User login
CASE 1
Lisa G, 31 years old, gravida 0, complains of severe dysmenorrhea that began when she discontinued an oral contraceptive (OC) one year ago. Prior to stopping the OC, she had been taking an OC without interruption since she was 28, during which time she continued to have moderate symptoms of dysmenorrhea. Before taking an OC, the patient had a trial of an etonogestrel implant, which was removed because of irregular bleeding, and depot medroxyprogesterone acetate (MPA) injection, which she discontinued because of associated weight gain and fatigue.
Ms. G is not sexually active and doesn’t want to start a family at this time, but is interested in having a diagnosis. She has no other medical problems, no surgical history, and no history of sexually transmitted infection. She reports that her mother and sister had endometriosis, including pain that resolved after definitive treatment.
Ms. G reports menstrual cycles that are exquisitely painful and occur regularly (every 28 days for 4 or 5 days), with a moderate volume of bleeding that requires a regular-size tampon change every 4 to 6 hours. She reports crampy abdominal pain as 10, on a scale of one to 10; dyschezia (without hematochezia); and generalized achy abdominal pain that is continuous during menses. Pain is partially controlled by ibuprofen, 800 mg every 8 hours. Ms. G also describes gastrointestinal symptoms of bloating, constipation preceding her menstrual cycle, diarrhea during her menses, and occasionally nausea and vomiting with the severe pain.
On examination (which is not performed during menses), Ms. G appears well and is not in acute distress. Abdominal examination is benign. There is no tenderness to palpation or distension; bowel sounds are normal. Pelvic examination reveals mild tenderness upon palpation of a small and mobile uterus. Rectal examination is normal. She has no signs of hyperandrogenism (eg, male-pattern body hair, central obesity).
CASE 2
Rhonda M, 42 years old, gravida 3, para 3003, reports continuous pelvic pain for 7 years that is exacerbated by defecation, intercourse, and insertion of a tampon. She has a low level of dull baseline pain (3, on scale of one to 10) that occasionally spikes up to sharp, knifelike pain (10 on the pain scale), which, she says, brings her to tears. Ms. M describes the pain as “deep inside,” central in her pelvis, and radiating to the left and right, particularly during pain flares.
The patient’s 3 children were born by spontaneous vaginal delivery; however, she recalls that her youngest son was born via a traumatic vaginal delivery 8 years ago (he “got stuck coming out,” she reports). The only other component of Ms. M’s medical history is an anxiety disorder, for which she takes citalopram. She has a family history of cervical cancer.
Ms. M’s past diagnostic work-up for pelvic pain includes pelvic ultrasonography, endometrial biopsy, Pap smear, and diagnostic laparoscopy—all normal. She had a negative gastrointestinal work-up, including upper- and lower-tract endoscopy. Medical therapy, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), did not provide significant relief of pain.
Despite the negative work-up, Ms. M is still concerned that the pain might be related to cancer. With her family history of cervical cancer, she says that she does not want to “miss anything.”
Ms. M is thin and appears anxious. The abdomen is mildly and diffusely tender to palpation with normal bowel sounds and no distension. Pelvic examination reveals some hyperesthesia upon single-digit palpation of the pelvic floor. Placement of the speculum is difficult because of discomfort.
How would you proceed with the care of these patients?
What is chronic pelvic pain? Why is management such a challenge?
Chronic pelvic pain (CPP) is defined as chronic or intermittent cyclic or noncyclic pelvic pain lasting longer than 6 months, localized to the pelvis, diminishing a woman’s quality of life, and requiring medical intervention.1 It’s estimated that CPP affects as many as 15% of women of reproductive age in the United States each year, at a cost to the health care system of approximately $2 billion annually.2,3
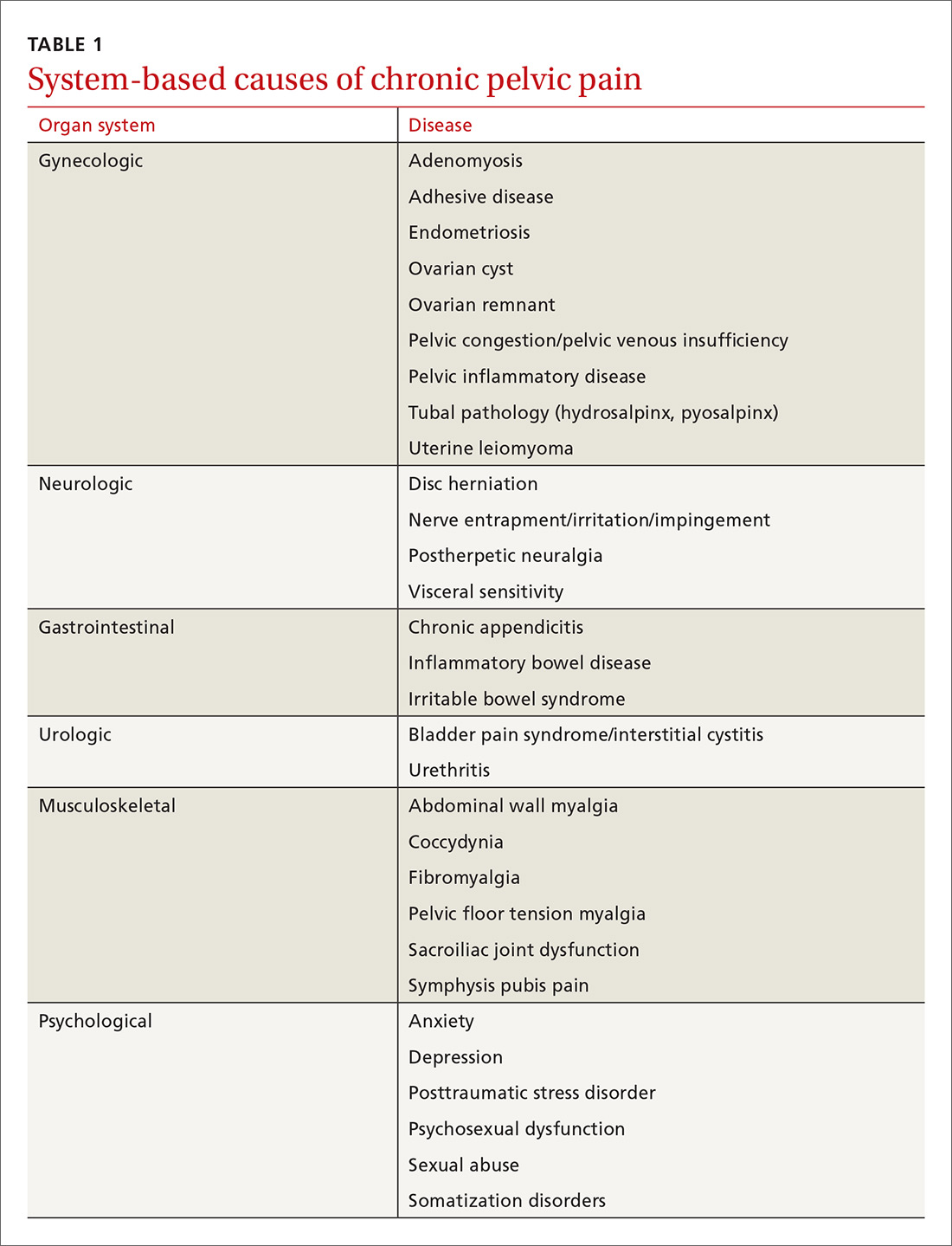
CPP can result from abnormal pain responses from multiple body systems, including gynecologic conditions such as endometriosis. Notably, a nongynecologic cause is more often the major pain generator, without significant identifiable pathology (TABLE 1). Like all chronic pain disorders, CPP can also result in central sensitization of the nervous system, altering how pain is processed at the level of the pain matrix in the brain.4
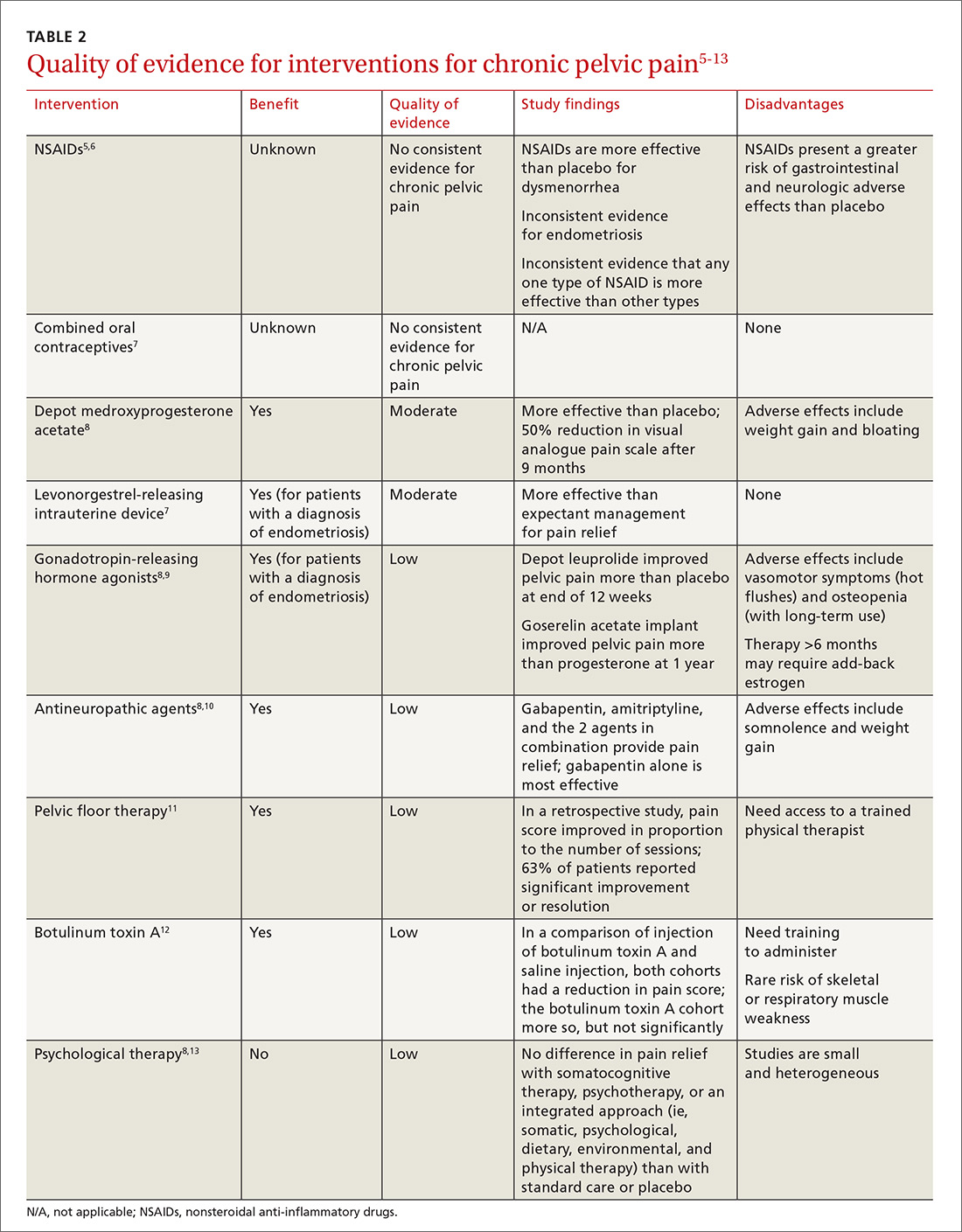
This article reviews the limited evidence for treating CPP and offers recommendations for the primary care physician on providing symptomatic relief in the absence of diagnosed pathology (TABLE 25-13).
Treatment
Analgesics
NSAIDs are frequently used as first-line treatment for any kind of pain, including CPP. There is some evidence of benefit from NSAIDs, compared to placebo, in cyclic CPP secondary to dysmenorrhea and endometriosis;5,6 however, evidence of effectiveness in noncyclic CPP is absent. Because of the low cost and availability of NSAIDs, a trial is reasonable as a first-line intervention, particularly in CPP suspected to be endometriosis or of musculoskeletal origin. NSAIDs can cause adverse effects, including nausea, vomiting, headache, and drowsiness in 11% to 14% of women, although these agents are generally well-tolerated on a short-term basis.5
Opioids bind to opioid receptors in the central and peripheral nervous systems, resulting in an analgesic effect. Guidelines issued in 2016 by the Centers for Disease Control and Prevention recommend safer prescribing through careful evaluation of the risks and benefits of opioids for pain not caused by cancer and for palliation as part of end-of-life care.14
The risks of opioid use are well known in the medical community; they include tolerance, physical dependence, misuse, and death, in addition to common adverse effects such as nausea and vomiting, itching, constipation, and fatigue.14,15 Because of those risks and limited long-term benefit in nonmalignant pain disorders, opioid therapy for CPP should be avoided.14 For patients already taking an opioid, discuss a strategy for weaning and, if possible, provide home naloxone therapy in the event of accidental overdose.14
Hormonal therapy
Hormonal therapies are the most common nonsurgical treatment of noncyclic CPP, with or without a definitive diagnosis of endometriosis, in reproductive-age women with CPP.
Combined OCs, despite a lack of quality evidence, are frequently the first hormonal treatment tried in both cyclic and noncyclic CPP. A low-dosage OC may decrease cyclic pain in endometriosis, although it can increase irregular bleeding and nausea.16 As many as 53% of women with CPP reported having undergone a trial of an OC for endometriosis, despite the absence of consistent evidence showing effectiveness in CPP.17
Depot MPA, in trials, decreased pain more than placebo. It can be tried as a treatment, but its use is often limited because of adverse effects, such as weight gain and bloating.8
A trial of a levonorgestrel-releasing intrauterine device (LNG-IUD) is supported by moderate-quality evidence for women whose CPP is thought to be a symptom of endometriosis or to have another uterine origin.7
Gonadotropin-releasing hormone agonists, such as depot leuprolide and goserelin acetate implant, may be considered in a woman with a diagnosis of endometriosis whose pelvic pain is not alleviated by MPA or an LNG-IUD.9
Nonhormonal therapies
CPP shares pain mechanisms with other pain syndromes, such as neuropathic pain. Antineuropathic medications, such as gabapentin and pregabalin, may, therefore, provide benefit. These medications also produce improvement in pain disorders of the musculoskeletal system, which may contribute to their analgesic effect.18
Gabapentin and amitriptyline have been studied in CPP; both were found successful in decreasing perceived pain. Of note, patients who received gabapentin, a gamma-aminobutyric acid analogue, with or without amitriptyline, had more pain relief than those treated with amitriptyline alone.10 Adverse effects of these medications may limit their use (TABLE 319-25).
