and may affect future choice of endografts.
Linda Harris, MD, FACS
Bariatric Surgery
Rosenthal RJ et al. Obesity in America. Surg Obes Relat Dis. 2017 Oct;13(10):1643-50.
Although much has been reported on the dramatic benefits of bariatric surgery, it remains a matter of deep disappointment that only 1%-2% of the eligible population is receiving this life-saving therapy. This is a paper that reports and analyzes the results of a national survey that was conducted on behalf of the American Society for Metabolic & Bariatric Surgery, in an attempt to identify barriers to access, public misconceptions on obesity and its consequences, and other pertinent factors. Survey results included the findings that, although 80% of Americans considered obesity as the most serious health risk problem, there was a clear overestimation of the effectiveness of diet and exercise alone. The importance of this paper lies in the persistent lack of recognition and/or awareness of proven, safe, and durable medical and surgical options in the lay population, highlighting the importance of aligning efforts and resources toward educating both the public and referring physicians.
Adams TD et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017 Sep 21;377(12):1143-55
This paper reports the results of an observational, prospective study that followed patients who received gastric bypass, in comparison with a group of patients who desired but did not receive gastric bypass, and a third group of obese patients who did not seek surgery. The authors concluded that gastric bypass provided durable, 12-year remission and prevention of such lethal diseases as diabetes, hypertension, and dyslipidemia. The importance of this study is in its detailed follow-up, the exceedingly high retention rate of 90% at 12 years, and the comparisons made between surgical and nonsurgical groups, demonstrating not only the benefits of gastric bypass, but as importantly, the hazards of not receiving this treatment.
Schauer PR et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med. 2017 Feb 16;376(7):641-51.This paper is the latest installment of the long-term results from the STAMPEDE trial conducted at the Cleveland Clinic. STAMPEDE is a randomized, controlled trial that compared the best, most “intensive” medical therapy for type 2 diabetes vs. bariatric surgery (comprising a mix of gastric bypass and sleeve gastrectomy). Prior publications from this group reported 1- and 3-year results, and this paper reported the 5-year results, demonstrating the persistent superiority of bariatric surgery over the most rigorous intensive medical therapy in the resolution or improvement of hyperglycemia in patients with BMI ranges of 27 kg/m2 to 43 kg/m2. Of further significance was the fact that there were no major late surgical complications except for one reoperation.
Samer Mattar, MD, FACS
Colon & Rectal Surgery
Jayne D et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The ROLARR randomized clinical trial. JAMA. 2017;318(16):1569-80.
This trial of 471 rectal cancer patients demonstrated similar conversion rates for robotic (8.1%) and laparoscopic (12.2%) surgery. Of the other secondary end points, including intraoperative complications, postoperative complications, plane of surgery, 30-day mortality, bladder dysfunction, and sexual dysfunction, none showed a statistically significant difference between groups.
Marshall JR et al. Laparoscopic lavage in the management of Hinchey grade III diverticulitis: A systematic review. Ann Surg. 2017;265(4):670-6.While there have been a number of groups using laparoscopic lavage in the setting of acute diverticulitis, including Hinchey grade III disease, several recent studies question this approach. This meta-analysis includes three recent randomized, controlled trials and analysis of 48 studies – demonstrating that rates of reintervention within 30 days to be 28.3% in the lavage group and 8.8% in the resection group. Other outcomes – including ICU admissions, 30- and 90-day mortality, or stoma rates at 12 months – were similar between groups.
Denost Q et al. To drain or not to drain infraperitoneal anastomosis after rectal excision for cancer: The GRECCAR 5 randomized trial. Ann Surg. 2017;365(3):474-80.
While many studies have confirmed infectiveness of drainage after colectomy, there is still some controversy of the role of pelvic drainage after rectal surgery. A multicenter randomized, controlled trial with two parallel arms (drain vs. no drain) was conducted in 469 patients after rectal surgery for cancer. Primary endpoint was postoperative pelvic sepsis within 30 postoperative days, including anastomotic leakage, pelvic abscess, and peritonitis. Rates of pelvic sepsis were similar between drain and no drain: 16.1% vs. 18.0% (P = .58), and there was no difference in surgical morbidity, rate of reoperation, length of hospital stay, and rate of stoma closure between groups. Overall, this trial suggests that the use of a pelvic drain after rectal excision for rectal cancer did not confer any benefit.
Genevieve Melton-Meaux, MD, FACS
Breast Surgery
Giuliano AE et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 randomized clinical trial. JAMA. 2017 Sep 12;318(10):918-926.
Long-term outcomes from the practice-changing ACOSOG Z0011 (Alliance) trial confirming the safety of omitting completion axillary lymph node dissection in women with T1/T2 tumors treated by lumpectomy and whole-breast radiation when metastatic disease is identified in one or two sentinel nodes.
Masuda N et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017 Jun 1;376(22):2147-59.
Breast cancer patients that achieve a complete pathologic response after receiving neoadjuvant chemotherapy have a survival advantage, and patients found to have residual disease represent a higher-risk population subset. This prospective randomized clinical trial (known as the CREATE-X study) revealed that adjuvant capecitabine can significantly mitigate this risk, especially for patients with triple-negative breast cancer.
Curigliano G et al. De-escalating and escalating treatments for early stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017 Aug 1;28(8):1700-12.
This summary of the 2017 St. Gallen Conference proceedings provides a comprehensive yet concise review of contemporary standards of care in managing early stage breast cancer. Issues reviewed include lumpectomy margins, extent of breast/axillary surgery following neoadjuvant chemotherapy, options for breast radiation schedules following lumpectomy, and application of currently available gene expression profiles.
Troester MA et al. Racial differences in PAM50 subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 2018 Feb 1;110(2);doi: 10.1093/jnci/djx135; epub ahead of print (Aug 2017).
Breast cancer outcome disparities related to racial/ethnic identity are well documented, with African American patients experiencing higher mortality rates, compared with White Americans. This disparity is partly explained by differences in tumor biology, since triple-negative breast cancer (TNBC) is twice as common in African American patients. Troester et al. conducted RNA expression-based PAM-50 tumor subtyping to demonstrate significantly higher rates of biologically aggressive tumor subtypes among African Americans breast cancer patients, compared with white Americans.
Lisa Newman, MD, FACS
Foregut
Teitelbaum EN et al. Clinical outcomes five years after POEM for treatment of primary esophageal motility disorders. Surg Endosc. 2017 Jun 29. doi: 10.1007/s00464-017-5699-2 ; epub ahead of print.
This provides the longest follow-up to date regarding clinical efficacy of peroral endoscopic myotomy (POEM) in the United States. Although not a panacea, POEM appears to provide substantial durable clinical improvement in patients suffering from esophageal motility disorders.
