User login
Reduce unnecessary imaging by refining clinical exam skills
“Good morning, Mr. Harris. What can I do for you today?”
“Dr. Hickner, I need an MRI of my right knee. I hurt it last week, and I need to find out if I tore something.”
We all know that too many patients request—and often get—costly (and unnecessary) magnetic resonance imaging (MRI) and computed tomography (CT) scans of their joints and backs. That’s why such imaging is targeted in the Choosing Wisely campaign, which aims to eliminate needless testing.1
But how can we confidently tell Mr. Harris that he doesn’t need an MRI or CT scan? One approach is to explain that imaging is generally reserved for those considering surgery, as it serves to inform the surgeon of the exact procedure needed. Another approach is to be skilled in physical exam techniques that increase our confidence in the clinical diagnosis.
Applying this to acute knee injuries. In this issue of JFP, Koster and colleagues explain that the Lachman test (and possibly the newer lever sign test) are maneuvers that have a high probability of ruling out complete anterior cruciate ligament (ACL) tears when performed properly. The Lachman test, for example, has a 96% sensitivity for complete ACL ruptures.2 (The anterior drawer test has too low a sensitivity to rule out ACL injuries, and the pivot shift test is a bit too challenging to be performed reliably.)
This is important information because early surgery for ACL tears leads to better outcomes for athletes, and a reliable physical exam to rule out an ACL tear reduces the need for imaging. Moreover, other than fractures near the knee, no other knee injuries require early surgery. So a thorough physical exam and selective plain x-rays are all that is needed for the initial evaluation of most knee injuries.
The same is true for back and shoulder injuries, where acute imaging with MRI or CT is rarely called for. A thorough and accurate physical examination is usually sufficient, supplemented with plain X-rays on a selective basis.
Going one step further, consider taking a look at the JAMA series called, “The Rational Clinical Examination,” which has been compiled into a single publication by the same name.3 It is an excellent guide to the sensitivity, specificity, and positive and negative likelihood ratios of a host of clinical findings and tests. It can help to greatly improve clinical skills and reduce unnecessary testing.
1. Choosing Wisely. Available at: http://www.choosingwisely.org. Accessed February 14, 2018.
2. Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;10:2805-2813.
3. The Rational Clinical Examination. Available at: https://medicinainternaucv.files.wordpress.com/2013/02/jama-the-rational-clinical-examination.pdf. Accessed February 14, 2018.
“Good morning, Mr. Harris. What can I do for you today?”
“Dr. Hickner, I need an MRI of my right knee. I hurt it last week, and I need to find out if I tore something.”
We all know that too many patients request—and often get—costly (and unnecessary) magnetic resonance imaging (MRI) and computed tomography (CT) scans of their joints and backs. That’s why such imaging is targeted in the Choosing Wisely campaign, which aims to eliminate needless testing.1
But how can we confidently tell Mr. Harris that he doesn’t need an MRI or CT scan? One approach is to explain that imaging is generally reserved for those considering surgery, as it serves to inform the surgeon of the exact procedure needed. Another approach is to be skilled in physical exam techniques that increase our confidence in the clinical diagnosis.
Applying this to acute knee injuries. In this issue of JFP, Koster and colleagues explain that the Lachman test (and possibly the newer lever sign test) are maneuvers that have a high probability of ruling out complete anterior cruciate ligament (ACL) tears when performed properly. The Lachman test, for example, has a 96% sensitivity for complete ACL ruptures.2 (The anterior drawer test has too low a sensitivity to rule out ACL injuries, and the pivot shift test is a bit too challenging to be performed reliably.)
This is important information because early surgery for ACL tears leads to better outcomes for athletes, and a reliable physical exam to rule out an ACL tear reduces the need for imaging. Moreover, other than fractures near the knee, no other knee injuries require early surgery. So a thorough physical exam and selective plain x-rays are all that is needed for the initial evaluation of most knee injuries.
The same is true for back and shoulder injuries, where acute imaging with MRI or CT is rarely called for. A thorough and accurate physical examination is usually sufficient, supplemented with plain X-rays on a selective basis.
Going one step further, consider taking a look at the JAMA series called, “The Rational Clinical Examination,” which has been compiled into a single publication by the same name.3 It is an excellent guide to the sensitivity, specificity, and positive and negative likelihood ratios of a host of clinical findings and tests. It can help to greatly improve clinical skills and reduce unnecessary testing.
“Good morning, Mr. Harris. What can I do for you today?”
“Dr. Hickner, I need an MRI of my right knee. I hurt it last week, and I need to find out if I tore something.”
We all know that too many patients request—and often get—costly (and unnecessary) magnetic resonance imaging (MRI) and computed tomography (CT) scans of their joints and backs. That’s why such imaging is targeted in the Choosing Wisely campaign, which aims to eliminate needless testing.1
But how can we confidently tell Mr. Harris that he doesn’t need an MRI or CT scan? One approach is to explain that imaging is generally reserved for those considering surgery, as it serves to inform the surgeon of the exact procedure needed. Another approach is to be skilled in physical exam techniques that increase our confidence in the clinical diagnosis.
Applying this to acute knee injuries. In this issue of JFP, Koster and colleagues explain that the Lachman test (and possibly the newer lever sign test) are maneuvers that have a high probability of ruling out complete anterior cruciate ligament (ACL) tears when performed properly. The Lachman test, for example, has a 96% sensitivity for complete ACL ruptures.2 (The anterior drawer test has too low a sensitivity to rule out ACL injuries, and the pivot shift test is a bit too challenging to be performed reliably.)
This is important information because early surgery for ACL tears leads to better outcomes for athletes, and a reliable physical exam to rule out an ACL tear reduces the need for imaging. Moreover, other than fractures near the knee, no other knee injuries require early surgery. So a thorough physical exam and selective plain x-rays are all that is needed for the initial evaluation of most knee injuries.
The same is true for back and shoulder injuries, where acute imaging with MRI or CT is rarely called for. A thorough and accurate physical examination is usually sufficient, supplemented with plain X-rays on a selective basis.
Going one step further, consider taking a look at the JAMA series called, “The Rational Clinical Examination,” which has been compiled into a single publication by the same name.3 It is an excellent guide to the sensitivity, specificity, and positive and negative likelihood ratios of a host of clinical findings and tests. It can help to greatly improve clinical skills and reduce unnecessary testing.
1. Choosing Wisely. Available at: http://www.choosingwisely.org. Accessed February 14, 2018.
2. Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;10:2805-2813.
3. The Rational Clinical Examination. Available at: https://medicinainternaucv.files.wordpress.com/2013/02/jama-the-rational-clinical-examination.pdf. Accessed February 14, 2018.
1. Choosing Wisely. Available at: http://www.choosingwisely.org. Accessed February 14, 2018.
2. Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;10:2805-2813.
3. The Rational Clinical Examination. Available at: https://medicinainternaucv.files.wordpress.com/2013/02/jama-the-rational-clinical-examination.pdf. Accessed February 14, 2018.
Bilateral wrist pain • limited range of motion • tenderness to palpation • Dx?
THE CASE
A 12-year-old girl presented to my office (JH) with bilateral wrist pain. She had fallen on both wrists palmar-flexed and then, while trying to get up, landed on both wrists dorsi-flexed. The patient did not hear any “pops,” but felt immediate pain when her wrists hyperextended. Hand, wrist, and forearm x-rays were negative bilaterally for fractures. She was placed in bilateral thumb spica splints.
At follow-up one week later, the patient reported 6/10 pain in her left wrist and 7/10 pain in her right wrist. The pain increased to 10/10 bilaterally with movement and was not relieved by icing or nonsteroidal anti-inflammatory drugs. On physical exam, there was bilateral swelling of the wrists without ecchymosis or erythema. The patient had limited passive and active range of motion, especially during wrist extension. She also had tenderness to palpation over the anatomical snuff box, extending proximally to the distal radius bilaterally. She had no tenderness over the ulna or metacarpals, no loss of sensation in any area nerves, and she was neurovascularly intact bilaterally.
Based on the mechanism of injury, undetected fracture or full thickness ligament tear were both possible. Because of this, and because magnetic resonance imaging (MRI) entails no radiation exposure, MRI was chosen for additional imaging of both wrists.
THE DIAGNOSIS
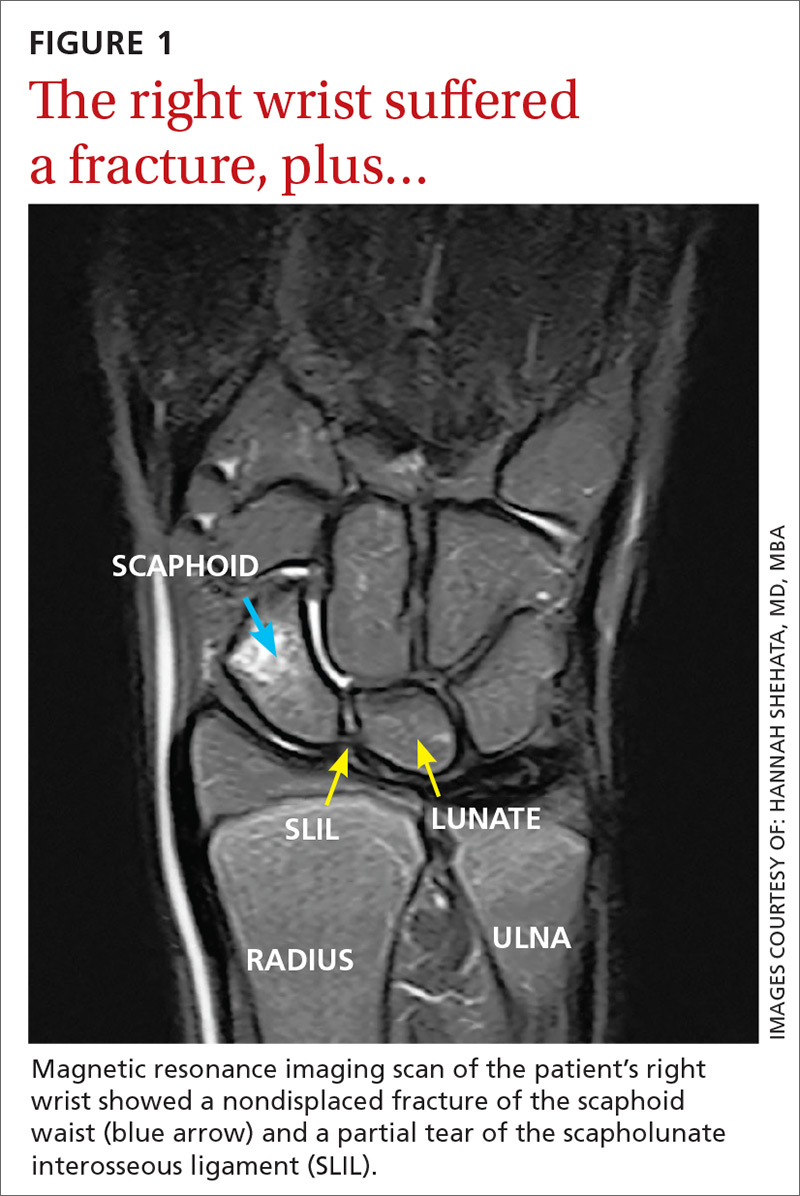
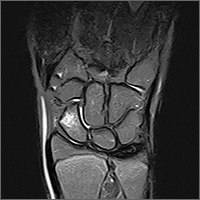
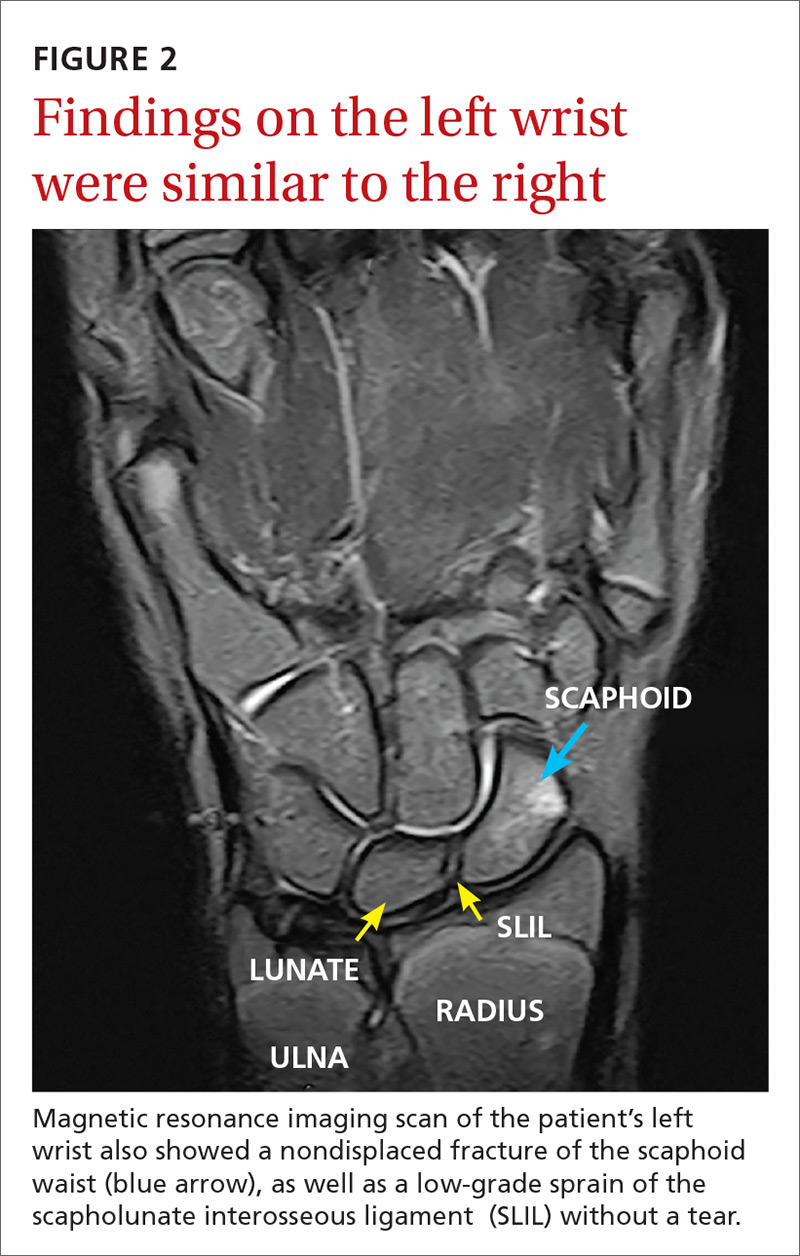
The MRI revealed bilateral, nondisplaced, extra-articular fractures extending through the scaphoid waist, with surrounding bone marrow edema. In the right wrist, the patient also had a low-grade partial tear of the membranous portion of the scapholunate interosseous ligament (SLIL) at the scaphoid attachment (FIGURE 1). In the left wrist, she also had a low-grade sprain of the SLIL without tear (FIGURE 2).
DISCUSSION
Carpal fractures account for 6% of all fractures.1 Scaphoid fractures are the most common carpal bone fracture among all age groups, but account for only 0.4% of all pediatric fractures.1-3 They’re commonly missed on x-rays because they are usually nondisplaced and hidden by other structures superimposed on the image.1,2,4 Undetected, scaphoid fractures can cause prolonged interruption to the bone’s architecture, leading to avascular necrosis of the proximal portion of the scaphoid bone.5,6
Bilateral scaphoid fractures are extremely rare and account for less than 1% of all scaphoid fractures.7 Very few of these cases have been published in the literature, and those that have been published have talked about the fractures being secondary to chronic stress fractures and as being treated with internal fixation (regardless of whether the fractures were nondisplaced or if the ligaments were intact).6-9
Our patient was placed in bilateral fiberglass short-arm thumb spica casts. We tried conservative treatment measures first because she had help with her activities of daily living (ADLs). At a follow-up visit 2 weeks later, we switched the casts to long-arm thumb spica casts because of the patient’s ability to pronate and supinate her wrists in the short-arm versions. After one month of wearing the long-arm casts, we placed her back in bilateral short-arm casts for 2 weeks. Eight weeks after the fall, we removed the short-arm casts for reevaluation.
We obtained x-rays to assess for any new changes to the wrist and specifically the scaphoid bones. The x-rays showed almost completely healed scaphoid bones with good alignment, but the patient still had 5/10 pain in the left wrist and 8/10 pain in the right wrist with movement. We placed her in adjustable thermoformable polymer braces, which were removed when she bathed.
Due to the uniqueness of her injuries, our patient had weekly visits with her primary care provider (PCP) for the first 2 months of treatment, followed by bimonthly visits for the remainder. At 10 weeks after the fall, her pain with movement was almost gone and she began physical therapy. She also began removing the braces during sedentary activity in order to practice range-of-motion exercises to prevent excessive stiffness in her wrists. Our patient regained full strength and range of motion one month later.
One other published case report describes the successful union of bilateral scaphoid fractures using bilateral long-arm casts followed by short-arm casts.7 Similar to our patient’s case, full union of the scaphoid bones was achieved within 12 weeks.7 Together, these cases suggest that conservative treatment methods are a viable alternative to surgery.
TAKEAWAY
For patients presenting with wrist pain after trauma to the wrists, assess anatomical snuffbox tenderness and obtain x-rays. Do not be falsely reassured by negative x-rays in the presence of a positive physical exam, however, as scaphoid fractures are often hidden on x-rays. If tenderness at the anatomical snuffbox is present and doesn’t subside within a few days, apply a short-arm thumb splint and obtain subsequent imaging.
If bilateral, nondisplaced, stable scaphoid fractures are diagnosed, conservative treatment with long-arm and short-arm casts is a viable alternative to surgery. This treatment decision should be made on an individual basis, however, as it requires the patient to have frequent PCP visits, assistance with ADLs, and complete adherence to the treatment plan.
1. Pillai A, Jain M. Management of clinical fractures of the scaphoid: results of an audit and literature review. Eur J Emerg Med. 2005;12:47-51.
2. Evenski AJ, Adamczyk MJ, Steiner RP, et al. Clinically suspected scaphoid fractures in children. J Pediatr Orthop. 2009;29:352-355.
3. Wulff R, Schmidt T. Carpal fractures in children. J Pediatr Orthop. 1998;18:462-465.
4. Nellans KW, Chung KC. Pediatric hand fractures. Hand Clin. 2013;29:569-578.
5. Jernigan EW, Smetana BS, Patterson JM. Pediatric scaphoid proximal pole nonunion with avascular necrosis. J Hand Surgery. 2017;42:299.e1-299.e4.
6. Pidemunt G, Torres-Claramunt R, Ginés A, et al. Bilateral stress fracture of the carpal scaphoid: report in a child and review of the literature. Clin J Sport Med. 2012;22:511-513.
7. Saglam F, Gulabi D, Baysal Ö, et al. Chronic wrist pain in a goalkeeper; bilateral scaphoid stress fracture: a case report. Int J Surg Case Rep. 2015;7:20-22.
8. Muzaffar N, Wani I, Ehsan M, et al. Simultaneous bilateral scaphoid fractures in a soldier managed conservatively by scaphoid casts. Arch Clin Exp Surg. 2016;5:63-64.
9. Mohamed Haflah NH, Mat Nor NF, Abdullah S, et al. Bilateral scaphoid stress fracture in a platform diver presenting with unilateral symptoms. Singapore Med J. 2014;55:e159-e161.
THE CASE
A 12-year-old girl presented to my office (JH) with bilateral wrist pain. She had fallen on both wrists palmar-flexed and then, while trying to get up, landed on both wrists dorsi-flexed. The patient did not hear any “pops,” but felt immediate pain when her wrists hyperextended. Hand, wrist, and forearm x-rays were negative bilaterally for fractures. She was placed in bilateral thumb spica splints.
At follow-up one week later, the patient reported 6/10 pain in her left wrist and 7/10 pain in her right wrist. The pain increased to 10/10 bilaterally with movement and was not relieved by icing or nonsteroidal anti-inflammatory drugs. On physical exam, there was bilateral swelling of the wrists without ecchymosis or erythema. The patient had limited passive and active range of motion, especially during wrist extension. She also had tenderness to palpation over the anatomical snuff box, extending proximally to the distal radius bilaterally. She had no tenderness over the ulna or metacarpals, no loss of sensation in any area nerves, and she was neurovascularly intact bilaterally.
Based on the mechanism of injury, undetected fracture or full thickness ligament tear were both possible. Because of this, and because magnetic resonance imaging (MRI) entails no radiation exposure, MRI was chosen for additional imaging of both wrists.
THE DIAGNOSIS

The MRI revealed bilateral, nondisplaced, extra-articular fractures extending through the scaphoid waist, with surrounding bone marrow edema. In the right wrist, the patient also had a low-grade partial tear of the membranous portion of the scapholunate interosseous ligament (SLIL) at the scaphoid attachment (FIGURE 1). In the left wrist, she also had a low-grade sprain of the SLIL without tear (FIGURE 2).

DISCUSSION
Carpal fractures account for 6% of all fractures.1 Scaphoid fractures are the most common carpal bone fracture among all age groups, but account for only 0.4% of all pediatric fractures.1-3 They’re commonly missed on x-rays because they are usually nondisplaced and hidden by other structures superimposed on the image.1,2,4 Undetected, scaphoid fractures can cause prolonged interruption to the bone’s architecture, leading to avascular necrosis of the proximal portion of the scaphoid bone.5,6
Bilateral scaphoid fractures are extremely rare and account for less than 1% of all scaphoid fractures.7 Very few of these cases have been published in the literature, and those that have been published have talked about the fractures being secondary to chronic stress fractures and as being treated with internal fixation (regardless of whether the fractures were nondisplaced or if the ligaments were intact).6-9
Our patient was placed in bilateral fiberglass short-arm thumb spica casts. We tried conservative treatment measures first because she had help with her activities of daily living (ADLs). At a follow-up visit 2 weeks later, we switched the casts to long-arm thumb spica casts because of the patient’s ability to pronate and supinate her wrists in the short-arm versions. After one month of wearing the long-arm casts, we placed her back in bilateral short-arm casts for 2 weeks. Eight weeks after the fall, we removed the short-arm casts for reevaluation.
We obtained x-rays to assess for any new changes to the wrist and specifically the scaphoid bones. The x-rays showed almost completely healed scaphoid bones with good alignment, but the patient still had 5/10 pain in the left wrist and 8/10 pain in the right wrist with movement. We placed her in adjustable thermoformable polymer braces, which were removed when she bathed.
Due to the uniqueness of her injuries, our patient had weekly visits with her primary care provider (PCP) for the first 2 months of treatment, followed by bimonthly visits for the remainder. At 10 weeks after the fall, her pain with movement was almost gone and she began physical therapy. She also began removing the braces during sedentary activity in order to practice range-of-motion exercises to prevent excessive stiffness in her wrists. Our patient regained full strength and range of motion one month later.
One other published case report describes the successful union of bilateral scaphoid fractures using bilateral long-arm casts followed by short-arm casts.7 Similar to our patient’s case, full union of the scaphoid bones was achieved within 12 weeks.7 Together, these cases suggest that conservative treatment methods are a viable alternative to surgery.
TAKEAWAY
For patients presenting with wrist pain after trauma to the wrists, assess anatomical snuffbox tenderness and obtain x-rays. Do not be falsely reassured by negative x-rays in the presence of a positive physical exam, however, as scaphoid fractures are often hidden on x-rays. If tenderness at the anatomical snuffbox is present and doesn’t subside within a few days, apply a short-arm thumb splint and obtain subsequent imaging.
If bilateral, nondisplaced, stable scaphoid fractures are diagnosed, conservative treatment with long-arm and short-arm casts is a viable alternative to surgery. This treatment decision should be made on an individual basis, however, as it requires the patient to have frequent PCP visits, assistance with ADLs, and complete adherence to the treatment plan.
THE CASE
A 12-year-old girl presented to my office (JH) with bilateral wrist pain. She had fallen on both wrists palmar-flexed and then, while trying to get up, landed on both wrists dorsi-flexed. The patient did not hear any “pops,” but felt immediate pain when her wrists hyperextended. Hand, wrist, and forearm x-rays were negative bilaterally for fractures. She was placed in bilateral thumb spica splints.
At follow-up one week later, the patient reported 6/10 pain in her left wrist and 7/10 pain in her right wrist. The pain increased to 10/10 bilaterally with movement and was not relieved by icing or nonsteroidal anti-inflammatory drugs. On physical exam, there was bilateral swelling of the wrists without ecchymosis or erythema. The patient had limited passive and active range of motion, especially during wrist extension. She also had tenderness to palpation over the anatomical snuff box, extending proximally to the distal radius bilaterally. She had no tenderness over the ulna or metacarpals, no loss of sensation in any area nerves, and she was neurovascularly intact bilaterally.
Based on the mechanism of injury, undetected fracture or full thickness ligament tear were both possible. Because of this, and because magnetic resonance imaging (MRI) entails no radiation exposure, MRI was chosen for additional imaging of both wrists.
THE DIAGNOSIS

The MRI revealed bilateral, nondisplaced, extra-articular fractures extending through the scaphoid waist, with surrounding bone marrow edema. In the right wrist, the patient also had a low-grade partial tear of the membranous portion of the scapholunate interosseous ligament (SLIL) at the scaphoid attachment (FIGURE 1). In the left wrist, she also had a low-grade sprain of the SLIL without tear (FIGURE 2).

