User login
After 6 weeks, HealthCare.gov activity still ahead of last year
compared with the total at the end of week 4, according to the Centers for Medicare & Medicaid Services.
The 1.89 million plans that consumers selected over the 2-week period ending Dec. 9 brought this year’s total to 4.68 million after 6 weeks. That’s 16.5% higher than last year’s 6-week total of 4.02 million, but the difference has been getting smaller: After week 2 (enrollment figures were released only biweekly last year), the 2018 open season’s tally was higher than the 2017 open season’s week 2 tally by almost 47%, but after 4 weeks, the difference was only 30%, the CMS data show.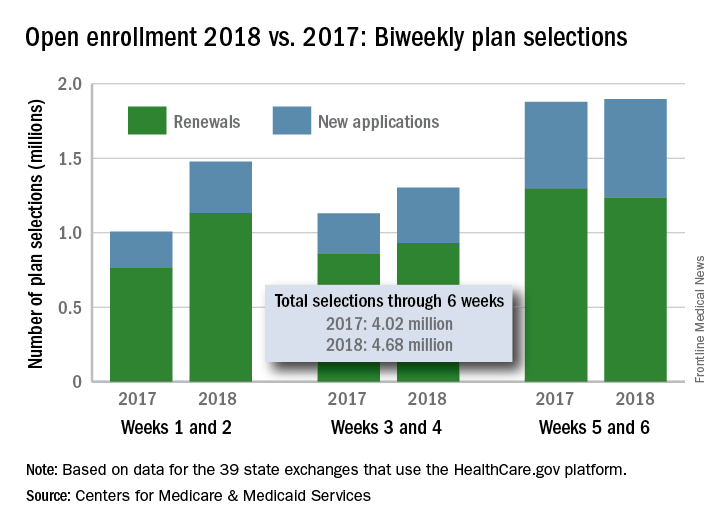
compared with the total at the end of week 4, according to the Centers for Medicare & Medicaid Services.
The 1.89 million plans that consumers selected over the 2-week period ending Dec. 9 brought this year’s total to 4.68 million after 6 weeks. That’s 16.5% higher than last year’s 6-week total of 4.02 million, but the difference has been getting smaller: After week 2 (enrollment figures were released only biweekly last year), the 2018 open season’s tally was higher than the 2017 open season’s week 2 tally by almost 47%, but after 4 weeks, the difference was only 30%, the CMS data show.
compared with the total at the end of week 4, according to the Centers for Medicare & Medicaid Services.
The 1.89 million plans that consumers selected over the 2-week period ending Dec. 9 brought this year’s total to 4.68 million after 6 weeks. That’s 16.5% higher than last year’s 6-week total of 4.02 million, but the difference has been getting smaller: After week 2 (enrollment figures were released only biweekly last year), the 2018 open season’s tally was higher than the 2017 open season’s week 2 tally by almost 47%, but after 4 weeks, the difference was only 30%, the CMS data show.
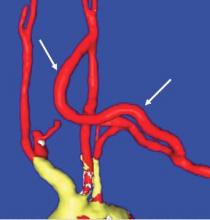
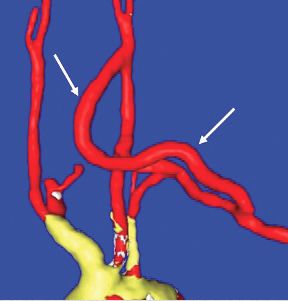
Carotid-axillary bypass for revascularization of the left subclavian artery in zone-2 TEVAR
Stent-graft coverage of the left subclavian artery (LSA) is often performed during TEVAR treatment of thoracic aortic pathologies and, consequently, debranching of the LSA is frequently performed in such settings. The carotid-subclavian bypass (CSB) is undoubtedly the cervical bypass option preferred by most surgeons for this purpose.1,2 The technique was first described by Lyons and Galbraith in 1957,3 and popularized by Diethrich et al. who reported their large experience in a well-known article published 10 years later.4 In the ensuing decades, CSB became the overwhelming favorite of surgeons everywhere performing LSA revascularization for management of arterial occlusive disease and, more recently, in the context of zone-2 TEVAR. Well- documented good results would seem to justify such preference,5 but some level of concern has been voiced consistently over the years about some technical complexities and potential complications such as phrenic nerve and thoracic duct injuries.6 My own personal experience substantiated these reservations early on, prompting adoption of an alternative operative solution with use of the carotid-axillary bypass (CAB),7 an operation first reported by Shumacker in 1973.8 In my hands, it has produced equivalent results to the carotid-subclavian technique in terms of efficacy and durability, and with the additional appeal of distinct practical advantages – mainly because the axillary artery tends to be an easier vessel to expose and handle, and through the avoidance of complications resulting from damage to anatomical structures that are often in harm’s way when exposing the LSA.
Technical aspects
End-to-side anastomoses proximally and distally are constructed in routine manner (Fig. 2). We do not use carotid shunting for this procedure.
Occasionally one may want to combine a carotid endarterectomy with the cervical bypass, in which case the proximal vascular graft anastomosis is constructed at the endarterectomy site (Fig. 3). Close attention must be paid to careful length-tailoring the conduit to achieve the desirable gently curving course without undue tension or redundancy.
Proximal ligation of the LSA, often performed during the CSB, cannot be a component of the carotid-axillary operation because of inaccessibility. Some experts look upon this as a disadvantage, but I tend to view such limitation as advantageous because it eliminates the potential for a misplaced ligation distal to the left vertebral artery origin which present-day CTA studies show it to be the case more frequently than previously suspected. If interruption of the LSA is deemed necessary, it is arguably best to use an endovascular (retrograde trans-brachial) approach with precise deployment of a vascular plug device under angiographic guidance (Fig. 4). ■
Dr. Criado is at MedStar Union Memorial Hospital, Baltimore.
References
1. J Endovasc Ther 2002;9(suppl 2):1132-1138.
2. Ann Cardiothorac Surg 2013;2:247-260.
3. Ann Surg 1957;146:487-494.
4. Am J Surg 1967;114:800-808.
5. J Vasc Surg 2008;48:555-560.
6. Ann Vasc Surg 2008;22:70-78.
7. J Vasc Surg 1995;22:717-723.
8. Surg Gynecol Obstet 1973;136:447-8.
9. J Vasc Surg 1999;30:1106-1112.
Stent-graft coverage of the left subclavian artery (LSA) is often performed during TEVAR treatment of thoracic aortic pathologies and, consequently, debranching of the LSA is frequently performed in such settings. The carotid-subclavian bypass (CSB) is undoubtedly the cervical bypass option preferred by most surgeons for this purpose.1,2 The technique was first described by Lyons and Galbraith in 1957,3 and popularized by Diethrich et al. who reported their large experience in a well-known article published 10 years later.4 In the ensuing decades, CSB became the overwhelming favorite of surgeons everywhere performing LSA revascularization for management of arterial occlusive disease and, more recently, in the context of zone-2 TEVAR. Well- documented good results would seem to justify such preference,5 but some level of concern has been voiced consistently over the years about some technical complexities and potential complications such as phrenic nerve and thoracic duct injuries.6 My own personal experience substantiated these reservations early on, prompting adoption of an alternative operative solution with use of the carotid-axillary bypass (CAB),7 an operation first reported by Shumacker in 1973.8 In my hands, it has produced equivalent results to the carotid-subclavian technique in terms of efficacy and durability, and with the additional appeal of distinct practical advantages – mainly because the axillary artery tends to be an easier vessel to expose and handle, and through the avoidance of complications resulting from damage to anatomical structures that are often in harm’s way when exposing the LSA.
Technical aspects
End-to-side anastomoses proximally and distally are constructed in routine manner (Fig. 2). We do not use carotid shunting for this procedure.
Occasionally one may want to combine a carotid endarterectomy with the cervical bypass, in which case the proximal vascular graft anastomosis is constructed at the endarterectomy site (Fig. 3). Close attention must be paid to careful length-tailoring the conduit to achieve the desirable gently curving course without undue tension or redundancy.
Proximal ligation of the LSA, often performed during the CSB, cannot be a component of the carotid-axillary operation because of inaccessibility. Some experts look upon this as a disadvantage, but I tend to view such limitation as advantageous because it eliminates the potential for a misplaced ligation distal to the left vertebral artery origin which present-day CTA studies show it to be the case more frequently than previously suspected. If interruption of the LSA is deemed necessary, it is arguably best to use an endovascular (retrograde trans-brachial) approach with precise deployment of a vascular plug device under angiographic guidance (Fig. 4). ■
Dr. Criado is at MedStar Union Memorial Hospital, Baltimore.
References
1. J Endovasc Ther 2002;9(suppl 2):1132-1138.
2. Ann Cardiothorac Surg 2013;2:247-260.
3. Ann Surg 1957;146:487-494.
4. Am J Surg 1967;114:800-808.
5. J Vasc Surg 2008;48:555-560.
6. Ann Vasc Surg 2008;22:70-78.
7. J Vasc Surg 1995;22:717-723.
8. Surg Gynecol Obstet 1973;136:447-8.
9. J Vasc Surg 1999;30:1106-1112.
Stent-graft coverage of the left subclavian artery (LSA) is often performed during TEVAR treatment of thoracic aortic pathologies and, consequently, debranching of the LSA is frequently performed in such settings. The carotid-subclavian bypass (CSB) is undoubtedly the cervical bypass option preferred by most surgeons for this purpose.1,2 The technique was first described by Lyons and Galbraith in 1957,3 and popularized by Diethrich et al. who reported their large experience in a well-known article published 10 years later.4 In the ensuing decades, CSB became the overwhelming favorite of surgeons everywhere performing LSA revascularization for management of arterial occlusive disease and, more recently, in the context of zone-2 TEVAR. Well- documented good results would seem to justify such preference,5 but some level of concern has been voiced consistently over the years about some technical complexities and potential complications such as phrenic nerve and thoracic duct injuries.6 My own personal experience substantiated these reservations early on, prompting adoption of an alternative operative solution with use of the carotid-axillary bypass (CAB),7 an operation first reported by Shumacker in 1973.8 In my hands, it has produced equivalent results to the carotid-subclavian technique in terms of efficacy and durability, and with the additional appeal of distinct practical advantages – mainly because the axillary artery tends to be an easier vessel to expose and handle, and through the avoidance of complications resulting from damage to anatomical structures that are often in harm’s way when exposing the LSA.
Technical aspects
End-to-side anastomoses proximally and distally are constructed in routine manner (Fig. 2). We do not use carotid shunting for this procedure.
Occasionally one may want to combine a carotid endarterectomy with the cervical bypass, in which case the proximal vascular graft anastomosis is constructed at the endarterectomy site (Fig. 3). Close attention must be paid to careful length-tailoring the conduit to achieve the desirable gently curving course without undue tension or redundancy.
Proximal ligation of the LSA, often performed during the CSB, cannot be a component of the carotid-axillary operation because of inaccessibility. Some experts look upon this as a disadvantage, but I tend to view such limitation as advantageous because it eliminates the potential for a misplaced ligation distal to the left vertebral artery origin which present-day CTA studies show it to be the case more frequently than previously suspected. If interruption of the LSA is deemed necessary, it is arguably best to use an endovascular (retrograde trans-brachial) approach with precise deployment of a vascular plug device under angiographic guidance (Fig. 4). ■
Dr. Criado is at MedStar Union Memorial Hospital, Baltimore.
References
1. J Endovasc Ther 2002;9(suppl 2):1132-1138.
2. Ann Cardiothorac Surg 2013;2:247-260.
3. Ann Surg 1957;146:487-494.
4. Am J Surg 1967;114:800-808.
5. J Vasc Surg 2008;48:555-560.
6. Ann Vasc Surg 2008;22:70-78.
7. J Vasc Surg 1995;22:717-723.
8. Surg Gynecol Obstet 1973;136:447-8.
9. J Vasc Surg 1999;30:1106-1112.
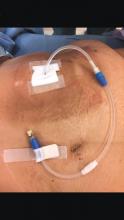
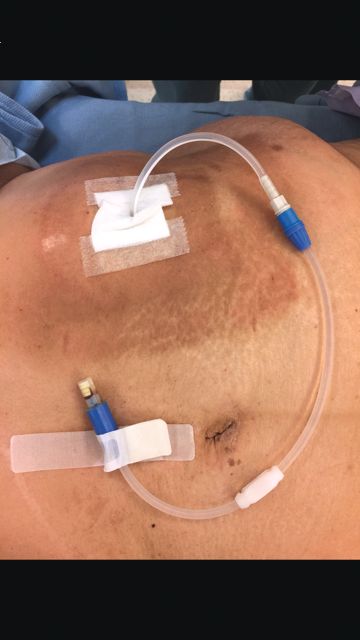
Tips and Tricks: Dealing with a troublesome peritoneal dialysis catheter
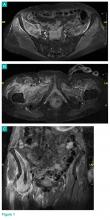
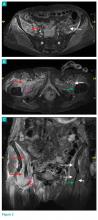
When faced with a poorly performing or nonfunctional peritoneal dialysis catheter, there is a very simple trick to make laparoscopic exploration easier. Prep the catheter into the field and take extra care to prep the cover of the catheter. It is usually easier to remove the extended portion of the catheter (A) and just leave the shorter piece (B).
As soon as the port is in, simply switch the CO2 over to it from the catheter. This technique comes in handy quite often, as many patients with difficult PD catheters have undergone multiple explorations or laparoscopies. Not rocket science, but it can definitely save you and the patient some difficulty!
Dr. Rigberg is Clinical Professor of Surgery and Program Director, University of California, Los Angeles, Division of Vascular Surgery, and an associate medical editor for Vascular Specialist.
When faced with a poorly performing or nonfunctional peritoneal dialysis catheter, there is a very simple trick to make laparoscopic exploration easier. Prep the catheter into the field and take extra care to prep the cover of the catheter. It is usually easier to remove the extended portion of the catheter (A) and just leave the shorter piece (B).
As soon as the port is in, simply switch the CO2 over to it from the catheter. This technique comes in handy quite often, as many patients with difficult PD catheters have undergone multiple explorations or laparoscopies. Not rocket science, but it can definitely save you and the patient some difficulty!
Dr. Rigberg is Clinical Professor of Surgery and Program Director, University of California, Los Angeles, Division of Vascular Surgery, and an associate medical editor for Vascular Specialist.
When faced with a poorly performing or nonfunctional peritoneal dialysis catheter, there is a very simple trick to make laparoscopic exploration easier. Prep the catheter into the field and take extra care to prep the cover of the catheter. It is usually easier to remove the extended portion of the catheter (A) and just leave the shorter piece (B).
As soon as the port is in, simply switch the CO2 over to it from the catheter. This technique comes in handy quite often, as many patients with difficult PD catheters have undergone multiple explorations or laparoscopies. Not rocket science, but it can definitely save you and the patient some difficulty!
Dr. Rigberg is Clinical Professor of Surgery and Program Director, University of California, Los Angeles, Division of Vascular Surgery, and an associate medical editor for Vascular Specialist.
The men and women of vascular surgery
From the Editor
Recent news events have detailed the many humiliations and abuses, both verbal and physical, that women, and some men, have to endure in the workforce. It would not surprise me if some vascular surgeons admit that they have heard of similar instances of egregious behavior occurring in our workplaces. The people that have been impacted have predominantly been women and have come from all walks of life. They have been patients, colleagues, our employees or those of the many institutions in which we work. Unfortunately, the demands of our profession and the pace of our lives may diminish our relationships with these persons. This facelessness and disconnection may allow some surgeons to justify their poor behavior whereas others may not realize that they are negatively impacting these individuals’ lives. The fact that these injustices persist is made more upsetting because we are so indebted for all that these nurses, technologists, office personnel, and even patients, do for us.
Just think how much we owe the nurses on the hospital floors. It is to nurses that we entrust the postoperative care of our patients. They make sure to call us when they detect that a pulse is weakening or suddenly absent, or that a neck is expanding as a hematoma threatens breathing. They timely diagnose a retroperitoneal bleed that may endanger the patient’s life. Dialysis nurses notify us that a puncture looks like it may suddenly bleed out. Our patients’ lives are often entirely dependent on the astute observation of an accomplished nurse.
And what about the operating nurses and scrub technicians? They lay out our surgical tray perfectly with all the tools that we are wont to use. They are there to assist when a sudden event requires the steady hand of an observant nurse who knows just what instrument we need without us having to ask. When you are in a difficult area, an encouraging word will often inspire the confidence required to accomplish a successful outcome. When a procedure is going poorly and tension mounts, their silence accepts our sometimes curt requests. There is a bond that develops between two professionals who recognize each other’s expertise.
Vascular technologists work tirelessly, often in darkened rooms, frequently under challenging positions straining eyes and limbs to detect pathology that may be life or limb saving. Their diagnostic acumen can be the difference between a subsequent procedure’s success or failure. Indeed, the vascular surgeon has to make the final interpretation, but if the technologist fails to show the pathology, even the most erudite physician may miss the diagnosis.
Front-desk personnel who sit at check-in and check-out in an office are the face of our practice. Their friendly attitude welcomes our patients and reassures them that they have come to a well-run, professional workplace. A smiling, personal greeting will calm even the most worried patient. Of course, their attention to detail assures that collections will not be misplaced.
