User login
Professional psychology
, the ability to figure out how to get people to accept what you’re trying to do for them.
Expertise and psychology: Every profession needs both, including our own. Over my years in practice, I’ve met people in many walks of life who develop the same combination. Here are some favorites:
1. Wedding planners
Venues, décor, dresses, floral arrangements, caterers, bands. Wedding planners must know about all of these. And that’s just the start.
Weddings make everyone a bit crazy, or more than a bit. There are parents trying not to let go, children trying to pull away (a Destination Wedding in Patagonia – perfect – none of the family can come!), cultural and taste gaps between the sides (tipplers from Tinseltown and teetotalers from Tupelo), culling the guest list (see Patagonia). Every wedding planner I’ve met could write a book, but won’t. Legal fees would be too high.
Given all this turmoil, some wedding planners might advise elopement and put themselves out of business. No fear of that happening.
2. Event planners
See Wedding Planners, only add: arbitrary and capricious bosses, incompetent implementers, acts of God, acts of man, and everything that goes wrong when there are too many moving parts. One close friend who organizes professional conventions says that every year one attendee posts this complaint on the message board: “Why is there no Diet Mountain Dew?!!!”
3. Dressmakers
Again, see Wedding Planners. Knowing how to design, create, and fit a dress demands a set of skills that earns my admiration and respect. Knowing how to deal with the people who are going to wear the dresses deserves not respect but awe. Even if I knew how to sew, I wouldn’t last a week in this business.
4. Financial planners
Every financial planner I meet describes what they do as “mostly psychology.” Of course, they need to recommend investments that suit the age, life status, and plans of their clients. That’s the easy part.
“When the market is dropping,” says Phil, “people call to scream that they’re losing their shirt. When the market is going up, they call to scream that they’re not making out as well as their friends claim they are.
“Either way, I just hold the phone far enough away from my ear to save my hearing until they’re done venting. Then I try to calm them down and assure them that investing is a long game, and over time they’ll do better staying the course we agreed on than jumping around with every market swing, up or down.”
“Do they listen?”
“Most of them. Eventually.”
4. Speakers’ booking agents
Matching clients with speakers can bring curious challenges. Celebrity speakers in particular may have unique requirements that the agents who book them must figure out and comply with. Or else.
For instance, one young man I met had to book a distinguished jurist. He needed to be picked up in a limo. The limo had to be a Bentley. And the Bentley had to be gray.
Well, excuse me.
5. Waiters
I think the favored term these days is “Servers.” Servers serve strangers. Some strangers are pleasant and courteous. Not all. Waiters mean to please, but to do that they have to put up with a lot. Always with a smile, of course.
Customers ask silly questions. (“What’s good here?”) Some don’t find the menu detailed enough. (“Can I have half of this and some of that, with the sauce on the side?”) They may find the food too hot. Or too cold. Or too spicy. Or too bland. After all that, they may tip a little. Or not.
But the server still has to ask, “Is everything satisfactory?”
6. Psychologists
Never mind.
7. Parking meter readers
Just kidding. Meter readers write parking tickets.
They know perfectly well that everyone knows exactly what they do. And they don’t care.
8. People who field complaints at call centers
Requirement: Patience, savvy, Xanax.
9. Dermatologists
You bet, in spades. I wrote a whole book on the subject. You can read it if you want to. If you disagree, don’t tell me.
I don’t need readers to disagree with me. I already have patients.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected] .
, the ability to figure out how to get people to accept what you’re trying to do for them.
Expertise and psychology: Every profession needs both, including our own. Over my years in practice, I’ve met people in many walks of life who develop the same combination. Here are some favorites:
1. Wedding planners
Venues, décor, dresses, floral arrangements, caterers, bands. Wedding planners must know about all of these. And that’s just the start.
Weddings make everyone a bit crazy, or more than a bit. There are parents trying not to let go, children trying to pull away (a Destination Wedding in Patagonia – perfect – none of the family can come!), cultural and taste gaps between the sides (tipplers from Tinseltown and teetotalers from Tupelo), culling the guest list (see Patagonia). Every wedding planner I’ve met could write a book, but won’t. Legal fees would be too high.
Given all this turmoil, some wedding planners might advise elopement and put themselves out of business. No fear of that happening.
2. Event planners
See Wedding Planners, only add: arbitrary and capricious bosses, incompetent implementers, acts of God, acts of man, and everything that goes wrong when there are too many moving parts. One close friend who organizes professional conventions says that every year one attendee posts this complaint on the message board: “Why is there no Diet Mountain Dew?!!!”
3. Dressmakers
Again, see Wedding Planners. Knowing how to design, create, and fit a dress demands a set of skills that earns my admiration and respect. Knowing how to deal with the people who are going to wear the dresses deserves not respect but awe. Even if I knew how to sew, I wouldn’t last a week in this business.
4. Financial planners
Every financial planner I meet describes what they do as “mostly psychology.” Of course, they need to recommend investments that suit the age, life status, and plans of their clients. That’s the easy part.
“When the market is dropping,” says Phil, “people call to scream that they’re losing their shirt. When the market is going up, they call to scream that they’re not making out as well as their friends claim they are.
“Either way, I just hold the phone far enough away from my ear to save my hearing until they’re done venting. Then I try to calm them down and assure them that investing is a long game, and over time they’ll do better staying the course we agreed on than jumping around with every market swing, up or down.”
“Do they listen?”
“Most of them. Eventually.”
4. Speakers’ booking agents
Matching clients with speakers can bring curious challenges. Celebrity speakers in particular may have unique requirements that the agents who book them must figure out and comply with. Or else.
For instance, one young man I met had to book a distinguished jurist. He needed to be picked up in a limo. The limo had to be a Bentley. And the Bentley had to be gray.
Well, excuse me.
5. Waiters
I think the favored term these days is “Servers.” Servers serve strangers. Some strangers are pleasant and courteous. Not all. Waiters mean to please, but to do that they have to put up with a lot. Always with a smile, of course.
Customers ask silly questions. (“What’s good here?”) Some don’t find the menu detailed enough. (“Can I have half of this and some of that, with the sauce on the side?”) They may find the food too hot. Or too cold. Or too spicy. Or too bland. After all that, they may tip a little. Or not.
But the server still has to ask, “Is everything satisfactory?”
6. Psychologists
Never mind.
7. Parking meter readers
Just kidding. Meter readers write parking tickets.
They know perfectly well that everyone knows exactly what they do. And they don’t care.
8. People who field complaints at call centers
Requirement: Patience, savvy, Xanax.
9. Dermatologists
You bet, in spades. I wrote a whole book on the subject. You can read it if you want to. If you disagree, don’t tell me.
I don’t need readers to disagree with me. I already have patients.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected] .
, the ability to figure out how to get people to accept what you’re trying to do for them.
Expertise and psychology: Every profession needs both, including our own. Over my years in practice, I’ve met people in many walks of life who develop the same combination. Here are some favorites:
1. Wedding planners
Venues, décor, dresses, floral arrangements, caterers, bands. Wedding planners must know about all of these. And that’s just the start.
Weddings make everyone a bit crazy, or more than a bit. There are parents trying not to let go, children trying to pull away (a Destination Wedding in Patagonia – perfect – none of the family can come!), cultural and taste gaps between the sides (tipplers from Tinseltown and teetotalers from Tupelo), culling the guest list (see Patagonia). Every wedding planner I’ve met could write a book, but won’t. Legal fees would be too high.
Given all this turmoil, some wedding planners might advise elopement and put themselves out of business. No fear of that happening.
2. Event planners
See Wedding Planners, only add: arbitrary and capricious bosses, incompetent implementers, acts of God, acts of man, and everything that goes wrong when there are too many moving parts. One close friend who organizes professional conventions says that every year one attendee posts this complaint on the message board: “Why is there no Diet Mountain Dew?!!!”
3. Dressmakers
Again, see Wedding Planners. Knowing how to design, create, and fit a dress demands a set of skills that earns my admiration and respect. Knowing how to deal with the people who are going to wear the dresses deserves not respect but awe. Even if I knew how to sew, I wouldn’t last a week in this business.
4. Financial planners
Every financial planner I meet describes what they do as “mostly psychology.” Of course, they need to recommend investments that suit the age, life status, and plans of their clients. That’s the easy part.
“When the market is dropping,” says Phil, “people call to scream that they’re losing their shirt. When the market is going up, they call to scream that they’re not making out as well as their friends claim they are.
“Either way, I just hold the phone far enough away from my ear to save my hearing until they’re done venting. Then I try to calm them down and assure them that investing is a long game, and over time they’ll do better staying the course we agreed on than jumping around with every market swing, up or down.”
“Do they listen?”
“Most of them. Eventually.”
4. Speakers’ booking agents
Matching clients with speakers can bring curious challenges. Celebrity speakers in particular may have unique requirements that the agents who book them must figure out and comply with. Or else.
For instance, one young man I met had to book a distinguished jurist. He needed to be picked up in a limo. The limo had to be a Bentley. And the Bentley had to be gray.
Well, excuse me.
5. Waiters
I think the favored term these days is “Servers.” Servers serve strangers. Some strangers are pleasant and courteous. Not all. Waiters mean to please, but to do that they have to put up with a lot. Always with a smile, of course.
Customers ask silly questions. (“What’s good here?”) Some don’t find the menu detailed enough. (“Can I have half of this and some of that, with the sauce on the side?”) They may find the food too hot. Or too cold. Or too spicy. Or too bland. After all that, they may tip a little. Or not.
But the server still has to ask, “Is everything satisfactory?”
6. Psychologists
Never mind.
7. Parking meter readers
Just kidding. Meter readers write parking tickets.
They know perfectly well that everyone knows exactly what they do. And they don’t care.
8. People who field complaints at call centers
Requirement: Patience, savvy, Xanax.
9. Dermatologists
You bet, in spades. I wrote a whole book on the subject. You can read it if you want to. If you disagree, don’t tell me.
I don’t need readers to disagree with me. I already have patients.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected] .
Telemedicine: Three fraud and abuse triggers
The practice of telemedicine is rapidly growing as more health professionals discover the value in treating patients via technology. Lisa S. Mazur, a Chicago-based health law attorney specializing in telemedicine, shares guidance on how to avoid running afoul of fraud and abuse regulations when using telehealth.
1. Improper coding. Incorrect billing for telemedicine services is a top trigger for federal fraud and abuse scrutiny. A 2018 Office of Inspector General (OIG) report found that 31% of a sample of 100 telehealth claims did not meet Medicare conditions for payment. Primary reasons for inaccurate billing included ineligible institutional providers; services provided by unacceptable means of communication; claims for noncovered services; and claims for patients who received services at nonrural originating sites. The inspector general estimated that Centers for Medicare & Medicaid Services wasted $3.7 million in improper telehealth payments during the audit period (2014 and 2015) and recommended that CMS conduct more audits going forward to identify telehealth overpayments.
“The error rate is shocking,” Ms. Mazur said in an interview. “The problem is the providers know what they need to do for traditional in-person services, but they don’t fully understand the complexities and nuances that can be implicated by telemedicine. [For instance], they know how to bill for an in-person E/M [evaluation and management] service, but they don’t know to bill for it properly for when it’s done virtually.”
To ensure correct billing, it’s critical for providers and billing staff to review CMS’ resources on requirements for telehealth payments and make sure they’re up to date with any changes.
2. Kickback skepticism in arrangements. Some telemedicine arrangements can raise kickback concerns if not properly defined. The federal Anti-Kickback Statute prohibits the exchange of anything of value in an effort to induce the referral of business in federal health care programs. For example, if a large hospital system purchases or leases telemedicine equipment at a discounted rate to a rural practice, the hospital could be accused of providing equipment at less than fair market value to secure referrals, Ms. Mazur explained.
Such arrangements should not raise alarm as long as certain conditions are met, according to a 2018 OIG advisory opinion. The opinion stemmed from an inquiry from a nonprofit, federally qualified health center that planned to provide free telemedicine equipment to a county clinic providing HIV testing and treatment. In his opinion, Robert K. DeConti, OIG assistant inspector general for legal affairs, wrote that the arrangement in question was low risk because it included safeguards to prevent inappropriate patient steering, it did not inappropriately increase costs to federal health programs, and it improved access to care.
Essentially, if health professionals can show that their telemedicine arrangement legitimately benefits patient care and improves patient outcomes, they are not likely to draw scrutiny, Ms. Mazur said. Because fraud and abuse laws can be complicated, she recommended having an attorney review telemedicine arrangements before they launch to spot any potential risks. Ensure the purpose of the arrangement can be clearly outlined should questions arise.
3. Free patient technology. The Civil Monetary Penalties Law is another fraud and abuse statute that can come into play in the telemedicine setting. Under this law, health professionals cannot knowingly solicit or receive remuneration for a patient referral nor can they induce patients to visit them via incentives such as free products. In the telemedicine context, the law can be triggered when practices offer patients free remote monitoring devices or apps that help track medical data.
“Hospitals and groups have very legitimate reasons to want to provide their patients with these types of tools for free,” Ms. Mazur said. “But anytime a health professional who is billing Medicare for services provides some to a patient for free, there’s a concern that you’re giving that service or product because you’re trying to induce them to come to you for care.”
The right parameters around free telemedicine tools can make all the difference, she said. For example, it’s important that practices do not market the free or discounted product to patients, according to Ms. Mazur. Also, make clear that the free products do not increase profits for the practice and that the offerings do not raise federal health care billings. Another way to go about it is to include the practice of providing a free telemedicine product or device under the scope of their charity policy by including language outlining when free or discounted products or services can be provided to underinsured patients, Ms. Mazur said.
Another good idea is for practices to integrate telemedicine into their corporate compliance programs. All health care entities are encouraged to have a corporate compliance program that outlines policies, training protocols, and standards of conduct to prevent, identify, and mitigate fraud and abuse.
Practices “need to make sure their existing compliance programs, including policies and procedures, take into account the nuances that are implicated by telemedicine,” Ms. Mazur said.
The practice of telemedicine is rapidly growing as more health professionals discover the value in treating patients via technology. Lisa S. Mazur, a Chicago-based health law attorney specializing in telemedicine, shares guidance on how to avoid running afoul of fraud and abuse regulations when using telehealth.
1. Improper coding. Incorrect billing for telemedicine services is a top trigger for federal fraud and abuse scrutiny. A 2018 Office of Inspector General (OIG) report found that 31% of a sample of 100 telehealth claims did not meet Medicare conditions for payment. Primary reasons for inaccurate billing included ineligible institutional providers; services provided by unacceptable means of communication; claims for noncovered services; and claims for patients who received services at nonrural originating sites. The inspector general estimated that Centers for Medicare & Medicaid Services wasted $3.7 million in improper telehealth payments during the audit period (2014 and 2015) and recommended that CMS conduct more audits going forward to identify telehealth overpayments.
“The error rate is shocking,” Ms. Mazur said in an interview. “The problem is the providers know what they need to do for traditional in-person services, but they don’t fully understand the complexities and nuances that can be implicated by telemedicine. [For instance], they know how to bill for an in-person E/M [evaluation and management] service, but they don’t know to bill for it properly for when it’s done virtually.”
To ensure correct billing, it’s critical for providers and billing staff to review CMS’ resources on requirements for telehealth payments and make sure they’re up to date with any changes.
2. Kickback skepticism in arrangements. Some telemedicine arrangements can raise kickback concerns if not properly defined. The federal Anti-Kickback Statute prohibits the exchange of anything of value in an effort to induce the referral of business in federal health care programs. For example, if a large hospital system purchases or leases telemedicine equipment at a discounted rate to a rural practice, the hospital could be accused of providing equipment at less than fair market value to secure referrals, Ms. Mazur explained.
Such arrangements should not raise alarm as long as certain conditions are met, according to a 2018 OIG advisory opinion. The opinion stemmed from an inquiry from a nonprofit, federally qualified health center that planned to provide free telemedicine equipment to a county clinic providing HIV testing and treatment. In his opinion, Robert K. DeConti, OIG assistant inspector general for legal affairs, wrote that the arrangement in question was low risk because it included safeguards to prevent inappropriate patient steering, it did not inappropriately increase costs to federal health programs, and it improved access to care.
Essentially, if health professionals can show that their telemedicine arrangement legitimately benefits patient care and improves patient outcomes, they are not likely to draw scrutiny, Ms. Mazur said. Because fraud and abuse laws can be complicated, she recommended having an attorney review telemedicine arrangements before they launch to spot any potential risks. Ensure the purpose of the arrangement can be clearly outlined should questions arise.
3. Free patient technology. The Civil Monetary Penalties Law is another fraud and abuse statute that can come into play in the telemedicine setting. Under this law, health professionals cannot knowingly solicit or receive remuneration for a patient referral nor can they induce patients to visit them via incentives such as free products. In the telemedicine context, the law can be triggered when practices offer patients free remote monitoring devices or apps that help track medical data.
“Hospitals and groups have very legitimate reasons to want to provide their patients with these types of tools for free,” Ms. Mazur said. “But anytime a health professional who is billing Medicare for services provides some to a patient for free, there’s a concern that you’re giving that service or product because you’re trying to induce them to come to you for care.”
The right parameters around free telemedicine tools can make all the difference, she said. For example, it’s important that practices do not market the free or discounted product to patients, according to Ms. Mazur. Also, make clear that the free products do not increase profits for the practice and that the offerings do not raise federal health care billings. Another way to go about it is to include the practice of providing a free telemedicine product or device under the scope of their charity policy by including language outlining when free or discounted products or services can be provided to underinsured patients, Ms. Mazur said.
