User login
CAR T Therapy: From Bench to Bedside and Back
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Migraine treatment insights offered for noninvasive vagus nerve stimulation
SAN FRANCISCO – Now that noninvasive vagus nerve stimulation using a small handheld device has earned clearance from the Food and Drug Administration for acute treatment of migraine attacks, investigators are pouring over data from the major clinical trials to gain post hoc insight into how to optimize use of the gammaCore device in routine clinical practice.
The answer is to use it early in the course of an attack and use it often, Eric Liebler said at the annual meeting of the American Headache Society.
“Our motto here in the U.S. is to get the device and use it as many times as you want during the month. There are no pharmacologic side effects, no drug-drug interactions, and no risk of overusing it that we are aware of at this point. So if you give it to a patient who’s able to say, ‘Wait a second – I don’t feel good, I have premonitory symptoms,’ if they use it, then they’re likely to feel better, with a clinically relevant reduction of at least 1 point when treating a migraine while it’s mild,” according to Mr. Liebler, senior vice president for neurology at electroCore, of Basking Ridge, N.J., which markets the noninvasive vagus nerve stimulation (nVNS) gammaCore device.
The device received FDA clearance for acute treatment of migraine in January 2018 following regulatory approval in the spring of 2017 for treatment of episodic cluster headache.
Mr. Liebler presented key highlights of the recently published pivotal PRESTO trial (Prospective Study of nVNS for the Acute Treatment of Migraine), a randomized, double-blind, multicenter, sham-controlled study of 243 adults under age 50 years who experienced 3-8 migraine attacks per month (Neurology. 2018 Jun 15. doi: 10.1212/WNL.0000000000005857).
“This study provides Class I evidence that, for patients with an episodic migraine, nVNS significantly increases the probability of having mild pain or being pain-free post stimulation,” Mr. Liebler declared.
During the 4-week, double-blind treatment period, 48% of the active nVNS group met the International Headache Society definition of pain relief, which meant at least a 1-point reduction in migraine severity on a 0-3 point pain scale at 120 minutes in at least 50% of treated migraine episodes without using rescue medications. This was a significantly better result than the 32% rate seen in patients in the control group who were given a sham device that emitted a perceptible but ineffective signal.
Similar results were documented during the subsequent 4-week, open-label treatment period, during which the control group was switched over to a functioning gammaCore.
“You can tell your patients they have a reliable chance of having a response more often than not,” according to Mr. Liebler.
What else can physicians tell their migraine patients to expect regarding efficacy? The pain-free responder rate at 120 minutes in PRESTO and other studies is similar to rates reported in meta-analyses of oral triptan therapy but without any drug side effects or limitations on frequency of use. Furthermore, the need for rescue medication at any time during a migraine attack was significantly lower with nVNS than it was with sham treatment, by a margin of 48%-63%.
“If they use this device, they’re often able to save that triptan for when they wake up in the morning and they’re already at a 3 in migraine severity,” he observed.
Also, nVNS is fast-acting: At 30 minutes from onset of pain in the first treated attack, 27% of the nVNS group had experienced pain relief, compared with 19% of controls.
The tolerability profile of the device therapy was outstanding, with no treatment discontinuations in the active treatment arm and only occasional reports of mild transient application site discomfort.
Asked about insurance coverage, Mr. Liebler said broad coverage is coming, but it’s not here yet.
“Insurers don’t really want to pay for anything, and devices confuse them even more than drugs. But we’re getting there because of the quality of the evidence. They were really having a good time telling us we didn’t have any published papers, and now that we’ve given them a class I study published in Neurology, they’ve said they had to review it. CVS will cover it starting Jan. 1,” he continued.
Planning is underway for additional clinical trials of the gammaCore for prevention of episodic migraine, acute treatment of attacks in adolescents, and for use in pregnancy.
Using the nVNS device
This nVNS device produces a proprietary, low-voltage electrical signal with a 24-volt peak voltage and a 60-mA peak output current. In the PRESTO study, patients were instructed to self-administer bilateral 120-second stimulations to the right and left sides of their necks within 20 minutes of pain onset. If the pain hadn’t improved within 15 minutes, they were to repeat the stimulations.
In a post hoc analysis of the PREVA (Prevention and Acute treatment of chronic cluster headache) study (Cephalalgia. 2016;36[6]:534-46), investigators determined that the mean reduction in the number of cluster headache attacks was significantly greater in patients who used the device to treat at least 77% of their attacks. That had a bigger preventive effect than did the number of stimulations a patient applied per day.
The PRESTO and PREVA trials were sponsored by electroCore, where Mr. Liebler is employed.
SAN FRANCISCO – Now that noninvasive vagus nerve stimulation using a small handheld device has earned clearance from the Food and Drug Administration for acute treatment of migraine attacks, investigators are pouring over data from the major clinical trials to gain post hoc insight into how to optimize use of the gammaCore device in routine clinical practice.
The answer is to use it early in the course of an attack and use it often, Eric Liebler said at the annual meeting of the American Headache Society.
“Our motto here in the U.S. is to get the device and use it as many times as you want during the month. There are no pharmacologic side effects, no drug-drug interactions, and no risk of overusing it that we are aware of at this point. So if you give it to a patient who’s able to say, ‘Wait a second – I don’t feel good, I have premonitory symptoms,’ if they use it, then they’re likely to feel better, with a clinically relevant reduction of at least 1 point when treating a migraine while it’s mild,” according to Mr. Liebler, senior vice president for neurology at electroCore, of Basking Ridge, N.J., which markets the noninvasive vagus nerve stimulation (nVNS) gammaCore device.
The device received FDA clearance for acute treatment of migraine in January 2018 following regulatory approval in the spring of 2017 for treatment of episodic cluster headache.
Mr. Liebler presented key highlights of the recently published pivotal PRESTO trial (Prospective Study of nVNS for the Acute Treatment of Migraine), a randomized, double-blind, multicenter, sham-controlled study of 243 adults under age 50 years who experienced 3-8 migraine attacks per month (Neurology. 2018 Jun 15. doi: 10.1212/WNL.0000000000005857).
“This study provides Class I evidence that, for patients with an episodic migraine, nVNS significantly increases the probability of having mild pain or being pain-free post stimulation,” Mr. Liebler declared.
During the 4-week, double-blind treatment period, 48% of the active nVNS group met the International Headache Society definition of pain relief, which meant at least a 1-point reduction in migraine severity on a 0-3 point pain scale at 120 minutes in at least 50% of treated migraine episodes without using rescue medications. This was a significantly better result than the 32% rate seen in patients in the control group who were given a sham device that emitted a perceptible but ineffective signal.
Similar results were documented during the subsequent 4-week, open-label treatment period, during which the control group was switched over to a functioning gammaCore.
“You can tell your patients they have a reliable chance of having a response more often than not,” according to Mr. Liebler.
What else can physicians tell their migraine patients to expect regarding efficacy? The pain-free responder rate at 120 minutes in PRESTO and other studies is similar to rates reported in meta-analyses of oral triptan therapy but without any drug side effects or limitations on frequency of use. Furthermore, the need for rescue medication at any time during a migraine attack was significantly lower with nVNS than it was with sham treatment, by a margin of 48%-63%.
“If they use this device, they’re often able to save that triptan for when they wake up in the morning and they’re already at a 3 in migraine severity,” he observed.
Also, nVNS is fast-acting: At 30 minutes from onset of pain in the first treated attack, 27% of the nVNS group had experienced pain relief, compared with 19% of controls.
The tolerability profile of the device therapy was outstanding, with no treatment discontinuations in the active treatment arm and only occasional reports of mild transient application site discomfort.
Asked about insurance coverage, Mr. Liebler said broad coverage is coming, but it’s not here yet.
“Insurers don’t really want to pay for anything, and devices confuse them even more than drugs. But we’re getting there because of the quality of the evidence. They were really having a good time telling us we didn’t have any published papers, and now that we’ve given them a class I study published in Neurology, they’ve said they had to review it. CVS will cover it starting Jan. 1,” he continued.
Planning is underway for additional clinical trials of the gammaCore for prevention of episodic migraine, acute treatment of attacks in adolescents, and for use in pregnancy.
Using the nVNS device
This nVNS device produces a proprietary, low-voltage electrical signal with a 24-volt peak voltage and a 60-mA peak output current. In the PRESTO study, patients were instructed to self-administer bilateral 120-second stimulations to the right and left sides of their necks within 20 minutes of pain onset. If the pain hadn’t improved within 15 minutes, they were to repeat the stimulations.
In a post hoc analysis of the PREVA (Prevention and Acute treatment of chronic cluster headache) study (Cephalalgia. 2016;36[6]:534-46), investigators determined that the mean reduction in the number of cluster headache attacks was significantly greater in patients who used the device to treat at least 77% of their attacks. That had a bigger preventive effect than did the number of stimulations a patient applied per day.
The PRESTO and PREVA trials were sponsored by electroCore, where Mr. Liebler is employed.
SAN FRANCISCO – Now that noninvasive vagus nerve stimulation using a small handheld device has earned clearance from the Food and Drug Administration for acute treatment of migraine attacks, investigators are pouring over data from the major clinical trials to gain post hoc insight into how to optimize use of the gammaCore device in routine clinical practice.
The answer is to use it early in the course of an attack and use it often, Eric Liebler said at the annual meeting of the American Headache Society.
“Our motto here in the U.S. is to get the device and use it as many times as you want during the month. There are no pharmacologic side effects, no drug-drug interactions, and no risk of overusing it that we are aware of at this point. So if you give it to a patient who’s able to say, ‘Wait a second – I don’t feel good, I have premonitory symptoms,’ if they use it, then they’re likely to feel better, with a clinically relevant reduction of at least 1 point when treating a migraine while it’s mild,” according to Mr. Liebler, senior vice president for neurology at electroCore, of Basking Ridge, N.J., which markets the noninvasive vagus nerve stimulation (nVNS) gammaCore device.
The device received FDA clearance for acute treatment of migraine in January 2018 following regulatory approval in the spring of 2017 for treatment of episodic cluster headache.
Mr. Liebler presented key highlights of the recently published pivotal PRESTO trial (Prospective Study of nVNS for the Acute Treatment of Migraine), a randomized, double-blind, multicenter, sham-controlled study of 243 adults under age 50 years who experienced 3-8 migraine attacks per month (Neurology. 2018 Jun 15. doi: 10.1212/WNL.0000000000005857).
“This study provides Class I evidence that, for patients with an episodic migraine, nVNS significantly increases the probability of having mild pain or being pain-free post stimulation,” Mr. Liebler declared.
During the 4-week, double-blind treatment period, 48% of the active nVNS group met the International Headache Society definition of pain relief, which meant at least a 1-point reduction in migraine severity on a 0-3 point pain scale at 120 minutes in at least 50% of treated migraine episodes without using rescue medications. This was a significantly better result than the 32% rate seen in patients in the control group who were given a sham device that emitted a perceptible but ineffective signal.
Similar results were documented during the subsequent 4-week, open-label treatment period, during which the control group was switched over to a functioning gammaCore.
“You can tell your patients they have a reliable chance of having a response more often than not,” according to Mr. Liebler.
What else can physicians tell their migraine patients to expect regarding efficacy? The pain-free responder rate at 120 minutes in PRESTO and other studies is similar to rates reported in meta-analyses of oral triptan therapy but without any drug side effects or limitations on frequency of use. Furthermore, the need for rescue medication at any time during a migraine attack was significantly lower with nVNS than it was with sham treatment, by a margin of 48%-63%.
“If they use this device, they’re often able to save that triptan for when they wake up in the morning and they’re already at a 3 in migraine severity,” he observed.
Also, nVNS is fast-acting: At 30 minutes from onset of pain in the first treated attack, 27% of the nVNS group had experienced pain relief, compared with 19% of controls.
The tolerability profile of the device therapy was outstanding, with no treatment discontinuations in the active treatment arm and only occasional reports of mild transient application site discomfort.
Asked about insurance coverage, Mr. Liebler said broad coverage is coming, but it’s not here yet.
“Insurers don’t really want to pay for anything, and devices confuse them even more than drugs. But we’re getting there because of the quality of the evidence. They were really having a good time telling us we didn’t have any published papers, and now that we’ve given them a class I study published in Neurology, they’ve said they had to review it. CVS will cover it starting Jan. 1,” he continued.
Planning is underway for additional clinical trials of the gammaCore for prevention of episodic migraine, acute treatment of attacks in adolescents, and for use in pregnancy.
Using the nVNS device
This nVNS device produces a proprietary, low-voltage electrical signal with a 24-volt peak voltage and a 60-mA peak output current. In the PRESTO study, patients were instructed to self-administer bilateral 120-second stimulations to the right and left sides of their necks within 20 minutes of pain onset. If the pain hadn’t improved within 15 minutes, they were to repeat the stimulations.
In a post hoc analysis of the PREVA (Prevention and Acute treatment of chronic cluster headache) study (Cephalalgia. 2016;36[6]:534-46), investigators determined that the mean reduction in the number of cluster headache attacks was significantly greater in patients who used the device to treat at least 77% of their attacks. That had a bigger preventive effect than did the number of stimulations a patient applied per day.
The PRESTO and PREVA trials were sponsored by electroCore, where Mr. Liebler is employed.
EXPERT ANALYSIS FROM THE AHS ANNUAL MEETING
For smokers, the ends may not justify the ENDS
Smokers who used e-cigarettes and other electronic nicotine delivery systems (ENDS) were less likely to quit than were those who did not use such products, according to a 2015 survey and a follow-up conducted a year later.
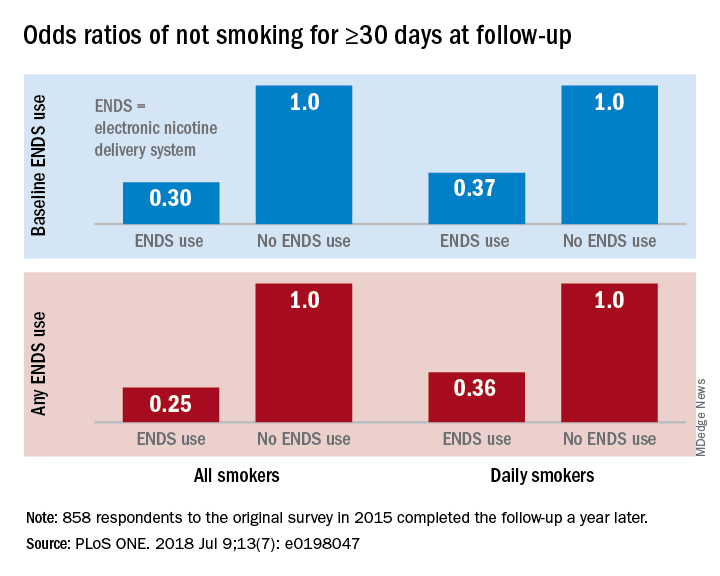
“Under ‘real world’ use and conditions [ENDS] may have suppressed or delayed quitting among some adult smokers,” Scott R. Weaver, PhD, and his associates at Georgia State University, Atlanta, wrote in PLoS One. The original survey, conducted in August and September of 2015, involved 1,284 U.S. adult smokers from the GfK KnowledgePanel, of whom 858 completed the follow-up survey in September 2016.
Smokers who used ENDS at baseline were slightly more likely to attempt to quit (53.7%) than were those who did not (48.6%) but were much less likely to have quit (defined as no smoking for at least 30 days at the time of follow-up): 9.4% vs. 18.9%, for an adjusted odds ratio of 0.30. Those who used ENDS at any time during the study were much more likely than were non-ENDS users to make an attempt (58.5% vs. 44.4%), but they were, again, much less likely to succeed (7.7% vs. 22.2%; AOR, 0.25), the investigators reported.
The results were similar for the subset of respondents who smoked every day: ENDS users were more likely to attempt to quit but less likely to succeed. Odds ratios for quitting were 0.37 for those using ENDS at baseline and 0.36 for those who used ENDS at any time since the first survey, Dr. Weaver and his associates said.
“Use of current ENDS products in real world conditions [does] not seem to improve the chances of quitting for smokers, and, under the current landscape, may not be the disruptive technology that increases the population quit rate and reduces the harm of combustibles,” they wrote.
The study was supported by the National Institute of Drug Abuse and the Food and Drug Administration’s Center for Tobacco Products. One of the investigators has received funding in the form of grant funding from Pfizer and the National Institutes of Health and another has served as a paid consultant to the Centers for Disease Control and Prevention.
SOURCE: Weaver SR et al. PLoS ONE. 2018 Jul 9;13(7): e0198047. doi: 10.1371/journal.pone.0198047.
Smokers who used e-cigarettes and other electronic nicotine delivery systems (ENDS) were less likely to quit than were those who did not use such products, according to a 2015 survey and a follow-up conducted a year later.

“Under ‘real world’ use and conditions [ENDS] may have suppressed or delayed quitting among some adult smokers,” Scott R. Weaver, PhD, and his associates at Georgia State University, Atlanta, wrote in PLoS One. The original survey, conducted in August and September of 2015, involved 1,284 U.S. adult smokers from the GfK KnowledgePanel, of whom 858 completed the follow-up survey in September 2016.
Smokers who used ENDS at baseline were slightly more likely to attempt to quit (53.7%) than were those who did not (48.6%) but were much less likely to have quit (defined as no smoking for at least 30 days at the time of follow-up): 9.4% vs. 18.9%, for an adjusted odds ratio of 0.30. Those who used ENDS at any time during the study were much more likely than were non-ENDS users to make an attempt (58.5% vs. 44.4%), but they were, again, much less likely to succeed (7.7% vs. 22.2%; AOR, 0.25), the investigators reported.
The results were similar for the subset of respondents who smoked every day: ENDS users were more likely to attempt to quit but less likely to succeed. Odds ratios for quitting were 0.37 for those using ENDS at baseline and 0.36 for those who used ENDS at any time since the first survey, Dr. Weaver and his associates said.
“Use of current ENDS products in real world conditions [does] not seem to improve the chances of quitting for smokers, and, under the current landscape, may not be the disruptive technology that increases the population quit rate and reduces the harm of combustibles,” they wrote.
The study was supported by the National Institute of Drug Abuse and the Food and Drug Administration’s Center for Tobacco Products. One of the investigators has received funding in the form of grant funding from Pfizer and the National Institutes of Health and another has served as a paid consultant to the Centers for Disease Control and Prevention.
SOURCE: Weaver SR et al. PLoS ONE. 2018 Jul 9;13(7): e0198047. doi: 10.1371/journal.pone.0198047.
Smokers who used e-cigarettes and other electronic nicotine delivery systems (ENDS) were less likely to quit than were those who did not use such products, according to a 2015 survey and a follow-up conducted a year later.

“Under ‘real world’ use and conditions [ENDS] may have suppressed or delayed quitting among some adult smokers,” Scott R. Weaver, PhD, and his associates at Georgia State University, Atlanta, wrote in PLoS One. The original survey, conducted in August and September of 2015, involved 1,284 U.S. adult smokers from the GfK KnowledgePanel, of whom 858 completed the follow-up survey in September 2016.
Smokers who used ENDS at baseline were slightly more likely to attempt to quit (53.7%) than were those who did not (48.6%) but were much less likely to have quit (defined as no smoking for at least 30 days at the time of follow-up): 9.4% vs. 18.9%, for an adjusted odds ratio of 0.30. Those who used ENDS at any time during the study were much more likely than were non-ENDS users to make an attempt (58.5% vs. 44.4%), but they were, again, much less likely to succeed (7.7% vs. 22.2%; AOR, 0.25), the investigators reported.
The results were similar for the subset of respondents who smoked every day: ENDS users were more likely to attempt to quit but less likely to succeed. Odds ratios for quitting were 0.37 for those using ENDS at baseline and 0.36 for those who used ENDS at any time since the first survey, Dr. Weaver and his associates said.
“Use of current ENDS products in real world conditions [does] not seem to improve the chances of quitting for smokers, and, under the current landscape, may not be the disruptive technology that increases the population quit rate and reduces the harm of combustibles,” they wrote.
The study was supported by the National Institute of Drug Abuse and the Food and Drug Administration’s Center for Tobacco Products. One of the investigators has received funding in the form of grant funding from Pfizer and the National Institutes of Health and another has served as a paid consultant to the Centers for Disease Control and Prevention.
SOURCE: Weaver SR et al. PLoS ONE. 2018 Jul 9;13(7): e0198047. doi: 10.1371/journal.pone.0198047.
FROM PLOS ONE
Fentanyl analogs nearly double their overdose death toll
, according to preliminary data from 10 states.
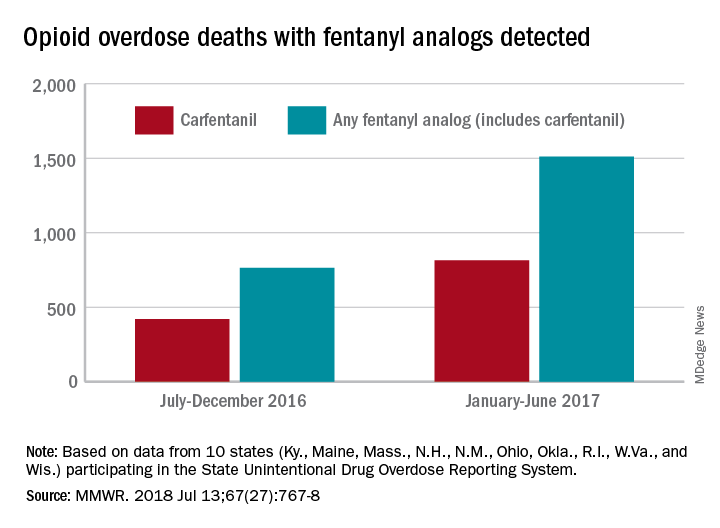
During July 2016 to December 2016, there were 764 opioid overdose deaths that tested positive for any fentanyl analog, with carfentanil being the most common (421 deaths). From January 2017 to June 2017, the respective numbers increased by 98% (1,511) and 94% (815), wrote Julie O’Donnell, PhD, and her associates at the Centers for Disease Control and Prevention’s National Center for Injury Prevention and Control. The report was published in the Morbidity and Mortality Weekly Report.
“The increasing array of fentanyl analogs highlights the need to build forensic toxicological testing capabilities to identify and report emerging threats, and to enhance capacity to rapidly respond to evolving drug trends,” Dr. O’Donnell and her associates said.
Along with carfentanil, 13 other analogs were detected in decedents during the 12-month period: 3-methylfentanyl, 4-fluorobutyrfentanyl, 4-fluorofentanyl, 4-fluoroisobutyrfentanyl, acetylfentanyl, acrylfentanyl, butyrylfentanyl, cyclopropylfentanyl, cyclopentylfentanyl, furanylethylfentanyl, furanylfentanyl, isobutyrylfentanyl, and tetrahydrofuranylfentanyl. Deaths may have involved “more than one analog, as well as ... other opioid and nonopioid substances,” they noted.
The 10 states reporting data to the State Unintentional Drug Overdose Reporting System (SUDORS) were Kentucky, Maine, Massachusetts, New Hampshire, New Mexico, Ohio, Oklahoma, Rhode Island, West Virginia, and Wisconsin. Two other SUDORS-reporting states – Missouri and Pennsylvania – did not have their data ready in time to be included in this analysis.
The increasing availability of fentanyl analogs hit Ohio especially hard: More deaths occurred there than in the other 10 states combined. Of the 421 carfentanil-related deaths in July 2016 to December 2016, nearly 400 were in Ohio, and there were 218 Ohio deaths in April 2017 alone. A look at the bigger picture shows that 3 of the 10 states reported carfentanil-related overdose deaths in the second half of 2016, compared with 7 in the first half of 2017, the investigators said.
Carfentanil, which is the most potent of the 14 fentanyl analogs that have been detected so far, “is intended for sedation of large animals, and is estimated to have 10,000 times the potency of morphine,” Dr. O’Donnell and her associates wrote.
SOURCE: O’Donnell J et al. MMWR. 2018 Jul 13;67(27):767-8.
, according to preliminary data from 10 states.

During July 2016 to December 2016, there were 764 opioid overdose deaths that tested positive for any fentanyl analog, with carfentanil being the most common (421 deaths). From January 2017 to June 2017, the respective numbers increased by 98% (1,511) and 94% (815), wrote Julie O’Donnell, PhD, and her associates at the Centers for Disease Control and Prevention’s National Center for Injury Prevention and Control. The report was published in the Morbidity and Mortality Weekly Report.
“The increasing array of fentanyl analogs highlights the need to build forensic toxicological testing capabilities to identify and report emerging threats, and to enhance capacity to rapidly respond to evolving drug trends,” Dr. O’Donnell and her associates said.
Along with carfentanil, 13 other analogs were detected in decedents during the 12-month period: 3-methylfentanyl, 4-fluorobutyrfentanyl, 4-fluorofentanyl, 4-fluoroisobutyrfentanyl, acetylfentanyl, acrylfentanyl, butyrylfentanyl, cyclopropylfentanyl, cyclopentylfentanyl, furanylethylfentanyl, furanylfentanyl, isobutyrylfentanyl, and tetrahydrofuranylfentanyl. Deaths may have involved “more than one analog, as well as ... other opioid and nonopioid substances,” they noted.
The 10 states reporting data to the State Unintentional Drug Overdose Reporting System (SUDORS) were Kentucky, Maine, Massachusetts, New Hampshire, New Mexico, Ohio, Oklahoma, Rhode Island, West Virginia, and Wisconsin. Two other SUDORS-reporting states – Missouri and Pennsylvania – did not have their data ready in time to be included in this analysis.
The increasing availability of fentanyl analogs hit Ohio especially hard: More deaths occurred there than in the other 10 states combined. Of the 421 carfentanil-related deaths in July 2016 to December 2016, nearly 400 were in Ohio, and there were 218 Ohio deaths in April 2017 alone. A look at the bigger picture shows that 3 of the 10 states reported carfentanil-related overdose deaths in the second half of 2016, compared with 7 in the first half of 2017, the investigators said.
Carfentanil, which is the most potent of the 14 fentanyl analogs that have been detected so far, “is intended for sedation of large animals, and is estimated to have 10,000 times the potency of morphine,” Dr. O’Donnell and her associates wrote.
SOURCE: O’Donnell J et al. MMWR. 2018 Jul 13;67(27):767-8.
, according to preliminary data from 10 states.

During July 2016 to December 2016, there were 764 opioid overdose deaths that tested positive for any fentanyl analog, with carfentanil being the most common (421 deaths). From January 2017 to June 2017, the respective numbers increased by 98% (1,511) and 94% (815), wrote Julie O’Donnell, PhD, and her associates at the Centers for Disease Control and Prevention’s National Center for Injury Prevention and Control. The report was published in the Morbidity and Mortality Weekly Report.
“The increasing array of fentanyl analogs highlights the need to build forensic toxicological testing capabilities to identify and report emerging threats, and to enhance capacity to rapidly respond to evolving drug trends,” Dr. O’Donnell and her associates said.
Along with carfentanil, 13 other analogs were detected in decedents during the 12-month period: 3-methylfentanyl, 4-fluorobutyrfentanyl, 4-fluorofentanyl, 4-fluoroisobutyrfentanyl, acetylfentanyl, acrylfentanyl, butyrylfentanyl, cyclopropylfentanyl, cyclopentylfentanyl, furanylethylfentanyl, furanylfentanyl, isobutyrylfentanyl, and tetrahydrofuranylfentanyl. Deaths may have involved “more than one analog, as well as ... other opioid and nonopioid substances,” they noted.
The 10 states reporting data to the State Unintentional Drug Overdose Reporting System (SUDORS) were Kentucky, Maine, Massachusetts, New Hampshire, New Mexico, Ohio, Oklahoma, Rhode Island, West Virginia, and Wisconsin. Two other SUDORS-reporting states – Missouri and Pennsylvania – did not have their data ready in time to be included in this analysis.
The increasing availability of fentanyl analogs hit Ohio especially hard: More deaths occurred there than in the other 10 states combined. Of the 421 carfentanil-related deaths in July 2016 to December 2016, nearly 400 were in Ohio, and there were 218 Ohio deaths in April 2017 alone. A look at the bigger picture shows that 3 of the 10 states reported carfentanil-related overdose deaths in the second half of 2016, compared with 7 in the first half of 2017, the investigators said.
Carfentanil, which is the most potent of the 14 fentanyl analogs that have been detected so far, “is intended for sedation of large animals, and is estimated to have 10,000 times the potency of morphine,” Dr. O’Donnell and her associates wrote.
SOURCE: O’Donnell J et al. MMWR. 2018 Jul 13;67(27):767-8.
FROM MMWR
New guideline for managing MCL
Rituximab should be included in first-line chemotherapy when treating mantle cell lymphoma, according to a new management guideline from the British Society for Haematology.
The best outcome data is for the R-CHOP regimen (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone) followed by maintenance treatment with rituximab, wrote Pamela McKay, MD, of Beatson West of Scotland Cancer Centre in Glasgow, and her colleagues. The report was published in the British Journal of Haematology. But the combination of rituximab and bendamustine is also effective and a more favorable safety profile, according to the guideline. Single agent rituximab is not recommended.
At relapse, the guideline calls on physicians to take an individualized approach based on age, comorbidities, performance status, and response to prior therapy. Some options to consider include ibrutinib as a single agent or rituximab plus chemotherapy. The authors cautioned that there is little evidence to support maintenance rituximab after relapse treatment.
The guideline also explores the role of autologous stem cell transplantation (ASCT) and allogeneic SCT (alloSCT). The authors recommend that ASCT be considered as consolidation of first-line therapy for patients who are fit for intensive therapy. AlloSCT is a viable option in second remission among fit patients who have an appropriate donor and it may also be effective as a rescue therapy for patients who relapse after ASCT. But alloSCT is appropriate only as a first-line therapy for high-risk patients and is best used as part of a clinical trial, according to the recommendations.
The British Society of Haematology previously issued guidance on mantle cell lymphoma in 2012, but the updated document includes new drug therapeutic options and transplant data. The guideline includes a therapeutic algorithm to assist physicians in choosing first-line therapy, options after first relapse, and management in the case of higher relapse.
The guideline authors reported having no conflicts of interest.
SOURCE: McKay P et al. Br J Haematol. 2018 Jul;182(1):46-62.
Rituximab should be included in first-line chemotherapy when treating mantle cell lymphoma, according to a new management guideline from the British Society for Haematology.
The best outcome data is for the R-CHOP regimen (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone) followed by maintenance treatment with rituximab, wrote Pamela McKay, MD, of Beatson West of Scotland Cancer Centre in Glasgow, and her colleagues. The report was published in the British Journal of Haematology. But the combination of rituximab and bendamustine is also effective and a more favorable safety profile, according to the guideline. Single agent rituximab is not recommended.
At relapse, the guideline calls on physicians to take an individualized approach based on age, comorbidities, performance status, and response to prior therapy. Some options to consider include ibrutinib as a single agent or rituximab plus chemotherapy. The authors cautioned that there is little evidence to support maintenance rituximab after relapse treatment.
The guideline also explores the role of autologous stem cell transplantation (ASCT) and allogeneic SCT (alloSCT). The authors recommend that ASCT be considered as consolidation of first-line therapy for patients who are fit for intensive therapy. AlloSCT is a viable option in second remission among fit patients who have an appropriate donor and it may also be effective as a rescue therapy for patients who relapse after ASCT. But alloSCT is appropriate only as a first-line therapy for high-risk patients and is best used as part of a clinical trial, according to the recommendations.
The British Society of Haematology previously issued guidance on mantle cell lymphoma in 2012, but the updated document includes new drug therapeutic options and transplant data. The guideline includes a therapeutic algorithm to assist physicians in choosing first-line therapy, options after first relapse, and management in the case of higher relapse.
The guideline authors reported having no conflicts of interest.
SOURCE: McKay P et al. Br J Haematol. 2018 Jul;182(1):46-62.
Rituximab should be included in first-line chemotherapy when treating mantle cell lymphoma, according to a new management guideline from the British Society for Haematology.
The best outcome data is for the R-CHOP regimen (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone) followed by maintenance treatment with rituximab, wrote Pamela McKay, MD, of Beatson West of Scotland Cancer Centre in Glasgow, and her colleagues. The report was published in the British Journal of Haematology. But the combination of rituximab and bendamustine is also effective and a more favorable safety profile, according to the guideline. Single agent rituximab is not recommended.
At relapse, the guideline calls on physicians to take an individualized approach based on age, comorbidities, performance status, and response to prior therapy. Some options to consider include ibrutinib as a single agent or rituximab plus chemotherapy. The authors cautioned that there is little evidence to support maintenance rituximab after relapse treatment.
The guideline also explores the role of autologous stem cell transplantation (ASCT) and allogeneic SCT (alloSCT). The authors recommend that ASCT be considered as consolidation of first-line therapy for patients who are fit for intensive therapy. AlloSCT is a viable option in second remission among fit patients who have an appropriate donor and it may also be effective as a rescue therapy for patients who relapse after ASCT. But alloSCT is appropriate only as a first-line therapy for high-risk patients and is best used as part of a clinical trial, according to the recommendations.
The British Society of Haematology previously issued guidance on mantle cell lymphoma in 2012, but the updated document includes new drug therapeutic options and transplant data. The guideline includes a therapeutic algorithm to assist physicians in choosing first-line therapy, options after first relapse, and management in the case of higher relapse.
The guideline authors reported having no conflicts of interest.
SOURCE: McKay P et al. Br J Haematol. 2018 Jul;182(1):46-62.
FROM THE BRITISH JOURNAL OF HAEMATOLOGY
Immunogenicity of two-dose Gardasil 9 persists at 36 months
MALMO, SWEDEN – Robust human papillomavirus antibody responses persist through 36 months in boys and girls who received two doses of the 9-valent HPV vaccine known as Gardasil 9 at age 9-14 years, according to an open-label randomized immunogenicity study conducted in 15 countries.
This is reassuring news supportive of regulatory decisions made in 2016/2017 to license the more convenient two-dose schedule in the United States, European Union, Canada, and other countries, Rosybel Drury, PhD, observed in reporting the results at the annual meeting of the European Society for Paediatric Infectious Diseases.
Moreover, HPV type-specific antibody levels at 36 months post vaccination in boys and girls who received two doses were similar to or greater than in the 36-month follow-up of adolescent and young adult females who received three doses at ages 16-26 years.
“This result supports the bridging of efficacy findings in young women receiving three doses to girls and boys receiving two doses,” according to Dr. Drury, a vaccine scientist at Merck Sharp & Dohme in Lyon, France.
She presented an update of a five-cohort study including roughly 1,500 recipients of either two doses of the 9-valent HPV vaccine given 6 or 12 months apart or three doses administered at 0, 2, and 6 months. Four cohorts were composed of 9- to 14-year-olds. The fifth consisted of adolescent girls and young women who got three doses of Gardasil 9 over the course of 6 months at ages 16-26 years. The previous report from this major study provided only short-term data based upon measurements of immunogenicity obtained 1 month after the last dose of vaccine (JAMA. 2016 Dec. 13;316(22):2411-21).
Anti-HPV geometric mean titers were highest 1 month after completing a two- or three-dose series, dropped off sharply during the next 6-12 months, then declined more slowly through 36 months.
While the demonstration of persistent immunogenicity over 3 years of follow-up was reassuring overall, there was a potential hitch: Significantly lower antibody levels for some HPV types were observed in girls who received two doses of the vaccine than in those who got three. Specifically, levels of anti-HPV antibodies to HPV types 18, 31, 45, and 52 were significantly lower in the girls who got two doses of the 9-valent HPV vaccine than in those who got three doses at the same age. Antibody levels directed against HPV types 6, 11, 16, 33, and 58 were similar in the two patient populations.
“The clinical significance of this finding remains unknown,” Dr. Drury said.
Challenged as to why the study didn’t include a single-dose vaccine arm, which would be the preferred preventive strategy in low-income countries where the burden of anogenital cancers and warts due to HPV is greatest, she replied that while there is great interest in this approach, and some modeling studies suggest it would be beneficial, the study she presented was designed to evaluate immunogenicity over time, not clinical efficacy, which needs to be assessed before single-dose public health programs are implemented.
The study was funded by Merck and presented by a company employee.
MALMO, SWEDEN – Robust human papillomavirus antibody responses persist through 36 months in boys and girls who received two doses of the 9-valent HPV vaccine known as Gardasil 9 at age 9-14 years, according to an open-label randomized immunogenicity study conducted in 15 countries.
This is reassuring news supportive of regulatory decisions made in 2016/2017 to license the more convenient two-dose schedule in the United States, European Union, Canada, and other countries, Rosybel Drury, PhD, observed in reporting the results at the annual meeting of the European Society for Paediatric Infectious Diseases.
Moreover, HPV type-specific antibody levels at 36 months post vaccination in boys and girls who received two doses were similar to or greater than in the 36-month follow-up of adolescent and young adult females who received three doses at ages 16-26 years.
“This result supports the bridging of efficacy findings in young women receiving three doses to girls and boys receiving two doses,” according to Dr. Drury, a vaccine scientist at Merck Sharp & Dohme in Lyon, France.
She presented an update of a five-cohort study including roughly 1,500 recipients of either two doses of the 9-valent HPV vaccine given 6 or 12 months apart or three doses administered at 0, 2, and 6 months. Four cohorts were composed of 9- to 14-year-olds. The fifth consisted of adolescent girls and young women who got three doses of Gardasil 9 over the course of 6 months at ages 16-26 years. The previous report from this major study provided only short-term data based upon measurements of immunogenicity obtained 1 month after the last dose of vaccine (JAMA. 2016 Dec. 13;316(22):2411-21).
Anti-HPV geometric mean titers were highest 1 month after completing a two- or three-dose series, dropped off sharply during the next 6-12 months, then declined more slowly through 36 months.
While the demonstration of persistent immunogenicity over 3 years of follow-up was reassuring overall, there was a potential hitch: Significantly lower antibody levels for some HPV types were observed in girls who received two doses of the vaccine than in those who got three. Specifically, levels of anti-HPV antibodies to HPV types 18, 31, 45, and 52 were significantly lower in the girls who got two doses of the 9-valent HPV vaccine than in those who got three doses at the same age. Antibody levels directed against HPV types 6, 11, 16, 33, and 58 were similar in the two patient populations.
“The clinical significance of this finding remains unknown,” Dr. Drury said.
Challenged as to why the study didn’t include a single-dose vaccine arm, which would be the preferred preventive strategy in low-income countries where the burden of anogenital cancers and warts due to HPV is greatest, she replied that while there is great interest in this approach, and some modeling studies suggest it would be beneficial, the study she presented was designed to evaluate immunogenicity over time, not clinical efficacy, which needs to be assessed before single-dose public health programs are implemented.
The study was funded by Merck and presented by a company employee.
MALMO, SWEDEN – Robust human papillomavirus antibody responses persist through 36 months in boys and girls who received two doses of the 9-valent HPV vaccine known as Gardasil 9 at age 9-14 years, according to an open-label randomized immunogenicity study conducted in 15 countries.
This is reassuring news supportive of regulatory decisions made in 2016/2017 to license the more convenient two-dose schedule in the United States, European Union, Canada, and other countries, Rosybel Drury, PhD, observed in reporting the results at the annual meeting of the European Society for Paediatric Infectious Diseases.
Moreover, HPV type-specific antibody levels at 36 months post vaccination in boys and girls who received two doses were similar to or greater than in the 36-month follow-up of adolescent and young adult females who received three doses at ages 16-26 years.
“This result supports the bridging of efficacy findings in young women receiving three doses to girls and boys receiving two doses,” according to Dr. Drury, a vaccine scientist at Merck Sharp & Dohme in Lyon, France.
She presented an update of a five-cohort study including roughly 1,500 recipients of either two doses of the 9-valent HPV vaccine given 6 or 12 months apart or three doses administered at 0, 2, and 6 months. Four cohorts were composed of 9- to 14-year-olds. The fifth consisted of adolescent girls and young women who got three doses of Gardasil 9 over the course of 6 months at ages 16-26 years. The previous report from this major study provided only short-term data based upon measurements of immunogenicity obtained 1 month after the last dose of vaccine (JAMA. 2016 Dec. 13;316(22):2411-21).
Anti-HPV geometric mean titers were highest 1 month after completing a two- or three-dose series, dropped off sharply during the next 6-12 months, then declined more slowly through 36 months.
While the demonstration of persistent immunogenicity over 3 years of follow-up was reassuring overall, there was a potential hitch: Significantly lower antibody levels for some HPV types were observed in girls who received two doses of the vaccine than in those who got three. Specifically, levels of anti-HPV antibodies to HPV types 18, 31, 45, and 52 were significantly lower in the girls who got two doses of the 9-valent HPV vaccine than in those who got three doses at the same age. Antibody levels directed against HPV types 6, 11, 16, 33, and 58 were similar in the two patient populations.
“The clinical significance of this finding remains unknown,” Dr. Drury said.
Challenged as to why the study didn’t include a single-dose vaccine arm, which would be the preferred preventive strategy in low-income countries where the burden of anogenital cancers and warts due to HPV is greatest, she replied that while there is great interest in this approach, and some modeling studies suggest it would be beneficial, the study she presented was designed to evaluate immunogenicity over time, not clinical efficacy, which needs to be assessed before single-dose public health programs are implemented.
The study was funded by Merck and presented by a company employee.
REPORTING FROM ESPID 2018
Key clinical point:
Major finding: HPV type-specific antibody responses to two doses given at age 9-14 years were as good as or better than in 16- to 26-year-olds who got three doses.
Study details: This prospective open-label immunogenicity study included roughly 1,500 subjects in 15 countries who received either two or three doses of the 9-valent HPV vaccine.
Disclosures: The study was funded by Merck and presented by a company employee.
AGA Clinical Practice Update: Statins are safe, effective, and important for most patients with liver disease and dyslipidemia
The medications are only contraindicated in patients with decompensated cirrhosis and statin-induced liver injury, Elizabeth Speliotes, MD, PhD, MPH, and her colleagues wrote in an expert review published in Clinical Gastroenterology and Hepatology. In these patients, statin treatment can compound liver damage and should be avoided, wrote Dr. Speliotes and her coauthors.
Because the liver plays a central role in cholesterol production, many clinicians shy away from treating hyperlipidemia in patients with liver disease. But studies consistently show that lipid-lowering drugs improve dyslipidemia in these patients, which significantly improves both high- and low-density lipoproteins and thereby reduces the long-term risk of cardiovascular disease, the authors wrote.
“Furthermore, the liver plays a role in the metabolism of many drugs, including those that are used to treat dyslipidemia,” wrote Dr. Speliotes of the University of Michigan, Ann Arbor. “It is not surprising, therefore, that many practitioners are hesitant to prescribe medicines to treat dyslipidemia in the setting of liver disease.”
Cholesterol targets described in the 2013 American College of Cardiology/American Heart Association guidelines can safely be applied to patients with liver disease. “The guidelines recommend that adults with cardiovascular disease or LDL of 190 mg/dL or higher be treated with high-intensity statins with the goal of reducing LDL levels by 50%,” they said. Patients whose LDL is 189 mg/dL or lower will benefit from moderate-intensity statins, with a target of a 30%-50% decrease in LDL.
The authors described best practice advice for dyslipidemia treatment in six liver diseases: drug-induced liver injury (DILI), nonalcoholic fatty liver disease (NAFLD), viral hepatitis B and C (HBV and HCV), primary biliary cholangitis (PBC), cirrhosis, and posttransplant dyslipidemia.
DILI
DILI is characterized by elevations of threefold or more in serum alanine aminotransferase (ALT) or aspartate aminotransferase and at least a doubling of total serum bilirubin with no other identifiable cause of these aberrations except the suspect drug. Statins rarely cause a DILI (1 in 100,000 patients), but can cause transient, benign ALT elevations. Statins should be discontinued if ALT or aspartate aminotransferase levels exceed a tripling of the upper limit of normal with concomitant bilirubin elevations. They should not be prescribed to patients with acute liver failure or decompensated liver disease, but otherwise they are safe for most patients with liver disease.
NAFLD
Many patients with NAFLD also have dyslipidemia. All NAFLD patients have an increased risk of cardiovascular disease, although NAFLD and nonalcoholic steatohepatitis are not traditional cardiovascular risk factors. Nevertheless, statins and the accompanying improvement in dyslipidemia have been shown to decrease cardiovascular mortality in these patients. The IDEAL study, for example, showed that moderate statin treatment with 80 mg atorvastatin was associated with a 44% decreased risk in secondary cardiovascular events. Other studies show similar results.
NAFLD patients with elevated LDL may benefit from ezetimibe as primary or add-on therapy. However, none of the drugs used to treat dyslipidemia will improve NAFLD or nonalcoholic steatohepatitis histology.
Viral hepatitis
Hepatitis C virus
Patients with HCV infection often experience decreased serum LDL and total cholesterol. However, these are virally mediated and don’t confer cardiovascular protection. In fact, HCV infections are associated with an increased risk of myocardial infarction. If the patient spontaneously clears the virus, lipids may rebound, so levels should be regularly monitored even if the patient does not need statin therapy.
Hepatitis B virus
HBV also interacts with lipid metabolism and can lead to hyperlipidemia. The American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment and statin therapy apply to these patients. Statins are safe in patients with either HCV or HBV, who tolerate them well.
PBC
PBC is a chronic autoimmune inflammatory cholestatic disease that is associated with dyslipidemia. These patients exhibit increased serum triglyceride and HDL levels that vary according to PBC stage. About 10% have a significant risk of cardiovascular disease. PBC patients with compensated liver disease can safely tolerate statin treatment, but the drugs should not be given to PBC patients with decompensated liver disease.
Obeticholic acid (OCA) is sometimes used as second-line therapy for PBC; it affects genes that regulate bile acid synthesis, transport, and action. However, the POISE study showed that, while OCA improved PBC symptoms, it was associated with an increase in LDL and total cholesterol and a decrease in HDL. No follow-up studies have determined cardiovascular implications of that change, but OCA should be avoided in patients with active cardiovascular disease or with cardiovascular risk factors.
Cirrhosis
Recent work suggests that patients with cirrhosis may face a higher risk of coronary artery disease than was previously thought, although that risk varies widely according to the etiology of the cirrhosis.
Statins are safe and effective in patients with Child-Pugh class A cirrhosis; there are few data on their safety in patients with decompensated cirrhosis. Some guidance for these patients exists in the 2014 recommendations of the Liver Expert Panel, which advised against statin use in patients with Child-Pugh class B or C cirrhosis.
There’s some evidence that statins reduce portal pressure and may reduce the risk of decompensation in patients whose cirrhosis is caused by HCV or HBV infections, but they should not be used for this purpose.
Posttransplant dyslipidemia
After liver transplant, more than 60% of patients will develop dyslipidemia; these patients often have obesity or diabetes.
Statins are safe for patients with liver transplant. Concomitant use of calcineurin inhibitors and statins that are metabolized by cytochrome P450 may increase the risk of statin-associated myopathy. Pravastatin and fluvastatin are preferable, because they are metabolized by cytochrome P450 34A.
Neither Dr. Speliotes nor her coauthors had any financial disclosures.
SOURCE: Speliotes EK et al. Clin Gastroenterol Hepatol. 2018 Apr 21. doi: 10.1016/j.cgh.2018.04.023.
The medications are only contraindicated in patients with decompensated cirrhosis and statin-induced liver injury, Elizabeth Speliotes, MD, PhD, MPH, and her colleagues wrote in an expert review published in Clinical Gastroenterology and Hepatology. In these patients, statin treatment can compound liver damage and should be avoided, wrote Dr. Speliotes and her coauthors.
Because the liver plays a central role in cholesterol production, many clinicians shy away from treating hyperlipidemia in patients with liver disease. But studies consistently show that lipid-lowering drugs improve dyslipidemia in these patients, which significantly improves both high- and low-density lipoproteins and thereby reduces the long-term risk of cardiovascular disease, the authors wrote.
“Furthermore, the liver plays a role in the metabolism of many drugs, including those that are used to treat dyslipidemia,” wrote Dr. Speliotes of the University of Michigan, Ann Arbor. “It is not surprising, therefore, that many practitioners are hesitant to prescribe medicines to treat dyslipidemia in the setting of liver disease.”
Cholesterol targets described in the 2013 American College of Cardiology/American Heart Association guidelines can safely be applied to patients with liver disease. “The guidelines recommend that adults with cardiovascular disease or LDL of 190 mg/dL or higher be treated with high-intensity statins with the goal of reducing LDL levels by 50%,” they said. Patients whose LDL is 189 mg/dL or lower will benefit from moderate-intensity statins, with a target of a 30%-50% decrease in LDL.
The authors described best practice advice for dyslipidemia treatment in six liver diseases: drug-induced liver injury (DILI), nonalcoholic fatty liver disease (NAFLD), viral hepatitis B and C (HBV and HCV), primary biliary cholangitis (PBC), cirrhosis, and posttransplant dyslipidemia.
DILI
DILI is characterized by elevations of threefold or more in serum alanine aminotransferase (ALT) or aspartate aminotransferase and at least a doubling of total serum bilirubin with no other identifiable cause of these aberrations except the suspect drug. Statins rarely cause a DILI (1 in 100,000 patients), but can cause transient, benign ALT elevations. Statins should be discontinued if ALT or aspartate aminotransferase levels exceed a tripling of the upper limit of normal with concomitant bilirubin elevations. They should not be prescribed to patients with acute liver failure or decompensated liver disease, but otherwise they are safe for most patients with liver disease.
NAFLD
Many patients with NAFLD also have dyslipidemia. All NAFLD patients have an increased risk of cardiovascular disease, although NAFLD and nonalcoholic steatohepatitis are not traditional cardiovascular risk factors. Nevertheless, statins and the accompanying improvement in dyslipidemia have been shown to decrease cardiovascular mortality in these patients. The IDEAL study, for example, showed that moderate statin treatment with 80 mg atorvastatin was associated with a 44% decreased risk in secondary cardiovascular events. Other studies show similar results.
NAFLD patients with elevated LDL may benefit from ezetimibe as primary or add-on therapy. However, none of the drugs used to treat dyslipidemia will improve NAFLD or nonalcoholic steatohepatitis histology.
Viral hepatitis
Hepatitis C virus
Patients with HCV infection often experience decreased serum LDL and total cholesterol. However, these are virally mediated and don’t confer cardiovascular protection. In fact, HCV infections are associated with an increased risk of myocardial infarction. If the patient spontaneously clears the virus, lipids may rebound, so levels should be regularly monitored even if the patient does not need statin therapy.
Hepatitis B virus
HBV also interacts with lipid metabolism and can lead to hyperlipidemia. The American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment and statin therapy apply to these patients. Statins are safe in patients with either HCV or HBV, who tolerate them well.
PBC
PBC is a chronic autoimmune inflammatory cholestatic disease that is associated with dyslipidemia. These patients exhibit increased serum triglyceride and HDL levels that vary according to PBC stage. About 10% have a significant risk of cardiovascular disease. PBC patients with compensated liver disease can safely tolerate statin treatment, but the drugs should not be given to PBC patients with decompensated liver disease.
Obeticholic acid (OCA) is sometimes used as second-line therapy for PBC; it affects genes that regulate bile acid synthesis, transport, and action. However, the POISE study showed that, while OCA improved PBC symptoms, it was associated with an increase in LDL and total cholesterol and a decrease in HDL. No follow-up studies have determined cardiovascular implications of that change, but OCA should be avoided in patients with active cardiovascular disease or with cardiovascular risk factors.
Cirrhosis
Recent work suggests that patients with cirrhosis may face a higher risk of coronary artery disease than was previously thought, although that risk varies widely according to the etiology of the cirrhosis.
Statins are safe and effective in patients with Child-Pugh class A cirrhosis; there are few data on their safety in patients with decompensated cirrhosis. Some guidance for these patients exists in the 2014 recommendations of the Liver Expert Panel, which advised against statin use in patients with Child-Pugh class B or C cirrhosis.
There’s some evidence that statins reduce portal pressure and may reduce the risk of decompensation in patients whose cirrhosis is caused by HCV or HBV infections, but they should not be used for this purpose.
Posttransplant dyslipidemia
After liver transplant, more than 60% of patients will develop dyslipidemia; these patients often have obesity or diabetes.
Statins are safe for patients with liver transplant. Concomitant use of calcineurin inhibitors and statins that are metabolized by cytochrome P450 may increase the risk of statin-associated myopathy. Pravastatin and fluvastatin are preferable, because they are metabolized by cytochrome P450 34A.
Neither Dr. Speliotes nor her coauthors had any financial disclosures.
SOURCE: Speliotes EK et al. Clin Gastroenterol Hepatol. 2018 Apr 21. doi: 10.1016/j.cgh.2018.04.023.
The medications are only contraindicated in patients with decompensated cirrhosis and statin-induced liver injury, Elizabeth Speliotes, MD, PhD, MPH, and her colleagues wrote in an expert review published in Clinical Gastroenterology and Hepatology. In these patients, statin treatment can compound liver damage and should be avoided, wrote Dr. Speliotes and her coauthors.
Because the liver plays a central role in cholesterol production, many clinicians shy away from treating hyperlipidemia in patients with liver disease. But studies consistently show that lipid-lowering drugs improve dyslipidemia in these patients, which significantly improves both high- and low-density lipoproteins and thereby reduces the long-term risk of cardiovascular disease, the authors wrote.
“Furthermore, the liver plays a role in the metabolism of many drugs, including those that are used to treat dyslipidemia,” wrote Dr. Speliotes of the University of Michigan, Ann Arbor. “It is not surprising, therefore, that many practitioners are hesitant to prescribe medicines to treat dyslipidemia in the setting of liver disease.”
Cholesterol targets described in the 2013 American College of Cardiology/American Heart Association guidelines can safely be applied to patients with liver disease. “The guidelines recommend that adults with cardiovascular disease or LDL of 190 mg/dL or higher be treated with high-intensity statins with the goal of reducing LDL levels by 50%,” they said. Patients whose LDL is 189 mg/dL or lower will benefit from moderate-intensity statins, with a target of a 30%-50% decrease in LDL.
The authors described best practice advice for dyslipidemia treatment in six liver diseases: drug-induced liver injury (DILI), nonalcoholic fatty liver disease (NAFLD), viral hepatitis B and C (HBV and HCV), primary biliary cholangitis (PBC), cirrhosis, and posttransplant dyslipidemia.
DILI
DILI is characterized by elevations of threefold or more in serum alanine aminotransferase (ALT) or aspartate aminotransferase and at least a doubling of total serum bilirubin with no other identifiable cause of these aberrations except the suspect drug. Statins rarely cause a DILI (1 in 100,000 patients), but can cause transient, benign ALT elevations. Statins should be discontinued if ALT or aspartate aminotransferase levels exceed a tripling of the upper limit of normal with concomitant bilirubin elevations. They should not be prescribed to patients with acute liver failure or decompensated liver disease, but otherwise they are safe for most patients with liver disease.
NAFLD
Many patients with NAFLD also have dyslipidemia. All NAFLD patients have an increased risk of cardiovascular disease, although NAFLD and nonalcoholic steatohepatitis are not traditional cardiovascular risk factors. Nevertheless, statins and the accompanying improvement in dyslipidemia have been shown to decrease cardiovascular mortality in these patients. The IDEAL study, for example, showed that moderate statin treatment with 80 mg atorvastatin was associated with a 44% decreased risk in secondary cardiovascular events. Other studies show similar results.
NAFLD patients with elevated LDL may benefit from ezetimibe as primary or add-on therapy. However, none of the drugs used to treat dyslipidemia will improve NAFLD or nonalcoholic steatohepatitis histology.
Viral hepatitis
Hepatitis C virus
Patients with HCV infection often experience decreased serum LDL and total cholesterol. However, these are virally mediated and don’t confer cardiovascular protection. In fact, HCV infections are associated with an increased risk of myocardial infarction. If the patient spontaneously clears the virus, lipids may rebound, so levels should be regularly monitored even if the patient does not need statin therapy.
Hepatitis B virus
HBV also interacts with lipid metabolism and can lead to hyperlipidemia. The American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment and statin therapy apply to these patients. Statins are safe in patients with either HCV or HBV, who tolerate them well.
PBC
PBC is a chronic autoimmune inflammatory cholestatic disease that is associated with dyslipidemia. These patients exhibit increased serum triglyceride and HDL levels that vary according to PBC stage. About 10% have a significant risk of cardiovascular disease. PBC patients with compensated liver disease can safely tolerate statin treatment, but the drugs should not be given to PBC patients with decompensated liver disease.
Obeticholic acid (OCA) is sometimes used as second-line therapy for PBC; it affects genes that regulate bile acid synthesis, transport, and action. However, the POISE study showed that, while OCA improved PBC symptoms, it was associated with an increase in LDL and total cholesterol and a decrease in HDL. No follow-up studies have determined cardiovascular implications of that change, but OCA should be avoided in patients with active cardiovascular disease or with cardiovascular risk factors.
Cirrhosis
Recent work suggests that patients with cirrhosis may face a higher risk of coronary artery disease than was previously thought, although that risk varies widely according to the etiology of the cirrhosis.
Statins are safe and effective in patients with Child-Pugh class A cirrhosis; there are few data on their safety in patients with decompensated cirrhosis. Some guidance for these patients exists in the 2014 recommendations of the Liver Expert Panel, which advised against statin use in patients with Child-Pugh class B or C cirrhosis.
There’s some evidence that statins reduce portal pressure and may reduce the risk of decompensation in patients whose cirrhosis is caused by HCV or HBV infections, but they should not be used for this purpose.
Posttransplant dyslipidemia
After liver transplant, more than 60% of patients will develop dyslipidemia; these patients often have obesity or diabetes.
Statins are safe for patients with liver transplant. Concomitant use of calcineurin inhibitors and statins that are metabolized by cytochrome P450 may increase the risk of statin-associated myopathy. Pravastatin and fluvastatin are preferable, because they are metabolized by cytochrome P450 34A.
Neither Dr. Speliotes nor her coauthors had any financial disclosures.
SOURCE: Speliotes EK et al. Clin Gastroenterol Hepatol. 2018 Apr 21. doi: 10.1016/j.cgh.2018.04.023.
EXPERT ANALYSIS FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Spironolactone effectively treats acne in adolescent females
LAKE TAHOE, CALIF. –
In an interview at the annual meeting of the Society for Pediatric Dermatology, study author Erin Roberts, MD, said that while spironolactone is widely used in dermatology for treating acne vulgaris in women, it is not approved by the Food and Drug Administration for the treatment of acne, likely because published data are lacking. In addition, she said, less is known about its use, safety, and efficacy in the pediatric population.
Dr. Roberts, a resident in the department of dermatology at the Mayo Clinic, Rochester, Minn., and her associates retrospectively reviewed 80 female patients younger than 21 years of age who were treated with spironolactone and topical therapies alone, or with spironolactone plus oral antibiotics and/or contraceptive pills. All patients were seen by clinicians at the Mayo department of dermatology and were followed for a mean of 11.2 months.
The mean age of patients was 19 years and 71.3% had acne flares with their menstrual cycles, 67.5% had acne located on the jawline, 58.8% had concomitant use of an estrogen-containing oral contraceptive, and 93.8% were unresponsive to other oral treatments prior to using spironolactone.
The median spironolactone daily dose was 100 mg, and ranged between 25 mg and 200 mg. Following acne score assessments, the researchers observed that 64 of the 80 patients (80%) experienced improvement of acne on treatment with spironolactone, while 16 (20%) did not respond and were subsequently escalated to oral isotretinoin therapy. Three patients (3.8%) experienced side effects, most commonly lightheadedness, headache, and fatigue, while five patients stopped taking the medication because of adverse effects, cost, or personal preference.
“It was nice to see that spironolactone did improve acne,” Dr. Roberts said. “We think of it as something to use for patients in their 20s, but not as much for patients in their teens. I think it could be a good option for them.” She also recommended starting patients on a dose of 100 mg daily. “We saw that it does have a dose response,” Dr. Roberts said. “It wasn’t until patients got to 100 mg daily that we started to see significant improvement.”
She reported having no financial disclosures.
LAKE TAHOE, CALIF. –
In an interview at the annual meeting of the Society for Pediatric Dermatology, study author Erin Roberts, MD, said that while spironolactone is widely used in dermatology for treating acne vulgaris in women, it is not approved by the Food and Drug Administration for the treatment of acne, likely because published data are lacking. In addition, she said, less is known about its use, safety, and efficacy in the pediatric population.
Dr. Roberts, a resident in the department of dermatology at the Mayo Clinic, Rochester, Minn., and her associates retrospectively reviewed 80 female patients younger than 21 years of age who were treated with spironolactone and topical therapies alone, or with spironolactone plus oral antibiotics and/or contraceptive pills. All patients were seen by clinicians at the Mayo department of dermatology and were followed for a mean of 11.2 months.
The mean age of patients was 19 years and 71.3% had acne flares with their menstrual cycles, 67.5% had acne located on the jawline, 58.8% had concomitant use of an estrogen-containing oral contraceptive, and 93.8% were unresponsive to other oral treatments prior to using spironolactone.
The median spironolactone daily dose was 100 mg, and ranged between 25 mg and 200 mg. Following acne score assessments, the researchers observed that 64 of the 80 patients (80%) experienced improvement of acne on treatment with spironolactone, while 16 (20%) did not respond and were subsequently escalated to oral isotretinoin therapy. Three patients (3.8%) experienced side effects, most commonly lightheadedness, headache, and fatigue, while five patients stopped taking the medication because of adverse effects, cost, or personal preference.
“It was nice to see that spironolactone did improve acne,” Dr. Roberts said. “We think of it as something to use for patients in their 20s, but not as much for patients in their teens. I think it could be a good option for them.” She also recommended starting patients on a dose of 100 mg daily. “We saw that it does have a dose response,” Dr. Roberts said. “It wasn’t until patients got to 100 mg daily that we started to see significant improvement.”
She reported having no financial disclosures.
LAKE TAHOE, CALIF. –
In an interview at the annual meeting of the Society for Pediatric Dermatology, study author Erin Roberts, MD, said that while spironolactone is widely used in dermatology for treating acne vulgaris in women, it is not approved by the Food and Drug Administration for the treatment of acne, likely because published data are lacking. In addition, she said, less is known about its use, safety, and efficacy in the pediatric population.
Dr. Roberts, a resident in the department of dermatology at the Mayo Clinic, Rochester, Minn., and her associates retrospectively reviewed 80 female patients younger than 21 years of age who were treated with spironolactone and topical therapies alone, or with spironolactone plus oral antibiotics and/or contraceptive pills. All patients were seen by clinicians at the Mayo department of dermatology and were followed for a mean of 11.2 months.
The mean age of patients was 19 years and 71.3% had acne flares with their menstrual cycles, 67.5% had acne located on the jawline, 58.8% had concomitant use of an estrogen-containing oral contraceptive, and 93.8% were unresponsive to other oral treatments prior to using spironolactone.
The median spironolactone daily dose was 100 mg, and ranged between 25 mg and 200 mg. Following acne score assessments, the researchers observed that 64 of the 80 patients (80%) experienced improvement of acne on treatment with spironolactone, while 16 (20%) did not respond and were subsequently escalated to oral isotretinoin therapy. Three patients (3.8%) experienced side effects, most commonly lightheadedness, headache, and fatigue, while five patients stopped taking the medication because of adverse effects, cost, or personal preference.
“It was nice to see that spironolactone did improve acne,” Dr. Roberts said. “We think of it as something to use for patients in their 20s, but not as much for patients in their teens. I think it could be a good option for them.” She also recommended starting patients on a dose of 100 mg daily. “We saw that it does have a dose response,” Dr. Roberts said. “It wasn’t until patients got to 100 mg daily that we started to see significant improvement.”
She reported having no financial disclosures.
AT SPD 2018
Key clinical point: Use of spironolactone for acne may be limited by side effects of lightheadedness, headache, and fatigue.
Major finding: Following acne score assessments, the researchers observed that 64 of the 80 patients (80%) experienced improvement of acne on treatment with spironolactone.
Study details: A retrospective review of 80 adolescent females who were treated with spironolactone and topical therapies alone, or with spironolactone plus oral antibiotics and/or contraceptive pills.
Disclosures: Dr. Roberts reported having no financial disclosures.
Maternal lifestyle affects child obesity
A study published in the British Medical Journal found that women who practiced five healthy habits had children who when they reached adolescence were 75% less likely to be overweight, compared with women who practiced none of the those healthy habits.
The healthy habits were maintaining a healthy weight, eating a nutritious diet, exercising regularly, not smoking, and consuming no more than a moderate amount of alcohol (BMJ 2018;362:k2486). I suspect you aren’t surprised by the core finding of this study of 16,945 female nurses and their 24,289 children. You’ve seen it scores of times. Mothers who lead unhealthy lifestyles seem to have children who are more likely to be obese. Now you have some numbers to support your decades of anecdotal observations. But the question is, what are we supposed to do with this new data? When and with whom should we share this unfortunate truth?
Evidence from previous studies makes it clear that by the time a child enters grade school the die is cast. Baby fat is neither cute nor temporary. This means that our target audience must be mothers-to-be and women whose children are infants and toddlers. On the other hand, telling the mother of an overweight teenager that her own unhealthy habits have probably contributed to her child’s weight problem is cruel and a waste of time. The mother already may have suspected her culpability. She also may feel that it is too late to do anything about it. While there have been some studies looking for an association between paternal body mass index and offspring BMI, I was unable to find any addressing paternal lifestyle and adolescent obesity.
This new study doesn’t address the unusual situation in which a mother of a teenager sheds all five of her unhealthy habits. I guess there may be examples in which a mother’s positive lifestyle change has helped reverse her adolescent child’s path to obesity. But I suspect these cases are rare.
So on one hand but on the other we must be careful to avoid playing the blame game and giving other mothers a one-way ticket on the guilt train. This is just one more example of the tightrope that we have been walking for generations. Every day in our offices we see children whose health is endangered by their parents’ behaviors and lifestyles. In cases in which the parental behavior is creating a serious short-term risk, such as failing to use an appropriate motor vehicle safety restraint system, we have no qualms about speaking out. We aren’t afraid to do a little shaming in hopes of sparing a family a serious guilt trip. When the threat to the child is more abstract and less dramatic – such as vaccine refusal – shaming and education don’t seem to be effective in changing parental behavior.
Obesity presents its own collection of complexities. It is like a car wreck seen in slow motion as the plots on the growth chart accumulate pound by pound. Unfortunately, parents often are among the last to notice or accept the reality. This new study doesn’t tell us whether we can make a difference. But it does suggest that when we first see the warning signs on the growth chart that we should engage the parents in a discussion of their lifestyle and its possible association with the child’s weight gain. The challenge, of course, is how one can cast the discussion without sounding judgmental.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A study published in the British Medical Journal found that women who practiced five healthy habits had children who when they reached adolescence were 75% less likely to be overweight, compared with women who practiced none of the those healthy habits.
The healthy habits were maintaining a healthy weight, eating a nutritious diet, exercising regularly, not smoking, and consuming no more than a moderate amount of alcohol (BMJ 2018;362:k2486). I suspect you aren’t surprised by the core finding of this study of 16,945 female nurses and their 24,289 children. You’ve seen it scores of times. Mothers who lead unhealthy lifestyles seem to have children who are more likely to be obese. Now you have some numbers to support your decades of anecdotal observations. But the question is, what are we supposed to do with this new data? When and with whom should we share this unfortunate truth?
Evidence from previous studies makes it clear that by the time a child enters grade school the die is cast. Baby fat is neither cute nor temporary. This means that our target audience must be mothers-to-be and women whose children are infants and toddlers. On the other hand, telling the mother of an overweight teenager that her own unhealthy habits have probably contributed to her child’s weight problem is cruel and a waste of time. The mother already may have suspected her culpability. She also may feel that it is too late to do anything about it. While there have been some studies looking for an association between paternal body mass index and offspring BMI, I was unable to find any addressing paternal lifestyle and adolescent obesity.
This new study doesn’t address the unusual situation in which a mother of a teenager sheds all five of her unhealthy habits. I guess there may be examples in which a mother’s positive lifestyle change has helped reverse her adolescent child’s path to obesity. But I suspect these cases are rare.
So on one hand but on the other we must be careful to avoid playing the blame game and giving other mothers a one-way ticket on the guilt train. This is just one more example of the tightrope that we have been walking for generations. Every day in our offices we see children whose health is endangered by their parents’ behaviors and lifestyles. In cases in which the parental behavior is creating a serious short-term risk, such as failing to use an appropriate motor vehicle safety restraint system, we have no qualms about speaking out. We aren’t afraid to do a little shaming in hopes of sparing a family a serious guilt trip. When the threat to the child is more abstract and less dramatic – such as vaccine refusal – shaming and education don’t seem to be effective in changing parental behavior.
Obesity presents its own collection of complexities. It is like a car wreck seen in slow motion as the plots on the growth chart accumulate pound by pound. Unfortunately, parents often are among the last to notice or accept the reality. This new study doesn’t tell us whether we can make a difference. But it does suggest that when we first see the warning signs on the growth chart that we should engage the parents in a discussion of their lifestyle and its possible association with the child’s weight gain. The challenge, of course, is how one can cast the discussion without sounding judgmental.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A study published in the British Medical Journal found that women who practiced five healthy habits had children who when they reached adolescence were 75% less likely to be overweight, compared with women who practiced none of the those healthy habits.
The healthy habits were maintaining a healthy weight, eating a nutritious diet, exercising regularly, not smoking, and consuming no more than a moderate amount of alcohol (BMJ 2018;362:k2486). I suspect you aren’t surprised by the core finding of this study of 16,945 female nurses and their 24,289 children. You’ve seen it scores of times. Mothers who lead unhealthy lifestyles seem to have children who are more likely to be obese. Now you have some numbers to support your decades of anecdotal observations. But the question is, what are we supposed to do with this new data? When and with whom should we share this unfortunate truth?
Evidence from previous studies makes it clear that by the time a child enters grade school the die is cast. Baby fat is neither cute nor temporary. This means that our target audience must be mothers-to-be and women whose children are infants and toddlers. On the other hand, telling the mother of an overweight teenager that her own unhealthy habits have probably contributed to her child’s weight problem is cruel and a waste of time. The mother already may have suspected her culpability. She also may feel that it is too late to do anything about it. While there have been some studies looking for an association between paternal body mass index and offspring BMI, I was unable to find any addressing paternal lifestyle and adolescent obesity.
This new study doesn’t address the unusual situation in which a mother of a teenager sheds all five of her unhealthy habits. I guess there may be examples in which a mother’s positive lifestyle change has helped reverse her adolescent child’s path to obesity. But I suspect these cases are rare.
So on one hand but on the other we must be careful to avoid playing the blame game and giving other mothers a one-way ticket on the guilt train. This is just one more example of the tightrope that we have been walking for generations. Every day in our offices we see children whose health is endangered by their parents’ behaviors and lifestyles. In cases in which the parental behavior is creating a serious short-term risk, such as failing to use an appropriate motor vehicle safety restraint system, we have no qualms about speaking out. We aren’t afraid to do a little shaming in hopes of sparing a family a serious guilt trip. When the threat to the child is more abstract and less dramatic – such as vaccine refusal – shaming and education don’t seem to be effective in changing parental behavior.
Obesity presents its own collection of complexities. It is like a car wreck seen in slow motion as the plots on the growth chart accumulate pound by pound. Unfortunately, parents often are among the last to notice or accept the reality. This new study doesn’t tell us whether we can make a difference. But it does suggest that when we first see the warning signs on the growth chart that we should engage the parents in a discussion of their lifestyle and its possible association with the child’s weight gain. The challenge, of course, is how one can cast the discussion without sounding judgmental.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
AHRQ National Guideline Clearinghouse shutting down
The Agency for Healthcare Research and Quality has announced that after July 16, 2018, its National Guideline Clearinghouse (NGC) website (guideline.gov) will no longer be available because its federal funding will be discontinued. According to the NGC website, its mission is to provide “an accessible mechanism for obtaining objective, detailed information on clinical practice guidelines and to further their dissemination, implementation, and use.”
Its services, all free to use, included providing summaries of each guideline, side-by-side comparisons of two or more guidelines, and syntheses of guidelines covering similar topics, highlighting areas of similarity and difference.
Update, July 17, 2018: Now that the guidelines are no longer available on the government site, ECRI Institute, the independent nonprofit that developed and maintained the National Guidelines Clearinghouse since its inception 20 years ago, “announced plans to continue providing this critical service to the healthcare community.” The ECRI Institute stated that they will launch an interim website this Fall “with many additional features planned for the near future.” Participating guideline developers will be able to access and contribute to the website free of charge, according to the company, while others will be charged a fee. In addition, as a temporary stop-gap, Fred Trotter, founder and CTO of CareSet Systems, a Medicare data use site, voluntarily cloned the complete guidelines before the government site shut down and made them available for public use here: https://github.com/CareSet/AHRQ_search_clone.
The Agency for Healthcare Research and Quality has announced that after July 16, 2018, its National Guideline Clearinghouse (NGC) website (guideline.gov) will no longer be available because its federal funding will be discontinued. According to the NGC website, its mission is to provide “an accessible mechanism for obtaining objective, detailed information on clinical practice guidelines and to further their dissemination, implementation, and use.”
Its services, all free to use, included providing summaries of each guideline, side-by-side comparisons of two or more guidelines, and syntheses of guidelines covering similar topics, highlighting areas of similarity and difference.
Update, July 17, 2018: Now that the guidelines are no longer available on the government site, ECRI Institute, the independent nonprofit that developed and maintained the National Guidelines Clearinghouse since its inception 20 years ago, “announced plans to continue providing this critical service to the healthcare community.” The ECRI Institute stated that they will launch an interim website this Fall “with many additional features planned for the near future.” Participating guideline developers will be able to access and contribute to the website free of charge, according to the company, while others will be charged a fee. In addition, as a temporary stop-gap, Fred Trotter, founder and CTO of CareSet Systems, a Medicare data use site, voluntarily cloned the complete guidelines before the government site shut down and made them available for public use here: https://github.com/CareSet/AHRQ_search_clone.
The Agency for Healthcare Research and Quality has announced that after July 16, 2018, its National Guideline Clearinghouse (NGC) website (guideline.gov) will no longer be available because its federal funding will be discontinued. According to the NGC website, its mission is to provide “an accessible mechanism for obtaining objective, detailed information on clinical practice guidelines and to further their dissemination, implementation, and use.”
Its services, all free to use, included providing summaries of each guideline, side-by-side comparisons of two or more guidelines, and syntheses of guidelines covering similar topics, highlighting areas of similarity and difference.
Update, July 17, 2018: Now that the guidelines are no longer available on the government site, ECRI Institute, the independent nonprofit that developed and maintained the National Guidelines Clearinghouse since its inception 20 years ago, “announced plans to continue providing this critical service to the healthcare community.” The ECRI Institute stated that they will launch an interim website this Fall “with many additional features planned for the near future.” Participating guideline developers will be able to access and contribute to the website free of charge, according to the company, while others will be charged a fee. In addition, as a temporary stop-gap, Fred Trotter, founder and CTO of CareSet Systems, a Medicare data use site, voluntarily cloned the complete guidelines before the government site shut down and made them available for public use here: https://github.com/CareSet/AHRQ_search_clone.