Our office nurses exude compassion for the many patients who face immense hurdles in living with vascular disease. They assist in teaching wound care, explain medications, and help in arranging social services. They cry with those that have recently lost a spouse or child and get excited to hear of the birth of a patient’s grandchild. Without their organizational skills, office hours would be interminable, and patients who are kept waiting would complain, or worse, leave the practice. They have learned to laugh at the same joke that they have heard us tell innumerable times, and to ignore the sometimes lousy mood we may bring into the office after a brutal night on call.
The spouses or significant others of our patients also play an important role since it is often from them that we get the most accurate history. They will ask to speak to us privately to make sure we do not cause despair when we discuss treatment options or to ensure that we firmly admonish their loved one to stop smoking, exercise or watch their weight. Unfortunately, they will sometimes have to accept a disparaging remark or gesture from their “spouse” to make sure that we are supplied all the necessary information to come to an appropriate diagnosis.
I can go on about other medical personnel that contribute to our success, but I believe I have made the point. The men and women with whom we interact as vascular surgeons deserve the same respect we grant ourselves. Any insult to them demeans not only the recipient but more so the abuser and those of us who stand by silently.
Finally, there are many female colleagues whose interest and drive has allowed them to not only break into but achieve leadership positions in a specialty that was almost uniformly male and unwelcoming. Their aptitudes and attitudes have broadened the specialty’s ability to help our patients. However, recently the news has been replete with evidence that women have been abused as they tried to enter other male-dominated professions and so it is likely that this has happened in ours.
Other recent news items suggest that these physical and emotional abuses are inflicted not only on women but also men. We may never know the scope of this mistreatment, but we must assure that it stops immediately.
Ethical behavior must be gender neutral. Further, condescending attitudes, cruel language, and a lack of appreciation sometimes can be as damaging as physical or sexual abuse and must be abolished from our workplace. ■
From the Editor
Recent news events have detailed the many humiliations and abuses, both verbal and physical, that women, and some men, have to endure in the workforce. It would not surprise me if some vascular surgeons admit that they have heard of similar instances of egregious behavior occurring in our workplaces. The people that have been impacted have predominantly been women and have come from all walks of life. They have been patients, colleagues, our employees or those of the many institutions in which we work. Unfortunately, the demands of our profession and the pace of our lives may diminish our relationships with these persons. This facelessness and disconnection may allow some surgeons to justify their poor behavior whereas others may not realize that they are negatively impacting these individuals’ lives. The fact that these injustices persist is made more upsetting because we are so indebted for all that these nurses, technologists, office personnel, and even patients, do for us.
Just think how much we owe the nurses on the hospital floors. It is to nurses that we entrust the postoperative care of our patients. They make sure to call us when they detect that a pulse is weakening or suddenly absent, or that a neck is expanding as a hematoma threatens breathing. They timely diagnose a retroperitoneal bleed that may endanger the patient’s life. Dialysis nurses notify us that a puncture looks like it may suddenly bleed out. Our patients’ lives are often entirely dependent on the astute observation of an accomplished nurse.
And what about the operating nurses and scrub technicians? They lay out our surgical tray perfectly with all the tools that we are wont to use. They are there to assist when a sudden event requires the steady hand of an observant nurse who knows just what instrument we need without us having to ask. When you are in a difficult area, an encouraging word will often inspire the confidence required to accomplish a successful outcome. When a procedure is going poorly and tension mounts, their silence accepts our sometimes curt requests. There is a bond that develops between two professionals who recognize each other’s expertise.
Vascular technologists work tirelessly, often in darkened rooms, frequently under challenging positions straining eyes and limbs to detect pathology that may be life or limb saving. Their diagnostic acumen can be the difference between a subsequent procedure’s success or failure. Indeed, the vascular surgeon has to make the final interpretation, but if the technologist fails to show the pathology, even the most erudite physician may miss the diagnosis.
Front-desk personnel who sit at check-in and check-out in an office are the face of our practice. Their friendly attitude welcomes our patients and reassures them that they have come to a well-run, professional workplace. A smiling, personal greeting will calm even the most worried patient. Of course, their attention to detail assures that collections will not be misplaced.
Our office nurses exude compassion for the many patients who face immense hurdles in living with vascular disease. They assist in teaching wound care, explain medications, and help in arranging social services. They cry with those that have recently lost a spouse or child and get excited to hear of the birth of a patient’s grandchild. Without their organizational skills, office hours would be interminable, and patients who are kept waiting would complain, or worse, leave the practice. They have learned to laugh at the same joke that they have heard us tell innumerable times, and to ignore the sometimes lousy mood we may bring into the office after a brutal night on call.
The spouses or significant others of our patients also play an important role since it is often from them that we get the most accurate history. They will ask to speak to us privately to make sure we do not cause despair when we discuss treatment options or to ensure that we firmly admonish their loved one to stop smoking, exercise or watch their weight. Unfortunately, they will sometimes have to accept a disparaging remark or gesture from their “spouse” to make sure that we are supplied all the necessary information to come to an appropriate diagnosis.
I can go on about other medical personnel that contribute to our success, but I believe I have made the point. The men and women with whom we interact as vascular surgeons deserve the same respect we grant ourselves. Any insult to them demeans not only the recipient but more so the abuser and those of us who stand by silently.
Finally, there are many female colleagues whose interest and drive has allowed them to not only break into but achieve leadership positions in a specialty that was almost uniformly male and unwelcoming. Their aptitudes and attitudes have broadened the specialty’s ability to help our patients. However, recently the news has been replete with evidence that women have been abused as they tried to enter other male-dominated professions and so it is likely that this has happened in ours.
Other recent news items suggest that these physical and emotional abuses are inflicted not only on women but also men. We may never know the scope of this mistreatment, but we must assure that it stops immediately.
Ethical behavior must be gender neutral. Further, condescending attitudes, cruel language, and a lack of appreciation sometimes can be as damaging as physical or sexual abuse and must be abolished from our workplace. ■
From the Editor
Recent news events have detailed the many humiliations and abuses, both verbal and physical, that women, and some men, have to endure in the workforce. It would not surprise me if some vascular surgeons admit that they have heard of similar instances of egregious behavior occurring in our workplaces. The people that have been impacted have predominantly been women and have come from all walks of life. They have been patients, colleagues, our employees or those of the many institutions in which we work. Unfortunately, the demands of our profession and the pace of our lives may diminish our relationships with these persons. This facelessness and disconnection may allow some surgeons to justify their poor behavior whereas others may not realize that they are negatively impacting these individuals’ lives. The fact that these injustices persist is made more upsetting because we are so indebted for all that these nurses, technologists, office personnel, and even patients, do for us.
Just think how much we owe the nurses on the hospital floors. It is to nurses that we entrust the postoperative care of our patients. They make sure to call us when they detect that a pulse is weakening or suddenly absent, or that a neck is expanding as a hematoma threatens breathing. They timely diagnose a retroperitoneal bleed that may endanger the patient’s life. Dialysis nurses notify us that a puncture looks like it may suddenly bleed out. Our patients’ lives are often entirely dependent on the astute observation of an accomplished nurse.
And what about the operating nurses and scrub technicians? They lay out our surgical tray perfectly with all the tools that we are wont to use. They are there to assist when a sudden event requires the steady hand of an observant nurse who knows just what instrument we need without us having to ask. When you are in a difficult area, an encouraging word will often inspire the confidence required to accomplish a successful outcome. When a procedure is going poorly and tension mounts, their silence accepts our sometimes curt requests. There is a bond that develops between two professionals who recognize each other’s expertise.
Vascular technologists work tirelessly, often in darkened rooms, frequently under challenging positions straining eyes and limbs to detect pathology that may be life or limb saving. Their diagnostic acumen can be the difference between a subsequent procedure’s success or failure. Indeed, the vascular surgeon has to make the final interpretation, but if the technologist fails to show the pathology, even the most erudite physician may miss the diagnosis.
Front-desk personnel who sit at check-in and check-out in an office are the face of our practice. Their friendly attitude welcomes our patients and reassures them that they have come to a well-run, professional workplace. A smiling, personal greeting will calm even the most worried patient. Of course, their attention to detail assures that collections will not be misplaced.
Our office nurses exude compassion for the many patients who face immense hurdles in living with vascular disease. They assist in teaching wound care, explain medications, and help in arranging social services. They cry with those that have recently lost a spouse or child and get excited to hear of the birth of a patient’s grandchild. Without their organizational skills, office hours would be interminable, and patients who are kept waiting would complain, or worse, leave the practice. They have learned to laugh at the same joke that they have heard us tell innumerable times, and to ignore the sometimes lousy mood we may bring into the office after a brutal night on call.
The spouses or significant others of our patients also play an important role since it is often from them that we get the most accurate history. They will ask to speak to us privately to make sure we do not cause despair when we discuss treatment options or to ensure that we firmly admonish their loved one to stop smoking, exercise or watch their weight. Unfortunately, they will sometimes have to accept a disparaging remark or gesture from their “spouse” to make sure that we are supplied all the necessary information to come to an appropriate diagnosis.
I can go on about other medical personnel that contribute to our success, but I believe I have made the point. The men and women with whom we interact as vascular surgeons deserve the same respect we grant ourselves. Any insult to them demeans not only the recipient but more so the abuser and those of us who stand by silently.
Finally, there are many female colleagues whose interest and drive has allowed them to not only break into but achieve leadership positions in a specialty that was almost uniformly male and unwelcoming. Their aptitudes and attitudes have broadened the specialty’s ability to help our patients. However, recently the news has been replete with evidence that women have been abused as they tried to enter other male-dominated professions and so it is likely that this has happened in ours.
Other recent news items suggest that these physical and emotional abuses are inflicted not only on women but also men. We may never know the scope of this mistreatment, but we must assure that it stops immediately.
Ethical behavior must be gender neutral. Further, condescending attitudes, cruel language, and a lack of appreciation sometimes can be as damaging as physical or sexual abuse and must be abolished from our workplace. ■
Should carotid body tumors be embolized preoperatively?
Preop embolization is safe and effective
To embolize or not to embolize ... that is the question when it comes to the management of carotid body tumors. Carotid body tumors (CBTs) are rare benign neoplasms that are almost always nonfunctional and account for up to 80% of all head and neck paragangliomas. Radiographic characteristics include tumor location at the carotid bifurcation, splaying apart the internal and external carotid arteries. Surgical resection is the mainstay of definitive treatment and is preferably performed at diagnosis. CBTs are categorized based upon their involvement with surrounding structures: Shamblin I tumors are small, without encasement of the carotid arteries; Shamblin II tumors partially encase the vessels; and Shamblin III tumors completely encase the vessels. When it comes to operative complications, advanced Shamblin stage is associated with a higher rate of cranial nerve injury when the tumor is resected.1
Pertinent to the technical aspects of surgical resection, CBTs are characteristically encased with an elaborate network of friable vessels which may contribute to significant intraoperative bleeding, especially with resection of larger tumors. The blood supply to CBTs typically originates from the branches of the external carotid artery. These branches may be sacrificed with minimal consequence using preoperative embolization. This common practice is felt by many to facilitate safe resection while minimizing intraoperative blood loss. Although the reduction of blood loss has not been observed universally, there is enough evidence of this in the literature to support the use of selective preoperative embolization. Several embolic agents have been described in this setting, including polyvinyl alcohol particles (150-1000 microns), polymerized glue (n-butyl cyanoacrylate), and more recently, ethylene-vinyl alcohol copolymer (Onyx, ev3, Irvine, Calif.). Risks of complications related to CBT embolization in experienced hands are quite low with no adverse events reported in a number of reports describing the technique.2,3
Although embolization is argued to decrease blood loss, its impact on rates of neurologic complications is admittedly insignificant. One might question the added cost of the embolization procedure in the current atmosphere of focus on cost-containment, but this may be defrayed by lower rates of postoperative hematoma and operative times. Although blood loss has been shown to be decreased in a number of reports, translating this to decreased need for transfusion has unfortunately yet to be demonstrated. Until large-volume prospective randomized studies of the outcomes associated with preoperative embolization of CBTs are available, its use should be considered mainly when risks of blood loss are significant in advanced stage tumors and only if the associated complication risks associated with its use can be kept to acceptably low rates. As with other adjunctive procedures whose merit has not yet been convincingly sorted out in the available data, personal experience and preference often play a role in the decision making process we all face. For those who have experienced the added challenge of the meticulous dissection of the CBT in the face of a bloodied field, having such tools as embolization at our fingertips is a reassuring adjunct to be considered.
References
1. J Vasc Surg. 2013;57:64-8S.
2. J Vasc Interv Neurol. 2008;1(2):37-41.
3. Am J Neuroradiol. 2009;30:1594-97.
4. J Vasc Surg. 2012;56:979-89.
5. Vasc Endovasc Surg. 2009;43:457-61.
Too many downsides
As vascular surgeons, we are frequently confronting situations where the “best” approach to a problem is far from established. The evidence to make a clinical decision may simply not exist, and we need to choose a course of therapy based more on personal preference than science. Such is the case with preoperative embolization for the resection of carotid body tumors (CBT).
It has been over 35 years since the technique for preoperative embolization of CBT was described, the aim being reduction in blood loss, decreased operative time, and improved visualization for safer tumor resection. This is based on the fact that most of the arterial supply to these tumors arises from external carotid branches (particularly the ascending pharyngeal), and these that can be safely sacrificed. Although this technique is certainly now a standard part of treatment for many vascular surgeons, the evidence of benefit is certainly not overwhelming, and like all interventions, nothing comes without risks.
So what are the downsides of preoperative embolization? The cost of the procedure itself is not insignificant, in some cases doubling the expense of the intervention. And there can also be organizational issues with coordinating the time in the endovascular suite versus the operating room. In my experience, we have taken patients directly from embolization for the CBT resection, and there are frequently delays and transport issues. Although advocates may claim decreased operative time as a benefit of embolization, this is attained at an overall increase in anesthetic time for the combined procedures. If a day or two separates the procedures, some patients do report the pain or fevers that can accompany any tumor embolization.
While the above may be nuisances, there are serious adverse effects from preoperative embolization as well. A review of 11 studies of preoperative embolization for CBT found a 2.5% rate of complications, including temporary aphasia, vocal cord paralysis, and arterial dissection. Stroke with permanent deficit has also been reported secondary to CBT embolization. Access site hematoma has also been reported as with any catheter-based intervention.
With regards to “selective” preoperative embolization, the literature again offers a mixed bag. Many advocates of embolization rely upon the Shamblin classification for selecting candidates, reserving embolization for Shamblin II and III tumors. However, the extent of carotid involvement is sometimes a surgical diagnosis. Although size of tumor generally correlates with Shamblin class, even smaller tumors can demonstrate arterial wall involvement. Abu-Ghanem provides a succinct review of the uncertainty regarding size, Shamblin class, and the impact these have on cranial nerve injury.2 The bottom line is that there is no data-based algorithm for deciding whom to select for preoperative embolization.
In the absence of compelling data for the use of preoperative embolization, this technique is difficult to recommend. There are undoubtedly cases with giant CBTs where a surgeon will want to take advantage of any preoperative adjuvant therapy that is available. However, for the vast majority of CBTs, the important principles are to stay in the correct plane, identify and protect the nerves at risk, and control the tumor blood supply in a systematic fashion. In the absence of a forthcoming randomized prospective study to evaluate preoperative embolization for CBT resection, I will rely on the wisdom of my mentor, Dr. Wesley Moore. He never utilized preoperative embolization for these cases. At least not that he will admit to me.
References
1. Surgery. 1980;87:459-464.
2. Head Neck. 2016;38:E2386-E2394.
3. J Vasc Surg. 2015;61:1081-91.
4. Otolaryngol Head Neck Surg. 2015;153:942-950.
Preop embolization is safe and effective
To embolize or not to embolize ... that is the question when it comes to the management of carotid body tumors. Carotid body tumors (CBTs) are rare benign neoplasms that are almost always nonfunctional and account for up to 80% of all head and neck paragangliomas. Radiographic characteristics include tumor location at the carotid bifurcation, splaying apart the internal and external carotid arteries. Surgical resection is the mainstay of definitive treatment and is preferably performed at diagnosis. CBTs are categorized based upon their involvement with surrounding structures: Shamblin I tumors are small, without encasement of the carotid arteries; Shamblin II tumors partially encase the vessels; and Shamblin III tumors completely encase the vessels. When it comes to operative complications, advanced Shamblin stage is associated with a higher rate of cranial nerve injury when the tumor is resected.1
Pertinent to the technical aspects of surgical resection, CBTs are characteristically encased with an elaborate network of friable vessels which may contribute to significant intraoperative bleeding, especially with resection of larger tumors. The blood supply to CBTs typically originates from the branches of the external carotid artery. These branches may be sacrificed with minimal consequence using preoperative embolization. This common practice is felt by many to facilitate safe resection while minimizing intraoperative blood loss. Although the reduction of blood loss has not been observed universally, there is enough evidence of this in the literature to support the use of selective preoperative embolization. Several embolic agents have been described in this setting, including polyvinyl alcohol particles (150-1000 microns), polymerized glue (n-butyl cyanoacrylate), and more recently, ethylene-vinyl alcohol copolymer (Onyx, ev3, Irvine, Calif.). Risks of complications related to CBT embolization in experienced hands are quite low with no adverse events reported in a number of reports describing the technique.2,3
Although embolization is argued to decrease blood loss, its impact on rates of neurologic complications is admittedly insignificant. One might question the added cost of the embolization procedure in the current atmosphere of focus on cost-containment, but this may be defrayed by lower rates of postoperative hematoma and operative times. Although blood loss has been shown to be decreased in a number of reports, translating this to decreased need for transfusion has unfortunately yet to be demonstrated. Until large-volume prospective randomized studies of the outcomes associated with preoperative embolization of CBTs are available, its use should be considered mainly when risks of blood loss are significant in advanced stage tumors and only if the associated complication risks associated with its use can be kept to acceptably low rates. As with other adjunctive procedures whose merit has not yet been convincingly sorted out in the available data, personal experience and preference often play a role in the decision making process we all face. For those who have experienced the added challenge of the meticulous dissection of the CBT in the face of a bloodied field, having such tools as embolization at our fingertips is a reassuring adjunct to be considered.
