User login
Hospitalists focus on POCUS
Point-of-care ultrasound (POCUS) is proving to be an increasing useful diagnostic tool for clinicians at the bedside, and many hospitalists have expressed interest in learning best practices in the use of the technology.
To that end, the Society of Hospital Medicine (SHM) is offering a half-day POCUS pre-course at Hospital Medicine 2018 this April in Orlando, with the intent of helping hospitalists learn how best to use POCUS in clinical settings.
“The agenda is really designed to teach people the basics of point-of-care ultrasound,” said Nilam J. Soni, MD, MSc, of the University of Texas at San Antonio and South Texas Veterans Health Care System, also in San Antonio, and a coinstructor of the POCUS pre-course. “It’s designed for the novice learner who has no prior experience in using ultrasound.”
Dr. Soni and his coinstructor, Ricardo Antonio Franco-Sadud, MD, of the Medical College of Wisconsin, Milwaukee, will lead attendees through the basics of “Point-of-Care Ultrasound for the Hospitalist” at HM18. Dr. Soni has taught a version of this course for almost a decade and is the assistant director of POCUS training programs with Veterans Affairs’ Simulation Learning Education and Research Network (SimLEARN). The pre-course is a 4.5-hour, multimodal class that involves hands-on training to teach the fundamentals of how to evaluate patients using POCUS.
With this course, Dr. Soni said, SHM is addressing training needs from “above and below.”
“Medical students, residents, fellows – basically doctors in training, whether they are student residents or getting ultrasound instruction in their basic training – when these kids graduate, they are pretty good,” he said. “But what about all the doctors who graduated long ago? They didn’t get any POCUS training. That’s where SHM, CHEST, and some of the other societies come in to play. We can offer these courses for training.”
Ultrasound training and credentialing has become a focus for SHM as interest has grown among clinicians. The Journal of Hospital Medicine recently released a consensus statement, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures,” which offers recommendations for credentialing hospitalists in ultrasound guidance and proposes initial and ongoing pathways to improve how hospitalists perform these procedures. The statement emphasizes how ultrasound guidance is increasingly essential to six bedside procedures that are core competencies of hospitalists: abdominal paracentesis, arterial catheter placement, arthrocentesis, central venous catheter placement, lumbar puncture, and thoracentesis.
According to Brian P. Lucas, MD, of Rush Medical College, Chicago, and lead author of the position statement, SHM’s Education Committee convened a POCUS Task Force to take on the project as American Board of Internal Medicine (ABIM) diplomates are no longer expected as part of their residency training to manually perform certain bedside procedures, with or without ultrasound guidance. SHM’s Board of Directors gave final approval on the statement in September 2017.
“There is much variation in the training and experience of both bedside procedures and point-of-care ultrasound. Many practicing hospitalists, for example, have no experience using ultrasound guidance for central lines,” Dr. Lucas said. “How then should hospitals initially, and then biennially, vet hospitalists’ competence in the performance of ultrasound-guided bedside procedures? This nationwide collaborative of experts, educators and front-line providers puts forth some recommendations to this very thorny problem.”
SHM also offers, in collaboration with the American College of Chest Physicians, a Point-of-Care Ultrasound Certificate of Completion for clinicians. The program is designed primarily to educate hospitalists and other providers caring for acutely ill patients, and requires attendance at a series of training programs within the course of 3 years, at the end of which a clinician must complete a comprehensive skills and knowledge assessment to obtain the certificate.
Focus on POCUS
Although medical societies recognize the growing demand, and are offering more ultrasound training, many hospitalists may not be familiar with POCUS specifically and the benefits of utilizing a bedside ultrasound exam, Dr. Soni noted.
“When we talk about point-of-care ultrasound, how is it different from what everyone else thinks about ultrasound? Specifically, it’s a point-of-care bedside exam” Dr. Soni explained. “So, the same provider – whether it’s a physician, nurse practitioner, or PA – who is taking care of a patient, can use ultrasound at the bedside to evaluate specific things in the patient’s body and answer very focused questions.”
The ability to have a hospitalist immediately use an imaging technique at a patient’s bedside can be invaluable, because it allows the clinician to provide a fairly accurate diagnosis in conjunction with patient input. This is much more effective than the traditional process of ultrasound imaging, Dr. Soni said.
“If you go to your primary care doctor [who orders] an ultrasound, you go to the radiology department and the technician captures the images,” he said. “Then the radiologist, who never even sees the patient, reads the ultrasound images with little to no clinical data.” The compartmentalization of the treating clinician, patient, and radiologist leaves the latter without critical information when reading an ultrasound. POCUS can potentially overcome this problematic situation, Dr. Soni suggested.
The HM18 POCUS pre-course has four objectives:
1. Recognize the fundamentals of ultrasound and the basic operation of an ultrasound machine (“knobology”).
2. Differentiate between the different types of ultrasound transducers and determine which is most appropriate for different POCUS applications.
3. Exhibit proper techniques on focused cardiac and lung ultrasound exams and be able to recognize thoracic pathologies from abnormal ultrasound results.
4. Identify and understand normal sonographic appearance of solid abdominal organs and vasculature of the neck and lower extremities and the ability to interpret abnormal ultrasounds to identify pathologies.
While all attendees can expect to learn a new skill that will improve their practice, POCUS training will specifically benefit hospitalists and the institutions in which they work, Dr. Soni said.
“Hospitalists, by nature, work for the hospital. In most cases, hospitalists are subsidized by the hospital. Because of that, the bigger gain from using ultrasound is not in the billing,” he said. “You can bill for focused ultrasound exams of the lungs, heart, abdomen, etc., and you might get a professional fee of $30-$40. But the bigger win in all of this, financially, is giving people more efficient health care. If we can prevent one complication from a bedside procedure or expedite a patient’s care and get them better sooner, we can save the hospital and the system money.”
The SHM is accredited to provide continuing medical education for physicians by the Accreditation Council for Continuing Medical Education. This live activity course will count for a maximum of 4.75 AMA PRA Category 1 Credits.
Point-of-Care Ultrasound for the Hospitalist
Sunday, April 8, 7:30 a.m.–12:15 p.m.
Point-of-care ultrasound (POCUS) is proving to be an increasing useful diagnostic tool for clinicians at the bedside, and many hospitalists have expressed interest in learning best practices in the use of the technology.
To that end, the Society of Hospital Medicine (SHM) is offering a half-day POCUS pre-course at Hospital Medicine 2018 this April in Orlando, with the intent of helping hospitalists learn how best to use POCUS in clinical settings.
“The agenda is really designed to teach people the basics of point-of-care ultrasound,” said Nilam J. Soni, MD, MSc, of the University of Texas at San Antonio and South Texas Veterans Health Care System, also in San Antonio, and a coinstructor of the POCUS pre-course. “It’s designed for the novice learner who has no prior experience in using ultrasound.”
Dr. Soni and his coinstructor, Ricardo Antonio Franco-Sadud, MD, of the Medical College of Wisconsin, Milwaukee, will lead attendees through the basics of “Point-of-Care Ultrasound for the Hospitalist” at HM18. Dr. Soni has taught a version of this course for almost a decade and is the assistant director of POCUS training programs with Veterans Affairs’ Simulation Learning Education and Research Network (SimLEARN). The pre-course is a 4.5-hour, multimodal class that involves hands-on training to teach the fundamentals of how to evaluate patients using POCUS.
With this course, Dr. Soni said, SHM is addressing training needs from “above and below.”
“Medical students, residents, fellows – basically doctors in training, whether they are student residents or getting ultrasound instruction in their basic training – when these kids graduate, they are pretty good,” he said. “But what about all the doctors who graduated long ago? They didn’t get any POCUS training. That’s where SHM, CHEST, and some of the other societies come in to play. We can offer these courses for training.”
Ultrasound training and credentialing has become a focus for SHM as interest has grown among clinicians. The Journal of Hospital Medicine recently released a consensus statement, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures,” which offers recommendations for credentialing hospitalists in ultrasound guidance and proposes initial and ongoing pathways to improve how hospitalists perform these procedures. The statement emphasizes how ultrasound guidance is increasingly essential to six bedside procedures that are core competencies of hospitalists: abdominal paracentesis, arterial catheter placement, arthrocentesis, central venous catheter placement, lumbar puncture, and thoracentesis.
According to Brian P. Lucas, MD, of Rush Medical College, Chicago, and lead author of the position statement, SHM’s Education Committee convened a POCUS Task Force to take on the project as American Board of Internal Medicine (ABIM) diplomates are no longer expected as part of their residency training to manually perform certain bedside procedures, with or without ultrasound guidance. SHM’s Board of Directors gave final approval on the statement in September 2017.
“There is much variation in the training and experience of both bedside procedures and point-of-care ultrasound. Many practicing hospitalists, for example, have no experience using ultrasound guidance for central lines,” Dr. Lucas said. “How then should hospitals initially, and then biennially, vet hospitalists’ competence in the performance of ultrasound-guided bedside procedures? This nationwide collaborative of experts, educators and front-line providers puts forth some recommendations to this very thorny problem.”
SHM also offers, in collaboration with the American College of Chest Physicians, a Point-of-Care Ultrasound Certificate of Completion for clinicians. The program is designed primarily to educate hospitalists and other providers caring for acutely ill patients, and requires attendance at a series of training programs within the course of 3 years, at the end of which a clinician must complete a comprehensive skills and knowledge assessment to obtain the certificate.
Focus on POCUS
Although medical societies recognize the growing demand, and are offering more ultrasound training, many hospitalists may not be familiar with POCUS specifically and the benefits of utilizing a bedside ultrasound exam, Dr. Soni noted.
“When we talk about point-of-care ultrasound, how is it different from what everyone else thinks about ultrasound? Specifically, it’s a point-of-care bedside exam” Dr. Soni explained. “So, the same provider – whether it’s a physician, nurse practitioner, or PA – who is taking care of a patient, can use ultrasound at the bedside to evaluate specific things in the patient’s body and answer very focused questions.”
The ability to have a hospitalist immediately use an imaging technique at a patient’s bedside can be invaluable, because it allows the clinician to provide a fairly accurate diagnosis in conjunction with patient input. This is much more effective than the traditional process of ultrasound imaging, Dr. Soni said.
“If you go to your primary care doctor [who orders] an ultrasound, you go to the radiology department and the technician captures the images,” he said. “Then the radiologist, who never even sees the patient, reads the ultrasound images with little to no clinical data.” The compartmentalization of the treating clinician, patient, and radiologist leaves the latter without critical information when reading an ultrasound. POCUS can potentially overcome this problematic situation, Dr. Soni suggested.
The HM18 POCUS pre-course has four objectives:
1. Recognize the fundamentals of ultrasound and the basic operation of an ultrasound machine (“knobology”).
2. Differentiate between the different types of ultrasound transducers and determine which is most appropriate for different POCUS applications.
3. Exhibit proper techniques on focused cardiac and lung ultrasound exams and be able to recognize thoracic pathologies from abnormal ultrasound results.
4. Identify and understand normal sonographic appearance of solid abdominal organs and vasculature of the neck and lower extremities and the ability to interpret abnormal ultrasounds to identify pathologies.
While all attendees can expect to learn a new skill that will improve their practice, POCUS training will specifically benefit hospitalists and the institutions in which they work, Dr. Soni said.
“Hospitalists, by nature, work for the hospital. In most cases, hospitalists are subsidized by the hospital. Because of that, the bigger gain from using ultrasound is not in the billing,” he said. “You can bill for focused ultrasound exams of the lungs, heart, abdomen, etc., and you might get a professional fee of $30-$40. But the bigger win in all of this, financially, is giving people more efficient health care. If we can prevent one complication from a bedside procedure or expedite a patient’s care and get them better sooner, we can save the hospital and the system money.”
The SHM is accredited to provide continuing medical education for physicians by the Accreditation Council for Continuing Medical Education. This live activity course will count for a maximum of 4.75 AMA PRA Category 1 Credits.
Point-of-Care Ultrasound for the Hospitalist
Sunday, April 8, 7:30 a.m.–12:15 p.m.
Point-of-care ultrasound (POCUS) is proving to be an increasing useful diagnostic tool for clinicians at the bedside, and many hospitalists have expressed interest in learning best practices in the use of the technology.
To that end, the Society of Hospital Medicine (SHM) is offering a half-day POCUS pre-course at Hospital Medicine 2018 this April in Orlando, with the intent of helping hospitalists learn how best to use POCUS in clinical settings.
“The agenda is really designed to teach people the basics of point-of-care ultrasound,” said Nilam J. Soni, MD, MSc, of the University of Texas at San Antonio and South Texas Veterans Health Care System, also in San Antonio, and a coinstructor of the POCUS pre-course. “It’s designed for the novice learner who has no prior experience in using ultrasound.”
Dr. Soni and his coinstructor, Ricardo Antonio Franco-Sadud, MD, of the Medical College of Wisconsin, Milwaukee, will lead attendees through the basics of “Point-of-Care Ultrasound for the Hospitalist” at HM18. Dr. Soni has taught a version of this course for almost a decade and is the assistant director of POCUS training programs with Veterans Affairs’ Simulation Learning Education and Research Network (SimLEARN). The pre-course is a 4.5-hour, multimodal class that involves hands-on training to teach the fundamentals of how to evaluate patients using POCUS.
With this course, Dr. Soni said, SHM is addressing training needs from “above and below.”
“Medical students, residents, fellows – basically doctors in training, whether they are student residents or getting ultrasound instruction in their basic training – when these kids graduate, they are pretty good,” he said. “But what about all the doctors who graduated long ago? They didn’t get any POCUS training. That’s where SHM, CHEST, and some of the other societies come in to play. We can offer these courses for training.”
Ultrasound training and credentialing has become a focus for SHM as interest has grown among clinicians. The Journal of Hospital Medicine recently released a consensus statement, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures,” which offers recommendations for credentialing hospitalists in ultrasound guidance and proposes initial and ongoing pathways to improve how hospitalists perform these procedures. The statement emphasizes how ultrasound guidance is increasingly essential to six bedside procedures that are core competencies of hospitalists: abdominal paracentesis, arterial catheter placement, arthrocentesis, central venous catheter placement, lumbar puncture, and thoracentesis.
According to Brian P. Lucas, MD, of Rush Medical College, Chicago, and lead author of the position statement, SHM’s Education Committee convened a POCUS Task Force to take on the project as American Board of Internal Medicine (ABIM) diplomates are no longer expected as part of their residency training to manually perform certain bedside procedures, with or without ultrasound guidance. SHM’s Board of Directors gave final approval on the statement in September 2017.
“There is much variation in the training and experience of both bedside procedures and point-of-care ultrasound. Many practicing hospitalists, for example, have no experience using ultrasound guidance for central lines,” Dr. Lucas said. “How then should hospitals initially, and then biennially, vet hospitalists’ competence in the performance of ultrasound-guided bedside procedures? This nationwide collaborative of experts, educators and front-line providers puts forth some recommendations to this very thorny problem.”
SHM also offers, in collaboration with the American College of Chest Physicians, a Point-of-Care Ultrasound Certificate of Completion for clinicians. The program is designed primarily to educate hospitalists and other providers caring for acutely ill patients, and requires attendance at a series of training programs within the course of 3 years, at the end of which a clinician must complete a comprehensive skills and knowledge assessment to obtain the certificate.
Focus on POCUS
Although medical societies recognize the growing demand, and are offering more ultrasound training, many hospitalists may not be familiar with POCUS specifically and the benefits of utilizing a bedside ultrasound exam, Dr. Soni noted.
“When we talk about point-of-care ultrasound, how is it different from what everyone else thinks about ultrasound? Specifically, it’s a point-of-care bedside exam” Dr. Soni explained. “So, the same provider – whether it’s a physician, nurse practitioner, or PA – who is taking care of a patient, can use ultrasound at the bedside to evaluate specific things in the patient’s body and answer very focused questions.”
The ability to have a hospitalist immediately use an imaging technique at a patient’s bedside can be invaluable, because it allows the clinician to provide a fairly accurate diagnosis in conjunction with patient input. This is much more effective than the traditional process of ultrasound imaging, Dr. Soni said.
“If you go to your primary care doctor [who orders] an ultrasound, you go to the radiology department and the technician captures the images,” he said. “Then the radiologist, who never even sees the patient, reads the ultrasound images with little to no clinical data.” The compartmentalization of the treating clinician, patient, and radiologist leaves the latter without critical information when reading an ultrasound. POCUS can potentially overcome this problematic situation, Dr. Soni suggested.
The HM18 POCUS pre-course has four objectives:
1. Recognize the fundamentals of ultrasound and the basic operation of an ultrasound machine (“knobology”).
2. Differentiate between the different types of ultrasound transducers and determine which is most appropriate for different POCUS applications.
3. Exhibit proper techniques on focused cardiac and lung ultrasound exams and be able to recognize thoracic pathologies from abnormal ultrasound results.
4. Identify and understand normal sonographic appearance of solid abdominal organs and vasculature of the neck and lower extremities and the ability to interpret abnormal ultrasounds to identify pathologies.
While all attendees can expect to learn a new skill that will improve their practice, POCUS training will specifically benefit hospitalists and the institutions in which they work, Dr. Soni said.
“Hospitalists, by nature, work for the hospital. In most cases, hospitalists are subsidized by the hospital. Because of that, the bigger gain from using ultrasound is not in the billing,” he said. “You can bill for focused ultrasound exams of the lungs, heart, abdomen, etc., and you might get a professional fee of $30-$40. But the bigger win in all of this, financially, is giving people more efficient health care. If we can prevent one complication from a bedside procedure or expedite a patient’s care and get them better sooner, we can save the hospital and the system money.”
The SHM is accredited to provide continuing medical education for physicians by the Accreditation Council for Continuing Medical Education. This live activity course will count for a maximum of 4.75 AMA PRA Category 1 Credits.
Point-of-Care Ultrasound for the Hospitalist
Sunday, April 8, 7:30 a.m.–12:15 p.m.
New tracks bring focus to HM18 program
At the 2018 annual meeting of the Society of Hospital Medicine – running from April 8 to 11 in Orlando – the theme could well be “in with the new, and in with the new.”
Planners for Hospital Medicine 2018 (HM18) have managed to pack the conference with five new tracks: Great Debate, Nurse Practitioner/Physician’s Assistant (NP/PA), Palliative Care, Seasoning Your Career, and a new Career Development workshop track. And they did this while eliminating only one track that was on the schedule last year – technology – and without adding any extra days to the meeting.
“We decided, since there were a bunch of themes that we really wanted to cover, we would do half-day tracks. The shorter tracks are also a way to gauge interest in a topic without making a big commitment to it,” Dr. Finn said. “The grouping of topics in smaller tracks in the Day-at-a-Glance helps people easily see a collection of lectures or a theme they might want to attend.”
While choosing themes for the meeting, the planners were trying to stay true to their own theme: timeliness.
“There’s pressure to make it a very relevant meeting,” Dr. Finn said. “We really want to have our finger on the pulse of what practicing hospitalists need and want to know and what is important to them. All the members of the committee feel very invested in figuring out: What is timely? What do we want to talk about right now? What are the active discussions and issues going on in health care that affect us in our practice?”
Assistant course director Dustin Smith, MD, FHM, an associate professor of medicine at Emory University, Atlanta, said much of the information for this year’s meeting came from the 2017 annual meeting, including attendance at sessions, speaker reviews, and session ratings.
“It’s building on momentum from the previous meeting,” he said. “Sometimes we choose things to offer that we know are going to go well, and sometimes we choose things that we hope go well, and all of a sudden we see [that they] go very, very well.” For instance, he said, the topic of sepsis was so popular last year that it has its own precourse this year.
The data on which the HM18 program is built doesn’t stop there. The 23 members of the planning committee – chosen strategically to represent a wide geographic range and array of practice types – all bring their own thoughts and experiences, as well as input from colleagues at their own centers. Then there are the submissions for workshop topics: Any SHM member can submit an idea, and – while just a few are chosen – those ideas help organizers see patterns of interest that can affect the planning of the rest of the sessions.
Here are more details on the new tracks:
Great debate
The annual meeting has traditionally had a “Great Debate” on perioperative medicine, but the format – with carefully chosen speakers who are dynamic and entertaining – will be used to cover pulmonary medicine and infectious diseases this year as well.
“It’s a hugely successful talk,” Dr. Finn said. “We can tell by our numbers that lots of people go, and it’s always funny, and it’s a very clever way of discussing the latest literature – by having two very dynamic speakers present a case and then debate the two options of the case and then use the literature to support the answer,” she said.
The hope is that the format will be more than just entertaining but will be an effective teaching tool, too.
“We think the high level of engagement and format of the talk leads to better overall education for those who attend,” Dr. Smith said.
NP/PA
This track includes topics chosen by the committee for advanced practice professionals.
“There are many hospitalist programs that include NP/PAs – this is what came through in all the feedback – and everybody is struggling with how do you best incorporate NPs and PAs into the group practice and have everybody work at the top of their license and work well together,” Dr. Finn said.
“The idea, too, is to be very inclusive of all providers and offering a track that focuses on NP/PAs but also includes physicians, physician leaders, and physician administrators,” Dr. Smith said. “It’s not designed for one type of practicing professional; it should be a good educational track for all.”
Palliative care
This was a topic that had been sprinkled throughout programs in previous years, but Dr. Finn and Dr. Smith said it was considered too important not to have its own track this year.
“I think hospitalists are often the doctors caring for patients at the end of their lives since many Americans die in the hospital,” Dr. Finn said. “So as a result, this is a skill set that as hospitalists we need to be very good at.”
Seasoning your career
This is a track geared toward one of this year’s themes: With “hospital medicine” now a concept that’s more than two decades old, how do hospitalists keep up the momentum in their careers, how do they take stock, how do they make the important decisions they face as they move ahead in their jobs?
“Hospital medicine is now over 20 years old – many hospitalists are now mid-career,” Dr. Finn said. “We picked an entire track on ‘seasoning your career’ to offer people ideas and skills to reflect on and rethink their career. Do you want to expand what you’re doing? Do you want to change it? How do you make this a lifelong career?”
Career development
There have always been workshops with a ‘career development’ focus, but this year six of them were chosen to be placed under the heading of an official “career development” workshop track.
“When you review the Day-at-a-Glance schedule, it really demarcates it,” Dr. Smith said. “This really helps attendees be able to quickly look through and find where they want to be for their next session.”
“Are there other skills you want to take on for the second half of your career?” Dr. Finn said. “Do you want to take on leadership? Do you want to learn how to better give your peers feedback? Do you want to promote women in your group? Do you want to prevent burnout or use emotional intelligence to improve your career? We cohorted these topics together.”
Aside from the new tracks, the course directors also drew attention to other new elements of the HM18 program.
For instance there are new topics in the Rapid Fire sessions. In the “Managing the patient on your service: Appendicitis, Bowel and biliary obstruction” session, a general surgeon will talk about how to manage these surgical issues when the patient is on a medical service. In “Interventional radiology: What every hospitalist needs to know,” an interventional radiologist will discuss when hospitalists may want to call in an interventional radiologist or refer to a hospital that has an interventional radiologist. And “Vulnerable populations and hospitalists” will continue with the theme of social determinants of health that was highlighted at last year’s meeting by keynote speaker Karen DeSalvo, MD, the national coordinator for health information technology.
Dr. Smith said that the program committee directors work with the Rapid Fire presenters so that the three or four questions discussed in the sessions are what attendees will want to learn most.
“We take an additional step: Once we recruit the speaker and have identified the topic, we have members of our committee work with the speakers,” he said.
“We don’t want them to come and give us an esoteric talk in an area that interests them. We want them to answer the clinical questions that hospitalists have,” Dr. Finn added.
Dr. Finn and Dr. Smith also highlighted sessions with a twist. For example, “Stupefy: EKGs for fun” is a session about EKGs that encourages hospitalists to “just go have fun reading them,” Dr. Finn said, while “Voldemort is on the plane: Airplane emergencies,” is scheduled for the final day of the conference, just before everyone flies back home.
As for catchy Disney-influenced titles, such as “The Mad Hatter: Updates in delirium” and “Waiting in line for ‘It’s a Small World’ and other things we do for no reason,” part of the credit can go to Dr. Finn’s niece. She said she “hired” her to come up with a list of Disney, Pixar, and Harry Potter movies and catchphrases. Then the committee worked them into the session titles.
Dr. Smith joked that part of his role was to veto some titles that were “a bit too cringe-worthy.”
“The theme of Orlando is making people happy,” Dr. Finn said. “One of the goals – the hopes – for me for at this meeting is that people bring their inner child and get curious again and explore new ideas and new topics and new career possibilities.”
At the 2018 annual meeting of the Society of Hospital Medicine – running from April 8 to 11 in Orlando – the theme could well be “in with the new, and in with the new.”
Planners for Hospital Medicine 2018 (HM18) have managed to pack the conference with five new tracks: Great Debate, Nurse Practitioner/Physician’s Assistant (NP/PA), Palliative Care, Seasoning Your Career, and a new Career Development workshop track. And they did this while eliminating only one track that was on the schedule last year – technology – and without adding any extra days to the meeting.
“We decided, since there were a bunch of themes that we really wanted to cover, we would do half-day tracks. The shorter tracks are also a way to gauge interest in a topic without making a big commitment to it,” Dr. Finn said. “The grouping of topics in smaller tracks in the Day-at-a-Glance helps people easily see a collection of lectures or a theme they might want to attend.”
While choosing themes for the meeting, the planners were trying to stay true to their own theme: timeliness.
“There’s pressure to make it a very relevant meeting,” Dr. Finn said. “We really want to have our finger on the pulse of what practicing hospitalists need and want to know and what is important to them. All the members of the committee feel very invested in figuring out: What is timely? What do we want to talk about right now? What are the active discussions and issues going on in health care that affect us in our practice?”
Assistant course director Dustin Smith, MD, FHM, an associate professor of medicine at Emory University, Atlanta, said much of the information for this year’s meeting came from the 2017 annual meeting, including attendance at sessions, speaker reviews, and session ratings.
“It’s building on momentum from the previous meeting,” he said. “Sometimes we choose things to offer that we know are going to go well, and sometimes we choose things that we hope go well, and all of a sudden we see [that they] go very, very well.” For instance, he said, the topic of sepsis was so popular last year that it has its own precourse this year.
The data on which the HM18 program is built doesn’t stop there. The 23 members of the planning committee – chosen strategically to represent a wide geographic range and array of practice types – all bring their own thoughts and experiences, as well as input from colleagues at their own centers. Then there are the submissions for workshop topics: Any SHM member can submit an idea, and – while just a few are chosen – those ideas help organizers see patterns of interest that can affect the planning of the rest of the sessions.
Here are more details on the new tracks:
Great debate
The annual meeting has traditionally had a “Great Debate” on perioperative medicine, but the format – with carefully chosen speakers who are dynamic and entertaining – will be used to cover pulmonary medicine and infectious diseases this year as well.
“It’s a hugely successful talk,” Dr. Finn said. “We can tell by our numbers that lots of people go, and it’s always funny, and it’s a very clever way of discussing the latest literature – by having two very dynamic speakers present a case and then debate the two options of the case and then use the literature to support the answer,” she said.
The hope is that the format will be more than just entertaining but will be an effective teaching tool, too.
“We think the high level of engagement and format of the talk leads to better overall education for those who attend,” Dr. Smith said.
NP/PA
This track includes topics chosen by the committee for advanced practice professionals.
“There are many hospitalist programs that include NP/PAs – this is what came through in all the feedback – and everybody is struggling with how do you best incorporate NPs and PAs into the group practice and have everybody work at the top of their license and work well together,” Dr. Finn said.
“The idea, too, is to be very inclusive of all providers and offering a track that focuses on NP/PAs but also includes physicians, physician leaders, and physician administrators,” Dr. Smith said. “It’s not designed for one type of practicing professional; it should be a good educational track for all.”
Palliative care
This was a topic that had been sprinkled throughout programs in previous years, but Dr. Finn and Dr. Smith said it was considered too important not to have its own track this year.
“I think hospitalists are often the doctors caring for patients at the end of their lives since many Americans die in the hospital,” Dr. Finn said. “So as a result, this is a skill set that as hospitalists we need to be very good at.”
Seasoning your career
This is a track geared toward one of this year’s themes: With “hospital medicine” now a concept that’s more than two decades old, how do hospitalists keep up the momentum in their careers, how do they take stock, how do they make the important decisions they face as they move ahead in their jobs?
“Hospital medicine is now over 20 years old – many hospitalists are now mid-career,” Dr. Finn said. “We picked an entire track on ‘seasoning your career’ to offer people ideas and skills to reflect on and rethink their career. Do you want to expand what you’re doing? Do you want to change it? How do you make this a lifelong career?”
Career development
There have always been workshops with a ‘career development’ focus, but this year six of them were chosen to be placed under the heading of an official “career development” workshop track.
“When you review the Day-at-a-Glance schedule, it really demarcates it,” Dr. Smith said. “This really helps attendees be able to quickly look through and find where they want to be for their next session.”
“Are there other skills you want to take on for the second half of your career?” Dr. Finn said. “Do you want to take on leadership? Do you want to learn how to better give your peers feedback? Do you want to promote women in your group? Do you want to prevent burnout or use emotional intelligence to improve your career? We cohorted these topics together.”
Aside from the new tracks, the course directors also drew attention to other new elements of the HM18 program.
For instance there are new topics in the Rapid Fire sessions. In the “Managing the patient on your service: Appendicitis, Bowel and biliary obstruction” session, a general surgeon will talk about how to manage these surgical issues when the patient is on a medical service. In “Interventional radiology: What every hospitalist needs to know,” an interventional radiologist will discuss when hospitalists may want to call in an interventional radiologist or refer to a hospital that has an interventional radiologist. And “Vulnerable populations and hospitalists” will continue with the theme of social determinants of health that was highlighted at last year’s meeting by keynote speaker Karen DeSalvo, MD, the national coordinator for health information technology.
Dr. Smith said that the program committee directors work with the Rapid Fire presenters so that the three or four questions discussed in the sessions are what attendees will want to learn most.
“We take an additional step: Once we recruit the speaker and have identified the topic, we have members of our committee work with the speakers,” he said.
“We don’t want them to come and give us an esoteric talk in an area that interests them. We want them to answer the clinical questions that hospitalists have,” Dr. Finn added.
Dr. Finn and Dr. Smith also highlighted sessions with a twist. For example, “Stupefy: EKGs for fun” is a session about EKGs that encourages hospitalists to “just go have fun reading them,” Dr. Finn said, while “Voldemort is on the plane: Airplane emergencies,” is scheduled for the final day of the conference, just before everyone flies back home.
As for catchy Disney-influenced titles, such as “The Mad Hatter: Updates in delirium” and “Waiting in line for ‘It’s a Small World’ and other things we do for no reason,” part of the credit can go to Dr. Finn’s niece. She said she “hired” her to come up with a list of Disney, Pixar, and Harry Potter movies and catchphrases. Then the committee worked them into the session titles.
Dr. Smith joked that part of his role was to veto some titles that were “a bit too cringe-worthy.”
“The theme of Orlando is making people happy,” Dr. Finn said. “One of the goals – the hopes – for me for at this meeting is that people bring their inner child and get curious again and explore new ideas and new topics and new career possibilities.”
At the 2018 annual meeting of the Society of Hospital Medicine – running from April 8 to 11 in Orlando – the theme could well be “in with the new, and in with the new.”
Planners for Hospital Medicine 2018 (HM18) have managed to pack the conference with five new tracks: Great Debate, Nurse Practitioner/Physician’s Assistant (NP/PA), Palliative Care, Seasoning Your Career, and a new Career Development workshop track. And they did this while eliminating only one track that was on the schedule last year – technology – and without adding any extra days to the meeting.
“We decided, since there were a bunch of themes that we really wanted to cover, we would do half-day tracks. The shorter tracks are also a way to gauge interest in a topic without making a big commitment to it,” Dr. Finn said. “The grouping of topics in smaller tracks in the Day-at-a-Glance helps people easily see a collection of lectures or a theme they might want to attend.”
While choosing themes for the meeting, the planners were trying to stay true to their own theme: timeliness.
“There’s pressure to make it a very relevant meeting,” Dr. Finn said. “We really want to have our finger on the pulse of what practicing hospitalists need and want to know and what is important to them. All the members of the committee feel very invested in figuring out: What is timely? What do we want to talk about right now? What are the active discussions and issues going on in health care that affect us in our practice?”
Assistant course director Dustin Smith, MD, FHM, an associate professor of medicine at Emory University, Atlanta, said much of the information for this year’s meeting came from the 2017 annual meeting, including attendance at sessions, speaker reviews, and session ratings.
“It’s building on momentum from the previous meeting,” he said. “Sometimes we choose things to offer that we know are going to go well, and sometimes we choose things that we hope go well, and all of a sudden we see [that they] go very, very well.” For instance, he said, the topic of sepsis was so popular last year that it has its own precourse this year.
The data on which the HM18 program is built doesn’t stop there. The 23 members of the planning committee – chosen strategically to represent a wide geographic range and array of practice types – all bring their own thoughts and experiences, as well as input from colleagues at their own centers. Then there are the submissions for workshop topics: Any SHM member can submit an idea, and – while just a few are chosen – those ideas help organizers see patterns of interest that can affect the planning of the rest of the sessions.
Here are more details on the new tracks:
Great debate
The annual meeting has traditionally had a “Great Debate” on perioperative medicine, but the format – with carefully chosen speakers who are dynamic and entertaining – will be used to cover pulmonary medicine and infectious diseases this year as well.
“It’s a hugely successful talk,” Dr. Finn said. “We can tell by our numbers that lots of people go, and it’s always funny, and it’s a very clever way of discussing the latest literature – by having two very dynamic speakers present a case and then debate the two options of the case and then use the literature to support the answer,” she said.
The hope is that the format will be more than just entertaining but will be an effective teaching tool, too.
“We think the high level of engagement and format of the talk leads to better overall education for those who attend,” Dr. Smith said.
NP/PA
This track includes topics chosen by the committee for advanced practice professionals.
“There are many hospitalist programs that include NP/PAs – this is what came through in all the feedback – and everybody is struggling with how do you best incorporate NPs and PAs into the group practice and have everybody work at the top of their license and work well together,” Dr. Finn said.
“The idea, too, is to be very inclusive of all providers and offering a track that focuses on NP/PAs but also includes physicians, physician leaders, and physician administrators,” Dr. Smith said. “It’s not designed for one type of practicing professional; it should be a good educational track for all.”
Palliative care
This was a topic that had been sprinkled throughout programs in previous years, but Dr. Finn and Dr. Smith said it was considered too important not to have its own track this year.
“I think hospitalists are often the doctors caring for patients at the end of their lives since many Americans die in the hospital,” Dr. Finn said. “So as a result, this is a skill set that as hospitalists we need to be very good at.”
Seasoning your career
This is a track geared toward one of this year’s themes: With “hospital medicine” now a concept that’s more than two decades old, how do hospitalists keep up the momentum in their careers, how do they take stock, how do they make the important decisions they face as they move ahead in their jobs?
“Hospital medicine is now over 20 years old – many hospitalists are now mid-career,” Dr. Finn said. “We picked an entire track on ‘seasoning your career’ to offer people ideas and skills to reflect on and rethink their career. Do you want to expand what you’re doing? Do you want to change it? How do you make this a lifelong career?”
Career development
There have always been workshops with a ‘career development’ focus, but this year six of them were chosen to be placed under the heading of an official “career development” workshop track.
“When you review the Day-at-a-Glance schedule, it really demarcates it,” Dr. Smith said. “This really helps attendees be able to quickly look through and find where they want to be for their next session.”
“Are there other skills you want to take on for the second half of your career?” Dr. Finn said. “Do you want to take on leadership? Do you want to learn how to better give your peers feedback? Do you want to promote women in your group? Do you want to prevent burnout or use emotional intelligence to improve your career? We cohorted these topics together.”
Aside from the new tracks, the course directors also drew attention to other new elements of the HM18 program.
For instance there are new topics in the Rapid Fire sessions. In the “Managing the patient on your service: Appendicitis, Bowel and biliary obstruction” session, a general surgeon will talk about how to manage these surgical issues when the patient is on a medical service. In “Interventional radiology: What every hospitalist needs to know,” an interventional radiologist will discuss when hospitalists may want to call in an interventional radiologist or refer to a hospital that has an interventional radiologist. And “Vulnerable populations and hospitalists” will continue with the theme of social determinants of health that was highlighted at last year’s meeting by keynote speaker Karen DeSalvo, MD, the national coordinator for health information technology.
Dr. Smith said that the program committee directors work with the Rapid Fire presenters so that the three or four questions discussed in the sessions are what attendees will want to learn most.
“We take an additional step: Once we recruit the speaker and have identified the topic, we have members of our committee work with the speakers,” he said.
“We don’t want them to come and give us an esoteric talk in an area that interests them. We want them to answer the clinical questions that hospitalists have,” Dr. Finn added.
Dr. Finn and Dr. Smith also highlighted sessions with a twist. For example, “Stupefy: EKGs for fun” is a session about EKGs that encourages hospitalists to “just go have fun reading them,” Dr. Finn said, while “Voldemort is on the plane: Airplane emergencies,” is scheduled for the final day of the conference, just before everyone flies back home.
As for catchy Disney-influenced titles, such as “The Mad Hatter: Updates in delirium” and “Waiting in line for ‘It’s a Small World’ and other things we do for no reason,” part of the credit can go to Dr. Finn’s niece. She said she “hired” her to come up with a list of Disney, Pixar, and Harry Potter movies and catchphrases. Then the committee worked them into the session titles.
Dr. Smith joked that part of his role was to veto some titles that were “a bit too cringe-worthy.”
“The theme of Orlando is making people happy,” Dr. Finn said. “One of the goals – the hopes – for me for at this meeting is that people bring their inner child and get curious again and explore new ideas and new topics and new career possibilities.”
‘Update in Hospital Medicine’ to highlight practice pearls
Barbara Slawski, MD, MS, SFHM, and Cynthia Cooper, MD, hadn’t met in person until early 2018. But that doesn’t mean they haven’t spent a lot of time together.
Once a month, usually on a Friday afternoon, the two hospitalists checked in with one another through relaxed, wide-ranging phone calls. Together they have combed the medical literature and conferred over the past year, making long lists of candidate studies for the “top 20” journal articles of 2018 for practicing hospitalists.
The two physicians are preparing for the “Update in Hospital Medicine” session they will comoderate at HM18 – historically one of the most popular at SHM annual meetings – where the research findings of these “Top 20” articles are summarized for conference attendees. Their hope, they said, is to present research that each attendee can bring home to improve patient outcomes on a daily basis, while making for a smoother and more efficient practice of hospital medicine.
Through a process each physician describes as collegial, Dr. Slawski and Dr. Cooper have winnowed their lists and are nearly ready to make their final calls. Since hospital medicine crosses so many disciplines, each physician said, in separate interviews, that doing justice to the literature has been time-consuming and intellectually challenging – but worthwhile.
Dr. Cooper, a hospitalist at Massachusetts General Hospital, Boston, said that although she and Dr. Slawski practice in geographically diverse areas, their practice settings – academic medical centers – have many similarities. She said that as she reviewed the medical literature over the past year, she gave considerable thought to the particular challenges and demands of hospitalists who practice in community hospitals and rural settings, where the level of support and access to subspecialty consults might be very different from the academic milieu where both she and Dr. Slawski practice.
“We hope that our unique approaches lend more breadth to the session,” said Dr. Slawski. “We want to make sure we have a good representation of SHM’s constituency, and that we present high-impact studies.”
In addition to her work at Mass General, Dr. Cooper also holds an appointment at Harvard Medical School. She said that the challenge over the past year has been to find the studies that are not focused just on primary care, but that really touch on the unique practice demands and skill set of physicians who practice hospital-based medicine.
Dr. Slawski said that cardiology is one of the areas that’s had relevant, practice-changing findings this past year: She expects to put at least one practice-changing cardiology article on the “Top 20” list. “How do we address patients with suspected acute coronary syndromes? Well, we have some new direction this year,” she said.
To hit the mark of articles that are relevant for all, Dr. Cooper said she wants to make sure to include a focus on research that touches on the practicalities of hospital-based practice – possible topics include prediction scores, hepatic encephalopathy, and the management of sepsis.
Dr. Slawski said that in addition to relevance, she and Dr. Cooper looked for methodological rigor in the studies they’ll be presenting; they agreed that having an adequate sample size for statistical power was a must.
The two presenters said they’re working hard to put together a session that’s as enjoyable as it is relevant. “I hope we’ll be able to inject some humor into the presentation, too,” Dr. Cooper said. Dr. Slawski agreed, noting that the bar has been set high at SHM. “It feels like a community,” she said. “There are always great speakers with a sense of humor.”
Dr. Slawski stressed that even though they’ll have their list ready to go for HM18, they will still be scouring journals until the week of the meeting so they can update their presentation with any late-breaking news of significance.
Neither Dr. Slawski nor Dr. Cooper reported any relevant conflicts of interest.
Barbara Slawski, MD, MS, SFHM, and Cynthia Cooper, MD, hadn’t met in person until early 2018. But that doesn’t mean they haven’t spent a lot of time together.
Once a month, usually on a Friday afternoon, the two hospitalists checked in with one another through relaxed, wide-ranging phone calls. Together they have combed the medical literature and conferred over the past year, making long lists of candidate studies for the “top 20” journal articles of 2018 for practicing hospitalists.
The two physicians are preparing for the “Update in Hospital Medicine” session they will comoderate at HM18 – historically one of the most popular at SHM annual meetings – where the research findings of these “Top 20” articles are summarized for conference attendees. Their hope, they said, is to present research that each attendee can bring home to improve patient outcomes on a daily basis, while making for a smoother and more efficient practice of hospital medicine.
Through a process each physician describes as collegial, Dr. Slawski and Dr. Cooper have winnowed their lists and are nearly ready to make their final calls. Since hospital medicine crosses so many disciplines, each physician said, in separate interviews, that doing justice to the literature has been time-consuming and intellectually challenging – but worthwhile.
Dr. Cooper, a hospitalist at Massachusetts General Hospital, Boston, said that although she and Dr. Slawski practice in geographically diverse areas, their practice settings – academic medical centers – have many similarities. She said that as she reviewed the medical literature over the past year, she gave considerable thought to the particular challenges and demands of hospitalists who practice in community hospitals and rural settings, where the level of support and access to subspecialty consults might be very different from the academic milieu where both she and Dr. Slawski practice.
“We hope that our unique approaches lend more breadth to the session,” said Dr. Slawski. “We want to make sure we have a good representation of SHM’s constituency, and that we present high-impact studies.”
In addition to her work at Mass General, Dr. Cooper also holds an appointment at Harvard Medical School. She said that the challenge over the past year has been to find the studies that are not focused just on primary care, but that really touch on the unique practice demands and skill set of physicians who practice hospital-based medicine.
Dr. Slawski said that cardiology is one of the areas that’s had relevant, practice-changing findings this past year: She expects to put at least one practice-changing cardiology article on the “Top 20” list. “How do we address patients with suspected acute coronary syndromes? Well, we have some new direction this year,” she said.
To hit the mark of articles that are relevant for all, Dr. Cooper said she wants to make sure to include a focus on research that touches on the practicalities of hospital-based practice – possible topics include prediction scores, hepatic encephalopathy, and the management of sepsis.
Dr. Slawski said that in addition to relevance, she and Dr. Cooper looked for methodological rigor in the studies they’ll be presenting; they agreed that having an adequate sample size for statistical power was a must.
The two presenters said they’re working hard to put together a session that’s as enjoyable as it is relevant. “I hope we’ll be able to inject some humor into the presentation, too,” Dr. Cooper said. Dr. Slawski agreed, noting that the bar has been set high at SHM. “It feels like a community,” she said. “There are always great speakers with a sense of humor.”
Dr. Slawski stressed that even though they’ll have their list ready to go for HM18, they will still be scouring journals until the week of the meeting so they can update their presentation with any late-breaking news of significance.
Neither Dr. Slawski nor Dr. Cooper reported any relevant conflicts of interest.
Barbara Slawski, MD, MS, SFHM, and Cynthia Cooper, MD, hadn’t met in person until early 2018. But that doesn’t mean they haven’t spent a lot of time together.
Once a month, usually on a Friday afternoon, the two hospitalists checked in with one another through relaxed, wide-ranging phone calls. Together they have combed the medical literature and conferred over the past year, making long lists of candidate studies for the “top 20” journal articles of 2018 for practicing hospitalists.
The two physicians are preparing for the “Update in Hospital Medicine” session they will comoderate at HM18 – historically one of the most popular at SHM annual meetings – where the research findings of these “Top 20” articles are summarized for conference attendees. Their hope, they said, is to present research that each attendee can bring home to improve patient outcomes on a daily basis, while making for a smoother and more efficient practice of hospital medicine.
Through a process each physician describes as collegial, Dr. Slawski and Dr. Cooper have winnowed their lists and are nearly ready to make their final calls. Since hospital medicine crosses so many disciplines, each physician said, in separate interviews, that doing justice to the literature has been time-consuming and intellectually challenging – but worthwhile.
Dr. Cooper, a hospitalist at Massachusetts General Hospital, Boston, said that although she and Dr. Slawski practice in geographically diverse areas, their practice settings – academic medical centers – have many similarities. She said that as she reviewed the medical literature over the past year, she gave considerable thought to the particular challenges and demands of hospitalists who practice in community hospitals and rural settings, where the level of support and access to subspecialty consults might be very different from the academic milieu where both she and Dr. Slawski practice.
“We hope that our unique approaches lend more breadth to the session,” said Dr. Slawski. “We want to make sure we have a good representation of SHM’s constituency, and that we present high-impact studies.”
In addition to her work at Mass General, Dr. Cooper also holds an appointment at Harvard Medical School. She said that the challenge over the past year has been to find the studies that are not focused just on primary care, but that really touch on the unique practice demands and skill set of physicians who practice hospital-based medicine.
Dr. Slawski said that cardiology is one of the areas that’s had relevant, practice-changing findings this past year: She expects to put at least one practice-changing cardiology article on the “Top 20” list. “How do we address patients with suspected acute coronary syndromes? Well, we have some new direction this year,” she said.
To hit the mark of articles that are relevant for all, Dr. Cooper said she wants to make sure to include a focus on research that touches on the practicalities of hospital-based practice – possible topics include prediction scores, hepatic encephalopathy, and the management of sepsis.
Dr. Slawski said that in addition to relevance, she and Dr. Cooper looked for methodological rigor in the studies they’ll be presenting; they agreed that having an adequate sample size for statistical power was a must.
The two presenters said they’re working hard to put together a session that’s as enjoyable as it is relevant. “I hope we’ll be able to inject some humor into the presentation, too,” Dr. Cooper said. Dr. Slawski agreed, noting that the bar has been set high at SHM. “It feels like a community,” she said. “There are always great speakers with a sense of humor.”
Dr. Slawski stressed that even though they’ll have their list ready to go for HM18, they will still be scouring journals until the week of the meeting so they can update their presentation with any late-breaking news of significance.
Neither Dr. Slawski nor Dr. Cooper reported any relevant conflicts of interest.
Study confirms higher risk of infection with CB transplant
SALT LAKE CITY—Results of a large, retrospective analysis support the notion that patients who receive cord blood (CB) transplants have a higher risk of infection than other hematopoietic stem cell transplant (HSCT) recipients.
Investigators found that CB recipients had a significantly higher risk of bacterial, viral, and fungal infections in the early post-transplant period than patients who received peripheral blood (PB) or bone marrow (BM) transplants.
In addition, CB recipients had longer hospital stays, higher inpatient costs, and greater inpatient mortality than PB and BM recipients.
Amandeep Godara, MD, of Tufts Medical Center in Boston, Massachusetts, presented these results at the 2018 BMT Tandem Meetings (abstract 30*).
“Infections are considered more common in cord blood transplant recipients based on some prior retrospective analyses,” Dr Godara noted. “But there is limited data comparing these infectious complications between cord blood transplant and peripheral blood/bone marrow stem cell transplants during the inpatient stay for the stem cell transplant.”
With this in mind, Dr Godara and his colleagues analyzed data from the Healthcare Cost and Utilization Project National Inpatient Sample. This database covers 46 US states and contains data from more than 7 million hospital stays each year.
The investigators searched the database for hospital admissions for HSCT from 2002 to 2014. They identified 2979 CB transplants and 56,845 PB or BM transplants.
The CB recipients had a median age of 48, and 55% were male. Fifty-nine percent were white, 18% Hispanic, 13% black, 5% Asian, and 5% “other.” Sixty-six percent of patients had acute leukemia, 18% non-Hodgkin lymphoma, 5% Hodgkin lymphoma, and 11% “other” diseases.
The PB/BM recipients had a median age of 45, and 58% were male. Seventy-nine percent were white, 8% Hispanic, 6% black, 3% Asian, and 4% “other.” Sixty-one percent of patients had acute leukemia, 16% non-Hodgkin lymphoma, 4% Hodgkin lymphoma, and 19% “other” diseases.
Results
Dr Godara and his colleagues compared the rates and types of infection from the time of HSCT to hospital discharge in CB and PB/BM recipients. The team also compared early inpatient mortality, the cost of hospitalization, and the length of hospital stay.
“[W]e observed a higher risk for infections in cord blood transplant patients compared to peripheral blood and bone marrow stem cell transplant patients, and this risk for infection extended through a wide spectrum of pathogens,” Dr Godara said.
“We also observed a higher all-cause inpatient mortality in cord blood transplant compared to peripheral blood and bone marrow transplant, especially in patients who had bacterial sepsis or invasive fungal infection.”
The rate of bacterial sepsis was 34.87% in CB recipients and 20.20% in PB/BM recipients (P<0.0001). Rates of viral infection were 20.05% and 8.19%, respectively (P<0.0001). And rates of invasive fungal infection were 12.87% and 7.89% (P<0.0001).
There was a similar distribution of bacterial infections in CB and PB/BM recipients. The most common was pneumonia (47% and 41%, respectively), followed by abdominal infections (29% and 31%, respectively), urinary tract infections (17% and 21%, respectively), central line-associated bloodstream infections (4% and 6%, respectively), and acute sinusitis (3% and 1%, respectively).
The rate of Clostridium difficile infection was significantly higher in CB recipients than PB/BM recipients—11.75% and 8.90%, respectively (P=0.02). However, there was no significant difference in mortality related to C. difficile—14% and 10%, respectively (P=0.3).
On the other hand, all-cause inpatient mortality was significantly higher in CB recipients than PB/BM recipients—16% and 7%, respectively (P<0.0001).
Inpatient mortality rates were significantly higher for CB recipients with bacterial sepsis (33% vs 23%, P=0.001) and invasive fungal infections (29% vs 16%, P=0.0045) but not viral infections (19% vs 17%, P=0.5).
The median length of hospital stay was 36 days for CB recipients and 25 days for PB/BM recipients. The mean inpatient charges were $448,892 and $250,437 respectively.
*Data in the abstract differ from the presentation.
SALT LAKE CITY—Results of a large, retrospective analysis support the notion that patients who receive cord blood (CB) transplants have a higher risk of infection than other hematopoietic stem cell transplant (HSCT) recipients.
Investigators found that CB recipients had a significantly higher risk of bacterial, viral, and fungal infections in the early post-transplant period than patients who received peripheral blood (PB) or bone marrow (BM) transplants.
In addition, CB recipients had longer hospital stays, higher inpatient costs, and greater inpatient mortality than PB and BM recipients.
Amandeep Godara, MD, of Tufts Medical Center in Boston, Massachusetts, presented these results at the 2018 BMT Tandem Meetings (abstract 30*).
“Infections are considered more common in cord blood transplant recipients based on some prior retrospective analyses,” Dr Godara noted. “But there is limited data comparing these infectious complications between cord blood transplant and peripheral blood/bone marrow stem cell transplants during the inpatient stay for the stem cell transplant.”
With this in mind, Dr Godara and his colleagues analyzed data from the Healthcare Cost and Utilization Project National Inpatient Sample. This database covers 46 US states and contains data from more than 7 million hospital stays each year.
The investigators searched the database for hospital admissions for HSCT from 2002 to 2014. They identified 2979 CB transplants and 56,845 PB or BM transplants.
The CB recipients had a median age of 48, and 55% were male. Fifty-nine percent were white, 18% Hispanic, 13% black, 5% Asian, and 5% “other.” Sixty-six percent of patients had acute leukemia, 18% non-Hodgkin lymphoma, 5% Hodgkin lymphoma, and 11% “other” diseases.
The PB/BM recipients had a median age of 45, and 58% were male. Seventy-nine percent were white, 8% Hispanic, 6% black, 3% Asian, and 4% “other.” Sixty-one percent of patients had acute leukemia, 16% non-Hodgkin lymphoma, 4% Hodgkin lymphoma, and 19% “other” diseases.
Results
Dr Godara and his colleagues compared the rates and types of infection from the time of HSCT to hospital discharge in CB and PB/BM recipients. The team also compared early inpatient mortality, the cost of hospitalization, and the length of hospital stay.
“[W]e observed a higher risk for infections in cord blood transplant patients compared to peripheral blood and bone marrow stem cell transplant patients, and this risk for infection extended through a wide spectrum of pathogens,” Dr Godara said.
“We also observed a higher all-cause inpatient mortality in cord blood transplant compared to peripheral blood and bone marrow transplant, especially in patients who had bacterial sepsis or invasive fungal infection.”
The rate of bacterial sepsis was 34.87% in CB recipients and 20.20% in PB/BM recipients (P<0.0001). Rates of viral infection were 20.05% and 8.19%, respectively (P<0.0001). And rates of invasive fungal infection were 12.87% and 7.89% (P<0.0001).
There was a similar distribution of bacterial infections in CB and PB/BM recipients. The most common was pneumonia (47% and 41%, respectively), followed by abdominal infections (29% and 31%, respectively), urinary tract infections (17% and 21%, respectively), central line-associated bloodstream infections (4% and 6%, respectively), and acute sinusitis (3% and 1%, respectively).
The rate of Clostridium difficile infection was significantly higher in CB recipients than PB/BM recipients—11.75% and 8.90%, respectively (P=0.02). However, there was no significant difference in mortality related to C. difficile—14% and 10%, respectively (P=0.3).
On the other hand, all-cause inpatient mortality was significantly higher in CB recipients than PB/BM recipients—16% and 7%, respectively (P<0.0001).
Inpatient mortality rates were significantly higher for CB recipients with bacterial sepsis (33% vs 23%, P=0.001) and invasive fungal infections (29% vs 16%, P=0.0045) but not viral infections (19% vs 17%, P=0.5).
The median length of hospital stay was 36 days for CB recipients and 25 days for PB/BM recipients. The mean inpatient charges were $448,892 and $250,437 respectively.
*Data in the abstract differ from the presentation.
SALT LAKE CITY—Results of a large, retrospective analysis support the notion that patients who receive cord blood (CB) transplants have a higher risk of infection than other hematopoietic stem cell transplant (HSCT) recipients.
Investigators found that CB recipients had a significantly higher risk of bacterial, viral, and fungal infections in the early post-transplant period than patients who received peripheral blood (PB) or bone marrow (BM) transplants.
In addition, CB recipients had longer hospital stays, higher inpatient costs, and greater inpatient mortality than PB and BM recipients.
Amandeep Godara, MD, of Tufts Medical Center in Boston, Massachusetts, presented these results at the 2018 BMT Tandem Meetings (abstract 30*).
“Infections are considered more common in cord blood transplant recipients based on some prior retrospective analyses,” Dr Godara noted. “But there is limited data comparing these infectious complications between cord blood transplant and peripheral blood/bone marrow stem cell transplants during the inpatient stay for the stem cell transplant.”
With this in mind, Dr Godara and his colleagues analyzed data from the Healthcare Cost and Utilization Project National Inpatient Sample. This database covers 46 US states and contains data from more than 7 million hospital stays each year.
The investigators searched the database for hospital admissions for HSCT from 2002 to 2014. They identified 2979 CB transplants and 56,845 PB or BM transplants.
The CB recipients had a median age of 48, and 55% were male. Fifty-nine percent were white, 18% Hispanic, 13% black, 5% Asian, and 5% “other.” Sixty-six percent of patients had acute leukemia, 18% non-Hodgkin lymphoma, 5% Hodgkin lymphoma, and 11% “other” diseases.
The PB/BM recipients had a median age of 45, and 58% were male. Seventy-nine percent were white, 8% Hispanic, 6% black, 3% Asian, and 4% “other.” Sixty-one percent of patients had acute leukemia, 16% non-Hodgkin lymphoma, 4% Hodgkin lymphoma, and 19% “other” diseases.
Results
Dr Godara and his colleagues compared the rates and types of infection from the time of HSCT to hospital discharge in CB and PB/BM recipients. The team also compared early inpatient mortality, the cost of hospitalization, and the length of hospital stay.
“[W]e observed a higher risk for infections in cord blood transplant patients compared to peripheral blood and bone marrow stem cell transplant patients, and this risk for infection extended through a wide spectrum of pathogens,” Dr Godara said.
“We also observed a higher all-cause inpatient mortality in cord blood transplant compared to peripheral blood and bone marrow transplant, especially in patients who had bacterial sepsis or invasive fungal infection.”
The rate of bacterial sepsis was 34.87% in CB recipients and 20.20% in PB/BM recipients (P<0.0001). Rates of viral infection were 20.05% and 8.19%, respectively (P<0.0001). And rates of invasive fungal infection were 12.87% and 7.89% (P<0.0001).
There was a similar distribution of bacterial infections in CB and PB/BM recipients. The most common was pneumonia (47% and 41%, respectively), followed by abdominal infections (29% and 31%, respectively), urinary tract infections (17% and 21%, respectively), central line-associated bloodstream infections (4% and 6%, respectively), and acute sinusitis (3% and 1%, respectively).
The rate of Clostridium difficile infection was significantly higher in CB recipients than PB/BM recipients—11.75% and 8.90%, respectively (P=0.02). However, there was no significant difference in mortality related to C. difficile—14% and 10%, respectively (P=0.3).
On the other hand, all-cause inpatient mortality was significantly higher in CB recipients than PB/BM recipients—16% and 7%, respectively (P<0.0001).
Inpatient mortality rates were significantly higher for CB recipients with bacterial sepsis (33% vs 23%, P=0.001) and invasive fungal infections (29% vs 16%, P=0.0045) but not viral infections (19% vs 17%, P=0.5).
The median length of hospital stay was 36 days for CB recipients and 25 days for PB/BM recipients. The mean inpatient charges were $448,892 and $250,437 respectively.
*Data in the abstract differ from the presentation.
CHMP recommends approval for GO in AML
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for gemtuzumab ozogamicin (GO, Mylotarg™).
The recommendation is for GO to be used in combination with daunorubicin and cytarabine to treat patients age 15 years and older with previously untreated, de novo, CD33-positive acute myeloid leukemia (AML) but not acute promyelocytic leukemia.
The CHMP’s opinion on GO will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
Previous rejection
The CHMP previously issued a negative opinion of GO (first in 2007, confirmed in 2008), saying the drug should not receive marketing authorization.
The proposed indication for GO at that time was as re-induction treatment in adults with CD33-positive AML in first relapse who were not candidates for other intensive re-induction chemotherapy regimens and were either older than 60 or had a duration of first remission lasting less than 12 months.
The CHMP said there was insufficient evidence to establish the effectiveness of GO in AML, and the drug’s benefits did not outweigh its risks.
Phase 3 trial
The current marketing authorization application for GO is supported by data from an investigator-led, phase 3, randomized trial known as ALFA-0701. Updated results from this trial are available in the US prescribing information for GO.
Patients and treatment
ALFA-0701 included 271 patients with newly diagnosed, de novo AML who were 50 to 70 years of age.
Patients were randomized (1:1) to receive induction consisting of daunorubicin (60 mg/m2 on days 1 to 3) and cytarabine (200 mg/m2 on days 1 to 7) with (n=135) or without (n=136) GO at 3 mg/m2 (up to maximum of 1 vial) on days 1, 4, and 7. Patients who did not achieve a response after first induction could receive a second induction with daunorubicin and cytarabine alone.
Patients with a response received consolidation therapy with 2 courses of treatment including daunorubicin (60 mg/m2 on day 1 of first consolidation course; 60 mg/m2 on days 1 and 2 of second consolidation course) and cytarabine (1 g/m2 every 12 hours on days 1 to 4) with or without GO at 3 mg/m2 (up to a maximum of 1 vial) on day 1 according to their initial randomization.
Patients who achieved remission were also eligible for allogeneic transplant. An interval of at least 2 months between the last dose of GO and transplant was recommended.
Baseline characteristics were largely well balanced between the treatment arms, but there was a higher percentage of males in the GO arm than the control arm—55% and 44%, respectively.
Results
The study’s primary endpoint was event-free survival. The median event-free survival was 17.3 months in the GO arm and 9.5 months in the control arm (hazard ratio=0.56; 95% CI: 0.42-0.76; P<0.001).
There was no significant difference in overall survival between the treatment arms. (Updated overall survival data have not been provided).
All patients in this trial developed severe neutropenia, thrombocytopenia, and anemia. However, the incidence of prolonged, grade 3–4 thrombocytopenia in the absence of active leukemia was higher in the GO arm.
Treatment-emergent adverse events (AEs) considered most important for understanding the safety profile of GO were hemorrhage, veno-occlusive liver disease (VOD), and severe infections.
Treatment discontinuation due to any AE occurred in 31% of patients in the GO arm and 7% of those in the control arm. The most frequent AEs leading to discontinuation for patients on GO were thrombocytopenia (15%), VOD (3%), and septic shock (2%).
Fatal AEs occurred in 8 patients (6%) in the GO arm and 3 (2%) in the control arm. In the GO arm, 3 patients died of VOD, 4 died of hemorrhage-related events, and 1 died of a suspected cardiac cause. All 3 fatal AEs in the control arm were sepsis.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for gemtuzumab ozogamicin (GO, Mylotarg™).
The recommendation is for GO to be used in combination with daunorubicin and cytarabine to treat patients age 15 years and older with previously untreated, de novo, CD33-positive acute myeloid leukemia (AML) but not acute promyelocytic leukemia.
The CHMP’s opinion on GO will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
Previous rejection
The CHMP previously issued a negative opinion of GO (first in 2007, confirmed in 2008), saying the drug should not receive marketing authorization.
The proposed indication for GO at that time was as re-induction treatment in adults with CD33-positive AML in first relapse who were not candidates for other intensive re-induction chemotherapy regimens and were either older than 60 or had a duration of first remission lasting less than 12 months.
The CHMP said there was insufficient evidence to establish the effectiveness of GO in AML, and the drug’s benefits did not outweigh its risks.
Phase 3 trial
The current marketing authorization application for GO is supported by data from an investigator-led, phase 3, randomized trial known as ALFA-0701. Updated results from this trial are available in the US prescribing information for GO.
Patients and treatment
ALFA-0701 included 271 patients with newly diagnosed, de novo AML who were 50 to 70 years of age.
Patients were randomized (1:1) to receive induction consisting of daunorubicin (60 mg/m2 on days 1 to 3) and cytarabine (200 mg/m2 on days 1 to 7) with (n=135) or without (n=136) GO at 3 mg/m2 (up to maximum of 1 vial) on days 1, 4, and 7. Patients who did not achieve a response after first induction could receive a second induction with daunorubicin and cytarabine alone.
Patients with a response received consolidation therapy with 2 courses of treatment including daunorubicin (60 mg/m2 on day 1 of first consolidation course; 60 mg/m2 on days 1 and 2 of second consolidation course) and cytarabine (1 g/m2 every 12 hours on days 1 to 4) with or without GO at 3 mg/m2 (up to a maximum of 1 vial) on day 1 according to their initial randomization.
Patients who achieved remission were also eligible for allogeneic transplant. An interval of at least 2 months between the last dose of GO and transplant was recommended.
Baseline characteristics were largely well balanced between the treatment arms, but there was a higher percentage of males in the GO arm than the control arm—55% and 44%, respectively.
Results
The study’s primary endpoint was event-free survival. The median event-free survival was 17.3 months in the GO arm and 9.5 months in the control arm (hazard ratio=0.56; 95% CI: 0.42-0.76; P<0.001).
There was no significant difference in overall survival between the treatment arms. (Updated overall survival data have not been provided).
All patients in this trial developed severe neutropenia, thrombocytopenia, and anemia. However, the incidence of prolonged, grade 3–4 thrombocytopenia in the absence of active leukemia was higher in the GO arm.
Treatment-emergent adverse events (AEs) considered most important for understanding the safety profile of GO were hemorrhage, veno-occlusive liver disease (VOD), and severe infections.
Treatment discontinuation due to any AE occurred in 31% of patients in the GO arm and 7% of those in the control arm. The most frequent AEs leading to discontinuation for patients on GO were thrombocytopenia (15%), VOD (3%), and septic shock (2%).
Fatal AEs occurred in 8 patients (6%) in the GO arm and 3 (2%) in the control arm. In the GO arm, 3 patients died of VOD, 4 died of hemorrhage-related events, and 1 died of a suspected cardiac cause. All 3 fatal AEs in the control arm were sepsis.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for gemtuzumab ozogamicin (GO, Mylotarg™).
The recommendation is for GO to be used in combination with daunorubicin and cytarabine to treat patients age 15 years and older with previously untreated, de novo, CD33-positive acute myeloid leukemia (AML) but not acute promyelocytic leukemia.
The CHMP’s opinion on GO will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
Previous rejection
The CHMP previously issued a negative opinion of GO (first in 2007, confirmed in 2008), saying the drug should not receive marketing authorization.
The proposed indication for GO at that time was as re-induction treatment in adults with CD33-positive AML in first relapse who were not candidates for other intensive re-induction chemotherapy regimens and were either older than 60 or had a duration of first remission lasting less than 12 months.
The CHMP said there was insufficient evidence to establish the effectiveness of GO in AML, and the drug’s benefits did not outweigh its risks.
Phase 3 trial
The current marketing authorization application for GO is supported by data from an investigator-led, phase 3, randomized trial known as ALFA-0701. Updated results from this trial are available in the US prescribing information for GO.
Patients and treatment
ALFA-0701 included 271 patients with newly diagnosed, de novo AML who were 50 to 70 years of age.
Patients were randomized (1:1) to receive induction consisting of daunorubicin (60 mg/m2 on days 1 to 3) and cytarabine (200 mg/m2 on days 1 to 7) with (n=135) or without (n=136) GO at 3 mg/m2 (up to maximum of 1 vial) on days 1, 4, and 7. Patients who did not achieve a response after first induction could receive a second induction with daunorubicin and cytarabine alone.
Patients with a response received consolidation therapy with 2 courses of treatment including daunorubicin (60 mg/m2 on day 1 of first consolidation course; 60 mg/m2 on days 1 and 2 of second consolidation course) and cytarabine (1 g/m2 every 12 hours on days 1 to 4) with or without GO at 3 mg/m2 (up to a maximum of 1 vial) on day 1 according to their initial randomization.
Patients who achieved remission were also eligible for allogeneic transplant. An interval of at least 2 months between the last dose of GO and transplant was recommended.
Baseline characteristics were largely well balanced between the treatment arms, but there was a higher percentage of males in the GO arm than the control arm—55% and 44%, respectively.
Results
The study’s primary endpoint was event-free survival. The median event-free survival was 17.3 months in the GO arm and 9.5 months in the control arm (hazard ratio=0.56; 95% CI: 0.42-0.76; P<0.001).
There was no significant difference in overall survival between the treatment arms. (Updated overall survival data have not been provided).
All patients in this trial developed severe neutropenia, thrombocytopenia, and anemia. However, the incidence of prolonged, grade 3–4 thrombocytopenia in the absence of active leukemia was higher in the GO arm.
Treatment-emergent adverse events (AEs) considered most important for understanding the safety profile of GO were hemorrhage, veno-occlusive liver disease (VOD), and severe infections.
Treatment discontinuation due to any AE occurred in 31% of patients in the GO arm and 7% of those in the control arm. The most frequent AEs leading to discontinuation for patients on GO were thrombocytopenia (15%), VOD (3%), and septic shock (2%).
Fatal AEs occurred in 8 patients (6%) in the GO arm and 3 (2%) in the control arm. In the GO arm, 3 patients died of VOD, 4 died of hemorrhage-related events, and 1 died of a suspected cardiac cause. All 3 fatal AEs in the control arm were sepsis.
Company withdraws application for idelalisib
Gilead Sciences International Ltd. recently withdrew its application for European approval of idelalisib (Zydelig) in combination with rituximab and bendamustine for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL).
Idelalisib is currently approved in the European Union for use in combination with an anti-CD20 monoclonal antibody (rituximab or ofatumumab) to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment of CLL in the presence of 17p deletion or TP53 mutation in patients who are not eligible for any other therapies.
Idelalisib is also approved as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment.
In seeking expanded approval for idelalisib, Gilead submitted data from a study (NCT01569295) comparing idelalisib plus bendamustine and rituximab to placebo plus bendamustine and rituximab.
Interim results from this study were published in The Lancet Oncology in March 2017.
Gilead withdrew the application for idelalisib after the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) had evaluated documentation provided by the company and formulated lists of questions.
Gilead had not responded to the last round of questions at the time of the withdrawal.
At that point, the CHMP was of the provisional opinion that idelalisib should not be approved for use in combination with rituximab and bendamustine in patients with relapsed/refractory CLL.
The CHMP said additional, longer-term data are needed to show the benefits of idelalisib plus rituximab and bendamustine outweigh the risks.
Gilead said its withdrawal of the application was based on the CHMP’s opinion that there was insufficient evidence of a favorable benefit-risk profile.
The company also said the withdrawal does not impact ongoing trials of idelalisib.
Gilead Sciences International Ltd. recently withdrew its application for European approval of idelalisib (Zydelig) in combination with rituximab and bendamustine for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL).
Idelalisib is currently approved in the European Union for use in combination with an anti-CD20 monoclonal antibody (rituximab or ofatumumab) to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment of CLL in the presence of 17p deletion or TP53 mutation in patients who are not eligible for any other therapies.
Idelalisib is also approved as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment.
In seeking expanded approval for idelalisib, Gilead submitted data from a study (NCT01569295) comparing idelalisib plus bendamustine and rituximab to placebo plus bendamustine and rituximab.
Interim results from this study were published in The Lancet Oncology in March 2017.
Gilead withdrew the application for idelalisib after the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) had evaluated documentation provided by the company and formulated lists of questions.
Gilead had not responded to the last round of questions at the time of the withdrawal.
At that point, the CHMP was of the provisional opinion that idelalisib should not be approved for use in combination with rituximab and bendamustine in patients with relapsed/refractory CLL.
The CHMP said additional, longer-term data are needed to show the benefits of idelalisib plus rituximab and bendamustine outweigh the risks.
Gilead said its withdrawal of the application was based on the CHMP’s opinion that there was insufficient evidence of a favorable benefit-risk profile.
The company also said the withdrawal does not impact ongoing trials of idelalisib.
Gilead Sciences International Ltd. recently withdrew its application for European approval of idelalisib (Zydelig) in combination with rituximab and bendamustine for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL).
Idelalisib is currently approved in the European Union for use in combination with an anti-CD20 monoclonal antibody (rituximab or ofatumumab) to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment of CLL in the presence of 17p deletion or TP53 mutation in patients who are not eligible for any other therapies.
Idelalisib is also approved as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment.
In seeking expanded approval for idelalisib, Gilead submitted data from a study (NCT01569295) comparing idelalisib plus bendamustine and rituximab to placebo plus bendamustine and rituximab.
Interim results from this study were published in The Lancet Oncology in March 2017.
Gilead withdrew the application for idelalisib after the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) had evaluated documentation provided by the company and formulated lists of questions.
Gilead had not responded to the last round of questions at the time of the withdrawal.
At that point, the CHMP was of the provisional opinion that idelalisib should not be approved for use in combination with rituximab and bendamustine in patients with relapsed/refractory CLL.
The CHMP said additional, longer-term data are needed to show the benefits of idelalisib plus rituximab and bendamustine outweigh the risks.
Gilead said its withdrawal of the application was based on the CHMP’s opinion that there was insufficient evidence of a favorable benefit-risk profile.
The company also said the withdrawal does not impact ongoing trials of idelalisib.
The agitated patient: Steps to take, how to stay safe
CASE A 40-year-old man came to our office slightly agitated. He had an acute illness that was minor in nature. However, he was not interested in answering my questions or undergoing a physical exam. The more I tried to proceed with the visit, the more agitated he became—pacing the room, muttering, avoiding eye contact. I was uncomfortable and knew that the situation could quickly escalate if it was not brought under control.
What steps would you take if this were your patient?
The scene described above occurred several years ago, but more recently, one of the institutions in my (TIM) area was affected by a shooter in the workplace. The apprehension felt by all of us who were on the periphery paled in comparison to what was experienced by those at the scene. The outcome was horrific. Communicating with those directly involved during, and immediately after, the event was heart-wrenching. The trauma that they continue to relive is unimaginable, and some are not yet able to return to work.
Situations involving agitated patients are not uncommon in health care settings, although ones that escalate to the level of a shooting are. And no matter where on the spectrum an incident involving an agitated patient falls, it can leave those involved with various levels of physical, emotional, and psychological harm. It can also leave everyone asking themselves: “How can I better prepare for such occurrences?”
This article offers some answers by providing tips and guidelines for handling agitated and/or violent patients in various settings.
[polldaddy:9948472]
Defining the problem, assessing its severity
Between 2011 and 2013, workplace assaults ranged from 23,540 and 25,630 annually, with 70% to 74% occurring in health care and social service settings.1,2
Agitation is defined as a state that may include inattention, disinhibition, emotional lability, impulsivity, motor restlessness, and aggression.3,4 Violence in a clinical setting may be seen as an extreme expression of agitation sufficient enough to cause harm to an individual or damage to an object.5,6
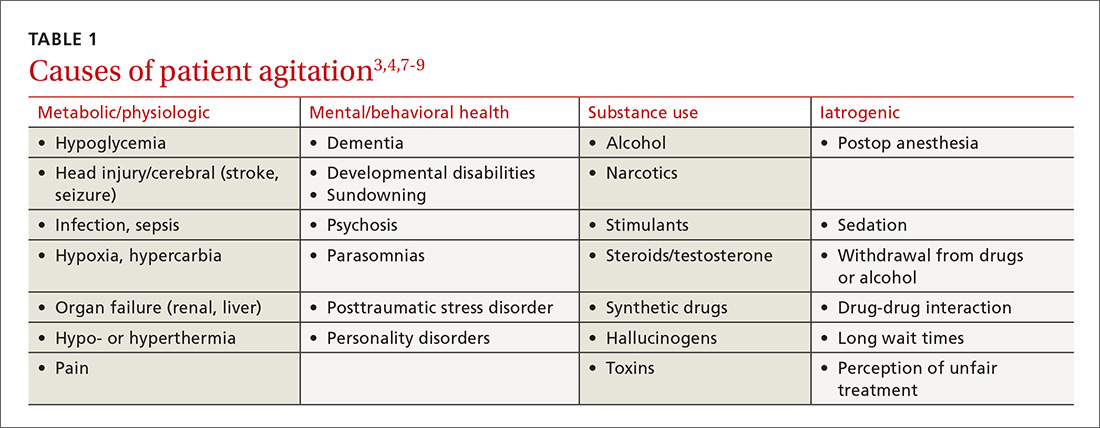
The causes of agitation can be grouped into categories: those due to a general medical condition, those due to a psychiatric condition, and those due to drug intoxication and/or withdrawal.7 We have chosen to add a fourth category—iatrogenic (see TABLE 13,4,7-9). They are not distinct categories, as there is sometimes overlap among areas.
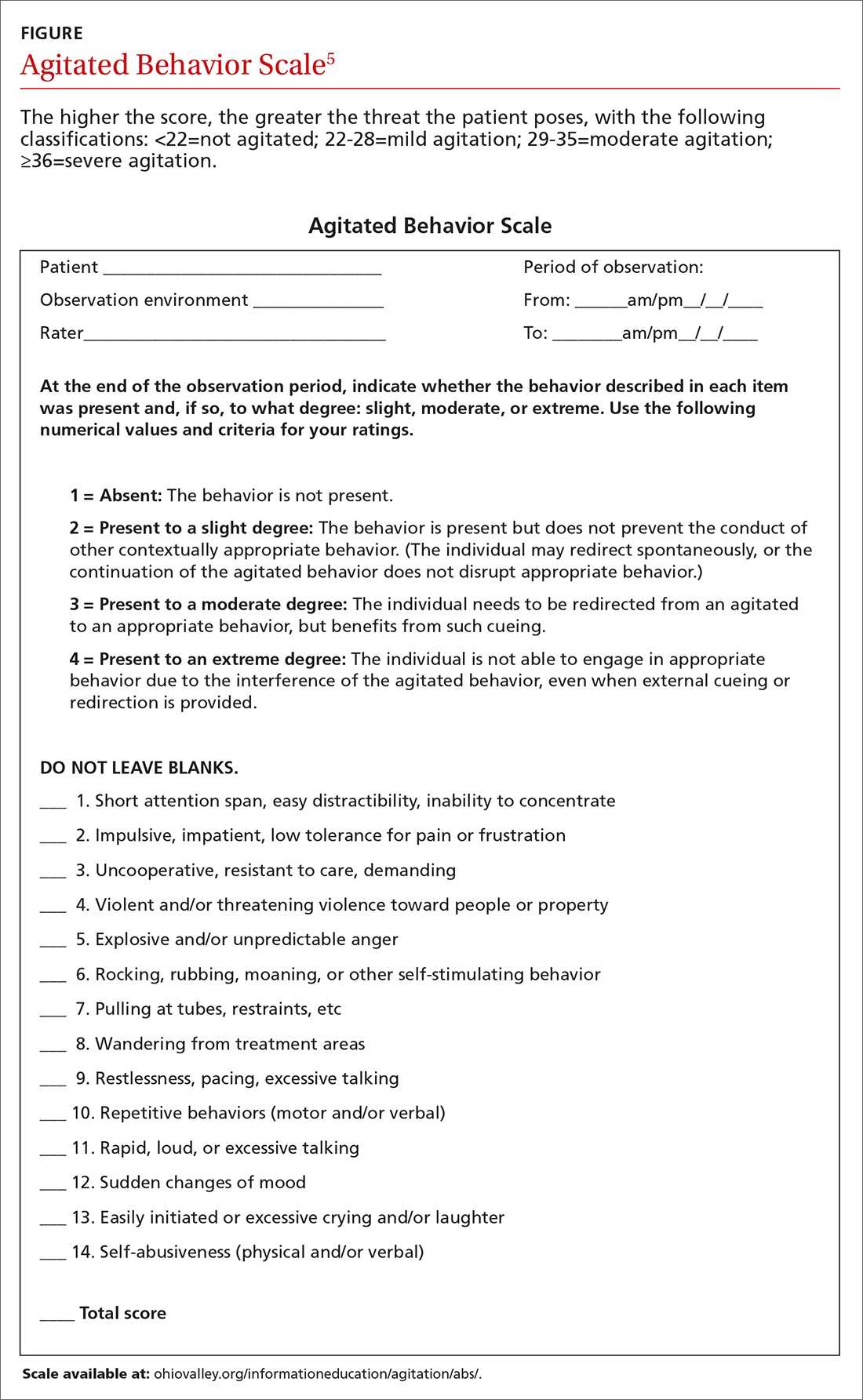
Determining the level of agitation. Various scales and approaches can help determine the level of agitation in a patient (eg, the Agitated Behavior Scale [ABS; FIGURE];5 the Behavioral Activity Rating Scale [BARS]10) and the risk for violence (eg, the ABC violence risk assessment,
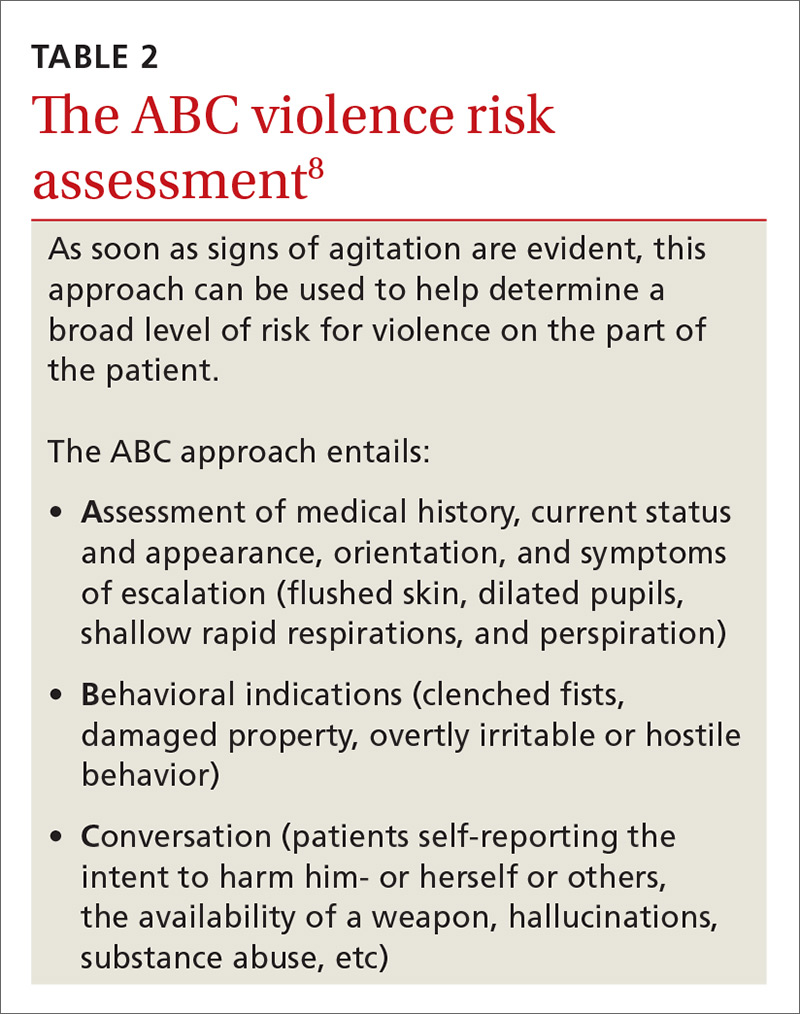
Scales like the ABS should be employed as soon as a patient shows signs of agitation sufficient to warrant intervention. The idea is for the family physician (FP) to be familiar enough with the tool to be able to mentally check it off, fill it out when time permits, and keep it in the patient’s chart. The first version of the form serves as a baseline so that if care is handed off to another provider, that provider can monitor whether signs and symptoms are improving or worsening.
Setting often drives the solution
Much of the evidence-based research on managing patient agitation and violence stems from inpatient psychiatric and emergency department (ED) settings. To make other health care providers aware of the experience gained in those settings, the American Association for Emergency Psychiatry created Project BETA (Best Practices in Evaluation and Treatment of Agitation). This project is designed to help promote consistency across health care settings and specialties in the way clinicians respond to agitated patients and to emphasize for all health care providers the availability of more than just pharmacologic approaches.7
De-escalating the situation. General tenets of de-escalation apply across practice settings. Among them:
- Stay calm. Avoid aggressive postures and prolonged eye contact.
- Be nonconfrontational. Acknowledge the patient’s frustration/perceptions and ask open-ended questions.
- Assess available resources such as clinical team members, family members, and silent alarms.
- Manage the situation and the patient’s underlying issues/diagnoses. This includes mobilizing other patients to avoid collateral damage and exploring solutions with the patient.
For more on de-escalation tools, see (TABLE 34,6,9,11).

Your setting matters. It’s worth noting that the settings in which clinicians practice greatly influence the resources available to de-escalate a situation and ensure the safety of the patient and others.7 The review that follows provides some issues—and tips—that are unique to different practice settings.
Ambulatory settings
Sim and colleagues9 noted that aggressive behavior in the general practice setting may stem not only from factors related to the patient’s own physical or psychological discomfort, but from patients feeling that they are being treated unfairly, whether it be because of wait times, uncomfortable waiting conditions, or something else. A number of international studies have shown high rates of abuse toward FPs.9,12 Of 831 primary care physicians surveyed in a German study, close to three-quarters indicated that within the last year, they had experienced aggression (ranging from verbal abuse and threats to physical violence and property damage) from a patient.12 This statistic increased to 91% when it included the length of their career.
Bell13 suggests that physicians be aware that transference and countertransference issues are often at play when dealing with hostile or potentially violent patients. Suggestions to prevent aggression include some practice-level approaches (eg, providing waiting room distractions, making patients aware of potential delays), as well as being aware of nonverbal cues suggesting increased agitation (eg, clenched fists, crossed arms, chin thrusts, finger pointing).9
Group practice
An FP who practices with other health care providers and clinical staff has a built-in team that can assist with de-escalation. When meeting with a patient who has a history of violence or agitation in an exam room or office, try to ensure that you can get to an exit quickly if necessary. Also, alert staff to any concerns, and have a system for at least one staff member to check in periodically during the visit.
It is also helpful to develop an evacuation plan and create a “panic room” or “safe zone” for emergencies.14,15 Such a space may be nothing more than an area or room for staff to gather. It should have access to the police or other emergency services via a land and/or cell phone line.
Solo practice
If you practice alone, institute safeguards whereby a colleague (at a different practice, building, or location) can be alerted if concerns arise. In addition, consider the following precautions: locking the door when alone after hours, screening potential patients, having a way to call for help (keep the number for the local police station and ED readily available), prohibiting potential weapons (as some states allow them to be carried), and learning some form of self-defense.15
Resources exist that offer guidelines for developing policies and procedures, checklists, and sample incident forms (eg, the International Association for Healthcare Security and Safety; iahss.org). Other organizations that can help with the development of a preparedness plan include the Occupational Safety and Health Administration (https://www.osha.gov/SLTC/workplaceviolence/evaluation.html), the Department of Homeland Security (https://www.dhs.gov/sites/default/files/publications/ISC%20Violence%20in%20%20the%20Federal%20Workplace%20Guide%20April%202013.pdf), and The Joint Commission (https://www.jointcommission.org/workplace_violence.aspx).
Long- and short-term care facilities
In long-term care settings, such as nursing homes, and shorter-term care settings, such as rehabilitation facilities, agitation may stem from causes related to a head injury or dementia or from living in an unfamiliar environment. Assessment can be accomplished using a formal scale (eg, the ABS), as well as by identifying potential underlying health-related factors that can lead to agitation, such as pain, an infection, bowel and bladder issues, seizures, wounds, endocrine anomalies, cardiac or pulmonary problems, gastrointestinal dysfunction, and metabolic abnormalities.3
Modify the environment. For this population, a primary approach involves modifying the environment to decrease the likelihood of agitation. This may involve decreasing noise or light or ensuring adequate levels of stimulation. Preventing disorientation can be addressed through verbal and visual reminders of the date, schedule, etc. If a particular situation or activity is identified as a source of agitation, attempts at modifications are called for.3
For patients with dementia, the American Psychiatric Association recommends using the lowest effective dose of an antipsychotic in conjunction with environmental and behavioral measures.16 A benzodiazepine (lorazepam, oxazepam) may be used for infrequent agitation. Trazodone or a selective serotonin reuptake inhibitor are alternatives for those without psychosis or who are intolerant to antipsychotics.16
For individuals in a rehabilitation setting, agitation can impede participation in therapy and has been associated with poorer functioning at the time of discharge.3 Agitation can also be disruptive and lead to distress for family members and caregivers, as well as for fellow patients. And because this environment has a greater likelihood of visitors unrelated to the patient being exposed to the aberrant behavior, it is especially important to have established policies and procedures for de-escalation in place.
Home care
More and more FPs and residents are conducting home visits. That’s because the Accreditation Council for Graduate Medical Education Program Requirements for Graduate Medical Education in Family Medicine now include integrating a patient’s care across settings—including the home.17 Those who do provide home care may find themselves in circumstances similar to those of domestic disputes.
The German study mentioned earlier of more than 800 primary care physicians found that while the vast majority of physicians felt safe in their offices, 66% of female doctors and 34% of male doctors did not feel safe making home visits.12
Know the neighborhood. There’s no doubt that working in the home health sector makes one vulnerable. More than 61% of home care workers report workplace violence annually.18,19 An action plan, as well as established policies and procedures, are essential when making home visits. Prior to the visit, be aware of the community and the location of the nearest police department and hospital.
Unwin and Tatum20 suggest not wearing a white coat or carrying a doctor’s bag so as not to stand out as a physician in neighborhoods where personal safety is an issue. Make sure that your cell phone is fully charged and that there is a GPS mechanism activated that allows others to locate you.21 Note the available exits in a patient’s home, and position yourself near them, if possible. Have someone call or text you at predetermined times so that the absence of a response from you will alert someone to send help.
In such situations, it is imperative to remain calm and to use the same verbal de-escalation techniques (TABLE 34,6,9,11) that would be used in any other health care setting. It is prudent to set expectations for the patient and family members prior to the home visit regarding the tools and services that will be provided in the home setting and the limitations in terms of scope of practice.
Emergency department
The ED is one of the most common settings for patient agitation and violence within the health care continuum.22 Providers must quickly determine the cause of the agitation while de-escalating the situation and ensuring that they do not miss a pertinent medical finding related to a time-sensitive issue, such as an intracerebral bleed or poisoning.7 In addition, the ED is usually heavily populated, providing an opportunity for tremendous collateral human damage should the violence escalate or weapons be deployed. The upside is that many EDs are now staffed with security personnel and, depending on the community, police officers may be on the premises or in the vicinity.22
Etiologies for agitation in the ED can range from ingestion of unknown or unidentified substances to psychiatric or medical conditions. Knowledge of etiology is necessary prior to initiation of treatment.4
As in other settings, the safety of the patient and others present is of utmost importance. Key recommendations for managing agitated patients in the ED include: 4
- Have an established plan for the management of agitated patients.
- Identify signs of agitation early, and complete an agitation rating scale.
- Attempt verbal de-escalation before using medication whenever possible.
- Employ a “show of concern” rather than “a show of force” in response to escalating agitation/violence. Doing so can strengthen the perception that interventions are coming from a place of caring.
- Use physical restraint as a last resort. When used, it should be with the intention of protecting the patient and those present, rather than as punishment.
Inpatient units
Unlike the ED, patients on units generally have a working diagnosis, and the provider has some background information with which to work, such as laboratory test results and radiology reports, facilitating more expedient and accurate situational assessment. However, the recommendations for assessment and early identification, as described for the ED, still apply.
If a provider finds him- or herself in an escalating situation, the call bells located in the rooms are of use. An alternative is to call out for help from someone in the hallway. One needs to be aware of the current policies and procedures for de-escalation, as some facilities have a specific “code” that is called for such occasions.19
Postop delirium is a common cause of agitation in the inpatient setting. Ng and colleagues11 recommend a cognitive assessment before surgery to establish a baseline in order to determine the risk for delirium after surgery. Additionally, the FP must remain aware of preexisting conditions that may surface during a hospital stay, such as dehydration or unrecognized alcohol or medication withdrawal.
Medication choice should be based on the type of delirium. Hyperactive delirium (restlessness, emotional lability, hallucinations) and mixed delirium (a combination of signs of hyperactive and hypoactive dementia) both hold the potential for agitation and even violence. The approach to hyperactive delirium includes consideration of an antipsychotic medication, although the efficacy of antipsychotics is considered controversial. In the case of mixed delirium, behavioral and environmental modifications are useful (eg, reducing noise and early ambulation).11
No medications are registered with the US Food and Drug Administration for the management of delirium, and it is suggested that antipsychotics be considered only when other, less invasive, strategies have been attempted.23
Addressing caregiver stress, anxiety disorders afterward
Regardless of the setting in which FPs work, witnessing or being directly involved in a traumatic event puts one at risk for symptoms—or a full diagnosis—of posttraumatic stress disorder (PTSD), acute stress disorder, or anxiety or mood disorders.24,25 Although findings vary, studies have found that as many as 12% of ED personnel meet the criteria for PTSD26,27 and 12% to 15% report having been threatened physically.28,29 More than half of physicians in another study had witnessed a physical attack.30
Physicians and other health care personnel who have experienced a traumatic incident, or offered help to another during an incident, may attempt to cope through avoidance, cutting down on work hours, leaving the work setting in which the event took place, or leaving the profession altogether.29,31,32
There is a paucity of methodologically sound research with regard to prevention and treatment of PTSD symptoms in this population.24 According to a 2002 Cochrane review, the effectiveness of individual, single-session debriefing does not have solid research support,33 and there are concerns about potential harms due to reliving the traumatic event when sessions are led by poorly trained debriefing staff.34-36
Critical incident stress debriefing (CISD), however, holds promise in terms of facilitating a return to pretrauma functioning based on studies of first responders.34,35 This may be because CISD follows a specific protocol and that group sessions may capitalize on the social support/camaraderie within a group that has undergone a traumatic event.34,35 It is important that those providing debriefing and support be well-trained.35
Debriefing, however, is not always sufficient, and those who appear to be affected on an ongoing basis may require individual treatment for PTSD symptoms. Evidence-based treatments for PTSD, such as trauma-focused psychotherapy and/or pharmacotherapy, may be considered37 (TABLE 424,34,38).
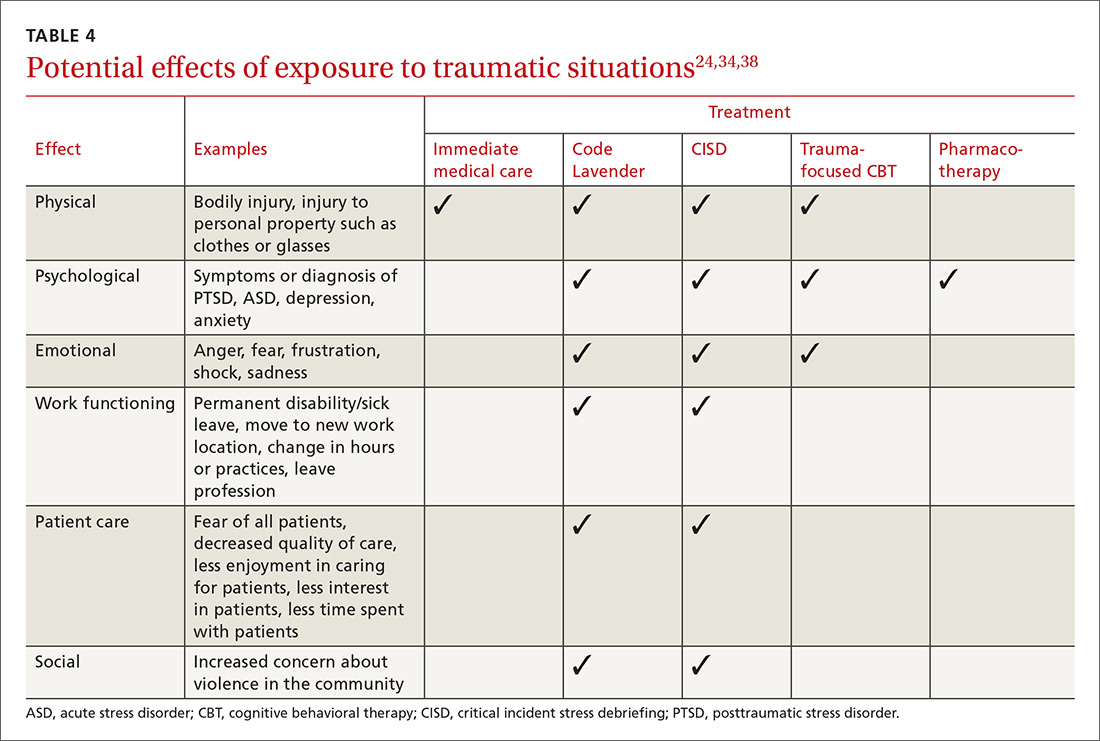
Ongoing support in the workplace. The Cleveland Clinic has developed a “Code Lavender” to combat stress in the workplace. Like a Code Blue for medical emergencies, a Code Lavender is called when a health care worker is in need of emotional or spiritual support.38 A provider who initiates the call is met by a team of holistic nurses within 30 minutes. The team provides Reiki and massage, healthy snacks and water, and lavender arm bands to remind the individual to relax for the rest of the day. Further opportunities for spiritual support, mindfulness training, counseling, and yoga may also be made available.
CASE Sensing that the situation with my patient might escalate, I lowered my voice, relaxed my shoulders, leaned casually against the desk, and asked him to tell me how I could best help him. As he spoke, I offered him a seat (by gesturing to the chair). I did this for 2 reasons: to move him away from blocking my exit from the room, and to put him at a lower level than me so that he was entirely in my view. I didn’t interrupt him as he spoke. I just nodded or tilted my head to show I was listening. In my mind, I played out the various scenarios that could ensue.
Fortunately, I was able to get him to relax enough for an assessment, which involved a more relevant history and the exam, which he agreed to once an aide had come into the room. He did not exhibit the concerning signs of flushed skin, dilated pupils, shallow rapid respirations, or perspiration. He did have a comorbid behavioral health issue, which we were able to address. His earlier behavioral indicators of agitation were controlled with verbal and physical cues on my part. Our conversation didn't reveal an intent to harm himself or others. In this case, physical restraints were not required. Throughout the encounter the door was left open, and the patient was reminded that we were there to help.
Once he left, I made the relevant notes in the chart regarding his agitated state at the start of the visit and his final state at the end of the visit so as to assist any other providers. We (TIM, MG) also held a quick debrief after the encounter with the office staff and decided that we needed to create a policy and protocol regarding how to handle such situations in the future.

CORRESPONDENCE
Tochi Iroku-Malize, MD, MPH, MBA, Family Medicine Department, Southside Hospital, 301 East Main Street, Bay Shore, NY 11706; [email protected].
1. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. December 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed February 8, 2018.
2. Occupational Safety and Health Administration. Guidelines for preventing workplace violence for healthcare and social service workers. 2016. Available at: https://www.osha.gov/Publications/osha3148.pdf. Accessed February 8, 2017.
3. Mortimer DS, Berg W. Agitation in patients recovering from traumatic brain injury: nursing management. J Neurosci Nurs. 2017;49:25-30.
4. Wilson MP, Nordstrom K, Vilke GM. The agitated patient in the emergency department. Curr Emerg Hosp Med Rep. 2015;3:188-194.
5. Bogner JA, Corrigan JD, Bode RK, et al. Rating scale analysis of the Agitated Behavior Scale. J Head Trauma Rehabil. 2000;15:656-669.
6. Gaynes BN, Brown CL, Lux LJ, et al. Preventing and de-escalating aggressive behavior among adult psychiatric patients: a systematic review of the evidence. Psychiatr Serv. 2017;68:819-831.
7. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project Beta Medical Evaluation Workgroup. West J Emerg Med. 2012;13:3-10.
8. Sands N. Mental health triage: towards a model for nursing practice. J Psychiatr Ment Health Nurs. 2007;14:243-249.
9. Sim MG, Wain T, Khong E. Aggressive behaviour - prevention and management in the general practice environment. Aust Fam Physician. 2011;40:866-872.
10. Swift RH, Harrigan EP, Cappelleri JC, et al. Validation of the behavioural activity rating scale (BARS): a novel measure of activity in agitated patients. J Psychiatr Res. 2002;36:87-95.
11. Ng J, Pan CX, Geube A, et al. Your postop patient is confused and agitated—next steps? J Fam Pract. 2015;64:361-366.
12. Vorderwülbecke F, Feistle M, Mehring M, et al. Aggression and violence against primary care physicians—a nationwide questionnaire survey. Dtsch Arztebl Int. 2015;112:159-165.
13. Bell HS. Curbside consultation—a potentially violent patient? Am Fam Physician. 2000;61:2237-2238.
14. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10:40-42.
15. Munsey C. How to stay safe in practice. APA Monitor. 2008;39:36.
16. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173:543-546.
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. Revised July 1, 2017. Available at: http://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_family_medicine_2017-07-01.pdf . Accessed October 30, 2017.
18. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374:1661-1669.
19. Hanson GC, Perrin NA, Moss H, et al. Workplace violence against homecare workers and its relationship with workers health outcomes: a cross-sectional study. BMC Public Health. 2015;15:11.
20. Unwin BK, Tatum PE 3rd. House calls. Am Fam Physician. 2011;83:925-938.
21. Victor P. Safety tips for home visits from a veteran NYC social worker. National Association of Social Workers, New York. Available at: http://www.naswnyc.org/?489. Accessed June 1, 2017.
22. Kansagra SM, Rao SR, Sullivan AF, et al. A survey of workplace violence across 65 U.S. emergency departments. Acad Emerg Med. 2008;15:1268-1274.
23. Meagher D, Agar MR, Teodorczuk A. Debate article: antipsychotic medications are clinically useful for the treatment of delirium. Int J Geriatr Psychiatry. 2017 Jul 30. doi: 10.1002/gps.4759. [Epub ahead of print].
24. Lanctot N, Guay S. The aftermath of workplace violence among healthcare workers: a systematic literature review of the consequences. Aggress Violent Behav. 2014;19:492-501.
25. Edward KL, Stephenson J, Ousey K, et al. A systematic review and meta-analysis of factors that relate to aggression perpetrated against nurses by patients/relatives or staff. J Clin Nurs. 2016;25:289-299.
26. Laposa JM, Alden LE. Posttraumatic stress disorder in the emergency room: exploration of a cognitive model. Behav Res Ther. 2003;41:49-65.
27. Mills LD, Mills TJ. Symptoms of post-traumatic stress disorder among emergency medicine residents. J Emerg Med. 2005;28:1-4.
28. Laposa JM, Alden LE, Fullerton LM. Work stress and posttraumatic stress disorder in ED nurses/personnel. J Emerg Nurs. 2003;29:23-28.
29. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed June 1, 2017.
30. Zafar W, Khan UR, Siddiqui SA, et al. Workplace violence and self-reported psychological health: coping with post-traumatic stress, mental distress, and burnout among physicians working in the emergency departments compared to other specialties in Pakistan. J Emerg Med. 2016;50:167-177.
31. de Boer J, Lok A, Van’t Verlaat E, et al. Work-related critical incidents in hospital-based health care providers and the risk of post-traumatic stress symptoms, anxiety, and depression: a meta-analysis. Soc Sci Med. 2011;73:316-326.
32. Shah L, Annamalai J, Aye SN, et al. Key components and strategies utilized by nurses for de-escalation of aggression in psychiatric in-patients: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2016;14:109-118.
33. Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002;(2):CD000560.
34. Tuckey MR, Scott JE. Group critical incident stress debriefing with emergency services personnel: a randomized controlled trial. Anxiety Stress Coping. 2014;27:38-54.
35. Pack MJ. Critical incident stress management: a review of the literature with implications for social work. Int Soc Work. 2012;56: 608-627.
36. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
37. Warner CH, Warner CM, Appenzeller GN, et al. Identifying and managing posttraumatic stress disorder. Am Fam Physician. 2013;88:827-834.
38. Johnson B. Code lavender: initiating holistic rapid response at the Cleveland Clinic. Beginnings. 2014;34:10-11.
CASE A 40-year-old man came to our office slightly agitated. He had an acute illness that was minor in nature. However, he was not interested in answering my questions or undergoing a physical exam. The more I tried to proceed with the visit, the more agitated he became—pacing the room, muttering, avoiding eye contact. I was uncomfortable and knew that the situation could quickly escalate if it was not brought under control.
What steps would you take if this were your patient?
The scene described above occurred several years ago, but more recently, one of the institutions in my (TIM) area was affected by a shooter in the workplace. The apprehension felt by all of us who were on the periphery paled in comparison to what was experienced by those at the scene. The outcome was horrific. Communicating with those directly involved during, and immediately after, the event was heart-wrenching. The trauma that they continue to relive is unimaginable, and some are not yet able to return to work.
Situations involving agitated patients are not uncommon in health care settings, although ones that escalate to the level of a shooting are. And no matter where on the spectrum an incident involving an agitated patient falls, it can leave those involved with various levels of physical, emotional, and psychological harm. It can also leave everyone asking themselves: “How can I better prepare for such occurrences?”
This article offers some answers by providing tips and guidelines for handling agitated and/or violent patients in various settings.
[polldaddy:9948472]
Defining the problem, assessing its severity
Between 2011 and 2013, workplace assaults ranged from 23,540 and 25,630 annually, with 70% to 74% occurring in health care and social service settings.1,2
Agitation is defined as a state that may include inattention, disinhibition, emotional lability, impulsivity, motor restlessness, and aggression.3,4 Violence in a clinical setting may be seen as an extreme expression of agitation sufficient enough to cause harm to an individual or damage to an object.5,6

The causes of agitation can be grouped into categories: those due to a general medical condition, those due to a psychiatric condition, and those due to drug intoxication and/or withdrawal.7 We have chosen to add a fourth category—iatrogenic (see TABLE 13,4,7-9). They are not distinct categories, as there is sometimes overlap among areas.

Determining the level of agitation. Various scales and approaches can help determine the level of agitation in a patient (eg, the Agitated Behavior Scale [ABS; FIGURE];5 the Behavioral Activity Rating Scale [BARS]10) and the risk for violence (eg, the ABC violence risk assessment,

Scales like the ABS should be employed as soon as a patient shows signs of agitation sufficient to warrant intervention. The idea is for the family physician (FP) to be familiar enough with the tool to be able to mentally check it off, fill it out when time permits, and keep it in the patient’s chart. The first version of the form serves as a baseline so that if care is handed off to another provider, that provider can monitor whether signs and symptoms are improving or worsening.
Setting often drives the solution
Much of the evidence-based research on managing patient agitation and violence stems from inpatient psychiatric and emergency department (ED) settings. To make other health care providers aware of the experience gained in those settings, the American Association for Emergency Psychiatry created Project BETA (Best Practices in Evaluation and Treatment of Agitation). This project is designed to help promote consistency across health care settings and specialties in the way clinicians respond to agitated patients and to emphasize for all health care providers the availability of more than just pharmacologic approaches.7
De-escalating the situation. General tenets of de-escalation apply across practice settings. Among them:
- Stay calm. Avoid aggressive postures and prolonged eye contact.
- Be nonconfrontational. Acknowledge the patient’s frustration/perceptions and ask open-ended questions.
- Assess available resources such as clinical team members, family members, and silent alarms.
- Manage the situation and the patient’s underlying issues/diagnoses. This includes mobilizing other patients to avoid collateral damage and exploring solutions with the patient.
For more on de-escalation tools, see (TABLE 34,6,9,11).

Your setting matters. It’s worth noting that the settings in which clinicians practice greatly influence the resources available to de-escalate a situation and ensure the safety of the patient and others.7 The review that follows provides some issues—and tips—that are unique to different practice settings.
Ambulatory settings
Sim and colleagues9 noted that aggressive behavior in the general practice setting may stem not only from factors related to the patient’s own physical or psychological discomfort, but from patients feeling that they are being treated unfairly, whether it be because of wait times, uncomfortable waiting conditions, or something else. A number of international studies have shown high rates of abuse toward FPs.9,12 Of 831 primary care physicians surveyed in a German study, close to three-quarters indicated that within the last year, they had experienced aggression (ranging from verbal abuse and threats to physical violence and property damage) from a patient.12 This statistic increased to 91% when it included the length of their career.
Bell13 suggests that physicians be aware that transference and countertransference issues are often at play when dealing with hostile or potentially violent patients. Suggestions to prevent aggression include some practice-level approaches (eg, providing waiting room distractions, making patients aware of potential delays), as well as being aware of nonverbal cues suggesting increased agitation (eg, clenched fists, crossed arms, chin thrusts, finger pointing).9
Group practice
An FP who practices with other health care providers and clinical staff has a built-in team that can assist with de-escalation. When meeting with a patient who has a history of violence or agitation in an exam room or office, try to ensure that you can get to an exit quickly if necessary. Also, alert staff to any concerns, and have a system for at least one staff member to check in periodically during the visit.
It is also helpful to develop an evacuation plan and create a “panic room” or “safe zone” for emergencies.14,15 Such a space may be nothing more than an area or room for staff to gather. It should have access to the police or other emergency services via a land and/or cell phone line.
Solo practice
If you practice alone, institute safeguards whereby a colleague (at a different practice, building, or location) can be alerted if concerns arise. In addition, consider the following precautions: locking the door when alone after hours, screening potential patients, having a way to call for help (keep the number for the local police station and ED readily available), prohibiting potential weapons (as some states allow them to be carried), and learning some form of self-defense.15
Resources exist that offer guidelines for developing policies and procedures, checklists, and sample incident forms (eg, the International Association for Healthcare Security and Safety; iahss.org). Other organizations that can help with the development of a preparedness plan include the Occupational Safety and Health Administration (https://www.osha.gov/SLTC/workplaceviolence/evaluation.html), the Department of Homeland Security (https://www.dhs.gov/sites/default/files/publications/ISC%20Violence%20in%20%20the%20Federal%20Workplace%20Guide%20April%202013.pdf), and The Joint Commission (https://www.jointcommission.org/workplace_violence.aspx).
Long- and short-term care facilities
In long-term care settings, such as nursing homes, and shorter-term care settings, such as rehabilitation facilities, agitation may stem from causes related to a head injury or dementia or from living in an unfamiliar environment. Assessment can be accomplished using a formal scale (eg, the ABS), as well as by identifying potential underlying health-related factors that can lead to agitation, such as pain, an infection, bowel and bladder issues, seizures, wounds, endocrine anomalies, cardiac or pulmonary problems, gastrointestinal dysfunction, and metabolic abnormalities.3
Modify the environment. For this population, a primary approach involves modifying the environment to decrease the likelihood of agitation. This may involve decreasing noise or light or ensuring adequate levels of stimulation. Preventing disorientation can be addressed through verbal and visual reminders of the date, schedule, etc. If a particular situation or activity is identified as a source of agitation, attempts at modifications are called for.3
For patients with dementia, the American Psychiatric Association recommends using the lowest effective dose of an antipsychotic in conjunction with environmental and behavioral measures.16 A benzodiazepine (lorazepam, oxazepam) may be used for infrequent agitation. Trazodone or a selective serotonin reuptake inhibitor are alternatives for those without psychosis or who are intolerant to antipsychotics.16
For individuals in a rehabilitation setting, agitation can impede participation in therapy and has been associated with poorer functioning at the time of discharge.3 Agitation can also be disruptive and lead to distress for family members and caregivers, as well as for fellow patients. And because this environment has a greater likelihood of visitors unrelated to the patient being exposed to the aberrant behavior, it is especially important to have established policies and procedures for de-escalation in place.
Home care
More and more FPs and residents are conducting home visits. That’s because the Accreditation Council for Graduate Medical Education Program Requirements for Graduate Medical Education in Family Medicine now include integrating a patient’s care across settings—including the home.17 Those who do provide home care may find themselves in circumstances similar to those of domestic disputes.
The German study mentioned earlier of more than 800 primary care physicians found that while the vast majority of physicians felt safe in their offices, 66% of female doctors and 34% of male doctors did not feel safe making home visits.12
Know the neighborhood. There’s no doubt that working in the home health sector makes one vulnerable. More than 61% of home care workers report workplace violence annually.18,19 An action plan, as well as established policies and procedures, are essential when making home visits. Prior to the visit, be aware of the community and the location of the nearest police department and hospital.
Unwin and Tatum20 suggest not wearing a white coat or carrying a doctor’s bag so as not to stand out as a physician in neighborhoods where personal safety is an issue. Make sure that your cell phone is fully charged and that there is a GPS mechanism activated that allows others to locate you.21 Note the available exits in a patient’s home, and position yourself near them, if possible. Have someone call or text you at predetermined times so that the absence of a response from you will alert someone to send help.
In such situations, it is imperative to remain calm and to use the same verbal de-escalation techniques (TABLE 34,6,9,11) that would be used in any other health care setting. It is prudent to set expectations for the patient and family members prior to the home visit regarding the tools and services that will be provided in the home setting and the limitations in terms of scope of practice.
Emergency department
The ED is one of the most common settings for patient agitation and violence within the health care continuum.22 Providers must quickly determine the cause of the agitation while de-escalating the situation and ensuring that they do not miss a pertinent medical finding related to a time-sensitive issue, such as an intracerebral bleed or poisoning.7 In addition, the ED is usually heavily populated, providing an opportunity for tremendous collateral human damage should the violence escalate or weapons be deployed. The upside is that many EDs are now staffed with security personnel and, depending on the community, police officers may be on the premises or in the vicinity.22
Etiologies for agitation in the ED can range from ingestion of unknown or unidentified substances to psychiatric or medical conditions. Knowledge of etiology is necessary prior to initiation of treatment.4
As in other settings, the safety of the patient and others present is of utmost importance. Key recommendations for managing agitated patients in the ED include: 4
- Have an established plan for the management of agitated patients.
- Identify signs of agitation early, and complete an agitation rating scale.
- Attempt verbal de-escalation before using medication whenever possible.
- Employ a “show of concern” rather than “a show of force” in response to escalating agitation/violence. Doing so can strengthen the perception that interventions are coming from a place of caring.
- Use physical restraint as a last resort. When used, it should be with the intention of protecting the patient and those present, rather than as punishment.
Inpatient units
Unlike the ED, patients on units generally have a working diagnosis, and the provider has some background information with which to work, such as laboratory test results and radiology reports, facilitating more expedient and accurate situational assessment. However, the recommendations for assessment and early identification, as described for the ED, still apply.
If a provider finds him- or herself in an escalating situation, the call bells located in the rooms are of use. An alternative is to call out for help from someone in the hallway. One needs to be aware of the current policies and procedures for de-escalation, as some facilities have a specific “code” that is called for such occasions.19
Postop delirium is a common cause of agitation in the inpatient setting. Ng and colleagues11 recommend a cognitive assessment before surgery to establish a baseline in order to determine the risk for delirium after surgery. Additionally, the FP must remain aware of preexisting conditions that may surface during a hospital stay, such as dehydration or unrecognized alcohol or medication withdrawal.
Medication choice should be based on the type of delirium. Hyperactive delirium (restlessness, emotional lability, hallucinations) and mixed delirium (a combination of signs of hyperactive and hypoactive dementia) both hold the potential for agitation and even violence. The approach to hyperactive delirium includes consideration of an antipsychotic medication, although the efficacy of antipsychotics is considered controversial. In the case of mixed delirium, behavioral and environmental modifications are useful (eg, reducing noise and early ambulation).11
No medications are registered with the US Food and Drug Administration for the management of delirium, and it is suggested that antipsychotics be considered only when other, less invasive, strategies have been attempted.23
Addressing caregiver stress, anxiety disorders afterward
Regardless of the setting in which FPs work, witnessing or being directly involved in a traumatic event puts one at risk for symptoms—or a full diagnosis—of posttraumatic stress disorder (PTSD), acute stress disorder, or anxiety or mood disorders.24,25 Although findings vary, studies have found that as many as 12% of ED personnel meet the criteria for PTSD26,27 and 12% to 15% report having been threatened physically.28,29 More than half of physicians in another study had witnessed a physical attack.30
Physicians and other health care personnel who have experienced a traumatic incident, or offered help to another during an incident, may attempt to cope through avoidance, cutting down on work hours, leaving the work setting in which the event took place, or leaving the profession altogether.29,31,32
There is a paucity of methodologically sound research with regard to prevention and treatment of PTSD symptoms in this population.24 According to a 2002 Cochrane review, the effectiveness of individual, single-session debriefing does not have solid research support,33 and there are concerns about potential harms due to reliving the traumatic event when sessions are led by poorly trained debriefing staff.34-36
Critical incident stress debriefing (CISD), however, holds promise in terms of facilitating a return to pretrauma functioning based on studies of first responders.34,35 This may be because CISD follows a specific protocol and that group sessions may capitalize on the social support/camaraderie within a group that has undergone a traumatic event.34,35 It is important that those providing debriefing and support be well-trained.35
Debriefing, however, is not always sufficient, and those who appear to be affected on an ongoing basis may require individual treatment for PTSD symptoms. Evidence-based treatments for PTSD, such as trauma-focused psychotherapy and/or pharmacotherapy, may be considered37 (TABLE 424,34,38).

Ongoing support in the workplace. The Cleveland Clinic has developed a “Code Lavender” to combat stress in the workplace. Like a Code Blue for medical emergencies, a Code Lavender is called when a health care worker is in need of emotional or spiritual support.38 A provider who initiates the call is met by a team of holistic nurses within 30 minutes. The team provides Reiki and massage, healthy snacks and water, and lavender arm bands to remind the individual to relax for the rest of the day. Further opportunities for spiritual support, mindfulness training, counseling, and yoga may also be made available.
CASE Sensing that the situation with my patient might escalate, I lowered my voice, relaxed my shoulders, leaned casually against the desk, and asked him to tell me how I could best help him. As he spoke, I offered him a seat (by gesturing to the chair). I did this for 2 reasons: to move him away from blocking my exit from the room, and to put him at a lower level than me so that he was entirely in my view. I didn’t interrupt him as he spoke. I just nodded or tilted my head to show I was listening. In my mind, I played out the various scenarios that could ensue.
Fortunately, I was able to get him to relax enough for an assessment, which involved a more relevant history and the exam, which he agreed to once an aide had come into the room. He did not exhibit the concerning signs of flushed skin, dilated pupils, shallow rapid respirations, or perspiration. He did have a comorbid behavioral health issue, which we were able to address. His earlier behavioral indicators of agitation were controlled with verbal and physical cues on my part. Our conversation didn't reveal an intent to harm himself or others. In this case, physical restraints were not required. Throughout the encounter the door was left open, and the patient was reminded that we were there to help.
Once he left, I made the relevant notes in the chart regarding his agitated state at the start of the visit and his final state at the end of the visit so as to assist any other providers. We (TIM, MG) also held a quick debrief after the encounter with the office staff and decided that we needed to create a policy and protocol regarding how to handle such situations in the future.

CORRESPONDENCE
Tochi Iroku-Malize, MD, MPH, MBA, Family Medicine Department, Southside Hospital, 301 East Main Street, Bay Shore, NY 11706; [email protected].
CASE A 40-year-old man came to our office slightly agitated. He had an acute illness that was minor in nature. However, he was not interested in answering my questions or undergoing a physical exam. The more I tried to proceed with the visit, the more agitated he became—pacing the room, muttering, avoiding eye contact. I was uncomfortable and knew that the situation could quickly escalate if it was not brought under control.
What steps would you take if this were your patient?
The scene described above occurred several years ago, but more recently, one of the institutions in my (TIM) area was affected by a shooter in the workplace. The apprehension felt by all of us who were on the periphery paled in comparison to what was experienced by those at the scene. The outcome was horrific. Communicating with those directly involved during, and immediately after, the event was heart-wrenching. The trauma that they continue to relive is unimaginable, and some are not yet able to return to work.
Situations involving agitated patients are not uncommon in health care settings, although ones that escalate to the level of a shooting are. And no matter where on the spectrum an incident involving an agitated patient falls, it can leave those involved with various levels of physical, emotional, and psychological harm. It can also leave everyone asking themselves: “How can I better prepare for such occurrences?”
This article offers some answers by providing tips and guidelines for handling agitated and/or violent patients in various settings.
[polldaddy:9948472]
Defining the problem, assessing its severity
Between 2011 and 2013, workplace assaults ranged from 23,540 and 25,630 annually, with 70% to 74% occurring in health care and social service settings.1,2
Agitation is defined as a state that may include inattention, disinhibition, emotional lability, impulsivity, motor restlessness, and aggression.3,4 Violence in a clinical setting may be seen as an extreme expression of agitation sufficient enough to cause harm to an individual or damage to an object.5,6

The causes of agitation can be grouped into categories: those due to a general medical condition, those due to a psychiatric condition, and those due to drug intoxication and/or withdrawal.7 We have chosen to add a fourth category—iatrogenic (see TABLE 13,4,7-9). They are not distinct categories, as there is sometimes overlap among areas.

Determining the level of agitation. Various scales and approaches can help determine the level of agitation in a patient (eg, the Agitated Behavior Scale [ABS; FIGURE];5 the Behavioral Activity Rating Scale [BARS]10) and the risk for violence (eg, the ABC violence risk assessment,

Scales like the ABS should be employed as soon as a patient shows signs of agitation sufficient to warrant intervention. The idea is for the family physician (FP) to be familiar enough with the tool to be able to mentally check it off, fill it out when time permits, and keep it in the patient’s chart. The first version of the form serves as a baseline so that if care is handed off to another provider, that provider can monitor whether signs and symptoms are improving or worsening.
Setting often drives the solution
Much of the evidence-based research on managing patient agitation and violence stems from inpatient psychiatric and emergency department (ED) settings. To make other health care providers aware of the experience gained in those settings, the American Association for Emergency Psychiatry created Project BETA (Best Practices in Evaluation and Treatment of Agitation). This project is designed to help promote consistency across health care settings and specialties in the way clinicians respond to agitated patients and to emphasize for all health care providers the availability of more than just pharmacologic approaches.7
De-escalating the situation. General tenets of de-escalation apply across practice settings. Among them:
- Stay calm. Avoid aggressive postures and prolonged eye contact.
- Be nonconfrontational. Acknowledge the patient’s frustration/perceptions and ask open-ended questions.
- Assess available resources such as clinical team members, family members, and silent alarms.
- Manage the situation and the patient’s underlying issues/diagnoses. This includes mobilizing other patients to avoid collateral damage and exploring solutions with the patient.
For more on de-escalation tools, see (TABLE 34,6,9,11).

Your setting matters. It’s worth noting that the settings in which clinicians practice greatly influence the resources available to de-escalate a situation and ensure the safety of the patient and others.7 The review that follows provides some issues—and tips—that are unique to different practice settings.
Ambulatory settings
Sim and colleagues9 noted that aggressive behavior in the general practice setting may stem not only from factors related to the patient’s own physical or psychological discomfort, but from patients feeling that they are being treated unfairly, whether it be because of wait times, uncomfortable waiting conditions, or something else. A number of international studies have shown high rates of abuse toward FPs.9,12 Of 831 primary care physicians surveyed in a German study, close to three-quarters indicated that within the last year, they had experienced aggression (ranging from verbal abuse and threats to physical violence and property damage) from a patient.12 This statistic increased to 91% when it included the length of their career.
Bell13 suggests that physicians be aware that transference and countertransference issues are often at play when dealing with hostile or potentially violent patients. Suggestions to prevent aggression include some practice-level approaches (eg, providing waiting room distractions, making patients aware of potential delays), as well as being aware of nonverbal cues suggesting increased agitation (eg, clenched fists, crossed arms, chin thrusts, finger pointing).9
Group practice
An FP who practices with other health care providers and clinical staff has a built-in team that can assist with de-escalation. When meeting with a patient who has a history of violence or agitation in an exam room or office, try to ensure that you can get to an exit quickly if necessary. Also, alert staff to any concerns, and have a system for at least one staff member to check in periodically during the visit.
It is also helpful to develop an evacuation plan and create a “panic room” or “safe zone” for emergencies.14,15 Such a space may be nothing more than an area or room for staff to gather. It should have access to the police or other emergency services via a land and/or cell phone line.
Solo practice
If you practice alone, institute safeguards whereby a colleague (at a different practice, building, or location) can be alerted if concerns arise. In addition, consider the following precautions: locking the door when alone after hours, screening potential patients, having a way to call for help (keep the number for the local police station and ED readily available), prohibiting potential weapons (as some states allow them to be carried), and learning some form of self-defense.15
Resources exist that offer guidelines for developing policies and procedures, checklists, and sample incident forms (eg, the International Association for Healthcare Security and Safety; iahss.org). Other organizations that can help with the development of a preparedness plan include the Occupational Safety and Health Administration (https://www.osha.gov/SLTC/workplaceviolence/evaluation.html), the Department of Homeland Security (https://www.dhs.gov/sites/default/files/publications/ISC%20Violence%20in%20%20the%20Federal%20Workplace%20Guide%20April%202013.pdf), and The Joint Commission (https://www.jointcommission.org/workplace_violence.aspx).
Long- and short-term care facilities
In long-term care settings, such as nursing homes, and shorter-term care settings, such as rehabilitation facilities, agitation may stem from causes related to a head injury or dementia or from living in an unfamiliar environment. Assessment can be accomplished using a formal scale (eg, the ABS), as well as by identifying potential underlying health-related factors that can lead to agitation, such as pain, an infection, bowel and bladder issues, seizures, wounds, endocrine anomalies, cardiac or pulmonary problems, gastrointestinal dysfunction, and metabolic abnormalities.3
Modify the environment. For this population, a primary approach involves modifying the environment to decrease the likelihood of agitation. This may involve decreasing noise or light or ensuring adequate levels of stimulation. Preventing disorientation can be addressed through verbal and visual reminders of the date, schedule, etc. If a particular situation or activity is identified as a source of agitation, attempts at modifications are called for.3
For patients with dementia, the American Psychiatric Association recommends using the lowest effective dose of an antipsychotic in conjunction with environmental and behavioral measures.16 A benzodiazepine (lorazepam, oxazepam) may be used for infrequent agitation. Trazodone or a selective serotonin reuptake inhibitor are alternatives for those without psychosis or who are intolerant to antipsychotics.16
For individuals in a rehabilitation setting, agitation can impede participation in therapy and has been associated with poorer functioning at the time of discharge.3 Agitation can also be disruptive and lead to distress for family members and caregivers, as well as for fellow patients. And because this environment has a greater likelihood of visitors unrelated to the patient being exposed to the aberrant behavior, it is especially important to have established policies and procedures for de-escalation in place.
Home care
More and more FPs and residents are conducting home visits. That’s because the Accreditation Council for Graduate Medical Education Program Requirements for Graduate Medical Education in Family Medicine now include integrating a patient’s care across settings—including the home.17 Those who do provide home care may find themselves in circumstances similar to those of domestic disputes.
The German study mentioned earlier of more than 800 primary care physicians found that while the vast majority of physicians felt safe in their offices, 66% of female doctors and 34% of male doctors did not feel safe making home visits.12
Know the neighborhood. There’s no doubt that working in the home health sector makes one vulnerable. More than 61% of home care workers report workplace violence annually.18,19 An action plan, as well as established policies and procedures, are essential when making home visits. Prior to the visit, be aware of the community and the location of the nearest police department and hospital.
Unwin and Tatum20 suggest not wearing a white coat or carrying a doctor’s bag so as not to stand out as a physician in neighborhoods where personal safety is an issue. Make sure that your cell phone is fully charged and that there is a GPS mechanism activated that allows others to locate you.21 Note the available exits in a patient’s home, and position yourself near them, if possible. Have someone call or text you at predetermined times so that the absence of a response from you will alert someone to send help.
In such situations, it is imperative to remain calm and to use the same verbal de-escalation techniques (TABLE 34,6,9,11) that would be used in any other health care setting. It is prudent to set expectations for the patient and family members prior to the home visit regarding the tools and services that will be provided in the home setting and the limitations in terms of scope of practice.
Emergency department
The ED is one of the most common settings for patient agitation and violence within the health care continuum.22 Providers must quickly determine the cause of the agitation while de-escalating the situation and ensuring that they do not miss a pertinent medical finding related to a time-sensitive issue, such as an intracerebral bleed or poisoning.7 In addition, the ED is usually heavily populated, providing an opportunity for tremendous collateral human damage should the violence escalate or weapons be deployed. The upside is that many EDs are now staffed with security personnel and, depending on the community, police officers may be on the premises or in the vicinity.22
Etiologies for agitation in the ED can range from ingestion of unknown or unidentified substances to psychiatric or medical conditions. Knowledge of etiology is necessary prior to initiation of treatment.4
As in other settings, the safety of the patient and others present is of utmost importance. Key recommendations for managing agitated patients in the ED include: 4
- Have an established plan for the management of agitated patients.
- Identify signs of agitation early, and complete an agitation rating scale.
- Attempt verbal de-escalation before using medication whenever possible.
- Employ a “show of concern” rather than “a show of force” in response to escalating agitation/violence. Doing so can strengthen the perception that interventions are coming from a place of caring.
- Use physical restraint as a last resort. When used, it should be with the intention of protecting the patient and those present, rather than as punishment.
Inpatient units
Unlike the ED, patients on units generally have a working diagnosis, and the provider has some background information with which to work, such as laboratory test results and radiology reports, facilitating more expedient and accurate situational assessment. However, the recommendations for assessment and early identification, as described for the ED, still apply.
If a provider finds him- or herself in an escalating situation, the call bells located in the rooms are of use. An alternative is to call out for help from someone in the hallway. One needs to be aware of the current policies and procedures for de-escalation, as some facilities have a specific “code” that is called for such occasions.19
Postop delirium is a common cause of agitation in the inpatient setting. Ng and colleagues11 recommend a cognitive assessment before surgery to establish a baseline in order to determine the risk for delirium after surgery. Additionally, the FP must remain aware of preexisting conditions that may surface during a hospital stay, such as dehydration or unrecognized alcohol or medication withdrawal.
Medication choice should be based on the type of delirium. Hyperactive delirium (restlessness, emotional lability, hallucinations) and mixed delirium (a combination of signs of hyperactive and hypoactive dementia) both hold the potential for agitation and even violence. The approach to hyperactive delirium includes consideration of an antipsychotic medication, although the efficacy of antipsychotics is considered controversial. In the case of mixed delirium, behavioral and environmental modifications are useful (eg, reducing noise and early ambulation).11
No medications are registered with the US Food and Drug Administration for the management of delirium, and it is suggested that antipsychotics be considered only when other, less invasive, strategies have been attempted.23
Addressing caregiver stress, anxiety disorders afterward
Regardless of the setting in which FPs work, witnessing or being directly involved in a traumatic event puts one at risk for symptoms—or a full diagnosis—of posttraumatic stress disorder (PTSD), acute stress disorder, or anxiety or mood disorders.24,25 Although findings vary, studies have found that as many as 12% of ED personnel meet the criteria for PTSD26,27 and 12% to 15% report having been threatened physically.28,29 More than half of physicians in another study had witnessed a physical attack.30
Physicians and other health care personnel who have experienced a traumatic incident, or offered help to another during an incident, may attempt to cope through avoidance, cutting down on work hours, leaving the work setting in which the event took place, or leaving the profession altogether.29,31,32
There is a paucity of methodologically sound research with regard to prevention and treatment of PTSD symptoms in this population.24 According to a 2002 Cochrane review, the effectiveness of individual, single-session debriefing does not have solid research support,33 and there are concerns about potential harms due to reliving the traumatic event when sessions are led by poorly trained debriefing staff.34-36
Critical incident stress debriefing (CISD), however, holds promise in terms of facilitating a return to pretrauma functioning based on studies of first responders.34,35 This may be because CISD follows a specific protocol and that group sessions may capitalize on the social support/camaraderie within a group that has undergone a traumatic event.34,35 It is important that those providing debriefing and support be well-trained.35
Debriefing, however, is not always sufficient, and those who appear to be affected on an ongoing basis may require individual treatment for PTSD symptoms. Evidence-based treatments for PTSD, such as trauma-focused psychotherapy and/or pharmacotherapy, may be considered37 (TABLE 424,34,38).

Ongoing support in the workplace. The Cleveland Clinic has developed a “Code Lavender” to combat stress in the workplace. Like a Code Blue for medical emergencies, a Code Lavender is called when a health care worker is in need of emotional or spiritual support.38 A provider who initiates the call is met by a team of holistic nurses within 30 minutes. The team provides Reiki and massage, healthy snacks and water, and lavender arm bands to remind the individual to relax for the rest of the day. Further opportunities for spiritual support, mindfulness training, counseling, and yoga may also be made available.
CASE Sensing that the situation with my patient might escalate, I lowered my voice, relaxed my shoulders, leaned casually against the desk, and asked him to tell me how I could best help him. As he spoke, I offered him a seat (by gesturing to the chair). I did this for 2 reasons: to move him away from blocking my exit from the room, and to put him at a lower level than me so that he was entirely in my view. I didn’t interrupt him as he spoke. I just nodded or tilted my head to show I was listening. In my mind, I played out the various scenarios that could ensue.
Fortunately, I was able to get him to relax enough for an assessment, which involved a more relevant history and the exam, which he agreed to once an aide had come into the room. He did not exhibit the concerning signs of flushed skin, dilated pupils, shallow rapid respirations, or perspiration. He did have a comorbid behavioral health issue, which we were able to address. His earlier behavioral indicators of agitation were controlled with verbal and physical cues on my part. Our conversation didn't reveal an intent to harm himself or others. In this case, physical restraints were not required. Throughout the encounter the door was left open, and the patient was reminded that we were there to help.
Once he left, I made the relevant notes in the chart regarding his agitated state at the start of the visit and his final state at the end of the visit so as to assist any other providers. We (TIM, MG) also held a quick debrief after the encounter with the office staff and decided that we needed to create a policy and protocol regarding how to handle such situations in the future.

CORRESPONDENCE
Tochi Iroku-Malize, MD, MPH, MBA, Family Medicine Department, Southside Hospital, 301 East Main Street, Bay Shore, NY 11706; [email protected].
1. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. December 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed February 8, 2018.
2. Occupational Safety and Health Administration. Guidelines for preventing workplace violence for healthcare and social service workers. 2016. Available at: https://www.osha.gov/Publications/osha3148.pdf. Accessed February 8, 2017.
3. Mortimer DS, Berg W. Agitation in patients recovering from traumatic brain injury: nursing management. J Neurosci Nurs. 2017;49:25-30.
4. Wilson MP, Nordstrom K, Vilke GM. The agitated patient in the emergency department. Curr Emerg Hosp Med Rep. 2015;3:188-194.
5. Bogner JA, Corrigan JD, Bode RK, et al. Rating scale analysis of the Agitated Behavior Scale. J Head Trauma Rehabil. 2000;15:656-669.
6. Gaynes BN, Brown CL, Lux LJ, et al. Preventing and de-escalating aggressive behavior among adult psychiatric patients: a systematic review of the evidence. Psychiatr Serv. 2017;68:819-831.
7. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project Beta Medical Evaluation Workgroup. West J Emerg Med. 2012;13:3-10.
8. Sands N. Mental health triage: towards a model for nursing practice. J Psychiatr Ment Health Nurs. 2007;14:243-249.
9. Sim MG, Wain T, Khong E. Aggressive behaviour - prevention and management in the general practice environment. Aust Fam Physician. 2011;40:866-872.
10. Swift RH, Harrigan EP, Cappelleri JC, et al. Validation of the behavioural activity rating scale (BARS): a novel measure of activity in agitated patients. J Psychiatr Res. 2002;36:87-95.
11. Ng J, Pan CX, Geube A, et al. Your postop patient is confused and agitated—next steps? J Fam Pract. 2015;64:361-366.
12. Vorderwülbecke F, Feistle M, Mehring M, et al. Aggression and violence against primary care physicians—a nationwide questionnaire survey. Dtsch Arztebl Int. 2015;112:159-165.
13. Bell HS. Curbside consultation—a potentially violent patient? Am Fam Physician. 2000;61:2237-2238.
14. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10:40-42.
15. Munsey C. How to stay safe in practice. APA Monitor. 2008;39:36.
16. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173:543-546.
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. Revised July 1, 2017. Available at: http://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_family_medicine_2017-07-01.pdf . Accessed October 30, 2017.
18. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374:1661-1669.
19. Hanson GC, Perrin NA, Moss H, et al. Workplace violence against homecare workers and its relationship with workers health outcomes: a cross-sectional study. BMC Public Health. 2015;15:11.
20. Unwin BK, Tatum PE 3rd. House calls. Am Fam Physician. 2011;83:925-938.
21. Victor P. Safety tips for home visits from a veteran NYC social worker. National Association of Social Workers, New York. Available at: http://www.naswnyc.org/?489. Accessed June 1, 2017.
22. Kansagra SM, Rao SR, Sullivan AF, et al. A survey of workplace violence across 65 U.S. emergency departments. Acad Emerg Med. 2008;15:1268-1274.
23. Meagher D, Agar MR, Teodorczuk A. Debate article: antipsychotic medications are clinically useful for the treatment of delirium. Int J Geriatr Psychiatry. 2017 Jul 30. doi: 10.1002/gps.4759. [Epub ahead of print].
24. Lanctot N, Guay S. The aftermath of workplace violence among healthcare workers: a systematic literature review of the consequences. Aggress Violent Behav. 2014;19:492-501.
25. Edward KL, Stephenson J, Ousey K, et al. A systematic review and meta-analysis of factors that relate to aggression perpetrated against nurses by patients/relatives or staff. J Clin Nurs. 2016;25:289-299.
26. Laposa JM, Alden LE. Posttraumatic stress disorder in the emergency room: exploration of a cognitive model. Behav Res Ther. 2003;41:49-65.
27. Mills LD, Mills TJ. Symptoms of post-traumatic stress disorder among emergency medicine residents. J Emerg Med. 2005;28:1-4.
28. Laposa JM, Alden LE, Fullerton LM. Work stress and posttraumatic stress disorder in ED nurses/personnel. J Emerg Nurs. 2003;29:23-28.
29. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed June 1, 2017.
30. Zafar W, Khan UR, Siddiqui SA, et al. Workplace violence and self-reported psychological health: coping with post-traumatic stress, mental distress, and burnout among physicians working in the emergency departments compared to other specialties in Pakistan. J Emerg Med. 2016;50:167-177.
31. de Boer J, Lok A, Van’t Verlaat E, et al. Work-related critical incidents in hospital-based health care providers and the risk of post-traumatic stress symptoms, anxiety, and depression: a meta-analysis. Soc Sci Med. 2011;73:316-326.
32. Shah L, Annamalai J, Aye SN, et al. Key components and strategies utilized by nurses for de-escalation of aggression in psychiatric in-patients: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2016;14:109-118.
33. Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002;(2):CD000560.
34. Tuckey MR, Scott JE. Group critical incident stress debriefing with emergency services personnel: a randomized controlled trial. Anxiety Stress Coping. 2014;27:38-54.
35. Pack MJ. Critical incident stress management: a review of the literature with implications for social work. Int Soc Work. 2012;56: 608-627.
36. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
37. Warner CH, Warner CM, Appenzeller GN, et al. Identifying and managing posttraumatic stress disorder. Am Fam Physician. 2013;88:827-834.
38. Johnson B. Code lavender: initiating holistic rapid response at the Cleveland Clinic. Beginnings. 2014;34:10-11.
1. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. December 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed February 8, 2018.
2. Occupational Safety and Health Administration. Guidelines for preventing workplace violence for healthcare and social service workers. 2016. Available at: https://www.osha.gov/Publications/osha3148.pdf. Accessed February 8, 2017.
3. Mortimer DS, Berg W. Agitation in patients recovering from traumatic brain injury: nursing management. J Neurosci Nurs. 2017;49:25-30.
4. Wilson MP, Nordstrom K, Vilke GM. The agitated patient in the emergency department. Curr Emerg Hosp Med Rep. 2015;3:188-194.
5. Bogner JA, Corrigan JD, Bode RK, et al. Rating scale analysis of the Agitated Behavior Scale. J Head Trauma Rehabil. 2000;15:656-669.
6. Gaynes BN, Brown CL, Lux LJ, et al. Preventing and de-escalating aggressive behavior among adult psychiatric patients: a systematic review of the evidence. Psychiatr Serv. 2017;68:819-831.
7. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project Beta Medical Evaluation Workgroup. West J Emerg Med. 2012;13:3-10.
8. Sands N. Mental health triage: towards a model for nursing practice. J Psychiatr Ment Health Nurs. 2007;14:243-249.
9. Sim MG, Wain T, Khong E. Aggressive behaviour - prevention and management in the general practice environment. Aust Fam Physician. 2011;40:866-872.
10. Swift RH, Harrigan EP, Cappelleri JC, et al. Validation of the behavioural activity rating scale (BARS): a novel measure of activity in agitated patients. J Psychiatr Res. 2002;36:87-95.
11. Ng J, Pan CX, Geube A, et al. Your postop patient is confused and agitated—next steps? J Fam Pract. 2015;64:361-366.
12. Vorderwülbecke F, Feistle M, Mehring M, et al. Aggression and violence against primary care physicians—a nationwide questionnaire survey. Dtsch Arztebl Int. 2015;112:159-165.
13. Bell HS. Curbside consultation—a potentially violent patient? Am Fam Physician. 2000;61:2237-2238.
14. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10:40-42.
15. Munsey C. How to stay safe in practice. APA Monitor. 2008;39:36.
16. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173:543-546.
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. Revised July 1, 2017. Available at: http://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_family_medicine_2017-07-01.pdf . Accessed October 30, 2017.
18. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374:1661-1669.
19. Hanson GC, Perrin NA, Moss H, et al. Workplace violence against homecare workers and its relationship with workers health outcomes: a cross-sectional study. BMC Public Health. 2015;15:11.
20. Unwin BK, Tatum PE 3rd. House calls. Am Fam Physician. 2011;83:925-938.
21. Victor P. Safety tips for home visits from a veteran NYC social worker. National Association of Social Workers, New York. Available at: http://www.naswnyc.org/?489. Accessed June 1, 2017.
22. Kansagra SM, Rao SR, Sullivan AF, et al. A survey of workplace violence across 65 U.S. emergency departments. Acad Emerg Med. 2008;15:1268-1274.
23. Meagher D, Agar MR, Teodorczuk A. Debate article: antipsychotic medications are clinically useful for the treatment of delirium. Int J Geriatr Psychiatry. 2017 Jul 30. doi: 10.1002/gps.4759. [Epub ahead of print].
24. Lanctot N, Guay S. The aftermath of workplace violence among healthcare workers: a systematic literature review of the consequences. Aggress Violent Behav. 2014;19:492-501.
25. Edward KL, Stephenson J, Ousey K, et al. A systematic review and meta-analysis of factors that relate to aggression perpetrated against nurses by patients/relatives or staff. J Clin Nurs. 2016;25:289-299.
26. Laposa JM, Alden LE. Posttraumatic stress disorder in the emergency room: exploration of a cognitive model. Behav Res Ther. 2003;41:49-65.
27. Mills LD, Mills TJ. Symptoms of post-traumatic stress disorder among emergency medicine residents. J Emerg Med. 2005;28:1-4.
28. Laposa JM, Alden LE, Fullerton LM. Work stress and posttraumatic stress disorder in ED nurses/personnel. J Emerg Nurs. 2003;29:23-28.
29. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed June 1, 2017.
30. Zafar W, Khan UR, Siddiqui SA, et al. Workplace violence and self-reported psychological health: coping with post-traumatic stress, mental distress, and burnout among physicians working in the emergency departments compared to other specialties in Pakistan. J Emerg Med. 2016;50:167-177.
31. de Boer J, Lok A, Van’t Verlaat E, et al. Work-related critical incidents in hospital-based health care providers and the risk of post-traumatic stress symptoms, anxiety, and depression: a meta-analysis. Soc Sci Med. 2011;73:316-326.
32. Shah L, Annamalai J, Aye SN, et al. Key components and strategies utilized by nurses for de-escalation of aggression in psychiatric in-patients: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2016;14:109-118.
33. Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002;(2):CD000560.
34. Tuckey MR, Scott JE. Group critical incident stress debriefing with emergency services personnel: a randomized controlled trial. Anxiety Stress Coping. 2014;27:38-54.
35. Pack MJ. Critical incident stress management: a review of the literature with implications for social work. Int Soc Work. 2012;56: 608-627.
36. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
37. Warner CH, Warner CM, Appenzeller GN, et al. Identifying and managing posttraumatic stress disorder. Am Fam Physician. 2013;88:827-834.
38. Johnson B. Code lavender: initiating holistic rapid response at the Cleveland Clinic. Beginnings. 2014;34:10-11.
PRACTICE RECOMMENDATIONS
› Be aware of signs of agitation and use verbal de-escalation and environmental modifications whenever possible. B
› Consider group-based critical incident debriefing with a trained provider after a traumatic event. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Mental health apps: What to tell patients
Have your patients asked you about smartphone apps? If they haven’t yet, they may soon, as interest in apps for mental health continues to expand. There are now >10,000 mental health–related smartphone apps.1 The rapid rise of these apps is partly due to their potential to transform a patient’s smartphone into a monitoring and therapeutic platform, capable of capturing mental health symptoms in real time and delivering on-the-go therapy. Setting aside questions about the potential of mobile health, 2 urgent questions remain for the busy psychiatrist in clinical practice: What is the current evidence base for mental health apps, and what should you tell your patients about them?
For most apps, evidence of efficacy is limited
While the evidence base for mental health smartphone apps continues to expand, for many of these apps, there is no evidence of effectiveness. The growing consensus is that most commercially available apps are not evidence-based and some are even dangerous. For example, researchers who examined >700 mindfulness apps on the iTunes and Google Play stores found that only 4% provided acceptable mindfulness training and education.2 Another study of 58 apps that claimed to offer sobriety assessments found that none had ever been formally evaluated.3 Evidence-based reviews of suicide prevention apps have identified potentially harmful apps,4 and studies evaluating apps for bipolar disorder5 and depression6 have yielded similar results—few have any evidence supporting their use, and some offer dangerous and harmful advice. For example, researchers found that one app for bipolar disorder advised patients who are experiencing a manic episode to drink alcohol.5 Currently, the vast majority of commercially available apps are not appropriate for clinical care. This finding is not unique to mental health; similar findings have been reported for apps for cancer.7 The bottom line is that the apps that your patients are finding, and perhaps already using, may not be useful or effective.
However, early studies have demonstrated efficacy of some apps for several conditions, including schizophrenia,8 depression,9 anxiety disorders,10 and suicidal ideation.11 Although many of the apps evaluated in these studies are not available to the public, or still require large-scale assessment before they are ready for mainstream clinical care, this research demonstrates that mental health apps can help improve treatment outcomes. As this research develops, a wave of evidence-based and effective mental health apps may be available in the near future.
Although it is unknown how many patients are presently using mental health apps, there is strong anecdotal evidence that an increasing number of patients who use these apps and other forms of digital technology are finding some benefits. In many cases, patients may actually be ahead of the research. For example, one study that conducted an online survey of patients with schizophrenia noted that some patients are using their smartphones to play music to help block auditory hallucinations.12
Why online reviews are of limited use
As this evidence continues to mature, and with an ever-growing number of mental health apps available on commercial marketplaces, busy psychiatrists need to navigate this complex space. Even psychiatrists who decide to not use apps as part of care still need to be knowledgeable about them, because patients are likely to ask about the benefits of using apps, and they will expect an informed response. How would you reply if your patient asked you about a new mood-tracking app he or she recently heard about? On what would you base your recommendation and opinion?
Reading online app reviews for guidance is not a good solution. A recent study found little relationship between the star ratings of health apps and the quality of those apps,13 which suggests that a 5-star rating on the app store is of limited use.
Unlike medications whose ingredients do not change over time, or manualized psychotherapies that use specific protocols, mental health apps are dynamic and constantly changing.14 Think of how often the apps on your smartphone update. Thus, the version of a mental health app that your patient downloads today may be very different from the version that received a favorable user review last month. And just as there is no single medication or therapy that is ideal for every patient, neither is there a single “best” app for all patients with the same disorder. Picking an app is a personal decision that cannot be made based on a single score or numeric rating. Furthermore, the validity of app rating systems is unclear. One study found a wide variation in the interrater reliability of measures used to evaluate apps from sources that included PsyberGuide, the Anxiety and Depression Association of America, and the research literature. Quality measures such as effectiveness, ease of use, and performance had relatively poor interrater reliability.15 This means that, for example, an app that one patient finds “easy to use” may be difficult to use for another. Thus, providing patients with suggestions based on an app’s ratings may result in providing information that sounds useful, but often is misleading.
A model for evaluating apps
One possible solution is a risk-based and personalized assessment approach to evaluating mental health apps. Although it does not offer scoring or recommendations of specific apps, the American Psychiatric Association (APA) App Evaluation Model (Figure) provides a framework to guide discussion and informed decision-making about apps. (The authors of this article helped create this model, but receive no compensation for that volunteer work.) The pyramid shape reflects the hierarchical nature of the model. To begin the process, start at the base of the pyramid and work upward.
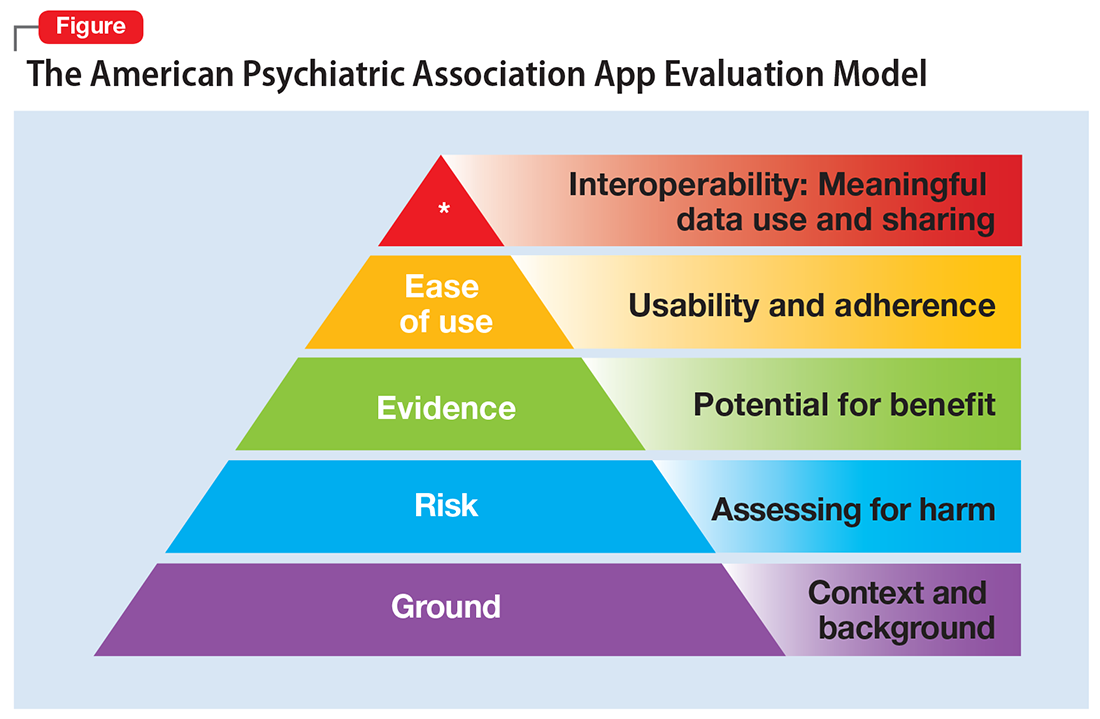
Ground. First, consider the context of the app by determining basic facts, such as who made it, how much it costs, and its technology requirements. This ground layer establishes the credibility of the app’s creator by questioning his or her reputation, ability to update the app, and funding sources. Understanding the app’s business model also will help you determine whether the app will stand the test of time: Will it continue to exist next month or next year, or will a lack of reliable funding lead the vendor to abandon it?
Risk. The next layer assesses the risk, privacy, and security features of the app. Many mental health apps actively aim to avoid falling under the jurisdiction of U.S. federal health care privacy rules, such as the Health Insurance Portability and Accountability Act of 1996, so there is no guarantee that sensitive data supplied to an app will be protected. The true cost of a “free” app often is your patient’s personal mental health information, which the app’s developer may accumulate and sell for profit. Thus, it is wise to check the privacy policy to learn where your patient’s data goes. Furthermore, patients and psychiatrists must be vigilant that malware-infected apps can be uploaded to the app store, which can further compromise privacy.16 You may be surprised to learn that many apps lack a privacy policy, which means there are no protections for personal information or safeguards against the misuse of mental health data.17 Checking that an app at least promises to digitally protect mental health data through encryption and secure storage also is a good step.
The goal of considering these factors is not to create a score, but rather to be aware of them and consider them in the context of the specific app, patient, and clinical situation. Doing so helps determine whether the app meets the appropriate risk, privacy, and security standards for your patient.
Evidence. The next layer of the evaluation framework is evidence. The goal is to seek an app with clinical evidence of effectiveness. Simply put, if a patient is going to use an app, he should use one that works. An app without formal evidence may be effective, but it is important to make sure the patient is aware that these claims have not been verified. Many apps claim that they offer cognitive-behavioral therapy or mindfulness therapy, but few deliver on such claims.18 It is wise to try an app before recommending it to a patient to ensure that it does what it claims it does, and does not offer dangerous or harmful recommendations.
Ease of use. Across all health apps, there is growing recognition that most downloaded apps are never used. Patient engagement with mental health apps appears to rapidly decline over the first week of use.19 There also is emerging evidence that many apps are not user-friendly. A recent study of several common mood-tracking apps found that patients with depression had difficulty entering and accessing their data.20 Because many psychiatric disorders are chronic or last at least several months, it is especially important to consider how engaging and usable the app will be for your patient. Usability varies from patient to patient, so it is best to check directly with your patient regarding his comfort with apps and mobile technology. Offering check-ins and support to help patients keep on track with apps may be critical for successful outcomes.
Interoperability. The final layer of the model is data sharing and interoperability. It is important to determine if the data collected or generated by the app are available to you, the patient, the treatment team, and others involved in the patient’s care. As mental health treatment moves toward integrated care, apps that fragment care (by not sharing information) impede care. Check if the app can share data with an electronic medical record, or if there is a plan to review and act on data from the app as part of your patient’s treatment plan.
More information about the APA App Evaluation Model, including additional factors to consider within each layer, is available from the APA for free at https://www.psychiatry.org/psychiatrists/practice/mental-health-apps/app-evaluation-model. For a sample of factors to consider when evaluating a mental health app, see the Table.
A reasonable strategy
Although the APA App Evaluation Model does not endorse any particular app, it can help guide more informed decision-making. As the evidence on mental health apps continues to evolve, it will become easier to make definitive statements on what constitutes a useful app. For now, the best strategy when discussing mental health apps with patients is to combine the use of this model with your clinical judgment.
1. Torous J, Roberts LW. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
2. Mani M, Kavanagh DJ, Hides L, et al. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3(3):e82. doi: 10.2196/mhealth.4328.
3. Wilson H, Stoyanov SR, Gandabhai S, et al. The quality and accuracy of mobile apps to prevent driving after drinking alcohol. JMIR Mhealth Uhealth. 2016;4(3):e98. doi: 10.2196/mhealth.5961.
4. Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PLoS One. 2016;11(4):e0152285. doi: 10.1371/journal.pone.0152285.
5. Nicholas J, Larsen ME, Proudfoot J, et al. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198. doi: 10.2196/jmir.4581.
6. Shen N, Levitan MJ, Johnson A, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713.
7. Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498-504.
8. Ben-Zeev D, Brenner CJ, Begale M, et al. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244-1253.
9. Mohr DC, Tomasino KN, Lattie EG, et al. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017;19(1):e10. doi: 10.2196/jmir.6645.
10. Tighe J, Shand F, Ridani R, et al. Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open. 2017;7(1):e013518. doi: 10.1136/bmjopen-2016-013518.
11. Firth J, Torous J, Nicholas J, et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017;218:15-22.
12. Gay K, Torous J, Joseph A, et al. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Mental Health. 2016;3(2):e15. doi: 10.2196/mental.5379.
13. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood). 2016;35(12):2310-2318.
14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth. 2016;4(3):e96. doi: 10.2196/mhealth.6020.
15. Powell AC, Torous J, Chan S, et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR Mhealth Uhealth. 2016;4(1):e15. doi: 10.2196/mhealth.5176.
16. Ducklin P. Apple’s XcodeGhost malware still in the machine…. https://nakedsecurity.sophos.com/2015/11/09/apples-xcodeghost-malware-still-in-the-machine. Published November 9, 2015. Accessed May 11, 2017.
17. Rosenfeld L, Torous J, Vahia IV. Data security and privacy in apps for dementia: an analysis of existing privacy policies. Am J Geriatr Psychiatry. 2017;25(8):873-877.
18. Torous J, Levin ME, Ahern DK, et al. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215-225.
19. Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Ment Health. 2015;2(1):e7. doi: 10.2196/mental.3935.
20. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med. 2016;31(12):1417-1426.
Have your patients asked you about smartphone apps? If they haven’t yet, they may soon, as interest in apps for mental health continues to expand. There are now >10,000 mental health–related smartphone apps.1 The rapid rise of these apps is partly due to their potential to transform a patient’s smartphone into a monitoring and therapeutic platform, capable of capturing mental health symptoms in real time and delivering on-the-go therapy. Setting aside questions about the potential of mobile health, 2 urgent questions remain for the busy psychiatrist in clinical practice: What is the current evidence base for mental health apps, and what should you tell your patients about them?
For most apps, evidence of efficacy is limited
While the evidence base for mental health smartphone apps continues to expand, for many of these apps, there is no evidence of effectiveness. The growing consensus is that most commercially available apps are not evidence-based and some are even dangerous. For example, researchers who examined >700 mindfulness apps on the iTunes and Google Play stores found that only 4% provided acceptable mindfulness training and education.2 Another study of 58 apps that claimed to offer sobriety assessments found that none had ever been formally evaluated.3 Evidence-based reviews of suicide prevention apps have identified potentially harmful apps,4 and studies evaluating apps for bipolar disorder5 and depression6 have yielded similar results—few have any evidence supporting their use, and some offer dangerous and harmful advice. For example, researchers found that one app for bipolar disorder advised patients who are experiencing a manic episode to drink alcohol.5 Currently, the vast majority of commercially available apps are not appropriate for clinical care. This finding is not unique to mental health; similar findings have been reported for apps for cancer.7 The bottom line is that the apps that your patients are finding, and perhaps already using, may not be useful or effective.
However, early studies have demonstrated efficacy of some apps for several conditions, including schizophrenia,8 depression,9 anxiety disorders,10 and suicidal ideation.11 Although many of the apps evaluated in these studies are not available to the public, or still require large-scale assessment before they are ready for mainstream clinical care, this research demonstrates that mental health apps can help improve treatment outcomes. As this research develops, a wave of evidence-based and effective mental health apps may be available in the near future.
Although it is unknown how many patients are presently using mental health apps, there is strong anecdotal evidence that an increasing number of patients who use these apps and other forms of digital technology are finding some benefits. In many cases, patients may actually be ahead of the research. For example, one study that conducted an online survey of patients with schizophrenia noted that some patients are using their smartphones to play music to help block auditory hallucinations.12
Why online reviews are of limited use
As this evidence continues to mature, and with an ever-growing number of mental health apps available on commercial marketplaces, busy psychiatrists need to navigate this complex space. Even psychiatrists who decide to not use apps as part of care still need to be knowledgeable about them, because patients are likely to ask about the benefits of using apps, and they will expect an informed response. How would you reply if your patient asked you about a new mood-tracking app he or she recently heard about? On what would you base your recommendation and opinion?
Reading online app reviews for guidance is not a good solution. A recent study found little relationship between the star ratings of health apps and the quality of those apps,13 which suggests that a 5-star rating on the app store is of limited use.
Unlike medications whose ingredients do not change over time, or manualized psychotherapies that use specific protocols, mental health apps are dynamic and constantly changing.14 Think of how often the apps on your smartphone update. Thus, the version of a mental health app that your patient downloads today may be very different from the version that received a favorable user review last month. And just as there is no single medication or therapy that is ideal for every patient, neither is there a single “best” app for all patients with the same disorder. Picking an app is a personal decision that cannot be made based on a single score or numeric rating. Furthermore, the validity of app rating systems is unclear. One study found a wide variation in the interrater reliability of measures used to evaluate apps from sources that included PsyberGuide, the Anxiety and Depression Association of America, and the research literature. Quality measures such as effectiveness, ease of use, and performance had relatively poor interrater reliability.15 This means that, for example, an app that one patient finds “easy to use” may be difficult to use for another. Thus, providing patients with suggestions based on an app’s ratings may result in providing information that sounds useful, but often is misleading.
A model for evaluating apps
One possible solution is a risk-based and personalized assessment approach to evaluating mental health apps. Although it does not offer scoring or recommendations of specific apps, the American Psychiatric Association (APA) App Evaluation Model (Figure) provides a framework to guide discussion and informed decision-making about apps. (The authors of this article helped create this model, but receive no compensation for that volunteer work.) The pyramid shape reflects the hierarchical nature of the model. To begin the process, start at the base of the pyramid and work upward.

Ground. First, consider the context of the app by determining basic facts, such as who made it, how much it costs, and its technology requirements. This ground layer establishes the credibility of the app’s creator by questioning his or her reputation, ability to update the app, and funding sources. Understanding the app’s business model also will help you determine whether the app will stand the test of time: Will it continue to exist next month or next year, or will a lack of reliable funding lead the vendor to abandon it?
Risk. The next layer assesses the risk, privacy, and security features of the app. Many mental health apps actively aim to avoid falling under the jurisdiction of U.S. federal health care privacy rules, such as the Health Insurance Portability and Accountability Act of 1996, so there is no guarantee that sensitive data supplied to an app will be protected. The true cost of a “free” app often is your patient’s personal mental health information, which the app’s developer may accumulate and sell for profit. Thus, it is wise to check the privacy policy to learn where your patient’s data goes. Furthermore, patients and psychiatrists must be vigilant that malware-infected apps can be uploaded to the app store, which can further compromise privacy.16 You may be surprised to learn that many apps lack a privacy policy, which means there are no protections for personal information or safeguards against the misuse of mental health data.17 Checking that an app at least promises to digitally protect mental health data through encryption and secure storage also is a good step.
The goal of considering these factors is not to create a score, but rather to be aware of them and consider them in the context of the specific app, patient, and clinical situation. Doing so helps determine whether the app meets the appropriate risk, privacy, and security standards for your patient.
Evidence. The next layer of the evaluation framework is evidence. The goal is to seek an app with clinical evidence of effectiveness. Simply put, if a patient is going to use an app, he should use one that works. An app without formal evidence may be effective, but it is important to make sure the patient is aware that these claims have not been verified. Many apps claim that they offer cognitive-behavioral therapy or mindfulness therapy, but few deliver on such claims.18 It is wise to try an app before recommending it to a patient to ensure that it does what it claims it does, and does not offer dangerous or harmful recommendations.
Ease of use. Across all health apps, there is growing recognition that most downloaded apps are never used. Patient engagement with mental health apps appears to rapidly decline over the first week of use.19 There also is emerging evidence that many apps are not user-friendly. A recent study of several common mood-tracking apps found that patients with depression had difficulty entering and accessing their data.20 Because many psychiatric disorders are chronic or last at least several months, it is especially important to consider how engaging and usable the app will be for your patient. Usability varies from patient to patient, so it is best to check directly with your patient regarding his comfort with apps and mobile technology. Offering check-ins and support to help patients keep on track with apps may be critical for successful outcomes.
Interoperability. The final layer of the model is data sharing and interoperability. It is important to determine if the data collected or generated by the app are available to you, the patient, the treatment team, and others involved in the patient’s care. As mental health treatment moves toward integrated care, apps that fragment care (by not sharing information) impede care. Check if the app can share data with an electronic medical record, or if there is a plan to review and act on data from the app as part of your patient’s treatment plan.
More information about the APA App Evaluation Model, including additional factors to consider within each layer, is available from the APA for free at https://www.psychiatry.org/psychiatrists/practice/mental-health-apps/app-evaluation-model. For a sample of factors to consider when evaluating a mental health app, see the Table.
A reasonable strategy
Although the APA App Evaluation Model does not endorse any particular app, it can help guide more informed decision-making. As the evidence on mental health apps continues to evolve, it will become easier to make definitive statements on what constitutes a useful app. For now, the best strategy when discussing mental health apps with patients is to combine the use of this model with your clinical judgment.
Have your patients asked you about smartphone apps? If they haven’t yet, they may soon, as interest in apps for mental health continues to expand. There are now >10,000 mental health–related smartphone apps.1 The rapid rise of these apps is partly due to their potential to transform a patient’s smartphone into a monitoring and therapeutic platform, capable of capturing mental health symptoms in real time and delivering on-the-go therapy. Setting aside questions about the potential of mobile health, 2 urgent questions remain for the busy psychiatrist in clinical practice: What is the current evidence base for mental health apps, and what should you tell your patients about them?
For most apps, evidence of efficacy is limited
While the evidence base for mental health smartphone apps continues to expand, for many of these apps, there is no evidence of effectiveness. The growing consensus is that most commercially available apps are not evidence-based and some are even dangerous. For example, researchers who examined >700 mindfulness apps on the iTunes and Google Play stores found that only 4% provided acceptable mindfulness training and education.2 Another study of 58 apps that claimed to offer sobriety assessments found that none had ever been formally evaluated.3 Evidence-based reviews of suicide prevention apps have identified potentially harmful apps,4 and studies evaluating apps for bipolar disorder5 and depression6 have yielded similar results—few have any evidence supporting their use, and some offer dangerous and harmful advice. For example, researchers found that one app for bipolar disorder advised patients who are experiencing a manic episode to drink alcohol.5 Currently, the vast majority of commercially available apps are not appropriate for clinical care. This finding is not unique to mental health; similar findings have been reported for apps for cancer.7 The bottom line is that the apps that your patients are finding, and perhaps already using, may not be useful or effective.
However, early studies have demonstrated efficacy of some apps for several conditions, including schizophrenia,8 depression,9 anxiety disorders,10 and suicidal ideation.11 Although many of the apps evaluated in these studies are not available to the public, or still require large-scale assessment before they are ready for mainstream clinical care, this research demonstrates that mental health apps can help improve treatment outcomes. As this research develops, a wave of evidence-based and effective mental health apps may be available in the near future.
Although it is unknown how many patients are presently using mental health apps, there is strong anecdotal evidence that an increasing number of patients who use these apps and other forms of digital technology are finding some benefits. In many cases, patients may actually be ahead of the research. For example, one study that conducted an online survey of patients with schizophrenia noted that some patients are using their smartphones to play music to help block auditory hallucinations.12
Why online reviews are of limited use
As this evidence continues to mature, and with an ever-growing number of mental health apps available on commercial marketplaces, busy psychiatrists need to navigate this complex space. Even psychiatrists who decide to not use apps as part of care still need to be knowledgeable about them, because patients are likely to ask about the benefits of using apps, and they will expect an informed response. How would you reply if your patient asked you about a new mood-tracking app he or she recently heard about? On what would you base your recommendation and opinion?
Reading online app reviews for guidance is not a good solution. A recent study found little relationship between the star ratings of health apps and the quality of those apps,13 which suggests that a 5-star rating on the app store is of limited use.
Unlike medications whose ingredients do not change over time, or manualized psychotherapies that use specific protocols, mental health apps are dynamic and constantly changing.14 Think of how often the apps on your smartphone update. Thus, the version of a mental health app that your patient downloads today may be very different from the version that received a favorable user review last month. And just as there is no single medication or therapy that is ideal for every patient, neither is there a single “best” app for all patients with the same disorder. Picking an app is a personal decision that cannot be made based on a single score or numeric rating. Furthermore, the validity of app rating systems is unclear. One study found a wide variation in the interrater reliability of measures used to evaluate apps from sources that included PsyberGuide, the Anxiety and Depression Association of America, and the research literature. Quality measures such as effectiveness, ease of use, and performance had relatively poor interrater reliability.15 This means that, for example, an app that one patient finds “easy to use” may be difficult to use for another. Thus, providing patients with suggestions based on an app’s ratings may result in providing information that sounds useful, but often is misleading.
A model for evaluating apps
One possible solution is a risk-based and personalized assessment approach to evaluating mental health apps. Although it does not offer scoring or recommendations of specific apps, the American Psychiatric Association (APA) App Evaluation Model (Figure) provides a framework to guide discussion and informed decision-making about apps. (The authors of this article helped create this model, but receive no compensation for that volunteer work.) The pyramid shape reflects the hierarchical nature of the model. To begin the process, start at the base of the pyramid and work upward.

Ground. First, consider the context of the app by determining basic facts, such as who made it, how much it costs, and its technology requirements. This ground layer establishes the credibility of the app’s creator by questioning his or her reputation, ability to update the app, and funding sources. Understanding the app’s business model also will help you determine whether the app will stand the test of time: Will it continue to exist next month or next year, or will a lack of reliable funding lead the vendor to abandon it?
Risk. The next layer assesses the risk, privacy, and security features of the app. Many mental health apps actively aim to avoid falling under the jurisdiction of U.S. federal health care privacy rules, such as the Health Insurance Portability and Accountability Act of 1996, so there is no guarantee that sensitive data supplied to an app will be protected. The true cost of a “free” app often is your patient’s personal mental health information, which the app’s developer may accumulate and sell for profit. Thus, it is wise to check the privacy policy to learn where your patient’s data goes. Furthermore, patients and psychiatrists must be vigilant that malware-infected apps can be uploaded to the app store, which can further compromise privacy.16 You may be surprised to learn that many apps lack a privacy policy, which means there are no protections for personal information or safeguards against the misuse of mental health data.17 Checking that an app at least promises to digitally protect mental health data through encryption and secure storage also is a good step.
The goal of considering these factors is not to create a score, but rather to be aware of them and consider them in the context of the specific app, patient, and clinical situation. Doing so helps determine whether the app meets the appropriate risk, privacy, and security standards for your patient.
Evidence. The next layer of the evaluation framework is evidence. The goal is to seek an app with clinical evidence of effectiveness. Simply put, if a patient is going to use an app, he should use one that works. An app without formal evidence may be effective, but it is important to make sure the patient is aware that these claims have not been verified. Many apps claim that they offer cognitive-behavioral therapy or mindfulness therapy, but few deliver on such claims.18 It is wise to try an app before recommending it to a patient to ensure that it does what it claims it does, and does not offer dangerous or harmful recommendations.
Ease of use. Across all health apps, there is growing recognition that most downloaded apps are never used. Patient engagement with mental health apps appears to rapidly decline over the first week of use.19 There also is emerging evidence that many apps are not user-friendly. A recent study of several common mood-tracking apps found that patients with depression had difficulty entering and accessing their data.20 Because many psychiatric disorders are chronic or last at least several months, it is especially important to consider how engaging and usable the app will be for your patient. Usability varies from patient to patient, so it is best to check directly with your patient regarding his comfort with apps and mobile technology. Offering check-ins and support to help patients keep on track with apps may be critical for successful outcomes.
Interoperability. The final layer of the model is data sharing and interoperability. It is important to determine if the data collected or generated by the app are available to you, the patient, the treatment team, and others involved in the patient’s care. As mental health treatment moves toward integrated care, apps that fragment care (by not sharing information) impede care. Check if the app can share data with an electronic medical record, or if there is a plan to review and act on data from the app as part of your patient’s treatment plan.
More information about the APA App Evaluation Model, including additional factors to consider within each layer, is available from the APA for free at https://www.psychiatry.org/psychiatrists/practice/mental-health-apps/app-evaluation-model. For a sample of factors to consider when evaluating a mental health app, see the Table.
A reasonable strategy
Although the APA App Evaluation Model does not endorse any particular app, it can help guide more informed decision-making. As the evidence on mental health apps continues to evolve, it will become easier to make definitive statements on what constitutes a useful app. For now, the best strategy when discussing mental health apps with patients is to combine the use of this model with your clinical judgment.
1. Torous J, Roberts LW. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
2. Mani M, Kavanagh DJ, Hides L, et al. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3(3):e82. doi: 10.2196/mhealth.4328.
3. Wilson H, Stoyanov SR, Gandabhai S, et al. The quality and accuracy of mobile apps to prevent driving after drinking alcohol. JMIR Mhealth Uhealth. 2016;4(3):e98. doi: 10.2196/mhealth.5961.
4. Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PLoS One. 2016;11(4):e0152285. doi: 10.1371/journal.pone.0152285.
5. Nicholas J, Larsen ME, Proudfoot J, et al. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198. doi: 10.2196/jmir.4581.
6. Shen N, Levitan MJ, Johnson A, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713.
7. Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498-504.
8. Ben-Zeev D, Brenner CJ, Begale M, et al. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244-1253.
9. Mohr DC, Tomasino KN, Lattie EG, et al. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017;19(1):e10. doi: 10.2196/jmir.6645.
10. Tighe J, Shand F, Ridani R, et al. Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open. 2017;7(1):e013518. doi: 10.1136/bmjopen-2016-013518.
11. Firth J, Torous J, Nicholas J, et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017;218:15-22.
12. Gay K, Torous J, Joseph A, et al. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Mental Health. 2016;3(2):e15. doi: 10.2196/mental.5379.
13. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood). 2016;35(12):2310-2318.
14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth. 2016;4(3):e96. doi: 10.2196/mhealth.6020.
15. Powell AC, Torous J, Chan S, et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR Mhealth Uhealth. 2016;4(1):e15. doi: 10.2196/mhealth.5176.
16. Ducklin P. Apple’s XcodeGhost malware still in the machine…. https://nakedsecurity.sophos.com/2015/11/09/apples-xcodeghost-malware-still-in-the-machine. Published November 9, 2015. Accessed May 11, 2017.
17. Rosenfeld L, Torous J, Vahia IV. Data security and privacy in apps for dementia: an analysis of existing privacy policies. Am J Geriatr Psychiatry. 2017;25(8):873-877.
18. Torous J, Levin ME, Ahern DK, et al. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215-225.
19. Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Ment Health. 2015;2(1):e7. doi: 10.2196/mental.3935.
20. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med. 2016;31(12):1417-1426.
1. Torous J, Roberts LW. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
2. Mani M, Kavanagh DJ, Hides L, et al. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3(3):e82. doi: 10.2196/mhealth.4328.
3. Wilson H, Stoyanov SR, Gandabhai S, et al. The quality and accuracy of mobile apps to prevent driving after drinking alcohol. JMIR Mhealth Uhealth. 2016;4(3):e98. doi: 10.2196/mhealth.5961.
4. Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PLoS One. 2016;11(4):e0152285. doi: 10.1371/journal.pone.0152285.
5. Nicholas J, Larsen ME, Proudfoot J, et al. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198. doi: 10.2196/jmir.4581.
6. Shen N, Levitan MJ, Johnson A, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713.
7. Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498-504.
8. Ben-Zeev D, Brenner CJ, Begale M, et al. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244-1253.
9. Mohr DC, Tomasino KN, Lattie EG, et al. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017;19(1):e10. doi: 10.2196/jmir.6645.
10. Tighe J, Shand F, Ridani R, et al. Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open. 2017;7(1):e013518. doi: 10.1136/bmjopen-2016-013518.
11. Firth J, Torous J, Nicholas J, et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017;218:15-22.
12. Gay K, Torous J, Joseph A, et al. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Mental Health. 2016;3(2):e15. doi: 10.2196/mental.5379.
13. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood). 2016;35(12):2310-2318.
14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth. 2016;4(3):e96. doi: 10.2196/mhealth.6020.
15. Powell AC, Torous J, Chan S, et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR Mhealth Uhealth. 2016;4(1):e15. doi: 10.2196/mhealth.5176.
16. Ducklin P. Apple’s XcodeGhost malware still in the machine…. https://nakedsecurity.sophos.com/2015/11/09/apples-xcodeghost-malware-still-in-the-machine. Published November 9, 2015. Accessed May 11, 2017.
17. Rosenfeld L, Torous J, Vahia IV. Data security and privacy in apps for dementia: an analysis of existing privacy policies. Am J Geriatr Psychiatry. 2017;25(8):873-877.
18. Torous J, Levin ME, Ahern DK, et al. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215-225.
19. Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Ment Health. 2015;2(1):e7. doi: 10.2196/mental.3935.
20. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med. 2016;31(12):1417-1426.
Neuromodulatory options for treatment-resistant depression
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.
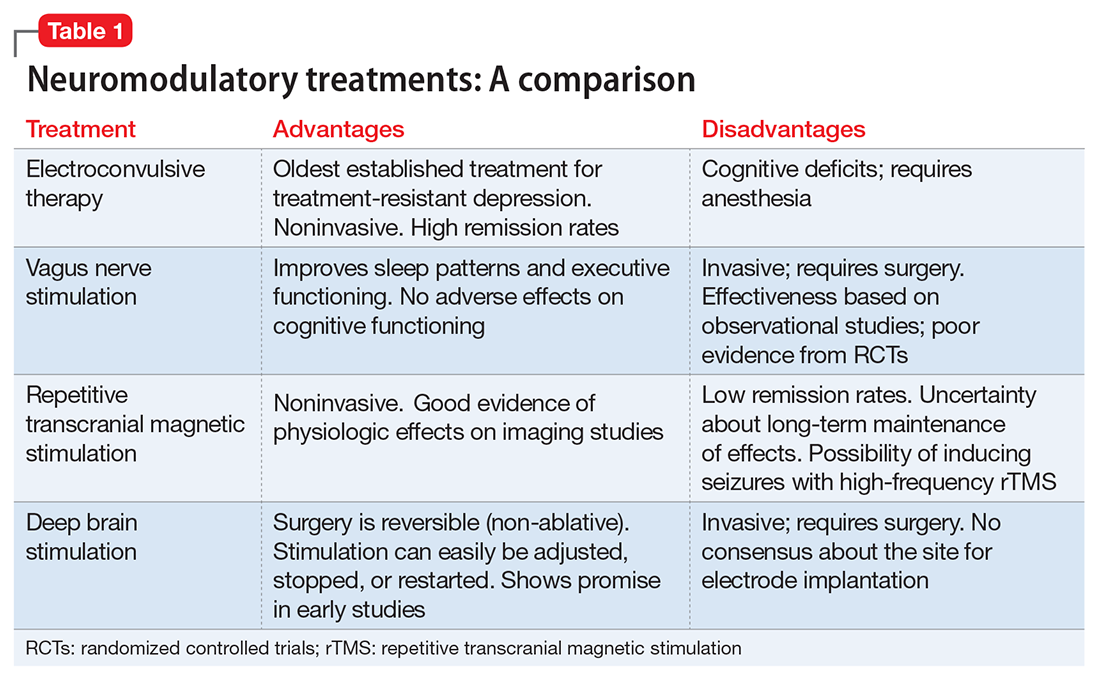
Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6
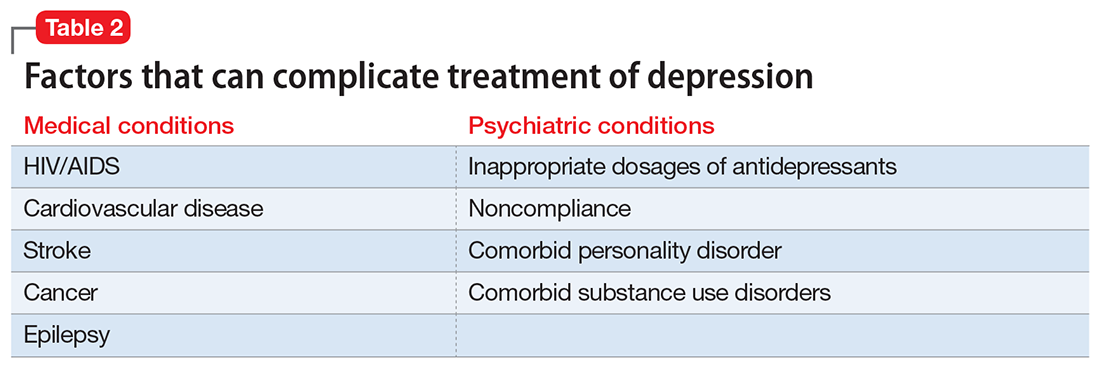
Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11
Some earlier trials found bilateral ECT to be more effective than unilateral ECT.12 Recent studies suggest that high-dose unilateral ECT (6 times the seizure threshold) is as effective as bilateral ECT.13 Studies have shown no significant differences in efficacy or treatment outcomes between twice- and thrice-weekly ECT regimens. Some studies suggest that twice-weekly ECT may be associated with a lower risk of short-term cognitive impairment compared with thrice-weekly ECT.14
In highly refractory cases, the effects of ECT can be augmented by using pre-treatment strategies such as hyperventilation, which may increase the duration of the seizure, and remifentanil, which helps reduce the anticonvulsant effect of agents used for anesthesia.15 Advanced age, psychotic features, resistance to pharmacotherapy, and comorbid personality disorders predict poor response to ECT.16
Adverse effects. Concerns about cognitive deficits secondary to ECT may curtail its use. Retrograde and anterograde amnesia are the most common deficits observed acutely after ECT.12 Other commonly affected cognitive functions include processing speed, attention/working memory, verbal and visual episodic memory, spatial problem solving, and executive functioning. The specific patterns of these deficits (in terms of duration and severity) vary between studies. In general, high-dose, thrice-weekly ECT and bilateral ECT are associated with greater cognitive deficits, whereas twice-weekly ECT and unilateral ECT are associated with a lower risk of cognitive adverse effects.12 A recent meta-analysis by Semkovska and McLoughlin17 found that most cognitive deficits seen after ECT are limited to the first 3 days after treatment. The authors of this meta-analysis concluded that these impairments improve over time and approach baseline 2 weeks after treatment. In fact, some of these impairments (processing speed, working memory, anterograde memory, and some aspects of executive function) improved beyond baseline after 15 days of treatment.17 The need for anesthesia and associated potential adverse effects also are a cause of concern with ECT.
Combining ECT with medication. Several patient-specific factors, including medication regimen and comorbid medical conditions, need to be considered before using ECT in combination with pharmacotherapy. Although most antipsychotics are safe to use with ECT, concomitant use of agents with higher antihistaminic properties may increase the risk of delirium. The risk of delirium also is increased with the use of anticonvulsants and mood stabilizers (eg, lithium) because these agents increase the seizure threshold. The potential for drug interactions may affect the choice of the anesthetic agents. Also, SSRIs and serotonin-norepinephrine reuptake inhibitors can increase the duration of induced seizures.18
Vagus nerve stimulation
VNS, in which an implanted device stimulates the vagus nerve with electrical impulses, initially was used to reduce the frequency of seizures in patients with epilepsy and treatment-resistant partial onset seizures.19 VNS was FDA-approved for TRD in July 2005.20 One VNS system, the NCP System, consists of an implantable, multi-programmable generator, known as a pulse generator, that is subcutaneously placed in the anterior chest wall during an outpatient surgical procedure. Separate bipolar nerve-stimulating electrodes are surgically wrapped around the left cervical vagus nerve, and then connected to the generator via a tunneling procedure. A telemetric wand is subsequently linked to a portable computer and used to adjust stimulation parameters.21,22
Support for using VNS for TRD came from a multitude of investigations and observations. Harden et al23 and Elger et al24 prospectively evaluated epileptic patients with standard depression symptom severity rating scales. They found that VNS was associated with statistically significant improvements in mood that were not related to reductions in seizures.23,24
The mechanism of action of VNS is not clear. Earlier researchers had found evidence that VNS affected brain regions associated with norepinephrine25 and serotonin systems26; both of these neurotransmitters have been implicated in the pathophysiology of depression. Positron emission tomography studies conducted during VNS treatment of epilepsy showed metabolic changes in cortical and subcortical areas of the brain, including the amygdala, hippocampus, and cingulate gyrus, all structures implicated in the pathophysiology of mood disorders.27
Most studies conducted to evaluate the efficacy of VNS have been observational, looking at depression ratings before and after treatment with VNS. The short-term studies measured the difference in depression rating scales at baseline and after 10 weeks of treatment. In most of these studies, treatment with VNS resulted in a statistically significant drop in depression rating scales scores, such as on the Hamilton Depression Rating Scale (HAM-D). Based on the study design and number of study participants, response rates have varied from 13%28 to 40%,29 whereas remission rates have varied from 15.3%30 to 28%.31 More than one-half of the reduction in symptoms occurred after 6 weeks of treatment.30 In longer-term follow-up studies, the antidepressant effect generally was sustained over time. Response rates remained essentially unchanged, but the remission rates increased to approximately 29%.29 Only 1 RCT has compared patients with controls; it found no significant differences in the response or remission rates between active VNS and sham VNS.32 In this study, all patients had VNS implanted, but in the control group, the VNS was never turned on.32 In a meta-analysis conducted by Martin and Martín-Sánchez,33 31.8% (95% confidence interval [CI], 23.2% to 41.8%; P < .001) of patients treated with VNS had a significant reduction in HAM-D scores. The response rate in patients with TRD ranged from 27% to 37% and the remission rate was approximately 13%. In studies that followed patients over longer periods, both the remission and response rates increased over time.34
Recent evidence suggests that the effectiveness of VNS may depend on the stimulation level. A multi-center double-blind study randomized patients to receive either a low (0.25 mA current, 130-millisecond pulse width), medium (0.5e1.0 mA, 250 millisecond), or high (1.25e1.5 mA, 250 millisecond) dose of VNS.35 Although all dose levels were associated with improvement in symptoms, a statistically significant durability in response was associated with the medium- and high-dose treatments.
Adverse effects. VNS has no major adverse effects on cognitive functioning, and some studies have found improvement in executive functioning that corresponded to improvement in depressive symptoms.30 VNS also may result in improved sleep patterns as evidenced by EEG changes.31 The most commonly reported adverse effects include pain in the incision site, hoarseness of voice, throat pain, and neck pain.36
Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that uses high-intensity magnetic impulses to stimulate cortical neurons. A magnetic field is produced when current passes through a coil, which in turn causes electrical stimulation in the cortical neurons that results in transient changes in the excitability of the cortical neurons.37 Although many stimulation parameters exist for TMS, high-frequency stimulation to the left prefrontal cortex (HFL-rTMS) and low-frequency stimulation to the right prefrontal cortex (LFR-rTMS) have been shown most efficacious for treating depression.38 High-frequency (5 Hz to 20 Hz) stimulation using rTMS increases cortical neuron excitability, whereas low-frequency (approximately 1 Hz) is associated with reduced cortical neuron excitability.39 The choice of targeting the DLPFC stems from a large body of functional neuroimaging studies that have shown reduction in activity/blood flow in the left DLPFC and abnormal activity/blood flow in the right DLPFC.40
There is no dearth of RCTs evaluating the efficacy of rTMS vs sham rTMS (where no magnetic stimulation was provided). In a meta-analysis of 8 RCTs, low-frequency rTMS applied to the right DLPFC was associated with a remission rate of approximately 34.6%, compared with a 9.7% remission rate with sham rTMS.41 A response rate of approximately 38.2% was observed with HFL-rTMS, compared with a response rate of 15.1% for sham rTMS.41
Gaynes et al42 conducted a meta-analysis to determine the efficacy of rTMS in TRD. They found that for patients with TRD, rTMs produced a response rate of 29% and a remission rate of 30%. In long-term, naturalistic, observational studies, the response rates and remission rates were much higher (58% and 37.1%, respectively).43 Over a 1-year follow-up, almost two-thirds of patients continued to meet criteria for response to treatment.44 Trials comparing HFL-rTMS and LFR-rTMS have found no significant differences in efficacy.45
Advanced age, psychotic symptoms, and a longer duration of the current depressive episode predict poor response to rTMS. Also, imaging studies have shown that a lower metabolism in cerebellar, temporal, anterior cingulate, and occipital parts of the brain correlate with better response to HFL-rTMS.46,47
Adverse effects. The major adverse effect associated with rTMS is the risk of inducing seizures, which is more commonly associated with high-frequency rTMS. Other common adverse effects include headache, facial muscle twitching, and tinnitus.37
Deep brain stimulation
DBS is an invasive stereotactic surgical procedure. It involves unilateral or bilateral placement of electrodes at neuroanatomical locations to deliver continuous stimulation from a subcutaneously implanted pulse generator.48 In the past, destructive surgical procedures were used to treat intractable depression. Surgeries such as anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy have been shown to effectively reduce depressive symptoms.49 The advantages of DBS over destructive procedures include the fact that DBS is reversible and that the stimulation levels can easily be adjusted, and the treatment can easily be stopped or restarted.
There is no consensus on the optimal anatomic locations for the electrode implantation in DBS. Electrodes have been implanted in the subcallosal cingulate gyrus, inferior thalamic peduncle, ventral capsule/ventral striatum, superolateral branch of the medial forebrain bundle (MFB), and nucleus accumbens.
The choice of anatomic locations stems from the large body of neuroimaging literature characterizing functional changes associated with acute depression and response to treatment. The electrode placement targets “nodes” that form an integral part of the affected neural circuits that are responsible for regulating depressive symptoms.50 Increased metabolic activity and blood flow to the subgenual cingulate gyrus and reduction in the blood flow to the DLPFC and the striatum have been associated with active depressed states. Response to antidepressant treatment has been associated with reversal of these findings.51 Functional magnetic resonance imaging studies have consistently shown increased activity in the amygdala in response to negative stimuli among patients with depression.
Regardless of the site of electrode placement, studies have reported symptomatic improvement among patients with depression who are treated with DBS. In 2 case reports, the electrode was implanted in the inferior thalamic peduncle.52,53 Each study had 1 participant, and each patient remitted.52,53
Placement of the electrodes in the nucleus accumbens resulted in a response rate of 45% in 1 study,54 whereas in a different study, all patients reported improvement in anhedonia.55 A response rate of 71% and a remission rate of 35% were observed in a study in which the electrode was implanted in the ventral capsule/ventral striatum area.56
Berlim et al57 published a systematic review and exploratory meta-analysis of studies in which the electrode had been implanted in the subgenual cingulate cortex. At 12 months, the response rate was 39.9% (95% CI, 28.4% to 52.8%), and 26.3% (95% CI, 13% to 45.9%) of patients achieved remission. The most significant drop in depression scores was observed 3 to 6 months after the surgery. No significant change in scores was observed between 6 to 12 months after surgery.57
The MFB, specifically the superolateral branch, is emerging as an exciting new target for electrode placement in DBS. Schlaepfer et al58 studied the effects of electrodes implanted bilaterally in the superolateral branch of the MFB. They observed an almost 50% reduction in symptoms by Day 7, and at the last follow-up visit (12 to 33 weeks) 4 of the 6 patients had achieved remission.58 In a recent systematic review, Gálvez et al59 found most studies had high response/remission rates without any significant adverse effects. In a recent study of DBS targeting the MFB, 3 of 4 patients had a >50% reduction in Montgomery-Åsberg Depression Rating Scale scores at the end of first week. Although 1 patient withdrew, 2 of the other 3 patients continued to report a >80% reduction in depressive symptoms, even at Week 26.60
Accurate localization of target areas (white matter tracts) and subsequent electrode placement might be an important factor governing treatment response. Riva-Posse et al61 found that clinical response was seen when the electrodes stimulated 3 specific white matter bundles. Interestingly, nonresponders were converted to responders simply by changing the position of the electrodes to include these white matter tracts.61
Adverse effects. The most common adverse effects noted during studies of DBS include pain at the site of implantation and wound infection. Other adverse effects include lead fracture, transient dysphagia, and other hardware-related problems.49
Sorting out the evidence
In the absence of head-to-head trials, it is difficult to establish a hierarchal algorithm for use of the 4 neuromodulatory treatments discussed in the article. If we were to base our decision solely on the current literature, ECT by far has the most evidence and highest remission rates.11 We can reduce the risk of cognitive deficits by using twice-weekly instead of thrice-weekly ECT, or by using unilateral instead of bilateral ECT.12 Another strategy for reducing adverse effects associated with long-term maintenance ECT is by using it in combination with VNS. ECT and VNS can be used safely concomitantly; ECT can be used to treat acutely worsening depression, and VNS for maintaining the antidepressant effect.62
Aside from ECT, rTMS is the only other treatment that has evidence from RCTs. Although the remission rates are not as high as ECT, its preferable adverse effects profile, noninvasive nature, and comparative low cost (compared with surgical procedures) make it a favorable choice. The Canadian Network for Mood and Anxiety Treatment guidelines suggest rTMS as the first-line treatment for patients who do not respond to pharmacologic treatments.63 ECT can be considered second-line treatment unless the patient has acute suicidal ideation, catatonia, psychotic features, greater treatment resistance, or physical deterioration, in which case ECT should be tried before TMS.63
Among the invasive options, VNS has more evidence and is FDA-approved for TRD. However, DBS has shown great promise in early studies, with remission rates as high as 35%.56 DBS has the advantage of being reversible, and the amount of stimulation can be adjusted easily. Despite early promise, more research is needed before DBS can be widely used in clinical settings.
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
3. Petersen T, Papakostas GI, Posternak MA, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336-341.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91.
6. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol. Psychiatry. 2003;54(3):177-180.
7. Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577-579.
8. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
9. Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27(18):4894-4901.
10. Abbott CC, Gallegos P, Rediske N et al. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33-46.
11. Heijnen WT, Birkenhäger TK, Wierdsma AI, et al. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619.
12. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
13. Semkovska M, Landau S, Dunne R et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. 2016;173(4):408-417.
14. Charlson F, Siskind D, Doi SA, et al. ECT efficacy and treatment course: a systematic review and meta-analysis of twice vs thrice weekly schedules. J Affect Disord. 2012;138(1-2):1-8.
15. Loo CK, Kaill A, Paton P, et al. The difficult-to-treat electroconvulsive therapy patient—strategies for augmenting outcomes. J Affect Disord. 2010;124(3):219-227.
16. de Vreede IM, Burger H, van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(2-3):323-327.
17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
18. Baghai TC, Marcuse A, Brosch M, et al. The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J Biol Psychiatry. 2006;7(2):82-90.
19. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616-626.
20. Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345-1355.
21. Matthews K, Eljamel MS. Vagus nerve stimulation and refractory depression: please can you switch me on doctor? Br J Psychiatry. 2003;183:181-183.
22. George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83.
23. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99.
24. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203-210.
25. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
26. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221-227.
27. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39(9):983-990.
28. O’Keane V, Dinan TG, Scott L, et al. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58(12):963-968.
29. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276-286.
30. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
31. Armitage R, Husain M, Hoffmann R, et al. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54(5):475-482.
32. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347-354.
33. Martin JL, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155.
34. Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83-93.
35. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631-640.
36. Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15.
37. Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241-258.
38. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621-631.
39. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-2596.
40. Fitzgerald PB, Oxley TJ, Laird AR, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33-45.
41. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551.
42. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
43. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
44. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014;75(12):1394-1401.
45. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234.
46. Dumas R, Padovani R, Richieri R, et al. Repetitive transcranial magnetic stimulation in major depression: response factor [in French]. Encephale. 2012;38(4):360-368.
47. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006;9(6):641-654.
48. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510.
49. Taghva AS, Malone DA, Rezai AR. Deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80(3-4):S27.e17-S27.e24.
50. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
51. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
52. Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(pt 2):393-398.
53. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585-593; discussion 585-593.
54. Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110-116.
55. Schlaepfer TE, Bewernick BH, Kayser S, et al. Deep brain stimulation of the human reward system for major depression—rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303-1314.
56. Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
57. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
58. Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204-1212.
59. Gálvez JF, Keser Z, Mwangi B, et al. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:59-70.
60. Fenoy AJ, Schulz P, Selvaraj. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143-151.
61. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969.
62. Burke MJ, Husain MM. Concomitant use of vagus nerve stimulation and electroconvulsive therapy for treatment-resistant depression. J ECT. 2006;22(3):218-222.
63. Milev R V, Giacobbe P, Kennedy SH, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561-575.
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.

Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6

Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11
Some earlier trials found bilateral ECT to be more effective than unilateral ECT.12 Recent studies suggest that high-dose unilateral ECT (6 times the seizure threshold) is as effective as bilateral ECT.13 Studies have shown no significant differences in efficacy or treatment outcomes between twice- and thrice-weekly ECT regimens. Some studies suggest that twice-weekly ECT may be associated with a lower risk of short-term cognitive impairment compared with thrice-weekly ECT.14
In highly refractory cases, the effects of ECT can be augmented by using pre-treatment strategies such as hyperventilation, which may increase the duration of the seizure, and remifentanil, which helps reduce the anticonvulsant effect of agents used for anesthesia.15 Advanced age, psychotic features, resistance to pharmacotherapy, and comorbid personality disorders predict poor response to ECT.16
Adverse effects. Concerns about cognitive deficits secondary to ECT may curtail its use. Retrograde and anterograde amnesia are the most common deficits observed acutely after ECT.12 Other commonly affected cognitive functions include processing speed, attention/working memory, verbal and visual episodic memory, spatial problem solving, and executive functioning. The specific patterns of these deficits (in terms of duration and severity) vary between studies. In general, high-dose, thrice-weekly ECT and bilateral ECT are associated with greater cognitive deficits, whereas twice-weekly ECT and unilateral ECT are associated with a lower risk of cognitive adverse effects.12 A recent meta-analysis by Semkovska and McLoughlin17 found that most cognitive deficits seen after ECT are limited to the first 3 days after treatment. The authors of this meta-analysis concluded that these impairments improve over time and approach baseline 2 weeks after treatment. In fact, some of these impairments (processing speed, working memory, anterograde memory, and some aspects of executive function) improved beyond baseline after 15 days of treatment.17 The need for anesthesia and associated potential adverse effects also are a cause of concern with ECT.
Combining ECT with medication. Several patient-specific factors, including medication regimen and comorbid medical conditions, need to be considered before using ECT in combination with pharmacotherapy. Although most antipsychotics are safe to use with ECT, concomitant use of agents with higher antihistaminic properties may increase the risk of delirium. The risk of delirium also is increased with the use of anticonvulsants and mood stabilizers (eg, lithium) because these agents increase the seizure threshold. The potential for drug interactions may affect the choice of the anesthetic agents. Also, SSRIs and serotonin-norepinephrine reuptake inhibitors can increase the duration of induced seizures.18
Vagus nerve stimulation
VNS, in which an implanted device stimulates the vagus nerve with electrical impulses, initially was used to reduce the frequency of seizures in patients with epilepsy and treatment-resistant partial onset seizures.19 VNS was FDA-approved for TRD in July 2005.20 One VNS system, the NCP System, consists of an implantable, multi-programmable generator, known as a pulse generator, that is subcutaneously placed in the anterior chest wall during an outpatient surgical procedure. Separate bipolar nerve-stimulating electrodes are surgically wrapped around the left cervical vagus nerve, and then connected to the generator via a tunneling procedure. A telemetric wand is subsequently linked to a portable computer and used to adjust stimulation parameters.21,22
Support for using VNS for TRD came from a multitude of investigations and observations. Harden et al23 and Elger et al24 prospectively evaluated epileptic patients with standard depression symptom severity rating scales. They found that VNS was associated with statistically significant improvements in mood that were not related to reductions in seizures.23,24
The mechanism of action of VNS is not clear. Earlier researchers had found evidence that VNS affected brain regions associated with norepinephrine25 and serotonin systems26; both of these neurotransmitters have been implicated in the pathophysiology of depression. Positron emission tomography studies conducted during VNS treatment of epilepsy showed metabolic changes in cortical and subcortical areas of the brain, including the amygdala, hippocampus, and cingulate gyrus, all structures implicated in the pathophysiology of mood disorders.27
Most studies conducted to evaluate the efficacy of VNS have been observational, looking at depression ratings before and after treatment with VNS. The short-term studies measured the difference in depression rating scales at baseline and after 10 weeks of treatment. In most of these studies, treatment with VNS resulted in a statistically significant drop in depression rating scales scores, such as on the Hamilton Depression Rating Scale (HAM-D). Based on the study design and number of study participants, response rates have varied from 13%28 to 40%,29 whereas remission rates have varied from 15.3%30 to 28%.31 More than one-half of the reduction in symptoms occurred after 6 weeks of treatment.30 In longer-term follow-up studies, the antidepressant effect generally was sustained over time. Response rates remained essentially unchanged, but the remission rates increased to approximately 29%.29 Only 1 RCT has compared patients with controls; it found no significant differences in the response or remission rates between active VNS and sham VNS.32 In this study, all patients had VNS implanted, but in the control group, the VNS was never turned on.32 In a meta-analysis conducted by Martin and Martín-Sánchez,33 31.8% (95% confidence interval [CI], 23.2% to 41.8%; P < .001) of patients treated with VNS had a significant reduction in HAM-D scores. The response rate in patients with TRD ranged from 27% to 37% and the remission rate was approximately 13%. In studies that followed patients over longer periods, both the remission and response rates increased over time.34
Recent evidence suggests that the effectiveness of VNS may depend on the stimulation level. A multi-center double-blind study randomized patients to receive either a low (0.25 mA current, 130-millisecond pulse width), medium (0.5e1.0 mA, 250 millisecond), or high (1.25e1.5 mA, 250 millisecond) dose of VNS.35 Although all dose levels were associated with improvement in symptoms, a statistically significant durability in response was associated with the medium- and high-dose treatments.
Adverse effects. VNS has no major adverse effects on cognitive functioning, and some studies have found improvement in executive functioning that corresponded to improvement in depressive symptoms.30 VNS also may result in improved sleep patterns as evidenced by EEG changes.31 The most commonly reported adverse effects include pain in the incision site, hoarseness of voice, throat pain, and neck pain.36
Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that uses high-intensity magnetic impulses to stimulate cortical neurons. A magnetic field is produced when current passes through a coil, which in turn causes electrical stimulation in the cortical neurons that results in transient changes in the excitability of the cortical neurons.37 Although many stimulation parameters exist for TMS, high-frequency stimulation to the left prefrontal cortex (HFL-rTMS) and low-frequency stimulation to the right prefrontal cortex (LFR-rTMS) have been shown most efficacious for treating depression.38 High-frequency (5 Hz to 20 Hz) stimulation using rTMS increases cortical neuron excitability, whereas low-frequency (approximately 1 Hz) is associated with reduced cortical neuron excitability.39 The choice of targeting the DLPFC stems from a large body of functional neuroimaging studies that have shown reduction in activity/blood flow in the left DLPFC and abnormal activity/blood flow in the right DLPFC.40
There is no dearth of RCTs evaluating the efficacy of rTMS vs sham rTMS (where no magnetic stimulation was provided). In a meta-analysis of 8 RCTs, low-frequency rTMS applied to the right DLPFC was associated with a remission rate of approximately 34.6%, compared with a 9.7% remission rate with sham rTMS.41 A response rate of approximately 38.2% was observed with HFL-rTMS, compared with a response rate of 15.1% for sham rTMS.41
Gaynes et al42 conducted a meta-analysis to determine the efficacy of rTMS in TRD. They found that for patients with TRD, rTMs produced a response rate of 29% and a remission rate of 30%. In long-term, naturalistic, observational studies, the response rates and remission rates were much higher (58% and 37.1%, respectively).43 Over a 1-year follow-up, almost two-thirds of patients continued to meet criteria for response to treatment.44 Trials comparing HFL-rTMS and LFR-rTMS have found no significant differences in efficacy.45
Advanced age, psychotic symptoms, and a longer duration of the current depressive episode predict poor response to rTMS. Also, imaging studies have shown that a lower metabolism in cerebellar, temporal, anterior cingulate, and occipital parts of the brain correlate with better response to HFL-rTMS.46,47
Adverse effects. The major adverse effect associated with rTMS is the risk of inducing seizures, which is more commonly associated with high-frequency rTMS. Other common adverse effects include headache, facial muscle twitching, and tinnitus.37
Deep brain stimulation
DBS is an invasive stereotactic surgical procedure. It involves unilateral or bilateral placement of electrodes at neuroanatomical locations to deliver continuous stimulation from a subcutaneously implanted pulse generator.48 In the past, destructive surgical procedures were used to treat intractable depression. Surgeries such as anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy have been shown to effectively reduce depressive symptoms.49 The advantages of DBS over destructive procedures include the fact that DBS is reversible and that the stimulation levels can easily be adjusted, and the treatment can easily be stopped or restarted.
There is no consensus on the optimal anatomic locations for the electrode implantation in DBS. Electrodes have been implanted in the subcallosal cingulate gyrus, inferior thalamic peduncle, ventral capsule/ventral striatum, superolateral branch of the medial forebrain bundle (MFB), and nucleus accumbens.
The choice of anatomic locations stems from the large body of neuroimaging literature characterizing functional changes associated with acute depression and response to treatment. The electrode placement targets “nodes” that form an integral part of the affected neural circuits that are responsible for regulating depressive symptoms.50 Increased metabolic activity and blood flow to the subgenual cingulate gyrus and reduction in the blood flow to the DLPFC and the striatum have been associated with active depressed states. Response to antidepressant treatment has been associated with reversal of these findings.51 Functional magnetic resonance imaging studies have consistently shown increased activity in the amygdala in response to negative stimuli among patients with depression.
Regardless of the site of electrode placement, studies have reported symptomatic improvement among patients with depression who are treated with DBS. In 2 case reports, the electrode was implanted in the inferior thalamic peduncle.52,53 Each study had 1 participant, and each patient remitted.52,53
Placement of the electrodes in the nucleus accumbens resulted in a response rate of 45% in 1 study,54 whereas in a different study, all patients reported improvement in anhedonia.55 A response rate of 71% and a remission rate of 35% were observed in a study in which the electrode was implanted in the ventral capsule/ventral striatum area.56
Berlim et al57 published a systematic review and exploratory meta-analysis of studies in which the electrode had been implanted in the subgenual cingulate cortex. At 12 months, the response rate was 39.9% (95% CI, 28.4% to 52.8%), and 26.3% (95% CI, 13% to 45.9%) of patients achieved remission. The most significant drop in depression scores was observed 3 to 6 months after the surgery. No significant change in scores was observed between 6 to 12 months after surgery.57
The MFB, specifically the superolateral branch, is emerging as an exciting new target for electrode placement in DBS. Schlaepfer et al58 studied the effects of electrodes implanted bilaterally in the superolateral branch of the MFB. They observed an almost 50% reduction in symptoms by Day 7, and at the last follow-up visit (12 to 33 weeks) 4 of the 6 patients had achieved remission.58 In a recent systematic review, Gálvez et al59 found most studies had high response/remission rates without any significant adverse effects. In a recent study of DBS targeting the MFB, 3 of 4 patients had a >50% reduction in Montgomery-Åsberg Depression Rating Scale scores at the end of first week. Although 1 patient withdrew, 2 of the other 3 patients continued to report a >80% reduction in depressive symptoms, even at Week 26.60
Accurate localization of target areas (white matter tracts) and subsequent electrode placement might be an important factor governing treatment response. Riva-Posse et al61 found that clinical response was seen when the electrodes stimulated 3 specific white matter bundles. Interestingly, nonresponders were converted to responders simply by changing the position of the electrodes to include these white matter tracts.61
Adverse effects. The most common adverse effects noted during studies of DBS include pain at the site of implantation and wound infection. Other adverse effects include lead fracture, transient dysphagia, and other hardware-related problems.49
Sorting out the evidence
In the absence of head-to-head trials, it is difficult to establish a hierarchal algorithm for use of the 4 neuromodulatory treatments discussed in the article. If we were to base our decision solely on the current literature, ECT by far has the most evidence and highest remission rates.11 We can reduce the risk of cognitive deficits by using twice-weekly instead of thrice-weekly ECT, or by using unilateral instead of bilateral ECT.12 Another strategy for reducing adverse effects associated with long-term maintenance ECT is by using it in combination with VNS. ECT and VNS can be used safely concomitantly; ECT can be used to treat acutely worsening depression, and VNS for maintaining the antidepressant effect.62
Aside from ECT, rTMS is the only other treatment that has evidence from RCTs. Although the remission rates are not as high as ECT, its preferable adverse effects profile, noninvasive nature, and comparative low cost (compared with surgical procedures) make it a favorable choice. The Canadian Network for Mood and Anxiety Treatment guidelines suggest rTMS as the first-line treatment for patients who do not respond to pharmacologic treatments.63 ECT can be considered second-line treatment unless the patient has acute suicidal ideation, catatonia, psychotic features, greater treatment resistance, or physical deterioration, in which case ECT should be tried before TMS.63
Among the invasive options, VNS has more evidence and is FDA-approved for TRD. However, DBS has shown great promise in early studies, with remission rates as high as 35%.56 DBS has the advantage of being reversible, and the amount of stimulation can be adjusted easily. Despite early promise, more research is needed before DBS can be widely used in clinical settings.
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.

Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6

Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11
Some earlier trials found bilateral ECT to be more effective than unilateral ECT.12 Recent studies suggest that high-dose unilateral ECT (6 times the seizure threshold) is as effective as bilateral ECT.13 Studies have shown no significant differences in efficacy or treatment outcomes between twice- and thrice-weekly ECT regimens. Some studies suggest that twice-weekly ECT may be associated with a lower risk of short-term cognitive impairment compared with thrice-weekly ECT.14
In highly refractory cases, the effects of ECT can be augmented by using pre-treatment strategies such as hyperventilation, which may increase the duration of the seizure, and remifentanil, which helps reduce the anticonvulsant effect of agents used for anesthesia.15 Advanced age, psychotic features, resistance to pharmacotherapy, and comorbid personality disorders predict poor response to ECT.16
Adverse effects. Concerns about cognitive deficits secondary to ECT may curtail its use. Retrograde and anterograde amnesia are the most common deficits observed acutely after ECT.12 Other commonly affected cognitive functions include processing speed, attention/working memory, verbal and visual episodic memory, spatial problem solving, and executive functioning. The specific patterns of these deficits (in terms of duration and severity) vary between studies. In general, high-dose, thrice-weekly ECT and bilateral ECT are associated with greater cognitive deficits, whereas twice-weekly ECT and unilateral ECT are associated with a lower risk of cognitive adverse effects.12 A recent meta-analysis by Semkovska and McLoughlin17 found that most cognitive deficits seen after ECT are limited to the first 3 days after treatment. The authors of this meta-analysis concluded that these impairments improve over time and approach baseline 2 weeks after treatment. In fact, some of these impairments (processing speed, working memory, anterograde memory, and some aspects of executive function) improved beyond baseline after 15 days of treatment.17 The need for anesthesia and associated potential adverse effects also are a cause of concern with ECT.
Combining ECT with medication. Several patient-specific factors, including medication regimen and comorbid medical conditions, need to be considered before using ECT in combination with pharmacotherapy. Although most antipsychotics are safe to use with ECT, concomitant use of agents with higher antihistaminic properties may increase the risk of delirium. The risk of delirium also is increased with the use of anticonvulsants and mood stabilizers (eg, lithium) because these agents increase the seizure threshold. The potential for drug interactions may affect the choice of the anesthetic agents. Also, SSRIs and serotonin-norepinephrine reuptake inhibitors can increase the duration of induced seizures.18
Vagus nerve stimulation
VNS, in which an implanted device stimulates the vagus nerve with electrical impulses, initially was used to reduce the frequency of seizures in patients with epilepsy and treatment-resistant partial onset seizures.19 VNS was FDA-approved for TRD in July 2005.20 One VNS system, the NCP System, consists of an implantable, multi-programmable generator, known as a pulse generator, that is subcutaneously placed in the anterior chest wall during an outpatient surgical procedure. Separate bipolar nerve-stimulating electrodes are surgically wrapped around the left cervical vagus nerve, and then connected to the generator via a tunneling procedure. A telemetric wand is subsequently linked to a portable computer and used to adjust stimulation parameters.21,22
Support for using VNS for TRD came from a multitude of investigations and observations. Harden et al23 and Elger et al24 prospectively evaluated epileptic patients with standard depression symptom severity rating scales. They found that VNS was associated with statistically significant improvements in mood that were not related to reductions in seizures.23,24
The mechanism of action of VNS is not clear. Earlier researchers had found evidence that VNS affected brain regions associated with norepinephrine25 and serotonin systems26; both of these neurotransmitters have been implicated in the pathophysiology of depression. Positron emission tomography studies conducted during VNS treatment of epilepsy showed metabolic changes in cortical and subcortical areas of the brain, including the amygdala, hippocampus, and cingulate gyrus, all structures implicated in the pathophysiology of mood disorders.27
Most studies conducted to evaluate the efficacy of VNS have been observational, looking at depression ratings before and after treatment with VNS. The short-term studies measured the difference in depression rating scales at baseline and after 10 weeks of treatment. In most of these studies, treatment with VNS resulted in a statistically significant drop in depression rating scales scores, such as on the Hamilton Depression Rating Scale (HAM-D). Based on the study design and number of study participants, response rates have varied from 13%28 to 40%,29 whereas remission rates have varied from 15.3%30 to 28%.31 More than one-half of the reduction in symptoms occurred after 6 weeks of treatment.30 In longer-term follow-up studies, the antidepressant effect generally was sustained over time. Response rates remained essentially unchanged, but the remission rates increased to approximately 29%.29 Only 1 RCT has compared patients with controls; it found no significant differences in the response or remission rates between active VNS and sham VNS.32 In this study, all patients had VNS implanted, but in the control group, the VNS was never turned on.32 In a meta-analysis conducted by Martin and Martín-Sánchez,33 31.8% (95% confidence interval [CI], 23.2% to 41.8%; P < .001) of patients treated with VNS had a significant reduction in HAM-D scores. The response rate in patients with TRD ranged from 27% to 37% and the remission rate was approximately 13%. In studies that followed patients over longer periods, both the remission and response rates increased over time.34
Recent evidence suggests that the effectiveness of VNS may depend on the stimulation level. A multi-center double-blind study randomized patients to receive either a low (0.25 mA current, 130-millisecond pulse width), medium (0.5e1.0 mA, 250 millisecond), or high (1.25e1.5 mA, 250 millisecond) dose of VNS.35 Although all dose levels were associated with improvement in symptoms, a statistically significant durability in response was associated with the medium- and high-dose treatments.
Adverse effects. VNS has no major adverse effects on cognitive functioning, and some studies have found improvement in executive functioning that corresponded to improvement in depressive symptoms.30 VNS also may result in improved sleep patterns as evidenced by EEG changes.31 The most commonly reported adverse effects include pain in the incision site, hoarseness of voice, throat pain, and neck pain.36
Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that uses high-intensity magnetic impulses to stimulate cortical neurons. A magnetic field is produced when current passes through a coil, which in turn causes electrical stimulation in the cortical neurons that results in transient changes in the excitability of the cortical neurons.37 Although many stimulation parameters exist for TMS, high-frequency stimulation to the left prefrontal cortex (HFL-rTMS) and low-frequency stimulation to the right prefrontal cortex (LFR-rTMS) have been shown most efficacious for treating depression.38 High-frequency (5 Hz to 20 Hz) stimulation using rTMS increases cortical neuron excitability, whereas low-frequency (approximately 1 Hz) is associated with reduced cortical neuron excitability.39 The choice of targeting the DLPFC stems from a large body of functional neuroimaging studies that have shown reduction in activity/blood flow in the left DLPFC and abnormal activity/blood flow in the right DLPFC.40
There is no dearth of RCTs evaluating the efficacy of rTMS vs sham rTMS (where no magnetic stimulation was provided). In a meta-analysis of 8 RCTs, low-frequency rTMS applied to the right DLPFC was associated with a remission rate of approximately 34.6%, compared with a 9.7% remission rate with sham rTMS.41 A response rate of approximately 38.2% was observed with HFL-rTMS, compared with a response rate of 15.1% for sham rTMS.41
Gaynes et al42 conducted a meta-analysis to determine the efficacy of rTMS in TRD. They found that for patients with TRD, rTMs produced a response rate of 29% and a remission rate of 30%. In long-term, naturalistic, observational studies, the response rates and remission rates were much higher (58% and 37.1%, respectively).43 Over a 1-year follow-up, almost two-thirds of patients continued to meet criteria for response to treatment.44 Trials comparing HFL-rTMS and LFR-rTMS have found no significant differences in efficacy.45
Advanced age, psychotic symptoms, and a longer duration of the current depressive episode predict poor response to rTMS. Also, imaging studies have shown that a lower metabolism in cerebellar, temporal, anterior cingulate, and occipital parts of the brain correlate with better response to HFL-rTMS.46,47
Adverse effects. The major adverse effect associated with rTMS is the risk of inducing seizures, which is more commonly associated with high-frequency rTMS. Other common adverse effects include headache, facial muscle twitching, and tinnitus.37
Deep brain stimulation
DBS is an invasive stereotactic surgical procedure. It involves unilateral or bilateral placement of electrodes at neuroanatomical locations to deliver continuous stimulation from a subcutaneously implanted pulse generator.48 In the past, destructive surgical procedures were used to treat intractable depression. Surgeries such as anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy have been shown to effectively reduce depressive symptoms.49 The advantages of DBS over destructive procedures include the fact that DBS is reversible and that the stimulation levels can easily be adjusted, and the treatment can easily be stopped or restarted.
There is no consensus on the optimal anatomic locations for the electrode implantation in DBS. Electrodes have been implanted in the subcallosal cingulate gyrus, inferior thalamic peduncle, ventral capsule/ventral striatum, superolateral branch of the medial forebrain bundle (MFB), and nucleus accumbens.
The choice of anatomic locations stems from the large body of neuroimaging literature characterizing functional changes associated with acute depression and response to treatment. The electrode placement targets “nodes” that form an integral part of the affected neural circuits that are responsible for regulating depressive symptoms.50 Increased metabolic activity and blood flow to the subgenual cingulate gyrus and reduction in the blood flow to the DLPFC and the striatum have been associated with active depressed states. Response to antidepressant treatment has been associated with reversal of these findings.51 Functional magnetic resonance imaging studies have consistently shown increased activity in the amygdala in response to negative stimuli among patients with depression.
Regardless of the site of electrode placement, studies have reported symptomatic improvement among patients with depression who are treated with DBS. In 2 case reports, the electrode was implanted in the inferior thalamic peduncle.52,53 Each study had 1 participant, and each patient remitted.52,53
Placement of the electrodes in the nucleus accumbens resulted in a response rate of 45% in 1 study,54 whereas in a different study, all patients reported improvement in anhedonia.55 A response rate of 71% and a remission rate of 35% were observed in a study in which the electrode was implanted in the ventral capsule/ventral striatum area.56
Berlim et al57 published a systematic review and exploratory meta-analysis of studies in which the electrode had been implanted in the subgenual cingulate cortex. At 12 months, the response rate was 39.9% (95% CI, 28.4% to 52.8%), and 26.3% (95% CI, 13% to 45.9%) of patients achieved remission. The most significant drop in depression scores was observed 3 to 6 months after the surgery. No significant change in scores was observed between 6 to 12 months after surgery.57
The MFB, specifically the superolateral branch, is emerging as an exciting new target for electrode placement in DBS. Schlaepfer et al58 studied the effects of electrodes implanted bilaterally in the superolateral branch of the MFB. They observed an almost 50% reduction in symptoms by Day 7, and at the last follow-up visit (12 to 33 weeks) 4 of the 6 patients had achieved remission.58 In a recent systematic review, Gálvez et al59 found most studies had high response/remission rates without any significant adverse effects. In a recent study of DBS targeting the MFB, 3 of 4 patients had a >50% reduction in Montgomery-Åsberg Depression Rating Scale scores at the end of first week. Although 1 patient withdrew, 2 of the other 3 patients continued to report a >80% reduction in depressive symptoms, even at Week 26.60
Accurate localization of target areas (white matter tracts) and subsequent electrode placement might be an important factor governing treatment response. Riva-Posse et al61 found that clinical response was seen when the electrodes stimulated 3 specific white matter bundles. Interestingly, nonresponders were converted to responders simply by changing the position of the electrodes to include these white matter tracts.61
Adverse effects. The most common adverse effects noted during studies of DBS include pain at the site of implantation and wound infection. Other adverse effects include lead fracture, transient dysphagia, and other hardware-related problems.49
Sorting out the evidence
In the absence of head-to-head trials, it is difficult to establish a hierarchal algorithm for use of the 4 neuromodulatory treatments discussed in the article. If we were to base our decision solely on the current literature, ECT by far has the most evidence and highest remission rates.11 We can reduce the risk of cognitive deficits by using twice-weekly instead of thrice-weekly ECT, or by using unilateral instead of bilateral ECT.12 Another strategy for reducing adverse effects associated with long-term maintenance ECT is by using it in combination with VNS. ECT and VNS can be used safely concomitantly; ECT can be used to treat acutely worsening depression, and VNS for maintaining the antidepressant effect.62
Aside from ECT, rTMS is the only other treatment that has evidence from RCTs. Although the remission rates are not as high as ECT, its preferable adverse effects profile, noninvasive nature, and comparative low cost (compared with surgical procedures) make it a favorable choice. The Canadian Network for Mood and Anxiety Treatment guidelines suggest rTMS as the first-line treatment for patients who do not respond to pharmacologic treatments.63 ECT can be considered second-line treatment unless the patient has acute suicidal ideation, catatonia, psychotic features, greater treatment resistance, or physical deterioration, in which case ECT should be tried before TMS.63
Among the invasive options, VNS has more evidence and is FDA-approved for TRD. However, DBS has shown great promise in early studies, with remission rates as high as 35%.56 DBS has the advantage of being reversible, and the amount of stimulation can be adjusted easily. Despite early promise, more research is needed before DBS can be widely used in clinical settings.
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
3. Petersen T, Papakostas GI, Posternak MA, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336-341.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91.
6. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol. Psychiatry. 2003;54(3):177-180.
7. Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577-579.
8. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
9. Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27(18):4894-4901.
10. Abbott CC, Gallegos P, Rediske N et al. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33-46.
11. Heijnen WT, Birkenhäger TK, Wierdsma AI, et al. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619.
12. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
13. Semkovska M, Landau S, Dunne R et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. 2016;173(4):408-417.
14. Charlson F, Siskind D, Doi SA, et al. ECT efficacy and treatment course: a systematic review and meta-analysis of twice vs thrice weekly schedules. J Affect Disord. 2012;138(1-2):1-8.
15. Loo CK, Kaill A, Paton P, et al. The difficult-to-treat electroconvulsive therapy patient—strategies for augmenting outcomes. J Affect Disord. 2010;124(3):219-227.
16. de Vreede IM, Burger H, van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(2-3):323-327.
17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
18. Baghai TC, Marcuse A, Brosch M, et al. The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J Biol Psychiatry. 2006;7(2):82-90.
19. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616-626.
20. Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345-1355.
21. Matthews K, Eljamel MS. Vagus nerve stimulation and refractory depression: please can you switch me on doctor? Br J Psychiatry. 2003;183:181-183.
22. George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83.
23. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99.
24. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203-210.
25. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
26. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221-227.
27. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39(9):983-990.
28. O’Keane V, Dinan TG, Scott L, et al. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58(12):963-968.
29. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276-286.
30. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
31. Armitage R, Husain M, Hoffmann R, et al. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54(5):475-482.
32. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347-354.
33. Martin JL, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155.
34. Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83-93.
35. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631-640.
36. Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15.
37. Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241-258.
38. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621-631.
39. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-2596.
40. Fitzgerald PB, Oxley TJ, Laird AR, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33-45.
41. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551.
42. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
43. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
44. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014;75(12):1394-1401.
45. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234.
46. Dumas R, Padovani R, Richieri R, et al. Repetitive transcranial magnetic stimulation in major depression: response factor [in French]. Encephale. 2012;38(4):360-368.
47. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006;9(6):641-654.
48. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510.
49. Taghva AS, Malone DA, Rezai AR. Deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80(3-4):S27.e17-S27.e24.
50. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
51. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
52. Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(pt 2):393-398.
53. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585-593; discussion 585-593.
54. Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110-116.
55. Schlaepfer TE, Bewernick BH, Kayser S, et al. Deep brain stimulation of the human reward system for major depression—rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303-1314.
56. Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
57. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
58. Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204-1212.
59. Gálvez JF, Keser Z, Mwangi B, et al. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:59-70.
60. Fenoy AJ, Schulz P, Selvaraj. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143-151.
61. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969.
62. Burke MJ, Husain MM. Concomitant use of vagus nerve stimulation and electroconvulsive therapy for treatment-resistant depression. J ECT. 2006;22(3):218-222.
63. Milev R V, Giacobbe P, Kennedy SH, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561-575.
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
3. Petersen T, Papakostas GI, Posternak MA, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336-341.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91.
6. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol. Psychiatry. 2003;54(3):177-180.
7. Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577-579.
8. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
9. Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27(18):4894-4901.
10. Abbott CC, Gallegos P, Rediske N et al. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33-46.
11. Heijnen WT, Birkenhäger TK, Wierdsma AI, et al. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619.
12. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
13. Semkovska M, Landau S, Dunne R et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. 2016;173(4):408-417.
14. Charlson F, Siskind D, Doi SA, et al. ECT efficacy and treatment course: a systematic review and meta-analysis of twice vs thrice weekly schedules. J Affect Disord. 2012;138(1-2):1-8.
15. Loo CK, Kaill A, Paton P, et al. The difficult-to-treat electroconvulsive therapy patient—strategies for augmenting outcomes. J Affect Disord. 2010;124(3):219-227.
16. de Vreede IM, Burger H, van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(2-3):323-327.
17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
18. Baghai TC, Marcuse A, Brosch M, et al. The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J Biol Psychiatry. 2006;7(2):82-90.
19. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616-626.
20. Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345-1355.
21. Matthews K, Eljamel MS. Vagus nerve stimulation and refractory depression: please can you switch me on doctor? Br J Psychiatry. 2003;183:181-183.
22. George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83.
23. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99.
24. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203-210.
25. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
26. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221-227.
27. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39(9):983-990.
28. O’Keane V, Dinan TG, Scott L, et al. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58(12):963-968.
29. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276-286.
30. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
31. Armitage R, Husain M, Hoffmann R, et al. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54(5):475-482.
32. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347-354.
33. Martin JL, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155.
34. Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83-93.
35. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631-640.
36. Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15.
37. Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241-258.
38. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621-631.
39. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-2596.
40. Fitzgerald PB, Oxley TJ, Laird AR, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33-45.
41. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551.
42. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
43. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
44. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014;75(12):1394-1401.
45. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234.
46. Dumas R, Padovani R, Richieri R, et al. Repetitive transcranial magnetic stimulation in major depression: response factor [in French]. Encephale. 2012;38(4):360-368.
47. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006;9(6):641-654.
48. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510.
49. Taghva AS, Malone DA, Rezai AR. Deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80(3-4):S27.e17-S27.e24.
50. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
51. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
52. Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(pt 2):393-398.
53. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585-593; discussion 585-593.
54. Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110-116.
55. Schlaepfer TE, Bewernick BH, Kayser S, et al. Deep brain stimulation of the human reward system for major depression—rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303-1314.
56. Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
57. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
58. Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204-1212.
59. Gálvez JF, Keser Z, Mwangi B, et al. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:59-70.
60. Fenoy AJ, Schulz P, Selvaraj. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143-151.
61. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969.
62. Burke MJ, Husain MM. Concomitant use of vagus nerve stimulation and electroconvulsive therapy for treatment-resistant depression. J ECT. 2006;22(3):218-222.
63. Milev R V, Giacobbe P, Kennedy SH, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561-575.