User login
Urgent care and retail clinics fuel inappropriate antibiotic prescribing
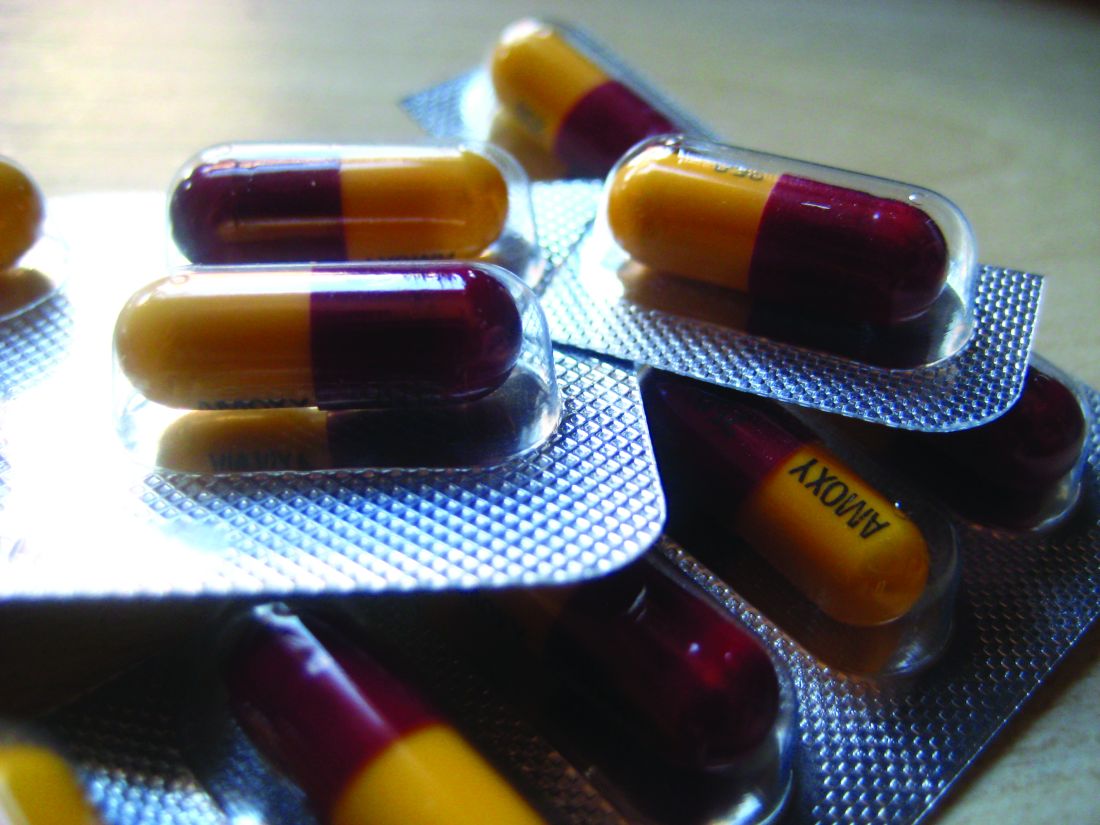
When it comes to inappropriate antibiotic prescribing, urgent care centers and retail clinics may have an outsized impact, a review of commercial insurance claims suggests.
Antibiotic prescription rates were at least twice as high in those settings, compared with emergency departments and medical office visits, according to the retrospective analysis.
The issue may be particularly pronounced in urgent care centers, based on this study, in which nearly half of visits for antibiotic-inappropriate respiratory diagnoses resulted in antibiotic prescribing.
Those findings suggest a need for “antibiotic stewardship interventions” to reduce unnecessary prescribing of antibiotics in ambulatory care settings, authors of the analysis reported in a research letter to JAMA Internal Medicine.
“Efforts targeting urgent care centers are urgently needed,” wrote Danielle L. Palms, MPH, of the Centers for Disease Control and Prevention, Atlanta, and her coauthors.
The retrospective study by Ms. Palms and her colleagues included claims from 2014 in a database of individuals 65 years of age or younger with employer-sponsored insurance.
The researchers included encounters in which medical and prescription coverage data were captured, including approximately 2.7 million urgent care center visits, 58,000 retail clinic visits, 4.8 million emergency department visits, and 148.5 million medical office visits.[[{"fid":"194045","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"1"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"}}}]]
They found antibiotic prescriptions linked to 39.0% of urgent care and 36.4% of retail clinic visits, compared with 13.8% of emergency department visits and 7.1% of medical office visits.
For respiratory diagnoses where antibiotics would be inappropriate, such as viral upper respiratory infections, antibiotics were nevertheless prescribed in 45.7% of urgent care visits, compared with 24.6% of emergency department, 17.0% of medical office visits, and 14.4% of retail clinic visits.
Those data show “substantial variability” that suggests case mix differences and evidence of antibiotic overuse, particularly in the urgent care setting, the researchers said in their letter.
In another recent study, looking at the 2010-2011 period, at least 30% of antibiotic prescriptions written in U.S. physician offices and emergency departments were unnecessary.
“The finding of the present study that antibiotic prescribing for antibiotic inappropriate respiratory diagnoses was highest in urgent care centers suggests that unnecessary antibiotic prescribing nationally in all outpatient settings may be higher than the estimated 30%,” wrote Ms. Palms and her coinvestigators.
The research was funded by the Centers for Disease Control and Prevention. Ms. Palms and her coauthors reported no conflicts of interest.
SOURCE: Palms DL et al. JAMA Intern Med. 2018 Jul 16.
This study suggests urgent care and retail clinics are “underrecognized” contributors to the ongoing problem of inappropriate antibiotic prescribing, according to authors of an invited commentary.
The urgent care sector, a $15 billion business representing more than 10,000 U.S. high-volume clinics, is growing very rapidly due to convenient locations, same-day access to care, and lower out-of-pocket expenditures versus emergency departments, the authors said.
“Lowering barriers for an office visit to such a degree may prompt frequent visits for mild self-resolving illnesses that would be better treated with rest and symptom management at home,” they wrote.
Innovations such as telephone triage lines could help reduce inappropriate antibiotic prescribing, but might “conflict with the business model” of urgent care and retail clinics, they added.
“Unfortunately, we all pay – in increased insurance premiums and increased antibiotic resistance – from the overprescribing of antibiotics for upper respiratory tract infections,” they wrote.
Michael A. Incze, MD, MSEd, and Rita F. Redberg, MD, MSc, are with the department of medicine, University of California, San Francisco. Mitchell H. Katz, MD, is with New York City Health and Hospitals. These comments are based on their invited commentary appearing in JAMA Internal Medicine . All three authors reported having no conflicts of interest.
This study suggests urgent care and retail clinics are “underrecognized” contributors to the ongoing problem of inappropriate antibiotic prescribing, according to authors of an invited commentary.
The urgent care sector, a $15 billion business representing more than 10,000 U.S. high-volume clinics, is growing very rapidly due to convenient locations, same-day access to care, and lower out-of-pocket expenditures versus emergency departments, the authors said.
“Lowering barriers for an office visit to such a degree may prompt frequent visits for mild self-resolving illnesses that would be better treated with rest and symptom management at home,” they wrote.
Innovations such as telephone triage lines could help reduce inappropriate antibiotic prescribing, but might “conflict with the business model” of urgent care and retail clinics, they added.
“Unfortunately, we all pay – in increased insurance premiums and increased antibiotic resistance – from the overprescribing of antibiotics for upper respiratory tract infections,” they wrote.
Michael A. Incze, MD, MSEd, and Rita F. Redberg, MD, MSc, are with the department of medicine, University of California, San Francisco. Mitchell H. Katz, MD, is with New York City Health and Hospitals. These comments are based on their invited commentary appearing in JAMA Internal Medicine . All three authors reported having no conflicts of interest.
This study suggests urgent care and retail clinics are “underrecognized” contributors to the ongoing problem of inappropriate antibiotic prescribing, according to authors of an invited commentary.
The urgent care sector, a $15 billion business representing more than 10,000 U.S. high-volume clinics, is growing very rapidly due to convenient locations, same-day access to care, and lower out-of-pocket expenditures versus emergency departments, the authors said.
“Lowering barriers for an office visit to such a degree may prompt frequent visits for mild self-resolving illnesses that would be better treated with rest and symptom management at home,” they wrote.
Innovations such as telephone triage lines could help reduce inappropriate antibiotic prescribing, but might “conflict with the business model” of urgent care and retail clinics, they added.
“Unfortunately, we all pay – in increased insurance premiums and increased antibiotic resistance – from the overprescribing of antibiotics for upper respiratory tract infections,” they wrote.
Michael A. Incze, MD, MSEd, and Rita F. Redberg, MD, MSc, are with the department of medicine, University of California, San Francisco. Mitchell H. Katz, MD, is with New York City Health and Hospitals. These comments are based on their invited commentary appearing in JAMA Internal Medicine . All three authors reported having no conflicts of interest.
When it comes to inappropriate antibiotic prescribing, urgent care centers and retail clinics may have an outsized impact, a review of commercial insurance claims suggests.
Antibiotic prescription rates were at least twice as high in those settings, compared with emergency departments and medical office visits, according to the retrospective analysis.
The issue may be particularly pronounced in urgent care centers, based on this study, in which nearly half of visits for antibiotic-inappropriate respiratory diagnoses resulted in antibiotic prescribing.
Those findings suggest a need for “antibiotic stewardship interventions” to reduce unnecessary prescribing of antibiotics in ambulatory care settings, authors of the analysis reported in a research letter to JAMA Internal Medicine.
“Efforts targeting urgent care centers are urgently needed,” wrote Danielle L. Palms, MPH, of the Centers for Disease Control and Prevention, Atlanta, and her coauthors.
The retrospective study by Ms. Palms and her colleagues included claims from 2014 in a database of individuals 65 years of age or younger with employer-sponsored insurance.
The researchers included encounters in which medical and prescription coverage data were captured, including approximately 2.7 million urgent care center visits, 58,000 retail clinic visits, 4.8 million emergency department visits, and 148.5 million medical office visits.[[{"fid":"194045","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"1"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"}}}]]
They found antibiotic prescriptions linked to 39.0% of urgent care and 36.4% of retail clinic visits, compared with 13.8% of emergency department visits and 7.1% of medical office visits.
For respiratory diagnoses where antibiotics would be inappropriate, such as viral upper respiratory infections, antibiotics were nevertheless prescribed in 45.7% of urgent care visits, compared with 24.6% of emergency department, 17.0% of medical office visits, and 14.4% of retail clinic visits.
Those data show “substantial variability” that suggests case mix differences and evidence of antibiotic overuse, particularly in the urgent care setting, the researchers said in their letter.
In another recent study, looking at the 2010-2011 period, at least 30% of antibiotic prescriptions written in U.S. physician offices and emergency departments were unnecessary.
“The finding of the present study that antibiotic prescribing for antibiotic inappropriate respiratory diagnoses was highest in urgent care centers suggests that unnecessary antibiotic prescribing nationally in all outpatient settings may be higher than the estimated 30%,” wrote Ms. Palms and her coinvestigators.
The research was funded by the Centers for Disease Control and Prevention. Ms. Palms and her coauthors reported no conflicts of interest.
SOURCE: Palms DL et al. JAMA Intern Med. 2018 Jul 16.
When it comes to inappropriate antibiotic prescribing, urgent care centers and retail clinics may have an outsized impact, a review of commercial insurance claims suggests.
Antibiotic prescription rates were at least twice as high in those settings, compared with emergency departments and medical office visits, according to the retrospective analysis.
The issue may be particularly pronounced in urgent care centers, based on this study, in which nearly half of visits for antibiotic-inappropriate respiratory diagnoses resulted in antibiotic prescribing.
Those findings suggest a need for “antibiotic stewardship interventions” to reduce unnecessary prescribing of antibiotics in ambulatory care settings, authors of the analysis reported in a research letter to JAMA Internal Medicine.
“Efforts targeting urgent care centers are urgently needed,” wrote Danielle L. Palms, MPH, of the Centers for Disease Control and Prevention, Atlanta, and her coauthors.
The retrospective study by Ms. Palms and her colleagues included claims from 2014 in a database of individuals 65 years of age or younger with employer-sponsored insurance.
The researchers included encounters in which medical and prescription coverage data were captured, including approximately 2.7 million urgent care center visits, 58,000 retail clinic visits, 4.8 million emergency department visits, and 148.5 million medical office visits.[[{"fid":"194045","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"1"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"}}}]]
They found antibiotic prescriptions linked to 39.0% of urgent care and 36.4% of retail clinic visits, compared with 13.8% of emergency department visits and 7.1% of medical office visits.
For respiratory diagnoses where antibiotics would be inappropriate, such as viral upper respiratory infections, antibiotics were nevertheless prescribed in 45.7% of urgent care visits, compared with 24.6% of emergency department, 17.0% of medical office visits, and 14.4% of retail clinic visits.
Those data show “substantial variability” that suggests case mix differences and evidence of antibiotic overuse, particularly in the urgent care setting, the researchers said in their letter.
In another recent study, looking at the 2010-2011 period, at least 30% of antibiotic prescriptions written in U.S. physician offices and emergency departments were unnecessary.
“The finding of the present study that antibiotic prescribing for antibiotic inappropriate respiratory diagnoses was highest in urgent care centers suggests that unnecessary antibiotic prescribing nationally in all outpatient settings may be higher than the estimated 30%,” wrote Ms. Palms and her coinvestigators.
The research was funded by the Centers for Disease Control and Prevention. Ms. Palms and her coauthors reported no conflicts of interest.
SOURCE: Palms DL et al. JAMA Intern Med. 2018 Jul 16.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: For respiratory diagnoses where antibiotics would be inappropriate, such as viral upper respiratory infections, antibiotics were nevertheless prescribed in 45.7% of urgent care visits.
Study details: A retrospective cohort study including claims from 2014 in a database for individuals 65 years of age or younger with employer-sponsored insurance.
Disclosures: The research was funded by the Centers for Disease Control and Prevention. The authors reported no conflicts of interest.
Source: Palms DL et al. JAMA Intern Med. 2018 Jul 16.
Preventing suicide: What should clinicians do differently?
“Suicide rates are increasing,” Dr. Igor Galynker said, “and I believe they will continue to rise. These are deaths of despair, and despair is increasing in our society.”
That said, I listened with interest to the May 16 MDedge Psychcast, “Approach assesses imminent suicide risk,” an interview with Igor Galynker, MD, PhD, author of “The Suicidal Crisis” and director of the Galynker Suicide Research Laboratory at the Icahn School of Medicine at Mount Sinai in New York. In the podcast, Dr. Galynker talked about techniques for identifying those at risk for suicide among the patients psychiatrists see for evaluation and treatment.
“Using suicidal ideation as a risk factor is flawed,” he contended. “Asking about suicidal thoughts leaves us to miss 75% of people who go on to die by suicide.” Dr. Galynker noted that suicidal thoughts are often absent or not endorsed at all and clinicians should view other factors – such as the patient’s sense of being entrapped and the clinician’s own emotional responses to the patient – as more sensitive measures of elevated suicide risk.
This informative podcast left me with more questions, so I called Dr. Galynker. Suicide remains a rare phenomenon, and most psychiatrists will have limited experience with completed suicide during the course of a career. Dr. Galynker’s interest in suicide as an area of research began after he had a patient die the year after he finished residency training. Since then, he’s had one more patient suicide, and he’s aware of eight people who have died after leaving his care. “It can be devastating,” he said.
I wanted to know what psychiatrists should be doing differently after we have identified a patient at risk. While it seems obvious that a depressed patient should be treated for major depression, it also seems obvious that our interventions are imprecisely targeted and not fully successful.
We talked about the role of hospitalization in preventing suicide. Dr. Galynker has mixed opinions on this. He noted that suicide rates skyrocket in the time right after psychiatric hospitalization. “For women, the rate is 250 times higher at the time of hospital discharge; for men it’s 100 times higher. But hospitalization may help someone to survive a transitional period and to gather their support systems.”
Dr. Galynker noted that since the podcast in May aired, the Centers for Disease Control and Prevention published findings on suicide rates in the United States. He summarized some of the key points from the findings.
“Suicide rates were going down until 1999. From 2000 to 2006, suicide rates increased by 1% per year. From 2006 until 2016, rates have increased by 2% per year. Most people who die by suicide don’t have a diagnosis of a mental illness. And finally – and what has gone unnoticed – most people who die by suicide do not express suicidal intent. In fact, in that study, suicide intent was disclosed by less than a quarter of persons both with and without known mental health conditions.”
Dr. Galynker talked about safety plans and emphasized means restriction as ways to prevent suicide, including limiting access to firearms, placing netting under bridges, and providing medications in smaller containers.
“Suicidal ideation comes late; it may happen 15 minutes before a suicidal act or attempt. We need to alert people that there are certainly things that put them at risk, and we need to look at the drivers.
“Sometimes, people die for trivial reasons.” He noted instances where a susceptible person might attempt or complete suicide after an argument or perceived slight. Work is being done to look at outreach interventions to those at risk, including phone contacts and postcards.
“We don’t have a suicide-specific diagnosis ... and people die for other reasons besides mental illness. Final romantic rejection, terminal illness, and humiliating failures in business all place people at elevated risk. We need to work to change the suicidal narrative for people away from one where life has no future; we need to help them open doors.”
Dr. Miller is the coauthor of “Committed: The Battle Over Involuntary Psychiatric Care,” (Baltimore: Johns Hopkins University Press, 2016).
“Suicide rates are increasing,” Dr. Igor Galynker said, “and I believe they will continue to rise. These are deaths of despair, and despair is increasing in our society.”
That said, I listened with interest to the May 16 MDedge Psychcast, “Approach assesses imminent suicide risk,” an interview with Igor Galynker, MD, PhD, author of “The Suicidal Crisis” and director of the Galynker Suicide Research Laboratory at the Icahn School of Medicine at Mount Sinai in New York. In the podcast, Dr. Galynker talked about techniques for identifying those at risk for suicide among the patients psychiatrists see for evaluation and treatment.
“Using suicidal ideation as a risk factor is flawed,” he contended. “Asking about suicidal thoughts leaves us to miss 75% of people who go on to die by suicide.” Dr. Galynker noted that suicidal thoughts are often absent or not endorsed at all and clinicians should view other factors – such as the patient’s sense of being entrapped and the clinician’s own emotional responses to the patient – as more sensitive measures of elevated suicide risk.
This informative podcast left me with more questions, so I called Dr. Galynker. Suicide remains a rare phenomenon, and most psychiatrists will have limited experience with completed suicide during the course of a career. Dr. Galynker’s interest in suicide as an area of research began after he had a patient die the year after he finished residency training. Since then, he’s had one more patient suicide, and he’s aware of eight people who have died after leaving his care. “It can be devastating,” he said.
I wanted to know what psychiatrists should be doing differently after we have identified a patient at risk. While it seems obvious that a depressed patient should be treated for major depression, it also seems obvious that our interventions are imprecisely targeted and not fully successful.
We talked about the role of hospitalization in preventing suicide. Dr. Galynker has mixed opinions on this. He noted that suicide rates skyrocket in the time right after psychiatric hospitalization. “For women, the rate is 250 times higher at the time of hospital discharge; for men it’s 100 times higher. But hospitalization may help someone to survive a transitional period and to gather their support systems.”
Dr. Galynker noted that since the podcast in May aired, the Centers for Disease Control and Prevention published findings on suicide rates in the United States. He summarized some of the key points from the findings.
“Suicide rates were going down until 1999. From 2000 to 2006, suicide rates increased by 1% per year. From 2006 until 2016, rates have increased by 2% per year. Most people who die by suicide don’t have a diagnosis of a mental illness. And finally – and what has gone unnoticed – most people who die by suicide do not express suicidal intent. In fact, in that study, suicide intent was disclosed by less than a quarter of persons both with and without known mental health conditions.”
Dr. Galynker talked about safety plans and emphasized means restriction as ways to prevent suicide, including limiting access to firearms, placing netting under bridges, and providing medications in smaller containers.
“Suicidal ideation comes late; it may happen 15 minutes before a suicidal act or attempt. We need to alert people that there are certainly things that put them at risk, and we need to look at the drivers.
“Sometimes, people die for trivial reasons.” He noted instances where a susceptible person might attempt or complete suicide after an argument or perceived slight. Work is being done to look at outreach interventions to those at risk, including phone contacts and postcards.
“We don’t have a suicide-specific diagnosis ... and people die for other reasons besides mental illness. Final romantic rejection, terminal illness, and humiliating failures in business all place people at elevated risk. We need to work to change the suicidal narrative for people away from one where life has no future; we need to help them open doors.”
Dr. Miller is the coauthor of “Committed: The Battle Over Involuntary Psychiatric Care,” (Baltimore: Johns Hopkins University Press, 2016).
“Suicide rates are increasing,” Dr. Igor Galynker said, “and I believe they will continue to rise. These are deaths of despair, and despair is increasing in our society.”
That said, I listened with interest to the May 16 MDedge Psychcast, “Approach assesses imminent suicide risk,” an interview with Igor Galynker, MD, PhD, author of “The Suicidal Crisis” and director of the Galynker Suicide Research Laboratory at the Icahn School of Medicine at Mount Sinai in New York. In the podcast, Dr. Galynker talked about techniques for identifying those at risk for suicide among the patients psychiatrists see for evaluation and treatment.
“Using suicidal ideation as a risk factor is flawed,” he contended. “Asking about suicidal thoughts leaves us to miss 75% of people who go on to die by suicide.” Dr. Galynker noted that suicidal thoughts are often absent or not endorsed at all and clinicians should view other factors – such as the patient’s sense of being entrapped and the clinician’s own emotional responses to the patient – as more sensitive measures of elevated suicide risk.
This informative podcast left me with more questions, so I called Dr. Galynker. Suicide remains a rare phenomenon, and most psychiatrists will have limited experience with completed suicide during the course of a career. Dr. Galynker’s interest in suicide as an area of research began after he had a patient die the year after he finished residency training. Since then, he’s had one more patient suicide, and he’s aware of eight people who have died after leaving his care. “It can be devastating,” he said.
I wanted to know what psychiatrists should be doing differently after we have identified a patient at risk. While it seems obvious that a depressed patient should be treated for major depression, it also seems obvious that our interventions are imprecisely targeted and not fully successful.
We talked about the role of hospitalization in preventing suicide. Dr. Galynker has mixed opinions on this. He noted that suicide rates skyrocket in the time right after psychiatric hospitalization. “For women, the rate is 250 times higher at the time of hospital discharge; for men it’s 100 times higher. But hospitalization may help someone to survive a transitional period and to gather their support systems.”
Dr. Galynker noted that since the podcast in May aired, the Centers for Disease Control and Prevention published findings on suicide rates in the United States. He summarized some of the key points from the findings.
“Suicide rates were going down until 1999. From 2000 to 2006, suicide rates increased by 1% per year. From 2006 until 2016, rates have increased by 2% per year. Most people who die by suicide don’t have a diagnosis of a mental illness. And finally – and what has gone unnoticed – most people who die by suicide do not express suicidal intent. In fact, in that study, suicide intent was disclosed by less than a quarter of persons both with and without known mental health conditions.”
Dr. Galynker talked about safety plans and emphasized means restriction as ways to prevent suicide, including limiting access to firearms, placing netting under bridges, and providing medications in smaller containers.
“Suicidal ideation comes late; it may happen 15 minutes before a suicidal act or attempt. We need to alert people that there are certainly things that put them at risk, and we need to look at the drivers.
“Sometimes, people die for trivial reasons.” He noted instances where a susceptible person might attempt or complete suicide after an argument or perceived slight. Work is being done to look at outreach interventions to those at risk, including phone contacts and postcards.
“We don’t have a suicide-specific diagnosis ... and people die for other reasons besides mental illness. Final romantic rejection, terminal illness, and humiliating failures in business all place people at elevated risk. We need to work to change the suicidal narrative for people away from one where life has no future; we need to help them open doors.”
Dr. Miller is the coauthor of “Committed: The Battle Over Involuntary Psychiatric Care,” (Baltimore: Johns Hopkins University Press, 2016).
Special care advised for HIV-infected patients with diabetes
ORLANDO – Research suggests that HIV-positive people who take the latest generations of AIDS medications are living almost as long as everyone else. But they still face special medical challenges, and an endocrinologist urged colleagues to adjust their approaches to diabetes in these patients.
said Todd T. Brown, MD, PhD, of Johns Hopkins Medicine, Baltimore, in a presentation at the annual scientific sessions of the American Diabetes Association.
It’s not just a matter of subbing in an alternate drug here or there. When it comes to diabetes, patients with HIV require significant adjustments to diagnosis and treatment, Dr. Brown said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In terms of diagnosis, treatment guidelines approved by the Infectious Diseases Society of America and ADA recommend that all HIV-positive patients be tested for diabetes before they begin taking antiretroviral therapy. Then, the guidelines suggest, they should be tested 4-6 weeks after initiation of therapy, and every 6-12 months going forward.
“It’s a bit of overkill to go every 6 months,” said Dr. Brown, who prefers an annual testing approach. He added that research has suggested that the 2-hour postload glucose test is more sensitive than the fasting glucose test in some HIV-positive populations. However, he believes that it’s generally fine to give a fasting glucose test before initiation of therapy – and on an annual basis afterward – rather than the more cumbersome postload test.
Still, he said, the postload test may be appropriate in a patient with impaired glucose tolerance “if you really want to make the diagnosis, and especially if you’ll change your treatment based on it.”
Ongoing treatment of HIV-positive patients also presents unique challenges, he said. For one, antiretroviral therapy seems to affect glucose metabolism and body fat, he said, and findings from a 2016 study suggest HIV-positive people who begin antiretroviral therapy face a higher risk of developing diabetes after weight gain (J Acquir Immune Defic Syndr. 2016 Oct 1;73[2]:228-36).
One option is to switch patients to integrase inhibitors, but findings from a 2017 study suggested that this may also lead to more weight gain, Dr. Brown said.
“This has been an evolving story,” he said. “The clinical consequences of this are unclear. This is a topic that’s being hotly investigated now in the HIV health world” (JAIDS. 2017 Dec 15;76[5]:527-31).
As for other diabetes management issues, Dr. Brown noted that hemoglobin A1c tests appear to underestimate glycemia in HIV-infected patients. He suggested that goal HbA1c levels should be lower in diabetic patients with HIV, especially those with CD4+ counts under 500 cells /mm3 and/or mean cell volume over 100 fL.
Research suggests that lifestyle changes seem to work well in HIV-positive patients, he said, and metformin is the ideal first-line drug treatment just as in the HIV-negative population. “It’s a good drug. We all love it,” he said. “It may improve lipohypertrophy and coronary plaque.”
He added that proteinuria and neuropathy are more common in HIV-positive patients with diabetes. He said levels of neuropathy and nephropathy could be related to AIDS drugs.
On the medication front, Dr. Brown cautioned about certain drugs in HIV-positive patients: The HIV drug dolutegravir increases metformin concentrations by about 80%, he said, and there are concerns about bone and cardiac health in HIV-positive patients who take the diabetes medications known as thiazolidinediones (glitazones).
He added that there are sparse data about the use of several types of diabetes drugs – DPP IV inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors – in HIV-positive patients.
Dr. Brown discloses consulting for Gilead Sciences, ViiV, BMS, Merck, Theratechnologies, and EMD Serono.
ORLANDO – Research suggests that HIV-positive people who take the latest generations of AIDS medications are living almost as long as everyone else. But they still face special medical challenges, and an endocrinologist urged colleagues to adjust their approaches to diabetes in these patients.
said Todd T. Brown, MD, PhD, of Johns Hopkins Medicine, Baltimore, in a presentation at the annual scientific sessions of the American Diabetes Association.
It’s not just a matter of subbing in an alternate drug here or there. When it comes to diabetes, patients with HIV require significant adjustments to diagnosis and treatment, Dr. Brown said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In terms of diagnosis, treatment guidelines approved by the Infectious Diseases Society of America and ADA recommend that all HIV-positive patients be tested for diabetes before they begin taking antiretroviral therapy. Then, the guidelines suggest, they should be tested 4-6 weeks after initiation of therapy, and every 6-12 months going forward.
“It’s a bit of overkill to go every 6 months,” said Dr. Brown, who prefers an annual testing approach. He added that research has suggested that the 2-hour postload glucose test is more sensitive than the fasting glucose test in some HIV-positive populations. However, he believes that it’s generally fine to give a fasting glucose test before initiation of therapy – and on an annual basis afterward – rather than the more cumbersome postload test.
Still, he said, the postload test may be appropriate in a patient with impaired glucose tolerance “if you really want to make the diagnosis, and especially if you’ll change your treatment based on it.”
Ongoing treatment of HIV-positive patients also presents unique challenges, he said. For one, antiretroviral therapy seems to affect glucose metabolism and body fat, he said, and findings from a 2016 study suggest HIV-positive people who begin antiretroviral therapy face a higher risk of developing diabetes after weight gain (J Acquir Immune Defic Syndr. 2016 Oct 1;73[2]:228-36).
One option is to switch patients to integrase inhibitors, but findings from a 2017 study suggested that this may also lead to more weight gain, Dr. Brown said.
“This has been an evolving story,” he said. “The clinical consequences of this are unclear. This is a topic that’s being hotly investigated now in the HIV health world” (JAIDS. 2017 Dec 15;76[5]:527-31).
As for other diabetes management issues, Dr. Brown noted that hemoglobin A1c tests appear to underestimate glycemia in HIV-infected patients. He suggested that goal HbA1c levels should be lower in diabetic patients with HIV, especially those with CD4+ counts under 500 cells /mm3 and/or mean cell volume over 100 fL.
Research suggests that lifestyle changes seem to work well in HIV-positive patients, he said, and metformin is the ideal first-line drug treatment just as in the HIV-negative population. “It’s a good drug. We all love it,” he said. “It may improve lipohypertrophy and coronary plaque.”
He added that proteinuria and neuropathy are more common in HIV-positive patients with diabetes. He said levels of neuropathy and nephropathy could be related to AIDS drugs.
On the medication front, Dr. Brown cautioned about certain drugs in HIV-positive patients: The HIV drug dolutegravir increases metformin concentrations by about 80%, he said, and there are concerns about bone and cardiac health in HIV-positive patients who take the diabetes medications known as thiazolidinediones (glitazones).
He added that there are sparse data about the use of several types of diabetes drugs – DPP IV inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors – in HIV-positive patients.
Dr. Brown discloses consulting for Gilead Sciences, ViiV, BMS, Merck, Theratechnologies, and EMD Serono.
ORLANDO – Research suggests that HIV-positive people who take the latest generations of AIDS medications are living almost as long as everyone else. But they still face special medical challenges, and an endocrinologist urged colleagues to adjust their approaches to diabetes in these patients.
said Todd T. Brown, MD, PhD, of Johns Hopkins Medicine, Baltimore, in a presentation at the annual scientific sessions of the American Diabetes Association.
It’s not just a matter of subbing in an alternate drug here or there. When it comes to diabetes, patients with HIV require significant adjustments to diagnosis and treatment, Dr. Brown said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In terms of diagnosis, treatment guidelines approved by the Infectious Diseases Society of America and ADA recommend that all HIV-positive patients be tested for diabetes before they begin taking antiretroviral therapy. Then, the guidelines suggest, they should be tested 4-6 weeks after initiation of therapy, and every 6-12 months going forward.
“It’s a bit of overkill to go every 6 months,” said Dr. Brown, who prefers an annual testing approach. He added that research has suggested that the 2-hour postload glucose test is more sensitive than the fasting glucose test in some HIV-positive populations. However, he believes that it’s generally fine to give a fasting glucose test before initiation of therapy – and on an annual basis afterward – rather than the more cumbersome postload test.
Still, he said, the postload test may be appropriate in a patient with impaired glucose tolerance “if you really want to make the diagnosis, and especially if you’ll change your treatment based on it.”
Ongoing treatment of HIV-positive patients also presents unique challenges, he said. For one, antiretroviral therapy seems to affect glucose metabolism and body fat, he said, and findings from a 2016 study suggest HIV-positive people who begin antiretroviral therapy face a higher risk of developing diabetes after weight gain (J Acquir Immune Defic Syndr. 2016 Oct 1;73[2]:228-36).
One option is to switch patients to integrase inhibitors, but findings from a 2017 study suggested that this may also lead to more weight gain, Dr. Brown said.
“This has been an evolving story,” he said. “The clinical consequences of this are unclear. This is a topic that’s being hotly investigated now in the HIV health world” (JAIDS. 2017 Dec 15;76[5]:527-31).
As for other diabetes management issues, Dr. Brown noted that hemoglobin A1c tests appear to underestimate glycemia in HIV-infected patients. He suggested that goal HbA1c levels should be lower in diabetic patients with HIV, especially those with CD4+ counts under 500 cells /mm3 and/or mean cell volume over 100 fL.
Research suggests that lifestyle changes seem to work well in HIV-positive patients, he said, and metformin is the ideal first-line drug treatment just as in the HIV-negative population. “It’s a good drug. We all love it,” he said. “It may improve lipohypertrophy and coronary plaque.”
He added that proteinuria and neuropathy are more common in HIV-positive patients with diabetes. He said levels of neuropathy and nephropathy could be related to AIDS drugs.
On the medication front, Dr. Brown cautioned about certain drugs in HIV-positive patients: The HIV drug dolutegravir increases metformin concentrations by about 80%, he said, and there are concerns about bone and cardiac health in HIV-positive patients who take the diabetes medications known as thiazolidinediones (glitazones).
He added that there are sparse data about the use of several types of diabetes drugs – DPP IV inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors – in HIV-positive patients.
Dr. Brown discloses consulting for Gilead Sciences, ViiV, BMS, Merck, Theratechnologies, and EMD Serono.
EXPERT ANALYSIS FROM ADA 2018
Newer cholangioscopy system safe and effective for difficult biliary stones
“D-SOC with either [electrohydraulic lithotripsy or laser lithotripsy] represents an efficient, highly valuable, safe and minimally invasive alternative to percutaneous or surgical approaches in the management of difficult biliary stones,” reported Olaya I. Brewer Gutierrez, MD, of Johns Hopkins Hospital, Baltimore, and her colleagues.
The study, published in Clinical Gastroenterology and Hepatology, involved 22 tertiary centers in the United States, the United Kingdom, and Korea. Patients had difficult biliary stones, defined as stones greater than 15 mm in size, multiple (more than three) stones, intrahepatic duct/cystic duct stones, impacted stones, and stones associated with Mirizzi syndrome or a difficult biliary anatomy such as stricture below the stone or duodenal diverticula.
Most patients (85.7%) had a prior failed treatment with endoscopic retrograde cholangiopancreatography (ERCP) for stone clearance at outside hospitals, with biliary sphincterotomy and the use of an extraction balloon attempted in 62.2% and 73.2% of these cases, respectively.
Of the 407 study patients, 306 (75.2%) were treated with electrohydraulic lithotripsy (EHL), and 101 (24.8%) were treated with laser lithotripsy (LL). Complete ductal clearance was achieved in 396 (97.3%) of patients with a median of one lithotripsy session (range of one to four sessions), and in 77.4% of patients in a single session. Stone clearance rates and procedure outcomes were similar between the two approaches, but the mean procedure time was significantly longer with EHL than with LL (74 vs. 50 minutes).
Eleven (2.7%) of the 407 patients failed EHL/LL and were treated either with surgery, extracorporeal shock-wave lithotripsy, or both. Adverse events were observed in 15 patients (3.7%), with cholangitis and abdominal pain most commonly reported.
In addition to evaluating the technical success of D-SOC for difficult bile duct stones and comparing the effectiveness of EHL and LL, the investigators looked for predictors of outcomes. On multivariate analysis, they found difficult anatomy/cannulation was associated with incomplete ductal clearance, and the duration of the procedure was a predictor of the need for more than one D-SOC.
The introduction of D-SOC several years ago has “improved the ease of SOC and markedly improved the image quality,” Dr. Gutierrez and her colleagues wrote. “These [improvements] have led to greater use of EHL or LL as part of the ERCP armamentarium to attempt stone clearance in difficult biliary or pancreatic stones.”
Still, given the retrospective nature of the study and subsequent selection bias, randomized controlled trials comparing D-SOC and EHL/LL with conventional techniques for stone extraction are needed, they say.
In an accompanying editorial, Dennis Yang, MD, of the University of Florida, and Jonathan M. Buscaglia, MD, AGAF, of State University of New York at Stony Brook commended the study for critically evaluating a new technology, but said that “it still remains unclear in whom D-SOC with EHL or LL should be considered over conventional ERCP with stone extraction techniques.”
Although most patients in the study had a prior failed ERCP, conventional stone extraction techniques such as endoscopic sphincterotomy with endoscopic papillary large balloon dilation (EPLBD) or mechanical lithotripsy were attempted in less than a quarter of the cases before D-SOC, they wrote. “In the era of high-cost health care, it is important that proven and effective measures for the treatment of stones ... be used adequately before moving up the ladder to cholangioscopy-based techniques.”
Eleven of the study authors reported serving as consultants for Boston Scientific, which manufactures the D-SOC system used at the study sites, and two of the investigators reported serving as speakers for the company. Dr. Gutierrez reported no conflicts of interest.
SOURCE: Brewer Gutierrez OI et al. Clin Gastroenterol Hepatol. 2018;16:918-26.
“D-SOC with either [electrohydraulic lithotripsy or laser lithotripsy] represents an efficient, highly valuable, safe and minimally invasive alternative to percutaneous or surgical approaches in the management of difficult biliary stones,” reported Olaya I. Brewer Gutierrez, MD, of Johns Hopkins Hospital, Baltimore, and her colleagues.
The study, published in Clinical Gastroenterology and Hepatology, involved 22 tertiary centers in the United States, the United Kingdom, and Korea. Patients had difficult biliary stones, defined as stones greater than 15 mm in size, multiple (more than three) stones, intrahepatic duct/cystic duct stones, impacted stones, and stones associated with Mirizzi syndrome or a difficult biliary anatomy such as stricture below the stone or duodenal diverticula.
Most patients (85.7%) had a prior failed treatment with endoscopic retrograde cholangiopancreatography (ERCP) for stone clearance at outside hospitals, with biliary sphincterotomy and the use of an extraction balloon attempted in 62.2% and 73.2% of these cases, respectively.
Of the 407 study patients, 306 (75.2%) were treated with electrohydraulic lithotripsy (EHL), and 101 (24.8%) were treated with laser lithotripsy (LL). Complete ductal clearance was achieved in 396 (97.3%) of patients with a median of one lithotripsy session (range of one to four sessions), and in 77.4% of patients in a single session. Stone clearance rates and procedure outcomes were similar between the two approaches, but the mean procedure time was significantly longer with EHL than with LL (74 vs. 50 minutes).
Eleven (2.7%) of the 407 patients failed EHL/LL and were treated either with surgery, extracorporeal shock-wave lithotripsy, or both. Adverse events were observed in 15 patients (3.7%), with cholangitis and abdominal pain most commonly reported.
In addition to evaluating the technical success of D-SOC for difficult bile duct stones and comparing the effectiveness of EHL and LL, the investigators looked for predictors of outcomes. On multivariate analysis, they found difficult anatomy/cannulation was associated with incomplete ductal clearance, and the duration of the procedure was a predictor of the need for more than one D-SOC.
The introduction of D-SOC several years ago has “improved the ease of SOC and markedly improved the image quality,” Dr. Gutierrez and her colleagues wrote. “These [improvements] have led to greater use of EHL or LL as part of the ERCP armamentarium to attempt stone clearance in difficult biliary or pancreatic stones.”
Still, given the retrospective nature of the study and subsequent selection bias, randomized controlled trials comparing D-SOC and EHL/LL with conventional techniques for stone extraction are needed, they say.
In an accompanying editorial, Dennis Yang, MD, of the University of Florida, and Jonathan M. Buscaglia, MD, AGAF, of State University of New York at Stony Brook commended the study for critically evaluating a new technology, but said that “it still remains unclear in whom D-SOC with EHL or LL should be considered over conventional ERCP with stone extraction techniques.”
Although most patients in the study had a prior failed ERCP, conventional stone extraction techniques such as endoscopic sphincterotomy with endoscopic papillary large balloon dilation (EPLBD) or mechanical lithotripsy were attempted in less than a quarter of the cases before D-SOC, they wrote. “In the era of high-cost health care, it is important that proven and effective measures for the treatment of stones ... be used adequately before moving up the ladder to cholangioscopy-based techniques.”
Eleven of the study authors reported serving as consultants for Boston Scientific, which manufactures the D-SOC system used at the study sites, and two of the investigators reported serving as speakers for the company. Dr. Gutierrez reported no conflicts of interest.
SOURCE: Brewer Gutierrez OI et al. Clin Gastroenterol Hepatol. 2018;16:918-26.
“D-SOC with either [electrohydraulic lithotripsy or laser lithotripsy] represents an efficient, highly valuable, safe and minimally invasive alternative to percutaneous or surgical approaches in the management of difficult biliary stones,” reported Olaya I. Brewer Gutierrez, MD, of Johns Hopkins Hospital, Baltimore, and her colleagues.
The study, published in Clinical Gastroenterology and Hepatology, involved 22 tertiary centers in the United States, the United Kingdom, and Korea. Patients had difficult biliary stones, defined as stones greater than 15 mm in size, multiple (more than three) stones, intrahepatic duct/cystic duct stones, impacted stones, and stones associated with Mirizzi syndrome or a difficult biliary anatomy such as stricture below the stone or duodenal diverticula.
Most patients (85.7%) had a prior failed treatment with endoscopic retrograde cholangiopancreatography (ERCP) for stone clearance at outside hospitals, with biliary sphincterotomy and the use of an extraction balloon attempted in 62.2% and 73.2% of these cases, respectively.
Of the 407 study patients, 306 (75.2%) were treated with electrohydraulic lithotripsy (EHL), and 101 (24.8%) were treated with laser lithotripsy (LL). Complete ductal clearance was achieved in 396 (97.3%) of patients with a median of one lithotripsy session (range of one to four sessions), and in 77.4% of patients in a single session. Stone clearance rates and procedure outcomes were similar between the two approaches, but the mean procedure time was significantly longer with EHL than with LL (74 vs. 50 minutes).
Eleven (2.7%) of the 407 patients failed EHL/LL and were treated either with surgery, extracorporeal shock-wave lithotripsy, or both. Adverse events were observed in 15 patients (3.7%), with cholangitis and abdominal pain most commonly reported.
In addition to evaluating the technical success of D-SOC for difficult bile duct stones and comparing the effectiveness of EHL and LL, the investigators looked for predictors of outcomes. On multivariate analysis, they found difficult anatomy/cannulation was associated with incomplete ductal clearance, and the duration of the procedure was a predictor of the need for more than one D-SOC.
The introduction of D-SOC several years ago has “improved the ease of SOC and markedly improved the image quality,” Dr. Gutierrez and her colleagues wrote. “These [improvements] have led to greater use of EHL or LL as part of the ERCP armamentarium to attempt stone clearance in difficult biliary or pancreatic stones.”
Still, given the retrospective nature of the study and subsequent selection bias, randomized controlled trials comparing D-SOC and EHL/LL with conventional techniques for stone extraction are needed, they say.
In an accompanying editorial, Dennis Yang, MD, of the University of Florida, and Jonathan M. Buscaglia, MD, AGAF, of State University of New York at Stony Brook commended the study for critically evaluating a new technology, but said that “it still remains unclear in whom D-SOC with EHL or LL should be considered over conventional ERCP with stone extraction techniques.”
Although most patients in the study had a prior failed ERCP, conventional stone extraction techniques such as endoscopic sphincterotomy with endoscopic papillary large balloon dilation (EPLBD) or mechanical lithotripsy were attempted in less than a quarter of the cases before D-SOC, they wrote. “In the era of high-cost health care, it is important that proven and effective measures for the treatment of stones ... be used adequately before moving up the ladder to cholangioscopy-based techniques.”
Eleven of the study authors reported serving as consultants for Boston Scientific, which manufactures the D-SOC system used at the study sites, and two of the investigators reported serving as speakers for the company. Dr. Gutierrez reported no conflicts of interest.
SOURCE: Brewer Gutierrez OI et al. Clin Gastroenterol Hepatol. 2018;16:918-26.
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Digital single-operator cholangioscopy (D-SOC) is effective and safe for difficult biliary stones
Major finding: More than 95% of 407 consecutive patients with difficult bile duct stones had complete bile duct clearance with the use of D-SOC with electrohydraulic or laser lithotripsy.
Study details: International, multicenter, retrospective analysis.
Disclosures: Eleven of the study authors reported serving as consultants for Boston Scientific, which manufactures the D-SOC system used at the study sites, and two of the investigators reported serving as speakers for the company. Dr. Gutierrez reported no conflicts of interest.
Source: Brewer Gutierrez OI et al. Clin Gastroenterol Hepatol. 2018;16:918-26.
Registration Open for SVS Coding Course
Learn all about coding at the SVS Coding & Reimbursement Workshop, set for Oct. 19 and 20 at the Renaissance Hotel in downtown Chicago. The intensive two-day program will address 2019 updates and proposed updates, the global surgical package and how it affects billing and reimbursement and applying modifiers for streamlined reimbursement. It is designed for vascular surgeons and their support staff. Learn more, see pricing and register here.
Learn all about coding at the SVS Coding & Reimbursement Workshop, set for Oct. 19 and 20 at the Renaissance Hotel in downtown Chicago. The intensive two-day program will address 2019 updates and proposed updates, the global surgical package and how it affects billing and reimbursement and applying modifiers for streamlined reimbursement. It is designed for vascular surgeons and their support staff. Learn more, see pricing and register here.
Learn all about coding at the SVS Coding & Reimbursement Workshop, set for Oct. 19 and 20 at the Renaissance Hotel in downtown Chicago. The intensive two-day program will address 2019 updates and proposed updates, the global surgical package and how it affects billing and reimbursement and applying modifiers for streamlined reimbursement. It is designed for vascular surgeons and their support staff. Learn more, see pricing and register here.
Travel Award Deadline is Aug. 15
The deadline to submit applications for the SVS Foundation Research Career Development Travel Award is Aug. 15. This award aims to develop strong leaders in vascular surgery. Awardees are assigned an SVS research mentor and are provided with funds to attend an establish research career development course.
The deadline to submit applications for the SVS Foundation Research Career Development Travel Award is Aug. 15. This award aims to develop strong leaders in vascular surgery. Awardees are assigned an SVS research mentor and are provided with funds to attend an establish research career development course.
The deadline to submit applications for the SVS Foundation Research Career Development Travel Award is Aug. 15. This award aims to develop strong leaders in vascular surgery. Awardees are assigned an SVS research mentor and are provided with funds to attend an establish research career development course.
International Scholars Program Applications Due Aug. 2
Applications are due by Aug. 2 for the SVS International Scholars Program, which provides up to four scholarships to qualified young vascular surgeons from countries other than the United States or Canada. Awardees receive $5,000 each, to attend the 2019 Vascular Annual Meeting and to visit clinical, teaching and research facilities in the U.S. and Canada.
Applications are due by Aug. 2 for the SVS International Scholars Program, which provides up to four scholarships to qualified young vascular surgeons from countries other than the United States or Canada. Awardees receive $5,000 each, to attend the 2019 Vascular Annual Meeting and to visit clinical, teaching and research facilities in the U.S. and Canada.
Applications are due by Aug. 2 for the SVS International Scholars Program, which provides up to four scholarships to qualified young vascular surgeons from countries other than the United States or Canada. Awardees receive $5,000 each, to attend the 2019 Vascular Annual Meeting and to visit clinical, teaching and research facilities in the U.S. and Canada.
Debunking Atopic Dermatitis Myths: Can Adults Develop Eczema?
Myth: Atopic Dermatitis Does Not Start in Adulthood
Atopic dermatitis (AD) typically first appears in childhood and tends to disappear before puberty begins; however, some patients experience AD that persists into adulthood or occurs de novo. Bannister and Freeman coined the term adult-onset atopic dermatitis after reviewing 2604 cases of AD and noting that 243 patients (9%) were first diagnosed with AD at 20 years of age or older. Adult-onset AD may be its own subset of AD or childhood AD that was simply not diagnosed until adulthood or was forgotten by the patient.
Characteristically, AD presents in adults as inflammatory eczema with areas of lichenification. It could occur after a change in residence to a cold dry climate or exposure to central heating, as patients who grew up in warm, sunny, humid climates might not have had diagnosable AD in childhood or adolescence. The more common forms of adult-onset AD are hand and neck dermatitis, hand eczema, nummular eczema, or prurigo, while childhood AD often manifests in a flexural distribution. Because it is difficult to detect, adult-onset AD is diagnosed after ruling out other diseases. Diagnostic procedures, such as patch tests, skin prick tests, biopsies, or blood screenings, usually are necessary to rule out other diseases or types of eczema. Contact eczema is the first diagnostic sign of AD in adults.
Maintaining AD in the differential diagnosis for patients with clinical symptoms of pruritus and eczema is essential due to the quality of life impact of the condition. Sleep disturbance is common in adults with severe AD and treatment may help to improve sleep quality.
Hanifin suggested the following when assessing adults for AD:
- Verify diagnosis (not allergic contact dermatitis or psoriasis)
- Determine patient's history of allergies (eg, food allergy) or childhood eczema
- Obtain family history of eczema/allergies
- Evaluate if patient's occupation may impact condition (eg, contact with irritants or known contact allergens)
- Inquire about patient's childhood residence (eg, tropical climate)
Adult-onset AD is a recalcitrant condition that can be difficult to treat, and appropriately labeling/diagnosing the condition will lead to better management.
Expert Commentary
Whereas once it was the rite of the pediatric AD patient to outgrow disease, it has now become clear that resolution is not as hard a stop in atopic disease as expected. Adult-onset and persistent disease in AD is clearly a significant problem, especially in developed nations and carries a host of comorbidites. Time and enhanced research will hopefully identify interventions to reverse the trend towards persistence into adulthood.
—Nanette B. Silverberg, MD (New York, New York)
Bannister MJ, Freeman S. Adult-onset atopic dermatitis. Australas J Dermatol. 2000;41:225-228.
Hanifin JM. Adult-onset atopic dermatitis: fact or fancy? Dermatol Clin. 2017;35:299-302.
Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27:78-88.
Myth: Atopic Dermatitis Does Not Start in Adulthood
Atopic dermatitis (AD) typically first appears in childhood and tends to disappear before puberty begins; however, some patients experience AD that persists into adulthood or occurs de novo. Bannister and Freeman coined the term adult-onset atopic dermatitis after reviewing 2604 cases of AD and noting that 243 patients (9%) were first diagnosed with AD at 20 years of age or older. Adult-onset AD may be its own subset of AD or childhood AD that was simply not diagnosed until adulthood or was forgotten by the patient.
Characteristically, AD presents in adults as inflammatory eczema with areas of lichenification. It could occur after a change in residence to a cold dry climate or exposure to central heating, as patients who grew up in warm, sunny, humid climates might not have had diagnosable AD in childhood or adolescence. The more common forms of adult-onset AD are hand and neck dermatitis, hand eczema, nummular eczema, or prurigo, while childhood AD often manifests in a flexural distribution. Because it is difficult to detect, adult-onset AD is diagnosed after ruling out other diseases. Diagnostic procedures, such as patch tests, skin prick tests, biopsies, or blood screenings, usually are necessary to rule out other diseases or types of eczema. Contact eczema is the first diagnostic sign of AD in adults.
Maintaining AD in the differential diagnosis for patients with clinical symptoms of pruritus and eczema is essential due to the quality of life impact of the condition. Sleep disturbance is common in adults with severe AD and treatment may help to improve sleep quality.
Hanifin suggested the following when assessing adults for AD:
- Verify diagnosis (not allergic contact dermatitis or psoriasis)
- Determine patient's history of allergies (eg, food allergy) or childhood eczema
- Obtain family history of eczema/allergies
- Evaluate if patient's occupation may impact condition (eg, contact with irritants or known contact allergens)
- Inquire about patient's childhood residence (eg, tropical climate)
Adult-onset AD is a recalcitrant condition that can be difficult to treat, and appropriately labeling/diagnosing the condition will lead to better management.
Expert Commentary
Whereas once it was the rite of the pediatric AD patient to outgrow disease, it has now become clear that resolution is not as hard a stop in atopic disease as expected. Adult-onset and persistent disease in AD is clearly a significant problem, especially in developed nations and carries a host of comorbidites. Time and enhanced research will hopefully identify interventions to reverse the trend towards persistence into adulthood.
—Nanette B. Silverberg, MD (New York, New York)
Myth: Atopic Dermatitis Does Not Start in Adulthood
Atopic dermatitis (AD) typically first appears in childhood and tends to disappear before puberty begins; however, some patients experience AD that persists into adulthood or occurs de novo. Bannister and Freeman coined the term adult-onset atopic dermatitis after reviewing 2604 cases of AD and noting that 243 patients (9%) were first diagnosed with AD at 20 years of age or older. Adult-onset AD may be its own subset of AD or childhood AD that was simply not diagnosed until adulthood or was forgotten by the patient.
Characteristically, AD presents in adults as inflammatory eczema with areas of lichenification. It could occur after a change in residence to a cold dry climate or exposure to central heating, as patients who grew up in warm, sunny, humid climates might not have had diagnosable AD in childhood or adolescence. The more common forms of adult-onset AD are hand and neck dermatitis, hand eczema, nummular eczema, or prurigo, while childhood AD often manifests in a flexural distribution. Because it is difficult to detect, adult-onset AD is diagnosed after ruling out other diseases. Diagnostic procedures, such as patch tests, skin prick tests, biopsies, or blood screenings, usually are necessary to rule out other diseases or types of eczema. Contact eczema is the first diagnostic sign of AD in adults.
Maintaining AD in the differential diagnosis for patients with clinical symptoms of pruritus and eczema is essential due to the quality of life impact of the condition. Sleep disturbance is common in adults with severe AD and treatment may help to improve sleep quality.
Hanifin suggested the following when assessing adults for AD:
- Verify diagnosis (not allergic contact dermatitis or psoriasis)
- Determine patient's history of allergies (eg, food allergy) or childhood eczema
- Obtain family history of eczema/allergies
- Evaluate if patient's occupation may impact condition (eg, contact with irritants or known contact allergens)
- Inquire about patient's childhood residence (eg, tropical climate)
Adult-onset AD is a recalcitrant condition that can be difficult to treat, and appropriately labeling/diagnosing the condition will lead to better management.
Expert Commentary
Whereas once it was the rite of the pediatric AD patient to outgrow disease, it has now become clear that resolution is not as hard a stop in atopic disease as expected. Adult-onset and persistent disease in AD is clearly a significant problem, especially in developed nations and carries a host of comorbidites. Time and enhanced research will hopefully identify interventions to reverse the trend towards persistence into adulthood.
—Nanette B. Silverberg, MD (New York, New York)
Bannister MJ, Freeman S. Adult-onset atopic dermatitis. Australas J Dermatol. 2000;41:225-228.
Hanifin JM. Adult-onset atopic dermatitis: fact or fancy? Dermatol Clin. 2017;35:299-302.
Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27:78-88.
Bannister MJ, Freeman S. Adult-onset atopic dermatitis. Australas J Dermatol. 2000;41:225-228.
Hanifin JM. Adult-onset atopic dermatitis: fact or fancy? Dermatol Clin. 2017;35:299-302.
Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27:78-88.
A Peek at Our July 2018 Issue
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Are We Beating Cancer—Finally?
Cancer death rates continue to decline in the US in all major racial and ethnic groups, according to the National Cancer Institute’s (NCI) latest Annual Report to the Nation on the Status of Cancer. The data are an “encouraging indicator of progress” in cancer research, says NCI Director Ned Sharpless, MD. “It’s clear that interventions are having an impact.”
Overall incidence, or rates of new cancers, dropped by 1.8% in men and 1.4% in women from 1999 to 2015. Between 2011 and 2015, death rates dropped for 11 of the 18 most common cancer types in men and 14 of the 20 most common types in women. The researchers say the “significant declines” also hold “significant differences” in rate by sex, race, and ethnicity. For example, black men and white women had the highest incidence rates, and black men and black women had the highest death rates.
However, over the same period, death rates for cancers of the liver, pancreas, and brain and nervous system rose in both men and women. Death rates for cancer of the uterus rose (the researchers say obesity is thought to be a contributing factor) and death rates for cancers of the oral cavity and pharynx and soft tissue increased in men, perhaps associated with human papillomavirus infection.
In a companion study, when researchers explored prostate cancer trends in more detail they found overall prostate cancer incidence rates declined an average of 6.5% each year between 2007 and 2014, from 163 new cases per 100,000 men to 104 new cases. Still, after a 2-decade steady decline, rates leveled off. Incidence of distant disease rose from 7.8 new cases per 100,000 to 9.2, but there was no increase in the rates of cases with aggressive histologic grade.
Interestingly, the researchers also report a decline in recent prostate-specific antigen screening between 2010 and 2013 national surveys. “The increase in late-stage disease and the flattening of the mortality trended occurred contemporaneously with the observed decrease in PSA screening,” said Serban Negoita, MD, DrPH, of NCI’s Surveillance Research Program. However, while “suggestive,” Negoita adds, their observation does not demonstrate causality: many factors contribute to incidence and mortality, such as improvements in staging and treating cancer.
Cancer death rates continue to decline in the US in all major racial and ethnic groups, according to the National Cancer Institute’s (NCI) latest Annual Report to the Nation on the Status of Cancer. The data are an “encouraging indicator of progress” in cancer research, says NCI Director Ned Sharpless, MD. “It’s clear that interventions are having an impact.”
Overall incidence, or rates of new cancers, dropped by 1.8% in men and 1.4% in women from 1999 to 2015. Between 2011 and 2015, death rates dropped for 11 of the 18 most common cancer types in men and 14 of the 20 most common types in women. The researchers say the “significant declines” also hold “significant differences” in rate by sex, race, and ethnicity. For example, black men and white women had the highest incidence rates, and black men and black women had the highest death rates.
However, over the same period, death rates for cancers of the liver, pancreas, and brain and nervous system rose in both men and women. Death rates for cancer of the uterus rose (the researchers say obesity is thought to be a contributing factor) and death rates for cancers of the oral cavity and pharynx and soft tissue increased in men, perhaps associated with human papillomavirus infection.
In a companion study, when researchers explored prostate cancer trends in more detail they found overall prostate cancer incidence rates declined an average of 6.5% each year between 2007 and 2014, from 163 new cases per 100,000 men to 104 new cases. Still, after a 2-decade steady decline, rates leveled off. Incidence of distant disease rose from 7.8 new cases per 100,000 to 9.2, but there was no increase in the rates of cases with aggressive histologic grade.
Interestingly, the researchers also report a decline in recent prostate-specific antigen screening between 2010 and 2013 national surveys. “The increase in late-stage disease and the flattening of the mortality trended occurred contemporaneously with the observed decrease in PSA screening,” said Serban Negoita, MD, DrPH, of NCI’s Surveillance Research Program. However, while “suggestive,” Negoita adds, their observation does not demonstrate causality: many factors contribute to incidence and mortality, such as improvements in staging and treating cancer.
Cancer death rates continue to decline in the US in all major racial and ethnic groups, according to the National Cancer Institute’s (NCI) latest Annual Report to the Nation on the Status of Cancer. The data are an “encouraging indicator of progress” in cancer research, says NCI Director Ned Sharpless, MD. “It’s clear that interventions are having an impact.”
Overall incidence, or rates of new cancers, dropped by 1.8% in men and 1.4% in women from 1999 to 2015. Between 2011 and 2015, death rates dropped for 11 of the 18 most common cancer types in men and 14 of the 20 most common types in women. The researchers say the “significant declines” also hold “significant differences” in rate by sex, race, and ethnicity. For example, black men and white women had the highest incidence rates, and black men and black women had the highest death rates.
However, over the same period, death rates for cancers of the liver, pancreas, and brain and nervous system rose in both men and women. Death rates for cancer of the uterus rose (the researchers say obesity is thought to be a contributing factor) and death rates for cancers of the oral cavity and pharynx and soft tissue increased in men, perhaps associated with human papillomavirus infection.
In a companion study, when researchers explored prostate cancer trends in more detail they found overall prostate cancer incidence rates declined an average of 6.5% each year between 2007 and 2014, from 163 new cases per 100,000 men to 104 new cases. Still, after a 2-decade steady decline, rates leveled off. Incidence of distant disease rose from 7.8 new cases per 100,000 to 9.2, but there was no increase in the rates of cases with aggressive histologic grade.
Interestingly, the researchers also report a decline in recent prostate-specific antigen screening between 2010 and 2013 national surveys. “The increase in late-stage disease and the flattening of the mortality trended occurred contemporaneously with the observed decrease in PSA screening,” said Serban Negoita, MD, DrPH, of NCI’s Surveillance Research Program. However, while “suggestive,” Negoita adds, their observation does not demonstrate causality: many factors contribute to incidence and mortality, such as improvements in staging and treating cancer.