References
1. J Vasc Surg. 2013;57:64-8S.
2. J Vasc Interv Neurol. 2008;1(2):37-41.
3. Am J Neuroradiol. 2009;30:1594-97.
4. J Vasc Surg. 2012;56:979-89.
5. Vasc Endovasc Surg. 2009;43:457-61.
Too many downsides
As vascular surgeons, we are frequently confronting situations where the “best” approach to a problem is far from established. The evidence to make a clinical decision may simply not exist, and we need to choose a course of therapy based more on personal preference than science. Such is the case with preoperative embolization for the resection of carotid body tumors (CBT).
It has been over 35 years since the technique for preoperative embolization of CBT was described, the aim being reduction in blood loss, decreased operative time, and improved visualization for safer tumor resection. This is based on the fact that most of the arterial supply to these tumors arises from external carotid branches (particularly the ascending pharyngeal), and these that can be safely sacrificed. Although this technique is certainly now a standard part of treatment for many vascular surgeons, the evidence of benefit is certainly not overwhelming, and like all interventions, nothing comes without risks.
So what are the downsides of preoperative embolization? The cost of the procedure itself is not insignificant, in some cases doubling the expense of the intervention. And there can also be organizational issues with coordinating the time in the endovascular suite versus the operating room. In my experience, we have taken patients directly from embolization for the CBT resection, and there are frequently delays and transport issues. Although advocates may claim decreased operative time as a benefit of embolization, this is attained at an overall increase in anesthetic time for the combined procedures. If a day or two separates the procedures, some patients do report the pain or fevers that can accompany any tumor embolization.
While the above may be nuisances, there are serious adverse effects from preoperative embolization as well. A review of 11 studies of preoperative embolization for CBT found a 2.5% rate of complications, including temporary aphasia, vocal cord paralysis, and arterial dissection. Stroke with permanent deficit has also been reported secondary to CBT embolization. Access site hematoma has also been reported as with any catheter-based intervention.
With regards to “selective” preoperative embolization, the literature again offers a mixed bag. Many advocates of embolization rely upon the Shamblin classification for selecting candidates, reserving embolization for Shamblin II and III tumors. However, the extent of carotid involvement is sometimes a surgical diagnosis. Although size of tumor generally correlates with Shamblin class, even smaller tumors can demonstrate arterial wall involvement. Abu-Ghanem provides a succinct review of the uncertainty regarding size, Shamblin class, and the impact these have on cranial nerve injury.2 The bottom line is that there is no data-based algorithm for deciding whom to select for preoperative embolization.
In the absence of compelling data for the use of preoperative embolization, this technique is difficult to recommend. There are undoubtedly cases with giant CBTs where a surgeon will want to take advantage of any preoperative adjuvant therapy that is available. However, for the vast majority of CBTs, the important principles are to stay in the correct plane, identify and protect the nerves at risk, and control the tumor blood supply in a systematic fashion. In the absence of a forthcoming randomized prospective study to evaluate preoperative embolization for CBT resection, I will rely on the wisdom of my mentor, Dr. Wesley Moore. He never utilized preoperative embolization for these cases. At least not that he will admit to me.
References
1. Surgery. 1980;87:459-464.
2. Head Neck. 2016;38:E2386-E2394.
3. J Vasc Surg. 2015;61:1081-91.
4. Otolaryngol Head Neck Surg. 2015;153:942-950.
Preop embolization is safe and effective
To embolize or not to embolize ... that is the question when it comes to the management of carotid body tumors. Carotid body tumors (CBTs) are rare benign neoplasms that are almost always nonfunctional and account for up to 80% of all head and neck paragangliomas. Radiographic characteristics include tumor location at the carotid bifurcation, splaying apart the internal and external carotid arteries. Surgical resection is the mainstay of definitive treatment and is preferably performed at diagnosis. CBTs are categorized based upon their involvement with surrounding structures: Shamblin I tumors are small, without encasement of the carotid arteries; Shamblin II tumors partially encase the vessels; and Shamblin III tumors completely encase the vessels. When it comes to operative complications, advanced Shamblin stage is associated with a higher rate of cranial nerve injury when the tumor is resected.1
Pertinent to the technical aspects of surgical resection, CBTs are characteristically encased with an elaborate network of friable vessels which may contribute to significant intraoperative bleeding, especially with resection of larger tumors. The blood supply to CBTs typically originates from the branches of the external carotid artery. These branches may be sacrificed with minimal consequence using preoperative embolization. This common practice is felt by many to facilitate safe resection while minimizing intraoperative blood loss. Although the reduction of blood loss has not been observed universally, there is enough evidence of this in the literature to support the use of selective preoperative embolization. Several embolic agents have been described in this setting, including polyvinyl alcohol particles (150-1000 microns), polymerized glue (n-butyl cyanoacrylate), and more recently, ethylene-vinyl alcohol copolymer (Onyx, ev3, Irvine, Calif.). Risks of complications related to CBT embolization in experienced hands are quite low with no adverse events reported in a number of reports describing the technique.2,3
Although embolization is argued to decrease blood loss, its impact on rates of neurologic complications is admittedly insignificant. One might question the added cost of the embolization procedure in the current atmosphere of focus on cost-containment, but this may be defrayed by lower rates of postoperative hematoma and operative times. Although blood loss has been shown to be decreased in a number of reports, translating this to decreased need for transfusion has unfortunately yet to be demonstrated. Until large-volume prospective randomized studies of the outcomes associated with preoperative embolization of CBTs are available, its use should be considered mainly when risks of blood loss are significant in advanced stage tumors and only if the associated complication risks associated with its use can be kept to acceptably low rates. As with other adjunctive procedures whose merit has not yet been convincingly sorted out in the available data, personal experience and preference often play a role in the decision making process we all face. For those who have experienced the added challenge of the meticulous dissection of the CBT in the face of a bloodied field, having such tools as embolization at our fingertips is a reassuring adjunct to be considered.
References
1. J Vasc Surg. 2013;57:64-8S.
2. J Vasc Interv Neurol. 2008;1(2):37-41.
3. Am J Neuroradiol. 2009;30:1594-97.
4. J Vasc Surg. 2012;56:979-89.
5. Vasc Endovasc Surg. 2009;43:457-61.
Too many downsides
As vascular surgeons, we are frequently confronting situations where the “best” approach to a problem is far from established. The evidence to make a clinical decision may simply not exist, and we need to choose a course of therapy based more on personal preference than science. Such is the case with preoperative embolization for the resection of carotid body tumors (CBT).
It has been over 35 years since the technique for preoperative embolization of CBT was described, the aim being reduction in blood loss, decreased operative time, and improved visualization for safer tumor resection. This is based on the fact that most of the arterial supply to these tumors arises from external carotid branches (particularly the ascending pharyngeal), and these that can be safely sacrificed. Although this technique is certainly now a standard part of treatment for many vascular surgeons, the evidence of benefit is certainly not overwhelming, and like all interventions, nothing comes without risks.
So what are the downsides of preoperative embolization? The cost of the procedure itself is not insignificant, in some cases doubling the expense of the intervention. And there can also be organizational issues with coordinating the time in the endovascular suite versus the operating room. In my experience, we have taken patients directly from embolization for the CBT resection, and there are frequently delays and transport issues. Although advocates may claim decreased operative time as a benefit of embolization, this is attained at an overall increase in anesthetic time for the combined procedures. If a day or two separates the procedures, some patients do report the pain or fevers that can accompany any tumor embolization.
While the above may be nuisances, there are serious adverse effects from preoperative embolization as well. A review of 11 studies of preoperative embolization for CBT found a 2.5% rate of complications, including temporary aphasia, vocal cord paralysis, and arterial dissection. Stroke with permanent deficit has also been reported secondary to CBT embolization. Access site hematoma has also been reported as with any catheter-based intervention.
With regards to “selective” preoperative embolization, the literature again offers a mixed bag. Many advocates of embolization rely upon the Shamblin classification for selecting candidates, reserving embolization for Shamblin II and III tumors. However, the extent of carotid involvement is sometimes a surgical diagnosis. Although size of tumor generally correlates with Shamblin class, even smaller tumors can demonstrate arterial wall involvement. Abu-Ghanem provides a succinct review of the uncertainty regarding size, Shamblin class, and the impact these have on cranial nerve injury.2 The bottom line is that there is no data-based algorithm for deciding whom to select for preoperative embolization.
In the absence of compelling data for the use of preoperative embolization, this technique is difficult to recommend. There are undoubtedly cases with giant CBTs where a surgeon will want to take advantage of any preoperative adjuvant therapy that is available. However, for the vast majority of CBTs, the important principles are to stay in the correct plane, identify and protect the nerves at risk, and control the tumor blood supply in a systematic fashion. In the absence of a forthcoming randomized prospective study to evaluate preoperative embolization for CBT resection, I will rely on the wisdom of my mentor, Dr. Wesley Moore. He never utilized preoperative embolization for these cases. At least not that he will admit to me.
References
1. Surgery. 1980;87:459-464.
2. Head Neck. 2016;38:E2386-E2394.
3. J Vasc Surg. 2015;61:1081-91.
4. Otolaryngol Head Neck Surg. 2015;153:942-950.
It’s time for us to talk about guns
Studies have shown that most of you already have deep-seated beliefs regarding guns. Some of you would frame the issue as Gun Rights, others as Gun Violence. I am not here to change your opinion. I am not in the habit of wasting my time. Logic has been drained from this discussion and emotion infused. As vascular surgeons, it is far past time to overcome these limitations and join the national discussion. Opinions and consensus statements have already been rendered from the American College of Surgeons, the American Medical Association, the American Academy of Pediatrics, the Society of Thoracic Surgeons, and even the American College of Phlebology. Where does the SVS stand?
In January 2013, the Board of Directors of the SVS voted to support the ACS Statement on Firearm Injuries. There is virtually no public record of this endorsement, it does not appear on the SVS website and it was essentially ignored by the public.
Even the diligent National Rifle Association (NRA) left the SVS off their list of “National Organizations with Anti-Gun Policies” (Ed note: for more information, see “The Evolution of the NRA and Our Modern Gun Debate” at www.vascularspecialistonline.com).
We need to do better. If we are truly an independent specialty it is time to behave as such. Vascular surgeons are on the front lines of this battle. We have cared for the injured, revived the dying, and bear witness to the dead. To not have a voice and be counted is a disservice to our patients and ourselves.
What can be done to reduce gun violence? In Australia, between 1979 and 1996, there were 13 mass shootings. After a semiautomatic weapon ban was instituted in 1996 there have been none. The U.S. ban on military style weapons lapsed in 2004. While it is difficult to characterize “mass shootings” in a country our size, there certainly seems to be an increase since then. If defined as “four or more shot and/or killed in a single event, at the same general time and location not including the shooter,” then we have seen 275 mass shootings this year as of Oct. 5, 2017.
The other statistics are familiar and sobering. More Americans have died from guns since 1968 than have died in all the wars since our country’s inception. The United States accounts for 91% of gun deaths of children among developed countries. Our casualty figures more closely mirror Somalia and Honduras, not Britain or Germany.
Contemporary, large-scale research in limiting gun violence is essentially nonexistent since a 1993 Centers for Disease Control and Prevention (CDC) funded study found a link between keeping a gun in the home with an increased risk of homicide. Quick to respond, Congress passed the 1996 Dickey Amendment that prohibits the CDC from funding efforts that “advocate or promote gun control.” This amendment has been renewed every year despite the author of the bill, Representative Jay Dickey, expressing regret for halting all gun research, stating that was not his intention. Rep. Dickey died earlier this year.
In the U.S., gun laws have actually relaxed over time. In 1988, 18 states had laws allowing civilians to carry concealed hand guns in public places, now this practice is legal in 40 states. In a 2008 landmark decision, the Supreme Court struck down a personal handgun ban in the District of Columbia. The Second Amendment rights afforded to a “well-regulated militia” to “keep and bear arms” were now extended to private individuals. Guns are clearly more prevalent and available in the U.S. than ever before.
The congressional ban on firearms research now extends to all Department of Health and Human Services agencies, including the NIH. We need the Dickey Amendment lifted so we can study the relationship of gun ownership and crime. As physicians, we need to deal from an informed, intelligent position and not an emotional one.
Over 50 medical societies, comprising essentially every physician in the U.S., have released statements on gun violence. The AMA has labeled it a “public health crisis.” The ACS stated, in the aftermath of the Las Vegas shooting, “It is important that the American College of Surgeons, whose Fellows care for the victims of these events, be part of the solution.”
Aside from ethical or moral obligations, why should we dive into this quagmire? Most of us are already represented in the discussion through other groups. The answer lies in our identity. If vascular surgery is to become a truly independent specialty, we can’t hide behind the ACS or the AMA when the politics become sticky.
To protect the 30,000 people who die from aneurysm rupture yearly we literally forced an act of Congress. Where do we stand on the more than 33,000 people who die yearly from gun violence? For vascular surgery to have a true public presence, we must be prepared to enter the most public of all discussions.
Luckily, there is already a pathway to consensus. The American College of Surgeons Committee on Trauma (ACS COT) surveyed its members and found that only 15% had no strong opinions on firearms. Just over 50% felt that guns were important for personal safety and defense, while 30% felt the large number of guns in the U.S. was a threat to safety.
Individuals who felt that firearms were important were most likely to associate guns with personal freedom, while those who felt they were a threat were most likely to associate guns with violence.
To further the discussion, the emotional battle between personal freedom and violence needed to be minimized. In doing so, the ACS COT was able to produce a consensus statement despite the seemingly diametrically opposed opinions of its members.
An independent specialty needs an independent voice. If we don’t know our own position, obviously the public doesn’t either.
As a starting point, here is the ACS Statement on Firearms Injuries:
Because violence inflicted by guns continues to be a daily event in the United States and mass casualties involving firearms threaten the health and safety of the public, the American College of Surgeons supports:
1. Legislation banning civilian access to assault weapons, large ammunition clips, and munitions designed for military and law enforcement agencies.
2. Enhancing mandatory background checks for the purchase of firearms to include gun shows and auctions.
3. Assuring that health care professionals can fulfill their role in preventing firearm injuries by health screening, patient counseling, and referral to mental health services for those with behavioral medical conditions. 4. Developing and promoting proactive programs directed at improving safe gun storage and the teaching of nonviolent conflict resolution for a culture that often glorifies guns and violence in media and gaming.
5. Evidence-based research on firearm injury and the creation of a national firearm injury database to inform federal health policy.
Selected References
1) Gun Violence Research: History of Federal Funding Freeze (www.apa.org/science/about/psa/2013/02/gun-violence.aspx)
2) Childhood Firearm Injuries in the United States (www.apa.org/science/about/psa/2013/02/gun-violence.aspx)
3) Gun Violence Letter to the U.S. House of Representatives (2013) (www.acponline.org/acp_policy/letters/gun_violence_letter_house_2013.pdf)
4) Survey of American College of Surgeons Committee on Trauma members on firearm injury: Consensus and opportunities (2016) (www.facs.org).
5) American College of Surgeons Statement on Firearm Injuries (www.facs.org)
Studies have shown that most of you already have deep-seated beliefs regarding guns. Some of you would frame the issue as Gun Rights, others as Gun Violence. I am not here to change your opinion. I am not in the habit of wasting my time. Logic has been drained from this discussion and emotion infused. As vascular surgeons, it is far past time to overcome these limitations and join the national discussion. Opinions and consensus statements have already been rendered from the American College of Surgeons, the American Medical Association, the American Academy of Pediatrics, the Society of Thoracic Surgeons, and even the American College of Phlebology. Where does the SVS stand?
In January 2013, the Board of Directors of the SVS voted to support the ACS Statement on Firearm Injuries. There is virtually no public record of this endorsement, it does not appear on the SVS website and it was essentially ignored by the public.
Even the diligent National Rifle Association (NRA) left the SVS off their list of “National Organizations with Anti-Gun Policies” (Ed note: for more information, see “The Evolution of the NRA and Our Modern Gun Debate” at www.vascularspecialistonline.com).