Kevin Reavis, MD, FACS
Yufei Chen et al. Primary lymph node gastrinoma: A single institution experience. Surgery 2017 Nov;162(5):1088-94
This article retrospectively review a rare neuroendocrine (gastrinoma) tumor over a 25-year period at a single institution, noting all demographics and outcomes. Great update and refresher. The article then went farther, evaluating an even rarer occurrence of a primary lymph node gastrinoma within this patient population and followed those patients outcomes as well. Two “values” for the “price of one.”
Haisley KR et al. Twenty-year trends in the utilization of Heller myotomy for achalasia in the United States. Am J Surg. 2017 Aug;214(2):299-302.
Gary Timmerman, MD, FACS
Recommended Reading lists are something of a tradition for ACS Surgery News. This feature has appeared several times over the years and it has always proved among the most popular items in the publication. But the project hinges on input from our Editorial Advisory Board, the members of which are already regularly called upon to help vet the publication’s content and give their advice. They have gone the extra mile and have once again chosen their “Best of 2017” studies in their own specialty areas, along with commentary on why their choices should be of interest to all surgeons. We hope our readers will find the list and the comments of interest.
General surgery
Cogbill TH et al. Rural general surgery: A 38-year experience with a regional network established by an integrated health system in the Midwestern United States. J Am Coll Surg. 2017;225(1):115-24.
This article is of particular interest because it provides details of an innovative, regional system of surgical care at the critical access hospitals and referral centers that cooperate seamlessly to improve quality of care and quality of practice for rural surgeons. It could serve as a model for similar independent hospitals and practices in a region to improve the practice lives of the surgeons in rural communities and preserve access to local care for rural patients.
Dimou FM et al. Outcomes in older patients with grade III cholecystitis and cholecystostomy tube placement: A propensity score analysis. J Am Coll Surg. 2017;224(4):502-14.This study is valuable because it sheds light on the current status of treatment of severe acute cholecystitis in the United States and reports outcomes of patients who get initial tube cholecystostomy. It demonstrates potential drawbacks of following the Tokyo Guidelines: fewer patients receiving definitive treatment (cholecystectomy) and higher mortality rates and readmissions.
Karen E. Deveney, MD, FACS
Palliative Care
Gani F et al. Palliative care utilization among patients admitted for gastrointestinal and thoracic cancers. J Palliat Med. 2017 Nov 3; doi: 10.1089/jpm.2017.0295; epub ahead of print.
Is this a matter of “too little too late”? This retrospective cross-sectional analysis of patients identified in the National Inpatient Sample database admitted with a primary diagnosis of gastrointestinal and/or thoracic cancer determined that only 8.5% of patients admitted received palliative care services. Surgical patients were 79% less likely to have received a palliative care consultation, and then only after a prolonged length of stay or postoperative complication. Is referral to palliative care services hindered by its stigmatization with these outcomes?
Taylor LJ et al. A framework to improve surgeon communication in high-stakes surgical decisions: Best Case/Worst Case. JAMA Surgery. 2017;152(6):531-8.
My chief used to say, “You might not be teachable, but you are trainable!” After surgeons received training in the Best Case/Worst Case framework described in this paper, they demonstrated that it was possible to successfully change the focus of decision-making conversations from an isolated surgical problem – with its menu of technical solutions – instead into a discussion about treatment alternatives and outcomes. This intervention is a useful tool for one of the most invasive procedures of all – an exploration of a patient’s preferences and values that is necessary for shared decision making within the acute setting.
Makhani SS et al. Cognitive impairment and overall survival in frail surgical patients. J Amer Coll Surg. 2017 Nov;225(5):590-600.
In my preoperative discussions with families of frail patients, it is often quite evident that the factor driving their decision is the cognitive state of the patient and the consequences of its further decline, even when they are willing to accept the risks of physical frailty. This study in a large multidisciplinary cohort of patients undergoing major operations determined that a combined frailty (Fried frailty score) and cognitive assessment score (Emory Clock Draw Test) has a more powerful potential to predict adult patients at higher risk of overall survival than does either measurement alone. Dual frailty and cognitive screening appears to be a promising adjunct to the shared decision-making process.
Geoffrey P. Dunn, MD, FACS
Wilson DG et al. Patterns of care in hospitalized vascular patients at end of life. JAMA Surg. 2017;152(2):183-90.
This thoughtful study and the excellent accompanying invited commentary by William Schecter, MD, FACS, address a major, difficult issue that faces all physicians as our patients become older and sicker and our ability to keep them alive expands: How do we speak honestly with patients about their prognosis and likely outcomes and honor their autonomy in decision making?
Karen E. Deveney, MD, FACS
Practice Management
Robinson JR et al. Complexity of medical decision making in care provided by surgeons through patient portals. Surg Res. 2017;214:93-101.
This article describes an analysis of the content of patient portal messages exchanged between surgical providers and patients. The study demonstrates that more than 90% of these exchanges involved the delivery of medical care, and more than two-thirds of the messages contained medical decision making, which might have generated charges if done in a face-to-face outpatient encounter. The articles argues that surgeons are providing substantial medical care to their patients through patient portal message exchanges and suggests that models for compensation of this type of online care should be developed.
Gretchen Purcell Jackson, MD, FACS
Vascular Surgery
Bennett KM et al. Carotid artery stenting is associated with a higher incidence of major adverse clinical events than carotid endarterectomy in female patients. J Vasc Surg. 2017 Sep;66(3):794-801.
This article uses the ACS NSQIP database to assess outcomes of women undergoing intervention for carotid stenosis in a real-world setting and finds that major adverse cardiac events in the first 30 days is higher for carotid artery stenting (12.2%), compared with carotid endarterectomy (5.2%). What we need to keep in mind is that the practice of any intervention for asymptomatic carotid stenosis is being reevaluated in the new CREST study, which will compare current best medical management with carotid stenting and carotid endarterectomy. The indications are likely to change for all, but because women had less relative risk reduction in the early studies, we can expect that the benefits for intervention for women will continue to be less than those for men, calling to question when we should truly intervene, and how best to do so.
Gargiulo M et al. Outcomes of endovascular aneurysm repair performed in abdominal aortic aneurysms with large infrarenal necks. J Vasc Surg. 2017 Oct;66(4);1065-72.
This study found that endovascular aneurysm repair, performed in patients with large necks (greater than 28 mm), was associated with further neck enlargement at 2 years, and a higher risk of proximal type I endoleak, with the need for reintervention. This is one of many recent studies, all with similar findings. The issue becomes how we can best address larger infrarenal necks, whether by use of fenestrated grafts, snorkels/chimneys with extension of the seal zone, aptus, or other technologies. The question of whether all grafts have equal impacts on these more dilated necks has still to be elucidated. Nonetheless, when we stretch the instructions for use, there is an increased likelihood for more interventions.
Zettervall SL et al. Renal complications after EVAR with suprarenal versus infrarenal fixation among all users and routine users. J Vasc Surg. 2017 Oct;66(4):1305.
This study found that endografts with suprarenal fixation were associated with a greater decline in renal function, compared with those with infrarenal fixation, as well as with a longer length of stay. The reasons for the renal function decline are not entirely clear, and there was a slight increase in contrast use for those with suprarenal fixation but were otherwise similar when comparing comorbidities. Clearly, assessment of any impact on long-term renal function is important, and may affect future choice of endografts.