DISCUSSION
Carpal fractures account for 6% of all fractures.1 Scaphoid fractures are the most common carpal bone fracture among all age groups, but account for only 0.4% of all pediatric fractures.1-3 They’re commonly missed on x-rays because they are usually nondisplaced and hidden by other structures superimposed on the image.1,2,4 Undetected, scaphoid fractures can cause prolonged interruption to the bone’s architecture, leading to avascular necrosis of the proximal portion of the scaphoid bone.5,6
Bilateral scaphoid fractures are extremely rare and account for less than 1% of all scaphoid fractures.7 Very few of these cases have been published in the literature, and those that have been published have talked about the fractures being secondary to chronic stress fractures and as being treated with internal fixation (regardless of whether the fractures were nondisplaced or if the ligaments were intact).6-9
Our patient was placed in bilateral fiberglass short-arm thumb spica casts. We tried conservative treatment measures first because she had help with her activities of daily living (ADLs). At a follow-up visit 2 weeks later, we switched the casts to long-arm thumb spica casts because of the patient’s ability to pronate and supinate her wrists in the short-arm versions. After one month of wearing the long-arm casts, we placed her back in bilateral short-arm casts for 2 weeks. Eight weeks after the fall, we removed the short-arm casts for reevaluation.
We obtained x-rays to assess for any new changes to the wrist and specifically the scaphoid bones. The x-rays showed almost completely healed scaphoid bones with good alignment, but the patient still had 5/10 pain in the left wrist and 8/10 pain in the right wrist with movement. We placed her in adjustable thermoformable polymer braces, which were removed when she bathed.
Due to the uniqueness of her injuries, our patient had weekly visits with her primary care provider (PCP) for the first 2 months of treatment, followed by bimonthly visits for the remainder. At 10 weeks after the fall, her pain with movement was almost gone and she began physical therapy. She also began removing the braces during sedentary activity in order to practice range-of-motion exercises to prevent excessive stiffness in her wrists. Our patient regained full strength and range of motion one month later.
One other published case report describes the successful union of bilateral scaphoid fractures using bilateral long-arm casts followed by short-arm casts.7 Similar to our patient’s case, full union of the scaphoid bones was achieved within 12 weeks.7 Together, these cases suggest that conservative treatment methods are a viable alternative to surgery.
TAKEAWAY
For patients presenting with wrist pain after trauma to the wrists, assess anatomical snuffbox tenderness and obtain x-rays. Do not be falsely reassured by negative x-rays in the presence of a positive physical exam, however, as scaphoid fractures are often hidden on x-rays. If tenderness at the anatomical snuffbox is present and doesn’t subside within a few days, apply a short-arm thumb splint and obtain subsequent imaging.
If bilateral, nondisplaced, stable scaphoid fractures are diagnosed, conservative treatment with long-arm and short-arm casts is a viable alternative to surgery. This treatment decision should be made on an individual basis, however, as it requires the patient to have frequent PCP visits, assistance with ADLs, and complete adherence to the treatment plan.
1. Pillai A, Jain M. Management of clinical fractures of the scaphoid: results of an audit and literature review. Eur J Emerg Med. 2005;12:47-51.
2. Evenski AJ, Adamczyk MJ, Steiner RP, et al. Clinically suspected scaphoid fractures in children. J Pediatr Orthop. 2009;29:352-355.
3. Wulff R, Schmidt T. Carpal fractures in children. J Pediatr Orthop. 1998;18:462-465.
4. Nellans KW, Chung KC. Pediatric hand fractures. Hand Clin. 2013;29:569-578.
5. Jernigan EW, Smetana BS, Patterson JM. Pediatric scaphoid proximal pole nonunion with avascular necrosis. J Hand Surgery. 2017;42:299.e1-299.e4.
6. Pidemunt G, Torres-Claramunt R, Ginés A, et al. Bilateral stress fracture of the carpal scaphoid: report in a child and review of the literature. Clin J Sport Med. 2012;22:511-513.
7. Saglam F, Gulabi D, Baysal Ö, et al. Chronic wrist pain in a goalkeeper; bilateral scaphoid stress fracture: a case report. Int J Surg Case Rep. 2015;7:20-22.
8. Muzaffar N, Wani I, Ehsan M, et al. Simultaneous bilateral scaphoid fractures in a soldier managed conservatively by scaphoid casts. Arch Clin Exp Surg. 2016;5:63-64.
9. Mohamed Haflah NH, Mat Nor NF, Abdullah S, et al. Bilateral scaphoid stress fracture in a platform diver presenting with unilateral symptoms. Singapore Med J. 2014;55:e159-e161.
1. Pillai A, Jain M. Management of clinical fractures of the scaphoid: results of an audit and literature review. Eur J Emerg Med. 2005;12:47-51.
2. Evenski AJ, Adamczyk MJ, Steiner RP, et al. Clinically suspected scaphoid fractures in children. J Pediatr Orthop. 2009;29:352-355.
3. Wulff R, Schmidt T. Carpal fractures in children. J Pediatr Orthop. 1998;18:462-465.
4. Nellans KW, Chung KC. Pediatric hand fractures. Hand Clin. 2013;29:569-578.
5. Jernigan EW, Smetana BS, Patterson JM. Pediatric scaphoid proximal pole nonunion with avascular necrosis. J Hand Surgery. 2017;42:299.e1-299.e4.
6. Pidemunt G, Torres-Claramunt R, Ginés A, et al. Bilateral stress fracture of the carpal scaphoid: report in a child and review of the literature. Clin J Sport Med. 2012;22:511-513.
7. Saglam F, Gulabi D, Baysal Ö, et al. Chronic wrist pain in a goalkeeper; bilateral scaphoid stress fracture: a case report. Int J Surg Case Rep. 2015;7:20-22.
8. Muzaffar N, Wani I, Ehsan M, et al. Simultaneous bilateral scaphoid fractures in a soldier managed conservatively by scaphoid casts. Arch Clin Exp Surg. 2016;5:63-64.
9. Mohamed Haflah NH, Mat Nor NF, Abdullah S, et al. Bilateral scaphoid stress fracture in a platform diver presenting with unilateral symptoms. Singapore Med J. 2014;55:e159-e161.
An easy approach to obtaining clean-catch urine from infants
ILLUSTRATIVE CASE
A fussy 6-month-old infant is brought into the emergency department (ED) with a rectal temperature of 101.5° F. She is consolable, breathing normally, and appears well hydrated. You find no clear etiology for her fever and suspect that a urinary tract infection (UTI) may be the source of her illness. How do you proceed with obtaining a urine sample?
A febrile infant in the family physician’s office or ED is a familiar clinical situation that may require an invasive diagnostic work-up. Up to 7% of infants ages 2 to 24 months with fever of unknown origin may have a UTI.2 Collecting a urine sample from pre-toilet-trained children can be time consuming. In fact, obtaining a clean-catch urine sample in this age group took an average of more than one hour in one randomized controlled trial (RCT).3 More convenient methods of urine collection, such as placing a cotton ball in the diaper or using a perineal collection bag, have contamination rates of up to 63%.4
The American Academy of Pediatrics (AAP) guidelines for evaluating possible UTI in a febrile child <2 years of age recommend obtaining a sample for urinalysis “through the most convenient means.”5 If urinalysis is positive, only urine obtained by catheterization or suprapubic aspiration should be cultured. Guidelines from the National Institute for Health and Care Excellence in the United Kingdom are similar, but allow for culture of clean-catch urine samples.6
A recent prospective cohort study examined a noninvasive alternating lumbar-bladder tapping method to stimulate voiding in infants ages 0 to 6 months.7 Within 5 minutes, 49% of the infants provided a clean-catch sample, with contamination rates similar to those of samples obtained using invasive methods.7 Younger infants were more likely to void within the time allotted. Another trial of bladder tapping conducted in hospitalized infants <30 days old showed similar results.8
There are, however, no previously reported randomized trials demonstrating the efficacy of a noninvasive urine collection technique in the outpatient setting.
Use of invasive collection methods requires skilled personnel and may cause significant discomfort for patients (and parents). Noninvasive methods, such as bag urine collection, have unacceptable contamination rates. In addition, waiting to catch a potentially cleaner urine sample is time-consuming, so better strategies to collect urine from infants are needed. This RCT is the first to examine the efficacy of a unique stimulation technique to obtain a clean-catch urine sample from infants ages 1 to 12 months.
STUDY SUMMARY
Noninvasive stimulation method triggers faster clean urine samples
A nonblinded, single-center RCT conducted in Australia compared 2 methods for obtaining a clean-catch urine sample within 5 minutes: the Quick-Wee method (suprapubic stimulation with gauze soaked in cold fluid) or usual care (waiting for spontaneous voiding with no stimulation).1 Three hundred fifty-four infants (ages 1-12 months) who required urine sample collection were randomized in a 1:1 ratio; allocation was concealed. Infants with anatomic or neurologic abnormalities and those needing immediate antibiotic therapy were excluded.
The most common reasons for obtaining the urine sample were fever of unknown origin and “unsettled baby,” followed by poor feeding and suspected UTI. The primary outcome was voiding within 5 minutes; secondary outcomes included time to void, whether urine was successfully caught, contamination rate, and parent/clinician satisfaction.
Study personnel removed the diaper, then cleaned the genitals of all patients with room temperature sterile water. A caregiver or clinician was ready and waiting to catch urine when the patient voided. In the Quick-Wee group, a clinician rubbed the patient’s suprapubic area in a circular fashion with gauze soaked in refrigerated saline (2.8° C). At 5 minutes, clinicians recorded the voiding status and decided how to proceed.
Using intention-to-treat analysis, 31% of the patients in the Quick-Wee group voided within 5 minutes, compared with 12% of the usual-care patients. Similarly, 30% of patients in the Quick-Wee group provided a successful clean-catch sample within 5 minutes compared with 9% in the usual-care group (P<.001; number needed to treat=4.7; 95% CI, 3.4-7.7). Contamination rates were no different between the Quick-Wee and usual-care samples. Both parents and clinicians were more satisfied with the Quick-Wee method than with usual care (median score of 2 vs 3 on a 5-point Likert scale, in which 1 is most satisfied; P<.001). There was no difference when results were adjusted for age or sex. No adverse events occurred.
WHAT’S NEW
New method could reduce the need for invasive sampling
A simple suprapubic stimulation technique increased the number of infants who provided a clean-catch voided urine sample within 5 minutes—a clinically relevant and satisfying outcome. In appropriate patients, use of the Quick-Wee method to obtain a clean-catch voided sample for initial urinalysis, rather than attempting methods with known high contamination rates, may potentially reduce the need for invasive sampling using catheterization or suprapubic aspiration.
CAVEATS
Complete age range and ideal storage temperature are unknown
Neonates and pre-continent children older than 12 months were not included in this trial, so these conclusions do not apply to those groups of patients. The intervention period lasted only 5 minutes, but other published studies suggest that this amount of time is adequate for voiding to occur.6,7 Although this study used soaking fluid stored at 2.8° C, the ideal storage temperature is unknown.
CHALLENGES TO IMPLEMENTATION
AAP doesn’t endorse clean-catch urine samples for culture
The Quick-Wee method is simple and easy to implement, and requires no specialized training or equipment. AAP guidelines do not endorse the use of clean-catch voided urine for culture, which may be a barrier to changing urine collection practices in some settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
2. Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302-308.
3. Davies P, Greenwood R, Benger J. Randomised trial of a vibrating bladder stimulator—the time to pee study. Arch Dis Child. 2008;93:423-424.
4. Al-Orifi F, McGillivray D, Tange S, et al. Urine culture from bag specimens in young children: are the risks too high? J Pediatr. 2000;137:221-226.
5. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138:e20163026.
6. National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management. Clinical guideline CG54. Published August 2007. Available at: https://www.nice.org.uk/guidance/cg54/chapter/1-guidance. Accessed May 30, 2017.
7. Labrosse M, Levy A, Autmizguine J, et al. Evaluation of a new strategy for clean-catch urine in infants. Pediatrics. 2016;138:e20160573.
8. Herreros Fernández ML, González Merino N, Tagarro García A, et al. A new technique for fast and safe collection of urine in newborns. Arch Dis Child. 2013;98:27-29.
ILLUSTRATIVE CASE
A fussy 6-month-old infant is brought into the emergency department (ED) with a rectal temperature of 101.5° F. She is consolable, breathing normally, and appears well hydrated. You find no clear etiology for her fever and suspect that a urinary tract infection (UTI) may be the source of her illness. How do you proceed with obtaining a urine sample?
A febrile infant in the family physician’s office or ED is a familiar clinical situation that may require an invasive diagnostic work-up. Up to 7% of infants ages 2 to 24 months with fever of unknown origin may have a UTI.2 Collecting a urine sample from pre-toilet-trained children can be time consuming. In fact, obtaining a clean-catch urine sample in this age group took an average of more than one hour in one randomized controlled trial (RCT).3 More convenient methods of urine collection, such as placing a cotton ball in the diaper or using a perineal collection bag, have contamination rates of up to 63%.4
The American Academy of Pediatrics (AAP) guidelines for evaluating possible UTI in a febrile child <2 years of age recommend obtaining a sample for urinalysis “through the most convenient means.”5 If urinalysis is positive, only urine obtained by catheterization or suprapubic aspiration should be cultured. Guidelines from the National Institute for Health and Care Excellence in the United Kingdom are similar, but allow for culture of clean-catch urine samples.6
A recent prospective cohort study examined a noninvasive alternating lumbar-bladder tapping method to stimulate voiding in infants ages 0 to 6 months.7 Within 5 minutes, 49% of the infants provided a clean-catch sample, with contamination rates similar to those of samples obtained using invasive methods.7 Younger infants were more likely to void within the time allotted. Another trial of bladder tapping conducted in hospitalized infants <30 days old showed similar results.8
There are, however, no previously reported randomized trials demonstrating the efficacy of a noninvasive urine collection technique in the outpatient setting.
Use of invasive collection methods requires skilled personnel and may cause significant discomfort for patients (and parents). Noninvasive methods, such as bag urine collection, have unacceptable contamination rates. In addition, waiting to catch a potentially cleaner urine sample is time-consuming, so better strategies to collect urine from infants are needed. This RCT is the first to examine the efficacy of a unique stimulation technique to obtain a clean-catch urine sample from infants ages 1 to 12 months.
STUDY SUMMARY
Noninvasive stimulation method triggers faster clean urine samples
A nonblinded, single-center RCT conducted in Australia compared 2 methods for obtaining a clean-catch urine sample within 5 minutes: the Quick-Wee method (suprapubic stimulation with gauze soaked in cold fluid) or usual care (waiting for spontaneous voiding with no stimulation).1 Three hundred fifty-four infants (ages 1-12 months) who required urine sample collection were randomized in a 1:1 ratio; allocation was concealed. Infants with anatomic or neurologic abnormalities and those needing immediate antibiotic therapy were excluded.
The most common reasons for obtaining the urine sample were fever of unknown origin and “unsettled baby,” followed by poor feeding and suspected UTI. The primary outcome was voiding within 5 minutes; secondary outcomes included time to void, whether urine was successfully caught, contamination rate, and parent/clinician satisfaction.
Study personnel removed the diaper, then cleaned the genitals of all patients with room temperature sterile water. A caregiver or clinician was ready and waiting to catch urine when the patient voided. In the Quick-Wee group, a clinician rubbed the patient’s suprapubic area in a circular fashion with gauze soaked in refrigerated saline (2.8° C). At 5 minutes, clinicians recorded the voiding status and decided how to proceed.
Using intention-to-treat analysis, 31% of the patients in the Quick-Wee group voided within 5 minutes, compared with 12% of the usual-care patients. Similarly, 30% of patients in the Quick-Wee group provided a successful clean-catch sample within 5 minutes compared with 9% in the usual-care group (P<.001; number needed to treat=4.7; 95% CI, 3.4-7.7). Contamination rates were no different between the Quick-Wee and usual-care samples. Both parents and clinicians were more satisfied with the Quick-Wee method than with usual care (median score of 2 vs 3 on a 5-point Likert scale, in which 1 is most satisfied; P<.001). There was no difference when results were adjusted for age or sex. No adverse events occurred.
WHAT’S NEW
New method could reduce the need for invasive sampling
A simple suprapubic stimulation technique increased the number of infants who provided a clean-catch voided urine sample within 5 minutes—a clinically relevant and satisfying outcome. In appropriate patients, use of the Quick-Wee method to obtain a clean-catch voided sample for initial urinalysis, rather than attempting methods with known high contamination rates, may potentially reduce the need for invasive sampling using catheterization or suprapubic aspiration.
CAVEATS
Complete age range and ideal storage temperature are unknown
Neonates and pre-continent children older than 12 months were not included in this trial, so these conclusions do not apply to those groups of patients. The intervention period lasted only 5 minutes, but other published studies suggest that this amount of time is adequate for voiding to occur.6,7 Although this study used soaking fluid stored at 2.8° C, the ideal storage temperature is unknown.
CHALLENGES TO IMPLEMENTATION
AAP doesn’t endorse clean-catch urine samples for culture
The Quick-Wee method is simple and easy to implement, and requires no specialized training or equipment. AAP guidelines do not endorse the use of clean-catch voided urine for culture, which may be a barrier to changing urine collection practices in some settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A fussy 6-month-old infant is brought into the emergency department (ED) with a rectal temperature of 101.5° F. She is consolable, breathing normally, and appears well hydrated. You find no clear etiology for her fever and suspect that a urinary tract infection (UTI) may be the source of her illness. How do you proceed with obtaining a urine sample?
A febrile infant in the family physician’s office or ED is a familiar clinical situation that may require an invasive diagnostic work-up. Up to 7% of infants ages 2 to 24 months with fever of unknown origin may have a UTI.2 Collecting a urine sample from pre-toilet-trained children can be time consuming. In fact, obtaining a clean-catch urine sample in this age group took an average of more than one hour in one randomized controlled trial (RCT).3 More convenient methods of urine collection, such as placing a cotton ball in the diaper or using a perineal collection bag, have contamination rates of up to 63%.4
The American Academy of Pediatrics (AAP) guidelines for evaluating possible UTI in a febrile child <2 years of age recommend obtaining a sample for urinalysis “through the most convenient means.”5 If urinalysis is positive, only urine obtained by catheterization or suprapubic aspiration should be cultured. Guidelines from the National Institute for Health and Care Excellence in the United Kingdom are similar, but allow for culture of clean-catch urine samples.6
A recent prospective cohort study examined a noninvasive alternating lumbar-bladder tapping method to stimulate voiding in infants ages 0 to 6 months.7 Within 5 minutes, 49% of the infants provided a clean-catch sample, with contamination rates similar to those of samples obtained using invasive methods.7 Younger infants were more likely to void within the time allotted. Another trial of bladder tapping conducted in hospitalized infants <30 days old showed similar results.8
There are, however, no previously reported randomized trials demonstrating the efficacy of a noninvasive urine collection technique in the outpatient setting.
Use of invasive collection methods requires skilled personnel and may cause significant discomfort for patients (and parents). Noninvasive methods, such as bag urine collection, have unacceptable contamination rates. In addition, waiting to catch a potentially cleaner urine sample is time-consuming, so better strategies to collect urine from infants are needed. This RCT is the first to examine the efficacy of a unique stimulation technique to obtain a clean-catch urine sample from infants ages 1 to 12 months.
STUDY SUMMARY
Noninvasive stimulation method triggers faster clean urine samples
A nonblinded, single-center RCT conducted in Australia compared 2 methods for obtaining a clean-catch urine sample within 5 minutes: the Quick-Wee method (suprapubic stimulation with gauze soaked in cold fluid) or usual care (waiting for spontaneous voiding with no stimulation).1 Three hundred fifty-four infants (ages 1-12 months) who required urine sample collection were randomized in a 1:1 ratio; allocation was concealed. Infants with anatomic or neurologic abnormalities and those needing immediate antibiotic therapy were excluded.
The most common reasons for obtaining the urine sample were fever of unknown origin and “unsettled baby,” followed by poor feeding and suspected UTI. The primary outcome was voiding within 5 minutes; secondary outcomes included time to void, whether urine was successfully caught, contamination rate, and parent/clinician satisfaction.
Study personnel removed the diaper, then cleaned the genitals of all patients with room temperature sterile water. A caregiver or clinician was ready and waiting to catch urine when the patient voided. In the Quick-Wee group, a clinician rubbed the patient’s suprapubic area in a circular fashion with gauze soaked in refrigerated saline (2.8° C). At 5 minutes, clinicians recorded the voiding status and decided how to proceed.
Using intention-to-treat analysis, 31% of the patients in the Quick-Wee group voided within 5 minutes, compared with 12% of the usual-care patients. Similarly, 30% of patients in the Quick-Wee group provided a successful clean-catch sample within 5 minutes compared with 9% in the usual-care group (P<.001; number needed to treat=4.7; 95% CI, 3.4-7.7). Contamination rates were no different between the Quick-Wee and usual-care samples. Both parents and clinicians were more satisfied with the Quick-Wee method than with usual care (median score of 2 vs 3 on a 5-point Likert scale, in which 1 is most satisfied; P<.001). There was no difference when results were adjusted for age or sex. No adverse events occurred.
WHAT’S NEW
New method could reduce the need for invasive sampling
A simple suprapubic stimulation technique increased the number of infants who provided a clean-catch voided urine sample within 5 minutes—a clinically relevant and satisfying outcome. In appropriate patients, use of the Quick-Wee method to obtain a clean-catch voided sample for initial urinalysis, rather than attempting methods with known high contamination rates, may potentially reduce the need for invasive sampling using catheterization or suprapubic aspiration.
CAVEATS
Complete age range and ideal storage temperature are unknown
Neonates and pre-continent children older than 12 months were not included in this trial, so these conclusions do not apply to those groups of patients. The intervention period lasted only 5 minutes, but other published studies suggest that this amount of time is adequate for voiding to occur.6,7 Although this study used soaking fluid stored at 2.8° C, the ideal storage temperature is unknown.
CHALLENGES TO IMPLEMENTATION
AAP doesn’t endorse clean-catch urine samples for culture
The Quick-Wee method is simple and easy to implement, and requires no specialized training or equipment. AAP guidelines do not endorse the use of clean-catch voided urine for culture, which may be a barrier to changing urine collection practices in some settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
2. Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302-308.
3. Davies P, Greenwood R, Benger J. Randomised trial of a vibrating bladder stimulator—the time to pee study. Arch Dis Child. 2008;93:423-424.
4. Al-Orifi F, McGillivray D, Tange S, et al. Urine culture from bag specimens in young children: are the risks too high? J Pediatr. 2000;137:221-226.
5. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138:e20163026.
6. National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management. Clinical guideline CG54. Published August 2007. Available at: https://www.nice.org.uk/guidance/cg54/chapter/1-guidance. Accessed May 30, 2017.
7. Labrosse M, Levy A, Autmizguine J, et al. Evaluation of a new strategy for clean-catch urine in infants. Pediatrics. 2016;138:e20160573.
8. Herreros Fernández ML, González Merino N, Tagarro García A, et al. A new technique for fast and safe collection of urine in newborns. Arch Dis Child. 2013;98:27-29.
1. Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
2. Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302-308.
3. Davies P, Greenwood R, Benger J. Randomised trial of a vibrating bladder stimulator—the time to pee study. Arch Dis Child. 2008;93:423-424.
4. Al-Orifi F, McGillivray D, Tange S, et al. Urine culture from bag specimens in young children: are the risks too high? J Pediatr. 2000;137:221-226.
5. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138:e20163026.
6. National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management. Clinical guideline CG54. Published August 2007. Available at: https://www.nice.org.uk/guidance/cg54/chapter/1-guidance. Accessed May 30, 2017.
7. Labrosse M, Levy A, Autmizguine J, et al. Evaluation of a new strategy for clean-catch urine in infants. Pediatrics. 2016;138:e20160573.
8. Herreros Fernández ML, González Merino N, Tagarro García A, et al. A new technique for fast and safe collection of urine in newborns. Arch Dis Child. 2013;98:27-29.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Apply gauze soaked in cold sterile saline to the suprapubic area to stimulate infants ages 1 to 12 months to provide a clean-catch urine sample. Doing so produces significantly more clean-catch urine samples within 5 minutes than simply waiting for the patient to void, with no difference in contamination and with increased parental and provider satisfaction.1
STRENGTH OF RECOMMENDATION
B: Based on a single good-quality, randomized controlled trial.
Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
ACIP vaccine update
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
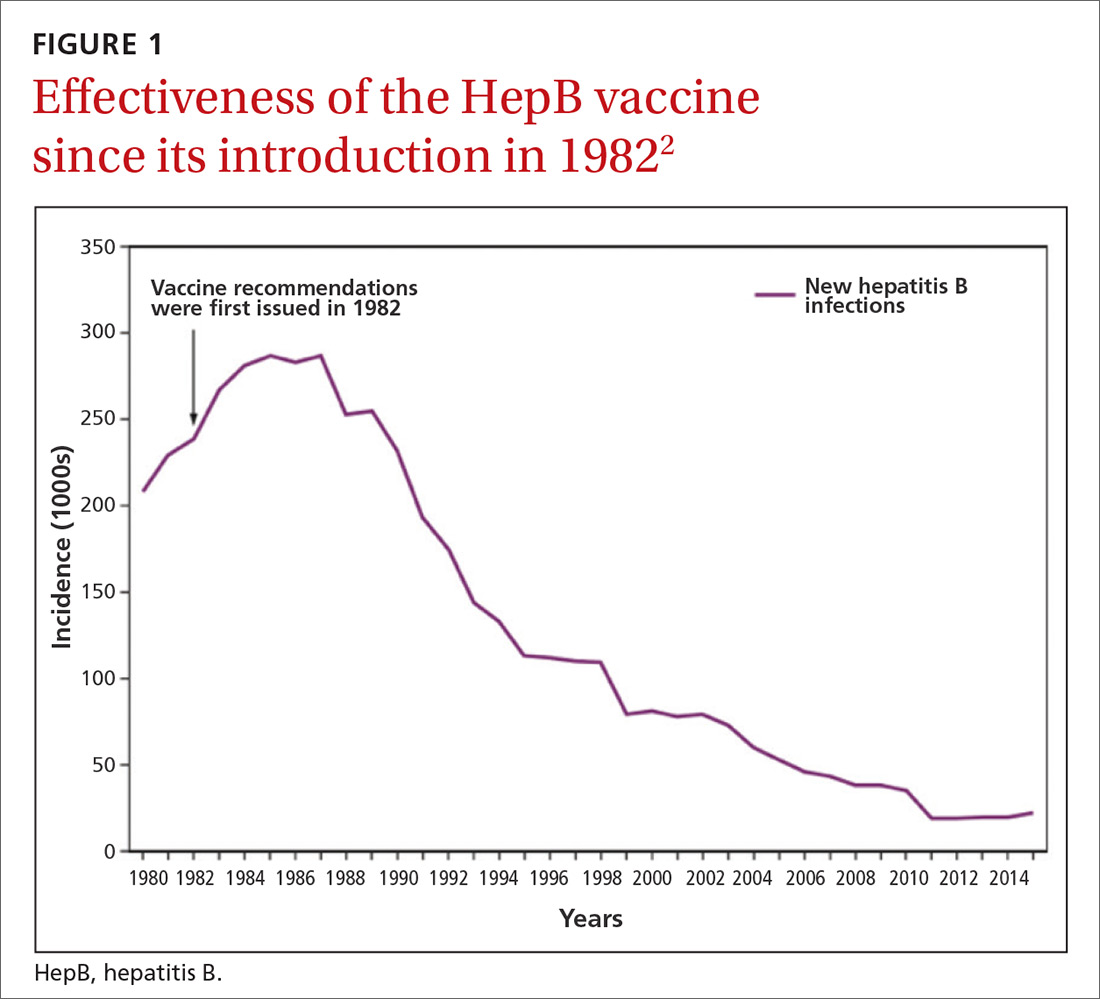
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.
Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
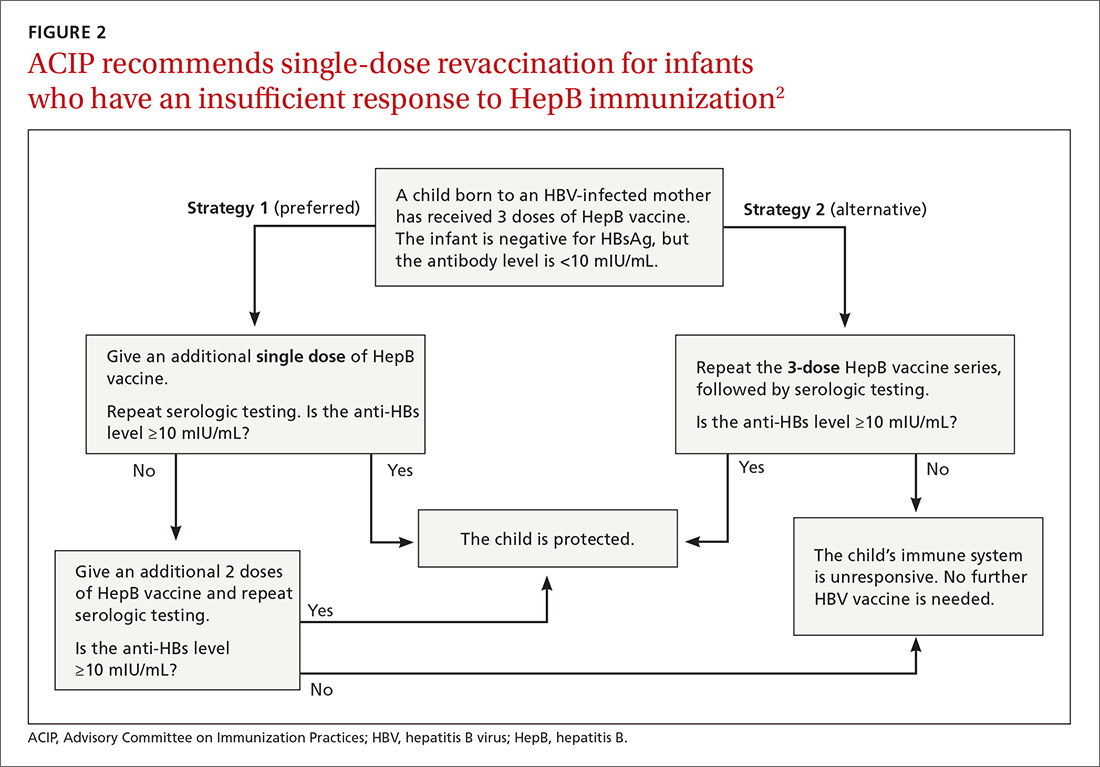
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).
(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.

Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).

(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.

Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).