We need to do better. If we are truly an independent specialty it is time to behave as such. Vascular surgeons are on the front lines of this battle. We have cared for the injured, revived the dying, and bear witness to the dead. To not have a voice and be counted is a disservice to our patients and ourselves.
What can be done to reduce gun violence? In Australia, between 1979 and 1996, there were 13 mass shootings. After a semiautomatic weapon ban was instituted in 1996 there have been none. The U.S. ban on military style weapons lapsed in 2004. While it is difficult to characterize “mass shootings” in a country our size, there certainly seems to be an increase since then. If defined as “four or more shot and/or killed in a single event, at the same general time and location not including the shooter,” then we have seen 275 mass shootings this year as of Oct. 5, 2017.
The other statistics are familiar and sobering. More Americans have died from guns since 1968 than have died in all the wars since our country’s inception. The United States accounts for 91% of gun deaths of children among developed countries. Our casualty figures more closely mirror Somalia and Honduras, not Britain or Germany.
Contemporary, large-scale research in limiting gun violence is essentially nonexistent since a 1993 Centers for Disease Control and Prevention (CDC) funded study found a link between keeping a gun in the home with an increased risk of homicide. Quick to respond, Congress passed the 1996 Dickey Amendment that prohibits the CDC from funding efforts that “advocate or promote gun control.” This amendment has been renewed every year despite the author of the bill, Representative Jay Dickey, expressing regret for halting all gun research, stating that was not his intention. Rep. Dickey died earlier this year.
In the U.S., gun laws have actually relaxed over time. In 1988, 18 states had laws allowing civilians to carry concealed hand guns in public places, now this practice is legal in 40 states. In a 2008 landmark decision, the Supreme Court struck down a personal handgun ban in the District of Columbia. The Second Amendment rights afforded to a “well-regulated militia” to “keep and bear arms” were now extended to private individuals. Guns are clearly more prevalent and available in the U.S. than ever before.
The congressional ban on firearms research now extends to all Department of Health and Human Services agencies, including the NIH. We need the Dickey Amendment lifted so we can study the relationship of gun ownership and crime. As physicians, we need to deal from an informed, intelligent position and not an emotional one.
Over 50 medical societies, comprising essentially every physician in the U.S., have released statements on gun violence. The AMA has labeled it a “public health crisis.” The ACS stated, in the aftermath of the Las Vegas shooting, “It is important that the American College of Surgeons, whose Fellows care for the victims of these events, be part of the solution.”
Aside from ethical or moral obligations, why should we dive into this quagmire? Most of us are already represented in the discussion through other groups. The answer lies in our identity. If vascular surgery is to become a truly independent specialty, we can’t hide behind the ACS or the AMA when the politics become sticky.
To protect the 30,000 people who die from aneurysm rupture yearly we literally forced an act of Congress. Where do we stand on the more than 33,000 people who die yearly from gun violence? For vascular surgery to have a true public presence, we must be prepared to enter the most public of all discussions.
Luckily, there is already a pathway to consensus. The American College of Surgeons Committee on Trauma (ACS COT) surveyed its members and found that only 15% had no strong opinions on firearms. Just over 50% felt that guns were important for personal safety and defense, while 30% felt the large number of guns in the U.S. was a threat to safety.
Individuals who felt that firearms were important were most likely to associate guns with personal freedom, while those who felt they were a threat were most likely to associate guns with violence.
To further the discussion, the emotional battle between personal freedom and violence needed to be minimized. In doing so, the ACS COT was able to produce a consensus statement despite the seemingly diametrically opposed opinions of its members.
An independent specialty needs an independent voice. If we don’t know our own position, obviously the public doesn’t either.
As a starting point, here is the ACS Statement on Firearms Injuries:
Because violence inflicted by guns continues to be a daily event in the United States and mass casualties involving firearms threaten the health and safety of the public, the American College of Surgeons supports:
1. Legislation banning civilian access to assault weapons, large ammunition clips, and munitions designed for military and law enforcement agencies.
2. Enhancing mandatory background checks for the purchase of firearms to include gun shows and auctions.
3. Assuring that health care professionals can fulfill their role in preventing firearm injuries by health screening, patient counseling, and referral to mental health services for those with behavioral medical conditions. 4. Developing and promoting proactive programs directed at improving safe gun storage and the teaching of nonviolent conflict resolution for a culture that often glorifies guns and violence in media and gaming.
5. Evidence-based research on firearm injury and the creation of a national firearm injury database to inform federal health policy.
Selected References
1) Gun Violence Research: History of Federal Funding Freeze (www.apa.org/science/about/psa/2013/02/gun-violence.aspx)
2) Childhood Firearm Injuries in the United States (www.apa.org/science/about/psa/2013/02/gun-violence.aspx)
3) Gun Violence Letter to the U.S. House of Representatives (2013) (www.acponline.org/acp_policy/letters/gun_violence_letter_house_2013.pdf)
4) Survey of American College of Surgeons Committee on Trauma members on firearm injury: Consensus and opportunities (2016) (www.facs.org).
5) American College of Surgeons Statement on Firearm Injuries (www.facs.org)
Studies have shown that most of you already have deep-seated beliefs regarding guns. Some of you would frame the issue as Gun Rights, others as Gun Violence. I am not here to change your opinion. I am not in the habit of wasting my time. Logic has been drained from this discussion and emotion infused. As vascular surgeons, it is far past time to overcome these limitations and join the national discussion. Opinions and consensus statements have already been rendered from the American College of Surgeons, the American Medical Association, the American Academy of Pediatrics, the Society of Thoracic Surgeons, and even the American College of Phlebology. Where does the SVS stand?
In January 2013, the Board of Directors of the SVS voted to support the ACS Statement on Firearm Injuries. There is virtually no public record of this endorsement, it does not appear on the SVS website and it was essentially ignored by the public.
Even the diligent National Rifle Association (NRA) left the SVS off their list of “National Organizations with Anti-Gun Policies” (Ed note: for more information, see “The Evolution of the NRA and Our Modern Gun Debate” at www.vascularspecialistonline.com).
We need to do better. If we are truly an independent specialty it is time to behave as such. Vascular surgeons are on the front lines of this battle. We have cared for the injured, revived the dying, and bear witness to the dead. To not have a voice and be counted is a disservice to our patients and ourselves.
What can be done to reduce gun violence? In Australia, between 1979 and 1996, there were 13 mass shootings. After a semiautomatic weapon ban was instituted in 1996 there have been none. The U.S. ban on military style weapons lapsed in 2004. While it is difficult to characterize “mass shootings” in a country our size, there certainly seems to be an increase since then. If defined as “four or more shot and/or killed in a single event, at the same general time and location not including the shooter,” then we have seen 275 mass shootings this year as of Oct. 5, 2017.
The other statistics are familiar and sobering. More Americans have died from guns since 1968 than have died in all the wars since our country’s inception. The United States accounts for 91% of gun deaths of children among developed countries. Our casualty figures more closely mirror Somalia and Honduras, not Britain or Germany.
Contemporary, large-scale research in limiting gun violence is essentially nonexistent since a 1993 Centers for Disease Control and Prevention (CDC) funded study found a link between keeping a gun in the home with an increased risk of homicide. Quick to respond, Congress passed the 1996 Dickey Amendment that prohibits the CDC from funding efforts that “advocate or promote gun control.” This amendment has been renewed every year despite the author of the bill, Representative Jay Dickey, expressing regret for halting all gun research, stating that was not his intention. Rep. Dickey died earlier this year.
In the U.S., gun laws have actually relaxed over time. In 1988, 18 states had laws allowing civilians to carry concealed hand guns in public places, now this practice is legal in 40 states. In a 2008 landmark decision, the Supreme Court struck down a personal handgun ban in the District of Columbia. The Second Amendment rights afforded to a “well-regulated militia” to “keep and bear arms” were now extended to private individuals. Guns are clearly more prevalent and available in the U.S. than ever before.
The congressional ban on firearms research now extends to all Department of Health and Human Services agencies, including the NIH. We need the Dickey Amendment lifted so we can study the relationship of gun ownership and crime. As physicians, we need to deal from an informed, intelligent position and not an emotional one.
Over 50 medical societies, comprising essentially every physician in the U.S., have released statements on gun violence. The AMA has labeled it a “public health crisis.” The ACS stated, in the aftermath of the Las Vegas shooting, “It is important that the American College of Surgeons, whose Fellows care for the victims of these events, be part of the solution.”
Aside from ethical or moral obligations, why should we dive into this quagmire? Most of us are already represented in the discussion through other groups. The answer lies in our identity. If vascular surgery is to become a truly independent specialty, we can’t hide behind the ACS or the AMA when the politics become sticky.
To protect the 30,000 people who die from aneurysm rupture yearly we literally forced an act of Congress. Where do we stand on the more than 33,000 people who die yearly from gun violence? For vascular surgery to have a true public presence, we must be prepared to enter the most public of all discussions.
Luckily, there is already a pathway to consensus. The American College of Surgeons Committee on Trauma (ACS COT) surveyed its members and found that only 15% had no strong opinions on firearms. Just over 50% felt that guns were important for personal safety and defense, while 30% felt the large number of guns in the U.S. was a threat to safety.
Individuals who felt that firearms were important were most likely to associate guns with personal freedom, while those who felt they were a threat were most likely to associate guns with violence.
To further the discussion, the emotional battle between personal freedom and violence needed to be minimized. In doing so, the ACS COT was able to produce a consensus statement despite the seemingly diametrically opposed opinions of its members.
An independent specialty needs an independent voice. If we don’t know our own position, obviously the public doesn’t either.
As a starting point, here is the ACS Statement on Firearms Injuries:
Because violence inflicted by guns continues to be a daily event in the United States and mass casualties involving firearms threaten the health and safety of the public, the American College of Surgeons supports:
1. Legislation banning civilian access to assault weapons, large ammunition clips, and munitions designed for military and law enforcement agencies.
2. Enhancing mandatory background checks for the purchase of firearms to include gun shows and auctions.
3. Assuring that health care professionals can fulfill their role in preventing firearm injuries by health screening, patient counseling, and referral to mental health services for those with behavioral medical conditions. 4. Developing and promoting proactive programs directed at improving safe gun storage and the teaching of nonviolent conflict resolution for a culture that often glorifies guns and violence in media and gaming.
5. Evidence-based research on firearm injury and the creation of a national firearm injury database to inform federal health policy.
Selected References
1) Gun Violence Research: History of Federal Funding Freeze (www.apa.org/science/about/psa/2013/02/gun-violence.aspx)
2) Childhood Firearm Injuries in the United States (www.apa.org/science/about/psa/2013/02/gun-violence.aspx)
3) Gun Violence Letter to the U.S. House of Representatives (2013) (www.acponline.org/acp_policy/letters/gun_violence_letter_house_2013.pdf)
4) Survey of American College of Surgeons Committee on Trauma members on firearm injury: Consensus and opportunities (2016) (www.facs.org).
5) American College of Surgeons Statement on Firearm Injuries (www.facs.org)
Open vs. endo repair for ruptured AAA
Open repair should be offered to all patients with rAAA
With the advent of endovascular repair of abdominal aortic aneurysms (EVAR), the treatment of elective AAAs was revolutionized. Since ruptured abdominal aortic aneurysms (rAAAs) carry a higher morbidity and mortality than elective AAA repair the use of EVAR has been advocated for these patients.1 Dr. Aziz contends that EVAR should be utilized in all patients presenting with a rAAA. It is my contention that endovascular repair cannot replace open aneurysm repair in all situations. The best treatment option should be offered taking patient and institutional considerations into account. Forcing a given procedure and trying to “make it work” is not best for the patient.
In a systematic literature review of patients presenting with rAAAs, selection bias regarding treatment choice (EVAR vs. open repair) was found consistently.2 In an effort to show that EVAR is superior to open repair for rAAA, Hinchliffe et al. published a randomized trial that showed no difference in 30-day mortality (53%) in each treatment group.3 The AJAX trial randomized 116 patients and did not show benefit for EVAR with respect to 30-day morbidity or mortality.4 The ECAR trial randomized 107 patients and also failed to show a difference in mortality between EVAR and open techniques for rAAA.5
Determining suitability for EVAR includes assessing diameter of the neck of the aneurysm, angulation, size of iliac arteries, presence of atherosclerotic occlusive disease, presence of accessory renal arteries, and presence of concurrent aneurysms of the iliac arteries.7,8 In the IMPROVE trial, patients were randomized depending on anatomic suitability for EVAR.9 Anatomy was determined by imaging in those patients stable enough to undergo preoperative computed tomography angiogram (CTA). Therefore, given anatomic differences, patients presenting with rAAA were not randomized without significant treatment bias.
A true pararenal or paravisceral rupture certainly elevates the complexity of the case. Open repair can deal with this difficult challenge by adapting additional established techniques to the situation. Although some authors have advocated EVAR for rAAA using snorkels and chimneys, these techniques may not be appropriate in the treatment of rAAA in most institutions.10 These procedures take longer, require considerable experience and resources and are therefore not appropriate in unstable patients. No randomized control trial exists at this time to address superiority of EVAR to open repair in patients with complex anatomy.
Patients presenting with rAAA that have had prior endovascular intervention also pose complex anatomic and technical challenges.11 These patients can present with hemodynamic instability and rupture but now are complicated by having device failure. Salvage using endovascular means may not be possible. The risk of re-intervention with endovascular repair for rAAA is much higher than with open repair.12
Emergency vascular surgery is best approached with protocols and systems in place no matter what approach is chosen.13 Skilled staff in the emergency department and in the operating room must be available without delay especially for rAAA, as time is of the essence. Skilled surgeons, skilled operating room staff, knowledgeable anesthesia staff are necessary to quickly move a patient from the emergency department to the operating room for definitive treatment. Other ancillary services, such as blood banks and laboratories are necessary to assist in the care for the patient intraoperatively. In endovascular surgery, trained technologists, fluoroscopy, a full armamentarium of interventional equipment (wires, balloons, stents, etc.), and a full stock of stent grafts and associated ancillary equipment are mandatory. Many hospitals may not have the variety of stent grafts available for emergency use. Regionalization of care has been suggested to improve outcomes in the treatment of rAAAs.14 In a study by Warner et al., mortality was decreased by 20% at the tertiary center compared to the smaller community hospitals in both EVAR and open repairs for rAAA. Zettervall et al. published data that showed that significant regional variation exists in perioperative outcomes and length of stay, and mortality in repair for rAAA approached with open or endovascular means.15 Low volume centers have worse outcomes, and teaching hospitals may have better outcomes treating AAA.16
EVAR is also reported to be associated with a 27-36% higher cost of care than open surgery for rAAA.17 Many institutions may not have the means to provide the resources needed to support such a program.
There is no question that EVAR has greatly impacted the treatment of aortic aneurysms. However, not every aneurysm is appropriate for endovascular repair at this time. For the many reasons cited, we cannot permit open repair to become an extinct skill. Vascular training programs must include open repair of rAAA just as it should for other vascular conditions.
I would agree with Dr. Aziz that we do not yet have a prospective, randomized study and Level 1 evidence to support either view and given the complexity and urgency associated with these patients, such a study may not be forthcoming. Till then, patients with a RAAA should have ‘all of the above’ approach including open surgery depending on the institutional experience and resources and urgency of the situation.
References
1. J Vasc Surg 2016; 2:297-305
2. J Vasc Surg 2009; 49:1077-1080.
3. Eur J Vasc Endovasc Surg 2006; 32:506-513
4. Ann Surg 2013. Aug 258 (2) 248-56
5. J Vasc Surg 2010; 51:267-70
6. Ann Vasc Surg 2017; 38:59-63
7. J Vasc Surg 2009: 50:243-50
8. J Endovasc Ther 2016; 23: 919-927
9. BJM 2014; 101:216-224
10. Radiographics 2015; 35:593-615.
11. Ann Vasc Surg 2013; 27:844-50
12. J Vasc Surg 2017; 65:52-57
13. Semin Vasc Surg 2016; 29:35-40
14. J Vasc Surg 2014; 59:1512-7
15. Ann Surg 2017; 264:538-43
16. J Vasc Surg 2016; 22 pii W0741-5214 (16) 31247-2
17. J Vasc Surg 2008; 47:1165-70
Dr. Ozsvatrh is a professor of surgery at Albany Medical College, Albany, N.Y., and a vascular attending, at Albany Medical Center Hospital, Albany, and chief, department of surgery, Samaritan Hospital, Troy, N.Y.
EVAR should be offered to all patients with rAAA
Since the inception of EVAR two decades ago, it has largely replaced open abdominal aortic aneurysm repair as the operation of choice for elective treatment of abdominal aortic aneurysms. Shorter duration of operation, avoiding general anesthesia and aortic cross clamping, ability to obtain balloon control of aorta, fast recovery time and reduced hospital length of hospital stay are among the most commonly cited reasons for this change in paradigm for treatment of abdominal aortic aneurysms. Since vascular surgeons have adopted the widespread use of EVAR, the total number of aneurysm related deaths has significantly reduced.1 While EVAR has become the most commonly used operation to treat AAAs electively, it’s utility to treat ruptured AAAs has been questioned. Recent analysis of nationwide trends for the operations for the diagnosis of ruptured AAA show that almost half of the patients with ruptured AAA are still treated with open surgical repair.2
Opponents of REVAR (EVAR for rAAA) cite comparable outcomes of open surgery and REVAR in randomized controlled trials, requirement of a specific REVAR protocol, increased cost and lack of resources and more importantly, lack of level I evidence to support it’s use in all cases of rAAA.
It is no surprise that REVAR is associated with significantly lower perioperative mortality (24%) as compared to open repair of ruptured AAA (44%).3 Mortality after open repair of ruptured AAA can vary anywhere from 30-70%.4,5 Improved mortality rates after REVAR are not only limited to stable patients, but also extend to those patients with ruptured AAA who are considered hemodynamically unstable at the time of initial presentation.6
The survival advantage among REVAR patients is not only evident in short term (30-days), but it also extends up to 5 years after the operation.3 Patients undergoing REVAR are less likely to require intra-operative blood transfusions, as compared to patients who undergo open repair of rAAA.7 Development of acute renal failure after surgical treatment of rAAAs is associated with significant mortality and REVAR has been associated with significantly lower incidence of acute renal failure as compared to open repair of ruptured AAA.2
Due to inherent issues of patient instability, emergent presentation and inequality among hospital resources and patient volumes, it is difficult to design a flawless, randomized controlled trial to compare REVAR with open surgical repair. The results from published randomized controlled trials on this topic should be interpreted with caution. The Nottingham trial from the United Kingdom8 showed similar mortality (53%) between REVAR and open surgical operation, however, out of the 203 patients enrolled in the trial, 70% were not randomized and REVAR was offered to only 15 patients. Likewise, the AJAX Trial from Netherlands9 showed that there was no significant difference in mortality between the two treatment groups (42% with REVAR and 47% with open repair), however almost 80% of enrolled patients were not randomized and there was a disagreement between the interpreters about CTA diagnosis of rAAA. In addition, the conversion rates from REVAR to open repair were unexpectedly high (14%), pointing to the surgeons’ inexperience.
Similarly, the IMPROVE trial from United Kingdom10 suffered from poor rates of randomization (50% of enrolled patients) and poor methodology (obtaining CTA after randomization, and making requirement of CTA optional for patients allocated to open surgery group). The trial showed equivalent mortality rates between the two surgical groups (35% with REVAR and 37% with open repair).
Due to flawed methodology and poor rates of randomization, results of these trials do not provide us with level I evidence, which is required to make any scientific recommendations about the treatment of choice for the treatment of rAAAs. Hence, the large body of evidence, obtained from retrospective studies should be used while comparing the outcomes of operations for ruptured AAAs. So far, all retrospective analyses, comparing REVAR with open repair in national registries, such as National Inpatient Sample (NIS) database11,12 American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database7 and Medicare database13 have clearly shown that the outcomes of REVAR are better than open surgical repair of ruptured AAA. Likewise, multi-center observational studies14,15 have demonstrated superiority of REVAR over open surgical repair.
To summarize, REVAR is a safe procedure, can be performed under local anesthetic, has shorter operative time and is associated with improved outcomes as compared to open surgery. REVAR should be the treatment of choice for all patients who meet the anatomic criteria for endovascular repair. With increasing experience of vascular surgeons with the endovascular technology, it is foreseeable that REVAR will soon become the treatment of choice for ruptured abdominal aortic aneurysms.
References
1. J Vasc Surg 2009;49:543-50; discussion 50-1.
2. Ann Vasc Surg 2016;35:147-55.
3. J Vasc Surg 2013;57:368-75.
4. J Vasc Surg 2001;34:41-6.
5. J Vasc Surg 1991;13:240-5; discussion 5-7.
6. J Vasc Surg 2014;60:1439-45.
7. J Vasc Surg 2010;51:305-9 e1.
8. Eur J Vasc Endovasc Surg 2006;32:506-13; discussion 14-5.
9. Ann Surg 2013;258:248-56.
10. BMJ 2014;348:f7661.
11. J Vasc Surg 2009;49:817-26.
12. J Vasc Surg 2008;47:1165-70; discussion 70-1.
13. J Vasc Surg 2014;59:575-82.
14. Ann Surg 2012;256:688-95; discussion 95-6.
15. Ann Surg 2009;250:818-24.
Dr. Aziz is in the division of vascular surgery, Penn State Heart and Vascular Institute, Pennsylvania State University College of Medicine, Hershey, Penn.
Open repair should be offered to all patients with rAAA
With the advent of endovascular repair of abdominal aortic aneurysms (EVAR), the treatment of elective AAAs was revolutionized. Since ruptured abdominal aortic aneurysms (rAAAs) carry a higher morbidity and mortality than elective AAA repair the use of EVAR has been advocated for these patients.1 Dr. Aziz contends that EVAR should be utilized in all patients presenting with a rAAA. It is my contention that endovascular repair cannot replace open aneurysm repair in all situations. The best treatment option should be offered taking patient and institutional considerations into account. Forcing a given procedure and trying to “make it work” is not best for the patient.
In a systematic literature review of patients presenting with rAAAs, selection bias regarding treatment choice (EVAR vs. open repair) was found consistently.2 In an effort to show that EVAR is superior to open repair for rAAA, Hinchliffe et al. published a randomized trial that showed no difference in 30-day mortality (53%) in each treatment group.3 The AJAX trial randomized 116 patients and did not show benefit for EVAR with respect to 30-day morbidity or mortality.4 The ECAR trial randomized 107 patients and also failed to show a difference in mortality between EVAR and open techniques for rAAA.5
Determining suitability for EVAR includes assessing diameter of the neck of the aneurysm, angulation, size of iliac arteries, presence of atherosclerotic occlusive disease, presence of accessory renal arteries, and presence of concurrent aneurysms of the iliac arteries.7,8 In the IMPROVE trial, patients were randomized depending on anatomic suitability for EVAR.9 Anatomy was determined by imaging in those patients stable enough to undergo preoperative computed tomography angiogram (CTA). Therefore, given anatomic differences, patients presenting with rAAA were not randomized without significant treatment bias.
A true pararenal or paravisceral rupture certainly elevates the complexity of the case. Open repair can deal with this difficult challenge by adapting additional established techniques to the situation. Although some authors have advocated EVAR for rAAA using snorkels and chimneys, these techniques may not be appropriate in the treatment of rAAA in most institutions.10 These procedures take longer, require considerable experience and resources and are therefore not appropriate in unstable patients. No randomized control trial exists at this time to address superiority of EVAR to open repair in patients with complex anatomy.
Patients presenting with rAAA that have had prior endovascular intervention also pose complex anatomic and technical challenges.11 These patients can present with hemodynamic instability and rupture but now are complicated by having device failure. Salvage using endovascular means may not be possible. The risk of re-intervention with endovascular repair for rAAA is much higher than with open repair.12
Emergency vascular surgery is best approached with protocols and systems in place no matter what approach is chosen.13 Skilled staff in the emergency department and in the operating room must be available without delay especially for rAAA, as time is of the essence. Skilled surgeons, skilled operating room staff, knowledgeable anesthesia staff are necessary to quickly move a patient from the emergency department to the operating room for definitive treatment. Other ancillary services, such as blood banks and laboratories are necessary to assist in the care for the patient intraoperatively. In endovascular surgery, trained technologists, fluoroscopy, a full armamentarium of interventional equipment (wires, balloons, stents, etc.), and a full stock of stent grafts and associated ancillary equipment are mandatory. Many hospitals may not have the variety of stent grafts available for emergency use. Regionalization of care has been suggested to improve outcomes in the treatment of rAAAs.14 In a study by Warner et al., mortality was decreased by 20% at the tertiary center compared to the smaller community hospitals in both EVAR and open repairs for rAAA. Zettervall et al. published data that showed that significant regional variation exists in perioperative outcomes and length of stay, and mortality in repair for rAAA approached with open or endovascular means.15 Low volume centers have worse outcomes, and teaching hospitals may have better outcomes treating AAA.16
EVAR is also reported to be associated with a 27-36% higher cost of care than open surgery for rAAA.17 Many institutions may not have the means to provide the resources needed to support such a program.
There is no question that EVAR has greatly impacted the treatment of aortic aneurysms. However, not every aneurysm is appropriate for endovascular repair at this time. For the many reasons cited, we cannot permit open repair to become an extinct skill. Vascular training programs must include open repair of rAAA just as it should for other vascular conditions.
I would agree with Dr. Aziz that we do not yet have a prospective, randomized study and Level 1 evidence to support either view and given the complexity and urgency associated with these patients, such a study may not be forthcoming. Till then, patients with a RAAA should have ‘all of the above’ approach including open surgery depending on the institutional experience and resources and urgency of the situation.
References
1. J Vasc Surg 2016; 2:297-305
2. J Vasc Surg 2009; 49:1077-1080.
3. Eur J Vasc Endovasc Surg 2006; 32:506-513
4. Ann Surg 2013. Aug 258 (2) 248-56
5. J Vasc Surg 2010; 51:267-70
6. Ann Vasc Surg 2017; 38:59-63
7. J Vasc Surg 2009: 50:243-50
8. J Endovasc Ther 2016; 23: 919-927
9. BJM 2014; 101:216-224
10. Radiographics 2015; 35:593-615.
11. Ann Vasc Surg 2013; 27:844-50
12. J Vasc Surg 2017; 65:52-57
13. Semin Vasc Surg 2016; 29:35-40
14. J Vasc Surg 2014; 59:1512-7
15. Ann Surg 2017; 264:538-43
16. J Vasc Surg 2016; 22 pii W0741-5214 (16) 31247-2
17. J Vasc Surg 2008; 47:1165-70
Dr. Ozsvatrh is a professor of surgery at Albany Medical College, Albany, N.Y., and a vascular attending, at Albany Medical Center Hospital, Albany, and chief, department of surgery, Samaritan Hospital, Troy, N.Y.
EVAR should be offered to all patients with rAAA
Since the inception of EVAR two decades ago, it has largely replaced open abdominal aortic aneurysm repair as the operation of choice for elective treatment of abdominal aortic aneurysms. Shorter duration of operation, avoiding general anesthesia and aortic cross clamping, ability to obtain balloon control of aorta, fast recovery time and reduced hospital length of hospital stay are among the most commonly cited reasons for this change in paradigm for treatment of abdominal aortic aneurysms. Since vascular surgeons have adopted the widespread use of EVAR, the total number of aneurysm related deaths has significantly reduced.1 While EVAR has become the most commonly used operation to treat AAAs electively, it’s utility to treat ruptured AAAs has been questioned. Recent analysis of nationwide trends for the operations for the diagnosis of ruptured AAA show that almost half of the patients with ruptured AAA are still treated with open surgical repair.2
Opponents of REVAR (EVAR for rAAA) cite comparable outcomes of open surgery and REVAR in randomized controlled trials, requirement of a specific REVAR protocol, increased cost and lack of resources and more importantly, lack of level I evidence to support it’s use in all cases of rAAA.
It is no surprise that REVAR is associated with significantly lower perioperative mortality (24%) as compared to open repair of ruptured AAA (44%).3 Mortality after open repair of ruptured AAA can vary anywhere from 30-70%.4,5 Improved mortality rates after REVAR are not only limited to stable patients, but also extend to those patients with ruptured AAA who are considered hemodynamically unstable at the time of initial presentation.6
The survival advantage among REVAR patients is not only evident in short term (30-days), but it also extends up to 5 years after the operation.3 Patients undergoing REVAR are less likely to require intra-operative blood transfusions, as compared to patients who undergo open repair of rAAA.7 Development of acute renal failure after surgical treatment of rAAAs is associated with significant mortality and REVAR has been associated with significantly lower incidence of acute renal failure as compared to open repair of ruptured AAA.2
Due to inherent issues of patient instability, emergent presentation and inequality among hospital resources and patient volumes, it is difficult to design a flawless, randomized controlled trial to compare REVAR with open surgical repair. The results from published randomized controlled trials on this topic should be interpreted with caution. The Nottingham trial from the United Kingdom8 showed similar mortality (53%) between REVAR and open surgical operation, however, out of the 203 patients enrolled in the trial, 70% were not randomized and REVAR was offered to only 15 patients. Likewise, the AJAX Trial from Netherlands9 showed that there was no significant difference in mortality between the two treatment groups (42% with REVAR and 47% with open repair), however almost 80% of enrolled patients were not randomized and there was a disagreement between the interpreters about CTA diagnosis of rAAA. In addition, the conversion rates from REVAR to open repair were unexpectedly high (14%), pointing to the surgeons’ inexperience.
Similarly, the IMPROVE trial from United Kingdom10 suffered from poor rates of randomization (50% of enrolled patients) and poor methodology (obtaining CTA after randomization, and making requirement of CTA optional for patients allocated to open surgery group). The trial showed equivalent mortality rates between the two surgical groups (35% with REVAR and 37% with open repair).
Due to flawed methodology and poor rates of randomization, results of these trials do not provide us with level I evidence, which is required to make any scientific recommendations about the treatment of choice for the treatment of rAAAs. Hence, the large body of evidence, obtained from retrospective studies should be used while comparing the outcomes of operations for ruptured AAAs. So far, all retrospective analyses, comparing REVAR with open repair in national registries, such as National Inpatient Sample (NIS) database11,12 American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database7 and Medicare database13 have clearly shown that the outcomes of REVAR are better than open surgical repair of ruptured AAA. Likewise, multi-center observational studies14,15 have demonstrated superiority of REVAR over open surgical repair.
To summarize, REVAR is a safe procedure, can be performed under local anesthetic, has shorter operative time and is associated with improved outcomes as compared to open surgery. REVAR should be the treatment of choice for all patients who meet the anatomic criteria for endovascular repair. With increasing experience of vascular surgeons with the endovascular technology, it is foreseeable that REVAR will soon become the treatment of choice for ruptured abdominal aortic aneurysms.
References
1. J Vasc Surg 2009;49:543-50; discussion 50-1.
2. Ann Vasc Surg 2016;35:147-55.
3. J Vasc Surg 2013;57:368-75.
4. J Vasc Surg 2001;34:41-6.
5. J Vasc Surg 1991;13:240-5; discussion 5-7.
6. J Vasc Surg 2014;60:1439-45.
7. J Vasc Surg 2010;51:305-9 e1.
8. Eur J Vasc Endovasc Surg 2006;32:506-13; discussion 14-5.
9. Ann Surg 2013;258:248-56.
10. BMJ 2014;348:f7661.
11. J Vasc Surg 2009;49:817-26.
12. J Vasc Surg 2008;47:1165-70; discussion 70-1.
13. J Vasc Surg 2014;59:575-82.
14. Ann Surg 2012;256:688-95; discussion 95-6.
15. Ann Surg 2009;250:818-24.
Dr. Aziz is in the division of vascular surgery, Penn State Heart and Vascular Institute, Pennsylvania State University College of Medicine, Hershey, Penn.
Open repair should be offered to all patients with rAAA
With the advent of endovascular repair of abdominal aortic aneurysms (EVAR), the treatment of elective AAAs was revolutionized. Since ruptured abdominal aortic aneurysms (rAAAs) carry a higher morbidity and mortality than elective AAA repair the use of EVAR has been advocated for these patients.1 Dr. Aziz contends that EVAR should be utilized in all patients presenting with a rAAA. It is my contention that endovascular repair cannot replace open aneurysm repair in all situations. The best treatment option should be offered taking patient and institutional considerations into account. Forcing a given procedure and trying to “make it work” is not best for the patient.
In a systematic literature review of patients presenting with rAAAs, selection bias regarding treatment choice (EVAR vs. open repair) was found consistently.2 In an effort to show that EVAR is superior to open repair for rAAA, Hinchliffe et al. published a randomized trial that showed no difference in 30-day mortality (53%) in each treatment group.3 The AJAX trial randomized 116 patients and did not show benefit for EVAR with respect to 30-day morbidity or mortality.4 The ECAR trial randomized 107 patients and also failed to show a difference in mortality between EVAR and open techniques for rAAA.5
Determining suitability for EVAR includes assessing diameter of the neck of the aneurysm, angulation, size of iliac arteries, presence of atherosclerotic occlusive disease, presence of accessory renal arteries, and presence of concurrent aneurysms of the iliac arteries.7,8 In the IMPROVE trial, patients were randomized depending on anatomic suitability for EVAR.9 Anatomy was determined by imaging in those patients stable enough to undergo preoperative computed tomography angiogram (CTA). Therefore, given anatomic differences, patients presenting with rAAA were not randomized without significant treatment bias.
A true pararenal or paravisceral rupture certainly elevates the complexity of the case. Open repair can deal with this difficult challenge by adapting additional established techniques to the situation. Although some authors have advocated EVAR for rAAA using snorkels and chimneys, these techniques may not be appropriate in the treatment of rAAA in most institutions.10 These procedures take longer, require considerable experience and resources and are therefore not appropriate in unstable patients. No randomized control trial exists at this time to address superiority of EVAR to open repair in patients with complex anatomy.
Patients presenting with rAAA that have had prior endovascular intervention also pose complex anatomic and technical challenges.11 These patients can present with hemodynamic instability and rupture but now are complicated by having device failure. Salvage using endovascular means may not be possible. The risk of re-intervention with endovascular repair for rAAA is much higher than with open repair.12
Emergency vascular surgery is best approached with protocols and systems in place no matter what approach is chosen.13 Skilled staff in the emergency department and in the operating room must be available without delay especially for rAAA, as time is of the essence. Skilled surgeons, skilled operating room staff, knowledgeable anesthesia staff are necessary to quickly move a patient from the emergency department to the operating room for definitive treatment. Other ancillary services, such as blood banks and laboratories are necessary to assist in the care for the patient intraoperatively. In endovascular surgery, trained technologists, fluoroscopy, a full armamentarium of interventional equipment (wires, balloons, stents, etc.), and a full stock of stent grafts and associated ancillary equipment are mandatory. Many hospitals may not have the variety of stent grafts available for emergency use. Regionalization of care has been suggested to improve outcomes in the treatment of rAAAs.14 In a study by Warner et al., mortality was decreased by 20% at the tertiary center compared to the smaller community hospitals in both EVAR and open repairs for rAAA. Zettervall et al. published data that showed that significant regional variation exists in perioperative outcomes and length of stay, and mortality in repair for rAAA approached with open or endovascular means.15 Low volume centers have worse outcomes, and teaching hospitals may have better outcomes treating AAA.16
EVAR is also reported to be associated with a 27-36% higher cost of care than open surgery for rAAA.17 Many institutions may not have the means to provide the resources needed to support such a program.
There is no question that EVAR has greatly impacted the treatment of aortic aneurysms. However, not every aneurysm is appropriate for endovascular repair at this time. For the many reasons cited, we cannot permit open repair to become an extinct skill. Vascular training programs must include open repair of rAAA just as it should for other vascular conditions.
I would agree with Dr. Aziz that we do not yet have a prospective, randomized study and Level 1 evidence to support either view and given the complexity and urgency associated with these patients, such a study may not be forthcoming. Till then, patients with a RAAA should have ‘all of the above’ approach including open surgery depending on the institutional experience and resources and urgency of the situation.
References
1. J Vasc Surg 2016; 2:297-305
2. J Vasc Surg 2009; 49:1077-1080.
3. Eur J Vasc Endovasc Surg 2006; 32:506-513
4. Ann Surg 2013. Aug 258 (2) 248-56
5. J Vasc Surg 2010; 51:267-70
6. Ann Vasc Surg 2017; 38:59-63
7. J Vasc Surg 2009: 50:243-50
8. J Endovasc Ther 2016; 23: 919-927
9. BJM 2014; 101:216-224
10. Radiographics 2015; 35:593-615.
11. Ann Vasc Surg 2013; 27:844-50
12. J Vasc Surg 2017; 65:52-57
13. Semin Vasc Surg 2016; 29:35-40
14. J Vasc Surg 2014; 59:1512-7
15. Ann Surg 2017; 264:538-43
16. J Vasc Surg 2016; 22 pii W0741-5214 (16) 31247-2
17. J Vasc Surg 2008; 47:1165-70
Dr. Ozsvatrh is a professor of surgery at Albany Medical College, Albany, N.Y., and a vascular attending, at Albany Medical Center Hospital, Albany, and chief, department of surgery, Samaritan Hospital, Troy, N.Y.
EVAR should be offered to all patients with rAAA
Since the inception of EVAR two decades ago, it has largely replaced open abdominal aortic aneurysm repair as the operation of choice for elective treatment of abdominal aortic aneurysms. Shorter duration of operation, avoiding general anesthesia and aortic cross clamping, ability to obtain balloon control of aorta, fast recovery time and reduced hospital length of hospital stay are among the most commonly cited reasons for this change in paradigm for treatment of abdominal aortic aneurysms. Since vascular surgeons have adopted the widespread use of EVAR, the total number of aneurysm related deaths has significantly reduced.1 While EVAR has become the most commonly used operation to treat AAAs electively, it’s utility to treat ruptured AAAs has been questioned. Recent analysis of nationwide trends for the operations for the diagnosis of ruptured AAA show that almost half of the patients with ruptured AAA are still treated with open surgical repair.2
Opponents of REVAR (EVAR for rAAA) cite comparable outcomes of open surgery and REVAR in randomized controlled trials, requirement of a specific REVAR protocol, increased cost and lack of resources and more importantly, lack of level I evidence to support it’s use in all cases of rAAA.
It is no surprise that REVAR is associated with significantly lower perioperative mortality (24%) as compared to open repair of ruptured AAA (44%).3 Mortality after open repair of ruptured AAA can vary anywhere from 30-70%.4,5 Improved mortality rates after REVAR are not only limited to stable patients, but also extend to those patients with ruptured AAA who are considered hemodynamically unstable at the time of initial presentation.6
The survival advantage among REVAR patients is not only evident in short term (30-days), but it also extends up to 5 years after the operation.3 Patients undergoing REVAR are less likely to require intra-operative blood transfusions, as compared to patients who undergo open repair of rAAA.7 Development of acute renal failure after surgical treatment of rAAAs is associated with significant mortality and REVAR has been associated with significantly lower incidence of acute renal failure as compared to open repair of ruptured AAA.2
Due to inherent issues of patient instability, emergent presentation and inequality among hospital resources and patient volumes, it is difficult to design a flawless, randomized controlled trial to compare REVAR with open surgical repair. The results from published randomized controlled trials on this topic should be interpreted with caution. The Nottingham trial from the United Kingdom8 showed similar mortality (53%) between REVAR and open surgical operation, however, out of the 203 patients enrolled in the trial, 70% were not randomized and REVAR was offered to only 15 patients. Likewise, the AJAX Trial from Netherlands9 showed that there was no significant difference in mortality between the two treatment groups (42% with REVAR and 47% with open repair), however almost 80% of enrolled patients were not randomized and there was a disagreement between the interpreters about CTA diagnosis of rAAA. In addition, the conversion rates from REVAR to open repair were unexpectedly high (14%), pointing to the surgeons’ inexperience.
Similarly, the IMPROVE trial from United Kingdom10 suffered from poor rates of randomization (50% of enrolled patients) and poor methodology (obtaining CTA after randomization, and making requirement of CTA optional for patients allocated to open surgery group). The trial showed equivalent mortality rates between the two surgical groups (35% with REVAR and 37% with open repair).
Due to flawed methodology and poor rates of randomization, results of these trials do not provide us with level I evidence, which is required to make any scientific recommendations about the treatment of choice for the treatment of rAAAs. Hence, the large body of evidence, obtained from retrospective studies should be used while comparing the outcomes of operations for ruptured AAAs. So far, all retrospective analyses, comparing REVAR with open repair in national registries, such as National Inpatient Sample (NIS) database11,12 American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database7 and Medicare database13 have clearly shown that the outcomes of REVAR are better than open surgical repair of ruptured AAA. Likewise, multi-center observational studies14,15 have demonstrated superiority of REVAR over open surgical repair.
To summarize, REVAR is a safe procedure, can be performed under local anesthetic, has shorter operative time and is associated with improved outcomes as compared to open surgery. REVAR should be the treatment of choice for all patients who meet the anatomic criteria for endovascular repair. With increasing experience of vascular surgeons with the endovascular technology, it is foreseeable that REVAR will soon become the treatment of choice for ruptured abdominal aortic aneurysms.
References
1. J Vasc Surg 2009;49:543-50; discussion 50-1.
2. Ann Vasc Surg 2016;35:147-55.
3. J Vasc Surg 2013;57:368-75.
4. J Vasc Surg 2001;34:41-6.
5. J Vasc Surg 1991;13:240-5; discussion 5-7.
6. J Vasc Surg 2014;60:1439-45.
7. J Vasc Surg 2010;51:305-9 e1.
8. Eur J Vasc Endovasc Surg 2006;32:506-13; discussion 14-5.
9. Ann Surg 2013;258:248-56.
10. BMJ 2014;348:f7661.
11. J Vasc Surg 2009;49:817-26.
12. J Vasc Surg 2008;47:1165-70; discussion 70-1.
13. J Vasc Surg 2014;59:575-82.
14. Ann Surg 2012;256:688-95; discussion 95-6.
15. Ann Surg 2009;250:818-24.
Dr. Aziz is in the division of vascular surgery, Penn State Heart and Vascular Institute, Pennsylvania State University College of Medicine, Hershey, Penn.
ABIM to allow do-overs for all subspecialties with Knowledge Check-In
ABIM previously announced that, beginning in 2018, physicians taking the Knowledge Check-In in 2018 would get another chance to take it in 2 years if they were unsuccessful, even if they were due to pass the maintenance of certification (MOC) exam later that year. In 2018, Knowledge Check-Ins will be offered in internal medicine and nephrology.
“Based on feedback ABIM has received from the physician community, we are happy to let you know that we are extending this policy to include all other internal medicine subspecialties in the future,” ABIM said in a Dec. 4 announcement on its website. “This means that if a physician takes the Knowledge Check-In in the first year it is offered in their subspecialty and is unsuccessful, they will get at least one additional opportunity to take and pass it 2 years later.”
The Knowledge Check-In is an alternative to the traditional MOC process, and is administered every 2 years rather than the standard decade between MOC exams. ABIM noted that a single failure on a Knowledge Check-In will not result in a status change to a physician’s certification status.
Separately, ABIM also announced that it will continue to make practice assessment activities (part IV of the MOC program) a part of the portfolio of options that can be used to satisfy MOC requirements but that practice assessment is not a required part of maintenance of certification.
“Our intent is to support physicians completing MOC activities that are most meaningful to their practice, including those that enhance and improve medical knowledge, as well as many existing quality improvement activities, and those that blend both,” ABIM said in its announcement.
AGA will continue to work with ABIM and advocate for a recertification pathway that reduces the burden of recertifying, emphasizes learning over testing and assesses diplomates in their areas of practice.
ABIM previously announced that, beginning in 2018, physicians taking the Knowledge Check-In in 2018 would get another chance to take it in 2 years if they were unsuccessful, even if they were due to pass the maintenance of certification (MOC) exam later that year. In 2018, Knowledge Check-Ins will be offered in internal medicine and nephrology.
“Based on feedback ABIM has received from the physician community, we are happy to let you know that we are extending this policy to include all other internal medicine subspecialties in the future,” ABIM said in a Dec. 4 announcement on its website. “This means that if a physician takes the Knowledge Check-In in the first year it is offered in their subspecialty and is unsuccessful, they will get at least one additional opportunity to take and pass it 2 years later.”
The Knowledge Check-In is an alternative to the traditional MOC process, and is administered every 2 years rather than the standard decade between MOC exams. ABIM noted that a single failure on a Knowledge Check-In will not result in a status change to a physician’s certification status.
Separately, ABIM also announced that it will continue to make practice assessment activities (part IV of the MOC program) a part of the portfolio of options that can be used to satisfy MOC requirements but that practice assessment is not a required part of maintenance of certification.
“Our intent is to support physicians completing MOC activities that are most meaningful to their practice, including those that enhance and improve medical knowledge, as well as many existing quality improvement activities, and those that blend both,” ABIM said in its announcement.
AGA will continue to work with ABIM and advocate for a recertification pathway that reduces the burden of recertifying, emphasizes learning over testing and assesses diplomates in their areas of practice.
ABIM previously announced that, beginning in 2018, physicians taking the Knowledge Check-In in 2018 would get another chance to take it in 2 years if they were unsuccessful, even if they were due to pass the maintenance of certification (MOC) exam later that year. In 2018, Knowledge Check-Ins will be offered in internal medicine and nephrology.
“Based on feedback ABIM has received from the physician community, we are happy to let you know that we are extending this policy to include all other internal medicine subspecialties in the future,” ABIM said in a Dec. 4 announcement on its website. “This means that if a physician takes the Knowledge Check-In in the first year it is offered in their subspecialty and is unsuccessful, they will get at least one additional opportunity to take and pass it 2 years later.”
The Knowledge Check-In is an alternative to the traditional MOC process, and is administered every 2 years rather than the standard decade between MOC exams. ABIM noted that a single failure on a Knowledge Check-In will not result in a status change to a physician’s certification status.
Separately, ABIM also announced that it will continue to make practice assessment activities (part IV of the MOC program) a part of the portfolio of options that can be used to satisfy MOC requirements but that practice assessment is not a required part of maintenance of certification.
“Our intent is to support physicians completing MOC activities that are most meaningful to their practice, including those that enhance and improve medical knowledge, as well as many existing quality improvement activities, and those that blend both,” ABIM said in its announcement.
AGA will continue to work with ABIM and advocate for a recertification pathway that reduces the burden of recertifying, emphasizes learning over testing and assesses diplomates in their areas of practice.
Emergency Imaging: Atraumatic Leg Pain
Case
A 96-year-old woman with a medical history of sciatica, vertigo, osteoporosis, and dementia presented with atraumatic right leg pain. She stated that the pain, which began 4 weeks prior to presentation, started in her right groin. The patient’s primary care physician diagnosed her with tendonitis, and prescribed acetaminophen/codeine and naproxen sodium for the pain. However, the patient’s pain progressively worsened to the point where she was no longer able to ambulate or bear weight on her right hip, prompting this visit to the ED.
On physical examination, the patient’s right hip was tender to palpation without any signs of physical deformity of the lower extremity. Upon hip flexion, she grimaced and communicated her pain.
Radiographs and computed tomography images taken of the right hip, femur, and pelvis demonstrated low-bone mineral density without fracture.
What is the diagnosis?
Answer
Axial and coronal edema-sensitive images of the pelvis demonstrated edema (increased signal) within the right psoas, iliacus, and iliopsoas muscles (red arrows, Figures 2a-2c), which were in contrast to the normal pelvic muscles on the left side (white arrows, Figures 2a-2c).
Iliopsoas Musculotendinous Unit
The iliopsoas musculotendinous unit consists of the psoas major, the psoas minor, and the iliacus, with the psoas minor absent in 40% to 50% of cases.1,2 The iliacus muscle arises from the iliac wing and inserts with the psoas tendon onto the lesser trochanter of the femur. These muscles function as primary flexors of the thigh and trunk, as well as lateral flexors of the lower vertebral column.2
Signs and Symptoms
In non-sports-related injuries, iliopsoas tendon tears typically occur in elderly female patients—even in the absence of any trauma or known predisposing factors. Patients with iliopsoas tears typically present with hip or groin pain, and weakness with hip flexion, which clinically may mimic hip or sacral fracture. An anterior thigh mass or ecchymosis may also be present. Complete tear of the iliopsoas tendon usually occurs at or near the distal insertion at the lesser trochanter, and is often associated with proximal retraction of the tendon to the level of the femoral head.1
Imaging Studies
Iliopsoas tendon injury is best evaluated with MRI, particularly with fluid-sensitive sequences. Patients with iliopsoas tendon tears have abnormal signal in the muscle belly, likely related to edema and hemorrhage, and hematoma or fluid around the torn tendon and at the site of retraction. In pediatric patients, iliopsoas injury is typically an avulsion of the lesser trochanter prior to fusion of the apophysis.3,4 In adult patients with avulsion of the lesser trochanter, this injury is regarded as a sign of metastatic disease until proven otherwise.5
Treatment
Patients with iliopsoas tendon rupture are treated conservatively with rest, ice, and physical therapy (PT). Preservation of the distal muscular insertion of the lateral portion of the iliacus muscle is thought to play a role in positive clinical outcomes.3
The patient in this case was admitted to the hospital and treated for pain with standing acetaminophen, tramadol as needed, and a lidocaine patch. After attending multiple inpatient PT sessions, she was discharged to a subacute rehabilitation facility.
1. Bergman G. MRI Web clinic – October 2015: Iliopsoas tendinopathy. Radsource. http://radsource.us/iliopsoas-tendinopathy/. Accessed November 22, 2017.
2. Van Dyke JA, Holley HC, Anderson SD. Review of iliopsoas anatomy and pathology. Radiographics. 1987;7(1):53-84. doi:10.1148/radiographics.7.1.3448631.
3. Lecouvet FE, Demondion X, Leemrijse T, Vande Berg BC, Devogelaer JP, Malghem J. Spontaneous rupture of the distal iliopsoas tendon: clinical and imaging findings, with anatomic correlations. Eur Radiol. 2005;15(11):2341-2346. doi:10.1007/s00330-005-2811-0.
4. Bui KL, Ilaslan H, Recht M, Sundaram M. Iliopsoas injury: an MRI study of patterns and prevalence correlated with clinical findings. Skeletal Radiol. 2008;37(3):245-249. doi:10.1007/s00256-007-0414-3.
5. James SL, Davies AM. Atraumatic avulsion of the lesser trochanter as an indicator of tumour infiltration. Eur Radiol. 2006;16(2):512-514.
Case
A 96-year-old woman with a medical history of sciatica, vertigo, osteoporosis, and dementia presented with atraumatic right leg pain. She stated that the pain, which began 4 weeks prior to presentation, started in her right groin. The patient’s primary care physician diagnosed her with tendonitis, and prescribed acetaminophen/codeine and naproxen sodium for the pain. However, the patient’s pain progressively worsened to the point where she was no longer able to ambulate or bear weight on her right hip, prompting this visit to the ED.
On physical examination, the patient’s right hip was tender to palpation without any signs of physical deformity of the lower extremity. Upon hip flexion, she grimaced and communicated her pain.
Radiographs and computed tomography images taken of the right hip, femur, and pelvis demonstrated low-bone mineral density without fracture.
What is the diagnosis?
Answer
Axial and coronal edema-sensitive images of the pelvis demonstrated edema (increased signal) within the right psoas, iliacus, and iliopsoas muscles (red arrows, Figures 2a-2c), which were in contrast to the normal pelvic muscles on the left side (white arrows, Figures 2a-2c).
Iliopsoas Musculotendinous Unit
The iliopsoas musculotendinous unit consists of the psoas major, the psoas minor, and the iliacus, with the psoas minor absent in 40% to 50% of cases.1,2 The iliacus muscle arises from the iliac wing and inserts with the psoas tendon onto the lesser trochanter of the femur. These muscles function as primary flexors of the thigh and trunk, as well as lateral flexors of the lower vertebral column.2
Signs and Symptoms
In non-sports-related injuries, iliopsoas tendon tears typically occur in elderly female patients—even in the absence of any trauma or known predisposing factors. Patients with iliopsoas tears typically present with hip or groin pain, and weakness with hip flexion, which clinically may mimic hip or sacral fracture. An anterior thigh mass or ecchymosis may also be present. Complete tear of the iliopsoas tendon usually occurs at or near the distal insertion at the lesser trochanter, and is often associated with proximal retraction of the tendon to the level of the femoral head.1
Imaging Studies
Iliopsoas tendon injury is best evaluated with MRI, particularly with fluid-sensitive sequences. Patients with iliopsoas tendon tears have abnormal signal in the muscle belly, likely related to edema and hemorrhage, and hematoma or fluid around the torn tendon and at the site of retraction. In pediatric patients, iliopsoas injury is typically an avulsion of the lesser trochanter prior to fusion of the apophysis.3,4 In adult patients with avulsion of the lesser trochanter, this injury is regarded as a sign of metastatic disease until proven otherwise.5
Treatment
Patients with iliopsoas tendon rupture are treated conservatively with rest, ice, and physical therapy (PT). Preservation of the distal muscular insertion of the lateral portion of the iliacus muscle is thought to play a role in positive clinical outcomes.3
The patient in this case was admitted to the hospital and treated for pain with standing acetaminophen, tramadol as needed, and a lidocaine patch. After attending multiple inpatient PT sessions, she was discharged to a subacute rehabilitation facility.
Case
A 96-year-old woman with a medical history of sciatica, vertigo, osteoporosis, and dementia presented with atraumatic right leg pain. She stated that the pain, which began 4 weeks prior to presentation, started in her right groin. The patient’s primary care physician diagnosed her with tendonitis, and prescribed acetaminophen/codeine and naproxen sodium for the pain. However, the patient’s pain progressively worsened to the point where she was no longer able to ambulate or bear weight on her right hip, prompting this visit to the ED.
On physical examination, the patient’s right hip was tender to palpation without any signs of physical deformity of the lower extremity. Upon hip flexion, she grimaced and communicated her pain.
Radiographs and computed tomography images taken of the right hip, femur, and pelvis demonstrated low-bone mineral density without fracture.
What is the diagnosis?
Answer
Axial and coronal edema-sensitive images of the pelvis demonstrated edema (increased signal) within the right psoas, iliacus, and iliopsoas muscles (red arrows, Figures 2a-2c), which were in contrast to the normal pelvic muscles on the left side (white arrows, Figures 2a-2c).
Iliopsoas Musculotendinous Unit
The iliopsoas musculotendinous unit consists of the psoas major, the psoas minor, and the iliacus, with the psoas minor absent in 40% to 50% of cases.1,2 The iliacus muscle arises from the iliac wing and inserts with the psoas tendon onto the lesser trochanter of the femur. These muscles function as primary flexors of the thigh and trunk, as well as lateral flexors of the lower vertebral column.2
Signs and Symptoms
In non-sports-related injuries, iliopsoas tendon tears typically occur in elderly female patients—even in the absence of any trauma or known predisposing factors. Patients with iliopsoas tears typically present with hip or groin pain, and weakness with hip flexion, which clinically may mimic hip or sacral fracture. An anterior thigh mass or ecchymosis may also be present. Complete tear of the iliopsoas tendon usually occurs at or near the distal insertion at the lesser trochanter, and is often associated with proximal retraction of the tendon to the level of the femoral head.1
Imaging Studies
Iliopsoas tendon injury is best evaluated with MRI, particularly with fluid-sensitive sequences. Patients with iliopsoas tendon tears have abnormal signal in the muscle belly, likely related to edema and hemorrhage, and hematoma or fluid around the torn tendon and at the site of retraction. In pediatric patients, iliopsoas injury is typically an avulsion of the lesser trochanter prior to fusion of the apophysis.3,4 In adult patients with avulsion of the lesser trochanter, this injury is regarded as a sign of metastatic disease until proven otherwise.5
Treatment
Patients with iliopsoas tendon rupture are treated conservatively with rest, ice, and physical therapy (PT). Preservation of the distal muscular insertion of the lateral portion of the iliacus muscle is thought to play a role in positive clinical outcomes.3
The patient in this case was admitted to the hospital and treated for pain with standing acetaminophen, tramadol as needed, and a lidocaine patch. After attending multiple inpatient PT sessions, she was discharged to a subacute rehabilitation facility.
1. Bergman G. MRI Web clinic – October 2015: Iliopsoas tendinopathy. Radsource. http://radsource.us/iliopsoas-tendinopathy/. Accessed November 22, 2017.
2. Van Dyke JA, Holley HC, Anderson SD. Review of iliopsoas anatomy and pathology. Radiographics. 1987;7(1):53-84. doi:10.1148/radiographics.7.1.3448631.
3. Lecouvet FE, Demondion X, Leemrijse T, Vande Berg BC, Devogelaer JP, Malghem J. Spontaneous rupture of the distal iliopsoas tendon: clinical and imaging findings, with anatomic correlations. Eur Radiol. 2005;15(11):2341-2346. doi:10.1007/s00330-005-2811-0.
4. Bui KL, Ilaslan H, Recht M, Sundaram M. Iliopsoas injury: an MRI study of patterns and prevalence correlated with clinical findings. Skeletal Radiol. 2008;37(3):245-249. doi:10.1007/s00256-007-0414-3.
5. James SL, Davies AM. Atraumatic avulsion of the lesser trochanter as an indicator of tumour infiltration. Eur Radiol. 2006;16(2):512-514.
1. Bergman G. MRI Web clinic – October 2015: Iliopsoas tendinopathy. Radsource. http://radsource.us/iliopsoas-tendinopathy/. Accessed November 22, 2017.
2. Van Dyke JA, Holley HC, Anderson SD. Review of iliopsoas anatomy and pathology. Radiographics. 1987;7(1):53-84. doi:10.1148/radiographics.7.1.3448631.
3. Lecouvet FE, Demondion X, Leemrijse T, Vande Berg BC, Devogelaer JP, Malghem J. Spontaneous rupture of the distal iliopsoas tendon: clinical and imaging findings, with anatomic correlations. Eur Radiol. 2005;15(11):2341-2346. doi:10.1007/s00330-005-2811-0.
4. Bui KL, Ilaslan H, Recht M, Sundaram M. Iliopsoas injury: an MRI study of patterns and prevalence correlated with clinical findings. Skeletal Radiol. 2008;37(3):245-249. doi:10.1007/s00256-007-0414-3.
5. James SL, Davies AM. Atraumatic avulsion of the lesser trochanter as an indicator of tumour infiltration. Eur Radiol. 2006;16(2):512-514.
Malpractice Counsel: Don’t Miss Popeye
A 42-year-old man presented to the ED with left arm pain secondary to an injury he sustained at work. The patient stated that he had been helping to lift a heavy steel beam at a construction site when he experienced abrupt onset of pain in his left arm. He further noted that his left arm felt slightly weaker than normal after the injury.
The patient was left-hand dominant, denied any other injury, was otherwise in good health, and on no medications. With the exception of an appendectomy at age 12 years, his medical history was unremarkable. Regarding his social history, he admitted to smoking one pack of cigarettes per day, and to occasional alcohol consumption. He had no known drug allergies.
On physical examination, the patient’s vital signs were: blood pressure, 125/76 mm Hg; heart rate, 78 beats/min; respiratory rate, 16 breaths/min; and temperature, 98.6°F. Oxygen saturation was 99% on room air.
Examination of the patient’s left shoulder revealed no swelling or tenderness; he was able to fully internally/externally rotate the left shoulder, and lift his left hand above his head. The patient did have tenderness along the biceps area of the left arm, but no tenderness in the triceps area. The left elbow was tender in the antecubital fossa, but without swelling. He had full range of motion of the left elbow but with some pain. He likewise had full range of motion in his left wrist, but no tenderness or swelling. The left radial pulse was 2+. The patient had 5/5 grip strength with the left hand and good capillary refill.
The physician assistant (PA) evaluating the patient diagnosed an arm strain. At discharge, he referred the patient to an occupational health physician (OHP) for follow-up. He also instructed the patient to take ibuprofen 400 mg every 6 to 8 hours, and to limit use of his left arm for 3 days.
The patient followed up with the OHP approximately 3 weeks after discharge from the ED. The OHP was concerned the patient had experienced a distal biceps tendon rupture and referred the patient emergently to an orthopedic surgeon. The orthopedic surgeon saw the patient the next day, agreed with the diagnosis of a distal biceps tendon rupture, and attempted surgical repair the following day. The orthopedic surgeon informed the patient prior to the surgery that the delay in the referral and surgery could result in a poor functional outcome. The patient did have a difficult recovery period, and a second surgery was required, which did not result in any significant functional improvement.
The plaintiff sued the treating PA and supervising emergency physician (EP) for failure to properly diagnose the biceps tendon rupture, failure to appreciate the existence of a 3-week window of opportunity to repair the distal biceps tendon rupture, and failure to obtain an urgent orthopedic referral. The experts for the defense argued that the poor outcome was not a consequence of any delay in diagnosis or surgical repair. In addition, the defense disputed the existence of a 3-week window of opportunity for successful repair of a distal biceps tendon rupture. The jury returned a defense verdict.
Discussion
Proximal and Distal Biceps Tendon Ruptures
While both proximal and distal biceps tendon ruptures involve the biceps brachii, they are managed differently and have the potential for very different outcomes.1 At its proximal attachment, the biceps has two distinct tendinous insertions—the long head and the short head. For the distal attachment, the two muscle bellies unite at the midshaft of the humerus and attach as a single tendon on the radial tuberosity. In general, 96% of biceps tendon ruptures involve the long head, 1% involve the short head, and only 3% involve the distal tendon.1 Biceps tendon ruptures occur more commonly in men, patients who use anabolic steroids, cigarette smokers, patient history of tendinopathy, or patients who have a rotator cuff tear.1 Biceps tendon ruptures have not been found to be associated with statin use.2 The mechanism of injury includes heavy-lifting activities, such as weight lifting and rock climbing. However, when associated with a tendinopathy, minimal force may be involved.1
Signs and Symptoms
For proximal biceps tendon rupture, patients usually present with an acute or gradual onset of pain, swelling, and bruising of the upper arm and shoulder. Occasionally, if there is an inciting event, the patient may describe hearing or feeling a “popping” or “snapping” sound. On physical examination, the patient may exhibit a “Popeye” sign—a bulge in the distal biceps area due to the retracted biceps muscle belly. There is also tenderness along the biceps.
On testing, it has been estimated that patients can experience strength loss of approximately 30% with elbow flexion.1 In contrast, patients with distal biceps tendon ruptures usually complain of pain, swelling, and possibly bruising in the antecubital fossa, as was the case with this patient. Similar to proximal ruptures, the patient may admit to hearing or feeling a “popping” sound if there is an inciting event. The patient may exhibit a “reverse Popeye” deformity, with a bulge in the proximal arm secondary to retraction of the biceps muscle belly proximally.1
Diagnosis
There are two tests that can be performed to assist in making the diagnosis—the biceps squeeze test and the hook test.
Biceps Squeeze Test. The first test to assess for distal biceps tendon rupture is the biceps squeeze test, in which the clinician forcefully squeezes the patient’s biceps muscle to observe for forearm flexion/supination. This test is similar in principle to the Thompson test for Achilles tendon rupture. If there is no forearm movement, the injury is suspicious for a complete distal biceps tendon rupture. In one observational study of this test, 21 of 22 patients with a positive biceps squeeze test were found to have a complete distal biceps tendon tear at surgery.3
Hook Test. The second test is the hook test. While the patient actively supinates with the elbow flexed at 900, an intact hook test permits the examiner to “hook” his or her index finger under the intact biceps tendon from the lateral side. The absence of a “hook” means that there is no cord-like structure under which the examiner can hook a finger, indicating distal avulsion.4 In one study comparing the hook test to magnetic resonance imaging (MRI) in 33 patients with this suspected injury, the hook test had 100% sensitivity and specificity, while MRI only demonstrated a 92% sensitivity and 85% specificity.4
Imaging Techniques
The need for diagnostic imaging is based somewhat on the location of the rupture—proximal or distal. Ultrasound has been shown to have a high sensitivity and specificity for identifying normal tendons and complete tears of the long head biceps tendon (ie, proximal). It is not sensitive at identifying proximal partial tears, however. For distal ruptures, ultrasound imaging of the distal biceps tendon is technically difficult and not reliable. For patients with suspected distal biceps tendon ruptures, the EP should consult with orthopedic services prior to ordering an MRI. While MRI is considered the gold standard imaging test, it is neither 100% sensitive nor specific. The bottom line is that the absence of pathologic findings on MRI is not sufficient enough to exclude biceps tendon pathology.5
Treatment and Management
Regarding management, the majority of patients with proximal biceps tendon ruptures tend to do well with conservative management. The exception is for younger, active patients who are less willing to accept the cosmetic deformity, or patients whose occupation makes them unable to tolerate minimal weakness or fatigue cramping (eg, carpenters), in which case referral for a surgical repair (tenodesis) may be appropriate.1 However, multiple systematic reviews examining tenotomy vs tenodesis have not shown any functional improvement, only cosmetic.1,6,7
Distal biceps tendon ruptures are usually treated surgically, since conservative management results in a decrease of 30% to 50% supination strength and 20% flexion strength.1,8 This surgery, however, is not without complications. Approximately 20% of the patients will have a minor complication and 5% will have major complications following surgery on the distal biceps tendon.9 It is preferable to operate on distal ruptures less than 4 weeks from the initial injury; otherwise, these injuries may be more difficult to fix, require a graft, and have less predictable outcomes.1 Nonoperative management should be reserved for the elderly or less active patients with multiple comorbidities, especially if the nondominant arm is involved.10
Summary
The PA clearly missed the correct diagnosis on this patient. A more thorough history and focused physical examination would have led to the correct diagnosis sooner, along with earlier surgical repair. It is impossible, however, to know if the outcome would have been any different in this uncommon injury.
1. Smith D. Proximal versus distal biceps tendon ruptures: when to refer. BCMJ. 2017;59(2):85.
2. Spoendlin J, Layton JB, Mundkur M, Meier C, Jick SS, Meier CR. The risk of achilles or biceps tendon rupture in new statin users: a propensity score-matched sequential cohort study. Drug Safety. 2016;39(12):1229-1237. doi:10.1007/s40264-016-0462-5.
3. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Relat Res. 2005;437:128-131.
4. O’Driscoll SW, Goncalves LBJ, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1969. doi:10.1177/0363546507305016.
5. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254. doi:10.1016/j.ejrad.2015.07.031.
6. Tangari M, Carbone S, Gallo M, Campi A. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20(3):409-413. doi:10.1016/j.jse.2010.08.008.
7. Eakin JL, Bailey JR, Dewing CB, Provencher MT. Subpectoral biceps tenodesis. Oper Tech Sports Med. 2012;20(3):244-252.
8. Thomas JR, Lawton JN. Biceps and triceps ruptures in athletes. Hand Clin. 2017;33(1):35-46. doi:10.1016/j.hcl.2016.08.019.
9. Beks RB, Claessen FM, Oh LS, Ring D, Chen NC. Factors associated with adverse events after distal biceps tendon repair or reconstruction. J Shoulder Elbow Surg. 2016;25(8):1229-1234. doi:10.1016/j.jse.2016.02.032.
10. Savin DD, Watson J, Youderian AR, et al. Surgical management of acute distal biceps tendon ruptures. J Bone Joint Surg. 2017;3(9):785-796. doi:0.2106/JBJS.17.00080.
A 42-year-old man presented to the ED with left arm pain secondary to an injury he sustained at work. The patient stated that he had been helping to lift a heavy steel beam at a construction site when he experienced abrupt onset of pain in his left arm. He further noted that his left arm felt slightly weaker than normal after the injury.
The patient was left-hand dominant, denied any other injury, was otherwise in good health, and on no medications. With the exception of an appendectomy at age 12 years, his medical history was unremarkable. Regarding his social history, he admitted to smoking one pack of cigarettes per day, and to occasional alcohol consumption. He had no known drug allergies.
On physical examination, the patient’s vital signs were: blood pressure, 125/76 mm Hg; heart rate, 78 beats/min; respiratory rate, 16 breaths/min; and temperature, 98.6°F. Oxygen saturation was 99% on room air.
Examination of the patient’s left shoulder revealed no swelling or tenderness; he was able to fully internally/externally rotate the left shoulder, and lift his left hand above his head. The patient did have tenderness along the biceps area of the left arm, but no tenderness in the triceps area. The left elbow was tender in the antecubital fossa, but without swelling. He had full range of motion of the left elbow but with some pain. He likewise had full range of motion in his left wrist, but no tenderness or swelling. The left radial pulse was 2+. The patient had 5/5 grip strength with the left hand and good capillary refill.
The physician assistant (PA) evaluating the patient diagnosed an arm strain. At discharge, he referred the patient to an occupational health physician (OHP) for follow-up. He also instructed the patient to take ibuprofen 400 mg every 6 to 8 hours, and to limit use of his left arm for 3 days.
The patient followed up with the OHP approximately 3 weeks after discharge from the ED. The OHP was concerned the patient had experienced a distal biceps tendon rupture and referred the patient emergently to an orthopedic surgeon. The orthopedic surgeon saw the patient the next day, agreed with the diagnosis of a distal biceps tendon rupture, and attempted surgical repair the following day. The orthopedic surgeon informed the patient prior to the surgery that the delay in the referral and surgery could result in a poor functional outcome. The patient did have a difficult recovery period, and a second surgery was required, which did not result in any significant functional improvement.
The plaintiff sued the treating PA and supervising emergency physician (EP) for failure to properly diagnose the biceps tendon rupture, failure to appreciate the existence of a 3-week window of opportunity to repair the distal biceps tendon rupture, and failure to obtain an urgent orthopedic referral. The experts for the defense argued that the poor outcome was not a consequence of any delay in diagnosis or surgical repair. In addition, the defense disputed the existence of a 3-week window of opportunity for successful repair of a distal biceps tendon rupture. The jury returned a defense verdict.
Discussion
Proximal and Distal Biceps Tendon Ruptures
While both proximal and distal biceps tendon ruptures involve the biceps brachii, they are managed differently and have the potential for very different outcomes.1 At its proximal attachment, the biceps has two distinct tendinous insertions—the long head and the short head. For the distal attachment, the two muscle bellies unite at the midshaft of the humerus and attach as a single tendon on the radial tuberosity. In general, 96% of biceps tendon ruptures involve the long head, 1% involve the short head, and only 3% involve the distal tendon.1 Biceps tendon ruptures occur more commonly in men, patients who use anabolic steroids, cigarette smokers, patient history of tendinopathy, or patients who have a rotator cuff tear.1 Biceps tendon ruptures have not been found to be associated with statin use.2 The mechanism of injury includes heavy-lifting activities, such as weight lifting and rock climbing. However, when associated with a tendinopathy, minimal force may be involved.1
Signs and Symptoms
For proximal biceps tendon rupture, patients usually present with an acute or gradual onset of pain, swelling, and bruising of the upper arm and shoulder. Occasionally, if there is an inciting event, the patient may describe hearing or feeling a “popping” or “snapping” sound. On physical examination, the patient may exhibit a “Popeye” sign—a bulge in the distal biceps area due to the retracted biceps muscle belly. There is also tenderness along the biceps.
On testing, it has been estimated that patients can experience strength loss of approximately 30% with elbow flexion.1 In contrast, patients with distal biceps tendon ruptures usually complain of pain, swelling, and possibly bruising in the antecubital fossa, as was the case with this patient. Similar to proximal ruptures, the patient may admit to hearing or feeling a “popping” sound if there is an inciting event. The patient may exhibit a “reverse Popeye” deformity, with a bulge in the proximal arm secondary to retraction of the biceps muscle belly proximally.1
Diagnosis
There are two tests that can be performed to assist in making the diagnosis—the biceps squeeze test and the hook test.
Biceps Squeeze Test. The first test to assess for distal biceps tendon rupture is the biceps squeeze test, in which the clinician forcefully squeezes the patient’s biceps muscle to observe for forearm flexion/supination. This test is similar in principle to the Thompson test for Achilles tendon rupture. If there is no forearm movement, the injury is suspicious for a complete distal biceps tendon rupture. In one observational study of this test, 21 of 22 patients with a positive biceps squeeze test were found to have a complete distal biceps tendon tear at surgery.3
Hook Test. The second test is the hook test. While the patient actively supinates with the elbow flexed at 900, an intact hook test permits the examiner to “hook” his or her index finger under the intact biceps tendon from the lateral side. The absence of a “hook” means that there is no cord-like structure under which the examiner can hook a finger, indicating distal avulsion.4 In one study comparing the hook test to magnetic resonance imaging (MRI) in 33 patients with this suspected injury, the hook test had 100% sensitivity and specificity, while MRI only demonstrated a 92% sensitivity and 85% specificity.4
Imaging Techniques
The need for diagnostic imaging is based somewhat on the location of the rupture—proximal or distal. Ultrasound has been shown to have a high sensitivity and specificity for identifying normal tendons and complete tears of the long head biceps tendon (ie, proximal). It is not sensitive at identifying proximal partial tears, however. For distal ruptures, ultrasound imaging of the distal biceps tendon is technically difficult and not reliable. For patients with suspected distal biceps tendon ruptures, the EP should consult with orthopedic services prior to ordering an MRI. While MRI is considered the gold standard imaging test, it is neither 100% sensitive nor specific. The bottom line is that the absence of pathologic findings on MRI is not sufficient enough to exclude biceps tendon pathology.5
Treatment and Management
Regarding management, the majority of patients with proximal biceps tendon ruptures tend to do well with conservative management. The exception is for younger, active patients who are less willing to accept the cosmetic deformity, or patients whose occupation makes them unable to tolerate minimal weakness or fatigue cramping (eg, carpenters), in which case referral for a surgical repair (tenodesis) may be appropriate.1 However, multiple systematic reviews examining tenotomy vs tenodesis have not shown any functional improvement, only cosmetic.1,6,7
Distal biceps tendon ruptures are usually treated surgically, since conservative management results in a decrease of 30% to 50% supination strength and 20% flexion strength.1,8 This surgery, however, is not without complications. Approximately 20% of the patients will have a minor complication and 5% will have major complications following surgery on the distal biceps tendon.9 It is preferable to operate on distal ruptures less than 4 weeks from the initial injury; otherwise, these injuries may be more difficult to fix, require a graft, and have less predictable outcomes.1 Nonoperative management should be reserved for the elderly or less active patients with multiple comorbidities, especially if the nondominant arm is involved.10
Summary
The PA clearly missed the correct diagnosis on this patient. A more thorough history and focused physical examination would have led to the correct diagnosis sooner, along with earlier surgical repair. It is impossible, however, to know if the outcome would have been any different in this uncommon injury.
A 42-year-old man presented to the ED with left arm pain secondary to an injury he sustained at work. The patient stated that he had been helping to lift a heavy steel beam at a construction site when he experienced abrupt onset of pain in his left arm. He further noted that his left arm felt slightly weaker than normal after the injury.
The patient was left-hand dominant, denied any other injury, was otherwise in good health, and on no medications. With the exception of an appendectomy at age 12 years, his medical history was unremarkable. Regarding his social history, he admitted to smoking one pack of cigarettes per day, and to occasional alcohol consumption. He had no known drug allergies.
On physical examination, the patient’s vital signs were: blood pressure, 125/76 mm Hg; heart rate, 78 beats/min; respiratory rate, 16 breaths/min; and temperature, 98.6°F. Oxygen saturation was 99% on room air.
Examination of the patient’s left shoulder revealed no swelling or tenderness; he was able to fully internally/externally rotate the left shoulder, and lift his left hand above his head. The patient did have tenderness along the biceps area of the left arm, but no tenderness in the triceps area. The left elbow was tender in the antecubital fossa, but without swelling. He had full range of motion of the left elbow but with some pain. He likewise had full range of motion in his left wrist, but no tenderness or swelling. The left radial pulse was 2+. The patient had 5/5 grip strength with the left hand and good capillary refill.
The physician assistant (PA) evaluating the patient diagnosed an arm strain. At discharge, he referred the patient to an occupational health physician (OHP) for follow-up. He also instructed the patient to take ibuprofen 400 mg every 6 to 8 hours, and to limit use of his left arm for 3 days.
The patient followed up with the OHP approximately 3 weeks after discharge from the ED. The OHP was concerned the patient had experienced a distal biceps tendon rupture and referred the patient emergently to an orthopedic surgeon. The orthopedic surgeon saw the patient the next day, agreed with the diagnosis of a distal biceps tendon rupture, and attempted surgical repair the following day. The orthopedic surgeon informed the patient prior to the surgery that the delay in the referral and surgery could result in a poor functional outcome. The patient did have a difficult recovery period, and a second surgery was required, which did not result in any significant functional improvement.
The plaintiff sued the treating PA and supervising emergency physician (EP) for failure to properly diagnose the biceps tendon rupture, failure to appreciate the existence of a 3-week window of opportunity to repair the distal biceps tendon rupture, and failure to obtain an urgent orthopedic referral. The experts for the defense argued that the poor outcome was not a consequence of any delay in diagnosis or surgical repair. In addition, the defense disputed the existence of a 3-week window of opportunity for successful repair of a distal biceps tendon rupture. The jury returned a defense verdict.
Discussion
Proximal and Distal Biceps Tendon Ruptures
While both proximal and distal biceps tendon ruptures involve the biceps brachii, they are managed differently and have the potential for very different outcomes.1 At its proximal attachment, the biceps has two distinct tendinous insertions—the long head and the short head. For the distal attachment, the two muscle bellies unite at the midshaft of the humerus and attach as a single tendon on the radial tuberosity. In general, 96% of biceps tendon ruptures involve the long head, 1% involve the short head, and only 3% involve the distal tendon.1 Biceps tendon ruptures occur more commonly in men, patients who use anabolic steroids, cigarette smokers, patient history of tendinopathy, or patients who have a rotator cuff tear.1 Biceps tendon ruptures have not been found to be associated with statin use.2 The mechanism of injury includes heavy-lifting activities, such as weight lifting and rock climbing. However, when associated with a tendinopathy, minimal force may be involved.1
Signs and Symptoms
For proximal biceps tendon rupture, patients usually present with an acute or gradual onset of pain, swelling, and bruising of the upper arm and shoulder. Occasionally, if there is an inciting event, the patient may describe hearing or feeling a “popping” or “snapping” sound. On physical examination, the patient may exhibit a “Popeye” sign—a bulge in the distal biceps area due to the retracted biceps muscle belly. There is also tenderness along the biceps.
On testing, it has been estimated that patients can experience strength loss of approximately 30% with elbow flexion.1 In contrast, patients with distal biceps tendon ruptures usually complain of pain, swelling, and possibly bruising in the antecubital fossa, as was the case with this patient. Similar to proximal ruptures, the patient may admit to hearing or feeling a “popping” sound if there is an inciting event. The patient may exhibit a “reverse Popeye” deformity, with a bulge in the proximal arm secondary to retraction of the biceps muscle belly proximally.1
Diagnosis
There are two tests that can be performed to assist in making the diagnosis—the biceps squeeze test and the hook test.
Biceps Squeeze Test. The first test to assess for distal biceps tendon rupture is the biceps squeeze test, in which the clinician forcefully squeezes the patient’s biceps muscle to observe for forearm flexion/supination. This test is similar in principle to the Thompson test for Achilles tendon rupture. If there is no forearm movement, the injury is suspicious for a complete distal biceps tendon rupture. In one observational study of this test, 21 of 22 patients with a positive biceps squeeze test were found to have a complete distal biceps tendon tear at surgery.3
Hook Test. The second test is the hook test. While the patient actively supinates with the elbow flexed at 900, an intact hook test permits the examiner to “hook” his or her index finger under the intact biceps tendon from the lateral side. The absence of a “hook” means that there is no cord-like structure under which the examiner can hook a finger, indicating distal avulsion.4 In one study comparing the hook test to magnetic resonance imaging (MRI) in 33 patients with this suspected injury, the hook test had 100% sensitivity and specificity, while MRI only demonstrated a 92% sensitivity and 85% specificity.4
Imaging Techniques
The need for diagnostic imaging is based somewhat on the location of the rupture—proximal or distal. Ultrasound has been shown to have a high sensitivity and specificity for identifying normal tendons and complete tears of the long head biceps tendon (ie, proximal). It is not sensitive at identifying proximal partial tears, however. For distal ruptures, ultrasound imaging of the distal biceps tendon is technically difficult and not reliable. For patients with suspected distal biceps tendon ruptures, the EP should consult with orthopedic services prior to ordering an MRI. While MRI is considered the gold standard imaging test, it is neither 100% sensitive nor specific. The bottom line is that the absence of pathologic findings on MRI is not sufficient enough to exclude biceps tendon pathology.5
Treatment and Management
Regarding management, the majority of patients with proximal biceps tendon ruptures tend to do well with conservative management. The exception is for younger, active patients who are less willing to accept the cosmetic deformity, or patients whose occupation makes them unable to tolerate minimal weakness or fatigue cramping (eg, carpenters), in which case referral for a surgical repair (tenodesis) may be appropriate.1 However, multiple systematic reviews examining tenotomy vs tenodesis have not shown any functional improvement, only cosmetic.1,6,7
Distal biceps tendon ruptures are usually treated surgically, since conservative management results in a decrease of 30% to 50% supination strength and 20% flexion strength.1,8 This surgery, however, is not without complications. Approximately 20% of the patients will have a minor complication and 5% will have major complications following surgery on the distal biceps tendon.9 It is preferable to operate on distal ruptures less than 4 weeks from the initial injury; otherwise, these injuries may be more difficult to fix, require a graft, and have less predictable outcomes.1 Nonoperative management should be reserved for the elderly or less active patients with multiple comorbidities, especially if the nondominant arm is involved.10
Summary
The PA clearly missed the correct diagnosis on this patient. A more thorough history and focused physical examination would have led to the correct diagnosis sooner, along with earlier surgical repair. It is impossible, however, to know if the outcome would have been any different in this uncommon injury.
1. Smith D. Proximal versus distal biceps tendon ruptures: when to refer. BCMJ. 2017;59(2):85.
2. Spoendlin J, Layton JB, Mundkur M, Meier C, Jick SS, Meier CR. The risk of achilles or biceps tendon rupture in new statin users: a propensity score-matched sequential cohort study. Drug Safety. 2016;39(12):1229-1237. doi:10.1007/s40264-016-0462-5.
3. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Relat Res. 2005;437:128-131.
4. O’Driscoll SW, Goncalves LBJ, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1969. doi:10.1177/0363546507305016.
5. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254. doi:10.1016/j.ejrad.2015.07.031.
6. Tangari M, Carbone S, Gallo M, Campi A. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20(3):409-413. doi:10.1016/j.jse.2010.08.008.
7. Eakin JL, Bailey JR, Dewing CB, Provencher MT. Subpectoral biceps tenodesis. Oper Tech Sports Med. 2012;20(3):244-252.
8. Thomas JR, Lawton JN. Biceps and triceps ruptures in athletes. Hand Clin. 2017;33(1):35-46. doi:10.1016/j.hcl.2016.08.019.
9. Beks RB, Claessen FM, Oh LS, Ring D, Chen NC. Factors associated with adverse events after distal biceps tendon repair or reconstruction. J Shoulder Elbow Surg. 2016;25(8):1229-1234. doi:10.1016/j.jse.2016.02.032.
10. Savin DD, Watson J, Youderian AR, et al. Surgical management of acute distal biceps tendon ruptures. J Bone Joint Surg. 2017;3(9):785-796. doi:0.2106/JBJS.17.00080.
1. Smith D. Proximal versus distal biceps tendon ruptures: when to refer. BCMJ. 2017;59(2):85.
2. Spoendlin J, Layton JB, Mundkur M, Meier C, Jick SS, Meier CR. The risk of achilles or biceps tendon rupture in new statin users: a propensity score-matched sequential cohort study. Drug Safety. 2016;39(12):1229-1237. doi:10.1007/s40264-016-0462-5.
3. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Relat Res. 2005;437:128-131.
4. O’Driscoll SW, Goncalves LBJ, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1969. doi:10.1177/0363546507305016.
5. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254. doi:10.1016/j.ejrad.2015.07.031.
6. Tangari M, Carbone S, Gallo M, Campi A. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20(3):409-413. doi:10.1016/j.jse.2010.08.008.
7. Eakin JL, Bailey JR, Dewing CB, Provencher MT. Subpectoral biceps tenodesis. Oper Tech Sports Med. 2012;20(3):244-252.
8. Thomas JR, Lawton JN. Biceps and triceps ruptures in athletes. Hand Clin. 2017;33(1):35-46. doi:10.1016/j.hcl.2016.08.019.
9. Beks RB, Claessen FM, Oh LS, Ring D, Chen NC. Factors associated with adverse events after distal biceps tendon repair or reconstruction. J Shoulder Elbow Surg. 2016;25(8):1229-1234. doi:10.1016/j.jse.2016.02.032.
10. Savin DD, Watson J, Youderian AR, et al. Surgical management of acute distal biceps tendon ruptures. J Bone Joint Surg. 2017;3(9):785-796. doi:0.2106/JBJS.17.00080.