User login
Prehospital plasma outperforms standard care
Prehospital administration of plasma to trauma patients at risk for hemorrhagic shock improves odds of survival, according to the Prehospital Air Medical Plasma (PAMPer) trial.
Patients who received plasma also had a decreased median prothrombin to time ratio, compared with those who received standard-care resuscitation alone.
Modern trauma patients benefit “from receiving less crystalloid-based therapy and early balanced blood component–based therapy once they arrive at a facility for definitive care,” Jason L. Sperry, MD, of the University of Pittsburgh and his coauthors wrote in the New England Journal of Medicine. These newer strategies aim to mitigate coagulopathy and its associated downstream complications.
Still, “a majority of deaths from traumatic hemorrhage continue to occur in the first hours after arrival at the trauma center, which underscores the importance of the prehospital environment for early interventions that provide benefit,” the investigators noted.
The PAMPer trial (NCT01818427) examined the efficacy and safety of prehospital plasma resuscitation, comparing it with standard-care resuscitation, in severely injured patients. The phase 3, randomized, superiority trial involved 501 trauma patients at risk for hemorrhagic shock who were transported from 27 air medical bases to nine trauma centers. In total, 230 patients received prehospital plasma and standard-care resuscitation, and 271 patients received standard-care alone. Standard-care included infusion of crystalloid fluids in a goal-directed manner. Air medical bases delivered each type of care in 1-month intervals.
Patients were eligible if they exhibited severe hypotension (systolic blood pressure less than 70 mm Hg) or both hypotension (systolic blood pressure less than 90 mm Hg) and tachycardia (greater than 108 beats per minute).
Eligible patients received two units of thawed plasma, which was completely delivered before administration of any other fluids. If infusion of plasma was not complete upon arrival at the trauma center, infusion was completed before any in-hospital fluids were administered. Following preexisting local protocols, 13 of 27 air transport teams carried 2U of red blood cells. If red blood cells were delivered, then this was performed after plasma administration if the patient was still hypotensive or obviously bleeding.
The 30-day mortality rate among all patients was 29.6%. Approximately one in three patients received a prehospital red blood cell transfusion, and nearly 60% underwent surgery within 24 hours of hospital admission.
A greater percentage of patients in the standard-care group were given red blood cell transfusions, compared with those in the plasma group. The standard-care patients also received greater volumes of crystalloid solution than did those receiving plasma.
The primary outcome, 30-day mortality, was 9.8% lower for patients who received plasma, compared with patients who received standard-care alone (23.2% vs. 33.0%; P = .03). Multivariate regression analysis revealed that plasma delivery accounted for a 39% lower risk of death within 30 days, compared with standard care (P = .02). Within 3 hours of randomization, Kaplan-Meier curves revealed a separation between the two treatment groups that persisted until 30 days.
Median prothrombin to time ratio, the only statistically significant secondary outcome, was lower in the plasma group than it was in the standard-care group (1.2 vs. 1.3; P less than .001).
Other measured parameters were similar between the two main treatment groups, including rates of nosocomial infections, allergic or transfusion-related reactions, acute lung injury/acute respiratory distress syndrome, and multiorgan failure.
“Although we cannot determine the independent or additive effects of prehospital administration of plasma and packed red cells, the survival benefits attributable to plasma administration persisted after adjustment for prehospital red-cell administration, and a subgroup analysis showed no heterogeneity of the treatment effect,” the investigators wrote.
The PAMPer trial was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
SOURCE: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
The Prehospital Air Medical Plasma (PAMPer) trial shows that prehospital plasma improves survival rates in trauma patients at risk for hemorrhagic shock, and its implementation should therefore be considered, according to Jeremy W. Cannon, MD.
“Severe hemorrhage from injury claims the lives of nearly 50,000 Americans every year,” Dr. Cannon wrote in an accompanying editorial. “Because many of these deaths occur in young, vital people, this number translates to an astounding loss of almost 2,000,000 years of productive life.”
Since most deaths occur in less than 2 hours, severe hemorrhage requires rapid intervention; however, the best methods of intervention are unclear. In 1994, Bickell et al. demonstrated that administration of crystalloid-based therapy was more beneficial in a goal-directed, operative setting than it was in transit to a trauma center. Other studies have shown that rapid transport and tourniquet application, when appropriate, are essential.
“But what about patients who have blunt injuries and longer transport times?” asked Dr. Cannon. Recently, damage-control resuscitation has proven effective in the trauma center – a combination of red cells, plasma, and platelets in approximately equal proportions, with minimal nonhemostatic crystalloid solution. Researchers now ask whether this intervention has a place in the prehospital setting.
The PAMPer trial suggests that the number needed to treat is 10 to save one life. These results “should motivate trauma center personnel and air medical crews across the country to consider implementing this lifesaving approach,” Dr. Cannon wrote.
Several blood products are candidates for prehospital resuscitation; their selection depends on both logistical and medical factors. Although fresh-frozen plasma was used in the PAMPer trial, it has a shelf life of only 5 days once thawed, leading to more frequent replenishment. Never-frozen plasma lasts 26 days, which could be easier to keep in stock. Beyond plasma, “refrigerated whole blood has a shelf life of 21 days and offers the benefit of both platelets and oxygen delivery, making it perhaps the ideal product for prehospital resuscitation,” Dr. Cannon wrote.
Jeremy W. Cannon, MD, is at the University of Pennsylvania in Philadelphia and the Uniformed Services University in Bethesda, Md. These comments are adapted from an accompanying editorial (N Engl J Med. 2018 Jul 26;379[4]:387-8). Dr. Cannon reported nonfinancial support from Prytime Medical Devices outside the submitted work and institutional participation in a research network funded by the Department of Defense.
The Prehospital Air Medical Plasma (PAMPer) trial shows that prehospital plasma improves survival rates in trauma patients at risk for hemorrhagic shock, and its implementation should therefore be considered, according to Jeremy W. Cannon, MD.
“Severe hemorrhage from injury claims the lives of nearly 50,000 Americans every year,” Dr. Cannon wrote in an accompanying editorial. “Because many of these deaths occur in young, vital people, this number translates to an astounding loss of almost 2,000,000 years of productive life.”
Since most deaths occur in less than 2 hours, severe hemorrhage requires rapid intervention; however, the best methods of intervention are unclear. In 1994, Bickell et al. demonstrated that administration of crystalloid-based therapy was more beneficial in a goal-directed, operative setting than it was in transit to a trauma center. Other studies have shown that rapid transport and tourniquet application, when appropriate, are essential.
“But what about patients who have blunt injuries and longer transport times?” asked Dr. Cannon. Recently, damage-control resuscitation has proven effective in the trauma center – a combination of red cells, plasma, and platelets in approximately equal proportions, with minimal nonhemostatic crystalloid solution. Researchers now ask whether this intervention has a place in the prehospital setting.
The PAMPer trial suggests that the number needed to treat is 10 to save one life. These results “should motivate trauma center personnel and air medical crews across the country to consider implementing this lifesaving approach,” Dr. Cannon wrote.
Several blood products are candidates for prehospital resuscitation; their selection depends on both logistical and medical factors. Although fresh-frozen plasma was used in the PAMPer trial, it has a shelf life of only 5 days once thawed, leading to more frequent replenishment. Never-frozen plasma lasts 26 days, which could be easier to keep in stock. Beyond plasma, “refrigerated whole blood has a shelf life of 21 days and offers the benefit of both platelets and oxygen delivery, making it perhaps the ideal product for prehospital resuscitation,” Dr. Cannon wrote.
Jeremy W. Cannon, MD, is at the University of Pennsylvania in Philadelphia and the Uniformed Services University in Bethesda, Md. These comments are adapted from an accompanying editorial (N Engl J Med. 2018 Jul 26;379[4]:387-8). Dr. Cannon reported nonfinancial support from Prytime Medical Devices outside the submitted work and institutional participation in a research network funded by the Department of Defense.
The Prehospital Air Medical Plasma (PAMPer) trial shows that prehospital plasma improves survival rates in trauma patients at risk for hemorrhagic shock, and its implementation should therefore be considered, according to Jeremy W. Cannon, MD.
“Severe hemorrhage from injury claims the lives of nearly 50,000 Americans every year,” Dr. Cannon wrote in an accompanying editorial. “Because many of these deaths occur in young, vital people, this number translates to an astounding loss of almost 2,000,000 years of productive life.”
Since most deaths occur in less than 2 hours, severe hemorrhage requires rapid intervention; however, the best methods of intervention are unclear. In 1994, Bickell et al. demonstrated that administration of crystalloid-based therapy was more beneficial in a goal-directed, operative setting than it was in transit to a trauma center. Other studies have shown that rapid transport and tourniquet application, when appropriate, are essential.
