User login
Deep Soft Tissue Mass of the Knee
The Diagnosis: Nodular Fasciitis
The diagnosis of spindle cell tumors can be challenging; however, by using a variety of immunoperoxidase stains and fluorescent in situ hybridization (FISH) testing in conjunction with histology, it often is possible to arrive at a definitive diagnosis. For this case, the histologic features in conjunction with the immunoperoxidase stains and FISH were consistent with a diagnosis of nodular fasciitis.
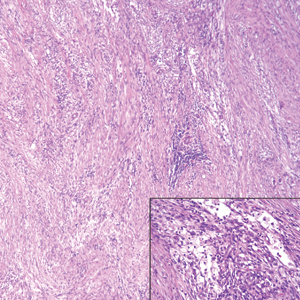
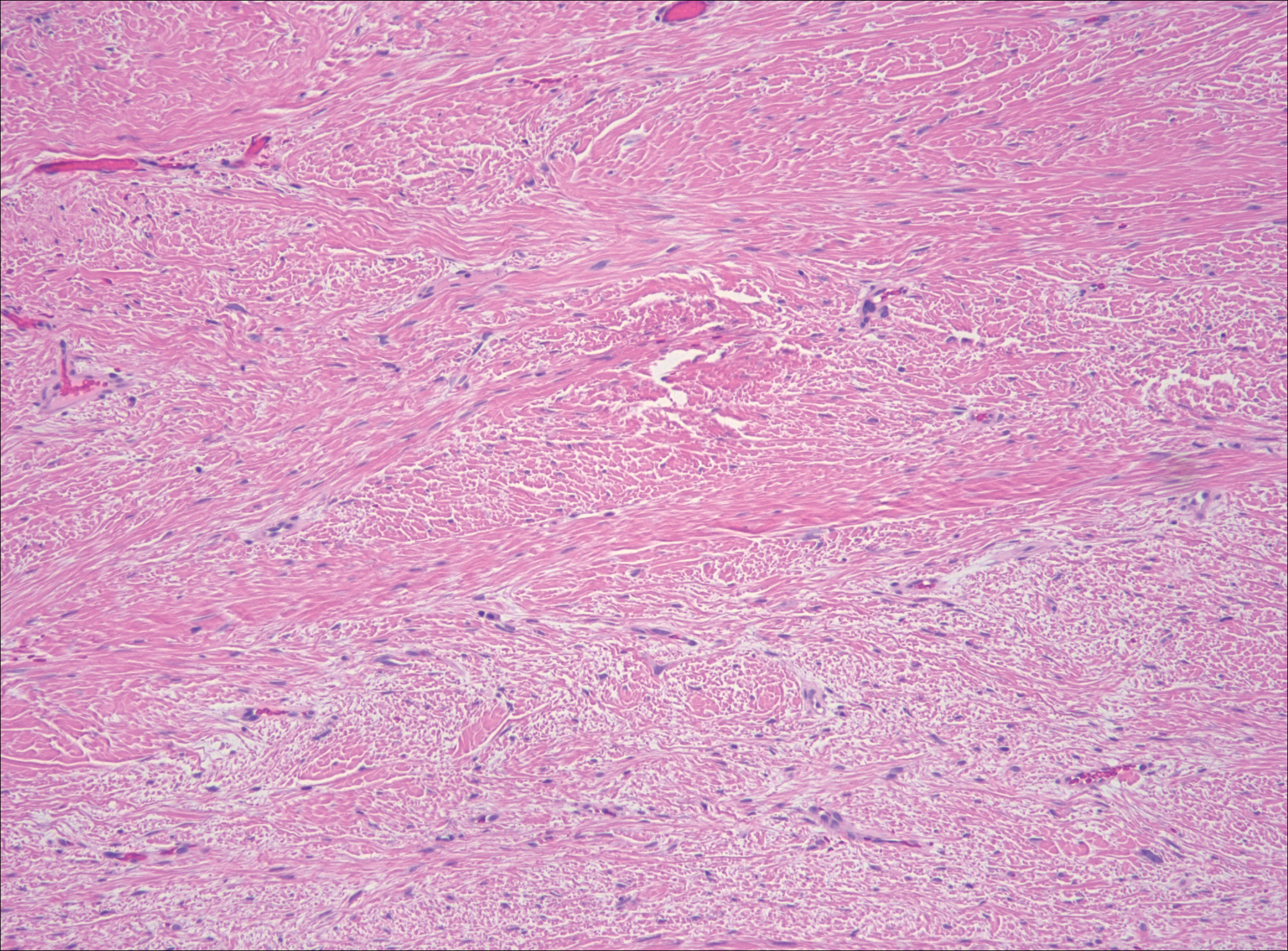
Nodular fasciitis is a benign, self-limiting, myofibroblastic, soft-tissue proliferation typically found in the subcutaneous tissue.1 It can be found anywhere on the body but most commonly on the upper arms and trunk. It most often is seen in young adults, and many cases have been reported in association with a history of trauma to the area.1,2 It typically measures less than 2 cm in diameter.3 The diagnosis of nodular fasciitis is particularly challenging because it mimics sarcoma, both in presentation and in histologic findings with rapid growth, high mitotic activity, and increased cellularity.1,4-7 In contrast to malignancy, nodular fasciitis has no atypical mitoses and little cytologic atypia.8,9 Rather, it contains plump myofibroblasts loosely arranged in a myxoid or fibrous stroma that also may contain lymphocytes, extravasated erythrocytes, and osteoclastlike giant cells distributed throughout.5,10,11 In this case, lymphocytes, extravasated red blood cells, and myxoid change are present, suggesting the diagnosis of nodular fasciitis. In other cases, however, these features may be much more limited, making the diagnosis more challenging. The spindle cells are arranged in poorly defined short fascicles. The tumor cells do not infiltrate between individual adipocytes. There is no notable cytologic atypia.
Because of the difficulty in making the diagnosis, overtreatment of this benign condition can be a problem, causing increased morbidity.1 Erickson-Johnson et al12 identified the role of an ubiquitin-specific peptidase 6, USP6, gene rearrangement on chromosome 17p13 in 92% (44/48) of cases of nodular fasciitis. The USP6 gene most often is rearranged with the myosin heavy chain 9 gene, MYH9, on chromosome 22q12.3. With this rearrangement, the MYH9 promoter leads to the overexpression of USP6, causing tumor formation.2,13 The use of multiple immunoperoxidase stains can be important in the identification of nodular fasciitis. Nodular fasciitis stains negative for S-100, epithelial membrane antigen, CD34, β-catenin, and cytokeratin, but typically stains positive for smooth muscle actin.9
Although dermatofibrosarcoma protuberans (DFSP) was in the differential diagnosis, these tumors tend to have greater cellularity than nodular fasciitis. In addition, the spindle cells of DFSP typically are arranged in a storiform pattern. Another characteristic feature of DFSP is that the tumor cells will infiltrate between adipose cells creating a lacelike or honeycomblike appearance within the subcutaneous tissue (Figure 1). Immunohistochemistry staining and FISH testing may be useful in making a diagnosis of DFSP. These tumors typically are positive for CD34 by immunoperoxidase staining and demonstrate a translocation t(17;22)(q21;q13) between platelet-derived growth factor subunit B gene, PDGFB, and collagen type I alpha 1 chain gene, COL1A1, by FISH.
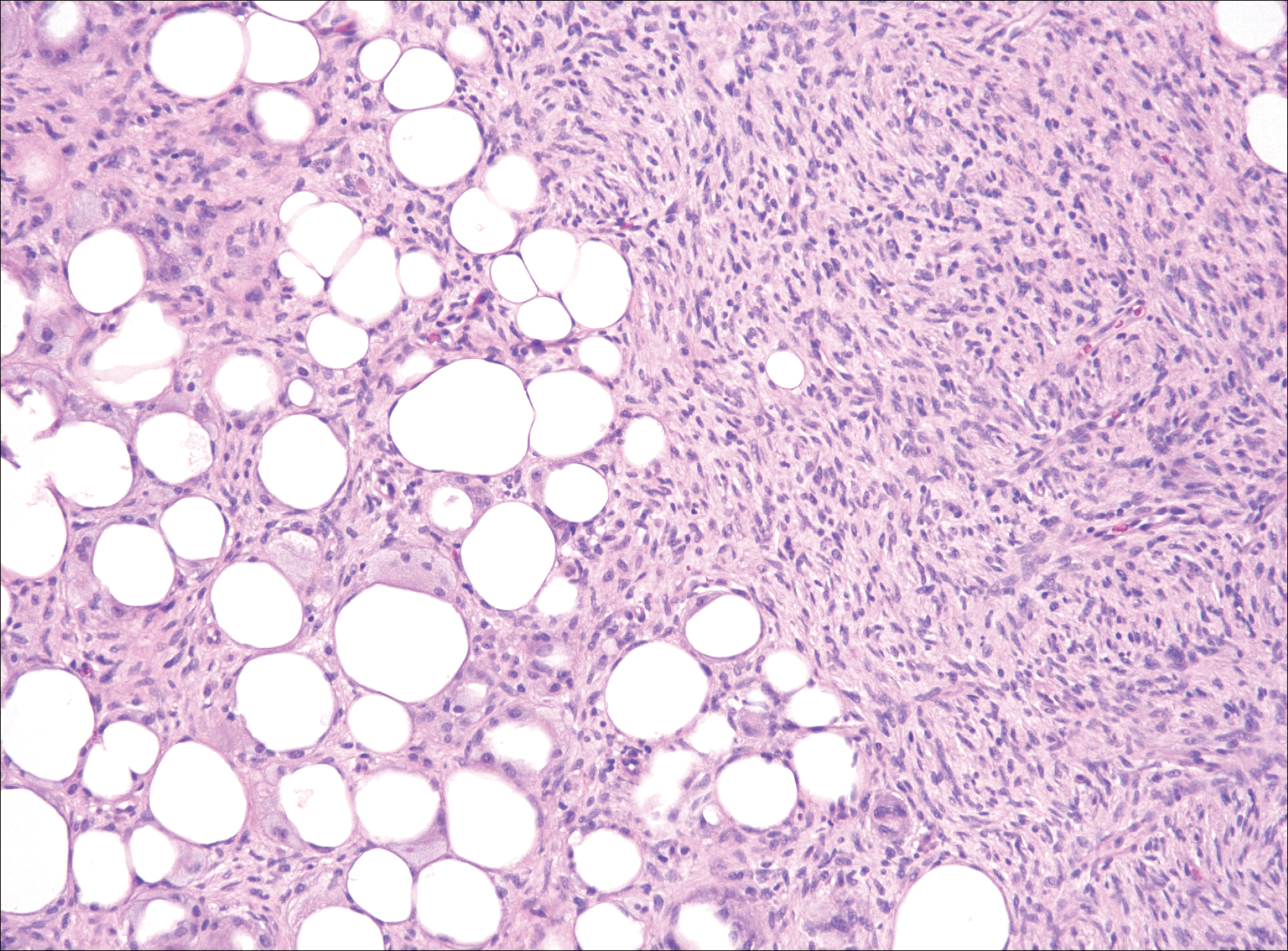
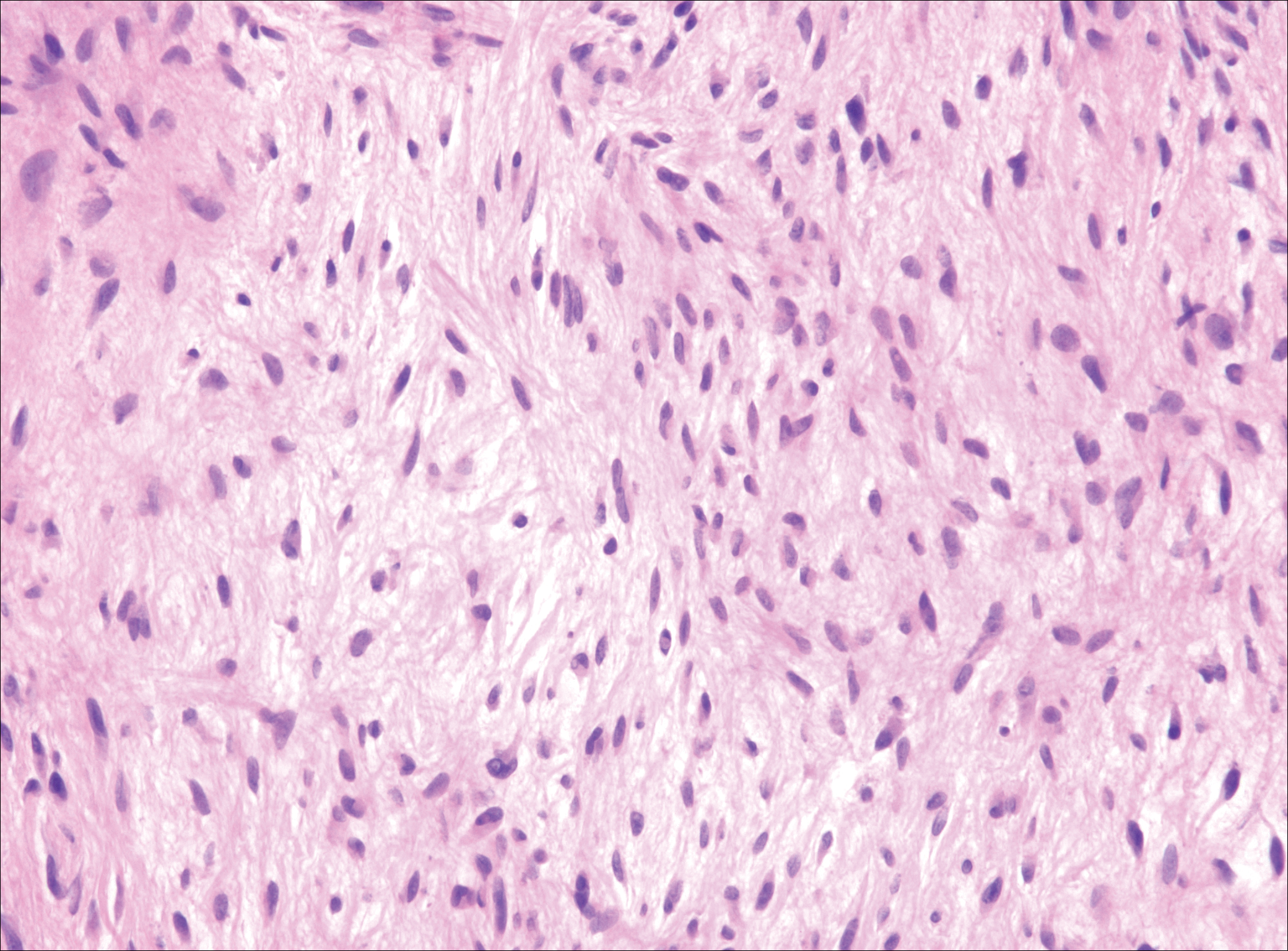
The distinction between the fibrous phase of nodular fasciitis and fibromatosis can be challenging. The size of the lesion may be helpful, with most lesions of nodular fasciitis being less than 3 cm, while lesions of fibromatosis have a mean diameter of 7 cm.5,14 Microscopically, both tumors demonstrate a fascicular growth pattern; however, the fascicles in nodular fasciitis tend to be short and irregular compared to the longer fascicles seen in fibromatosis (Figure 2). Immunohistochemistry staining has limited utility with only 56% (14/25) of superficial fibromatoses having positive nuclear staining for β-catenin.15
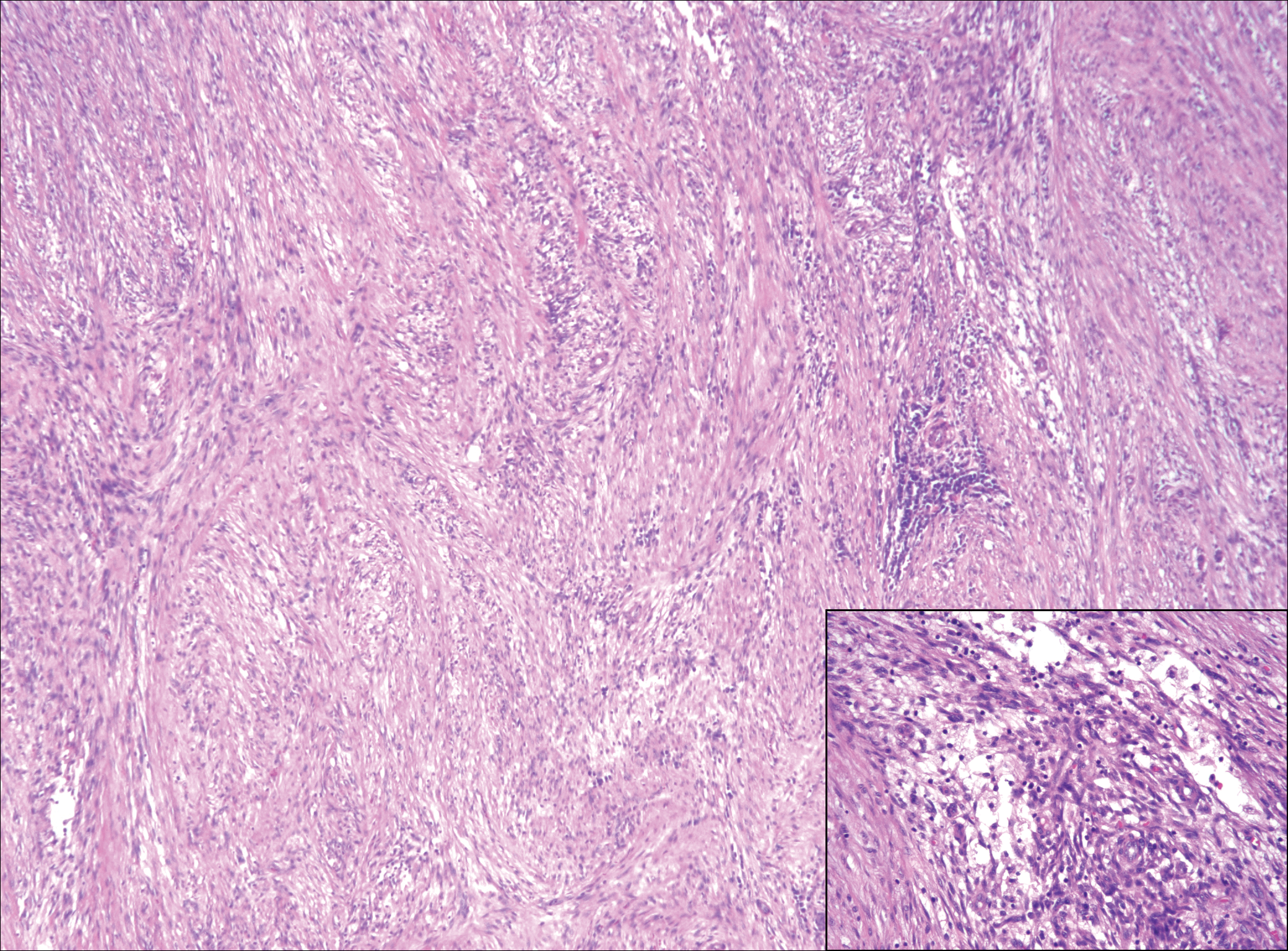
Low-grade fibromyxoid sarcoma (LGFMS) would be unusual in this clinical scenario. Only 13% to 19% of cases present in patients younger than 18 years (mean age, 33 years).16 In LGFMS there are cytologically bland spindle cells that are typically arranged in a patternless or whorled pattern (Figure 3), though fascicular architecture may be seen. There are alternating areas of fibrous and myxoid stroma. A curvilinear vasculature network and lack of lymphocytes and extravasated red blood cells are histologic features favoring LGFMS over nodular fasciitis. Immunohistochemistry staining and FISH testing can be useful in making the diagnosis of LGFMS. These tumors are characterized by a translocation t(7;16)(q34;p11) involving the fusion in sarcoma, FUS, and cAMP responsive element binding protein 3 like 2, CREB3L2, genes.16 Positive immunohistochemistry staining for MUC4 can be seen in up to 100% of LGFMS and is absent in many other spindle cell tumors.16
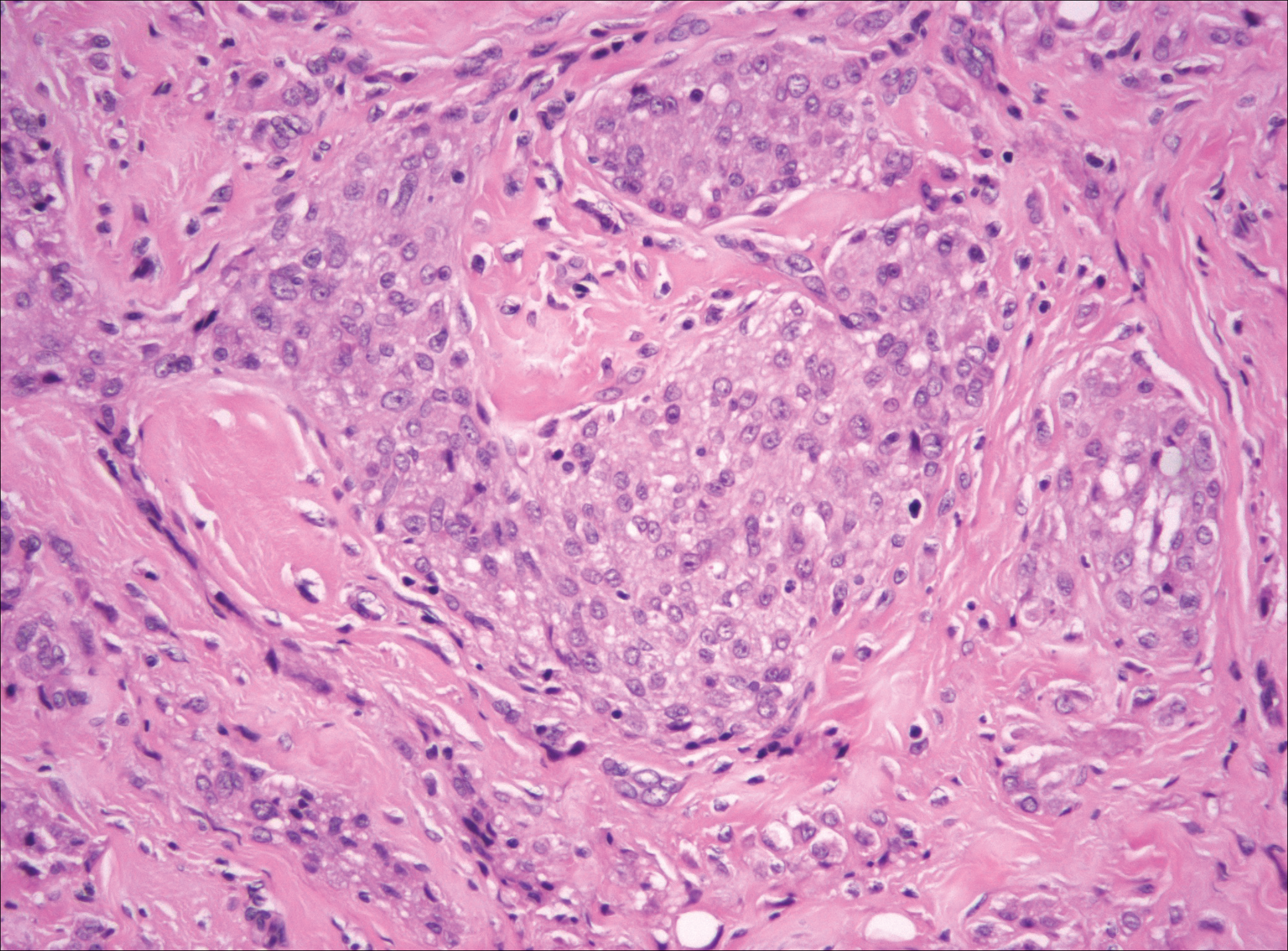
Plexiform fibrohistiocytic tumor (PFT) is least likely to be confused with nodular fasciitis. Histologically these tumors are characterized by multiple small nodules arranged in a plexiform pattern (Figure 4). Within the nodules, 3 cell types may be noted: spindle fibroblast-like cells, mononuclear histiocyte-like cells, and osteoclastlike cells.17 Either the spindle cells or the mononuclear cells may predominate in cases of PFT. Immunohistochemistry staining of PFT is nonspecific and there are no molecular/FISH studies that can be used to help confirm the diagnosis.
- Shin C, Low I, Ng D, et al. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784-791.
- Kumar E, Patel NR, Demicco EG, et al. Cutaneous nodular fasciitis with genetic analysis: a case series. J Cutan Pathol. 2016;43:1143-1149.
- Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12-18.
- Lin X, Wang L, Zhang Y, et al. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn Pathol. 2013;8:50.
- Goldstein J, Cates J. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol. 2015;22:260-266.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyons, France: IARC Press; 2013.
- Bridge JA, Cushman-Vokoun AM. Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med. 2011;135:588-601.
- Anzeljc AJ, Oliveira AM, Grossniklaus HE, et al. Nodular fasciitis of the orbit: a case report confirmed by molecular cytogenetic analysis. Ophthalmic Plast Reconstr Surg. 2017;33(3S suppl 1):S152-S155.
- de Paula SA, Cruz AA, de Alencar VM, et al. Nodular fasciitis presenting as a large mass in the upper eyelid. Ophthalmic Plast Reconstr Surg. 2006;22:494-495.
- Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678.
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Amary MF, Ye H, Berisha F, et al. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98.
- Wirth L, Klein A, Baur-Melnyk A. Desmoid tumors of the extremity and trunk. a retrospective study of 44 patients. BMC Musculoskelet Disord. 2018;19:2.
- Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cells lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509-514.
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60-67.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138.
The Diagnosis: Nodular Fasciitis
The diagnosis of spindle cell tumors can be challenging; however, by using a variety of immunoperoxidase stains and fluorescent in situ hybridization (FISH) testing in conjunction with histology, it often is possible to arrive at a definitive diagnosis. For this case, the histologic features in conjunction with the immunoperoxidase stains and FISH were consistent with a diagnosis of nodular fasciitis.
Nodular fasciitis is a benign, self-limiting, myofibroblastic, soft-tissue proliferation typically found in the subcutaneous tissue.1 It can be found anywhere on the body but most commonly on the upper arms and trunk. It most often is seen in young adults, and many cases have been reported in association with a history of trauma to the area.1,2 It typically measures less than 2 cm in diameter.3 The diagnosis of nodular fasciitis is particularly challenging because it mimics sarcoma, both in presentation and in histologic findings with rapid growth, high mitotic activity, and increased cellularity.1,4-7 In contrast to malignancy, nodular fasciitis has no atypical mitoses and little cytologic atypia.8,9 Rather, it contains plump myofibroblasts loosely arranged in a myxoid or fibrous stroma that also may contain lymphocytes, extravasated erythrocytes, and osteoclastlike giant cells distributed throughout.5,10,11 In this case, lymphocytes, extravasated red blood cells, and myxoid change are present, suggesting the diagnosis of nodular fasciitis. In other cases, however, these features may be much more limited, making the diagnosis more challenging. The spindle cells are arranged in poorly defined short fascicles. The tumor cells do not infiltrate between individual adipocytes. There is no notable cytologic atypia.
Because of the difficulty in making the diagnosis, overtreatment of this benign condition can be a problem, causing increased morbidity.1 Erickson-Johnson et al12 identified the role of an ubiquitin-specific peptidase 6, USP6, gene rearrangement on chromosome 17p13 in 92% (44/48) of cases of nodular fasciitis. The USP6 gene most often is rearranged with the myosin heavy chain 9 gene, MYH9, on chromosome 22q12.3. With this rearrangement, the MYH9 promoter leads to the overexpression of USP6, causing tumor formation.2,13 The use of multiple immunoperoxidase stains can be important in the identification of nodular fasciitis. Nodular fasciitis stains negative for S-100, epithelial membrane antigen, CD34, β-catenin, and cytokeratin, but typically stains positive for smooth muscle actin.9
Although dermatofibrosarcoma protuberans (DFSP) was in the differential diagnosis, these tumors tend to have greater cellularity than nodular fasciitis. In addition, the spindle cells of DFSP typically are arranged in a storiform pattern. Another characteristic feature of DFSP is that the tumor cells will infiltrate between adipose cells creating a lacelike or honeycomblike appearance within the subcutaneous tissue (Figure 1). Immunohistochemistry staining and FISH testing may be useful in making a diagnosis of DFSP. These tumors typically are positive for CD34 by immunoperoxidase staining and demonstrate a translocation t(17;22)(q21;q13) between platelet-derived growth factor subunit B gene, PDGFB, and collagen type I alpha 1 chain gene, COL1A1, by FISH.

The distinction between the fibrous phase of nodular fasciitis and fibromatosis can be challenging. The size of the lesion may be helpful, with most lesions of nodular fasciitis being less than 3 cm, while lesions of fibromatosis have a mean diameter of 7 cm.5,14 Microscopically, both tumors demonstrate a fascicular growth pattern; however, the fascicles in nodular fasciitis tend to be short and irregular compared to the longer fascicles seen in fibromatosis (Figure 2). Immunohistochemistry staining has limited utility with only 56% (14/25) of superficial fibromatoses having positive nuclear staining for β-catenin.15

Low-grade fibromyxoid sarcoma (LGFMS) would be unusual in this clinical scenario. Only 13% to 19% of cases present in patients younger than 18 years (mean age, 33 years).16 In LGFMS there are cytologically bland spindle cells that are typically arranged in a patternless or whorled pattern (Figure 3), though fascicular architecture may be seen. There are alternating areas of fibrous and myxoid stroma. A curvilinear vasculature network and lack of lymphocytes and extravasated red blood cells are histologic features favoring LGFMS over nodular fasciitis. Immunohistochemistry staining and FISH testing can be useful in making the diagnosis of LGFMS. These tumors are characterized by a translocation t(7;16)(q34;p11) involving the fusion in sarcoma, FUS, and cAMP responsive element binding protein 3 like 2, CREB3L2, genes.16 Positive immunohistochemistry staining for MUC4 can be seen in up to 100% of LGFMS and is absent in many other spindle cell tumors.16

Plexiform fibrohistiocytic tumor (PFT) is least likely to be confused with nodular fasciitis. Histologically these tumors are characterized by multiple small nodules arranged in a plexiform pattern (Figure 4). Within the nodules, 3 cell types may be noted: spindle fibroblast-like cells, mononuclear histiocyte-like cells, and osteoclastlike cells.17 Either the spindle cells or the mononuclear cells may predominate in cases of PFT. Immunohistochemistry staining of PFT is nonspecific and there are no molecular/FISH studies that can be used to help confirm the diagnosis.

The Diagnosis: Nodular Fasciitis
The diagnosis of spindle cell tumors can be challenging; however, by using a variety of immunoperoxidase stains and fluorescent in situ hybridization (FISH) testing in conjunction with histology, it often is possible to arrive at a definitive diagnosis. For this case, the histologic features in conjunction with the immunoperoxidase stains and FISH were consistent with a diagnosis of nodular fasciitis.
Nodular fasciitis is a benign, self-limiting, myofibroblastic, soft-tissue proliferation typically found in the subcutaneous tissue.1 It can be found anywhere on the body but most commonly on the upper arms and trunk. It most often is seen in young adults, and many cases have been reported in association with a history of trauma to the area.1,2 It typically measures less than 2 cm in diameter.3 The diagnosis of nodular fasciitis is particularly challenging because it mimics sarcoma, both in presentation and in histologic findings with rapid growth, high mitotic activity, and increased cellularity.1,4-7 In contrast to malignancy, nodular fasciitis has no atypical mitoses and little cytologic atypia.8,9 Rather, it contains plump myofibroblasts loosely arranged in a myxoid or fibrous stroma that also may contain lymphocytes, extravasated erythrocytes, and osteoclastlike giant cells distributed throughout.5,10,11 In this case, lymphocytes, extravasated red blood cells, and myxoid change are present, suggesting the diagnosis of nodular fasciitis. In other cases, however, these features may be much more limited, making the diagnosis more challenging. The spindle cells are arranged in poorly defined short fascicles. The tumor cells do not infiltrate between individual adipocytes. There is no notable cytologic atypia.
Because of the difficulty in making the diagnosis, overtreatment of this benign condition can be a problem, causing increased morbidity.1 Erickson-Johnson et al12 identified the role of an ubiquitin-specific peptidase 6, USP6, gene rearrangement on chromosome 17p13 in 92% (44/48) of cases of nodular fasciitis. The USP6 gene most often is rearranged with the myosin heavy chain 9 gene, MYH9, on chromosome 22q12.3. With this rearrangement, the MYH9 promoter leads to the overexpression of USP6, causing tumor formation.2,13 The use of multiple immunoperoxidase stains can be important in the identification of nodular fasciitis. Nodular fasciitis stains negative for S-100, epithelial membrane antigen, CD34, β-catenin, and cytokeratin, but typically stains positive for smooth muscle actin.9
Although dermatofibrosarcoma protuberans (DFSP) was in the differential diagnosis, these tumors tend to have greater cellularity than nodular fasciitis. In addition, the spindle cells of DFSP typically are arranged in a storiform pattern. Another characteristic feature of DFSP is that the tumor cells will infiltrate between adipose cells creating a lacelike or honeycomblike appearance within the subcutaneous tissue (Figure 1). Immunohistochemistry staining and FISH testing may be useful in making a diagnosis of DFSP. These tumors typically are positive for CD34 by immunoperoxidase staining and demonstrate a translocation t(17;22)(q21;q13) between platelet-derived growth factor subunit B gene, PDGFB, and collagen type I alpha 1 chain gene, COL1A1, by FISH.

The distinction between the fibrous phase of nodular fasciitis and fibromatosis can be challenging. The size of the lesion may be helpful, with most lesions of nodular fasciitis being less than 3 cm, while lesions of fibromatosis have a mean diameter of 7 cm.5,14 Microscopically, both tumors demonstrate a fascicular growth pattern; however, the fascicles in nodular fasciitis tend to be short and irregular compared to the longer fascicles seen in fibromatosis (Figure 2). Immunohistochemistry staining has limited utility with only 56% (14/25) of superficial fibromatoses having positive nuclear staining for β-catenin.15

Low-grade fibromyxoid sarcoma (LGFMS) would be unusual in this clinical scenario. Only 13% to 19% of cases present in patients younger than 18 years (mean age, 33 years).16 In LGFMS there are cytologically bland spindle cells that are typically arranged in a patternless or whorled pattern (Figure 3), though fascicular architecture may be seen. There are alternating areas of fibrous and myxoid stroma. A curvilinear vasculature network and lack of lymphocytes and extravasated red blood cells are histologic features favoring LGFMS over nodular fasciitis. Immunohistochemistry staining and FISH testing can be useful in making the diagnosis of LGFMS. These tumors are characterized by a translocation t(7;16)(q34;p11) involving the fusion in sarcoma, FUS, and cAMP responsive element binding protein 3 like 2, CREB3L2, genes.16 Positive immunohistochemistry staining for MUC4 can be seen in up to 100% of LGFMS and is absent in many other spindle cell tumors.16

Plexiform fibrohistiocytic tumor (PFT) is least likely to be confused with nodular fasciitis. Histologically these tumors are characterized by multiple small nodules arranged in a plexiform pattern (Figure 4). Within the nodules, 3 cell types may be noted: spindle fibroblast-like cells, mononuclear histiocyte-like cells, and osteoclastlike cells.17 Either the spindle cells or the mononuclear cells may predominate in cases of PFT. Immunohistochemistry staining of PFT is nonspecific and there are no molecular/FISH studies that can be used to help confirm the diagnosis.

- Shin C, Low I, Ng D, et al. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784-791.
- Kumar E, Patel NR, Demicco EG, et al. Cutaneous nodular fasciitis with genetic analysis: a case series. J Cutan Pathol. 2016;43:1143-1149.
- Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12-18.
- Lin X, Wang L, Zhang Y, et al. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn Pathol. 2013;8:50.
- Goldstein J, Cates J. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol. 2015;22:260-266.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyons, France: IARC Press; 2013.
- Bridge JA, Cushman-Vokoun AM. Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med. 2011;135:588-601.
- Anzeljc AJ, Oliveira AM, Grossniklaus HE, et al. Nodular fasciitis of the orbit: a case report confirmed by molecular cytogenetic analysis. Ophthalmic Plast Reconstr Surg. 2017;33(3S suppl 1):S152-S155.
- de Paula SA, Cruz AA, de Alencar VM, et al. Nodular fasciitis presenting as a large mass in the upper eyelid. Ophthalmic Plast Reconstr Surg. 2006;22:494-495.
- Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678.
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Amary MF, Ye H, Berisha F, et al. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98.
- Wirth L, Klein A, Baur-Melnyk A. Desmoid tumors of the extremity and trunk. a retrospective study of 44 patients. BMC Musculoskelet Disord. 2018;19:2.
- Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cells lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509-514.
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60-67.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138.
- Shin C, Low I, Ng D, et al. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784-791.
- Kumar E, Patel NR, Demicco EG, et al. Cutaneous nodular fasciitis with genetic analysis: a case series. J Cutan Pathol. 2016;43:1143-1149.
- Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12-18.
- Lin X, Wang L, Zhang Y, et al. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn Pathol. 2013;8:50.
- Goldstein J, Cates J. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol. 2015;22:260-266.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyons, France: IARC Press; 2013.
- Bridge JA, Cushman-Vokoun AM. Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med. 2011;135:588-601.
- Anzeljc AJ, Oliveira AM, Grossniklaus HE, et al. Nodular fasciitis of the orbit: a case report confirmed by molecular cytogenetic analysis. Ophthalmic Plast Reconstr Surg. 2017;33(3S suppl 1):S152-S155.
- de Paula SA, Cruz AA, de Alencar VM, et al. Nodular fasciitis presenting as a large mass in the upper eyelid. Ophthalmic Plast Reconstr Surg. 2006;22:494-495.
- Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678.
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Amary MF, Ye H, Berisha F, et al. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98.
- Wirth L, Klein A, Baur-Melnyk A. Desmoid tumors of the extremity and trunk. a retrospective study of 44 patients. BMC Musculoskelet Disord. 2018;19:2.
- Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cells lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509-514.
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60-67.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138.
A 16-year-old adolescent girl presented with a bump over the left posterior knee of 1 month's duration. Her medical history was unremarkable. She denied recent trauma or injury to the area. On physical examination there was a visible and palpable tense nontender mass the size of an egg over the left posterior knee. Magnetic resonance imaging showed a lobulated mass-like focus of T2 hyperintensity centered at the subcutaneous tissues and superficial myofascial plane of the gastrocnemius on the posterior knee. Complete excision of the lesion was performed and demonstrated a 2.6.2 ×2.9.2 ×2.1-cm mass within subcutaneous adipose tissue. There was no microscopic involvement of skeletal muscle. Immunohistochemistry staining of the tumor was performed that was positive for smooth muscle actin and negative for desmin, S-100, CD34, pan-cytokeratin, and β-catenin. Fluorescent in situ hybridization testing demonstrated rearrangement of the ubiquitin-specific peptidase 6 gene, USP6, locus (17p13).
Submit Comments on VTE Guidelines by July 25
The American Society of Hematology (ASH) seeks comments by July 25 on draft clinical practice guidelines on venous thromboembolism: VTE Prevention in Surgical Hospitalized Patients.
The draft recommendations and a link to the online survey where comments are collected are available here.
The American Society of Hematology (ASH) seeks comments by July 25 on draft clinical practice guidelines on venous thromboembolism: VTE Prevention in Surgical Hospitalized Patients.
The draft recommendations and a link to the online survey where comments are collected are available here.
The American Society of Hematology (ASH) seeks comments by July 25 on draft clinical practice guidelines on venous thromboembolism: VTE Prevention in Surgical Hospitalized Patients.
The draft recommendations and a link to the online survey where comments are collected are available here.
New and Noteworthy Information—July 2018
Adequate Sleep Associated With Lower Dementia Risk
Short and long daily sleep duration are risk factors for dementia and death in adults age 60 and older, according to a study published online ahead of print June 6 in the Journal of the American Geriatrics Society. In a prospective cohort study, researchers followed 1,517 adults without dementia for 10 years. Self-reported daily sleep durations were grouped into five categories. The association between daily sleep duration and risk of dementia and death was determined using Cox proportional hazards models. During follow-up, 294 participants developed dementia, and 282 died. Age- and sex-adjusted incidence rates of dementia and all-cause mortality were significantly greater in subjects who slept less than 5.0 hours/day or 10.0 or more hours/day than in people who slept from 5.0 to 6.9 hours/day.
Ohara T, Honda T, Hata J, et al. Association between daily sleep duration and risk of dementia and mortality in a Japanese community. J Am Geriatr Soc. 2018 Jun 6 [Epub ahead of print].
Rivaroxaban Not Superior to Aspirin for Stroke Prevention
Rivaroxaban is not superior to aspirin in the prevention of recurrent stroke, according to a study published June 7 in the New England Journal of Medicine. Researchers compared the efficacy and safety of rivaroxaban at a daily dose of 15 mg with aspirin at a daily dose of 100 mg for the prevention of recurrent stroke in patients with recent ischemic stroke that was presumed to be from cerebral embolism. The primary outcome was the first recurrence of ischemic or hemorrhagic stroke or systemic embolism in a time-to-event analysis. At 459 sites, 3,609 patients were randomly assigned to receive rivaroxaban, and 3,604 were randomized to aspirin. Recurrent ischemic stroke occurred in 172 patients in the rivaroxaban group and in 160 in the aspirin group.
Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191-2201.
Disintegrating Brain Lesions May Indicate Worsening MS
Atrophied lesion volume may indicate increasing disability in patients with multiple sclerosis (MS), according to a study published online ahead of print June 1 in the Journal of Neuroimaging. A total of 192 patients with clinically isolated syndrome or MS received 3T MRI at baseline and at five years. Investigators quantified lesions at baseline and calculated new and enlarging lesion volumes during the study interval. Atrophied lesion volume was calculated by combining baseline lesion masks with follow-up SIENAX-derived CSF partial volume maps. The researchers evaluated correlations between these measures and disability, as measured by the Expanded Disability Status Scale (EDSS). Atrophied lesion volume was different between MS subtypes and exceeded new lesion volume accumulation in progressive MS. Atrophied lesion volume was the only significant correlate of EDSS change.
Dwyer MG, Bergsland N, Ramasamy DP, et al. Atrophied brain lesion volume: a new imaging biomarker in multiple sclerosis. J Neuroimaging. 2018 Jun 1 [Epub ahead of print].
Do Migraineurs Seek Behavioral Treatment After a Referral?
A significant number of migraineurs are not using effective behavioral treatments for migraine, according to a study published online ahead of print June 5 in Pain Medicine. In a prospective cohort study, researchers tracked 234 patients with migraine who presented to an academic headache center and referred 69 of them for behavioral treatment with an appropriately trained therapist. Fifty-three of the referred patients completed a follow-up interview within three months of their initial appointment and were included in the analysis. Of the patients referred for behavioral treatment, 30 made an appointment. Investigators found no differences between people who started behavioral therapy and people who did not. Study authors did find that people who had previously seen a psychologist for migraine were more likely to initiate therapy.
Minen MT, Azarchi S, Sobolev R, et al. Factors related to migraine patients’ decisions to initiate behavioral migraine treatment following a headache specialist’s recommendation: a prospective observational study. Pain Med. 2018 Jun 5 [Epub ahead of print].
TIA Associated With Increased Five-Year Risk of Stroke
People with transient ischemic attack (TIA) are at risk for a cardiovascular event in the following five years, according to a study published June 7 in the New England Journal of Medicine. Researchers evaluated patients who had had a TIA within seven days before enrollment in a registry of TIA clinics. Of 61 sites, 42 had follow-up data on more than 50% of their enrolled patients at five years. The study’s primary outcome was a composite of stroke, acute coronary syndrome, or death from cardiovascular causes, with an emphasis on events that occurred in the second through fifth years. At five years, stroke had occurred in 345 of the 3,847 patients included in the follow-up study, and 149 of them had a stroke during the second through fifth years of follow-up.
Amarenco P, Lavallée PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378(23):2182-2190.
Follow-Up Care for TBI Is Not Delivered Adequately
Follow-up care for patients with traumatic brain injury (TBI) is not delivered optimally, according to a study published May 25 in JAMA Network Open. In a cohort study, researchers surveyed 831 participants in the Transforming Research and Clinical Knowledge in TBI initiative about their care after hospital discharge. Follow-up care was defined as providing TBI-related educational materials at discharge, calling patients within two weeks after release, seeing a healthcare provider within two weeks, and seeing a healthcare provider within three months. Approximately 42% of participants reported receiving TBI-related educational material at discharge, and 44% reported seeing a physician or other medical practitioner within three months after injury. Of patients with a positive finding on CT, 39% had not seen a medical practitioner at three months after injury.
Seabury SA, Gaudette E, Goldman DP, et al. Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: results from the TRACK-TBI study. JAMA Network Open. 2018;1(1):e180210.
Researchers Examine Mortality Rate of Pediatric Stroke
In-hospital mortality occurs in 2.6% of children with arterial ischemic stroke, according to a study published in the May issue of Pediatrics. The retrospective study included 915 infants younger than 1 month and 2,273 children age 1 month to 18 years with stroke at 87 hospitals in 24 countries. Death during hospitalization and cause of death were ascertained from medical records. A total of 14 neonates and 70 children died during hospitalization. Of 48 cases with reported causes of death, 31 were stroke-related. Remaining deaths were attributed to medical disease. In multivariable analysis, congenital heart disease, posterior plus anterior circulation stroke, and stroke presentation without seizures were associated with in-hospital mortality for neonates. Hispanic ethnicity, congenital heart disease, and posterior plus anterior circulation stroke were associated with in-hospital mortality for children.
Beslow LA, Dowling MM, Hassanein SMA, et al. Mortality after pediatric arterial ischemic stroke. Pediatrics. 2018;141(5).
FDA Approves zEEG Dry Electrode Headset
The FDA has approved the zEEG dry electrode EEG headset for clinical use. The zEEG headset is backed by a cloud platform that allows users to upload data instantly, provides tools for analysis, and enables remote interpretation by neurologists. A clinical study found that the zEEG headset provided EEG signal quality that was comparable to that of an approved, traditional EEG system. In two study cohorts, a total of 30 patients were studied for time periods of as long as two hours, and the zEEG device performed at least as well as the reference device, based on predefined acceptance criteria. Study results will be published in the coming months. Zeto, headquartered in Santa Clara, California, markets the device.
South Asian Americans Have High Cardiovascular Mortality
South Asians living in the United States have higher mortality from heart conditions caused by atherosclerosis, such as heart attack and stroke, according to a study published online ahead of print May 24 in Circulation. Investigators reviewed the literature relevant to South Asian populations’ demographics and risk factors, health behaviors, and interventions, including physical activity, diet, medications, and community strategies. South Asians have higher proportional mortality rates from atherosclerotic cardiovascular disease, compared with other Asian groups, largely because of the lower risk observed in East Asian populations. A majority of the risk in South Asians can be explained by the increased prevalence of known risk factors, especially factors related to insulin resistance. The authors found no unique risk factors in this population.
Volgman AS, Palaniappan LS, Aggarwal NT, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation. 2018 May 24 [Epub ahead of print].
How Much Exercise Improves Cognition in Older Adults?
Exercising for at least 52 hours over six months is associated with improved cognitive performance in older adults with and without cognitive impairment, according to a study published online ahead of print May 30 in Neurology. Researchers reviewed data for 98 randomized, controlled exercise trials including 11,061 participants with an average age of 73. About 59% of the participants were healthy adults, 26% had mild cognitive impairment, and 15% had dementia. Researchers collected data on exercise session length, intensity, weekly frequency, and amount of exercise over time. Aerobic exercise was the most common form of exercise. In healthy people and people with cognitive impairment, longer term exposure to exercise, at least 52 hours conducted over an average of about six months, improved the brain’s processing speed.
Gomes-Osman J, Cabral DF, Morris TP, et al. Exercise for cognitive brain health in aging. Neurology. 2018 May 30 [Epub ahead of print].
Blood Biomarkers Detect Subconcussive Head Trauma
Blood biomarkers can detect the neurologic injury associated with repetitive subconcussive head trauma, according to a study published online ahead of print May 29 in the Journal of Neurosurgery. A total of 35 National Collegiate Athletic Association football players underwent blood sampling throughout the 2016 football season. Samples were analyzed for plasma concentrations of tau and serum concentrations of neurofilament light. Athletes were categorized as starters or nonstarters, and the investigators assessed between-group differences and time-course differences. In nonstarters, plasma concentrations of tau decreased over the season. Starters had lower plasma concentrations of tau. Plasma concentrations of tau could not be used to distinguish between starters and nonstarters. Serum concentrations of neurofilament light increased as head impacts increased, specifically in starters. Serum neurofilament light distinguished starters from nonstarters.
Oliver JM, Anzalone AJ, Stone JD, et al. Fluctuations in blood biomarkers of head trauma in NCAA football athletes over the course of a season. J Neurosurg. 2018 May 29 [Epub ahead of print].
Model Estimates Risk of Alzheimer’s Disease Dementia
Most people with preclinical Alzheimer’s disease will not develop dementia during their lifetimes, according to a study published online ahead of print May 7 in Alzheimer’s & Dementia. Researchers used a multistate model for Alzheimer’s disease along with US death rates to estimate lifetime and 10-year risks of Alzheimer’s disease dementia. Lifetime risks of Alzheimer’s disease dementia vary by age, gender, and preclinical or clinical disease state. A 70-year-old male with amyloid but no signs of neurodegeneration and no memory loss has a lifetime risk of 19.9%. The lifetime risks for a female with amyloidosis are 8.4% at age 90 and 29.3% at age 65. People younger than 85 with mild cognitive impairment, amyloidosis, and neurodegeneration have lifetime risks of Alzheimer’s disease dementia greater than 50%.
Brookmeyer R, Abdalla N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 2018 May 7 [Epub ahead of print].
Depression Is Associated With Brain and Memory Outcomes
In a sample of mostly Caribbean Hispanic, stroke-free older adults, greater depressive symptoms were associated with worse episodic memory, smaller cerebral volume, and silent infarcts, according to a study published online ahead of print May 9 in Neurology. Researchers analyzed data from the Northern Manhattan Study. A total of 1,111 participants underwent baseline evaluations of depressive symptoms, MRI markers, and cognitive function. At baseline, 22% of participants had greater depressive symptoms. Greater depressive symptoms were significantly associated with worse baseline episodic memory in models adjusted for sociodemographics, vascular risk factors, behavioral factors, and antidepressant medications. Furthermore, greater depressive symptoms were associated with smaller cerebral parenchymal fraction and increased odds of subclinical brain infarcts, after adjustment for sociodemographics, behavioral factors, and vascular risk factors.
Al Hazzouri AZ, Caunca MR, Nobrega JC. Greater depressive symptoms, cognition, and markers of brain aging. Neurology. 2018 May 9 [Epub ahead of print].
—Kimberly Williams
Adequate Sleep Associated With Lower Dementia Risk
Short and long daily sleep duration are risk factors for dementia and death in adults age 60 and older, according to a study published online ahead of print June 6 in the Journal of the American Geriatrics Society. In a prospective cohort study, researchers followed 1,517 adults without dementia for 10 years. Self-reported daily sleep durations were grouped into five categories. The association between daily sleep duration and risk of dementia and death was determined using Cox proportional hazards models. During follow-up, 294 participants developed dementia, and 282 died. Age- and sex-adjusted incidence rates of dementia and all-cause mortality were significantly greater in subjects who slept less than 5.0 hours/day or 10.0 or more hours/day than in people who slept from 5.0 to 6.9 hours/day.
Ohara T, Honda T, Hata J, et al. Association between daily sleep duration and risk of dementia and mortality in a Japanese community. J Am Geriatr Soc. 2018 Jun 6 [Epub ahead of print].
Rivaroxaban Not Superior to Aspirin for Stroke Prevention
Rivaroxaban is not superior to aspirin in the prevention of recurrent stroke, according to a study published June 7 in the New England Journal of Medicine. Researchers compared the efficacy and safety of rivaroxaban at a daily dose of 15 mg with aspirin at a daily dose of 100 mg for the prevention of recurrent stroke in patients with recent ischemic stroke that was presumed to be from cerebral embolism. The primary outcome was the first recurrence of ischemic or hemorrhagic stroke or systemic embolism in a time-to-event analysis. At 459 sites, 3,609 patients were randomly assigned to receive rivaroxaban, and 3,604 were randomized to aspirin. Recurrent ischemic stroke occurred in 172 patients in the rivaroxaban group and in 160 in the aspirin group.
Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191-2201.
Disintegrating Brain Lesions May Indicate Worsening MS
Atrophied lesion volume may indicate increasing disability in patients with multiple sclerosis (MS), according to a study published online ahead of print June 1 in the Journal of Neuroimaging. A total of 192 patients with clinically isolated syndrome or MS received 3T MRI at baseline and at five years. Investigators quantified lesions at baseline and calculated new and enlarging lesion volumes during the study interval. Atrophied lesion volume was calculated by combining baseline lesion masks with follow-up SIENAX-derived CSF partial volume maps. The researchers evaluated correlations between these measures and disability, as measured by the Expanded Disability Status Scale (EDSS). Atrophied lesion volume was different between MS subtypes and exceeded new lesion volume accumulation in progressive MS. Atrophied lesion volume was the only significant correlate of EDSS change.
Dwyer MG, Bergsland N, Ramasamy DP, et al. Atrophied brain lesion volume: a new imaging biomarker in multiple sclerosis. J Neuroimaging. 2018 Jun 1 [Epub ahead of print].
Do Migraineurs Seek Behavioral Treatment After a Referral?
A significant number of migraineurs are not using effective behavioral treatments for migraine, according to a study published online ahead of print June 5 in Pain Medicine. In a prospective cohort study, researchers tracked 234 patients with migraine who presented to an academic headache center and referred 69 of them for behavioral treatment with an appropriately trained therapist. Fifty-three of the referred patients completed a follow-up interview within three months of their initial appointment and were included in the analysis. Of the patients referred for behavioral treatment, 30 made an appointment. Investigators found no differences between people who started behavioral therapy and people who did not. Study authors did find that people who had previously seen a psychologist for migraine were more likely to initiate therapy.
Minen MT, Azarchi S, Sobolev R, et al. Factors related to migraine patients’ decisions to initiate behavioral migraine treatment following a headache specialist’s recommendation: a prospective observational study. Pain Med. 2018 Jun 5 [Epub ahead of print].
TIA Associated With Increased Five-Year Risk of Stroke
People with transient ischemic attack (TIA) are at risk for a cardiovascular event in the following five years, according to a study published June 7 in the New England Journal of Medicine. Researchers evaluated patients who had had a TIA within seven days before enrollment in a registry of TIA clinics. Of 61 sites, 42 had follow-up data on more than 50% of their enrolled patients at five years. The study’s primary outcome was a composite of stroke, acute coronary syndrome, or death from cardiovascular causes, with an emphasis on events that occurred in the second through fifth years. At five years, stroke had occurred in 345 of the 3,847 patients included in the follow-up study, and 149 of them had a stroke during the second through fifth years of follow-up.
Amarenco P, Lavallée PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378(23):2182-2190.
Follow-Up Care for TBI Is Not Delivered Adequately
Follow-up care for patients with traumatic brain injury (TBI) is not delivered optimally, according to a study published May 25 in JAMA Network Open. In a cohort study, researchers surveyed 831 participants in the Transforming Research and Clinical Knowledge in TBI initiative about their care after hospital discharge. Follow-up care was defined as providing TBI-related educational materials at discharge, calling patients within two weeks after release, seeing a healthcare provider within two weeks, and seeing a healthcare provider within three months. Approximately 42% of participants reported receiving TBI-related educational material at discharge, and 44% reported seeing a physician or other medical practitioner within three months after injury. Of patients with a positive finding on CT, 39% had not seen a medical practitioner at three months after injury.
Seabury SA, Gaudette E, Goldman DP, et al. Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: results from the TRACK-TBI study. JAMA Network Open. 2018;1(1):e180210.
Researchers Examine Mortality Rate of Pediatric Stroke
In-hospital mortality occurs in 2.6% of children with arterial ischemic stroke, according to a study published in the May issue of Pediatrics. The retrospective study included 915 infants younger than 1 month and 2,273 children age 1 month to 18 years with stroke at 87 hospitals in 24 countries. Death during hospitalization and cause of death were ascertained from medical records. A total of 14 neonates and 70 children died during hospitalization. Of 48 cases with reported causes of death, 31 were stroke-related. Remaining deaths were attributed to medical disease. In multivariable analysis, congenital heart disease, posterior plus anterior circulation stroke, and stroke presentation without seizures were associated with in-hospital mortality for neonates. Hispanic ethnicity, congenital heart disease, and posterior plus anterior circulation stroke were associated with in-hospital mortality for children.
Beslow LA, Dowling MM, Hassanein SMA, et al. Mortality after pediatric arterial ischemic stroke. Pediatrics. 2018;141(5).
FDA Approves zEEG Dry Electrode Headset
The FDA has approved the zEEG dry electrode EEG headset for clinical use. The zEEG headset is backed by a cloud platform that allows users to upload data instantly, provides tools for analysis, and enables remote interpretation by neurologists. A clinical study found that the zEEG headset provided EEG signal quality that was comparable to that of an approved, traditional EEG system. In two study cohorts, a total of 30 patients were studied for time periods of as long as two hours, and the zEEG device performed at least as well as the reference device, based on predefined acceptance criteria. Study results will be published in the coming months. Zeto, headquartered in Santa Clara, California, markets the device.
South Asian Americans Have High Cardiovascular Mortality
South Asians living in the United States have higher mortality from heart conditions caused by atherosclerosis, such as heart attack and stroke, according to a study published online ahead of print May 24 in Circulation. Investigators reviewed the literature relevant to South Asian populations’ demographics and risk factors, health behaviors, and interventions, including physical activity, diet, medications, and community strategies. South Asians have higher proportional mortality rates from atherosclerotic cardiovascular disease, compared with other Asian groups, largely because of the lower risk observed in East Asian populations. A majority of the risk in South Asians can be explained by the increased prevalence of known risk factors, especially factors related to insulin resistance. The authors found no unique risk factors in this population.
Volgman AS, Palaniappan LS, Aggarwal NT, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation. 2018 May 24 [Epub ahead of print].
How Much Exercise Improves Cognition in Older Adults?
Exercising for at least 52 hours over six months is associated with improved cognitive performance in older adults with and without cognitive impairment, according to a study published online ahead of print May 30 in Neurology. Researchers reviewed data for 98 randomized, controlled exercise trials including 11,061 participants with an average age of 73. About 59% of the participants were healthy adults, 26% had mild cognitive impairment, and 15% had dementia. Researchers collected data on exercise session length, intensity, weekly frequency, and amount of exercise over time. Aerobic exercise was the most common form of exercise. In healthy people and people with cognitive impairment, longer term exposure to exercise, at least 52 hours conducted over an average of about six months, improved the brain’s processing speed.
Gomes-Osman J, Cabral DF, Morris TP, et al. Exercise for cognitive brain health in aging. Neurology. 2018 May 30 [Epub ahead of print].
Blood Biomarkers Detect Subconcussive Head Trauma
Blood biomarkers can detect the neurologic injury associated with repetitive subconcussive head trauma, according to a study published online ahead of print May 29 in the Journal of Neurosurgery. A total of 35 National Collegiate Athletic Association football players underwent blood sampling throughout the 2016 football season. Samples were analyzed for plasma concentrations of tau and serum concentrations of neurofilament light. Athletes were categorized as starters or nonstarters, and the investigators assessed between-group differences and time-course differences. In nonstarters, plasma concentrations of tau decreased over the season. Starters had lower plasma concentrations of tau. Plasma concentrations of tau could not be used to distinguish between starters and nonstarters. Serum concentrations of neurofilament light increased as head impacts increased, specifically in starters. Serum neurofilament light distinguished starters from nonstarters.
Oliver JM, Anzalone AJ, Stone JD, et al. Fluctuations in blood biomarkers of head trauma in NCAA football athletes over the course of a season. J Neurosurg. 2018 May 29 [Epub ahead of print].
Model Estimates Risk of Alzheimer’s Disease Dementia
Most people with preclinical Alzheimer’s disease will not develop dementia during their lifetimes, according to a study published online ahead of print May 7 in Alzheimer’s & Dementia. Researchers used a multistate model for Alzheimer’s disease along with US death rates to estimate lifetime and 10-year risks of Alzheimer’s disease dementia. Lifetime risks of Alzheimer’s disease dementia vary by age, gender, and preclinical or clinical disease state. A 70-year-old male with amyloid but no signs of neurodegeneration and no memory loss has a lifetime risk of 19.9%. The lifetime risks for a female with amyloidosis are 8.4% at age 90 and 29.3% at age 65. People younger than 85 with mild cognitive impairment, amyloidosis, and neurodegeneration have lifetime risks of Alzheimer’s disease dementia greater than 50%.
Brookmeyer R, Abdalla N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 2018 May 7 [Epub ahead of print].
Depression Is Associated With Brain and Memory Outcomes
In a sample of mostly Caribbean Hispanic, stroke-free older adults, greater depressive symptoms were associated with worse episodic memory, smaller cerebral volume, and silent infarcts, according to a study published online ahead of print May 9 in Neurology. Researchers analyzed data from the Northern Manhattan Study. A total of 1,111 participants underwent baseline evaluations of depressive symptoms, MRI markers, and cognitive function. At baseline, 22% of participants had greater depressive symptoms. Greater depressive symptoms were significantly associated with worse baseline episodic memory in models adjusted for sociodemographics, vascular risk factors, behavioral factors, and antidepressant medications. Furthermore, greater depressive symptoms were associated with smaller cerebral parenchymal fraction and increased odds of subclinical brain infarcts, after adjustment for sociodemographics, behavioral factors, and vascular risk factors.
Al Hazzouri AZ, Caunca MR, Nobrega JC. Greater depressive symptoms, cognition, and markers of brain aging. Neurology. 2018 May 9 [Epub ahead of print].
—Kimberly Williams
Adequate Sleep Associated With Lower Dementia Risk
Short and long daily sleep duration are risk factors for dementia and death in adults age 60 and older, according to a study published online ahead of print June 6 in the Journal of the American Geriatrics Society. In a prospective cohort study, researchers followed 1,517 adults without dementia for 10 years. Self-reported daily sleep durations were grouped into five categories. The association between daily sleep duration and risk of dementia and death was determined using Cox proportional hazards models. During follow-up, 294 participants developed dementia, and 282 died. Age- and sex-adjusted incidence rates of dementia and all-cause mortality were significantly greater in subjects who slept less than 5.0 hours/day or 10.0 or more hours/day than in people who slept from 5.0 to 6.9 hours/day.
Ohara T, Honda T, Hata J, et al. Association between daily sleep duration and risk of dementia and mortality in a Japanese community. J Am Geriatr Soc. 2018 Jun 6 [Epub ahead of print].
Rivaroxaban Not Superior to Aspirin for Stroke Prevention
Rivaroxaban is not superior to aspirin in the prevention of recurrent stroke, according to a study published June 7 in the New England Journal of Medicine. Researchers compared the efficacy and safety of rivaroxaban at a daily dose of 15 mg with aspirin at a daily dose of 100 mg for the prevention of recurrent stroke in patients with recent ischemic stroke that was presumed to be from cerebral embolism. The primary outcome was the first recurrence of ischemic or hemorrhagic stroke or systemic embolism in a time-to-event analysis. At 459 sites, 3,609 patients were randomly assigned to receive rivaroxaban, and 3,604 were randomized to aspirin. Recurrent ischemic stroke occurred in 172 patients in the rivaroxaban group and in 160 in the aspirin group.
Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191-2201.
Disintegrating Brain Lesions May Indicate Worsening MS
Atrophied lesion volume may indicate increasing disability in patients with multiple sclerosis (MS), according to a study published online ahead of print June 1 in the Journal of Neuroimaging. A total of 192 patients with clinically isolated syndrome or MS received 3T MRI at baseline and at five years. Investigators quantified lesions at baseline and calculated new and enlarging lesion volumes during the study interval. Atrophied lesion volume was calculated by combining baseline lesion masks with follow-up SIENAX-derived CSF partial volume maps. The researchers evaluated correlations between these measures and disability, as measured by the Expanded Disability Status Scale (EDSS). Atrophied lesion volume was different between MS subtypes and exceeded new lesion volume accumulation in progressive MS. Atrophied lesion volume was the only significant correlate of EDSS change.
Dwyer MG, Bergsland N, Ramasamy DP, et al. Atrophied brain lesion volume: a new imaging biomarker in multiple sclerosis. J Neuroimaging. 2018 Jun 1 [Epub ahead of print].
Do Migraineurs Seek Behavioral Treatment After a Referral?
A significant number of migraineurs are not using effective behavioral treatments for migraine, according to a study published online ahead of print June 5 in Pain Medicine. In a prospective cohort study, researchers tracked 234 patients with migraine who presented to an academic headache center and referred 69 of them for behavioral treatment with an appropriately trained therapist. Fifty-three of the referred patients completed a follow-up interview within three months of their initial appointment and were included in the analysis. Of the patients referred for behavioral treatment, 30 made an appointment. Investigators found no differences between people who started behavioral therapy and people who did not. Study authors did find that people who had previously seen a psychologist for migraine were more likely to initiate therapy.
Minen MT, Azarchi S, Sobolev R, et al. Factors related to migraine patients’ decisions to initiate behavioral migraine treatment following a headache specialist’s recommendation: a prospective observational study. Pain Med. 2018 Jun 5 [Epub ahead of print].
TIA Associated With Increased Five-Year Risk of Stroke
People with transient ischemic attack (TIA) are at risk for a cardiovascular event in the following five years, according to a study published June 7 in the New England Journal of Medicine. Researchers evaluated patients who had had a TIA within seven days before enrollment in a registry of TIA clinics. Of 61 sites, 42 had follow-up data on more than 50% of their enrolled patients at five years. The study’s primary outcome was a composite of stroke, acute coronary syndrome, or death from cardiovascular causes, with an emphasis on events that occurred in the second through fifth years. At five years, stroke had occurred in 345 of the 3,847 patients included in the follow-up study, and 149 of them had a stroke during the second through fifth years of follow-up.
Amarenco P, Lavallée PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378(23):2182-2190.
Follow-Up Care for TBI Is Not Delivered Adequately
Follow-up care for patients with traumatic brain injury (TBI) is not delivered optimally, according to a study published May 25 in JAMA Network Open. In a cohort study, researchers surveyed 831 participants in the Transforming Research and Clinical Knowledge in TBI initiative about their care after hospital discharge. Follow-up care was defined as providing TBI-related educational materials at discharge, calling patients within two weeks after release, seeing a healthcare provider within two weeks, and seeing a healthcare provider within three months. Approximately 42% of participants reported receiving TBI-related educational material at discharge, and 44% reported seeing a physician or other medical practitioner within three months after injury. Of patients with a positive finding on CT, 39% had not seen a medical practitioner at three months after injury.
Seabury SA, Gaudette E, Goldman DP, et al. Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: results from the TRACK-TBI study. JAMA Network Open. 2018;1(1):e180210.
Researchers Examine Mortality Rate of Pediatric Stroke
In-hospital mortality occurs in 2.6% of children with arterial ischemic stroke, according to a study published in the May issue of Pediatrics. The retrospective study included 915 infants younger than 1 month and 2,273 children age 1 month to 18 years with stroke at 87 hospitals in 24 countries. Death during hospitalization and cause of death were ascertained from medical records. A total of 14 neonates and 70 children died during hospitalization. Of 48 cases with reported causes of death, 31 were stroke-related. Remaining deaths were attributed to medical disease. In multivariable analysis, congenital heart disease, posterior plus anterior circulation stroke, and stroke presentation without seizures were associated with in-hospital mortality for neonates. Hispanic ethnicity, congenital heart disease, and posterior plus anterior circulation stroke were associated with in-hospital mortality for children.
Beslow LA, Dowling MM, Hassanein SMA, et al. Mortality after pediatric arterial ischemic stroke. Pediatrics. 2018;141(5).
