User login
What gastroenterologists need to know about the 2024 Medicare payment rules
Medicare Physician Fee Schedule (MPFS) Final Rule
Cuts to physician payments continue: The final calendar year (CY) 2024 MPFS conversion factor will be $32.7442, a cut of approximately 3.4% from CY 2023, unless Congress acts. The reduction is the result of several factors, including the statutory base payment update of 0 percent, the reduction in assistance provided by the Consolidated Appropriations Act, 2023 (from 2.5% for 2023 to 1.25% for 2024), and budget neutrality adjustments of –2.18 percent resulting from CMS’ finalized policies.
New add-on code for complex care: CMS is finalizing complexity add-on code, G2211 (Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition or a complex condition), that it originally proposed in 2018 rulemaking. CMS noted that G2211 cannot be used with an office and outpatient E/M procedure reported with modifier –25. CMS further clarified that the add-on code “is not intended for use by a professional whose relationship with the patient is of a discrete, routine, or time-limited nature ...” CMS further stated, “The inherent complexity that this code (G2211) captures is not in the clinical condition itself ... but rather the cognitive load of the continued responsibility of being the focal point for all needed services for this patient.” For gastroenterologists, it is reasonable to assume G2211 could be reported for care of patients with complex, chronic conditions such as inflammatory bowel disease (IBD), celiac disease, and/or chronic liver disease.
CMS to align split (or shared) visit policy with CPT rules: Originally, CMS proposed to again delay “through at least December 31, 2024” its planned implementation of defining the “substantive portion” of a split/shared visit as more than half of the total time. However, after the American Medical Association’s CPT Editorial Panel, the body responsible for maintaining the CPT code set, issued new guidelines for split (or shared) services CMS decided to finalize the following policy to align with those guidelines: “Substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit, or a substantive part of the medical decision making except as otherwise provided in this paragraph. For critical care visits, substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit.”
While the CPT guidance states, “If code selection is based on total time on the date of the encounter, the service is reported by the professional who spent the majority of the face-to-face or non-face-to-face time performing the service,” this direction does not appear in the finalized CMS language.
CMS has extended Telehealth flexibility provisions through Dec. 31, 2024:
- Reporting of Home Address — CMS will continue to permit distant site practitioners to use their currently enrolled practice location instead of their home address when providing telehealth services from their home through CY 2024.
- Place of Service (POS) for Medicare Telehealth Services — Beginning in CY 2024, claims billed with POS 10 (Telehealth Provided in Patient’s Home) will be paid at the non-facility rate, and claims billed with POS 02 (Telehealth Provided Other than in Patient’s Home) will be paid at the facility rate. CMS also clarified that modifier –95 should be used when the clinician is in the hospital and the patient is at home.
- Direct Supervision with Virtual Presence — CMS will continue to define direct supervision to permit the presence and “immediate availability” of the supervising practitioner through real-time audio and visual interactive telecommunications through CY 2024.
- Supervision of Residents in Teaching Settings — CMS will allow teaching physicians to have a virtual presence (to continue to include real-time audio and video observation by the teaching physician) in all teaching settings, but only in clinical instances when the service is furnished virtually, through CY 2024.
- Telephone E/M Services — CMS will continue to pay for CPT codes for telephone assessment and management services (99441-99443) through CY 2024.
Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgery Center (ASC) Final Rule
Hospital and ASC payments will increase: Conversion factors will increase 3.1% to $87.38 for hospitals and $53.51 for ASCs that meet applicable quality reporting requirements.
Hospital payments for Peroral Endoscopic Myotomy (POEM) increase: The GI societies successfully advocated for a 67% increase to the facility payment for POEM. To better align with the procedure’s cost, CMS will place CPT code 43497 for POEM into a higher-level Ambulatory Payment Classification (APC) (5331 — Complex GI procedures) with a facility payment of $5,435.83.
Cuts to hospital payments for some Level 3 upper GI procedures: CMS has finalized moving the following GI CPT codes that had previously been assigned to APC 5303 (Level 3 Upper GI Procedures — $3,260.69) to APC 5302 (Level 2 Upper GI Procedures — $1,814.88) without explanation and against advice from AGA and the GI societies. This will result in payment cuts of 44% to hospitals.
- 43252 (EGD, flexible transoral with optical microscopy)
- 43263 (ERCP with pressure measurement, sphincter of Oddi)
- 43275 (ERCP, remove foreign body/stent biliary/pancreatic duct)
GI Comprehensive APC complexity adjustments: Based on a cost and volume threshold, CMS sometimes makes payment adjustments for Comprehensive APCs when two procedures are performed together. In response to comments received, CMS is adding the following procedures to the list of code combinations eligible for an increased payment via the Complexity Adjustment.
- CPT 43270 (EGD, ablate tumor polyp/lesion with dilation and wire)
- CPT 43252 (EGD, flexible transoral with optical microscopy)
For more information, see 2024 the payment rules summary and payment tables at https://gastro.org/practice-resources/reimbursement.
The Coverage and Reimbursement Subcommittee members have no conflicts of interest.
Medicare Physician Fee Schedule (MPFS) Final Rule
Cuts to physician payments continue: The final calendar year (CY) 2024 MPFS conversion factor will be $32.7442, a cut of approximately 3.4% from CY 2023, unless Congress acts. The reduction is the result of several factors, including the statutory base payment update of 0 percent, the reduction in assistance provided by the Consolidated Appropriations Act, 2023 (from 2.5% for 2023 to 1.25% for 2024), and budget neutrality adjustments of –2.18 percent resulting from CMS’ finalized policies.
New add-on code for complex care: CMS is finalizing complexity add-on code, G2211 (Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition or a complex condition), that it originally proposed in 2018 rulemaking. CMS noted that G2211 cannot be used with an office and outpatient E/M procedure reported with modifier –25. CMS further clarified that the add-on code “is not intended for use by a professional whose relationship with the patient is of a discrete, routine, or time-limited nature ...” CMS further stated, “The inherent complexity that this code (G2211) captures is not in the clinical condition itself ... but rather the cognitive load of the continued responsibility of being the focal point for all needed services for this patient.” For gastroenterologists, it is reasonable to assume G2211 could be reported for care of patients with complex, chronic conditions such as inflammatory bowel disease (IBD), celiac disease, and/or chronic liver disease.
CMS to align split (or shared) visit policy with CPT rules: Originally, CMS proposed to again delay “through at least December 31, 2024” its planned implementation of defining the “substantive portion” of a split/shared visit as more than half of the total time. However, after the American Medical Association’s CPT Editorial Panel, the body responsible for maintaining the CPT code set, issued new guidelines for split (or shared) services CMS decided to finalize the following policy to align with those guidelines: “Substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit, or a substantive part of the medical decision making except as otherwise provided in this paragraph. For critical care visits, substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit.”
While the CPT guidance states, “If code selection is based on total time on the date of the encounter, the service is reported by the professional who spent the majority of the face-to-face or non-face-to-face time performing the service,” this direction does not appear in the finalized CMS language.
CMS has extended Telehealth flexibility provisions through Dec. 31, 2024:
- Reporting of Home Address — CMS will continue to permit distant site practitioners to use their currently enrolled practice location instead of their home address when providing telehealth services from their home through CY 2024.
- Place of Service (POS) for Medicare Telehealth Services — Beginning in CY 2024, claims billed with POS 10 (Telehealth Provided in Patient’s Home) will be paid at the non-facility rate, and claims billed with POS 02 (Telehealth Provided Other than in Patient’s Home) will be paid at the facility rate. CMS also clarified that modifier –95 should be used when the clinician is in the hospital and the patient is at home.
- Direct Supervision with Virtual Presence — CMS will continue to define direct supervision to permit the presence and “immediate availability” of the supervising practitioner through real-time audio and visual interactive telecommunications through CY 2024.
- Supervision of Residents in Teaching Settings — CMS will allow teaching physicians to have a virtual presence (to continue to include real-time audio and video observation by the teaching physician) in all teaching settings, but only in clinical instances when the service is furnished virtually, through CY 2024.
- Telephone E/M Services — CMS will continue to pay for CPT codes for telephone assessment and management services (99441-99443) through CY 2024.
Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgery Center (ASC) Final Rule
Hospital and ASC payments will increase: Conversion factors will increase 3.1% to $87.38 for hospitals and $53.51 for ASCs that meet applicable quality reporting requirements.
Hospital payments for Peroral Endoscopic Myotomy (POEM) increase: The GI societies successfully advocated for a 67% increase to the facility payment for POEM. To better align with the procedure’s cost, CMS will place CPT code 43497 for POEM into a higher-level Ambulatory Payment Classification (APC) (5331 — Complex GI procedures) with a facility payment of $5,435.83.
Cuts to hospital payments for some Level 3 upper GI procedures: CMS has finalized moving the following GI CPT codes that had previously been assigned to APC 5303 (Level 3 Upper GI Procedures — $3,260.69) to APC 5302 (Level 2 Upper GI Procedures — $1,814.88) without explanation and against advice from AGA and the GI societies. This will result in payment cuts of 44% to hospitals.
- 43252 (EGD, flexible transoral with optical microscopy)
- 43263 (ERCP with pressure measurement, sphincter of Oddi)
- 43275 (ERCP, remove foreign body/stent biliary/pancreatic duct)
GI Comprehensive APC complexity adjustments: Based on a cost and volume threshold, CMS sometimes makes payment adjustments for Comprehensive APCs when two procedures are performed together. In response to comments received, CMS is adding the following procedures to the list of code combinations eligible for an increased payment via the Complexity Adjustment.
- CPT 43270 (EGD, ablate tumor polyp/lesion with dilation and wire)
- CPT 43252 (EGD, flexible transoral with optical microscopy)
For more information, see 2024 the payment rules summary and payment tables at https://gastro.org/practice-resources/reimbursement.
The Coverage and Reimbursement Subcommittee members have no conflicts of interest.
Medicare Physician Fee Schedule (MPFS) Final Rule
Cuts to physician payments continue: The final calendar year (CY) 2024 MPFS conversion factor will be $32.7442, a cut of approximately 3.4% from CY 2023, unless Congress acts. The reduction is the result of several factors, including the statutory base payment update of 0 percent, the reduction in assistance provided by the Consolidated Appropriations Act, 2023 (from 2.5% for 2023 to 1.25% for 2024), and budget neutrality adjustments of –2.18 percent resulting from CMS’ finalized policies.
New add-on code for complex care: CMS is finalizing complexity add-on code, G2211 (Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition or a complex condition), that it originally proposed in 2018 rulemaking. CMS noted that G2211 cannot be used with an office and outpatient E/M procedure reported with modifier –25. CMS further clarified that the add-on code “is not intended for use by a professional whose relationship with the patient is of a discrete, routine, or time-limited nature ...” CMS further stated, “The inherent complexity that this code (G2211) captures is not in the clinical condition itself ... but rather the cognitive load of the continued responsibility of being the focal point for all needed services for this patient.” For gastroenterologists, it is reasonable to assume G2211 could be reported for care of patients with complex, chronic conditions such as inflammatory bowel disease (IBD), celiac disease, and/or chronic liver disease.
CMS to align split (or shared) visit policy with CPT rules: Originally, CMS proposed to again delay “through at least December 31, 2024” its planned implementation of defining the “substantive portion” of a split/shared visit as more than half of the total time. However, after the American Medical Association’s CPT Editorial Panel, the body responsible for maintaining the CPT code set, issued new guidelines for split (or shared) services CMS decided to finalize the following policy to align with those guidelines: “Substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit, or a substantive part of the medical decision making except as otherwise provided in this paragraph. For critical care visits, substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit.”
While the CPT guidance states, “If code selection is based on total time on the date of the encounter, the service is reported by the professional who spent the majority of the face-to-face or non-face-to-face time performing the service,” this direction does not appear in the finalized CMS language.
CMS has extended Telehealth flexibility provisions through Dec. 31, 2024:
- Reporting of Home Address — CMS will continue to permit distant site practitioners to use their currently enrolled practice location instead of their home address when providing telehealth services from their home through CY 2024.
- Place of Service (POS) for Medicare Telehealth Services — Beginning in CY 2024, claims billed with POS 10 (Telehealth Provided in Patient’s Home) will be paid at the non-facility rate, and claims billed with POS 02 (Telehealth Provided Other than in Patient’s Home) will be paid at the facility rate. CMS also clarified that modifier –95 should be used when the clinician is in the hospital and the patient is at home.
- Direct Supervision with Virtual Presence — CMS will continue to define direct supervision to permit the presence and “immediate availability” of the supervising practitioner through real-time audio and visual interactive telecommunications through CY 2024.
- Supervision of Residents in Teaching Settings — CMS will allow teaching physicians to have a virtual presence (to continue to include real-time audio and video observation by the teaching physician) in all teaching settings, but only in clinical instances when the service is furnished virtually, through CY 2024.
- Telephone E/M Services — CMS will continue to pay for CPT codes for telephone assessment and management services (99441-99443) through CY 2024.
Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgery Center (ASC) Final Rule
Hospital and ASC payments will increase: Conversion factors will increase 3.1% to $87.38 for hospitals and $53.51 for ASCs that meet applicable quality reporting requirements.
Hospital payments for Peroral Endoscopic Myotomy (POEM) increase: The GI societies successfully advocated for a 67% increase to the facility payment for POEM. To better align with the procedure’s cost, CMS will place CPT code 43497 for POEM into a higher-level Ambulatory Payment Classification (APC) (5331 — Complex GI procedures) with a facility payment of $5,435.83.
Cuts to hospital payments for some Level 3 upper GI procedures: CMS has finalized moving the following GI CPT codes that had previously been assigned to APC 5303 (Level 3 Upper GI Procedures — $3,260.69) to APC 5302 (Level 2 Upper GI Procedures — $1,814.88) without explanation and against advice from AGA and the GI societies. This will result in payment cuts of 44% to hospitals.
- 43252 (EGD, flexible transoral with optical microscopy)
- 43263 (ERCP with pressure measurement, sphincter of Oddi)
- 43275 (ERCP, remove foreign body/stent biliary/pancreatic duct)
GI Comprehensive APC complexity adjustments: Based on a cost and volume threshold, CMS sometimes makes payment adjustments for Comprehensive APCs when two procedures are performed together. In response to comments received, CMS is adding the following procedures to the list of code combinations eligible for an increased payment via the Complexity Adjustment.
- CPT 43270 (EGD, ablate tumor polyp/lesion with dilation and wire)
- CPT 43252 (EGD, flexible transoral with optical microscopy)
For more information, see 2024 the payment rules summary and payment tables at https://gastro.org/practice-resources/reimbursement.
The Coverage and Reimbursement Subcommittee members have no conflicts of interest.
Painful Growing Nodule on the Right Calf
The Diagnosis: Merkel Cell Carcinoma
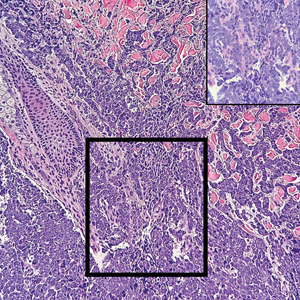
Multiple diagnoses should be considered for a small, round, blue cell neoplasm of the skin, including both primary and metastatic entities. In our patient, histopathology revealed sheets and nests of infiltrative neoplastic cells with dispersed chromatin, minimal cytoplasm, and multiple mitoses (quiz image 1).1 The lesional cells were in the dermis and superficial subcutaneous tissue but did not appear to be arising from the epidermis. Lymphovascular invasion also was evident on additional sections. Metastatic disease was identified in 3 sentinel lymph nodes from the right inguinal and right iliac regions. These features were compatible with a diagnosis of Merkel cell carcinoma (MCC).
Merkel cell carcinoma is a rare malignant neuroendocrine cutaneous tumor with a worldwide incidence of 0.1 to 1.6 cases per 100,000 individuals annually.2 The typical patient is older than 75 years with fair skin and a history of extensive sun exposure. Immunocompromised individuals are predisposed and more susceptible to infection with the Merkel cell polyomavirus, which promotes oncogenesis in the majority of MCCs. Our patient’s history of combined variable immunodeficiency likely explains her presentation at a younger age.
The prognosis in patients with MCC is poor, with 5-year survival rates of 51% for local disease, 35% for nodal disease, and 14% for systemic metastases. Survival also is reduced in cases with head/ neck primary tumors and polyomavirus-negative tumors, as well as in immunocompromised patients.2 Treatment of resectable MCC consists of Mohs micrographic surgery or wide local excision depending on the patient’s cosmetic concerns. Radiation therapy is recommended for cases with increased risk for recurrence or positive surgical margins, as well as when additional resection is impossible. A study investigating immunotherapy with nivolumab demonstrated complete pathologic response and radiographic tumor regression in nearly half of patients when given 4 weeks prior to surgery.3
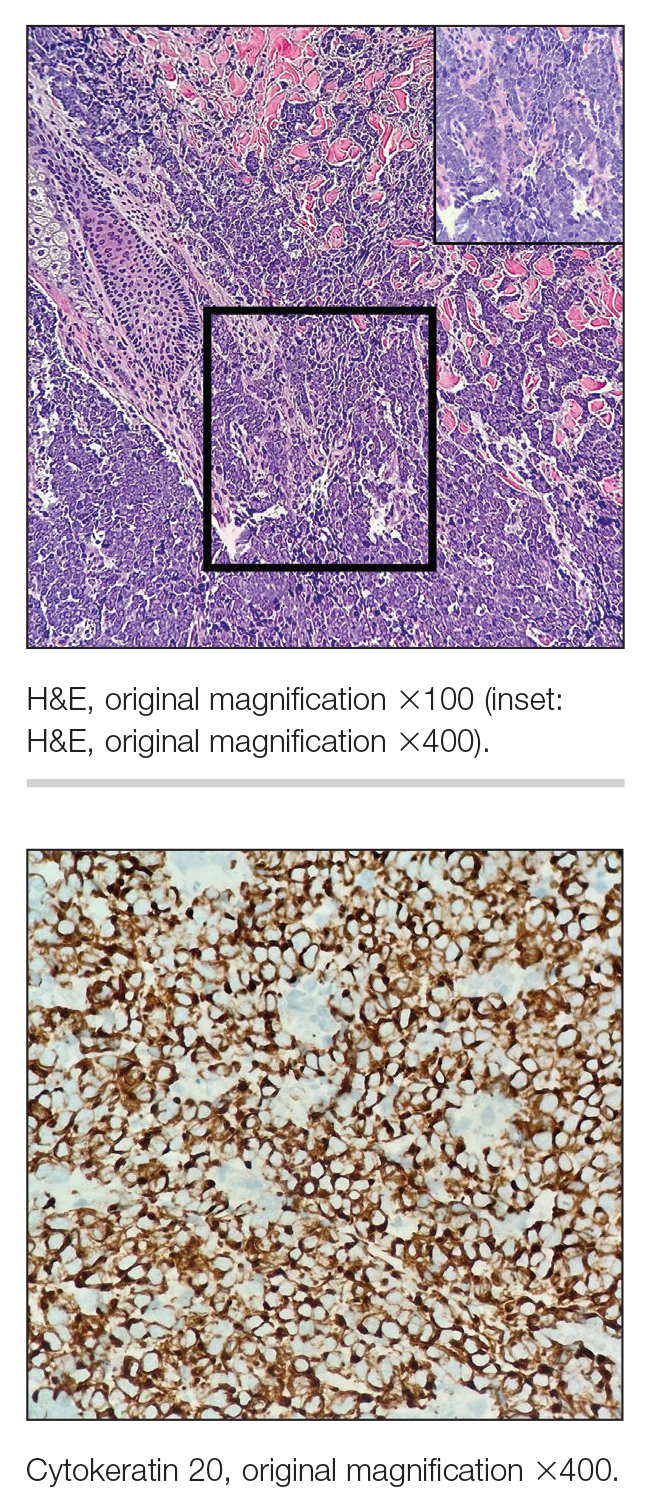
Immunohistochemistry is essential in discerning MCC from other small blue cell tumors. Most MCC cases show positive expression of neuroendocrine markers such as synaptophysin, chromogranin, and insulinomaassociated protein 1. Perinuclear dotlike staining with cytokeratin (CK) 20 (quiz image 2) commonly is seen, but up to 15% of cases may be CK20 negative. Many of these CK20-negative cases also express CK7. This tumor also may stain with paired box 5 (PAX-5), CD99, terminal deoxynucleotidyl transferase, Ber-EP4, and CD1171,4; melanoma stains (ie, human melanoma black [HMB] 45, SRYrelated HMB-box 10 [SOX-10], S-100, melanoma antigen recognized by T-cells 1 [MART-1]) should be negative. However, PAX-5 expression may be a potential pitfall given that B-cell lymphomas also would express that marker and could mimic MCC histologically. Therefore, other universal lymphoid markers such as CD45 should be ordered to rule out this entity. Even with one or a few aberrant stains, a diagnosis of MCC still can be rendered using the histomorphology and the overall staining profile.4 Of prognostic significance, p63 expression is associated with more aggressive tumors, while Bcl-2 expression is favorable, as it offers an additional targeted treatment option.5,6
Basal cell carcinoma (BCC) is linked to excessive sun exposure and is the most common skin cancer. Similar to MCC, it typically is mitotically active and hyperchromatic; however, lymphovascular invasion or metastasis almost never is observed in BCC, whereas approximately one-third of MCC cases have metastasized by the time of diagnosis. Additionally, BCC lacks the perinuclear dotlike staining seen with CK20.2,7 Features present in BCC that are unusual for MCC include peripheral nuclear palisading, mucin, and retraction artifact on paraffin-embedded sections (Figure 1).7
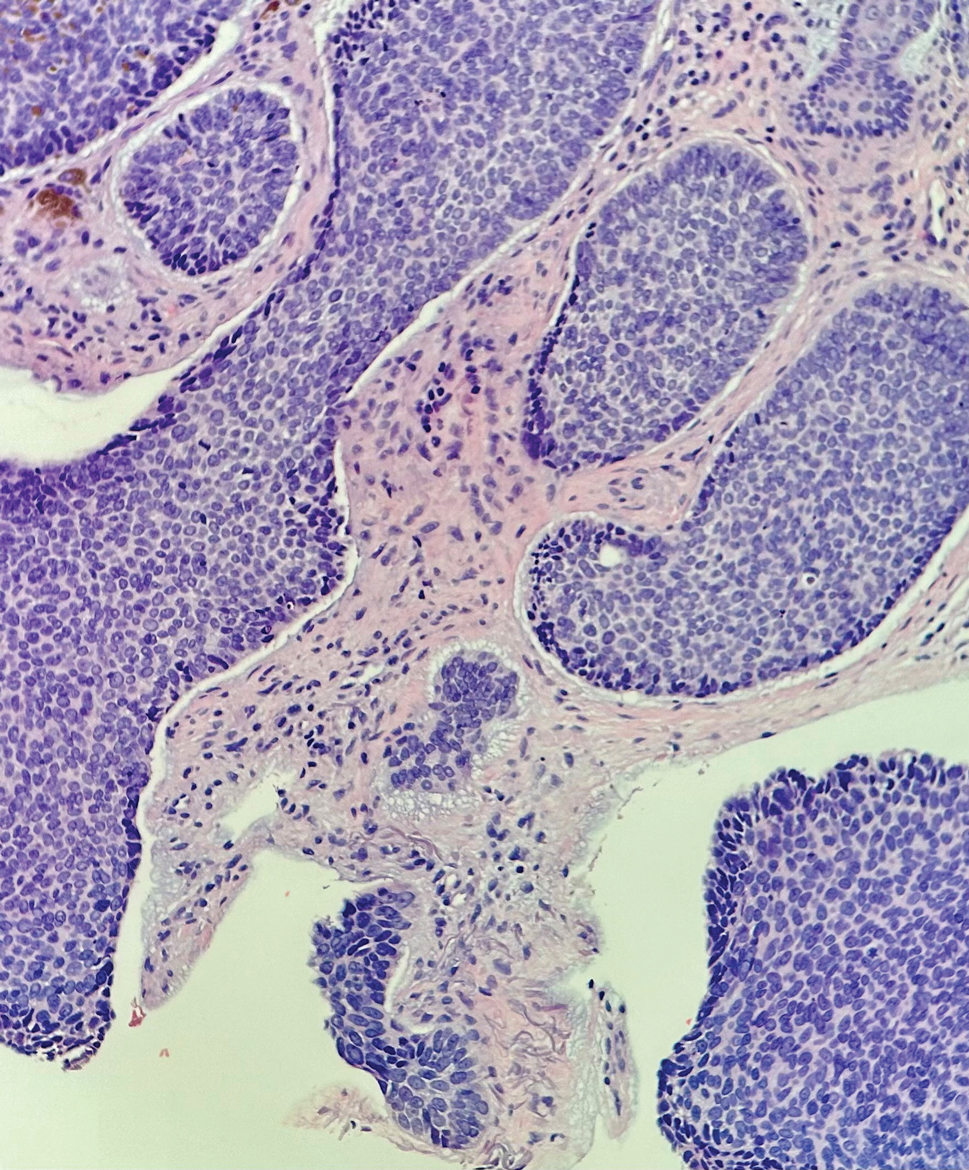
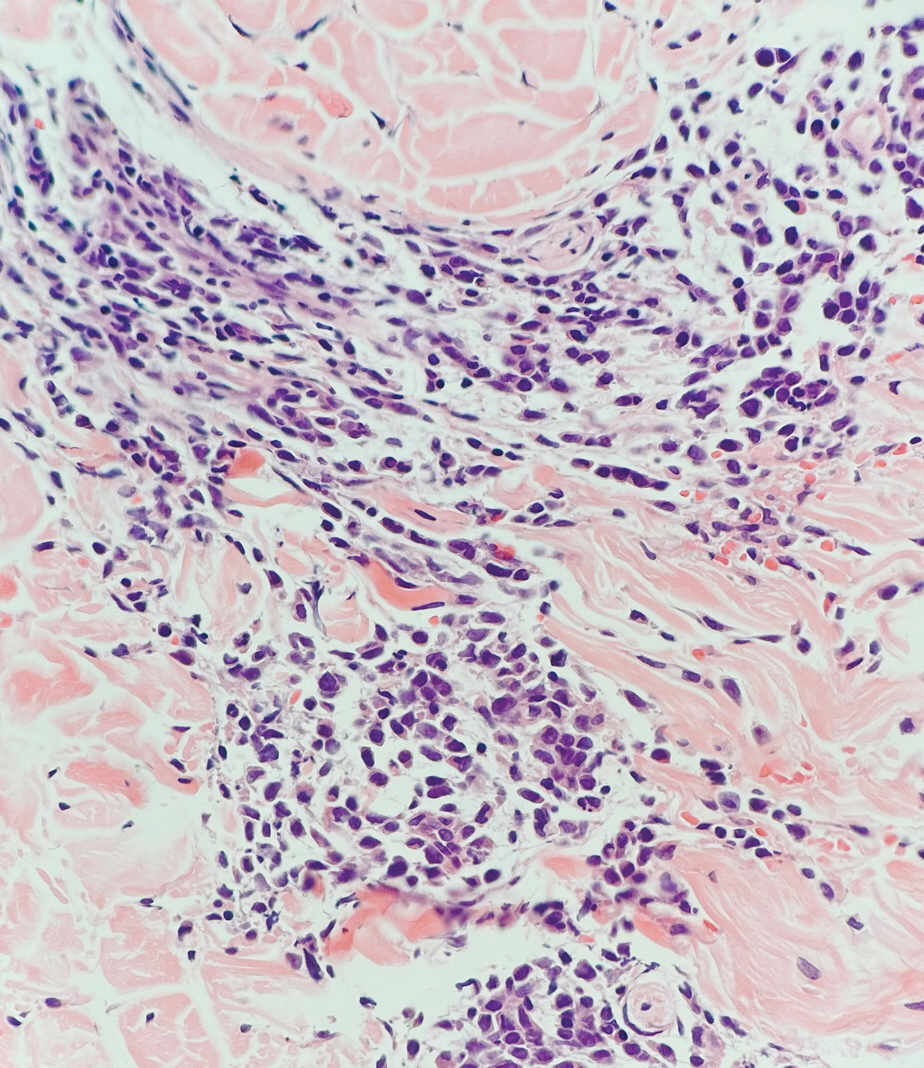
Leukemia cutis (or cutaneous infiltrates of leukemia) commonly displays a perivascular and periadnexal pattern in the dermis and subcutis. These infiltrates of neoplastic leukocytes can congregate into sheets, sometimes with an overlying Grenz zone, or form single-file infiltrates (Figure 2).1,4 The neoplastic cells can be monomorphic or atypical and commonly are susceptible to crush artifact.4 Although the immunohistochemical profile varies depending on the etiology of the underlying leukemia, broad hematologic markers such as CD43 and CD45 are helpful to discern these malignancies from MCC.4
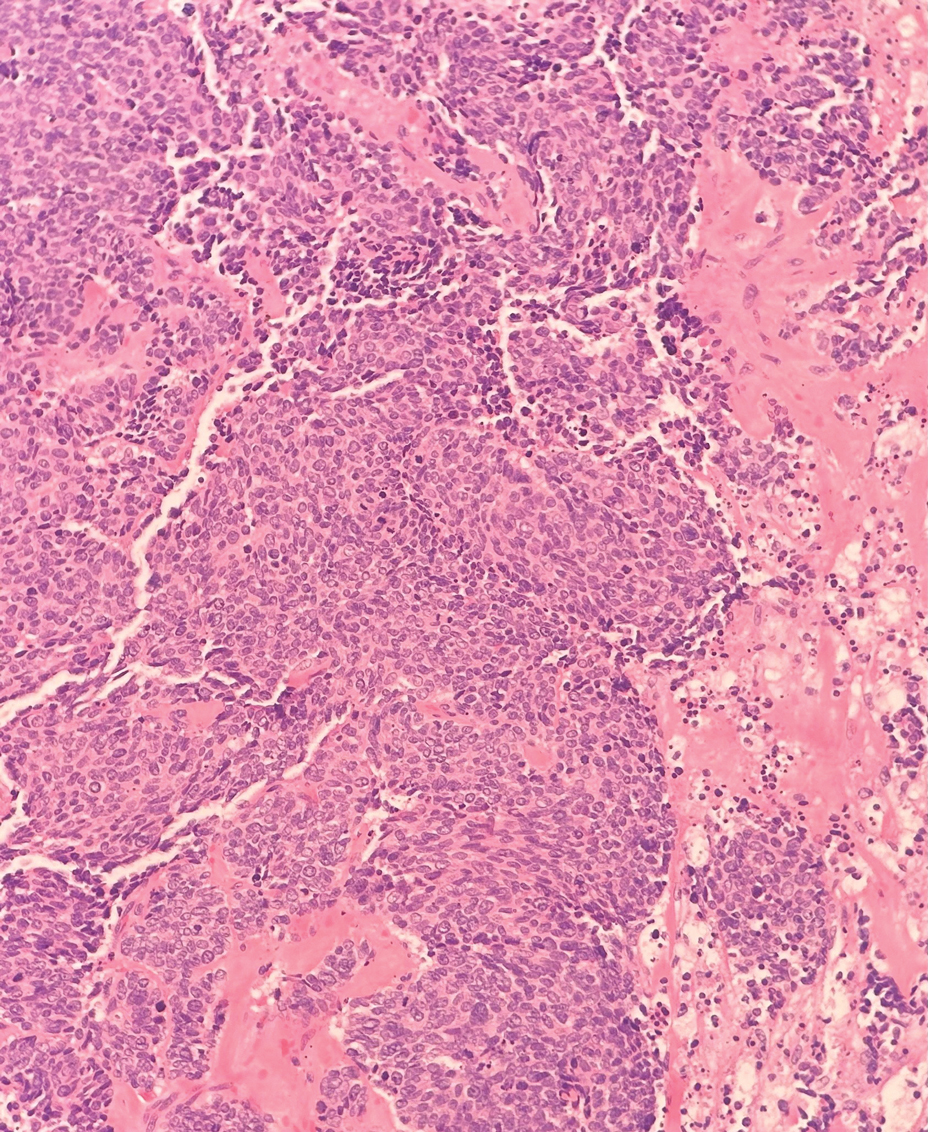
Being neuroendocrine in origin, metastatic small cell carcinoma (Figure 3) strongly mimics MCC histologically and usually stains with synaptophysin, chromogranin, and insulinoma-associated protein 1. Both tumor cells typically exhibit nuclear molding and high mitotic rates. Although small cell carcinoma is more likely to stain with high-molecular-weight cytokeratins (ie, CK7), it is not uncommon for these tumors to express lowmolecular- weight cytokeratins such as CK20. Because most cases originate from the lungs, these lesions should be positive for thyroid transcription factor 1 and negative for PAX-5, whereas MCC would show the reverse for those stains.1 Ultimately, however, clinical correlation with imaging results is the single best methodology for differentiation.
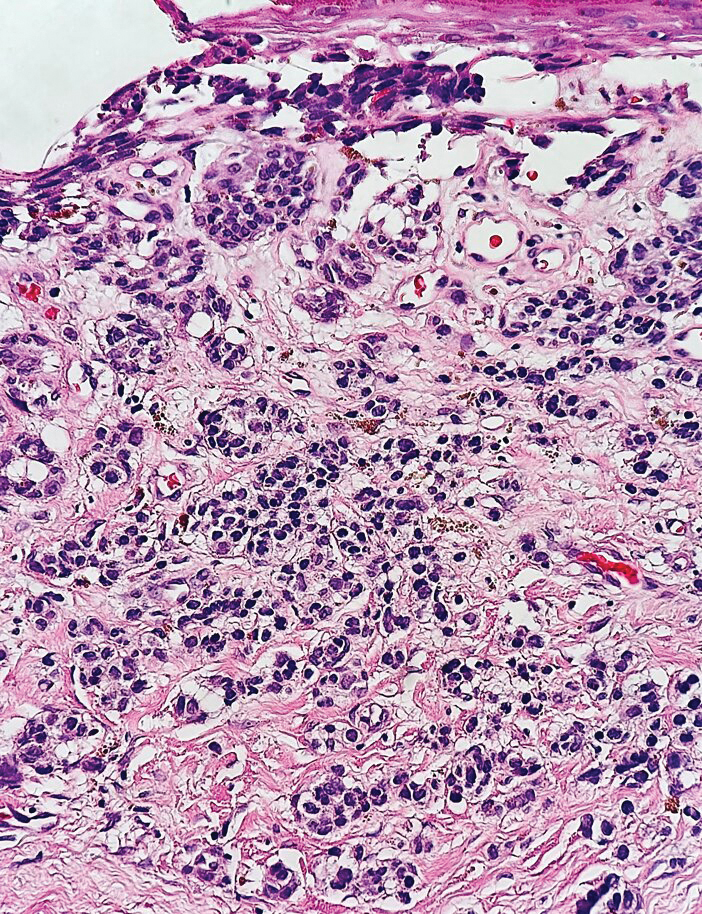
Small cell melanoma, a variant of nevoid melanoma, can strongly resemble an MCC or a lymphoma. Usually located on the scalp or arising from a congenital nevus, small cell melanomas are aggressive and confer an unfavorable prognosis. Histologically, they consist of nests to sheets of atypical cells within the epidermis and dermis. These cells typically exhibit hyperchromatic nuclei, minimal cytoplasm, and frequent mitoses (Figure 4). Furthermore, the cells do not display maturation based on depth.8 These tumors usually are positive for HMB45, S-100, MART-1, SOX-10, and tyrosinase, all of which are extremely unlikely to stain an MCC.1
- Patterson JW, Hosler GA. Weedon’s Skin Pathology. 4th ed. Churchill Livingstone/Elsevier; 2016.
- Walsh NM, Cerroni L. Merkel cell carcinoma: a review. J Cutan Pathol. 2021;48:411-421.
- Topalian SL, Bhatia S, Amin A, et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 Trial. J Clin Oncol. 2020;38:2476-2488.
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier; 2021.
- Asioli S, Righi A, Volante M, et al. p63 expression as a new prognostic marker in Merkel cell carcinoma. Cancer. 2007;110:640-647.
- Verhaegen ME, Mangelberger D, Weick JW, et al. Merkel cell carcinoma dependence on Bcl-2 family members for survival. J Invest Dermatol. 2014;134:2241-2250.
- Le MD, O’Steen LH, Cassarino DS. A rare case of CK20/CK7 double negative Merkel cell carcinoma. Am J Dermatopathol. 2017;39:208-211.
- North JP, Bastian BC, Lazar AJ. Melanoma. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin With Clinical Correlations. 5th ed. Elsevier; 2020.
The Diagnosis: Merkel Cell Carcinoma
Multiple diagnoses should be considered for a small, round, blue cell neoplasm of the skin, including both primary and metastatic entities. In our patient, histopathology revealed sheets and nests of infiltrative neoplastic cells with dispersed chromatin, minimal cytoplasm, and multiple mitoses (quiz image 1).1 The lesional cells were in the dermis and superficial subcutaneous tissue but did not appear to be arising from the epidermis. Lymphovascular invasion also was evident on additional sections. Metastatic disease was identified in 3 sentinel lymph nodes from the right inguinal and right iliac regions. These features were compatible with a diagnosis of Merkel cell carcinoma (MCC).
Merkel cell carcinoma is a rare malignant neuroendocrine cutaneous tumor with a worldwide incidence of 0.1 to 1.6 cases per 100,000 individuals annually.2 The typical patient is older than 75 years with fair skin and a history of extensive sun exposure. Immunocompromised individuals are predisposed and more susceptible to infection with the Merkel cell polyomavirus, which promotes oncogenesis in the majority of MCCs. Our patient’s history of combined variable immunodeficiency likely explains her presentation at a younger age.
The prognosis in patients with MCC is poor, with 5-year survival rates of 51% for local disease, 35% for nodal disease, and 14% for systemic metastases. Survival also is reduced in cases with head/ neck primary tumors and polyomavirus-negative tumors, as well as in immunocompromised patients.2 Treatment of resectable MCC consists of Mohs micrographic surgery or wide local excision depending on the patient’s cosmetic concerns. Radiation therapy is recommended for cases with increased risk for recurrence or positive surgical margins, as well as when additional resection is impossible. A study investigating immunotherapy with nivolumab demonstrated complete pathologic response and radiographic tumor regression in nearly half of patients when given 4 weeks prior to surgery.3
Immunohistochemistry is essential in discerning MCC from other small blue cell tumors. Most MCC cases show positive expression of neuroendocrine markers such as synaptophysin, chromogranin, and insulinomaassociated protein 1. Perinuclear dotlike staining with cytokeratin (CK) 20 (quiz image 2) commonly is seen, but up to 15% of cases may be CK20 negative. Many of these CK20-negative cases also express CK7. This tumor also may stain with paired box 5 (PAX-5), CD99, terminal deoxynucleotidyl transferase, Ber-EP4, and CD1171,4; melanoma stains (ie, human melanoma black [HMB] 45, SRYrelated HMB-box 10 [SOX-10], S-100, melanoma antigen recognized by T-cells 1 [MART-1]) should be negative. However, PAX-5 expression may be a potential pitfall given that B-cell lymphomas also would express that marker and could mimic MCC histologically. Therefore, other universal lymphoid markers such as CD45 should be ordered to rule out this entity. Even with one or a few aberrant stains, a diagnosis of MCC still can be rendered using the histomorphology and the overall staining profile.4 Of prognostic significance, p63 expression is associated with more aggressive tumors, while Bcl-2 expression is favorable, as it offers an additional targeted treatment option.5,6
Basal cell carcinoma (BCC) is linked to excessive sun exposure and is the most common skin cancer. Similar to MCC, it typically is mitotically active and hyperchromatic; however, lymphovascular invasion or metastasis almost never is observed in BCC, whereas approximately one-third of MCC cases have metastasized by the time of diagnosis. Additionally, BCC lacks the perinuclear dotlike staining seen with CK20.2,7 Features present in BCC that are unusual for MCC include peripheral nuclear palisading, mucin, and retraction artifact on paraffin-embedded sections (Figure 1).7

Leukemia cutis (or cutaneous infiltrates of leukemia) commonly displays a perivascular and periadnexal pattern in the dermis and subcutis. These infiltrates of neoplastic leukocytes can congregate into sheets, sometimes with an overlying Grenz zone, or form single-file infiltrates (Figure 2).1,4 The neoplastic cells can be monomorphic or atypical and commonly are susceptible to crush artifact.4 Although the immunohistochemical profile varies depending on the etiology of the underlying leukemia, broad hematologic markers such as CD43 and CD45 are helpful to discern these malignancies from MCC.4

Being neuroendocrine in origin, metastatic small cell carcinoma (Figure 3) strongly mimics MCC histologically and usually stains with synaptophysin, chromogranin, and insulinoma-associated protein 1. Both tumor cells typically exhibit nuclear molding and high mitotic rates. Although small cell carcinoma is more likely to stain with high-molecular-weight cytokeratins (ie, CK7), it is not uncommon for these tumors to express lowmolecular- weight cytokeratins such as CK20. Because most cases originate from the lungs, these lesions should be positive for thyroid transcription factor 1 and negative for PAX-5, whereas MCC would show the reverse for those stains.1 Ultimately, however, clinical correlation with imaging results is the single best methodology for differentiation.

Small cell melanoma, a variant of nevoid melanoma, can strongly resemble an MCC or a lymphoma. Usually located on the scalp or arising from a congenital nevus, small cell melanomas are aggressive and confer an unfavorable prognosis. Histologically, they consist of nests to sheets of atypical cells within the epidermis and dermis. These cells typically exhibit hyperchromatic nuclei, minimal cytoplasm, and frequent mitoses (Figure 4). Furthermore, the cells do not display maturation based on depth.8 These tumors usually are positive for HMB45, S-100, MART-1, SOX-10, and tyrosinase, all of which are extremely unlikely to stain an MCC.1

The Diagnosis: Merkel Cell Carcinoma
Multiple diagnoses should be considered for a small, round, blue cell neoplasm of the skin, including both primary and metastatic entities. In our patient, histopathology revealed sheets and nests of infiltrative neoplastic cells with dispersed chromatin, minimal cytoplasm, and multiple mitoses (quiz image 1).1 The lesional cells were in the dermis and superficial subcutaneous tissue but did not appear to be arising from the epidermis. Lymphovascular invasion also was evident on additional sections. Metastatic disease was identified in 3 sentinel lymph nodes from the right inguinal and right iliac regions. These features were compatible with a diagnosis of Merkel cell carcinoma (MCC).
Merkel cell carcinoma is a rare malignant neuroendocrine cutaneous tumor with a worldwide incidence of 0.1 to 1.6 cases per 100,000 individuals annually.2 The typical patient is older than 75 years with fair skin and a history of extensive sun exposure. Immunocompromised individuals are predisposed and more susceptible to infection with the Merkel cell polyomavirus, which promotes oncogenesis in the majority of MCCs. Our patient’s history of combined variable immunodeficiency likely explains her presentation at a younger age.
The prognosis in patients with MCC is poor, with 5-year survival rates of 51% for local disease, 35% for nodal disease, and 14% for systemic metastases. Survival also is reduced in cases with head/ neck primary tumors and polyomavirus-negative tumors, as well as in immunocompromised patients.2 Treatment of resectable MCC consists of Mohs micrographic surgery or wide local excision depending on the patient’s cosmetic concerns. Radiation therapy is recommended for cases with increased risk for recurrence or positive surgical margins, as well as when additional resection is impossible. A study investigating immunotherapy with nivolumab demonstrated complete pathologic response and radiographic tumor regression in nearly half of patients when given 4 weeks prior to surgery.3
Immunohistochemistry is essential in discerning MCC from other small blue cell tumors. Most MCC cases show positive expression of neuroendocrine markers such as synaptophysin, chromogranin, and insulinomaassociated protein 1. Perinuclear dotlike staining with cytokeratin (CK) 20 (quiz image 2) commonly is seen, but up to 15% of cases may be CK20 negative. Many of these CK20-negative cases also express CK7. This tumor also may stain with paired box 5 (PAX-5), CD99, terminal deoxynucleotidyl transferase, Ber-EP4, and CD1171,4; melanoma stains (ie, human melanoma black [HMB] 45, SRYrelated HMB-box 10 [SOX-10], S-100, melanoma antigen recognized by T-cells 1 [MART-1]) should be negative. However, PAX-5 expression may be a potential pitfall given that B-cell lymphomas also would express that marker and could mimic MCC histologically. Therefore, other universal lymphoid markers such as CD45 should be ordered to rule out this entity. Even with one or a few aberrant stains, a diagnosis of MCC still can be rendered using the histomorphology and the overall staining profile.4 Of prognostic significance, p63 expression is associated with more aggressive tumors, while Bcl-2 expression is favorable, as it offers an additional targeted treatment option.5,6
Basal cell carcinoma (BCC) is linked to excessive sun exposure and is the most common skin cancer. Similar to MCC, it typically is mitotically active and hyperchromatic; however, lymphovascular invasion or metastasis almost never is observed in BCC, whereas approximately one-third of MCC cases have metastasized by the time of diagnosis. Additionally, BCC lacks the perinuclear dotlike staining seen with CK20.2,7 Features present in BCC that are unusual for MCC include peripheral nuclear palisading, mucin, and retraction artifact on paraffin-embedded sections (Figure 1).7

Leukemia cutis (or cutaneous infiltrates of leukemia) commonly displays a perivascular and periadnexal pattern in the dermis and subcutis. These infiltrates of neoplastic leukocytes can congregate into sheets, sometimes with an overlying Grenz zone, or form single-file infiltrates (Figure 2).1,4 The neoplastic cells can be monomorphic or atypical and commonly are susceptible to crush artifact.4 Although the immunohistochemical profile varies depending on the etiology of the underlying leukemia, broad hematologic markers such as CD43 and CD45 are helpful to discern these malignancies from MCC.4

Being neuroendocrine in origin, metastatic small cell carcinoma (Figure 3) strongly mimics MCC histologically and usually stains with synaptophysin, chromogranin, and insulinoma-associated protein 1. Both tumor cells typically exhibit nuclear molding and high mitotic rates. Although small cell carcinoma is more likely to stain with high-molecular-weight cytokeratins (ie, CK7), it is not uncommon for these tumors to express lowmolecular- weight cytokeratins such as CK20. Because most cases originate from the lungs, these lesions should be positive for thyroid transcription factor 1 and negative for PAX-5, whereas MCC would show the reverse for those stains.1 Ultimately, however, clinical correlation with imaging results is the single best methodology for differentiation.

Small cell melanoma, a variant of nevoid melanoma, can strongly resemble an MCC or a lymphoma. Usually located on the scalp or arising from a congenital nevus, small cell melanomas are aggressive and confer an unfavorable prognosis. Histologically, they consist of nests to sheets of atypical cells within the epidermis and dermis. These cells typically exhibit hyperchromatic nuclei, minimal cytoplasm, and frequent mitoses (Figure 4). Furthermore, the cells do not display maturation based on depth.8 These tumors usually are positive for HMB45, S-100, MART-1, SOX-10, and tyrosinase, all of which are extremely unlikely to stain an MCC.1

- Patterson JW, Hosler GA. Weedon’s Skin Pathology. 4th ed. Churchill Livingstone/Elsevier; 2016.
- Walsh NM, Cerroni L. Merkel cell carcinoma: a review. J Cutan Pathol. 2021;48:411-421.
- Topalian SL, Bhatia S, Amin A, et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 Trial. J Clin Oncol. 2020;38:2476-2488.
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier; 2021.
- Asioli S, Righi A, Volante M, et al. p63 expression as a new prognostic marker in Merkel cell carcinoma. Cancer. 2007;110:640-647.
- Verhaegen ME, Mangelberger D, Weick JW, et al. Merkel cell carcinoma dependence on Bcl-2 family members for survival. J Invest Dermatol. 2014;134:2241-2250.
- Le MD, O’Steen LH, Cassarino DS. A rare case of CK20/CK7 double negative Merkel cell carcinoma. Am J Dermatopathol. 2017;39:208-211.
- North JP, Bastian BC, Lazar AJ. Melanoma. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin With Clinical Correlations. 5th ed. Elsevier; 2020.
- Patterson JW, Hosler GA. Weedon’s Skin Pathology. 4th ed. Churchill Livingstone/Elsevier; 2016.
- Walsh NM, Cerroni L. Merkel cell carcinoma: a review. J Cutan Pathol. 2021;48:411-421.
- Topalian SL, Bhatia S, Amin A, et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 Trial. J Clin Oncol. 2020;38:2476-2488.
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier; 2021.
- Asioli S, Righi A, Volante M, et al. p63 expression as a new prognostic marker in Merkel cell carcinoma. Cancer. 2007;110:640-647.
- Verhaegen ME, Mangelberger D, Weick JW, et al. Merkel cell carcinoma dependence on Bcl-2 family members for survival. J Invest Dermatol. 2014;134:2241-2250.
- Le MD, O’Steen LH, Cassarino DS. A rare case of CK20/CK7 double negative Merkel cell carcinoma. Am J Dermatopathol. 2017;39:208-211.
- North JP, Bastian BC, Lazar AJ. Melanoma. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin With Clinical Correlations. 5th ed. Elsevier; 2020.
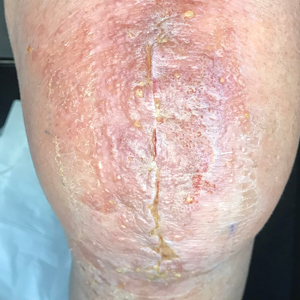
A 47-year-old woman with a history of combined variable immunodeficiency presented with a 2.6×2.4-cm nodule on the lateral aspect of the right calf that was first noticed 2 years prior as a smaller nodule. It increased in size and became painful to touch over the last 3 to 4 months. Following diagnostic biopsy, the nodule was removed by wide local excision and was tan-brown on gross dissection. The lesion showed dotlike perinuclear positivity with cytokeratin 20 immunostaining. Positron emission tomography–computed tomography showed no evidence of lung lesions. A complete blood cell count was within reference range.
Memorial and Honorary Gifts: A Special Tribute
Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation in honor of your loved one.
- AGA Institute program naming opportunities. Individuals interested in receiving name recognition for selected AGA Institute program can do so by contributing a new, unrestricted gift totaling a designated amount to the AGA Research Foundation.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.foundation.gastro.org.
Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation in honor of your loved one.
- AGA Institute program naming opportunities. Individuals interested in receiving name recognition for selected AGA Institute program can do so by contributing a new, unrestricted gift totaling a designated amount to the AGA Research Foundation.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.foundation.gastro.org.
Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation in honor of your loved one.
- AGA Institute program naming opportunities. Individuals interested in receiving name recognition for selected AGA Institute program can do so by contributing a new, unrestricted gift totaling a designated amount to the AGA Research Foundation.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.foundation.gastro.org.
How to address chemo-related amenorrhea in early breast cancer to help improve quality of life
in a large multicenter French cohort study.
The findings, which showed a particularly increased risk of persistent CRA in older women and those who received adjuvant tamoxifen, can help inform communication, personalized counseling, and supportive care, according to the investigators.
At 1 year after treatment, CRA occurred in 1242 of 1497 women (83.0%) from the prospective, longitudinal Cancers Toxicity Study (CANTO). The rates at years 2 and 4 after treatment were 72.5% and 66.1%, respectively, Rayan Kabirian, MD, of Gustave Roussy, Villejuif, France, and Sorbonne University, Paris, and colleagues reported.
In a quality-of-life analysis conducted among 729 women from the cohort, 416 (57.1%) had persistent CRA, although 11 of 21 women aged 18-34 years who had no menses at year 2 had late menses recovery between years 2 and 4. Those with persistent CRA at year 4, compared with those who had menses recovery at any time, had significantly worse insomnia (mean difference, 9.9 points), worse systemic therapy-related adverse effects (mean difference, 3.0 points), and worse sexual functioning (mean difference, -9.2 points).
Factors associated with greater risk of persistent CRA included receipt versus non-receipt of adjuvant tamoxifen (adjusted odds ratio, 1.97), and hot flashes at diagnosis (aOR, 1.83, and older age versus age 18-34 (aORs, 1.84 for those aged 35-39 years; 5.90 for those aged 40-44 years, and 21.29 for those 45 or older).
The findings were published online November 16 in JAMA Network Open.
The study cohort included 1636 women under age 50 years (mean age of 42.2 years) at the time of diagnosis of stage I to III breast cancer. Outcomes at up to 4 years after diagnosis and enrollment between 2012 and 2017 were reported. QOL was assessed using the European Organization for Research and Treatment of Cancer (EORTC) QOL questionnaires c30 and br23.
“Breast cancer is the most commonly diagnosed tumor in women, and approximately 20% of women with breast cancer are younger than 50 years at diagnosis,” the investigators note, explaining that younger survivors have higher risk of cancer-related symptoms and quality-of-life deterioration. “In particular, treatment-related symptoms linked to the menopausal transition (ie, vasomotor symptoms and sexual problems) represent an important source of distress during and after treatment, highlighting a need to monitor and address survivorship-related problems that are specific to this population.”
The current analysis “helps answer several clinical questions about long-term trajectories of CRA and menses recovery rates by age and about factors associated with higher likelihood of CRA,” they added, noting that the findings have several clinical implications.
For example, premenopausal women should be made aware of the risks associated with chemotherapy-related premature ovarian failure and persistent CRA, and should and receive systematic oncofertility counseling, they argue.
“In addition, in light of data showing possible late [menses] recoveries, contraceptive options should also be clearly discussed,” and “[d]edicated gynecological counseling may help patients who have an inaccurate perception of infertility due to previous exposure to chemotherapy and long-term absence of menses.”
Given that a late menses recovery pattern was also observed in older age groups in the cohort, the investigators noted that choosing the optimal adjuvant endocrine treatment can pose a challenge.
“The absence of menses after completion of chemotherapy should not be used as a proxy for permanent transition to menopause, because it does not represent a reliable surrogate of gonadotoxicity,” they warned. “Adjuvant endocrine treatment choices should be based on a more thorough and comprehensive evaluation, combining absence of menses, assessments of circulating hormone levels, and gynecological ultrasonographic imaging.”
These findings “can inform personalized care pathways targeting patients at higher risk of QOL deterioration associated with a permanent menopausal transition,” they noted, concluding that “[r]isk and duration of CRA, including potential late resumption of menses and its downstream implications for QOL, should be approached using a coordinated biopsychosocial model addressing multiple dimensions of physical, psychological, and social health.
“Proactive management of premenopausal women with early breast cancer undergoing chemotherapy should also include adapted strategies for risk communication, as well as personalized counseling and early supportive care referrals.”
The CANTO study is supported by the French government under the Investment for the Future program managed by the National Research Agency, the Prism project, and the MYPROBE Program. Dr. Kabirian reported having no disclosures.
in a large multicenter French cohort study.
The findings, which showed a particularly increased risk of persistent CRA in older women and those who received adjuvant tamoxifen, can help inform communication, personalized counseling, and supportive care, according to the investigators.
At 1 year after treatment, CRA occurred in 1242 of 1497 women (83.0%) from the prospective, longitudinal Cancers Toxicity Study (CANTO). The rates at years 2 and 4 after treatment were 72.5% and 66.1%, respectively, Rayan Kabirian, MD, of Gustave Roussy, Villejuif, France, and Sorbonne University, Paris, and colleagues reported.
In a quality-of-life analysis conducted among 729 women from the cohort, 416 (57.1%) had persistent CRA, although 11 of 21 women aged 18-34 years who had no menses at year 2 had late menses recovery between years 2 and 4. Those with persistent CRA at year 4, compared with those who had menses recovery at any time, had significantly worse insomnia (mean difference, 9.9 points), worse systemic therapy-related adverse effects (mean difference, 3.0 points), and worse sexual functioning (mean difference, -9.2 points).
Factors associated with greater risk of persistent CRA included receipt versus non-receipt of adjuvant tamoxifen (adjusted odds ratio, 1.97), and hot flashes at diagnosis (aOR, 1.83, and older age versus age 18-34 (aORs, 1.84 for those aged 35-39 years; 5.90 for those aged 40-44 years, and 21.29 for those 45 or older).
The findings were published online November 16 in JAMA Network Open.
The study cohort included 1636 women under age 50 years (mean age of 42.2 years) at the time of diagnosis of stage I to III breast cancer. Outcomes at up to 4 years after diagnosis and enrollment between 2012 and 2017 were reported. QOL was assessed using the European Organization for Research and Treatment of Cancer (EORTC) QOL questionnaires c30 and br23.
“Breast cancer is the most commonly diagnosed tumor in women, and approximately 20% of women with breast cancer are younger than 50 years at diagnosis,” the investigators note, explaining that younger survivors have higher risk of cancer-related symptoms and quality-of-life deterioration. “In particular, treatment-related symptoms linked to the menopausal transition (ie, vasomotor symptoms and sexual problems) represent an important source of distress during and after treatment, highlighting a need to monitor and address survivorship-related problems that are specific to this population.”
The current analysis “helps answer several clinical questions about long-term trajectories of CRA and menses recovery rates by age and about factors associated with higher likelihood of CRA,” they added, noting that the findings have several clinical implications.
For example, premenopausal women should be made aware of the risks associated with chemotherapy-related premature ovarian failure and persistent CRA, and should and receive systematic oncofertility counseling, they argue.
“In addition, in light of data showing possible late [menses] recoveries, contraceptive options should also be clearly discussed,” and “[d]edicated gynecological counseling may help patients who have an inaccurate perception of infertility due to previous exposure to chemotherapy and long-term absence of menses.”
Given that a late menses recovery pattern was also observed in older age groups in the cohort, the investigators noted that choosing the optimal adjuvant endocrine treatment can pose a challenge.
“The absence of menses after completion of chemotherapy should not be used as a proxy for permanent transition to menopause, because it does not represent a reliable surrogate of gonadotoxicity,” they warned. “Adjuvant endocrine treatment choices should be based on a more thorough and comprehensive evaluation, combining absence of menses, assessments of circulating hormone levels, and gynecological ultrasonographic imaging.”
These findings “can inform personalized care pathways targeting patients at higher risk of QOL deterioration associated with a permanent menopausal transition,” they noted, concluding that “[r]isk and duration of CRA, including potential late resumption of menses and its downstream implications for QOL, should be approached using a coordinated biopsychosocial model addressing multiple dimensions of physical, psychological, and social health.
“Proactive management of premenopausal women with early breast cancer undergoing chemotherapy should also include adapted strategies for risk communication, as well as personalized counseling and early supportive care referrals.”
The CANTO study is supported by the French government under the Investment for the Future program managed by the National Research Agency, the Prism project, and the MYPROBE Program. Dr. Kabirian reported having no disclosures.
in a large multicenter French cohort study.
The findings, which showed a particularly increased risk of persistent CRA in older women and those who received adjuvant tamoxifen, can help inform communication, personalized counseling, and supportive care, according to the investigators.
At 1 year after treatment, CRA occurred in 1242 of 1497 women (83.0%) from the prospective, longitudinal Cancers Toxicity Study (CANTO). The rates at years 2 and 4 after treatment were 72.5% and 66.1%, respectively, Rayan Kabirian, MD, of Gustave Roussy, Villejuif, France, and Sorbonne University, Paris, and colleagues reported.
In a quality-of-life analysis conducted among 729 women from the cohort, 416 (57.1%) had persistent CRA, although 11 of 21 women aged 18-34 years who had no menses at year 2 had late menses recovery between years 2 and 4. Those with persistent CRA at year 4, compared with those who had menses recovery at any time, had significantly worse insomnia (mean difference, 9.9 points), worse systemic therapy-related adverse effects (mean difference, 3.0 points), and worse sexual functioning (mean difference, -9.2 points).
Factors associated with greater risk of persistent CRA included receipt versus non-receipt of adjuvant tamoxifen (adjusted odds ratio, 1.97), and hot flashes at diagnosis (aOR, 1.83, and older age versus age 18-34 (aORs, 1.84 for those aged 35-39 years; 5.90 for those aged 40-44 years, and 21.29 for those 45 or older).
The findings were published online November 16 in JAMA Network Open.
The study cohort included 1636 women under age 50 years (mean age of 42.2 years) at the time of diagnosis of stage I to III breast cancer. Outcomes at up to 4 years after diagnosis and enrollment between 2012 and 2017 were reported. QOL was assessed using the European Organization for Research and Treatment of Cancer (EORTC) QOL questionnaires c30 and br23.
“Breast cancer is the most commonly diagnosed tumor in women, and approximately 20% of women with breast cancer are younger than 50 years at diagnosis,” the investigators note, explaining that younger survivors have higher risk of cancer-related symptoms and quality-of-life deterioration. “In particular, treatment-related symptoms linked to the menopausal transition (ie, vasomotor symptoms and sexual problems) represent an important source of distress during and after treatment, highlighting a need to monitor and address survivorship-related problems that are specific to this population.”
The current analysis “helps answer several clinical questions about long-term trajectories of CRA and menses recovery rates by age and about factors associated with higher likelihood of CRA,” they added, noting that the findings have several clinical implications.
For example, premenopausal women should be made aware of the risks associated with chemotherapy-related premature ovarian failure and persistent CRA, and should and receive systematic oncofertility counseling, they argue.
“In addition, in light of data showing possible late [menses] recoveries, contraceptive options should also be clearly discussed,” and “[d]edicated gynecological counseling may help patients who have an inaccurate perception of infertility due to previous exposure to chemotherapy and long-term absence of menses.”
Given that a late menses recovery pattern was also observed in older age groups in the cohort, the investigators noted that choosing the optimal adjuvant endocrine treatment can pose a challenge.
“The absence of menses after completion of chemotherapy should not be used as a proxy for permanent transition to menopause, because it does not represent a reliable surrogate of gonadotoxicity,” they warned. “Adjuvant endocrine treatment choices should be based on a more thorough and comprehensive evaluation, combining absence of menses, assessments of circulating hormone levels, and gynecological ultrasonographic imaging.”
These findings “can inform personalized care pathways targeting patients at higher risk of QOL deterioration associated with a permanent menopausal transition,” they noted, concluding that “[r]isk and duration of CRA, including potential late resumption of menses and its downstream implications for QOL, should be approached using a coordinated biopsychosocial model addressing multiple dimensions of physical, psychological, and social health.
“Proactive management of premenopausal women with early breast cancer undergoing chemotherapy should also include adapted strategies for risk communication, as well as personalized counseling and early supportive care referrals.”
The CANTO study is supported by the French government under the Investment for the Future program managed by the National Research Agency, the Prism project, and the MYPROBE Program. Dr. Kabirian reported having no disclosures.
FROM JAMA NETWORK OPEN
Procedures may ease postmenopausal pain better than drugs
a new study shows.
“This study provides us a better understanding of pain management strategies for pre versus postmenopausal women,” said Tian Yu, MD, who presented the research at the annual pain medicine meeting of the American Society of Regional Anesthesia and Pain Medicine. “With our postmenopausal patients, we may no longer jump the gun and give them a lot of medications; we may first turn to physical therapy or procedural intervention, which they seem to benefit much more from than pharmacological therapy.”Pain perception is a multifaceted phenomenon influenced by age, gender, individual variations, and hormonal changes. Pain management in women, particularly in the context of menopausal status, still lacks consensus.
Menopause primarily results from diminished production of estrogen by the ovaries, leading to spinal and joint pain, hot flashes, night sweats, chronic fatigue, increased osteoclastic activity with a heightened risk for osteoporosis, psychological symptoms, and elevated risk for cardiovascular disease.
For their retrospective cohort study, Dr. Yu, department of anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, and his colleagues looked at 1215 women who had been treated for different chronic pain conditions for at least 3 months. The researchers used a predefined age cutoff of 51 years (considered the national average) to categorize participants as either premenopausal (n = 248) or postmenopausal (n = 967). Pain scores and subjective improvement were assessed after pharmacological and procedural interventions.
According to Dr. Yu, the results revealed distinct patterns in pain scores and response to interventions between the two groups.
Although postmenopausal women initially reported higher mean pain scores upon presentation (8.037 vs 7.613 in premenopausal women), they reported more improvement following intervention (63% vs 59%; P = .029). They responded more favorably to both procedural and pharmacological interventions, but were prescribed muscle relaxants, tricyclic antidepressants, and benzodiazepines less frequently than premenopausal women, Dr. Yu’s group found.
“So even though postmenopausal women had a higher initial pain score, they had better pain improvement after procedural intervention, although they were prescribed fewer pharmacological interventions,” Dr. Yu said.
The fact that postmenopausal women typically are older than women who have not reached menopause could act as a confounding factor in this study in terms of disease prevalence and intervention, Dr. Yu said. Additionally, the study’s reliance on a broad menopausal age cutoff of 51 years may limit the true characterization of menopausal status.
While acknowledging study limitations, the findings suggest a potential shift toward prioritizing nonpharmacological interventions in postmenopausal women. Further investigation into physical therapy and other approaches could provide a more comprehensive understanding of pain management strategies in this population.
“We hope to take these findings into consideration during our practice to better individualize care,” Yu said.
Robert Wenham, MD, MS, chair of gynecologic oncology, Moffitt Cancer Center, Tampa, Florida, who was not involved in the study, said: “Despite the many methodological challenges it has, including using age as a surrogate for menopause, I applaud the authors for investigating how pain and pain management may be individualized for women.”
Dr. Wenham added that he hoped the findings would prompt additional studies “that specifically address populations based on hormonal status and other confounding factors, so that interventional avenues may be identified for clinical trials.”
Dr. Yu and Dr. Wenham report no relevant financial relationships.
A version of this article appeared on Medscape.com.
a new study shows.
“This study provides us a better understanding of pain management strategies for pre versus postmenopausal women,” said Tian Yu, MD, who presented the research at the annual pain medicine meeting of the American Society of Regional Anesthesia and Pain Medicine. “With our postmenopausal patients, we may no longer jump the gun and give them a lot of medications; we may first turn to physical therapy or procedural intervention, which they seem to benefit much more from than pharmacological therapy.”Pain perception is a multifaceted phenomenon influenced by age, gender, individual variations, and hormonal changes. Pain management in women, particularly in the context of menopausal status, still lacks consensus.
Menopause primarily results from diminished production of estrogen by the ovaries, leading to spinal and joint pain, hot flashes, night sweats, chronic fatigue, increased osteoclastic activity with a heightened risk for osteoporosis, psychological symptoms, and elevated risk for cardiovascular disease.
For their retrospective cohort study, Dr. Yu, department of anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, and his colleagues looked at 1215 women who had been treated for different chronic pain conditions for at least 3 months. The researchers used a predefined age cutoff of 51 years (considered the national average) to categorize participants as either premenopausal (n = 248) or postmenopausal (n = 967). Pain scores and subjective improvement were assessed after pharmacological and procedural interventions.
According to Dr. Yu, the results revealed distinct patterns in pain scores and response to interventions between the two groups.
Although postmenopausal women initially reported higher mean pain scores upon presentation (8.037 vs 7.613 in premenopausal women), they reported more improvement following intervention (63% vs 59%; P = .029). They responded more favorably to both procedural and pharmacological interventions, but were prescribed muscle relaxants, tricyclic antidepressants, and benzodiazepines less frequently than premenopausal women, Dr. Yu’s group found.
“So even though postmenopausal women had a higher initial pain score, they had better pain improvement after procedural intervention, although they were prescribed fewer pharmacological interventions,” Dr. Yu said.
The fact that postmenopausal women typically are older than women who have not reached menopause could act as a confounding factor in this study in terms of disease prevalence and intervention, Dr. Yu said. Additionally, the study’s reliance on a broad menopausal age cutoff of 51 years may limit the true characterization of menopausal status.
While acknowledging study limitations, the findings suggest a potential shift toward prioritizing nonpharmacological interventions in postmenopausal women. Further investigation into physical therapy and other approaches could provide a more comprehensive understanding of pain management strategies in this population.
“We hope to take these findings into consideration during our practice to better individualize care,” Yu said.
Robert Wenham, MD, MS, chair of gynecologic oncology, Moffitt Cancer Center, Tampa, Florida, who was not involved in the study, said: “Despite the many methodological challenges it has, including using age as a surrogate for menopause, I applaud the authors for investigating how pain and pain management may be individualized for women.”
Dr. Wenham added that he hoped the findings would prompt additional studies “that specifically address populations based on hormonal status and other confounding factors, so that interventional avenues may be identified for clinical trials.”
Dr. Yu and Dr. Wenham report no relevant financial relationships.
A version of this article appeared on Medscape.com.
a new study shows.
“This study provides us a better understanding of pain management strategies for pre versus postmenopausal women,” said Tian Yu, MD, who presented the research at the annual pain medicine meeting of the American Society of Regional Anesthesia and Pain Medicine. “With our postmenopausal patients, we may no longer jump the gun and give them a lot of medications; we may first turn to physical therapy or procedural intervention, which they seem to benefit much more from than pharmacological therapy.”Pain perception is a multifaceted phenomenon influenced by age, gender, individual variations, and hormonal changes. Pain management in women, particularly in the context of menopausal status, still lacks consensus.
Menopause primarily results from diminished production of estrogen by the ovaries, leading to spinal and joint pain, hot flashes, night sweats, chronic fatigue, increased osteoclastic activity with a heightened risk for osteoporosis, psychological symptoms, and elevated risk for cardiovascular disease.
For their retrospective cohort study, Dr. Yu, department of anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, and his colleagues looked at 1215 women who had been treated for different chronic pain conditions for at least 3 months. The researchers used a predefined age cutoff of 51 years (considered the national average) to categorize participants as either premenopausal (n = 248) or postmenopausal (n = 967). Pain scores and subjective improvement were assessed after pharmacological and procedural interventions.
According to Dr. Yu, the results revealed distinct patterns in pain scores and response to interventions between the two groups.
Although postmenopausal women initially reported higher mean pain scores upon presentation (8.037 vs 7.613 in premenopausal women), they reported more improvement following intervention (63% vs 59%; P = .029). They responded more favorably to both procedural and pharmacological interventions, but were prescribed muscle relaxants, tricyclic antidepressants, and benzodiazepines less frequently than premenopausal women, Dr. Yu’s group found.
“So even though postmenopausal women had a higher initial pain score, they had better pain improvement after procedural intervention, although they were prescribed fewer pharmacological interventions,” Dr. Yu said.
The fact that postmenopausal women typically are older than women who have not reached menopause could act as a confounding factor in this study in terms of disease prevalence and intervention, Dr. Yu said. Additionally, the study’s reliance on a broad menopausal age cutoff of 51 years may limit the true characterization of menopausal status.
While acknowledging study limitations, the findings suggest a potential shift toward prioritizing nonpharmacological interventions in postmenopausal women. Further investigation into physical therapy and other approaches could provide a more comprehensive understanding of pain management strategies in this population.
“We hope to take these findings into consideration during our practice to better individualize care,” Yu said.
Robert Wenham, MD, MS, chair of gynecologic oncology, Moffitt Cancer Center, Tampa, Florida, who was not involved in the study, said: “Despite the many methodological challenges it has, including using age as a surrogate for menopause, I applaud the authors for investigating how pain and pain management may be individualized for women.”
Dr. Wenham added that he hoped the findings would prompt additional studies “that specifically address populations based on hormonal status and other confounding factors, so that interventional avenues may be identified for clinical trials.”
Dr. Yu and Dr. Wenham report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Antireflux surgery may not reduce cancer risk in Barrett’s esophagus
, according to a Nordic retrospective study.
Risk of EAC was higher among patients who underwent surgery, and risk appeared to increase over time, suggesting that postoperative patients should continue to participate in surveillance programs, reported lead author Jesper Lagergren, MD, PhD, of the Karolinska Institutet, Stockholm, and colleagues.
“Antireflux surgery with fundoplication increases the ability of the gastroesophageal anatomic and physiological barrier to prevent reflux, and can thus prevent any carcinogenic gastric content from reaching the esophagus, including both acid and bile,” the investigators wrote in Gastroenterology, noting that surgery reduces esophageal acid exposure to a greater degree than medication. “Antireflux surgery may thus prevent esophageal adenocarcinoma better than antireflux medication.”
Three meta-analyses to date, however, have failed to provide consistent support for this hypothesis.
“Most of the studies included in these meta-analyses came from single centers, were of small sample size, examined only one treatment arm, and had a short or incomplete follow-up, and ... were hampered by heterogeneity among the included studies,” they noted.
For the present study, Dr. Lagergren and colleagues analyzed national registry data from 33,939 patients with Barrett’s esophagus in Denmark, Finland, Norway, and Sweden. Out of this group, 542 patients (1.6%) had undergone antireflux surgery, while the remainder were managed with antireflux medication.
In both groups, approximately two-thirds of the patients were men. The median age at enrollment was about a decade higher in the medication group (66 vs. 54 years), and this group also tended to have more comorbidities.
After a follow-up period as long as 32 years, the absolute rates of EAC were 1.3% and 2.6% in the medication and surgery groups, respectively. Multivariate analysis, with adjustments for sex, age, year, comorbidities, and age, revealed that postsurgical patients had a 90% increased risk of EAC (hazard ratio [HR], 1.9; 95% CI, 1.1-3.5), versus patients treated with antireflux medication alone.
The relatively higher risk of EAC appeared to increase over time, based on a nonsignificant hazard ratio of 1.8 during the 1- to 4-year follow-up period (HR, 1.8; 95% CI, 0.6-5.0), versus a significant, fourfold risk elevation during the 10- to 32-year follow-up period (HR, 4.4; 95% CI, 1.4-13.5).
“In this cohort of patients with Barrett’s esophagus, the risk of esophageal adenocarcinoma did not decrease after antireflux surgery compared with antireflux medication,” the investigators wrote. “Instead, the risk was increased throughout the follow-up among patients having undergone antireflux surgery.”
Dr. Lagergren and colleagues suggested that the reason for relatively higher cancer risk in the group that underwent surgery likely stems from early and prolonged acid exposure.
“[P]erforming antireflux surgery after years of GERD may be too late to enable a cancer-preventative effect, and most of the patients first diagnosed with Barrett’s esophagus reported a history of many years of GERD symptoms,” they wrote, suggesting that carcinogenic processes had already been set in motion by the time surgery was performed.
“[P]atients with Barrett’s esophagus who undergo antireflux surgery remain at an increased risk of esophageal adenocarcinoma and should continue taking part in surveillance programs,” the investigators concluded.
The study was funded by the Swedish Cancer Society, Swedish Research Council, and Stockholm County Council. The investigators disclosed no conflicts of interest.
Esophageal adenocarcinoma (EAC) has been increasing in frequency for decades. EAC’s only known precursor is Barrett’s esophagus (BE), a complication of GERD with chronic esophageal inflammation (reflux esophagitis). Chronic inflammation can predispose to cancer and refluxed acid itself can cause potentially carcinogenic double-strand DNA breaks in Barrett’s metaplasia. PPIs, which block secretion of the gastric acid that causes reflux esophagitis and DNA damage, are recommended to BE patients for cancer prevention. Logical as that practice may seem, meta-analyses have reached contradictory conclusions regarding the cancer-preventive benefits of PPIs. PPIs do not stop the reflux of other potential carcinogens such as bile salts, and thus it has been argued that fundoplication, which blocks the reflux of all gastric material, should be superior to PPIs for cancer prevention. Plausible as that argument sounds, meta-analyses of the generally small and heterogeneous studies on this issue have not found consistently that antireflux surgery is superior to medical therapy for cancer prevention in BE.
Now, a large, population-based cohort study by Åkerström et al. of Nordic BE patients followed for up to 32 years has found that the overall risk of EAC was higher for patients treated with fundoplication than for those treated with medication (adjusted HR 1.9, 95%CI 1.1-3.5). Furthermore, the EAC risk increased over time in the surgical patients. Well done as this study was, it has important limitations. The overall BE population was large (n=33,939), but only 1.6% (542 patients) had antireflux surgery, and only 14 of those developed EAC during follow-up. Those small numbers limit statistical power. Moreover, important residual confounding cannot be excluded. The surgical patients might have had more severe GERD than medical patients, and it is difficult to make a plausible argument for why fundoplication should increase EAC risk. Nevertheless, this study provides a good lesson on why a plausible argument needs supportive evidence before acting on it in clinical practice. While there may be some excellent reasons for recommending antireflux surgery over medication for patients with severe GERD, better esophageal cancer prevention does not appear to be one of them.
Stuart Jon Spechler, MD, is chief of the division of gastroenterology and codirector of the Center for Esophageal Diseases at Baylor University Medical Center, and codirector of the Center for Esophageal Research at Baylor Scott & White Research Institute, Dallas, Texas. Dr. Spechler is a consultant for Phathom Pharmaceuticals and ISOThrive, LLC.
Esophageal adenocarcinoma (EAC) has been increasing in frequency for decades. EAC’s only known precursor is Barrett’s esophagus (BE), a complication of GERD with chronic esophageal inflammation (reflux esophagitis). Chronic inflammation can predispose to cancer and refluxed acid itself can cause potentially carcinogenic double-strand DNA breaks in Barrett’s metaplasia. PPIs, which block secretion of the gastric acid that causes reflux esophagitis and DNA damage, are recommended to BE patients for cancer prevention. Logical as that practice may seem, meta-analyses have reached contradictory conclusions regarding the cancer-preventive benefits of PPIs. PPIs do not stop the reflux of other potential carcinogens such as bile salts, and thus it has been argued that fundoplication, which blocks the reflux of all gastric material, should be superior to PPIs for cancer prevention. Plausible as that argument sounds, meta-analyses of the generally small and heterogeneous studies on this issue have not found consistently that antireflux surgery is superior to medical therapy for cancer prevention in BE.
Now, a large, population-based cohort study by Åkerström et al. of Nordic BE patients followed for up to 32 years has found that the overall risk of EAC was higher for patients treated with fundoplication than for those treated with medication (adjusted HR 1.9, 95%CI 1.1-3.5). Furthermore, the EAC risk increased over time in the surgical patients. Well done as this study was, it has important limitations. The overall BE population was large (n=33,939), but only 1.6% (542 patients) had antireflux surgery, and only 14 of those developed EAC during follow-up. Those small numbers limit statistical power. Moreover, important residual confounding cannot be excluded. The surgical patients might have had more severe GERD than medical patients, and it is difficult to make a plausible argument for why fundoplication should increase EAC risk. Nevertheless, this study provides a good lesson on why a plausible argument needs supportive evidence before acting on it in clinical practice. While there may be some excellent reasons for recommending antireflux surgery over medication for patients with severe GERD, better esophageal cancer prevention does not appear to be one of them.
Stuart Jon Spechler, MD, is chief of the division of gastroenterology and codirector of the Center for Esophageal Diseases at Baylor University Medical Center, and codirector of the Center for Esophageal Research at Baylor Scott & White Research Institute, Dallas, Texas. Dr. Spechler is a consultant for Phathom Pharmaceuticals and ISOThrive, LLC.
Esophageal adenocarcinoma (EAC) has been increasing in frequency for decades. EAC’s only known precursor is Barrett’s esophagus (BE), a complication of GERD with chronic esophageal inflammation (reflux esophagitis). Chronic inflammation can predispose to cancer and refluxed acid itself can cause potentially carcinogenic double-strand DNA breaks in Barrett’s metaplasia. PPIs, which block secretion of the gastric acid that causes reflux esophagitis and DNA damage, are recommended to BE patients for cancer prevention. Logical as that practice may seem, meta-analyses have reached contradictory conclusions regarding the cancer-preventive benefits of PPIs. PPIs do not stop the reflux of other potential carcinogens such as bile salts, and thus it has been argued that fundoplication, which blocks the reflux of all gastric material, should be superior to PPIs for cancer prevention. Plausible as that argument sounds, meta-analyses of the generally small and heterogeneous studies on this issue have not found consistently that antireflux surgery is superior to medical therapy for cancer prevention in BE.
Now, a large, population-based cohort study by Åkerström et al. of Nordic BE patients followed for up to 32 years has found that the overall risk of EAC was higher for patients treated with fundoplication than for those treated with medication (adjusted HR 1.9, 95%CI 1.1-3.5). Furthermore, the EAC risk increased over time in the surgical patients. Well done as this study was, it has important limitations. The overall BE population was large (n=33,939), but only 1.6% (542 patients) had antireflux surgery, and only 14 of those developed EAC during follow-up. Those small numbers limit statistical power. Moreover, important residual confounding cannot be excluded. The surgical patients might have had more severe GERD than medical patients, and it is difficult to make a plausible argument for why fundoplication should increase EAC risk. Nevertheless, this study provides a good lesson on why a plausible argument needs supportive evidence before acting on it in clinical practice. While there may be some excellent reasons for recommending antireflux surgery over medication for patients with severe GERD, better esophageal cancer prevention does not appear to be one of them.
Stuart Jon Spechler, MD, is chief of the division of gastroenterology and codirector of the Center for Esophageal Diseases at Baylor University Medical Center, and codirector of the Center for Esophageal Research at Baylor Scott & White Research Institute, Dallas, Texas. Dr. Spechler is a consultant for Phathom Pharmaceuticals and ISOThrive, LLC.
, according to a Nordic retrospective study.
Risk of EAC was higher among patients who underwent surgery, and risk appeared to increase over time, suggesting that postoperative patients should continue to participate in surveillance programs, reported lead author Jesper Lagergren, MD, PhD, of the Karolinska Institutet, Stockholm, and colleagues.
“Antireflux surgery with fundoplication increases the ability of the gastroesophageal anatomic and physiological barrier to prevent reflux, and can thus prevent any carcinogenic gastric content from reaching the esophagus, including both acid and bile,” the investigators wrote in Gastroenterology, noting that surgery reduces esophageal acid exposure to a greater degree than medication. “Antireflux surgery may thus prevent esophageal adenocarcinoma better than antireflux medication.”
Three meta-analyses to date, however, have failed to provide consistent support for this hypothesis.
“Most of the studies included in these meta-analyses came from single centers, were of small sample size, examined only one treatment arm, and had a short or incomplete follow-up, and ... were hampered by heterogeneity among the included studies,” they noted.
For the present study, Dr. Lagergren and colleagues analyzed national registry data from 33,939 patients with Barrett’s esophagus in Denmark, Finland, Norway, and Sweden. Out of this group, 542 patients (1.6%) had undergone antireflux surgery, while the remainder were managed with antireflux medication.
In both groups, approximately two-thirds of the patients were men. The median age at enrollment was about a decade higher in the medication group (66 vs. 54 years), and this group also tended to have more comorbidities.
After a follow-up period as long as 32 years, the absolute rates of EAC were 1.3% and 2.6% in the medication and surgery groups, respectively. Multivariate analysis, with adjustments for sex, age, year, comorbidities, and age, revealed that postsurgical patients had a 90% increased risk of EAC (hazard ratio [HR], 1.9; 95% CI, 1.1-3.5), versus patients treated with antireflux medication alone.
The relatively higher risk of EAC appeared to increase over time, based on a nonsignificant hazard ratio of 1.8 during the 1- to 4-year follow-up period (HR, 1.8; 95% CI, 0.6-5.0), versus a significant, fourfold risk elevation during the 10- to 32-year follow-up period (HR, 4.4; 95% CI, 1.4-13.5).
“In this cohort of patients with Barrett’s esophagus, the risk of esophageal adenocarcinoma did not decrease after antireflux surgery compared with antireflux medication,” the investigators wrote. “Instead, the risk was increased throughout the follow-up among patients having undergone antireflux surgery.”
Dr. Lagergren and colleagues suggested that the reason for relatively higher cancer risk in the group that underwent surgery likely stems from early and prolonged acid exposure.
“[P]erforming antireflux surgery after years of GERD may be too late to enable a cancer-preventative effect, and most of the patients first diagnosed with Barrett’s esophagus reported a history of many years of GERD symptoms,” they wrote, suggesting that carcinogenic processes had already been set in motion by the time surgery was performed.
“[P]atients with Barrett’s esophagus who undergo antireflux surgery remain at an increased risk of esophageal adenocarcinoma and should continue taking part in surveillance programs,” the investigators concluded.
The study was funded by the Swedish Cancer Society, Swedish Research Council, and Stockholm County Council. The investigators disclosed no conflicts of interest.
, according to a Nordic retrospective study.
Risk of EAC was higher among patients who underwent surgery, and risk appeared to increase over time, suggesting that postoperative patients should continue to participate in surveillance programs, reported lead author Jesper Lagergren, MD, PhD, of the Karolinska Institutet, Stockholm, and colleagues.
“Antireflux surgery with fundoplication increases the ability of the gastroesophageal anatomic and physiological barrier to prevent reflux, and can thus prevent any carcinogenic gastric content from reaching the esophagus, including both acid and bile,” the investigators wrote in Gastroenterology, noting that surgery reduces esophageal acid exposure to a greater degree than medication. “Antireflux surgery may thus prevent esophageal adenocarcinoma better than antireflux medication.”
Three meta-analyses to date, however, have failed to provide consistent support for this hypothesis.
“Most of the studies included in these meta-analyses came from single centers, were of small sample size, examined only one treatment arm, and had a short or incomplete follow-up, and ... were hampered by heterogeneity among the included studies,” they noted.
For the present study, Dr. Lagergren and colleagues analyzed national registry data from 33,939 patients with Barrett’s esophagus in Denmark, Finland, Norway, and Sweden. Out of this group, 542 patients (1.6%) had undergone antireflux surgery, while the remainder were managed with antireflux medication.
In both groups, approximately two-thirds of the patients were men. The median age at enrollment was about a decade higher in the medication group (66 vs. 54 years), and this group also tended to have more comorbidities.
After a follow-up period as long as 32 years, the absolute rates of EAC were 1.3% and 2.6% in the medication and surgery groups, respectively. Multivariate analysis, with adjustments for sex, age, year, comorbidities, and age, revealed that postsurgical patients had a 90% increased risk of EAC (hazard ratio [HR], 1.9; 95% CI, 1.1-3.5), versus patients treated with antireflux medication alone.
The relatively higher risk of EAC appeared to increase over time, based on a nonsignificant hazard ratio of 1.8 during the 1- to 4-year follow-up period (HR, 1.8; 95% CI, 0.6-5.0), versus a significant, fourfold risk elevation during the 10- to 32-year follow-up period (HR, 4.4; 95% CI, 1.4-13.5).
“In this cohort of patients with Barrett’s esophagus, the risk of esophageal adenocarcinoma did not decrease after antireflux surgery compared with antireflux medication,” the investigators wrote. “Instead, the risk was increased throughout the follow-up among patients having undergone antireflux surgery.”
Dr. Lagergren and colleagues suggested that the reason for relatively higher cancer risk in the group that underwent surgery likely stems from early and prolonged acid exposure.
“[P]erforming antireflux surgery after years of GERD may be too late to enable a cancer-preventative effect, and most of the patients first diagnosed with Barrett’s esophagus reported a history of many years of GERD symptoms,” they wrote, suggesting that carcinogenic processes had already been set in motion by the time surgery was performed.
“[P]atients with Barrett’s esophagus who undergo antireflux surgery remain at an increased risk of esophageal adenocarcinoma and should continue taking part in surveillance programs,” the investigators concluded.
The study was funded by the Swedish Cancer Society, Swedish Research Council, and Stockholm County Council. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Treatment and Current Policies on Pseudofolliculitis Barbae in the US Military
Pseudofolliculitis barbae (PFB)(also referred to as razor bumps) is a skin disease of the face and neck caused by shaving and remains prevalent in the US Military. As the sharpened ends of curly hair strands penetrate back into the epidermis, they can trigger inflammatory reactions, leading to papules and pustules as well as hyperpigmentation and scarring.1 Although anyone with thick curly hair can develop PFB, Black individuals are disproportionately affected, with 45% to 83% reporting PFB symptoms compared with 18% of White individuals.2 In this article, we review the treatments and current policies on PFB in the military.
Treatment Options
Shaving Guidelines—Daily shaving remains the grooming standard for US service members who are encouraged to follow prescribed grooming techniques to prevent mild cases of PFB, defined as having “few, scattered papules with scant hair growth of the beard area,” according to the technical bulletin of the US Army, which provides the most detailed guidelines among the branches.3 The bulletin recommends hydrating the face with warm water, followed by a preshave lotion and shaving with a single pass superiorly to inferiorly. Following shaving, postrazor hydration lotion is recommended. Single-bladed razors are preferred, as there is less trauma to existing PFB and less potential for hair retraction under the epidermis, though multibladed razors can be used with adequate preshave and postrazor hydration.4 Shaving can be undertaken in the evening to ensure adequate time for preshave preparation and postshave hydration. Waterless shaving uses waterless soaps or lotions containing α-hydroxy acid just prior to shaving in lieu of preshaving and postshaving procedures.4
Topical Medications—For PFB cases that are recalcitrant to management by changes in shaving, topical retinoids are commonly prescribed, as they reduce follicular hyperkeratosis that may lead to PFB.5 The Army medical bulletin recommends a pea-sized amount of tretinoin cream or gel 0.025%, 0.05%, or 0.1% for moderate cases, defined as “heavier beard growth, more scattered papules, no evidence of pustules or denudation.”3 Adapalene cream 0.1% may be used instead of tretinoin for sensitive skin. Oral doxycycline or topical benzoyl peroxide–clindamycin may be added for secondary bacterial skin infections. Clinical trials have demonstrated that combination benzoyl peroxide–clindamycin significantly reduces papules and pustules in up to 63% of patients with PFB (P<.029).6 Azelaic acid can be prescribed for prominent postinflammatory hyperpigmentation. The bulletin also suggests depilatories such as barium sulfide to obtund the hair ends and make them less likely to re-enter the skin surface, though it notes low compliance rates due to strong sulfur odor, messy application, and irritation and reactions to ingredients in the preparations.4
Shaving Waivers and Laser Hair Removal—The definitive treatment of PFB is to not shave, and a shaving waiver or laser hair removal (LHR) are the best options for severe PFB or PFB refractory to other treatments. A shaving waiver (or shaving profile) allows for growth of up to 0.25 inches of facial hair with maintenance of the length using clippers. The shaving profile typically is issued by the referring primary care manager (PCM) but also can be recommended by a dermatologist. Each military branch implements different regulations on shaving profiles, which complicates care delivery at joint-service military treatment facilities (MTFs). The Table provides guidelines that govern the management of PFB by the US Army, Air Force, Navy, and Marine Corps. The issuance and duration of shaving waivers vary by service.
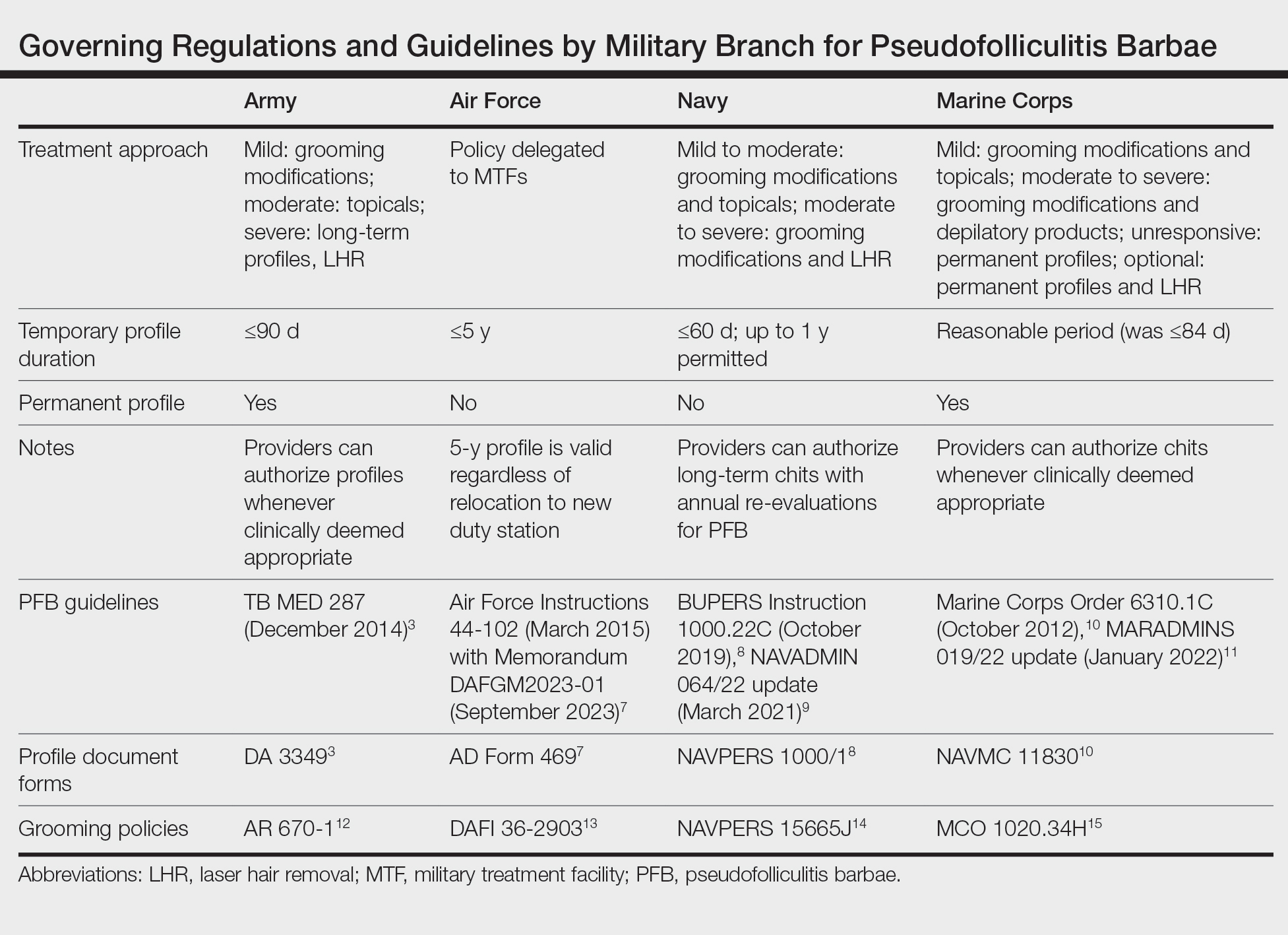
Laser hair removal therapy uses high-wavelength lasers that largely bypass the melanocyte-containing basal layer and selectively target hair follicles located deeper in the skin, which results in precise hair reduction with relative sparing of the epidermis.16 Clinical trials at military clinics have demonstrated that treatments with the 1064-nm long-pulse Nd:YAG laser generally are safe and effective in impeding hair growth in Fitzpatrick skin types IV, V, and VI.17 This laser, along with the Alexandrite 755-nm long-pulse laser for Fitzpatrick skin types I to III, is widely available and used for LHR at MTFs that house dermatologists. Eflornithine cream 13.9%, which is approved by the US Food and Drug Administration to treat hirsutism, can be used as monotherapy for treatment of PFB and has a synergistic depilatory effect in PFB patients when used in conjunction with LHR.18,19 Laser hair removal treatments can induce a permanent change in facial hair density and pattern of growth. Side effects and complications of LHR include discomfort during treatment and, in rare instances, blistering and dyspigmentation of the skin as well as paradoxical hair growth.17
TRICARE, the uniformed health care program, covers LHR in the civilian sector if the following criteria are met: candidates must work in an environment that may require breathing protection, and they must have failed conservative therapy; an MTF dermatologist must evaluate each case and attempt LHR at an MTF to limit outside referrals; and the MTF dermatologist must process each outside referral claim to completion and ensure that the LHR is rendered by a civilian dermatologist and is consistent with branch-specific policies.20
Service Policies on PFB
Army—
The technical bulletin also allows a permanent shaving profile for soldiers who demonstrate a severe adverse reaction to treatment or progression of the disease despite a trial of all these methods.3 The regulation stipulates that 0.125 to 0.25 inches of beard growth usually is sufficient to prevent PFB. Patients on profiles must be re-evaluated by a PCM or a dermatologist at least once a year.3
Air Force—Air Force Instruction 44-102 delegates PFB treatment and management strategies to each individual MTF, which allows for decentralized management of PFB, resulting in treatment protocols that can differ from one MTF to another.7 Since 2020, waivers have been valid for 5 years regardless of deployment or permanent change of station location. Previously, shaving profiles required annual renewals.7 Special duties, such as Honor Guard, Thunderbirds, Special Warfare Mission Support, recruiters, and the Air Force Band, often follow the professional appearance standards more strictly. Until recently, the Honor Guard used to reassign those with long-term medical shaving waivers but now allows airmen with shaving profiles to serve with exceptions (eg, shaving before ceremonies).21
Navy—BUPERS (Bureau of Naval Personnel) Instruction 1000.22C divides PFB severity into 2 categories.8 For mild to moderate PFB cases, topical tretinoin and adapalene are recommended, along with improved shaving hygiene practices. As an alternative to topical steroids, topical eflornithine monotherapy can be used twice daily for 60 days. For moderate to severe PFB cases, continued grooming modifications and LHR at military clinics with dermatologic services are expected.8
Naval administrative memorandum NAVADMIN 064/22 (released in 2022) no longer requires sailors with a shaving “chit,” or shaving waiver, to fully grow out their beards.9 Sailors may now outline or edge their beards as long as doing so does not trigger a skin irritation or outbreak. Furthermore, sailors are no longer required to carry a physical copy of their shaving chit at all times. Laser hair removal for sailors with PFB is now considered optional, whereas sailors with severe PFB were previously expected to receive LHR.9
Marine Corps—The Marine Corps endorses a 4-phase treatment algorithm (Table). As of January 2022, permanent shaving chits are authorized. Marines no longer need to carry physical copies of their chits at all times and cannot be separated from service because of PFB.10 New updates explicitly state that medical officers, not the commanding officers, now have final authority for granting shaving chits.11
Final Thoughts
The Army provides the most detailed bulletin, which defines the clinical features and treatments expected for each stage of PFB. All 4 service branches permit temporary profiles, albeit for different lengths of time. However, only the Army and the Marine Corps currently authorize permanent shaving waivers if all treatments mentioned in their respective bulletins have failed.
The Air Force has adopted the most decentralized approach, in which each MTF is responsible for implementing its own treatment protocols and definitions. Air Force regulations now authorize a 5-year shaving profile for medical reasons, including PFB. The Air Force also has spearheaded efforts to create more inclusive policies. A study of 10,000 active-duty male Air Force members conducted by Air Force physicians found that shaving waivers were associated with longer times to promotion. Although self-identified race was not independently linked to longer promotion times, more Black service members were affected because of a higher prevalence of PFB and shaving profiles.22
The Navy has outlined the most specific timeline for therapy for PFB. The regulations allow a 60-day temporary shaving chit that expires on the day of the appointment with the dermatologist or PCM. Although sailors were previously mandated to fully grow out their beards without modifications during the 60-day shaving chit period, Navy leadership recently overturned these requirements. However, permanent shaving chits are still not authorized in the Navy.
Service members are trying to destigmatize shaving profiles and facial hair in our military. A Facebook group called DoD Beard Action Initiative has more than 17,000 members and was created in 2021 to compile testimonies and data regarding the effects of PFB on airmen.23 Soldiers also have petitioned for growing beards in the garrison environment with more than 100,000 signatures, citing that North Atlantic Treaty Organization allied nations permit beard growth in their respective ranks.24 A Sikh marine captain recently won a lawsuit against the US Department of the Navy to maintain a beard with a turban in uniform on religious grounds.25
The clean-shaven look remains standard across the military, not only for uniformity of appearance but also for safety concerns. The Naval Safety Center’s ALSAFE report concluded that any facial hair impedes a tight fit of gas masks, which can be lethal in chemical warfare. However, the report did not explore how different hair lengths would affect the seal of gas masks.26 It remains unknown how 0.25 inch of facial hair, the maximum hair length authorized for most PFB patients, affects the seal. Department of Defense occupational health researchers currently are assessing how each specific facial hair length diminishes the effectiveness of gas masks.27
Furthermore, the COVID-19 pandemic has led to frequent N95 respirator wear in the military. It is likely that growing a long beard disrupts the fitting of N95 respirators and could endanger service members, especially in clinical settings. However, one study confirmed that 0.125 inch of facial hair still results in 98% effectiveness in filtering particles for the respirator wearers.28 Although unverified, it is surmisable that 0.25 inch of facial hair will likely not render all respirators useless. However, current Occupational Safety and Health Administration guidelines require fit tests to be conducted only on clean-shaven faces.29 Effectively, service members with facial hair cannot be fit-tested for N95 respirators.
More research is needed to optimize treatment protocols and regulations for PFB in our military. As long as the current grooming standards remain in place, treatment of PFB will be a controversial topic. Guidelines will need to be continuously updated to balance the needs of our service members and to minimize risk to unit safety and mission success. Department of Defense Instruction 6130.03, Volume 1, revised in late 2022, now no longer designates PFB as a condition that disqualifies a candidate from entering service in any military branch.30 The Department of Defense is demonstrating active research and adoption of policies regarding PFB that will benefit our service members.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27.
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. Published December 10, 2014. Accessed November 16, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed287.pdf
- Tshudy M, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:52-57.
- Kligman AM, Mills OH. Pseudofolliculitis of the beard and topically applied tretinoin. J Am Acad Dermatol. 1973;107:551-552.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- US Department of the Air Force. Air Force Instruction 44-102. Medical Care Management. March 17, 2015. Updated July 13, 2022. Accessed October 1, 2022. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-102/afi44-102.pdf
- Chief of Naval Personnel, Department of the Navy. BUPERS Instruction 1000.22C. Management of Navy Uniformed Personnel Diagnosed With Pseudofolliculitis Barbae. October 8, 2019. Accessed November 16, 2023. https://www.mynavyhr.navy.mil/Portals/55/Reference/Instructions/BUPERS/BUPERSINST%201000.22C%20Signed.pdf?ver=iby4-mqcxYCTM1t3AOsqxA%3D%3D
- Chief of Naval Operations, Department of the Navy. NAVADMIN 064/22. BUPERSINST 1000,22C Management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 9, 2022. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064.txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Commandant of the Marine Corps, Department of the Navy. Marine Corps Order 6310.1C. Pseudofolliculitis Barbae. October 9, 2012. Accessed November 16, 2023. https://www.marines.mil/Portals/1/Publications/MCO%206310.1C.pdf
- US Marine Corps. Advance Notification of Change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). January 21, 2022. Accessed November 16, 2023. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900
- Department of the Army. Army Regulation 670-1. Uniform and Insignia. Wear and Appearance of Army Uniforms and Insignia. January 26, 2021. Accessed November 19, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Department of the Air Force. Department of the Air Force Guidance Memorandum to DAFI 36-2903, Dress and Personal Appearance of United States Air Force and United States Space Force Personnel. Published March 31, 2023. Accessed November 20, 2023. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR website. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- US Marine Corps. Marine Corps Uniform Regulations. Published May 1, 2018. Accessed November 20, 2023. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Xia Y, Cho SC, Howard RS, et al. Topical eflornithine hydrochloride improves effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: a randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012;67:694-699.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525. doi:10.1111/jocd.14027
- TRICARE operations manual 6010.59-M. Supplemental Health Care Program (SHCP)—chapter 17. Contractor responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed November 16, 2023. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
- Air Force Honor Guard: Recruiting. Accessed November 16, 2023. https://www.honorguard.af.mil/About-Us/Recruiting/
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- DoD Beard Action Initiative Facebook group. Accessed November 5, 2023. https://www.facebook.com/groups/326068578791063/
- Geske R. Petition gets 95K signatures in push for facial hair for soldiers. KWTX. February 4, 2021. Accessed November 16, 2023. https://www.kwtx.com/2021/02/04/petition-gets-95k-signatures-in-push-for-facial-hair-for-soldiers/
- Athey P. A Sikh marine is now allowed to wear a turban in uniform. Marine Corps Times. October 5, 2021. Accessed November 16, 2023. https://www.marinecorpstimes.com/news/your-marine-corps/2021/10/05/a-sikh-marine-is-now-allowed-to-wear-a-turban-in-uniform
- US Department of the Navy. Face Seal Guidance update (ALSAFE 18-008). Naval Safety Center. Published November 18, 2018. Accessed October 22, 2022. https://navalsafetycommand.navy.mil/Portals/29/ALSAFE18-008.pdf
- Garland C. Navy and Marine Corps to study facial hair’s effect on gas masks, lawsuit reveals. Stars and Stripes. January 25, 2022. Accessed November 16, 2023. https://www.stripes.com/branches/navy/2022-01-25/court-oversee-navy-marine-gas-mask-facial-hair-study-4410015.html
- Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334-340.
- Occupational Safety and Health Administration. Occupational Safety and Health Standards 1910.134 App A. Fit Testing Procedures—General Requirements. US Department of Labor. April 23, 1998. Updated August 4, 2004. Accessed November 16, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. November 16, 2022. Accessed November 16, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
Pseudofolliculitis barbae (PFB)(also referred to as razor bumps) is a skin disease of the face and neck caused by shaving and remains prevalent in the US Military. As the sharpened ends of curly hair strands penetrate back into the epidermis, they can trigger inflammatory reactions, leading to papules and pustules as well as hyperpigmentation and scarring.1 Although anyone with thick curly hair can develop PFB, Black individuals are disproportionately affected, with 45% to 83% reporting PFB symptoms compared with 18% of White individuals.2 In this article, we review the treatments and current policies on PFB in the military.
Treatment Options
Shaving Guidelines—Daily shaving remains the grooming standard for US service members who are encouraged to follow prescribed grooming techniques to prevent mild cases of PFB, defined as having “few, scattered papules with scant hair growth of the beard area,” according to the technical bulletin of the US Army, which provides the most detailed guidelines among the branches.3 The bulletin recommends hydrating the face with warm water, followed by a preshave lotion and shaving with a single pass superiorly to inferiorly. Following shaving, postrazor hydration lotion is recommended. Single-bladed razors are preferred, as there is less trauma to existing PFB and less potential for hair retraction under the epidermis, though multibladed razors can be used with adequate preshave and postrazor hydration.4 Shaving can be undertaken in the evening to ensure adequate time for preshave preparation and postshave hydration. Waterless shaving uses waterless soaps or lotions containing α-hydroxy acid just prior to shaving in lieu of preshaving and postshaving procedures.4
Topical Medications—For PFB cases that are recalcitrant to management by changes in shaving, topical retinoids are commonly prescribed, as they reduce follicular hyperkeratosis that may lead to PFB.5 The Army medical bulletin recommends a pea-sized amount of tretinoin cream or gel 0.025%, 0.05%, or 0.1% for moderate cases, defined as “heavier beard growth, more scattered papules, no evidence of pustules or denudation.”3 Adapalene cream 0.1% may be used instead of tretinoin for sensitive skin. Oral doxycycline or topical benzoyl peroxide–clindamycin may be added for secondary bacterial skin infections. Clinical trials have demonstrated that combination benzoyl peroxide–clindamycin significantly reduces papules and pustules in up to 63% of patients with PFB (P<.029).6 Azelaic acid can be prescribed for prominent postinflammatory hyperpigmentation. The bulletin also suggests depilatories such as barium sulfide to obtund the hair ends and make them less likely to re-enter the skin surface, though it notes low compliance rates due to strong sulfur odor, messy application, and irritation and reactions to ingredients in the preparations.4
Shaving Waivers and Laser Hair Removal—The definitive treatment of PFB is to not shave, and a shaving waiver or laser hair removal (LHR) are the best options for severe PFB or PFB refractory to other treatments. A shaving waiver (or shaving profile) allows for growth of up to 0.25 inches of facial hair with maintenance of the length using clippers. The shaving profile typically is issued by the referring primary care manager (PCM) but also can be recommended by a dermatologist. Each military branch implements different regulations on shaving profiles, which complicates care delivery at joint-service military treatment facilities (MTFs). The Table provides guidelines that govern the management of PFB by the US Army, Air Force, Navy, and Marine Corps. The issuance and duration of shaving waivers vary by service.

Laser hair removal therapy uses high-wavelength lasers that largely bypass the melanocyte-containing basal layer and selectively target hair follicles located deeper in the skin, which results in precise hair reduction with relative sparing of the epidermis.16 Clinical trials at military clinics have demonstrated that treatments with the 1064-nm long-pulse Nd:YAG laser generally are safe and effective in impeding hair growth in Fitzpatrick skin types IV, V, and VI.17 This laser, along with the Alexandrite 755-nm long-pulse laser for Fitzpatrick skin types I to III, is widely available and used for LHR at MTFs that house dermatologists. Eflornithine cream 13.9%, which is approved by the US Food and Drug Administration to treat hirsutism, can be used as monotherapy for treatment of PFB and has a synergistic depilatory effect in PFB patients when used in conjunction with LHR.18,19 Laser hair removal treatments can induce a permanent change in facial hair density and pattern of growth. Side effects and complications of LHR include discomfort during treatment and, in rare instances, blistering and dyspigmentation of the skin as well as paradoxical hair growth.17
TRICARE, the uniformed health care program, covers LHR in the civilian sector if the following criteria are met: candidates must work in an environment that may require breathing protection, and they must have failed conservative therapy; an MTF dermatologist must evaluate each case and attempt LHR at an MTF to limit outside referrals; and the MTF dermatologist must process each outside referral claim to completion and ensure that the LHR is rendered by a civilian dermatologist and is consistent with branch-specific policies.20
Service Policies on PFB
Army—
The technical bulletin also allows a permanent shaving profile for soldiers who demonstrate a severe adverse reaction to treatment or progression of the disease despite a trial of all these methods.3 The regulation stipulates that 0.125 to 0.25 inches of beard growth usually is sufficient to prevent PFB. Patients on profiles must be re-evaluated by a PCM or a dermatologist at least once a year.3
Air Force—Air Force Instruction 44-102 delegates PFB treatment and management strategies to each individual MTF, which allows for decentralized management of PFB, resulting in treatment protocols that can differ from one MTF to another.7 Since 2020, waivers have been valid for 5 years regardless of deployment or permanent change of station location. Previously, shaving profiles required annual renewals.7 Special duties, such as Honor Guard, Thunderbirds, Special Warfare Mission Support, recruiters, and the Air Force Band, often follow the professional appearance standards more strictly. Until recently, the Honor Guard used to reassign those with long-term medical shaving waivers but now allows airmen with shaving profiles to serve with exceptions (eg, shaving before ceremonies).21
Navy—BUPERS (Bureau of Naval Personnel) Instruction 1000.22C divides PFB severity into 2 categories.8 For mild to moderate PFB cases, topical tretinoin and adapalene are recommended, along with improved shaving hygiene practices. As an alternative to topical steroids, topical eflornithine monotherapy can be used twice daily for 60 days. For moderate to severe PFB cases, continued grooming modifications and LHR at military clinics with dermatologic services are expected.8
Naval administrative memorandum NAVADMIN 064/22 (released in 2022) no longer requires sailors with a shaving “chit,” or shaving waiver, to fully grow out their beards.9 Sailors may now outline or edge their beards as long as doing so does not trigger a skin irritation or outbreak. Furthermore, sailors are no longer required to carry a physical copy of their shaving chit at all times. Laser hair removal for sailors with PFB is now considered optional, whereas sailors with severe PFB were previously expected to receive LHR.9
Marine Corps—The Marine Corps endorses a 4-phase treatment algorithm (Table). As of January 2022, permanent shaving chits are authorized. Marines no longer need to carry physical copies of their chits at all times and cannot be separated from service because of PFB.10 New updates explicitly state that medical officers, not the commanding officers, now have final authority for granting shaving chits.11
Final Thoughts
The Army provides the most detailed bulletin, which defines the clinical features and treatments expected for each stage of PFB. All 4 service branches permit temporary profiles, albeit for different lengths of time. However, only the Army and the Marine Corps currently authorize permanent shaving waivers if all treatments mentioned in their respective bulletins have failed.
The Air Force has adopted the most decentralized approach, in which each MTF is responsible for implementing its own treatment protocols and definitions. Air Force regulations now authorize a 5-year shaving profile for medical reasons, including PFB. The Air Force also has spearheaded efforts to create more inclusive policies. A study of 10,000 active-duty male Air Force members conducted by Air Force physicians found that shaving waivers were associated with longer times to promotion. Although self-identified race was not independently linked to longer promotion times, more Black service members were affected because of a higher prevalence of PFB and shaving profiles.22
The Navy has outlined the most specific timeline for therapy for PFB. The regulations allow a 60-day temporary shaving chit that expires on the day of the appointment with the dermatologist or PCM. Although sailors were previously mandated to fully grow out their beards without modifications during the 60-day shaving chit period, Navy leadership recently overturned these requirements. However, permanent shaving chits are still not authorized in the Navy.
Service members are trying to destigmatize shaving profiles and facial hair in our military. A Facebook group called DoD Beard Action Initiative has more than 17,000 members and was created in 2021 to compile testimonies and data regarding the effects of PFB on airmen.23 Soldiers also have petitioned for growing beards in the garrison environment with more than 100,000 signatures, citing that North Atlantic Treaty Organization allied nations permit beard growth in their respective ranks.24 A Sikh marine captain recently won a lawsuit against the US Department of the Navy to maintain a beard with a turban in uniform on religious grounds.25
The clean-shaven look remains standard across the military, not only for uniformity of appearance but also for safety concerns. The Naval Safety Center’s ALSAFE report concluded that any facial hair impedes a tight fit of gas masks, which can be lethal in chemical warfare. However, the report did not explore how different hair lengths would affect the seal of gas masks.26 It remains unknown how 0.25 inch of facial hair, the maximum hair length authorized for most PFB patients, affects the seal. Department of Defense occupational health researchers currently are assessing how each specific facial hair length diminishes the effectiveness of gas masks.27
Furthermore, the COVID-19 pandemic has led to frequent N95 respirator wear in the military. It is likely that growing a long beard disrupts the fitting of N95 respirators and could endanger service members, especially in clinical settings. However, one study confirmed that 0.125 inch of facial hair still results in 98% effectiveness in filtering particles for the respirator wearers.28 Although unverified, it is surmisable that 0.25 inch of facial hair will likely not render all respirators useless. However, current Occupational Safety and Health Administration guidelines require fit tests to be conducted only on clean-shaven faces.29 Effectively, service members with facial hair cannot be fit-tested for N95 respirators.
More research is needed to optimize treatment protocols and regulations for PFB in our military. As long as the current grooming standards remain in place, treatment of PFB will be a controversial topic. Guidelines will need to be continuously updated to balance the needs of our service members and to minimize risk to unit safety and mission success. Department of Defense Instruction 6130.03, Volume 1, revised in late 2022, now no longer designates PFB as a condition that disqualifies a candidate from entering service in any military branch.30 The Department of Defense is demonstrating active research and adoption of policies regarding PFB that will benefit our service members.
Pseudofolliculitis barbae (PFB)(also referred to as razor bumps) is a skin disease of the face and neck caused by shaving and remains prevalent in the US Military. As the sharpened ends of curly hair strands penetrate back into the epidermis, they can trigger inflammatory reactions, leading to papules and pustules as well as hyperpigmentation and scarring.1 Although anyone with thick curly hair can develop PFB, Black individuals are disproportionately affected, with 45% to 83% reporting PFB symptoms compared with 18% of White individuals.2 In this article, we review the treatments and current policies on PFB in the military.
Treatment Options
Shaving Guidelines—Daily shaving remains the grooming standard for US service members who are encouraged to follow prescribed grooming techniques to prevent mild cases of PFB, defined as having “few, scattered papules with scant hair growth of the beard area,” according to the technical bulletin of the US Army, which provides the most detailed guidelines among the branches.3 The bulletin recommends hydrating the face with warm water, followed by a preshave lotion and shaving with a single pass superiorly to inferiorly. Following shaving, postrazor hydration lotion is recommended. Single-bladed razors are preferred, as there is less trauma to existing PFB and less potential for hair retraction under the epidermis, though multibladed razors can be used with adequate preshave and postrazor hydration.4 Shaving can be undertaken in the evening to ensure adequate time for preshave preparation and postshave hydration. Waterless shaving uses waterless soaps or lotions containing α-hydroxy acid just prior to shaving in lieu of preshaving and postshaving procedures.4
Topical Medications—For PFB cases that are recalcitrant to management by changes in shaving, topical retinoids are commonly prescribed, as they reduce follicular hyperkeratosis that may lead to PFB.5 The Army medical bulletin recommends a pea-sized amount of tretinoin cream or gel 0.025%, 0.05%, or 0.1% for moderate cases, defined as “heavier beard growth, more scattered papules, no evidence of pustules or denudation.”3 Adapalene cream 0.1% may be used instead of tretinoin for sensitive skin. Oral doxycycline or topical benzoyl peroxide–clindamycin may be added for secondary bacterial skin infections. Clinical trials have demonstrated that combination benzoyl peroxide–clindamycin significantly reduces papules and pustules in up to 63% of patients with PFB (P<.029).6 Azelaic acid can be prescribed for prominent postinflammatory hyperpigmentation. The bulletin also suggests depilatories such as barium sulfide to obtund the hair ends and make them less likely to re-enter the skin surface, though it notes low compliance rates due to strong sulfur odor, messy application, and irritation and reactions to ingredients in the preparations.4
Shaving Waivers and Laser Hair Removal—The definitive treatment of PFB is to not shave, and a shaving waiver or laser hair removal (LHR) are the best options for severe PFB or PFB refractory to other treatments. A shaving waiver (or shaving profile) allows for growth of up to 0.25 inches of facial hair with maintenance of the length using clippers. The shaving profile typically is issued by the referring primary care manager (PCM) but also can be recommended by a dermatologist. Each military branch implements different regulations on shaving profiles, which complicates care delivery at joint-service military treatment facilities (MTFs). The Table provides guidelines that govern the management of PFB by the US Army, Air Force, Navy, and Marine Corps. The issuance and duration of shaving waivers vary by service.

Laser hair removal therapy uses high-wavelength lasers that largely bypass the melanocyte-containing basal layer and selectively target hair follicles located deeper in the skin, which results in precise hair reduction with relative sparing of the epidermis.16 Clinical trials at military clinics have demonstrated that treatments with the 1064-nm long-pulse Nd:YAG laser generally are safe and effective in impeding hair growth in Fitzpatrick skin types IV, V, and VI.17 This laser, along with the Alexandrite 755-nm long-pulse laser for Fitzpatrick skin types I to III, is widely available and used for LHR at MTFs that house dermatologists. Eflornithine cream 13.9%, which is approved by the US Food and Drug Administration to treat hirsutism, can be used as monotherapy for treatment of PFB and has a synergistic depilatory effect in PFB patients when used in conjunction with LHR.18,19 Laser hair removal treatments can induce a permanent change in facial hair density and pattern of growth. Side effects and complications of LHR include discomfort during treatment and, in rare instances, blistering and dyspigmentation of the skin as well as paradoxical hair growth.17
TRICARE, the uniformed health care program, covers LHR in the civilian sector if the following criteria are met: candidates must work in an environment that may require breathing protection, and they must have failed conservative therapy; an MTF dermatologist must evaluate each case and attempt LHR at an MTF to limit outside referrals; and the MTF dermatologist must process each outside referral claim to completion and ensure that the LHR is rendered by a civilian dermatologist and is consistent with branch-specific policies.20
Service Policies on PFB
Army—
The technical bulletin also allows a permanent shaving profile for soldiers who demonstrate a severe adverse reaction to treatment or progression of the disease despite a trial of all these methods.3 The regulation stipulates that 0.125 to 0.25 inches of beard growth usually is sufficient to prevent PFB. Patients on profiles must be re-evaluated by a PCM or a dermatologist at least once a year.3
Air Force—Air Force Instruction 44-102 delegates PFB treatment and management strategies to each individual MTF, which allows for decentralized management of PFB, resulting in treatment protocols that can differ from one MTF to another.7 Since 2020, waivers have been valid for 5 years regardless of deployment or permanent change of station location. Previously, shaving profiles required annual renewals.7 Special duties, such as Honor Guard, Thunderbirds, Special Warfare Mission Support, recruiters, and the Air Force Band, often follow the professional appearance standards more strictly. Until recently, the Honor Guard used to reassign those with long-term medical shaving waivers but now allows airmen with shaving profiles to serve with exceptions (eg, shaving before ceremonies).21
Navy—BUPERS (Bureau of Naval Personnel) Instruction 1000.22C divides PFB severity into 2 categories.8 For mild to moderate PFB cases, topical tretinoin and adapalene are recommended, along with improved shaving hygiene practices. As an alternative to topical steroids, topical eflornithine monotherapy can be used twice daily for 60 days. For moderate to severe PFB cases, continued grooming modifications and LHR at military clinics with dermatologic services are expected.8
Naval administrative memorandum NAVADMIN 064/22 (released in 2022) no longer requires sailors with a shaving “chit,” or shaving waiver, to fully grow out their beards.9 Sailors may now outline or edge their beards as long as doing so does not trigger a skin irritation or outbreak. Furthermore, sailors are no longer required to carry a physical copy of their shaving chit at all times. Laser hair removal for sailors with PFB is now considered optional, whereas sailors with severe PFB were previously expected to receive LHR.9
Marine Corps—The Marine Corps endorses a 4-phase treatment algorithm (Table). As of January 2022, permanent shaving chits are authorized. Marines no longer need to carry physical copies of their chits at all times and cannot be separated from service because of PFB.10 New updates explicitly state that medical officers, not the commanding officers, now have final authority for granting shaving chits.11
Final Thoughts
The Army provides the most detailed bulletin, which defines the clinical features and treatments expected for each stage of PFB. All 4 service branches permit temporary profiles, albeit for different lengths of time. However, only the Army and the Marine Corps currently authorize permanent shaving waivers if all treatments mentioned in their respective bulletins have failed.
The Air Force has adopted the most decentralized approach, in which each MTF is responsible for implementing its own treatment protocols and definitions. Air Force regulations now authorize a 5-year shaving profile for medical reasons, including PFB. The Air Force also has spearheaded efforts to create more inclusive policies. A study of 10,000 active-duty male Air Force members conducted by Air Force physicians found that shaving waivers were associated with longer times to promotion. Although self-identified race was not independently linked to longer promotion times, more Black service members were affected because of a higher prevalence of PFB and shaving profiles.22
The Navy has outlined the most specific timeline for therapy for PFB. The regulations allow a 60-day temporary shaving chit that expires on the day of the appointment with the dermatologist or PCM. Although sailors were previously mandated to fully grow out their beards without modifications during the 60-day shaving chit period, Navy leadership recently overturned these requirements. However, permanent shaving chits are still not authorized in the Navy.
Service members are trying to destigmatize shaving profiles and facial hair in our military. A Facebook group called DoD Beard Action Initiative has more than 17,000 members and was created in 2021 to compile testimonies and data regarding the effects of PFB on airmen.23 Soldiers also have petitioned for growing beards in the garrison environment with more than 100,000 signatures, citing that North Atlantic Treaty Organization allied nations permit beard growth in their respective ranks.24 A Sikh marine captain recently won a lawsuit against the US Department of the Navy to maintain a beard with a turban in uniform on religious grounds.25
The clean-shaven look remains standard across the military, not only for uniformity of appearance but also for safety concerns. The Naval Safety Center’s ALSAFE report concluded that any facial hair impedes a tight fit of gas masks, which can be lethal in chemical warfare. However, the report did not explore how different hair lengths would affect the seal of gas masks.26 It remains unknown how 0.25 inch of facial hair, the maximum hair length authorized for most PFB patients, affects the seal. Department of Defense occupational health researchers currently are assessing how each specific facial hair length diminishes the effectiveness of gas masks.27
Furthermore, the COVID-19 pandemic has led to frequent N95 respirator wear in the military. It is likely that growing a long beard disrupts the fitting of N95 respirators and could endanger service members, especially in clinical settings. However, one study confirmed that 0.125 inch of facial hair still results in 98% effectiveness in filtering particles for the respirator wearers.28 Although unverified, it is surmisable that 0.25 inch of facial hair will likely not render all respirators useless. However, current Occupational Safety and Health Administration guidelines require fit tests to be conducted only on clean-shaven faces.29 Effectively, service members with facial hair cannot be fit-tested for N95 respirators.
More research is needed to optimize treatment protocols and regulations for PFB in our military. As long as the current grooming standards remain in place, treatment of PFB will be a controversial topic. Guidelines will need to be continuously updated to balance the needs of our service members and to minimize risk to unit safety and mission success. Department of Defense Instruction 6130.03, Volume 1, revised in late 2022, now no longer designates PFB as a condition that disqualifies a candidate from entering service in any military branch.30 The Department of Defense is demonstrating active research and adoption of policies regarding PFB that will benefit our service members.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27.
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. Published December 10, 2014. Accessed November 16, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed287.pdf
- Tshudy M, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:52-57.
- Kligman AM, Mills OH. Pseudofolliculitis of the beard and topically applied tretinoin. J Am Acad Dermatol. 1973;107:551-552.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- US Department of the Air Force. Air Force Instruction 44-102. Medical Care Management. March 17, 2015. Updated July 13, 2022. Accessed October 1, 2022. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-102/afi44-102.pdf
- Chief of Naval Personnel, Department of the Navy. BUPERS Instruction 1000.22C. Management of Navy Uniformed Personnel Diagnosed With Pseudofolliculitis Barbae. October 8, 2019. Accessed November 16, 2023. https://www.mynavyhr.navy.mil/Portals/55/Reference/Instructions/BUPERS/BUPERSINST%201000.22C%20Signed.pdf?ver=iby4-mqcxYCTM1t3AOsqxA%3D%3D
- Chief of Naval Operations, Department of the Navy. NAVADMIN 064/22. BUPERSINST 1000,22C Management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 9, 2022. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064.txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Commandant of the Marine Corps, Department of the Navy. Marine Corps Order 6310.1C. Pseudofolliculitis Barbae. October 9, 2012. Accessed November 16, 2023. https://www.marines.mil/Portals/1/Publications/MCO%206310.1C.pdf
- US Marine Corps. Advance Notification of Change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). January 21, 2022. Accessed November 16, 2023. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900
- Department of the Army. Army Regulation 670-1. Uniform and Insignia. Wear and Appearance of Army Uniforms and Insignia. January 26, 2021. Accessed November 19, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Department of the Air Force. Department of the Air Force Guidance Memorandum to DAFI 36-2903, Dress and Personal Appearance of United States Air Force and United States Space Force Personnel. Published March 31, 2023. Accessed November 20, 2023. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR website. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- US Marine Corps. Marine Corps Uniform Regulations. Published May 1, 2018. Accessed November 20, 2023. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Xia Y, Cho SC, Howard RS, et al. Topical eflornithine hydrochloride improves effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: a randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012;67:694-699.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525. doi:10.1111/jocd.14027
- TRICARE operations manual 6010.59-M. Supplemental Health Care Program (SHCP)—chapter 17. Contractor responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed November 16, 2023. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
- Air Force Honor Guard: Recruiting. Accessed November 16, 2023. https://www.honorguard.af.mil/About-Us/Recruiting/
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- DoD Beard Action Initiative Facebook group. Accessed November 5, 2023. https://www.facebook.com/groups/326068578791063/
- Geske R. Petition gets 95K signatures in push for facial hair for soldiers. KWTX. February 4, 2021. Accessed November 16, 2023. https://www.kwtx.com/2021/02/04/petition-gets-95k-signatures-in-push-for-facial-hair-for-soldiers/
- Athey P. A Sikh marine is now allowed to wear a turban in uniform. Marine Corps Times. October 5, 2021. Accessed November 16, 2023. https://www.marinecorpstimes.com/news/your-marine-corps/2021/10/05/a-sikh-marine-is-now-allowed-to-wear-a-turban-in-uniform
- US Department of the Navy. Face Seal Guidance update (ALSAFE 18-008). Naval Safety Center. Published November 18, 2018. Accessed October 22, 2022. https://navalsafetycommand.navy.mil/Portals/29/ALSAFE18-008.pdf
- Garland C. Navy and Marine Corps to study facial hair’s effect on gas masks, lawsuit reveals. Stars and Stripes. January 25, 2022. Accessed November 16, 2023. https://www.stripes.com/branches/navy/2022-01-25/court-oversee-navy-marine-gas-mask-facial-hair-study-4410015.html
- Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334-340.
- Occupational Safety and Health Administration. Occupational Safety and Health Standards 1910.134 App A. Fit Testing Procedures—General Requirements. US Department of Labor. April 23, 1998. Updated August 4, 2004. Accessed November 16, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. November 16, 2022. Accessed November 16, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27.
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. Published December 10, 2014. Accessed November 16, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed287.pdf
- Tshudy M, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:52-57.
- Kligman AM, Mills OH. Pseudofolliculitis of the beard and topically applied tretinoin. J Am Acad Dermatol. 1973;107:551-552.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- US Department of the Air Force. Air Force Instruction 44-102. Medical Care Management. March 17, 2015. Updated July 13, 2022. Accessed October 1, 2022. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-102/afi44-102.pdf
- Chief of Naval Personnel, Department of the Navy. BUPERS Instruction 1000.22C. Management of Navy Uniformed Personnel Diagnosed With Pseudofolliculitis Barbae. October 8, 2019. Accessed November 16, 2023. https://www.mynavyhr.navy.mil/Portals/55/Reference/Instructions/BUPERS/BUPERSINST%201000.22C%20Signed.pdf?ver=iby4-mqcxYCTM1t3AOsqxA%3D%3D
- Chief of Naval Operations, Department of the Navy. NAVADMIN 064/22. BUPERSINST 1000,22C Management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 9, 2022. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064.txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Commandant of the Marine Corps, Department of the Navy. Marine Corps Order 6310.1C. Pseudofolliculitis Barbae. October 9, 2012. Accessed November 16, 2023. https://www.marines.mil/Portals/1/Publications/MCO%206310.1C.pdf
- US Marine Corps. Advance Notification of Change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). January 21, 2022. Accessed November 16, 2023. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900
- Department of the Army. Army Regulation 670-1. Uniform and Insignia. Wear and Appearance of Army Uniforms and Insignia. January 26, 2021. Accessed November 19, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Department of the Air Force. Department of the Air Force Guidance Memorandum to DAFI 36-2903, Dress and Personal Appearance of United States Air Force and United States Space Force Personnel. Published March 31, 2023. Accessed November 20, 2023. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR website. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- US Marine Corps. Marine Corps Uniform Regulations. Published May 1, 2018. Accessed November 20, 2023. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Xia Y, Cho SC, Howard RS, et al. Topical eflornithine hydrochloride improves effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: a randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012;67:694-699.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525. doi:10.1111/jocd.14027
- TRICARE operations manual 6010.59-M. Supplemental Health Care Program (SHCP)—chapter 17. Contractor responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed November 16, 2023. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
- Air Force Honor Guard: Recruiting. Accessed November 16, 2023. https://www.honorguard.af.mil/About-Us/Recruiting/
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- DoD Beard Action Initiative Facebook group. Accessed November 5, 2023. https://www.facebook.com/groups/326068578791063/
- Geske R. Petition gets 95K signatures in push for facial hair for soldiers. KWTX. February 4, 2021. Accessed November 16, 2023. https://www.kwtx.com/2021/02/04/petition-gets-95k-signatures-in-push-for-facial-hair-for-soldiers/
- Athey P. A Sikh marine is now allowed to wear a turban in uniform. Marine Corps Times. October 5, 2021. Accessed November 16, 2023. https://www.marinecorpstimes.com/news/your-marine-corps/2021/10/05/a-sikh-marine-is-now-allowed-to-wear-a-turban-in-uniform
- US Department of the Navy. Face Seal Guidance update (ALSAFE 18-008). Naval Safety Center. Published November 18, 2018. Accessed October 22, 2022. https://navalsafetycommand.navy.mil/Portals/29/ALSAFE18-008.pdf
- Garland C. Navy and Marine Corps to study facial hair’s effect on gas masks, lawsuit reveals. Stars and Stripes. January 25, 2022. Accessed November 16, 2023. https://www.stripes.com/branches/navy/2022-01-25/court-oversee-navy-marine-gas-mask-facial-hair-study-4410015.html
- Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334-340.
- Occupational Safety and Health Administration. Occupational Safety and Health Standards 1910.134 App A. Fit Testing Procedures—General Requirements. US Department of Labor. April 23, 1998. Updated August 4, 2004. Accessed November 16, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. November 16, 2022. Accessed November 16, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
Practice Points
- Pseudofolliculitis barbae (PFB) is common among US service members due to grooming standards in the military.
- Each military branch follows separate yet related guidelines to treat PFB.
- The best treatment for severe or refractory cases of PFB is a long-term shaving restriction or laser hair removal.
Tackling Acrylate Allergy: The Sticky Truth
Acrylates are a ubiquitous family of synthetic thermoplastic resins that are employed in a wide array of products. Since the discovery of acrylic acid in 1843 and its industrialization in the early 20th century, acrylates have been used by many different sectors of industry.1 Today, acrylates can be found in diverse sources such as adhesives, coatings, electronics, nail cosmetics, dental materials, and medical devices. Although these versatile compounds have revolutionized numerous sectors, their potential to trigger allergic contact dermatitis (ACD) has garnered considerable attention in recent years. In 2012, acrylates as a group were named Allergen of the Year by the American Contact Dermatitis Society,2 and one member—isobornyl acrylate—also was given the infamous award in 2020.3 In this article, we highlight the chemistry of acrylates, the growing prevalence of acrylate contact allergy, common sources of exposure, patch testing considerations, and management/prevention strategies.
Chemistry and Uses of Acrylates
Acrylates are widely used due to their pliable and resilient properties.4 They begin as liquid monomers of (meth)acrylic acid or cyanoacrylic acid that are molded to the desired application before being cured or hardened by one of several means: spontaneously, using chemical catalysts, or with heat, UV light, or a light-emitting diode. Once cured, the final polymers (ie, [meth]acrylates, cyanoacrylates) serve a myriad of different purposes. Table 1 includes some of the more clinically relevant sources of acrylate exposure. Although this list is not comprehensive, it offers a glimpse into the vast array of uses for acrylates.
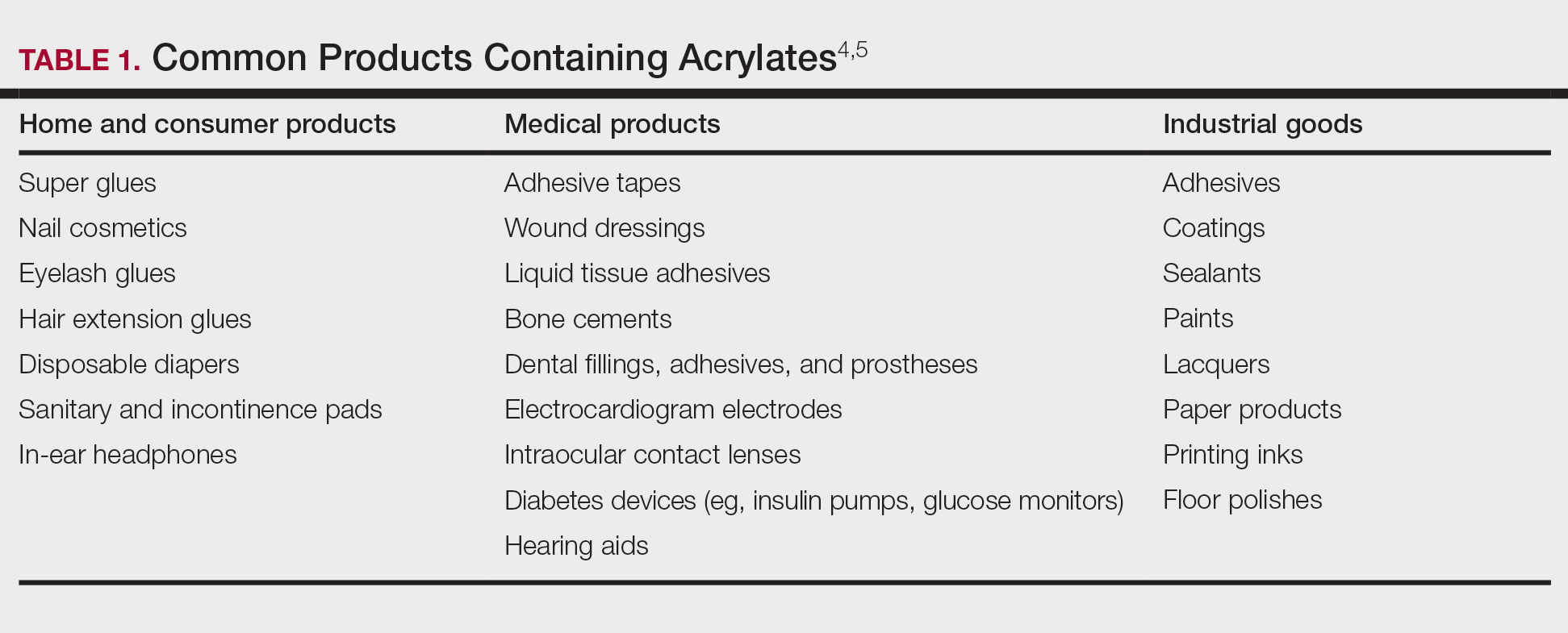
Acrylate Contact Allergy
Acrylic monomers are potent contact allergens, but the polymerized final products are not considered allergenic, assuming they are completely cured; however, ACD can occur with incomplete curing.6 It is of clinical importance that once an individual becomes sensitized to one type of acrylate, they may develop cross-reactions to others contained in different products. Notably, cyanoacrylates generally do not cross-react with (meth)acrylates; this has important implications for choosing safe alternative products in sensitized patients, though independent sensitization to cyanoacrylates is possible.7,8
Epidemiology and Risk Factors
The prevalence of acrylate allergy in the general population is unknown; however, there is a trend of increased patch test positivity in studies of patients referred for patch testing. A 2018 study by the European Environmental Contact Dermatitis Research Group reported positive patch tests to acrylates in 1.1% of 18,228 patients tested from 2013 to 2015.9 More recently, a multicenter European study (2019-2020) reported a 2.3% patch test positivity to 2-hydroxyethyl methacrylate (HEMA) among 7675 tested individuals,10 and even higher HEMA positivity was reported in Spain (3.7% of 1884 patients in 2019-2020).11 In addition, the North American Contact Dermatitis Group (NACDG) reported positive patch test reactions to HEMA in 3.2% of 4111 patients tested from 2019 to 2020, a statistically significant increase compared with those tested in 2009 to 2018 (odds ratio, 1.25 [95% CI, 1.03-1.51]; P=.02).12
Historically, acrylate sensitization primarily stemmed from occupational exposure. A retrospective analysis of occupational dermatitis performed by the NACDG (2001-2016) showed that HEMA was among the top 10 most common occupational allergens (3.4% positivity [83/2461]) and had the fifth highest percentage of occupationally relevant reactions (73.5% [83/113]).13 High-risk occupations include dental providers and nail technicians. Dentistry utilizes many materials containing acrylates, including uncured plastic resins used in dental prostheses, dentin bonding materials, and glass ionomers.14 A retrospective analysis of 585 dental personnel who were patch tested by the NACDG (2001-2018) found that more than 20% of occupational ACD cases were related to acrylates.15 Nail technicians are another group routinely exposed to acrylates through a variety of modern nail cosmetics. In a 7-year study from Portugal evaluating acrylate ACD, 68% (25/37) of cases were attributed to occupation, 80% (20/25) of which were in nail technicians.16 Likewise, among 28 nail technicians in Sweden who were referred for patch testing, 57% (16/28) tested positive for at least 1 acrylate.17
Modern Sources of Acrylate Exposure
Once thought to be a predominantly occupational exposure, acrylates have rapidly made their way into everyday consumer products. Clinicians should be aware of several sources of clinically relevant acrylate exposure, including nail cosmetics, consumer electronics, and medical/surgical adhesives.
A 2016 study found a shift to nail cosmetics as the most common source of acrylate sensitization.18 Nail cosmetics that contain acrylates include traditional acrylic, gel (shellac), dipped, and press-on (false) nails.19 The NACDG found that the most common allergen in patients experiencing ACD associated with nail products (2001-2016) was HEMA (56.6% [273/482]), far ahead of the traditional nail polish allergen tosylamide (36.2% [273/755]). Over the study period, the frequency of positive patch tests statistically increased for HEMA (P=.0069) and decreased for tosylamide (P<.0001).20 There is concern that the use of home gel nail kits, which can be purchased online at the click of a button, may be associated with a risk for acrylate sensitization.21,22 A recent study surveyed a Facebook support group for individuals with self-reported reactions to nail cosmetics, finding that 78% of the 199 individuals had used at-home gel nail kits, and more than 80% of them first developed skin reactions after starting to use at-home kits.23 The risks for sensitization are thought to be greater when self-applying nail acrylates compared to having them done professionally because individuals are more likely to spill allergenic monomers onto the skin at home; it also is possible that home techniques could lead to incomplete curing. Table 2 reviews the different types of acrylic nail cosmetics.
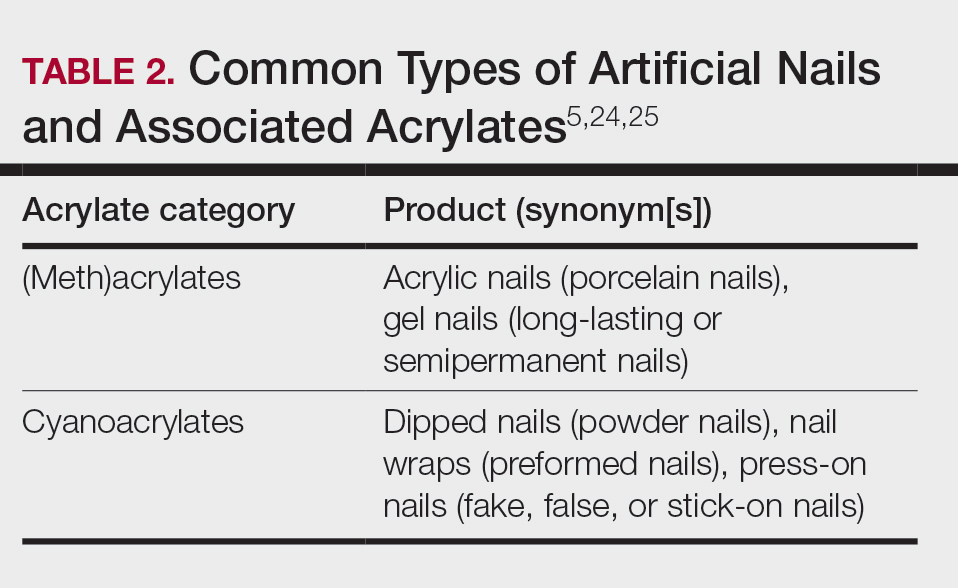
Medical adhesives and equipment are other important areas where acrylates can be encountered in abundance. A review by Spencer et al18 cautioned wound dressings as an up-and-coming source of sensitization, and this has been demonstrated in the literature as coming to fruition.26 Another study identified acrylates in 15 of 16 (94%) tested medical adhesives; among 7 medical adhesives labeled as hypoallergenic, 100% still contained acrylates and/or abietic acid.27 Multiple case reports have described ACD to adhesives of electrocardiogram electrodes containing acrylates.28-31 Physicians providing care to patients with diabetes mellitus also must be aware of acrylates in glucose monitors and insulin pumps, either found in the adhesives or leaching from the inside of the device to reach the skin.32 Isobornyl acrylate in particular has made quite the name for itself in this sector, being crowned the 2020 Allergen of the Year owing to its key role in cases of ACD to diabetes devices.3
Cyanoacrylate-based tissue adhesives (eg, 2‐octyl cyanoacrylate) are now well documented to cause postoperative ACD.33,34 Although robust prospective data are limited, studies suggest that 2% to 14% of patients develop postoperative skin reactions following 2-octyl cyanoacrylate application.35-37 It has been shown that sensitization to tissue adhesives often occurs after the first application, followed by an eruption of ACD as long as a month later, which can create confusion about the nature of the rash for patients and health care providers alike, who may for instance attribute it to infection rather than allergy.38 In the orthopedic literature, a woman with a known history of acrylic nail ACD had knee arthroplasty failure attributed to acrylic bone cement with resolution of the joint symptoms after changing to a cementless device.39
Awareness of the common use of acrylates is important to identify the cause of reactions from products that would otherwise seem nonallergenic. A case of occupational ACD to isobornyl acrylate in UV-cured phone screen protectors has been reported40; several cases of ACD to acrylates in headphones41,42 as well as one related to a wearable fitness device also have been reported.43 Given all these possible sources of exposure, ACD to acrylates should be on your radar.
When to Consider Acrylate ACD
When working up a patient with dermatitis, it is essential to ask about occupational history and hobbies to get a sense of potential contact allergen exposures. The typical presentation of occupational acrylate-associated ACD is hand eczema, specifically involving the fingertips.5,24,25,44 Acrylate ACD should be considered in patients with nail dystrophy and a history of wearing acrylic nails.45 There can even be involvement of the face and eyelids secondary to airborne contact or ectopic spread from the hands.24 Spreading vesicular eruptions associated with adhesives also should raise concern. The Figure depicts several possible presentations of ACD to acrylates. In a time of abundant access to products containing acrylates, dermatologists should consider this allergy in their differential diagnosis and consider patch testing.
Patch Testing to Acrylates
The gold standard for ACD diagnosis is patch testing. It should be noted that no acrylates are included in the thin-layer rapid use epicutaneous (T.R.U.E.) test series. Several acrylates are tested in expanded patch test series including the American Contact Dermatitis Society Core Allergen series and North American 80 Comprehensive Series. 2-Hydroxyethyl methacrylate is thought to be the most important screening allergen to test. Ramos et al16 reported a positive patch test to HEMA in 81% (30/37) of patients who had any type of acrylate allergy.
If initial testing to a limited number of acrylates is negative but clinical suspicion remains high, expanded acrylates/plastics and glue series also are available from commercial patch test suppliers. Testing to an expanded panel of acrylates is especially pertinent to consider in suspected occupational cases given the risk of workplace absenteeism and even disability that come with continued exposure to the allergen. Of note, isobornyl acrylate is not included in the baseline patch test series and must be tested separately, particularly because it usually does not cross-react with other acrylates, and therefore allergy could be missed if not tested on its own.
Acrylates are volatile substances that have been shown to degrade at room temperature and to a lesser degree when refrigerated. Ideally, they should be stored in a freezer and not used beyond their expiration date. Furthermore, it is advised that acrylate patch tests be prepared immediately prior to placement on the patient and to discard the initial extrusion from the syringe, as the concentration at the tip may be decreased.46,47
With regard to tissue adhesives, the actual product should be tested as-is because these are not commercially available patch test substances.48 Occasionally, patients who are sensitized to the tissue adhesive will not react when patch tested on intact skin. If clinical suspicion remains high, scratch patch testing may confirm contact allergy in cases of negative testing on intact skin.49
Management and Prevention
Once a diagnosis of ACD secondary to acrylates has been established, counseling patients on allergen avoidance strategies is essential. For (meth)acrylate-allergic patients who want to continue using modern nail products, cyanoacrylate-based options (eg, dipped, press-on nails) can be considered as an alternative, as they do not cross-react, though independent sensitization is still possible. However, traditional nail polish is the safest option to recommend.
The concern with acrylate sensitization extends beyond the immediate issue that brought the patient into your clinic. Dermatologists must counsel patients who are sensitized to acrylates on the possible sequelae of acrylate-containing dental or orthopedic procedures. Oral lichenoid lesions, denture stomatitis, burning mouth syndrome, or even acute facial swelling have been reported following dental work in patients with acrylate allergy.50-53 Dentists of patients with acrylate ACD should be informed of the diagnosis so acrylates can be avoided during dental work; if unavoidable, all possible steps should be taken to ensure complete curing of the monomers. In the surgical setting, patients sensitized to cyanoacrylate-based tissue adhesives should be offered wound closure alternatives such as sutures or staples.34
In patients with diabetes mellitus who develop ACD to their glucose monitor or insulin pump, ideally they should be switched to a device that does not contain acrylates. Problematically, these devices are constantly being reformulated, and manufacturers do not always divulge their components, which can make it challenging to determine safe alternative options.32,54 Various barrier products may help on a case-by-case basis.55Preventative measures should be implemented in workplaces that utilize acrylates, including dental practices and nail salons. Acrylic monomers have been shown to penetrate most gloves within minutes of exposure.56,57 Double gloving with nitrile gloves affords some protection for no longer than 60 minutes.6 4H gloves have been shown to provide true protection but result in a loss of dexterity.58 The fingerstall technique involves removing the fingers from a 4H glove, inserting them on the fingers, and applying a more flexible glove on top to hold them in place; this offers a hybrid between protection and finger dexterity.59
Final Interpretation
In a world characterized by technological advancements and increasing accessibility to acrylate-containing products, we hope this brief review serves as a resource and reminder to dermatologists to consider acrylates as a potential cause of ACD with diverse presentations and important future implications for affected individuals. The rising trend of acrylate allergy necessitates comprehensive assessment and shared decision-making between physicians and patients. As we navigate the ever-changing landscape of materials and technologies, clinicians must remain vigilant to avoid some potentially sticky situations for patients.
- Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent. 2021;23:397-406.
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society Allergens of the Year 2000 to 2020. Dermatol Clin. 2020;38:309-320.
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Society Allergen of the Year. Cutis. 2020;105:283-285.
- Ajekwene KK. Properties and applications of acrylates. In: Serrano-Aroca A, Deb S, eds. Acrylate Polymers for Advanced Applications. IntechOpen; 2020:35-46. https://doi.org/10.5772/intechopen.89867
- Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45:277-283.
- Sasseville D. Acrylates in contact dermatitis. Dermat Contact Atopic Occup Drug. 2012;23:6-16.
- Gardeen S, Hylwa S. A review of acrylates: super glue, nail adhesives, and diabetic pump adhesives increasing sensitization risk in women and children. Int J Womens Dermatol. 2020;6:263-267.
- Chou M, Dhingra N, Strugar TL. Contact sensitization to allergens in nail cosmetics. Dermat Contact Atopic Occup Drug. 2017;28:231-240.
- Gonçalo M, Pinho A, Agner T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2018;78:254-260.
- Uter W, Wilkinson SM, Aerts O, et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermatitis. 2022;87:343-355.
- Hernández-Fernández CP, Mercader-García P, Silvestre Salvador JF, et al. Candidate allergens for inclusion in the Spanish standard series based on data from the Spanish Contact Dermatitis Registry. Actas Dermosifiliogr. 2021;112:798-805.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermat Contact Atopic Occup Drug. 2023;34:90-104.
- DeKoven JG, DeKoven BM, Warshaw EM, et al. Occupational contact dermatitis: retrospective analysis of North American Contact Dermatitis Group Data, 2001 to 2016. J Am Acad Dermatol. 2022;86:782-790.
- Heratizadeh A, Werfel T, Schubert S, et al. Contact sensitization in dental technicians with occupational contact dermatitis. data of the Information Network of Departments of Dermatology (IVDK) 2001-2015. Contact Dermatitis. 2018;78:266-273.
- Warshaw EM, Ruggiero JL, Atwater AR, et al. Occupational contact dermatitis in dental personnel: a retrospective analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermat Contact Atopic Occup Drug. 2022;33:80-90.
- Ramos L, Cabral R, Gonçalo M. Allergic contact dermatitis caused by acrylates and methacrylates—a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study. Contact Dermatitis. 2019;81:58-60.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review. Contact Dermatitis. 2016;75:157-164.
- DeKoven S, DeKoven J, Holness DL. (Meth)acrylate occupational contact dermatitis in nail salon workers: a case series. J Cutan Med Surg. 2017;21:340-344.
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermat Contact Atopic Occup Drug. 2020;31:191-201.
- Le Q, Cahill J, Palmer-Le A, et al. The rising trend in allergic contact dermatitis to acrylic nail products. Australas J Dermatol. 2015;56:221-223.
- Gatica-Ortega ME, Pastor-Nieto M. The present and future burden of contact dermatitis from acrylates in manicure. Curr Treat Options Allergy. 2020;7:1-21.
- Guenther J, Norman T, Wee C, et al. A survey of skin reactions associated with acrylic nail cosmetics, with a focus on home kits: is there a need for regulation [published online October 16, 2023]? Dermatitis. doi:10.1089/derm.2023.0204
- Calado R, Gomes T, Matos A, et al. Contact dermatitis to nail cosmetics. Curr Dermatol Rep. 2021;10:173-181.
- Draelos ZD. Nail cosmetics and adornment. Dermatol Clin. 2021;39:351-359.
- Mestach L, Huygens S, Goossens A, et al. Allergic contact dermatitis caused by acrylic-based medical dressings and adhesives. Contact Dermatitis. 2018;79:81-84.
- Tam I, Wang JX, Yu JD. Identifying acrylates in medical adhesives. Dermat Contact Atopic Occup Drug. 2020;31:E40-E42.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermatitis. 2015;73:44-48.
- Ozkaya E, Kavlak Bozkurt P. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: a rare case with concomitant roles of nickel and acrylates. Contact Dermatitis. 2014;70:121-123.
- Lyons G, Nixon R. Allergic contact dermatitis to methacrylates in ECG electrode dots. Australas J Dermatol. 2013;54:39-40.
- Jelen G. Acrylate, a hidden allergen of electrocardiogram electrodes. Contact Dermatitis. 2001;45:315-316.
- Bembry R, Brys AK, Atwater AR. Medical device contact allergy: glucose monitors and insulin pumps. Curr Dermatol Rep. 2022;11:13-20.
- Liu T, Wan J, McKenna RA, et al. Allergic contact dermatitis caused by Dermabond in a paediatric patient undergoing skin surgery. Contact Dermatitis. 2019;80:61-62.
- Ricciardo BM, Nixon RL, Tam MM, et al. Allergic contact dermatitis to Dermabond Prineo after elective orthopedic surgery. Orthopedics. 2020;43:E515-E522.
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? the incidence of dermatitis after 2-octyl cyanoacrylate exposure in 102 consecutive breast cases. Plast Reconstr Surg. 2020;145:32-37.
- Alotaibi NN, Ahmad T, Rabah SM, et al. Type IV hypersensitivity reaction to Dermabond (2-octyl cyanoacrylate) in plastic surgical patients: a retrospective study. Plast Surg Oakv Ont. 2022;30:222-226.
- Durando D, Porubsky C, Winter S, et al. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermat Contact Atopic Occup Drug. 2014;25:99-100.
- Asai C, Inomata N, Sato M, et al. Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure. Contact Dermatitis. 2021;84:103-108.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59:S123-S124.
- Amat-Samaranch V, Garcia-Melendo C, Tubau C, et al. Occupational allergic contact dermatitis to isobornyl acrylate present in cell phone screen protectors. Contact Dermatitis. 2021;84:352-354.
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112.
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461.
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560.
- Nanda S. Nail salon safety: from nail dystrophy to acrylate contact allergies. Cutis. 2022;110:E32-E33.
- Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermat Contact Atopic Occup Drug. 2013;24:227-236.
- Jou PC, Siegel PD, Warshaw EM. Vapor pressure and predicted stability of American Contact Dermatitis Society core allergens. Dermat Contact Atopic Occup Drug. 2016;27:193-201.
- Cook KA, White AA, Shaw DW. Patch testing ingredients of Dermabond and other cyanoacrylate-containing adhesives. Dermat Contact Atopic Occup Drug. 2019;30:314-322.
- Patel K, Nixon R. Scratch patch testing to Dermabond in a patient with suspected allergic contact dermatitis. Dermat Contact Atopic Occup Drug. 2023;34:250-251.
- Ditrichova D, Kapralova S, Tichy M, et al. Oral lichenoid lesions and allergy to dental materials. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2007;151:333-339.
- Chen AYY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6:92-94.
- Marino R, Capaccio P, Pignataro L, et al. Burning mouth syndrome: the role of contact hypersensitivity. Oral Dis. 2009;15:255-258.
- Obayashi N, Shintani T, Kamegashira A, et al. A case report of allergic reaction with acute facial swelling: a rare complication of dental acrylic resin. J Int Med Res. 2023;51:3000605231187819.
- Cameli N, Silvestri M, Mariano M, et al. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermat Contact Atopic Occup Drug. 20221;33:110-115.
- Ng KL, Nixon RL, Grills C, et al. Solution using Stomahesive® wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Australas J Dermatol. 2022;63:E56-E59.
- Lönnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci. 2003;111:440-446.
- Sananez A, Sanchez A, Davis L, et al. Allergic reaction from dental bonding material through nitrile gloves: clinical case study and glove permeability testing. J Esthet Restor Dent. 2020;32:371-379.
- Andersson T, Bruze M, Björkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis. 1999;41:254-259.
- Roche E, Cuadra J, Alegre V. Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases. Actas Dermo-Sifiliográficas. 2009;99:788-794.
Acrylates are a ubiquitous family of synthetic thermoplastic resins that are employed in a wide array of products. Since the discovery of acrylic acid in 1843 and its industrialization in the early 20th century, acrylates have been used by many different sectors of industry.1 Today, acrylates can be found in diverse sources such as adhesives, coatings, electronics, nail cosmetics, dental materials, and medical devices. Although these versatile compounds have revolutionized numerous sectors, their potential to trigger allergic contact dermatitis (ACD) has garnered considerable attention in recent years. In 2012, acrylates as a group were named Allergen of the Year by the American Contact Dermatitis Society,2 and one member—isobornyl acrylate—also was given the infamous award in 2020.3 In this article, we highlight the chemistry of acrylates, the growing prevalence of acrylate contact allergy, common sources of exposure, patch testing considerations, and management/prevention strategies.
Chemistry and Uses of Acrylates
Acrylates are widely used due to their pliable and resilient properties.4 They begin as liquid monomers of (meth)acrylic acid or cyanoacrylic acid that are molded to the desired application before being cured or hardened by one of several means: spontaneously, using chemical catalysts, or with heat, UV light, or a light-emitting diode. Once cured, the final polymers (ie, [meth]acrylates, cyanoacrylates) serve a myriad of different purposes. Table 1 includes some of the more clinically relevant sources of acrylate exposure. Although this list is not comprehensive, it offers a glimpse into the vast array of uses for acrylates.

Acrylate Contact Allergy
Acrylic monomers are potent contact allergens, but the polymerized final products are not considered allergenic, assuming they are completely cured; however, ACD can occur with incomplete curing.6 It is of clinical importance that once an individual becomes sensitized to one type of acrylate, they may develop cross-reactions to others contained in different products. Notably, cyanoacrylates generally do not cross-react with (meth)acrylates; this has important implications for choosing safe alternative products in sensitized patients, though independent sensitization to cyanoacrylates is possible.7,8
Epidemiology and Risk Factors
The prevalence of acrylate allergy in the general population is unknown; however, there is a trend of increased patch test positivity in studies of patients referred for patch testing. A 2018 study by the European Environmental Contact Dermatitis Research Group reported positive patch tests to acrylates in 1.1% of 18,228 patients tested from 2013 to 2015.9 More recently, a multicenter European study (2019-2020) reported a 2.3% patch test positivity to 2-hydroxyethyl methacrylate (HEMA) among 7675 tested individuals,10 and even higher HEMA positivity was reported in Spain (3.7% of 1884 patients in 2019-2020).11 In addition, the North American Contact Dermatitis Group (NACDG) reported positive patch test reactions to HEMA in 3.2% of 4111 patients tested from 2019 to 2020, a statistically significant increase compared with those tested in 2009 to 2018 (odds ratio, 1.25 [95% CI, 1.03-1.51]; P=.02).12
Historically, acrylate sensitization primarily stemmed from occupational exposure. A retrospective analysis of occupational dermatitis performed by the NACDG (2001-2016) showed that HEMA was among the top 10 most common occupational allergens (3.4% positivity [83/2461]) and had the fifth highest percentage of occupationally relevant reactions (73.5% [83/113]).13 High-risk occupations include dental providers and nail technicians. Dentistry utilizes many materials containing acrylates, including uncured plastic resins used in dental prostheses, dentin bonding materials, and glass ionomers.14 A retrospective analysis of 585 dental personnel who were patch tested by the NACDG (2001-2018) found that more than 20% of occupational ACD cases were related to acrylates.15 Nail technicians are another group routinely exposed to acrylates through a variety of modern nail cosmetics. In a 7-year study from Portugal evaluating acrylate ACD, 68% (25/37) of cases were attributed to occupation, 80% (20/25) of which were in nail technicians.16 Likewise, among 28 nail technicians in Sweden who were referred for patch testing, 57% (16/28) tested positive for at least 1 acrylate.17
Modern Sources of Acrylate Exposure
Once thought to be a predominantly occupational exposure, acrylates have rapidly made their way into everyday consumer products. Clinicians should be aware of several sources of clinically relevant acrylate exposure, including nail cosmetics, consumer electronics, and medical/surgical adhesives.
A 2016 study found a shift to nail cosmetics as the most common source of acrylate sensitization.18 Nail cosmetics that contain acrylates include traditional acrylic, gel (shellac), dipped, and press-on (false) nails.19 The NACDG found that the most common allergen in patients experiencing ACD associated with nail products (2001-2016) was HEMA (56.6% [273/482]), far ahead of the traditional nail polish allergen tosylamide (36.2% [273/755]). Over the study period, the frequency of positive patch tests statistically increased for HEMA (P=.0069) and decreased for tosylamide (P<.0001).20 There is concern that the use of home gel nail kits, which can be purchased online at the click of a button, may be associated with a risk for acrylate sensitization.21,22 A recent study surveyed a Facebook support group for individuals with self-reported reactions to nail cosmetics, finding that 78% of the 199 individuals had used at-home gel nail kits, and more than 80% of them first developed skin reactions after starting to use at-home kits.23 The risks for sensitization are thought to be greater when self-applying nail acrylates compared to having them done professionally because individuals are more likely to spill allergenic monomers onto the skin at home; it also is possible that home techniques could lead to incomplete curing. Table 2 reviews the different types of acrylic nail cosmetics.

Medical adhesives and equipment are other important areas where acrylates can be encountered in abundance. A review by Spencer et al18 cautioned wound dressings as an up-and-coming source of sensitization, and this has been demonstrated in the literature as coming to fruition.26 Another study identified acrylates in 15 of 16 (94%) tested medical adhesives; among 7 medical adhesives labeled as hypoallergenic, 100% still contained acrylates and/or abietic acid.27 Multiple case reports have described ACD to adhesives of electrocardiogram electrodes containing acrylates.28-31 Physicians providing care to patients with diabetes mellitus also must be aware of acrylates in glucose monitors and insulin pumps, either found in the adhesives or leaching from the inside of the device to reach the skin.32 Isobornyl acrylate in particular has made quite the name for itself in this sector, being crowned the 2020 Allergen of the Year owing to its key role in cases of ACD to diabetes devices.3
Cyanoacrylate-based tissue adhesives (eg, 2‐octyl cyanoacrylate) are now well documented to cause postoperative ACD.33,34 Although robust prospective data are limited, studies suggest that 2% to 14% of patients develop postoperative skin reactions following 2-octyl cyanoacrylate application.35-37 It has been shown that sensitization to tissue adhesives often occurs after the first application, followed by an eruption of ACD as long as a month later, which can create confusion about the nature of the rash for patients and health care providers alike, who may for instance attribute it to infection rather than allergy.38 In the orthopedic literature, a woman with a known history of acrylic nail ACD had knee arthroplasty failure attributed to acrylic bone cement with resolution of the joint symptoms after changing to a cementless device.39
Awareness of the common use of acrylates is important to identify the cause of reactions from products that would otherwise seem nonallergenic. A case of occupational ACD to isobornyl acrylate in UV-cured phone screen protectors has been reported40; several cases of ACD to acrylates in headphones41,42 as well as one related to a wearable fitness device also have been reported.43 Given all these possible sources of exposure, ACD to acrylates should be on your radar.
When to Consider Acrylate ACD
When working up a patient with dermatitis, it is essential to ask about occupational history and hobbies to get a sense of potential contact allergen exposures. The typical presentation of occupational acrylate-associated ACD is hand eczema, specifically involving the fingertips.5,24,25,44 Acrylate ACD should be considered in patients with nail dystrophy and a history of wearing acrylic nails.45 There can even be involvement of the face and eyelids secondary to airborne contact or ectopic spread from the hands.24 Spreading vesicular eruptions associated with adhesives also should raise concern. The Figure depicts several possible presentations of ACD to acrylates. In a time of abundant access to products containing acrylates, dermatologists should consider this allergy in their differential diagnosis and consider patch testing.

Patch Testing to Acrylates
The gold standard for ACD diagnosis is patch testing. It should be noted that no acrylates are included in the thin-layer rapid use epicutaneous (T.R.U.E.) test series. Several acrylates are tested in expanded patch test series including the American Contact Dermatitis Society Core Allergen series and North American 80 Comprehensive Series. 2-Hydroxyethyl methacrylate is thought to be the most important screening allergen to test. Ramos et al16 reported a positive patch test to HEMA in 81% (30/37) of patients who had any type of acrylate allergy.
If initial testing to a limited number of acrylates is negative but clinical suspicion remains high, expanded acrylates/plastics and glue series also are available from commercial patch test suppliers. Testing to an expanded panel of acrylates is especially pertinent to consider in suspected occupational cases given the risk of workplace absenteeism and even disability that come with continued exposure to the allergen. Of note, isobornyl acrylate is not included in the baseline patch test series and must be tested separately, particularly because it usually does not cross-react with other acrylates, and therefore allergy could be missed if not tested on its own.
Acrylates are volatile substances that have been shown to degrade at room temperature and to a lesser degree when refrigerated. Ideally, they should be stored in a freezer and not used beyond their expiration date. Furthermore, it is advised that acrylate patch tests be prepared immediately prior to placement on the patient and to discard the initial extrusion from the syringe, as the concentration at the tip may be decreased.46,47
With regard to tissue adhesives, the actual product should be tested as-is because these are not commercially available patch test substances.48 Occasionally, patients who are sensitized to the tissue adhesive will not react when patch tested on intact skin. If clinical suspicion remains high, scratch patch testing may confirm contact allergy in cases of negative testing on intact skin.49
Management and Prevention
Once a diagnosis of ACD secondary to acrylates has been established, counseling patients on allergen avoidance strategies is essential. For (meth)acrylate-allergic patients who want to continue using modern nail products, cyanoacrylate-based options (eg, dipped, press-on nails) can be considered as an alternative, as they do not cross-react, though independent sensitization is still possible. However, traditional nail polish is the safest option to recommend.
The concern with acrylate sensitization extends beyond the immediate issue that brought the patient into your clinic. Dermatologists must counsel patients who are sensitized to acrylates on the possible sequelae of acrylate-containing dental or orthopedic procedures. Oral lichenoid lesions, denture stomatitis, burning mouth syndrome, or even acute facial swelling have been reported following dental work in patients with acrylate allergy.50-53 Dentists of patients with acrylate ACD should be informed of the diagnosis so acrylates can be avoided during dental work; if unavoidable, all possible steps should be taken to ensure complete curing of the monomers. In the surgical setting, patients sensitized to cyanoacrylate-based tissue adhesives should be offered wound closure alternatives such as sutures or staples.34
In patients with diabetes mellitus who develop ACD to their glucose monitor or insulin pump, ideally they should be switched to a device that does not contain acrylates. Problematically, these devices are constantly being reformulated, and manufacturers do not always divulge their components, which can make it challenging to determine safe alternative options.32,54 Various barrier products may help on a case-by-case basis.55Preventative measures should be implemented in workplaces that utilize acrylates, including dental practices and nail salons. Acrylic monomers have been shown to penetrate most gloves within minutes of exposure.56,57 Double gloving with nitrile gloves affords some protection for no longer than 60 minutes.6 4H gloves have been shown to provide true protection but result in a loss of dexterity.58 The fingerstall technique involves removing the fingers from a 4H glove, inserting them on the fingers, and applying a more flexible glove on top to hold them in place; this offers a hybrid between protection and finger dexterity.59
Final Interpretation
In a world characterized by technological advancements and increasing accessibility to acrylate-containing products, we hope this brief review serves as a resource and reminder to dermatologists to consider acrylates as a potential cause of ACD with diverse presentations and important future implications for affected individuals. The rising trend of acrylate allergy necessitates comprehensive assessment and shared decision-making between physicians and patients. As we navigate the ever-changing landscape of materials and technologies, clinicians must remain vigilant to avoid some potentially sticky situations for patients.
Acrylates are a ubiquitous family of synthetic thermoplastic resins that are employed in a wide array of products. Since the discovery of acrylic acid in 1843 and its industrialization in the early 20th century, acrylates have been used by many different sectors of industry.1 Today, acrylates can be found in diverse sources such as adhesives, coatings, electronics, nail cosmetics, dental materials, and medical devices. Although these versatile compounds have revolutionized numerous sectors, their potential to trigger allergic contact dermatitis (ACD) has garnered considerable attention in recent years. In 2012, acrylates as a group were named Allergen of the Year by the American Contact Dermatitis Society,2 and one member—isobornyl acrylate—also was given the infamous award in 2020.3 In this article, we highlight the chemistry of acrylates, the growing prevalence of acrylate contact allergy, common sources of exposure, patch testing considerations, and management/prevention strategies.
Chemistry and Uses of Acrylates
Acrylates are widely used due to their pliable and resilient properties.4 They begin as liquid monomers of (meth)acrylic acid or cyanoacrylic acid that are molded to the desired application before being cured or hardened by one of several means: spontaneously, using chemical catalysts, or with heat, UV light, or a light-emitting diode. Once cured, the final polymers (ie, [meth]acrylates, cyanoacrylates) serve a myriad of different purposes. Table 1 includes some of the more clinically relevant sources of acrylate exposure. Although this list is not comprehensive, it offers a glimpse into the vast array of uses for acrylates.

Acrylate Contact Allergy
Acrylic monomers are potent contact allergens, but the polymerized final products are not considered allergenic, assuming they are completely cured; however, ACD can occur with incomplete curing.6 It is of clinical importance that once an individual becomes sensitized to one type of acrylate, they may develop cross-reactions to others contained in different products. Notably, cyanoacrylates generally do not cross-react with (meth)acrylates; this has important implications for choosing safe alternative products in sensitized patients, though independent sensitization to cyanoacrylates is possible.7,8
Epidemiology and Risk Factors
The prevalence of acrylate allergy in the general population is unknown; however, there is a trend of increased patch test positivity in studies of patients referred for patch testing. A 2018 study by the European Environmental Contact Dermatitis Research Group reported positive patch tests to acrylates in 1.1% of 18,228 patients tested from 2013 to 2015.9 More recently, a multicenter European study (2019-2020) reported a 2.3% patch test positivity to 2-hydroxyethyl methacrylate (HEMA) among 7675 tested individuals,10 and even higher HEMA positivity was reported in Spain (3.7% of 1884 patients in 2019-2020).11 In addition, the North American Contact Dermatitis Group (NACDG) reported positive patch test reactions to HEMA in 3.2% of 4111 patients tested from 2019 to 2020, a statistically significant increase compared with those tested in 2009 to 2018 (odds ratio, 1.25 [95% CI, 1.03-1.51]; P=.02).12
Historically, acrylate sensitization primarily stemmed from occupational exposure. A retrospective analysis of occupational dermatitis performed by the NACDG (2001-2016) showed that HEMA was among the top 10 most common occupational allergens (3.4% positivity [83/2461]) and had the fifth highest percentage of occupationally relevant reactions (73.5% [83/113]).13 High-risk occupations include dental providers and nail technicians. Dentistry utilizes many materials containing acrylates, including uncured plastic resins used in dental prostheses, dentin bonding materials, and glass ionomers.14 A retrospective analysis of 585 dental personnel who were patch tested by the NACDG (2001-2018) found that more than 20% of occupational ACD cases were related to acrylates.15 Nail technicians are another group routinely exposed to acrylates through a variety of modern nail cosmetics. In a 7-year study from Portugal evaluating acrylate ACD, 68% (25/37) of cases were attributed to occupation, 80% (20/25) of which were in nail technicians.16 Likewise, among 28 nail technicians in Sweden who were referred for patch testing, 57% (16/28) tested positive for at least 1 acrylate.17
Modern Sources of Acrylate Exposure
Once thought to be a predominantly occupational exposure, acrylates have rapidly made their way into everyday consumer products. Clinicians should be aware of several sources of clinically relevant acrylate exposure, including nail cosmetics, consumer electronics, and medical/surgical adhesives.
A 2016 study found a shift to nail cosmetics as the most common source of acrylate sensitization.18 Nail cosmetics that contain acrylates include traditional acrylic, gel (shellac), dipped, and press-on (false) nails.19 The NACDG found that the most common allergen in patients experiencing ACD associated with nail products (2001-2016) was HEMA (56.6% [273/482]), far ahead of the traditional nail polish allergen tosylamide (36.2% [273/755]). Over the study period, the frequency of positive patch tests statistically increased for HEMA (P=.0069) and decreased for tosylamide (P<.0001).20 There is concern that the use of home gel nail kits, which can be purchased online at the click of a button, may be associated with a risk for acrylate sensitization.21,22 A recent study surveyed a Facebook support group for individuals with self-reported reactions to nail cosmetics, finding that 78% of the 199 individuals had used at-home gel nail kits, and more than 80% of them first developed skin reactions after starting to use at-home kits.23 The risks for sensitization are thought to be greater when self-applying nail acrylates compared to having them done professionally because individuals are more likely to spill allergenic monomers onto the skin at home; it also is possible that home techniques could lead to incomplete curing. Table 2 reviews the different types of acrylic nail cosmetics.

Medical adhesives and equipment are other important areas where acrylates can be encountered in abundance. A review by Spencer et al18 cautioned wound dressings as an up-and-coming source of sensitization, and this has been demonstrated in the literature as coming to fruition.26 Another study identified acrylates in 15 of 16 (94%) tested medical adhesives; among 7 medical adhesives labeled as hypoallergenic, 100% still contained acrylates and/or abietic acid.27 Multiple case reports have described ACD to adhesives of electrocardiogram electrodes containing acrylates.28-31 Physicians providing care to patients with diabetes mellitus also must be aware of acrylates in glucose monitors and insulin pumps, either found in the adhesives or leaching from the inside of the device to reach the skin.32 Isobornyl acrylate in particular has made quite the name for itself in this sector, being crowned the 2020 Allergen of the Year owing to its key role in cases of ACD to diabetes devices.3
Cyanoacrylate-based tissue adhesives (eg, 2‐octyl cyanoacrylate) are now well documented to cause postoperative ACD.33,34 Although robust prospective data are limited, studies suggest that 2% to 14% of patients develop postoperative skin reactions following 2-octyl cyanoacrylate application.35-37 It has been shown that sensitization to tissue adhesives often occurs after the first application, followed by an eruption of ACD as long as a month later, which can create confusion about the nature of the rash for patients and health care providers alike, who may for instance attribute it to infection rather than allergy.38 In the orthopedic literature, a woman with a known history of acrylic nail ACD had knee arthroplasty failure attributed to acrylic bone cement with resolution of the joint symptoms after changing to a cementless device.39
Awareness of the common use of acrylates is important to identify the cause of reactions from products that would otherwise seem nonallergenic. A case of occupational ACD to isobornyl acrylate in UV-cured phone screen protectors has been reported40; several cases of ACD to acrylates in headphones41,42 as well as one related to a wearable fitness device also have been reported.43 Given all these possible sources of exposure, ACD to acrylates should be on your radar.
When to Consider Acrylate ACD
When working up a patient with dermatitis, it is essential to ask about occupational history and hobbies to get a sense of potential contact allergen exposures. The typical presentation of occupational acrylate-associated ACD is hand eczema, specifically involving the fingertips.5,24,25,44 Acrylate ACD should be considered in patients with nail dystrophy and a history of wearing acrylic nails.45 There can even be involvement of the face and eyelids secondary to airborne contact or ectopic spread from the hands.24 Spreading vesicular eruptions associated with adhesives also should raise concern. The Figure depicts several possible presentations of ACD to acrylates. In a time of abundant access to products containing acrylates, dermatologists should consider this allergy in their differential diagnosis and consider patch testing.

Patch Testing to Acrylates
The gold standard for ACD diagnosis is patch testing. It should be noted that no acrylates are included in the thin-layer rapid use epicutaneous (T.R.U.E.) test series. Several acrylates are tested in expanded patch test series including the American Contact Dermatitis Society Core Allergen series and North American 80 Comprehensive Series. 2-Hydroxyethyl methacrylate is thought to be the most important screening allergen to test. Ramos et al16 reported a positive patch test to HEMA in 81% (30/37) of patients who had any type of acrylate allergy.
If initial testing to a limited number of acrylates is negative but clinical suspicion remains high, expanded acrylates/plastics and glue series also are available from commercial patch test suppliers. Testing to an expanded panel of acrylates is especially pertinent to consider in suspected occupational cases given the risk of workplace absenteeism and even disability that come with continued exposure to the allergen. Of note, isobornyl acrylate is not included in the baseline patch test series and must be tested separately, particularly because it usually does not cross-react with other acrylates, and therefore allergy could be missed if not tested on its own.
Acrylates are volatile substances that have been shown to degrade at room temperature and to a lesser degree when refrigerated. Ideally, they should be stored in a freezer and not used beyond their expiration date. Furthermore, it is advised that acrylate patch tests be prepared immediately prior to placement on the patient and to discard the initial extrusion from the syringe, as the concentration at the tip may be decreased.46,47
With regard to tissue adhesives, the actual product should be tested as-is because these are not commercially available patch test substances.48 Occasionally, patients who are sensitized to the tissue adhesive will not react when patch tested on intact skin. If clinical suspicion remains high, scratch patch testing may confirm contact allergy in cases of negative testing on intact skin.49
Management and Prevention
Once a diagnosis of ACD secondary to acrylates has been established, counseling patients on allergen avoidance strategies is essential. For (meth)acrylate-allergic patients who want to continue using modern nail products, cyanoacrylate-based options (eg, dipped, press-on nails) can be considered as an alternative, as they do not cross-react, though independent sensitization is still possible. However, traditional nail polish is the safest option to recommend.
The concern with acrylate sensitization extends beyond the immediate issue that brought the patient into your clinic. Dermatologists must counsel patients who are sensitized to acrylates on the possible sequelae of acrylate-containing dental or orthopedic procedures. Oral lichenoid lesions, denture stomatitis, burning mouth syndrome, or even acute facial swelling have been reported following dental work in patients with acrylate allergy.50-53 Dentists of patients with acrylate ACD should be informed of the diagnosis so acrylates can be avoided during dental work; if unavoidable, all possible steps should be taken to ensure complete curing of the monomers. In the surgical setting, patients sensitized to cyanoacrylate-based tissue adhesives should be offered wound closure alternatives such as sutures or staples.34
In patients with diabetes mellitus who develop ACD to their glucose monitor or insulin pump, ideally they should be switched to a device that does not contain acrylates. Problematically, these devices are constantly being reformulated, and manufacturers do not always divulge their components, which can make it challenging to determine safe alternative options.32,54 Various barrier products may help on a case-by-case basis.55Preventative measures should be implemented in workplaces that utilize acrylates, including dental practices and nail salons. Acrylic monomers have been shown to penetrate most gloves within minutes of exposure.56,57 Double gloving with nitrile gloves affords some protection for no longer than 60 minutes.6 4H gloves have been shown to provide true protection but result in a loss of dexterity.58 The fingerstall technique involves removing the fingers from a 4H glove, inserting them on the fingers, and applying a more flexible glove on top to hold them in place; this offers a hybrid between protection and finger dexterity.59
Final Interpretation
In a world characterized by technological advancements and increasing accessibility to acrylate-containing products, we hope this brief review serves as a resource and reminder to dermatologists to consider acrylates as a potential cause of ACD with diverse presentations and important future implications for affected individuals. The rising trend of acrylate allergy necessitates comprehensive assessment and shared decision-making between physicians and patients. As we navigate the ever-changing landscape of materials and technologies, clinicians must remain vigilant to avoid some potentially sticky situations for patients.
- Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent. 2021;23:397-406.
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society Allergens of the Year 2000 to 2020. Dermatol Clin. 2020;38:309-320.
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Society Allergen of the Year. Cutis. 2020;105:283-285.
- Ajekwene KK. Properties and applications of acrylates. In: Serrano-Aroca A, Deb S, eds. Acrylate Polymers for Advanced Applications. IntechOpen; 2020:35-46. https://doi.org/10.5772/intechopen.89867
- Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45:277-283.
- Sasseville D. Acrylates in contact dermatitis. Dermat Contact Atopic Occup Drug. 2012;23:6-16.
- Gardeen S, Hylwa S. A review of acrylates: super glue, nail adhesives, and diabetic pump adhesives increasing sensitization risk in women and children. Int J Womens Dermatol. 2020;6:263-267.
- Chou M, Dhingra N, Strugar TL. Contact sensitization to allergens in nail cosmetics. Dermat Contact Atopic Occup Drug. 2017;28:231-240.
- Gonçalo M, Pinho A, Agner T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2018;78:254-260.
- Uter W, Wilkinson SM, Aerts O, et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermatitis. 2022;87:343-355.
- Hernández-Fernández CP, Mercader-García P, Silvestre Salvador JF, et al. Candidate allergens for inclusion in the Spanish standard series based on data from the Spanish Contact Dermatitis Registry. Actas Dermosifiliogr. 2021;112:798-805.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermat Contact Atopic Occup Drug. 2023;34:90-104.
- DeKoven JG, DeKoven BM, Warshaw EM, et al. Occupational contact dermatitis: retrospective analysis of North American Contact Dermatitis Group Data, 2001 to 2016. J Am Acad Dermatol. 2022;86:782-790.
- Heratizadeh A, Werfel T, Schubert S, et al. Contact sensitization in dental technicians with occupational contact dermatitis. data of the Information Network of Departments of Dermatology (IVDK) 2001-2015. Contact Dermatitis. 2018;78:266-273.
- Warshaw EM, Ruggiero JL, Atwater AR, et al. Occupational contact dermatitis in dental personnel: a retrospective analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermat Contact Atopic Occup Drug. 2022;33:80-90.
- Ramos L, Cabral R, Gonçalo M. Allergic contact dermatitis caused by acrylates and methacrylates—a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study. Contact Dermatitis. 2019;81:58-60.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review. Contact Dermatitis. 2016;75:157-164.
- DeKoven S, DeKoven J, Holness DL. (Meth)acrylate occupational contact dermatitis in nail salon workers: a case series. J Cutan Med Surg. 2017;21:340-344.
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermat Contact Atopic Occup Drug. 2020;31:191-201.
- Le Q, Cahill J, Palmer-Le A, et al. The rising trend in allergic contact dermatitis to acrylic nail products. Australas J Dermatol. 2015;56:221-223.
- Gatica-Ortega ME, Pastor-Nieto M. The present and future burden of contact dermatitis from acrylates in manicure. Curr Treat Options Allergy. 2020;7:1-21.
- Guenther J, Norman T, Wee C, et al. A survey of skin reactions associated with acrylic nail cosmetics, with a focus on home kits: is there a need for regulation [published online October 16, 2023]? Dermatitis. doi:10.1089/derm.2023.0204
- Calado R, Gomes T, Matos A, et al. Contact dermatitis to nail cosmetics. Curr Dermatol Rep. 2021;10:173-181.
- Draelos ZD. Nail cosmetics and adornment. Dermatol Clin. 2021;39:351-359.
- Mestach L, Huygens S, Goossens A, et al. Allergic contact dermatitis caused by acrylic-based medical dressings and adhesives. Contact Dermatitis. 2018;79:81-84.
- Tam I, Wang JX, Yu JD. Identifying acrylates in medical adhesives. Dermat Contact Atopic Occup Drug. 2020;31:E40-E42.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermatitis. 2015;73:44-48.
- Ozkaya E, Kavlak Bozkurt P. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: a rare case with concomitant roles of nickel and acrylates. Contact Dermatitis. 2014;70:121-123.
- Lyons G, Nixon R. Allergic contact dermatitis to methacrylates in ECG electrode dots. Australas J Dermatol. 2013;54:39-40.
- Jelen G. Acrylate, a hidden allergen of electrocardiogram electrodes. Contact Dermatitis. 2001;45:315-316.
- Bembry R, Brys AK, Atwater AR. Medical device contact allergy: glucose monitors and insulin pumps. Curr Dermatol Rep. 2022;11:13-20.
- Liu T, Wan J, McKenna RA, et al. Allergic contact dermatitis caused by Dermabond in a paediatric patient undergoing skin surgery. Contact Dermatitis. 2019;80:61-62.
- Ricciardo BM, Nixon RL, Tam MM, et al. Allergic contact dermatitis to Dermabond Prineo after elective orthopedic surgery. Orthopedics. 2020;43:E515-E522.
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? the incidence of dermatitis after 2-octyl cyanoacrylate exposure in 102 consecutive breast cases. Plast Reconstr Surg. 2020;145:32-37.
- Alotaibi NN, Ahmad T, Rabah SM, et al. Type IV hypersensitivity reaction to Dermabond (2-octyl cyanoacrylate) in plastic surgical patients: a retrospective study. Plast Surg Oakv Ont. 2022;30:222-226.
- Durando D, Porubsky C, Winter S, et al. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermat Contact Atopic Occup Drug. 2014;25:99-100.
- Asai C, Inomata N, Sato M, et al. Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure. Contact Dermatitis. 2021;84:103-108.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59:S123-S124.
- Amat-Samaranch V, Garcia-Melendo C, Tubau C, et al. Occupational allergic contact dermatitis to isobornyl acrylate present in cell phone screen protectors. Contact Dermatitis. 2021;84:352-354.
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112.
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461.
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560.
- Nanda S. Nail salon safety: from nail dystrophy to acrylate contact allergies. Cutis. 2022;110:E32-E33.
- Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermat Contact Atopic Occup Drug. 2013;24:227-236.
- Jou PC, Siegel PD, Warshaw EM. Vapor pressure and predicted stability of American Contact Dermatitis Society core allergens. Dermat Contact Atopic Occup Drug. 2016;27:193-201.
- Cook KA, White AA, Shaw DW. Patch testing ingredients of Dermabond and other cyanoacrylate-containing adhesives. Dermat Contact Atopic Occup Drug. 2019;30:314-322.
- Patel K, Nixon R. Scratch patch testing to Dermabond in a patient with suspected allergic contact dermatitis. Dermat Contact Atopic Occup Drug. 2023;34:250-251.
- Ditrichova D, Kapralova S, Tichy M, et al. Oral lichenoid lesions and allergy to dental materials. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2007;151:333-339.
- Chen AYY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6:92-94.
- Marino R, Capaccio P, Pignataro L, et al. Burning mouth syndrome: the role of contact hypersensitivity. Oral Dis. 2009;15:255-258.
- Obayashi N, Shintani T, Kamegashira A, et al. A case report of allergic reaction with acute facial swelling: a rare complication of dental acrylic resin. J Int Med Res. 2023;51:3000605231187819.
- Cameli N, Silvestri M, Mariano M, et al. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermat Contact Atopic Occup Drug. 20221;33:110-115.
- Ng KL, Nixon RL, Grills C, et al. Solution using Stomahesive® wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Australas J Dermatol. 2022;63:E56-E59.
- Lönnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci. 2003;111:440-446.
- Sananez A, Sanchez A, Davis L, et al. Allergic reaction from dental bonding material through nitrile gloves: clinical case study and glove permeability testing. J Esthet Restor Dent. 2020;32:371-379.
- Andersson T, Bruze M, Björkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis. 1999;41:254-259.
- Roche E, Cuadra J, Alegre V. Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases. Actas Dermo-Sifiliográficas. 2009;99:788-794.
- Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent. 2021;23:397-406.
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society Allergens of the Year 2000 to 2020. Dermatol Clin. 2020;38:309-320.
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Society Allergen of the Year. Cutis. 2020;105:283-285.
- Ajekwene KK. Properties and applications of acrylates. In: Serrano-Aroca A, Deb S, eds. Acrylate Polymers for Advanced Applications. IntechOpen; 2020:35-46. https://doi.org/10.5772/intechopen.89867
- Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45:277-283.
- Sasseville D. Acrylates in contact dermatitis. Dermat Contact Atopic Occup Drug. 2012;23:6-16.
- Gardeen S, Hylwa S. A review of acrylates: super glue, nail adhesives, and diabetic pump adhesives increasing sensitization risk in women and children. Int J Womens Dermatol. 2020;6:263-267.
- Chou M, Dhingra N, Strugar TL. Contact sensitization to allergens in nail cosmetics. Dermat Contact Atopic Occup Drug. 2017;28:231-240.
- Gonçalo M, Pinho A, Agner T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2018;78:254-260.
- Uter W, Wilkinson SM, Aerts O, et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermatitis. 2022;87:343-355.
- Hernández-Fernández CP, Mercader-García P, Silvestre Salvador JF, et al. Candidate allergens for inclusion in the Spanish standard series based on data from the Spanish Contact Dermatitis Registry. Actas Dermosifiliogr. 2021;112:798-805.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermat Contact Atopic Occup Drug. 2023;34:90-104.
- DeKoven JG, DeKoven BM, Warshaw EM, et al. Occupational contact dermatitis: retrospective analysis of North American Contact Dermatitis Group Data, 2001 to 2016. J Am Acad Dermatol. 2022;86:782-790.
- Heratizadeh A, Werfel T, Schubert S, et al. Contact sensitization in dental technicians with occupational contact dermatitis. data of the Information Network of Departments of Dermatology (IVDK) 2001-2015. Contact Dermatitis. 2018;78:266-273.
- Warshaw EM, Ruggiero JL, Atwater AR, et al. Occupational contact dermatitis in dental personnel: a retrospective analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermat Contact Atopic Occup Drug. 2022;33:80-90.
- Ramos L, Cabral R, Gonçalo M. Allergic contact dermatitis caused by acrylates and methacrylates—a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study. Contact Dermatitis. 2019;81:58-60.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review. Contact Dermatitis. 2016;75:157-164.
- DeKoven S, DeKoven J, Holness DL. (Meth)acrylate occupational contact dermatitis in nail salon workers: a case series. J Cutan Med Surg. 2017;21:340-344.
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermat Contact Atopic Occup Drug. 2020;31:191-201.
- Le Q, Cahill J, Palmer-Le A, et al. The rising trend in allergic contact dermatitis to acrylic nail products. Australas J Dermatol. 2015;56:221-223.
- Gatica-Ortega ME, Pastor-Nieto M. The present and future burden of contact dermatitis from acrylates in manicure. Curr Treat Options Allergy. 2020;7:1-21.
- Guenther J, Norman T, Wee C, et al. A survey of skin reactions associated with acrylic nail cosmetics, with a focus on home kits: is there a need for regulation [published online October 16, 2023]? Dermatitis. doi:10.1089/derm.2023.0204
- Calado R, Gomes T, Matos A, et al. Contact dermatitis to nail cosmetics. Curr Dermatol Rep. 2021;10:173-181.
- Draelos ZD. Nail cosmetics and adornment. Dermatol Clin. 2021;39:351-359.
- Mestach L, Huygens S, Goossens A, et al. Allergic contact dermatitis caused by acrylic-based medical dressings and adhesives. Contact Dermatitis. 2018;79:81-84.
- Tam I, Wang JX, Yu JD. Identifying acrylates in medical adhesives. Dermat Contact Atopic Occup Drug. 2020;31:E40-E42.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermatitis. 2015;73:44-48.
- Ozkaya E, Kavlak Bozkurt P. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: a rare case with concomitant roles of nickel and acrylates. Contact Dermatitis. 2014;70:121-123.
- Lyons G, Nixon R. Allergic contact dermatitis to methacrylates in ECG electrode dots. Australas J Dermatol. 2013;54:39-40.
- Jelen G. Acrylate, a hidden allergen of electrocardiogram electrodes. Contact Dermatitis. 2001;45:315-316.
- Bembry R, Brys AK, Atwater AR. Medical device contact allergy: glucose monitors and insulin pumps. Curr Dermatol Rep. 2022;11:13-20.
- Liu T, Wan J, McKenna RA, et al. Allergic contact dermatitis caused by Dermabond in a paediatric patient undergoing skin surgery. Contact Dermatitis. 2019;80:61-62.
- Ricciardo BM, Nixon RL, Tam MM, et al. Allergic contact dermatitis to Dermabond Prineo after elective orthopedic surgery. Orthopedics. 2020;43:E515-E522.
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? the incidence of dermatitis after 2-octyl cyanoacrylate exposure in 102 consecutive breast cases. Plast Reconstr Surg. 2020;145:32-37.
- Alotaibi NN, Ahmad T, Rabah SM, et al. Type IV hypersensitivity reaction to Dermabond (2-octyl cyanoacrylate) in plastic surgical patients: a retrospective study. Plast Surg Oakv Ont. 2022;30:222-226.
- Durando D, Porubsky C, Winter S, et al. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermat Contact Atopic Occup Drug. 2014;25:99-100.
- Asai C, Inomata N, Sato M, et al. Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure. Contact Dermatitis. 2021;84:103-108.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59:S123-S124.
- Amat-Samaranch V, Garcia-Melendo C, Tubau C, et al. Occupational allergic contact dermatitis to isobornyl acrylate present in cell phone screen protectors. Contact Dermatitis. 2021;84:352-354.
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112.
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461.
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560.
- Nanda S. Nail salon safety: from nail dystrophy to acrylate contact allergies. Cutis. 2022;110:E32-E33.
- Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermat Contact Atopic Occup Drug. 2013;24:227-236.
- Jou PC, Siegel PD, Warshaw EM. Vapor pressure and predicted stability of American Contact Dermatitis Society core allergens. Dermat Contact Atopic Occup Drug. 2016;27:193-201.
- Cook KA, White AA, Shaw DW. Patch testing ingredients of Dermabond and other cyanoacrylate-containing adhesives. Dermat Contact Atopic Occup Drug. 2019;30:314-322.
- Patel K, Nixon R. Scratch patch testing to Dermabond in a patient with suspected allergic contact dermatitis. Dermat Contact Atopic Occup Drug. 2023;34:250-251.
- Ditrichova D, Kapralova S, Tichy M, et al. Oral lichenoid lesions and allergy to dental materials. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2007;151:333-339.
- Chen AYY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6:92-94.
- Marino R, Capaccio P, Pignataro L, et al. Burning mouth syndrome: the role of contact hypersensitivity. Oral Dis. 2009;15:255-258.
- Obayashi N, Shintani T, Kamegashira A, et al. A case report of allergic reaction with acute facial swelling: a rare complication of dental acrylic resin. J Int Med Res. 2023;51:3000605231187819.
- Cameli N, Silvestri M, Mariano M, et al. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermat Contact Atopic Occup Drug. 20221;33:110-115.
- Ng KL, Nixon RL, Grills C, et al. Solution using Stomahesive® wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Australas J Dermatol. 2022;63:E56-E59.
- Lönnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci. 2003;111:440-446.
- Sananez A, Sanchez A, Davis L, et al. Allergic reaction from dental bonding material through nitrile gloves: clinical case study and glove permeability testing. J Esthet Restor Dent. 2020;32:371-379.
- Andersson T, Bruze M, Björkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis. 1999;41:254-259.
- Roche E, Cuadra J, Alegre V. Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases. Actas Dermo-Sifiliográficas. 2009;99:788-794.
Practice Points
- Acrylates are thermoplastic resins used in a variety of products ranging from cosmetics to adhesives and industrial materials. Acrylic monomers are strong contact allergens, whereas fully polymerized forms are inert, provided they are completely cured.
- The use of home gel nail kits may increase the risk for sensitization to acrylates, which are the most common modern nail cosmetic allergens.
- When patch testing for suspected acrylate allergy, 2-hydroxyethyl methacrylate (HEMA) is the most important screening allergen. Expanded testing to additional acrylates should be considered depending on the clinical scenario.
Association Between Atopic Dermatitis and Chronic Obstructive Pulmonary Disease Among US Adults in the 1999-2006 NHANES Survey
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).
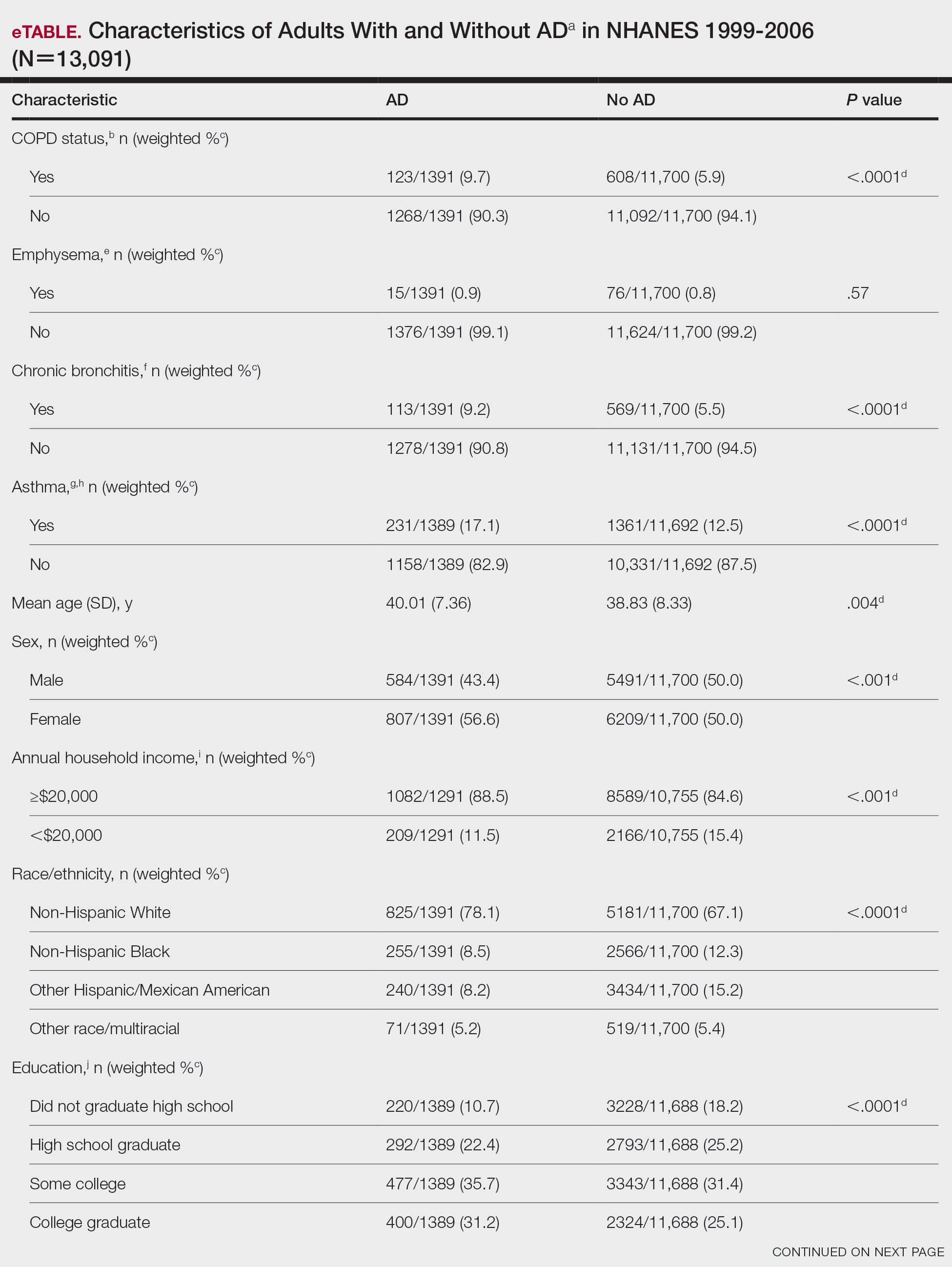
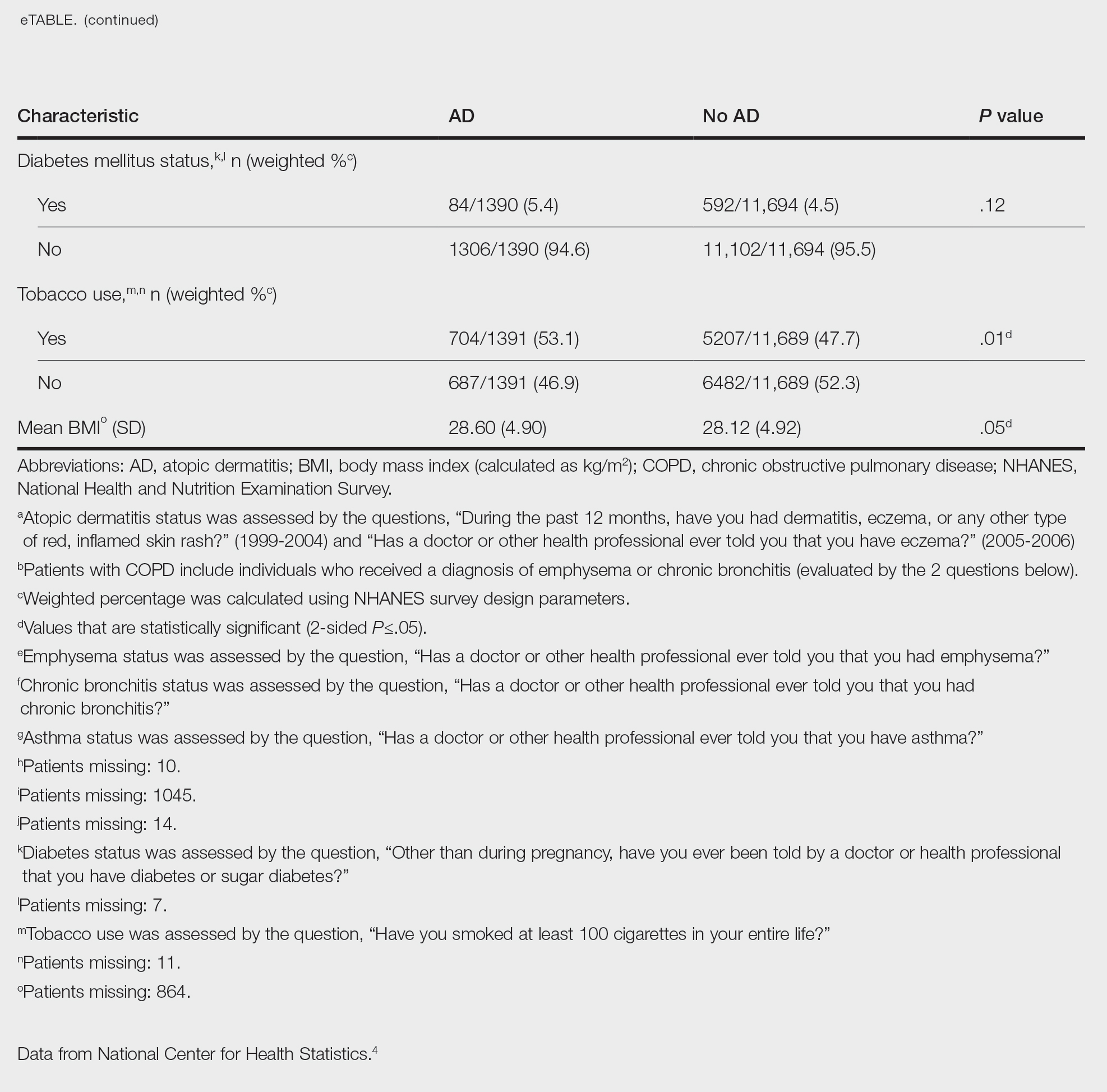
Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).
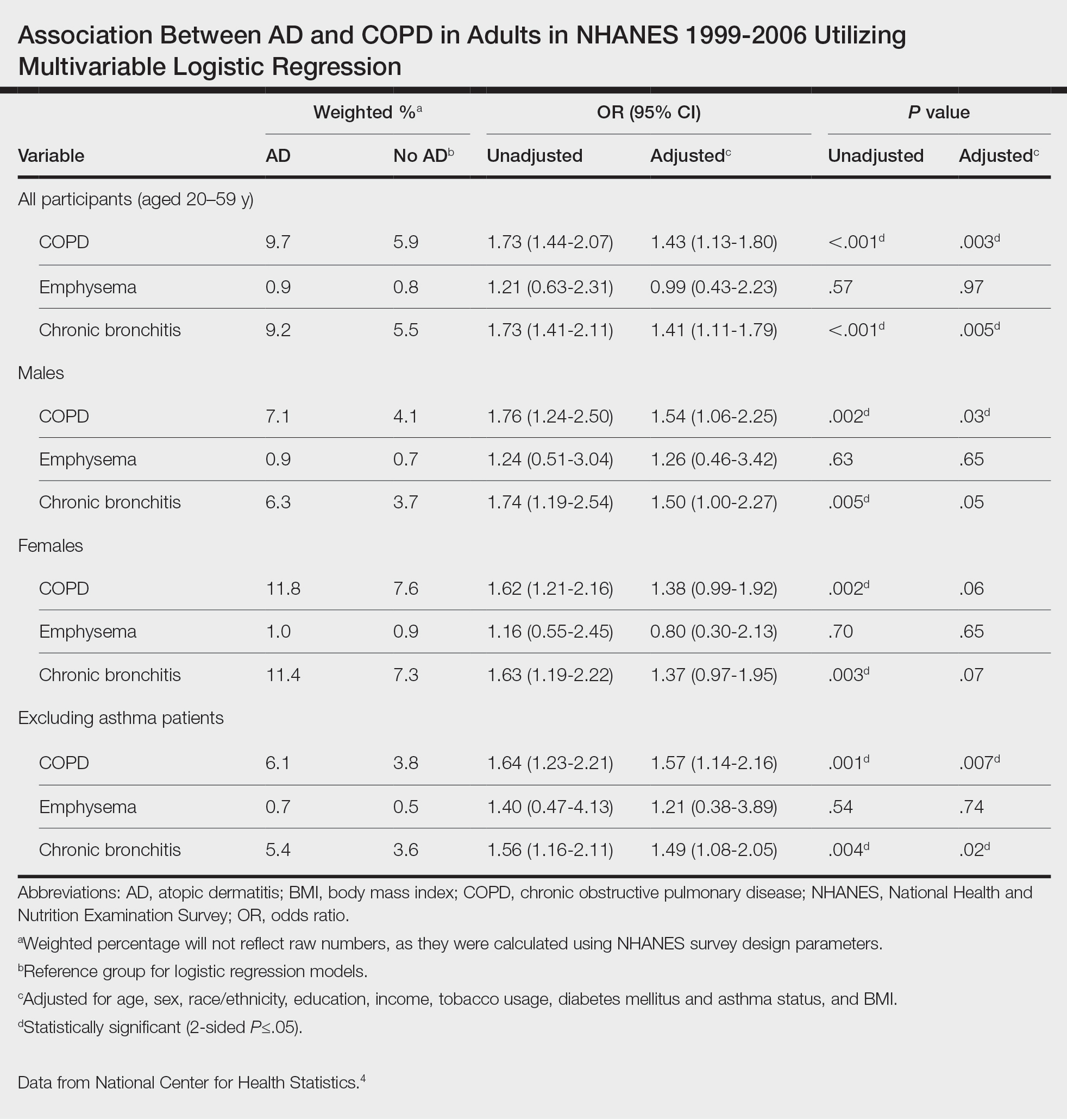
Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
Practice Points
- Various comorbidities are associated with atopic dermatitis (AD). Currently, research exploring the association between AD and chronic obstructive pulmonary disease is limited.
- Understanding the systemic diseases associated with inflammatory skin diseases can assist with adequate patient management.
Culprits of Medication-Induced Telogen Effluvium, Part 1
Alopecia is a commonly reported side effect of various medications. Anagen effluvium and telogen effluvium (TE) are considered the most common mechanisms underlying medication-related hair loss. Anagen effluvium is associated with chemotherapeutic agents and radiation therapy, with anagen shedding typically occurring within 2 weeks of medication administration.1,2 Medication-induced TE is a diffuse nonscarring alopecia that is a reversible reactive process.3-5 Telogen effluvium is clinically apparent as a generalized shedding of scalp hair 1 to 6 months after an inciting cause.6 The underlying cause of TE may be multifactorial and difficult to identify given the delay between the trigger and the onset of clinically apparent hair loss. Other known triggers of TE include acute illness,7,8 nutritional deficiencies,4,9 and/or major surgery.10
Each hair follicle independently and sequentially progresses through anagen growth, catagen transition, and telogen resting phases. In the human scalp, the telogen phase typically lasts 3 months, at the end of which the telogen hair is extruded from the scalp. Anagen and telogen follicles typically account for an average of 90% and 10% of follicles on the human scalp, respectively.11 Immediate anagen release is hypothesized to be the mechanism underlying medication-induced TE.12 This theory suggests that an increased percentage of anagen follicles prematurely enter the telogen phase, with a notable increase in hair shedding at the conclusion of the telogen phase approximately 1 to 6 months later.12 First-line management of medication-induced TE is identification and cessation of the causative agent, if possible. Notable regrowth of hair is expected several months after removal of the inciting medication. In part 1 of this 2-part series, we review the existing literature to identify common culprits of medication-induced TE, including retinoids, antifungals, and psychotropic medications.
Retinoids
Retinoids are vitamin A derivatives used in the treatment of a myriad of dermatologic and nondermatologic conditions.13,14 Retinoids modulate sebum production,15 keratinocyte proliferation,16 and epithelial differentiation through signal transduction downstream of the ligand-activated nuclear retinoic acid receptors and retinoid X receptors.13,14,17 The recommended daily dosage of retinol is 900 µg retinol activity equivalent (3000 IU) for men and 700 µg retinol activity equivalent (2333 IU) for women. Retinoids are used in the treatment of acne vulgaris,18 psoriasis,19 and ichthyosis.20 The most commonly reported adverse effects of systemic retinoid therapy include cheilitis, alopecia, and xerosis.21 Retinoid-associated alopecia is dose and duration dependent.19,21-24 A prospective study of acitretin therapy in plaque psoriasis reported that more than 63% (42/66) of patients on 50 mg or more of acitretin daily for 6 months or longer experienced alopecia that reversed with discontinuation.23 A systematic review of isotretinoin use in acne showed alopecia was seen in 3.2% (18/565) of patients on less than 0.5 mg/kg/d of isotretinoin and in 5.7% (192/3375) of patients on 0.5 mg/kg/d or less of isotretinoin.24 In a phase 2 clinical trial of orally administered 9-cis-retinoic acid (alitretinoin) in the treatment of Kaposi sarcoma related to AIDS, 42% (24/57) of adult male patients receiving 60, 100, or 140 mg/m2 alitretinoin daily (median treatment duration, 15.1 weeks) reported alopecia as an adverse effect of treatment.25 In one case report, a patient who ingested 500,000 IU of vitamin A daily for 4 months and then 100,000 IU monthly for 6 months experienced diffusely increased shedding of scalp hair along with muscle soreness, nail dystrophy, diffuse skin rash, and refractory ascites; he was found to have severe liver damage secondary to hypervitaminosis A that required liver transplantation.26 Regarding the pathomechanism of retinoid-induced alopecia, animal and in vitro studies similarly have demonstrated that all-trans-retinoic acid appears to exert its inhibitory effects on hair follicle growth via the influence of the transforming growth factor β2 and SMAD2/3 pathway influence on dermal papillae cells.14,27 Development of hair loss secondary to systemic retinoid therapy may be managed with dose reduction or cessation.
Antifungals
Azole medications have broad-spectrum fungistatic activity against a wide range of yeast and filamentous fungi. Azoles inhibit sterol 14α-demethylase activity, impairing ergosterol synthesis and thereby disrupting plasma membrane synthesis and activity of membrane-bound enzymes.28 Fluconazole is a systemic oral agent in this class that was first approved by the US Food and Drug Administration (FDA) for use in the 1990s.29 A retrospective study by the National Institute of Allergy and Infectious Disease Mycoses Study Group followed the clinical course of 33 patients who developed alopecia while receiving fluconazole therapy for various mycoses.30 The majority (88% [29/33]) of patients received 400 mg or more of fluconazole daily. The median time to hair loss after starting fluconazole was 3 months, and the scalp was involved in all cases. In 97% (32/33) of patients, resolution of alopecia was noted following discontinuation of fluconazole or a dose reduction of 50% or more. In 85% (28/33) of patients, complete resolution of alopecia occurred within 6 months of fluconazole cessation or dose reduction.30 Fluconazole-induced TE was reproducible in an animal model using Wistar rats31; however, further studies are required to clarify the molecular pathways of its effect on hair growth.
Voriconazole is an azole approved for the treatment of invasive aspergillosis, candidemia, and fungal infections caused by Scedosporium apiospermum and Fusarium species. A retrospective survey study of patients who received voriconazole for 1 month or longer found a considerable proportion of patients developed diffuse reversible hair loss.32 Scalp alopecia was noted in 79% (120/152) of patients who completed the survey, with a mean (SD) time to alopecia of 75 (54) days after initiation of voriconazole. Notable regrowth was reported in 69% (79/114) of patients who discontinued voriconazole for at least 3 months. A subgroup of 32 patients were changed to itraconazole or posaconazole, and hair loss stopped in 84% (27/32) with regrowth noted in 69% (22/32) of patients.32 Voriconazole and fluconazole share structural similarity not present with other triazoles.33,34 Because voriconazole-associated alopecia was reversed in the majority of patients who switched to itraconazole or posaconazole, the authors hypothesized that structural similarity of fluconazole and voriconazole may underly the greater risk for TE that is not a class effect of azole medications.31
Psychotropic Medications
Various psychotropic medications have been associated with hair loss. Valproic acid (or sodium valproate) is an anticonvulsant and mood-stabilizing agent used for the treatment of seizures, bipolar disorder (BD), migraines, and neuropathic pain.35,36 Divalproex sodium (or divalproex) is an enteric-coated formulation of sodium valproate and valproic acid with similar indications. Valproate is a notorious culprit of medication-induced hair loss, with alopecia listed among the most common adverse reactions (reported >5%) on its structure product labeling document.37 A systemic review and meta-analysis by Wang et al38 estimated the overall incidence of valproate-related alopecia to be 11% (95% CI, 0.08-0.13). Although this meta-analysis did not find an association between incidence of alopecia and dose or duration of valproate therapy,38 a separate review suggested that valproate-induced alopecia is dose dependent and can be managed with dose reduction.39 A 12-month, randomized, double-blind study of treatment of BD with divalproex (valproate derivative), lithium, or placebo (2:1:1 ratio) showed a significantly higher frequency of alopecia in the divalproex group compared with placebo (16% [30/187] vs 6% [6/94]; P=.03).40 Valproate-related hair loss is characteristically diffuse and nonscarring, often noted 3 to 6 months following initiation of valproate.41,42 The proposed mechanism of valproate-induced alopecia includes chelation of zinc and selenium,43 and a reduction in serum biotinidase activity, thereby decreasing the availability of these essential micronutrients required for hair growth.41 Studies examining the effects of valproate administration and serum biotinidase activity in patients have yielded conflicting results.44-46 In a study of children with seizures including 57 patients treated with valproic acid, 17 treated with carbamazepine, and 75 age- and sex-matched healthy controls, the authors found no significant differences in serum biotinidase enzyme activity across the 3 groups.44 In contrast, a study of 75 children with seizures on valproic acid therapy stratified by dose (mean [SD])—group A: 28.7 [8.5] mg/kg/d; group B: 41.6 [4.9] mg/kg/d; group C: 64.5 [5.8] mg/kg/d—found that patients receiving higher doses (groups B and C) had significantly reduced serum biotinidase activity (1.22
Lithium carbonate (lithium) is used in the treatment of BD. Despite its efficacy and low cost, its potential for adverse effects, narrow therapeutic index, and subsequent need for routine monitoring are factors that limit its use.48 Some reported dermatologic adverse reactions on its structure product labeling include xerosis, thinning of hair, alopecia, xerosis cutis, psoriasis onset/exacerbation, and generalized pruritus.49 A systematic review and meta-analysis of 385 studies identified 24 publications reporting adverse effects of lithium on hair with no significantly increased risk of alopecia overall.50 The analysis included 2 randomized controlled trials comparing the effects of lithium and placebo on hair loss in patients with BD. Hair loss was reported in 7% (7/94) of patients taking lithium and 6% (6/94) of the placebo group in the 12-month study40 and in 3% (1/32) of the lithium group and 0% (0/28) of the divalproex group in the 20-month study.51 Despite anecdotal reports of alopecia associated with lithium, there is a lack of high-quality evidence to support this claim. Of note, hypothyroidism is a known complication of lithium use, and serum testing of thyroid function at 6-month intervals is recommended for patients on lithium treatment.52 Because thyroid abnormalities can cause alopecia distinct from TE, new-onset alopecia during lithium use should prompt serum testing of thyroid function. The development of hypothyroidism secondary to lithium is not a direct contraindication to its use53; rather, treatment should be focused on correction with thyroid replacement therapy (eg, supplementation with thyroxine).54
Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs) and bupropion. Selective serotonin reuptake inhibitors affect the neuronal serotonin transporter, increasing the concentration of serotonin in the synaptic cleft available for stimulation of postsynaptic serotonin receptors55,56; bupropion is an antidepressant medication that inhibits norepinephrine and dopamine reuptake at the synaptic cleft.57 Alopecia is an infrequent (1 in 100 to 1 in 1000 patients) adverse effect for several SSRIs.58-62 A recent systematic review identified a total of 71 cases of alopecia associated with SSRI use including citalopram (n=11), escitalopram (n=7), fluoxetine (n=27), fluoxvamine (n=5), paroxetine (n=4), and sertraline (n=20), with a median time to onset of hair shedding of 8.6 weeks (range, 3 days to 5 years). Discontinuation of the suspected culprit SSRI led to improvement and/or resolution in 63% (51/81) episodes of alopecia, with a median time to improvement and/or resolution of 4 weeks.63 A comparative retrospective cohort study using a large US health claims database from 2006 to 2014 included more than 1 million new and mutually exclusive patients taking fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, paroxetine, duloxetine, venlafaxine, desvenlafaxine, and bupropion.64 Overall, 1% (1569/150,404) of patients treated with bupropion received 1 or more physician visits for alopecia. Patients on SSRIs generally had a lower risk for hair loss compared with patients using bupropion (citalopram: hazard ratio [HR], 0.80 [95% CI, 0.74-0.86]; escitalopram: HR, 0.79 [95% CI, 0.74-0.86]; fluoxetine: HR, 0.68 [95% CI, 0.63-0.74]; paroxetine: HR, 0.68 [95% CI, 0.62-0.74]; sertraline: HR, 0.74 [95% CI, 0.69-0.79]), with the exception of fluvoxamine (HR, 0.93 [95% CI, 0.64-1.37]). However, the type of alopecia, time to onset, and time to resolution were not reported, making it difficult to assess whether the reported hair loss was consistent with medication-induced TE. Additionally, the authors acknowledged that bupropion may have been prescribed for smoking cessation, which may carry a different risk profile for the development of alopecia.64 Several other case reports have described alopecia following treatment with SSRIs, including sertraline,65 fluvoxamine,66 paroxetine,67 fluoxetine,68 and escitalopram.69
Overall, it appears that the use of SSRIs portends relatively low risk for alopecia and medication-induced TE. Little is known regarding the molecular effects of SSRIs on hair growth and the pathomechanism of SSRI-induced TE. The potential benefits of discontinuing a suspected culprit medication should be carefully weighed against the risks of medication cessation, and consideration should be given to alternative medications in the same class that also may be associated with TE. In patients requiring antidepressant therapy with suspected medication-induced TE, consider transitioning to a different class of medication with lower risk of medication-induced alopecia; for example, discontinuing bupropion in favor of an SSRI.
Final Thoughts
Medication-induced alopecia is an undesired side effect of many commonly used drugs and drug classes, including retinoids, azole antifungals, and mood stabilizers. Although the precise pathomechanisms of medication-induced TE remain unclear, the recommended management often requires identification of the likely causative agent and its discontinuation, if possible. Suspicion for medication-induced TE should prompt a thorough history of recent changes to medications, risk factors for nutritional deficiencies, underlying illnesses, and recent surgical procedures. Underlying nutritional, electrolyte, and/or metabolic disturbances should be corrected. In part 2 of this series, we will discuss medication-induced alopecia associated with anticoagulant and antihypertensive medications.
- Saleh D, Nassereddin A, Cook C. Anagen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482293/
- Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, et al. Anagen effluvium after neurointerventional radiation: trichoscopy as a diagnostic ally. Skin Appendage Disord. 2021;8:102-107. doi:10.1159/000518743
- Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67-73. doi:https://doi.org/10.1016/j.det.2012.08.002
- Chen V, Strazzulla L, Asbeck SM, et al. Etiology, management, and outcomes of pediatric telogen effluvium: a single-center study in the United States. Pediatr Dermatol. 2023;40:120-124. doi:10.1111/pde.15154
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Hughes EC, Saleh D. Telogen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK430848/
- Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77. doi:10.1016/j.jdin.2022.02.006
- Starace M, Piraccini BM, Evangelista V, et al. Acute telogen effluvium due to dengue fever mimicking androgenetic alopecia. Ital J Dermatol Venerol. 2023;158:66-67. doi:10.23736/s2784-8671.22.07369-8
- Patel KV, Farrant P, Sanderson JD, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. doi:10.1097/MIB.0b013e31828132de
- Cohen-Kurzrock RA, Cohen PR. Bariatric surgery–induced telogen effluvium (bar site): case report and a review of hair loss following weight loss surgery. Cureus. 2021;13:E14617. doi:10.7759/cureus.14617
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964-973. doi:10.1056/nejm199909233411307
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129:356-363. doi:10.1001/arcderm.1993.01680240096017
- Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427-439. doi:10.1002/jcp.21784
- Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119-1126. doi:10.1111/j.0022-202X.2005.23686.x
- Karlsson T, Vahlquist A, Kedishvili N, et al. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273-278. doi:10.1016/s0006-291x(03)00332-2
- Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402-3413. doi:10.1093/emboj/cdf331
- Geiger JM. Retinoids and sebaceous gland activity. Dermatology. 1995;191:305-310. doi:10.1159/000246581
- Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100:475-484.
- Pilkington T, Brogden RN. Acitretin. Drugs. 1992;43:597-627. doi:10.2165/00003495-199243040-00010
- Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38:164-180. doi:10.1111/pde.14408
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 suppl):S7-S12. doi:10.1016/s0190-9622(99)70359-2
- Tran PT, Evron E, Goh C. Characteristics of patients with hair loss after isotretinoin treatment: a retrospective review study. Int J Trichology. 2022;14:125-127. doi:10.4103/ijt.ijt_80_20
- Gupta AK, Goldfarb MT, Ellis CN, et al. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088-1093. doi:10.1016/s0190-9622(89)70138-9
- Lytvyn Y, McDonald K, Mufti A, et al. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125-142. doi:10.1016/j.jdin.2022.01.002
- Aboulafia DM, Norris D, Henry D, et al. 9-cis-Retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178-186. doi:10.1001/archderm.139.2.178
- Cheruvattath R, Orrego M, Gautam M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl. 2006;12:1888-1891. doi:10.1002/lt.21007
- Nan W, Li G, Si H, et al. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-β2/Smad2/3 pathway. Acta Histochem. 2020;122:151603. doi:10.1016/j.acthis.2020.151603
- Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279-291. doi:10.1128/aac.40.2.279
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40-79. doi:10.1128/cmr.12.1.40
- Pappas PG, Kauffman CA, Perfect J, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354-357. doi:10.7326/0003-4819-123-5-199509010-00006
- Thompson GR 3rd, Krois CR, Affolter VK, et al. Examination of fluconazole-induced alopecia in an animal model and human cohort. Antimicrob Agents Chemother. 2019;63:e01384-18. doi:10.1128/aac.01384-18
- Malani AN, Kerr L, Obear J, et al. Alopecia and nail changes associated with voriconazole therapy. Clin Infect Dis. 2014;59:E61-E65. doi:10.1093/cid/ciu275
- Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241-248. doi:10.1080/08998280.2003.11927910
- Drabin´ska B, Dettlaff K, Kossakowski K, et al. Structural and spectroscopic properties of voriconazole and fluconazole—experimental and theoretical studies. Open Chemistry. 2022;20:1575-1590. doi:10.1515/chem-2022-0253
- Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. doi:10.1016/s0301-0082(98)00075-6
- Gill D, Derry S, Wiffen PJ, et al. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011:CD009183. doi:10.1002/14651858.CD009183.pub2
- Depakote, Prescribing information. Abbott Laboratories; 2011. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018723s037lbl.pdf
- Wang X, Wang H, Xu D, et al. Risk of valproic acid-related alopecia: a systematic review and meta-analysis. Seizure. 2019;69:61-69. doi:10.1016/j.seizure.2019.04.003
- Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12:35-42. doi:10.1023/a:1009074926921
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481-489. doi:10.1001/archpsyc.57.5.481
- Praharaj SK, Munoli RN, Udupa ST, et al. Valproate-associated hair abnormalities: pathophysiology and management strategies. Hum Psychopharmacol. 2022;37:E2814. doi:10.1002/hup.2814
- Wilting I, van Laarhoven JH, de Koning-Verest IF, et al. Valproic acid-induced hair-texture changes in a white woman. Epilepsia. 2007;48:400-401. doi:10.1111/j.1528-1167.2006.00933.x
- Potter WZ, Ketter TA. Pharmacological issues in the treatment of bipolar disorder: focus on mood-stabilizing compounds. Can J Psychiatry. 1993;38(3 suppl 2):S51-S56.
- Castro-Gago M, Gómez-Lado C, Eirís-Pun´al J, et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2009;25:32-35. doi:10.1177/0883073809336118
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. doi:10.1046/j.1528-1157.2001.47000.x
- Yilmaz Y, Tasdemir HA, Paksu MS. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur J Paediatr Neurol. 2009;13:439-443. doi:10.1016/j.ejpn.2008.08.007
- Henriksen O, Johannessen SI. Clinical and pharmacokinetic observations on sodium valproate—a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand. 1982;65:504-523. doi:10.1111/j.1600-0404.1982.tb03106.x
- Fountoulakis KN, Tohen M, Zarate CA Jr. Lithium treatment of bipolar disorder in adults: a systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100-115. doi:10.1016/j.euroneuro.2021.10.003
- Lithium carbonate. Prescribing information. West-Ward Pharmaceuticals; 2018. Accessed November 20, 2023. https://ww.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf
- McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721-728. doi:10.1016/s0140-6736(11)61516-x
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152-2161. doi:10.1176/appi.ajp.162.11.2152.
- Duce HL, Duff CJ, Zaidi S, et al. Evaluation of thyroid function monitoring in people treated with lithium: advice based on real-world data. Bipolar Disord. 2023;25:402-409. doi:10.1111/bdi.13298
- Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi:10.1186/1745-0179-2-23.
- Joffe RT. How should lithium-induced thyroid dysfunction be managed in patients with bipolar disorder? J Psychiatry Neurosci. 2002;27:392.
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1-21. doi:10.2165/00003088-199700321-00003
- Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. StatPearls Publishing; 2023.
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159-166. doi:10.4088/pcc.v06n0403
- Escitalopram. Prescribing information. Solco Healthcare US, LLC; 2022. Accessed November 20, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2ffc6ec3-830f-46bc-9b3f-7c42cefa39b2/spl-doc
- Fluoxetine. Eli Lilly & Company; 2017. Prescribing information. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Paxil. Prescribing information. GlaxoSmithKline; 2012. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020031s067,020710s031.pdf
- Zoloft. Prescribing information. Pfizer; 2016. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf
- Celexa. Prescribing information. Allergan; 2022. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: systematic review. Psychiatry Res. 2022;313:114620. 10.1016/j.psychres.2022.114620
- Etminan M, Sodhi M, Procyshyn RM, et al. Risk of hair loss with different antidepressants: a comparative retrospective cohort study. Int Clin Psychopharmacol. 2018;33:44-48.
- Ghanizadeh A. Sertraline-associated hair loss. J Drugs Dermatol. 2008;7:693-694.
- Parameshwar E. Hair loss associated with fluvoxamine use. Am J Psychiatry. 1996;153:581-582. doi:10.1176/ajp.153.4.581
- Zalsman G, Sever J, Munitz H. Hair loss associated with paroxetine treatment: a case report. Clin Neuropharmacol. 1999;22:246-247.
- Ananth J, Elmishaugh A. Hair loss associated with fluoxetinetreatment. Can J Psychiatry. 1991;36:621. doi:10.1177/070674379103600824
- Tirmazi SI, Imran H, Rasheed A, et al. Escitalopram-induced hair loss. Prim Care Companion CNS Disord. 2020;22:19l02496. doi:10.4088/PCC.19l02496
Alopecia is a commonly reported side effect of various medications. Anagen effluvium and telogen effluvium (TE) are considered the most common mechanisms underlying medication-related hair loss. Anagen effluvium is associated with chemotherapeutic agents and radiation therapy, with anagen shedding typically occurring within 2 weeks of medication administration.1,2 Medication-induced TE is a diffuse nonscarring alopecia that is a reversible reactive process.3-5 Telogen effluvium is clinically apparent as a generalized shedding of scalp hair 1 to 6 months after an inciting cause.6 The underlying cause of TE may be multifactorial and difficult to identify given the delay between the trigger and the onset of clinically apparent hair loss. Other known triggers of TE include acute illness,7,8 nutritional deficiencies,4,9 and/or major surgery.10
Each hair follicle independently and sequentially progresses through anagen growth, catagen transition, and telogen resting phases. In the human scalp, the telogen phase typically lasts 3 months, at the end of which the telogen hair is extruded from the scalp. Anagen and telogen follicles typically account for an average of 90% and 10% of follicles on the human scalp, respectively.11 Immediate anagen release is hypothesized to be the mechanism underlying medication-induced TE.12 This theory suggests that an increased percentage of anagen follicles prematurely enter the telogen phase, with a notable increase in hair shedding at the conclusion of the telogen phase approximately 1 to 6 months later.12 First-line management of medication-induced TE is identification and cessation of the causative agent, if possible. Notable regrowth of hair is expected several months after removal of the inciting medication. In part 1 of this 2-part series, we review the existing literature to identify common culprits of medication-induced TE, including retinoids, antifungals, and psychotropic medications.
Retinoids
Retinoids are vitamin A derivatives used in the treatment of a myriad of dermatologic and nondermatologic conditions.13,14 Retinoids modulate sebum production,15 keratinocyte proliferation,16 and epithelial differentiation through signal transduction downstream of the ligand-activated nuclear retinoic acid receptors and retinoid X receptors.13,14,17 The recommended daily dosage of retinol is 900 µg retinol activity equivalent (3000 IU) for men and 700 µg retinol activity equivalent (2333 IU) for women. Retinoids are used in the treatment of acne vulgaris,18 psoriasis,19 and ichthyosis.20 The most commonly reported adverse effects of systemic retinoid therapy include cheilitis, alopecia, and xerosis.21 Retinoid-associated alopecia is dose and duration dependent.19,21-24 A prospective study of acitretin therapy in plaque psoriasis reported that more than 63% (42/66) of patients on 50 mg or more of acitretin daily for 6 months or longer experienced alopecia that reversed with discontinuation.23 A systematic review of isotretinoin use in acne showed alopecia was seen in 3.2% (18/565) of patients on less than 0.5 mg/kg/d of isotretinoin and in 5.7% (192/3375) of patients on 0.5 mg/kg/d or less of isotretinoin.24 In a phase 2 clinical trial of orally administered 9-cis-retinoic acid (alitretinoin) in the treatment of Kaposi sarcoma related to AIDS, 42% (24/57) of adult male patients receiving 60, 100, or 140 mg/m2 alitretinoin daily (median treatment duration, 15.1 weeks) reported alopecia as an adverse effect of treatment.25 In one case report, a patient who ingested 500,000 IU of vitamin A daily for 4 months and then 100,000 IU monthly for 6 months experienced diffusely increased shedding of scalp hair along with muscle soreness, nail dystrophy, diffuse skin rash, and refractory ascites; he was found to have severe liver damage secondary to hypervitaminosis A that required liver transplantation.26 Regarding the pathomechanism of retinoid-induced alopecia, animal and in vitro studies similarly have demonstrated that all-trans-retinoic acid appears to exert its inhibitory effects on hair follicle growth via the influence of the transforming growth factor β2 and SMAD2/3 pathway influence on dermal papillae cells.14,27 Development of hair loss secondary to systemic retinoid therapy may be managed with dose reduction or cessation.
Antifungals
Azole medications have broad-spectrum fungistatic activity against a wide range of yeast and filamentous fungi. Azoles inhibit sterol 14α-demethylase activity, impairing ergosterol synthesis and thereby disrupting plasma membrane synthesis and activity of membrane-bound enzymes.28 Fluconazole is a systemic oral agent in this class that was first approved by the US Food and Drug Administration (FDA) for use in the 1990s.29 A retrospective study by the National Institute of Allergy and Infectious Disease Mycoses Study Group followed the clinical course of 33 patients who developed alopecia while receiving fluconazole therapy for various mycoses.30 The majority (88% [29/33]) of patients received 400 mg or more of fluconazole daily. The median time to hair loss after starting fluconazole was 3 months, and the scalp was involved in all cases. In 97% (32/33) of patients, resolution of alopecia was noted following discontinuation of fluconazole or a dose reduction of 50% or more. In 85% (28/33) of patients, complete resolution of alopecia occurred within 6 months of fluconazole cessation or dose reduction.30 Fluconazole-induced TE was reproducible in an animal model using Wistar rats31; however, further studies are required to clarify the molecular pathways of its effect on hair growth.
Voriconazole is an azole approved for the treatment of invasive aspergillosis, candidemia, and fungal infections caused by Scedosporium apiospermum and Fusarium species. A retrospective survey study of patients who received voriconazole for 1 month or longer found a considerable proportion of patients developed diffuse reversible hair loss.32 Scalp alopecia was noted in 79% (120/152) of patients who completed the survey, with a mean (SD) time to alopecia of 75 (54) days after initiation of voriconazole. Notable regrowth was reported in 69% (79/114) of patients who discontinued voriconazole for at least 3 months. A subgroup of 32 patients were changed to itraconazole or posaconazole, and hair loss stopped in 84% (27/32) with regrowth noted in 69% (22/32) of patients.32 Voriconazole and fluconazole share structural similarity not present with other triazoles.33,34 Because voriconazole-associated alopecia was reversed in the majority of patients who switched to itraconazole or posaconazole, the authors hypothesized that structural similarity of fluconazole and voriconazole may underly the greater risk for TE that is not a class effect of azole medications.31
Psychotropic Medications
Various psychotropic medications have been associated with hair loss. Valproic acid (or sodium valproate) is an anticonvulsant and mood-stabilizing agent used for the treatment of seizures, bipolar disorder (BD), migraines, and neuropathic pain.35,36 Divalproex sodium (or divalproex) is an enteric-coated formulation of sodium valproate and valproic acid with similar indications. Valproate is a notorious culprit of medication-induced hair loss, with alopecia listed among the most common adverse reactions (reported >5%) on its structure product labeling document.37 A systemic review and meta-analysis by Wang et al38 estimated the overall incidence of valproate-related alopecia to be 11% (95% CI, 0.08-0.13). Although this meta-analysis did not find an association between incidence of alopecia and dose or duration of valproate therapy,38 a separate review suggested that valproate-induced alopecia is dose dependent and can be managed with dose reduction.39 A 12-month, randomized, double-blind study of treatment of BD with divalproex (valproate derivative), lithium, or placebo (2:1:1 ratio) showed a significantly higher frequency of alopecia in the divalproex group compared with placebo (16% [30/187] vs 6% [6/94]; P=.03).40 Valproate-related hair loss is characteristically diffuse and nonscarring, often noted 3 to 6 months following initiation of valproate.41,42 The proposed mechanism of valproate-induced alopecia includes chelation of zinc and selenium,43 and a reduction in serum biotinidase activity, thereby decreasing the availability of these essential micronutrients required for hair growth.41 Studies examining the effects of valproate administration and serum biotinidase activity in patients have yielded conflicting results.44-46 In a study of children with seizures including 57 patients treated with valproic acid, 17 treated with carbamazepine, and 75 age- and sex-matched healthy controls, the authors found no significant differences in serum biotinidase enzyme activity across the 3 groups.44 In contrast, a study of 75 children with seizures on valproic acid therapy stratified by dose (mean [SD])—group A: 28.7 [8.5] mg/kg/d; group B: 41.6 [4.9] mg/kg/d; group C: 64.5 [5.8] mg/kg/d—found that patients receiving higher doses (groups B and C) had significantly reduced serum biotinidase activity (1.22
Lithium carbonate (lithium) is used in the treatment of BD. Despite its efficacy and low cost, its potential for adverse effects, narrow therapeutic index, and subsequent need for routine monitoring are factors that limit its use.48 Some reported dermatologic adverse reactions on its structure product labeling include xerosis, thinning of hair, alopecia, xerosis cutis, psoriasis onset/exacerbation, and generalized pruritus.49 A systematic review and meta-analysis of 385 studies identified 24 publications reporting adverse effects of lithium on hair with no significantly increased risk of alopecia overall.50 The analysis included 2 randomized controlled trials comparing the effects of lithium and placebo on hair loss in patients with BD. Hair loss was reported in 7% (7/94) of patients taking lithium and 6% (6/94) of the placebo group in the 12-month study40 and in 3% (1/32) of the lithium group and 0% (0/28) of the divalproex group in the 20-month study.51 Despite anecdotal reports of alopecia associated with lithium, there is a lack of high-quality evidence to support this claim. Of note, hypothyroidism is a known complication of lithium use, and serum testing of thyroid function at 6-month intervals is recommended for patients on lithium treatment.52 Because thyroid abnormalities can cause alopecia distinct from TE, new-onset alopecia during lithium use should prompt serum testing of thyroid function. The development of hypothyroidism secondary to lithium is not a direct contraindication to its use53; rather, treatment should be focused on correction with thyroid replacement therapy (eg, supplementation with thyroxine).54
Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs) and bupropion. Selective serotonin reuptake inhibitors affect the neuronal serotonin transporter, increasing the concentration of serotonin in the synaptic cleft available for stimulation of postsynaptic serotonin receptors55,56; bupropion is an antidepressant medication that inhibits norepinephrine and dopamine reuptake at the synaptic cleft.57 Alopecia is an infrequent (1 in 100 to 1 in 1000 patients) adverse effect for several SSRIs.58-62 A recent systematic review identified a total of 71 cases of alopecia associated with SSRI use including citalopram (n=11), escitalopram (n=7), fluoxetine (n=27), fluoxvamine (n=5), paroxetine (n=4), and sertraline (n=20), with a median time to onset of hair shedding of 8.6 weeks (range, 3 days to 5 years). Discontinuation of the suspected culprit SSRI led to improvement and/or resolution in 63% (51/81) episodes of alopecia, with a median time to improvement and/or resolution of 4 weeks.63 A comparative retrospective cohort study using a large US health claims database from 2006 to 2014 included more than 1 million new and mutually exclusive patients taking fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, paroxetine, duloxetine, venlafaxine, desvenlafaxine, and bupropion.64 Overall, 1% (1569/150,404) of patients treated with bupropion received 1 or more physician visits for alopecia. Patients on SSRIs generally had a lower risk for hair loss compared with patients using bupropion (citalopram: hazard ratio [HR], 0.80 [95% CI, 0.74-0.86]; escitalopram: HR, 0.79 [95% CI, 0.74-0.86]; fluoxetine: HR, 0.68 [95% CI, 0.63-0.74]; paroxetine: HR, 0.68 [95% CI, 0.62-0.74]; sertraline: HR, 0.74 [95% CI, 0.69-0.79]), with the exception of fluvoxamine (HR, 0.93 [95% CI, 0.64-1.37]). However, the type of alopecia, time to onset, and time to resolution were not reported, making it difficult to assess whether the reported hair loss was consistent with medication-induced TE. Additionally, the authors acknowledged that bupropion may have been prescribed for smoking cessation, which may carry a different risk profile for the development of alopecia.64 Several other case reports have described alopecia following treatment with SSRIs, including sertraline,65 fluvoxamine,66 paroxetine,67 fluoxetine,68 and escitalopram.69
Overall, it appears that the use of SSRIs portends relatively low risk for alopecia and medication-induced TE. Little is known regarding the molecular effects of SSRIs on hair growth and the pathomechanism of SSRI-induced TE. The potential benefits of discontinuing a suspected culprit medication should be carefully weighed against the risks of medication cessation, and consideration should be given to alternative medications in the same class that also may be associated with TE. In patients requiring antidepressant therapy with suspected medication-induced TE, consider transitioning to a different class of medication with lower risk of medication-induced alopecia; for example, discontinuing bupropion in favor of an SSRI.
Final Thoughts
Medication-induced alopecia is an undesired side effect of many commonly used drugs and drug classes, including retinoids, azole antifungals, and mood stabilizers. Although the precise pathomechanisms of medication-induced TE remain unclear, the recommended management often requires identification of the likely causative agent and its discontinuation, if possible. Suspicion for medication-induced TE should prompt a thorough history of recent changes to medications, risk factors for nutritional deficiencies, underlying illnesses, and recent surgical procedures. Underlying nutritional, electrolyte, and/or metabolic disturbances should be corrected. In part 2 of this series, we will discuss medication-induced alopecia associated with anticoagulant and antihypertensive medications.
Alopecia is a commonly reported side effect of various medications. Anagen effluvium and telogen effluvium (TE) are considered the most common mechanisms underlying medication-related hair loss. Anagen effluvium is associated with chemotherapeutic agents and radiation therapy, with anagen shedding typically occurring within 2 weeks of medication administration.1,2 Medication-induced TE is a diffuse nonscarring alopecia that is a reversible reactive process.3-5 Telogen effluvium is clinically apparent as a generalized shedding of scalp hair 1 to 6 months after an inciting cause.6 The underlying cause of TE may be multifactorial and difficult to identify given the delay between the trigger and the onset of clinically apparent hair loss. Other known triggers of TE include acute illness,7,8 nutritional deficiencies,4,9 and/or major surgery.10
Each hair follicle independently and sequentially progresses through anagen growth, catagen transition, and telogen resting phases. In the human scalp, the telogen phase typically lasts 3 months, at the end of which the telogen hair is extruded from the scalp. Anagen and telogen follicles typically account for an average of 90% and 10% of follicles on the human scalp, respectively.11 Immediate anagen release is hypothesized to be the mechanism underlying medication-induced TE.12 This theory suggests that an increased percentage of anagen follicles prematurely enter the telogen phase, with a notable increase in hair shedding at the conclusion of the telogen phase approximately 1 to 6 months later.12 First-line management of medication-induced TE is identification and cessation of the causative agent, if possible. Notable regrowth of hair is expected several months after removal of the inciting medication. In part 1 of this 2-part series, we review the existing literature to identify common culprits of medication-induced TE, including retinoids, antifungals, and psychotropic medications.
Retinoids
Retinoids are vitamin A derivatives used in the treatment of a myriad of dermatologic and nondermatologic conditions.13,14 Retinoids modulate sebum production,15 keratinocyte proliferation,16 and epithelial differentiation through signal transduction downstream of the ligand-activated nuclear retinoic acid receptors and retinoid X receptors.13,14,17 The recommended daily dosage of retinol is 900 µg retinol activity equivalent (3000 IU) for men and 700 µg retinol activity equivalent (2333 IU) for women. Retinoids are used in the treatment of acne vulgaris,18 psoriasis,19 and ichthyosis.20 The most commonly reported adverse effects of systemic retinoid therapy include cheilitis, alopecia, and xerosis.21 Retinoid-associated alopecia is dose and duration dependent.19,21-24 A prospective study of acitretin therapy in plaque psoriasis reported that more than 63% (42/66) of patients on 50 mg or more of acitretin daily for 6 months or longer experienced alopecia that reversed with discontinuation.23 A systematic review of isotretinoin use in acne showed alopecia was seen in 3.2% (18/565) of patients on less than 0.5 mg/kg/d of isotretinoin and in 5.7% (192/3375) of patients on 0.5 mg/kg/d or less of isotretinoin.24 In a phase 2 clinical trial of orally administered 9-cis-retinoic acid (alitretinoin) in the treatment of Kaposi sarcoma related to AIDS, 42% (24/57) of adult male patients receiving 60, 100, or 140 mg/m2 alitretinoin daily (median treatment duration, 15.1 weeks) reported alopecia as an adverse effect of treatment.25 In one case report, a patient who ingested 500,000 IU of vitamin A daily for 4 months and then 100,000 IU monthly for 6 months experienced diffusely increased shedding of scalp hair along with muscle soreness, nail dystrophy, diffuse skin rash, and refractory ascites; he was found to have severe liver damage secondary to hypervitaminosis A that required liver transplantation.26 Regarding the pathomechanism of retinoid-induced alopecia, animal and in vitro studies similarly have demonstrated that all-trans-retinoic acid appears to exert its inhibitory effects on hair follicle growth via the influence of the transforming growth factor β2 and SMAD2/3 pathway influence on dermal papillae cells.14,27 Development of hair loss secondary to systemic retinoid therapy may be managed with dose reduction or cessation.
Antifungals
Azole medications have broad-spectrum fungistatic activity against a wide range of yeast and filamentous fungi. Azoles inhibit sterol 14α-demethylase activity, impairing ergosterol synthesis and thereby disrupting plasma membrane synthesis and activity of membrane-bound enzymes.28 Fluconazole is a systemic oral agent in this class that was first approved by the US Food and Drug Administration (FDA) for use in the 1990s.29 A retrospective study by the National Institute of Allergy and Infectious Disease Mycoses Study Group followed the clinical course of 33 patients who developed alopecia while receiving fluconazole therapy for various mycoses.30 The majority (88% [29/33]) of patients received 400 mg or more of fluconazole daily. The median time to hair loss after starting fluconazole was 3 months, and the scalp was involved in all cases. In 97% (32/33) of patients, resolution of alopecia was noted following discontinuation of fluconazole or a dose reduction of 50% or more. In 85% (28/33) of patients, complete resolution of alopecia occurred within 6 months of fluconazole cessation or dose reduction.30 Fluconazole-induced TE was reproducible in an animal model using Wistar rats31; however, further studies are required to clarify the molecular pathways of its effect on hair growth.
Voriconazole is an azole approved for the treatment of invasive aspergillosis, candidemia, and fungal infections caused by Scedosporium apiospermum and Fusarium species. A retrospective survey study of patients who received voriconazole for 1 month or longer found a considerable proportion of patients developed diffuse reversible hair loss.32 Scalp alopecia was noted in 79% (120/152) of patients who completed the survey, with a mean (SD) time to alopecia of 75 (54) days after initiation of voriconazole. Notable regrowth was reported in 69% (79/114) of patients who discontinued voriconazole for at least 3 months. A subgroup of 32 patients were changed to itraconazole or posaconazole, and hair loss stopped in 84% (27/32) with regrowth noted in 69% (22/32) of patients.32 Voriconazole and fluconazole share structural similarity not present with other triazoles.33,34 Because voriconazole-associated alopecia was reversed in the majority of patients who switched to itraconazole or posaconazole, the authors hypothesized that structural similarity of fluconazole and voriconazole may underly the greater risk for TE that is not a class effect of azole medications.31
Psychotropic Medications
Various psychotropic medications have been associated with hair loss. Valproic acid (or sodium valproate) is an anticonvulsant and mood-stabilizing agent used for the treatment of seizures, bipolar disorder (BD), migraines, and neuropathic pain.35,36 Divalproex sodium (or divalproex) is an enteric-coated formulation of sodium valproate and valproic acid with similar indications. Valproate is a notorious culprit of medication-induced hair loss, with alopecia listed among the most common adverse reactions (reported >5%) on its structure product labeling document.37 A systemic review and meta-analysis by Wang et al38 estimated the overall incidence of valproate-related alopecia to be 11% (95% CI, 0.08-0.13). Although this meta-analysis did not find an association between incidence of alopecia and dose or duration of valproate therapy,38 a separate review suggested that valproate-induced alopecia is dose dependent and can be managed with dose reduction.39 A 12-month, randomized, double-blind study of treatment of BD with divalproex (valproate derivative), lithium, or placebo (2:1:1 ratio) showed a significantly higher frequency of alopecia in the divalproex group compared with placebo (16% [30/187] vs 6% [6/94]; P=.03).40 Valproate-related hair loss is characteristically diffuse and nonscarring, often noted 3 to 6 months following initiation of valproate.41,42 The proposed mechanism of valproate-induced alopecia includes chelation of zinc and selenium,43 and a reduction in serum biotinidase activity, thereby decreasing the availability of these essential micronutrients required for hair growth.41 Studies examining the effects of valproate administration and serum biotinidase activity in patients have yielded conflicting results.44-46 In a study of children with seizures including 57 patients treated with valproic acid, 17 treated with carbamazepine, and 75 age- and sex-matched healthy controls, the authors found no significant differences in serum biotinidase enzyme activity across the 3 groups.44 In contrast, a study of 75 children with seizures on valproic acid therapy stratified by dose (mean [SD])—group A: 28.7 [8.5] mg/kg/d; group B: 41.6 [4.9] mg/kg/d; group C: 64.5 [5.8] mg/kg/d—found that patients receiving higher doses (groups B and C) had significantly reduced serum biotinidase activity (1.22
Lithium carbonate (lithium) is used in the treatment of BD. Despite its efficacy and low cost, its potential for adverse effects, narrow therapeutic index, and subsequent need for routine monitoring are factors that limit its use.48 Some reported dermatologic adverse reactions on its structure product labeling include xerosis, thinning of hair, alopecia, xerosis cutis, psoriasis onset/exacerbation, and generalized pruritus.49 A systematic review and meta-analysis of 385 studies identified 24 publications reporting adverse effects of lithium on hair with no significantly increased risk of alopecia overall.50 The analysis included 2 randomized controlled trials comparing the effects of lithium and placebo on hair loss in patients with BD. Hair loss was reported in 7% (7/94) of patients taking lithium and 6% (6/94) of the placebo group in the 12-month study40 and in 3% (1/32) of the lithium group and 0% (0/28) of the divalproex group in the 20-month study.51 Despite anecdotal reports of alopecia associated with lithium, there is a lack of high-quality evidence to support this claim. Of note, hypothyroidism is a known complication of lithium use, and serum testing of thyroid function at 6-month intervals is recommended for patients on lithium treatment.52 Because thyroid abnormalities can cause alopecia distinct from TE, new-onset alopecia during lithium use should prompt serum testing of thyroid function. The development of hypothyroidism secondary to lithium is not a direct contraindication to its use53; rather, treatment should be focused on correction with thyroid replacement therapy (eg, supplementation with thyroxine).54
Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs) and bupropion. Selective serotonin reuptake inhibitors affect the neuronal serotonin transporter, increasing the concentration of serotonin in the synaptic cleft available for stimulation of postsynaptic serotonin receptors55,56; bupropion is an antidepressant medication that inhibits norepinephrine and dopamine reuptake at the synaptic cleft.57 Alopecia is an infrequent (1 in 100 to 1 in 1000 patients) adverse effect for several SSRIs.58-62 A recent systematic review identified a total of 71 cases of alopecia associated with SSRI use including citalopram (n=11), escitalopram (n=7), fluoxetine (n=27), fluoxvamine (n=5), paroxetine (n=4), and sertraline (n=20), with a median time to onset of hair shedding of 8.6 weeks (range, 3 days to 5 years). Discontinuation of the suspected culprit SSRI led to improvement and/or resolution in 63% (51/81) episodes of alopecia, with a median time to improvement and/or resolution of 4 weeks.63 A comparative retrospective cohort study using a large US health claims database from 2006 to 2014 included more than 1 million new and mutually exclusive patients taking fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, paroxetine, duloxetine, venlafaxine, desvenlafaxine, and bupropion.64 Overall, 1% (1569/150,404) of patients treated with bupropion received 1 or more physician visits for alopecia. Patients on SSRIs generally had a lower risk for hair loss compared with patients using bupropion (citalopram: hazard ratio [HR], 0.80 [95% CI, 0.74-0.86]; escitalopram: HR, 0.79 [95% CI, 0.74-0.86]; fluoxetine: HR, 0.68 [95% CI, 0.63-0.74]; paroxetine: HR, 0.68 [95% CI, 0.62-0.74]; sertraline: HR, 0.74 [95% CI, 0.69-0.79]), with the exception of fluvoxamine (HR, 0.93 [95% CI, 0.64-1.37]). However, the type of alopecia, time to onset, and time to resolution were not reported, making it difficult to assess whether the reported hair loss was consistent with medication-induced TE. Additionally, the authors acknowledged that bupropion may have been prescribed for smoking cessation, which may carry a different risk profile for the development of alopecia.64 Several other case reports have described alopecia following treatment with SSRIs, including sertraline,65 fluvoxamine,66 paroxetine,67 fluoxetine,68 and escitalopram.69
Overall, it appears that the use of SSRIs portends relatively low risk for alopecia and medication-induced TE. Little is known regarding the molecular effects of SSRIs on hair growth and the pathomechanism of SSRI-induced TE. The potential benefits of discontinuing a suspected culprit medication should be carefully weighed against the risks of medication cessation, and consideration should be given to alternative medications in the same class that also may be associated with TE. In patients requiring antidepressant therapy with suspected medication-induced TE, consider transitioning to a different class of medication with lower risk of medication-induced alopecia; for example, discontinuing bupropion in favor of an SSRI.
Final Thoughts
Medication-induced alopecia is an undesired side effect of many commonly used drugs and drug classes, including retinoids, azole antifungals, and mood stabilizers. Although the precise pathomechanisms of medication-induced TE remain unclear, the recommended management often requires identification of the likely causative agent and its discontinuation, if possible. Suspicion for medication-induced TE should prompt a thorough history of recent changes to medications, risk factors for nutritional deficiencies, underlying illnesses, and recent surgical procedures. Underlying nutritional, electrolyte, and/or metabolic disturbances should be corrected. In part 2 of this series, we will discuss medication-induced alopecia associated with anticoagulant and antihypertensive medications.
- Saleh D, Nassereddin A, Cook C. Anagen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482293/
- Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, et al. Anagen effluvium after neurointerventional radiation: trichoscopy as a diagnostic ally. Skin Appendage Disord. 2021;8:102-107. doi:10.1159/000518743
- Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67-73. doi:https://doi.org/10.1016/j.det.2012.08.002
- Chen V, Strazzulla L, Asbeck SM, et al. Etiology, management, and outcomes of pediatric telogen effluvium: a single-center study in the United States. Pediatr Dermatol. 2023;40:120-124. doi:10.1111/pde.15154
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Hughes EC, Saleh D. Telogen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK430848/
- Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77. doi:10.1016/j.jdin.2022.02.006
- Starace M, Piraccini BM, Evangelista V, et al. Acute telogen effluvium due to dengue fever mimicking androgenetic alopecia. Ital J Dermatol Venerol. 2023;158:66-67. doi:10.23736/s2784-8671.22.07369-8
- Patel KV, Farrant P, Sanderson JD, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. doi:10.1097/MIB.0b013e31828132de
- Cohen-Kurzrock RA, Cohen PR. Bariatric surgery–induced telogen effluvium (bar site): case report and a review of hair loss following weight loss surgery. Cureus. 2021;13:E14617. doi:10.7759/cureus.14617
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964-973. doi:10.1056/nejm199909233411307
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129:356-363. doi:10.1001/arcderm.1993.01680240096017
- Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427-439. doi:10.1002/jcp.21784
- Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119-1126. doi:10.1111/j.0022-202X.2005.23686.x
- Karlsson T, Vahlquist A, Kedishvili N, et al. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273-278. doi:10.1016/s0006-291x(03)00332-2
- Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402-3413. doi:10.1093/emboj/cdf331
- Geiger JM. Retinoids and sebaceous gland activity. Dermatology. 1995;191:305-310. doi:10.1159/000246581
- Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100:475-484.
- Pilkington T, Brogden RN. Acitretin. Drugs. 1992;43:597-627. doi:10.2165/00003495-199243040-00010
- Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38:164-180. doi:10.1111/pde.14408
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 suppl):S7-S12. doi:10.1016/s0190-9622(99)70359-2
- Tran PT, Evron E, Goh C. Characteristics of patients with hair loss after isotretinoin treatment: a retrospective review study. Int J Trichology. 2022;14:125-127. doi:10.4103/ijt.ijt_80_20
- Gupta AK, Goldfarb MT, Ellis CN, et al. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088-1093. doi:10.1016/s0190-9622(89)70138-9
- Lytvyn Y, McDonald K, Mufti A, et al. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125-142. doi:10.1016/j.jdin.2022.01.002
- Aboulafia DM, Norris D, Henry D, et al. 9-cis-Retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178-186. doi:10.1001/archderm.139.2.178
- Cheruvattath R, Orrego M, Gautam M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl. 2006;12:1888-1891. doi:10.1002/lt.21007
- Nan W, Li G, Si H, et al. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-β2/Smad2/3 pathway. Acta Histochem. 2020;122:151603. doi:10.1016/j.acthis.2020.151603
- Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279-291. doi:10.1128/aac.40.2.279
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40-79. doi:10.1128/cmr.12.1.40
- Pappas PG, Kauffman CA, Perfect J, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354-357. doi:10.7326/0003-4819-123-5-199509010-00006
- Thompson GR 3rd, Krois CR, Affolter VK, et al. Examination of fluconazole-induced alopecia in an animal model and human cohort. Antimicrob Agents Chemother. 2019;63:e01384-18. doi:10.1128/aac.01384-18
- Malani AN, Kerr L, Obear J, et al. Alopecia and nail changes associated with voriconazole therapy. Clin Infect Dis. 2014;59:E61-E65. doi:10.1093/cid/ciu275
- Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241-248. doi:10.1080/08998280.2003.11927910
- Drabin´ska B, Dettlaff K, Kossakowski K, et al. Structural and spectroscopic properties of voriconazole and fluconazole—experimental and theoretical studies. Open Chemistry. 2022;20:1575-1590. doi:10.1515/chem-2022-0253
- Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. doi:10.1016/s0301-0082(98)00075-6
- Gill D, Derry S, Wiffen PJ, et al. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011:CD009183. doi:10.1002/14651858.CD009183.pub2
- Depakote, Prescribing information. Abbott Laboratories; 2011. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018723s037lbl.pdf
- Wang X, Wang H, Xu D, et al. Risk of valproic acid-related alopecia: a systematic review and meta-analysis. Seizure. 2019;69:61-69. doi:10.1016/j.seizure.2019.04.003
- Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12:35-42. doi:10.1023/a:1009074926921
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481-489. doi:10.1001/archpsyc.57.5.481
- Praharaj SK, Munoli RN, Udupa ST, et al. Valproate-associated hair abnormalities: pathophysiology and management strategies. Hum Psychopharmacol. 2022;37:E2814. doi:10.1002/hup.2814
- Wilting I, van Laarhoven JH, de Koning-Verest IF, et al. Valproic acid-induced hair-texture changes in a white woman. Epilepsia. 2007;48:400-401. doi:10.1111/j.1528-1167.2006.00933.x
- Potter WZ, Ketter TA. Pharmacological issues in the treatment of bipolar disorder: focus on mood-stabilizing compounds. Can J Psychiatry. 1993;38(3 suppl 2):S51-S56.
- Castro-Gago M, Gómez-Lado C, Eirís-Pun´al J, et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2009;25:32-35. doi:10.1177/0883073809336118
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. doi:10.1046/j.1528-1157.2001.47000.x
- Yilmaz Y, Tasdemir HA, Paksu MS. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur J Paediatr Neurol. 2009;13:439-443. doi:10.1016/j.ejpn.2008.08.007
- Henriksen O, Johannessen SI. Clinical and pharmacokinetic observations on sodium valproate—a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand. 1982;65:504-523. doi:10.1111/j.1600-0404.1982.tb03106.x
- Fountoulakis KN, Tohen M, Zarate CA Jr. Lithium treatment of bipolar disorder in adults: a systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100-115. doi:10.1016/j.euroneuro.2021.10.003
- Lithium carbonate. Prescribing information. West-Ward Pharmaceuticals; 2018. Accessed November 20, 2023. https://ww.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf
- McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721-728. doi:10.1016/s0140-6736(11)61516-x
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152-2161. doi:10.1176/appi.ajp.162.11.2152.
- Duce HL, Duff CJ, Zaidi S, et al. Evaluation of thyroid function monitoring in people treated with lithium: advice based on real-world data. Bipolar Disord. 2023;25:402-409. doi:10.1111/bdi.13298
- Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi:10.1186/1745-0179-2-23.
- Joffe RT. How should lithium-induced thyroid dysfunction be managed in patients with bipolar disorder? J Psychiatry Neurosci. 2002;27:392.
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1-21. doi:10.2165/00003088-199700321-00003
- Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. StatPearls Publishing; 2023.
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159-166. doi:10.4088/pcc.v06n0403
- Escitalopram. Prescribing information. Solco Healthcare US, LLC; 2022. Accessed November 20, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2ffc6ec3-830f-46bc-9b3f-7c42cefa39b2/spl-doc
- Fluoxetine. Eli Lilly & Company; 2017. Prescribing information. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Paxil. Prescribing information. GlaxoSmithKline; 2012. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020031s067,020710s031.pdf
- Zoloft. Prescribing information. Pfizer; 2016. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf
- Celexa. Prescribing information. Allergan; 2022. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: systematic review. Psychiatry Res. 2022;313:114620. 10.1016/j.psychres.2022.114620
- Etminan M, Sodhi M, Procyshyn RM, et al. Risk of hair loss with different antidepressants: a comparative retrospective cohort study. Int Clin Psychopharmacol. 2018;33:44-48.
- Ghanizadeh A. Sertraline-associated hair loss. J Drugs Dermatol. 2008;7:693-694.
- Parameshwar E. Hair loss associated with fluvoxamine use. Am J Psychiatry. 1996;153:581-582. doi:10.1176/ajp.153.4.581
- Zalsman G, Sever J, Munitz H. Hair loss associated with paroxetine treatment: a case report. Clin Neuropharmacol. 1999;22:246-247.
- Ananth J, Elmishaugh A. Hair loss associated with fluoxetinetreatment. Can J Psychiatry. 1991;36:621. doi:10.1177/070674379103600824
- Tirmazi SI, Imran H, Rasheed A, et al. Escitalopram-induced hair loss. Prim Care Companion CNS Disord. 2020;22:19l02496. doi:10.4088/PCC.19l02496
- Saleh D, Nassereddin A, Cook C. Anagen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482293/
- Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, et al. Anagen effluvium after neurointerventional radiation: trichoscopy as a diagnostic ally. Skin Appendage Disord. 2021;8:102-107. doi:10.1159/000518743
- Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67-73. doi:https://doi.org/10.1016/j.det.2012.08.002
- Chen V, Strazzulla L, Asbeck SM, et al. Etiology, management, and outcomes of pediatric telogen effluvium: a single-center study in the United States. Pediatr Dermatol. 2023;40:120-124. doi:10.1111/pde.15154
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Hughes EC, Saleh D. Telogen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK430848/
- Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77. doi:10.1016/j.jdin.2022.02.006
- Starace M, Piraccini BM, Evangelista V, et al. Acute telogen effluvium due to dengue fever mimicking androgenetic alopecia. Ital J Dermatol Venerol. 2023;158:66-67. doi:10.23736/s2784-8671.22.07369-8
- Patel KV, Farrant P, Sanderson JD, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. doi:10.1097/MIB.0b013e31828132de
- Cohen-Kurzrock RA, Cohen PR. Bariatric surgery–induced telogen effluvium (bar site): case report and a review of hair loss following weight loss surgery. Cureus. 2021;13:E14617. doi:10.7759/cureus.14617
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964-973. doi:10.1056/nejm199909233411307
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129:356-363. doi:10.1001/arcderm.1993.01680240096017
- Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427-439. doi:10.1002/jcp.21784
- Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119-1126. doi:10.1111/j.0022-202X.2005.23686.x
- Karlsson T, Vahlquist A, Kedishvili N, et al. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273-278. doi:10.1016/s0006-291x(03)00332-2
- Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402-3413. doi:10.1093/emboj/cdf331
- Geiger JM. Retinoids and sebaceous gland activity. Dermatology. 1995;191:305-310. doi:10.1159/000246581
- Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100:475-484.
- Pilkington T, Brogden RN. Acitretin. Drugs. 1992;43:597-627. doi:10.2165/00003495-199243040-00010
- Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38:164-180. doi:10.1111/pde.14408
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 suppl):S7-S12. doi:10.1016/s0190-9622(99)70359-2
- Tran PT, Evron E, Goh C. Characteristics of patients with hair loss after isotretinoin treatment: a retrospective review study. Int J Trichology. 2022;14:125-127. doi:10.4103/ijt.ijt_80_20
- Gupta AK, Goldfarb MT, Ellis CN, et al. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088-1093. doi:10.1016/s0190-9622(89)70138-9
- Lytvyn Y, McDonald K, Mufti A, et al. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125-142. doi:10.1016/j.jdin.2022.01.002
- Aboulafia DM, Norris D, Henry D, et al. 9-cis-Retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178-186. doi:10.1001/archderm.139.2.178
- Cheruvattath R, Orrego M, Gautam M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl. 2006;12:1888-1891. doi:10.1002/lt.21007
- Nan W, Li G, Si H, et al. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-β2/Smad2/3 pathway. Acta Histochem. 2020;122:151603. doi:10.1016/j.acthis.2020.151603
- Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279-291. doi:10.1128/aac.40.2.279
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40-79. doi:10.1128/cmr.12.1.40
- Pappas PG, Kauffman CA, Perfect J, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354-357. doi:10.7326/0003-4819-123-5-199509010-00006
- Thompson GR 3rd, Krois CR, Affolter VK, et al. Examination of fluconazole-induced alopecia in an animal model and human cohort. Antimicrob Agents Chemother. 2019;63:e01384-18. doi:10.1128/aac.01384-18
- Malani AN, Kerr L, Obear J, et al. Alopecia and nail changes associated with voriconazole therapy. Clin Infect Dis. 2014;59:E61-E65. doi:10.1093/cid/ciu275
- Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241-248. doi:10.1080/08998280.2003.11927910
- Drabin´ska B, Dettlaff K, Kossakowski K, et al. Structural and spectroscopic properties of voriconazole and fluconazole—experimental and theoretical studies. Open Chemistry. 2022;20:1575-1590. doi:10.1515/chem-2022-0253
- Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. doi:10.1016/s0301-0082(98)00075-6
- Gill D, Derry S, Wiffen PJ, et al. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011:CD009183. doi:10.1002/14651858.CD009183.pub2
- Depakote, Prescribing information. Abbott Laboratories; 2011. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018723s037lbl.pdf
- Wang X, Wang H, Xu D, et al. Risk of valproic acid-related alopecia: a systematic review and meta-analysis. Seizure. 2019;69:61-69. doi:10.1016/j.seizure.2019.04.003
- Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12:35-42. doi:10.1023/a:1009074926921
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481-489. doi:10.1001/archpsyc.57.5.481
- Praharaj SK, Munoli RN, Udupa ST, et al. Valproate-associated hair abnormalities: pathophysiology and management strategies. Hum Psychopharmacol. 2022;37:E2814. doi:10.1002/hup.2814
- Wilting I, van Laarhoven JH, de Koning-Verest IF, et al. Valproic acid-induced hair-texture changes in a white woman. Epilepsia. 2007;48:400-401. doi:10.1111/j.1528-1167.2006.00933.x
- Potter WZ, Ketter TA. Pharmacological issues in the treatment of bipolar disorder: focus on mood-stabilizing compounds. Can J Psychiatry. 1993;38(3 suppl 2):S51-S56.
- Castro-Gago M, Gómez-Lado C, Eirís-Pun´al J, et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2009;25:32-35. doi:10.1177/0883073809336118
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. doi:10.1046/j.1528-1157.2001.47000.x
- Yilmaz Y, Tasdemir HA, Paksu MS. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur J Paediatr Neurol. 2009;13:439-443. doi:10.1016/j.ejpn.2008.08.007
- Henriksen O, Johannessen SI. Clinical and pharmacokinetic observations on sodium valproate—a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand. 1982;65:504-523. doi:10.1111/j.1600-0404.1982.tb03106.x
- Fountoulakis KN, Tohen M, Zarate CA Jr. Lithium treatment of bipolar disorder in adults: a systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100-115. doi:10.1016/j.euroneuro.2021.10.003
- Lithium carbonate. Prescribing information. West-Ward Pharmaceuticals; 2018. Accessed November 20, 2023. https://ww.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf
- McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721-728. doi:10.1016/s0140-6736(11)61516-x
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152-2161. doi:10.1176/appi.ajp.162.11.2152.
- Duce HL, Duff CJ, Zaidi S, et al. Evaluation of thyroid function monitoring in people treated with lithium: advice based on real-world data. Bipolar Disord. 2023;25:402-409. doi:10.1111/bdi.13298
- Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi:10.1186/1745-0179-2-23.
- Joffe RT. How should lithium-induced thyroid dysfunction be managed in patients with bipolar disorder? J Psychiatry Neurosci. 2002;27:392.
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1-21. doi:10.2165/00003088-199700321-00003
- Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. StatPearls Publishing; 2023.
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159-166. doi:10.4088/pcc.v06n0403
- Escitalopram. Prescribing information. Solco Healthcare US, LLC; 2022. Accessed November 20, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2ffc6ec3-830f-46bc-9b3f-7c42cefa39b2/spl-doc
- Fluoxetine. Eli Lilly & Company; 2017. Prescribing information. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Paxil. Prescribing information. GlaxoSmithKline; 2012. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020031s067,020710s031.pdf
- Zoloft. Prescribing information. Pfizer; 2016. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf
- Celexa. Prescribing information. Allergan; 2022. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: systematic review. Psychiatry Res. 2022;313:114620. 10.1016/j.psychres.2022.114620
- Etminan M, Sodhi M, Procyshyn RM, et al. Risk of hair loss with different antidepressants: a comparative retrospective cohort study. Int Clin Psychopharmacol. 2018;33:44-48.
- Ghanizadeh A. Sertraline-associated hair loss. J Drugs Dermatol. 2008;7:693-694.
- Parameshwar E. Hair loss associated with fluvoxamine use. Am J Psychiatry. 1996;153:581-582. doi:10.1176/ajp.153.4.581
- Zalsman G, Sever J, Munitz H. Hair loss associated with paroxetine treatment: a case report. Clin Neuropharmacol. 1999;22:246-247.
- Ananth J, Elmishaugh A. Hair loss associated with fluoxetinetreatment. Can J Psychiatry. 1991;36:621. doi:10.1177/070674379103600824
- Tirmazi SI, Imran H, Rasheed A, et al. Escitalopram-induced hair loss. Prim Care Companion CNS Disord. 2020;22:19l02496. doi:10.4088/PCC.19l02496
Practice Points
- Medications are a common culprit of telogen effluvium (TE), and medication-induced TE should be suspected in patients presenting with diffuse nonscarring alopecia who are taking systemic medication(s).
- A careful history of new medications and dose adjustments 1 to 6 months prior to notable hair loss may identify the most likely inciting cause.
- Medication-induced TE often improves with cessation or dose reduction of the culprit medication.