User login
Correction: Update on VTE
In the article, “Update on the management of venous thromboembolism” (Bartholomew JR, Cleve Clin J Med 2017; 84[suppl 3]:39–46), 2 sentences in the text regarding dose reduction for body weight have errors. The corrected sentences follow:
On page 42, left column, the last 5 lines should read: “The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater.”
And on page 42, right column, the sentence 10 lines from the top should read: “Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors.”
In the article, “Update on the management of venous thromboembolism” (Bartholomew JR, Cleve Clin J Med 2017; 84[suppl 3]:39–46), 2 sentences in the text regarding dose reduction for body weight have errors. The corrected sentences follow:
On page 42, left column, the last 5 lines should read: “The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater.”
And on page 42, right column, the sentence 10 lines from the top should read: “Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors.”
In the article, “Update on the management of venous thromboembolism” (Bartholomew JR, Cleve Clin J Med 2017; 84[suppl 3]:39–46), 2 sentences in the text regarding dose reduction for body weight have errors. The corrected sentences follow:
On page 42, left column, the last 5 lines should read: “The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater.”
And on page 42, right column, the sentence 10 lines from the top should read: “Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors.”
MDedge Daily News: Medical students keep their DACA protection
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Medical students remain protected by DACA – for now. The AIDS epidemic’s skin villains are back, there’s a new leading cause of liver cancer, and how storage of firearms at home affects suicidal teens.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Medical students remain protected by DACA – for now. The AIDS epidemic’s skin villains are back, there’s a new leading cause of liver cancer, and how storage of firearms at home affects suicidal teens.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Medical students remain protected by DACA – for now. The AIDS epidemic’s skin villains are back, there’s a new leading cause of liver cancer, and how storage of firearms at home affects suicidal teens.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
March 2018 Digital Edition
Click here to access the March 2018 Digital Edition.
Table of Contents
- A Nationwide Survey and Needs Assessment of Colonoscopy Quality Assurance Programs
- Incidence and Management of Asymptomatic Hypertensive Urgency at a VA Emergency Department
- Gabapentin Use in Acute Alcohol Withdrawal Management
- Medical Marijuana Redux
- Choice Program Expansion Jeopardizes High-Quality VHA Mental Health Services
- Brigadier General Carl Rogers Darnall: Saving Lives on a Massive Scale

Click here to access the March 2018 Digital Edition.
Table of Contents
- A Nationwide Survey and Needs Assessment of Colonoscopy Quality Assurance Programs
- Incidence and Management of Asymptomatic Hypertensive Urgency at a VA Emergency Department
- Gabapentin Use in Acute Alcohol Withdrawal Management
- Medical Marijuana Redux
- Choice Program Expansion Jeopardizes High-Quality VHA Mental Health Services
- Brigadier General Carl Rogers Darnall: Saving Lives on a Massive Scale

Click here to access the March 2018 Digital Edition.
Table of Contents
- A Nationwide Survey and Needs Assessment of Colonoscopy Quality Assurance Programs
- Incidence and Management of Asymptomatic Hypertensive Urgency at a VA Emergency Department
- Gabapentin Use in Acute Alcohol Withdrawal Management
- Medical Marijuana Redux
- Choice Program Expansion Jeopardizes High-Quality VHA Mental Health Services
- Brigadier General Carl Rogers Darnall: Saving Lives on a Massive Scale

Combating Public Pathogens in Federal Health Care Systems (March 2018)
Click here to access Combating Public Pathogens in Federal Health Care Systems
Table of Contents
- Current State of Hepatitis C Care in the VA
- Integrating Care for Patients With Chronic Liver Disease and Mental Health and Substance Use Disorders
- Hepatitis A Virus Prevention and Vaccination Within and Outside the VHA in Light of Recent Outbreaks
- Accessibility and Uptake of Pre-Exposure Prophylaxis for HIV Prevention in the VHA
- Achieving Excellence in Hepatitis B Virus Care for Veterans in the VHA

Click here to access Combating Public Pathogens in Federal Health Care Systems
Table of Contents
- Current State of Hepatitis C Care in the VA
- Integrating Care for Patients With Chronic Liver Disease and Mental Health and Substance Use Disorders
- Hepatitis A Virus Prevention and Vaccination Within and Outside the VHA in Light of Recent Outbreaks
- Accessibility and Uptake of Pre-Exposure Prophylaxis for HIV Prevention in the VHA
- Achieving Excellence in Hepatitis B Virus Care for Veterans in the VHA

Click here to access Combating Public Pathogens in Federal Health Care Systems
Table of Contents
- Current State of Hepatitis C Care in the VA
- Integrating Care for Patients With Chronic Liver Disease and Mental Health and Substance Use Disorders
- Hepatitis A Virus Prevention and Vaccination Within and Outside the VHA in Light of Recent Outbreaks
- Accessibility and Uptake of Pre-Exposure Prophylaxis for HIV Prevention in the VHA
- Achieving Excellence in Hepatitis B Virus Care for Veterans in the VHA

Digging for the Diagnosis
The lesions on this 41-year-old African-American woman’s skin have waxed and waned over the years—but they’re always on her mind. They are most prominent on her arms and trunk but crop up almost anywhere on her body.
When they manifest—for no apparent reason—they itch, creating an irresistible urge for the patient to pick at them. This provides some relief, both from the itching and from her feeling that there is “something in there” that she needs to remove. Fairly often, her digging (with fingernails) results in “finding” white bumps at the ends of tiny hairs.
As these small excoriations heal, the wounds itch—compelling her to pick the site open again. She knows she is caught in a vicious cycle but doesn’t know how to stop. Selective serotonin reuptake inhibitors have been tried, with little to no effect.
EXAMINATION
The patient’s type IV skin is covered with dark brown, maculopapular lesions that are so numerous and large (average diameter, 2 to 3 cm) that they are impossible to ignore. Her palms, soles, face, and midback are spared.

Only a few of the newer lesions are palpable, showing faint signs of central excoriation. Previous biopsies failed to show significant pathology.
The patient appears ill at ease during history-taking. She admits to picking her skin for many years but doesn’t believe she is inhabited by any kind of bug. The skin on her wrists and between her fingers is clear. Her 3-year-old daughter’s skin is free of notable changes.
What is the diagnosis?
DISCUSSION
Known in the DSM-5 and ICD-10 as skin-picking disorder, this condition has also been called dermatillomania. For unknown reasons, its incidence is far greater among women than men.
While it has been posited as a form of obsessive compulsive disorder (OCD), dermatillomania responds poorly, if at all, to standard OCD treatments. It is considered by others to more closely resemble addiction because, despite knowing its harm, patients persistently pick at the skin and often report a subsequent sense of relief.
This patient’s type IV skin lent itself to postinflammatory hyperpigmentation upon injury. Although she knew this, she still felt that she could somehow pick the darkness away.
Bloodwork was done to rule out other conditions, such as porphyria, hematologic disease, and renal or liver disease. Had a recent biopsy not been performed, this would have been included to rule out systemic disease.
The patient was given a topical steroid cream to put on any itchy lesions and counseled to avoid picking or scratching them, since this was the only way her skin could ever clear.
TAKE-HOME LEARNING POINTS
- One common term for this patient’s disorder is dermatillomania, though the DSM-5 and ICD-10 refer to it as skin-picking disorder.
- This patient experienced postinflammatory hyperpigmentation, which caused her considerable embarrassment.
- Many affected patients have unresolved underlying psychologic issues that contribute to their problem.
- The solution (which may require extensive counseling): Stop picking, and the dark lesions will eventually resolve.
The lesions on this 41-year-old African-American woman’s skin have waxed and waned over the years—but they’re always on her mind. They are most prominent on her arms and trunk but crop up almost anywhere on her body.
When they manifest—for no apparent reason—they itch, creating an irresistible urge for the patient to pick at them. This provides some relief, both from the itching and from her feeling that there is “something in there” that she needs to remove. Fairly often, her digging (with fingernails) results in “finding” white bumps at the ends of tiny hairs.
As these small excoriations heal, the wounds itch—compelling her to pick the site open again. She knows she is caught in a vicious cycle but doesn’t know how to stop. Selective serotonin reuptake inhibitors have been tried, with little to no effect.
EXAMINATION
The patient’s type IV skin is covered with dark brown, maculopapular lesions that are so numerous and large (average diameter, 2 to 3 cm) that they are impossible to ignore. Her palms, soles, face, and midback are spared.

Only a few of the newer lesions are palpable, showing faint signs of central excoriation. Previous biopsies failed to show significant pathology.
The patient appears ill at ease during history-taking. She admits to picking her skin for many years but doesn’t believe she is inhabited by any kind of bug. The skin on her wrists and between her fingers is clear. Her 3-year-old daughter’s skin is free of notable changes.
What is the diagnosis?
DISCUSSION
Known in the DSM-5 and ICD-10 as skin-picking disorder, this condition has also been called dermatillomania. For unknown reasons, its incidence is far greater among women than men.
While it has been posited as a form of obsessive compulsive disorder (OCD), dermatillomania responds poorly, if at all, to standard OCD treatments. It is considered by others to more closely resemble addiction because, despite knowing its harm, patients persistently pick at the skin and often report a subsequent sense of relief.
This patient’s type IV skin lent itself to postinflammatory hyperpigmentation upon injury. Although she knew this, she still felt that she could somehow pick the darkness away.
Bloodwork was done to rule out other conditions, such as porphyria, hematologic disease, and renal or liver disease. Had a recent biopsy not been performed, this would have been included to rule out systemic disease.
The patient was given a topical steroid cream to put on any itchy lesions and counseled to avoid picking or scratching them, since this was the only way her skin could ever clear.
TAKE-HOME LEARNING POINTS
- One common term for this patient’s disorder is dermatillomania, though the DSM-5 and ICD-10 refer to it as skin-picking disorder.
- This patient experienced postinflammatory hyperpigmentation, which caused her considerable embarrassment.
- Many affected patients have unresolved underlying psychologic issues that contribute to their problem.
- The solution (which may require extensive counseling): Stop picking, and the dark lesions will eventually resolve.
The lesions on this 41-year-old African-American woman’s skin have waxed and waned over the years—but they’re always on her mind. They are most prominent on her arms and trunk but crop up almost anywhere on her body.
When they manifest—for no apparent reason—they itch, creating an irresistible urge for the patient to pick at them. This provides some relief, both from the itching and from her feeling that there is “something in there” that she needs to remove. Fairly often, her digging (with fingernails) results in “finding” white bumps at the ends of tiny hairs.
As these small excoriations heal, the wounds itch—compelling her to pick the site open again. She knows she is caught in a vicious cycle but doesn’t know how to stop. Selective serotonin reuptake inhibitors have been tried, with little to no effect.
EXAMINATION
The patient’s type IV skin is covered with dark brown, maculopapular lesions that are so numerous and large (average diameter, 2 to 3 cm) that they are impossible to ignore. Her palms, soles, face, and midback are spared.

Only a few of the newer lesions are palpable, showing faint signs of central excoriation. Previous biopsies failed to show significant pathology.
The patient appears ill at ease during history-taking. She admits to picking her skin for many years but doesn’t believe she is inhabited by any kind of bug. The skin on her wrists and between her fingers is clear. Her 3-year-old daughter’s skin is free of notable changes.
What is the diagnosis?
DISCUSSION
Known in the DSM-5 and ICD-10 as skin-picking disorder, this condition has also been called dermatillomania. For unknown reasons, its incidence is far greater among women than men.
While it has been posited as a form of obsessive compulsive disorder (OCD), dermatillomania responds poorly, if at all, to standard OCD treatments. It is considered by others to more closely resemble addiction because, despite knowing its harm, patients persistently pick at the skin and often report a subsequent sense of relief.
This patient’s type IV skin lent itself to postinflammatory hyperpigmentation upon injury. Although she knew this, she still felt that she could somehow pick the darkness away.
Bloodwork was done to rule out other conditions, such as porphyria, hematologic disease, and renal or liver disease. Had a recent biopsy not been performed, this would have been included to rule out systemic disease.
The patient was given a topical steroid cream to put on any itchy lesions and counseled to avoid picking or scratching them, since this was the only way her skin could ever clear.
TAKE-HOME LEARNING POINTS
- One common term for this patient’s disorder is dermatillomania, though the DSM-5 and ICD-10 refer to it as skin-picking disorder.
- This patient experienced postinflammatory hyperpigmentation, which caused her considerable embarrassment.
- Many affected patients have unresolved underlying psychologic issues that contribute to their problem.
- The solution (which may require extensive counseling): Stop picking, and the dark lesions will eventually resolve.
Engineered liver models to study human hepatotropic pathogens
Recently, exciting clinical progress has been made in the study of hepatotropic pathogens in the context of liver-dependent infectious diseases. This is crucial for the development and validation of therapeutic interventions, such as drug and vaccine candidates that may act on the liver cells. The engineered models range from two-dimensional (2-D) cultures of primary human hepatocytes (HH) and stem cell–derived progeny to three-dimensional (3-D) organoid cultures and humanized rodent models. A review by Nil Gural and colleagues, published in Cellular and Molecular Gastroenterology and Hepatology, described these unique models. Furthermore, the progress made in combining individual approaches and pairing the most appropriate model system and readout modality was discussed.
The major human hepatotropic pathogens include hepatitis C virus (HCV), hepatitis B virus (HBV), and the protozoan parasites Plasmodium falciparum and P. vivax. While HBV and HCV can cause chronic liver diseases such as cirrhosis and hepatocellular carcinoma, Plasmodium parasites cause malaria. The use of cancer cell lines and animal models to study host-pathogen interactions is limited by uncontrolled proliferation, abnormal liver-specific functions, and stringent host dependency of the hepatotropic pathogens. HHs are thus the only ideal system to study these pathogens, however, maintaining these cells ex vivo is challenging.
For instance, 2D monolayers of human hepatoma-derived cell lines (such as HepG2-A16 and HepaRG) are easier to maintain, to amplify for scaling up, and to use for drug screening, thus representing a renewable alternative to primary hepatocytes. These model systems have been useful to study short-term infections of human Plasmodium parasites (P. vivax and P. falciparum); other hepatotropic pathogens such as Ebola, Lassa, human cytomegalovirus, and dengue viruses; and to generate virion stocks (HCV, HBV). For long-term scientific analyses and cultures, as well as clinical isolates of pathogens that do not infect hepatoma cells, immortalized cell lines have been engineered to differentiate and maintain HH functions for a longer duration. Additionally, cocultivation of primary hepatocytes with nonparenchymal cells or hepatocytes with mouse fibroblasts preserves hepatocyte phenotype. The latter is a self-assembling coculture system that could potentially maintain an infection for over 30 days and be used for testing anti-HBV drugs. A micropatterned coculture system, in which hepatocytes are positioned in “islands” via photolithographic patterning of collagen, surrounded by mouse embryonic fibroblasts, can maintain hepatocyte phenotypes for 4-6 weeks, and remain permissive to P. falciparum, P. vivax, HBV, and HCV infections. Furthermore, micropatterned coculture systems support full developmental liver stages of both P. falciparum and P. vivax, with the release of merozoites from hepatocytes and their subsequent infection of overlaid human red blood cells.
Alternatively, embryonic stem cells and induced pluripotent stem cells of human origin can be differentiated into hepatocytelike cells that enable investigation of host genetics within the context of host-pathogen interactions, and can also be used for target identification for drug development. However, stem cell cultures require significant culture expertise and may not represent a fully differentiated adult hepatocyte phenotype.
Although 2D cultures offer ease of use and monitoring of infection, they often lack the complexity of the liver microenvironment and impact of different cell types on liver infections. A 3D radial-flow bioreactor (cylindrical matrix) was able to maintain and amplify human hepatoma cells (for example, Huh7 cells), by providing sufficient oxygen and nutrient supply, supporting productive HCV infection for months. Other 3D cultures of hepatoma cells using polyethylene glycol–based hydrogels, thermoreversible gelatin polymers, alginate, galactosylated cellulosic sponges, matrigel, and collagen have been developed and shown to be permissive to HCV or HBV infections. Although 3D coculture systems exhibit better hepatic function and differential gene expression profiles in comparison to 2D counterparts, they require a large quantity of cells and are a challenge to scale up. Recently, several liver-on-a-chip models have been created that mimic shear stress, blood flow, and the extracellular environment within a tissue, holding great potential for modeling liver-specific pathogens.
Humanized mouse models with ectopic human liver structures have been developed in which primary HHs are transplanted following liver injury. Chimeric mouse models including Alb-uPA/SCID (HHs transplanted into urokinase-type plasminogen activator-transgenic severe combined immunodeficient mice), FNRG/FRG (HHs transplanted into Fah[-/-], Rag2[-/-], and Il2rg[-/-] mice with or without a nonobese diabetic background), and TK-NOG (HHs transplanted into herpes simplex virus type-1 thymidine kinase mice) were validated for HCV, HBV, P. falciparum, and P. vivax infections. It is, however, laborious to create and maintain chimeric mouse models and monitor infection processes in them.
It is important to note that the selection of model system and the readout modality to monitor infection will vary based on the experimental question at hand. Tissue engineering has thus far made significant contributions to the knowledge of hepatotropic pathogens; a continued effort to develop better liver models is envisioned.
