User login
Third-line avelumab for gastric cancer safer than chemotherapy
according to a recent study.
Although no primary or secondary endpoints were met, avelumab demonstrated similar antitumor activity compared with chemotherapy, reported Yung-Jue Bang, MD, PhD, of Seoul National University College of Medicine, South Korea, and his coauthors.
“There currently are no internationally recognized treatment guidelines for patients with advanced gastric cancer/gastroesophageal junction cancer [GC/GEJC] in whom two prior lines of therapy have failed,” the investigators wrote in Annals of Oncology.
The phase 3, randomized JAVELIN Gastric 300 trial compared avelumab, an anti–programmed death ligand 1 (PD-L1) monoclonal antibody, with physician’s choice of chemotherapy in 371 patients with metastatic GC/GEJC who had received two previous lines of systemic therapy. A total of 185 patients received avelumab and 186 patients received chemotherapy. Of those in the chemotherapy group, 120 (64.5%) were given irinotecan, 50 (29.0%) were given paclitaxel, and 3 (1.6%) received best supportive care only. The primary endpoint was overall survival, and secondary endpoints were objective response rate and progression-free survival. Safety and tolerability were also evaluated.
Neither primary nor secondary endpoints were met, and no significant difference in efficacy was found between treatment arms. However, tolerability was notably better in the avelumab treatment group. Treatment-related adverse events occurred in 90 patients (48.9%) in the avelumab group, compared with 131 patients (74.0%) in the chemotherapy group. Grade 3 or higher treatment-related adverse events were also less common in the avelumab group (9.2% vs. 31.6%). In the avelumab treatment arm, 4 patients (2.2%) had grade 3 or higher immune-related adverse events (autoimmune hypothyroidism, autoimmune hepatitis, elevated AST, and colitis).
“These results demonstrate that avelumab is better tolerated than chemotherapy in patients with heavily pretreated GC/GEJC, supporting the potential of avelumab for combination or maintenance therapy, even in later stages of disease,” the investigators wrote. “Nevertheless, the optimal strategy for incorporating checkpoint inhibitors into the continuum of care for patients with advanced GC/GEJC is still unknown, and studies of alternative anti–PD-1/PD-L1 treatment strategies in earlier lines of therapy are warranted.”
The study was funded by Merck and Pfizer. Authors reported compensation from AstraZeneca, Eli Lilly, Novartis, and others.
SOURCE: Bang YJ et al. Ann Oncol. 2018 Jul 24. doi:10.1093/annonc/mdy264.
according to a recent study.
Although no primary or secondary endpoints were met, avelumab demonstrated similar antitumor activity compared with chemotherapy, reported Yung-Jue Bang, MD, PhD, of Seoul National University College of Medicine, South Korea, and his coauthors.
“There currently are no internationally recognized treatment guidelines for patients with advanced gastric cancer/gastroesophageal junction cancer [GC/GEJC] in whom two prior lines of therapy have failed,” the investigators wrote in Annals of Oncology.
The phase 3, randomized JAVELIN Gastric 300 trial compared avelumab, an anti–programmed death ligand 1 (PD-L1) monoclonal antibody, with physician’s choice of chemotherapy in 371 patients with metastatic GC/GEJC who had received two previous lines of systemic therapy. A total of 185 patients received avelumab and 186 patients received chemotherapy. Of those in the chemotherapy group, 120 (64.5%) were given irinotecan, 50 (29.0%) were given paclitaxel, and 3 (1.6%) received best supportive care only. The primary endpoint was overall survival, and secondary endpoints were objective response rate and progression-free survival. Safety and tolerability were also evaluated.
Neither primary nor secondary endpoints were met, and no significant difference in efficacy was found between treatment arms. However, tolerability was notably better in the avelumab treatment group. Treatment-related adverse events occurred in 90 patients (48.9%) in the avelumab group, compared with 131 patients (74.0%) in the chemotherapy group. Grade 3 or higher treatment-related adverse events were also less common in the avelumab group (9.2% vs. 31.6%). In the avelumab treatment arm, 4 patients (2.2%) had grade 3 or higher immune-related adverse events (autoimmune hypothyroidism, autoimmune hepatitis, elevated AST, and colitis).
“These results demonstrate that avelumab is better tolerated than chemotherapy in patients with heavily pretreated GC/GEJC, supporting the potential of avelumab for combination or maintenance therapy, even in later stages of disease,” the investigators wrote. “Nevertheless, the optimal strategy for incorporating checkpoint inhibitors into the continuum of care for patients with advanced GC/GEJC is still unknown, and studies of alternative anti–PD-1/PD-L1 treatment strategies in earlier lines of therapy are warranted.”
The study was funded by Merck and Pfizer. Authors reported compensation from AstraZeneca, Eli Lilly, Novartis, and others.
SOURCE: Bang YJ et al. Ann Oncol. 2018 Jul 24. doi:10.1093/annonc/mdy264.
according to a recent study.
Although no primary or secondary endpoints were met, avelumab demonstrated similar antitumor activity compared with chemotherapy, reported Yung-Jue Bang, MD, PhD, of Seoul National University College of Medicine, South Korea, and his coauthors.
“There currently are no internationally recognized treatment guidelines for patients with advanced gastric cancer/gastroesophageal junction cancer [GC/GEJC] in whom two prior lines of therapy have failed,” the investigators wrote in Annals of Oncology.
The phase 3, randomized JAVELIN Gastric 300 trial compared avelumab, an anti–programmed death ligand 1 (PD-L1) monoclonal antibody, with physician’s choice of chemotherapy in 371 patients with metastatic GC/GEJC who had received two previous lines of systemic therapy. A total of 185 patients received avelumab and 186 patients received chemotherapy. Of those in the chemotherapy group, 120 (64.5%) were given irinotecan, 50 (29.0%) were given paclitaxel, and 3 (1.6%) received best supportive care only. The primary endpoint was overall survival, and secondary endpoints were objective response rate and progression-free survival. Safety and tolerability were also evaluated.
Neither primary nor secondary endpoints were met, and no significant difference in efficacy was found between treatment arms. However, tolerability was notably better in the avelumab treatment group. Treatment-related adverse events occurred in 90 patients (48.9%) in the avelumab group, compared with 131 patients (74.0%) in the chemotherapy group. Grade 3 or higher treatment-related adverse events were also less common in the avelumab group (9.2% vs. 31.6%). In the avelumab treatment arm, 4 patients (2.2%) had grade 3 or higher immune-related adverse events (autoimmune hypothyroidism, autoimmune hepatitis, elevated AST, and colitis).
“These results demonstrate that avelumab is better tolerated than chemotherapy in patients with heavily pretreated GC/GEJC, supporting the potential of avelumab for combination or maintenance therapy, even in later stages of disease,” the investigators wrote. “Nevertheless, the optimal strategy for incorporating checkpoint inhibitors into the continuum of care for patients with advanced GC/GEJC is still unknown, and studies of alternative anti–PD-1/PD-L1 treatment strategies in earlier lines of therapy are warranted.”
The study was funded by Merck and Pfizer. Authors reported compensation from AstraZeneca, Eli Lilly, Novartis, and others.
SOURCE: Bang YJ et al. Ann Oncol. 2018 Jul 24. doi:10.1093/annonc/mdy264.
FROM ANNALS OF ONCOLOGY
Key clinical point: Third-line avelumab for patients with gastric cancer is safer, but no more effective, than chemotherapy.
Major finding: Grade 3 or higher treatment-related adverse events occurred in 9.2% of patients treated with avelumab, compared with 31.6% of patients treated with chemotherapy.
Study details: The phase 3 JAVELIN Gastric 300 trial involved 371 patients with metastatic, nonresectable gastric cancer who had received two previous lines of systemic therapy.
Disclosures: The study was funded by Merck and Pfizer. Authors reported compensation from AstraZeneca, Eli Lilly, Novartis, and others.
Source: Bang YJ et al. Ann Oncol. 2018 Jul 24. doi:10.1093/annonc/mdy264.
Asthma medication ratio identifies high-risk pediatric patients
ATLANTA – An according to researchers from the Medical University of South Carolina (MUSC), Charleston.
The asthma medication ratio (AMR) – the number of prescriptions for controller medications divided by the number of prescriptions for both controller and rescue medications – has been around for a while, but it’s mostly been used as a quality metric. The new study shows that it’s also useful in the clinic to identify children who could benefit from extra attention.
A perfect ratio of 1 means that control is good without rescue inhalers. The ratio falls as the number of rescue inhalers goes up, signaling poorer control. Children with a ratio below 0.5 are considered high risk; they’d hit that mark if, for instance, they were prescribed one control medication such as fluticasone propionate (Flovent) and two albuterol rescue inhalers in a month.
If control is good, “you should only need a rescue inhaler very, very sporadically;” high-risk children probably need a higher dose of their controller, or help with compliance, explained lead investigator Annie L. Andrews, MD, associate professor of pediatrics at MUSC.
The university uses the EPIC records system, which incorporates prescription data from Surescripts, so the number of asthma medication fills is already available. The system just needs to be adjusted to calculate and report AMRs monthly, something Dr. Andrews and her team are working on. “The information is right there, but it’s an untapped resource,” she said. “We just need to crunch the numbers, and operationalize it. Why are we waiting until kids are in the hospital” to intervene?
Dr. Andrews presented a proof-of-concept study at the Pediatric Hospital Medicine meeting. Her team identified 214,452 asthma patients aged 2-17 years with at least one claim for an inhaled corticosteroid in the Truven MarketScan Medicaid database from 2013-14.
They calculated AMRs for each child every 3 months over a 15-month period. About 9% of children at any given time had AMRs below 0.5.
The first AMR was at or above 0.5 in 93,512 children; 18.1% had a subsequent asthma-related event, meaning an ED visit or hospitalization, during the course of the study. Among the 17,635 children with an initial AMR below 0.5, 25% had asthma-related events. The initial AMR couldn’t be calculated in 103,305 children, which likely meant they had less-active disease. Those children had the lowest proportion of asthma events, at 13.9%.
An AMR below 0.5 nearly doubled the risk of an asthma-related hospitalization or ED visit in the subsequent 3 months, with an odds ratios ranging from 1.7 to 1.9, compared with other children. The findings were statistically significant.
In short, serial AMRs helped predict exacerbations among Medicaid children. The team showed the same trend among commercially insured children in a recently published study. The only difference was that Medicaid children had a higher proportion of high-risk AMRs, and a higher number of asthma events (Am J Manag Care. 2018 Jun;24[6]:294-300). Together, the studies validate “the rolling 3-month AMR as an appropriate method for identifying children at high risk for imminent exacerbation,” the investigators concluded.