Linda Harris, MD, FACS
Bariatric Surgery
Rosenthal RJ et al. Obesity in America. Surg Obes Relat Dis. 2017 Oct;13(10):1643-50.
Although much has been reported on the dramatic benefits of bariatric surgery, it remains a matter of deep disappointment that only 1%-2% of the eligible population is receiving this life-saving therapy. This is a paper that reports and analyzes the results of a national survey that was conducted on behalf of the American Society for Metabolic & Bariatric Surgery, in an attempt to identify barriers to access, public misconceptions on obesity and its consequences, and other pertinent factors. Survey results included the findings that, although 80% of Americans considered obesity as the most serious health risk problem, there was a clear overestimation of the effectiveness of diet and exercise alone. The importance of this paper lies in the persistent lack of recognition and/or awareness of proven, safe, and durable medical and surgical options in the lay population, highlighting the importance of aligning efforts and resources toward educating both the public and referring physicians.
Adams TD et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017 Sep 21;377(12):1143-55
This paper reports the results of an observational, prospective study that followed patients who received gastric bypass, in comparison with a group of patients who desired but did not receive gastric bypass, and a third group of obese patients who did not seek surgery. The authors concluded that gastric bypass provided durable, 12-year remission and prevention of such lethal diseases as diabetes, hypertension, and dyslipidemia. The importance of this study is in its detailed follow-up, the exceedingly high retention rate of 90% at 12 years, and the comparisons made between surgical and nonsurgical groups, demonstrating not only the benefits of gastric bypass, but as importantly, the hazards of not receiving this treatment.
Schauer PR et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med. 2017 Feb 16;376(7):641-51.This paper is the latest installment of the long-term results from the STAMPEDE trial conducted at the Cleveland Clinic. STAMPEDE is a randomized, controlled trial that compared the best, most “intensive” medical therapy for type 2 diabetes vs. bariatric surgery (comprising a mix of gastric bypass and sleeve gastrectomy). Prior publications from this group reported 1- and 3-year results, and this paper reported the 5-year results, demonstrating the persistent superiority of bariatric surgery over the most rigorous intensive medical therapy in the resolution or improvement of hyperglycemia in patients with BMI ranges of 27 kg/m2 to 43 kg/m2. Of further significance was the fact that there were no major late surgical complications except for one reoperation.
Samer Mattar, MD, FACS
Colon & Rectal Surgery
Jayne D et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The ROLARR randomized clinical trial. JAMA. 2017;318(16):1569-80.
This trial of 471 rectal cancer patients demonstrated similar conversion rates for robotic (8.1%) and laparoscopic (12.2%) surgery. Of the other secondary end points, including intraoperative complications, postoperative complications, plane of surgery, 30-day mortality, bladder dysfunction, and sexual dysfunction, none showed a statistically significant difference between groups.
Marshall JR et al. Laparoscopic lavage in the management of Hinchey grade III diverticulitis: A systematic review. Ann Surg. 2017;265(4):670-6.While there have been a number of groups using laparoscopic lavage in the setting of acute diverticulitis, including Hinchey grade III disease, several recent studies question this approach. This meta-analysis includes three recent randomized, controlled trials and analysis of 48 studies – demonstrating that rates of reintervention within 30 days to be 28.3% in the lavage group and 8.8% in the resection group. Other outcomes – including ICU admissions, 30- and 90-day mortality, or stoma rates at 12 months – were similar between groups.
Denost Q et al. To drain or not to drain infraperitoneal anastomosis after rectal excision for cancer: The GRECCAR 5 randomized trial. Ann Surg. 2017;365(3):474-80.
While many studies have confirmed infectiveness of drainage after colectomy, there is still some controversy of the role of pelvic drainage after rectal surgery. A multicenter randomized, controlled trial with two parallel arms (drain vs. no drain) was conducted in 469 patients after rectal surgery for cancer. Primary endpoint was postoperative pelvic sepsis within 30 postoperative days, including anastomotic leakage, pelvic abscess, and peritonitis. Rates of pelvic sepsis were similar between drain and no drain: 16.1% vs. 18.0% (P = .58), and there was no difference in surgical morbidity, rate of reoperation, length of hospital stay, and rate of stoma closure between groups. Overall, this trial suggests that the use of a pelvic drain after rectal excision for rectal cancer did not confer any benefit.
Genevieve Melton-Meaux, MD, FACS
Breast Surgery
Giuliano AE et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 randomized clinical trial. JAMA. 2017 Sep 12;318(10):918-926.
Long-term outcomes from the practice-changing ACOSOG Z0011 (Alliance) trial confirming the safety of omitting completion axillary lymph node dissection in women with T1/T2 tumors treated by lumpectomy and whole-breast radiation when metastatic disease is identified in one or two sentinel nodes.
Masuda N et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017 Jun 1;376(22):2147-59.
Breast cancer patients that achieve a complete pathologic response after receiving neoadjuvant chemotherapy have a survival advantage, and patients found to have residual disease represent a higher-risk population subset. This prospective randomized clinical trial (known as the CREATE-X study) revealed that adjuvant capecitabine can significantly mitigate this risk, especially for patients with triple-negative breast cancer.
Curigliano G et al. De-escalating and escalating treatments for early stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017 Aug 1;28(8):1700-12.
This summary of the 2017 St. Gallen Conference proceedings provides a comprehensive yet concise review of contemporary standards of care in managing early stage breast cancer. Issues reviewed include lumpectomy margins, extent of breast/axillary surgery following neoadjuvant chemotherapy, options for breast radiation schedules following lumpectomy, and application of currently available gene expression profiles.
Troester MA et al. Racial differences in PAM50 subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 2018 Feb 1;110(2);doi: 10.1093/jnci/djx135; epub ahead of print (Aug 2017).
Breast cancer outcome disparities related to racial/ethnic identity are well documented, with African American patients experiencing higher mortality rates, compared with White Americans. This disparity is partly explained by differences in tumor biology, since triple-negative breast cancer (TNBC) is twice as common in African American patients. Troester et al. conducted RNA expression-based PAM-50 tumor subtyping to demonstrate significantly higher rates of biologically aggressive tumor subtypes among African Americans breast cancer patients, compared with white Americans.
Lisa Newman, MD, FACS
Foregut
Teitelbaum EN et al. Clinical outcomes five years after POEM for treatment of primary esophageal motility disorders. Surg Endosc. 2017 Jun 29. doi: 10.1007/s00464-017-5699-2 ; epub ahead of print.
This provides the longest follow-up to date regarding clinical efficacy of peroral endoscopic myotomy (POEM) in the United States. Although not a panacea, POEM appears to provide substantial durable clinical improvement in patients suffering from esophageal motility disorders.
Kevin Reavis, MD, FACS
Yufei Chen et al. Primary lymph node gastrinoma: A single institution experience. Surgery 2017 Nov;162(5):1088-94
This article retrospectively review a rare neuroendocrine (gastrinoma) tumor over a 25-year period at a single institution, noting all demographics and outcomes. Great update and refresher. The article then went farther, evaluating an even rarer occurrence of a primary lymph node gastrinoma within this patient population and followed those patients outcomes as well. Two “values” for the “price of one.”
Haisley KR et al. Twenty-year trends in the utilization of Heller myotomy for achalasia in the United States. Am J Surg. 2017 Aug;214(2):299-302.
Gary Timmerman, MD, FACS
Recommended Reading lists are something of a tradition for ACS Surgery News. This feature has appeared several times over the years and it has always proved among the most popular items in the publication. But the project hinges on input from our Editorial Advisory Board, the members of which are already regularly called upon to help vet the publication’s content and give their advice. They have gone the extra mile and have once again chosen their “Best of 2017” studies in their own specialty areas, along with commentary on why their choices should be of interest to all surgeons. We hope our readers will find the list and the comments of interest.
General surgery
Cogbill TH et al. Rural general surgery: A 38-year experience with a regional network established by an integrated health system in the Midwestern United States. J Am Coll Surg. 2017;225(1):115-24.
This article is of particular interest because it provides details of an innovative, regional system of surgical care at the critical access hospitals and referral centers that cooperate seamlessly to improve quality of care and quality of practice for rural surgeons. It could serve as a model for similar independent hospitals and practices in a region to improve the practice lives of the surgeons in rural communities and preserve access to local care for rural patients.
Dimou FM et al. Outcomes in older patients with grade III cholecystitis and cholecystostomy tube placement: A propensity score analysis. J Am Coll Surg. 2017;224(4):502-14.This study is valuable because it sheds light on the current status of treatment of severe acute cholecystitis in the United States and reports outcomes of patients who get initial tube cholecystostomy. It demonstrates potential drawbacks of following the Tokyo Guidelines: fewer patients receiving definitive treatment (cholecystectomy) and higher mortality rates and readmissions.
Karen E. Deveney, MD, FACS
Palliative Care
Gani F et al. Palliative care utilization among patients admitted for gastrointestinal and thoracic cancers. J Palliat Med. 2017 Nov 3; doi: 10.1089/jpm.2017.0295; epub ahead of print.
Is this a matter of “too little too late”? This retrospective cross-sectional analysis of patients identified in the National Inpatient Sample database admitted with a primary diagnosis of gastrointestinal and/or thoracic cancer determined that only 8.5% of patients admitted received palliative care services. Surgical patients were 79% less likely to have received a palliative care consultation, and then only after a prolonged length of stay or postoperative complication. Is referral to palliative care services hindered by its stigmatization with these outcomes?
Taylor LJ et al. A framework to improve surgeon communication in high-stakes surgical decisions: Best Case/Worst Case. JAMA Surgery. 2017;152(6):531-8.
My chief used to say, “You might not be teachable, but you are trainable!” After surgeons received training in the Best Case/Worst Case framework described in this paper, they demonstrated that it was possible to successfully change the focus of decision-making conversations from an isolated surgical problem – with its menu of technical solutions – instead into a discussion about treatment alternatives and outcomes. This intervention is a useful tool for one of the most invasive procedures of all – an exploration of a patient’s preferences and values that is necessary for shared decision making within the acute setting.
Makhani SS et al. Cognitive impairment and overall survival in frail surgical patients. J Amer Coll Surg. 2017 Nov;225(5):590-600.
In my preoperative discussions with families of frail patients, it is often quite evident that the factor driving their decision is the cognitive state of the patient and the consequences of its further decline, even when they are willing to accept the risks of physical frailty. This study in a large multidisciplinary cohort of patients undergoing major operations determined that a combined frailty (Fried frailty score) and cognitive assessment score (Emory Clock Draw Test) has a more powerful potential to predict adult patients at higher risk of overall survival than does either measurement alone. Dual frailty and cognitive screening appears to be a promising adjunct to the shared decision-making process.
Geoffrey P. Dunn, MD, FACS
Wilson DG et al. Patterns of care in hospitalized vascular patients at end of life. JAMA Surg. 2017;152(2):183-90.
This thoughtful study and the excellent accompanying invited commentary by William Schecter, MD, FACS, address a major, difficult issue that faces all physicians as our patients become older and sicker and our ability to keep them alive expands: How do we speak honestly with patients about their prognosis and likely outcomes and honor their autonomy in decision making?
Karen E. Deveney, MD, FACS
Practice Management
Robinson JR et al. Complexity of medical decision making in care provided by surgeons through patient portals. Surg Res. 2017;214:93-101.
This article describes an analysis of the content of patient portal messages exchanged between surgical providers and patients. The study demonstrates that more than 90% of these exchanges involved the delivery of medical care, and more than two-thirds of the messages contained medical decision making, which might have generated charges if done in a face-to-face outpatient encounter. The articles argues that surgeons are providing substantial medical care to their patients through patient portal message exchanges and suggests that models for compensation of this type of online care should be developed.
Gretchen Purcell Jackson, MD, FACS
Vascular Surgery
Bennett KM et al. Carotid artery stenting is associated with a higher incidence of major adverse clinical events than carotid endarterectomy in female patients. J Vasc Surg. 2017 Sep;66(3):794-801.
This article uses the ACS NSQIP database to assess outcomes of women undergoing intervention for carotid stenosis in a real-world setting and finds that major adverse cardiac events in the first 30 days is higher for carotid artery stenting (12.2%), compared with carotid endarterectomy (5.2%). What we need to keep in mind is that the practice of any intervention for asymptomatic carotid stenosis is being reevaluated in the new CREST study, which will compare current best medical management with carotid stenting and carotid endarterectomy. The indications are likely to change for all, but because women had less relative risk reduction in the early studies, we can expect that the benefits for intervention for women will continue to be less than those for men, calling to question when we should truly intervene, and how best to do so.
Gargiulo M et al. Outcomes of endovascular aneurysm repair performed in abdominal aortic aneurysms with large infrarenal necks. J Vasc Surg. 2017 Oct;66(4);1065-72.
This study found that endovascular aneurysm repair, performed in patients with large necks (greater than 28 mm), was associated with further neck enlargement at 2 years, and a higher risk of proximal type I endoleak, with the need for reintervention. This is one of many recent studies, all with similar findings. The issue becomes how we can best address larger infrarenal necks, whether by use of fenestrated grafts, snorkels/chimneys with extension of the seal zone, aptus, or other technologies. The question of whether all grafts have equal impacts on these more dilated necks has still to be elucidated. Nonetheless, when we stretch the instructions for use, there is an increased likelihood for more interventions.
Zettervall SL et al. Renal complications after EVAR with suprarenal versus infrarenal fixation among all users and routine users. J Vasc Surg. 2017 Oct;66(4):1305.
