User login
Plasma transfusion during air transport can reduce mortality
Receiving a plasma transfusion during emergency air transport can improve survival for trauma patients, according to a study published in NEJM.
Trauma patients with severe bleeding had a significant decrease in 30-day mortality when they received a plasma transfusion while being airlifted to a hospital.
Transfusion-related reactions and allergic reactions were more common among plasma recipients than patients who only received standard care.
However, this difference was not significant, and most of these reactions were considered minor.
“These results have the power to significantly alter trauma resuscitation, and their importance to the trauma community cannot be overstated,” said study author Jason Sperry, MD, of the University of Pittsburgh School of Medicine in Pennsylvania.
“This is the first trial in a quarter century to have the potential to alter prehospital care so considerably.”
Patients and intervention
This trial, known as PAMPer (Prehospital Air Medical Plasma), was a phase 3, randomized study enrolling 501 trauma patients at risk of hemorrhagic shock.
Most patients were male (72.7%), and most had suffered blunt trauma (82.4%). About half of patients (51.1%) had prehospital intubation, and more than a third (34.7%) received a prehospital transfusion of red blood cells.
Air medical bases participating in this study were randomized to administer plasma or standard care to eligible patients for 1-month intervals. When the air transport teams were in their plasma interval, they’d begin administering 2 units of thawed plasma to a patient as soon as trial eligibility was confirmed.
If the 2 units were completed during the flight, the team would revert to standard care. If the transfusions weren’t completed, the plasma would continue to be administered when the patient arrived at the trauma center.
The teams administered the assigned treatment 99% of the time (496/501).
In the plasma group, there were 205 patients (89.1%) who received 2 units of plasma, 21 (9.1%) who received 1 unit, and 4 patients (1.7%) who did not receive plasma due to logistical challenges.
In 84.4% of the patients, the plasma infusion was completed during air transport. The remaining patients completed their plasma transfusions at the trauma center.
There was 1 patient (0.4%) in the standard-care group who received plasma before transport began.
Primary outcome
The study’s primary outcome was 30-day mortality. Ninety-six percent of patients (n=481) had data for this outcome—220 patients in the plasma group and 261 in the standard-care group.
Thirty-day mortality was significantly lower in the plasma group than the standard-care group—23.2% and 33.0%, respectively (P=0.03).
In an adjusted analysis, the administration of prehospital plasma was associated with a 39% lower risk for 30-day mortality than standard care (adjusted odds ratio, 0.61; P=0.02).
Secondary outcomes
Initially, there were significant differences between the plasma (n=230) and standard-care groups (n=271) when it came to:
- Mortality at 24 hours—13.9% and 22.1%, respectively (P=0.02)
- In-hospital mortality—22.2% and 32.5%, respectively (P=0.01)
- Median volume of blood components transfused in the first 24 hours—3 and 4 units, respectively (P=0.02)
- Median volume of red cells transfused in the first 24 hours—3 and 4 units, respectively (P=0.03).
- Median prothrombin-time ratio at first blood sampling—1.2 and 1.3, respectively (P<0.001).
When the researchers adjusted P values for multiple comparisons, the between-group difference in prothrombin-time ratio remained significant (P<0.001).
However, the differences in 24-hour mortality (P=0.55), in-hospital mortality (P=0.33), blood components transfused (P=0.41), and red cells transfused (P=0.69) did not retain significance.
Likewise, there were no significant between-group differences (in adjusted or unadjusted analyses) when it came to multi-organ failure, acute lung injury/acute respiratory distress syndrome, nosocomial infections, or allergic/transfusion-related reactions.
There were 10 adverse events (AEs) considered related to the trial regimen. In the standard-care group, the 4 AEs were sepsis (a serious AE), adult respiratory distress syndrome (a serious AE), fever, and pain.
In the plasma group, the 6 AEs were 2 allergic reactions, 1 case of anaphylaxis, 1 case of hypotension, 1 case of urticaria, and 1 transfusion-related reaction (a serious AE).
Receiving a plasma transfusion during emergency air transport can improve survival for trauma patients, according to a study published in NEJM.
Trauma patients with severe bleeding had a significant decrease in 30-day mortality when they received a plasma transfusion while being airlifted to a hospital.
Transfusion-related reactions and allergic reactions were more common among plasma recipients than patients who only received standard care.
However, this difference was not significant, and most of these reactions were considered minor.
“These results have the power to significantly alter trauma resuscitation, and their importance to the trauma community cannot be overstated,” said study author Jason Sperry, MD, of the University of Pittsburgh School of Medicine in Pennsylvania.
“This is the first trial in a quarter century to have the potential to alter prehospital care so considerably.”
Patients and intervention
This trial, known as PAMPer (Prehospital Air Medical Plasma), was a phase 3, randomized study enrolling 501 trauma patients at risk of hemorrhagic shock.
Most patients were male (72.7%), and most had suffered blunt trauma (82.4%). About half of patients (51.1%) had prehospital intubation, and more than a third (34.7%) received a prehospital transfusion of red blood cells.
Air medical bases participating in this study were randomized to administer plasma or standard care to eligible patients for 1-month intervals. When the air transport teams were in their plasma interval, they’d begin administering 2 units of thawed plasma to a patient as soon as trial eligibility was confirmed.
If the 2 units were completed during the flight, the team would revert to standard care. If the transfusions weren’t completed, the plasma would continue to be administered when the patient arrived at the trauma center.
The teams administered the assigned treatment 99% of the time (496/501).
In the plasma group, there were 205 patients (89.1%) who received 2 units of plasma, 21 (9.1%) who received 1 unit, and 4 patients (1.7%) who did not receive plasma due to logistical challenges.
In 84.4% of the patients, the plasma infusion was completed during air transport. The remaining patients completed their plasma transfusions at the trauma center.
There was 1 patient (0.4%) in the standard-care group who received plasma before transport began.
Primary outcome
The study’s primary outcome was 30-day mortality. Ninety-six percent of patients (n=481) had data for this outcome—220 patients in the plasma group and 261 in the standard-care group.
Thirty-day mortality was significantly lower in the plasma group than the standard-care group—23.2% and 33.0%, respectively (P=0.03).
In an adjusted analysis, the administration of prehospital plasma was associated with a 39% lower risk for 30-day mortality than standard care (adjusted odds ratio, 0.61; P=0.02).
Secondary outcomes
Initially, there were significant differences between the plasma (n=230) and standard-care groups (n=271) when it came to:
- Mortality at 24 hours—13.9% and 22.1%, respectively (P=0.02)
- In-hospital mortality—22.2% and 32.5%, respectively (P=0.01)
- Median volume of blood components transfused in the first 24 hours—3 and 4 units, respectively (P=0.02)
- Median volume of red cells transfused in the first 24 hours—3 and 4 units, respectively (P=0.03).
- Median prothrombin-time ratio at first blood sampling—1.2 and 1.3, respectively (P<0.001).
When the researchers adjusted P values for multiple comparisons, the between-group difference in prothrombin-time ratio remained significant (P<0.001).
However, the differences in 24-hour mortality (P=0.55), in-hospital mortality (P=0.33), blood components transfused (P=0.41), and red cells transfused (P=0.69) did not retain significance.
Likewise, there were no significant between-group differences (in adjusted or unadjusted analyses) when it came to multi-organ failure, acute lung injury/acute respiratory distress syndrome, nosocomial infections, or allergic/transfusion-related reactions.
There were 10 adverse events (AEs) considered related to the trial regimen. In the standard-care group, the 4 AEs were sepsis (a serious AE), adult respiratory distress syndrome (a serious AE), fever, and pain.
In the plasma group, the 6 AEs were 2 allergic reactions, 1 case of anaphylaxis, 1 case of hypotension, 1 case of urticaria, and 1 transfusion-related reaction (a serious AE).
Receiving a plasma transfusion during emergency air transport can improve survival for trauma patients, according to a study published in NEJM.
Trauma patients with severe bleeding had a significant decrease in 30-day mortality when they received a plasma transfusion while being airlifted to a hospital.
Transfusion-related reactions and allergic reactions were more common among plasma recipients than patients who only received standard care.
However, this difference was not significant, and most of these reactions were considered minor.
“These results have the power to significantly alter trauma resuscitation, and their importance to the trauma community cannot be overstated,” said study author Jason Sperry, MD, of the University of Pittsburgh School of Medicine in Pennsylvania.
“This is the first trial in a quarter century to have the potential to alter prehospital care so considerably.”
Patients and intervention
This trial, known as PAMPer (Prehospital Air Medical Plasma), was a phase 3, randomized study enrolling 501 trauma patients at risk of hemorrhagic shock.
Most patients were male (72.7%), and most had suffered blunt trauma (82.4%). About half of patients (51.1%) had prehospital intubation, and more than a third (34.7%) received a prehospital transfusion of red blood cells.
Air medical bases participating in this study were randomized to administer plasma or standard care to eligible patients for 1-month intervals. When the air transport teams were in their plasma interval, they’d begin administering 2 units of thawed plasma to a patient as soon as trial eligibility was confirmed.
If the 2 units were completed during the flight, the team would revert to standard care. If the transfusions weren’t completed, the plasma would continue to be administered when the patient arrived at the trauma center.
The teams administered the assigned treatment 99% of the time (496/501).
In the plasma group, there were 205 patients (89.1%) who received 2 units of plasma, 21 (9.1%) who received 1 unit, and 4 patients (1.7%) who did not receive plasma due to logistical challenges.
In 84.4% of the patients, the plasma infusion was completed during air transport. The remaining patients completed their plasma transfusions at the trauma center.
There was 1 patient (0.4%) in the standard-care group who received plasma before transport began.
Primary outcome
The study’s primary outcome was 30-day mortality. Ninety-six percent of patients (n=481) had data for this outcome—220 patients in the plasma group and 261 in the standard-care group.
Thirty-day mortality was significantly lower in the plasma group than the standard-care group—23.2% and 33.0%, respectively (P=0.03).
In an adjusted analysis, the administration of prehospital plasma was associated with a 39% lower risk for 30-day mortality than standard care (adjusted odds ratio, 0.61; P=0.02).
