User login
Metabolic Complications of HIV Infection
From the University of Connecticut School of Medicine, Farmington, CT.
Abstract
- Objective: To review the metabolic complications of HIV infection.
- Methods: Review of the literature in the context of 3 clinical cases.
- Results: People with HIV infection are living longer thanks to the advent of potent antiretroviral therapy. This has led to increased incidence of age-related metabolic complications, including a higher risk of cardiovascular disease, hyperlipidemia, metabolic syndrome, and osteoporosis. Appropriate management of these complications requires an understanding of disease-related and drug-related side effects as well as the potential for drug-drug interactions. A multidisciplinary approach to patient management is most effective.
- Conclusion: Awareness of the metabolic complications frequently encountered in HIV infection, drug interactions, and possible toxicities is critical to the successful management of HIV-infected individuals.
Key words: HIV; antiretroviral therapy; hyperlipidemia; metabolic syndrome; diabetes; hypogonadism.
According to the most recent data from the Joint United Nations Programme on HIV/AIDS (UNAIDS), 36 million people worldwide are living with HIV/AIDS, with 18 million accessing effective antiretroviral therapy (ART) [1]. The past 2 decades have witnessed enormous advances in the field from prevention to diagnosis and therapeutics, and modern ART largely allows HIV-infected persons to live near-normal life spans [2,3]. However, from the beginning of the epidemic, HIV-infected persons on effective therapy have suffered from myriad metabolic consequences, many of which affect quality of life and result in excess mortality [4]. It is also true that untreated HIV infection portends an increased risk of metabolic complications, likely related to abnormal immune activation, as demonstrated in structured interruption trials [5,6]. It is worth noting, however, that while many of these metabolic dyscrasias and associated risks have historically been attributed primarily to the treatment of HIV infection with ART, data from cohort studies and randomized clinical trials have repeatedly demonstrated significant reductions in morbidity and mortality when ART is initiated early [7]. In this paper, we review HIV-related metabolic complications frequently encountered in clinical practice (hyperlipidemia, diabetes, and bone disease) and best practice considerations in the context of 3 clinical cases.
Case Patient 1
Initial Presentation and History
A 58-year-old male with a history of hypertension and mixed hyperlipidemia is referred for evaluation of newly diagnosed HIV infection. He has no history of intravenous drug use but has had multiple male and female sex partners in the past few years, and requested testing after a partner tested positive. His last negative test was 2 years ago. The patient does not smoke cigarettes. Overall he feels well and tolerates his regimen of lisinopril 10 mg and simvastatin 20 mg daily. On initial evaluation, his exam is unremarkable other than subtle white plaques on the dorsal surface of the tongue and buccal mucosa, and moderate central obesity. Vital signs including blood pressure are normal. Initial laboratory evaluation reveals a CD4 cell count of 150 cells/mm3 and an HIV RNA level of 200,000 copies/mL. Fasting serum total cholesterol is 220 mg/dL, triglycerides 250 mg/dL, LDL 170 mg/dL, and HDL 35 mg/dL. Serum BUN, creatinine, and liver function testing results are normal.
What initial regimen might be recommended based on the status of his HIV infection and comorbidities?
The most recent iteration of the US Department of Health and Human Services (DHHS) guidelines on use of antiretroviral agents (ARVs) in HIV recommends an initial ART regimen that includes a backbone of 2 nucleoside reverse transcriptase inhibitors (NRTIs), generally tenofovir disoproxil fumarate or tenofovir alafenamide, abacavir (ABC), emtricitabine (FTC), or lamivudine (3TC) [2]. To this backbone should be added a third agent; the majority of those currently recommended are integrase strand transfer inhibitors (INSTIs) (dolutegravir, elvitegravir, raltegravir); one recommended protease inhibitor (PI) (ritonavir-boosted darunavir) is also an option. Some of these initial recommended regimens are available as fixed-dosed combinations in 1 pill, making them attractive options.
The latest guidelines also clearly recommend starting ART in all HIV-infected individuals, irrespective of CD4 count. The patient described above has a very low CD4 count, so there is no question he needs to begin therapy promptly. Given his low CD4 count and relatively high viral load, one may consider a ritonavir-boosted PI as perhaps the most robust option and with a relatively high barrier to resistance development, in contrast to other options. Assuming the patient’s baseline resistance testing reveals a fully sensitive wild-type virus without meaningful resistance mutations, he will begin a regimen of TDF/FTC plus ritonavir-boosted darunavir, 3 pills once daily. Given his low CD4 count (below 200 cells/mm3), he will also need prophylaxis for Pneumocystis jirovecii pneumonia, in the form of trimethoprim/sulfamethoxazole (TMP/SMX) daily. Given the potential for interaction between the boosted PI and simvastatin, his lipid-lowering agent is switched to atorvastatin 10 mg daily.
What is the association between hyperlipidemia and HIV infection and treatment?
Hyperlipidemia represents a key modifiable risk factor for the development of cardiovascular disease (CVD) in HIV-infected individuals [8]. Indeed, a multicenter cross-sectional study of older HIV-infected individuals performed in Spain revealed a 54% prevalence of dyslipidemia and 23% CVD [9]. Most experts believe that metabolic abnormalities observed in HIV-infected individuals are the result of a combination of factors: those resulting from abnormal immune activation and inflammation related to viral replication, and those related to certain ARVs [10].
Early after HIV seroconversion, decline in HDL is one of the first proatherogenic changes observed. This, along with increased triglyceride and LDL levels, likely contribute to increased risk of CVD in this population. Increased microbial translocation, evidenced by increased levels of lipopolysaccharide (LPS), may drive immune activation, leading to dyslipidemia via a multitude of hypothesized mechanisms [4]. It has been theorized that HDL lipoproteins are less stable on ART, leading to potentially impaired plasma lipolytic activities or hepatic cholesteryl ester uptake [6,11]. Increased VLDL from release of free fatty acids may lead to higher triglyceride levels and triglyceride-rich LDL and HDL, all associated with increased risk of CVD [11].
In terms of effects of specific ARV classes, although newer agents have less of a propensity to cause dyslipidemia, the PI class arguably remains most problematic. In comparison to other classes, the PIs tend to result in greater increases in triglycerides, total cholesterol, and LDL, and have frequent drug-drug interactions with lipid-lowering agents [10,12]. Estimated prevalence of dyslipidemia in patients receiving PI therapy varies from 28% to 80% [13]. The prospective multinational cohort Data collection on Adverse events of Anti-HIV Drugs (DAD) study found significantly higher rates of hypertriglyceridemia, hypercholesterolemia, and low HDL in patients on PIs in comparison to non–nucleoside reverse transcriptase inhibitors (NNRTIs) [14]. Various mechanisms have been proposed to explain the PIs adverse effects on lipids, including inhibition of lipogenesis and adipocyte differentiation, decreased hepatocyte clearance of chylomicrons and VLDL, and increased hepatic synthesis of triglycerides [15]. Of the available PIs, atazanavir and darunavir have less potential to lead to dyslipidemia [10], while lopinavir/ritonavir, fosamprenavir, and tipranavir may have the highest [13]. Of the NNRTIs, efavirenz is most frequently associated with dyslipidemia, specifically increased triglycerides and total cholesterol [13]. However, these increased values seen on efavirenz therapy may be offset by relative increases in HDL, with little resultant effect on the total cholesterol:HDL ratio. Rilpivirine, etravirine, and nevirapine are relatively less likely to drive lipid changes, although certain drug interactions are important to recognize in clinical practice, such as the interaction between rilpivirine and proton pump inhibitors [2,13,16]. It is also worth noting that no NNRTIs are included in current guidelines as preferred therapy [2].
Historically, the thymidine analogue NRTIs (stavudine, didanosine, zidovudine) have been associated with lipid dyscrasias and lipoatrophy, but fortunately these are no longer used frequenty except in cases requiring deep salvage therapy for highly treatment-experienced patients. Two newer NRTIs, tenofovir and abacavir, have relatively neutral to favorable effects on lipids. The combination of tenofovir disoproxil (TDF) and emtricitabine (trade name Truvada) was associated with significantly lower triglycerides, total cholesterol and LDL than other NRTI pairs [6]. TDF has been postulated to have lipid-lowering effects. Switch studies in which patients were taken off thymidine analogues and placed on TDF, demonstrated recovery of limb fat in patients with lipoatrophy, and those switched off abacavir-based ART to TDF showed statistically significant lower fasting total cholesterol at week 12, without differences of viral suppression [8]. Tenofovir alafenamide (TAF) is a next-generation prodrug of tenofovir that results in improved stability in plasma and higher intracellular levels in comparison to TDF [17]. Although randomized controlled trials of TAF vs TDF-based ARV regimens have suggested statistically higher total cholesterol, serum HDL is also increased resulting in unchanged total:HDL ratios and no differences in risk classifications [18]. Integrase inhibitors (INSTI) now represent first-line therapy in combination with an NRTI backbone, and since their availability in 2007 have been evaluated in comparison to various PIs and NNRTIs. Both raltegravir and dolutegravir have consistently shown broad neutral effects on lipids and are among the most metabolically friendly agents available [19–21]. Because it is given in fixed-dose combination with non-ritonavir pharmacologic booster cobicistat, elvitegravir has effects similar to ritonavir-boosted PIs on lipids [6].
What are management considerations in the treatment of hyperlipidemia in HIV-infected patients?
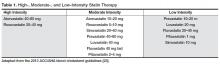
Patients with HIV and hyperlipidemia may benefit from lipid-lowering therapy in addition to ART, although in certain cases appropriate switches may make a difference. Careful consideration of drug interactions between ARVs and lipid-lowering agents, in addition to ARV history and known drug resistance, is warranted prior to selecting a regimen in these patients. In addition, the latest American College of Cardiology/American Heart Association guidelines suggest evaluating 10-year risk of atherosclerotic cardiovascular disease (ASCVD) using the pooled cohort equation to determine the type and dose of statin required (moderate vs high intensity) [22]. It is noteworthy that HIV infection and its therapies are not taken into account as potential risk factors in this model. Primary prevention in non-diabetic patients with a statin is recommended for patients with a 10-year absolute risk of ≥ 7.5% [22]. This patient’s risk is estimated at between 12% and 13% based on this equation, so primary prevention with a moderate-or high-intensity statin is recommended (Table 1) [23]. Data from more than 80,000 patients in the Veterans Aging Cohort Study (VACS) showed that HIV-infected patients with no baseline ASCVD had 50% increased risk of acute myocardial infarction when compared to HIV-uninfected patients over 6 years of follow-up [24]. Thus, consideration of the virus itself or its therapy as an additional risk factor may be valid.
Screening and Monitoring of Hyperlipidemia
The most recent iteration of the DHHS primary care guidelines for the management of HIV-infected individuals recommends obtaining fasting (ideally 12 hours) lipid profiles upon initiation of care, and within 1 to 3 months of beginning therapy [12,13]. These initial levels, along with other elements of the patient’s history and calculation of risk may help determine whether lipid-lowering therapy is indicated, and if so, which therapy would be best. In general, after regimen switches or additions of either ARV or statin therapy, repeating fasting lipid levels 6 weeks later is recommended to gauge the effects of the switch. This is especially critical when interactions between ARVs and lipid-lowering therapies are possible. Some experts recommend performing annual screening of patients with normal baseline lipids or with well-controlled hyperlipidemia on therapy. Assessment of 10-year ASCVD risk is also recommended annually, in addition to baseline risk assessment, to determine the need and appropriateness of statin therapy [25]. The question of primary prevention in HIV has yet to be definitively answered. Small studies in this population have demonstrated that statins have the potential to slow progression of carotid intima media thickness and reduce noncalcified plaque volume [24]. An NIH/AIDS Clinical Trial Group–sponsored randomized clinical trial (“REPRIEVE”) is currently underway to address this question. More than 6000 HIV-infected men and women with no history of ASCVD at 100 sites in several countries are enrolled to assess the benefit of pitavastatin as primary prevention in this risk group [24]. Metabolized via glucuronidation primarily, as opposed to cytochrome p450 (CYP 3A4 isoenzyme), pitavastatin is thought to have fewer drug interactions with ARVs in general [6] (Table 2).
Relevant Drug-Drug Interactions
Deciding which statin to begin in HIV-infected patients depends on whether moderate- or high-intensity therapy is warranted and whether the potential for drug interaction with ARVs exists. Table 2 [6,12] depicts available statins and the potential for pharmacokinetic interaction with the primary ARV classes. Simvastatin and lovastatin are heavily metabolized via the CYP 3A4 pathway, resulting in the highest potential risk of interaction with CYP 3A4 inhibitors, such as the PIs, or inducers (eg, NNRTIs, in particular efavirenz) [6]. The former may inhibit metabolism of these statins, resulting in increased risk of toxicity, while co-administration with efavirenz, for example, may result in inadequate serum concentration and therefore inadequate lipid-lowering effects. Although less lipophilic, atorvastatin results in similar interactions with PIs and NNRTIs, and therefore low starting doses with close monitoring is recommended [6]. Fewer interactions have been noted with rosuvastatin, pravastatin, and pitavastatin, as these do not require CYP 3A4 for their metabolism and are thus less likely to be affected by ARVs. These therefore represent potentially safer first choices for certain patients on ARVs, although of these, only rosuvastatin is classified as a high-intensity statin [22,23] (Table 1). When compared directly to pravastatin 40 mg daily in patients receiving ritonavir-boosted PIs, rosuvastatin performed superiorly at 10 mg per day, resulting in more significant reductions in LDL and triglyceride levels [15]. Although it is eliminated largely unchanged through the kidney and liver, pravastatin has been reported to idiosyncratically interact with darunavir, resulting in potentially increased pravastatin levels and associated toxicity [25]. Treatment of pure hypertriglyceridemia in HIV-infected patients should begin with fibrates, which have little to no risk of interaction with most clinically relevant ARVs [6,10]. Alternatives to lower triglycerides include niacin and N-3 polyunsaturated fatty acids [25].
Case 1 Continued
The patient has an impressive response to his initial regimen of TDF/FTC plus boosted darunavir, with repeat CD4 count after 12 weeks of 275 (18%) cells/mm3 and an undetectable viral load (< 20 copies/mL). Other lab parameters are favorable and he is tolerating the regimen well without notable side effects. However, at his next visit, although his viral load remains undetectable, his triglyceride level has increased to 350 mg/dL, although other lipid parameters are comparable to the prior result. He complains of diffuse body aches, concentrated in large muscle groups of the extremities, and dark-colored urine. A creatine phosphokinase (CPK) level is elevated at 300 IU/L (normal, 22–269, negative MB fraction). Serum creatinine is 1.4 mg/dL (had been 1.1 mg/dL at baseline). Given he has done so well otherwise on these ARVs, he is reluctant to make any changes.
What drug-drug interaction is most likely causing this patient's problem, and how should it be managed?
This scenario is not uncommon in clinical practice, and changes to regimens are sometimes necessary in order to avoid drug interactions. Care must be taken to thoroughly review antiretroviral history and available resistance testing (in this case a relatively short history) in order to ensure a fully active and suppressive regimen is chosen. This description could be the result of an interaction between lipid-lowering therapy and ARVs resulting in increased relative concentrations of one drug or the other and therefore leading to toxicity. Given this possibility, and suboptimal control of hyperlipidemia, consideration should be given to switching both his ART and his statin therapy.
Safety and Potential Toxicities of Lipid-Lowering Therapy
Increased serum concentration of certain statins when co-administered with CYP 3A4 inhibitors like the PIs leads to heightened risk of statin-associated toxicities. In general, this includes muscle inflammation, leading to increases in serum CPK level and associated symptoms, including myalgias, myositis, or in extreme cases, rhabdomyolysis [6]. Although rare, this toxicity can be serious and may lead to acute renal injury if not recognized and managed appropriately. In theory, the potential for statin-associated hepatotoxicity may also be increased in patients receiving PIs, although this has not been borne out in clinical trials [26]. In fact, quite the opposite may be true, in that statins have been shown to improve liver function in patients with hepatitis C virus (HCV) coinfection and with nonalcoholic fatty liver disease [6,15].
Case 1 Conclusion
The patient does well on his new ARV regimen of TAF/FTC and dolutegravir, 2 pills once daily. He no longer requires TMP/SMX, as his CD4 count has been reliably above 200 cells/mm3 on several occasions. Serum creatinine is back down to baseline and CPK has normalized. Fasting lipids have improved since the switch, and he no longer has symptoms of myositis on rosuvastatin 10 mg daily.
Summary
Consideration of statin therapy is complicated by potential drug interactions with ARVs and associated toxicity. However, given known effects of ARVs on lipids, and of immune activation and inflammation related to the virus itself, these patients should be carefully evaluated for statin therapy for their anti-inflammatory and immune modulatory effects as much as for their lipid-lowering ability. Utilization of HIV infection and its therapies as additional cardiovascular risk factors when calculating 10-year risk deserves further consideration; forthcoming results of the REPRIEVE trial are certain to contribute valuable information to this field of study.
Case Patient 2
Initial Presentation and History
A 45-year-old female with history of HIV infection since 2008 presents to the office for new-onset diabetes, diagnosed 2 weeks ago. She has had symptoms of polyuria and polydipsia for the last 1 month. She denies diarrhea, nausea, vomiting or weight loss. She is currently on a regimen consisting of zidovudine/lamivudine plus lopinavir/ritonavir. There is no family history of diabetes. Her examination is unremarkable, including normal vital signs (weight 150 lb, blood pressure 114/70, heart rate 76) and no evidence of insulin resistance, including acanthosis nigricans or striae. Glycosylated hemoglobin level (HbA1c) is 8%. Creatinine and liver function tests are within reference ranges.
Do HIV-infected patients have a higher incidence of type 2 diabetes mellitus (DM)?
Prevalence of type 2 DM in HIV-infected patients varies between 2% to 14% [27]. This variation is due to the different cutoffs used for diagnosis, differences in cohorts studied, and how risk factors are analyzed [28–31]. In a recent nationally representative estimate of DM prevalence among HIV-infected adults receiving medical care in the United States in 2009–2010, the prevalence of DM was noted to be 10.3%. In comparison to the general adult US population, HIV-infected individuals have a 3.8% higher prevalence of DM after adjusting for age, sex, race/ethnicity, education, poverty-level, obesity, and HCV infection [27].
There is controversy over whether HIV infection itself increases the risk of type 2 DM, with some studies showing increased risk [28,32,33] and others showing no independent effect or an inverse effect [30,34,35]. Studies on the impact of ethnicity and race on prevalence of DM are limited [36].
Certain traditional risk factors (age, ethnicity, obesity) are still responsible for most of the increased risk of diabetes in the HIV-infected population [35,37]. HIV infection itself is associated with metabolic dysfunction, independent of ARV. In HIV-infected patients, impaired glucose metabolism is associated with altered levels of adipokines, increased adiponectin and soluble-tumor necrosis factor receptor 1 (sTNFR1) and decreased leptin [38,39]. HIV-associated alterations in CD4+ and CD8+ T cell function also impair glycolysis, which may adversely impact glucose metabolism [40].
Other contributing factors in HIV-infected patients are HCV co-infection [41], medications (atypical antipsychotics, corticosteroids), opiates, and low testosterone [42]. HCV co-infection may lead to hepatic steatosis and liver fibrosis, and increasing insulin resistance.
Recent genomic studies show several common single-nucleotide polymorphisms (SNPs) associated with diabetes in the general population. In the Swiss HIV Cohort Study, SNPs accounted for 14% of type 2 DM risk variability, whereas ARV exposure accounted for 3% and age for 19% of the variability in DM [43].
ARVs also increase the risk of type 2 DM by both direct and indirect effects. Certain ARVs causes lipoatrophy [30] and visceral fat accumulation/lipohypertrophy [29,44]. PIs increase insulin resistance via effects on GLUT-4 transporter and decrease insulin secretion through effects on B cell function [45]. NRTIs (eg, stavudine, zidovudine and didanosine) can cause direct mitochondrial toxicity [46–48]. Utilization of newer ARV agents has decreased the prevalence of severe lipoatrophy, but lipohypertrophy and the underlying metabolic abnormalities persist. The DHHS “preferred” nucleoside analogues, tenofovir and abacavir, do not induce mitochondrial toxicity and have more favorable metabolic profiles [49,50]. In ACTG Study 5142, thymidine-sparing regimens were found to cause less lipoatrophy [51]. In addition, darunavir and atazanavir, the preferred and alternative PIs and the integrase strand transfer inhibitor have limited or modest impact on insulin sensitivity [20,52,53]. This has led to a recent decline in the incidence of type 2 DM in HIV-infected patients.
Statins can also increase insulin resistance and DM [54], although studies have shown mixed results [55–57]. The benefits of statin therapy likely outweigh the risk of DM since there is a significant cardiovascular event reduction with their use [58,59].
How is diabetes diagnosed in HIV-infected patients?
Optimal diabetes screening guidelines have not been established specifically for HIV-infected patients. The American Diabetes Association (ADA) guidelines recommend that diabetes in the general population be diagnosed by 2 elevated fasting blood glucose levels, HbA1c, oral glucose tolerance test (OGTT), or high random glucose with classic symptoms of hyperglycemia [60]. Repeat testing is recommended every 3 years. The OGTT is recommended for diagnosis in pregnant women.
HbA1c may underestimate glycemic burden in HIV-infected individual due to higher mean corpuscular volume, NRTI use (specifically abacavir), or lower CD4 count [61–65]. The Infectious Diseases Society of America (IDSA) 2013 primary care guidelines for HIV-infected patients recommends obtaining a fasting glucose and/or HbA1c prior to and within 1–3 months after starting ARV [12]. Use of HbA1c threshold cutoff of 5.8% for the diagnosis of DM and testing every 6–12 months are recommended.
How should this patient’s diabetes be managed?
The ADA guidelines suggest a patient-centered approach to management of diabetes [66]. All patients should be educated about lifestyle modifications with medical nutrition therapy and moderate-intensity aerobic activity and weight loss [67]. If a patient is on lopinavir/ritonavir or a thymidine analogue (zidovudine, stavudine), one should consider switching the ARV regimen [2].
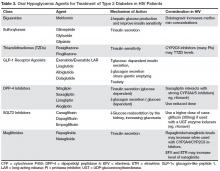
There are currently no randomized controlled trials of diabetes treatment specific to patients with HIV infection. Metformin is the first-line agent. It improves insulin sensitivity by reducing hepatic glucose production and improving peripheral glucose uptake and lipid parameters [68,69]. Other oral hypoglycemic agents used in the treatment of type 2 diabetes are shown in Table 3.
Case 2 Continued
The patient is switched to TAF/FTC plus dolutegravir with improvement in blood sugars. She is also started on metformin. Co-administration of metformin and dolutegravir will be carefully monitored since dolutegravir increases metformin concentration [70]. When dolutegravir is used with metformin, the total daily dose of metformin should be limited to 1000 mg.
• How should this patient be followed?
If the patient is still not at goal HbAb1c at follow-up, there are multiple other treatment options, including use of insulin. Goal HbA1c for most patients with type 2 DM is < 7%; however, this goal should be individualized for each patient in accordance with the ADA guidelines [12]. A longitudinal cohort study of 11,346 veterans with type 2 diabetes compared the glycemic effectiveness of oral diabetic medications ( metformin, sulfonylurea and a thiazolidinedione) among veterans with and without HIV infection. This study did not find any significant difference in HbA1c based on different diabetes medications. However the HBA1c reduction was less in black and Hispanic patients. The mechanism for the poorer response among these patients need to be evaluated further [71]. In addition to management of blood sugar, other CVD risk factors, hyperlipidemia, hypertension, smoking, etc, should be assessed and managed aggressively.
Case Patient 3
Initial Presentation and History
A 45-year-old male with a history of HIV infection diagnosed 10 years ago, on TDF/FTC/efavirenz (trade name Atripla) for the last 7 years, presents with a left femoral neck fracture after he missed the pavement and fell on his left hip. His history is significant for IV drug abuse for 10 years prior to diagnosis of HIV, and he has been on methadone for the last 6 years.
Is HIV infection associated with increased prevalence of osteopenia and osteoporosis and increased risk of fractures?
With recent advancements in antiretroviral therapy and improved survival of the HIV-infected population, osteoporosis and increased fracture risk have become important causes of morbidity and mortality. Osteoporosis is a skeletal disorder characterized by compromised bone strength, which predisposes to an increased risk of fracture. The World Health Organization defines osteoporosis as a bone mineral density (BMD) measurement by dual X-ray absorptiometry (DXA) at the spine, hip, or forearm that is more than 2.5 standard deviations below that of a "young normal" adult (T-score < –2.5) or a history of one or more fragility fractures. Fragility fractures result from mechanical forces that would not ordinarily result in fracture, such as fall from standing height [40]. Osteopenia is characterized by low BMD (T-score between –1.0 and –2.5) and can be a precursor to osteoporosis.
Several observational, retrospective, and prospective studies have shown lower bone density and an increased risk of fractures in the HIV-infected population compared to age-, race- and sex-matched HIV-negative adults. In a large meta-analysis of pooled prevalence data on 884 HIV-infected patients compared with 654 HIV-uninfected age- and sex-matched controls [72], overall, HIV-infected patients had a significant 6.4-fold increased odds of reduced BMD and a 3.7-fold increased odds of osteoporosis compared to the control population. This meta-analysis also compared ARV-treated subjects to ARV-naive subjects and showed that ARV-treated subjects (n = 824) had a higher prevalence of reduced BMD compared with ARV-naive subjects (n= 202; odds ratio 2.5, 95% CI 1.8–3.7). The odds of osteoporosis was increased 2.4 times (95% CI 1.2 – 4.8) in ARV-treated subjects compared with ARV-naive subjects. None of the studies adjusted for potentially important confounding factors, such as age or duration of HIV infection. PI-treated patients (n = 791) were also found to have a higher prevalence of reduced BMD compared with PI-untreated patients (n = 410; OR 1.5, 95% CI 1.1–2.0). The odds of osteoporosis in PI-treated patients (n = 666) was also 1.6-fold greater (95% CI 1.1–2.3) than those not treated with PI (n = 367).
Low bone density has also been reported in HIV- positive premenopausal women irrespective of ARV status. In a recent study of 89 premenopausal women (mean age, 37 years) predominantly of African origin with HIV infection, osteopenia and osteoporosis were prevalent in one-third of these women, irrespective of ARV use and were associated with low BMI [73]. In a sub-study of the INSIGHT trial evaluating prevalence of and risk factors for low BMD in untreated HIV infection, performed at several sites across 6 continents involving 424 subjects, osteopenia was present in a third of this relatively young predominantly non-white ART-naive population (mean age 34 + 10 years) with normal CD4 cell counts, while only 2% had osteoporosis. Factors independently associated with lower BMD at the hip and spine were female sex, Latino/Hispanic ethnicity, lower BMI, and higher estimated glomerular filtration rate. Longer duration of HIV infection was also associated with lower hip BMD. Current or nadir CD4 cell count and HIV viral load were not associated with low BMD [74].
Many studies have reported increased fracture prevalence in the HIV population. In a retrospective study of fracture prevalence in a large US health care system, a significantly higher rate of fractures was reported in HIV-infected men and women compared to non-HIV-infected controls (2.87 vs. 1.77 fractures per 100 persons, P < 0.001). The difference in the increased fracture prevalence was greater in HIV positive men compared to women (3.08 vs. 1.83; P < 0.001). Vertebral, wrist and hip fractures were more prevalent in men compared to vertebral and wrist fractures only in women. Fracture prevalence was higher in both Caucasian females and males and only in African-American women [75].
In the HIV Outpatient Study (HOPS) [76], age-adjusted fracture rates in the HIV population were noted to be 1.98 to 3.69 times higher than rates in the general population. The HOPS was an open prospective cohort study of HIV-infected adults who were followed at 10 US HIV clinics. Rates of first fractures at any anatomic site from 2000–2008 were assessed among 5826 active HOPS patients (median age 40 years, 79% male, 52% Caucasian, and 73% exposed to ART). Among persons aged 25–54 years, both fracture rates and relative proportion of fragility fractures were higher among HOPS patients than among outpatient controls. Older age, substance abuse, nadir CD4+ cell count <200 cells/mm, HCV infection and DM were associated with incident fractures [76].
What factors contribute to poor bone health in the HIV population?
Several factors that contribute to low bone density are present at a higher rate in the HIV population (Table 4). These include poor nutritional status in terms of suboptimal calcium and vitamin D intake, hypogonadism, low body weight, and alcohol, tobacco and substance abuse.
Vitamin D deficiency is very common in HIV-infected patients, with a prevalence of up to 60% to 75% [77]. Hypogonadism is also relatively common among HIV population [78], contributing to lower bone density. Co-infection with HCV is also associated with increased risk of fractures. In a large cohort of Medicaid beneficiaries, a significant increase in the risk of hip fracture was demonstrated in HCV/HIV co-infected subjects compared either with HCV mono-infected, HIV mono-infected or non-infected individuals [79]. In another large database study, a significantly higher risk of osteoporotic fracture (closed wrist, vertebral or hip fracture) was reported in HCV/HIV co-infected versus HIV mono-infected individuals [80] with fracture rates of 2.57 and 2.07/1000 patient-years (P < 0.001). Dual treatment for HIV/hepatitis B co-infection has also been shown to be associated with a higher risk of hip fracture compared to treatment of HIV mono-infected individuals [81].
HIV infection itself can increase bone loss and reduce bone formation through direct effects related to the HIV antigen load or indirect effects related to activation of the pro-inflammatory cytokines resulting in bone resorption and loss [82]. Co-infection with HCV and/or hepatitis B also contributes to lower bone density in this population. Certain ARVs may also contribute to low bone density in the HIV population. Lipoatrophy related to HIV may also mediate bone loss through complex relationship between central signaling of adipocyte hormones [82,83].
Direct Viral Effects
Several HIV viral proteins have been shown to promote osteoclast activity (vpr and gp120), suppress osteoblast activity (p55-gag) and increase osteoblast apoptosis [84], resulting in increased bone resorption and reduced bone formation, leading to low bone mass. High HIV RNA viral load and T-cell activation are also associated with elevated levels of receptor activator of nuclear factor kappa-B ligand (RANKL), which results in osteoclast formation and increased bone resorption [85]. Other endogenous physiological inhibitors of osteoclastogenesis such as osteoprotegrin and interferon-γ levels are also remarkably downregulated in advanced HIV infection, resulting in increased bone resorption [86]. At a cellular level, HIV proteins including Tat and Nef reduce the number of available mesenchymal stem cell (MSC) precursors that proliferate into osteoblasts by inducing MSC senescence, due to increased oxidative stress and mitochondrial dysfunction resulting in reduced proliferation of osteoblasts and lower rates of bone formation [87]. Collectively, these mechanisms result in significant uncoupling of bone formation and resorption, resulting in less bone formation and greater rate of bone loss and lower bone density.
Pro-inflammatory Pathways
Cytokines and other soluble immune factors play a major role in the physiology of osteoblast maturation and osteoclastic bone resorption [88,89]. Immune dysfunction and persistent inflammation in HIV result in increased levels of several inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), and RANKL, resulting in stimulation of osteoclastogenesis and bone resorption [90]. Due to a disruption between T and B cells in HIV and decreased osteoprotegrin (OPG) production and increased RANKL level, RANKL/OPG ratio is elevated, favoring osteoclastogenesis [91].
Is antiretroviral therapy associated with bone loss?
The initiation of ART has been reported to cause 2% to 6% bone loss irrespective of the regimen used, similar to that sustained in the first 2 years after menopause [92]. Certain NRTIs and PIs are associated with higher rates of bone loss than others. TDF has been associated most commonly with decreased bone mineral density, which usually stabilizes with continued use [93]. In a randomized trial comparing 4 treatment arms of ABC/3TC or TDF/FTC with EFV or ATV/ritonavir, TDF was associated with a greater reduction in BMD compared to abacavir-based regimens [94]. The likely cause of this may be TDF-mediated renal toxicity, including proximal tubular dysfunction and hypophosphatemia, resulting in increased PTH and bone resorption, and nephrogenic diabetes insipidus [95]. TAF is another prodrug of tenofovir diphosphate associated with less renal and bone toxicity compared with TDF. TAF has been associated with significantly less decrease in bone mineral density and renal dysfunction in randomized studies compared to regimen using TDF [17]. Vitamin D deficiency and hypophosphatemia associated with TDF therapy may present with osteomalacia, which predisposes to bone pain and fractures. Treatment with TDF may rarely be associated with the development of Fanconi syndrome and osteomalacia [96]. BMD is often severely reduced and bone pain and pathological fractures are characteristic features. Certain PI regimens containing ritonavir-boosted atazanavir have also been associated with greater bone loss in the spine than the hip, compared to efavirenz-containing regimens [97].
The universal bone loss associated with ART is thought to be a result of the "immune reconstitution inflammatory syndrome" (IRIS). This occurs as a result of rapid improvement in immune function after the commencement of ARV as a result of systemic or local inflammation, resulting in increased levels of cytokines that may contribute to bone loss. This has been shown in animal studies where T cell transplantation into immunocompromised mice to mimic ARV-induced T-cell expansion resulted in increased RANKL and TNF-α production by B cells and/or T cells, accompanied by enhanced bone resorption and BMD loss. When TNF-α or RANKL-null T-cells or TNF-α antagonists were used instead, the loss of cortical bone was prevented [98]. In a prospective study evaluating changes in bone turnover markers and inflammatory cytokines with ARV therapy in HIV infected subjects, a significant increase in bone resorption markers, RANKL and TNF-α were seen after initiation of ARV. The magnitude of CD4-cell recovery correlated with the increase in markers of bone resorption [99], suggesting that recovery of the immune system contributes to the increase in cytokine-mediated bone resorption.
How is bone health and fracture risk assessed in the HIV-positive population?
The predictive value of low BMD for fracture risk assessment in the HIV-positive population has not been established. In the absence of definitive data, the fracture risk assessment and standard methods of measuring bone density using DXA are utilized. In a large study of 1000 men and women, osteoporosis defined as a BMD T-score –2.5 as measured by DXA, was associated with a significantly increased risk of incident fractures but was not a good predictor of morphometric vertebral fractures [100]. In the absence of prospective longitudinal studies evaluating the bone density parameters at which fracture risk is significantly increased in the HIV population, it is reasonable to follow the guidelines used in the non-HIV population.
The approach to treatment of osteopenia and osteoporosis is similar to that in non HIV-infected population and is directed at lifestyle changes and treatment of secondary causes of osteoporosis [101], followed by initiation of antiresorptive therapy.
Management of Bone Disease
There are several guidelines available for the management of bone disease in the HIV population. The most recent guidelines from the IDSA [12] recommend assessing the risk of fragility fracture using the Fracture Risk Assessment Tool (FRAX), without DXA, in all HIV-infected men aged 40–49 years and HIV-infected premenopausal women aged ≥ 40 years. DXA should be performed in men aged ≥ 50 years, postmenopausal women, patients with a history of fragility fracture, patients receiving chronic glucocorticoid treatment, and patients at high risk of falls. In resource-limited settings, FRAX without bone mineral density can be substituted for DXA. ART guidelines should be followed. TDF and boosted PIs should be avoided if possible in at-risk patients. Dietary and lifestyle management strategies for high-risk patients should be employed and anti-osteoporosis treatment initiated if indicated [102].
The FRAX tool is available at www.shef.ac.uk/FRAX/ and is used to calculate 10-year fracture risk using patient clinical data, including presence of risk factors for osteoporosis. The tool is population-specific by race and region. It has not been validated for the HIV-positive population and may underestimate fracture risk [103]. HIV status is considered a secondary cause of osteoporosis in FRAX calculation.
The National Osteoporosis Foundation recommends screening with DXA for all women > 65 years of age, all men > 70 years of age, and adults > 50 years of age with additional risk factors for osteoporosis. Evaluation for secondary causes for low BMD should always be considered in the HIV-positive population including evaluation of calcium and vitamin D intake. Laboratory testing may include complete blood count, calcium, phosphate, albumin, creatinine, PTH, 25 hydroxy vitamin D (25,OHD) and 24 hour urine for evaluation of calcium, creatinine and phosphate (especially if on TDF) excretion. Testosterone level can be checked in men and estradiol, prolactin, FSH and LH in women for evaluation of hypogonadism. Bone turnover markers (bone specific alkaline phosphatase and serum C-terminal telopeptide) can also be assessed at baseline.
Studies using high-resolution peripheral quantitative computed tomography (HSPQCT) have shown significant reductions in tibial trabecular bone density and trabecular number in pre-menopausal and postmenopausal HIV-infected women [104], with reduced bone stiffness measured using finite element analysis [105]. Co-infection with HCV is also associated with significantly lower trabecular volumetric BMD and smaller cortical dimensions in the tibia, compared to healthy subjects [106]. HSPQCT is not widely available for clinical use at this time. Lateral imaging of the spine or vertebral morphometric analysis may be done in cases of height loss to assess for occult vertebral compression fractures.
There is a high prevalence of vitamin D deficiency in the HIV-infected population [107]. Treatment goal is to have a vitamin D level of at least 30 ng/mL, based on Endocrine Society practice guidelines [108], and may require supplementation with 1000–2000 units of vitamin D daily. Calcium intake should be optimized, averaging 1000 mg per day including diet and supplements, to be taken in divided amounts through the day for optimal absorption. Secondary causes of low bone density as mentioned in Table 4 should also be addressed. Patients should be counseled on tobacco and alcohol abuse. Corticosteroids should be dosed at the lowest dose needed. Medications such as proton pump inhibitors can impair the absorption of calcium carbonate, in which case calcium citrate supplements should be used if there is suboptimal calcium intake in the diet.
Which medications have been shown to be effective in treatment of osteoporosis in the HIV population?
Bisphosphonates are the mainstay of therapy for osteoporosis in the HIV-infected population. Only alendronate and zoledronate have substantial evidence of safety and effectiveness in the HIV-infected population, but these studies have been small and of limited duration.
Bisphosphonates are pyrophosphate analogues that inhibit bone resorption by binding to the hydroxyapatite crystals in the bone. Several prospective studies have shown alendronate to increase bone density compared to calcium and vitamin D alone in the HIV infected patients with reduced bone density [109,110], with significant reduction in markers of bone resorption [111].
Zoledronic acid (ZA), an amino-bisphosphonate which is infused intravenously, has also been used in smaller studies in HIV-infected persons. In a prospective study evaluating yearly ZA infusion to biennial ZA infusion in subjects with HIV and low bone density [112], biennial ZA infusions were found to be effective in improving and maintaining bone density in the HIV population. In another prospective study evaluating the effects of ZA in HIV-positive men, ZA infusion was given at baseline and at 12 months. Compared to placebo, treatment group had significantly higher bone density and lower bone turnover markers till 5 years after the last infusion [113].
In a meta-analysis evaluating the effect of bisphosphonates on bone mineral density in 328 adults with HIV infection from 8 randomized controlled trials (5 with alendronate and 3 with ZA as the intervention), a significant increase in BMD at the lumbar spine and hip was observed in the treatment groups at 48 and 96 weeks. However, these studies were not long enough to detect the impact of bisphosphonates on fracture risk [114]. ZA has also been shown to be effective in preventing ARV induced bone loss after a single infusion [115].
These studies confirm that both alendronate and ZA are effective in improving BMD in the HIV-infected population, with early studies showing a beneficial effect of ZA in mitigating ARV-induced bone loss as well. DXA may be repeated 1 to 2 years after initiation of osteoporosis therapy and less often subsequently if BMD is stable to improved [116].
Although these studies show significant improvement in bone density with treatment, longitudinal data on fracture reduction with these medications in the HIV-infected population are not available. Additionally, these patients have onset of osteoporosis at a younger age and the need for osteoporosis treatment needs to be assessed carefully before initiating treatment. There are other medications available for the treatment of osteoporosis in the non-HIV population such as raloxifene, teriparatide and denosumab, but no randomized controlled studies of these agents are available in the HIV-infected population.
Summary
The advent of highly potent antiretroviral therapy capable of early and prolonged viral suppression in HIV-infected patients has resulted in significant increases in life span. As we have already seen, this will likely lead to a rising incidence of various metabolic complications of HIV and ARV, including hyperlipidemia and diabetes with associated cardiovascular disease risk. A keen awareness of these potential complications, drug interactions, and possible toxicities will be paramount to their successful management. Appropriate care of HIV-infected individuals going forward will likely require multidisciplinary collaboration as the epidemic evolves to allow our patients to live not only longer, but healthier lives.
Corresponding author: Lisa M. Chirch, MD, UCONN Health, Farmington, CT 06030, [email protected].
Financial disclosures: None
Author contributions: All authors contributed equally to this article
1. UNAIDS. Fact sheet - Latest statistics on the status of the AIDS epidemic. Accessed 10 Nov 2017 at http://www.unaids.org/en/resources/fact-sheet.
2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 10 Nov 2017 at https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
3. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One 2013;8:e81355.
4. Pedersen KK, Pedersen M, Troseid M, et al. Microbial translocation in HIV infection is associated with dyslipidemia, insulin resistance, and risk of myocardial infarction. J Acquir Immune Defic Syndr 2013;64:425–33.
5. Strategies for Management of Antiretroviral Therapy Study Group, El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–96.
6. Chastain DB, Henderson H, Stover KR. Epidemiology and management of antiretroviral-associated cardiovascular disease. Open AIDS J 2015;9:23–37.
7. Group ISS, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
8. Moyle GJ, Orkin C, Fisher M, et al. A randomized comparative trial of continued abacavir/lamivudine plus efavirenz or replacement with efavirenz/emtricitabine/tenofovir DF in hypercholesterolemic HIV-1 infected individuals. PloS One 2015;10:e0116297.
9. Mothe B, Perez I, Domingo P, et al. HIV-1 infection in subjects older than 70: a multicenter cross-sectional assessment in Catalonia, Spain. Curr HIV Res 2009;7:597–600.
10. Calvo M, Martinez E. Update on metabolic issues in HIV patients. Curr Opin HIV AIDS 2014;9:332–9.
11. Gillard BK, Raya JL, Ruiz-Esponda R, et al. Impaired lipoprotein processing in HIV patients on antiretroviral therapy: aberrant high-density lipoprotein lipids, stability, and function. Arterioscler Thromb Vasc Biol 2013;33:1714-21.
12. Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58:1–10.
13. Calza L, Colangeli V, Manfredi R, et al. Clinical management of dyslipidaemia associated with combination antiretroviral therapy in HIV-infected patients. J Antimicrob Chemother 2016;71:1451–65.
14. Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS 2003;17:1179–93.
15. Husain NE, Ahmed MH. Managing dyslipidemia in HIV/AIDS patients: challenges and solutions. HIV/AIDS (Auckl) 2015;7:1–10.
16. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. Accessed at www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
17. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015;385:2606–15.
18. Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2014;67:52–8.
19. Eron JJ, Young B, Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 2010;375:396–407.
20. Quercia R, Roberts J, Martin-Carpenter L, Zala C. Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks. Clin Drug Investig 2015;35:211–9.
21. Lennox JL, DeJesus E, Berger DS, et al. Raltegravir versus efavirenz regimens in treatment-naive hiv-1–infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010;55:39–48.
22. Nayor M, Vasan RS. Recent update to the US cholesterol treatment guidelines. a comparison with international guidelines. Circulation 2016;133:1795–806.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934.
24. Mitka M. Exploring statins to decrease HIV-related heart disease risk. JAMA 2015;314:657–9.
25. Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Diseases Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003;37:613–27.
26. Milazzo L, Menzaghi B, Corvasce S, et al. Safety of statin therapy in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr 2007;46:258–60.
27. Hernandez-Romieu AC, Garg S, Rosenberg ES, et al. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diab Res Care 2017;5:e000304.
28. Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–84.
29. De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008;31:1224–9.
30. Rasmussen LD, Mathiesen ER, Kronborg G, et al. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PloS One 2012;7:e44575.
31. Polsky S, Floris-Moore M, Schoenbaum EE, et al. Incident hyperglycaemia among older adults with or at-risk for HIV infection. Antivir Ther 2011;16:181–8.
32. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–12.
33. Galli L, Salpietro S, Pellicciotta G, et al. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur J Epidemiol 2012;27:657–65.
34. Howard AA, Hoover DR, Anastos K, et al. The effects of opiate use and hepatitis C virus infection on risk of diabetes mellitus in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr 2010;54:152–9.
35. Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–34.
36. Hadigan C, Kattakuzhy S. Diabetes mellitus type 2 and abnormal glucose metabolism in the setting of human immunodeficiency virus. Endocrin Metab Clin North Am 2014;43:685–96.
37. Butt AA, Fultz SL, Kwoh CK, Kelley D, et al. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology 2004;40:115–9.
38. Veloso S, Escote X, Ceperuelo-Mallafre V, et al. Leptin and adiponectin, but not IL18, are related with insulin resistance in treated HIV-1-infected patients with lipodystrophy. Cytokine 2012;58:253–60.
39. Vigouroux C, Maachi M, Nguyen TH, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS 2003;17:1503–11.
40. Palmer CS, Hussain T, Duette G, et al. Regulators of glucose metabolism in CD4+ and CD8+ T cells. Int Rev Immunol 2016;35:477–88.
41. Mehta SH, Moore RD, Thomas DL, et al. The effect of HAART and HCV infection on the development of hyperglycemia among HIV-infected persons. J Acquir Immune Defic Syndr 2003;33:577–84.
42. Monroe AK, Dobs AS, Xu X, et al. Sex hormones, insulin resistance, and diabetes mellitus among men with or at risk for HIV infection. J Acquir Immune Defic Syndr 2011;58:173–80.
43. Rotger M, Gsponer T, Martinez R, et al. Impact of single nucleotide polymorphisms and of clinical risk factors on new-onset diabetes mellitus in HIV-infected individuals. Clin Infect Dis 2010;51:1090–8.
44. Ledergerber B, Furrer H, Rickenbach M, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007;45:111–9.
45. Hruz PW. Molecular mechanisms for insulin resistance in treated HIV-infection. Best practice & research. Clin Endocrin Metab 2011;25:459–68.
46. Brown TT, Li X, Cole SR, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS 2005;19:1375–83.
47. Cossarizza A, Moyle G. Antiretroviral nucleoside and nucleotide analogues and mitochondria. AIDS 2004;18:137–51.
48. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 2005;307:384-7.
49. McComsey GA, Paulsen DM, Lonergan JT, et al. Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. AIDS 2005;19:15–23.
50. Venhoff N, Setzer B, Melkaoui K, Walker UA. Mitochondrial toxicity of tenofovir, emtricitabine and abacavir alone and in combination with additional nucleoside reverse transcriptase inhibitors. Antivir Ther 2007;12:1075–85.
51. Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 2009;23:1109–18.
52. Aberg JA, Tebas P, Overton ET, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retrovir 2012;28:1184–95.
53. Overton ET, Tebas P, Coate B, et al. Effects of once-daily darunavir/ritonavir versus atazanavir/ritonavir on insulin sensitivity in HIV-infected persons over 48 weeks: results of an exploratory substudy of METABOLIK, a phase 4, randomized trial. HIV Clin Trials 2016;17:72–7.
54. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–42.
55. McComsey G, Jiang Y, Erlandson KM, et al. Rosuvastatin improves hip bone mineral density but worsens insulin resistance. Boston, MA: Conference on Retroviruses and Opportunistic Infections; 2014.
56. Lichtenstein K DR, Wood K, et al. Statin use is associated with incident diabetes mellitus among patients in the HIV Outpatient Study. Atlanta, GA: Conference on Retroviruses and Opportunistic Infections; 2013.
57. Spagnuolo V GL, Poli A, et al. Association between statin use and type 2 diabetes mellitus occurrence among HIV-1+ patients receiving ART. Atlanta, GA: Conference on Retroviruses and Opportunistic Infections; 2013.
58. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol 2012;60:1231–8.
59. Ridker PM, Pradhan A, MacFadyen JG, et al. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–71.
60. Bloomgarden ZT, Handelsman Y. Approaches to treatment 2: Comparison of American Association of Clinical Endocrinologists (AACE) and American Diabetes Association (ADA) type 2 diabetes treatment guidelines. J Diabetes 2016;8:4–6.
61. Kim PS, Woods C, Georgoff P, et al. A1C underestimates glycemia in HIV infection. Diabetes Care 2009;32:1591–3.
62. Diop ME, Bastard JP, Meunier N, et al. Inappropriately low glycated hemoglobin values and hemolysis in HIV-infected patients. AIDS Res Hum Retrovir 2006;22:1242–7.
63. Polgreen PM, Putz D, Stapleton JT. Inaccurate glycosylated hemoglobin A1C measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infect Dis 2003;37:e53–56.
64. Glesby MJ, Hoover DR, Shi Q, et al. Glycated haemoglobin in diabetic women with and without HIV infection: data from the Women’s Interagency HIV Study. Antivir Ther 2010;15:571–7.
65. Slama L, Palella FJ Jr, Abraham AG, et al. Inaccuracy of haemoglobin A1c among HIV-infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J Antimicrob Chemother 2014;69:3360–7.
66. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–96.
67. Look ARG, Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–83.
68. Hajjar J, Habra MA, Naing A. Metformin: an old drug with new potential. Expert Opin Investig Drugs 2013;22:1511–7.
69. Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med 2007;8:420–6.
70. Tivicay prescribing information. Accessed at www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tivicay/pdf/TIVICAY-PI-PIL.PDF.
71. Han JH, Gordon K, Womack JA, et al. Comparative effectiveness of diabetic oral medications among HIV-infected and HIV-uninfected veterans. Diabetes Care 2017;40:218–25.
72. Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006;20:2165–74.
73. Libois A, Clumeck N, Kabeya K, et al. Risk factors of osteopenia in HIV-infected women: no role of antiretroviral therapy. Maturitas 2010;65:51–4.
74. Carr A, Grund B, Neuhaus J, et al. Prevalence of and risk factors for low bone mineral density in untreated HIV infection: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med 2015;16 Suppl 1:137–46.
75. Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 2008;93:3499–504.
76. Young B, Dao CN, Buchacz K, Baker R, Brooks JT, Investigators HIVOS. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000-2006. Clin Infect Dis 2011;52:1061–8.
77. Rodriguez M, Daniels B, Gunawardene S, Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-Positive patients. AIDS Res Hum Retrovir 2009;25:9–14.
78. Teichmann J, Lange U, Discher T, et al. Bone mineral density in human immunodeficiency virus-1 infected men with hypogonadism prior to highly-active-antiretroviral-therapy (HAART). Eur J Med Res 2009;14:59–64.
79. Lo Re V 3rd, Volk J, Newcomb CW, et al. Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/human immunodeficiency virus coinfection. Hepatology 2012;56:1688–98.
80. Maalouf NM, Zhang S, Drechsler H, et al. Hepatitis C co-infection and severity of liver disease as risk factors for osteoporotic fractures among HIV-infected patients. J Bone Miner Res 2013;28:2577–83.
81. Byrne DD, Newcomb CW, Carbonari DM, et al. Increased risk of hip fracture associated with dually treated HIV/hepatitis B virus coinfection. J Viral Hepat 2015;22:936–47.
82. Gilsanz V, Chalfant J, Mo AO, et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 2009;94:3387–93.
83. Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 2009;122:409–14.
84. Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem 2003;278:48251–8.
85. Gazzola L, Bellistri GM, Tincati C, et al. Association between peripheral T-Lymphocyte activation and impaired bone mineral density in HIV-infected patients. J Transl Med 2013;11:51.
86. Clerici M, Shearer GM. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today 1993;14:107–11.
87. Chew N, Tan E, Li L, Lim R. HIV-1 tat and rev upregulates osteoclast bone resorption. J Int AIDS Soc 2014;17(4 Suppl 3):19724.
88. McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis 2010;51:937–46.
89. Panayiotopoulos A, Bhat N, Bhangoo A. Bone and vitamin D metabolism in HIV. Rev Endocr Metab Disord 2013;14:119–25.
90. Fakruddin JM, Laurence J. Interactions among human immunodeficiency virus (HIV)-1, interferon-gamma and receptor of activated NF-kappa B ligand (RANKL): implications for HIV pathogenesis. Clin Exp Immunol 2004;137:538–45.
91. Li Y, Toraldo G, Li A, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 2007;109:3839–48.
92. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008;93:861–8.
93. Huang JS, Hughes MD, Riddler SA, Haubrich RH, Aids Clinical Trials Group AST. Bone mineral density effects of randomized regimen and nucleoside reverse transcriptase inhibitor selection from ACTG A5142. HIV Clin Trials 2013;145:224–34.
94. McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011;203:1791–801.
95. Schafer JJ, Manlangit K, Squires KE. Bone health and human immunodeficiency virus infection. Pharmacotherapy 2013;33:665–82.
96. Mateo L, Holgado S, Marinoso ML, et al. Hypophosphatemic osteomalacia induced by tenofovir in HIV-infected patients. Clin Rheumatol 2016;35:1271–9.
97. Brown TT, Moser C, Currier JS, et al. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis 2015;212:1241–9.
98. Ofotokun I, Titanji K, Vikulina T, et al. Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun 2015;6:8282.
99. Ofotokun I, Titanji K, Vunnava A, et al. Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS 2016;30:405–14.
100. Stephens KI, Rubinsztain L, Payan J, et al. Dual-energy x-ray absorptiometry and calculated FRAX risk scores may underestimate osteoporotic fracture risk in vitamin d-deficient veterans with HIV infection. Endocr Pract 2016;22:440–6.
101. Mirza F, Canalis E. Management of endocrine disease: Secondary osteoporosis: pathophysiology and management. Eur J Endocrinol 2015;173:R131–151.
102. Brown TT, Hoy J, Borderi M, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis 2015;60:1242–51.
103. Mazzotta E, Ursini T, Agostinone A, et al. Prevalence and predictors of low bone mineral density and fragility fractures among HIV-infected patients at one Italian center after universal DXA screening: sensitivity and specificity of current guidelines on bone mineral density management. AIDS Patient Care STDS 2015;29:169–80.
104. Calmy A, Chevalley T, Delhumeau C, et al. Long-term HIV infection and antiretroviral therapy are associated with bone microstructure alterations in premenopausal women. Osteoporos Int 2013;24:1843–52.
105. Yin MT, Lund E, Shah J, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS 2014;28:345–53.
106. Lo Re V, 3rd, Lynn K, Stumm ER, et al. Structural bone deficits in HIV/HCV-coinfected, HCV-monoinfected, and HIV-monoinfected women. J Infect Dis 2015;212:924–33.
107. Allavena C, Delpierre C, Cuzin L, et al. High frequency of vitamin D deficiency in HIV-infected patients: effects of HIV-related factors and antiretroviral drugs. J Antimicrob Chemother 2012;67:2222–30.
108. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30.
109. Mondy K, Powderly WG, Claxton SA, et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr 2005;38:426–31.
110. McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007;21:2473–82.
111. Guaraldi G, Orlando G, Madeddu G, et al. Alendronate reduces bone resorption in HIV-associated osteopenia/osteoporosis. HIV Clin Trials 2004;5:269–77.
112. Negredo E, Bonjoch A, Perez-Alvarez N, et al. Comparison of two different strategies of treatment with zoledronate in HIV-infected patients with low bone mineral density: single dose versus two doses in 2 years. HIV Med 2015;16:441–8.
113. Bolland MJ, Grey A, Horne AM, et al. Effects of intravenous zoledronate on bone turnover and bone density persist for at least five years in HIV-infected men. J Clin Endocrinol Metab 2012;97:1922–8.
114. Pinzone MR, Moreno S, Cacopardo B, Nunnari G. Is there enough evidence to use bisphosphonates in HIV-infected patients? A systematic review and meta-analysis. AIDS Rev 2014;16:213–22.
115. Ofotokun I, Titanji K, Lahiri CD, et al. A Single-dose zoledronic acid infusion prevents antiretroviral therapy-induced bone loss in treatment-naive HIV-infected patients: a phase IIb trial. Clin Infect Dis 2016;63:663–71.
116. Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 2008;43:1115–21.
From the University of Connecticut School of Medicine, Farmington, CT.
Abstract
- Objective: To review the metabolic complications of HIV infection.
- Methods: Review of the literature in the context of 3 clinical cases.
- Results: People with HIV infection are living longer thanks to the advent of potent antiretroviral therapy. This has led to increased incidence of age-related metabolic complications, including a higher risk of cardiovascular disease, hyperlipidemia, metabolic syndrome, and osteoporosis. Appropriate management of these complications requires an understanding of disease-related and drug-related side effects as well as the potential for drug-drug interactions. A multidisciplinary approach to patient management is most effective.
- Conclusion: Awareness of the metabolic complications frequently encountered in HIV infection, drug interactions, and possible toxicities is critical to the successful management of HIV-infected individuals.
Key words: HIV; antiretroviral therapy; hyperlipidemia; metabolic syndrome; diabetes; hypogonadism.
According to the most recent data from the Joint United Nations Programme on HIV/AIDS (UNAIDS), 36 million people worldwide are living with HIV/AIDS, with 18 million accessing effective antiretroviral therapy (ART) [1]. The past 2 decades have witnessed enormous advances in the field from prevention to diagnosis and therapeutics, and modern ART largely allows HIV-infected persons to live near-normal life spans [2,3]. However, from the beginning of the epidemic, HIV-infected persons on effective therapy have suffered from myriad metabolic consequences, many of which affect quality of life and result in excess mortality [4]. It is also true that untreated HIV infection portends an increased risk of metabolic complications, likely related to abnormal immune activation, as demonstrated in structured interruption trials [5,6]. It is worth noting, however, that while many of these metabolic dyscrasias and associated risks have historically been attributed primarily to the treatment of HIV infection with ART, data from cohort studies and randomized clinical trials have repeatedly demonstrated significant reductions in morbidity and mortality when ART is initiated early [7]. In this paper, we review HIV-related metabolic complications frequently encountered in clinical practice (hyperlipidemia, diabetes, and bone disease) and best practice considerations in the context of 3 clinical cases.
Case Patient 1
Initial Presentation and History
A 58-year-old male with a history of hypertension and mixed hyperlipidemia is referred for evaluation of newly diagnosed HIV infection. He has no history of intravenous drug use but has had multiple male and female sex partners in the past few years, and requested testing after a partner tested positive. His last negative test was 2 years ago. The patient does not smoke cigarettes. Overall he feels well and tolerates his regimen of lisinopril 10 mg and simvastatin 20 mg daily. On initial evaluation, his exam is unremarkable other than subtle white plaques on the dorsal surface of the tongue and buccal mucosa, and moderate central obesity. Vital signs including blood pressure are normal. Initial laboratory evaluation reveals a CD4 cell count of 150 cells/mm3 and an HIV RNA level of 200,000 copies/mL. Fasting serum total cholesterol is 220 mg/dL, triglycerides 250 mg/dL, LDL 170 mg/dL, and HDL 35 mg/dL. Serum BUN, creatinine, and liver function testing results are normal.
What initial regimen might be recommended based on the status of his HIV infection and comorbidities?
The most recent iteration of the US Department of Health and Human Services (DHHS) guidelines on use of antiretroviral agents (ARVs) in HIV recommends an initial ART regimen that includes a backbone of 2 nucleoside reverse transcriptase inhibitors (NRTIs), generally tenofovir disoproxil fumarate or tenofovir alafenamide, abacavir (ABC), emtricitabine (FTC), or lamivudine (3TC) [2]. To this backbone should be added a third agent; the majority of those currently recommended are integrase strand transfer inhibitors (INSTIs) (dolutegravir, elvitegravir, raltegravir); one recommended protease inhibitor (PI) (ritonavir-boosted darunavir) is also an option. Some of these initial recommended regimens are available as fixed-dosed combinations in 1 pill, making them attractive options.
The latest guidelines also clearly recommend starting ART in all HIV-infected individuals, irrespective of CD4 count. The patient described above has a very low CD4 count, so there is no question he needs to begin therapy promptly. Given his low CD4 count and relatively high viral load, one may consider a ritonavir-boosted PI as perhaps the most robust option and with a relatively high barrier to resistance development, in contrast to other options. Assuming the patient’s baseline resistance testing reveals a fully sensitive wild-type virus without meaningful resistance mutations, he will begin a regimen of TDF/FTC plus ritonavir-boosted darunavir, 3 pills once daily. Given his low CD4 count (below 200 cells/mm3), he will also need prophylaxis for Pneumocystis jirovecii pneumonia, in the form of trimethoprim/sulfamethoxazole (TMP/SMX) daily. Given the potential for interaction between the boosted PI and simvastatin, his lipid-lowering agent is switched to atorvastatin 10 mg daily.
What is the association between hyperlipidemia and HIV infection and treatment?
Hyperlipidemia represents a key modifiable risk factor for the development of cardiovascular disease (CVD) in HIV-infected individuals [8]. Indeed, a multicenter cross-sectional study of older HIV-infected individuals performed in Spain revealed a 54% prevalence of dyslipidemia and 23% CVD [9]. Most experts believe that metabolic abnormalities observed in HIV-infected individuals are the result of a combination of factors: those resulting from abnormal immune activation and inflammation related to viral replication, and those related to certain ARVs [10].
Early after HIV seroconversion, decline in HDL is one of the first proatherogenic changes observed. This, along with increased triglyceride and LDL levels, likely contribute to increased risk of CVD in this population. Increased microbial translocation, evidenced by increased levels of lipopolysaccharide (LPS), may drive immune activation, leading to dyslipidemia via a multitude of hypothesized mechanisms [4]. It has been theorized that HDL lipoproteins are less stable on ART, leading to potentially impaired plasma lipolytic activities or hepatic cholesteryl ester uptake [6,11]. Increased VLDL from release of free fatty acids may lead to higher triglyceride levels and triglyceride-rich LDL and HDL, all associated with increased risk of CVD [11].
In terms of effects of specific ARV classes, although newer agents have less of a propensity to cause dyslipidemia, the PI class arguably remains most problematic. In comparison to other classes, the PIs tend to result in greater increases in triglycerides, total cholesterol, and LDL, and have frequent drug-drug interactions with lipid-lowering agents [10,12]. Estimated prevalence of dyslipidemia in patients receiving PI therapy varies from 28% to 80% [13]. The prospective multinational cohort Data collection on Adverse events of Anti-HIV Drugs (DAD) study found significantly higher rates of hypertriglyceridemia, hypercholesterolemia, and low HDL in patients on PIs in comparison to non–nucleoside reverse transcriptase inhibitors (NNRTIs) [14]. Various mechanisms have been proposed to explain the PIs adverse effects on lipids, including inhibition of lipogenesis and adipocyte differentiation, decreased hepatocyte clearance of chylomicrons and VLDL, and increased hepatic synthesis of triglycerides [15]. Of the available PIs, atazanavir and darunavir have less potential to lead to dyslipidemia [10], while lopinavir/ritonavir, fosamprenavir, and tipranavir may have the highest [13]. Of the NNRTIs, efavirenz is most frequently associated with dyslipidemia, specifically increased triglycerides and total cholesterol [13]. However, these increased values seen on efavirenz therapy may be offset by relative increases in HDL, with little resultant effect on the total cholesterol:HDL ratio. Rilpivirine, etravirine, and nevirapine are relatively less likely to drive lipid changes, although certain drug interactions are important to recognize in clinical practice, such as the interaction between rilpivirine and proton pump inhibitors [2,13,16]. It is also worth noting that no NNRTIs are included in current guidelines as preferred therapy [2].
Historically, the thymidine analogue NRTIs (stavudine, didanosine, zidovudine) have been associated with lipid dyscrasias and lipoatrophy, but fortunately these are no longer used frequenty except in cases requiring deep salvage therapy for highly treatment-experienced patients. Two newer NRTIs, tenofovir and abacavir, have relatively neutral to favorable effects on lipids. The combination of tenofovir disoproxil (TDF) and emtricitabine (trade name Truvada) was associated with significantly lower triglycerides, total cholesterol and LDL than other NRTI pairs [6]. TDF has been postulated to have lipid-lowering effects. Switch studies in which patients were taken off thymidine analogues and placed on TDF, demonstrated recovery of limb fat in patients with lipoatrophy, and those switched off abacavir-based ART to TDF showed statistically significant lower fasting total cholesterol at week 12, without differences of viral suppression [8]. Tenofovir alafenamide (TAF) is a next-generation prodrug of tenofovir that results in improved stability in plasma and higher intracellular levels in comparison to TDF [17]. Although randomized controlled trials of TAF vs TDF-based ARV regimens have suggested statistically higher total cholesterol, serum HDL is also increased resulting in unchanged total:HDL ratios and no differences in risk classifications [18]. Integrase inhibitors (INSTI) now represent first-line therapy in combination with an NRTI backbone, and since their availability in 2007 have been evaluated in comparison to various PIs and NNRTIs. Both raltegravir and dolutegravir have consistently shown broad neutral effects on lipids and are among the most metabolically friendly agents available [19–21]. Because it is given in fixed-dose combination with non-ritonavir pharmacologic booster cobicistat, elvitegravir has effects similar to ritonavir-boosted PIs on lipids [6].
What are management considerations in the treatment of hyperlipidemia in HIV-infected patients?
Patients with HIV and hyperlipidemia may benefit from lipid-lowering therapy in addition to ART, although in certain cases appropriate switches may make a difference. Careful consideration of drug interactions between ARVs and lipid-lowering agents, in addition to ARV history and known drug resistance, is warranted prior to selecting a regimen in these patients. In addition, the latest American College of Cardiology/American Heart Association guidelines suggest evaluating 10-year risk of atherosclerotic cardiovascular disease (ASCVD) using the pooled cohort equation to determine the type and dose of statin required (moderate vs high intensity) [22]. It is noteworthy that HIV infection and its therapies are not taken into account as potential risk factors in this model. Primary prevention in non-diabetic patients with a statin is recommended for patients with a 10-year absolute risk of ≥ 7.5% [22]. This patient’s risk is estimated at between 12% and 13% based on this equation, so primary prevention with a moderate-or high-intensity statin is recommended (Table 1) [23]. Data from more than 80,000 patients in the Veterans Aging Cohort Study (VACS) showed that HIV-infected patients with no baseline ASCVD had 50% increased risk of acute myocardial infarction when compared to HIV-uninfected patients over 6 years of follow-up [24]. Thus, consideration of the virus itself or its therapy as an additional risk factor may be valid.
Screening and Monitoring of Hyperlipidemia
The most recent iteration of the DHHS primary care guidelines for the management of HIV-infected individuals recommends obtaining fasting (ideally 12 hours) lipid profiles upon initiation of care, and within 1 to 3 months of beginning therapy [12,13]. These initial levels, along with other elements of the patient’s history and calculation of risk may help determine whether lipid-lowering therapy is indicated, and if so, which therapy would be best. In general, after regimen switches or additions of either ARV or statin therapy, repeating fasting lipid levels 6 weeks later is recommended to gauge the effects of the switch. This is especially critical when interactions between ARVs and lipid-lowering therapies are possible. Some experts recommend performing annual screening of patients with normal baseline lipids or with well-controlled hyperlipidemia on therapy. Assessment of 10-year ASCVD risk is also recommended annually, in addition to baseline risk assessment, to determine the need and appropriateness of statin therapy [25]. The question of primary prevention in HIV has yet to be definitively answered. Small studies in this population have demonstrated that statins have the potential to slow progression of carotid intima media thickness and reduce noncalcified plaque volume [24]. An NIH/AIDS Clinical Trial Group–sponsored randomized clinical trial (“REPRIEVE”) is currently underway to address this question. More than 6000 HIV-infected men and women with no history of ASCVD at 100 sites in several countries are enrolled to assess the benefit of pitavastatin as primary prevention in this risk group [24]. Metabolized via glucuronidation primarily, as opposed to cytochrome p450 (CYP 3A4 isoenzyme), pitavastatin is thought to have fewer drug interactions with ARVs in general [6] (Table 2).
Relevant Drug-Drug Interactions
Deciding which statin to begin in HIV-infected patients depends on whether moderate- or high-intensity therapy is warranted and whether the potential for drug interaction with ARVs exists. Table 2 [6,12] depicts available statins and the potential for pharmacokinetic interaction with the primary ARV classes. Simvastatin and lovastatin are heavily metabolized via the CYP 3A4 pathway, resulting in the highest potential risk of interaction with CYP 3A4 inhibitors, such as the PIs, or inducers (eg, NNRTIs, in particular efavirenz) [6]. The former may inhibit metabolism of these statins, resulting in increased risk of toxicity, while co-administration with efavirenz, for example, may result in inadequate serum concentration and therefore inadequate lipid-lowering effects. Although less lipophilic, atorvastatin results in similar interactions with PIs and NNRTIs, and therefore low starting doses with close monitoring is recommended [6]. Fewer interactions have been noted with rosuvastatin, pravastatin, and pitavastatin, as these do not require CYP 3A4 for their metabolism and are thus less likely to be affected by ARVs. These therefore represent potentially safer first choices for certain patients on ARVs, although of these, only rosuvastatin is classified as a high-intensity statin [22,23] (Table 1). When compared directly to pravastatin 40 mg daily in patients receiving ritonavir-boosted PIs, rosuvastatin performed superiorly at 10 mg per day, resulting in more significant reductions in LDL and triglyceride levels [15]. Although it is eliminated largely unchanged through the kidney and liver, pravastatin has been reported to idiosyncratically interact with darunavir, resulting in potentially increased pravastatin levels and associated toxicity [25]. Treatment of pure hypertriglyceridemia in HIV-infected patients should begin with fibrates, which have little to no risk of interaction with most clinically relevant ARVs [6,10]. Alternatives to lower triglycerides include niacin and N-3 polyunsaturated fatty acids [25].
Case 1 Continued
The patient has an impressive response to his initial regimen of TDF/FTC plus boosted darunavir, with repeat CD4 count after 12 weeks of 275 (18%) cells/mm3 and an undetectable viral load (< 20 copies/mL). Other lab parameters are favorable and he is tolerating the regimen well without notable side effects. However, at his next visit, although his viral load remains undetectable, his triglyceride level has increased to 350 mg/dL, although other lipid parameters are comparable to the prior result. He complains of diffuse body aches, concentrated in large muscle groups of the extremities, and dark-colored urine. A creatine phosphokinase (CPK) level is elevated at 300 IU/L (normal, 22–269, negative MB fraction). Serum creatinine is 1.4 mg/dL (had been 1.1 mg/dL at baseline). Given he has done so well otherwise on these ARVs, he is reluctant to make any changes.
What drug-drug interaction is most likely causing this patient's problem, and how should it be managed?
This scenario is not uncommon in clinical practice, and changes to regimens are sometimes necessary in order to avoid drug interactions. Care must be taken to thoroughly review antiretroviral history and available resistance testing (in this case a relatively short history) in order to ensure a fully active and suppressive regimen is chosen. This description could be the result of an interaction between lipid-lowering therapy and ARVs resulting in increased relative concentrations of one drug or the other and therefore leading to toxicity. Given this possibility, and suboptimal control of hyperlipidemia, consideration should be given to switching both his ART and his statin therapy.
Safety and Potential Toxicities of Lipid-Lowering Therapy
Increased serum concentration of certain statins when co-administered with CYP 3A4 inhibitors like the PIs leads to heightened risk of statin-associated toxicities. In general, this includes muscle inflammation, leading to increases in serum CPK level and associated symptoms, including myalgias, myositis, or in extreme cases, rhabdomyolysis [6]. Although rare, this toxicity can be serious and may lead to acute renal injury if not recognized and managed appropriately. In theory, the potential for statin-associated hepatotoxicity may also be increased in patients receiving PIs, although this has not been borne out in clinical trials [26]. In fact, quite the opposite may be true, in that statins have been shown to improve liver function in patients with hepatitis C virus (HCV) coinfection and with nonalcoholic fatty liver disease [6,15].
Case 1 Conclusion
The patient does well on his new ARV regimen of TAF/FTC and dolutegravir, 2 pills once daily. He no longer requires TMP/SMX, as his CD4 count has been reliably above 200 cells/mm3 on several occasions. Serum creatinine is back down to baseline and CPK has normalized. Fasting lipids have improved since the switch, and he no longer has symptoms of myositis on rosuvastatin 10 mg daily.
Summary
Consideration of statin therapy is complicated by potential drug interactions with ARVs and associated toxicity. However, given known effects of ARVs on lipids, and of immune activation and inflammation related to the virus itself, these patients should be carefully evaluated for statin therapy for their anti-inflammatory and immune modulatory effects as much as for their lipid-lowering ability. Utilization of HIV infection and its therapies as additional cardiovascular risk factors when calculating 10-year risk deserves further consideration; forthcoming results of the REPRIEVE trial are certain to contribute valuable information to this field of study.
Case Patient 2
Initial Presentation and History
A 45-year-old female with history of HIV infection since 2008 presents to the office for new-onset diabetes, diagnosed 2 weeks ago. She has had symptoms of polyuria and polydipsia for the last 1 month. She denies diarrhea, nausea, vomiting or weight loss. She is currently on a regimen consisting of zidovudine/lamivudine plus lopinavir/ritonavir. There is no family history of diabetes. Her examination is unremarkable, including normal vital signs (weight 150 lb, blood pressure 114/70, heart rate 76) and no evidence of insulin resistance, including acanthosis nigricans or striae. Glycosylated hemoglobin level (HbA1c) is 8%. Creatinine and liver function tests are within reference ranges.
Do HIV-infected patients have a higher incidence of type 2 diabetes mellitus (DM)?
Prevalence of type 2 DM in HIV-infected patients varies between 2% to 14% [27]. This variation is due to the different cutoffs used for diagnosis, differences in cohorts studied, and how risk factors are analyzed [28–31]. In a recent nationally representative estimate of DM prevalence among HIV-infected adults receiving medical care in the United States in 2009–2010, the prevalence of DM was noted to be 10.3%. In comparison to the general adult US population, HIV-infected individuals have a 3.8% higher prevalence of DM after adjusting for age, sex, race/ethnicity, education, poverty-level, obesity, and HCV infection [27].
There is controversy over whether HIV infection itself increases the risk of type 2 DM, with some studies showing increased risk [28,32,33] and others showing no independent effect or an inverse effect [30,34,35]. Studies on the impact of ethnicity and race on prevalence of DM are limited [36].
Certain traditional risk factors (age, ethnicity, obesity) are still responsible for most of the increased risk of diabetes in the HIV-infected population [35,37]. HIV infection itself is associated with metabolic dysfunction, independent of ARV. In HIV-infected patients, impaired glucose metabolism is associated with altered levels of adipokines, increased adiponectin and soluble-tumor necrosis factor receptor 1 (sTNFR1) and decreased leptin [38,39]. HIV-associated alterations in CD4+ and CD8+ T cell function also impair glycolysis, which may adversely impact glucose metabolism [40].
Other contributing factors in HIV-infected patients are HCV co-infection [41], medications (atypical antipsychotics, corticosteroids), opiates, and low testosterone [42]. HCV co-infection may lead to hepatic steatosis and liver fibrosis, and increasing insulin resistance.
Recent genomic studies show several common single-nucleotide polymorphisms (SNPs) associated with diabetes in the general population. In the Swiss HIV Cohort Study, SNPs accounted for 14% of type 2 DM risk variability, whereas ARV exposure accounted for 3% and age for 19% of the variability in DM [43].
ARVs also increase the risk of type 2 DM by both direct and indirect effects. Certain ARVs causes lipoatrophy [30] and visceral fat accumulation/lipohypertrophy [29,44]. PIs increase insulin resistance via effects on GLUT-4 transporter and decrease insulin secretion through effects on B cell function [45]. NRTIs (eg, stavudine, zidovudine and didanosine) can cause direct mitochondrial toxicity [46–48]. Utilization of newer ARV agents has decreased the prevalence of severe lipoatrophy, but lipohypertrophy and the underlying metabolic abnormalities persist. The DHHS “preferred” nucleoside analogues, tenofovir and abacavir, do not induce mitochondrial toxicity and have more favorable metabolic profiles [49,50]. In ACTG Study 5142, thymidine-sparing regimens were found to cause less lipoatrophy [51]. In addition, darunavir and atazanavir, the preferred and alternative PIs and the integrase strand transfer inhibitor have limited or modest impact on insulin sensitivity [20,52,53]. This has led to a recent decline in the incidence of type 2 DM in HIV-infected patients.
Statins can also increase insulin resistance and DM [54], although studies have shown mixed results [55–57]. The benefits of statin therapy likely outweigh the risk of DM since there is a significant cardiovascular event reduction with their use [58,59].
How is diabetes diagnosed in HIV-infected patients?
Optimal diabetes screening guidelines have not been established specifically for HIV-infected patients. The American Diabetes Association (ADA) guidelines recommend that diabetes in the general population be diagnosed by 2 elevated fasting blood glucose levels, HbA1c, oral glucose tolerance test (OGTT), or high random glucose with classic symptoms of hyperglycemia [60]. Repeat testing is recommended every 3 years. The OGTT is recommended for diagnosis in pregnant women.
HbA1c may underestimate glycemic burden in HIV-infected individual due to higher mean corpuscular volume, NRTI use (specifically abacavir), or lower CD4 count [61–65]. The Infectious Diseases Society of America (IDSA) 2013 primary care guidelines for HIV-infected patients recommends obtaining a fasting glucose and/or HbA1c prior to and within 1–3 months after starting ARV [12]. Use of HbA1c threshold cutoff of 5.8% for the diagnosis of DM and testing every 6–12 months are recommended.
How should this patient’s diabetes be managed?
The ADA guidelines suggest a patient-centered approach to management of diabetes [66]. All patients should be educated about lifestyle modifications with medical nutrition therapy and moderate-intensity aerobic activity and weight loss [67]. If a patient is on lopinavir/ritonavir or a thymidine analogue (zidovudine, stavudine), one should consider switching the ARV regimen [2].
There are currently no randomized controlled trials of diabetes treatment specific to patients with HIV infection. Metformin is the first-line agent. It improves insulin sensitivity by reducing hepatic glucose production and improving peripheral glucose uptake and lipid parameters [68,69]. Other oral hypoglycemic agents used in the treatment of type 2 diabetes are shown in Table 3.
Case 2 Continued
The patient is switched to TAF/FTC plus dolutegravir with improvement in blood sugars. She is also started on metformin. Co-administration of metformin and dolutegravir will be carefully monitored since dolutegravir increases metformin concentration [70]. When dolutegravir is used with metformin, the total daily dose of metformin should be limited to 1000 mg.
• How should this patient be followed?
If the patient is still not at goal HbAb1c at follow-up, there are multiple other treatment options, including use of insulin. Goal HbA1c for most patients with type 2 DM is < 7%; however, this goal should be individualized for each patient in accordance with the ADA guidelines [12]. A longitudinal cohort study of 11,346 veterans with type 2 diabetes compared the glycemic effectiveness of oral diabetic medications ( metformin, sulfonylurea and a thiazolidinedione) among veterans with and without HIV infection. This study did not find any significant difference in HbA1c based on different diabetes medications. However the HBA1c reduction was less in black and Hispanic patients. The mechanism for the poorer response among these patients need to be evaluated further [71]. In addition to management of blood sugar, other CVD risk factors, hyperlipidemia, hypertension, smoking, etc, should be assessed and managed aggressively.
Case Patient 3
Initial Presentation and History
A 45-year-old male with a history of HIV infection diagnosed 10 years ago, on TDF/FTC/efavirenz (trade name Atripla) for the last 7 years, presents with a left femoral neck fracture after he missed the pavement and fell on his left hip. His history is significant for IV drug abuse for 10 years prior to diagnosis of HIV, and he has been on methadone for the last 6 years.
Is HIV infection associated with increased prevalence of osteopenia and osteoporosis and increased risk of fractures?
With recent advancements in antiretroviral therapy and improved survival of the HIV-infected population, osteoporosis and increased fracture risk have become important causes of morbidity and mortality. Osteoporosis is a skeletal disorder characterized by compromised bone strength, which predisposes to an increased risk of fracture. The World Health Organization defines osteoporosis as a bone mineral density (BMD) measurement by dual X-ray absorptiometry (DXA) at the spine, hip, or forearm that is more than 2.5 standard deviations below that of a "young normal" adult (T-score < –2.5) or a history of one or more fragility fractures. Fragility fractures result from mechanical forces that would not ordinarily result in fracture, such as fall from standing height [40]. Osteopenia is characterized by low BMD (T-score between –1.0 and –2.5) and can be a precursor to osteoporosis.
Several observational, retrospective, and prospective studies have shown lower bone density and an increased risk of fractures in the HIV-infected population compared to age-, race- and sex-matched HIV-negative adults. In a large meta-analysis of pooled prevalence data on 884 HIV-infected patients compared with 654 HIV-uninfected age- and sex-matched controls [72], overall, HIV-infected patients had a significant 6.4-fold increased odds of reduced BMD and a 3.7-fold increased odds of osteoporosis compared to the control population. This meta-analysis also compared ARV-treated subjects to ARV-naive subjects and showed that ARV-treated subjects (n = 824) had a higher prevalence of reduced BMD compared with ARV-naive subjects (n= 202; odds ratio 2.5, 95% CI 1.8–3.7). The odds of osteoporosis was increased 2.4 times (95% CI 1.2 – 4.8) in ARV-treated subjects compared with ARV-naive subjects. None of the studies adjusted for potentially important confounding factors, such as age or duration of HIV infection. PI-treated patients (n = 791) were also found to have a higher prevalence of reduced BMD compared with PI-untreated patients (n = 410; OR 1.5, 95% CI 1.1–2.0). The odds of osteoporosis in PI-treated patients (n = 666) was also 1.6-fold greater (95% CI 1.1–2.3) than those not treated with PI (n = 367).
Low bone density has also been reported in HIV- positive premenopausal women irrespective of ARV status. In a recent study of 89 premenopausal women (mean age, 37 years) predominantly of African origin with HIV infection, osteopenia and osteoporosis were prevalent in one-third of these women, irrespective of ARV use and were associated with low BMI [73]. In a sub-study of the INSIGHT trial evaluating prevalence of and risk factors for low BMD in untreated HIV infection, performed at several sites across 6 continents involving 424 subjects, osteopenia was present in a third of this relatively young predominantly non-white ART-naive population (mean age 34 + 10 years) with normal CD4 cell counts, while only 2% had osteoporosis. Factors independently associated with lower BMD at the hip and spine were female sex, Latino/Hispanic ethnicity, lower BMI, and higher estimated glomerular filtration rate. Longer duration of HIV infection was also associated with lower hip BMD. Current or nadir CD4 cell count and HIV viral load were not associated with low BMD [74].
Many studies have reported increased fracture prevalence in the HIV population. In a retrospective study of fracture prevalence in a large US health care system, a significantly higher rate of fractures was reported in HIV-infected men and women compared to non-HIV-infected controls (2.87 vs. 1.77 fractures per 100 persons, P < 0.001). The difference in the increased fracture prevalence was greater in HIV positive men compared to women (3.08 vs. 1.83; P < 0.001). Vertebral, wrist and hip fractures were more prevalent in men compared to vertebral and wrist fractures only in women. Fracture prevalence was higher in both Caucasian females and males and only in African-American women [75].
In the HIV Outpatient Study (HOPS) [76], age-adjusted fracture rates in the HIV population were noted to be 1.98 to 3.69 times higher than rates in the general population. The HOPS was an open prospective cohort study of HIV-infected adults who were followed at 10 US HIV clinics. Rates of first fractures at any anatomic site from 2000–2008 were assessed among 5826 active HOPS patients (median age 40 years, 79% male, 52% Caucasian, and 73% exposed to ART). Among persons aged 25–54 years, both fracture rates and relative proportion of fragility fractures were higher among HOPS patients than among outpatient controls. Older age, substance abuse, nadir CD4+ cell count <200 cells/mm, HCV infection and DM were associated with incident fractures [76].
What factors contribute to poor bone health in the HIV population?
Several factors that contribute to low bone density are present at a higher rate in the HIV population (Table 4). These include poor nutritional status in terms of suboptimal calcium and vitamin D intake, hypogonadism, low body weight, and alcohol, tobacco and substance abuse.
Vitamin D deficiency is very common in HIV-infected patients, with a prevalence of up to 60% to 75% [77]. Hypogonadism is also relatively common among HIV population [78], contributing to lower bone density. Co-infection with HCV is also associated with increased risk of fractures. In a large cohort of Medicaid beneficiaries, a significant increase in the risk of hip fracture was demonstrated in HCV/HIV co-infected subjects compared either with HCV mono-infected, HIV mono-infected or non-infected individuals [79]. In another large database study, a significantly higher risk of osteoporotic fracture (closed wrist, vertebral or hip fracture) was reported in HCV/HIV co-infected versus HIV mono-infected individuals [80] with fracture rates of 2.57 and 2.07/1000 patient-years (P < 0.001). Dual treatment for HIV/hepatitis B co-infection has also been shown to be associated with a higher risk of hip fracture compared to treatment of HIV mono-infected individuals [81].
HIV infection itself can increase bone loss and reduce bone formation through direct effects related to the HIV antigen load or indirect effects related to activation of the pro-inflammatory cytokines resulting in bone resorption and loss [82]. Co-infection with HCV and/or hepatitis B also contributes to lower bone density in this population. Certain ARVs may also contribute to low bone density in the HIV population. Lipoatrophy related to HIV may also mediate bone loss through complex relationship between central signaling of adipocyte hormones [82,83].
Direct Viral Effects
Several HIV viral proteins have been shown to promote osteoclast activity (vpr and gp120), suppress osteoblast activity (p55-gag) and increase osteoblast apoptosis [84], resulting in increased bone resorption and reduced bone formation, leading to low bone mass. High HIV RNA viral load and T-cell activation are also associated with elevated levels of receptor activator of nuclear factor kappa-B ligand (RANKL), which results in osteoclast formation and increased bone resorption [85]. Other endogenous physiological inhibitors of osteoclastogenesis such as osteoprotegrin and interferon-γ levels are also remarkably downregulated in advanced HIV infection, resulting in increased bone resorption [86]. At a cellular level, HIV proteins including Tat and Nef reduce the number of available mesenchymal stem cell (MSC) precursors that proliferate into osteoblasts by inducing MSC senescence, due to increased oxidative stress and mitochondrial dysfunction resulting in reduced proliferation of osteoblasts and lower rates of bone formation [87]. Collectively, these mechanisms result in significant uncoupling of bone formation and resorption, resulting in less bone formation and greater rate of bone loss and lower bone density.
Pro-inflammatory Pathways
Cytokines and other soluble immune factors play a major role in the physiology of osteoblast maturation and osteoclastic bone resorption [88,89]. Immune dysfunction and persistent inflammation in HIV result in increased levels of several inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), and RANKL, resulting in stimulation of osteoclastogenesis and bone resorption [90]. Due to a disruption between T and B cells in HIV and decreased osteoprotegrin (OPG) production and increased RANKL level, RANKL/OPG ratio is elevated, favoring osteoclastogenesis [91].
Is antiretroviral therapy associated with bone loss?
The initiation of ART has been reported to cause 2% to 6% bone loss irrespective of the regimen used, similar to that sustained in the first 2 years after menopause [92]. Certain NRTIs and PIs are associated with higher rates of bone loss than others. TDF has been associated most commonly with decreased bone mineral density, which usually stabilizes with continued use [93]. In a randomized trial comparing 4 treatment arms of ABC/3TC or TDF/FTC with EFV or ATV/ritonavir, TDF was associated with a greater reduction in BMD compared to abacavir-based regimens [94]. The likely cause of this may be TDF-mediated renal toxicity, including proximal tubular dysfunction and hypophosphatemia, resulting in increased PTH and bone resorption, and nephrogenic diabetes insipidus [95]. TAF is another prodrug of tenofovir diphosphate associated with less renal and bone toxicity compared with TDF. TAF has been associated with significantly less decrease in bone mineral density and renal dysfunction in randomized studies compared to regimen using TDF [17]. Vitamin D deficiency and hypophosphatemia associated with TDF therapy may present with osteomalacia, which predisposes to bone pain and fractures. Treatment with TDF may rarely be associated with the development of Fanconi syndrome and osteomalacia [96]. BMD is often severely reduced and bone pain and pathological fractures are characteristic features. Certain PI regimens containing ritonavir-boosted atazanavir have also been associated with greater bone loss in the spine than the hip, compared to efavirenz-containing regimens [97].
The universal bone loss associated with ART is thought to be a result of the "immune reconstitution inflammatory syndrome" (IRIS). This occurs as a result of rapid improvement in immune function after the commencement of ARV as a result of systemic or local inflammation, resulting in increased levels of cytokines that may contribute to bone loss. This has been shown in animal studies where T cell transplantation into immunocompromised mice to mimic ARV-induced T-cell expansion resulted in increased RANKL and TNF-α production by B cells and/or T cells, accompanied by enhanced bone resorption and BMD loss. When TNF-α or RANKL-null T-cells or TNF-α antagonists were used instead, the loss of cortical bone was prevented [98]. In a prospective study evaluating changes in bone turnover markers and inflammatory cytokines with ARV therapy in HIV infected subjects, a significant increase in bone resorption markers, RANKL and TNF-α were seen after initiation of ARV. The magnitude of CD4-cell recovery correlated with the increase in markers of bone resorption [99], suggesting that recovery of the immune system contributes to the increase in cytokine-mediated bone resorption.
How is bone health and fracture risk assessed in the HIV-positive population?
The predictive value of low BMD for fracture risk assessment in the HIV-positive population has not been established. In the absence of definitive data, the fracture risk assessment and standard methods of measuring bone density using DXA are utilized. In a large study of 1000 men and women, osteoporosis defined as a BMD T-score –2.5 as measured by DXA, was associated with a significantly increased risk of incident fractures but was not a good predictor of morphometric vertebral fractures [100]. In the absence of prospective longitudinal studies evaluating the bone density parameters at which fracture risk is significantly increased in the HIV population, it is reasonable to follow the guidelines used in the non-HIV population.
The approach to treatment of osteopenia and osteoporosis is similar to that in non HIV-infected population and is directed at lifestyle changes and treatment of secondary causes of osteoporosis [101], followed by initiation of antiresorptive therapy.
Management of Bone Disease
There are several guidelines available for the management of bone disease in the HIV population. The most recent guidelines from the IDSA [12] recommend assessing the risk of fragility fracture using the Fracture Risk Assessment Tool (FRAX), without DXA, in all HIV-infected men aged 40–49 years and HIV-infected premenopausal women aged ≥ 40 years. DXA should be performed in men aged ≥ 50 years, postmenopausal women, patients with a history of fragility fracture, patients receiving chronic glucocorticoid treatment, and patients at high risk of falls. In resource-limited settings, FRAX without bone mineral density can be substituted for DXA. ART guidelines should be followed. TDF and boosted PIs should be avoided if possible in at-risk patients. Dietary and lifestyle management strategies for high-risk patients should be employed and anti-osteoporosis treatment initiated if indicated [102].
The FRAX tool is available at www.shef.ac.uk/FRAX/ and is used to calculate 10-year fracture risk using patient clinical data, including presence of risk factors for osteoporosis. The tool is population-specific by race and region. It has not been validated for the HIV-positive population and may underestimate fracture risk [103]. HIV status is considered a secondary cause of osteoporosis in FRAX calculation.
The National Osteoporosis Foundation recommends screening with DXA for all women > 65 years of age, all men > 70 years of age, and adults > 50 years of age with additional risk factors for osteoporosis. Evaluation for secondary causes for low BMD should always be considered in the HIV-positive population including evaluation of calcium and vitamin D intake. Laboratory testing may include complete blood count, calcium, phosphate, albumin, creatinine, PTH, 25 hydroxy vitamin D (25,OHD) and 24 hour urine for evaluation of calcium, creatinine and phosphate (especially if on TDF) excretion. Testosterone level can be checked in men and estradiol, prolactin, FSH and LH in women for evaluation of hypogonadism. Bone turnover markers (bone specific alkaline phosphatase and serum C-terminal telopeptide) can also be assessed at baseline.
Studies using high-resolution peripheral quantitative computed tomography (HSPQCT) have shown significant reductions in tibial trabecular bone density and trabecular number in pre-menopausal and postmenopausal HIV-infected women [104], with reduced bone stiffness measured using finite element analysis [105]. Co-infection with HCV is also associated with significantly lower trabecular volumetric BMD and smaller cortical dimensions in the tibia, compared to healthy subjects [106]. HSPQCT is not widely available for clinical use at this time. Lateral imaging of the spine or vertebral morphometric analysis may be done in cases of height loss to assess for occult vertebral compression fractures.
There is a high prevalence of vitamin D deficiency in the HIV-infected population [107]. Treatment goal is to have a vitamin D level of at least 30 ng/mL, based on Endocrine Society practice guidelines [108], and may require supplementation with 1000–2000 units of vitamin D daily. Calcium intake should be optimized, averaging 1000 mg per day including diet and supplements, to be taken in divided amounts through the day for optimal absorption. Secondary causes of low bone density as mentioned in Table 4 should also be addressed. Patients should be counseled on tobacco and alcohol abuse. Corticosteroids should be dosed at the lowest dose needed. Medications such as proton pump inhibitors can impair the absorption of calcium carbonate, in which case calcium citrate supplements should be used if there is suboptimal calcium intake in the diet.
Which medications have been shown to be effective in treatment of osteoporosis in the HIV population?
Bisphosphonates are the mainstay of therapy for osteoporosis in the HIV-infected population. Only alendronate and zoledronate have substantial evidence of safety and effectiveness in the HIV-infected population, but these studies have been small and of limited duration.
Bisphosphonates are pyrophosphate analogues that inhibit bone resorption by binding to the hydroxyapatite crystals in the bone. Several prospective studies have shown alendronate to increase bone density compared to calcium and vitamin D alone in the HIV infected patients with reduced bone density [109,110], with significant reduction in markers of bone resorption [111].
Zoledronic acid (ZA), an amino-bisphosphonate which is infused intravenously, has also been used in smaller studies in HIV-infected persons. In a prospective study evaluating yearly ZA infusion to biennial ZA infusion in subjects with HIV and low bone density [112], biennial ZA infusions were found to be effective in improving and maintaining bone density in the HIV population. In another prospective study evaluating the effects of ZA in HIV-positive men, ZA infusion was given at baseline and at 12 months. Compared to placebo, treatment group had significantly higher bone density and lower bone turnover markers till 5 years after the last infusion [113].
In a meta-analysis evaluating the effect of bisphosphonates on bone mineral density in 328 adults with HIV infection from 8 randomized controlled trials (5 with alendronate and 3 with ZA as the intervention), a significant increase in BMD at the lumbar spine and hip was observed in the treatment groups at 48 and 96 weeks. However, these studies were not long enough to detect the impact of bisphosphonates on fracture risk [114]. ZA has also been shown to be effective in preventing ARV induced bone loss after a single infusion [115].
These studies confirm that both alendronate and ZA are effective in improving BMD in the HIV-infected population, with early studies showing a beneficial effect of ZA in mitigating ARV-induced bone loss as well. DXA may be repeated 1 to 2 years after initiation of osteoporosis therapy and less often subsequently if BMD is stable to improved [116].
Although these studies show significant improvement in bone density with treatment, longitudinal data on fracture reduction with these medications in the HIV-infected population are not available. Additionally, these patients have onset of osteoporosis at a younger age and the need for osteoporosis treatment needs to be assessed carefully before initiating treatment. There are other medications available for the treatment of osteoporosis in the non-HIV population such as raloxifene, teriparatide and denosumab, but no randomized controlled studies of these agents are available in the HIV-infected population.
Summary
The advent of highly potent antiretroviral therapy capable of early and prolonged viral suppression in HIV-infected patients has resulted in significant increases in life span. As we have already seen, this will likely lead to a rising incidence of various metabolic complications of HIV and ARV, including hyperlipidemia and diabetes with associated cardiovascular disease risk. A keen awareness of these potential complications, drug interactions, and possible toxicities will be paramount to their successful management. Appropriate care of HIV-infected individuals going forward will likely require multidisciplinary collaboration as the epidemic evolves to allow our patients to live not only longer, but healthier lives.
Corresponding author: Lisa M. Chirch, MD, UCONN Health, Farmington, CT 06030, [email protected].
Financial disclosures: None
Author contributions: All authors contributed equally to this article
From the University of Connecticut School of Medicine, Farmington, CT.
Abstract
- Objective: To review the metabolic complications of HIV infection.
- Methods: Review of the literature in the context of 3 clinical cases.
- Results: People with HIV infection are living longer thanks to the advent of potent antiretroviral therapy. This has led to increased incidence of age-related metabolic complications, including a higher risk of cardiovascular disease, hyperlipidemia, metabolic syndrome, and osteoporosis. Appropriate management of these complications requires an understanding of disease-related and drug-related side effects as well as the potential for drug-drug interactions. A multidisciplinary approach to patient management is most effective.
- Conclusion: Awareness of the metabolic complications frequently encountered in HIV infection, drug interactions, and possible toxicities is critical to the successful management of HIV-infected individuals.
Key words: HIV; antiretroviral therapy; hyperlipidemia; metabolic syndrome; diabetes; hypogonadism.
According to the most recent data from the Joint United Nations Programme on HIV/AIDS (UNAIDS), 36 million people worldwide are living with HIV/AIDS, with 18 million accessing effective antiretroviral therapy (ART) [1]. The past 2 decades have witnessed enormous advances in the field from prevention to diagnosis and therapeutics, and modern ART largely allows HIV-infected persons to live near-normal life spans [2,3]. However, from the beginning of the epidemic, HIV-infected persons on effective therapy have suffered from myriad metabolic consequences, many of which affect quality of life and result in excess mortality [4]. It is also true that untreated HIV infection portends an increased risk of metabolic complications, likely related to abnormal immune activation, as demonstrated in structured interruption trials [5,6]. It is worth noting, however, that while many of these metabolic dyscrasias and associated risks have historically been attributed primarily to the treatment of HIV infection with ART, data from cohort studies and randomized clinical trials have repeatedly demonstrated significant reductions in morbidity and mortality when ART is initiated early [7]. In this paper, we review HIV-related metabolic complications frequently encountered in clinical practice (hyperlipidemia, diabetes, and bone disease) and best practice considerations in the context of 3 clinical cases.
Case Patient 1
Initial Presentation and History
A 58-year-old male with a history of hypertension and mixed hyperlipidemia is referred for evaluation of newly diagnosed HIV infection. He has no history of intravenous drug use but has had multiple male and female sex partners in the past few years, and requested testing after a partner tested positive. His last negative test was 2 years ago. The patient does not smoke cigarettes. Overall he feels well and tolerates his regimen of lisinopril 10 mg and simvastatin 20 mg daily. On initial evaluation, his exam is unremarkable other than subtle white plaques on the dorsal surface of the tongue and buccal mucosa, and moderate central obesity. Vital signs including blood pressure are normal. Initial laboratory evaluation reveals a CD4 cell count of 150 cells/mm3 and an HIV RNA level of 200,000 copies/mL. Fasting serum total cholesterol is 220 mg/dL, triglycerides 250 mg/dL, LDL 170 mg/dL, and HDL 35 mg/dL. Serum BUN, creatinine, and liver function testing results are normal.
What initial regimen might be recommended based on the status of his HIV infection and comorbidities?
The most recent iteration of the US Department of Health and Human Services (DHHS) guidelines on use of antiretroviral agents (ARVs) in HIV recommends an initial ART regimen that includes a backbone of 2 nucleoside reverse transcriptase inhibitors (NRTIs), generally tenofovir disoproxil fumarate or tenofovir alafenamide, abacavir (ABC), emtricitabine (FTC), or lamivudine (3TC) [2]. To this backbone should be added a third agent; the majority of those currently recommended are integrase strand transfer inhibitors (INSTIs) (dolutegravir, elvitegravir, raltegravir); one recommended protease inhibitor (PI) (ritonavir-boosted darunavir) is also an option. Some of these initial recommended regimens are available as fixed-dosed combinations in 1 pill, making them attractive options.
The latest guidelines also clearly recommend starting ART in all HIV-infected individuals, irrespective of CD4 count. The patient described above has a very low CD4 count, so there is no question he needs to begin therapy promptly. Given his low CD4 count and relatively high viral load, one may consider a ritonavir-boosted PI as perhaps the most robust option and with a relatively high barrier to resistance development, in contrast to other options. Assuming the patient’s baseline resistance testing reveals a fully sensitive wild-type virus without meaningful resistance mutations, he will begin a regimen of TDF/FTC plus ritonavir-boosted darunavir, 3 pills once daily. Given his low CD4 count (below 200 cells/mm3), he will also need prophylaxis for Pneumocystis jirovecii pneumonia, in the form of trimethoprim/sulfamethoxazole (TMP/SMX) daily. Given the potential for interaction between the boosted PI and simvastatin, his lipid-lowering agent is switched to atorvastatin 10 mg daily.
What is the association between hyperlipidemia and HIV infection and treatment?
Hyperlipidemia represents a key modifiable risk factor for the development of cardiovascular disease (CVD) in HIV-infected individuals [8]. Indeed, a multicenter cross-sectional study of older HIV-infected individuals performed in Spain revealed a 54% prevalence of dyslipidemia and 23% CVD [9]. Most experts believe that metabolic abnormalities observed in HIV-infected individuals are the result of a combination of factors: those resulting from abnormal immune activation and inflammation related to viral replication, and those related to certain ARVs [10].
Early after HIV seroconversion, decline in HDL is one of the first proatherogenic changes observed. This, along with increased triglyceride and LDL levels, likely contribute to increased risk of CVD in this population. Increased microbial translocation, evidenced by increased levels of lipopolysaccharide (LPS), may drive immune activation, leading to dyslipidemia via a multitude of hypothesized mechanisms [4]. It has been theorized that HDL lipoproteins are less stable on ART, leading to potentially impaired plasma lipolytic activities or hepatic cholesteryl ester uptake [6,11]. Increased VLDL from release of free fatty acids may lead to higher triglyceride levels and triglyceride-rich LDL and HDL, all associated with increased risk of CVD [11].
In terms of effects of specific ARV classes, although newer agents have less of a propensity to cause dyslipidemia, the PI class arguably remains most problematic. In comparison to other classes, the PIs tend to result in greater increases in triglycerides, total cholesterol, and LDL, and have frequent drug-drug interactions with lipid-lowering agents [10,12]. Estimated prevalence of dyslipidemia in patients receiving PI therapy varies from 28% to 80% [13]. The prospective multinational cohort Data collection on Adverse events of Anti-HIV Drugs (DAD) study found significantly higher rates of hypertriglyceridemia, hypercholesterolemia, and low HDL in patients on PIs in comparison to non–nucleoside reverse transcriptase inhibitors (NNRTIs) [14]. Various mechanisms have been proposed to explain the PIs adverse effects on lipids, including inhibition of lipogenesis and adipocyte differentiation, decreased hepatocyte clearance of chylomicrons and VLDL, and increased hepatic synthesis of triglycerides [15]. Of the available PIs, atazanavir and darunavir have less potential to lead to dyslipidemia [10], while lopinavir/ritonavir, fosamprenavir, and tipranavir may have the highest [13]. Of the NNRTIs, efavirenz is most frequently associated with dyslipidemia, specifically increased triglycerides and total cholesterol [13]. However, these increased values seen on efavirenz therapy may be offset by relative increases in HDL, with little resultant effect on the total cholesterol:HDL ratio. Rilpivirine, etravirine, and nevirapine are relatively less likely to drive lipid changes, although certain drug interactions are important to recognize in clinical practice, such as the interaction between rilpivirine and proton pump inhibitors [2,13,16]. It is also worth noting that no NNRTIs are included in current guidelines as preferred therapy [2].
Historically, the thymidine analogue NRTIs (stavudine, didanosine, zidovudine) have been associated with lipid dyscrasias and lipoatrophy, but fortunately these are no longer used frequenty except in cases requiring deep salvage therapy for highly treatment-experienced patients. Two newer NRTIs, tenofovir and abacavir, have relatively neutral to favorable effects on lipids. The combination of tenofovir disoproxil (TDF) and emtricitabine (trade name Truvada) was associated with significantly lower triglycerides, total cholesterol and LDL than other NRTI pairs [6]. TDF has been postulated to have lipid-lowering effects. Switch studies in which patients were taken off thymidine analogues and placed on TDF, demonstrated recovery of limb fat in patients with lipoatrophy, and those switched off abacavir-based ART to TDF showed statistically significant lower fasting total cholesterol at week 12, without differences of viral suppression [8]. Tenofovir alafenamide (TAF) is a next-generation prodrug of tenofovir that results in improved stability in plasma and higher intracellular levels in comparison to TDF [17]. Although randomized controlled trials of TAF vs TDF-based ARV regimens have suggested statistically higher total cholesterol, serum HDL is also increased resulting in unchanged total:HDL ratios and no differences in risk classifications [18]. Integrase inhibitors (INSTI) now represent first-line therapy in combination with an NRTI backbone, and since their availability in 2007 have been evaluated in comparison to various PIs and NNRTIs. Both raltegravir and dolutegravir have consistently shown broad neutral effects on lipids and are among the most metabolically friendly agents available [19–21]. Because it is given in fixed-dose combination with non-ritonavir pharmacologic booster cobicistat, elvitegravir has effects similar to ritonavir-boosted PIs on lipids [6].
What are management considerations in the treatment of hyperlipidemia in HIV-infected patients?
Patients with HIV and hyperlipidemia may benefit from lipid-lowering therapy in addition to ART, although in certain cases appropriate switches may make a difference. Careful consideration of drug interactions between ARVs and lipid-lowering agents, in addition to ARV history and known drug resistance, is warranted prior to selecting a regimen in these patients. In addition, the latest American College of Cardiology/American Heart Association guidelines suggest evaluating 10-year risk of atherosclerotic cardiovascular disease (ASCVD) using the pooled cohort equation to determine the type and dose of statin required (moderate vs high intensity) [22]. It is noteworthy that HIV infection and its therapies are not taken into account as potential risk factors in this model. Primary prevention in non-diabetic patients with a statin is recommended for patients with a 10-year absolute risk of ≥ 7.5% [22]. This patient’s risk is estimated at between 12% and 13% based on this equation, so primary prevention with a moderate-or high-intensity statin is recommended (Table 1) [23]. Data from more than 80,000 patients in the Veterans Aging Cohort Study (VACS) showed that HIV-infected patients with no baseline ASCVD had 50% increased risk of acute myocardial infarction when compared to HIV-uninfected patients over 6 years of follow-up [24]. Thus, consideration of the virus itself or its therapy as an additional risk factor may be valid.
Screening and Monitoring of Hyperlipidemia
The most recent iteration of the DHHS primary care guidelines for the management of HIV-infected individuals recommends obtaining fasting (ideally 12 hours) lipid profiles upon initiation of care, and within 1 to 3 months of beginning therapy [12,13]. These initial levels, along with other elements of the patient’s history and calculation of risk may help determine whether lipid-lowering therapy is indicated, and if so, which therapy would be best. In general, after regimen switches or additions of either ARV or statin therapy, repeating fasting lipid levels 6 weeks later is recommended to gauge the effects of the switch. This is especially critical when interactions between ARVs and lipid-lowering therapies are possible. Some experts recommend performing annual screening of patients with normal baseline lipids or with well-controlled hyperlipidemia on therapy. Assessment of 10-year ASCVD risk is also recommended annually, in addition to baseline risk assessment, to determine the need and appropriateness of statin therapy [25]. The question of primary prevention in HIV has yet to be definitively answered. Small studies in this population have demonstrated that statins have the potential to slow progression of carotid intima media thickness and reduce noncalcified plaque volume [24]. An NIH/AIDS Clinical Trial Group–sponsored randomized clinical trial (“REPRIEVE”) is currently underway to address this question. More than 6000 HIV-infected men and women with no history of ASCVD at 100 sites in several countries are enrolled to assess the benefit of pitavastatin as primary prevention in this risk group [24]. Metabolized via glucuronidation primarily, as opposed to cytochrome p450 (CYP 3A4 isoenzyme), pitavastatin is thought to have fewer drug interactions with ARVs in general [6] (Table 2).
Relevant Drug-Drug Interactions
Deciding which statin to begin in HIV-infected patients depends on whether moderate- or high-intensity therapy is warranted and whether the potential for drug interaction with ARVs exists. Table 2 [6,12] depicts available statins and the potential for pharmacokinetic interaction with the primary ARV classes. Simvastatin and lovastatin are heavily metabolized via the CYP 3A4 pathway, resulting in the highest potential risk of interaction with CYP 3A4 inhibitors, such as the PIs, or inducers (eg, NNRTIs, in particular efavirenz) [6]. The former may inhibit metabolism of these statins, resulting in increased risk of toxicity, while co-administration with efavirenz, for example, may result in inadequate serum concentration and therefore inadequate lipid-lowering effects. Although less lipophilic, atorvastatin results in similar interactions with PIs and NNRTIs, and therefore low starting doses with close monitoring is recommended [6]. Fewer interactions have been noted with rosuvastatin, pravastatin, and pitavastatin, as these do not require CYP 3A4 for their metabolism and are thus less likely to be affected by ARVs. These therefore represent potentially safer first choices for certain patients on ARVs, although of these, only rosuvastatin is classified as a high-intensity statin [22,23] (Table 1). When compared directly to pravastatin 40 mg daily in patients receiving ritonavir-boosted PIs, rosuvastatin performed superiorly at 10 mg per day, resulting in more significant reductions in LDL and triglyceride levels [15]. Although it is eliminated largely unchanged through the kidney and liver, pravastatin has been reported to idiosyncratically interact with darunavir, resulting in potentially increased pravastatin levels and associated toxicity [25]. Treatment of pure hypertriglyceridemia in HIV-infected patients should begin with fibrates, which have little to no risk of interaction with most clinically relevant ARVs [6,10]. Alternatives to lower triglycerides include niacin and N-3 polyunsaturated fatty acids [25].
Case 1 Continued
The patient has an impressive response to his initial regimen of TDF/FTC plus boosted darunavir, with repeat CD4 count after 12 weeks of 275 (18%) cells/mm3 and an undetectable viral load (< 20 copies/mL). Other lab parameters are favorable and he is tolerating the regimen well without notable side effects. However, at his next visit, although his viral load remains undetectable, his triglyceride level has increased to 350 mg/dL, although other lipid parameters are comparable to the prior result. He complains of diffuse body aches, concentrated in large muscle groups of the extremities, and dark-colored urine. A creatine phosphokinase (CPK) level is elevated at 300 IU/L (normal, 22–269, negative MB fraction). Serum creatinine is 1.4 mg/dL (had been 1.1 mg/dL at baseline). Given he has done so well otherwise on these ARVs, he is reluctant to make any changes.
What drug-drug interaction is most likely causing this patient's problem, and how should it be managed?
This scenario is not uncommon in clinical practice, and changes to regimens are sometimes necessary in order to avoid drug interactions. Care must be taken to thoroughly review antiretroviral history and available resistance testing (in this case a relatively short history) in order to ensure a fully active and suppressive regimen is chosen. This description could be the result of an interaction between lipid-lowering therapy and ARVs resulting in increased relative concentrations of one drug or the other and therefore leading to toxicity. Given this possibility, and suboptimal control of hyperlipidemia, consideration should be given to switching both his ART and his statin therapy.
Safety and Potential Toxicities of Lipid-Lowering Therapy
Increased serum concentration of certain statins when co-administered with CYP 3A4 inhibitors like the PIs leads to heightened risk of statin-associated toxicities. In general, this includes muscle inflammation, leading to increases in serum CPK level and associated symptoms, including myalgias, myositis, or in extreme cases, rhabdomyolysis [6]. Although rare, this toxicity can be serious and may lead to acute renal injury if not recognized and managed appropriately. In theory, the potential for statin-associated hepatotoxicity may also be increased in patients receiving PIs, although this has not been borne out in clinical trials [26]. In fact, quite the opposite may be true, in that statins have been shown to improve liver function in patients with hepatitis C virus (HCV) coinfection and with nonalcoholic fatty liver disease [6,15].
Case 1 Conclusion
The patient does well on his new ARV regimen of TAF/FTC and dolutegravir, 2 pills once daily. He no longer requires TMP/SMX, as his CD4 count has been reliably above 200 cells/mm3 on several occasions. Serum creatinine is back down to baseline and CPK has normalized. Fasting lipids have improved since the switch, and he no longer has symptoms of myositis on rosuvastatin 10 mg daily.
Summary
Consideration of statin therapy is complicated by potential drug interactions with ARVs and associated toxicity. However, given known effects of ARVs on lipids, and of immune activation and inflammation related to the virus itself, these patients should be carefully evaluated for statin therapy for their anti-inflammatory and immune modulatory effects as much as for their lipid-lowering ability. Utilization of HIV infection and its therapies as additional cardiovascular risk factors when calculating 10-year risk deserves further consideration; forthcoming results of the REPRIEVE trial are certain to contribute valuable information to this field of study.
Case Patient 2
Initial Presentation and History
A 45-year-old female with history of HIV infection since 2008 presents to the office for new-onset diabetes, diagnosed 2 weeks ago. She has had symptoms of polyuria and polydipsia for the last 1 month. She denies diarrhea, nausea, vomiting or weight loss. She is currently on a regimen consisting of zidovudine/lamivudine plus lopinavir/ritonavir. There is no family history of diabetes. Her examination is unremarkable, including normal vital signs (weight 150 lb, blood pressure 114/70, heart rate 76) and no evidence of insulin resistance, including acanthosis nigricans or striae. Glycosylated hemoglobin level (HbA1c) is 8%. Creatinine and liver function tests are within reference ranges.
Do HIV-infected patients have a higher incidence of type 2 diabetes mellitus (DM)?
Prevalence of type 2 DM in HIV-infected patients varies between 2% to 14% [27]. This variation is due to the different cutoffs used for diagnosis, differences in cohorts studied, and how risk factors are analyzed [28–31]. In a recent nationally representative estimate of DM prevalence among HIV-infected adults receiving medical care in the United States in 2009–2010, the prevalence of DM was noted to be 10.3%. In comparison to the general adult US population, HIV-infected individuals have a 3.8% higher prevalence of DM after adjusting for age, sex, race/ethnicity, education, poverty-level, obesity, and HCV infection [27].
There is controversy over whether HIV infection itself increases the risk of type 2 DM, with some studies showing increased risk [28,32,33] and others showing no independent effect or an inverse effect [30,34,35]. Studies on the impact of ethnicity and race on prevalence of DM are limited [36].
Certain traditional risk factors (age, ethnicity, obesity) are still responsible for most of the increased risk of diabetes in the HIV-infected population [35,37]. HIV infection itself is associated with metabolic dysfunction, independent of ARV. In HIV-infected patients, impaired glucose metabolism is associated with altered levels of adipokines, increased adiponectin and soluble-tumor necrosis factor receptor 1 (sTNFR1) and decreased leptin [38,39]. HIV-associated alterations in CD4+ and CD8+ T cell function also impair glycolysis, which may adversely impact glucose metabolism [40].
Other contributing factors in HIV-infected patients are HCV co-infection [41], medications (atypical antipsychotics, corticosteroids), opiates, and low testosterone [42]. HCV co-infection may lead to hepatic steatosis and liver fibrosis, and increasing insulin resistance.
Recent genomic studies show several common single-nucleotide polymorphisms (SNPs) associated with diabetes in the general population. In the Swiss HIV Cohort Study, SNPs accounted for 14% of type 2 DM risk variability, whereas ARV exposure accounted for 3% and age for 19% of the variability in DM [43].
ARVs also increase the risk of type 2 DM by both direct and indirect effects. Certain ARVs causes lipoatrophy [30] and visceral fat accumulation/lipohypertrophy [29,44]. PIs increase insulin resistance via effects on GLUT-4 transporter and decrease insulin secretion through effects on B cell function [45]. NRTIs (eg, stavudine, zidovudine and didanosine) can cause direct mitochondrial toxicity [46–48]. Utilization of newer ARV agents has decreased the prevalence of severe lipoatrophy, but lipohypertrophy and the underlying metabolic abnormalities persist. The DHHS “preferred” nucleoside analogues, tenofovir and abacavir, do not induce mitochondrial toxicity and have more favorable metabolic profiles [49,50]. In ACTG Study 5142, thymidine-sparing regimens were found to cause less lipoatrophy [51]. In addition, darunavir and atazanavir, the preferred and alternative PIs and the integrase strand transfer inhibitor have limited or modest impact on insulin sensitivity [20,52,53]. This has led to a recent decline in the incidence of type 2 DM in HIV-infected patients.
Statins can also increase insulin resistance and DM [54], although studies have shown mixed results [55–57]. The benefits of statin therapy likely outweigh the risk of DM since there is a significant cardiovascular event reduction with their use [58,59].
How is diabetes diagnosed in HIV-infected patients?
Optimal diabetes screening guidelines have not been established specifically for HIV-infected patients. The American Diabetes Association (ADA) guidelines recommend that diabetes in the general population be diagnosed by 2 elevated fasting blood glucose levels, HbA1c, oral glucose tolerance test (OGTT), or high random glucose with classic symptoms of hyperglycemia [60]. Repeat testing is recommended every 3 years. The OGTT is recommended for diagnosis in pregnant women.
HbA1c may underestimate glycemic burden in HIV-infected individual due to higher mean corpuscular volume, NRTI use (specifically abacavir), or lower CD4 count [61–65]. The Infectious Diseases Society of America (IDSA) 2013 primary care guidelines for HIV-infected patients recommends obtaining a fasting glucose and/or HbA1c prior to and within 1–3 months after starting ARV [12]. Use of HbA1c threshold cutoff of 5.8% for the diagnosis of DM and testing every 6–12 months are recommended.
How should this patient’s diabetes be managed?
The ADA guidelines suggest a patient-centered approach to management of diabetes [66]. All patients should be educated about lifestyle modifications with medical nutrition therapy and moderate-intensity aerobic activity and weight loss [67]. If a patient is on lopinavir/ritonavir or a thymidine analogue (zidovudine, stavudine), one should consider switching the ARV regimen [2].
There are currently no randomized controlled trials of diabetes treatment specific to patients with HIV infection. Metformin is the first-line agent. It improves insulin sensitivity by reducing hepatic glucose production and improving peripheral glucose uptake and lipid parameters [68,69]. Other oral hypoglycemic agents used in the treatment of type 2 diabetes are shown in Table 3.
Case 2 Continued
The patient is switched to TAF/FTC plus dolutegravir with improvement in blood sugars. She is also started on metformin. Co-administration of metformin and dolutegravir will be carefully monitored since dolutegravir increases metformin concentration [70]. When dolutegravir is used with metformin, the total daily dose of metformin should be limited to 1000 mg.
• How should this patient be followed?
If the patient is still not at goal HbAb1c at follow-up, there are multiple other treatment options, including use of insulin. Goal HbA1c for most patients with type 2 DM is < 7%; however, this goal should be individualized for each patient in accordance with the ADA guidelines [12]. A longitudinal cohort study of 11,346 veterans with type 2 diabetes compared the glycemic effectiveness of oral diabetic medications ( metformin, sulfonylurea and a thiazolidinedione) among veterans with and without HIV infection. This study did not find any significant difference in HbA1c based on different diabetes medications. However the HBA1c reduction was less in black and Hispanic patients. The mechanism for the poorer response among these patients need to be evaluated further [71]. In addition to management of blood sugar, other CVD risk factors, hyperlipidemia, hypertension, smoking, etc, should be assessed and managed aggressively.
Case Patient 3
Initial Presentation and History
A 45-year-old male with a history of HIV infection diagnosed 10 years ago, on TDF/FTC/efavirenz (trade name Atripla) for the last 7 years, presents with a left femoral neck fracture after he missed the pavement and fell on his left hip. His history is significant for IV drug abuse for 10 years prior to diagnosis of HIV, and he has been on methadone for the last 6 years.
Is HIV infection associated with increased prevalence of osteopenia and osteoporosis and increased risk of fractures?
With recent advancements in antiretroviral therapy and improved survival of the HIV-infected population, osteoporosis and increased fracture risk have become important causes of morbidity and mortality. Osteoporosis is a skeletal disorder characterized by compromised bone strength, which predisposes to an increased risk of fracture. The World Health Organization defines osteoporosis as a bone mineral density (BMD) measurement by dual X-ray absorptiometry (DXA) at the spine, hip, or forearm that is more than 2.5 standard deviations below that of a "young normal" adult (T-score < –2.5) or a history of one or more fragility fractures. Fragility fractures result from mechanical forces that would not ordinarily result in fracture, such as fall from standing height [40]. Osteopenia is characterized by low BMD (T-score between –1.0 and –2.5) and can be a precursor to osteoporosis.
Several observational, retrospective, and prospective studies have shown lower bone density and an increased risk of fractures in the HIV-infected population compared to age-, race- and sex-matched HIV-negative adults. In a large meta-analysis of pooled prevalence data on 884 HIV-infected patients compared with 654 HIV-uninfected age- and sex-matched controls [72], overall, HIV-infected patients had a significant 6.4-fold increased odds of reduced BMD and a 3.7-fold increased odds of osteoporosis compared to the control population. This meta-analysis also compared ARV-treated subjects to ARV-naive subjects and showed that ARV-treated subjects (n = 824) had a higher prevalence of reduced BMD compared with ARV-naive subjects (n= 202; odds ratio 2.5, 95% CI 1.8–3.7). The odds of osteoporosis was increased 2.4 times (95% CI 1.2 – 4.8) in ARV-treated subjects compared with ARV-naive subjects. None of the studies adjusted for potentially important confounding factors, such as age or duration of HIV infection. PI-treated patients (n = 791) were also found to have a higher prevalence of reduced BMD compared with PI-untreated patients (n = 410; OR 1.5, 95% CI 1.1–2.0). The odds of osteoporosis in PI-treated patients (n = 666) was also 1.6-fold greater (95% CI 1.1–2.3) than those not treated with PI (n = 367).
Low bone density has also been reported in HIV- positive premenopausal women irrespective of ARV status. In a recent study of 89 premenopausal women (mean age, 37 years) predominantly of African origin with HIV infection, osteopenia and osteoporosis were prevalent in one-third of these women, irrespective of ARV use and were associated with low BMI [73]. In a sub-study of the INSIGHT trial evaluating prevalence of and risk factors for low BMD in untreated HIV infection, performed at several sites across 6 continents involving 424 subjects, osteopenia was present in a third of this relatively young predominantly non-white ART-naive population (mean age 34 + 10 years) with normal CD4 cell counts, while only 2% had osteoporosis. Factors independently associated with lower BMD at the hip and spine were female sex, Latino/Hispanic ethnicity, lower BMI, and higher estimated glomerular filtration rate. Longer duration of HIV infection was also associated with lower hip BMD. Current or nadir CD4 cell count and HIV viral load were not associated with low BMD [74].
Many studies have reported increased fracture prevalence in the HIV population. In a retrospective study of fracture prevalence in a large US health care system, a significantly higher rate of fractures was reported in HIV-infected men and women compared to non-HIV-infected controls (2.87 vs. 1.77 fractures per 100 persons, P < 0.001). The difference in the increased fracture prevalence was greater in HIV positive men compared to women (3.08 vs. 1.83; P < 0.001). Vertebral, wrist and hip fractures were more prevalent in men compared to vertebral and wrist fractures only in women. Fracture prevalence was higher in both Caucasian females and males and only in African-American women [75].
In the HIV Outpatient Study (HOPS) [76], age-adjusted fracture rates in the HIV population were noted to be 1.98 to 3.69 times higher than rates in the general population. The HOPS was an open prospective cohort study of HIV-infected adults who were followed at 10 US HIV clinics. Rates of first fractures at any anatomic site from 2000–2008 were assessed among 5826 active HOPS patients (median age 40 years, 79% male, 52% Caucasian, and 73% exposed to ART). Among persons aged 25–54 years, both fracture rates and relative proportion of fragility fractures were higher among HOPS patients than among outpatient controls. Older age, substance abuse, nadir CD4+ cell count <200 cells/mm, HCV infection and DM were associated with incident fractures [76].
What factors contribute to poor bone health in the HIV population?
Several factors that contribute to low bone density are present at a higher rate in the HIV population (Table 4). These include poor nutritional status in terms of suboptimal calcium and vitamin D intake, hypogonadism, low body weight, and alcohol, tobacco and substance abuse.
Vitamin D deficiency is very common in HIV-infected patients, with a prevalence of up to 60% to 75% [77]. Hypogonadism is also relatively common among HIV population [78], contributing to lower bone density. Co-infection with HCV is also associated with increased risk of fractures. In a large cohort of Medicaid beneficiaries, a significant increase in the risk of hip fracture was demonstrated in HCV/HIV co-infected subjects compared either with HCV mono-infected, HIV mono-infected or non-infected individuals [79]. In another large database study, a significantly higher risk of osteoporotic fracture (closed wrist, vertebral or hip fracture) was reported in HCV/HIV co-infected versus HIV mono-infected individuals [80] with fracture rates of 2.57 and 2.07/1000 patient-years (P < 0.001). Dual treatment for HIV/hepatitis B co-infection has also been shown to be associated with a higher risk of hip fracture compared to treatment of HIV mono-infected individuals [81].
HIV infection itself can increase bone loss and reduce bone formation through direct effects related to the HIV antigen load or indirect effects related to activation of the pro-inflammatory cytokines resulting in bone resorption and loss [82]. Co-infection with HCV and/or hepatitis B also contributes to lower bone density in this population. Certain ARVs may also contribute to low bone density in the HIV population. Lipoatrophy related to HIV may also mediate bone loss through complex relationship between central signaling of adipocyte hormones [82,83].
Direct Viral Effects
Several HIV viral proteins have been shown to promote osteoclast activity (vpr and gp120), suppress osteoblast activity (p55-gag) and increase osteoblast apoptosis [84], resulting in increased bone resorption and reduced bone formation, leading to low bone mass. High HIV RNA viral load and T-cell activation are also associated with elevated levels of receptor activator of nuclear factor kappa-B ligand (RANKL), which results in osteoclast formation and increased bone resorption [85]. Other endogenous physiological inhibitors of osteoclastogenesis such as osteoprotegrin and interferon-γ levels are also remarkably downregulated in advanced HIV infection, resulting in increased bone resorption [86]. At a cellular level, HIV proteins including Tat and Nef reduce the number of available mesenchymal stem cell (MSC) precursors that proliferate into osteoblasts by inducing MSC senescence, due to increased oxidative stress and mitochondrial dysfunction resulting in reduced proliferation of osteoblasts and lower rates of bone formation [87]. Collectively, these mechanisms result in significant uncoupling of bone formation and resorption, resulting in less bone formation and greater rate of bone loss and lower bone density.
Pro-inflammatory Pathways
Cytokines and other soluble immune factors play a major role in the physiology of osteoblast maturation and osteoclastic bone resorption [88,89]. Immune dysfunction and persistent inflammation in HIV result in increased levels of several inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), and RANKL, resulting in stimulation of osteoclastogenesis and bone resorption [90]. Due to a disruption between T and B cells in HIV and decreased osteoprotegrin (OPG) production and increased RANKL level, RANKL/OPG ratio is elevated, favoring osteoclastogenesis [91].
Is antiretroviral therapy associated with bone loss?
The initiation of ART has been reported to cause 2% to 6% bone loss irrespective of the regimen used, similar to that sustained in the first 2 years after menopause [92]. Certain NRTIs and PIs are associated with higher rates of bone loss than others. TDF has been associated most commonly with decreased bone mineral density, which usually stabilizes with continued use [93]. In a randomized trial comparing 4 treatment arms of ABC/3TC or TDF/FTC with EFV or ATV/ritonavir, TDF was associated with a greater reduction in BMD compared to abacavir-based regimens [94]. The likely cause of this may be TDF-mediated renal toxicity, including proximal tubular dysfunction and hypophosphatemia, resulting in increased PTH and bone resorption, and nephrogenic diabetes insipidus [95]. TAF is another prodrug of tenofovir diphosphate associated with less renal and bone toxicity compared with TDF. TAF has been associated with significantly less decrease in bone mineral density and renal dysfunction in randomized studies compared to regimen using TDF [17]. Vitamin D deficiency and hypophosphatemia associated with TDF therapy may present with osteomalacia, which predisposes to bone pain and fractures. Treatment with TDF may rarely be associated with the development of Fanconi syndrome and osteomalacia [96]. BMD is often severely reduced and bone pain and pathological fractures are characteristic features. Certain PI regimens containing ritonavir-boosted atazanavir have also been associated with greater bone loss in the spine than the hip, compared to efavirenz-containing regimens [97].
The universal bone loss associated with ART is thought to be a result of the "immune reconstitution inflammatory syndrome" (IRIS). This occurs as a result of rapid improvement in immune function after the commencement of ARV as a result of systemic or local inflammation, resulting in increased levels of cytokines that may contribute to bone loss. This has been shown in animal studies where T cell transplantation into immunocompromised mice to mimic ARV-induced T-cell expansion resulted in increased RANKL and TNF-α production by B cells and/or T cells, accompanied by enhanced bone resorption and BMD loss. When TNF-α or RANKL-null T-cells or TNF-α antagonists were used instead, the loss of cortical bone was prevented [98]. In a prospective study evaluating changes in bone turnover markers and inflammatory cytokines with ARV therapy in HIV infected subjects, a significant increase in bone resorption markers, RANKL and TNF-α were seen after initiation of ARV. The magnitude of CD4-cell recovery correlated with the increase in markers of bone resorption [99], suggesting that recovery of the immune system contributes to the increase in cytokine-mediated bone resorption.
How is bone health and fracture risk assessed in the HIV-positive population?
The predictive value of low BMD for fracture risk assessment in the HIV-positive population has not been established. In the absence of definitive data, the fracture risk assessment and standard methods of measuring bone density using DXA are utilized. In a large study of 1000 men and women, osteoporosis defined as a BMD T-score –2.5 as measured by DXA, was associated with a significantly increased risk of incident fractures but was not a good predictor of morphometric vertebral fractures [100]. In the absence of prospective longitudinal studies evaluating the bone density parameters at which fracture risk is significantly increased in the HIV population, it is reasonable to follow the guidelines used in the non-HIV population.
The approach to treatment of osteopenia and osteoporosis is similar to that in non HIV-infected population and is directed at lifestyle changes and treatment of secondary causes of osteoporosis [101], followed by initiation of antiresorptive therapy.
Management of Bone Disease
There are several guidelines available for the management of bone disease in the HIV population. The most recent guidelines from the IDSA [12] recommend assessing the risk of fragility fracture using the Fracture Risk Assessment Tool (FRAX), without DXA, in all HIV-infected men aged 40–49 years and HIV-infected premenopausal women aged ≥ 40 years. DXA should be performed in men aged ≥ 50 years, postmenopausal women, patients with a history of fragility fracture, patients receiving chronic glucocorticoid treatment, and patients at high risk of falls. In resource-limited settings, FRAX without bone mineral density can be substituted for DXA. ART guidelines should be followed. TDF and boosted PIs should be avoided if possible in at-risk patients. Dietary and lifestyle management strategies for high-risk patients should be employed and anti-osteoporosis treatment initiated if indicated [102].
The FRAX tool is available at www.shef.ac.uk/FRAX/ and is used to calculate 10-year fracture risk using patient clinical data, including presence of risk factors for osteoporosis. The tool is population-specific by race and region. It has not been validated for the HIV-positive population and may underestimate fracture risk [103]. HIV status is considered a secondary cause of osteoporosis in FRAX calculation.
The National Osteoporosis Foundation recommends screening with DXA for all women > 65 years of age, all men > 70 years of age, and adults > 50 years of age with additional risk factors for osteoporosis. Evaluation for secondary causes for low BMD should always be considered in the HIV-positive population including evaluation of calcium and vitamin D intake. Laboratory testing may include complete blood count, calcium, phosphate, albumin, creatinine, PTH, 25 hydroxy vitamin D (25,OHD) and 24 hour urine for evaluation of calcium, creatinine and phosphate (especially if on TDF) excretion. Testosterone level can be checked in men and estradiol, prolactin, FSH and LH in women for evaluation of hypogonadism. Bone turnover markers (bone specific alkaline phosphatase and serum C-terminal telopeptide) can also be assessed at baseline.
Studies using high-resolution peripheral quantitative computed tomography (HSPQCT) have shown significant reductions in tibial trabecular bone density and trabecular number in pre-menopausal and postmenopausal HIV-infected women [104], with reduced bone stiffness measured using finite element analysis [105]. Co-infection with HCV is also associated with significantly lower trabecular volumetric BMD and smaller cortical dimensions in the tibia, compared to healthy subjects [106]. HSPQCT is not widely available for clinical use at this time. Lateral imaging of the spine or vertebral morphometric analysis may be done in cases of height loss to assess for occult vertebral compression fractures.
There is a high prevalence of vitamin D deficiency in the HIV-infected population [107]. Treatment goal is to have a vitamin D level of at least 30 ng/mL, based on Endocrine Society practice guidelines [108], and may require supplementation with 1000–2000 units of vitamin D daily. Calcium intake should be optimized, averaging 1000 mg per day including diet and supplements, to be taken in divided amounts through the day for optimal absorption. Secondary causes of low bone density as mentioned in Table 4 should also be addressed. Patients should be counseled on tobacco and alcohol abuse. Corticosteroids should be dosed at the lowest dose needed. Medications such as proton pump inhibitors can impair the absorption of calcium carbonate, in which case calcium citrate supplements should be used if there is suboptimal calcium intake in the diet.
Which medications have been shown to be effective in treatment of osteoporosis in the HIV population?
Bisphosphonates are the mainstay of therapy for osteoporosis in the HIV-infected population. Only alendronate and zoledronate have substantial evidence of safety and effectiveness in the HIV-infected population, but these studies have been small and of limited duration.
Bisphosphonates are pyrophosphate analogues that inhibit bone resorption by binding to the hydroxyapatite crystals in the bone. Several prospective studies have shown alendronate to increase bone density compared to calcium and vitamin D alone in the HIV infected patients with reduced bone density [109,110], with significant reduction in markers of bone resorption [111].
Zoledronic acid (ZA), an amino-bisphosphonate which is infused intravenously, has also been used in smaller studies in HIV-infected persons. In a prospective study evaluating yearly ZA infusion to biennial ZA infusion in subjects with HIV and low bone density [112], biennial ZA infusions were found to be effective in improving and maintaining bone density in the HIV population. In another prospective study evaluating the effects of ZA in HIV-positive men, ZA infusion was given at baseline and at 12 months. Compared to placebo, treatment group had significantly higher bone density and lower bone turnover markers till 5 years after the last infusion [113].
In a meta-analysis evaluating the effect of bisphosphonates on bone mineral density in 328 adults with HIV infection from 8 randomized controlled trials (5 with alendronate and 3 with ZA as the intervention), a significant increase in BMD at the lumbar spine and hip was observed in the treatment groups at 48 and 96 weeks. However, these studies were not long enough to detect the impact of bisphosphonates on fracture risk [114]. ZA has also been shown to be effective in preventing ARV induced bone loss after a single infusion [115].
These studies confirm that both alendronate and ZA are effective in improving BMD in the HIV-infected population, with early studies showing a beneficial effect of ZA in mitigating ARV-induced bone loss as well. DXA may be repeated 1 to 2 years after initiation of osteoporosis therapy and less often subsequently if BMD is stable to improved [116].
Although these studies show significant improvement in bone density with treatment, longitudinal data on fracture reduction with these medications in the HIV-infected population are not available. Additionally, these patients have onset of osteoporosis at a younger age and the need for osteoporosis treatment needs to be assessed carefully before initiating treatment. There are other medications available for the treatment of osteoporosis in the non-HIV population such as raloxifene, teriparatide and denosumab, but no randomized controlled studies of these agents are available in the HIV-infected population.
Summary
The advent of highly potent antiretroviral therapy capable of early and prolonged viral suppression in HIV-infected patients has resulted in significant increases in life span. As we have already seen, this will likely lead to a rising incidence of various metabolic complications of HIV and ARV, including hyperlipidemia and diabetes with associated cardiovascular disease risk. A keen awareness of these potential complications, drug interactions, and possible toxicities will be paramount to their successful management. Appropriate care of HIV-infected individuals going forward will likely require multidisciplinary collaboration as the epidemic evolves to allow our patients to live not only longer, but healthier lives.
Corresponding author: Lisa M. Chirch, MD, UCONN Health, Farmington, CT 06030, [email protected].
Financial disclosures: None
Author contributions: All authors contributed equally to this article
1. UNAIDS. Fact sheet - Latest statistics on the status of the AIDS epidemic. Accessed 10 Nov 2017 at http://www.unaids.org/en/resources/fact-sheet.
2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 10 Nov 2017 at https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
3. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One 2013;8:e81355.
4. Pedersen KK, Pedersen M, Troseid M, et al. Microbial translocation in HIV infection is associated with dyslipidemia, insulin resistance, and risk of myocardial infarction. J Acquir Immune Defic Syndr 2013;64:425–33.
5. Strategies for Management of Antiretroviral Therapy Study Group, El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–96.
6. Chastain DB, Henderson H, Stover KR. Epidemiology and management of antiretroviral-associated cardiovascular disease. Open AIDS J 2015;9:23–37.
7. Group ISS, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
8. Moyle GJ, Orkin C, Fisher M, et al. A randomized comparative trial of continued abacavir/lamivudine plus efavirenz or replacement with efavirenz/emtricitabine/tenofovir DF in hypercholesterolemic HIV-1 infected individuals. PloS One 2015;10:e0116297.
9. Mothe B, Perez I, Domingo P, et al. HIV-1 infection in subjects older than 70: a multicenter cross-sectional assessment in Catalonia, Spain. Curr HIV Res 2009;7:597–600.
10. Calvo M, Martinez E. Update on metabolic issues in HIV patients. Curr Opin HIV AIDS 2014;9:332–9.
11. Gillard BK, Raya JL, Ruiz-Esponda R, et al. Impaired lipoprotein processing in HIV patients on antiretroviral therapy: aberrant high-density lipoprotein lipids, stability, and function. Arterioscler Thromb Vasc Biol 2013;33:1714-21.
12. Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58:1–10.
13. Calza L, Colangeli V, Manfredi R, et al. Clinical management of dyslipidaemia associated with combination antiretroviral therapy in HIV-infected patients. J Antimicrob Chemother 2016;71:1451–65.
14. Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS 2003;17:1179–93.
15. Husain NE, Ahmed MH. Managing dyslipidemia in HIV/AIDS patients: challenges and solutions. HIV/AIDS (Auckl) 2015;7:1–10.
16. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. Accessed at www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
17. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015;385:2606–15.
18. Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2014;67:52–8.
19. Eron JJ, Young B, Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 2010;375:396–407.
20. Quercia R, Roberts J, Martin-Carpenter L, Zala C. Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks. Clin Drug Investig 2015;35:211–9.
21. Lennox JL, DeJesus E, Berger DS, et al. Raltegravir versus efavirenz regimens in treatment-naive hiv-1–infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010;55:39–48.
22. Nayor M, Vasan RS. Recent update to the US cholesterol treatment guidelines. a comparison with international guidelines. Circulation 2016;133:1795–806.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934.
24. Mitka M. Exploring statins to decrease HIV-related heart disease risk. JAMA 2015;314:657–9.
25. Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Diseases Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003;37:613–27.
26. Milazzo L, Menzaghi B, Corvasce S, et al. Safety of statin therapy in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr 2007;46:258–60.
27. Hernandez-Romieu AC, Garg S, Rosenberg ES, et al. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diab Res Care 2017;5:e000304.
28. Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–84.
29. De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008;31:1224–9.
30. Rasmussen LD, Mathiesen ER, Kronborg G, et al. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PloS One 2012;7:e44575.
31. Polsky S, Floris-Moore M, Schoenbaum EE, et al. Incident hyperglycaemia among older adults with or at-risk for HIV infection. Antivir Ther 2011;16:181–8.
32. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–12.
33. Galli L, Salpietro S, Pellicciotta G, et al. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur J Epidemiol 2012;27:657–65.
34. Howard AA, Hoover DR, Anastos K, et al. The effects of opiate use and hepatitis C virus infection on risk of diabetes mellitus in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr 2010;54:152–9.
35. Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–34.
36. Hadigan C, Kattakuzhy S. Diabetes mellitus type 2 and abnormal glucose metabolism in the setting of human immunodeficiency virus. Endocrin Metab Clin North Am 2014;43:685–96.
37. Butt AA, Fultz SL, Kwoh CK, Kelley D, et al. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology 2004;40:115–9.
38. Veloso S, Escote X, Ceperuelo-Mallafre V, et al. Leptin and adiponectin, but not IL18, are related with insulin resistance in treated HIV-1-infected patients with lipodystrophy. Cytokine 2012;58:253–60.
39. Vigouroux C, Maachi M, Nguyen TH, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS 2003;17:1503–11.
40. Palmer CS, Hussain T, Duette G, et al. Regulators of glucose metabolism in CD4+ and CD8+ T cells. Int Rev Immunol 2016;35:477–88.
41. Mehta SH, Moore RD, Thomas DL, et al. The effect of HAART and HCV infection on the development of hyperglycemia among HIV-infected persons. J Acquir Immune Defic Syndr 2003;33:577–84.
42. Monroe AK, Dobs AS, Xu X, et al. Sex hormones, insulin resistance, and diabetes mellitus among men with or at risk for HIV infection. J Acquir Immune Defic Syndr 2011;58:173–80.
43. Rotger M, Gsponer T, Martinez R, et al. Impact of single nucleotide polymorphisms and of clinical risk factors on new-onset diabetes mellitus in HIV-infected individuals. Clin Infect Dis 2010;51:1090–8.
44. Ledergerber B, Furrer H, Rickenbach M, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007;45:111–9.
45. Hruz PW. Molecular mechanisms for insulin resistance in treated HIV-infection. Best practice & research. Clin Endocrin Metab 2011;25:459–68.
46. Brown TT, Li X, Cole SR, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS 2005;19:1375–83.
47. Cossarizza A, Moyle G. Antiretroviral nucleoside and nucleotide analogues and mitochondria. AIDS 2004;18:137–51.
48. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 2005;307:384-7.
49. McComsey GA, Paulsen DM, Lonergan JT, et al. Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. AIDS 2005;19:15–23.
50. Venhoff N, Setzer B, Melkaoui K, Walker UA. Mitochondrial toxicity of tenofovir, emtricitabine and abacavir alone and in combination with additional nucleoside reverse transcriptase inhibitors. Antivir Ther 2007;12:1075–85.
51. Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 2009;23:1109–18.
52. Aberg JA, Tebas P, Overton ET, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retrovir 2012;28:1184–95.
53. Overton ET, Tebas P, Coate B, et al. Effects of once-daily darunavir/ritonavir versus atazanavir/ritonavir on insulin sensitivity in HIV-infected persons over 48 weeks: results of an exploratory substudy of METABOLIK, a phase 4, randomized trial. HIV Clin Trials 2016;17:72–7.
54. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–42.
55. McComsey G, Jiang Y, Erlandson KM, et al. Rosuvastatin improves hip bone mineral density but worsens insulin resistance. Boston, MA: Conference on Retroviruses and Opportunistic Infections; 2014.
56. Lichtenstein K DR, Wood K, et al. Statin use is associated with incident diabetes mellitus among patients in the HIV Outpatient Study. Atlanta, GA: Conference on Retroviruses and Opportunistic Infections; 2013.
57. Spagnuolo V GL, Poli A, et al. Association between statin use and type 2 diabetes mellitus occurrence among HIV-1+ patients receiving ART. Atlanta, GA: Conference on Retroviruses and Opportunistic Infections; 2013.
58. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol 2012;60:1231–8.
59. Ridker PM, Pradhan A, MacFadyen JG, et al. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–71.
60. Bloomgarden ZT, Handelsman Y. Approaches to treatment 2: Comparison of American Association of Clinical Endocrinologists (AACE) and American Diabetes Association (ADA) type 2 diabetes treatment guidelines. J Diabetes 2016;8:4–6.
61. Kim PS, Woods C, Georgoff P, et al. A1C underestimates glycemia in HIV infection. Diabetes Care 2009;32:1591–3.
62. Diop ME, Bastard JP, Meunier N, et al. Inappropriately low glycated hemoglobin values and hemolysis in HIV-infected patients. AIDS Res Hum Retrovir 2006;22:1242–7.
63. Polgreen PM, Putz D, Stapleton JT. Inaccurate glycosylated hemoglobin A1C measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infect Dis 2003;37:e53–56.
64. Glesby MJ, Hoover DR, Shi Q, et al. Glycated haemoglobin in diabetic women with and without HIV infection: data from the Women’s Interagency HIV Study. Antivir Ther 2010;15:571–7.
65. Slama L, Palella FJ Jr, Abraham AG, et al. Inaccuracy of haemoglobin A1c among HIV-infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J Antimicrob Chemother 2014;69:3360–7.
66. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–96.
67. Look ARG, Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–83.
68. Hajjar J, Habra MA, Naing A. Metformin: an old drug with new potential. Expert Opin Investig Drugs 2013;22:1511–7.
69. Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med 2007;8:420–6.
70. Tivicay prescribing information. Accessed at www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tivicay/pdf/TIVICAY-PI-PIL.PDF.
71. Han JH, Gordon K, Womack JA, et al. Comparative effectiveness of diabetic oral medications among HIV-infected and HIV-uninfected veterans. Diabetes Care 2017;40:218–25.
72. Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006;20:2165–74.
73. Libois A, Clumeck N, Kabeya K, et al. Risk factors of osteopenia in HIV-infected women: no role of antiretroviral therapy. Maturitas 2010;65:51–4.
74. Carr A, Grund B, Neuhaus J, et al. Prevalence of and risk factors for low bone mineral density in untreated HIV infection: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med 2015;16 Suppl 1:137–46.
75. Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 2008;93:3499–504.
76. Young B, Dao CN, Buchacz K, Baker R, Brooks JT, Investigators HIVOS. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000-2006. Clin Infect Dis 2011;52:1061–8.
77. Rodriguez M, Daniels B, Gunawardene S, Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-Positive patients. AIDS Res Hum Retrovir 2009;25:9–14.
78. Teichmann J, Lange U, Discher T, et al. Bone mineral density in human immunodeficiency virus-1 infected men with hypogonadism prior to highly-active-antiretroviral-therapy (HAART). Eur J Med Res 2009;14:59–64.
79. Lo Re V 3rd, Volk J, Newcomb CW, et al. Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/human immunodeficiency virus coinfection. Hepatology 2012;56:1688–98.
80. Maalouf NM, Zhang S, Drechsler H, et al. Hepatitis C co-infection and severity of liver disease as risk factors for osteoporotic fractures among HIV-infected patients. J Bone Miner Res 2013;28:2577–83.
81. Byrne DD, Newcomb CW, Carbonari DM, et al. Increased risk of hip fracture associated with dually treated HIV/hepatitis B virus coinfection. J Viral Hepat 2015;22:936–47.
82. Gilsanz V, Chalfant J, Mo AO, et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 2009;94:3387–93.
83. Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 2009;122:409–14.
84. Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem 2003;278:48251–8.
85. Gazzola L, Bellistri GM, Tincati C, et al. Association between peripheral T-Lymphocyte activation and impaired bone mineral density in HIV-infected patients. J Transl Med 2013;11:51.
86. Clerici M, Shearer GM. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today 1993;14:107–11.
87. Chew N, Tan E, Li L, Lim R. HIV-1 tat and rev upregulates osteoclast bone resorption. J Int AIDS Soc 2014;17(4 Suppl 3):19724.
88. McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis 2010;51:937–46.
89. Panayiotopoulos A, Bhat N, Bhangoo A. Bone and vitamin D metabolism in HIV. Rev Endocr Metab Disord 2013;14:119–25.
90. Fakruddin JM, Laurence J. Interactions among human immunodeficiency virus (HIV)-1, interferon-gamma and receptor of activated NF-kappa B ligand (RANKL): implications for HIV pathogenesis. Clin Exp Immunol 2004;137:538–45.
91. Li Y, Toraldo G, Li A, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 2007;109:3839–48.
92. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008;93:861–8.
93. Huang JS, Hughes MD, Riddler SA, Haubrich RH, Aids Clinical Trials Group AST. Bone mineral density effects of randomized regimen and nucleoside reverse transcriptase inhibitor selection from ACTG A5142. HIV Clin Trials 2013;145:224–34.
94. McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011;203:1791–801.
95. Schafer JJ, Manlangit K, Squires KE. Bone health and human immunodeficiency virus infection. Pharmacotherapy 2013;33:665–82.
96. Mateo L, Holgado S, Marinoso ML, et al. Hypophosphatemic osteomalacia induced by tenofovir in HIV-infected patients. Clin Rheumatol 2016;35:1271–9.
97. Brown TT, Moser C, Currier JS, et al. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis 2015;212:1241–9.
98. Ofotokun I, Titanji K, Vikulina T, et al. Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun 2015;6:8282.
99. Ofotokun I, Titanji K, Vunnava A, et al. Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS 2016;30:405–14.
100. Stephens KI, Rubinsztain L, Payan J, et al. Dual-energy x-ray absorptiometry and calculated FRAX risk scores may underestimate osteoporotic fracture risk in vitamin d-deficient veterans with HIV infection. Endocr Pract 2016;22:440–6.
101. Mirza F, Canalis E. Management of endocrine disease: Secondary osteoporosis: pathophysiology and management. Eur J Endocrinol 2015;173:R131–151.
102. Brown TT, Hoy J, Borderi M, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis 2015;60:1242–51.
103. Mazzotta E, Ursini T, Agostinone A, et al. Prevalence and predictors of low bone mineral density and fragility fractures among HIV-infected patients at one Italian center after universal DXA screening: sensitivity and specificity of current guidelines on bone mineral density management. AIDS Patient Care STDS 2015;29:169–80.
104. Calmy A, Chevalley T, Delhumeau C, et al. Long-term HIV infection and antiretroviral therapy are associated with bone microstructure alterations in premenopausal women. Osteoporos Int 2013;24:1843–52.
105. Yin MT, Lund E, Shah J, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS 2014;28:345–53.
106. Lo Re V, 3rd, Lynn K, Stumm ER, et al. Structural bone deficits in HIV/HCV-coinfected, HCV-monoinfected, and HIV-monoinfected women. J Infect Dis 2015;212:924–33.
107. Allavena C, Delpierre C, Cuzin L, et al. High frequency of vitamin D deficiency in HIV-infected patients: effects of HIV-related factors and antiretroviral drugs. J Antimicrob Chemother 2012;67:2222–30.
108. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30.
109. Mondy K, Powderly WG, Claxton SA, et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr 2005;38:426–31.
110. McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007;21:2473–82.
111. Guaraldi G, Orlando G, Madeddu G, et al. Alendronate reduces bone resorption in HIV-associated osteopenia/osteoporosis. HIV Clin Trials 2004;5:269–77.
112. Negredo E, Bonjoch A, Perez-Alvarez N, et al. Comparison of two different strategies of treatment with zoledronate in HIV-infected patients with low bone mineral density: single dose versus two doses in 2 years. HIV Med 2015;16:441–8.
113. Bolland MJ, Grey A, Horne AM, et al. Effects of intravenous zoledronate on bone turnover and bone density persist for at least five years in HIV-infected men. J Clin Endocrinol Metab 2012;97:1922–8.
114. Pinzone MR, Moreno S, Cacopardo B, Nunnari G. Is there enough evidence to use bisphosphonates in HIV-infected patients? A systematic review and meta-analysis. AIDS Rev 2014;16:213–22.
115. Ofotokun I, Titanji K, Lahiri CD, et al. A Single-dose zoledronic acid infusion prevents antiretroviral therapy-induced bone loss in treatment-naive HIV-infected patients: a phase IIb trial. Clin Infect Dis 2016;63:663–71.
116. Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 2008;43:1115–21.
1. UNAIDS. Fact sheet - Latest statistics on the status of the AIDS epidemic. Accessed 10 Nov 2017 at http://www.unaids.org/en/resources/fact-sheet.
2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 10 Nov 2017 at https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
3. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One 2013;8:e81355.
4. Pedersen KK, Pedersen M, Troseid M, et al. Microbial translocation in HIV infection is associated with dyslipidemia, insulin resistance, and risk of myocardial infarction. J Acquir Immune Defic Syndr 2013;64:425–33.
5. Strategies for Management of Antiretroviral Therapy Study Group, El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–96.
6. Chastain DB, Henderson H, Stover KR. Epidemiology and management of antiretroviral-associated cardiovascular disease. Open AIDS J 2015;9:23–37.
7. Group ISS, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
8. Moyle GJ, Orkin C, Fisher M, et al. A randomized comparative trial of continued abacavir/lamivudine plus efavirenz or replacement with efavirenz/emtricitabine/tenofovir DF in hypercholesterolemic HIV-1 infected individuals. PloS One 2015;10:e0116297.
9. Mothe B, Perez I, Domingo P, et al. HIV-1 infection in subjects older than 70: a multicenter cross-sectional assessment in Catalonia, Spain. Curr HIV Res 2009;7:597–600.
10. Calvo M, Martinez E. Update on metabolic issues in HIV patients. Curr Opin HIV AIDS 2014;9:332–9.
11. Gillard BK, Raya JL, Ruiz-Esponda R, et al. Impaired lipoprotein processing in HIV patients on antiretroviral therapy: aberrant high-density lipoprotein lipids, stability, and function. Arterioscler Thromb Vasc Biol 2013;33:1714-21.
12. Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58:1–10.
13. Calza L, Colangeli V, Manfredi R, et al. Clinical management of dyslipidaemia associated with combination antiretroviral therapy in HIV-infected patients. J Antimicrob Chemother 2016;71:1451–65.
14. Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS 2003;17:1179–93.
15. Husain NE, Ahmed MH. Managing dyslipidemia in HIV/AIDS patients: challenges and solutions. HIV/AIDS (Auckl) 2015;7:1–10.
16. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. Accessed at www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
17. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015;385:2606–15.
18. Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2014;67:52–8.
19. Eron JJ, Young B, Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 2010;375:396–407.
20. Quercia R, Roberts J, Martin-Carpenter L, Zala C. Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks. Clin Drug Investig 2015;35:211–9.
21. Lennox JL, DeJesus E, Berger DS, et al. Raltegravir versus efavirenz regimens in treatment-naive hiv-1–infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010;55:39–48.
22. Nayor M, Vasan RS. Recent update to the US cholesterol treatment guidelines. a comparison with international guidelines. Circulation 2016;133:1795–806.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934.
24. Mitka M. Exploring statins to decrease HIV-related heart disease risk. JAMA 2015;314:657–9.
25. Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Diseases Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003;37:613–27.
26. Milazzo L, Menzaghi B, Corvasce S, et al. Safety of statin therapy in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr 2007;46:258–60.
27. Hernandez-Romieu AC, Garg S, Rosenberg ES, et al. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diab Res Care 2017;5:e000304.
28. Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–84.
29. De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008;31:1224–9.
30. Rasmussen LD, Mathiesen ER, Kronborg G, et al. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PloS One 2012;7:e44575.
31. Polsky S, Floris-Moore M, Schoenbaum EE, et al. Incident hyperglycaemia among older adults with or at-risk for HIV infection. Antivir Ther 2011;16:181–8.
32. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–12.
33. Galli L, Salpietro S, Pellicciotta G, et al. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur J Epidemiol 2012;27:657–65.
34. Howard AA, Hoover DR, Anastos K, et al. The effects of opiate use and hepatitis C virus infection on risk of diabetes mellitus in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr 2010;54:152–9.
35. Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–34.
36. Hadigan C, Kattakuzhy S. Diabetes mellitus type 2 and abnormal glucose metabolism in the setting of human immunodeficiency virus. Endocrin Metab Clin North Am 2014;43:685–96.
37. Butt AA, Fultz SL, Kwoh CK, Kelley D, et al. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology 2004;40:115–9.
38. Veloso S, Escote X, Ceperuelo-Mallafre V, et al. Leptin and adiponectin, but not IL18, are related with insulin resistance in treated HIV-1-infected patients with lipodystrophy. Cytokine 2012;58:253–60.
39. Vigouroux C, Maachi M, Nguyen TH, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS 2003;17:1503–11.
40. Palmer CS, Hussain T, Duette G, et al. Regulators of glucose metabolism in CD4+ and CD8+ T cells. Int Rev Immunol 2016;35:477–88.
41. Mehta SH, Moore RD, Thomas DL, et al. The effect of HAART and HCV infection on the development of hyperglycemia among HIV-infected persons. J Acquir Immune Defic Syndr 2003;33:577–84.
42. Monroe AK, Dobs AS, Xu X, et al. Sex hormones, insulin resistance, and diabetes mellitus among men with or at risk for HIV infection. J Acquir Immune Defic Syndr 2011;58:173–80.
43. Rotger M, Gsponer T, Martinez R, et al. Impact of single nucleotide polymorphisms and of clinical risk factors on new-onset diabetes mellitus in HIV-infected individuals. Clin Infect Dis 2010;51:1090–8.
44. Ledergerber B, Furrer H, Rickenbach M, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007;45:111–9.
45. Hruz PW. Molecular mechanisms for insulin resistance in treated HIV-infection. Best practice & research. Clin Endocrin Metab 2011;25:459–68.
46. Brown TT, Li X, Cole SR, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS 2005;19:1375–83.
47. Cossarizza A, Moyle G. Antiretroviral nucleoside and nucleotide analogues and mitochondria. AIDS 2004;18:137–51.
48. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 2005;307:384-7.
49. McComsey GA, Paulsen DM, Lonergan JT, et al. Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. AIDS 2005;19:15–23.
50. Venhoff N, Setzer B, Melkaoui K, Walker UA. Mitochondrial toxicity of tenofovir, emtricitabine and abacavir alone and in combination with additional nucleoside reverse transcriptase inhibitors. Antivir Ther 2007;12:1075–85.
51. Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 2009;23:1109–18.
52. Aberg JA, Tebas P, Overton ET, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retrovir 2012;28:1184–95.
53. Overton ET, Tebas P, Coate B, et al. Effects of once-daily darunavir/ritonavir versus atazanavir/ritonavir on insulin sensitivity in HIV-infected persons over 48 weeks: results of an exploratory substudy of METABOLIK, a phase 4, randomized trial. HIV Clin Trials 2016;17:72–7.
54. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–42.
55. McComsey G, Jiang Y, Erlandson KM, et al. Rosuvastatin improves hip bone mineral density but worsens insulin resistance. Boston, MA: Conference on Retroviruses and Opportunistic Infections; 2014.
56. Lichtenstein K DR, Wood K, et al. Statin use is associated with incident diabetes mellitus among patients in the HIV Outpatient Study. Atlanta, GA: Conference on Retroviruses and Opportunistic Infections; 2013.
57. Spagnuolo V GL, Poli A, et al. Association between statin use and type 2 diabetes mellitus occurrence among HIV-1+ patients receiving ART. Atlanta, GA: Conference on Retroviruses and Opportunistic Infections; 2013.
58. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol 2012;60:1231–8.
59. Ridker PM, Pradhan A, MacFadyen JG, et al. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–71.
60. Bloomgarden ZT, Handelsman Y. Approaches to treatment 2: Comparison of American Association of Clinical Endocrinologists (AACE) and American Diabetes Association (ADA) type 2 diabetes treatment guidelines. J Diabetes 2016;8:4–6.
61. Kim PS, Woods C, Georgoff P, et al. A1C underestimates glycemia in HIV infection. Diabetes Care 2009;32:1591–3.
62. Diop ME, Bastard JP, Meunier N, et al. Inappropriately low glycated hemoglobin values and hemolysis in HIV-infected patients. AIDS Res Hum Retrovir 2006;22:1242–7.
63. Polgreen PM, Putz D, Stapleton JT. Inaccurate glycosylated hemoglobin A1C measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infect Dis 2003;37:e53–56.
64. Glesby MJ, Hoover DR, Shi Q, et al. Glycated haemoglobin in diabetic women with and without HIV infection: data from the Women’s Interagency HIV Study. Antivir Ther 2010;15:571–7.
65. Slama L, Palella FJ Jr, Abraham AG, et al. Inaccuracy of haemoglobin A1c among HIV-infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J Antimicrob Chemother 2014;69:3360–7.
66. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–96.
67. Look ARG, Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–83.
68. Hajjar J, Habra MA, Naing A. Metformin: an old drug with new potential. Expert Opin Investig Drugs 2013;22:1511–7.
69. Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med 2007;8:420–6.
70. Tivicay prescribing information. Accessed at www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tivicay/pdf/TIVICAY-PI-PIL.PDF.
71. Han JH, Gordon K, Womack JA, et al. Comparative effectiveness of diabetic oral medications among HIV-infected and HIV-uninfected veterans. Diabetes Care 2017;40:218–25.
72. Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006;20:2165–74.
73. Libois A, Clumeck N, Kabeya K, et al. Risk factors of osteopenia in HIV-infected women: no role of antiretroviral therapy. Maturitas 2010;65:51–4.
74. Carr A, Grund B, Neuhaus J, et al. Prevalence of and risk factors for low bone mineral density in untreated HIV infection: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med 2015;16 Suppl 1:137–46.
75. Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 2008;93:3499–504.
76. Young B, Dao CN, Buchacz K, Baker R, Brooks JT, Investigators HIVOS. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000-2006. Clin Infect Dis 2011;52:1061–8.
77. Rodriguez M, Daniels B, Gunawardene S, Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-Positive patients. AIDS Res Hum Retrovir 2009;25:9–14.
78. Teichmann J, Lange U, Discher T, et al. Bone mineral density in human immunodeficiency virus-1 infected men with hypogonadism prior to highly-active-antiretroviral-therapy (HAART). Eur J Med Res 2009;14:59–64.
79. Lo Re V 3rd, Volk J, Newcomb CW, et al. Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/human immunodeficiency virus coinfection. Hepatology 2012;56:1688–98.
80. Maalouf NM, Zhang S, Drechsler H, et al. Hepatitis C co-infection and severity of liver disease as risk factors for osteoporotic fractures among HIV-infected patients. J Bone Miner Res 2013;28:2577–83.
81. Byrne DD, Newcomb CW, Carbonari DM, et al. Increased risk of hip fracture associated with dually treated HIV/hepatitis B virus coinfection. J Viral Hepat 2015;22:936–47.
82. Gilsanz V, Chalfant J, Mo AO, et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 2009;94:3387–93.
83. Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 2009;122:409–14.
84. Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem 2003;278:48251–8.
85. Gazzola L, Bellistri GM, Tincati C, et al. Association between peripheral T-Lymphocyte activation and impaired bone mineral density in HIV-infected patients. J Transl Med 2013;11:51.
86. Clerici M, Shearer GM. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today 1993;14:107–11.
87. Chew N, Tan E, Li L, Lim R. HIV-1 tat and rev upregulates osteoclast bone resorption. J Int AIDS Soc 2014;17(4 Suppl 3):19724.
88. McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis 2010;51:937–46.
89. Panayiotopoulos A, Bhat N, Bhangoo A. Bone and vitamin D metabolism in HIV. Rev Endocr Metab Disord 2013;14:119–25.
90. Fakruddin JM, Laurence J. Interactions among human immunodeficiency virus (HIV)-1, interferon-gamma and receptor of activated NF-kappa B ligand (RANKL): implications for HIV pathogenesis. Clin Exp Immunol 2004;137:538–45.
91. Li Y, Toraldo G, Li A, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 2007;109:3839–48.
92. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008;93:861–8.
93. Huang JS, Hughes MD, Riddler SA, Haubrich RH, Aids Clinical Trials Group AST. Bone mineral density effects of randomized regimen and nucleoside reverse transcriptase inhibitor selection from ACTG A5142. HIV Clin Trials 2013;145:224–34.
94. McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011;203:1791–801.
95. Schafer JJ, Manlangit K, Squires KE. Bone health and human immunodeficiency virus infection. Pharmacotherapy 2013;33:665–82.
96. Mateo L, Holgado S, Marinoso ML, et al. Hypophosphatemic osteomalacia induced by tenofovir in HIV-infected patients. Clin Rheumatol 2016;35:1271–9.
97. Brown TT, Moser C, Currier JS, et al. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis 2015;212:1241–9.
98. Ofotokun I, Titanji K, Vikulina T, et al. Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun 2015;6:8282.
99. Ofotokun I, Titanji K, Vunnava A, et al. Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS 2016;30:405–14.
100. Stephens KI, Rubinsztain L, Payan J, et al. Dual-energy x-ray absorptiometry and calculated FRAX risk scores may underestimate osteoporotic fracture risk in vitamin d-deficient veterans with HIV infection. Endocr Pract 2016;22:440–6.
101. Mirza F, Canalis E. Management of endocrine disease: Secondary osteoporosis: pathophysiology and management. Eur J Endocrinol 2015;173:R131–151.
102. Brown TT, Hoy J, Borderi M, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis 2015;60:1242–51.
103. Mazzotta E, Ursini T, Agostinone A, et al. Prevalence and predictors of low bone mineral density and fragility fractures among HIV-infected patients at one Italian center after universal DXA screening: sensitivity and specificity of current guidelines on bone mineral density management. AIDS Patient Care STDS 2015;29:169–80.
104. Calmy A, Chevalley T, Delhumeau C, et al. Long-term HIV infection and antiretroviral therapy are associated with bone microstructure alterations in premenopausal women. Osteoporos Int 2013;24:1843–52.
105. Yin MT, Lund E, Shah J, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS 2014;28:345–53.
106. Lo Re V, 3rd, Lynn K, Stumm ER, et al. Structural bone deficits in HIV/HCV-coinfected, HCV-monoinfected, and HIV-monoinfected women. J Infect Dis 2015;212:924–33.
107. Allavena C, Delpierre C, Cuzin L, et al. High frequency of vitamin D deficiency in HIV-infected patients: effects of HIV-related factors and antiretroviral drugs. J Antimicrob Chemother 2012;67:2222–30.
108. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30.
109. Mondy K, Powderly WG, Claxton SA, et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr 2005;38:426–31.
110. McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007;21:2473–82.
111. Guaraldi G, Orlando G, Madeddu G, et al. Alendronate reduces bone resorption in HIV-associated osteopenia/osteoporosis. HIV Clin Trials 2004;5:269–77.
112. Negredo E, Bonjoch A, Perez-Alvarez N, et al. Comparison of two different strategies of treatment with zoledronate in HIV-infected patients with low bone mineral density: single dose versus two doses in 2 years. HIV Med 2015;16:441–8.
113. Bolland MJ, Grey A, Horne AM, et al. Effects of intravenous zoledronate on bone turnover and bone density persist for at least five years in HIV-infected men. J Clin Endocrinol Metab 2012;97:1922–8.
114. Pinzone MR, Moreno S, Cacopardo B, Nunnari G. Is there enough evidence to use bisphosphonates in HIV-infected patients? A systematic review and meta-analysis. AIDS Rev 2014;16:213–22.
115. Ofotokun I, Titanji K, Lahiri CD, et al. A Single-dose zoledronic acid infusion prevents antiretroviral therapy-induced bone loss in treatment-naive HIV-infected patients: a phase IIb trial. Clin Infect Dis 2016;63:663–71.
116. Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 2008;43:1115–21.