FDA Approves zEEG Dry Electrode Headset
The FDA has approved the zEEG dry electrode EEG headset for clinical use. The zEEG headset is backed by a cloud platform that allows users to upload data instantly, provides tools for analysis, and enables remote interpretation by neurologists. A clinical study found that the zEEG headset provided EEG signal quality that was comparable to that of an approved, traditional EEG system. In two study cohorts, a total of 30 patients were studied for time periods of as long as two hours, and the zEEG device performed at least as well as the reference device, based on predefined acceptance criteria. Study results will be published in the coming months. Zeto, headquartered in Santa Clara, California, markets the device.
South Asian Americans Have High Cardiovascular Mortality
South Asians living in the United States have higher mortality from heart conditions caused by atherosclerosis, such as heart attack and stroke, according to a study published online ahead of print May 24 in Circulation. Investigators reviewed the literature relevant to South Asian populations’ demographics and risk factors, health behaviors, and interventions, including physical activity, diet, medications, and community strategies. South Asians have higher proportional mortality rates from atherosclerotic cardiovascular disease, compared with other Asian groups, largely because of the lower risk observed in East Asian populations. A majority of the risk in South Asians can be explained by the increased prevalence of known risk factors, especially factors related to insulin resistance. The authors found no unique risk factors in this population.
Volgman AS, Palaniappan LS, Aggarwal NT, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation. 2018 May 24 [Epub ahead of print].
How Much Exercise Improves Cognition in Older Adults?
Exercising for at least 52 hours over six months is associated with improved cognitive performance in older adults with and without cognitive impairment, according to a study published online ahead of print May 30 in Neurology. Researchers reviewed data for 98 randomized, controlled exercise trials including 11,061 participants with an average age of 73. About 59% of the participants were healthy adults, 26% had mild cognitive impairment, and 15% had dementia. Researchers collected data on exercise session length, intensity, weekly frequency, and amount of exercise over time. Aerobic exercise was the most common form of exercise. In healthy people and people with cognitive impairment, longer term exposure to exercise, at least 52 hours conducted over an average of about six months, improved the brain’s processing speed.
Gomes-Osman J, Cabral DF, Morris TP, et al. Exercise for cognitive brain health in aging. Neurology. 2018 May 30 [Epub ahead of print].
Blood Biomarkers Detect Subconcussive Head Trauma
Blood biomarkers can detect the neurologic injury associated with repetitive subconcussive head trauma, according to a study published online ahead of print May 29 in the Journal of Neurosurgery. A total of 35 National Collegiate Athletic Association football players underwent blood sampling throughout the 2016 football season. Samples were analyzed for plasma concentrations of tau and serum concentrations of neurofilament light. Athletes were categorized as starters or nonstarters, and the investigators assessed between-group differences and time-course differences. In nonstarters, plasma concentrations of tau decreased over the season. Starters had lower plasma concentrations of tau. Plasma concentrations of tau could not be used to distinguish between starters and nonstarters. Serum concentrations of neurofilament light increased as head impacts increased, specifically in starters. Serum neurofilament light distinguished starters from nonstarters.
Oliver JM, Anzalone AJ, Stone JD, et al. Fluctuations in blood biomarkers of head trauma in NCAA football athletes over the course of a season. J Neurosurg. 2018 May 29 [Epub ahead of print].
Model Estimates Risk of Alzheimer’s Disease Dementia
Most people with preclinical Alzheimer’s disease will not develop dementia during their lifetimes, according to a study published online ahead of print May 7 in Alzheimer’s & Dementia. Researchers used a multistate model for Alzheimer’s disease along with US death rates to estimate lifetime and 10-year risks of Alzheimer’s disease dementia. Lifetime risks of Alzheimer’s disease dementia vary by age, gender, and preclinical or clinical disease state. A 70-year-old male with amyloid but no signs of neurodegeneration and no memory loss has a lifetime risk of 19.9%. The lifetime risks for a female with amyloidosis are 8.4% at age 90 and 29.3% at age 65. People younger than 85 with mild cognitive impairment, amyloidosis, and neurodegeneration have lifetime risks of Alzheimer’s disease dementia greater than 50%.
Brookmeyer R, Abdalla N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 2018 May 7 [Epub ahead of print].
Depression Is Associated With Brain and Memory Outcomes
In a sample of mostly Caribbean Hispanic, stroke-free older adults, greater depressive symptoms were associated with worse episodic memory, smaller cerebral volume, and silent infarcts, according to a study published online ahead of print May 9 in Neurology. Researchers analyzed data from the Northern Manhattan Study. A total of 1,111 participants underwent baseline evaluations of depressive symptoms, MRI markers, and cognitive function. At baseline, 22% of participants had greater depressive symptoms. Greater depressive symptoms were significantly associated with worse baseline episodic memory in models adjusted for sociodemographics, vascular risk factors, behavioral factors, and antidepressant medications. Furthermore, greater depressive symptoms were associated with smaller cerebral parenchymal fraction and increased odds of subclinical brain infarcts, after adjustment for sociodemographics, behavioral factors, and vascular risk factors.
Al Hazzouri AZ, Caunca MR, Nobrega JC. Greater depressive symptoms, cognition, and markers of brain aging. Neurology. 2018 May 9 [Epub ahead of print].
—Kimberly Williams
Study spotlights risk factors for albuminuria in youth with T2DM
TORONTO – When Brandy Wicklow, MD, began her pediatric endocrinology fellowship at McGill University in 2006, about 12 per 100,000 children in Manitoba, Canada, were diagnosed with type 2 diabetes mellitus each year. By 2016 that rate had more than doubled, to 26 per 100,000 children.
“If you look just at indigenous youth in our province, it’s probably one of the highest rates ever reported, with 95 per 100,000 Manitoba First Nation children diagnosed with type 2 diabetes,” said Dr. Wicklow, a pediatric endocrinologist at the University of Manitoba and the Children’s Hospital Research Institute of Manitoba.
Many indigenous populations also face an increased risk for primary renal disease. One study reviewed the charts 90 of Canadian First Nation children and adolescents with T2DM (Diabetes Care. 2009;32[5]:786-90). Of 10 who had renal biopsies performed, nine had immune complex disease/glomerulosclerosis, two had mild diabetes-related lesions, and seven had focal segmental glomerulosclerosis (FSGS); yet none had classic nephropathy. An analysis of Chinese youth that included 216 renal biopsies yielded similar findings (Intl Urol Nephrol. 2012;45[1]:173-9).
It’s also known that early-onset T2DM is associated with substantially increased incidence of end-stage renal disease (ESRD) and mortality in middle age. For example, one study of Pima Indians found that those who were diagnosed with T2DM earlier than 20 years of age had a one in five chance of developing ESRD, while those who were diagnosed at age 20 years or older had a one in two chance of ESRD (JAMA. 2006;296[4]:421-6). In a separate analysis, researchers estimated the remaining lifetime risks for ESRD among Aboriginal people in Australia with and without diabetes (Diabetes Res Clin Pract. 2014;103[3]:e24-6). The value for young adults with diabetes was high, about one in two at the age of 30 years, while it decreased with age to one in seven at 60 years.
“One of the first biomarkers we see in terms of renal disease in kids with T2DM is albuminuria,” Dr. Wicklow said at the Pediatric Academic Societies meeting. “The question is, why do kids with type 2 get more renal disease than kids with type 1 diabetes?” The SEARCH for Diabetes in Youth (SEARCH) study from 2006 found that hypertension, increased body mass index, increased weight circumference, and increased lipids were factors, while the SEARCH study from 2015 found that ethnicity, increased weight to height ratio, and mean arterial pressure were factors.
“Insulin resistance is significantly associated with albuminuria,” Dr. Wicklow continued. “It’s also been shown to be associated with hyperfiltration. Some of the markers of insulin resistance are important but they make up about 19% of the variance between type 1 and type 2, which means there are other variables that we’re not measuring.”
Enter ICARE (Improving Renal Complications in Adolescents with Type 2 Diabetes through Research), an ongoing prospective cohort study that Dr. Wicklow and her associates launched in 2014 at eight centers in Canada. It aims to examine the biopsychosocial risk factors for albuminuria in youth with T2DM and the mechanisms for renal injury. “Our theoretical framework was that biological exposures that we are aware of, such as glycemic control, hypertension, and lipids, would all be important in the development of albuminuria and renal disease in kids,” said Dr. Wicklow, who is the study’s coprimary investigator along with Allison Dart, MD. “But what we thought was novel was that psychological exposures either as socioeconomic status or as mental health factors would also directly impinge on renal health with respect to chronic inflammation in the body, inflammation in the kidneys, and long-term kidney damage.”
The first phase of ICARE involved a detailed phenotypic assessment of youth, including anthropometrics, biochemistry, 24-hour ambulatory blood pressure monitoring, overnight urine collections for albumin excretion, renal ultrasound, and iohexol-derived glomerular filtration rate (GFR). Phase 2 included an evaluation of psychological factors, including hair-derived cortisol; validated questionnaires for perceived stress, distress, and resiliency; and a detailed evaluation of systemic and urine inflammatory biomarkers. Annual follow-up is carried out to assess temporal associations between clinical risk factors and renal outcomes, including progression of albuminuria.
At the meeting, Dr. Wicklow reported on 187 youth enrolled to date. Of these, 96% were of indigenous ethnicity, 57 had albuminuria and 130 did not, and the mean ages of the groups were 16 years and 15 years, respectively. At baseline, a higher proportion of those in the albuminuria group were female (74% vs. 64% of those in the no albuminuria group, respectively), had a higher mean hemoglobin A1c (11% vs. 9%), and had hypertension (94% vs. 72%). She noted that upon presentation to the clinic, only 23% of participants had HbA1c levels less than 7%, only 26% had ranges between 7% and 9%, and about 40% did not have any hypertension. Of those who did, 27% had nighttime-only hypertension, and only 2% had daytime-only hypertension.
“The other risk factor these kids have for developing ESRD is that the majority were exposed to diabetes in pregnancy,” Dr. Wicklow said. “Murine models of maternal diabetes exposure have demonstrated that offspring have small kidneys, less ureteric bud branching, and a lower number of nephrons. Most of the human clinical cohort studies look at associations between development of diabetes and parental hypertension, maternal smoking, and maternal education. There is likely an impact at birth that sets these kids up for development of type 2 diabetes.”
In addition, results from clinical cohort studies have found that depression, mental stress, and distress are high in youth with T2DM. “Preliminary data suggest that if you have positive mental health, or coping strategies, or someone has worked through this with you and you are resilient, you might benefit in terms of overall glycemic control,” she said. For example, ICARE investigators have found that the higher the score on the Kessler Psychological Distress Scale (K6), the greater the risk of renal inflammation as measured by monocyte chemotactic protein-1 (MCP-1; P = .02). “Mental health seems to be something that can directly impact your health from a biological standpoint, and we might be able to find biomarkers of that risk,” Dr. Wicklow said. “Where does the stress come from? Most of my patients are indigenous, so it’s not surprising that the history in Canada of colonization of residential schools has left a lasting impression on these families and communities in terms of loss of language, loss of culture, and loss of land. There’s a community-based stress and a family-based stress that these children feel.”
Social factors also play a big role. She presented baseline findings from 196 youth with T2DM and 456 with T1DM, including measures such as the Socioeconomic Factor Index-Version 2 (SEFI-2), a way to assess socioeconomic characteristics based on Canadian Census data that reflects nonmedical social determinants of health. “It looks at factors like number of rooms in the house, single-parent households, maternal education attainment, and family income,” Dr. Wicklow explained. “The higher the SEFI-2 score, the lower your socioeconomic status is for the area you live in. Kids with T2DM generally live in areas of lower SES and lower socioeconomic index. They often live far away from health care providers. Many do not attend school and many are not with their biologic families, so we’ve had a lot of issues addressing child and family services, in particular in the phase of a chronic illness where our expectation is one thing and the family’s and community’s expectations of what’s realistic in terms of treatment and goals is another. We also have a lot of adolescent pregnancies.”
To date, about 80% of youth with T1D have seen a health care provider within the first year after transition from the pediatric diabetes clinic, compared with just over 50% of kids with T2D. “We transition youth with T1DM to internists, while our youth with T2DM go to itinerant physicians often back in their communities and/or rural family physicians,” she said. Between baseline and year 2, the rate of hospital admissions remained similar among T1DM at 11.6 and 11.8 admissions per 100 patient-years, respectively, but the number of hospital admissions for T2DM patients jumped from 20.1 to 25.5 admissions per 100 patient-years. “Kids with type 2 are showing up in the hospital a lot more than those with type 1 diabetes, but not for diabetes-related diagnoses,” Dr. Wicklow said. “We’re starting to look through the data now, and most of our kids are showing up with mental health complaints and issues. That’s why they’re getting hospitalized.”
Among ICARE study participants who have completed 3 years of follow-up, about 52% had albuminuria at their baseline visit and 48% sustained albuminuria throughout the study. About 26% progressed from normal levels of albuminuria to microalbuminuria, from microalbuminuria to macroalbuminuria, or from normal levels of albuminuria to macroalbuminuria. In addition, 16% persisted in the category that they were in, and 10% regressed. “The good news is, some of our kids get better over time,” Dr. Wicklow said. “The bad news is that the majority do not.”
Going forward, Dr. Wicklow and her associates work with an ICARE advisory group composed of children and families “who sit with us and talk about what mental health needs might be important, and how we should organize our study in a follow-up of the kids, to try and answer some of the questions that are important,” she said. “Working with the concept of the study’s theoretical framework, they acknowledged that the biological exposures are important, but they were also concerned about food security, finding strength/resilience within the community, and finding coping factors in terms of keeping themselves healthy with their diabetes. For some communities, they are concerned with basic needs. We’re working with them to help them progress, and to figure out how to best study children with type 2 diabetes.”
ICARE has received support from Diabetes Canada, Research Manitoba, the Canadian Institutes of Health Research, the Children’s Hospital Research Institute of Manitoba (specifically the Diabetes Research Envisioned and Accomplished in Manitoba (DREAM) theme), and the University of Manitoba. Dr. Wicklow reported having no financial disclosures.
TORONTO – When Brandy Wicklow, MD, began her pediatric endocrinology fellowship at McGill University in 2006, about 12 per 100,000 children in Manitoba, Canada, were diagnosed with type 2 diabetes mellitus each year. By 2016 that rate had more than doubled, to 26 per 100,000 children.
“If you look just at indigenous youth in our province, it’s probably one of the highest rates ever reported, with 95 per 100,000 Manitoba First Nation children diagnosed with type 2 diabetes,” said Dr. Wicklow, a pediatric endocrinologist at the University of Manitoba and the Children’s Hospital Research Institute of Manitoba.
Many indigenous populations also face an increased risk for primary renal disease. One study reviewed the charts 90 of Canadian First Nation children and adolescents with T2DM (Diabetes Care. 2009;32[5]:786-90). Of 10 who had renal biopsies performed, nine had immune complex disease/glomerulosclerosis, two had mild diabetes-related lesions, and seven had focal segmental glomerulosclerosis (FSGS); yet none had classic nephropathy. An analysis of Chinese youth that included 216 renal biopsies yielded similar findings (Intl Urol Nephrol. 2012;45[1]:173-9).
It’s also known that early-onset T2DM is associated with substantially increased incidence of end-stage renal disease (ESRD) and mortality in middle age. For example, one study of Pima Indians found that those who were diagnosed with T2DM earlier than 20 years of age had a one in five chance of developing ESRD, while those who were diagnosed at age 20 years or older had a one in two chance of ESRD (JAMA. 2006;296[4]:421-6). In a separate analysis, researchers estimated the remaining lifetime risks for ESRD among Aboriginal people in Australia with and without diabetes (Diabetes Res Clin Pract. 2014;103[3]:e24-6). The value for young adults with diabetes was high, about one in two at the age of 30 years, while it decreased with age to one in seven at 60 years.
“One of the first biomarkers we see in terms of renal disease in kids with T2DM is albuminuria,” Dr. Wicklow said at the Pediatric Academic Societies meeting. “The question is, why do kids with type 2 get more renal disease than kids with type 1 diabetes?” The SEARCH for Diabetes in Youth (SEARCH) study from 2006 found that hypertension, increased body mass index, increased weight circumference, and increased lipids were factors, while the SEARCH study from 2015 found that ethnicity, increased weight to height ratio, and mean arterial pressure were factors.
“Insulin resistance is significantly associated with albuminuria,” Dr. Wicklow continued. “It’s also been shown to be associated with hyperfiltration. Some of the markers of insulin resistance are important but they make up about 19% of the variance between type 1 and type 2, which means there are other variables that we’re not measuring.”
Enter ICARE (Improving Renal Complications in Adolescents with Type 2 Diabetes through Research), an ongoing prospective cohort study that Dr. Wicklow and her associates launched in 2014 at eight centers in Canada. It aims to examine the biopsychosocial risk factors for albuminuria in youth with T2DM and the mechanisms for renal injury. “Our theoretical framework was that biological exposures that we are aware of, such as glycemic control, hypertension, and lipids, would all be important in the development of albuminuria and renal disease in kids,” said Dr. Wicklow, who is the study’s coprimary investigator along with Allison Dart, MD. “But what we thought was novel was that psychological exposures either as socioeconomic status or as mental health factors would also directly impinge on renal health with respect to chronic inflammation in the body, inflammation in the kidneys, and long-term kidney damage.”
The first phase of ICARE involved a detailed phenotypic assessment of youth, including anthropometrics, biochemistry, 24-hour ambulatory blood pressure monitoring, overnight urine collections for albumin excretion, renal ultrasound, and iohexol-derived glomerular filtration rate (GFR). Phase 2 included an evaluation of psychological factors, including hair-derived cortisol; validated questionnaires for perceived stress, distress, and resiliency; and a detailed evaluation of systemic and urine inflammatory biomarkers. Annual follow-up is carried out to assess temporal associations between clinical risk factors and renal outcomes, including progression of albuminuria.
At the meeting, Dr. Wicklow reported on 187 youth enrolled to date. Of these, 96% were of indigenous ethnicity, 57 had albuminuria and 130 did not, and the mean ages of the groups were 16 years and 15 years, respectively. At baseline, a higher proportion of those in the albuminuria group were female (74% vs. 64% of those in the no albuminuria group, respectively), had a higher mean hemoglobin A1c (11% vs. 9%), and had hypertension (94% vs. 72%). She noted that upon presentation to the clinic, only 23% of participants had HbA1c levels less than 7%, only 26% had ranges between 7% and 9%, and about 40% did not have any hypertension. Of those who did, 27% had nighttime-only hypertension, and only 2% had daytime-only hypertension.
“The other risk factor these kids have for developing ESRD is that the majority were exposed to diabetes in pregnancy,” Dr. Wicklow said. “Murine models of maternal diabetes exposure have demonstrated that offspring have small kidneys, less ureteric bud branching, and a lower number of nephrons. Most of the human clinical cohort studies look at associations between development of diabetes and parental hypertension, maternal smoking, and maternal education. There is likely an impact at birth that sets these kids up for development of type 2 diabetes.”
In addition, results from clinical cohort studies have found that depression, mental stress, and distress are high in youth with T2DM. “Preliminary data suggest that if you have positive mental health, or coping strategies, or someone has worked through this with you and you are resilient, you might benefit in terms of overall glycemic control,” she said. For example, ICARE investigators have found that the higher the score on the Kessler Psychological Distress Scale (K6), the greater the risk of renal inflammation as measured by monocyte chemotactic protein-1 (MCP-1; P = .02). “Mental health seems to be something that can directly impact your health from a biological standpoint, and we might be able to find biomarkers of that risk,” Dr. Wicklow said. “Where does the stress come from? Most of my patients are indigenous, so it’s not surprising that the history in Canada of colonization of residential schools has left a lasting impression on these families and communities in terms of loss of language, loss of culture, and loss of land. There’s a community-based stress and a family-based stress that these children feel.”
Social factors also play a big role. She presented baseline findings from 196 youth with T2DM and 456 with T1DM, including measures such as the Socioeconomic Factor Index-Version 2 (SEFI-2), a way to assess socioeconomic characteristics based on Canadian Census data that reflects nonmedical social determinants of health. “It looks at factors like number of rooms in the house, single-parent households, maternal education attainment, and family income,” Dr. Wicklow explained. “The higher the SEFI-2 score, the lower your socioeconomic status is for the area you live in. Kids with T2DM generally live in areas of lower SES and lower socioeconomic index. They often live far away from health care providers. Many do not attend school and many are not with their biologic families, so we’ve had a lot of issues addressing child and family services, in particular in the phase of a chronic illness where our expectation is one thing and the family’s and community’s expectations of what’s realistic in terms of treatment and goals is another. We also have a lot of adolescent pregnancies.”
To date, about 80% of youth with T1D have seen a health care provider within the first year after transition from the pediatric diabetes clinic, compared with just over 50% of kids with T2D. “We transition youth with T1DM to internists, while our youth with T2DM go to itinerant physicians often back in their communities and/or rural family physicians,” she said. Between baseline and year 2, the rate of hospital admissions remained similar among T1DM at 11.6 and 11.8 admissions per 100 patient-years, respectively, but the number of hospital admissions for T2DM patients jumped from 20.1 to 25.5 admissions per 100 patient-years. “Kids with type 2 are showing up in the hospital a lot more than those with type 1 diabetes, but not for diabetes-related diagnoses,” Dr. Wicklow said. “We’re starting to look through the data now, and most of our kids are showing up with mental health complaints and issues. That’s why they’re getting hospitalized.”
Among ICARE study participants who have completed 3 years of follow-up, about 52% had albuminuria at their baseline visit and 48% sustained albuminuria throughout the study. About 26% progressed from normal levels of albuminuria to microalbuminuria, from microalbuminuria to macroalbuminuria, or from normal levels of albuminuria to macroalbuminuria. In addition, 16% persisted in the category that they were in, and 10% regressed. “The good news is, some of our kids get better over time,” Dr. Wicklow said. “The bad news is that the majority do not.”
Going forward, Dr. Wicklow and her associates work with an ICARE advisory group composed of children and families “who sit with us and talk about what mental health needs might be important, and how we should organize our study in a follow-up of the kids, to try and answer some of the questions that are important,” she said. “Working with the concept of the study’s theoretical framework, they acknowledged that the biological exposures are important, but they were also concerned about food security, finding strength/resilience within the community, and finding coping factors in terms of keeping themselves healthy with their diabetes. For some communities, they are concerned with basic needs. We’re working with them to help them progress, and to figure out how to best study children with type 2 diabetes.”
ICARE has received support from Diabetes Canada, Research Manitoba, the Canadian Institutes of Health Research, the Children’s Hospital Research Institute of Manitoba (specifically the Diabetes Research Envisioned and Accomplished in Manitoba (DREAM) theme), and the University of Manitoba. Dr. Wicklow reported having no financial disclosures.
TORONTO – When Brandy Wicklow, MD, began her pediatric endocrinology fellowship at McGill University in 2006, about 12 per 100,000 children in Manitoba, Canada, were diagnosed with type 2 diabetes mellitus each year. By 2016 that rate had more than doubled, to 26 per 100,000 children.
“If you look just at indigenous youth in our province, it’s probably one of the highest rates ever reported, with 95 per 100,000 Manitoba First Nation children diagnosed with type 2 diabetes,” said Dr. Wicklow, a pediatric endocrinologist at the University of Manitoba and the Children’s Hospital Research Institute of Manitoba.
Many indigenous populations also face an increased risk for primary renal disease. One study reviewed the charts 90 of Canadian First Nation children and adolescents with T2DM (Diabetes Care. 2009;32[5]:786-90). Of 10 who had renal biopsies performed, nine had immune complex disease/glomerulosclerosis, two had mild diabetes-related lesions, and seven had focal segmental glomerulosclerosis (FSGS); yet none had classic nephropathy. An analysis of Chinese youth that included 216 renal biopsies yielded similar findings (Intl Urol Nephrol. 2012;45[1]:173-9).
It’s also known that early-onset T2DM is associated with substantially increased incidence of end-stage renal disease (ESRD) and mortality in middle age. For example, one study of Pima Indians found that those who were diagnosed with T2DM earlier than 20 years of age had a one in five chance of developing ESRD, while those who were diagnosed at age 20 years or older had a one in two chance of ESRD (JAMA. 2006;296[4]:421-6). In a separate analysis, researchers estimated the remaining lifetime risks for ESRD among Aboriginal people in Australia with and without diabetes (Diabetes Res Clin Pract. 2014;103[3]:e24-6). The value for young adults with diabetes was high, about one in two at the age of 30 years, while it decreased with age to one in seven at 60 years.
“One of the first biomarkers we see in terms of renal disease in kids with T2DM is albuminuria,” Dr. Wicklow said at the Pediatric Academic Societies meeting. “The question is, why do kids with type 2 get more renal disease than kids with type 1 diabetes?” The SEARCH for Diabetes in Youth (SEARCH) study from 2006 found that hypertension, increased body mass index, increased weight circumference, and increased lipids were factors, while the SEARCH study from 2015 found that ethnicity, increased weight to height ratio, and mean arterial pressure were factors.
“Insulin resistance is significantly associated with albuminuria,” Dr. Wicklow continued. “It’s also been shown to be associated with hyperfiltration. Some of the markers of insulin resistance are important but they make up about 19% of the variance between type 1 and type 2, which means there are other variables that we’re not measuring.”
Enter ICARE (Improving Renal Complications in Adolescents with Type 2 Diabetes through Research), an ongoing prospective cohort study that Dr. Wicklow and her associates launched in 2014 at eight centers in Canada. It aims to examine the biopsychosocial risk factors for albuminuria in youth with T2DM and the mechanisms for renal injury. “Our theoretical framework was that biological exposures that we are aware of, such as glycemic control, hypertension, and lipids, would all be important in the development of albuminuria and renal disease in kids,” said Dr. Wicklow, who is the study’s coprimary investigator along with Allison Dart, MD. “But what we thought was novel was that psychological exposures either as socioeconomic status or as mental health factors would also directly impinge on renal health with respect to chronic inflammation in the body, inflammation in the kidneys, and long-term kidney damage.”
The first phase of ICARE involved a detailed phenotypic assessment of youth, including anthropometrics, biochemistry, 24-hour ambulatory blood pressure monitoring, overnight urine collections for albumin excretion, renal ultrasound, and iohexol-derived glomerular filtration rate (GFR). Phase 2 included an evaluation of psychological factors, including hair-derived cortisol; validated questionnaires for perceived stress, distress, and resiliency; and a detailed evaluation of systemic and urine inflammatory biomarkers. Annual follow-up is carried out to assess temporal associations between clinical risk factors and renal outcomes, including progression of albuminuria.
At the meeting, Dr. Wicklow reported on 187 youth enrolled to date. Of these, 96% were of indigenous ethnicity, 57 had albuminuria and 130 did not, and the mean ages of the groups were 16 years and 15 years, respectively. At baseline, a higher proportion of those in the albuminuria group were female (74% vs. 64% of those in the no albuminuria group, respectively), had a higher mean hemoglobin A1c (11% vs. 9%), and had hypertension (94% vs. 72%). She noted that upon presentation to the clinic, only 23% of participants had HbA1c levels less than 7%, only 26% had ranges between 7% and 9%, and about 40% did not have any hypertension. Of those who did, 27% had nighttime-only hypertension, and only 2% had daytime-only hypertension.
“The other risk factor these kids have for developing ESRD is that the majority were exposed to diabetes in pregnancy,” Dr. Wicklow said. “Murine models of maternal diabetes exposure have demonstrated that offspring have small kidneys, less ureteric bud branching, and a lower number of nephrons. Most of the human clinical cohort studies look at associations between development of diabetes and parental hypertension, maternal smoking, and maternal education. There is likely an impact at birth that sets these kids up for development of type 2 diabetes.”
In addition, results from clinical cohort studies have found that depression, mental stress, and distress are high in youth with T2DM. “Preliminary data suggest that if you have positive mental health, or coping strategies, or someone has worked through this with you and you are resilient, you might benefit in terms of overall glycemic control,” she said. For example, ICARE investigators have found that the higher the score on the Kessler Psychological Distress Scale (K6), the greater the risk of renal inflammation as measured by monocyte chemotactic protein-1 (MCP-1; P = .02). “Mental health seems to be something that can directly impact your health from a biological standpoint, and we might be able to find biomarkers of that risk,” Dr. Wicklow said. “Where does the stress come from? Most of my patients are indigenous, so it’s not surprising that the history in Canada of colonization of residential schools has left a lasting impression on these families and communities in terms of loss of language, loss of culture, and loss of land. There’s a community-based stress and a family-based stress that these children feel.”
Social factors also play a big role. She presented baseline findings from 196 youth with T2DM and 456 with T1DM, including measures such as the Socioeconomic Factor Index-Version 2 (SEFI-2), a way to assess socioeconomic characteristics based on Canadian Census data that reflects nonmedical social determinants of health. “It looks at factors like number of rooms in the house, single-parent households, maternal education attainment, and family income,” Dr. Wicklow explained. “The higher the SEFI-2 score, the lower your socioeconomic status is for the area you live in. Kids with T2DM generally live in areas of lower SES and lower socioeconomic index. They often live far away from health care providers. Many do not attend school and many are not with their biologic families, so we’ve had a lot of issues addressing child and family services, in particular in the phase of a chronic illness where our expectation is one thing and the family’s and community’s expectations of what’s realistic in terms of treatment and goals is another. We also have a lot of adolescent pregnancies.”
To date, about 80% of youth with T1D have seen a health care provider within the first year after transition from the pediatric diabetes clinic, compared with just over 50% of kids with T2D. “We transition youth with T1DM to internists, while our youth with T2DM go to itinerant physicians often back in their communities and/or rural family physicians,” she said. Between baseline and year 2, the rate of hospital admissions remained similar among T1DM at 11.6 and 11.8 admissions per 100 patient-years, respectively, but the number of hospital admissions for T2DM patients jumped from 20.1 to 25.5 admissions per 100 patient-years. “Kids with type 2 are showing up in the hospital a lot more than those with type 1 diabetes, but not for diabetes-related diagnoses,” Dr. Wicklow said. “We’re starting to look through the data now, and most of our kids are showing up with mental health complaints and issues. That’s why they’re getting hospitalized.”
Among ICARE study participants who have completed 3 years of follow-up, about 52% had albuminuria at their baseline visit and 48% sustained albuminuria throughout the study. About 26% progressed from normal levels of albuminuria to microalbuminuria, from microalbuminuria to macroalbuminuria, or from normal levels of albuminuria to macroalbuminuria. In addition, 16% persisted in the category that they were in, and 10% regressed. “The good news is, some of our kids get better over time,” Dr. Wicklow said. “The bad news is that the majority do not.”
Going forward, Dr. Wicklow and her associates work with an ICARE advisory group composed of children and families “who sit with us and talk about what mental health needs might be important, and how we should organize our study in a follow-up of the kids, to try and answer some of the questions that are important,” she said. “Working with the concept of the study’s theoretical framework, they acknowledged that the biological exposures are important, but they were also concerned about food security, finding strength/resilience within the community, and finding coping factors in terms of keeping themselves healthy with their diabetes. For some communities, they are concerned with basic needs. We’re working with them to help them progress, and to figure out how to best study children with type 2 diabetes.”
ICARE has received support from Diabetes Canada, Research Manitoba, the Canadian Institutes of Health Research, the Children’s Hospital Research Institute of Manitoba (specifically the Diabetes Research Envisioned and Accomplished in Manitoba (DREAM) theme), and the University of Manitoba. Dr. Wicklow reported having no financial disclosures.
AT PAS 18
Key clinical point: Mental health may indirectly increase inflammation, which contributes to kidney health.
Major finding: The higher the score on the Kessler Psychological Distress Scale (K6), the greater the risk of renal inflammation as measured by MCP-1 (P = .02).
Study details: Preliminary results from ICARE (Improving Renal Complications in Adolescents with Type 2 Diabetes through Research), an ongoing prospective cohort study.
Disclosures: ICARE has received support from Diabetes Canada, Research Manitoba, the Canadian Institutes of Health Research, the Children’s Hospital Research Institute of Manitoba (specifically the Diabetes Research Envisioned and Accomplished in Manitoba [DREAM] theme), and the University of Manitoba. Dr. Wicklow reported having no financial disclosures.
Primary cirrhotic prophylaxis of bacterial peritonitis falls short
WASHINGTON –
The mortality rate during follow-up of cirrhotic patients hospitalized while on primary prophylaxis against spontaneous bacterial peritonitis (SBP) was 19%, compared with a 9% death rate among cirrhotic patients hospitalized while on secondary prophylaxis, Jasmohan S. Bajaj, MD, said at the annual Digestive Disease Week®.
Although the findings raised questions about the value of primary prophylaxis with an antibiotic in cirrhotic patients for preventing a first episode of SBP, secondary prophylaxis remains an important precaution.
“There is clear benefit from secondary prophylaxis; please use it. The data supporting it are robust,” said Dr. Bajaj, a hepatologist at Virginia Commonwealth University, Richmond. In contrast, the evidence supporting benefits from primary prophylaxis is weaker, he said. The findings were also counterintuitive, because patients who experience repeat episodes of SBP might be expected to fare worse than those hit by SBP just once.
Dr. Bajaj also acknowledged the substantial confounding that distinguishes patients with cirrhosis receiving primary or secondary prophylaxis, and the difficulty of fully adjusting for all this confounding by statistical analyses. “There is selective bias for secondary prevention, and there is no way to correct for this,” he explained. Patients who need secondary prophylaxis have “weathered the storm” of a first episode of SBP, which might have exerted selection pressure, and might have triggered important immunologic changes, Dr. Bajaj suggested.
The findings also raised concerns about the appropriateness of existing antibiotic prophylaxis for SBP. The patients included in the study all received similar regimens regardless of whether they were on primary or secondary prophylaxis. Three-quarters of primary prophylaxis patients received a fluoroquinolone, as did 81% on secondary prophylaxis. All other patients received trimethoprim-sulfamethoxazole. These regimens are aimed at preventing gram-negative infections; however, an increasing number of SBP episodes are caused by either gram-positive pathogens or strains of gram-negative bacteria or fungi resistant to standard antibiotics.
Clinicians “absolutely” need to rethink their approach to both primary and secondary prophylaxis, Dr. Bajaj said. “As fast as treatment evolves, bacteria evolve 20 times faster. We need to find ways to prevent infections without antibiotic prophylaxis, whatever that might be.”
The study used data collected prospectively from patients with cirrhosis at any of 12 U.S. and 2 Canadian centers that belonged to the North American Consortium for the Study of End-Stage Liver Disease. Among 2,731 cirrhotic patients admitted nonelectively, 492 (18%) were on antibiotic prophylaxis at the time of their admission, 305 for primary prophylaxis and 187 for secondary prophylaxis. Dr. Bajaj and his associates used both the baseline model for end-stage liver disease score and serum albumin level of each patient to focus on a group of 154 primary prophylaxis and 154 secondary prophylaxis patients who were similar by these two criteria. Despite this matching, the two subgroups showed statistically significant differences at the time of their index hospitalization for several important clinical measures.
The secondary prophylaxis patients were significantly more likely to have been hospitalized within the previous 6 months, significantly more likely to be on treatment for hepatic encephalopathy at the time of their index admission, and significantly less likely to have systemic inflammatory response syndrome on admission.
Also, at the time of admission, secondary prophylaxis patients were significantly more likely to have an infection of any type at a rate of 40%, compared with 24% among those on primary prophylaxis, as well as a significantly higher rate of SBP at 16%, compared with a 9% rate among the primary prophylaxis patients. During hospitalization, nosocomial SBP occurred significantly more often among the secondary prophylaxis patients at a rate of 6%, compared with a 0.5% rate among those on primary prophylaxis.
Despite these between-group differences, the average duration of hospitalization, and the average incidence of acute-on-chronic liver failure during follow-up out to 30 days post discharge was similar in the two subgroups. And the patients on secondary prophylaxis showed better outcomes by two important parameters: mortality during hospitalization and 30 days post discharge; and the incidence of ICU admission during hospitalization, which was significantly greater for primary prophylaxis patients at 31%, compared with 21% among the secondary prophylaxis patients, Dr. Bajaj reported.
Dr. Bajaj has been a consultant for Norgine and Salix Pharmaceuticals and has received research support from Grifols and Salix Pharmaceuticals.
WASHINGTON –
The mortality rate during follow-up of cirrhotic patients hospitalized while on primary prophylaxis against spontaneous bacterial peritonitis (SBP) was 19%, compared with a 9% death rate among cirrhotic patients hospitalized while on secondary prophylaxis, Jasmohan S. Bajaj, MD, said at the annual Digestive Disease Week®.
Although the findings raised questions about the value of primary prophylaxis with an antibiotic in cirrhotic patients for preventing a first episode of SBP, secondary prophylaxis remains an important precaution.
“There is clear benefit from secondary prophylaxis; please use it. The data supporting it are robust,” said Dr. Bajaj, a hepatologist at Virginia Commonwealth University, Richmond. In contrast, the evidence supporting benefits from primary prophylaxis is weaker, he said. The findings were also counterintuitive, because patients who experience repeat episodes of SBP might be expected to fare worse than those hit by SBP just once.
Dr. Bajaj also acknowledged the substantial confounding that distinguishes patients with cirrhosis receiving primary or secondary prophylaxis, and the difficulty of fully adjusting for all this confounding by statistical analyses. “There is selective bias for secondary prevention, and there is no way to correct for this,” he explained. Patients who need secondary prophylaxis have “weathered the storm” of a first episode of SBP, which might have exerted selection pressure, and might have triggered important immunologic changes, Dr. Bajaj suggested.
The findings also raised concerns about the appropriateness of existing antibiotic prophylaxis for SBP. The patients included in the study all received similar regimens regardless of whether they were on primary or secondary prophylaxis. Three-quarters of primary prophylaxis patients received a fluoroquinolone, as did 81% on secondary prophylaxis. All other patients received trimethoprim-sulfamethoxazole. These regimens are aimed at preventing gram-negative infections; however, an increasing number of SBP episodes are caused by either gram-positive pathogens or strains of gram-negative bacteria or fungi resistant to standard antibiotics.
Clinicians “absolutely” need to rethink their approach to both primary and secondary prophylaxis, Dr. Bajaj said. “As fast as treatment evolves, bacteria evolve 20 times faster. We need to find ways to prevent infections without antibiotic prophylaxis, whatever that might be.”
The study used data collected prospectively from patients with cirrhosis at any of 12 U.S. and 2 Canadian centers that belonged to the North American Consortium for the Study of End-Stage Liver Disease. Among 2,731 cirrhotic patients admitted nonelectively, 492 (18%) were on antibiotic prophylaxis at the time of their admission, 305 for primary prophylaxis and 187 for secondary prophylaxis. Dr. Bajaj and his associates used both the baseline model for end-stage liver disease score and serum albumin level of each patient to focus on a group of 154 primary prophylaxis and 154 secondary prophylaxis patients who were similar by these two criteria. Despite this matching, the two subgroups showed statistically significant differences at the time of their index hospitalization for several important clinical measures.
The secondary prophylaxis patients were significantly more likely to have been hospitalized within the previous 6 months, significantly more likely to be on treatment for hepatic encephalopathy at the time of their index admission, and significantly less likely to have systemic inflammatory response syndrome on admission.
Also, at the time of admission, secondary prophylaxis patients were significantly more likely to have an infection of any type at a rate of 40%, compared with 24% among those on primary prophylaxis, as well as a significantly higher rate of SBP at 16%, compared with a 9% rate among the primary prophylaxis patients. During hospitalization, nosocomial SBP occurred significantly more often among the secondary prophylaxis patients at a rate of 6%, compared with a 0.5% rate among those on primary prophylaxis.
Despite these between-group differences, the average duration of hospitalization, and the average incidence of acute-on-chronic liver failure during follow-up out to 30 days post discharge was similar in the two subgroups. And the patients on secondary prophylaxis showed better outcomes by two important parameters: mortality during hospitalization and 30 days post discharge; and the incidence of ICU admission during hospitalization, which was significantly greater for primary prophylaxis patients at 31%, compared with 21% among the secondary prophylaxis patients, Dr. Bajaj reported.
Dr. Bajaj has been a consultant for Norgine and Salix Pharmaceuticals and has received research support from Grifols and Salix Pharmaceuticals.
WASHINGTON –
The mortality rate during follow-up of cirrhotic patients hospitalized while on primary prophylaxis against spontaneous bacterial peritonitis (SBP) was 19%, compared with a 9% death rate among cirrhotic patients hospitalized while on secondary prophylaxis, Jasmohan S. Bajaj, MD, said at the annual Digestive Disease Week®.
Although the findings raised questions about the value of primary prophylaxis with an antibiotic in cirrhotic patients for preventing a first episode of SBP, secondary prophylaxis remains an important precaution.
“There is clear benefit from secondary prophylaxis; please use it. The data supporting it are robust,” said Dr. Bajaj, a hepatologist at Virginia Commonwealth University, Richmond. In contrast, the evidence supporting benefits from primary prophylaxis is weaker, he said. The findings were also counterintuitive, because patients who experience repeat episodes of SBP might be expected to fare worse than those hit by SBP just once.
Dr. Bajaj also acknowledged the substantial confounding that distinguishes patients with cirrhosis receiving primary or secondary prophylaxis, and the difficulty of fully adjusting for all this confounding by statistical analyses. “There is selective bias for secondary prevention, and there is no way to correct for this,” he explained. Patients who need secondary prophylaxis have “weathered the storm” of a first episode of SBP, which might have exerted selection pressure, and might have triggered important immunologic changes, Dr. Bajaj suggested.
The findings also raised concerns about the appropriateness of existing antibiotic prophylaxis for SBP. The patients included in the study all received similar regimens regardless of whether they were on primary or secondary prophylaxis. Three-quarters of primary prophylaxis patients received a fluoroquinolone, as did 81% on secondary prophylaxis. All other patients received trimethoprim-sulfamethoxazole. These regimens are aimed at preventing gram-negative infections; however, an increasing number of SBP episodes are caused by either gram-positive pathogens or strains of gram-negative bacteria or fungi resistant to standard antibiotics.
Clinicians “absolutely” need to rethink their approach to both primary and secondary prophylaxis, Dr. Bajaj said. “As fast as treatment evolves, bacteria evolve 20 times faster. We need to find ways to prevent infections without antibiotic prophylaxis, whatever that might be.”
The study used data collected prospectively from patients with cirrhosis at any of 12 U.S. and 2 Canadian centers that belonged to the North American Consortium for the Study of End-Stage Liver Disease. Among 2,731 cirrhotic patients admitted nonelectively, 492 (18%) were on antibiotic prophylaxis at the time of their admission, 305 for primary prophylaxis and 187 for secondary prophylaxis. Dr. Bajaj and his associates used both the baseline model for end-stage liver disease score and serum albumin level of each patient to focus on a group of 154 primary prophylaxis and 154 secondary prophylaxis patients who were similar by these two criteria. Despite this matching, the two subgroups showed statistically significant differences at the time of their index hospitalization for several important clinical measures.
The secondary prophylaxis patients were significantly more likely to have been hospitalized within the previous 6 months, significantly more likely to be on treatment for hepatic encephalopathy at the time of their index admission, and significantly less likely to have systemic inflammatory response syndrome on admission.
Also, at the time of admission, secondary prophylaxis patients were significantly more likely to have an infection of any type at a rate of 40%, compared with 24% among those on primary prophylaxis, as well as a significantly higher rate of SBP at 16%, compared with a 9% rate among the primary prophylaxis patients. During hospitalization, nosocomial SBP occurred significantly more often among the secondary prophylaxis patients at a rate of 6%, compared with a 0.5% rate among those on primary prophylaxis.
Despite these between-group differences, the average duration of hospitalization, and the average incidence of acute-on-chronic liver failure during follow-up out to 30 days post discharge was similar in the two subgroups. And the patients on secondary prophylaxis showed better outcomes by two important parameters: mortality during hospitalization and 30 days post discharge; and the incidence of ICU admission during hospitalization, which was significantly greater for primary prophylaxis patients at 31%, compared with 21% among the secondary prophylaxis patients, Dr. Bajaj reported.
Dr. Bajaj has been a consultant for Norgine and Salix Pharmaceuticals and has received research support from Grifols and Salix Pharmaceuticals.
REPORTING FROM DDW 2018
Key clinical point: Antibiotic prophylaxis for bacterial peritonitis showed limitations, especially for primary prophylaxis.
Major finding: Mortality was 19% among primary prophylaxis patients and 9% among secondary prophylaxis patients during hospitalization and 30 days following.
Study details: An analysis of data from 308 cirrhotic patients on antibiotic prophylaxis at 14 North American centers.
Disclosures: Dr. Bajaj has been a consultant for Norgine and Salix Pharmaceuticals and has received research support from Grifols and Salix Pharmaceuticals.
25+ Years of Migraine
R. Allan Purdy, MD, FRCPC
Dr. Purdy is Professor of Medicine (Neurology) at Dalhousie University, Halifax, Nova Scotia.
Although migraine has been with humans since antiquity, it is truly amazing that over the past 25 years and a bit longer there have been remarkable advances in our clinical understanding of migraine and its variations, along with sophisticated epidemiologic evidence and basic research into the neurobiological basis of migraine. These advances have brought this disorder/disease to the forefront as a serious neurologic condition deserving of attention. Migraine is a major cause of neurologic disability in the world. This fact has only been fully recognized in recent years.
From the 1980s through the 1990s, a series of events led to a seminal study of a medication that would truly alter the vector for migraine in the future. Studies coming out of Glaxo in the United Kingdom, under the direction of Pat Humphrey, OBE, DSc, PhD, led to the discovery of sumatriptan, the first truly designer medication for the treatment of acute migraine. The story of how this medication biologically affects the brain serotonin receptors on blood vessels and brain tissue to abort a migraine attack is well known today. Early observations on patients receiving subcutaneous sumatriptan clearly showed how powerful this agent was in shutting down the migraine attack. Patients having migraine in extremis, with severe throbbing headache, pallor, nausea and vomiting, appearing markedly distressed and ill, within minutes to an hour would return to a normal state. Nothing that preceded sumatriptan demonstrated such a remarkable clinical response in patients with headache.
In the past century, sumatriptan came to be one of the most important therapeutic advances in neurology. After its discovery, six other triptans entered the market over time. All of them had minor tweaks on the original molecule’s pharmacology and pharmacokinetics, including various modes of delivery with different results in subgroups of patients. Nevertheless, on balance, the triptans acted in a similar manner to produce similar outcomes. Today, many patients take triptans regularly for help with acute migraine attacks; however, other patients with migraine remain undiagnosed and undertreated and do not receive optimal care.
In the past 20 years, sophisticated laboratory and neuroimaging research allowed in-depth analysis of the migraine attack and spectrum of the migraine disorder. The brain areas that subserve the migraine attack have been mapped neuroanatomically throughout the nervous system, with input from the brainstem, hypothalamus, thalamus, and cortical structures. Cortical-initiated electrical events possibly trigger the trigeminal vascular system, and/or peripheral or central activation mechanisms produce the symptoms of migraine. The migraine story is not complete, but evidence clearly shows migraine to be a valid neurobiological disorder and disease. For some people with migraine, it is occasional aggravation, but for others it can be life-altering and, rarely, life-threatening.
In the past few years, new targets for migraine therapy have been pursued. A CGRP receptor antagonist (or “gepant”) showed benefit in early trials; however, because of potential hepatic side effects, other gepants and CGRP monoclonal antibodies have been studied in clinical trials.
Results of recent trials—one in episodic migraine and the other in chronic migraine—show that anti-CGRP monoclonal antibodies appear to be quite efficacious, have few side effects, and are well tolerated. Time will determine whether there are long-term consequences of their use, and what is the effectiveness of using these large molecules to treat migraine, but current results appear promising. Another triptan-like agent, a ditan, which activates receptors of serotonin without vasoconstrictor properties, has shown promise in acute migraine. Neuromodulation devices are also showing promise in migraine therapy and appear to be safe and well tolerated. Nonpharmacological therapies are more often utilized with benefits and help to avoid problems with medications and their side effects.
Increasing knowledge of migraine epidemiology has shown that there is a transition from acute to more frequent, and from high frequency to subsequent chronic migraine attacks. This transformation, or chronification, appears unique in patients with migraine. This process appears to be bidirectional and reversible in migraine. Whether migraine becomes chronic as a process over time or whether migraine is a chronic disease with episodic fluctuations is something to be further explored in research and clinical practice. Nevertheless, these concepts can only lead to better understanding and, hopefully, new therapeutic interventions that will reduce the frequency and severity of this unique neurologic disease.
As migraine progresses or evolves it can be associated with multiple comorbid disorders, including stroke, depression, seizures, and medication overuse. New preventive therapies in development can be modifications of medications for other neurologic conditions, such as the antiepileptic medications, for example. There are many other potential targets that will be explored for the management of migraine in this century. The future looks promising in that regard.
For decades, and now centuries, neurologists have been interested in migraine and related disorders. Sumatriptan jump-started the modern revolution and evolution of therapeutic options to manage migraine pharmacologically. Since its discovery and use in clinical medicine, the world of migraine has changed dramatically. As headache clinicians are being trained in the latest advances in migraine and other headache disorders, they are showing increasing interest and knowledge,which is provided by some of the most unique and relevant research involving the brain in the world. A cure for migraine may not be possible, but a better understanding and control of all of migraine’s myriad of symptoms is probably within reach in this century.
Suggested Reading
Bigal ME, Lipton RB. Clinical course in migraine: conceptualizing migraine transformation. Neurology. 2008;71(11):848-855.
Deen M, Correnti E, Kamm K, et al. Blocking CGRP in migraine patients – a review of pros and cons. J Headache Pain. 2017;18(1):96.
Dodick DW. Migraine. Lancet. 2018;391(10127):1315-1330.
Färkkilä M, Diener HC, Géraud G, et al. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurology. 2012;11(5):405-413.
Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132.
Humphrey PP. The discovery and development of the triptans, a major therapeutic breakthrough. Headache. 2008;48(5):685-687.
Puledda F, Goadsby PJ. An update on non-pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57(4):685-691.
Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113-2122.
Wietecha LA, Kuca B, Case MG, et al. Phase 3 study (SPARTAN) of lasmiditan compared to placebo for acute treatment of migraine. Cephalalgia. 2017;37 (suppl 1):367-68 (abstr).
R. Allan Purdy, MD, FRCPC
Dr. Purdy is Professor of Medicine (Neurology) at Dalhousie University, Halifax, Nova Scotia.
Although migraine has been with humans since antiquity, it is truly amazing that over the past 25 years and a bit longer there have been remarkable advances in our clinical understanding of migraine and its variations, along with sophisticated epidemiologic evidence and basic research into the neurobiological basis of migraine. These advances have brought this disorder/disease to the forefront as a serious neurologic condition deserving of attention. Migraine is a major cause of neurologic disability in the world. This fact has only been fully recognized in recent years.
From the 1980s through the 1990s, a series of events led to a seminal study of a medication that would truly alter the vector for migraine in the future. Studies coming out of Glaxo in the United Kingdom, under the direction of Pat Humphrey, OBE, DSc, PhD, led to the discovery of sumatriptan, the first truly designer medication for the treatment of acute migraine. The story of how this medication biologically affects the brain serotonin receptors on blood vessels and brain tissue to abort a migraine attack is well known today. Early observations on patients receiving subcutaneous sumatriptan clearly showed how powerful this agent was in shutting down the migraine attack. Patients having migraine in extremis, with severe throbbing headache, pallor, nausea and vomiting, appearing markedly distressed and ill, within minutes to an hour would return to a normal state. Nothing that preceded sumatriptan demonstrated such a remarkable clinical response in patients with headache.
In the past century, sumatriptan came to be one of the most important therapeutic advances in neurology. After its discovery, six other triptans entered the market over time. All of them had minor tweaks on the original molecule’s pharmacology and pharmacokinetics, including various modes of delivery with different results in subgroups of patients. Nevertheless, on balance, the triptans acted in a similar manner to produce similar outcomes. Today, many patients take triptans regularly for help with acute migraine attacks; however, other patients with migraine remain undiagnosed and undertreated and do not receive optimal care.
In the past 20 years, sophisticated laboratory and neuroimaging research allowed in-depth analysis of the migraine attack and spectrum of the migraine disorder. The brain areas that subserve the migraine attack have been mapped neuroanatomically throughout the nervous system, with input from the brainstem, hypothalamus, thalamus, and cortical structures. Cortical-initiated electrical events possibly trigger the trigeminal vascular system, and/or peripheral or central activation mechanisms produce the symptoms of migraine. The migraine story is not complete, but evidence clearly shows migraine to be a valid neurobiological disorder and disease. For some people with migraine, it is occasional aggravation, but for others it can be life-altering and, rarely, life-threatening.
In the past few years, new targets for migraine therapy have been pursued. A CGRP receptor antagonist (or “gepant”) showed benefit in early trials; however, because of potential hepatic side effects, other gepants and CGRP monoclonal antibodies have been studied in clinical trials.
Results of recent trials—one in episodic migraine and the other in chronic migraine—show that anti-CGRP monoclonal antibodies appear to be quite efficacious, have few side effects, and are well tolerated. Time will determine whether there are long-term consequences of their use, and what is the effectiveness of using these large molecules to treat migraine, but current results appear promising. Another triptan-like agent, a ditan, which activates receptors of serotonin without vasoconstrictor properties, has shown promise in acute migraine. Neuromodulation devices are also showing promise in migraine therapy and appear to be safe and well tolerated. Nonpharmacological therapies are more often utilized with benefits and help to avoid problems with medications and their side effects.
Increasing knowledge of migraine epidemiology has shown that there is a transition from acute to more frequent, and from high frequency to subsequent chronic migraine attacks. This transformation, or chronification, appears unique in patients with migraine. This process appears to be bidirectional and reversible in migraine. Whether migraine becomes chronic as a process over time or whether migraine is a chronic disease with episodic fluctuations is something to be further explored in research and clinical practice. Nevertheless, these concepts can only lead to better understanding and, hopefully, new therapeutic interventions that will reduce the frequency and severity of this unique neurologic disease.
As migraine progresses or evolves it can be associated with multiple comorbid disorders, including stroke, depression, seizures, and medication overuse. New preventive therapies in development can be modifications of medications for other neurologic conditions, such as the antiepileptic medications, for example. There are many other potential targets that will be explored for the management of migraine in this century. The future looks promising in that regard.
For decades, and now centuries, neurologists have been interested in migraine and related disorders. Sumatriptan jump-started the modern revolution and evolution of therapeutic options to manage migraine pharmacologically. Since its discovery and use in clinical medicine, the world of migraine has changed dramatically. As headache clinicians are being trained in the latest advances in migraine and other headache disorders, they are showing increasing interest and knowledge,which is provided by some of the most unique and relevant research involving the brain in the world. A cure for migraine may not be possible, but a better understanding and control of all of migraine’s myriad of symptoms is probably within reach in this century.
Suggested Reading
Bigal ME, Lipton RB. Clinical course in migraine: conceptualizing migraine transformation. Neurology. 2008;71(11):848-855.
Deen M, Correnti E, Kamm K, et al. Blocking CGRP in migraine patients – a review of pros and cons. J Headache Pain. 2017;18(1):96.
Dodick DW. Migraine. Lancet. 2018;391(10127):1315-1330.
Färkkilä M, Diener HC, Géraud G, et al. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurology. 2012;11(5):405-413.
Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132.
Humphrey PP. The discovery and development of the triptans, a major therapeutic breakthrough. Headache. 2008;48(5):685-687.
Puledda F, Goadsby PJ. An update on non-pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57(4):685-691.
Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113-2122.
Wietecha LA, Kuca B, Case MG, et al. Phase 3 study (SPARTAN) of lasmiditan compared to placebo for acute treatment of migraine. Cephalalgia. 2017;37 (suppl 1):367-68 (abstr).
R. Allan Purdy, MD, FRCPC
Dr. Purdy is Professor of Medicine (Neurology) at Dalhousie University, Halifax, Nova Scotia.
Although migraine has been with humans since antiquity, it is truly amazing that over the past 25 years and a bit longer there have been remarkable advances in our clinical understanding of migraine and its variations, along with sophisticated epidemiologic evidence and basic research into the neurobiological basis of migraine. These advances have brought this disorder/disease to the forefront as a serious neurologic condition deserving of attention. Migraine is a major cause of neurologic disability in the world. This fact has only been fully recognized in recent years.
From the 1980s through the 1990s, a series of events led to a seminal study of a medication that would truly alter the vector for migraine in the future. Studies coming out of Glaxo in the United Kingdom, under the direction of Pat Humphrey, OBE, DSc, PhD, led to the discovery of sumatriptan, the first truly designer medication for the treatment of acute migraine. The story of how this medication biologically affects the brain serotonin receptors on blood vessels and brain tissue to abort a migraine attack is well known today. Early observations on patients receiving subcutaneous sumatriptan clearly showed how powerful this agent was in shutting down the migraine attack. Patients having migraine in extremis, with severe throbbing headache, pallor, nausea and vomiting, appearing markedly distressed and ill, within minutes to an hour would return to a normal state. Nothing that preceded sumatriptan demonstrated such a remarkable clinical response in patients with headache.
In the past century, sumatriptan came to be one of the most important therapeutic advances in neurology. After its discovery, six other triptans entered the market over time. All of them had minor tweaks on the original molecule’s pharmacology and pharmacokinetics, including various modes of delivery with different results in subgroups of patients. Nevertheless, on balance, the triptans acted in a similar manner to produce similar outcomes. Today, many patients take triptans regularly for help with acute migraine attacks; however, other patients with migraine remain undiagnosed and undertreated and do not receive optimal care.
In the past 20 years, sophisticated laboratory and neuroimaging research allowed in-depth analysis of the migraine attack and spectrum of the migraine disorder. The brain areas that subserve the migraine attack have been mapped neuroanatomically throughout the nervous system, with input from the brainstem, hypothalamus, thalamus, and cortical structures. Cortical-initiated electrical events possibly trigger the trigeminal vascular system, and/or peripheral or central activation mechanisms produce the symptoms of migraine. The migraine story is not complete, but evidence clearly shows migraine to be a valid neurobiological disorder and disease. For some people with migraine, it is occasional aggravation, but for others it can be life-altering and, rarely, life-threatening.
In the past few years, new targets for migraine therapy have been pursued. A CGRP receptor antagonist (or “gepant”) showed benefit in early trials; however, because of potential hepatic side effects, other gepants and CGRP monoclonal antibodies have been studied in clinical trials.
Results of recent trials—one in episodic migraine and the other in chronic migraine—show that anti-CGRP monoclonal antibodies appear to be quite efficacious, have few side effects, and are well tolerated. Time will determine whether there are long-term consequences of their use, and what is the effectiveness of using these large molecules to treat migraine, but current results appear promising. Another triptan-like agent, a ditan, which activates receptors of serotonin without vasoconstrictor properties, has shown promise in acute migraine. Neuromodulation devices are also showing promise in migraine therapy and appear to be safe and well tolerated. Nonpharmacological therapies are more often utilized with benefits and help to avoid problems with medications and their side effects.
Increasing knowledge of migraine epidemiology has shown that there is a transition from acute to more frequent, and from high frequency to subsequent chronic migraine attacks. This transformation, or chronification, appears unique in patients with migraine. This process appears to be bidirectional and reversible in migraine. Whether migraine becomes chronic as a process over time or whether migraine is a chronic disease with episodic fluctuations is something to be further explored in research and clinical practice. Nevertheless, these concepts can only lead to better understanding and, hopefully, new therapeutic interventions that will reduce the frequency and severity of this unique neurologic disease.
As migraine progresses or evolves it can be associated with multiple comorbid disorders, including stroke, depression, seizures, and medication overuse. New preventive therapies in development can be modifications of medications for other neurologic conditions, such as the antiepileptic medications, for example. There are many other potential targets that will be explored for the management of migraine in this century. The future looks promising in that regard.
For decades, and now centuries, neurologists have been interested in migraine and related disorders. Sumatriptan jump-started the modern revolution and evolution of therapeutic options to manage migraine pharmacologically. Since its discovery and use in clinical medicine, the world of migraine has changed dramatically. As headache clinicians are being trained in the latest advances in migraine and other headache disorders, they are showing increasing interest and knowledge,which is provided by some of the most unique and relevant research involving the brain in the world. A cure for migraine may not be possible, but a better understanding and control of all of migraine’s myriad of symptoms is probably within reach in this century.
Suggested Reading
Bigal ME, Lipton RB. Clinical course in migraine: conceptualizing migraine transformation. Neurology. 2008;71(11):848-855.
Deen M, Correnti E, Kamm K, et al. Blocking CGRP in migraine patients – a review of pros and cons. J Headache Pain. 2017;18(1):96.
Dodick DW. Migraine. Lancet. 2018;391(10127):1315-1330.
Färkkilä M, Diener HC, Géraud G, et al. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurology. 2012;11(5):405-413.
Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132.
Humphrey PP. The discovery and development of the triptans, a major therapeutic breakthrough. Headache. 2008;48(5):685-687.
Puledda F, Goadsby PJ. An update on non-pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57(4):685-691.
Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113-2122.
Wietecha LA, Kuca B, Case MG, et al. Phase 3 study (SPARTAN) of lasmiditan compared to placebo for acute treatment of migraine. Cephalalgia. 2017;37 (suppl 1):367-68 (abstr).
Drug price transparency laws gain ground
Connecticut is the latest state to enact a so-called drug price transparency law that imposes reporting requirements on drug makers, health insurers, and pharmacy benefit managers (PBMs).
The new requirements, signed into law by Connecticut Governor Dannel Malloy (D) on May 31, call on drug manufacturers to provide information about significant drug cost increases, including the factors that triggered the price hike and information about the drug’s development costs and capital expenditures. As part of the law, PBMs must report the volume of formulary rebates received from drug makers, including the portion provided to health insurers.
Connecticut’s law is the first to require that health insurers submit data about the most frequently prescribed and highest-cost drugs, as well as information about the impact of drug costs on the plan and its members.
Connecticut Comptroller Kevin Lembo called the law “groundbreaking” and said enactment of the measure is a victory for patients who pay outrageous prices while corporations are “enriched by big discounts.
“The extreme wealth exchange between corporate giants from pharmaceutical manufacturers to pharmacy benefit managers to insurance companies, will no longer happen in the dark,” Mr. Lembo said in a statement. “This legislative victory is a groundbreaking step, but the fight for fairness has only just begun as we continue the fight for relief at the pharmacy counter.”
Priscilla VanderVeer, a spokeswoman for Pharmaceutical Research and Manufacturers of America (PhRMA), said Connecticut’s law has some positive features, but does not go far enough in ensuring savings are passed along to patients.
“While we are glad that this legislation will require middlemen to report what portion of rebates are being passed on to consumers, we are disappointed that the final version of the legislation does not include provisions that would ensure steep rebates given to middlemen are passed on to consumers,” Ms. VanderVeer said in an interview. “Making sure that patients who share the cost of their prescription medicines also share the savings is one of the most important things we can do to provide relief for patients facing higher out-of-pocket costs at the pharmacy counter. We are committed to working with Connecticut lawmakers and other health care stakeholders to craft a solution that will provide patients with the solutions that matter the most to them.”
At least seven other states have passed similar laws that aim to expose questionable medication pricing and compel drug makers to provide the reasoning behind their cost decisions. Between 2016 and 2018, drug price transparency laws were enacted in California, Louisiana, Nevada, New York, Oregon, Maryland, and Vermont. Maine meanwhile, has enacted legislation that requires the development of a plan to collect data from manufacturers.
The majority of drug price transparency laws require drug makers to report and justify dramatic drug price increases to the state. Maryland however, went a step further by allowing the state attorney general to take legal action against drug makers that price gouge and to obtain restitution for state health programs and patients. In April, a federal appeals court struck down Maryland’s law as unconstitutional, ruling that the measure violates the federal commerce clause because it attempts to regulate price transactions. The law remains in limbo while the legal challenge continues.
The recent drug price transparency laws are necessary first steps to enable states to better understand and anticipate price increases, said Jennifer Reck, project director for the National Academy for State Health Policy.
“Faced with unsustainable prescription drug price increases, states are passing laws to create greater transparency and accountability around pricing,” Ms. Reck said in an interview.
However, with the exception of Maryland’s measure, the laws are limited because they do not empower states to take action when companies dramatically increase drug prices, she said. It’s also unclear what impact the greater transparency requirements will have on the marketplace, she added.
Gerard F. Anderson, PhD, a health policy and management professor at Johns Hopkins University in Baltimore, agreed that the drug price transparency laws are a good start. But a second component is needed so that states can take effective action, he said in an interview.
“Price transparency, alone, doesn’t do anything,” he said. “What you need to do is couple price transparency with some kind of other activity that would allow you to actually lower the price.”
Some of those other activities include prohibiting rebates by PBMs, rate setting, or establishing a maximum amount that patients should pay for certain drugs, he suggested.
Some states are already exploring policies that go beyond transparency to allow states to take action against overpricing of medications, Ms. Reck noted. New Jersey and Minnesota, for example, have introduced rate-setting bills that would create cost commissions with the authority to establish payment rates for drugs determined to be unjustifiably priced.
To truly lower drug costs for patients, state laws must be comprehensive and address the various rungs of the pharmaceutical supply chain, Ms. VanderVeer said. PhRMA supported Louisiana’s recent drug price transparency law, but has opposed laws in Vermont, California, and Nevada.
“If it is transparency legislation and other policies that actually help patients afford their medicines and make sure that they are getting access to the same discounts and rebates their insurers and PMBs are getting, then yes, we support it,” Ms. VanderVeer said in an interview. “Unfortunately, a lot of the so-called ‘transparency’ bills that have passed over the last few years do no such thing. All they do is look at one part of the supply chain – the inventors and manufacturers of the medicines – and completely leave out those in the middle and have no provisions in them that will help patients access or afford their medicines.”
Connecticut’s law goes into effect in January 2020.
Connecticut is the latest state to enact a so-called drug price transparency law that imposes reporting requirements on drug makers, health insurers, and pharmacy benefit managers (PBMs).
The new requirements, signed into law by Connecticut Governor Dannel Malloy (D) on May 31, call on drug manufacturers to provide information about significant drug cost increases, including the factors that triggered the price hike and information about the drug’s development costs and capital expenditures. As part of the law, PBMs must report the volume of formulary rebates received from drug makers, including the portion provided to health insurers.
Connecticut’s law is the first to require that health insurers submit data about the most frequently prescribed and highest-cost drugs, as well as information about the impact of drug costs on the plan and its members.
Connecticut Comptroller Kevin Lembo called the law “groundbreaking” and said enactment of the measure is a victory for patients who pay outrageous prices while corporations are “enriched by big discounts.
“The extreme wealth exchange between corporate giants from pharmaceutical manufacturers to pharmacy benefit managers to insurance companies, will no longer happen in the dark,” Mr. Lembo said in a statement. “This legislative victory is a groundbreaking step, but the fight for fairness has only just begun as we continue the fight for relief at the pharmacy counter.”
Priscilla VanderVeer, a spokeswoman for Pharmaceutical Research and Manufacturers of America (PhRMA), said Connecticut’s law has some positive features, but does not go far enough in ensuring savings are passed along to patients.
“While we are glad that this legislation will require middlemen to report what portion of rebates are being passed on to consumers, we are disappointed that the final version of the legislation does not include provisions that would ensure steep rebates given to middlemen are passed on to consumers,” Ms. VanderVeer said in an interview. “Making sure that patients who share the cost of their prescription medicines also share the savings is one of the most important things we can do to provide relief for patients facing higher out-of-pocket costs at the pharmacy counter. We are committed to working with Connecticut lawmakers and other health care stakeholders to craft a solution that will provide patients with the solutions that matter the most to them.”
At least seven other states have passed similar laws that aim to expose questionable medication pricing and compel drug makers to provide the reasoning behind their cost decisions. Between 2016 and 2018, drug price transparency laws were enacted in California, Louisiana, Nevada, New York, Oregon, Maryland, and Vermont. Maine meanwhile, has enacted legislation that requires the development of a plan to collect data from manufacturers.
The majority of drug price transparency laws require drug makers to report and justify dramatic drug price increases to the state. Maryland however, went a step further by allowing the state attorney general to take legal action against drug makers that price gouge and to obtain restitution for state health programs and patients. In April, a federal appeals court struck down Maryland’s law as unconstitutional, ruling that the measure violates the federal commerce clause because it attempts to regulate price transactions. The law remains in limbo while the legal challenge continues.
The recent drug price transparency laws are necessary first steps to enable states to better understand and anticipate price increases, said Jennifer Reck, project director for the National Academy for State Health Policy.
“Faced with unsustainable prescription drug price increases, states are passing laws to create greater transparency and accountability around pricing,” Ms. Reck said in an interview.
However, with the exception of Maryland’s measure, the laws are limited because they do not empower states to take action when companies dramatically increase drug prices, she said. It’s also unclear what impact the greater transparency requirements will have on the marketplace, she added.
Gerard F. Anderson, PhD, a health policy and management professor at Johns Hopkins University in Baltimore, agreed that the drug price transparency laws are a good start. But a second component is needed so that states can take effective action, he said in an interview.
“Price transparency, alone, doesn’t do anything,” he said. “What you need to do is couple price transparency with some kind of other activity that would allow you to actually lower the price.”
Some of those other activities include prohibiting rebates by PBMs, rate setting, or establishing a maximum amount that patients should pay for certain drugs, he suggested.
Some states are already exploring policies that go beyond transparency to allow states to take action against overpricing of medications, Ms. Reck noted. New Jersey and Minnesota, for example, have introduced rate-setting bills that would create cost commissions with the authority to establish payment rates for drugs determined to be unjustifiably priced.
To truly lower drug costs for patients, state laws must be comprehensive and address the various rungs of the pharmaceutical supply chain, Ms. VanderVeer said. PhRMA supported Louisiana’s recent drug price transparency law, but has opposed laws in Vermont, California, and Nevada.
“If it is transparency legislation and other policies that actually help patients afford their medicines and make sure that they are getting access to the same discounts and rebates their insurers and PMBs are getting, then yes, we support it,” Ms. VanderVeer said in an interview. “Unfortunately, a lot of the so-called ‘transparency’ bills that have passed over the last few years do no such thing. All they do is look at one part of the supply chain – the inventors and manufacturers of the medicines – and completely leave out those in the middle and have no provisions in them that will help patients access or afford their medicines.”
Connecticut’s law goes into effect in January 2020.
Connecticut is the latest state to enact a so-called drug price transparency law that imposes reporting requirements on drug makers, health insurers, and pharmacy benefit managers (PBMs).
The new requirements, signed into law by Connecticut Governor Dannel Malloy (D) on May 31, call on drug manufacturers to provide information about significant drug cost increases, including the factors that triggered the price hike and information about the drug’s development costs and capital expenditures. As part of the law, PBMs must report the volume of formulary rebates received from drug makers, including the portion provided to health insurers.
Connecticut’s law is the first to require that health insurers submit data about the most frequently prescribed and highest-cost drugs, as well as information about the impact of drug costs on the plan and its members.
Connecticut Comptroller Kevin Lembo called the law “groundbreaking” and said enactment of the measure is a victory for patients who pay outrageous prices while corporations are “enriched by big discounts.
“The extreme wealth exchange between corporate giants from pharmaceutical manufacturers to pharmacy benefit managers to insurance companies, will no longer happen in the dark,” Mr. Lembo said in a statement. “This legislative victory is a groundbreaking step, but the fight for fairness has only just begun as we continue the fight for relief at the pharmacy counter.”
Priscilla VanderVeer, a spokeswoman for Pharmaceutical Research and Manufacturers of America (PhRMA), said Connecticut’s law has some positive features, but does not go far enough in ensuring savings are passed along to patients.
“While we are glad that this legislation will require middlemen to report what portion of rebates are being passed on to consumers, we are disappointed that the final version of the legislation does not include provisions that would ensure steep rebates given to middlemen are passed on to consumers,” Ms. VanderVeer said in an interview. “Making sure that patients who share the cost of their prescription medicines also share the savings is one of the most important things we can do to provide relief for patients facing higher out-of-pocket costs at the pharmacy counter. We are committed to working with Connecticut lawmakers and other health care stakeholders to craft a solution that will provide patients with the solutions that matter the most to them.”
At least seven other states have passed similar laws that aim to expose questionable medication pricing and compel drug makers to provide the reasoning behind their cost decisions. Between 2016 and 2018, drug price transparency laws were enacted in California, Louisiana, Nevada, New York, Oregon, Maryland, and Vermont. Maine meanwhile, has enacted legislation that requires the development of a plan to collect data from manufacturers.
The majority of drug price transparency laws require drug makers to report and justify dramatic drug price increases to the state. Maryland however, went a step further by allowing the state attorney general to take legal action against drug makers that price gouge and to obtain restitution for state health programs and patients. In April, a federal appeals court struck down Maryland’s law as unconstitutional, ruling that the measure violates the federal commerce clause because it attempts to regulate price transactions. The law remains in limbo while the legal challenge continues.
The recent drug price transparency laws are necessary first steps to enable states to better understand and anticipate price increases, said Jennifer Reck, project director for the National Academy for State Health Policy.
“Faced with unsustainable prescription drug price increases, states are passing laws to create greater transparency and accountability around pricing,” Ms. Reck said in an interview.
However, with the exception of Maryland’s measure, the laws are limited because they do not empower states to take action when companies dramatically increase drug prices, she said. It’s also unclear what impact the greater transparency requirements will have on the marketplace, she added.
Gerard F. Anderson, PhD, a health policy and management professor at Johns Hopkins University in Baltimore, agreed that the drug price transparency laws are a good start. But a second component is needed so that states can take effective action, he said in an interview.
“Price transparency, alone, doesn’t do anything,” he said. “What you need to do is couple price transparency with some kind of other activity that would allow you to actually lower the price.”
Some of those other activities include prohibiting rebates by PBMs, rate setting, or establishing a maximum amount that patients should pay for certain drugs, he suggested.
Some states are already exploring policies that go beyond transparency to allow states to take action against overpricing of medications, Ms. Reck noted. New Jersey and Minnesota, for example, have introduced rate-setting bills that would create cost commissions with the authority to establish payment rates for drugs determined to be unjustifiably priced.
To truly lower drug costs for patients, state laws must be comprehensive and address the various rungs of the pharmaceutical supply chain, Ms. VanderVeer said. PhRMA supported Louisiana’s recent drug price transparency law, but has opposed laws in Vermont, California, and Nevada.
“If it is transparency legislation and other policies that actually help patients afford their medicines and make sure that they are getting access to the same discounts and rebates their insurers and PMBs are getting, then yes, we support it,” Ms. VanderVeer said in an interview. “Unfortunately, a lot of the so-called ‘transparency’ bills that have passed over the last few years do no such thing. All they do is look at one part of the supply chain – the inventors and manufacturers of the medicines – and completely leave out those in the middle and have no provisions in them that will help patients access or afford their medicines.”
Connecticut’s law goes into effect in January 2020.
Using Stroke Order Sets to Improve Compliance With Quality Measures for Ischemic Stroke Admissions
Stroke and cardiovascular disease (CVD) create a heavy economic burden on the health care system in the US.1 About 795,000 people have a stroke in the US each year. In 2013, stroke was the cause of 1 in every 20 deaths in the US.2 On average, someone in the US has a stroke every 40 seconds, and someone dies of one about every 4 minutes.3 Stroke also accounts for 889,000 hospitalizations per year.4,5
Stroke has been studied widely, and evidence-based guidelines have been created for the management of stroke. Despite these published guidelines for stroke care, inconsistencies in stroke management of veterans still exist. These inconsistencies led to the creation of guidelines that include quality measurements for the care of veterans with stroke.
Several campaigns have been mounted to bolster quality care for veterans with ischemic stroke. These include the Primary Stroke Center Certification by The Joint Commission (JC),6 Get With the Guidelines by the American Stroke Association,7 the Paul Coverdell Registry by the Centers for Disease Control and Prevention,8 and other efforts by the National Quality Forum (NQF) and the Centers for Medicare and Medicaid Services.9 These organizations have independently and collaboratively established quality metrics associated with health care delivery for the care of veterans with stroke. Some of these metrics have been distinguished as performance measures, or metrics that are suitable for public reporting, and may be used for comparing institutions and rewarding those who meet specific thresholds (ie, pay for performance).10
The aim of this project was to increase compliance at the Atlanta VA Medical Center (VAMC) in Decatur, Georgia, with JC National Quality Measures for the care of veterans with ischemic stroke, thus providing optimal care for veterans admitted for ischemic stroke management.
There are 3 phases in the management of a patient with a stroke: stroke presentation, admission/management, and discharge. This project focused on the admission/management phase. The stroke presentation phase is completed in the emergency department (ED), and the discharge phase has a check list for stroke, including atrial fibrillation (AF) and counseling prior to discharge. Data from the check list and counseling were not included in this project.
Specific attention was given to the following JC measures: stroke (STK) 1, STK 5, and STK 10 because the Atlanta VAMC was below the national average for these core measures for fiscal year 2015. Compliance was accomplished by creating order sets for the admission and subsequent care of veterans with ischemic stroke, tracking order set usage, and reporting regularly to the medicine/admitting team members on use rates and meeting quality measures. This project underwent the quality vs research review process and was determined to be a quality improvement (QI) project, so the project did not require institutional review board approval.
Methods
At the Atlanta VAMC, all patients admitted for stroke workup or management are admitted to the medicine service. The medicine admitting teams are composed of an attending physician, a medicine resident, a nurse practitioner (NP), a pharmacist, and 2 interns; and the hospitalist team composed of a hospitalist. The project began January 1, 2016, and ended December 31, 2016.
The hospitalist created evidence-based admission orders for all patients admitted for stroke or transient ischemic attack (TIA).The measures used were from the JC Specification Manual for Joint Commission National Quality as well as The American College of Cardiology/American Heart Association classification of care metrics.5
The order sets were reviewed and confirmed by a neurologist. The JC quality measures required for the care of patients admitted for stroke management were embedded in these order sets. These order sets were placed directly under the general admission orders in the Computerized Patient Record System (CPRS)
The quality measures included:
- STK 1: Veteran admitted for stroke received venous thromboembolic (VTE) prophylaxis in a timely manner. Pharmacologic management for VTE prophylaxis with subcutaneous low-molecular weight heparin and/or application of bilateral sequential compression devices were tracked.
- STK 5: Veteran admitted for stroke administered antithrombotic therapy by end of hospital day 2. Aspirin, aspirin/dipyridamole, and ticlopidine were tracked.
- STK 10: Veteran admitted for stroke assessed for rehabilitation services during admission. Physical therapy and occupational therapy consult placements were tracked. Quality measures, such as administration of tissue plasminogen activator (tPA), were not embedded in the order set because veterans who met the criteria for tPA were immediately administered tPA in the ED or transferred to the closest stroke center.
In this QI project, only quality measures that had to be completed in the inpatient setting were included. Quality measures such as tPA administration, National Institutes of Health (NIH) Stroke Scale timely documentation, swallow screen prior to po intake, and stroke transfers were completed in the ED prior to clearance for admission, so these were not included in the project. The Atlanta VAMC ED has protocols to care for these patients, but they do not have order sets with markers that could trace their usage.
All admission orders placed were reviewed by a QI team to check whether the stroke order set had been used. The ability to determine order set use was accomplished by adding the unique identifier Stroke Order Set Marker, which allowed for querying using structured query language (SQL) within the Corporate Data Warehouse.
Next, all admissions were checked through chart review for compliance with quality measures. Admissions that had not been completed for all quality measures were identified, and the physicians or NPs caring for those veterans were alerted. These order sets were supposed to be used during admission of all patients admitted for stroke management or workup; however, some patients were admitted without the use of the order sets.
The successful completion of the quality measures were then compared between the groups of patients admitted using the order set and the group of patients in which the order set was not used at their admission. The physicians were provided acceptable reasons, including contraindications to certain medications such as patient history of allergy. The admitting physician made decisions on the antiplatelet medications to use or on neurology recommendations. The neurology department was consulted on all patients who had acute or subacute ischemic stroke findings on magnetic resonance imaging (MRI).
At the beginning of the month, internal medicine residents from Emory University and Morehouse School of Medicine received orientation on the use of the stroke order set from the team NP and chief resident. Tips on how to use the CPRS and how to access the stroke order sets also were created.
One challenge the project faced was the continuous change in the admitting team pool: Some residents did not remember to use the stroke order sets.
Results
Of 323 admitted patients with stroke, 93 admissions were entered using the stroke order set. Out of these completed orders, 85 (91%) veterans admitted for ischemic stroke or TIA management received timely VTE prophylaxis, and 8 (9%) veterans did not. Of the 230 admissions completed without using the stroke order set, 167 (73%) veterans received timely VTE prophylaxis, and 63 (28%) veterans did not. Additionally out of the 93 veterans admitted using the stroke order set, 74 (80%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by end of hospital day 2, whereas 19 (20%) veterans did not, and there were no clear contraindications documented as to why.
For veterans admitted without using the order set, 167 (73%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by the end of hospital day 2, whereas 76 (33%) veterans did not. Last, 90 (97%) of the 93 veterans admitted for stroke workup using the order set were assessed for rehabilitation services during admission, whereas 3 (3%) were not. For the veterans who were admitted without using the stroke order set, 162 (70%) were assessed for rehabilitation services during admission, whereas 68 (30%) were not.
Out of 969 compliance measures looked at, 237 measures were missed and 732 measures were appropriately completed irrespective of whether the stroke order set was used. Out of the 279 admissions where the stroke order set was used, 249 (89%) quality measures were met.
The study threshold for meeting the standards was the national average for 2015, which was 91.1% for the administration of VTE prophylaxis in a timely manner, 97.9% for administering antithrombotic therapy by end of hospital day 2, and 94.2% for assessment of the patient by rehabilitation services during the admission.
Discussion
Despite the repeated training and orientation, compliance to the order set usage was not optimal, likely secondary to a frequent change in the pool of admitting physicians using the order set. Also, the order set was new to staff, thus, admitting physicians sometimes forgot to use them. The next step in this project will be to create an order set for the ED with markers for tracing usage. These order sets will include all quality measures that need to be completed in the ED, such as the NIH Stroke Scale timely documentation, tPA administration data, swallow screen prior to po intake, and stroke transfers.
This QI project also streamlined the process for stroke admissions. With the creation of the order set, all orders needed for stroke were available to the admitting physician, resulting in less need for searching the order individually from a large pool of orders (Figures 3 and 4).
Several reputable institutions have quality metrics and performance measures typically focused on processes of care based on specific clinical guidelines recommendations. Clinical guidelines are usually based on sufficient evidence that failure to provide the recommended care is likely to result in suboptimal clinical outcomes. Stroke quality measure compliance is part of the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) initiative, and most hospitals will be required to report these measures in order to receive full Medicare payments.11
Limitations
Limitations of this study relate to CPRS functions, which must be specifically activated at different VA sites in order to enable the use of these functions. Also, the successful creation of these order sets depended on the information specialist’s knowledge of the capabilities of the CPRS.
Conclusion
Gaps in practice and recommended guidelines can be bridged by creating standardized admission orders embedded with required quality measures. The Atlanta VAMC project showed that the use of a standardized stroke admission order set significantly improved compliance to quality measures for veterans admitted for ischemic stroke management. This is consistent with a study completed in the ED, which showed that for veterans hospitalized for acute ischemic stroke, electronic order set use was associated with increased use of IV tPA.12 Creating order sets can be challenging, but if these barriers can be overcome, with the first order set, similar templates can be used to create order sets for other clinical conditions, such as heart failure, sepsis, and chronic obstructive pulmonary disease exacerbation.
1. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447-454.
2. Centers for Disease Control and Prevention. Vital signs: recent trends in stroke death rates–United States, 2000-2015. https://www.cdc.gov/mmwr/volumes/66/wr/mm6635e1.htm. Published September 8, 2017. Accessed June 14, 2018.
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e229-e445.
4. Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480-486.
5. Poisson SN, Josephson SA. Quality measures in stroke. Neurohospitalist. 2011;1(2):71-77.
6. The Joint Commission. Primary Stroke Centers—Stroke Performance Measurement. https://www.jointcommission.org/performance_ measurement.aspx. Accessed June 14, 2018.
7. American Stroke Association. Get with the guidelines–stroke. http://www.heart.org/HEARTORG/Professional/GetWithTheGuidelines/GetWithTheGuidelines-Stroke/Get-With-The-Guidelines-Stroke-Overview_UCM_308021_Article.jsp#.WyKre1VKiUk. Accessed June 14, 2018.
8. Centers for Disease Control and Prevention. The Paul Coverdell National Acute Stroke Registry. www.cdc.gov/DHDSP/stroke_registry.htm. Published March 13, 2008.
9. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
10. American College of Cardiology/American Heart Association Task Force on Performance Measures, Bonow RO, Masoudi FA, et al. ACC/AHA classification of care metrics: performance measures and quality metrics: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. Circulation. 2008;118(24):2662-2666.
11. Centers for Medicare and Medicaid Services. Reporting Hospital Quality Data for Annual Payment Update https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalFactSheetAP.pdf. Published November 2004. Accessed June 14, 2018.
12. Ballard DW, Kim AS, Huang J, et al; KP CREST Network Investigators. Implementation of computerized physician order entry is associated with increased thrombolytic administration for emergency department veterans with acute ischemic stroke. Ann Emerg Med. 2015;66(6):601-610.
Stroke and cardiovascular disease (CVD) create a heavy economic burden on the health care system in the US.1 About 795,000 people have a stroke in the US each year. In 2013, stroke was the cause of 1 in every 20 deaths in the US.2 On average, someone in the US has a stroke every 40 seconds, and someone dies of one about every 4 minutes.3 Stroke also accounts for 889,000 hospitalizations per year.4,5
Stroke has been studied widely, and evidence-based guidelines have been created for the management of stroke. Despite these published guidelines for stroke care, inconsistencies in stroke management of veterans still exist. These inconsistencies led to the creation of guidelines that include quality measurements for the care of veterans with stroke.
Several campaigns have been mounted to bolster quality care for veterans with ischemic stroke. These include the Primary Stroke Center Certification by The Joint Commission (JC),6 Get With the Guidelines by the American Stroke Association,7 the Paul Coverdell Registry by the Centers for Disease Control and Prevention,8 and other efforts by the National Quality Forum (NQF) and the Centers for Medicare and Medicaid Services.9 These organizations have independently and collaboratively established quality metrics associated with health care delivery for the care of veterans with stroke. Some of these metrics have been distinguished as performance measures, or metrics that are suitable for public reporting, and may be used for comparing institutions and rewarding those who meet specific thresholds (ie, pay for performance).10
The aim of this project was to increase compliance at the Atlanta VA Medical Center (VAMC) in Decatur, Georgia, with JC National Quality Measures for the care of veterans with ischemic stroke, thus providing optimal care for veterans admitted for ischemic stroke management.
There are 3 phases in the management of a patient with a stroke: stroke presentation, admission/management, and discharge. This project focused on the admission/management phase. The stroke presentation phase is completed in the emergency department (ED), and the discharge phase has a check list for stroke, including atrial fibrillation (AF) and counseling prior to discharge. Data from the check list and counseling were not included in this project.
Specific attention was given to the following JC measures: stroke (STK) 1, STK 5, and STK 10 because the Atlanta VAMC was below the national average for these core measures for fiscal year 2015. Compliance was accomplished by creating order sets for the admission and subsequent care of veterans with ischemic stroke, tracking order set usage, and reporting regularly to the medicine/admitting team members on use rates and meeting quality measures. This project underwent the quality vs research review process and was determined to be a quality improvement (QI) project, so the project did not require institutional review board approval.
Methods
At the Atlanta VAMC, all patients admitted for stroke workup or management are admitted to the medicine service. The medicine admitting teams are composed of an attending physician, a medicine resident, a nurse practitioner (NP), a pharmacist, and 2 interns; and the hospitalist team composed of a hospitalist. The project began January 1, 2016, and ended December 31, 2016.
The hospitalist created evidence-based admission orders for all patients admitted for stroke or transient ischemic attack (TIA).The measures used were from the JC Specification Manual for Joint Commission National Quality as well as The American College of Cardiology/American Heart Association classification of care metrics.5
The order sets were reviewed and confirmed by a neurologist. The JC quality measures required for the care of patients admitted for stroke management were embedded in these order sets. These order sets were placed directly under the general admission orders in the Computerized Patient Record System (CPRS)
The quality measures included:
- STK 1: Veteran admitted for stroke received venous thromboembolic (VTE) prophylaxis in a timely manner. Pharmacologic management for VTE prophylaxis with subcutaneous low-molecular weight heparin and/or application of bilateral sequential compression devices were tracked.
- STK 5: Veteran admitted for stroke administered antithrombotic therapy by end of hospital day 2. Aspirin, aspirin/dipyridamole, and ticlopidine were tracked.
- STK 10: Veteran admitted for stroke assessed for rehabilitation services during admission. Physical therapy and occupational therapy consult placements were tracked. Quality measures, such as administration of tissue plasminogen activator (tPA), were not embedded in the order set because veterans who met the criteria for tPA were immediately administered tPA in the ED or transferred to the closest stroke center.
In this QI project, only quality measures that had to be completed in the inpatient setting were included. Quality measures such as tPA administration, National Institutes of Health (NIH) Stroke Scale timely documentation, swallow screen prior to po intake, and stroke transfers were completed in the ED prior to clearance for admission, so these were not included in the project. The Atlanta VAMC ED has protocols to care for these patients, but they do not have order sets with markers that could trace their usage.
All admission orders placed were reviewed by a QI team to check whether the stroke order set had been used. The ability to determine order set use was accomplished by adding the unique identifier Stroke Order Set Marker, which allowed for querying using structured query language (SQL) within the Corporate Data Warehouse.
Next, all admissions were checked through chart review for compliance with quality measures. Admissions that had not been completed for all quality measures were identified, and the physicians or NPs caring for those veterans were alerted. These order sets were supposed to be used during admission of all patients admitted for stroke management or workup; however, some patients were admitted without the use of the order sets.
The successful completion of the quality measures were then compared between the groups of patients admitted using the order set and the group of patients in which the order set was not used at their admission. The physicians were provided acceptable reasons, including contraindications to certain medications such as patient history of allergy. The admitting physician made decisions on the antiplatelet medications to use or on neurology recommendations. The neurology department was consulted on all patients who had acute or subacute ischemic stroke findings on magnetic resonance imaging (MRI).
At the beginning of the month, internal medicine residents from Emory University and Morehouse School of Medicine received orientation on the use of the stroke order set from the team NP and chief resident. Tips on how to use the CPRS and how to access the stroke order sets also were created.
One challenge the project faced was the continuous change in the admitting team pool: Some residents did not remember to use the stroke order sets.
Results
Of 323 admitted patients with stroke, 93 admissions were entered using the stroke order set. Out of these completed orders, 85 (91%) veterans admitted for ischemic stroke or TIA management received timely VTE prophylaxis, and 8 (9%) veterans did not. Of the 230 admissions completed without using the stroke order set, 167 (73%) veterans received timely VTE prophylaxis, and 63 (28%) veterans did not. Additionally out of the 93 veterans admitted using the stroke order set, 74 (80%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by end of hospital day 2, whereas 19 (20%) veterans did not, and there were no clear contraindications documented as to why.
For veterans admitted without using the order set, 167 (73%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by the end of hospital day 2, whereas 76 (33%) veterans did not. Last, 90 (97%) of the 93 veterans admitted for stroke workup using the order set were assessed for rehabilitation services during admission, whereas 3 (3%) were not. For the veterans who were admitted without using the stroke order set, 162 (70%) were assessed for rehabilitation services during admission, whereas 68 (30%) were not.
Out of 969 compliance measures looked at, 237 measures were missed and 732 measures were appropriately completed irrespective of whether the stroke order set was used. Out of the 279 admissions where the stroke order set was used, 249 (89%) quality measures were met.
The study threshold for meeting the standards was the national average for 2015, which was 91.1% for the administration of VTE prophylaxis in a timely manner, 97.9% for administering antithrombotic therapy by end of hospital day 2, and 94.2% for assessment of the patient by rehabilitation services during the admission.
Discussion
Despite the repeated training and orientation, compliance to the order set usage was not optimal, likely secondary to a frequent change in the pool of admitting physicians using the order set. Also, the order set was new to staff, thus, admitting physicians sometimes forgot to use them. The next step in this project will be to create an order set for the ED with markers for tracing usage. These order sets will include all quality measures that need to be completed in the ED, such as the NIH Stroke Scale timely documentation, tPA administration data, swallow screen prior to po intake, and stroke transfers.
This QI project also streamlined the process for stroke admissions. With the creation of the order set, all orders needed for stroke were available to the admitting physician, resulting in less need for searching the order individually from a large pool of orders (Figures 3 and 4).
Several reputable institutions have quality metrics and performance measures typically focused on processes of care based on specific clinical guidelines recommendations. Clinical guidelines are usually based on sufficient evidence that failure to provide the recommended care is likely to result in suboptimal clinical outcomes. Stroke quality measure compliance is part of the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) initiative, and most hospitals will be required to report these measures in order to receive full Medicare payments.11
Limitations
Limitations of this study relate to CPRS functions, which must be specifically activated at different VA sites in order to enable the use of these functions. Also, the successful creation of these order sets depended on the information specialist’s knowledge of the capabilities of the CPRS.
Conclusion
Gaps in practice and recommended guidelines can be bridged by creating standardized admission orders embedded with required quality measures. The Atlanta VAMC project showed that the use of a standardized stroke admission order set significantly improved compliance to quality measures for veterans admitted for ischemic stroke management. This is consistent with a study completed in the ED, which showed that for veterans hospitalized for acute ischemic stroke, electronic order set use was associated with increased use of IV tPA.12 Creating order sets can be challenging, but if these barriers can be overcome, with the first order set, similar templates can be used to create order sets for other clinical conditions, such as heart failure, sepsis, and chronic obstructive pulmonary disease exacerbation.
Stroke and cardiovascular disease (CVD) create a heavy economic burden on the health care system in the US.1 About 795,000 people have a stroke in the US each year. In 2013, stroke was the cause of 1 in every 20 deaths in the US.2 On average, someone in the US has a stroke every 40 seconds, and someone dies of one about every 4 minutes.3 Stroke also accounts for 889,000 hospitalizations per year.4,5
Stroke has been studied widely, and evidence-based guidelines have been created for the management of stroke. Despite these published guidelines for stroke care, inconsistencies in stroke management of veterans still exist. These inconsistencies led to the creation of guidelines that include quality measurements for the care of veterans with stroke.
Several campaigns have been mounted to bolster quality care for veterans with ischemic stroke. These include the Primary Stroke Center Certification by The Joint Commission (JC),6 Get With the Guidelines by the American Stroke Association,7 the Paul Coverdell Registry by the Centers for Disease Control and Prevention,8 and other efforts by the National Quality Forum (NQF) and the Centers for Medicare and Medicaid Services.9 These organizations have independently and collaboratively established quality metrics associated with health care delivery for the care of veterans with stroke. Some of these metrics have been distinguished as performance measures, or metrics that are suitable for public reporting, and may be used for comparing institutions and rewarding those who meet specific thresholds (ie, pay for performance).10
The aim of this project was to increase compliance at the Atlanta VA Medical Center (VAMC) in Decatur, Georgia, with JC National Quality Measures for the care of veterans with ischemic stroke, thus providing optimal care for veterans admitted for ischemic stroke management.
There are 3 phases in the management of a patient with a stroke: stroke presentation, admission/management, and discharge. This project focused on the admission/management phase. The stroke presentation phase is completed in the emergency department (ED), and the discharge phase has a check list for stroke, including atrial fibrillation (AF) and counseling prior to discharge. Data from the check list and counseling were not included in this project.
Specific attention was given to the following JC measures: stroke (STK) 1, STK 5, and STK 10 because the Atlanta VAMC was below the national average for these core measures for fiscal year 2015. Compliance was accomplished by creating order sets for the admission and subsequent care of veterans with ischemic stroke, tracking order set usage, and reporting regularly to the medicine/admitting team members on use rates and meeting quality measures. This project underwent the quality vs research review process and was determined to be a quality improvement (QI) project, so the project did not require institutional review board approval.
Methods
At the Atlanta VAMC, all patients admitted for stroke workup or management are admitted to the medicine service. The medicine admitting teams are composed of an attending physician, a medicine resident, a nurse practitioner (NP), a pharmacist, and 2 interns; and the hospitalist team composed of a hospitalist. The project began January 1, 2016, and ended December 31, 2016.
The hospitalist created evidence-based admission orders for all patients admitted for stroke or transient ischemic attack (TIA).The measures used were from the JC Specification Manual for Joint Commission National Quality as well as The American College of Cardiology/American Heart Association classification of care metrics.5
The order sets were reviewed and confirmed by a neurologist. The JC quality measures required for the care of patients admitted for stroke management were embedded in these order sets. These order sets were placed directly under the general admission orders in the Computerized Patient Record System (CPRS)
The quality measures included:
- STK 1: Veteran admitted for stroke received venous thromboembolic (VTE) prophylaxis in a timely manner. Pharmacologic management for VTE prophylaxis with subcutaneous low-molecular weight heparin and/or application of bilateral sequential compression devices were tracked.
- STK 5: Veteran admitted for stroke administered antithrombotic therapy by end of hospital day 2. Aspirin, aspirin/dipyridamole, and ticlopidine were tracked.
- STK 10: Veteran admitted for stroke assessed for rehabilitation services during admission. Physical therapy and occupational therapy consult placements were tracked. Quality measures, such as administration of tissue plasminogen activator (tPA), were not embedded in the order set because veterans who met the criteria for tPA were immediately administered tPA in the ED or transferred to the closest stroke center.
In this QI project, only quality measures that had to be completed in the inpatient setting were included. Quality measures such as tPA administration, National Institutes of Health (NIH) Stroke Scale timely documentation, swallow screen prior to po intake, and stroke transfers were completed in the ED prior to clearance for admission, so these were not included in the project. The Atlanta VAMC ED has protocols to care for these patients, but they do not have order sets with markers that could trace their usage.
All admission orders placed were reviewed by a QI team to check whether the stroke order set had been used. The ability to determine order set use was accomplished by adding the unique identifier Stroke Order Set Marker, which allowed for querying using structured query language (SQL) within the Corporate Data Warehouse.
Next, all admissions were checked through chart review for compliance with quality measures. Admissions that had not been completed for all quality measures were identified, and the physicians or NPs caring for those veterans were alerted. These order sets were supposed to be used during admission of all patients admitted for stroke management or workup; however, some patients were admitted without the use of the order sets.
The successful completion of the quality measures were then compared between the groups of patients admitted using the order set and the group of patients in which the order set was not used at their admission. The physicians were provided acceptable reasons, including contraindications to certain medications such as patient history of allergy. The admitting physician made decisions on the antiplatelet medications to use or on neurology recommendations. The neurology department was consulted on all patients who had acute or subacute ischemic stroke findings on magnetic resonance imaging (MRI).
At the beginning of the month, internal medicine residents from Emory University and Morehouse School of Medicine received orientation on the use of the stroke order set from the team NP and chief resident. Tips on how to use the CPRS and how to access the stroke order sets also were created.
One challenge the project faced was the continuous change in the admitting team pool: Some residents did not remember to use the stroke order sets.
Results
Of 323 admitted patients with stroke, 93 admissions were entered using the stroke order set. Out of these completed orders, 85 (91%) veterans admitted for ischemic stroke or TIA management received timely VTE prophylaxis, and 8 (9%) veterans did not. Of the 230 admissions completed without using the stroke order set, 167 (73%) veterans received timely VTE prophylaxis, and 63 (28%) veterans did not. Additionally out of the 93 veterans admitted using the stroke order set, 74 (80%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by end of hospital day 2, whereas 19 (20%) veterans did not, and there were no clear contraindications documented as to why.
For veterans admitted without using the order set, 167 (73%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by the end of hospital day 2, whereas 76 (33%) veterans did not. Last, 90 (97%) of the 93 veterans admitted for stroke workup using the order set were assessed for rehabilitation services during admission, whereas 3 (3%) were not. For the veterans who were admitted without using the stroke order set, 162 (70%) were assessed for rehabilitation services during admission, whereas 68 (30%) were not.
Out of 969 compliance measures looked at, 237 measures were missed and 732 measures were appropriately completed irrespective of whether the stroke order set was used. Out of the 279 admissions where the stroke order set was used, 249 (89%) quality measures were met.
The study threshold for meeting the standards was the national average for 2015, which was 91.1% for the administration of VTE prophylaxis in a timely manner, 97.9% for administering antithrombotic therapy by end of hospital day 2, and 94.2% for assessment of the patient by rehabilitation services during the admission.
Discussion
Despite the repeated training and orientation, compliance to the order set usage was not optimal, likely secondary to a frequent change in the pool of admitting physicians using the order set. Also, the order set was new to staff, thus, admitting physicians sometimes forgot to use them. The next step in this project will be to create an order set for the ED with markers for tracing usage. These order sets will include all quality measures that need to be completed in the ED, such as the NIH Stroke Scale timely documentation, tPA administration data, swallow screen prior to po intake, and stroke transfers.
This QI project also streamlined the process for stroke admissions. With the creation of the order set, all orders needed for stroke were available to the admitting physician, resulting in less need for searching the order individually from a large pool of orders (Figures 3 and 4).
Several reputable institutions have quality metrics and performance measures typically focused on processes of care based on specific clinical guidelines recommendations. Clinical guidelines are usually based on sufficient evidence that failure to provide the recommended care is likely to result in suboptimal clinical outcomes. Stroke quality measure compliance is part of the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) initiative, and most hospitals will be required to report these measures in order to receive full Medicare payments.11
Limitations
Limitations of this study relate to CPRS functions, which must be specifically activated at different VA sites in order to enable the use of these functions. Also, the successful creation of these order sets depended on the information specialist’s knowledge of the capabilities of the CPRS.
Conclusion
Gaps in practice and recommended guidelines can be bridged by creating standardized admission orders embedded with required quality measures. The Atlanta VAMC project showed that the use of a standardized stroke admission order set significantly improved compliance to quality measures for veterans admitted for ischemic stroke management. This is consistent with a study completed in the ED, which showed that for veterans hospitalized for acute ischemic stroke, electronic order set use was associated with increased use of IV tPA.12 Creating order sets can be challenging, but if these barriers can be overcome, with the first order set, similar templates can be used to create order sets for other clinical conditions, such as heart failure, sepsis, and chronic obstructive pulmonary disease exacerbation.
1. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447-454.
2. Centers for Disease Control and Prevention. Vital signs: recent trends in stroke death rates–United States, 2000-2015. https://www.cdc.gov/mmwr/volumes/66/wr/mm6635e1.htm. Published September 8, 2017. Accessed June 14, 2018.
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e229-e445.
4. Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480-486.
5. Poisson SN, Josephson SA. Quality measures in stroke. Neurohospitalist. 2011;1(2):71-77.
6. The Joint Commission. Primary Stroke Centers—Stroke Performance Measurement. https://www.jointcommission.org/performance_ measurement.aspx. Accessed June 14, 2018.
7. American Stroke Association. Get with the guidelines–stroke. http://www.heart.org/HEARTORG/Professional/GetWithTheGuidelines/GetWithTheGuidelines-Stroke/Get-With-The-Guidelines-Stroke-Overview_UCM_308021_Article.jsp#.WyKre1VKiUk. Accessed June 14, 2018.
8. Centers for Disease Control and Prevention. The Paul Coverdell National Acute Stroke Registry. www.cdc.gov/DHDSP/stroke_registry.htm. Published March 13, 2008.
9. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
10. American College of Cardiology/American Heart Association Task Force on Performance Measures, Bonow RO, Masoudi FA, et al. ACC/AHA classification of care metrics: performance measures and quality metrics: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. Circulation. 2008;118(24):2662-2666.
11. Centers for Medicare and Medicaid Services. Reporting Hospital Quality Data for Annual Payment Update https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalFactSheetAP.pdf. Published November 2004. Accessed June 14, 2018.
12. Ballard DW, Kim AS, Huang J, et al; KP CREST Network Investigators. Implementation of computerized physician order entry is associated with increased thrombolytic administration for emergency department veterans with acute ischemic stroke. Ann Emerg Med. 2015;66(6):601-610.
1. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447-454.
2. Centers for Disease Control and Prevention. Vital signs: recent trends in stroke death rates–United States, 2000-2015. https://www.cdc.gov/mmwr/volumes/66/wr/mm6635e1.htm. Published September 8, 2017. Accessed June 14, 2018.
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e229-e445.
4. Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480-486.
5. Poisson SN, Josephson SA. Quality measures in stroke. Neurohospitalist. 2011;1(2):71-77.
6. The Joint Commission. Primary Stroke Centers—Stroke Performance Measurement. https://www.jointcommission.org/performance_ measurement.aspx. Accessed June 14, 2018.
7. American Stroke Association. Get with the guidelines–stroke. http://www.heart.org/HEARTORG/Professional/GetWithTheGuidelines/GetWithTheGuidelines-Stroke/Get-With-The-Guidelines-Stroke-Overview_UCM_308021_Article.jsp#.WyKre1VKiUk. Accessed June 14, 2018.
8. Centers for Disease Control and Prevention. The Paul Coverdell National Acute Stroke Registry. www.cdc.gov/DHDSP/stroke_registry.htm. Published March 13, 2008.
9. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
10. American College of Cardiology/American Heart Association Task Force on Performance Measures, Bonow RO, Masoudi FA, et al. ACC/AHA classification of care metrics: performance measures and quality metrics: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. Circulation. 2008;118(24):2662-2666.
11. Centers for Medicare and Medicaid Services. Reporting Hospital Quality Data for Annual Payment Update https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalFactSheetAP.pdf. Published November 2004. Accessed June 14, 2018.
12. Ballard DW, Kim AS, Huang J, et al; KP CREST Network Investigators. Implementation of computerized physician order entry is associated with increased thrombolytic administration for emergency department veterans with acute ischemic stroke. Ann Emerg Med. 2015;66(6):601-610.
Federal Health Care Data Trends: Gulf War & Post-9/11 Veterans
Currently, the largest growing percentage of veterans are those who served during the Gulf War and Post-9/11 era. Over the next few years, nearly every state in the US can expect growth in this veteran population greater than 20%. Although this group of 4.1 million veterans tend to use their VA health benefits less than do the veterans of previous eras, these veterans also exhibit some unique qualities not previously seen in VA patient populations. These veterans already have been identified to be at an increased risk of acquiring several diseases, which include arthritis, chronic fatigue syndrome, and sleep apnea. But perhaps the biggest shift, and the one that could require the most change in the way the Veterans Health Administration provides care, is along gender lines.
Click here to continue reading.
Currently, the largest growing percentage of veterans are those who served during the Gulf War and Post-9/11 era. Over the next few years, nearly every state in the US can expect growth in this veteran population greater than 20%. Although this group of 4.1 million veterans tend to use their VA health benefits less than do the veterans of previous eras, these veterans also exhibit some unique qualities not previously seen in VA patient populations. These veterans already have been identified to be at an increased risk of acquiring several diseases, which include arthritis, chronic fatigue syndrome, and sleep apnea. But perhaps the biggest shift, and the one that could require the most change in the way the Veterans Health Administration provides care, is along gender lines.
Click here to continue reading.
Currently, the largest growing percentage of veterans are those who served during the Gulf War and Post-9/11 era. Over the next few years, nearly every state in the US can expect growth in this veteran population greater than 20%. Although this group of 4.1 million veterans tend to use their VA health benefits less than do the veterans of previous eras, these veterans also exhibit some unique qualities not previously seen in VA patient populations. These veterans already have been identified to be at an increased risk of acquiring several diseases, which include arthritis, chronic fatigue syndrome, and sleep apnea. But perhaps the biggest shift, and the one that could require the most change in the way the Veterans Health Administration provides care, is along gender lines.
Click here to continue reading.
Inhibitor could be repurposed for MM
Tofacitinib, a pan-JAK inhibitor approved to treat rheumatoid arthritis, may advance as a potential treatment for multiple myeloma (MM) based on results from preclinical studies.
In these studies, tofacitinib was able to reverse proliferative effects in stromal-responsive human MM cell lines and reduce tumor growth in mouse models of MM.
Christine Lam, of University of California, San Francisco, and her colleagues conducted this research and reported the results in haematologica.
The researchers showed that, in co-cultures of MM cell lines and bone marrow stromal cells (BMSCs), tofacitinib inhibited the growth of MM cells in a dose-dependent manner.
RNA sequencing and phosphoproteonomics revealed an upregulation of 67 transcripts in MM cell lines co-cultured with BMSCs—most related to JAK-STAT and interleukin signaling.
Additional cell culture experiments showed that tofacitinib inhibited the downstream signaling molecule STAT3, which is responsible for proliferation through the JAK/STAT pathway.
The JAK1/2 inhibitor ruxolitinib did not replicate results seen with tofacitinib.
Further experiments showed that carfilzomib did not have synergistic effects with tofacitinib.
Venetoclax did demonstrate synergy with tofacitinib but only in MM cells cocultured with BMSCs, not in MM cells alone.
The researchers also tested tofacitinib in vivo. They injected mice with an MM cell line, and, after 2 weeks, mice were treated with tofacitinib for 4 weeks.
Mice treated with tofacitinib had lower tumor burden and a significant improvement in survival compared to untreated control mice.
Finally, the researchers tested tofacitinib in bone marrow mononuclear cells from patients. After stimulation with IL-6, the cells were exposed to tofacitinib.
The researchers observed “modest” viability against malignant plasma cells. They noted that because ex vivo MM plasma cells are minimally proliferative even with added cytokines or stromal stimulations, “these results may not fully reflect the potential therapeutic efficacy of tofacitinib in MM patients, where plasma cells are constantly proliferating within the [bone marrow].”
The researchers concluded that “tofacitinib is a promising agent to reverse the tumor-proliferative effects of the [bone marrow] microenvironment that can be rapidly repurposed to benefit MM patients.”
Tofacitinib, a pan-JAK inhibitor approved to treat rheumatoid arthritis, may advance as a potential treatment for multiple myeloma (MM) based on results from preclinical studies.
In these studies, tofacitinib was able to reverse proliferative effects in stromal-responsive human MM cell lines and reduce tumor growth in mouse models of MM.
Christine Lam, of University of California, San Francisco, and her colleagues conducted this research and reported the results in haematologica.
The researchers showed that, in co-cultures of MM cell lines and bone marrow stromal cells (BMSCs), tofacitinib inhibited the growth of MM cells in a dose-dependent manner.
RNA sequencing and phosphoproteonomics revealed an upregulation of 67 transcripts in MM cell lines co-cultured with BMSCs—most related to JAK-STAT and interleukin signaling.
Additional cell culture experiments showed that tofacitinib inhibited the downstream signaling molecule STAT3, which is responsible for proliferation through the JAK/STAT pathway.
The JAK1/2 inhibitor ruxolitinib did not replicate results seen with tofacitinib.
Further experiments showed that carfilzomib did not have synergistic effects with tofacitinib.
Venetoclax did demonstrate synergy with tofacitinib but only in MM cells cocultured with BMSCs, not in MM cells alone.
The researchers also tested tofacitinib in vivo. They injected mice with an MM cell line, and, after 2 weeks, mice were treated with tofacitinib for 4 weeks.
Mice treated with tofacitinib had lower tumor burden and a significant improvement in survival compared to untreated control mice.
Finally, the researchers tested tofacitinib in bone marrow mononuclear cells from patients. After stimulation with IL-6, the cells were exposed to tofacitinib.
The researchers observed “modest” viability against malignant plasma cells. They noted that because ex vivo MM plasma cells are minimally proliferative even with added cytokines or stromal stimulations, “these results may not fully reflect the potential therapeutic efficacy of tofacitinib in MM patients, where plasma cells are constantly proliferating within the [bone marrow].”
The researchers concluded that “tofacitinib is a promising agent to reverse the tumor-proliferative effects of the [bone marrow] microenvironment that can be rapidly repurposed to benefit MM patients.”
Tofacitinib, a pan-JAK inhibitor approved to treat rheumatoid arthritis, may advance as a potential treatment for multiple myeloma (MM) based on results from preclinical studies.
In these studies, tofacitinib was able to reverse proliferative effects in stromal-responsive human MM cell lines and reduce tumor growth in mouse models of MM.
Christine Lam, of University of California, San Francisco, and her colleagues conducted this research and reported the results in haematologica.
The researchers showed that, in co-cultures of MM cell lines and bone marrow stromal cells (BMSCs), tofacitinib inhibited the growth of MM cells in a dose-dependent manner.
RNA sequencing and phosphoproteonomics revealed an upregulation of 67 transcripts in MM cell lines co-cultured with BMSCs—most related to JAK-STAT and interleukin signaling.
Additional cell culture experiments showed that tofacitinib inhibited the downstream signaling molecule STAT3, which is responsible for proliferation through the JAK/STAT pathway.
The JAK1/2 inhibitor ruxolitinib did not replicate results seen with tofacitinib.
Further experiments showed that carfilzomib did not have synergistic effects with tofacitinib.
Venetoclax did demonstrate synergy with tofacitinib but only in MM cells cocultured with BMSCs, not in MM cells alone.
The researchers also tested tofacitinib in vivo. They injected mice with an MM cell line, and, after 2 weeks, mice were treated with tofacitinib for 4 weeks.
Mice treated with tofacitinib had lower tumor burden and a significant improvement in survival compared to untreated control mice.
Finally, the researchers tested tofacitinib in bone marrow mononuclear cells from patients. After stimulation with IL-6, the cells were exposed to tofacitinib.
The researchers observed “modest” viability against malignant plasma cells. They noted that because ex vivo MM plasma cells are minimally proliferative even with added cytokines or stromal stimulations, “these results may not fully reflect the potential therapeutic efficacy of tofacitinib in MM patients, where plasma cells are constantly proliferating within the [bone marrow].”
The researchers concluded that “tofacitinib is a promising agent to reverse the tumor-proliferative effects of the [bone marrow] microenvironment that can be rapidly repurposed to benefit MM patients.”