“But what about patients who have blunt injuries and longer transport times?” asked Dr. Cannon. Recently, damage-control resuscitation has proven effective in the trauma center – a combination of red cells, plasma, and platelets in approximately equal proportions, with minimal nonhemostatic crystalloid solution. Researchers now ask whether this intervention has a place in the prehospital setting.
The PAMPer trial suggests that the number needed to treat is 10 to save one life. These results “should motivate trauma center personnel and air medical crews across the country to consider implementing this lifesaving approach,” Dr. Cannon wrote.
Several blood products are candidates for prehospital resuscitation; their selection depends on both logistical and medical factors. Although fresh-frozen plasma was used in the PAMPer trial, it has a shelf life of only 5 days once thawed, leading to more frequent replenishment. Never-frozen plasma lasts 26 days, which could be easier to keep in stock. Beyond plasma, “refrigerated whole blood has a shelf life of 21 days and offers the benefit of both platelets and oxygen delivery, making it perhaps the ideal product for prehospital resuscitation,” Dr. Cannon wrote.
Jeremy W. Cannon, MD, is at the University of Pennsylvania in Philadelphia and the Uniformed Services University in Bethesda, Md. These comments are adapted from an accompanying editorial (N Engl J Med. 2018 Jul 26;379[4]:387-8). Dr. Cannon reported nonfinancial support from Prytime Medical Devices outside the submitted work and institutional participation in a research network funded by the Department of Defense.
Prehospital administration of plasma to trauma patients at risk for hemorrhagic shock improves odds of survival, according to the Prehospital Air Medical Plasma (PAMPer) trial.
Patients who received plasma also had a decreased median prothrombin to time ratio, compared with those who received standard-care resuscitation alone.
Modern trauma patients benefit “from receiving less crystalloid-based therapy and early balanced blood component–based therapy once they arrive at a facility for definitive care,” Jason L. Sperry, MD, of the University of Pittsburgh and his coauthors wrote in the New England Journal of Medicine. These newer strategies aim to mitigate coagulopathy and its associated downstream complications.
Still, “a majority of deaths from traumatic hemorrhage continue to occur in the first hours after arrival at the trauma center, which underscores the importance of the prehospital environment for early interventions that provide benefit,” the investigators noted.
The PAMPer trial (NCT01818427) examined the efficacy and safety of prehospital plasma resuscitation, comparing it with standard-care resuscitation, in severely injured patients. The phase 3, randomized, superiority trial involved 501 trauma patients at risk for hemorrhagic shock who were transported from 27 air medical bases to nine trauma centers. In total, 230 patients received prehospital plasma and standard-care resuscitation, and 271 patients received standard-care alone. Standard-care included infusion of crystalloid fluids in a goal-directed manner. Air medical bases delivered each type of care in 1-month intervals.
Patients were eligible if they exhibited severe hypotension (systolic blood pressure less than 70 mm Hg) or both hypotension (systolic blood pressure less than 90 mm Hg) and tachycardia (greater than 108 beats per minute).
Eligible patients received two units of thawed plasma, which was completely delivered before administration of any other fluids. If infusion of plasma was not complete upon arrival at the trauma center, infusion was completed before any in-hospital fluids were administered. Following preexisting local protocols, 13 of 27 air transport teams carried 2U of red blood cells. If red blood cells were delivered, then this was performed after plasma administration if the patient was still hypotensive or obviously bleeding.
The 30-day mortality rate among all patients was 29.6%. Approximately one in three patients received a prehospital red blood cell transfusion, and nearly 60% underwent surgery within 24 hours of hospital admission.
A greater percentage of patients in the standard-care group were given red blood cell transfusions, compared with those in the plasma group. The standard-care patients also received greater volumes of crystalloid solution than did those receiving plasma.
The primary outcome, 30-day mortality, was 9.8% lower for patients who received plasma, compared with patients who received standard-care alone (23.2% vs. 33.0%; P = .03). Multivariate regression analysis revealed that plasma delivery accounted for a 39% lower risk of death within 30 days, compared with standard care (P = .02). Within 3 hours of randomization, Kaplan-Meier curves revealed a separation between the two treatment groups that persisted until 30 days.
Median prothrombin to time ratio, the only statistically significant secondary outcome, was lower in the plasma group than it was in the standard-care group (1.2 vs. 1.3; P less than .001).
Other measured parameters were similar between the two main treatment groups, including rates of nosocomial infections, allergic or transfusion-related reactions, acute lung injury/acute respiratory distress syndrome, and multiorgan failure.
“Although we cannot determine the independent or additive effects of prehospital administration of plasma and packed red cells, the survival benefits attributable to plasma administration persisted after adjustment for prehospital red-cell administration, and a subgroup analysis showed no heterogeneity of the treatment effect,” the investigators wrote.
The PAMPer trial was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
SOURCE: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
Prehospital administration of plasma to trauma patients at risk for hemorrhagic shock improves odds of survival, according to the Prehospital Air Medical Plasma (PAMPer) trial.
Patients who received plasma also had a decreased median prothrombin to time ratio, compared with those who received standard-care resuscitation alone.
Modern trauma patients benefit “from receiving less crystalloid-based therapy and early balanced blood component–based therapy once they arrive at a facility for definitive care,” Jason L. Sperry, MD, of the University of Pittsburgh and his coauthors wrote in the New England Journal of Medicine. These newer strategies aim to mitigate coagulopathy and its associated downstream complications.
Still, “a majority of deaths from traumatic hemorrhage continue to occur in the first hours after arrival at the trauma center, which underscores the importance of the prehospital environment for early interventions that provide benefit,” the investigators noted.
The PAMPer trial (NCT01818427) examined the efficacy and safety of prehospital plasma resuscitation, comparing it with standard-care resuscitation, in severely injured patients. The phase 3, randomized, superiority trial involved 501 trauma patients at risk for hemorrhagic shock who were transported from 27 air medical bases to nine trauma centers. In total, 230 patients received prehospital plasma and standard-care resuscitation, and 271 patients received standard-care alone. Standard-care included infusion of crystalloid fluids in a goal-directed manner. Air medical bases delivered each type of care in 1-month intervals.
Patients were eligible if they exhibited severe hypotension (systolic blood pressure less than 70 mm Hg) or both hypotension (systolic blood pressure less than 90 mm Hg) and tachycardia (greater than 108 beats per minute).
Eligible patients received two units of thawed plasma, which was completely delivered before administration of any other fluids. If infusion of plasma was not complete upon arrival at the trauma center, infusion was completed before any in-hospital fluids were administered. Following preexisting local protocols, 13 of 27 air transport teams carried 2U of red blood cells. If red blood cells were delivered, then this was performed after plasma administration if the patient was still hypotensive or obviously bleeding.
The 30-day mortality rate among all patients was 29.6%. Approximately one in three patients received a prehospital red blood cell transfusion, and nearly 60% underwent surgery within 24 hours of hospital admission.
A greater percentage of patients in the standard-care group were given red blood cell transfusions, compared with those in the plasma group. The standard-care patients also received greater volumes of crystalloid solution than did those receiving plasma.
The primary outcome, 30-day mortality, was 9.8% lower for patients who received plasma, compared with patients who received standard-care alone (23.2% vs. 33.0%; P = .03). Multivariate regression analysis revealed that plasma delivery accounted for a 39% lower risk of death within 30 days, compared with standard care (P = .02). Within 3 hours of randomization, Kaplan-Meier curves revealed a separation between the two treatment groups that persisted until 30 days.
Median prothrombin to time ratio, the only statistically significant secondary outcome, was lower in the plasma group than it was in the standard-care group (1.2 vs. 1.3; P less than .001).
Other measured parameters were similar between the two main treatment groups, including rates of nosocomial infections, allergic or transfusion-related reactions, acute lung injury/acute respiratory distress syndrome, and multiorgan failure.
“Although we cannot determine the independent or additive effects of prehospital administration of plasma and packed red cells, the survival benefits attributable to plasma administration persisted after adjustment for prehospital red-cell administration, and a subgroup analysis showed no heterogeneity of the treatment effect,” the investigators wrote.
The PAMPer trial was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
SOURCE: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Thirty-day mortality was 9.8 percentage points lower for patients receiving plasma, compared with patients receiving standard care (23.2% vs. 33.0%; P = .03).
Study details: A randomized, controlled superiority trial involving 501 trauma patients at risk for hemorrhagic shock, with 230 patients receiving plasma and 271 receiving standard-care during air medical transport.
Disclosures: The study was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
Source: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
Monoclonal antibody slowed cognitive decline, cleared Alzheimer’s plaques in phase 2 trial
CHICAGO – BAN2401, a monoclonal antibody that targets soluble amyloid beta oligomers, slowed cognitive decline in patients with mild cognitive impairment or early Alzheimer’s dementia on two measures, while clearing brain amyloid in 81% of patients in a phase 2 study.
“The majority of subjects receiving the top dose [10 mg/kg intravenously, biweekly] went from being amyloid positive to amyloid negative,” by the end of the 18-month study, Lynn Kramer, MD, said at the Alzheimer’s Association International Conference.
At that dose, BAN2401 also slowed cognitive decline by 30% relative to placebo on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, which is codeveloping the drug with Biogen. The antibody also hit statistical significance on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), with a 47% slowing of decline. A third measure, the Clinical Dementia Rating Sum of Boxes (CDR-sb), showed a 26% reduction, but this was not statistically significant.
There were also significant changes in cerebrospinal fluid amyloid beta and total tau, said Dr. Kramer, chief medical officer of Eisai’s neurology division.
“In our opinion, we have fairly conclusive results,” he said at a press briefing. “We view this as robust enough to approach regulatory authorities [around the world] and discuss the next steps with them, and what we need to do to register this product.”
In addition to planning larger, longer phase 3 studies, Dr. Kramer said that Eisai might try to obtain FDA breakthrough therapy status.
BAN2401 selectively binds to amyloid-beta protofibrils – large, soluble amyloid-beta oligomers – and targets them for clearance. It was originally developed by Swedish biopharma company BioArctic; Eisai acquired the molecule in 2007 and entered a deal with Biogen in 2014.
This is a developing story. Please check back for details.
CHICAGO – BAN2401, a monoclonal antibody that targets soluble amyloid beta oligomers, slowed cognitive decline in patients with mild cognitive impairment or early Alzheimer’s dementia on two measures, while clearing brain amyloid in 81% of patients in a phase 2 study.
“The majority of subjects receiving the top dose [10 mg/kg intravenously, biweekly] went from being amyloid positive to amyloid negative,” by the end of the 18-month study, Lynn Kramer, MD, said at the Alzheimer’s Association International Conference.
At that dose, BAN2401 also slowed cognitive decline by 30% relative to placebo on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, which is codeveloping the drug with Biogen. The antibody also hit statistical significance on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), with a 47% slowing of decline. A third measure, the Clinical Dementia Rating Sum of Boxes (CDR-sb), showed a 26% reduction, but this was not statistically significant.
There were also significant changes in cerebrospinal fluid amyloid beta and total tau, said Dr. Kramer, chief medical officer of Eisai’s neurology division.
“In our opinion, we have fairly conclusive results,” he said at a press briefing. “We view this as robust enough to approach regulatory authorities [around the world] and discuss the next steps with them, and what we need to do to register this product.”
In addition to planning larger, longer phase 3 studies, Dr. Kramer said that Eisai might try to obtain FDA breakthrough therapy status.
BAN2401 selectively binds to amyloid-beta protofibrils – large, soluble amyloid-beta oligomers – and targets them for clearance. It was originally developed by Swedish biopharma company BioArctic; Eisai acquired the molecule in 2007 and entered a deal with Biogen in 2014.
This is a developing story. Please check back for details.
CHICAGO – BAN2401, a monoclonal antibody that targets soluble amyloid beta oligomers, slowed cognitive decline in patients with mild cognitive impairment or early Alzheimer’s dementia on two measures, while clearing brain amyloid in 81% of patients in a phase 2 study.
“The majority of subjects receiving the top dose [10 mg/kg intravenously, biweekly] went from being amyloid positive to amyloid negative,” by the end of the 18-month study, Lynn Kramer, MD, said at the Alzheimer’s Association International Conference.
At that dose, BAN2401 also slowed cognitive decline by 30% relative to placebo on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, which is codeveloping the drug with Biogen. The antibody also hit statistical significance on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), with a 47% slowing of decline. A third measure, the Clinical Dementia Rating Sum of Boxes (CDR-sb), showed a 26% reduction, but this was not statistically significant.
There were also significant changes in cerebrospinal fluid amyloid beta and total tau, said Dr. Kramer, chief medical officer of Eisai’s neurology division.
“In our opinion, we have fairly conclusive results,” he said at a press briefing. “We view this as robust enough to approach regulatory authorities [around the world] and discuss the next steps with them, and what we need to do to register this product.”
In addition to planning larger, longer phase 3 studies, Dr. Kramer said that Eisai might try to obtain FDA breakthrough therapy status.
BAN2401 selectively binds to amyloid-beta protofibrils – large, soluble amyloid-beta oligomers – and targets them for clearance. It was originally developed by Swedish biopharma company BioArctic; Eisai acquired the molecule in 2007 and entered a deal with Biogen in 2014.
This is a developing story. Please check back for details.
REPORTING FROM AAIC 2018
FDA Approves Deep Brain Stimulation System
The Food and Drug Administration has approved a deep brain stimulation system that has been shown to reduce seizures in a select group of patients with epilepsy.
- Medtronics DBS System for Epilepsy has been cleared as adjunct treatment for patients with partial onset seizures with or without secondary generalization.
- The system is only indicated for patients who have not responded to 3 or more antiepileptic drugs and who have experienced an average of 6 or more seizures each month for the last 3 months.
- The FDA approval also stipulates that the patients’ seizures be no more than 30 days apart.
- The DBS System includes a pulse generator that is implanted in a patient’s chest and 2 lead wires implanted in the brain.
Voelker R. Electrical stimulation for epilepsy. JAMA; 2018;319(21):2164.
The Food and Drug Administration has approved a deep brain stimulation system that has been shown to reduce seizures in a select group of patients with epilepsy.
- Medtronics DBS System for Epilepsy has been cleared as adjunct treatment for patients with partial onset seizures with or without secondary generalization.
- The system is only indicated for patients who have not responded to 3 or more antiepileptic drugs and who have experienced an average of 6 or more seizures each month for the last 3 months.
- The FDA approval also stipulates that the patients’ seizures be no more than 30 days apart.
- The DBS System includes a pulse generator that is implanted in a patient’s chest and 2 lead wires implanted in the brain.
Voelker R. Electrical stimulation for epilepsy. JAMA; 2018;319(21):2164.
The Food and Drug Administration has approved a deep brain stimulation system that has been shown to reduce seizures in a select group of patients with epilepsy.
- Medtronics DBS System for Epilepsy has been cleared as adjunct treatment for patients with partial onset seizures with or without secondary generalization.
- The system is only indicated for patients who have not responded to 3 or more antiepileptic drugs and who have experienced an average of 6 or more seizures each month for the last 3 months.
- The FDA approval also stipulates that the patients’ seizures be no more than 30 days apart.
- The DBS System includes a pulse generator that is implanted in a patient’s chest and 2 lead wires implanted in the brain.
Voelker R. Electrical stimulation for epilepsy. JAMA; 2018;319(21):2164.
Psychiatric Interventions Important for Patients with Epilepsy
Clinicians need to pay more attention to the psychological impact of epilepsy and its treatment according to a report from the International League Against Epilepsy Psychology Task Force.
- The task force identified the best interventions for depression, neurocognitive problems, and medication adherence.
- Several psychological strategies are worth consideration according to the task force, including cognitive behavioral therapy and mindfulness-based treatment.
- These interventions have the potential to improve health-related quality of life among adults and children.
- The recommendations outlined by the League are based primarily on evidence discussed in a recent Cochrane review of randomized clinical trials that evaluated psychological treatment of patients with epilepsy.
Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
Clinicians need to pay more attention to the psychological impact of epilepsy and its treatment according to a report from the International League Against Epilepsy Psychology Task Force.
- The task force identified the best interventions for depression, neurocognitive problems, and medication adherence.
- Several psychological strategies are worth consideration according to the task force, including cognitive behavioral therapy and mindfulness-based treatment.
- These interventions have the potential to improve health-related quality of life among adults and children.
- The recommendations outlined by the League are based primarily on evidence discussed in a recent Cochrane review of randomized clinical trials that evaluated psychological treatment of patients with epilepsy.
Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
Clinicians need to pay more attention to the psychological impact of epilepsy and its treatment according to a report from the International League Against Epilepsy Psychology Task Force.
- The task force identified the best interventions for depression, neurocognitive problems, and medication adherence.
- Several psychological strategies are worth consideration according to the task force, including cognitive behavioral therapy and mindfulness-based treatment.
- These interventions have the potential to improve health-related quality of life among adults and children.
- The recommendations outlined by the League are based primarily on evidence discussed in a recent Cochrane review of randomized clinical trials that evaluated psychological treatment of patients with epilepsy.
Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
No Differences Found Between Generic/Brand Name Epileptic Medication
Switching patients from brand name antiepileptic drugs to generics is generally safe and cost effective according to an analysis published in Epilepsia.
- Researchers looked at data on bioequivalence, health care utilization, and clinical studies on the safety of antiepileptic agents, including a comparison of area under the plasma concentration-time curve (AUC) and peak plasma concentration.
- For most of the drugs that were evaluated, there were negligible differences in AUC and peak plasma concentration between generic drugs and brand name equivalents.
- There were significant increases in health care usage when patients were switched from brand name to generic versions.
- Clinical studies were unable to detect differences in seizure frequency or tolerability.
Holtkamp M, Theodore WH. Generic antiepileptic drugs—safe or harmful in patients with epilepsy? Epilepsia. 2018;59(7):1273-1281.
Switching patients from brand name antiepileptic drugs to generics is generally safe and cost effective according to an analysis published in Epilepsia.
- Researchers looked at data on bioequivalence, health care utilization, and clinical studies on the safety of antiepileptic agents, including a comparison of area under the plasma concentration-time curve (AUC) and peak plasma concentration.
- For most of the drugs that were evaluated, there were negligible differences in AUC and peak plasma concentration between generic drugs and brand name equivalents.
- There were significant increases in health care usage when patients were switched from brand name to generic versions.
- Clinical studies were unable to detect differences in seizure frequency or tolerability.
Holtkamp M, Theodore WH. Generic antiepileptic drugs—safe or harmful in patients with epilepsy? Epilepsia. 2018;59(7):1273-1281.
Switching patients from brand name antiepileptic drugs to generics is generally safe and cost effective according to an analysis published in Epilepsia.
- Researchers looked at data on bioequivalence, health care utilization, and clinical studies on the safety of antiepileptic agents, including a comparison of area under the plasma concentration-time curve (AUC) and peak plasma concentration.
- For most of the drugs that were evaluated, there were negligible differences in AUC and peak plasma concentration between generic drugs and brand name equivalents.
- There were significant increases in health care usage when patients were switched from brand name to generic versions.
- Clinical studies were unable to detect differences in seizure frequency or tolerability.
Holtkamp M, Theodore WH. Generic antiepileptic drugs—safe or harmful in patients with epilepsy? Epilepsia. 2018;59(7):1273-1281.
In Ghana, SCD research is meeting patients on home turf
WASHINGTON – Sometimes, the hardest part of solving a problem is figuring out how to work around misaligned resources, and so it has been with sickle cell disease research.
“From my point of view, what I call the geographical disparity in sickle cell disease research can be explained by the fact that the majority of affected individuals are living in the East, and the overwhelming majority of the research takes place in the West,” said Solomon Ofori-Acquah, PhD. He and three physician collaborators from Ghana shared their roadmap to conducting clinical trials in West Africa during an “East Meets West” session of the annual symposium of the Foundation for Sickle Cell Disease Research.
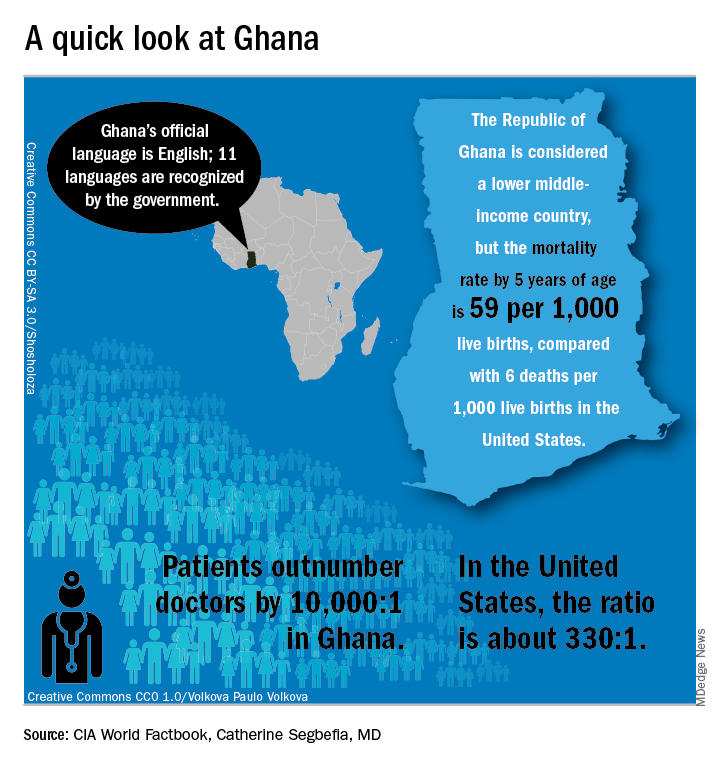
In Ghana, not far from where scientists now believe the hemoglobin sickling mutation originated, fully 2% of newborns have SCD; this translates into 16,000 new cases per year in a population of just 28 million, compared with the 2,000 new SCD cases seen annually in the entire United States. And access even to proven therapies can be limited; historically, little to no clinical drug development work has been conducted in this part of the world.
In the United States, half of the SCD trials that were withdrawn or terminated listed recruitment and retention of study participants as a factor in the study’s discontinuation, said Amma Owusu-Ansah, MD. “I see what we are doing as a very feasible solution to the problem of inadequate accrual to studies in the U.S.,” said Dr. Owusu-Ansah, a hematologist at the University of Pittsburgh’s Center for Translational and International Hematology (CTIH), where she serves as clinical director.
From the African perspective, hosting clinical trials – and building a robust infrastructure to do so – may help alleviate the delay in translation of disease-modifying therapies for SCD to Africa, where most people with the disease live, she said.
An existing example of resource sharing is the Human Heredity & Health in Africa (H3Africa) initiative, said Dr. Ofori-Acquah, who directs the CTIH and also holds an appointment at the University of Ghana. The project, funded by the National Institutes of Health and the Wellcome Trust, “aims to facilitate a contemporary research approach to the study of genomics and environmental determinants of common diseases with the goal of improving the health of African populations,” according to the H3Africa website. Within this framework of 40 research centers conducting genomics research and biobanking, several discrete projects aim to expand knowledge of sickle cell disease.
“All of these networks are going to study thousands of patients,” said Dr. Ofori-Acquah. “I see the H3 as a mechanism to accelerate genomics research in sickle cell disease.”
“We created a research team and built capacity for future work…. Ghana, and Africa, are capable of conducting clinical trials to global standards and producing quality data,” she said.
The story of one clinical trial is illustrative of the challenges and strengths of the multinational approach.
The phase 1b trial of a novel treatment for sickle cell disease, NVX-508, began with an initial hurdle of lack of access to emergency care at the study site, said Dr. Owusu-Ansah, a study investigator. Her first reaction, she said, was, “Well, we can’t do this, because we don’t have access to a big staff and emergency facilities.”
But after consulting with colleagues, she realized a shift in mindset was needed: “Rather than focus on what we don’t have, what do we actually have available? We have relationships we have built with institutions,” including the oldest SCD clinic in Ghana, the Ghana Institute of Clinical Genetics (GICG). This facility sits next door to a hospital with 24-hour care, Korle Bu Teaching Hospital (KBTH), a major tertiary care and referral center.
Open since 1974, the KBTH-allied GICG provides comprehensive outpatient health care to teens and adult with SCD. Currently, more than 25,000 SCD patients are registered at GICG; about half have the HbSS genotype, and another 40% have the HbSC genotype, said Yvonne Dei-Adomakoh, MD. Dr. Dei-Adomakoh of the University of Ghana is an investigator for an upcoming phase 3 trial to test voxelotor against placebo in SCD.
The GICG is working hard to become a site where clinical trials, as well as research and development, are embedded into clinic functions. In this way, not only will research be advanced for all those with SCD, but advances will be more easily incorporated into clinical care, said Dr. Dei-Adomakoh.
Dr. Owusu-Ansah noted that the facility offers a pharmacy, a laboratory, exam rooms, and information technology and medical record resources. Importantly, GICG is already staffed with physicians and allied health personnel with SCD expertise.
The University of Ghana campus is home to one of Africa’s leading biomedical research facilities, a sophisticated 11,000-square-foot laboratory that can perform testing ranging from polymerase chain reactions to DNA sequencing to genotyping and flow cytometry; it also houses a laboratory animal facility. This laboratory, the Noguchi Memorial Institute for Medical Research, also offers administrative, scientific, and research support, and houses an institutional review board.
The problem of the Noguchi laboratory site’s distance from the 24-hour support of KBTH has been solved by arranging to have an ambulance with paramedics available on site during the clinical trials.
Some other challenges the investigators discovered highlighted less-obvious infrastructure deficits; keeping a refrigerated chain of custody for biological samples, for example, can be difficult. In preparation for the trials, much basic laboratory and clinical equipment has been updated.
Conducting a U.S.-registered clinical trial in Ghana doesn’t obviate the need to meet that country’s considerable regulatory hurdles, said Dr. Owusu-Ansah. Requirements include a full regulatory submission to, and physical inspection by, Ghana’s FDA. Ghana also requires that the principal investigator must live in Ghana for the duration of the trial and that key study personnel complete Ghanaian good clinical practices training, she said.
The University of Pittsburgh is a U.S. partner in the NVX-508 study, and it was non-negotiable for that institution that a clinical trial monitor visit the African study sites. The institution’s institutional review board was sensitive to the importance of protecting vulnerable populations, and needed to hear complete plans for risk assessment, data protection, and compensation for Ghanaian study participants, Dr. Owusu-Ansah said.
But, in a turn of events typical of the ups and downs of drug development, the phase 1 trial had passed most of the administrative hurdles when in July the drug’s sponsor, NuvOx Pharma, suspended the NVX-508 trial to focus on other areas. For now, the trial registration has been withdrawn on clinicaltrials.gov and the new drug application is inactive. But Dr. Owusu-Ansah said study preparations could resume in the future, if the drug is made available to investigators.
Dr. Owusu-Ansah reported that she has received salary support from NuvOx Pharma. Dr. Segbefia reported that she has received support from Daiichi-Sankyo and Eli Lilly and Company.
WASHINGTON – Sometimes, the hardest part of solving a problem is figuring out how to work around misaligned resources, and so it has been with sickle cell disease research.

“From my point of view, what I call the geographical disparity in sickle cell disease research can be explained by the fact that the majority of affected individuals are living in the East, and the overwhelming majority of the research takes place in the West,” said Solomon Ofori-Acquah, PhD. He and three physician collaborators from Ghana shared their roadmap to conducting clinical trials in West Africa during an “East Meets West” session of the annual symposium of the Foundation for Sickle Cell Disease Research.
In Ghana, not far from where scientists now believe the hemoglobin sickling mutation originated, fully 2% of newborns have SCD; this translates into 16,000 new cases per year in a population of just 28 million, compared with the 2,000 new SCD cases seen annually in the entire United States. And access even to proven therapies can be limited; historically, little to no clinical drug development work has been conducted in this part of the world.
In the United States, half of the SCD trials that were withdrawn or terminated listed recruitment and retention of study participants as a factor in the study’s discontinuation, said Amma Owusu-Ansah, MD. “I see what we are doing as a very feasible solution to the problem of inadequate accrual to studies in the U.S.,” said Dr. Owusu-Ansah, a hematologist at the University of Pittsburgh’s Center for Translational and International Hematology (CTIH), where she serves as clinical director.
From the African perspective, hosting clinical trials – and building a robust infrastructure to do so – may help alleviate the delay in translation of disease-modifying therapies for SCD to Africa, where most people with the disease live, she said.
An existing example of resource sharing is the Human Heredity & Health in Africa (H3Africa) initiative, said Dr. Ofori-Acquah, who directs the CTIH and also holds an appointment at the University of Ghana. The project, funded by the National Institutes of Health and the Wellcome Trust, “aims to facilitate a contemporary research approach to the study of genomics and environmental determinants of common diseases with the goal of improving the health of African populations,” according to the H3Africa website. Within this framework of 40 research centers conducting genomics research and biobanking, several discrete projects aim to expand knowledge of sickle cell disease.
“All of these networks are going to study thousands of patients,” said Dr. Ofori-Acquah. “I see the H3 as a mechanism to accelerate genomics research in sickle cell disease.”
“We created a research team and built capacity for future work…. Ghana, and Africa, are capable of conducting clinical trials to global standards and producing quality data,” she said.
The story of one clinical trial is illustrative of the challenges and strengths of the multinational approach.
The phase 1b trial of a novel treatment for sickle cell disease, NVX-508, began with an initial hurdle of lack of access to emergency care at the study site, said Dr. Owusu-Ansah, a study investigator. Her first reaction, she said, was, “Well, we can’t do this, because we don’t have access to a big staff and emergency facilities.”
But after consulting with colleagues, she realized a shift in mindset was needed: “Rather than focus on what we don’t have, what do we actually have available? We have relationships we have built with institutions,” including the oldest SCD clinic in Ghana, the Ghana Institute of Clinical Genetics (GICG). This facility sits next door to a hospital with 24-hour care, Korle Bu Teaching Hospital (KBTH), a major tertiary care and referral center.
Open since 1974, the KBTH-allied GICG provides comprehensive outpatient health care to teens and adult with SCD. Currently, more than 25,000 SCD patients are registered at GICG; about half have the HbSS genotype, and another 40% have the HbSC genotype, said Yvonne Dei-Adomakoh, MD. Dr. Dei-Adomakoh of the University of Ghana is an investigator for an upcoming phase 3 trial to test voxelotor against placebo in SCD.
The GICG is working hard to become a site where clinical trials, as well as research and development, are embedded into clinic functions. In this way, not only will research be advanced for all those with SCD, but advances will be more easily incorporated into clinical care, said Dr. Dei-Adomakoh.
Dr. Owusu-Ansah noted that the facility offers a pharmacy, a laboratory, exam rooms, and information technology and medical record resources. Importantly, GICG is already staffed with physicians and allied health personnel with SCD expertise.
The University of Ghana campus is home to one of Africa’s leading biomedical research facilities, a sophisticated 11,000-square-foot laboratory that can perform testing ranging from polymerase chain reactions to DNA sequencing to genotyping and flow cytometry; it also houses a laboratory animal facility. This laboratory, the Noguchi Memorial Institute for Medical Research, also offers administrative, scientific, and research support, and houses an institutional review board.
The problem of the Noguchi laboratory site’s distance from the 24-hour support of KBTH has been solved by arranging to have an ambulance with paramedics available on site during the clinical trials.
Some other challenges the investigators discovered highlighted less-obvious infrastructure deficits; keeping a refrigerated chain of custody for biological samples, for example, can be difficult. In preparation for the trials, much basic laboratory and clinical equipment has been updated.
Conducting a U.S.-registered clinical trial in Ghana doesn’t obviate the need to meet that country’s considerable regulatory hurdles, said Dr. Owusu-Ansah. Requirements include a full regulatory submission to, and physical inspection by, Ghana’s FDA. Ghana also requires that the principal investigator must live in Ghana for the duration of the trial and that key study personnel complete Ghanaian good clinical practices training, she said.
The University of Pittsburgh is a U.S. partner in the NVX-508 study, and it was non-negotiable for that institution that a clinical trial monitor visit the African study sites. The institution’s institutional review board was sensitive to the importance of protecting vulnerable populations, and needed to hear complete plans for risk assessment, data protection, and compensation for Ghanaian study participants, Dr. Owusu-Ansah said.
But, in a turn of events typical of the ups and downs of drug development, the phase 1 trial had passed most of the administrative hurdles when in July the drug’s sponsor, NuvOx Pharma, suspended the NVX-508 trial to focus on other areas. For now, the trial registration has been withdrawn on clinicaltrials.gov and the new drug application is inactive. But Dr. Owusu-Ansah said study preparations could resume in the future, if the drug is made available to investigators.
Dr. Owusu-Ansah reported that she has received salary support from NuvOx Pharma. Dr. Segbefia reported that she has received support from Daiichi-Sankyo and Eli Lilly and Company.
WASHINGTON – Sometimes, the hardest part of solving a problem is figuring out how to work around misaligned resources, and so it has been with sickle cell disease research.

“From my point of view, what I call the geographical disparity in sickle cell disease research can be explained by the fact that the majority of affected individuals are living in the East, and the overwhelming majority of the research takes place in the West,” said Solomon Ofori-Acquah, PhD. He and three physician collaborators from Ghana shared their roadmap to conducting clinical trials in West Africa during an “East Meets West” session of the annual symposium of the Foundation for Sickle Cell Disease Research.
In Ghana, not far from where scientists now believe the hemoglobin sickling mutation originated, fully 2% of newborns have SCD; this translates into 16,000 new cases per year in a population of just 28 million, compared with the 2,000 new SCD cases seen annually in the entire United States. And access even to proven therapies can be limited; historically, little to no clinical drug development work has been conducted in this part of the world.
In the United States, half of the SCD trials that were withdrawn or terminated listed recruitment and retention of study participants as a factor in the study’s discontinuation, said Amma Owusu-Ansah, MD. “I see what we are doing as a very feasible solution to the problem of inadequate accrual to studies in the U.S.,” said Dr. Owusu-Ansah, a hematologist at the University of Pittsburgh’s Center for Translational and International Hematology (CTIH), where she serves as clinical director.
From the African perspective, hosting clinical trials – and building a robust infrastructure to do so – may help alleviate the delay in translation of disease-modifying therapies for SCD to Africa, where most people with the disease live, she said.
An existing example of resource sharing is the Human Heredity & Health in Africa (H3Africa) initiative, said Dr. Ofori-Acquah, who directs the CTIH and also holds an appointment at the University of Ghana. The project, funded by the National Institutes of Health and the Wellcome Trust, “aims to facilitate a contemporary research approach to the study of genomics and environmental determinants of common diseases with the goal of improving the health of African populations,” according to the H3Africa website. Within this framework of 40 research centers conducting genomics research and biobanking, several discrete projects aim to expand knowledge of sickle cell disease.
“All of these networks are going to study thousands of patients,” said Dr. Ofori-Acquah. “I see the H3 as a mechanism to accelerate genomics research in sickle cell disease.”
“We created a research team and built capacity for future work…. Ghana, and Africa, are capable of conducting clinical trials to global standards and producing quality data,” she said.
The story of one clinical trial is illustrative of the challenges and strengths of the multinational approach.
The phase 1b trial of a novel treatment for sickle cell disease, NVX-508, began with an initial hurdle of lack of access to emergency care at the study site, said Dr. Owusu-Ansah, a study investigator. Her first reaction, she said, was, “Well, we can’t do this, because we don’t have access to a big staff and emergency facilities.”
But after consulting with colleagues, she realized a shift in mindset was needed: “Rather than focus on what we don’t have, what do we actually have available? We have relationships we have built with institutions,” including the oldest SCD clinic in Ghana, the Ghana Institute of Clinical Genetics (GICG). This facility sits next door to a hospital with 24-hour care, Korle Bu Teaching Hospital (KBTH), a major tertiary care and referral center.
Open since 1974, the KBTH-allied GICG provides comprehensive outpatient health care to teens and adult with SCD. Currently, more than 25,000 SCD patients are registered at GICG; about half have the HbSS genotype, and another 40% have the HbSC genotype, said Yvonne Dei-Adomakoh, MD. Dr. Dei-Adomakoh of the University of Ghana is an investigator for an upcoming phase 3 trial to test voxelotor against placebo in SCD.
The GICG is working hard to become a site where clinical trials, as well as research and development, are embedded into clinic functions. In this way, not only will research be advanced for all those with SCD, but advances will be more easily incorporated into clinical care, said Dr. Dei-Adomakoh.
Dr. Owusu-Ansah noted that the facility offers a pharmacy, a laboratory, exam rooms, and information technology and medical record resources. Importantly, GICG is already staffed with physicians and allied health personnel with SCD expertise.
The University of Ghana campus is home to one of Africa’s leading biomedical research facilities, a sophisticated 11,000-square-foot laboratory that can perform testing ranging from polymerase chain reactions to DNA sequencing to genotyping and flow cytometry; it also houses a laboratory animal facility. This laboratory, the Noguchi Memorial Institute for Medical Research, also offers administrative, scientific, and research support, and houses an institutional review board.
The problem of the Noguchi laboratory site’s distance from the 24-hour support of KBTH has been solved by arranging to have an ambulance with paramedics available on site during the clinical trials.
Some other challenges the investigators discovered highlighted less-obvious infrastructure deficits; keeping a refrigerated chain of custody for biological samples, for example, can be difficult. In preparation for the trials, much basic laboratory and clinical equipment has been updated.
Conducting a U.S.-registered clinical trial in Ghana doesn’t obviate the need to meet that country’s considerable regulatory hurdles, said Dr. Owusu-Ansah. Requirements include a full regulatory submission to, and physical inspection by, Ghana’s FDA. Ghana also requires that the principal investigator must live in Ghana for the duration of the trial and that key study personnel complete Ghanaian good clinical practices training, she said.
The University of Pittsburgh is a U.S. partner in the NVX-508 study, and it was non-negotiable for that institution that a clinical trial monitor visit the African study sites. The institution’s institutional review board was sensitive to the importance of protecting vulnerable populations, and needed to hear complete plans for risk assessment, data protection, and compensation for Ghanaian study participants, Dr. Owusu-Ansah said.
But, in a turn of events typical of the ups and downs of drug development, the phase 1 trial had passed most of the administrative hurdles when in July the drug’s sponsor, NuvOx Pharma, suspended the NVX-508 trial to focus on other areas. For now, the trial registration has been withdrawn on clinicaltrials.gov and the new drug application is inactive. But Dr. Owusu-Ansah said study preparations could resume in the future, if the drug is made available to investigators.
Dr. Owusu-Ansah reported that she has received salary support from NuvOx Pharma. Dr. Segbefia reported that she has received support from Daiichi-Sankyo and Eli Lilly and Company.
EXPERT ANALYSIS FROM FSCDR 2018
Myeloma frailty index predicts survival based on biological age
A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than chronological age alone, investigators report. A 16% increased risk of death was seen for each 10% increase in the deficit-accumulation frailty index (DAFI), which includes 25 variables related health, function, and activities of daily living.
There was only a weak correlation between chronological age and increase in deficits tracked by the index, in contrast to a cohort without cancer, in which age and frailty were strongly correlated, the investigators reported in JCO Clinical Cancer Informatics.
“Our results demonstrate that, for patients with multiple myeloma, chronological age alone is not a good measure for assessing overall health,” study author Tanya M. Wildes, MD, of Washington University, St. Louis, said in a news release from the American Society of Clinical Oncology.
Existing tools to assess frailty include an index proposed by the International Myeloma Working Group that looks at age plus other indexes related to comorbidities and activities of daily living, and the revised Myeloma Comorbidity Index that incorporates age with other prognostic factors.
“Although both tools provide prognostic information, chronological age automatically increases frailty without taking biologic or functional age into account,” Dr. Wildes and her coauthors wrote in their report.
By contrast, the DAFI is based on the concept of biologic age, in which the health status of an individual is measured based on the proportion of aging-associated deficits they have accumulated, according to the authors.
To create the DAFI, Dr. Wildes and her colleagues analyzed nearly 2.7 million records of noncancer patients aged 66 years or older in the SEER Medicare Health Outcomes Survey (MHOS) database. They identified 25 variables in the database representing chronic health conditions, activities of daily living, functioning, mental health, and general health.
An individual’s DAFI score was calculated as the sum of scores for each of the 25 variables as 0 for absent, 0.5 for limited, and 1 for present. Predicted DAFI means were calculated for each year of age and used to create age-specific cut points to determine whether an individual would be considered frail or not versus others of the same age.
“In other words, the same frailty score may qualify an 80-year-old individual as fit and a 70-year-old as frail, depending on the cutoff for their respective age group,” investigators explained in their report.
They applied the index to 305 patients with newly diagnosed myeloma in the SEER-MHOS database who were 66 years of age or older (median age, 76 years) and had completed the survey within 1 year of diagnosis.
The DAFI classified 52% of the myeloma patients as frail, and for that group, median overall survival was 26.8 months, versus 43.7 months for nonfrail patients (P = .015), according to the reported data. For each 10% increase in score, the risk of death increased by 16% (P less than .001).
Notably, advancing age was very weakly correlated with increased age-related deficits in the myeloma cohort (r2 = 0.15; P = .010), according to investigators, but very strongly correlated with deficits in the cohort of noncancer patients (r2 = 0.98; P less than .001).
“This suggests that, in patients with multiple myeloma, the prevalence of impairments across domains of function, chronic comorbidities, general health, and mental health are more related to the overall burden of myeloma rather than chronological age alone,” the investigators wrote.
The information used to calculate a DAFI score is easily obtainable during a clinic visit, according to the authors, who provided an overview of all 25 variables in the journal article.
Further development of a computerized program would further enhance usability in the clinic, allowing for real-time calculation during a patient visit, they said.
Survivorship expert Merry Jennifer Markham, MD, said in the ASCO news release that this frailty index is notable because it accounts for more than just chronological age. “Knowing this information can help oncologists have more informed discussions with patients about their prognosis, which in turn can empower patients and families as they weigh treatment options,” she said.
The research was supported by National Cancer Institute. Dr. Wildes reported honoraria from Carevive Systems and research funding from Janssen Oncology. Another coauthor reported honoraria from Celgene and Janssen, and a consulting or advisory role with Amgen and Takeda.
SOURCE: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than chronological age alone, investigators report. A 16% increased risk of death was seen for each 10% increase in the deficit-accumulation frailty index (DAFI), which includes 25 variables related health, function, and activities of daily living.
There was only a weak correlation between chronological age and increase in deficits tracked by the index, in contrast to a cohort without cancer, in which age and frailty were strongly correlated, the investigators reported in JCO Clinical Cancer Informatics.
“Our results demonstrate that, for patients with multiple myeloma, chronological age alone is not a good measure for assessing overall health,” study author Tanya M. Wildes, MD, of Washington University, St. Louis, said in a news release from the American Society of Clinical Oncology.
Existing tools to assess frailty include an index proposed by the International Myeloma Working Group that looks at age plus other indexes related to comorbidities and activities of daily living, and the revised Myeloma Comorbidity Index that incorporates age with other prognostic factors.
“Although both tools provide prognostic information, chronological age automatically increases frailty without taking biologic or functional age into account,” Dr. Wildes and her coauthors wrote in their report.
By contrast, the DAFI is based on the concept of biologic age, in which the health status of an individual is measured based on the proportion of aging-associated deficits they have accumulated, according to the authors.
To create the DAFI, Dr. Wildes and her colleagues analyzed nearly 2.7 million records of noncancer patients aged 66 years or older in the SEER Medicare Health Outcomes Survey (MHOS) database. They identified 25 variables in the database representing chronic health conditions, activities of daily living, functioning, mental health, and general health.
An individual’s DAFI score was calculated as the sum of scores for each of the 25 variables as 0 for absent, 0.5 for limited, and 1 for present. Predicted DAFI means were calculated for each year of age and used to create age-specific cut points to determine whether an individual would be considered frail or not versus others of the same age.
“In other words, the same frailty score may qualify an 80-year-old individual as fit and a 70-year-old as frail, depending on the cutoff for their respective age group,” investigators explained in their report.
They applied the index to 305 patients with newly diagnosed myeloma in the SEER-MHOS database who were 66 years of age or older (median age, 76 years) and had completed the survey within 1 year of diagnosis.
The DAFI classified 52% of the myeloma patients as frail, and for that group, median overall survival was 26.8 months, versus 43.7 months for nonfrail patients (P = .015), according to the reported data. For each 10% increase in score, the risk of death increased by 16% (P less than .001).
Notably, advancing age was very weakly correlated with increased age-related deficits in the myeloma cohort (r2 = 0.15; P = .010), according to investigators, but very strongly correlated with deficits in the cohort of noncancer patients (r2 = 0.98; P less than .001).
“This suggests that, in patients with multiple myeloma, the prevalence of impairments across domains of function, chronic comorbidities, general health, and mental health are more related to the overall burden of myeloma rather than chronological age alone,” the investigators wrote.
The information used to calculate a DAFI score is easily obtainable during a clinic visit, according to the authors, who provided an overview of all 25 variables in the journal article.
Further development of a computerized program would further enhance usability in the clinic, allowing for real-time calculation during a patient visit, they said.
Survivorship expert Merry Jennifer Markham, MD, said in the ASCO news release that this frailty index is notable because it accounts for more than just chronological age. “Knowing this information can help oncologists have more informed discussions with patients about their prognosis, which in turn can empower patients and families as they weigh treatment options,” she said.
The research was supported by National Cancer Institute. Dr. Wildes reported honoraria from Carevive Systems and research funding from Janssen Oncology. Another coauthor reported honoraria from Celgene and Janssen, and a consulting or advisory role with Amgen and Takeda.
SOURCE: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than chronological age alone, investigators report. A 16% increased risk of death was seen for each 10% increase in the deficit-accumulation frailty index (DAFI), which includes 25 variables related health, function, and activities of daily living.
There was only a weak correlation between chronological age and increase in deficits tracked by the index, in contrast to a cohort without cancer, in which age and frailty were strongly correlated, the investigators reported in JCO Clinical Cancer Informatics.
“Our results demonstrate that, for patients with multiple myeloma, chronological age alone is not a good measure for assessing overall health,” study author Tanya M. Wildes, MD, of Washington University, St. Louis, said in a news release from the American Society of Clinical Oncology.
Existing tools to assess frailty include an index proposed by the International Myeloma Working Group that looks at age plus other indexes related to comorbidities and activities of daily living, and the revised Myeloma Comorbidity Index that incorporates age with other prognostic factors.
“Although both tools provide prognostic information, chronological age automatically increases frailty without taking biologic or functional age into account,” Dr. Wildes and her coauthors wrote in their report.
By contrast, the DAFI is based on the concept of biologic age, in which the health status of an individual is measured based on the proportion of aging-associated deficits they have accumulated, according to the authors.
To create the DAFI, Dr. Wildes and her colleagues analyzed nearly 2.7 million records of noncancer patients aged 66 years or older in the SEER Medicare Health Outcomes Survey (MHOS) database. They identified 25 variables in the database representing chronic health conditions, activities of daily living, functioning, mental health, and general health.
An individual’s DAFI score was calculated as the sum of scores for each of the 25 variables as 0 for absent, 0.5 for limited, and 1 for present. Predicted DAFI means were calculated for each year of age and used to create age-specific cut points to determine whether an individual would be considered frail or not versus others of the same age.
“In other words, the same frailty score may qualify an 80-year-old individual as fit and a 70-year-old as frail, depending on the cutoff for their respective age group,” investigators explained in their report.
They applied the index to 305 patients with newly diagnosed myeloma in the SEER-MHOS database who were 66 years of age or older (median age, 76 years) and had completed the survey within 1 year of diagnosis.
The DAFI classified 52% of the myeloma patients as frail, and for that group, median overall survival was 26.8 months, versus 43.7 months for nonfrail patients (P = .015), according to the reported data. For each 10% increase in score, the risk of death increased by 16% (P less than .001).
Notably, advancing age was very weakly correlated with increased age-related deficits in the myeloma cohort (r2 = 0.15; P = .010), according to investigators, but very strongly correlated with deficits in the cohort of noncancer patients (r2 = 0.98; P less than .001).
“This suggests that, in patients with multiple myeloma, the prevalence of impairments across domains of function, chronic comorbidities, general health, and mental health are more related to the overall burden of myeloma rather than chronological age alone,” the investigators wrote.
The information used to calculate a DAFI score is easily obtainable during a clinic visit, according to the authors, who provided an overview of all 25 variables in the journal article.
Further development of a computerized program would further enhance usability in the clinic, allowing for real-time calculation during a patient visit, they said.
Survivorship expert Merry Jennifer Markham, MD, said in the ASCO news release that this frailty index is notable because it accounts for more than just chronological age. “Knowing this information can help oncologists have more informed discussions with patients about their prognosis, which in turn can empower patients and families as they weigh treatment options,” she said.
The research was supported by National Cancer Institute. Dr. Wildes reported honoraria from Carevive Systems and research funding from Janssen Oncology. Another coauthor reported honoraria from Celgene and Janssen, and a consulting or advisory role with Amgen and Takeda.
SOURCE: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
FROM JCO CLINICAL CANCER INFORMATICS
Key clinical point: A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than on chronological age alone.
Major finding: Median overall survival was 26.8 months for patients classified as frail, vs. 43.7 months for nonfrail patients (P = .015).
Study details: Retrospective analysis of 2.7 million records of noncancer patients to create an index subsequently validated in records for 305 patients with newly diagnosed multiple myeloma (aged 66 years and older).
Disclosures: The research was supported by National Cancer Institute. Authors reported disclosures related to Celgene, Janssen, Amgen, Takeda, and Carevive Systems.
Source: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
Benzodiazepines for anxious depression
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
VIDEO: Look for Signs of Depression in Rosacea Patients
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Cannabis falls short for chronic noncancer pain
Cannabis did not improve outcomes or reduce prescription opioid use among 1,514 Australians with noncancer pain, according to a recent report in Lancet Public Health.
Almost a quarter of patients reported using cannabis, mostly illicitly since most of the data were collected before Australia legalized medical marijuana in 2016. About 9% reported marijuana use in the previous month at baseline; 13% reported use in the past month at the final interview.
Overall, users rated the degree of relief they got from pain and pain-related distress as 7 out of 10, but the study findings did not support their impression.
At 4 years, cannabis users, compared with nonusers, reported greater pain severity (for daily or near-daily use: risk ratio, 1.17; 95% confidence interval, 1.03-1.32; for less frequent use: RR, 1.14; 95% CI, 1.01-1.29), more interference from pain in their daily lives (for daily or near-daily use: RR, 1.14; 95% CI, 1.03-1.26; for less frequent use: RR, 1.21; 95% CI, 1.09-1.35 ), less ability to cope with pain (for daily or near-daily use: RR, 0.98; 95% CI, 0.96-1.00; for less frequent use: RR, 0.97; 95% CI, 0.96-1.00), and greater generalized anxiety (for daily or near-daily use: RR, 1.10; 95% CI, 1.06-1.15; for less frequent use: RR, 1.07; 95% CI, 1.03-1.12). Results were adjusted for age, sex, pain duration, and other factors.
Pain severity scores on the 10-point Brief Pain Inventory, for instance, were 4.7 points at the end of the study among nonusers, compared with 5.3 among daily or near-daily users.
Few differences were reported in oral morphine equivalents between the groups. People who reported using marijuana 1-19 days a month were less likely to have discontinued opioids at 4 years (9%) than were those reporting no use (21%).
“Interest in the use of cannabis and cannabinoids to treat chronic noncancer pain is increasing because of their potential to reduce opioid dose requirements.” However, “we found no evidence that cannabis use improved patient outcomes” or that cannabis “exerted an opioid-sparing effect,” said the investigators, led by Gabrielle Campbell, PhD, of the National Drug and Alcohol Research Centre, Sydney.
The findings are not a slam dunk against cannabis for chronic pain. The investigators noted that people might have used cannabis because they had more pain to begin with and poorer coping mechanisms. Had they not been using marijuana, perhaps they would have been worse off.
However, “to date, evidence that cannabinoids are effective for chronic noncancer pain and aid in reducing opioid use is lacking. they said.
Dr. Campbell and her associates cited several important limitations. Since cannabis use was primarily illicit, it’s unlikely that it was used under medical supervision. Also, the study only gauged frequency of use on a per-day basis. “We do not know if some people used once in a day or more than once. Likewise, we do not know what type of cannabis the participants used. ... This fact matters, as cannabis varies in strength and, as with any analgesia, the dose needs to be matched to the severity of pain experienced,” the team said.
The subjects were a median age of 58 years at baseline, and 56% were women. They had been prescribed a strong opioid for a median of 4 years at study entry and were on a median oral morphine equivalent dose of 75 mg/day, which fell to 57 mg/day at the study’s conclusion. About 62% of the subjects reported neuropathic pain.
The work was funded by the Australian government, and the National Health and Medical Research Council. Dr. Campbell reported grants from Reckitt Benckiser.
SOURCE: Campbell G et al. Lancet Public Health. 2018 Jul. doi: 10.1016/S2468-2667(18)30110-5.
Cannabis did not improve outcomes or reduce prescription opioid use among 1,514 Australians with noncancer pain, according to a recent report in Lancet Public Health.
Almost a quarter of patients reported using cannabis, mostly illicitly since most of the data were collected before Australia legalized medical marijuana in 2016. About 9% reported marijuana use in the previous month at baseline; 13% reported use in the past month at the final interview.
Overall, users rated the degree of relief they got from pain and pain-related distress as 7 out of 10, but the study findings did not support their impression.
At 4 years, cannabis users, compared with nonusers, reported greater pain severity (for daily or near-daily use: risk ratio, 1.17; 95% confidence interval, 1.03-1.32; for less frequent use: RR, 1.14; 95% CI, 1.01-1.29), more interference from pain in their daily lives (for daily or near-daily use: RR, 1.14; 95% CI, 1.03-1.26; for less frequent use: RR, 1.21; 95% CI, 1.09-1.35 ), less ability to cope with pain (for daily or near-daily use: RR, 0.98; 95% CI, 0.96-1.00; for less frequent use: RR, 0.97; 95% CI, 0.96-1.00), and greater generalized anxiety (for daily or near-daily use: RR, 1.10; 95% CI, 1.06-1.15; for less frequent use: RR, 1.07; 95% CI, 1.03-1.12). Results were adjusted for age, sex, pain duration, and other factors.
Pain severity scores on the 10-point Brief Pain Inventory, for instance, were 4.7 points at the end of the study among nonusers, compared with 5.3 among daily or near-daily users.
Few differences were reported in oral morphine equivalents between the groups. People who reported using marijuana 1-19 days a month were less likely to have discontinued opioids at 4 years (9%) than were those reporting no use (21%).
“Interest in the use of cannabis and cannabinoids to treat chronic noncancer pain is increasing because of their potential to reduce opioid dose requirements.” However, “we found no evidence that cannabis use improved patient outcomes” or that cannabis “exerted an opioid-sparing effect,” said the investigators, led by Gabrielle Campbell, PhD, of the National Drug and Alcohol Research Centre, Sydney.
The findings are not a slam dunk against cannabis for chronic pain. The investigators noted that people might have used cannabis because they had more pain to begin with and poorer coping mechanisms. Had they not been using marijuana, perhaps they would have been worse off.
However, “to date, evidence that cannabinoids are effective for chronic noncancer pain and aid in reducing opioid use is lacking. they said.
Dr. Campbell and her associates cited several important limitations. Since cannabis use was primarily illicit, it’s unlikely that it was used under medical supervision. Also, the study only gauged frequency of use on a per-day basis. “We do not know if some people used once in a day or more than once. Likewise, we do not know what type of cannabis the participants used. ... This fact matters, as cannabis varies in strength and, as with any analgesia, the dose needs to be matched to the severity of pain experienced,” the team said.
The subjects were a median age of 58 years at baseline, and 56% were women. They had been prescribed a strong opioid for a median of 4 years at study entry and were on a median oral morphine equivalent dose of 75 mg/day, which fell to 57 mg/day at the study’s conclusion. About 62% of the subjects reported neuropathic pain.
The work was funded by the Australian government, and the National Health and Medical Research Council. Dr. Campbell reported grants from Reckitt Benckiser.
SOURCE: Campbell G et al. Lancet Public Health. 2018 Jul. doi: 10.1016/S2468-2667(18)30110-5.
Cannabis did not improve outcomes or reduce prescription opioid use among 1,514 Australians with noncancer pain, according to a recent report in Lancet Public Health.
Almost a quarter of patients reported using cannabis, mostly illicitly since most of the data were collected before Australia legalized medical marijuana in 2016. About 9% reported marijuana use in the previous month at baseline; 13% reported use in the past month at the final interview.
Overall, users rated the degree of relief they got from pain and pain-related distress as 7 out of 10, but the study findings did not support their impression.
At 4 years, cannabis users, compared with nonusers, reported greater pain severity (for daily or near-daily use: risk ratio, 1.17; 95% confidence interval, 1.03-1.32; for less frequent use: RR, 1.14; 95% CI, 1.01-1.29), more interference from pain in their daily lives (for daily or near-daily use: RR, 1.14; 95% CI, 1.03-1.26; for less frequent use: RR, 1.21; 95% CI, 1.09-1.35 ), less ability to cope with pain (for daily or near-daily use: RR, 0.98; 95% CI, 0.96-1.00; for less frequent use: RR, 0.97; 95% CI, 0.96-1.00), and greater generalized anxiety (for daily or near-daily use: RR, 1.10; 95% CI, 1.06-1.15; for less frequent use: RR, 1.07; 95% CI, 1.03-1.12). Results were adjusted for age, sex, pain duration, and other factors.
Pain severity scores on the 10-point Brief Pain Inventory, for instance, were 4.7 points at the end of the study among nonusers, compared with 5.3 among daily or near-daily users.
Few differences were reported in oral morphine equivalents between the groups. People who reported using marijuana 1-19 days a month were less likely to have discontinued opioids at 4 years (9%) than were those reporting no use (21%).
“Interest in the use of cannabis and cannabinoids to treat chronic noncancer pain is increasing because of their potential to reduce opioid dose requirements.” However, “we found no evidence that cannabis use improved patient outcomes” or that cannabis “exerted an opioid-sparing effect,” said the investigators, led by Gabrielle Campbell, PhD, of the National Drug and Alcohol Research Centre, Sydney.
The findings are not a slam dunk against cannabis for chronic pain. The investigators noted that people might have used cannabis because they had more pain to begin with and poorer coping mechanisms. Had they not been using marijuana, perhaps they would have been worse off.
However, “to date, evidence that cannabinoids are effective for chronic noncancer pain and aid in reducing opioid use is lacking. they said.
Dr. Campbell and her associates cited several important limitations. Since cannabis use was primarily illicit, it’s unlikely that it was used under medical supervision. Also, the study only gauged frequency of use on a per-day basis. “We do not know if some people used once in a day or more than once. Likewise, we do not know what type of cannabis the participants used. ... This fact matters, as cannabis varies in strength and, as with any analgesia, the dose needs to be matched to the severity of pain experienced,” the team said.
The subjects were a median age of 58 years at baseline, and 56% were women. They had been prescribed a strong opioid for a median of 4 years at study entry and were on a median oral morphine equivalent dose of 75 mg/day, which fell to 57 mg/day at the study’s conclusion. About 62% of the subjects reported neuropathic pain.
The work was funded by the Australian government, and the National Health and Medical Research Council. Dr. Campbell reported grants from Reckitt Benckiser.
SOURCE: Campbell G et al. Lancet Public Health. 2018 Jul. doi: 10.1016/S2468-2667(18)30110-5.
FROM THE LANCET PUBLIC HEALTH
Key clinical point: Cannabis did not improve outcomes or reduce prescription opioid use among 1,514 Australians with chronic noncancer pain.
Major finding: Pain severity scores on the 10-point Brief Pain Inventory were 4.7 points at the end of the study among nonusers, compared with 5.3 among daily or near-daily users.
Study details: A 4-year observational, prospective investigation.
Disclosures: The work was funded by the Australian government and the National Health and Medical Research Council. The study lead reported grants from Reckitt Benckiser.
Source: Campbell G et al. Lancet Public Health. 2018 Jul. doi: 10.1016/S2468-2667(18)30110-5.