With automatic AMR reporting already in the works at MUSC, “we are now trying to figure out how to intervene. Do we just tell providers who their high-risk kids are and let them figure out how to contact families, or do we use this information to contact families directly? That’s kind of what I favor: ‘Hey, your kid just popped up as high risk, so let’s figure out what you need. Do you need a new prescription or a reminder to see your doctor?’ ” Dr. Andrews said.
Her team is developing a mobile app to communicate with families.
The mean age in the study was 7.9 years; 59% of the children were boys, and 41% were black.
The work was funded by the National Institutes of Health, among others. Dr. Andrews had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
ATLANTA – An according to researchers from the Medical University of South Carolina (MUSC), Charleston.
The asthma medication ratio (AMR) – the number of prescriptions for controller medications divided by the number of prescriptions for both controller and rescue medications – has been around for a while, but it’s mostly been used as a quality metric. The new study shows that it’s also useful in the clinic to identify children who could benefit from extra attention.
A perfect ratio of 1 means that control is good without rescue inhalers. The ratio falls as the number of rescue inhalers goes up, signaling poorer control. Children with a ratio below 0.5 are considered high risk; they’d hit that mark if, for instance, they were prescribed one control medication such as fluticasone propionate (Flovent) and two albuterol rescue inhalers in a month.
If control is good, “you should only need a rescue inhaler very, very sporadically;” high-risk children probably need a higher dose of their controller, or help with compliance, explained lead investigator Annie L. Andrews, MD, associate professor of pediatrics at MUSC.
The university uses the EPIC records system, which incorporates prescription data from Surescripts, so the number of asthma medication fills is already available. The system just needs to be adjusted to calculate and report AMRs monthly, something Dr. Andrews and her team are working on. “The information is right there, but it’s an untapped resource,” she said. “We just need to crunch the numbers, and operationalize it. Why are we waiting until kids are in the hospital” to intervene?
Dr. Andrews presented a proof-of-concept study at the Pediatric Hospital Medicine meeting. Her team identified 214,452 asthma patients aged 2-17 years with at least one claim for an inhaled corticosteroid in the Truven MarketScan Medicaid database from 2013-14.
They calculated AMRs for each child every 3 months over a 15-month period. About 9% of children at any given time had AMRs below 0.5.
The first AMR was at or above 0.5 in 93,512 children; 18.1% had a subsequent asthma-related event, meaning an ED visit or hospitalization, during the course of the study. Among the 17,635 children with an initial AMR below 0.5, 25% had asthma-related events. The initial AMR couldn’t be calculated in 103,305 children, which likely meant they had less-active disease. Those children had the lowest proportion of asthma events, at 13.9%.
An AMR below 0.5 nearly doubled the risk of an asthma-related hospitalization or ED visit in the subsequent 3 months, with an odds ratios ranging from 1.7 to 1.9, compared with other children. The findings were statistically significant.
In short, serial AMRs helped predict exacerbations among Medicaid children. The team showed the same trend among commercially insured children in a recently published study. The only difference was that Medicaid children had a higher proportion of high-risk AMRs, and a higher number of asthma events (Am J Manag Care. 2018 Jun;24[6]:294-300). Together, the studies validate “the rolling 3-month AMR as an appropriate method for identifying children at high risk for imminent exacerbation,” the investigators concluded.
With automatic AMR reporting already in the works at MUSC, “we are now trying to figure out how to intervene. Do we just tell providers who their high-risk kids are and let them figure out how to contact families, or do we use this information to contact families directly? That’s kind of what I favor: ‘Hey, your kid just popped up as high risk, so let’s figure out what you need. Do you need a new prescription or a reminder to see your doctor?’ ” Dr. Andrews said.
Her team is developing a mobile app to communicate with families.
The mean age in the study was 7.9 years; 59% of the children were boys, and 41% were black.
The work was funded by the National Institutes of Health, among others. Dr. Andrews had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
ATLANTA – An according to researchers from the Medical University of South Carolina (MUSC), Charleston.
The asthma medication ratio (AMR) – the number of prescriptions for controller medications divided by the number of prescriptions for both controller and rescue medications – has been around for a while, but it’s mostly been used as a quality metric. The new study shows that it’s also useful in the clinic to identify children who could benefit from extra attention.
A perfect ratio of 1 means that control is good without rescue inhalers. The ratio falls as the number of rescue inhalers goes up, signaling poorer control. Children with a ratio below 0.5 are considered high risk; they’d hit that mark if, for instance, they were prescribed one control medication such as fluticasone propionate (Flovent) and two albuterol rescue inhalers in a month.
If control is good, “you should only need a rescue inhaler very, very sporadically;” high-risk children probably need a higher dose of their controller, or help with compliance, explained lead investigator Annie L. Andrews, MD, associate professor of pediatrics at MUSC.
The university uses the EPIC records system, which incorporates prescription data from Surescripts, so the number of asthma medication fills is already available. The system just needs to be adjusted to calculate and report AMRs monthly, something Dr. Andrews and her team are working on. “The information is right there, but it’s an untapped resource,” she said. “We just need to crunch the numbers, and operationalize it. Why are we waiting until kids are in the hospital” to intervene?
Dr. Andrews presented a proof-of-concept study at the Pediatric Hospital Medicine meeting. Her team identified 214,452 asthma patients aged 2-17 years with at least one claim for an inhaled corticosteroid in the Truven MarketScan Medicaid database from 2013-14.
They calculated AMRs for each child every 3 months over a 15-month period. About 9% of children at any given time had AMRs below 0.5.
The first AMR was at or above 0.5 in 93,512 children; 18.1% had a subsequent asthma-related event, meaning an ED visit or hospitalization, during the course of the study. Among the 17,635 children with an initial AMR below 0.5, 25% had asthma-related events. The initial AMR couldn’t be calculated in 103,305 children, which likely meant they had less-active disease. Those children had the lowest proportion of asthma events, at 13.9%.
An AMR below 0.5 nearly doubled the risk of an asthma-related hospitalization or ED visit in the subsequent 3 months, with an odds ratios ranging from 1.7 to 1.9, compared with other children. The findings were statistically significant.
In short, serial AMRs helped predict exacerbations among Medicaid children. The team showed the same trend among commercially insured children in a recently published study. The only difference was that Medicaid children had a higher proportion of high-risk AMRs, and a higher number of asthma events (Am J Manag Care. 2018 Jun;24[6]:294-300). Together, the studies validate “the rolling 3-month AMR as an appropriate method for identifying children at high risk for imminent exacerbation,” the investigators concluded.
With automatic AMR reporting already in the works at MUSC, “we are now trying to figure out how to intervene. Do we just tell providers who their high-risk kids are and let them figure out how to contact families, or do we use this information to contact families directly? That’s kind of what I favor: ‘Hey, your kid just popped up as high risk, so let’s figure out what you need. Do you need a new prescription or a reminder to see your doctor?’ ” Dr. Andrews said.
Her team is developing a mobile app to communicate with families.
The mean age in the study was 7.9 years; 59% of the children were boys, and 41% were black.
The work was funded by the National Institutes of Health, among others. Dr. Andrews had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2018
Key clinical point: The asthma medication ratio is useful in the clinic to identify children who could benefit from extra attention.
Major finding: About 9% of children at any given time had AMRs below 0.5, meaning they were at high risk for acute exacerbations.
Study details: Review of more than 200,000 pediatric asthma patients on Medicaid
Disclosures: The work was funded by the National Institutes of Health, among others. The study lead had no disclosures.
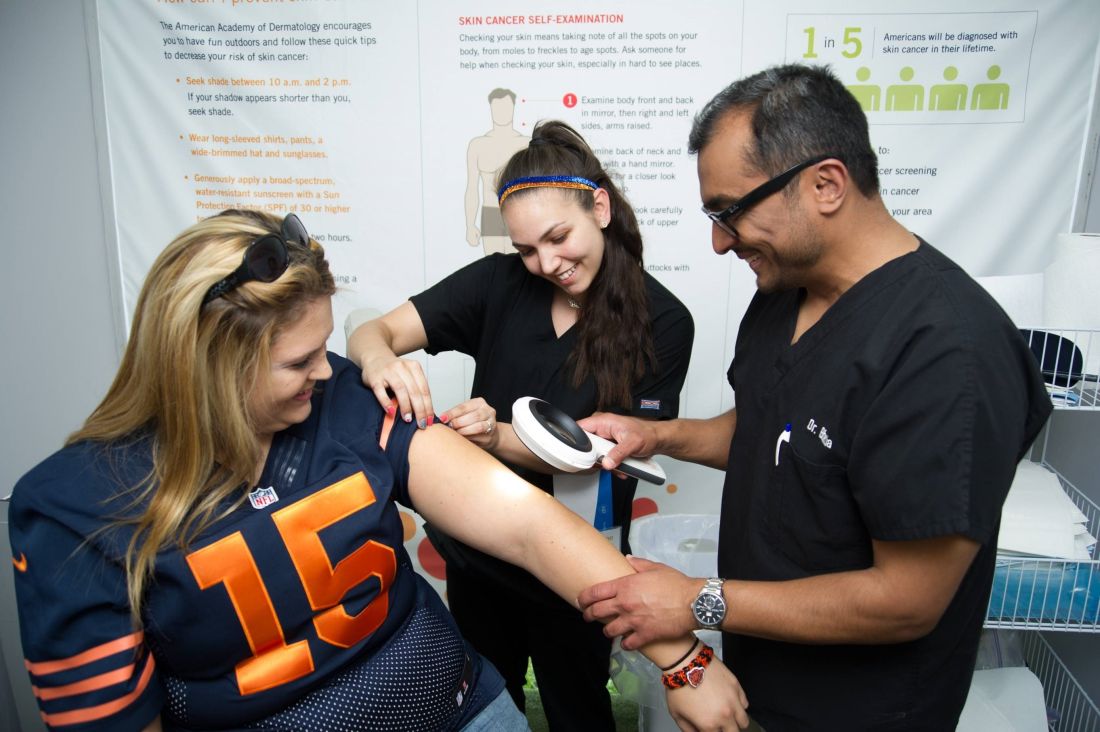
SPOTme addresses unmet need for skin cancer screening
Almost half of the individuals diagnosed with melanoma in a free skin cancer screening program otherwise would not have gone to a doctor to have their skin examined, according to an analysis of the American Academy of Dermatology’s national skin cancer screening program, during 1986-2014.
