User login
Myeloma frailty index predicts survival based on biological age
A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than chronological age alone, investigators report. A 16% increased risk of death was seen for each 10% increase in the deficit-accumulation frailty index (DAFI), which includes 25 variables related health, function, and activities of daily living.
There was only a weak correlation between chronological age and increase in deficits tracked by the index, in contrast to a cohort without cancer, in which age and frailty were strongly correlated, the investigators reported in JCO Clinical Cancer Informatics.
“Our results demonstrate that, for patients with multiple myeloma, chronological age alone is not a good measure for assessing overall health,” study author Tanya M. Wildes, MD, of Washington University, St. Louis, said in a news release from the American Society of Clinical Oncology.
Existing tools to assess frailty include an index proposed by the International Myeloma Working Group that looks at age plus other indexes related to comorbidities and activities of daily living, and the revised Myeloma Comorbidity Index that incorporates age with other prognostic factors.
“Although both tools provide prognostic information, chronological age automatically increases frailty without taking biologic or functional age into account,” Dr. Wildes and her coauthors wrote in their report.
By contrast, the DAFI is based on the concept of biologic age, in which the health status of an individual is measured based on the proportion of aging-associated deficits they have accumulated, according to the authors.
To create the DAFI, Dr. Wildes and her colleagues analyzed nearly 2.7 million records of noncancer patients aged 66 years or older in the SEER Medicare Health Outcomes Survey (MHOS) database. They identified 25 variables in the database representing chronic health conditions, activities of daily living, functioning, mental health, and general health.
An individual’s DAFI score was calculated as the sum of scores for each of the 25 variables as 0 for absent, 0.5 for limited, and 1 for present. Predicted DAFI means were calculated for each year of age and used to create age-specific cut points to determine whether an individual would be considered frail or not versus others of the same age.
“In other words, the same frailty score may qualify an 80-year-old individual as fit and a 70-year-old as frail, depending on the cutoff for their respective age group,” investigators explained in their report.
They applied the index to 305 patients with newly diagnosed myeloma in the SEER-MHOS database who were 66 years of age or older (median age, 76 years) and had completed the survey within 1 year of diagnosis.
The DAFI classified 52% of the myeloma patients as frail, and for that group, median overall survival was 26.8 months, versus 43.7 months for nonfrail patients (P = .015), according to the reported data. For each 10% increase in score, the risk of death increased by 16% (P less than .001).
Notably, advancing age was very weakly correlated with increased age-related deficits in the myeloma cohort (r2 = 0.15; P = .010), according to investigators, but very strongly correlated with deficits in the cohort of noncancer patients (r2 = 0.98; P less than .001).
“This suggests that, in patients with multiple myeloma, the prevalence of impairments across domains of function, chronic comorbidities, general health, and mental health are more related to the overall burden of myeloma rather than chronological age alone,” the investigators wrote.
The information used to calculate a DAFI score is easily obtainable during a clinic visit, according to the authors, who provided an overview of all 25 variables in the journal article.
Further development of a computerized program would further enhance usability in the clinic, allowing for real-time calculation during a patient visit, they said.
Survivorship expert Merry Jennifer Markham, MD, said in the ASCO news release that this frailty index is notable because it accounts for more than just chronological age. “Knowing this information can help oncologists have more informed discussions with patients about their prognosis, which in turn can empower patients and families as they weigh treatment options,” she said.
The research was supported by National Cancer Institute. Dr. Wildes reported honoraria from Carevive Systems and research funding from Janssen Oncology. Another coauthor reported honoraria from Celgene and Janssen, and a consulting or advisory role with Amgen and Takeda.
SOURCE: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than chronological age alone, investigators report. A 16% increased risk of death was seen for each 10% increase in the deficit-accumulation frailty index (DAFI), which includes 25 variables related health, function, and activities of daily living.
There was only a weak correlation between chronological age and increase in deficits tracked by the index, in contrast to a cohort without cancer, in which age and frailty were strongly correlated, the investigators reported in JCO Clinical Cancer Informatics.
“Our results demonstrate that, for patients with multiple myeloma, chronological age alone is not a good measure for assessing overall health,” study author Tanya M. Wildes, MD, of Washington University, St. Louis, said in a news release from the American Society of Clinical Oncology.
Existing tools to assess frailty include an index proposed by the International Myeloma Working Group that looks at age plus other indexes related to comorbidities and activities of daily living, and the revised Myeloma Comorbidity Index that incorporates age with other prognostic factors.
“Although both tools provide prognostic information, chronological age automatically increases frailty without taking biologic or functional age into account,” Dr. Wildes and her coauthors wrote in their report.
By contrast, the DAFI is based on the concept of biologic age, in which the health status of an individual is measured based on the proportion of aging-associated deficits they have accumulated, according to the authors.
To create the DAFI, Dr. Wildes and her colleagues analyzed nearly 2.7 million records of noncancer patients aged 66 years or older in the SEER Medicare Health Outcomes Survey (MHOS) database. They identified 25 variables in the database representing chronic health conditions, activities of daily living, functioning, mental health, and general health.
An individual’s DAFI score was calculated as the sum of scores for each of the 25 variables as 0 for absent, 0.5 for limited, and 1 for present. Predicted DAFI means were calculated for each year of age and used to create age-specific cut points to determine whether an individual would be considered frail or not versus others of the same age.
“In other words, the same frailty score may qualify an 80-year-old individual as fit and a 70-year-old as frail, depending on the cutoff for their respective age group,” investigators explained in their report.
They applied the index to 305 patients with newly diagnosed myeloma in the SEER-MHOS database who were 66 years of age or older (median age, 76 years) and had completed the survey within 1 year of diagnosis.
The DAFI classified 52% of the myeloma patients as frail, and for that group, median overall survival was 26.8 months, versus 43.7 months for nonfrail patients (P = .015), according to the reported data. For each 10% increase in score, the risk of death increased by 16% (P less than .001).
Notably, advancing age was very weakly correlated with increased age-related deficits in the myeloma cohort (r2 = 0.15; P = .010), according to investigators, but very strongly correlated with deficits in the cohort of noncancer patients (r2 = 0.98; P less than .001).
“This suggests that, in patients with multiple myeloma, the prevalence of impairments across domains of function, chronic comorbidities, general health, and mental health are more related to the overall burden of myeloma rather than chronological age alone,” the investigators wrote.
The information used to calculate a DAFI score is easily obtainable during a clinic visit, according to the authors, who provided an overview of all 25 variables in the journal article.
Further development of a computerized program would further enhance usability in the clinic, allowing for real-time calculation during a patient visit, they said.
Survivorship expert Merry Jennifer Markham, MD, said in the ASCO news release that this frailty index is notable because it accounts for more than just chronological age. “Knowing this information can help oncologists have more informed discussions with patients about their prognosis, which in turn can empower patients and families as they weigh treatment options,” she said.
The research was supported by National Cancer Institute. Dr. Wildes reported honoraria from Carevive Systems and research funding from Janssen Oncology. Another coauthor reported honoraria from Celgene and Janssen, and a consulting or advisory role with Amgen and Takeda.
SOURCE: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than chronological age alone, investigators report. A 16% increased risk of death was seen for each 10% increase in the deficit-accumulation frailty index (DAFI), which includes 25 variables related health, function, and activities of daily living.
There was only a weak correlation between chronological age and increase in deficits tracked by the index, in contrast to a cohort without cancer, in which age and frailty were strongly correlated, the investigators reported in JCO Clinical Cancer Informatics.
“Our results demonstrate that, for patients with multiple myeloma, chronological age alone is not a good measure for assessing overall health,” study author Tanya M. Wildes, MD, of Washington University, St. Louis, said in a news release from the American Society of Clinical Oncology.
Existing tools to assess frailty include an index proposed by the International Myeloma Working Group that looks at age plus other indexes related to comorbidities and activities of daily living, and the revised Myeloma Comorbidity Index that incorporates age with other prognostic factors.
“Although both tools provide prognostic information, chronological age automatically increases frailty without taking biologic or functional age into account,” Dr. Wildes and her coauthors wrote in their report.
By contrast, the DAFI is based on the concept of biologic age, in which the health status of an individual is measured based on the proportion of aging-associated deficits they have accumulated, according to the authors.
To create the DAFI, Dr. Wildes and her colleagues analyzed nearly 2.7 million records of noncancer patients aged 66 years or older in the SEER Medicare Health Outcomes Survey (MHOS) database. They identified 25 variables in the database representing chronic health conditions, activities of daily living, functioning, mental health, and general health.
An individual’s DAFI score was calculated as the sum of scores for each of the 25 variables as 0 for absent, 0.5 for limited, and 1 for present. Predicted DAFI means were calculated for each year of age and used to create age-specific cut points to determine whether an individual would be considered frail or not versus others of the same age.
“In other words, the same frailty score may qualify an 80-year-old individual as fit and a 70-year-old as frail, depending on the cutoff for their respective age group,” investigators explained in their report.
They applied the index to 305 patients with newly diagnosed myeloma in the SEER-MHOS database who were 66 years of age or older (median age, 76 years) and had completed the survey within 1 year of diagnosis.
The DAFI classified 52% of the myeloma patients as frail, and for that group, median overall survival was 26.8 months, versus 43.7 months for nonfrail patients (P = .015), according to the reported data. For each 10% increase in score, the risk of death increased by 16% (P less than .001).
Notably, advancing age was very weakly correlated with increased age-related deficits in the myeloma cohort (r2 = 0.15; P = .010), according to investigators, but very strongly correlated with deficits in the cohort of noncancer patients (r2 = 0.98; P less than .001).
“This suggests that, in patients with multiple myeloma, the prevalence of impairments across domains of function, chronic comorbidities, general health, and mental health are more related to the overall burden of myeloma rather than chronological age alone,” the investigators wrote.
The information used to calculate a DAFI score is easily obtainable during a clinic visit, according to the authors, who provided an overview of all 25 variables in the journal article.
Further development of a computerized program would further enhance usability in the clinic, allowing for real-time calculation during a patient visit, they said.
Survivorship expert Merry Jennifer Markham, MD, said in the ASCO news release that this frailty index is notable because it accounts for more than just chronological age. “Knowing this information can help oncologists have more informed discussions with patients about their prognosis, which in turn can empower patients and families as they weigh treatment options,” she said.
The research was supported by National Cancer Institute. Dr. Wildes reported honoraria from Carevive Systems and research funding from Janssen Oncology. Another coauthor reported honoraria from Celgene and Janssen, and a consulting or advisory role with Amgen and Takeda.
SOURCE: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
FROM JCO CLINICAL CANCER INFORMATICS
Key clinical point: A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than on chronological age alone.
Major finding: Median overall survival was 26.8 months for patients classified as frail, vs. 43.7 months for nonfrail patients (P = .015).
Study details: Retrospective analysis of 2.7 million records of noncancer patients to create an index subsequently validated in records for 305 patients with newly diagnosed multiple myeloma (aged 66 years and older).
Disclosures: The research was supported by National Cancer Institute. Authors reported disclosures related to Celgene, Janssen, Amgen, Takeda, and Carevive Systems.
Source: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
Benzodiazepines for anxious depression
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
VIDEO: Look for Signs of Depression in Rosacea Patients
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Cannabis falls short for chronic noncancer pain
Cannabis did not improve outcomes or reduce prescription opioid use among 1,514 Australians with noncancer pain, according to a recent report in Lancet Public Health.
Almost a quarter of patients reported using cannabis, mostly illicitly since most of the data were collected before Australia legalized medical marijuana in 2016. About 9% reported marijuana use in the previous month at baseline; 13% reported use in the past month at the final interview.
Overall, users rated the degree of relief they got from pain and pain-related distress as 7 out of 10, but the study findings did not support their impression.
At 4 years, cannabis users, compared with nonusers, reported greater pain severity (for daily or near-daily use: risk ratio, 1.17; 95% confidence interval, 1.03-1.32; for less frequent use: RR, 1.14; 95% CI, 1.01-1.29), more interference from pain in their daily lives (for daily or near-daily use: RR, 1.14; 95% CI, 1.03-1.26; for less frequent use: RR, 1.21; 95% CI, 1.09-1.35 ), less ability to cope with pain (for daily or near-daily use: RR, 0.98; 95% CI, 0.96-1.00; for less frequent use: RR, 0.97; 95% CI, 0.96-1.00), and greater generalized anxiety (for daily or near-daily use: RR, 1.10; 95% CI, 1.06-1.15; for less frequent use: RR, 1.07; 95% CI, 1.03-1.12). Results were adjusted for age, sex, pain duration, and other factors.
Pain severity scores on the 10-point Brief Pain Inventory, for instance, were 4.7 points at the end of the study among nonusers, compared with 5.3 among daily or near-daily users.
Few differences were reported in oral morphine equivalents between the groups. People who reported using marijuana 1-19 days a month were less likely to have discontinued opioids at 4 years (9%) than were those reporting no use (21%).
“Interest in the use of cannabis and cannabinoids to treat chronic noncancer pain is increasing because of their potential to reduce opioid dose requirements.” However, “we found no evidence that cannabis use improved patient outcomes” or that cannabis “exerted an opioid-sparing effect,” said the investigators, led by Gabrielle Campbell, PhD, of the National Drug and Alcohol Research Centre, Sydney.
The findings are not a slam dunk against cannabis for chronic pain. The investigators noted that people might have used cannabis because they had more pain to begin with and poorer coping mechanisms. Had they not been using marijuana, perhaps they would have been worse off.
However, “to date, evidence that cannabinoids are effective for chronic noncancer pain and aid in reducing opioid use is lacking. they said.
Dr. Campbell and her associates cited several important limitations. Since cannabis use was primarily illicit, it’s unlikely that it was used under medical supervision. Also, the study only gauged frequency of use on a per-day basis. “We do not know if some people used once in a day or more than once. Likewise, we do not know what type of cannabis the participants used. ... This fact matters, as cannabis varies in strength and, as with any analgesia, the dose needs to be matched to the severity of pain experienced,” the team said.
The subjects were a median age of 58 years at baseline, and 56% were women. They had been prescribed a strong opioid for a median of 4 years at study entry and were on a median oral morphine equivalent dose of 75 mg/day, which fell to 57 mg/day at the study’s conclusion. About 62% of the subjects reported neuropathic pain.
The work was funded by the Australian government, and the National Health and Medical Research Council. Dr. Campbell reported grants from Reckitt Benckiser.
SOURCE: Campbell G et al. Lancet Public Health. 2018 Jul. doi: 10.1016/S2468-2667(18)30110-5.
Cannabis did not improve outcomes or reduce prescription opioid use among 1,514 Australians with noncancer pain, according to a recent report in Lancet Public Health.
Almost a quarter of patients reported using cannabis, mostly illicitly since most of the data were collected before Australia legalized medical marijuana in 2016. About 9% reported marijuana use in the previous month at baseline; 13% reported use in the past month at the final interview.
Overall, users rated the degree of relief they got from pain and pain-related distress as 7 out of 10, but the study findings did not support their impression.
At 4 years, cannabis users, compared with nonusers, reported greater pain severity (for daily or near-daily use: risk ratio, 1.17; 95% confidence interval, 1.03-1.32; for less frequent use: RR, 1.14; 95% CI, 1.01-1.29), more interference from pain in their daily lives (for daily or near-daily use: RR, 1.14; 95% CI, 1.03-1.26; for less frequent use: RR, 1.21; 95% CI, 1.09-1.35 ), less ability to cope with pain (for daily or near-daily use: RR, 0.98; 95% CI, 0.96-1.00; for less frequent use: RR, 0.97; 95% CI, 0.96-1.00), and greater generalized anxiety (for daily or near-daily use: RR, 1.10; 95% CI, 1.06-1.15; for less frequent use: RR, 1.07; 95% CI, 1.03-1.12). Results were adjusted for age, sex, pain duration, and other factors.
Pain severity scores on the 10-point Brief Pain Inventory, for instance, were 4.7 points at the end of the study among nonusers, compared with 5.3 among daily or near-daily users.
Few differences were reported in oral morphine equivalents between the groups. People who reported using marijuana 1-19 days a month were less likely to have discontinued opioids at 4 years (9%) than were those reporting no use (21%).
“Interest in the use of cannabis and cannabinoids to treat chronic noncancer pain is increasing because of their potential to reduce opioid dose requirements.” However, “we found no evidence that cannabis use improved patient outcomes” or that cannabis “exerted an opioid-sparing effect,” said the investigators, led by Gabrielle Campbell, PhD, of the National Drug and Alcohol Research Centre, Sydney.
The findings are not a slam dunk against cannabis for chronic pain. The investigators noted that people might have used cannabis because they had more pain to begin with and poorer coping mechanisms. Had they not been using marijuana, perhaps they would have been worse off.
However, “to date, evidence that cannabinoids are effective for chronic noncancer pain and aid in reducing opioid use is lacking. they said.
Dr. Campbell and her associates cited several important limitations. Since cannabis use was primarily illicit, it’s unlikely that it was used under medical supervision. Also, the study only gauged frequency of use on a per-day basis. “We do not know if some people used once in a day or more than once. Likewise, we do not know what type of cannabis the participants used. ... This fact matters, as cannabis varies in strength and, as with any analgesia, the dose needs to be matched to the severity of pain experienced,” the team said.
The subjects were a median age of 58 years at baseline, and 56% were women. They had been prescribed a strong opioid for a median of 4 years at study entry and were on a median oral morphine equivalent dose of 75 mg/day, which fell to 57 mg/day at the study’s conclusion. About 62% of the subjects reported neuropathic pain.
The work was funded by the Australian government, and the National Health and Medical Research Council. Dr. Campbell reported grants from Reckitt Benckiser.
SOURCE: Campbell G et al. Lancet Public Health. 2018 Jul. doi: 10.1016/S2468-2667(18)30110-5.
Cannabis did not improve outcomes or reduce prescription opioid use among 1,514 Australians with noncancer pain, according to a recent report in Lancet Public Health.
Almost a quarter of patients reported using cannabis, mostly illicitly since most of the data were collected before Australia legalized medical marijuana in 2016. About 9% reported marijuana use in the previous month at baseline; 13% reported use in the past month at the final interview.
Overall, users rated the degree of relief they got from pain and pain-related distress as 7 out of 10, but the study findings did not support their impression.
At 4 years, cannabis users, compared with nonusers, reported greater pain severity (for daily or near-daily use: risk ratio, 1.17; 95% confidence interval, 1.03-1.32; for less frequent use: RR, 1.14; 95% CI, 1.01-1.29), more interference from pain in their daily lives (for daily or near-daily use: RR, 1.14; 95% CI, 1.03-1.26; for less frequent use: RR, 1.21; 95% CI, 1.09-1.35 ), less ability to cope with pain (for daily or near-daily use: RR, 0.98; 95% CI, 0.96-1.00; for less frequent use: RR, 0.97; 95% CI, 0.96-1.00), and greater generalized anxiety (for daily or near-daily use: RR, 1.10; 95% CI, 1.06-1.15; for less frequent use: RR, 1.07; 95% CI, 1.03-1.12). Results were adjusted for age, sex, pain duration, and other factors.
Pain severity scores on the 10-point Brief Pain Inventory, for instance, were 4.7 points at the end of the study among nonusers, compared with 5.3 among daily or near-daily users.
Few differences were reported in oral morphine equivalents between the groups. People who reported using marijuana 1-19 days a month were less likely to have discontinued opioids at 4 years (9%) than were those reporting no use (21%).
“Interest in the use of cannabis and cannabinoids to treat chronic noncancer pain is increasing because of their potential to reduce opioid dose requirements.” However, “we found no evidence that cannabis use improved patient outcomes” or that cannabis “exerted an opioid-sparing effect,” said the investigators, led by Gabrielle Campbell, PhD, of the National Drug and Alcohol Research Centre, Sydney.
The findings are not a slam dunk against cannabis for chronic pain. The investigators noted that people might have used cannabis because they had more pain to begin with and poorer coping mechanisms. Had they not been using marijuana, perhaps they would have been worse off.
However, “to date, evidence that cannabinoids are effective for chronic noncancer pain and aid in reducing opioid use is lacking. they said.
Dr. Campbell and her associates cited several important limitations. Since cannabis use was primarily illicit, it’s unlikely that it was used under medical supervision. Also, the study only gauged frequency of use on a per-day basis. “We do not know if some people used once in a day or more than once. Likewise, we do not know what type of cannabis the participants used. ... This fact matters, as cannabis varies in strength and, as with any analgesia, the dose needs to be matched to the severity of pain experienced,” the team said.
The subjects were a median age of 58 years at baseline, and 56% were women. They had been prescribed a strong opioid for a median of 4 years at study entry and were on a median oral morphine equivalent dose of 75 mg/day, which fell to 57 mg/day at the study’s conclusion. About 62% of the subjects reported neuropathic pain.
The work was funded by the Australian government, and the National Health and Medical Research Council. Dr. Campbell reported grants from Reckitt Benckiser.
SOURCE: Campbell G et al. Lancet Public Health. 2018 Jul. doi: 10.1016/S2468-2667(18)30110-5.
FROM THE LANCET PUBLIC HEALTH
Key clinical point: Cannabis did not improve outcomes or reduce prescription opioid use among 1,514 Australians with chronic noncancer pain.
Major finding: Pain severity scores on the 10-point Brief Pain Inventory were 4.7 points at the end of the study among nonusers, compared with 5.3 among daily or near-daily users.
Study details: A 4-year observational, prospective investigation.
Disclosures: The work was funded by the Australian government and the National Health and Medical Research Council. The study lead reported grants from Reckitt Benckiser.
Source: Campbell G et al. Lancet Public Health. 2018 Jul. doi: 10.1016/S2468-2667(18)30110-5.
Risk Stratification for Cellulitis Versus Noncellulitic Conditions of the Lower Extremity: A Retrospective Review of the NEW HAvUN Criteria
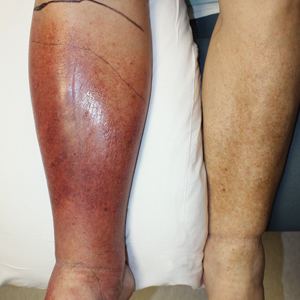
Cellulitis is defined as an acute or subacute, bacterial-induced inflammation of subcutaneous tissue that can extend superficially. The inciting incident often is assumed to be invasion of bacteria through loose connective tissue.1 Although cellulitis is bacterial in origin, it often is difficult to culture the offending microorganism from biopsy sites, swabs, or blood. Erythema, fever, induration, and tenderness are largely seen as clinical manifestations. Moderate and severe cases may be accompanied by fever, malaise, and leukocytosis. The lower extremity is the most common location of involvement (Figure 1), and usually a wound, ulcer, or interdigital superficial infection can be identified and implicated as the source of entry.
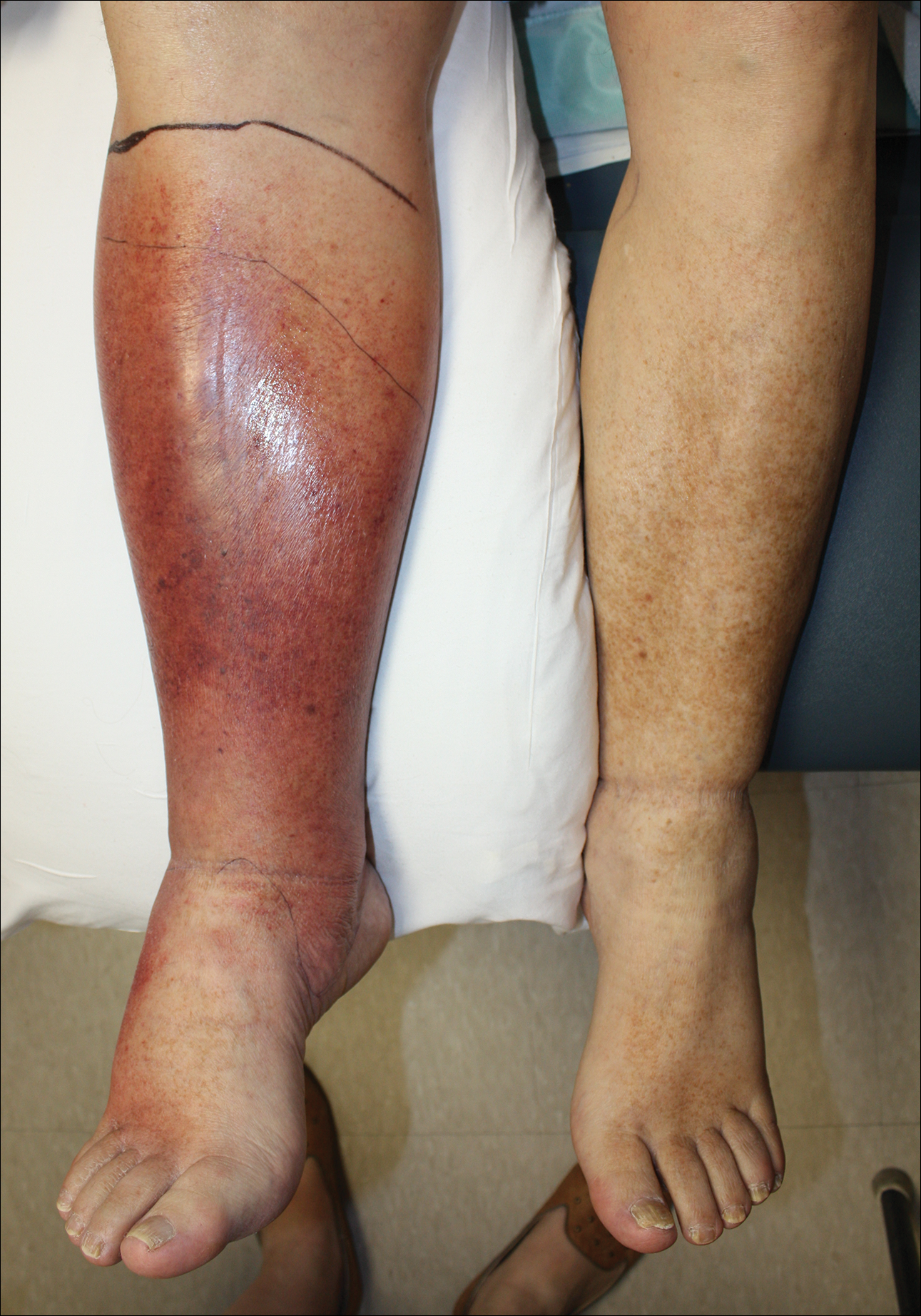
Effective treatment of cellulitis is necessary because complications such as abscesses, underlying fascia or muscle involvement, and septicemia can develop, leading to poor outcomes. Antibiotics should be administered intravenously in patients with suspected fascial involvement, septicemia, or dermal necrosis, or in those with an immunological comorbidity.2
The differential diagnosis of lower extremity cellulitis is wide due to the existence of several mimicking dermatologic conditions. These so-called pseudocellulitis conditions include stasis dermatitis, venous ulceration, acute lipodermatosclerosis, pigmented purpura, vasculopathy, contact dermatitis, adverse medication reaction, and arthropod bite. Stasis dermatitis and lipodermatosclerosis, both arising from venous insufficiency, are by far 2 of the most common skin conditions that imitate cellulitis.
Stasis dermatitis is a common condition in the United States and Europe, usually manifesting as a pigmented purpuric dermatosis on anterior tibial surfaces, around the ankle, or overlying dependent varicosities. Skin changes can include hyperpigmentation, edema, mild scaling, eczematous patches, and even ulceration.3
Lipodermatosclerosis is a disorder of progressive fibrosis of subcutaneous fat. It is more common in middle-aged women who have a high body mass index and a venous abnormality.4 This form of panniculitis typically affects the lower extremities bilaterally, manifesting as erythematous and indurated skin changes, sometimes described as inverted champagne bottles (Figure 2). At times, there can be accompanying painful ulceration on the erythematous areas, features that closely resemble cellulitis.5,6 Lipodermatosclerosis is commonly misdiagnosed as cellulitis, leading to inappropriate prescription of antibiotics.7

Distinguishing cellulitis from noncellulitic conditions of the lower extremity is paramount to effective patient management in the emergent setting. With a reported incidence of 24.6 per 100 person-years, cellulitis constitutes 1% to 14% of emergency department visits and 4% to 7% of hospital admissions.Therefore, prompt appropriate diagnosis and treatment can avoid life-threatening complications associated with infection such as sepsis, abscess, lymphangitis, and necrotizing fasciitis.8-11
It is estimated that 10% to 20% of patients who have been given a diagnosis of cellulitis do not actually have the disease.2,12 This discrepancy consumes a remarkable amount of hospital resources and can lead to inappropriate or excessive use of antibiotics.13 Although the true incidence of adverse antibiotic reactions is unknown, it is estimated that they are the cause of 3% to 6% of acute hospital admissions and occur in 10% to 15% of inpatients admitted for other primary reasons.14 These findings illustrate the potential for an increased risk for morbidity and increased length of stay for patients beginning an antibiotic regimen, especially when the agents are administered unnecessarily. In addition, inappropriate antibiotic use contributes to antibiotic resistance, which continues to be a major problem, especially in hospitalized patients.
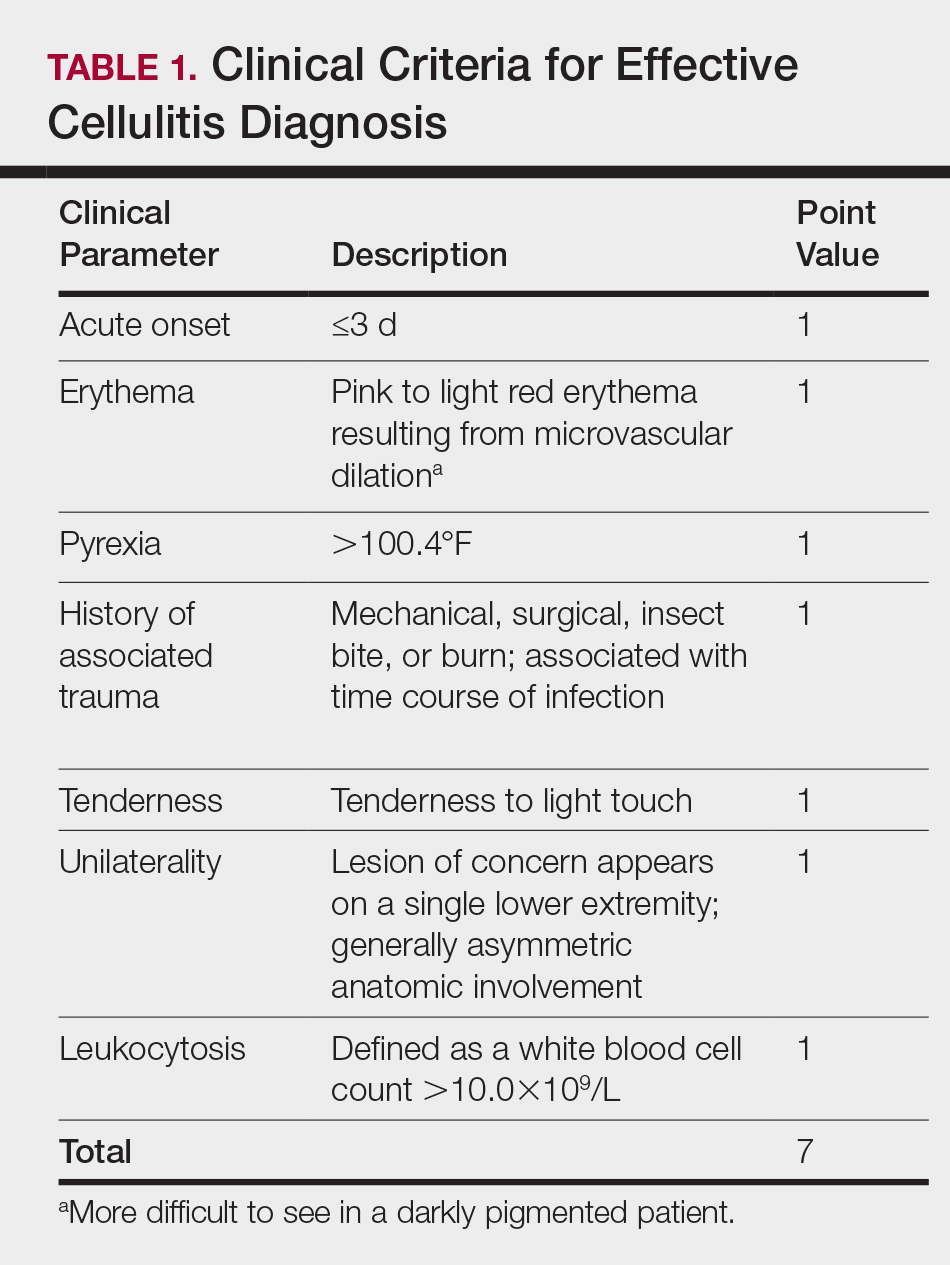
There is a lack of consensus in the literature about methods to risk stratify patients who present with acute dermatologic conditions that include and resemble cellulitis. We sought to identify clinical features based on available clinical literature-derived variables. We tested our scheme in a series of patients with a known diagnosis of cellulitis or other dermatologic pathology of the lower extremity to assess the validity of the following 7 clinical criteria: acute onset, erythema, pyrexia, history of associated trauma, tenderness, unilaterality, and leukocytosis.
Materials and Methods
This retrospective chart review was approved by the Yale University (New Haven, Connecticut) institutional review board (HIC#1409014533). Final diagnosis, demographic data, clinical manifestations, and relevant diagnostic laboratory values of 57 patients were obtained from a database in the dermatology department’s consultation log and electronic medical record database (December 2011 to December 2014). The presence of each clinical symptom—acute onset, erythema, pyrexia, history of associated trauma, tenderness, unilaterality, and leukocytosis—was assigned a score equal to 1; values were tallied to achieve a final score for each patient (Table 1). Patients who were seen initially as a consultation for possible cellulitis but given a final diagnosis of stasis dermatitis or lipodermatosclerosis were included (Table 2).

Clinical Criteria
The clinical criteria were developed based largely on clinical experience and relevant secondary literature.15-17 At the patient encounter, presence of each of the variables (Table 1) was assessed according to the following definitions:
- acute onset: within the prior 72 hours and more indicative of an acute infective process than a gradual and chronic consequence of venous stasis
- erythema: a subjective clinical marker for inflammation that can be associated with cellulitis, though darker, erythematous-appearing discolorations also can be seen in patients with chronic venous hypertension or valvular incompetence4,15
- pyrexia: body temperature greater than 100.4°F
- history of associated trauma: encompassing mechanical wounds, surgical incisions, burns, and insect bites that correlate closely to the time course of symptomatic development
- tenderness: tenderness to light touch, which may be more common in patients afflicted with cellulitis than in those with venous insufficiency
- unilaterality: a helpful distinguishing feature that points the diagnosis away from a dermatitislike clinical picture, especially because bilateral cellulitis is rare and regarded as a diagnostic pitfall18
- leukocytosis: white blood cell count greater than 10.0×109/L and is reasonably considered a cardinal metric of inflammatory processes, though it can be confounded by immunocompromise (low count) or steroid use (high count)
Statistical Analysis
Odds ratios (ORs) were calculated and χ2 analysis was performed for each presenting symptom using JMP 10.0 analytical software (SAS Institute Inc). Each patient was rated separately by means of the clinical feature–based scoring system for the calculation of a total score. After application of the score to the patient population, receiver operating characteristic curves were constructed to identify the optimal score threshold for discriminating cellulitis from dermatitis in this group. For each clinical feature, P<.05 was considered significant.
Results
Our cohort included 32 male and 25 female patients with a mean age of 63 and 61 years, respectively. The final clinical diagnosis of cellulitis was made in 20 patients (35%). An established diagnosis of cellulitis was assigned based on a dermatology evaluation located within our electronic medical record database (Table 2).
Each clinical parameter was evaluated separately for each patient; combined results are summarized in Table 3. Acute onset (≤3 days) was a clinical characteristic seen in 80% (16/20) of cellulitis cases and 22% (8/37) of noncellulitis cases (OR, 14.5; P<.001). Erythema had similar significance (OR, 10.3; prevalence, 95% [19/20] vs 65% [24/37]; P=.012). Pyrexia possessed an OR of 99.2 for cellulitis and was seen in 85% (17/20) of cellulitis cases and only 5% (2/37) of noncellulitis cases (P<.001).

A history of associated trauma had an OR of 36.0 for cellulitis, with 50% (10/20) and 3% (1/37) prevalence in cellulitis cases and noncellulitis cases, respectively (P<.001). Tenderness, documented in 90% (18/20) of cellulitis cases and 43% (16/37) of noncellulitis cases, had an OR of 11.8 (P<.001).
Unilaterality had 100% (20/20) prevalence in our cellulitis cohort and was the only characteristic within the algorithm that yielded an incalculable OR. Noncellulitis or stasis dermatitis of the lower extremity exhibited a unilateral lesion in 11 cases (30%), of which 1 case resulted from a unilateral tibial fracture. Leukocytosis was seen in 65% (13/20) of cellulitis cases and 8% (3/37) of noncellulitis cases, with an OR for cellulitis of 21.0 (P<.001).
All parameters were significant by χ2 analysis (Table 3).
Comment
We found that testing positive for 4 of 7 clinical criteria for assessing cellulitis was highly specific (95%) and sensitive (100%) for a diagnosis of cellulitis among its range of mimics (Figure 3). These cellulitis criteria can be remembered, with some modification, using NEW HAvUN as a mnemonic device (New onset,
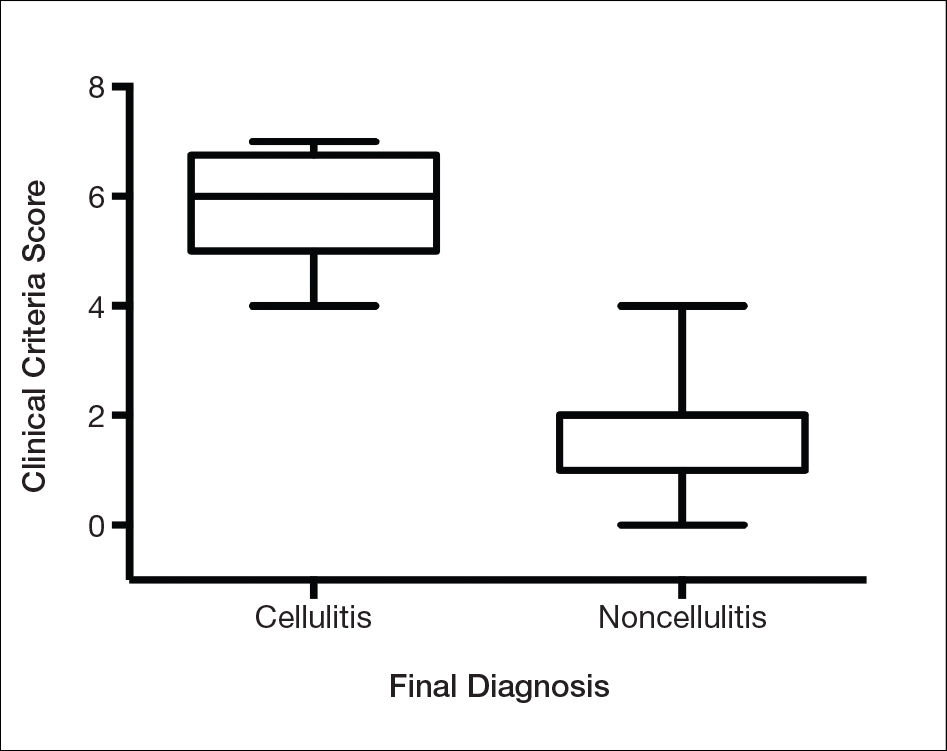
Consistent with the literature, pyrexia, history of associated trauma, and unilaterality also were predictors of cellulitis diagnosis. Unilaterality often is used as a diagnostic tool by dermatologist consultants when a patient lacks other criteria for cellulitis, so these findings are intuitive and consistent with our institutional experience. Interestingly, leukocytosis was seen in only 65% of cellulitis cases and 8% of noncellulitis cases and therefore might not serve as a sensitive independent predictor of a diagnosis of cellulitis, emphasizing the importance of the multifactorial scoring system we have put forward. Additionally, acuity of onset, erythema, and tenderness are not independently associated with cellulitis when assessing a patient because several of those findings are present in other dermatologic conditions of the lower extremity; when combined with the other criteria, however, these 3 findings can play a role in diagnosis.
Effective cellulitis diagnosis provides well-recognized challenges in the acute medical setting because many clinical mimics exist. The estimated rate of misdiagnosed cellulitis is certainly well-established: 30% to 75% in independent and multi-institutional studies. These studies also revealed that patients admitted for bilateral “cellulitis” overwhelmingly tended to be stasis clinical pictures.13,19
Cost implications from inappropriate diagnosis largely regard inappropriate antibiotic use and the potential for microbial resistance, with associated costs estimated to be more than $50 billion (2004 dollars).20,21 The true cost burden is extremely difficult to model or predict due to remarkable variations in the institutional misdiagnosis rate, prescribing pattern, and antibiotic cost and could represent avenues of further study. Misappropriation of antibiotics includes not only a monetary cost that encompasses all aspects of acute treatment and hospitalization but also an unquantifiable cost: human lives associated with the consequences of antibiotic resistance.
Conclusion
There is a lack of consensus or criteria for differentiating cellulitis from its most common clinical counterparts. Here, we propose a convenient clinical correlation system that we hope will lead to more efficient allocation of clinical resources, including antibiotics and hospital admissions, while lowering the incidence of adverse events and leading to better patient outcomes. We recognize that the small sample size of our study may limit broad application of these criteria, though we anticipate that further prospective studies can improve the diagnostic relevance and risk-assessment power of the NEW HAvUN criteria put forth here for assessing cellulitis in the acute medical setting.
Acknowledgement—Author H.H.E. recognizes the loving memory of Nadia Ezaldein for her profound influence on and motivation behind this research.
- Lep
pard BJ, Seal DV, Colman G, et al. The value of bacteriology and serology in the diagnosis of cellulitis and erysipelas. Br J Dermatol. 1985;112:559-567. - Hep
burn MJ, Dooley DP, Skidmore PJ, et al. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Int Med. 2004;164:1669-1674. - Bergan JJ, Schmid-Schönbein GW, Smith PD, et al. Chronic venous disease. N Engl J Med. 2006;355:488-498.
- Bruc
e AJ, Bennett DD, Lohse CM, et al. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol. 2002;46:187-192. - Heym
ann WR. Lipodermatosclerosis. J Am Acad Dermatol. 2009;60:1022-1023. - Vesi
ć S, Vuković J, Medenica LJ, et al. Acute lipodermatosclerosis: an open clinical trial of stanozolol in patients unable to sustain compression therapy. Dermatol Online J. 2008;14:1. - Keller
EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-552. - Dong SL, Kelly KD, Oland RC, et al. ED management of cellulitis: a review of five urban centers. Am J Emerg Med. 2001;19:535-540.
- Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134:293-299.
- Manfredi R, Calza L, Chiodo F. Epidemiology and microbiology of cellulitis and bacterial soft tissue infection during HIV disease: a 10-year survey. J Cutan Pathol. 2002;29:168-172.
- Pascarella L, Schonbein GW, Bergan JJ. Microcirculation and venous ulcers: a review. Ann Vasc Surg. 2005;19:921-927.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician. 2003;67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hay RJ, Adriaans BM. Bacterial infections. In: Thong BY, Tan TC. Epidemiology and risk factors for drug allergy. 8th ed. Br J Clin Pharmacol. 2011;71:684-700.
- Hay RJ, Adriaans BM. Bacterial infections. In: Burns T, Breathnach S, Cox N, et al. Rook’s Textbook of Dermatology. 8th ed. Hoboken, NJ: John Wiley & Sons, Inc; 2004:1345-1426.
- Wolff K, Goldsmith LA, Katz SI, et al. Fitzpatrick’s Dermatology In General Medicine. 7th ed. New York, NY: McGraw-Hill; 2003.
- Sommer LL, Reboli AC, Heymann WR. Bacterial infections. In: Bolognia J, Schaffer J, Cerroni L, et al. Dermatology. Vol 4. Philadelphia, PA: Elsevier Saunders; 2012:1462-1502.
- Cox NH. Management of lower leg cellulitis. Clin Med. 2002;2:23-27.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Pinder R, Sallis A, Berry D, et al. Behaviour change and antibiotic prescribing in healthcare settings: literature review and behavioural analysis. London, UK: Public Health England; February 2015. https://assets.publishing.service.gov.uk/government/
uploads/system/uploads/attachment_data/file/405031
/Behaviour_Change_for_Antibiotic_Prescribing_-_FINAL.pdf. Accessed May 7, 2018. - Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346:f1493.
Cellulitis is defined as an acute or subacute, bacterial-induced inflammation of subcutaneous tissue that can extend superficially. The inciting incident often is assumed to be invasion of bacteria through loose connective tissue.1 Although cellulitis is bacterial in origin, it often is difficult to culture the offending microorganism from biopsy sites, swabs, or blood. Erythema, fever, induration, and tenderness are largely seen as clinical manifestations. Moderate and severe cases may be accompanied by fever, malaise, and leukocytosis. The lower extremity is the most common location of involvement (Figure 1), and usually a wound, ulcer, or interdigital superficial infection can be identified and implicated as the source of entry.

Effective treatment of cellulitis is necessary because complications such as abscesses, underlying fascia or muscle involvement, and septicemia can develop, leading to poor outcomes. Antibiotics should be administered intravenously in patients with suspected fascial involvement, septicemia, or dermal necrosis, or in those with an immunological comorbidity.2
The differential diagnosis of lower extremity cellulitis is wide due to the existence of several mimicking dermatologic conditions. These so-called pseudocellulitis conditions include stasis dermatitis, venous ulceration, acute lipodermatosclerosis, pigmented purpura, vasculopathy, contact dermatitis, adverse medication reaction, and arthropod bite. Stasis dermatitis and lipodermatosclerosis, both arising from venous insufficiency, are by far 2 of the most common skin conditions that imitate cellulitis.
Stasis dermatitis is a common condition in the United States and Europe, usually manifesting as a pigmented purpuric dermatosis on anterior tibial surfaces, around the ankle, or overlying dependent varicosities. Skin changes can include hyperpigmentation, edema, mild scaling, eczematous patches, and even ulceration.3
Lipodermatosclerosis is a disorder of progressive fibrosis of subcutaneous fat. It is more common in middle-aged women who have a high body mass index and a venous abnormality.4 This form of panniculitis typically affects the lower extremities bilaterally, manifesting as erythematous and indurated skin changes, sometimes described as inverted champagne bottles (Figure 2). At times, there can be accompanying painful ulceration on the erythematous areas, features that closely resemble cellulitis.5,6 Lipodermatosclerosis is commonly misdiagnosed as cellulitis, leading to inappropriate prescription of antibiotics.7

Distinguishing cellulitis from noncellulitic conditions of the lower extremity is paramount to effective patient management in the emergent setting. With a reported incidence of 24.6 per 100 person-years, cellulitis constitutes 1% to 14% of emergency department visits and 4% to 7% of hospital admissions.Therefore, prompt appropriate diagnosis and treatment can avoid life-threatening complications associated with infection such as sepsis, abscess, lymphangitis, and necrotizing fasciitis.8-11
It is estimated that 10% to 20% of patients who have been given a diagnosis of cellulitis do not actually have the disease.2,12 This discrepancy consumes a remarkable amount of hospital resources and can lead to inappropriate or excessive use of antibiotics.13 Although the true incidence of adverse antibiotic reactions is unknown, it is estimated that they are the cause of 3% to 6% of acute hospital admissions and occur in 10% to 15% of inpatients admitted for other primary reasons.14 These findings illustrate the potential for an increased risk for morbidity and increased length of stay for patients beginning an antibiotic regimen, especially when the agents are administered unnecessarily. In addition, inappropriate antibiotic use contributes to antibiotic resistance, which continues to be a major problem, especially in hospitalized patients.
There is a lack of consensus in the literature about methods to risk stratify patients who present with acute dermatologic conditions that include and resemble cellulitis. We sought to identify clinical features based on available clinical literature-derived variables. We tested our scheme in a series of patients with a known diagnosis of cellulitis or other dermatologic pathology of the lower extremity to assess the validity of the following 7 clinical criteria: acute onset, erythema, pyrexia, history of associated trauma, tenderness, unilaterality, and leukocytosis.
Materials and Methods
This retrospective chart review was approved by the Yale University (New Haven, Connecticut) institutional review board (HIC#1409014533). Final diagnosis, demographic data, clinical manifestations, and relevant diagnostic laboratory values of 57 patients were obtained from a database in the dermatology department’s consultation log and electronic medical record database (December 2011 to December 2014). The presence of each clinical symptom—acute onset, erythema, pyrexia, history of associated trauma, tenderness, unilaterality, and leukocytosis—was assigned a score equal to 1; values were tallied to achieve a final score for each patient (Table 1). Patients who were seen initially as a consultation for possible cellulitis but given a final diagnosis of stasis dermatitis or lipodermatosclerosis were included (Table 2).


Clinical Criteria
The clinical criteria were developed based largely on clinical experience and relevant secondary literature.15-17 At the patient encounter, presence of each of the variables (Table 1) was assessed according to the following definitions:
- acute onset: within the prior 72 hours and more indicative of an acute infective process than a gradual and chronic consequence of venous stasis
- erythema: a subjective clinical marker for inflammation that can be associated with cellulitis, though darker, erythematous-appearing discolorations also can be seen in patients with chronic venous hypertension or valvular incompetence4,15
- pyrexia: body temperature greater than 100.4°F
- history of associated trauma: encompassing mechanical wounds, surgical incisions, burns, and insect bites that correlate closely to the time course of symptomatic development
- tenderness: tenderness to light touch, which may be more common in patients afflicted with cellulitis than in those with venous insufficiency
- unilaterality: a helpful distinguishing feature that points the diagnosis away from a dermatitislike clinical picture, especially because bilateral cellulitis is rare and regarded as a diagnostic pitfall18
- leukocytosis: white blood cell count greater than 10.0×109/L and is reasonably considered a cardinal metric of inflammatory processes, though it can be confounded by immunocompromise (low count) or steroid use (high count)
Statistical Analysis
Odds ratios (ORs) were calculated and χ2 analysis was performed for each presenting symptom using JMP 10.0 analytical software (SAS Institute Inc). Each patient was rated separately by means of the clinical feature–based scoring system for the calculation of a total score. After application of the score to the patient population, receiver operating characteristic curves were constructed to identify the optimal score threshold for discriminating cellulitis from dermatitis in this group. For each clinical feature, P<.05 was considered significant.
Results
Our cohort included 32 male and 25 female patients with a mean age of 63 and 61 years, respectively. The final clinical diagnosis of cellulitis was made in 20 patients (35%). An established diagnosis of cellulitis was assigned based on a dermatology evaluation located within our electronic medical record database (Table 2).
Each clinical parameter was evaluated separately for each patient; combined results are summarized in Table 3. Acute onset (≤3 days) was a clinical characteristic seen in 80% (16/20) of cellulitis cases and 22% (8/37) of noncellulitis cases (OR, 14.5; P<.001). Erythema had similar significance (OR, 10.3; prevalence, 95% [19/20] vs 65% [24/37]; P=.012). Pyrexia possessed an OR of 99.2 for cellulitis and was seen in 85% (17/20) of cellulitis cases and only 5% (2/37) of noncellulitis cases (P<.001).

A history of associated trauma had an OR of 36.0 for cellulitis, with 50% (10/20) and 3% (1/37) prevalence in cellulitis cases and noncellulitis cases, respectively (P<.001). Tenderness, documented in 90% (18/20) of cellulitis cases and 43% (16/37) of noncellulitis cases, had an OR of 11.8 (P<.001).
Unilaterality had 100% (20/20) prevalence in our cellulitis cohort and was the only characteristic within the algorithm that yielded an incalculable OR. Noncellulitis or stasis dermatitis of the lower extremity exhibited a unilateral lesion in 11 cases (30%), of which 1 case resulted from a unilateral tibial fracture. Leukocytosis was seen in 65% (13/20) of cellulitis cases and 8% (3/37) of noncellulitis cases, with an OR for cellulitis of 21.0 (P<.001).
All parameters were significant by χ2 analysis (Table 3).
Comment
We found that testing positive for 4 of 7 clinical criteria for assessing cellulitis was highly specific (95%) and sensitive (100%) for a diagnosis of cellulitis among its range of mimics (Figure 3). These cellulitis criteria can be remembered, with some modification, using NEW HAvUN as a mnemonic device (New onset,

Consistent with the literature, pyrexia, history of associated trauma, and unilaterality also were predictors of cellulitis diagnosis. Unilaterality often is used as a diagnostic tool by dermatologist consultants when a patient lacks other criteria for cellulitis, so these findings are intuitive and consistent with our institutional experience. Interestingly, leukocytosis was seen in only 65% of cellulitis cases and 8% of noncellulitis cases and therefore might not serve as a sensitive independent predictor of a diagnosis of cellulitis, emphasizing the importance of the multifactorial scoring system we have put forward. Additionally, acuity of onset, erythema, and tenderness are not independently associated with cellulitis when assessing a patient because several of those findings are present in other dermatologic conditions of the lower extremity; when combined with the other criteria, however, these 3 findings can play a role in diagnosis.
Effective cellulitis diagnosis provides well-recognized challenges in the acute medical setting because many clinical mimics exist. The estimated rate of misdiagnosed cellulitis is certainly well-established: 30% to 75% in independent and multi-institutional studies. These studies also revealed that patients admitted for bilateral “cellulitis” overwhelmingly tended to be stasis clinical pictures.13,19
Cost implications from inappropriate diagnosis largely regard inappropriate antibiotic use and the potential for microbial resistance, with associated costs estimated to be more than $50 billion (2004 dollars).20,21 The true cost burden is extremely difficult to model or predict due to remarkable variations in the institutional misdiagnosis rate, prescribing pattern, and antibiotic cost and could represent avenues of further study. Misappropriation of antibiotics includes not only a monetary cost that encompasses all aspects of acute treatment and hospitalization but also an unquantifiable cost: human lives associated with the consequences of antibiotic resistance.
Conclusion
There is a lack of consensus or criteria for differentiating cellulitis from its most common clinical counterparts. Here, we propose a convenient clinical correlation system that we hope will lead to more efficient allocation of clinical resources, including antibiotics and hospital admissions, while lowering the incidence of adverse events and leading to better patient outcomes. We recognize that the small sample size of our study may limit broad application of these criteria, though we anticipate that further prospective studies can improve the diagnostic relevance and risk-assessment power of the NEW HAvUN criteria put forth here for assessing cellulitis in the acute medical setting.
Acknowledgement—Author H.H.E. recognizes the loving memory of Nadia Ezaldein for her profound influence on and motivation behind this research.
Cellulitis is defined as an acute or subacute, bacterial-induced inflammation of subcutaneous tissue that can extend superficially. The inciting incident often is assumed to be invasion of bacteria through loose connective tissue.1 Although cellulitis is bacterial in origin, it often is difficult to culture the offending microorganism from biopsy sites, swabs, or blood. Erythema, fever, induration, and tenderness are largely seen as clinical manifestations. Moderate and severe cases may be accompanied by fever, malaise, and leukocytosis. The lower extremity is the most common location of involvement (Figure 1), and usually a wound, ulcer, or interdigital superficial infection can be identified and implicated as the source of entry.

Effective treatment of cellulitis is necessary because complications such as abscesses, underlying fascia or muscle involvement, and septicemia can develop, leading to poor outcomes. Antibiotics should be administered intravenously in patients with suspected fascial involvement, septicemia, or dermal necrosis, or in those with an immunological comorbidity.2
The differential diagnosis of lower extremity cellulitis is wide due to the existence of several mimicking dermatologic conditions. These so-called pseudocellulitis conditions include stasis dermatitis, venous ulceration, acute lipodermatosclerosis, pigmented purpura, vasculopathy, contact dermatitis, adverse medication reaction, and arthropod bite. Stasis dermatitis and lipodermatosclerosis, both arising from venous insufficiency, are by far 2 of the most common skin conditions that imitate cellulitis.
Stasis dermatitis is a common condition in the United States and Europe, usually manifesting as a pigmented purpuric dermatosis on anterior tibial surfaces, around the ankle, or overlying dependent varicosities. Skin changes can include hyperpigmentation, edema, mild scaling, eczematous patches, and even ulceration.3
Lipodermatosclerosis is a disorder of progressive fibrosis of subcutaneous fat. It is more common in middle-aged women who have a high body mass index and a venous abnormality.4 This form of panniculitis typically affects the lower extremities bilaterally, manifesting as erythematous and indurated skin changes, sometimes described as inverted champagne bottles (Figure 2). At times, there can be accompanying painful ulceration on the erythematous areas, features that closely resemble cellulitis.5,6 Lipodermatosclerosis is commonly misdiagnosed as cellulitis, leading to inappropriate prescription of antibiotics.7

Distinguishing cellulitis from noncellulitic conditions of the lower extremity is paramount to effective patient management in the emergent setting. With a reported incidence of 24.6 per 100 person-years, cellulitis constitutes 1% to 14% of emergency department visits and 4% to 7% of hospital admissions.Therefore, prompt appropriate diagnosis and treatment can avoid life-threatening complications associated with infection such as sepsis, abscess, lymphangitis, and necrotizing fasciitis.8-11
It is estimated that 10% to 20% of patients who have been given a diagnosis of cellulitis do not actually have the disease.2,12 This discrepancy consumes a remarkable amount of hospital resources and can lead to inappropriate or excessive use of antibiotics.13 Although the true incidence of adverse antibiotic reactions is unknown, it is estimated that they are the cause of 3% to 6% of acute hospital admissions and occur in 10% to 15% of inpatients admitted for other primary reasons.14 These findings illustrate the potential for an increased risk for morbidity and increased length of stay for patients beginning an antibiotic regimen, especially when the agents are administered unnecessarily. In addition, inappropriate antibiotic use contributes to antibiotic resistance, which continues to be a major problem, especially in hospitalized patients.
There is a lack of consensus in the literature about methods to risk stratify patients who present with acute dermatologic conditions that include and resemble cellulitis. We sought to identify clinical features based on available clinical literature-derived variables. We tested our scheme in a series of patients with a known diagnosis of cellulitis or other dermatologic pathology of the lower extremity to assess the validity of the following 7 clinical criteria: acute onset, erythema, pyrexia, history of associated trauma, tenderness, unilaterality, and leukocytosis.
Materials and Methods
This retrospective chart review was approved by the Yale University (New Haven, Connecticut) institutional review board (HIC#1409014533). Final diagnosis, demographic data, clinical manifestations, and relevant diagnostic laboratory values of 57 patients were obtained from a database in the dermatology department’s consultation log and electronic medical record database (December 2011 to December 2014). The presence of each clinical symptom—acute onset, erythema, pyrexia, history of associated trauma, tenderness, unilaterality, and leukocytosis—was assigned a score equal to 1; values were tallied to achieve a final score for each patient (Table 1). Patients who were seen initially as a consultation for possible cellulitis but given a final diagnosis of stasis dermatitis or lipodermatosclerosis were included (Table 2).


Clinical Criteria
The clinical criteria were developed based largely on clinical experience and relevant secondary literature.15-17 At the patient encounter, presence of each of the variables (Table 1) was assessed according to the following definitions:
- acute onset: within the prior 72 hours and more indicative of an acute infective process than a gradual and chronic consequence of venous stasis
- erythema: a subjective clinical marker for inflammation that can be associated with cellulitis, though darker, erythematous-appearing discolorations also can be seen in patients with chronic venous hypertension or valvular incompetence4,15
- pyrexia: body temperature greater than 100.4°F
- history of associated trauma: encompassing mechanical wounds, surgical incisions, burns, and insect bites that correlate closely to the time course of symptomatic development
- tenderness: tenderness to light touch, which may be more common in patients afflicted with cellulitis than in those with venous insufficiency
- unilaterality: a helpful distinguishing feature that points the diagnosis away from a dermatitislike clinical picture, especially because bilateral cellulitis is rare and regarded as a diagnostic pitfall18
- leukocytosis: white blood cell count greater than 10.0×109/L and is reasonably considered a cardinal metric of inflammatory processes, though it can be confounded by immunocompromise (low count) or steroid use (high count)
Statistical Analysis
Odds ratios (ORs) were calculated and χ2 analysis was performed for each presenting symptom using JMP 10.0 analytical software (SAS Institute Inc). Each patient was rated separately by means of the clinical feature–based scoring system for the calculation of a total score. After application of the score to the patient population, receiver operating characteristic curves were constructed to identify the optimal score threshold for discriminating cellulitis from dermatitis in this group. For each clinical feature, P<.05 was considered significant.
Results
Our cohort included 32 male and 25 female patients with a mean age of 63 and 61 years, respectively. The final clinical diagnosis of cellulitis was made in 20 patients (35%). An established diagnosis of cellulitis was assigned based on a dermatology evaluation located within our electronic medical record database (Table 2).
Each clinical parameter was evaluated separately for each patient; combined results are summarized in Table 3. Acute onset (≤3 days) was a clinical characteristic seen in 80% (16/20) of cellulitis cases and 22% (8/37) of noncellulitis cases (OR, 14.5; P<.001). Erythema had similar significance (OR, 10.3; prevalence, 95% [19/20] vs 65% [24/37]; P=.012). Pyrexia possessed an OR of 99.2 for cellulitis and was seen in 85% (17/20) of cellulitis cases and only 5% (2/37) of noncellulitis cases (P<.001).

A history of associated trauma had an OR of 36.0 for cellulitis, with 50% (10/20) and 3% (1/37) prevalence in cellulitis cases and noncellulitis cases, respectively (P<.001). Tenderness, documented in 90% (18/20) of cellulitis cases and 43% (16/37) of noncellulitis cases, had an OR of 11.8 (P<.001).
Unilaterality had 100% (20/20) prevalence in our cellulitis cohort and was the only characteristic within the algorithm that yielded an incalculable OR. Noncellulitis or stasis dermatitis of the lower extremity exhibited a unilateral lesion in 11 cases (30%), of which 1 case resulted from a unilateral tibial fracture. Leukocytosis was seen in 65% (13/20) of cellulitis cases and 8% (3/37) of noncellulitis cases, with an OR for cellulitis of 21.0 (P<.001).
All parameters were significant by χ2 analysis (Table 3).
Comment
We found that testing positive for 4 of 7 clinical criteria for assessing cellulitis was highly specific (95%) and sensitive (100%) for a diagnosis of cellulitis among its range of mimics (Figure 3). These cellulitis criteria can be remembered, with some modification, using NEW HAvUN as a mnemonic device (New onset,

Consistent with the literature, pyrexia, history of associated trauma, and unilaterality also were predictors of cellulitis diagnosis. Unilaterality often is used as a diagnostic tool by dermatologist consultants when a patient lacks other criteria for cellulitis, so these findings are intuitive and consistent with our institutional experience. Interestingly, leukocytosis was seen in only 65% of cellulitis cases and 8% of noncellulitis cases and therefore might not serve as a sensitive independent predictor of a diagnosis of cellulitis, emphasizing the importance of the multifactorial scoring system we have put forward. Additionally, acuity of onset, erythema, and tenderness are not independently associated with cellulitis when assessing a patient because several of those findings are present in other dermatologic conditions of the lower extremity; when combined with the other criteria, however, these 3 findings can play a role in diagnosis.
Effective cellulitis diagnosis provides well-recognized challenges in the acute medical setting because many clinical mimics exist. The estimated rate of misdiagnosed cellulitis is certainly well-established: 30% to 75% in independent and multi-institutional studies. These studies also revealed that patients admitted for bilateral “cellulitis” overwhelmingly tended to be stasis clinical pictures.13,19
Cost implications from inappropriate diagnosis largely regard inappropriate antibiotic use and the potential for microbial resistance, with associated costs estimated to be more than $50 billion (2004 dollars).20,21 The true cost burden is extremely difficult to model or predict due to remarkable variations in the institutional misdiagnosis rate, prescribing pattern, and antibiotic cost and could represent avenues of further study. Misappropriation of antibiotics includes not only a monetary cost that encompasses all aspects of acute treatment and hospitalization but also an unquantifiable cost: human lives associated with the consequences of antibiotic resistance.
Conclusion
There is a lack of consensus or criteria for differentiating cellulitis from its most common clinical counterparts. Here, we propose a convenient clinical correlation system that we hope will lead to more efficient allocation of clinical resources, including antibiotics and hospital admissions, while lowering the incidence of adverse events and leading to better patient outcomes. We recognize that the small sample size of our study may limit broad application of these criteria, though we anticipate that further prospective studies can improve the diagnostic relevance and risk-assessment power of the NEW HAvUN criteria put forth here for assessing cellulitis in the acute medical setting.
Acknowledgement—Author H.H.E. recognizes the loving memory of Nadia Ezaldein for her profound influence on and motivation behind this research.
- Lep
pard BJ, Seal DV, Colman G, et al. The value of bacteriology and serology in the diagnosis of cellulitis and erysipelas. Br J Dermatol. 1985;112:559-567. - Hep
burn MJ, Dooley DP, Skidmore PJ, et al. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Int Med. 2004;164:1669-1674. - Bergan JJ, Schmid-Schönbein GW, Smith PD, et al. Chronic venous disease. N Engl J Med. 2006;355:488-498.
- Bruc
e AJ, Bennett DD, Lohse CM, et al. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol. 2002;46:187-192. - Heym
ann WR. Lipodermatosclerosis. J Am Acad Dermatol. 2009;60:1022-1023. - Vesi
ć S, Vuković J, Medenica LJ, et al. Acute lipodermatosclerosis: an open clinical trial of stanozolol in patients unable to sustain compression therapy. Dermatol Online J. 2008;14:1. - Keller
EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-552. - Dong SL, Kelly KD, Oland RC, et al. ED management of cellulitis: a review of five urban centers. Am J Emerg Med. 2001;19:535-540.
- Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134:293-299.
- Manfredi R, Calza L, Chiodo F. Epidemiology and microbiology of cellulitis and bacterial soft tissue infection during HIV disease: a 10-year survey. J Cutan Pathol. 2002;29:168-172.
- Pascarella L, Schonbein GW, Bergan JJ. Microcirculation and venous ulcers: a review. Ann Vasc Surg. 2005;19:921-927.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician. 2003;67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hay RJ, Adriaans BM. Bacterial infections. In: Thong BY, Tan TC. Epidemiology and risk factors for drug allergy. 8th ed. Br J Clin Pharmacol. 2011;71:684-700.
- Hay RJ, Adriaans BM. Bacterial infections. In: Burns T, Breathnach S, Cox N, et al. Rook’s Textbook of Dermatology. 8th ed. Hoboken, NJ: John Wiley & Sons, Inc; 2004:1345-1426.
- Wolff K, Goldsmith LA, Katz SI, et al. Fitzpatrick’s Dermatology In General Medicine. 7th ed. New York, NY: McGraw-Hill; 2003.
- Sommer LL, Reboli AC, Heymann WR. Bacterial infections. In: Bolognia J, Schaffer J, Cerroni L, et al. Dermatology. Vol 4. Philadelphia, PA: Elsevier Saunders; 2012:1462-1502.
- Cox NH. Management of lower leg cellulitis. Clin Med. 2002;2:23-27.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Pinder R, Sallis A, Berry D, et al. Behaviour change and antibiotic prescribing in healthcare settings: literature review and behavioural analysis. London, UK: Public Health England; February 2015. https://assets.publishing.service.gov.uk/government/
uploads/system/uploads/attachment_data/file/405031
/Behaviour_Change_for_Antibiotic_Prescribing_-_FINAL.pdf. Accessed May 7, 2018. - Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346:f1493.
- Lep
pard BJ, Seal DV, Colman G, et al. The value of bacteriology and serology in the diagnosis of cellulitis and erysipelas. Br J Dermatol. 1985;112:559-567. - Hep
burn MJ, Dooley DP, Skidmore PJ, et al. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Int Med. 2004;164:1669-1674. - Bergan JJ, Schmid-Schönbein GW, Smith PD, et al. Chronic venous disease. N Engl J Med. 2006;355:488-498.
- Bruc
e AJ, Bennett DD, Lohse CM, et al. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol. 2002;46:187-192. - Heym
ann WR. Lipodermatosclerosis. J Am Acad Dermatol. 2009;60:1022-1023. - Vesi
ć S, Vuković J, Medenica LJ, et al. Acute lipodermatosclerosis: an open clinical trial of stanozolol in patients unable to sustain compression therapy. Dermatol Online J. 2008;14:1. - Keller
EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-552. - Dong SL, Kelly KD, Oland RC, et al. ED management of cellulitis: a review of five urban centers. Am J Emerg Med. 2001;19:535-540.
- Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134:293-299.
- Manfredi R, Calza L, Chiodo F. Epidemiology and microbiology of cellulitis and bacterial soft tissue infection during HIV disease: a 10-year survey. J Cutan Pathol. 2002;29:168-172.
- Pascarella L, Schonbein GW, Bergan JJ. Microcirculation and venous ulcers: a review. Ann Vasc Surg. 2005;19:921-927.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician. 2003;67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hay RJ, Adriaans BM. Bacterial infections. In: Thong BY, Tan TC. Epidemiology and risk factors for drug allergy. 8th ed. Br J Clin Pharmacol. 2011;71:684-700.
- Hay RJ, Adriaans BM. Bacterial infections. In: Burns T, Breathnach S, Cox N, et al. Rook’s Textbook of Dermatology. 8th ed. Hoboken, NJ: John Wiley & Sons, Inc; 2004:1345-1426.
- Wolff K, Goldsmith LA, Katz SI, et al. Fitzpatrick’s Dermatology In General Medicine. 7th ed. New York, NY: McGraw-Hill; 2003.
- Sommer LL, Reboli AC, Heymann WR. Bacterial infections. In: Bolognia J, Schaffer J, Cerroni L, et al. Dermatology. Vol 4. Philadelphia, PA: Elsevier Saunders; 2012:1462-1502.
- Cox NH. Management of lower leg cellulitis. Clin Med. 2002;2:23-27.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Pinder R, Sallis A, Berry D, et al. Behaviour change and antibiotic prescribing in healthcare settings: literature review and behavioural analysis. London, UK: Public Health England; February 2015. https://assets.publishing.service.gov.uk/government/
uploads/system/uploads/attachment_data/file/405031
/Behaviour_Change_for_Antibiotic_Prescribing_-_FINAL.pdf. Accessed May 7, 2018. - Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346:f1493.
Practice Points
- Distinguishing cellulitis from noncellulitic conditions of the lower extremity is paramount to effective patient management in the emergent setting, given that misdiagnosis consumes hospital resources and can lead to inappropriate or excessive use of antibiotics.
- We evaluated the specificity and sensitivity of the following 7 clinical criteria: acute onset, erythema, pyrexia, history of associated trauma, tenderness, unilaterality, and leukocytosis.
Gene assays reveal some ‘unknown primary’ cancers as RCC
Gene expression profiling and/or immunohistochemistry can identify occult renal cell carcinoma (RCC) in a subset of patients diagnosed with carcinoma of unknown primary (CUP), suggesting that these patients could benefit from RCC-specific targeted therapy or immunotherapy, investigators contend.
Of 539 patients presenting at a single center with CUP, a 92-gene reverse transcription polymerase chain reaction molecular cancer classifier assay (MCCA) performed on biopsy specimens identified 24 as having RCC. All of the patients had clinical characteristics typical of advanced RCC, but none had suspicious renal lesions on CT scans, reported F. Anthony Greco, MD and John D. Hainsworth, MD, of the Sarah Cannon Cancer Center and Research Institute in Nashville, Tenn.
“Although further experience is necessary, these patients responded to RCC-specific therapy in a manner consistent with advanced RCC. These patients are unlikely to benefit from treatment with empiric chemotherapy. The reliable identification of RCC patients within the heterogeneous CUP population is possible using MCCA, and has potentially important therapeutic implications,” they wrote. The report was published in Clinical Genitourinary Cancer.
They noted that previously the only therapeutic option for patients with CUP suspected of being renal in origin was ineffective systemic chemotherapy, making a specific diagnosis of more academic interest than clinical importance.
“This situation has now changed because of the introduction of several targeted agents and immune checkpoint blockers that improve survival in patients with advanced RCC. It is likely that these new RCC treatments are also more effective than empiric chemotherapy for patients with CUP who have an occult renal primary site. Therefore, recognition of the RCC subset of patients within the CUP population has practical therapeutic importance,” they wrote.
Dr. Greco and Dr. Hainsworth conducted a retrospective review of patients at their center with CUP from 2008 through 2013 who had RCC predicted by MCCA.
A total of 539 patients presented with CUP during the study period, and of this group, 24 (4.4%) had RCC identified by MCCA.
The patients, 18 men and 6 women, with a median age of 61 years, all had abdominal CT scans that failed to show renal lesions suggestive of a primary RCC. Nine of the 24 patients had baseline MCCA performed as part of a prospective phase 2 clinical trial; the other 15 were patients treated at the center who had MCCA performed later in the clinical course, usually during or after first-line empiric chemotherapy.
Sixteen patients had metastases in the retroperitoneum, 10 in the mediastinum, 6 in bone, 5 in the liver, and 5 in lungs and/or pleura.
Pathologic studies using light microscopy showed poorly differentiated carcinomas in eight patients, poorly differentiated adenocarcinomas in nine, and well or moderately differentiated adenocarcinomas in seven patients.
A pathologist identified RCC as the possible primary in only 4 of the 24 patients. Immunohistochemistry tests in these patients were consistent with a diagnosis of RCC. Only 5 of the 24 had focal features suggestive of RCC, including one clear-cell and four papillary histologies.
Sixteen of the 24 patients received first-line treatment for advanced RCC, including sunitinib, temsirolimus, bevacizumab, and/or interleukin 1. Four other patients received RCC-specific therapy following empiric chemotherapy (three patients who received RCC-specific therapies in the first line also received it in the second line).
Among the 16 patients who received first-line RCC-specific therapies there were 3 partial responses (PR), 10 cases of stable disease (SD), 2 of progressive disease (PD), and 1 patient was not evaluable. The median duration of both PR and SD was 8 months.
Of the eight patients who received first-line empiric chemotherapy, one had a PR, two had SD, and five had PD.
For the seven patients who received second-line RCC-specific therapy after either first-line chemotherapy or site-specific therapy, responses included one PR, two SD, two PD, and two not evaluable.
Median survival for all 24 patients was 12 months (range 2 to more than 43 months). Median survival of the 16 patients who received first-line RCC-specific treatment was 14 months (range 2-25 months).
Median survival for all 20 patients who received RCC-specific treatment at some time during their course was 16 months (range, 2 to more than 43 months).
The authors called for further prospective studies of this subset of patients with CUP.
SOURCE: Greco FA, Hainsworth JD. Clin Genitourin Cancer. 2018 Aug;16(4):e893-8.
Gene expression profiling and/or immunohistochemistry can identify occult renal cell carcinoma (RCC) in a subset of patients diagnosed with carcinoma of unknown primary (CUP), suggesting that these patients could benefit from RCC-specific targeted therapy or immunotherapy, investigators contend.
Of 539 patients presenting at a single center with CUP, a 92-gene reverse transcription polymerase chain reaction molecular cancer classifier assay (MCCA) performed on biopsy specimens identified 24 as having RCC. All of the patients had clinical characteristics typical of advanced RCC, but none had suspicious renal lesions on CT scans, reported F. Anthony Greco, MD and John D. Hainsworth, MD, of the Sarah Cannon Cancer Center and Research Institute in Nashville, Tenn.
“Although further experience is necessary, these patients responded to RCC-specific therapy in a manner consistent with advanced RCC. These patients are unlikely to benefit from treatment with empiric chemotherapy. The reliable identification of RCC patients within the heterogeneous CUP population is possible using MCCA, and has potentially important therapeutic implications,” they wrote. The report was published in Clinical Genitourinary Cancer.
They noted that previously the only therapeutic option for patients with CUP suspected of being renal in origin was ineffective systemic chemotherapy, making a specific diagnosis of more academic interest than clinical importance.
“This situation has now changed because of the introduction of several targeted agents and immune checkpoint blockers that improve survival in patients with advanced RCC. It is likely that these new RCC treatments are also more effective than empiric chemotherapy for patients with CUP who have an occult renal primary site. Therefore, recognition of the RCC subset of patients within the CUP population has practical therapeutic importance,” they wrote.
Dr. Greco and Dr. Hainsworth conducted a retrospective review of patients at their center with CUP from 2008 through 2013 who had RCC predicted by MCCA.
A total of 539 patients presented with CUP during the study period, and of this group, 24 (4.4%) had RCC identified by MCCA.
The patients, 18 men and 6 women, with a median age of 61 years, all had abdominal CT scans that failed to show renal lesions suggestive of a primary RCC. Nine of the 24 patients had baseline MCCA performed as part of a prospective phase 2 clinical trial; the other 15 were patients treated at the center who had MCCA performed later in the clinical course, usually during or after first-line empiric chemotherapy.
Sixteen patients had metastases in the retroperitoneum, 10 in the mediastinum, 6 in bone, 5 in the liver, and 5 in lungs and/or pleura.
Pathologic studies using light microscopy showed poorly differentiated carcinomas in eight patients, poorly differentiated adenocarcinomas in nine, and well or moderately differentiated adenocarcinomas in seven patients.
A pathologist identified RCC as the possible primary in only 4 of the 24 patients. Immunohistochemistry tests in these patients were consistent with a diagnosis of RCC. Only 5 of the 24 had focal features suggestive of RCC, including one clear-cell and four papillary histologies.
Sixteen of the 24 patients received first-line treatment for advanced RCC, including sunitinib, temsirolimus, bevacizumab, and/or interleukin 1. Four other patients received RCC-specific therapy following empiric chemotherapy (three patients who received RCC-specific therapies in the first line also received it in the second line).
Among the 16 patients who received first-line RCC-specific therapies there were 3 partial responses (PR), 10 cases of stable disease (SD), 2 of progressive disease (PD), and 1 patient was not evaluable. The median duration of both PR and SD was 8 months.
Of the eight patients who received first-line empiric chemotherapy, one had a PR, two had SD, and five had PD.
For the seven patients who received second-line RCC-specific therapy after either first-line chemotherapy or site-specific therapy, responses included one PR, two SD, two PD, and two not evaluable.
Median survival for all 24 patients was 12 months (range 2 to more than 43 months). Median survival of the 16 patients who received first-line RCC-specific treatment was 14 months (range 2-25 months).
Median survival for all 20 patients who received RCC-specific treatment at some time during their course was 16 months (range, 2 to more than 43 months).
The authors called for further prospective studies of this subset of patients with CUP.
SOURCE: Greco FA, Hainsworth JD. Clin Genitourin Cancer. 2018 Aug;16(4):e893-8.
Gene expression profiling and/or immunohistochemistry can identify occult renal cell carcinoma (RCC) in a subset of patients diagnosed with carcinoma of unknown primary (CUP), suggesting that these patients could benefit from RCC-specific targeted therapy or immunotherapy, investigators contend.
Of 539 patients presenting at a single center with CUP, a 92-gene reverse transcription polymerase chain reaction molecular cancer classifier assay (MCCA) performed on biopsy specimens identified 24 as having RCC. All of the patients had clinical characteristics typical of advanced RCC, but none had suspicious renal lesions on CT scans, reported F. Anthony Greco, MD and John D. Hainsworth, MD, of the Sarah Cannon Cancer Center and Research Institute in Nashville, Tenn.
“Although further experience is necessary, these patients responded to RCC-specific therapy in a manner consistent with advanced RCC. These patients are unlikely to benefit from treatment with empiric chemotherapy. The reliable identification of RCC patients within the heterogeneous CUP population is possible using MCCA, and has potentially important therapeutic implications,” they wrote. The report was published in Clinical Genitourinary Cancer.
They noted that previously the only therapeutic option for patients with CUP suspected of being renal in origin was ineffective systemic chemotherapy, making a specific diagnosis of more academic interest than clinical importance.
“This situation has now changed because of the introduction of several targeted agents and immune checkpoint blockers that improve survival in patients with advanced RCC. It is likely that these new RCC treatments are also more effective than empiric chemotherapy for patients with CUP who have an occult renal primary site. Therefore, recognition of the RCC subset of patients within the CUP population has practical therapeutic importance,” they wrote.
Dr. Greco and Dr. Hainsworth conducted a retrospective review of patients at their center with CUP from 2008 through 2013 who had RCC predicted by MCCA.
A total of 539 patients presented with CUP during the study period, and of this group, 24 (4.4%) had RCC identified by MCCA.
The patients, 18 men and 6 women, with a median age of 61 years, all had abdominal CT scans that failed to show renal lesions suggestive of a primary RCC. Nine of the 24 patients had baseline MCCA performed as part of a prospective phase 2 clinical trial; the other 15 were patients treated at the center who had MCCA performed later in the clinical course, usually during or after first-line empiric chemotherapy.
Sixteen patients had metastases in the retroperitoneum, 10 in the mediastinum, 6 in bone, 5 in the liver, and 5 in lungs and/or pleura.
Pathologic studies using light microscopy showed poorly differentiated carcinomas in eight patients, poorly differentiated adenocarcinomas in nine, and well or moderately differentiated adenocarcinomas in seven patients.
A pathologist identified RCC as the possible primary in only 4 of the 24 patients. Immunohistochemistry tests in these patients were consistent with a diagnosis of RCC. Only 5 of the 24 had focal features suggestive of RCC, including one clear-cell and four papillary histologies.
Sixteen of the 24 patients received first-line treatment for advanced RCC, including sunitinib, temsirolimus, bevacizumab, and/or interleukin 1. Four other patients received RCC-specific therapy following empiric chemotherapy (three patients who received RCC-specific therapies in the first line also received it in the second line).
Among the 16 patients who received first-line RCC-specific therapies there were 3 partial responses (PR), 10 cases of stable disease (SD), 2 of progressive disease (PD), and 1 patient was not evaluable. The median duration of both PR and SD was 8 months.
Of the eight patients who received first-line empiric chemotherapy, one had a PR, two had SD, and five had PD.
For the seven patients who received second-line RCC-specific therapy after either first-line chemotherapy or site-specific therapy, responses included one PR, two SD, two PD, and two not evaluable.
Median survival for all 24 patients was 12 months (range 2 to more than 43 months). Median survival of the 16 patients who received first-line RCC-specific treatment was 14 months (range 2-25 months).
Median survival for all 20 patients who received RCC-specific treatment at some time during their course was 16 months (range, 2 to more than 43 months).
The authors called for further prospective studies of this subset of patients with CUP.
SOURCE: Greco FA, Hainsworth JD. Clin Genitourin Cancer. 2018 Aug;16(4):e893-8.
FROM CLINICAL GENITOURINARY CANCER
Key clinical point: Some carcinomas of unknown primary (CUP) can be identified as renal in origin by molecular assays and treated accordingly.
Major finding: Molecular assays identified RCC as the primary in 24 of 539 patients with CUP.
Study details: Retrospective review of patients with CUP presenting at a single center from 2008 through 2013.
Disclosures: The Minnie Pearl Cancer Research Foundation supported the study. Dr. Greco disclosed a consultant role and speakers bureau activities for bioTheranostics.
Source: Greco FA, Hainsworth JD. Clinical Genitourinary Cancer 16(4): e893-8.
Rituximab reduces risk of follicular lymphoma transformation
Rituximab-based chemotherapy can significantly reduce the risk of transformation of follicular lymphoma (FL) from an indolent to an aggressive histology, such as diffuse large B-cell lymphoma, results of a retrospective pooled analysis have suggested.
“Despite the intrinsic limitations related to the retrospective nature of our study, we confirmed that the cumulative hazard of histological transformation as a first event in follicular lymphoma can be reduced significantly by introducing rituximab to a backbone therapy. Moreover, our data also confirm that histological transformation still has an adverse effect on patient outcome, although it is less catastrophic than the pre-rituximab regimens,” they wrote in the Lancet Haematology.
These investigators, from 11 cooperative groups or institutions across Europe, pooled data on patients aged 18 years and older who had a histologically confirmed diagnosis of grade 1, 2, or 3a FL between Jan. 2, 1997, and Dec. 20, 2013.
They defined histologic transformation as a biopsy-proven aggressive lymphoma that occurred as a first event after first-line therapy.
Data on a total of 8,116 patients were available for analysis; 509 of these patients had had histologic transformations. After a median follow-up of 87 months, the 10-year cumulative hazard for all patients was 7.7%. The 10-year cumulative hazard – one of two primary endpoints – was 5.2% for patients who had received any rituximab versus 8.7% for those who did not, which translated into a hazard ratio of 0.73 (P = .004).
Among patients who received rituximab during induction only, the 10-year cumulative hazard was 5.9%, and it was 3.6% among those who received rituximab during induction and maintenance phases of treatment. This difference translated into a HR of 0.55 (P = .003).
The benefit of rituximab induction and maintenance – compared with induction only – held up in a multivariate analysis controlling for age at diagnosis, sex, FLIPI (Follicular Lymphoma International Prognostic Index) score, active surveillance vs. treatment, and FL grade (HR, 0.55; P = .016).
There were 287 deaths among the 509 patients with transformation, resulting in a 10-year survival after transformation of 32%.
The 5-year survival after transformation was 38% for patients who were not exposed to rituximab, 42% for patients who received induction rituximab, and 43% for those who received both induction and maintenance rituximab, but the differences between the three groups were not statistically significant.
“More comprehensive knowledge of the biological risk factors for follicular lymphoma transformation and the molecular pathways involved is likely to help clinicians make more accurate prognostic assessments and also inform the potential usefulness of novel drugs for the treatment of follicular lymphoma,” the researchers wrote.
The study was funded by the European Lymphoma Institute and other research groups. The researchers reported having no financial disclosures.
SOURCE: Federico M et al. Lancet Haematol. 2018 Jul 4. doi: 10.1016/S2352-3026(18)30090-5.
Rituximab-based chemotherapy can significantly reduce the risk of transformation of follicular lymphoma (FL) from an indolent to an aggressive histology, such as diffuse large B-cell lymphoma, results of a retrospective pooled analysis have suggested.
“Despite the intrinsic limitations related to the retrospective nature of our study, we confirmed that the cumulative hazard of histological transformation as a first event in follicular lymphoma can be reduced significantly by introducing rituximab to a backbone therapy. Moreover, our data also confirm that histological transformation still has an adverse effect on patient outcome, although it is less catastrophic than the pre-rituximab regimens,” they wrote in the Lancet Haematology.
These investigators, from 11 cooperative groups or institutions across Europe, pooled data on patients aged 18 years and older who had a histologically confirmed diagnosis of grade 1, 2, or 3a FL between Jan. 2, 1997, and Dec. 20, 2013.
They defined histologic transformation as a biopsy-proven aggressive lymphoma that occurred as a first event after first-line therapy.
Data on a total of 8,116 patients were available for analysis; 509 of these patients had had histologic transformations. After a median follow-up of 87 months, the 10-year cumulative hazard for all patients was 7.7%. The 10-year cumulative hazard – one of two primary endpoints – was 5.2% for patients who had received any rituximab versus 8.7% for those who did not, which translated into a hazard ratio of 0.73 (P = .004).
Among patients who received rituximab during induction only, the 10-year cumulative hazard was 5.9%, and it was 3.6% among those who received rituximab during induction and maintenance phases of treatment. This difference translated into a HR of 0.55 (P = .003).
The benefit of rituximab induction and maintenance – compared with induction only – held up in a multivariate analysis controlling for age at diagnosis, sex, FLIPI (Follicular Lymphoma International Prognostic Index) score, active surveillance vs. treatment, and FL grade (HR, 0.55; P = .016).
There were 287 deaths among the 509 patients with transformation, resulting in a 10-year survival after transformation of 32%.
The 5-year survival after transformation was 38% for patients who were not exposed to rituximab, 42% for patients who received induction rituximab, and 43% for those who received both induction and maintenance rituximab, but the differences between the three groups were not statistically significant.
“More comprehensive knowledge of the biological risk factors for follicular lymphoma transformation and the molecular pathways involved is likely to help clinicians make more accurate prognostic assessments and also inform the potential usefulness of novel drugs for the treatment of follicular lymphoma,” the researchers wrote.
The study was funded by the European Lymphoma Institute and other research groups. The researchers reported having no financial disclosures.
SOURCE: Federico M et al. Lancet Haematol. 2018 Jul 4. doi: 10.1016/S2352-3026(18)30090-5.
Rituximab-based chemotherapy can significantly reduce the risk of transformation of follicular lymphoma (FL) from an indolent to an aggressive histology, such as diffuse large B-cell lymphoma, results of a retrospective pooled analysis have suggested.
“Despite the intrinsic limitations related to the retrospective nature of our study, we confirmed that the cumulative hazard of histological transformation as a first event in follicular lymphoma can be reduced significantly by introducing rituximab to a backbone therapy. Moreover, our data also confirm that histological transformation still has an adverse effect on patient outcome, although it is less catastrophic than the pre-rituximab regimens,” they wrote in the Lancet Haematology.
These investigators, from 11 cooperative groups or institutions across Europe, pooled data on patients aged 18 years and older who had a histologically confirmed diagnosis of grade 1, 2, or 3a FL between Jan. 2, 1997, and Dec. 20, 2013.
They defined histologic transformation as a biopsy-proven aggressive lymphoma that occurred as a first event after first-line therapy.
Data on a total of 8,116 patients were available for analysis; 509 of these patients had had histologic transformations. After a median follow-up of 87 months, the 10-year cumulative hazard for all patients was 7.7%. The 10-year cumulative hazard – one of two primary endpoints – was 5.2% for patients who had received any rituximab versus 8.7% for those who did not, which translated into a hazard ratio of 0.73 (P = .004).
Among patients who received rituximab during induction only, the 10-year cumulative hazard was 5.9%, and it was 3.6% among those who received rituximab during induction and maintenance phases of treatment. This difference translated into a HR of 0.55 (P = .003).
The benefit of rituximab induction and maintenance – compared with induction only – held up in a multivariate analysis controlling for age at diagnosis, sex, FLIPI (Follicular Lymphoma International Prognostic Index) score, active surveillance vs. treatment, and FL grade (HR, 0.55; P = .016).
There were 287 deaths among the 509 patients with transformation, resulting in a 10-year survival after transformation of 32%.
The 5-year survival after transformation was 38% for patients who were not exposed to rituximab, 42% for patients who received induction rituximab, and 43% for those who received both induction and maintenance rituximab, but the differences between the three groups were not statistically significant.
“More comprehensive knowledge of the biological risk factors for follicular lymphoma transformation and the molecular pathways involved is likely to help clinicians make more accurate prognostic assessments and also inform the potential usefulness of novel drugs for the treatment of follicular lymphoma,” the researchers wrote.
The study was funded by the European Lymphoma Institute and other research groups. The researchers reported having no financial disclosures.
SOURCE: Federico M et al. Lancet Haematol. 2018 Jul 4. doi: 10.1016/S2352-3026(18)30090-5.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: The 10-year cumulative hazard of histologic transformation was 5.2% for patients who had received rituximab and 8.7% for those who had not.
Study details: Retrospective pooled analysis of 8,116 patients with FL, 509 of whom had transformation over a 10-year period.
Disclosures: The study was funded by Associazione Angela Serra per la Ricerca sul Cancro, European Lymphoma Institute, European Hematology Association Lymphoma Group, Fondazione Italiana Linfomi, and the Spanish Group of Lymphoma and Bone Marrow Transplantation. The researchers reported having no financial disclosures.
Source: Federico M et al. Lancet Haematol. 2018 Jul 4. doi: 10.1016/S2352-3026(18)30090-5.
ACS NSQIP project collected patient-reported data on surgery outcomes
ORLANDO – A pilot survey to generate had a high response rate and yielded clinically meaningful data, an investigator reported at the American College of Surgeons Quality and Safety Conference.
The 45-question electronic survey, conducted as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) had 1,300 respondents with a response rate of 20%, according to Jason B. Liu, MD, an ACS Clinical Scholar-in-Residence and general surgery resident at the University of Chicago.
Results to date have demonstrated that in patients undergoing total knee arthroplasty (TKA), pain had a greater impact on daily activities than for other procedures, Dr. Liu said in a general session presentation the conference.
“Overall, the lesson learned is that in the current health care landscape, with its regulations and privacy issues, it is indeed both feasible and acceptable to electronically measure patient-reported outcomes using the ACS NSQIP platform,” Dr. Liu said at the meeting.
Eighteen hospitals in the United States and Canada participated in the pilot survey, which elicited responses from patients with a median age of 63 years, representing more than 340 types of operations.
The survey incorporates measurements from the PROMIS Pain Interference instrument, which measures how much pain hinders daily activities; PROMIS Global Health, which measures physical and mental health; and aspects of the Consumer Assessment of Healthcare Providers and Systems Surgical Care Survey (S-CAHPS), Dr. Liu said.
The TKA finding is just one example of the data obtained through the pilot, he said. Looking at PROMIS Pain Interference, pain had more impact in TKA patients compared with open GI, breast hernia, and laparoscopic GI procedures. Difference between means ranged from 3.2 to 9.4 for TKA, compared with those procedures.
Conducting the pilot has been an “uphill battle,” according to Dr. Liu, citing critics who wondered if the program would generate meaningful data, whether older patients would respond to an electronic survey, and whether patients would take time to fill out a 45-question survey.
In fact, the average completion time for the survey was just 6.4 minutes, and the median number of items missing was zero, meaning that patients who started the survey tended to finish it, he said.
“We really hope to expand what we’ve learned across all of the [ACS] quality programs so that we can begin to really incorporate the patients’ perspective in improving national surgical quality,” he said.
Dr. Liu had no disclosures to report.
ORLANDO – A pilot survey to generate had a high response rate and yielded clinically meaningful data, an investigator reported at the American College of Surgeons Quality and Safety Conference.
The 45-question electronic survey, conducted as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) had 1,300 respondents with a response rate of 20%, according to Jason B. Liu, MD, an ACS Clinical Scholar-in-Residence and general surgery resident at the University of Chicago.
Results to date have demonstrated that in patients undergoing total knee arthroplasty (TKA), pain had a greater impact on daily activities than for other procedures, Dr. Liu said in a general session presentation the conference.
“Overall, the lesson learned is that in the current health care landscape, with its regulations and privacy issues, it is indeed both feasible and acceptable to electronically measure patient-reported outcomes using the ACS NSQIP platform,” Dr. Liu said at the meeting.
Eighteen hospitals in the United States and Canada participated in the pilot survey, which elicited responses from patients with a median age of 63 years, representing more than 340 types of operations.
The survey incorporates measurements from the PROMIS Pain Interference instrument, which measures how much pain hinders daily activities; PROMIS Global Health, which measures physical and mental health; and aspects of the Consumer Assessment of Healthcare Providers and Systems Surgical Care Survey (S-CAHPS), Dr. Liu said.
The TKA finding is just one example of the data obtained through the pilot, he said. Looking at PROMIS Pain Interference, pain had more impact in TKA patients compared with open GI, breast hernia, and laparoscopic GI procedures. Difference between means ranged from 3.2 to 9.4 for TKA, compared with those procedures.
Conducting the pilot has been an “uphill battle,” according to Dr. Liu, citing critics who wondered if the program would generate meaningful data, whether older patients would respond to an electronic survey, and whether patients would take time to fill out a 45-question survey.
In fact, the average completion time for the survey was just 6.4 minutes, and the median number of items missing was zero, meaning that patients who started the survey tended to finish it, he said.
“We really hope to expand what we’ve learned across all of the [ACS] quality programs so that we can begin to really incorporate the patients’ perspective in improving national surgical quality,” he said.
Dr. Liu had no disclosures to report.
ORLANDO – A pilot survey to generate had a high response rate and yielded clinically meaningful data, an investigator reported at the American College of Surgeons Quality and Safety Conference.
The 45-question electronic survey, conducted as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) had 1,300 respondents with a response rate of 20%, according to Jason B. Liu, MD, an ACS Clinical Scholar-in-Residence and general surgery resident at the University of Chicago.
Results to date have demonstrated that in patients undergoing total knee arthroplasty (TKA), pain had a greater impact on daily activities than for other procedures, Dr. Liu said in a general session presentation the conference.
“Overall, the lesson learned is that in the current health care landscape, with its regulations and privacy issues, it is indeed both feasible and acceptable to electronically measure patient-reported outcomes using the ACS NSQIP platform,” Dr. Liu said at the meeting.
Eighteen hospitals in the United States and Canada participated in the pilot survey, which elicited responses from patients with a median age of 63 years, representing more than 340 types of operations.
The survey incorporates measurements from the PROMIS Pain Interference instrument, which measures how much pain hinders daily activities; PROMIS Global Health, which measures physical and mental health; and aspects of the Consumer Assessment of Healthcare Providers and Systems Surgical Care Survey (S-CAHPS), Dr. Liu said.
The TKA finding is just one example of the data obtained through the pilot, he said. Looking at PROMIS Pain Interference, pain had more impact in TKA patients compared with open GI, breast hernia, and laparoscopic GI procedures. Difference between means ranged from 3.2 to 9.4 for TKA, compared with those procedures.
Conducting the pilot has been an “uphill battle,” according to Dr. Liu, citing critics who wondered if the program would generate meaningful data, whether older patients would respond to an electronic survey, and whether patients would take time to fill out a 45-question survey.
In fact, the average completion time for the survey was just 6.4 minutes, and the median number of items missing was zero, meaning that patients who started the survey tended to finish it, he said.
“We really hope to expand what we’ve learned across all of the [ACS] quality programs so that we can begin to really incorporate the patients’ perspective in improving national surgical quality,” he said.
Dr. Liu had no disclosures to report.
REPORTING FROM ACSQSC 2018
Key clinical point: Clinically meaningful data on patient-reported outcomes can be obtained using the ACS NSQIP platform.
Major finding: The average completion time for the survey was 6.4 minutes, and the median number of items missing was zero.
Study details: A 45-question electronic survey of 1,300 patients treated at 18 hospitals for 340 different types of surgical procedures.
Disclosures: Dr. Liu had no disclosures to report.
Pseudotumor cerebri pediatric rates are rising
Pseudotumor cerebri, benign intracranial hypertension, and idiopathic intracranial hypertension are all terms to describe a syndrome of increased intracranial pressure, headaches, vision loss, or changes without an associated mass lesion.1 The condition was considered relatively rare, presenting most commonly in obese women in childbearing years. Surprisingly, 2
Obesity is the fastest growing morbidity among adolescents. The Centers for Disease Control and Prevention reported 32% of children 2-19 years were obese.1 This reality is impacting many areas of an adolescent’s health, but it also is changing the landscape of diseases that present in this age group. Although pediatric and adult pseudotumor cerebri always have had slightly varied features, many features were similar such as the papilledema, vision loss, headaches, and sixth nerve palsy. Obesity and female predominance tended to present more in the adult population, as many pediatric patients were not obese,2 and had fewer associated symptoms at the time of diagnosis, and the cause was thought to idiopathic.
Now, with the increase in obesity, more adolescents and more male patients are presenting with pseudotumor cerebri as a cause for their headache, and 57%-100% are obese, making it a compounding factor.3
Pediatric populations also are at risk of secondary pseudotumor cerebri, which is an increase in intracranial pressure from the use of medication, or other disease states such as anemia, kidney disease, or Down syndrome. Minocycline use is the most common medication cause and usually presents 1-2 months after normal use.4 Discontinuing the drug does lead to resolution. Retinoids, vitamin A products, growth hormone, and steroids also have been implicated. Given that acne is a common complaint amongst teens, knowledge of these side effects is important.4
In 2013, the criteria for diagnosis of pseudotumor cerebri was revised. Currently, the presence of papilledema, normal neurologic exam except for abnormal sixth cranial nerve, normal cerebral spinal fluid, elevated lumbar opening pressure, and normal imaging are needed for a definitive diagnosis. A probable diagnosis can be made if papilledema is not present but there abducens nerve palsy.2
In a routine physical exam, when I questioned a patient on any medication that was used daily, she replied she took ibuprofen daily for headaches and that she had been doing this for several months. Headaches were not in her chief complaints as she had learned to live with and ignore this symptom. Upon further evaluation, she was slightly overweight and has a questionable fundoscopic exam. After further evaluation by an ophthalmologist and a neurologist, pseudotumor cerebri was diagnosed.
Index of suspicion is key in correctly diagnosing patients, and understanding the changing landscape of medicine will lead to more thoughtful questioning during routine health exams and better outcomes for your patients.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Am J Ophthalmol. 2015 Feb;159(2):344-52.e1.
2. Horm Res Paediatr. 2014;81(4):217-25.
3. Clin Imaging. 2018 May 24. doi: 10.1016/j.clinimag.2018.05.020.
4. Am J Ophthalmol. 1998 Jul;126(1):116-21.
5. Glob Pediatr Health. 2018. doi:10.1177/2333794X18785550.
Pseudotumor cerebri, benign intracranial hypertension, and idiopathic intracranial hypertension are all terms to describe a syndrome of increased intracranial pressure, headaches, vision loss, or changes without an associated mass lesion.1 The condition was considered relatively rare, presenting most commonly in obese women in childbearing years. Surprisingly, 2
Obesity is the fastest growing morbidity among adolescents. The Centers for Disease Control and Prevention reported 32% of children 2-19 years were obese.1 This reality is impacting many areas of an adolescent’s health, but it also is changing the landscape of diseases that present in this age group. Although pediatric and adult pseudotumor cerebri always have had slightly varied features, many features were similar such as the papilledema, vision loss, headaches, and sixth nerve palsy. Obesity and female predominance tended to present more in the adult population, as many pediatric patients were not obese,2 and had fewer associated symptoms at the time of diagnosis, and the cause was thought to idiopathic.
Now, with the increase in obesity, more adolescents and more male patients are presenting with pseudotumor cerebri as a cause for their headache, and 57%-100% are obese, making it a compounding factor.3
Pediatric populations also are at risk of secondary pseudotumor cerebri, which is an increase in intracranial pressure from the use of medication, or other disease states such as anemia, kidney disease, or Down syndrome. Minocycline use is the most common medication cause and usually presents 1-2 months after normal use.4 Discontinuing the drug does lead to resolution. Retinoids, vitamin A products, growth hormone, and steroids also have been implicated. Given that acne is a common complaint amongst teens, knowledge of these side effects is important.4
In 2013, the criteria for diagnosis of pseudotumor cerebri was revised. Currently, the presence of papilledema, normal neurologic exam except for abnormal sixth cranial nerve, normal cerebral spinal fluid, elevated lumbar opening pressure, and normal imaging are needed for a definitive diagnosis. A probable diagnosis can be made if papilledema is not present but there abducens nerve palsy.2
In a routine physical exam, when I questioned a patient on any medication that was used daily, she replied she took ibuprofen daily for headaches and that she had been doing this for several months. Headaches were not in her chief complaints as she had learned to live with and ignore this symptom. Upon further evaluation, she was slightly overweight and has a questionable fundoscopic exam. After further evaluation by an ophthalmologist and a neurologist, pseudotumor cerebri was diagnosed.
Index of suspicion is key in correctly diagnosing patients, and understanding the changing landscape of medicine will lead to more thoughtful questioning during routine health exams and better outcomes for your patients.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Am J Ophthalmol. 2015 Feb;159(2):344-52.e1.
2. Horm Res Paediatr. 2014;81(4):217-25.
3. Clin Imaging. 2018 May 24. doi: 10.1016/j.clinimag.2018.05.020.
4. Am J Ophthalmol. 1998 Jul;126(1):116-21.
5. Glob Pediatr Health. 2018. doi:10.1177/2333794X18785550.
Pseudotumor cerebri, benign intracranial hypertension, and idiopathic intracranial hypertension are all terms to describe a syndrome of increased intracranial pressure, headaches, vision loss, or changes without an associated mass lesion.1 The condition was considered relatively rare, presenting most commonly in obese women in childbearing years. Surprisingly, 2
Obesity is the fastest growing morbidity among adolescents. The Centers for Disease Control and Prevention reported 32% of children 2-19 years were obese.1 This reality is impacting many areas of an adolescent’s health, but it also is changing the landscape of diseases that present in this age group. Although pediatric and adult pseudotumor cerebri always have had slightly varied features, many features were similar such as the papilledema, vision loss, headaches, and sixth nerve palsy. Obesity and female predominance tended to present more in the adult population, as many pediatric patients were not obese,2 and had fewer associated symptoms at the time of diagnosis, and the cause was thought to idiopathic.
Now, with the increase in obesity, more adolescents and more male patients are presenting with pseudotumor cerebri as a cause for their headache, and 57%-100% are obese, making it a compounding factor.3
Pediatric populations also are at risk of secondary pseudotumor cerebri, which is an increase in intracranial pressure from the use of medication, or other disease states such as anemia, kidney disease, or Down syndrome. Minocycline use is the most common medication cause and usually presents 1-2 months after normal use.4 Discontinuing the drug does lead to resolution. Retinoids, vitamin A products, growth hormone, and steroids also have been implicated. Given that acne is a common complaint amongst teens, knowledge of these side effects is important.4
In 2013, the criteria for diagnosis of pseudotumor cerebri was revised. Currently, the presence of papilledema, normal neurologic exam except for abnormal sixth cranial nerve, normal cerebral spinal fluid, elevated lumbar opening pressure, and normal imaging are needed for a definitive diagnosis. A probable diagnosis can be made if papilledema is not present but there abducens nerve palsy.2
In a routine physical exam, when I questioned a patient on any medication that was used daily, she replied she took ibuprofen daily for headaches and that she had been doing this for several months. Headaches were not in her chief complaints as she had learned to live with and ignore this symptom. Upon further evaluation, she was slightly overweight and has a questionable fundoscopic exam. After further evaluation by an ophthalmologist and a neurologist, pseudotumor cerebri was diagnosed.
Index of suspicion is key in correctly diagnosing patients, and understanding the changing landscape of medicine will lead to more thoughtful questioning during routine health exams and better outcomes for your patients.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Am J Ophthalmol. 2015 Feb;159(2):344-52.e1.
2. Horm Res Paediatr. 2014;81(4):217-25.
3. Clin Imaging. 2018 May 24. doi: 10.1016/j.clinimag.2018.05.020.
4. Am J Ophthalmol. 1998 Jul;126(1):116-21.
5. Glob Pediatr Health. 2018. doi:10.1177/2333794X18785550.
New guidance offered for managing poorly controlled asthma in children
published in the Annals of Allergy, Asthma & Immunology.
“Although many children with asthma achieve symptom control with appropriate management, a substantial subset does not,” Bradley E. Chipps, MD, from the Capital Allergy & Respiratory Disease Center in Sacramento, Calif., and his colleagues wrote in the recommendations sponsored by the American College of Allergy, Asthma, and Immunology. “These children should undergo a step-up in care, but when and how to do that is not always straightforward. The Pediatric Asthma Yardstick is a practical resource for starting or adjusting controller therapy based on the options that are currently available for children, from infants to 18 years of age.”
In their recommendations, the authors grouped patients into age ranges of adolescent (12-18 years), school aged (6-11 years), and young children (5 years and under) as well as severity classifications.
Adolescents and school-aged children
For adolescents and school-aged children, step 1 was classified as intermittent asthma that can be controlled with low-dose inhaled corticosteroids (ICS) with short-acting beta2-agonist (SABA) for as-needed relief. Children considered for stepping up to the next therapy should show symptoms of mild persistent asthma that the authors recommended controlling with low-dose ICS, leukotriene receptor antagonist (LTRA), or low-dose theophylline with as-needed SABA.
In children 12-18 years with moderate persistent asthma (step 3), the authors recommended a combination of low-dose ICA and a long-acting beta2-agonist (LABA), while children 6-11 years should receive a medium dose of ICS; other considerations for school-aged children include a medium-high dose of ICS, a low-dose combination of ICS and LTRA, or low-dose ICS together with theophylline.
Adolescent or school-aged children with severe persistent asthma (step 4) should take a medium or high dose of ICS together with LABA, with the authors recommending adding tiotropium to a soft mist inhaler, combination high-dose ICS and LTRA, or a combination high-dose ICS and theophylline.
Dr. Chipps and his coauthors recommended children stepping up therapy beyond severe persistent asthma (step 5) should add on treatment such as low-dose oral corticosteroids, anti-immunoglobulin E therapy, and adding tiotropium to a soft-mist inhaler.
For adolescent and school-aged children going to steps 3-5, Dr. Chipps and his coauthors recommended prescribing as needed a short-acting beta2 agonist or low-dose ICS/LABA.
Children 5 years and younger
In children 5 years and younger, intermittent asthma (step 1) should be considered if the child has infrequent or viral wheezing but few or no symptoms in the interim that can be controlled with as-needed SABA. These young children who show symptoms of mild persistent asthma (step 2) can be treated with daily low-dose ICS, with other controller options of LTRA or intermittent ICS.
Stepping up therapy from mild to moderate persistent asthma (step 3), young children should receive double the daily dose of low-dose ICS from the previous step or use the low-dose ICS together with LTRA; if children show symptoms of severe persistent asthma (step 4), they should continue their daily controller and be referred to a specialist; other considerations for controllers at this step included adding LTRA, adding intermittent ICS, or increasing ICS frequency.
Other factors to consider

Inconsistencies in response to medication can occur because of comorbid conditions such as obesity, rhinosinusitis, respiratory infection or gastroesophageal reflux; suboptimal inhaled drug delivery; or failure to comply with treatment because of not wanting to take medication (common in adolescents), belief that even controller medicine can be taken intermittently, family stress, cost including lack of insurance or medication not covered by insurance. “Before adjusting therapy, it is important to ensure that the child’s change in symptoms is due to asthma and not to any of these factors that need to be addressed,” Dr. Chipps and his colleagues wrote.
Collaboration among children, their parents, and clinicians is needed to achieve good asthma control because of the “variable presentation within individuals and within the population of children affected” with asthma, they wrote.
The article summarizing the guidelines was sponsored by the American College of Allergy, Asthma, and Immunology. Most of the authors report various financial relationships with companies including AstraZeneca, Aerocrine, Aviragen, Boehringer Ingelheim, Cephalon, Circassia, Commense, Genentech, GlaxoSmithKline, Greer, Meda, Merck, Mylan, Novartis, Patara, Regeneron, Sanofi, TEVA, Theravance, and Vectura Group. Dr. Farrar and Dr. Szefler had no financial interests to disclose.
SOURCE: Chipps BE et al. Ann Allergy Asthma Immunol. 2018 Apr. doi: 1010.1016/j.anai.2018.04.002.
published in the Annals of Allergy, Asthma & Immunology.
“Although many children with asthma achieve symptom control with appropriate management, a substantial subset does not,” Bradley E. Chipps, MD, from the Capital Allergy & Respiratory Disease Center in Sacramento, Calif., and his colleagues wrote in the recommendations sponsored by the American College of Allergy, Asthma, and Immunology. “These children should undergo a step-up in care, but when and how to do that is not always straightforward. The Pediatric Asthma Yardstick is a practical resource for starting or adjusting controller therapy based on the options that are currently available for children, from infants to 18 years of age.”
In their recommendations, the authors grouped patients into age ranges of adolescent (12-18 years), school aged (6-11 years), and young children (5 years and under) as well as severity classifications.
Adolescents and school-aged children
For adolescents and school-aged children, step 1 was classified as intermittent asthma that can be controlled with low-dose inhaled corticosteroids (ICS) with short-acting beta2-agonist (SABA) for as-needed relief. Children considered for stepping up to the next therapy should show symptoms of mild persistent asthma that the authors recommended controlling with low-dose ICS, leukotriene receptor antagonist (LTRA), or low-dose theophylline with as-needed SABA.
In children 12-18 years with moderate persistent asthma (step 3), the authors recommended a combination of low-dose ICA and a long-acting beta2-agonist (LABA), while children 6-11 years should receive a medium dose of ICS; other considerations for school-aged children include a medium-high dose of ICS, a low-dose combination of ICS and LTRA, or low-dose ICS together with theophylline.
Adolescent or school-aged children with severe persistent asthma (step 4) should take a medium or high dose of ICS together with LABA, with the authors recommending adding tiotropium to a soft mist inhaler, combination high-dose ICS and LTRA, or a combination high-dose ICS and theophylline.
Dr. Chipps and his coauthors recommended children stepping up therapy beyond severe persistent asthma (step 5) should add on treatment such as low-dose oral corticosteroids, anti-immunoglobulin E therapy, and adding tiotropium to a soft-mist inhaler.
For adolescent and school-aged children going to steps 3-5, Dr. Chipps and his coauthors recommended prescribing as needed a short-acting beta2 agonist or low-dose ICS/LABA.
Children 5 years and younger
In children 5 years and younger, intermittent asthma (step 1) should be considered if the child has infrequent or viral wheezing but few or no symptoms in the interim that can be controlled with as-needed SABA. These young children who show symptoms of mild persistent asthma (step 2) can be treated with daily low-dose ICS, with other controller options of LTRA or intermittent ICS.
Stepping up therapy from mild to moderate persistent asthma (step 3), young children should receive double the daily dose of low-dose ICS from the previous step or use the low-dose ICS together with LTRA; if children show symptoms of severe persistent asthma (step 4), they should continue their daily controller and be referred to a specialist; other considerations for controllers at this step included adding LTRA, adding intermittent ICS, or increasing ICS frequency.
Other factors to consider

Inconsistencies in response to medication can occur because of comorbid conditions such as obesity, rhinosinusitis, respiratory infection or gastroesophageal reflux; suboptimal inhaled drug delivery; or failure to comply with treatment because of not wanting to take medication (common in adolescents), belief that even controller medicine can be taken intermittently, family stress, cost including lack of insurance or medication not covered by insurance. “Before adjusting therapy, it is important to ensure that the child’s change in symptoms is due to asthma and not to any of these factors that need to be addressed,” Dr. Chipps and his colleagues wrote.
Collaboration among children, their parents, and clinicians is needed to achieve good asthma control because of the “variable presentation within individuals and within the population of children affected” with asthma, they wrote.
The article summarizing the guidelines was sponsored by the American College of Allergy, Asthma, and Immunology. Most of the authors report various financial relationships with companies including AstraZeneca, Aerocrine, Aviragen, Boehringer Ingelheim, Cephalon, Circassia, Commense, Genentech, GlaxoSmithKline, Greer, Meda, Merck, Mylan, Novartis, Patara, Regeneron, Sanofi, TEVA, Theravance, and Vectura Group. Dr. Farrar and Dr. Szefler had no financial interests to disclose.
SOURCE: Chipps BE et al. Ann Allergy Asthma Immunol. 2018 Apr. doi: 1010.1016/j.anai.2018.04.002.
published in the Annals of Allergy, Asthma & Immunology.
“Although many children with asthma achieve symptom control with appropriate management, a substantial subset does not,” Bradley E. Chipps, MD, from the Capital Allergy & Respiratory Disease Center in Sacramento, Calif., and his colleagues wrote in the recommendations sponsored by the American College of Allergy, Asthma, and Immunology. “These children should undergo a step-up in care, but when and how to do that is not always straightforward. The Pediatric Asthma Yardstick is a practical resource for starting or adjusting controller therapy based on the options that are currently available for children, from infants to 18 years of age.”
In their recommendations, the authors grouped patients into age ranges of adolescent (12-18 years), school aged (6-11 years), and young children (5 years and under) as well as severity classifications.
Adolescents and school-aged children
For adolescents and school-aged children, step 1 was classified as intermittent asthma that can be controlled with low-dose inhaled corticosteroids (ICS) with short-acting beta2-agonist (SABA) for as-needed relief. Children considered for stepping up to the next therapy should show symptoms of mild persistent asthma that the authors recommended controlling with low-dose ICS, leukotriene receptor antagonist (LTRA), or low-dose theophylline with as-needed SABA.
In children 12-18 years with moderate persistent asthma (step 3), the authors recommended a combination of low-dose ICA and a long-acting beta2-agonist (LABA), while children 6-11 years should receive a medium dose of ICS; other considerations for school-aged children include a medium-high dose of ICS, a low-dose combination of ICS and LTRA, or low-dose ICS together with theophylline.
Adolescent or school-aged children with severe persistent asthma (step 4) should take a medium or high dose of ICS together with LABA, with the authors recommending adding tiotropium to a soft mist inhaler, combination high-dose ICS and LTRA, or a combination high-dose ICS and theophylline.
Dr. Chipps and his coauthors recommended children stepping up therapy beyond severe persistent asthma (step 5) should add on treatment such as low-dose oral corticosteroids, anti-immunoglobulin E therapy, and adding tiotropium to a soft-mist inhaler.
For adolescent and school-aged children going to steps 3-5, Dr. Chipps and his coauthors recommended prescribing as needed a short-acting beta2 agonist or low-dose ICS/LABA.
Children 5 years and younger
In children 5 years and younger, intermittent asthma (step 1) should be considered if the child has infrequent or viral wheezing but few or no symptoms in the interim that can be controlled with as-needed SABA. These young children who show symptoms of mild persistent asthma (step 2) can be treated with daily low-dose ICS, with other controller options of LTRA or intermittent ICS.
Stepping up therapy from mild to moderate persistent asthma (step 3), young children should receive double the daily dose of low-dose ICS from the previous step or use the low-dose ICS together with LTRA; if children show symptoms of severe persistent asthma (step 4), they should continue their daily controller and be referred to a specialist; other considerations for controllers at this step included adding LTRA, adding intermittent ICS, or increasing ICS frequency.
Other factors to consider

Inconsistencies in response to medication can occur because of comorbid conditions such as obesity, rhinosinusitis, respiratory infection or gastroesophageal reflux; suboptimal inhaled drug delivery; or failure to comply with treatment because of not wanting to take medication (common in adolescents), belief that even controller medicine can be taken intermittently, family stress, cost including lack of insurance or medication not covered by insurance. “Before adjusting therapy, it is important to ensure that the child’s change in symptoms is due to asthma and not to any of these factors that need to be addressed,” Dr. Chipps and his colleagues wrote.
Collaboration among children, their parents, and clinicians is needed to achieve good asthma control because of the “variable presentation within individuals and within the population of children affected” with asthma, they wrote.
The article summarizing the guidelines was sponsored by the American College of Allergy, Asthma, and Immunology. Most of the authors report various financial relationships with companies including AstraZeneca, Aerocrine, Aviragen, Boehringer Ingelheim, Cephalon, Circassia, Commense, Genentech, GlaxoSmithKline, Greer, Meda, Merck, Mylan, Novartis, Patara, Regeneron, Sanofi, TEVA, Theravance, and Vectura Group. Dr. Farrar and Dr. Szefler had no financial interests to disclose.
SOURCE: Chipps BE et al. Ann Allergy Asthma Immunol. 2018 Apr. doi: 1010.1016/j.anai.2018.04.002.
FROM ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY