User login
Let Low-risk Moms Eat During Labor?
A 23-year-old nulliparous woman at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the past three hours and says she’s hungry. What type of diet should you order for this patient? Should you place any restrictions in the order?
Since the first reports of Mendelson syndrome (aspiration during general anesthesia) in the early 1940s, many health care providers managing laboring women restrict their diets to clear liquids or less, with little evidence to support the decision.2 In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a general-population study of 172,334 adults who underwent a total of 215,488 surgeries with general anesthesia, the risk for aspiration was 1:895 for emergency procedures and 1:3886 for elective procedures.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less-restrictive diet during labor.
STUDY SUMMARY
Not one case of aspiration
This meta-analysis of 10 RCTs, including 3,982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less-restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥ 37 weeks, two studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥ 30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Continue to: Results
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes. Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal ICU were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk, 1.00). None of the 3,982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
An outdated practice, per the data
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less-restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk for complications/surgery during labor are not justified based on current data.
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less-restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[6]:379-380).
1. Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38(11):1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14(4):171-175.
6. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.

A 23-year-old nulliparous woman at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the past three hours and says she’s hungry. What type of diet should you order for this patient? Should you place any restrictions in the order?
Since the first reports of Mendelson syndrome (aspiration during general anesthesia) in the early 1940s, many health care providers managing laboring women restrict their diets to clear liquids or less, with little evidence to support the decision.2 In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a general-population study of 172,334 adults who underwent a total of 215,488 surgeries with general anesthesia, the risk for aspiration was 1:895 for emergency procedures and 1:3886 for elective procedures.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less-restrictive diet during labor.
STUDY SUMMARY
Not one case of aspiration
This meta-analysis of 10 RCTs, including 3,982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less-restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥ 37 weeks, two studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥ 30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Continue to: Results
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes. Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal ICU were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk, 1.00). None of the 3,982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
An outdated practice, per the data
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less-restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk for complications/surgery during labor are not justified based on current data.
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less-restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[6]:379-380).

A 23-year-old nulliparous woman at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the past three hours and says she’s hungry. What type of diet should you order for this patient? Should you place any restrictions in the order?
Since the first reports of Mendelson syndrome (aspiration during general anesthesia) in the early 1940s, many health care providers managing laboring women restrict their diets to clear liquids or less, with little evidence to support the decision.2 In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a general-population study of 172,334 adults who underwent a total of 215,488 surgeries with general anesthesia, the risk for aspiration was 1:895 for emergency procedures and 1:3886 for elective procedures.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less-restrictive diet during labor.
STUDY SUMMARY
Not one case of aspiration
This meta-analysis of 10 RCTs, including 3,982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less-restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥ 37 weeks, two studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥ 30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Continue to: Results
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes. Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal ICU were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk, 1.00). None of the 3,982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
An outdated practice, per the data
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less-restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk for complications/surgery during labor are not justified based on current data.
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less-restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[6]:379-380).
1. Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38(11):1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14(4):171-175.
6. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
1. Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38(11):1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14(4):171-175.
6. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
Make The Diagnosis - August 2018
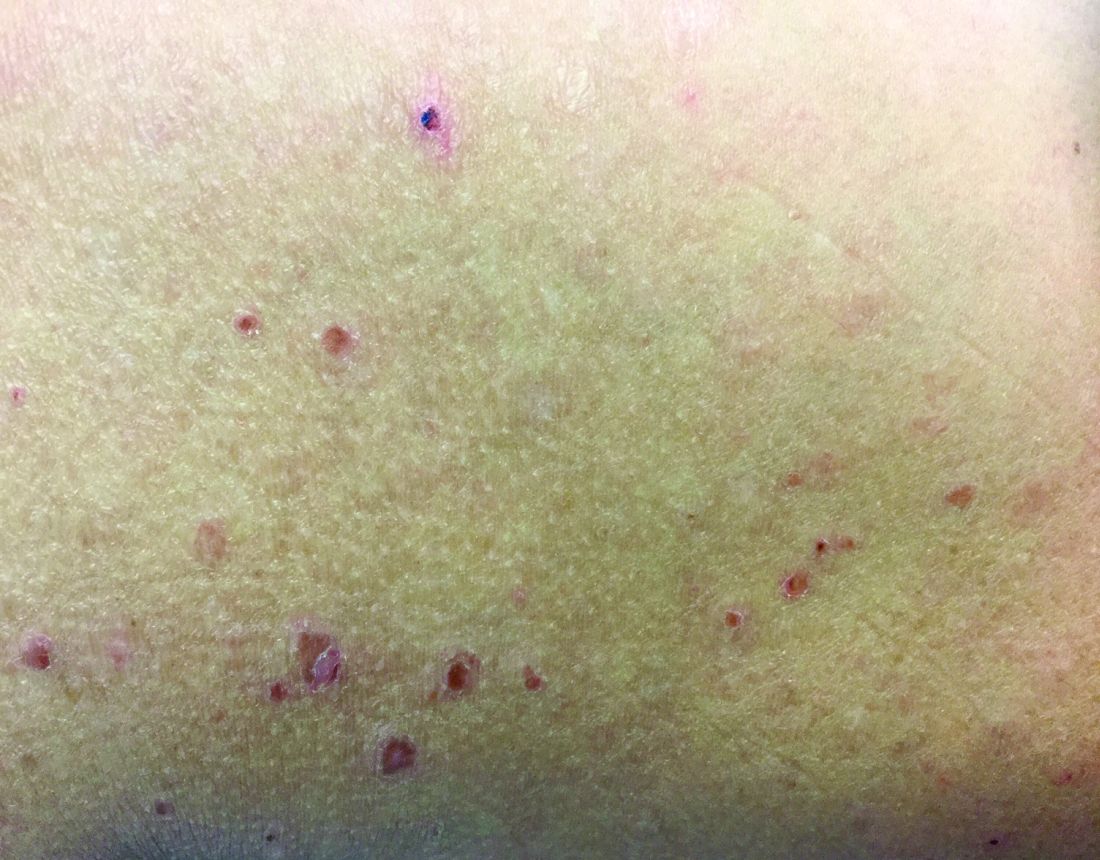
respectively, of pityriasis lichenoides, an uncommon clonal T-cell disorder. The cause is unknown, although associations with infections have been reported. Pityriasis lichenoides more commonly affects children or young adults, usually before age 30 years. The disease can occur in all races.
In PLEVA, erythematous to brown papules and macules, in various stages of evolution, appear suddenly and in crops. The trunk and flexural areas are most often affected, but lesions may become widespread and may be pruritic or painful. Lesions may crust, ulcerate, or become necrotic and can heal with scarring. In general, patients don’t have constitutional symptoms. Lesions tend to resolve spontaneously over 1-3 years.
Rarely, PLEVA may develop into a more severe form called febrile ulceronecrotic Mucha-Habermann disease, a dermatologic emergency. Patients (more commonly, young males) may present with high fever, malaise, and lymphadenopathy. Lesions become very painful, ulcerated, and necrotic, and extensive necrosis may be present. Changes in mental status, breathing difficulties, anemia, arthritis, abdominal pain, and sepsis may occur. Patients require hospitalization. There is a 25% mortality rate.
PLC is at the other end of this disease spectrum, representing the chronic, more mild stage of the disorder. Lesions present as indolent, asymptomatic, scaly macules and erythematous papules, favoring the trunk and proximal extremities. Lesions tend to be fewer in number than seen in PLEVA. They resolve over several months and may result in hypopigmentation, but usually don’t cause scarring. Patients may have long periods of remission between outbreaks. T-cell gene rearrangement may demonstrate monoclonality. PLC is generally considered a benign disease, although there are patients who have developed cutaneous T-cell lymphoma. For this reason, patients should be followed carefully for signs of malignant transformation.
Both forms share a common histologic picture. In PLEVA, focal parakeratosis and crusting is present. A dense, wedge-shaped infiltrate can be seen with prominent lymphocytic exocystosis in the epidermis. Necrotic keratinocytes are often seen. There may be spongiosis and intraepidermal vesicles. Extravasation of erythrocytes often occurs in the epidermis. PLC is histologically similar but far more subtle. There is less crusting, less spongiosis, fewer vesicles, and fewer necrotic keratinocytes. Generally, atypia of lymphocytes is absent.
Mucha-Habermann requires treatment with systemic steroids. Methotrexate, cyclosporine, or dapsone may be used as steroid-sparing agents. Upon treatment, lesions may resolve or revert back to more typical lesions of PLEVA. Treatment for PLEVA and PLC includes oral tetracycline or erythromycin, antihistamines (if pruritus is present), topical steroids, topical tacrolimus or pimecrolimus, or phototherapy. Low-dose weekly methotrexate may be helpful.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
respectively, of pityriasis lichenoides, an uncommon clonal T-cell disorder. The cause is unknown, although associations with infections have been reported. Pityriasis lichenoides more commonly affects children or young adults, usually before age 30 years. The disease can occur in all races.
In PLEVA, erythematous to brown papules and macules, in various stages of evolution, appear suddenly and in crops. The trunk and flexural areas are most often affected, but lesions may become widespread and may be pruritic or painful. Lesions may crust, ulcerate, or become necrotic and can heal with scarring. In general, patients don’t have constitutional symptoms. Lesions tend to resolve spontaneously over 1-3 years.
Rarely, PLEVA may develop into a more severe form called febrile ulceronecrotic Mucha-Habermann disease, a dermatologic emergency. Patients (more commonly, young males) may present with high fever, malaise, and lymphadenopathy. Lesions become very painful, ulcerated, and necrotic, and extensive necrosis may be present. Changes in mental status, breathing difficulties, anemia, arthritis, abdominal pain, and sepsis may occur. Patients require hospitalization. There is a 25% mortality rate.
PLC is at the other end of this disease spectrum, representing the chronic, more mild stage of the disorder. Lesions present as indolent, asymptomatic, scaly macules and erythematous papules, favoring the trunk and proximal extremities. Lesions tend to be fewer in number than seen in PLEVA. They resolve over several months and may result in hypopigmentation, but usually don’t cause scarring. Patients may have long periods of remission between outbreaks. T-cell gene rearrangement may demonstrate monoclonality. PLC is generally considered a benign disease, although there are patients who have developed cutaneous T-cell lymphoma. For this reason, patients should be followed carefully for signs of malignant transformation.
Both forms share a common histologic picture. In PLEVA, focal parakeratosis and crusting is present. A dense, wedge-shaped infiltrate can be seen with prominent lymphocytic exocystosis in the epidermis. Necrotic keratinocytes are often seen. There may be spongiosis and intraepidermal vesicles. Extravasation of erythrocytes often occurs in the epidermis. PLC is histologically similar but far more subtle. There is less crusting, less spongiosis, fewer vesicles, and fewer necrotic keratinocytes. Generally, atypia of lymphocytes is absent.
Mucha-Habermann requires treatment with systemic steroids. Methotrexate, cyclosporine, or dapsone may be used as steroid-sparing agents. Upon treatment, lesions may resolve or revert back to more typical lesions of PLEVA. Treatment for PLEVA and PLC includes oral tetracycline or erythromycin, antihistamines (if pruritus is present), topical steroids, topical tacrolimus or pimecrolimus, or phototherapy. Low-dose weekly methotrexate may be helpful.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
respectively, of pityriasis lichenoides, an uncommon clonal T-cell disorder. The cause is unknown, although associations with infections have been reported. Pityriasis lichenoides more commonly affects children or young adults, usually before age 30 years. The disease can occur in all races.
In PLEVA, erythematous to brown papules and macules, in various stages of evolution, appear suddenly and in crops. The trunk and flexural areas are most often affected, but lesions may become widespread and may be pruritic or painful. Lesions may crust, ulcerate, or become necrotic and can heal with scarring. In general, patients don’t have constitutional symptoms. Lesions tend to resolve spontaneously over 1-3 years.
Rarely, PLEVA may develop into a more severe form called febrile ulceronecrotic Mucha-Habermann disease, a dermatologic emergency. Patients (more commonly, young males) may present with high fever, malaise, and lymphadenopathy. Lesions become very painful, ulcerated, and necrotic, and extensive necrosis may be present. Changes in mental status, breathing difficulties, anemia, arthritis, abdominal pain, and sepsis may occur. Patients require hospitalization. There is a 25% mortality rate.
PLC is at the other end of this disease spectrum, representing the chronic, more mild stage of the disorder. Lesions present as indolent, asymptomatic, scaly macules and erythematous papules, favoring the trunk and proximal extremities. Lesions tend to be fewer in number than seen in PLEVA. They resolve over several months and may result in hypopigmentation, but usually don’t cause scarring. Patients may have long periods of remission between outbreaks. T-cell gene rearrangement may demonstrate monoclonality. PLC is generally considered a benign disease, although there are patients who have developed cutaneous T-cell lymphoma. For this reason, patients should be followed carefully for signs of malignant transformation.
Both forms share a common histologic picture. In PLEVA, focal parakeratosis and crusting is present. A dense, wedge-shaped infiltrate can be seen with prominent lymphocytic exocystosis in the epidermis. Necrotic keratinocytes are often seen. There may be spongiosis and intraepidermal vesicles. Extravasation of erythrocytes often occurs in the epidermis. PLC is histologically similar but far more subtle. There is less crusting, less spongiosis, fewer vesicles, and fewer necrotic keratinocytes. Generally, atypia of lymphocytes is absent.
Mucha-Habermann requires treatment with systemic steroids. Methotrexate, cyclosporine, or dapsone may be used as steroid-sparing agents. Upon treatment, lesions may resolve or revert back to more typical lesions of PLEVA. Treatment for PLEVA and PLC includes oral tetracycline or erythromycin, antihistamines (if pruritus is present), topical steroids, topical tacrolimus or pimecrolimus, or phototherapy. Low-dose weekly methotrexate may be helpful.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
A 28-year-old white female with no significant past medical history presents with a 10-year history of asymptomatic erythematous papules and scaly patches that come and go. She has used topical steroids in the past.
Assessing adverse events tied to outpatient opioid use in children
Nearly three-quarters of opioid-related adverse events seen in children were related to therapeutic opioid use, based on data from more than a million prescriptions.
Prescription of opioids to children for outpatient conditions may have risen along with the increased opioid prescriptions for adults, but most of the literature focuses on opioid toxicity in children, and “the incidence of adverse opioid effects for children during appropriate medical use for relatively minor conditions is unknown,” wrote Cecilia P. Chung, MD, of Vanderbilt University in Nashville, Tenn., and her colleagues.
In a retrospective study published in Pediatrics, the researchers reviewed data from 401,972 children and adolescents aged 2-17 years with no chronic or severe conditions. The patients filled a total of 1,362,503 prescriptions for opioids, with a mean 15% filling one or more opioid prescriptions a year, and 1 in every 2,611 prescriptions was followed by an emergency department visit, hospitalization, or death related to an adverse event associated with opioid use.
Approximately 20% of the prescriptions were for children aged 2-5 years, 28% for ages 6-11 years, and 52% for ages 12-17 years. The patients were enrolled in Medicaid between Jan. 1, 1999, and Dec. 31, 2014, in Tennessee, and were seen at outpatient centers. Dental procedures were the most common reasons for opioid prescriptions in the study population (31%), followed by outpatient procedures or surgeries (25%), trauma (18%), and infections (16%).
Overall, 437 cases of opioid-related adverse events were confirmed by medical record review; 89% of these were deemed related to the prescription, and 71% were related to proper therapeutic use, the researchers said. The remainder were considered to be related to unintentional overdose, abuse, self-harm, or the circumstances were not indicated.
The opioid-related symptoms most frequently were gastrointestinal, neuropsychiatric, dermatologic, and central nervous system depression.
“The incidence of opioid-related adverse events increased for children and adolescents 12-17 years of age, during current opioid use, and with higher opioid doses,” the researchers said.
The study findings were limited by several factors including the use of Medicaid patients only, the lack of clinical details such as patient weight, and the potential for incomplete medical records, Dr. Chung and her associates noted. However, the results support the need for more comprehensive guidelines in treating acute, self-limited conditions in children to reduce unnecessary opioid exposure, they said.
The researchers had no relevant financial conflicts to disclose. The study was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development and funded by the National Institutes of Health. Dr. Chung received grant support from the National Institutes of Health and the Rheumatology Research Foundation Career Development Research K-supplement.
SOURCE: Chung C et al. Pediatrics. 2018 Jul 16. doi: 10.1542/peds.2017-2156.
“We know that opioids are associated with many untoward side effects and are potentially lethal. But we believe there is a reason why opioids have been used to treat pain since the Sumerians 5,000 years ago,” Elliot J. Krane, MD, Steven J. Weisman, MD, and Gary A. Walco, PhD, wrote in an accompanying editorial.
The editorialists noted that they are not blanket advocates of opioid prescriptions for children, but they do believe in the importance of pain management for conditions including postsurgical pain, burns, physical trauma, and medical illnesses. In many cases, opioids are the most effective treatment option.
In addition, data on opioid-related deaths in the United States have been shown inaccurate for various reasons including the coding of deaths as opioid related if opioids were present among a number of other drugs, even if the cause of death was another substance or an act such as suicide, the writers noted. Even the Centers for Disease Control and Prevention has admitted overestimating the prevalence of opioid-related deaths by as much as 100%. Consequently, the opioid epidemic portrayed in the media, “pales in comparison with other public health hazards and causes of deaths in America such as tobacco-related deaths, alcoholic hepatic disease, and even hospital-acquired infections,” Dr. Krane, Dr. Weisman, and Dr. Walco said.
“The data as presented cannot be considered causal for associating opioid prescribing with severe morbidity, more hospital emergency department visits, and even death,” the editorialists concluded. They emphasized the need for good judgment on the part of clinicians when prescribing opioids to children and advocated always making good use of nonopioid alternatives, but Dr. Krane, Dr. Weisman, and Dr. Walco added that the findings of this study should not deter doctors from prescribing an opioid when they think it is the most effective and appropriate option for moderately to severely painful conditions.
“Too often, consideration of the need to prevent and treat pain can be lost in the national discussion,” they said.
Dr. Krane is affiliated with Stanford University in Palo Alto, Calif., Dr. Weisman is affiliated with the Medical College of Milwaukee, Wisc., and Dr. Walco is affiliated with the University of Washington, Seattle. Dr. Krane disclosed consulting for Collegium Pharmaceuticals and honoraria for lecturing on pain and analgesia. Dr. Weisman disclosed consulting for Grünenthal Pharmaceuticals and Pfizer Pharmaceuticals and has conducted clinical trials for Grünenthal Pharmaceuticals, Cadence Pharmaceuticals, and The Medicines Company. Their editorial accompanying the article by Chung et al. appeared in Pediatrics (2018 Jul 16. doi: 10.1542/peds.2018-1623).
“We know that opioids are associated with many untoward side effects and are potentially lethal. But we believe there is a reason why opioids have been used to treat pain since the Sumerians 5,000 years ago,” Elliot J. Krane, MD, Steven J. Weisman, MD, and Gary A. Walco, PhD, wrote in an accompanying editorial.
The editorialists noted that they are not blanket advocates of opioid prescriptions for children, but they do believe in the importance of pain management for conditions including postsurgical pain, burns, physical trauma, and medical illnesses. In many cases, opioids are the most effective treatment option.
In addition, data on opioid-related deaths in the United States have been shown inaccurate for various reasons including the coding of deaths as opioid related if opioids were present among a number of other drugs, even if the cause of death was another substance or an act such as suicide, the writers noted. Even the Centers for Disease Control and Prevention has admitted overestimating the prevalence of opioid-related deaths by as much as 100%. Consequently, the opioid epidemic portrayed in the media, “pales in comparison with other public health hazards and causes of deaths in America such as tobacco-related deaths, alcoholic hepatic disease, and even hospital-acquired infections,” Dr. Krane, Dr. Weisman, and Dr. Walco said.
“The data as presented cannot be considered causal for associating opioid prescribing with severe morbidity, more hospital emergency department visits, and even death,” the editorialists concluded. They emphasized the need for good judgment on the part of clinicians when prescribing opioids to children and advocated always making good use of nonopioid alternatives, but Dr. Krane, Dr. Weisman, and Dr. Walco added that the findings of this study should not deter doctors from prescribing an opioid when they think it is the most effective and appropriate option for moderately to severely painful conditions.
“Too often, consideration of the need to prevent and treat pain can be lost in the national discussion,” they said.
Dr. Krane is affiliated with Stanford University in Palo Alto, Calif., Dr. Weisman is affiliated with the Medical College of Milwaukee, Wisc., and Dr. Walco is affiliated with the University of Washington, Seattle. Dr. Krane disclosed consulting for Collegium Pharmaceuticals and honoraria for lecturing on pain and analgesia. Dr. Weisman disclosed consulting for Grünenthal Pharmaceuticals and Pfizer Pharmaceuticals and has conducted clinical trials for Grünenthal Pharmaceuticals, Cadence Pharmaceuticals, and The Medicines Company. Their editorial accompanying the article by Chung et al. appeared in Pediatrics (2018 Jul 16. doi: 10.1542/peds.2018-1623).
“We know that opioids are associated with many untoward side effects and are potentially lethal. But we believe there is a reason why opioids have been used to treat pain since the Sumerians 5,000 years ago,” Elliot J. Krane, MD, Steven J. Weisman, MD, and Gary A. Walco, PhD, wrote in an accompanying editorial.
The editorialists noted that they are not blanket advocates of opioid prescriptions for children, but they do believe in the importance of pain management for conditions including postsurgical pain, burns, physical trauma, and medical illnesses. In many cases, opioids are the most effective treatment option.
In addition, data on opioid-related deaths in the United States have been shown inaccurate for various reasons including the coding of deaths as opioid related if opioids were present among a number of other drugs, even if the cause of death was another substance or an act such as suicide, the writers noted. Even the Centers for Disease Control and Prevention has admitted overestimating the prevalence of opioid-related deaths by as much as 100%. Consequently, the opioid epidemic portrayed in the media, “pales in comparison with other public health hazards and causes of deaths in America such as tobacco-related deaths, alcoholic hepatic disease, and even hospital-acquired infections,” Dr. Krane, Dr. Weisman, and Dr. Walco said.
“The data as presented cannot be considered causal for associating opioid prescribing with severe morbidity, more hospital emergency department visits, and even death,” the editorialists concluded. They emphasized the need for good judgment on the part of clinicians when prescribing opioids to children and advocated always making good use of nonopioid alternatives, but Dr. Krane, Dr. Weisman, and Dr. Walco added that the findings of this study should not deter doctors from prescribing an opioid when they think it is the most effective and appropriate option for moderately to severely painful conditions.
“Too often, consideration of the need to prevent and treat pain can be lost in the national discussion,” they said.
Dr. Krane is affiliated with Stanford University in Palo Alto, Calif., Dr. Weisman is affiliated with the Medical College of Milwaukee, Wisc., and Dr. Walco is affiliated with the University of Washington, Seattle. Dr. Krane disclosed consulting for Collegium Pharmaceuticals and honoraria for lecturing on pain and analgesia. Dr. Weisman disclosed consulting for Grünenthal Pharmaceuticals and Pfizer Pharmaceuticals and has conducted clinical trials for Grünenthal Pharmaceuticals, Cadence Pharmaceuticals, and The Medicines Company. Their editorial accompanying the article by Chung et al. appeared in Pediatrics (2018 Jul 16. doi: 10.1542/peds.2018-1623).
Nearly three-quarters of opioid-related adverse events seen in children were related to therapeutic opioid use, based on data from more than a million prescriptions.
Prescription of opioids to children for outpatient conditions may have risen along with the increased opioid prescriptions for adults, but most of the literature focuses on opioid toxicity in children, and “the incidence of adverse opioid effects for children during appropriate medical use for relatively minor conditions is unknown,” wrote Cecilia P. Chung, MD, of Vanderbilt University in Nashville, Tenn., and her colleagues.
In a retrospective study published in Pediatrics, the researchers reviewed data from 401,972 children and adolescents aged 2-17 years with no chronic or severe conditions. The patients filled a total of 1,362,503 prescriptions for opioids, with a mean 15% filling one or more opioid prescriptions a year, and 1 in every 2,611 prescriptions was followed by an emergency department visit, hospitalization, or death related to an adverse event associated with opioid use.
Approximately 20% of the prescriptions were for children aged 2-5 years, 28% for ages 6-11 years, and 52% for ages 12-17 years. The patients were enrolled in Medicaid between Jan. 1, 1999, and Dec. 31, 2014, in Tennessee, and were seen at outpatient centers. Dental procedures were the most common reasons for opioid prescriptions in the study population (31%), followed by outpatient procedures or surgeries (25%), trauma (18%), and infections (16%).
Overall, 437 cases of opioid-related adverse events were confirmed by medical record review; 89% of these were deemed related to the prescription, and 71% were related to proper therapeutic use, the researchers said. The remainder were considered to be related to unintentional overdose, abuse, self-harm, or the circumstances were not indicated.
The opioid-related symptoms most frequently were gastrointestinal, neuropsychiatric, dermatologic, and central nervous system depression.
“The incidence of opioid-related adverse events increased for children and adolescents 12-17 years of age, during current opioid use, and with higher opioid doses,” the researchers said.
The study findings were limited by several factors including the use of Medicaid patients only, the lack of clinical details such as patient weight, and the potential for incomplete medical records, Dr. Chung and her associates noted. However, the results support the need for more comprehensive guidelines in treating acute, self-limited conditions in children to reduce unnecessary opioid exposure, they said.
The researchers had no relevant financial conflicts to disclose. The study was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development and funded by the National Institutes of Health. Dr. Chung received grant support from the National Institutes of Health and the Rheumatology Research Foundation Career Development Research K-supplement.
SOURCE: Chung C et al. Pediatrics. 2018 Jul 16. doi: 10.1542/peds.2017-2156.
Nearly three-quarters of opioid-related adverse events seen in children were related to therapeutic opioid use, based on data from more than a million prescriptions.
Prescription of opioids to children for outpatient conditions may have risen along with the increased opioid prescriptions for adults, but most of the literature focuses on opioid toxicity in children, and “the incidence of adverse opioid effects for children during appropriate medical use for relatively minor conditions is unknown,” wrote Cecilia P. Chung, MD, of Vanderbilt University in Nashville, Tenn., and her colleagues.
In a retrospective study published in Pediatrics, the researchers reviewed data from 401,972 children and adolescents aged 2-17 years with no chronic or severe conditions. The patients filled a total of 1,362,503 prescriptions for opioids, with a mean 15% filling one or more opioid prescriptions a year, and 1 in every 2,611 prescriptions was followed by an emergency department visit, hospitalization, or death related to an adverse event associated with opioid use.
Approximately 20% of the prescriptions were for children aged 2-5 years, 28% for ages 6-11 years, and 52% for ages 12-17 years. The patients were enrolled in Medicaid between Jan. 1, 1999, and Dec. 31, 2014, in Tennessee, and were seen at outpatient centers. Dental procedures were the most common reasons for opioid prescriptions in the study population (31%), followed by outpatient procedures or surgeries (25%), trauma (18%), and infections (16%).
Overall, 437 cases of opioid-related adverse events were confirmed by medical record review; 89% of these were deemed related to the prescription, and 71% were related to proper therapeutic use, the researchers said. The remainder were considered to be related to unintentional overdose, abuse, self-harm, or the circumstances were not indicated.
The opioid-related symptoms most frequently were gastrointestinal, neuropsychiatric, dermatologic, and central nervous system depression.
“The incidence of opioid-related adverse events increased for children and adolescents 12-17 years of age, during current opioid use, and with higher opioid doses,” the researchers said.
The study findings were limited by several factors including the use of Medicaid patients only, the lack of clinical details such as patient weight, and the potential for incomplete medical records, Dr. Chung and her associates noted. However, the results support the need for more comprehensive guidelines in treating acute, self-limited conditions in children to reduce unnecessary opioid exposure, they said.
The researchers had no relevant financial conflicts to disclose. The study was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development and funded by the National Institutes of Health. Dr. Chung received grant support from the National Institutes of Health and the Rheumatology Research Foundation Career Development Research K-supplement.
SOURCE: Chung C et al. Pediatrics. 2018 Jul 16. doi: 10.1542/peds.2017-2156.
FROM PEDIATRICS
Key clinical point: One in every 2,611 opioid prescriptions in children and teens was followed by an ED visit, hospitalization, or death related to an adverse event associated with opioid use.
Major finding: A total of 437 cases of opioid-related adverse events were confirmed; most were associated with therapeutic medication use.
Study details: The data come from a retrospective cohort study of 401,972 children aged 2-17 years enrolled in Medicaid between Jan. 1, 1999, and Dec. 31, 2014.
Disclosures: The researchers had no relevant financial conflicts to disclose. The study was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development, and funded by the National Institutes of Health. Dr. Chung received grant support from the National Institutes of Health and the Rheumatology Research Foundation Career Development Research K-supplement.
Source: Chung C et al. Pediatrics. 2018 Jul 16. doi: 10.1542/peds.2017-2156
Occult blood in feces linked to more than just colorectal cancer mortality
Occult blood in the feces was associated not only with colorectal cancer mortality, but also mortality from other causes, Scottish investigators have reported based on findings of a large, retrospective study.
A positive guaiac fecal occult blood test (gFOBT) was associated with all-cause mortality excluding colorectal cancer in the study, which included data on individuals screened in Scotland during 2000-2016.
The findings might have important clinical implications beyond colorectal cancer if corroborated by prospective studies in the future, wrote investigator Gillian Libby of the Bowel Screening Research Unit at Ninewells Hospital and Medical School, Dundee, Scotland, and coinvestigators.
“If hemoglobin in feces is a risk factor for all-cause death, it may have the potential as a modifiable biomarker that could be used to assess the efficacy of both lifestyle and drug interventions to reduce the risk of premature mortality,” investigators wrote in a report on the study released in the journal Gut.
The investigators linked gFOBT results for 133,921 screened individuals who ranged in age from 50 to 74 years to mortality data from the National Records of Scotland Database.
As expected, individuals with positive results had a considerably higher risk of death not only from colorectal cancer (hazard ratio, 7.79; 95% confidence interval, 6.13-9.89; P less than .0001) but also for noncolorectal cancer causes combined (HR, 1.58; 95% CI, 1.45-1.73; P less than .0001) after adjustment for age, sex, deprivation, and prescribed medicines.
The higher risk of death held for mortality related to circulatory, respiratory, digestive, endocrine, neuropsychological, and other causes, as reported.
“It is clear from this study that, in the Scottish population, the presence of hemoglobin in the faeces as detected by gFOBT is associated with a number of non-CRC causes of death,” investigators wrote.
These results do corroborate those of one other recent study in Taiwan that showed a relationship between positive gFOBT tests and all-cause mortality.
“In contrast to the Taiwanese study, we were able to examine this association broken down by disease categories and adjusting for confounding factors,” they noted in their discussion of the results.
Funding for the study came from the Chief Scientist Office of the Scottish Government Health Directorates. One study coauthor reported a consultancy with Immunostics, and no other disclosures were reported.
SOURCE: Libby G et al. Gut. 2018. doi: 10.1136/gutjnl-2018-316483.
This study makes the “provocative” suggestion that occult blood in feces may reveal more than was previously thought, Uri Ladabaum, MD, of Stanford (Calif.) University wrote in a commentary.
“If the eye is the window to the soul, is a fecal test the window to general health?” he asked in the commentary appearing in the journal Gut.
The initial surprise that a positive guaiac fecal occult blood test is associated with more than just colorectal cancer (CRC) mortality might be tempered, however, after considering that risk factors for many diseases are integrated, Dr. Ladabaum said. In other words, risk factors for CRC, such as obesity, inactivity, poor diet, or diabetes, may be at least partly responsible for the effects on non-CRC death seen in this study, although authors did try to control for some of those factors in their analyses, he said.
Whether a positive guaiac fecal occult blood test should prompt any additional interventions or alerts to the patient remains a question to be resolved, according to Dr. Ladabaum. “For now, I believe that our enthusiasm for the established CRC screening methods should not be affected, and that the focus after an abnormal fecal occult blood test should be to ensure prompt delivery of a follow-up colonoscopy,” he wrote.
Dr. Ladabaum is with the division of gastroenterology and hepatology and the department of medicine at Stanford (Calif.) University. These comments are from his commentary in Gut (2018. doi: 10.1136/gutjnl-2018-316762). Dr. Ladabaum reported being a consultant for Medtronic and Motus and an advisory board member of Universal Dx.
This study makes the “provocative” suggestion that occult blood in feces may reveal more than was previously thought, Uri Ladabaum, MD, of Stanford (Calif.) University wrote in a commentary.
“If the eye is the window to the soul, is a fecal test the window to general health?” he asked in the commentary appearing in the journal Gut.
The initial surprise that a positive guaiac fecal occult blood test is associated with more than just colorectal cancer (CRC) mortality might be tempered, however, after considering that risk factors for many diseases are integrated, Dr. Ladabaum said. In other words, risk factors for CRC, such as obesity, inactivity, poor diet, or diabetes, may be at least partly responsible for the effects on non-CRC death seen in this study, although authors did try to control for some of those factors in their analyses, he said.
Whether a positive guaiac fecal occult blood test should prompt any additional interventions or alerts to the patient remains a question to be resolved, according to Dr. Ladabaum. “For now, I believe that our enthusiasm for the established CRC screening methods should not be affected, and that the focus after an abnormal fecal occult blood test should be to ensure prompt delivery of a follow-up colonoscopy,” he wrote.
Dr. Ladabaum is with the division of gastroenterology and hepatology and the department of medicine at Stanford (Calif.) University. These comments are from his commentary in Gut (2018. doi: 10.1136/gutjnl-2018-316762). Dr. Ladabaum reported being a consultant for Medtronic and Motus and an advisory board member of Universal Dx.
This study makes the “provocative” suggestion that occult blood in feces may reveal more than was previously thought, Uri Ladabaum, MD, of Stanford (Calif.) University wrote in a commentary.
“If the eye is the window to the soul, is a fecal test the window to general health?” he asked in the commentary appearing in the journal Gut.
The initial surprise that a positive guaiac fecal occult blood test is associated with more than just colorectal cancer (CRC) mortality might be tempered, however, after considering that risk factors for many diseases are integrated, Dr. Ladabaum said. In other words, risk factors for CRC, such as obesity, inactivity, poor diet, or diabetes, may be at least partly responsible for the effects on non-CRC death seen in this study, although authors did try to control for some of those factors in their analyses, he said.
Whether a positive guaiac fecal occult blood test should prompt any additional interventions or alerts to the patient remains a question to be resolved, according to Dr. Ladabaum. “For now, I believe that our enthusiasm for the established CRC screening methods should not be affected, and that the focus after an abnormal fecal occult blood test should be to ensure prompt delivery of a follow-up colonoscopy,” he wrote.
Dr. Ladabaum is with the division of gastroenterology and hepatology and the department of medicine at Stanford (Calif.) University. These comments are from his commentary in Gut (2018. doi: 10.1136/gutjnl-2018-316762). Dr. Ladabaum reported being a consultant for Medtronic and Motus and an advisory board member of Universal Dx.
Occult blood in the feces was associated not only with colorectal cancer mortality, but also mortality from other causes, Scottish investigators have reported based on findings of a large, retrospective study.
A positive guaiac fecal occult blood test (gFOBT) was associated with all-cause mortality excluding colorectal cancer in the study, which included data on individuals screened in Scotland during 2000-2016.
The findings might have important clinical implications beyond colorectal cancer if corroborated by prospective studies in the future, wrote investigator Gillian Libby of the Bowel Screening Research Unit at Ninewells Hospital and Medical School, Dundee, Scotland, and coinvestigators.
“If hemoglobin in feces is a risk factor for all-cause death, it may have the potential as a modifiable biomarker that could be used to assess the efficacy of both lifestyle and drug interventions to reduce the risk of premature mortality,” investigators wrote in a report on the study released in the journal Gut.
The investigators linked gFOBT results for 133,921 screened individuals who ranged in age from 50 to 74 years to mortality data from the National Records of Scotland Database.
As expected, individuals with positive results had a considerably higher risk of death not only from colorectal cancer (hazard ratio, 7.79; 95% confidence interval, 6.13-9.89; P less than .0001) but also for noncolorectal cancer causes combined (HR, 1.58; 95% CI, 1.45-1.73; P less than .0001) after adjustment for age, sex, deprivation, and prescribed medicines.
The higher risk of death held for mortality related to circulatory, respiratory, digestive, endocrine, neuropsychological, and other causes, as reported.
“It is clear from this study that, in the Scottish population, the presence of hemoglobin in the faeces as detected by gFOBT is associated with a number of non-CRC causes of death,” investigators wrote.
These results do corroborate those of one other recent study in Taiwan that showed a relationship between positive gFOBT tests and all-cause mortality.
“In contrast to the Taiwanese study, we were able to examine this association broken down by disease categories and adjusting for confounding factors,” they noted in their discussion of the results.
Funding for the study came from the Chief Scientist Office of the Scottish Government Health Directorates. One study coauthor reported a consultancy with Immunostics, and no other disclosures were reported.
SOURCE: Libby G et al. Gut. 2018. doi: 10.1136/gutjnl-2018-316483.
Occult blood in the feces was associated not only with colorectal cancer mortality, but also mortality from other causes, Scottish investigators have reported based on findings of a large, retrospective study.
A positive guaiac fecal occult blood test (gFOBT) was associated with all-cause mortality excluding colorectal cancer in the study, which included data on individuals screened in Scotland during 2000-2016.
The findings might have important clinical implications beyond colorectal cancer if corroborated by prospective studies in the future, wrote investigator Gillian Libby of the Bowel Screening Research Unit at Ninewells Hospital and Medical School, Dundee, Scotland, and coinvestigators.
“If hemoglobin in feces is a risk factor for all-cause death, it may have the potential as a modifiable biomarker that could be used to assess the efficacy of both lifestyle and drug interventions to reduce the risk of premature mortality,” investigators wrote in a report on the study released in the journal Gut.
The investigators linked gFOBT results for 133,921 screened individuals who ranged in age from 50 to 74 years to mortality data from the National Records of Scotland Database.
As expected, individuals with positive results had a considerably higher risk of death not only from colorectal cancer (hazard ratio, 7.79; 95% confidence interval, 6.13-9.89; P less than .0001) but also for noncolorectal cancer causes combined (HR, 1.58; 95% CI, 1.45-1.73; P less than .0001) after adjustment for age, sex, deprivation, and prescribed medicines.
The higher risk of death held for mortality related to circulatory, respiratory, digestive, endocrine, neuropsychological, and other causes, as reported.
“It is clear from this study that, in the Scottish population, the presence of hemoglobin in the faeces as detected by gFOBT is associated with a number of non-CRC causes of death,” investigators wrote.
These results do corroborate those of one other recent study in Taiwan that showed a relationship between positive gFOBT tests and all-cause mortality.
“In contrast to the Taiwanese study, we were able to examine this association broken down by disease categories and adjusting for confounding factors,” they noted in their discussion of the results.
Funding for the study came from the Chief Scientist Office of the Scottish Government Health Directorates. One study coauthor reported a consultancy with Immunostics, and no other disclosures were reported.
SOURCE: Libby G et al. Gut. 2018. doi: 10.1136/gutjnl-2018-316483.
FROM GUT
Key clinical point: Occult blood in the feces may be associated with not only colorectal cancer mortality, but also mortality from other causes.
Major finding: A positive fecal occult blood test was associated with a higher risk of death from noncolorectal cancer causes (adjusted hazard ratio, 1.58; 95% confidence interval, 1.45-1.73; P less than .0001).
Study details: A retrospective study in Scotland based on 133,921 screened individuals with linked mortality data.
Disclosures: Funding for the study came from the Chief Scientist Office of the Scottish Government Health Directorates. One study coauthor reported a consultancy with Immunostics.
Source: Libby G et al. Gut. 2018. doi: 10.1136/gutjnl-2018-316483.
Probiotics RCTs lack needed safety data, report says
, authors of a systematic review have concluded.
Out of 384 randomized clinical trials, nearly a third gave no information on potential harms associated with probiotics, prebiotics, or products that combine the two, according to authors of the review, which appears in Annals of Internal Medicine.
Only 2% of the trials adequately reported all key safety components, said the authors, led by Aïda Bafeta, PhD, Centre d’Épidémiologie Clinique, Hôpital Hôtel-Dieu, Paris.
“The inadequacy in reporting harms-related results may lead to an inaccurate safety profile and erroneous decision making, with major consequences for patients,” Dr. Bafeta and her colleagues wrote in their review.
While some may assume detailed safety evaluation is unnecessary, caution is especially needed when considering use of probiotics and prebiotics in patients who are vulnerable or critically ill, according to the authors.
“More worrying is that potential risks have been described in case reports and clinical trial results,” they wrote.
Dr. Bafeta and her coauthors cited a 2008 randomized, placebo-controlled trial published in The Lancet showing an increased risk of mortality associated with a particular combination of probiotic strains administered as prophylaxis in patients with predicted severe acute pancreatitis.
A 2011 report by the Agency for Healthcare Research and Quality found that current literature at that time was “not well equipped” to answer with confidence on the safety of probiotics as administered in clinical trials.
In the present report, Dr. Bafeta and her colleagues conducted a systematic review of 384 published randomized trials assessing probiotics, prebiotics, or synbiotics, which are products that combine probiotics and probiotics.
They found that 28% (106 trials) gave no information at all related to harms, while 90% (347) failed to define adverse events, and 97% (372) left out any mention of methods for collecting harms-related information.
Out of 53 studies including hospitalized or critical care patients, 7 reported the number of serious adverse events per study group, they said.
When safety data were included, reporting was often inadequate, according to Dr. Bafeta and her colleagues. Generic statements were used in 37% of the randomized trials, while 5% gave only global statistical comparisons.
Only 2% (nine trials) reported all safety-related parameters recommended by guidelines, such as adverse event definitions, seriousness of adverse events, and number of participant withdrawals due to harms, the reviewers found.
“The safety profile of an intervention should never be presumed,” they wrote. “Rather, it should be rigorously evaluated and reported.”
Probiotics and prebiotics are of increasing interest as treatments that may modify gut microbiota, potentially resulting in health benefits, they said. Probiotics are live microorganisms administered to confer a health benefit in the gut, while prebiotics are ingredients that change the composition or activity of gut microbiota.
No reviews previous to this one have assessed the adequacy of reporting adverse effects in trials of probiotics, prebiotics, and synbiotics, according to Dr. Bafeta and her colleagues, who reported no conflicts of interest associated with their review.
SOURCE: Bafeta A et al. Ann Intern Med. 2018 Jul 17.
, authors of a systematic review have concluded.
Out of 384 randomized clinical trials, nearly a third gave no information on potential harms associated with probiotics, prebiotics, or products that combine the two, according to authors of the review, which appears in Annals of Internal Medicine.
Only 2% of the trials adequately reported all key safety components, said the authors, led by Aïda Bafeta, PhD, Centre d’Épidémiologie Clinique, Hôpital Hôtel-Dieu, Paris.
“The inadequacy in reporting harms-related results may lead to an inaccurate safety profile and erroneous decision making, with major consequences for patients,” Dr. Bafeta and her colleagues wrote in their review.
While some may assume detailed safety evaluation is unnecessary, caution is especially needed when considering use of probiotics and prebiotics in patients who are vulnerable or critically ill, according to the authors.
“More worrying is that potential risks have been described in case reports and clinical trial results,” they wrote.
Dr. Bafeta and her coauthors cited a 2008 randomized, placebo-controlled trial published in The Lancet showing an increased risk of mortality associated with a particular combination of probiotic strains administered as prophylaxis in patients with predicted severe acute pancreatitis.
A 2011 report by the Agency for Healthcare Research and Quality found that current literature at that time was “not well equipped” to answer with confidence on the safety of probiotics as administered in clinical trials.
In the present report, Dr. Bafeta and her colleagues conducted a systematic review of 384 published randomized trials assessing probiotics, prebiotics, or synbiotics, which are products that combine probiotics and probiotics.
They found that 28% (106 trials) gave no information at all related to harms, while 90% (347) failed to define adverse events, and 97% (372) left out any mention of methods for collecting harms-related information.
Out of 53 studies including hospitalized or critical care patients, 7 reported the number of serious adverse events per study group, they said.
When safety data were included, reporting was often inadequate, according to Dr. Bafeta and her colleagues. Generic statements were used in 37% of the randomized trials, while 5% gave only global statistical comparisons.
Only 2% (nine trials) reported all safety-related parameters recommended by guidelines, such as adverse event definitions, seriousness of adverse events, and number of participant withdrawals due to harms, the reviewers found.
“The safety profile of an intervention should never be presumed,” they wrote. “Rather, it should be rigorously evaluated and reported.”
Probiotics and prebiotics are of increasing interest as treatments that may modify gut microbiota, potentially resulting in health benefits, they said. Probiotics are live microorganisms administered to confer a health benefit in the gut, while prebiotics are ingredients that change the composition or activity of gut microbiota.
No reviews previous to this one have assessed the adequacy of reporting adverse effects in trials of probiotics, prebiotics, and synbiotics, according to Dr. Bafeta and her colleagues, who reported no conflicts of interest associated with their review.
SOURCE: Bafeta A et al. Ann Intern Med. 2018 Jul 17.
, authors of a systematic review have concluded.
Out of 384 randomized clinical trials, nearly a third gave no information on potential harms associated with probiotics, prebiotics, or products that combine the two, according to authors of the review, which appears in Annals of Internal Medicine.
Only 2% of the trials adequately reported all key safety components, said the authors, led by Aïda Bafeta, PhD, Centre d’Épidémiologie Clinique, Hôpital Hôtel-Dieu, Paris.
“The inadequacy in reporting harms-related results may lead to an inaccurate safety profile and erroneous decision making, with major consequences for patients,” Dr. Bafeta and her colleagues wrote in their review.
While some may assume detailed safety evaluation is unnecessary, caution is especially needed when considering use of probiotics and prebiotics in patients who are vulnerable or critically ill, according to the authors.
“More worrying is that potential risks have been described in case reports and clinical trial results,” they wrote.
Dr. Bafeta and her coauthors cited a 2008 randomized, placebo-controlled trial published in The Lancet showing an increased risk of mortality associated with a particular combination of probiotic strains administered as prophylaxis in patients with predicted severe acute pancreatitis.
A 2011 report by the Agency for Healthcare Research and Quality found that current literature at that time was “not well equipped” to answer with confidence on the safety of probiotics as administered in clinical trials.
In the present report, Dr. Bafeta and her colleagues conducted a systematic review of 384 published randomized trials assessing probiotics, prebiotics, or synbiotics, which are products that combine probiotics and probiotics.
They found that 28% (106 trials) gave no information at all related to harms, while 90% (347) failed to define adverse events, and 97% (372) left out any mention of methods for collecting harms-related information.
Out of 53 studies including hospitalized or critical care patients, 7 reported the number of serious adverse events per study group, they said.
When safety data were included, reporting was often inadequate, according to Dr. Bafeta and her colleagues. Generic statements were used in 37% of the randomized trials, while 5% gave only global statistical comparisons.
Only 2% (nine trials) reported all safety-related parameters recommended by guidelines, such as adverse event definitions, seriousness of adverse events, and number of participant withdrawals due to harms, the reviewers found.
“The safety profile of an intervention should never be presumed,” they wrote. “Rather, it should be rigorously evaluated and reported.”
Probiotics and prebiotics are of increasing interest as treatments that may modify gut microbiota, potentially resulting in health benefits, they said. Probiotics are live microorganisms administered to confer a health benefit in the gut, while prebiotics are ingredients that change the composition or activity of gut microbiota.
No reviews previous to this one have assessed the adequacy of reporting adverse effects in trials of probiotics, prebiotics, and synbiotics, according to Dr. Bafeta and her colleagues, who reported no conflicts of interest associated with their review.
SOURCE: Bafeta A et al. Ann Intern Med. 2018 Jul 17.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Clinical trials of interventions designed to modify gut microbiota are often lacking adverse event data, making broad conclusions about their safety impossible.
Major finding: Nearly one-third of trials gave no information on potential harms associated with the probiotics, prebiotics, or synbiotics under study, and only 2% reported all key safety components.
Study details: A systematic review of 384 published randomized trials assessing probiotics, prebiotics, or synbiotics,
Disclosures: The study authors reported no conflicts of interest.
Source: Bafeta A et al. Ann Intern Med. 2018 Jul 17.
What the (HM) world needs now
Practice compassion to rise to the challenges of HM
If you are in the business of health care – whether as a direct care provider who is doing their best in an increasingly complex system with an increasingly complex panel of patients; a hospital medicine group leader who is trying to keep a group afloat and lead people through this rocky terrain; or a hospital system leader or chief medical officer dealing with the arcane and ever-changing landscape – there is one universal truth: This business is hard.
You can call it “challenging.” You can say there are “opportunities for improvement.” You can put all kinds of sugar on top, but at times, it is a bitter drink to swallow.
So why, as hospitalists, do we keep doing this?
I always joke that I’m going to open a “fro-yo” stand on the beach, but of course, I never do. And that constancy is one huge reason why I love hospitalists. We are always trying to decode, unlock, and solve some of these seemingly unsolvable problems. But at the same time, this plethora of constant change and instability at all kinds of levels can be a bit, well, impossible.
How do we do it every day? You can change jobs, change patient panels, and change medical systems, but no matter what, you will be confronted on some level with a gap of clearly defined solutions to your “challenges.”
One thing in my arsenal of coping, beyond my fro-yo fantasy, is simply this: compassion. When one of your providers comes to you and is complaining about their workload, don’t tell them about how you used to see three times as many patients at your last job. Instead, put your hand on their shoulder, look them in the eye, and say “It is hard. It is.”
When the CEO of your hospital tells you that the already tiny margin of the hospital is shrinking, and she has to cut a service you feel is indispensable, reflect her pain. Believe me – she feels it.
To practice compassion in hospital medicine is to accept that medicine is hard on everyone. It’s not “us” versus “them.” It’s not just “us” that hurts and “them” that are immune. We all struggle.
We need – I need – to acknowledge the pain this profession often elicits. It can be burnout, resentment, overarching grief, or incredible frustration with broken systems and sometimes broken people. When we deny it, when we try to shove those feelings deep down, then people – good people who feel these things – perceive they are flawed or somehow not cut out for this profession. So they end up leaving. Or imploding.
Instead, if we practice compassion for ourselves and each other, we may find strength and restoration in these relationships with others. We will normalize these very normal responses to the challenges we face every day. And we may then survive all these “opportunities for improvement.”
I challenge everyone to practice this simple compassionate meditation. It will take less than five minutes. As you lay in bed at night, your mind racing, concentrate on feeling compassion for four different people. Start with the person you don’t know well, such as the person who works at the dry cleaner. Breathe deeply. Pick a sentence – a gift to give. I always think, “I wish you happy and healthy, wealthy and wise.” Do this for three or four deep breaths.
Next, using this same technique, choose someone that is hard to feel compassion for – perhaps that difficult family member, or the co-worker that gets under your skin.
Then feel that compassion. Breathe deeply – for yourself, with all your human frailties. You don’t have to be perfect to be loved or lovable. Feel that.
Finally, take a deep breath, feel your chest opening, expanding. Feel that compassion for the whole world – the whole crummy mixed-up world that’s just doing its best. The world needs our compassion, too.
While you were at HM18, I hope you were able to look into the eyes of the others you see. These are your fellow hospitalists. People who feel your joys, your frustrations. Some of those eyes will be bright and excited; others will be worn and tired. But revel in this shared and universal knowledge.
It is hard. But with compassion and understanding, we can make it a bit better. For all of us.
Read the full post at hospitalleader.org.
Ms. Cardin, ACNP-BC, SFHM is vice president, Advanced Practice Providers, at Sound Physicians, and also serves on SHM’s Board of Directors.
Also in The Hospital Leader
- How Can Hospitalists Improve Their HCAHPS Scores? by Leslie Flores, MHA, SFHM
- “Harper’s Index” of Hospital Medicine 2018 by Jordan Messler, MD, SFHM
- What’s a Cost, Charge, and Price? by Brad Flansbaum, DO, MPH, MHM
Practice compassion to rise to the challenges of HM
Practice compassion to rise to the challenges of HM
If you are in the business of health care – whether as a direct care provider who is doing their best in an increasingly complex system with an increasingly complex panel of patients; a hospital medicine group leader who is trying to keep a group afloat and lead people through this rocky terrain; or a hospital system leader or chief medical officer dealing with the arcane and ever-changing landscape – there is one universal truth: This business is hard.
You can call it “challenging.” You can say there are “opportunities for improvement.” You can put all kinds of sugar on top, but at times, it is a bitter drink to swallow.
So why, as hospitalists, do we keep doing this?
I always joke that I’m going to open a “fro-yo” stand on the beach, but of course, I never do. And that constancy is one huge reason why I love hospitalists. We are always trying to decode, unlock, and solve some of these seemingly unsolvable problems. But at the same time, this plethora of constant change and instability at all kinds of levels can be a bit, well, impossible.
How do we do it every day? You can change jobs, change patient panels, and change medical systems, but no matter what, you will be confronted on some level with a gap of clearly defined solutions to your “challenges.”
One thing in my arsenal of coping, beyond my fro-yo fantasy, is simply this: compassion. When one of your providers comes to you and is complaining about their workload, don’t tell them about how you used to see three times as many patients at your last job. Instead, put your hand on their shoulder, look them in the eye, and say “It is hard. It is.”
When the CEO of your hospital tells you that the already tiny margin of the hospital is shrinking, and she has to cut a service you feel is indispensable, reflect her pain. Believe me – she feels it.
To practice compassion in hospital medicine is to accept that medicine is hard on everyone. It’s not “us” versus “them.” It’s not just “us” that hurts and “them” that are immune. We all struggle.
We need – I need – to acknowledge the pain this profession often elicits. It can be burnout, resentment, overarching grief, or incredible frustration with broken systems and sometimes broken people. When we deny it, when we try to shove those feelings deep down, then people – good people who feel these things – perceive they are flawed or somehow not cut out for this profession. So they end up leaving. Or imploding.
Instead, if we practice compassion for ourselves and each other, we may find strength and restoration in these relationships with others. We will normalize these very normal responses to the challenges we face every day. And we may then survive all these “opportunities for improvement.”
I challenge everyone to practice this simple compassionate meditation. It will take less than five minutes. As you lay in bed at night, your mind racing, concentrate on feeling compassion for four different people. Start with the person you don’t know well, such as the person who works at the dry cleaner. Breathe deeply. Pick a sentence – a gift to give. I always think, “I wish you happy and healthy, wealthy and wise.” Do this for three or four deep breaths.
Next, using this same technique, choose someone that is hard to feel compassion for – perhaps that difficult family member, or the co-worker that gets under your skin.
Then feel that compassion. Breathe deeply – for yourself, with all your human frailties. You don’t have to be perfect to be loved or lovable. Feel that.
Finally, take a deep breath, feel your chest opening, expanding. Feel that compassion for the whole world – the whole crummy mixed-up world that’s just doing its best. The world needs our compassion, too.
While you were at HM18, I hope you were able to look into the eyes of the others you see. These are your fellow hospitalists. People who feel your joys, your frustrations. Some of those eyes will be bright and excited; others will be worn and tired. But revel in this shared and universal knowledge.
It is hard. But with compassion and understanding, we can make it a bit better. For all of us.
Read the full post at hospitalleader.org.
Ms. Cardin, ACNP-BC, SFHM is vice president, Advanced Practice Providers, at Sound Physicians, and also serves on SHM’s Board of Directors.
Also in The Hospital Leader
- How Can Hospitalists Improve Their HCAHPS Scores? by Leslie Flores, MHA, SFHM
- “Harper’s Index” of Hospital Medicine 2018 by Jordan Messler, MD, SFHM
- What’s a Cost, Charge, and Price? by Brad Flansbaum, DO, MPH, MHM
If you are in the business of health care – whether as a direct care provider who is doing their best in an increasingly complex system with an increasingly complex panel of patients; a hospital medicine group leader who is trying to keep a group afloat and lead people through this rocky terrain; or a hospital system leader or chief medical officer dealing with the arcane and ever-changing landscape – there is one universal truth: This business is hard.
You can call it “challenging.” You can say there are “opportunities for improvement.” You can put all kinds of sugar on top, but at times, it is a bitter drink to swallow.
So why, as hospitalists, do we keep doing this?
I always joke that I’m going to open a “fro-yo” stand on the beach, but of course, I never do. And that constancy is one huge reason why I love hospitalists. We are always trying to decode, unlock, and solve some of these seemingly unsolvable problems. But at the same time, this plethora of constant change and instability at all kinds of levels can be a bit, well, impossible.
How do we do it every day? You can change jobs, change patient panels, and change medical systems, but no matter what, you will be confronted on some level with a gap of clearly defined solutions to your “challenges.”
One thing in my arsenal of coping, beyond my fro-yo fantasy, is simply this: compassion. When one of your providers comes to you and is complaining about their workload, don’t tell them about how you used to see three times as many patients at your last job. Instead, put your hand on their shoulder, look them in the eye, and say “It is hard. It is.”
When the CEO of your hospital tells you that the already tiny margin of the hospital is shrinking, and she has to cut a service you feel is indispensable, reflect her pain. Believe me – she feels it.
To practice compassion in hospital medicine is to accept that medicine is hard on everyone. It’s not “us” versus “them.” It’s not just “us” that hurts and “them” that are immune. We all struggle.
We need – I need – to acknowledge the pain this profession often elicits. It can be burnout, resentment, overarching grief, or incredible frustration with broken systems and sometimes broken people. When we deny it, when we try to shove those feelings deep down, then people – good people who feel these things – perceive they are flawed or somehow not cut out for this profession. So they end up leaving. Or imploding.
Instead, if we practice compassion for ourselves and each other, we may find strength and restoration in these relationships with others. We will normalize these very normal responses to the challenges we face every day. And we may then survive all these “opportunities for improvement.”
I challenge everyone to practice this simple compassionate meditation. It will take less than five minutes. As you lay in bed at night, your mind racing, concentrate on feeling compassion for four different people. Start with the person you don’t know well, such as the person who works at the dry cleaner. Breathe deeply. Pick a sentence – a gift to give. I always think, “I wish you happy and healthy, wealthy and wise.” Do this for three or four deep breaths.
Next, using this same technique, choose someone that is hard to feel compassion for – perhaps that difficult family member, or the co-worker that gets under your skin.
Then feel that compassion. Breathe deeply – for yourself, with all your human frailties. You don’t have to be perfect to be loved or lovable. Feel that.
Finally, take a deep breath, feel your chest opening, expanding. Feel that compassion for the whole world – the whole crummy mixed-up world that’s just doing its best. The world needs our compassion, too.
While you were at HM18, I hope you were able to look into the eyes of the others you see. These are your fellow hospitalists. People who feel your joys, your frustrations. Some of those eyes will be bright and excited; others will be worn and tired. But revel in this shared and universal knowledge.
It is hard. But with compassion and understanding, we can make it a bit better. For all of us.
Read the full post at hospitalleader.org.
Ms. Cardin, ACNP-BC, SFHM is vice president, Advanced Practice Providers, at Sound Physicians, and also serves on SHM’s Board of Directors.
Also in The Hospital Leader
- How Can Hospitalists Improve Their HCAHPS Scores? by Leslie Flores, MHA, SFHM
- “Harper’s Index” of Hospital Medicine 2018 by Jordan Messler, MD, SFHM
- What’s a Cost, Charge, and Price? by Brad Flansbaum, DO, MPH, MHM
No gender bias found in ABS exam
, an analysis found.
Lead author Thai Q. Ong of the James Madison University Center for Assessment and Research Studies, Harrisonburg, Va., and colleagues examined data from the 2016-2017 ABS general surgery certifying exam (CE), which included 1,341 examinees and 216 examiners. Of examinees, 61% were male and of examiners, 82% were male. Investigators used factorial analysis of variance and logistic regression analyses to evaluate the effect of examinee and examiner gender on CE ratings and likelihood of passing the CE.
Results showed that gender was not a factor in rates received, and the gender of examiners had no bearing on the ratings they gave examinees of either gender, according to the study, published in the Journal of Surgical Research. In addition, examiner teams of different gender combinations did not affect their ratings of examinees. The investigators found also that examinee gender was not a significant predictor of session pass rates nor was examiner gender a factor in session pass rates.
The study authors concluded that there is no evidence of gender bias in ABS certifying exam rates or the likelihood of passing the exam. “Although these findings are favorable, the ABS continues to undertake efforts to minimize potential examiner bias in future examinations. All examiners are required to participate in rater training as well as implicit bias training in advance of the general surgery CE. The ABS has also added information on preventing implicit bias in the instructions that examiners receive in preparation for these exams.”
However, they noted, further studies should be conducted to replicate the results and explore other possible sources of examiner bias in CE ratings.
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
SOURCE: Ong et al. J Surg Res. 2018 June doi: 10.1016/j.jss.2018.06.014.
, an analysis found.
Lead author Thai Q. Ong of the James Madison University Center for Assessment and Research Studies, Harrisonburg, Va., and colleagues examined data from the 2016-2017 ABS general surgery certifying exam (CE), which included 1,341 examinees and 216 examiners. Of examinees, 61% were male and of examiners, 82% were male. Investigators used factorial analysis of variance and logistic regression analyses to evaluate the effect of examinee and examiner gender on CE ratings and likelihood of passing the CE.
Results showed that gender was not a factor in rates received, and the gender of examiners had no bearing on the ratings they gave examinees of either gender, according to the study, published in the Journal of Surgical Research. In addition, examiner teams of different gender combinations did not affect their ratings of examinees. The investigators found also that examinee gender was not a significant predictor of session pass rates nor was examiner gender a factor in session pass rates.
The study authors concluded that there is no evidence of gender bias in ABS certifying exam rates or the likelihood of passing the exam. “Although these findings are favorable, the ABS continues to undertake efforts to minimize potential examiner bias in future examinations. All examiners are required to participate in rater training as well as implicit bias training in advance of the general surgery CE. The ABS has also added information on preventing implicit bias in the instructions that examiners receive in preparation for these exams.”
However, they noted, further studies should be conducted to replicate the results and explore other possible sources of examiner bias in CE ratings.
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
SOURCE: Ong et al. J Surg Res. 2018 June doi: 10.1016/j.jss.2018.06.014.
, an analysis found.
Lead author Thai Q. Ong of the James Madison University Center for Assessment and Research Studies, Harrisonburg, Va., and colleagues examined data from the 2016-2017 ABS general surgery certifying exam (CE), which included 1,341 examinees and 216 examiners. Of examinees, 61% were male and of examiners, 82% were male. Investigators used factorial analysis of variance and logistic regression analyses to evaluate the effect of examinee and examiner gender on CE ratings and likelihood of passing the CE.
Results showed that gender was not a factor in rates received, and the gender of examiners had no bearing on the ratings they gave examinees of either gender, according to the study, published in the Journal of Surgical Research. In addition, examiner teams of different gender combinations did not affect their ratings of examinees. The investigators found also that examinee gender was not a significant predictor of session pass rates nor was examiner gender a factor in session pass rates.
The study authors concluded that there is no evidence of gender bias in ABS certifying exam rates or the likelihood of passing the exam. “Although these findings are favorable, the ABS continues to undertake efforts to minimize potential examiner bias in future examinations. All examiners are required to participate in rater training as well as implicit bias training in advance of the general surgery CE. The ABS has also added information on preventing implicit bias in the instructions that examiners receive in preparation for these exams.”
However, they noted, further studies should be conducted to replicate the results and explore other possible sources of examiner bias in CE ratings.
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
SOURCE: Ong et al. J Surg Res. 2018 June doi: 10.1016/j.jss.2018.06.014.
Key clinical point: The gender of examinees and the gender of examiners do not affect ABS exam outcomes.
Major finding: No correlation was found between surgeon gender and CE ratings received.
Study details: Investigators examined data from the 2016-2017 ABS general surgery certifying exam, which consisted of 1,341 examinees and 216 examiners.
Disclosures: The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Source: Ong et al. J Surg Res. 2018 Jun. doi: 10.1016/j.jss.2018.06.014.
More testing of febrile infants at teaching vs. community hospitals, but similar outcomes
TORONTO – according to a study presented at the Pediatric Academic Societies annual meeting.
“The community hospitals are doing less procedures on the infants, but with basically the exact same outcomes,” said Beth C. Natt, MD, MPH, director of pediatric hospital medicine at Bridgeport (Conn.) Hospital.
Babies who presented to university-affiliated hospitals were more likely to be hospitalized (70% vs. 67%; P = .001) than were those at community hospitals, but had a similar likelihood of being diagnosed with bacteremia, meningitis, or urinary tract infection. The rates of missed bacterial infection were 0.8% for teaching hospitals and 1% for community hospitals (P = .346).
“There is some thought that in community settings, because we’re not completing the workup in the standard, protocolized way seen at teaching hospitals, we might be doing wrong by the children, but these data show we’re actually doing just fine,” Dr. Natt said in an interview.
She and her colleagues reviewed 9,884 febrile infant evaluations occurring at 132 hospitals participating in the Reducing Excessive Variation in the Infant Sepsis Evaluation (REVISE) quality improvement project. Two-thirds of the infants (n = 6,479) were evaluated across 78 university-affiliated hospitals and 3,405 (or 34%) were seen at 54 community hospitals. Hospital status was self-reported.
The teaching hospitals more often had at least one pediatric emergency medicine provider, compared with community hospitals (90% vs. 57%; P = .001) and were more likely to see babies between 7 and 30 days old (90% vs. 57%; P = .001). They also were more likely to obtain urine cultures (92% vs. 88%; P = 0.001), blood cultures (84% vs. 80%; P = .001), and cerebral spinal fluid cultures (62% vs. 57%; P = .001).
On the other hand, community hospitals were significantly more likely to see children presenting with respiratory symptoms (39% vs. 36% for teaching hospitals; P = .014), and were more likely to order chest x-rays on febrile infants (32% vs. 24% for university-affiliated hospitals; P = .001).
“As a community hospitalist, the results weren’t that surprising to me,” said Dr. Natt. “If anything was surprising it was how often we were doing chest x-rays, but I think that had to do with the fact that we had more children with respiratory symptoms coming to community hospitals.
“The American Academy of Pediatrics guidelines for fever were written last in 1993, when I was in high school, so they are very due to be revised,” said Dr. Natt. “I suspect the new guidelines will have us doing fewer spinal taps in children and more watchful waiting.”
TORONTO – according to a study presented at the Pediatric Academic Societies annual meeting.
“The community hospitals are doing less procedures on the infants, but with basically the exact same outcomes,” said Beth C. Natt, MD, MPH, director of pediatric hospital medicine at Bridgeport (Conn.) Hospital.
Babies who presented to university-affiliated hospitals were more likely to be hospitalized (70% vs. 67%; P = .001) than were those at community hospitals, but had a similar likelihood of being diagnosed with bacteremia, meningitis, or urinary tract infection. The rates of missed bacterial infection were 0.8% for teaching hospitals and 1% for community hospitals (P = .346).
“There is some thought that in community settings, because we’re not completing the workup in the standard, protocolized way seen at teaching hospitals, we might be doing wrong by the children, but these data show we’re actually doing just fine,” Dr. Natt said in an interview.
She and her colleagues reviewed 9,884 febrile infant evaluations occurring at 132 hospitals participating in the Reducing Excessive Variation in the Infant Sepsis Evaluation (REVISE) quality improvement project. Two-thirds of the infants (n = 6,479) were evaluated across 78 university-affiliated hospitals and 3,405 (or 34%) were seen at 54 community hospitals. Hospital status was self-reported.
The teaching hospitals more often had at least one pediatric emergency medicine provider, compared with community hospitals (90% vs. 57%; P = .001) and were more likely to see babies between 7 and 30 days old (90% vs. 57%; P = .001). They also were more likely to obtain urine cultures (92% vs. 88%; P = 0.001), blood cultures (84% vs. 80%; P = .001), and cerebral spinal fluid cultures (62% vs. 57%; P = .001).
On the other hand, community hospitals were significantly more likely to see children presenting with respiratory symptoms (39% vs. 36% for teaching hospitals; P = .014), and were more likely to order chest x-rays on febrile infants (32% vs. 24% for university-affiliated hospitals; P = .001).
“As a community hospitalist, the results weren’t that surprising to me,” said Dr. Natt. “If anything was surprising it was how often we were doing chest x-rays, but I think that had to do with the fact that we had more children with respiratory symptoms coming to community hospitals.
“The American Academy of Pediatrics guidelines for fever were written last in 1993, when I was in high school, so they are very due to be revised,” said Dr. Natt. “I suspect the new guidelines will have us doing fewer spinal taps in children and more watchful waiting.”
TORONTO – according to a study presented at the Pediatric Academic Societies annual meeting.
“The community hospitals are doing less procedures on the infants, but with basically the exact same outcomes,” said Beth C. Natt, MD, MPH, director of pediatric hospital medicine at Bridgeport (Conn.) Hospital.
Babies who presented to university-affiliated hospitals were more likely to be hospitalized (70% vs. 67%; P = .001) than were those at community hospitals, but had a similar likelihood of being diagnosed with bacteremia, meningitis, or urinary tract infection. The rates of missed bacterial infection were 0.8% for teaching hospitals and 1% for community hospitals (P = .346).
“There is some thought that in community settings, because we’re not completing the workup in the standard, protocolized way seen at teaching hospitals, we might be doing wrong by the children, but these data show we’re actually doing just fine,” Dr. Natt said in an interview.
She and her colleagues reviewed 9,884 febrile infant evaluations occurring at 132 hospitals participating in the Reducing Excessive Variation in the Infant Sepsis Evaluation (REVISE) quality improvement project. Two-thirds of the infants (n = 6,479) were evaluated across 78 university-affiliated hospitals and 3,405 (or 34%) were seen at 54 community hospitals. Hospital status was self-reported.
The teaching hospitals more often had at least one pediatric emergency medicine provider, compared with community hospitals (90% vs. 57%; P = .001) and were more likely to see babies between 7 and 30 days old (90% vs. 57%; P = .001). They also were more likely to obtain urine cultures (92% vs. 88%; P = 0.001), blood cultures (84% vs. 80%; P = .001), and cerebral spinal fluid cultures (62% vs. 57%; P = .001).
On the other hand, community hospitals were significantly more likely to see children presenting with respiratory symptoms (39% vs. 36% for teaching hospitals; P = .014), and were more likely to order chest x-rays on febrile infants (32% vs. 24% for university-affiliated hospitals; P = .001).
“As a community hospitalist, the results weren’t that surprising to me,” said Dr. Natt. “If anything was surprising it was how often we were doing chest x-rays, but I think that had to do with the fact that we had more children with respiratory symptoms coming to community hospitals.
“The American Academy of Pediatrics guidelines for fever were written last in 1993, when I was in high school, so they are very due to be revised,” said Dr. Natt. “I suspect the new guidelines will have us doing fewer spinal taps in children and more watchful waiting.”
AT PAS 18
Key clinical point: University-affiliated hospitals do more invasive testing in febrile infants, but have outcomes similar to those of community hospitals.
Major finding: The rate of missed bacterial infection did not differ between hospital types: 0.8% for teaching hospitals and 1% for community hospitals (P = .346).
Study details: Review of 9,884 febrile infant evaluations occurring at 132 hospitals, 66% of which were university-affiliated hospitals and 34% of which were community hospitals.
Disclosures: The investigators reported no conflicts of interest.
Later diagnosis predicts poorer outcomes in adult-diagnosed cystic fibrosis
Older age at diagnosis, diabetes, and poorer lung function are all , new research suggests.
A growing number of people with cystic fibrosis are diagnosed in adulthood, partly because of increased awareness among physicians of variations in disease presentation, more accessible genotyping, and easier diagnostic criteria.
Adult-diagnosed cystic fibrosis patients generally have a milder form of the disease than that of those diagnosed in childhood; however, less is known about their prognosis and life expectancy.
Researchers reported the outcomes of a retrospective cohort study of 362 adults diagnosed with cystic fibrosis at age 18 years or older. The median age at diagnosis was 34.3 years, and 71% of patients presented with pulmonary and/or gastrointestinal symptoms. The study was published in Annals of the American Thoracic Society.
The patients were followed for a median of 7.7 years, during which time there were 15 lung transplants and 33 deaths without transplant. Overall, 10-year lung transplant–free survival was 87.7%, and 15-year survival was 86.1%.
Those who were diagnosed young and who had higher lung function had the best median survival times. For each 5-year increase in age at diagnosis, the risk of death or transplant increased by 24%, and for each 5% decrease in forced expiratory volume in one second (FEV1), the risk was 35% higher.
Individuals who had diabetes at baseline had a more than fourfold higher risk of death or transplant than did those without diabetes.
“While newborn screening programs will reduce the rate of missed diagnoses in the future, clinicians still need to consider CF as a possible diagnosis if individuals are presenting with suspicious CF symptoms (e.g. GI or pulmonary symptoms) during adulthood, particularly if born prior to the introduction of newborn screening in their jurisdiction,” wrote Sameer Desai, of the University of British Columbia, Vancouver, and his coauthors.
Commenting on the association with diabetes, the authors noted that this finding had some uncertainty but suggested the additional inflammatory burden could increase the risk of death in individuals with cystic fibrosis.
The authors highlighted that fewer than 5% of people with adult-diagnosed cystic fibrosis had two copies of the F508del mutation, which is associated with severe, early-onset disease. However, those who were homozygous for that mutation tended to be diagnosed at a younger adult age, had worse nutritional status and a lower FEV1 percent predicted, compared with the overall adult-diagnosed population.
“This finding suggests potential delays in CF diagnosis for these people leading to worse outcomes,” the authors wrote.
The researchers also identified 25 individuals who had a possible unconfirmed diagnosis based on the most recent cystic fibrosis diagnostic guidelines. These individuals were either asymptomatic or had unknown symptoms, had sweat chlorides at or below 60 mmol/L (where available), and either unknown or two non–cystic fibrosis–causing mutations. They were also more likely to be male, to be nonwhite, to have increased unknown mutations, and to be pancreatic sufficient, compared with individuals with a confirmed diagnosis.
The study looked at whether Pseudomonas aeruginosa and Burkholderia cepacia complex increased the risk of transplant or death, but found these did not significantly predict survival.
“Adult CF clinicians can use this information to educate newly diagnosed adults with CF about their prognosis and to guide treatment decisions, specifically those at high-risk for a worse prognosis,” the authors wrote.
The study was partly funded by the Rare Disease Foundation. Two authors declared support from Cystic Fibrosis Canada, but no other conflicts of interest were declared.
SOURCE: Desai A et al. Ann Am Thorac Soc. 2018 Jun 26. doi: 10.1513/AnnalsATS.201801-037OC.
Older age at diagnosis, diabetes, and poorer lung function are all , new research suggests.
A growing number of people with cystic fibrosis are diagnosed in adulthood, partly because of increased awareness among physicians of variations in disease presentation, more accessible genotyping, and easier diagnostic criteria.
Adult-diagnosed cystic fibrosis patients generally have a milder form of the disease than that of those diagnosed in childhood; however, less is known about their prognosis and life expectancy.
Researchers reported the outcomes of a retrospective cohort study of 362 adults diagnosed with cystic fibrosis at age 18 years or older. The median age at diagnosis was 34.3 years, and 71% of patients presented with pulmonary and/or gastrointestinal symptoms. The study was published in Annals of the American Thoracic Society.
The patients were followed for a median of 7.7 years, during which time there were 15 lung transplants and 33 deaths without transplant. Overall, 10-year lung transplant–free survival was 87.7%, and 15-year survival was 86.1%.
Those who were diagnosed young and who had higher lung function had the best median survival times. For each 5-year increase in age at diagnosis, the risk of death or transplant increased by 24%, and for each 5% decrease in forced expiratory volume in one second (FEV1), the risk was 35% higher.
Individuals who had diabetes at baseline had a more than fourfold higher risk of death or transplant than did those without diabetes.
“While newborn screening programs will reduce the rate of missed diagnoses in the future, clinicians still need to consider CF as a possible diagnosis if individuals are presenting with suspicious CF symptoms (e.g. GI or pulmonary symptoms) during adulthood, particularly if born prior to the introduction of newborn screening in their jurisdiction,” wrote Sameer Desai, of the University of British Columbia, Vancouver, and his coauthors.
Commenting on the association with diabetes, the authors noted that this finding had some uncertainty but suggested the additional inflammatory burden could increase the risk of death in individuals with cystic fibrosis.
The authors highlighted that fewer than 5% of people with adult-diagnosed cystic fibrosis had two copies of the F508del mutation, which is associated with severe, early-onset disease. However, those who were homozygous for that mutation tended to be diagnosed at a younger adult age, had worse nutritional status and a lower FEV1 percent predicted, compared with the overall adult-diagnosed population.
“This finding suggests potential delays in CF diagnosis for these people leading to worse outcomes,” the authors wrote.
The researchers also identified 25 individuals who had a possible unconfirmed diagnosis based on the most recent cystic fibrosis diagnostic guidelines. These individuals were either asymptomatic or had unknown symptoms, had sweat chlorides at or below 60 mmol/L (where available), and either unknown or two non–cystic fibrosis–causing mutations. They were also more likely to be male, to be nonwhite, to have increased unknown mutations, and to be pancreatic sufficient, compared with individuals with a confirmed diagnosis.
The study looked at whether Pseudomonas aeruginosa and Burkholderia cepacia complex increased the risk of transplant or death, but found these did not significantly predict survival.
“Adult CF clinicians can use this information to educate newly diagnosed adults with CF about their prognosis and to guide treatment decisions, specifically those at high-risk for a worse prognosis,” the authors wrote.
The study was partly funded by the Rare Disease Foundation. Two authors declared support from Cystic Fibrosis Canada, but no other conflicts of interest were declared.
SOURCE: Desai A et al. Ann Am Thorac Soc. 2018 Jun 26. doi: 10.1513/AnnalsATS.201801-037OC.
Older age at diagnosis, diabetes, and poorer lung function are all , new research suggests.
A growing number of people with cystic fibrosis are diagnosed in adulthood, partly because of increased awareness among physicians of variations in disease presentation, more accessible genotyping, and easier diagnostic criteria.
Adult-diagnosed cystic fibrosis patients generally have a milder form of the disease than that of those diagnosed in childhood; however, less is known about their prognosis and life expectancy.
Researchers reported the outcomes of a retrospective cohort study of 362 adults diagnosed with cystic fibrosis at age 18 years or older. The median age at diagnosis was 34.3 years, and 71% of patients presented with pulmonary and/or gastrointestinal symptoms. The study was published in Annals of the American Thoracic Society.
The patients were followed for a median of 7.7 years, during which time there were 15 lung transplants and 33 deaths without transplant. Overall, 10-year lung transplant–free survival was 87.7%, and 15-year survival was 86.1%.
Those who were diagnosed young and who had higher lung function had the best median survival times. For each 5-year increase in age at diagnosis, the risk of death or transplant increased by 24%, and for each 5% decrease in forced expiratory volume in one second (FEV1), the risk was 35% higher.
Individuals who had diabetes at baseline had a more than fourfold higher risk of death or transplant than did those without diabetes.
“While newborn screening programs will reduce the rate of missed diagnoses in the future, clinicians still need to consider CF as a possible diagnosis if individuals are presenting with suspicious CF symptoms (e.g. GI or pulmonary symptoms) during adulthood, particularly if born prior to the introduction of newborn screening in their jurisdiction,” wrote Sameer Desai, of the University of British Columbia, Vancouver, and his coauthors.
Commenting on the association with diabetes, the authors noted that this finding had some uncertainty but suggested the additional inflammatory burden could increase the risk of death in individuals with cystic fibrosis.
The authors highlighted that fewer than 5% of people with adult-diagnosed cystic fibrosis had two copies of the F508del mutation, which is associated with severe, early-onset disease. However, those who were homozygous for that mutation tended to be diagnosed at a younger adult age, had worse nutritional status and a lower FEV1 percent predicted, compared with the overall adult-diagnosed population.
“This finding suggests potential delays in CF diagnosis for these people leading to worse outcomes,” the authors wrote.
The researchers also identified 25 individuals who had a possible unconfirmed diagnosis based on the most recent cystic fibrosis diagnostic guidelines. These individuals were either asymptomatic or had unknown symptoms, had sweat chlorides at or below 60 mmol/L (where available), and either unknown or two non–cystic fibrosis–causing mutations. They were also more likely to be male, to be nonwhite, to have increased unknown mutations, and to be pancreatic sufficient, compared with individuals with a confirmed diagnosis.
The study looked at whether Pseudomonas aeruginosa and Burkholderia cepacia complex increased the risk of transplant or death, but found these did not significantly predict survival.
“Adult CF clinicians can use this information to educate newly diagnosed adults with CF about their prognosis and to guide treatment decisions, specifically those at high-risk for a worse prognosis,” the authors wrote.
The study was partly funded by the Rare Disease Foundation. Two authors declared support from Cystic Fibrosis Canada, but no other conflicts of interest were declared.
SOURCE: Desai A et al. Ann Am Thorac Soc. 2018 Jun 26. doi: 10.1513/AnnalsATS.201801-037OC.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Key clinical point: Older age at diagnosis is a risk factor for death or transplant in adult-diagnosed cystic fibrosis.
Major finding: The risk of death or transplant increases by 24% per 5-year increase in age at diagnosis in adult-diagnosed cystic fibrosis.
Study details: A retrospective cohort study of 362 adults diagnosed with cystic fibrosis.
Disclosures: The study was partly funded by The Rare Disease Foundation. Two authors declared support from Cystic Fibrosis Canada, but no other conflicts of interest were declared.
Source: Desai A et al. Ann Am Thorac Soc. 2018 Jun 26. doi: 10.1513/AnnalsATS.201801-037OC.
FDA approves enzalutamide for non-metastatic CRPC
The Food and Drug Administration has expanded the prostate cancer indication for enzalutamide to include nonmetastatic castration-resistant prostate cancer (CRPC). The androgen-receptor inhibitor was first approved in 2012 for the treatment of patients with metastatic CRPC who had previously received chemotherapy and was granted approval in 2014 for men with metastatic CRPC who had not received chemotherapy.
The current approval was based on a statistically significant improvement in metastasis-free survival for patients receiving enzalutamide in the phase 3 PROSPER trial, a trial that randomized 1,401 patients (2:1) with nonmetastatic CRPC to 160 mg of oral enzalutamide daily or to placebo. Median metastasis-free survival was 36.6 months for those receiving enzalutamide versus 14.7 months for those receiving placebo (hazard ratio, 0.29; 95% confidence interval, 0.24-0.35; P less than .0001), the FDA said in a press statement.
The most common adverse events were asthenia/fatigue, hot flush, hypertension, dizziness, nausea, and falls.
The recommended dose for enzalutamide, marketed as Xtandi by Astellas Pharma US, is 160 mg (four 40-mg capsules) administered orally once daily.
The Food and Drug Administration has expanded the prostate cancer indication for enzalutamide to include nonmetastatic castration-resistant prostate cancer (CRPC). The androgen-receptor inhibitor was first approved in 2012 for the treatment of patients with metastatic CRPC who had previously received chemotherapy and was granted approval in 2014 for men with metastatic CRPC who had not received chemotherapy.
The current approval was based on a statistically significant improvement in metastasis-free survival for patients receiving enzalutamide in the phase 3 PROSPER trial, a trial that randomized 1,401 patients (2:1) with nonmetastatic CRPC to 160 mg of oral enzalutamide daily or to placebo. Median metastasis-free survival was 36.6 months for those receiving enzalutamide versus 14.7 months for those receiving placebo (hazard ratio, 0.29; 95% confidence interval, 0.24-0.35; P less than .0001), the FDA said in a press statement.
The most common adverse events were asthenia/fatigue, hot flush, hypertension, dizziness, nausea, and falls.
The recommended dose for enzalutamide, marketed as Xtandi by Astellas Pharma US, is 160 mg (four 40-mg capsules) administered orally once daily.
The Food and Drug Administration has expanded the prostate cancer indication for enzalutamide to include nonmetastatic castration-resistant prostate cancer (CRPC). The androgen-receptor inhibitor was first approved in 2012 for the treatment of patients with metastatic CRPC who had previously received chemotherapy and was granted approval in 2014 for men with metastatic CRPC who had not received chemotherapy.
The current approval was based on a statistically significant improvement in metastasis-free survival for patients receiving enzalutamide in the phase 3 PROSPER trial, a trial that randomized 1,401 patients (2:1) with nonmetastatic CRPC to 160 mg of oral enzalutamide daily or to placebo. Median metastasis-free survival was 36.6 months for those receiving enzalutamide versus 14.7 months for those receiving placebo (hazard ratio, 0.29; 95% confidence interval, 0.24-0.35; P less than .0001), the FDA said in a press statement.
The most common adverse events were asthenia/fatigue, hot flush, hypertension, dizziness, nausea, and falls.
The recommended dose for enzalutamide, marketed as Xtandi by Astellas Pharma US, is 160 mg (four 40-mg capsules) administered orally once daily.