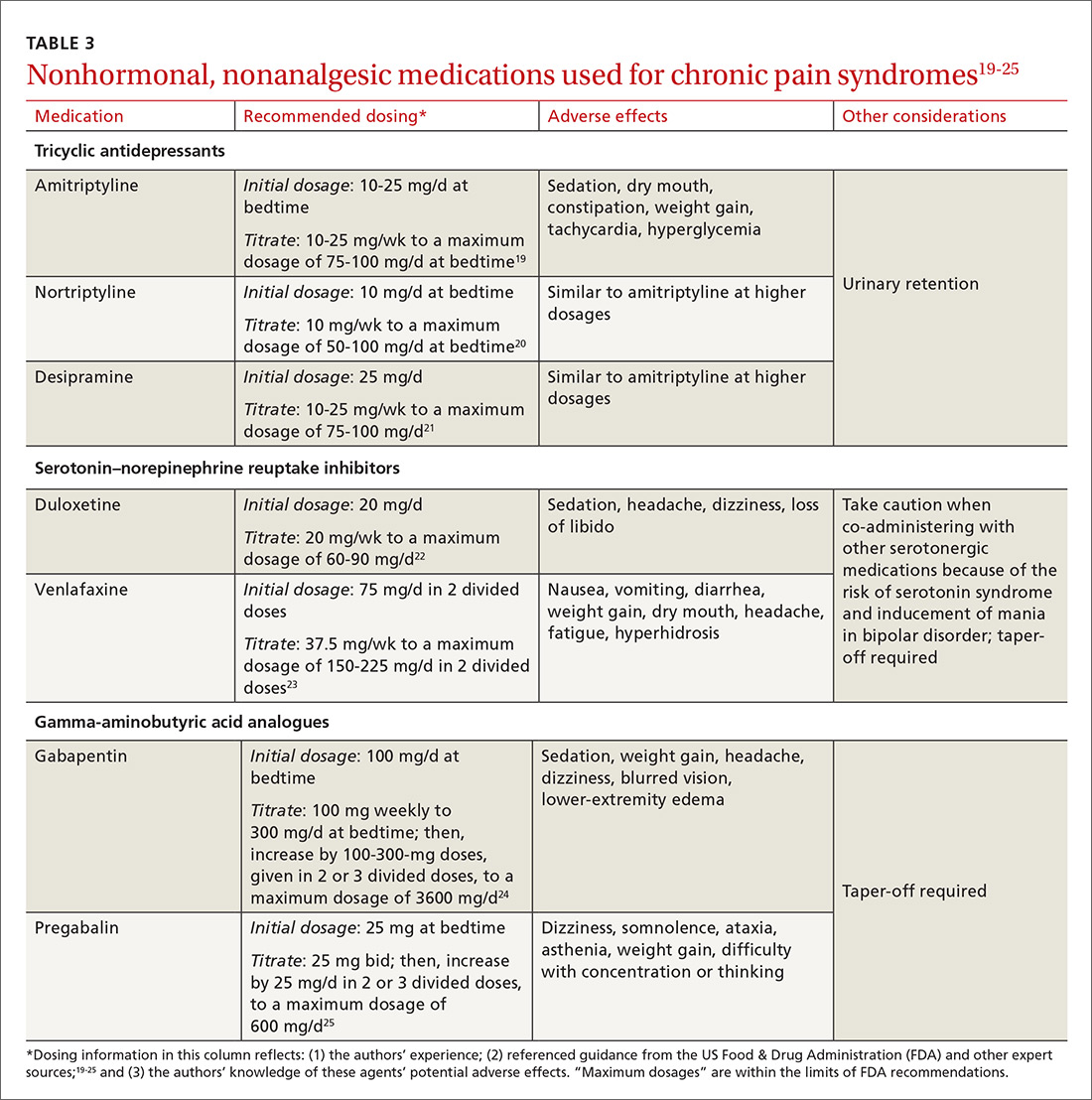
Tricyclic antidepressants are well-supported, effective treatments for chronic pain through the central increase of norepinephrine. Beginning at a low dosage to diminish adverse effects (TABLE 319-25) and increasing the dosage slowly to an effective level may increase adherence. A trial of at least 6 to 8 weeks, at a moderate dosage, is recommended before discontinuing the medication. Although amitriptyline has the most evidence for value in the management of CPP disorders,10 second-generation tricyclic antidepressants nortriptyline and desipramine have also been used for pain control, and may be better tolerated.
Duloxetine and venlafaxine—serotonin–norepinephrine reuptake inhibitors—increase serotonin in addition to norepinephrine, which is believed to result in pain control. Although a systematic review of trials of duloxetine for chronic pain showed some improvement in diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis, the review excluded CPP in its analysis.26
In our opinion, a selective neurotransmitter reuptake inhibitor can be attempted to diminish the central pain sensitization of CPP. As with all drugs that increase the availability of serotonin, serotonin syndrome is a rare risk. Additionally, when stopping duloxetine, a prolonged taper may be required.
Pelvic floor dysfunction therapy
Pelvic floor dysfunction of the musculature within the bony pelvis may contribute to, or cause, CPP. The pelvic floor musculature may be hypertonic or hypotonic, and trigger points may exist. Despite the frequency of pelvic floor dysfunction, detailed examination of the pelvic floor is not routinely performed during a pelvic exam.
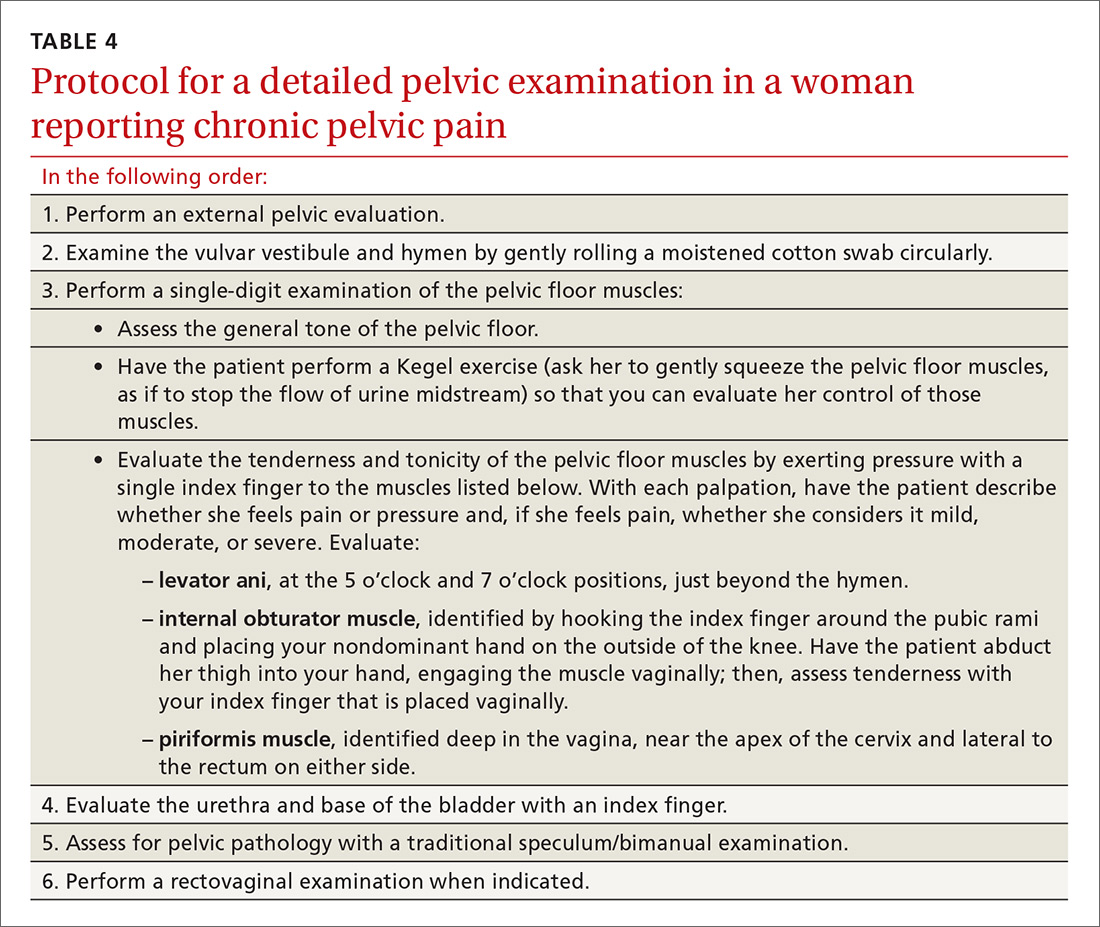
Because of the high prevalence of pelvic floor dysfunction in women with CPP, evaluation of the pelvic floor muscles is warranted.27 (A protocol for this evaluation is detailed in TABLE 4.) Pelvic dynamometry may indicate muscle spasm or chronic tension; palpation of the pelvic floor during the exam can also identify a pain generator.