Gural et al. present a timely and outstanding review of the advances made in the engineering of human-relevant liver culture platforms for investigating the molecular mechanisms of infectious diseases (e.g., hepatitis B/C viruses and Plasmodium parasites that cause malaria) and developing better drugs or vaccines against such diseases. The authors cover a continuum of platforms with increasing physiological complexity, such as 2-D hepatocyte monocultures on collagen-coated plastic, 2-D cocultures of hepatocytes and nonparenchymal cells, (both randomly distributed and patterned into microdomains to optimize cell-cell contact), 3-D cultures/cocultures housed in biomaterial-based scaffolds, perfusion-based bioreactors to induce cell growth and phenotypic stability, and finally rodents with humanized livers. Cell sourcing considerations for building human-relevant platforms are discussed, including cancerous cell lines, primary human hepatocytes, and stem cell–derived hepatocytes (e.g., induced pluripotent stem cells).
From the discussions of various studies, it is clear that this field has benefitted tremendously from advances in tissue engineering, including microfabrication tools adapted from the semiconductor industry, to construct human liver platforms that last for several weeks in vitro, can be infected with hepatitis B/C virus and Plasmodium parasites with high efficiencies, and are very useful for high-throughput and high-content drug screening applications. The latest protocols in isolating and cryopreserving primary human hepatocytes and differentiating stem cells into hepatocyte-like cells with adult functions help reduce the reliance on abnormal or cancerous cell lines for building platforms with higher relevance to the clinic. Ultimately, continued advances in microfabricated human liver platforms can aid our understanding of liver infections and spur further drug/vaccine development.
Salman R. Khetani, PhD, is associate professor, department of bioengineering, University of Illinois at Chicago. He has no conflicts of interest.
Gural et al. present a timely and outstanding review of the advances made in the engineering of human-relevant liver culture platforms for investigating the molecular mechanisms of infectious diseases (e.g., hepatitis B/C viruses and Plasmodium parasites that cause malaria) and developing better drugs or vaccines against such diseases. The authors cover a continuum of platforms with increasing physiological complexity, such as 2-D hepatocyte monocultures on collagen-coated plastic, 2-D cocultures of hepatocytes and nonparenchymal cells, (both randomly distributed and patterned into microdomains to optimize cell-cell contact), 3-D cultures/cocultures housed in biomaterial-based scaffolds, perfusion-based bioreactors to induce cell growth and phenotypic stability, and finally rodents with humanized livers. Cell sourcing considerations for building human-relevant platforms are discussed, including cancerous cell lines, primary human hepatocytes, and stem cell–derived hepatocytes (e.g., induced pluripotent stem cells).
From the discussions of various studies, it is clear that this field has benefitted tremendously from advances in tissue engineering, including microfabrication tools adapted from the semiconductor industry, to construct human liver platforms that last for several weeks in vitro, can be infected with hepatitis B/C virus and Plasmodium parasites with high efficiencies, and are very useful for high-throughput and high-content drug screening applications. The latest protocols in isolating and cryopreserving primary human hepatocytes and differentiating stem cells into hepatocyte-like cells with adult functions help reduce the reliance on abnormal or cancerous cell lines for building platforms with higher relevance to the clinic. Ultimately, continued advances in microfabricated human liver platforms can aid our understanding of liver infections and spur further drug/vaccine development.
Salman R. Khetani, PhD, is associate professor, department of bioengineering, University of Illinois at Chicago. He has no conflicts of interest.
Gural et al. present a timely and outstanding review of the advances made in the engineering of human-relevant liver culture platforms for investigating the molecular mechanisms of infectious diseases (e.g., hepatitis B/C viruses and Plasmodium parasites that cause malaria) and developing better drugs or vaccines against such diseases. The authors cover a continuum of platforms with increasing physiological complexity, such as 2-D hepatocyte monocultures on collagen-coated plastic, 2-D cocultures of hepatocytes and nonparenchymal cells, (both randomly distributed and patterned into microdomains to optimize cell-cell contact), 3-D cultures/cocultures housed in biomaterial-based scaffolds, perfusion-based bioreactors to induce cell growth and phenotypic stability, and finally rodents with humanized livers. Cell sourcing considerations for building human-relevant platforms are discussed, including cancerous cell lines, primary human hepatocytes, and stem cell–derived hepatocytes (e.g., induced pluripotent stem cells).
From the discussions of various studies, it is clear that this field has benefitted tremendously from advances in tissue engineering, including microfabrication tools adapted from the semiconductor industry, to construct human liver platforms that last for several weeks in vitro, can be infected with hepatitis B/C virus and Plasmodium parasites with high efficiencies, and are very useful for high-throughput and high-content drug screening applications. The latest protocols in isolating and cryopreserving primary human hepatocytes and differentiating stem cells into hepatocyte-like cells with adult functions help reduce the reliance on abnormal or cancerous cell lines for building platforms with higher relevance to the clinic. Ultimately, continued advances in microfabricated human liver platforms can aid our understanding of liver infections and spur further drug/vaccine development.
Salman R. Khetani, PhD, is associate professor, department of bioengineering, University of Illinois at Chicago. He has no conflicts of interest.
Recently, exciting clinical progress has been made in the study of hepatotropic pathogens in the context of liver-dependent infectious diseases. This is crucial for the development and validation of therapeutic interventions, such as drug and vaccine candidates that may act on the liver cells. The engineered models range from two-dimensional (2-D) cultures of primary human hepatocytes (HH) and stem cell–derived progeny to three-dimensional (3-D) organoid cultures and humanized rodent models. A review by Nil Gural and colleagues, published in Cellular and Molecular Gastroenterology and Hepatology, described these unique models. Furthermore, the progress made in combining individual approaches and pairing the most appropriate model system and readout modality was discussed.
The major human hepatotropic pathogens include hepatitis C virus (HCV), hepatitis B virus (HBV), and the protozoan parasites Plasmodium falciparum and P. vivax. While HBV and HCV can cause chronic liver diseases such as cirrhosis and hepatocellular carcinoma, Plasmodium parasites cause malaria. The use of cancer cell lines and animal models to study host-pathogen interactions is limited by uncontrolled proliferation, abnormal liver-specific functions, and stringent host dependency of the hepatotropic pathogens. HHs are thus the only ideal system to study these pathogens, however, maintaining these cells ex vivo is challenging.
For instance, 2D monolayers of human hepatoma-derived cell lines (such as HepG2-A16 and HepaRG) are easier to maintain, to amplify for scaling up, and to use for drug screening, thus representing a renewable alternative to primary hepatocytes. These model systems have been useful to study short-term infections of human Plasmodium parasites (P. vivax and P. falciparum); other hepatotropic pathogens such as Ebola, Lassa, human cytomegalovirus, and dengue viruses; and to generate virion stocks (HCV, HBV). For long-term scientific analyses and cultures, as well as clinical isolates of pathogens that do not infect hepatoma cells, immortalized cell lines have been engineered to differentiate and maintain HH functions for a longer duration. Additionally, cocultivation of primary hepatocytes with nonparenchymal cells or hepatocytes with mouse fibroblasts preserves hepatocyte phenotype. The latter is a self-assembling coculture system that could potentially maintain an infection for over 30 days and be used for testing anti-HBV drugs. A micropatterned coculture system, in which hepatocytes are positioned in “islands” via photolithographic patterning of collagen, surrounded by mouse embryonic fibroblasts, can maintain hepatocyte phenotypes for 4-6 weeks, and remain permissive to P. falciparum, P. vivax, HBV, and HCV infections. Furthermore, micropatterned coculture systems support full developmental liver stages of both P. falciparum and P. vivax, with the release of merozoites from hepatocytes and their subsequent infection of overlaid human red blood cells.
Alternatively, embryonic stem cells and induced pluripotent stem cells of human origin can be differentiated into hepatocytelike cells that enable investigation of host genetics within the context of host-pathogen interactions, and can also be used for target identification for drug development. However, stem cell cultures require significant culture expertise and may not represent a fully differentiated adult hepatocyte phenotype.
Although 2D cultures offer ease of use and monitoring of infection, they often lack the complexity of the liver microenvironment and impact of different cell types on liver infections. A 3D radial-flow bioreactor (cylindrical matrix) was able to maintain and amplify human hepatoma cells (for example, Huh7 cells), by providing sufficient oxygen and nutrient supply, supporting productive HCV infection for months. Other 3D cultures of hepatoma cells using polyethylene glycol–based hydrogels, thermoreversible gelatin polymers, alginate, galactosylated cellulosic sponges, matrigel, and collagen have been developed and shown to be permissive to HCV or HBV infections. Although 3D coculture systems exhibit better hepatic function and differential gene expression profiles in comparison to 2D counterparts, they require a large quantity of cells and are a challenge to scale up. Recently, several liver-on-a-chip models have been created that mimic shear stress, blood flow, and the extracellular environment within a tissue, holding great potential for modeling liver-specific pathogens.
Humanized mouse models with ectopic human liver structures have been developed in which primary HHs are transplanted following liver injury. Chimeric mouse models including Alb-uPA/SCID (HHs transplanted into urokinase-type plasminogen activator-transgenic severe combined immunodeficient mice), FNRG/FRG (HHs transplanted into Fah[-/-], Rag2[-/-], and Il2rg[-/-] mice with or without a nonobese diabetic background), and TK-NOG (HHs transplanted into herpes simplex virus type-1 thymidine kinase mice) were validated for HCV, HBV, P. falciparum, and P. vivax infections. It is, however, laborious to create and maintain chimeric mouse models and monitor infection processes in them.
It is important to note that the selection of model system and the readout modality to monitor infection will vary based on the experimental question at hand. Tissue engineering has thus far made significant contributions to the knowledge of hepatotropic pathogens; a continued effort to develop better liver models is envisioned.
Recently, exciting clinical progress has been made in the study of hepatotropic pathogens in the context of liver-dependent infectious diseases. This is crucial for the development and validation of therapeutic interventions, such as drug and vaccine candidates that may act on the liver cells. The engineered models range from two-dimensional (2-D) cultures of primary human hepatocytes (HH) and stem cell–derived progeny to three-dimensional (3-D) organoid cultures and humanized rodent models. A review by Nil Gural and colleagues, published in Cellular and Molecular Gastroenterology and Hepatology, described these unique models. Furthermore, the progress made in combining individual approaches and pairing the most appropriate model system and readout modality was discussed.
The major human hepatotropic pathogens include hepatitis C virus (HCV), hepatitis B virus (HBV), and the protozoan parasites Plasmodium falciparum and P. vivax. While HBV and HCV can cause chronic liver diseases such as cirrhosis and hepatocellular carcinoma, Plasmodium parasites cause malaria. The use of cancer cell lines and animal models to study host-pathogen interactions is limited by uncontrolled proliferation, abnormal liver-specific functions, and stringent host dependency of the hepatotropic pathogens. HHs are thus the only ideal system to study these pathogens, however, maintaining these cells ex vivo is challenging.
For instance, 2D monolayers of human hepatoma-derived cell lines (such as HepG2-A16 and HepaRG) are easier to maintain, to amplify for scaling up, and to use for drug screening, thus representing a renewable alternative to primary hepatocytes. These model systems have been useful to study short-term infections of human Plasmodium parasites (P. vivax and P. falciparum); other hepatotropic pathogens such as Ebola, Lassa, human cytomegalovirus, and dengue viruses; and to generate virion stocks (HCV, HBV). For long-term scientific analyses and cultures, as well as clinical isolates of pathogens that do not infect hepatoma cells, immortalized cell lines have been engineered to differentiate and maintain HH functions for a longer duration. Additionally, cocultivation of primary hepatocytes with nonparenchymal cells or hepatocytes with mouse fibroblasts preserves hepatocyte phenotype. The latter is a self-assembling coculture system that could potentially maintain an infection for over 30 days and be used for testing anti-HBV drugs. A micropatterned coculture system, in which hepatocytes are positioned in “islands” via photolithographic patterning of collagen, surrounded by mouse embryonic fibroblasts, can maintain hepatocyte phenotypes for 4-6 weeks, and remain permissive to P. falciparum, P. vivax, HBV, and HCV infections. Furthermore, micropatterned coculture systems support full developmental liver stages of both P. falciparum and P. vivax, with the release of merozoites from hepatocytes and their subsequent infection of overlaid human red blood cells.
Alternatively, embryonic stem cells and induced pluripotent stem cells of human origin can be differentiated into hepatocytelike cells that enable investigation of host genetics within the context of host-pathogen interactions, and can also be used for target identification for drug development. However, stem cell cultures require significant culture expertise and may not represent a fully differentiated adult hepatocyte phenotype.
Although 2D cultures offer ease of use and monitoring of infection, they often lack the complexity of the liver microenvironment and impact of different cell types on liver infections. A 3D radial-flow bioreactor (cylindrical matrix) was able to maintain and amplify human hepatoma cells (for example, Huh7 cells), by providing sufficient oxygen and nutrient supply, supporting productive HCV infection for months. Other 3D cultures of hepatoma cells using polyethylene glycol–based hydrogels, thermoreversible gelatin polymers, alginate, galactosylated cellulosic sponges, matrigel, and collagen have been developed and shown to be permissive to HCV or HBV infections. Although 3D coculture systems exhibit better hepatic function and differential gene expression profiles in comparison to 2D counterparts, they require a large quantity of cells and are a challenge to scale up. Recently, several liver-on-a-chip models have been created that mimic shear stress, blood flow, and the extracellular environment within a tissue, holding great potential for modeling liver-specific pathogens.
Humanized mouse models with ectopic human liver structures have been developed in which primary HHs are transplanted following liver injury. Chimeric mouse models including Alb-uPA/SCID (HHs transplanted into urokinase-type plasminogen activator-transgenic severe combined immunodeficient mice), FNRG/FRG (HHs transplanted into Fah[-/-], Rag2[-/-], and Il2rg[-/-] mice with or without a nonobese diabetic background), and TK-NOG (HHs transplanted into herpes simplex virus type-1 thymidine kinase mice) were validated for HCV, HBV, P. falciparum, and P. vivax infections. It is, however, laborious to create and maintain chimeric mouse models and monitor infection processes in them.
It is important to note that the selection of model system and the readout modality to monitor infection will vary based on the experimental question at hand. Tissue engineering has thus far made significant contributions to the knowledge of hepatotropic pathogens; a continued effort to develop better liver models is envisioned.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Substance abuse among older adults: A growing problem
Baby Boomers—a term used to refer to individuals born in the United States between 1946 and 1964—are now approaching old age. Surprisingly, these older adults are using illicit substances in a pattern not seen in prior generations of older adults, including developing substance use disorders (SUDs) at increasingly higher rates; in previous generations, the prevalence of such disorders typically lowered with advancing age.
This article discusses how to recognize and treat SUDs in older adults. Alcohol is the most commonly used substance among older adults,1 and there is a largebody of literature describing the identification and treatment of alcohol-related disorders in these patients. Therefore, this article will instead focus on older adults’ use of illicit substances, including marijuana, cocaine, and heroin.
Epidemiology
Prior clinical data regarding substance abuse in older adults focused on alcohol, prescription drugs, nicotine, and caffeine.2 In the past, compared with younger adults, older adults had lower rates of alcohol and other illicit drug use.3,4 Baby Boomers appear to be defying this trend.
A 2013 Substance Abuse and Mental Health Services Administration survey found that the percentage of adults ages 50 to 64 who used illicit substances increased from 2.7% in 2002 to 6.0% in 2013.5 Specifically, during that time, past-month illicit substance use increased from 3.4% to 7.9% among those ages 50 to 54, from 1.9% to 5.7% among those ages 55 to 59, and from 2.5% to 3.9% among those ages 60 to 64.5
More recently, a 2014 study of geriatric patients found that of the 1,302 patients age ≥65 admitted to a Level 1 trauma center, 48.3% had a positive urine drug screen.6 Someresearchers have estimated that 5.7 million older adults will require treatment for a substance use disorder in 2020, which is roughly double the 2.8 million who had an SUD in 2002 to 2006.7
Risk factors and patterns of substance abuse
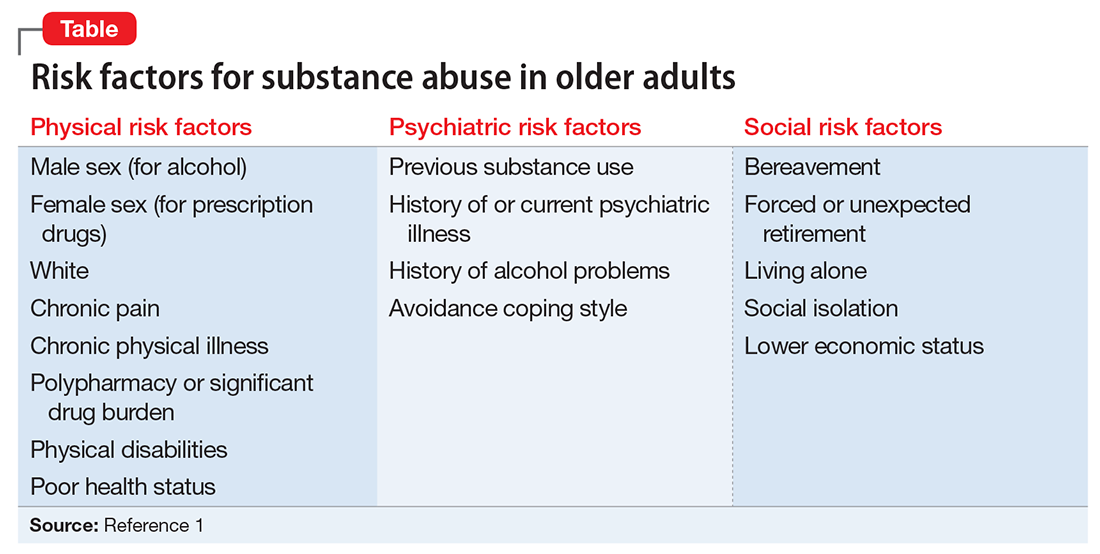
Individual, social, and familial factors can contribute to substance use and abuse in late life. The Table1 outlines some of the potential risk factors for older adults associated with the use of illicit substances. Substance abuse among older adults can be divided into 2 broad categories: early onset (starting before age 50) and late onset (starting after age 50).8 While data are limited, in general, early-onset use is a more common pattern; late-onset use represents an estimated <10% of substance use among older adults. The factors that lead some adults to continue substance use in late life, or to begin substance use later in life, have not been thoroughly evaluated.