The SPOTme program, a national skin cancer screening and education program conducted by volunteer dermatologists, was launched in 1985. More than 2 million free screenings have been provided by the program in a “predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants,” according to first authors Jean-Phillip Okhovat, MD, of Beth Israel Deaconess Medical Center, and Derek Beaulieu, MD, of Tufts University, both in Boston, and their colleagues.
The analysis was published online in the Journal of the American Academy of Dermatology on July 26.
Their study analyzed data on almost 2 million people screened through the program from 1986-2014. About 62% were women; 90% were white, about 2% were black, and almost 4% were Hispanic. Almost 80% had no regular dermatologist, almost 73% had not been screened previously, almost 45% had never had a skin cancer check, and 9% were uninsured. Almost 31% reported a mole that had recently change in size, color, or shape; almost 34% said they had a family history of skin cancer, and about 14% said they had a personal history of skin cancer.
Participants were asked about demographics and risk factors, although some questions changed from year to year (for example, in 2009 and 2010, participants were asked about melanoma risk factors, and from 1992 through 2010, participants were asked about their access to dermatologic care).
During 1991-2014 (which did not include data for 1995, 1996, and 2000, which were not available), the screening program resulted in 20,628 clinical melanoma diagnoses, 156,087 clinical dysplastic nevi diagnoses, 32,893 clinical squamous cell carcinoma diagnoses, and 129,848 clinical basal cell carcinoma diagnoses.
Of those clinically diagnosed with melanoma during 1992-2010, 83% said they did not have a regular dermatologist, 77% said they had not been screened previously, and 47% said they would not have seen a doctor for a skin exam if the SPOTme program had not been available.
Of those screened in 2009 and 2010 , 72% were considered at high risk for melanoma (older than age 65 years, having a history of sunburns, a family history of skin cancer, and/or more than 50 moles or unusual moles).
Among the other findings was that from 1992 to 2010, about 12% of those with a clinical melanoma diagnosis were not insured, which increased over time, from almost 11% during 1992-1999 to almost 16% during 2007-2010.
The “consistently high rates” of multiple skin cancer risk factors among those newly screened in the study are consistent with previously reported data, “suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer,” the authors wrote. “While the SPOTme program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened,” they added.
Limitations of the study included the inability to confirm the clinical diagnoses with histopathology, and no data from the providers were available.
The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
SOURCE: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
Almost half of the individuals diagnosed with melanoma in a free skin cancer screening program otherwise would not have gone to a doctor to have their skin examined, according to an analysis of the American Academy of Dermatology’s national skin cancer screening program, during 1986-2014.
The SPOTme program, a national skin cancer screening and education program conducted by volunteer dermatologists, was launched in 1985. More than 2 million free screenings have been provided by the program in a “predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants,” according to first authors Jean-Phillip Okhovat, MD, of Beth Israel Deaconess Medical Center, and Derek Beaulieu, MD, of Tufts University, both in Boston, and their colleagues.
The analysis was published online in the Journal of the American Academy of Dermatology on July 26.
Their study analyzed data on almost 2 million people screened through the program from 1986-2014. About 62% were women; 90% were white, about 2% were black, and almost 4% were Hispanic. Almost 80% had no regular dermatologist, almost 73% had not been screened previously, almost 45% had never had a skin cancer check, and 9% were uninsured. Almost 31% reported a mole that had recently change in size, color, or shape; almost 34% said they had a family history of skin cancer, and about 14% said they had a personal history of skin cancer.
Participants were asked about demographics and risk factors, although some questions changed from year to year (for example, in 2009 and 2010, participants were asked about melanoma risk factors, and from 1992 through 2010, participants were asked about their access to dermatologic care).
During 1991-2014 (which did not include data for 1995, 1996, and 2000, which were not available), the screening program resulted in 20,628 clinical melanoma diagnoses, 156,087 clinical dysplastic nevi diagnoses, 32,893 clinical squamous cell carcinoma diagnoses, and 129,848 clinical basal cell carcinoma diagnoses.
Of those clinically diagnosed with melanoma during 1992-2010, 83% said they did not have a regular dermatologist, 77% said they had not been screened previously, and 47% said they would not have seen a doctor for a skin exam if the SPOTme program had not been available.
Of those screened in 2009 and 2010 , 72% were considered at high risk for melanoma (older than age 65 years, having a history of sunburns, a family history of skin cancer, and/or more than 50 moles or unusual moles).
Among the other findings was that from 1992 to 2010, about 12% of those with a clinical melanoma diagnosis were not insured, which increased over time, from almost 11% during 1992-1999 to almost 16% during 2007-2010.
The “consistently high rates” of multiple skin cancer risk factors among those newly screened in the study are consistent with previously reported data, “suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer,” the authors wrote. “While the SPOTme program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened,” they added.
Limitations of the study included the inability to confirm the clinical diagnoses with histopathology, and no data from the providers were available.
The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
SOURCE: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
Almost half of the individuals diagnosed with melanoma in a free skin cancer screening program otherwise would not have gone to a doctor to have their skin examined, according to an analysis of the American Academy of Dermatology’s national skin cancer screening program, during 1986-2014.
The SPOTme program, a national skin cancer screening and education program conducted by volunteer dermatologists, was launched in 1985. More than 2 million free screenings have been provided by the program in a “predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants,” according to first authors Jean-Phillip Okhovat, MD, of Beth Israel Deaconess Medical Center, and Derek Beaulieu, MD, of Tufts University, both in Boston, and their colleagues.
The analysis was published online in the Journal of the American Academy of Dermatology on July 26.
Their study analyzed data on almost 2 million people screened through the program from 1986-2014. About 62% were women; 90% were white, about 2% were black, and almost 4% were Hispanic. Almost 80% had no regular dermatologist, almost 73% had not been screened previously, almost 45% had never had a skin cancer check, and 9% were uninsured. Almost 31% reported a mole that had recently change in size, color, or shape; almost 34% said they had a family history of skin cancer, and about 14% said they had a personal history of skin cancer.
Participants were asked about demographics and risk factors, although some questions changed from year to year (for example, in 2009 and 2010, participants were asked about melanoma risk factors, and from 1992 through 2010, participants were asked about their access to dermatologic care).
During 1991-2014 (which did not include data for 1995, 1996, and 2000, which were not available), the screening program resulted in 20,628 clinical melanoma diagnoses, 156,087 clinical dysplastic nevi diagnoses, 32,893 clinical squamous cell carcinoma diagnoses, and 129,848 clinical basal cell carcinoma diagnoses.
Of those clinically diagnosed with melanoma during 1992-2010, 83% said they did not have a regular dermatologist, 77% said they had not been screened previously, and 47% said they would not have seen a doctor for a skin exam if the SPOTme program had not been available.
Of those screened in 2009 and 2010 , 72% were considered at high risk for melanoma (older than age 65 years, having a history of sunburns, a family history of skin cancer, and/or more than 50 moles or unusual moles).
Among the other findings was that from 1992 to 2010, about 12% of those with a clinical melanoma diagnosis were not insured, which increased over time, from almost 11% during 1992-1999 to almost 16% during 2007-2010.
The “consistently high rates” of multiple skin cancer risk factors among those newly screened in the study are consistent with previously reported data, “suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer,” the authors wrote. “While the SPOTme program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened,” they added.
Limitations of the study included the inability to confirm the clinical diagnoses with histopathology, and no data from the providers were available.
The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
SOURCE: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Free skin cancer screening programs help meet an unmet need for people at high risk for skin cancer.
Major finding: Of those who received a clinical diagnosis of melanoma during 1992-2010, 47% said they would not have seen a doctor for a skin exam if the free program had not been available.
Study details: The study analyzed data on almost 2 million people screened through the free SPOTme skin cancer screening program during 1986-2014.
Disclosures: The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
Source: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
AGA continues to ‘push the envelope,’ President says
“AGA is pushing the envelope in a number of areas,” said outgoing AGA President Sheila E. Crowe MD, AGAF, during the AGA Presidential Plenary at Digestive Disease Week® (DDW). “And in a changing world, there are battlefronts where we must continue to work toward innovative solutions.”
The association continues to push reform in maintenance of certification (MOC). The Gastroenterologist-accountable Professionalism in Practice (G-APP) alternative certification pathway introduced 2 years ago created a strong platform to continue guiding the American Board of Internal Medicine (ABIM) to adapt its own MOC process to be more flexible, less costly, and more reflective of the realities of clinical practice.
“ABIM has responded to our pressure by unveiling the 2-year check-in,” Dr. Crowe said. “This is progress, but it still fails to address all the concerns we have expressed to ABIM. AGA will continue to work collaboratively, but forcefully, to make sure that recertification is convenient, relevant, and meaningful.”
AGA has successfully pushed for improvements to the Medicare Quality Payments program. Dr. Crowe said the association remains committed to reducing the regulatory hoops, red tape, and associated costs that practices must navigate.
The continuing push to ease regulatory burdens of practice is buoyed by successes in other areas.
AGA built a new partnership with the Crohn’s and Colitis Foundation and launched the first annual Crohn’s & Colitis Congress™ earlier this year. Registration for the 2019 Congress opens later this month.
The AGA Community (community.gastro.org) became a vibrant hub for clinicians to discuss their most difficult cases. Most recently, AGA made its patient education materials freely available online through the AGA GI Patient Center (patient.gastro.org). All educational pieces are available in both English and Spanish.
Research has been a top priority since AGA was founded 121 years ago, Dr. Crowe added.
“We are proud to be part of a coalition that aggressively advocated for increases at NIH and were pleased that a $3 billion increase was secured in the budget,” she said. “It was a big win in a tough environment.”
To help bridge the continuing shortfall in federal funding, the AGA Research Foundation launched an active year of fundraising and funding. The Foundation provided $2 million in research funding to 41 young investigators in the past year. An expanded awards program provided more pilot awards and more research scholar awards (RSA).
The past year also saw the launch of the AGA Fecal Microbiota Transplantation (FMT) National Registry. The new registry will assess the short- and long-term patient outcomes associated with FMT. In addition, AGA created a clinical research registry for endoscopic suturing procedures. The annual AGA Tech Summit continues to push for innovation in all areas of GI. The ultimate goal, Dr. Crowe said, is to put the most effective innovations into the hands of clinicians as quickly as possible.
During the plenary, Dr. Crowe presented the annual Julius Friedenwald Medal to Loren Laine, MD, AGAF. In addition to a distinguished academic career, Dr. Laine helped create the AGA Center for Gut Microbiome Research and Education while he was AGA president and helped establish AGA’s guideline development process.
“AGA is pushing the envelope in a number of areas,” said outgoing AGA President Sheila E. Crowe MD, AGAF, during the AGA Presidential Plenary at Digestive Disease Week® (DDW). “And in a changing world, there are battlefronts where we must continue to work toward innovative solutions.”
The association continues to push reform in maintenance of certification (MOC). The Gastroenterologist-accountable Professionalism in Practice (G-APP) alternative certification pathway introduced 2 years ago created a strong platform to continue guiding the American Board of Internal Medicine (ABIM) to adapt its own MOC process to be more flexible, less costly, and more reflective of the realities of clinical practice.
“ABIM has responded to our pressure by unveiling the 2-year check-in,” Dr. Crowe said. “This is progress, but it still fails to address all the concerns we have expressed to ABIM. AGA will continue to work collaboratively, but forcefully, to make sure that recertification is convenient, relevant, and meaningful.”
AGA has successfully pushed for improvements to the Medicare Quality Payments program. Dr. Crowe said the association remains committed to reducing the regulatory hoops, red tape, and associated costs that practices must navigate.
The continuing push to ease regulatory burdens of practice is buoyed by successes in other areas.
AGA built a new partnership with the Crohn’s and Colitis Foundation and launched the first annual Crohn’s & Colitis Congress™ earlier this year. Registration for the 2019 Congress opens later this month.
The AGA Community (community.gastro.org) became a vibrant hub for clinicians to discuss their most difficult cases. Most recently, AGA made its patient education materials freely available online through the AGA GI Patient Center (patient.gastro.org). All educational pieces are available in both English and Spanish.
Research has been a top priority since AGA was founded 121 years ago, Dr. Crowe added.
“We are proud to be part of a coalition that aggressively advocated for increases at NIH and were pleased that a $3 billion increase was secured in the budget,” she said. “It was a big win in a tough environment.”
To help bridge the continuing shortfall in federal funding, the AGA Research Foundation launched an active year of fundraising and funding. The Foundation provided $2 million in research funding to 41 young investigators in the past year. An expanded awards program provided more pilot awards and more research scholar awards (RSA).
The past year also saw the launch of the AGA Fecal Microbiota Transplantation (FMT) National Registry. The new registry will assess the short- and long-term patient outcomes associated with FMT. In addition, AGA created a clinical research registry for endoscopic suturing procedures. The annual AGA Tech Summit continues to push for innovation in all areas of GI. The ultimate goal, Dr. Crowe said, is to put the most effective innovations into the hands of clinicians as quickly as possible.
During the plenary, Dr. Crowe presented the annual Julius Friedenwald Medal to Loren Laine, MD, AGAF. In addition to a distinguished academic career, Dr. Laine helped create the AGA Center for Gut Microbiome Research and Education while he was AGA president and helped establish AGA’s guideline development process.
“AGA is pushing the envelope in a number of areas,” said outgoing AGA President Sheila E. Crowe MD, AGAF, during the AGA Presidential Plenary at Digestive Disease Week® (DDW). “And in a changing world, there are battlefronts where we must continue to work toward innovative solutions.”
The association continues to push reform in maintenance of certification (MOC). The Gastroenterologist-accountable Professionalism in Practice (G-APP) alternative certification pathway introduced 2 years ago created a strong platform to continue guiding the American Board of Internal Medicine (ABIM) to adapt its own MOC process to be more flexible, less costly, and more reflective of the realities of clinical practice.
“ABIM has responded to our pressure by unveiling the 2-year check-in,” Dr. Crowe said. “This is progress, but it still fails to address all the concerns we have expressed to ABIM. AGA will continue to work collaboratively, but forcefully, to make sure that recertification is convenient, relevant, and meaningful.”
AGA has successfully pushed for improvements to the Medicare Quality Payments program. Dr. Crowe said the association remains committed to reducing the regulatory hoops, red tape, and associated costs that practices must navigate.
The continuing push to ease regulatory burdens of practice is buoyed by successes in other areas.
AGA built a new partnership with the Crohn’s and Colitis Foundation and launched the first annual Crohn’s & Colitis Congress™ earlier this year. Registration for the 2019 Congress opens later this month.
The AGA Community (community.gastro.org) became a vibrant hub for clinicians to discuss their most difficult cases. Most recently, AGA made its patient education materials freely available online through the AGA GI Patient Center (patient.gastro.org). All educational pieces are available in both English and Spanish.
Research has been a top priority since AGA was founded 121 years ago, Dr. Crowe added.
“We are proud to be part of a coalition that aggressively advocated for increases at NIH and were pleased that a $3 billion increase was secured in the budget,” she said. “It was a big win in a tough environment.”
To help bridge the continuing shortfall in federal funding, the AGA Research Foundation launched an active year of fundraising and funding. The Foundation provided $2 million in research funding to 41 young investigators in the past year. An expanded awards program provided more pilot awards and more research scholar awards (RSA).
The past year also saw the launch of the AGA Fecal Microbiota Transplantation (FMT) National Registry. The new registry will assess the short- and long-term patient outcomes associated with FMT. In addition, AGA created a clinical research registry for endoscopic suturing procedures. The annual AGA Tech Summit continues to push for innovation in all areas of GI. The ultimate goal, Dr. Crowe said, is to put the most effective innovations into the hands of clinicians as quickly as possible.
During the plenary, Dr. Crowe presented the annual Julius Friedenwald Medal to Loren Laine, MD, AGAF. In addition to a distinguished academic career, Dr. Laine helped create the AGA Center for Gut Microbiome Research and Education while he was AGA president and helped establish AGA’s guideline development process.
Oral drug seen preventing angioedema attacks
An experimental agent reduced swelling episodes markedly in patients with hereditary angioedema, according to results from a phase 2 randomized, dose-ranging, placebo-controlled trial.
The drug BCX7353, developed by BioCryst Pharmaceuticals, is taken orally and works by inhibiting plasma kallikrein, an enzyme overexpressed in hereditary angioedema, a rare genetic disease that causes severe tissue swelling. In research published July 26 in the New England Journal of Medicine (N Engl J Med. 2018;379:352-62), Emel Aygören-Pürsün, MD, of Goethe University in Frankfurt, Germany, and colleagues, randomized 77 patients with type 1 or II hereditary angioedema and a pattern of frequent attacks to one of four doses of daily BCX7353, or placebo, for 28 days.
Dr. Aygören-Pürsün’s group found significant reductions in the number of monthly attacks for the three doses used in the study, with the best response seen in the group receiving the second-lowest dose of 125 mg. These patients saw a reduction of 73.8% (P less than .001) in monthly attacks from baseline, and 43% of patients receiving that dose had no attacks during the study period. The higher-dose groups saw more adverse events and apparently less efficacy, with the 250-mg dose associated with a reduction in attacks of 44.6% (P = .01), and for the 350-mg group, a 45.5% reduction (P = .006).
Patients receiving the lowest dose in the study, 62.5 mg, saw a small (about 10%) reduction in attacks that did not reach statistical significance. Gastrointestinal adverse events were reported in the two highest dose groups, and three patients in the 350-mg group dropped out after reporting serious adverse events, including one liver disorder considered likely related to the trial regimen.
The efficacy of the highest doses “was probably masked by gastrointestinal adverse events that may have been misattributed as early symptoms of abdominal angioedema attacks,” the investigators wrote in their analysis. Improvements in angioedema-related quality of life scores, a secondary trial endpoint, reached statistical significance for the 125- and 250-mg doses.
The authors cautioned that the safety of long-term dosing would need to be studied in longer-term trials.
The study was sponsored by the drug manufacturer, BioCryst Pharmaceuticals. All of the study’s authors, including the lead author, disclosed financial relationships in the form of grant support, fees, or employment with the study sponsor.
SOURCE: Aygören-Pürsün et al. N Engl J Med. 2018;379:352-62.
An experimental agent reduced swelling episodes markedly in patients with hereditary angioedema, according to results from a phase 2 randomized, dose-ranging, placebo-controlled trial.
The drug BCX7353, developed by BioCryst Pharmaceuticals, is taken orally and works by inhibiting plasma kallikrein, an enzyme overexpressed in hereditary angioedema, a rare genetic disease that causes severe tissue swelling. In research published July 26 in the New England Journal of Medicine (N Engl J Med. 2018;379:352-62), Emel Aygören-Pürsün, MD, of Goethe University in Frankfurt, Germany, and colleagues, randomized 77 patients with type 1 or II hereditary angioedema and a pattern of frequent attacks to one of four doses of daily BCX7353, or placebo, for 28 days.
Dr. Aygören-Pürsün’s group found significant reductions in the number of monthly attacks for the three doses used in the study, with the best response seen in the group receiving the second-lowest dose of 125 mg. These patients saw a reduction of 73.8% (P less than .001) in monthly attacks from baseline, and 43% of patients receiving that dose had no attacks during the study period. The higher-dose groups saw more adverse events and apparently less efficacy, with the 250-mg dose associated with a reduction in attacks of 44.6% (P = .01), and for the 350-mg group, a 45.5% reduction (P = .006).
Patients receiving the lowest dose in the study, 62.5 mg, saw a small (about 10%) reduction in attacks that did not reach statistical significance. Gastrointestinal adverse events were reported in the two highest dose groups, and three patients in the 350-mg group dropped out after reporting serious adverse events, including one liver disorder considered likely related to the trial regimen.
The efficacy of the highest doses “was probably masked by gastrointestinal adverse events that may have been misattributed as early symptoms of abdominal angioedema attacks,” the investigators wrote in their analysis. Improvements in angioedema-related quality of life scores, a secondary trial endpoint, reached statistical significance for the 125- and 250-mg doses.
The authors cautioned that the safety of long-term dosing would need to be studied in longer-term trials.
The study was sponsored by the drug manufacturer, BioCryst Pharmaceuticals. All of the study’s authors, including the lead author, disclosed financial relationships in the form of grant support, fees, or employment with the study sponsor.
SOURCE: Aygören-Pürsün et al. N Engl J Med. 2018;379:352-62.
An experimental agent reduced swelling episodes markedly in patients with hereditary angioedema, according to results from a phase 2 randomized, dose-ranging, placebo-controlled trial.
The drug BCX7353, developed by BioCryst Pharmaceuticals, is taken orally and works by inhibiting plasma kallikrein, an enzyme overexpressed in hereditary angioedema, a rare genetic disease that causes severe tissue swelling. In research published July 26 in the New England Journal of Medicine (N Engl J Med. 2018;379:352-62), Emel Aygören-Pürsün, MD, of Goethe University in Frankfurt, Germany, and colleagues, randomized 77 patients with type 1 or II hereditary angioedema and a pattern of frequent attacks to one of four doses of daily BCX7353, or placebo, for 28 days.
Dr. Aygören-Pürsün’s group found significant reductions in the number of monthly attacks for the three doses used in the study, with the best response seen in the group receiving the second-lowest dose of 125 mg. These patients saw a reduction of 73.8% (P less than .001) in monthly attacks from baseline, and 43% of patients receiving that dose had no attacks during the study period. The higher-dose groups saw more adverse events and apparently less efficacy, with the 250-mg dose associated with a reduction in attacks of 44.6% (P = .01), and for the 350-mg group, a 45.5% reduction (P = .006).
Patients receiving the lowest dose in the study, 62.5 mg, saw a small (about 10%) reduction in attacks that did not reach statistical significance. Gastrointestinal adverse events were reported in the two highest dose groups, and three patients in the 350-mg group dropped out after reporting serious adverse events, including one liver disorder considered likely related to the trial regimen.
The efficacy of the highest doses “was probably masked by gastrointestinal adverse events that may have been misattributed as early symptoms of abdominal angioedema attacks,” the investigators wrote in their analysis. Improvements in angioedema-related quality of life scores, a secondary trial endpoint, reached statistical significance for the 125- and 250-mg doses.
The authors cautioned that the safety of long-term dosing would need to be studied in longer-term trials.
The study was sponsored by the drug manufacturer, BioCryst Pharmaceuticals. All of the study’s authors, including the lead author, disclosed financial relationships in the form of grant support, fees, or employment with the study sponsor.
SOURCE: Aygören-Pürsün et al. N Engl J Med. 2018;379:352-62.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: An experimental kallikrein inhibitor, taken daily, prevented swelling attacks in people with hereditary angioedema.
Major finding: Patients in a moderate-dose group saw a 73.8% reduction in monthly swelling attacks from baseline, and 43% of saw no attacks.
Study details: A phase 2 study randomizing 77 patients with hereditary angioedema in three countries to one of four escalating doses or placebo.
Disclosures: All authors disclosed some relationship (current or past grant or fee support, or employment) with sponsor, BioCryst Pharmaceuticals.
Source: Aygören-Pürsün et. al. N Engl J Med. 2018;379:352-62.
Lower CTC count IDs indolent MBC disease subset
CHICAGO – A circulating tumor cell (CTC) count less than 5 per 7.5 mL of blood in patients with metastatic breast cancer indicates an indolent disease subset, according to a pooled analysis of individual patient data from two large cohorts.
The findings, which were independent of molecular subtype, disease location, or line of treatment, have important implications for CTC-based staging of metastatic breast cancer (MBC), which in turn could guide treatment decision making and drug development, Andrew A. Davis, MD, of Northwestern University, Chicago, and his colleagues reported in a poster at the annual meeting of the American Society of Clinical Oncology.
In 1,944 patients from the European Pooled Analysis Consortium (EPAC) and 492 from MD Anderson Cancer Center, CTC counts of 5 per 7.5mL or greater were associated with worse outcomes overall (hazard ratio, 2.43), the investigators said.
Median overall survival (OS) among all patients with CTC counts less than 5, who were considered to have stage IV indolent disease (stage IVindolent), was 36.3 months, and OS among those with de novo disease and CDC counts less than 5 was greater than 5.5 years, they said, noting that the survival benefit persisted across all disease subtypes.
For example, median OS in patients with stage IVindolent vs. stage IVaggressive (those with CTC counts of 5 or greater ) was 44.0 vs. 17.3 months in patients with hormone receptor–positive disease, 23.8 vs. 9.0 months in triple negative breast cancer patients, and 36.7 vs. 20.4 months in patients with HER2+ disease, respectively, they explained.They also noted that stage IVindolent disease could discriminate a less aggressive cohort both for patients with and without prior treatment; the hazard ratios were 0.40 and 0.42 favoring indolent disease for both first-line treatment and treatment beyond the first line, respectively.
In early-stage breast cancer, diagnostic tools have been incorporated into practice to help identify patients who will benefit from conservative vs. aggressive therapy, and the current findings suggest that CTC counts could be used in that manner for staging MBC.
“We propose a CTC-based staging system for MBC based on indolent and aggressive disease to incorporate into the American Joint Committee on Cancer [tumor node metastasis] staging classification,” they wrote, adding that prospective studies of single-agent, cost-effective treatments for stage IVindolent disease in the first-line setting are needed.
This study was supported by the Lynn Sage Breast Cancer Research OncoSET Program at Robert H. Lurie Cancer Center. Dr. Davis reported having no disclosures.
SOURCE: Davis A et al., ASCO 2018 Poster 1019.
CHICAGO – A circulating tumor cell (CTC) count less than 5 per 7.5 mL of blood in patients with metastatic breast cancer indicates an indolent disease subset, according to a pooled analysis of individual patient data from two large cohorts.
The findings, which were independent of molecular subtype, disease location, or line of treatment, have important implications for CTC-based staging of metastatic breast cancer (MBC), which in turn could guide treatment decision making and drug development, Andrew A. Davis, MD, of Northwestern University, Chicago, and his colleagues reported in a poster at the annual meeting of the American Society of Clinical Oncology.
In 1,944 patients from the European Pooled Analysis Consortium (EPAC) and 492 from MD Anderson Cancer Center, CTC counts of 5 per 7.5mL or greater were associated with worse outcomes overall (hazard ratio, 2.43), the investigators said.
Median overall survival (OS) among all patients with CTC counts less than 5, who were considered to have stage IV indolent disease (stage IVindolent), was 36.3 months, and OS among those with de novo disease and CDC counts less than 5 was greater than 5.5 years, they said, noting that the survival benefit persisted across all disease subtypes.
For example, median OS in patients with stage IVindolent vs. stage IVaggressive (those with CTC counts of 5 or greater ) was 44.0 vs. 17.3 months in patients with hormone receptor–positive disease, 23.8 vs. 9.0 months in triple negative breast cancer patients, and 36.7 vs. 20.4 months in patients with HER2+ disease, respectively, they explained.They also noted that stage IVindolent disease could discriminate a less aggressive cohort both for patients with and without prior treatment; the hazard ratios were 0.40 and 0.42 favoring indolent disease for both first-line treatment and treatment beyond the first line, respectively.
In early-stage breast cancer, diagnostic tools have been incorporated into practice to help identify patients who will benefit from conservative vs. aggressive therapy, and the current findings suggest that CTC counts could be used in that manner for staging MBC.
“We propose a CTC-based staging system for MBC based on indolent and aggressive disease to incorporate into the American Joint Committee on Cancer [tumor node metastasis] staging classification,” they wrote, adding that prospective studies of single-agent, cost-effective treatments for stage IVindolent disease in the first-line setting are needed.
This study was supported by the Lynn Sage Breast Cancer Research OncoSET Program at Robert H. Lurie Cancer Center. Dr. Davis reported having no disclosures.
SOURCE: Davis A et al., ASCO 2018 Poster 1019.
CHICAGO – A circulating tumor cell (CTC) count less than 5 per 7.5 mL of blood in patients with metastatic breast cancer indicates an indolent disease subset, according to a pooled analysis of individual patient data from two large cohorts.
The findings, which were independent of molecular subtype, disease location, or line of treatment, have important implications for CTC-based staging of metastatic breast cancer (MBC), which in turn could guide treatment decision making and drug development, Andrew A. Davis, MD, of Northwestern University, Chicago, and his colleagues reported in a poster at the annual meeting of the American Society of Clinical Oncology.
In 1,944 patients from the European Pooled Analysis Consortium (EPAC) and 492 from MD Anderson Cancer Center, CTC counts of 5 per 7.5mL or greater were associated with worse outcomes overall (hazard ratio, 2.43), the investigators said.
Median overall survival (OS) among all patients with CTC counts less than 5, who were considered to have stage IV indolent disease (stage IVindolent), was 36.3 months, and OS among those with de novo disease and CDC counts less than 5 was greater than 5.5 years, they said, noting that the survival benefit persisted across all disease subtypes.
For example, median OS in patients with stage IVindolent vs. stage IVaggressive (those with CTC counts of 5 or greater ) was 44.0 vs. 17.3 months in patients with hormone receptor–positive disease, 23.8 vs. 9.0 months in triple negative breast cancer patients, and 36.7 vs. 20.4 months in patients with HER2+ disease, respectively, they explained.They also noted that stage IVindolent disease could discriminate a less aggressive cohort both for patients with and without prior treatment; the hazard ratios were 0.40 and 0.42 favoring indolent disease for both first-line treatment and treatment beyond the first line, respectively.
In early-stage breast cancer, diagnostic tools have been incorporated into practice to help identify patients who will benefit from conservative vs. aggressive therapy, and the current findings suggest that CTC counts could be used in that manner for staging MBC.
“We propose a CTC-based staging system for MBC based on indolent and aggressive disease to incorporate into the American Joint Committee on Cancer [tumor node metastasis] staging classification,” they wrote, adding that prospective studies of single-agent, cost-effective treatments for stage IVindolent disease in the first-line setting are needed.
This study was supported by the Lynn Sage Breast Cancer Research OncoSET Program at Robert H. Lurie Cancer Center. Dr. Davis reported having no disclosures.
SOURCE: Davis A et al., ASCO 2018 Poster 1019.
REPORTING FROM ASCO 2018
Key clinical point: A CTC count less than 5 per 7.5 mL of blood in patients with MBC indicates an indolent disease subset.
Major finding: Median OS for stage IVindolent vs. stage IVaggressive disease was 4.0 vs. 17.3 months in HER2-negative patients, 23.8 vs. 9.0 months in TNBC patients, and 36.7 vs. 20.4 months in HER2-positive disease.
Study details: A pooled analysis of data from two cohort studies including 2,436 patients.
Disclosures: This study was supported by the Lynn Sage Breast Cancer Research OncoSET Program at Robert H. Lurie Cancer Center. Dr. Davis reported having no disclosures.
Source: Davis A et al. ASCO 2018 Poster 1019.
After initial rivaroxaban, aspirin is noninferior to rivaroxaban for thromboprophylaxis following joint arthroplasty
Background: While there is a consensus on the need for chemoprophylaxis to reduce the rates of postoperative VTE, there is wide variation in choice of agents recommended. Aspirin, while cheap and widely available, has never been directly compared with a direct oral anticoagulant in randomized, controlled trials.
Study design: Multicenter, double-blind, randomized, controlled noninferiority trial.
Setting: 15 university-affiliated health centers in Canada from January 2013 through April 2016.
Synopsis: 3,224 patients who received daily rivaroxaban for 5 days following joint arthroplasty were randomized to either receive aspirin 81 mg daily or continue daily rivaroxaban. Duration of therapy was determined by type of surgery (9 days for knee, 17 days for hip). The primary effectiveness outcome was defined as symptomatic pulmonary embolism or proximal deep venous thrombosis diagnosed in the 90-day follow-up period. The primary outcome results met the predetermined criterion for noninferiority with similar rates of symptomatic VTE in the aspirin and rivaroxaban group (0.64% vs. 0.7%; P less than .001). There was no significant difference in bleeding rates between the groups. Given that patients with prior VTE, morbid obesity, or cancer were not well represented in this study, these results should not be extrapolated to those populations felt to be at highest risk for VTE.
Bottom line: For thromboprophylaxis after joint arthroplasty, rivaroxaban followed by aspirin may be noninferior to extended rivaroxaban.
Citation: Anderson D et al. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Eng J Med. 2018 Feb 22;378(8):699-707.
Dr. Abdallah is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: While there is a consensus on the need for chemoprophylaxis to reduce the rates of postoperative VTE, there is wide variation in choice of agents recommended. Aspirin, while cheap and widely available, has never been directly compared with a direct oral anticoagulant in randomized, controlled trials.
Study design: Multicenter, double-blind, randomized, controlled noninferiority trial.
Setting: 15 university-affiliated health centers in Canada from January 2013 through April 2016.
Synopsis: 3,224 patients who received daily rivaroxaban for 5 days following joint arthroplasty were randomized to either receive aspirin 81 mg daily or continue daily rivaroxaban. Duration of therapy was determined by type of surgery (9 days for knee, 17 days for hip). The primary effectiveness outcome was defined as symptomatic pulmonary embolism or proximal deep venous thrombosis diagnosed in the 90-day follow-up period. The primary outcome results met the predetermined criterion for noninferiority with similar rates of symptomatic VTE in the aspirin and rivaroxaban group (0.64% vs. 0.7%; P less than .001). There was no significant difference in bleeding rates between the groups. Given that patients with prior VTE, morbid obesity, or cancer were not well represented in this study, these results should not be extrapolated to those populations felt to be at highest risk for VTE.
Bottom line: For thromboprophylaxis after joint arthroplasty, rivaroxaban followed by aspirin may be noninferior to extended rivaroxaban.
Citation: Anderson D et al. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Eng J Med. 2018 Feb 22;378(8):699-707.
Dr. Abdallah is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: While there is a consensus on the need for chemoprophylaxis to reduce the rates of postoperative VTE, there is wide variation in choice of agents recommended. Aspirin, while cheap and widely available, has never been directly compared with a direct oral anticoagulant in randomized, controlled trials.
Study design: Multicenter, double-blind, randomized, controlled noninferiority trial.
Setting: 15 university-affiliated health centers in Canada from January 2013 through April 2016.
Synopsis: 3,224 patients who received daily rivaroxaban for 5 days following joint arthroplasty were randomized to either receive aspirin 81 mg daily or continue daily rivaroxaban. Duration of therapy was determined by type of surgery (9 days for knee, 17 days for hip). The primary effectiveness outcome was defined as symptomatic pulmonary embolism or proximal deep venous thrombosis diagnosed in the 90-day follow-up period. The primary outcome results met the predetermined criterion for noninferiority with similar rates of symptomatic VTE in the aspirin and rivaroxaban group (0.64% vs. 0.7%; P less than .001). There was no significant difference in bleeding rates between the groups. Given that patients with prior VTE, morbid obesity, or cancer were not well represented in this study, these results should not be extrapolated to those populations felt to be at highest risk for VTE.
Bottom line: For thromboprophylaxis after joint arthroplasty, rivaroxaban followed by aspirin may be noninferior to extended rivaroxaban.
Citation: Anderson D et al. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Eng J Med. 2018 Feb 22;378(8):699-707.
Dr. Abdallah is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Short-course IV antibiotics okay for newborn bacteremic UTI
ATLANTA – A short course of IV antibiotics – 7 days or less – is fine for most infants with uncomplicated bacteremic urinary tract infections, according to a review of 116 children younger than 60 days.
How long to treat bacteremic UTIs in the very young has been debated in pediatrics for a while, with some centers opting for a few days and others for 2 weeks or more. Shorter courses reduce length of stay, costs, and complications, but there hasn’t been much research to see whether they work as well.
The new investigation has suggested they do. “Young infants with bacteremic UTI who received less than or equal to 7 days of IV antibiotic therapy did not have more recurrent UTIs,” compared “to infants who received longer courses. Short course IV therapy with early conversion to oral antibiotics may be considered in this population,” said lead investigator Sanyukta Desai, MD, at the Pediatric Hospital Medicine meeting.
The team compared outcomes of 58 infants treated for 7 days or less to outcomes of 58 infants treated for more than 7 days at 11 children’s hospitals scattered across the United States.
Urine was collected by catheter, and each child grew the same organism in their blood and urine cultures, confirming the diagnosis of bacteremic UTI. Children with bacterial meningitis, or suspected of having it, were excluded. The subjects had all been admitted through the ED.
There was quite a bit of variation among the 11 hospitals, with the proportion of children treated with short courses ranging from 10% to 81%.
As for the results, two patients in the short-course group (3%) and four in the long-course group (7%) had recurrent UTIs within 30 days. None of them developed meningitis, and none required ICU admission. Propensity-score matching revealed an odds ratio for recurrence that favored shorter treatment, but it wasn’t statistically significant.
The mean length of stay was 5 days in the short-course arm and 11 days in the long-course arm. There were no serious adverse events within 30 days of the index admission in either group.
Among the recurrences, the two children in the short-course arm were initially treated for 3 and 5 days. Both were older than 28 days at their initial presentation, and both had vesicoureteral reflux of at least grade 2, which was not diagnosed in one child until after the recurrence. The other child had been on prophylactic trimethoprim/sulfamethoxazole before the recurrence.
The four recurrent cases in the long arm initially received either 10 or 14 days of IV antibiotics. Two children had grade 4 vesicoureteral reflux and had been on prophylactic amoxicillin.
Infants treated with longer antibiotic courses were more likely to be under 28 days old, appear ill at presentation, have had bacteremia for more than 24 hours, and have and grow out pathogens other than Escherichia coli. The two groups were otherwise balanced for sex, prematurity, complex chronic conditions, and known genitourinary anomalies.
With such low event rates, the study wasn’t powered to detect small but potentially meaningful differences in outcomes, and further work is needed to define which children would benefit from longer treatment courses. Even so, “it was reassuring that patients did well in both arms,” said Dr. Desai, a clinical fellow in the division of hospital medicine at Cincinnati Children’s Hospital.
“At our institution with uncomplicated UTI, we wait to see what the culture grows.” If there’s an oral antibiotic that will work, “we send [infants] home in 3-4 days. We haven’t had any poor outcomes, even when they’re bacteremic,” she said.
The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
ATLANTA – A short course of IV antibiotics – 7 days or less – is fine for most infants with uncomplicated bacteremic urinary tract infections, according to a review of 116 children younger than 60 days.
How long to treat bacteremic UTIs in the very young has been debated in pediatrics for a while, with some centers opting for a few days and others for 2 weeks or more. Shorter courses reduce length of stay, costs, and complications, but there hasn’t been much research to see whether they work as well.
The new investigation has suggested they do. “Young infants with bacteremic UTI who received less than or equal to 7 days of IV antibiotic therapy did not have more recurrent UTIs,” compared “to infants who received longer courses. Short course IV therapy with early conversion to oral antibiotics may be considered in this population,” said lead investigator Sanyukta Desai, MD, at the Pediatric Hospital Medicine meeting.
The team compared outcomes of 58 infants treated for 7 days or less to outcomes of 58 infants treated for more than 7 days at 11 children’s hospitals scattered across the United States.
Urine was collected by catheter, and each child grew the same organism in their blood and urine cultures, confirming the diagnosis of bacteremic UTI. Children with bacterial meningitis, or suspected of having it, were excluded. The subjects had all been admitted through the ED.
There was quite a bit of variation among the 11 hospitals, with the proportion of children treated with short courses ranging from 10% to 81%.
As for the results, two patients in the short-course group (3%) and four in the long-course group (7%) had recurrent UTIs within 30 days. None of them developed meningitis, and none required ICU admission. Propensity-score matching revealed an odds ratio for recurrence that favored shorter treatment, but it wasn’t statistically significant.
The mean length of stay was 5 days in the short-course arm and 11 days in the long-course arm. There were no serious adverse events within 30 days of the index admission in either group.
Among the recurrences, the two children in the short-course arm were initially treated for 3 and 5 days. Both were older than 28 days at their initial presentation, and both had vesicoureteral reflux of at least grade 2, which was not diagnosed in one child until after the recurrence. The other child had been on prophylactic trimethoprim/sulfamethoxazole before the recurrence.
The four recurrent cases in the long arm initially received either 10 or 14 days of IV antibiotics. Two children had grade 4 vesicoureteral reflux and had been on prophylactic amoxicillin.
Infants treated with longer antibiotic courses were more likely to be under 28 days old, appear ill at presentation, have had bacteremia for more than 24 hours, and have and grow out pathogens other than Escherichia coli. The two groups were otherwise balanced for sex, prematurity, complex chronic conditions, and known genitourinary anomalies.
With such low event rates, the study wasn’t powered to detect small but potentially meaningful differences in outcomes, and further work is needed to define which children would benefit from longer treatment courses. Even so, “it was reassuring that patients did well in both arms,” said Dr. Desai, a clinical fellow in the division of hospital medicine at Cincinnati Children’s Hospital.
“At our institution with uncomplicated UTI, we wait to see what the culture grows.” If there’s an oral antibiotic that will work, “we send [infants] home in 3-4 days. We haven’t had any poor outcomes, even when they’re bacteremic,” she said.
The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
ATLANTA – A short course of IV antibiotics – 7 days or less – is fine for most infants with uncomplicated bacteremic urinary tract infections, according to a review of 116 children younger than 60 days.
How long to treat bacteremic UTIs in the very young has been debated in pediatrics for a while, with some centers opting for a few days and others for 2 weeks or more. Shorter courses reduce length of stay, costs, and complications, but there hasn’t been much research to see whether they work as well.
The new investigation has suggested they do. “Young infants with bacteremic UTI who received less than or equal to 7 days of IV antibiotic therapy did not have more recurrent UTIs,” compared “to infants who received longer courses. Short course IV therapy with early conversion to oral antibiotics may be considered in this population,” said lead investigator Sanyukta Desai, MD, at the Pediatric Hospital Medicine meeting.
The team compared outcomes of 58 infants treated for 7 days or less to outcomes of 58 infants treated for more than 7 days at 11 children’s hospitals scattered across the United States.
Urine was collected by catheter, and each child grew the same organism in their blood and urine cultures, confirming the diagnosis of bacteremic UTI. Children with bacterial meningitis, or suspected of having it, were excluded. The subjects had all been admitted through the ED.
There was quite a bit of variation among the 11 hospitals, with the proportion of children treated with short courses ranging from 10% to 81%.
As for the results, two patients in the short-course group (3%) and four in the long-course group (7%) had recurrent UTIs within 30 days. None of them developed meningitis, and none required ICU admission. Propensity-score matching revealed an odds ratio for recurrence that favored shorter treatment, but it wasn’t statistically significant.
The mean length of stay was 5 days in the short-course arm and 11 days in the long-course arm. There were no serious adverse events within 30 days of the index admission in either group.
Among the recurrences, the two children in the short-course arm were initially treated for 3 and 5 days. Both were older than 28 days at their initial presentation, and both had vesicoureteral reflux of at least grade 2, which was not diagnosed in one child until after the recurrence. The other child had been on prophylactic trimethoprim/sulfamethoxazole before the recurrence.
The four recurrent cases in the long arm initially received either 10 or 14 days of IV antibiotics. Two children had grade 4 vesicoureteral reflux and had been on prophylactic amoxicillin.
Infants treated with longer antibiotic courses were more likely to be under 28 days old, appear ill at presentation, have had bacteremia for more than 24 hours, and have and grow out pathogens other than Escherichia coli. The two groups were otherwise balanced for sex, prematurity, complex chronic conditions, and known genitourinary anomalies.
With such low event rates, the study wasn’t powered to detect small but potentially meaningful differences in outcomes, and further work is needed to define which children would benefit from longer treatment courses. Even so, “it was reassuring that patients did well in both arms,” said Dr. Desai, a clinical fellow in the division of hospital medicine at Cincinnati Children’s Hospital.
“At our institution with uncomplicated UTI, we wait to see what the culture grows.” If there’s an oral antibiotic that will work, “we send [infants] home in 3-4 days. We haven’t had any poor outcomes, even when they’re bacteremic,” she said.
The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
REPORTING FROM PHM 2018
Key clinical point:
Major finding: Two patients in the short-course group (3%) and four in the long-course group (7%) had recurrent UTIs within 30 days.
Study details: Review of 116 infants.
Disclosures: The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
Study explores adolescents’ views on their skin tone, pressure to tan
LAKE TAHOE, CALIF. –
During an interview at the annual meeting of the Society for Pediatric Dermatology, lead study author Shivani Patel, MD, said that prior research on skin color had focused mainly on adults and its impact on self-esteem and perceived attractiveness, yet little data are available on perceptions of skin color among adolescents.
“During puberty, adolescents receive pressure from friends, family, and social media to conform to a certain acceptable standard of skin tone,” said Dr. Patel, a chief resident in the department of dermatology at Johns Hopkins University, Baltimore. “They will often engage in risky behaviors such as tanning bed use, suntanning, and use of skin lightening creams.”
In an effort to characterize the attitudes of adolescents about their skin tone, she and her associates recruited 50 patients aged 12-19 years who were seen at the Johns Hopkins dermatology clinics. Slightly more than half (56%) were female. They were asked to complete surveys on their use of sunscreen, tanning beds, and skin-lightening creams, as well as to report any family or friends who have used these interventions.
Next, the researchers used Pantone’s Capsure device to record each subject’s skin tone according to a palate of 110 skin colors available from Pantone’s SkinTone Guide, which is intended to match and reproduce lifelike skin tones in a variety of industries. The adolescents were then given the palette and asked which skin tone they felt best represented their skin and which skin tone they wished they had. These differences were compared with their objective measurement by the study team.
Of all respondents, 20% indicated that they felt pressure to have tan skin and were likely to engage in suntanning (P less than .001), a feeling they said started around the age of 12 years and stemmed from perceived pressure from friends and celebrity figures. Those who suntanned were more likely to wear sunscreen (P less than .01), a finding “that was reassuring and showed that they are aware of sunscreen and sun safety,” Dr. Patel said. However, about half of the respondents reported never wearing sunscreen and only two reported wearing sunscreen daily. No one reported using tanning beds, but 8% reported that a family member used them. One adolescent reported using skin lightening creams, and three reported that their mothers used them.
The researchers also found that black and Asian study participants were significantly more likely to desire a skin tone lighter than what they perceived their skin tone to be, while white participants were significantly more likely to desire a darker skin tone (P less than .011 for both associations).
The findings suggest that sun safety initiatives should target prepubertal patients before they engage in risky behaviors, Dr. Patel said. She acknowledged that the small sample size is a limitation of the study, but said that she and her associates hope to conduct a larger-scale analysis.
She reported having no financial disclosures.
LAKE TAHOE, CALIF. –
During an interview at the annual meeting of the Society for Pediatric Dermatology, lead study author Shivani Patel, MD, said that prior research on skin color had focused mainly on adults and its impact on self-esteem and perceived attractiveness, yet little data are available on perceptions of skin color among adolescents.
“During puberty, adolescents receive pressure from friends, family, and social media to conform to a certain acceptable standard of skin tone,” said Dr. Patel, a chief resident in the department of dermatology at Johns Hopkins University, Baltimore. “They will often engage in risky behaviors such as tanning bed use, suntanning, and use of skin lightening creams.”
In an effort to characterize the attitudes of adolescents about their skin tone, she and her associates recruited 50 patients aged 12-19 years who were seen at the Johns Hopkins dermatology clinics. Slightly more than half (56%) were female. They were asked to complete surveys on their use of sunscreen, tanning beds, and skin-lightening creams, as well as to report any family or friends who have used these interventions.
Next, the researchers used Pantone’s Capsure device to record each subject’s skin tone according to a palate of 110 skin colors available from Pantone’s SkinTone Guide, which is intended to match and reproduce lifelike skin tones in a variety of industries. The adolescents were then given the palette and asked which skin tone they felt best represented their skin and which skin tone they wished they had. These differences were compared with their objective measurement by the study team.
Of all respondents, 20% indicated that they felt pressure to have tan skin and were likely to engage in suntanning (P less than .001), a feeling they said started around the age of 12 years and stemmed from perceived pressure from friends and celebrity figures. Those who suntanned were more likely to wear sunscreen (P less than .01), a finding “that was reassuring and showed that they are aware of sunscreen and sun safety,” Dr. Patel said. However, about half of the respondents reported never wearing sunscreen and only two reported wearing sunscreen daily. No one reported using tanning beds, but 8% reported that a family member used them. One adolescent reported using skin lightening creams, and three reported that their mothers used them.
The researchers also found that black and Asian study participants were significantly more likely to desire a skin tone lighter than what they perceived their skin tone to be, while white participants were significantly more likely to desire a darker skin tone (P less than .011 for both associations).
The findings suggest that sun safety initiatives should target prepubertal patients before they engage in risky behaviors, Dr. Patel said. She acknowledged that the small sample size is a limitation of the study, but said that she and her associates hope to conduct a larger-scale analysis.
She reported having no financial disclosures.
LAKE TAHOE, CALIF. –
During an interview at the annual meeting of the Society for Pediatric Dermatology, lead study author Shivani Patel, MD, said that prior research on skin color had focused mainly on adults and its impact on self-esteem and perceived attractiveness, yet little data are available on perceptions of skin color among adolescents.
“During puberty, adolescents receive pressure from friends, family, and social media to conform to a certain acceptable standard of skin tone,” said Dr. Patel, a chief resident in the department of dermatology at Johns Hopkins University, Baltimore. “They will often engage in risky behaviors such as tanning bed use, suntanning, and use of skin lightening creams.”
In an effort to characterize the attitudes of adolescents about their skin tone, she and her associates recruited 50 patients aged 12-19 years who were seen at the Johns Hopkins dermatology clinics. Slightly more than half (56%) were female. They were asked to complete surveys on their use of sunscreen, tanning beds, and skin-lightening creams, as well as to report any family or friends who have used these interventions.
Next, the researchers used Pantone’s Capsure device to record each subject’s skin tone according to a palate of 110 skin colors available from Pantone’s SkinTone Guide, which is intended to match and reproduce lifelike skin tones in a variety of industries. The adolescents were then given the palette and asked which skin tone they felt best represented their skin and which skin tone they wished they had. These differences were compared with their objective measurement by the study team.
Of all respondents, 20% indicated that they felt pressure to have tan skin and were likely to engage in suntanning (P less than .001), a feeling they said started around the age of 12 years and stemmed from perceived pressure from friends and celebrity figures. Those who suntanned were more likely to wear sunscreen (P less than .01), a finding “that was reassuring and showed that they are aware of sunscreen and sun safety,” Dr. Patel said. However, about half of the respondents reported never wearing sunscreen and only two reported wearing sunscreen daily. No one reported using tanning beds, but 8% reported that a family member used them. One adolescent reported using skin lightening creams, and three reported that their mothers used them.
The researchers also found that black and Asian study participants were significantly more likely to desire a skin tone lighter than what they perceived their skin tone to be, while white participants were significantly more likely to desire a darker skin tone (P less than .011 for both associations).
The findings suggest that sun safety initiatives should target prepubertal patients before they engage in risky behaviors, Dr. Patel said. She acknowledged that the small sample size is a limitation of the study, but said that she and her associates hope to conduct a larger-scale analysis.
She reported having no financial disclosures.
REPORTING FROM SPD 2018
Key clinical point: Sun safety initiatives should target prepubertal patients before they engage in risky behaviors.
Major finding: One in five adolescents indicated that they felt pressure to have tan skin and were likely to engage in suntanning (P less than .001).
Study details: A survey of 50 patients aged 12-19 years.
Disclosures: Dr. Patel reported having no financial disclosures.
Title X and proposed changes: Take action now
The facts
Title X, a bill originally passed in 1970 under President Nixon, is the only federal grant program dedicated to providing family planning services as well as other preventive health care to primarily low-income patients. It is estimated that 70% of patients using Title X services are below the federal poverty level and more than 60% are uninsured or underinsured.1
In 2015 alone, Title X clinics served 3.8 million women, preventing 822,300 unintended pregnancies and 277,800 abortions.2 These clinics provide comprehensive family planning services including information, counseling, and referrals for abortion services. Title X clinics do not use the funding to provide abortion care, and no federal funding from Title X has ever been used to pay for abortions.

Proposed rule changes
The Trump Administration has proposed several new rules for Title X grant recipients.
Here are the main changes3:
- There must be a “financial and physical” separation between a clinic that is a Title X grant recipient and a facility where “abortion is a method of family planning.” This would prevent health centers that receive Title X funding from providing abortions at the same facility. This rule would predominantly affect health centers like Planned Parenthood. Although these clinics already have a financial separation from abortion care, there would not be a physical one in most situations and these clinics would lose Title X funding or be forced to stop providing abortion services.
- Providers who work at a clinic that receives Title X funding but provides abortions at a completely different facility may be ineligible for ongoing Title X grant money. In the new changes, “funds may not be used…to support the separate abortion business of Title X grant subrecipient.” The changes also propose to “protect Title X providers” from choosing between the health of their patients and their consciences. It plans to do this by removing the requirement to provide abortion counseling and referral and allows “non-directive” counseling.
- There would also be a requirement to encourage more parental involvement in minors’ decision making. While clinics already discuss parental involvement, the change would seek to increase the encouragement to young patients to involve parents. Most young patients do involve a parent or guardian in their care; however, many Title X clinics serve young patients who seek care confidentially. Patients seek confidential care due to a multitude of reasons, including history of abuse, lack of trust, and intimate partner violence.
- “A Title X project may not perform, promote, refer for, or support abortion as a method of family planning.” Although the rule does not prevent providers from discussing abortions, clinicians could offer little guidance if a patient opts for an abortion. Providers can give a list of “qualified, comprehensive health service providers” but may not disclose which, if any, of the providers perform abortions.
Take action
Title X provides important health care services to low-income, uninsured, and underinsured patients. These proposals put access to comprehensive health care for vulnerable populations at risk. Medical organizations including the American Medical Association and American College of Obstetricians and Gynecologists have made statements against the proposed changes to Title X. As ObGyns, we need to ensure our patients are fully informed and have access to all family planning and preventive health services.
Call or email your local representative and tell them you oppose the changes to Title X. Find your representatives here.
Follow ACOG’s Action Center on protecting Title X, which includes a flyer for your waiting room.
Send a message to the Health and Human Services Secretary. Submit a formal comment through July 31, 2018, on the Federal Registrar website expressing your thoughts with these proposed changes.
- Title X: Helping ensure access to high-quality care. National family planning website. https://www.nationalfamilyplanning.org/document.doc?id=514. Accessed July 25, 2018.
- Publicly Funded Contraceptive Services at U.S. Clinics, 2015. Guttmacher website. https://www.guttmacher.org/article/2018/06/domestic-gag-rule-and-more-administrations-proposed-changes-title-x. Accessed July 25, 2018.
- Compliance with statutory program integrity requirements. Federal register website. https://www.federalregister.gov/documents/2018/06/01/2018-11673/compliance-with-statutory-program-integrity-requirements. Accessed July 25, 2018.
The facts
Title X, a bill originally passed in 1970 under President Nixon, is the only federal grant program dedicated to providing family planning services as well as other preventive health care to primarily low-income patients. It is estimated that 70% of patients using Title X services are below the federal poverty level and more than 60% are uninsured or underinsured.1
In 2015 alone, Title X clinics served 3.8 million women, preventing 822,300 unintended pregnancies and 277,800 abortions.2 These clinics provide comprehensive family planning services including information, counseling, and referrals for abortion services. Title X clinics do not use the funding to provide abortion care, and no federal funding from Title X has ever been used to pay for abortions.

Proposed rule changes
The Trump Administration has proposed several new rules for Title X grant recipients.
Here are the main changes3:
- There must be a “financial and physical” separation between a clinic that is a Title X grant recipient and a facility where “abortion is a method of family planning.” This would prevent health centers that receive Title X funding from providing abortions at the same facility. This rule would predominantly affect health centers like Planned Parenthood. Although these clinics already have a financial separation from abortion care, there would not be a physical one in most situations and these clinics would lose Title X funding or be forced to stop providing abortion services.
- Providers who work at a clinic that receives Title X funding but provides abortions at a completely different facility may be ineligible for ongoing Title X grant money. In the new changes, “funds may not be used…to support the separate abortion business of Title X grant subrecipient.” The changes also propose to “protect Title X providers” from choosing between the health of their patients and their consciences. It plans to do this by removing the requirement to provide abortion counseling and referral and allows “non-directive” counseling.
- There would also be a requirement to encourage more parental involvement in minors’ decision making. While clinics already discuss parental involvement, the change would seek to increase the encouragement to young patients to involve parents. Most young patients do involve a parent or guardian in their care; however, many Title X clinics serve young patients who seek care confidentially. Patients seek confidential care due to a multitude of reasons, including history of abuse, lack of trust, and intimate partner violence.
- “A Title X project may not perform, promote, refer for, or support abortion as a method of family planning.” Although the rule does not prevent providers from discussing abortions, clinicians could offer little guidance if a patient opts for an abortion. Providers can give a list of “qualified, comprehensive health service providers” but may not disclose which, if any, of the providers perform abortions.
Take action
Title X provides important health care services to low-income, uninsured, and underinsured patients. These proposals put access to comprehensive health care for vulnerable populations at risk. Medical organizations including the American Medical Association and American College of Obstetricians and Gynecologists have made statements against the proposed changes to Title X. As ObGyns, we need to ensure our patients are fully informed and have access to all family planning and preventive health services.
Call or email your local representative and tell them you oppose the changes to Title X. Find your representatives here.
Follow ACOG’s Action Center on protecting Title X, which includes a flyer for your waiting room.
Send a message to the Health and Human Services Secretary. Submit a formal comment through July 31, 2018, on the Federal Registrar website expressing your thoughts with these proposed changes.
The facts
Title X, a bill originally passed in 1970 under President Nixon, is the only federal grant program dedicated to providing family planning services as well as other preventive health care to primarily low-income patients. It is estimated that 70% of patients using Title X services are below the federal poverty level and more than 60% are uninsured or underinsured.1
In 2015 alone, Title X clinics served 3.8 million women, preventing 822,300 unintended pregnancies and 277,800 abortions.2 These clinics provide comprehensive family planning services including information, counseling, and referrals for abortion services. Title X clinics do not use the funding to provide abortion care, and no federal funding from Title X has ever been used to pay for abortions.

Proposed rule changes
The Trump Administration has proposed several new rules for Title X grant recipients.
Here are the main changes3:
- There must be a “financial and physical” separation between a clinic that is a Title X grant recipient and a facility where “abortion is a method of family planning.” This would prevent health centers that receive Title X funding from providing abortions at the same facility. This rule would predominantly affect health centers like Planned Parenthood. Although these clinics already have a financial separation from abortion care, there would not be a physical one in most situations and these clinics would lose Title X funding or be forced to stop providing abortion services.
- Providers who work at a clinic that receives Title X funding but provides abortions at a completely different facility may be ineligible for ongoing Title X grant money. In the new changes, “funds may not be used…to support the separate abortion business of Title X grant subrecipient.” The changes also propose to “protect Title X providers” from choosing between the health of their patients and their consciences. It plans to do this by removing the requirement to provide abortion counseling and referral and allows “non-directive” counseling.
- There would also be a requirement to encourage more parental involvement in minors’ decision making. While clinics already discuss parental involvement, the change would seek to increase the encouragement to young patients to involve parents. Most young patients do involve a parent or guardian in their care; however, many Title X clinics serve young patients who seek care confidentially. Patients seek confidential care due to a multitude of reasons, including history of abuse, lack of trust, and intimate partner violence.
- “A Title X project may not perform, promote, refer for, or support abortion as a method of family planning.” Although the rule does not prevent providers from discussing abortions, clinicians could offer little guidance if a patient opts for an abortion. Providers can give a list of “qualified, comprehensive health service providers” but may not disclose which, if any, of the providers perform abortions.
Take action
Title X provides important health care services to low-income, uninsured, and underinsured patients. These proposals put access to comprehensive health care for vulnerable populations at risk. Medical organizations including the American Medical Association and American College of Obstetricians and Gynecologists have made statements against the proposed changes to Title X. As ObGyns, we need to ensure our patients are fully informed and have access to all family planning and preventive health services.
Call or email your local representative and tell them you oppose the changes to Title X. Find your representatives here.
Follow ACOG’s Action Center on protecting Title X, which includes a flyer for your waiting room.
Send a message to the Health and Human Services Secretary. Submit a formal comment through July 31, 2018, on the Federal Registrar website expressing your thoughts with these proposed changes.
- Title X: Helping ensure access to high-quality care. National family planning website. https://www.nationalfamilyplanning.org/document.doc?id=514. Accessed July 25, 2018.
- Publicly Funded Contraceptive Services at U.S. Clinics, 2015. Guttmacher website. https://www.guttmacher.org/article/2018/06/domestic-gag-rule-and-more-administrations-proposed-changes-title-x. Accessed July 25, 2018.
- Compliance with statutory program integrity requirements. Federal register website. https://www.federalregister.gov/documents/2018/06/01/2018-11673/compliance-with-statutory-program-integrity-requirements. Accessed July 25, 2018.
- Title X: Helping ensure access to high-quality care. National family planning website. https://www.nationalfamilyplanning.org/document.doc?id=514. Accessed July 25, 2018.
- Publicly Funded Contraceptive Services at U.S. Clinics, 2015. Guttmacher website. https://www.guttmacher.org/article/2018/06/domestic-gag-rule-and-more-administrations-proposed-changes-title-x. Accessed July 25, 2018.
- Compliance with statutory program integrity requirements. Federal register website. https://www.federalregister.gov/documents/2018/06/01/2018-11673/compliance-with-statutory-program-integrity-requirements. Accessed July 25, 2018.