Although it might be difficult to distinguish pelvic floor myofascial pain as the primary or secondary cause of pain, pelvic floor physical therapy may clarify the role of the pelvic floor response (depending on the patient’s clinical exam and history). A low-quality retrospective case study on pelvic floor physical therapy reported significant improvement in pain that was proportional to the number of sessions completed.11 Trigger-point injections and injections of botulinum toxin A have been used with reported improvement in the pelvic floor pain profile, and there is evidence to support the benefit of such injections in pelvic muscle dysfunction.12
Psychotherapy
Cognitive behavioral therapy (CBT) is well established as an option to manage a patient’s response to pain, including teaching coping skills for a chronic pain disorder and pain flares. Evidence supports using CBT or mindfulness techniques over usual care in reducing the intensity of pain in chronic low back pain,28 and may be helpful in CPP. Patients with CPP who received 10 treatments of Mensendieck somatocognitive therapy (a mind–body therapy technique popular in Europe) over 90 days, compared with standard treatment alone, demonstrated improvement in pain, motor function, and psychological distress that persisted 9 months after treatment.13
Lifestyle changes, complementary and alternative therapies
Although medical and nonpharmacotherapeutic treatments are often important in the
Diet modifications may relieve pain in some women with CPP. Although a systematic review in 2011 highlighted the lack of data available for the efficacy of dietary therapies for treating CPP, the authors did present data that a diet rich in antioxidants might alleviate pain sysmptoms.29 Also, a gluten-free diet might reduce the symptoms of pain related to endometriosis and, thus, improve physical functioning, among other health domains.30
Exercise can be an important factor in the management of CPP, as with other chronic pain syndromes. In functional pain syndromes, the addition or maintenance of an exercise program has been shown to decrease the amount of pain medications required, improve depressive symptoms, increase energy, and decrease stress. Exercise also improves sleep quality and one’s ability to cope with pain.31
Yoga provides a good balance of aerobic and muscle-building activity and, in the authors’ experience, is tolerated by most women with CPP.
Acupuncture has limited evidence in the treatment of pelvic pain in women. Of the available studies, most are limited to pain related to endometriosis.32
Sleep hygiene may be an important consideration in managing CPP. Sleep disturbances are reported in more than 80% of women with CPP,33 including excessive time in bed and frequent napping, resulting in daytime fatigue and feeling generally unrested. A recent meta-analysis reported mild-to-moderate immediate improvement in patients’ pain after nonpharmacotherapeutic sleep interventions.34 The National Sleep Foundation has produced a patient guide to assist in sleep hygiene.35
Devising a management strategy despite sparse evidence
Because the cause of noncyclic CPP may be multifactorial, and because the literature on the etiology of CPP is limited (and, when there is research, it is inconclusive or of poor quality36), there are few evidence-based recommendations for treating CPP. Given the paucity of quality evidence, physicians should treat patients empirically, based on their experience and their familiarity with the range of medical and nonpharmacotherapeutic options used to manage other chronic pain syndromes.
CASE 1
Ms. G’s cyclic pelvic pain was present only during menses. The dyschezia, severe pain that began only after she discontinued a combined OC, aching pain, and severe menstrual cramps are, taken together, suggestive of endometriosis, despite a normal physical exam.
Medical and surgical options were reviewed with Ms. G. She elected to undergo diagnostic laparoscopy. Several extrauterine foci of endometrial tissue were noted and excised; an LNG-IUD was inserted. Her pain improved significantly after surgery.
CASE 2
Ms. M was found to have significant pain on single-digit examination of the pelvic floor muscles, indicating likely pelvic floor muscle dysfunction. Pelvic dynamometry revealed significant tightness and spasm in the pelvic floor muscles—specifically, the levator ani complex.
Ms. M was started on gabapentin to reduce baseline pain and was referred for pelvic floor physical therapy. She felt reassured that her risk of cancer was low, considering her negative work-up, and that cancer was not the cause of her pain. Her symptoms improved greatly with a regimen of medical and physical therapy, although she continues to experience pain flares.
CORRESPONDENCE
Wendy S. Biggs, MD, Central Michigan University College of Medicine, 1632 Stone St., Saginaw, MI 48602; [email protected].
1. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 51. Chronic pelvic pain. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2004;103:589-605.
2. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327.
3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys. 2014;17:e141-e147.
4. Rodriguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence of overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
5. Allen C, Hopewell S, Prentice A, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
6. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;(7):CD001751.
7. Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(3):CD009590.
8. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
9. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
10. Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761-768.
11. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
12. Abbott JA, Jarvis SK, Lyons SC, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
13. Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am J Obstet Gynecol. 2008;199:615.e1-e8.
14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645.
15. Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13:1181-1211.
16. Harada T, Momoeda M, Taketani Y, et al. Low-dose contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008:90:1583-1588.
17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human Reprod. 2013;28:2677-2685.
18. Haviv Y, Rettman A, Aframian D, et al. Myofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J Oral Facial Pain Headache. 2015;29:144-151.
19. Drugs.com. Amitriptyline dosing information. Available at: https://www.drugs.com/amitriptyline.html. Accessed January 4, 2018.
20. Drugs.com. Nortriptyline dosage. Available at: https://www.drugs.com/dosage/nortriptyline.html. Accessed January 4, 2018.
21. Drugs.com. Desipramine (oral route). Available at: https://www.drugs.com/cons/desipramine.html. Accessed January 4, 2018.
22. Drugs.com. Duloxetine capsules. Available at: https://www.drugs.com/pro/duloxetine-capsules.html. Accessed January 4, 2018.
23. Drugs.com. Venlafaxine. Available at: https://www.drugs.com/pro/venlafaxine.html. Accessed January 4, 2018.
24. Drugs.com. Gabapentin. Available at: https://www.drugs.com/pro/gabapentin.html. Accessed January 4, 2018.
25. Drugs.com. Pregabalin. Available at: https://www.drugs.com/monograph/pregabalin.html. Accessed January 4, 2018.
26. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
27. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594-611.
28. Cherkin DC, Sheman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
29. Sesti F, Capozzolo T, Pietropolli A, et al. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev. 2011;24:31-38.
30. Marziali M, Venza M, Lazzaro A, et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. 2012;67:499-504.
31. Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946-955.
32. Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database Syst Rev. 2011;(9):CD007864.
33. Cosar E, Çakır Güngör A, Gencer M, et.al. Sleep disturbance among women with chronic pelvic pain. Int J Gynaecol Obstet. 2014;126:232-234.
34. Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751-1764.
35. National Sleep Foundation. Healthy sleep tips. Available at: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. Accessed December 26, 2017.
36. Yunker A, Sathe NA, Reynolds WS, et al. Systematic review of therapies for noncyclic chronic pelvic pain in women. Obstet Gynecol Survey. 2012;67:417-425.
CASE 1
Lisa G, 31 years old, gravida 0, complains of severe dysmenorrhea that began when she discontinued an oral contraceptive (OC) one year ago. Prior to stopping the OC, she had been taking an OC without interruption since she was 28, during which time she continued to have moderate symptoms of dysmenorrhea. Before taking an OC, the patient had a trial of an etonogestrel implant, which was removed because of irregular bleeding, and depot medroxyprogesterone acetate (MPA) injection, which she discontinued because of associated weight gain and fatigue.
Ms. G is not sexually active and doesn’t want to start a family at this time, but is interested in having a diagnosis. She has no other medical problems, no surgical history, and no history of sexually transmitted infection. She reports that her mother and sister had endometriosis, including pain that resolved after definitive treatment.
Ms. G reports menstrual cycles that are exquisitely painful and occur regularly (every 28 days for 4 or 5 days), with a moderate volume of bleeding that requires a regular-size tampon change every 4 to 6 hours. She reports crampy abdominal pain as 10, on a scale of one to 10; dyschezia (without hematochezia); and generalized achy abdominal pain that is continuous during menses. Pain is partially controlled by ibuprofen, 800 mg every 8 hours. Ms. G also describes gastrointestinal symptoms of bloating, constipation preceding her menstrual cycle, diarrhea during her menses, and occasionally nausea and vomiting with the severe pain.
On examination (which is not performed during menses), Ms. G appears well and is not in acute distress. Abdominal examination is benign. There is no tenderness to palpation or distension; bowel sounds are normal. Pelvic examination reveals mild tenderness upon palpation of a small and mobile uterus. Rectal examination is normal. She has no signs of hyperandrogenism (eg, male-pattern body hair, central obesity).
CASE 2
Rhonda M, 42 years old, gravida 3, para 3003, reports continuous pelvic pain for 7 years that is exacerbated by defecation, intercourse, and insertion of a tampon. She has a low level of dull baseline pain (3, on scale of one to 10) that occasionally spikes up to sharp, knifelike pain (10 on the pain scale), which, she says, brings her to tears. Ms. M describes the pain as “deep inside,” central in her pelvis, and radiating to the left and right, particularly during pain flares.
The patient’s 3 children were born by spontaneous vaginal delivery; however, she recalls that her youngest son was born via a traumatic vaginal delivery 8 years ago (he “got stuck coming out,” she reports). The only other component of Ms. M’s medical history is an anxiety disorder, for which she takes citalopram. She has a family history of cervical cancer.
Ms. M’s past diagnostic work-up for pelvic pain includes pelvic ultrasonography, endometrial biopsy, Pap smear, and diagnostic laparoscopy—all normal. She had a negative gastrointestinal work-up, including upper- and lower-tract endoscopy. Medical therapy, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), did not provide significant relief of pain.
Despite the negative work-up, Ms. M is still concerned that the pain might be related to cancer. With her family history of cervical cancer, she says that she does not want to “miss anything.”
Ms. M is thin and appears anxious. The abdomen is mildly and diffusely tender to palpation with normal bowel sounds and no distension. Pelvic examination reveals some hyperesthesia upon single-digit palpation of the pelvic floor. Placement of the speculum is difficult because of discomfort.
How would you proceed with the care of these patients?
What is chronic pelvic pain? Why is management such a challenge?
Chronic pelvic pain (CPP) is defined as chronic or intermittent cyclic or noncyclic pelvic pain lasting longer than 6 months, localized to the pelvis, diminishing a woman’s quality of life, and requiring medical intervention.1 It’s estimated that CPP affects as many as 15% of women of reproductive age in the United States each year, at a cost to the health care system of approximately $2 billion annually.2,3
CPP can result from abnormal pain responses from multiple body systems, including gynecologic conditions such as endometriosis. Notably, a nongynecologic cause is more often the major pain generator, without significant identifiable pathology (TABLE 1). Like all chronic pain disorders, CPP can also result in central sensitization of the nervous system, altering how pain is processed at the level of the pain matrix in the brain.4

This article reviews the limited evidence for treating CPP and offers recommendations for the primary care physician on providing symptomatic relief in the absence of diagnosed pathology (TABLE 25-13).

Treatment
Analgesics
NSAIDs are frequently used as first-line treatment for any kind of pain, including CPP. There is some evidence of benefit from NSAIDs, compared to placebo, in cyclic CPP secondary to dysmenorrhea and endometriosis;5,6 however, evidence of effectiveness in noncyclic CPP is absent. Because of the low cost and availability of NSAIDs, a trial is reasonable as a first-line intervention, particularly in CPP suspected to be endometriosis or of musculoskeletal origin. NSAIDs can cause adverse effects, including nausea, vomiting, headache, and drowsiness in 11% to 14% of women, although these agents are generally well-tolerated on a short-term basis.5
Opioids bind to opioid receptors in the central and peripheral nervous systems, resulting in an analgesic effect. Guidelines issued in 2016 by the Centers for Disease Control and Prevention recommend safer prescribing through careful evaluation of the risks and benefits of opioids for pain not caused by cancer and for palliation as part of end-of-life care.14
The risks of opioid use are well known in the medical community; they include tolerance, physical dependence, misuse, and death, in addition to common adverse effects such as nausea and vomiting, itching, constipation, and fatigue.14,15 Because of those risks and limited long-term benefit in nonmalignant pain disorders, opioid therapy for CPP should be avoided.14 For patients already taking an opioid, discuss a strategy for weaning and, if possible, provide home naloxone therapy in the event of accidental overdose.14
Hormonal therapy
Hormonal therapies are the most common nonsurgical treatment of noncyclic CPP, with or without a definitive diagnosis of endometriosis, in reproductive-age women with CPP.
Combined OCs, despite a lack of quality evidence, are frequently the first hormonal treatment tried in both cyclic and noncyclic CPP. A low-dosage OC may decrease cyclic pain in endometriosis, although it can increase irregular bleeding and nausea.16 As many as 53% of women with CPP reported having undergone a trial of an OC for endometriosis, despite the absence of consistent evidence showing effectiveness in CPP.17
Depot MPA, in trials, decreased pain more than placebo. It can be tried as a treatment, but its use is often limited because of adverse effects, such as weight gain and bloating.8
A trial of a levonorgestrel-releasing intrauterine device (LNG-IUD) is supported by moderate-quality evidence for women whose CPP is thought to be a symptom of endometriosis or to have another uterine origin.7
Gonadotropin-releasing hormone agonists, such as depot leuprolide and goserelin acetate implant, may be considered in a woman with a diagnosis of endometriosis whose pelvic pain is not alleviated by MPA or an LNG-IUD.9
Nonhormonal therapies
CPP shares pain mechanisms with other pain syndromes, such as neuropathic pain. Antineuropathic medications, such as gabapentin and pregabalin, may, therefore, provide benefit. These medications also produce improvement in pain disorders of the musculoskeletal system, which may contribute to their analgesic effect.18
Gabapentin and amitriptyline have been studied in CPP; both were found successful in decreasing perceived pain. Of note, patients who received gabapentin, a gamma-aminobutyric acid analogue, with or without amitriptyline, had more pain relief than those treated with amitriptyline alone.10 Adverse effects of these medications may limit their use (TABLE 319-25).

Tricyclic antidepressants are well-supported, effective treatments for chronic pain through the central increase of norepinephrine. Beginning at a low dosage to diminish adverse effects (TABLE 319-25) and increasing the dosage slowly to an effective level may increase adherence. A trial of at least 6 to 8 weeks, at a moderate dosage, is recommended before discontinuing the medication. Although amitriptyline has the most evidence for value in the management of CPP disorders,10 second-generation tricyclic antidepressants nortriptyline and desipramine have also been used for pain control, and may be better tolerated.
Duloxetine and venlafaxine—serotonin–norepinephrine reuptake inhibitors—increase serotonin in addition to norepinephrine, which is believed to result in pain control. Although a systematic review of trials of duloxetine for chronic pain showed some improvement in diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis, the review excluded CPP in its analysis.26
In our opinion, a selective neurotransmitter reuptake inhibitor can be attempted to diminish the central pain sensitization of CPP. As with all drugs that increase the availability of serotonin, serotonin syndrome is a rare risk. Additionally, when stopping duloxetine, a prolonged taper may be required.
Pelvic floor dysfunction therapy
Pelvic floor dysfunction of the musculature within the bony pelvis may contribute to, or cause, CPP. The pelvic floor musculature may be hypertonic or hypotonic, and trigger points may exist. Despite the frequency of pelvic floor dysfunction, detailed examination of the pelvic floor is not routinely performed during a pelvic exam.
Because of the high prevalence of pelvic floor dysfunction in women with CPP, evaluation of the pelvic floor muscles is warranted.27 (A protocol for this evaluation is detailed in TABLE 4.) Pelvic dynamometry may indicate muscle spasm or chronic tension; palpation of the pelvic floor during the exam can also identify a pain generator.

Although it might be difficult to distinguish pelvic floor myofascial pain as the primary or secondary cause of pain, pelvic floor physical therapy may clarify the role of the pelvic floor response (depending on the patient’s clinical exam and history). A low-quality retrospective case study on pelvic floor physical therapy reported significant improvement in pain that was proportional to the number of sessions completed.11 Trigger-point injections and injections of botulinum toxin A have been used with reported improvement in the pelvic floor pain profile, and there is evidence to support the benefit of such injections in pelvic muscle dysfunction.12
Psychotherapy
Cognitive behavioral therapy (CBT) is well established as an option to manage a patient’s response to pain, including teaching coping skills for a chronic pain disorder and pain flares. Evidence supports using CBT or mindfulness techniques over usual care in reducing the intensity of pain in chronic low back pain,28 and may be helpful in CPP. Patients with CPP who received 10 treatments of Mensendieck somatocognitive therapy (a mind–body therapy technique popular in Europe) over 90 days, compared with standard treatment alone, demonstrated improvement in pain, motor function, and psychological distress that persisted 9 months after treatment.13
Lifestyle changes, complementary and alternative therapies
Although medical and nonpharmacotherapeutic treatments are often important in the
Diet modifications may relieve pain in some women with CPP. Although a systematic review in 2011 highlighted the lack of data available for the efficacy of dietary therapies for treating CPP, the authors did present data that a diet rich in antioxidants might alleviate pain sysmptoms.29 Also, a gluten-free diet might reduce the symptoms of pain related to endometriosis and, thus, improve physical functioning, among other health domains.30
Exercise can be an important factor in the management of CPP, as with other chronic pain syndromes. In functional pain syndromes, the addition or maintenance of an exercise program has been shown to decrease the amount of pain medications required, improve depressive symptoms, increase energy, and decrease stress. Exercise also improves sleep quality and one’s ability to cope with pain.31
Yoga provides a good balance of aerobic and muscle-building activity and, in the authors’ experience, is tolerated by most women with CPP.
Acupuncture has limited evidence in the treatment of pelvic pain in women. Of the available studies, most are limited to pain related to endometriosis.32
Sleep hygiene may be an important consideration in managing CPP. Sleep disturbances are reported in more than 80% of women with CPP,33 including excessive time in bed and frequent napping, resulting in daytime fatigue and feeling generally unrested. A recent meta-analysis reported mild-to-moderate immediate improvement in patients’ pain after nonpharmacotherapeutic sleep interventions.34 The National Sleep Foundation has produced a patient guide to assist in sleep hygiene.35
Devising a management strategy despite sparse evidence
Because the cause of noncyclic CPP may be multifactorial, and because the literature on the etiology of CPP is limited (and, when there is research, it is inconclusive or of poor quality36), there are few evidence-based recommendations for treating CPP. Given the paucity of quality evidence, physicians should treat patients empirically, based on their experience and their familiarity with the range of medical and nonpharmacotherapeutic options used to manage other chronic pain syndromes.
CASE 1
Ms. G’s cyclic pelvic pain was present only during menses. The dyschezia, severe pain that began only after she discontinued a combined OC, aching pain, and severe menstrual cramps are, taken together, suggestive of endometriosis, despite a normal physical exam.
Medical and surgical options were reviewed with Ms. G. She elected to undergo diagnostic laparoscopy. Several extrauterine foci of endometrial tissue were noted and excised; an LNG-IUD was inserted. Her pain improved significantly after surgery.
CASE 2
Ms. M was found to have significant pain on single-digit examination of the pelvic floor muscles, indicating likely pelvic floor muscle dysfunction. Pelvic dynamometry revealed significant tightness and spasm in the pelvic floor muscles—specifically, the levator ani complex.
Ms. M was started on gabapentin to reduce baseline pain and was referred for pelvic floor physical therapy. She felt reassured that her risk of cancer was low, considering her negative work-up, and that cancer was not the cause of her pain. Her symptoms improved greatly with a regimen of medical and physical therapy, although she continues to experience pain flares.
CORRESPONDENCE
Wendy S. Biggs, MD, Central Michigan University College of Medicine, 1632 Stone St., Saginaw, MI 48602; [email protected].
CASE 1
Lisa G, 31 years old, gravida 0, complains of severe dysmenorrhea that began when she discontinued an oral contraceptive (OC) one year ago. Prior to stopping the OC, she had been taking an OC without interruption since she was 28, during which time she continued to have moderate symptoms of dysmenorrhea. Before taking an OC, the patient had a trial of an etonogestrel implant, which was removed because of irregular bleeding, and depot medroxyprogesterone acetate (MPA) injection, which she discontinued because of associated weight gain and fatigue.
Ms. G is not sexually active and doesn’t want to start a family at this time, but is interested in having a diagnosis. She has no other medical problems, no surgical history, and no history of sexually transmitted infection. She reports that her mother and sister had endometriosis, including pain that resolved after definitive treatment.
Ms. G reports menstrual cycles that are exquisitely painful and occur regularly (every 28 days for 4 or 5 days), with a moderate volume of bleeding that requires a regular-size tampon change every 4 to 6 hours. She reports crampy abdominal pain as 10, on a scale of one to 10; dyschezia (without hematochezia); and generalized achy abdominal pain that is continuous during menses. Pain is partially controlled by ibuprofen, 800 mg every 8 hours. Ms. G also describes gastrointestinal symptoms of bloating, constipation preceding her menstrual cycle, diarrhea during her menses, and occasionally nausea and vomiting with the severe pain.
On examination (which is not performed during menses), Ms. G appears well and is not in acute distress. Abdominal examination is benign. There is no tenderness to palpation or distension; bowel sounds are normal. Pelvic examination reveals mild tenderness upon palpation of a small and mobile uterus. Rectal examination is normal. She has no signs of hyperandrogenism (eg, male-pattern body hair, central obesity).
CASE 2
Rhonda M, 42 years old, gravida 3, para 3003, reports continuous pelvic pain for 7 years that is exacerbated by defecation, intercourse, and insertion of a tampon. She has a low level of dull baseline pain (3, on scale of one to 10) that occasionally spikes up to sharp, knifelike pain (10 on the pain scale), which, she says, brings her to tears. Ms. M describes the pain as “deep inside,” central in her pelvis, and radiating to the left and right, particularly during pain flares.
The patient’s 3 children were born by spontaneous vaginal delivery; however, she recalls that her youngest son was born via a traumatic vaginal delivery 8 years ago (he “got stuck coming out,” she reports). The only other component of Ms. M’s medical history is an anxiety disorder, for which she takes citalopram. She has a family history of cervical cancer.
Ms. M’s past diagnostic work-up for pelvic pain includes pelvic ultrasonography, endometrial biopsy, Pap smear, and diagnostic laparoscopy—all normal. She had a negative gastrointestinal work-up, including upper- and lower-tract endoscopy. Medical therapy, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), did not provide significant relief of pain.
Despite the negative work-up, Ms. M is still concerned that the pain might be related to cancer. With her family history of cervical cancer, she says that she does not want to “miss anything.”
Ms. M is thin and appears anxious. The abdomen is mildly and diffusely tender to palpation with normal bowel sounds and no distension. Pelvic examination reveals some hyperesthesia upon single-digit palpation of the pelvic floor. Placement of the speculum is difficult because of discomfort.
How would you proceed with the care of these patients?
What is chronic pelvic pain? Why is management such a challenge?
Chronic pelvic pain (CPP) is defined as chronic or intermittent cyclic or noncyclic pelvic pain lasting longer than 6 months, localized to the pelvis, diminishing a woman’s quality of life, and requiring medical intervention.1 It’s estimated that CPP affects as many as 15% of women of reproductive age in the United States each year, at a cost to the health care system of approximately $2 billion annually.2,3
CPP can result from abnormal pain responses from multiple body systems, including gynecologic conditions such as endometriosis. Notably, a nongynecologic cause is more often the major pain generator, without significant identifiable pathology (TABLE 1). Like all chronic pain disorders, CPP can also result in central sensitization of the nervous system, altering how pain is processed at the level of the pain matrix in the brain.4

This article reviews the limited evidence for treating CPP and offers recommendations for the primary care physician on providing symptomatic relief in the absence of diagnosed pathology (TABLE 25-13).

Treatment
Analgesics
NSAIDs are frequently used as first-line treatment for any kind of pain, including CPP. There is some evidence of benefit from NSAIDs, compared to placebo, in cyclic CPP secondary to dysmenorrhea and endometriosis;5,6 however, evidence of effectiveness in noncyclic CPP is absent. Because of the low cost and availability of NSAIDs, a trial is reasonable as a first-line intervention, particularly in CPP suspected to be endometriosis or of musculoskeletal origin. NSAIDs can cause adverse effects, including nausea, vomiting, headache, and drowsiness in 11% to 14% of women, although these agents are generally well-tolerated on a short-term basis.5
Opioids bind to opioid receptors in the central and peripheral nervous systems, resulting in an analgesic effect. Guidelines issued in 2016 by the Centers for Disease Control and Prevention recommend safer prescribing through careful evaluation of the risks and benefits of opioids for pain not caused by cancer and for palliation as part of end-of-life care.14
The risks of opioid use are well known in the medical community; they include tolerance, physical dependence, misuse, and death, in addition to common adverse effects such as nausea and vomiting, itching, constipation, and fatigue.14,15 Because of those risks and limited long-term benefit in nonmalignant pain disorders, opioid therapy for CPP should be avoided.14 For patients already taking an opioid, discuss a strategy for weaning and, if possible, provide home naloxone therapy in the event of accidental overdose.14
Hormonal therapy
Hormonal therapies are the most common nonsurgical treatment of noncyclic CPP, with or without a definitive diagnosis of endometriosis, in reproductive-age women with CPP.
Combined OCs, despite a lack of quality evidence, are frequently the first hormonal treatment tried in both cyclic and noncyclic CPP. A low-dosage OC may decrease cyclic pain in endometriosis, although it can increase irregular bleeding and nausea.16 As many as 53% of women with CPP reported having undergone a trial of an OC for endometriosis, despite the absence of consistent evidence showing effectiveness in CPP.17
Depot MPA, in trials, decreased pain more than placebo. It can be tried as a treatment, but its use is often limited because of adverse effects, such as weight gain and bloating.8
A trial of a levonorgestrel-releasing intrauterine device (LNG-IUD) is supported by moderate-quality evidence for women whose CPP is thought to be a symptom of endometriosis or to have another uterine origin.7
Gonadotropin-releasing hormone agonists, such as depot leuprolide and goserelin acetate implant, may be considered in a woman with a diagnosis of endometriosis whose pelvic pain is not alleviated by MPA or an LNG-IUD.9
Nonhormonal therapies
CPP shares pain mechanisms with other pain syndromes, such as neuropathic pain. Antineuropathic medications, such as gabapentin and pregabalin, may, therefore, provide benefit. These medications also produce improvement in pain disorders of the musculoskeletal system, which may contribute to their analgesic effect.18
Gabapentin and amitriptyline have been studied in CPP; both were found successful in decreasing perceived pain. Of note, patients who received gabapentin, a gamma-aminobutyric acid analogue, with or without amitriptyline, had more pain relief than those treated with amitriptyline alone.10 Adverse effects of these medications may limit their use (TABLE 319-25).

Tricyclic antidepressants are well-supported, effective treatments for chronic pain through the central increase of norepinephrine. Beginning at a low dosage to diminish adverse effects (TABLE 319-25) and increasing the dosage slowly to an effective level may increase adherence. A trial of at least 6 to 8 weeks, at a moderate dosage, is recommended before discontinuing the medication. Although amitriptyline has the most evidence for value in the management of CPP disorders,10 second-generation tricyclic antidepressants nortriptyline and desipramine have also been used for pain control, and may be better tolerated.
Duloxetine and venlafaxine—serotonin–norepinephrine reuptake inhibitors—increase serotonin in addition to norepinephrine, which is believed to result in pain control. Although a systematic review of trials of duloxetine for chronic pain showed some improvement in diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis, the review excluded CPP in its analysis.26
In our opinion, a selective neurotransmitter reuptake inhibitor can be attempted to diminish the central pain sensitization of CPP. As with all drugs that increase the availability of serotonin, serotonin syndrome is a rare risk. Additionally, when stopping duloxetine, a prolonged taper may be required.
Pelvic floor dysfunction therapy
Pelvic floor dysfunction of the musculature within the bony pelvis may contribute to, or cause, CPP. The pelvic floor musculature may be hypertonic or hypotonic, and trigger points may exist. Despite the frequency of pelvic floor dysfunction, detailed examination of the pelvic floor is not routinely performed during a pelvic exam.
Because of the high prevalence of pelvic floor dysfunction in women with CPP, evaluation of the pelvic floor muscles is warranted.27 (A protocol for this evaluation is detailed in TABLE 4.) Pelvic dynamometry may indicate muscle spasm or chronic tension; palpation of the pelvic floor during the exam can also identify a pain generator.

Although it might be difficult to distinguish pelvic floor myofascial pain as the primary or secondary cause of pain, pelvic floor physical therapy may clarify the role of the pelvic floor response (depending on the patient’s clinical exam and history). A low-quality retrospective case study on pelvic floor physical therapy reported significant improvement in pain that was proportional to the number of sessions completed.11 Trigger-point injections and injections of botulinum toxin A have been used with reported improvement in the pelvic floor pain profile, and there is evidence to support the benefit of such injections in pelvic muscle dysfunction.12
Psychotherapy
Cognitive behavioral therapy (CBT) is well established as an option to manage a patient’s response to pain, including teaching coping skills for a chronic pain disorder and pain flares. Evidence supports using CBT or mindfulness techniques over usual care in reducing the intensity of pain in chronic low back pain,28 and may be helpful in CPP. Patients with CPP who received 10 treatments of Mensendieck somatocognitive therapy (a mind–body therapy technique popular in Europe) over 90 days, compared with standard treatment alone, demonstrated improvement in pain, motor function, and psychological distress that persisted 9 months after treatment.13
Lifestyle changes, complementary and alternative therapies
Although medical and nonpharmacotherapeutic treatments are often important in the
Diet modifications may relieve pain in some women with CPP. Although a systematic review in 2011 highlighted the lack of data available for the efficacy of dietary therapies for treating CPP, the authors did present data that a diet rich in antioxidants might alleviate pain sysmptoms.29 Also, a gluten-free diet might reduce the symptoms of pain related to endometriosis and, thus, improve physical functioning, among other health domains.30
Exercise can be an important factor in the management of CPP, as with other chronic pain syndromes. In functional pain syndromes, the addition or maintenance of an exercise program has been shown to decrease the amount of pain medications required, improve depressive symptoms, increase energy, and decrease stress. Exercise also improves sleep quality and one’s ability to cope with pain.31
Yoga provides a good balance of aerobic and muscle-building activity and, in the authors’ experience, is tolerated by most women with CPP.
Acupuncture has limited evidence in the treatment of pelvic pain in women. Of the available studies, most are limited to pain related to endometriosis.32
Sleep hygiene may be an important consideration in managing CPP. Sleep disturbances are reported in more than 80% of women with CPP,33 including excessive time in bed and frequent napping, resulting in daytime fatigue and feeling generally unrested. A recent meta-analysis reported mild-to-moderate immediate improvement in patients’ pain after nonpharmacotherapeutic sleep interventions.34 The National Sleep Foundation has produced a patient guide to assist in sleep hygiene.35
Devising a management strategy despite sparse evidence
Because the cause of noncyclic CPP may be multifactorial, and because the literature on the etiology of CPP is limited (and, when there is research, it is inconclusive or of poor quality36), there are few evidence-based recommendations for treating CPP. Given the paucity of quality evidence, physicians should treat patients empirically, based on their experience and their familiarity with the range of medical and nonpharmacotherapeutic options used to manage other chronic pain syndromes.
CASE 1
Ms. G’s cyclic pelvic pain was present only during menses. The dyschezia, severe pain that began only after she discontinued a combined OC, aching pain, and severe menstrual cramps are, taken together, suggestive of endometriosis, despite a normal physical exam.
Medical and surgical options were reviewed with Ms. G. She elected to undergo diagnostic laparoscopy. Several extrauterine foci of endometrial tissue were noted and excised; an LNG-IUD was inserted. Her pain improved significantly after surgery.
CASE 2
Ms. M was found to have significant pain on single-digit examination of the pelvic floor muscles, indicating likely pelvic floor muscle dysfunction. Pelvic dynamometry revealed significant tightness and spasm in the pelvic floor muscles—specifically, the levator ani complex.
Ms. M was started on gabapentin to reduce baseline pain and was referred for pelvic floor physical therapy. She felt reassured that her risk of cancer was low, considering her negative work-up, and that cancer was not the cause of her pain. Her symptoms improved greatly with a regimen of medical and physical therapy, although she continues to experience pain flares.
CORRESPONDENCE
Wendy S. Biggs, MD, Central Michigan University College of Medicine, 1632 Stone St., Saginaw, MI 48602; [email protected].
1. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 51. Chronic pelvic pain. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2004;103:589-605.
2. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327.
3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys. 2014;17:e141-e147.
4. Rodriguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence of overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
5. Allen C, Hopewell S, Prentice A, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
6. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;(7):CD001751.
7. Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(3):CD009590.
8. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
9. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
10. Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761-768.
11. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
12. Abbott JA, Jarvis SK, Lyons SC, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
13. Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am J Obstet Gynecol. 2008;199:615.e1-e8.
14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645.
15. Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13:1181-1211.
16. Harada T, Momoeda M, Taketani Y, et al. Low-dose contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008:90:1583-1588.
17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human Reprod. 2013;28:2677-2685.
18. Haviv Y, Rettman A, Aframian D, et al. Myofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J Oral Facial Pain Headache. 2015;29:144-151.
19. Drugs.com. Amitriptyline dosing information. Available at: https://www.drugs.com/amitriptyline.html. Accessed January 4, 2018.
20. Drugs.com. Nortriptyline dosage. Available at: https://www.drugs.com/dosage/nortriptyline.html. Accessed January 4, 2018.
21. Drugs.com. Desipramine (oral route). Available at: https://www.drugs.com/cons/desipramine.html. Accessed January 4, 2018.
22. Drugs.com. Duloxetine capsules. Available at: https://www.drugs.com/pro/duloxetine-capsules.html. Accessed January 4, 2018.
23. Drugs.com. Venlafaxine. Available at: https://www.drugs.com/pro/venlafaxine.html. Accessed January 4, 2018.
24. Drugs.com. Gabapentin. Available at: https://www.drugs.com/pro/gabapentin.html. Accessed January 4, 2018.
25. Drugs.com. Pregabalin. Available at: https://www.drugs.com/monograph/pregabalin.html. Accessed January 4, 2018.
26. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
27. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594-611.
28. Cherkin DC, Sheman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
29. Sesti F, Capozzolo T, Pietropolli A, et al. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev. 2011;24:31-38.
30. Marziali M, Venza M, Lazzaro A, et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. 2012;67:499-504.
31. Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946-955.
32. Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database Syst Rev. 2011;(9):CD007864.
33. Cosar E, Çakır Güngör A, Gencer M, et.al. Sleep disturbance among women with chronic pelvic pain. Int J Gynaecol Obstet. 2014;126:232-234.
34. Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751-1764.
35. National Sleep Foundation. Healthy sleep tips. Available at: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. Accessed December 26, 2017.
36. Yunker A, Sathe NA, Reynolds WS, et al. Systematic review of therapies for noncyclic chronic pelvic pain in women. Obstet Gynecol Survey. 2012;67:417-425.
1. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 51. Chronic pelvic pain. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2004;103:589-605.
2. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327.
3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys. 2014;17:e141-e147.
4. Rodriguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence of overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
5. Allen C, Hopewell S, Prentice A, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
6. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;(7):CD001751.
7. Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(3):CD009590.
8. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
9. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
10. Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761-768.
11. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
12. Abbott JA, Jarvis SK, Lyons SC, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
13. Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am J Obstet Gynecol. 2008;199:615.e1-e8.
14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645.
15. Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13:1181-1211.
16. Harada T, Momoeda M, Taketani Y, et al. Low-dose contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008:90:1583-1588.
17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human Reprod. 2013;28:2677-2685.
18. Haviv Y, Rettman A, Aframian D, et al. Myofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J Oral Facial Pain Headache. 2015;29:144-151.
19. Drugs.com. Amitriptyline dosing information. Available at: https://www.drugs.com/amitriptyline.html. Accessed January 4, 2018.
20. Drugs.com. Nortriptyline dosage. Available at: https://www.drugs.com/dosage/nortriptyline.html. Accessed January 4, 2018.
21. Drugs.com. Desipramine (oral route). Available at: https://www.drugs.com/cons/desipramine.html. Accessed January 4, 2018.
22. Drugs.com. Duloxetine capsules. Available at: https://www.drugs.com/pro/duloxetine-capsules.html. Accessed January 4, 2018.
23. Drugs.com. Venlafaxine. Available at: https://www.drugs.com/pro/venlafaxine.html. Accessed January 4, 2018.
24. Drugs.com. Gabapentin. Available at: https://www.drugs.com/pro/gabapentin.html. Accessed January 4, 2018.
25. Drugs.com. Pregabalin. Available at: https://www.drugs.com/monograph/pregabalin.html. Accessed January 4, 2018.
26. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
27. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594-611.
28. Cherkin DC, Sheman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
29. Sesti F, Capozzolo T, Pietropolli A, et al. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev. 2011;24:31-38.
30. Marziali M, Venza M, Lazzaro A, et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. 2012;67:499-504.
31. Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946-955.
32. Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database Syst Rev. 2011;(9):CD007864.
33. Cosar E, Çakır Güngör A, Gencer M, et.al. Sleep disturbance among women with chronic pelvic pain. Int J Gynaecol Obstet. 2014;126:232-234.
34. Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751-1764.
35. National Sleep Foundation. Healthy sleep tips. Available at: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. Accessed December 26, 2017.
36. Yunker A, Sathe NA, Reynolds WS, et al. Systematic review of therapies for noncyclic chronic pelvic pain in women. Obstet Gynecol Survey. 2012;67:417-425.
PRACTICE RECOMMENDATIONS
› Consider the levonorgestrel-releasing intrauterine device for relief of chronic pelvic pain (CPP) from endometriosis; it’s been found to be more effective than expectant management. B
› Prescribe a trial of depot medroxyprogesterone acetate, which was more effective than placebo for CPP for as long as 9 months. B
› Use gabapentin—with or without amitriptyline—to provide greater relief of CPP than amitriptyline alone. B
› Recommend pelvic physical therapy for CPP; the pelvic pain score can be reduced in proportion to the number of sessions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