Although older adults may abuse a wide variety of illicit substances, here we describe their use of marijuana, cocaine, and heroin.
Marijuana use has changed substantially in the last decade. While marijuana is illegal under federal law, as of November 2017, 29 states had legalized marijuana for medicinal purposes and 7 states and the District of Columbia had legalized it for recreational use. The increased legal and social acceptance of marijuana has led to new businesses and methods of use beyond smoking. New types of marijuana products include edible substances, tinctures, and oils that can be vaporized and inhaled.
In addition to euphoria and relaxation, the effects of marijuana use include increased latency time and decreased ability to respond to stimuli.2 Nonpsychiatric effects of marijuana include shallow breathing, weakened immune system, and increasing cardiac workload.2 The latter effect is especially important for older adults, many of whom may have preexisting cardiac illness and may be more likely to experience an adverse cardiac event as a result of marijuana use.2 Older adults who begin to use marijuana in late life may do so not primarily as a social activity, but more likely to experience the drug’s potentially beneficial effects on pain or appetite.2 For more on theuse of marijuana for these reasons, see “Medical marijuana: Do the benefits outweigh the risks?” in
Cocaine. Although cocaine is a CNS stimulant that causes a short-lived euphoria, its adverse effects impact many body systems.9 Myocardial infarction (MI) secondary to coronary artery vasospasms, stroke (hemorrhagic and ischemic), seizures, psychosis, aortic dissection, and acute renal injury are some of the most severe complications. Acute MI is the most frequent and severe cardiovascular complication seen among abusers.10 Cocaine use can cause dizziness, restlessness, headache, mydriasis, and anxiety.
In a pilot study, Kalapatapu et al11 compared the effects of cocaine abuse in younger vs older users. They found that older users had similar patterns of cocaine abuse in terms of the amount of cocaine used and frequency of use.11 They also found that specific cognitive functions, including psychomotor speed, attention, and short-term memory, are particularly sensitive to the combined effects of aging and cocaine abuse.11
Heroin is an opioid and a CNS depressant. Common effects include slowed heart rate, decreased blood pressure, and decreased respiration rate. Chronic heroin users show an overall decrease in immune system functioning12; this deficit might be particularly pronounced in an older person whose immune system functioning has already begun to decline as a result of aging. In recent years, as is the case with younger substance users, prescription opioids have replaced heroin as the opioid of choice among older users. However, for some early-onset heroin users, the use of this particular drug becomes well entrenched and unlikely to change, even in late life. Each year of heroin use increases the likelihood of continued use the next year by approximately 3%.2 Some research suggests that older heroin users do not decrease their use over time, and face many of the same risks as younger users, including poorer physical and mental health, severe physical disability, and mortality.13
Challenges to recognizing the problem
There are no screening protocols in the clinical setting that are designed specifically for detecting illicit substance abuse among older adults. Furthermore, diagnosis can be easily overlooked because the signs and symptoms of illicit substance use can be mistaken for other illnesses. To complicate matters further, older adults often do not disclose their substance use, understate it, or even try to explain away their symptoms.1 Many older adults live alone, which may increase their risk of receiving no treatment.14
Older adults generally experience reduced tolerance to the effects of illicit substances because of age-related physiologic changes, such as decreases in renal functioning, motor functioning, and cardiac output; altered liver metabolism of certain drugs; and elevated blood glucose levels.15 As a result, symptoms of illicit substance use could be mistaken for dementia or other forms of cognitive impairment.1,16
Although not designed specifically for older adults, an evidence-based screening instrument, such as the CAGE Questionnaire Adapted to Include Drugs, may be helpful in identifying substance abuse in these patients. Urine and/or serum drug screening, along with obtaining a comprehensive history from a trustworthy source, is useful for diagnosis.
Pharmacologic treatments
Research evaluating the use of medication for treating substance abuse specifically in older adults is extremely limited; studies have focused primarily on younger patients or mixed-age populations. Treatments that have been shown to be effective for younger patients may or may not be effective for older adults.
Marijuana. There are no FDA-approved treatments for marijuana abuse. An open-label study found that N-acetylcysteine, 1,200 mg twice a day, resulted in a significant reduction in marijuana craving as measured by the 12-item version of the Marijuana Craving Questionnaire.17 In a double-blinded placebo-controlled study, adolescents who were dependent on marijuana who received N-acetylcysteine, 1,200 mg twice a day, were more than twice likely to stop marijuana use compared with those who received placebo.18 Some researchers have proposed that N-acetylcysteine may prevent continued use of marijuana via glutamate modulation in the nucleus accumbens. Animal models have demonstrated that chronic drug self-administration downregulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine upregulates this exchanger, which reduces reinstatement of drug seeking.Further studies are needed to verify this speculation.
Cocaine. There are no FDA-approved treatments for cocaine abuse. No specific treatment approach has been found to be consistently effective.
A potential “cocaine vaccine” called TA-CD, which is made from succinyl norcocaine conjugated to cholera toxin, is being evaluated. An initial study had promising results, finding a significant reduction in cocaine use among those who received TA-CD.19 A later double-blinded placebo-controlled study only partially replicated the efficacy found in the initial study.20
Currently, other cocaine treatments are also being investigated. An enzyme to rapidly metabolize cocaine is being evaluated.21 So far, none of these treatments have targeted older adults, and there may be age-specific issues to consider if these approaches eventually receive FDA approval.
Heroin. Several FDA-approved medications are available for treating dependency to heroin and other opioids, including naltrexone, buprenorphine, and methadone, but none have been studied specifically in older adults. Some studies of transdermal buprenorphine for treating chronic pain in older adults have concluded that this formulation may offer advantages for older patients.22,23 Compared with oral or sublingual buprenorphine, the transdermal formulation avoids the first-pass effect in the liver, thus greatly increasing bioavailability of the drug; avoids renal metabolism; and offers greater tolerability in patients with mild to moderate hepatic impairment.22,23 However, transdermal buprenorphine has been approved only for the treatment of pain. These beneficial aspects of transdermal buprenorphine may be applicable to older opioid users, but no age-specific studies of buprenorphine for treating opioid abuse have been conducted.
Nonpharmacologic treatments
The same psychotherapeutic treatments used to treat younger patients with SUDs may be appropriate for older adults. Older patients may experience feelings of isolation and shame related to needing treatment for substance abuse. These factors in treatment of older patients often are overcome by group psychotherapy. Self-help programs, such as Narcotics Anonymous or Alcoholics Anonymous, and group therapy also may be options.
On the other hand, individual psychotherapy, such as cognitive-behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapy, can provide a private and confidential environment for older adults who are less social.24
The highly structured nature of CBT may be well suited to older adults who have memory difficulties.1 A study of 110 older veterans with substance abuse problems found evidence for the effectiveness of group CBT among these patients.25 All but 8 participants in this study were age ≥65. The intervention consisted of 16 weekly group sessions that began with analysis of substance use behavior to determine high-risk situations for use, followed by a series of modules to teach skills for coping with social pressure, being at home and alone, feelings of depression and loneliness, anxiety and tension, anger and frustration, cues for substance use, and other factors. Approximately 44% (49 of 110) completed treatment (≥13 sessions). Approximately 55% of those who completed the treatment were abstinent at 6-month follow-up.25
Don’t assume your older patient is not using illicit substances
It is a myth that older adults do not use and abuse illicit substances. Illicit drug use among older adults is increasing. Older adults with SUDs may not present with the same symptoms as their younger counterparts, and thus it may be difficult to identify the problem. Maintain a high index of suspicion regarding the use of illicit substances in these patients.
Treatment options are generally limited and health care settings offer few interventions designed specifically for older adults. In general, proper identification of SUDs and targeted treatment can highly improve outcomes.
1. Kuerbis A, Sacco P, Blazer DG, et al. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629-654.
2. Taylor MH, Grossberg GT. (2012). The growing problem of illicit substance abuse in the elderly: a review. Prim Care Companion CNS Disord. 2012;14(4):PCC.11r01320. doi: 10.4088/PCC.11r01320.
3. Cummings SM, Bride B, Rawlings-Shaw AM. Alcohol abuse treatment for older adults: a review of recent empirical research. J Evid Based Soc Work. 2006;3(1):79-99.
4. Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: summary of national findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
5. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
6. Ekeh AP, Parikh P, Walusimbi MS, et al. The prevalence of positive drug and alcohol screens in elderly trauma patients. Subst Abus. 2014;35(1):51-55.
7. Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23(3):481-504.
8. Roe B, Beynon C, Pickering L, et al. Experiences of drug use and ageing: health, quality of life, relationship and service implications. J Adv Nurs. 2010;66(9):1968-1979.
9. Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012;28(4):517-526.
10. Weber JE, Chudnofsky CR, Boczar M, et al. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med. 2000;7(8):873-877.
11. Kalapatapu RK, Vadhan NP, Rubin E, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228-239.
12. Edelman EJ, Cheng DM, Krupitsky EM, et al. Heroin use and HIV disease progression: results from a pilot study of a Russian cohort. AIDS Behav. 2015;19(6):1089-1097.
13. Darke S, Mills KL, Ross J, et al. The ageing heroin user: career length, clinical profile and outcomes across 36 months. Drug Alcohol Rev. 2009;28(3):243-249.
14. West LA, Cole S, Goodkind D, et al. U.S. Census Bureau, P23-212. 65+ in the United States: 2010. Washington, DC: United States Census Bureau; 2014.
15. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434-440.
16. Ruiz P, Strain EC, Langrod JG. The substance abuse handbook. Philadelphia, PA: Wolters Kluwer Health; 2007.
17. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
18. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
19. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123
20. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47.
21. Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310(3):1046-1052.
22. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 2008;3(3):421-430.
23. Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143-149.
24. Schultz SK, Arndt S, Liesveld J. Locations of facilities with special programs for older substance abuse clients in the US. Int J Geriatr Psychiatry. 2003;18(9):839-843.
25. Schonfeld L, Dupree LW, Dickson-Fuhrman E, et al. Cognitive-behavioral treatment of older veterans with substance abuse problems. J Geriatr Psychiatry Neurol. 2000;13(3):124-129.
Baby Boomers—a term used to refer to individuals born in the United States between 1946 and 1964—are now approaching old age. Surprisingly, these older adults are using illicit substances in a pattern not seen in prior generations of older adults, including developing substance use disorders (SUDs) at increasingly higher rates; in previous generations, the prevalence of such disorders typically lowered with advancing age.
This article discusses how to recognize and treat SUDs in older adults. Alcohol is the most commonly used substance among older adults,1 and there is a largebody of literature describing the identification and treatment of alcohol-related disorders in these patients. Therefore, this article will instead focus on older adults’ use of illicit substances, including marijuana, cocaine, and heroin.
Epidemiology
Prior clinical data regarding substance abuse in older adults focused on alcohol, prescription drugs, nicotine, and caffeine.2 In the past, compared with younger adults, older adults had lower rates of alcohol and other illicit drug use.3,4 Baby Boomers appear to be defying this trend.
A 2013 Substance Abuse and Mental Health Services Administration survey found that the percentage of adults ages 50 to 64 who used illicit substances increased from 2.7% in 2002 to 6.0% in 2013.5 Specifically, during that time, past-month illicit substance use increased from 3.4% to 7.9% among those ages 50 to 54, from 1.9% to 5.7% among those ages 55 to 59, and from 2.5% to 3.9% among those ages 60 to 64.5
More recently, a 2014 study of geriatric patients found that of the 1,302 patients age ≥65 admitted to a Level 1 trauma center, 48.3% had a positive urine drug screen.6 Someresearchers have estimated that 5.7 million older adults will require treatment for a substance use disorder in 2020, which is roughly double the 2.8 million who had an SUD in 2002 to 2006.7
Risk factors and patterns of substance abuse
Individual, social, and familial factors can contribute to substance use and abuse in late life. The Table1 outlines some of the potential risk factors for older adults associated with the use of illicit substances. Substance abuse among older adults can be divided into 2 broad categories: early onset (starting before age 50) and late onset (starting after age 50).8 While data are limited, in general, early-onset use is a more common pattern; late-onset use represents an estimated <10% of substance use among older adults. The factors that lead some adults to continue substance use in late life, or to begin substance use later in life, have not been thoroughly evaluated.

Although older adults may abuse a wide variety of illicit substances, here we describe their use of marijuana, cocaine, and heroin.
Marijuana use has changed substantially in the last decade. While marijuana is illegal under federal law, as of November 2017, 29 states had legalized marijuana for medicinal purposes and 7 states and the District of Columbia had legalized it for recreational use. The increased legal and social acceptance of marijuana has led to new businesses and methods of use beyond smoking. New types of marijuana products include edible substances, tinctures, and oils that can be vaporized and inhaled.
In addition to euphoria and relaxation, the effects of marijuana use include increased latency time and decreased ability to respond to stimuli.2 Nonpsychiatric effects of marijuana include shallow breathing, weakened immune system, and increasing cardiac workload.2 The latter effect is especially important for older adults, many of whom may have preexisting cardiac illness and may be more likely to experience an adverse cardiac event as a result of marijuana use.2 Older adults who begin to use marijuana in late life may do so not primarily as a social activity, but more likely to experience the drug’s potentially beneficial effects on pain or appetite.2 For more on theuse of marijuana for these reasons, see “Medical marijuana: Do the benefits outweigh the risks?” in
Cocaine. Although cocaine is a CNS stimulant that causes a short-lived euphoria, its adverse effects impact many body systems.9 Myocardial infarction (MI) secondary to coronary artery vasospasms, stroke (hemorrhagic and ischemic), seizures, psychosis, aortic dissection, and acute renal injury are some of the most severe complications. Acute MI is the most frequent and severe cardiovascular complication seen among abusers.10 Cocaine use can cause dizziness, restlessness, headache, mydriasis, and anxiety.
In a pilot study, Kalapatapu et al11 compared the effects of cocaine abuse in younger vs older users. They found that older users had similar patterns of cocaine abuse in terms of the amount of cocaine used and frequency of use.11 They also found that specific cognitive functions, including psychomotor speed, attention, and short-term memory, are particularly sensitive to the combined effects of aging and cocaine abuse.11
Heroin is an opioid and a CNS depressant. Common effects include slowed heart rate, decreased blood pressure, and decreased respiration rate. Chronic heroin users show an overall decrease in immune system functioning12; this deficit might be particularly pronounced in an older person whose immune system functioning has already begun to decline as a result of aging. In recent years, as is the case with younger substance users, prescription opioids have replaced heroin as the opioid of choice among older users. However, for some early-onset heroin users, the use of this particular drug becomes well entrenched and unlikely to change, even in late life. Each year of heroin use increases the likelihood of continued use the next year by approximately 3%.2 Some research suggests that older heroin users do not decrease their use over time, and face many of the same risks as younger users, including poorer physical and mental health, severe physical disability, and mortality.13
Challenges to recognizing the problem
There are no screening protocols in the clinical setting that are designed specifically for detecting illicit substance abuse among older adults. Furthermore, diagnosis can be easily overlooked because the signs and symptoms of illicit substance use can be mistaken for other illnesses. To complicate matters further, older adults often do not disclose their substance use, understate it, or even try to explain away their symptoms.1 Many older adults live alone, which may increase their risk of receiving no treatment.14
Older adults generally experience reduced tolerance to the effects of illicit substances because of age-related physiologic changes, such as decreases in renal functioning, motor functioning, and cardiac output; altered liver metabolism of certain drugs; and elevated blood glucose levels.15 As a result, symptoms of illicit substance use could be mistaken for dementia or other forms of cognitive impairment.1,16
Although not designed specifically for older adults, an evidence-based screening instrument, such as the CAGE Questionnaire Adapted to Include Drugs, may be helpful in identifying substance abuse in these patients. Urine and/or serum drug screening, along with obtaining a comprehensive history from a trustworthy source, is useful for diagnosis.
Pharmacologic treatments
Research evaluating the use of medication for treating substance abuse specifically in older adults is extremely limited; studies have focused primarily on younger patients or mixed-age populations. Treatments that have been shown to be effective for younger patients may or may not be effective for older adults.
Marijuana. There are no FDA-approved treatments for marijuana abuse. An open-label study found that N-acetylcysteine, 1,200 mg twice a day, resulted in a significant reduction in marijuana craving as measured by the 12-item version of the Marijuana Craving Questionnaire.17 In a double-blinded placebo-controlled study, adolescents who were dependent on marijuana who received N-acetylcysteine, 1,200 mg twice a day, were more than twice likely to stop marijuana use compared with those who received placebo.18 Some researchers have proposed that N-acetylcysteine may prevent continued use of marijuana via glutamate modulation in the nucleus accumbens. Animal models have demonstrated that chronic drug self-administration downregulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine upregulates this exchanger, which reduces reinstatement of drug seeking.Further studies are needed to verify this speculation.
Cocaine. There are no FDA-approved treatments for cocaine abuse. No specific treatment approach has been found to be consistently effective.
A potential “cocaine vaccine” called TA-CD, which is made from succinyl norcocaine conjugated to cholera toxin, is being evaluated. An initial study had promising results, finding a significant reduction in cocaine use among those who received TA-CD.19 A later double-blinded placebo-controlled study only partially replicated the efficacy found in the initial study.20
Currently, other cocaine treatments are also being investigated. An enzyme to rapidly metabolize cocaine is being evaluated.21 So far, none of these treatments have targeted older adults, and there may be age-specific issues to consider if these approaches eventually receive FDA approval.
Heroin. Several FDA-approved medications are available for treating dependency to heroin and other opioids, including naltrexone, buprenorphine, and methadone, but none have been studied specifically in older adults. Some studies of transdermal buprenorphine for treating chronic pain in older adults have concluded that this formulation may offer advantages for older patients.22,23 Compared with oral or sublingual buprenorphine, the transdermal formulation avoids the first-pass effect in the liver, thus greatly increasing bioavailability of the drug; avoids renal metabolism; and offers greater tolerability in patients with mild to moderate hepatic impairment.22,23 However, transdermal buprenorphine has been approved only for the treatment of pain. These beneficial aspects of transdermal buprenorphine may be applicable to older opioid users, but no age-specific studies of buprenorphine for treating opioid abuse have been conducted.
Nonpharmacologic treatments
The same psychotherapeutic treatments used to treat younger patients with SUDs may be appropriate for older adults. Older patients may experience feelings of isolation and shame related to needing treatment for substance abuse. These factors in treatment of older patients often are overcome by group psychotherapy. Self-help programs, such as Narcotics Anonymous or Alcoholics Anonymous, and group therapy also may be options.
On the other hand, individual psychotherapy, such as cognitive-behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapy, can provide a private and confidential environment for older adults who are less social.24
The highly structured nature of CBT may be well suited to older adults who have memory difficulties.1 A study of 110 older veterans with substance abuse problems found evidence for the effectiveness of group CBT among these patients.25 All but 8 participants in this study were age ≥65. The intervention consisted of 16 weekly group sessions that began with analysis of substance use behavior to determine high-risk situations for use, followed by a series of modules to teach skills for coping with social pressure, being at home and alone, feelings of depression and loneliness, anxiety and tension, anger and frustration, cues for substance use, and other factors. Approximately 44% (49 of 110) completed treatment (≥13 sessions). Approximately 55% of those who completed the treatment were abstinent at 6-month follow-up.25
Don’t assume your older patient is not using illicit substances
It is a myth that older adults do not use and abuse illicit substances. Illicit drug use among older adults is increasing. Older adults with SUDs may not present with the same symptoms as their younger counterparts, and thus it may be difficult to identify the problem. Maintain a high index of suspicion regarding the use of illicit substances in these patients.
Treatment options are generally limited and health care settings offer few interventions designed specifically for older adults. In general, proper identification of SUDs and targeted treatment can highly improve outcomes.
Baby Boomers—a term used to refer to individuals born in the United States between 1946 and 1964—are now approaching old age. Surprisingly, these older adults are using illicit substances in a pattern not seen in prior generations of older adults, including developing substance use disorders (SUDs) at increasingly higher rates; in previous generations, the prevalence of such disorders typically lowered with advancing age.
This article discusses how to recognize and treat SUDs in older adults. Alcohol is the most commonly used substance among older adults,1 and there is a largebody of literature describing the identification and treatment of alcohol-related disorders in these patients. Therefore, this article will instead focus on older adults’ use of illicit substances, including marijuana, cocaine, and heroin.
Epidemiology
Prior clinical data regarding substance abuse in older adults focused on alcohol, prescription drugs, nicotine, and caffeine.2 In the past, compared with younger adults, older adults had lower rates of alcohol and other illicit drug use.3,4 Baby Boomers appear to be defying this trend.
A 2013 Substance Abuse and Mental Health Services Administration survey found that the percentage of adults ages 50 to 64 who used illicit substances increased from 2.7% in 2002 to 6.0% in 2013.5 Specifically, during that time, past-month illicit substance use increased from 3.4% to 7.9% among those ages 50 to 54, from 1.9% to 5.7% among those ages 55 to 59, and from 2.5% to 3.9% among those ages 60 to 64.5
More recently, a 2014 study of geriatric patients found that of the 1,302 patients age ≥65 admitted to a Level 1 trauma center, 48.3% had a positive urine drug screen.6 Someresearchers have estimated that 5.7 million older adults will require treatment for a substance use disorder in 2020, which is roughly double the 2.8 million who had an SUD in 2002 to 2006.7
Risk factors and patterns of substance abuse
Individual, social, and familial factors can contribute to substance use and abuse in late life. The Table1 outlines some of the potential risk factors for older adults associated with the use of illicit substances. Substance abuse among older adults can be divided into 2 broad categories: early onset (starting before age 50) and late onset (starting after age 50).8 While data are limited, in general, early-onset use is a more common pattern; late-onset use represents an estimated <10% of substance use among older adults. The factors that lead some adults to continue substance use in late life, or to begin substance use later in life, have not been thoroughly evaluated.

Although older adults may abuse a wide variety of illicit substances, here we describe their use of marijuana, cocaine, and heroin.
Marijuana use has changed substantially in the last decade. While marijuana is illegal under federal law, as of November 2017, 29 states had legalized marijuana for medicinal purposes and 7 states and the District of Columbia had legalized it for recreational use. The increased legal and social acceptance of marijuana has led to new businesses and methods of use beyond smoking. New types of marijuana products include edible substances, tinctures, and oils that can be vaporized and inhaled.
In addition to euphoria and relaxation, the effects of marijuana use include increased latency time and decreased ability to respond to stimuli.2 Nonpsychiatric effects of marijuana include shallow breathing, weakened immune system, and increasing cardiac workload.2 The latter effect is especially important for older adults, many of whom may have preexisting cardiac illness and may be more likely to experience an adverse cardiac event as a result of marijuana use.2 Older adults who begin to use marijuana in late life may do so not primarily as a social activity, but more likely to experience the drug’s potentially beneficial effects on pain or appetite.2 For more on theuse of marijuana for these reasons, see “Medical marijuana: Do the benefits outweigh the risks?” in
Cocaine. Although cocaine is a CNS stimulant that causes a short-lived euphoria, its adverse effects impact many body systems.9 Myocardial infarction (MI) secondary to coronary artery vasospasms, stroke (hemorrhagic and ischemic), seizures, psychosis, aortic dissection, and acute renal injury are some of the most severe complications. Acute MI is the most frequent and severe cardiovascular complication seen among abusers.10 Cocaine use can cause dizziness, restlessness, headache, mydriasis, and anxiety.
In a pilot study, Kalapatapu et al11 compared the effects of cocaine abuse in younger vs older users. They found that older users had similar patterns of cocaine abuse in terms of the amount of cocaine used and frequency of use.11 They also found that specific cognitive functions, including psychomotor speed, attention, and short-term memory, are particularly sensitive to the combined effects of aging and cocaine abuse.11
Heroin is an opioid and a CNS depressant. Common effects include slowed heart rate, decreased blood pressure, and decreased respiration rate. Chronic heroin users show an overall decrease in immune system functioning12; this deficit might be particularly pronounced in an older person whose immune system functioning has already begun to decline as a result of aging. In recent years, as is the case with younger substance users, prescription opioids have replaced heroin as the opioid of choice among older users. However, for some early-onset heroin users, the use of this particular drug becomes well entrenched and unlikely to change, even in late life. Each year of heroin use increases the likelihood of continued use the next year by approximately 3%.2 Some research suggests that older heroin users do not decrease their use over time, and face many of the same risks as younger users, including poorer physical and mental health, severe physical disability, and mortality.13
Challenges to recognizing the problem
There are no screening protocols in the clinical setting that are designed specifically for detecting illicit substance abuse among older adults. Furthermore, diagnosis can be easily overlooked because the signs and symptoms of illicit substance use can be mistaken for other illnesses. To complicate matters further, older adults often do not disclose their substance use, understate it, or even try to explain away their symptoms.1 Many older adults live alone, which may increase their risk of receiving no treatment.14
Older adults generally experience reduced tolerance to the effects of illicit substances because of age-related physiologic changes, such as decreases in renal functioning, motor functioning, and cardiac output; altered liver metabolism of certain drugs; and elevated blood glucose levels.15 As a result, symptoms of illicit substance use could be mistaken for dementia or other forms of cognitive impairment.1,16
Although not designed specifically for older adults, an evidence-based screening instrument, such as the CAGE Questionnaire Adapted to Include Drugs, may be helpful in identifying substance abuse in these patients. Urine and/or serum drug screening, along with obtaining a comprehensive history from a trustworthy source, is useful for diagnosis.
Pharmacologic treatments
Research evaluating the use of medication for treating substance abuse specifically in older adults is extremely limited; studies have focused primarily on younger patients or mixed-age populations. Treatments that have been shown to be effective for younger patients may or may not be effective for older adults.
Marijuana. There are no FDA-approved treatments for marijuana abuse. An open-label study found that N-acetylcysteine, 1,200 mg twice a day, resulted in a significant reduction in marijuana craving as measured by the 12-item version of the Marijuana Craving Questionnaire.17 In a double-blinded placebo-controlled study, adolescents who were dependent on marijuana who received N-acetylcysteine, 1,200 mg twice a day, were more than twice likely to stop marijuana use compared with those who received placebo.18 Some researchers have proposed that N-acetylcysteine may prevent continued use of marijuana via glutamate modulation in the nucleus accumbens. Animal models have demonstrated that chronic drug self-administration downregulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine upregulates this exchanger, which reduces reinstatement of drug seeking.Further studies are needed to verify this speculation.
Cocaine. There are no FDA-approved treatments for cocaine abuse. No specific treatment approach has been found to be consistently effective.
A potential “cocaine vaccine” called TA-CD, which is made from succinyl norcocaine conjugated to cholera toxin, is being evaluated. An initial study had promising results, finding a significant reduction in cocaine use among those who received TA-CD.19 A later double-blinded placebo-controlled study only partially replicated the efficacy found in the initial study.20
Currently, other cocaine treatments are also being investigated. An enzyme to rapidly metabolize cocaine is being evaluated.21 So far, none of these treatments have targeted older adults, and there may be age-specific issues to consider if these approaches eventually receive FDA approval.
Heroin. Several FDA-approved medications are available for treating dependency to heroin and other opioids, including naltrexone, buprenorphine, and methadone, but none have been studied specifically in older adults. Some studies of transdermal buprenorphine for treating chronic pain in older adults have concluded that this formulation may offer advantages for older patients.22,23 Compared with oral or sublingual buprenorphine, the transdermal formulation avoids the first-pass effect in the liver, thus greatly increasing bioavailability of the drug; avoids renal metabolism; and offers greater tolerability in patients with mild to moderate hepatic impairment.22,23 However, transdermal buprenorphine has been approved only for the treatment of pain. These beneficial aspects of transdermal buprenorphine may be applicable to older opioid users, but no age-specific studies of buprenorphine for treating opioid abuse have been conducted.
Nonpharmacologic treatments
The same psychotherapeutic treatments used to treat younger patients with SUDs may be appropriate for older adults. Older patients may experience feelings of isolation and shame related to needing treatment for substance abuse. These factors in treatment of older patients often are overcome by group psychotherapy. Self-help programs, such as Narcotics Anonymous or Alcoholics Anonymous, and group therapy also may be options.
On the other hand, individual psychotherapy, such as cognitive-behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapy, can provide a private and confidential environment for older adults who are less social.24
The highly structured nature of CBT may be well suited to older adults who have memory difficulties.1 A study of 110 older veterans with substance abuse problems found evidence for the effectiveness of group CBT among these patients.25 All but 8 participants in this study were age ≥65. The intervention consisted of 16 weekly group sessions that began with analysis of substance use behavior to determine high-risk situations for use, followed by a series of modules to teach skills for coping with social pressure, being at home and alone, feelings of depression and loneliness, anxiety and tension, anger and frustration, cues for substance use, and other factors. Approximately 44% (49 of 110) completed treatment (≥13 sessions). Approximately 55% of those who completed the treatment were abstinent at 6-month follow-up.25
Don’t assume your older patient is not using illicit substances
It is a myth that older adults do not use and abuse illicit substances. Illicit drug use among older adults is increasing. Older adults with SUDs may not present with the same symptoms as their younger counterparts, and thus it may be difficult to identify the problem. Maintain a high index of suspicion regarding the use of illicit substances in these patients.
Treatment options are generally limited and health care settings offer few interventions designed specifically for older adults. In general, proper identification of SUDs and targeted treatment can highly improve outcomes.
1. Kuerbis A, Sacco P, Blazer DG, et al. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629-654.
2. Taylor MH, Grossberg GT. (2012). The growing problem of illicit substance abuse in the elderly: a review. Prim Care Companion CNS Disord. 2012;14(4):PCC.11r01320. doi: 10.4088/PCC.11r01320.
3. Cummings SM, Bride B, Rawlings-Shaw AM. Alcohol abuse treatment for older adults: a review of recent empirical research. J Evid Based Soc Work. 2006;3(1):79-99.
4. Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: summary of national findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
5. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
6. Ekeh AP, Parikh P, Walusimbi MS, et al. The prevalence of positive drug and alcohol screens in elderly trauma patients. Subst Abus. 2014;35(1):51-55.
7. Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23(3):481-504.
8. Roe B, Beynon C, Pickering L, et al. Experiences of drug use and ageing: health, quality of life, relationship and service implications. J Adv Nurs. 2010;66(9):1968-1979.
9. Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012;28(4):517-526.
10. Weber JE, Chudnofsky CR, Boczar M, et al. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med. 2000;7(8):873-877.
11. Kalapatapu RK, Vadhan NP, Rubin E, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228-239.
12. Edelman EJ, Cheng DM, Krupitsky EM, et al. Heroin use and HIV disease progression: results from a pilot study of a Russian cohort. AIDS Behav. 2015;19(6):1089-1097.
13. Darke S, Mills KL, Ross J, et al. The ageing heroin user: career length, clinical profile and outcomes across 36 months. Drug Alcohol Rev. 2009;28(3):243-249.
14. West LA, Cole S, Goodkind D, et al. U.S. Census Bureau, P23-212. 65+ in the United States: 2010. Washington, DC: United States Census Bureau; 2014.
15. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434-440.
16. Ruiz P, Strain EC, Langrod JG. The substance abuse handbook. Philadelphia, PA: Wolters Kluwer Health; 2007.
17. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
18. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
19. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123
20. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47.
21. Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310(3):1046-1052.
22. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 2008;3(3):421-430.
23. Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143-149.
24. Schultz SK, Arndt S, Liesveld J. Locations of facilities with special programs for older substance abuse clients in the US. Int J Geriatr Psychiatry. 2003;18(9):839-843.
25. Schonfeld L, Dupree LW, Dickson-Fuhrman E, et al. Cognitive-behavioral treatment of older veterans with substance abuse problems. J Geriatr Psychiatry Neurol. 2000;13(3):124-129.
1. Kuerbis A, Sacco P, Blazer DG, et al. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629-654.
2. Taylor MH, Grossberg GT. (2012). The growing problem of illicit substance abuse in the elderly: a review. Prim Care Companion CNS Disord. 2012;14(4):PCC.11r01320. doi: 10.4088/PCC.11r01320.
3. Cummings SM, Bride B, Rawlings-Shaw AM. Alcohol abuse treatment for older adults: a review of recent empirical research. J Evid Based Soc Work. 2006;3(1):79-99.
4. Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: summary of national findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
5. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
6. Ekeh AP, Parikh P, Walusimbi MS, et al. The prevalence of positive drug and alcohol screens in elderly trauma patients. Subst Abus. 2014;35(1):51-55.
7. Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23(3):481-504.
8. Roe B, Beynon C, Pickering L, et al. Experiences of drug use and ageing: health, quality of life, relationship and service implications. J Adv Nurs. 2010;66(9):1968-1979.
9. Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012;28(4):517-526.
10. Weber JE, Chudnofsky CR, Boczar M, et al. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med. 2000;7(8):873-877.
11. Kalapatapu RK, Vadhan NP, Rubin E, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228-239.
12. Edelman EJ, Cheng DM, Krupitsky EM, et al. Heroin use and HIV disease progression: results from a pilot study of a Russian cohort. AIDS Behav. 2015;19(6):1089-1097.
13. Darke S, Mills KL, Ross J, et al. The ageing heroin user: career length, clinical profile and outcomes across 36 months. Drug Alcohol Rev. 2009;28(3):243-249.
14. West LA, Cole S, Goodkind D, et al. U.S. Census Bureau, P23-212. 65+ in the United States: 2010. Washington, DC: United States Census Bureau; 2014.
15. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434-440.
16. Ruiz P, Strain EC, Langrod JG. The substance abuse handbook. Philadelphia, PA: Wolters Kluwer Health; 2007.
17. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
18. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
19. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123
20. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47.
21. Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310(3):1046-1052.
22. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 2008;3(3):421-430.
23. Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143-149.
24. Schultz SK, Arndt S, Liesveld J. Locations of facilities with special programs for older substance abuse clients in the US. Int J Geriatr Psychiatry. 2003;18(9):839-843.
25. Schonfeld L, Dupree LW, Dickson-Fuhrman E, et al. Cognitive-behavioral treatment of older veterans with substance abuse problems. J Geriatr Psychiatry Neurol. 2000;13(3):124-129.
Enhancing the effects of microfocused ultrasound with cosmeceuticals
The use of microfocused ultrasound (MFUS) emerged in dermatology in 2009 as a minimally invasive approach to treating mild to moderate facial and neck laxity. Microfocused ultrasound with visualization (MFU-V), as represented by the device Ultherapy, adds high-resolution ultrasound imaging so that the user can see the targets for MFUS energy. Visualization also allows the user to choose the appropriate treatment depth and transducer.1 Since its introduction, Ultherapy has been investigated for efficacy and safety in tightening, lifting, and wrinkle reduction beyond the face and neck, specifically including the décolletage, abdomen, arms/elbows, knees, medial thighs, and buttocks.1-8 This column will focus on using cosmeceuticals to improve the skin-tightening outcomes of microfocused ultrasound.
Two weeks before the procedure
Ingredients that should be used prior to MFU-V include retinoids, such as tretinoin and retinol. Various studies have demonstrated that pretreatment with tretinoin increases collagen production and speeds wound healing.12-14 Kligman et al. assessed wound healing after punch biopsy and found that arm wounds pretreated with tretinoin cream 0.05%-0.1% were significantly smaller by 35%-37% on days 1 and 4 and by 47%-50% on days 6, 8, and 11 than were wounds on the untreated arms.15 The majority of studies on the subject recommend a 2- to 4-week tretinoin pretreatment regimen because peak epidermal hypertrophy occurs after 7 days of tretinoin application and normalizes after 14 days of continued treatment.16,17 This time frame allows for the skin to recover from any retinoid dermatitis prior to the procedure. Adapalene should be started 5-6 weeks prior to procedures because it has a longer half-life and requires an earlier initiation period.18 Although studies have not been performed evaluating the efficacy of topical ascorbic acid and hydroxy acids, pretreating skin with them also may accelerate collagen production after procedures such as MFU-V.19,20
Postprocedure skin care
Because no incision or ablation occurs with MFUS, the use of ascorbic acid, hydroxy acids, and retinoids can and should be continued after the procedure in addition to before the procedure. The combination of topical Arnica montana and Rhododendron tomentosum in a gel pad has been shown to mitigate postoperative ecchymosis and edema after oculofacial surgery.24 Topical curcumin has been shown to facilitate wound healing in animals, but its smell and color make it difficult to use topically. I recommend adding turmeric to food pre- and postprocedure.25
Adverse events
Late in 2017, Friedmann et al. offered a report on the nature of the rare complications from MFUS, which have included mild and fleeting ecchymosis, edema, erythema, and nerve paralysis. In this retrospective multicenter case series of five patients seen in the authors’ practice who experienced serious adverse reactions to Ultherapy, the authors reported that single sessions of MFUS yielded blistering, erosion/ulceration, or cutaneous or subcutaneous tissue edema with resulting atrophy and/or cutaneous necrosis. The authors concluded that while serious adverse events following MFUS are rare, such reactions might be underreported and should be prepared for with early management to diminish inflammation.26 Other adverse events, which are transient and rare, may include discomfort and mild bruising.
Educating patients on pre- and postprocedure instructions can help minimize adverse events. Avoiding foods that decrease platelet function, like ginger, green tea, alcohol (red wine), salmon, and flax seeds, can reduce the risk of bruising. Use of topical and oral antioxidants before and after treatments also may help reduce inflammation and edema.
Conclusion
Please email me at [email protected] if you have any comments, suggestions, or anecdotal reports to share on using cosmeceuticals and nutraceuticals before and after procedures. I will share your responses on my LinkedIn account.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also wrote a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
References
1. Clin Cosmet Investig Dermatol. 2017 Oct 30;10:423-9.
2. J Am Acad Dermatol. 2013 Dec;69(6):965-71.
3. Dermatol Surg. 2015 Mar;41(3):327-35.
4. J Cosmet Dermatol Sci Appl. 2012;2(2A):108-16.
5. Dermatol Surg. 2015 Jul;41(7):821-6.
6. J Cosmet Laser Ther. 2014 Oct;16(5):225-9.
7. Dermatol Surg. 2012 May;38(5):754-9.
8. Dermatol Surg. 2014 Oct;40(10):1113-7.
9. Clin Cosmet Investig Dermatol. 2015; 8: 47-52.
10. Dermatol Surg. 2017 Sep 8. doi: 10.1097/DSS.0000000000001216. [Epub ahead of print]
11. Arch Dermatol. 2004 Feb;140(2):204-9.
12. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
13. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
14. J Am Acad Dermatol. 1998 Aug;39(2 Pt 3):S79-81.
15. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
16. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
17. J Korean Med Sci. 1996 Aug;11(4):335-41.
18. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
19. Proc Natl Acad Sci U S A. 1981 May; 78(5): 2879-82.
20. Exp Dermatol. 2003;12 Suppl 2:57-63.
21. Dermatol Surg. 2001 Feb;27(2):137-42.
22. Br J Dermatol. 1992 Sep;127(3):247-53.
23. J Invest Dermatol. 1991;96:587.
24. Ophthal Plast Reconstr Surg. 2017 Jan/Feb;33(1):47-52.
25. Wound Repair Regen. 1998 Mar-Apr;6(2):167-77.
26. Lasers Surg Med. 2018 Jan;50(1):13-9.
The use of microfocused ultrasound (MFUS) emerged in dermatology in 2009 as a minimally invasive approach to treating mild to moderate facial and neck laxity. Microfocused ultrasound with visualization (MFU-V), as represented by the device Ultherapy, adds high-resolution ultrasound imaging so that the user can see the targets for MFUS energy. Visualization also allows the user to choose the appropriate treatment depth and transducer.1 Since its introduction, Ultherapy has been investigated for efficacy and safety in tightening, lifting, and wrinkle reduction beyond the face and neck, specifically including the décolletage, abdomen, arms/elbows, knees, medial thighs, and buttocks.1-8 This column will focus on using cosmeceuticals to improve the skin-tightening outcomes of microfocused ultrasound.
Two weeks before the procedure
Ingredients that should be used prior to MFU-V include retinoids, such as tretinoin and retinol. Various studies have demonstrated that pretreatment with tretinoin increases collagen production and speeds wound healing.12-14 Kligman et al. assessed wound healing after punch biopsy and found that arm wounds pretreated with tretinoin cream 0.05%-0.1% were significantly smaller by 35%-37% on days 1 and 4 and by 47%-50% on days 6, 8, and 11 than were wounds on the untreated arms.15 The majority of studies on the subject recommend a 2- to 4-week tretinoin pretreatment regimen because peak epidermal hypertrophy occurs after 7 days of tretinoin application and normalizes after 14 days of continued treatment.16,17 This time frame allows for the skin to recover from any retinoid dermatitis prior to the procedure. Adapalene should be started 5-6 weeks prior to procedures because it has a longer half-life and requires an earlier initiation period.18 Although studies have not been performed evaluating the efficacy of topical ascorbic acid and hydroxy acids, pretreating skin with them also may accelerate collagen production after procedures such as MFU-V.19,20
Postprocedure skin care
Because no incision or ablation occurs with MFUS, the use of ascorbic acid, hydroxy acids, and retinoids can and should be continued after the procedure in addition to before the procedure. The combination of topical Arnica montana and Rhododendron tomentosum in a gel pad has been shown to mitigate postoperative ecchymosis and edema after oculofacial surgery.24 Topical curcumin has been shown to facilitate wound healing in animals, but its smell and color make it difficult to use topically. I recommend adding turmeric to food pre- and postprocedure.25
Adverse events
Late in 2017, Friedmann et al. offered a report on the nature of the rare complications from MFUS, which have included mild and fleeting ecchymosis, edema, erythema, and nerve paralysis. In this retrospective multicenter case series of five patients seen in the authors’ practice who experienced serious adverse reactions to Ultherapy, the authors reported that single sessions of MFUS yielded blistering, erosion/ulceration, or cutaneous or subcutaneous tissue edema with resulting atrophy and/or cutaneous necrosis. The authors concluded that while serious adverse events following MFUS are rare, such reactions might be underreported and should be prepared for with early management to diminish inflammation.26 Other adverse events, which are transient and rare, may include discomfort and mild bruising.
Educating patients on pre- and postprocedure instructions can help minimize adverse events. Avoiding foods that decrease platelet function, like ginger, green tea, alcohol (red wine), salmon, and flax seeds, can reduce the risk of bruising. Use of topical and oral antioxidants before and after treatments also may help reduce inflammation and edema.
Conclusion
Please email me at [email protected] if you have any comments, suggestions, or anecdotal reports to share on using cosmeceuticals and nutraceuticals before and after procedures. I will share your responses on my LinkedIn account.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also wrote a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
References
1. Clin Cosmet Investig Dermatol. 2017 Oct 30;10:423-9.
2. J Am Acad Dermatol. 2013 Dec;69(6):965-71.
3. Dermatol Surg. 2015 Mar;41(3):327-35.
4. J Cosmet Dermatol Sci Appl. 2012;2(2A):108-16.
5. Dermatol Surg. 2015 Jul;41(7):821-6.
6. J Cosmet Laser Ther. 2014 Oct;16(5):225-9.
7. Dermatol Surg. 2012 May;38(5):754-9.
8. Dermatol Surg. 2014 Oct;40(10):1113-7.
9. Clin Cosmet Investig Dermatol. 2015; 8: 47-52.
10. Dermatol Surg. 2017 Sep 8. doi: 10.1097/DSS.0000000000001216. [Epub ahead of print]
11. Arch Dermatol. 2004 Feb;140(2):204-9.
12. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
13. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
14. J Am Acad Dermatol. 1998 Aug;39(2 Pt 3):S79-81.
15. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
16. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
17. J Korean Med Sci. 1996 Aug;11(4):335-41.
18. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
19. Proc Natl Acad Sci U S A. 1981 May; 78(5): 2879-82.
20. Exp Dermatol. 2003;12 Suppl 2:57-63.
21. Dermatol Surg. 2001 Feb;27(2):137-42.
22. Br J Dermatol. 1992 Sep;127(3):247-53.
23. J Invest Dermatol. 1991;96:587.
24. Ophthal Plast Reconstr Surg. 2017 Jan/Feb;33(1):47-52.
25. Wound Repair Regen. 1998 Mar-Apr;6(2):167-77.
26. Lasers Surg Med. 2018 Jan;50(1):13-9.
The use of microfocused ultrasound (MFUS) emerged in dermatology in 2009 as a minimally invasive approach to treating mild to moderate facial and neck laxity. Microfocused ultrasound with visualization (MFU-V), as represented by the device Ultherapy, adds high-resolution ultrasound imaging so that the user can see the targets for MFUS energy. Visualization also allows the user to choose the appropriate treatment depth and transducer.1 Since its introduction, Ultherapy has been investigated for efficacy and safety in tightening, lifting, and wrinkle reduction beyond the face and neck, specifically including the décolletage, abdomen, arms/elbows, knees, medial thighs, and buttocks.1-8 This column will focus on using cosmeceuticals to improve the skin-tightening outcomes of microfocused ultrasound.
Two weeks before the procedure
Ingredients that should be used prior to MFU-V include retinoids, such as tretinoin and retinol. Various studies have demonstrated that pretreatment with tretinoin increases collagen production and speeds wound healing.12-14 Kligman et al. assessed wound healing after punch biopsy and found that arm wounds pretreated with tretinoin cream 0.05%-0.1% were significantly smaller by 35%-37% on days 1 and 4 and by 47%-50% on days 6, 8, and 11 than were wounds on the untreated arms.15 The majority of studies on the subject recommend a 2- to 4-week tretinoin pretreatment regimen because peak epidermal hypertrophy occurs after 7 days of tretinoin application and normalizes after 14 days of continued treatment.16,17 This time frame allows for the skin to recover from any retinoid dermatitis prior to the procedure. Adapalene should be started 5-6 weeks prior to procedures because it has a longer half-life and requires an earlier initiation period.18 Although studies have not been performed evaluating the efficacy of topical ascorbic acid and hydroxy acids, pretreating skin with them also may accelerate collagen production after procedures such as MFU-V.19,20
Postprocedure skin care
Because no incision or ablation occurs with MFUS, the use of ascorbic acid, hydroxy acids, and retinoids can and should be continued after the procedure in addition to before the procedure. The combination of topical Arnica montana and Rhododendron tomentosum in a gel pad has been shown to mitigate postoperative ecchymosis and edema after oculofacial surgery.24 Topical curcumin has been shown to facilitate wound healing in animals, but its smell and color make it difficult to use topically. I recommend adding turmeric to food pre- and postprocedure.25
Adverse events
Late in 2017, Friedmann et al. offered a report on the nature of the rare complications from MFUS, which have included mild and fleeting ecchymosis, edema, erythema, and nerve paralysis. In this retrospective multicenter case series of five patients seen in the authors’ practice who experienced serious adverse reactions to Ultherapy, the authors reported that single sessions of MFUS yielded blistering, erosion/ulceration, or cutaneous or subcutaneous tissue edema with resulting atrophy and/or cutaneous necrosis. The authors concluded that while serious adverse events following MFUS are rare, such reactions might be underreported and should be prepared for with early management to diminish inflammation.26 Other adverse events, which are transient and rare, may include discomfort and mild bruising.
Educating patients on pre- and postprocedure instructions can help minimize adverse events. Avoiding foods that decrease platelet function, like ginger, green tea, alcohol (red wine), salmon, and flax seeds, can reduce the risk of bruising. Use of topical and oral antioxidants before and after treatments also may help reduce inflammation and edema.
Conclusion
Please email me at [email protected] if you have any comments, suggestions, or anecdotal reports to share on using cosmeceuticals and nutraceuticals before and after procedures. I will share your responses on my LinkedIn account.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also wrote a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems.
References
1. Clin Cosmet Investig Dermatol. 2017 Oct 30;10:423-9.
2. J Am Acad Dermatol. 2013 Dec;69(6):965-71.
3. Dermatol Surg. 2015 Mar;41(3):327-35.
4. J Cosmet Dermatol Sci Appl. 2012;2(2A):108-16.
5. Dermatol Surg. 2015 Jul;41(7):821-6.
6. J Cosmet Laser Ther. 2014 Oct;16(5):225-9.
7. Dermatol Surg. 2012 May;38(5):754-9.
8. Dermatol Surg. 2014 Oct;40(10):1113-7.
9. Clin Cosmet Investig Dermatol. 2015; 8: 47-52.
10. Dermatol Surg. 2017 Sep 8. doi: 10.1097/DSS.0000000000001216. [Epub ahead of print]
11. Arch Dermatol. 2004 Feb;140(2):204-9.
12. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
13. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
14. J Am Acad Dermatol. 1998 Aug;39(2 Pt 3):S79-81.
15. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
16. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
17. J Korean Med Sci. 1996 Aug;11(4):335-41.
18. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
19. Proc Natl Acad Sci U S A. 1981 May; 78(5): 2879-82.
20. Exp Dermatol. 2003;12 Suppl 2:57-63.
21. Dermatol Surg. 2001 Feb;27(2):137-42.
22. Br J Dermatol. 1992 Sep;127(3):247-53.
23. J Invest Dermatol. 1991;96:587.
24. Ophthal Plast Reconstr Surg. 2017 Jan/Feb;33(1):47-52.
25. Wound Repair Regen. 1998 Mar-Apr;6(2):167-77.
26. Lasers Surg Med. 2018 Jan;50(1):13-9.
RIV takes center stage at HM18
If prior SHM annual meetings are any guide, a highlight of the upcoming HM18 conference will be the Scientific Abstract and Poster Competition. This event, also known as the Research, Innovations, and Clinical Vignettes (RIV), has become one of the annual meeting’s most popular events. Crowds of attendees cluster around posters to read abstracts summarizing some of the most exciting, cutting-edge research in hospital medicine.
Networking in that crowd is a major factor in the RIV’s popularity, says the HM18 Innovations chair, Benji K. Mathews, MD, FACP, SFHM, CLHM, section head of hospital medicine at Regions Hospital, St. Paul, Minn., which is part of HealthPartners, the largest consumer-governed, non-profit health care organization in the U.S.
“From my standpoint, the power of this Innovations RIV competition is the opportunity to network,” said Dr. Mathews, who is also assistant professor of medicine at the University of Minnesota, Minneapolis. “In addition to primary authors, often these posters have several different people involved, and then there’s the foot traffic: We’re expecting thousands of people to walk through. The hope is to create the opportunity to network, to collaborate intergenerationally and also cross-institutionally.”
The RIV competition features some 1,000 posters this year, Dr. Mathews said. Plenary and oral sessions are chosen from the pool of abstracts prior to the meeting, and their authors are invited to present on-site at HM18.
But in spirit, the RIV is not really a competition, said RIV chair Ethan Cumbler, MD, FACP, FHM, professor of medicine at the University of Colorado.
“It’s really about sharing the latest science and the cases and innovations that are going to change practice tomorrow. The RIV is about sharing with our colleagues and moving the science of hospital medicine forward,” he said.
“Hospitalists can share and discuss their work and exchange ideas in a nonthreatening, collegial manner,” Dr. Mathews added. “In the end, we understand it’s not all about winning. We try to make sure it’s an atmosphere where people can engage and collaborate with each other.”
As far as the competitive element goes, the judges’ decisions are driven by an abstract’s content, organization, and style, Dr. Mathews said. “When we look at abstracts, affecting patient care in the authors’ own hospital is a beautiful thing, but is there a potential in this abstract to reach the masses? Is it able to be implemented beyond their local microcosm to affect people regionally, nationally, internationally? If there’s potential for that, that’s usually a good abstract.”
What’s new in 2018
The more than 1,000 posters and oral presentations at HM18 is a new record, and it demonstrates the growth of hospital medicine as a scientific field, Dr. Cumbler said.
“We received a huge number of submissions,” he revealed. “We see that trend rise, year over year, and the quality has been going up as well.”
New this year is a Trainee Award category for resident and student authors. Another difference in 2018: The top 15 advances in Research and Innovations have been given a special track on day 2 of the conference, with oral presentations by the authors sharing their work.
The Vignettes are being featured in a new way as well. “We have so many incredible cases that we’re going to have a clinical vignette luncheon on two different days of the conference,” Dr. Cumbler said. “These are cases that we want to highlight, so that the experience of a hospitalist in one part of the country could help a hospitalist provide the right diagnosis for a patient on the other side of the country. There are lessons to be learned in clinical medicine, and our clinical vignettes is a fantastic way of sharing them.”
In making their selections in the different categories, the judges aimed to highlight some negative studies this year, Dr. Mathews said, which is a slight departure from previous years. “Sometimes you try something and it didn’t work, and it’s important to share that so we don’t just try the same thing over and over.”
This year, Research and Innovations abstracts will be grouped by theme, making it easier for attendees to navigate the posters. “If you’ve got a particular interest in a topic like transitions or communication, you’ll be able to find that portion of the poster session and talk to some of the people who are doing groundbreaking work in that topic,” Dr. Cumbler said.
He also noted that he expects to see a strong expression of RIV content on social media from HM18, as judges encounter some of the best and most interesting work at RIV. Dr. Mathews is similarly enthusiastic about that amplification of the work.
“I love that the conversation continues into social media platforms such as Twitter,” he said. “People are engaging back and forth, saying, ‘Hey, take a look at this poster.’ Being in a room with countless people interested in research innovations for a field that’s still relatively young – I love that there’s movement toward that.”
Exciting research
By definition, the research on display at the RIV is the best of the best. “It’s difficult to get your work accepted at a national meeting, and it’s a high honor to be selected as a finalist. The poster abstracts or oral presentations that win are always remarkable pieces of work,” Dr. Cumbler said.
Some of this year’s most exciting projects examine prediction models and scoring systems for patients with infections such as sepsis or influenza, he said. “One of the most fascinating abstracts looked at deep learning, or machine learning, to create algorithms to predict sepsis and decompensation in ways that simplistic models might not. Many of our current prediction rules are designed around simple acronyms, because they’re easy to remember: the ABCD score, the CURB-65 score. But if you looked at the source code of the Google search algorithm – not that they’d let you – you’d discover that it doesn’t translate to a simple four-variable prediction model. It’s incredibly complex; it looks at interactions between variables.”
This research attempts to move medical prediction models in that direction, Dr. Cumbler said. “Examining deep learning models, or neural networks, to help clinicians make more accurate predictions and take better care of patients – we are getting a taste of the future of clinical medicine at HM18.”
Several research projects highlighted at RIV this year examine ways to make better use of the data in the electronic health record.
“One of the pieces of research I’m particularly excited to hear more about looks at how the vast data that exists within electronic health records is actually used,” Dr. Cumbler said. “With electronic health records, we have all of the information in a patient’s record at our fingertips, yet this creates incredible new challenges for the hospitalist needing to make decisions in real time, with the limitations of our organic neural networks.” Dr. Cumbler revealed that one of the research teams sharing their work at HM18 explored how hospitalists interact with the volume of information that exists within the health record at the time of admission. “The results are pretty amazing,” he said.
Another project Dr. Cumbler found fascinating examines the impact of delivery of real-time performance data to hospitalists on their phones, and how it affected practice across a number of different performance metrics.
“We will see a project using game theory to teach quality improvement and another sharing important quality improvement work occurring at the intersection of evidence-based medicine and patient experience – like looking at how to keep patients NPO for less unnecessary time,” he said. “It makes perfect sense that we don’t want to keep people hungry in the hospital longer than we need to. It’s really interesting seeing how one team worked to make that happen and what they found.”
The importance of the RIV
The influence of the RIV program extends far beyond the conference itself; there are implications for the field of hospital medicine today and into the future.
“The RIV competition allows the field in hospital medicine to mature and evolve, so we remain cutting edge,” Dr. Mathews said. “That’s the beauty of the innovation field: Research is built off of it.”
Dr. Cumbler said that the growth and evolution of the RIV is reflective of the maturation of hospital medicine as a specialty. “It’s transitioning from a different way to organize patient care to learning more, in a scientific way, about how care can and should be delivered.”
At its heart, the RIV is really about community, he added. “The community of hospitalists is sharing knowledge, graciously and unselfishly, so that we can all improve the quality of care that we’re providing and give patients safer care, a better experience, and improved outcomes.”
Finally, RIV offers a way for hospitalists to be engaged in lifelong learning. “The presenters are teaching from their experience, and the hospitalists who come to the RIV get to leave better clinicians, researchers, and leaders as a result,” Dr. Cumbler said. “These things, to me, are about our evolution as a profession.”
If prior SHM annual meetings are any guide, a highlight of the upcoming HM18 conference will be the Scientific Abstract and Poster Competition. This event, also known as the Research, Innovations, and Clinical Vignettes (RIV), has become one of the annual meeting’s most popular events. Crowds of attendees cluster around posters to read abstracts summarizing some of the most exciting, cutting-edge research in hospital medicine.
Networking in that crowd is a major factor in the RIV’s popularity, says the HM18 Innovations chair, Benji K. Mathews, MD, FACP, SFHM, CLHM, section head of hospital medicine at Regions Hospital, St. Paul, Minn., which is part of HealthPartners, the largest consumer-governed, non-profit health care organization in the U.S.
“From my standpoint, the power of this Innovations RIV competition is the opportunity to network,” said Dr. Mathews, who is also assistant professor of medicine at the University of Minnesota, Minneapolis. “In addition to primary authors, often these posters have several different people involved, and then there’s the foot traffic: We’re expecting thousands of people to walk through. The hope is to create the opportunity to network, to collaborate intergenerationally and also cross-institutionally.”
The RIV competition features some 1,000 posters this year, Dr. Mathews said. Plenary and oral sessions are chosen from the pool of abstracts prior to the meeting, and their authors are invited to present on-site at HM18.
But in spirit, the RIV is not really a competition, said RIV chair Ethan Cumbler, MD, FACP, FHM, professor of medicine at the University of Colorado.
“It’s really about sharing the latest science and the cases and innovations that are going to change practice tomorrow. The RIV is about sharing with our colleagues and moving the science of hospital medicine forward,” he said.
“Hospitalists can share and discuss their work and exchange ideas in a nonthreatening, collegial manner,” Dr. Mathews added. “In the end, we understand it’s not all about winning. We try to make sure it’s an atmosphere where people can engage and collaborate with each other.”
As far as the competitive element goes, the judges’ decisions are driven by an abstract’s content, organization, and style, Dr. Mathews said. “When we look at abstracts, affecting patient care in the authors’ own hospital is a beautiful thing, but is there a potential in this abstract to reach the masses? Is it able to be implemented beyond their local microcosm to affect people regionally, nationally, internationally? If there’s potential for that, that’s usually a good abstract.”
What’s new in 2018
The more than 1,000 posters and oral presentations at HM18 is a new record, and it demonstrates the growth of hospital medicine as a scientific field, Dr. Cumbler said.
“We received a huge number of submissions,” he revealed. “We see that trend rise, year over year, and the quality has been going up as well.”
New this year is a Trainee Award category for resident and student authors. Another difference in 2018: The top 15 advances in Research and Innovations have been given a special track on day 2 of the conference, with oral presentations by the authors sharing their work.
The Vignettes are being featured in a new way as well. “We have so many incredible cases that we’re going to have a clinical vignette luncheon on two different days of the conference,” Dr. Cumbler said. “These are cases that we want to highlight, so that the experience of a hospitalist in one part of the country could help a hospitalist provide the right diagnosis for a patient on the other side of the country. There are lessons to be learned in clinical medicine, and our clinical vignettes is a fantastic way of sharing them.”
In making their selections in the different categories, the judges aimed to highlight some negative studies this year, Dr. Mathews said, which is a slight departure from previous years. “Sometimes you try something and it didn’t work, and it’s important to share that so we don’t just try the same thing over and over.”
This year, Research and Innovations abstracts will be grouped by theme, making it easier for attendees to navigate the posters. “If you’ve got a particular interest in a topic like transitions or communication, you’ll be able to find that portion of the poster session and talk to some of the people who are doing groundbreaking work in that topic,” Dr. Cumbler said.
He also noted that he expects to see a strong expression of RIV content on social media from HM18, as judges encounter some of the best and most interesting work at RIV. Dr. Mathews is similarly enthusiastic about that amplification of the work.
“I love that the conversation continues into social media platforms such as Twitter,” he said. “People are engaging back and forth, saying, ‘Hey, take a look at this poster.’ Being in a room with countless people interested in research innovations for a field that’s still relatively young – I love that there’s movement toward that.”
Exciting research
By definition, the research on display at the RIV is the best of the best. “It’s difficult to get your work accepted at a national meeting, and it’s a high honor to be selected as a finalist. The poster abstracts or oral presentations that win are always remarkable pieces of work,” Dr. Cumbler said.
Some of this year’s most exciting projects examine prediction models and scoring systems for patients with infections such as sepsis or influenza, he said. “One of the most fascinating abstracts looked at deep learning, or machine learning, to create algorithms to predict sepsis and decompensation in ways that simplistic models might not. Many of our current prediction rules are designed around simple acronyms, because they’re easy to remember: the ABCD score, the CURB-65 score. But if you looked at the source code of the Google search algorithm – not that they’d let you – you’d discover that it doesn’t translate to a simple four-variable prediction model. It’s incredibly complex; it looks at interactions between variables.”
This research attempts to move medical prediction models in that direction, Dr. Cumbler said. “Examining deep learning models, or neural networks, to help clinicians make more accurate predictions and take better care of patients – we are getting a taste of the future of clinical medicine at HM18.”
Several research projects highlighted at RIV this year examine ways to make better use of the data in the electronic health record.
“One of the pieces of research I’m particularly excited to hear more about looks at how the vast data that exists within electronic health records is actually used,” Dr. Cumbler said. “With electronic health records, we have all of the information in a patient’s record at our fingertips, yet this creates incredible new challenges for the hospitalist needing to make decisions in real time, with the limitations of our organic neural networks.” Dr. Cumbler revealed that one of the research teams sharing their work at HM18 explored how hospitalists interact with the volume of information that exists within the health record at the time of admission. “The results are pretty amazing,” he said.
Another project Dr. Cumbler found fascinating examines the impact of delivery of real-time performance data to hospitalists on their phones, and how it affected practice across a number of different performance metrics.
“We will see a project using game theory to teach quality improvement and another sharing important quality improvement work occurring at the intersection of evidence-based medicine and patient experience – like looking at how to keep patients NPO for less unnecessary time,” he said. “It makes perfect sense that we don’t want to keep people hungry in the hospital longer than we need to. It’s really interesting seeing how one team worked to make that happen and what they found.”
The importance of the RIV
The influence of the RIV program extends far beyond the conference itself; there are implications for the field of hospital medicine today and into the future.
“The RIV competition allows the field in hospital medicine to mature and evolve, so we remain cutting edge,” Dr. Mathews said. “That’s the beauty of the innovation field: Research is built off of it.”
Dr. Cumbler said that the growth and evolution of the RIV is reflective of the maturation of hospital medicine as a specialty. “It’s transitioning from a different way to organize patient care to learning more, in a scientific way, about how care can and should be delivered.”
At its heart, the RIV is really about community, he added. “The community of hospitalists is sharing knowledge, graciously and unselfishly, so that we can all improve the quality of care that we’re providing and give patients safer care, a better experience, and improved outcomes.”
Finally, RIV offers a way for hospitalists to be engaged in lifelong learning. “The presenters are teaching from their experience, and the hospitalists who come to the RIV get to leave better clinicians, researchers, and leaders as a result,” Dr. Cumbler said. “These things, to me, are about our evolution as a profession.”
If prior SHM annual meetings are any guide, a highlight of the upcoming HM18 conference will be the Scientific Abstract and Poster Competition. This event, also known as the Research, Innovations, and Clinical Vignettes (RIV), has become one of the annual meeting’s most popular events. Crowds of attendees cluster around posters to read abstracts summarizing some of the most exciting, cutting-edge research in hospital medicine.
Networking in that crowd is a major factor in the RIV’s popularity, says the HM18 Innovations chair, Benji K. Mathews, MD, FACP, SFHM, CLHM, section head of hospital medicine at Regions Hospital, St. Paul, Minn., which is part of HealthPartners, the largest consumer-governed, non-profit health care organization in the U.S.
“From my standpoint, the power of this Innovations RIV competition is the opportunity to network,” said Dr. Mathews, who is also assistant professor of medicine at the University of Minnesota, Minneapolis. “In addition to primary authors, often these posters have several different people involved, and then there’s the foot traffic: We’re expecting thousands of people to walk through. The hope is to create the opportunity to network, to collaborate intergenerationally and also cross-institutionally.”
The RIV competition features some 1,000 posters this year, Dr. Mathews said. Plenary and oral sessions are chosen from the pool of abstracts prior to the meeting, and their authors are invited to present on-site at HM18.
But in spirit, the RIV is not really a competition, said RIV chair Ethan Cumbler, MD, FACP, FHM, professor of medicine at the University of Colorado.
“It’s really about sharing the latest science and the cases and innovations that are going to change practice tomorrow. The RIV is about sharing with our colleagues and moving the science of hospital medicine forward,” he said.
“Hospitalists can share and discuss their work and exchange ideas in a nonthreatening, collegial manner,” Dr. Mathews added. “In the end, we understand it’s not all about winning. We try to make sure it’s an atmosphere where people can engage and collaborate with each other.”
As far as the competitive element goes, the judges’ decisions are driven by an abstract’s content, organization, and style, Dr. Mathews said. “When we look at abstracts, affecting patient care in the authors’ own hospital is a beautiful thing, but is there a potential in this abstract to reach the masses? Is it able to be implemented beyond their local microcosm to affect people regionally, nationally, internationally? If there’s potential for that, that’s usually a good abstract.”
What’s new in 2018
The more than 1,000 posters and oral presentations at HM18 is a new record, and it demonstrates the growth of hospital medicine as a scientific field, Dr. Cumbler said.
“We received a huge number of submissions,” he revealed. “We see that trend rise, year over year, and the quality has been going up as well.”
New this year is a Trainee Award category for resident and student authors. Another difference in 2018: The top 15 advances in Research and Innovations have been given a special track on day 2 of the conference, with oral presentations by the authors sharing their work.
The Vignettes are being featured in a new way as well. “We have so many incredible cases that we’re going to have a clinical vignette luncheon on two different days of the conference,” Dr. Cumbler said. “These are cases that we want to highlight, so that the experience of a hospitalist in one part of the country could help a hospitalist provide the right diagnosis for a patient on the other side of the country. There are lessons to be learned in clinical medicine, and our clinical vignettes is a fantastic way of sharing them.”
In making their selections in the different categories, the judges aimed to highlight some negative studies this year, Dr. Mathews said, which is a slight departure from previous years. “Sometimes you try something and it didn’t work, and it’s important to share that so we don’t just try the same thing over and over.”
This year, Research and Innovations abstracts will be grouped by theme, making it easier for attendees to navigate the posters. “If you’ve got a particular interest in a topic like transitions or communication, you’ll be able to find that portion of the poster session and talk to some of the people who are doing groundbreaking work in that topic,” Dr. Cumbler said.
He also noted that he expects to see a strong expression of RIV content on social media from HM18, as judges encounter some of the best and most interesting work at RIV. Dr. Mathews is similarly enthusiastic about that amplification of the work.
“I love that the conversation continues into social media platforms such as Twitter,” he said. “People are engaging back and forth, saying, ‘Hey, take a look at this poster.’ Being in a room with countless people interested in research innovations for a field that’s still relatively young – I love that there’s movement toward that.”
Exciting research
By definition, the research on display at the RIV is the best of the best. “It’s difficult to get your work accepted at a national meeting, and it’s a high honor to be selected as a finalist. The poster abstracts or oral presentations that win are always remarkable pieces of work,” Dr. Cumbler said.
Some of this year’s most exciting projects examine prediction models and scoring systems for patients with infections such as sepsis or influenza, he said. “One of the most fascinating abstracts looked at deep learning, or machine learning, to create algorithms to predict sepsis and decompensation in ways that simplistic models might not. Many of our current prediction rules are designed around simple acronyms, because they’re easy to remember: the ABCD score, the CURB-65 score. But if you looked at the source code of the Google search algorithm – not that they’d let you – you’d discover that it doesn’t translate to a simple four-variable prediction model. It’s incredibly complex; it looks at interactions between variables.”
This research attempts to move medical prediction models in that direction, Dr. Cumbler said. “Examining deep learning models, or neural networks, to help clinicians make more accurate predictions and take better care of patients – we are getting a taste of the future of clinical medicine at HM18.”
Several research projects highlighted at RIV this year examine ways to make better use of the data in the electronic health record.
“One of the pieces of research I’m particularly excited to hear more about looks at how the vast data that exists within electronic health records is actually used,” Dr. Cumbler said. “With electronic health records, we have all of the information in a patient’s record at our fingertips, yet this creates incredible new challenges for the hospitalist needing to make decisions in real time, with the limitations of our organic neural networks.” Dr. Cumbler revealed that one of the research teams sharing their work at HM18 explored how hospitalists interact with the volume of information that exists within the health record at the time of admission. “The results are pretty amazing,” he said.
Another project Dr. Cumbler found fascinating examines the impact of delivery of real-time performance data to hospitalists on their phones, and how it affected practice across a number of different performance metrics.
“We will see a project using game theory to teach quality improvement and another sharing important quality improvement work occurring at the intersection of evidence-based medicine and patient experience – like looking at how to keep patients NPO for less unnecessary time,” he said. “It makes perfect sense that we don’t want to keep people hungry in the hospital longer than we need to. It’s really interesting seeing how one team worked to make that happen and what they found.”
The importance of the RIV
The influence of the RIV program extends far beyond the conference itself; there are implications for the field of hospital medicine today and into the future.
“The RIV competition allows the field in hospital medicine to mature and evolve, so we remain cutting edge,” Dr. Mathews said. “That’s the beauty of the innovation field: Research is built off of it.”
Dr. Cumbler said that the growth and evolution of the RIV is reflective of the maturation of hospital medicine as a specialty. “It’s transitioning from a different way to organize patient care to learning more, in a scientific way, about how care can and should be delivered.”
At its heart, the RIV is really about community, he added. “The community of hospitalists is sharing knowledge, graciously and unselfishly, so that we can all improve the quality of care that we’re providing and give patients safer care, a better experience, and improved outcomes.”
Finally, RIV offers a way for hospitalists to be engaged in lifelong learning. “The presenters are teaching from their experience, and the hospitalists who come to the RIV get to leave better clinicians, researchers, and leaders as a result,” Dr. Cumbler said. “These things, to me, are about our evolution as a profession.”
Here are the ‘must-see’ sessions at HM18
Welcome to Hospital Medicine 2018, the second-happiest place in Orlando – at least for hospitalists who want to be in the know.
The 2018 education program is a ride through the diverse world of hospital medicine, with sessions ranging from clinical updates to cutting-edge techniques, communication tools, building a satisfying career, and finding your way through tangles of red tape and policy.
Two tracks new for 2018 hone in on managing alternative providers and palliative care.
The half-day NP/PA track (beginning April 11 at 7:30 a.m.) recognizes these practitioners for their crucial roles in hospital medicine care delivery. Among the discussions aimed at hospitalists: Best practices in provider utilization and collaboration; supervision vs. collaboration; and challenging situations when working with mid-level providers.
The palliative care track (also a half day, starting April 11 at 10 a.m.) recognizes the crucial role hospitalists play in optimizing end-of-life care. Sessions will help hospitalists understand that role, and guide them in managing pain and other symptoms commonly encountered during this transitional time.
As for the rest of the meeting, picking favorites is as tough as picking between Disney’s Big Thunder Railroad and Splash Mountain, said HM18 course director Dustin Smith, MD, SFHM, of Emory University, Atlanta. “We feel strongly that all offerings at the conference are ‘must-sees,’ and it’s why we offer repeat sessions of what we predict will be the most popular talks overall. Since there are so many good sessions competing for attendees at the same time, we wanted to make sure we offered these repeat sessions of common, high-yield clinical topics.”
The Repeated Sessions track is set for April 10, and runs a full day. The track includes these dynamic sessions:
- Updates in congestive heart failure: Pablo Quintero, MD; 11-11:40 a.m.
- He-who-shall-not-be-named: Updates in sepsis and critical care: Patricia Kritek, MD, EdM; 11:50 a.m.-12:30 p.m.
- Not true love’s kiss? Updates in infectious disease: John Sanders, MD, MPHTM; 2:50-3:30 p.m.
- Updates in acute coronary syndrome: Jeff Trost, MD; 3:40-4:20 p.m.
- Waiting in line for ‘It’s a Small World’ and other things we do for no reason: Tony Breu, MD, FHM; 4:30-5:10 p.m.
- “The Mad Hatter”: Updates in delirium: Ethan Cumbler, MD, FHM; 5:20-6:00 p.m.
In addition to the sepsis update in the Repeated Sessions track, Dr. Smith noted that sepsis will also be the topic of a pre-course offering (April 8, 8:15 a.m.-4:50 p.m.). “The topic of sepsis remains a hot item in hospital medicine,” he said.
“I’d also like to highlight a new pre-course offering this year – ‘Keep your finger on the pulse: Cardiology update for the hospitalist’ (April 8, 8:30 a.m.-4:50 p.m.),” he said. “Many of our pre-course offerings are carry-overs from previous years due to ongoing great success with the individual pre-courses themselves. Although we have had a cardiology pre-course in our lineup of offerings in the past, we chose to offer a freshly redesigned pre-course in cardiology this year to round out the lineup of pre-course offerings and to keep things fresh.”
The “Stump the attentive (not absent-minded) professor” sessions on clinical unknowns in the Diagnostics Reasoning track are also must-sees, Dr. Smith said. So much so, that SHM is offering two of them this year (April 9, 2:00-2:40 p.m.; 3:45-4:25 p.m.).
Dr. Smith’s codirector Kathleen Finn, MD, MPhil, SFHM, also has a few personal favorites on the education program.
“I know the talks in the ‘Seasoning your career track’ will be great,” said Dr. Finn, a hospitalist at Massachusetts General Hospital, Boston. “This new track provides mid-career hospitalists (and new hospitalists) ideas in how to continue to make their career enjoyable and stimulating. It includes talks on how to advance in a leadership position, use emotional intelligence to achieve success, prevent burnout or design your groups schedule so it doesn’t rule your life.”
The board weighs in
The 2018 HM18 line-up garnered an enthusiastic thumbs-up from The Hospitalist’s editorial advisory board. We polled these experts for their 2018 “must-see” sessions, and they responded with a selection that spans the meeting’s wide-ranging offerings.
1. Leadership essentials for success in hospital medicine (April 9, 10:35 a.m.-12:05 p.m.)
Amit Vashist, MD, MBA, FHM, system chair, hospitalist division, Mountain State Health Alliance, Virginia/Tennessee, is especially excited about this session, intended to help hospitalists assume leadership roles.
“Given the ever-expanding footprint of hospitalists inside the hospitals and beyond, and the way they are being called upon to be the drivers of an increasingly value-based care, I believe it is imperative for every hospitalist provider – regardless of being in a leadership role or not – to have a fundamental understanding of the leadership nuances pertaining specifically to hospital medicine in order to optimally leverage their skill set to drive transformational changes in the health care arena,” he said. “This primer on leadership essentials should pique the interest of the hospitalists further towards developing a deeper appreciation of some of the leadership dimensions must-haves in the realm of hospital medicine.”
Raj Sehgal, MD, FHM, clinical associate professor of medicine, University of Texas Health Sciences Center at San Antonio, pegged communication and behavioral medicine as two top picks.
2. Do you have a minute to talk? Peer-to-peer feedback (April 9, 2:50-4:20 p.m.)
“Those of us in academic settings spend a lot of time thinking about giving feedback to – and receiving feedback from – students and residents, but some of the most valuable feedback we can get is from our coworkers,” he said. “Many hospitalist groups are actively working on ways for their providers to learn from each other, such as peer observations, and this session should help in guiding some of those programs.”
3. Through the looking glass: A psychiatrist’s tricks for inpatient acute behavioral emergencies (April 10, 2:50-3:50 p.m.)
“Even for a seasoned hospitalist who never breaks a sweat treating the most acutely medically ill patients, the acutely psychotic (or agitated, or suicidal) patient can provoke significant anxiety,” Dr. Sehgal said. “The opportunity to gain another couple of ‘tools’ to add to our kit for these patients should help alleviate that feeling.”
No need for an academic meeting to be boring, said Weijen Chang, MD, SFHM, chief of pediatric hospital medicine at Baystate Children’s Hospital, Springfield, Mass.
4. Can we just stick to the “Bare Necessities”? – Things we do for no reason (April 9, 10:35-11:35 a.m.)
5. “Mirror, Mirror on the Wall”: Which articles are the fairest of them all? Top pediatric updates (April 10, 5:45-6:45 p.m.)
“I’d say Dr. Lenny Feldman’s [SFHM] ‘Things we do for no reason’ is a must-see. Lenny is a master at simplifying complex issues and communicating them in an easily understood manner, and he’s quite entertaining,” Dr. Chang said. “And of course, another must-see is Top Pediatric Updates. It is entertaining, educational, and we almost got thrown out last year for bringing beer!”
Sarah Stella, MD, FHM, a hospitalist at Denver Health, had a hard time choosing between the many interesting offerings. “There are quite a few great sessions this year that I’m interested in, but these are my top picks:”
6. Convert your everyday work into scholarship (and get it funded) (April 9, 1:35-2:35 p.m.)
“By virtue of their daily clinical and quality improvement/committee work, many hospitalists are well on their way to generating scholarship and funding, but are unsure how to make this conversion,” she said. “This workshop is a must for academic hospitalists working toward promotion who want a framework and tangible steps on how to get credit for what they are already doing.”
7. “Heigh ho, heigh ho,” it’s off to changing roles mid-career we go (April 11, 8:20-9:00 a.m.)
“Part of what attracts many of us to hospital medicine in the first place is the versatility of what we do and the ability to diversify based on our interests. I think this is a must-see for mid-career hospitalists like myself, or really any hospitalist dreaming of reinventing oneself.”
8. Winning hearts and minds at the bedside: Battling unconscious bias through cultural humility (April 11, 9:10-9:50 a.m.)
“Recognizing and confronting our implicit biases and how they affect patient-physician interactions is hard but incredibly important work,” Dr. Stella said. “I’ll definitely be attending this session by Aziz Ansari, DO, SFHM, to learn how to improve my relationship (and hence outcomes) with my patients.”
Harry (Hyung) Cho, MD, FHM, assistant professor of medicine and director of quality, safety, and value, division of hospital medicine, Mount Sinai Hospital, New York, had some diverse choices.
9. Being female in hospital medicine: Overcoming individual and institutional barriers in the workplace (April 9, 12:40-2:15 p.m.)
“This is a very timely, very important topic in the news and I think it will draw a lot of people,” he said.
10. Every patient tells a story and the art of diagnosis (April 9, 2:55-3:35 p.m.)
“The presenter is Dr. Lisa Sanders, who writes the ‘Diagnosis’ column for the New York Times and is a Yale University faculty member. She’s a great speaker and, incidentally, was a consultant on the TV show, ‘House, MD.’ ”
Raman Palabindala, MD, FHM, a hospitalist at the University of Mississippi Medical Center, Jackson, thinks the most important session at HM18 is the annual update.
11. Update in hospital medicine (April 10, 1:40-2:40 p.m.)“Almost every year, this is the most high energy presentation, and I don’t think I ever missed this session, no matter who is the presenter is,” he said. “As physicians, I think we need this update every year, and this is the best single hour where we can learn a lot as a hospitalist related to hospital medicine. This is the most concentrated extract of the entire meeting. What I learned about the behind scenes efforts up to 50-100 hours of work – why not we take advantage of this session.”
Lonika Sood, MD, FHM of the department of hospital medicine, Aurora BayCare Medical Center, Green Bay, Wis., has a passion for both leadership and scholarship, and her choices reflect that interest.
12. How to write a winning abstract (April 11, 7:30-8:30 a.m.)
13. Leadership positions in medical education: How to break into the field (April 11, 11:40 a.m.-12:20 p.m.)
14. Serious illness communication: A skills-based workshop (April 11, 8:00-9:30 a.m.)
“I would recommend all of those, especially for early-career hospitalists. And, having enjoyed and learned a lot from the workshops at HM17, I would highly recommend checking out a few that will help polish your communications – a much-needed skill in hospital medicine,” she said.
Finally, don’t just pick up another embroidered mouse ear hat on your way out. The best HM18 souvenir is taking back the knowledge you gained and – as Dr. Sood said – there’s a session for that.
15. How to bring the things you learn at SHM back to your institution: Advocating for high value care on hospital committees (April 11, 8:00-9:30 a.m.).
For more information on the HM18 education sessions, check the latest version of the conference schedule at http://shmannualconference.org/conference-schedule.
Welcome to Hospital Medicine 2018, the second-happiest place in Orlando – at least for hospitalists who want to be in the know.
The 2018 education program is a ride through the diverse world of hospital medicine, with sessions ranging from clinical updates to cutting-edge techniques, communication tools, building a satisfying career, and finding your way through tangles of red tape and policy.
Two tracks new for 2018 hone in on managing alternative providers and palliative care.
The half-day NP/PA track (beginning April 11 at 7:30 a.m.) recognizes these practitioners for their crucial roles in hospital medicine care delivery. Among the discussions aimed at hospitalists: Best practices in provider utilization and collaboration; supervision vs. collaboration; and challenging situations when working with mid-level providers.
The palliative care track (also a half day, starting April 11 at 10 a.m.) recognizes the crucial role hospitalists play in optimizing end-of-life care. Sessions will help hospitalists understand that role, and guide them in managing pain and other symptoms commonly encountered during this transitional time.
As for the rest of the meeting, picking favorites is as tough as picking between Disney’s Big Thunder Railroad and Splash Mountain, said HM18 course director Dustin Smith, MD, SFHM, of Emory University, Atlanta. “We feel strongly that all offerings at the conference are ‘must-sees,’ and it’s why we offer repeat sessions of what we predict will be the most popular talks overall. Since there are so many good sessions competing for attendees at the same time, we wanted to make sure we offered these repeat sessions of common, high-yield clinical topics.”
The Repeated Sessions track is set for April 10, and runs a full day. The track includes these dynamic sessions:
- Updates in congestive heart failure: Pablo Quintero, MD; 11-11:40 a.m.
- He-who-shall-not-be-named: Updates in sepsis and critical care: Patricia Kritek, MD, EdM; 11:50 a.m.-12:30 p.m.
- Not true love’s kiss? Updates in infectious disease: John Sanders, MD, MPHTM; 2:50-3:30 p.m.
- Updates in acute coronary syndrome: Jeff Trost, MD; 3:40-4:20 p.m.
- Waiting in line for ‘It’s a Small World’ and other things we do for no reason: Tony Breu, MD, FHM; 4:30-5:10 p.m.
- “The Mad Hatter”: Updates in delirium: Ethan Cumbler, MD, FHM; 5:20-6:00 p.m.
In addition to the sepsis update in the Repeated Sessions track, Dr. Smith noted that sepsis will also be the topic of a pre-course offering (April 8, 8:15 a.m.-4:50 p.m.). “The topic of sepsis remains a hot item in hospital medicine,” he said.
“I’d also like to highlight a new pre-course offering this year – ‘Keep your finger on the pulse: Cardiology update for the hospitalist’ (April 8, 8:30 a.m.-4:50 p.m.),” he said. “Many of our pre-course offerings are carry-overs from previous years due to ongoing great success with the individual pre-courses themselves. Although we have had a cardiology pre-course in our lineup of offerings in the past, we chose to offer a freshly redesigned pre-course in cardiology this year to round out the lineup of pre-course offerings and to keep things fresh.”
The “Stump the attentive (not absent-minded) professor” sessions on clinical unknowns in the Diagnostics Reasoning track are also must-sees, Dr. Smith said. So much so, that SHM is offering two of them this year (April 9, 2:00-2:40 p.m.; 3:45-4:25 p.m.).
Dr. Smith’s codirector Kathleen Finn, MD, MPhil, SFHM, also has a few personal favorites on the education program.
“I know the talks in the ‘Seasoning your career track’ will be great,” said Dr. Finn, a hospitalist at Massachusetts General Hospital, Boston. “This new track provides mid-career hospitalists (and new hospitalists) ideas in how to continue to make their career enjoyable and stimulating. It includes talks on how to advance in a leadership position, use emotional intelligence to achieve success, prevent burnout or design your groups schedule so it doesn’t rule your life.”
The board weighs in
The 2018 HM18 line-up garnered an enthusiastic thumbs-up from The Hospitalist’s editorial advisory board. We polled these experts for their 2018 “must-see” sessions, and they responded with a selection that spans the meeting’s wide-ranging offerings.
1. Leadership essentials for success in hospital medicine (April 9, 10:35 a.m.-12:05 p.m.)
Amit Vashist, MD, MBA, FHM, system chair, hospitalist division, Mountain State Health Alliance, Virginia/Tennessee, is especially excited about this session, intended to help hospitalists assume leadership roles.
“Given the ever-expanding footprint of hospitalists inside the hospitals and beyond, and the way they are being called upon to be the drivers of an increasingly value-based care, I believe it is imperative for every hospitalist provider – regardless of being in a leadership role or not – to have a fundamental understanding of the leadership nuances pertaining specifically to hospital medicine in order to optimally leverage their skill set to drive transformational changes in the health care arena,” he said. “This primer on leadership essentials should pique the interest of the hospitalists further towards developing a deeper appreciation of some of the leadership dimensions must-haves in the realm of hospital medicine.”
Raj Sehgal, MD, FHM, clinical associate professor of medicine, University of Texas Health Sciences Center at San Antonio, pegged communication and behavioral medicine as two top picks.
2. Do you have a minute to talk? Peer-to-peer feedback (April 9, 2:50-4:20 p.m.)
“Those of us in academic settings spend a lot of time thinking about giving feedback to – and receiving feedback from – students and residents, but some of the most valuable feedback we can get is from our coworkers,” he said. “Many hospitalist groups are actively working on ways for their providers to learn from each other, such as peer observations, and this session should help in guiding some of those programs.”
3. Through the looking glass: A psychiatrist’s tricks for inpatient acute behavioral emergencies (April 10, 2:50-3:50 p.m.)
“Even for a seasoned hospitalist who never breaks a sweat treating the most acutely medically ill patients, the acutely psychotic (or agitated, or suicidal) patient can provoke significant anxiety,” Dr. Sehgal said. “The opportunity to gain another couple of ‘tools’ to add to our kit for these patients should help alleviate that feeling.”
No need for an academic meeting to be boring, said Weijen Chang, MD, SFHM, chief of pediatric hospital medicine at Baystate Children’s Hospital, Springfield, Mass.
4. Can we just stick to the “Bare Necessities”? – Things we do for no reason (April 9, 10:35-11:35 a.m.)
5. “Mirror, Mirror on the Wall”: Which articles are the fairest of them all? Top pediatric updates (April 10, 5:45-6:45 p.m.)
“I’d say Dr. Lenny Feldman’s [SFHM] ‘Things we do for no reason’ is a must-see. Lenny is a master at simplifying complex issues and communicating them in an easily understood manner, and he’s quite entertaining,” Dr. Chang said. “And of course, another must-see is Top Pediatric Updates. It is entertaining, educational, and we almost got thrown out last year for bringing beer!”
Sarah Stella, MD, FHM, a hospitalist at Denver Health, had a hard time choosing between the many interesting offerings. “There are quite a few great sessions this year that I’m interested in, but these are my top picks:”
6. Convert your everyday work into scholarship (and get it funded) (April 9, 1:35-2:35 p.m.)
“By virtue of their daily clinical and quality improvement/committee work, many hospitalists are well on their way to generating scholarship and funding, but are unsure how to make this conversion,” she said. “This workshop is a must for academic hospitalists working toward promotion who want a framework and tangible steps on how to get credit for what they are already doing.”
7. “Heigh ho, heigh ho,” it’s off to changing roles mid-career we go (April 11, 8:20-9:00 a.m.)
“Part of what attracts many of us to hospital medicine in the first place is the versatility of what we do and the ability to diversify based on our interests. I think this is a must-see for mid-career hospitalists like myself, or really any hospitalist dreaming of reinventing oneself.”
8. Winning hearts and minds at the bedside: Battling unconscious bias through cultural humility (April 11, 9:10-9:50 a.m.)
“Recognizing and confronting our implicit biases and how they affect patient-physician interactions is hard but incredibly important work,” Dr. Stella said. “I’ll definitely be attending this session by Aziz Ansari, DO, SFHM, to learn how to improve my relationship (and hence outcomes) with my patients.”
Harry (Hyung) Cho, MD, FHM, assistant professor of medicine and director of quality, safety, and value, division of hospital medicine, Mount Sinai Hospital, New York, had some diverse choices.
9. Being female in hospital medicine: Overcoming individual and institutional barriers in the workplace (April 9, 12:40-2:15 p.m.)
“This is a very timely, very important topic in the news and I think it will draw a lot of people,” he said.
10. Every patient tells a story and the art of diagnosis (April 9, 2:55-3:35 p.m.)
“The presenter is Dr. Lisa Sanders, who writes the ‘Diagnosis’ column for the New York Times and is a Yale University faculty member. She’s a great speaker and, incidentally, was a consultant on the TV show, ‘House, MD.’ ”
Raman Palabindala, MD, FHM, a hospitalist at the University of Mississippi Medical Center, Jackson, thinks the most important session at HM18 is the annual update.
11. Update in hospital medicine (April 10, 1:40-2:40 p.m.)“Almost every year, this is the most high energy presentation, and I don’t think I ever missed this session, no matter who is the presenter is,” he said. “As physicians, I think we need this update every year, and this is the best single hour where we can learn a lot as a hospitalist related to hospital medicine. This is the most concentrated extract of the entire meeting. What I learned about the behind scenes efforts up to 50-100 hours of work – why not we take advantage of this session.”
Lonika Sood, MD, FHM of the department of hospital medicine, Aurora BayCare Medical Center, Green Bay, Wis., has a passion for both leadership and scholarship, and her choices reflect that interest.
12. How to write a winning abstract (April 11, 7:30-8:30 a.m.)
13. Leadership positions in medical education: How to break into the field (April 11, 11:40 a.m.-12:20 p.m.)
14. Serious illness communication: A skills-based workshop (April 11, 8:00-9:30 a.m.)
“I would recommend all of those, especially for early-career hospitalists. And, having enjoyed and learned a lot from the workshops at HM17, I would highly recommend checking out a few that will help polish your communications – a much-needed skill in hospital medicine,” she said.
Finally, don’t just pick up another embroidered mouse ear hat on your way out. The best HM18 souvenir is taking back the knowledge you gained and – as Dr. Sood said – there’s a session for that.
15. How to bring the things you learn at SHM back to your institution: Advocating for high value care on hospital committees (April 11, 8:00-9:30 a.m.).
For more information on the HM18 education sessions, check the latest version of the conference schedule at http://shmannualconference.org/conference-schedule.
Welcome to Hospital Medicine 2018, the second-happiest place in Orlando – at least for hospitalists who want to be in the know.
The 2018 education program is a ride through the diverse world of hospital medicine, with sessions ranging from clinical updates to cutting-edge techniques, communication tools, building a satisfying career, and finding your way through tangles of red tape and policy.
Two tracks new for 2018 hone in on managing alternative providers and palliative care.
The half-day NP/PA track (beginning April 11 at 7:30 a.m.) recognizes these practitioners for their crucial roles in hospital medicine care delivery. Among the discussions aimed at hospitalists: Best practices in provider utilization and collaboration; supervision vs. collaboration; and challenging situations when working with mid-level providers.
The palliative care track (also a half day, starting April 11 at 10 a.m.) recognizes the crucial role hospitalists play in optimizing end-of-life care. Sessions will help hospitalists understand that role, and guide them in managing pain and other symptoms commonly encountered during this transitional time.
As for the rest of the meeting, picking favorites is as tough as picking between Disney’s Big Thunder Railroad and Splash Mountain, said HM18 course director Dustin Smith, MD, SFHM, of Emory University, Atlanta. “We feel strongly that all offerings at the conference are ‘must-sees,’ and it’s why we offer repeat sessions of what we predict will be the most popular talks overall. Since there are so many good sessions competing for attendees at the same time, we wanted to make sure we offered these repeat sessions of common, high-yield clinical topics.”
The Repeated Sessions track is set for April 10, and runs a full day. The track includes these dynamic sessions:
- Updates in congestive heart failure: Pablo Quintero, MD; 11-11:40 a.m.
- He-who-shall-not-be-named: Updates in sepsis and critical care: Patricia Kritek, MD, EdM; 11:50 a.m.-12:30 p.m.
- Not true love’s kiss? Updates in infectious disease: John Sanders, MD, MPHTM; 2:50-3:30 p.m.
- Updates in acute coronary syndrome: Jeff Trost, MD; 3:40-4:20 p.m.
- Waiting in line for ‘It’s a Small World’ and other things we do for no reason: Tony Breu, MD, FHM; 4:30-5:10 p.m.
- “The Mad Hatter”: Updates in delirium: Ethan Cumbler, MD, FHM; 5:20-6:00 p.m.
In addition to the sepsis update in the Repeated Sessions track, Dr. Smith noted that sepsis will also be the topic of a pre-course offering (April 8, 8:15 a.m.-4:50 p.m.). “The topic of sepsis remains a hot item in hospital medicine,” he said.
“I’d also like to highlight a new pre-course offering this year – ‘Keep your finger on the pulse: Cardiology update for the hospitalist’ (April 8, 8:30 a.m.-4:50 p.m.),” he said. “Many of our pre-course offerings are carry-overs from previous years due to ongoing great success with the individual pre-courses themselves. Although we have had a cardiology pre-course in our lineup of offerings in the past, we chose to offer a freshly redesigned pre-course in cardiology this year to round out the lineup of pre-course offerings and to keep things fresh.”
The “Stump the attentive (not absent-minded) professor” sessions on clinical unknowns in the Diagnostics Reasoning track are also must-sees, Dr. Smith said. So much so, that SHM is offering two of them this year (April 9, 2:00-2:40 p.m.; 3:45-4:25 p.m.).
Dr. Smith’s codirector Kathleen Finn, MD, MPhil, SFHM, also has a few personal favorites on the education program.
“I know the talks in the ‘Seasoning your career track’ will be great,” said Dr. Finn, a hospitalist at Massachusetts General Hospital, Boston. “This new track provides mid-career hospitalists (and new hospitalists) ideas in how to continue to make their career enjoyable and stimulating. It includes talks on how to advance in a leadership position, use emotional intelligence to achieve success, prevent burnout or design your groups schedule so it doesn’t rule your life.”
The board weighs in
The 2018 HM18 line-up garnered an enthusiastic thumbs-up from The Hospitalist’s editorial advisory board. We polled these experts for their 2018 “must-see” sessions, and they responded with a selection that spans the meeting’s wide-ranging offerings.
1. Leadership essentials for success in hospital medicine (April 9, 10:35 a.m.-12:05 p.m.)
Amit Vashist, MD, MBA, FHM, system chair, hospitalist division, Mountain State Health Alliance, Virginia/Tennessee, is especially excited about this session, intended to help hospitalists assume leadership roles.
“Given the ever-expanding footprint of hospitalists inside the hospitals and beyond, and the way they are being called upon to be the drivers of an increasingly value-based care, I believe it is imperative for every hospitalist provider – regardless of being in a leadership role or not – to have a fundamental understanding of the leadership nuances pertaining specifically to hospital medicine in order to optimally leverage their skill set to drive transformational changes in the health care arena,” he said. “This primer on leadership essentials should pique the interest of the hospitalists further towards developing a deeper appreciation of some of the leadership dimensions must-haves in the realm of hospital medicine.”
Raj Sehgal, MD, FHM, clinical associate professor of medicine, University of Texas Health Sciences Center at San Antonio, pegged communication and behavioral medicine as two top picks.
2. Do you have a minute to talk? Peer-to-peer feedback (April 9, 2:50-4:20 p.m.)
“Those of us in academic settings spend a lot of time thinking about giving feedback to – and receiving feedback from – students and residents, but some of the most valuable feedback we can get is from our coworkers,” he said. “Many hospitalist groups are actively working on ways for their providers to learn from each other, such as peer observations, and this session should help in guiding some of those programs.”
3. Through the looking glass: A psychiatrist’s tricks for inpatient acute behavioral emergencies (April 10, 2:50-3:50 p.m.)
“Even for a seasoned hospitalist who never breaks a sweat treating the most acutely medically ill patients, the acutely psychotic (or agitated, or suicidal) patient can provoke significant anxiety,” Dr. Sehgal said. “The opportunity to gain another couple of ‘tools’ to add to our kit for these patients should help alleviate that feeling.”
No need for an academic meeting to be boring, said Weijen Chang, MD, SFHM, chief of pediatric hospital medicine at Baystate Children’s Hospital, Springfield, Mass.
4. Can we just stick to the “Bare Necessities”? – Things we do for no reason (April 9, 10:35-11:35 a.m.)
5. “Mirror, Mirror on the Wall”: Which articles are the fairest of them all? Top pediatric updates (April 10, 5:45-6:45 p.m.)
“I’d say Dr. Lenny Feldman’s [SFHM] ‘Things we do for no reason’ is a must-see. Lenny is a master at simplifying complex issues and communicating them in an easily understood manner, and he’s quite entertaining,” Dr. Chang said. “And of course, another must-see is Top Pediatric Updates. It is entertaining, educational, and we almost got thrown out last year for bringing beer!”
Sarah Stella, MD, FHM, a hospitalist at Denver Health, had a hard time choosing between the many interesting offerings. “There are quite a few great sessions this year that I’m interested in, but these are my top picks:”
6. Convert your everyday work into scholarship (and get it funded) (April 9, 1:35-2:35 p.m.)
“By virtue of their daily clinical and quality improvement/committee work, many hospitalists are well on their way to generating scholarship and funding, but are unsure how to make this conversion,” she said. “This workshop is a must for academic hospitalists working toward promotion who want a framework and tangible steps on how to get credit for what they are already doing.”
7. “Heigh ho, heigh ho,” it’s off to changing roles mid-career we go (April 11, 8:20-9:00 a.m.)
“Part of what attracts many of us to hospital medicine in the first place is the versatility of what we do and the ability to diversify based on our interests. I think this is a must-see for mid-career hospitalists like myself, or really any hospitalist dreaming of reinventing oneself.”
8. Winning hearts and minds at the bedside: Battling unconscious bias through cultural humility (April 11, 9:10-9:50 a.m.)
“Recognizing and confronting our implicit biases and how they affect patient-physician interactions is hard but incredibly important work,” Dr. Stella said. “I’ll definitely be attending this session by Aziz Ansari, DO, SFHM, to learn how to improve my relationship (and hence outcomes) with my patients.”
Harry (Hyung) Cho, MD, FHM, assistant professor of medicine and director of quality, safety, and value, division of hospital medicine, Mount Sinai Hospital, New York, had some diverse choices.
9. Being female in hospital medicine: Overcoming individual and institutional barriers in the workplace (April 9, 12:40-2:15 p.m.)
“This is a very timely, very important topic in the news and I think it will draw a lot of people,” he said.
10. Every patient tells a story and the art of diagnosis (April 9, 2:55-3:35 p.m.)
“The presenter is Dr. Lisa Sanders, who writes the ‘Diagnosis’ column for the New York Times and is a Yale University faculty member. She’s a great speaker and, incidentally, was a consultant on the TV show, ‘House, MD.’ ”
Raman Palabindala, MD, FHM, a hospitalist at the University of Mississippi Medical Center, Jackson, thinks the most important session at HM18 is the annual update.
11. Update in hospital medicine (April 10, 1:40-2:40 p.m.)“Almost every year, this is the most high energy presentation, and I don’t think I ever missed this session, no matter who is the presenter is,” he said. “As physicians, I think we need this update every year, and this is the best single hour where we can learn a lot as a hospitalist related to hospital medicine. This is the most concentrated extract of the entire meeting. What I learned about the behind scenes efforts up to 50-100 hours of work – why not we take advantage of this session.”
Lonika Sood, MD, FHM of the department of hospital medicine, Aurora BayCare Medical Center, Green Bay, Wis., has a passion for both leadership and scholarship, and her choices reflect that interest.
12. How to write a winning abstract (April 11, 7:30-8:30 a.m.)
13. Leadership positions in medical education: How to break into the field (April 11, 11:40 a.m.-12:20 p.m.)
14. Serious illness communication: A skills-based workshop (April 11, 8:00-9:30 a.m.)
“I would recommend all of those, especially for early-career hospitalists. And, having enjoyed and learned a lot from the workshops at HM17, I would highly recommend checking out a few that will help polish your communications – a much-needed skill in hospital medicine,” she said.
Finally, don’t just pick up another embroidered mouse ear hat on your way out. The best HM18 souvenir is taking back the knowledge you gained and – as Dr. Sood said – there’s a session for that.
15. How to bring the things you learn at SHM back to your institution: Advocating for high value care on hospital committees (April 11, 8:00-9:30 a.m.).
For more information on the HM18 education sessions, check the latest version of the conference schedule at http://shmannualconference.org/conference-schedule.

















