User login
DOACs’ safety affirmed in real-world setting
Direct oral anticoagulants were associated with decreased bleeding risk versus warfarin in a recent retrospective analysis of primary care databases.
Apixaban (Eliquis) was associated with decreased risk of major bleeding events versus warfarin both in patients with atrial fibrillation (AF) and those prescribed anticoagulants for other causes, according to study results.
Rivaroxaban (Xarelto) was associated with a decrease in risk of intracranial bleeding, compared with warfarin in patients without AF, as was dabigatran (Pradaxa), reported Yana Vinogradova, a research statistician in the division of primary care at the University of Nottingham, England, and her coauthors.
An increased risk of all-cause mortality was seen with both rivaroxaban and low-dose apixaban, possibly because more patients died of age-related causes while on these direct oral anticoagulants (DOACs), they reported.
“This large observational study, based on a general population in a primary care setting, provides reassurance about the safety of DOACs as an alternative to warfarin across all new incident users,” Ms. Vinogradova and her colleagues said in the BMJ.
Evidence establishing the noninferiority of DOACs to warfarin comes mostly from controlled trials in AF leaving “residual concerns” about the safety of these newer agents in real world settings, where a broader range of patients may receive them, they added.
Accordingly, they conducted an analysis based on patient data from two U.K. primary care databases that were representative of the national population, according to the researchers.
A total of 196,061 patients were represented in the study, including 103,270 (53%) with AF and 92,791 (47%) who received anticoagulants for other reasons.
A total of 67% of patients received warfarin, though its use declined from 98% in 2011, the beginning of the study period, to 23% in 2016, the end of the study period. Over that same time period, use of rivaroxaban rose from 1% to 42%, and use of apixaban rose from 0% to 31%, while dabigatran use peaked in 2013 at 10%, dropping to 3% by 2016.
Edoxaban was excluded from the study because it was not licensed in the United Kingdom until the end of 2015, investigators said.
For patients with AF, apixaban was linked to a lower major bleeding risk, both versus warfarin (adjusted hazard ratio, 0.66; 95% confidence interval, 0.54-0.79) and versus rivaroxaban, the published data show. Apixaban was associated with a lower risk of intracranial bleed versus warfarin in patients with AF (aHR, 0.40; 95% CI, 0.25-0.64) as was dabigatran (aHR, 0.45; 95% CI, 0.26-0.77).
For patients without AF, apixaban was again associated with a lower risk of major bleeding versus warfarin and versus rivaroxaban, while rivaroxaban was associated with lower intracranial bleeding risk versus warfarin, and apixaban with lower risks for gastrointestinal bleeds.
Compared with apixaban, rivaroxaban and dabigatran were associated with higher risks of certain bleeding events, further analyses show.
Rivaroxaban and lower-dose apixaban were both associated with increased all-cause mortality risk versus warfarin, both in the atrial fibrillation and non-AF groups, Ms. Vinogradova and her coinvestigators noted.
“A greater proportion of the older patients on apixaban and rivaroxaban may have died while still taking anticoagulants but from age-related causes other than ischemic stroke or venous thromboembolism,” they wrote.
Compared with patients on higher doses of DOACs, patients receiving lower doses were older and had more comorbidities and more previous events, they added.
Between DOACs, results of this particular analysis were most favorable for apixaban, according to investigators.
“Our study has shown that the risk of major bleeding is lower in patients taking apixaban regardless of the reason for prescribing,” they wrote. “This was most pronounced for intracranial bleeding in patients with atrial fibrillation and for gastrointestinal bleeding in patients without atrial fibrillation, appearing, in general, to show apixaban to be the safest drug.”
The study was supported by a grant from the National Institute for Health Research. The investigators had no relevant disclosures.
SOURCE: Vinogradova Y et al. BMJ 2018; 362:K2505.
Direct oral anticoagulants were associated with decreased bleeding risk versus warfarin in a recent retrospective analysis of primary care databases.
Apixaban (Eliquis) was associated with decreased risk of major bleeding events versus warfarin both in patients with atrial fibrillation (AF) and those prescribed anticoagulants for other causes, according to study results.
Rivaroxaban (Xarelto) was associated with a decrease in risk of intracranial bleeding, compared with warfarin in patients without AF, as was dabigatran (Pradaxa), reported Yana Vinogradova, a research statistician in the division of primary care at the University of Nottingham, England, and her coauthors.
An increased risk of all-cause mortality was seen with both rivaroxaban and low-dose apixaban, possibly because more patients died of age-related causes while on these direct oral anticoagulants (DOACs), they reported.
“This large observational study, based on a general population in a primary care setting, provides reassurance about the safety of DOACs as an alternative to warfarin across all new incident users,” Ms. Vinogradova and her colleagues said in the BMJ.
Evidence establishing the noninferiority of DOACs to warfarin comes mostly from controlled trials in AF leaving “residual concerns” about the safety of these newer agents in real world settings, where a broader range of patients may receive them, they added.
Accordingly, they conducted an analysis based on patient data from two U.K. primary care databases that were representative of the national population, according to the researchers.
A total of 196,061 patients were represented in the study, including 103,270 (53%) with AF and 92,791 (47%) who received anticoagulants for other reasons.
A total of 67% of patients received warfarin, though its use declined from 98% in 2011, the beginning of the study period, to 23% in 2016, the end of the study period. Over that same time period, use of rivaroxaban rose from 1% to 42%, and use of apixaban rose from 0% to 31%, while dabigatran use peaked in 2013 at 10%, dropping to 3% by 2016.
Edoxaban was excluded from the study because it was not licensed in the United Kingdom until the end of 2015, investigators said.
For patients with AF, apixaban was linked to a lower major bleeding risk, both versus warfarin (adjusted hazard ratio, 0.66; 95% confidence interval, 0.54-0.79) and versus rivaroxaban, the published data show. Apixaban was associated with a lower risk of intracranial bleed versus warfarin in patients with AF (aHR, 0.40; 95% CI, 0.25-0.64) as was dabigatran (aHR, 0.45; 95% CI, 0.26-0.77).
For patients without AF, apixaban was again associated with a lower risk of major bleeding versus warfarin and versus rivaroxaban, while rivaroxaban was associated with lower intracranial bleeding risk versus warfarin, and apixaban with lower risks for gastrointestinal bleeds.
Compared with apixaban, rivaroxaban and dabigatran were associated with higher risks of certain bleeding events, further analyses show.
Rivaroxaban and lower-dose apixaban were both associated with increased all-cause mortality risk versus warfarin, both in the atrial fibrillation and non-AF groups, Ms. Vinogradova and her coinvestigators noted.
“A greater proportion of the older patients on apixaban and rivaroxaban may have died while still taking anticoagulants but from age-related causes other than ischemic stroke or venous thromboembolism,” they wrote.
Compared with patients on higher doses of DOACs, patients receiving lower doses were older and had more comorbidities and more previous events, they added.
Between DOACs, results of this particular analysis were most favorable for apixaban, according to investigators.
“Our study has shown that the risk of major bleeding is lower in patients taking apixaban regardless of the reason for prescribing,” they wrote. “This was most pronounced for intracranial bleeding in patients with atrial fibrillation and for gastrointestinal bleeding in patients without atrial fibrillation, appearing, in general, to show apixaban to be the safest drug.”
The study was supported by a grant from the National Institute for Health Research. The investigators had no relevant disclosures.
SOURCE: Vinogradova Y et al. BMJ 2018; 362:K2505.
Direct oral anticoagulants were associated with decreased bleeding risk versus warfarin in a recent retrospective analysis of primary care databases.
Apixaban (Eliquis) was associated with decreased risk of major bleeding events versus warfarin both in patients with atrial fibrillation (AF) and those prescribed anticoagulants for other causes, according to study results.
Rivaroxaban (Xarelto) was associated with a decrease in risk of intracranial bleeding, compared with warfarin in patients without AF, as was dabigatran (Pradaxa), reported Yana Vinogradova, a research statistician in the division of primary care at the University of Nottingham, England, and her coauthors.
An increased risk of all-cause mortality was seen with both rivaroxaban and low-dose apixaban, possibly because more patients died of age-related causes while on these direct oral anticoagulants (DOACs), they reported.
“This large observational study, based on a general population in a primary care setting, provides reassurance about the safety of DOACs as an alternative to warfarin across all new incident users,” Ms. Vinogradova and her colleagues said in the BMJ.
Evidence establishing the noninferiority of DOACs to warfarin comes mostly from controlled trials in AF leaving “residual concerns” about the safety of these newer agents in real world settings, where a broader range of patients may receive them, they added.
Accordingly, they conducted an analysis based on patient data from two U.K. primary care databases that were representative of the national population, according to the researchers.
A total of 196,061 patients were represented in the study, including 103,270 (53%) with AF and 92,791 (47%) who received anticoagulants for other reasons.
A total of 67% of patients received warfarin, though its use declined from 98% in 2011, the beginning of the study period, to 23% in 2016, the end of the study period. Over that same time period, use of rivaroxaban rose from 1% to 42%, and use of apixaban rose from 0% to 31%, while dabigatran use peaked in 2013 at 10%, dropping to 3% by 2016.
Edoxaban was excluded from the study because it was not licensed in the United Kingdom until the end of 2015, investigators said.
For patients with AF, apixaban was linked to a lower major bleeding risk, both versus warfarin (adjusted hazard ratio, 0.66; 95% confidence interval, 0.54-0.79) and versus rivaroxaban, the published data show. Apixaban was associated with a lower risk of intracranial bleed versus warfarin in patients with AF (aHR, 0.40; 95% CI, 0.25-0.64) as was dabigatran (aHR, 0.45; 95% CI, 0.26-0.77).
For patients without AF, apixaban was again associated with a lower risk of major bleeding versus warfarin and versus rivaroxaban, while rivaroxaban was associated with lower intracranial bleeding risk versus warfarin, and apixaban with lower risks for gastrointestinal bleeds.
Compared with apixaban, rivaroxaban and dabigatran were associated with higher risks of certain bleeding events, further analyses show.
Rivaroxaban and lower-dose apixaban were both associated with increased all-cause mortality risk versus warfarin, both in the atrial fibrillation and non-AF groups, Ms. Vinogradova and her coinvestigators noted.
“A greater proportion of the older patients on apixaban and rivaroxaban may have died while still taking anticoagulants but from age-related causes other than ischemic stroke or venous thromboembolism,” they wrote.
Compared with patients on higher doses of DOACs, patients receiving lower doses were older and had more comorbidities and more previous events, they added.
Between DOACs, results of this particular analysis were most favorable for apixaban, according to investigators.
“Our study has shown that the risk of major bleeding is lower in patients taking apixaban regardless of the reason for prescribing,” they wrote. “This was most pronounced for intracranial bleeding in patients with atrial fibrillation and for gastrointestinal bleeding in patients without atrial fibrillation, appearing, in general, to show apixaban to be the safest drug.”
The study was supported by a grant from the National Institute for Health Research. The investigators had no relevant disclosures.
SOURCE: Vinogradova Y et al. BMJ 2018; 362:K2505.
FROM THE BMJ
Key clinical point: .
Major finding: Apixaban was linked with an adjusted 34% decreased risk of major bleeding in patients with AF and a 40% lower risk in those prescribed anticoagulants for other causes, compared with warfarin.
Study details: A retrospective cohort study representing 196,061 patients from two U.K. primary care databases.
Disclosures: The study was supported by a grant from the National Institute for Health Research. The investigators had no relevant disclosures.
Source: Vinogradova Y et al. BMJ 2018;362:k2505.
Screening for Depression in Rosacea Patients
Rosacea is a chronic skin condition that can be classified into 4 subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular. Erythematotelangiectatic rosacea is characterized by redness of the face and excessive blushing. Papulopustular rosacea is a more severe form of disease that is characterized by papules and pustules of the central face. If left untreated, these subtypes may progress to phymatous rosacea, which is characterized by skin thickening, fibrosis, and cosmetic disfigurement. Ocular rosacea is characterized by redness and irritation of the eyes.1 Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
The Patient Health Questionnaire 9 (PHQ-9) is a validated and reliable self-administered tool for diagnosis of depression and designation of depression severity. This instrument could prove useful in screening for depression in rosacea patients given the high incidence of psychiatric comorbidities in this patient population.2 The PHQ-9 consists of 9 questions that assess for criteria used to define depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).3 The questionnaire is brief, easy to administer, and has 88% specificity and sensitivity.4
Other studies have evaluated the relationship between rosacea and psychiatric illness, but the PHQ-9 was not used as a screening tool.7,8 Rosacea patients are at increased risk for having psychiatrist-diagnosed depression.5 In one assessment, a positive correlation between rosacea and psychiatric illness was noted using the Dermatology Life Quality Index, the rejection scale of the Questionnaire on Experience with Skin Complaints, and the German version of the Hospital Anxiety and Depression Scale.6 Interpretation of Rosacea Quality of Life and Dermatology Life Quality Index scores indicated that rosacea has a negative impact on quality of life.7
The purpose of this study was to examine the relationship between self-assessed rosacea severity scores and level of depression using the validated rosacea self-assessment tool and the PHQ-9 questionnaire, respectively.
Methods
Study Population
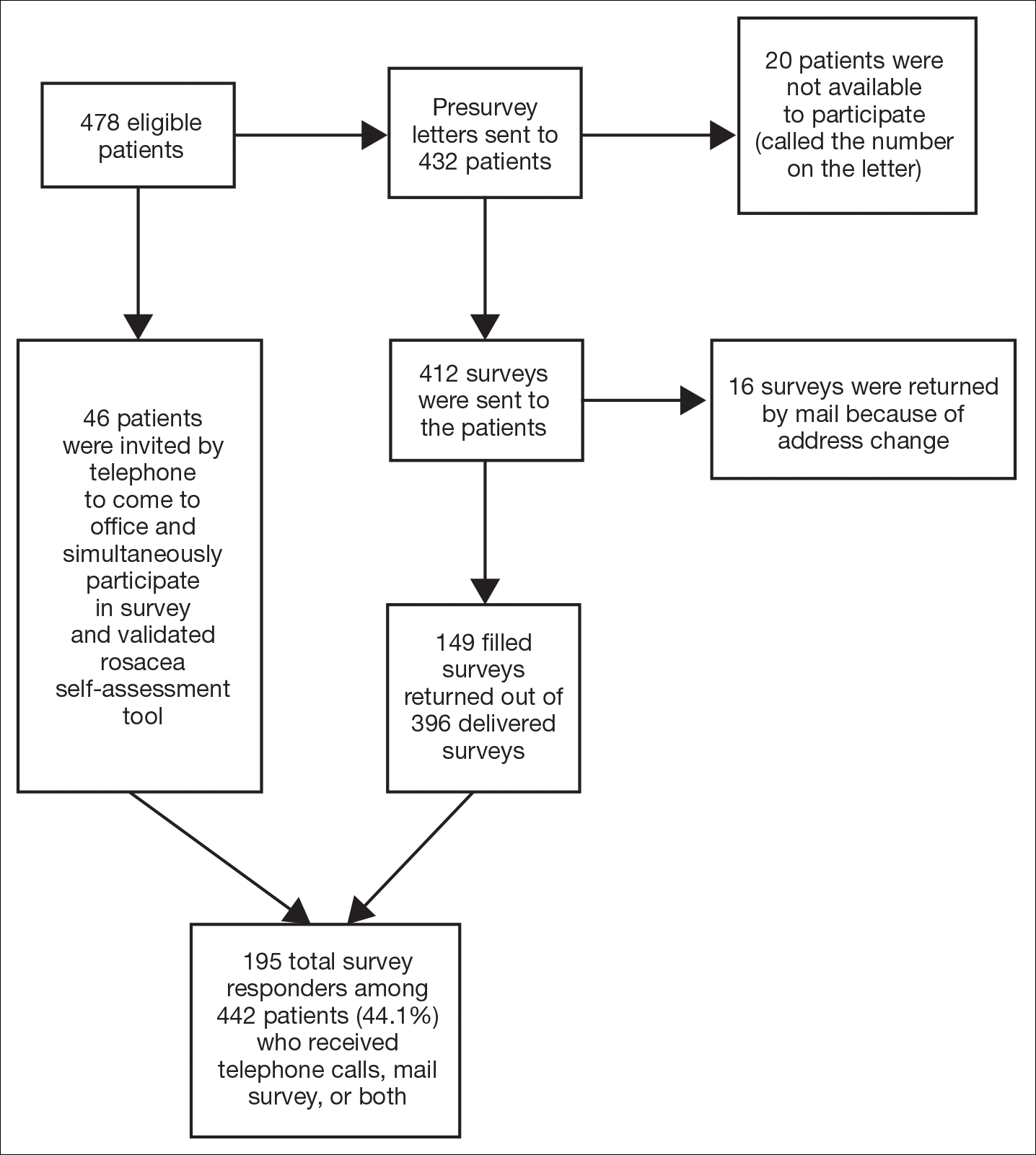
Study participants were adult patients from the Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) dermatology clinic from January 2011 to December 2014 who had received a diagnosis of rosacea (International Classification of Diseases, Ninth Revision [ICD-9] code 695.3) from a Wake Forest dermatologist. Institutional review board approval was obtained prior to initiation of the study. Data collection occurred from October 2014 through February 2015. A total of 478 patients met criteria for participation in the study and were identified from the Wake Forest Baptist Hospital Transitional Data Warehouse and the electronic medical record. Because rosacea typically is not diagnosed in children and the data measures are not validated in children, this demographic group was excluded from participation.
Of 478 eligible patients who were invited to participate via mail or telephone, 46 completed the rosacea self-assessment tool and PHQ-9 survey in person. A total of 432 patients were mailed a presurvey recruitment letter notifying them that they would be receiving a survey in the mail unless they contacted the study team to decline participation. An email address and telephone number for the study team was provided. Twenty patients declined to participate in the study; surveys were then mailed to the remaining 412 patients. Sixteen of the mailed surveys were returned by the post office due to an incorrect address.
Self-assessments
Patients selected images to self-identify the severity of their rosacea symptoms, including erythema, papulopustular lesions, ocular symptoms, and nasal involvement by looking at photographs on the self-assessment tool, which showed various rosacea severity levels. Scores ranged from 2 (least severe) to 8 (most severe). The PHQ-9 survey was completed by participants to assess mental health and mood.
Statistical Analysis
Results were reported using descriptive statistics. Regression analysis was performed to identify independent outcome predictors. To study the relationship between age and demographic variables, the population was divided into 2 groups: patients aged 60 years and older and patients younger than 60 years. Correlation of variables with duration of disease also was studied by creating 2 groups: patients with a disease duration of 11 years or longer and patients with a disease duration of less than 11 years. Comparisons were completed between groups using χ2 tests for proportions and t tests or analysis of variance for continuous variables. Analysis of variance was applied among all patients classified according to the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe).
Results
There is a direct relationship between rosacea severity and depression when comparing across the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe)(P=.006; F=5.18; N=183)
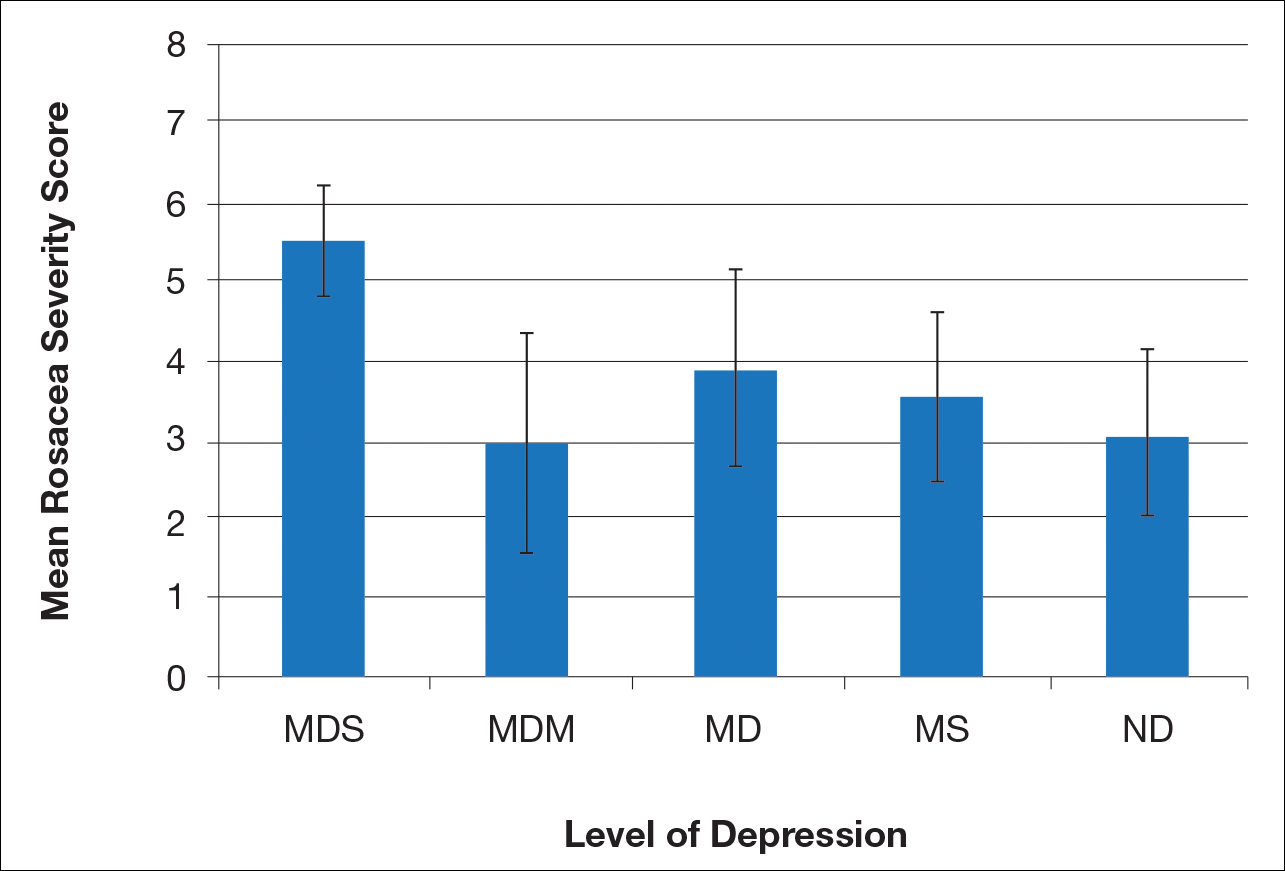
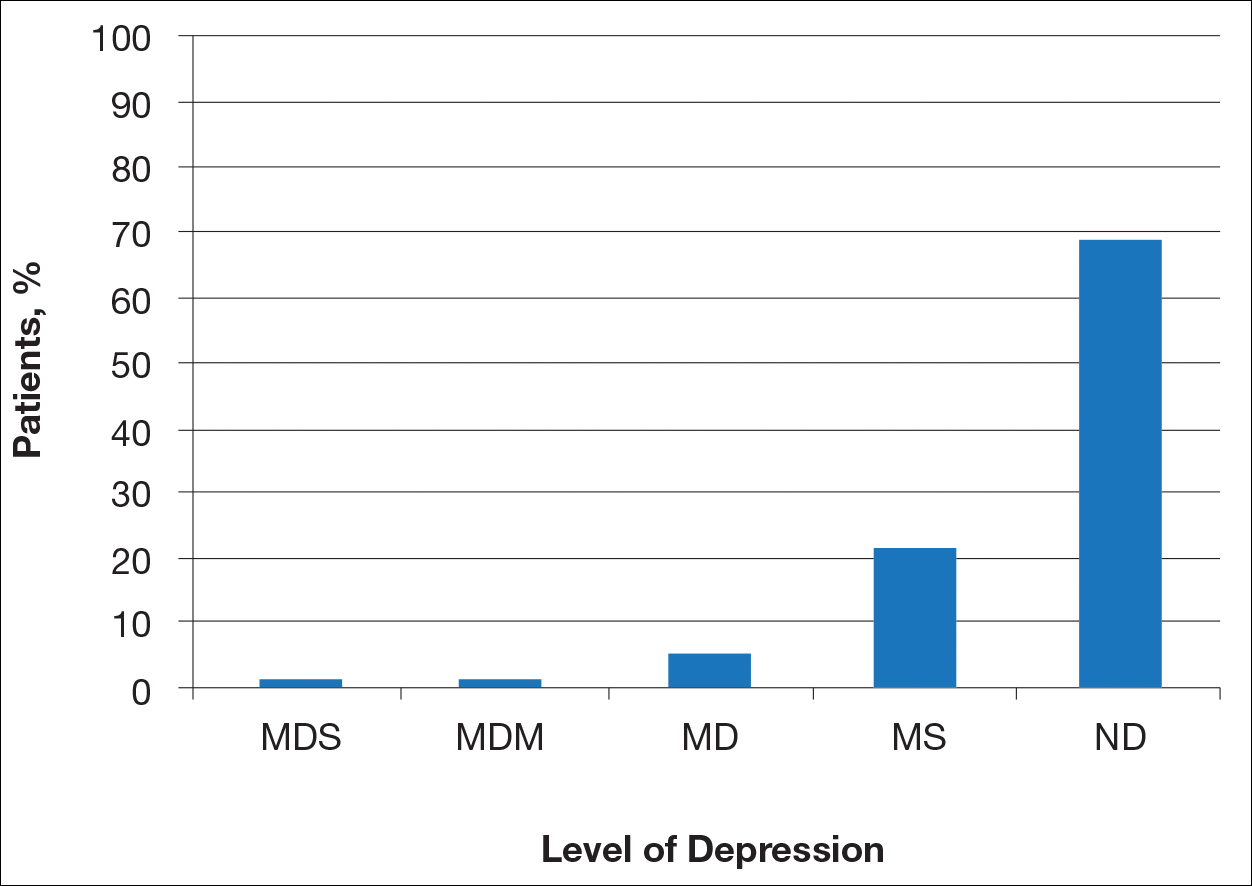
Most patients reported they were nondepressed (68.9%). As measured by the PHQ-9, 31.1% of patients experienced some level of depression: 21.9% reported minimal depression symptoms, 7.1% reported minor depression, 1.1% reported major depression (moderate), and 1.1% reported major depression (severe)(Table).

Comment
There is a direct relationship between rosacea severity and level of depression. In our study, nearly one-third of patients reported some degree of depression. The reason for this correlation may be due to disease stigmatization and decreased quality of life due to the somatic symptoms of rosacea. Our study reinforced the results of other studies evaluating the psychosocial impact of rosacea.8,9 Depression is associated with poor treatment adherence and poor outcomes in rosacea patients; therefore, depression may serve as an important outcome measure.10 The psychosocial impact of rosacea can be severe, but with disease improvement, there often is an improvement in the patient’s psychosocial status.7
There are several limitations to our study. The study population consisted of patients at a university dermatology clinic who may not be representative of patients in the general population; however, our hospital system does not require referral and provides care to a large percentage of the surrounding community.
Clinical implementation of the validated rosacea self-assessment tool and PHQ-9 may have several benefits. Patient-assessed rosacea severity and psychosocial impact obtained via use of these tools would provide physicians with information to fine-tune rosacea treatment regimens. Patients with the greatest social impact may require a more aggressive treatment approach. Early detection of depression in the rosacea population is important in informing treatment strategy and improving outcomes. Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated. The correlation between rosacea and depression underscores the importance of treating this highly impactful disease; however, the low number of responders from the major depression (moderate) subgroup prevented us from making any strong conclusion about this specific subgroup.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychol Ann. 2002;32:509-515.
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Gupta MA, Gupta AK, Chen SJ, et al. Comorbidity of rosacea and depression: an analysis of the National Ambulatory Medical Care Survey and National Hospital Ambulatory Care Survey—outpatient department data collected by the US National Center for Health Statistics from 1995 to 2002. Br J Dermatol. 2005;153:1176-1181.
- Böhm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Halioua B, Cribier B, Frey M, et al. Feelings of stigmatization in patients with rosacea [published online June 21, 2016]. J Eur Acad Dermatol Venereol. 2016;31:163-168.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis [published online March 16, 2016]. Dermatol Ther (Heidelb). 2016;6:237-247.
- Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients [published online January 4, 2016]. Expert Opin Pharmacother. 2016;17:147-152.
Rosacea is a chronic skin condition that can be classified into 4 subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular. Erythematotelangiectatic rosacea is characterized by redness of the face and excessive blushing. Papulopustular rosacea is a more severe form of disease that is characterized by papules and pustules of the central face. If left untreated, these subtypes may progress to phymatous rosacea, which is characterized by skin thickening, fibrosis, and cosmetic disfigurement. Ocular rosacea is characterized by redness and irritation of the eyes.1 Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
The Patient Health Questionnaire 9 (PHQ-9) is a validated and reliable self-administered tool for diagnosis of depression and designation of depression severity. This instrument could prove useful in screening for depression in rosacea patients given the high incidence of psychiatric comorbidities in this patient population.2 The PHQ-9 consists of 9 questions that assess for criteria used to define depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).3 The questionnaire is brief, easy to administer, and has 88% specificity and sensitivity.4
Other studies have evaluated the relationship between rosacea and psychiatric illness, but the PHQ-9 was not used as a screening tool.7,8 Rosacea patients are at increased risk for having psychiatrist-diagnosed depression.5 In one assessment, a positive correlation between rosacea and psychiatric illness was noted using the Dermatology Life Quality Index, the rejection scale of the Questionnaire on Experience with Skin Complaints, and the German version of the Hospital Anxiety and Depression Scale.6 Interpretation of Rosacea Quality of Life and Dermatology Life Quality Index scores indicated that rosacea has a negative impact on quality of life.7
The purpose of this study was to examine the relationship between self-assessed rosacea severity scores and level of depression using the validated rosacea self-assessment tool and the PHQ-9 questionnaire, respectively.
Methods
Study Population
Study participants were adult patients from the Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) dermatology clinic from January 2011 to December 2014 who had received a diagnosis of rosacea (International Classification of Diseases, Ninth Revision [ICD-9] code 695.3) from a Wake Forest dermatologist. Institutional review board approval was obtained prior to initiation of the study. Data collection occurred from October 2014 through February 2015. A total of 478 patients met criteria for participation in the study and were identified from the Wake Forest Baptist Hospital Transitional Data Warehouse and the electronic medical record. Because rosacea typically is not diagnosed in children and the data measures are not validated in children, this demographic group was excluded from participation.
Of 478 eligible patients who were invited to participate via mail or telephone, 46 completed the rosacea self-assessment tool and PHQ-9 survey in person. A total of 432 patients were mailed a presurvey recruitment letter notifying them that they would be receiving a survey in the mail unless they contacted the study team to decline participation. An email address and telephone number for the study team was provided. Twenty patients declined to participate in the study; surveys were then mailed to the remaining 412 patients. Sixteen of the mailed surveys were returned by the post office due to an incorrect address.

Self-assessments
Patients selected images to self-identify the severity of their rosacea symptoms, including erythema, papulopustular lesions, ocular symptoms, and nasal involvement by looking at photographs on the self-assessment tool, which showed various rosacea severity levels. Scores ranged from 2 (least severe) to 8 (most severe). The PHQ-9 survey was completed by participants to assess mental health and mood.
Statistical Analysis
Results were reported using descriptive statistics. Regression analysis was performed to identify independent outcome predictors. To study the relationship between age and demographic variables, the population was divided into 2 groups: patients aged 60 years and older and patients younger than 60 years. Correlation of variables with duration of disease also was studied by creating 2 groups: patients with a disease duration of 11 years or longer and patients with a disease duration of less than 11 years. Comparisons were completed between groups using χ2 tests for proportions and t tests or analysis of variance for continuous variables. Analysis of variance was applied among all patients classified according to the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe).
Results
There is a direct relationship between rosacea severity and depression when comparing across the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe)(P=.006; F=5.18; N=183)


Most patients reported they were nondepressed (68.9%). As measured by the PHQ-9, 31.1% of patients experienced some level of depression: 21.9% reported minimal depression symptoms, 7.1% reported minor depression, 1.1% reported major depression (moderate), and 1.1% reported major depression (severe)(Table).

Comment
There is a direct relationship between rosacea severity and level of depression. In our study, nearly one-third of patients reported some degree of depression. The reason for this correlation may be due to disease stigmatization and decreased quality of life due to the somatic symptoms of rosacea. Our study reinforced the results of other studies evaluating the psychosocial impact of rosacea.8,9 Depression is associated with poor treatment adherence and poor outcomes in rosacea patients; therefore, depression may serve as an important outcome measure.10 The psychosocial impact of rosacea can be severe, but with disease improvement, there often is an improvement in the patient’s psychosocial status.7
There are several limitations to our study. The study population consisted of patients at a university dermatology clinic who may not be representative of patients in the general population; however, our hospital system does not require referral and provides care to a large percentage of the surrounding community.
Clinical implementation of the validated rosacea self-assessment tool and PHQ-9 may have several benefits. Patient-assessed rosacea severity and psychosocial impact obtained via use of these tools would provide physicians with information to fine-tune rosacea treatment regimens. Patients with the greatest social impact may require a more aggressive treatment approach. Early detection of depression in the rosacea population is important in informing treatment strategy and improving outcomes. Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated. The correlation between rosacea and depression underscores the importance of treating this highly impactful disease; however, the low number of responders from the major depression (moderate) subgroup prevented us from making any strong conclusion about this specific subgroup.
Rosacea is a chronic skin condition that can be classified into 4 subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular. Erythematotelangiectatic rosacea is characterized by redness of the face and excessive blushing. Papulopustular rosacea is a more severe form of disease that is characterized by papules and pustules of the central face. If left untreated, these subtypes may progress to phymatous rosacea, which is characterized by skin thickening, fibrosis, and cosmetic disfigurement. Ocular rosacea is characterized by redness and irritation of the eyes.1 Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
The Patient Health Questionnaire 9 (PHQ-9) is a validated and reliable self-administered tool for diagnosis of depression and designation of depression severity. This instrument could prove useful in screening for depression in rosacea patients given the high incidence of psychiatric comorbidities in this patient population.2 The PHQ-9 consists of 9 questions that assess for criteria used to define depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).3 The questionnaire is brief, easy to administer, and has 88% specificity and sensitivity.4
Other studies have evaluated the relationship between rosacea and psychiatric illness, but the PHQ-9 was not used as a screening tool.7,8 Rosacea patients are at increased risk for having psychiatrist-diagnosed depression.5 In one assessment, a positive correlation between rosacea and psychiatric illness was noted using the Dermatology Life Quality Index, the rejection scale of the Questionnaire on Experience with Skin Complaints, and the German version of the Hospital Anxiety and Depression Scale.6 Interpretation of Rosacea Quality of Life and Dermatology Life Quality Index scores indicated that rosacea has a negative impact on quality of life.7
The purpose of this study was to examine the relationship between self-assessed rosacea severity scores and level of depression using the validated rosacea self-assessment tool and the PHQ-9 questionnaire, respectively.
Methods
Study Population
Study participants were adult patients from the Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) dermatology clinic from January 2011 to December 2014 who had received a diagnosis of rosacea (International Classification of Diseases, Ninth Revision [ICD-9] code 695.3) from a Wake Forest dermatologist. Institutional review board approval was obtained prior to initiation of the study. Data collection occurred from October 2014 through February 2015. A total of 478 patients met criteria for participation in the study and were identified from the Wake Forest Baptist Hospital Transitional Data Warehouse and the electronic medical record. Because rosacea typically is not diagnosed in children and the data measures are not validated in children, this demographic group was excluded from participation.
Of 478 eligible patients who were invited to participate via mail or telephone, 46 completed the rosacea self-assessment tool and PHQ-9 survey in person. A total of 432 patients were mailed a presurvey recruitment letter notifying them that they would be receiving a survey in the mail unless they contacted the study team to decline participation. An email address and telephone number for the study team was provided. Twenty patients declined to participate in the study; surveys were then mailed to the remaining 412 patients. Sixteen of the mailed surveys were returned by the post office due to an incorrect address.

Self-assessments
Patients selected images to self-identify the severity of their rosacea symptoms, including erythema, papulopustular lesions, ocular symptoms, and nasal involvement by looking at photographs on the self-assessment tool, which showed various rosacea severity levels. Scores ranged from 2 (least severe) to 8 (most severe). The PHQ-9 survey was completed by participants to assess mental health and mood.
Statistical Analysis
Results were reported using descriptive statistics. Regression analysis was performed to identify independent outcome predictors. To study the relationship between age and demographic variables, the population was divided into 2 groups: patients aged 60 years and older and patients younger than 60 years. Correlation of variables with duration of disease also was studied by creating 2 groups: patients with a disease duration of 11 years or longer and patients with a disease duration of less than 11 years. Comparisons were completed between groups using χ2 tests for proportions and t tests or analysis of variance for continuous variables. Analysis of variance was applied among all patients classified according to the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe).
Results
There is a direct relationship between rosacea severity and depression when comparing across the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe)(P=.006; F=5.18; N=183)


Most patients reported they were nondepressed (68.9%). As measured by the PHQ-9, 31.1% of patients experienced some level of depression: 21.9% reported minimal depression symptoms, 7.1% reported minor depression, 1.1% reported major depression (moderate), and 1.1% reported major depression (severe)(Table).

Comment
There is a direct relationship between rosacea severity and level of depression. In our study, nearly one-third of patients reported some degree of depression. The reason for this correlation may be due to disease stigmatization and decreased quality of life due to the somatic symptoms of rosacea. Our study reinforced the results of other studies evaluating the psychosocial impact of rosacea.8,9 Depression is associated with poor treatment adherence and poor outcomes in rosacea patients; therefore, depression may serve as an important outcome measure.10 The psychosocial impact of rosacea can be severe, but with disease improvement, there often is an improvement in the patient’s psychosocial status.7
There are several limitations to our study. The study population consisted of patients at a university dermatology clinic who may not be representative of patients in the general population; however, our hospital system does not require referral and provides care to a large percentage of the surrounding community.
Clinical implementation of the validated rosacea self-assessment tool and PHQ-9 may have several benefits. Patient-assessed rosacea severity and psychosocial impact obtained via use of these tools would provide physicians with information to fine-tune rosacea treatment regimens. Patients with the greatest social impact may require a more aggressive treatment approach. Early detection of depression in the rosacea population is important in informing treatment strategy and improving outcomes. Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated. The correlation between rosacea and depression underscores the importance of treating this highly impactful disease; however, the low number of responders from the major depression (moderate) subgroup prevented us from making any strong conclusion about this specific subgroup.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychol Ann. 2002;32:509-515.
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Gupta MA, Gupta AK, Chen SJ, et al. Comorbidity of rosacea and depression: an analysis of the National Ambulatory Medical Care Survey and National Hospital Ambulatory Care Survey—outpatient department data collected by the US National Center for Health Statistics from 1995 to 2002. Br J Dermatol. 2005;153:1176-1181.
- Böhm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Halioua B, Cribier B, Frey M, et al. Feelings of stigmatization in patients with rosacea [published online June 21, 2016]. J Eur Acad Dermatol Venereol. 2016;31:163-168.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis [published online March 16, 2016]. Dermatol Ther (Heidelb). 2016;6:237-247.
- Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients [published online January 4, 2016]. Expert Opin Pharmacother. 2016;17:147-152.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychol Ann. 2002;32:509-515.
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Gupta MA, Gupta AK, Chen SJ, et al. Comorbidity of rosacea and depression: an analysis of the National Ambulatory Medical Care Survey and National Hospital Ambulatory Care Survey—outpatient department data collected by the US National Center for Health Statistics from 1995 to 2002. Br J Dermatol. 2005;153:1176-1181.
- Böhm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Halioua B, Cribier B, Frey M, et al. Feelings of stigmatization in patients with rosacea [published online June 21, 2016]. J Eur Acad Dermatol Venereol. 2016;31:163-168.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis [published online March 16, 2016]. Dermatol Ther (Heidelb). 2016;6:237-247.
- Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients [published online January 4, 2016]. Expert Opin Pharmacother. 2016;17:147-152.
Practice Points
- Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
- There is a direct relationship between rosacea severity and degree of depression.
- Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated.
Venetoclax with 5+2 chemo looks effective in older AML patients
STOCKHOLM – For fit, older patients with acute myeloid leukemia (AML), a combination of venetoclax and attenuated-dose induction chemotherapy is tolerable and associated with high response rates, results of the phase 1b CAVEAT trial have suggested.
“Venetoclax up to 600 mg in combination with a 5 plus 2 intensive chemotherapy approach is indeed feasible, with very reasonable count recovery times not unexpected for intensive chemotherapy,” Dr. Wei said at the annual congress of the European Hematology Association.
“A high response rate was observed, and we had very low levels of TLS [tumor lysis syndrome] – in fact no evidence of clinical TLS – and early mortality was also low,” he added.
Previous studies have shown that venetoclax in combination with hypomethylating agents or low-dose cytarabine has promising efficacy for the treatment of elderly patients with AML who are considered to be not fit enough to withstand the rigors of intensive chemotherapy.
“We know that intensive chemotherapy in older patients delivers a remission rate of approximately 60%, and the French group [Acute Leukemia French Association] have demonstrated that low-dose ambulatory approaches are as good as, if not even superior to, intensive consolidation,” he said.
Additionally, other studies have suggested that attenuated-dose or “5+2” induction chemotherapy in patients aged 65 years or older is associated with a combined complete remission (CR) and CR with incomplete recovery of counts (CRi) rate approaching 60%, Dr. Wei noted.
The investigator-initiated CAVEAT study, conducted at four hospitals in Melbourne, is designed to test whether use of a conservative intensive chemotherapy backbone with 5+2 induction could reduce the risk of severe marrow hypoplasia in elderly treatment-naive patients, and minimize the occurrence of TLS with a venetoclax ramp-up prephase and staggered introduction of chemotherapy.
Patients 65 years and older with de novo, secondary, or therapy-related AML with no prior exposure to induction chemotherapy were enrolled. Patients for whom previous therapy with hydroxyurea, low-dose cytarabine, hypomethylating agents, or nonchemotherapy investigational agents had failed could be included in the study. Also eligible for inclusion were patients 60 years and older with a monosomal AML karyotype.
Prior to induction, there was a 7-day venetoclax prephase with a dose ramp-up to achieve a steady state. The trial contains five dose-escalation cohorts, with venetoclax started at doses of either 50 mg (cohort A), 100 mg (B), 200 mg (C), 400 mg (D), or 600 mg (E).
Also during induction, chemotherapy was staggered and doses were attenuated, beginning with the addition of continuous intravenous infusion of cytarabine 100 mg/m2 per day on days 8 through 12 and idarubicin 12 mg/m2 IV on days 9 and 10 of each cycle.
For those patients who achieved a remission, there was a venetoclax-free phase after day 14 to allow for hematopoietic recovery. Patients in remission can receive further therapy with four cycles of “continuation,” each of which was 14 days of venetoclax at the cohort-prescribed dose plus bolus cytarabine 100 mg/m2 IV on days 8 and 9 and idarubicin 12 mg/m2 IV on day 8. After the continuation phase, up to seven cycles of venetoclax monotherapy maintenance can be given.
There was one dose-limiting toxicity in a patient in cohort E (600 mg venetoclax). There were three deaths, all from sepsis, during the induction period (within 42 days) and one after 42 days. The deaths occurred in cohorts C, D, and E.
At the time of data cutoff, two patients had completed treatment, six were continuing, and 33 had discontinued. The primary reason for discontinuation was disease relapse, followed by refractory disease, adverse events, dose-limiting toxicity, or physician/patient decision.
Other adverse events included infections, including grade 3 infections in all 16 patients treated at the 400 mg and 600 mg levels, as well as sepsis, febrile neutropenia, and grade 3 rapid atrial fibrillation in two patients treated in the 400 mg and 600 mg venetoclax cohorts.“Overall, the impression from the investigators was that this is a very deliverable and well-tolerated regimen,” Dr. Wei said.
The overall combined CR/CRi rate was 71%, including CR/CRi in all 9 patients in the 200 mg venetoclax dose cohort.
“Even just with 1 week of monotherapy venetoclax exposure, 25% of patients had a 50% reduction in their bone marrow blasts,” Dr. Wei said.
Median overall survival among the 37 evaluable patients was 7.7 months. Among 12 patients who achieved a CR, the median overall survival was 18.5 months, and among the 12 patients with a CRi, the median overall survival was 7.7 months. For the remaining 13 patients, the median overall survival was 6.3 months.
Survival was significantly better for patients who were treatment-naive prior to venetoclax and chemotherapy, at a median of 18.6 months, compared with 3.8 months for patients who had previously received a hypomethylating agent and/or low-dose cytarabine (P = .0018).
Dose expansion of the 600-mg cohort is ongoing to provide better perspectives on efficacy.
The findings provide evidence that venetoclax monotherapy has cytoreductive potential and support future exploration of venetoclax in combination with 7+3 chemotherapy in younger, fit adults with AML, Dr. Wei said.
The study was supported by AbbVie/Genentech, the Victorian Cancer Agency, and the National Health and Medical Research Council of Australia. Dr. Wei reported research support and advisory board activities with AbbVie and other companies.
SOURCE: Wei AH et al. EHA Congress, Abstract S1564.
STOCKHOLM – For fit, older patients with acute myeloid leukemia (AML), a combination of venetoclax and attenuated-dose induction chemotherapy is tolerable and associated with high response rates, results of the phase 1b CAVEAT trial have suggested.
“Venetoclax up to 600 mg in combination with a 5 plus 2 intensive chemotherapy approach is indeed feasible, with very reasonable count recovery times not unexpected for intensive chemotherapy,” Dr. Wei said at the annual congress of the European Hematology Association.
“A high response rate was observed, and we had very low levels of TLS [tumor lysis syndrome] – in fact no evidence of clinical TLS – and early mortality was also low,” he added.
Previous studies have shown that venetoclax in combination with hypomethylating agents or low-dose cytarabine has promising efficacy for the treatment of elderly patients with AML who are considered to be not fit enough to withstand the rigors of intensive chemotherapy.
“We know that intensive chemotherapy in older patients delivers a remission rate of approximately 60%, and the French group [Acute Leukemia French Association] have demonstrated that low-dose ambulatory approaches are as good as, if not even superior to, intensive consolidation,” he said.
Additionally, other studies have suggested that attenuated-dose or “5+2” induction chemotherapy in patients aged 65 years or older is associated with a combined complete remission (CR) and CR with incomplete recovery of counts (CRi) rate approaching 60%, Dr. Wei noted.
The investigator-initiated CAVEAT study, conducted at four hospitals in Melbourne, is designed to test whether use of a conservative intensive chemotherapy backbone with 5+2 induction could reduce the risk of severe marrow hypoplasia in elderly treatment-naive patients, and minimize the occurrence of TLS with a venetoclax ramp-up prephase and staggered introduction of chemotherapy.
Patients 65 years and older with de novo, secondary, or therapy-related AML with no prior exposure to induction chemotherapy were enrolled. Patients for whom previous therapy with hydroxyurea, low-dose cytarabine, hypomethylating agents, or nonchemotherapy investigational agents had failed could be included in the study. Also eligible for inclusion were patients 60 years and older with a monosomal AML karyotype.
Prior to induction, there was a 7-day venetoclax prephase with a dose ramp-up to achieve a steady state. The trial contains five dose-escalation cohorts, with venetoclax started at doses of either 50 mg (cohort A), 100 mg (B), 200 mg (C), 400 mg (D), or 600 mg (E).
Also during induction, chemotherapy was staggered and doses were attenuated, beginning with the addition of continuous intravenous infusion of cytarabine 100 mg/m2 per day on days 8 through 12 and idarubicin 12 mg/m2 IV on days 9 and 10 of each cycle.
For those patients who achieved a remission, there was a venetoclax-free phase after day 14 to allow for hematopoietic recovery. Patients in remission can receive further therapy with four cycles of “continuation,” each of which was 14 days of venetoclax at the cohort-prescribed dose plus bolus cytarabine 100 mg/m2 IV on days 8 and 9 and idarubicin 12 mg/m2 IV on day 8. After the continuation phase, up to seven cycles of venetoclax monotherapy maintenance can be given.
There was one dose-limiting toxicity in a patient in cohort E (600 mg venetoclax). There were three deaths, all from sepsis, during the induction period (within 42 days) and one after 42 days. The deaths occurred in cohorts C, D, and E.
At the time of data cutoff, two patients had completed treatment, six were continuing, and 33 had discontinued. The primary reason for discontinuation was disease relapse, followed by refractory disease, adverse events, dose-limiting toxicity, or physician/patient decision.
Other adverse events included infections, including grade 3 infections in all 16 patients treated at the 400 mg and 600 mg levels, as well as sepsis, febrile neutropenia, and grade 3 rapid atrial fibrillation in two patients treated in the 400 mg and 600 mg venetoclax cohorts.“Overall, the impression from the investigators was that this is a very deliverable and well-tolerated regimen,” Dr. Wei said.
The overall combined CR/CRi rate was 71%, including CR/CRi in all 9 patients in the 200 mg venetoclax dose cohort.
“Even just with 1 week of monotherapy venetoclax exposure, 25% of patients had a 50% reduction in their bone marrow blasts,” Dr. Wei said.
Median overall survival among the 37 evaluable patients was 7.7 months. Among 12 patients who achieved a CR, the median overall survival was 18.5 months, and among the 12 patients with a CRi, the median overall survival was 7.7 months. For the remaining 13 patients, the median overall survival was 6.3 months.
Survival was significantly better for patients who were treatment-naive prior to venetoclax and chemotherapy, at a median of 18.6 months, compared with 3.8 months for patients who had previously received a hypomethylating agent and/or low-dose cytarabine (P = .0018).
Dose expansion of the 600-mg cohort is ongoing to provide better perspectives on efficacy.
The findings provide evidence that venetoclax monotherapy has cytoreductive potential and support future exploration of venetoclax in combination with 7+3 chemotherapy in younger, fit adults with AML, Dr. Wei said.
The study was supported by AbbVie/Genentech, the Victorian Cancer Agency, and the National Health and Medical Research Council of Australia. Dr. Wei reported research support and advisory board activities with AbbVie and other companies.
SOURCE: Wei AH et al. EHA Congress, Abstract S1564.
STOCKHOLM – For fit, older patients with acute myeloid leukemia (AML), a combination of venetoclax and attenuated-dose induction chemotherapy is tolerable and associated with high response rates, results of the phase 1b CAVEAT trial have suggested.
“Venetoclax up to 600 mg in combination with a 5 plus 2 intensive chemotherapy approach is indeed feasible, with very reasonable count recovery times not unexpected for intensive chemotherapy,” Dr. Wei said at the annual congress of the European Hematology Association.
“A high response rate was observed, and we had very low levels of TLS [tumor lysis syndrome] – in fact no evidence of clinical TLS – and early mortality was also low,” he added.
Previous studies have shown that venetoclax in combination with hypomethylating agents or low-dose cytarabine has promising efficacy for the treatment of elderly patients with AML who are considered to be not fit enough to withstand the rigors of intensive chemotherapy.
“We know that intensive chemotherapy in older patients delivers a remission rate of approximately 60%, and the French group [Acute Leukemia French Association] have demonstrated that low-dose ambulatory approaches are as good as, if not even superior to, intensive consolidation,” he said.
Additionally, other studies have suggested that attenuated-dose or “5+2” induction chemotherapy in patients aged 65 years or older is associated with a combined complete remission (CR) and CR with incomplete recovery of counts (CRi) rate approaching 60%, Dr. Wei noted.
The investigator-initiated CAVEAT study, conducted at four hospitals in Melbourne, is designed to test whether use of a conservative intensive chemotherapy backbone with 5+2 induction could reduce the risk of severe marrow hypoplasia in elderly treatment-naive patients, and minimize the occurrence of TLS with a venetoclax ramp-up prephase and staggered introduction of chemotherapy.
Patients 65 years and older with de novo, secondary, or therapy-related AML with no prior exposure to induction chemotherapy were enrolled. Patients for whom previous therapy with hydroxyurea, low-dose cytarabine, hypomethylating agents, or nonchemotherapy investigational agents had failed could be included in the study. Also eligible for inclusion were patients 60 years and older with a monosomal AML karyotype.
Prior to induction, there was a 7-day venetoclax prephase with a dose ramp-up to achieve a steady state. The trial contains five dose-escalation cohorts, with venetoclax started at doses of either 50 mg (cohort A), 100 mg (B), 200 mg (C), 400 mg (D), or 600 mg (E).
Also during induction, chemotherapy was staggered and doses were attenuated, beginning with the addition of continuous intravenous infusion of cytarabine 100 mg/m2 per day on days 8 through 12 and idarubicin 12 mg/m2 IV on days 9 and 10 of each cycle.
For those patients who achieved a remission, there was a venetoclax-free phase after day 14 to allow for hematopoietic recovery. Patients in remission can receive further therapy with four cycles of “continuation,” each of which was 14 days of venetoclax at the cohort-prescribed dose plus bolus cytarabine 100 mg/m2 IV on days 8 and 9 and idarubicin 12 mg/m2 IV on day 8. After the continuation phase, up to seven cycles of venetoclax monotherapy maintenance can be given.
There was one dose-limiting toxicity in a patient in cohort E (600 mg venetoclax). There were three deaths, all from sepsis, during the induction period (within 42 days) and one after 42 days. The deaths occurred in cohorts C, D, and E.
At the time of data cutoff, two patients had completed treatment, six were continuing, and 33 had discontinued. The primary reason for discontinuation was disease relapse, followed by refractory disease, adverse events, dose-limiting toxicity, or physician/patient decision.
Other adverse events included infections, including grade 3 infections in all 16 patients treated at the 400 mg and 600 mg levels, as well as sepsis, febrile neutropenia, and grade 3 rapid atrial fibrillation in two patients treated in the 400 mg and 600 mg venetoclax cohorts.“Overall, the impression from the investigators was that this is a very deliverable and well-tolerated regimen,” Dr. Wei said.
The overall combined CR/CRi rate was 71%, including CR/CRi in all 9 patients in the 200 mg venetoclax dose cohort.
“Even just with 1 week of monotherapy venetoclax exposure, 25% of patients had a 50% reduction in their bone marrow blasts,” Dr. Wei said.
Median overall survival among the 37 evaluable patients was 7.7 months. Among 12 patients who achieved a CR, the median overall survival was 18.5 months, and among the 12 patients with a CRi, the median overall survival was 7.7 months. For the remaining 13 patients, the median overall survival was 6.3 months.
Survival was significantly better for patients who were treatment-naive prior to venetoclax and chemotherapy, at a median of 18.6 months, compared with 3.8 months for patients who had previously received a hypomethylating agent and/or low-dose cytarabine (P = .0018).
Dose expansion of the 600-mg cohort is ongoing to provide better perspectives on efficacy.
The findings provide evidence that venetoclax monotherapy has cytoreductive potential and support future exploration of venetoclax in combination with 7+3 chemotherapy in younger, fit adults with AML, Dr. Wei said.
The study was supported by AbbVie/Genentech, the Victorian Cancer Agency, and the National Health and Medical Research Council of Australia. Dr. Wei reported research support and advisory board activities with AbbVie and other companies.
SOURCE: Wei AH et al. EHA Congress, Abstract S1564.
REPORTING FROM EHA 2018
Key clinical point:
Major finding: The overall response rate was 71%.
Study details: Phase 1b dose-expansion study in 41 adults with AML.
Disclosures: The study was supported by AbbVie/Genentech, the Victorian Cancer Agency, and the National Health and Medical Research Council of Australia. Dr. Wei reported research support and advisory board activities with AbbVie and other companies.
Source: Wei AH et al. EHA Congress, Abstract S1564.
Many actionable mutations may be missed in current testing of advanced RCC
Germline mutations in patients with advanced renal cell carcinoma may be more common than previously suspected.
In a single-center cohort of 254 patients with advanced renal cell carcinoma (RCC) who received matched tumor-germline DNA sequencing, over a third (35.7%) of patients who had mutations in genes associated with RCC had not met current clinical criteria for testing.
In all, pathogenic germline mutations were identified in 41 patients (16.1%), with 14 patients’ mutations (5.5%) in genes known to be associated with RCC. For the remaining 27 patients (10.5%), the mutations were in non–RCC-associated genes, investigators reported in JAMA Oncology.
Of the non–RCC-associated mutations, CHEK2 was particularly common among patients with clear cell RCC (ccRCC), occurring in eight patients with ccRCC and two with non ccRCC (nccRCC). The overall odds ratio for this mutation among the study cohort was 3.0, compared with the general population (95% confidence interval 1.3-5.8; P = .003). “Although there are currently no RCC-specific screening recommendations for individuals with CHEK2 mutations, there may be incremental screening for other cancers, justifying including this gene on RCC panel tests,” wrote Maria Carlo, MD, and her coauthors.
Germline FH mutations were seen in seven patients, all with nccRCC. This higher rate of hereditary leiomyomatosis and RCC (HLRCC) was higher than previously reported in the literature, and clinical cues to the diagnosis were few among the study patients. Even though clues pointing to HLRCC were seen when tumor samples were submitted for histopathology to the genitourinary specialists at the study site, “it is unclear whether nonspecialist pathologists would be able to draw the same conclusions,” wrote Dr. Carlo and her colleagues.
Renal cell cancer–associated mutations were significantly more common in patients with nccRCC than in the ccRCC group: 9/74 (11.7%) nccRCC patients had an RCC-associated mutation, compared with 3/177 of the ccRCC group (P = .001).
The patient’s course of therapy could be guided by the mutation identified in 10% (eight) of the nccRCC patients, “none of which would have been identified with somatic-only sequencing,” wrote Dr. Carlo and associates. “Our results suggest that germline mutations in cancer-associated genes in patients with advanced RCC may be prevalent, and many of these mutations can be used to guide therapy.”
The 254 patients (median age 56 years, 70.5% male, 83.1% non-Hispanic white) were drawn from 267 patients with American Joint Committee on Cancer (AJCC) stage III or IV RCC participating in clinical trials at Memorial Sloan Kettering Cancer Center, New York, where Dr. Carlo practices as an oncologist. The patients included in the cohort were those who consented to germline sequencing and results disclosure.
To determine which pathogenic variants were identified by the study protocol that would have been missed by current testing standards, the investigators assumed that for those who met guidelines, the multigene test panel would probe for VHL, VH, FLCN, MET, SDHB, SDHD, BAP1, TSC1, TSC2, TP53, and MITF. If another mutation was picked up by the next-generation sequencing used in the study, or if a mutation was found in an individual who otherwise would not have been tested, the finding was considered incremental and attributable to the study protocol.
Implications of the additional mutations picked up by the tumor-germline sequencing approach go beyond the patient, said the researchers, who have seen several of the study participants’ family members receive positive test results for cancer-associated mutations as well. “Relatives who are also found to carry FH mutations should be considered for RCC screening. Early detection may increase the likelihood of cure and survivorship,” wrote Dr. Carlo and her coinvestigators.
Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J. Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
SOURCE: Carlo M et al. JAMA Oncol. 2018 Jul 5. doi: 10.1001/jamaoncol.2018.1986.
In the present study, the number of patients who did not meet current criteria for genetic testing, but who had germline RCC-associated mutations, should prompt reevaluation of testing criteria for individuals with advanced RCC.
Particularly for patients with advanced nccRCC, a genetic referral should be considered to weigh germline testing as well as testing for an expanded set of mutations. Patients with advanced ccRCC may also benefit from a broader testing panel that may include some non-RCC related genes.
Further research is needed to elucidate the genotype-phenotype association in some of the non-RCC mutations seen in this cohort, particularly in CHEK2 mutations. There are currently no screening guidelines for CHEK2 in regard to RCC, and the risk for RCC among those with these mutations is not known. RCC patients who have biallelic loss of DNA damage repair genes such as CHEK2 may benefit from treatment that targets these pathways, though these therapies are not currently offered for RCC.
The study population were individuals with advanced RCC, and the increased numbers of pathogenic germline mutations seen in this population are consistent with other studies finding higher rates of these mutations in patients who have other cancer with advanced disease. As this body of knowledge accumulates, interdisciplinary teams will be able to give more accurate information about risk and prognosis to patients and families and, increasingly, offer optimized care.
Dr. Patrick Pilié is an oncologist at the University of Texas MD Anderson Cancer Center, Houston; Dr. Kathleen Cooney is chair of the department of internal medicine and H.A. and Edna Benning Presidential Endowed Chair at the University of Utah, Salt Lake City. These remarks are drawn from a jointly authored editorial accompanying the study’s publication.
In the present study, the number of patients who did not meet current criteria for genetic testing, but who had germline RCC-associated mutations, should prompt reevaluation of testing criteria for individuals with advanced RCC.
Particularly for patients with advanced nccRCC, a genetic referral should be considered to weigh germline testing as well as testing for an expanded set of mutations. Patients with advanced ccRCC may also benefit from a broader testing panel that may include some non-RCC related genes.
Further research is needed to elucidate the genotype-phenotype association in some of the non-RCC mutations seen in this cohort, particularly in CHEK2 mutations. There are currently no screening guidelines for CHEK2 in regard to RCC, and the risk for RCC among those with these mutations is not known. RCC patients who have biallelic loss of DNA damage repair genes such as CHEK2 may benefit from treatment that targets these pathways, though these therapies are not currently offered for RCC.
The study population were individuals with advanced RCC, and the increased numbers of pathogenic germline mutations seen in this population are consistent with other studies finding higher rates of these mutations in patients who have other cancer with advanced disease. As this body of knowledge accumulates, interdisciplinary teams will be able to give more accurate information about risk and prognosis to patients and families and, increasingly, offer optimized care.
Dr. Patrick Pilié is an oncologist at the University of Texas MD Anderson Cancer Center, Houston; Dr. Kathleen Cooney is chair of the department of internal medicine and H.A. and Edna Benning Presidential Endowed Chair at the University of Utah, Salt Lake City. These remarks are drawn from a jointly authored editorial accompanying the study’s publication.
In the present study, the number of patients who did not meet current criteria for genetic testing, but who had germline RCC-associated mutations, should prompt reevaluation of testing criteria for individuals with advanced RCC.
Particularly for patients with advanced nccRCC, a genetic referral should be considered to weigh germline testing as well as testing for an expanded set of mutations. Patients with advanced ccRCC may also benefit from a broader testing panel that may include some non-RCC related genes.
Further research is needed to elucidate the genotype-phenotype association in some of the non-RCC mutations seen in this cohort, particularly in CHEK2 mutations. There are currently no screening guidelines for CHEK2 in regard to RCC, and the risk for RCC among those with these mutations is not known. RCC patients who have biallelic loss of DNA damage repair genes such as CHEK2 may benefit from treatment that targets these pathways, though these therapies are not currently offered for RCC.
The study population were individuals with advanced RCC, and the increased numbers of pathogenic germline mutations seen in this population are consistent with other studies finding higher rates of these mutations in patients who have other cancer with advanced disease. As this body of knowledge accumulates, interdisciplinary teams will be able to give more accurate information about risk and prognosis to patients and families and, increasingly, offer optimized care.
Dr. Patrick Pilié is an oncologist at the University of Texas MD Anderson Cancer Center, Houston; Dr. Kathleen Cooney is chair of the department of internal medicine and H.A. and Edna Benning Presidential Endowed Chair at the University of Utah, Salt Lake City. These remarks are drawn from a jointly authored editorial accompanying the study’s publication.
Germline mutations in patients with advanced renal cell carcinoma may be more common than previously suspected.
In a single-center cohort of 254 patients with advanced renal cell carcinoma (RCC) who received matched tumor-germline DNA sequencing, over a third (35.7%) of patients who had mutations in genes associated with RCC had not met current clinical criteria for testing.
In all, pathogenic germline mutations were identified in 41 patients (16.1%), with 14 patients’ mutations (5.5%) in genes known to be associated with RCC. For the remaining 27 patients (10.5%), the mutations were in non–RCC-associated genes, investigators reported in JAMA Oncology.
Of the non–RCC-associated mutations, CHEK2 was particularly common among patients with clear cell RCC (ccRCC), occurring in eight patients with ccRCC and two with non ccRCC (nccRCC). The overall odds ratio for this mutation among the study cohort was 3.0, compared with the general population (95% confidence interval 1.3-5.8; P = .003). “Although there are currently no RCC-specific screening recommendations for individuals with CHEK2 mutations, there may be incremental screening for other cancers, justifying including this gene on RCC panel tests,” wrote Maria Carlo, MD, and her coauthors.
Germline FH mutations were seen in seven patients, all with nccRCC. This higher rate of hereditary leiomyomatosis and RCC (HLRCC) was higher than previously reported in the literature, and clinical cues to the diagnosis were few among the study patients. Even though clues pointing to HLRCC were seen when tumor samples were submitted for histopathology to the genitourinary specialists at the study site, “it is unclear whether nonspecialist pathologists would be able to draw the same conclusions,” wrote Dr. Carlo and her colleagues.
Renal cell cancer–associated mutations were significantly more common in patients with nccRCC than in the ccRCC group: 9/74 (11.7%) nccRCC patients had an RCC-associated mutation, compared with 3/177 of the ccRCC group (P = .001).
The patient’s course of therapy could be guided by the mutation identified in 10% (eight) of the nccRCC patients, “none of which would have been identified with somatic-only sequencing,” wrote Dr. Carlo and associates. “Our results suggest that germline mutations in cancer-associated genes in patients with advanced RCC may be prevalent, and many of these mutations can be used to guide therapy.”
The 254 patients (median age 56 years, 70.5% male, 83.1% non-Hispanic white) were drawn from 267 patients with American Joint Committee on Cancer (AJCC) stage III or IV RCC participating in clinical trials at Memorial Sloan Kettering Cancer Center, New York, where Dr. Carlo practices as an oncologist. The patients included in the cohort were those who consented to germline sequencing and results disclosure.
To determine which pathogenic variants were identified by the study protocol that would have been missed by current testing standards, the investigators assumed that for those who met guidelines, the multigene test panel would probe for VHL, VH, FLCN, MET, SDHB, SDHD, BAP1, TSC1, TSC2, TP53, and MITF. If another mutation was picked up by the next-generation sequencing used in the study, or if a mutation was found in an individual who otherwise would not have been tested, the finding was considered incremental and attributable to the study protocol.
Implications of the additional mutations picked up by the tumor-germline sequencing approach go beyond the patient, said the researchers, who have seen several of the study participants’ family members receive positive test results for cancer-associated mutations as well. “Relatives who are also found to carry FH mutations should be considered for RCC screening. Early detection may increase the likelihood of cure and survivorship,” wrote Dr. Carlo and her coinvestigators.
Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J. Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
SOURCE: Carlo M et al. JAMA Oncol. 2018 Jul 5. doi: 10.1001/jamaoncol.2018.1986.
Germline mutations in patients with advanced renal cell carcinoma may be more common than previously suspected.
In a single-center cohort of 254 patients with advanced renal cell carcinoma (RCC) who received matched tumor-germline DNA sequencing, over a third (35.7%) of patients who had mutations in genes associated with RCC had not met current clinical criteria for testing.
In all, pathogenic germline mutations were identified in 41 patients (16.1%), with 14 patients’ mutations (5.5%) in genes known to be associated with RCC. For the remaining 27 patients (10.5%), the mutations were in non–RCC-associated genes, investigators reported in JAMA Oncology.
Of the non–RCC-associated mutations, CHEK2 was particularly common among patients with clear cell RCC (ccRCC), occurring in eight patients with ccRCC and two with non ccRCC (nccRCC). The overall odds ratio for this mutation among the study cohort was 3.0, compared with the general population (95% confidence interval 1.3-5.8; P = .003). “Although there are currently no RCC-specific screening recommendations for individuals with CHEK2 mutations, there may be incremental screening for other cancers, justifying including this gene on RCC panel tests,” wrote Maria Carlo, MD, and her coauthors.
Germline FH mutations were seen in seven patients, all with nccRCC. This higher rate of hereditary leiomyomatosis and RCC (HLRCC) was higher than previously reported in the literature, and clinical cues to the diagnosis were few among the study patients. Even though clues pointing to HLRCC were seen when tumor samples were submitted for histopathology to the genitourinary specialists at the study site, “it is unclear whether nonspecialist pathologists would be able to draw the same conclusions,” wrote Dr. Carlo and her colleagues.
Renal cell cancer–associated mutations were significantly more common in patients with nccRCC than in the ccRCC group: 9/74 (11.7%) nccRCC patients had an RCC-associated mutation, compared with 3/177 of the ccRCC group (P = .001).
The patient’s course of therapy could be guided by the mutation identified in 10% (eight) of the nccRCC patients, “none of which would have been identified with somatic-only sequencing,” wrote Dr. Carlo and associates. “Our results suggest that germline mutations in cancer-associated genes in patients with advanced RCC may be prevalent, and many of these mutations can be used to guide therapy.”
The 254 patients (median age 56 years, 70.5% male, 83.1% non-Hispanic white) were drawn from 267 patients with American Joint Committee on Cancer (AJCC) stage III or IV RCC participating in clinical trials at Memorial Sloan Kettering Cancer Center, New York, where Dr. Carlo practices as an oncologist. The patients included in the cohort were those who consented to germline sequencing and results disclosure.
To determine which pathogenic variants were identified by the study protocol that would have been missed by current testing standards, the investigators assumed that for those who met guidelines, the multigene test panel would probe for VHL, VH, FLCN, MET, SDHB, SDHD, BAP1, TSC1, TSC2, TP53, and MITF. If another mutation was picked up by the next-generation sequencing used in the study, or if a mutation was found in an individual who otherwise would not have been tested, the finding was considered incremental and attributable to the study protocol.
Implications of the additional mutations picked up by the tumor-germline sequencing approach go beyond the patient, said the researchers, who have seen several of the study participants’ family members receive positive test results for cancer-associated mutations as well. “Relatives who are also found to carry FH mutations should be considered for RCC screening. Early detection may increase the likelihood of cure and survivorship,” wrote Dr. Carlo and her coinvestigators.
Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J. Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
SOURCE: Carlo M et al. JAMA Oncol. 2018 Jul 5. doi: 10.1001/jamaoncol.2018.1986.
FROM JAMA ONCOLOGY
Key clinical point: A broader approach to sequencing of patients with advanced RCC may identify patients for targeted therapy.
Major finding: Pathogenic germline mutations were seen in 16% of patients with advanced RCC
Study details: Prospective single-center cohort study of 254 patients with advanced RCC.
Disclosures: Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J.Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
Source: Carlo M et al. JAMA Oncol. 2018 July 5. doi: 10.1001/jamaoncol.2018.1986.
Update on Acne Scar Treatment
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26
Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33
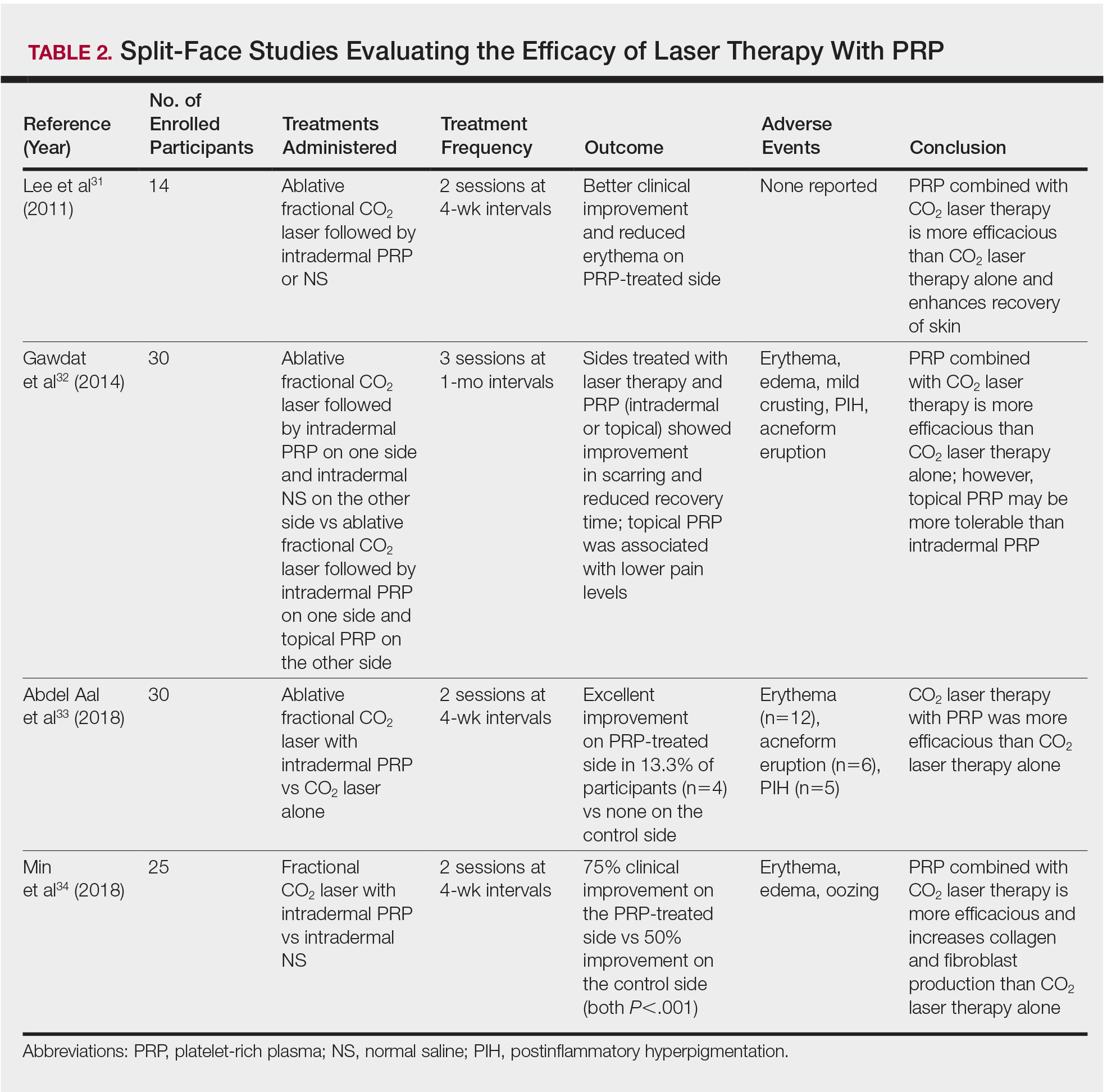
In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26


Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33

In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26


Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33

In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
Practice Points
- Acne scarring affects millions of Americans and can lead to poor psychological sequelae such as low self-esteem.
- Multiple modalities for acne scarring treatment exist including microneedling, lasers, chemical peels, and dermal fillers.
- Consider patient-desired outcome, cost, and adverse events when choosing a specific treatment modality.
U.S. pancreatic insufficiency patients often get inadequate enzyme replacement
WASHINGTON – according to a recent analysis of insurance claims data from more than 48 million Americans.
Amid concerns that some people with nonspecific symptoms are overdiagnosed with exocrine pancreatic insufficiency (EPI) and hence getting unneeded treatment with pancreatic enzyme replacement therapy (PERT), it seems like substantial numbers of patients with legitimate pancreatic morbidity are often missed and are going untreated, Chris E. Forsmark, MD, said at the annual Digestive Disease Week®. This includes patients with chronic pancreatitis, pancreatic cancer, and patients who underwent pancreatic resection surgery.
Dr. Forsmark cited still-unpublished evidence that he has reported at meetings during the past year. At DDW 2017 he and his associates reported findings from an analysis of health insurance claims data collected in the PharMetrics database for more than 48 million Americans during 2006-2013, which included 37,061 insured adults diagnosed with chronic pancreatitis. Analysis of these data showed that just 7% had ever undergone testing for EPI and 30% had received a prescription for PERT, of which only 31% received an appropriate dosage (Gastroenterology. 2017 Apr;152[5, suppl 1]:S677). In other words, a scant 9% of insured U.S. adults with chronic pancreatitis during the studied period had received a minimally effective dosage of PERT.
Dr. Forsmark and his associates ran a second analysis using the same 2006-2013 insurance database, but this time looked at 32,461 insured American adults diagnosed with pancreatic cancer and reported similar findings: Fewer than 2% of patients underwent testing for EPI, 22% were prescribed PERT, and of these, 22% of patients per quarter received a minimally effective dosage of PERT, meaning that, overall, only 6% of pancreatic cancer patients received treatment that could be expected to resolve their presumed enzyme deficiency. Dr. Forsmark and his associates presented this report at the annual meeting of the American Pancreatic Association in San Diego in November 2017 (Pancreas. 2017 Nov/Dec;46[10]:1386-1448).
An irony is that PERT underuse comes at a time when some Internet sites promote PERT as a treatment for patients with nonspecific symptoms of EPI such as bloating, dyspepsia, and loose stools, Dr. Forsmark noted. “There is a possibility that patients with nonspecific gastrointestinal symptoms may request or receive PERT. Some patients may receive PERT who do not have EPI.” In 2015, clinicians had written roughly 746,000 prescriptions for PERT to U.S. patients, with the number of prescriptions steadily increasing during 2010-2015. Five different formulations for PERT are currently on the U.S. market, and a typical course of treatment costs about $1,500-$2,000 per month, he added.
Dr. Forsmark had no disclosures to report.
WASHINGTON – according to a recent analysis of insurance claims data from more than 48 million Americans.
Amid concerns that some people with nonspecific symptoms are overdiagnosed with exocrine pancreatic insufficiency (EPI) and hence getting unneeded treatment with pancreatic enzyme replacement therapy (PERT), it seems like substantial numbers of patients with legitimate pancreatic morbidity are often missed and are going untreated, Chris E. Forsmark, MD, said at the annual Digestive Disease Week®. This includes patients with chronic pancreatitis, pancreatic cancer, and patients who underwent pancreatic resection surgery.
Dr. Forsmark cited still-unpublished evidence that he has reported at meetings during the past year. At DDW 2017 he and his associates reported findings from an analysis of health insurance claims data collected in the PharMetrics database for more than 48 million Americans during 2006-2013, which included 37,061 insured adults diagnosed with chronic pancreatitis. Analysis of these data showed that just 7% had ever undergone testing for EPI and 30% had received a prescription for PERT, of which only 31% received an appropriate dosage (Gastroenterology. 2017 Apr;152[5, suppl 1]:S677). In other words, a scant 9% of insured U.S. adults with chronic pancreatitis during the studied period had received a minimally effective dosage of PERT.
Dr. Forsmark and his associates ran a second analysis using the same 2006-2013 insurance database, but this time looked at 32,461 insured American adults diagnosed with pancreatic cancer and reported similar findings: Fewer than 2% of patients underwent testing for EPI, 22% were prescribed PERT, and of these, 22% of patients per quarter received a minimally effective dosage of PERT, meaning that, overall, only 6% of pancreatic cancer patients received treatment that could be expected to resolve their presumed enzyme deficiency. Dr. Forsmark and his associates presented this report at the annual meeting of the American Pancreatic Association in San Diego in November 2017 (Pancreas. 2017 Nov/Dec;46[10]:1386-1448).
An irony is that PERT underuse comes at a time when some Internet sites promote PERT as a treatment for patients with nonspecific symptoms of EPI such as bloating, dyspepsia, and loose stools, Dr. Forsmark noted. “There is a possibility that patients with nonspecific gastrointestinal symptoms may request or receive PERT. Some patients may receive PERT who do not have EPI.” In 2015, clinicians had written roughly 746,000 prescriptions for PERT to U.S. patients, with the number of prescriptions steadily increasing during 2010-2015. Five different formulations for PERT are currently on the U.S. market, and a typical course of treatment costs about $1,500-$2,000 per month, he added.
Dr. Forsmark had no disclosures to report.
WASHINGTON – according to a recent analysis of insurance claims data from more than 48 million Americans.
Amid concerns that some people with nonspecific symptoms are overdiagnosed with exocrine pancreatic insufficiency (EPI) and hence getting unneeded treatment with pancreatic enzyme replacement therapy (PERT), it seems like substantial numbers of patients with legitimate pancreatic morbidity are often missed and are going untreated, Chris E. Forsmark, MD, said at the annual Digestive Disease Week®. This includes patients with chronic pancreatitis, pancreatic cancer, and patients who underwent pancreatic resection surgery.
Dr. Forsmark cited still-unpublished evidence that he has reported at meetings during the past year. At DDW 2017 he and his associates reported findings from an analysis of health insurance claims data collected in the PharMetrics database for more than 48 million Americans during 2006-2013, which included 37,061 insured adults diagnosed with chronic pancreatitis. Analysis of these data showed that just 7% had ever undergone testing for EPI and 30% had received a prescription for PERT, of which only 31% received an appropriate dosage (Gastroenterology. 2017 Apr;152[5, suppl 1]:S677). In other words, a scant 9% of insured U.S. adults with chronic pancreatitis during the studied period had received a minimally effective dosage of PERT.
Dr. Forsmark and his associates ran a second analysis using the same 2006-2013 insurance database, but this time looked at 32,461 insured American adults diagnosed with pancreatic cancer and reported similar findings: Fewer than 2% of patients underwent testing for EPI, 22% were prescribed PERT, and of these, 22% of patients per quarter received a minimally effective dosage of PERT, meaning that, overall, only 6% of pancreatic cancer patients received treatment that could be expected to resolve their presumed enzyme deficiency. Dr. Forsmark and his associates presented this report at the annual meeting of the American Pancreatic Association in San Diego in November 2017 (Pancreas. 2017 Nov/Dec;46[10]:1386-1448).
An irony is that PERT underuse comes at a time when some Internet sites promote PERT as a treatment for patients with nonspecific symptoms of EPI such as bloating, dyspepsia, and loose stools, Dr. Forsmark noted. “There is a possibility that patients with nonspecific gastrointestinal symptoms may request or receive PERT. Some patients may receive PERT who do not have EPI.” In 2015, clinicians had written roughly 746,000 prescriptions for PERT to U.S. patients, with the number of prescriptions steadily increasing during 2010-2015. Five different formulations for PERT are currently on the U.S. market, and a typical course of treatment costs about $1,500-$2,000 per month, he added.
Dr. Forsmark had no disclosures to report.
REPORTING FROM DDW 2018
Key clinical point: U.S. patients with presumed exocrine pancreatic insufficiency often appear undertreated.
Major finding: During 2006-2013, only 9% of U.S. adults with chronic pancreatitis and 6% with pancreatic cancer received adequate enzyme replacement.
Study details: A review of diagnosis and claims data from 48.67 million insured U.S. adults during 2006-2013.
Disclosures: Dr. Forsmark had no disclosures to report.
EULAR nears first recommendations for managing Sjögren’s syndrome
AMSTERDAM – , and they divide the treatment targets into sicca syndrome and systemic manifestations of the disease.
“In Sjögren’s, we always have two subtypes of patients: those who have sicca syndrome only, and those with sicca syndrome plus systemic disease,” explained Soledad Retamozo, MD, who presented the current version of the recommendations at the European Congress of Rheumatology. “We wanted to highlight that there are two types of patients,” said Dr. Retamozo, a rheumatologist at the University of Córdoba (Argentina). “It’s hard to treat patients with sicca syndrome plus fatigue and pain because there is no high-level evidence on how to do this; all we have is expert opinion,” Dr. Retamozo said in an interview.
In fact, roughly half of the recommendations have no supporting evidence base, as presented by Dr. Retamozo. That starts with all three general recommendations she presented:
• Patients with Sjögren’s should be managed at a center of expertise using a multidisciplinary approach, which she said should include ophthalmologists and dentists to help address the mouth and ocular manifestations of sicca syndrome.
• Patients with sicca syndrome should receive symptomatic relief with topical treatments.
• Systemic treatments – glucocorticoids, immunosuppressants, and biologicals – can be considered for patients with active systemic disease.
The statement’s specific recommendations start with managing oral dryness, an intervention that should begin by measuring salivary gland (SG) dysfunction. The document next recommends nonpharmacologic interventions for mild SG dysfunction, pharmacological stimulation for moderate SG dysfunction, and a saliva substitute for severe SG dysfunction. All three recommendations are evidence based, relying on results from either randomized trials or controlled studies.
The second target for topical treatments is ocular dryness, which starts with artificial tears, or ocular gels or ointments, recommendations based on randomized trials. Refractory or severe ocular dryness should receive eye drops that contain a nonsteroidal anti-inflammatory drug or a glucocorticoid, based on controlled study results, or autologous serum eye drops, a strategy tested in a randomized trial.
The recommendations then shift to dealing with systemic manifestations, starting with fatigue and pain, offering the expert recommendation to evaluate the contribution of comorbid diseases and assess their severity with tools such as the Eular Sjögren’s Syndrome Patient-Reported Index (ESSPRI) (Ann Rheum Dis. 2011 June;70[6]:968-72), the Profile of Fatigue, and the Brief Pain Inventory.
Using evidence from randomized trials, the recommendations tell clinicians to consider treatment with analgesics or pain-modifying agents for musculoskeletal pain by weighing the potential benefits and adverse effects from this treatment.
For other forms of systemic disease, the recommendations offer the expert opinion to tailor treatment to the organ-specific severity using the ESSPRI definitions. If using glucocorticoids to treat systemic disease, they should be given at the minimum effective dose and for the shortest period of time needed to control active systemic disease, a recommendation based on retrospective or descriptive studies. Expert opinion called for using immunosuppressive treatments as glucocorticoid-sparing options for systemic disease, and this recommendation added that no particular immunosuppressive agent stands out as best compared with all available agents. In more than 95% of reported cases of systemic disease treatment in Sjögren’s patients, clinicians used the immunosuppressive drugs in association with glucocorticoids, Dr. Retamozo noted.
Finally, for systemic disease the recommendations cited evidence from controlled studies that B-cell targeted therapies, such as rituximab (Rituxan) and belimumab (Benlysta), may be considered in patients with severe, refractory systemic disease. An additional expert opinion was that the systemic, organ-specific approach should sequence treatments by using glucocorticoids first, followed by immunosuppressants, and finally biological drugs.
The recommendations finish with an entry that treatment of B-cell lymphoma be individualized based on the specific histopathologic subtype involved and the level of disease extension, an approach based on results from retrospective or descriptive studies.
The recommendations must still undergo final EULAR review and endorsement, with publication on track to occur before the end of 2018, Dr. Retamozo said.
She had no disclosures.
SOURCE: Retamozo S al. Ann Rheum Dis. 2018;77(Suppl 2):42. Abstract SP0159.
AMSTERDAM – , and they divide the treatment targets into sicca syndrome and systemic manifestations of the disease.
“In Sjögren’s, we always have two subtypes of patients: those who have sicca syndrome only, and those with sicca syndrome plus systemic disease,” explained Soledad Retamozo, MD, who presented the current version of the recommendations at the European Congress of Rheumatology. “We wanted to highlight that there are two types of patients,” said Dr. Retamozo, a rheumatologist at the University of Córdoba (Argentina). “It’s hard to treat patients with sicca syndrome plus fatigue and pain because there is no high-level evidence on how to do this; all we have is expert opinion,” Dr. Retamozo said in an interview.
In fact, roughly half of the recommendations have no supporting evidence base, as presented by Dr. Retamozo. That starts with all three general recommendations she presented:
• Patients with Sjögren’s should be managed at a center of expertise using a multidisciplinary approach, which she said should include ophthalmologists and dentists to help address the mouth and ocular manifestations of sicca syndrome.
• Patients with sicca syndrome should receive symptomatic relief with topical treatments.
• Systemic treatments – glucocorticoids, immunosuppressants, and biologicals – can be considered for patients with active systemic disease.
The statement’s specific recommendations start with managing oral dryness, an intervention that should begin by measuring salivary gland (SG) dysfunction. The document next recommends nonpharmacologic interventions for mild SG dysfunction, pharmacological stimulation for moderate SG dysfunction, and a saliva substitute for severe SG dysfunction. All three recommendations are evidence based, relying on results from either randomized trials or controlled studies.
The second target for topical treatments is ocular dryness, which starts with artificial tears, or ocular gels or ointments, recommendations based on randomized trials. Refractory or severe ocular dryness should receive eye drops that contain a nonsteroidal anti-inflammatory drug or a glucocorticoid, based on controlled study results, or autologous serum eye drops, a strategy tested in a randomized trial.
The recommendations then shift to dealing with systemic manifestations, starting with fatigue and pain, offering the expert recommendation to evaluate the contribution of comorbid diseases and assess their severity with tools such as the Eular Sjögren’s Syndrome Patient-Reported Index (ESSPRI) (Ann Rheum Dis. 2011 June;70[6]:968-72), the Profile of Fatigue, and the Brief Pain Inventory.
Using evidence from randomized trials, the recommendations tell clinicians to consider treatment with analgesics or pain-modifying agents for musculoskeletal pain by weighing the potential benefits and adverse effects from this treatment.
For other forms of systemic disease, the recommendations offer the expert opinion to tailor treatment to the organ-specific severity using the ESSPRI definitions. If using glucocorticoids to treat systemic disease, they should be given at the minimum effective dose and for the shortest period of time needed to control active systemic disease, a recommendation based on retrospective or descriptive studies. Expert opinion called for using immunosuppressive treatments as glucocorticoid-sparing options for systemic disease, and this recommendation added that no particular immunosuppressive agent stands out as best compared with all available agents. In more than 95% of reported cases of systemic disease treatment in Sjögren’s patients, clinicians used the immunosuppressive drugs in association with glucocorticoids, Dr. Retamozo noted.
Finally, for systemic disease the recommendations cited evidence from controlled studies that B-cell targeted therapies, such as rituximab (Rituxan) and belimumab (Benlysta), may be considered in patients with severe, refractory systemic disease. An additional expert opinion was that the systemic, organ-specific approach should sequence treatments by using glucocorticoids first, followed by immunosuppressants, and finally biological drugs.
The recommendations finish with an entry that treatment of B-cell lymphoma be individualized based on the specific histopathologic subtype involved and the level of disease extension, an approach based on results from retrospective or descriptive studies.
The recommendations must still undergo final EULAR review and endorsement, with publication on track to occur before the end of 2018, Dr. Retamozo said.
She had no disclosures.
SOURCE: Retamozo S al. Ann Rheum Dis. 2018;77(Suppl 2):42. Abstract SP0159.
AMSTERDAM – , and they divide the treatment targets into sicca syndrome and systemic manifestations of the disease.
“In Sjögren’s, we always have two subtypes of patients: those who have sicca syndrome only, and those with sicca syndrome plus systemic disease,” explained Soledad Retamozo, MD, who presented the current version of the recommendations at the European Congress of Rheumatology. “We wanted to highlight that there are two types of patients,” said Dr. Retamozo, a rheumatologist at the University of Córdoba (Argentina). “It’s hard to treat patients with sicca syndrome plus fatigue and pain because there is no high-level evidence on how to do this; all we have is expert opinion,” Dr. Retamozo said in an interview.
In fact, roughly half of the recommendations have no supporting evidence base, as presented by Dr. Retamozo. That starts with all three general recommendations she presented:
• Patients with Sjögren’s should be managed at a center of expertise using a multidisciplinary approach, which she said should include ophthalmologists and dentists to help address the mouth and ocular manifestations of sicca syndrome.
• Patients with sicca syndrome should receive symptomatic relief with topical treatments.
• Systemic treatments – glucocorticoids, immunosuppressants, and biologicals – can be considered for patients with active systemic disease.
The statement’s specific recommendations start with managing oral dryness, an intervention that should begin by measuring salivary gland (SG) dysfunction. The document next recommends nonpharmacologic interventions for mild SG dysfunction, pharmacological stimulation for moderate SG dysfunction, and a saliva substitute for severe SG dysfunction. All three recommendations are evidence based, relying on results from either randomized trials or controlled studies.
The second target for topical treatments is ocular dryness, which starts with artificial tears, or ocular gels or ointments, recommendations based on randomized trials. Refractory or severe ocular dryness should receive eye drops that contain a nonsteroidal anti-inflammatory drug or a glucocorticoid, based on controlled study results, or autologous serum eye drops, a strategy tested in a randomized trial.
The recommendations then shift to dealing with systemic manifestations, starting with fatigue and pain, offering the expert recommendation to evaluate the contribution of comorbid diseases and assess their severity with tools such as the Eular Sjögren’s Syndrome Patient-Reported Index (ESSPRI) (Ann Rheum Dis. 2011 June;70[6]:968-72), the Profile of Fatigue, and the Brief Pain Inventory.
Using evidence from randomized trials, the recommendations tell clinicians to consider treatment with analgesics or pain-modifying agents for musculoskeletal pain by weighing the potential benefits and adverse effects from this treatment.
For other forms of systemic disease, the recommendations offer the expert opinion to tailor treatment to the organ-specific severity using the ESSPRI definitions. If using glucocorticoids to treat systemic disease, they should be given at the minimum effective dose and for the shortest period of time needed to control active systemic disease, a recommendation based on retrospective or descriptive studies. Expert opinion called for using immunosuppressive treatments as glucocorticoid-sparing options for systemic disease, and this recommendation added that no particular immunosuppressive agent stands out as best compared with all available agents. In more than 95% of reported cases of systemic disease treatment in Sjögren’s patients, clinicians used the immunosuppressive drugs in association with glucocorticoids, Dr. Retamozo noted.
Finally, for systemic disease the recommendations cited evidence from controlled studies that B-cell targeted therapies, such as rituximab (Rituxan) and belimumab (Benlysta), may be considered in patients with severe, refractory systemic disease. An additional expert opinion was that the systemic, organ-specific approach should sequence treatments by using glucocorticoids first, followed by immunosuppressants, and finally biological drugs.
The recommendations finish with an entry that treatment of B-cell lymphoma be individualized based on the specific histopathologic subtype involved and the level of disease extension, an approach based on results from retrospective or descriptive studies.
The recommendations must still undergo final EULAR review and endorsement, with publication on track to occur before the end of 2018, Dr. Retamozo said.
She had no disclosures.
SOURCE: Retamozo S al. Ann Rheum Dis. 2018;77(Suppl 2):42. Abstract SP0159.
REPORTING FROM THE EULAR 2018 CONGRESS
Trastuzumab biosimilar is equivalent on central review
CHICAGO – according to a new analysis of the phase 3 LILAC trial.
The 725 women in the multinational trial received run-in, anthracycline-based chemotherapy and were then evenly randomized to receive ABP 980 or trastuzumab, each with paclitaxel, followed by surgery.
The difference in pathologic complete response (pCR) rate assessed by local pathologists has been previously reported (Lancet Oncol. 2018 Jun 4. doi: 10.1016/S1470-2045(18)30241-9); those findings established non-inferiority of the biosimilar but left the matter of non-superiority inconclusive. However, in the new analysis, reported in a poster session at the ASCO Annual Meeting, the difference in pCR rate when instead assessed by a central pathologist fell within all bounds for equivalence.
“This is part of the totality of evidence in the course of approval of ABP 980,” lead author Hans-Christian Kolberg, MD, head of the department of gynecology and obstetrics of the Breast Cancer Center of the Gynecologic Cancer Center at Marien Hospital Bottrop (Germany), commented in an interview.
The new data prompted European regulators to authorize marketing of the biosimilar (branded as Kanjinti) for HER2+ early breast cancer and metastatic breast cancer, as well as HER2+ metastatic gastric cancer. (In the United States, the Food and Drug Administration recently rejected the application for ABP 980 market approval.)
“Breast cancer therapy is getting more and more expensive, and we somehow have to raise the money to pay for it. If we have a chance to make an antibody that is 20%-30% cheaper, which is what we hope it will be in Europe, we have that money for other things,” Dr. Kolberg said, reflecting on the bigger picture.
“I am also a visiting professor at a university in China, where patients who are HER2+ don’t get Herceptin because they can’t afford it. We always have to remember that in Europe and the U.S., we are kind of living on an island. If you look at Africa, Asia, and South America, making things affordable is important,” he added. “I hope and believe that this is just the beginning of the price fight. I hope that the biosimilar companies really will fight to see who will have the lowest price because that will be good for the patients. The lower the price, the better for the patients.”
Study details
Research leading up to the LILAC trial established that ABP 980 had analytic characteristics, nonclinical attributes, and pharmacokinetics similar to those of trastuzumab. The trial, conducted in 97 centers in 20 countries in western Europe, eastern Europe, and other world regions, assessed clinical similarity.
“I think central review was done in the study because we had so many centers all over the world that it was questionable as to how we could monitor the quality in dozens and dozens of pathology labs,” Dr. Kolberg explained. “So the idea was that we make it a little bit more difficult, a little bit more expensive, but more reliable if we use one pathologist.”
The central review was not without logistical issues, he acknowledged. In particular, it was challenging to ensure that all centers – including some doing so for the first time – followed a standardized procedure for sending tissue to the central lab.
The previously reported locally assessed pCR rates in breast tissue and axillary lymph nodes were 48.0% with ABP 980 and 40.5% with trastuzumab. The risk difference was 7.3% (90% confidence interval, 1.2%-13.4%) and the risk ratio was 1.188 (90% CI, 1.033-1.366), with the upper bounds of the confidence intervals exceeding the predefined equivalence margins of 13% and 1.318, respectively.
The centrally assessed pCR rates were 47.8% with ABP 980 and 41.8% with trastuzumab. The risk difference was 5.8% (90% CI, –0.5% to 12.0%), and the risk ratio was 1.14 (90% CI, 0.993 to 1.312), with the upper bounds of the confidence intervals now falling within the equivalence margins.
“This is the first study ever that used central pathology review for pCR in a neoadjuvant breast cancer study. We were really skeptical at the beginning as to whether that would work because we had a lot of centers all over the world, from Russia, Brazil, the U.S., Germany,” Dr. Kolberg commented.
“It worked, and we were very lucky that it worked because in the local review, we did not reach our biosimilar margins, our equivalence margins. In the central review, we were well within the margins,” he said. “So if we had not in the beginning planned a coprimary endpoint with local and central pathology review, the medication would never have been approved.”
Dr. Kolberg disclosed that he is a consultant for Amgen, Carl Zeiss Meditec, Genomic Health, GlaxoSmithKline, Janssen, LIV Pharma, Novartis, Pfizer, Roche, SurgVision, Teva Pharmaceutical Industries, and Theraclion. The trial was sponsored by Amgen.
SOURCE: Kolberg HC et al. ASCO Annual Meeting, Abstract 583.
CHICAGO – according to a new analysis of the phase 3 LILAC trial.
The 725 women in the multinational trial received run-in, anthracycline-based chemotherapy and were then evenly randomized to receive ABP 980 or trastuzumab, each with paclitaxel, followed by surgery.
The difference in pathologic complete response (pCR) rate assessed by local pathologists has been previously reported (Lancet Oncol. 2018 Jun 4. doi: 10.1016/S1470-2045(18)30241-9); those findings established non-inferiority of the biosimilar but left the matter of non-superiority inconclusive. However, in the new analysis, reported in a poster session at the ASCO Annual Meeting, the difference in pCR rate when instead assessed by a central pathologist fell within all bounds for equivalence.
“This is part of the totality of evidence in the course of approval of ABP 980,” lead author Hans-Christian Kolberg, MD, head of the department of gynecology and obstetrics of the Breast Cancer Center of the Gynecologic Cancer Center at Marien Hospital Bottrop (Germany), commented in an interview.
The new data prompted European regulators to authorize marketing of the biosimilar (branded as Kanjinti) for HER2+ early breast cancer and metastatic breast cancer, as well as HER2+ metastatic gastric cancer. (In the United States, the Food and Drug Administration recently rejected the application for ABP 980 market approval.)
“Breast cancer therapy is getting more and more expensive, and we somehow have to raise the money to pay for it. If we have a chance to make an antibody that is 20%-30% cheaper, which is what we hope it will be in Europe, we have that money for other things,” Dr. Kolberg said, reflecting on the bigger picture.
“I am also a visiting professor at a university in China, where patients who are HER2+ don’t get Herceptin because they can’t afford it. We always have to remember that in Europe and the U.S., we are kind of living on an island. If you look at Africa, Asia, and South America, making things affordable is important,” he added. “I hope and believe that this is just the beginning of the price fight. I hope that the biosimilar companies really will fight to see who will have the lowest price because that will be good for the patients. The lower the price, the better for the patients.”
Study details
Research leading up to the LILAC trial established that ABP 980 had analytic characteristics, nonclinical attributes, and pharmacokinetics similar to those of trastuzumab. The trial, conducted in 97 centers in 20 countries in western Europe, eastern Europe, and other world regions, assessed clinical similarity.
“I think central review was done in the study because we had so many centers all over the world that it was questionable as to how we could monitor the quality in dozens and dozens of pathology labs,” Dr. Kolberg explained. “So the idea was that we make it a little bit more difficult, a little bit more expensive, but more reliable if we use one pathologist.”
The central review was not without logistical issues, he acknowledged. In particular, it was challenging to ensure that all centers – including some doing so for the first time – followed a standardized procedure for sending tissue to the central lab.
The previously reported locally assessed pCR rates in breast tissue and axillary lymph nodes were 48.0% with ABP 980 and 40.5% with trastuzumab. The risk difference was 7.3% (90% confidence interval, 1.2%-13.4%) and the risk ratio was 1.188 (90% CI, 1.033-1.366), with the upper bounds of the confidence intervals exceeding the predefined equivalence margins of 13% and 1.318, respectively.
The centrally assessed pCR rates were 47.8% with ABP 980 and 41.8% with trastuzumab. The risk difference was 5.8% (90% CI, –0.5% to 12.0%), and the risk ratio was 1.14 (90% CI, 0.993 to 1.312), with the upper bounds of the confidence intervals now falling within the equivalence margins.
“This is the first study ever that used central pathology review for pCR in a neoadjuvant breast cancer study. We were really skeptical at the beginning as to whether that would work because we had a lot of centers all over the world, from Russia, Brazil, the U.S., Germany,” Dr. Kolberg commented.
“It worked, and we were very lucky that it worked because in the local review, we did not reach our biosimilar margins, our equivalence margins. In the central review, we were well within the margins,” he said. “So if we had not in the beginning planned a coprimary endpoint with local and central pathology review, the medication would never have been approved.”
Dr. Kolberg disclosed that he is a consultant for Amgen, Carl Zeiss Meditec, Genomic Health, GlaxoSmithKline, Janssen, LIV Pharma, Novartis, Pfizer, Roche, SurgVision, Teva Pharmaceutical Industries, and Theraclion. The trial was sponsored by Amgen.
SOURCE: Kolberg HC et al. ASCO Annual Meeting, Abstract 583.
CHICAGO – according to a new analysis of the phase 3 LILAC trial.
The 725 women in the multinational trial received run-in, anthracycline-based chemotherapy and were then evenly randomized to receive ABP 980 or trastuzumab, each with paclitaxel, followed by surgery.
The difference in pathologic complete response (pCR) rate assessed by local pathologists has been previously reported (Lancet Oncol. 2018 Jun 4. doi: 10.1016/S1470-2045(18)30241-9); those findings established non-inferiority of the biosimilar but left the matter of non-superiority inconclusive. However, in the new analysis, reported in a poster session at the ASCO Annual Meeting, the difference in pCR rate when instead assessed by a central pathologist fell within all bounds for equivalence.
“This is part of the totality of evidence in the course of approval of ABP 980,” lead author Hans-Christian Kolberg, MD, head of the department of gynecology and obstetrics of the Breast Cancer Center of the Gynecologic Cancer Center at Marien Hospital Bottrop (Germany), commented in an interview.
The new data prompted European regulators to authorize marketing of the biosimilar (branded as Kanjinti) for HER2+ early breast cancer and metastatic breast cancer, as well as HER2+ metastatic gastric cancer. (In the United States, the Food and Drug Administration recently rejected the application for ABP 980 market approval.)
“Breast cancer therapy is getting more and more expensive, and we somehow have to raise the money to pay for it. If we have a chance to make an antibody that is 20%-30% cheaper, which is what we hope it will be in Europe, we have that money for other things,” Dr. Kolberg said, reflecting on the bigger picture.
“I am also a visiting professor at a university in China, where patients who are HER2+ don’t get Herceptin because they can’t afford it. We always have to remember that in Europe and the U.S., we are kind of living on an island. If you look at Africa, Asia, and South America, making things affordable is important,” he added. “I hope and believe that this is just the beginning of the price fight. I hope that the biosimilar companies really will fight to see who will have the lowest price because that will be good for the patients. The lower the price, the better for the patients.”
Study details
Research leading up to the LILAC trial established that ABP 980 had analytic characteristics, nonclinical attributes, and pharmacokinetics similar to those of trastuzumab. The trial, conducted in 97 centers in 20 countries in western Europe, eastern Europe, and other world regions, assessed clinical similarity.
“I think central review was done in the study because we had so many centers all over the world that it was questionable as to how we could monitor the quality in dozens and dozens of pathology labs,” Dr. Kolberg explained. “So the idea was that we make it a little bit more difficult, a little bit more expensive, but more reliable if we use one pathologist.”
The central review was not without logistical issues, he acknowledged. In particular, it was challenging to ensure that all centers – including some doing so for the first time – followed a standardized procedure for sending tissue to the central lab.
The previously reported locally assessed pCR rates in breast tissue and axillary lymph nodes were 48.0% with ABP 980 and 40.5% with trastuzumab. The risk difference was 7.3% (90% confidence interval, 1.2%-13.4%) and the risk ratio was 1.188 (90% CI, 1.033-1.366), with the upper bounds of the confidence intervals exceeding the predefined equivalence margins of 13% and 1.318, respectively.
The centrally assessed pCR rates were 47.8% with ABP 980 and 41.8% with trastuzumab. The risk difference was 5.8% (90% CI, –0.5% to 12.0%), and the risk ratio was 1.14 (90% CI, 0.993 to 1.312), with the upper bounds of the confidence intervals now falling within the equivalence margins.
“This is the first study ever that used central pathology review for pCR in a neoadjuvant breast cancer study. We were really skeptical at the beginning as to whether that would work because we had a lot of centers all over the world, from Russia, Brazil, the U.S., Germany,” Dr. Kolberg commented.
“It worked, and we were very lucky that it worked because in the local review, we did not reach our biosimilar margins, our equivalence margins. In the central review, we were well within the margins,” he said. “So if we had not in the beginning planned a coprimary endpoint with local and central pathology review, the medication would never have been approved.”
Dr. Kolberg disclosed that he is a consultant for Amgen, Carl Zeiss Meditec, Genomic Health, GlaxoSmithKline, Janssen, LIV Pharma, Novartis, Pfizer, Roche, SurgVision, Teva Pharmaceutical Industries, and Theraclion. The trial was sponsored by Amgen.
SOURCE: Kolberg HC et al. ASCO Annual Meeting, Abstract 583.
REPORTING FROM THE ASCO ANNUAL MEETING
Key clinical point: Central review determined that ABP 980 was neither inferior nor superior to trastuzumab in breast cancer patients.
Major finding: The centrally determined pCR rates were 47.8% with ABP 980 and 41.8% with trastuzumab, with bounds of the confidence intervals for risk difference and for risk ratio falling within the predefined equivalence margins.
Study details: An analysis of a phase 3 randomized controlled trial of neoadjuvant (and adjuvant) therapy among 725 patients with HER2+ early breast cancer (LILAC trial).
Disclosures: Dr. Kolberg disclosed that he is a consultant for Amgen, Carl Zeiss Meditec, Genomic Health, GlaxoSmithKline, Janssen, LIV Pharma, Novartis, Pfizer, Roche, SurgVision, Teva Pharmaceutical Industries, and Theraclion. The trial was sponsored by Amgen.
Source: Kolberg HC et al. ASCO Annual Meeting, Abstract 583.
My experience with the 2017 Gastroenterology Editorial Fellowship
When I entered the Gastroenterology Editorial Fellowship last year, many of my cofellows asked, “What exactly is an editorial fellowship?” After completing the program, I can now reflect on what was truly a fantastic year-long experience that complemented my final year of fellowship training.
Manuscripts certainly don’t review and accept themselves into journals, and fellowship training usually gives little insight into how manuscripts move through the submission, peer review, and production processes. What happens while authors wait for an editorial decision?
Since its first publication in 1943, Gastroenterology has importantly affected clinical care and the direction of research in our field. The quality of the American Gastroenterological Association’s flagship journal is derived from the sweat and muscle put in daily by gastroenterology-oriented and hepatology-oriented professionals who strive to transform the steady stream of cutting-edge manuscript submissions into an influential monthly publication read by a broad audience of clinicians, trainees, academic researchers, and policy makers. Without a doubt, this fellowship provided me with a sincere appreciation for the dedication that the board of editors puts into the peer review process and into maintaining the quality of monthly publications.
Near the beginning of my editorial fellowship, I spent a week at Vanderbilt University with the on-site editors. This was an irreplaceable opportunity for a trainee like myself to meet with both clinical- and research-oriented academic gastroenterologists who integrate demanding editorial roles into busy and fulfilling professional careers. Throughout my week there, I met with editors and staff who held various roles within the journal. Overall, this experience taught me about what metrics the journal uses to ensure quality, how manuscripts move from submission to publication, and how the direction and content of the journal is directed toward both AGA members and a broader readership.
At its core, the fellowship was focused on teaching the fundamental process of peer review. High-quality reviews for Gastroenterology provide consultative content and methodological expertise to editors who can then provide direction and make editorial recommendations to the authors. During my fellowship, I learned how to write a structured and nuanced review on the basis of novelty, clinical relevance and effects, and methodological rigor. I was paired with one of the associate editors on the basis of my primary content area of interest and regularly provided reviews for original article submissions. As the year progressed, I become more comfortable with reviewing beyond my immediate knowledge base. I also became more adept at providing detailed comments that would be insightful and accessible to both authors and editors.
Each week, I participated in a phone call with the board of editors, which was composed of thought leaders with content expertise in both gastroenterology and hepatology. During the call, we would thoughtfully critique some of the most cutting-edge research in our field; each manuscript often represented the culmination of years of meticulous work by research groups and multinational collaborations. From a fellow’s perspective, these calls gave me access to what may be the most insightful discussions taking place in our field, discussions which could have potential implications on future disease management principles and clinical practice guidelines. Through our meetings, it became apparent how much work goes into finding quality reviewers and how much time goes into assimilating the resulting recommendations into a cohesive discussion. This was an opportunity to learn how associate editors walk the entire board through a manuscript: from a basis of current knowledge and practice, through the conduct and findings of a particular study, and ultimately, to how study findings might affect the field.
What I came away with the most from the Gastroenterology Editorial Fellowship was an appreciation for the importance of the editorial and peer review process in maintaining the integrity and detail needed in high-quality research. Ultimately, this fellowship gave me a meaningful and immediate way to give back to the field that I can continue over the course of my professional career. I am certain that this unique program will continue to give future editorial fellows the skills and motivation they need to become actively involved in the editorial and peer review processes when they are beginning their independent careers.
Dr. Shah, MD, MBA, is an assistant professor; he is also the director of the Center for Gastrointestinal Motility in the division of gastroenterology in the department of internal medicine at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
When I entered the Gastroenterology Editorial Fellowship last year, many of my cofellows asked, “What exactly is an editorial fellowship?” After completing the program, I can now reflect on what was truly a fantastic year-long experience that complemented my final year of fellowship training.
Manuscripts certainly don’t review and accept themselves into journals, and fellowship training usually gives little insight into how manuscripts move through the submission, peer review, and production processes. What happens while authors wait for an editorial decision?
Since its first publication in 1943, Gastroenterology has importantly affected clinical care and the direction of research in our field. The quality of the American Gastroenterological Association’s flagship journal is derived from the sweat and muscle put in daily by gastroenterology-oriented and hepatology-oriented professionals who strive to transform the steady stream of cutting-edge manuscript submissions into an influential monthly publication read by a broad audience of clinicians, trainees, academic researchers, and policy makers. Without a doubt, this fellowship provided me with a sincere appreciation for the dedication that the board of editors puts into the peer review process and into maintaining the quality of monthly publications.
Near the beginning of my editorial fellowship, I spent a week at Vanderbilt University with the on-site editors. This was an irreplaceable opportunity for a trainee like myself to meet with both clinical- and research-oriented academic gastroenterologists who integrate demanding editorial roles into busy and fulfilling professional careers. Throughout my week there, I met with editors and staff who held various roles within the journal. Overall, this experience taught me about what metrics the journal uses to ensure quality, how manuscripts move from submission to publication, and how the direction and content of the journal is directed toward both AGA members and a broader readership.
At its core, the fellowship was focused on teaching the fundamental process of peer review. High-quality reviews for Gastroenterology provide consultative content and methodological expertise to editors who can then provide direction and make editorial recommendations to the authors. During my fellowship, I learned how to write a structured and nuanced review on the basis of novelty, clinical relevance and effects, and methodological rigor. I was paired with one of the associate editors on the basis of my primary content area of interest and regularly provided reviews for original article submissions. As the year progressed, I become more comfortable with reviewing beyond my immediate knowledge base. I also became more adept at providing detailed comments that would be insightful and accessible to both authors and editors.
Each week, I participated in a phone call with the board of editors, which was composed of thought leaders with content expertise in both gastroenterology and hepatology. During the call, we would thoughtfully critique some of the most cutting-edge research in our field; each manuscript often represented the culmination of years of meticulous work by research groups and multinational collaborations. From a fellow’s perspective, these calls gave me access to what may be the most insightful discussions taking place in our field, discussions which could have potential implications on future disease management principles and clinical practice guidelines. Through our meetings, it became apparent how much work goes into finding quality reviewers and how much time goes into assimilating the resulting recommendations into a cohesive discussion. This was an opportunity to learn how associate editors walk the entire board through a manuscript: from a basis of current knowledge and practice, through the conduct and findings of a particular study, and ultimately, to how study findings might affect the field.
What I came away with the most from the Gastroenterology Editorial Fellowship was an appreciation for the importance of the editorial and peer review process in maintaining the integrity and detail needed in high-quality research. Ultimately, this fellowship gave me a meaningful and immediate way to give back to the field that I can continue over the course of my professional career. I am certain that this unique program will continue to give future editorial fellows the skills and motivation they need to become actively involved in the editorial and peer review processes when they are beginning their independent careers.
Dr. Shah, MD, MBA, is an assistant professor; he is also the director of the Center for Gastrointestinal Motility in the division of gastroenterology in the department of internal medicine at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
When I entered the Gastroenterology Editorial Fellowship last year, many of my cofellows asked, “What exactly is an editorial fellowship?” After completing the program, I can now reflect on what was truly a fantastic year-long experience that complemented my final year of fellowship training.
Manuscripts certainly don’t review and accept themselves into journals, and fellowship training usually gives little insight into how manuscripts move through the submission, peer review, and production processes. What happens while authors wait for an editorial decision?
Since its first publication in 1943, Gastroenterology has importantly affected clinical care and the direction of research in our field. The quality of the American Gastroenterological Association’s flagship journal is derived from the sweat and muscle put in daily by gastroenterology-oriented and hepatology-oriented professionals who strive to transform the steady stream of cutting-edge manuscript submissions into an influential monthly publication read by a broad audience of clinicians, trainees, academic researchers, and policy makers. Without a doubt, this fellowship provided me with a sincere appreciation for the dedication that the board of editors puts into the peer review process and into maintaining the quality of monthly publications.
Near the beginning of my editorial fellowship, I spent a week at Vanderbilt University with the on-site editors. This was an irreplaceable opportunity for a trainee like myself to meet with both clinical- and research-oriented academic gastroenterologists who integrate demanding editorial roles into busy and fulfilling professional careers. Throughout my week there, I met with editors and staff who held various roles within the journal. Overall, this experience taught me about what metrics the journal uses to ensure quality, how manuscripts move from submission to publication, and how the direction and content of the journal is directed toward both AGA members and a broader readership.
At its core, the fellowship was focused on teaching the fundamental process of peer review. High-quality reviews for Gastroenterology provide consultative content and methodological expertise to editors who can then provide direction and make editorial recommendations to the authors. During my fellowship, I learned how to write a structured and nuanced review on the basis of novelty, clinical relevance and effects, and methodological rigor. I was paired with one of the associate editors on the basis of my primary content area of interest and regularly provided reviews for original article submissions. As the year progressed, I become more comfortable with reviewing beyond my immediate knowledge base. I also became more adept at providing detailed comments that would be insightful and accessible to both authors and editors.
Each week, I participated in a phone call with the board of editors, which was composed of thought leaders with content expertise in both gastroenterology and hepatology. During the call, we would thoughtfully critique some of the most cutting-edge research in our field; each manuscript often represented the culmination of years of meticulous work by research groups and multinational collaborations. From a fellow’s perspective, these calls gave me access to what may be the most insightful discussions taking place in our field, discussions which could have potential implications on future disease management principles and clinical practice guidelines. Through our meetings, it became apparent how much work goes into finding quality reviewers and how much time goes into assimilating the resulting recommendations into a cohesive discussion. This was an opportunity to learn how associate editors walk the entire board through a manuscript: from a basis of current knowledge and practice, through the conduct and findings of a particular study, and ultimately, to how study findings might affect the field.
What I came away with the most from the Gastroenterology Editorial Fellowship was an appreciation for the importance of the editorial and peer review process in maintaining the integrity and detail needed in high-quality research. Ultimately, this fellowship gave me a meaningful and immediate way to give back to the field that I can continue over the course of my professional career. I am certain that this unique program will continue to give future editorial fellows the skills and motivation they need to become actively involved in the editorial and peer review processes when they are beginning their independent careers.
Dr. Shah, MD, MBA, is an assistant professor; he is also the director of the Center for Gastrointestinal Motility in the division of gastroenterology in the department of internal medicine at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
Female authorship trends in academic gastroenterology over 4 decades
WASHINGTON – Gastroenterology is still a majority male specialty, although women are entering the field at higher and higher rates. Female first authorship tripled from 1995 to 2010 (from 11% to 32%) and female senior authorship tripled from 2000 to 2010 (from 7% to 24%), but gains have not been equal in all areas and have not continued in all areas.
Eileen J. Benz, MD, of Beaumont Hospital, Dublin, described in a video interview at the annual Digestive Disease Week® a study she and her colleagues conducted to analyze published research in the journal Gastroenterology for the changing prevalence of female authorship over 4 decades.
The researchers reviewed all research published in the January and July issues of Gastroenterology during 1971-2010 (865 abstracts); animal trials were excluded. The sex of the first author and the last author (considered the senior author) of each paper was recorded, as was the type of study (basic science, clinical trials, or epidemiologic research). The increase in female senior authorship lagged behind the increase in first authorship, which likely reflects the promotion of female gastroenterologists over time into senior academic positions.
Also noted was that basic science and epidemiology research have the highest number of female authors overall, and these areas seem to continue to add female authors, whereas the number of female authors in clinical trials research seems to have stagnated since 1996. Dr. Benz hypothesizes that both bench science and epidemiology have research time built in, but that for a physician who may have other demands on her time, clinical trials research is an add-on for which there may not be protected time.
WASHINGTON – Gastroenterology is still a majority male specialty, although women are entering the field at higher and higher rates. Female first authorship tripled from 1995 to 2010 (from 11% to 32%) and female senior authorship tripled from 2000 to 2010 (from 7% to 24%), but gains have not been equal in all areas and have not continued in all areas.
Eileen J. Benz, MD, of Beaumont Hospital, Dublin, described in a video interview at the annual Digestive Disease Week® a study she and her colleagues conducted to analyze published research in the journal Gastroenterology for the changing prevalence of female authorship over 4 decades.
The researchers reviewed all research published in the January and July issues of Gastroenterology during 1971-2010 (865 abstracts); animal trials were excluded. The sex of the first author and the last author (considered the senior author) of each paper was recorded, as was the type of study (basic science, clinical trials, or epidemiologic research). The increase in female senior authorship lagged behind the increase in first authorship, which likely reflects the promotion of female gastroenterologists over time into senior academic positions.
Also noted was that basic science and epidemiology research have the highest number of female authors overall, and these areas seem to continue to add female authors, whereas the number of female authors in clinical trials research seems to have stagnated since 1996. Dr. Benz hypothesizes that both bench science and epidemiology have research time built in, but that for a physician who may have other demands on her time, clinical trials research is an add-on for which there may not be protected time.
WASHINGTON – Gastroenterology is still a majority male specialty, although women are entering the field at higher and higher rates. Female first authorship tripled from 1995 to 2010 (from 11% to 32%) and female senior authorship tripled from 2000 to 2010 (from 7% to 24%), but gains have not been equal in all areas and have not continued in all areas.
Eileen J. Benz, MD, of Beaumont Hospital, Dublin, described in a video interview at the annual Digestive Disease Week® a study she and her colleagues conducted to analyze published research in the journal Gastroenterology for the changing prevalence of female authorship over 4 decades.
The researchers reviewed all research published in the January and July issues of Gastroenterology during 1971-2010 (865 abstracts); animal trials were excluded. The sex of the first author and the last author (considered the senior author) of each paper was recorded, as was the type of study (basic science, clinical trials, or epidemiologic research). The increase in female senior authorship lagged behind the increase in first authorship, which likely reflects the promotion of female gastroenterologists over time into senior academic positions.
Also noted was that basic science and epidemiology research have the highest number of female authors overall, and these areas seem to continue to add female authors, whereas the number of female authors in clinical trials research seems to have stagnated since 1996. Dr. Benz hypothesizes that both bench science and epidemiology have research time built in, but that for a physician who may have other demands on her time, clinical trials research is an add-on for which there may not be protected time.
Reporting from DDW 2018