(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
Depigmented plaques on vulva
A mother brought her 8-year-old daughter to our office for evaluation of vitiligo “down there” (FIGURE). The skin eruption first appeared on her vulva a year earlier and was intermittently pruritic. The lesions were initially smaller and red, but had since lightened in color, coalesced, and had begun to spread to the perianal area. The patient’s mother had received a call from her daughter’s teacher who observed that her daughter was scratching the area and might be masturbating in class.
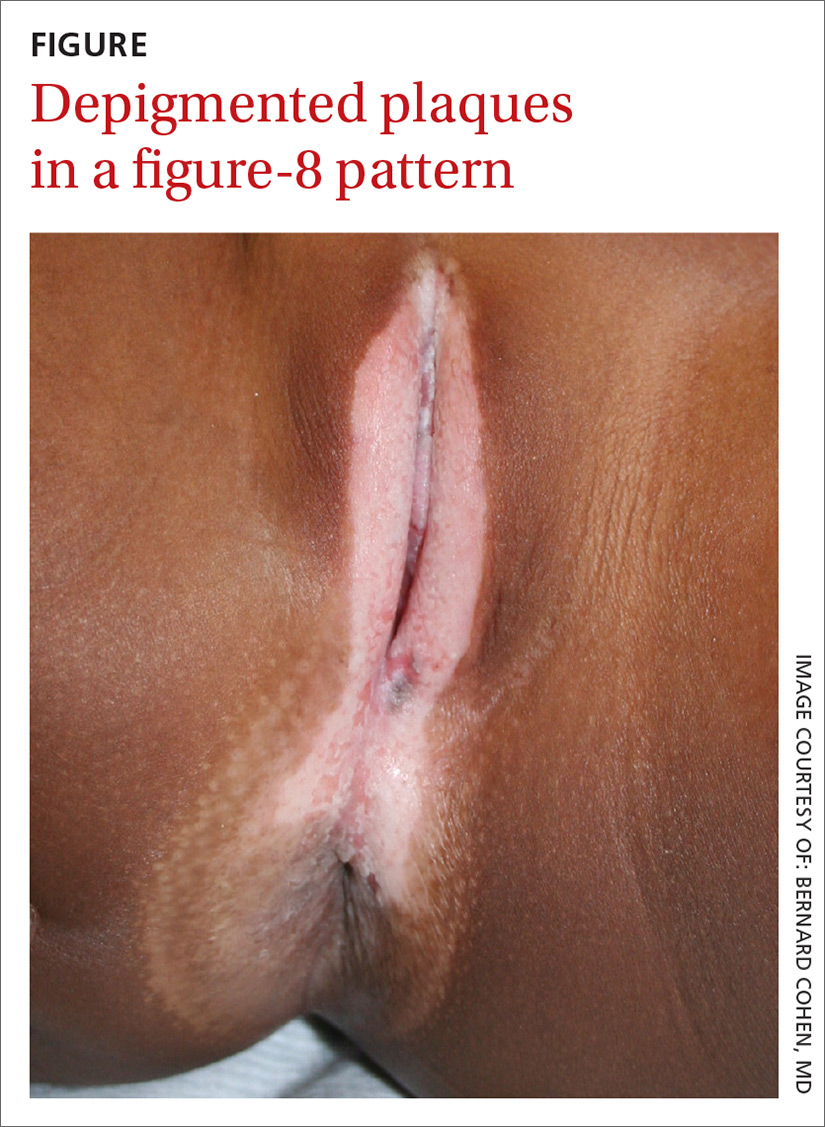
The mother reported that 6 months earlier, her daughter had experienced bloody spots in her underwear accompanied by dysuria. The mother brought her to the emergency department, where she was treated with antibiotics for a urinary tract infection.
Our physical examination revealed well-circumscribed, symmetric, depigmented, confluent, crinkled, parchment-like plaques with small hemorrhagic erosions on the medial labia majora and minora. The lesions had spread to the perianal area with depigmentation superiorly and hypopigmentation inferiorly, creating a figure-8 pattern.
A review of systems was negative for pruritus, pain, dysuria, dyschezia, constipation, and vaginal discharge. The patient denied sexual activity, depression, or anxiety. Her mother denied behavioral changes in her daughter and said that her daughter hadn’t had any one-on-one time alone with any adults besides herself. Her mother was concerned that the white spots might spread to the rest of her daughter’s body, which could affect her socially.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen sclerosus
Based on the history and clinical findings, including the classic figure-8 pattern, we diagnosed childhood lichen sclerosus (LS) in this patient. LS is a chronic inflammatory skin disorder that primarily affects the genital mucosa. The disorder can present at any age, but is most common among postmenopausal women, with a prevalence estimated to be as high as one in 30.1-3 A second incidence peak is observed in prepubescent girls, with a prevalence of one in 900.3,4 LS is less common in men and boys, with a female-to-male ratio that can reach 10:1.5 The classic symptoms of LS are pruritus and pain, which may be intermittent or persistent.
In girls, initial manifestations may be constipation, dysuria, or even behavioral symptoms such as night fears, which can occur because children are less active at night and become more aware of urinary discomfort.1,2,6 Typical signs of LS are thin atrophic plaques that spare the vagina and cervix. The plaques can be ivory-white, erythematous, or violaceous. Some patients have perianal lesions as well, and can display the pathognomonic figure-8 pattern of porcelain plaques around the vulva and anus.5
With more advanced disease, erosions, lichenification, and even distortion of vulvar architecture may occur.2,4,7 In severe cases, labia resorption and clitoral phimosis may develop.5 Complications include secondary infection, dyspareunia, and psychosexual distress. The most worrisome sequela of LS is squamous cell carcinoma of the vulva (SCCV), which occurs in 5% of female patients with LS.4
In men and boys, LS typically involves the foreskin and the glans, while sparing the perianal region.5 Scarring of the foreskin can lead to phimosis, and patients may complain of painful erections and difficulty urinating. LS can also occur away from the genitalia in both males and females.
Autoimmune mechanisms, genetics, and hormones play a role
The exact pathogenesis of LS remains unknown, but multiple factors are likely at work.
Autoimmune mechanisms. Up to 60% of women with LS have an autoimmune disorder, which is most commonly vitiligo, alopecia areata, or thyroid disease.5 In addition, 67% of patients have autoantibodies against extracellular matrix protein 1, and 30% have them against bullous pemphigoid antigen 180.1,8
Genetics. LS is associated with certain human leukocyte antigen class II haplotypes (especially DQ7) and with polymorphisms at the interleukin-1 receptor antagonist gene locus.5,6,9
Hormones. The clear peaks of incidence during times of low estrogen, and a higher incidence in patients with Turner syndrome or kidney disease, suggest that low estrogen may play a role in the development of LS, as well.1,5,6
While it is generally accepted that trauma may trigger LS via the Koebner phenomenon (the appearance of lesions at the site of injury), there is debate as to whether microbes—especially Borrelia burgdorferi and human papillomavirus (HPV)—might play a role.1,5
Diagnosis is often delayed, misdiagnosis is common
The average delay from symptom onset to diagnosis of LS is 1.3 years, and up to 84% of childhood LS is misdiagnosed before referral.2,9 The differential diagnosis includes:
Sexual abuse. In prepubertal girls presenting with genital redness, the can’t-miss diagnosis is sexual abuse, which occurs in more than 25% of children in the United States.10 Initial manifestations may be regression in developmental milestones, such as new-onset bedwetting, or behavioral changes such as social withdrawal or declining academic performance.11
However, physicians must be conscientious about ruling out medical etiologies before prematurely diagnosing abuse. Fourteen percent of girls with LS are incorrectly diagnosed as having been sexually abused.2 A clinical pearl is that while LS may resemble abuse on exam, it rarely affects the hymenal structure.12 It is also important to keep in mind that the 2 entities are not incompatible, as sexual abuse leading to LS via Koebnerization is a well-described phenomenon.12
Lichen planus. LP, which is also an immune-mediated inflammatory disorder affecting the vulva, classically presents with the 6 Ps: pruritic, polygonal, planar, purple papules and plaques.4 LP is distinguished from LS by being rare in childhood, having a predilection for the flexor wrists, and involving the oral and vaginal mucosa.4
Lichen simplex chronicus (LSC) is a chronic, circumscribed, pruritic, eczematous condition that becomes lichenified with thickened skin secondary to repeated scratching.13 Children with atopic dermatitis can develop LSC, but other children can also develop the scratch-itch cycle that results in the thickened plaques of LSC. Like LS, LSC can occur in areas other than the genitalia, including the neck and feet.14
Allergic contact dermatitis can occur in the genital area from diaper creams, soaps, and perfumes. Irritant contact dermatitis can occur from exposure to diarrhea, bedwetting, and other irritants. Contact dermatitis is less likely to have the classic figure-8 pattern seen in LS.
Psoriasis in the genital area can be confused with LS. However, psoriasis favors the groin creases in what is called inverse psoriasis. In addition, psoriasis tends to involve multiple areas, including the extensor surfaces of the elbows and knees, the nails, and the scalp.
Vitiligo can present on the genitals as circumscribed hypopigmented and depigmented patches that are flat. Vitiligo is asymptomatic, and the only pathology is the change in skin color. With LS, there is lichenification, atrophy, and sclerosis.4 Vitiligo often occurs with bilateral symmetric involvement in areas of trauma including the face, neck, scalp, elbows, wrists, hands, knees, ankles, and feet.
Treatment aims to improve symptoms
LS is usually diagnosed clinically (especially in children, as a biopsy is a great challenge to perform). However, when the clinical presentation is unclear, a skin biopsy will demonstrate the diagnostic findings of thinning of the epidermis, loss of rete pegs, hyperkeratosis, and dermal fibrosis with a T-lymphocyte-dominant inflammatory infiltrate.1,2,4,5
LS is a remitting and relapsing condition with no cure. The goals of treatment are to provide symptom relief and minimize scarring and atrophy,2 but it is unknown whether treatment reduces the risk of malignancy.9
First-line treatment for both genders and all ages is ultrapotent topical corticosteroids; clobetasol propionate 0.05% is most commonly used.1,6 Regimens vary, but the vast majority of patients improve within 3 months of once-daily treatment.4
For refractory LS, calcineurin inhibitors such as tacrolimus may be used. Although it has a black box warning regarding a potential cancer risk, long-term studies of children using tacrolimus for atopic dermatitis have not demonstrated an increased risk of malignancy.6,9 Because of a considerable adverse effect profile, oral retinoids are limited to refractory cases in adults.6 Surgery is reserved for scarring and adhesions.4
Follow-up plays an important role in management
Historically, it was believed that pediatric LS had an excellent prognosis, with patients achieving complete resolution after puberty.1,4 Recent findings have shown mixed results, with LS persisting in many patients beyond puberty.2,4 Therefore, regular follow-up is recommended every 6 to 12 months.
For uncomplicated LS, specialist follow-up is not indicated. Female patients should regularly conduct self-examinations and, at a minimum, undergo annual examinations by their primary care physician. Those who require specialist follow-up include patients with difficult-to-control symptoms, hypertrophic lesions, a history of SCCV or differentiated vulvar intraepithelial neoplasia (dVIN), or pathology showing possible dVIN.15
Our patient. We prescribed clobetasol propionate 0.05% ointment to be used once daily for 8 weeks. We stressed the importance of genital self-examinations using a mirror to monitor for any concerning changes such as skin thickening. We showed the patient and her mother photos of normal female genitalia to help normalize the genital exam, and taught the patient how to find her plaques in the mirror. We set expectations by emphasizing the chronic nature of LS and the likelihood of recurrence. We also encouraged HPV vaccination in the upcoming years to prevent both cervical cancer and HPV-related SCCV.
CORRESPONDENCE
Somya Abubucker, MD, University of Hawaii, 1356 Lusitana Street, 7th floor, Honolulu, HI 96813; [email protected].
1. Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707-715.
2. Dendrinos ML, Quint EH. Lichen sclerosus in children and adolescents. Curr Opin Obstet Gynecol. 2013;25:370-374.
3. Eva LJ. Screening and follow up of vulval skin disorders. Best Pract Res Clin Obstet Gynaecol. 2012;26:175-188.
4. Focseneanu MA, Gupta M, Squires KC, et al. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26:153-155.
5. Funaro D. Lichen sclerosus: a review and practical approach. Dermatol Ther. 2004;17:28-37.
6. Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683-684.
7. Tong LX, Sun GS, Teng JM. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32:593-599.
8. Lagerstedt M, Karvinen K, Joki-Erkkilä M, et al. Childhood lichen sclerosus—a challenge for clinicians. Pediatr Dermatol. 2013;30:444-450.
9. Keith PJ, Wolz MM, Peters MS. Eosinophils in lichen sclerosus et atrophicus. J Cutan Pathol. 2015;42:693-698.
10. National Sexual Violence Resource Center. Child sexual abuse prevention. 2011. Available at: https://www.nsvrc.org/sites/default/files/Publications_NSVRC_Overview_Child-sexual-abuse-prevention_0.pdf. Accessed February 8, 2018.
11. Dubowitz H, Lane WG. Abused and neglected children. In: Kliegman RM, Stanton BF, St. Geme JW, et al, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016:236-249.
12. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803-806.
13. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
14. Warshaw E, Hook K. Dermatitis. In: Soutor C, Hordinsky MK, eds. Clinical Dermatology. 1st ed. New York, NY: McGraw-Hill; 2013.
15. Jones RW, Scurry J, Neill S, et al. Guidelines for the follow-up of women with vulvar lichen sclerosus in specialist clinics. Am J Obstet Gynecol. 2008;198:496.e1-e3.
A mother brought her 8-year-old daughter to our office for evaluation of vitiligo “down there” (FIGURE). The skin eruption first appeared on her vulva a year earlier and was intermittently pruritic. The lesions were initially smaller and red, but had since lightened in color, coalesced, and had begun to spread to the perianal area. The patient’s mother had received a call from her daughter’s teacher who observed that her daughter was scratching the area and might be masturbating in class.

The mother reported that 6 months earlier, her daughter had experienced bloody spots in her underwear accompanied by dysuria. The mother brought her to the emergency department, where she was treated with antibiotics for a urinary tract infection.
Our physical examination revealed well-circumscribed, symmetric, depigmented, confluent, crinkled, parchment-like plaques with small hemorrhagic erosions on the medial labia majora and minora. The lesions had spread to the perianal area with depigmentation superiorly and hypopigmentation inferiorly, creating a figure-8 pattern.
A review of systems was negative for pruritus, pain, dysuria, dyschezia, constipation, and vaginal discharge. The patient denied sexual activity, depression, or anxiety. Her mother denied behavioral changes in her daughter and said that her daughter hadn’t had any one-on-one time alone with any adults besides herself. Her mother was concerned that the white spots might spread to the rest of her daughter’s body, which could affect her socially.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen sclerosus
Based on the history and clinical findings, including the classic figure-8 pattern, we diagnosed childhood lichen sclerosus (LS) in this patient. LS is a chronic inflammatory skin disorder that primarily affects the genital mucosa. The disorder can present at any age, but is most common among postmenopausal women, with a prevalence estimated to be as high as one in 30.1-3 A second incidence peak is observed in prepubescent girls, with a prevalence of one in 900.3,4 LS is less common in men and boys, with a female-to-male ratio that can reach 10:1.5 The classic symptoms of LS are pruritus and pain, which may be intermittent or persistent.
In girls, initial manifestations may be constipation, dysuria, or even behavioral symptoms such as night fears, which can occur because children are less active at night and become more aware of urinary discomfort.1,2,6 Typical signs of LS are thin atrophic plaques that spare the vagina and cervix. The plaques can be ivory-white, erythematous, or violaceous. Some patients have perianal lesions as well, and can display the pathognomonic figure-8 pattern of porcelain plaques around the vulva and anus.5
With more advanced disease, erosions, lichenification, and even distortion of vulvar architecture may occur.2,4,7 In severe cases, labia resorption and clitoral phimosis may develop.5 Complications include secondary infection, dyspareunia, and psychosexual distress. The most worrisome sequela of LS is squamous cell carcinoma of the vulva (SCCV), which occurs in 5% of female patients with LS.4
In men and boys, LS typically involves the foreskin and the glans, while sparing the perianal region.5 Scarring of the foreskin can lead to phimosis, and patients may complain of painful erections and difficulty urinating. LS can also occur away from the genitalia in both males and females.
Autoimmune mechanisms, genetics, and hormones play a role
The exact pathogenesis of LS remains unknown, but multiple factors are likely at work.
Autoimmune mechanisms. Up to 60% of women with LS have an autoimmune disorder, which is most commonly vitiligo, alopecia areata, or thyroid disease.5 In addition, 67% of patients have autoantibodies against extracellular matrix protein 1, and 30% have them against bullous pemphigoid antigen 180.1,8
Genetics. LS is associated with certain human leukocyte antigen class II haplotypes (especially DQ7) and with polymorphisms at the interleukin-1 receptor antagonist gene locus.5,6,9
Hormones. The clear peaks of incidence during times of low estrogen, and a higher incidence in patients with Turner syndrome or kidney disease, suggest that low estrogen may play a role in the development of LS, as well.1,5,6
While it is generally accepted that trauma may trigger LS via the Koebner phenomenon (the appearance of lesions at the site of injury), there is debate as to whether microbes—especially Borrelia burgdorferi and human papillomavirus (HPV)—might play a role.1,5
Diagnosis is often delayed, misdiagnosis is common
The average delay from symptom onset to diagnosis of LS is 1.3 years, and up to 84% of childhood LS is misdiagnosed before referral.2,9 The differential diagnosis includes:
Sexual abuse. In prepubertal girls presenting with genital redness, the can’t-miss diagnosis is sexual abuse, which occurs in more than 25% of children in the United States.10 Initial manifestations may be regression in developmental milestones, such as new-onset bedwetting, or behavioral changes such as social withdrawal or declining academic performance.11
However, physicians must be conscientious about ruling out medical etiologies before prematurely diagnosing abuse. Fourteen percent of girls with LS are incorrectly diagnosed as having been sexually abused.2 A clinical pearl is that while LS may resemble abuse on exam, it rarely affects the hymenal structure.12 It is also important to keep in mind that the 2 entities are not incompatible, as sexual abuse leading to LS via Koebnerization is a well-described phenomenon.12
Lichen planus. LP, which is also an immune-mediated inflammatory disorder affecting the vulva, classically presents with the 6 Ps: pruritic, polygonal, planar, purple papules and plaques.4 LP is distinguished from LS by being rare in childhood, having a predilection for the flexor wrists, and involving the oral and vaginal mucosa.4
Lichen simplex chronicus (LSC) is a chronic, circumscribed, pruritic, eczematous condition that becomes lichenified with thickened skin secondary to repeated scratching.13 Children with atopic dermatitis can develop LSC, but other children can also develop the scratch-itch cycle that results in the thickened plaques of LSC. Like LS, LSC can occur in areas other than the genitalia, including the neck and feet.14
Allergic contact dermatitis can occur in the genital area from diaper creams, soaps, and perfumes. Irritant contact dermatitis can occur from exposure to diarrhea, bedwetting, and other irritants. Contact dermatitis is less likely to have the classic figure-8 pattern seen in LS.
Psoriasis in the genital area can be confused with LS. However, psoriasis favors the groin creases in what is called inverse psoriasis. In addition, psoriasis tends to involve multiple areas, including the extensor surfaces of the elbows and knees, the nails, and the scalp.
Vitiligo can present on the genitals as circumscribed hypopigmented and depigmented patches that are flat. Vitiligo is asymptomatic, and the only pathology is the change in skin color. With LS, there is lichenification, atrophy, and sclerosis.4 Vitiligo often occurs with bilateral symmetric involvement in areas of trauma including the face, neck, scalp, elbows, wrists, hands, knees, ankles, and feet.
Treatment aims to improve symptoms
LS is usually diagnosed clinically (especially in children, as a biopsy is a great challenge to perform). However, when the clinical presentation is unclear, a skin biopsy will demonstrate the diagnostic findings of thinning of the epidermis, loss of rete pegs, hyperkeratosis, and dermal fibrosis with a T-lymphocyte-dominant inflammatory infiltrate.1,2,4,5
LS is a remitting and relapsing condition with no cure. The goals of treatment are to provide symptom relief and minimize scarring and atrophy,2 but it is unknown whether treatment reduces the risk of malignancy.9
First-line treatment for both genders and all ages is ultrapotent topical corticosteroids; clobetasol propionate 0.05% is most commonly used.1,6 Regimens vary, but the vast majority of patients improve within 3 months of once-daily treatment.4
For refractory LS, calcineurin inhibitors such as tacrolimus may be used. Although it has a black box warning regarding a potential cancer risk, long-term studies of children using tacrolimus for atopic dermatitis have not demonstrated an increased risk of malignancy.6,9 Because of a considerable adverse effect profile, oral retinoids are limited to refractory cases in adults.6 Surgery is reserved for scarring and adhesions.4
Follow-up plays an important role in management
Historically, it was believed that pediatric LS had an excellent prognosis, with patients achieving complete resolution after puberty.1,4 Recent findings have shown mixed results, with LS persisting in many patients beyond puberty.2,4 Therefore, regular follow-up is recommended every 6 to 12 months.
For uncomplicated LS, specialist follow-up is not indicated. Female patients should regularly conduct self-examinations and, at a minimum, undergo annual examinations by their primary care physician. Those who require specialist follow-up include patients with difficult-to-control symptoms, hypertrophic lesions, a history of SCCV or differentiated vulvar intraepithelial neoplasia (dVIN), or pathology showing possible dVIN.15
Our patient. We prescribed clobetasol propionate 0.05% ointment to be used once daily for 8 weeks. We stressed the importance of genital self-examinations using a mirror to monitor for any concerning changes such as skin thickening. We showed the patient and her mother photos of normal female genitalia to help normalize the genital exam, and taught the patient how to find her plaques in the mirror. We set expectations by emphasizing the chronic nature of LS and the likelihood of recurrence. We also encouraged HPV vaccination in the upcoming years to prevent both cervical cancer and HPV-related SCCV.
CORRESPONDENCE
Somya Abubucker, MD, University of Hawaii, 1356 Lusitana Street, 7th floor, Honolulu, HI 96813; [email protected].
A mother brought her 8-year-old daughter to our office for evaluation of vitiligo “down there” (FIGURE). The skin eruption first appeared on her vulva a year earlier and was intermittently pruritic. The lesions were initially smaller and red, but had since lightened in color, coalesced, and had begun to spread to the perianal area. The patient’s mother had received a call from her daughter’s teacher who observed that her daughter was scratching the area and might be masturbating in class.

