User login
Adding elotuzumab improves myeloma PFS over pom/dex alone
STOCKHOLM –Adding the monoclonal antibody elotuzumab to pomalidomide and dexamethasone nearly doubled the overall response rate and median progression-free survival in patients with relapsed/refractory multiple myeloma compared with pomalidomide and dexamethasone alone, results of the phase 2 ELOQUENT-3 trial showed.
After a minimum follow-up of 9.1 months, median progression-free survival (PFS) for 60 patients assigned to receive elotuzumab (Empliciti), pomalidomide (Pomalyst), and dexamethasone (EPd) was 10.3 months, compared with 4.7 months for 60 patients assigned to pomalidomide and dexamethasone (Pd). This difference translated into a hazard ratio (HR) of 0.54 (P = .0078) favoring EPd, reported Meletios A. Dimopoulos, MD, of the National and Kapodistrian University of Athens (Greece).
“The study met its primary endpoint, which was specifically designed to detect a large treatment effect in a relatively small sample of patients. Elotuzumab with pomalidomide and dexamethasone showed a significant and clinically meaningful 46% reduction in the risk of progression or death,” he said at the annual congress of the European Hematology Association.
Elotuzumab is an immunoglobulin G (IgG) monoclonal antibody that targets signaling lymphocytic activation molecule F7 (SLAMF7) expressed on multiple myeloma cells. Pomalidomide, an immunomodulator, may act synergistically with elotuzumab through several different mechanisms to increase killing of multiple myeloma cells, Dr. Dimopoulos said.
In ELOQUENT-3, patients with relapsed or refractory multiple myeloma after 2 or more prior lines of therapy, including lenalidomide (Revlimid) and a proteasome inhibitor and no prior pomalidomide were enrolled and randomly assigned to receive either pomalidomide 4 mg orally on days 1-21 of each 28-day cycle plus oral dexamethasone 40 mg equivalent weekly, or to the same regimen plus intravenous elotuzumab 10 mg/kg weekly for cycles 1 and 2, and 20 mg/kg every 4 weeks for cycle 3 and subsequent cycles.
The trial met its primary endpoint of investigator-assessed PFS, with a 46% reduction in the risk of progression or death with EPd compared with Pd.
An analysis of PFS by subgroups showed that EPd was significantly superior to Pd for patients younger than 65 years, those with International Staging System stage I-II at study entry, patients with lactate dehydrogenase levels below 300 IU/L at baseline, patients who had two or three prior lines of therapy vs. four or more, and those who had disease that was refractory to both lenalidomide and a proteasome inhibitor.
EPd was also associated with a trend toward better PFS in an analysis combining patients with high-risk cytogenetics (deletion 17p or translocation 14;16) or high LDH levels, with a median of 7.7 months compared with 3.6 months for Pd. However, the HR, 0.55, was not statistically significant, likely because of the small sample size.
Similarly, the elotuzumab-containing combination showed a nonsignificant trend toward better PFS among patients without high risk disease, with a median PFS not reached, vs. not reached, vs. 4.7 months for patients treated with Pd.
The overall response rate with EPd was 53%, compared with 26% for Pd (odds ratio 3.5, P = .0029). The responses in the elotuzumab arm consisted of 8% complete response, 12% very good partial responses, and 33% partial responses. The respective rates in the Pd group were 2%, 7%, and 18%.
The median duration of response with EPd was not reached at the time of the database lock, compared with 8.3 months with Pd.
A preliminary analysis of overall survival showed a trend favoring EPd (13 deaths out to 22 months of follow-up, compared with 18 deaths out to 20 months in the Pd arm; HR 0.62, nonsignificant).
There were five treatment-related deaths in the EPd arm, and eight in the Pd arm. Grade 1 or 2 infusion reactions occurred in three patients in the EPd arm.
Other adverse events were comparable between the arms, with 57% of patients in the EPd arm and 60% in the Pd arm having at least one grade 3 or 4 adverse event.
“The hematologic toxicity was driven by pomalidomide and low-dose dexamethasone. For unclear reasons, there was less grade 3 or 4 neutropenia with the addition of elotuzumab to pomalidomide/dexamethasone, and also the infection rate was lower in the EPd arm,” Dr. Dimopoulos said.
SOURCE: Dimopoulos MA et al. EHA Congress, Abstract LB2606.
STOCKHOLM –Adding the monoclonal antibody elotuzumab to pomalidomide and dexamethasone nearly doubled the overall response rate and median progression-free survival in patients with relapsed/refractory multiple myeloma compared with pomalidomide and dexamethasone alone, results of the phase 2 ELOQUENT-3 trial showed.
After a minimum follow-up of 9.1 months, median progression-free survival (PFS) for 60 patients assigned to receive elotuzumab (Empliciti), pomalidomide (Pomalyst), and dexamethasone (EPd) was 10.3 months, compared with 4.7 months for 60 patients assigned to pomalidomide and dexamethasone (Pd). This difference translated into a hazard ratio (HR) of 0.54 (P = .0078) favoring EPd, reported Meletios A. Dimopoulos, MD, of the National and Kapodistrian University of Athens (Greece).
“The study met its primary endpoint, which was specifically designed to detect a large treatment effect in a relatively small sample of patients. Elotuzumab with pomalidomide and dexamethasone showed a significant and clinically meaningful 46% reduction in the risk of progression or death,” he said at the annual congress of the European Hematology Association.
Elotuzumab is an immunoglobulin G (IgG) monoclonal antibody that targets signaling lymphocytic activation molecule F7 (SLAMF7) expressed on multiple myeloma cells. Pomalidomide, an immunomodulator, may act synergistically with elotuzumab through several different mechanisms to increase killing of multiple myeloma cells, Dr. Dimopoulos said.
In ELOQUENT-3, patients with relapsed or refractory multiple myeloma after 2 or more prior lines of therapy, including lenalidomide (Revlimid) and a proteasome inhibitor and no prior pomalidomide were enrolled and randomly assigned to receive either pomalidomide 4 mg orally on days 1-21 of each 28-day cycle plus oral dexamethasone 40 mg equivalent weekly, or to the same regimen plus intravenous elotuzumab 10 mg/kg weekly for cycles 1 and 2, and 20 mg/kg every 4 weeks for cycle 3 and subsequent cycles.
The trial met its primary endpoint of investigator-assessed PFS, with a 46% reduction in the risk of progression or death with EPd compared with Pd.
An analysis of PFS by subgroups showed that EPd was significantly superior to Pd for patients younger than 65 years, those with International Staging System stage I-II at study entry, patients with lactate dehydrogenase levels below 300 IU/L at baseline, patients who had two or three prior lines of therapy vs. four or more, and those who had disease that was refractory to both lenalidomide and a proteasome inhibitor.
EPd was also associated with a trend toward better PFS in an analysis combining patients with high-risk cytogenetics (deletion 17p or translocation 14;16) or high LDH levels, with a median of 7.7 months compared with 3.6 months for Pd. However, the HR, 0.55, was not statistically significant, likely because of the small sample size.
Similarly, the elotuzumab-containing combination showed a nonsignificant trend toward better PFS among patients without high risk disease, with a median PFS not reached, vs. not reached, vs. 4.7 months for patients treated with Pd.
The overall response rate with EPd was 53%, compared with 26% for Pd (odds ratio 3.5, P = .0029). The responses in the elotuzumab arm consisted of 8% complete response, 12% very good partial responses, and 33% partial responses. The respective rates in the Pd group were 2%, 7%, and 18%.
The median duration of response with EPd was not reached at the time of the database lock, compared with 8.3 months with Pd.
A preliminary analysis of overall survival showed a trend favoring EPd (13 deaths out to 22 months of follow-up, compared with 18 deaths out to 20 months in the Pd arm; HR 0.62, nonsignificant).
There were five treatment-related deaths in the EPd arm, and eight in the Pd arm. Grade 1 or 2 infusion reactions occurred in three patients in the EPd arm.
Other adverse events were comparable between the arms, with 57% of patients in the EPd arm and 60% in the Pd arm having at least one grade 3 or 4 adverse event.
“The hematologic toxicity was driven by pomalidomide and low-dose dexamethasone. For unclear reasons, there was less grade 3 or 4 neutropenia with the addition of elotuzumab to pomalidomide/dexamethasone, and also the infection rate was lower in the EPd arm,” Dr. Dimopoulos said.
SOURCE: Dimopoulos MA et al. EHA Congress, Abstract LB2606.
STOCKHOLM –Adding the monoclonal antibody elotuzumab to pomalidomide and dexamethasone nearly doubled the overall response rate and median progression-free survival in patients with relapsed/refractory multiple myeloma compared with pomalidomide and dexamethasone alone, results of the phase 2 ELOQUENT-3 trial showed.
After a minimum follow-up of 9.1 months, median progression-free survival (PFS) for 60 patients assigned to receive elotuzumab (Empliciti), pomalidomide (Pomalyst), and dexamethasone (EPd) was 10.3 months, compared with 4.7 months for 60 patients assigned to pomalidomide and dexamethasone (Pd). This difference translated into a hazard ratio (HR) of 0.54 (P = .0078) favoring EPd, reported Meletios A. Dimopoulos, MD, of the National and Kapodistrian University of Athens (Greece).
“The study met its primary endpoint, which was specifically designed to detect a large treatment effect in a relatively small sample of patients. Elotuzumab with pomalidomide and dexamethasone showed a significant and clinically meaningful 46% reduction in the risk of progression or death,” he said at the annual congress of the European Hematology Association.
Elotuzumab is an immunoglobulin G (IgG) monoclonal antibody that targets signaling lymphocytic activation molecule F7 (SLAMF7) expressed on multiple myeloma cells. Pomalidomide, an immunomodulator, may act synergistically with elotuzumab through several different mechanisms to increase killing of multiple myeloma cells, Dr. Dimopoulos said.
In ELOQUENT-3, patients with relapsed or refractory multiple myeloma after 2 or more prior lines of therapy, including lenalidomide (Revlimid) and a proteasome inhibitor and no prior pomalidomide were enrolled and randomly assigned to receive either pomalidomide 4 mg orally on days 1-21 of each 28-day cycle plus oral dexamethasone 40 mg equivalent weekly, or to the same regimen plus intravenous elotuzumab 10 mg/kg weekly for cycles 1 and 2, and 20 mg/kg every 4 weeks for cycle 3 and subsequent cycles.
The trial met its primary endpoint of investigator-assessed PFS, with a 46% reduction in the risk of progression or death with EPd compared with Pd.
An analysis of PFS by subgroups showed that EPd was significantly superior to Pd for patients younger than 65 years, those with International Staging System stage I-II at study entry, patients with lactate dehydrogenase levels below 300 IU/L at baseline, patients who had two or three prior lines of therapy vs. four or more, and those who had disease that was refractory to both lenalidomide and a proteasome inhibitor.
EPd was also associated with a trend toward better PFS in an analysis combining patients with high-risk cytogenetics (deletion 17p or translocation 14;16) or high LDH levels, with a median of 7.7 months compared with 3.6 months for Pd. However, the HR, 0.55, was not statistically significant, likely because of the small sample size.
Similarly, the elotuzumab-containing combination showed a nonsignificant trend toward better PFS among patients without high risk disease, with a median PFS not reached, vs. not reached, vs. 4.7 months for patients treated with Pd.
The overall response rate with EPd was 53%, compared with 26% for Pd (odds ratio 3.5, P = .0029). The responses in the elotuzumab arm consisted of 8% complete response, 12% very good partial responses, and 33% partial responses. The respective rates in the Pd group were 2%, 7%, and 18%.
The median duration of response with EPd was not reached at the time of the database lock, compared with 8.3 months with Pd.
A preliminary analysis of overall survival showed a trend favoring EPd (13 deaths out to 22 months of follow-up, compared with 18 deaths out to 20 months in the Pd arm; HR 0.62, nonsignificant).
There were five treatment-related deaths in the EPd arm, and eight in the Pd arm. Grade 1 or 2 infusion reactions occurred in three patients in the EPd arm.
Other adverse events were comparable between the arms, with 57% of patients in the EPd arm and 60% in the Pd arm having at least one grade 3 or 4 adverse event.
“The hematologic toxicity was driven by pomalidomide and low-dose dexamethasone. For unclear reasons, there was less grade 3 or 4 neutropenia with the addition of elotuzumab to pomalidomide/dexamethasone, and also the infection rate was lower in the EPd arm,” Dr. Dimopoulos said.
SOURCE: Dimopoulos MA et al. EHA Congress, Abstract LB2606.
REPORTING FROM THE EHA CONGRESS
Key clinical point: Elotuzumab may have synergistic clinical activity with pomalidomide against multiple myeloma.
Major finding: Median PFS was 10.3 months with elotuzumab, pomalidomide, and dexamethasone vs. 4.7 months with pomalidomide and dexamethasone.
Study details: Randomized open-label phase 2 trial of 120 patients with multiple myeloma relapsed or refractory after 2 or more prior lines of therapy.
Disclosures: Bristol-Myers Squibb and AbbVie Biotherapeutics funded the study. Dr. Dimopoulos disclosed honoraria and/or consulting fees from Amgen, BMS, Celgene, Janssen and Takeda.
Source: Dimopoulos MA et al. EHA Congress, Abstract LB2606.
TAVR requirements tweaked in a 6-year update
Transcatheter aortic valve replacement has entered a new stage of development, and so needed a tweaked set of standards for how existing programs operate and what new program need to open, said a panel of experts formed by the four U.S. societies with the closest links to this procedure.
U.S. transcatheter aortic valve replacement (TAVR) programs have “matured as a therapeutic option” since its commercial U.S. introduction in 2012, said a revised statement of operator and institutional recommendations and requirements issued on July 18 by the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. A writing panel formed by these four groups prepared the revision, published online in the Journal of the American College of Cardiology on July 18, to replace the first set of recommendations for running U.S. TAVR programs that came out in 2012 (J Am Coll Cardiol. 2012 May 29;59[22]:2028-42).
“The main thrust is to ensure and allow for the metrics of quality TAVR,” said Joseph E. Bavaria, MD, cochair of the writing panel and professor of surgery and codirector of the transcatheter valve program at the University of Pennsylvania in Philadelphia. “We’re trying to force continuous quality improvement across U.S. TAVR teams,” Dr. Bavaria explained in an interview.
The key to this change will be the data collected on every U.S. TAVR patient in the Transcatheter Valve Therapy registry maintained by the American College of Cardiology and the Society of Thoracic Surgeons, which now has data on more than 120,000 patients who have undergone TAVR at what are now 582 active U.S. TAVR programs, noted Carl L. Tommaso, MD, an interventional cardiologist with NorthShore Medical Group in Bannockburn, Ill., and cochair of the writing panel. “You need to do risk adjustment to measure quality of care,” and the robust database that now exists has begun to make this possible, said Dr. Tommaso, who neither performs TAVR procedures nor participates on a TAVR team. Statistical analyses based on this substantial and always-expanding database of TAVR patients now allows for risk-adjusted assessment of in hospital and 30-day mortality, and risk-adjusted evaluation of 1-year mortality and quality-of-life outcomes are expected within the next couple of years.
“We’re still not yet at the point of having good, risk-adjusted models” for all these measures, but our hope is that in the next 2-7 years we can move completely to quality measures, as has already been done for percutaneous coronary interventions” and away from procedure volume, which currently serves as a surrogate marker for a TAVR program’s competence.
The new document continues to call for TAVR programs to average at least 50 TAVR procedures a year or at least 100 every 2 years, but primarily to insure that each TAVR program can generate enough data about its performance to produce statistically reliable numbers.
“The volume floors are only there because you can’t measure quality without volume,” said Dr. Bavaria. “It’s impossible to measure quality without a certain procedure volume.”
Dr. Bavaria stressed, however, that the goal of the new document is not to limit TAVR programs based on their procedure volume, especially because another goal of the document is to ensure reasonable geographic access to TAVR for U.S. patients. “This document does not advocate for any program to shut down,” he declared. On top of that, “we have no problem with new programs,” although the document noted that “the major threat to low volume sites growing and achieving higher levels of experience is the opening of additional sites in the same geographic region.”
In 2017, 204 of the 525 sites (39%) performing TAVR at that time were performing fewer than 50 procedures annually, the document said, but added that many TAVR centers now operate in what are predominantly rural regions “and it is important that they remain active if they can document acceptable quality even if they should fall below volume thresholds to maintain patient access to care.”
Dr. Tommaso said that perhaps a TAVR center in Alaska, for example, might not meet the 50 cases/year standard, “but I don’t think anyone would worry if the volume was low because we’re serving patients in Alaska.” Currently, 84% of TAVR centers that have been operating for more than 2 years meet the 50 procedures/year threshold, he added. And TAVR centers now operate in 49 of the 50 states, with only Wyoming lacking a TAVR facility within its borders. Despite this, use of TAVR among Wyoming residents is comparable to the rate in Illinois, Dr. Bavaria said.
Both cochairs also highlighted that, with TAVR now approved for patients at moderate risk for aortic-valve surgery, the number of patients who are TAVR candidates has grown, and it’s possible that pending trial results will soon broaden TAVR’s availability to low-risk patients, a step that would greatly further expand the potential patient pool for the procedure.
The revised recommendations and requirements will make it “a little easier to start a new program, except now, for the first time, you need to start with an operator who is already experienced with TAVR,” noted Dr. Bavaria. “The TAVR technology is now mature enough that it’s inappropriate to have learning-curve mortality.” But aside from this the new standards lower the bar a bit for a center’s volume of percutaneous coronary interventions and surgical aortic valve replacements. The revision also maintains that an examination by and consultation with a single cardiac surgeon by a prospective TAVR patient is adequate, similar to the 2012 document, although the Center for Medicare & Medicaid Services mandated in its coverage decision that prospective TAVR patients consult with two cardiac surgeons, the so-called “two-surgeon rule.” If CMS eliminated the two-surgeon rule it would “streamline” the process that patients go through when being assessed for TAVR, Dr. Tommaso observed, and both he and Dr. Bavaria expressed hope that the new document might prompt CMS to reconsider this guidance.
“We felt that two surgeons weren’t needed,” but the document specifies that both the surgeon and the cardiologist whom a prospective patient consults before finalizing plans for the intervention should both be members of the multidisciplinary team that performs the procedure. Until now, these clinicians weren’t specified as necessarily members of the TAVR team, Dr. Bavaria said.
One additional new element in the revised document is specification of shared decision making as the mechanism patients should go through when considering TAVR relative to their other management options, Dr. Tommaso said.Dr. Bavaria and Dr. Tommaso had no disclosures.
SOURCE: Bavaria JE et al. JACC. 2018 Jul18. doi:10.1016/j.jacc.2018.07.002.
Transcatheter aortic valve replacement has entered a new stage of development, and so needed a tweaked set of standards for how existing programs operate and what new program need to open, said a panel of experts formed by the four U.S. societies with the closest links to this procedure.
U.S. transcatheter aortic valve replacement (TAVR) programs have “matured as a therapeutic option” since its commercial U.S. introduction in 2012, said a revised statement of operator and institutional recommendations and requirements issued on July 18 by the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. A writing panel formed by these four groups prepared the revision, published online in the Journal of the American College of Cardiology on July 18, to replace the first set of recommendations for running U.S. TAVR programs that came out in 2012 (J Am Coll Cardiol. 2012 May 29;59[22]:2028-42).
“The main thrust is to ensure and allow for the metrics of quality TAVR,” said Joseph E. Bavaria, MD, cochair of the writing panel and professor of surgery and codirector of the transcatheter valve program at the University of Pennsylvania in Philadelphia. “We’re trying to force continuous quality improvement across U.S. TAVR teams,” Dr. Bavaria explained in an interview.
The key to this change will be the data collected on every U.S. TAVR patient in the Transcatheter Valve Therapy registry maintained by the American College of Cardiology and the Society of Thoracic Surgeons, which now has data on more than 120,000 patients who have undergone TAVR at what are now 582 active U.S. TAVR programs, noted Carl L. Tommaso, MD, an interventional cardiologist with NorthShore Medical Group in Bannockburn, Ill., and cochair of the writing panel. “You need to do risk adjustment to measure quality of care,” and the robust database that now exists has begun to make this possible, said Dr. Tommaso, who neither performs TAVR procedures nor participates on a TAVR team. Statistical analyses based on this substantial and always-expanding database of TAVR patients now allows for risk-adjusted assessment of in hospital and 30-day mortality, and risk-adjusted evaluation of 1-year mortality and quality-of-life outcomes are expected within the next couple of years.
“We’re still not yet at the point of having good, risk-adjusted models” for all these measures, but our hope is that in the next 2-7 years we can move completely to quality measures, as has already been done for percutaneous coronary interventions” and away from procedure volume, which currently serves as a surrogate marker for a TAVR program’s competence.
The new document continues to call for TAVR programs to average at least 50 TAVR procedures a year or at least 100 every 2 years, but primarily to insure that each TAVR program can generate enough data about its performance to produce statistically reliable numbers.
“The volume floors are only there because you can’t measure quality without volume,” said Dr. Bavaria. “It’s impossible to measure quality without a certain procedure volume.”
Dr. Bavaria stressed, however, that the goal of the new document is not to limit TAVR programs based on their procedure volume, especially because another goal of the document is to ensure reasonable geographic access to TAVR for U.S. patients. “This document does not advocate for any program to shut down,” he declared. On top of that, “we have no problem with new programs,” although the document noted that “the major threat to low volume sites growing and achieving higher levels of experience is the opening of additional sites in the same geographic region.”
In 2017, 204 of the 525 sites (39%) performing TAVR at that time were performing fewer than 50 procedures annually, the document said, but added that many TAVR centers now operate in what are predominantly rural regions “and it is important that they remain active if they can document acceptable quality even if they should fall below volume thresholds to maintain patient access to care.”
Dr. Tommaso said that perhaps a TAVR center in Alaska, for example, might not meet the 50 cases/year standard, “but I don’t think anyone would worry if the volume was low because we’re serving patients in Alaska.” Currently, 84% of TAVR centers that have been operating for more than 2 years meet the 50 procedures/year threshold, he added. And TAVR centers now operate in 49 of the 50 states, with only Wyoming lacking a TAVR facility within its borders. Despite this, use of TAVR among Wyoming residents is comparable to the rate in Illinois, Dr. Bavaria said.
Both cochairs also highlighted that, with TAVR now approved for patients at moderate risk for aortic-valve surgery, the number of patients who are TAVR candidates has grown, and it’s possible that pending trial results will soon broaden TAVR’s availability to low-risk patients, a step that would greatly further expand the potential patient pool for the procedure.
The revised recommendations and requirements will make it “a little easier to start a new program, except now, for the first time, you need to start with an operator who is already experienced with TAVR,” noted Dr. Bavaria. “The TAVR technology is now mature enough that it’s inappropriate to have learning-curve mortality.” But aside from this the new standards lower the bar a bit for a center’s volume of percutaneous coronary interventions and surgical aortic valve replacements. The revision also maintains that an examination by and consultation with a single cardiac surgeon by a prospective TAVR patient is adequate, similar to the 2012 document, although the Center for Medicare & Medicaid Services mandated in its coverage decision that prospective TAVR patients consult with two cardiac surgeons, the so-called “two-surgeon rule.” If CMS eliminated the two-surgeon rule it would “streamline” the process that patients go through when being assessed for TAVR, Dr. Tommaso observed, and both he and Dr. Bavaria expressed hope that the new document might prompt CMS to reconsider this guidance.
“We felt that two surgeons weren’t needed,” but the document specifies that both the surgeon and the cardiologist whom a prospective patient consults before finalizing plans for the intervention should both be members of the multidisciplinary team that performs the procedure. Until now, these clinicians weren’t specified as necessarily members of the TAVR team, Dr. Bavaria said.
One additional new element in the revised document is specification of shared decision making as the mechanism patients should go through when considering TAVR relative to their other management options, Dr. Tommaso said.Dr. Bavaria and Dr. Tommaso had no disclosures.
SOURCE: Bavaria JE et al. JACC. 2018 Jul18. doi:10.1016/j.jacc.2018.07.002.
Transcatheter aortic valve replacement has entered a new stage of development, and so needed a tweaked set of standards for how existing programs operate and what new program need to open, said a panel of experts formed by the four U.S. societies with the closest links to this procedure.
U.S. transcatheter aortic valve replacement (TAVR) programs have “matured as a therapeutic option” since its commercial U.S. introduction in 2012, said a revised statement of operator and institutional recommendations and requirements issued on July 18 by the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. A writing panel formed by these four groups prepared the revision, published online in the Journal of the American College of Cardiology on July 18, to replace the first set of recommendations for running U.S. TAVR programs that came out in 2012 (J Am Coll Cardiol. 2012 May 29;59[22]:2028-42).
“The main thrust is to ensure and allow for the metrics of quality TAVR,” said Joseph E. Bavaria, MD, cochair of the writing panel and professor of surgery and codirector of the transcatheter valve program at the University of Pennsylvania in Philadelphia. “We’re trying to force continuous quality improvement across U.S. TAVR teams,” Dr. Bavaria explained in an interview.
The key to this change will be the data collected on every U.S. TAVR patient in the Transcatheter Valve Therapy registry maintained by the American College of Cardiology and the Society of Thoracic Surgeons, which now has data on more than 120,000 patients who have undergone TAVR at what are now 582 active U.S. TAVR programs, noted Carl L. Tommaso, MD, an interventional cardiologist with NorthShore Medical Group in Bannockburn, Ill., and cochair of the writing panel. “You need to do risk adjustment to measure quality of care,” and the robust database that now exists has begun to make this possible, said Dr. Tommaso, who neither performs TAVR procedures nor participates on a TAVR team. Statistical analyses based on this substantial and always-expanding database of TAVR patients now allows for risk-adjusted assessment of in hospital and 30-day mortality, and risk-adjusted evaluation of 1-year mortality and quality-of-life outcomes are expected within the next couple of years.
“We’re still not yet at the point of having good, risk-adjusted models” for all these measures, but our hope is that in the next 2-7 years we can move completely to quality measures, as has already been done for percutaneous coronary interventions” and away from procedure volume, which currently serves as a surrogate marker for a TAVR program’s competence.
The new document continues to call for TAVR programs to average at least 50 TAVR procedures a year or at least 100 every 2 years, but primarily to insure that each TAVR program can generate enough data about its performance to produce statistically reliable numbers.
“The volume floors are only there because you can’t measure quality without volume,” said Dr. Bavaria. “It’s impossible to measure quality without a certain procedure volume.”
Dr. Bavaria stressed, however, that the goal of the new document is not to limit TAVR programs based on their procedure volume, especially because another goal of the document is to ensure reasonable geographic access to TAVR for U.S. patients. “This document does not advocate for any program to shut down,” he declared. On top of that, “we have no problem with new programs,” although the document noted that “the major threat to low volume sites growing and achieving higher levels of experience is the opening of additional sites in the same geographic region.”
In 2017, 204 of the 525 sites (39%) performing TAVR at that time were performing fewer than 50 procedures annually, the document said, but added that many TAVR centers now operate in what are predominantly rural regions “and it is important that they remain active if they can document acceptable quality even if they should fall below volume thresholds to maintain patient access to care.”
Dr. Tommaso said that perhaps a TAVR center in Alaska, for example, might not meet the 50 cases/year standard, “but I don’t think anyone would worry if the volume was low because we’re serving patients in Alaska.” Currently, 84% of TAVR centers that have been operating for more than 2 years meet the 50 procedures/year threshold, he added. And TAVR centers now operate in 49 of the 50 states, with only Wyoming lacking a TAVR facility within its borders. Despite this, use of TAVR among Wyoming residents is comparable to the rate in Illinois, Dr. Bavaria said.
Both cochairs also highlighted that, with TAVR now approved for patients at moderate risk for aortic-valve surgery, the number of patients who are TAVR candidates has grown, and it’s possible that pending trial results will soon broaden TAVR’s availability to low-risk patients, a step that would greatly further expand the potential patient pool for the procedure.
The revised recommendations and requirements will make it “a little easier to start a new program, except now, for the first time, you need to start with an operator who is already experienced with TAVR,” noted Dr. Bavaria. “The TAVR technology is now mature enough that it’s inappropriate to have learning-curve mortality.” But aside from this the new standards lower the bar a bit for a center’s volume of percutaneous coronary interventions and surgical aortic valve replacements. The revision also maintains that an examination by and consultation with a single cardiac surgeon by a prospective TAVR patient is adequate, similar to the 2012 document, although the Center for Medicare & Medicaid Services mandated in its coverage decision that prospective TAVR patients consult with two cardiac surgeons, the so-called “two-surgeon rule.” If CMS eliminated the two-surgeon rule it would “streamline” the process that patients go through when being assessed for TAVR, Dr. Tommaso observed, and both he and Dr. Bavaria expressed hope that the new document might prompt CMS to reconsider this guidance.
“We felt that two surgeons weren’t needed,” but the document specifies that both the surgeon and the cardiologist whom a prospective patient consults before finalizing plans for the intervention should both be members of the multidisciplinary team that performs the procedure. Until now, these clinicians weren’t specified as necessarily members of the TAVR team, Dr. Bavaria said.
One additional new element in the revised document is specification of shared decision making as the mechanism patients should go through when considering TAVR relative to their other management options, Dr. Tommaso said.Dr. Bavaria and Dr. Tommaso had no disclosures.
SOURCE: Bavaria JE et al. JACC. 2018 Jul18. doi:10.1016/j.jacc.2018.07.002.
FROM JACC
Spinal muscular atrophy added to newborn screening panel recommendations
Spinal muscular atrophy (SMA) is now among the disorders officially included in the Recommended Uniform Screening Panel (RUSP), which is used by state public health departments to screen newborns for genetic disorders.
Secretary of the Department of Health and Human Services Alex M. Azar II formally added SMA to the panel July 2 on the recommendation of the Advisory Committee on Heritable Disorders in Newborns and Children.
“Adding SMA to the list will help ensure that babies born with SMA are identified, so that they have the opportunity to benefit from early treatment and intervention,” according to a statement from the Muscular Dystrophy Association about the decision. “This testing can also provide families with a genetic diagnosis – information that often is required to determine whether their child is eligible to participate in clinical trials.”
Adding SMA to the RUSP does not mean states must screen newborns for the disorder. Each state’s public health apparatus decides independently whether to accept the recommendation and which disorders on the RUSP to screen for. Most states screen for most disorders on the RUSP. Evidence compiled by the advisory committee suggested wide variation in resources, infrastructure, funding, and time to implementation among states.
An estimated 1 in 11,000 newborns have SMA, a disorder caused by mutations in the survival motor neuron 1 (SMN1) gene. SMA affects motor neurons in the brain stem and spinal cord leading to motor weakness and atrophy. The only treatment for SMA had been palliative care until the Food and Drug Administration approved nusinersen (Spinraza) for the disorder in December 2016, although the drug’s approval has raised some ethical questions.1-3
After reviewing the evidence at their February 8, 2018 meeting, the advisory committee recommended the addition of spinal muscular atrophy screening to the RUSP in a March 8, 2018, letter from committee chair Joseph A. Bocchini Jr., MD, who is a professor and the chairman of the department of pediatrics at Louisiana State University Health in Shreveport.
Secretary Azar accepted the recommendation based on the evidence the committee provided; he also requested a follow-up report within 2 years “describing the status of implementing newborn screening for SMA and clinical outcomes of early treatment, including any potential harms, for infants diagnosed with SMA.”
The advisory committee makes its recommendations to the HHS on which heritable disorders to include in the RUSP after they have assessed a systematic, evidence-based review assigned by the committee to an external independent group. Alex R. Kemper, MD, MPH, a professor of pediatrics at the Ohio State University and division chief of ambulatory pediatrics at Nationwide Children’s Hospital, both in Columbus, led the review group for SMA. Dr. Kemper is also deputy editor of the journal Pediatrics and a member of the U.S. Preventive Services Task Force.
According to Secretary Azar’s summary in his July 2, 2018, letter of acceptance, the evidence review suggested that “early screening and treatment can lead to decreased mortality for individuals with SMA and improved motor milestones.”
Dr. Kemper elaborated in an interview that, “SMA can be detected through newborn screening, and treatment is now available that can not only reduce the risk of death but decrease the development of neurologic impairment. As with adding any condition to newborn screening, public health laboratories will need to develop strategies to incorporate the screening test. The current FDA-approved treatment, nusinersen, is delivered by lumbar puncture into the spinal fluid. In addition, there are exciting advances in gene therapy leading to new treatment approaches.”
Approximately 95% of SMA cases result from the deletion of exon 7 from both alleles of SMN1. (Other rarer cases are caused by mutations in different genes.) Without the SMN protein produced by SMN1, a person gradually loses muscle function.
A similar gene, SMN2, also can produce the SMN protein but in much lower amounts, typically less than 10% of what a person needs. People can, however, have multiple copies of SMN2, which can produce slightly more SMN protein for a slower disease process.
The five types of spinal muscular atrophy are determined according to symptom onset, which directly correlates with disorder severity and prognosis. Just over half (54%) of SMA cases are Type I, in which progressive weakness occurs over the first 6 months of life and results in early death. Only 18% of children with Type I live past age 4 years, and 68% die by age 2 years. Type 0 is rarer but more severe, usually causing fetal loss or early infant death.
Type II represents 18% of SMA cases and causes progressive weakness by age 15 months. Most people with Type II survive to their 30s but then experience respiratory failure and rarely reach their fourth decade. Individuals with Types III and IV typically have a normal lifespan and only begin to see progressive muscle weakness after 1 year old or in adulthood.
Dr. Kemper’s group focused on the three types diagnosed in infancy: types I, II, and III.
Dr. Kemper emphasized in an interview that “it will be critical to make sure that infants diagnosed with SMA through newborn screening receive follow-up shortly afterward to determine whether they would benefit from nusinersen. More information is needed about the long-term outcomes of those infants who begin treatment following newborn screening so we not only know about outcomes in later childhood and adolescence but treatment approaches can be further refined and personalized.”
Nusinersen works by altering the splicing of precursor messenger RNA in SMN2 so that the mRNA strands are longer, which thereby increases how much SMN protein is produced. Concerns about the medication, however, have included its cost – $750,000 in the first year and $375,000 every following year for life – and potential adverse events from repeated administration. Nusinersen is injected into the spinal canal four times in the first year and then once annually, and the painful injections require patient immobilization. Potential adverse events include thrombocytopenia and nephrotoxicity, along with potential complications from repeated lumbar punctures over time.2
Other concerns about the drug include its limited evidence base, lack of long-term data, associated costs with administration (for example, travel costs), the potential for patients taking nusinersen to be excluded from future clinical trials on other treatments, and ensuring parents have enough information on the drug’s limitations and potential risks to provide adequate informed consent.2
Yet evidence to date is favorable in children with early onset. Dr. Bocchini wrote in the letter to Secretary Azar that “limited data suggest that treatment effect is greater when the treatment is initiated before symptoms develop and when the individual has more copies of SMN2.”
Dr. Kemper’s group concluded that screening can detect SMA in newborns and that treatment can modify disease course. “Grey literature suggests those with total disease duration less than or equal to 12 weeks before nusinersen treatment were more likely to have better outcomes than those with longer periods of disease duration.”
“Presymptomatic treatment alters the natural history” of the disorder, the group found, although outcome data past 1 year of age are not yet available. Based on findings from a New York pilot program, they predicted that nationwide newborn screening would avert 33 deaths and 48 cases of children who were dependent on a ventilator among an annual cohort of 4 million births.
At the time of the evidence review, Massachusetts, Minnesota, Missouri, North Carolina, New York, Utah, and Wisconsin initiated pilot programs or whole-population mandated screening for SMA. Of the three states that reported costs, all reported costs at $1 or less per screen.
The research for the evidence review was funded by a Health Resources and Services Administration grant to Duke University, Durham, N.C. No disclosures were provided for evidence review group members.
References
1. Gene Ther. 2017 Sep;24(9):534-8.
2. JAMA Intern Med. 2018 Jun 1;178(6):743-44.
3. JAMA Pediatr. 2018 Feb 1;172(2):188-92.
Spinal muscular atrophy (SMA) is now among the disorders officially included in the Recommended Uniform Screening Panel (RUSP), which is used by state public health departments to screen newborns for genetic disorders.
Secretary of the Department of Health and Human Services Alex M. Azar II formally added SMA to the panel July 2 on the recommendation of the Advisory Committee on Heritable Disorders in Newborns and Children.
“Adding SMA to the list will help ensure that babies born with SMA are identified, so that they have the opportunity to benefit from early treatment and intervention,” according to a statement from the Muscular Dystrophy Association about the decision. “This testing can also provide families with a genetic diagnosis – information that often is required to determine whether their child is eligible to participate in clinical trials.”
Adding SMA to the RUSP does not mean states must screen newborns for the disorder. Each state’s public health apparatus decides independently whether to accept the recommendation and which disorders on the RUSP to screen for. Most states screen for most disorders on the RUSP. Evidence compiled by the advisory committee suggested wide variation in resources, infrastructure, funding, and time to implementation among states.
An estimated 1 in 11,000 newborns have SMA, a disorder caused by mutations in the survival motor neuron 1 (SMN1) gene. SMA affects motor neurons in the brain stem and spinal cord leading to motor weakness and atrophy. The only treatment for SMA had been palliative care until the Food and Drug Administration approved nusinersen (Spinraza) for the disorder in December 2016, although the drug’s approval has raised some ethical questions.1-3
After reviewing the evidence at their February 8, 2018 meeting, the advisory committee recommended the addition of spinal muscular atrophy screening to the RUSP in a March 8, 2018, letter from committee chair Joseph A. Bocchini Jr., MD, who is a professor and the chairman of the department of pediatrics at Louisiana State University Health in Shreveport.
Secretary Azar accepted the recommendation based on the evidence the committee provided; he also requested a follow-up report within 2 years “describing the status of implementing newborn screening for SMA and clinical outcomes of early treatment, including any potential harms, for infants diagnosed with SMA.”
The advisory committee makes its recommendations to the HHS on which heritable disorders to include in the RUSP after they have assessed a systematic, evidence-based review assigned by the committee to an external independent group. Alex R. Kemper, MD, MPH, a professor of pediatrics at the Ohio State University and division chief of ambulatory pediatrics at Nationwide Children’s Hospital, both in Columbus, led the review group for SMA. Dr. Kemper is also deputy editor of the journal Pediatrics and a member of the U.S. Preventive Services Task Force.
According to Secretary Azar’s summary in his July 2, 2018, letter of acceptance, the evidence review suggested that “early screening and treatment can lead to decreased mortality for individuals with SMA and improved motor milestones.”
Dr. Kemper elaborated in an interview that, “SMA can be detected through newborn screening, and treatment is now available that can not only reduce the risk of death but decrease the development of neurologic impairment. As with adding any condition to newborn screening, public health laboratories will need to develop strategies to incorporate the screening test. The current FDA-approved treatment, nusinersen, is delivered by lumbar puncture into the spinal fluid. In addition, there are exciting advances in gene therapy leading to new treatment approaches.”
Approximately 95% of SMA cases result from the deletion of exon 7 from both alleles of SMN1. (Other rarer cases are caused by mutations in different genes.) Without the SMN protein produced by SMN1, a person gradually loses muscle function.
A similar gene, SMN2, also can produce the SMN protein but in much lower amounts, typically less than 10% of what a person needs. People can, however, have multiple copies of SMN2, which can produce slightly more SMN protein for a slower disease process.
The five types of spinal muscular atrophy are determined according to symptom onset, which directly correlates with disorder severity and prognosis. Just over half (54%) of SMA cases are Type I, in which progressive weakness occurs over the first 6 months of life and results in early death. Only 18% of children with Type I live past age 4 years, and 68% die by age 2 years. Type 0 is rarer but more severe, usually causing fetal loss or early infant death.
Type II represents 18% of SMA cases and causes progressive weakness by age 15 months. Most people with Type II survive to their 30s but then experience respiratory failure and rarely reach their fourth decade. Individuals with Types III and IV typically have a normal lifespan and only begin to see progressive muscle weakness after 1 year old or in adulthood.
Dr. Kemper’s group focused on the three types diagnosed in infancy: types I, II, and III.
Dr. Kemper emphasized in an interview that “it will be critical to make sure that infants diagnosed with SMA through newborn screening receive follow-up shortly afterward to determine whether they would benefit from nusinersen. More information is needed about the long-term outcomes of those infants who begin treatment following newborn screening so we not only know about outcomes in later childhood and adolescence but treatment approaches can be further refined and personalized.”
Nusinersen works by altering the splicing of precursor messenger RNA in SMN2 so that the mRNA strands are longer, which thereby increases how much SMN protein is produced. Concerns about the medication, however, have included its cost – $750,000 in the first year and $375,000 every following year for life – and potential adverse events from repeated administration. Nusinersen is injected into the spinal canal four times in the first year and then once annually, and the painful injections require patient immobilization. Potential adverse events include thrombocytopenia and nephrotoxicity, along with potential complications from repeated lumbar punctures over time.2
Other concerns about the drug include its limited evidence base, lack of long-term data, associated costs with administration (for example, travel costs), the potential for patients taking nusinersen to be excluded from future clinical trials on other treatments, and ensuring parents have enough information on the drug’s limitations and potential risks to provide adequate informed consent.2
Yet evidence to date is favorable in children with early onset. Dr. Bocchini wrote in the letter to Secretary Azar that “limited data suggest that treatment effect is greater when the treatment is initiated before symptoms develop and when the individual has more copies of SMN2.”
Dr. Kemper’s group concluded that screening can detect SMA in newborns and that treatment can modify disease course. “Grey literature suggests those with total disease duration less than or equal to 12 weeks before nusinersen treatment were more likely to have better outcomes than those with longer periods of disease duration.”
“Presymptomatic treatment alters the natural history” of the disorder, the group found, although outcome data past 1 year of age are not yet available. Based on findings from a New York pilot program, they predicted that nationwide newborn screening would avert 33 deaths and 48 cases of children who were dependent on a ventilator among an annual cohort of 4 million births.
At the time of the evidence review, Massachusetts, Minnesota, Missouri, North Carolina, New York, Utah, and Wisconsin initiated pilot programs or whole-population mandated screening for SMA. Of the three states that reported costs, all reported costs at $1 or less per screen.
The research for the evidence review was funded by a Health Resources and Services Administration grant to Duke University, Durham, N.C. No disclosures were provided for evidence review group members.
References
1. Gene Ther. 2017 Sep;24(9):534-8.
2. JAMA Intern Med. 2018 Jun 1;178(6):743-44.
3. JAMA Pediatr. 2018 Feb 1;172(2):188-92.
Spinal muscular atrophy (SMA) is now among the disorders officially included in the Recommended Uniform Screening Panel (RUSP), which is used by state public health departments to screen newborns for genetic disorders.
Secretary of the Department of Health and Human Services Alex M. Azar II formally added SMA to the panel July 2 on the recommendation of the Advisory Committee on Heritable Disorders in Newborns and Children.
“Adding SMA to the list will help ensure that babies born with SMA are identified, so that they have the opportunity to benefit from early treatment and intervention,” according to a statement from the Muscular Dystrophy Association about the decision. “This testing can also provide families with a genetic diagnosis – information that often is required to determine whether their child is eligible to participate in clinical trials.”
Adding SMA to the RUSP does not mean states must screen newborns for the disorder. Each state’s public health apparatus decides independently whether to accept the recommendation and which disorders on the RUSP to screen for. Most states screen for most disorders on the RUSP. Evidence compiled by the advisory committee suggested wide variation in resources, infrastructure, funding, and time to implementation among states.
An estimated 1 in 11,000 newborns have SMA, a disorder caused by mutations in the survival motor neuron 1 (SMN1) gene. SMA affects motor neurons in the brain stem and spinal cord leading to motor weakness and atrophy. The only treatment for SMA had been palliative care until the Food and Drug Administration approved nusinersen (Spinraza) for the disorder in December 2016, although the drug’s approval has raised some ethical questions.1-3
After reviewing the evidence at their February 8, 2018 meeting, the advisory committee recommended the addition of spinal muscular atrophy screening to the RUSP in a March 8, 2018, letter from committee chair Joseph A. Bocchini Jr., MD, who is a professor and the chairman of the department of pediatrics at Louisiana State University Health in Shreveport.
Secretary Azar accepted the recommendation based on the evidence the committee provided; he also requested a follow-up report within 2 years “describing the status of implementing newborn screening for SMA and clinical outcomes of early treatment, including any potential harms, for infants diagnosed with SMA.”
The advisory committee makes its recommendations to the HHS on which heritable disorders to include in the RUSP after they have assessed a systematic, evidence-based review assigned by the committee to an external independent group. Alex R. Kemper, MD, MPH, a professor of pediatrics at the Ohio State University and division chief of ambulatory pediatrics at Nationwide Children’s Hospital, both in Columbus, led the review group for SMA. Dr. Kemper is also deputy editor of the journal Pediatrics and a member of the U.S. Preventive Services Task Force.
According to Secretary Azar’s summary in his July 2, 2018, letter of acceptance, the evidence review suggested that “early screening and treatment can lead to decreased mortality for individuals with SMA and improved motor milestones.”
Dr. Kemper elaborated in an interview that, “SMA can be detected through newborn screening, and treatment is now available that can not only reduce the risk of death but decrease the development of neurologic impairment. As with adding any condition to newborn screening, public health laboratories will need to develop strategies to incorporate the screening test. The current FDA-approved treatment, nusinersen, is delivered by lumbar puncture into the spinal fluid. In addition, there are exciting advances in gene therapy leading to new treatment approaches.”
Approximately 95% of SMA cases result from the deletion of exon 7 from both alleles of SMN1. (Other rarer cases are caused by mutations in different genes.) Without the SMN protein produced by SMN1, a person gradually loses muscle function.
A similar gene, SMN2, also can produce the SMN protein but in much lower amounts, typically less than 10% of what a person needs. People can, however, have multiple copies of SMN2, which can produce slightly more SMN protein for a slower disease process.
The five types of spinal muscular atrophy are determined according to symptom onset, which directly correlates with disorder severity and prognosis. Just over half (54%) of SMA cases are Type I, in which progressive weakness occurs over the first 6 months of life and results in early death. Only 18% of children with Type I live past age 4 years, and 68% die by age 2 years. Type 0 is rarer but more severe, usually causing fetal loss or early infant death.
Type II represents 18% of SMA cases and causes progressive weakness by age 15 months. Most people with Type II survive to their 30s but then experience respiratory failure and rarely reach their fourth decade. Individuals with Types III and IV typically have a normal lifespan and only begin to see progressive muscle weakness after 1 year old or in adulthood.
Dr. Kemper’s group focused on the three types diagnosed in infancy: types I, II, and III.
Dr. Kemper emphasized in an interview that “it will be critical to make sure that infants diagnosed with SMA through newborn screening receive follow-up shortly afterward to determine whether they would benefit from nusinersen. More information is needed about the long-term outcomes of those infants who begin treatment following newborn screening so we not only know about outcomes in later childhood and adolescence but treatment approaches can be further refined and personalized.”
Nusinersen works by altering the splicing of precursor messenger RNA in SMN2 so that the mRNA strands are longer, which thereby increases how much SMN protein is produced. Concerns about the medication, however, have included its cost – $750,000 in the first year and $375,000 every following year for life – and potential adverse events from repeated administration. Nusinersen is injected into the spinal canal four times in the first year and then once annually, and the painful injections require patient immobilization. Potential adverse events include thrombocytopenia and nephrotoxicity, along with potential complications from repeated lumbar punctures over time.2
Other concerns about the drug include its limited evidence base, lack of long-term data, associated costs with administration (for example, travel costs), the potential for patients taking nusinersen to be excluded from future clinical trials on other treatments, and ensuring parents have enough information on the drug’s limitations and potential risks to provide adequate informed consent.2
Yet evidence to date is favorable in children with early onset. Dr. Bocchini wrote in the letter to Secretary Azar that “limited data suggest that treatment effect is greater when the treatment is initiated before symptoms develop and when the individual has more copies of SMN2.”
Dr. Kemper’s group concluded that screening can detect SMA in newborns and that treatment can modify disease course. “Grey literature suggests those with total disease duration less than or equal to 12 weeks before nusinersen treatment were more likely to have better outcomes than those with longer periods of disease duration.”
“Presymptomatic treatment alters the natural history” of the disorder, the group found, although outcome data past 1 year of age are not yet available. Based on findings from a New York pilot program, they predicted that nationwide newborn screening would avert 33 deaths and 48 cases of children who were dependent on a ventilator among an annual cohort of 4 million births.
At the time of the evidence review, Massachusetts, Minnesota, Missouri, North Carolina, New York, Utah, and Wisconsin initiated pilot programs or whole-population mandated screening for SMA. Of the three states that reported costs, all reported costs at $1 or less per screen.
The research for the evidence review was funded by a Health Resources and Services Administration grant to Duke University, Durham, N.C. No disclosures were provided for evidence review group members.
References
1. Gene Ther. 2017 Sep;24(9):534-8.
2. JAMA Intern Med. 2018 Jun 1;178(6):743-44.
3. JAMA Pediatr. 2018 Feb 1;172(2):188-92.
No survival boost with atezolizumab vs. regorafenib in mCRC
BARCELONA – There was no overall survival advantage for patients with metastatic colorectal cancer treated with the immune checkpoint inhibitor atezolizumab (Tencentriq) either in combination with cobimetinib (Cotellic) or as monotherapy compared with regorafenib (Stivarga), results of the randomized phase 3 IMblaze370 trial showed.
Median overall survival (OS, the primary endpoint) for 183 patients treated with atezolizumab and cobimetinib was 8.9 months, compared with 7.1 months for 90 patients treated with atezolizumab monotherapy, and 8.5 months for 90 patients treated with regorafenib. None of the differences were statistically significant, reported Johanna C. Bendell, MD, of the Sarah Cannon Research Institute in Nashville, Tenn.
“The lack of clinical activity may be due to the immune-excluded phenotype of metastatic colorectal cancer. The dual inhibition of the PD-L1 immune checkpoint and MAP kinase–mediated immune suppression may not be sufficient to generate that immune response that we need for antitumor activity,” she said at the European Society for Medical Oncology World Congress on Gastrointestinal Cancer.
In an interview, Dr. Bendell said that “we were all hoping that we were going to see some benefit, and we had some preliminary data suggesting that we would. What we still need to see is whether there are potentially populations of patients who might have benefited more, which hopefully will come out of the biomarker analysis.”
The results demonstrate the need for controlled comparison trials to detect true clinical benefits from a specific agent or combination, she added.
Microsatellite-stable disease, which accounts for approximately 95% of cases of metastatic colorectal cancer (MSS mCRC) is considered to be an immune-excluded or “cold” tumor type due to a lack of tumor-infiltrating lymphocytes. Single agent inhibitors of the programmed death 1 protein and its ligand (PD1/PD-L1) have only minimal activity in MSS mCRC, she noted.
In preclinical studies, there was evidence to suggest that the combination of atezolizumab and cobimetinib could augment antitumor T-cell responses. The combination also was shown to be safe and to demonstrate “promising” clinical activity in a phase 1b trial conducted by Dr. Bendell and her colleagues.
To see whether the initial promise of atezolizumab in this population held up under closer scrutiny, the investigators enrolled patients with MSS mCRC refractory to chemotherapy and randomly assigned them on a 2:1:1 basis to receive either atezolizumab plus cobimetinib, atezolizumab monotherapy, or regorafenib.
Atezolizumab was administered intravenously at 840 mg every 2 weeks in the combination arm, and at 1,200 mg every 3 weeks in the monotherapy arm. Cobimetinib was administered orally at 60 mg on a 21-days-on/7-days-off schedule. Regorafenib was administered orally at 160 mg on a 21-days-on/7-days-off schedule.
As noted, the trial did not meet its primary endpoint of an OS benefit in the intention-to-treat population. In addition, there were no significant differences in either median progression-free survival, overall response rates, or duration of response.
Response rates for both the atezolizumab/cobimetinib combination and atezolizumab monotherapy arms were consistent with published data for other PD-1/PD-L1 inhibitors, and the safety of the combination was consistent with the known safety profiles of the individual agents, Dr. Bendell said.
The investigators are conducting extensive biomarker evaluations and gene expression profiles, and hope to present the results of these studies at a future conference, she added.
SOURCE: Bendell J et al. ESMO GI 2018. Abstract LBA-004.
BARCELONA – There was no overall survival advantage for patients with metastatic colorectal cancer treated with the immune checkpoint inhibitor atezolizumab (Tencentriq) either in combination with cobimetinib (Cotellic) or as monotherapy compared with regorafenib (Stivarga), results of the randomized phase 3 IMblaze370 trial showed.
Median overall survival (OS, the primary endpoint) for 183 patients treated with atezolizumab and cobimetinib was 8.9 months, compared with 7.1 months for 90 patients treated with atezolizumab monotherapy, and 8.5 months for 90 patients treated with regorafenib. None of the differences were statistically significant, reported Johanna C. Bendell, MD, of the Sarah Cannon Research Institute in Nashville, Tenn.
“The lack of clinical activity may be due to the immune-excluded phenotype of metastatic colorectal cancer. The dual inhibition of the PD-L1 immune checkpoint and MAP kinase–mediated immune suppression may not be sufficient to generate that immune response that we need for antitumor activity,” she said at the European Society for Medical Oncology World Congress on Gastrointestinal Cancer.
In an interview, Dr. Bendell said that “we were all hoping that we were going to see some benefit, and we had some preliminary data suggesting that we would. What we still need to see is whether there are potentially populations of patients who might have benefited more, which hopefully will come out of the biomarker analysis.”
The results demonstrate the need for controlled comparison trials to detect true clinical benefits from a specific agent or combination, she added.
Microsatellite-stable disease, which accounts for approximately 95% of cases of metastatic colorectal cancer (MSS mCRC) is considered to be an immune-excluded or “cold” tumor type due to a lack of tumor-infiltrating lymphocytes. Single agent inhibitors of the programmed death 1 protein and its ligand (PD1/PD-L1) have only minimal activity in MSS mCRC, she noted.
In preclinical studies, there was evidence to suggest that the combination of atezolizumab and cobimetinib could augment antitumor T-cell responses. The combination also was shown to be safe and to demonstrate “promising” clinical activity in a phase 1b trial conducted by Dr. Bendell and her colleagues.
To see whether the initial promise of atezolizumab in this population held up under closer scrutiny, the investigators enrolled patients with MSS mCRC refractory to chemotherapy and randomly assigned them on a 2:1:1 basis to receive either atezolizumab plus cobimetinib, atezolizumab monotherapy, or regorafenib.
Atezolizumab was administered intravenously at 840 mg every 2 weeks in the combination arm, and at 1,200 mg every 3 weeks in the monotherapy arm. Cobimetinib was administered orally at 60 mg on a 21-days-on/7-days-off schedule. Regorafenib was administered orally at 160 mg on a 21-days-on/7-days-off schedule.
As noted, the trial did not meet its primary endpoint of an OS benefit in the intention-to-treat population. In addition, there were no significant differences in either median progression-free survival, overall response rates, or duration of response.
Response rates for both the atezolizumab/cobimetinib combination and atezolizumab monotherapy arms were consistent with published data for other PD-1/PD-L1 inhibitors, and the safety of the combination was consistent with the known safety profiles of the individual agents, Dr. Bendell said.
The investigators are conducting extensive biomarker evaluations and gene expression profiles, and hope to present the results of these studies at a future conference, she added.
SOURCE: Bendell J et al. ESMO GI 2018. Abstract LBA-004.
BARCELONA – There was no overall survival advantage for patients with metastatic colorectal cancer treated with the immune checkpoint inhibitor atezolizumab (Tencentriq) either in combination with cobimetinib (Cotellic) or as monotherapy compared with regorafenib (Stivarga), results of the randomized phase 3 IMblaze370 trial showed.
Median overall survival (OS, the primary endpoint) for 183 patients treated with atezolizumab and cobimetinib was 8.9 months, compared with 7.1 months for 90 patients treated with atezolizumab monotherapy, and 8.5 months for 90 patients treated with regorafenib. None of the differences were statistically significant, reported Johanna C. Bendell, MD, of the Sarah Cannon Research Institute in Nashville, Tenn.
“The lack of clinical activity may be due to the immune-excluded phenotype of metastatic colorectal cancer. The dual inhibition of the PD-L1 immune checkpoint and MAP kinase–mediated immune suppression may not be sufficient to generate that immune response that we need for antitumor activity,” she said at the European Society for Medical Oncology World Congress on Gastrointestinal Cancer.
In an interview, Dr. Bendell said that “we were all hoping that we were going to see some benefit, and we had some preliminary data suggesting that we would. What we still need to see is whether there are potentially populations of patients who might have benefited more, which hopefully will come out of the biomarker analysis.”
The results demonstrate the need for controlled comparison trials to detect true clinical benefits from a specific agent or combination, she added.
Microsatellite-stable disease, which accounts for approximately 95% of cases of metastatic colorectal cancer (MSS mCRC) is considered to be an immune-excluded or “cold” tumor type due to a lack of tumor-infiltrating lymphocytes. Single agent inhibitors of the programmed death 1 protein and its ligand (PD1/PD-L1) have only minimal activity in MSS mCRC, she noted.
In preclinical studies, there was evidence to suggest that the combination of atezolizumab and cobimetinib could augment antitumor T-cell responses. The combination also was shown to be safe and to demonstrate “promising” clinical activity in a phase 1b trial conducted by Dr. Bendell and her colleagues.
To see whether the initial promise of atezolizumab in this population held up under closer scrutiny, the investigators enrolled patients with MSS mCRC refractory to chemotherapy and randomly assigned them on a 2:1:1 basis to receive either atezolizumab plus cobimetinib, atezolizumab monotherapy, or regorafenib.
Atezolizumab was administered intravenously at 840 mg every 2 weeks in the combination arm, and at 1,200 mg every 3 weeks in the monotherapy arm. Cobimetinib was administered orally at 60 mg on a 21-days-on/7-days-off schedule. Regorafenib was administered orally at 160 mg on a 21-days-on/7-days-off schedule.
As noted, the trial did not meet its primary endpoint of an OS benefit in the intention-to-treat population. In addition, there were no significant differences in either median progression-free survival, overall response rates, or duration of response.
Response rates for both the atezolizumab/cobimetinib combination and atezolizumab monotherapy arms were consistent with published data for other PD-1/PD-L1 inhibitors, and the safety of the combination was consistent with the known safety profiles of the individual agents, Dr. Bendell said.
The investigators are conducting extensive biomarker evaluations and gene expression profiles, and hope to present the results of these studies at a future conference, she added.
SOURCE: Bendell J et al. ESMO GI 2018. Abstract LBA-004.
REPORTING FROM ESMO GI 2018
Key clinical point: Microsatellite stable metastatic colorectal cancer (mCRC) has a poor survival prognosis; better agents or combinations are needed.
Major finding: There were no significant differences in overall or progression-free survival, overall response rates, or duration of responses between atezolizumab with or without cobimetinib vs. regorafenib.
Study details: Randomized phase 3 trial of 363 patients with mCRC.
Disclosures: Hoffman-La Roche sponsored the study. Dr. Bendell reported no relevant disclosures. She is a member of the Oncology Practice editorial advisory board.
Source: Bendell J et al. ESMO GI 2018, Abstract LBA-004.
Stress balls, hand-holding no help during dermatology procedures
according to a randomized trial of 135 patients at Northwestern University, Chicago.
In all three groups, anxiety levels were a little over 3 points on a 10-point Visual Analog Scale (VAS) before surgery and around 2 points during it. The 6-item State Trait Anxiety Inventory score was just under 9 in all three groups right after the procedure, meaning patients weren’t very anxious. Physiological measures did not change from before to after the procedure or between groups. Postoperative pain scores were all under 1 on a 10-point scale, and patients in all three groups were highly satisfied with their encounter, the researchers said in JAMA Dermatology.
“Many patients commented anecdotally on the calming effect of hand-holding or stress ball use,” so “it was surprising that the total data did not show these interventions to preferentially decrease anxiety or alleviate pain,” Arianna F. Yanes, a medical student at Northwestern University, and her coinvestigators said.
It could be that standard measures – giving patients an opportunity to ask questions, making sure they feel comfortable, and the like – are enough. However, “hand-holding and stress balls may still provide stress relief in patients who are particularly anxious before the procedure.” Perhaps patients would have preferred having their hand held by a loved one instead of a stranger, the investigators said.
Meanwhile, patients who researched their operation online beforehand had higher preoperative anxiety scores (3.84 vs. 2.62 points on the VAS; P = .04), but they could have been more anxious from the start.
The mean subject age was 66 years, and 62% were men.
The work was funded by Northwestern University and a grant from Merz. The investigators had no relevant disclosures.
SOURCE: Yanes AF et al. JAMA Dermatol. 2018 Jul 18. doi:10.1001/jamadermatol.2018.1783.
according to a randomized trial of 135 patients at Northwestern University, Chicago.
In all three groups, anxiety levels were a little over 3 points on a 10-point Visual Analog Scale (VAS) before surgery and around 2 points during it. The 6-item State Trait Anxiety Inventory score was just under 9 in all three groups right after the procedure, meaning patients weren’t very anxious. Physiological measures did not change from before to after the procedure or between groups. Postoperative pain scores were all under 1 on a 10-point scale, and patients in all three groups were highly satisfied with their encounter, the researchers said in JAMA Dermatology.
“Many patients commented anecdotally on the calming effect of hand-holding or stress ball use,” so “it was surprising that the total data did not show these interventions to preferentially decrease anxiety or alleviate pain,” Arianna F. Yanes, a medical student at Northwestern University, and her coinvestigators said.
It could be that standard measures – giving patients an opportunity to ask questions, making sure they feel comfortable, and the like – are enough. However, “hand-holding and stress balls may still provide stress relief in patients who are particularly anxious before the procedure.” Perhaps patients would have preferred having their hand held by a loved one instead of a stranger, the investigators said.
Meanwhile, patients who researched their operation online beforehand had higher preoperative anxiety scores (3.84 vs. 2.62 points on the VAS; P = .04), but they could have been more anxious from the start.
The mean subject age was 66 years, and 62% were men.
The work was funded by Northwestern University and a grant from Merz. The investigators had no relevant disclosures.
SOURCE: Yanes AF et al. JAMA Dermatol. 2018 Jul 18. doi:10.1001/jamadermatol.2018.1783.
according to a randomized trial of 135 patients at Northwestern University, Chicago.
In all three groups, anxiety levels were a little over 3 points on a 10-point Visual Analog Scale (VAS) before surgery and around 2 points during it. The 6-item State Trait Anxiety Inventory score was just under 9 in all three groups right after the procedure, meaning patients weren’t very anxious. Physiological measures did not change from before to after the procedure or between groups. Postoperative pain scores were all under 1 on a 10-point scale, and patients in all three groups were highly satisfied with their encounter, the researchers said in JAMA Dermatology.
“Many patients commented anecdotally on the calming effect of hand-holding or stress ball use,” so “it was surprising that the total data did not show these interventions to preferentially decrease anxiety or alleviate pain,” Arianna F. Yanes, a medical student at Northwestern University, and her coinvestigators said.
It could be that standard measures – giving patients an opportunity to ask questions, making sure they feel comfortable, and the like – are enough. However, “hand-holding and stress balls may still provide stress relief in patients who are particularly anxious before the procedure.” Perhaps patients would have preferred having their hand held by a loved one instead of a stranger, the investigators said.
Meanwhile, patients who researched their operation online beforehand had higher preoperative anxiety scores (3.84 vs. 2.62 points on the VAS; P = .04), but they could have been more anxious from the start.
The mean subject age was 66 years, and 62% were men.
The work was funded by Northwestern University and a grant from Merz. The investigators had no relevant disclosures.
SOURCE: Yanes AF et al. JAMA Dermatol. 2018 Jul 18. doi:10.1001/jamadermatol.2018.1783.
FROM JAMA DERMATOLOGY
Unlikely mentors
Mentorship is a hot topic. Mentors are generally perceived as knowledgeable, kind, generous souls who will guide mentees through tough challenges and be your pal. I suggest to you that while such encounters are marvelous, and to be sought out, some of the most important lessons are taught by members of the opposite cast.
The presiding secretary of the Ohio state medical board was a brigadier general, still active reserve, tall with a bristling countenance. I was president of the Ohio Dermatological Association and was accompanied by Mark Bechtel, MD, who was our chair of state legislation. We had been invited to , and that there had not been any deaths related to local anesthesia administered by dermatologists in Ohio (or anywhere else). We expected to tell the medical board there was nothing to worry about, and we could all go home. This was 17 years ago.
It quickly became apparent that there had been an extensive prior dialogue between the medical board and representatives of the American College of Surgeons and the American Society of Anesthesiologists. They sat at the head table with the secretary of the medical board.
“In our experience, there are really some dangerous procedures going on in the office setting under local anesthesia, and this area desperately needs regulation,” the anesthesiologist’s representative said. The surgeon’s representative chimed in: “From what I’ve heard, office surgery is a ticking time bomb and needs regulation, and as soon as possible.” This prattle went on and on, with the medical board secretary sympathetically nodding his head. I raised my hand and was ignored – and ignored. It became apparent that this was a show trial, and our opinion was not really wanted, just our attendance noted in the minutes. Finally, I stood up and protested, and pointed out that all of this “testimony” was conjecture and personal opinion, and that there was no factual basis for their claims. The president stiffened, stood up, and started barking orders.He told me to “sit down and not speak unless I was called on.” I sat down and shut up. And I was never called on. Mark Bechtel put a calming hand on my arm. Goodness, I had not been treated like that since junior high.
I soon realized that dermatologists were not at all important to the medical board, and that the medical board had no idea about our safety and efficiency and really did not care.
Following the meeting, I was told by Larry Lanier (the American Academy of Dermatology state legislative liaison at the time) that Ohio was to be the test state to restrict local anesthesia and tumescent anesthesia nationwide. He explained that some widely reported liposuction-related deaths in Florida had given the medical board the “justification” to act. He went on to explain that yes, there were no data either way, but we had better hire a lobbyist and hope for the best.
I was stunned by what I now call (in this case, rough) “mentorship” by the medical board secretary. I understood I could meekly go along – or get angry. I chose the latter, and it has greatly changed my career.
Now, this was not a hot, red, foam-at-the-mouth mad, but a slow burn, the kind that sustains, not consumes.
I went home and did a literature review and was disheartened to find absolutely nothing in the literature on the safety record of dermatologists in the office setting or on the safety of office surgery in general under local anesthesia. We had nothing to back us up.
I looked up the liposuction deaths in Florida and discovered the procedures were all done under general anesthesia or deep sedation by surgeons of one type or another. I also discovered that Florida had enacted mandatory reporting, and the reports could be had for copying costs. I ordered all available, 9 months of data.
We dermatologists passed a special assessment and hired a lobbyist who told us we were too late to have any impact on the impending restrictions. We took a resolution opposing the medical board rules – which would have eliminated using any sedation in the office, even haloperidol and tumescent anesthesia – to the state medical society and got it passed despite the medical board secretary (who was a former president of the society) testifying against us. The Florida data showed no deaths or injuries from using local anesthesia in the office by anyone, and I succeeded in getting a letter addressing that data published expeditiously in the Journal of the American Medical Association (JAMA 2001;285[20]:2582).
The president of the American Society for Dermatologic Surgery at that time, Stephen Mandy, MD, came to town and testified against the proposed restrictions along with about 60 physicians, including dermatologists, plastic surgeons, and other physicians who perform office-based surgery who we had rallied to join us from across the state. So many colleagues joined us, in fact, that some of us had to sit on the floor during the proceedings.
The proposed restrictions evaporated. I and many others have since devoted our research efforts to solidifying dermatology’s safety and quality record. Dr. Bechtel, professor of dermatology at Ohio State University, Columbus, is now secretary of the state medical board. At the last annual state meeting, I thanked the brigadier general, the former secretary of the medical board, for his unlikely mentorship. He smiled and we got our picture taken together.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Mentorship is a hot topic. Mentors are generally perceived as knowledgeable, kind, generous souls who will guide mentees through tough challenges and be your pal. I suggest to you that while such encounters are marvelous, and to be sought out, some of the most important lessons are taught by members of the opposite cast.
The presiding secretary of the Ohio state medical board was a brigadier general, still active reserve, tall with a bristling countenance. I was president of the Ohio Dermatological Association and was accompanied by Mark Bechtel, MD, who was our chair of state legislation. We had been invited to , and that there had not been any deaths related to local anesthesia administered by dermatologists in Ohio (or anywhere else). We expected to tell the medical board there was nothing to worry about, and we could all go home. This was 17 years ago.
It quickly became apparent that there had been an extensive prior dialogue between the medical board and representatives of the American College of Surgeons and the American Society of Anesthesiologists. They sat at the head table with the secretary of the medical board.
“In our experience, there are really some dangerous procedures going on in the office setting under local anesthesia, and this area desperately needs regulation,” the anesthesiologist’s representative said. The surgeon’s representative chimed in: “From what I’ve heard, office surgery is a ticking time bomb and needs regulation, and as soon as possible.” This prattle went on and on, with the medical board secretary sympathetically nodding his head. I raised my hand and was ignored – and ignored. It became apparent that this was a show trial, and our opinion was not really wanted, just our attendance noted in the minutes. Finally, I stood up and protested, and pointed out that all of this “testimony” was conjecture and personal opinion, and that there was no factual basis for their claims. The president stiffened, stood up, and started barking orders.He told me to “sit down and not speak unless I was called on.” I sat down and shut up. And I was never called on. Mark Bechtel put a calming hand on my arm. Goodness, I had not been treated like that since junior high.
I soon realized that dermatologists were not at all important to the medical board, and that the medical board had no idea about our safety and efficiency and really did not care.
Following the meeting, I was told by Larry Lanier (the American Academy of Dermatology state legislative liaison at the time) that Ohio was to be the test state to restrict local anesthesia and tumescent anesthesia nationwide. He explained that some widely reported liposuction-related deaths in Florida had given the medical board the “justification” to act. He went on to explain that yes, there were no data either way, but we had better hire a lobbyist and hope for the best.
I was stunned by what I now call (in this case, rough) “mentorship” by the medical board secretary. I understood I could meekly go along – or get angry. I chose the latter, and it has greatly changed my career.
Now, this was not a hot, red, foam-at-the-mouth mad, but a slow burn, the kind that sustains, not consumes.
I went home and did a literature review and was disheartened to find absolutely nothing in the literature on the safety record of dermatologists in the office setting or on the safety of office surgery in general under local anesthesia. We had nothing to back us up.
I looked up the liposuction deaths in Florida and discovered the procedures were all done under general anesthesia or deep sedation by surgeons of one type or another. I also discovered that Florida had enacted mandatory reporting, and the reports could be had for copying costs. I ordered all available, 9 months of data.
We dermatologists passed a special assessment and hired a lobbyist who told us we were too late to have any impact on the impending restrictions. We took a resolution opposing the medical board rules – which would have eliminated using any sedation in the office, even haloperidol and tumescent anesthesia – to the state medical society and got it passed despite the medical board secretary (who was a former president of the society) testifying against us. The Florida data showed no deaths or injuries from using local anesthesia in the office by anyone, and I succeeded in getting a letter addressing that data published expeditiously in the Journal of the American Medical Association (JAMA 2001;285[20]:2582).
The president of the American Society for Dermatologic Surgery at that time, Stephen Mandy, MD, came to town and testified against the proposed restrictions along with about 60 physicians, including dermatologists, plastic surgeons, and other physicians who perform office-based surgery who we had rallied to join us from across the state. So many colleagues joined us, in fact, that some of us had to sit on the floor during the proceedings.
The proposed restrictions evaporated. I and many others have since devoted our research efforts to solidifying dermatology’s safety and quality record. Dr. Bechtel, professor of dermatology at Ohio State University, Columbus, is now secretary of the state medical board. At the last annual state meeting, I thanked the brigadier general, the former secretary of the medical board, for his unlikely mentorship. He smiled and we got our picture taken together.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Mentorship is a hot topic. Mentors are generally perceived as knowledgeable, kind, generous souls who will guide mentees through tough challenges and be your pal. I suggest to you that while such encounters are marvelous, and to be sought out, some of the most important lessons are taught by members of the opposite cast.
The presiding secretary of the Ohio state medical board was a brigadier general, still active reserve, tall with a bristling countenance. I was president of the Ohio Dermatological Association and was accompanied by Mark Bechtel, MD, who was our chair of state legislation. We had been invited to , and that there had not been any deaths related to local anesthesia administered by dermatologists in Ohio (or anywhere else). We expected to tell the medical board there was nothing to worry about, and we could all go home. This was 17 years ago.
It quickly became apparent that there had been an extensive prior dialogue between the medical board and representatives of the American College of Surgeons and the American Society of Anesthesiologists. They sat at the head table with the secretary of the medical board.
“In our experience, there are really some dangerous procedures going on in the office setting under local anesthesia, and this area desperately needs regulation,” the anesthesiologist’s representative said. The surgeon’s representative chimed in: “From what I’ve heard, office surgery is a ticking time bomb and needs regulation, and as soon as possible.” This prattle went on and on, with the medical board secretary sympathetically nodding his head. I raised my hand and was ignored – and ignored. It became apparent that this was a show trial, and our opinion was not really wanted, just our attendance noted in the minutes. Finally, I stood up and protested, and pointed out that all of this “testimony” was conjecture and personal opinion, and that there was no factual basis for their claims. The president stiffened, stood up, and started barking orders.He told me to “sit down and not speak unless I was called on.” I sat down and shut up. And I was never called on. Mark Bechtel put a calming hand on my arm. Goodness, I had not been treated like that since junior high.
I soon realized that dermatologists were not at all important to the medical board, and that the medical board had no idea about our safety and efficiency and really did not care.
Following the meeting, I was told by Larry Lanier (the American Academy of Dermatology state legislative liaison at the time) that Ohio was to be the test state to restrict local anesthesia and tumescent anesthesia nationwide. He explained that some widely reported liposuction-related deaths in Florida had given the medical board the “justification” to act. He went on to explain that yes, there were no data either way, but we had better hire a lobbyist and hope for the best.
I was stunned by what I now call (in this case, rough) “mentorship” by the medical board secretary. I understood I could meekly go along – or get angry. I chose the latter, and it has greatly changed my career.
Now, this was not a hot, red, foam-at-the-mouth mad, but a slow burn, the kind that sustains, not consumes.
I went home and did a literature review and was disheartened to find absolutely nothing in the literature on the safety record of dermatologists in the office setting or on the safety of office surgery in general under local anesthesia. We had nothing to back us up.
I looked up the liposuction deaths in Florida and discovered the procedures were all done under general anesthesia or deep sedation by surgeons of one type or another. I also discovered that Florida had enacted mandatory reporting, and the reports could be had for copying costs. I ordered all available, 9 months of data.
We dermatologists passed a special assessment and hired a lobbyist who told us we were too late to have any impact on the impending restrictions. We took a resolution opposing the medical board rules – which would have eliminated using any sedation in the office, even haloperidol and tumescent anesthesia – to the state medical society and got it passed despite the medical board secretary (who was a former president of the society) testifying against us. The Florida data showed no deaths or injuries from using local anesthesia in the office by anyone, and I succeeded in getting a letter addressing that data published expeditiously in the Journal of the American Medical Association (JAMA 2001;285[20]:2582).
The president of the American Society for Dermatologic Surgery at that time, Stephen Mandy, MD, came to town and testified against the proposed restrictions along with about 60 physicians, including dermatologists, plastic surgeons, and other physicians who perform office-based surgery who we had rallied to join us from across the state. So many colleagues joined us, in fact, that some of us had to sit on the floor during the proceedings.
The proposed restrictions evaporated. I and many others have since devoted our research efforts to solidifying dermatology’s safety and quality record. Dr. Bechtel, professor of dermatology at Ohio State University, Columbus, is now secretary of the state medical board. At the last annual state meeting, I thanked the brigadier general, the former secretary of the medical board, for his unlikely mentorship. He smiled and we got our picture taken together.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
One practice’s experience with obesity treatment
WASHINGTON – “We have heard about a lot of really interesting new things in therapy, but the reality is that they are just not economically viable at present,” said David Feldshon, MD, of Minnesota Gastroenterology (MNGI), speaking about his experience with offering obesity treatment in his practice.
One problem that Dr. Feldshon and MNGI faced was that they could never get enough patients into their weight loss program, a meal-replacement program priced at about $200 a month. Part of this program, Optifast, a commercially available low-calorie diet and behavior modification program, cost about $360 a month. Most patients found the cost to be prohibitive according to Dr. Feldshon. The program that MNGI offered was fairly competitive when compared with a number of other commercial diet programs like Nutrisystem and Medifast, which cost $300 and $329 a month, respectively.
Because of the economic pressure these programs can exert on patients, Dr. Feldshon spoke to the difficulty of recruiting patients with major metabolic and digestive disorders. “I work in the liver clinic, so I see people with NASH, NAFLD, cirrhosis, near-cirrhosis, diabetes. These are people that would really benefit from this type of program, and I could not get a single patient to sign up.”
For Dr. Feldshon’s program, patients were required to come to MNGI once a week, which led to higher dropout rates. This is troubling because it is important that patients meet with doctors and other patients as part of a weight loss program. But the inconvenience for patients and doctors is not just in physically attending meetings, but in the medical billing. The Affordable Care Act and Medicare cover obesity screening and counseling, but this is only for primary care physicians, nurse practitioners, physician assistants, or clinical nurse specialists. These limits may not extend into private insurance, but they remain a barrier for those covered by Medicare, according to Dr. Feldshon.
Another effective but ultimately expensive option is weight loss drugs. The cost of drugs can vary wildly. Even well-established drugs like phentermine can cost anywhere from $5 to $35 out of pocket a month, according to Dr. Feldshon. Some drugs, like Saxenda, can cost as much as $1,414 per month if paid for out of pocket. While Saxenda is effective, there is a catch in the prescribing and billing for this drug: “The moment you go above 2.4 mg per day, the insurance company says, ‘Aha! He’s treating obesity,’ and they stop covering it.” One drug that Dr. Feldshon recommends is topiramate because it is a multiuse drug. “Often, the insurance company can’t figure out why you’re prescribing it, so they’ll okay it,” said Dr. Feldshon.
One of the most effective nonsurgical methods of weight loss is intragastric balloon. From 2016 to 2017, MNGI used gastric balloons in 22 patients, resulting in 11.4% total body weight loss in these patients. Unfortunately, it was a “financial loser,” according to Dr. Feldshon. The global case rate charge for one balloon is $8,200. MNGI then incurred $2,000 per balloon, $3,000 in hospital charges, $750 for the medical weight loss program, $1,350 for office personnel and visits, and a calculated opportunity cost of $3,140 resulting in a net loss of $2,040 per balloon. The most important factor in this calculation is the opportunity cost, which includes travel to and from the hospital and phone encounters with patients, which took away Dr. Feldshon’s ability to conduct colonoscopies and GI consults.
In an attempt to make balloons cost effective, Dr. Feldshon committed to doing these procedures on his day off, which reduced the opportunity cost to $0. This made the balloon procedure profitable, at $1,100 per balloon, but the volume was too low to make it worthwhile.
Despite the challenges his group faced with treating obesity, Dr. Feldshon offered some cost-saving solutions to help keep costs down for both patients and doctors. He suggested avoiding manufacturer weight loss programs. Identify an internal program that is reasonably priced or an external program like Weight Watchers. Physicians can utilize video conferencing for weekly meetings; this helps patients interact with doctors, and products like AdobeConnect cost physicians only about $50 a month. Patients can use free online journaling products like MyFitnessPal to track diet and exercise. Physicians can also recommend using generic and over-the-counter drugs and consider enlisting the help of a life coach or dietitian.
“All obese patients benefit from weight loss but we should be targeting those with metabolic syndrome, diabetes, heart disease, hypercholesterolemia, hyperlipidemia, and increased abdominal girth,” said Dr. Feldshon.
Dr. Feldshon has served on advisory committees and review panels and has worked with United Health Group as well as Prime Therapeutics.
AGA Resource
AGA has created an Obesity Practice Guide to provide gastroenterologists with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management, including a model for how to operationalize business issues.
The increase in the proportion of people who are overweight and obese presents gastroenterologists with new challenges and opportunities. Our internal medicine background, experience in nutrition, and role as endoscopists puts us in a unique position to manage obesity. In addition, many GI conditions are directly affected by obesity, including NASH, GERD, pancreatic diseases, and colon cancer. Good nutrition will always be the cornerstone of healthy weight, but nutritional advice alone results in modest (2%-3%) total weight loss. This can be augmented with medications, meal replacement, endobariatrics, and combinations of these.
Having said this, there are significant challenges to managing obesity as a gastroenterologist, and these stem almost entirely from the fact that there is poor coverage for these therapeutic options, as emphasized by Dr. Feldshon. However, it is still important to bring weight loss interventions into our clinical practice – for many reasons.
First, unlike other obesity management programs, we are typically not managing obesity in isolation. Usually, we are managing obesity in the setting of a disease state such as NASH. When we manage patients with NASH and stage 3 fibrosis, the patients’ decision making on how much to invest to prevent further progression is different; they’re more likely to take on some costs. Second, the degree of coverage for medications is improving. Similarly, although endobariatrics is not currently covered, with time it likely will be under certain criteria.
We need to build the clinical experience necessary to manage obesity and do so now, or other specialties will have become the main providers of weight loss interventions. This will become a lost opportunity for both medical and endobariatric management of these patients by us.
So despite the challenges raised by Dr. Feldshon, I would suggest that a practicing gastroenterologist interested in weight loss management focus on patients with obesity-related diseases first and expand their focus incrementally.
Wahajat Mehal, MD, DPhil, is a hepatologist and director of the Yale Weight Loss Program at Yale University, New Haven, Conn. He is an associate editor for GI & Hepatology News.
The increase in the proportion of people who are overweight and obese presents gastroenterologists with new challenges and opportunities. Our internal medicine background, experience in nutrition, and role as endoscopists puts us in a unique position to manage obesity. In addition, many GI conditions are directly affected by obesity, including NASH, GERD, pancreatic diseases, and colon cancer. Good nutrition will always be the cornerstone of healthy weight, but nutritional advice alone results in modest (2%-3%) total weight loss. This can be augmented with medications, meal replacement, endobariatrics, and combinations of these.
Having said this, there are significant challenges to managing obesity as a gastroenterologist, and these stem almost entirely from the fact that there is poor coverage for these therapeutic options, as emphasized by Dr. Feldshon. However, it is still important to bring weight loss interventions into our clinical practice – for many reasons.
First, unlike other obesity management programs, we are typically not managing obesity in isolation. Usually, we are managing obesity in the setting of a disease state such as NASH. When we manage patients with NASH and stage 3 fibrosis, the patients’ decision making on how much to invest to prevent further progression is different; they’re more likely to take on some costs. Second, the degree of coverage for medications is improving. Similarly, although endobariatrics is not currently covered, with time it likely will be under certain criteria.
We need to build the clinical experience necessary to manage obesity and do so now, or other specialties will have become the main providers of weight loss interventions. This will become a lost opportunity for both medical and endobariatric management of these patients by us.
So despite the challenges raised by Dr. Feldshon, I would suggest that a practicing gastroenterologist interested in weight loss management focus on patients with obesity-related diseases first and expand their focus incrementally.
Wahajat Mehal, MD, DPhil, is a hepatologist and director of the Yale Weight Loss Program at Yale University, New Haven, Conn. He is an associate editor for GI & Hepatology News.
The increase in the proportion of people who are overweight and obese presents gastroenterologists with new challenges and opportunities. Our internal medicine background, experience in nutrition, and role as endoscopists puts us in a unique position to manage obesity. In addition, many GI conditions are directly affected by obesity, including NASH, GERD, pancreatic diseases, and colon cancer. Good nutrition will always be the cornerstone of healthy weight, but nutritional advice alone results in modest (2%-3%) total weight loss. This can be augmented with medications, meal replacement, endobariatrics, and combinations of these.
Having said this, there are significant challenges to managing obesity as a gastroenterologist, and these stem almost entirely from the fact that there is poor coverage for these therapeutic options, as emphasized by Dr. Feldshon. However, it is still important to bring weight loss interventions into our clinical practice – for many reasons.
First, unlike other obesity management programs, we are typically not managing obesity in isolation. Usually, we are managing obesity in the setting of a disease state such as NASH. When we manage patients with NASH and stage 3 fibrosis, the patients’ decision making on how much to invest to prevent further progression is different; they’re more likely to take on some costs. Second, the degree of coverage for medications is improving. Similarly, although endobariatrics is not currently covered, with time it likely will be under certain criteria.
We need to build the clinical experience necessary to manage obesity and do so now, or other specialties will have become the main providers of weight loss interventions. This will become a lost opportunity for both medical and endobariatric management of these patients by us.
So despite the challenges raised by Dr. Feldshon, I would suggest that a practicing gastroenterologist interested in weight loss management focus on patients with obesity-related diseases first and expand their focus incrementally.
Wahajat Mehal, MD, DPhil, is a hepatologist and director of the Yale Weight Loss Program at Yale University, New Haven, Conn. He is an associate editor for GI & Hepatology News.
WASHINGTON – “We have heard about a lot of really interesting new things in therapy, but the reality is that they are just not economically viable at present,” said David Feldshon, MD, of Minnesota Gastroenterology (MNGI), speaking about his experience with offering obesity treatment in his practice.
One problem that Dr. Feldshon and MNGI faced was that they could never get enough patients into their weight loss program, a meal-replacement program priced at about $200 a month. Part of this program, Optifast, a commercially available low-calorie diet and behavior modification program, cost about $360 a month. Most patients found the cost to be prohibitive according to Dr. Feldshon. The program that MNGI offered was fairly competitive when compared with a number of other commercial diet programs like Nutrisystem and Medifast, which cost $300 and $329 a month, respectively.
Because of the economic pressure these programs can exert on patients, Dr. Feldshon spoke to the difficulty of recruiting patients with major metabolic and digestive disorders. “I work in the liver clinic, so I see people with NASH, NAFLD, cirrhosis, near-cirrhosis, diabetes. These are people that would really benefit from this type of program, and I could not get a single patient to sign up.”
For Dr. Feldshon’s program, patients were required to come to MNGI once a week, which led to higher dropout rates. This is troubling because it is important that patients meet with doctors and other patients as part of a weight loss program. But the inconvenience for patients and doctors is not just in physically attending meetings, but in the medical billing. The Affordable Care Act and Medicare cover obesity screening and counseling, but this is only for primary care physicians, nurse practitioners, physician assistants, or clinical nurse specialists. These limits may not extend into private insurance, but they remain a barrier for those covered by Medicare, according to Dr. Feldshon.
Another effective but ultimately expensive option is weight loss drugs. The cost of drugs can vary wildly. Even well-established drugs like phentermine can cost anywhere from $5 to $35 out of pocket a month, according to Dr. Feldshon. Some drugs, like Saxenda, can cost as much as $1,414 per month if paid for out of pocket. While Saxenda is effective, there is a catch in the prescribing and billing for this drug: “The moment you go above 2.4 mg per day, the insurance company says, ‘Aha! He’s treating obesity,’ and they stop covering it.” One drug that Dr. Feldshon recommends is topiramate because it is a multiuse drug. “Often, the insurance company can’t figure out why you’re prescribing it, so they’ll okay it,” said Dr. Feldshon.
One of the most effective nonsurgical methods of weight loss is intragastric balloon. From 2016 to 2017, MNGI used gastric balloons in 22 patients, resulting in 11.4% total body weight loss in these patients. Unfortunately, it was a “financial loser,” according to Dr. Feldshon. The global case rate charge for one balloon is $8,200. MNGI then incurred $2,000 per balloon, $3,000 in hospital charges, $750 for the medical weight loss program, $1,350 for office personnel and visits, and a calculated opportunity cost of $3,140 resulting in a net loss of $2,040 per balloon. The most important factor in this calculation is the opportunity cost, which includes travel to and from the hospital and phone encounters with patients, which took away Dr. Feldshon’s ability to conduct colonoscopies and GI consults.
In an attempt to make balloons cost effective, Dr. Feldshon committed to doing these procedures on his day off, which reduced the opportunity cost to $0. This made the balloon procedure profitable, at $1,100 per balloon, but the volume was too low to make it worthwhile.
Despite the challenges his group faced with treating obesity, Dr. Feldshon offered some cost-saving solutions to help keep costs down for both patients and doctors. He suggested avoiding manufacturer weight loss programs. Identify an internal program that is reasonably priced or an external program like Weight Watchers. Physicians can utilize video conferencing for weekly meetings; this helps patients interact with doctors, and products like AdobeConnect cost physicians only about $50 a month. Patients can use free online journaling products like MyFitnessPal to track diet and exercise. Physicians can also recommend using generic and over-the-counter drugs and consider enlisting the help of a life coach or dietitian.
“All obese patients benefit from weight loss but we should be targeting those with metabolic syndrome, diabetes, heart disease, hypercholesterolemia, hyperlipidemia, and increased abdominal girth,” said Dr. Feldshon.
Dr. Feldshon has served on advisory committees and review panels and has worked with United Health Group as well as Prime Therapeutics.
AGA Resource
AGA has created an Obesity Practice Guide to provide gastroenterologists with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management, including a model for how to operationalize business issues.
WASHINGTON – “We have heard about a lot of really interesting new things in therapy, but the reality is that they are just not economically viable at present,” said David Feldshon, MD, of Minnesota Gastroenterology (MNGI), speaking about his experience with offering obesity treatment in his practice.
One problem that Dr. Feldshon and MNGI faced was that they could never get enough patients into their weight loss program, a meal-replacement program priced at about $200 a month. Part of this program, Optifast, a commercially available low-calorie diet and behavior modification program, cost about $360 a month. Most patients found the cost to be prohibitive according to Dr. Feldshon. The program that MNGI offered was fairly competitive when compared with a number of other commercial diet programs like Nutrisystem and Medifast, which cost $300 and $329 a month, respectively.
Because of the economic pressure these programs can exert on patients, Dr. Feldshon spoke to the difficulty of recruiting patients with major metabolic and digestive disorders. “I work in the liver clinic, so I see people with NASH, NAFLD, cirrhosis, near-cirrhosis, diabetes. These are people that would really benefit from this type of program, and I could not get a single patient to sign up.”
For Dr. Feldshon’s program, patients were required to come to MNGI once a week, which led to higher dropout rates. This is troubling because it is important that patients meet with doctors and other patients as part of a weight loss program. But the inconvenience for patients and doctors is not just in physically attending meetings, but in the medical billing. The Affordable Care Act and Medicare cover obesity screening and counseling, but this is only for primary care physicians, nurse practitioners, physician assistants, or clinical nurse specialists. These limits may not extend into private insurance, but they remain a barrier for those covered by Medicare, according to Dr. Feldshon.
Another effective but ultimately expensive option is weight loss drugs. The cost of drugs can vary wildly. Even well-established drugs like phentermine can cost anywhere from $5 to $35 out of pocket a month, according to Dr. Feldshon. Some drugs, like Saxenda, can cost as much as $1,414 per month if paid for out of pocket. While Saxenda is effective, there is a catch in the prescribing and billing for this drug: “The moment you go above 2.4 mg per day, the insurance company says, ‘Aha! He’s treating obesity,’ and they stop covering it.” One drug that Dr. Feldshon recommends is topiramate because it is a multiuse drug. “Often, the insurance company can’t figure out why you’re prescribing it, so they’ll okay it,” said Dr. Feldshon.
One of the most effective nonsurgical methods of weight loss is intragastric balloon. From 2016 to 2017, MNGI used gastric balloons in 22 patients, resulting in 11.4% total body weight loss in these patients. Unfortunately, it was a “financial loser,” according to Dr. Feldshon. The global case rate charge for one balloon is $8,200. MNGI then incurred $2,000 per balloon, $3,000 in hospital charges, $750 for the medical weight loss program, $1,350 for office personnel and visits, and a calculated opportunity cost of $3,140 resulting in a net loss of $2,040 per balloon. The most important factor in this calculation is the opportunity cost, which includes travel to and from the hospital and phone encounters with patients, which took away Dr. Feldshon’s ability to conduct colonoscopies and GI consults.
In an attempt to make balloons cost effective, Dr. Feldshon committed to doing these procedures on his day off, which reduced the opportunity cost to $0. This made the balloon procedure profitable, at $1,100 per balloon, but the volume was too low to make it worthwhile.
Despite the challenges his group faced with treating obesity, Dr. Feldshon offered some cost-saving solutions to help keep costs down for both patients and doctors. He suggested avoiding manufacturer weight loss programs. Identify an internal program that is reasonably priced or an external program like Weight Watchers. Physicians can utilize video conferencing for weekly meetings; this helps patients interact with doctors, and products like AdobeConnect cost physicians only about $50 a month. Patients can use free online journaling products like MyFitnessPal to track diet and exercise. Physicians can also recommend using generic and over-the-counter drugs and consider enlisting the help of a life coach or dietitian.
“All obese patients benefit from weight loss but we should be targeting those with metabolic syndrome, diabetes, heart disease, hypercholesterolemia, hyperlipidemia, and increased abdominal girth,” said Dr. Feldshon.
Dr. Feldshon has served on advisory committees and review panels and has worked with United Health Group as well as Prime Therapeutics.
AGA Resource
AGA has created an Obesity Practice Guide to provide gastroenterologists with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management, including a model for how to operationalize business issues.
EXPERT ANALYSIS FROM DDW 2018
Bivalent HPV vaccine brings no significant increase in 38 potential adverse outcomes
MALMO, SWEDEN – in a nationwide, retrospective cohort study.
“Anxiety and fear regarding adverse events are still very much alive and are associated with lack of acceptance of HPV vaccination,” Jozica Skufca, DVM, MIPH, observed while presenting the study findings at the annual meeting of the European Society for Paediatric Infectious Diseases.
“These results contribute valid evidence to combat public skepticism, anxiety, fears of adverse events, and possible opposition to HPV vaccination and consequently can contribute to increase HPV vaccination coverage in Finland as well as elsewhere,” added Dr. Skufca, who conducted this research while at the Finnish National Institute for Health and Welfare but is now an epidemiologist at P95, a privately held epidemiology and pharmacovigilance consulting firm in Heverlee, Belgium.
Finland introduced the bivalent HPV vaccine known as Cervarix, which is directed against HPV types 16 and 18, into the nation’s vaccination program in November 2013. The target population were girls aged 11-13 years, with catch-up vaccination at ages 14-15 years until 2015.
Dr. Skufca utilized Finland’s comprehensive system of national health care registries to conduct a study of all 240,605 Finnish girls aged 11-15 years who were eligible for the bivalent HPV vaccine from November 2013 to December 2016. Only 56% of the target population, or 134,615 girls, were actually vaccinated. All were subsequently followed for more than a year.
The investigators compared vaccinated and unvaccinated groups in terms of the subsequent rates of 38 selected outcomes of national and international interest. These included celiac disease, chronic fatigue syndrome, type 1 diabetes, Guillain-Barré syndrome, postural orthostatic tachycardia syndrome, ulcerative colitis, venous thromboembolism, psoriasis, vitiligo, scleroderma, autism, poyarteritis nodosa, asthma, Raynaud’s disease, systemic lupus erythematosus, Bell’s palsy, polycystic ovaries, juvenile arthritis, Hashimoto’s disease, and many others.
HPV vaccination rates varied considerably between hospital districts, so the results were adjusted for that potential confounder. Risks also were adjusted for the number of hospital visits 1-2 years prior to vaccination, because healthier girls – those with fewer hospital visits – turned out to be more likely to get vaccinated.
The study’s main result was that HPV vaccination wasn’t associated with a significantly higher risk of any of the 38 adverse outcomes. To the contrary, vaccination was associated with significantly reduced risk of several of them: The risk of chronic fatigue syndrome was reduced by 25%, epilepsy and recurrent seizures were reduced by 28%, the risk of Henoch-Schönlein purpura was an adjusted 52% lower in children vaccinated against HPV, and the risk of malaise and fatigue was reduced by 24%.
Dr. Skufca drew particular attention to the 400% increased risk of Guillain-Barré syndrome in vaccinated children. While this may look impressive, it wasn’t a statistically significant difference. Guillain-Barré was a rare event, with only six cases occurring during 186,934 person-years of follow-up of vaccinated individuals and a single case in unvaccinated girls. Two cases occurred within 180 days after vaccination, for an adjusted 200% increase in risk, which was nonsignificant.
Another two cases occurred more than 365 days postvaccination, for a whopping 3100% increased risk, compared with unvaccinated children; however, this too was a nonstatistically significant finding, with extraordinarily broad confidence intervals of 1.59-652.4. And there is no plausible mechanism to account for a true association between HPV vaccination and a complication, such as this, occurring more than 1 year later. It appeared to be a matter of chance, she added.
However, session cochair Ron Dagan, MD, commented that vaccine skeptics won’t find the Guillain-Barré findings reassuring.
“With HPV vaccination, there are a lot of issues related to mass perception. You say it’s not statistically significant, but some people will say, ‘Forget about the statistics – look at the confidence intervals, the risk could be more than 600 times increased.’ How do you answer this? It’s not easy, especially when we’re talking about a vaccine which has a strong adjuvant,” said Dr. Dagan, director of the pediatric infectious disease unit at Soroka University Medical Center and professor of pediatrics and infectious diseases at the Ben-Gurion University of the Negev, both in Beer-Sheva, Israel.
“I completely agree, how to communicate results to the public is one of the most challenging things. They don’t understand about statistics,” Dr. Skufca replied.
The ball is now in the court of the Finnish health ministry, she added. These study results are brand new. Ministry officials are now going over the details and developing a strategy to communicate the results to the media, the public, and affected families.
The study was funded by the Finnish National Institute for Health and Welfare. Dr. Skufca reported receiving research grants from GlaxoSmithKline and Pfizer.
MALMO, SWEDEN – in a nationwide, retrospective cohort study.
“Anxiety and fear regarding adverse events are still very much alive and are associated with lack of acceptance of HPV vaccination,” Jozica Skufca, DVM, MIPH, observed while presenting the study findings at the annual meeting of the European Society for Paediatric Infectious Diseases.
“These results contribute valid evidence to combat public skepticism, anxiety, fears of adverse events, and possible opposition to HPV vaccination and consequently can contribute to increase HPV vaccination coverage in Finland as well as elsewhere,” added Dr. Skufca, who conducted this research while at the Finnish National Institute for Health and Welfare but is now an epidemiologist at P95, a privately held epidemiology and pharmacovigilance consulting firm in Heverlee, Belgium.
Finland introduced the bivalent HPV vaccine known as Cervarix, which is directed against HPV types 16 and 18, into the nation’s vaccination program in November 2013. The target population were girls aged 11-13 years, with catch-up vaccination at ages 14-15 years until 2015.
Dr. Skufca utilized Finland’s comprehensive system of national health care registries to conduct a study of all 240,605 Finnish girls aged 11-15 years who were eligible for the bivalent HPV vaccine from November 2013 to December 2016. Only 56% of the target population, or 134,615 girls, were actually vaccinated. All were subsequently followed for more than a year.
The investigators compared vaccinated and unvaccinated groups in terms of the subsequent rates of 38 selected outcomes of national and international interest. These included celiac disease, chronic fatigue syndrome, type 1 diabetes, Guillain-Barré syndrome, postural orthostatic tachycardia syndrome, ulcerative colitis, venous thromboembolism, psoriasis, vitiligo, scleroderma, autism, poyarteritis nodosa, asthma, Raynaud’s disease, systemic lupus erythematosus, Bell’s palsy, polycystic ovaries, juvenile arthritis, Hashimoto’s disease, and many others.
HPV vaccination rates varied considerably between hospital districts, so the results were adjusted for that potential confounder. Risks also were adjusted for the number of hospital visits 1-2 years prior to vaccination, because healthier girls – those with fewer hospital visits – turned out to be more likely to get vaccinated.
The study’s main result was that HPV vaccination wasn’t associated with a significantly higher risk of any of the 38 adverse outcomes. To the contrary, vaccination was associated with significantly reduced risk of several of them: The risk of chronic fatigue syndrome was reduced by 25%, epilepsy and recurrent seizures were reduced by 28%, the risk of Henoch-Schönlein purpura was an adjusted 52% lower in children vaccinated against HPV, and the risk of malaise and fatigue was reduced by 24%.
Dr. Skufca drew particular attention to the 400% increased risk of Guillain-Barré syndrome in vaccinated children. While this may look impressive, it wasn’t a statistically significant difference. Guillain-Barré was a rare event, with only six cases occurring during 186,934 person-years of follow-up of vaccinated individuals and a single case in unvaccinated girls. Two cases occurred within 180 days after vaccination, for an adjusted 200% increase in risk, which was nonsignificant.
Another two cases occurred more than 365 days postvaccination, for a whopping 3100% increased risk, compared with unvaccinated children; however, this too was a nonstatistically significant finding, with extraordinarily broad confidence intervals of 1.59-652.4. And there is no plausible mechanism to account for a true association between HPV vaccination and a complication, such as this, occurring more than 1 year later. It appeared to be a matter of chance, she added.
However, session cochair Ron Dagan, MD, commented that vaccine skeptics won’t find the Guillain-Barré findings reassuring.
“With HPV vaccination, there are a lot of issues related to mass perception. You say it’s not statistically significant, but some people will say, ‘Forget about the statistics – look at the confidence intervals, the risk could be more than 600 times increased.’ How do you answer this? It’s not easy, especially when we’re talking about a vaccine which has a strong adjuvant,” said Dr. Dagan, director of the pediatric infectious disease unit at Soroka University Medical Center and professor of pediatrics and infectious diseases at the Ben-Gurion University of the Negev, both in Beer-Sheva, Israel.
“I completely agree, how to communicate results to the public is one of the most challenging things. They don’t understand about statistics,” Dr. Skufca replied.
The ball is now in the court of the Finnish health ministry, she added. These study results are brand new. Ministry officials are now going over the details and developing a strategy to communicate the results to the media, the public, and affected families.
The study was funded by the Finnish National Institute for Health and Welfare. Dr. Skufca reported receiving research grants from GlaxoSmithKline and Pfizer.
MALMO, SWEDEN – in a nationwide, retrospective cohort study.
“Anxiety and fear regarding adverse events are still very much alive and are associated with lack of acceptance of HPV vaccination,” Jozica Skufca, DVM, MIPH, observed while presenting the study findings at the annual meeting of the European Society for Paediatric Infectious Diseases.
“These results contribute valid evidence to combat public skepticism, anxiety, fears of adverse events, and possible opposition to HPV vaccination and consequently can contribute to increase HPV vaccination coverage in Finland as well as elsewhere,” added Dr. Skufca, who conducted this research while at the Finnish National Institute for Health and Welfare but is now an epidemiologist at P95, a privately held epidemiology and pharmacovigilance consulting firm in Heverlee, Belgium.
Finland introduced the bivalent HPV vaccine known as Cervarix, which is directed against HPV types 16 and 18, into the nation’s vaccination program in November 2013. The target population were girls aged 11-13 years, with catch-up vaccination at ages 14-15 years until 2015.
Dr. Skufca utilized Finland’s comprehensive system of national health care registries to conduct a study of all 240,605 Finnish girls aged 11-15 years who were eligible for the bivalent HPV vaccine from November 2013 to December 2016. Only 56% of the target population, or 134,615 girls, were actually vaccinated. All were subsequently followed for more than a year.
The investigators compared vaccinated and unvaccinated groups in terms of the subsequent rates of 38 selected outcomes of national and international interest. These included celiac disease, chronic fatigue syndrome, type 1 diabetes, Guillain-Barré syndrome, postural orthostatic tachycardia syndrome, ulcerative colitis, venous thromboembolism, psoriasis, vitiligo, scleroderma, autism, poyarteritis nodosa, asthma, Raynaud’s disease, systemic lupus erythematosus, Bell’s palsy, polycystic ovaries, juvenile arthritis, Hashimoto’s disease, and many others.
HPV vaccination rates varied considerably between hospital districts, so the results were adjusted for that potential confounder. Risks also were adjusted for the number of hospital visits 1-2 years prior to vaccination, because healthier girls – those with fewer hospital visits – turned out to be more likely to get vaccinated.
The study’s main result was that HPV vaccination wasn’t associated with a significantly higher risk of any of the 38 adverse outcomes. To the contrary, vaccination was associated with significantly reduced risk of several of them: The risk of chronic fatigue syndrome was reduced by 25%, epilepsy and recurrent seizures were reduced by 28%, the risk of Henoch-Schönlein purpura was an adjusted 52% lower in children vaccinated against HPV, and the risk of malaise and fatigue was reduced by 24%.
Dr. Skufca drew particular attention to the 400% increased risk of Guillain-Barré syndrome in vaccinated children. While this may look impressive, it wasn’t a statistically significant difference. Guillain-Barré was a rare event, with only six cases occurring during 186,934 person-years of follow-up of vaccinated individuals and a single case in unvaccinated girls. Two cases occurred within 180 days after vaccination, for an adjusted 200% increase in risk, which was nonsignificant.
Another two cases occurred more than 365 days postvaccination, for a whopping 3100% increased risk, compared with unvaccinated children; however, this too was a nonstatistically significant finding, with extraordinarily broad confidence intervals of 1.59-652.4. And there is no plausible mechanism to account for a true association between HPV vaccination and a complication, such as this, occurring more than 1 year later. It appeared to be a matter of chance, she added.
However, session cochair Ron Dagan, MD, commented that vaccine skeptics won’t find the Guillain-Barré findings reassuring.
“With HPV vaccination, there are a lot of issues related to mass perception. You say it’s not statistically significant, but some people will say, ‘Forget about the statistics – look at the confidence intervals, the risk could be more than 600 times increased.’ How do you answer this? It’s not easy, especially when we’re talking about a vaccine which has a strong adjuvant,” said Dr. Dagan, director of the pediatric infectious disease unit at Soroka University Medical Center and professor of pediatrics and infectious diseases at the Ben-Gurion University of the Negev, both in Beer-Sheva, Israel.
“I completely agree, how to communicate results to the public is one of the most challenging things. They don’t understand about statistics,” Dr. Skufca replied.
The ball is now in the court of the Finnish health ministry, she added. These study results are brand new. Ministry officials are now going over the details and developing a strategy to communicate the results to the media, the public, and affected families.
The study was funded by the Finnish National Institute for Health and Welfare. Dr. Skufca reported receiving research grants from GlaxoSmithKline and Pfizer.
REPORTING FROM ESPID 2018
Key clinical point: Human papillomavirus vaccination poses no significant increased risk of numerous potential adverse outcomes.
Major finding: There was no significantly increased risk of any of 38 potential adverse outcomes in girls aged 11-15 years following administration of a bivalent HPV vaccine.
Study details: This was a retrospective cohort study including more than 240,000 Finnish girls eligible for HPV vaccination following its introduction into the national immunization program.
Disclosures: The study was sponsored by the Finnish National Institute for Health and Welfare. The presenter reported receiving research grants from GlaxoSmithKline and Pfizer.
Hospitalist movers and shakers – July 2018
Steven Pantilat, MD, MHM, has been named the first chief of the newly established division of palliative medicine at University of California, San Francisco Health. Dr. Pantilat’s new role commenced on May 1st, with the division launch anticipated for July 1st.
Dr. Pantilat is a Master of the Society of Hospital Medicine and a former president of the society (2005-2006).
Gary J. Carver, MD, recently was named the chief medical officer at Coshocton (Ohio) Regional Medical Center. Dr. Carver has been the hospital’s director of hospital medicine since 2013 and will continue in that role in addition to his duties as CMO.
In his new position, Dr. Carver joins Coshocton Medical Center’s senior leadership team, providing medical oversight, as well as clinical direction and leadership as the facility seeks accreditation, quality improvement, and service line development.
Lisa Shah, MD, has been hired by Sound Physicians as the group’s chief innovation officer. Dr. Shah had been working as senior vice president of Evolent Health’s clinical operations and network. With Sound Physicians, Dr. Shah will lead clinical innovation and transformation for the nationwide organization of physicians providing emergency medical, critical care, and hospital medicine services at more than 180 hospitals.
Dr. Shah will be tasked with developing innovative care models, tech-centered clinical workflows, and telemedicine strategies. She brings a robust hospital medicine background, having served in a 2-year Hospitalist Scholars Fellowship at the University of Chicago, while simultaneously earning a master’s degree in public health.
BUSINESS MOVES
The University of Mississippi Medical Center Children’s of Mississippi, Hattiesburg, branch is joining forces with Memorial Hospital at Gulfport (Miss.) to provide care throughout southern Mississippi.
The highlight of the merger is the acquisition of six pediatric clinics into the UMMC family, with UMMC assuming control of the pediatric hospitalist program at each of the locations. The acquired clinics all have been branded as Children’s of Mississippi as of March 26th.
Sound Physicians’ parent company Fresenius Medical Care, which has held a controlling interest in Sound since 2014, has sold that interest to Germany-based Summit Partners for a reported $2.15 billion. The acquisition is expected to be finalized later this calendar year.
Sound, which reported revenues of approximately $1.5 billion in 2017, is optimistic that it can tap into new markets while under the Summit umbrella.
The Ob Hospitalist Group, Greenville, S.C., the nation’s largest Ob/Gyn hospitalist organization, recently announced the rollout of its CARE (Clinician Assistance, Recovery, and Encourage) program. CARE uses peer support to assist clinicians facing psychological and emotional impacts from adverse Ob events.
CARE peer counseling focuses on confidentiality, empathy, trust, and respect for colleagues suffering from a negative patient-care event. The program is available to more than 600 Ob hospitalist clinicians at more than 120 hospitals nationwide.
Steven Pantilat, MD, MHM, has been named the first chief of the newly established division of palliative medicine at University of California, San Francisco Health. Dr. Pantilat’s new role commenced on May 1st, with the division launch anticipated for July 1st.
Dr. Pantilat is a Master of the Society of Hospital Medicine and a former president of the society (2005-2006).
Gary J. Carver, MD, recently was named the chief medical officer at Coshocton (Ohio) Regional Medical Center. Dr. Carver has been the hospital’s director of hospital medicine since 2013 and will continue in that role in addition to his duties as CMO.
In his new position, Dr. Carver joins Coshocton Medical Center’s senior leadership team, providing medical oversight, as well as clinical direction and leadership as the facility seeks accreditation, quality improvement, and service line development.
Lisa Shah, MD, has been hired by Sound Physicians as the group’s chief innovation officer. Dr. Shah had been working as senior vice president of Evolent Health’s clinical operations and network. With Sound Physicians, Dr. Shah will lead clinical innovation and transformation for the nationwide organization of physicians providing emergency medical, critical care, and hospital medicine services at more than 180 hospitals.
Dr. Shah will be tasked with developing innovative care models, tech-centered clinical workflows, and telemedicine strategies. She brings a robust hospital medicine background, having served in a 2-year Hospitalist Scholars Fellowship at the University of Chicago, while simultaneously earning a master’s degree in public health.
BUSINESS MOVES
The University of Mississippi Medical Center Children’s of Mississippi, Hattiesburg, branch is joining forces with Memorial Hospital at Gulfport (Miss.) to provide care throughout southern Mississippi.
The highlight of the merger is the acquisition of six pediatric clinics into the UMMC family, with UMMC assuming control of the pediatric hospitalist program at each of the locations. The acquired clinics all have been branded as Children’s of Mississippi as of March 26th.
Sound Physicians’ parent company Fresenius Medical Care, which has held a controlling interest in Sound since 2014, has sold that interest to Germany-based Summit Partners for a reported $2.15 billion. The acquisition is expected to be finalized later this calendar year.
Sound, which reported revenues of approximately $1.5 billion in 2017, is optimistic that it can tap into new markets while under the Summit umbrella.
The Ob Hospitalist Group, Greenville, S.C., the nation’s largest Ob/Gyn hospitalist organization, recently announced the rollout of its CARE (Clinician Assistance, Recovery, and Encourage) program. CARE uses peer support to assist clinicians facing psychological and emotional impacts from adverse Ob events.
CARE peer counseling focuses on confidentiality, empathy, trust, and respect for colleagues suffering from a negative patient-care event. The program is available to more than 600 Ob hospitalist clinicians at more than 120 hospitals nationwide.
Steven Pantilat, MD, MHM, has been named the first chief of the newly established division of palliative medicine at University of California, San Francisco Health. Dr. Pantilat’s new role commenced on May 1st, with the division launch anticipated for July 1st.
Dr. Pantilat is a Master of the Society of Hospital Medicine and a former president of the society (2005-2006).
Gary J. Carver, MD, recently was named the chief medical officer at Coshocton (Ohio) Regional Medical Center. Dr. Carver has been the hospital’s director of hospital medicine since 2013 and will continue in that role in addition to his duties as CMO.
In his new position, Dr. Carver joins Coshocton Medical Center’s senior leadership team, providing medical oversight, as well as clinical direction and leadership as the facility seeks accreditation, quality improvement, and service line development.
Lisa Shah, MD, has been hired by Sound Physicians as the group’s chief innovation officer. Dr. Shah had been working as senior vice president of Evolent Health’s clinical operations and network. With Sound Physicians, Dr. Shah will lead clinical innovation and transformation for the nationwide organization of physicians providing emergency medical, critical care, and hospital medicine services at more than 180 hospitals.
Dr. Shah will be tasked with developing innovative care models, tech-centered clinical workflows, and telemedicine strategies. She brings a robust hospital medicine background, having served in a 2-year Hospitalist Scholars Fellowship at the University of Chicago, while simultaneously earning a master’s degree in public health.
BUSINESS MOVES
The University of Mississippi Medical Center Children’s of Mississippi, Hattiesburg, branch is joining forces with Memorial Hospital at Gulfport (Miss.) to provide care throughout southern Mississippi.
The highlight of the merger is the acquisition of six pediatric clinics into the UMMC family, with UMMC assuming control of the pediatric hospitalist program at each of the locations. The acquired clinics all have been branded as Children’s of Mississippi as of March 26th.
Sound Physicians’ parent company Fresenius Medical Care, which has held a controlling interest in Sound since 2014, has sold that interest to Germany-based Summit Partners for a reported $2.15 billion. The acquisition is expected to be finalized later this calendar year.
Sound, which reported revenues of approximately $1.5 billion in 2017, is optimistic that it can tap into new markets while under the Summit umbrella.
The Ob Hospitalist Group, Greenville, S.C., the nation’s largest Ob/Gyn hospitalist organization, recently announced the rollout of its CARE (Clinician Assistance, Recovery, and Encourage) program. CARE uses peer support to assist clinicians facing psychological and emotional impacts from adverse Ob events.
CARE peer counseling focuses on confidentiality, empathy, trust, and respect for colleagues suffering from a negative patient-care event. The program is available to more than 600 Ob hospitalist clinicians at more than 120 hospitals nationwide.
Nearly one-quarter of presurgery patients already using opioids
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.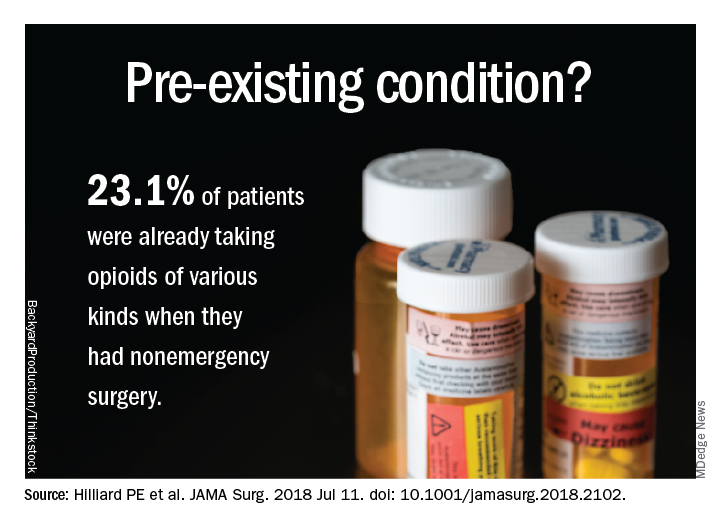
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.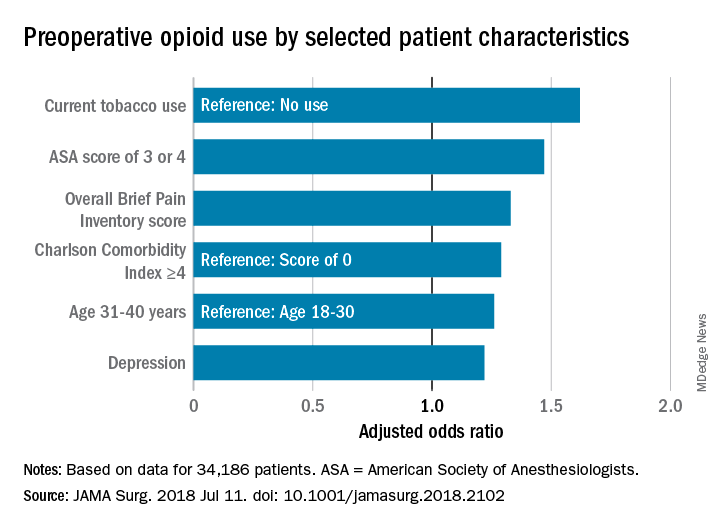
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
FROM JAMA SURGERY
Key clinical point: Preoperative opioid use is prevalent in patients who are having spinal surgery and have depression.
Major finding:
Study details: An observational study of 34,186 surgical patients in the University of Michigan Health system.
Disclosures: The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
Source: Hilliard P E et al. JAMA Surg. 2018 Jul 11;. doi:10.1001/jamasurg.2018.2102.