Secondary outcomes
Initially, there were significant differences between the plasma (n=230) and standard-care groups (n=271) when it came to:
- Mortality at 24 hours—13.9% and 22.1%, respectively (P=0.02)
- In-hospital mortality—22.2% and 32.5%, respectively (P=0.01)
- Median volume of blood components transfused in the first 24 hours—3 and 4 units, respectively (P=0.02)
- Median volume of red cells transfused in the first 24 hours—3 and 4 units, respectively (P=0.03).
- Median prothrombin-time ratio at first blood sampling—1.2 and 1.3, respectively (P<0.001).
When the researchers adjusted P values for multiple comparisons, the between-group difference in prothrombin-time ratio remained significant (P<0.001).
However, the differences in 24-hour mortality (P=0.55), in-hospital mortality (P=0.33), blood components transfused (P=0.41), and red cells transfused (P=0.69) did not retain significance.
Likewise, there were no significant between-group differences (in adjusted or unadjusted analyses) when it came to multi-organ failure, acute lung injury/acute respiratory distress syndrome, nosocomial infections, or allergic/transfusion-related reactions.
There were 10 adverse events (AEs) considered related to the trial regimen. In the standard-care group, the 4 AEs were sepsis (a serious AE), adult respiratory distress syndrome (a serious AE), fever, and pain.
In the plasma group, the 6 AEs were 2 allergic reactions, 1 case of anaphylaxis, 1 case of hypotension, 1 case of urticaria, and 1 transfusion-related reaction (a serious AE).
Drug receives fast track designation for WM
The US Food and Drug Administration (FDA) has granted fast track designation to zanubrutinib for the treatment of Waldenström’s macroglobulinemia (WM).
Zanubrutinib (BGB-3111) is a BTK inhibitor being developed by BeiGene to treat various B-cell malignancies.
BeiGene is preparing to submit to the FDA, in the first half of 2019, a new drug application seeking accelerated approval of zanubrutinib for patients with WM.
The application will be supported by results from a phase 1 study. Results from this trial were presented at the 14th International Conference on Malignant Lymphoma (14-ICML) last year.
Researchers are also evaluating zanubrutinib in phase 2 (NCT03332173) and phase 3 (NCT03053440) trials of WM patients. In the phase 3 trial, researchers are comparing zanubrutinib to the BTK inhibitor ibrutinib.
Phase 1 results
As of March 31, 2017, 48 WM patients were enrolled in the phase 1 study. Thirty-eight patients had relapsed/refractory disease, and 10 patients were treatment-naïve.
There was a dose-escalation phase and a dose-expansion phase. The dose-expansion phase included doses of 160 mg twice a day or 320 mg once a day.
The most common (>10%) adverse events, (AEs) of any attribution were petechiae/purpura/contusion (35%), upper respiratory tract infection (31%), constipation (25%), diarrhea (19%), epistaxis (19%), nausea (17%), cough (15%), anemia (15%), headache (15%), neutropenia (13%), and rash (13%).
Most of these events were grade 1 or 2 in severity. The exceptions were grade 3/4 anemia and neutropenia (8% each) as well as grade 3/4 diarrhea and headache (2% each).
Five serious AEs were considered possibly related to zanubrutinib—1 case each of hemothorax, atrial fibrillation, colitis, febrile neutropenia, and headache. Three AEs led to treatment discontinuation—1 case each of bronchiectasis, prostate adenocarcinoma, and adenocarcinoma of pylorus.
At the time of the data cutoff, 42 patients were evaluable for response. At a median follow-up of 12.3 months (range, 4.4 to 30.5 months), the overall response rate was 90% (38/42).
The major response rate was 76% (32/42), with very good partial responses in 43% (18/42) of patients and partial responses in 33% (14/42) of patients. There were no complete responses and 2 cases of disease progression.
About fast track designation
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
The US Food and Drug Administration (FDA) has granted fast track designation to zanubrutinib for the treatment of Waldenström’s macroglobulinemia (WM).
Zanubrutinib (BGB-3111) is a BTK inhibitor being developed by BeiGene to treat various B-cell malignancies.
BeiGene is preparing to submit to the FDA, in the first half of 2019, a new drug application seeking accelerated approval of zanubrutinib for patients with WM.
The application will be supported by results from a phase 1 study. Results from this trial were presented at the 14th International Conference on Malignant Lymphoma (14-ICML) last year.
Researchers are also evaluating zanubrutinib in phase 2 (NCT03332173) and phase 3 (NCT03053440) trials of WM patients. In the phase 3 trial, researchers are comparing zanubrutinib to the BTK inhibitor ibrutinib.
Phase 1 results
As of March 31, 2017, 48 WM patients were enrolled in the phase 1 study. Thirty-eight patients had relapsed/refractory disease, and 10 patients were treatment-naïve.
There was a dose-escalation phase and a dose-expansion phase. The dose-expansion phase included doses of 160 mg twice a day or 320 mg once a day.
The most common (>10%) adverse events, (AEs) of any attribution were petechiae/purpura/contusion (35%), upper respiratory tract infection (31%), constipation (25%), diarrhea (19%), epistaxis (19%), nausea (17%), cough (15%), anemia (15%), headache (15%), neutropenia (13%), and rash (13%).
Most of these events were grade 1 or 2 in severity. The exceptions were grade 3/4 anemia and neutropenia (8% each) as well as grade 3/4 diarrhea and headache (2% each).
Five serious AEs were considered possibly related to zanubrutinib—1 case each of hemothorax, atrial fibrillation, colitis, febrile neutropenia, and headache. Three AEs led to treatment discontinuation—1 case each of bronchiectasis, prostate adenocarcinoma, and adenocarcinoma of pylorus.
At the time of the data cutoff, 42 patients were evaluable for response. At a median follow-up of 12.3 months (range, 4.4 to 30.5 months), the overall response rate was 90% (38/42).
The major response rate was 76% (32/42), with very good partial responses in 43% (18/42) of patients and partial responses in 33% (14/42) of patients. There were no complete responses and 2 cases of disease progression.
About fast track designation
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
The US Food and Drug Administration (FDA) has granted fast track designation to zanubrutinib for the treatment of Waldenström’s macroglobulinemia (WM).
Zanubrutinib (BGB-3111) is a BTK inhibitor being developed by BeiGene to treat various B-cell malignancies.
BeiGene is preparing to submit to the FDA, in the first half of 2019, a new drug application seeking accelerated approval of zanubrutinib for patients with WM.
The application will be supported by results from a phase 1 study. Results from this trial were presented at the 14th International Conference on Malignant Lymphoma (14-ICML) last year.
Researchers are also evaluating zanubrutinib in phase 2 (NCT03332173) and phase 3 (NCT03053440) trials of WM patients. In the phase 3 trial, researchers are comparing zanubrutinib to the BTK inhibitor ibrutinib.
Phase 1 results
As of March 31, 2017, 48 WM patients were enrolled in the phase 1 study. Thirty-eight patients had relapsed/refractory disease, and 10 patients were treatment-naïve.
There was a dose-escalation phase and a dose-expansion phase. The dose-expansion phase included doses of 160 mg twice a day or 320 mg once a day.
The most common (>10%) adverse events, (AEs) of any attribution were petechiae/purpura/contusion (35%), upper respiratory tract infection (31%), constipation (25%), diarrhea (19%), epistaxis (19%), nausea (17%), cough (15%), anemia (15%), headache (15%), neutropenia (13%), and rash (13%).
Most of these events were grade 1 or 2 in severity. The exceptions were grade 3/4 anemia and neutropenia (8% each) as well as grade 3/4 diarrhea and headache (2% each).
Five serious AEs were considered possibly related to zanubrutinib—1 case each of hemothorax, atrial fibrillation, colitis, febrile neutropenia, and headache. Three AEs led to treatment discontinuation—1 case each of bronchiectasis, prostate adenocarcinoma, and adenocarcinoma of pylorus.
At the time of the data cutoff, 42 patients were evaluable for response. At a median follow-up of 12.3 months (range, 4.4 to 30.5 months), the overall response rate was 90% (38/42).
The major response rate was 76% (32/42), with very good partial responses in 43% (18/42) of patients and partial responses in 33% (14/42) of patients. There were no complete responses and 2 cases of disease progression.
About fast track designation
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
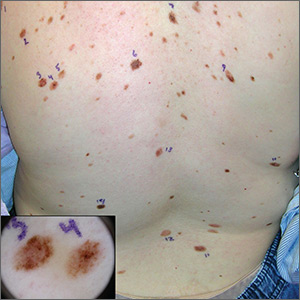
Atypical pigmented lesions on back
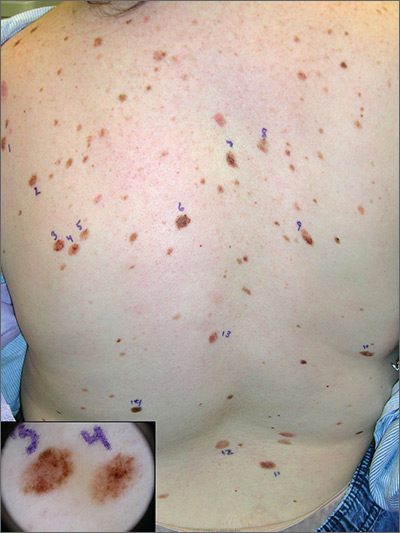
The FP recognized that this patient had multiple dysplastic nevi (DN) and was concerned about a possible melanoma.
Although this patient did not have the familial atypical mole and melanoma (FAMM) syndrome, she was at higher risk of developing a melanoma based on the numerous DN that were so visibly apparent on her trunk. (A diagnosis of FAMM requires the occurrence of melanoma in one or more first- or second-degree relatives.)
Total body photography is the current state-of-the-art technology for monitoring patients with multiple atypical moles at higher risk for skin cancer. Small changes in DN and new nevi can be seen best with this technology. Individual flat lesions also can be monitored with dermoscopy over 3 to 4 months.
It is not recommended, however, to follow a suspicious raised lesion over time with dermoscopy and/or photography because a raised lesion might be a nodular melanoma and a delay of diagnosis of over 3 months may worsen the prognosis.
In this case, the patient was happy to be referred to a dermatologist with total body photography. Fortunately, the patient reported back to her FP that the dermatologist did not detect any melanomas, and 2 biopsies that were performed came back as DN only.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized that this patient had multiple dysplastic nevi (DN) and was concerned about a possible melanoma.
Although this patient did not have the familial atypical mole and melanoma (FAMM) syndrome, she was at higher risk of developing a melanoma based on the numerous DN that were so visibly apparent on her trunk. (A diagnosis of FAMM requires the occurrence of melanoma in one or more first- or second-degree relatives.)
Total body photography is the current state-of-the-art technology for monitoring patients with multiple atypical moles at higher risk for skin cancer. Small changes in DN and new nevi can be seen best with this technology. Individual flat lesions also can be monitored with dermoscopy over 3 to 4 months.
It is not recommended, however, to follow a suspicious raised lesion over time with dermoscopy and/or photography because a raised lesion might be a nodular melanoma and a delay of diagnosis of over 3 months may worsen the prognosis.
In this case, the patient was happy to be referred to a dermatologist with total body photography. Fortunately, the patient reported back to her FP that the dermatologist did not detect any melanomas, and 2 biopsies that were performed came back as DN only.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized that this patient had multiple dysplastic nevi (DN) and was concerned about a possible melanoma.
Although this patient did not have the familial atypical mole and melanoma (FAMM) syndrome, she was at higher risk of developing a melanoma based on the numerous DN that were so visibly apparent on her trunk. (A diagnosis of FAMM requires the occurrence of melanoma in one or more first- or second-degree relatives.)
Total body photography is the current state-of-the-art technology for monitoring patients with multiple atypical moles at higher risk for skin cancer. Small changes in DN and new nevi can be seen best with this technology. Individual flat lesions also can be monitored with dermoscopy over 3 to 4 months.
It is not recommended, however, to follow a suspicious raised lesion over time with dermoscopy and/or photography because a raised lesion might be a nodular melanoma and a delay of diagnosis of over 3 months may worsen the prognosis.
In this case, the patient was happy to be referred to a dermatologist with total body photography. Fortunately, the patient reported back to her FP that the dermatologist did not detect any melanomas, and 2 biopsies that were performed came back as DN only.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Guideline: PFO closure best bet for recurrent stroke prevention
In patients younger than 60 years, patent foramen ovale (PFO) closure plus antiplatelet therapy is a better strategy for preventing recurrent ischemic stroke than indefinite anticoagulant therapy without closure, according to a recommendation from an expert panel after a systematic literature review.
When compared with the alternative strategy of indefinite anticoagulant therapy for those patients “open to all options,” the recommendation was labeled only “weak” on the basis of the GRADE classification system used by the panel to rate treatment strategies. However, the recommendation for PFO closure plus antiplatelets becomes “strong” if anticoagulation therapy is contraindicated or declined as a treatment option.
The new recommendations were largely driven by three multicenter studies of that were published simultaneously last year. (N Engl J Med. 2017 Sep 14;377:1011-21; 1022-32; 1033-42). However, these and previous studies did not provide adequate data on risks and benefits for all options, which the authors emphasized in guidelines meant to help clinicians weigh options.
Weak means that clinicians “should recognize that different choices will be appropriate for different patients,” explained Frederick A. Spencer, MD, professor, division of cardiology, McMaster University, Hamilton, Ontario. Quoting from GRADE definitions, Dr. Spencer, who was the guideline panel chair, explained that weak recommendations are the product of uncertainties that may affect choice in specific individuals.
In addition to the recommendation for PFO closure plus antiplatelets as either a weak or strong recommendation in relation to the availability of anticoagulation therapy, the guidelines offered one other recommendation. For patients contraindicated or unwilling to undergo PFO closure, anticoagulation therapy was preferred over antiplatelet therapy. This was also labeled a “weak” recommendation.
These guidelines were the product of collaboration between the BMJ and the nonprofit MAGIC (Making GRADE the Irresistible Choice) project. In addition to providing a methodology and structure to organize guideline deliberations among the participating international experts, MAGIC facilitated communication and collaboration through Web-based technology.
This approach was particularly useful for the detailed analyses and debate about relative risks and benefits of treatment alternatives in which direct comparative data were limited. For example, the authors noted that the only randomized comparison of PFO closure to anticoagulation involved just 353 patients. Indirect evidence was therefore added to improve the precision of the estimated benefits and risks. The panel selected PFO closure plus antiplatelet therapy over anticoagulation therapy, because “the most serious complications of PFO closure are usually short term, whereas anticoagulation imposes a long-term burden and increased risk of major bleeding.”
The estimated rate of adverse events associated with PFO is 3.6%, according to data cited in the expert guidelines. Atrial fibrillation accounts for about half of these events. At 5 years, the absolute reduction in stroke from PFO closure versus no intervention is an estimated 8.7%. The panel assumed that most patients would place greater weight on stroke prevention than the largely reversible adverse events associated with PFO closure.
The newly published guidelines include a detailed review of the available data to allow clinicians to counsel patients appropriately. Dr. Spencer cautioned that no recommendation, strong or weak, should be uniformly applied without considering individual patient factors and preferences.
According to Christopher J. White, MD, system chairman for cardiology, Ochsner Medical Center, New Orleans, the guideline committee had no choice but to label PFO closure plus antiplatelet therapy as a “weak” recommendation within the requirements of GRADE. However, Dr. White believes that it should be considered the best option in most patients despite this terminology.
“What I tell patients is that this is a one-and-done procedure,” Dr. White explained. “Without closure, patients must remain on anticoagulation therapy, which involves refilling prescriptions, remaining compliant with daily therapy for life, and accepting an increased risk of bleeding. Once the PFO is closed and endothelialized, no additional anticoagulation treatment is needed.”
There are adverse events associated with PFO closure, but Dr. White called these uncommon and more acceptable than the risks of indefinite anticoagulation therapy, particularly if patients are not fully compliant.
“When I explain the options to patients, most will opt for PFO closure,” Dr. White said.
He also emphasized that the advantage of PFO closure accrues over time.
“When these patients have a stroke at a relatively young age, they may need to be on anticoagulation for decades. Once the PFO is closed, you are saving the patient the burden of lifelong compliance to anticoagulation therapy as well as the costs,” he added. Although the guidelines compared relative benefits of a given strategy over 5 years, the advantage of PFO closure over anticoagulation will keep increasing beyond this point.
Source: Kuipers et al. BMJ 2018 Jul 25;362:K2515
In patients younger than 60 years, patent foramen ovale (PFO) closure plus antiplatelet therapy is a better strategy for preventing recurrent ischemic stroke than indefinite anticoagulant therapy without closure, according to a recommendation from an expert panel after a systematic literature review.
When compared with the alternative strategy of indefinite anticoagulant therapy for those patients “open to all options,” the recommendation was labeled only “weak” on the basis of the GRADE classification system used by the panel to rate treatment strategies. However, the recommendation for PFO closure plus antiplatelets becomes “strong” if anticoagulation therapy is contraindicated or declined as a treatment option.
The new recommendations were largely driven by three multicenter studies of that were published simultaneously last year. (N Engl J Med. 2017 Sep 14;377:1011-21; 1022-32; 1033-42). However, these and previous studies did not provide adequate data on risks and benefits for all options, which the authors emphasized in guidelines meant to help clinicians weigh options.
Weak means that clinicians “should recognize that different choices will be appropriate for different patients,” explained Frederick A. Spencer, MD, professor, division of cardiology, McMaster University, Hamilton, Ontario. Quoting from GRADE definitions, Dr. Spencer, who was the guideline panel chair, explained that weak recommendations are the product of uncertainties that may affect choice in specific individuals.
In addition to the recommendation for PFO closure plus antiplatelets as either a weak or strong recommendation in relation to the availability of anticoagulation therapy, the guidelines offered one other recommendation. For patients contraindicated or unwilling to undergo PFO closure, anticoagulation therapy was preferred over antiplatelet therapy. This was also labeled a “weak” recommendation.
These guidelines were the product of collaboration between the BMJ and the nonprofit MAGIC (Making GRADE the Irresistible Choice) project. In addition to providing a methodology and structure to organize guideline deliberations among the participating international experts, MAGIC facilitated communication and collaboration through Web-based technology.
This approach was particularly useful for the detailed analyses and debate about relative risks and benefits of treatment alternatives in which direct comparative data were limited. For example, the authors noted that the only randomized comparison of PFO closure to anticoagulation involved just 353 patients. Indirect evidence was therefore added to improve the precision of the estimated benefits and risks. The panel selected PFO closure plus antiplatelet therapy over anticoagulation therapy, because “the most serious complications of PFO closure are usually short term, whereas anticoagulation imposes a long-term burden and increased risk of major bleeding.”
The estimated rate of adverse events associated with PFO is 3.6%, according to data cited in the expert guidelines. Atrial fibrillation accounts for about half of these events. At 5 years, the absolute reduction in stroke from PFO closure versus no intervention is an estimated 8.7%. The panel assumed that most patients would place greater weight on stroke prevention than the largely reversible adverse events associated with PFO closure.
The newly published guidelines include a detailed review of the available data to allow clinicians to counsel patients appropriately. Dr. Spencer cautioned that no recommendation, strong or weak, should be uniformly applied without considering individual patient factors and preferences.
According to Christopher J. White, MD, system chairman for cardiology, Ochsner Medical Center, New Orleans, the guideline committee had no choice but to label PFO closure plus antiplatelet therapy as a “weak” recommendation within the requirements of GRADE. However, Dr. White believes that it should be considered the best option in most patients despite this terminology.
“What I tell patients is that this is a one-and-done procedure,” Dr. White explained. “Without closure, patients must remain on anticoagulation therapy, which involves refilling prescriptions, remaining compliant with daily therapy for life, and accepting an increased risk of bleeding. Once the PFO is closed and endothelialized, no additional anticoagulation treatment is needed.”
There are adverse events associated with PFO closure, but Dr. White called these uncommon and more acceptable than the risks of indefinite anticoagulation therapy, particularly if patients are not fully compliant.
“When I explain the options to patients, most will opt for PFO closure,” Dr. White said.
He also emphasized that the advantage of PFO closure accrues over time.
“When these patients have a stroke at a relatively young age, they may need to be on anticoagulation for decades. Once the PFO is closed, you are saving the patient the burden of lifelong compliance to anticoagulation therapy as well as the costs,” he added. Although the guidelines compared relative benefits of a given strategy over 5 years, the advantage of PFO closure over anticoagulation will keep increasing beyond this point.
Source: Kuipers et al. BMJ 2018 Jul 25;362:K2515
In patients younger than 60 years, patent foramen ovale (PFO) closure plus antiplatelet therapy is a better strategy for preventing recurrent ischemic stroke than indefinite anticoagulant therapy without closure, according to a recommendation from an expert panel after a systematic literature review.
When compared with the alternative strategy of indefinite anticoagulant therapy for those patients “open to all options,” the recommendation was labeled only “weak” on the basis of the GRADE classification system used by the panel to rate treatment strategies. However, the recommendation for PFO closure plus antiplatelets becomes “strong” if anticoagulation therapy is contraindicated or declined as a treatment option.
The new recommendations were largely driven by three multicenter studies of that were published simultaneously last year. (N Engl J Med. 2017 Sep 14;377:1011-21; 1022-32; 1033-42). However, these and previous studies did not provide adequate data on risks and benefits for all options, which the authors emphasized in guidelines meant to help clinicians weigh options.
Weak means that clinicians “should recognize that different choices will be appropriate for different patients,” explained Frederick A. Spencer, MD, professor, division of cardiology, McMaster University, Hamilton, Ontario. Quoting from GRADE definitions, Dr. Spencer, who was the guideline panel chair, explained that weak recommendations are the product of uncertainties that may affect choice in specific individuals.
In addition to the recommendation for PFO closure plus antiplatelets as either a weak or strong recommendation in relation to the availability of anticoagulation therapy, the guidelines offered one other recommendation. For patients contraindicated or unwilling to undergo PFO closure, anticoagulation therapy was preferred over antiplatelet therapy. This was also labeled a “weak” recommendation.
These guidelines were the product of collaboration between the BMJ and the nonprofit MAGIC (Making GRADE the Irresistible Choice) project. In addition to providing a methodology and structure to organize guideline deliberations among the participating international experts, MAGIC facilitated communication and collaboration through Web-based technology.
This approach was particularly useful for the detailed analyses and debate about relative risks and benefits of treatment alternatives in which direct comparative data were limited. For example, the authors noted that the only randomized comparison of PFO closure to anticoagulation involved just 353 patients. Indirect evidence was therefore added to improve the precision of the estimated benefits and risks. The panel selected PFO closure plus antiplatelet therapy over anticoagulation therapy, because “the most serious complications of PFO closure are usually short term, whereas anticoagulation imposes a long-term burden and increased risk of major bleeding.”
The estimated rate of adverse events associated with PFO is 3.6%, according to data cited in the expert guidelines. Atrial fibrillation accounts for about half of these events. At 5 years, the absolute reduction in stroke from PFO closure versus no intervention is an estimated 8.7%. The panel assumed that most patients would place greater weight on stroke prevention than the largely reversible adverse events associated with PFO closure.
The newly published guidelines include a detailed review of the available data to allow clinicians to counsel patients appropriately. Dr. Spencer cautioned that no recommendation, strong or weak, should be uniformly applied without considering individual patient factors and preferences.
According to Christopher J. White, MD, system chairman for cardiology, Ochsner Medical Center, New Orleans, the guideline committee had no choice but to label PFO closure plus antiplatelet therapy as a “weak” recommendation within the requirements of GRADE. However, Dr. White believes that it should be considered the best option in most patients despite this terminology.
“What I tell patients is that this is a one-and-done procedure,” Dr. White explained. “Without closure, patients must remain on anticoagulation therapy, which involves refilling prescriptions, remaining compliant with daily therapy for life, and accepting an increased risk of bleeding. Once the PFO is closed and endothelialized, no additional anticoagulation treatment is needed.”
There are adverse events associated with PFO closure, but Dr. White called these uncommon and more acceptable than the risks of indefinite anticoagulation therapy, particularly if patients are not fully compliant.
“When I explain the options to patients, most will opt for PFO closure,” Dr. White said.
He also emphasized that the advantage of PFO closure accrues over time.
“When these patients have a stroke at a relatively young age, they may need to be on anticoagulation for decades. Once the PFO is closed, you are saving the patient the burden of lifelong compliance to anticoagulation therapy as well as the costs,” he added. Although the guidelines compared relative benefits of a given strategy over 5 years, the advantage of PFO closure over anticoagulation will keep increasing beyond this point.
Source: Kuipers et al. BMJ 2018 Jul 25;362:K2515
FROM BMJ
Prehospital plasma outperforms standard care
Prehospital administration of plasma to trauma patients at risk for hemorrhagic shock improves odds of survival, according to the Prehospital Air Medical Plasma (PAMPer) trial.
Patients who received plasma also had a decreased median prothrombin to time ratio, compared with those who received standard-care resuscitation alone.
Modern trauma patients benefit “from receiving less crystalloid-based therapy and early balanced blood component–based therapy once they arrive at a facility for definitive care,” Jason L. Sperry, MD, of the University of Pittsburgh and his coauthors wrote in the New England Journal of Medicine. These newer strategies aim to mitigate coagulopathy and its associated downstream complications.
Still, “a majority of deaths from traumatic hemorrhage continue to occur in the first hours after arrival at the trauma center, which underscores the importance of the prehospital environment for early interventions that provide benefit,” the investigators noted.
The PAMPer trial (NCT01818427) examined the efficacy and safety of prehospital plasma resuscitation, comparing it with standard-care resuscitation, in severely injured patients. The phase 3, randomized, superiority trial involved 501 trauma patients at risk for hemorrhagic shock who were transported from 27 air medical bases to nine trauma centers. In total, 230 patients received prehospital plasma and standard-care resuscitation, and 271 patients received standard-care alone. Standard-care included infusion of crystalloid fluids in a goal-directed manner. Air medical bases delivered each type of care in 1-month intervals.
Patients were eligible if they exhibited severe hypotension (systolic blood pressure less than 70 mm Hg) or both hypotension (systolic blood pressure less than 90 mm Hg) and tachycardia (greater than 108 beats per minute).
Eligible patients received two units of thawed plasma, which was completely delivered before administration of any other fluids. If infusion of plasma was not complete upon arrival at the trauma center, infusion was completed before any in-hospital fluids were administered. Following preexisting local protocols, 13 of 27 air transport teams carried 2U of red blood cells. If red blood cells were delivered, then this was performed after plasma administration if the patient was still hypotensive or obviously bleeding.
The 30-day mortality rate among all patients was 29.6%. Approximately one in three patients received a prehospital red blood cell transfusion, and nearly 60% underwent surgery within 24 hours of hospital admission.
A greater percentage of patients in the standard-care group were given red blood cell transfusions, compared with those in the plasma group. The standard-care patients also received greater volumes of crystalloid solution than did those receiving plasma.
The primary outcome, 30-day mortality, was 9.8% lower for patients who received plasma, compared with patients who received standard-care alone (23.2% vs. 33.0%; P = .03). Multivariate regression analysis revealed that plasma delivery accounted for a 39% lower risk of death within 30 days, compared with standard care (P = .02). Within 3 hours of randomization, Kaplan-Meier curves revealed a separation between the two treatment groups that persisted until 30 days.
Median prothrombin to time ratio, the only statistically significant secondary outcome, was lower in the plasma group than it was in the standard-care group (1.2 vs. 1.3; P less than .001).
Other measured parameters were similar between the two main treatment groups, including rates of nosocomial infections, allergic or transfusion-related reactions, acute lung injury/acute respiratory distress syndrome, and multiorgan failure.
“Although we cannot determine the independent or additive effects of prehospital administration of plasma and packed red cells, the survival benefits attributable to plasma administration persisted after adjustment for prehospital red-cell administration, and a subgroup analysis showed no heterogeneity of the treatment effect,” the investigators wrote.
The PAMPer trial was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
SOURCE: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
The Prehospital Air Medical Plasma (PAMPer) trial shows that prehospital plasma improves survival rates in trauma patients at risk for hemorrhagic shock, and its implementation should therefore be considered, according to Jeremy W. Cannon, MD.
“Severe hemorrhage from injury claims the lives of nearly 50,000 Americans every year,” Dr. Cannon wrote in an accompanying editorial. “Because many of these deaths occur in young, vital people, this number translates to an astounding loss of almost 2,000,000 years of productive life.”
Since most deaths occur in less than 2 hours, severe hemorrhage requires rapid intervention; however, the best methods of intervention are unclear. In 1994, Bickell et al. demonstrated that administration of crystalloid-based therapy was more beneficial in a goal-directed, operative setting than it was in transit to a trauma center. Other studies have shown that rapid transport and tourniquet application, when appropriate, are essential.
“But what about patients who have blunt injuries and longer transport times?” asked Dr. Cannon. Recently, damage-control resuscitation has proven effective in the trauma center – a combination of red cells, plasma, and platelets in approximately equal proportions, with minimal nonhemostatic crystalloid solution. Researchers now ask whether this intervention has a place in the prehospital setting.
The PAMPer trial suggests that the number needed to treat is 10 to save one life. These results “should motivate trauma center personnel and air medical crews across the country to consider implementing this lifesaving approach,” Dr. Cannon wrote.
Several blood products are candidates for prehospital resuscitation; their selection depends on both logistical and medical factors. Although fresh-frozen plasma was used in the PAMPer trial, it has a shelf life of only 5 days once thawed, leading to more frequent replenishment. Never-frozen plasma lasts 26 days, which could be easier to keep in stock. Beyond plasma, “refrigerated whole blood has a shelf life of 21 days and offers the benefit of both platelets and oxygen delivery, making it perhaps the ideal product for prehospital resuscitation,” Dr. Cannon wrote.
Jeremy W. Cannon, MD, is at the University of Pennsylvania in Philadelphia and the Uniformed Services University in Bethesda, Md. These comments are adapted from an accompanying editorial (N Engl J Med. 2018 Jul 26;379[4]:387-8). Dr. Cannon reported nonfinancial support from Prytime Medical Devices outside the submitted work and institutional participation in a research network funded by the Department of Defense.
The Prehospital Air Medical Plasma (PAMPer) trial shows that prehospital plasma improves survival rates in trauma patients at risk for hemorrhagic shock, and its implementation should therefore be considered, according to Jeremy W. Cannon, MD.
“Severe hemorrhage from injury claims the lives of nearly 50,000 Americans every year,” Dr. Cannon wrote in an accompanying editorial. “Because many of these deaths occur in young, vital people, this number translates to an astounding loss of almost 2,000,000 years of productive life.”
Since most deaths occur in less than 2 hours, severe hemorrhage requires rapid intervention; however, the best methods of intervention are unclear. In 1994, Bickell et al. demonstrated that administration of crystalloid-based therapy was more beneficial in a goal-directed, operative setting than it was in transit to a trauma center. Other studies have shown that rapid transport and tourniquet application, when appropriate, are essential.
“But what about patients who have blunt injuries and longer transport times?” asked Dr. Cannon. Recently, damage-control resuscitation has proven effective in the trauma center – a combination of red cells, plasma, and platelets in approximately equal proportions, with minimal nonhemostatic crystalloid solution. Researchers now ask whether this intervention has a place in the prehospital setting.
The PAMPer trial suggests that the number needed to treat is 10 to save one life. These results “should motivate trauma center personnel and air medical crews across the country to consider implementing this lifesaving approach,” Dr. Cannon wrote.
Several blood products are candidates for prehospital resuscitation; their selection depends on both logistical and medical factors. Although fresh-frozen plasma was used in the PAMPer trial, it has a shelf life of only 5 days once thawed, leading to more frequent replenishment. Never-frozen plasma lasts 26 days, which could be easier to keep in stock. Beyond plasma, “refrigerated whole blood has a shelf life of 21 days and offers the benefit of both platelets and oxygen delivery, making it perhaps the ideal product for prehospital resuscitation,” Dr. Cannon wrote.
Jeremy W. Cannon, MD, is at the University of Pennsylvania in Philadelphia and the Uniformed Services University in Bethesda, Md. These comments are adapted from an accompanying editorial (N Engl J Med. 2018 Jul 26;379[4]:387-8). Dr. Cannon reported nonfinancial support from Prytime Medical Devices outside the submitted work and institutional participation in a research network funded by the Department of Defense.
The Prehospital Air Medical Plasma (PAMPer) trial shows that prehospital plasma improves survival rates in trauma patients at risk for hemorrhagic shock, and its implementation should therefore be considered, according to Jeremy W. Cannon, MD.
“Severe hemorrhage from injury claims the lives of nearly 50,000 Americans every year,” Dr. Cannon wrote in an accompanying editorial. “Because many of these deaths occur in young, vital people, this number translates to an astounding loss of almost 2,000,000 years of productive life.”
Since most deaths occur in less than 2 hours, severe hemorrhage requires rapid intervention; however, the best methods of intervention are unclear. In 1994, Bickell et al. demonstrated that administration of crystalloid-based therapy was more beneficial in a goal-directed, operative setting than it was in transit to a trauma center. Other studies have shown that rapid transport and tourniquet application, when appropriate, are essential.
“But what about patients who have blunt injuries and longer transport times?” asked Dr. Cannon. Recently, damage-control resuscitation has proven effective in the trauma center – a combination of red cells, plasma, and platelets in approximately equal proportions, with minimal nonhemostatic crystalloid solution. Researchers now ask whether this intervention has a place in the prehospital setting.
The PAMPer trial suggests that the number needed to treat is 10 to save one life. These results “should motivate trauma center personnel and air medical crews across the country to consider implementing this lifesaving approach,” Dr. Cannon wrote.
Several blood products are candidates for prehospital resuscitation; their selection depends on both logistical and medical factors. Although fresh-frozen plasma was used in the PAMPer trial, it has a shelf life of only 5 days once thawed, leading to more frequent replenishment. Never-frozen plasma lasts 26 days, which could be easier to keep in stock. Beyond plasma, “refrigerated whole blood has a shelf life of 21 days and offers the benefit of both platelets and oxygen delivery, making it perhaps the ideal product for prehospital resuscitation,” Dr. Cannon wrote.
Jeremy W. Cannon, MD, is at the University of Pennsylvania in Philadelphia and the Uniformed Services University in Bethesda, Md. These comments are adapted from an accompanying editorial (N Engl J Med. 2018 Jul 26;379[4]:387-8). Dr. Cannon reported nonfinancial support from Prytime Medical Devices outside the submitted work and institutional participation in a research network funded by the Department of Defense.
Prehospital administration of plasma to trauma patients at risk for hemorrhagic shock improves odds of survival, according to the Prehospital Air Medical Plasma (PAMPer) trial.
Patients who received plasma also had a decreased median prothrombin to time ratio, compared with those who received standard-care resuscitation alone.
Modern trauma patients benefit “from receiving less crystalloid-based therapy and early balanced blood component–based therapy once they arrive at a facility for definitive care,” Jason L. Sperry, MD, of the University of Pittsburgh and his coauthors wrote in the New England Journal of Medicine. These newer strategies aim to mitigate coagulopathy and its associated downstream complications.
Still, “a majority of deaths from traumatic hemorrhage continue to occur in the first hours after arrival at the trauma center, which underscores the importance of the prehospital environment for early interventions that provide benefit,” the investigators noted.
The PAMPer trial (NCT01818427) examined the efficacy and safety of prehospital plasma resuscitation, comparing it with standard-care resuscitation, in severely injured patients. The phase 3, randomized, superiority trial involved 501 trauma patients at risk for hemorrhagic shock who were transported from 27 air medical bases to nine trauma centers. In total, 230 patients received prehospital plasma and standard-care resuscitation, and 271 patients received standard-care alone. Standard-care included infusion of crystalloid fluids in a goal-directed manner. Air medical bases delivered each type of care in 1-month intervals.
Patients were eligible if they exhibited severe hypotension (systolic blood pressure less than 70 mm Hg) or both hypotension (systolic blood pressure less than 90 mm Hg) and tachycardia (greater than 108 beats per minute).
Eligible patients received two units of thawed plasma, which was completely delivered before administration of any other fluids. If infusion of plasma was not complete upon arrival at the trauma center, infusion was completed before any in-hospital fluids were administered. Following preexisting local protocols, 13 of 27 air transport teams carried 2U of red blood cells. If red blood cells were delivered, then this was performed after plasma administration if the patient was still hypotensive or obviously bleeding.
The 30-day mortality rate among all patients was 29.6%. Approximately one in three patients received a prehospital red blood cell transfusion, and nearly 60% underwent surgery within 24 hours of hospital admission.
A greater percentage of patients in the standard-care group were given red blood cell transfusions, compared with those in the plasma group. The standard-care patients also received greater volumes of crystalloid solution than did those receiving plasma.
The primary outcome, 30-day mortality, was 9.8% lower for patients who received plasma, compared with patients who received standard-care alone (23.2% vs. 33.0%; P = .03). Multivariate regression analysis revealed that plasma delivery accounted for a 39% lower risk of death within 30 days, compared with standard care (P = .02). Within 3 hours of randomization, Kaplan-Meier curves revealed a separation between the two treatment groups that persisted until 30 days.
Median prothrombin to time ratio, the only statistically significant secondary outcome, was lower in the plasma group than it was in the standard-care group (1.2 vs. 1.3; P less than .001).
Other measured parameters were similar between the two main treatment groups, including rates of nosocomial infections, allergic or transfusion-related reactions, acute lung injury/acute respiratory distress syndrome, and multiorgan failure.
“Although we cannot determine the independent or additive effects of prehospital administration of plasma and packed red cells, the survival benefits attributable to plasma administration persisted after adjustment for prehospital red-cell administration, and a subgroup analysis showed no heterogeneity of the treatment effect,” the investigators wrote.
The PAMPer trial was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
SOURCE: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
Prehospital administration of plasma to trauma patients at risk for hemorrhagic shock improves odds of survival, according to the Prehospital Air Medical Plasma (PAMPer) trial.
Patients who received plasma also had a decreased median prothrombin to time ratio, compared with those who received standard-care resuscitation alone.
Modern trauma patients benefit “from receiving less crystalloid-based therapy and early balanced blood component–based therapy once they arrive at a facility for definitive care,” Jason L. Sperry, MD, of the University of Pittsburgh and his coauthors wrote in the New England Journal of Medicine. These newer strategies aim to mitigate coagulopathy and its associated downstream complications.
Still, “a majority of deaths from traumatic hemorrhage continue to occur in the first hours after arrival at the trauma center, which underscores the importance of the prehospital environment for early interventions that provide benefit,” the investigators noted.
The PAMPer trial (NCT01818427) examined the efficacy and safety of prehospital plasma resuscitation, comparing it with standard-care resuscitation, in severely injured patients. The phase 3, randomized, superiority trial involved 501 trauma patients at risk for hemorrhagic shock who were transported from 27 air medical bases to nine trauma centers. In total, 230 patients received prehospital plasma and standard-care resuscitation, and 271 patients received standard-care alone. Standard-care included infusion of crystalloid fluids in a goal-directed manner. Air medical bases delivered each type of care in 1-month intervals.
Patients were eligible if they exhibited severe hypotension (systolic blood pressure less than 70 mm Hg) or both hypotension (systolic blood pressure less than 90 mm Hg) and tachycardia (greater than 108 beats per minute).
Eligible patients received two units of thawed plasma, which was completely delivered before administration of any other fluids. If infusion of plasma was not complete upon arrival at the trauma center, infusion was completed before any in-hospital fluids were administered. Following preexisting local protocols, 13 of 27 air transport teams carried 2U of red blood cells. If red blood cells were delivered, then this was performed after plasma administration if the patient was still hypotensive or obviously bleeding.
The 30-day mortality rate among all patients was 29.6%. Approximately one in three patients received a prehospital red blood cell transfusion, and nearly 60% underwent surgery within 24 hours of hospital admission.
A greater percentage of patients in the standard-care group were given red blood cell transfusions, compared with those in the plasma group. The standard-care patients also received greater volumes of crystalloid solution than did those receiving plasma.
The primary outcome, 30-day mortality, was 9.8% lower for patients who received plasma, compared with patients who received standard-care alone (23.2% vs. 33.0%; P = .03). Multivariate regression analysis revealed that plasma delivery accounted for a 39% lower risk of death within 30 days, compared with standard care (P = .02). Within 3 hours of randomization, Kaplan-Meier curves revealed a separation between the two treatment groups that persisted until 30 days.
Median prothrombin to time ratio, the only statistically significant secondary outcome, was lower in the plasma group than it was in the standard-care group (1.2 vs. 1.3; P less than .001).
Other measured parameters were similar between the two main treatment groups, including rates of nosocomial infections, allergic or transfusion-related reactions, acute lung injury/acute respiratory distress syndrome, and multiorgan failure.
“Although we cannot determine the independent or additive effects of prehospital administration of plasma and packed red cells, the survival benefits attributable to plasma administration persisted after adjustment for prehospital red-cell administration, and a subgroup analysis showed no heterogeneity of the treatment effect,” the investigators wrote.
The PAMPer trial was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
SOURCE: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Thirty-day mortality was 9.8 percentage points lower for patients receiving plasma, compared with patients receiving standard care (23.2% vs. 33.0%; P = .03).
Study details: A randomized, controlled superiority trial involving 501 trauma patients at risk for hemorrhagic shock, with 230 patients receiving plasma and 271 receiving standard-care during air medical transport.
Disclosures: The study was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
Source: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
Monoclonal antibody slowed cognitive decline, cleared Alzheimer’s plaques in phase 2 trial
CHICAGO – BAN2401, a monoclonal antibody that targets soluble amyloid beta oligomers, slowed cognitive decline in patients with mild cognitive impairment or early Alzheimer’s dementia on two measures, while clearing brain amyloid in 81% of patients in a phase 2 study.
“The majority of subjects receiving the top dose [10 mg/kg intravenously, biweekly] went from being amyloid positive to amyloid negative,” by the end of the 18-month study, Lynn Kramer, MD, said at the Alzheimer’s Association International Conference.
At that dose, BAN2401 also slowed cognitive decline by 30% relative to placebo on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, which is codeveloping the drug with Biogen. The antibody also hit statistical significance on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), with a 47% slowing of decline. A third measure, the Clinical Dementia Rating Sum of Boxes (CDR-sb), showed a 26% reduction, but this was not statistically significant.
There were also significant changes in cerebrospinal fluid amyloid beta and total tau, said Dr. Kramer, chief medical officer of Eisai’s neurology division.
“In our opinion, we have fairly conclusive results,” he said at a press briefing. “We view this as robust enough to approach regulatory authorities [around the world] and discuss the next steps with them, and what we need to do to register this product.”
In addition to planning larger, longer phase 3 studies, Dr. Kramer said that Eisai might try to obtain FDA breakthrough therapy status.
BAN2401 selectively binds to amyloid-beta protofibrils – large, soluble amyloid-beta oligomers – and targets them for clearance. It was originally developed by Swedish biopharma company BioArctic; Eisai acquired the molecule in 2007 and entered a deal with Biogen in 2014.
This is a developing story. Please check back for details.
CHICAGO – BAN2401, a monoclonal antibody that targets soluble amyloid beta oligomers, slowed cognitive decline in patients with mild cognitive impairment or early Alzheimer’s dementia on two measures, while clearing brain amyloid in 81% of patients in a phase 2 study.
“The majority of subjects receiving the top dose [10 mg/kg intravenously, biweekly] went from being amyloid positive to amyloid negative,” by the end of the 18-month study, Lynn Kramer, MD, said at the Alzheimer’s Association International Conference.
At that dose, BAN2401 also slowed cognitive decline by 30% relative to placebo on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, which is codeveloping the drug with Biogen. The antibody also hit statistical significance on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), with a 47% slowing of decline. A third measure, the Clinical Dementia Rating Sum of Boxes (CDR-sb), showed a 26% reduction, but this was not statistically significant.
There were also significant changes in cerebrospinal fluid amyloid beta and total tau, said Dr. Kramer, chief medical officer of Eisai’s neurology division.
“In our opinion, we have fairly conclusive results,” he said at a press briefing. “We view this as robust enough to approach regulatory authorities [around the world] and discuss the next steps with them, and what we need to do to register this product.”
In addition to planning larger, longer phase 3 studies, Dr. Kramer said that Eisai might try to obtain FDA breakthrough therapy status.
BAN2401 selectively binds to amyloid-beta protofibrils – large, soluble amyloid-beta oligomers – and targets them for clearance. It was originally developed by Swedish biopharma company BioArctic; Eisai acquired the molecule in 2007 and entered a deal with Biogen in 2014.
This is a developing story. Please check back for details.
CHICAGO – BAN2401, a monoclonal antibody that targets soluble amyloid beta oligomers, slowed cognitive decline in patients with mild cognitive impairment or early Alzheimer’s dementia on two measures, while clearing brain amyloid in 81% of patients in a phase 2 study.
“The majority of subjects receiving the top dose [10 mg/kg intravenously, biweekly] went from being amyloid positive to amyloid negative,” by the end of the 18-month study, Lynn Kramer, MD, said at the Alzheimer’s Association International Conference.
At that dose, BAN2401 also slowed cognitive decline by 30% relative to placebo on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, which is codeveloping the drug with Biogen. The antibody also hit statistical significance on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), with a 47% slowing of decline. A third measure, the Clinical Dementia Rating Sum of Boxes (CDR-sb), showed a 26% reduction, but this was not statistically significant.
There were also significant changes in cerebrospinal fluid amyloid beta and total tau, said Dr. Kramer, chief medical officer of Eisai’s neurology division.
“In our opinion, we have fairly conclusive results,” he said at a press briefing. “We view this as robust enough to approach regulatory authorities [around the world] and discuss the next steps with them, and what we need to do to register this product.”
In addition to planning larger, longer phase 3 studies, Dr. Kramer said that Eisai might try to obtain FDA breakthrough therapy status.
BAN2401 selectively binds to amyloid-beta protofibrils – large, soluble amyloid-beta oligomers – and targets them for clearance. It was originally developed by Swedish biopharma company BioArctic; Eisai acquired the molecule in 2007 and entered a deal with Biogen in 2014.
This is a developing story. Please check back for details.
REPORTING FROM AAIC 2018
FDA Approves Deep Brain Stimulation System
The Food and Drug Administration has approved a deep brain stimulation system that has been shown to reduce seizures in a select group of patients with epilepsy.
- Medtronics DBS System for Epilepsy has been cleared as adjunct treatment for patients with partial onset seizures with or without secondary generalization.
- The system is only indicated for patients who have not responded to 3 or more antiepileptic drugs and who have experienced an average of 6 or more seizures each month for the last 3 months.
- The FDA approval also stipulates that the patients’ seizures be no more than 30 days apart.
- The DBS System includes a pulse generator that is implanted in a patient’s chest and 2 lead wires implanted in the brain.
Voelker R. Electrical stimulation for epilepsy. JAMA; 2018;319(21):2164.
The Food and Drug Administration has approved a deep brain stimulation system that has been shown to reduce seizures in a select group of patients with epilepsy.
- Medtronics DBS System for Epilepsy has been cleared as adjunct treatment for patients with partial onset seizures with or without secondary generalization.
- The system is only indicated for patients who have not responded to 3 or more antiepileptic drugs and who have experienced an average of 6 or more seizures each month for the last 3 months.
- The FDA approval also stipulates that the patients’ seizures be no more than 30 days apart.
- The DBS System includes a pulse generator that is implanted in a patient’s chest and 2 lead wires implanted in the brain.
Voelker R. Electrical stimulation for epilepsy. JAMA; 2018;319(21):2164.
The Food and Drug Administration has approved a deep brain stimulation system that has been shown to reduce seizures in a select group of patients with epilepsy.
- Medtronics DBS System for Epilepsy has been cleared as adjunct treatment for patients with partial onset seizures with or without secondary generalization.
- The system is only indicated for patients who have not responded to 3 or more antiepileptic drugs and who have experienced an average of 6 or more seizures each month for the last 3 months.
- The FDA approval also stipulates that the patients’ seizures be no more than 30 days apart.
- The DBS System includes a pulse generator that is implanted in a patient’s chest and 2 lead wires implanted in the brain.
Voelker R. Electrical stimulation for epilepsy. JAMA; 2018;319(21):2164.
Psychiatric Interventions Important for Patients with Epilepsy
Clinicians need to pay more attention to the psychological impact of epilepsy and its treatment according to a report from the International League Against Epilepsy Psychology Task Force.
- The task force identified the best interventions for depression, neurocognitive problems, and medication adherence.
- Several psychological strategies are worth consideration according to the task force, including cognitive behavioral therapy and mindfulness-based treatment.
- These interventions have the potential to improve health-related quality of life among adults and children.
- The recommendations outlined by the League are based primarily on evidence discussed in a recent Cochrane review of randomized clinical trials that evaluated psychological treatment of patients with epilepsy.
Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
Clinicians need to pay more attention to the psychological impact of epilepsy and its treatment according to a report from the International League Against Epilepsy Psychology Task Force.
- The task force identified the best interventions for depression, neurocognitive problems, and medication adherence.
- Several psychological strategies are worth consideration according to the task force, including cognitive behavioral therapy and mindfulness-based treatment.
- These interventions have the potential to improve health-related quality of life among adults and children.
- The recommendations outlined by the League are based primarily on evidence discussed in a recent Cochrane review of randomized clinical trials that evaluated psychological treatment of patients with epilepsy.
Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
Clinicians need to pay more attention to the psychological impact of epilepsy and its treatment according to a report from the International League Against Epilepsy Psychology Task Force.
- The task force identified the best interventions for depression, neurocognitive problems, and medication adherence.
- Several psychological strategies are worth consideration according to the task force, including cognitive behavioral therapy and mindfulness-based treatment.
- These interventions have the potential to improve health-related quality of life among adults and children.
- The recommendations outlined by the League are based primarily on evidence discussed in a recent Cochrane review of randomized clinical trials that evaluated psychological treatment of patients with epilepsy.
Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
No Differences Found Between Generic/Brand Name Epileptic Medication
Switching patients from brand name antiepileptic drugs to generics is generally safe and cost effective according to an analysis published in Epilepsia.
- Researchers looked at data on bioequivalence, health care utilization, and clinical studies on the safety of antiepileptic agents, including a comparison of area under the plasma concentration-time curve (AUC) and peak plasma concentration.
- For most of the drugs that were evaluated, there were negligible differences in AUC and peak plasma concentration between generic drugs and brand name equivalents.
- There were significant increases in health care usage when patients were switched from brand name to generic versions.
- Clinical studies were unable to detect differences in seizure frequency or tolerability.
Holtkamp M, Theodore WH. Generic antiepileptic drugs—safe or harmful in patients with epilepsy? Epilepsia. 2018;59(7):1273-1281.
Switching patients from brand name antiepileptic drugs to generics is generally safe and cost effective according to an analysis published in Epilepsia.
- Researchers looked at data on bioequivalence, health care utilization, and clinical studies on the safety of antiepileptic agents, including a comparison of area under the plasma concentration-time curve (AUC) and peak plasma concentration.
- For most of the drugs that were evaluated, there were negligible differences in AUC and peak plasma concentration between generic drugs and brand name equivalents.
- There were significant increases in health care usage when patients were switched from brand name to generic versions.
- Clinical studies were unable to detect differences in seizure frequency or tolerability.
Holtkamp M, Theodore WH. Generic antiepileptic drugs—safe or harmful in patients with epilepsy? Epilepsia. 2018;59(7):1273-1281.
Switching patients from brand name antiepileptic drugs to generics is generally safe and cost effective according to an analysis published in Epilepsia.
- Researchers looked at data on bioequivalence, health care utilization, and clinical studies on the safety of antiepileptic agents, including a comparison of area under the plasma concentration-time curve (AUC) and peak plasma concentration.
- For most of the drugs that were evaluated, there were negligible differences in AUC and peak plasma concentration between generic drugs and brand name equivalents.
- There were significant increases in health care usage when patients were switched from brand name to generic versions.
- Clinical studies were unable to detect differences in seizure frequency or tolerability.
Holtkamp M, Theodore WH. Generic antiepileptic drugs—safe or harmful in patients with epilepsy? Epilepsia. 2018;59(7):1273-1281.
In Ghana, SCD research is meeting patients on home turf
WASHINGTON – Sometimes, the hardest part of solving a problem is figuring out how to work around misaligned resources, and so it has been with sickle cell disease research.
“From my point of view, what I call the geographical disparity in sickle cell disease research can be explained by the fact that the majority of affected individuals are living in the East, and the overwhelming majority of the research takes place in the West,” said Solomon Ofori-Acquah, PhD. He and three physician collaborators from Ghana shared their roadmap to conducting clinical trials in West Africa during an “East Meets West” session of the annual symposium of the Foundation for Sickle Cell Disease Research.
In Ghana, not far from where scientists now believe the hemoglobin sickling mutation originated, fully 2% of newborns have SCD; this translates into 16,000 new cases per year in a population of just 28 million, compared with the 2,000 new SCD cases seen annually in the entire United States. And access even to proven therapies can be limited; historically, little to no clinical drug development work has been conducted in this part of the world.
In the United States, half of the SCD trials that were withdrawn or terminated listed recruitment and retention of study participants as a factor in the study’s discontinuation, said Amma Owusu-Ansah, MD. “I see what we are doing as a very feasible solution to the problem of inadequate accrual to studies in the U.S.,” said Dr. Owusu-Ansah, a hematologist at the University of Pittsburgh’s Center for Translational and International Hematology (CTIH), where she serves as clinical director.
From the African perspective, hosting clinical trials – and building a robust infrastructure to do so – may help alleviate the delay in translation of disease-modifying therapies for SCD to Africa, where most people with the disease live, she said.
An existing example of resource sharing is the Human Heredity & Health in Africa (H3Africa) initiative, said Dr. Ofori-Acquah, who directs the CTIH and also holds an appointment at the University of Ghana. The project, funded by the National Institutes of Health and the Wellcome Trust, “aims to facilitate a contemporary research approach to the study of genomics and environmental determinants of common diseases with the goal of improving the health of African populations,” according to the H3Africa website. Within this framework of 40 research centers conducting genomics research and biobanking, several discrete projects aim to expand knowledge of sickle cell disease.
“All of these networks are going to study thousands of patients,” said Dr. Ofori-Acquah. “I see the H3 as a mechanism to accelerate genomics research in sickle cell disease.”
“We created a research team and built capacity for future work…. Ghana, and Africa, are capable of conducting clinical trials to global standards and producing quality data,” she said.
The story of one clinical trial is illustrative of the challenges and strengths of the multinational approach.
The phase 1b trial of a novel treatment for sickle cell disease, NVX-508, began with an initial hurdle of lack of access to emergency care at the study site, said Dr. Owusu-Ansah, a study investigator. Her first reaction, she said, was, “Well, we can’t do this, because we don’t have access to a big staff and emergency facilities.”
But after consulting with colleagues, she realized a shift in mindset was needed: “Rather than focus on what we don’t have, what do we actually have available? We have relationships we have built with institutions,” including the oldest SCD clinic in Ghana, the Ghana Institute of Clinical Genetics (GICG). This facility sits next door to a hospital with 24-hour care, Korle Bu Teaching Hospital (KBTH), a major tertiary care and referral center.
Open since 1974, the KBTH-allied GICG provides comprehensive outpatient health care to teens and adult with SCD. Currently, more than 25,000 SCD patients are registered at GICG; about half have the HbSS genotype, and another 40% have the HbSC genotype, said Yvonne Dei-Adomakoh, MD. Dr. Dei-Adomakoh of the University of Ghana is an investigator for an upcoming phase 3 trial to test voxelotor against placebo in SCD.
The GICG is working hard to become a site where clinical trials, as well as research and development, are embedded into clinic functions. In this way, not only will research be advanced for all those with SCD, but advances will be more easily incorporated into clinical care, said Dr. Dei-Adomakoh.
Dr. Owusu-Ansah noted that the facility offers a pharmacy, a laboratory, exam rooms, and information technology and medical record resources. Importantly, GICG is already staffed with physicians and allied health personnel with SCD expertise.
The University of Ghana campus is home to one of Africa’s leading biomedical research facilities, a sophisticated 11,000-square-foot laboratory that can perform testing ranging from polymerase chain reactions to DNA sequencing to genotyping and flow cytometry; it also houses a laboratory animal facility. This laboratory, the Noguchi Memorial Institute for Medical Research, also offers administrative, scientific, and research support, and houses an institutional review board.
The problem of the Noguchi laboratory site’s distance from the 24-hour support of KBTH has been solved by arranging to have an ambulance with paramedics available on site during the clinical trials.
Some other challenges the investigators discovered highlighted less-obvious infrastructure deficits; keeping a refrigerated chain of custody for biological samples, for example, can be difficult. In preparation for the trials, much basic laboratory and clinical equipment has been updated.
Conducting a U.S.-registered clinical trial in Ghana doesn’t obviate the need to meet that country’s considerable regulatory hurdles, said Dr. Owusu-Ansah. Requirements include a full regulatory submission to, and physical inspection by, Ghana’s FDA. Ghana also requires that the principal investigator must live in Ghana for the duration of the trial and that key study personnel complete Ghanaian good clinical practices training, she said.
The University of Pittsburgh is a U.S. partner in the NVX-508 study, and it was non-negotiable for that institution that a clinical trial monitor visit the African study sites. The institution’s institutional review board was sensitive to the importance of protecting vulnerable populations, and needed to hear complete plans for risk assessment, data protection, and compensation for Ghanaian study participants, Dr. Owusu-Ansah said.
But, in a turn of events typical of the ups and downs of drug development, the phase 1 trial had passed most of the administrative hurdles when in July the drug’s sponsor, NuvOx Pharma, suspended the NVX-508 trial to focus on other areas. For now, the trial registration has been withdrawn on clinicaltrials.gov and the new drug application is inactive. But Dr. Owusu-Ansah said study preparations could resume in the future, if the drug is made available to investigators.
Dr. Owusu-Ansah reported that she has received salary support from NuvOx Pharma. Dr. Segbefia reported that she has received support from Daiichi-Sankyo and Eli Lilly and Company.
WASHINGTON – Sometimes, the hardest part of solving a problem is figuring out how to work around misaligned resources, and so it has been with sickle cell disease research.

“From my point of view, what I call the geographical disparity in sickle cell disease research can be explained by the fact that the majority of affected individuals are living in the East, and the overwhelming majority of the research takes place in the West,” said Solomon Ofori-Acquah, PhD. He and three physician collaborators from Ghana shared their roadmap to conducting clinical trials in West Africa during an “East Meets West” session of the annual symposium of the Foundation for Sickle Cell Disease Research.
In Ghana, not far from where scientists now believe the hemoglobin sickling mutation originated, fully 2% of newborns have SCD; this translates into 16,000 new cases per year in a population of just 28 million, compared with the 2,000 new SCD cases seen annually in the entire United States. And access even to proven therapies can be limited; historically, little to no clinical drug development work has been conducted in this part of the world.
In the United States, half of the SCD trials that were withdrawn or terminated listed recruitment and retention of study participants as a factor in the study’s discontinuation, said Amma Owusu-Ansah, MD. “I see what we are doing as a very feasible solution to the problem of inadequate accrual to studies in the U.S.,” said Dr. Owusu-Ansah, a hematologist at the University of Pittsburgh’s Center for Translational and International Hematology (CTIH), where she serves as clinical director.
From the African perspective, hosting clinical trials – and building a robust infrastructure to do so – may help alleviate the delay in translation of disease-modifying therapies for SCD to Africa, where most people with the disease live, she said.
An existing example of resource sharing is the Human Heredity & Health in Africa (H3Africa) initiative, said Dr. Ofori-Acquah, who directs the CTIH and also holds an appointment at the University of Ghana. The project, funded by the National Institutes of Health and the Wellcome Trust, “aims to facilitate a contemporary research approach to the study of genomics and environmental determinants of common diseases with the goal of improving the health of African populations,” according to the H3Africa website. Within this framework of 40 research centers conducting genomics research and biobanking, several discrete projects aim to expand knowledge of sickle cell disease.
“All of these networks are going to study thousands of patients,” said Dr. Ofori-Acquah. “I see the H3 as a mechanism to accelerate genomics research in sickle cell disease.”
“We created a research team and built capacity for future work…. Ghana, and Africa, are capable of conducting clinical trials to global standards and producing quality data,” she said.
The story of one clinical trial is illustrative of the challenges and strengths of the multinational approach.
The phase 1b trial of a novel treatment for sickle cell disease, NVX-508, began with an initial hurdle of lack of access to emergency care at the study site, said Dr. Owusu-Ansah, a study investigator. Her first reaction, she said, was, “Well, we can’t do this, because we don’t have access to a big staff and emergency facilities.”
But after consulting with colleagues, she realized a shift in mindset was needed: “Rather than focus on what we don’t have, what do we actually have available? We have relationships we have built with institutions,” including the oldest SCD clinic in Ghana, the Ghana Institute of Clinical Genetics (GICG). This facility sits next door to a hospital with 24-hour care, Korle Bu Teaching Hospital (KBTH), a major tertiary care and referral center.
Open since 1974, the KBTH-allied GICG provides comprehensive outpatient health care to teens and adult with SCD. Currently, more than 25,000 SCD patients are registered at GICG; about half have the HbSS genotype, and another 40% have the HbSC genotype, said Yvonne Dei-Adomakoh, MD. Dr. Dei-Adomakoh of the University of Ghana is an investigator for an upcoming phase 3 trial to test voxelotor against placebo in SCD.
The GICG is working hard to become a site where clinical trials, as well as research and development, are embedded into clinic functions. In this way, not only will research be advanced for all those with SCD, but advances will be more easily incorporated into clinical care, said Dr. Dei-Adomakoh.
Dr. Owusu-Ansah noted that the facility offers a pharmacy, a laboratory, exam rooms, and information technology and medical record resources. Importantly, GICG is already staffed with physicians and allied health personnel with SCD expertise.
The University of Ghana campus is home to one of Africa’s leading biomedical research facilities, a sophisticated 11,000-square-foot laboratory that can perform testing ranging from polymerase chain reactions to DNA sequencing to genotyping and flow cytometry; it also houses a laboratory animal facility. This laboratory, the Noguchi Memorial Institute for Medical Research, also offers administrative, scientific, and research support, and houses an institutional review board.
The problem of the Noguchi laboratory site’s distance from the 24-hour support of KBTH has been solved by arranging to have an ambulance with paramedics available on site during the clinical trials.
Some other challenges the investigators discovered highlighted less-obvious infrastructure deficits; keeping a refrigerated chain of custody for biological samples, for example, can be difficult. In preparation for the trials, much basic laboratory and clinical equipment has been updated.
Conducting a U.S.-registered clinical trial in Ghana doesn’t obviate the need to meet that country’s considerable regulatory hurdles, said Dr. Owusu-Ansah. Requirements include a full regulatory submission to, and physical inspection by, Ghana’s FDA. Ghana also requires that the principal investigator must live in Ghana for the duration of the trial and that key study personnel complete Ghanaian good clinical practices training, she said.
The University of Pittsburgh is a U.S. partner in the NVX-508 study, and it was non-negotiable for that institution that a clinical trial monitor visit the African study sites. The institution’s institutional review board was sensitive to the importance of protecting vulnerable populations, and needed to hear complete plans for risk assessment, data protection, and compensation for Ghanaian study participants, Dr. Owusu-Ansah said.
But, in a turn of events typical of the ups and downs of drug development, the phase 1 trial had passed most of the administrative hurdles when in July the drug’s sponsor, NuvOx Pharma, suspended the NVX-508 trial to focus on other areas. For now, the trial registration has been withdrawn on clinicaltrials.gov and the new drug application is inactive. But Dr. Owusu-Ansah said study preparations could resume in the future, if the drug is made available to investigators.
Dr. Owusu-Ansah reported that she has received salary support from NuvOx Pharma. Dr. Segbefia reported that she has received support from Daiichi-Sankyo and Eli Lilly and Company.
WASHINGTON – Sometimes, the hardest part of solving a problem is figuring out how to work around misaligned resources, and so it has been with sickle cell disease research.

“From my point of view, what I call the geographical disparity in sickle cell disease research can be explained by the fact that the majority of affected individuals are living in the East, and the overwhelming majority of the research takes place in the West,” said Solomon Ofori-Acquah, PhD. He and three physician collaborators from Ghana shared their roadmap to conducting clinical trials in West Africa during an “East Meets West” session of the annual symposium of the Foundation for Sickle Cell Disease Research.
In Ghana, not far from where scientists now believe the hemoglobin sickling mutation originated, fully 2% of newborns have SCD; this translates into 16,000 new cases per year in a population of just 28 million, compared with the 2,000 new SCD cases seen annually in the entire United States. And access even to proven therapies can be limited; historically, little to no clinical drug development work has been conducted in this part of the world.
In the United States, half of the SCD trials that were withdrawn or terminated listed recruitment and retention of study participants as a factor in the study’s discontinuation, said Amma Owusu-Ansah, MD. “I see what we are doing as a very feasible solution to the problem of inadequate accrual to studies in the U.S.,” said Dr. Owusu-Ansah, a hematologist at the University of Pittsburgh’s Center for Translational and International Hematology (CTIH), where she serves as clinical director.
From the African perspective, hosting clinical trials – and building a robust infrastructure to do so – may help alleviate the delay in translation of disease-modifying therapies for SCD to Africa, where most people with the disease live, she said.
An existing example of resource sharing is the Human Heredity & Health in Africa (H3Africa) initiative, said Dr. Ofori-Acquah, who directs the CTIH and also holds an appointment at the University of Ghana. The project, funded by the National Institutes of Health and the Wellcome Trust, “aims to facilitate a contemporary research approach to the study of genomics and environmental determinants of common diseases with the goal of improving the health of African populations,” according to the H3Africa website. Within this framework of 40 research centers conducting genomics research and biobanking, several discrete projects aim to expand knowledge of sickle cell disease.
“All of these networks are going to study thousands of patients,” said Dr. Ofori-Acquah. “I see the H3 as a mechanism to accelerate genomics research in sickle cell disease.”
“We created a research team and built capacity for future work…. Ghana, and Africa, are capable of conducting clinical trials to global standards and producing quality data,” she said.
The story of one clinical trial is illustrative of the challenges and strengths of the multinational approach.
The phase 1b trial of a novel treatment for sickle cell disease, NVX-508, began with an initial hurdle of lack of access to emergency care at the study site, said Dr. Owusu-Ansah, a study investigator. Her first reaction, she said, was, “Well, we can’t do this, because we don’t have access to a big staff and emergency facilities.”
But after consulting with colleagues, she realized a shift in mindset was needed: “Rather than focus on what we don’t have, what do we actually have available? We have relationships we have built with institutions,” including the oldest SCD clinic in Ghana, the Ghana Institute of Clinical Genetics (GICG). This facility sits next door to a hospital with 24-hour care, Korle Bu Teaching Hospital (KBTH), a major tertiary care and referral center.
Open since 1974, the KBTH-allied GICG provides comprehensive outpatient health care to teens and adult with SCD. Currently, more than 25,000 SCD patients are registered at GICG; about half have the HbSS genotype, and another 40% have the HbSC genotype, said Yvonne Dei-Adomakoh, MD. Dr. Dei-Adomakoh of the University of Ghana is an investigator for an upcoming phase 3 trial to test voxelotor against placebo in SCD.
The GICG is working hard to become a site where clinical trials, as well as research and development, are embedded into clinic functions. In this way, not only will research be advanced for all those with SCD, but advances will be more easily incorporated into clinical care, said Dr. Dei-Adomakoh.
Dr. Owusu-Ansah noted that the facility offers a pharmacy, a laboratory, exam rooms, and information technology and medical record resources. Importantly, GICG is already staffed with physicians and allied health personnel with SCD expertise.
The University of Ghana campus is home to one of Africa’s leading biomedical research facilities, a sophisticated 11,000-square-foot laboratory that can perform testing ranging from polymerase chain reactions to DNA sequencing to genotyping and flow cytometry; it also houses a laboratory animal facility. This laboratory, the Noguchi Memorial Institute for Medical Research, also offers administrative, scientific, and research support, and houses an institutional review board.
The problem of the Noguchi laboratory site’s distance from the 24-hour support of KBTH has been solved by arranging to have an ambulance with paramedics available on site during the clinical trials.
Some other challenges the investigators discovered highlighted less-obvious infrastructure deficits; keeping a refrigerated chain of custody for biological samples, for example, can be difficult. In preparation for the trials, much basic laboratory and clinical equipment has been updated.
Conducting a U.S.-registered clinical trial in Ghana doesn’t obviate the need to meet that country’s considerable regulatory hurdles, said Dr. Owusu-Ansah. Requirements include a full regulatory submission to, and physical inspection by, Ghana’s FDA. Ghana also requires that the principal investigator must live in Ghana for the duration of the trial and that key study personnel complete Ghanaian good clinical practices training, she said.
The University of Pittsburgh is a U.S. partner in the NVX-508 study, and it was non-negotiable for that institution that a clinical trial monitor visit the African study sites. The institution’s institutional review board was sensitive to the importance of protecting vulnerable populations, and needed to hear complete plans for risk assessment, data protection, and compensation for Ghanaian study participants, Dr. Owusu-Ansah said.
But, in a turn of events typical of the ups and downs of drug development, the phase 1 trial had passed most of the administrative hurdles when in July the drug’s sponsor, NuvOx Pharma, suspended the NVX-508 trial to focus on other areas. For now, the trial registration has been withdrawn on clinicaltrials.gov and the new drug application is inactive. But Dr. Owusu-Ansah said study preparations could resume in the future, if the drug is made available to investigators.
Dr. Owusu-Ansah reported that she has received salary support from NuvOx Pharma. Dr. Segbefia reported that she has received support from Daiichi-Sankyo and Eli Lilly and Company.
EXPERT ANALYSIS FROM FSCDR 2018