User login
Recombinant poliovirus appears safe, active as recurrent glioblastoma treatment
Treatment with the recombinant poliovirus vaccine PVSRIPO in patients with recurrent glioblastoma can be delivered at a safe dose with efficacy that compares favorably with historical data, recently reported results of a phase 1, nonrandomized study suggest.
The survival rate at 36 months after intratumoral infusion of PVSRIPO was 21%, versus 4% in a control group of patients who would have met the study’s eligibility criteria, investigators wrote in the New England Journal of Medicine.
There was no evidence of virus shedding or viral neuropathogenicity in the study, which included 61 patients with recurrent World Health Organization grade IV malignant glioma. “Further investigations are warranted,” wrote Annick Desjardins, MD, of Duke University, Durham, N.C., and her coauthors.
The prognosis of WHO grade IV malignant glioma remains dismal despite aggressive therapy and decades of research focused on advanced surgery, radiation, chemotherapy, and targeted agents, Dr. Desjardins and her colleagues said.
Accordingly, they sought to evaluate the potential of PVSRIPO, a live-attenuated poliovirus type 1 vaccine with its viral internal ribosome entry site replaced by one of human rhinovirus type. The engineered virus gains entry via the CD155 receptor, which is upregulated in solid tumors such as glioblastomas and expressed in antigen-presenting cells.
“Tumor cytotoxic effects, interferon-dominant activation of antigen-presenting cells, and the profound inflammatory response to poliovirus may counter tumor-induced immunosuppression and instigate antitumor immunity,” the investigators wrote.
With a median follow-up of 27.6 months, the median overall survival for PVSRIPO-treated patients was 12.5 months, longer than the 11.3 months seen in the historical control group. It was also longer than the 6.6 months found in a second comparison group of patients who underwent therapy with tumor-treating fields, which involves application of alternating electrical current to the head.
Survival hit a “plateau” in the PVSRIPO-treated patients, investigators said, with an overall survival rate of 21% at both 24 and 36 months. That stood in contrast to a decline in the historical control group from 14% at 24 months to 4% at 36 months, and a decline from 8% to 3% in the tumor-treating-fields group.
The phase 1 study had a dose-escalation phase including 9 patients and a dose-expansion phase with 52 patients. In the dose-expansion phase, 19% of patients had grade 3 or greater adverse events attributable to PVSRIPO, according to the report.
Of all 61 patients, 69% had a vaccine-related grade 1 or 2 event as their most severe adverse event.
One patient death caused by complications from an intracranial hemorrhage was attributed to bevacizumab. As part of a study protocol amendment, bevacizumab at half the standard dose was allowed to control symptoms of locoregional inflammation, investigators said.
In an ongoing, phase 2, randomized trial, PVSRIPO is being evaluated alone or with lomustine in patients with recurrent WHO grade IV malignant glioma. The Food and Drug Administration granted breakthrough therapy designation to PVSRIPO in May 2016.
Seven study authors reported equity in Istari Oncology, a biotechnology company that is developing PVSRIPO. Authors also reported disclosures related to Genentech/Roche, Celgene, Celldex, and Eli Lilly, among other entities. The study was supported by grants from the Brain Tumor Research Charity, the Tisch family through the Jewish Communal Fund, the National Institutes of Health, and others.
SOURCE: Desjardins A et al .N Engl J Med. 2018 Jun 26. doi: 10.1056/NEJMoa1716435.
The potentially useful anticancer properties of viruses are just starting to be recognized and exploited, Dan L. Longo, MD, and Lindsey R. Baden, MD, both with the Dana-Farber Cancer Institute at Brigham and Women’s Hospital, Boston, said in an editorial.
One approach is the development of oncolytic viruses that can not only directly kill tumor cells, but can also prompt an immune response against viable tumor cells, they wrote. The study by Dr. Desjardins and her colleagues describes clinical experience with PVSRIPO, a recombinant, nonpathogenic polio-rhinovirus chimera. This engineered virus targets glioblastoma by gaining cell entry through the CD155 receptor, which is expressed on solid tumors.
The survival data showed a plateau, with a 36-month survival rate of 21%, compared with 4% for a historical control cohort of patients, Dr. Longo and Dr. Baden noted.
In this study, PVSRIPO was delivered into intracranial tumors using an indwelling catheter. One of the outstanding questions with viral approaches to cancer treatment, according to the editorialists, is how local administration impacts systemic immunity in terms of recognition and elimination of remote lesions.
“Much more needs to be learned, but the clinical results to date encourage further exploration of this new treatment approach,” Dr. Longo and Dr. Baden wrote.
This summary is based on an editorial written by Dr. Longo and Dr. Baden that appeared in the New England Journal of Medicine. Dr. Baden and Longo both reported employment by the New England Journal of Medicine as deputy editor. Dr. Baden reported grant support from the Ragon Institute, the National Institutes of Health and the National Institute of Allergy and Infectious Disease, and the Gates Foundation outside the submitted work and also reported involvement in HIV vaccine trials done in collaboration with NIH, HIV Vaccine Trials Network, and others.
The potentially useful anticancer properties of viruses are just starting to be recognized and exploited, Dan L. Longo, MD, and Lindsey R. Baden, MD, both with the Dana-Farber Cancer Institute at Brigham and Women’s Hospital, Boston, said in an editorial.
One approach is the development of oncolytic viruses that can not only directly kill tumor cells, but can also prompt an immune response against viable tumor cells, they wrote. The study by Dr. Desjardins and her colleagues describes clinical experience with PVSRIPO, a recombinant, nonpathogenic polio-rhinovirus chimera. This engineered virus targets glioblastoma by gaining cell entry through the CD155 receptor, which is expressed on solid tumors.
The survival data showed a plateau, with a 36-month survival rate of 21%, compared with 4% for a historical control cohort of patients, Dr. Longo and Dr. Baden noted.
In this study, PVSRIPO was delivered into intracranial tumors using an indwelling catheter. One of the outstanding questions with viral approaches to cancer treatment, according to the editorialists, is how local administration impacts systemic immunity in terms of recognition and elimination of remote lesions.
“Much more needs to be learned, but the clinical results to date encourage further exploration of this new treatment approach,” Dr. Longo and Dr. Baden wrote.
This summary is based on an editorial written by Dr. Longo and Dr. Baden that appeared in the New England Journal of Medicine. Dr. Baden and Longo both reported employment by the New England Journal of Medicine as deputy editor. Dr. Baden reported grant support from the Ragon Institute, the National Institutes of Health and the National Institute of Allergy and Infectious Disease, and the Gates Foundation outside the submitted work and also reported involvement in HIV vaccine trials done in collaboration with NIH, HIV Vaccine Trials Network, and others.
The potentially useful anticancer properties of viruses are just starting to be recognized and exploited, Dan L. Longo, MD, and Lindsey R. Baden, MD, both with the Dana-Farber Cancer Institute at Brigham and Women’s Hospital, Boston, said in an editorial.
One approach is the development of oncolytic viruses that can not only directly kill tumor cells, but can also prompt an immune response against viable tumor cells, they wrote. The study by Dr. Desjardins and her colleagues describes clinical experience with PVSRIPO, a recombinant, nonpathogenic polio-rhinovirus chimera. This engineered virus targets glioblastoma by gaining cell entry through the CD155 receptor, which is expressed on solid tumors.
The survival data showed a plateau, with a 36-month survival rate of 21%, compared with 4% for a historical control cohort of patients, Dr. Longo and Dr. Baden noted.
In this study, PVSRIPO was delivered into intracranial tumors using an indwelling catheter. One of the outstanding questions with viral approaches to cancer treatment, according to the editorialists, is how local administration impacts systemic immunity in terms of recognition and elimination of remote lesions.
“Much more needs to be learned, but the clinical results to date encourage further exploration of this new treatment approach,” Dr. Longo and Dr. Baden wrote.
This summary is based on an editorial written by Dr. Longo and Dr. Baden that appeared in the New England Journal of Medicine. Dr. Baden and Longo both reported employment by the New England Journal of Medicine as deputy editor. Dr. Baden reported grant support from the Ragon Institute, the National Institutes of Health and the National Institute of Allergy and Infectious Disease, and the Gates Foundation outside the submitted work and also reported involvement in HIV vaccine trials done in collaboration with NIH, HIV Vaccine Trials Network, and others.
Treatment with the recombinant poliovirus vaccine PVSRIPO in patients with recurrent glioblastoma can be delivered at a safe dose with efficacy that compares favorably with historical data, recently reported results of a phase 1, nonrandomized study suggest.
The survival rate at 36 months after intratumoral infusion of PVSRIPO was 21%, versus 4% in a control group of patients who would have met the study’s eligibility criteria, investigators wrote in the New England Journal of Medicine.
There was no evidence of virus shedding or viral neuropathogenicity in the study, which included 61 patients with recurrent World Health Organization grade IV malignant glioma. “Further investigations are warranted,” wrote Annick Desjardins, MD, of Duke University, Durham, N.C., and her coauthors.
The prognosis of WHO grade IV malignant glioma remains dismal despite aggressive therapy and decades of research focused on advanced surgery, radiation, chemotherapy, and targeted agents, Dr. Desjardins and her colleagues said.
Accordingly, they sought to evaluate the potential of PVSRIPO, a live-attenuated poliovirus type 1 vaccine with its viral internal ribosome entry site replaced by one of human rhinovirus type. The engineered virus gains entry via the CD155 receptor, which is upregulated in solid tumors such as glioblastomas and expressed in antigen-presenting cells.
“Tumor cytotoxic effects, interferon-dominant activation of antigen-presenting cells, and the profound inflammatory response to poliovirus may counter tumor-induced immunosuppression and instigate antitumor immunity,” the investigators wrote.
With a median follow-up of 27.6 months, the median overall survival for PVSRIPO-treated patients was 12.5 months, longer than the 11.3 months seen in the historical control group. It was also longer than the 6.6 months found in a second comparison group of patients who underwent therapy with tumor-treating fields, which involves application of alternating electrical current to the head.
Survival hit a “plateau” in the PVSRIPO-treated patients, investigators said, with an overall survival rate of 21% at both 24 and 36 months. That stood in contrast to a decline in the historical control group from 14% at 24 months to 4% at 36 months, and a decline from 8% to 3% in the tumor-treating-fields group.
The phase 1 study had a dose-escalation phase including 9 patients and a dose-expansion phase with 52 patients. In the dose-expansion phase, 19% of patients had grade 3 or greater adverse events attributable to PVSRIPO, according to the report.
Of all 61 patients, 69% had a vaccine-related grade 1 or 2 event as their most severe adverse event.
One patient death caused by complications from an intracranial hemorrhage was attributed to bevacizumab. As part of a study protocol amendment, bevacizumab at half the standard dose was allowed to control symptoms of locoregional inflammation, investigators said.
In an ongoing, phase 2, randomized trial, PVSRIPO is being evaluated alone or with lomustine in patients with recurrent WHO grade IV malignant glioma. The Food and Drug Administration granted breakthrough therapy designation to PVSRIPO in May 2016.
Seven study authors reported equity in Istari Oncology, a biotechnology company that is developing PVSRIPO. Authors also reported disclosures related to Genentech/Roche, Celgene, Celldex, and Eli Lilly, among other entities. The study was supported by grants from the Brain Tumor Research Charity, the Tisch family through the Jewish Communal Fund, the National Institutes of Health, and others.
SOURCE: Desjardins A et al .N Engl J Med. 2018 Jun 26. doi: 10.1056/NEJMoa1716435.
Treatment with the recombinant poliovirus vaccine PVSRIPO in patients with recurrent glioblastoma can be delivered at a safe dose with efficacy that compares favorably with historical data, recently reported results of a phase 1, nonrandomized study suggest.
The survival rate at 36 months after intratumoral infusion of PVSRIPO was 21%, versus 4% in a control group of patients who would have met the study’s eligibility criteria, investigators wrote in the New England Journal of Medicine.
There was no evidence of virus shedding or viral neuropathogenicity in the study, which included 61 patients with recurrent World Health Organization grade IV malignant glioma. “Further investigations are warranted,” wrote Annick Desjardins, MD, of Duke University, Durham, N.C., and her coauthors.
The prognosis of WHO grade IV malignant glioma remains dismal despite aggressive therapy and decades of research focused on advanced surgery, radiation, chemotherapy, and targeted agents, Dr. Desjardins and her colleagues said.
Accordingly, they sought to evaluate the potential of PVSRIPO, a live-attenuated poliovirus type 1 vaccine with its viral internal ribosome entry site replaced by one of human rhinovirus type. The engineered virus gains entry via the CD155 receptor, which is upregulated in solid tumors such as glioblastomas and expressed in antigen-presenting cells.
“Tumor cytotoxic effects, interferon-dominant activation of antigen-presenting cells, and the profound inflammatory response to poliovirus may counter tumor-induced immunosuppression and instigate antitumor immunity,” the investigators wrote.
With a median follow-up of 27.6 months, the median overall survival for PVSRIPO-treated patients was 12.5 months, longer than the 11.3 months seen in the historical control group. It was also longer than the 6.6 months found in a second comparison group of patients who underwent therapy with tumor-treating fields, which involves application of alternating electrical current to the head.
Survival hit a “plateau” in the PVSRIPO-treated patients, investigators said, with an overall survival rate of 21% at both 24 and 36 months. That stood in contrast to a decline in the historical control group from 14% at 24 months to 4% at 36 months, and a decline from 8% to 3% in the tumor-treating-fields group.
The phase 1 study had a dose-escalation phase including 9 patients and a dose-expansion phase with 52 patients. In the dose-expansion phase, 19% of patients had grade 3 or greater adverse events attributable to PVSRIPO, according to the report.
Of all 61 patients, 69% had a vaccine-related grade 1 or 2 event as their most severe adverse event.
One patient death caused by complications from an intracranial hemorrhage was attributed to bevacizumab. As part of a study protocol amendment, bevacizumab at half the standard dose was allowed to control symptoms of locoregional inflammation, investigators said.
In an ongoing, phase 2, randomized trial, PVSRIPO is being evaluated alone or with lomustine in patients with recurrent WHO grade IV malignant glioma. The Food and Drug Administration granted breakthrough therapy designation to PVSRIPO in May 2016.
Seven study authors reported equity in Istari Oncology, a biotechnology company that is developing PVSRIPO. Authors also reported disclosures related to Genentech/Roche, Celgene, Celldex, and Eli Lilly, among other entities. The study was supported by grants from the Brain Tumor Research Charity, the Tisch family through the Jewish Communal Fund, the National Institutes of Health, and others.
SOURCE: Desjardins A et al .N Engl J Med. 2018 Jun 26. doi: 10.1056/NEJMoa1716435.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Delivery of PVSRIPO was safe, with efficacy comparing favorably with historical data.
Major finding: Overall survival reached 21% at 24 months and remained at 21% at 36 months.
Study details: A phase 1 study including 61 patients with recurrent World Health Organization grade IV glioma.
Disclosures: Seven study authors reported equity in Istari Oncology, a biotechnology company that is developing PVSRIPO. Study authors also reported disclosures related to Genentech/Roche, Celgene, Celldex, and Eli Lilly, among other entities. The study was supported by grants from the Brain Tumor Research Charity, the Tisch family through the Jewish Communal Fund, the National Institutes of Health, and others.
Source: Desjardins A et al. N Engl J Med. 2018 Jun 26. doi: 10.1056/NEJMoa1716435.
Patch-based AF screening boosts diagnosis rate
People at increased risk for atrial fibrillation who wore a screening ECG patch for about 2 weeks had their arrhythmia diagnosis rate boosted by 200%-800% during 4 months of follow-up, compared with conventionally followed adults in a randomized, novel-design trial with more than 2,600 randomized participants.
The patients who wore an ECG patch had a 3.9% rate of atrial fibrillation (AF) diagnosis in the study’s intention-to-treat analysis, and a 5.1% rate in the per protocol analysis that were the coprimary endpoints for the study, compared with rates of 0.9% and 0.6%, respectively, among people followed with usual care and diagnosed with AF based only on clinical findings.
Patients who underwent ECG screening for AF using a patch, compared with those followed with usual care, had more AF diagnoses, greater treatment with anticoagulation over the following year, and increased use of health care resources after 1 year, Steven R. Steinhubl, MD, and his associates reported in JAMA.
The mSToPS (mHealth Screening to Prevent Strokes) trial enrolled adults covered by an Aetna commercial or Medicare health plan who fell into a high-risk group for AF onset: Those aged 75 years or older or with at least one of several specified comorbidities. This identified more than 359,000 eligible insured patients. Dr. Steinhubl and his associates invited more than 100,000 people to participate, of whom 2,659 consented and met further eligibility screens. They randomized these people to either undergo immediate ECG patch screening, or have their screening delayed for 4 months while undergoing clinical follow-up.
The researchers sent two commercially available patches to the 1,366 people randomized to immediate screening, with instructions that they wear one patch for 2 weeks immediately, and wear the second patch for 2 weeks starting 3 months after they removed the first patch. Participants mailed their patches to a central site for analysis. Diagnosis of AF was based on an adjudicated episode of at least 30 seconds, and the researchers alerted participants and their individual physicians about diagnostic positives.
Among the 1,366 immediate patch recipients, a third never wore a patch for at least 30 minutes and were excluded from the per protocol analysis. The 908 patch users from the immediate screening subgroup as well as the patch users from the delayed subgroup wore each patch for an average of nearly 12 days, and about two-thirds wore both assigned patches. People diagnosed with AF had, on average, nearly 10 discrete episodes during screening, with a median episode duration of 186 minutes. The median AF burden among those who screened positive was 0.9%, reported Dr. Steinhubl, a cardiologist and director of digital medicine at the Scripps Translational Science Institute in La Jolla, Calif.
The researchers also compared medical interventions during the year following entry among all 1,738 screened patients (from both the immediate and delayed screening subgroups) and a matched group of 3,476 unscreened people who had consented to participate in the study. This showed that AF screening was linked to a doubled rate of anticoagulant treatment initiation. The ECG patch screening also identified 70 additional people with various other potentially actionable cardiac arrhythmias.
Of the 1,738 people who wore at least one patch for more than 30 minutes, 40 (2%) had skin irritation, 32 stopped using the patch prematurely because of irritation, and 2 people sought medical treatment for their irritation, which involved topical treatment.
mSToPS was funded by Janssen. Dr. Steinhubl has received research funding from Janssen, DynoSense, EasyG, Spry Health, and Striiv.
SOURCE: Steinhubl SR et al. JAMA. 2018 July 10;320[2]:149-55.
The results from mSToPS provide strong support for the use of continuous rhythm monitoring to screen for atrial fibrillation (AF) in at-risk populations, showing a detection rate as high as 5.1% after 4 months in the per protocol analysis. Screening people using a wearable ECG patch for a 2-week interval appears to have detected a substantial proportion of patients who might otherwise be detected with more inconvenient, invasive, costly, or longer monitoring approaches.
Existing epidemiologic and outcomes data support interventions for risk factors and symptoms of AF early in the disease process, but clinical trials demonstrating improved cardiovascular outcomes such as reduced stroke occurrence will be necessary to take action and screen for AF at the population level. Before the findings of mSToPS can be incorporated into clinical practice, clinicians need to consider two major questions about structured AF screening: Does earlier or more sensitive detection of AF improve clinical outcomes? And is this approach cost effective?
Benjamin A. Steinberg, MD , an electrophysiologist at the University of Utah, Salt Lake City, and Jonathan P. Piccini, MD , an electrophysiologist at Duke University, Durham, N.C., made these comments in an accompanying editorial ( JAMA. 2018 Jul 10;320[2]:139-41 ). Dr. Steinberg reported receiving research grants or personal fees from Boston Scientific, Biosense Webster, and Janssen. Dr. Piccini reported consulting with Allergen, Bayer, Johnson & Johnson, Medtronic, Sanofi, and Phillips, and receiving research funding from Abbott, ARCA Biopharma, Boston Scientific, Gilead, Janssen, and Verily.
The results from mSToPS provide strong support for the use of continuous rhythm monitoring to screen for atrial fibrillation (AF) in at-risk populations, showing a detection rate as high as 5.1% after 4 months in the per protocol analysis. Screening people using a wearable ECG patch for a 2-week interval appears to have detected a substantial proportion of patients who might otherwise be detected with more inconvenient, invasive, costly, or longer monitoring approaches.
Existing epidemiologic and outcomes data support interventions for risk factors and symptoms of AF early in the disease process, but clinical trials demonstrating improved cardiovascular outcomes such as reduced stroke occurrence will be necessary to take action and screen for AF at the population level. Before the findings of mSToPS can be incorporated into clinical practice, clinicians need to consider two major questions about structured AF screening: Does earlier or more sensitive detection of AF improve clinical outcomes? And is this approach cost effective?
Benjamin A. Steinberg, MD , an electrophysiologist at the University of Utah, Salt Lake City, and Jonathan P. Piccini, MD , an electrophysiologist at Duke University, Durham, N.C., made these comments in an accompanying editorial ( JAMA. 2018 Jul 10;320[2]:139-41 ). Dr. Steinberg reported receiving research grants or personal fees from Boston Scientific, Biosense Webster, and Janssen. Dr. Piccini reported consulting with Allergen, Bayer, Johnson & Johnson, Medtronic, Sanofi, and Phillips, and receiving research funding from Abbott, ARCA Biopharma, Boston Scientific, Gilead, Janssen, and Verily.
The results from mSToPS provide strong support for the use of continuous rhythm monitoring to screen for atrial fibrillation (AF) in at-risk populations, showing a detection rate as high as 5.1% after 4 months in the per protocol analysis. Screening people using a wearable ECG patch for a 2-week interval appears to have detected a substantial proportion of patients who might otherwise be detected with more inconvenient, invasive, costly, or longer monitoring approaches.
Existing epidemiologic and outcomes data support interventions for risk factors and symptoms of AF early in the disease process, but clinical trials demonstrating improved cardiovascular outcomes such as reduced stroke occurrence will be necessary to take action and screen for AF at the population level. Before the findings of mSToPS can be incorporated into clinical practice, clinicians need to consider two major questions about structured AF screening: Does earlier or more sensitive detection of AF improve clinical outcomes? And is this approach cost effective?
Benjamin A. Steinberg, MD , an electrophysiologist at the University of Utah, Salt Lake City, and Jonathan P. Piccini, MD , an electrophysiologist at Duke University, Durham, N.C., made these comments in an accompanying editorial ( JAMA. 2018 Jul 10;320[2]:139-41 ). Dr. Steinberg reported receiving research grants or personal fees from Boston Scientific, Biosense Webster, and Janssen. Dr. Piccini reported consulting with Allergen, Bayer, Johnson & Johnson, Medtronic, Sanofi, and Phillips, and receiving research funding from Abbott, ARCA Biopharma, Boston Scientific, Gilead, Janssen, and Verily.
People at increased risk for atrial fibrillation who wore a screening ECG patch for about 2 weeks had their arrhythmia diagnosis rate boosted by 200%-800% during 4 months of follow-up, compared with conventionally followed adults in a randomized, novel-design trial with more than 2,600 randomized participants.
The patients who wore an ECG patch had a 3.9% rate of atrial fibrillation (AF) diagnosis in the study’s intention-to-treat analysis, and a 5.1% rate in the per protocol analysis that were the coprimary endpoints for the study, compared with rates of 0.9% and 0.6%, respectively, among people followed with usual care and diagnosed with AF based only on clinical findings.
Patients who underwent ECG screening for AF using a patch, compared with those followed with usual care, had more AF diagnoses, greater treatment with anticoagulation over the following year, and increased use of health care resources after 1 year, Steven R. Steinhubl, MD, and his associates reported in JAMA.
The mSToPS (mHealth Screening to Prevent Strokes) trial enrolled adults covered by an Aetna commercial or Medicare health plan who fell into a high-risk group for AF onset: Those aged 75 years or older or with at least one of several specified comorbidities. This identified more than 359,000 eligible insured patients. Dr. Steinhubl and his associates invited more than 100,000 people to participate, of whom 2,659 consented and met further eligibility screens. They randomized these people to either undergo immediate ECG patch screening, or have their screening delayed for 4 months while undergoing clinical follow-up.
The researchers sent two commercially available patches to the 1,366 people randomized to immediate screening, with instructions that they wear one patch for 2 weeks immediately, and wear the second patch for 2 weeks starting 3 months after they removed the first patch. Participants mailed their patches to a central site for analysis. Diagnosis of AF was based on an adjudicated episode of at least 30 seconds, and the researchers alerted participants and their individual physicians about diagnostic positives.
Among the 1,366 immediate patch recipients, a third never wore a patch for at least 30 minutes and were excluded from the per protocol analysis. The 908 patch users from the immediate screening subgroup as well as the patch users from the delayed subgroup wore each patch for an average of nearly 12 days, and about two-thirds wore both assigned patches. People diagnosed with AF had, on average, nearly 10 discrete episodes during screening, with a median episode duration of 186 minutes. The median AF burden among those who screened positive was 0.9%, reported Dr. Steinhubl, a cardiologist and director of digital medicine at the Scripps Translational Science Institute in La Jolla, Calif.
The researchers also compared medical interventions during the year following entry among all 1,738 screened patients (from both the immediate and delayed screening subgroups) and a matched group of 3,476 unscreened people who had consented to participate in the study. This showed that AF screening was linked to a doubled rate of anticoagulant treatment initiation. The ECG patch screening also identified 70 additional people with various other potentially actionable cardiac arrhythmias.
Of the 1,738 people who wore at least one patch for more than 30 minutes, 40 (2%) had skin irritation, 32 stopped using the patch prematurely because of irritation, and 2 people sought medical treatment for their irritation, which involved topical treatment.
mSToPS was funded by Janssen. Dr. Steinhubl has received research funding from Janssen, DynoSense, EasyG, Spry Health, and Striiv.
SOURCE: Steinhubl SR et al. JAMA. 2018 July 10;320[2]:149-55.
People at increased risk for atrial fibrillation who wore a screening ECG patch for about 2 weeks had their arrhythmia diagnosis rate boosted by 200%-800% during 4 months of follow-up, compared with conventionally followed adults in a randomized, novel-design trial with more than 2,600 randomized participants.
The patients who wore an ECG patch had a 3.9% rate of atrial fibrillation (AF) diagnosis in the study’s intention-to-treat analysis, and a 5.1% rate in the per protocol analysis that were the coprimary endpoints for the study, compared with rates of 0.9% and 0.6%, respectively, among people followed with usual care and diagnosed with AF based only on clinical findings.
Patients who underwent ECG screening for AF using a patch, compared with those followed with usual care, had more AF diagnoses, greater treatment with anticoagulation over the following year, and increased use of health care resources after 1 year, Steven R. Steinhubl, MD, and his associates reported in JAMA.
The mSToPS (mHealth Screening to Prevent Strokes) trial enrolled adults covered by an Aetna commercial or Medicare health plan who fell into a high-risk group for AF onset: Those aged 75 years or older or with at least one of several specified comorbidities. This identified more than 359,000 eligible insured patients. Dr. Steinhubl and his associates invited more than 100,000 people to participate, of whom 2,659 consented and met further eligibility screens. They randomized these people to either undergo immediate ECG patch screening, or have their screening delayed for 4 months while undergoing clinical follow-up.
The researchers sent two commercially available patches to the 1,366 people randomized to immediate screening, with instructions that they wear one patch for 2 weeks immediately, and wear the second patch for 2 weeks starting 3 months after they removed the first patch. Participants mailed their patches to a central site for analysis. Diagnosis of AF was based on an adjudicated episode of at least 30 seconds, and the researchers alerted participants and their individual physicians about diagnostic positives.
Among the 1,366 immediate patch recipients, a third never wore a patch for at least 30 minutes and were excluded from the per protocol analysis. The 908 patch users from the immediate screening subgroup as well as the patch users from the delayed subgroup wore each patch for an average of nearly 12 days, and about two-thirds wore both assigned patches. People diagnosed with AF had, on average, nearly 10 discrete episodes during screening, with a median episode duration of 186 minutes. The median AF burden among those who screened positive was 0.9%, reported Dr. Steinhubl, a cardiologist and director of digital medicine at the Scripps Translational Science Institute in La Jolla, Calif.
The researchers also compared medical interventions during the year following entry among all 1,738 screened patients (from both the immediate and delayed screening subgroups) and a matched group of 3,476 unscreened people who had consented to participate in the study. This showed that AF screening was linked to a doubled rate of anticoagulant treatment initiation. The ECG patch screening also identified 70 additional people with various other potentially actionable cardiac arrhythmias.
Of the 1,738 people who wore at least one patch for more than 30 minutes, 40 (2%) had skin irritation, 32 stopped using the patch prematurely because of irritation, and 2 people sought medical treatment for their irritation, which involved topical treatment.
mSToPS was funded by Janssen. Dr. Steinhubl has received research funding from Janssen, DynoSense, EasyG, Spry Health, and Striiv.
SOURCE: Steinhubl SR et al. JAMA. 2018 July 10;320[2]:149-55.
FROM JAMA
Key clinical point: An ECG patch detected atrial fibrillation in high-risk people 200%-800% more often than clinical diagnosis.
Major finding: ECG patches identified a 3.9% incidence of atrial fibrillation during 4 months, compared with a 0.9% rate using clinical diagnoses.
Study details: A randomized trial with 2,659 people at high risk for incident atrial fibrillation.
Disclosures: mSToPS was funded by Janssen. Dr. Steinhubl has received research funding from Janssen, DynoSense, EasyG, Spry Health, and Striiv.
Source: Steinhubl SR et al. JAMA. 2018 Jul 10;320[2]:149-55.
SHM expresses support for Fairness for High-Skilled Immigrants Act
Without immigrant hospitalists, health care accessibility would decrease tremendously.
The Society of Hospital Medicine recently expressed its support for the Fairness for High-Skilled Immigrants Act (H.R. 392). This legislation will ensure that highly-skilled medical professionals and their families will not be turned away from working in the United States based on per-country limitations.
What inspired the PPC – and more broadly, SHM – to express support for this bill?
SHM and the PPC have always taken pride in assuming a leadership role when it comes to policy issues affecting hospitalists and the patients they serve, ranging from observation status to addressing the opiate epidemic and now, immigration reform. We are one of the first medical societies to support this bill.
What inspired us to take action is that there are country-specific caps when applying for a green card for those immigrants currently in the United States on an H1B visa. In the current green card pool, no country can occupy more than 7% of applications. For more populated countries like India and China, two significant countries of origin for hospitalists practicing in the U.S., this creates a significant backlog. At the moment, the projected wait time for applicants from countries in this situation to receive their green cards could easily exceed 25 years.
What impact would this have on hospital medicine providers and patients?
The number of hospitalists trained in the U.S. who have come on visas from other countries is astounding. By virtue of what we do as hospital medicine providers, we are leaders in health care. We own major QI initiatives across the hospital and oversee health care outcomes that many other providers never become involved with. By stifling the ability of people to enter the country and stay here long-term, it would have a devastating impact on our communities. A large chunk of hospitalist staffing companies employ providers who are international medical graduates who completed their residencies in the U.S. Without them, health care accessibility would decrease tremendously – especially in rural areas like those in which I work.
This is more than just an issue of citizenship – these caps have a major impact on quality of life and morale for those affected by them. The high level of uncertainty surrounding the current process affects large-scale decision-making. For example, people who are waiting to be approved for their green cards often ask questions like, “Should I buy a house?” and “Can I visit my family abroad and still be able to get back into the U.S. without any unwarranted delays or hassles?” This demoralizes quality providers personally, and if they feel this way, I can’t see how it wouldn’t affect their performance professionally as hospital medicine providers.
How have the existing restrictions affected you?
I graduated from medical school in India and came to the U.S..initially as a student and eventually transitioned to an H1B visa. After waiting for many years and having participated in numerous QI initiatives, I was fortunate enough to have my green card petition approved under a higher application category termed “Aliens of Extraordinary Ability” with a lesser wait time. However, by nature of the work that they perform, most hospitalists usually are eligible to apply for their green cards under the “Exceptional Ability” or “Advanced Degree” category, the wait times of which are excruciatingly long, and that is what we at the PPC and at the SHM level are striving to address and correct.
If someone is reading and says, “I want to do more and help advocate,” what can they do?
You don’t have to be a member of the PPC to have an impact on policy. Every member of SHM can contact their local representatives and be informed using SHM’s Grassroots Network. I have even gone so far as to meet and talk with local representatives to help them understand how policy issues affect both me and my patients. It is imperative that we are on the right side of history for those affected by this bill, and all bills affecting our fellow providers in the future.
Without immigrant hospitalists, health care accessibility would decrease tremendously.
Without immigrant hospitalists, health care accessibility would decrease tremendously.
The Society of Hospital Medicine recently expressed its support for the Fairness for High-Skilled Immigrants Act (H.R. 392). This legislation will ensure that highly-skilled medical professionals and their families will not be turned away from working in the United States based on per-country limitations.
What inspired the PPC – and more broadly, SHM – to express support for this bill?
SHM and the PPC have always taken pride in assuming a leadership role when it comes to policy issues affecting hospitalists and the patients they serve, ranging from observation status to addressing the opiate epidemic and now, immigration reform. We are one of the first medical societies to support this bill.
What inspired us to take action is that there are country-specific caps when applying for a green card for those immigrants currently in the United States on an H1B visa. In the current green card pool, no country can occupy more than 7% of applications. For more populated countries like India and China, two significant countries of origin for hospitalists practicing in the U.S., this creates a significant backlog. At the moment, the projected wait time for applicants from countries in this situation to receive their green cards could easily exceed 25 years.
What impact would this have on hospital medicine providers and patients?
The number of hospitalists trained in the U.S. who have come on visas from other countries is astounding. By virtue of what we do as hospital medicine providers, we are leaders in health care. We own major QI initiatives across the hospital and oversee health care outcomes that many other providers never become involved with. By stifling the ability of people to enter the country and stay here long-term, it would have a devastating impact on our communities. A large chunk of hospitalist staffing companies employ providers who are international medical graduates who completed their residencies in the U.S. Without them, health care accessibility would decrease tremendously – especially in rural areas like those in which I work.
This is more than just an issue of citizenship – these caps have a major impact on quality of life and morale for those affected by them. The high level of uncertainty surrounding the current process affects large-scale decision-making. For example, people who are waiting to be approved for their green cards often ask questions like, “Should I buy a house?” and “Can I visit my family abroad and still be able to get back into the U.S. without any unwarranted delays or hassles?” This demoralizes quality providers personally, and if they feel this way, I can’t see how it wouldn’t affect their performance professionally as hospital medicine providers.
How have the existing restrictions affected you?
I graduated from medical school in India and came to the U.S..initially as a student and eventually transitioned to an H1B visa. After waiting for many years and having participated in numerous QI initiatives, I was fortunate enough to have my green card petition approved under a higher application category termed “Aliens of Extraordinary Ability” with a lesser wait time. However, by nature of the work that they perform, most hospitalists usually are eligible to apply for their green cards under the “Exceptional Ability” or “Advanced Degree” category, the wait times of which are excruciatingly long, and that is what we at the PPC and at the SHM level are striving to address and correct.
If someone is reading and says, “I want to do more and help advocate,” what can they do?
You don’t have to be a member of the PPC to have an impact on policy. Every member of SHM can contact their local representatives and be informed using SHM’s Grassroots Network. I have even gone so far as to meet and talk with local representatives to help them understand how policy issues affect both me and my patients. It is imperative that we are on the right side of history for those affected by this bill, and all bills affecting our fellow providers in the future.
The Society of Hospital Medicine recently expressed its support for the Fairness for High-Skilled Immigrants Act (H.R. 392). This legislation will ensure that highly-skilled medical professionals and their families will not be turned away from working in the United States based on per-country limitations.
What inspired the PPC – and more broadly, SHM – to express support for this bill?
SHM and the PPC have always taken pride in assuming a leadership role when it comes to policy issues affecting hospitalists and the patients they serve, ranging from observation status to addressing the opiate epidemic and now, immigration reform. We are one of the first medical societies to support this bill.
What inspired us to take action is that there are country-specific caps when applying for a green card for those immigrants currently in the United States on an H1B visa. In the current green card pool, no country can occupy more than 7% of applications. For more populated countries like India and China, two significant countries of origin for hospitalists practicing in the U.S., this creates a significant backlog. At the moment, the projected wait time for applicants from countries in this situation to receive their green cards could easily exceed 25 years.
What impact would this have on hospital medicine providers and patients?
The number of hospitalists trained in the U.S. who have come on visas from other countries is astounding. By virtue of what we do as hospital medicine providers, we are leaders in health care. We own major QI initiatives across the hospital and oversee health care outcomes that many other providers never become involved with. By stifling the ability of people to enter the country and stay here long-term, it would have a devastating impact on our communities. A large chunk of hospitalist staffing companies employ providers who are international medical graduates who completed their residencies in the U.S. Without them, health care accessibility would decrease tremendously – especially in rural areas like those in which I work.
This is more than just an issue of citizenship – these caps have a major impact on quality of life and morale for those affected by them. The high level of uncertainty surrounding the current process affects large-scale decision-making. For example, people who are waiting to be approved for their green cards often ask questions like, “Should I buy a house?” and “Can I visit my family abroad and still be able to get back into the U.S. without any unwarranted delays or hassles?” This demoralizes quality providers personally, and if they feel this way, I can’t see how it wouldn’t affect their performance professionally as hospital medicine providers.
How have the existing restrictions affected you?
I graduated from medical school in India and came to the U.S..initially as a student and eventually transitioned to an H1B visa. After waiting for many years and having participated in numerous QI initiatives, I was fortunate enough to have my green card petition approved under a higher application category termed “Aliens of Extraordinary Ability” with a lesser wait time. However, by nature of the work that they perform, most hospitalists usually are eligible to apply for their green cards under the “Exceptional Ability” or “Advanced Degree” category, the wait times of which are excruciatingly long, and that is what we at the PPC and at the SHM level are striving to address and correct.
If someone is reading and says, “I want to do more and help advocate,” what can they do?
You don’t have to be a member of the PPC to have an impact on policy. Every member of SHM can contact their local representatives and be informed using SHM’s Grassroots Network. I have even gone so far as to meet and talk with local representatives to help them understand how policy issues affect both me and my patients. It is imperative that we are on the right side of history for those affected by this bill, and all bills affecting our fellow providers in the future.
ABP 980 similar to trastuzumab in HER2+ breast cancer in all but name
In women with HER2-positive early breast cancer, the anti-HER2 biosimilar agent ABP-980 was clinically similar in efficacy and safety to the original drug trastuzumab (Herceptin).
Although ABP 980 was associated with a higher pathologic complete response (pCR) rate in breast tissues and axillary lymph nodes compared with trastuzumab, the trial technically failed to meet its coprimary endpoints of risk ratio and risk difference because of a statistical nicety involving local lab review of tissue samples vs. centralized review, reported Gunter von Minckwitz, MD, PhD, of the German Breast Group in Neu-Isenburg, Germany, and his colleagues.
“In our sensitivity analyses based on central laboratory evaluation of tumor samples, estimates for the two drugs were contained within the predefined equivalence margins, indicating similar efficacy. ABP 980 and trastuzumab had similar safety outcomes in both the neoadjuvant and adjuvant phases of the study,” the researchers wrote. The report was published in The Lancet Oncology.
ABP 980 is one of several contenders for trastuzumab biosimilar making their way through clinical trials. In phase 1 studies, it was shown to be similar in its structure, pharmacodynamics, and pharmacokinetics to the reference agent trastuzumab. In the LILAC trial Dr. von Minckwitz and his associates put the biosimilar through its paces to see whether it would also be equivalent in efficacy and safety, including in patients switched from the original drug to the copy-cat agent.
Investigators for the randomized phase 3 trial, conducted in 97 centers in 20 countries in Europe, South America, and Canada, enrolled 827 women age and 18 and older with HER2-positive breast cancer, 725 of whom were randomly assigned to neoadjuvant therapy with either ABP 980 or trastuzumab plus paclitaxel after a four-cycle run-in of anthracycline-based chemotherapy,
Neoadjuvant therapy was followed 3-7 weeks later by surgery and adjuvant therapy with either of the HER2 inhibitors. At baseline, patients were randomly assigned to either continue adjuvant therapy with their original HER2 inhibitor, or to switch from trastuzumab in the neoadjuvant setting to ABP 980 in the adjuvant setting.
In all, 696 patients were evaluable for the primary endpoint, 358 of whom received the biosimilar, and 338 of whom received trastuzumab. In all, 48% of patients randomly assigned to ABP 980 had a pCR in breast and axillary lymph node tissues assessed at a local laboratory, compared with 41% assigned to trastuzumab.
The risk difference was 7.3%, (90% confidence interval [CI] 1.2-13.4), The risk ratio was 1.188 (90% CI, 1.033-1.366). Although the lower bounds of the confidence intervals showed that ABP 980 was noninferior to trastuzumab, the upper bounds exceeded the predefined equivalence margins of a 13% risk difference and 1.318 risk ratio, respectively, meaning that technically the trial did not meet its coprimary endpoints.
However, in central laboratory review pCR was seen in 48% of patients assigned to ABP 980 at baseline and 42% of those assigned to trastuzumab at baseline. The risk difference was 5.8% (90% CI, –0.5-12.0), and risk ratio was 1.142 (90% CI, 0.993-1.312), and both the lower and upper bounds of the confidence intervals fell within prespecified limits.
The safety analysis showed a similar incidence of grade 3 or greater adverse events during neoadjuvant therapy (15% of patients on ABP 980 vs. 14% on trastuzumab). Grade 3 or greater neutropenia occurred in 6% of patients in each group.
During adjuvant therapy, grade 3 or greater adverse events occurred in 9% of patients continuing ABP 980, 6% continuing trastuzumab, and 8% of these switched from trastuzumab to ABP 980. The most frequent grade 3 or greater events of interest were infections and neutropenia, all occurring in 1% of patients in each arm, and infusion reaction, which occurred in 1% of patients who stayed on the assigned HER2 inhibitor and in 2% of patients who were switched to ABP 980.
There were two patient deaths from adverse events, each deemed to be unrelated to treatment. One patient died from pneumonia during neoadjuvant ABP 980 therapy, and one died from septic shock during adjuvant therapy with ABP 980 after being switch from trastuzumab.
“To our knowledge, this is the first study of a trastuzumab biosimilar encompassing a single-switch design from the reference product to a biosimilar, which allowed us to assess the clinical safety and immunogenicity of this approach to treatment. Safety and immunogenicity were similar in patients who were switched and in those who continued to receive trastuzumab as adjuvant therapy,” the investigators wrote.
SOURCE: von Minckwitz G et al. Lancet Oncol 2018 Jun 4. doi: 10.1016/S1470-2045(18)30241-9.
The LILAC trial has some strengths and weaknesses and raises a curious regulatory issue. To begin with the weaknesses, only 696 of 725 randomized patients were evaluable for pathological complete response after surgery. No data about the outcomes, characteristics, or allocated treatment of the patients who did not reach surgery were provided. These lost patients should have been included in the intention-to-treat analysis and their responses classified when possible (e.g., those who did not reach surgery due to progressive disease should have been classified as nonpathological complete response). The effect of these few patients on the overall results is unknown, although it is possibly small.
Among the strengths of LILAC were that the trial was done in a sensitive population (i.e., a population in which differences in safety, immunogenicity, and efficacy could be attributed to the biosimilar or reference drug rather than patient-related or disease-related factors). Two chemotherapy choices were included that are broadly used worldwide, and thus mimicked routine clinical practice, and the study had a sensitive primary endpoint (pathological complete response). The aim of clinical trials in the regulatory pathway of biosimilars is to show an acceptable degree of similarity in clinical efficacy and safety to the reference product. For original products, endpoints in clinical trials must show benefits to patients, such as progression-free survival, disease-free survival, or overall survival, whereas for biosimilars, surrogate endpoints, such as the proportion of patients with pathological response in breast cancer neoadjuvant trials, are appropriate. The study design of LILAC, therefore, meets the main clinical requirements demanded by medicine agencies for the registration of biosimilars.
Miguel Martin, MD, PhD is with Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense, Madrid. Dr. Martin’s remarks are adapted and condensed from an editorial in The Lancet Oncology accompanying the study by von Minckwitz G et al. He disclosed grants from Novartis and Roche and personal fees from AstraZeneca, Lilly, Pfizer, and Roche.
The LILAC trial has some strengths and weaknesses and raises a curious regulatory issue. To begin with the weaknesses, only 696 of 725 randomized patients were evaluable for pathological complete response after surgery. No data about the outcomes, characteristics, or allocated treatment of the patients who did not reach surgery were provided. These lost patients should have been included in the intention-to-treat analysis and their responses classified when possible (e.g., those who did not reach surgery due to progressive disease should have been classified as nonpathological complete response). The effect of these few patients on the overall results is unknown, although it is possibly small.
Among the strengths of LILAC were that the trial was done in a sensitive population (i.e., a population in which differences in safety, immunogenicity, and efficacy could be attributed to the biosimilar or reference drug rather than patient-related or disease-related factors). Two chemotherapy choices were included that are broadly used worldwide, and thus mimicked routine clinical practice, and the study had a sensitive primary endpoint (pathological complete response). The aim of clinical trials in the regulatory pathway of biosimilars is to show an acceptable degree of similarity in clinical efficacy and safety to the reference product. For original products, endpoints in clinical trials must show benefits to patients, such as progression-free survival, disease-free survival, or overall survival, whereas for biosimilars, surrogate endpoints, such as the proportion of patients with pathological response in breast cancer neoadjuvant trials, are appropriate. The study design of LILAC, therefore, meets the main clinical requirements demanded by medicine agencies for the registration of biosimilars.
Miguel Martin, MD, PhD is with Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense, Madrid. Dr. Martin’s remarks are adapted and condensed from an editorial in The Lancet Oncology accompanying the study by von Minckwitz G et al. He disclosed grants from Novartis and Roche and personal fees from AstraZeneca, Lilly, Pfizer, and Roche.
The LILAC trial has some strengths and weaknesses and raises a curious regulatory issue. To begin with the weaknesses, only 696 of 725 randomized patients were evaluable for pathological complete response after surgery. No data about the outcomes, characteristics, or allocated treatment of the patients who did not reach surgery were provided. These lost patients should have been included in the intention-to-treat analysis and their responses classified when possible (e.g., those who did not reach surgery due to progressive disease should have been classified as nonpathological complete response). The effect of these few patients on the overall results is unknown, although it is possibly small.
Among the strengths of LILAC were that the trial was done in a sensitive population (i.e., a population in which differences in safety, immunogenicity, and efficacy could be attributed to the biosimilar or reference drug rather than patient-related or disease-related factors). Two chemotherapy choices were included that are broadly used worldwide, and thus mimicked routine clinical practice, and the study had a sensitive primary endpoint (pathological complete response). The aim of clinical trials in the regulatory pathway of biosimilars is to show an acceptable degree of similarity in clinical efficacy and safety to the reference product. For original products, endpoints in clinical trials must show benefits to patients, such as progression-free survival, disease-free survival, or overall survival, whereas for biosimilars, surrogate endpoints, such as the proportion of patients with pathological response in breast cancer neoadjuvant trials, are appropriate. The study design of LILAC, therefore, meets the main clinical requirements demanded by medicine agencies for the registration of biosimilars.
Miguel Martin, MD, PhD is with Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense, Madrid. Dr. Martin’s remarks are adapted and condensed from an editorial in The Lancet Oncology accompanying the study by von Minckwitz G et al. He disclosed grants from Novartis and Roche and personal fees from AstraZeneca, Lilly, Pfizer, and Roche.
In women with HER2-positive early breast cancer, the anti-HER2 biosimilar agent ABP-980 was clinically similar in efficacy and safety to the original drug trastuzumab (Herceptin).
Although ABP 980 was associated with a higher pathologic complete response (pCR) rate in breast tissues and axillary lymph nodes compared with trastuzumab, the trial technically failed to meet its coprimary endpoints of risk ratio and risk difference because of a statistical nicety involving local lab review of tissue samples vs. centralized review, reported Gunter von Minckwitz, MD, PhD, of the German Breast Group in Neu-Isenburg, Germany, and his colleagues.
“In our sensitivity analyses based on central laboratory evaluation of tumor samples, estimates for the two drugs were contained within the predefined equivalence margins, indicating similar efficacy. ABP 980 and trastuzumab had similar safety outcomes in both the neoadjuvant and adjuvant phases of the study,” the researchers wrote. The report was published in The Lancet Oncology.
ABP 980 is one of several contenders for trastuzumab biosimilar making their way through clinical trials. In phase 1 studies, it was shown to be similar in its structure, pharmacodynamics, and pharmacokinetics to the reference agent trastuzumab. In the LILAC trial Dr. von Minckwitz and his associates put the biosimilar through its paces to see whether it would also be equivalent in efficacy and safety, including in patients switched from the original drug to the copy-cat agent.
Investigators for the randomized phase 3 trial, conducted in 97 centers in 20 countries in Europe, South America, and Canada, enrolled 827 women age and 18 and older with HER2-positive breast cancer, 725 of whom were randomly assigned to neoadjuvant therapy with either ABP 980 or trastuzumab plus paclitaxel after a four-cycle run-in of anthracycline-based chemotherapy,
Neoadjuvant therapy was followed 3-7 weeks later by surgery and adjuvant therapy with either of the HER2 inhibitors. At baseline, patients were randomly assigned to either continue adjuvant therapy with their original HER2 inhibitor, or to switch from trastuzumab in the neoadjuvant setting to ABP 980 in the adjuvant setting.
In all, 696 patients were evaluable for the primary endpoint, 358 of whom received the biosimilar, and 338 of whom received trastuzumab. In all, 48% of patients randomly assigned to ABP 980 had a pCR in breast and axillary lymph node tissues assessed at a local laboratory, compared with 41% assigned to trastuzumab.
The risk difference was 7.3%, (90% confidence interval [CI] 1.2-13.4), The risk ratio was 1.188 (90% CI, 1.033-1.366). Although the lower bounds of the confidence intervals showed that ABP 980 was noninferior to trastuzumab, the upper bounds exceeded the predefined equivalence margins of a 13% risk difference and 1.318 risk ratio, respectively, meaning that technically the trial did not meet its coprimary endpoints.
However, in central laboratory review pCR was seen in 48% of patients assigned to ABP 980 at baseline and 42% of those assigned to trastuzumab at baseline. The risk difference was 5.8% (90% CI, –0.5-12.0), and risk ratio was 1.142 (90% CI, 0.993-1.312), and both the lower and upper bounds of the confidence intervals fell within prespecified limits.
The safety analysis showed a similar incidence of grade 3 or greater adverse events during neoadjuvant therapy (15% of patients on ABP 980 vs. 14% on trastuzumab). Grade 3 or greater neutropenia occurred in 6% of patients in each group.
During adjuvant therapy, grade 3 or greater adverse events occurred in 9% of patients continuing ABP 980, 6% continuing trastuzumab, and 8% of these switched from trastuzumab to ABP 980. The most frequent grade 3 or greater events of interest were infections and neutropenia, all occurring in 1% of patients in each arm, and infusion reaction, which occurred in 1% of patients who stayed on the assigned HER2 inhibitor and in 2% of patients who were switched to ABP 980.
There were two patient deaths from adverse events, each deemed to be unrelated to treatment. One patient died from pneumonia during neoadjuvant ABP 980 therapy, and one died from septic shock during adjuvant therapy with ABP 980 after being switch from trastuzumab.
“To our knowledge, this is the first study of a trastuzumab biosimilar encompassing a single-switch design from the reference product to a biosimilar, which allowed us to assess the clinical safety and immunogenicity of this approach to treatment. Safety and immunogenicity were similar in patients who were switched and in those who continued to receive trastuzumab as adjuvant therapy,” the investigators wrote.
SOURCE: von Minckwitz G et al. Lancet Oncol 2018 Jun 4. doi: 10.1016/S1470-2045(18)30241-9.
In women with HER2-positive early breast cancer, the anti-HER2 biosimilar agent ABP-980 was clinically similar in efficacy and safety to the original drug trastuzumab (Herceptin).
Although ABP 980 was associated with a higher pathologic complete response (pCR) rate in breast tissues and axillary lymph nodes compared with trastuzumab, the trial technically failed to meet its coprimary endpoints of risk ratio and risk difference because of a statistical nicety involving local lab review of tissue samples vs. centralized review, reported Gunter von Minckwitz, MD, PhD, of the German Breast Group in Neu-Isenburg, Germany, and his colleagues.
“In our sensitivity analyses based on central laboratory evaluation of tumor samples, estimates for the two drugs were contained within the predefined equivalence margins, indicating similar efficacy. ABP 980 and trastuzumab had similar safety outcomes in both the neoadjuvant and adjuvant phases of the study,” the researchers wrote. The report was published in The Lancet Oncology.
ABP 980 is one of several contenders for trastuzumab biosimilar making their way through clinical trials. In phase 1 studies, it was shown to be similar in its structure, pharmacodynamics, and pharmacokinetics to the reference agent trastuzumab. In the LILAC trial Dr. von Minckwitz and his associates put the biosimilar through its paces to see whether it would also be equivalent in efficacy and safety, including in patients switched from the original drug to the copy-cat agent.
Investigators for the randomized phase 3 trial, conducted in 97 centers in 20 countries in Europe, South America, and Canada, enrolled 827 women age and 18 and older with HER2-positive breast cancer, 725 of whom were randomly assigned to neoadjuvant therapy with either ABP 980 or trastuzumab plus paclitaxel after a four-cycle run-in of anthracycline-based chemotherapy,
Neoadjuvant therapy was followed 3-7 weeks later by surgery and adjuvant therapy with either of the HER2 inhibitors. At baseline, patients were randomly assigned to either continue adjuvant therapy with their original HER2 inhibitor, or to switch from trastuzumab in the neoadjuvant setting to ABP 980 in the adjuvant setting.
In all, 696 patients were evaluable for the primary endpoint, 358 of whom received the biosimilar, and 338 of whom received trastuzumab. In all, 48% of patients randomly assigned to ABP 980 had a pCR in breast and axillary lymph node tissues assessed at a local laboratory, compared with 41% assigned to trastuzumab.
The risk difference was 7.3%, (90% confidence interval [CI] 1.2-13.4), The risk ratio was 1.188 (90% CI, 1.033-1.366). Although the lower bounds of the confidence intervals showed that ABP 980 was noninferior to trastuzumab, the upper bounds exceeded the predefined equivalence margins of a 13% risk difference and 1.318 risk ratio, respectively, meaning that technically the trial did not meet its coprimary endpoints.
However, in central laboratory review pCR was seen in 48% of patients assigned to ABP 980 at baseline and 42% of those assigned to trastuzumab at baseline. The risk difference was 5.8% (90% CI, –0.5-12.0), and risk ratio was 1.142 (90% CI, 0.993-1.312), and both the lower and upper bounds of the confidence intervals fell within prespecified limits.
The safety analysis showed a similar incidence of grade 3 or greater adverse events during neoadjuvant therapy (15% of patients on ABP 980 vs. 14% on trastuzumab). Grade 3 or greater neutropenia occurred in 6% of patients in each group.
During adjuvant therapy, grade 3 or greater adverse events occurred in 9% of patients continuing ABP 980, 6% continuing trastuzumab, and 8% of these switched from trastuzumab to ABP 980. The most frequent grade 3 or greater events of interest were infections and neutropenia, all occurring in 1% of patients in each arm, and infusion reaction, which occurred in 1% of patients who stayed on the assigned HER2 inhibitor and in 2% of patients who were switched to ABP 980.
There were two patient deaths from adverse events, each deemed to be unrelated to treatment. One patient died from pneumonia during neoadjuvant ABP 980 therapy, and one died from septic shock during adjuvant therapy with ABP 980 after being switch from trastuzumab.
“To our knowledge, this is the first study of a trastuzumab biosimilar encompassing a single-switch design from the reference product to a biosimilar, which allowed us to assess the clinical safety and immunogenicity of this approach to treatment. Safety and immunogenicity were similar in patients who were switched and in those who continued to receive trastuzumab as adjuvant therapy,” the investigators wrote.
SOURCE: von Minckwitz G et al. Lancet Oncol 2018 Jun 4. doi: 10.1016/S1470-2045(18)30241-9.
FROM THE LANCET ONCOLOGY
Key clinical point: The biosimilar ABP 980 appears to be comparable in efficacy and safety to trastuzumab in women with early HER2-positive breast cancer.
Major finding: According to local lab assessments, 48% of patients assigned to ABP 980 had a pathologic complete response, compared with 41% assigned to trastuzumab.
Study details: Randomized, double-blind, phase 3 trial of 696 adult women with HER2-positive breast cancer.
Disclosures: Dr. von Minckwitz is a consultant for Amgen, which funded the study. Two coauthors are employees of the company and stockholders. Other coauthors disclosed relationships with various companies.
Source: von Minckwitz G et al. Lancet Oncol 2018 Jun 4. doi: 10.1016/S1470-2045(18)30241-9.
mSToPS breaks ground as a ‘pragmatic’ randomized trial
The mSToPS study “represents an innovative example of the potential (and challenges) inherent in a pragmatic information technology trial. The trial “represents a brave new world for clinical research: an innovative, highly commendable, contemporary pragmatic health care information technology study that tested an important question and yielded significant clinical findings,” wrote two leaders in trial design in an editorial about the study.
In addition, the mHealth Screening to Prevent Strokes (mSToPS) trial tested the utility of a wearable ECG patch to detect new-onset episodes of atrial fibrillation. Thus the study also served as one of the first examples of a trial designed to examine whether a wearable, digital device can transform health care by improving clinical outcomes, an advance that crosses the current “chasm between the technology and clinical worlds,” wrote Eric D. Peterson, MD, and Robert A. Harrington, MD. Their editorial framed mSToPS as a breakthrough in a new type of information technology–based, pragmatic clinical trial (JAMA. 2018 July 10;320[2]:137-8).
Future trials with similar designs and novel health information technology methods could tap into the enormous information contained in electronic health records, wrote Dr. Peterson, a cardiologist, professor of medicine, and executive director of the Duke Clinical Research Institute at Duke University in Durham, N.C., and Dr. Harrington, a cardiologist, professor, and chairman of medicine at Stanford (Calif.) University.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
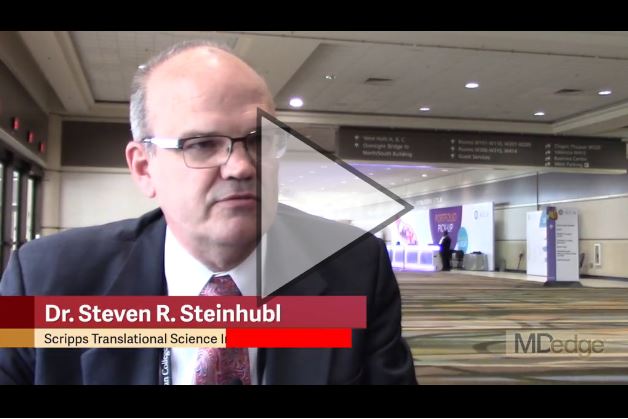
Dr. Steven R. Steinhubl, lead investigator for the mSToPS trial, agreed with this assessment. Speaking in a video interview in March 2018 during the annual scientific sessions of the American College of Cardiology, where Dr. Steinhubl first reported the mSToPS results, he characterized the trial as “completely reimagining how clinical trials are done,” by making them less expensive and more convenient for participants. In this way, mSToPS is a break from the traditional randomized clinical trial format, which creates an “artificial treatment environment and artificial patient behaviors,” said Dr. Steinhubl, a cardiologist and director of digital medicine at the Scripps Translational Science Institute in La Jolla, Calif.
Dr. Peterson has received personal fees from Livogo and has received research funding from Akili, RefleXion Medical, and Verily Life Sciences. Dr. Harrington has been a consultant to Amgen, Element Science, Gilead Sciences, MyoKardia, and WebMD; has served on the board of directors of Signal Path and Scanadu; has received personal fees from Bayer; and has received research funding from Apple, AstraZeneca, Bristol-Myers Squibb, CSL, Janssen, Novartis, Portola, Sanofi, and the Medicines Company. Dr. Steinhubl has received research funding from Janssen, DynoSense, EasyG, SpryHealth, and Striiv.
SOURCE: Peterson ED et al. JAMA. 2018 July 10;320[2]:138-9.
The mSToPS study “represents an innovative example of the potential (and challenges) inherent in a pragmatic information technology trial. The trial “represents a brave new world for clinical research: an innovative, highly commendable, contemporary pragmatic health care information technology study that tested an important question and yielded significant clinical findings,” wrote two leaders in trial design in an editorial about the study.
In addition, the mHealth Screening to Prevent Strokes (mSToPS) trial tested the utility of a wearable ECG patch to detect new-onset episodes of atrial fibrillation. Thus the study also served as one of the first examples of a trial designed to examine whether a wearable, digital device can transform health care by improving clinical outcomes, an advance that crosses the current “chasm between the technology and clinical worlds,” wrote Eric D. Peterson, MD, and Robert A. Harrington, MD. Their editorial framed mSToPS as a breakthrough in a new type of information technology–based, pragmatic clinical trial (JAMA. 2018 July 10;320[2]:137-8).
Future trials with similar designs and novel health information technology methods could tap into the enormous information contained in electronic health records, wrote Dr. Peterson, a cardiologist, professor of medicine, and executive director of the Duke Clinical Research Institute at Duke University in Durham, N.C., and Dr. Harrington, a cardiologist, professor, and chairman of medicine at Stanford (Calif.) University.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Steven R. Steinhubl, lead investigator for the mSToPS trial, agreed with this assessment. Speaking in a video interview in March 2018 during the annual scientific sessions of the American College of Cardiology, where Dr. Steinhubl first reported the mSToPS results, he characterized the trial as “completely reimagining how clinical trials are done,” by making them less expensive and more convenient for participants. In this way, mSToPS is a break from the traditional randomized clinical trial format, which creates an “artificial treatment environment and artificial patient behaviors,” said Dr. Steinhubl, a cardiologist and director of digital medicine at the Scripps Translational Science Institute in La Jolla, Calif.
Dr. Peterson has received personal fees from Livogo and has received research funding from Akili, RefleXion Medical, and Verily Life Sciences. Dr. Harrington has been a consultant to Amgen, Element Science, Gilead Sciences, MyoKardia, and WebMD; has served on the board of directors of Signal Path and Scanadu; has received personal fees from Bayer; and has received research funding from Apple, AstraZeneca, Bristol-Myers Squibb, CSL, Janssen, Novartis, Portola, Sanofi, and the Medicines Company. Dr. Steinhubl has received research funding from Janssen, DynoSense, EasyG, SpryHealth, and Striiv.
SOURCE: Peterson ED et al. JAMA. 2018 July 10;320[2]:138-9.
The mSToPS study “represents an innovative example of the potential (and challenges) inherent in a pragmatic information technology trial. The trial “represents a brave new world for clinical research: an innovative, highly commendable, contemporary pragmatic health care information technology study that tested an important question and yielded significant clinical findings,” wrote two leaders in trial design in an editorial about the study.
In addition, the mHealth Screening to Prevent Strokes (mSToPS) trial tested the utility of a wearable ECG patch to detect new-onset episodes of atrial fibrillation. Thus the study also served as one of the first examples of a trial designed to examine whether a wearable, digital device can transform health care by improving clinical outcomes, an advance that crosses the current “chasm between the technology and clinical worlds,” wrote Eric D. Peterson, MD, and Robert A. Harrington, MD. Their editorial framed mSToPS as a breakthrough in a new type of information technology–based, pragmatic clinical trial (JAMA. 2018 July 10;320[2]:137-8).
Future trials with similar designs and novel health information technology methods could tap into the enormous information contained in electronic health records, wrote Dr. Peterson, a cardiologist, professor of medicine, and executive director of the Duke Clinical Research Institute at Duke University in Durham, N.C., and Dr. Harrington, a cardiologist, professor, and chairman of medicine at Stanford (Calif.) University.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Steven R. Steinhubl, lead investigator for the mSToPS trial, agreed with this assessment. Speaking in a video interview in March 2018 during the annual scientific sessions of the American College of Cardiology, where Dr. Steinhubl first reported the mSToPS results, he characterized the trial as “completely reimagining how clinical trials are done,” by making them less expensive and more convenient for participants. In this way, mSToPS is a break from the traditional randomized clinical trial format, which creates an “artificial treatment environment and artificial patient behaviors,” said Dr. Steinhubl, a cardiologist and director of digital medicine at the Scripps Translational Science Institute in La Jolla, Calif.
Dr. Peterson has received personal fees from Livogo and has received research funding from Akili, RefleXion Medical, and Verily Life Sciences. Dr. Harrington has been a consultant to Amgen, Element Science, Gilead Sciences, MyoKardia, and WebMD; has served on the board of directors of Signal Path and Scanadu; has received personal fees from Bayer; and has received research funding from Apple, AstraZeneca, Bristol-Myers Squibb, CSL, Janssen, Novartis, Portola, Sanofi, and the Medicines Company. Dr. Steinhubl has received research funding from Janssen, DynoSense, EasyG, SpryHealth, and Striiv.
SOURCE: Peterson ED et al. JAMA. 2018 July 10;320[2]:138-9.
FROM JAMA
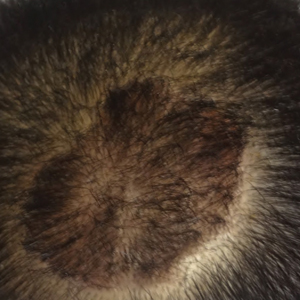
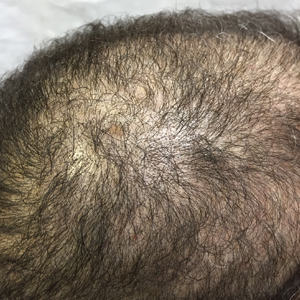
Scalp Psoriasis With Increased Hair Density
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Practice Points
- Scalp psoriasis may present with hair loss or increased hair density.
- Psoriasis with increased hair density may make topical medications more difficult to apply.
Reflectance Confocal Microscopy as a First-Line Diagnostic Technique for Mycosis Fungoides
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.
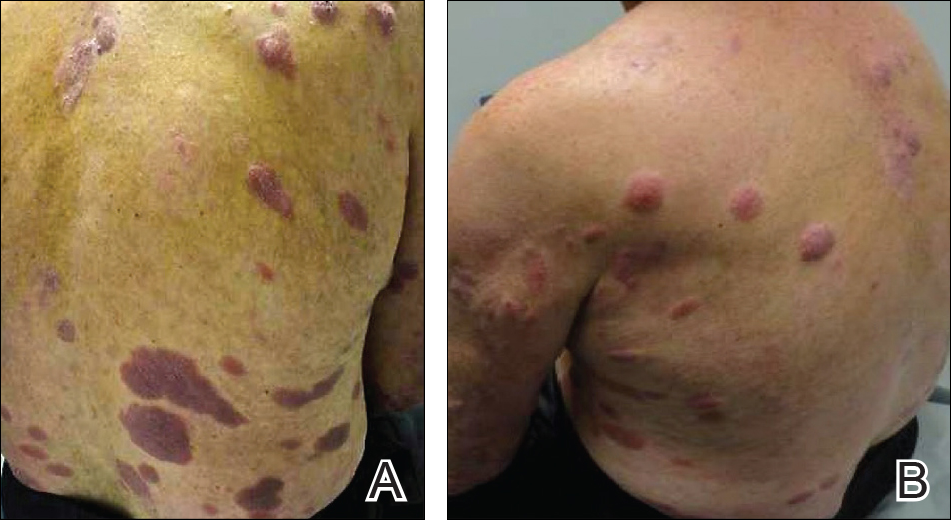
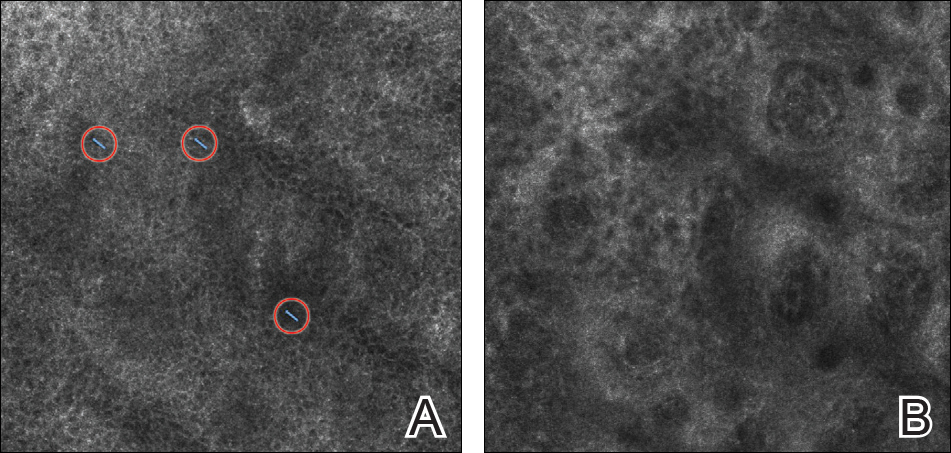
A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.


A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.


A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
Practice Points
- Mycosis fungoides (MF) can be a challenging diagnosis to establish and often requires multiple biopsies.
- Reflectance confocal microscopy (RCM) may be helpful as a bedside noninvasive diagnostic technique.
- In suspected MF cases, RCM may assist in selecting the optimal biopsy site for better yield of histopathologic results.
Biomechanical Analysis of a Novel Buried Fixation Technique Using Headless Compression Screws for the Treatment of Patella Fractures
ABSTRACT
The traditional technique for patella fracture fixation utilizes prominent hardware. Prominent hardware use, however, results in a high rate of reoperation for symptomatic implant removal. This biomechanical study evaluates the effectiveness of a novel patella fixation technique that minimizes implant prominence.
Patellar transverse osteotomies were created in 13 pairs of cadaveric knees. Paired knees were assigned to either standard fixation (SF) using cannulated partially threaded screws and stainless steel wire tension band, or buried fixation (BF) using headless compression screws with a No. 2 FiberWire tension band and a No. 5 FiberWire cerclage suture. Quadriceps tendons were cyclically loaded to full extension followed by load to failure. The gap across the fracture site, stiffness, and load to failure were measured.
The differences in stiffness and load to failure between the 2 groups were not statistically significant. During cyclic loading, significantly greater gapping was observed across the fracture site in the BF group compared with SF group (P < .05).
Both constructs failed under loads that exceeded typical loads experienced during the postoperative rehabilitation period. Nevertheless, the BF technique demonstrated larger gap formation and a reduced load to failure than the SF technique. Further clinical studies are therefore underway to determine whether the use of constructs with decreased stability but increased patient comfort could improve clinical outcomes and reduce reoperation rates.
Continue to: Patella fractures are common...
Patella fractures are common injuries that can cause considerable disability to the knee extensor apparatus.1-3 Transverse patella fractures are the most common fracture pattern associated with patella fractures.{Harrell, 2003 #3}2 Given that the patella plays a crucial role in knee extensor biomechanics, its proper integrity is vital for physiological knee motion and ambulation.4 Traditionally, patella fractures with >2 mm of displacement have been managed with cannulated screws or Kirschner wires (K-wires) and a stainless-steel wire tension band.5-9 The goal in the treatment of patellar fractures is to reduce fracture fragments accurately and to minimize additional insults to the articular cartilage.10
Despite advances in surgical protocols and acceptable radiographic outcomes, functional impairment remains common after the treatment of patella fractures. Functional impairment includes knee pain, screw head pain, implant removal, wire breakage, and patella baja.1 The need for implant removal is one of the most common complications following the open reduction internal fixation of patella fractures.2,11 The subcutaneous and exposed nature of the patella in conjunction with soft tissue irritation resulting from standard fixation (SF) predisposes the patient toward prominence and discomfort with the retained implant. Although nonunion rates are low, the rate of implant removal can reach as high as 52%.2,10-12 To overcome some of these complications, we designed a novel buried fixation (BF) method for the treatment of transverse fractures. Our method minimizes the amount of exposed implant to improve patient comfort and potentially reduce the need for future implant removal. These effects are achieved by using headless compression screws and nonabsorbable sutures to attenuate the soft tissue irritation associated with traditional fixation.13 While our novel technique has demonstrated improved clinical results, it has not been tested biomechanically against a traditional fixation technique. Therefore, this study aims to evaluate and compare the structural integrity of our novel BF technique with that of the standard technique that uses cannulated screws and wire tension band. We hypothesized that the stability provided by our technique would be similar to that provided by SF for transverse patella fractures.
MATERIALS AND METHODS
SPECIMEN PREPARATION
Thirteen matched pairs of fresh-frozen human cadaveric knees were obtained from a Cedars-Sinai approved tissue bank. Specimens were cut midfemur and were intact to the foot. Legs with major structural bony or ligamentous abnormalities, extensor mechanism disruption, or septic knees were excluded from testing. To assess the bone quality of each specimen prior to testing, dual-energy X-ray absorptiometry was performed using a GE Lunar iDXA scanner (GE Healthcare). Specimens were stored at −30°C and thawed at room temperature for 24 hours prior to biomechanical testing.
A midline anterior approach to the patella was performed, and the extensor retinaculum, quadriceps tendon, and patellar tendon were exposed. A digital caliper was used to measure the craniocaudal and mediolateral dimensions of the patella, and a transverse osteotomy (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association [AO/OTA] type 34-C1) was created at the midway point between superior and inferior poles by using an oscillating saw. The retinaculum was then incised to the level of the midaxial line of the femur. One leg from each matched pair was allocated to the SF group, and the other was allocated to the BF group. Left and right legs were alternately assigned to each group to ensure that laterality was balanced between the 2 groups.
SURGICAL TECHNIQUE
The repair of the specimens in the SF group involved the use of 2 parallel 4.0-mm partially threaded cannulated screws (Acumed) and an 18-gauge monofilament steel wire (Ethicon) in a figure-eight tension band (Figure 1A). The repair of the specimens in the BF group involved the use of 2 parallel standard Acutrak headless compression screws (Acumed), a No. 2 FiberWire (Arthrex) in a figure-eight tension band, and a No. 5 FiberWire (Arthrex) was applied as cerclage around the patella (Figure 1B).
Continue to: Mechanical testing...
MECHANICAL TESTING
Mechanical testing was performed on a biaxial 370.02 Bionix Testing System (MTS Systems Corp.). The femur was rigidly and horizontally secured to a custom-built test frame, and the lower leg was left free to move. The quadriceps tendon was secured in a freeze clamp and was attached to the MTS actuator for loading via a pulley system such that raising the actuator was translated into a simulated quadriceps extensor force.
A differential variable reluctance transducer (DVRT) (Lord MicroStrain) was placed across the osteotomy site to measure the distraction, or gap, across the fracture line. The minimum load to full extension for each specimen was then determined under a slow, controlled increase in load until the leg was in a fully extended position. Any distraction across the fracture line during the initial loading phase was determined by using digital calipers. The specimen was then subjected to a preconditioning phase with 10 cycles from 0 N to full extension under the previously determined load, which was applied at the rate of 5 N/s. Meanwhile, displacement across the fracture site was recorded via the DVRT. Following the preconditioning phase, each specimen was then tested to failure in displacement control at the rate of 1.5 mm/s. Failure was defined as implant failure (screw pullout) or DVRT gapping across the osteotomy site >3 mm.10,14
Outcome measures included stiffness (N/mm), which was calculated as the slope of the linear change in load from full extension to failure vs DVRT displacement during the final loading phase; failure load (N); gapping (mm) across the osteotomy site at each cycle during the preconditioning phase; and failure mode (pullout vs >3.0 mm gap).
STATISTICAL ANALYSIS
An a priori power analysis revealed that 13 knees per group would be required to obtain an α of 0.05 and a power of 0.80. This calculation was based on a 20% difference in fracture displacement calculated by using the standard deviation and mean previously reported for cannulated screws with nonabsorbable sutures.14
Means and standard deviations for all dependent outcome measures were computed and compared across the independent measure of fixation type (BF vs SF) through repeated measures Analysis of variance (ANOVA-GLM, SAS 9.3, SAS Institute, Inc.) after controlling for bone mineral density (BMD), gender, and age. Multivariate repeated-measures ANOVA with Tukey's studentized range was applied to cyclic gap data. The mode of failure was compared across fixation type (BF vs SF) for matched data using McNemar’s test. Intracorrelations were computed and examined over all data and separately on the basis of screw fixation type (BF vs SF). All tests were considered statistically significant when P < .05.
Continue to: Results...
RESULTS
Specimen donors were 46% (6/13) male with an average age of 78.5 years (±13.77; range, 56-91 years) and 54% (7/13) female with an average age of 76.57 years (±14.37; range, 59-102 years). Average BMD was significantly lower in female (0.71 ± 0.18) than in male specimens (1.15 ± 0.33) (P < .05).
The average load to full extension across all specimens was 272 N (±54; range, 160-360 N) and was well balanced across matched pairs (270 ± 56 N for BF and 273 ± 54 N for SF). Of the 13 BF specimens, 4 experienced distraction across the fracture line during the determination of the minimum load to full extension. This initial pretest gap was measured with digital calipers (average, 1.5 mm; range, 0.90-1.85 mm) and added as an offset to the respective DVRT displacement data recorded during testing.
The total number of specimens included in the displacement data calculations decreased from 13 to 11 per group because DVRT data were not recorded during cyclic loading for 1 specimen and were considered unreliable in another. The maximum displacement measured across the fracture site during cyclic loading was significantly higher in the BF (0.94 ± 1.21) group than in the SF group (0.19 ± 0.26) as shown in the Table. The average slope of the gap per cycle for each specimen was calculated and compared between the BF and SF groups. The BF group demonstrated a significantly greater increase in gap per cycle than the SF group (Figure 2). Stiffness during load to failure was calculated for all but 1 specimen that did not display any measurable displacement during the final loading cycle. The average final stiffness and failure load between the BF and SF groups were not significantly different (Table). An equal number of specimens in both groups failed through gapping (6/13) and pullout (7/13).
Table. Means and Standard Deviations of the Main Outcome Measures
| Standard Fixation | Buried Fixation | N | P-value |
Load at Failure (N) | 1112.78 ± 457.25 | 973.20 ± 321.38 | 13 | 0.265 |
Final Stiffness (N/mm) | 358.42 ± 165.45 | 445.33 ± 310.09 | 11 | 0.175 |
Max Cyclic Gap (mm) | 0.19 ± 0.26 | 0.94 ± 1.21 | 11 | 0.026a |
Pullout: Gap Failure (ratio) | 7:6 | 7:6 | 13 | NS |
aIndicates statistical significance (P < .05).
Abbreviation: NS, not significant.
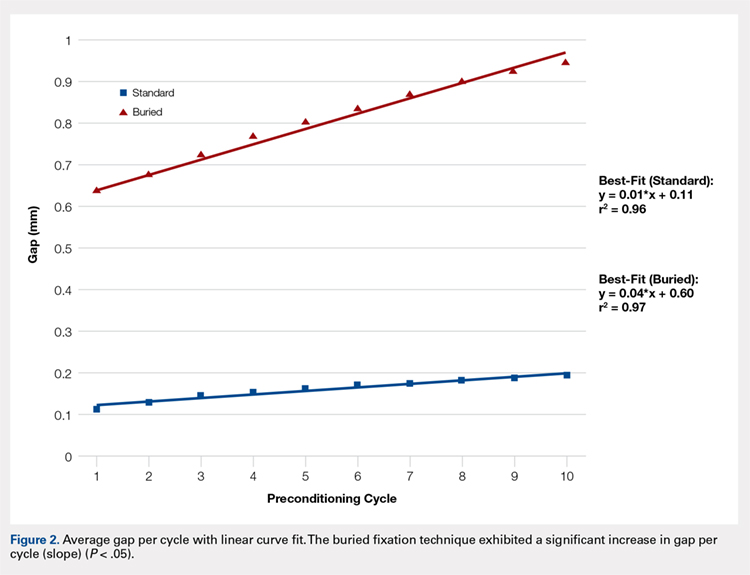
Failure load was significantly positively correlated with BMD (R = 0.62, P < .001) when all specimens were grouped together. When analyzed separately, the SF group was significantly correlated with BMD (P < .01), whereas the BF group had a marginally significant correlation (P = .06) with BMD (Figure 3). In both groups, BMD was positively correlated with stiffness and negatively correlated with gapping. Neither of these trends, however, was significant.
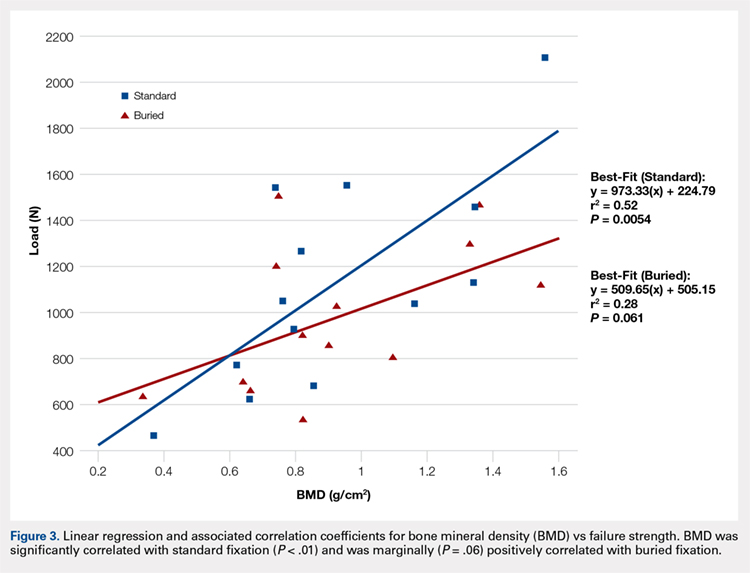
Continue to: Discussion...
DISCUSSION
We proposed a novel BF technique for the treatment of noncomminuted transverse patella fractures. Our technique utilizes headless cannulated compression screws and nonabsorbable suture tension bands. We then biomechanically compared our proposed technique with an established fixation technique that uses partially threaded cannulated screws and stainless steel wire tension bands. We hypothesized that the mechanical response of the BF technique to cyclic and failure loading would be similar to that of the SF technique. Our results demonstrate a significant increase in gap formation across the fracture site among knees and an overall reduced load to failure in the BF group (Figure 2). Whether these inferior results manifest clinically is not yet established. Both constructs could withstand forces that are typically experienced during the postoperative period. Given the high rate of symptomatic implant removal associated with the traditional technique, the low-profile buried technique might be an attractive alternative that provides increased patient comfort but may require an extended period of postoperative protection against bony ingrowths.
Patellar fixation constructs that use a combination of cannulated screws and a wire tension band provide the best resistance to patella fracture displacement when compared with screws or wires alone.4,15 Although this combination is biomechanically favorable, the steel wire often causes the painful irritation of the surrounding soft tissues and can break or migrate, thus increasing the rates of implant removal surgery to as high as 52%.4,10,12,15 We developed our novel BF technique, which uses headless compression screws and a No. 2 FiberWire tension band, to address the high rates of reoperation and patient dissatisfaction associated with the SF technique.
Headless compression screws have been successfully used in the reduction and fixation of scaphoid fractures and sesamoid fractures.16,17 The pull-out strengths of these screws are comparable with those of other commonly used screws, such as Twinfix and Herbert-Whipple screws.16 Similarly, the strength of a No. 5 FiberWire is comparable with that of an 18-gauge stainless-steel wire.14,18 Several studies have also obtained good outcomes with nonmetallic constructs that use nonabsorbable sutures alone.19,20 In this study, we utilized a No. 2 FiberWire as the tension band. The use of the No. 2 FiberWire facilitated threading through headless cannulated screws and created a low-profile knot. However, the use of thin FiberWire, despite a No. 5 FiberWire cerclage, likely contributed to the increase in distraction across the fracture.
The highest patellofemoral joint reaction force during level walking is approximately 35 kg (half body weight), which is equivalent to 350 N.15,21,22 This force is similar to the average cyclic load used in this experiment (272 ± 54 N). Gapping increased in the BF group but did not reach the defined failure value of 3 mm, and the ultimate load to failure was relatively high across both groups (SF, 1123 N; BF, 973 N). These results suggest that both fixation methods can withstand the typical patellofemoral joint forces that are experienced during the postoperative period.4 In addition, in a clinical setting, patients are placed in hinged knee braces for at least 2 weeks to limit their flexion angle and to allow for healing and bony ingrowth. Postoperative knee-brace protection presumably increases the overall strength of the fixation.
The number of specimens (n = 26) evaluated in this study was greater than that used in other biomechanical patella fracture studies.14 Furthermore, none of our specimens were reused. Our study design was further strengthened given that fellowship-trained trauma surgeons performed all surgical procedures. Finally, the data collection and analysis of numerous clinically relevant factors, such as BMD, age, and cyclical loading, contributed to the comprehensive description of each technique with respect to patient-specific criteria.
Similar to all cadaveric studies, our data only represent the immediate postoperative condition and does not represent any healing that would occur during postoperative rehabilitation. Postoperative knee-brace protection and bone healing across the fracture site would likely strengthen both constructs in a clinical setting. In addition, the average age of our specimens is 77.5 years, and therefore does not best represent the age range (20-50 years) of the typical adult population affected by patella fractures.3,23,24 Finally, postsurgical reduction was confirmed through visual inspection and not through fluoroscopy as in a clinical setting. Radiographic images were obtained after each experiment only to confirm screw placement post facto (Figures 4A, 4B).
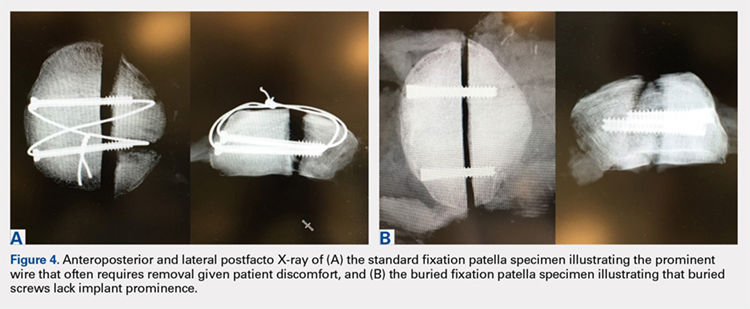
CONCLUSION
This study demonstrates the utility of a novel BF technique. Nevertheless, the proposed technique exhibited increased gapping and a lower load to failure than the current gold standard. The significance of these inferior results in clinical and functional settings has not been established. The proposed BF technique may be an appealing alternative to the SF technique given its low profile and potential to reduce the rates of future implant removal. Further studies on the long-term outcomes of patients treated through the BF technique are currently under way and will ultimately determine the utility of the proposed construct.
This paper will be judged for the Resident Writer’s Award.
- Lazaro LE, Wellman DS, Sauro G, et al. Outcomes after operative fixation of complete articular patellar fractures: assessment of functional impairment. J Bone Joint Surg Am. 2013;95(14):e96 1-8. doi:10.2106/JBJS.L.00012.
- Bostman O, Kiviluoto O, Santavirta S, Nirhamo J, Wilppula E. Fractures of the patella treated by operation. Arch Orthop Trauma Surg. 1983;102(2):78-81.
- Gwinner C, Märdian S, Schwabe P, Schaser KD, Krapohl BD, Jung TM. Current concepts review: fractures of the patella. GMS Interdiscip Plast Reconstr Surg DGPW. 2016;5:Doc01. doi:10.3205/iprs000080.
- Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
- Patel VR, Parks BG, Wang Y, Ebert FR, Jinnah RH. Fixation of patella fractures with braided polyester suture: a biomechanical study. Injury. 2000;31(1):1-6.
- Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
- Banks KE, Ambrose CG, Wheeless JS, Tissue CM, Sen M. An alternative patellar fracture fixation: a biomechanical study. J Orthop Trauma. 2013;27(6):345-351. doi:10.1097/BOT.0b013e31826623eb.
- Thelen S, Schneppendahl J, Baumgartner R, et al. Cyclic long-term loading of a bilateral fixed-angle plate in comparison with tension band wiring with K-wires or cannulated screws in transverse patella fractures. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):311-317. doi:10.1007/s00167-012-1999-1.
- Thelen S, Schneppendahl J, Jopen E, et al. Biomechanical cadaver testing of a fixed-angle plate in comparison to tension wiring and screw fixation in transverse patella fractures. Injury. 2012;43(8):1290-1295. doi:10.1016/j.injury.2012.04.020.
- LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426. doi:10.1097/BOT.0b013e318228c1a1.
- Dy CJ, Little MT, Berkes MB, et al. Meta-analysis of re-operation, nonunion, and infection after open reduction and internal fixation of patella fractures. J Trauma Acute Care Surg. 2012;73(4):928-932. doi:10.1097/TA.0b013e31825168b6.
- Smith ST, Cramer KE, Karges DE, Watson JT, Moed BR. Early complications in the operative treatment of patella fractures. J Orthop Trauma. 1997;11(3):183-187.
- Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
- Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: A pilot study. J Orthop. 2015;12(2):92-96. doi:10.1016/j.jor.2014.04.011.
- Burvant JG, Thomas KA, Alexander R, Harris MB. Evaluation of methods of internal fixation of transverse patella fractures: a biomechanical study. J Orthop Trauma. 1994;8(2):147-153.
- Crawford LA, Powell ES, Trail IA. The fixation strength of scaphoid bone screws: an in vitro investigation using polyurethane foam. J Hand Surg Am. 2012;37(2):255-260. doi:10.1016/j.jhsa.2011.10.021.
- Eddy AL, Galuppo LD, Stover SM, Taylor KT, Jensen DG. A biomechanical comparison of headless tapered variable pitch compression and ao cortical bone screws for fixation of a simulated midbody transverse fracture of the proximal sesamoid bone in horses. Vet Surg. 2004;33(3):253-262. doi:10.1111/j.1532-950X.2004.04037.x.
- Camarda L, La Gattuta A, Butera M, Siragusa F, D'Arienzo M. FiberWire tension band for patellar fractures. J Orthop Traumatol. 2016;17(1):75-80. doi:10.1007/s10195-015-0359-6.
- Camarda L, Morello S, Balistreri F, D'Arienzo A, D'Arienzo M. Non-metallic implant for patellar fracture fixation: A systematic review. Injury. 2016;47(8):1613-1617. doi:10.1016/j.injury.2016.05.039.
- Han F, Pearce CJ, Ng DQ, et al. A double button adjustable loop device is biomechanically equivalent to tension band wire in the fixation of transverse patellar fractures-A cadaveric study. Injury. 2017;48(2):270-276. doi:10.1016/j.injury.2016.11.013.
- Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126-137. doi:10.1016/j.injury.2016.11.013.
- Buff HU, Jones LC, Hungerford DS. Experimental determination of forces transmitted through the patello-femoral joint. J Biomech. 1988;21(1):17-23.
- Bostrom A. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37(8):691-697. doi:10.1111/iwj.12675.
ABSTRACT
The traditional technique for patella fracture fixation utilizes prominent hardware. Prominent hardware use, however, results in a high rate of reoperation for symptomatic implant removal. This biomechanical study evaluates the effectiveness of a novel patella fixation technique that minimizes implant prominence.
Patellar transverse osteotomies were created in 13 pairs of cadaveric knees. Paired knees were assigned to either standard fixation (SF) using cannulated partially threaded screws and stainless steel wire tension band, or buried fixation (BF) using headless compression screws with a No. 2 FiberWire tension band and a No. 5 FiberWire cerclage suture. Quadriceps tendons were cyclically loaded to full extension followed by load to failure. The gap across the fracture site, stiffness, and load to failure were measured.
The differences in stiffness and load to failure between the 2 groups were not statistically significant. During cyclic loading, significantly greater gapping was observed across the fracture site in the BF group compared with SF group (P < .05).
Both constructs failed under loads that exceeded typical loads experienced during the postoperative rehabilitation period. Nevertheless, the BF technique demonstrated larger gap formation and a reduced load to failure than the SF technique. Further clinical studies are therefore underway to determine whether the use of constructs with decreased stability but increased patient comfort could improve clinical outcomes and reduce reoperation rates.
Continue to: Patella fractures are common...
Patella fractures are common injuries that can cause considerable disability to the knee extensor apparatus.1-3 Transverse patella fractures are the most common fracture pattern associated with patella fractures.{Harrell, 2003 #3}2 Given that the patella plays a crucial role in knee extensor biomechanics, its proper integrity is vital for physiological knee motion and ambulation.4 Traditionally, patella fractures with >2 mm of displacement have been managed with cannulated screws or Kirschner wires (K-wires) and a stainless-steel wire tension band.5-9 The goal in the treatment of patellar fractures is to reduce fracture fragments accurately and to minimize additional insults to the articular cartilage.10
Despite advances in surgical protocols and acceptable radiographic outcomes, functional impairment remains common after the treatment of patella fractures. Functional impairment includes knee pain, screw head pain, implant removal, wire breakage, and patella baja.1 The need for implant removal is one of the most common complications following the open reduction internal fixation of patella fractures.2,11 The subcutaneous and exposed nature of the patella in conjunction with soft tissue irritation resulting from standard fixation (SF) predisposes the patient toward prominence and discomfort with the retained implant. Although nonunion rates are low, the rate of implant removal can reach as high as 52%.2,10-12 To overcome some of these complications, we designed a novel buried fixation (BF) method for the treatment of transverse fractures. Our method minimizes the amount of exposed implant to improve patient comfort and potentially reduce the need for future implant removal. These effects are achieved by using headless compression screws and nonabsorbable sutures to attenuate the soft tissue irritation associated with traditional fixation.13 While our novel technique has demonstrated improved clinical results, it has not been tested biomechanically against a traditional fixation technique. Therefore, this study aims to evaluate and compare the structural integrity of our novel BF technique with that of the standard technique that uses cannulated screws and wire tension band. We hypothesized that the stability provided by our technique would be similar to that provided by SF for transverse patella fractures.
MATERIALS AND METHODS
SPECIMEN PREPARATION
Thirteen matched pairs of fresh-frozen human cadaveric knees were obtained from a Cedars-Sinai approved tissue bank. Specimens were cut midfemur and were intact to the foot. Legs with major structural bony or ligamentous abnormalities, extensor mechanism disruption, or septic knees were excluded from testing. To assess the bone quality of each specimen prior to testing, dual-energy X-ray absorptiometry was performed using a GE Lunar iDXA scanner (GE Healthcare). Specimens were stored at −30°C and thawed at room temperature for 24 hours prior to biomechanical testing.
A midline anterior approach to the patella was performed, and the extensor retinaculum, quadriceps tendon, and patellar tendon were exposed. A digital caliper was used to measure the craniocaudal and mediolateral dimensions of the patella, and a transverse osteotomy (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association [AO/OTA] type 34-C1) was created at the midway point between superior and inferior poles by using an oscillating saw. The retinaculum was then incised to the level of the midaxial line of the femur. One leg from each matched pair was allocated to the SF group, and the other was allocated to the BF group. Left and right legs were alternately assigned to each group to ensure that laterality was balanced between the 2 groups.
SURGICAL TECHNIQUE
The repair of the specimens in the SF group involved the use of 2 parallel 4.0-mm partially threaded cannulated screws (Acumed) and an 18-gauge monofilament steel wire (Ethicon) in a figure-eight tension band (Figure 1A). The repair of the specimens in the BF group involved the use of 2 parallel standard Acutrak headless compression screws (Acumed), a No. 2 FiberWire (Arthrex) in a figure-eight tension band, and a No. 5 FiberWire (Arthrex) was applied as cerclage around the patella (Figure 1B).

Continue to: Mechanical testing...
MECHANICAL TESTING
Mechanical testing was performed on a biaxial 370.02 Bionix Testing System (MTS Systems Corp.). The femur was rigidly and horizontally secured to a custom-built test frame, and the lower leg was left free to move. The quadriceps tendon was secured in a freeze clamp and was attached to the MTS actuator for loading via a pulley system such that raising the actuator was translated into a simulated quadriceps extensor force.
A differential variable reluctance transducer (DVRT) (Lord MicroStrain) was placed across the osteotomy site to measure the distraction, or gap, across the fracture line. The minimum load to full extension for each specimen was then determined under a slow, controlled increase in load until the leg was in a fully extended position. Any distraction across the fracture line during the initial loading phase was determined by using digital calipers. The specimen was then subjected to a preconditioning phase with 10 cycles from 0 N to full extension under the previously determined load, which was applied at the rate of 5 N/s. Meanwhile, displacement across the fracture site was recorded via the DVRT. Following the preconditioning phase, each specimen was then tested to failure in displacement control at the rate of 1.5 mm/s. Failure was defined as implant failure (screw pullout) or DVRT gapping across the osteotomy site >3 mm.10,14
Outcome measures included stiffness (N/mm), which was calculated as the slope of the linear change in load from full extension to failure vs DVRT displacement during the final loading phase; failure load (N); gapping (mm) across the osteotomy site at each cycle during the preconditioning phase; and failure mode (pullout vs >3.0 mm gap).
STATISTICAL ANALYSIS
An a priori power analysis revealed that 13 knees per group would be required to obtain an α of 0.05 and a power of 0.80. This calculation was based on a 20% difference in fracture displacement calculated by using the standard deviation and mean previously reported for cannulated screws with nonabsorbable sutures.14
Means and standard deviations for all dependent outcome measures were computed and compared across the independent measure of fixation type (BF vs SF) through repeated measures Analysis of variance (ANOVA-GLM, SAS 9.3, SAS Institute, Inc.) after controlling for bone mineral density (BMD), gender, and age. Multivariate repeated-measures ANOVA with Tukey's studentized range was applied to cyclic gap data. The mode of failure was compared across fixation type (BF vs SF) for matched data using McNemar’s test. Intracorrelations were computed and examined over all data and separately on the basis of screw fixation type (BF vs SF). All tests were considered statistically significant when P < .05.
Continue to: Results...
RESULTS
Specimen donors were 46% (6/13) male with an average age of 78.5 years (±13.77; range, 56-91 years) and 54% (7/13) female with an average age of 76.57 years (±14.37; range, 59-102 years). Average BMD was significantly lower in female (0.71 ± 0.18) than in male specimens (1.15 ± 0.33) (P < .05).
The average load to full extension across all specimens was 272 N (±54; range, 160-360 N) and was well balanced across matched pairs (270 ± 56 N for BF and 273 ± 54 N for SF). Of the 13 BF specimens, 4 experienced distraction across the fracture line during the determination of the minimum load to full extension. This initial pretest gap was measured with digital calipers (average, 1.5 mm; range, 0.90-1.85 mm) and added as an offset to the respective DVRT displacement data recorded during testing.
The total number of specimens included in the displacement data calculations decreased from 13 to 11 per group because DVRT data were not recorded during cyclic loading for 1 specimen and were considered unreliable in another. The maximum displacement measured across the fracture site during cyclic loading was significantly higher in the BF (0.94 ± 1.21) group than in the SF group (0.19 ± 0.26) as shown in the Table. The average slope of the gap per cycle for each specimen was calculated and compared between the BF and SF groups. The BF group demonstrated a significantly greater increase in gap per cycle than the SF group (Figure 2). Stiffness during load to failure was calculated for all but 1 specimen that did not display any measurable displacement during the final loading cycle. The average final stiffness and failure load between the BF and SF groups were not significantly different (Table). An equal number of specimens in both groups failed through gapping (6/13) and pullout (7/13).
Table. Means and Standard Deviations of the Main Outcome Measures
| Standard Fixation | Buried Fixation | N | P-value |
Load at Failure (N) | 1112.78 ± 457.25 | 973.20 ± 321.38 | 13 | 0.265 |
Final Stiffness (N/mm) | 358.42 ± 165.45 | 445.33 ± 310.09 | 11 | 0.175 |
Max Cyclic Gap (mm) | 0.19 ± 0.26 | 0.94 ± 1.21 | 11 | 0.026a |
Pullout: Gap Failure (ratio) | 7:6 | 7:6 | 13 | NS |
aIndicates statistical significance (P < .05).
Abbreviation: NS, not significant.

Failure load was significantly positively correlated with BMD (R = 0.62, P < .001) when all specimens were grouped together. When analyzed separately, the SF group was significantly correlated with BMD (P < .01), whereas the BF group had a marginally significant correlation (P = .06) with BMD (Figure 3). In both groups, BMD was positively correlated with stiffness and negatively correlated with gapping. Neither of these trends, however, was significant.

Continue to: Discussion...
DISCUSSION
We proposed a novel BF technique for the treatment of noncomminuted transverse patella fractures. Our technique utilizes headless cannulated compression screws and nonabsorbable suture tension bands. We then biomechanically compared our proposed technique with an established fixation technique that uses partially threaded cannulated screws and stainless steel wire tension bands. We hypothesized that the mechanical response of the BF technique to cyclic and failure loading would be similar to that of the SF technique. Our results demonstrate a significant increase in gap formation across the fracture site among knees and an overall reduced load to failure in the BF group (Figure 2). Whether these inferior results manifest clinically is not yet established. Both constructs could withstand forces that are typically experienced during the postoperative period. Given the high rate of symptomatic implant removal associated with the traditional technique, the low-profile buried technique might be an attractive alternative that provides increased patient comfort but may require an extended period of postoperative protection against bony ingrowths.
Patellar fixation constructs that use a combination of cannulated screws and a wire tension band provide the best resistance to patella fracture displacement when compared with screws or wires alone.4,15 Although this combination is biomechanically favorable, the steel wire often causes the painful irritation of the surrounding soft tissues and can break or migrate, thus increasing the rates of implant removal surgery to as high as 52%.4,10,12,15 We developed our novel BF technique, which uses headless compression screws and a No. 2 FiberWire tension band, to address the high rates of reoperation and patient dissatisfaction associated with the SF technique.
Headless compression screws have been successfully used in the reduction and fixation of scaphoid fractures and sesamoid fractures.16,17 The pull-out strengths of these screws are comparable with those of other commonly used screws, such as Twinfix and Herbert-Whipple screws.16 Similarly, the strength of a No. 5 FiberWire is comparable with that of an 18-gauge stainless-steel wire.14,18 Several studies have also obtained good outcomes with nonmetallic constructs that use nonabsorbable sutures alone.19,20 In this study, we utilized a No. 2 FiberWire as the tension band. The use of the No. 2 FiberWire facilitated threading through headless cannulated screws and created a low-profile knot. However, the use of thin FiberWire, despite a No. 5 FiberWire cerclage, likely contributed to the increase in distraction across the fracture.
The highest patellofemoral joint reaction force during level walking is approximately 35 kg (half body weight), which is equivalent to 350 N.15,21,22 This force is similar to the average cyclic load used in this experiment (272 ± 54 N). Gapping increased in the BF group but did not reach the defined failure value of 3 mm, and the ultimate load to failure was relatively high across both groups (SF, 1123 N; BF, 973 N). These results suggest that both fixation methods can withstand the typical patellofemoral joint forces that are experienced during the postoperative period.4 In addition, in a clinical setting, patients are placed in hinged knee braces for at least 2 weeks to limit their flexion angle and to allow for healing and bony ingrowth. Postoperative knee-brace protection presumably increases the overall strength of the fixation.
The number of specimens (n = 26) evaluated in this study was greater than that used in other biomechanical patella fracture studies.14 Furthermore, none of our specimens were reused. Our study design was further strengthened given that fellowship-trained trauma surgeons performed all surgical procedures. Finally, the data collection and analysis of numerous clinically relevant factors, such as BMD, age, and cyclical loading, contributed to the comprehensive description of each technique with respect to patient-specific criteria.
Similar to all cadaveric studies, our data only represent the immediate postoperative condition and does not represent any healing that would occur during postoperative rehabilitation. Postoperative knee-brace protection and bone healing across the fracture site would likely strengthen both constructs in a clinical setting. In addition, the average age of our specimens is 77.5 years, and therefore does not best represent the age range (20-50 years) of the typical adult population affected by patella fractures.3,23,24 Finally, postsurgical reduction was confirmed through visual inspection and not through fluoroscopy as in a clinical setting. Radiographic images were obtained after each experiment only to confirm screw placement post facto (Figures 4A, 4B).

CONCLUSION
This study demonstrates the utility of a novel BF technique. Nevertheless, the proposed technique exhibited increased gapping and a lower load to failure than the current gold standard. The significance of these inferior results in clinical and functional settings has not been established. The proposed BF technique may be an appealing alternative to the SF technique given its low profile and potential to reduce the rates of future implant removal. Further studies on the long-term outcomes of patients treated through the BF technique are currently under way and will ultimately determine the utility of the proposed construct.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The traditional technique for patella fracture fixation utilizes prominent hardware. Prominent hardware use, however, results in a high rate of reoperation for symptomatic implant removal. This biomechanical study evaluates the effectiveness of a novel patella fixation technique that minimizes implant prominence.
Patellar transverse osteotomies were created in 13 pairs of cadaveric knees. Paired knees were assigned to either standard fixation (SF) using cannulated partially threaded screws and stainless steel wire tension band, or buried fixation (BF) using headless compression screws with a No. 2 FiberWire tension band and a No. 5 FiberWire cerclage suture. Quadriceps tendons were cyclically loaded to full extension followed by load to failure. The gap across the fracture site, stiffness, and load to failure were measured.
The differences in stiffness and load to failure between the 2 groups were not statistically significant. During cyclic loading, significantly greater gapping was observed across the fracture site in the BF group compared with SF group (P < .05).
Both constructs failed under loads that exceeded typical loads experienced during the postoperative rehabilitation period. Nevertheless, the BF technique demonstrated larger gap formation and a reduced load to failure than the SF technique. Further clinical studies are therefore underway to determine whether the use of constructs with decreased stability but increased patient comfort could improve clinical outcomes and reduce reoperation rates.
Continue to: Patella fractures are common...
Patella fractures are common injuries that can cause considerable disability to the knee extensor apparatus.1-3 Transverse patella fractures are the most common fracture pattern associated with patella fractures.{Harrell, 2003 #3}2 Given that the patella plays a crucial role in knee extensor biomechanics, its proper integrity is vital for physiological knee motion and ambulation.4 Traditionally, patella fractures with >2 mm of displacement have been managed with cannulated screws or Kirschner wires (K-wires) and a stainless-steel wire tension band.5-9 The goal in the treatment of patellar fractures is to reduce fracture fragments accurately and to minimize additional insults to the articular cartilage.10
Despite advances in surgical protocols and acceptable radiographic outcomes, functional impairment remains common after the treatment of patella fractures. Functional impairment includes knee pain, screw head pain, implant removal, wire breakage, and patella baja.1 The need for implant removal is one of the most common complications following the open reduction internal fixation of patella fractures.2,11 The subcutaneous and exposed nature of the patella in conjunction with soft tissue irritation resulting from standard fixation (SF) predisposes the patient toward prominence and discomfort with the retained implant. Although nonunion rates are low, the rate of implant removal can reach as high as 52%.2,10-12 To overcome some of these complications, we designed a novel buried fixation (BF) method for the treatment of transverse fractures. Our method minimizes the amount of exposed implant to improve patient comfort and potentially reduce the need for future implant removal. These effects are achieved by using headless compression screws and nonabsorbable sutures to attenuate the soft tissue irritation associated with traditional fixation.13 While our novel technique has demonstrated improved clinical results, it has not been tested biomechanically against a traditional fixation technique. Therefore, this study aims to evaluate and compare the structural integrity of our novel BF technique with that of the standard technique that uses cannulated screws and wire tension band. We hypothesized that the stability provided by our technique would be similar to that provided by SF for transverse patella fractures.
MATERIALS AND METHODS
SPECIMEN PREPARATION
Thirteen matched pairs of fresh-frozen human cadaveric knees were obtained from a Cedars-Sinai approved tissue bank. Specimens were cut midfemur and were intact to the foot. Legs with major structural bony or ligamentous abnormalities, extensor mechanism disruption, or septic knees were excluded from testing. To assess the bone quality of each specimen prior to testing, dual-energy X-ray absorptiometry was performed using a GE Lunar iDXA scanner (GE Healthcare). Specimens were stored at −30°C and thawed at room temperature for 24 hours prior to biomechanical testing.
A midline anterior approach to the patella was performed, and the extensor retinaculum, quadriceps tendon, and patellar tendon were exposed. A digital caliper was used to measure the craniocaudal and mediolateral dimensions of the patella, and a transverse osteotomy (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association [AO/OTA] type 34-C1) was created at the midway point between superior and inferior poles by using an oscillating saw. The retinaculum was then incised to the level of the midaxial line of the femur. One leg from each matched pair was allocated to the SF group, and the other was allocated to the BF group. Left and right legs were alternately assigned to each group to ensure that laterality was balanced between the 2 groups.
SURGICAL TECHNIQUE
The repair of the specimens in the SF group involved the use of 2 parallel 4.0-mm partially threaded cannulated screws (Acumed) and an 18-gauge monofilament steel wire (Ethicon) in a figure-eight tension band (Figure 1A). The repair of the specimens in the BF group involved the use of 2 parallel standard Acutrak headless compression screws (Acumed), a No. 2 FiberWire (Arthrex) in a figure-eight tension band, and a No. 5 FiberWire (Arthrex) was applied as cerclage around the patella (Figure 1B).

Continue to: Mechanical testing...
MECHANICAL TESTING
Mechanical testing was performed on a biaxial 370.02 Bionix Testing System (MTS Systems Corp.). The femur was rigidly and horizontally secured to a custom-built test frame, and the lower leg was left free to move. The quadriceps tendon was secured in a freeze clamp and was attached to the MTS actuator for loading via a pulley system such that raising the actuator was translated into a simulated quadriceps extensor force.
A differential variable reluctance transducer (DVRT) (Lord MicroStrain) was placed across the osteotomy site to measure the distraction, or gap, across the fracture line. The minimum load to full extension for each specimen was then determined under a slow, controlled increase in load until the leg was in a fully extended position. Any distraction across the fracture line during the initial loading phase was determined by using digital calipers. The specimen was then subjected to a preconditioning phase with 10 cycles from 0 N to full extension under the previously determined load, which was applied at the rate of 5 N/s. Meanwhile, displacement across the fracture site was recorded via the DVRT. Following the preconditioning phase, each specimen was then tested to failure in displacement control at the rate of 1.5 mm/s. Failure was defined as implant failure (screw pullout) or DVRT gapping across the osteotomy site >3 mm.10,14
Outcome measures included stiffness (N/mm), which was calculated as the slope of the linear change in load from full extension to failure vs DVRT displacement during the final loading phase; failure load (N); gapping (mm) across the osteotomy site at each cycle during the preconditioning phase; and failure mode (pullout vs >3.0 mm gap).
STATISTICAL ANALYSIS
An a priori power analysis revealed that 13 knees per group would be required to obtain an α of 0.05 and a power of 0.80. This calculation was based on a 20% difference in fracture displacement calculated by using the standard deviation and mean previously reported for cannulated screws with nonabsorbable sutures.14
Means and standard deviations for all dependent outcome measures were computed and compared across the independent measure of fixation type (BF vs SF) through repeated measures Analysis of variance (ANOVA-GLM, SAS 9.3, SAS Institute, Inc.) after controlling for bone mineral density (BMD), gender, and age. Multivariate repeated-measures ANOVA with Tukey's studentized range was applied to cyclic gap data. The mode of failure was compared across fixation type (BF vs SF) for matched data using McNemar’s test. Intracorrelations were computed and examined over all data and separately on the basis of screw fixation type (BF vs SF). All tests were considered statistically significant when P < .05.
Continue to: Results...
RESULTS
Specimen donors were 46% (6/13) male with an average age of 78.5 years (±13.77; range, 56-91 years) and 54% (7/13) female with an average age of 76.57 years (±14.37; range, 59-102 years). Average BMD was significantly lower in female (0.71 ± 0.18) than in male specimens (1.15 ± 0.33) (P < .05).
The average load to full extension across all specimens was 272 N (±54; range, 160-360 N) and was well balanced across matched pairs (270 ± 56 N for BF and 273 ± 54 N for SF). Of the 13 BF specimens, 4 experienced distraction across the fracture line during the determination of the minimum load to full extension. This initial pretest gap was measured with digital calipers (average, 1.5 mm; range, 0.90-1.85 mm) and added as an offset to the respective DVRT displacement data recorded during testing.
The total number of specimens included in the displacement data calculations decreased from 13 to 11 per group because DVRT data were not recorded during cyclic loading for 1 specimen and were considered unreliable in another. The maximum displacement measured across the fracture site during cyclic loading was significantly higher in the BF (0.94 ± 1.21) group than in the SF group (0.19 ± 0.26) as shown in the Table. The average slope of the gap per cycle for each specimen was calculated and compared between the BF and SF groups. The BF group demonstrated a significantly greater increase in gap per cycle than the SF group (Figure 2). Stiffness during load to failure was calculated for all but 1 specimen that did not display any measurable displacement during the final loading cycle. The average final stiffness and failure load between the BF and SF groups were not significantly different (Table). An equal number of specimens in both groups failed through gapping (6/13) and pullout (7/13).
Table. Means and Standard Deviations of the Main Outcome Measures
| Standard Fixation | Buried Fixation | N | P-value |
Load at Failure (N) | 1112.78 ± 457.25 | 973.20 ± 321.38 | 13 | 0.265 |
Final Stiffness (N/mm) | 358.42 ± 165.45 | 445.33 ± 310.09 | 11 | 0.175 |
Max Cyclic Gap (mm) | 0.19 ± 0.26 | 0.94 ± 1.21 | 11 | 0.026a |
Pullout: Gap Failure (ratio) | 7:6 | 7:6 | 13 | NS |
aIndicates statistical significance (P < .05).
Abbreviation: NS, not significant.

Failure load was significantly positively correlated with BMD (R = 0.62, P < .001) when all specimens were grouped together. When analyzed separately, the SF group was significantly correlated with BMD (P < .01), whereas the BF group had a marginally significant correlation (P = .06) with BMD (Figure 3). In both groups, BMD was positively correlated with stiffness and negatively correlated with gapping. Neither of these trends, however, was significant.

Continue to: Discussion...
DISCUSSION
We proposed a novel BF technique for the treatment of noncomminuted transverse patella fractures. Our technique utilizes headless cannulated compression screws and nonabsorbable suture tension bands. We then biomechanically compared our proposed technique with an established fixation technique that uses partially threaded cannulated screws and stainless steel wire tension bands. We hypothesized that the mechanical response of the BF technique to cyclic and failure loading would be similar to that of the SF technique. Our results demonstrate a significant increase in gap formation across the fracture site among knees and an overall reduced load to failure in the BF group (Figure 2). Whether these inferior results manifest clinically is not yet established. Both constructs could withstand forces that are typically experienced during the postoperative period. Given the high rate of symptomatic implant removal associated with the traditional technique, the low-profile buried technique might be an attractive alternative that provides increased patient comfort but may require an extended period of postoperative protection against bony ingrowths.
Patellar fixation constructs that use a combination of cannulated screws and a wire tension band provide the best resistance to patella fracture displacement when compared with screws or wires alone.4,15 Although this combination is biomechanically favorable, the steel wire often causes the painful irritation of the surrounding soft tissues and can break or migrate, thus increasing the rates of implant removal surgery to as high as 52%.4,10,12,15 We developed our novel BF technique, which uses headless compression screws and a No. 2 FiberWire tension band, to address the high rates of reoperation and patient dissatisfaction associated with the SF technique.
Headless compression screws have been successfully used in the reduction and fixation of scaphoid fractures and sesamoid fractures.16,17 The pull-out strengths of these screws are comparable with those of other commonly used screws, such as Twinfix and Herbert-Whipple screws.16 Similarly, the strength of a No. 5 FiberWire is comparable with that of an 18-gauge stainless-steel wire.14,18 Several studies have also obtained good outcomes with nonmetallic constructs that use nonabsorbable sutures alone.19,20 In this study, we utilized a No. 2 FiberWire as the tension band. The use of the No. 2 FiberWire facilitated threading through headless cannulated screws and created a low-profile knot. However, the use of thin FiberWire, despite a No. 5 FiberWire cerclage, likely contributed to the increase in distraction across the fracture.
The highest patellofemoral joint reaction force during level walking is approximately 35 kg (half body weight), which is equivalent to 350 N.15,21,22 This force is similar to the average cyclic load used in this experiment (272 ± 54 N). Gapping increased in the BF group but did not reach the defined failure value of 3 mm, and the ultimate load to failure was relatively high across both groups (SF, 1123 N; BF, 973 N). These results suggest that both fixation methods can withstand the typical patellofemoral joint forces that are experienced during the postoperative period.4 In addition, in a clinical setting, patients are placed in hinged knee braces for at least 2 weeks to limit their flexion angle and to allow for healing and bony ingrowth. Postoperative knee-brace protection presumably increases the overall strength of the fixation.
The number of specimens (n = 26) evaluated in this study was greater than that used in other biomechanical patella fracture studies.14 Furthermore, none of our specimens were reused. Our study design was further strengthened given that fellowship-trained trauma surgeons performed all surgical procedures. Finally, the data collection and analysis of numerous clinically relevant factors, such as BMD, age, and cyclical loading, contributed to the comprehensive description of each technique with respect to patient-specific criteria.
Similar to all cadaveric studies, our data only represent the immediate postoperative condition and does not represent any healing that would occur during postoperative rehabilitation. Postoperative knee-brace protection and bone healing across the fracture site would likely strengthen both constructs in a clinical setting. In addition, the average age of our specimens is 77.5 years, and therefore does not best represent the age range (20-50 years) of the typical adult population affected by patella fractures.3,23,24 Finally, postsurgical reduction was confirmed through visual inspection and not through fluoroscopy as in a clinical setting. Radiographic images were obtained after each experiment only to confirm screw placement post facto (Figures 4A, 4B).

CONCLUSION
This study demonstrates the utility of a novel BF technique. Nevertheless, the proposed technique exhibited increased gapping and a lower load to failure than the current gold standard. The significance of these inferior results in clinical and functional settings has not been established. The proposed BF technique may be an appealing alternative to the SF technique given its low profile and potential to reduce the rates of future implant removal. Further studies on the long-term outcomes of patients treated through the BF technique are currently under way and will ultimately determine the utility of the proposed construct.
This paper will be judged for the Resident Writer’s Award.
- Lazaro LE, Wellman DS, Sauro G, et al. Outcomes after operative fixation of complete articular patellar fractures: assessment of functional impairment. J Bone Joint Surg Am. 2013;95(14):e96 1-8. doi:10.2106/JBJS.L.00012.
- Bostman O, Kiviluoto O, Santavirta S, Nirhamo J, Wilppula E. Fractures of the patella treated by operation. Arch Orthop Trauma Surg. 1983;102(2):78-81.
- Gwinner C, Märdian S, Schwabe P, Schaser KD, Krapohl BD, Jung TM. Current concepts review: fractures of the patella. GMS Interdiscip Plast Reconstr Surg DGPW. 2016;5:Doc01. doi:10.3205/iprs000080.
- Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
- Patel VR, Parks BG, Wang Y, Ebert FR, Jinnah RH. Fixation of patella fractures with braided polyester suture: a biomechanical study. Injury. 2000;31(1):1-6.
- Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
- Banks KE, Ambrose CG, Wheeless JS, Tissue CM, Sen M. An alternative patellar fracture fixation: a biomechanical study. J Orthop Trauma. 2013;27(6):345-351. doi:10.1097/BOT.0b013e31826623eb.
- Thelen S, Schneppendahl J, Baumgartner R, et al. Cyclic long-term loading of a bilateral fixed-angle plate in comparison with tension band wiring with K-wires or cannulated screws in transverse patella fractures. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):311-317. doi:10.1007/s00167-012-1999-1.
- Thelen S, Schneppendahl J, Jopen E, et al. Biomechanical cadaver testing of a fixed-angle plate in comparison to tension wiring and screw fixation in transverse patella fractures. Injury. 2012;43(8):1290-1295. doi:10.1016/j.injury.2012.04.020.
- LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426. doi:10.1097/BOT.0b013e318228c1a1.
- Dy CJ, Little MT, Berkes MB, et al. Meta-analysis of re-operation, nonunion, and infection after open reduction and internal fixation of patella fractures. J Trauma Acute Care Surg. 2012;73(4):928-932. doi:10.1097/TA.0b013e31825168b6.
- Smith ST, Cramer KE, Karges DE, Watson JT, Moed BR. Early complications in the operative treatment of patella fractures. J Orthop Trauma. 1997;11(3):183-187.
- Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
- Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: A pilot study. J Orthop. 2015;12(2):92-96. doi:10.1016/j.jor.2014.04.011.
- Burvant JG, Thomas KA, Alexander R, Harris MB. Evaluation of methods of internal fixation of transverse patella fractures: a biomechanical study. J Orthop Trauma. 1994;8(2):147-153.
- Crawford LA, Powell ES, Trail IA. The fixation strength of scaphoid bone screws: an in vitro investigation using polyurethane foam. J Hand Surg Am. 2012;37(2):255-260. doi:10.1016/j.jhsa.2011.10.021.
- Eddy AL, Galuppo LD, Stover SM, Taylor KT, Jensen DG. A biomechanical comparison of headless tapered variable pitch compression and ao cortical bone screws for fixation of a simulated midbody transverse fracture of the proximal sesamoid bone in horses. Vet Surg. 2004;33(3):253-262. doi:10.1111/j.1532-950X.2004.04037.x.
- Camarda L, La Gattuta A, Butera M, Siragusa F, D'Arienzo M. FiberWire tension band for patellar fractures. J Orthop Traumatol. 2016;17(1):75-80. doi:10.1007/s10195-015-0359-6.
- Camarda L, Morello S, Balistreri F, D'Arienzo A, D'Arienzo M. Non-metallic implant for patellar fracture fixation: A systematic review. Injury. 2016;47(8):1613-1617. doi:10.1016/j.injury.2016.05.039.
- Han F, Pearce CJ, Ng DQ, et al. A double button adjustable loop device is biomechanically equivalent to tension band wire in the fixation of transverse patellar fractures-A cadaveric study. Injury. 2017;48(2):270-276. doi:10.1016/j.injury.2016.11.013.
- Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126-137. doi:10.1016/j.injury.2016.11.013.
- Buff HU, Jones LC, Hungerford DS. Experimental determination of forces transmitted through the patello-femoral joint. J Biomech. 1988;21(1):17-23.
- Bostrom A. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37(8):691-697. doi:10.1111/iwj.12675.
- Lazaro LE, Wellman DS, Sauro G, et al. Outcomes after operative fixation of complete articular patellar fractures: assessment of functional impairment. J Bone Joint Surg Am. 2013;95(14):e96 1-8. doi:10.2106/JBJS.L.00012.
- Bostman O, Kiviluoto O, Santavirta S, Nirhamo J, Wilppula E. Fractures of the patella treated by operation. Arch Orthop Trauma Surg. 1983;102(2):78-81.
- Gwinner C, Märdian S, Schwabe P, Schaser KD, Krapohl BD, Jung TM. Current concepts review: fractures of the patella. GMS Interdiscip Plast Reconstr Surg DGPW. 2016;5:Doc01. doi:10.3205/iprs000080.
- Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
- Patel VR, Parks BG, Wang Y, Ebert FR, Jinnah RH. Fixation of patella fractures with braided polyester suture: a biomechanical study. Injury. 2000;31(1):1-6.
- Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
- Banks KE, Ambrose CG, Wheeless JS, Tissue CM, Sen M. An alternative patellar fracture fixation: a biomechanical study. J Orthop Trauma. 2013;27(6):345-351. doi:10.1097/BOT.0b013e31826623eb.
- Thelen S, Schneppendahl J, Baumgartner R, et al. Cyclic long-term loading of a bilateral fixed-angle plate in comparison with tension band wiring with K-wires or cannulated screws in transverse patella fractures. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):311-317. doi:10.1007/s00167-012-1999-1.
- Thelen S, Schneppendahl J, Jopen E, et al. Biomechanical cadaver testing of a fixed-angle plate in comparison to tension wiring and screw fixation in transverse patella fractures. Injury. 2012;43(8):1290-1295. doi:10.1016/j.injury.2012.04.020.
- LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426. doi:10.1097/BOT.0b013e318228c1a1.
- Dy CJ, Little MT, Berkes MB, et al. Meta-analysis of re-operation, nonunion, and infection after open reduction and internal fixation of patella fractures. J Trauma Acute Care Surg. 2012;73(4):928-932. doi:10.1097/TA.0b013e31825168b6.
- Smith ST, Cramer KE, Karges DE, Watson JT, Moed BR. Early complications in the operative treatment of patella fractures. J Orthop Trauma. 1997;11(3):183-187.
- Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
- Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: A pilot study. J Orthop. 2015;12(2):92-96. doi:10.1016/j.jor.2014.04.011.
- Burvant JG, Thomas KA, Alexander R, Harris MB. Evaluation of methods of internal fixation of transverse patella fractures: a biomechanical study. J Orthop Trauma. 1994;8(2):147-153.
- Crawford LA, Powell ES, Trail IA. The fixation strength of scaphoid bone screws: an in vitro investigation using polyurethane foam. J Hand Surg Am. 2012;37(2):255-260. doi:10.1016/j.jhsa.2011.10.021.
- Eddy AL, Galuppo LD, Stover SM, Taylor KT, Jensen DG. A biomechanical comparison of headless tapered variable pitch compression and ao cortical bone screws for fixation of a simulated midbody transverse fracture of the proximal sesamoid bone in horses. Vet Surg. 2004;33(3):253-262. doi:10.1111/j.1532-950X.2004.04037.x.
- Camarda L, La Gattuta A, Butera M, Siragusa F, D'Arienzo M. FiberWire tension band for patellar fractures. J Orthop Traumatol. 2016;17(1):75-80. doi:10.1007/s10195-015-0359-6.
- Camarda L, Morello S, Balistreri F, D'Arienzo A, D'Arienzo M. Non-metallic implant for patellar fracture fixation: A systematic review. Injury. 2016;47(8):1613-1617. doi:10.1016/j.injury.2016.05.039.
- Han F, Pearce CJ, Ng DQ, et al. A double button adjustable loop device is biomechanically equivalent to tension band wire in the fixation of transverse patellar fractures-A cadaveric study. Injury. 2017;48(2):270-276. doi:10.1016/j.injury.2016.11.013.
- Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126-137. doi:10.1016/j.injury.2016.11.013.
- Buff HU, Jones LC, Hungerford DS. Experimental determination of forces transmitted through the patello-femoral joint. J Biomech. 1988;21(1):17-23.
- Bostrom A. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37(8):691-697. doi:10.1111/iwj.12675.
TAKE-HOME POINTS
- Symptomatic implant removal rates are high after patella fixation with standard techniques.
- Novel buried technique may address the issue of symptomatic implants and is an attractive alternative.
- Both techniques withstand physiologic loads, but the buried technique had overall increased gapping and lower load to failure.
- The significance of these inferior results in clinical and functional settings has not been established.
- Long-term functional outcome studies will delineate the utility of the proposed new construct.
Uric acid tied to pediatric diabetic kidney disease
ORLANDO – , according to a 7-year investigation of 539 children.
Every 1-mg/dL climb in baseline serum uric acid increased the risk of subsequent elevated urine albumin excretion 1.23 fold, after adjustment for potential confounders (P = .02).
The finding adds to growing evidence that serum uric acid (SUA) isn’t just a marker of diabetic kidney disease, but a contributor to it. “There is definitely” cross-talk between gout and diabetes, said lead investigator Petter Bjornstad, MD, assistant professor of pediatric endocrinology at the University of Colorado, Aurora.
Elevated SUA is common in both conditions and a risk factor for kidney disease. Newer studies have linked higher levels to nephron number decline and other pathologies, perhaps through renal inflammation. Allopurinol, the traditional uric acid lowering agent in gout, is already under investigation to prevent kidney decline in adults with type 1 diabetes mellitus. There’s also evidence that the potent uric acid lowering agent, febuxostat (Uloric), attenuates hypofiltration in early diabetic kidney disease.
The 539 children, all part of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) trial, were assessed annually over a mean of 5.7 years. At baseline, they were 13.9 years old and had T2DM for 7.9 months, on average. The mean body mass index was 34.6 kg/m2, mean hemoglobin A1c was 6%.
Almost 20% of the children were hypertensive at baseline (130/80 mm Hg or higher); 26% were hyperuricemic (6.8 mg/dL or higher); and 6.1% had elevated urine albumin excretion (urine albumin creatinine ratio of at least 30 mg/g), a marker of renal pathology. At the end of follow-up, 18% had elevated albumin excretion and 37.4% were hypertensive.
“Hyperuricemia was common in youth with type 2 diabetes,” just as it’s been shown in adults with the disease. “Higher baseline SUA independently increase[s] risk for onset of hypertension and elevated urine albumin excretion,” Dr. Bjornstad said.
However, the association between SUA and elevated albumin excretion was statistically significant only in boys – 36% of the study population – and non-Hispanic whites, 20% of the subjects, after adjustment for BMI, hemoglobin A1c, estimated glomerular filtration rate, and use of ACE inhibitors and angiotensin II receptor blockers.
The National Institutes of Health funded the work. Dr. Bjornstad is a consultant for Boehringer Ingelheim.
SOURCE: Bjornstad P et al. ADA 2018, abstract 339-OR.
ORLANDO – , according to a 7-year investigation of 539 children.
Every 1-mg/dL climb in baseline serum uric acid increased the risk of subsequent elevated urine albumin excretion 1.23 fold, after adjustment for potential confounders (P = .02).
The finding adds to growing evidence that serum uric acid (SUA) isn’t just a marker of diabetic kidney disease, but a contributor to it. “There is definitely” cross-talk between gout and diabetes, said lead investigator Petter Bjornstad, MD, assistant professor of pediatric endocrinology at the University of Colorado, Aurora.
Elevated SUA is common in both conditions and a risk factor for kidney disease. Newer studies have linked higher levels to nephron number decline and other pathologies, perhaps through renal inflammation. Allopurinol, the traditional uric acid lowering agent in gout, is already under investigation to prevent kidney decline in adults with type 1 diabetes mellitus. There’s also evidence that the potent uric acid lowering agent, febuxostat (Uloric), attenuates hypofiltration in early diabetic kidney disease.
The 539 children, all part of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) trial, were assessed annually over a mean of 5.7 years. At baseline, they were 13.9 years old and had T2DM for 7.9 months, on average. The mean body mass index was 34.6 kg/m2, mean hemoglobin A1c was 6%.
Almost 20% of the children were hypertensive at baseline (130/80 mm Hg or higher); 26% were hyperuricemic (6.8 mg/dL or higher); and 6.1% had elevated urine albumin excretion (urine albumin creatinine ratio of at least 30 mg/g), a marker of renal pathology. At the end of follow-up, 18% had elevated albumin excretion and 37.4% were hypertensive.
“Hyperuricemia was common in youth with type 2 diabetes,” just as it’s been shown in adults with the disease. “Higher baseline SUA independently increase[s] risk for onset of hypertension and elevated urine albumin excretion,” Dr. Bjornstad said.
However, the association between SUA and elevated albumin excretion was statistically significant only in boys – 36% of the study population – and non-Hispanic whites, 20% of the subjects, after adjustment for BMI, hemoglobin A1c, estimated glomerular filtration rate, and use of ACE inhibitors and angiotensin II receptor blockers.
The National Institutes of Health funded the work. Dr. Bjornstad is a consultant for Boehringer Ingelheim.
SOURCE: Bjornstad P et al. ADA 2018, abstract 339-OR.
ORLANDO – , according to a 7-year investigation of 539 children.
Every 1-mg/dL climb in baseline serum uric acid increased the risk of subsequent elevated urine albumin excretion 1.23 fold, after adjustment for potential confounders (P = .02).
The finding adds to growing evidence that serum uric acid (SUA) isn’t just a marker of diabetic kidney disease, but a contributor to it. “There is definitely” cross-talk between gout and diabetes, said lead investigator Petter Bjornstad, MD, assistant professor of pediatric endocrinology at the University of Colorado, Aurora.
Elevated SUA is common in both conditions and a risk factor for kidney disease. Newer studies have linked higher levels to nephron number decline and other pathologies, perhaps through renal inflammation. Allopurinol, the traditional uric acid lowering agent in gout, is already under investigation to prevent kidney decline in adults with type 1 diabetes mellitus. There’s also evidence that the potent uric acid lowering agent, febuxostat (Uloric), attenuates hypofiltration in early diabetic kidney disease.
The 539 children, all part of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) trial, were assessed annually over a mean of 5.7 years. At baseline, they were 13.9 years old and had T2DM for 7.9 months, on average. The mean body mass index was 34.6 kg/m2, mean hemoglobin A1c was 6%.
Almost 20% of the children were hypertensive at baseline (130/80 mm Hg or higher); 26% were hyperuricemic (6.8 mg/dL or higher); and 6.1% had elevated urine albumin excretion (urine albumin creatinine ratio of at least 30 mg/g), a marker of renal pathology. At the end of follow-up, 18% had elevated albumin excretion and 37.4% were hypertensive.
“Hyperuricemia was common in youth with type 2 diabetes,” just as it’s been shown in adults with the disease. “Higher baseline SUA independently increase[s] risk for onset of hypertension and elevated urine albumin excretion,” Dr. Bjornstad said.
However, the association between SUA and elevated albumin excretion was statistically significant only in boys – 36% of the study population – and non-Hispanic whites, 20% of the subjects, after adjustment for BMI, hemoglobin A1c, estimated glomerular filtration rate, and use of ACE inhibitors and angiotensin II receptor blockers.
The National Institutes of Health funded the work. Dr. Bjornstad is a consultant for Boehringer Ingelheim.
SOURCE: Bjornstad P et al. ADA 2018, abstract 339-OR.
REPORTING FROM ADA 2018
Key clinical point: Serum uric acid lowering might help prevent kidney disease in children with T2DM.
Major finding: Every1-mg/dL climb in baseline serum uric acid increased the risk of subsequent elevated urine albumin excretion 1.23 fold, after adjustment for potential confounders (P = .02)
Study details: Seven-year investigation of 539 children with new-onset T2DM.
Disclosures: The National Institutes of Health funded the work. The study lead is a consultant for Boehringer Ingelheim.
Source: Bjornstad P et al. ADA 2018 Abstract 339-OR.
Nonscarring Alopecia Associated With Vitamin D Deficiency
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.
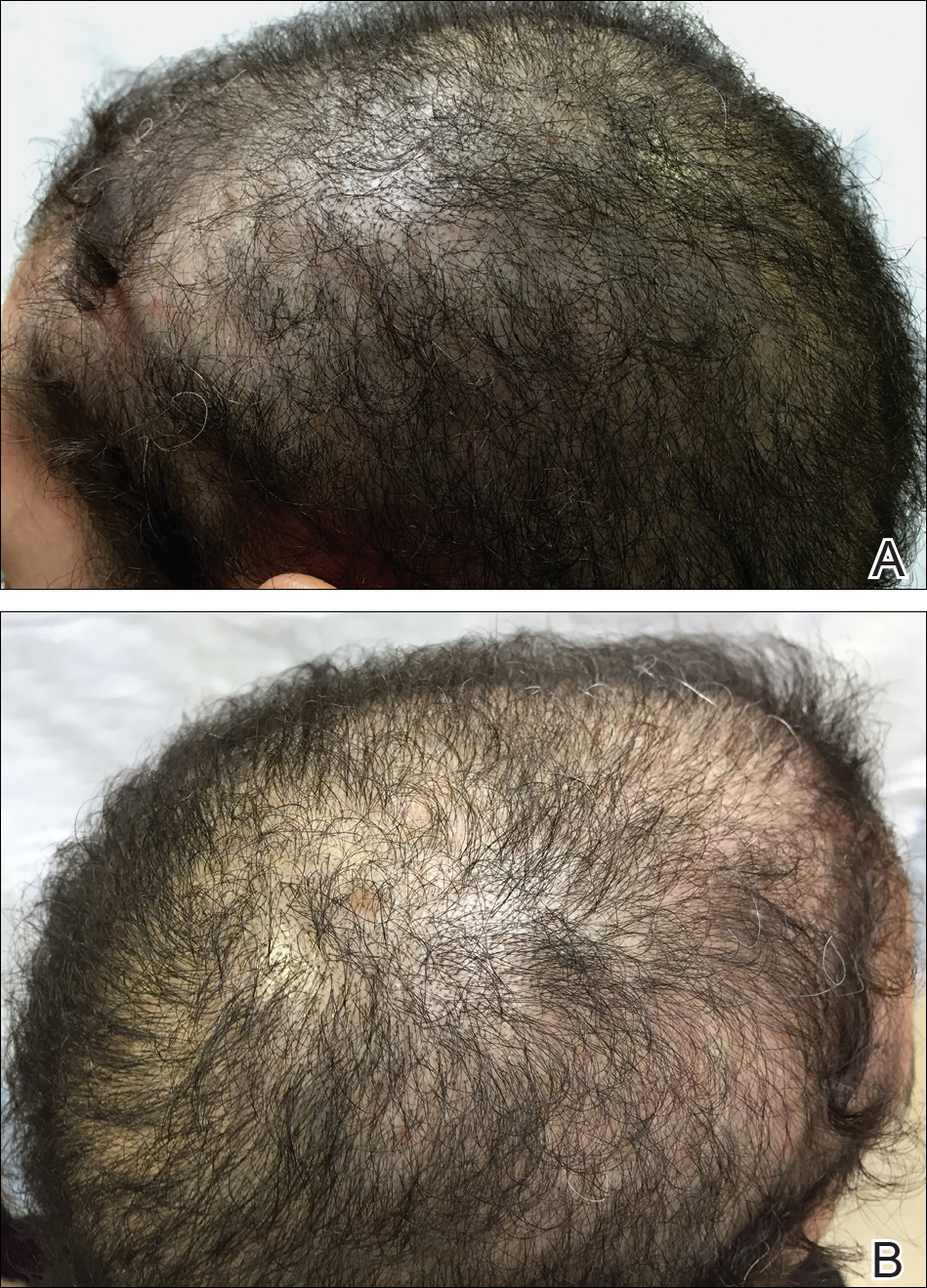
On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.
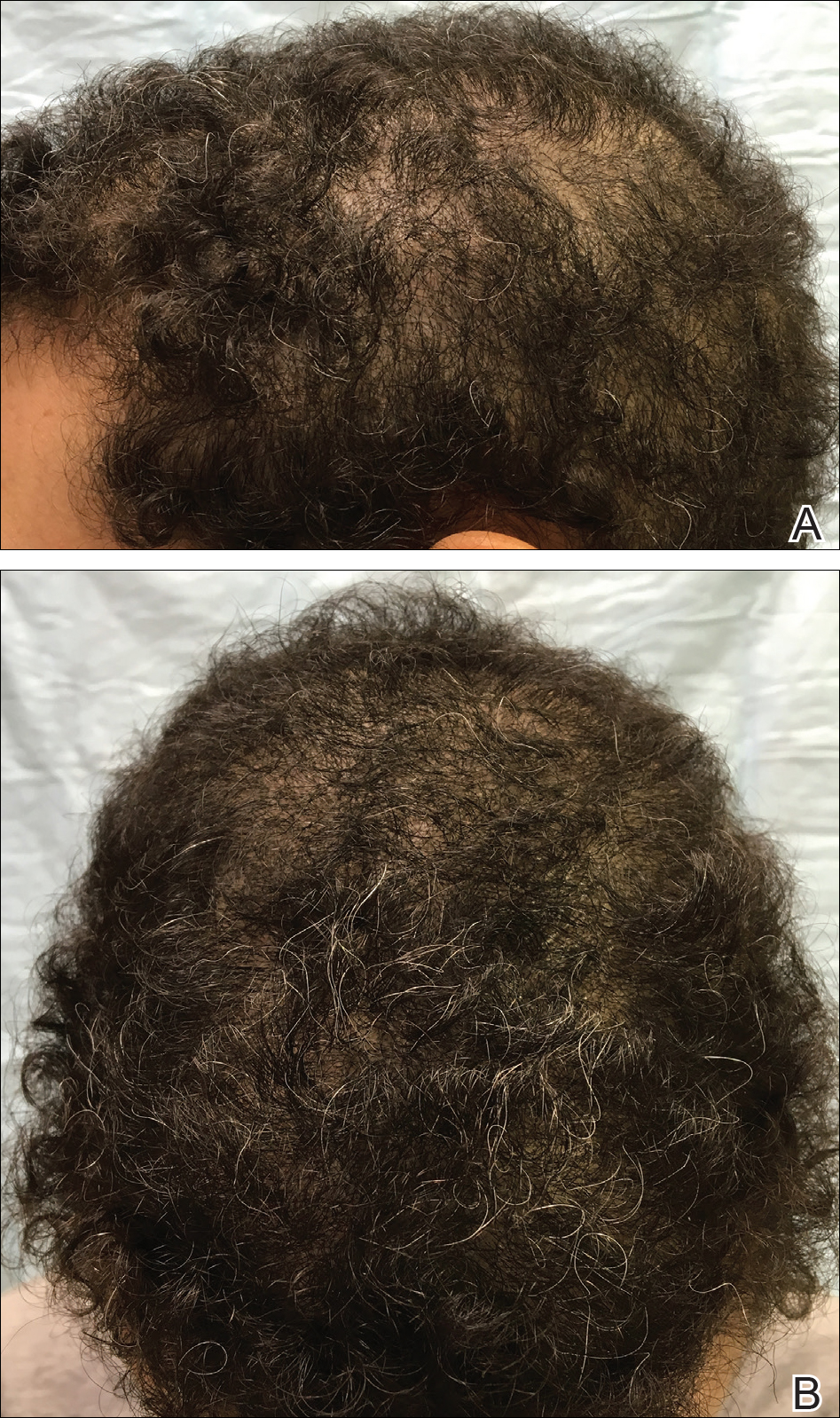
Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.

On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.

Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
Vitamin D receptors are found in every cell of the body and have been shown to play a role in bone, neural, and cardiovascular health; immune regulation; and possibly cancer prevention via the regulation of cell differentiation, proliferation, and apoptosis.1 Although it is controversial, vitamin D deficiency has been associated with various forms of nonscarring hair loss,2-4 including telogen effluvium, androgenetic alopecia, and alopecia areata. We describe a notable case of nonscarring alopecia associated with vitamin D deficiency in which vitamin D replacement therapy promoted hair regrowth.
Case Report
An otherwise healthy 34-year-old black woman presented to the Hair and Nail Clinic at the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania) for evaluation of progressive hair loss of 4 years’ duration that began shortly after her fourth child was born. Although she denied any history of excessive shedding, she stated that she used to have shoulder-length hair and somehow it had become extremely short without shaving or cutting the hair (Figure 1). Her current medications included a progestin intrauterine device and biotin 10 mg once daily, the latter of which she had taken for several months for the hair loss without any improvement.

On physical examination, the patient was noted to have diffusely thinning, short, brittle hair. Trichoscopy was notable for hairs of varying diameters, with some fractured at the level of the follicular ostia but no yellow dots at the follicular openings or exclamation point hairs. No scarring or erythema was seen on the scalp. The patient refused several of our team’s recommendations for scalp biopsy due to needle phobia. A hair growth window was made that showed good regrowth at 2 weeks after the initial presentation. Initial blood work revealed a total serum 25-hydroxyvitamin D level of 12 ng/mL (optimal, >30 ng/mL). Complete blood cell count, hormonal panel, zinc level, iron level, and thyroid studies were all normal.
The patient was started on vitamin D3 replacement therapy 50,000 IU once weekly for 4 weeks followed by 1000 IU once daily for 6 months. No other topical or systemic treatments were administered for the nonscarring alopecia. At a follow-up visit 6 months later, the patient’s vitamin D level was 36 ng/mL, and she had noticeable hair regrowth (Figure 2). At this time, the diagnosis of nonscarring alopecia associated with vitamin D deficiency was made.

Comment
Vitamin D is a fat-soluble vitamin that can be obtained via sun exposure, food sources (eg, fish, vitamin D–fortified foods), and direct supplementation.5 It has been estimated that nearly 1 billion individuals worldwide6 and approximately 41.6% of US adults are vitamin D deficient.7 Certainly not all of these individuals will present with alopecia, but in patients with hair loss, we suggest that vitamin D deficiency is an important factor to consider. Risk factors for vitamin D deficiency include older age, obesity, darker skin types, residence in northern latitudes, and malabsorption syndromes.7
Pathogenesis
Vitamin D is thought to play a role in the normal initiation and completion of the hair cycle as well as the differentiation of the follicular and interfollicular epidermis. The vitamin D receptor (VDR) is thought to induce the development of mature anagen hairs via the canonical WNT-β-catenin and hedgehog signaling pathways.8 In the absence of VDRs, the stem cells in the bulge of the hair follicle have an impaired ability to replicate, and as a result, VDR-deficient mice have shown near-total hair loss.9-12 We propose that vitamin D deficiency can not only be a trigger for hair loss but also can perpetuate hair loss and poor regrowth.
Diagnosis and Prevention of Vitamin D Deficiency
In the skin, 7-dehydrocholesterol is converted to previtamin D3 via UVB light, followed by subsequent conversion to vitamin D3. Dietary sources are in the form of either vitamin D2 or D3, both of which are converted in the liver to 25-hydroxyvitamin D, the major circulating metabolite. In the kidneys, 25-hydroxyvitamin D is then converted to 1,25-dihydroxyvitamin D, the biologically active form. Paradoxically, serum levels of 1,25-dihydroxyvitamin D can be normal or high in the setting of vitamin D deficiency; therefore, serum total 25-hydroxyvitamin D is the best way to assess a patient’s vitamin D status.5,13
The optimal serum 25-hydroxyvitamin D level is controversial. Recommendations range between 20 to 40 ng/mL14 and 30 to 50 ng/mL.13,15,16 Vitamin D levels higher than 50 ng/mL have been correlated with an increased risk of bone fractures and certain cancers.16-18 Vitamin D toxicity usually is noted in serum levels greater than 88 ng/mL; symptoms of toxicity include hypercalcemia, nausea, vomiting, and muscle weakness. For nondeficient patients, the National Academy of Medicine (formerly the Institute of Medicine) recommended an upper limit of 4000 IU daily.14 The optimal dose in preventing vitamin D deficiency ranges from 600 to 1000 IU daily.13-15
Treatment of Vitamin D Deficiency
In the setting of vitamin D deficiency, the amount required for repletion often is dependent on each individual’s ability to absorb and convert to 25-hydroxyvitamin D. Typically every 100 IU of vitamin D correlates with a 0.7 to 1.0 ng/mL increase in serum 25-hydroxyvitamin D levels.19 There are multiple dosing regimens used to achieve the desired serum 25-hydroxyvitamin D levels in deficient patients. One recommendation from the Endocrine Society is 50,000 IU once weekly for 6 to 8 weeks (single doses >50,000 IU typically are not recommended due to increased risk for toxicity), followed by 600 to 1000 IU once daily in children and 1500 to 2000 IU once daily in adults thereafter.13 In patients with vitamin D deficiency, reassessment of serum 25-hydroxyvitamin D levels is recommended after 3 to 4 months of treatment, and adjustments to the repletion regimen should be made as needed.15,16 Generally, vitamin D3 is recommended over vitamin D2 due to enhanced efficacy in raising serum 25-hydroxyvitamin D levels.20
Vitamin D Deficiency in Alopecia
Although most recommendations are given in the interest of optimizing bone health, in the setting of alopecia, we set a similar serum 25-hydroxyvitamin D goal of greater than 30 ng/mL. We recommend treatment with vitamin D3 and practice the following repletion protocol: 50,000 IU once weekly for 4 weeks, followed by 1000 IU once daily for at least 8 weeks for serum 25-hydroxyvitamin D levels less than 20 ng/mL. For serum hydroxyvitamin D levels between 20 and 29 ng/mL, we recommend 1000 IU once daily for at least 12 weeks. We recheck blood levels again in 3 months. If levels fail to normalize, we will refer the patient to endocrinology. If levels return to normal, we transition to a daily multivitamin with vitamin D (400–800 IU) once daily and refer the patient back to the primary care physician for long-term monitoring.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687.
- Cheung EJ, Sink JR, English III JC. Vitamin and mineral deficiencies in patients with telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. 2016;15:1235-1237.
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107.
- Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S-564S.
- Lisse TS, Saini V, Zhao H, et al. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698-1706.
- Cianferotti L, Cox M, Skorjia K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function [published online May 17, 2007]. Porc Natl Acad Sci U S A. 2007;104:9428-9433.
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16.
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631-638.
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340-353.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-1154.
- Judge J, Birge S, Gloth F 3rd; American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Ahn J, Peters U, Albanes D, et al; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;4:100:796-804.
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers [published online June 18, 2010]. Am J Epidemiol. 2010;172:81-93.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204-210. Erratum in: 2003;78:1047.
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364.
Practice Points
- The evaluation of vitamin D levels is important in the management of nonscarring alopecia.
- Vitamin D deficiency can present as nonscarring alopecia not associated with alopecia areata, androgenetic alopecia, or telogen effluvium.