This study found that endografts with suprarenal fixation were associated with a greater decline in renal function, compared with those with infrarenal fixation, as well as with a longer length of stay. The reasons for the renal function decline are not entirely clear, and there was a slight increase in contrast use for those with suprarenal fixation but were otherwise similar when comparing comorbidities. Clearly, assessment of any impact on long-term renal function is important, and may affect future choice of endografts.
Linda Harris, MD, FACS
Bariatric Surgery
Rosenthal RJ et al. Obesity in America. Surg Obes Relat Dis. 2017 Oct;13(10):1643-50.
Although much has been reported on the dramatic benefits of bariatric surgery, it remains a matter of deep disappointment that only 1%-2% of the eligible population is receiving this life-saving therapy. This is a paper that reports and analyzes the results of a national survey that was conducted on behalf of the American Society for Metabolic & Bariatric Surgery, in an attempt to identify barriers to access, public misconceptions on obesity and its consequences, and other pertinent factors. Survey results included the findings that, although 80% of Americans considered obesity as the most serious health risk problem, there was a clear overestimation of the effectiveness of diet and exercise alone. The importance of this paper lies in the persistent lack of recognition and/or awareness of proven, safe, and durable medical and surgical options in the lay population, highlighting the importance of aligning efforts and resources toward educating both the public and referring physicians.
Adams TD et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017 Sep 21;377(12):1143-55
This paper reports the results of an observational, prospective study that followed patients who received gastric bypass, in comparison with a group of patients who desired but did not receive gastric bypass, and a third group of obese patients who did not seek surgery. The authors concluded that gastric bypass provided durable, 12-year remission and prevention of such lethal diseases as diabetes, hypertension, and dyslipidemia. The importance of this study is in its detailed follow-up, the exceedingly high retention rate of 90% at 12 years, and the comparisons made between surgical and nonsurgical groups, demonstrating not only the benefits of gastric bypass, but as importantly, the hazards of not receiving this treatment.
Schauer PR et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med. 2017 Feb 16;376(7):641-51.This paper is the latest installment of the long-term results from the STAMPEDE trial conducted at the Cleveland Clinic. STAMPEDE is a randomized, controlled trial that compared the best, most “intensive” medical therapy for type 2 diabetes vs. bariatric surgery (comprising a mix of gastric bypass and sleeve gastrectomy). Prior publications from this group reported 1- and 3-year results, and this paper reported the 5-year results, demonstrating the persistent superiority of bariatric surgery over the most rigorous intensive medical therapy in the resolution or improvement of hyperglycemia in patients with BMI ranges of 27 kg/m2 to 43 kg/m2. Of further significance was the fact that there were no major late surgical complications except for one reoperation.
Samer Mattar, MD, FACS
Colon & Rectal Surgery
Jayne D et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The ROLARR randomized clinical trial. JAMA. 2017;318(16):1569-80.
This trial of 471 rectal cancer patients demonstrated similar conversion rates for robotic (8.1%) and laparoscopic (12.2%) surgery. Of the other secondary end points, including intraoperative complications, postoperative complications, plane of surgery, 30-day mortality, bladder dysfunction, and sexual dysfunction, none showed a statistically significant difference between groups.
Marshall JR et al. Laparoscopic lavage in the management of Hinchey grade III diverticulitis: A systematic review. Ann Surg. 2017;265(4):670-6.While there have been a number of groups using laparoscopic lavage in the setting of acute diverticulitis, including Hinchey grade III disease, several recent studies question this approach. This meta-analysis includes three recent randomized, controlled trials and analysis of 48 studies – demonstrating that rates of reintervention within 30 days to be 28.3% in the lavage group and 8.8% in the resection group. Other outcomes – including ICU admissions, 30- and 90-day mortality, or stoma rates at 12 months – were similar between groups.
Denost Q et al. To drain or not to drain infraperitoneal anastomosis after rectal excision for cancer: The GRECCAR 5 randomized trial. Ann Surg. 2017;365(3):474-80.
While many studies have confirmed infectiveness of drainage after colectomy, there is still some controversy of the role of pelvic drainage after rectal surgery. A multicenter randomized, controlled trial with two parallel arms (drain vs. no drain) was conducted in 469 patients after rectal surgery for cancer. Primary endpoint was postoperative pelvic sepsis within 30 postoperative days, including anastomotic leakage, pelvic abscess, and peritonitis. Rates of pelvic sepsis were similar between drain and no drain: 16.1% vs. 18.0% (P = .58), and there was no difference in surgical morbidity, rate of reoperation, length of hospital stay, and rate of stoma closure between groups. Overall, this trial suggests that the use of a pelvic drain after rectal excision for rectal cancer did not confer any benefit.
Genevieve Melton-Meaux, MD, FACS
Breast Surgery
Giuliano AE et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 randomized clinical trial. JAMA. 2017 Sep 12;318(10):918-926.
Long-term outcomes from the practice-changing ACOSOG Z0011 (Alliance) trial confirming the safety of omitting completion axillary lymph node dissection in women with T1/T2 tumors treated by lumpectomy and whole-breast radiation when metastatic disease is identified in one or two sentinel nodes.
Masuda N et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017 Jun 1;376(22):2147-59.
Breast cancer patients that achieve a complete pathologic response after receiving neoadjuvant chemotherapy have a survival advantage, and patients found to have residual disease represent a higher-risk population subset. This prospective randomized clinical trial (known as the CREATE-X study) revealed that adjuvant capecitabine can significantly mitigate this risk, especially for patients with triple-negative breast cancer.
Curigliano G et al. De-escalating and escalating treatments for early stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017 Aug 1;28(8):1700-12.
This summary of the 2017 St. Gallen Conference proceedings provides a comprehensive yet concise review of contemporary standards of care in managing early stage breast cancer. Issues reviewed include lumpectomy margins, extent of breast/axillary surgery following neoadjuvant chemotherapy, options for breast radiation schedules following lumpectomy, and application of currently available gene expression profiles.
Troester MA et al. Racial differences in PAM50 subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 2018 Feb 1;110(2);doi: 10.1093/jnci/djx135; epub ahead of print (Aug 2017).
Breast cancer outcome disparities related to racial/ethnic identity are well documented, with African American patients experiencing higher mortality rates, compared with White Americans. This disparity is partly explained by differences in tumor biology, since triple-negative breast cancer (TNBC) is twice as common in African American patients. Troester et al. conducted RNA expression-based PAM-50 tumor subtyping to demonstrate significantly higher rates of biologically aggressive tumor subtypes among African Americans breast cancer patients, compared with white Americans.
Lisa Newman, MD, FACS
Foregut
Teitelbaum EN et al. Clinical outcomes five years after POEM for treatment of primary esophageal motility disorders. Surg Endosc. 2017 Jun 29. doi: 10.1007/s00464-017-5699-2 ; epub ahead of print.
This provides the longest follow-up to date regarding clinical efficacy of peroral endoscopic myotomy (POEM) in the United States. Although not a panacea, POEM appears to provide substantial durable clinical improvement in patients suffering from esophageal motility disorders.
Kevin Reavis, MD, FACS
Yufei Chen et al. Primary lymph node gastrinoma: A single institution experience. Surgery 2017 Nov;162(5):1088-94
This article retrospectively review a rare neuroendocrine (gastrinoma) tumor over a 25-year period at a single institution, noting all demographics and outcomes. Great update and refresher. The article then went farther, evaluating an even rarer occurrence of a primary lymph node gastrinoma within this patient population and followed those patients outcomes as well. Two “values” for the “price of one.”
Haisley KR et al. Twenty-year trends in the utilization of Heller myotomy for achalasia in the United States. Am J Surg. 2017 Aug;214(2):299-302.
Gary Timmerman, MD, FACS
Testing for latent tuberculosis infection
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
A broadly effective meningitis B vaccine has been proved effective in a Danish study
MenB-FHbp, a licensed meningococcal B vaccine that targets factor H-binding protein, a surface-exposed bacterial lipoprotein, has been shown to provide broad immunological protection against 4 primary and 10 additional strains of meningococcal B disease in teens and young adults, suggesting the potential to reduce the number of shots needed to protect this population, according to two phase 3 randomized, controlled, observer-blinded, multicenter trials.
After the third dose of MenB-FHbp, the percentage of patients whose antibody titers of serum bactericidal assays with human complement (hSBA) had increased by a factor of four rose as high as 78.8%-90.2% in adolescents and 78.9%-89.7% in young adults in the modified intention-to-treat population, depending on which strain was tested. This population was made up of randomized patients who had at least one valid and determinate assay. Among participants, 82% of adolescents and 84.5% of young adults had the composite response, which was defined as the proportion of patients who had an hSBA titer response that reached or exceeded the limit of quantitation for all primary test strains combined at 1 month after the third MenB-FHbp vaccine dose was administered.
Using the hSBA responses to the primary strains, a positive predictive value analysis predicted the immune responses to other meningitis strains with H-binding protein. For subfamily A strains among adolescents, the positive predictive values for patient responses were 64.4%-100% after dose 2 and 75.6%-99.6% after dose 3. Among young adults, the positive predictive values after dose 2 and 3 were 61.6%-100% and 72.2%-100%, respectively.
A similar pattern was observed for subfamily B strains.* Among adolescents the positive predictive values for patient responses were 78.9%-100% and 86.4%-99.6% after doses 2 and 3, respectively. Young adults also had high positive predictive values after doses 2 and 3, with values of 70%-100% and 80.5%-98.8%, respectively.
Injection site reactions and systemic events were both observed in response to MenB-FHbp in both adolescents and young adults. The most common injection site reaction was pain, which often occurred occurring after the first dose. Six adolescents and three young adults, one of whom received a saline injection, withdrew from the study because of injection site reactions. The most common systemic events among adolescents were headaches and fatigue. The events that caused patients to withdraw were associated with fever, chills, and mild arthralgia and moder myalgia.
“By demonstrating with positive–predictive value analyses the ability of 4 primary test strains to predict coverage with the use of 10 additional test strains, our findings provide assurance that observed immune responses to the primary strains are representative and indicative of vaccine responses to diverse disease-causing meningococcal B strains,” wrote Lars Ostergaard, MD, of the Aarhus (Denmark) University Hospital and his colleagues.
The studies were supported by Pfizer, and all researchers reported having received various forms of support from that company. Although many of the researchers hold patents, or have patents pending, that are relevant to these studies, they had no other.
SOURCE: Ostergaard L et al. N Engl J Med. 2017 Dec 14;377(24):2350-62.
Correction 12/14/17: An earlier version of this story misstated the findings concerning this subfamily.
MenB-FHbp, a licensed meningococcal B vaccine that targets factor H-binding protein, a surface-exposed bacterial lipoprotein, has been shown to provide broad immunological protection against 4 primary and 10 additional strains of meningococcal B disease in teens and young adults, suggesting the potential to reduce the number of shots needed to protect this population, according to two phase 3 randomized, controlled, observer-blinded, multicenter trials.
After the third dose of MenB-FHbp, the percentage of patients whose antibody titers of serum bactericidal assays with human complement (hSBA) had increased by a factor of four rose as high as 78.8%-90.2% in adolescents and 78.9%-89.7% in young adults in the modified intention-to-treat population, depending on which strain was tested. This population was made up of randomized patients who had at least one valid and determinate assay. Among participants, 82% of adolescents and 84.5% of young adults had the composite response, which was defined as the proportion of patients who had an hSBA titer response that reached or exceeded the limit of quantitation for all primary test strains combined at 1 month after the third MenB-FHbp vaccine dose was administered.
Using the hSBA responses to the primary strains, a positive predictive value analysis predicted the immune responses to other meningitis strains with H-binding protein. For subfamily A strains among adolescents, the positive predictive values for patient responses were 64.4%-100% after dose 2 and 75.6%-99.6% after dose 3. Among young adults, the positive predictive values after dose 2 and 3 were 61.6%-100% and 72.2%-100%, respectively.
A similar pattern was observed for subfamily B strains.* Among adolescents the positive predictive values for patient responses were 78.9%-100% and 86.4%-99.6% after doses 2 and 3, respectively. Young adults also had high positive predictive values after doses 2 and 3, with values of 70%-100% and 80.5%-98.8%, respectively.
Injection site reactions and systemic events were both observed in response to MenB-FHbp in both adolescents and young adults. The most common injection site reaction was pain, which often occurred occurring after the first dose. Six adolescents and three young adults, one of whom received a saline injection, withdrew from the study because of injection site reactions. The most common systemic events among adolescents were headaches and fatigue. The events that caused patients to withdraw were associated with fever, chills, and mild arthralgia and moder myalgia.
“By demonstrating with positive–predictive value analyses the ability of 4 primary test strains to predict coverage with the use of 10 additional test strains, our findings provide assurance that observed immune responses to the primary strains are representative and indicative of vaccine responses to diverse disease-causing meningococcal B strains,” wrote Lars Ostergaard, MD, of the Aarhus (Denmark) University Hospital and his colleagues.
The studies were supported by Pfizer, and all researchers reported having received various forms of support from that company. Although many of the researchers hold patents, or have patents pending, that are relevant to these studies, they had no other.
SOURCE: Ostergaard L et al. N Engl J Med. 2017 Dec 14;377(24):2350-62.
Correction 12/14/17: An earlier version of this story misstated the findings concerning this subfamily.
MenB-FHbp, a licensed meningococcal B vaccine that targets factor H-binding protein, a surface-exposed bacterial lipoprotein, has been shown to provide broad immunological protection against 4 primary and 10 additional strains of meningococcal B disease in teens and young adults, suggesting the potential to reduce the number of shots needed to protect this population, according to two phase 3 randomized, controlled, observer-blinded, multicenter trials.
After the third dose of MenB-FHbp, the percentage of patients whose antibody titers of serum bactericidal assays with human complement (hSBA) had increased by a factor of four rose as high as 78.8%-90.2% in adolescents and 78.9%-89.7% in young adults in the modified intention-to-treat population, depending on which strain was tested. This population was made up of randomized patients who had at least one valid and determinate assay. Among participants, 82% of adolescents and 84.5% of young adults had the composite response, which was defined as the proportion of patients who had an hSBA titer response that reached or exceeded the limit of quantitation for all primary test strains combined at 1 month after the third MenB-FHbp vaccine dose was administered.
Using the hSBA responses to the primary strains, a positive predictive value analysis predicted the immune responses to other meningitis strains with H-binding protein. For subfamily A strains among adolescents, the positive predictive values for patient responses were 64.4%-100% after dose 2 and 75.6%-99.6% after dose 3. Among young adults, the positive predictive values after dose 2 and 3 were 61.6%-100% and 72.2%-100%, respectively.
A similar pattern was observed for subfamily B strains.* Among adolescents the positive predictive values for patient responses were 78.9%-100% and 86.4%-99.6% after doses 2 and 3, respectively. Young adults also had high positive predictive values after doses 2 and 3, with values of 70%-100% and 80.5%-98.8%, respectively.
Injection site reactions and systemic events were both observed in response to MenB-FHbp in both adolescents and young adults. The most common injection site reaction was pain, which often occurred occurring after the first dose. Six adolescents and three young adults, one of whom received a saline injection, withdrew from the study because of injection site reactions. The most common systemic events among adolescents were headaches and fatigue. The events that caused patients to withdraw were associated with fever, chills, and mild arthralgia and moder myalgia.
“By demonstrating with positive–predictive value analyses the ability of 4 primary test strains to predict coverage with the use of 10 additional test strains, our findings provide assurance that observed immune responses to the primary strains are representative and indicative of vaccine responses to diverse disease-causing meningococcal B strains,” wrote Lars Ostergaard, MD, of the Aarhus (Denmark) University Hospital and his colleagues.
The studies were supported by Pfizer, and all researchers reported having received various forms of support from that company. Although many of the researchers hold patents, or have patents pending, that are relevant to these studies, they had no other.
SOURCE: Ostergaard L et al. N Engl J Med. 2017 Dec 14;377(24):2350-62.
Correction 12/14/17: An earlier version of this story misstated the findings concerning this subfamily.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A surface protein–focused vaccine is broadly effective against meningitis B.
Major finding: After the third dose of MenB-FHbp, hSBA titers against each primary strain increased by a factor of four or more in 78.8%-90.2% of adolescents and 78.9%-89.7% of young adults in the modified intention-to-treat population, depending on which strain was tested.
Study details: Two phase 3 randomized, controlled, observer-blinded, multicenter trials of 3,596 adolescents recruited between April 18, 2013, and June 17, 2015, and 3,304 young adults recruited between May 3, 2013, and July 9, 2015.
Disclosures: The studies were supported by Pfizer, and all researchers reported having received various forms of support from that company. Although many of the researchers hold patents, or have patents pending, that are relevant to these studies, they had no other.
Source: Ostergaard L et al. N Engl J Med. 2017 Dec 14;377(24):2350-62.
Intractable VT halted by noninvasive radiation ablation
who had failed to respond to medical management and, in some cases, catheter ablation, according to a report in the New England Journal of Medicine.
During the 3 months before the procedure, the five subjects had 6,577 episodes of VT, ranging from 5 to 4,312 per patient. In addition to failing to respond to medications, three had failed catheter ablation; that procedure was contraindicated in the other two.
During the 6-week postablation blanking period, when inflammation can trigger arrhythmias, there were 680 VT episodes. After the first 6 weeks, there were four episodes over 46 patient-months, for a reduction from baseline of 99.9%.
The oldest subject, an 83-year-old woman who had had 4,312 pretreatment episodes, had a fatal stroke 3 weeks after the procedure, with no clear relationship to treatment on autopsy. In the 3 weeks before she died, her VT burden was reduced 82%.
Among the rest – all men aged 60-65 years, with pretreatment episodes numbering from 5 to 2,210 – there was no decline in left-ventricular ejection fraction during the 12-month follow-up; mild adjacent lung inflammatory changes noted at 3 months resolved during that follow-up period.
The investigators have launched a phase 1-2 trial called ENCORE-VT to further evaluate the technique.
“Although catheter ablation is the current state-of-the-art treatment for drug-refractory ventricular arrhythmias in patients with structural heart disease, it is not curative for many patients” because of inadequate ablation and other problems. Radiation ablation “has the potential to overcome these challenges. ... If a noninvasive approach to ablation of ventricular tachycardia is shown to be safe and effective, it would be a potentially important therapeutic advance,” said investigators led by Washington University cardiologist Phillip Cuculich, MD. However, “because of the novelty of noninvasive radioablation, its potential for harm, and the limited number of patients who were included in this analysis, this procedure should not be considered to be suitable for clinical use, pending the results of further research studies,” they said.
The long-term effects of high-dose radiation to previously injured heart tissue is unknown. “The volumes of myocardium that are subjected to radiotherapy in these patients (from 17 to 81 mL) are large enough that effects on specialized cardiac structures (papillary muscles, coronary arteries, conduction system, and valves) are of potential concern, as is the risk of overall effects on ventricular function, although no such effects were seen during the 12-month follow-up,” they said.
Of the four patients who were alive at 12 months, three were no longer on antiarrhythmic medications. One restarted amiodarone at 9 months after the first posttreatment episode of antitachycardia pacing. One patient had catheter ablation 4 weeks after treatment because of incomplete VT cessation, with no further episodes during follow-up.
In the 3 months before treatment, patients had an aggregate number of 55 implantable cardioverter defibrillator shocks and 6,577 episodes of antitachycardia pacing. Over the following 12 months, there was just one shock and three pacing episodes. After the blanking period, one patient had three VT episodes, one had one, and two didn’t have any, including a man who had 2,210 in the 3 months before treatment; he was the subject who had the secondary catheter ablation.
The work was funded by the Barnes-Jewish Hospital Foundation, Washington University, and the National Institutes of Health. Dr. Cuculich and another author have a patent pending on electrocardiographic imaging and stereotactic body radiation therapy for cardiac arrhythmia. Several authors reported receiving personal fees from and other involvement with a number of companies, including ViewRay, Varian, Elekta, and Medtronic.
SOURCE: Cuculich P et al. N Engl J Med. 2017 Dec 13. doi: 10.1056/NEJMoa1613773.
who had failed to respond to medical management and, in some cases, catheter ablation, according to a report in the New England Journal of Medicine.
During the 3 months before the procedure, the five subjects had 6,577 episodes of VT, ranging from 5 to 4,312 per patient. In addition to failing to respond to medications, three had failed catheter ablation; that procedure was contraindicated in the other two.
During the 6-week postablation blanking period, when inflammation can trigger arrhythmias, there were 680 VT episodes. After the first 6 weeks, there were four episodes over 46 patient-months, for a reduction from baseline of 99.9%.
The oldest subject, an 83-year-old woman who had had 4,312 pretreatment episodes, had a fatal stroke 3 weeks after the procedure, with no clear relationship to treatment on autopsy. In the 3 weeks before she died, her VT burden was reduced 82%.
Among the rest – all men aged 60-65 years, with pretreatment episodes numbering from 5 to 2,210 – there was no decline in left-ventricular ejection fraction during the 12-month follow-up; mild adjacent lung inflammatory changes noted at 3 months resolved during that follow-up period.
The investigators have launched a phase 1-2 trial called ENCORE-VT to further evaluate the technique.
“Although catheter ablation is the current state-of-the-art treatment for drug-refractory ventricular arrhythmias in patients with structural heart disease, it is not curative for many patients” because of inadequate ablation and other problems. Radiation ablation “has the potential to overcome these challenges. ... If a noninvasive approach to ablation of ventricular tachycardia is shown to be safe and effective, it would be a potentially important therapeutic advance,” said investigators led by Washington University cardiologist Phillip Cuculich, MD. However, “because of the novelty of noninvasive radioablation, its potential for harm, and the limited number of patients who were included in this analysis, this procedure should not be considered to be suitable for clinical use, pending the results of further research studies,” they said.
The long-term effects of high-dose radiation to previously injured heart tissue is unknown. “The volumes of myocardium that are subjected to radiotherapy in these patients (from 17 to 81 mL) are large enough that effects on specialized cardiac structures (papillary muscles, coronary arteries, conduction system, and valves) are of potential concern, as is the risk of overall effects on ventricular function, although no such effects were seen during the 12-month follow-up,” they said.
Of the four patients who were alive at 12 months, three were no longer on antiarrhythmic medications. One restarted amiodarone at 9 months after the first posttreatment episode of antitachycardia pacing. One patient had catheter ablation 4 weeks after treatment because of incomplete VT cessation, with no further episodes during follow-up.
In the 3 months before treatment, patients had an aggregate number of 55 implantable cardioverter defibrillator shocks and 6,577 episodes of antitachycardia pacing. Over the following 12 months, there was just one shock and three pacing episodes. After the blanking period, one patient had three VT episodes, one had one, and two didn’t have any, including a man who had 2,210 in the 3 months before treatment; he was the subject who had the secondary catheter ablation.
The work was funded by the Barnes-Jewish Hospital Foundation, Washington University, and the National Institutes of Health. Dr. Cuculich and another author have a patent pending on electrocardiographic imaging and stereotactic body radiation therapy for cardiac arrhythmia. Several authors reported receiving personal fees from and other involvement with a number of companies, including ViewRay, Varian, Elekta, and Medtronic.
SOURCE: Cuculich P et al. N Engl J Med. 2017 Dec 13. doi: 10.1056/NEJMoa1613773.
who had failed to respond to medical management and, in some cases, catheter ablation, according to a report in the New England Journal of Medicine.
During the 3 months before the procedure, the five subjects had 6,577 episodes of VT, ranging from 5 to 4,312 per patient. In addition to failing to respond to medications, three had failed catheter ablation; that procedure was contraindicated in the other two.
During the 6-week postablation blanking period, when inflammation can trigger arrhythmias, there were 680 VT episodes. After the first 6 weeks, there were four episodes over 46 patient-months, for a reduction from baseline of 99.9%.
The oldest subject, an 83-year-old woman who had had 4,312 pretreatment episodes, had a fatal stroke 3 weeks after the procedure, with no clear relationship to treatment on autopsy. In the 3 weeks before she died, her VT burden was reduced 82%.
Among the rest – all men aged 60-65 years, with pretreatment episodes numbering from 5 to 2,210 – there was no decline in left-ventricular ejection fraction during the 12-month follow-up; mild adjacent lung inflammatory changes noted at 3 months resolved during that follow-up period.
The investigators have launched a phase 1-2 trial called ENCORE-VT to further evaluate the technique.
“Although catheter ablation is the current state-of-the-art treatment for drug-refractory ventricular arrhythmias in patients with structural heart disease, it is not curative for many patients” because of inadequate ablation and other problems. Radiation ablation “has the potential to overcome these challenges. ... If a noninvasive approach to ablation of ventricular tachycardia is shown to be safe and effective, it would be a potentially important therapeutic advance,” said investigators led by Washington University cardiologist Phillip Cuculich, MD. However, “because of the novelty of noninvasive radioablation, its potential for harm, and the limited number of patients who were included in this analysis, this procedure should not be considered to be suitable for clinical use, pending the results of further research studies,” they said.
The long-term effects of high-dose radiation to previously injured heart tissue is unknown. “The volumes of myocardium that are subjected to radiotherapy in these patients (from 17 to 81 mL) are large enough that effects on specialized cardiac structures (papillary muscles, coronary arteries, conduction system, and valves) are of potential concern, as is the risk of overall effects on ventricular function, although no such effects were seen during the 12-month follow-up,” they said.
Of the four patients who were alive at 12 months, three were no longer on antiarrhythmic medications. One restarted amiodarone at 9 months after the first posttreatment episode of antitachycardia pacing. One patient had catheter ablation 4 weeks after treatment because of incomplete VT cessation, with no further episodes during follow-up.
In the 3 months before treatment, patients had an aggregate number of 55 implantable cardioverter defibrillator shocks and 6,577 episodes of antitachycardia pacing. Over the following 12 months, there was just one shock and three pacing episodes. After the blanking period, one patient had three VT episodes, one had one, and two didn’t have any, including a man who had 2,210 in the 3 months before treatment; he was the subject who had the secondary catheter ablation.
The work was funded by the Barnes-Jewish Hospital Foundation, Washington University, and the National Institutes of Health. Dr. Cuculich and another author have a patent pending on electrocardiographic imaging and stereotactic body radiation therapy for cardiac arrhythmia. Several authors reported receiving personal fees from and other involvement with a number of companies, including ViewRay, Varian, Elekta, and Medtronic.
SOURCE: Cuculich P et al. N Engl J Med. 2017 Dec 13. doi: 10.1056/NEJMoa1613773.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A one-time dose of radiation to arrhythmogenic scars nearly eliminated ventricular tachycardia in five patients who had failed to respond to medical management and, in some cases, catheter ablation.
Major finding: VT episodes were slashed by 99.9% from baseline.
Study details: Case series in 5 patients
Disclosures: The work was funded by the Barnes-Jewish Hospital Foundation, Washington University in St. Louis, and the National Institutes of Health. Dr. Cuculich and another author have a patent pending on electrocardiographic imaging and stereotactic body radiation therapy for cardiac arrhythmia. Several authors reported receiving personal fees from and other involvement with a number of companies, including ViewRay, Varian, Elekta, and Medtronic.
Source: Cuculich P et al. N Engl J Med. 2017 Dec 13. doi: 10.1056/NEJMoa1613773