User login
Just Ride It Out?
ANSWER
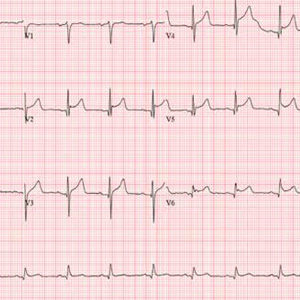
This ECG shows evidence of an acute anterior ST-elevation myocardial infarction (STEMI) and inferolateral ischemia.
STEMI criteria include a new ST-elevation in the anterior chest leads (V2–V4) at the J point (the junction of the termination of the QRS complex and the beginning of the ST segment). The American College of Cardiology/American Heart Association guidelines define cut-points for STEMI as follows: in women, 1.5 mm in leads V2 and V3 and 1 mm in all other leads; in men, 2 mm (those 40 and older) or 2.5 mm (those younger than 40) in leads V2 and V3 and 1 mm in all other leads. In this ECG, the classic “tombstone” pattern can be seen in the T wave in leads V3 and V4.
Inferolateral ischemia is evidenced by the ST elevation and T-wave flattening in the inferior leads II, III, and aVF, as well as in lateral chest leads V5 and V6.
The patient was transferred via ACLS ambulance to a tertiary care hospital for cardiac intervention
ANSWER
This ECG shows evidence of an acute anterior ST-elevation myocardial infarction (STEMI) and inferolateral ischemia.
STEMI criteria include a new ST-elevation in the anterior chest leads (V2–V4) at the J point (the junction of the termination of the QRS complex and the beginning of the ST segment). The American College of Cardiology/American Heart Association guidelines define cut-points for STEMI as follows: in women, 1.5 mm in leads V2 and V3 and 1 mm in all other leads; in men, 2 mm (those 40 and older) or 2.5 mm (those younger than 40) in leads V2 and V3 and 1 mm in all other leads. In this ECG, the classic “tombstone” pattern can be seen in the T wave in leads V3 and V4.
Inferolateral ischemia is evidenced by the ST elevation and T-wave flattening in the inferior leads II, III, and aVF, as well as in lateral chest leads V5 and V6.
The patient was transferred via ACLS ambulance to a tertiary care hospital for cardiac intervention
ANSWER
This ECG shows evidence of an acute anterior ST-elevation myocardial infarction (STEMI) and inferolateral ischemia.
STEMI criteria include a new ST-elevation in the anterior chest leads (V2–V4) at the J point (the junction of the termination of the QRS complex and the beginning of the ST segment). The American College of Cardiology/American Heart Association guidelines define cut-points for STEMI as follows: in women, 1.5 mm in leads V2 and V3 and 1 mm in all other leads; in men, 2 mm (those 40 and older) or 2.5 mm (those younger than 40) in leads V2 and V3 and 1 mm in all other leads. In this ECG, the classic “tombstone” pattern can be seen in the T wave in leads V3 and V4.
Inferolateral ischemia is evidenced by the ST elevation and T-wave flattening in the inferior leads II, III, and aVF, as well as in lateral chest leads V5 and V6.
The patient was transferred via ACLS ambulance to a tertiary care hospital for cardiac intervention
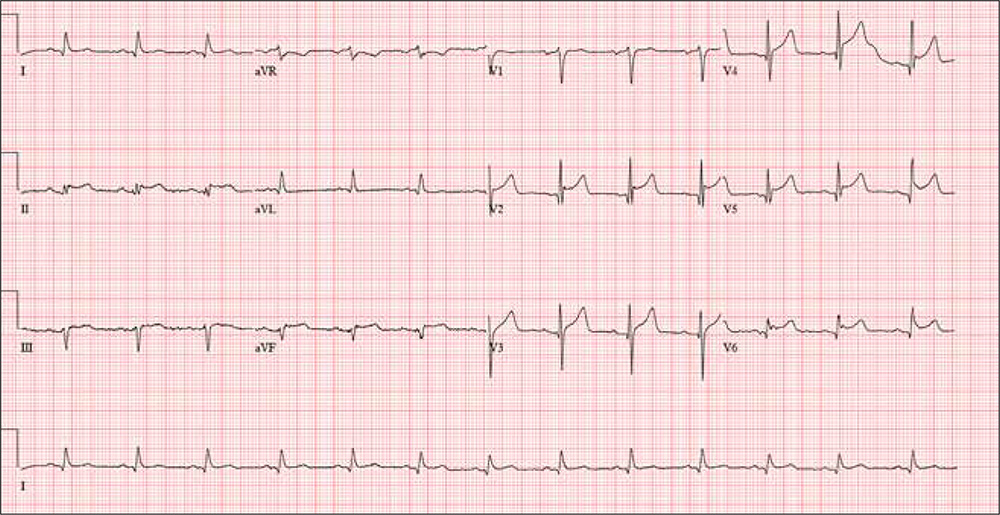
A 61-year-old avid cyclist is training for his third triathlon this year. About a week ago, after completing a 25-mile bike ride, he developed substernal chest discomfort, which he attributed to his longstanding history of GERD and his discontinuation of omeprazole. The discomfort resolved quickly with rest, and he was symptom free until two days ago. At that time, he “just rode through” the discomfort.
Today, however, about five miles into his ride, the discomfort was so severe that he stopped to wait for it to resolve. When it did not, he slowly rode another three miles to the urgent care center to request antacids and a prescription for a proton pump inhibitor.
He describes the problem as a combination of a burning sensation and a dull ache. It does not radiate beyond the substernal area. He denies shortness of breath and is not diaphoretic. He has never had hypertension, angina, dyspnea, syncope, or near-syncope.
His medical history is remarkable only for GERD. As a child, he sustained a nondisplaced fracture of his left clavicle. He has never had a surgical procedure.
The patient, an architect, is married with two adult children. He and his wife split a bottle of wine every other day, and he says he drinks 8 to 10 cups of coffee per day on average. He has never smoked tobacco or used recreational drugs.
Family history is remarkable for type 2 diabetes (father) and hypothyroidism (mother). Both parents are alive and otherwise well. The patient’s one sibling, a brother, died in a private plane accident.
Current medications include ibuprofen and chewable antacids. He took OTC omeprazole daily for about four months, but he recently ran out and hasn’t purchased more. He has a true anaphylactic allergy to sulfa.
Review of systems is unremarkable, other than typical muscle fatigue from cycling.
Vital signs include a blood pressure of 122/64 mm Hg; pulse, 80 beats/min, respiratory rate, 12 breaths/min-1; and temperature, 97.4°F. His height is 74 in and his weight, 184 lb.
The physical exam reveals an otherwise healthy-appearing male in no acute distress. He appears frustrated that he cannot get back out on his bike. There are no pertinent findings on physical exam. The lungs are clear, and the cardiac exam reveals a regular rate and rhythm with no murmurs, gallops, rubs, or extra heart sounds. The abdomen is soft and nontender, and there is no hepatojugular reflux. Peripheral pulses are strong bilaterally, and there are no neurologic focal signs.
The ECG reveals a ventricular rate of 80 beats/min; PR interval, 162 ms; QRS duration, 106 ms; QT/QTc interval, 370/426 ms; Q axis, 51°; R axis, –20°; and T axis, 70°. What is your interpretation?
Rebleeding and mortality after lower-GI bleeding in patients taking antiplatelets or anticoagulants
Clinical question: Is there a difference in lower GI rebleeding risk in patients on antiplatelet medications versus those on anticoagulation medications?
Background: It is estimated that 29%-37% of patient with GI bleeds are also on antiplatelet or anticoagulation medications. Minimal research has looked at outcomes for these populations and the comparative risk of rebleeding.
Study design: A retrospective study.
Setting: Multicenter study in the United Kingdom.
Synopsis: The study followed 2,528 patients with lower GI bleeds, 917 of whom were on antiplatelet or anticoagulation medications. Of these, 504 were on single-antiplatelet therapy, 79 on dual-antiplatelet therapy, 232 on warfarin, and 102 on direct-acting oral anticoagulants (DOACs). Patients on single-antiplatelet agents had a threefold increased risk of rebleeding (hazard ratio, 3.57), those on dual-antiplatelet agents had a fivefold increased risk of rebleeding (HR, 5.38), and patients taking warfarin or DOACs had no increased risk of rebleeding.
In addition, the authors concluded that there was no significant difference in rebleeding risk if antiplatelet medications were held for less than 5 days during hospitalization versus if they were continued. The risk of rebleeding with antiplatelet agents is likely caused by the relatively long half-lives of these therapies. In contrast, warfarin and DOACs have available reversal agents, and DOACs have comparatively shorter half-lives.
Bottom line: The risk of rebleeding from a lower-GI bleed is higher in patients on antiplatelet medications than it is in patients on warfarin or DOACs.
Citation: Oakland K et al. Rebleeding and mortality after lower gastrointestinal bleeding in patients taking antiplatelets or anticoagulants. Clin Gastroent Hepatol. 2017 Dec 23. doi: 10.1016/j.cgh.2017.12.032.
Dr. Thota is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: Is there a difference in lower GI rebleeding risk in patients on antiplatelet medications versus those on anticoagulation medications?
Background: It is estimated that 29%-37% of patient with GI bleeds are also on antiplatelet or anticoagulation medications. Minimal research has looked at outcomes for these populations and the comparative risk of rebleeding.
Study design: A retrospective study.
Setting: Multicenter study in the United Kingdom.
Synopsis: The study followed 2,528 patients with lower GI bleeds, 917 of whom were on antiplatelet or anticoagulation medications. Of these, 504 were on single-antiplatelet therapy, 79 on dual-antiplatelet therapy, 232 on warfarin, and 102 on direct-acting oral anticoagulants (DOACs). Patients on single-antiplatelet agents had a threefold increased risk of rebleeding (hazard ratio, 3.57), those on dual-antiplatelet agents had a fivefold increased risk of rebleeding (HR, 5.38), and patients taking warfarin or DOACs had no increased risk of rebleeding.
In addition, the authors concluded that there was no significant difference in rebleeding risk if antiplatelet medications were held for less than 5 days during hospitalization versus if they were continued. The risk of rebleeding with antiplatelet agents is likely caused by the relatively long half-lives of these therapies. In contrast, warfarin and DOACs have available reversal agents, and DOACs have comparatively shorter half-lives.
Bottom line: The risk of rebleeding from a lower-GI bleed is higher in patients on antiplatelet medications than it is in patients on warfarin or DOACs.
Citation: Oakland K et al. Rebleeding and mortality after lower gastrointestinal bleeding in patients taking antiplatelets or anticoagulants. Clin Gastroent Hepatol. 2017 Dec 23. doi: 10.1016/j.cgh.2017.12.032.
Dr. Thota is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: Is there a difference in lower GI rebleeding risk in patients on antiplatelet medications versus those on anticoagulation medications?
Background: It is estimated that 29%-37% of patient with GI bleeds are also on antiplatelet or anticoagulation medications. Minimal research has looked at outcomes for these populations and the comparative risk of rebleeding.
Study design: A retrospective study.
Setting: Multicenter study in the United Kingdom.
Synopsis: The study followed 2,528 patients with lower GI bleeds, 917 of whom were on antiplatelet or anticoagulation medications. Of these, 504 were on single-antiplatelet therapy, 79 on dual-antiplatelet therapy, 232 on warfarin, and 102 on direct-acting oral anticoagulants (DOACs). Patients on single-antiplatelet agents had a threefold increased risk of rebleeding (hazard ratio, 3.57), those on dual-antiplatelet agents had a fivefold increased risk of rebleeding (HR, 5.38), and patients taking warfarin or DOACs had no increased risk of rebleeding.
In addition, the authors concluded that there was no significant difference in rebleeding risk if antiplatelet medications were held for less than 5 days during hospitalization versus if they were continued. The risk of rebleeding with antiplatelet agents is likely caused by the relatively long half-lives of these therapies. In contrast, warfarin and DOACs have available reversal agents, and DOACs have comparatively shorter half-lives.
Bottom line: The risk of rebleeding from a lower-GI bleed is higher in patients on antiplatelet medications than it is in patients on warfarin or DOACs.
Citation: Oakland K et al. Rebleeding and mortality after lower gastrointestinal bleeding in patients taking antiplatelets or anticoagulants. Clin Gastroent Hepatol. 2017 Dec 23. doi: 10.1016/j.cgh.2017.12.032.
Dr. Thota is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
USPSTF: Insufficient evidence for ABI screening in asymptomatic adults
in asymptomatic adults without known cardiovascular or chronic kidney disease, according to the U.S. Preventive Services Task Force.
“A substantial number of asymptomatic persons with low ABI may never develop clinical signs or symptoms of CVD or PAD but would still be subjected to the harms of testing,” including false positives, exposure to gadolinium or contrast dye with subsequent imaging, and others, the Task Force wrote in JAMA.
In short, it found “inadequate evidence to assess whether screening for and treatment of PAD in asymptomatic patients leads to clinically important benefits in either preventing the progression of PAD or preventing CVD events. ... The current evidence is insufficient to assess the balance of benefits and harms of screening for PAD and CVD risk with the ABI in asymptomatic adults.”
The group made no recommendation, and issued an I statement, for insufficient evidence, July 10. The new work replaces the Task Force’s last visitation in 2013, which was also an “I statement.”
ABI is systolic blood pressure at the ankle divided by the systolic blood pressure in the arm while the patient is lying down. A ratio below 1 is considered abnormal.
ABI is low in perhaps about 6% of adults over 40 years old but “the natural history of screen-detected PAD, including the development of morbidity and mortality directly related to atherosclerosis in the lower limbs, is not well known. ... Large, population-based, randomized trials of screening [versus] no screening are needed to determine whether screening for PAD with the ABI improves clinical outcomes,” the task force said.
Among the many studies it reviewed were two large trials of asymptomatic women with low ABI treated with aspirin 100 mg/d for several years. Neither study showed any significant difference in CVD events, mortality, or development of intermittent claudication.
Even in high-risk people – diabetes, high blood pressure, high cholesterol, current tobacco use – there was “no compelling evidence” to support routine screening, so long as they have no symptoms.
The new review is broader than the group’s 2013 effort, and includes a broader population and range of interventions. Even so, “the recommendation remains an I statement,” it said.
The USPSTF is supported by the U.S. Agency for Healthcare Research and Quality.
SOURCE: JAMA. 2018 Jul 10;320(2):177-83
The USPSTF’s conclusion that current evidence is insufficient to recommend screening for PAD and cardiovascular risk with the ABI in asymptomatic adults should not be misconstrued as a determination that PAD is not common, clinically important, or associated with significant adverse outcomes.
The recommendation does not apply to people with ischemic symptoms during walking activity, who should be tested for PAD with the ABI. The ABI is important for diagnosing nonspecific leg symptoms, common in older people at risk for PAD who frequently have comorbidities such as spinal stenosis, arthritis, and neuropathy that contribute to leg symptoms. The USPSTF recommendation does not apply to these people.
Because most people with PAD do not have classic symptoms of intermittent claudication, the ABI is an important clinical tool for diagnosing PAD and identifying people at increased risk of cardiovascular events and functional decline.
Mary McDermott, MD , is a professor of internal medicine, geriatrics, and preventive medicine at Northwestern University, Chicago, and a JAMA senior editor. Michael Criqui, MD , is a professor of family medicine and public health at the University of California, San Diego. They made their comments in an editorial. Dr. McDermott disclosed research funding from Novartis and Regeneron ( JAMA. 2018 Jul 10;320[2]:143-5 ).
The USPSTF’s conclusion that current evidence is insufficient to recommend screening for PAD and cardiovascular risk with the ABI in asymptomatic adults should not be misconstrued as a determination that PAD is not common, clinically important, or associated with significant adverse outcomes.
The recommendation does not apply to people with ischemic symptoms during walking activity, who should be tested for PAD with the ABI. The ABI is important for diagnosing nonspecific leg symptoms, common in older people at risk for PAD who frequently have comorbidities such as spinal stenosis, arthritis, and neuropathy that contribute to leg symptoms. The USPSTF recommendation does not apply to these people.
Because most people with PAD do not have classic symptoms of intermittent claudication, the ABI is an important clinical tool for diagnosing PAD and identifying people at increased risk of cardiovascular events and functional decline.
Mary McDermott, MD , is a professor of internal medicine, geriatrics, and preventive medicine at Northwestern University, Chicago, and a JAMA senior editor. Michael Criqui, MD , is a professor of family medicine and public health at the University of California, San Diego. They made their comments in an editorial. Dr. McDermott disclosed research funding from Novartis and Regeneron ( JAMA. 2018 Jul 10;320[2]:143-5 ).
The USPSTF’s conclusion that current evidence is insufficient to recommend screening for PAD and cardiovascular risk with the ABI in asymptomatic adults should not be misconstrued as a determination that PAD is not common, clinically important, or associated with significant adverse outcomes.
The recommendation does not apply to people with ischemic symptoms during walking activity, who should be tested for PAD with the ABI. The ABI is important for diagnosing nonspecific leg symptoms, common in older people at risk for PAD who frequently have comorbidities such as spinal stenosis, arthritis, and neuropathy that contribute to leg symptoms. The USPSTF recommendation does not apply to these people.
Because most people with PAD do not have classic symptoms of intermittent claudication, the ABI is an important clinical tool for diagnosing PAD and identifying people at increased risk of cardiovascular events and functional decline.
Mary McDermott, MD , is a professor of internal medicine, geriatrics, and preventive medicine at Northwestern University, Chicago, and a JAMA senior editor. Michael Criqui, MD , is a professor of family medicine and public health at the University of California, San Diego. They made their comments in an editorial. Dr. McDermott disclosed research funding from Novartis and Regeneron ( JAMA. 2018 Jul 10;320[2]:143-5 ).
in asymptomatic adults without known cardiovascular or chronic kidney disease, according to the U.S. Preventive Services Task Force.
“A substantial number of asymptomatic persons with low ABI may never develop clinical signs or symptoms of CVD or PAD but would still be subjected to the harms of testing,” including false positives, exposure to gadolinium or contrast dye with subsequent imaging, and others, the Task Force wrote in JAMA.
In short, it found “inadequate evidence to assess whether screening for and treatment of PAD in asymptomatic patients leads to clinically important benefits in either preventing the progression of PAD or preventing CVD events. ... The current evidence is insufficient to assess the balance of benefits and harms of screening for PAD and CVD risk with the ABI in asymptomatic adults.”
The group made no recommendation, and issued an I statement, for insufficient evidence, July 10. The new work replaces the Task Force’s last visitation in 2013, which was also an “I statement.”
ABI is systolic blood pressure at the ankle divided by the systolic blood pressure in the arm while the patient is lying down. A ratio below 1 is considered abnormal.
ABI is low in perhaps about 6% of adults over 40 years old but “the natural history of screen-detected PAD, including the development of morbidity and mortality directly related to atherosclerosis in the lower limbs, is not well known. ... Large, population-based, randomized trials of screening [versus] no screening are needed to determine whether screening for PAD with the ABI improves clinical outcomes,” the task force said.
Among the many studies it reviewed were two large trials of asymptomatic women with low ABI treated with aspirin 100 mg/d for several years. Neither study showed any significant difference in CVD events, mortality, or development of intermittent claudication.
Even in high-risk people – diabetes, high blood pressure, high cholesterol, current tobacco use – there was “no compelling evidence” to support routine screening, so long as they have no symptoms.
The new review is broader than the group’s 2013 effort, and includes a broader population and range of interventions. Even so, “the recommendation remains an I statement,” it said.
The USPSTF is supported by the U.S. Agency for Healthcare Research and Quality.
SOURCE: JAMA. 2018 Jul 10;320(2):177-83
in asymptomatic adults without known cardiovascular or chronic kidney disease, according to the U.S. Preventive Services Task Force.
“A substantial number of asymptomatic persons with low ABI may never develop clinical signs or symptoms of CVD or PAD but would still be subjected to the harms of testing,” including false positives, exposure to gadolinium or contrast dye with subsequent imaging, and others, the Task Force wrote in JAMA.
In short, it found “inadequate evidence to assess whether screening for and treatment of PAD in asymptomatic patients leads to clinically important benefits in either preventing the progression of PAD or preventing CVD events. ... The current evidence is insufficient to assess the balance of benefits and harms of screening for PAD and CVD risk with the ABI in asymptomatic adults.”
The group made no recommendation, and issued an I statement, for insufficient evidence, July 10. The new work replaces the Task Force’s last visitation in 2013, which was also an “I statement.”
ABI is systolic blood pressure at the ankle divided by the systolic blood pressure in the arm while the patient is lying down. A ratio below 1 is considered abnormal.
ABI is low in perhaps about 6% of adults over 40 years old but “the natural history of screen-detected PAD, including the development of morbidity and mortality directly related to atherosclerosis in the lower limbs, is not well known. ... Large, population-based, randomized trials of screening [versus] no screening are needed to determine whether screening for PAD with the ABI improves clinical outcomes,” the task force said.
Among the many studies it reviewed were two large trials of asymptomatic women with low ABI treated with aspirin 100 mg/d for several years. Neither study showed any significant difference in CVD events, mortality, or development of intermittent claudication.
Even in high-risk people – diabetes, high blood pressure, high cholesterol, current tobacco use – there was “no compelling evidence” to support routine screening, so long as they have no symptoms.
The new review is broader than the group’s 2013 effort, and includes a broader population and range of interventions. Even so, “the recommendation remains an I statement,” it said.
The USPSTF is supported by the U.S. Agency for Healthcare Research and Quality.
SOURCE: JAMA. 2018 Jul 10;320(2):177-83
FROM JAMA
Antiamyloid antibody slowed Alzheimer’s progression while clearing brain amyloid
An antibody that targets clumps of soluble amyloid-beta before they aggregate into plaques has passed its phase 2 challenge, posting statistically significant results on a combined measure of cognition and function while decreasing amyloid deposition in the brain.
Full data won’t be released until a late-breaker session on July 25 at the Alzheimer’s Association International Conference in Chicago, so it’s still impossible to fully dissect the 18-month, dose exploration study. But according to codevelopers Eisai and Biogen, the significant clinical improvements accrued to the highest dose tested (10 mg/kg intravenously, twice a month) and were evident as early as 6 months. Amyloid cleared in a dose-dependent manner as well.
Although there is no available information on P values or effect sizes, the trial design designated success as at least a 25% reduction in the rate of decline over 1 year, relative to placebo.
In an interview, Lynn Kramer, MD, of Eisai declined to give further details. “I will say, however, that we believe these changes are clinically meaningful,” said Dr. Kramer, chief medical officer of the company’s neurology division.
These results make BAN2401 the first antiamyloid antibody to score significant results in both cognition and amyloid brain imaging a clinical trial, and warrant tempered optimism, according to Keith Fargo, PhD, director of scientific programs for the Alzheimer’s Association.
“We can’t know whether this is a breakthrough until we get past phase 3,” Dr. Fargo said in an interview. “In drug development, you’re never done until you’re done.”
Richard J. Caselli, MD, agreed with the tempered enthusiasm.
“This is very encouraging, but we await the full data and ultimately the phase 3 trial,” said Dr. Caselli, professor of neurology the Mayo Clinic Arizona in Scottsdale and is also associate director and clinical core director of the Arizona Alzheimer’s Disease Center. “I would also say that 25% slowing in disease progression is nice and unprecedented but clinically not exactly earth shattering. If it holds up in phase 3, the cost-benefit will need to be carefully weighed.”
Nevertheless, the publicly available data seem to lay a firm foundation for future studies, he said. “It’s unusual to see this kind of clarity in both clinical and biomarker results in a phase 2. There’s a history of large drug companies taking things from phase 2 to phase 3 on data that have been less clear than this, teasing out results from subgroups or using biomarker but not clinical data to make a decision on moving forward.”
The BAN2401 research team staked this commitment in the 2016 paper describing the study methodology. “They wanted to have clear evidence on both biomarker and clinical efficacy to allow them to make a decision [to move into phase 3],” Dr. Fargo said. “I don’t know if they are or not, but I’d be surprised if they don’t, and I think they would be on solid footing.”
BAN2401 selectively binds to amyloid-beta protofibrils – large AB oligomers that are still soluble – and targets them for clearance. It was originally developed by Swedish biopharma company BioArctic; Eisai acquired the molecule in 2007 and entered the Biogen deal in 2014.
The study randomized 856 patients with mild cognitive impairment or early Alzheimer’s dementia to six treatment arms: BAN2401 2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, and 10 mg/kg biweekly, or placebo. All patients had PET-confirmed amyloid brain pathology, a key baseline requirement; only one other antiamyloid agent (Biogen’s antiamyloid antibody aducanumab) has completed a phase 2 study in a purely amyloid-positive cohort. Before PET imaging, patients with non-Alzheimer’s pathology comprised up to 30% of Alzheimer’s drug studies, which researchers say confounded results and likely contributed to the long string of antiamyloid failures.
The coprimary endpoints were reduction of brain amyloid on PET scan and slowing of progression as measured by the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai. ADCOMS combines measures from the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog), Clinical Dementia Rating Sum of Boxes (CDR-sb), and the Mini-Mental State Examination (MMSE). In devising ADCOMS, researchers chose individual components of each tool that are most likely to change in very-early-stage disease. The scale was validated retrospectively in 1,160 patients who were included in four datasets. This is the first time it’s been used in an Alzheimer’s study.
The BAN2401 trial employed a Bayesian adaptive randomization, a computer-driven algorithm designed to drive patients to the two most effective doses. Often seen in trials of faster-progressing diseases, like cancer, it is unusual in Alzheimer’s studies, Dr. Fargo said.
“I would say it’s unorthodox, but it doesn’t give me heartburn. It’s an adaptive trial design that’s been considered hard to do in the AD field, because the changes between placebo and treatment groups tend to be very slow. In studies that have a very clear biomarker endpoint, like tumor size for example, that everyone can agree on, you can quickly see which treatment arm is most effective. In general, we haven’t been able to do this in AD because the signal changes so slowly.”
The BAN2401 algorithm assessed outcomes monthly, and reallocated incoming subjects according to the most recent efficacy data. But the 12-month primary endpoint assessment, a widely publicized failure, was “unfortunate,” Dr. Fargo said.
“This study had a built-in 12-month endpoint, at which time the sponsor could declare either futility or early success, and stop it for either one.” If neither was achieved, the study was to continue for 18 months and reassess outcomes. This was the situation Eisai and Biogen encountered in December when they announced that BAN2401 had failed to achieve its clinical and imaging endpoints but that the study would continue as planned. That news generated a large amount of negative press for the companies – which saw stock prices plunge – and contributed to media and consumer confusion about the trial design’s validity, Dr. Fargo said.
The 18-month data released on July 5 also included some information on adverse events. Amyloid-related imaging abnormalities, both edematous and hemorrhagic (ARIA-E and ARIA-H) occurred in an undisclosed number of patients. Dr. Kramer said ARIA occurred in “not more than 10% in any of the treatment arms” and in less than 15% of patients who carried an apolipoprotein E e4 allele. Only a portion of these patients were included in the final analysis, and this may have influenced the results somewhat negatively, Dr. Kramer said. In the first years of the study, before ARIA was understood to be largely subclinical and self-resolving, any patient who developed it was dropped.
“We initially dropped every patient who had ARIA, and this is one of the issues that actually make the observed effect [of BAN2401] probably a little bit conservative. Patients with ARIA are typically responding to amyloid removal, and they could be expected to do better.”
An antibody that targets clumps of soluble amyloid-beta before they aggregate into plaques has passed its phase 2 challenge, posting statistically significant results on a combined measure of cognition and function while decreasing amyloid deposition in the brain.
Full data won’t be released until a late-breaker session on July 25 at the Alzheimer’s Association International Conference in Chicago, so it’s still impossible to fully dissect the 18-month, dose exploration study. But according to codevelopers Eisai and Biogen, the significant clinical improvements accrued to the highest dose tested (10 mg/kg intravenously, twice a month) and were evident as early as 6 months. Amyloid cleared in a dose-dependent manner as well.
Although there is no available information on P values or effect sizes, the trial design designated success as at least a 25% reduction in the rate of decline over 1 year, relative to placebo.
In an interview, Lynn Kramer, MD, of Eisai declined to give further details. “I will say, however, that we believe these changes are clinically meaningful,” said Dr. Kramer, chief medical officer of the company’s neurology division.
These results make BAN2401 the first antiamyloid antibody to score significant results in both cognition and amyloid brain imaging a clinical trial, and warrant tempered optimism, according to Keith Fargo, PhD, director of scientific programs for the Alzheimer’s Association.
“We can’t know whether this is a breakthrough until we get past phase 3,” Dr. Fargo said in an interview. “In drug development, you’re never done until you’re done.”
Richard J. Caselli, MD, agreed with the tempered enthusiasm.
“This is very encouraging, but we await the full data and ultimately the phase 3 trial,” said Dr. Caselli, professor of neurology the Mayo Clinic Arizona in Scottsdale and is also associate director and clinical core director of the Arizona Alzheimer’s Disease Center. “I would also say that 25% slowing in disease progression is nice and unprecedented but clinically not exactly earth shattering. If it holds up in phase 3, the cost-benefit will need to be carefully weighed.”
Nevertheless, the publicly available data seem to lay a firm foundation for future studies, he said. “It’s unusual to see this kind of clarity in both clinical and biomarker results in a phase 2. There’s a history of large drug companies taking things from phase 2 to phase 3 on data that have been less clear than this, teasing out results from subgroups or using biomarker but not clinical data to make a decision on moving forward.”
The BAN2401 research team staked this commitment in the 2016 paper describing the study methodology. “They wanted to have clear evidence on both biomarker and clinical efficacy to allow them to make a decision [to move into phase 3],” Dr. Fargo said. “I don’t know if they are or not, but I’d be surprised if they don’t, and I think they would be on solid footing.”
BAN2401 selectively binds to amyloid-beta protofibrils – large AB oligomers that are still soluble – and targets them for clearance. It was originally developed by Swedish biopharma company BioArctic; Eisai acquired the molecule in 2007 and entered the Biogen deal in 2014.
The study randomized 856 patients with mild cognitive impairment or early Alzheimer’s dementia to six treatment arms: BAN2401 2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, and 10 mg/kg biweekly, or placebo. All patients had PET-confirmed amyloid brain pathology, a key baseline requirement; only one other antiamyloid agent (Biogen’s antiamyloid antibody aducanumab) has completed a phase 2 study in a purely amyloid-positive cohort. Before PET imaging, patients with non-Alzheimer’s pathology comprised up to 30% of Alzheimer’s drug studies, which researchers say confounded results and likely contributed to the long string of antiamyloid failures.
The coprimary endpoints were reduction of brain amyloid on PET scan and slowing of progression as measured by the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai. ADCOMS combines measures from the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog), Clinical Dementia Rating Sum of Boxes (CDR-sb), and the Mini-Mental State Examination (MMSE). In devising ADCOMS, researchers chose individual components of each tool that are most likely to change in very-early-stage disease. The scale was validated retrospectively in 1,160 patients who were included in four datasets. This is the first time it’s been used in an Alzheimer’s study.
The BAN2401 trial employed a Bayesian adaptive randomization, a computer-driven algorithm designed to drive patients to the two most effective doses. Often seen in trials of faster-progressing diseases, like cancer, it is unusual in Alzheimer’s studies, Dr. Fargo said.
“I would say it’s unorthodox, but it doesn’t give me heartburn. It’s an adaptive trial design that’s been considered hard to do in the AD field, because the changes between placebo and treatment groups tend to be very slow. In studies that have a very clear biomarker endpoint, like tumor size for example, that everyone can agree on, you can quickly see which treatment arm is most effective. In general, we haven’t been able to do this in AD because the signal changes so slowly.”
The BAN2401 algorithm assessed outcomes monthly, and reallocated incoming subjects according to the most recent efficacy data. But the 12-month primary endpoint assessment, a widely publicized failure, was “unfortunate,” Dr. Fargo said.
“This study had a built-in 12-month endpoint, at which time the sponsor could declare either futility or early success, and stop it for either one.” If neither was achieved, the study was to continue for 18 months and reassess outcomes. This was the situation Eisai and Biogen encountered in December when they announced that BAN2401 had failed to achieve its clinical and imaging endpoints but that the study would continue as planned. That news generated a large amount of negative press for the companies – which saw stock prices plunge – and contributed to media and consumer confusion about the trial design’s validity, Dr. Fargo said.
The 18-month data released on July 5 also included some information on adverse events. Amyloid-related imaging abnormalities, both edematous and hemorrhagic (ARIA-E and ARIA-H) occurred in an undisclosed number of patients. Dr. Kramer said ARIA occurred in “not more than 10% in any of the treatment arms” and in less than 15% of patients who carried an apolipoprotein E e4 allele. Only a portion of these patients were included in the final analysis, and this may have influenced the results somewhat negatively, Dr. Kramer said. In the first years of the study, before ARIA was understood to be largely subclinical and self-resolving, any patient who developed it was dropped.
“We initially dropped every patient who had ARIA, and this is one of the issues that actually make the observed effect [of BAN2401] probably a little bit conservative. Patients with ARIA are typically responding to amyloid removal, and they could be expected to do better.”
An antibody that targets clumps of soluble amyloid-beta before they aggregate into plaques has passed its phase 2 challenge, posting statistically significant results on a combined measure of cognition and function while decreasing amyloid deposition in the brain.
Full data won’t be released until a late-breaker session on July 25 at the Alzheimer’s Association International Conference in Chicago, so it’s still impossible to fully dissect the 18-month, dose exploration study. But according to codevelopers Eisai and Biogen, the significant clinical improvements accrued to the highest dose tested (10 mg/kg intravenously, twice a month) and were evident as early as 6 months. Amyloid cleared in a dose-dependent manner as well.
Although there is no available information on P values or effect sizes, the trial design designated success as at least a 25% reduction in the rate of decline over 1 year, relative to placebo.
In an interview, Lynn Kramer, MD, of Eisai declined to give further details. “I will say, however, that we believe these changes are clinically meaningful,” said Dr. Kramer, chief medical officer of the company’s neurology division.
These results make BAN2401 the first antiamyloid antibody to score significant results in both cognition and amyloid brain imaging a clinical trial, and warrant tempered optimism, according to Keith Fargo, PhD, director of scientific programs for the Alzheimer’s Association.
“We can’t know whether this is a breakthrough until we get past phase 3,” Dr. Fargo said in an interview. “In drug development, you’re never done until you’re done.”
Richard J. Caselli, MD, agreed with the tempered enthusiasm.
“This is very encouraging, but we await the full data and ultimately the phase 3 trial,” said Dr. Caselli, professor of neurology the Mayo Clinic Arizona in Scottsdale and is also associate director and clinical core director of the Arizona Alzheimer’s Disease Center. “I would also say that 25% slowing in disease progression is nice and unprecedented but clinically not exactly earth shattering. If it holds up in phase 3, the cost-benefit will need to be carefully weighed.”
Nevertheless, the publicly available data seem to lay a firm foundation for future studies, he said. “It’s unusual to see this kind of clarity in both clinical and biomarker results in a phase 2. There’s a history of large drug companies taking things from phase 2 to phase 3 on data that have been less clear than this, teasing out results from subgroups or using biomarker but not clinical data to make a decision on moving forward.”
The BAN2401 research team staked this commitment in the 2016 paper describing the study methodology. “They wanted to have clear evidence on both biomarker and clinical efficacy to allow them to make a decision [to move into phase 3],” Dr. Fargo said. “I don’t know if they are or not, but I’d be surprised if they don’t, and I think they would be on solid footing.”
BAN2401 selectively binds to amyloid-beta protofibrils – large AB oligomers that are still soluble – and targets them for clearance. It was originally developed by Swedish biopharma company BioArctic; Eisai acquired the molecule in 2007 and entered the Biogen deal in 2014.
The study randomized 856 patients with mild cognitive impairment or early Alzheimer’s dementia to six treatment arms: BAN2401 2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, and 10 mg/kg biweekly, or placebo. All patients had PET-confirmed amyloid brain pathology, a key baseline requirement; only one other antiamyloid agent (Biogen’s antiamyloid antibody aducanumab) has completed a phase 2 study in a purely amyloid-positive cohort. Before PET imaging, patients with non-Alzheimer’s pathology comprised up to 30% of Alzheimer’s drug studies, which researchers say confounded results and likely contributed to the long string of antiamyloid failures.
The coprimary endpoints were reduction of brain amyloid on PET scan and slowing of progression as measured by the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai. ADCOMS combines measures from the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog), Clinical Dementia Rating Sum of Boxes (CDR-sb), and the Mini-Mental State Examination (MMSE). In devising ADCOMS, researchers chose individual components of each tool that are most likely to change in very-early-stage disease. The scale was validated retrospectively in 1,160 patients who were included in four datasets. This is the first time it’s been used in an Alzheimer’s study.
The BAN2401 trial employed a Bayesian adaptive randomization, a computer-driven algorithm designed to drive patients to the two most effective doses. Often seen in trials of faster-progressing diseases, like cancer, it is unusual in Alzheimer’s studies, Dr. Fargo said.
“I would say it’s unorthodox, but it doesn’t give me heartburn. It’s an adaptive trial design that’s been considered hard to do in the AD field, because the changes between placebo and treatment groups tend to be very slow. In studies that have a very clear biomarker endpoint, like tumor size for example, that everyone can agree on, you can quickly see which treatment arm is most effective. In general, we haven’t been able to do this in AD because the signal changes so slowly.”
The BAN2401 algorithm assessed outcomes monthly, and reallocated incoming subjects according to the most recent efficacy data. But the 12-month primary endpoint assessment, a widely publicized failure, was “unfortunate,” Dr. Fargo said.
“This study had a built-in 12-month endpoint, at which time the sponsor could declare either futility or early success, and stop it for either one.” If neither was achieved, the study was to continue for 18 months and reassess outcomes. This was the situation Eisai and Biogen encountered in December when they announced that BAN2401 had failed to achieve its clinical and imaging endpoints but that the study would continue as planned. That news generated a large amount of negative press for the companies – which saw stock prices plunge – and contributed to media and consumer confusion about the trial design’s validity, Dr. Fargo said.
The 18-month data released on July 5 also included some information on adverse events. Amyloid-related imaging abnormalities, both edematous and hemorrhagic (ARIA-E and ARIA-H) occurred in an undisclosed number of patients. Dr. Kramer said ARIA occurred in “not more than 10% in any of the treatment arms” and in less than 15% of patients who carried an apolipoprotein E e4 allele. Only a portion of these patients were included in the final analysis, and this may have influenced the results somewhat negatively, Dr. Kramer said. In the first years of the study, before ARIA was understood to be largely subclinical and self-resolving, any patient who developed it was dropped.
“We initially dropped every patient who had ARIA, and this is one of the issues that actually make the observed effect [of BAN2401] probably a little bit conservative. Patients with ARIA are typically responding to amyloid removal, and they could be expected to do better.”
Countdown to launch: Health care IT primed for disruption
On Friday, June 29, at 5:42 a.m., I stood with my family on a Florida shore overlooking Kennedy Space Center. We had gathered with about a hundred other people to watch a rocket launch and were overwhelmed with excitement as the coastline erupted in fire, and the spacecraft lifted off toward the heavens. Standing there watching the spectacle, I couldn’t help but be caught up in the irony of the moment. Here we were, at the place where NASA sent the first Americans into space – on the very shores where the Apollo astronauts set off for the moon to plant our nation’s flag in the lunar dust in July of 1969. Yet now, almost 50 years later, this launch was profoundly different. The rocket wasn’t built by NASA, and the intention of its builders wasn’t exploration. This was a Falcon 9, built by SpaceX, a for-profit company founded by an enterprising billionaire. Most surprisingly, this relatively routine launch was intended to accomplish something that NASA – the United States’ own space agency – currently can’t do on its own: Launch rockets.
Since retiring the Space Shuttle in 2011, the United States has had to rely on others – including even Roscosmos (the Russian space agency) – to ferry passengers, satellites, and cargo into space. Seeing this opportunity in a multibillion-dollar industry, private enterprise has risen to the challenge, innovating more quickly and at a lower cost than “the establishment” has ever been capable of. As a result, space travel has been disrupted by corporations competing in a new “space race.” Instead of national pride or scientific dominance, this race has been fueled by profit and is quite similar to one being run in another industry: health care.
Just 1 day prior to watching the launch – on June 28 – we learned that Amazon had purchased PillPack, a prescription drug home delivery service. The stock market responded to the news, and the establishment (in this case CVS, Walgreen’s, and WalMart, among others) collectively lost $17.5 billion in one day. This isn’t the first time Amazon has disrupted the health care world; in January of this year, they, along with Berkshire Hathaway and JPMorgan Chase, announced a health care partnership to cut costs and improve care delivery for their employees. This move also sent shivers through the market, as health insurers and providers such as Aetna and United Health lost big on expectations that Amazon et al. wouldn’t stop with their own employees. Those of us watching this play out from the sidelines realized we were witnessing a revolution that would mean the end of health care delivery as we know it – and that’s not necessarily a bad thing, especially in the world of Electronic Health Records.
As you’ve probably noticed, it is quite rare to find physicians nowadays who love computers. Once an exciting novelty in health care, PCs have become a burdensome necessity and providers often feel enslaved to the EHRs that run on them. There are numerous reasons for this, but one primary cause is that the hundreds of disparate EHRs currently available sprouted out of health care – a centuries-old and very provincial industry – prior to the development of technical and regulatory standards to govern them. As they’ve grown larger and larger from their primitive underpinnings, these EHRs have become more cumbersome to navigate, and vendors have simply “bolted-on” additional features without significant changes to their near-obsolete software architecture.
It’s worth noting that a few EHR companies purport to be true innovators in platform usability, such as industry giant, Epic. According to CEO Judy Faulkner, Epic pours 50% of their revenue back into research and development (though, as Epic is a privately held company, this number can’t be verified). If accurate, Epic is truly an exception, as most electronic record companies spend about 10%-30% on improving their products – far less than they spend on recruiting new customers. Regardless, the outcome is this: Physician expectations for user interface and user experience have far outpaced the current state of the art of EHRs, and this has left a gap that new players outside the health care establishment are apt to fill.
Like Amazon, other software giants have made significant investments in health care over the past several years. According to their website, Apple has been working with hospitals, scientists, and developers to “help health care providers streamline their work, deliver better care, and conduct medical research.” Similarly, Google claims to be “making a number of big bets in health care and life sciences,” by leveraging their artificial intelligence technology to assist in clinical diagnosis and scientific discovery. In spite of a few false starts in the past, these companies are poised to do more than simply disrupt health care. As experts in user interface and design, they could truly change the way physicians interact with health care technology, and it seems like it’s no longer a question of if, but when we’ll see that happen.
The effort of SpaceX and others to change the way we launch rockets tells a story that transcends space travel – It’s a story of how new thinking, more efficient processes, and better design can disrupt the establishment. It’s worth pointing out that NASA hasn’t given up – they are continuing to develop the Space Launch System, which, when completed, will be the most powerful rocket in the world and be capable of carrying astronauts into deep space. In the meantime, however, NASA is embracing the efforts of private industry to help pave a better way forward and make space travel safer and more accessible for everyone. We are hopeful that EHR vendors and other establishment health care institutions are taking note, adapting to meet the needs of the current generation of physicians and patients, and innovating a better way to launch health care into the future.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on twitter (@doctornotte). Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
On Friday, June 29, at 5:42 a.m., I stood with my family on a Florida shore overlooking Kennedy Space Center. We had gathered with about a hundred other people to watch a rocket launch and were overwhelmed with excitement as the coastline erupted in fire, and the spacecraft lifted off toward the heavens. Standing there watching the spectacle, I couldn’t help but be caught up in the irony of the moment. Here we were, at the place where NASA sent the first Americans into space – on the very shores where the Apollo astronauts set off for the moon to plant our nation’s flag in the lunar dust in July of 1969. Yet now, almost 50 years later, this launch was profoundly different. The rocket wasn’t built by NASA, and the intention of its builders wasn’t exploration. This was a Falcon 9, built by SpaceX, a for-profit company founded by an enterprising billionaire. Most surprisingly, this relatively routine launch was intended to accomplish something that NASA – the United States’ own space agency – currently can’t do on its own: Launch rockets.
Since retiring the Space Shuttle in 2011, the United States has had to rely on others – including even Roscosmos (the Russian space agency) – to ferry passengers, satellites, and cargo into space. Seeing this opportunity in a multibillion-dollar industry, private enterprise has risen to the challenge, innovating more quickly and at a lower cost than “the establishment” has ever been capable of. As a result, space travel has been disrupted by corporations competing in a new “space race.” Instead of national pride or scientific dominance, this race has been fueled by profit and is quite similar to one being run in another industry: health care.
Just 1 day prior to watching the launch – on June 28 – we learned that Amazon had purchased PillPack, a prescription drug home delivery service. The stock market responded to the news, and the establishment (in this case CVS, Walgreen’s, and WalMart, among others) collectively lost $17.5 billion in one day. This isn’t the first time Amazon has disrupted the health care world; in January of this year, they, along with Berkshire Hathaway and JPMorgan Chase, announced a health care partnership to cut costs and improve care delivery for their employees. This move also sent shivers through the market, as health insurers and providers such as Aetna and United Health lost big on expectations that Amazon et al. wouldn’t stop with their own employees. Those of us watching this play out from the sidelines realized we were witnessing a revolution that would mean the end of health care delivery as we know it – and that’s not necessarily a bad thing, especially in the world of Electronic Health Records.
As you’ve probably noticed, it is quite rare to find physicians nowadays who love computers. Once an exciting novelty in health care, PCs have become a burdensome necessity and providers often feel enslaved to the EHRs that run on them. There are numerous reasons for this, but one primary cause is that the hundreds of disparate EHRs currently available sprouted out of health care – a centuries-old and very provincial industry – prior to the development of technical and regulatory standards to govern them. As they’ve grown larger and larger from their primitive underpinnings, these EHRs have become more cumbersome to navigate, and vendors have simply “bolted-on” additional features without significant changes to their near-obsolete software architecture.
It’s worth noting that a few EHR companies purport to be true innovators in platform usability, such as industry giant, Epic. According to CEO Judy Faulkner, Epic pours 50% of their revenue back into research and development (though, as Epic is a privately held company, this number can’t be verified). If accurate, Epic is truly an exception, as most electronic record companies spend about 10%-30% on improving their products – far less than they spend on recruiting new customers. Regardless, the outcome is this: Physician expectations for user interface and user experience have far outpaced the current state of the art of EHRs, and this has left a gap that new players outside the health care establishment are apt to fill.
Like Amazon, other software giants have made significant investments in health care over the past several years. According to their website, Apple has been working with hospitals, scientists, and developers to “help health care providers streamline their work, deliver better care, and conduct medical research.” Similarly, Google claims to be “making a number of big bets in health care and life sciences,” by leveraging their artificial intelligence technology to assist in clinical diagnosis and scientific discovery. In spite of a few false starts in the past, these companies are poised to do more than simply disrupt health care. As experts in user interface and design, they could truly change the way physicians interact with health care technology, and it seems like it’s no longer a question of if, but when we’ll see that happen.
The effort of SpaceX and others to change the way we launch rockets tells a story that transcends space travel – It’s a story of how new thinking, more efficient processes, and better design can disrupt the establishment. It’s worth pointing out that NASA hasn’t given up – they are continuing to develop the Space Launch System, which, when completed, will be the most powerful rocket in the world and be capable of carrying astronauts into deep space. In the meantime, however, NASA is embracing the efforts of private industry to help pave a better way forward and make space travel safer and more accessible for everyone. We are hopeful that EHR vendors and other establishment health care institutions are taking note, adapting to meet the needs of the current generation of physicians and patients, and innovating a better way to launch health care into the future.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on twitter (@doctornotte). Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
On Friday, June 29, at 5:42 a.m., I stood with my family on a Florida shore overlooking Kennedy Space Center. We had gathered with about a hundred other people to watch a rocket launch and were overwhelmed with excitement as the coastline erupted in fire, and the spacecraft lifted off toward the heavens. Standing there watching the spectacle, I couldn’t help but be caught up in the irony of the moment. Here we were, at the place where NASA sent the first Americans into space – on the very shores where the Apollo astronauts set off for the moon to plant our nation’s flag in the lunar dust in July of 1969. Yet now, almost 50 years later, this launch was profoundly different. The rocket wasn’t built by NASA, and the intention of its builders wasn’t exploration. This was a Falcon 9, built by SpaceX, a for-profit company founded by an enterprising billionaire. Most surprisingly, this relatively routine launch was intended to accomplish something that NASA – the United States’ own space agency – currently can’t do on its own: Launch rockets.
Since retiring the Space Shuttle in 2011, the United States has had to rely on others – including even Roscosmos (the Russian space agency) – to ferry passengers, satellites, and cargo into space. Seeing this opportunity in a multibillion-dollar industry, private enterprise has risen to the challenge, innovating more quickly and at a lower cost than “the establishment” has ever been capable of. As a result, space travel has been disrupted by corporations competing in a new “space race.” Instead of national pride or scientific dominance, this race has been fueled by profit and is quite similar to one being run in another industry: health care.
Just 1 day prior to watching the launch – on June 28 – we learned that Amazon had purchased PillPack, a prescription drug home delivery service. The stock market responded to the news, and the establishment (in this case CVS, Walgreen’s, and WalMart, among others) collectively lost $17.5 billion in one day. This isn’t the first time Amazon has disrupted the health care world; in January of this year, they, along with Berkshire Hathaway and JPMorgan Chase, announced a health care partnership to cut costs and improve care delivery for their employees. This move also sent shivers through the market, as health insurers and providers such as Aetna and United Health lost big on expectations that Amazon et al. wouldn’t stop with their own employees. Those of us watching this play out from the sidelines realized we were witnessing a revolution that would mean the end of health care delivery as we know it – and that’s not necessarily a bad thing, especially in the world of Electronic Health Records.
As you’ve probably noticed, it is quite rare to find physicians nowadays who love computers. Once an exciting novelty in health care, PCs have become a burdensome necessity and providers often feel enslaved to the EHRs that run on them. There are numerous reasons for this, but one primary cause is that the hundreds of disparate EHRs currently available sprouted out of health care – a centuries-old and very provincial industry – prior to the development of technical and regulatory standards to govern them. As they’ve grown larger and larger from their primitive underpinnings, these EHRs have become more cumbersome to navigate, and vendors have simply “bolted-on” additional features without significant changes to their near-obsolete software architecture.
It’s worth noting that a few EHR companies purport to be true innovators in platform usability, such as industry giant, Epic. According to CEO Judy Faulkner, Epic pours 50% of their revenue back into research and development (though, as Epic is a privately held company, this number can’t be verified). If accurate, Epic is truly an exception, as most electronic record companies spend about 10%-30% on improving their products – far less than they spend on recruiting new customers. Regardless, the outcome is this: Physician expectations for user interface and user experience have far outpaced the current state of the art of EHRs, and this has left a gap that new players outside the health care establishment are apt to fill.
Like Amazon, other software giants have made significant investments in health care over the past several years. According to their website, Apple has been working with hospitals, scientists, and developers to “help health care providers streamline their work, deliver better care, and conduct medical research.” Similarly, Google claims to be “making a number of big bets in health care and life sciences,” by leveraging their artificial intelligence technology to assist in clinical diagnosis and scientific discovery. In spite of a few false starts in the past, these companies are poised to do more than simply disrupt health care. As experts in user interface and design, they could truly change the way physicians interact with health care technology, and it seems like it’s no longer a question of if, but when we’ll see that happen.
The effort of SpaceX and others to change the way we launch rockets tells a story that transcends space travel – It’s a story of how new thinking, more efficient processes, and better design can disrupt the establishment. It’s worth pointing out that NASA hasn’t given up – they are continuing to develop the Space Launch System, which, when completed, will be the most powerful rocket in the world and be capable of carrying astronauts into deep space. In the meantime, however, NASA is embracing the efforts of private industry to help pave a better way forward and make space travel safer and more accessible for everyone. We are hopeful that EHR vendors and other establishment health care institutions are taking note, adapting to meet the needs of the current generation of physicians and patients, and innovating a better way to launch health care into the future.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on twitter (@doctornotte). Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
What might Kavanaugh confirmation mean for ACA, abortion access?
In 2011, Judge Kavanaugh wrote a dissenting opinion on Seven-Sky v. Holder, noting that the ACA’s individual mandate was in fact a tax and that the court could not hear questions about the law’s constitutionality because the tax had not been levied.
That dissenting opinion could become germane in the currently debated Texas v. Azar, which looks destined to reach the high court.
In Texas v. Azar, the Department of Justice has refused to defend the ACA and is siding with Texas and 19 other states. Texas and 19 other states in the case contend that the penalty for not carrying health insurance under the individual mandate is a tax; however, since the penalty was reduced to zero by the Tax Cuts and Jobs Act of 2017, the plaintiffs argue that the individual mandate is unconstitutional. They further contend that the ACA provisions of guaranteed issue and community rating are inseverable and should be eliminated as well.
If confirmed, Judge Kavanaugh’s views that the penalty for the individual mandate is in fact a tax could shift the Supreme Court’s majority view, further putting the law in jeopardy.
Judge Kavanaugh currently serves on the U.S. Court of Appeals for the District of Columbia Circuit, he was appointed by President George W. Bush and has held the position since May 30, 2006. He previously served as White House staff secretary for nearly 3 years.
As an attorney, he had a lead role in drafting the Starr Report, which recommended impeachment of President Bill Clinton.
Judge Kavanaugh will face a contentious confirmation process. In addition to health care, abortion rights will be front and center to the debate as moderate Republican senators who are proponents of abortion rights may not support his nomination. The GOP currently holds a slim 51-49 majority in the Senate, but with the medical absence of Sen. John McCain (R-Ariz.), the majority party may only have 50 members present to vote, meaning only one GOP vote against Judge Kavanaugh could deny his confirmation.
Judge Kavanaugh does not have a stated position on Roe v. Wade; however, his dissenting opinion in Garza v. Hargan questioned whether the government should be involved in provisioning abortions for undocumented immigrant minors.
That opinion is a reason Physicians for Reproductive Health have come out against Judge Kavanaugh’s nomination.
“The Trump administration has already enacted numerous policies restricting access to vital reproductive health services, including contraception, abortion, and maternity care, and will continue to chip away at health care access,” Willie Parker, MD, board chair of Physicians for Reproductive Health, said in a statement. “This nominee will harm not only abortion rights, but will imperil other fundamental rights as well.”
Senate Majority Leader Mitch McConnell said in a statement that Judge Kavanaugh “understands that, in the United States of America, judges are not unelected super-legislators whom we select for their personal views or policy preferences. A judge’s duty is to interpret the plain meaning of our laws and our Constitution according to how they are written. Judges need to be unbiased. They need to treat all parties fairly. They need to approach every case with open ears and an open mind. Judges’ decisions must turn on the facts of each case and be based on the texts that it is their job to interpret. By all accounts, Judge Kavanaugh is precisely that sort of judge.”
Sen. Susan Collins (R-Maine), thought to be one of the potential swing voters that will determine whether Judge Kavanaugh’s appointment is approved, said in a statement that he “has impressive credentials and extensive experience, having served more than a decade on the D.C. Circuit Court of Appeals. I will conduct a careful, thorough vetting of the President’s nominee to the Supreme Court, as I have done with the five previous Supreme Court Justices whom I have considered. I look forward to Judge Kavanaugh’s public hearing before the Senate Judiciary Committee and to questioning him in a meeting in my office.”
President Trump nominated Judge Kavanaugh to the Supreme Court on July 9 to replace the retiring Justice Anthony Kennedy.
In 2011, Judge Kavanaugh wrote a dissenting opinion on Seven-Sky v. Holder, noting that the ACA’s individual mandate was in fact a tax and that the court could not hear questions about the law’s constitutionality because the tax had not been levied.
That dissenting opinion could become germane in the currently debated Texas v. Azar, which looks destined to reach the high court.
In Texas v. Azar, the Department of Justice has refused to defend the ACA and is siding with Texas and 19 other states. Texas and 19 other states in the case contend that the penalty for not carrying health insurance under the individual mandate is a tax; however, since the penalty was reduced to zero by the Tax Cuts and Jobs Act of 2017, the plaintiffs argue that the individual mandate is unconstitutional. They further contend that the ACA provisions of guaranteed issue and community rating are inseverable and should be eliminated as well.
If confirmed, Judge Kavanaugh’s views that the penalty for the individual mandate is in fact a tax could shift the Supreme Court’s majority view, further putting the law in jeopardy.
Judge Kavanaugh currently serves on the U.S. Court of Appeals for the District of Columbia Circuit, he was appointed by President George W. Bush and has held the position since May 30, 2006. He previously served as White House staff secretary for nearly 3 years.
As an attorney, he had a lead role in drafting the Starr Report, which recommended impeachment of President Bill Clinton.
Judge Kavanaugh will face a contentious confirmation process. In addition to health care, abortion rights will be front and center to the debate as moderate Republican senators who are proponents of abortion rights may not support his nomination. The GOP currently holds a slim 51-49 majority in the Senate, but with the medical absence of Sen. John McCain (R-Ariz.), the majority party may only have 50 members present to vote, meaning only one GOP vote against Judge Kavanaugh could deny his confirmation.
Judge Kavanaugh does not have a stated position on Roe v. Wade; however, his dissenting opinion in Garza v. Hargan questioned whether the government should be involved in provisioning abortions for undocumented immigrant minors.
That opinion is a reason Physicians for Reproductive Health have come out against Judge Kavanaugh’s nomination.
“The Trump administration has already enacted numerous policies restricting access to vital reproductive health services, including contraception, abortion, and maternity care, and will continue to chip away at health care access,” Willie Parker, MD, board chair of Physicians for Reproductive Health, said in a statement. “This nominee will harm not only abortion rights, but will imperil other fundamental rights as well.”
Senate Majority Leader Mitch McConnell said in a statement that Judge Kavanaugh “understands that, in the United States of America, judges are not unelected super-legislators whom we select for their personal views or policy preferences. A judge’s duty is to interpret the plain meaning of our laws and our Constitution according to how they are written. Judges need to be unbiased. They need to treat all parties fairly. They need to approach every case with open ears and an open mind. Judges’ decisions must turn on the facts of each case and be based on the texts that it is their job to interpret. By all accounts, Judge Kavanaugh is precisely that sort of judge.”
Sen. Susan Collins (R-Maine), thought to be one of the potential swing voters that will determine whether Judge Kavanaugh’s appointment is approved, said in a statement that he “has impressive credentials and extensive experience, having served more than a decade on the D.C. Circuit Court of Appeals. I will conduct a careful, thorough vetting of the President’s nominee to the Supreme Court, as I have done with the five previous Supreme Court Justices whom I have considered. I look forward to Judge Kavanaugh’s public hearing before the Senate Judiciary Committee and to questioning him in a meeting in my office.”
President Trump nominated Judge Kavanaugh to the Supreme Court on July 9 to replace the retiring Justice Anthony Kennedy.
In 2011, Judge Kavanaugh wrote a dissenting opinion on Seven-Sky v. Holder, noting that the ACA’s individual mandate was in fact a tax and that the court could not hear questions about the law’s constitutionality because the tax had not been levied.
That dissenting opinion could become germane in the currently debated Texas v. Azar, which looks destined to reach the high court.
In Texas v. Azar, the Department of Justice has refused to defend the ACA and is siding with Texas and 19 other states. Texas and 19 other states in the case contend that the penalty for not carrying health insurance under the individual mandate is a tax; however, since the penalty was reduced to zero by the Tax Cuts and Jobs Act of 2017, the plaintiffs argue that the individual mandate is unconstitutional. They further contend that the ACA provisions of guaranteed issue and community rating are inseverable and should be eliminated as well.
If confirmed, Judge Kavanaugh’s views that the penalty for the individual mandate is in fact a tax could shift the Supreme Court’s majority view, further putting the law in jeopardy.
Judge Kavanaugh currently serves on the U.S. Court of Appeals for the District of Columbia Circuit, he was appointed by President George W. Bush and has held the position since May 30, 2006. He previously served as White House staff secretary for nearly 3 years.
As an attorney, he had a lead role in drafting the Starr Report, which recommended impeachment of President Bill Clinton.
Judge Kavanaugh will face a contentious confirmation process. In addition to health care, abortion rights will be front and center to the debate as moderate Republican senators who are proponents of abortion rights may not support his nomination. The GOP currently holds a slim 51-49 majority in the Senate, but with the medical absence of Sen. John McCain (R-Ariz.), the majority party may only have 50 members present to vote, meaning only one GOP vote against Judge Kavanaugh could deny his confirmation.
Judge Kavanaugh does not have a stated position on Roe v. Wade; however, his dissenting opinion in Garza v. Hargan questioned whether the government should be involved in provisioning abortions for undocumented immigrant minors.
That opinion is a reason Physicians for Reproductive Health have come out against Judge Kavanaugh’s nomination.
“The Trump administration has already enacted numerous policies restricting access to vital reproductive health services, including contraception, abortion, and maternity care, and will continue to chip away at health care access,” Willie Parker, MD, board chair of Physicians for Reproductive Health, said in a statement. “This nominee will harm not only abortion rights, but will imperil other fundamental rights as well.”
Senate Majority Leader Mitch McConnell said in a statement that Judge Kavanaugh “understands that, in the United States of America, judges are not unelected super-legislators whom we select for their personal views or policy preferences. A judge’s duty is to interpret the plain meaning of our laws and our Constitution according to how they are written. Judges need to be unbiased. They need to treat all parties fairly. They need to approach every case with open ears and an open mind. Judges’ decisions must turn on the facts of each case and be based on the texts that it is their job to interpret. By all accounts, Judge Kavanaugh is precisely that sort of judge.”
Sen. Susan Collins (R-Maine), thought to be one of the potential swing voters that will determine whether Judge Kavanaugh’s appointment is approved, said in a statement that he “has impressive credentials and extensive experience, having served more than a decade on the D.C. Circuit Court of Appeals. I will conduct a careful, thorough vetting of the President’s nominee to the Supreme Court, as I have done with the five previous Supreme Court Justices whom I have considered. I look forward to Judge Kavanaugh’s public hearing before the Senate Judiciary Committee and to questioning him in a meeting in my office.”
President Trump nominated Judge Kavanaugh to the Supreme Court on July 9 to replace the retiring Justice Anthony Kennedy.
Migraine with Aura Linked with TIA Readmission
In a large, nationally representative retrospective cohort study, migraine admission with aura was independently associated with transient ischemic attack (TIA) readmission, and status migrainosus was independently associated with subarachnoid hemorrhage. The Nationwide Readmissions Database was designed to analyze readmissions for all payers and uninsured, with data on more than 14 million US admissions in 2013. Researchers identified index migraine admissions with and without aura or status migrainosus, and readmissions for cerebrovascular events. Cox proportional hazards regression was performed for each outcome with aura and status migrainosus as main predictors, adjusting for age and vascular risk factors. They found:
- Out of 12,448 index admissions for migraine, 9972 (80.1%) were women, mean age was 45.5 ± 14.8 years, aura was present in 3038 (24.41%), and status migrainosus in 1798 (14.44%).
- The 30‐day readmission rate (per 100,000 index admissions) was 154 for ischemic stroke, 86 for TIA, 42 for subarachnoid hemorrhage, and 17 for intracranial hemorrhage.
- In unadjusted models, aura was significantly associated with TIA (hazard ratio 2.43), but not acute ischemic stroke (1.26), intracranial hemorrhage (1.86), or subarachnoid hemorrhage (1.85).
Velickovic Osotjic L, Liang JW, Sheikh HU, Dhamoon MS. Impact of aura and status migrainosus on readmissions for vascular events after migraine admission. [Published online ahead of print June 22, 2018]. Headache. doi:10.1111/head.13347.
In a large, nationally representative retrospective cohort study, migraine admission with aura was independently associated with transient ischemic attack (TIA) readmission, and status migrainosus was independently associated with subarachnoid hemorrhage. The Nationwide Readmissions Database was designed to analyze readmissions for all payers and uninsured, with data on more than 14 million US admissions in 2013. Researchers identified index migraine admissions with and without aura or status migrainosus, and readmissions for cerebrovascular events. Cox proportional hazards regression was performed for each outcome with aura and status migrainosus as main predictors, adjusting for age and vascular risk factors. They found:
- Out of 12,448 index admissions for migraine, 9972 (80.1%) were women, mean age was 45.5 ± 14.8 years, aura was present in 3038 (24.41%), and status migrainosus in 1798 (14.44%).
- The 30‐day readmission rate (per 100,000 index admissions) was 154 for ischemic stroke, 86 for TIA, 42 for subarachnoid hemorrhage, and 17 for intracranial hemorrhage.
- In unadjusted models, aura was significantly associated with TIA (hazard ratio 2.43), but not acute ischemic stroke (1.26), intracranial hemorrhage (1.86), or subarachnoid hemorrhage (1.85).
Velickovic Osotjic L, Liang JW, Sheikh HU, Dhamoon MS. Impact of aura and status migrainosus on readmissions for vascular events after migraine admission. [Published online ahead of print June 22, 2018]. Headache. doi:10.1111/head.13347.
In a large, nationally representative retrospective cohort study, migraine admission with aura was independently associated with transient ischemic attack (TIA) readmission, and status migrainosus was independently associated with subarachnoid hemorrhage. The Nationwide Readmissions Database was designed to analyze readmissions for all payers and uninsured, with data on more than 14 million US admissions in 2013. Researchers identified index migraine admissions with and without aura or status migrainosus, and readmissions for cerebrovascular events. Cox proportional hazards regression was performed for each outcome with aura and status migrainosus as main predictors, adjusting for age and vascular risk factors. They found:
- Out of 12,448 index admissions for migraine, 9972 (80.1%) were women, mean age was 45.5 ± 14.8 years, aura was present in 3038 (24.41%), and status migrainosus in 1798 (14.44%).
- The 30‐day readmission rate (per 100,000 index admissions) was 154 for ischemic stroke, 86 for TIA, 42 for subarachnoid hemorrhage, and 17 for intracranial hemorrhage.
- In unadjusted models, aura was significantly associated with TIA (hazard ratio 2.43), but not acute ischemic stroke (1.26), intracranial hemorrhage (1.86), or subarachnoid hemorrhage (1.85).
Velickovic Osotjic L, Liang JW, Sheikh HU, Dhamoon MS. Impact of aura and status migrainosus on readmissions for vascular events after migraine admission. [Published online ahead of print June 22, 2018]. Headache. doi:10.1111/head.13347.
Headache/Migraine Apps Sharing Information
Headache smartphone applications (apps) shared information with third parties, posing privacy risks partly because there are few legal protections against the sale or disclosure of data from medical apps to third parties. This is according to a recent study that sought to assess whether there are privacy issues surrounding apps and the potential privacy implications of how the app companies (and other third parties) might use that information. Researchers conducted a systematic search of the most popular “headache” and “migraine” apps and developed a database of the types of data the apps requested for input by the user, and whether the apps had clear privacy policies. They also examined the content of the privacy policies and found:
- Twenty-nine apps were examined (14 diary apps, 15 relaxation apps).
- Of the diary applications, 79% (11/14) had visible privacy policies.
- Of the diary apps with privacy policies, all (11/11) stated whether or not the app collects and stores information remotely.
- A total of 55% (6/11) stated that some user data were used to serve targeted advertisements.
- A total of 11/15 (73%) of the relaxation apps had privacy policies.
Minen MT, Stieglitz EJ, Sciortino R, Torous J. Privacy issues in smartphone applications: An analysis of headache/migraine applications. [Published online ahead of print July 4, 2018]. Headache. doi:10.1111/head.13341.
Headache smartphone applications (apps) shared information with third parties, posing privacy risks partly because there are few legal protections against the sale or disclosure of data from medical apps to third parties. This is according to a recent study that sought to assess whether there are privacy issues surrounding apps and the potential privacy implications of how the app companies (and other third parties) might use that information. Researchers conducted a systematic search of the most popular “headache” and “migraine” apps and developed a database of the types of data the apps requested for input by the user, and whether the apps had clear privacy policies. They also examined the content of the privacy policies and found:
- Twenty-nine apps were examined (14 diary apps, 15 relaxation apps).
- Of the diary applications, 79% (11/14) had visible privacy policies.
- Of the diary apps with privacy policies, all (11/11) stated whether or not the app collects and stores information remotely.
- A total of 55% (6/11) stated that some user data were used to serve targeted advertisements.
- A total of 11/15 (73%) of the relaxation apps had privacy policies.
Minen MT, Stieglitz EJ, Sciortino R, Torous J. Privacy issues in smartphone applications: An analysis of headache/migraine applications. [Published online ahead of print July 4, 2018]. Headache. doi:10.1111/head.13341.
Headache smartphone applications (apps) shared information with third parties, posing privacy risks partly because there are few legal protections against the sale or disclosure of data from medical apps to third parties. This is according to a recent study that sought to assess whether there are privacy issues surrounding apps and the potential privacy implications of how the app companies (and other third parties) might use that information. Researchers conducted a systematic search of the most popular “headache” and “migraine” apps and developed a database of the types of data the apps requested for input by the user, and whether the apps had clear privacy policies. They also examined the content of the privacy policies and found:
- Twenty-nine apps were examined (14 diary apps, 15 relaxation apps).
- Of the diary applications, 79% (11/14) had visible privacy policies.
- Of the diary apps with privacy policies, all (11/11) stated whether or not the app collects and stores information remotely.
- A total of 55% (6/11) stated that some user data were used to serve targeted advertisements.
- A total of 11/15 (73%) of the relaxation apps had privacy policies.
Minen MT, Stieglitz EJ, Sciortino R, Torous J. Privacy issues in smartphone applications: An analysis of headache/migraine applications. [Published online ahead of print July 4, 2018]. Headache. doi:10.1111/head.13341.
Migraine, Post-Surgery Pain, and Re-hospitalization
Patients with migraine undergoing surgery are at increased risk of 30-day hospital readmission due to pain, a recent study found. This hospital registry study examined 150,710 patients, aged 18 years or older, who underwent surgery with general anesthesia and mechanical ventilation between 2007 and 2015 at a tertiary care center and 2 affiliated community hospitals in Massachusetts. Researchers found:
- Migraine was associated with an increased risk of 30-day pain-related readmission after surgery (adjusted odds ratio [OR] 1.42).
- The association was stronger for migraine with aura (compared to migraine without aura: adjusted OR 1.69; compared to no migraine: adjusted OR 2.20).
- The predicted adjusted risk of pain-related 30-day readmissions was 9.1 in 1000 surgical patients with migraine with aura and 5.4 in 1,000 patients with migraine without aura, compared to 4.2 in 1000 patients with no migraine.
- Furthermore, migraine was associated with an increased risk of postsurgical 30-day readmission due to a priori defined migraine-related pain (headache or abdominal pain) (adjusted OR 1.55).
Platzbecker K, Zhang MB, Kurth T, et al. The association between migraine and hospital readmission due to pain after surgery: A hospital registry study. [Published online ahead of print July 8, 2018]. Cephalalgia. doi:10.1177/0333102418786457.
Patients with migraine undergoing surgery are at increased risk of 30-day hospital readmission due to pain, a recent study found. This hospital registry study examined 150,710 patients, aged 18 years or older, who underwent surgery with general anesthesia and mechanical ventilation between 2007 and 2015 at a tertiary care center and 2 affiliated community hospitals in Massachusetts. Researchers found:
- Migraine was associated with an increased risk of 30-day pain-related readmission after surgery (adjusted odds ratio [OR] 1.42).
- The association was stronger for migraine with aura (compared to migraine without aura: adjusted OR 1.69; compared to no migraine: adjusted OR 2.20).
- The predicted adjusted risk of pain-related 30-day readmissions was 9.1 in 1000 surgical patients with migraine with aura and 5.4 in 1,000 patients with migraine without aura, compared to 4.2 in 1000 patients with no migraine.
- Furthermore, migraine was associated with an increased risk of postsurgical 30-day readmission due to a priori defined migraine-related pain (headache or abdominal pain) (adjusted OR 1.55).
Platzbecker K, Zhang MB, Kurth T, et al. The association between migraine and hospital readmission due to pain after surgery: A hospital registry study. [Published online ahead of print July 8, 2018]. Cephalalgia. doi:10.1177/0333102418786457.
Patients with migraine undergoing surgery are at increased risk of 30-day hospital readmission due to pain, a recent study found. This hospital registry study examined 150,710 patients, aged 18 years or older, who underwent surgery with general anesthesia and mechanical ventilation between 2007 and 2015 at a tertiary care center and 2 affiliated community hospitals in Massachusetts. Researchers found:
- Migraine was associated with an increased risk of 30-day pain-related readmission after surgery (adjusted odds ratio [OR] 1.42).
- The association was stronger for migraine with aura (compared to migraine without aura: adjusted OR 1.69; compared to no migraine: adjusted OR 2.20).
- The predicted adjusted risk of pain-related 30-day readmissions was 9.1 in 1000 surgical patients with migraine with aura and 5.4 in 1,000 patients with migraine without aura, compared to 4.2 in 1000 patients with no migraine.
- Furthermore, migraine was associated with an increased risk of postsurgical 30-day readmission due to a priori defined migraine-related pain (headache or abdominal pain) (adjusted OR 1.55).
Platzbecker K, Zhang MB, Kurth T, et al. The association between migraine and hospital readmission due to pain after surgery: A hospital registry study. [Published online ahead of print July 8, 2018]. Cephalalgia. doi:10.1177/0333102418786457.
Prolia tops risedronate at 2 years in steroid-induced osteoporosis
AMSTERDAM – Greater increases in bone strength were achieved with denosumab (Prolia) than with risedronate in patients receiving glucocorticoid treatment in a phase 3 trial, suggesting it may prove to be an effective means of preventing osteoporosis in this at-risk population of patients.
At 2 years, the percentage change in bone mineral density (BMD) from baseline with denosumab compared to risedronate in patients initiating steroid treatment was significantly higher (all P values less than .001) at the lumbar spine (6% vs. 2%), total hip (3% vs. 0%), and the femoral neck (1.5% vs. –1%). Similar results were seen in patients who were continuing steroid treatment when they entered the randomized, multicenter, double blind trial.
These data build on the 1-year data that have just been published (Lancet Diabetes Endocrinol. 2018;6[6]:445-54), Willem F. Lems, MD, the presenting study investigator, said at the European Congress of Rheumatology.
“Denosumab continued to increased BMD significantly more then risedronate through 24 months,” said Dr. Lems of VU University Medical Center, Amsterdam. He added that “the treatment differences at all skeletal sites were larger at 24 months than as 12 months.”
The Food and Drug Administration approved an additional indication in May of 2018 for denosumab to treat glucocorticoid-induced osteoporosis in adults at high risk of fracture. The biologic, a monoclonal antibody that targets the RANK ligand, was already approved to treat postmenopausal women with osteoporosis at high fracture risk, to increase bone mass in men with osteoporosis at high fracture risk, and to increase bone mass in women receiving adjuvant aromatase inhibitor therapy for breast cancer and in men receiving androgen deprivation therapy for nonmetastatic prostate cancer.
Secondary osteoporosis is a well known downside of glucocorticoid treatment, and despite there being approved therapies, many patients do not receive such treatment, Dr. Lems noted.
“This 24-month study assessed the safety and efficacy of denosumab versus risedronate in glucocorticoid-treated individuals, in whom guidelines advocate treatment,” Dr. Lems said.
In all, 795 adults who were taking glucocorticoids (7.5 mg prednisone or more daily, or equivalent) were enrolled at 79 sites in Europe, North America, Latin America, and Asia. Patients were subdivided into two populations based on their duration of glucocorticoid use, with 290 designated “glucocorticoid initiating” as they had been treated for less than 3 months, and 505 as “glucocorticoid continuing” as they had been treated for 3 or more months. Within these groups, approximately half were randomized to receive denosumab at a 60-mg subcutaneous dose every 6 months and half to receive risedronate 5 mg orally every day. All participants received calcium and vitamin D supplementation.
Bone turnover markers also declined “significantly more” with denosumab than with risedronate, with the exception of procollagen type 1 N-terminal telopeptide on day 10 and at 2 years and serum C-telopeptide of type 1 collagen at 2 years.
As for safety, a similar percentage of adverse events, including serious adverse events, was seen, Dr. Lems said. So far, he added, “data seem to be reassuring” regarding the risk for serious infections in high-risk subgroups of patients, such as those also taking biologic or immunosuppressant medications. There were no infections reported in patients taking biologics (vs. 12.1% for risedronate) and fewer infections in those taking immunosuppressant or biologic medication (3.6% vs. 6.8%).
Although the study was not powered to look at the rate of fractures between the two treatments, Dr. Lems noted that there was no significant difference in the rates of either new vertebral (4.1% for denosumab; 5.8% risedronate) or clinical (5.8% vs. 6.3%) fractures at 2 years.
“Additional studies are needed to confirm if denosumab is superior to other bisphosphonates for BMD improvement and fracture reduction,” Dr. Lems said. “Denosumab may offer a useful osteoporosis treatment option for patients receiving glucocorticoids,” he concluded.
Amgen sponsored the study. Dr. Lems acknowledged ties to Amgen, Eli Lilly, and Merck: being on the speakers bureau, receiving honoraria for advisory board meetings, and acting as a consultant. All but one other author disclosed ties to Amgen.
SOURCE: Saag KG et al. Ann Rheum Dis. 2018;77(Suppl 2):218. Abstract OP0345.
AMSTERDAM – Greater increases in bone strength were achieved with denosumab (Prolia) than with risedronate in patients receiving glucocorticoid treatment in a phase 3 trial, suggesting it may prove to be an effective means of preventing osteoporosis in this at-risk population of patients.
At 2 years, the percentage change in bone mineral density (BMD) from baseline with denosumab compared to risedronate in patients initiating steroid treatment was significantly higher (all P values less than .001) at the lumbar spine (6% vs. 2%), total hip (3% vs. 0%), and the femoral neck (1.5% vs. –1%). Similar results were seen in patients who were continuing steroid treatment when they entered the randomized, multicenter, double blind trial.
These data build on the 1-year data that have just been published (Lancet Diabetes Endocrinol. 2018;6[6]:445-54), Willem F. Lems, MD, the presenting study investigator, said at the European Congress of Rheumatology.
“Denosumab continued to increased BMD significantly more then risedronate through 24 months,” said Dr. Lems of VU University Medical Center, Amsterdam. He added that “the treatment differences at all skeletal sites were larger at 24 months than as 12 months.”
The Food and Drug Administration approved an additional indication in May of 2018 for denosumab to treat glucocorticoid-induced osteoporosis in adults at high risk of fracture. The biologic, a monoclonal antibody that targets the RANK ligand, was already approved to treat postmenopausal women with osteoporosis at high fracture risk, to increase bone mass in men with osteoporosis at high fracture risk, and to increase bone mass in women receiving adjuvant aromatase inhibitor therapy for breast cancer and in men receiving androgen deprivation therapy for nonmetastatic prostate cancer.
Secondary osteoporosis is a well known downside of glucocorticoid treatment, and despite there being approved therapies, many patients do not receive such treatment, Dr. Lems noted.
“This 24-month study assessed the safety and efficacy of denosumab versus risedronate in glucocorticoid-treated individuals, in whom guidelines advocate treatment,” Dr. Lems said.
In all, 795 adults who were taking glucocorticoids (7.5 mg prednisone or more daily, or equivalent) were enrolled at 79 sites in Europe, North America, Latin America, and Asia. Patients were subdivided into two populations based on their duration of glucocorticoid use, with 290 designated “glucocorticoid initiating” as they had been treated for less than 3 months, and 505 as “glucocorticoid continuing” as they had been treated for 3 or more months. Within these groups, approximately half were randomized to receive denosumab at a 60-mg subcutaneous dose every 6 months and half to receive risedronate 5 mg orally every day. All participants received calcium and vitamin D supplementation.
Bone turnover markers also declined “significantly more” with denosumab than with risedronate, with the exception of procollagen type 1 N-terminal telopeptide on day 10 and at 2 years and serum C-telopeptide of type 1 collagen at 2 years.
As for safety, a similar percentage of adverse events, including serious adverse events, was seen, Dr. Lems said. So far, he added, “data seem to be reassuring” regarding the risk for serious infections in high-risk subgroups of patients, such as those also taking biologic or immunosuppressant medications. There were no infections reported in patients taking biologics (vs. 12.1% for risedronate) and fewer infections in those taking immunosuppressant or biologic medication (3.6% vs. 6.8%).
Although the study was not powered to look at the rate of fractures between the two treatments, Dr. Lems noted that there was no significant difference in the rates of either new vertebral (4.1% for denosumab; 5.8% risedronate) or clinical (5.8% vs. 6.3%) fractures at 2 years.
“Additional studies are needed to confirm if denosumab is superior to other bisphosphonates for BMD improvement and fracture reduction,” Dr. Lems said. “Denosumab may offer a useful osteoporosis treatment option for patients receiving glucocorticoids,” he concluded.
Amgen sponsored the study. Dr. Lems acknowledged ties to Amgen, Eli Lilly, and Merck: being on the speakers bureau, receiving honoraria for advisory board meetings, and acting as a consultant. All but one other author disclosed ties to Amgen.
SOURCE: Saag KG et al. Ann Rheum Dis. 2018;77(Suppl 2):218. Abstract OP0345.
AMSTERDAM – Greater increases in bone strength were achieved with denosumab (Prolia) than with risedronate in patients receiving glucocorticoid treatment in a phase 3 trial, suggesting it may prove to be an effective means of preventing osteoporosis in this at-risk population of patients.
At 2 years, the percentage change in bone mineral density (BMD) from baseline with denosumab compared to risedronate in patients initiating steroid treatment was significantly higher (all P values less than .001) at the lumbar spine (6% vs. 2%), total hip (3% vs. 0%), and the femoral neck (1.5% vs. –1%). Similar results were seen in patients who were continuing steroid treatment when they entered the randomized, multicenter, double blind trial.
These data build on the 1-year data that have just been published (Lancet Diabetes Endocrinol. 2018;6[6]:445-54), Willem F. Lems, MD, the presenting study investigator, said at the European Congress of Rheumatology.
“Denosumab continued to increased BMD significantly more then risedronate through 24 months,” said Dr. Lems of VU University Medical Center, Amsterdam. He added that “the treatment differences at all skeletal sites were larger at 24 months than as 12 months.”
The Food and Drug Administration approved an additional indication in May of 2018 for denosumab to treat glucocorticoid-induced osteoporosis in adults at high risk of fracture. The biologic, a monoclonal antibody that targets the RANK ligand, was already approved to treat postmenopausal women with osteoporosis at high fracture risk, to increase bone mass in men with osteoporosis at high fracture risk, and to increase bone mass in women receiving adjuvant aromatase inhibitor therapy for breast cancer and in men receiving androgen deprivation therapy for nonmetastatic prostate cancer.
Secondary osteoporosis is a well known downside of glucocorticoid treatment, and despite there being approved therapies, many patients do not receive such treatment, Dr. Lems noted.
“This 24-month study assessed the safety and efficacy of denosumab versus risedronate in glucocorticoid-treated individuals, in whom guidelines advocate treatment,” Dr. Lems said.
In all, 795 adults who were taking glucocorticoids (7.5 mg prednisone or more daily, or equivalent) were enrolled at 79 sites in Europe, North America, Latin America, and Asia. Patients were subdivided into two populations based on their duration of glucocorticoid use, with 290 designated “glucocorticoid initiating” as they had been treated for less than 3 months, and 505 as “glucocorticoid continuing” as they had been treated for 3 or more months. Within these groups, approximately half were randomized to receive denosumab at a 60-mg subcutaneous dose every 6 months and half to receive risedronate 5 mg orally every day. All participants received calcium and vitamin D supplementation.
Bone turnover markers also declined “significantly more” with denosumab than with risedronate, with the exception of procollagen type 1 N-terminal telopeptide on day 10 and at 2 years and serum C-telopeptide of type 1 collagen at 2 years.
As for safety, a similar percentage of adverse events, including serious adverse events, was seen, Dr. Lems said. So far, he added, “data seem to be reassuring” regarding the risk for serious infections in high-risk subgroups of patients, such as those also taking biologic or immunosuppressant medications. There were no infections reported in patients taking biologics (vs. 12.1% for risedronate) and fewer infections in those taking immunosuppressant or biologic medication (3.6% vs. 6.8%).
Although the study was not powered to look at the rate of fractures between the two treatments, Dr. Lems noted that there was no significant difference in the rates of either new vertebral (4.1% for denosumab; 5.8% risedronate) or clinical (5.8% vs. 6.3%) fractures at 2 years.
“Additional studies are needed to confirm if denosumab is superior to other bisphosphonates for BMD improvement and fracture reduction,” Dr. Lems said. “Denosumab may offer a useful osteoporosis treatment option for patients receiving glucocorticoids,” he concluded.
Amgen sponsored the study. Dr. Lems acknowledged ties to Amgen, Eli Lilly, and Merck: being on the speakers bureau, receiving honoraria for advisory board meetings, and acting as a consultant. All but one other author disclosed ties to Amgen.
SOURCE: Saag KG et al. Ann Rheum Dis. 2018;77(Suppl 2):218. Abstract OP0345.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Denosumab may offer a useful alternative to risedronate in patients at risk for steroid-induced osteoporosis.
Major finding: Treatment differences were greater at 2 years than at 1 year at all skeletal sites assessed.
Study details: A phase 3, randomized, double-blind, active-controlled study to evaluate the efficacy and safety of denosumab versus risedronate in 795 glucocorticoid-treated individuals.
Disclosures: Amgen sponsored the study. Dr. Lems acknowledged ties to Amgen, Eli Lilly, and Merck: being on the speakers bureau, receiving honoraria for advisory board meetings, and acting as a consultant. All but one other author disclosed ties to Amgen.
Source: Saag KG et al. Ann Rheum Dis. 2018;77(Suppl 2):218. Abstract OP0345.