User login
GPS receives fast track designation for MM
The US Food and Drug Administration (FDA) has granted fast track designation to the cancer vaccine galinpepimut-S (GPS) for the treatment of multiple myeloma (MM).
GPS consists of 4 modified peptide chains that induce an innate immune response (CD4+/CD8+ T cells) against the Wilms’ tumor 1 (WT1) antigen.
GPS is administered in combination with an adjuvant and an immune modulator to improve the immune response to the target.
GPS has been tested in a phase 2 trial of patients with MM. Results from this trial were presented at the 44th Annual Meeting of the EBMT in March.
Phase 2 trial
The study enrolled 19 MM patients. Fifteen of them had high-risk cytogenetics at diagnosis, and 18 were at least minimal residual disease-positive after autologous stem cell transplant (ASCT).
Patients began receiving GPS within 22 days of ASCT. Initially, they received 6 doses (administered subcutaneously with the oil emulsifier montanide) every 2 weeks. Injection sites were pre-stimulated with granulocyte-macrophage colony-stimulating factor (70 μg) on days -2 (± 1 day) and 0 of each GPS vaccination.
The patients underwent assessment 2 to 4 weeks after the 6th GPS dose. Then, they received 6 additional monthly doses of GPS in conjunction with lenalidomide maintenance (10 mg daily), starting on day 100 post-ASCT. Patients were assessed 2 to 4 weeks after the 12th GPS dose.
The researchers found that GPS stimulated time-dependent CD4+ or CD8+ T-cell immune responses specific for all 4 WT1 peptides within GPS, 2 of which are heteroclitic.
Immune responses were confirmed in up to 91% of patients, with multivalent immune responses in up to 64% of patients. Three-quarters of patients had multifunctional cross-epitope T-cell reactivity to antigenic epitopes against which the hosts were not specifically immunized, in a pattern akin to epitope spreading.
In patients who received all 12 doses of GPS (n=12), there was a “strong” association between clinical benefit—defined as complete response (CR) or very good partial response (VGPR)—and frequency of CD4/CD8 immune responses.
Of those patients who had achieved CR/VGPR upon completion of GPS treatment, 100% (n=11) had CD4 immune responses, and 81.8% (n=9) had CD8 immune responses.
The median progression-free survival was 23.6 months, and the median overall survival was not reached. At 18 months, the rate of progression-free survival was 62%, and the rate of overall survival was 88%.
About fast track designation
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
The US Food and Drug Administration (FDA) has granted fast track designation to the cancer vaccine galinpepimut-S (GPS) for the treatment of multiple myeloma (MM).
GPS consists of 4 modified peptide chains that induce an innate immune response (CD4+/CD8+ T cells) against the Wilms’ tumor 1 (WT1) antigen.
GPS is administered in combination with an adjuvant and an immune modulator to improve the immune response to the target.
GPS has been tested in a phase 2 trial of patients with MM. Results from this trial were presented at the 44th Annual Meeting of the EBMT in March.
Phase 2 trial
The study enrolled 19 MM patients. Fifteen of them had high-risk cytogenetics at diagnosis, and 18 were at least minimal residual disease-positive after autologous stem cell transplant (ASCT).
Patients began receiving GPS within 22 days of ASCT. Initially, they received 6 doses (administered subcutaneously with the oil emulsifier montanide) every 2 weeks. Injection sites were pre-stimulated with granulocyte-macrophage colony-stimulating factor (70 μg) on days -2 (± 1 day) and 0 of each GPS vaccination.
The patients underwent assessment 2 to 4 weeks after the 6th GPS dose. Then, they received 6 additional monthly doses of GPS in conjunction with lenalidomide maintenance (10 mg daily), starting on day 100 post-ASCT. Patients were assessed 2 to 4 weeks after the 12th GPS dose.
The researchers found that GPS stimulated time-dependent CD4+ or CD8+ T-cell immune responses specific for all 4 WT1 peptides within GPS, 2 of which are heteroclitic.
Immune responses were confirmed in up to 91% of patients, with multivalent immune responses in up to 64% of patients. Three-quarters of patients had multifunctional cross-epitope T-cell reactivity to antigenic epitopes against which the hosts were not specifically immunized, in a pattern akin to epitope spreading.
In patients who received all 12 doses of GPS (n=12), there was a “strong” association between clinical benefit—defined as complete response (CR) or very good partial response (VGPR)—and frequency of CD4/CD8 immune responses.
Of those patients who had achieved CR/VGPR upon completion of GPS treatment, 100% (n=11) had CD4 immune responses, and 81.8% (n=9) had CD8 immune responses.
The median progression-free survival was 23.6 months, and the median overall survival was not reached. At 18 months, the rate of progression-free survival was 62%, and the rate of overall survival was 88%.
About fast track designation
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
The US Food and Drug Administration (FDA) has granted fast track designation to the cancer vaccine galinpepimut-S (GPS) for the treatment of multiple myeloma (MM).
GPS consists of 4 modified peptide chains that induce an innate immune response (CD4+/CD8+ T cells) against the Wilms’ tumor 1 (WT1) antigen.
GPS is administered in combination with an adjuvant and an immune modulator to improve the immune response to the target.
GPS has been tested in a phase 2 trial of patients with MM. Results from this trial were presented at the 44th Annual Meeting of the EBMT in March.
Phase 2 trial
The study enrolled 19 MM patients. Fifteen of them had high-risk cytogenetics at diagnosis, and 18 were at least minimal residual disease-positive after autologous stem cell transplant (ASCT).
Patients began receiving GPS within 22 days of ASCT. Initially, they received 6 doses (administered subcutaneously with the oil emulsifier montanide) every 2 weeks. Injection sites were pre-stimulated with granulocyte-macrophage colony-stimulating factor (70 μg) on days -2 (± 1 day) and 0 of each GPS vaccination.
The patients underwent assessment 2 to 4 weeks after the 6th GPS dose. Then, they received 6 additional monthly doses of GPS in conjunction with lenalidomide maintenance (10 mg daily), starting on day 100 post-ASCT. Patients were assessed 2 to 4 weeks after the 12th GPS dose.
The researchers found that GPS stimulated time-dependent CD4+ or CD8+ T-cell immune responses specific for all 4 WT1 peptides within GPS, 2 of which are heteroclitic.
Immune responses were confirmed in up to 91% of patients, with multivalent immune responses in up to 64% of patients. Three-quarters of patients had multifunctional cross-epitope T-cell reactivity to antigenic epitopes against which the hosts were not specifically immunized, in a pattern akin to epitope spreading.
In patients who received all 12 doses of GPS (n=12), there was a “strong” association between clinical benefit—defined as complete response (CR) or very good partial response (VGPR)—and frequency of CD4/CD8 immune responses.
Of those patients who had achieved CR/VGPR upon completion of GPS treatment, 100% (n=11) had CD4 immune responses, and 81.8% (n=9) had CD8 immune responses.
The median progression-free survival was 23.6 months, and the median overall survival was not reached. At 18 months, the rate of progression-free survival was 62%, and the rate of overall survival was 88%.
About fast track designation
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
Pregnancy and years of reproductive capability linked to dementia risk
CHICAGO – More pregnancies and a longer span of reproductive years appear to confer some level of protection against dementia, suggesting that lifetime estrogen exposure may be an important modulator of long-term cognitive health.
The study, presented at the Alzheimer’s Association International Conference, is the first to examine this question in a large cohort, comprising more than 14,500 women with up to 50 years of follow-up data. It is one of the first explorations in a renewed interest in the link between hormones and cognition, said Suzanne Craft, PhD, who moderated a press briefing on the topic.
“WHI [Women’s Heath Initiative] had a completely chilling effect on research into estrogen and cognition completely,” Dr. Craft of Wake Forest University, Winston-Salem, N.C., said in an interview. “Many well-regarded researchers simply couldn’t get funding and had to close their programs and move on to other areas. But now I think the pendulum is slowly moving back. It’s clear that something is going on, that there is a link. I’m glad we’re starting to explore this again.”
The investigation of women’s total reproductive experience affects dementia risk found several intriguing associations, said Paola Gilsanz, ScD, of Kaiser Permanente, Oakland, Calif. Earlier age of menarche, later menopause, and more completed pregnancies all independently reduced the risk of dementia in women.
Her study comprised 14,595 women, all members of the Kaiser Permanente health care database. All of them had completed a comprehensive health checkup sometime between 1964 and 1973, when they were 40-55 years old. They reported their number of miscarriages, number of children, ages at first and last menstrual period, and the total number of years in their reproductive period. Most of the group (68%) was white, but 16% were black, 6% Asian, and 5% Hispanic. Dr. Gilsanz looked at rates of dementia during 1996-2017, when the women were 62-86 years old.
A multivariate regression model controlled for age, race, education, midlife health issues (hypertension, smoking, and body mass index), hysterectomy, and late-life health issues (stroke, heart failure, and diabetes).
Half of the cohort (50%) had at least three children, and 75% had experienced at least one miscarriage. The average age at menarche was 13 years, and the average age at last natural menstrual period was 47 years. This equated to an average reproductive period of 34 years.
At the end of follow-up, 36% of the cohort had developed some type of dementia.
Women with at least three children were 12% less likely to develop dementia, compared with those with one child (hazard ratio, 0.88). The association remained significant even after researchers controlled for age, race, educational level, and hysterectomy.
Miscarriages also influenced the risk of dementia. Those who didn’t report one were 20% less likely to develop dementia than were those who had experienced at least one (HR, 0.81). The benefit of no miscarriage was even greater among women with at least three children, conferring a 28% reduced risk (HR, 0.72).
A shortened reproductive period increased the risk of dementia. Those who experienced menarche at 16 years or older had a 31% increased risk of dementia (HR, 1.31), and those who experienced their last period at age 45 or younger faced a 28% greater risk (HR, 1.28). Each additional year of reproductive capability was associated with a 2% decreased risk (HR, 0.98).
Women with a reproductive period of 21-30 years were 33% more likely to develop dementia than were those with a longer period (HR, 1.33)
“Reproductive events that signal different exposures to estrogen, like pregnancy and reproductive period may play a role in modulating dementia risk,” Dr. Gilsanz said. “Women who are less likely to have a miscarriage may have different hormonal milieu that may be neuroprotective. Underlying health conditions increasing the risk of miscarriages may also elevate risk of dementia.”
She had no financial disclosures.
SOURCE: Gilsanz P et al. AAIC 2018, Abstract P3-587
CHICAGO – More pregnancies and a longer span of reproductive years appear to confer some level of protection against dementia, suggesting that lifetime estrogen exposure may be an important modulator of long-term cognitive health.
The study, presented at the Alzheimer’s Association International Conference, is the first to examine this question in a large cohort, comprising more than 14,500 women with up to 50 years of follow-up data. It is one of the first explorations in a renewed interest in the link between hormones and cognition, said Suzanne Craft, PhD, who moderated a press briefing on the topic.
“WHI [Women’s Heath Initiative] had a completely chilling effect on research into estrogen and cognition completely,” Dr. Craft of Wake Forest University, Winston-Salem, N.C., said in an interview. “Many well-regarded researchers simply couldn’t get funding and had to close their programs and move on to other areas. But now I think the pendulum is slowly moving back. It’s clear that something is going on, that there is a link. I’m glad we’re starting to explore this again.”
The investigation of women’s total reproductive experience affects dementia risk found several intriguing associations, said Paola Gilsanz, ScD, of Kaiser Permanente, Oakland, Calif. Earlier age of menarche, later menopause, and more completed pregnancies all independently reduced the risk of dementia in women.
Her study comprised 14,595 women, all members of the Kaiser Permanente health care database. All of them had completed a comprehensive health checkup sometime between 1964 and 1973, when they were 40-55 years old. They reported their number of miscarriages, number of children, ages at first and last menstrual period, and the total number of years in their reproductive period. Most of the group (68%) was white, but 16% were black, 6% Asian, and 5% Hispanic. Dr. Gilsanz looked at rates of dementia during 1996-2017, when the women were 62-86 years old.
A multivariate regression model controlled for age, race, education, midlife health issues (hypertension, smoking, and body mass index), hysterectomy, and late-life health issues (stroke, heart failure, and diabetes).
Half of the cohort (50%) had at least three children, and 75% had experienced at least one miscarriage. The average age at menarche was 13 years, and the average age at last natural menstrual period was 47 years. This equated to an average reproductive period of 34 years.
At the end of follow-up, 36% of the cohort had developed some type of dementia.
Women with at least three children were 12% less likely to develop dementia, compared with those with one child (hazard ratio, 0.88). The association remained significant even after researchers controlled for age, race, educational level, and hysterectomy.
Miscarriages also influenced the risk of dementia. Those who didn’t report one were 20% less likely to develop dementia than were those who had experienced at least one (HR, 0.81). The benefit of no miscarriage was even greater among women with at least three children, conferring a 28% reduced risk (HR, 0.72).
A shortened reproductive period increased the risk of dementia. Those who experienced menarche at 16 years or older had a 31% increased risk of dementia (HR, 1.31), and those who experienced their last period at age 45 or younger faced a 28% greater risk (HR, 1.28). Each additional year of reproductive capability was associated with a 2% decreased risk (HR, 0.98).
Women with a reproductive period of 21-30 years were 33% more likely to develop dementia than were those with a longer period (HR, 1.33)
“Reproductive events that signal different exposures to estrogen, like pregnancy and reproductive period may play a role in modulating dementia risk,” Dr. Gilsanz said. “Women who are less likely to have a miscarriage may have different hormonal milieu that may be neuroprotective. Underlying health conditions increasing the risk of miscarriages may also elevate risk of dementia.”
She had no financial disclosures.
SOURCE: Gilsanz P et al. AAIC 2018, Abstract P3-587
CHICAGO – More pregnancies and a longer span of reproductive years appear to confer some level of protection against dementia, suggesting that lifetime estrogen exposure may be an important modulator of long-term cognitive health.
The study, presented at the Alzheimer’s Association International Conference, is the first to examine this question in a large cohort, comprising more than 14,500 women with up to 50 years of follow-up data. It is one of the first explorations in a renewed interest in the link between hormones and cognition, said Suzanne Craft, PhD, who moderated a press briefing on the topic.
“WHI [Women’s Heath Initiative] had a completely chilling effect on research into estrogen and cognition completely,” Dr. Craft of Wake Forest University, Winston-Salem, N.C., said in an interview. “Many well-regarded researchers simply couldn’t get funding and had to close their programs and move on to other areas. But now I think the pendulum is slowly moving back. It’s clear that something is going on, that there is a link. I’m glad we’re starting to explore this again.”
The investigation of women’s total reproductive experience affects dementia risk found several intriguing associations, said Paola Gilsanz, ScD, of Kaiser Permanente, Oakland, Calif. Earlier age of menarche, later menopause, and more completed pregnancies all independently reduced the risk of dementia in women.
Her study comprised 14,595 women, all members of the Kaiser Permanente health care database. All of them had completed a comprehensive health checkup sometime between 1964 and 1973, when they were 40-55 years old. They reported their number of miscarriages, number of children, ages at first and last menstrual period, and the total number of years in their reproductive period. Most of the group (68%) was white, but 16% were black, 6% Asian, and 5% Hispanic. Dr. Gilsanz looked at rates of dementia during 1996-2017, when the women were 62-86 years old.
A multivariate regression model controlled for age, race, education, midlife health issues (hypertension, smoking, and body mass index), hysterectomy, and late-life health issues (stroke, heart failure, and diabetes).
Half of the cohort (50%) had at least three children, and 75% had experienced at least one miscarriage. The average age at menarche was 13 years, and the average age at last natural menstrual period was 47 years. This equated to an average reproductive period of 34 years.
At the end of follow-up, 36% of the cohort had developed some type of dementia.
Women with at least three children were 12% less likely to develop dementia, compared with those with one child (hazard ratio, 0.88). The association remained significant even after researchers controlled for age, race, educational level, and hysterectomy.
Miscarriages also influenced the risk of dementia. Those who didn’t report one were 20% less likely to develop dementia than were those who had experienced at least one (HR, 0.81). The benefit of no miscarriage was even greater among women with at least three children, conferring a 28% reduced risk (HR, 0.72).
A shortened reproductive period increased the risk of dementia. Those who experienced menarche at 16 years or older had a 31% increased risk of dementia (HR, 1.31), and those who experienced their last period at age 45 or younger faced a 28% greater risk (HR, 1.28). Each additional year of reproductive capability was associated with a 2% decreased risk (HR, 0.98).
Women with a reproductive period of 21-30 years were 33% more likely to develop dementia than were those with a longer period (HR, 1.33)
“Reproductive events that signal different exposures to estrogen, like pregnancy and reproductive period may play a role in modulating dementia risk,” Dr. Gilsanz said. “Women who are less likely to have a miscarriage may have different hormonal milieu that may be neuroprotective. Underlying health conditions increasing the risk of miscarriages may also elevate risk of dementia.”
She had no financial disclosures.
SOURCE: Gilsanz P et al. AAIC 2018, Abstract P3-587
AT AAIC 2018
Key clinical point: More pregnancies and a longer reproductive period were associated with a significantly reduced risk of late-life dementia among women.
Major finding: Women with at least three children were 12% less likely to develop dementia than were those who had just one child.
Study details: The retrospective observational study comprised 14,595 women.
Disclosures: Dr. Gilsanz had no financial disclosures.
Source: Gilsanz P et al. AAIC 2018, Abstract P3-587.
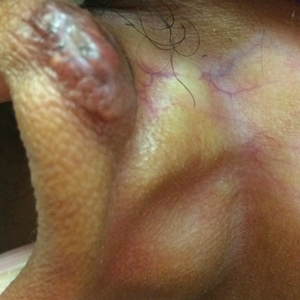
Hypopigmentation on the Ear
The Diagnosis: Corticosteroid-Induced Hypopigmentation
This patient received several intralesional injections of triamcinolone acetonide once monthly for treatment of the keloid scar on the left ear at an outside institution. There was improvement in the size of the keloid over time. On physical examination during the most recent visit there was a prominent streak of hypopigmentation and atrophy near the corticosteroid injection site with extension to the postauricular region. There also was telangiectasia noted within the area of hypopigmentation. Intralesional triamcinolone injections were discontinued and the patient was advised to return for monitoring.
Intra-articular and intralesional corticosteroid injections frequently are used by clinicians. Cutaneous complications associated with these injections include atrophy, pigmentary changes, hypersensitivity reactions, flushing, cellulitis, and necrotizing fasciitis. Tendon rupture also has been reported.1
There are several case reports in the literature describing hypopigmentation and/or subcutaneous atrophy after intralesional or intra-articular corticosteroid injections. A variety of underlying conditions were treated including alopecia areata, keloids, rheumatoid arthritis, de Quervain tendonitis, and psoriasis.2-6 The lesions typically are described as linear rays of atrophy and hypopigmentation at or near the injection site, with some cases noting extension along lymph channels and proximal veins.4,6 There usually is no associated pruritus or pain.3 This phenomenon can be seen after single or multiple injections.4,6
Extension of hypopigmentation from the site of injection has been postulated to be due to venous or lymphatic uptake.2,4-6 The mechanism of hypopigmentation is not known. Biopsy of a previously described case showed intact melanocytes along the dermoepidermal junction.2 Biopsy from another case revealed a decrease in melanin staining, which suggests a decrease in number or activity of melanocytes.4 It was proposed that hypopigmentation was secondary to loss of melanocyte function instead of loss of melanocytes.2 Spontaneous improvement or resolution of the hypopigmentation were noted in some cases ranging from 1 month to 1 year after initial presentation, but the hypopigmentation also can be persistent.3-6
Hypopigmented sarcoidosis and hypopigmented mycosis fungoides, both often present on dark-skinned individuals, are included in the differential diagnosis. Hypopigmented sarcoidosis presents with hypopigmented macules or patches, some with central papules, and hypopigmented mycosis fungoides presents with hypopigmented patches or plaques with fine scale and onset often in childhood or adolescence.7,8 Morphea can present with an initial inflammatory stage that develops into a sclerotic firm plaque or nodule with hyperpigmentation or hypopigmentation.9 Vitiligo usually presents with depigmented macules or patches and depigmented hair within the lesion.10
- Brinks A, Koes BW, Volkers AC, et al. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010;11:206.
- Venkatesan P, Fangman WL. Linear hypopigmentation and cutaneous atrophy following intra-articular steroid injections for de Quervain's tendonitis. J Drugs Dermatol. 2009;8:492-493.
- Evans AV, McGibbon DH. Symmetrical hypopigmentation following triamcinolone injection for de Quervain's tenosynovitis. Clin Exp Dermatol. 2002;27:247-251.
- Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-541.
- van Vendeloo SN, Ettema HB. Skin depigmentation along lymph vessels of the lower leg following local corticosteroid injection for interdigital neuroma. Foot Ankle Surg. 2016;22:139-141.
- Kumar P, Adolph S. Hypopigmentation along subcutaneous veins following intrakeloid triamcinolone injection: a case report and review of literature. Burns. 1998;24:487-488.
- Elgart ML. Cutaneous sarcoidosis: definitions and types of lesions. Clin Dermatol. 1986;4:35-45.
- El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, et al. Hypopigmented mycosis fungoides: frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002;26:450-457.
- Marzano AV, Menni S, Parodi A, et al. Localized scleroderma in adults and children. clinical and laboratory investigations on 239 cases. Eur J Dermatol. 2003;13:171-176.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
The Diagnosis: Corticosteroid-Induced Hypopigmentation
This patient received several intralesional injections of triamcinolone acetonide once monthly for treatment of the keloid scar on the left ear at an outside institution. There was improvement in the size of the keloid over time. On physical examination during the most recent visit there was a prominent streak of hypopigmentation and atrophy near the corticosteroid injection site with extension to the postauricular region. There also was telangiectasia noted within the area of hypopigmentation. Intralesional triamcinolone injections were discontinued and the patient was advised to return for monitoring.
Intra-articular and intralesional corticosteroid injections frequently are used by clinicians. Cutaneous complications associated with these injections include atrophy, pigmentary changes, hypersensitivity reactions, flushing, cellulitis, and necrotizing fasciitis. Tendon rupture also has been reported.1
There are several case reports in the literature describing hypopigmentation and/or subcutaneous atrophy after intralesional or intra-articular corticosteroid injections. A variety of underlying conditions were treated including alopecia areata, keloids, rheumatoid arthritis, de Quervain tendonitis, and psoriasis.2-6 The lesions typically are described as linear rays of atrophy and hypopigmentation at or near the injection site, with some cases noting extension along lymph channels and proximal veins.4,6 There usually is no associated pruritus or pain.3 This phenomenon can be seen after single or multiple injections.4,6
Extension of hypopigmentation from the site of injection has been postulated to be due to venous or lymphatic uptake.2,4-6 The mechanism of hypopigmentation is not known. Biopsy of a previously described case showed intact melanocytes along the dermoepidermal junction.2 Biopsy from another case revealed a decrease in melanin staining, which suggests a decrease in number or activity of melanocytes.4 It was proposed that hypopigmentation was secondary to loss of melanocyte function instead of loss of melanocytes.2 Spontaneous improvement or resolution of the hypopigmentation were noted in some cases ranging from 1 month to 1 year after initial presentation, but the hypopigmentation also can be persistent.3-6
Hypopigmented sarcoidosis and hypopigmented mycosis fungoides, both often present on dark-skinned individuals, are included in the differential diagnosis. Hypopigmented sarcoidosis presents with hypopigmented macules or patches, some with central papules, and hypopigmented mycosis fungoides presents with hypopigmented patches or plaques with fine scale and onset often in childhood or adolescence.7,8 Morphea can present with an initial inflammatory stage that develops into a sclerotic firm plaque or nodule with hyperpigmentation or hypopigmentation.9 Vitiligo usually presents with depigmented macules or patches and depigmented hair within the lesion.10
The Diagnosis: Corticosteroid-Induced Hypopigmentation
This patient received several intralesional injections of triamcinolone acetonide once monthly for treatment of the keloid scar on the left ear at an outside institution. There was improvement in the size of the keloid over time. On physical examination during the most recent visit there was a prominent streak of hypopigmentation and atrophy near the corticosteroid injection site with extension to the postauricular region. There also was telangiectasia noted within the area of hypopigmentation. Intralesional triamcinolone injections were discontinued and the patient was advised to return for monitoring.
Intra-articular and intralesional corticosteroid injections frequently are used by clinicians. Cutaneous complications associated with these injections include atrophy, pigmentary changes, hypersensitivity reactions, flushing, cellulitis, and necrotizing fasciitis. Tendon rupture also has been reported.1
There are several case reports in the literature describing hypopigmentation and/or subcutaneous atrophy after intralesional or intra-articular corticosteroid injections. A variety of underlying conditions were treated including alopecia areata, keloids, rheumatoid arthritis, de Quervain tendonitis, and psoriasis.2-6 The lesions typically are described as linear rays of atrophy and hypopigmentation at or near the injection site, with some cases noting extension along lymph channels and proximal veins.4,6 There usually is no associated pruritus or pain.3 This phenomenon can be seen after single or multiple injections.4,6
Extension of hypopigmentation from the site of injection has been postulated to be due to venous or lymphatic uptake.2,4-6 The mechanism of hypopigmentation is not known. Biopsy of a previously described case showed intact melanocytes along the dermoepidermal junction.2 Biopsy from another case revealed a decrease in melanin staining, which suggests a decrease in number or activity of melanocytes.4 It was proposed that hypopigmentation was secondary to loss of melanocyte function instead of loss of melanocytes.2 Spontaneous improvement or resolution of the hypopigmentation were noted in some cases ranging from 1 month to 1 year after initial presentation, but the hypopigmentation also can be persistent.3-6
Hypopigmented sarcoidosis and hypopigmented mycosis fungoides, both often present on dark-skinned individuals, are included in the differential diagnosis. Hypopigmented sarcoidosis presents with hypopigmented macules or patches, some with central papules, and hypopigmented mycosis fungoides presents with hypopigmented patches or plaques with fine scale and onset often in childhood or adolescence.7,8 Morphea can present with an initial inflammatory stage that develops into a sclerotic firm plaque or nodule with hyperpigmentation or hypopigmentation.9 Vitiligo usually presents with depigmented macules or patches and depigmented hair within the lesion.10
- Brinks A, Koes BW, Volkers AC, et al. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010;11:206.
- Venkatesan P, Fangman WL. Linear hypopigmentation and cutaneous atrophy following intra-articular steroid injections for de Quervain's tendonitis. J Drugs Dermatol. 2009;8:492-493.
- Evans AV, McGibbon DH. Symmetrical hypopigmentation following triamcinolone injection for de Quervain's tenosynovitis. Clin Exp Dermatol. 2002;27:247-251.
- Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-541.
- van Vendeloo SN, Ettema HB. Skin depigmentation along lymph vessels of the lower leg following local corticosteroid injection for interdigital neuroma. Foot Ankle Surg. 2016;22:139-141.
- Kumar P, Adolph S. Hypopigmentation along subcutaneous veins following intrakeloid triamcinolone injection: a case report and review of literature. Burns. 1998;24:487-488.
- Elgart ML. Cutaneous sarcoidosis: definitions and types of lesions. Clin Dermatol. 1986;4:35-45.
- El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, et al. Hypopigmented mycosis fungoides: frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002;26:450-457.
- Marzano AV, Menni S, Parodi A, et al. Localized scleroderma in adults and children. clinical and laboratory investigations on 239 cases. Eur J Dermatol. 2003;13:171-176.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Brinks A, Koes BW, Volkers AC, et al. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010;11:206.
- Venkatesan P, Fangman WL. Linear hypopigmentation and cutaneous atrophy following intra-articular steroid injections for de Quervain's tendonitis. J Drugs Dermatol. 2009;8:492-493.
- Evans AV, McGibbon DH. Symmetrical hypopigmentation following triamcinolone injection for de Quervain's tenosynovitis. Clin Exp Dermatol. 2002;27:247-251.
- Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537-541.
- van Vendeloo SN, Ettema HB. Skin depigmentation along lymph vessels of the lower leg following local corticosteroid injection for interdigital neuroma. Foot Ankle Surg. 2016;22:139-141.
- Kumar P, Adolph S. Hypopigmentation along subcutaneous veins following intrakeloid triamcinolone injection: a case report and review of literature. Burns. 1998;24:487-488.
- Elgart ML. Cutaneous sarcoidosis: definitions and types of lesions. Clin Dermatol. 1986;4:35-45.
- El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, et al. Hypopigmented mycosis fungoides: frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002;26:450-457.
- Marzano AV, Menni S, Parodi A, et al. Localized scleroderma in adults and children. clinical and laboratory investigations on 239 cases. Eur J Dermatol. 2003;13:171-176.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
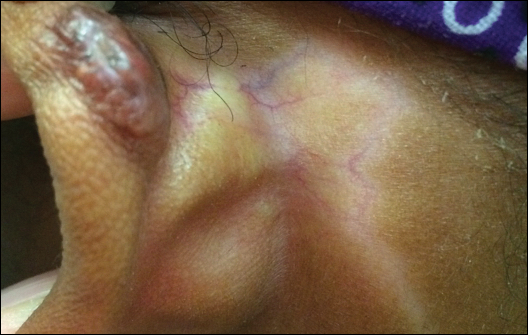
A 20-year-old black woman underwent multiple intralesional corticosteroid injections for treatment of a keloid on the superior aspect of the left helix and subsequently presented with a streak of atrophy and hypopigmentation in the postauricular region of unknown duration due to the lesion location.
FDA: Krintafel approved as ‘radical cure’ for preventing malaria relapse
The Food and Drug Administration has approved, under priority review, single-dose tafenoquine (to be marketed as Krintafel) for the prevention of relapse in patients aged 16 years and older who are receiving appropriate antimalarial therapy for acute Plasmodium vivax infection, according to an announcement by research partners GSK and the nonprofit Medicines for Malaria Venture.
Clinical efficacy and safety of the 300-mg single-dose tablet was provided by three randomized, double-blind studies: DETECTIVE Part 1 and Part 2 (TAF112582) and GATHER (TAF116564). The results of the two phase III studies were announced in June 2017.
Tafenoquine is referred to as a “radical cure,” because it targets the dormant liver forms of P. vivax and is coadministered with currently available antimalarials such as chloroquine or artemisinin-based combination therapies.
“The world has waited decades for a new medicine to counter P. vivax malaria relapse. Today, we can say the wait is over. Moreover, as the first ever single-dose for this indication, Krintafel will help improve patient compliance,” David Reddy, MD, CEO of MMV stated.
The FDA approval letter and the Krintafel label information are available online.
The Food and Drug Administration has approved, under priority review, single-dose tafenoquine (to be marketed as Krintafel) for the prevention of relapse in patients aged 16 years and older who are receiving appropriate antimalarial therapy for acute Plasmodium vivax infection, according to an announcement by research partners GSK and the nonprofit Medicines for Malaria Venture.
Clinical efficacy and safety of the 300-mg single-dose tablet was provided by three randomized, double-blind studies: DETECTIVE Part 1 and Part 2 (TAF112582) and GATHER (TAF116564). The results of the two phase III studies were announced in June 2017.
Tafenoquine is referred to as a “radical cure,” because it targets the dormant liver forms of P. vivax and is coadministered with currently available antimalarials such as chloroquine or artemisinin-based combination therapies.
“The world has waited decades for a new medicine to counter P. vivax malaria relapse. Today, we can say the wait is over. Moreover, as the first ever single-dose for this indication, Krintafel will help improve patient compliance,” David Reddy, MD, CEO of MMV stated.
The FDA approval letter and the Krintafel label information are available online.
The Food and Drug Administration has approved, under priority review, single-dose tafenoquine (to be marketed as Krintafel) for the prevention of relapse in patients aged 16 years and older who are receiving appropriate antimalarial therapy for acute Plasmodium vivax infection, according to an announcement by research partners GSK and the nonprofit Medicines for Malaria Venture.
Clinical efficacy and safety of the 300-mg single-dose tablet was provided by three randomized, double-blind studies: DETECTIVE Part 1 and Part 2 (TAF112582) and GATHER (TAF116564). The results of the two phase III studies were announced in June 2017.
Tafenoquine is referred to as a “radical cure,” because it targets the dormant liver forms of P. vivax and is coadministered with currently available antimalarials such as chloroquine or artemisinin-based combination therapies.
“The world has waited decades for a new medicine to counter P. vivax malaria relapse. Today, we can say the wait is over. Moreover, as the first ever single-dose for this indication, Krintafel will help improve patient compliance,” David Reddy, MD, CEO of MMV stated.
The FDA approval letter and the Krintafel label information are available online.
Gates Foundation, Lauder family launch $30 million Alzheimer’s biomarker search
Two billionaires have joined forces in a $30 million effort to develop peripheral biomarkers for the early detection of Alzheimer’s disease and other dementias.
Bill Gates and Leonard Lauder, cofounder of the Alzheimer’s Drug Discovery Foundation (ADDF) and chairman emeritus of Estée Lauder, provided initial funding for the project, dubbed Diagnostics Accelerator. Support from other philanthropists, including the Dolby family and the Charles and Helen Schwab Foundation, will bring the initiative up to its full funding capacity, according to an ADDF press statement.
“Over the next 3 years, we will provide more than $30 million in grants to researchers who are working on the most promising and innovative ideas to help diagnose Alzheimer’s disease [AD] early before the more devastating symptoms occur,” Mr. Lauder said in the statement.
Researchers at academic centers and nonprofit organizations are eligible to apply for the program; biotech companies and startups are also invited. Letters of intent for the first round of funding are due by Sept. 14, and the final proposals are due Nov. 16. Conducted under the auspices of the ADDF, the program will employ strict scientific review of all proposals and grant priority to blood and other peripheral markers, including saliva, urine, and ocular biomarkers. Neuroimaging and cerebrospinal fluid biomarkers won’t be considered, although researchers may use these as validation tests for their investigational markers.
Target areas include, but aren’t limited to:
- Neuroprotection
- Neurodegeneration
- Protein misfolding
- Synaptic integrity and/or activity
- Vascular injury and blood-brain barrier integrity
- Mitochondria and metabolic function
- Oxidative stress
- White matter changes
The initiative reflects recent emphasis on the critical role of biomarkers in drug development; this is especially important because emerging data paint a clear picture of a long AD prodrome during which the disease might be more amenable to treatment, ADDF’s Howard Fillit, MD, said in the press statement.
“The significance of biomarkers in Alzheimer’s disease research is underscored by recent FDA [Food and Drug Administration] guidelines that recognize the critical role of biomarkers in drug development and shift the research definition of the early stages of the disease to include biomarkers, even before clinical symptoms become apparent. ... Like in cancer today, using the biomarker-specific model of precision medicine, we will be able to predict more accurately which treatment and prevention strategies will work in different at-risk populations of people who have Alzheimer’s disease or other forms of dementia.”
Diagnostics Accelerator will award two general types of grants. Proof-of-principle awards of up to $500,000 will support exploratory analyses of biomarkers in smaller human sample sizes of 50-100. Proposals must be supported by human data that demonstrate that the candidate markers correspond with disease pathophysiology. Preliminary assay validation data for the proposed studies should be included. Successful proof-of principle projects may be eligible for follow-on funding in the form of a validation award.
Validation awards will support exploring biomarkers that need to be tested at a larger scale (500-1,000 samples). These must be supported by a significant extant body of human data that demonstrate that the biomarker corresponds to disease pathophysiology. Validation studies should compare peripheral analytes to quantitative measurements using PET imaging and/or cerebrospinal fluid testing, and not cognition alone. Award amounts will be based on stage and scope of research.
The announcement drew praise from researchers and clinicians.
“I think this is a terrific initiative,” said Michael S. Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. “It’s so important to identify those at high risk of developing AD well before the onset of symptoms. All clinical trials for disease-modifying therapies have failed because these have enrolled subjects who already have AD or MCI. Preventing or delaying disease onset [i.e., prophylaxis] is the way forward, but this will require specific predictive biomarkers.”
Richard J. Caselli, MD, a professor of neurology at the Mayo Clinic Arizona in Scottsdale and clinical core director of the Arizona Alzheimer’s Disease Center, agreed.
“I would add that we are still trying to understand how various ‘experiences’ might push us toward Alzheimer’s disease; head injury, diabetes, and air pollution, for example. We know already, based on existing genomic and acquired risk factors, that AD is a complex disease and that many disparate things can influence our risk. Are each of these equally important in each of us or do we each have our own unique vulnerability profile? New biomarkers can help us determine that, and if we are in fact each unique, then maybe prevention strategies need to be personalized.”
Two billionaires have joined forces in a $30 million effort to develop peripheral biomarkers for the early detection of Alzheimer’s disease and other dementias.
Bill Gates and Leonard Lauder, cofounder of the Alzheimer’s Drug Discovery Foundation (ADDF) and chairman emeritus of Estée Lauder, provided initial funding for the project, dubbed Diagnostics Accelerator. Support from other philanthropists, including the Dolby family and the Charles and Helen Schwab Foundation, will bring the initiative up to its full funding capacity, according to an ADDF press statement.
“Over the next 3 years, we will provide more than $30 million in grants to researchers who are working on the most promising and innovative ideas to help diagnose Alzheimer’s disease [AD] early before the more devastating symptoms occur,” Mr. Lauder said in the statement.
Researchers at academic centers and nonprofit organizations are eligible to apply for the program; biotech companies and startups are also invited. Letters of intent for the first round of funding are due by Sept. 14, and the final proposals are due Nov. 16. Conducted under the auspices of the ADDF, the program will employ strict scientific review of all proposals and grant priority to blood and other peripheral markers, including saliva, urine, and ocular biomarkers. Neuroimaging and cerebrospinal fluid biomarkers won’t be considered, although researchers may use these as validation tests for their investigational markers.
Target areas include, but aren’t limited to:
- Neuroprotection
- Neurodegeneration
- Protein misfolding
- Synaptic integrity and/or activity
- Vascular injury and blood-brain barrier integrity
- Mitochondria and metabolic function
- Oxidative stress
- White matter changes
The initiative reflects recent emphasis on the critical role of biomarkers in drug development; this is especially important because emerging data paint a clear picture of a long AD prodrome during which the disease might be more amenable to treatment, ADDF’s Howard Fillit, MD, said in the press statement.
“The significance of biomarkers in Alzheimer’s disease research is underscored by recent FDA [Food and Drug Administration] guidelines that recognize the critical role of biomarkers in drug development and shift the research definition of the early stages of the disease to include biomarkers, even before clinical symptoms become apparent. ... Like in cancer today, using the biomarker-specific model of precision medicine, we will be able to predict more accurately which treatment and prevention strategies will work in different at-risk populations of people who have Alzheimer’s disease or other forms of dementia.”
Diagnostics Accelerator will award two general types of grants. Proof-of-principle awards of up to $500,000 will support exploratory analyses of biomarkers in smaller human sample sizes of 50-100. Proposals must be supported by human data that demonstrate that the candidate markers correspond with disease pathophysiology. Preliminary assay validation data for the proposed studies should be included. Successful proof-of principle projects may be eligible for follow-on funding in the form of a validation award.
Validation awards will support exploring biomarkers that need to be tested at a larger scale (500-1,000 samples). These must be supported by a significant extant body of human data that demonstrate that the biomarker corresponds to disease pathophysiology. Validation studies should compare peripheral analytes to quantitative measurements using PET imaging and/or cerebrospinal fluid testing, and not cognition alone. Award amounts will be based on stage and scope of research.
The announcement drew praise from researchers and clinicians.
“I think this is a terrific initiative,” said Michael S. Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. “It’s so important to identify those at high risk of developing AD well before the onset of symptoms. All clinical trials for disease-modifying therapies have failed because these have enrolled subjects who already have AD or MCI. Preventing or delaying disease onset [i.e., prophylaxis] is the way forward, but this will require specific predictive biomarkers.”
Richard J. Caselli, MD, a professor of neurology at the Mayo Clinic Arizona in Scottsdale and clinical core director of the Arizona Alzheimer’s Disease Center, agreed.
“I would add that we are still trying to understand how various ‘experiences’ might push us toward Alzheimer’s disease; head injury, diabetes, and air pollution, for example. We know already, based on existing genomic and acquired risk factors, that AD is a complex disease and that many disparate things can influence our risk. Are each of these equally important in each of us or do we each have our own unique vulnerability profile? New biomarkers can help us determine that, and if we are in fact each unique, then maybe prevention strategies need to be personalized.”
Two billionaires have joined forces in a $30 million effort to develop peripheral biomarkers for the early detection of Alzheimer’s disease and other dementias.
Bill Gates and Leonard Lauder, cofounder of the Alzheimer’s Drug Discovery Foundation (ADDF) and chairman emeritus of Estée Lauder, provided initial funding for the project, dubbed Diagnostics Accelerator. Support from other philanthropists, including the Dolby family and the Charles and Helen Schwab Foundation, will bring the initiative up to its full funding capacity, according to an ADDF press statement.
“Over the next 3 years, we will provide more than $30 million in grants to researchers who are working on the most promising and innovative ideas to help diagnose Alzheimer’s disease [AD] early before the more devastating symptoms occur,” Mr. Lauder said in the statement.
Researchers at academic centers and nonprofit organizations are eligible to apply for the program; biotech companies and startups are also invited. Letters of intent for the first round of funding are due by Sept. 14, and the final proposals are due Nov. 16. Conducted under the auspices of the ADDF, the program will employ strict scientific review of all proposals and grant priority to blood and other peripheral markers, including saliva, urine, and ocular biomarkers. Neuroimaging and cerebrospinal fluid biomarkers won’t be considered, although researchers may use these as validation tests for their investigational markers.
Target areas include, but aren’t limited to:
- Neuroprotection
- Neurodegeneration
- Protein misfolding
- Synaptic integrity and/or activity
- Vascular injury and blood-brain barrier integrity
- Mitochondria and metabolic function
- Oxidative stress
- White matter changes
The initiative reflects recent emphasis on the critical role of biomarkers in drug development; this is especially important because emerging data paint a clear picture of a long AD prodrome during which the disease might be more amenable to treatment, ADDF’s Howard Fillit, MD, said in the press statement.
“The significance of biomarkers in Alzheimer’s disease research is underscored by recent FDA [Food and Drug Administration] guidelines that recognize the critical role of biomarkers in drug development and shift the research definition of the early stages of the disease to include biomarkers, even before clinical symptoms become apparent. ... Like in cancer today, using the biomarker-specific model of precision medicine, we will be able to predict more accurately which treatment and prevention strategies will work in different at-risk populations of people who have Alzheimer’s disease or other forms of dementia.”
Diagnostics Accelerator will award two general types of grants. Proof-of-principle awards of up to $500,000 will support exploratory analyses of biomarkers in smaller human sample sizes of 50-100. Proposals must be supported by human data that demonstrate that the candidate markers correspond with disease pathophysiology. Preliminary assay validation data for the proposed studies should be included. Successful proof-of principle projects may be eligible for follow-on funding in the form of a validation award.
Validation awards will support exploring biomarkers that need to be tested at a larger scale (500-1,000 samples). These must be supported by a significant extant body of human data that demonstrate that the biomarker corresponds to disease pathophysiology. Validation studies should compare peripheral analytes to quantitative measurements using PET imaging and/or cerebrospinal fluid testing, and not cognition alone. Award amounts will be based on stage and scope of research.
The announcement drew praise from researchers and clinicians.
“I think this is a terrific initiative,” said Michael S. Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence. “It’s so important to identify those at high risk of developing AD well before the onset of symptoms. All clinical trials for disease-modifying therapies have failed because these have enrolled subjects who already have AD or MCI. Preventing or delaying disease onset [i.e., prophylaxis] is the way forward, but this will require specific predictive biomarkers.”
Richard J. Caselli, MD, a professor of neurology at the Mayo Clinic Arizona in Scottsdale and clinical core director of the Arizona Alzheimer’s Disease Center, agreed.
“I would add that we are still trying to understand how various ‘experiences’ might push us toward Alzheimer’s disease; head injury, diabetes, and air pollution, for example. We know already, based on existing genomic and acquired risk factors, that AD is a complex disease and that many disparate things can influence our risk. Are each of these equally important in each of us or do we each have our own unique vulnerability profile? New biomarkers can help us determine that, and if we are in fact each unique, then maybe prevention strategies need to be personalized.”
Simplified formula validated for MRI Crohn’s disease assessment
WASHINGTON – The same Spanish research team who introduced an MRI-based formula for scoring the activity of luminal Crohn’s disease in 2011 have developed and validated a new, simplified version of their MRI score that speeds assessment.
“The simplified version of the MaRIA [Magnetic Resonance Index of Activity] score allows a faster and easier assessment of inflammation and quantification of severity in Crohn’s disease by keeping high accuracy for diagnosis and therapeutic response,” Ingrid Ordás, MD, said at the annual Digestive Disease Week®. The main advantage of the simplified MaRIA is that it is a “less time-consuming calculation that is not confounded by missing segments,” said Dr. Ordás, a gastroenterologist at the Hospital Clinic of Barcelona.
Although the data reported by Dr. Ordás included the derivation results, which used 98 patients enrolled in two separate prospective studies, and a separate prospective validation cohort of 37 patients, all these patients were evaluated by clinicians at the Hospital Clinic of Barcelona, and hence further validation with patients enrolled at other sites is now needed, Dr. Ordás said in an interview. Further accumulation of evidence for high sensitivity and specificity of Crohn’s disease assessment using the simplified MaRIA could allow it to replace endoscopy as the standard tool for assessing disease activity and severity in patients with luminal Crohn’s disease.
The derivation phase of the study identified four features that significantly correlated with disease activity and severity: bowel wall thickening to more than 3 mm, mural edema, perienteric fat stranding, and mucosal ulcerations. Limiting assessment to these four features cut in half the elements in the original MaRIA (Inflamm Bowel Dis. 2011 Aug;17[8];1759-68). Fat stranding – loss of the usual sharp interface between the wall and mesentery because of fluid – is a new parameter in the simplified MaRIA. The other three elements had been in the original index, but several other elements are now gone, including relative contrast enhancement wall signal intensity and consideration of lymph nodes.
In the validation phase, the researchers compared the MaRIA findings of the validation cohort with endoscopy findings both at baseline and then after they had received treatment. The sensitivity and specificity of the simplified MaRIA depended on the cutoff used, but as an example, a patient with a simplified MaRIA of 1 or greater as having active disease had a sensitivity of 90%, specificity of 81%, and an area under the receiver operator characteristic curve of 0.91. Using a simplified MaRIA of at least 2 as indicative of severe disease had a sensitivity of 85%, a specificity of 92%, and an AUROC of 0.94, Dr. Ordás reported.
Further assessment in patients who underwent treatment showed that reductions in the simplified MaRIA significantly correlated with treatment responses and remained essentially unchanged in patients who did not have clinical response to treatment. The analysis also showed a strong, positive correlation coefficient of 0.83 when the simplified MaRIA of an individual patient, compared with the patient’s Crohn’s disease endoscopy index of severity, and a correlation coefficient of 0.94 when a patient’s simplified MaRIA determined by one clinician, compared with the index score calculated by a second clinician.
Dr. Ordás had no disclosures to report.
SOURCE: Ordás I et al. DDW 2018, Presentation 437.
WASHINGTON – The same Spanish research team who introduced an MRI-based formula for scoring the activity of luminal Crohn’s disease in 2011 have developed and validated a new, simplified version of their MRI score that speeds assessment.
“The simplified version of the MaRIA [Magnetic Resonance Index of Activity] score allows a faster and easier assessment of inflammation and quantification of severity in Crohn’s disease by keeping high accuracy for diagnosis and therapeutic response,” Ingrid Ordás, MD, said at the annual Digestive Disease Week®. The main advantage of the simplified MaRIA is that it is a “less time-consuming calculation that is not confounded by missing segments,” said Dr. Ordás, a gastroenterologist at the Hospital Clinic of Barcelona.
Although the data reported by Dr. Ordás included the derivation results, which used 98 patients enrolled in two separate prospective studies, and a separate prospective validation cohort of 37 patients, all these patients were evaluated by clinicians at the Hospital Clinic of Barcelona, and hence further validation with patients enrolled at other sites is now needed, Dr. Ordás said in an interview. Further accumulation of evidence for high sensitivity and specificity of Crohn’s disease assessment using the simplified MaRIA could allow it to replace endoscopy as the standard tool for assessing disease activity and severity in patients with luminal Crohn’s disease.
The derivation phase of the study identified four features that significantly correlated with disease activity and severity: bowel wall thickening to more than 3 mm, mural edema, perienteric fat stranding, and mucosal ulcerations. Limiting assessment to these four features cut in half the elements in the original MaRIA (Inflamm Bowel Dis. 2011 Aug;17[8];1759-68). Fat stranding – loss of the usual sharp interface between the wall and mesentery because of fluid – is a new parameter in the simplified MaRIA. The other three elements had been in the original index, but several other elements are now gone, including relative contrast enhancement wall signal intensity and consideration of lymph nodes.
In the validation phase, the researchers compared the MaRIA findings of the validation cohort with endoscopy findings both at baseline and then after they had received treatment. The sensitivity and specificity of the simplified MaRIA depended on the cutoff used, but as an example, a patient with a simplified MaRIA of 1 or greater as having active disease had a sensitivity of 90%, specificity of 81%, and an area under the receiver operator characteristic curve of 0.91. Using a simplified MaRIA of at least 2 as indicative of severe disease had a sensitivity of 85%, a specificity of 92%, and an AUROC of 0.94, Dr. Ordás reported.
Further assessment in patients who underwent treatment showed that reductions in the simplified MaRIA significantly correlated with treatment responses and remained essentially unchanged in patients who did not have clinical response to treatment. The analysis also showed a strong, positive correlation coefficient of 0.83 when the simplified MaRIA of an individual patient, compared with the patient’s Crohn’s disease endoscopy index of severity, and a correlation coefficient of 0.94 when a patient’s simplified MaRIA determined by one clinician, compared with the index score calculated by a second clinician.
Dr. Ordás had no disclosures to report.
SOURCE: Ordás I et al. DDW 2018, Presentation 437.
WASHINGTON – The same Spanish research team who introduced an MRI-based formula for scoring the activity of luminal Crohn’s disease in 2011 have developed and validated a new, simplified version of their MRI score that speeds assessment.
“The simplified version of the MaRIA [Magnetic Resonance Index of Activity] score allows a faster and easier assessment of inflammation and quantification of severity in Crohn’s disease by keeping high accuracy for diagnosis and therapeutic response,” Ingrid Ordás, MD, said at the annual Digestive Disease Week®. The main advantage of the simplified MaRIA is that it is a “less time-consuming calculation that is not confounded by missing segments,” said Dr. Ordás, a gastroenterologist at the Hospital Clinic of Barcelona.
Although the data reported by Dr. Ordás included the derivation results, which used 98 patients enrolled in two separate prospective studies, and a separate prospective validation cohort of 37 patients, all these patients were evaluated by clinicians at the Hospital Clinic of Barcelona, and hence further validation with patients enrolled at other sites is now needed, Dr. Ordás said in an interview. Further accumulation of evidence for high sensitivity and specificity of Crohn’s disease assessment using the simplified MaRIA could allow it to replace endoscopy as the standard tool for assessing disease activity and severity in patients with luminal Crohn’s disease.
The derivation phase of the study identified four features that significantly correlated with disease activity and severity: bowel wall thickening to more than 3 mm, mural edema, perienteric fat stranding, and mucosal ulcerations. Limiting assessment to these four features cut in half the elements in the original MaRIA (Inflamm Bowel Dis. 2011 Aug;17[8];1759-68). Fat stranding – loss of the usual sharp interface between the wall and mesentery because of fluid – is a new parameter in the simplified MaRIA. The other three elements had been in the original index, but several other elements are now gone, including relative contrast enhancement wall signal intensity and consideration of lymph nodes.
In the validation phase, the researchers compared the MaRIA findings of the validation cohort with endoscopy findings both at baseline and then after they had received treatment. The sensitivity and specificity of the simplified MaRIA depended on the cutoff used, but as an example, a patient with a simplified MaRIA of 1 or greater as having active disease had a sensitivity of 90%, specificity of 81%, and an area under the receiver operator characteristic curve of 0.91. Using a simplified MaRIA of at least 2 as indicative of severe disease had a sensitivity of 85%, a specificity of 92%, and an AUROC of 0.94, Dr. Ordás reported.
Further assessment in patients who underwent treatment showed that reductions in the simplified MaRIA significantly correlated with treatment responses and remained essentially unchanged in patients who did not have clinical response to treatment. The analysis also showed a strong, positive correlation coefficient of 0.83 when the simplified MaRIA of an individual patient, compared with the patient’s Crohn’s disease endoscopy index of severity, and a correlation coefficient of 0.94 when a patient’s simplified MaRIA determined by one clinician, compared with the index score calculated by a second clinician.
Dr. Ordás had no disclosures to report.
SOURCE: Ordás I et al. DDW 2018, Presentation 437.
REPORTING FROM DDW 2018
Key clinical point: Researchers devised a simplified way to use MRI to noninvasively assess Crohn’s disease activity.
Major finding: The simplified, MRI-based formula identified Crohn’s disease activity with 90% sensitivity and 81% specificity.
Study details: The validation study included 37 patients with luminal Crohn’s disease at a single center in Barcelona.
Disclosures: Dr. Ordás had no disclosures to report.
Source: Ordás I et al. DDW 2018, Presentation 437.
Endoscopic weight loss interventions need lifestyle component
WASHINGTON – and used in the context of a multidimensional lifestyle intervention, Shelby Sullivan, MD, said at the annual Digestive Disease Week.®
Regardless of which endoscopic intervention a clinician uses, the chances for successful and complication-free weight loss highly depends on enlisting adjunctive care by specialists, including a dietitian, behavior coach, psychologist, exercise specialist, and an endocrine or obesity-specialist physician, said Dr. Sullivan, director of the gastroenterology metabolic and bariatric program at the University of Colorado, Aurora. Often it’s more cost effective to arrange for collaboration with these adjunctive specialists as consultants rather than having them on staff, she noted.
A weight loss program that provides at least 14 interventions with the patient over a 6-month period has led to a 5% greater increase in weight loss compared with a moderate-intensity program that includes 6-13 encounters with members of the weight-loss team, Dr. Sullivan said. These intervention episodes need not all be individual or one on one, but can include group sessions, telephone consults, and even online coaching sessions, according to 2013 recommendations from The Obesity Society, the American College of Cardiology, and the American Heart Association (Obesity. 2014 Jun 24;22[S2]:S5-S39). “Patient contact is the key to success with weight loss,” Dr. Sullivan said. She also strongly suggested that clinicians who wish to offer an obesity intervention “get training in delivering basic obesity education.”
Another tip for providers is to have protocols in place to both prevent and, when necessary, manage potential complications. This can involve administration of additional antibiotics beyond what’s used for prophylaxis, treatment with additional IV fluid, and imaging. Complication prevention and management of complications when they occur are two of the most important steps to take to make sure that an elective obesity intervention practice runs smoothly, Dr. Sullivan said. “Make sure you can manage these patients safely,” she admonished. Also, be sure to arrange in advance for institutional approval for using whatever devices the procedure requires, and make sure you have malpractice coverage for any novel devices or procedures. Approval for use of a novel device often requires documentation of specialized training or certification.
Endoscopic weight loss procedures often are not fully or even partially covered by health insurance, which means that patients will pay most or all of the costs out of pocket and, hence, the clinician should look on this practice as a “concierge service.” Therefore, the clinician should be especially attuned to ensuring that the staff is uniformly courteous, and be alert for any overt or covert obesity bias the staff may have that could mar a patient’s experience. You need a “reliable and compassionate” staff, Dr. Sullivan advised, and the staff should schedule patient appointments that minimize wait times.
Marketing and procedure pricing are other concerns for the physician who is contemplating an obestiy-intervention practice. A great marketing tool is delivering seminars to patients, either in person or on the Internet. The general format for such a seminar addresses the health risks of obesity, the range of intervention options in addition to what you are offering, and the objective risks and benefits for each of the intervention options. Prospective patients who respond to your presentation and contact you should receive very prompt callbacks. Regarding pricing, Dr. Sullivan recommended making sure that the price you charge will fully cover all costs, including the potential cost of complications.
WASHINGTON – and used in the context of a multidimensional lifestyle intervention, Shelby Sullivan, MD, said at the annual Digestive Disease Week.®
Regardless of which endoscopic intervention a clinician uses, the chances for successful and complication-free weight loss highly depends on enlisting adjunctive care by specialists, including a dietitian, behavior coach, psychologist, exercise specialist, and an endocrine or obesity-specialist physician, said Dr. Sullivan, director of the gastroenterology metabolic and bariatric program at the University of Colorado, Aurora. Often it’s more cost effective to arrange for collaboration with these adjunctive specialists as consultants rather than having them on staff, she noted.
A weight loss program that provides at least 14 interventions with the patient over a 6-month period has led to a 5% greater increase in weight loss compared with a moderate-intensity program that includes 6-13 encounters with members of the weight-loss team, Dr. Sullivan said. These intervention episodes need not all be individual or one on one, but can include group sessions, telephone consults, and even online coaching sessions, according to 2013 recommendations from The Obesity Society, the American College of Cardiology, and the American Heart Association (Obesity. 2014 Jun 24;22[S2]:S5-S39). “Patient contact is the key to success with weight loss,” Dr. Sullivan said. She also strongly suggested that clinicians who wish to offer an obesity intervention “get training in delivering basic obesity education.”
Another tip for providers is to have protocols in place to both prevent and, when necessary, manage potential complications. This can involve administration of additional antibiotics beyond what’s used for prophylaxis, treatment with additional IV fluid, and imaging. Complication prevention and management of complications when they occur are two of the most important steps to take to make sure that an elective obesity intervention practice runs smoothly, Dr. Sullivan said. “Make sure you can manage these patients safely,” she admonished. Also, be sure to arrange in advance for institutional approval for using whatever devices the procedure requires, and make sure you have malpractice coverage for any novel devices or procedures. Approval for use of a novel device often requires documentation of specialized training or certification.
Endoscopic weight loss procedures often are not fully or even partially covered by health insurance, which means that patients will pay most or all of the costs out of pocket and, hence, the clinician should look on this practice as a “concierge service.” Therefore, the clinician should be especially attuned to ensuring that the staff is uniformly courteous, and be alert for any overt or covert obesity bias the staff may have that could mar a patient’s experience. You need a “reliable and compassionate” staff, Dr. Sullivan advised, and the staff should schedule patient appointments that minimize wait times.
Marketing and procedure pricing are other concerns for the physician who is contemplating an obestiy-intervention practice. A great marketing tool is delivering seminars to patients, either in person or on the Internet. The general format for such a seminar addresses the health risks of obesity, the range of intervention options in addition to what you are offering, and the objective risks and benefits for each of the intervention options. Prospective patients who respond to your presentation and contact you should receive very prompt callbacks. Regarding pricing, Dr. Sullivan recommended making sure that the price you charge will fully cover all costs, including the potential cost of complications.
WASHINGTON – and used in the context of a multidimensional lifestyle intervention, Shelby Sullivan, MD, said at the annual Digestive Disease Week.®
Regardless of which endoscopic intervention a clinician uses, the chances for successful and complication-free weight loss highly depends on enlisting adjunctive care by specialists, including a dietitian, behavior coach, psychologist, exercise specialist, and an endocrine or obesity-specialist physician, said Dr. Sullivan, director of the gastroenterology metabolic and bariatric program at the University of Colorado, Aurora. Often it’s more cost effective to arrange for collaboration with these adjunctive specialists as consultants rather than having them on staff, she noted.
A weight loss program that provides at least 14 interventions with the patient over a 6-month period has led to a 5% greater increase in weight loss compared with a moderate-intensity program that includes 6-13 encounters with members of the weight-loss team, Dr. Sullivan said. These intervention episodes need not all be individual or one on one, but can include group sessions, telephone consults, and even online coaching sessions, according to 2013 recommendations from The Obesity Society, the American College of Cardiology, and the American Heart Association (Obesity. 2014 Jun 24;22[S2]:S5-S39). “Patient contact is the key to success with weight loss,” Dr. Sullivan said. She also strongly suggested that clinicians who wish to offer an obesity intervention “get training in delivering basic obesity education.”
Another tip for providers is to have protocols in place to both prevent and, when necessary, manage potential complications. This can involve administration of additional antibiotics beyond what’s used for prophylaxis, treatment with additional IV fluid, and imaging. Complication prevention and management of complications when they occur are two of the most important steps to take to make sure that an elective obesity intervention practice runs smoothly, Dr. Sullivan said. “Make sure you can manage these patients safely,” she admonished. Also, be sure to arrange in advance for institutional approval for using whatever devices the procedure requires, and make sure you have malpractice coverage for any novel devices or procedures. Approval for use of a novel device often requires documentation of specialized training or certification.
Endoscopic weight loss procedures often are not fully or even partially covered by health insurance, which means that patients will pay most or all of the costs out of pocket and, hence, the clinician should look on this practice as a “concierge service.” Therefore, the clinician should be especially attuned to ensuring that the staff is uniformly courteous, and be alert for any overt or covert obesity bias the staff may have that could mar a patient’s experience. You need a “reliable and compassionate” staff, Dr. Sullivan advised, and the staff should schedule patient appointments that minimize wait times.
Marketing and procedure pricing are other concerns for the physician who is contemplating an obestiy-intervention practice. A great marketing tool is delivering seminars to patients, either in person or on the Internet. The general format for such a seminar addresses the health risks of obesity, the range of intervention options in addition to what you are offering, and the objective risks and benefits for each of the intervention options. Prospective patients who respond to your presentation and contact you should receive very prompt callbacks. Regarding pricing, Dr. Sullivan recommended making sure that the price you charge will fully cover all costs, including the potential cost of complications.
EXPERT ANALYSIS FROM DDW 2018
The diagnosis and surgical repair of vesicovaginal fistula
Vesicovaginal fistulas (VVFs) are the most common type of urogenital fistulas – approximately three times more common than ureterovaginal fistulas – and can be a debilitating problem for women.
Most of the research published in recent years on VVFs and other urogenital fistulas comes from developing countries where these abnormal communications are a common complication of obstructed labor. In the United States, despite a relative paucity of data, VVFs are known to occur most often as a sequelae of gynecologic surgery, usually hysterectomy. Estimates of the incidence of VVF and other urogenital fistula formation are debated but have ranged from 0.5% or less after simple hysterectomy to as high as 2% after radical hysterectomy. Most VVFs are believed to occur after hysterectomy performed for benign disease, and many – but not all – are caused by inadvertent bladder injury that was not recognized intraoperatively.
Women who have had one or more cesarean deliveries and those who have had prior pelvic or vaginal surgery are at increased risk. In addition, both radiation therapy and inflammation that occur with diseases such as pelvic inflammatory disease or inflammatory bowel disease can negatively affect tissue quality and healing from surgical procedures – and can lead ultimately to the development of urogenital fistulas – although even less is known about incidence in these cases.
Prevention
Intraoperatively, VVFs may best be prevented through careful mobilization of the bladder off the vaginal wall, the use of delayed absorbable sutures (preferably Vicryl sutures), and the use of cystoscopy to assess the bladder for injury. If cystoscopy is not available, retrograde filling with a Foley catheter will still be helpful.
An overly aggressive approach to creating the bladder flap during hysterectomy and other surgeries can increase the risk of devascularization and the subsequent formation of fistulas. When the blood supply is found to have been compromised, affected tissue can be strengthened by oversewing with imbrication. When an inadvertent cystotomy is identified, repair is often best achieved with omental tissue interposed between the bladder and vagina. If there is any doubt about bladder integrity, an interposition graft between the bladder flap and the vaginal cuff will help reduce the incidence of fistula formation. Whenever overlapping suture lines occur (the vaginal cuff and the cystotomy repair), the risk of VVF formation will increase. Other than that using omentum, peritoneal grafts will also work well.
VVF formation may still occur, however, despite recognition and repair of an injury – and despite normal findings on cystoscopy. In patients who have had prior cesarean deliveries or other prior pelvic surgery, for example, tissue devascularization may cause a delayed injury, with the process of tissue necrosis and VVF formation occurring up to a month after surgery. It is important to appreciate the factors that predispose patients to VVF and to anticipate an increased risk, but in many cases of delayed VVF, it’s quite possible that nothing could have been done to prevent the problem.
Work-up
Vesicovaginal fistulas typically present as painless, continuous urine leakage from the vagina. The medical history should include standard questions about pelvic health history and symptom characteristics (in order to exclude hematuria or leakage of fluid other than urine), as well as questions aimed at differentiating symptoms of VVF from other causes of urinary incontinence, such as stress incontinence. In my experience, urine leakage is often incorrectly dismissed as stress incontinence when it is actually VVF. A high index of suspicion will help make an earlier diagnosis. This does not usually change the management, but helps manage the anxiety, expectations, and needs of the patient.
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.
A VVF that is larger may require the use of additional sources of tissue. A graft called the Martius graft, or labial fibrofatty tissue graft, is sometimes used to reinforce repairs of larger fistulas, even those that are high in the vaginal vault. The procedure involves a vertical incision on the inner side of the labium majus and detachment of fibroadipose tissue from its underlying bulbocavernosus muscle. This fat-pad flap is vascularized and thus serves as a pedicled graft. It can be tunneled under the vaginal epithelium to reach the site of closure. The procedure has limited use with the vaginal approach to VVF, but is important to be aware of.
Other sources of grafts or flaps that can sometimes be used with the vaginal approach include the gracilis muscle, the gluteal muscle and peritoneum, and fasciocutaneous tissue from the inner thigh.
The avoidance of overlapping suture lines and multiple layers of closure will help ensure a water-tight closure. If there is any leakage upon testing the integrity of the repair, particularly one that is vaginally approached, such leakage will continue and the repair will have been unsuccessful. In an abdominal surgery for VVF, a small amount of remaining leakage will probably resolve on its own after 10-14 days of catheter placement.
Placement of a Jackson-Pratt (JP) drain is controversial. It has been suggested that a JP drain placed on continuous suction will pull urine out of the bladder and increase the risk of a fistula. I don’t place a JP drain in my repairs as I find them to not be helpful. A cystogram can be done 1 week after repair to confirm healing, but there is some debate about whether or not the procedure is useful at that point. In my experience, if the patient does not have a cystogram and gets postrepair leakage, I have the same information as I would have obtained through a positive finding on a cystogram.
Dr. Garely is chair of obstetrics and gynecology and director of urogynecology and pelvic reconstructive surgery at the South Nassau Communities Hospital, Oceanside, N.Y., and a clinical professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York. He has no disclosures related to this column.
Vesicovaginal fistulas (VVFs) are the most common type of urogenital fistulas – approximately three times more common than ureterovaginal fistulas – and can be a debilitating problem for women.
Most of the research published in recent years on VVFs and other urogenital fistulas comes from developing countries where these abnormal communications are a common complication of obstructed labor. In the United States, despite a relative paucity of data, VVFs are known to occur most often as a sequelae of gynecologic surgery, usually hysterectomy. Estimates of the incidence of VVF and other urogenital fistula formation are debated but have ranged from 0.5% or less after simple hysterectomy to as high as 2% after radical hysterectomy. Most VVFs are believed to occur after hysterectomy performed for benign disease, and many – but not all – are caused by inadvertent bladder injury that was not recognized intraoperatively.
Women who have had one or more cesarean deliveries and those who have had prior pelvic or vaginal surgery are at increased risk. In addition, both radiation therapy and inflammation that occur with diseases such as pelvic inflammatory disease or inflammatory bowel disease can negatively affect tissue quality and healing from surgical procedures – and can lead ultimately to the development of urogenital fistulas – although even less is known about incidence in these cases.
Prevention
Intraoperatively, VVFs may best be prevented through careful mobilization of the bladder off the vaginal wall, the use of delayed absorbable sutures (preferably Vicryl sutures), and the use of cystoscopy to assess the bladder for injury. If cystoscopy is not available, retrograde filling with a Foley catheter will still be helpful.
An overly aggressive approach to creating the bladder flap during hysterectomy and other surgeries can increase the risk of devascularization and the subsequent formation of fistulas. When the blood supply is found to have been compromised, affected tissue can be strengthened by oversewing with imbrication. When an inadvertent cystotomy is identified, repair is often best achieved with omental tissue interposed between the bladder and vagina. If there is any doubt about bladder integrity, an interposition graft between the bladder flap and the vaginal cuff will help reduce the incidence of fistula formation. Whenever overlapping suture lines occur (the vaginal cuff and the cystotomy repair), the risk of VVF formation will increase. Other than that using omentum, peritoneal grafts will also work well.
VVF formation may still occur, however, despite recognition and repair of an injury – and despite normal findings on cystoscopy. In patients who have had prior cesarean deliveries or other prior pelvic surgery, for example, tissue devascularization may cause a delayed injury, with the process of tissue necrosis and VVF formation occurring up to a month after surgery. It is important to appreciate the factors that predispose patients to VVF and to anticipate an increased risk, but in many cases of delayed VVF, it’s quite possible that nothing could have been done to prevent the problem.
Work-up
Vesicovaginal fistulas typically present as painless, continuous urine leakage from the vagina. The medical history should include standard questions about pelvic health history and symptom characteristics (in order to exclude hematuria or leakage of fluid other than urine), as well as questions aimed at differentiating symptoms of VVF from other causes of urinary incontinence, such as stress incontinence. In my experience, urine leakage is often incorrectly dismissed as stress incontinence when it is actually VVF. A high index of suspicion will help make an earlier diagnosis. This does not usually change the management, but helps manage the anxiety, expectations, and needs of the patient.
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.
A VVF that is larger may require the use of additional sources of tissue. A graft called the Martius graft, or labial fibrofatty tissue graft, is sometimes used to reinforce repairs of larger fistulas, even those that are high in the vaginal vault. The procedure involves a vertical incision on the inner side of the labium majus and detachment of fibroadipose tissue from its underlying bulbocavernosus muscle. This fat-pad flap is vascularized and thus serves as a pedicled graft. It can be tunneled under the vaginal epithelium to reach the site of closure. The procedure has limited use with the vaginal approach to VVF, but is important to be aware of.
Other sources of grafts or flaps that can sometimes be used with the vaginal approach include the gracilis muscle, the gluteal muscle and peritoneum, and fasciocutaneous tissue from the inner thigh.
The avoidance of overlapping suture lines and multiple layers of closure will help ensure a water-tight closure. If there is any leakage upon testing the integrity of the repair, particularly one that is vaginally approached, such leakage will continue and the repair will have been unsuccessful. In an abdominal surgery for VVF, a small amount of remaining leakage will probably resolve on its own after 10-14 days of catheter placement.
Placement of a Jackson-Pratt (JP) drain is controversial. It has been suggested that a JP drain placed on continuous suction will pull urine out of the bladder and increase the risk of a fistula. I don’t place a JP drain in my repairs as I find them to not be helpful. A cystogram can be done 1 week after repair to confirm healing, but there is some debate about whether or not the procedure is useful at that point. In my experience, if the patient does not have a cystogram and gets postrepair leakage, I have the same information as I would have obtained through a positive finding on a cystogram.
Dr. Garely is chair of obstetrics and gynecology and director of urogynecology and pelvic reconstructive surgery at the South Nassau Communities Hospital, Oceanside, N.Y., and a clinical professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York. He has no disclosures related to this column.
Vesicovaginal fistulas (VVFs) are the most common type of urogenital fistulas – approximately three times more common than ureterovaginal fistulas – and can be a debilitating problem for women.
Most of the research published in recent years on VVFs and other urogenital fistulas comes from developing countries where these abnormal communications are a common complication of obstructed labor. In the United States, despite a relative paucity of data, VVFs are known to occur most often as a sequelae of gynecologic surgery, usually hysterectomy. Estimates of the incidence of VVF and other urogenital fistula formation are debated but have ranged from 0.5% or less after simple hysterectomy to as high as 2% after radical hysterectomy. Most VVFs are believed to occur after hysterectomy performed for benign disease, and many – but not all – are caused by inadvertent bladder injury that was not recognized intraoperatively.
Women who have had one or more cesarean deliveries and those who have had prior pelvic or vaginal surgery are at increased risk. In addition, both radiation therapy and inflammation that occur with diseases such as pelvic inflammatory disease or inflammatory bowel disease can negatively affect tissue quality and healing from surgical procedures – and can lead ultimately to the development of urogenital fistulas – although even less is known about incidence in these cases.
Prevention
Intraoperatively, VVFs may best be prevented through careful mobilization of the bladder off the vaginal wall, the use of delayed absorbable sutures (preferably Vicryl sutures), and the use of cystoscopy to assess the bladder for injury. If cystoscopy is not available, retrograde filling with a Foley catheter will still be helpful.
An overly aggressive approach to creating the bladder flap during hysterectomy and other surgeries can increase the risk of devascularization and the subsequent formation of fistulas. When the blood supply is found to have been compromised, affected tissue can be strengthened by oversewing with imbrication. When an inadvertent cystotomy is identified, repair is often best achieved with omental tissue interposed between the bladder and vagina. If there is any doubt about bladder integrity, an interposition graft between the bladder flap and the vaginal cuff will help reduce the incidence of fistula formation. Whenever overlapping suture lines occur (the vaginal cuff and the cystotomy repair), the risk of VVF formation will increase. Other than that using omentum, peritoneal grafts will also work well.
VVF formation may still occur, however, despite recognition and repair of an injury – and despite normal findings on cystoscopy. In patients who have had prior cesarean deliveries or other prior pelvic surgery, for example, tissue devascularization may cause a delayed injury, with the process of tissue necrosis and VVF formation occurring up to a month after surgery. It is important to appreciate the factors that predispose patients to VVF and to anticipate an increased risk, but in many cases of delayed VVF, it’s quite possible that nothing could have been done to prevent the problem.
Work-up
Vesicovaginal fistulas typically present as painless, continuous urine leakage from the vagina. The medical history should include standard questions about pelvic health history and symptom characteristics (in order to exclude hematuria or leakage of fluid other than urine), as well as questions aimed at differentiating symptoms of VVF from other causes of urinary incontinence, such as stress incontinence. In my experience, urine leakage is often incorrectly dismissed as stress incontinence when it is actually VVF. A high index of suspicion will help make an earlier diagnosis. This does not usually change the management, but helps manage the anxiety, expectations, and needs of the patient.
I recommend beginning the work-up for a suspected VVF with a thorough cystoscopic evaluation of the bladder for injury. An irregular appearance of the bladder, signs of inflammation, and poor or absent ureteral efflux are often indicative of VVF in the presence of vaginal leakage. Following cystoscopy, I perform a split speculum examination of the vagina. Most injuries will be on the anterior wall or the apex (cuff). A recently formed fistula may appear as a hole or as a small, red area of granulation tissue with no visible opening.
It can be difficult to visualize the vaginal fistula opening of more mature fistulas; similarly, very small fistulas may be difficult to find because of their size and the anatomy of the vagina. When a prior hysterectomy has led to a fistula, the vaginal fistula opening is typically located in the upper third of the vagina or at the vaginal cuff. If cuff sutures are still intact, this may also make localization of the fistula more difficult.
Leakage in the vagina can sometimes be detected with a retrograde filling of the bladder; other times, it is possible to detect leakage without filling the bladder. In all cases, it’s important to remember that more than one fistula – and more than one fistula type – may be present. A VVF and ureterovaginal fistula will sometimes occur together, which means that abnormal cystoscopy findings in a patient who experiences leakage does not necessarily rule out the presence of a concurrent ureterovaginal fistula.
Phenazopyridine (Pyridium) administered orally will turn the urine orange and can help visualize the leakage of urine into the vagina. When used in combination with the use of blue dye (methylene blue) infused into the bladder, a VVF may be distinguished from a ureterovaginal fistula. To completely evaluate the number and location of fistulas, however, imaging studies are necessary. In my experience, a CT urogram with IV contrast can also help localize ureteral injuries.
Surgical treatment
VVFs can almost always be repaired vaginally. If the fistula is too high in location or too complex, then an abdominal approach, either robotic, laparoscopic, or open, may be necessary. I prefer a vaginal approach to VVF repair whenever feasible because of its straightforward nature, lower morbidity, and high rate of success on the first attempt. Failure rates are between 5% and 20% for each attempt, so more than one surgery may be required. It is not unreasonable to attempt two or three vaginal approach repairs if each successive attempt results in a smaller fistula. A decision to go abdominal must be made based on the chances of a successful vaginal approach and on the patient’s wishes.
Successful fistula repair requires tension-free suture lines, no overlapping suture lines, and good vascular supply to the tissue. The timing of repair has long been controversial, but barring the presence of active pelvic infection, which may require an immediate surgical approach, the timing of fistula repair depends almost solely on the quality of the surrounding tissue. This relates to the need for a good vascular supply.
Early repair can be done if the tissue is pliable and healthy. But in general, if surgery is performed too close to the time of injury, the surrounding tissue will be erythematous and likely to break down with closure. The goal is to wait until the granulation tissue has dissipated and the area is no longer inflamed; after gynecologic surgery, this generally occurs within 6-12 weeks.
Regular vaginal exams about every 2 weeks can be used to monitor progress. During the waiting period, catheterization of the bladder can improve comfort for the patient and may even allow for spontaneous closure of the fistula. In fact, I usually tell patients who are diagnosed with a VVF within the first few weeks after surgery that spontaneous closure is a possible outcome given continuous urinary drainage for up to 30 days, provided that the VVF is small enough. This may be optimistic thinking on the part of the surgeon and the patient, but there is little downside to this approach.
The Latzko technique described in 1992 is still widely used for vaginal repair of VVFs. With this approach, the vaginal epithelium is incised around the fistula, and vaginal epithelial flaps are raised and removed around the fistula tract (in a circle of about 2-3 cm in diameter) for a multilayer approximation of healthy tissues. Several layers are sometimes needed, but in most cases, two layers are sufficient.
In my experience, a modified approach to the traditional Latzko procedure is more successful. Prior to closure, either anterior or posterior to the VVF, a small rim of vaginal epithelium is removed and, on the other side, the epithelium is mobilized at least 1 cm lateral to the fistula on both sides, and about 2 cm distal. This allows for the creation of a small, modified, thumbnail flap that completely patches the fistula closure without tension and without the need for any overlapping suture lines. The key is to secure flap tissue from the side where there appears to be more vaginal tissue. The tissue should be loose; if there appears to be any strain, the repair is likely to fail.
There are not enough data from the United States or other developed countries to demonstrate the superiority of this modified approach, but data from the obstetric population in Africa – and my own experience – suggest that it yields better outcomes.
A VVF that is larger may require the use of additional sources of tissue. A graft called the Martius graft, or labial fibrofatty tissue graft, is sometimes used to reinforce repairs of larger fistulas, even those that are high in the vaginal vault. The procedure involves a vertical incision on the inner side of the labium majus and detachment of fibroadipose tissue from its underlying bulbocavernosus muscle. This fat-pad flap is vascularized and thus serves as a pedicled graft. It can be tunneled under the vaginal epithelium to reach the site of closure. The procedure has limited use with the vaginal approach to VVF, but is important to be aware of.
Other sources of grafts or flaps that can sometimes be used with the vaginal approach include the gracilis muscle, the gluteal muscle and peritoneum, and fasciocutaneous tissue from the inner thigh.
The avoidance of overlapping suture lines and multiple layers of closure will help ensure a water-tight closure. If there is any leakage upon testing the integrity of the repair, particularly one that is vaginally approached, such leakage will continue and the repair will have been unsuccessful. In an abdominal surgery for VVF, a small amount of remaining leakage will probably resolve on its own after 10-14 days of catheter placement.
Placement of a Jackson-Pratt (JP) drain is controversial. It has been suggested that a JP drain placed on continuous suction will pull urine out of the bladder and increase the risk of a fistula. I don’t place a JP drain in my repairs as I find them to not be helpful. A cystogram can be done 1 week after repair to confirm healing, but there is some debate about whether or not the procedure is useful at that point. In my experience, if the patient does not have a cystogram and gets postrepair leakage, I have the same information as I would have obtained through a positive finding on a cystogram.
Dr. Garely is chair of obstetrics and gynecology and director of urogynecology and pelvic reconstructive surgery at the South Nassau Communities Hospital, Oceanside, N.Y., and a clinical professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York. He has no disclosures related to this column.
Lessons learned from the AGA Future Leaders Program
I have been a member of the American Gastroenterological Association since my first year of GI fellowship. Out of all the GI professional societies, I have always considered it to be my home. AGA is the organization I turn to for direction on providing high-value and quality care to my patients, up-to-date information on research and technology innovations in our specialty, education and training programs for the next generation of gastroenterology and hepatology providers, and for my own continued learning. I have received so many benefits from my membership and am always looking to pay back my appreciation. A wonderful way to do this was through my participation in the Future Leaders Program, which “provides a pathway within the organization for selected participants to network, connect with mentors, develop leadership skills, and learn about AGA’s governance and operations while advancing their careers and supporting the profession.” I can honestly say that the program delivers on all of these promises.
The program started with a meeting at AGA headquarters in Bethesda, Md., where we were introduced to key AGA staff, learned about the core mission and goals of AGA, and received training in leadership skills. I was able to gain invaluable insight into the leadership and governance of the AGA and now have a better understanding of the AGA’s commitment to maintaining a vital organization. At this meeting, we were assigned two mentors – one for career coaching and one to guide us through our main project. We worked closely with these mentors throughout the program, and I have no doubt these relationships will continue well into the future. For the main project we were assigned to a team with another participant to research and develop a project that aligned with the overall strategic plan of the AGA and fulfilled a need or gap within the organization. The projects covered topics such as ways to engage early-career professionals in the AGA Community, enhance access to the AGA guidelines, increase membership, highlight top publications from the AGA journals, expand networking among fellows and early career members, develop education metrics, and provide educational opportunities for international members. We worked on our main project remotely with our teams over 6 months, culminating in a final presentation that was shared with all participants in the Future Leaders Program, as well as AGA committees. Some of these projects already are being implemented. It was a truly amazing experience to work closely with the knowledgeable and dedicated AGA staff, gain skills in teamwork and strategic planning, and hear about the innovative ideas from all the participants in the Future Leaders Program.

In addition to the initial meeting in Bethesda, we attended a reception at Digestive Disease Week® with alumni from the prior class of Future Leaders, a face-to-face meeting in Washington held in conjunction with the AGA Joint Committee Meetings, participated in Advocacy Day after the fall meeting, and attended some virtual roundtable discussions. One of the virtual discussions focused on our final project, which allowed us to apply our skills in strategic planning on a smaller scale to proposals for microvolunteerism. These proposals allow for convenient, short-term volunteer activities that move forward the mission of the AGA and cultivate local leaders. The microvolunteerism proposals are a fantastic example of AGA leadership’s understanding that utilizing and engaging members is one of the best ways to ensure a vital and relevant organization. I am excited to see some of the proposals come to fruition over the next few years.
Through the Future Leaders Program, I not only learned a lot about the AGA but also a great deal about myself. I learned about my own leadership style, which for those of you who know me would not be surprised to find out is “harmony.” I see the possibilities in others and try to capitalize on this potential to achieve our mutual goals. I learned how this leadership style can contribute to organizations as a whole, but also the importance of incorporating multiple leadership styles in all projects. This is valuable information that I have no doubt will help throughout my career. This program also provided an opportunity for me to connect and network with colleagues across the country whom I may not have otherwise met. These connections resulted in new research collaborations, as well as new friendships. As the 2018 AGA Future Leaders Program came to a close, I found myself with a deeper understanding of the mission and vision of the AGA, opportunities for more involvement, and a stronger commitment to our organization. I hope to one day participate as a mentor to other future leaders in the program. I encourage everyone interested in gaining leadership skills and more insight into the governance and operations of the AGA to apply.
Since the AGA Future Leaders Program began in the spring of 2015, two classes of 18 participants and nine mentors have participated in the 18-month comprehensive leadership development program. Nearly all of the Future Leaders Program alumni are serving the AGA in a variety of leadership capacities including serving on committees, the editorial boards, or volunteering as speakers and authors for AGA publications and events.
The next Future Leaders Program will begin in the spring of 2019 and the opportunity to apply will launch this fall.
Dr. Weiss is assistant professor, division of gastroenterology and hepatology; director, UW Health Gastrointestinal Genetics Clinic, University of Wisconsin School of Medicine and Public Health, Madison.
I have been a member of the American Gastroenterological Association since my first year of GI fellowship. Out of all the GI professional societies, I have always considered it to be my home. AGA is the organization I turn to for direction on providing high-value and quality care to my patients, up-to-date information on research and technology innovations in our specialty, education and training programs for the next generation of gastroenterology and hepatology providers, and for my own continued learning. I have received so many benefits from my membership and am always looking to pay back my appreciation. A wonderful way to do this was through my participation in the Future Leaders Program, which “provides a pathway within the organization for selected participants to network, connect with mentors, develop leadership skills, and learn about AGA’s governance and operations while advancing their careers and supporting the profession.” I can honestly say that the program delivers on all of these promises.
The program started with a meeting at AGA headquarters in Bethesda, Md., where we were introduced to key AGA staff, learned about the core mission and goals of AGA, and received training in leadership skills. I was able to gain invaluable insight into the leadership and governance of the AGA and now have a better understanding of the AGA’s commitment to maintaining a vital organization. At this meeting, we were assigned two mentors – one for career coaching and one to guide us through our main project. We worked closely with these mentors throughout the program, and I have no doubt these relationships will continue well into the future. For the main project we were assigned to a team with another participant to research and develop a project that aligned with the overall strategic plan of the AGA and fulfilled a need or gap within the organization. The projects covered topics such as ways to engage early-career professionals in the AGA Community, enhance access to the AGA guidelines, increase membership, highlight top publications from the AGA journals, expand networking among fellows and early career members, develop education metrics, and provide educational opportunities for international members. We worked on our main project remotely with our teams over 6 months, culminating in a final presentation that was shared with all participants in the Future Leaders Program, as well as AGA committees. Some of these projects already are being implemented. It was a truly amazing experience to work closely with the knowledgeable and dedicated AGA staff, gain skills in teamwork and strategic planning, and hear about the innovative ideas from all the participants in the Future Leaders Program.

In addition to the initial meeting in Bethesda, we attended a reception at Digestive Disease Week® with alumni from the prior class of Future Leaders, a face-to-face meeting in Washington held in conjunction with the AGA Joint Committee Meetings, participated in Advocacy Day after the fall meeting, and attended some virtual roundtable discussions. One of the virtual discussions focused on our final project, which allowed us to apply our skills in strategic planning on a smaller scale to proposals for microvolunteerism. These proposals allow for convenient, short-term volunteer activities that move forward the mission of the AGA and cultivate local leaders. The microvolunteerism proposals are a fantastic example of AGA leadership’s understanding that utilizing and engaging members is one of the best ways to ensure a vital and relevant organization. I am excited to see some of the proposals come to fruition over the next few years.
Through the Future Leaders Program, I not only learned a lot about the AGA but also a great deal about myself. I learned about my own leadership style, which for those of you who know me would not be surprised to find out is “harmony.” I see the possibilities in others and try to capitalize on this potential to achieve our mutual goals. I learned how this leadership style can contribute to organizations as a whole, but also the importance of incorporating multiple leadership styles in all projects. This is valuable information that I have no doubt will help throughout my career. This program also provided an opportunity for me to connect and network with colleagues across the country whom I may not have otherwise met. These connections resulted in new research collaborations, as well as new friendships. As the 2018 AGA Future Leaders Program came to a close, I found myself with a deeper understanding of the mission and vision of the AGA, opportunities for more involvement, and a stronger commitment to our organization. I hope to one day participate as a mentor to other future leaders in the program. I encourage everyone interested in gaining leadership skills and more insight into the governance and operations of the AGA to apply.
Since the AGA Future Leaders Program began in the spring of 2015, two classes of 18 participants and nine mentors have participated in the 18-month comprehensive leadership development program. Nearly all of the Future Leaders Program alumni are serving the AGA in a variety of leadership capacities including serving on committees, the editorial boards, or volunteering as speakers and authors for AGA publications and events.
The next Future Leaders Program will begin in the spring of 2019 and the opportunity to apply will launch this fall.
Dr. Weiss is assistant professor, division of gastroenterology and hepatology; director, UW Health Gastrointestinal Genetics Clinic, University of Wisconsin School of Medicine and Public Health, Madison.
I have been a member of the American Gastroenterological Association since my first year of GI fellowship. Out of all the GI professional societies, I have always considered it to be my home. AGA is the organization I turn to for direction on providing high-value and quality care to my patients, up-to-date information on research and technology innovations in our specialty, education and training programs for the next generation of gastroenterology and hepatology providers, and for my own continued learning. I have received so many benefits from my membership and am always looking to pay back my appreciation. A wonderful way to do this was through my participation in the Future Leaders Program, which “provides a pathway within the organization for selected participants to network, connect with mentors, develop leadership skills, and learn about AGA’s governance and operations while advancing their careers and supporting the profession.” I can honestly say that the program delivers on all of these promises.
The program started with a meeting at AGA headquarters in Bethesda, Md., where we were introduced to key AGA staff, learned about the core mission and goals of AGA, and received training in leadership skills. I was able to gain invaluable insight into the leadership and governance of the AGA and now have a better understanding of the AGA’s commitment to maintaining a vital organization. At this meeting, we were assigned two mentors – one for career coaching and one to guide us through our main project. We worked closely with these mentors throughout the program, and I have no doubt these relationships will continue well into the future. For the main project we were assigned to a team with another participant to research and develop a project that aligned with the overall strategic plan of the AGA and fulfilled a need or gap within the organization. The projects covered topics such as ways to engage early-career professionals in the AGA Community, enhance access to the AGA guidelines, increase membership, highlight top publications from the AGA journals, expand networking among fellows and early career members, develop education metrics, and provide educational opportunities for international members. We worked on our main project remotely with our teams over 6 months, culminating in a final presentation that was shared with all participants in the Future Leaders Program, as well as AGA committees. Some of these projects already are being implemented. It was a truly amazing experience to work closely with the knowledgeable and dedicated AGA staff, gain skills in teamwork and strategic planning, and hear about the innovative ideas from all the participants in the Future Leaders Program.

In addition to the initial meeting in Bethesda, we attended a reception at Digestive Disease Week® with alumni from the prior class of Future Leaders, a face-to-face meeting in Washington held in conjunction with the AGA Joint Committee Meetings, participated in Advocacy Day after the fall meeting, and attended some virtual roundtable discussions. One of the virtual discussions focused on our final project, which allowed us to apply our skills in strategic planning on a smaller scale to proposals for microvolunteerism. These proposals allow for convenient, short-term volunteer activities that move forward the mission of the AGA and cultivate local leaders. The microvolunteerism proposals are a fantastic example of AGA leadership’s understanding that utilizing and engaging members is one of the best ways to ensure a vital and relevant organization. I am excited to see some of the proposals come to fruition over the next few years.
Through the Future Leaders Program, I not only learned a lot about the AGA but also a great deal about myself. I learned about my own leadership style, which for those of you who know me would not be surprised to find out is “harmony.” I see the possibilities in others and try to capitalize on this potential to achieve our mutual goals. I learned how this leadership style can contribute to organizations as a whole, but also the importance of incorporating multiple leadership styles in all projects. This is valuable information that I have no doubt will help throughout my career. This program also provided an opportunity for me to connect and network with colleagues across the country whom I may not have otherwise met. These connections resulted in new research collaborations, as well as new friendships. As the 2018 AGA Future Leaders Program came to a close, I found myself with a deeper understanding of the mission and vision of the AGA, opportunities for more involvement, and a stronger commitment to our organization. I hope to one day participate as a mentor to other future leaders in the program. I encourage everyone interested in gaining leadership skills and more insight into the governance and operations of the AGA to apply.
Since the AGA Future Leaders Program began in the spring of 2015, two classes of 18 participants and nine mentors have participated in the 18-month comprehensive leadership development program. Nearly all of the Future Leaders Program alumni are serving the AGA in a variety of leadership capacities including serving on committees, the editorial boards, or volunteering as speakers and authors for AGA publications and events.
The next Future Leaders Program will begin in the spring of 2019 and the opportunity to apply will launch this fall.
Dr. Weiss is assistant professor, division of gastroenterology and hepatology; director, UW Health Gastrointestinal Genetics Clinic, University of Wisconsin School of Medicine and Public Health, Madison.
A rare but debilitating diagnosis in developed countries
Vesicovaginal fistula continues to be the most common form of genitourinary fistula, with resultant diminishment in quality of life secondary to physical and psychosocial distress. While it has been reported that 1 million women in Sub-Saharan Africa have untreated vesicovaginal fistula secondary to obstetric trauma, vesicovaginal fistulas are relatively rare in the United States. Per the United States National Hospital Discharge Survey, in 2007, fewer than 5,000 vesicovaginal fistula repairs were performed out of over 2.3 million procedures involving the female urinary and genital system.
The rarity of the diagnosis is also reflected in data collected from the English National Health Service, where vesicovaginal fistula occurred in 1 in 788 hysterectomies (although more common in radical hysterectomy, at 1 in 87).
In a recent systematic review and meta-analysis on the management of vesicovaginal fistulas in women following benign gynecologic surgery, Bodner-Adler et al. evaluated 282 full-text articles to identify 124 studies for inclusion (PLoS One. 2017 Feb 22;12[2]:e0171554). Only ten studies involved solely conservative management with prolonged bladder drainage. Dismal success was noted: 8%. Surgery was performed in 96.4% of cases (1379/1430); transvaginal in 39%, transabdominal/transvesical in 36%, laparoscopic/robotic approach in 15%, and transabdominal/transvaginal in 3%. Overall success rate in these surgical cases was 97.98% (95% confidence interval, 96.13%-99.29%); with similar procedural success: transvaginal, 89.96%-97.49%; transabdominal/transvesical, 94.55%-99.18%; and laparoscopic/robotic, 96.85%-99.99%. Studies are very limited comparing the various surgical techniques, with only one study comparing transvaginal, transabdominal, and laparoscopic approaches. Interestingly, in this study, the laparoscopic approach was noted to have the least morbidity (Ou CS et al. J Lapraoendosc Adv Surg Tech A. 2004 Feb;14(1):17-21).
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Alan D. Garely, MD, FACOG, FACS, of the Icahn School of Medicine at Mount Sinai, New York. Dr. Garely has served on the board of directors for the American Urogynecologic Society, serves as chair of the gynecology and obstetrics advisory board for the American College of Surgeons, and has published numerous papers and book chapters.
It is a pleasure to welcome Dr. Garely to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He has no disclosures related to this column.
Vesicovaginal fistula continues to be the most common form of genitourinary fistula, with resultant diminishment in quality of life secondary to physical and psychosocial distress. While it has been reported that 1 million women in Sub-Saharan Africa have untreated vesicovaginal fistula secondary to obstetric trauma, vesicovaginal fistulas are relatively rare in the United States. Per the United States National Hospital Discharge Survey, in 2007, fewer than 5,000 vesicovaginal fistula repairs were performed out of over 2.3 million procedures involving the female urinary and genital system.
The rarity of the diagnosis is also reflected in data collected from the English National Health Service, where vesicovaginal fistula occurred in 1 in 788 hysterectomies (although more common in radical hysterectomy, at 1 in 87).
In a recent systematic review and meta-analysis on the management of vesicovaginal fistulas in women following benign gynecologic surgery, Bodner-Adler et al. evaluated 282 full-text articles to identify 124 studies for inclusion (PLoS One. 2017 Feb 22;12[2]:e0171554). Only ten studies involved solely conservative management with prolonged bladder drainage. Dismal success was noted: 8%. Surgery was performed in 96.4% of cases (1379/1430); transvaginal in 39%, transabdominal/transvesical in 36%, laparoscopic/robotic approach in 15%, and transabdominal/transvaginal in 3%. Overall success rate in these surgical cases was 97.98% (95% confidence interval, 96.13%-99.29%); with similar procedural success: transvaginal, 89.96%-97.49%; transabdominal/transvesical, 94.55%-99.18%; and laparoscopic/robotic, 96.85%-99.99%. Studies are very limited comparing the various surgical techniques, with only one study comparing transvaginal, transabdominal, and laparoscopic approaches. Interestingly, in this study, the laparoscopic approach was noted to have the least morbidity (Ou CS et al. J Lapraoendosc Adv Surg Tech A. 2004 Feb;14(1):17-21).
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Alan D. Garely, MD, FACOG, FACS, of the Icahn School of Medicine at Mount Sinai, New York. Dr. Garely has served on the board of directors for the American Urogynecologic Society, serves as chair of the gynecology and obstetrics advisory board for the American College of Surgeons, and has published numerous papers and book chapters.
It is a pleasure to welcome Dr. Garely to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He has no disclosures related to this column.
Vesicovaginal fistula continues to be the most common form of genitourinary fistula, with resultant diminishment in quality of life secondary to physical and psychosocial distress. While it has been reported that 1 million women in Sub-Saharan Africa have untreated vesicovaginal fistula secondary to obstetric trauma, vesicovaginal fistulas are relatively rare in the United States. Per the United States National Hospital Discharge Survey, in 2007, fewer than 5,000 vesicovaginal fistula repairs were performed out of over 2.3 million procedures involving the female urinary and genital system.
The rarity of the diagnosis is also reflected in data collected from the English National Health Service, where vesicovaginal fistula occurred in 1 in 788 hysterectomies (although more common in radical hysterectomy, at 1 in 87).
In a recent systematic review and meta-analysis on the management of vesicovaginal fistulas in women following benign gynecologic surgery, Bodner-Adler et al. evaluated 282 full-text articles to identify 124 studies for inclusion (PLoS One. 2017 Feb 22;12[2]:e0171554). Only ten studies involved solely conservative management with prolonged bladder drainage. Dismal success was noted: 8%. Surgery was performed in 96.4% of cases (1379/1430); transvaginal in 39%, transabdominal/transvesical in 36%, laparoscopic/robotic approach in 15%, and transabdominal/transvaginal in 3%. Overall success rate in these surgical cases was 97.98% (95% confidence interval, 96.13%-99.29%); with similar procedural success: transvaginal, 89.96%-97.49%; transabdominal/transvesical, 94.55%-99.18%; and laparoscopic/robotic, 96.85%-99.99%. Studies are very limited comparing the various surgical techniques, with only one study comparing transvaginal, transabdominal, and laparoscopic approaches. Interestingly, in this study, the laparoscopic approach was noted to have the least morbidity (Ou CS et al. J Lapraoendosc Adv Surg Tech A. 2004 Feb;14(1):17-21).
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Alan D. Garely, MD, FACOG, FACS, of the Icahn School of Medicine at Mount Sinai, New York. Dr. Garely has served on the board of directors for the American Urogynecologic Society, serves as chair of the gynecology and obstetrics advisory board for the American College of Surgeons, and has published numerous papers and book chapters.
It is a pleasure to welcome Dr. Garely to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He has no disclosures related to this column.