Another good idea is for practices to integrate telemedicine into their corporate compliance programs. All health care entities are encouraged to have a corporate compliance program that outlines policies, training protocols, and standards of conduct to prevent, identify, and mitigate fraud and abuse.
Practices “need to make sure their existing compliance programs, including policies and procedures, take into account the nuances that are implicated by telemedicine,” Ms. Mazur said.
The practice of telemedicine is rapidly growing as more health professionals discover the value in treating patients via technology. Lisa S. Mazur, a Chicago-based health law attorney specializing in telemedicine, shares guidance on how to avoid running afoul of fraud and abuse regulations when using telehealth.
1. Improper coding. Incorrect billing for telemedicine services is a top trigger for federal fraud and abuse scrutiny. A 2018 Office of Inspector General (OIG) report found that 31% of a sample of 100 telehealth claims did not meet Medicare conditions for payment. Primary reasons for inaccurate billing included ineligible institutional providers; services provided by unacceptable means of communication; claims for noncovered services; and claims for patients who received services at nonrural originating sites. The inspector general estimated that Centers for Medicare & Medicaid Services wasted $3.7 million in improper telehealth payments during the audit period (2014 and 2015) and recommended that CMS conduct more audits going forward to identify telehealth overpayments.
“The error rate is shocking,” Ms. Mazur said in an interview. “The problem is the providers know what they need to do for traditional in-person services, but they don’t fully understand the complexities and nuances that can be implicated by telemedicine. [For instance], they know how to bill for an in-person E/M [evaluation and management] service, but they don’t know to bill for it properly for when it’s done virtually.”
To ensure correct billing, it’s critical for providers and billing staff to review CMS’ resources on requirements for telehealth payments and make sure they’re up to date with any changes.
2. Kickback skepticism in arrangements. Some telemedicine arrangements can raise kickback concerns if not properly defined. The federal Anti-Kickback Statute prohibits the exchange of anything of value in an effort to induce the referral of business in federal health care programs. For example, if a large hospital system purchases or leases telemedicine equipment at a discounted rate to a rural practice, the hospital could be accused of providing equipment at less than fair market value to secure referrals, Ms. Mazur explained.
Such arrangements should not raise alarm as long as certain conditions are met, according to a 2018 OIG advisory opinion. The opinion stemmed from an inquiry from a nonprofit, federally qualified health center that planned to provide free telemedicine equipment to a county clinic providing HIV testing and treatment. In his opinion, Robert K. DeConti, OIG assistant inspector general for legal affairs, wrote that the arrangement in question was low risk because it included safeguards to prevent inappropriate patient steering, it did not inappropriately increase costs to federal health programs, and it improved access to care.
Essentially, if health professionals can show that their telemedicine arrangement legitimately benefits patient care and improves patient outcomes, they are not likely to draw scrutiny, Ms. Mazur said. Because fraud and abuse laws can be complicated, she recommended having an attorney review telemedicine arrangements before they launch to spot any potential risks. Ensure the purpose of the arrangement can be clearly outlined should questions arise.
3. Free patient technology. The Civil Monetary Penalties Law is another fraud and abuse statute that can come into play in the telemedicine setting. Under this law, health professionals cannot knowingly solicit or receive remuneration for a patient referral nor can they induce patients to visit them via incentives such as free products. In the telemedicine context, the law can be triggered when practices offer patients free remote monitoring devices or apps that help track medical data.
“Hospitals and groups have very legitimate reasons to want to provide their patients with these types of tools for free,” Ms. Mazur said. “But anytime a health professional who is billing Medicare for services provides some to a patient for free, there’s a concern that you’re giving that service or product because you’re trying to induce them to come to you for care.”
The right parameters around free telemedicine tools can make all the difference, she said. For example, it’s important that practices do not market the free or discounted product to patients, according to Ms. Mazur. Also, make clear that the free products do not increase profits for the practice and that the offerings do not raise federal health care billings. Another way to go about it is to include the practice of providing a free telemedicine product or device under the scope of their charity policy by including language outlining when free or discounted products or services can be provided to underinsured patients, Ms. Mazur said.
Another good idea is for practices to integrate telemedicine into their corporate compliance programs. All health care entities are encouraged to have a corporate compliance program that outlines policies, training protocols, and standards of conduct to prevent, identify, and mitigate fraud and abuse.
Practices “need to make sure their existing compliance programs, including policies and procedures, take into account the nuances that are implicated by telemedicine,” Ms. Mazur said.
Preappointment consults: Evidence builds for boosting access, revenue
About 15 years ago, rheumatologists at the University of Colorado School of Medicine, Aurora, implemented a preappointment consult triage system as a way to identify rheumatology patients who require timely evaluation and treatment.
Over time, the endeavor caused some soul searching. They wondered how effective their consult triage system was in identifying all patients with inflammatory rheumatic diseases and in ensuring they were seen promptly. They also wondered about the revenue implications of routine outpatient care of autoimmune and inflammatory rheumatic disease (AIRD) patients, compared with that of non-AIRD patients.
“Hospital leadership is very interested in making sure that all patients have access to specialty care in a timely manner,” said Sterling G. West, MD, one of the study authors who is also professor of medicine at the university. “However, there are not enough rheumatologists to see all patients with rheumatic complaints, and this deficit is likely to get worse. Although all patients with a rheumatic complaint would likely benefit from a rheumatology consultation, it is clear that timely access to rheumatologic care is most beneficial for patients with inflammatory rheumatic diseases to prevent future morbidity and disability.”
Year-long follow-up finds high sensitivity, more revenue
What started out as a quality improvement project morphed into a robust study that was published online July 12, 2018, in Arthritis Care & Research. Using data recorded in the electronic medical record, Dr. West and his colleagues retrospectively reviewed 961 new outpatient rheumatology consults sent during a 9-month period for final diagnosis and revenue generation for routine outpatient care over 1 year following consult review or initial evaluation. The first step of the consult management involves an intake access coordinator, Ryan Goecker, obtaining information about the patient from the referring clinician. Next, one of three experienced rheumatologists at the university – Duane W. Pearson, MD, Christopher C. Striebich, MD, or Jason R. Kolfenbach, MD – reviews the information about the case. “We do request that labs and x-rays be done ahead of time, depending on what the consult question is, so that we have the data to be able to decide whether a patient likely has an inflammatory process that we need to see or not,” Dr. West explained. “It takes somewhere between 5 and 20 minutes per consult. In total, it takes our rheumatologists about 5 hours a week to do the consults. However, even after subtracting the physician time spent screening consults, the time saved by the consult triage system enabled over 200 more time slots per year to be available to see new AIRD patients than would have been possible without the triage system.”
Following review of the data supplied about the case, patients who may have acute inflammatory monoarticular arthritis or some other rheumatologic emergency are seen within 24-48 hours, while consults with a possible AIRD are approved and seen within 1-4 weeks. Priority is given to those with suspected vasculitis, systemic lupus erythematosus, myositis, and disabling inflammatory arthritis. Patients with probable noninflammatory conditions such as osteoarthritis, fibromyalgia, and mechanical low back pain are not scheduled for an in-person visit. “Instead of simply declining the consult, we try to give some direction, or we say, ‘Why don’t you try this, this, and this, sort of a miniconsult,” Dr. West noted. “One of the potential adverse reactions to doing consult triage would be that the referring provider would be upset if we decline their consult. We acknowledge that. But over time we’ve been able to convince them that this is best for all concerned, so that the inflammatory disease group gets in to see us in an appropriate amount of time.”
Of the 961 consults, 673 were scheduled for an in-person AIRD evaluation and 288 consults were not scheduled. Patients were seen an average of 13 days after consult review. Of the 673 approved consults, 597 (89%) came for evaluation. Of these, 357 were diagnosed as having an AIRD, while 240 were diagnosed as having a non-AIRD. Among the patients not scheduled for a rheumatology visit, 128 had 1-year follow-up data, with 6 patients eventually diagnosed as having an AIRD. This translated into a consult triage sensitivity of 98% and a positive predictive value of 60%. “There’s a fair number of people with noninflammatory disease who do get into see us,” Dr. West said. “That has to do with our desire to not be overly rigid. The sensitivity, specificity, and positive predictive value were not quite as high as some people would like to see, but we really weren’t missing people who really needed to be seen.”
In their conservative cost analysis, revenue data for outpatient care was available for 318 of the 357 AIRD patients and 192 of 240 non-AIRD patients. It demonstrated that care of AIRD patients generates 44 times more revenue, compared with non-AIRD patients ($5,877 vs. $134 per patient, respectively; P less than .001).
“Our consult triage protocol appears to be an effective method to assure that patients with inflammatory rheumatic diseases get expedited access to appropriate rheumatologic care,” Dr. West concluded. “Using conservative measures, caring for patients with inflammatory rheumatic diseases results in significantly more revenue generation.” Generalizability of such a protocol to other practice settings depends on the time rheumatologists are willing to commit to it. “That’s the big thing,” he said. “The question comes up, could a nurse or a nurse practitioner learn the skills over time to be able to do efficient consult triage, to free up the doc from that? Yes. I think that’s absolutely possible.”
Going forward, Dr. Pearson and Dr. Kolfenbach, who are authors on the paper, have launched a pilot project in which the rheumatologist is compensated for doing a record review and e-consult on patients not recommended to be seen in the rheumatology clinic. “For consults who do not have inflammatory disease, we can say [to the referring clinician], ‘If we did see them, this is what we would be doing,’ ” Dr. West said. “Sometimes if there are still questions, we’ll say something like, ‘Once you get the results back, let us know and we can look at it again.’ That way they are getting similar compensation as telemedicine and other forms of consultation.”
At the Zuckerberg San Francisco General Hospital (ZSFG), use of a novel electronic consultation and referral system that was implemented in 2007 by University of California, San Francisco, providers continues to thrive for rheumatology and other specialty services. With this electronic system, previously called e-referral, all provider communication is captured in real time and recorded within the electronic health record. In a 2015 article from Arthritis Care & Research, researchers led by Jinoos Yazdany, MD, MPH, reviewed 2,105 e-referrals made between 2008 and 2012 (Arthritis Care Res. 2015;67[8]:1158-63). The main outcome of interest was use of preconsultation exchange, defined as back-and-forth communication between referring and specialty care providers, facilitating triage of referrals, requests for more information, or resolution of questions without a visit. The researchers found that about 25% of e-consults were resolved without a clinic visit, and that the proportion of e-consults undergoing preconsultation exchange increased over time, from 55% in 2008 to 74% in 2011.
“We’ve had situations in which somebody within our hospital system has requested a rheumatology referral but did not realize the urgency of the patients’ clinical signs and symptoms,” Dr. Yazdany, a rheumatologist and health services researcher at UCSF, said in an interview. “E-referrals are often responded to within that day. When we see an e-consult for a young woman with a rash and protein in her urine, a rheumatologist will recognize that is very concerning for a serious diagnosis like lupus of the kidney or systemic vasculitis. In that situation, we would pick up the phone and sometimes even call the patient and have them come to the emergency room for an urgent evaluation. Over the years, there have been many situations in which we’ve been able to intervene much earlier than we otherwise would have. In some cases that early intervention was lifesaving, or at least organ-preserving for the patient.”
Two studies from the early 2000s examined the use of preappointment management in rheumatology (see Arthritis Care Res. 2001;45:295-300 and Arthritis Rheum. 2004;51:253-7), but this is among the first to incorporate use of an electronic medical record to provide real-time exchange between the specialist and the referring provider. Clinicians at UCSF are reimbursed for reviewing e-consults in one of two ways. Those at ZSFG have protected and compensated time for the task, “so it’s basically part of their job to spend a half day a week on e-consults,” Dr. Yazdany said. “The department of public health and our health care system fold that into operational costs. That’s absolutely critical for success. To be done well and thoughtfully, managing consults and referrals takes time. It takes a lot of expertise.” Meanwhile, clinicians at UCSF’s main university hospital receive a small payment for each e-consult they review. “If it’s a complex consult it’s a higher reimbursement,” she said. “If it’s a simple one, it’s slightly less reimbursement, so it’s a fee-for-service model. It’s something that the health system funds, because it creates efficiencies and access for patients.”
Gaining popularity across specialties
Delphine S. Tuot, MD, a nephrologist who directs the ZSFG e-consult system, said that the notion of preconsult triage is gaining popularity in all medical specialties. For example, the Blue Shield of California Foundation is funding implementation of e-consult systems across many of California’s safety net health care delivery systems. “We have many health care plans that are interested in this process as well, because it improves access to specialty care, particularly in rural areas or in areas where the specialist workforce is limited,” said Dr. Tuot, who is codirector of the UCSF Center for Innovation in Access and Quality at ZSFG. Such efforts are also being promoted by the Public Hospital Redesign and Incentives in Medi-Cal Program (PRIME), which is part of California’s Medicaid waiver. PRIME “is encouraging health systems to look at innovative ways to deliver specialty care,” Dr. Tuot said. “E-consults and other non–face-to-face ways to deliver specialty care, including telemedicine encounters, count toward that metric for the Medicaid waiver.”
At the national level, the Association of American Medical Colleges has collaborated with more than 20 academic medical centers in 14 states, including Dartmouth-Hitchcock and Yale University, to implement tools built into the electronic medical record system through a program known as Project CORE (Coordinating Optimal Referral Experiences). According to Scott Shipman, MD, MPH, director of clinical innovations for the AAMC, nearly all of the current CORE sites have either gone live with the model in rheumatology or are planning to do so. “Better communication and coordination between primary care providers and specialists is important for all specialties, but because of the complexity of evaluation and management of problems in rheumatology, there is a tremendous opportunity for the CORE model to help get providers on the same page,” Dr. Shipman said in an interview. “We do this through simple decision support that we build into the referral order in the EMR, available at the point of care. Additionally, given the workforce challenges facing rheumatology in most regions of the country and consequent access barriers, offloading some of the demand via e-consults holds great promise.” Current focus areas for Project CORE, he said, include continued support of current CORE sites in their implementation and scaling efforts to maximize impact, advocacy to promote payer engagement in support of e-consult reimbursements, and working to extend the model to additional academic medical centers.
Dr. Tuot emphasized that performing e-consults “takes time and effort on behalf of specialists, so if you’re by yourself in solo practice it probably does not make sense to implement,” she said. “You need to spend your time seeing patients as much as possible. For primary care providers who are asking for curbside consults, it’s probably best to have things in writing from the specialist, such as in an e-consult, to make sure there’s no misunderstanding.”
As demand for rheumatology services increases, clinicians “have to figure out a way to see patients in most urgent need of our services,” Dr. Yazdany said. “That requires that we use technology like the e-consult system to prioritize the patients that we’re seeing. As we look at the rheumatology workforce shortage, especially in some geographic regions, it’s going to be absolutely critical. There are some diseases that no other specialists have experience caring for. In those situations, those patients need to get in to see rheumatologists in a timely fashion.”
Paper-based preconsult triage system
At Essentia Health, an integrated health care system with facilities in Minnesota, Wisconsin, and North Dakota, Meghan Scheibe, MD, a rheumatologist, is currently working with a rheumatologist colleague and a registered nurse to implement a paper-based preconsult triage system, “because our wait times have unfortunately skyrocketed,” she said. “We receive a lot of outside referrals from other health care groups and large health care systems that don’t have access to rheumatology.” Currently, the triage system is comprised of a referral note which includes the reason for consultation. Dr. Scheibe and her colleague rank the referral as expedited, intermediate concern, or low priority based on information provided by the referring clinician. “We mark those for our nurse to help schedule, and we let the referring provider know what the wait time is,” Dr. Scheibe said. “Then they have an opportunity to communicate back to us or say, ‘Yes, that’s fine,’ or, ‘I’m really worried about this patient. Could you get her in sooner?’ ”
It’s early in the process, but so far, implementation of the preconsult triage protocol “is allowing us to focus on the consultations on which we can be most impactful and not overwhelm the rheumatology workforce that we have,” she said.
Research reported in the UCSF study was supported by the American College of Rheumatology’s Ephraim P. Engleman Endowed Resident Research Preceptorship and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. None of the sources interviewed for this story reported having relevant financial disclosures.
About 15 years ago, rheumatologists at the University of Colorado School of Medicine, Aurora, implemented a preappointment consult triage system as a way to identify rheumatology patients who require timely evaluation and treatment.
Over time, the endeavor caused some soul searching. They wondered how effective their consult triage system was in identifying all patients with inflammatory rheumatic diseases and in ensuring they were seen promptly. They also wondered about the revenue implications of routine outpatient care of autoimmune and inflammatory rheumatic disease (AIRD) patients, compared with that of non-AIRD patients.
“Hospital leadership is very interested in making sure that all patients have access to specialty care in a timely manner,” said Sterling G. West, MD, one of the study authors who is also professor of medicine at the university. “However, there are not enough rheumatologists to see all patients with rheumatic complaints, and this deficit is likely to get worse. Although all patients with a rheumatic complaint would likely benefit from a rheumatology consultation, it is clear that timely access to rheumatologic care is most beneficial for patients with inflammatory rheumatic diseases to prevent future morbidity and disability.”
Year-long follow-up finds high sensitivity, more revenue
What started out as a quality improvement project morphed into a robust study that was published online July 12, 2018, in Arthritis Care & Research. Using data recorded in the electronic medical record, Dr. West and his colleagues retrospectively reviewed 961 new outpatient rheumatology consults sent during a 9-month period for final diagnosis and revenue generation for routine outpatient care over 1 year following consult review or initial evaluation. The first step of the consult management involves an intake access coordinator, Ryan Goecker, obtaining information about the patient from the referring clinician. Next, one of three experienced rheumatologists at the university – Duane W. Pearson, MD, Christopher C. Striebich, MD, or Jason R. Kolfenbach, MD – reviews the information about the case. “We do request that labs and x-rays be done ahead of time, depending on what the consult question is, so that we have the data to be able to decide whether a patient likely has an inflammatory process that we need to see or not,” Dr. West explained. “It takes somewhere between 5 and 20 minutes per consult. In total, it takes our rheumatologists about 5 hours a week to do the consults. However, even after subtracting the physician time spent screening consults, the time saved by the consult triage system enabled over 200 more time slots per year to be available to see new AIRD patients than would have been possible without the triage system.”
Following review of the data supplied about the case, patients who may have acute inflammatory monoarticular arthritis or some other rheumatologic emergency are seen within 24-48 hours, while consults with a possible AIRD are approved and seen within 1-4 weeks. Priority is given to those with suspected vasculitis, systemic lupus erythematosus, myositis, and disabling inflammatory arthritis. Patients with probable noninflammatory conditions such as osteoarthritis, fibromyalgia, and mechanical low back pain are not scheduled for an in-person visit. “Instead of simply declining the consult, we try to give some direction, or we say, ‘Why don’t you try this, this, and this, sort of a miniconsult,” Dr. West noted. “One of the potential adverse reactions to doing consult triage would be that the referring provider would be upset if we decline their consult. We acknowledge that. But over time we’ve been able to convince them that this is best for all concerned, so that the inflammatory disease group gets in to see us in an appropriate amount of time.”
Of the 961 consults, 673 were scheduled for an in-person AIRD evaluation and 288 consults were not scheduled. Patients were seen an average of 13 days after consult review. Of the 673 approved consults, 597 (89%) came for evaluation. Of these, 357 were diagnosed as having an AIRD, while 240 were diagnosed as having a non-AIRD. Among the patients not scheduled for a rheumatology visit, 128 had 1-year follow-up data, with 6 patients eventually diagnosed as having an AIRD. This translated into a consult triage sensitivity of 98% and a positive predictive value of 60%. “There’s a fair number of people with noninflammatory disease who do get into see us,” Dr. West said. “That has to do with our desire to not be overly rigid. The sensitivity, specificity, and positive predictive value were not quite as high as some people would like to see, but we really weren’t missing people who really needed to be seen.”
In their conservative cost analysis, revenue data for outpatient care was available for 318 of the 357 AIRD patients and 192 of 240 non-AIRD patients. It demonstrated that care of AIRD patients generates 44 times more revenue, compared with non-AIRD patients ($5,877 vs. $134 per patient, respectively; P less than .001).
“Our consult triage protocol appears to be an effective method to assure that patients with inflammatory rheumatic diseases get expedited access to appropriate rheumatologic care,” Dr. West concluded. “Using conservative measures, caring for patients with inflammatory rheumatic diseases results in significantly more revenue generation.” Generalizability of such a protocol to other practice settings depends on the time rheumatologists are willing to commit to it. “That’s the big thing,” he said. “The question comes up, could a nurse or a nurse practitioner learn the skills over time to be able to do efficient consult triage, to free up the doc from that? Yes. I think that’s absolutely possible.”
Going forward, Dr. Pearson and Dr. Kolfenbach, who are authors on the paper, have launched a pilot project in which the rheumatologist is compensated for doing a record review and e-consult on patients not recommended to be seen in the rheumatology clinic. “For consults who do not have inflammatory disease, we can say [to the referring clinician], ‘If we did see them, this is what we would be doing,’ ” Dr. West said. “Sometimes if there are still questions, we’ll say something like, ‘Once you get the results back, let us know and we can look at it again.’ That way they are getting similar compensation as telemedicine and other forms of consultation.”
At the Zuckerberg San Francisco General Hospital (ZSFG), use of a novel electronic consultation and referral system that was implemented in 2007 by University of California, San Francisco, providers continues to thrive for rheumatology and other specialty services. With this electronic system, previously called e-referral, all provider communication is captured in real time and recorded within the electronic health record. In a 2015 article from Arthritis Care & Research, researchers led by Jinoos Yazdany, MD, MPH, reviewed 2,105 e-referrals made between 2008 and 2012 (Arthritis Care Res. 2015;67[8]:1158-63). The main outcome of interest was use of preconsultation exchange, defined as back-and-forth communication between referring and specialty care providers, facilitating triage of referrals, requests for more information, or resolution of questions without a visit. The researchers found that about 25% of e-consults were resolved without a clinic visit, and that the proportion of e-consults undergoing preconsultation exchange increased over time, from 55% in 2008 to 74% in 2011.
“We’ve had situations in which somebody within our hospital system has requested a rheumatology referral but did not realize the urgency of the patients’ clinical signs and symptoms,” Dr. Yazdany, a rheumatologist and health services researcher at UCSF, said in an interview. “E-referrals are often responded to within that day. When we see an e-consult for a young woman with a rash and protein in her urine, a rheumatologist will recognize that is very concerning for a serious diagnosis like lupus of the kidney or systemic vasculitis. In that situation, we would pick up the phone and sometimes even call the patient and have them come to the emergency room for an urgent evaluation. Over the years, there have been many situations in which we’ve been able to intervene much earlier than we otherwise would have. In some cases that early intervention was lifesaving, or at least organ-preserving for the patient.”
Two studies from the early 2000s examined the use of preappointment management in rheumatology (see Arthritis Care Res. 2001;45:295-300 and Arthritis Rheum. 2004;51:253-7), but this is among the first to incorporate use of an electronic medical record to provide real-time exchange between the specialist and the referring provider. Clinicians at UCSF are reimbursed for reviewing e-consults in one of two ways. Those at ZSFG have protected and compensated time for the task, “so it’s basically part of their job to spend a half day a week on e-consults,” Dr. Yazdany said. “The department of public health and our health care system fold that into operational costs. That’s absolutely critical for success. To be done well and thoughtfully, managing consults and referrals takes time. It takes a lot of expertise.” Meanwhile, clinicians at UCSF’s main university hospital receive a small payment for each e-consult they review. “If it’s a complex consult it’s a higher reimbursement,” she said. “If it’s a simple one, it’s slightly less reimbursement, so it’s a fee-for-service model. It’s something that the health system funds, because it creates efficiencies and access for patients.”
Gaining popularity across specialties
Delphine S. Tuot, MD, a nephrologist who directs the ZSFG e-consult system, said that the notion of preconsult triage is gaining popularity in all medical specialties. For example, the Blue Shield of California Foundation is funding implementation of e-consult systems across many of California’s safety net health care delivery systems. “We have many health care plans that are interested in this process as well, because it improves access to specialty care, particularly in rural areas or in areas where the specialist workforce is limited,” said Dr. Tuot, who is codirector of the UCSF Center for Innovation in Access and Quality at ZSFG. Such efforts are also being promoted by the Public Hospital Redesign and Incentives in Medi-Cal Program (PRIME), which is part of California’s Medicaid waiver. PRIME “is encouraging health systems to look at innovative ways to deliver specialty care,” Dr. Tuot said. “E-consults and other non–face-to-face ways to deliver specialty care, including telemedicine encounters, count toward that metric for the Medicaid waiver.”
At the national level, the Association of American Medical Colleges has collaborated with more than 20 academic medical centers in 14 states, including Dartmouth-Hitchcock and Yale University, to implement tools built into the electronic medical record system through a program known as Project CORE (Coordinating Optimal Referral Experiences). According to Scott Shipman, MD, MPH, director of clinical innovations for the AAMC, nearly all of the current CORE sites have either gone live with the model in rheumatology or are planning to do so. “Better communication and coordination between primary care providers and specialists is important for all specialties, but because of the complexity of evaluation and management of problems in rheumatology, there is a tremendous opportunity for the CORE model to help get providers on the same page,” Dr. Shipman said in an interview. “We do this through simple decision support that we build into the referral order in the EMR, available at the point of care. Additionally, given the workforce challenges facing rheumatology in most regions of the country and consequent access barriers, offloading some of the demand via e-consults holds great promise.” Current focus areas for Project CORE, he said, include continued support of current CORE sites in their implementation and scaling efforts to maximize impact, advocacy to promote payer engagement in support of e-consult reimbursements, and working to extend the model to additional academic medical centers.
Dr. Tuot emphasized that performing e-consults “takes time and effort on behalf of specialists, so if you’re by yourself in solo practice it probably does not make sense to implement,” she said. “You need to spend your time seeing patients as much as possible. For primary care providers who are asking for curbside consults, it’s probably best to have things in writing from the specialist, such as in an e-consult, to make sure there’s no misunderstanding.”
As demand for rheumatology services increases, clinicians “have to figure out a way to see patients in most urgent need of our services,” Dr. Yazdany said. “That requires that we use technology like the e-consult system to prioritize the patients that we’re seeing. As we look at the rheumatology workforce shortage, especially in some geographic regions, it’s going to be absolutely critical. There are some diseases that no other specialists have experience caring for. In those situations, those patients need to get in to see rheumatologists in a timely fashion.”
Paper-based preconsult triage system
At Essentia Health, an integrated health care system with facilities in Minnesota, Wisconsin, and North Dakota, Meghan Scheibe, MD, a rheumatologist, is currently working with a rheumatologist colleague and a registered nurse to implement a paper-based preconsult triage system, “because our wait times have unfortunately skyrocketed,” she said. “We receive a lot of outside referrals from other health care groups and large health care systems that don’t have access to rheumatology.” Currently, the triage system is comprised of a referral note which includes the reason for consultation. Dr. Scheibe and her colleague rank the referral as expedited, intermediate concern, or low priority based on information provided by the referring clinician. “We mark those for our nurse to help schedule, and we let the referring provider know what the wait time is,” Dr. Scheibe said. “Then they have an opportunity to communicate back to us or say, ‘Yes, that’s fine,’ or, ‘I’m really worried about this patient. Could you get her in sooner?’ ”
It’s early in the process, but so far, implementation of the preconsult triage protocol “is allowing us to focus on the consultations on which we can be most impactful and not overwhelm the rheumatology workforce that we have,” she said.
Research reported in the UCSF study was supported by the American College of Rheumatology’s Ephraim P. Engleman Endowed Resident Research Preceptorship and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. None of the sources interviewed for this story reported having relevant financial disclosures.
About 15 years ago, rheumatologists at the University of Colorado School of Medicine, Aurora, implemented a preappointment consult triage system as a way to identify rheumatology patients who require timely evaluation and treatment.
Over time, the endeavor caused some soul searching. They wondered how effective their consult triage system was in identifying all patients with inflammatory rheumatic diseases and in ensuring they were seen promptly. They also wondered about the revenue implications of routine outpatient care of autoimmune and inflammatory rheumatic disease (AIRD) patients, compared with that of non-AIRD patients.
“Hospital leadership is very interested in making sure that all patients have access to specialty care in a timely manner,” said Sterling G. West, MD, one of the study authors who is also professor of medicine at the university. “However, there are not enough rheumatologists to see all patients with rheumatic complaints, and this deficit is likely to get worse. Although all patients with a rheumatic complaint would likely benefit from a rheumatology consultation, it is clear that timely access to rheumatologic care is most beneficial for patients with inflammatory rheumatic diseases to prevent future morbidity and disability.”
Year-long follow-up finds high sensitivity, more revenue
What started out as a quality improvement project morphed into a robust study that was published online July 12, 2018, in Arthritis Care & Research. Using data recorded in the electronic medical record, Dr. West and his colleagues retrospectively reviewed 961 new outpatient rheumatology consults sent during a 9-month period for final diagnosis and revenue generation for routine outpatient care over 1 year following consult review or initial evaluation. The first step of the consult management involves an intake access coordinator, Ryan Goecker, obtaining information about the patient from the referring clinician. Next, one of three experienced rheumatologists at the university – Duane W. Pearson, MD, Christopher C. Striebich, MD, or Jason R. Kolfenbach, MD – reviews the information about the case. “We do request that labs and x-rays be done ahead of time, depending on what the consult question is, so that we have the data to be able to decide whether a patient likely has an inflammatory process that we need to see or not,” Dr. West explained. “It takes somewhere between 5 and 20 minutes per consult. In total, it takes our rheumatologists about 5 hours a week to do the consults. However, even after subtracting the physician time spent screening consults, the time saved by the consult triage system enabled over 200 more time slots per year to be available to see new AIRD patients than would have been possible without the triage system.”
Following review of the data supplied about the case, patients who may have acute inflammatory monoarticular arthritis or some other rheumatologic emergency are seen within 24-48 hours, while consults with a possible AIRD are approved and seen within 1-4 weeks. Priority is given to those with suspected vasculitis, systemic lupus erythematosus, myositis, and disabling inflammatory arthritis. Patients with probable noninflammatory conditions such as osteoarthritis, fibromyalgia, and mechanical low back pain are not scheduled for an in-person visit. “Instead of simply declining the consult, we try to give some direction, or we say, ‘Why don’t you try this, this, and this, sort of a miniconsult,” Dr. West noted. “One of the potential adverse reactions to doing consult triage would be that the referring provider would be upset if we decline their consult. We acknowledge that. But over time we’ve been able to convince them that this is best for all concerned, so that the inflammatory disease group gets in to see us in an appropriate amount of time.”
Of the 961 consults, 673 were scheduled for an in-person AIRD evaluation and 288 consults were not scheduled. Patients were seen an average of 13 days after consult review. Of the 673 approved consults, 597 (89%) came for evaluation. Of these, 357 were diagnosed as having an AIRD, while 240 were diagnosed as having a non-AIRD. Among the patients not scheduled for a rheumatology visit, 128 had 1-year follow-up data, with 6 patients eventually diagnosed as having an AIRD. This translated into a consult triage sensitivity of 98% and a positive predictive value of 60%. “There’s a fair number of people with noninflammatory disease who do get into see us,” Dr. West said. “That has to do with our desire to not be overly rigid. The sensitivity, specificity, and positive predictive value were not quite as high as some people would like to see, but we really weren’t missing people who really needed to be seen.”
In their conservative cost analysis, revenue data for outpatient care was available for 318 of the 357 AIRD patients and 192 of 240 non-AIRD patients. It demonstrated that care of AIRD patients generates 44 times more revenue, compared with non-AIRD patients ($5,877 vs. $134 per patient, respectively; P less than .001).
“Our consult triage protocol appears to be an effective method to assure that patients with inflammatory rheumatic diseases get expedited access to appropriate rheumatologic care,” Dr. West concluded. “Using conservative measures, caring for patients with inflammatory rheumatic diseases results in significantly more revenue generation.” Generalizability of such a protocol to other practice settings depends on the time rheumatologists are willing to commit to it. “That’s the big thing,” he said. “The question comes up, could a nurse or a nurse practitioner learn the skills over time to be able to do efficient consult triage, to free up the doc from that? Yes. I think that’s absolutely possible.”
Going forward, Dr. Pearson and Dr. Kolfenbach, who are authors on the paper, have launched a pilot project in which the rheumatologist is compensated for doing a record review and e-consult on patients not recommended to be seen in the rheumatology clinic. “For consults who do not have inflammatory disease, we can say [to the referring clinician], ‘If we did see them, this is what we would be doing,’ ” Dr. West said. “Sometimes if there are still questions, we’ll say something like, ‘Once you get the results back, let us know and we can look at it again.’ That way they are getting similar compensation as telemedicine and other forms of consultation.”
At the Zuckerberg San Francisco General Hospital (ZSFG), use of a novel electronic consultation and referral system that was implemented in 2007 by University of California, San Francisco, providers continues to thrive for rheumatology and other specialty services. With this electronic system, previously called e-referral, all provider communication is captured in real time and recorded within the electronic health record. In a 2015 article from Arthritis Care & Research, researchers led by Jinoos Yazdany, MD, MPH, reviewed 2,105 e-referrals made between 2008 and 2012 (Arthritis Care Res. 2015;67[8]:1158-63). The main outcome of interest was use of preconsultation exchange, defined as back-and-forth communication between referring and specialty care providers, facilitating triage of referrals, requests for more information, or resolution of questions without a visit. The researchers found that about 25% of e-consults were resolved without a clinic visit, and that the proportion of e-consults undergoing preconsultation exchange increased over time, from 55% in 2008 to 74% in 2011.
“We’ve had situations in which somebody within our hospital system has requested a rheumatology referral but did not realize the urgency of the patients’ clinical signs and symptoms,” Dr. Yazdany, a rheumatologist and health services researcher at UCSF, said in an interview. “E-referrals are often responded to within that day. When we see an e-consult for a young woman with a rash and protein in her urine, a rheumatologist will recognize that is very concerning for a serious diagnosis like lupus of the kidney or systemic vasculitis. In that situation, we would pick up the phone and sometimes even call the patient and have them come to the emergency room for an urgent evaluation. Over the years, there have been many situations in which we’ve been able to intervene much earlier than we otherwise would have. In some cases that early intervention was lifesaving, or at least organ-preserving for the patient.”
Two studies from the early 2000s examined the use of preappointment management in rheumatology (see Arthritis Care Res. 2001;45:295-300 and Arthritis Rheum. 2004;51:253-7), but this is among the first to incorporate use of an electronic medical record to provide real-time exchange between the specialist and the referring provider. Clinicians at UCSF are reimbursed for reviewing e-consults in one of two ways. Those at ZSFG have protected and compensated time for the task, “so it’s basically part of their job to spend a half day a week on e-consults,” Dr. Yazdany said. “The department of public health and our health care system fold that into operational costs. That’s absolutely critical for success. To be done well and thoughtfully, managing consults and referrals takes time. It takes a lot of expertise.” Meanwhile, clinicians at UCSF’s main university hospital receive a small payment for each e-consult they review. “If it’s a complex consult it’s a higher reimbursement,” she said. “If it’s a simple one, it’s slightly less reimbursement, so it’s a fee-for-service model. It’s something that the health system funds, because it creates efficiencies and access for patients.”
Gaining popularity across specialties
Delphine S. Tuot, MD, a nephrologist who directs the ZSFG e-consult system, said that the notion of preconsult triage is gaining popularity in all medical specialties. For example, the Blue Shield of California Foundation is funding implementation of e-consult systems across many of California’s safety net health care delivery systems. “We have many health care plans that are interested in this process as well, because it improves access to specialty care, particularly in rural areas or in areas where the specialist workforce is limited,” said Dr. Tuot, who is codirector of the UCSF Center for Innovation in Access and Quality at ZSFG. Such efforts are also being promoted by the Public Hospital Redesign and Incentives in Medi-Cal Program (PRIME), which is part of California’s Medicaid waiver. PRIME “is encouraging health systems to look at innovative ways to deliver specialty care,” Dr. Tuot said. “E-consults and other non–face-to-face ways to deliver specialty care, including telemedicine encounters, count toward that metric for the Medicaid waiver.”
At the national level, the Association of American Medical Colleges has collaborated with more than 20 academic medical centers in 14 states, including Dartmouth-Hitchcock and Yale University, to implement tools built into the electronic medical record system through a program known as Project CORE (Coordinating Optimal Referral Experiences). According to Scott Shipman, MD, MPH, director of clinical innovations for the AAMC, nearly all of the current CORE sites have either gone live with the model in rheumatology or are planning to do so. “Better communication and coordination between primary care providers and specialists is important for all specialties, but because of the complexity of evaluation and management of problems in rheumatology, there is a tremendous opportunity for the CORE model to help get providers on the same page,” Dr. Shipman said in an interview. “We do this through simple decision support that we build into the referral order in the EMR, available at the point of care. Additionally, given the workforce challenges facing rheumatology in most regions of the country and consequent access barriers, offloading some of the demand via e-consults holds great promise.” Current focus areas for Project CORE, he said, include continued support of current CORE sites in their implementation and scaling efforts to maximize impact, advocacy to promote payer engagement in support of e-consult reimbursements, and working to extend the model to additional academic medical centers.
Dr. Tuot emphasized that performing e-consults “takes time and effort on behalf of specialists, so if you’re by yourself in solo practice it probably does not make sense to implement,” she said. “You need to spend your time seeing patients as much as possible. For primary care providers who are asking for curbside consults, it’s probably best to have things in writing from the specialist, such as in an e-consult, to make sure there’s no misunderstanding.”
As demand for rheumatology services increases, clinicians “have to figure out a way to see patients in most urgent need of our services,” Dr. Yazdany said. “That requires that we use technology like the e-consult system to prioritize the patients that we’re seeing. As we look at the rheumatology workforce shortage, especially in some geographic regions, it’s going to be absolutely critical. There are some diseases that no other specialists have experience caring for. In those situations, those patients need to get in to see rheumatologists in a timely fashion.”
Paper-based preconsult triage system
At Essentia Health, an integrated health care system with facilities in Minnesota, Wisconsin, and North Dakota, Meghan Scheibe, MD, a rheumatologist, is currently working with a rheumatologist colleague and a registered nurse to implement a paper-based preconsult triage system, “because our wait times have unfortunately skyrocketed,” she said. “We receive a lot of outside referrals from other health care groups and large health care systems that don’t have access to rheumatology.” Currently, the triage system is comprised of a referral note which includes the reason for consultation. Dr. Scheibe and her colleague rank the referral as expedited, intermediate concern, or low priority based on information provided by the referring clinician. “We mark those for our nurse to help schedule, and we let the referring provider know what the wait time is,” Dr. Scheibe said. “Then they have an opportunity to communicate back to us or say, ‘Yes, that’s fine,’ or, ‘I’m really worried about this patient. Could you get her in sooner?’ ”
It’s early in the process, but so far, implementation of the preconsult triage protocol “is allowing us to focus on the consultations on which we can be most impactful and not overwhelm the rheumatology workforce that we have,” she said.
Research reported in the UCSF study was supported by the American College of Rheumatology’s Ephraim P. Engleman Endowed Resident Research Preceptorship and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. None of the sources interviewed for this story reported having relevant financial disclosures.
Flexible Bronchoscopic Removal of 3 Foreign Objects
Consider flexible bronchoscopy as an option to retrieve aspirated foreign bodies in the airway.
Airway foreign-body aspiration may cause no symptoms, although it can produce acute and life-threatening central airway obstruction.1 In the US, at least 2,700 people, including more than 300 children, die of foreign-body aspiration each year.2 Most foreign-body aspirations occur in children and elderly patients.3 In adults, dementia, drug intoxication, strokes, seizures, and neurologic disorders may predispose patients to aspiration.3 Some of the consequences of an aspirated object are complete or partial airway obstruction, respiratory distress and failure, pneumothorax, and hemorrhage.2 In addition, inadvertent aspiration of foreign objects in asymptomatic patients may not be evident for months, resulting in late complications as postobstructive pneumonia, bronchiectasis, or lung abscess.2
We present a case of a patient with documented schizophrenia with nonadherence to his antipsychotic medications who aspirated different objects. Flexible bronchoscopy was performed since rigid bronchoscopy is not available at our institution. Several bronchoscopy tools were required to successfully remove the objects and avoid further invasive interventions, such as cardiothoracic surgery.
Case Presentation
A 55-year-old man with schizophrenia on antipsychotics developed cough, shortness of breath, and dysphagia of 1-month of evolution. Because his symptoms worsened, his mother brought him to the emergency department. Peripheral oxygen saturation was 97% at room air. Lung auscultation was remarkable for bilateral scattered rhonchi and wheezes.
Laboratory results showed leukocytosis with neutrophilia and hypotonic hypovolemic hyponatremia.
The patient stated that he did not remember swallowing any objects, although his mother confirmed that he was not adherent with his antipsychotic medications, which could have predisposed him to aspiration secondary to possible psychotic episodes.
Piperacillin/tazobactam 4.5 g every 8 hours was started to cover anaerobic bacterial organisms causing abscess, and IV fluids were given for hypovolemia. Flexible bronchoscopy (rigid bronchoscopy is superior although not available at our institution) was planned to be performed in the operating room (OR) because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects.
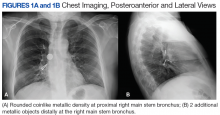
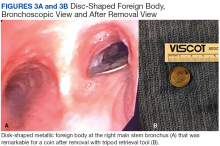
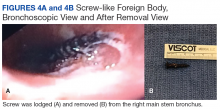
A bronchoscopy was performed, showing a disk-shaped metallic foreign body at the right main stem bronchus. After multiple attempts using the tripod retrieval tool, a coin was removed (Figures 3A and 3B).
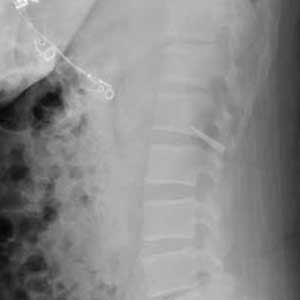
The patient was reintubated without any complications. A postprocedure chest radiograph showed the absence of foreign bodies and no pneumothorax. The patient completed IV antibiotic with piperacillin/tazobactam and supportive therapy with clinical improvement and successful extubation within 2 days. Cardiothoracic surgery was not required. Psychiatry service recommended to continue the same antipsychotic medications, administered only by his mother to assure adherence and to avoid similar future events. The patient was discharged home without any immediate complications despite having had a coin, nail, and screw aspiration (Figure 6).
Discussion
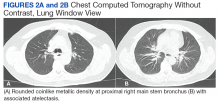
More than 50% of foreign bodies lodge at the right main stem bronchus due to the trachea’s anatomical position.2,4 In adults, foreign-body aspiration may present with nonspecific symptoms, such as cough and dyspnea.4 Other symptoms might include wheezes, chest discomfort, and sputum production. A chest radiograph is helpful as part of the initial diagnostic workup. A chest CT scan without contrast should be performed to confirm the diagnosis and to plan possible foreign-body retrieval.
Bronchoscopy is the gold standard for diagnosis and management of foreign-body aspiration.1 Rigid bronchoscopy is superior to flexible bronchoscopy in removal of large airway foreign bodies.1 The rigid bronchoscopy provides the ability to function as an endotracheal tube, thus allowing control of the airway and a conduit through which foreign bodies can be removed.1 Nonetheless, sometimes retrieval of foreign bodies deeper into the subsegmental bronchi cannot be achieved.1 Moreover, the required equipment or knowledgeable staff is not always available.1 Therefore, flexible bronchoscopy is an option to retrieve airway foreign bodies especially those located distal in the airway and for those medical centers without rigid bronchoscopy as is the case in our institution.
In our case, flexible bronchoscopy was performed in the OR because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects. Anesthesia Service assistance was requested anticipating need for patient sedation and intubation. We used the tripod and snare retrieval tools to remove 3 foreign objects located at the right main stem bronchus. Even though multiple attempts were made and endotracheal intubation was required, a successful retrieval with flexible bronchoscopy was performed. Moreover, cardiothoracic surgery was not required avoiding more invasive interventions with subsequent morbidity and mortality.
Conclusion
Flexible bronchoscopy is an important tool within the arsenal of the Pulmonology Service. The management of the underlying etiology also should be performed. In our case, the Psychiatry Service recommended that the patient’s medications should be administered by his mother to avoid similar events in the future. Flexible bronchoscopy can be a valuable option for foreign objects removal, especially those distally located in the lung segments as well as in those medical centers where rigid bronchoscopy is not available.
1. Mehta D, Mehta C, Bansal S, Singla S, Tangri N. Flexible bronchoscopic removal of a three piece foreign body from a child’s bronchus. Australas Med J. 2012;5(4):227-230.
2. Mercado JA, Rodríguez W. Occult aspiration of a chicken wishbone as a cause of hemoptysis. P R Health Sci J. 1999;18(1):71-73.
3. Robles-Arias CM, Campos-Santiago Z, Vega MT, Rosa-Cruz F, Rodríguez-Cintrón W. Aspiration of a dental tool during a crown placement procedure. Fed Pract. 2014;31(6):12-14.
4. Blanco-Ramos M, Botana-Rial M, García-Fontán E, Fernández-Villar A, Gallas-Torreira M. Update in the extraction of airway foreign bodies in adults. J Thorac Dis. 2016;8(11):3452-3456.
Consider flexible bronchoscopy as an option to retrieve aspirated foreign bodies in the airway.
Consider flexible bronchoscopy as an option to retrieve aspirated foreign bodies in the airway.
Airway foreign-body aspiration may cause no symptoms, although it can produce acute and life-threatening central airway obstruction.1 In the US, at least 2,700 people, including more than 300 children, die of foreign-body aspiration each year.2 Most foreign-body aspirations occur in children and elderly patients.3 In adults, dementia, drug intoxication, strokes, seizures, and neurologic disorders may predispose patients to aspiration.3 Some of the consequences of an aspirated object are complete or partial airway obstruction, respiratory distress and failure, pneumothorax, and hemorrhage.2 In addition, inadvertent aspiration of foreign objects in asymptomatic patients may not be evident for months, resulting in late complications as postobstructive pneumonia, bronchiectasis, or lung abscess.2
We present a case of a patient with documented schizophrenia with nonadherence to his antipsychotic medications who aspirated different objects. Flexible bronchoscopy was performed since rigid bronchoscopy is not available at our institution. Several bronchoscopy tools were required to successfully remove the objects and avoid further invasive interventions, such as cardiothoracic surgery.
Case Presentation
A 55-year-old man with schizophrenia on antipsychotics developed cough, shortness of breath, and dysphagia of 1-month of evolution. Because his symptoms worsened, his mother brought him to the emergency department. Peripheral oxygen saturation was 97% at room air. Lung auscultation was remarkable for bilateral scattered rhonchi and wheezes.
Laboratory results showed leukocytosis with neutrophilia and hypotonic hypovolemic hyponatremia.
The patient stated that he did not remember swallowing any objects, although his mother confirmed that he was not adherent with his antipsychotic medications, which could have predisposed him to aspiration secondary to possible psychotic episodes.
Piperacillin/tazobactam 4.5 g every 8 hours was started to cover anaerobic bacterial organisms causing abscess, and IV fluids were given for hypovolemia. Flexible bronchoscopy (rigid bronchoscopy is superior although not available at our institution) was planned to be performed in the operating room (OR) because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects.
A bronchoscopy was performed, showing a disk-shaped metallic foreign body at the right main stem bronchus. After multiple attempts using the tripod retrieval tool, a coin was removed (Figures 3A and 3B).
The patient was reintubated without any complications. A postprocedure chest radiograph showed the absence of foreign bodies and no pneumothorax. The patient completed IV antibiotic with piperacillin/tazobactam and supportive therapy with clinical improvement and successful extubation within 2 days. Cardiothoracic surgery was not required. Psychiatry service recommended to continue the same antipsychotic medications, administered only by his mother to assure adherence and to avoid similar future events. The patient was discharged home without any immediate complications despite having had a coin, nail, and screw aspiration (Figure 6).
Discussion
More than 50% of foreign bodies lodge at the right main stem bronchus due to the trachea’s anatomical position.2,4 In adults, foreign-body aspiration may present with nonspecific symptoms, such as cough and dyspnea.4 Other symptoms might include wheezes, chest discomfort, and sputum production. A chest radiograph is helpful as part of the initial diagnostic workup. A chest CT scan without contrast should be performed to confirm the diagnosis and to plan possible foreign-body retrieval.
Bronchoscopy is the gold standard for diagnosis and management of foreign-body aspiration.1 Rigid bronchoscopy is superior to flexible bronchoscopy in removal of large airway foreign bodies.1 The rigid bronchoscopy provides the ability to function as an endotracheal tube, thus allowing control of the airway and a conduit through which foreign bodies can be removed.1 Nonetheless, sometimes retrieval of foreign bodies deeper into the subsegmental bronchi cannot be achieved.1 Moreover, the required equipment or knowledgeable staff is not always available.1 Therefore, flexible bronchoscopy is an option to retrieve airway foreign bodies especially those located distal in the airway and for those medical centers without rigid bronchoscopy as is the case in our institution.
In our case, flexible bronchoscopy was performed in the OR because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects. Anesthesia Service assistance was requested anticipating need for patient sedation and intubation. We used the tripod and snare retrieval tools to remove 3 foreign objects located at the right main stem bronchus. Even though multiple attempts were made and endotracheal intubation was required, a successful retrieval with flexible bronchoscopy was performed. Moreover, cardiothoracic surgery was not required avoiding more invasive interventions with subsequent morbidity and mortality.
Conclusion
Flexible bronchoscopy is an important tool within the arsenal of the Pulmonology Service. The management of the underlying etiology also should be performed. In our case, the Psychiatry Service recommended that the patient’s medications should be administered by his mother to avoid similar events in the future. Flexible bronchoscopy can be a valuable option for foreign objects removal, especially those distally located in the lung segments as well as in those medical centers where rigid bronchoscopy is not available.
Airway foreign-body aspiration may cause no symptoms, although it can produce acute and life-threatening central airway obstruction.1 In the US, at least 2,700 people, including more than 300 children, die of foreign-body aspiration each year.2 Most foreign-body aspirations occur in children and elderly patients.3 In adults, dementia, drug intoxication, strokes, seizures, and neurologic disorders may predispose patients to aspiration.3 Some of the consequences of an aspirated object are complete or partial airway obstruction, respiratory distress and failure, pneumothorax, and hemorrhage.2 In addition, inadvertent aspiration of foreign objects in asymptomatic patients may not be evident for months, resulting in late complications as postobstructive pneumonia, bronchiectasis, or lung abscess.2
We present a case of a patient with documented schizophrenia with nonadherence to his antipsychotic medications who aspirated different objects. Flexible bronchoscopy was performed since rigid bronchoscopy is not available at our institution. Several bronchoscopy tools were required to successfully remove the objects and avoid further invasive interventions, such as cardiothoracic surgery.
Case Presentation
A 55-year-old man with schizophrenia on antipsychotics developed cough, shortness of breath, and dysphagia of 1-month of evolution. Because his symptoms worsened, his mother brought him to the emergency department. Peripheral oxygen saturation was 97% at room air. Lung auscultation was remarkable for bilateral scattered rhonchi and wheezes.
Laboratory results showed leukocytosis with neutrophilia and hypotonic hypovolemic hyponatremia.
The patient stated that he did not remember swallowing any objects, although his mother confirmed that he was not adherent with his antipsychotic medications, which could have predisposed him to aspiration secondary to possible psychotic episodes.
Piperacillin/tazobactam 4.5 g every 8 hours was started to cover anaerobic bacterial organisms causing abscess, and IV fluids were given for hypovolemia. Flexible bronchoscopy (rigid bronchoscopy is superior although not available at our institution) was planned to be performed in the operating room (OR) because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects.
A bronchoscopy was performed, showing a disk-shaped metallic foreign body at the right main stem bronchus. After multiple attempts using the tripod retrieval tool, a coin was removed (Figures 3A and 3B).
The patient was reintubated without any complications. A postprocedure chest radiograph showed the absence of foreign bodies and no pneumothorax. The patient completed IV antibiotic with piperacillin/tazobactam and supportive therapy with clinical improvement and successful extubation within 2 days. Cardiothoracic surgery was not required. Psychiatry service recommended to continue the same antipsychotic medications, administered only by his mother to assure adherence and to avoid similar future events. The patient was discharged home without any immediate complications despite having had a coin, nail, and screw aspiration (Figure 6).
Discussion
More than 50% of foreign bodies lodge at the right main stem bronchus due to the trachea’s anatomical position.2,4 In adults, foreign-body aspiration may present with nonspecific symptoms, such as cough and dyspnea.4 Other symptoms might include wheezes, chest discomfort, and sputum production. A chest radiograph is helpful as part of the initial diagnostic workup. A chest CT scan without contrast should be performed to confirm the diagnosis and to plan possible foreign-body retrieval.
Bronchoscopy is the gold standard for diagnosis and management of foreign-body aspiration.1 Rigid bronchoscopy is superior to flexible bronchoscopy in removal of large airway foreign bodies.1 The rigid bronchoscopy provides the ability to function as an endotracheal tube, thus allowing control of the airway and a conduit through which foreign bodies can be removed.1 Nonetheless, sometimes retrieval of foreign bodies deeper into the subsegmental bronchi cannot be achieved.1 Moreover, the required equipment or knowledgeable staff is not always available.1 Therefore, flexible bronchoscopy is an option to retrieve airway foreign bodies especially those located distal in the airway and for those medical centers without rigid bronchoscopy as is the case in our institution.
In our case, flexible bronchoscopy was performed in the OR because we predicted a difficult and prolonged retrieval in view of multiple and different-sized objects. Anesthesia Service assistance was requested anticipating need for patient sedation and intubation. We used the tripod and snare retrieval tools to remove 3 foreign objects located at the right main stem bronchus. Even though multiple attempts were made and endotracheal intubation was required, a successful retrieval with flexible bronchoscopy was performed. Moreover, cardiothoracic surgery was not required avoiding more invasive interventions with subsequent morbidity and mortality.
Conclusion
Flexible bronchoscopy is an important tool within the arsenal of the Pulmonology Service. The management of the underlying etiology also should be performed. In our case, the Psychiatry Service recommended that the patient’s medications should be administered by his mother to avoid similar events in the future. Flexible bronchoscopy can be a valuable option for foreign objects removal, especially those distally located in the lung segments as well as in those medical centers where rigid bronchoscopy is not available.
1. Mehta D, Mehta C, Bansal S, Singla S, Tangri N. Flexible bronchoscopic removal of a three piece foreign body from a child’s bronchus. Australas Med J. 2012;5(4):227-230.
2. Mercado JA, Rodríguez W. Occult aspiration of a chicken wishbone as a cause of hemoptysis. P R Health Sci J. 1999;18(1):71-73.
3. Robles-Arias CM, Campos-Santiago Z, Vega MT, Rosa-Cruz F, Rodríguez-Cintrón W. Aspiration of a dental tool during a crown placement procedure. Fed Pract. 2014;31(6):12-14.
4. Blanco-Ramos M, Botana-Rial M, García-Fontán E, Fernández-Villar A, Gallas-Torreira M. Update in the extraction of airway foreign bodies in adults. J Thorac Dis. 2016;8(11):3452-3456.
1. Mehta D, Mehta C, Bansal S, Singla S, Tangri N. Flexible bronchoscopic removal of a three piece foreign body from a child’s bronchus. Australas Med J. 2012;5(4):227-230.
2. Mercado JA, Rodríguez W. Occult aspiration of a chicken wishbone as a cause of hemoptysis. P R Health Sci J. 1999;18(1):71-73.
3. Robles-Arias CM, Campos-Santiago Z, Vega MT, Rosa-Cruz F, Rodríguez-Cintrón W. Aspiration of a dental tool during a crown placement procedure. Fed Pract. 2014;31(6):12-14.
4. Blanco-Ramos M, Botana-Rial M, García-Fontán E, Fernández-Villar A, Gallas-Torreira M. Update in the extraction of airway foreign bodies in adults. J Thorac Dis. 2016;8(11):3452-3456.
What’s The Impact of Occult HBV in Chronic HCV?
The reported prevalence of occult hepatitis B infection (OBI) varies widely: from < 1% to as high as 89.5% in HIV patients. Among patients with chronic hepatitis, the prevalence—again—ranges widely, from 0% to 52% but is highest in patients with chronic hepatitis C (CHC).
The clinical impact of OBI on patients with CHC has been extensively investigated, say researchers from the Institute of Liver and Biliary Sciences in New Delhi, India, but the available data are conflicting. In fact, when they conducted their study to assess the prevalence of OBI and evaluate its impact on clinical outcomes and response to antiviral therapy in CHC, the findings were “largely inconclusive.”
The study included 80 patients, of whom 32 (40%) had seropositive OBI. Hepatitis C virus genotype information was available for 59 patients, revealing that genotype 3 was most common.
However, analysis of clinical, biochemical, histopathologic and treatment response based on seropositivity and semiquantitative estimate of anti-HBc did not yield statistically significant results. Plasma samples of 14 were reactive for anti-HBc, 12 for anti-HBs, and 6 for both antibodies. Hepatitis B virus DNA (34 IU/mL) was detected in the plasma sample of only 1 patient by quantitative polymerase chain reaction. Therefore, the researchers say, the prevalence of OBI was 1.25%.
Anti-HBc total antibody levels did not influence clinical outcomes and response to directly acting antiviral therapy. Nor did genotype make a significant difference: 90.7% of genotype 3 patients and 92.8% of genotype 1 patients attained sustained virologic response.
More prospective studies should be conducted, the researchers urge, to further explore “this seemingly enigmatic issue.”
Source:
Bhatia M, Gupta E, Choudhary MC, Jindal A, Sarin SK. J Lab Physicians. 2018;10(3):304-308.
doi: 10.4103/JLP.JLP_12_18.
The reported prevalence of occult hepatitis B infection (OBI) varies widely: from < 1% to as high as 89.5% in HIV patients. Among patients with chronic hepatitis, the prevalence—again—ranges widely, from 0% to 52% but is highest in patients with chronic hepatitis C (CHC).
The clinical impact of OBI on patients with CHC has been extensively investigated, say researchers from the Institute of Liver and Biliary Sciences in New Delhi, India, but the available data are conflicting. In fact, when they conducted their study to assess the prevalence of OBI and evaluate its impact on clinical outcomes and response to antiviral therapy in CHC, the findings were “largely inconclusive.”
The study included 80 patients, of whom 32 (40%) had seropositive OBI. Hepatitis C virus genotype information was available for 59 patients, revealing that genotype 3 was most common.
However, analysis of clinical, biochemical, histopathologic and treatment response based on seropositivity and semiquantitative estimate of anti-HBc did not yield statistically significant results. Plasma samples of 14 were reactive for anti-HBc, 12 for anti-HBs, and 6 for both antibodies. Hepatitis B virus DNA (34 IU/mL) was detected in the plasma sample of only 1 patient by quantitative polymerase chain reaction. Therefore, the researchers say, the prevalence of OBI was 1.25%.
Anti-HBc total antibody levels did not influence clinical outcomes and response to directly acting antiviral therapy. Nor did genotype make a significant difference: 90.7% of genotype 3 patients and 92.8% of genotype 1 patients attained sustained virologic response.
More prospective studies should be conducted, the researchers urge, to further explore “this seemingly enigmatic issue.”
Source:
Bhatia M, Gupta E, Choudhary MC, Jindal A, Sarin SK. J Lab Physicians. 2018;10(3):304-308.
doi: 10.4103/JLP.JLP_12_18.
The reported prevalence of occult hepatitis B infection (OBI) varies widely: from < 1% to as high as 89.5% in HIV patients. Among patients with chronic hepatitis, the prevalence—again—ranges widely, from 0% to 52% but is highest in patients with chronic hepatitis C (CHC).
The clinical impact of OBI on patients with CHC has been extensively investigated, say researchers from the Institute of Liver and Biliary Sciences in New Delhi, India, but the available data are conflicting. In fact, when they conducted their study to assess the prevalence of OBI and evaluate its impact on clinical outcomes and response to antiviral therapy in CHC, the findings were “largely inconclusive.”
The study included 80 patients, of whom 32 (40%) had seropositive OBI. Hepatitis C virus genotype information was available for 59 patients, revealing that genotype 3 was most common.
However, analysis of clinical, biochemical, histopathologic and treatment response based on seropositivity and semiquantitative estimate of anti-HBc did not yield statistically significant results. Plasma samples of 14 were reactive for anti-HBc, 12 for anti-HBs, and 6 for both antibodies. Hepatitis B virus DNA (34 IU/mL) was detected in the plasma sample of only 1 patient by quantitative polymerase chain reaction. Therefore, the researchers say, the prevalence of OBI was 1.25%.
Anti-HBc total antibody levels did not influence clinical outcomes and response to directly acting antiviral therapy. Nor did genotype make a significant difference: 90.7% of genotype 3 patients and 92.8% of genotype 1 patients attained sustained virologic response.
More prospective studies should be conducted, the researchers urge, to further explore “this seemingly enigmatic issue.”
Source:
Bhatia M, Gupta E, Choudhary MC, Jindal A, Sarin SK. J Lab Physicians. 2018;10(3):304-308.
doi: 10.4103/JLP.JLP_12_18.
Team identifies potential immunotherapy target for AML
New research could aid the development of immunotherapies tailored to patients with acute myeloid leukemia (AML) who are undergoing stem cell transplant (SCT).
Researchers found they could use genetic sequencing and computer software to identify minor histocompatibility antigens (mHAs) known to occur in AML.
The team used this method to predict novel graft-versus-leukemia (GVL) mHAs and demonstrated that one of these mHAs could be a “potentially useful” therapeutic target.
Ben Vincent, MD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, and his colleagues reported these findings in Blood Advances.
In their retrospective study, the researchers tested whether their software could predict antigenic targets in 101 SCT donor-recipient pairs.
The researchers found they could correctly identify 14 of 18 mHAs known to occur in AML, but they were also able to predict 102 new GVL mHAs.
The researchers then confirmed one of these GVL mHAs, called UNC-GRK4-V, as a potential target for immunotherapy. The team observed immune responses to UNC-GRK4-V in four of nine AML patients who had undergone SCT.
Looking ahead, the researchers want to optimize their software to predict the most common AML-associated mHAs present in the U.S. population and confirm these predicted antigens as valid immunotherapy targets.
The team believes they could potentially use their predictions to engineer donor immune cells to specifically target the cancer cell antigens while preventing graft-versus-host disease.
“We’ve developed a software package that predicts leukemia-specific immune targets in any leukemia patient undergoing a stem cell transplant based on DNA and RNA sequencing and demonstrated that these data can lead to actual targets expressed on leukemia cells,” Dr. Vincent said.
“The next step of our work is to use that information for patient-specific therapies to try to improve cure rates without making graft-versus-host disease worse.”
The current research was supported by a National Cancer Institute grant, an ASCO Young Investigator Award, the North Carolina University Cancer Research Fund, and the Scott Neil Schwirck Fellowship.
New research could aid the development of immunotherapies tailored to patients with acute myeloid leukemia (AML) who are undergoing stem cell transplant (SCT).
Researchers found they could use genetic sequencing and computer software to identify minor histocompatibility antigens (mHAs) known to occur in AML.
The team used this method to predict novel graft-versus-leukemia (GVL) mHAs and demonstrated that one of these mHAs could be a “potentially useful” therapeutic target.
Ben Vincent, MD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, and his colleagues reported these findings in Blood Advances.
In their retrospective study, the researchers tested whether their software could predict antigenic targets in 101 SCT donor-recipient pairs.
The researchers found they could correctly identify 14 of 18 mHAs known to occur in AML, but they were also able to predict 102 new GVL mHAs.
The researchers then confirmed one of these GVL mHAs, called UNC-GRK4-V, as a potential target for immunotherapy. The team observed immune responses to UNC-GRK4-V in four of nine AML patients who had undergone SCT.
Looking ahead, the researchers want to optimize their software to predict the most common AML-associated mHAs present in the U.S. population and confirm these predicted antigens as valid immunotherapy targets.
The team believes they could potentially use their predictions to engineer donor immune cells to specifically target the cancer cell antigens while preventing graft-versus-host disease.
“We’ve developed a software package that predicts leukemia-specific immune targets in any leukemia patient undergoing a stem cell transplant based on DNA and RNA sequencing and demonstrated that these data can lead to actual targets expressed on leukemia cells,” Dr. Vincent said.
“The next step of our work is to use that information for patient-specific therapies to try to improve cure rates without making graft-versus-host disease worse.”
The current research was supported by a National Cancer Institute grant, an ASCO Young Investigator Award, the North Carolina University Cancer Research Fund, and the Scott Neil Schwirck Fellowship.
New research could aid the development of immunotherapies tailored to patients with acute myeloid leukemia (AML) who are undergoing stem cell transplant (SCT).
Researchers found they could use genetic sequencing and computer software to identify minor histocompatibility antigens (mHAs) known to occur in AML.
The team used this method to predict novel graft-versus-leukemia (GVL) mHAs and demonstrated that one of these mHAs could be a “potentially useful” therapeutic target.
Ben Vincent, MD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, and his colleagues reported these findings in Blood Advances.
In their retrospective study, the researchers tested whether their software could predict antigenic targets in 101 SCT donor-recipient pairs.
The researchers found they could correctly identify 14 of 18 mHAs known to occur in AML, but they were also able to predict 102 new GVL mHAs.
The researchers then confirmed one of these GVL mHAs, called UNC-GRK4-V, as a potential target for immunotherapy. The team observed immune responses to UNC-GRK4-V in four of nine AML patients who had undergone SCT.
Looking ahead, the researchers want to optimize their software to predict the most common AML-associated mHAs present in the U.S. population and confirm these predicted antigens as valid immunotherapy targets.
The team believes they could potentially use their predictions to engineer donor immune cells to specifically target the cancer cell antigens while preventing graft-versus-host disease.
“We’ve developed a software package that predicts leukemia-specific immune targets in any leukemia patient undergoing a stem cell transplant based on DNA and RNA sequencing and demonstrated that these data can lead to actual targets expressed on leukemia cells,” Dr. Vincent said.
“The next step of our work is to use that information for patient-specific therapies to try to improve cure rates without making graft-versus-host disease worse.”
The current research was supported by a National Cancer Institute grant, an ASCO Young Investigator Award, the North Carolina University Cancer Research Fund, and the Scott Neil Schwirck Fellowship.
Taking a Stab in the Dark
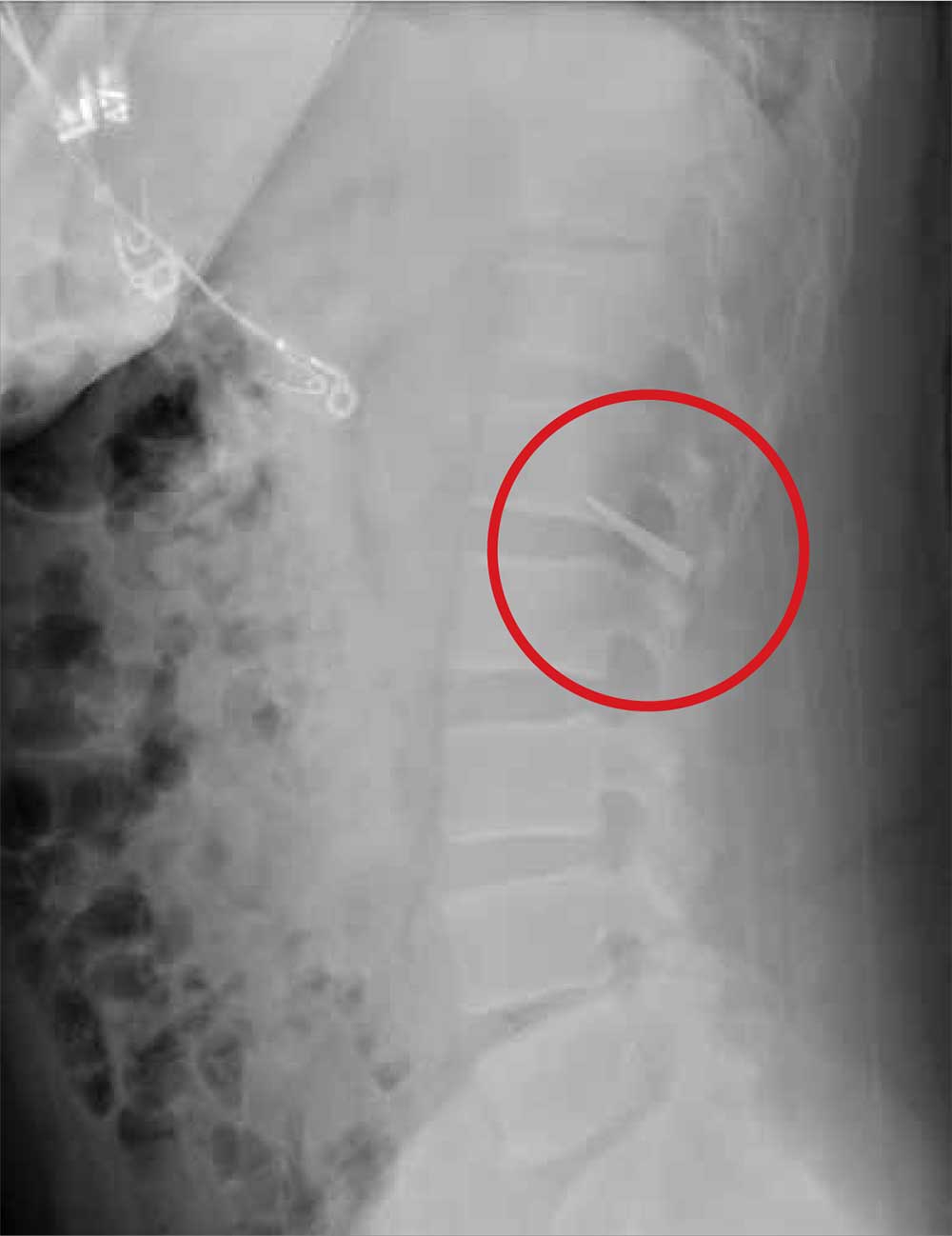
ANSWER
The radiograph shows an obvious metallic foreign body that appears to be lodged within the first and second lumbar disc space. This is likely the tip of the knife, which presumably broke off when the patient was stabbed.
The patient was promptly transferred to a trauma center with neurosurgery coverage. Subsequent CT showed that the blade had penetrated the spinal canal, but remarkably, the patient remained neurologically intact. He underwent successful removal without any neurologic compromise.
This case highlights several points for clinicians: First, provider-to-provider sign-out of patients should be complete and detailed. Second, obtaining a thorough history is essential. And third, you should maintain a low threshold for obtaining radiographs of wounds, to rule out a foreign body.

ANSWER
The radiograph shows an obvious metallic foreign body that appears to be lodged within the first and second lumbar disc space. This is likely the tip of the knife, which presumably broke off when the patient was stabbed.
The patient was promptly transferred to a trauma center with neurosurgery coverage. Subsequent CT showed that the blade had penetrated the spinal canal, but remarkably, the patient remained neurologically intact. He underwent successful removal without any neurologic compromise.
This case highlights several points for clinicians: First, provider-to-provider sign-out of patients should be complete and detailed. Second, obtaining a thorough history is essential. And third, you should maintain a low threshold for obtaining radiographs of wounds, to rule out a foreign body.

ANSWER
The radiograph shows an obvious metallic foreign body that appears to be lodged within the first and second lumbar disc space. This is likely the tip of the knife, which presumably broke off when the patient was stabbed.
The patient was promptly transferred to a trauma center with neurosurgery coverage. Subsequent CT showed that the blade had penetrated the spinal canal, but remarkably, the patient remained neurologically intact. He underwent successful removal without any neurologic compromise.
This case highlights several points for clinicians: First, provider-to-provider sign-out of patients should be complete and detailed. Second, obtaining a thorough history is essential. And third, you should maintain a low threshold for obtaining radiographs of wounds, to rule out a foreign body.
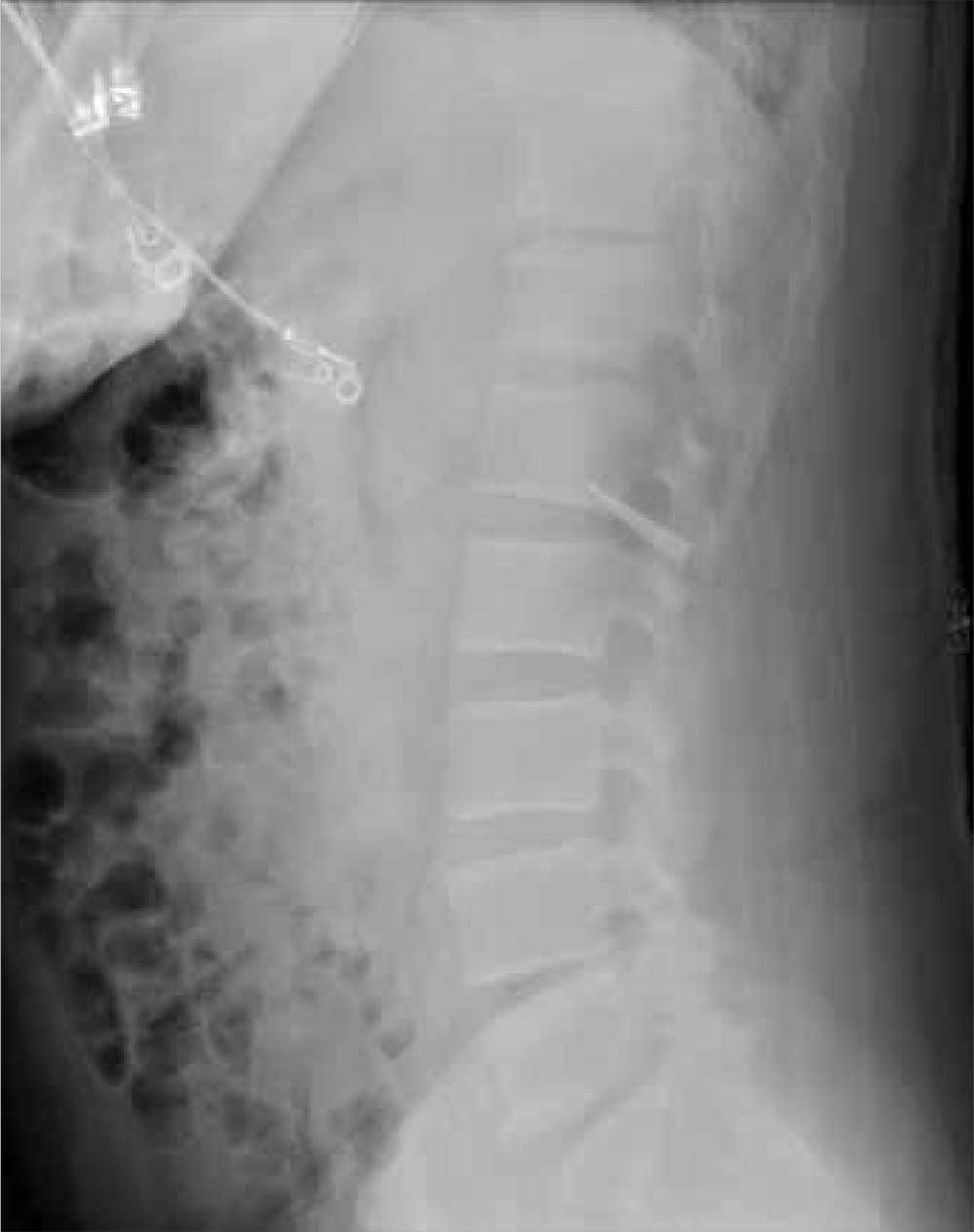
As you arrive for your shift in the emergency department, the outgoing provider asks if you would mind checking a laceration that his student is stapling. “The discharge paperwork is all done,” he says, as he waves goodbye and walks out the door.
You find that the student has just about completed his task: sterilely stapling a 2.5-cm laceration on the left lumbar area of a man in his early 40s.
You ask the student for the basic history, and he informs you that the patient was drinking with friends and “accidentally got cut” when they started roughhousing. You turn your attention to the patient, who appears intoxicated but in no obvious distress; he confirms the story as presented.
A quick review of the chart shows no significant medial history, stable vital signs, and up-to-date tetanus status. The patient can move all extremities well and appears neurovascularly intact. But some instinct prompts you to probe further.
On additional questioning, the patient reveals that he was accidentally stabbed. When you inquire about the object he was stabbed with, he describes it as a knife, “pretty long, sort of like a dagger.”
With this information, you decide to order some laboratory studies and abdominal radiographs (lateral view shown). What is your impression?
Expert provides antibiotic stewardship tips for dermatologists
LAKE TAHOE, CALIF. – Dermatologists prescribe more antibiotics than any other physician group, a statistic that George G. Zhanel, PhD, would like to see go by the wayside.
After all, the World Health Organization projects that the number of annual deaths in North America attributable to antibiotic resistance will reach 317,000 by the year 2050.
Dr. Zhanel, a microbiologist at the College of Medicine, University of Manitoba, Winnipeg, Canada, said at the annual meeting of the Society for Pediatric Dermatology. “Many of us are very concerned about this. Countries have put together an optimal action plan. What are we going to do about this? The plans are quite similar from country to country. They talk about surveillance, finding where these pathogens are. They talk about infection control such as washing your hands in the clinic so you’re not moving antibiotic-resistant organisms around. They talk about diagnostic and treatment guidelines, new antibiotic therapies, probiotics, and vaccination strategies. My own group is doing research on all of these areas, but today I’m going to focus on antibiotic stewardship: Using antibiotics wisely, trying to optimize efficacy while trying to minimize the development of resistant organisms.”
Dr. Zhanel, who is also director of the Canadian Antimicrobial Resistance Alliance (CARA) at the College of Medicine, University of Manitoba, described dermatologists as “big players” when it comes to antibiotic use. According to a 2016 report from the Scientific Panel on Antibiotic Use in Dermatology, dermatologists order 8.2 million oral antibiotic prescriptions each year, which is more common than any other physician group based on the prescribing rate per clinician (J Clin Aesthet Dermatol. 2016;9[4]:18-24). In addition, the prescribed duration of antibiotic therapy is often markedly longer with therapies treated by dermatologists, especially acne and rosacea. One study of general practitioners in the United Kingdom found that the mean duration of oral antibiotic use for treating acne was 175 days (J Am Acad Dermatol 2016;75:1142-50). “For some patients it went on much longer,” said Dr. Zhanel, who was not affiliated with the study.
“You are important players when it comes to antibiotics. How you use them and if you use them wisely impacts not only your patients, but the world.”
The correlation between antibiotic use and resistance is widely established, he continued. “We have known for 30 to 40 years that if you treat patients with tetracyclines, the Staphylococcus epidermidis that we all have on our skin develop tetracycline resistance,” he said. “The tetracycline resistance genes from S. epidermidis can then transfer to putative pathogens such as Staphylococcus aureus, and potentially [methicillin-resistant S. aureus]. That’s why we need to try to minimize oral tetracycline exposure on the normal microbiome.” In addition, tetracycline use can help create multidrug resistant organisms.
Next, Dr. Zhanel discussed potential solutions to antimicrobial usage/resistance in dermatology. According to recent guidelines on the care for the management of acne vulgaris, systemic antibiotic use should be limited to the shortest possible duration, typically 90 days (J Am Acad Dermatol. 2016;74[5]:945-73). A common treatment for moderate to-severe acne is to combine a topical retinoid with an oral or topical antimicrobial (J Am Acad Dermatol. 2009;60(5 suppl):S1-S50). If the addition of an oral antibiotic is required, limit its use to 3 or 4 months and co-prescribe with a product that contains benzoyl peroxide (BPO), or use as a washout. “Ideally, that’s your exit strategy,” he said. “Once you finish the oral antibiotic, in about 3 months if possible, continue with the topical retinoids plus BPO to maintain that particular remission.”
Why add benzoyl peroxide to topical retinoids for maintenance therapy? “Benzoyl peroxide and topical retinoids affect multiple targets in your acne strategy, and when you use them together they are powerful,” Dr. Zhanel said. He advises dermatologists not to prescribe oral or topical clindamycin unless they have to, because that drug is one of the main drivers of Clostridium difficile infection.
Dr. Zhanel’s stewardship tips for topical antibiotics involve not using topical tetracyclines/clindamycin/macrolides, in favor of using a topical antimicrobial such as BPO. “We think that benzoyl peroxide is less likely to drive resistance than are the traditional topical antibiotics like tetracyclines and clindamycin,” he said. “Use topical retinoids and benzoyl peroxide, if possible.”
Subtherapeutic oral doses of tetracyclines such as doxycycline 40 mg modified release “look very powerful for treating rosacea and do not affect the normal microbiome or select for resistance,” he said. In the meantime, Dr. Zhanel and other researchers are working to develop narrow spectrum tetracyclines with less impact on the GI flora, such as sarecycline. “So there is the potential for more eco-friendly tetracyclines,” he said.
Going forward, many questions remain about optimal antibiotic stewardship in dermatology, Dr. Zhanel said. For example, if you combine a topical antibiotic with benzoyl peroxide, are you less likely to get resistance to that topical antibiotic? “I think the answer is yes, but the literature isn’t very strong on that,” he said. “Also, is benzoyl peroxide plus a topical retinoid better than benzoyl peroxide plus a topical antibiotic in terms of resistance? I think the answer is yes, but again there is very little data on this.”
Dr. Zhanel disclosed having numerous financial ties to the pharmaceutical industry.
LAKE TAHOE, CALIF. – Dermatologists prescribe more antibiotics than any other physician group, a statistic that George G. Zhanel, PhD, would like to see go by the wayside.
After all, the World Health Organization projects that the number of annual deaths in North America attributable to antibiotic resistance will reach 317,000 by the year 2050.
Dr. Zhanel, a microbiologist at the College of Medicine, University of Manitoba, Winnipeg, Canada, said at the annual meeting of the Society for Pediatric Dermatology. “Many of us are very concerned about this. Countries have put together an optimal action plan. What are we going to do about this? The plans are quite similar from country to country. They talk about surveillance, finding where these pathogens are. They talk about infection control such as washing your hands in the clinic so you’re not moving antibiotic-resistant organisms around. They talk about diagnostic and treatment guidelines, new antibiotic therapies, probiotics, and vaccination strategies. My own group is doing research on all of these areas, but today I’m going to focus on antibiotic stewardship: Using antibiotics wisely, trying to optimize efficacy while trying to minimize the development of resistant organisms.”
Dr. Zhanel, who is also director of the Canadian Antimicrobial Resistance Alliance (CARA) at the College of Medicine, University of Manitoba, described dermatologists as “big players” when it comes to antibiotic use. According to a 2016 report from the Scientific Panel on Antibiotic Use in Dermatology, dermatologists order 8.2 million oral antibiotic prescriptions each year, which is more common than any other physician group based on the prescribing rate per clinician (J Clin Aesthet Dermatol. 2016;9[4]:18-24). In addition, the prescribed duration of antibiotic therapy is often markedly longer with therapies treated by dermatologists, especially acne and rosacea. One study of general practitioners in the United Kingdom found that the mean duration of oral antibiotic use for treating acne was 175 days (J Am Acad Dermatol 2016;75:1142-50). “For some patients it went on much longer,” said Dr. Zhanel, who was not affiliated with the study.
“You are important players when it comes to antibiotics. How you use them and if you use them wisely impacts not only your patients, but the world.”
The correlation between antibiotic use and resistance is widely established, he continued. “We have known for 30 to 40 years that if you treat patients with tetracyclines, the Staphylococcus epidermidis that we all have on our skin develop tetracycline resistance,” he said. “The tetracycline resistance genes from S. epidermidis can then transfer to putative pathogens such as Staphylococcus aureus, and potentially [methicillin-resistant S. aureus]. That’s why we need to try to minimize oral tetracycline exposure on the normal microbiome.” In addition, tetracycline use can help create multidrug resistant organisms.
Next, Dr. Zhanel discussed potential solutions to antimicrobial usage/resistance in dermatology. According to recent guidelines on the care for the management of acne vulgaris, systemic antibiotic use should be limited to the shortest possible duration, typically 90 days (J Am Acad Dermatol. 2016;74[5]:945-73). A common treatment for moderate to-severe acne is to combine a topical retinoid with an oral or topical antimicrobial (J Am Acad Dermatol. 2009;60(5 suppl):S1-S50). If the addition of an oral antibiotic is required, limit its use to 3 or 4 months and co-prescribe with a product that contains benzoyl peroxide (BPO), or use as a washout. “Ideally, that’s your exit strategy,” he said. “Once you finish the oral antibiotic, in about 3 months if possible, continue with the topical retinoids plus BPO to maintain that particular remission.”
Why add benzoyl peroxide to topical retinoids for maintenance therapy? “Benzoyl peroxide and topical retinoids affect multiple targets in your acne strategy, and when you use them together they are powerful,” Dr. Zhanel said. He advises dermatologists not to prescribe oral or topical clindamycin unless they have to, because that drug is one of the main drivers of Clostridium difficile infection.
Dr. Zhanel’s stewardship tips for topical antibiotics involve not using topical tetracyclines/clindamycin/macrolides, in favor of using a topical antimicrobial such as BPO. “We think that benzoyl peroxide is less likely to drive resistance than are the traditional topical antibiotics like tetracyclines and clindamycin,” he said. “Use topical retinoids and benzoyl peroxide, if possible.”
Subtherapeutic oral doses of tetracyclines such as doxycycline 40 mg modified release “look very powerful for treating rosacea and do not affect the normal microbiome or select for resistance,” he said. In the meantime, Dr. Zhanel and other researchers are working to develop narrow spectrum tetracyclines with less impact on the GI flora, such as sarecycline. “So there is the potential for more eco-friendly tetracyclines,” he said.
Going forward, many questions remain about optimal antibiotic stewardship in dermatology, Dr. Zhanel said. For example, if you combine a topical antibiotic with benzoyl peroxide, are you less likely to get resistance to that topical antibiotic? “I think the answer is yes, but the literature isn’t very strong on that,” he said. “Also, is benzoyl peroxide plus a topical retinoid better than benzoyl peroxide plus a topical antibiotic in terms of resistance? I think the answer is yes, but again there is very little data on this.”
Dr. Zhanel disclosed having numerous financial ties to the pharmaceutical industry.
LAKE TAHOE, CALIF. – Dermatologists prescribe more antibiotics than any other physician group, a statistic that George G. Zhanel, PhD, would like to see go by the wayside.
After all, the World Health Organization projects that the number of annual deaths in North America attributable to antibiotic resistance will reach 317,000 by the year 2050.
Dr. Zhanel, a microbiologist at the College of Medicine, University of Manitoba, Winnipeg, Canada, said at the annual meeting of the Society for Pediatric Dermatology. “Many of us are very concerned about this. Countries have put together an optimal action plan. What are we going to do about this? The plans are quite similar from country to country. They talk about surveillance, finding where these pathogens are. They talk about infection control such as washing your hands in the clinic so you’re not moving antibiotic-resistant organisms around. They talk about diagnostic and treatment guidelines, new antibiotic therapies, probiotics, and vaccination strategies. My own group is doing research on all of these areas, but today I’m going to focus on antibiotic stewardship: Using antibiotics wisely, trying to optimize efficacy while trying to minimize the development of resistant organisms.”
Dr. Zhanel, who is also director of the Canadian Antimicrobial Resistance Alliance (CARA) at the College of Medicine, University of Manitoba, described dermatologists as “big players” when it comes to antibiotic use. According to a 2016 report from the Scientific Panel on Antibiotic Use in Dermatology, dermatologists order 8.2 million oral antibiotic prescriptions each year, which is more common than any other physician group based on the prescribing rate per clinician (J Clin Aesthet Dermatol. 2016;9[4]:18-24). In addition, the prescribed duration of antibiotic therapy is often markedly longer with therapies treated by dermatologists, especially acne and rosacea. One study of general practitioners in the United Kingdom found that the mean duration of oral antibiotic use for treating acne was 175 days (J Am Acad Dermatol 2016;75:1142-50). “For some patients it went on much longer,” said Dr. Zhanel, who was not affiliated with the study.
“You are important players when it comes to antibiotics. How you use them and if you use them wisely impacts not only your patients, but the world.”
The correlation between antibiotic use and resistance is widely established, he continued. “We have known for 30 to 40 years that if you treat patients with tetracyclines, the Staphylococcus epidermidis that we all have on our skin develop tetracycline resistance,” he said. “The tetracycline resistance genes from S. epidermidis can then transfer to putative pathogens such as Staphylococcus aureus, and potentially [methicillin-resistant S. aureus]. That’s why we need to try to minimize oral tetracycline exposure on the normal microbiome.” In addition, tetracycline use can help create multidrug resistant organisms.
Next, Dr. Zhanel discussed potential solutions to antimicrobial usage/resistance in dermatology. According to recent guidelines on the care for the management of acne vulgaris, systemic antibiotic use should be limited to the shortest possible duration, typically 90 days (J Am Acad Dermatol. 2016;74[5]:945-73). A common treatment for moderate to-severe acne is to combine a topical retinoid with an oral or topical antimicrobial (J Am Acad Dermatol. 2009;60(5 suppl):S1-S50). If the addition of an oral antibiotic is required, limit its use to 3 or 4 months and co-prescribe with a product that contains benzoyl peroxide (BPO), or use as a washout. “Ideally, that’s your exit strategy,” he said. “Once you finish the oral antibiotic, in about 3 months if possible, continue with the topical retinoids plus BPO to maintain that particular remission.”
Why add benzoyl peroxide to topical retinoids for maintenance therapy? “Benzoyl peroxide and topical retinoids affect multiple targets in your acne strategy, and when you use them together they are powerful,” Dr. Zhanel said. He advises dermatologists not to prescribe oral or topical clindamycin unless they have to, because that drug is one of the main drivers of Clostridium difficile infection.
Dr. Zhanel’s stewardship tips for topical antibiotics involve not using topical tetracyclines/clindamycin/macrolides, in favor of using a topical antimicrobial such as BPO. “We think that benzoyl peroxide is less likely to drive resistance than are the traditional topical antibiotics like tetracyclines and clindamycin,” he said. “Use topical retinoids and benzoyl peroxide, if possible.”
Subtherapeutic oral doses of tetracyclines such as doxycycline 40 mg modified release “look very powerful for treating rosacea and do not affect the normal microbiome or select for resistance,” he said. In the meantime, Dr. Zhanel and other researchers are working to develop narrow spectrum tetracyclines with less impact on the GI flora, such as sarecycline. “So there is the potential for more eco-friendly tetracyclines,” he said.
Going forward, many questions remain about optimal antibiotic stewardship in dermatology, Dr. Zhanel said. For example, if you combine a topical antibiotic with benzoyl peroxide, are you less likely to get resistance to that topical antibiotic? “I think the answer is yes, but the literature isn’t very strong on that,” he said. “Also, is benzoyl peroxide plus a topical retinoid better than benzoyl peroxide plus a topical antibiotic in terms of resistance? I think the answer is yes, but again there is very little data on this.”
Dr. Zhanel disclosed having numerous financial ties to the pharmaceutical industry.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Certain skin conditions signal potential overgrowth disorder
LAKE TAHOE, CALIF. – and during human development, Leslie G. Biesecker, MD said at the annual meeting of the Society for Pediatric Dermatology.
Dr. Biesecker, senior investigator and head of the clinical genomics section of the National Human Genome Research Institute’s Medical Genomics and Metabolic Genetics Branch, discussed mosaicism and a number of overgrowth syndromes that he and his associates have been studying that have clinical relevance for pediatric dermatologists. He noted that mosaicism can affect any tissue, anywhere, in any pattern. “If an affected cell cannot survive gametogenesis, fertilization, or survive early development, this generates Happle-type mosaicism,” explained Dr. Biesecker, who is trained in pediatrics and in clinical and molecular genetics.
“This is characterized by patchy manifestations, and no parent-to-child transmission or recurrence. You must always be careful here, though, because Mother Nature does what she wants to. Mosaic mutations can happen more than once, but it’s a very unlikely outcome. Happle-type mosaicism is also characterized by discordant monozygotic twins,” he noted.
The prototype for Happle-type mosaicism is Proteus syndrome, formerly known as Elephant Man disease, which is caused by a mutation in the AKT1 gene. Patients with Proteus syndrome undergo severe, relentless overgrowth, and about 25% of them die during childhood. “If you see one of these patients, you have a serious clinical problem on your hands,” he said. “There is enormous individual variability, but it is ultra rare.”
Dermatologic lesions that are characteristic of Proteus syndrome include cerebriform connective tissue nevus, which typically presents on the hands and feet. “A wide range of vascular malformations have also been associated with this, even patients with arteriovenous malformations,” Dr. Biesecker said. “They are a serious problem.” Linear verrucous epidermal nevus is another characteristic lesion of Proteus syndrome. It can present in a number of ways and in various body sites. “The natural history of these lesions is important,” he commented. “Over time, are they stable, or do they spread and expand over time? These lesions do not ever spontaneously regress. This does enable molecular diagnosis, but don’t bother sampling their blood, because it will be negative. You have to have a biopsy sample.”
Overgrowth syndromes that do not meet criteria for Proteus syndrome fall into a category known as PIK3CA-related overgrowth spectrum, which Dr. Biesecker characterized as “a bunch of clinical designations all caused by the same underlying somatic mutation in a gene called PIK3CA. There is an enormous variability in these patients, ranging from those who have profound overgrowth, including malformations, truncal overgrowth, and vascular malformations, and digital overgrowth in all sorts of patterns. We designate this as PIK3CA-related overgrowth spectrum (PROS), because we can’t clinically separate these things from one another.”
These conditions include what used to be called CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal/spinal anomalies), facial infiltrating lipomatosis, and megalencephaly-capillary malformation syndrome. PROS is about 100 times more common than Proteus syndrome. “There are no rational boundaries to distinguish these entities,” Dr. Biesecker said. “They are rationalized under a combined clinical-molecular PROS framework, meaning that the molecular diagnosis is absolutely key to correctly diagnosing these patients.”
In this way, mosaicism challenges the traditional concept of diagnosing overgrowth disorders. “What we thought were separate disorders are in fact many manifestations of a single disorder,” he continued. “When I was doing my genetics training, we were taught that it would turn out that there was one gene for every disease, and one disease for every gene. That is completely wrong; it’s much more complicated than that. Mosaicism is also important for us as biologists, because it gives us a window into biology we otherwise would not see. Without a mosaicism, Proteus syndrome cannot exist biologically. So if I want to understand that gene product, I have to study patients who are mosaics. Mosaicism can happen in any tissue, whether it’s visible or not.”
Dr. Biesecker, who has been elected to serve as president of the American Society of Human Genetics for 2019, noted that most of the gene mutations that cause overgrowth disorders are the same ones implicated in cancer. “It makes sense, because cancer is a disorder of uncontrolled proliferation and differentiation,” he said. “These overgrowth disorders are similar but less severe manifestations of the same problem. It turns out that these mosaic patients are single gene model systems of cancer biology.” Therefore, when a drug company develops an anti-cancer drug, he continued, it also can be useful for PROS or Proteus syndrome. It’s much easier to inhibit a protein that’s overactive than it is to replace the activity of a gene that has lost its function.
But in PROS and Proteus, “we have very different treatment objectives than oncologists do,” he said. “Our goal is to reduce the signaling caused by these mutations; we do not want to kill the cells. Some of my patients with these disorders have pretty close to 50% of cells in their body carrying these mutations. If I were thinking like an oncologist, the oncologist wants to kill cancer cells; that’s their objective. If I were to kill all of the mutant cells in my patients, I’m certain that would kill them.”
One promising development is the investigational oral agent ARQ 092, which is an inhibitor of AKT1. Dr. Biesecker and his colleagues at the NIH have been working to figure out what dosing should be used in humans based on mouse data, lab data, and data from cancer patients. They started with about one-twelvth the dose that oncologists use. After treating the first patient with overgrowth syndrome, on day 15 that person’s AKT1 level dropped to about 20% of normal, while on day 75 it moved to around 60% of normal. “We are right in that zone where we want to drive the activity of that protein to about half of what it should be,” Dr. Biesecker said. He and his colleagues also have observed regression of lesions in a patient with cerebriform connective tissue nevus who was treated with ARQ 092. “We’ve never seen this before.”
Dr. Biesecker disclosed that he is a member of the Illumina medical ethics board. He has received royalties from Genentech and in-kind research support from ArQule and Pfizer.
[email protected]
LAKE TAHOE, CALIF. – and during human development, Leslie G. Biesecker, MD said at the annual meeting of the Society for Pediatric Dermatology.
Dr. Biesecker, senior investigator and head of the clinical genomics section of the National Human Genome Research Institute’s Medical Genomics and Metabolic Genetics Branch, discussed mosaicism and a number of overgrowth syndromes that he and his associates have been studying that have clinical relevance for pediatric dermatologists. He noted that mosaicism can affect any tissue, anywhere, in any pattern. “If an affected cell cannot survive gametogenesis, fertilization, or survive early development, this generates Happle-type mosaicism,” explained Dr. Biesecker, who is trained in pediatrics and in clinical and molecular genetics.
“This is characterized by patchy manifestations, and no parent-to-child transmission or recurrence. You must always be careful here, though, because Mother Nature does what she wants to. Mosaic mutations can happen more than once, but it’s a very unlikely outcome. Happle-type mosaicism is also characterized by discordant monozygotic twins,” he noted.
The prototype for Happle-type mosaicism is Proteus syndrome, formerly known as Elephant Man disease, which is caused by a mutation in the AKT1 gene. Patients with Proteus syndrome undergo severe, relentless overgrowth, and about 25% of them die during childhood. “If you see one of these patients, you have a serious clinical problem on your hands,” he said. “There is enormous individual variability, but it is ultra rare.”
Dermatologic lesions that are characteristic of Proteus syndrome include cerebriform connective tissue nevus, which typically presents on the hands and feet. “A wide range of vascular malformations have also been associated with this, even patients with arteriovenous malformations,” Dr. Biesecker said. “They are a serious problem.” Linear verrucous epidermal nevus is another characteristic lesion of Proteus syndrome. It can present in a number of ways and in various body sites. “The natural history of these lesions is important,” he commented. “Over time, are they stable, or do they spread and expand over time? These lesions do not ever spontaneously regress. This does enable molecular diagnosis, but don’t bother sampling their blood, because it will be negative. You have to have a biopsy sample.”
Overgrowth syndromes that do not meet criteria for Proteus syndrome fall into a category known as PIK3CA-related overgrowth spectrum, which Dr. Biesecker characterized as “a bunch of clinical designations all caused by the same underlying somatic mutation in a gene called PIK3CA. There is an enormous variability in these patients, ranging from those who have profound overgrowth, including malformations, truncal overgrowth, and vascular malformations, and digital overgrowth in all sorts of patterns. We designate this as PIK3CA-related overgrowth spectrum (PROS), because we can’t clinically separate these things from one another.”
These conditions include what used to be called CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal/spinal anomalies), facial infiltrating lipomatosis, and megalencephaly-capillary malformation syndrome. PROS is about 100 times more common than Proteus syndrome. “There are no rational boundaries to distinguish these entities,” Dr. Biesecker said. “They are rationalized under a combined clinical-molecular PROS framework, meaning that the molecular diagnosis is absolutely key to correctly diagnosing these patients.”
In this way, mosaicism challenges the traditional concept of diagnosing overgrowth disorders. “What we thought were separate disorders are in fact many manifestations of a single disorder,” he continued. “When I was doing my genetics training, we were taught that it would turn out that there was one gene for every disease, and one disease for every gene. That is completely wrong; it’s much more complicated than that. Mosaicism is also important for us as biologists, because it gives us a window into biology we otherwise would not see. Without a mosaicism, Proteus syndrome cannot exist biologically. So if I want to understand that gene product, I have to study patients who are mosaics. Mosaicism can happen in any tissue, whether it’s visible or not.”
Dr. Biesecker, who has been elected to serve as president of the American Society of Human Genetics for 2019, noted that most of the gene mutations that cause overgrowth disorders are the same ones implicated in cancer. “It makes sense, because cancer is a disorder of uncontrolled proliferation and differentiation,” he said. “These overgrowth disorders are similar but less severe manifestations of the same problem. It turns out that these mosaic patients are single gene model systems of cancer biology.” Therefore, when a drug company develops an anti-cancer drug, he continued, it also can be useful for PROS or Proteus syndrome. It’s much easier to inhibit a protein that’s overactive than it is to replace the activity of a gene that has lost its function.
But in PROS and Proteus, “we have very different treatment objectives than oncologists do,” he said. “Our goal is to reduce the signaling caused by these mutations; we do not want to kill the cells. Some of my patients with these disorders have pretty close to 50% of cells in their body carrying these mutations. If I were thinking like an oncologist, the oncologist wants to kill cancer cells; that’s their objective. If I were to kill all of the mutant cells in my patients, I’m certain that would kill them.”
One promising development is the investigational oral agent ARQ 092, which is an inhibitor of AKT1. Dr. Biesecker and his colleagues at the NIH have been working to figure out what dosing should be used in humans based on mouse data, lab data, and data from cancer patients. They started with about one-twelvth the dose that oncologists use. After treating the first patient with overgrowth syndrome, on day 15 that person’s AKT1 level dropped to about 20% of normal, while on day 75 it moved to around 60% of normal. “We are right in that zone where we want to drive the activity of that protein to about half of what it should be,” Dr. Biesecker said. He and his colleagues also have observed regression of lesions in a patient with cerebriform connective tissue nevus who was treated with ARQ 092. “We’ve never seen this before.”
Dr. Biesecker disclosed that he is a member of the Illumina medical ethics board. He has received royalties from Genentech and in-kind research support from ArQule and Pfizer.
[email protected]
LAKE TAHOE, CALIF. – and during human development, Leslie G. Biesecker, MD said at the annual meeting of the Society for Pediatric Dermatology.
Dr. Biesecker, senior investigator and head of the clinical genomics section of the National Human Genome Research Institute’s Medical Genomics and Metabolic Genetics Branch, discussed mosaicism and a number of overgrowth syndromes that he and his associates have been studying that have clinical relevance for pediatric dermatologists. He noted that mosaicism can affect any tissue, anywhere, in any pattern. “If an affected cell cannot survive gametogenesis, fertilization, or survive early development, this generates Happle-type mosaicism,” explained Dr. Biesecker, who is trained in pediatrics and in clinical and molecular genetics.
“This is characterized by patchy manifestations, and no parent-to-child transmission or recurrence. You must always be careful here, though, because Mother Nature does what she wants to. Mosaic mutations can happen more than once, but it’s a very unlikely outcome. Happle-type mosaicism is also characterized by discordant monozygotic twins,” he noted.
The prototype for Happle-type mosaicism is Proteus syndrome, formerly known as Elephant Man disease, which is caused by a mutation in the AKT1 gene. Patients with Proteus syndrome undergo severe, relentless overgrowth, and about 25% of them die during childhood. “If you see one of these patients, you have a serious clinical problem on your hands,” he said. “There is enormous individual variability, but it is ultra rare.”
Dermatologic lesions that are characteristic of Proteus syndrome include cerebriform connective tissue nevus, which typically presents on the hands and feet. “A wide range of vascular malformations have also been associated with this, even patients with arteriovenous malformations,” Dr. Biesecker said. “They are a serious problem.” Linear verrucous epidermal nevus is another characteristic lesion of Proteus syndrome. It can present in a number of ways and in various body sites. “The natural history of these lesions is important,” he commented. “Over time, are they stable, or do they spread and expand over time? These lesions do not ever spontaneously regress. This does enable molecular diagnosis, but don’t bother sampling their blood, because it will be negative. You have to have a biopsy sample.”
Overgrowth syndromes that do not meet criteria for Proteus syndrome fall into a category known as PIK3CA-related overgrowth spectrum, which Dr. Biesecker characterized as “a bunch of clinical designations all caused by the same underlying somatic mutation in a gene called PIK3CA. There is an enormous variability in these patients, ranging from those who have profound overgrowth, including malformations, truncal overgrowth, and vascular malformations, and digital overgrowth in all sorts of patterns. We designate this as PIK3CA-related overgrowth spectrum (PROS), because we can’t clinically separate these things from one another.”
These conditions include what used to be called CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal/spinal anomalies), facial infiltrating lipomatosis, and megalencephaly-capillary malformation syndrome. PROS is about 100 times more common than Proteus syndrome. “There are no rational boundaries to distinguish these entities,” Dr. Biesecker said. “They are rationalized under a combined clinical-molecular PROS framework, meaning that the molecular diagnosis is absolutely key to correctly diagnosing these patients.”
In this way, mosaicism challenges the traditional concept of diagnosing overgrowth disorders. “What we thought were separate disorders are in fact many manifestations of a single disorder,” he continued. “When I was doing my genetics training, we were taught that it would turn out that there was one gene for every disease, and one disease for every gene. That is completely wrong; it’s much more complicated than that. Mosaicism is also important for us as biologists, because it gives us a window into biology we otherwise would not see. Without a mosaicism, Proteus syndrome cannot exist biologically. So if I want to understand that gene product, I have to study patients who are mosaics. Mosaicism can happen in any tissue, whether it’s visible or not.”
Dr. Biesecker, who has been elected to serve as president of the American Society of Human Genetics for 2019, noted that most of the gene mutations that cause overgrowth disorders are the same ones implicated in cancer. “It makes sense, because cancer is a disorder of uncontrolled proliferation and differentiation,” he said. “These overgrowth disorders are similar but less severe manifestations of the same problem. It turns out that these mosaic patients are single gene model systems of cancer biology.” Therefore, when a drug company develops an anti-cancer drug, he continued, it also can be useful for PROS or Proteus syndrome. It’s much easier to inhibit a protein that’s overactive than it is to replace the activity of a gene that has lost its function.
But in PROS and Proteus, “we have very different treatment objectives than oncologists do,” he said. “Our goal is to reduce the signaling caused by these mutations; we do not want to kill the cells. Some of my patients with these disorders have pretty close to 50% of cells in their body carrying these mutations. If I were thinking like an oncologist, the oncologist wants to kill cancer cells; that’s their objective. If I were to kill all of the mutant cells in my patients, I’m certain that would kill them.”
One promising development is the investigational oral agent ARQ 092, which is an inhibitor of AKT1. Dr. Biesecker and his colleagues at the NIH have been working to figure out what dosing should be used in humans based on mouse data, lab data, and data from cancer patients. They started with about one-twelvth the dose that oncologists use. After treating the first patient with overgrowth syndrome, on day 15 that person’s AKT1 level dropped to about 20% of normal, while on day 75 it moved to around 60% of normal. “We are right in that zone where we want to drive the activity of that protein to about half of what it should be,” Dr. Biesecker said. He and his colleagues also have observed regression of lesions in a patient with cerebriform connective tissue nevus who was treated with ARQ 092. “We’ve never seen this before.”
Dr. Biesecker disclosed that he is a member of the Illumina medical ethics board. He has received royalties from Genentech and in-kind research support from ArQule and Pfizer.
[email protected]
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
JAK inhibitors emerge as promising alopecia treatment
LAKE TAHOE, CALIF. – After Brett King, MD, PhD, and his wife and collaborator, Brittany G. Craiglow, MD, published an index case of oral tofacitinib reversing alopecia universalis in a 25-year-old male patient back in 2014 (J Invest Dermatol. 2014;134:2988-90), they received hundreds of e-mails and phone calls from clinicians and patients.
“We also received quite a bit of media attention from around the world,” Dr. King recalled at the annual meeting of the Society for Pediatric Dermatology.
After all, alopecia areata and its variants affect 1%-2% of the population and have a marked impact on health-related quality of life, with high rates of concomitant generalized anxiety disorder and major depressive disorder. “The health-related quality of life is similar to that of atopic dermatitis and psoriasis, and there are no reliably effective therapies, especially for severe disease,” he said. “”
Currently available Janus kinase (JAK) inhibitors include tofacitinib (Xeljanz), ruxolitinib (Jakafi), and baricitinib (Olumiant). “These medicines are not [Food and Drug Administration] approved for alopecia areata, though tofacitinib was recently approved for psoriatic arthritis, and so we have formal entry of this medicine into dermatology for the first time,” said Dr. King, who is a dermatologist at Yale University, New Haven, Conn.
Potential adverse effects of JAKs include nasopharyngitis, headache, diarrhea, elevated cholesterol, uncommonly herpes zoster, cytopenias, transaminitis, and rarely non-melanoma skin cancer, solid organ malignancy and lymphoma, and GI perforation. Tofacitinib has an FDA black box warning regarding serious infections and malignancies, and baricitinib has these plus an additional warning about thrombosis.
In an open label, two-center trial that followed the index patient report, Dr. King and his associates enrolled 66 patients aged 19-35 years who had greater than 50% scalp hair loss for at least 6 months to receive tofacitinib 5 mg twice daily for 3 months (JCI Insight. 2016; 1[15]:e89776). A primary outcome of interest was regrowth of hair as measured by the percent change in Severity of Alopecia Tool (SALT) score. A SALT score of 100 indicates baldness, while a score of zero indicates no hair loss. Following 3 months of treatment, 32% of patients had a greater than 50% change in their SALT score, 32% had a change in the range of 5%-50%, while 36% had a change that was less than 5%.
“One of the interesting findings was that long duration of current episode of complete scalp hair loss was a negative predictor of treatment response, especially for those who have had hair loss greater than 10 years,” Dr. King said. Adverse events were “pretty bland,” with the most common being upper respiratory infection (17%), headache (8%), abdominal pain (8%), and acne (8%). Weight gain occurred in 1.5% of patients.
Next, Dr. King and colleagues reviewed the records of 90 patients aged 18 years or older who were treated with tofacitinib for at least 4 months (J Am Acad Dermatol. 2017;76[1]:22-8). Patients had greater than 40% scalp hair loss, and the tofacitinib dose was up to 10 mg per day at the discretion of the physician. “About 43% of patients were treated with tofacitinib 5 mg” twice daily, Dr. King said. “Other patients had higher doses or the addition of prednisone for three doses to see if that would help.”
After treatment, 20% of patients had a greater than 90% change in their SALT score (complete scalp hair regrowth), while 38.4% had a change that ranged from 51%-90%. At the same time, 18% had a change in their SALT score that ranged from 6%-50%, while 23% had a change that was 5% or less. As observed in the earlier trial, researchers saw a negative correlation between duration of current episode of hair loss and latest percent change in SALT score.
“We believe that you have to catch people before they get to more than 10 years of complete scalp hair loss,” Dr. King said. “This is important for the pediatric age group. I just saw somebody who’s 13, and they’ve been bald for 8 years. You might make the argument that this person deserves treatment, at least for a period of time long enough to regrow their hair in order to reset the clock.”
The most common adverse events were acne and weight gain.
In a separate analysis, Dr. King, Dr. Craiglow, and Lucy Y. Liu, evaluated the use of tofacitinib for at least 2 months in 13 alopecia areata patients aged 12-17 years (J Am Acad Dermatol. 2017;76[1]:29-32). They limited the analysis to those who had greater than 20% scalp hair loss, alopecia totalis, or alopecia universalis that was stable or worsening for 6 months or longer. Of the 13 patients, 9 (69%) were responders. Of the four non-responders, one had a very long duration of baldness. The percent change in SALT score was 93% overall, including 100% in the responder group over a median of 5 months and just 1% in the non-responder group over a median of 4 months. “This does not work every time,” Dr. King said.
While some preliminary studies of topical JAK inhibitors for alopecia areata show promise, it remains unclear if this approach will translate in a clinically meaningful way, he said. Clinical trials are currently under way.
Dr. King disclosed that he has received honoraria or consulting fees from Aclaris Therapeutics, Celgene, Concert Pharmaceuticals, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, and Dermavant Sciences. He has also received funding support from The Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research.
[email protected]
LAKE TAHOE, CALIF. – After Brett King, MD, PhD, and his wife and collaborator, Brittany G. Craiglow, MD, published an index case of oral tofacitinib reversing alopecia universalis in a 25-year-old male patient back in 2014 (J Invest Dermatol. 2014;134:2988-90), they received hundreds of e-mails and phone calls from clinicians and patients.
“We also received quite a bit of media attention from around the world,” Dr. King recalled at the annual meeting of the Society for Pediatric Dermatology.
After all, alopecia areata and its variants affect 1%-2% of the population and have a marked impact on health-related quality of life, with high rates of concomitant generalized anxiety disorder and major depressive disorder. “The health-related quality of life is similar to that of atopic dermatitis and psoriasis, and there are no reliably effective therapies, especially for severe disease,” he said. “”
Currently available Janus kinase (JAK) inhibitors include tofacitinib (Xeljanz), ruxolitinib (Jakafi), and baricitinib (Olumiant). “These medicines are not [Food and Drug Administration] approved for alopecia areata, though tofacitinib was recently approved for psoriatic arthritis, and so we have formal entry of this medicine into dermatology for the first time,” said Dr. King, who is a dermatologist at Yale University, New Haven, Conn.
Potential adverse effects of JAKs include nasopharyngitis, headache, diarrhea, elevated cholesterol, uncommonly herpes zoster, cytopenias, transaminitis, and rarely non-melanoma skin cancer, solid organ malignancy and lymphoma, and GI perforation. Tofacitinib has an FDA black box warning regarding serious infections and malignancies, and baricitinib has these plus an additional warning about thrombosis.
In an open label, two-center trial that followed the index patient report, Dr. King and his associates enrolled 66 patients aged 19-35 years who had greater than 50% scalp hair loss for at least 6 months to receive tofacitinib 5 mg twice daily for 3 months (JCI Insight. 2016; 1[15]:e89776). A primary outcome of interest was regrowth of hair as measured by the percent change in Severity of Alopecia Tool (SALT) score. A SALT score of 100 indicates baldness, while a score of zero indicates no hair loss. Following 3 months of treatment, 32% of patients had a greater than 50% change in their SALT score, 32% had a change in the range of 5%-50%, while 36% had a change that was less than 5%.
“One of the interesting findings was that long duration of current episode of complete scalp hair loss was a negative predictor of treatment response, especially for those who have had hair loss greater than 10 years,” Dr. King said. Adverse events were “pretty bland,” with the most common being upper respiratory infection (17%), headache (8%), abdominal pain (8%), and acne (8%). Weight gain occurred in 1.5% of patients.
Next, Dr. King and colleagues reviewed the records of 90 patients aged 18 years or older who were treated with tofacitinib for at least 4 months (J Am Acad Dermatol. 2017;76[1]:22-8). Patients had greater than 40% scalp hair loss, and the tofacitinib dose was up to 10 mg per day at the discretion of the physician. “About 43% of patients were treated with tofacitinib 5 mg” twice daily, Dr. King said. “Other patients had higher doses or the addition of prednisone for three doses to see if that would help.”
After treatment, 20% of patients had a greater than 90% change in their SALT score (complete scalp hair regrowth), while 38.4% had a change that ranged from 51%-90%. At the same time, 18% had a change in their SALT score that ranged from 6%-50%, while 23% had a change that was 5% or less. As observed in the earlier trial, researchers saw a negative correlation between duration of current episode of hair loss and latest percent change in SALT score.
“We believe that you have to catch people before they get to more than 10 years of complete scalp hair loss,” Dr. King said. “This is important for the pediatric age group. I just saw somebody who’s 13, and they’ve been bald for 8 years. You might make the argument that this person deserves treatment, at least for a period of time long enough to regrow their hair in order to reset the clock.”
The most common adverse events were acne and weight gain.
In a separate analysis, Dr. King, Dr. Craiglow, and Lucy Y. Liu, evaluated the use of tofacitinib for at least 2 months in 13 alopecia areata patients aged 12-17 years (J Am Acad Dermatol. 2017;76[1]:29-32). They limited the analysis to those who had greater than 20% scalp hair loss, alopecia totalis, or alopecia universalis that was stable or worsening for 6 months or longer. Of the 13 patients, 9 (69%) were responders. Of the four non-responders, one had a very long duration of baldness. The percent change in SALT score was 93% overall, including 100% in the responder group over a median of 5 months and just 1% in the non-responder group over a median of 4 months. “This does not work every time,” Dr. King said.
While some preliminary studies of topical JAK inhibitors for alopecia areata show promise, it remains unclear if this approach will translate in a clinically meaningful way, he said. Clinical trials are currently under way.
Dr. King disclosed that he has received honoraria or consulting fees from Aclaris Therapeutics, Celgene, Concert Pharmaceuticals, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, and Dermavant Sciences. He has also received funding support from The Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research.
[email protected]
LAKE TAHOE, CALIF. – After Brett King, MD, PhD, and his wife and collaborator, Brittany G. Craiglow, MD, published an index case of oral tofacitinib reversing alopecia universalis in a 25-year-old male patient back in 2014 (J Invest Dermatol. 2014;134:2988-90), they received hundreds of e-mails and phone calls from clinicians and patients.
“We also received quite a bit of media attention from around the world,” Dr. King recalled at the annual meeting of the Society for Pediatric Dermatology.
After all, alopecia areata and its variants affect 1%-2% of the population and have a marked impact on health-related quality of life, with high rates of concomitant generalized anxiety disorder and major depressive disorder. “The health-related quality of life is similar to that of atopic dermatitis and psoriasis, and there are no reliably effective therapies, especially for severe disease,” he said. “”
Currently available Janus kinase (JAK) inhibitors include tofacitinib (Xeljanz), ruxolitinib (Jakafi), and baricitinib (Olumiant). “These medicines are not [Food and Drug Administration] approved for alopecia areata, though tofacitinib was recently approved for psoriatic arthritis, and so we have formal entry of this medicine into dermatology for the first time,” said Dr. King, who is a dermatologist at Yale University, New Haven, Conn.
Potential adverse effects of JAKs include nasopharyngitis, headache, diarrhea, elevated cholesterol, uncommonly herpes zoster, cytopenias, transaminitis, and rarely non-melanoma skin cancer, solid organ malignancy and lymphoma, and GI perforation. Tofacitinib has an FDA black box warning regarding serious infections and malignancies, and baricitinib has these plus an additional warning about thrombosis.
In an open label, two-center trial that followed the index patient report, Dr. King and his associates enrolled 66 patients aged 19-35 years who had greater than 50% scalp hair loss for at least 6 months to receive tofacitinib 5 mg twice daily for 3 months (JCI Insight. 2016; 1[15]:e89776). A primary outcome of interest was regrowth of hair as measured by the percent change in Severity of Alopecia Tool (SALT) score. A SALT score of 100 indicates baldness, while a score of zero indicates no hair loss. Following 3 months of treatment, 32% of patients had a greater than 50% change in their SALT score, 32% had a change in the range of 5%-50%, while 36% had a change that was less than 5%.
“One of the interesting findings was that long duration of current episode of complete scalp hair loss was a negative predictor of treatment response, especially for those who have had hair loss greater than 10 years,” Dr. King said. Adverse events were “pretty bland,” with the most common being upper respiratory infection (17%), headache (8%), abdominal pain (8%), and acne (8%). Weight gain occurred in 1.5% of patients.
Next, Dr. King and colleagues reviewed the records of 90 patients aged 18 years or older who were treated with tofacitinib for at least 4 months (J Am Acad Dermatol. 2017;76[1]:22-8). Patients had greater than 40% scalp hair loss, and the tofacitinib dose was up to 10 mg per day at the discretion of the physician. “About 43% of patients were treated with tofacitinib 5 mg” twice daily, Dr. King said. “Other patients had higher doses or the addition of prednisone for three doses to see if that would help.”
After treatment, 20% of patients had a greater than 90% change in their SALT score (complete scalp hair regrowth), while 38.4% had a change that ranged from 51%-90%. At the same time, 18% had a change in their SALT score that ranged from 6%-50%, while 23% had a change that was 5% or less. As observed in the earlier trial, researchers saw a negative correlation between duration of current episode of hair loss and latest percent change in SALT score.
“We believe that you have to catch people before they get to more than 10 years of complete scalp hair loss,” Dr. King said. “This is important for the pediatric age group. I just saw somebody who’s 13, and they’ve been bald for 8 years. You might make the argument that this person deserves treatment, at least for a period of time long enough to regrow their hair in order to reset the clock.”
The most common adverse events were acne and weight gain.
In a separate analysis, Dr. King, Dr. Craiglow, and Lucy Y. Liu, evaluated the use of tofacitinib for at least 2 months in 13 alopecia areata patients aged 12-17 years (J Am Acad Dermatol. 2017;76[1]:29-32). They limited the analysis to those who had greater than 20% scalp hair loss, alopecia totalis, or alopecia universalis that was stable or worsening for 6 months or longer. Of the 13 patients, 9 (69%) were responders. Of the four non-responders, one had a very long duration of baldness. The percent change in SALT score was 93% overall, including 100% in the responder group over a median of 5 months and just 1% in the non-responder group over a median of 4 months. “This does not work every time,” Dr. King said.
While some preliminary studies of topical JAK inhibitors for alopecia areata show promise, it remains unclear if this approach will translate in a clinically meaningful way, he said. Clinical trials are currently under way.
Dr. King disclosed that he has received honoraria or consulting fees from Aclaris Therapeutics, Celgene, Concert Pharmaceuticals, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, and Dermavant Sciences. He has also received funding support from The Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research.
[email protected]
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING