User login
Epinephrine for cardiac arrest: Better survival, more brain damage
Using epinephrine for cardiac arrest improves 30-day survival by less than 1%, and nearly doubles the risk of severe brain damage among survivors, according to PARAMEDIC2, a randomized, double-blind trial in more than 8,000 patients in Great Britain.
It’s clear what patients want. “Our own work with patients and the public before starting the trial identified survival without brain damage [as] more important to patients than survival alone. The findings of this trial will require careful consideration by the wider community and those responsible for clinical practice guidelines for cardiac arrest,” lead investigator Gavin D. Perkins, MD, professor of critical care medicine at the University of Warwick, Coventry, England, and lead author of the study published in the New England Journal of Medicine, wrote in a statement.
In PARAMEDIC2, after initial attempts with CPR and defibrillation failed, 4,012 patients were given epinephrine 1 mg by intravenous or intraosseous infusion every 3-5 minutes for a maximum of 10 doses, and 3,995 were given a saline placebo in the same fashion. The median time from emergency call to ambulance arrival was just over 6 minutes in both groups, with a further 14 minutes until drug administration.
The heart restarted in a higher proportion of epinephrine patients (36.3% vs. 11.7%), and 3.2% of epinephrine patients were alive at 30 days, versus 2.4% in the placebo arm, a 39% increase.
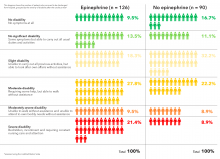
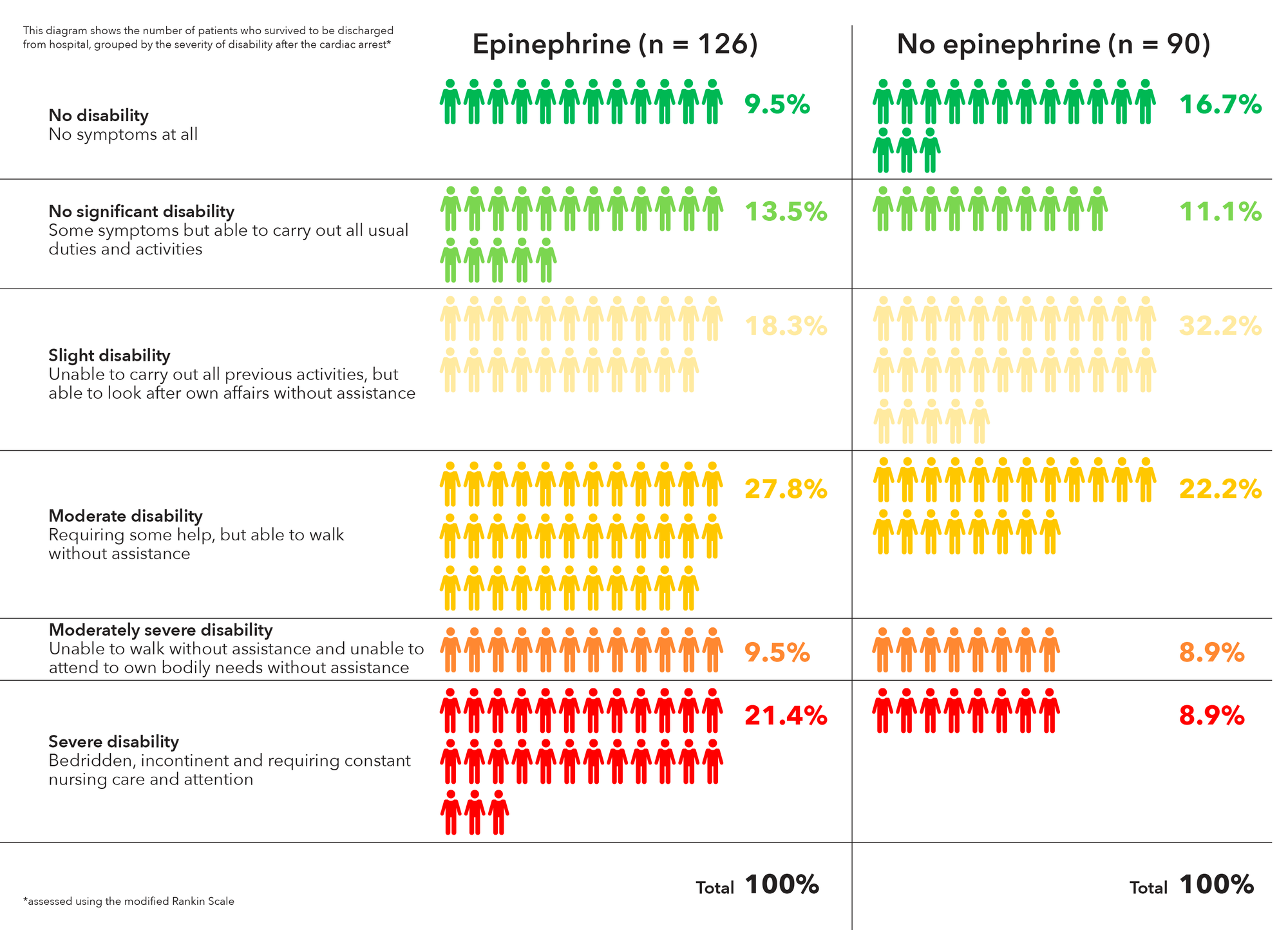
However, that slight benefit came at a significant cost. Of the 126 epinephrine patients who survived to hospital discharge, 39 (31%) had severe brain damage, compared with 16 (17.8%) among the 90 placebo survivors. Severe brain damage meant inability to walk and tend to bodily functions, or a persistent vegetative state (modified Rankin scale grade 4 or 5).
The trial addresses a long-standing question in resuscitation medicine, the role of epinephrine in cardiac arrest. It’s a devil’s bargain: Epinephrine increases blood flow to the heart, so helps with resuscitation, but it also reduces blood flow in the brain’s microvasculature, increasing the risk of brain damage.
“The benefit of epinephrine on survival demonstrated in this trial should be considered in comparison with other treatments in the chain of survival.” Early cardiac arrest recognition saves 1 in every 11 patients, bystander CPR saves 1 in every 15, and early defibrillation saves 1 in 5, the investigators noted.
The trial did not collect data on prearrest neurologic status, but the number of subjects with impaired function was probably very small and balanced between the groups, according to the report.
On average, patients were aged just under 70 years, 65% were men, and bystander CPR was performed in about 60% in both groups. They were enrolled by five ambulance services in England and Wales. Informed consent was obtained, when possible, after resuscitation.
The trial was funded by the U.K. National Institute for Health Research. The researchers had no relevant disclosures to report.
SOURCE: Perkins GD et al. N Engl J Med. 2018 Jul 18. doi:10.1056/NEJMoa1806842.
Epinephrine has been used in resuscitation efforts since the 1960s, yet no reliable evidence on the practice has been collected. Now, PARAMEDIC2 provides the most rigorous data on patient-centered outcomes with respect to epinephrine to date.
Epinephrine increased 30-day survival in patients with nonshockable rhythms by more than 100%, but the benefit was less clear in those with shockable rhythms. Shockable rhythms are more likely to occur in patients with cardiac or cardiovascular causes of arrest, which epinephrine may exacerbate. The results underscore the principle that drug administration should not compete with or delay defibrillation, and that epinephrine may have different effects in patients with different ECG rhythms.
The PARAMEDIC2 results leave us with several questions: Could other, additional treatments after a return of spontaneous circulation improve functional recovery, should drug use differ on the basis of cardiac rhythm, and would lower doses of epinephrine be superior to higher doses among patients with out-of-hospital cardiac arrest?
Clifton W. Callaway, MD, PhD, of the University of Pittsburgh, and Michael W. Donnino, MD, of Beth Israel Deaconess Medical Center, Boston, made these comments in an accompanying editorial (N Engl J Med. 2018 Jul 18. doi: 10.1056/NEJMe1808255). They had no relevant disclosures.
Epinephrine has been used in resuscitation efforts since the 1960s, yet no reliable evidence on the practice has been collected. Now, PARAMEDIC2 provides the most rigorous data on patient-centered outcomes with respect to epinephrine to date.
Epinephrine increased 30-day survival in patients with nonshockable rhythms by more than 100%, but the benefit was less clear in those with shockable rhythms. Shockable rhythms are more likely to occur in patients with cardiac or cardiovascular causes of arrest, which epinephrine may exacerbate. The results underscore the principle that drug administration should not compete with or delay defibrillation, and that epinephrine may have different effects in patients with different ECG rhythms.
The PARAMEDIC2 results leave us with several questions: Could other, additional treatments after a return of spontaneous circulation improve functional recovery, should drug use differ on the basis of cardiac rhythm, and would lower doses of epinephrine be superior to higher doses among patients with out-of-hospital cardiac arrest?
Clifton W. Callaway, MD, PhD, of the University of Pittsburgh, and Michael W. Donnino, MD, of Beth Israel Deaconess Medical Center, Boston, made these comments in an accompanying editorial (N Engl J Med. 2018 Jul 18. doi: 10.1056/NEJMe1808255). They had no relevant disclosures.
Epinephrine has been used in resuscitation efforts since the 1960s, yet no reliable evidence on the practice has been collected. Now, PARAMEDIC2 provides the most rigorous data on patient-centered outcomes with respect to epinephrine to date.
Epinephrine increased 30-day survival in patients with nonshockable rhythms by more than 100%, but the benefit was less clear in those with shockable rhythms. Shockable rhythms are more likely to occur in patients with cardiac or cardiovascular causes of arrest, which epinephrine may exacerbate. The results underscore the principle that drug administration should not compete with or delay defibrillation, and that epinephrine may have different effects in patients with different ECG rhythms.
The PARAMEDIC2 results leave us with several questions: Could other, additional treatments after a return of spontaneous circulation improve functional recovery, should drug use differ on the basis of cardiac rhythm, and would lower doses of epinephrine be superior to higher doses among patients with out-of-hospital cardiac arrest?
Clifton W. Callaway, MD, PhD, of the University of Pittsburgh, and Michael W. Donnino, MD, of Beth Israel Deaconess Medical Center, Boston, made these comments in an accompanying editorial (N Engl J Med. 2018 Jul 18. doi: 10.1056/NEJMe1808255). They had no relevant disclosures.
Using epinephrine for cardiac arrest improves 30-day survival by less than 1%, and nearly doubles the risk of severe brain damage among survivors, according to PARAMEDIC2, a randomized, double-blind trial in more than 8,000 patients in Great Britain.
It’s clear what patients want. “Our own work with patients and the public before starting the trial identified survival without brain damage [as] more important to patients than survival alone. The findings of this trial will require careful consideration by the wider community and those responsible for clinical practice guidelines for cardiac arrest,” lead investigator Gavin D. Perkins, MD, professor of critical care medicine at the University of Warwick, Coventry, England, and lead author of the study published in the New England Journal of Medicine, wrote in a statement.
In PARAMEDIC2, after initial attempts with CPR and defibrillation failed, 4,012 patients were given epinephrine 1 mg by intravenous or intraosseous infusion every 3-5 minutes for a maximum of 10 doses, and 3,995 were given a saline placebo in the same fashion. The median time from emergency call to ambulance arrival was just over 6 minutes in both groups, with a further 14 minutes until drug administration.
The heart restarted in a higher proportion of epinephrine patients (36.3% vs. 11.7%), and 3.2% of epinephrine patients were alive at 30 days, versus 2.4% in the placebo arm, a 39% increase.
However, that slight benefit came at a significant cost. Of the 126 epinephrine patients who survived to hospital discharge, 39 (31%) had severe brain damage, compared with 16 (17.8%) among the 90 placebo survivors. Severe brain damage meant inability to walk and tend to bodily functions, or a persistent vegetative state (modified Rankin scale grade 4 or 5).
The trial addresses a long-standing question in resuscitation medicine, the role of epinephrine in cardiac arrest. It’s a devil’s bargain: Epinephrine increases blood flow to the heart, so helps with resuscitation, but it also reduces blood flow in the brain’s microvasculature, increasing the risk of brain damage.
“The benefit of epinephrine on survival demonstrated in this trial should be considered in comparison with other treatments in the chain of survival.” Early cardiac arrest recognition saves 1 in every 11 patients, bystander CPR saves 1 in every 15, and early defibrillation saves 1 in 5, the investigators noted.
The trial did not collect data on prearrest neurologic status, but the number of subjects with impaired function was probably very small and balanced between the groups, according to the report.
On average, patients were aged just under 70 years, 65% were men, and bystander CPR was performed in about 60% in both groups. They were enrolled by five ambulance services in England and Wales. Informed consent was obtained, when possible, after resuscitation.
The trial was funded by the U.K. National Institute for Health Research. The researchers had no relevant disclosures to report.
SOURCE: Perkins GD et al. N Engl J Med. 2018 Jul 18. doi:10.1056/NEJMoa1806842.
Using epinephrine for cardiac arrest improves 30-day survival by less than 1%, and nearly doubles the risk of severe brain damage among survivors, according to PARAMEDIC2, a randomized, double-blind trial in more than 8,000 patients in Great Britain.
It’s clear what patients want. “Our own work with patients and the public before starting the trial identified survival without brain damage [as] more important to patients than survival alone. The findings of this trial will require careful consideration by the wider community and those responsible for clinical practice guidelines for cardiac arrest,” lead investigator Gavin D. Perkins, MD, professor of critical care medicine at the University of Warwick, Coventry, England, and lead author of the study published in the New England Journal of Medicine, wrote in a statement.
In PARAMEDIC2, after initial attempts with CPR and defibrillation failed, 4,012 patients were given epinephrine 1 mg by intravenous or intraosseous infusion every 3-5 minutes for a maximum of 10 doses, and 3,995 were given a saline placebo in the same fashion. The median time from emergency call to ambulance arrival was just over 6 minutes in both groups, with a further 14 minutes until drug administration.
The heart restarted in a higher proportion of epinephrine patients (36.3% vs. 11.7%), and 3.2% of epinephrine patients were alive at 30 days, versus 2.4% in the placebo arm, a 39% increase.
However, that slight benefit came at a significant cost. Of the 126 epinephrine patients who survived to hospital discharge, 39 (31%) had severe brain damage, compared with 16 (17.8%) among the 90 placebo survivors. Severe brain damage meant inability to walk and tend to bodily functions, or a persistent vegetative state (modified Rankin scale grade 4 or 5).
The trial addresses a long-standing question in resuscitation medicine, the role of epinephrine in cardiac arrest. It’s a devil’s bargain: Epinephrine increases blood flow to the heart, so helps with resuscitation, but it also reduces blood flow in the brain’s microvasculature, increasing the risk of brain damage.
“The benefit of epinephrine on survival demonstrated in this trial should be considered in comparison with other treatments in the chain of survival.” Early cardiac arrest recognition saves 1 in every 11 patients, bystander CPR saves 1 in every 15, and early defibrillation saves 1 in 5, the investigators noted.
The trial did not collect data on prearrest neurologic status, but the number of subjects with impaired function was probably very small and balanced between the groups, according to the report.
On average, patients were aged just under 70 years, 65% were men, and bystander CPR was performed in about 60% in both groups. They were enrolled by five ambulance services in England and Wales. Informed consent was obtained, when possible, after resuscitation.
The trial was funded by the U.K. National Institute for Health Research. The researchers had no relevant disclosures to report.
SOURCE: Perkins GD et al. N Engl J Med. 2018 Jul 18. doi:10.1056/NEJMoa1806842.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Of the 128 epinephrine patients who survived to hospital discharge, 39 (30.1%) had severe brain damage, compared with 16 (18.7%) among the 91 placebo survivors.
Study details: A randomized, double-blind trial of over 8,000 U.K. patients experiencing an out-of-hospital cardiac arrest.
Disclosures: The trial was funded by the U.K. National Institute for Health Research. The researchers had no relevant disclosures to report.
Source: Perkins GD et al. N Engl J Med. 2018 Jul 18. doi:10.1056/NEJMoa1806842.
National Academies issues 5-step plan to address infections linked to opioid use disorder
Widespread opioid use disorder (OUD) has spawned new epidemics of hepatitis C virus (HCV) and HIV infections as well as increased hospitalizations for bacteremia, endocarditis, skin and soft tissue infections, and osteomyelitis, according to a report arising from a National Academies of Science, Engineering and Medicine (NASEM) workshop titled Integrating Infectious Disease Considerations with Response to the Opioid Epidemic.
Optimal treatment of these infections is often impeded by untreated OUD, Sandra A. Springer, MD, and her colleagues wrote in an article published online in the Annals of Internal Medicine. Failing to address OUD can result in longer hospital stays; frequent readmissions because of a lack of adherence to antibiotic regimens; or reinfection, morbidity, and high costs. “Medical settings that manage such infections offer a potential means of engaging people in treatment of OUD; however, few providers and hospitals treating such infections have the needed resources and capabilities,” Dr. Springer, director, infectious disease outpatient clinic, Veterans Administration, Newington, and of Yale University, New Haven, both in Conn., and her colleagues wrote.
The authors outlined five action steps resulting from the NASEM workshop:
- Implement screening for OUD in all relevant health care settings.
- For patients with positive screening results, immediately prescribe effective medication for OUD and/or opioid withdrawal symptoms.
- Develop hospital-based protocols that facilitate OUD treatment initiation and linkage to community-based treatment upon discharge.
- Hospitals, medical schools, physician assistant schools, nursing schools, and residency programs should increase training to identify and treat OUD.
- Increase access to addiction care and funding to states to provide effective medications to treat OUD.
Opioid withdrawal and pain syndromes should be addressed with opioid agonist therapies to optimize infectious disease (ID) treatment and relieve pain, according to Dr. Springer and her colleagues. In addition, “Because ID specialists are likely to be consulted for anyone requiring long-term antibiotic therapy or patients with HIV and HCV infection, OUD screening should be a standard part of an ID consult assessment,” the authors wrote.
“All health care providers have a role in combating the OUD epidemic and its ID consequences. Those who treat infectious complications of OUD are well suited to screen for OUD and begin treatment with effective FDA-approved medications,” the authors concluded.
The workshop was held in March 2018 in Washington and videos and slide presentations from the meeting are available.
Dr. Springer and her colleagues reported grant funding from the National Institutes of Health, but no commercial conflicts.
SOURCE: Springer SA et al. Ann Intern Med. 2018 Jul 13. doi: 10.7326/M18-1203.
Widespread opioid use disorder (OUD) has spawned new epidemics of hepatitis C virus (HCV) and HIV infections as well as increased hospitalizations for bacteremia, endocarditis, skin and soft tissue infections, and osteomyelitis, according to a report arising from a National Academies of Science, Engineering and Medicine (NASEM) workshop titled Integrating Infectious Disease Considerations with Response to the Opioid Epidemic.
Optimal treatment of these infections is often impeded by untreated OUD, Sandra A. Springer, MD, and her colleagues wrote in an article published online in the Annals of Internal Medicine. Failing to address OUD can result in longer hospital stays; frequent readmissions because of a lack of adherence to antibiotic regimens; or reinfection, morbidity, and high costs. “Medical settings that manage such infections offer a potential means of engaging people in treatment of OUD; however, few providers and hospitals treating such infections have the needed resources and capabilities,” Dr. Springer, director, infectious disease outpatient clinic, Veterans Administration, Newington, and of Yale University, New Haven, both in Conn., and her colleagues wrote.
The authors outlined five action steps resulting from the NASEM workshop:
- Implement screening for OUD in all relevant health care settings.
- For patients with positive screening results, immediately prescribe effective medication for OUD and/or opioid withdrawal symptoms.
- Develop hospital-based protocols that facilitate OUD treatment initiation and linkage to community-based treatment upon discharge.
- Hospitals, medical schools, physician assistant schools, nursing schools, and residency programs should increase training to identify and treat OUD.
- Increase access to addiction care and funding to states to provide effective medications to treat OUD.
Opioid withdrawal and pain syndromes should be addressed with opioid agonist therapies to optimize infectious disease (ID) treatment and relieve pain, according to Dr. Springer and her colleagues. In addition, “Because ID specialists are likely to be consulted for anyone requiring long-term antibiotic therapy or patients with HIV and HCV infection, OUD screening should be a standard part of an ID consult assessment,” the authors wrote.
“All health care providers have a role in combating the OUD epidemic and its ID consequences. Those who treat infectious complications of OUD are well suited to screen for OUD and begin treatment with effective FDA-approved medications,” the authors concluded.
The workshop was held in March 2018 in Washington and videos and slide presentations from the meeting are available.
Dr. Springer and her colleagues reported grant funding from the National Institutes of Health, but no commercial conflicts.
SOURCE: Springer SA et al. Ann Intern Med. 2018 Jul 13. doi: 10.7326/M18-1203.
Widespread opioid use disorder (OUD) has spawned new epidemics of hepatitis C virus (HCV) and HIV infections as well as increased hospitalizations for bacteremia, endocarditis, skin and soft tissue infections, and osteomyelitis, according to a report arising from a National Academies of Science, Engineering and Medicine (NASEM) workshop titled Integrating Infectious Disease Considerations with Response to the Opioid Epidemic.
Optimal treatment of these infections is often impeded by untreated OUD, Sandra A. Springer, MD, and her colleagues wrote in an article published online in the Annals of Internal Medicine. Failing to address OUD can result in longer hospital stays; frequent readmissions because of a lack of adherence to antibiotic regimens; or reinfection, morbidity, and high costs. “Medical settings that manage such infections offer a potential means of engaging people in treatment of OUD; however, few providers and hospitals treating such infections have the needed resources and capabilities,” Dr. Springer, director, infectious disease outpatient clinic, Veterans Administration, Newington, and of Yale University, New Haven, both in Conn., and her colleagues wrote.
The authors outlined five action steps resulting from the NASEM workshop:
- Implement screening for OUD in all relevant health care settings.
- For patients with positive screening results, immediately prescribe effective medication for OUD and/or opioid withdrawal symptoms.
- Develop hospital-based protocols that facilitate OUD treatment initiation and linkage to community-based treatment upon discharge.
- Hospitals, medical schools, physician assistant schools, nursing schools, and residency programs should increase training to identify and treat OUD.
- Increase access to addiction care and funding to states to provide effective medications to treat OUD.
Opioid withdrawal and pain syndromes should be addressed with opioid agonist therapies to optimize infectious disease (ID) treatment and relieve pain, according to Dr. Springer and her colleagues. In addition, “Because ID specialists are likely to be consulted for anyone requiring long-term antibiotic therapy or patients with HIV and HCV infection, OUD screening should be a standard part of an ID consult assessment,” the authors wrote.
“All health care providers have a role in combating the OUD epidemic and its ID consequences. Those who treat infectious complications of OUD are well suited to screen for OUD and begin treatment with effective FDA-approved medications,” the authors concluded.
The workshop was held in March 2018 in Washington and videos and slide presentations from the meeting are available.
Dr. Springer and her colleagues reported grant funding from the National Institutes of Health, but no commercial conflicts.
SOURCE: Springer SA et al. Ann Intern Med. 2018 Jul 13. doi: 10.7326/M18-1203.
FROM ANNALS OF INTERNAL MEDICINE
Development of a Pharmacist-Led Emergency Department Antimicrobial Surveillance Program
On September 18, 2014, President Barack Obama signed an executive order that made addressing antibiotic-resistant bacteria a national security policy.1 This legislation resulted in the creation of a large multidepartment task force to combat the global and domestic problem of antimicrobial resistance. The order required hospitals and other inpatient health care delivery facilities, including the Department of Veterans Affairs (VA), to implement robust antimicrobial stewardship programs that adhere to best practices, such as those identified by the Centers for Disease Control and Prevention (CDC). More specifically, the VA was mandated to take steps to encourage other areas of health care, such as ambulatory surgery centers and outpatient clinics, to adopt antimicrobial stewardship programs.1 This order also reinforced the importance for VA facilities to continue to develop, improve, and sustain efforts in antimicrobial stewardship.
Prior to the order, in 2012 the Richard L. Roudebush VA Medical Center (RRVAMC) in Indianapolis, Indiana, implemented an inpatient antimicrobial stewardship program that included thrice-weekly meetings to review inpatient records and make stewardship recommendations with an infectious diseases physician champion and clinical pharmacists. These efforts led to the improved use of antimicrobial agents on the inpatient side of the medical center. During the first 4 years of implementation, the program helped to decrease the defined daily doses of broad-spectrum antibiotics per 1,000 patient days nearly 36%, from 532 in 2012 to 343 in 2015, as well as decrease the days of therapy of fluoroquinolones per 1,000 patient days 28.75%, from 80 in 2012 to 57 in 2015. Additionally, the program showed a significant decrease in the standardized antimicrobial administration ratio, a benchmark measure developed by the CDC to reflect a facility’s actual antimicrobial use to the expected use of a similar facility based on bed size, number of intensive care unit beds, location type, and medical school affiliation.2
While the RRVAMC antimicrobial stewardship team has been able to intervene on most of the inpatients admitted to the medical center, the outpatient arena has had few antimicrobial stewardship interventions. Recognizing a need to establish and expand pharmacy services and for improvement of outpatient antimicrobial stewardship, RRVAMC leadership decided to establish a pharmacist-led outpatient antimicrobial surveillance program, starting specifically within the emergency department (ED).
Clinical pharmacists in the ED setting are uniquely positioned to improve patient care and encourage the judicious use of antimicrobials for empiric treatment of urinary tract infections (UTIs). The CDC’s Core Elements of Outpatient Antibiotic Stewardship recommends pharmacist availability in the ED setting, and previous literature has demonstrated pharmacist utility in ED postdischarge culture monitoring and surveillance.3-5
This article will highlight one such program review at the RRVAMC and demonstrate the need for pharmacist-led antimicrobial stewardship and monitoring in the ED. The purpose of this study was to test the hypothesis that pharmacist intervention would be necessary to prospectively check for “bug-drug mismatch” and assure proper follow-up of urine cultures at this institution. The project was deemed to be quality improvement and thereby granted exemption by the RRVAMC Institutional Review Board.
Methods
This project took place at the RRVAMC, a 229-bed tertiary academic medical center that serves > 60,000 patients annually. The RRVAMC ED has 20 beds and received about 29,000 visits in 2014. Patients were eligible for initial evaluation if they had a urine culture collected in the ED within the 91-day period from September 1, 2015 to November 30, 2015. Patients were included for data analysis if it was documented that they were treated for actual or clinically suspected, based on signs and symptoms, uncomplicated UTI, complicated UTI, or UTI with pyelonephritis. Patients did not need to have a positive urine culture for inclusion, as infections could still be present despite negative culture results.6 Patients with positive cultures who were not clinically symptomatic of a UTI and were not treated as such by the ED provider (ie, asymptomatic bacteriuria) were excluded from the study.
Data collection took place via daily chart review of patient records in both the Computerized Patient Record System and Decentralized Hospital Computer Program medical applications as urine cultures were performed. Data were gathered and assessed by a postgraduate year-2 internal medicine pharmacy resident on rotation in the ED who reviewed cultures daily and made interventions based on the results as needed. The pharmacy resident was physically present within the ED during the first 30 days of the project. The pharmacy resident was not within the direct practice area during the final 61 days of the project but was in a different area of the hospital and available for consultation.
Primary data collected included urine culture results and susceptibilities, empiric antimicrobial choices, and admission status. Other data collected included duration of treatment and secondary antibiotics chosen, each of which specifically evaluated those patients who were not admitted to the hospital and were thus treated as outpatients. Additional data generated from this study were used to identify empiric antibiotics utilized for the treatment of UTIs and assess for appropriate selection and duration of therapy within this institution.
Results
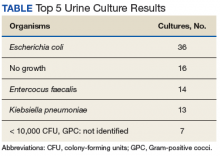
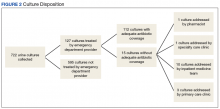
During the study period, 722 urine cultures were collected in the ED and were included for initial evaluation. Of these, 127 were treated by the ED provider pursuant to one of the indications specified and were included in the data analysis. Treatment with an antimicrobial agent provided adequate coverage for the identified pathogen in 112 patients, yielding a match rate of 88%. As all included cultures were collected in suspicion of an infection, those cultures yielding no growth were considered to have been adequately covered.
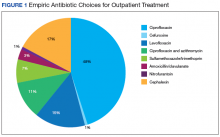
Nearly half (45%) of treatment plans included a fluoroquinolone. Of those treated on an outpatient basis, fluoroquinolones were even more frequently used, comprising 50 of 82 (61%) courses. Ciprofloxacin was the most frequently used treatment, used in 39 of the 82 outpatient regimens (48%). Cephalexin was the second most common and was used in 14 outpatient regimens (17%), followed by levofloxacin (15%) (Figure 1).
Mismatched cultures, or those where the prescribed antibiotic did not provide adequate coverage of the identified pathogens based on susceptibilities, occurred at a rate of 12%. Follow-up on these cultures was determined largely by the patient’s admission status. The majority of mismatched cultures were addressed by the inpatient team (10/15) upon admission.
Discussion
Empiric antibiotic selection for the treatment of UTIs continues to be the cornerstone of antibiotic management for the treatment of such a disease state.7 The noted drug-bug match rate of 88% in this study demonstrates effective initial empiric coverage and ensures a vast majority of veterans receive adequate coverage for identified pathogens. Additionally, this rate shows that the current system seems to be functioning appropriately and refutes the author’s preconceived ideas that the mismatch rate was higher at RRVAMC. However, these findings also demonstrate a predominant use of fluoroquinolones for empiric treatment in a majority of patients who could be better served with narrower spectrum agents. Only 2 of the outpatient regimens were for the treatment of pyelonephritis, the only indication in which a fluoroquinolone would be the standard of care per guideline recommendations.7
These findings were consistent with a similar study in which 83% of ED collected urine cultures ultimately grew bacteria susceptibleto empiric treatment.8 This number was similar to the current study despite the latter study consisting of predominantly female patients (93%) and excluding patients with a history of benign prostatic hypertrophy, catheter use, or history of genitourinary cancer, which are frequently found within the VA population. Thus, despite having a differing patient population at the current study’s facility with characteristics that would classify most to be treated as a complicated UTI, empiric coverage rates remained similar. The lower than anticipated intervention rate by the pharmacist on rotation in the ED can be directly attributed to this high empiric match rate, which could in turn be attributed to the extensive use of broad-spectrum antibiotics for treatment.
Empiric antimicrobial selection is based largely on local resistance patterns.7 Of particular importance is the resistance patterns of E coli, as it is the primary isolate responsible for UTIs worldwide. Thus, it is not unexpected that the most frequently isolated pathogen in the current study also was E coli. While clinical practice guidelines state that hospital-wide antibiograms often are skewed by cultures collected from inpatients or those with complicated infection, the current study found hospital-wide E coli resistance patterns, specifically those related to fluoroquinolone use, to be similar to those collected in the ED alone (78.5% hospital-wide susceptibility vs. 75% ED susceptibility). This was expected, as similar studies comparing E coli resistance patterns from ED-collected urine cultures to those institution-wide also have found similar rates of resistance.8,9 These findings are of particular importance as E coli resistance is noted to be increasing, varies with geographic area, and local resistance patterns are rarely known.7 Thus, these findings may aid ED providers in their empiric antimicrobial selections.
Ciprofloxacin was the most frequently used medication for the treatment of UTIs. While overall empiric selections were found to have favorable resistance patterns, it is difficult to interpret the appropriateness of ciprofloxacin’s use in the present study. First, there is a distinct lack of US-based clinical practice guidelines for the treatment of complicated UTIs. As the majority of this study population was male, it is difficult to directly extrapolate from the current Infectious Diseases Society of America treatment guidelines for uncomplicated cystitis and apply to the study population. Although recommended for the treatment of pyelonephritis, it is unclear whether ciprofloxacin should be utilized as a first-line empiric option for the treatment of UTIs in males.
Despite the lack of disease-specific recommendations for ciprofloxacin, recommendations exist regarding its use when local resistance patterns are known.7 It is currently recommended that these agents not be used when resistance rates of E coli exceed 20% for trimethoprim-sulfamethoxazole or 10% for fluoroquinolones. As this study demonstrated a nearly 25% resistance rate for E coli to fluoroquinolones in both the ED and institution-wide sample populations, it could potentially be ascertained that ciprofloxacin is an inappropriate choice for the empiric treatment of UTIs in this patient population. However, as noted, it is unknown whether this recommendation would still be applicable when applied to the treatment of complicated cystitis and greater male population, as overall rates of susceptible cultures to all organisms was similar to other published studies.8,9
While there is scant specific guidance related to the treatment of complicated UTIs, there is emerging guidance on the use of fluoroquinolones, both in general and specifically related to the treatment of UTIs. In July 2016, the FDA issued a drug safety communication regarding the use of and warnings for fluoroquinolones, which explicitly stated that “health care professionals should not prescribe systemic fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs because the risks outweigh the benefits in these patients.”10
This guidance has the potential to impact fluoroquinolone prescribing significantly at RRVAMC. Given the large number of fluoroquinolones prescribed for UTIs, the downstream effects that this shift in prescribing would have is unknown. As most nonfluoroquinolones used for UTI typically are narrower in antimicrobial spectrum (eg, trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, etc) the possibility exists that the match rate for empiric therapy may decrease. Thus, a larger need for closer follow-up to assure adequate coverage may arise, posing a more expanded role for an ED-based pharmacist than was demonstrated in the current study.
This new guidance also may place providers in an area of larger uncertainty with regards to treating both complicated and uncomplicated cystitis. Given the enhanced warnings on fluoroquinolone use, it is unknown whether prescribers would gravitate to utilizing similar options as their peers as alternatives to fluoroquinolones. Similarly, duration of therapy with nonfluoroquinolone agents is unclear as well; as the present study demonstrated a large range in treatment duration of outpatients (3-14 days). While the average observed duration of 8.3 days is intuitively fitting, as the majority of cases were in males, no published guideline exists that affirms the appropriateness of this finding. Such uncertainty and potential inconsistency between providers affords a large opportunity for developing a standardized treatment pathway for the treatment of UTIs to ensure both effective and guideline concordant treatment for patients, specifically with regards to antimicrobial selection and duration of treatment.
It is noteworthy to mention that all follow-ups on positive cultures inadequately covered by empiric therapy took place on the day organism identification and susceptibility data were released. This finding was somewhat surprising, as it was originally theorized that most ED-collected urine cultures were not monitored to completion by a pharmacist and that would be necessary in order to ensure proper follow-up of culture results. What is not clear is whether there is a robust process for the follow-up of urine cultures in the ED. Most of the bug-drug mismatches coincidentally were admitted to the inpatient teams where there were appropriate personnel to follow up and adjust the antibiotic selection. If there was a bug-drug mismatch, and the patient was not admitted, it is unclear whether there is a consistent process for follow-up.
Given the limited number of mismatched cultures that required change in therapy, it is unknown if this role would expand if more narrow-spectrum agents were utilized, theoretically leading to a higher mismatch rate and necessitating closer follow-up. Furthermore, given the common practice of mailed prescriptions at the VA, it is all the more imperative that the cultures be acted upon on the day they were identified, as the mailing and processing time of prescriptions may limit the clinical utility in switching from a more broad-spectrum agent, to one more targeted for an identified organism. While a patient traveling back to the medical center for expedited prescription pickup at the pharmacy would alleviate this problem, many patients at the facility travel great distances or may not have readily available travel means to return to the medical center.
Future Directions
While minimal follow-up was required after a patient had left the ED, this study demonstrated a fundamental need for further refinements in antimicrobial stewardship activities within the ED. Duration of therapy, empiric selection, and proper dosing are key areas where the ED-based pharmacy resident was able to intervene during the time physically stationed in the ED. The data collected from this study demonstrated this and was ultimately combined with other ED-based interventions and utilized as supporting evidence in the pharmacy service business plan, outlining the necessity of a full-time pharmacy presence in the ED. The business plan submission, along with other ongoing RRVAMC initiatives, ultimately led to the approval for clinical pharmacy specialists to expand practice into the ED. These positions will continue to advance pharmacy practice within the ED, while affording opportunities for pharmacists to practice at the top of their licensure, provide individualized provider education, and deliver real-time antimicrobial stewardship interventions. Furthermore, as the majority of the study period was monitored outside of the ED, the project may provide a model for other VA institutions without full-time ED pharmacists to implement as a means to improve antimicrobial stewardship and further build an evidence base for expanding their pharmacy services to the ED.
Given the large number of fluoroquinolones utilized in the ED, this study has raised the question of what prescribing patterns look like with regards to outpatient UTI treatment within the realm of primary care at RRVAMC. Despite the great strides made with regards to antimicrobial stewardship at this facility on the inpatient side, no formal antimicrobial stewardship program exists for review in the outpatient setting, where literature suggests the majority of antibiotics are prescribed.3,11 While more robust protocols are in place for follow-up of culture data in the primary care realm at this facility, the prescribing patterns are relatively unknown.
A recent study completed at a similar VA facility found that 60% of antibiotics prescribed for cystitis, pharyngitis, or sinusitis on an outpatient basis were guideline-discordant, and CDC guidance has further recommended specific focus should be undertaken with regards to outpatient stewardship practices in the treatment of genitourinary infections.3,12 These findings highlight the need for outpatient antimicrobial stewardship and presents a compelling reason to further investigate outpatient prescribing within primary care at RRVAMC.
Strengths and Limitations
Strengths of the current study include the ability to monitor urine cultures in real time and to provide timely interventions in the event of a rare bug-drug mismatch. The evaluation of cultures in this study shows that the majority of cases had a drug selected with adequate coverage. The study did assure ED providers that, even though guidelines may suggest otherwise, urine cultures drawn in the ED at RRVAMC followed similar resistance patterns seen for the facility as a whole. Moreover, it is valuable as it captures data that are directly applicable to the VA patient population, in which there is little published data with regards to UTI treatment and no formal VA guidance.
A primary limitation of this study is the lack of differentiation between cultures collected from patients with or without indwelling catheters. However, only including patients who presented with signs and/or symptoms of a UTI limits the number of cultures that could potentially be deemed as colonization, thus minimizing the potential for nonpathogenic organisms to confound the results. This study also did not differentiate the setting from which the patient presented (eg, community, extended care facility, etc) that could have potentially provided guidance on resistance patterns for community-acquired UTIs and whether this may have differed from hospital-acquired or facility-acquired UTIs. Another limitation was the relatively short time frame for data collection. A data collection period greater than 91 days would allow for a larger sample size, thus making the data more robust and potentially allowing for the identification of other trends not seen in the current study. A longer data collection period also would have afforded the opportunity to track more robust clinical outcomes throughout the study, identifying whether treatment failure may have been linked to the use of certain classes or spectrums of activity of antibiotics.
Conclusion
Despite the E coli resistance rate to ciprofloxacin (> 20%), the empiric treatments chosen were > 85% effective, needing minimal follow-up once a patient left the ED. Nonetheless, a change in prescribing patterns based on recent national recommendations may provide expanded opportunities in antimicrobial stewardship for ED-based pharmacists. Further research is needed in antimicrobial stewardship within this facility’s outpatient primary care realm, potentially uncovering other opportunities for pharmacist intervention to assure guideline concordant care for the treatment of UTIs as well as other infections treated in primary care patients.
1. Obama B. Executive order–combating antibiotic-resistant bacteria. https://www.whitehouse.gov/the -press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Published September 18, 2014. Accessed June 7, 2018.
2. Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017;38(6):721-723.
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hick LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
4. Wymore ES, Casanova TJ, Broekenmeier RL, Martin JK Jr. Clinical pharmacist’s daily role in the emergency department of a community hospital. Am J Health-Syst Pharm. 2008;65(5):395-396, 398-399.
5. Frandzel S. ED pharmacists’ value on display at ASHP Midyear. http://www.pharmacypracticenews.com/ViewArticle.aspx?ses=ogst&d_id=53&a_id=22524. Published February 14, 2013. Accessed June 15, 2018.
6. Heytens S, DeSutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017;23(9)647-652.
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120.
8. Lingenfelter E, Drapkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient urinary tract infection. Am J Emerg Med. 2016;34(8):1600-1603.
9. Zatorski C, Jordan JA, Cosgrove SE, Zocchi M, May L. Comparison of antibiotic susceptibility of Escherichia coli in urinary isolates from an emergency department with other institutional susceptibility data. Am J Health-Syst Pharm. 2015;72(24):2176-2180.
10. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Updated March 8, 2018. Accessed June 13, 2018.
11. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
12. Meyer HE, Lund BC, Heintz BH, Alexander B, Egge JA, Livorsi DJ. Identifying opportunities to improve guideline-concordant antibiotic prescribing in veterans with acute respiratory infections or cystitis. Infect Control Hosp Epidemiol. 2017;38(6):724-728.
On September 18, 2014, President Barack Obama signed an executive order that made addressing antibiotic-resistant bacteria a national security policy.1 This legislation resulted in the creation of a large multidepartment task force to combat the global and domestic problem of antimicrobial resistance. The order required hospitals and other inpatient health care delivery facilities, including the Department of Veterans Affairs (VA), to implement robust antimicrobial stewardship programs that adhere to best practices, such as those identified by the Centers for Disease Control and Prevention (CDC). More specifically, the VA was mandated to take steps to encourage other areas of health care, such as ambulatory surgery centers and outpatient clinics, to adopt antimicrobial stewardship programs.1 This order also reinforced the importance for VA facilities to continue to develop, improve, and sustain efforts in antimicrobial stewardship.
Prior to the order, in 2012 the Richard L. Roudebush VA Medical Center (RRVAMC) in Indianapolis, Indiana, implemented an inpatient antimicrobial stewardship program that included thrice-weekly meetings to review inpatient records and make stewardship recommendations with an infectious diseases physician champion and clinical pharmacists. These efforts led to the improved use of antimicrobial agents on the inpatient side of the medical center. During the first 4 years of implementation, the program helped to decrease the defined daily doses of broad-spectrum antibiotics per 1,000 patient days nearly 36%, from 532 in 2012 to 343 in 2015, as well as decrease the days of therapy of fluoroquinolones per 1,000 patient days 28.75%, from 80 in 2012 to 57 in 2015. Additionally, the program showed a significant decrease in the standardized antimicrobial administration ratio, a benchmark measure developed by the CDC to reflect a facility’s actual antimicrobial use to the expected use of a similar facility based on bed size, number of intensive care unit beds, location type, and medical school affiliation.2
While the RRVAMC antimicrobial stewardship team has been able to intervene on most of the inpatients admitted to the medical center, the outpatient arena has had few antimicrobial stewardship interventions. Recognizing a need to establish and expand pharmacy services and for improvement of outpatient antimicrobial stewardship, RRVAMC leadership decided to establish a pharmacist-led outpatient antimicrobial surveillance program, starting specifically within the emergency department (ED).
Clinical pharmacists in the ED setting are uniquely positioned to improve patient care and encourage the judicious use of antimicrobials for empiric treatment of urinary tract infections (UTIs). The CDC’s Core Elements of Outpatient Antibiotic Stewardship recommends pharmacist availability in the ED setting, and previous literature has demonstrated pharmacist utility in ED postdischarge culture monitoring and surveillance.3-5
This article will highlight one such program review at the RRVAMC and demonstrate the need for pharmacist-led antimicrobial stewardship and monitoring in the ED. The purpose of this study was to test the hypothesis that pharmacist intervention would be necessary to prospectively check for “bug-drug mismatch” and assure proper follow-up of urine cultures at this institution. The project was deemed to be quality improvement and thereby granted exemption by the RRVAMC Institutional Review Board.
Methods
This project took place at the RRVAMC, a 229-bed tertiary academic medical center that serves > 60,000 patients annually. The RRVAMC ED has 20 beds and received about 29,000 visits in 2014. Patients were eligible for initial evaluation if they had a urine culture collected in the ED within the 91-day period from September 1, 2015 to November 30, 2015. Patients were included for data analysis if it was documented that they were treated for actual or clinically suspected, based on signs and symptoms, uncomplicated UTI, complicated UTI, or UTI with pyelonephritis. Patients did not need to have a positive urine culture for inclusion, as infections could still be present despite negative culture results.6 Patients with positive cultures who were not clinically symptomatic of a UTI and were not treated as such by the ED provider (ie, asymptomatic bacteriuria) were excluded from the study.
Data collection took place via daily chart review of patient records in both the Computerized Patient Record System and Decentralized Hospital Computer Program medical applications as urine cultures were performed. Data were gathered and assessed by a postgraduate year-2 internal medicine pharmacy resident on rotation in the ED who reviewed cultures daily and made interventions based on the results as needed. The pharmacy resident was physically present within the ED during the first 30 days of the project. The pharmacy resident was not within the direct practice area during the final 61 days of the project but was in a different area of the hospital and available for consultation.
Primary data collected included urine culture results and susceptibilities, empiric antimicrobial choices, and admission status. Other data collected included duration of treatment and secondary antibiotics chosen, each of which specifically evaluated those patients who were not admitted to the hospital and were thus treated as outpatients. Additional data generated from this study were used to identify empiric antibiotics utilized for the treatment of UTIs and assess for appropriate selection and duration of therapy within this institution.
Results
During the study period, 722 urine cultures were collected in the ED and were included for initial evaluation. Of these, 127 were treated by the ED provider pursuant to one of the indications specified and were included in the data analysis. Treatment with an antimicrobial agent provided adequate coverage for the identified pathogen in 112 patients, yielding a match rate of 88%. As all included cultures were collected in suspicion of an infection, those cultures yielding no growth were considered to have been adequately covered.
Nearly half (45%) of treatment plans included a fluoroquinolone. Of those treated on an outpatient basis, fluoroquinolones were even more frequently used, comprising 50 of 82 (61%) courses. Ciprofloxacin was the most frequently used treatment, used in 39 of the 82 outpatient regimens (48%). Cephalexin was the second most common and was used in 14 outpatient regimens (17%), followed by levofloxacin (15%) (Figure 1).
Mismatched cultures, or those where the prescribed antibiotic did not provide adequate coverage of the identified pathogens based on susceptibilities, occurred at a rate of 12%. Follow-up on these cultures was determined largely by the patient’s admission status. The majority of mismatched cultures were addressed by the inpatient team (10/15) upon admission.
Discussion
Empiric antibiotic selection for the treatment of UTIs continues to be the cornerstone of antibiotic management for the treatment of such a disease state.7 The noted drug-bug match rate of 88% in this study demonstrates effective initial empiric coverage and ensures a vast majority of veterans receive adequate coverage for identified pathogens. Additionally, this rate shows that the current system seems to be functioning appropriately and refutes the author’s preconceived ideas that the mismatch rate was higher at RRVAMC. However, these findings also demonstrate a predominant use of fluoroquinolones for empiric treatment in a majority of patients who could be better served with narrower spectrum agents. Only 2 of the outpatient regimens were for the treatment of pyelonephritis, the only indication in which a fluoroquinolone would be the standard of care per guideline recommendations.7
These findings were consistent with a similar study in which 83% of ED collected urine cultures ultimately grew bacteria susceptibleto empiric treatment.8 This number was similar to the current study despite the latter study consisting of predominantly female patients (93%) and excluding patients with a history of benign prostatic hypertrophy, catheter use, or history of genitourinary cancer, which are frequently found within the VA population. Thus, despite having a differing patient population at the current study’s facility with characteristics that would classify most to be treated as a complicated UTI, empiric coverage rates remained similar. The lower than anticipated intervention rate by the pharmacist on rotation in the ED can be directly attributed to this high empiric match rate, which could in turn be attributed to the extensive use of broad-spectrum antibiotics for treatment.
Empiric antimicrobial selection is based largely on local resistance patterns.7 Of particular importance is the resistance patterns of E coli, as it is the primary isolate responsible for UTIs worldwide. Thus, it is not unexpected that the most frequently isolated pathogen in the current study also was E coli. While clinical practice guidelines state that hospital-wide antibiograms often are skewed by cultures collected from inpatients or those with complicated infection, the current study found hospital-wide E coli resistance patterns, specifically those related to fluoroquinolone use, to be similar to those collected in the ED alone (78.5% hospital-wide susceptibility vs. 75% ED susceptibility). This was expected, as similar studies comparing E coli resistance patterns from ED-collected urine cultures to those institution-wide also have found similar rates of resistance.8,9 These findings are of particular importance as E coli resistance is noted to be increasing, varies with geographic area, and local resistance patterns are rarely known.7 Thus, these findings may aid ED providers in their empiric antimicrobial selections.
Ciprofloxacin was the most frequently used medication for the treatment of UTIs. While overall empiric selections were found to have favorable resistance patterns, it is difficult to interpret the appropriateness of ciprofloxacin’s use in the present study. First, there is a distinct lack of US-based clinical practice guidelines for the treatment of complicated UTIs. As the majority of this study population was male, it is difficult to directly extrapolate from the current Infectious Diseases Society of America treatment guidelines for uncomplicated cystitis and apply to the study population. Although recommended for the treatment of pyelonephritis, it is unclear whether ciprofloxacin should be utilized as a first-line empiric option for the treatment of UTIs in males.
Despite the lack of disease-specific recommendations for ciprofloxacin, recommendations exist regarding its use when local resistance patterns are known.7 It is currently recommended that these agents not be used when resistance rates of E coli exceed 20% for trimethoprim-sulfamethoxazole or 10% for fluoroquinolones. As this study demonstrated a nearly 25% resistance rate for E coli to fluoroquinolones in both the ED and institution-wide sample populations, it could potentially be ascertained that ciprofloxacin is an inappropriate choice for the empiric treatment of UTIs in this patient population. However, as noted, it is unknown whether this recommendation would still be applicable when applied to the treatment of complicated cystitis and greater male population, as overall rates of susceptible cultures to all organisms was similar to other published studies.8,9
While there is scant specific guidance related to the treatment of complicated UTIs, there is emerging guidance on the use of fluoroquinolones, both in general and specifically related to the treatment of UTIs. In July 2016, the FDA issued a drug safety communication regarding the use of and warnings for fluoroquinolones, which explicitly stated that “health care professionals should not prescribe systemic fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs because the risks outweigh the benefits in these patients.”10
This guidance has the potential to impact fluoroquinolone prescribing significantly at RRVAMC. Given the large number of fluoroquinolones prescribed for UTIs, the downstream effects that this shift in prescribing would have is unknown. As most nonfluoroquinolones used for UTI typically are narrower in antimicrobial spectrum (eg, trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, etc) the possibility exists that the match rate for empiric therapy may decrease. Thus, a larger need for closer follow-up to assure adequate coverage may arise, posing a more expanded role for an ED-based pharmacist than was demonstrated in the current study.
This new guidance also may place providers in an area of larger uncertainty with regards to treating both complicated and uncomplicated cystitis. Given the enhanced warnings on fluoroquinolone use, it is unknown whether prescribers would gravitate to utilizing similar options as their peers as alternatives to fluoroquinolones. Similarly, duration of therapy with nonfluoroquinolone agents is unclear as well; as the present study demonstrated a large range in treatment duration of outpatients (3-14 days). While the average observed duration of 8.3 days is intuitively fitting, as the majority of cases were in males, no published guideline exists that affirms the appropriateness of this finding. Such uncertainty and potential inconsistency between providers affords a large opportunity for developing a standardized treatment pathway for the treatment of UTIs to ensure both effective and guideline concordant treatment for patients, specifically with regards to antimicrobial selection and duration of treatment.
It is noteworthy to mention that all follow-ups on positive cultures inadequately covered by empiric therapy took place on the day organism identification and susceptibility data were released. This finding was somewhat surprising, as it was originally theorized that most ED-collected urine cultures were not monitored to completion by a pharmacist and that would be necessary in order to ensure proper follow-up of culture results. What is not clear is whether there is a robust process for the follow-up of urine cultures in the ED. Most of the bug-drug mismatches coincidentally were admitted to the inpatient teams where there were appropriate personnel to follow up and adjust the antibiotic selection. If there was a bug-drug mismatch, and the patient was not admitted, it is unclear whether there is a consistent process for follow-up.
Given the limited number of mismatched cultures that required change in therapy, it is unknown if this role would expand if more narrow-spectrum agents were utilized, theoretically leading to a higher mismatch rate and necessitating closer follow-up. Furthermore, given the common practice of mailed prescriptions at the VA, it is all the more imperative that the cultures be acted upon on the day they were identified, as the mailing and processing time of prescriptions may limit the clinical utility in switching from a more broad-spectrum agent, to one more targeted for an identified organism. While a patient traveling back to the medical center for expedited prescription pickup at the pharmacy would alleviate this problem, many patients at the facility travel great distances or may not have readily available travel means to return to the medical center.
Future Directions
While minimal follow-up was required after a patient had left the ED, this study demonstrated a fundamental need for further refinements in antimicrobial stewardship activities within the ED. Duration of therapy, empiric selection, and proper dosing are key areas where the ED-based pharmacy resident was able to intervene during the time physically stationed in the ED. The data collected from this study demonstrated this and was ultimately combined with other ED-based interventions and utilized as supporting evidence in the pharmacy service business plan, outlining the necessity of a full-time pharmacy presence in the ED. The business plan submission, along with other ongoing RRVAMC initiatives, ultimately led to the approval for clinical pharmacy specialists to expand practice into the ED. These positions will continue to advance pharmacy practice within the ED, while affording opportunities for pharmacists to practice at the top of their licensure, provide individualized provider education, and deliver real-time antimicrobial stewardship interventions. Furthermore, as the majority of the study period was monitored outside of the ED, the project may provide a model for other VA institutions without full-time ED pharmacists to implement as a means to improve antimicrobial stewardship and further build an evidence base for expanding their pharmacy services to the ED.
Given the large number of fluoroquinolones utilized in the ED, this study has raised the question of what prescribing patterns look like with regards to outpatient UTI treatment within the realm of primary care at RRVAMC. Despite the great strides made with regards to antimicrobial stewardship at this facility on the inpatient side, no formal antimicrobial stewardship program exists for review in the outpatient setting, where literature suggests the majority of antibiotics are prescribed.3,11 While more robust protocols are in place for follow-up of culture data in the primary care realm at this facility, the prescribing patterns are relatively unknown.
A recent study completed at a similar VA facility found that 60% of antibiotics prescribed for cystitis, pharyngitis, or sinusitis on an outpatient basis were guideline-discordant, and CDC guidance has further recommended specific focus should be undertaken with regards to outpatient stewardship practices in the treatment of genitourinary infections.3,12 These findings highlight the need for outpatient antimicrobial stewardship and presents a compelling reason to further investigate outpatient prescribing within primary care at RRVAMC.
Strengths and Limitations
Strengths of the current study include the ability to monitor urine cultures in real time and to provide timely interventions in the event of a rare bug-drug mismatch. The evaluation of cultures in this study shows that the majority of cases had a drug selected with adequate coverage. The study did assure ED providers that, even though guidelines may suggest otherwise, urine cultures drawn in the ED at RRVAMC followed similar resistance patterns seen for the facility as a whole. Moreover, it is valuable as it captures data that are directly applicable to the VA patient population, in which there is little published data with regards to UTI treatment and no formal VA guidance.
A primary limitation of this study is the lack of differentiation between cultures collected from patients with or without indwelling catheters. However, only including patients who presented with signs and/or symptoms of a UTI limits the number of cultures that could potentially be deemed as colonization, thus minimizing the potential for nonpathogenic organisms to confound the results. This study also did not differentiate the setting from which the patient presented (eg, community, extended care facility, etc) that could have potentially provided guidance on resistance patterns for community-acquired UTIs and whether this may have differed from hospital-acquired or facility-acquired UTIs. Another limitation was the relatively short time frame for data collection. A data collection period greater than 91 days would allow for a larger sample size, thus making the data more robust and potentially allowing for the identification of other trends not seen in the current study. A longer data collection period also would have afforded the opportunity to track more robust clinical outcomes throughout the study, identifying whether treatment failure may have been linked to the use of certain classes or spectrums of activity of antibiotics.
Conclusion
Despite the E coli resistance rate to ciprofloxacin (> 20%), the empiric treatments chosen were > 85% effective, needing minimal follow-up once a patient left the ED. Nonetheless, a change in prescribing patterns based on recent national recommendations may provide expanded opportunities in antimicrobial stewardship for ED-based pharmacists. Further research is needed in antimicrobial stewardship within this facility’s outpatient primary care realm, potentially uncovering other opportunities for pharmacist intervention to assure guideline concordant care for the treatment of UTIs as well as other infections treated in primary care patients.
On September 18, 2014, President Barack Obama signed an executive order that made addressing antibiotic-resistant bacteria a national security policy.1 This legislation resulted in the creation of a large multidepartment task force to combat the global and domestic problem of antimicrobial resistance. The order required hospitals and other inpatient health care delivery facilities, including the Department of Veterans Affairs (VA), to implement robust antimicrobial stewardship programs that adhere to best practices, such as those identified by the Centers for Disease Control and Prevention (CDC). More specifically, the VA was mandated to take steps to encourage other areas of health care, such as ambulatory surgery centers and outpatient clinics, to adopt antimicrobial stewardship programs.1 This order also reinforced the importance for VA facilities to continue to develop, improve, and sustain efforts in antimicrobial stewardship.
Prior to the order, in 2012 the Richard L. Roudebush VA Medical Center (RRVAMC) in Indianapolis, Indiana, implemented an inpatient antimicrobial stewardship program that included thrice-weekly meetings to review inpatient records and make stewardship recommendations with an infectious diseases physician champion and clinical pharmacists. These efforts led to the improved use of antimicrobial agents on the inpatient side of the medical center. During the first 4 years of implementation, the program helped to decrease the defined daily doses of broad-spectrum antibiotics per 1,000 patient days nearly 36%, from 532 in 2012 to 343 in 2015, as well as decrease the days of therapy of fluoroquinolones per 1,000 patient days 28.75%, from 80 in 2012 to 57 in 2015. Additionally, the program showed a significant decrease in the standardized antimicrobial administration ratio, a benchmark measure developed by the CDC to reflect a facility’s actual antimicrobial use to the expected use of a similar facility based on bed size, number of intensive care unit beds, location type, and medical school affiliation.2
While the RRVAMC antimicrobial stewardship team has been able to intervene on most of the inpatients admitted to the medical center, the outpatient arena has had few antimicrobial stewardship interventions. Recognizing a need to establish and expand pharmacy services and for improvement of outpatient antimicrobial stewardship, RRVAMC leadership decided to establish a pharmacist-led outpatient antimicrobial surveillance program, starting specifically within the emergency department (ED).
Clinical pharmacists in the ED setting are uniquely positioned to improve patient care and encourage the judicious use of antimicrobials for empiric treatment of urinary tract infections (UTIs). The CDC’s Core Elements of Outpatient Antibiotic Stewardship recommends pharmacist availability in the ED setting, and previous literature has demonstrated pharmacist utility in ED postdischarge culture monitoring and surveillance.3-5
This article will highlight one such program review at the RRVAMC and demonstrate the need for pharmacist-led antimicrobial stewardship and monitoring in the ED. The purpose of this study was to test the hypothesis that pharmacist intervention would be necessary to prospectively check for “bug-drug mismatch” and assure proper follow-up of urine cultures at this institution. The project was deemed to be quality improvement and thereby granted exemption by the RRVAMC Institutional Review Board.
Methods
This project took place at the RRVAMC, a 229-bed tertiary academic medical center that serves > 60,000 patients annually. The RRVAMC ED has 20 beds and received about 29,000 visits in 2014. Patients were eligible for initial evaluation if they had a urine culture collected in the ED within the 91-day period from September 1, 2015 to November 30, 2015. Patients were included for data analysis if it was documented that they were treated for actual or clinically suspected, based on signs and symptoms, uncomplicated UTI, complicated UTI, or UTI with pyelonephritis. Patients did not need to have a positive urine culture for inclusion, as infections could still be present despite negative culture results.6 Patients with positive cultures who were not clinically symptomatic of a UTI and were not treated as such by the ED provider (ie, asymptomatic bacteriuria) were excluded from the study.
Data collection took place via daily chart review of patient records in both the Computerized Patient Record System and Decentralized Hospital Computer Program medical applications as urine cultures were performed. Data were gathered and assessed by a postgraduate year-2 internal medicine pharmacy resident on rotation in the ED who reviewed cultures daily and made interventions based on the results as needed. The pharmacy resident was physically present within the ED during the first 30 days of the project. The pharmacy resident was not within the direct practice area during the final 61 days of the project but was in a different area of the hospital and available for consultation.
Primary data collected included urine culture results and susceptibilities, empiric antimicrobial choices, and admission status. Other data collected included duration of treatment and secondary antibiotics chosen, each of which specifically evaluated those patients who were not admitted to the hospital and were thus treated as outpatients. Additional data generated from this study were used to identify empiric antibiotics utilized for the treatment of UTIs and assess for appropriate selection and duration of therapy within this institution.
Results
During the study period, 722 urine cultures were collected in the ED and were included for initial evaluation. Of these, 127 were treated by the ED provider pursuant to one of the indications specified and were included in the data analysis. Treatment with an antimicrobial agent provided adequate coverage for the identified pathogen in 112 patients, yielding a match rate of 88%. As all included cultures were collected in suspicion of an infection, those cultures yielding no growth were considered to have been adequately covered.
Nearly half (45%) of treatment plans included a fluoroquinolone. Of those treated on an outpatient basis, fluoroquinolones were even more frequently used, comprising 50 of 82 (61%) courses. Ciprofloxacin was the most frequently used treatment, used in 39 of the 82 outpatient regimens (48%). Cephalexin was the second most common and was used in 14 outpatient regimens (17%), followed by levofloxacin (15%) (Figure 1).
Mismatched cultures, or those where the prescribed antibiotic did not provide adequate coverage of the identified pathogens based on susceptibilities, occurred at a rate of 12%. Follow-up on these cultures was determined largely by the patient’s admission status. The majority of mismatched cultures were addressed by the inpatient team (10/15) upon admission.
Discussion
Empiric antibiotic selection for the treatment of UTIs continues to be the cornerstone of antibiotic management for the treatment of such a disease state.7 The noted drug-bug match rate of 88% in this study demonstrates effective initial empiric coverage and ensures a vast majority of veterans receive adequate coverage for identified pathogens. Additionally, this rate shows that the current system seems to be functioning appropriately and refutes the author’s preconceived ideas that the mismatch rate was higher at RRVAMC. However, these findings also demonstrate a predominant use of fluoroquinolones for empiric treatment in a majority of patients who could be better served with narrower spectrum agents. Only 2 of the outpatient regimens were for the treatment of pyelonephritis, the only indication in which a fluoroquinolone would be the standard of care per guideline recommendations.7
These findings were consistent with a similar study in which 83% of ED collected urine cultures ultimately grew bacteria susceptibleto empiric treatment.8 This number was similar to the current study despite the latter study consisting of predominantly female patients (93%) and excluding patients with a history of benign prostatic hypertrophy, catheter use, or history of genitourinary cancer, which are frequently found within the VA population. Thus, despite having a differing patient population at the current study’s facility with characteristics that would classify most to be treated as a complicated UTI, empiric coverage rates remained similar. The lower than anticipated intervention rate by the pharmacist on rotation in the ED can be directly attributed to this high empiric match rate, which could in turn be attributed to the extensive use of broad-spectrum antibiotics for treatment.
Empiric antimicrobial selection is based largely on local resistance patterns.7 Of particular importance is the resistance patterns of E coli, as it is the primary isolate responsible for UTIs worldwide. Thus, it is not unexpected that the most frequently isolated pathogen in the current study also was E coli. While clinical practice guidelines state that hospital-wide antibiograms often are skewed by cultures collected from inpatients or those with complicated infection, the current study found hospital-wide E coli resistance patterns, specifically those related to fluoroquinolone use, to be similar to those collected in the ED alone (78.5% hospital-wide susceptibility vs. 75% ED susceptibility). This was expected, as similar studies comparing E coli resistance patterns from ED-collected urine cultures to those institution-wide also have found similar rates of resistance.8,9 These findings are of particular importance as E coli resistance is noted to be increasing, varies with geographic area, and local resistance patterns are rarely known.7 Thus, these findings may aid ED providers in their empiric antimicrobial selections.
Ciprofloxacin was the most frequently used medication for the treatment of UTIs. While overall empiric selections were found to have favorable resistance patterns, it is difficult to interpret the appropriateness of ciprofloxacin’s use in the present study. First, there is a distinct lack of US-based clinical practice guidelines for the treatment of complicated UTIs. As the majority of this study population was male, it is difficult to directly extrapolate from the current Infectious Diseases Society of America treatment guidelines for uncomplicated cystitis and apply to the study population. Although recommended for the treatment of pyelonephritis, it is unclear whether ciprofloxacin should be utilized as a first-line empiric option for the treatment of UTIs in males.
Despite the lack of disease-specific recommendations for ciprofloxacin, recommendations exist regarding its use when local resistance patterns are known.7 It is currently recommended that these agents not be used when resistance rates of E coli exceed 20% for trimethoprim-sulfamethoxazole or 10% for fluoroquinolones. As this study demonstrated a nearly 25% resistance rate for E coli to fluoroquinolones in both the ED and institution-wide sample populations, it could potentially be ascertained that ciprofloxacin is an inappropriate choice for the empiric treatment of UTIs in this patient population. However, as noted, it is unknown whether this recommendation would still be applicable when applied to the treatment of complicated cystitis and greater male population, as overall rates of susceptible cultures to all organisms was similar to other published studies.8,9
While there is scant specific guidance related to the treatment of complicated UTIs, there is emerging guidance on the use of fluoroquinolones, both in general and specifically related to the treatment of UTIs. In July 2016, the FDA issued a drug safety communication regarding the use of and warnings for fluoroquinolones, which explicitly stated that “health care professionals should not prescribe systemic fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs because the risks outweigh the benefits in these patients.”10
This guidance has the potential to impact fluoroquinolone prescribing significantly at RRVAMC. Given the large number of fluoroquinolones prescribed for UTIs, the downstream effects that this shift in prescribing would have is unknown. As most nonfluoroquinolones used for UTI typically are narrower in antimicrobial spectrum (eg, trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, etc) the possibility exists that the match rate for empiric therapy may decrease. Thus, a larger need for closer follow-up to assure adequate coverage may arise, posing a more expanded role for an ED-based pharmacist than was demonstrated in the current study.
This new guidance also may place providers in an area of larger uncertainty with regards to treating both complicated and uncomplicated cystitis. Given the enhanced warnings on fluoroquinolone use, it is unknown whether prescribers would gravitate to utilizing similar options as their peers as alternatives to fluoroquinolones. Similarly, duration of therapy with nonfluoroquinolone agents is unclear as well; as the present study demonstrated a large range in treatment duration of outpatients (3-14 days). While the average observed duration of 8.3 days is intuitively fitting, as the majority of cases were in males, no published guideline exists that affirms the appropriateness of this finding. Such uncertainty and potential inconsistency between providers affords a large opportunity for developing a standardized treatment pathway for the treatment of UTIs to ensure both effective and guideline concordant treatment for patients, specifically with regards to antimicrobial selection and duration of treatment.
It is noteworthy to mention that all follow-ups on positive cultures inadequately covered by empiric therapy took place on the day organism identification and susceptibility data were released. This finding was somewhat surprising, as it was originally theorized that most ED-collected urine cultures were not monitored to completion by a pharmacist and that would be necessary in order to ensure proper follow-up of culture results. What is not clear is whether there is a robust process for the follow-up of urine cultures in the ED. Most of the bug-drug mismatches coincidentally were admitted to the inpatient teams where there were appropriate personnel to follow up and adjust the antibiotic selection. If there was a bug-drug mismatch, and the patient was not admitted, it is unclear whether there is a consistent process for follow-up.
Given the limited number of mismatched cultures that required change in therapy, it is unknown if this role would expand if more narrow-spectrum agents were utilized, theoretically leading to a higher mismatch rate and necessitating closer follow-up. Furthermore, given the common practice of mailed prescriptions at the VA, it is all the more imperative that the cultures be acted upon on the day they were identified, as the mailing and processing time of prescriptions may limit the clinical utility in switching from a more broad-spectrum agent, to one more targeted for an identified organism. While a patient traveling back to the medical center for expedited prescription pickup at the pharmacy would alleviate this problem, many patients at the facility travel great distances or may not have readily available travel means to return to the medical center.
Future Directions
While minimal follow-up was required after a patient had left the ED, this study demonstrated a fundamental need for further refinements in antimicrobial stewardship activities within the ED. Duration of therapy, empiric selection, and proper dosing are key areas where the ED-based pharmacy resident was able to intervene during the time physically stationed in the ED. The data collected from this study demonstrated this and was ultimately combined with other ED-based interventions and utilized as supporting evidence in the pharmacy service business plan, outlining the necessity of a full-time pharmacy presence in the ED. The business plan submission, along with other ongoing RRVAMC initiatives, ultimately led to the approval for clinical pharmacy specialists to expand practice into the ED. These positions will continue to advance pharmacy practice within the ED, while affording opportunities for pharmacists to practice at the top of their licensure, provide individualized provider education, and deliver real-time antimicrobial stewardship interventions. Furthermore, as the majority of the study period was monitored outside of the ED, the project may provide a model for other VA institutions without full-time ED pharmacists to implement as a means to improve antimicrobial stewardship and further build an evidence base for expanding their pharmacy services to the ED.
Given the large number of fluoroquinolones utilized in the ED, this study has raised the question of what prescribing patterns look like with regards to outpatient UTI treatment within the realm of primary care at RRVAMC. Despite the great strides made with regards to antimicrobial stewardship at this facility on the inpatient side, no formal antimicrobial stewardship program exists for review in the outpatient setting, where literature suggests the majority of antibiotics are prescribed.3,11 While more robust protocols are in place for follow-up of culture data in the primary care realm at this facility, the prescribing patterns are relatively unknown.
A recent study completed at a similar VA facility found that 60% of antibiotics prescribed for cystitis, pharyngitis, or sinusitis on an outpatient basis were guideline-discordant, and CDC guidance has further recommended specific focus should be undertaken with regards to outpatient stewardship practices in the treatment of genitourinary infections.3,12 These findings highlight the need for outpatient antimicrobial stewardship and presents a compelling reason to further investigate outpatient prescribing within primary care at RRVAMC.
Strengths and Limitations
Strengths of the current study include the ability to monitor urine cultures in real time and to provide timely interventions in the event of a rare bug-drug mismatch. The evaluation of cultures in this study shows that the majority of cases had a drug selected with adequate coverage. The study did assure ED providers that, even though guidelines may suggest otherwise, urine cultures drawn in the ED at RRVAMC followed similar resistance patterns seen for the facility as a whole. Moreover, it is valuable as it captures data that are directly applicable to the VA patient population, in which there is little published data with regards to UTI treatment and no formal VA guidance.
A primary limitation of this study is the lack of differentiation between cultures collected from patients with or without indwelling catheters. However, only including patients who presented with signs and/or symptoms of a UTI limits the number of cultures that could potentially be deemed as colonization, thus minimizing the potential for nonpathogenic organisms to confound the results. This study also did not differentiate the setting from which the patient presented (eg, community, extended care facility, etc) that could have potentially provided guidance on resistance patterns for community-acquired UTIs and whether this may have differed from hospital-acquired or facility-acquired UTIs. Another limitation was the relatively short time frame for data collection. A data collection period greater than 91 days would allow for a larger sample size, thus making the data more robust and potentially allowing for the identification of other trends not seen in the current study. A longer data collection period also would have afforded the opportunity to track more robust clinical outcomes throughout the study, identifying whether treatment failure may have been linked to the use of certain classes or spectrums of activity of antibiotics.
Conclusion
Despite the E coli resistance rate to ciprofloxacin (> 20%), the empiric treatments chosen were > 85% effective, needing minimal follow-up once a patient left the ED. Nonetheless, a change in prescribing patterns based on recent national recommendations may provide expanded opportunities in antimicrobial stewardship for ED-based pharmacists. Further research is needed in antimicrobial stewardship within this facility’s outpatient primary care realm, potentially uncovering other opportunities for pharmacist intervention to assure guideline concordant care for the treatment of UTIs as well as other infections treated in primary care patients.
1. Obama B. Executive order–combating antibiotic-resistant bacteria. https://www.whitehouse.gov/the -press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Published September 18, 2014. Accessed June 7, 2018.
2. Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017;38(6):721-723.
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hick LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
4. Wymore ES, Casanova TJ, Broekenmeier RL, Martin JK Jr. Clinical pharmacist’s daily role in the emergency department of a community hospital. Am J Health-Syst Pharm. 2008;65(5):395-396, 398-399.
5. Frandzel S. ED pharmacists’ value on display at ASHP Midyear. http://www.pharmacypracticenews.com/ViewArticle.aspx?ses=ogst&d_id=53&a_id=22524. Published February 14, 2013. Accessed June 15, 2018.
6. Heytens S, DeSutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017;23(9)647-652.
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120.
8. Lingenfelter E, Drapkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient urinary tract infection. Am J Emerg Med. 2016;34(8):1600-1603.
9. Zatorski C, Jordan JA, Cosgrove SE, Zocchi M, May L. Comparison of antibiotic susceptibility of Escherichia coli in urinary isolates from an emergency department with other institutional susceptibility data. Am J Health-Syst Pharm. 2015;72(24):2176-2180.
10. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Updated March 8, 2018. Accessed June 13, 2018.
11. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
12. Meyer HE, Lund BC, Heintz BH, Alexander B, Egge JA, Livorsi DJ. Identifying opportunities to improve guideline-concordant antibiotic prescribing in veterans with acute respiratory infections or cystitis. Infect Control Hosp Epidemiol. 2017;38(6):724-728.
1. Obama B. Executive order–combating antibiotic-resistant bacteria. https://www.whitehouse.gov/the -press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Published September 18, 2014. Accessed June 7, 2018.
2. Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017;38(6):721-723.
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hick LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
4. Wymore ES, Casanova TJ, Broekenmeier RL, Martin JK Jr. Clinical pharmacist’s daily role in the emergency department of a community hospital. Am J Health-Syst Pharm. 2008;65(5):395-396, 398-399.
5. Frandzel S. ED pharmacists’ value on display at ASHP Midyear. http://www.pharmacypracticenews.com/ViewArticle.aspx?ses=ogst&d_id=53&a_id=22524. Published February 14, 2013. Accessed June 15, 2018.
6. Heytens S, DeSutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017;23(9)647-652.
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120.
8. Lingenfelter E, Drapkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient urinary tract infection. Am J Emerg Med. 2016;34(8):1600-1603.
9. Zatorski C, Jordan JA, Cosgrove SE, Zocchi M, May L. Comparison of antibiotic susceptibility of Escherichia coli in urinary isolates from an emergency department with other institutional susceptibility data. Am J Health-Syst Pharm. 2015;72(24):2176-2180.
10. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Updated March 8, 2018. Accessed June 13, 2018.
11. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
12. Meyer HE, Lund BC, Heintz BH, Alexander B, Egge JA, Livorsi DJ. Identifying opportunities to improve guideline-concordant antibiotic prescribing in veterans with acute respiratory infections or cystitis. Infect Control Hosp Epidemiol. 2017;38(6):724-728.
Could High BMI Reduce Premenopausal Breast Cancer Risk?
Young women may not want to hear it, but fat could be their friend. Researchers from the Premenopausal Breast Cancer Collaborative Group have found that women aged 18 – 24 years with high body fat have a lower risk of developing breast cancer before menopause.
The researchers pooled data from 19 different studies, involving about 800,000 women from around the world. Overall, 1.7% of the women developed breast cancer. The researchers found that the relative risk of premenopausal breast cancer dropped 12% to 23% for each 5-unit increase in body mass index, depending on age. They saw the strongest effect at ages 18 – 24 years: Very obese women in this age group were 4.2 times less likely to develop premenopausal breast cancer than women with low body mass index (BMI) at the same age.
The researchers do not know why high BMI might protect against breast cancer in some women. Breast cancer is relatively rare before menopause, although previous studies have suggested that the risk factors might be different for younger vs older women, says Dale Sandler, PhD, co-author of the group and head of the Epidemiology Branch at the National Institute of Environmental Health Sciences. For instance, it is well known that women who gain weight, particularly after menopause, have a higher risk. The fact that this study found that the risk not only is not increased, but actually decreased, in younger women points to the possibility that different biologic mechanisms are at work, Sandler says.
Nonetheless, the researchers caution that young women should not intentionally gain weight to offset the risk.
Source:
National Institutes of Health. https://www.nih.gov/news-events/news-releases/nih-study-associates-obesity-lower-breast-cancer-risk-young-women. Published June 27, 2018. Accessed July 18, 2018.
Young women may not want to hear it, but fat could be their friend. Researchers from the Premenopausal Breast Cancer Collaborative Group have found that women aged 18 – 24 years with high body fat have a lower risk of developing breast cancer before menopause.
The researchers pooled data from 19 different studies, involving about 800,000 women from around the world. Overall, 1.7% of the women developed breast cancer. The researchers found that the relative risk of premenopausal breast cancer dropped 12% to 23% for each 5-unit increase in body mass index, depending on age. They saw the strongest effect at ages 18 – 24 years: Very obese women in this age group were 4.2 times less likely to develop premenopausal breast cancer than women with low body mass index (BMI) at the same age.
The researchers do not know why high BMI might protect against breast cancer in some women. Breast cancer is relatively rare before menopause, although previous studies have suggested that the risk factors might be different for younger vs older women, says Dale Sandler, PhD, co-author of the group and head of the Epidemiology Branch at the National Institute of Environmental Health Sciences. For instance, it is well known that women who gain weight, particularly after menopause, have a higher risk. The fact that this study found that the risk not only is not increased, but actually decreased, in younger women points to the possibility that different biologic mechanisms are at work, Sandler says.
Nonetheless, the researchers caution that young women should not intentionally gain weight to offset the risk.
Source:
National Institutes of Health. https://www.nih.gov/news-events/news-releases/nih-study-associates-obesity-lower-breast-cancer-risk-young-women. Published June 27, 2018. Accessed July 18, 2018.
Young women may not want to hear it, but fat could be their friend. Researchers from the Premenopausal Breast Cancer Collaborative Group have found that women aged 18 – 24 years with high body fat have a lower risk of developing breast cancer before menopause.
The researchers pooled data from 19 different studies, involving about 800,000 women from around the world. Overall, 1.7% of the women developed breast cancer. The researchers found that the relative risk of premenopausal breast cancer dropped 12% to 23% for each 5-unit increase in body mass index, depending on age. They saw the strongest effect at ages 18 – 24 years: Very obese women in this age group were 4.2 times less likely to develop premenopausal breast cancer than women with low body mass index (BMI) at the same age.
The researchers do not know why high BMI might protect against breast cancer in some women. Breast cancer is relatively rare before menopause, although previous studies have suggested that the risk factors might be different for younger vs older women, says Dale Sandler, PhD, co-author of the group and head of the Epidemiology Branch at the National Institute of Environmental Health Sciences. For instance, it is well known that women who gain weight, particularly after menopause, have a higher risk. The fact that this study found that the risk not only is not increased, but actually decreased, in younger women points to the possibility that different biologic mechanisms are at work, Sandler says.
Nonetheless, the researchers caution that young women should not intentionally gain weight to offset the risk.
Source:
National Institutes of Health. https://www.nih.gov/news-events/news-releases/nih-study-associates-obesity-lower-breast-cancer-risk-young-women. Published June 27, 2018. Accessed July 18, 2018.
L-glutamine reduces complications of SCD
Results of a phase 3 trial showed that L-glutamine can reduce complications of sickle cell disease (SCD).
SCD patients who received pharmaceutical-grade L-glutamine (with or without hydroxyurea) had a reduction in sickle cell crises, hospitalizations, and acute chest syndrome (ACS) when compared to patients who received placebo (with or without hydroxyurea).
Gastrointestinal events and pain in the chest (noncardiac), back, and extremities were more frequent in L-glutamine recipients than controls.
Yutaka Niihara, MD, of Emmaus Medical, Inc., in Torrance, California, and his colleagues reported these results in NEJM. The research was sponsored by Emmaus Medical.
Dr Niihara noted that L-glutamine (Endari), which was approved by the US Food and Drug Administration last summer, is the first treatment approved to treat SCD in pediatric patients age 5 and older and the first SCD treatment approved for adults in nearly 20 years.
“Our hope in sharing the results [of the phase 3 trial] is to aid in increasing the awareness of sickle cell disease, a life-long hereditary blood disorder which commonly affects those of African descent, as well as those from Central and South America and people of Middle Eastern, Asian, Indian, and Mediterranean descent,” Dr Niihara said.
He and his colleagues enrolled 230 patients on the trial. One hundred and fifty-two were randomized to receive L-glutamine at 0.3 g/kg twice daily (maximum 30 g/day), and 78 were randomized to placebo (maltodextrin) at 0.3 g/kg twice daily for 48 weeks.
Baseline characteristics were similar between the treatment arms. Most patients had sickle cell anemia—89.5% (n=136) in the L-glutamine arm and 91% (n=71) in the placebo arm. Several patients had sickle B0 thalassemia—9.2% (n=14) and 9.0% (n=7), respectively. Two patients in the L-glutamine arm had B+ thalassemia, but 1 of these patients did not receive L-glutamine.
The median age was 19 (range, 5-57) in the L-glutamine arm and 17 (range, 5-58) in the placebo arm. There were more females than males in both arms—52% (n=79) and 57.7% (n=45), respectively.
Most patients received concomitant hydroxyurea—66.4% (n=101) in the L-glutamine arm and 66.7% (n=52) in the placebo arm.
Efficacy
Patients in the L-glutamine arm had 25% fewer pain crises than patients in the placebo arm. The median number of crises was 3 and 4, respectively (P=0.005).
The median time to a first crisis was 84 days in the L-glutamine arm and 54 days in the placebo arm (P=0.02). The median time to a second crisis was 212 days and 133 days, respectively (P=0.03).
The median number of hospitalizations was 2 in the L-glutamine arm and 3 in the placebo arm, which is a difference of 33% (P=0.005). The median cumulative number of days in the hospital was 6.5 and 11, respectively (P=0.02).
The incidence of ACS was significantly lower in the L-glutamine arm, with 8.6% of patients in that group having at least 1 episode of ACS and 23.1% of the placebo group having at least 1 episode (P=0.003).
Safety
The rate of adverse events (AEs) was 98% in the L-glutamine arm and 100% in the placebo arm. The rate of serious AEs was 78.2% and 87.1%, respectively.
AEs with a higher incidence in the L-glutamine arm (at least 5%) are listed in the table below.
| AE | L-glutamine (n=151) |
Placebo (n=78) |
| Constipation | 38 (25.2%) | 19 (24.4%) |
| Nausea | 34 (22.5%) | 13 (16.7%) |
| Headache | 32 (21.2%) | 14 (17.9%) |
| Pain in extremity | 24 (15.9%) | 6 (7.7%) |
| Vomiting | 22 (14.6%) | 10 (12.8%) |
| Chest pain (noncardiac) | 21 (13.9%) | 7 (9.0%) |
| Back pain | 20 (13.2%) | 5 (6.4%) |
| Upper abdominal pain | 16 (10.6%) | 6 (7.7%) |
| Diarrhea | 12 (7.9%) | 5 (6.4%) |
| Nasal congestion | 11 (7.3%) | 5 (6.4%) |
| Urinary tract infection | 10 (6.6%) | 3 (3.8%) |
| Fatigue | 9 (6.0%) | 1 (1.3%) |
| Tachycardia | 8 (5.3%) | 4 (5.1%) |
| Dizziness | 8 (5.3%) | 4 (5.1%) |
![]()
Results of a phase 3 trial showed that L-glutamine can reduce complications of sickle cell disease (SCD).
SCD patients who received pharmaceutical-grade L-glutamine (with or without hydroxyurea) had a reduction in sickle cell crises, hospitalizations, and acute chest syndrome (ACS) when compared to patients who received placebo (with or without hydroxyurea).
Gastrointestinal events and pain in the chest (noncardiac), back, and extremities were more frequent in L-glutamine recipients than controls.
Yutaka Niihara, MD, of Emmaus Medical, Inc., in Torrance, California, and his colleagues reported these results in NEJM. The research was sponsored by Emmaus Medical.
Dr Niihara noted that L-glutamine (Endari), which was approved by the US Food and Drug Administration last summer, is the first treatment approved to treat SCD in pediatric patients age 5 and older and the first SCD treatment approved for adults in nearly 20 years.
“Our hope in sharing the results [of the phase 3 trial] is to aid in increasing the awareness of sickle cell disease, a life-long hereditary blood disorder which commonly affects those of African descent, as well as those from Central and South America and people of Middle Eastern, Asian, Indian, and Mediterranean descent,” Dr Niihara said.
He and his colleagues enrolled 230 patients on the trial. One hundred and fifty-two were randomized to receive L-glutamine at 0.3 g/kg twice daily (maximum 30 g/day), and 78 were randomized to placebo (maltodextrin) at 0.3 g/kg twice daily for 48 weeks.
Baseline characteristics were similar between the treatment arms. Most patients had sickle cell anemia—89.5% (n=136) in the L-glutamine arm and 91% (n=71) in the placebo arm. Several patients had sickle B0 thalassemia—9.2% (n=14) and 9.0% (n=7), respectively. Two patients in the L-glutamine arm had B+ thalassemia, but 1 of these patients did not receive L-glutamine.
The median age was 19 (range, 5-57) in the L-glutamine arm and 17 (range, 5-58) in the placebo arm. There were more females than males in both arms—52% (n=79) and 57.7% (n=45), respectively.
Most patients received concomitant hydroxyurea—66.4% (n=101) in the L-glutamine arm and 66.7% (n=52) in the placebo arm.
Efficacy
Patients in the L-glutamine arm had 25% fewer pain crises than patients in the placebo arm. The median number of crises was 3 and 4, respectively (P=0.005).
The median time to a first crisis was 84 days in the L-glutamine arm and 54 days in the placebo arm (P=0.02). The median time to a second crisis was 212 days and 133 days, respectively (P=0.03).
The median number of hospitalizations was 2 in the L-glutamine arm and 3 in the placebo arm, which is a difference of 33% (P=0.005). The median cumulative number of days in the hospital was 6.5 and 11, respectively (P=0.02).
The incidence of ACS was significantly lower in the L-glutamine arm, with 8.6% of patients in that group having at least 1 episode of ACS and 23.1% of the placebo group having at least 1 episode (P=0.003).
Safety
The rate of adverse events (AEs) was 98% in the L-glutamine arm and 100% in the placebo arm. The rate of serious AEs was 78.2% and 87.1%, respectively.
AEs with a higher incidence in the L-glutamine arm (at least 5%) are listed in the table below.
| AE | L-glutamine (n=151) |
Placebo (n=78) |
| Constipation | 38 (25.2%) | 19 (24.4%) |
| Nausea | 34 (22.5%) | 13 (16.7%) |
| Headache | 32 (21.2%) | 14 (17.9%) |
| Pain in extremity | 24 (15.9%) | 6 (7.7%) |
| Vomiting | 22 (14.6%) | 10 (12.8%) |
| Chest pain (noncardiac) | 21 (13.9%) | 7 (9.0%) |
| Back pain | 20 (13.2%) | 5 (6.4%) |
| Upper abdominal pain | 16 (10.6%) | 6 (7.7%) |
| Diarrhea | 12 (7.9%) | 5 (6.4%) |
| Nasal congestion | 11 (7.3%) | 5 (6.4%) |
| Urinary tract infection | 10 (6.6%) | 3 (3.8%) |
| Fatigue | 9 (6.0%) | 1 (1.3%) |
| Tachycardia | 8 (5.3%) | 4 (5.1%) |
| Dizziness | 8 (5.3%) | 4 (5.1%) |
![]()
Results of a phase 3 trial showed that L-glutamine can reduce complications of sickle cell disease (SCD).
SCD patients who received pharmaceutical-grade L-glutamine (with or without hydroxyurea) had a reduction in sickle cell crises, hospitalizations, and acute chest syndrome (ACS) when compared to patients who received placebo (with or without hydroxyurea).
Gastrointestinal events and pain in the chest (noncardiac), back, and extremities were more frequent in L-glutamine recipients than controls.
Yutaka Niihara, MD, of Emmaus Medical, Inc., in Torrance, California, and his colleagues reported these results in NEJM. The research was sponsored by Emmaus Medical.
Dr Niihara noted that L-glutamine (Endari), which was approved by the US Food and Drug Administration last summer, is the first treatment approved to treat SCD in pediatric patients age 5 and older and the first SCD treatment approved for adults in nearly 20 years.
“Our hope in sharing the results [of the phase 3 trial] is to aid in increasing the awareness of sickle cell disease, a life-long hereditary blood disorder which commonly affects those of African descent, as well as those from Central and South America and people of Middle Eastern, Asian, Indian, and Mediterranean descent,” Dr Niihara said.
He and his colleagues enrolled 230 patients on the trial. One hundred and fifty-two were randomized to receive L-glutamine at 0.3 g/kg twice daily (maximum 30 g/day), and 78 were randomized to placebo (maltodextrin) at 0.3 g/kg twice daily for 48 weeks.
Baseline characteristics were similar between the treatment arms. Most patients had sickle cell anemia—89.5% (n=136) in the L-glutamine arm and 91% (n=71) in the placebo arm. Several patients had sickle B0 thalassemia—9.2% (n=14) and 9.0% (n=7), respectively. Two patients in the L-glutamine arm had B+ thalassemia, but 1 of these patients did not receive L-glutamine.
The median age was 19 (range, 5-57) in the L-glutamine arm and 17 (range, 5-58) in the placebo arm. There were more females than males in both arms—52% (n=79) and 57.7% (n=45), respectively.
Most patients received concomitant hydroxyurea—66.4% (n=101) in the L-glutamine arm and 66.7% (n=52) in the placebo arm.
Efficacy
Patients in the L-glutamine arm had 25% fewer pain crises than patients in the placebo arm. The median number of crises was 3 and 4, respectively (P=0.005).
The median time to a first crisis was 84 days in the L-glutamine arm and 54 days in the placebo arm (P=0.02). The median time to a second crisis was 212 days and 133 days, respectively (P=0.03).
The median number of hospitalizations was 2 in the L-glutamine arm and 3 in the placebo arm, which is a difference of 33% (P=0.005). The median cumulative number of days in the hospital was 6.5 and 11, respectively (P=0.02).
The incidence of ACS was significantly lower in the L-glutamine arm, with 8.6% of patients in that group having at least 1 episode of ACS and 23.1% of the placebo group having at least 1 episode (P=0.003).
Safety
The rate of adverse events (AEs) was 98% in the L-glutamine arm and 100% in the placebo arm. The rate of serious AEs was 78.2% and 87.1%, respectively.
AEs with a higher incidence in the L-glutamine arm (at least 5%) are listed in the table below.
| AE | L-glutamine (n=151) |
Placebo (n=78) |
| Constipation | 38 (25.2%) | 19 (24.4%) |
| Nausea | 34 (22.5%) | 13 (16.7%) |
| Headache | 32 (21.2%) | 14 (17.9%) |
| Pain in extremity | 24 (15.9%) | 6 (7.7%) |
| Vomiting | 22 (14.6%) | 10 (12.8%) |
| Chest pain (noncardiac) | 21 (13.9%) | 7 (9.0%) |
| Back pain | 20 (13.2%) | 5 (6.4%) |
| Upper abdominal pain | 16 (10.6%) | 6 (7.7%) |
| Diarrhea | 12 (7.9%) | 5 (6.4%) |
| Nasal congestion | 11 (7.3%) | 5 (6.4%) |
| Urinary tract infection | 10 (6.6%) | 3 (3.8%) |
| Fatigue | 9 (6.0%) | 1 (1.3%) |
| Tachycardia | 8 (5.3%) | 4 (5.1%) |
| Dizziness | 8 (5.3%) | 4 (5.1%) |
![]()
How ALL invades the CNS
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Breakthrough drugs approved with less stringent criteria
Clinical trials supporting the approval of drugs with breakthrough therapy designation1 do not meet the same standards as trials for non-breakthrough drugs, according to researchers.
Between 2012 and 2017, the US Food and Drug Administration (FDA) approved 46 breakthrough therapeutics on the basis of 89 pivotal trials.
Researchers found these trials “commonly lacked randomization, double-blinding, and control groups, used surrogate markers as primary endpoints, and enrolled small numbers of patients.”
Joseph S. Ross, MD, of the Yale School of Medicine in New Haven, Connecticut, and his colleagues detailed these findings in a letter to JAMA.
“To be clear, I think the FDA, as directed by Congress, is doing everything it can to expedite the development and review of drugs that treat serious and life-threatening conditions,” Dr Ross said.
“Our research suggests that FDA approval of these breakthrough therapies is generally based on shorter and smaller clinical trials than those that support FDA approval of non-breakthrough therapy drugs.”
Analyzing the approvals
More than half of the 46 approvals analyzed were for cancer therapeutics (n=25; 54.3%), and an equal number were considered first-in-class.
All 46 products received priority review2, 30 (65.2%) received orphan designation3, 24 qualified for fast track4 review (52.2%), and 18 received accelerated approval5 (39.1%).
The median time from an investigational new drug activation to final FDA approval was 4.9 years. The median time from the submission of the new drug application to FDA approval was 6.9 months.
The median number of pivotal trials per indication was 1, and the median number of patients supporting an indication was 222.
Of all the approvals, 27 (58.7%) were made based on randomized trials, 21 (45.7%) were based on double-blind allocation, and 25 (54.3%) used an active or placebo comparator. Only 10 (21.7%) used a clinical primary endpoint.
The analysis also showed that trials supporting breakthrough drugs with accelerated approval were significantly less likely to be randomized, double-blinded, or have an active/placebo control group.
Of 18 trials that were used to grant drugs accelerated approval, 3 (16.7%) trials were randomized, 1 (5.6%) was double-blinded, and 3 (16.7%) had an active/placebo control group.
Of 28 trials supporting drugs without accelerated approval, 24 (85.7%) trials were randomized, 20 (71.4%) were double-blinded, and 22 (78.6%) had an active/placebo control group.
All 18 accelerated approvals had at least 1 safety analysis or efficacy-focused postmarketing requirement.
Dr Ross pointed out that when approvals are based on shorter and smaller clinical trials, there is greater uncertainty at the time of approval.
For example, will effects observed in a small, single trial be observed in a larger population or in another independent study? Will effects observed over a short period persist over time? Will new risks (or benefits) be observed over a longer period? And will the effect observed on the outcomes used in these shorter trials—usually surrogate endpoints believed to predict a clinical benefit—be confirmed by clinical outcomes?
“If we are going to be making this trade-off to allow novel drugs to come to market on the basis of evidence that is generally accompanied by greater uncertainty, we must be committed as a clinical and scientific community to ensuring that high-quality, rigorous postmarketing trials are conducted within a reasonable period of time,” Dr Ross said.
He noted that postmarketing trials will resolve some of the uncertainty and will ensure that drugs are associated with the benefit/safety profile that is expected based on the initial clinical studies.
“This will allow clinicians and patients to make fully informed decisions about whether to use these novel treatments,” he said.
1. The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions. Breakthrough designation entitles sponsors to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review. To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
2. The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
3. The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US. Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
4. The FDA’s fast track program is designed to expedite clinical development and submission of applications for drugs with the potential to treat serious or life-threatening conditions and address unmet medical needs. Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the drug’s development plan and written communications about issues such as trial design and use of biomarkers. Drugs that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Fast track drugs may also be eligible for rolling review, which allows a developer to submit individual sections of a drug’s application for review as they are ready, rather than waiting until all sections are complete.
5. The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition. Accelerated approval is based on surrogate or intermediate endpoints that are reasonably likely to predict clinical benefit. Continued approval of drugs granted accelerated approval may be contingent upon verification of clinical benefit in confirmatory trials.
Clinical trials supporting the approval of drugs with breakthrough therapy designation1 do not meet the same standards as trials for non-breakthrough drugs, according to researchers.
Between 2012 and 2017, the US Food and Drug Administration (FDA) approved 46 breakthrough therapeutics on the basis of 89 pivotal trials.
Researchers found these trials “commonly lacked randomization, double-blinding, and control groups, used surrogate markers as primary endpoints, and enrolled small numbers of patients.”
Joseph S. Ross, MD, of the Yale School of Medicine in New Haven, Connecticut, and his colleagues detailed these findings in a letter to JAMA.
“To be clear, I think the FDA, as directed by Congress, is doing everything it can to expedite the development and review of drugs that treat serious and life-threatening conditions,” Dr Ross said.
“Our research suggests that FDA approval of these breakthrough therapies is generally based on shorter and smaller clinical trials than those that support FDA approval of non-breakthrough therapy drugs.”
Analyzing the approvals
More than half of the 46 approvals analyzed were for cancer therapeutics (n=25; 54.3%), and an equal number were considered first-in-class.
All 46 products received priority review2, 30 (65.2%) received orphan designation3, 24 qualified for fast track4 review (52.2%), and 18 received accelerated approval5 (39.1%).
The median time from an investigational new drug activation to final FDA approval was 4.9 years. The median time from the submission of the new drug application to FDA approval was 6.9 months.
The median number of pivotal trials per indication was 1, and the median number of patients supporting an indication was 222.
Of all the approvals, 27 (58.7%) were made based on randomized trials, 21 (45.7%) were based on double-blind allocation, and 25 (54.3%) used an active or placebo comparator. Only 10 (21.7%) used a clinical primary endpoint.
The analysis also showed that trials supporting breakthrough drugs with accelerated approval were significantly less likely to be randomized, double-blinded, or have an active/placebo control group.
Of 18 trials that were used to grant drugs accelerated approval, 3 (16.7%) trials were randomized, 1 (5.6%) was double-blinded, and 3 (16.7%) had an active/placebo control group.
Of 28 trials supporting drugs without accelerated approval, 24 (85.7%) trials were randomized, 20 (71.4%) were double-blinded, and 22 (78.6%) had an active/placebo control group.
All 18 accelerated approvals had at least 1 safety analysis or efficacy-focused postmarketing requirement.
Dr Ross pointed out that when approvals are based on shorter and smaller clinical trials, there is greater uncertainty at the time of approval.
For example, will effects observed in a small, single trial be observed in a larger population or in another independent study? Will effects observed over a short period persist over time? Will new risks (or benefits) be observed over a longer period? And will the effect observed on the outcomes used in these shorter trials—usually surrogate endpoints believed to predict a clinical benefit—be confirmed by clinical outcomes?
“If we are going to be making this trade-off to allow novel drugs to come to market on the basis of evidence that is generally accompanied by greater uncertainty, we must be committed as a clinical and scientific community to ensuring that high-quality, rigorous postmarketing trials are conducted within a reasonable period of time,” Dr Ross said.
He noted that postmarketing trials will resolve some of the uncertainty and will ensure that drugs are associated with the benefit/safety profile that is expected based on the initial clinical studies.
“This will allow clinicians and patients to make fully informed decisions about whether to use these novel treatments,” he said.
1. The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions. Breakthrough designation entitles sponsors to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review. To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
2. The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
3. The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US. Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
4. The FDA’s fast track program is designed to expedite clinical development and submission of applications for drugs with the potential to treat serious or life-threatening conditions and address unmet medical needs. Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the drug’s development plan and written communications about issues such as trial design and use of biomarkers. Drugs that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Fast track drugs may also be eligible for rolling review, which allows a developer to submit individual sections of a drug’s application for review as they are ready, rather than waiting until all sections are complete.
5. The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition. Accelerated approval is based on surrogate or intermediate endpoints that are reasonably likely to predict clinical benefit. Continued approval of drugs granted accelerated approval may be contingent upon verification of clinical benefit in confirmatory trials.
Clinical trials supporting the approval of drugs with breakthrough therapy designation1 do not meet the same standards as trials for non-breakthrough drugs, according to researchers.
Between 2012 and 2017, the US Food and Drug Administration (FDA) approved 46 breakthrough therapeutics on the basis of 89 pivotal trials.
Researchers found these trials “commonly lacked randomization, double-blinding, and control groups, used surrogate markers as primary endpoints, and enrolled small numbers of patients.”
Joseph S. Ross, MD, of the Yale School of Medicine in New Haven, Connecticut, and his colleagues detailed these findings in a letter to JAMA.
“To be clear, I think the FDA, as directed by Congress, is doing everything it can to expedite the development and review of drugs that treat serious and life-threatening conditions,” Dr Ross said.
“Our research suggests that FDA approval of these breakthrough therapies is generally based on shorter and smaller clinical trials than those that support FDA approval of non-breakthrough therapy drugs.”
Analyzing the approvals
More than half of the 46 approvals analyzed were for cancer therapeutics (n=25; 54.3%), and an equal number were considered first-in-class.
All 46 products received priority review2, 30 (65.2%) received orphan designation3, 24 qualified for fast track4 review (52.2%), and 18 received accelerated approval5 (39.1%).
The median time from an investigational new drug activation to final FDA approval was 4.9 years. The median time from the submission of the new drug application to FDA approval was 6.9 months.
The median number of pivotal trials per indication was 1, and the median number of patients supporting an indication was 222.
Of all the approvals, 27 (58.7%) were made based on randomized trials, 21 (45.7%) were based on double-blind allocation, and 25 (54.3%) used an active or placebo comparator. Only 10 (21.7%) used a clinical primary endpoint.
The analysis also showed that trials supporting breakthrough drugs with accelerated approval were significantly less likely to be randomized, double-blinded, or have an active/placebo control group.
Of 18 trials that were used to grant drugs accelerated approval, 3 (16.7%) trials were randomized, 1 (5.6%) was double-blinded, and 3 (16.7%) had an active/placebo control group.
Of 28 trials supporting drugs without accelerated approval, 24 (85.7%) trials were randomized, 20 (71.4%) were double-blinded, and 22 (78.6%) had an active/placebo control group.
All 18 accelerated approvals had at least 1 safety analysis or efficacy-focused postmarketing requirement.
Dr Ross pointed out that when approvals are based on shorter and smaller clinical trials, there is greater uncertainty at the time of approval.
For example, will effects observed in a small, single trial be observed in a larger population or in another independent study? Will effects observed over a short period persist over time? Will new risks (or benefits) be observed over a longer period? And will the effect observed on the outcomes used in these shorter trials—usually surrogate endpoints believed to predict a clinical benefit—be confirmed by clinical outcomes?
“If we are going to be making this trade-off to allow novel drugs to come to market on the basis of evidence that is generally accompanied by greater uncertainty, we must be committed as a clinical and scientific community to ensuring that high-quality, rigorous postmarketing trials are conducted within a reasonable period of time,” Dr Ross said.
He noted that postmarketing trials will resolve some of the uncertainty and will ensure that drugs are associated with the benefit/safety profile that is expected based on the initial clinical studies.
“This will allow clinicians and patients to make fully informed decisions about whether to use these novel treatments,” he said.
1. The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions. Breakthrough designation entitles sponsors to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review. To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
2. The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
3. The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US. Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
4. The FDA’s fast track program is designed to expedite clinical development and submission of applications for drugs with the potential to treat serious or life-threatening conditions and address unmet medical needs. Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the drug’s development plan and written communications about issues such as trial design and use of biomarkers. Drugs that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Fast track drugs may also be eligible for rolling review, which allows a developer to submit individual sections of a drug’s application for review as they are ready, rather than waiting until all sections are complete.
5. The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition. Accelerated approval is based on surrogate or intermediate endpoints that are reasonably likely to predict clinical benefit. Continued approval of drugs granted accelerated approval may be contingent upon verification of clinical benefit in confirmatory trials.
Dark spot on back
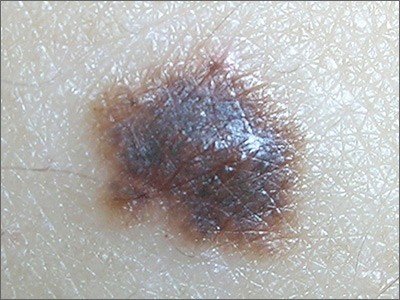
A biopsy revealed a compound dysplastic nevus (DN) with no signs of malignancy. There was only mild atypia (if severe atypia is reported, then the lesion often is treated as a melanoma in-situ).
The FP was initially concerned about melanoma, given the size, growth, and other characteristics of the lesion. He performed dermoscopy and noted an irregular network with multiple asymmetrically placed dots off the network. His differential diagnosis included melanoma, melanoma in-situ, and dysplastic nevus. After informed consent, he performed a saucerization (deep shave) with a DermaBlade, taking 2-mm margins of clinically normal skin, which revealed the DN. (See the Watch & Learn video on “Shave biopsy.”)
Dysplastic nevi (with mild to moderate atypia) are benign acquired melanocytic lesions of the skin. Most are compound nevi possessing a junctional and intradermal component. While dysplastic nevi are not premalignant lesions, they do have some (small) potential for malignant transformation and patients with multiple DN have an increased risk for melanoma. Cutting out all the DN does not change that risk of melanoma.
The FP explained that no further treatment was needed and offered yearly skin exams to monitor for melanoma.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:958-962
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

A biopsy revealed a compound dysplastic nevus (DN) with no signs of malignancy. There was only mild atypia (if severe atypia is reported, then the lesion often is treated as a melanoma in-situ).
The FP was initially concerned about melanoma, given the size, growth, and other characteristics of the lesion. He performed dermoscopy and noted an irregular network with multiple asymmetrically placed dots off the network. His differential diagnosis included melanoma, melanoma in-situ, and dysplastic nevus. After informed consent, he performed a saucerization (deep shave) with a DermaBlade, taking 2-mm margins of clinically normal skin, which revealed the DN. (See the Watch & Learn video on “Shave biopsy.”)
Dysplastic nevi (with mild to moderate atypia) are benign acquired melanocytic lesions of the skin. Most are compound nevi possessing a junctional and intradermal component. While dysplastic nevi are not premalignant lesions, they do have some (small) potential for malignant transformation and patients with multiple DN have an increased risk for melanoma. Cutting out all the DN does not change that risk of melanoma.
The FP explained that no further treatment was needed and offered yearly skin exams to monitor for melanoma.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:958-962
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

A biopsy revealed a compound dysplastic nevus (DN) with no signs of malignancy. There was only mild atypia (if severe atypia is reported, then the lesion often is treated as a melanoma in-situ).
The FP was initially concerned about melanoma, given the size, growth, and other characteristics of the lesion. He performed dermoscopy and noted an irregular network with multiple asymmetrically placed dots off the network. His differential diagnosis included melanoma, melanoma in-situ, and dysplastic nevus. After informed consent, he performed a saucerization (deep shave) with a DermaBlade, taking 2-mm margins of clinically normal skin, which revealed the DN. (See the Watch & Learn video on “Shave biopsy.”)
Dysplastic nevi (with mild to moderate atypia) are benign acquired melanocytic lesions of the skin. Most are compound nevi possessing a junctional and intradermal component. While dysplastic nevi are not premalignant lesions, they do have some (small) potential for malignant transformation and patients with multiple DN have an increased risk for melanoma. Cutting out all the DN does not change that risk of melanoma.
The FP explained that no further treatment was needed and offered yearly skin exams to monitor for melanoma.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:958-962
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
FDA expands indication for ribociclib for advanced breast cancer
The Food and Drug Administration has approved ribociclib (Kisqali) in combination with an aromatase inhibitor (AI) for the treatment of pre/perimenopausal or postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)–negative advanced or metastatic breast cancer, as initial endocrine-based therapy.
Ribociclib was first approved in March 2017 for use with an AI to treat HR-positive, HER2-negative advanced breast cancer in postmenopausal women.
Approval for ribociclib in combination with an AI for pre/perimenopausal women was based on progression-free survival (PFS) in MONALEESA-7, a trial of premenopausal women with HR-positive, HER2-negative, advanced breast cancer. The women received either ribociclib and an AI, or placebo and an AI, and all also received ovarian suppression with goserelin (Zoladex). Of 495 women who received nonsteroidal AIs, median PFS was 27.5 months for women also receiving ribociclib, versus 13.8 months for women who received placebo plus the AI.
Approval for ribociclib in combination with fulvestrant in treating advanced or metastatic breast cancer was based on PFS results from MONALEESA-3, which enrolled 726 women with HR-positive, HER2-negative, advanced breast cancer who received no or up to one line of prior endocrine therapy. Median PFS was 20.5 months for women randomized to receive ribociclib and fulvestrant, compared with 12.8 months for women randomized to receive placebo plus fulvestrant.
The common side effects of ribociclib are infections, neutropenia, leukopenia, headache, cough, nausea, fatigue, diarrhea, vomiting, constipation, hair loss, and rash. Warnings include the risk of QT prolongation, serious liver problems, low white blood cell counts, and fetal harm, the FDA said.
This is the first FDA approval as part of two new pilot programs announced earlier this year: Real-Time Oncology Review allows for the FDA to review much of the data earlier, before the information is formally submitted to the FDA, and the Assessment Aid is a structured template that offers a more streamlined approach.
“With today’s approval, the FDA used these new approaches to allow the review team to start analyzing data before the actual submission of the application and help guide the sponsor’s analysis of the top-line data to tease out the most relevant information,” FDA Commissioner Scott Gottlieb, MD, said in the press statement. “This enabled our approval less than 1 month after the June 28 submission date and several months ahead of the goal date.”
The two pilot programs are currently being used for supplemental applications for already approved cancer drugs and could later be expanded to original drugs and biologics, the FDA said.
Ribociclib is marketed as Kisqali by Novartis Pharmaceuticals Corporation.
The Food and Drug Administration has approved ribociclib (Kisqali) in combination with an aromatase inhibitor (AI) for the treatment of pre/perimenopausal or postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)–negative advanced or metastatic breast cancer, as initial endocrine-based therapy.
Ribociclib was first approved in March 2017 for use with an AI to treat HR-positive, HER2-negative advanced breast cancer in postmenopausal women.
Approval for ribociclib in combination with an AI for pre/perimenopausal women was based on progression-free survival (PFS) in MONALEESA-7, a trial of premenopausal women with HR-positive, HER2-negative, advanced breast cancer. The women received either ribociclib and an AI, or placebo and an AI, and all also received ovarian suppression with goserelin (Zoladex). Of 495 women who received nonsteroidal AIs, median PFS was 27.5 months for women also receiving ribociclib, versus 13.8 months for women who received placebo plus the AI.
Approval for ribociclib in combination with fulvestrant in treating advanced or metastatic breast cancer was based on PFS results from MONALEESA-3, which enrolled 726 women with HR-positive, HER2-negative, advanced breast cancer who received no or up to one line of prior endocrine therapy. Median PFS was 20.5 months for women randomized to receive ribociclib and fulvestrant, compared with 12.8 months for women randomized to receive placebo plus fulvestrant.
The common side effects of ribociclib are infections, neutropenia, leukopenia, headache, cough, nausea, fatigue, diarrhea, vomiting, constipation, hair loss, and rash. Warnings include the risk of QT prolongation, serious liver problems, low white blood cell counts, and fetal harm, the FDA said.
This is the first FDA approval as part of two new pilot programs announced earlier this year: Real-Time Oncology Review allows for the FDA to review much of the data earlier, before the information is formally submitted to the FDA, and the Assessment Aid is a structured template that offers a more streamlined approach.
“With today’s approval, the FDA used these new approaches to allow the review team to start analyzing data before the actual submission of the application and help guide the sponsor’s analysis of the top-line data to tease out the most relevant information,” FDA Commissioner Scott Gottlieb, MD, said in the press statement. “This enabled our approval less than 1 month after the June 28 submission date and several months ahead of the goal date.”
The two pilot programs are currently being used for supplemental applications for already approved cancer drugs and could later be expanded to original drugs and biologics, the FDA said.
Ribociclib is marketed as Kisqali by Novartis Pharmaceuticals Corporation.
The Food and Drug Administration has approved ribociclib (Kisqali) in combination with an aromatase inhibitor (AI) for the treatment of pre/perimenopausal or postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)–negative advanced or metastatic breast cancer, as initial endocrine-based therapy.
Ribociclib was first approved in March 2017 for use with an AI to treat HR-positive, HER2-negative advanced breast cancer in postmenopausal women.
Approval for ribociclib in combination with an AI for pre/perimenopausal women was based on progression-free survival (PFS) in MONALEESA-7, a trial of premenopausal women with HR-positive, HER2-negative, advanced breast cancer. The women received either ribociclib and an AI, or placebo and an AI, and all also received ovarian suppression with goserelin (Zoladex). Of 495 women who received nonsteroidal AIs, median PFS was 27.5 months for women also receiving ribociclib, versus 13.8 months for women who received placebo plus the AI.
Approval for ribociclib in combination with fulvestrant in treating advanced or metastatic breast cancer was based on PFS results from MONALEESA-3, which enrolled 726 women with HR-positive, HER2-negative, advanced breast cancer who received no or up to one line of prior endocrine therapy. Median PFS was 20.5 months for women randomized to receive ribociclib and fulvestrant, compared with 12.8 months for women randomized to receive placebo plus fulvestrant.
The common side effects of ribociclib are infections, neutropenia, leukopenia, headache, cough, nausea, fatigue, diarrhea, vomiting, constipation, hair loss, and rash. Warnings include the risk of QT prolongation, serious liver problems, low white blood cell counts, and fetal harm, the FDA said.
This is the first FDA approval as part of two new pilot programs announced earlier this year: Real-Time Oncology Review allows for the FDA to review much of the data earlier, before the information is formally submitted to the FDA, and the Assessment Aid is a structured template that offers a more streamlined approach.
“With today’s approval, the FDA used these new approaches to allow the review team to start analyzing data before the actual submission of the application and help guide the sponsor’s analysis of the top-line data to tease out the most relevant information,” FDA Commissioner Scott Gottlieb, MD, said in the press statement. “This enabled our approval less than 1 month after the June 28 submission date and several months ahead of the goal date.”
The two pilot programs are currently being used for supplemental applications for already approved cancer drugs and could later be expanded to original drugs and biologics, the FDA said.
Ribociclib is marketed as Kisqali by Novartis Pharmaceuticals Corporation.
NAFLD less common, more severe in black children
ORLANDO – according to a review of 503 adolescents at the Yale University pediatric obesity clinic in New Haven, Conn.
As childhood obesity rates have climbed – the prevalence is now estimated to be around 20% – there’s been a corresponding increase in pediatric NAFLD, but it’s not very well characterized in children, and “there are many gaps in our knowledge,” said Nicola Santoro, MD, PhD, an assistant professor of pediatric endocrinology at Yale, and senior author of the review.
The goal of the work was to begin to plug the gaps. The children had baseline abdominal MRIs to quantify their hepatic fat content, along with oral glucose tolerance tests and genotyping for three single nucleotide polymorphisms (SNPs) strongly associated with the condition (PNPLA3 rs738409, GCKR rs1260326, and TM6SF2 rs58542926). MRI and metabolic testing were repeated at a mean of 2.27 years in 133 children.
The subjects were 13 years old on average, with a mean body mass index z-score of 2.52; 191 were white, 134 black, and 178 Hispanic. NAFLD was defined as a hepatic fat content of at least 5.5%.
The prevalence of fatty liver was 41.6% but ranged widely by ethnicity, with NAFLD diagnosed in 60% of Hispanic, 43% of white, but only 16% of black children. Among all three groups, prevalence was higher among boys.
Although NAFLD was least common among black children, when it was present, it was worse. Black children with NAFLD, compared with others, had the highest fasting glucose and 2-hour glucose levels; the highest insulin and C-peptide levels, and the highest hemoglobin A1c, despite similar age and gender distribution across the groups.
The findings translated to a higher prevalence of prediabetes and type 2 diabetes mellitus (66.6%), compared with white (24.4%) and Hispanic children (31.1%) with NAFLD.
Among 76 children who didn’t have NAFLD at baseline, 17 were diagnosed with the condition at follow-up. Progressors, compared with nonprogressors, showed higher baseline C-peptide levels (about 1,250 pmol/L versus 1,000 pmol/L) and greater weight gain (increase, versus a loss of, about 0.1 point on body mass index z-scores). Black children were the least likely to progress to NAFLD.
Increasing BMI z-score, higher baseline fasting C-peptide levels, and nonblack race strongly predicted progression (area under the curve = 0.887). The risk of progression was even higher when a NAFLD SNP was on board (AUC equal to or greater than 0.96).
Of 57 children with NAFLD at baseline, 13 didn’t meet the definition at follow-up, but regression turned out to be harder to predict. Regressors showed lower intrahepatic fat fractions at baseline (about 10% versus 20%), and a lowering of BMI z-scores at follow-up. Adding SNPs didn’t improve the model (AUC = 0.756).
As in adults, weight loss is the single most important factor to reverse NAFLD. “Even if you lose only a few kilos, fatty liver can go away. The liver cleans up pretty easily, but if you keep your weight, or you gain even a little bit, the disease keeps progressing,” Dr. Santoro said at the annual scientific sessions of the American Diabetes Association.
The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
*This story was updated on 7/20/2018.
SOURCE: Trico D et al. ADA 2018, Abstract 313-OR.
ORLANDO – according to a review of 503 adolescents at the Yale University pediatric obesity clinic in New Haven, Conn.
As childhood obesity rates have climbed – the prevalence is now estimated to be around 20% – there’s been a corresponding increase in pediatric NAFLD, but it’s not very well characterized in children, and “there are many gaps in our knowledge,” said Nicola Santoro, MD, PhD, an assistant professor of pediatric endocrinology at Yale, and senior author of the review.
The goal of the work was to begin to plug the gaps. The children had baseline abdominal MRIs to quantify their hepatic fat content, along with oral glucose tolerance tests and genotyping for three single nucleotide polymorphisms (SNPs) strongly associated with the condition (PNPLA3 rs738409, GCKR rs1260326, and TM6SF2 rs58542926). MRI and metabolic testing were repeated at a mean of 2.27 years in 133 children.
The subjects were 13 years old on average, with a mean body mass index z-score of 2.52; 191 were white, 134 black, and 178 Hispanic. NAFLD was defined as a hepatic fat content of at least 5.5%.
The prevalence of fatty liver was 41.6% but ranged widely by ethnicity, with NAFLD diagnosed in 60% of Hispanic, 43% of white, but only 16% of black children. Among all three groups, prevalence was higher among boys.
Although NAFLD was least common among black children, when it was present, it was worse. Black children with NAFLD, compared with others, had the highest fasting glucose and 2-hour glucose levels; the highest insulin and C-peptide levels, and the highest hemoglobin A1c, despite similar age and gender distribution across the groups.
The findings translated to a higher prevalence of prediabetes and type 2 diabetes mellitus (66.6%), compared with white (24.4%) and Hispanic children (31.1%) with NAFLD.
Among 76 children who didn’t have NAFLD at baseline, 17 were diagnosed with the condition at follow-up. Progressors, compared with nonprogressors, showed higher baseline C-peptide levels (about 1,250 pmol/L versus 1,000 pmol/L) and greater weight gain (increase, versus a loss of, about 0.1 point on body mass index z-scores). Black children were the least likely to progress to NAFLD.
Increasing BMI z-score, higher baseline fasting C-peptide levels, and nonblack race strongly predicted progression (area under the curve = 0.887). The risk of progression was even higher when a NAFLD SNP was on board (AUC equal to or greater than 0.96).
Of 57 children with NAFLD at baseline, 13 didn’t meet the definition at follow-up, but regression turned out to be harder to predict. Regressors showed lower intrahepatic fat fractions at baseline (about 10% versus 20%), and a lowering of BMI z-scores at follow-up. Adding SNPs didn’t improve the model (AUC = 0.756).
As in adults, weight loss is the single most important factor to reverse NAFLD. “Even if you lose only a few kilos, fatty liver can go away. The liver cleans up pretty easily, but if you keep your weight, or you gain even a little bit, the disease keeps progressing,” Dr. Santoro said at the annual scientific sessions of the American Diabetes Association.
The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
*This story was updated on 7/20/2018.
SOURCE: Trico D et al. ADA 2018, Abstract 313-OR.
ORLANDO – according to a review of 503 adolescents at the Yale University pediatric obesity clinic in New Haven, Conn.
As childhood obesity rates have climbed – the prevalence is now estimated to be around 20% – there’s been a corresponding increase in pediatric NAFLD, but it’s not very well characterized in children, and “there are many gaps in our knowledge,” said Nicola Santoro, MD, PhD, an assistant professor of pediatric endocrinology at Yale, and senior author of the review.
The goal of the work was to begin to plug the gaps. The children had baseline abdominal MRIs to quantify their hepatic fat content, along with oral glucose tolerance tests and genotyping for three single nucleotide polymorphisms (SNPs) strongly associated with the condition (PNPLA3 rs738409, GCKR rs1260326, and TM6SF2 rs58542926). MRI and metabolic testing were repeated at a mean of 2.27 years in 133 children.
The subjects were 13 years old on average, with a mean body mass index z-score of 2.52; 191 were white, 134 black, and 178 Hispanic. NAFLD was defined as a hepatic fat content of at least 5.5%.
The prevalence of fatty liver was 41.6% but ranged widely by ethnicity, with NAFLD diagnosed in 60% of Hispanic, 43% of white, but only 16% of black children. Among all three groups, prevalence was higher among boys.
Although NAFLD was least common among black children, when it was present, it was worse. Black children with NAFLD, compared with others, had the highest fasting glucose and 2-hour glucose levels; the highest insulin and C-peptide levels, and the highest hemoglobin A1c, despite similar age and gender distribution across the groups.
The findings translated to a higher prevalence of prediabetes and type 2 diabetes mellitus (66.6%), compared with white (24.4%) and Hispanic children (31.1%) with NAFLD.
Among 76 children who didn’t have NAFLD at baseline, 17 were diagnosed with the condition at follow-up. Progressors, compared with nonprogressors, showed higher baseline C-peptide levels (about 1,250 pmol/L versus 1,000 pmol/L) and greater weight gain (increase, versus a loss of, about 0.1 point on body mass index z-scores). Black children were the least likely to progress to NAFLD.
Increasing BMI z-score, higher baseline fasting C-peptide levels, and nonblack race strongly predicted progression (area under the curve = 0.887). The risk of progression was even higher when a NAFLD SNP was on board (AUC equal to or greater than 0.96).
Of 57 children with NAFLD at baseline, 13 didn’t meet the definition at follow-up, but regression turned out to be harder to predict. Regressors showed lower intrahepatic fat fractions at baseline (about 10% versus 20%), and a lowering of BMI z-scores at follow-up. Adding SNPs didn’t improve the model (AUC = 0.756).
As in adults, weight loss is the single most important factor to reverse NAFLD. “Even if you lose only a few kilos, fatty liver can go away. The liver cleans up pretty easily, but if you keep your weight, or you gain even a little bit, the disease keeps progressing,” Dr. Santoro said at the annual scientific sessions of the American Diabetes Association.
The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
*This story was updated on 7/20/2018.
SOURCE: Trico D et al. ADA 2018, Abstract 313-OR.
REPORTING FROM ADA 2018
Key clinical point: Obese black children are less likely than others to develop non-alcoholic fatty liver disease, but more likely to suffer its consequences if they do.
Major finding: Black children with NAFLD had a higher prevalence of prediabetes and type 2 diabetes (66.6%), compared with white (24.4%) and Hispanic children (31.1%).
Study details: Review of 503 obese adolescents
Disclosures: The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
Source: Trico D et al. ADA 2018, Abstract 313-OR.