The mother reported that 6 months earlier, her daughter had experienced bloody spots in her underwear accompanied by dysuria. The mother brought her to the emergency department, where she was treated with antibiotics for a urinary tract infection.
Our physical examination revealed well-circumscribed, symmetric, depigmented, confluent, crinkled, parchment-like plaques with small hemorrhagic erosions on the medial labia majora and minora. The lesions had spread to the perianal area with depigmentation superiorly and hypopigmentation inferiorly, creating a figure-8 pattern.
A review of systems was negative for pruritus, pain, dysuria, dyschezia, constipation, and vaginal discharge. The patient denied sexual activity, depression, or anxiety. Her mother denied behavioral changes in her daughter and said that her daughter hadn’t had any one-on-one time alone with any adults besides herself. Her mother was concerned that the white spots might spread to the rest of her daughter’s body, which could affect her socially.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen sclerosus
Based on the history and clinical findings, including the classic figure-8 pattern, we diagnosed childhood lichen sclerosus (LS) in this patient. LS is a chronic inflammatory skin disorder that primarily affects the genital mucosa. The disorder can present at any age, but is most common among postmenopausal women, with a prevalence estimated to be as high as one in 30.1-3 A second incidence peak is observed in prepubescent girls, with a prevalence of one in 900.3,4 LS is less common in men and boys, with a female-to-male ratio that can reach 10:1.5 The classic symptoms of LS are pruritus and pain, which may be intermittent or persistent.
In girls, initial manifestations may be constipation, dysuria, or even behavioral symptoms such as night fears, which can occur because children are less active at night and become more aware of urinary discomfort.1,2,6 Typical signs of LS are thin atrophic plaques that spare the vagina and cervix. The plaques can be ivory-white, erythematous, or violaceous. Some patients have perianal lesions as well, and can display the pathognomonic figure-8 pattern of porcelain plaques around the vulva and anus.5
With more advanced disease, erosions, lichenification, and even distortion of vulvar architecture may occur.2,4,7 In severe cases, labia resorption and clitoral phimosis may develop.5 Complications include secondary infection, dyspareunia, and psychosexual distress. The most worrisome sequela of LS is squamous cell carcinoma of the vulva (SCCV), which occurs in 5% of female patients with LS.4
In men and boys, LS typically involves the foreskin and the glans, while sparing the perianal region.5 Scarring of the foreskin can lead to phimosis, and patients may complain of painful erections and difficulty urinating. LS can also occur away from the genitalia in both males and females.
Autoimmune mechanisms, genetics, and hormones play a role
The exact pathogenesis of LS remains unknown, but multiple factors are likely at work.
Autoimmune mechanisms. Up to 60% of women with LS have an autoimmune disorder, which is most commonly vitiligo, alopecia areata, or thyroid disease.5 In addition, 67% of patients have autoantibodies against extracellular matrix protein 1, and 30% have them against bullous pemphigoid antigen 180.1,8
Genetics. LS is associated with certain human leukocyte antigen class II haplotypes (especially DQ7) and with polymorphisms at the interleukin-1 receptor antagonist gene locus.5,6,9
Hormones. The clear peaks of incidence during times of low estrogen, and a higher incidence in patients with Turner syndrome or kidney disease, suggest that low estrogen may play a role in the development of LS, as well.1,5,6
While it is generally accepted that trauma may trigger LS via the Koebner phenomenon (the appearance of lesions at the site of injury), there is debate as to whether microbes—especially Borrelia burgdorferi and human papillomavirus (HPV)—might play a role.1,5
Diagnosis is often delayed, misdiagnosis is common
The average delay from symptom onset to diagnosis of LS is 1.3 years, and up to 84% of childhood LS is misdiagnosed before referral.2,9 The differential diagnosis includes:
Sexual abuse. In prepubertal girls presenting with genital redness, the can’t-miss diagnosis is sexual abuse, which occurs in more than 25% of children in the United States.10 Initial manifestations may be regression in developmental milestones, such as new-onset bedwetting, or behavioral changes such as social withdrawal or declining academic performance.11
However, physicians must be conscientious about ruling out medical etiologies before prematurely diagnosing abuse. Fourteen percent of girls with LS are incorrectly diagnosed as having been sexually abused.2 A clinical pearl is that while LS may resemble abuse on exam, it rarely affects the hymenal structure.12 It is also important to keep in mind that the 2 entities are not incompatible, as sexual abuse leading to LS via Koebnerization is a well-described phenomenon.12
Lichen planus. LP, which is also an immune-mediated inflammatory disorder affecting the vulva, classically presents with the 6 Ps: pruritic, polygonal, planar, purple papules and plaques.4 LP is distinguished from LS by being rare in childhood, having a predilection for the flexor wrists, and involving the oral and vaginal mucosa.4
Lichen simplex chronicus (LSC) is a chronic, circumscribed, pruritic, eczematous condition that becomes lichenified with thickened skin secondary to repeated scratching.13 Children with atopic dermatitis can develop LSC, but other children can also develop the scratch-itch cycle that results in the thickened plaques of LSC. Like LS, LSC can occur in areas other than the genitalia, including the neck and feet.14
Allergic contact dermatitis can occur in the genital area from diaper creams, soaps, and perfumes. Irritant contact dermatitis can occur from exposure to diarrhea, bedwetting, and other irritants. Contact dermatitis is less likely to have the classic figure-8 pattern seen in LS.
Psoriasis in the genital area can be confused with LS. However, psoriasis favors the groin creases in what is called inverse psoriasis. In addition, psoriasis tends to involve multiple areas, including the extensor surfaces of the elbows and knees, the nails, and the scalp.
Vitiligo can present on the genitals as circumscribed hypopigmented and depigmented patches that are flat. Vitiligo is asymptomatic, and the only pathology is the change in skin color. With LS, there is lichenification, atrophy, and sclerosis.4 Vitiligo often occurs with bilateral symmetric involvement in areas of trauma including the face, neck, scalp, elbows, wrists, hands, knees, ankles, and feet.
Treatment aims to improve symptoms
LS is usually diagnosed clinically (especially in children, as a biopsy is a great challenge to perform). However, when the clinical presentation is unclear, a skin biopsy will demonstrate the diagnostic findings of thinning of the epidermis, loss of rete pegs, hyperkeratosis, and dermal fibrosis with a T-lymphocyte-dominant inflammatory infiltrate.1,2,4,5
LS is a remitting and relapsing condition with no cure. The goals of treatment are to provide symptom relief and minimize scarring and atrophy,2 but it is unknown whether treatment reduces the risk of malignancy.9
First-line treatment for both genders and all ages is ultrapotent topical corticosteroids; clobetasol propionate 0.05% is most commonly used.1,6 Regimens vary, but the vast majority of patients improve within 3 months of once-daily treatment.4
For refractory LS, calcineurin inhibitors such as tacrolimus may be used. Although it has a black box warning regarding a potential cancer risk, long-term studies of children using tacrolimus for atopic dermatitis have not demonstrated an increased risk of malignancy.6,9 Because of a considerable adverse effect profile, oral retinoids are limited to refractory cases in adults.6 Surgery is reserved for scarring and adhesions.4
Follow-up plays an important role in management
Historically, it was believed that pediatric LS had an excellent prognosis, with patients achieving complete resolution after puberty.1,4 Recent findings have shown mixed results, with LS persisting in many patients beyond puberty.2,4 Therefore, regular follow-up is recommended every 6 to 12 months.
For uncomplicated LS, specialist follow-up is not indicated. Female patients should regularly conduct self-examinations and, at a minimum, undergo annual examinations by their primary care physician. Those who require specialist follow-up include patients with difficult-to-control symptoms, hypertrophic lesions, a history of SCCV or differentiated vulvar intraepithelial neoplasia (dVIN), or pathology showing possible dVIN.15
Our patient. We prescribed clobetasol propionate 0.05% ointment to be used once daily for 8 weeks. We stressed the importance of genital self-examinations using a mirror to monitor for any concerning changes such as skin thickening. We showed the patient and her mother photos of normal female genitalia to help normalize the genital exam, and taught the patient how to find her plaques in the mirror. We set expectations by emphasizing the chronic nature of LS and the likelihood of recurrence. We also encouraged HPV vaccination in the upcoming years to prevent both cervical cancer and HPV-related SCCV.
CORRESPONDENCE
Somya Abubucker, MD, University of Hawaii, 1356 Lusitana Street, 7th floor, Honolulu, HI 96813; [email protected].
1. Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707-715.
2. Dendrinos ML, Quint EH. Lichen sclerosus in children and adolescents. Curr Opin Obstet Gynecol. 2013;25:370-374.
3. Eva LJ. Screening and follow up of vulval skin disorders. Best Pract Res Clin Obstet Gynaecol. 2012;26:175-188.
4. Focseneanu MA, Gupta M, Squires KC, et al. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26:153-155.
5. Funaro D. Lichen sclerosus: a review and practical approach. Dermatol Ther. 2004;17:28-37.
6. Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683-684.
7. Tong LX, Sun GS, Teng JM. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32:593-599.
8. Lagerstedt M, Karvinen K, Joki-Erkkilä M, et al. Childhood lichen sclerosus—a challenge for clinicians. Pediatr Dermatol. 2013;30:444-450.
9. Keith PJ, Wolz MM, Peters MS. Eosinophils in lichen sclerosus et atrophicus. J Cutan Pathol. 2015;42:693-698.
10. National Sexual Violence Resource Center. Child sexual abuse prevention. 2011. Available at: https://www.nsvrc.org/sites/default/files/Publications_NSVRC_Overview_Child-sexual-abuse-prevention_0.pdf. Accessed February 8, 2018.
11. Dubowitz H, Lane WG. Abused and neglected children. In: Kliegman RM, Stanton BF, St. Geme JW, et al, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016:236-249.
12. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803-806.
13. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
14. Warshaw E, Hook K. Dermatitis. In: Soutor C, Hordinsky MK, eds. Clinical Dermatology. 1st ed. New York, NY: McGraw-Hill; 2013.
15. Jones RW, Scurry J, Neill S, et al. Guidelines for the follow-up of women with vulvar lichen sclerosus in specialist clinics. Am J Obstet Gynecol. 2008;198:496.e1-e3.
1. Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707-715.
2. Dendrinos ML, Quint EH. Lichen sclerosus in children and adolescents. Curr Opin Obstet Gynecol. 2013;25:370-374.
3. Eva LJ. Screening and follow up of vulval skin disorders. Best Pract Res Clin Obstet Gynaecol. 2012;26:175-188.
4. Focseneanu MA, Gupta M, Squires KC, et al. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26:153-155.
5. Funaro D. Lichen sclerosus: a review and practical approach. Dermatol Ther. 2004;17:28-37.
6. Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683-684.
7. Tong LX, Sun GS, Teng JM. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32:593-599.
8. Lagerstedt M, Karvinen K, Joki-Erkkilä M, et al. Childhood lichen sclerosus—a challenge for clinicians. Pediatr Dermatol. 2013;30:444-450.
9. Keith PJ, Wolz MM, Peters MS. Eosinophils in lichen sclerosus et atrophicus. J Cutan Pathol. 2015;42:693-698.
10. National Sexual Violence Resource Center. Child sexual abuse prevention. 2011. Available at: https://www.nsvrc.org/sites/default/files/Publications_NSVRC_Overview_Child-sexual-abuse-prevention_0.pdf. Accessed February 8, 2018.
11. Dubowitz H, Lane WG. Abused and neglected children. In: Kliegman RM, Stanton BF, St. Geme JW, et al, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016:236-249.
12. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803-806.
13. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
14. Warshaw E, Hook K. Dermatitis. In: Soutor C, Hordinsky MK, eds. Clinical Dermatology. 1st ed. New York, NY: McGraw-Hill; 2013.
15. Jones RW, Scurry J, Neill S, et al. Guidelines for the follow-up of women with vulvar lichen sclerosus in specialist clinics. Am J Obstet Gynecol. 2008;198:496.e1-e3.
Does exercise relieve vasomotor menopausal symptoms?
EVIDENCE SUMMARY
A 2014 Cochrane meta-analysis of 5 RCTs with a total of 733 patients examined the effectiveness of any type of exercise in decreasing vasomotor symptoms in perimenopausal and postmenopausal women.1 The studies compared exercise—defined as structured exercise or physical activity through active living—with no active treatment, yoga, or hormone therapy (HT) over a 3- to 24-month follow-up period.
Three trials of 454 women that compared exercise with no active treatment found no difference between groups in frequency or intensity of vasomotor symptoms (standard mean difference [SMD]= -0.10; 95% confidence interval [CI], -0.33 to 0.13).
Two trials with 279 women that compared exercise with yoga didn’t find a difference in reported frequency or intensity of vasomotor symptoms between the groups (SMD= -0.03; 95% CI, -0.45 to 0.38).
One small trial (14 women) of exercise and HT found that HT patients reported decreased frequency of flushes over 24 hours compared with the exercise group (mean difference [MD]=5.8; 95% CI, 3.17-8.43).
Overall, the evidence was of low quality because of heterogeneity in study design.1
Two exercise interventions fail to reduce symptoms
A 2014 RCT, published after the Cochrane search date, investigated exercise as a treatment for VMS in 261 perimenopausal and postmenopausal women ages 48 to 57 years.2 Patients had a history of at least 5 hot flashes or night sweats per day and hadn’t taken HT in the previous 3 months.
The women were randomized to one of 2 exercise interventions or a control group. The exercise interventions both entailed 2 one-on-one consultations with a physical activity facilitator and use of a pedometer. Patients were encouraged to perform 30 minutes of moderate-intensity exercise 3 days a week during Weeks 1 through 12, then increase the frequency to 3 to 5 days a week during Weeks 13 through 24. In one intervention arm, the women also received an informational DVD and 5 educational leaflets.
In the other arm, they were invited to attend 3 exercise support groups in their local community. The control group was offered an opportunity for exercise consultation and given a pedometer at the end of the study.
At the end of the 6-month intervention, neither exercise intervention significantly decreased self-reported hot flashes/night sweats per week compared with the control group (DVD exercise arm vs control: MD= -8.9; 95% CI, -20 to 2.2; social support exercise arm vs control: MD= -5.2; 95% CI, -16.7 to 6.3). The study also found no difference in hot flashes/night sweats per week at 12-month follow-up between the DVD exercise arm and controls (MD= -3.2; 95% CI, -12.7 to 6.4) and the social-support group and controls (MD= -3.5; 95% CI, -13.2 to 6.1).
Drug therapy relieves symptoms, but other methods—not so much
An analysis of pooled individual data from 3 RCTs compared exercise with 5 other interventions for VMS in 899 perimenopausal and postmenopausal women.3 Patients had at least 14 bothersome symptoms per week.
The 6 interventions ranged from nonpharmacologic therapies, such as aerobic exercise and yoga, to pharmacologic treatments, including escitalopram 10 to 20 mg/d, venlafaxine 75 mg/d, oral estradiol (E2) 0.5 mg/d, and omega-3 supplementation 1.8 g/d. The primary outcome was a change in VMS frequency and bother as assessed by a symptom diary over the 4- to 12-week follow-up.
The analysis found a significant 6-week reduction in daily VMS frequency relative to placebo for escitalopram (MD= -1.4; 95% CI, -2.7 to -0.2), low-dose E2 (MD= -1.9; 95% CI, -2.9 to -0.9), and venlafaxine (MD= -1.3; 95% CI, -2.3 to -0.3). However, no difference in VMS frequency or bother was found with exercise (MD= -0.4; 95% CI, -1.1 to 0.3), yoga (MD= -0.6; 95% CI, -1.3 to 0.1), or omega-3 supplementation (MD= 0.2; 95% CI, -0.4 to 0.8).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) doesn’t offer specific recommendations regarding exercise as a treatment for symptoms of menopause. The 2014 ACOG guidelines for managing symptoms report that data don’t support phytoestrogens, supplements, or lifestyle modifications (Level B, based on limited or inconsistent evidence). ACOG recommends basic palliative measures such as drinking cool drinks and decreasing layers of clothing (Level B).4
The American Association of Clinical Endocrinologists’ recommendations don’t mention exercise as a menopause therapy.5
The North American Menopause Society’s 2015 statement regarding the nonhormonal treatment of menopause symptoms doesn’t recommend exercise as an effective therapy because of insufficient or inconclusive data.6
1. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;(11):CD006108.
2. Daley AJ, Thomas A, Roalfe AK, et al. The effectiveness of exercise as treatment for vasomotor menopausal symptoms: randomized controlled trial. BJOG. 2015;122:565-575.
3. Guthrie KA, LaCroix AZ, Ensrud KE, et al. Pooled analysis of six pharmacologic and nonpharmacologic interventions for vasomotor symptoms. Obstet Gynecol. 2015;126:413-422.
4. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
5. Goodman NF, Cobin RH, Ginzburg SB, et al; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause: executive summary of recommendations. Endocr Pract. 2011;17:949-954.
6. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22:1155-1172.
EVIDENCE SUMMARY
A 2014 Cochrane meta-analysis of 5 RCTs with a total of 733 patients examined the effectiveness of any type of exercise in decreasing vasomotor symptoms in perimenopausal and postmenopausal women.1 The studies compared exercise—defined as structured exercise or physical activity through active living—with no active treatment, yoga, or hormone therapy (HT) over a 3- to 24-month follow-up period.
Three trials of 454 women that compared exercise with no active treatment found no difference between groups in frequency or intensity of vasomotor symptoms (standard mean difference [SMD]= -0.10; 95% confidence interval [CI], -0.33 to 0.13).
Two trials with 279 women that compared exercise with yoga didn’t find a difference in reported frequency or intensity of vasomotor symptoms between the groups (SMD= -0.03; 95% CI, -0.45 to 0.38).
One small trial (14 women) of exercise and HT found that HT patients reported decreased frequency of flushes over 24 hours compared with the exercise group (mean difference [MD]=5.8; 95% CI, 3.17-8.43).
Overall, the evidence was of low quality because of heterogeneity in study design.1
Two exercise interventions fail to reduce symptoms
A 2014 RCT, published after the Cochrane search date, investigated exercise as a treatment for VMS in 261 perimenopausal and postmenopausal women ages 48 to 57 years.2 Patients had a history of at least 5 hot flashes or night sweats per day and hadn’t taken HT in the previous 3 months.
The women were randomized to one of 2 exercise interventions or a control group. The exercise interventions both entailed 2 one-on-one consultations with a physical activity facilitator and use of a pedometer. Patients were encouraged to perform 30 minutes of moderate-intensity exercise 3 days a week during Weeks 1 through 12, then increase the frequency to 3 to 5 days a week during Weeks 13 through 24. In one intervention arm, the women also received an informational DVD and 5 educational leaflets.
In the other arm, they were invited to attend 3 exercise support groups in their local community. The control group was offered an opportunity for exercise consultation and given a pedometer at the end of the study.
At the end of the 6-month intervention, neither exercise intervention significantly decreased self-reported hot flashes/night sweats per week compared with the control group (DVD exercise arm vs control: MD= -8.9; 95% CI, -20 to 2.2; social support exercise arm vs control: MD= -5.2; 95% CI, -16.7 to 6.3). The study also found no difference in hot flashes/night sweats per week at 12-month follow-up between the DVD exercise arm and controls (MD= -3.2; 95% CI, -12.7 to 6.4) and the social-support group and controls (MD= -3.5; 95% CI, -13.2 to 6.1).
Drug therapy relieves symptoms, but other methods—not so much
An analysis of pooled individual data from 3 RCTs compared exercise with 5 other interventions for VMS in 899 perimenopausal and postmenopausal women.3 Patients had at least 14 bothersome symptoms per week.
The 6 interventions ranged from nonpharmacologic therapies, such as aerobic exercise and yoga, to pharmacologic treatments, including escitalopram 10 to 20 mg/d, venlafaxine 75 mg/d, oral estradiol (E2) 0.5 mg/d, and omega-3 supplementation 1.8 g/d. The primary outcome was a change in VMS frequency and bother as assessed by a symptom diary over the 4- to 12-week follow-up.
The analysis found a significant 6-week reduction in daily VMS frequency relative to placebo for escitalopram (MD= -1.4; 95% CI, -2.7 to -0.2), low-dose E2 (MD= -1.9; 95% CI, -2.9 to -0.9), and venlafaxine (MD= -1.3; 95% CI, -2.3 to -0.3). However, no difference in VMS frequency or bother was found with exercise (MD= -0.4; 95% CI, -1.1 to 0.3), yoga (MD= -0.6; 95% CI, -1.3 to 0.1), or omega-3 supplementation (MD= 0.2; 95% CI, -0.4 to 0.8).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) doesn’t offer specific recommendations regarding exercise as a treatment for symptoms of menopause. The 2014 ACOG guidelines for managing symptoms report that data don’t support phytoestrogens, supplements, or lifestyle modifications (Level B, based on limited or inconsistent evidence). ACOG recommends basic palliative measures such as drinking cool drinks and decreasing layers of clothing (Level B).4
The American Association of Clinical Endocrinologists’ recommendations don’t mention exercise as a menopause therapy.5
The North American Menopause Society’s 2015 statement regarding the nonhormonal treatment of menopause symptoms doesn’t recommend exercise as an effective therapy because of insufficient or inconclusive data.6
EVIDENCE SUMMARY
A 2014 Cochrane meta-analysis of 5 RCTs with a total of 733 patients examined the effectiveness of any type of exercise in decreasing vasomotor symptoms in perimenopausal and postmenopausal women.1 The studies compared exercise—defined as structured exercise or physical activity through active living—with no active treatment, yoga, or hormone therapy (HT) over a 3- to 24-month follow-up period.
Three trials of 454 women that compared exercise with no active treatment found no difference between groups in frequency or intensity of vasomotor symptoms (standard mean difference [SMD]= -0.10; 95% confidence interval [CI], -0.33 to 0.13).
Two trials with 279 women that compared exercise with yoga didn’t find a difference in reported frequency or intensity of vasomotor symptoms between the groups (SMD= -0.03; 95% CI, -0.45 to 0.38).
One small trial (14 women) of exercise and HT found that HT patients reported decreased frequency of flushes over 24 hours compared with the exercise group (mean difference [MD]=5.8; 95% CI, 3.17-8.43).
Overall, the evidence was of low quality because of heterogeneity in study design.1
Two exercise interventions fail to reduce symptoms
A 2014 RCT, published after the Cochrane search date, investigated exercise as a treatment for VMS in 261 perimenopausal and postmenopausal women ages 48 to 57 years.2 Patients had a history of at least 5 hot flashes or night sweats per day and hadn’t taken HT in the previous 3 months.
The women were randomized to one of 2 exercise interventions or a control group. The exercise interventions both entailed 2 one-on-one consultations with a physical activity facilitator and use of a pedometer. Patients were encouraged to perform 30 minutes of moderate-intensity exercise 3 days a week during Weeks 1 through 12, then increase the frequency to 3 to 5 days a week during Weeks 13 through 24. In one intervention arm, the women also received an informational DVD and 5 educational leaflets.
In the other arm, they were invited to attend 3 exercise support groups in their local community. The control group was offered an opportunity for exercise consultation and given a pedometer at the end of the study.
At the end of the 6-month intervention, neither exercise intervention significantly decreased self-reported hot flashes/night sweats per week compared with the control group (DVD exercise arm vs control: MD= -8.9; 95% CI, -20 to 2.2; social support exercise arm vs control: MD= -5.2; 95% CI, -16.7 to 6.3). The study also found no difference in hot flashes/night sweats per week at 12-month follow-up between the DVD exercise arm and controls (MD= -3.2; 95% CI, -12.7 to 6.4) and the social-support group and controls (MD= -3.5; 95% CI, -13.2 to 6.1).
Drug therapy relieves symptoms, but other methods—not so much
An analysis of pooled individual data from 3 RCTs compared exercise with 5 other interventions for VMS in 899 perimenopausal and postmenopausal women.3 Patients had at least 14 bothersome symptoms per week.
The 6 interventions ranged from nonpharmacologic therapies, such as aerobic exercise and yoga, to pharmacologic treatments, including escitalopram 10 to 20 mg/d, venlafaxine 75 mg/d, oral estradiol (E2) 0.5 mg/d, and omega-3 supplementation 1.8 g/d. The primary outcome was a change in VMS frequency and bother as assessed by a symptom diary over the 4- to 12-week follow-up.
The analysis found a significant 6-week reduction in daily VMS frequency relative to placebo for escitalopram (MD= -1.4; 95% CI, -2.7 to -0.2), low-dose E2 (MD= -1.9; 95% CI, -2.9 to -0.9), and venlafaxine (MD= -1.3; 95% CI, -2.3 to -0.3). However, no difference in VMS frequency or bother was found with exercise (MD= -0.4; 95% CI, -1.1 to 0.3), yoga (MD= -0.6; 95% CI, -1.3 to 0.1), or omega-3 supplementation (MD= 0.2; 95% CI, -0.4 to 0.8).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) doesn’t offer specific recommendations regarding exercise as a treatment for symptoms of menopause. The 2014 ACOG guidelines for managing symptoms report that data don’t support phytoestrogens, supplements, or lifestyle modifications (Level B, based on limited or inconsistent evidence). ACOG recommends basic palliative measures such as drinking cool drinks and decreasing layers of clothing (Level B).4
The American Association of Clinical Endocrinologists’ recommendations don’t mention exercise as a menopause therapy.5
The North American Menopause Society’s 2015 statement regarding the nonhormonal treatment of menopause symptoms doesn’t recommend exercise as an effective therapy because of insufficient or inconclusive data.6
1. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;(11):CD006108.
2. Daley AJ, Thomas A, Roalfe AK, et al. The effectiveness of exercise as treatment for vasomotor menopausal symptoms: randomized controlled trial. BJOG. 2015;122:565-575.
3. Guthrie KA, LaCroix AZ, Ensrud KE, et al. Pooled analysis of six pharmacologic and nonpharmacologic interventions for vasomotor symptoms. Obstet Gynecol. 2015;126:413-422.
4. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
5. Goodman NF, Cobin RH, Ginzburg SB, et al; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause: executive summary of recommendations. Endocr Pract. 2011;17:949-954.
6. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22:1155-1172.
1. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;(11):CD006108.
2. Daley AJ, Thomas A, Roalfe AK, et al. The effectiveness of exercise as treatment for vasomotor menopausal symptoms: randomized controlled trial. BJOG. 2015;122:565-575.
3. Guthrie KA, LaCroix AZ, Ensrud KE, et al. Pooled analysis of six pharmacologic and nonpharmacologic interventions for vasomotor symptoms. Obstet Gynecol. 2015;126:413-422.
4. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
5. Goodman NF, Cobin RH, Ginzburg SB, et al; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause: executive summary of recommendations. Endocr Pract. 2011;17:949-954.
6. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22:1155-1172.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
No. Exercise doesn’t decrease the frequency or severity of vasomotor menopausal symptoms (VMS) in perimenopausal and postmenopausal women (strength of recommendation: A, systematic review of randomized controlled trials [RCTs] and consistent RCT).
How accurate are point-of-care urine drug screens in patients taking chronic opioid therapy?
EVIDENCE SUMMARY
A 2011 blinded diagnostic accuracy study of 1000 adult chronic pain patients in an interventional pain management program in the United States compared POC immunoassay urine drug testing with LC-MS.1 The immunoassay index test can be performed in the office with rapid results. The LC-MS reference test requires that the urine sample be sent to a lab.
Study participants were 37% male and 63% female, average age 51 years. Of the 1000 patients, 920 were prescribed opioids. Morphine, hydrocodone, codeine, and hydromorphone (morphine group) were tested with cutoff values of 300 ng/mL for POC testing and 50 ng/mL for LC-MS. Cutoffs for methadone were 300 ng/mL for POC and 100 ng/mL for LC-MS. For oxycodone, they were 100 ng/mL for POC and 50 ng/mL for LC-MS.
Methadone had the highest sensitivity and specificity at 96% and 99%, with a false-negative rate of 3.9% and a false-positive rate of 1.2%. It also had the highest agreement between the 2 testing methods at 99%. The morphine group had a sensitivity of 92%, specificity of 93%, false-negative rate of 7.8%, false-positive rate of 6.9%, and 93% test agreement. Oxycodone showed the lowest sensitivity at 75%; it had a specificity of 92%, a false-negative rate of 25%, a false-positive rate of 7.7%, and 90% test agreement.
More false negatives than with LC-MS
A 2010 blinded diagnostic accuracy study of 4200 adults treated with opioids for chronic pain compared immunoassay urine testing with LC-MS for opioids, benzodiazepines, marijuana, cocaine, and methamphetamine between October and November 2008.2 Urine samples were tested using both methods simultaneously on split specimens. Cutoff values for methadone, codeine, hydrocodone, hydromorphone, and morphine were 50 ng/mL on LC-MS. Immunoassay relative activity—the difference between the immunoassay and the LC-MS cutoffs—was 300 for methadone, 180 for codeine, 1700 for hydrocodone, 4000 for hydromorphone, and 300 for morphine.
Of the 3414 samples submitted for opiate testing, 2191 tested positive using immunoassay and 2233 tested positive using LC-MS for a total of 42 false-negative results with immunoassay. The positive rate (percentage of samples testing positive by LC-MS) was 65%, and the false-negative rate was 1.9%. Methadone testing produced 17 false-negative results; the positive rate was 10%, and the false-negative rate was 6.1%. The immunoassay false-positive results occurred in patients taking hydromorphone and hydrocodone.
The study was limited by lack of demographic information on the participants.
1. Manchikanti L, Malla Y, Wargo B, et al. Comparative evaluation of the accuracy of immunoassay with liquid chromatography tandem mass spectrometry of urine drug testing opioids and illicit drugs in chronic pain patients. Pain Physician. 2011;14:175–187.
2. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectrometry versus immunoassay drug testing in pain patients. Pain Physician. 2010;13:273–281.
EVIDENCE SUMMARY
A 2011 blinded diagnostic accuracy study of 1000 adult chronic pain patients in an interventional pain management program in the United States compared POC immunoassay urine drug testing with LC-MS.1 The immunoassay index test can be performed in the office with rapid results. The LC-MS reference test requires that the urine sample be sent to a lab.
Study participants were 37% male and 63% female, average age 51 years. Of the 1000 patients, 920 were prescribed opioids. Morphine, hydrocodone, codeine, and hydromorphone (morphine group) were tested with cutoff values of 300 ng/mL for POC testing and 50 ng/mL for LC-MS. Cutoffs for methadone were 300 ng/mL for POC and 100 ng/mL for LC-MS. For oxycodone, they were 100 ng/mL for POC and 50 ng/mL for LC-MS.
Methadone had the highest sensitivity and specificity at 96% and 99%, with a false-negative rate of 3.9% and a false-positive rate of 1.2%. It also had the highest agreement between the 2 testing methods at 99%. The morphine group had a sensitivity of 92%, specificity of 93%, false-negative rate of 7.8%, false-positive rate of 6.9%, and 93% test agreement. Oxycodone showed the lowest sensitivity at 75%; it had a specificity of 92%, a false-negative rate of 25%, a false-positive rate of 7.7%, and 90% test agreement.
More false negatives than with LC-MS
A 2010 blinded diagnostic accuracy study of 4200 adults treated with opioids for chronic pain compared immunoassay urine testing with LC-MS for opioids, benzodiazepines, marijuana, cocaine, and methamphetamine between October and November 2008.2 Urine samples were tested using both methods simultaneously on split specimens. Cutoff values for methadone, codeine, hydrocodone, hydromorphone, and morphine were 50 ng/mL on LC-MS. Immunoassay relative activity—the difference between the immunoassay and the LC-MS cutoffs—was 300 for methadone, 180 for codeine, 1700 for hydrocodone, 4000 for hydromorphone, and 300 for morphine.
Of the 3414 samples submitted for opiate testing, 2191 tested positive using immunoassay and 2233 tested positive using LC-MS for a total of 42 false-negative results with immunoassay. The positive rate (percentage of samples testing positive by LC-MS) was 65%, and the false-negative rate was 1.9%. Methadone testing produced 17 false-negative results; the positive rate was 10%, and the false-negative rate was 6.1%. The immunoassay false-positive results occurred in patients taking hydromorphone and hydrocodone.
The study was limited by lack of demographic information on the participants.
EVIDENCE SUMMARY
A 2011 blinded diagnostic accuracy study of 1000 adult chronic pain patients in an interventional pain management program in the United States compared POC immunoassay urine drug testing with LC-MS.1 The immunoassay index test can be performed in the office with rapid results. The LC-MS reference test requires that the urine sample be sent to a lab.
Study participants were 37% male and 63% female, average age 51 years. Of the 1000 patients, 920 were prescribed opioids. Morphine, hydrocodone, codeine, and hydromorphone (morphine group) were tested with cutoff values of 300 ng/mL for POC testing and 50 ng/mL for LC-MS. Cutoffs for methadone were 300 ng/mL for POC and 100 ng/mL for LC-MS. For oxycodone, they were 100 ng/mL for POC and 50 ng/mL for LC-MS.
Methadone had the highest sensitivity and specificity at 96% and 99%, with a false-negative rate of 3.9% and a false-positive rate of 1.2%. It also had the highest agreement between the 2 testing methods at 99%. The morphine group had a sensitivity of 92%, specificity of 93%, false-negative rate of 7.8%, false-positive rate of 6.9%, and 93% test agreement. Oxycodone showed the lowest sensitivity at 75%; it had a specificity of 92%, a false-negative rate of 25%, a false-positive rate of 7.7%, and 90% test agreement.
More false negatives than with LC-MS
A 2010 blinded diagnostic accuracy study of 4200 adults treated with opioids for chronic pain compared immunoassay urine testing with LC-MS for opioids, benzodiazepines, marijuana, cocaine, and methamphetamine between October and November 2008.2 Urine samples were tested using both methods simultaneously on split specimens. Cutoff values for methadone, codeine, hydrocodone, hydromorphone, and morphine were 50 ng/mL on LC-MS. Immunoassay relative activity—the difference between the immunoassay and the LC-MS cutoffs—was 300 for methadone, 180 for codeine, 1700 for hydrocodone, 4000 for hydromorphone, and 300 for morphine.
Of the 3414 samples submitted for opiate testing, 2191 tested positive using immunoassay and 2233 tested positive using LC-MS for a total of 42 false-negative results with immunoassay. The positive rate (percentage of samples testing positive by LC-MS) was 65%, and the false-negative rate was 1.9%. Methadone testing produced 17 false-negative results; the positive rate was 10%, and the false-negative rate was 6.1%. The immunoassay false-positive results occurred in patients taking hydromorphone and hydrocodone.
The study was limited by lack of demographic information on the participants.
1. Manchikanti L, Malla Y, Wargo B, et al. Comparative evaluation of the accuracy of immunoassay with liquid chromatography tandem mass spectrometry of urine drug testing opioids and illicit drugs in chronic pain patients. Pain Physician. 2011;14:175–187.
2. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectrometry versus immunoassay drug testing in pain patients. Pain Physician. 2010;13:273–281.
1. Manchikanti L, Malla Y, Wargo B, et al. Comparative evaluation of the accuracy of immunoassay with liquid chromatography tandem mass spectrometry of urine drug testing opioids and illicit drugs in chronic pain patients. Pain Physician. 2011;14:175–187.
2. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectrometry versus immunoassay drug testing in pain patients. Pain Physician. 2010;13:273–281.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
In adults treated with opioids for chronic pain, point-of-care (POC) urine drug screens (immunoassays) for detecting opioids show a false-negative rate of 1.9%, a sensitivity of 92%, and a specificity of 93% compared with the gold-standard liquid chromatography tandem mass spectrometry (LC-MS). Oxycodone has the highest rate of false-negative results at 25%; methadone has the lowest rate at 4% to 6% (strength of recommendation [SOR]: A, 2 blinded diagnostic accuracy studies with similar results).
Limited evidence guides empiric Tx of female chronic pelvic pain
CASE 1
Lisa G, 31 years old, gravida 0, complains of severe dysmenorrhea that began when she discontinued an oral contraceptive (OC) one year ago. Prior to stopping the OC, she had been taking an OC without interruption since she was 28, during which time she continued to have moderate symptoms of dysmenorrhea. Before taking an OC, the patient had a trial of an etonogestrel implant, which was removed because of irregular bleeding, and depot medroxyprogesterone acetate (MPA) injection, which she discontinued because of associated weight gain and fatigue.
Ms. G is not sexually active and doesn’t want to start a family at this time, but is interested in having a diagnosis. She has no other medical problems, no surgical history, and no history of sexually transmitted infection. She reports that her mother and sister had endometriosis, including pain that resolved after definitive treatment.
Ms. G reports menstrual cycles that are exquisitely painful and occur regularly (every 28 days for 4 or 5 days), with a moderate volume of bleeding that requires a regular-size tampon change every 4 to 6 hours. She reports crampy abdominal pain as 10, on a scale of one to 10; dyschezia (without hematochezia); and generalized achy abdominal pain that is continuous during menses. Pain is partially controlled by ibuprofen, 800 mg every 8 hours. Ms. G also describes gastrointestinal symptoms of bloating, constipation preceding her menstrual cycle, diarrhea during her menses, and occasionally nausea and vomiting with the severe pain.
On examination (which is not performed during menses), Ms. G appears well and is not in acute distress. Abdominal examination is benign. There is no tenderness to palpation or distension; bowel sounds are normal. Pelvic examination reveals mild tenderness upon palpation of a small and mobile uterus. Rectal examination is normal. She has no signs of hyperandrogenism (eg, male-pattern body hair, central obesity).
CASE 2
Rhonda M, 42 years old, gravida 3, para 3003, reports continuous pelvic pain for 7 years that is exacerbated by defecation, intercourse, and insertion of a tampon. She has a low level of dull baseline pain (3, on scale of one to 10) that occasionally spikes up to sharp, knifelike pain (10 on the pain scale), which, she says, brings her to tears. Ms. M describes the pain as “deep inside,” central in her pelvis, and radiating to the left and right, particularly during pain flares.
The patient’s 3 children were born by spontaneous vaginal delivery; however, she recalls that her youngest son was born via a traumatic vaginal delivery 8 years ago (he “got stuck coming out,” she reports). The only other component of Ms. M’s medical history is an anxiety disorder, for which she takes citalopram. She has a family history of cervical cancer.
Ms. M’s past diagnostic work-up for pelvic pain includes pelvic ultrasonography, endometrial biopsy, Pap smear, and diagnostic laparoscopy—all normal. She had a negative gastrointestinal work-up, including upper- and lower-tract endoscopy. Medical therapy, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), did not provide significant relief of pain.
Despite the negative work-up, Ms. M is still concerned that the pain might be related to cancer. With her family history of cervical cancer, she says that she does not want to “miss anything.”
Ms. M is thin and appears anxious. The abdomen is mildly and diffusely tender to palpation with normal bowel sounds and no distension. Pelvic examination reveals some hyperesthesia upon single-digit palpation of the pelvic floor. Placement of the speculum is difficult because of discomfort.
How would you proceed with the care of these patients?
What is chronic pelvic pain? Why is management such a challenge?
Chronic pelvic pain (CPP) is defined as chronic or intermittent cyclic or noncyclic pelvic pain lasting longer than 6 months, localized to the pelvis, diminishing a woman’s quality of life, and requiring medical intervention.1 It’s estimated that CPP affects as many as 15% of women of reproductive age in the United States each year, at a cost to the health care system of approximately $2 billion annually.2,3
CPP can result from abnormal pain responses from multiple body systems, including gynecologic conditions such as endometriosis. Notably, a nongynecologic cause is more often the major pain generator, without significant identifiable pathology (TABLE 1). Like all chronic pain disorders, CPP can also result in central sensitization of the nervous system, altering how pain is processed at the level of the pain matrix in the brain.4
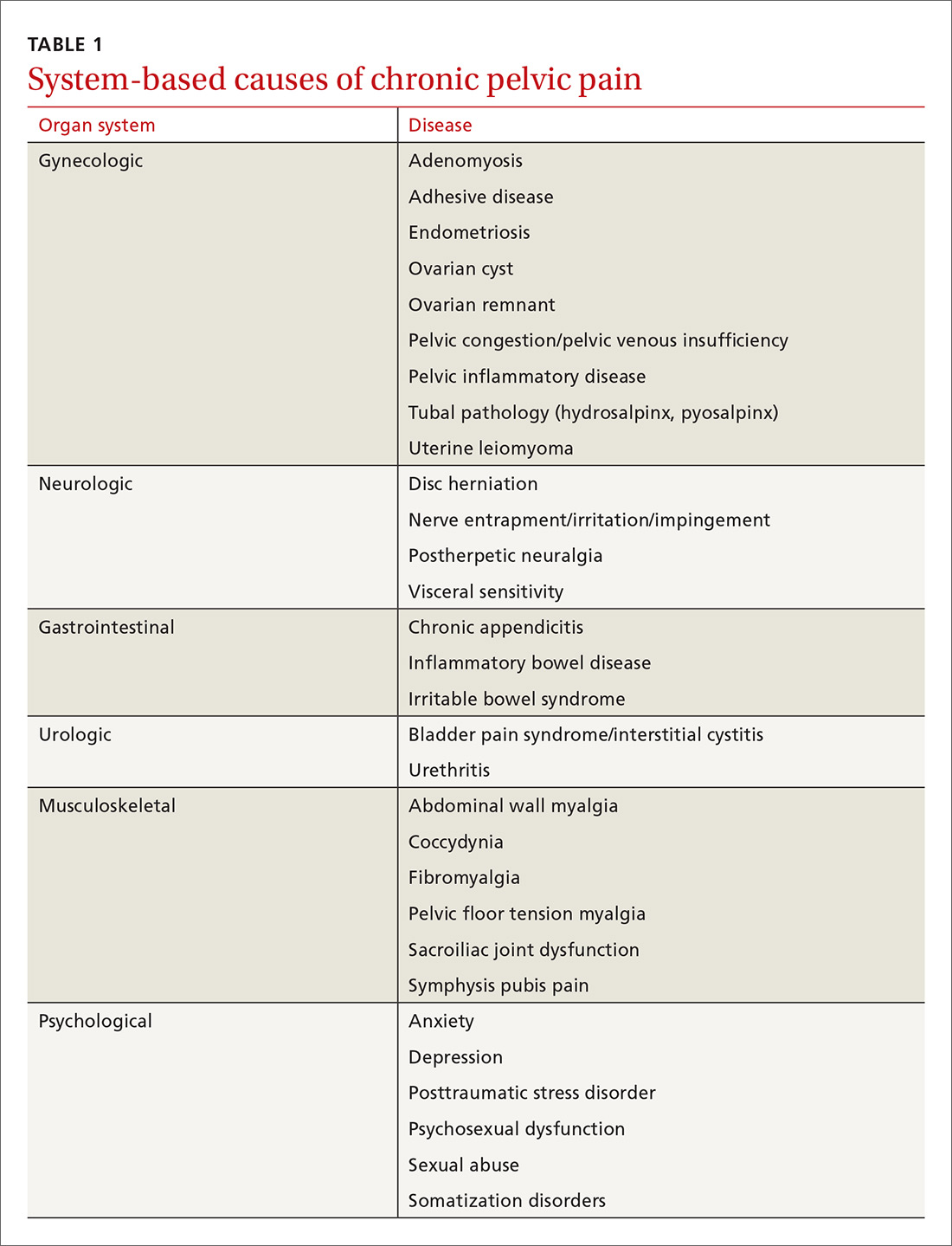
This article reviews the limited evidence for treating CPP and offers recommendations for the primary care physician on providing symptomatic relief in the absence of diagnosed pathology (TABLE 25-13).
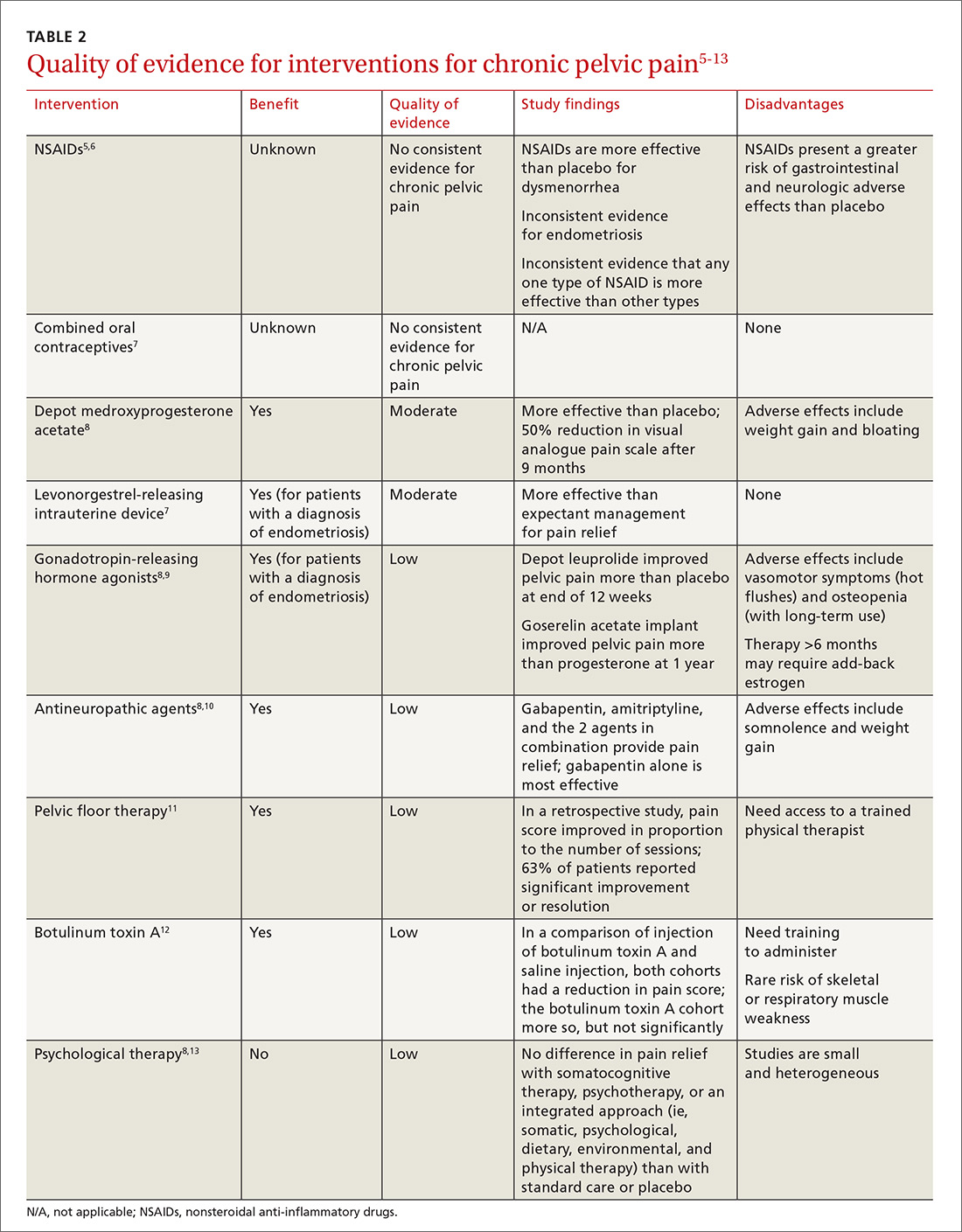
Treatment
Analgesics
NSAIDs are frequently used as first-line treatment for any kind of pain, including CPP. There is some evidence of benefit from NSAIDs, compared to placebo, in cyclic CPP secondary to dysmenorrhea and endometriosis;5,6 however, evidence of effectiveness in noncyclic CPP is absent. Because of the low cost and availability of NSAIDs, a trial is reasonable as a first-line intervention, particularly in CPP suspected to be endometriosis or of musculoskeletal origin. NSAIDs can cause adverse effects, including nausea, vomiting, headache, and drowsiness in 11% to 14% of women, although these agents are generally well-tolerated on a short-term basis.5
Opioids bind to opioid receptors in the central and peripheral nervous systems, resulting in an analgesic effect. Guidelines issued in 2016 by the Centers for Disease Control and Prevention recommend safer prescribing through careful evaluation of the risks and benefits of opioids for pain not caused by cancer and for palliation as part of end-of-life care.14
The risks of opioid use are well known in the medical community; they include tolerance, physical dependence, misuse, and death, in addition to common adverse effects such as nausea and vomiting, itching, constipation, and fatigue.14,15 Because of those risks and limited long-term benefit in nonmalignant pain disorders, opioid therapy for CPP should be avoided.14 For patients already taking an opioid, discuss a strategy for weaning and, if possible, provide home naloxone therapy in the event of accidental overdose.14
Hormonal therapy
Hormonal therapies are the most common nonsurgical treatment of noncyclic CPP, with or without a definitive diagnosis of endometriosis, in reproductive-age women with CPP.
Combined OCs, despite a lack of quality evidence, are frequently the first hormonal treatment tried in both cyclic and noncyclic CPP. A low-dosage OC may decrease cyclic pain in endometriosis, although it can increase irregular bleeding and nausea.16 As many as 53% of women with CPP reported having undergone a trial of an OC for endometriosis, despite the absence of consistent evidence showing effectiveness in CPP.17
Depot MPA, in trials, decreased pain more than placebo. It can be tried as a treatment, but its use is often limited because of adverse effects, such as weight gain and bloating.8
A trial of a levonorgestrel-releasing intrauterine device (LNG-IUD) is supported by moderate-quality evidence for women whose CPP is thought to be a symptom of endometriosis or to have another uterine origin.7
Gonadotropin-releasing hormone agonists, such as depot leuprolide and goserelin acetate implant, may be considered in a woman with a diagnosis of endometriosis whose pelvic pain is not alleviated by MPA or an LNG-IUD.9
Nonhormonal therapies
CPP shares pain mechanisms with other pain syndromes, such as neuropathic pain. Antineuropathic medications, such as gabapentin and pregabalin, may, therefore, provide benefit. These medications also produce improvement in pain disorders of the musculoskeletal system, which may contribute to their analgesic effect.18
Gabapentin and amitriptyline have been studied in CPP; both were found successful in decreasing perceived pain. Of note, patients who received gabapentin, a gamma-aminobutyric acid analogue, with or without amitriptyline, had more pain relief than those treated with amitriptyline alone.10 Adverse effects of these medications may limit their use (TABLE 319-25).
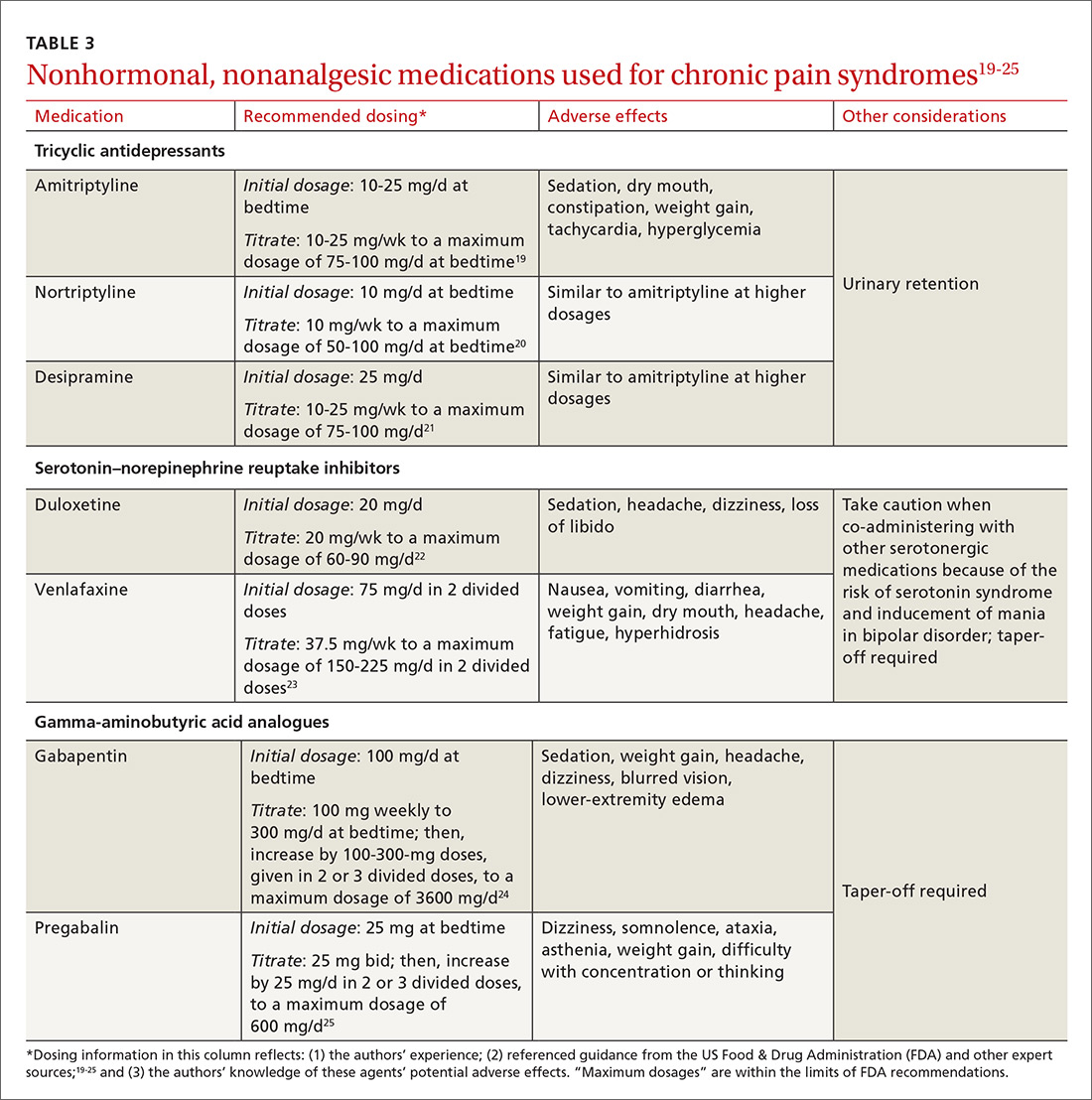
Tricyclic antidepressants are well-supported, effective treatments for chronic pain through the central increase of norepinephrine. Beginning at a low dosage to diminish adverse effects (TABLE 319-25) and increasing the dosage slowly to an effective level may increase adherence. A trial of at least 6 to 8 weeks, at a moderate dosage, is recommended before discontinuing the medication. Although amitriptyline has the most evidence for value in the management of CPP disorders,10 second-generation tricyclic antidepressants nortriptyline and desipramine have also been used for pain control, and may be better tolerated.
Duloxetine and venlafaxine—serotonin–norepinephrine reuptake inhibitors—increase serotonin in addition to norepinephrine, which is believed to result in pain control. Although a systematic review of trials of duloxetine for chronic pain showed some improvement in diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis, the review excluded CPP in its analysis.26
In our opinion, a selective neurotransmitter reuptake inhibitor can be attempted to diminish the central pain sensitization of CPP. As with all drugs that increase the availability of serotonin, serotonin syndrome is a rare risk. Additionally, when stopping duloxetine, a prolonged taper may be required.
Pelvic floor dysfunction therapy
Pelvic floor dysfunction of the musculature within the bony pelvis may contribute to, or cause, CPP. The pelvic floor musculature may be hypertonic or hypotonic, and trigger points may exist. Despite the frequency of pelvic floor dysfunction, detailed examination of the pelvic floor is not routinely performed during a pelvic exam.
Because of the high prevalence of pelvic floor dysfunction in women with CPP, evaluation of the pelvic floor muscles is warranted.27 (A protocol for this evaluation is detailed in TABLE 4.) Pelvic dynamometry may indicate muscle spasm or chronic tension; palpation of the pelvic floor during the exam can also identify a pain generator.
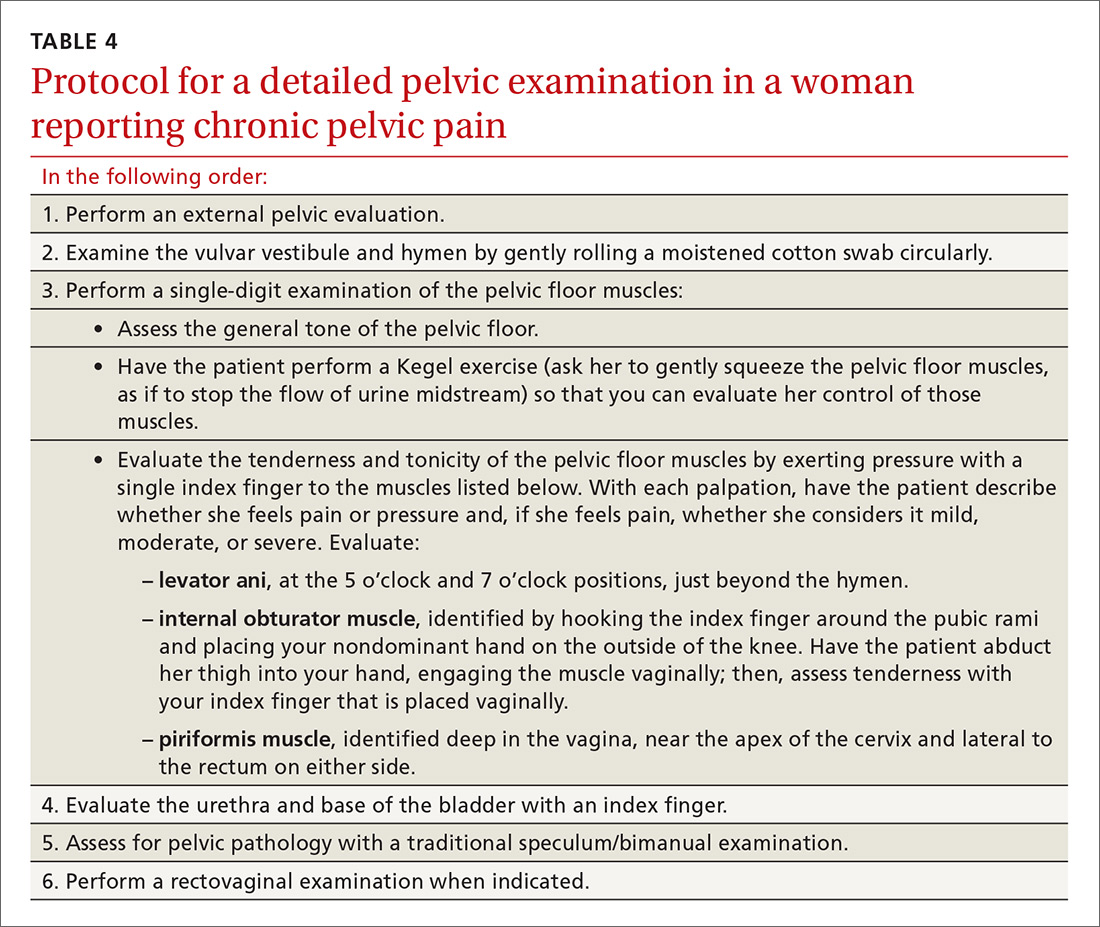
Although it might be difficult to distinguish pelvic floor myofascial pain as the primary or secondary cause of pain, pelvic floor physical therapy may clarify the role of the pelvic floor response (depending on the patient’s clinical exam and history). A low-quality retrospective case study on pelvic floor physical therapy reported significant improvement in pain that was proportional to the number of sessions completed.11 Trigger-point injections and injections of botulinum toxin A have been used with reported improvement in the pelvic floor pain profile, and there is evidence to support the benefit of such injections in pelvic muscle dysfunction.12
Psychotherapy
Cognitive behavioral therapy (CBT) is well established as an option to manage a patient’s response to pain, including teaching coping skills for a chronic pain disorder and pain flares. Evidence supports using CBT or mindfulness techniques over usual care in reducing the intensity of pain in chronic low back pain,28 and may be helpful in CPP. Patients with CPP who received 10 treatments of Mensendieck somatocognitive therapy (a mind–body therapy technique popular in Europe) over 90 days, compared with standard treatment alone, demonstrated improvement in pain, motor function, and psychological distress that persisted 9 months after treatment.13
Lifestyle changes, complementary and alternative therapies
Although medical and nonpharmacotherapeutic treatments are often important in the
Diet modifications may relieve pain in some women with CPP. Although a systematic review in 2011 highlighted the lack of data available for the efficacy of dietary therapies for treating CPP, the authors did present data that a diet rich in antioxidants might alleviate pain sysmptoms.29 Also, a gluten-free diet might reduce the symptoms of pain related to endometriosis and, thus, improve physical functioning, among other health domains.30
Exercise can be an important factor in the management of CPP, as with other chronic pain syndromes. In functional pain syndromes, the addition or maintenance of an exercise program has been shown to decrease the amount of pain medications required, improve depressive symptoms, increase energy, and decrease stress. Exercise also improves sleep quality and one’s ability to cope with pain.31
Yoga provides a good balance of aerobic and muscle-building activity and, in the authors’ experience, is tolerated by most women with CPP.
Acupuncture has limited evidence in the treatment of pelvic pain in women. Of the available studies, most are limited to pain related to endometriosis.32
Sleep hygiene may be an important consideration in managing CPP. Sleep disturbances are reported in more than 80% of women with CPP,33 including excessive time in bed and frequent napping, resulting in daytime fatigue and feeling generally unrested. A recent meta-analysis reported mild-to-moderate immediate improvement in patients’ pain after nonpharmacotherapeutic sleep interventions.34 The National Sleep Foundation has produced a patient guide to assist in sleep hygiene.35
Devising a management strategy despite sparse evidence
Because the cause of noncyclic CPP may be multifactorial, and because the literature on the etiology of CPP is limited (and, when there is research, it is inconclusive or of poor quality36), there are few evidence-based recommendations for treating CPP. Given the paucity of quality evidence, physicians should treat patients empirically, based on their experience and their familiarity with the range of medical and nonpharmacotherapeutic options used to manage other chronic pain syndromes.
CASE 1
Ms. G’s cyclic pelvic pain was present only during menses. The dyschezia, severe pain that began only after she discontinued a combined OC, aching pain, and severe menstrual cramps are, taken together, suggestive of endometriosis, despite a normal physical exam.
Medical and surgical options were reviewed with Ms. G. She elected to undergo diagnostic laparoscopy. Several extrauterine foci of endometrial tissue were noted and excised; an LNG-IUD was inserted. Her pain improved significantly after surgery.
CASE 2
Ms. M was found to have significant pain on single-digit examination of the pelvic floor muscles, indicating likely pelvic floor muscle dysfunction. Pelvic dynamometry revealed significant tightness and spasm in the pelvic floor muscles—specifically, the levator ani complex.
Ms. M was started on gabapentin to reduce baseline pain and was referred for pelvic floor physical therapy. She felt reassured that her risk of cancer was low, considering her negative work-up, and that cancer was not the cause of her pain. Her symptoms improved greatly with a regimen of medical and physical therapy, although she continues to experience pain flares.
CORRESPONDENCE
Wendy S. Biggs, MD, Central Michigan University College of Medicine, 1632 Stone St., Saginaw, MI 48602; [email protected].
1. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 51. Chronic pelvic pain. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2004;103:589-605.
2. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327.
3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys. 2014;17:e141-e147.
4. Rodriguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence of overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
5. Allen C, Hopewell S, Prentice A, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
6. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;(7):CD001751.
7. Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(3):CD009590.
8. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
9. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
10. Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761-768.
11. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
12. Abbott JA, Jarvis SK, Lyons SC, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
13. Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am J Obstet Gynecol. 2008;199:615.e1-e8.
14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645.
15. Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13:1181-1211.
16. Harada T, Momoeda M, Taketani Y, et al. Low-dose contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008:90:1583-1588.
17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human Reprod. 2013;28:2677-2685.
18. Haviv Y, Rettman A, Aframian D, et al. Myofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J Oral Facial Pain Headache. 2015;29:144-151.
19. Drugs.com. Amitriptyline dosing information. Available at: https://www.drugs.com/amitriptyline.html. Accessed January 4, 2018.
20. Drugs.com. Nortriptyline dosage. Available at: https://www.drugs.com/dosage/nortriptyline.html. Accessed January 4, 2018.
21. Drugs.com. Desipramine (oral route). Available at: https://www.drugs.com/cons/desipramine.html. Accessed January 4, 2018.
22. Drugs.com. Duloxetine capsules. Available at: https://www.drugs.com/pro/duloxetine-capsules.html. Accessed January 4, 2018.
23. Drugs.com. Venlafaxine. Available at: https://www.drugs.com/pro/venlafaxine.html. Accessed January 4, 2018.
24. Drugs.com. Gabapentin. Available at: https://www.drugs.com/pro/gabapentin.html. Accessed January 4, 2018.
25. Drugs.com. Pregabalin. Available at: https://www.drugs.com/monograph/pregabalin.html. Accessed January 4, 2018.
26. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
27. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594-611.
28. Cherkin DC, Sheman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
29. Sesti F, Capozzolo T, Pietropolli A, et al. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev. 2011;24:31-38.
30. Marziali M, Venza M, Lazzaro A, et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. 2012;67:499-504.
31. Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946-955.
32. Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database Syst Rev. 2011;(9):CD007864.
33. Cosar E, Çakır Güngör A, Gencer M, et.al. Sleep disturbance among women with chronic pelvic pain. Int J Gynaecol Obstet. 2014;126:232-234.
34. Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751-1764.
35. National Sleep Foundation. Healthy sleep tips. Available at: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. Accessed December 26, 2017.
36. Yunker A, Sathe NA, Reynolds WS, et al. Systematic review of therapies for noncyclic chronic pelvic pain in women. Obstet Gynecol Survey. 2012;67:417-425.
CASE 1
Lisa G, 31 years old, gravida 0, complains of severe dysmenorrhea that began when she discontinued an oral contraceptive (OC) one year ago. Prior to stopping the OC, she had been taking an OC without interruption since she was 28, during which time she continued to have moderate symptoms of dysmenorrhea. Before taking an OC, the patient had a trial of an etonogestrel implant, which was removed because of irregular bleeding, and depot medroxyprogesterone acetate (MPA) injection, which she discontinued because of associated weight gain and fatigue.
Ms. G is not sexually active and doesn’t want to start a family at this time, but is interested in having a diagnosis. She has no other medical problems, no surgical history, and no history of sexually transmitted infection. She reports that her mother and sister had endometriosis, including pain that resolved after definitive treatment.
Ms. G reports menstrual cycles that are exquisitely painful and occur regularly (every 28 days for 4 or 5 days), with a moderate volume of bleeding that requires a regular-size tampon change every 4 to 6 hours. She reports crampy abdominal pain as 10, on a scale of one to 10; dyschezia (without hematochezia); and generalized achy abdominal pain that is continuous during menses. Pain is partially controlled by ibuprofen, 800 mg every 8 hours. Ms. G also describes gastrointestinal symptoms of bloating, constipation preceding her menstrual cycle, diarrhea during her menses, and occasionally nausea and vomiting with the severe pain.
On examination (which is not performed during menses), Ms. G appears well and is not in acute distress. Abdominal examination is benign. There is no tenderness to palpation or distension; bowel sounds are normal. Pelvic examination reveals mild tenderness upon palpation of a small and mobile uterus. Rectal examination is normal. She has no signs of hyperandrogenism (eg, male-pattern body hair, central obesity).
CASE 2
Rhonda M, 42 years old, gravida 3, para 3003, reports continuous pelvic pain for 7 years that is exacerbated by defecation, intercourse, and insertion of a tampon. She has a low level of dull baseline pain (3, on scale of one to 10) that occasionally spikes up to sharp, knifelike pain (10 on the pain scale), which, she says, brings her to tears. Ms. M describes the pain as “deep inside,” central in her pelvis, and radiating to the left and right, particularly during pain flares.
The patient’s 3 children were born by spontaneous vaginal delivery; however, she recalls that her youngest son was born via a traumatic vaginal delivery 8 years ago (he “got stuck coming out,” she reports). The only other component of Ms. M’s medical history is an anxiety disorder, for which she takes citalopram. She has a family history of cervical cancer.
Ms. M’s past diagnostic work-up for pelvic pain includes pelvic ultrasonography, endometrial biopsy, Pap smear, and diagnostic laparoscopy—all normal. She had a negative gastrointestinal work-up, including upper- and lower-tract endoscopy. Medical therapy, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), did not provide significant relief of pain.
Despite the negative work-up, Ms. M is still concerned that the pain might be related to cancer. With her family history of cervical cancer, she says that she does not want to “miss anything.”
Ms. M is thin and appears anxious. The abdomen is mildly and diffusely tender to palpation with normal bowel sounds and no distension. Pelvic examination reveals some hyperesthesia upon single-digit palpation of the pelvic floor. Placement of the speculum is difficult because of discomfort.
How would you proceed with the care of these patients?
What is chronic pelvic pain? Why is management such a challenge?
Chronic pelvic pain (CPP) is defined as chronic or intermittent cyclic or noncyclic pelvic pain lasting longer than 6 months, localized to the pelvis, diminishing a woman’s quality of life, and requiring medical intervention.1 It’s estimated that CPP affects as many as 15% of women of reproductive age in the United States each year, at a cost to the health care system of approximately $2 billion annually.2,3
CPP can result from abnormal pain responses from multiple body systems, including gynecologic conditions such as endometriosis. Notably, a nongynecologic cause is more often the major pain generator, without significant identifiable pathology (TABLE 1). Like all chronic pain disorders, CPP can also result in central sensitization of the nervous system, altering how pain is processed at the level of the pain matrix in the brain.4

This article reviews the limited evidence for treating CPP and offers recommendations for the primary care physician on providing symptomatic relief in the absence of diagnosed pathology (TABLE 25-13).

Treatment
Analgesics
NSAIDs are frequently used as first-line treatment for any kind of pain, including CPP. There is some evidence of benefit from NSAIDs, compared to placebo, in cyclic CPP secondary to dysmenorrhea and endometriosis;5,6 however, evidence of effectiveness in noncyclic CPP is absent. Because of the low cost and availability of NSAIDs, a trial is reasonable as a first-line intervention, particularly in CPP suspected to be endometriosis or of musculoskeletal origin. NSAIDs can cause adverse effects, including nausea, vomiting, headache, and drowsiness in 11% to 14% of women, although these agents are generally well-tolerated on a short-term basis.5
Opioids bind to opioid receptors in the central and peripheral nervous systems, resulting in an analgesic effect. Guidelines issued in 2016 by the Centers for Disease Control and Prevention recommend safer prescribing through careful evaluation of the risks and benefits of opioids for pain not caused by cancer and for palliation as part of end-of-life care.14
The risks of opioid use are well known in the medical community; they include tolerance, physical dependence, misuse, and death, in addition to common adverse effects such as nausea and vomiting, itching, constipation, and fatigue.14,15 Because of those risks and limited long-term benefit in nonmalignant pain disorders, opioid therapy for CPP should be avoided.14 For patients already taking an opioid, discuss a strategy for weaning and, if possible, provide home naloxone therapy in the event of accidental overdose.14
Hormonal therapy
Hormonal therapies are the most common nonsurgical treatment of noncyclic CPP, with or without a definitive diagnosis of endometriosis, in reproductive-age women with CPP.
Combined OCs, despite a lack of quality evidence, are frequently the first hormonal treatment tried in both cyclic and noncyclic CPP. A low-dosage OC may decrease cyclic pain in endometriosis, although it can increase irregular bleeding and nausea.16 As many as 53% of women with CPP reported having undergone a trial of an OC for endometriosis, despite the absence of consistent evidence showing effectiveness in CPP.17
Depot MPA, in trials, decreased pain more than placebo. It can be tried as a treatment, but its use is often limited because of adverse effects, such as weight gain and bloating.8
A trial of a levonorgestrel-releasing intrauterine device (LNG-IUD) is supported by moderate-quality evidence for women whose CPP is thought to be a symptom of endometriosis or to have another uterine origin.7
Gonadotropin-releasing hormone agonists, such as depot leuprolide and goserelin acetate implant, may be considered in a woman with a diagnosis of endometriosis whose pelvic pain is not alleviated by MPA or an LNG-IUD.9
Nonhormonal therapies
CPP shares pain mechanisms with other pain syndromes, such as neuropathic pain. Antineuropathic medications, such as gabapentin and pregabalin, may, therefore, provide benefit. These medications also produce improvement in pain disorders of the musculoskeletal system, which may contribute to their analgesic effect.18
Gabapentin and amitriptyline have been studied in CPP; both were found successful in decreasing perceived pain. Of note, patients who received gabapentin, a gamma-aminobutyric acid analogue, with or without amitriptyline, had more pain relief than those treated with amitriptyline alone.10 Adverse effects of these medications may limit their use (TABLE 319-25).

Tricyclic antidepressants are well-supported, effective treatments for chronic pain through the central increase of norepinephrine. Beginning at a low dosage to diminish adverse effects (TABLE 319-25) and increasing the dosage slowly to an effective level may increase adherence. A trial of at least 6 to 8 weeks, at a moderate dosage, is recommended before discontinuing the medication. Although amitriptyline has the most evidence for value in the management of CPP disorders,10 second-generation tricyclic antidepressants nortriptyline and desipramine have also been used for pain control, and may be better tolerated.
Duloxetine and venlafaxine—serotonin–norepinephrine reuptake inhibitors—increase serotonin in addition to norepinephrine, which is believed to result in pain control. Although a systematic review of trials of duloxetine for chronic pain showed some improvement in diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis, the review excluded CPP in its analysis.26
In our opinion, a selective neurotransmitter reuptake inhibitor can be attempted to diminish the central pain sensitization of CPP. As with all drugs that increase the availability of serotonin, serotonin syndrome is a rare risk. Additionally, when stopping duloxetine, a prolonged taper may be required.
Pelvic floor dysfunction therapy
Pelvic floor dysfunction of the musculature within the bony pelvis may contribute to, or cause, CPP. The pelvic floor musculature may be hypertonic or hypotonic, and trigger points may exist. Despite the frequency of pelvic floor dysfunction, detailed examination of the pelvic floor is not routinely performed during a pelvic exam.
Because of the high prevalence of pelvic floor dysfunction in women with CPP, evaluation of the pelvic floor muscles is warranted.27 (A protocol for this evaluation is detailed in TABLE 4.) Pelvic dynamometry may indicate muscle spasm or chronic tension; palpation of the pelvic floor during the exam can also identify a pain generator.

Although it might be difficult to distinguish pelvic floor myofascial pain as the primary or secondary cause of pain, pelvic floor physical therapy may clarify the role of the pelvic floor response (depending on the patient’s clinical exam and history). A low-quality retrospective case study on pelvic floor physical therapy reported significant improvement in pain that was proportional to the number of sessions completed.11 Trigger-point injections and injections of botulinum toxin A have been used with reported improvement in the pelvic floor pain profile, and there is evidence to support the benefit of such injections in pelvic muscle dysfunction.12
Psychotherapy
Cognitive behavioral therapy (CBT) is well established as an option to manage a patient’s response to pain, including teaching coping skills for a chronic pain disorder and pain flares. Evidence supports using CBT or mindfulness techniques over usual care in reducing the intensity of pain in chronic low back pain,28 and may be helpful in CPP. Patients with CPP who received 10 treatments of Mensendieck somatocognitive therapy (a mind–body therapy technique popular in Europe) over 90 days, compared with standard treatment alone, demonstrated improvement in pain, motor function, and psychological distress that persisted 9 months after treatment.13
Lifestyle changes, complementary and alternative therapies
Although medical and nonpharmacotherapeutic treatments are often important in the
Diet modifications may relieve pain in some women with CPP. Although a systematic review in 2011 highlighted the lack of data available for the efficacy of dietary therapies for treating CPP, the authors did present data that a diet rich in antioxidants might alleviate pain sysmptoms.29 Also, a gluten-free diet might reduce the symptoms of pain related to endometriosis and, thus, improve physical functioning, among other health domains.30
Exercise can be an important factor in the management of CPP, as with other chronic pain syndromes. In functional pain syndromes, the addition or maintenance of an exercise program has been shown to decrease the amount of pain medications required, improve depressive symptoms, increase energy, and decrease stress. Exercise also improves sleep quality and one’s ability to cope with pain.31
Yoga provides a good balance of aerobic and muscle-building activity and, in the authors’ experience, is tolerated by most women with CPP.
Acupuncture has limited evidence in the treatment of pelvic pain in women. Of the available studies, most are limited to pain related to endometriosis.32
Sleep hygiene may be an important consideration in managing CPP. Sleep disturbances are reported in more than 80% of women with CPP,33 including excessive time in bed and frequent napping, resulting in daytime fatigue and feeling generally unrested. A recent meta-analysis reported mild-to-moderate immediate improvement in patients’ pain after nonpharmacotherapeutic sleep interventions.34 The National Sleep Foundation has produced a patient guide to assist in sleep hygiene.35
Devising a management strategy despite sparse evidence
Because the cause of noncyclic CPP may be multifactorial, and because the literature on the etiology of CPP is limited (and, when there is research, it is inconclusive or of poor quality36), there are few evidence-based recommendations for treating CPP. Given the paucity of quality evidence, physicians should treat patients empirically, based on their experience and their familiarity with the range of medical and nonpharmacotherapeutic options used to manage other chronic pain syndromes.
CASE 1
Ms. G’s cyclic pelvic pain was present only during menses. The dyschezia, severe pain that began only after she discontinued a combined OC, aching pain, and severe menstrual cramps are, taken together, suggestive of endometriosis, despite a normal physical exam.
Medical and surgical options were reviewed with Ms. G. She elected to undergo diagnostic laparoscopy. Several extrauterine foci of endometrial tissue were noted and excised; an LNG-IUD was inserted. Her pain improved significantly after surgery.
CASE 2
Ms. M was found to have significant pain on single-digit examination of the pelvic floor muscles, indicating likely pelvic floor muscle dysfunction. Pelvic dynamometry revealed significant tightness and spasm in the pelvic floor muscles—specifically, the levator ani complex.
Ms. M was started on gabapentin to reduce baseline pain and was referred for pelvic floor physical therapy. She felt reassured that her risk of cancer was low, considering her negative work-up, and that cancer was not the cause of her pain. Her symptoms improved greatly with a regimen of medical and physical therapy, although she continues to experience pain flares.
CORRESPONDENCE
Wendy S. Biggs, MD, Central Michigan University College of Medicine, 1632 Stone St., Saginaw, MI 48602; [email protected].
CASE 1
Lisa G, 31 years old, gravida 0, complains of severe dysmenorrhea that began when she discontinued an oral contraceptive (OC) one year ago. Prior to stopping the OC, she had been taking an OC without interruption since she was 28, during which time she continued to have moderate symptoms of dysmenorrhea. Before taking an OC, the patient had a trial of an etonogestrel implant, which was removed because of irregular bleeding, and depot medroxyprogesterone acetate (MPA) injection, which she discontinued because of associated weight gain and fatigue.
Ms. G is not sexually active and doesn’t want to start a family at this time, but is interested in having a diagnosis. She has no other medical problems, no surgical history, and no history of sexually transmitted infection. She reports that her mother and sister had endometriosis, including pain that resolved after definitive treatment.
Ms. G reports menstrual cycles that are exquisitely painful and occur regularly (every 28 days for 4 or 5 days), with a moderate volume of bleeding that requires a regular-size tampon change every 4 to 6 hours. She reports crampy abdominal pain as 10, on a scale of one to 10; dyschezia (without hematochezia); and generalized achy abdominal pain that is continuous during menses. Pain is partially controlled by ibuprofen, 800 mg every 8 hours. Ms. G also describes gastrointestinal symptoms of bloating, constipation preceding her menstrual cycle, diarrhea during her menses, and occasionally nausea and vomiting with the severe pain.
On examination (which is not performed during menses), Ms. G appears well and is not in acute distress. Abdominal examination is benign. There is no tenderness to palpation or distension; bowel sounds are normal. Pelvic examination reveals mild tenderness upon palpation of a small and mobile uterus. Rectal examination is normal. She has no signs of hyperandrogenism (eg, male-pattern body hair, central obesity).
CASE 2
Rhonda M, 42 years old, gravida 3, para 3003, reports continuous pelvic pain for 7 years that is exacerbated by defecation, intercourse, and insertion of a tampon. She has a low level of dull baseline pain (3, on scale of one to 10) that occasionally spikes up to sharp, knifelike pain (10 on the pain scale), which, she says, brings her to tears. Ms. M describes the pain as “deep inside,” central in her pelvis, and radiating to the left and right, particularly during pain flares.
The patient’s 3 children were born by spontaneous vaginal delivery; however, she recalls that her youngest son was born via a traumatic vaginal delivery 8 years ago (he “got stuck coming out,” she reports). The only other component of Ms. M’s medical history is an anxiety disorder, for which she takes citalopram. She has a family history of cervical cancer.
Ms. M’s past diagnostic work-up for pelvic pain includes pelvic ultrasonography, endometrial biopsy, Pap smear, and diagnostic laparoscopy—all normal. She had a negative gastrointestinal work-up, including upper- and lower-tract endoscopy. Medical therapy, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), did not provide significant relief of pain.
Despite the negative work-up, Ms. M is still concerned that the pain might be related to cancer. With her family history of cervical cancer, she says that she does not want to “miss anything.”
Ms. M is thin and appears anxious. The abdomen is mildly and diffusely tender to palpation with normal bowel sounds and no distension. Pelvic examination reveals some hyperesthesia upon single-digit palpation of the pelvic floor. Placement of the speculum is difficult because of discomfort.
How would you proceed with the care of these patients?
What is chronic pelvic pain? Why is management such a challenge?
Chronic pelvic pain (CPP) is defined as chronic or intermittent cyclic or noncyclic pelvic pain lasting longer than 6 months, localized to the pelvis, diminishing a woman’s quality of life, and requiring medical intervention.1 It’s estimated that CPP affects as many as 15% of women of reproductive age in the United States each year, at a cost to the health care system of approximately $2 billion annually.2,3
CPP can result from abnormal pain responses from multiple body systems, including gynecologic conditions such as endometriosis. Notably, a nongynecologic cause is more often the major pain generator, without significant identifiable pathology (TABLE 1). Like all chronic pain disorders, CPP can also result in central sensitization of the nervous system, altering how pain is processed at the level of the pain matrix in the brain.4

This article reviews the limited evidence for treating CPP and offers recommendations for the primary care physician on providing symptomatic relief in the absence of diagnosed pathology (TABLE 25-13).

Treatment
Analgesics
NSAIDs are frequently used as first-line treatment for any kind of pain, including CPP. There is some evidence of benefit from NSAIDs, compared to placebo, in cyclic CPP secondary to dysmenorrhea and endometriosis;5,6 however, evidence of effectiveness in noncyclic CPP is absent. Because of the low cost and availability of NSAIDs, a trial is reasonable as a first-line intervention, particularly in CPP suspected to be endometriosis or of musculoskeletal origin. NSAIDs can cause adverse effects, including nausea, vomiting, headache, and drowsiness in 11% to 14% of women, although these agents are generally well-tolerated on a short-term basis.5
Opioids bind to opioid receptors in the central and peripheral nervous systems, resulting in an analgesic effect. Guidelines issued in 2016 by the Centers for Disease Control and Prevention recommend safer prescribing through careful evaluation of the risks and benefits of opioids for pain not caused by cancer and for palliation as part of end-of-life care.14
The risks of opioid use are well known in the medical community; they include tolerance, physical dependence, misuse, and death, in addition to common adverse effects such as nausea and vomiting, itching, constipation, and fatigue.14,15 Because of those risks and limited long-term benefit in nonmalignant pain disorders, opioid therapy for CPP should be avoided.14 For patients already taking an opioid, discuss a strategy for weaning and, if possible, provide home naloxone therapy in the event of accidental overdose.14
Hormonal therapy
Hormonal therapies are the most common nonsurgical treatment of noncyclic CPP, with or without a definitive diagnosis of endometriosis, in reproductive-age women with CPP.
Combined OCs, despite a lack of quality evidence, are frequently the first hormonal treatment tried in both cyclic and noncyclic CPP. A low-dosage OC may decrease cyclic pain in endometriosis, although it can increase irregular bleeding and nausea.16 As many as 53% of women with CPP reported having undergone a trial of an OC for endometriosis, despite the absence of consistent evidence showing effectiveness in CPP.17
Depot MPA, in trials, decreased pain more than placebo. It can be tried as a treatment, but its use is often limited because of adverse effects, such as weight gain and bloating.8
A trial of a levonorgestrel-releasing intrauterine device (LNG-IUD) is supported by moderate-quality evidence for women whose CPP is thought to be a symptom of endometriosis or to have another uterine origin.7
Gonadotropin-releasing hormone agonists, such as depot leuprolide and goserelin acetate implant, may be considered in a woman with a diagnosis of endometriosis whose pelvic pain is not alleviated by MPA or an LNG-IUD.9
Nonhormonal therapies
CPP shares pain mechanisms with other pain syndromes, such as neuropathic pain. Antineuropathic medications, such as gabapentin and pregabalin, may, therefore, provide benefit. These medications also produce improvement in pain disorders of the musculoskeletal system, which may contribute to their analgesic effect.18
Gabapentin and amitriptyline have been studied in CPP; both were found successful in decreasing perceived pain. Of note, patients who received gabapentin, a gamma-aminobutyric acid analogue, with or without amitriptyline, had more pain relief than those treated with amitriptyline alone.10 Adverse effects of these medications may limit their use (TABLE 319-25).

Tricyclic antidepressants are well-supported, effective treatments for chronic pain through the central increase of norepinephrine. Beginning at a low dosage to diminish adverse effects (TABLE 319-25) and increasing the dosage slowly to an effective level may increase adherence. A trial of at least 6 to 8 weeks, at a moderate dosage, is recommended before discontinuing the medication. Although amitriptyline has the most evidence for value in the management of CPP disorders,10 second-generation tricyclic antidepressants nortriptyline and desipramine have also been used for pain control, and may be better tolerated.
Duloxetine and venlafaxine—serotonin–norepinephrine reuptake inhibitors—increase serotonin in addition to norepinephrine, which is believed to result in pain control. Although a systematic review of trials of duloxetine for chronic pain showed some improvement in diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis, the review excluded CPP in its analysis.26
In our opinion, a selective neurotransmitter reuptake inhibitor can be attempted to diminish the central pain sensitization of CPP. As with all drugs that increase the availability of serotonin, serotonin syndrome is a rare risk. Additionally, when stopping duloxetine, a prolonged taper may be required.
Pelvic floor dysfunction therapy
Pelvic floor dysfunction of the musculature within the bony pelvis may contribute to, or cause, CPP. The pelvic floor musculature may be hypertonic or hypotonic, and trigger points may exist. Despite the frequency of pelvic floor dysfunction, detailed examination of the pelvic floor is not routinely performed during a pelvic exam.
Because of the high prevalence of pelvic floor dysfunction in women with CPP, evaluation of the pelvic floor muscles is warranted.27 (A protocol for this evaluation is detailed in TABLE 4.) Pelvic dynamometry may indicate muscle spasm or chronic tension; palpation of the pelvic floor during the exam can also identify a pain generator.

Although it might be difficult to distinguish pelvic floor myofascial pain as the primary or secondary cause of pain, pelvic floor physical therapy may clarify the role of the pelvic floor response (depending on the patient’s clinical exam and history). A low-quality retrospective case study on pelvic floor physical therapy reported significant improvement in pain that was proportional to the number of sessions completed.11 Trigger-point injections and injections of botulinum toxin A have been used with reported improvement in the pelvic floor pain profile, and there is evidence to support the benefit of such injections in pelvic muscle dysfunction.12
Psychotherapy
Cognitive behavioral therapy (CBT) is well established as an option to manage a patient’s response to pain, including teaching coping skills for a chronic pain disorder and pain flares. Evidence supports using CBT or mindfulness techniques over usual care in reducing the intensity of pain in chronic low back pain,28 and may be helpful in CPP. Patients with CPP who received 10 treatments of Mensendieck somatocognitive therapy (a mind–body therapy technique popular in Europe) over 90 days, compared with standard treatment alone, demonstrated improvement in pain, motor function, and psychological distress that persisted 9 months after treatment.13
Lifestyle changes, complementary and alternative therapies
Although medical and nonpharmacotherapeutic treatments are often important in the
Diet modifications may relieve pain in some women with CPP. Although a systematic review in 2011 highlighted the lack of data available for the efficacy of dietary therapies for treating CPP, the authors did present data that a diet rich in antioxidants might alleviate pain sysmptoms.29 Also, a gluten-free diet might reduce the symptoms of pain related to endometriosis and, thus, improve physical functioning, among other health domains.30
Exercise can be an important factor in the management of CPP, as with other chronic pain syndromes. In functional pain syndromes, the addition or maintenance of an exercise program has been shown to decrease the amount of pain medications required, improve depressive symptoms, increase energy, and decrease stress. Exercise also improves sleep quality and one’s ability to cope with pain.31
Yoga provides a good balance of aerobic and muscle-building activity and, in the authors’ experience, is tolerated by most women with CPP.
Acupuncture has limited evidence in the treatment of pelvic pain in women. Of the available studies, most are limited to pain related to endometriosis.32
Sleep hygiene may be an important consideration in managing CPP. Sleep disturbances are reported in more than 80% of women with CPP,33 including excessive time in bed and frequent napping, resulting in daytime fatigue and feeling generally unrested. A recent meta-analysis reported mild-to-moderate immediate improvement in patients’ pain after nonpharmacotherapeutic sleep interventions.34 The National Sleep Foundation has produced a patient guide to assist in sleep hygiene.35
Devising a management strategy despite sparse evidence
Because the cause of noncyclic CPP may be multifactorial, and because the literature on the etiology of CPP is limited (and, when there is research, it is inconclusive or of poor quality36), there are few evidence-based recommendations for treating CPP. Given the paucity of quality evidence, physicians should treat patients empirically, based on their experience and their familiarity with the range of medical and nonpharmacotherapeutic options used to manage other chronic pain syndromes.
CASE 1
Ms. G’s cyclic pelvic pain was present only during menses. The dyschezia, severe pain that began only after she discontinued a combined OC, aching pain, and severe menstrual cramps are, taken together, suggestive of endometriosis, despite a normal physical exam.
Medical and surgical options were reviewed with Ms. G. She elected to undergo diagnostic laparoscopy. Several extrauterine foci of endometrial tissue were noted and excised; an LNG-IUD was inserted. Her pain improved significantly after surgery.
CASE 2
Ms. M was found to have significant pain on single-digit examination of the pelvic floor muscles, indicating likely pelvic floor muscle dysfunction. Pelvic dynamometry revealed significant tightness and spasm in the pelvic floor muscles—specifically, the levator ani complex.
Ms. M was started on gabapentin to reduce baseline pain and was referred for pelvic floor physical therapy. She felt reassured that her risk of cancer was low, considering her negative work-up, and that cancer was not the cause of her pain. Her symptoms improved greatly with a regimen of medical and physical therapy, although she continues to experience pain flares.
CORRESPONDENCE
Wendy S. Biggs, MD, Central Michigan University College of Medicine, 1632 Stone St., Saginaw, MI 48602; [email protected].
1. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 51. Chronic pelvic pain. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2004;103:589-605.
2. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327.
3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys. 2014;17:e141-e147.
4. Rodriguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence of overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
5. Allen C, Hopewell S, Prentice A, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
6. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;(7):CD001751.
7. Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(3):CD009590.
8. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
9. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
10. Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761-768.
11. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
12. Abbott JA, Jarvis SK, Lyons SC, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
13. Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am J Obstet Gynecol. 2008;199:615.e1-e8.
14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645.
15. Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13:1181-1211.
16. Harada T, Momoeda M, Taketani Y, et al. Low-dose contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008:90:1583-1588.
17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human Reprod. 2013;28:2677-2685.
18. Haviv Y, Rettman A, Aframian D, et al. Myofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J Oral Facial Pain Headache. 2015;29:144-151.
19. Drugs.com. Amitriptyline dosing information. Available at: https://www.drugs.com/amitriptyline.html. Accessed January 4, 2018.
20. Drugs.com. Nortriptyline dosage. Available at: https://www.drugs.com/dosage/nortriptyline.html. Accessed January 4, 2018.
21. Drugs.com. Desipramine (oral route). Available at: https://www.drugs.com/cons/desipramine.html. Accessed January 4, 2018.
22. Drugs.com. Duloxetine capsules. Available at: https://www.drugs.com/pro/duloxetine-capsules.html. Accessed January 4, 2018.
23. Drugs.com. Venlafaxine. Available at: https://www.drugs.com/pro/venlafaxine.html. Accessed January 4, 2018.
24. Drugs.com. Gabapentin. Available at: https://www.drugs.com/pro/gabapentin.html. Accessed January 4, 2018.
25. Drugs.com. Pregabalin. Available at: https://www.drugs.com/monograph/pregabalin.html. Accessed January 4, 2018.
26. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
27. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594-611.
28. Cherkin DC, Sheman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
29. Sesti F, Capozzolo T, Pietropolli A, et al. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev. 2011;24:31-38.
30. Marziali M, Venza M, Lazzaro A, et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. 2012;67:499-504.
31. Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946-955.
32. Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database Syst Rev. 2011;(9):CD007864.
33. Cosar E, Çakır Güngör A, Gencer M, et.al. Sleep disturbance among women with chronic pelvic pain. Int J Gynaecol Obstet. 2014;126:232-234.
34. Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751-1764.
35. National Sleep Foundation. Healthy sleep tips. Available at: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. Accessed December 26, 2017.
36. Yunker A, Sathe NA, Reynolds WS, et al. Systematic review of therapies for noncyclic chronic pelvic pain in women. Obstet Gynecol Survey. 2012;67:417-425.
1. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 51. Chronic pelvic pain. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2004;103:589-605.
2. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327.
3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys. 2014;17:e141-e147.
4. Rodriguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence of overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
5. Allen C, Hopewell S, Prentice A, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
6. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;(7):CD001751.
7. Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(3):CD009590.
8. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
9. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
10. Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761-768.
11. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
12. Abbott JA, Jarvis SK, Lyons SC, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
13. Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am J Obstet Gynecol. 2008;199:615.e1-e8.
14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645.
15. Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13:1181-1211.
16. Harada T, Momoeda M, Taketani Y, et al. Low-dose contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008:90:1583-1588.
17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human Reprod. 2013;28:2677-2685.
18. Haviv Y, Rettman A, Aframian D, et al. Myofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J Oral Facial Pain Headache. 2015;29:144-151.
19. Drugs.com. Amitriptyline dosing information. Available at: https://www.drugs.com/amitriptyline.html. Accessed January 4, 2018.
20. Drugs.com. Nortriptyline dosage. Available at: https://www.drugs.com/dosage/nortriptyline.html. Accessed January 4, 2018.
21. Drugs.com. Desipramine (oral route). Available at: https://www.drugs.com/cons/desipramine.html. Accessed January 4, 2018.
22. Drugs.com. Duloxetine capsules. Available at: https://www.drugs.com/pro/duloxetine-capsules.html. Accessed January 4, 2018.
23. Drugs.com. Venlafaxine. Available at: https://www.drugs.com/pro/venlafaxine.html. Accessed January 4, 2018.
24. Drugs.com. Gabapentin. Available at: https://www.drugs.com/pro/gabapentin.html. Accessed January 4, 2018.
25. Drugs.com. Pregabalin. Available at: https://www.drugs.com/monograph/pregabalin.html. Accessed January 4, 2018.
26. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
27. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594-611.
28. Cherkin DC, Sheman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
29. Sesti F, Capozzolo T, Pietropolli A, et al. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev. 2011;24:31-38.
30. Marziali M, Venza M, Lazzaro A, et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. 2012;67:499-504.
31. Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946-955.
32. Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database Syst Rev. 2011;(9):CD007864.
33. Cosar E, Çakır Güngör A, Gencer M, et.al. Sleep disturbance among women with chronic pelvic pain. Int J Gynaecol Obstet. 2014;126:232-234.
34. Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751-1764.
35. National Sleep Foundation. Healthy sleep tips. Available at: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. Accessed December 26, 2017.
36. Yunker A, Sathe NA, Reynolds WS, et al. Systematic review of therapies for noncyclic chronic pelvic pain in women. Obstet Gynecol Survey. 2012;67:417-425.
PRACTICE RECOMMENDATIONS
› Consider the levonorgestrel-releasing intrauterine device for relief of chronic pelvic pain (CPP) from endometriosis; it’s been found to be more effective than expectant management. B
› Prescribe a trial of depot medroxyprogesterone acetate, which was more effective than placebo for CPP for as long as 9 months. B
› Use gabapentin—with or without amitriptyline—to provide greater relief of CPP than amitriptyline alone. B
› Recommend pelvic physical therapy for CPP; the pelvic pain score can be reduced in proportion to the number of sessions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
ACL injury: How do the physical examination tests compare?
CASE An athletic 25-year-old woman presents to her family physician complaining of a painful and swollen knee. She says that she injured the knee the day before during a judo match. The injury occurred when her upper body suddenly changed direction while her foot remained planted and her knee rotated medially. A cruciate ligament injury immediately comes to mind, but other potential diagnoses include meniscal injury, collateral ligament injury, and patellar instability. The first step in determining an accurate diagnosis is to evaluate the stability of the knee by physical examination—often a difficult task immediately following an injury.
How would you proceed?
Rupture of the anterior cruciate ligament (ACL), partial or complete, is a common injury, especially in athletes who hurt their knee in a pivoting movement.1 The number of patients who present with ACL injury is estimated at 252,000 per year.2 Cruciate ligament injury may lead to complaints of instability with subsequent inability to engage in sports activities. Cruciate ligament injury is also associated with premature development of osteoarthritis later in life.3 Operative treatment seems to be superior to conservative treatment in improving both subjective and objective measures of knee instability and in helping athletes return to their former level of activity.4
Because early detection is key to achieving the best clinical outcome, it is essential that the most accurate physical examination tests are performed during the acute phase. Primary care physicians, emergency room doctors, physical therapists, and athletic trainers are the ones who most often see these patients immediately following the injury, and they often have only the physical examination with which to assess ACL injury. Their task is to identify the patient with potential ACL injury and to refer the patient swiftly.
Three physical examination tests are most commonly used to evaluate cruciate ligament injury. The best known and most frequently used technique is the anterior drawer test. The other 2 tests, the Lachman test and the pivot shift test, are more difficult to perform and are used less often, especially by physicians untrained in their use. In addition, there is a relatively new diagnostic test: the lever sign test. The aim of our article is to provide a short, clinically relevant overview of the literature and to assess the diagnostic value of physical examination for the primary care physician.
Anterior drawer test
How it’s done. In this test, the patient lies supine on the examination table with hips flexed to 45 degrees and knees flexed to 90 degrees (FIGURE 1).5 The examiner sits on the table with a leg resting on the patient's foot, grasps the tibia of the injured leg just below the knee, and draws the tibia forward. If the tibia, compared with the tibia of the uninjured leg, moves farther anteriorly, or if the endpoint feels softened or is absent, the result is positive for an ACL injury.
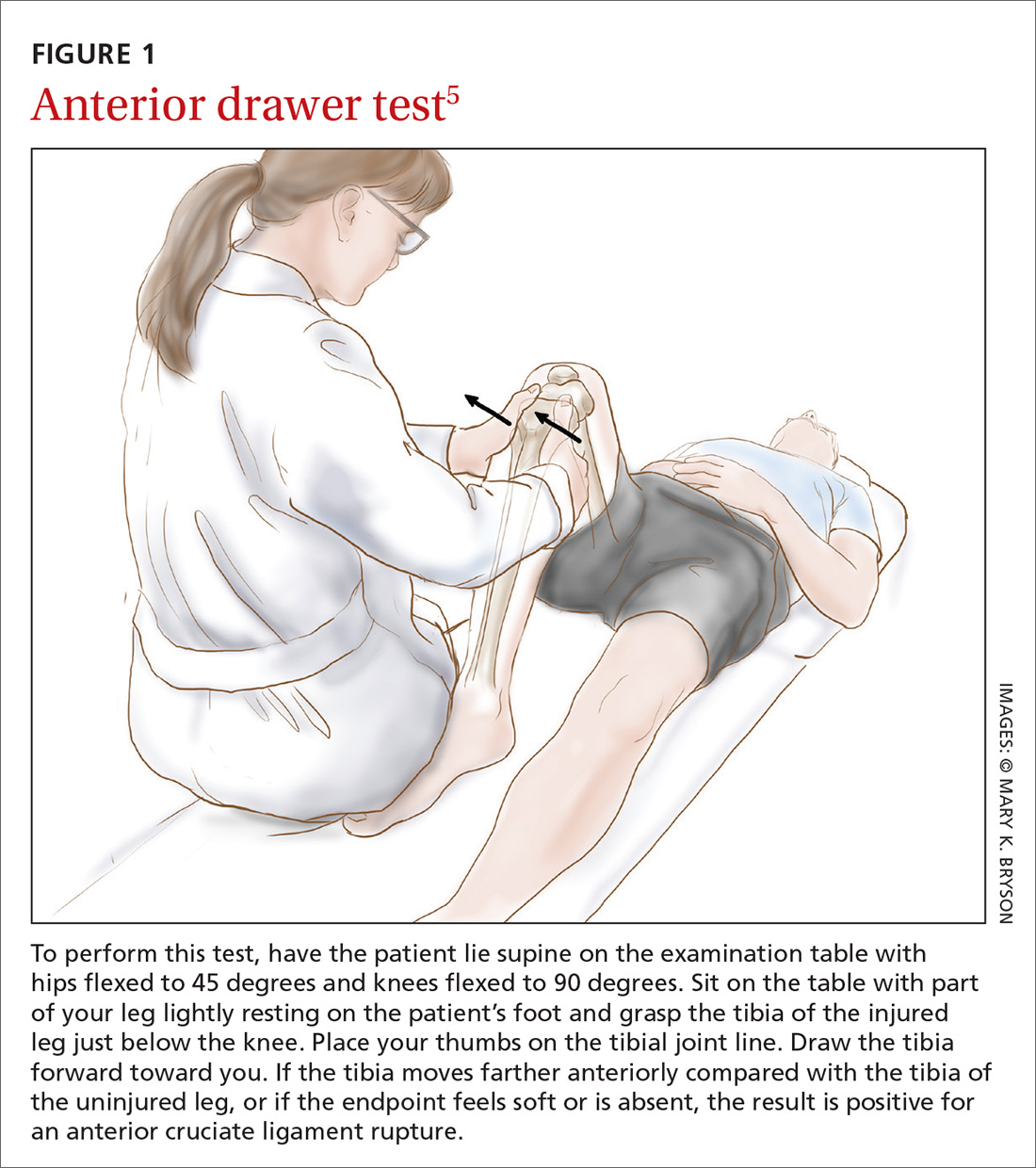
The literature. Nine systematic reviews conclude that the anterior drawer test is inferior to the Lachman test,6-14 which we’ll describe in a moment. This is due, in part, to the anterior drawer test’s unacceptably low sensitivity and specificity in the clinical setting—especially during the acute phase.10 The most recent meta-analysis on the anterior drawer test reports a sensitivity of 38% and a specificity of 81%.9 In other words, out of 100 ruptured ligaments, only 38 will test positive with the anterior drawer test.
The literature offers possible explanations for findings on the test’s validity. First, rupture of the ACL is often accompanied by swelling of the knee caused by hemarthrosis and reactive synovitis that can prevent the patient from flexing the knee to 90 degrees. Second, the joint pain may induce a protective muscle action, also called guarding of the hamstrings, that creates a vector opposing the passive anterior translation.15
Apart from the matter of a test’s validity, it's also important to consider the test’s inter- and intra-rater reliability.16 Compared with the Lachman test, the anterior drawer test is inferior in reliability.7
Lachman test
How it’s done. The Lachman test is performed with the patient supine on the table and the injured knee flexed at 20 to 30 degrees (FIGURE 2).5 The examiner holds the patient’s thigh with one hand and places the other hand beneath the tibia with the thumb of that hand on the tibial joint line. As the tibia is pulled forward, firm resistance suggests an uninjured ACL. Free movement without a hard endpoint, compared with the uninjured knee, indicates ACL injury.
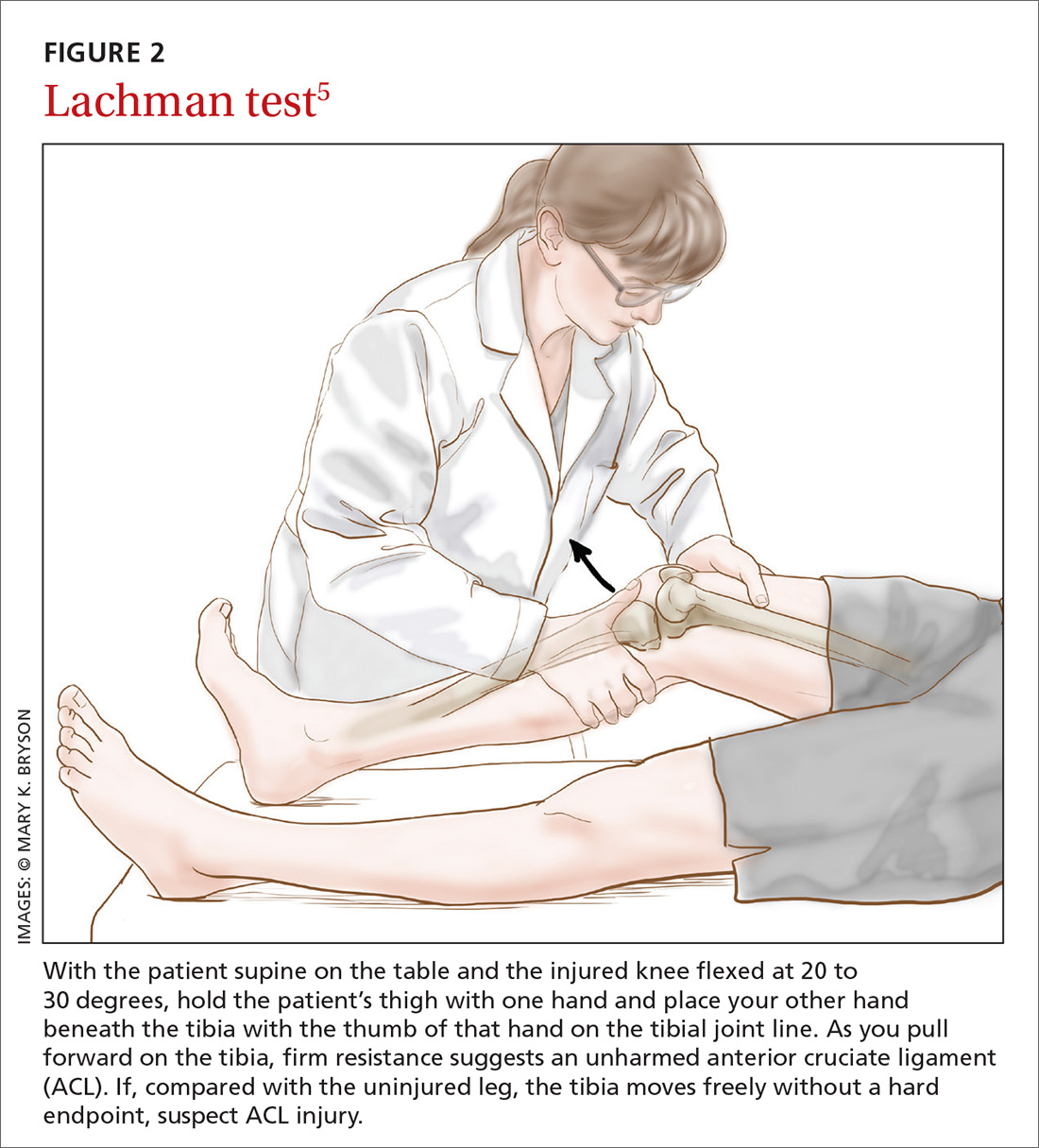
The literature. The Lachman test is the most accurate of the 3 diagnostic physical procedures. The most recent meta-analysis reports a sensitivity of 68% for partial ruptures and 96% for complete ACL ruptures.6 According to a recently published overview of systematic reviews, the Lachman test has high diagnostic value in confirming or ruling out an ACL injury.17
Two factors are important when assessing results of the Lachman test. The quantity of anterior translation of the tibia relative to the femur is as important as the quality of the endpoint of the anterior translation. Quantity of translation must always be compared with the unaffected knee. Quality of the endpoint in passive anterior translation should be assessed as “firm” or “sudden,” indicating an intact ACL, or as “absent, ill-defined, or softened,” indicating ACL pathology (TABLE).18
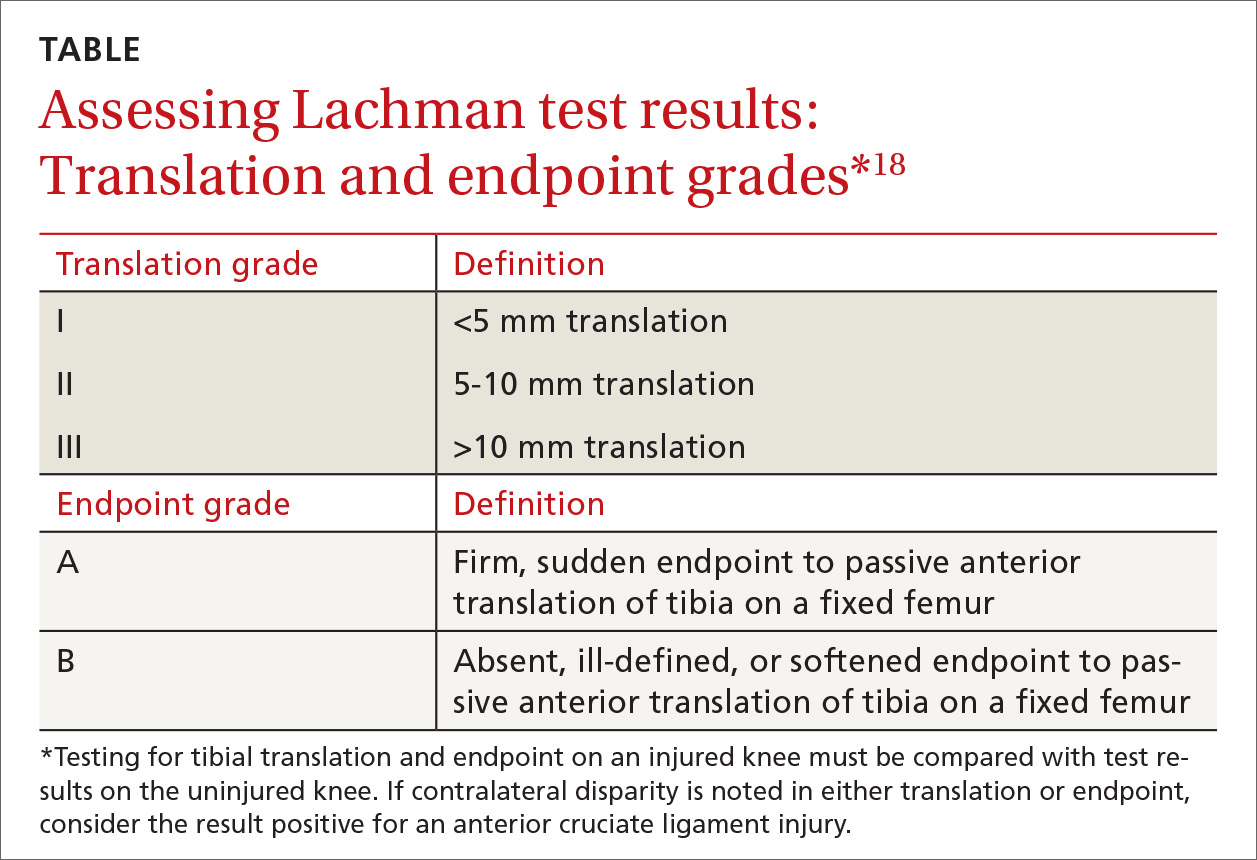
A drawback of the Lachman test is that it is challenging to perform correctly.19 The patient’s ability to relax the upper leg musculature is critically important. It is also essential to stabilize the distal femur, which can be problematic if the examiner has small hands relative to the size of the patient's leg musculature.10 These difficulties might be resolved by conducting the Lachman test with the patient in the prone position, known as the Prone Lachman.19 However, good evidence is not yet available to support this proposed solution. One systematic review, though, reports that the Prone Lachman test has the highest inter-rater reliability of all commonly used physical examination tests.7
The Lachman test is known as the test with highest validity on physical examination. When the outcome of a correctly performed Lachman test is negative, a rupture of the ACL is very unlikely.
Pivot shift test
How it’s done.
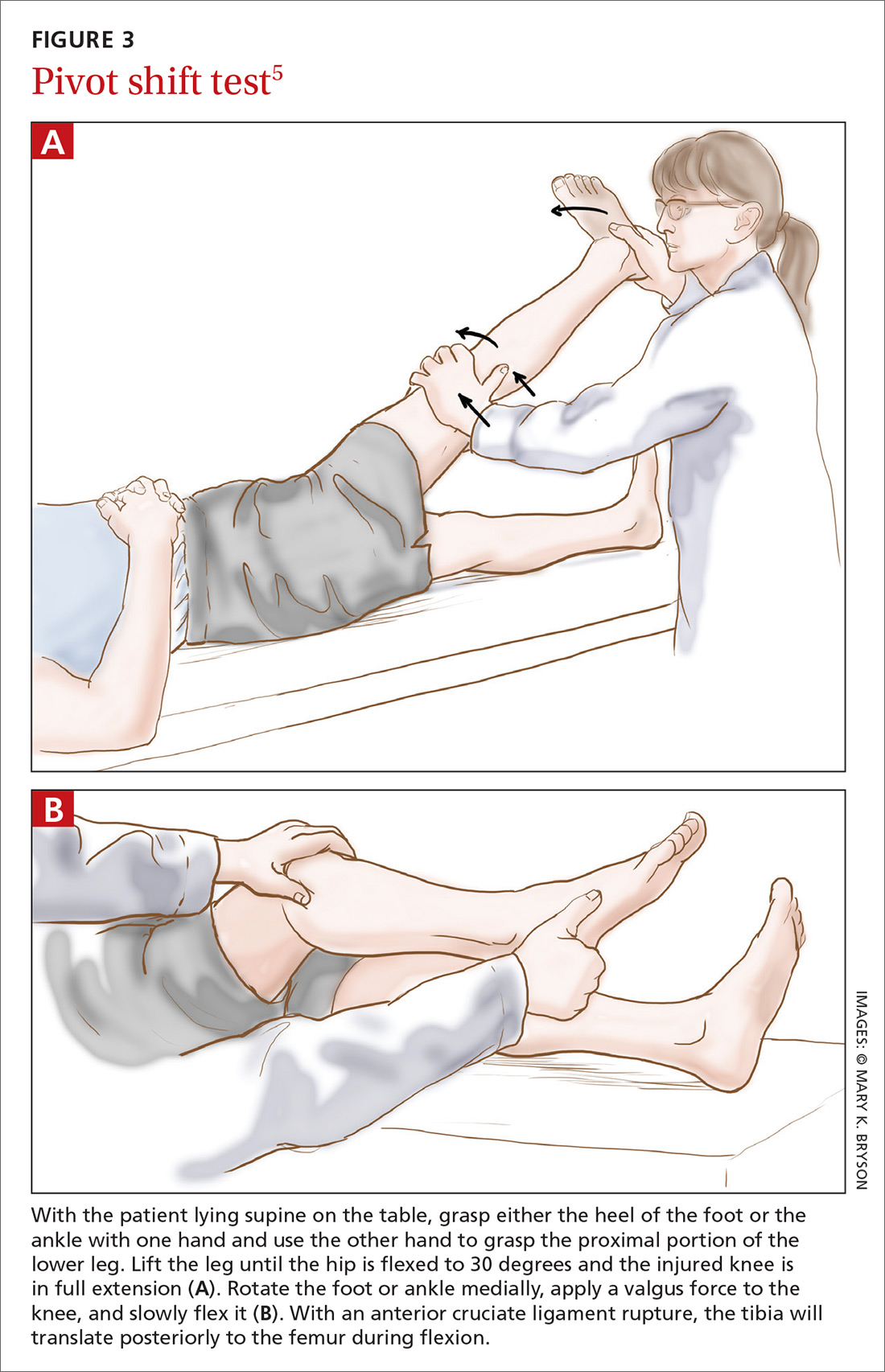
The literature. The pivot shift test is technically more challenging to perform than the other 2 tests and is, therefore, less practical in the primary care setting. However, when this test is done correctly, a positive result is highly specific for ACL injury.9,10 Reported sensitivity values are contradictory. The most recent meta-analysis reports a sensitivity of 85%.6 Two other studies cite much lower values: 24% and 28%.9,10 These data suggest that the pivot shift test, when carried out correctly, can be of use in confirming a possible ACL rupture. However, the test should not be used alone in ruling out a possible ACL injury.
New diagnostic test: Lever sign test
How it’s done. The lever sign test (FIGURE 4),20 introduced in the mid-2010s, is also performed with the patient lying in the supine position. The examiner stands at the side of the affected knee of the patient, places a closed fist just beneath the proximal third of the patient’s tibia, creating a slight flexion of the knee joint. With the other hand, the examiner applies a downward directed force to the distal third of the femur. With an intact ACL, the patient’s foot should rise from the table due to the induced lever mechanism. With a ruptured ACL, the lever effect is absent and the foot will not rise.
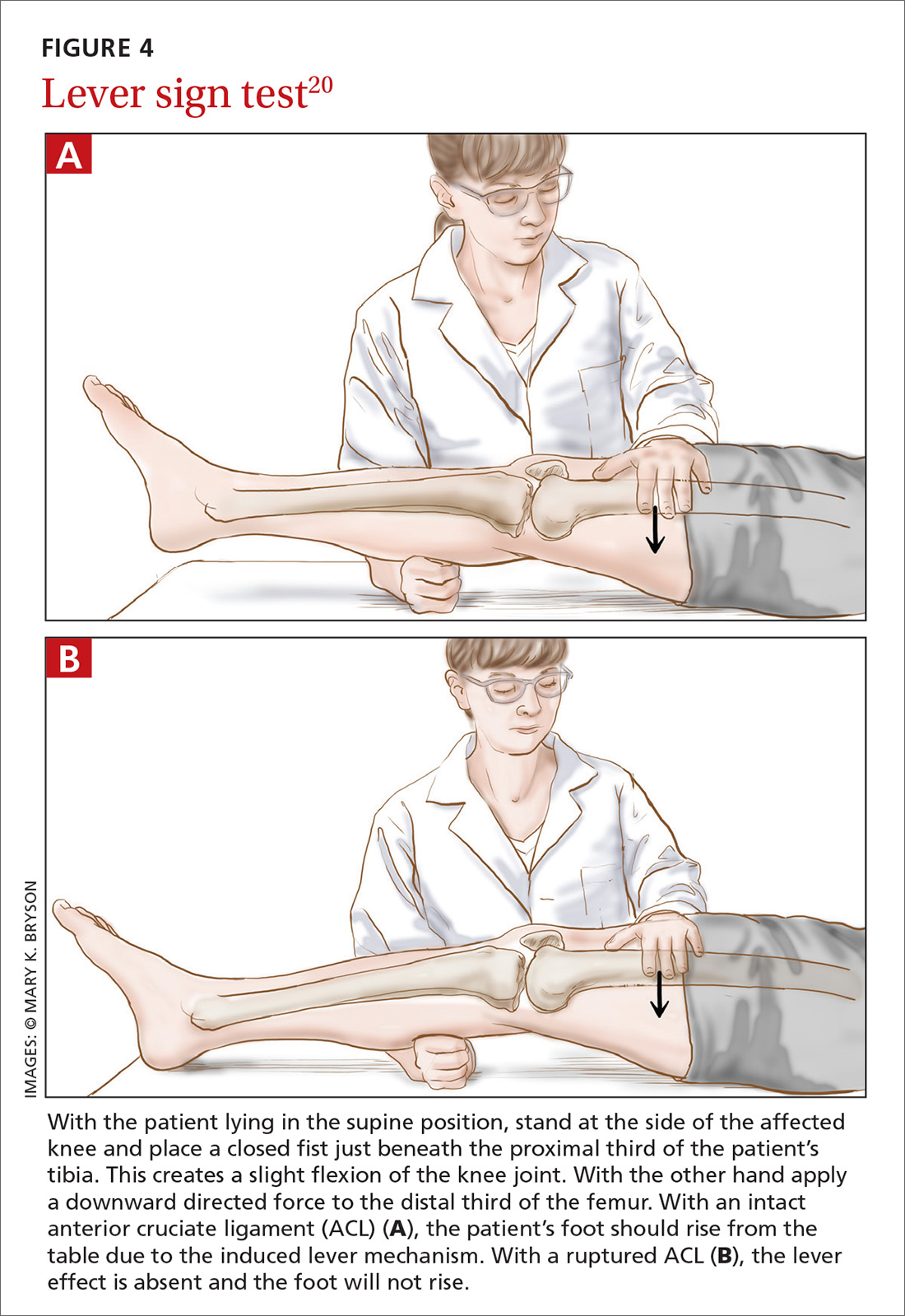
The literature. In the prospective clinical study that introduced the lever sign test, the sensitivity rate was reported at 100%—higher than that seen with the other commonly used tests.20 Another study has reported that the lever sign test was easily adopted in clinical practice and showed higher sensitivity than the Lachman test (94% vs 80% in pre-anesthesia assessment).21 However, a more recent study has shown a sensitivity of 77% for the lever sign.22 The lever sign test is relatively easy to perform and requires less examiner strength than does the Lachman test. These factors enhance applicability of the lever sign test in the primary care office and in other settings such as physical therapy centers and emergency departments.
Applying this information in primary care
Given the importance of physical examination in diagnosing ACL injury, how can the current evidence best be applied in primary care practice? Based on its good test properties and feasibility, the Lachman test is preferred in primary care. The anterior drawer test can be used, but its low accuracy must be considered in making an assessment. The pivot shift test, given its difficulty of execution, should not be used by physicians unacquainted with it.
If future research supports early reports of the lever sign test’s accuracy, it could be very helpful in family practice. Going forward, research should aim at developing a constructive strategy for applying these physical examination tests in both primary care and specialty settings.
CORRESPONDENCE
Christiaan H. Koster, Department of Trauma Surgery, VU University Medical Centre, P.O. Box 7057, 1081 HV Amsterdam, The Netherlands; [email protected].
ACKNOWLEDGEMENTS
We thank Frits Oosterveld, PhD, for critically reviewing the manuscript and Ralph de Vries for his assistance in the literature search.
1. Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141-50.
2. American Academy of Orthopedic Surgeons. Management of anterior cruciate ligament injuries. Evidence-based clinical practice guideline. 2014. Available at: http://www.aaos.org/research/guidelines/ACLGuidelineFINAL.pdf. Accessed January 26, 2018.
3. Simon D, Mascarenhas R, Saltzman BM, et al. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv Orthop. 2015;2015:928301. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4410751/. Accessed January 26, 2018.
4. Hinterwimmer S, Engelschalk M, Sauerland S, et al. [Operative or conservative treatment of anterior cruciate ligament rupture: a systematic review of the literature.] Unfallchirurg. 2003;106:374-379.
5. Brown JR, Trojian TH. Anterior and posterior cruciate ligament injuries. Prim Care. 2004;31:925-956.
6. Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;10:2805-2813.
7. Lange T, Freiberg A, Dröge P, et al. The reliability of physical examination tests for the diagnosis of anterior cruciate ligament rupture – a systematic review. Man Ther. 2015;20:402-411.
8. Swain MS, Henschke N, Kamper SJ, et al. Accuracy of clinical tests in the diagnosis of anterior cruciate ligament injury: a systematic review. Chiropr Man Therap. 2014;22:25. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4152763/. Accessed January 26, 2018.
9. van Eck CF, van den Bekerom MP, Fu FH, et al. Methods to diagnose acute anterior cruciate ligament rupture: a meta-analysis of physical examinations with and without anaesthesia. Knee Surg Sports Traumatol Arthrosc. 2013;21:1895-1903.
10. Benjaminse A, Gokeler A, van der Schans CP. Clinical diagnosis of an anterior cruciate ligament rupture: a meta-analysis. J Orthop Sports Phys Ther. 2006;36:267-288.
11. Jackson J, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med. 2003;139:575-588.
12. Malanga GA, Andrus S, Nadler SF, et al. Physical examination of the knee: a review of the original test description and scientific validity of common orthopedic tests. Arch Phys Med Rehabil. 2003;84:592-603.
13. Scholten RJ, Opstelten W, van der Plas CG, et al. Accuracy of physical diagnostic tests for assessing ruptures of the anterior cruciate ligament: a meta-analysis. J Fam. Pract. 2003;52:689-694.
14. Solomon DH, Simel DL, Bates DW, et al. The rational clinical examination. Does this patient have a torn meniscus or ligament of the knee? Value of the physical examination. JAMA. 2001;286:1610-1620.
15. Gurtler RA, Stine R, Torg JS. Lachman test evaluated. Quantification of a clinical observation. Clin Orthop Relat Res. 1987;216:141-150.
16. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217-238.
17. Décary S, Ouellet P, Vendittoli PA, et al. Diagnostic validity of physical examination tests for common knee disorders: an overview of systematic reviews and meta-analysis. Phys Ther Sport. 2017;23:143-155.
18. Mulligan EP, McGuffie DQ, Coyner K, et al. The reliability and diagnostic accuracy of assessing the translation endpoint during the Lachman test. Int J Sports Phys Ther. 2015;10:52-61.
19. Floyd RT, Peery DS, Andrews JR. Advantages of the prone Lachman versus the traditional Lachman. Orthopedics. 2008;31:671-675.
20. Lelli A, Di Turi RP, Spenciner DB, et al. The "Lever Sign": a new clinical test for the diagnosis of anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2016;24:2794-2797.
21. Deveci A, Cankaya D, Yilmaz S, et al. The arthroscopical and radiological corelation of lever sign test for the diagnosis of anterior cruciate ligament rupture. Springerplus. 2015;4:830. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4695483/. Accessed January 26, 2018.
22. Jarbo KA, Hartigan DE, Scott KL, et al. Accuracy of the Lever Sign Test in the diagnosis of anterior cruciate ligament injuries. Orthop J Sports Med. 2017;5(10):2325967117729809. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5639970/. Accessed January 26, 2018.
CASE An athletic 25-year-old woman presents to her family physician complaining of a painful and swollen knee. She says that she injured the knee the day before during a judo match. The injury occurred when her upper body suddenly changed direction while her foot remained planted and her knee rotated medially. A cruciate ligament injury immediately comes to mind, but other potential diagnoses include meniscal injury, collateral ligament injury, and patellar instability. The first step in determining an accurate diagnosis is to evaluate the stability of the knee by physical examination—often a difficult task immediately following an injury.
How would you proceed?
Rupture of the anterior cruciate ligament (ACL), partial or complete, is a common injury, especially in athletes who hurt their knee in a pivoting movement.1 The number of patients who present with ACL injury is estimated at 252,000 per year.2 Cruciate ligament injury may lead to complaints of instability with subsequent inability to engage in sports activities. Cruciate ligament injury is also associated with premature development of osteoarthritis later in life.3 Operative treatment seems to be superior to conservative treatment in improving both subjective and objective measures of knee instability and in helping athletes return to their former level of activity.4
Because early detection is key to achieving the best clinical outcome, it is essential that the most accurate physical examination tests are performed during the acute phase. Primary care physicians, emergency room doctors, physical therapists, and athletic trainers are the ones who most often see these patients immediately following the injury, and they often have only the physical examination with which to assess ACL injury. Their task is to identify the patient with potential ACL injury and to refer the patient swiftly.
Three physical examination tests are most commonly used to evaluate cruciate ligament injury. The best known and most frequently used technique is the anterior drawer test. The other 2 tests, the Lachman test and the pivot shift test, are more difficult to perform and are used less often, especially by physicians untrained in their use. In addition, there is a relatively new diagnostic test: the lever sign test. The aim of our article is to provide a short, clinically relevant overview of the literature and to assess the diagnostic value of physical examination for the primary care physician.
Anterior drawer test
How it’s done. In this test, the patient lies supine on the examination table with hips flexed to 45 degrees and knees flexed to 90 degrees (FIGURE 1).5 The examiner sits on the table with a leg resting on the patient's foot, grasps the tibia of the injured leg just below the knee, and draws the tibia forward. If the tibia, compared with the tibia of the uninjured leg, moves farther anteriorly, or if the endpoint feels softened or is absent, the result is positive for an ACL injury.

The literature. Nine systematic reviews conclude that the anterior drawer test is inferior to the Lachman test,6-14 which we’ll describe in a moment. This is due, in part, to the anterior drawer test’s unacceptably low sensitivity and specificity in the clinical setting—especially during the acute phase.10 The most recent meta-analysis on the anterior drawer test reports a sensitivity of 38% and a specificity of 81%.9 In other words, out of 100 ruptured ligaments, only 38 will test positive with the anterior drawer test.
The literature offers possible explanations for findings on the test’s validity. First, rupture of the ACL is often accompanied by swelling of the knee caused by hemarthrosis and reactive synovitis that can prevent the patient from flexing the knee to 90 degrees. Second, the joint pain may induce a protective muscle action, also called guarding of the hamstrings, that creates a vector opposing the passive anterior translation.15
Apart from the matter of a test’s validity, it's also important to consider the test’s inter- and intra-rater reliability.16 Compared with the Lachman test, the anterior drawer test is inferior in reliability.7
Lachman test
How it’s done. The Lachman test is performed with the patient supine on the table and the injured knee flexed at 20 to 30 degrees (FIGURE 2).5 The examiner holds the patient’s thigh with one hand and places the other hand beneath the tibia with the thumb of that hand on the tibial joint line. As the tibia is pulled forward, firm resistance suggests an uninjured ACL. Free movement without a hard endpoint, compared with the uninjured knee, indicates ACL injury.

The literature. The Lachman test is the most accurate of the 3 diagnostic physical procedures. The most recent meta-analysis reports a sensitivity of 68% for partial ruptures and 96% for complete ACL ruptures.6 According to a recently published overview of systematic reviews, the Lachman test has high diagnostic value in confirming or ruling out an ACL injury.17
Two factors are important when assessing results of the Lachman test. The quantity of anterior translation of the tibia relative to the femur is as important as the quality of the endpoint of the anterior translation. Quantity of translation must always be compared with the unaffected knee. Quality of the endpoint in passive anterior translation should be assessed as “firm” or “sudden,” indicating an intact ACL, or as “absent, ill-defined, or softened,” indicating ACL pathology (TABLE).18

A drawback of the Lachman test is that it is challenging to perform correctly.19 The patient’s ability to relax the upper leg musculature is critically important. It is also essential to stabilize the distal femur, which can be problematic if the examiner has small hands relative to the size of the patient's leg musculature.10 These difficulties might be resolved by conducting the Lachman test with the patient in the prone position, known as the Prone Lachman.19 However, good evidence is not yet available to support this proposed solution. One systematic review, though, reports that the Prone Lachman test has the highest inter-rater reliability of all commonly used physical examination tests.7
The Lachman test is known as the test with highest validity on physical examination. When the outcome of a correctly performed Lachman test is negative, a rupture of the ACL is very unlikely.
Pivot shift test
How it’s done.

The literature. The pivot shift test is technically more challenging to perform than the other 2 tests and is, therefore, less practical in the primary care setting. However, when this test is done correctly, a positive result is highly specific for ACL injury.9,10 Reported sensitivity values are contradictory. The most recent meta-analysis reports a sensitivity of 85%.6 Two other studies cite much lower values: 24% and 28%.9,10 These data suggest that the pivot shift test, when carried out correctly, can be of use in confirming a possible ACL rupture. However, the test should not be used alone in ruling out a possible ACL injury.
New diagnostic test: Lever sign test
How it’s done. The lever sign test (FIGURE 4),20 introduced in the mid-2010s, is also performed with the patient lying in the supine position. The examiner stands at the side of the affected knee of the patient, places a closed fist just beneath the proximal third of the patient’s tibia, creating a slight flexion of the knee joint. With the other hand, the examiner applies a downward directed force to the distal third of the femur. With an intact ACL, the patient’s foot should rise from the table due to the induced lever mechanism. With a ruptured ACL, the lever effect is absent and the foot will not rise.

The literature. In the prospective clinical study that introduced the lever sign test, the sensitivity rate was reported at 100%—higher than that seen with the other commonly used tests.20 Another study has reported that the lever sign test was easily adopted in clinical practice and showed higher sensitivity than the Lachman test (94% vs 80% in pre-anesthesia assessment).21 However, a more recent study has shown a sensitivity of 77% for the lever sign.22 The lever sign test is relatively easy to perform and requires less examiner strength than does the Lachman test. These factors enhance applicability of the lever sign test in the primary care office and in other settings such as physical therapy centers and emergency departments.
Applying this information in primary care
Given the importance of physical examination in diagnosing ACL injury, how can the current evidence best be applied in primary care practice? Based on its good test properties and feasibility, the Lachman test is preferred in primary care. The anterior drawer test can be used, but its low accuracy must be considered in making an assessment. The pivot shift test, given its difficulty of execution, should not be used by physicians unacquainted with it.
If future research supports early reports of the lever sign test’s accuracy, it could be very helpful in family practice. Going forward, research should aim at developing a constructive strategy for applying these physical examination tests in both primary care and specialty settings.
CORRESPONDENCE
Christiaan H. Koster, Department of Trauma Surgery, VU University Medical Centre, P.O. Box 7057, 1081 HV Amsterdam, The Netherlands; [email protected].
ACKNOWLEDGEMENTS
We thank Frits Oosterveld, PhD, for critically reviewing the manuscript and Ralph de Vries for his assistance in the literature search.
CASE An athletic 25-year-old woman presents to her family physician complaining of a painful and swollen knee. She says that she injured the knee the day before during a judo match. The injury occurred when her upper body suddenly changed direction while her foot remained planted and her knee rotated medially. A cruciate ligament injury immediately comes to mind, but other potential diagnoses include meniscal injury, collateral ligament injury, and patellar instability. The first step in determining an accurate diagnosis is to evaluate the stability of the knee by physical examination—often a difficult task immediately following an injury.
How would you proceed?
Rupture of the anterior cruciate ligament (ACL), partial or complete, is a common injury, especially in athletes who hurt their knee in a pivoting movement.1 The number of patients who present with ACL injury is estimated at 252,000 per year.2 Cruciate ligament injury may lead to complaints of instability with subsequent inability to engage in sports activities. Cruciate ligament injury is also associated with premature development of osteoarthritis later in life.3 Operative treatment seems to be superior to conservative treatment in improving both subjective and objective measures of knee instability and in helping athletes return to their former level of activity.4
Because early detection is key to achieving the best clinical outcome, it is essential that the most accurate physical examination tests are performed during the acute phase. Primary care physicians, emergency room doctors, physical therapists, and athletic trainers are the ones who most often see these patients immediately following the injury, and they often have only the physical examination with which to assess ACL injury. Their task is to identify the patient with potential ACL injury and to refer the patient swiftly.
Three physical examination tests are most commonly used to evaluate cruciate ligament injury. The best known and most frequently used technique is the anterior drawer test. The other 2 tests, the Lachman test and the pivot shift test, are more difficult to perform and are used less often, especially by physicians untrained in their use. In addition, there is a relatively new diagnostic test: the lever sign test. The aim of our article is to provide a short, clinically relevant overview of the literature and to assess the diagnostic value of physical examination for the primary care physician.
Anterior drawer test
How it’s done. In this test, the patient lies supine on the examination table with hips flexed to 45 degrees and knees flexed to 90 degrees (FIGURE 1).5 The examiner sits on the table with a leg resting on the patient's foot, grasps the tibia of the injured leg just below the knee, and draws the tibia forward. If the tibia, compared with the tibia of the uninjured leg, moves farther anteriorly, or if the endpoint feels softened or is absent, the result is positive for an ACL injury.

The literature. Nine systematic reviews conclude that the anterior drawer test is inferior to the Lachman test,6-14 which we’ll describe in a moment. This is due, in part, to the anterior drawer test’s unacceptably low sensitivity and specificity in the clinical setting—especially during the acute phase.10 The most recent meta-analysis on the anterior drawer test reports a sensitivity of 38% and a specificity of 81%.9 In other words, out of 100 ruptured ligaments, only 38 will test positive with the anterior drawer test.
The literature offers possible explanations for findings on the test’s validity. First, rupture of the ACL is often accompanied by swelling of the knee caused by hemarthrosis and reactive synovitis that can prevent the patient from flexing the knee to 90 degrees. Second, the joint pain may induce a protective muscle action, also called guarding of the hamstrings, that creates a vector opposing the passive anterior translation.15
Apart from the matter of a test’s validity, it's also important to consider the test’s inter- and intra-rater reliability.16 Compared with the Lachman test, the anterior drawer test is inferior in reliability.7
Lachman test
How it’s done. The Lachman test is performed with the patient supine on the table and the injured knee flexed at 20 to 30 degrees (FIGURE 2).5 The examiner holds the patient’s thigh with one hand and places the other hand beneath the tibia with the thumb of that hand on the tibial joint line. As the tibia is pulled forward, firm resistance suggests an uninjured ACL. Free movement without a hard endpoint, compared with the uninjured knee, indicates ACL injury.

The literature. The Lachman test is the most accurate of the 3 diagnostic physical procedures. The most recent meta-analysis reports a sensitivity of 68% for partial ruptures and 96% for complete ACL ruptures.6 According to a recently published overview of systematic reviews, the Lachman test has high diagnostic value in confirming or ruling out an ACL injury.17
Two factors are important when assessing results of the Lachman test. The quantity of anterior translation of the tibia relative to the femur is as important as the quality of the endpoint of the anterior translation. Quantity of translation must always be compared with the unaffected knee. Quality of the endpoint in passive anterior translation should be assessed as “firm” or “sudden,” indicating an intact ACL, or as “absent, ill-defined, or softened,” indicating ACL pathology (TABLE).18

A drawback of the Lachman test is that it is challenging to perform correctly.19 The patient’s ability to relax the upper leg musculature is critically important. It is also essential to stabilize the distal femur, which can be problematic if the examiner has small hands relative to the size of the patient's leg musculature.10 These difficulties might be resolved by conducting the Lachman test with the patient in the prone position, known as the Prone Lachman.19 However, good evidence is not yet available to support this proposed solution. One systematic review, though, reports that the Prone Lachman test has the highest inter-rater reliability of all commonly used physical examination tests.7
The Lachman test is known as the test with highest validity on physical examination. When the outcome of a correctly performed Lachman test is negative, a rupture of the ACL is very unlikely.
Pivot shift test
How it’s done.

The literature. The pivot shift test is technically more challenging to perform than the other 2 tests and is, therefore, less practical in the primary care setting. However, when this test is done correctly, a positive result is highly specific for ACL injury.9,10 Reported sensitivity values are contradictory. The most recent meta-analysis reports a sensitivity of 85%.6 Two other studies cite much lower values: 24% and 28%.9,10 These data suggest that the pivot shift test, when carried out correctly, can be of use in confirming a possible ACL rupture. However, the test should not be used alone in ruling out a possible ACL injury.
New diagnostic test: Lever sign test
How it’s done. The lever sign test (FIGURE 4),20 introduced in the mid-2010s, is also performed with the patient lying in the supine position. The examiner stands at the side of the affected knee of the patient, places a closed fist just beneath the proximal third of the patient’s tibia, creating a slight flexion of the knee joint. With the other hand, the examiner applies a downward directed force to the distal third of the femur. With an intact ACL, the patient’s foot should rise from the table due to the induced lever mechanism. With a ruptured ACL, the lever effect is absent and the foot will not rise.

The literature. In the prospective clinical study that introduced the lever sign test, the sensitivity rate was reported at 100%—higher than that seen with the other commonly used tests.20 Another study has reported that the lever sign test was easily adopted in clinical practice and showed higher sensitivity than the Lachman test (94% vs 80% in pre-anesthesia assessment).21 However, a more recent study has shown a sensitivity of 77% for the lever sign.22 The lever sign test is relatively easy to perform and requires less examiner strength than does the Lachman test. These factors enhance applicability of the lever sign test in the primary care office and in other settings such as physical therapy centers and emergency departments.
Applying this information in primary care
Given the importance of physical examination in diagnosing ACL injury, how can the current evidence best be applied in primary care practice? Based on its good test properties and feasibility, the Lachman test is preferred in primary care. The anterior drawer test can be used, but its low accuracy must be considered in making an assessment. The pivot shift test, given its difficulty of execution, should not be used by physicians unacquainted with it.
If future research supports early reports of the lever sign test’s accuracy, it could be very helpful in family practice. Going forward, research should aim at developing a constructive strategy for applying these physical examination tests in both primary care and specialty settings.
CORRESPONDENCE
Christiaan H. Koster, Department of Trauma Surgery, VU University Medical Centre, P.O. Box 7057, 1081 HV Amsterdam, The Netherlands; [email protected].
ACKNOWLEDGEMENTS
We thank Frits Oosterveld, PhD, for critically reviewing the manuscript and Ralph de Vries for his assistance in the literature search.
1. Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141-50.
2. American Academy of Orthopedic Surgeons. Management of anterior cruciate ligament injuries. Evidence-based clinical practice guideline. 2014. Available at: http://www.aaos.org/research/guidelines/ACLGuidelineFINAL.pdf. Accessed January 26, 2018.
3. Simon D, Mascarenhas R, Saltzman BM, et al. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv Orthop. 2015;2015:928301. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4410751/. Accessed January 26, 2018.
4. Hinterwimmer S, Engelschalk M, Sauerland S, et al. [Operative or conservative treatment of anterior cruciate ligament rupture: a systematic review of the literature.] Unfallchirurg. 2003;106:374-379.
5. Brown JR, Trojian TH. Anterior and posterior cruciate ligament injuries. Prim Care. 2004;31:925-956.
6. Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;10:2805-2813.
7. Lange T, Freiberg A, Dröge P, et al. The reliability of physical examination tests for the diagnosis of anterior cruciate ligament rupture – a systematic review. Man Ther. 2015;20:402-411.
8. Swain MS, Henschke N, Kamper SJ, et al. Accuracy of clinical tests in the diagnosis of anterior cruciate ligament injury: a systematic review. Chiropr Man Therap. 2014;22:25. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4152763/. Accessed January 26, 2018.
9. van Eck CF, van den Bekerom MP, Fu FH, et al. Methods to diagnose acute anterior cruciate ligament rupture: a meta-analysis of physical examinations with and without anaesthesia. Knee Surg Sports Traumatol Arthrosc. 2013;21:1895-1903.
10. Benjaminse A, Gokeler A, van der Schans CP. Clinical diagnosis of an anterior cruciate ligament rupture: a meta-analysis. J Orthop Sports Phys Ther. 2006;36:267-288.
11. Jackson J, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med. 2003;139:575-588.
12. Malanga GA, Andrus S, Nadler SF, et al. Physical examination of the knee: a review of the original test description and scientific validity of common orthopedic tests. Arch Phys Med Rehabil. 2003;84:592-603.
13. Scholten RJ, Opstelten W, van der Plas CG, et al. Accuracy of physical diagnostic tests for assessing ruptures of the anterior cruciate ligament: a meta-analysis. J Fam. Pract. 2003;52:689-694.
14. Solomon DH, Simel DL, Bates DW, et al. The rational clinical examination. Does this patient have a torn meniscus or ligament of the knee? Value of the physical examination. JAMA. 2001;286:1610-1620.
15. Gurtler RA, Stine R, Torg JS. Lachman test evaluated. Quantification of a clinical observation. Clin Orthop Relat Res. 1987;216:141-150.
16. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217-238.
17. Décary S, Ouellet P, Vendittoli PA, et al. Diagnostic validity of physical examination tests for common knee disorders: an overview of systematic reviews and meta-analysis. Phys Ther Sport. 2017;23:143-155.
18. Mulligan EP, McGuffie DQ, Coyner K, et al. The reliability and diagnostic accuracy of assessing the translation endpoint during the Lachman test. Int J Sports Phys Ther. 2015;10:52-61.
19. Floyd RT, Peery DS, Andrews JR. Advantages of the prone Lachman versus the traditional Lachman. Orthopedics. 2008;31:671-675.
20. Lelli A, Di Turi RP, Spenciner DB, et al. The "Lever Sign": a new clinical test for the diagnosis of anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2016;24:2794-2797.
21. Deveci A, Cankaya D, Yilmaz S, et al. The arthroscopical and radiological corelation of lever sign test for the diagnosis of anterior cruciate ligament rupture. Springerplus. 2015;4:830. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4695483/. Accessed January 26, 2018.
22. Jarbo KA, Hartigan DE, Scott KL, et al. Accuracy of the Lever Sign Test in the diagnosis of anterior cruciate ligament injuries. Orthop J Sports Med. 2017;5(10):2325967117729809. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5639970/. Accessed January 26, 2018.
1. Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141-50.
2. American Academy of Orthopedic Surgeons. Management of anterior cruciate ligament injuries. Evidence-based clinical practice guideline. 2014. Available at: http://www.aaos.org/research/guidelines/ACLGuidelineFINAL.pdf. Accessed January 26, 2018.
3. Simon D, Mascarenhas R, Saltzman BM, et al. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv Orthop. 2015;2015:928301. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4410751/. Accessed January 26, 2018.
4. Hinterwimmer S, Engelschalk M, Sauerland S, et al. [Operative or conservative treatment of anterior cruciate ligament rupture: a systematic review of the literature.] Unfallchirurg. 2003;106:374-379.
5. Brown JR, Trojian TH. Anterior and posterior cruciate ligament injuries. Prim Care. 2004;31:925-956.
6. Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;10:2805-2813.
7. Lange T, Freiberg A, Dröge P, et al. The reliability of physical examination tests for the diagnosis of anterior cruciate ligament rupture – a systematic review. Man Ther. 2015;20:402-411.
8. Swain MS, Henschke N, Kamper SJ, et al. Accuracy of clinical tests in the diagnosis of anterior cruciate ligament injury: a systematic review. Chiropr Man Therap. 2014;22:25. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4152763/. Accessed January 26, 2018.
9. van Eck CF, van den Bekerom MP, Fu FH, et al. Methods to diagnose acute anterior cruciate ligament rupture: a meta-analysis of physical examinations with and without anaesthesia. Knee Surg Sports Traumatol Arthrosc. 2013;21:1895-1903.
10. Benjaminse A, Gokeler A, van der Schans CP. Clinical diagnosis of an anterior cruciate ligament rupture: a meta-analysis. J Orthop Sports Phys Ther. 2006;36:267-288.
11. Jackson J, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med. 2003;139:575-588.
12. Malanga GA, Andrus S, Nadler SF, et al. Physical examination of the knee: a review of the original test description and scientific validity of common orthopedic tests. Arch Phys Med Rehabil. 2003;84:592-603.
13. Scholten RJ, Opstelten W, van der Plas CG, et al. Accuracy of physical diagnostic tests for assessing ruptures of the anterior cruciate ligament: a meta-analysis. J Fam. Pract. 2003;52:689-694.
14. Solomon DH, Simel DL, Bates DW, et al. The rational clinical examination. Does this patient have a torn meniscus or ligament of the knee? Value of the physical examination. JAMA. 2001;286:1610-1620.
15. Gurtler RA, Stine R, Torg JS. Lachman test evaluated. Quantification of a clinical observation. Clin Orthop Relat Res. 1987;216:141-150.
16. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217-238.
17. Décary S, Ouellet P, Vendittoli PA, et al. Diagnostic validity of physical examination tests for common knee disorders: an overview of systematic reviews and meta-analysis. Phys Ther Sport. 2017;23:143-155.
18. Mulligan EP, McGuffie DQ, Coyner K, et al. The reliability and diagnostic accuracy of assessing the translation endpoint during the Lachman test. Int J Sports Phys Ther. 2015;10:52-61.
19. Floyd RT, Peery DS, Andrews JR. Advantages of the prone Lachman versus the traditional Lachman. Orthopedics. 2008;31:671-675.
20. Lelli A, Di Turi RP, Spenciner DB, et al. The "Lever Sign": a new clinical test for the diagnosis of anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2016;24:2794-2797.
21. Deveci A, Cankaya D, Yilmaz S, et al. The arthroscopical and radiological corelation of lever sign test for the diagnosis of anterior cruciate ligament rupture. Springerplus. 2015;4:830. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4695483/. Accessed January 26, 2018.
22. Jarbo KA, Hartigan DE, Scott KL, et al. Accuracy of the Lever Sign Test in the diagnosis of anterior cruciate ligament injuries. Orthop J Sports Med. 2017;5(10):2325967117729809. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5639970/. Accessed January 26, 2018.
PRACTICE RECOMMENDATIONS
› Consider using the Lachman test, known to have higher validity than other anterior cruciate ligament (ACL) physical examination tests. When the outcome of a correctly performed test is negative, a rupture of the ACL is unlikely. A
› Use the pivot shift test to confirm a possible ACL rupture only if good execution is assured. Do not use the pivot shift test alone to rule out a possible ACL injury. A
› Familiarize yourself with the lever sign test, which is easy to perform but has yielded varying reports on sensitivity and specificity for ACL rupture. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
March 2018: Click for Credit
Here are 4 articles in the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Prenatal Maternal Anxiety Linked to Hyperactivity in Offspring as Teenagers
To take the posttest, go to: http://bit.ly/2BLXsRs
Expires November 15, 2018
2. The Better Mammogram: Experts Explore Sensitivity of New Modalities
To take the posttest, go to: http://bit.ly/2nQaJii
Expires November 14, 2018
3. Large Database Analysis Suggests Safety of Bariatric Surgery in Seniors
To take the posttest, go to: http://bit.ly/2E3tcmJ
Expires November 14, 2018
4. Salivary Biomarker for Huntington Disease Identified
To take the posttest, go to: http://bit.ly/2BGQpJP
Expires November 13, 2018
Here are 4 articles in the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Prenatal Maternal Anxiety Linked to Hyperactivity in Offspring as Teenagers
To take the posttest, go to: http://bit.ly/2BLXsRs
Expires November 15, 2018
2. The Better Mammogram: Experts Explore Sensitivity of New Modalities
To take the posttest, go to: http://bit.ly/2nQaJii
Expires November 14, 2018
3. Large Database Analysis Suggests Safety of Bariatric Surgery in Seniors
To take the posttest, go to: http://bit.ly/2E3tcmJ
Expires November 14, 2018
4. Salivary Biomarker for Huntington Disease Identified
To take the posttest, go to: http://bit.ly/2BGQpJP
Expires November 13, 2018
Here are 4 articles in the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Prenatal Maternal Anxiety Linked to Hyperactivity in Offspring as Teenagers
To take the posttest, go to: http://bit.ly/2BLXsRs
Expires November 15, 2018
2. The Better Mammogram: Experts Explore Sensitivity of New Modalities
To take the posttest, go to: http://bit.ly/2nQaJii
Expires November 14, 2018
3. Large Database Analysis Suggests Safety of Bariatric Surgery in Seniors
To take the posttest, go to: http://bit.ly/2E3tcmJ
Expires November 14, 2018
4. Salivary Biomarker for Huntington Disease Identified
To take the posttest, go to: http://bit.ly/2BGQpJP
Expires November 13, 2018