User login
Hot Topics in Primary Care 2018
Click here to read Hot Topics in Primary Care.

This supplement offers the opportunity to earn a total of 5 CME credits.
Credit is awarded for successful completion of the online evaluations at the links below. These links may also be found within the supplement on the first page of each article.
- On the Front Lines: Hepatitis C Infection in Primary Care
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/hepC.
- Long-term Treatment of Gout: New Opportunities for Improved Outcomes
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/gout.
- Recognition and Management of Orthostatic Hypotension in Primary Care
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/orthostatic.
- Practical Evaluation and Management of Irritable Bowel Syndrome with Diarrhea: A Case Study Approach
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/ibs.
- Differentiating Among the SGLT-2 Inhibitors: Considering Cardiovascular and Other Safety Outcomes
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go www.pceconsortium.org/sglt2.
Click here to read Hot Topics in Primary Care.

This supplement offers the opportunity to earn a total of 5 CME credits.
Credit is awarded for successful completion of the online evaluations at the links below. These links may also be found within the supplement on the first page of each article.
- On the Front Lines: Hepatitis C Infection in Primary Care
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/hepC.
- Long-term Treatment of Gout: New Opportunities for Improved Outcomes
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/gout.
- Recognition and Management of Orthostatic Hypotension in Primary Care
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/orthostatic.
- Practical Evaluation and Management of Irritable Bowel Syndrome with Diarrhea: A Case Study Approach
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/ibs.
- Differentiating Among the SGLT-2 Inhibitors: Considering Cardiovascular and Other Safety Outcomes
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go www.pceconsortium.org/sglt2.
Click here to read Hot Topics in Primary Care.

This supplement offers the opportunity to earn a total of 5 CME credits.
Credit is awarded for successful completion of the online evaluations at the links below. These links may also be found within the supplement on the first page of each article.
- On the Front Lines: Hepatitis C Infection in Primary Care
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/hepC.
- Long-term Treatment of Gout: New Opportunities for Improved Outcomes
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/gout.
- Recognition and Management of Orthostatic Hypotension in Primary Care
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/orthostatic.
- Practical Evaluation and Management of Irritable Bowel Syndrome with Diarrhea: A Case Study Approach
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/ibs.
- Differentiating Among the SGLT-2 Inhibitors: Considering Cardiovascular and Other Safety Outcomes
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go www.pceconsortium.org/sglt2.
Outcomes similar for concurrent versus sequential treatment in HER2-positive breast cancers
Outcomes for women with operable HER2-positive breast cancer were similar whether they received standard combination chemotherapy with either concurrent or sequential paclitaxel/trastuzumab, long-term results of the phase 3, randomized American College of Surgeons Oncology Group Z1041 trial showed.
Among 280 women with HER-2 positive breast cancer followed for a median of 5.1 years, there were no significant differences in either pathological complete response rates (pCR), disease-free survival (DFS), or overall survival with either concurrent or sequential therapy, wrote Aman U. Buzdar, MD, from the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
“A previous publication of this study’s primary analysis reported that breast pCR in patients treated with paclitaxel and trastuzumab followed by FEC [fluorouracil, epirubicin, cyclophosphamide] and trastuzumab did not differ significantly from that of patients receiving FEC followed by paclitaxel and trastuzumab. We now report the findings concerning the secondary outcomes, that is, with a median follow-up of approximately 5 years, DFS is similar among the two treatment arms,” they wrote in JAMA Oncology.
The purpose of the current analysis was to evaluate long-term outcomes associated with the two treatment approaches.
In the trial, conducted at 36 centers in the continental United States and Puerto Rico, 280 women (median age, 50 years; range, 28-76 years) were treated with 500 mg/m2 of fluorouracil, 75 mg/m2 epirubicin, and 500 mg/m2 cyclophosphamide every 3 weeks for 12 weeks with concurrent weekly paclitaxel at 80 mg/m2 and trastuzumab at 2 mg/kg – after an initial dose of 4 mg/kg – or the same paclitaxel/trastuzumab combination delivered weekly for 12 weeks, followed by FEC every 3 weeks with weekly trastuzumab for 12 weeks.
Women who also had hormone receptor–positive disease received endocrine therapy. Radiotherapy was delivered at the discretion of the attending physician.
As noted, there were no differences in either DFS rates (adjusted hazard ratio, 1.02; P = .96) or overall survival rates (adjusted HR, 1.17; P = .73) between the trial arms.
The authors concluded that “concurrent administration of trastuzumab with FEC was not found to offer additional clinical benefit and is not warranted.”
The study was supported by grants to participating institutions from the National Cancer Institute. Dr. Buzdar reported no conflicts of interest. Three coauthors reported research support, consulting fees, travel support, and/or other relationships with multiple companies.
SOURCE: Buzdar AU et al. JAMA Oncol. 2018 Sept 6. doi: 10.1001/jamaoncol.2018.3691.
Outcomes for women with operable HER2-positive breast cancer were similar whether they received standard combination chemotherapy with either concurrent or sequential paclitaxel/trastuzumab, long-term results of the phase 3, randomized American College of Surgeons Oncology Group Z1041 trial showed.
Among 280 women with HER-2 positive breast cancer followed for a median of 5.1 years, there were no significant differences in either pathological complete response rates (pCR), disease-free survival (DFS), or overall survival with either concurrent or sequential therapy, wrote Aman U. Buzdar, MD, from the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
“A previous publication of this study’s primary analysis reported that breast pCR in patients treated with paclitaxel and trastuzumab followed by FEC [fluorouracil, epirubicin, cyclophosphamide] and trastuzumab did not differ significantly from that of patients receiving FEC followed by paclitaxel and trastuzumab. We now report the findings concerning the secondary outcomes, that is, with a median follow-up of approximately 5 years, DFS is similar among the two treatment arms,” they wrote in JAMA Oncology.
The purpose of the current analysis was to evaluate long-term outcomes associated with the two treatment approaches.
In the trial, conducted at 36 centers in the continental United States and Puerto Rico, 280 women (median age, 50 years; range, 28-76 years) were treated with 500 mg/m2 of fluorouracil, 75 mg/m2 epirubicin, and 500 mg/m2 cyclophosphamide every 3 weeks for 12 weeks with concurrent weekly paclitaxel at 80 mg/m2 and trastuzumab at 2 mg/kg – after an initial dose of 4 mg/kg – or the same paclitaxel/trastuzumab combination delivered weekly for 12 weeks, followed by FEC every 3 weeks with weekly trastuzumab for 12 weeks.
Women who also had hormone receptor–positive disease received endocrine therapy. Radiotherapy was delivered at the discretion of the attending physician.
As noted, there were no differences in either DFS rates (adjusted hazard ratio, 1.02; P = .96) or overall survival rates (adjusted HR, 1.17; P = .73) between the trial arms.
The authors concluded that “concurrent administration of trastuzumab with FEC was not found to offer additional clinical benefit and is not warranted.”
The study was supported by grants to participating institutions from the National Cancer Institute. Dr. Buzdar reported no conflicts of interest. Three coauthors reported research support, consulting fees, travel support, and/or other relationships with multiple companies.
SOURCE: Buzdar AU et al. JAMA Oncol. 2018 Sept 6. doi: 10.1001/jamaoncol.2018.3691.
Outcomes for women with operable HER2-positive breast cancer were similar whether they received standard combination chemotherapy with either concurrent or sequential paclitaxel/trastuzumab, long-term results of the phase 3, randomized American College of Surgeons Oncology Group Z1041 trial showed.
Among 280 women with HER-2 positive breast cancer followed for a median of 5.1 years, there were no significant differences in either pathological complete response rates (pCR), disease-free survival (DFS), or overall survival with either concurrent or sequential therapy, wrote Aman U. Buzdar, MD, from the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
“A previous publication of this study’s primary analysis reported that breast pCR in patients treated with paclitaxel and trastuzumab followed by FEC [fluorouracil, epirubicin, cyclophosphamide] and trastuzumab did not differ significantly from that of patients receiving FEC followed by paclitaxel and trastuzumab. We now report the findings concerning the secondary outcomes, that is, with a median follow-up of approximately 5 years, DFS is similar among the two treatment arms,” they wrote in JAMA Oncology.
The purpose of the current analysis was to evaluate long-term outcomes associated with the two treatment approaches.
In the trial, conducted at 36 centers in the continental United States and Puerto Rico, 280 women (median age, 50 years; range, 28-76 years) were treated with 500 mg/m2 of fluorouracil, 75 mg/m2 epirubicin, and 500 mg/m2 cyclophosphamide every 3 weeks for 12 weeks with concurrent weekly paclitaxel at 80 mg/m2 and trastuzumab at 2 mg/kg – after an initial dose of 4 mg/kg – or the same paclitaxel/trastuzumab combination delivered weekly for 12 weeks, followed by FEC every 3 weeks with weekly trastuzumab for 12 weeks.
Women who also had hormone receptor–positive disease received endocrine therapy. Radiotherapy was delivered at the discretion of the attending physician.
As noted, there were no differences in either DFS rates (adjusted hazard ratio, 1.02; P = .96) or overall survival rates (adjusted HR, 1.17; P = .73) between the trial arms.
The authors concluded that “concurrent administration of trastuzumab with FEC was not found to offer additional clinical benefit and is not warranted.”
The study was supported by grants to participating institutions from the National Cancer Institute. Dr. Buzdar reported no conflicts of interest. Three coauthors reported research support, consulting fees, travel support, and/or other relationships with multiple companies.
SOURCE: Buzdar AU et al. JAMA Oncol. 2018 Sept 6. doi: 10.1001/jamaoncol.2018.3691.
FROM JAMA ONCOLOGY
Key clinical point: Sequencing of chemotherapy, paclitaxel, and trastuzumab did not affect outcomes in women with HER2-positive breast cancers.
Major finding: There were no significant differences in disease-free survival or overall survival among treated with concurrent or sequential therapy.
Study details: A phase 3, randomized trial in 280 women with operable HER2-positive breast cancers.
Disclosures: The study was supported by grants to participating institutions from the National Cancer Institute. Dr. Buzdar reported no conflicts of interest. Three coauthors reported research support, consulting fees, travel support, and/or other relationships with multiple companies.
Source: Buzdar AU et al. JAMA Oncol. 2018 Sept 6. doi: 10.1001/jamaoncol.2018.3691.
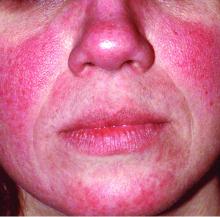
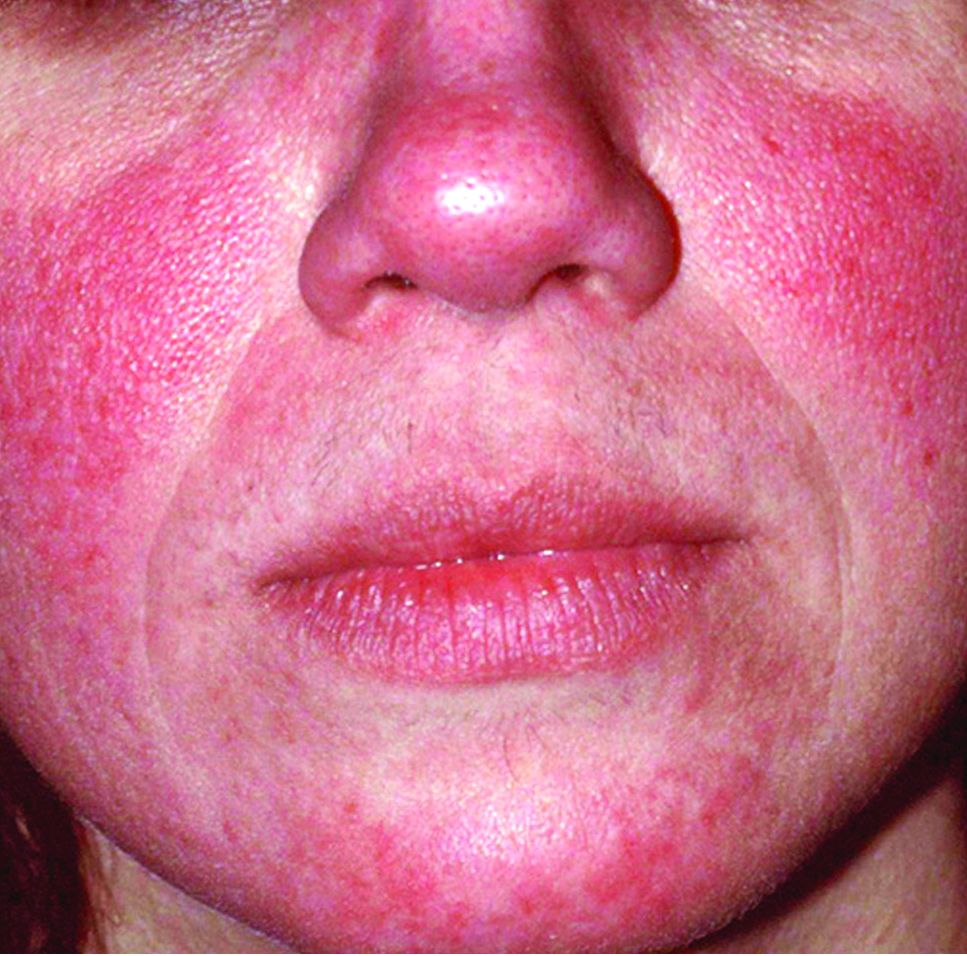
Global incidence of rosacea estimated to be over 5%
The global incidence of rosacea among adults is approximately 5%, based on data from a meta-analysis and systematic review.
The National Rosacea Society Expert Committee recently updated its phenotype-based classification system, but the global prevalence and incidence of rosacea remain unknown, wrote Lise Gether, MD, of the University of Copenhagen, Denmark, and her colleagues.
In a review published in the British Journal of Dermatology, the researchers examined the prevalence of rosacea among dermatology patients and in the general population worldwide by analyzing 32 studies including 41 populations and 26,538,319 individuals. The study populations comprised 22 from Europe, 9 from North America, 4 from Asia, 3 from South America, and 3 from Africa. Of the 32 studies, 18 included the general population and 14 included only dermatology patients.
Overall, the pooled proportion of individuals with rosacea was 5.46% in the general population and 2.39% among dermatology patients. Of note, the pooled proportions varied when the studies were grouped by diagnostic method: self-diagnosis (9.7%), physician diagnosis (5.5%), and health care database estimate (1.05%).
Rosacea prevalence by age was highest among individuals aged 45-60 years, but “based on the available data, it was not possible to make useful stratified estimates,” the researchers said.
The researchers estimated rosacea prevalence by gender using data from the 10 studies that reported numbers from both genders. Based on a population of 5,601,642 women and 3,529,559 men, the pooled proportions were similar; 5.4% for women and 3.9% for men.
“The methods used to study the prevalence of rosacea are of great importance as misclassification may be a concern,” the researchers noted. Individuals with mild to moderate rosacea might not seek medical treatment, which might contribute to the low prevalence from health care database estimates. Conversely, the high prevalence with self-reports might suggest a lack of specificity in the questionnaires.
There were no external funding sources. Dr. Gether had no relevant financial disclosures. Coauthors disclosed relationships with companies including Galderma, Pfizer, Eli Lilly, Novartis, and Janssen.
SOURCE: Gether L et al. Br J Dermatol. 2018. doi: 10.1111/bjd.16481
The global incidence of rosacea among adults is approximately 5%, based on data from a meta-analysis and systematic review.
The National Rosacea Society Expert Committee recently updated its phenotype-based classification system, but the global prevalence and incidence of rosacea remain unknown, wrote Lise Gether, MD, of the University of Copenhagen, Denmark, and her colleagues.
In a review published in the British Journal of Dermatology, the researchers examined the prevalence of rosacea among dermatology patients and in the general population worldwide by analyzing 32 studies including 41 populations and 26,538,319 individuals. The study populations comprised 22 from Europe, 9 from North America, 4 from Asia, 3 from South America, and 3 from Africa. Of the 32 studies, 18 included the general population and 14 included only dermatology patients.
Overall, the pooled proportion of individuals with rosacea was 5.46% in the general population and 2.39% among dermatology patients. Of note, the pooled proportions varied when the studies were grouped by diagnostic method: self-diagnosis (9.7%), physician diagnosis (5.5%), and health care database estimate (1.05%).
Rosacea prevalence by age was highest among individuals aged 45-60 years, but “based on the available data, it was not possible to make useful stratified estimates,” the researchers said.
The researchers estimated rosacea prevalence by gender using data from the 10 studies that reported numbers from both genders. Based on a population of 5,601,642 women and 3,529,559 men, the pooled proportions were similar; 5.4% for women and 3.9% for men.
“The methods used to study the prevalence of rosacea are of great importance as misclassification may be a concern,” the researchers noted. Individuals with mild to moderate rosacea might not seek medical treatment, which might contribute to the low prevalence from health care database estimates. Conversely, the high prevalence with self-reports might suggest a lack of specificity in the questionnaires.
There were no external funding sources. Dr. Gether had no relevant financial disclosures. Coauthors disclosed relationships with companies including Galderma, Pfizer, Eli Lilly, Novartis, and Janssen.
SOURCE: Gether L et al. Br J Dermatol. 2018. doi: 10.1111/bjd.16481
The global incidence of rosacea among adults is approximately 5%, based on data from a meta-analysis and systematic review.
The National Rosacea Society Expert Committee recently updated its phenotype-based classification system, but the global prevalence and incidence of rosacea remain unknown, wrote Lise Gether, MD, of the University of Copenhagen, Denmark, and her colleagues.
In a review published in the British Journal of Dermatology, the researchers examined the prevalence of rosacea among dermatology patients and in the general population worldwide by analyzing 32 studies including 41 populations and 26,538,319 individuals. The study populations comprised 22 from Europe, 9 from North America, 4 from Asia, 3 from South America, and 3 from Africa. Of the 32 studies, 18 included the general population and 14 included only dermatology patients.
Overall, the pooled proportion of individuals with rosacea was 5.46% in the general population and 2.39% among dermatology patients. Of note, the pooled proportions varied when the studies were grouped by diagnostic method: self-diagnosis (9.7%), physician diagnosis (5.5%), and health care database estimate (1.05%).
Rosacea prevalence by age was highest among individuals aged 45-60 years, but “based on the available data, it was not possible to make useful stratified estimates,” the researchers said.
The researchers estimated rosacea prevalence by gender using data from the 10 studies that reported numbers from both genders. Based on a population of 5,601,642 women and 3,529,559 men, the pooled proportions were similar; 5.4% for women and 3.9% for men.
“The methods used to study the prevalence of rosacea are of great importance as misclassification may be a concern,” the researchers noted. Individuals with mild to moderate rosacea might not seek medical treatment, which might contribute to the low prevalence from health care database estimates. Conversely, the high prevalence with self-reports might suggest a lack of specificity in the questionnaires.
There were no external funding sources. Dr. Gether had no relevant financial disclosures. Coauthors disclosed relationships with companies including Galderma, Pfizer, Eli Lilly, Novartis, and Janssen.
SOURCE: Gether L et al. Br J Dermatol. 2018. doi: 10.1111/bjd.16481
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Rosacea occurs worldwide in both men and women, but diagnosis remains inconsistent.
Major finding:
Study details: A meta-analysis of 32 studies and 26,538,319 individuals.
Disclosures: There were no external funding sources. Dr. Gether had no relevant financial disclosures. Coauthors disclosed relationships with companies including Galderma, Pfizer, Eli Lilly, Novartis, and Janssen.
Source: Gether L et al. Br J Dermatol. 2018. doi: 10.1111/bjd.16481.
Top cancer researcher fails to disclose corporate financial ties in major research journals
This article was produced in partnership with The New York Times.
One of the world’s top breast cancer doctors failed to disclose millions of dollars in payments from drug and health care companies in recent years, omitting his financial ties from dozens of research articles in prestigious publications like the New England Journal of Medicine and the Lancet.
The researcher, José Baselga, MD, a towering figure in the cancer world, is the chief medical officer at Memorial Sloan Kettering Cancer Center in New York. He has held board memberships or advisory roles with Roche and Bristol-Myers Squibb, among other corporations; has had a stake in start-ups testing cancer therapies; and played a key role in the development of breakthrough drugs that have revolutionized treatments for breast cancer.
According to an analysis by ProPublica and the New York Times, Dr. Baselga did not follow financial disclosure rules set by the American Association for Cancer Research when he was president of the group. He also left out payments he received from companies connected to cancer research in his articles published in the group’s journal, Cancer Discovery. At the same time, he has been one of the journal’s two editors in chief.
At a conference this year and before analysts in 2017, he put a positive spin on the results of two Roche-sponsored clinical trials that many others considered disappointments, without disclosing his relationship to the company. Since 2014, he has received more than $3 million from Roche in consulting fees and for his stake in a company it acquired.
Dr. Baselga did not dispute his relationships with at least a dozen companies. In an interview, he said the disclosure lapses were unintentional.
He stressed that much of his industry work was publicly known although he declined to provide payment figures from his involvement with some biotech start-ups. “I acknowledge that there have been inconsistencies, but that’s what it is,” he said. “It’s not that I do not appreciate the importance.”
Dr. Baselga’s extensive corporate relationships – and his frequent failure to disclose them – illustrate how permeable the boundaries remain between academic research and industry, and how weakly reporting requirements are enforced by the medical journals and professional societies charged with policing them.
A decade ago, a series of scandals involving the secret influence of the pharmaceutical industry on drug research prompted the medical community to beef up its conflict-of-interest disclosure requirements. Ethicists worry that outside entanglements can shape the way studies are designed and medications are prescribed to patients, allowing bias to influence medical practice. Disclosing those connections allows the public, other scientists, and doctors to evaluate the research and weigh potential conflicts.
If leaders don’t follow the rules, then we don’t really have rules,” said Walid Gellad, MD, an of the department of medicine at the University of Pittsburgh and director of its Center for Pharmaceutical Policy and Prescribing. “It says that the rules don’t matter.”
The penalties for such ethical lapses are not severe. The cancer research group, the American Association for Cancer Research, warns authors who fill out disclosure forms for its journals that they face a 3-year ban on publishing if they are found to have financial relationships that they did not disclose. But the ban is not included in the conflict-of-interest policy posted on its website, and the group said no author had ever been barred.
Many journals and professional societies do not check conflicts and simply require authors to correct the record.
Officials at the AACR, the American Society of Clinical Oncology and the New England Journal of Medicine said they were looking into Dr. Baselga’s omissions after inquiries from the Times and ProPublica. The Lancet declined to say whether it would look into the matter.
Christine Hickey, a spokeswoman for Memorial Sloan Kettering, said that Dr. Baselga had properly informed the hospital of his outside industry work and that it was Dr. Baselga’s responsibility to disclose such relationships to entities such as medical journals. The cancer center, she said, “has a rigorous and comprehensive compliance program in place to promote honesty and objectivity in scientific research.”
Asked if he planned to correct his disclosures, Dr. Baselga asked reporters what they would recommend. In a statement several days later, he said he would correct his conflict-of-interest reporting for 17 articles, including in the New England Journal of Medicine, the Lancet, and the publication he edits, Cancer Discovery. He said that he did not believe disclosure was required for dozens of other articles detailing early stages of research.
“I have spent my career caring for cancer patients and bringing new therapies to the clinic with the goal of extending and saving lives,” Dr. Baselga said in the statement. “While I have been inconsistent with disclosures and acknowledge that fact, that is a far cry from compromising my responsibilities as a physician, as a scientist and as a clinical leader.”
The corporate imprint on cancer research
Dr. Baselga, 59, supervises clinical operations at Memorial Sloan Kettering, one of the nation’s top cancer centers and wields influence over the lives of patients and companies wishing to conduct trials there. He was paid more than $1.5 million in compensation by the cancer center in 2016, according to the hospital’s latest available tax disclosures, but that does not include his consulting or board fees from outside companies.
Many top medical researchers have ties to the for-profit health care industry, and some overlap is seen as a good thing – after all, these are the companies charged with developing the drugs, medical devices and diagnostic tests of the future.
Dr. Baselga’s relationship to industry is extensive. In addition to sitting on the board of Bristol-Myers Squibb, he is a director of Varian Medical Systems, which sells radiation equipment and for whom Memorial Sloan Kettering is a client.
In all, Dr. Baselga has served on the boards of at least six companies since 2013, positions that have required him to assume a fiduciary responsibility to protect the interests of those companies, even as he oversees the cancer center’s medical operations.
The hospital and Dr. Baselga said steps had been taken to prevent him from having a say in any business between the cancer center and the companies on whose boards he sits.
The chief executive of Memorial Sloan Kettering, Craig B. Thompson, MD, settled lawsuits several years ago that were filed by the University of Pennsylvania, Philadelphia, and an affiliated research center. They contended that he hid research conducted while he was at Penn to start a new company, Agios Pharmaceuticals, and did not share the earnings. Dr. Thompson disputed the allegations. He now sits on the board of Merck, which manufactures Keytruda, a blockbuster cancer therapy.
Ms. Hickey said the cancer center cannot fulfill its charitable mission without working with industry. “We encourage collaboration and are proud that our work has led to the approval of novel, lifesaving cancer treatments for patients around the world,” she said.
Some disclosures are required; others aren’t
After the scandals a decade ago over lack of disclosure, the federal government began requiring drug and device manufacturers to publicly disclose payments to doctors in 2013.
From August 2013 through 2017, Dr. Baselga received nearly $3.5 million from nine companies, according to the federal Open Payments database, which compiles disclosures filed by drug and device companies.
Dr. Baselga has disclosed in other forums investments and advisory roles in biotech start-ups, but he declined to provide a tally of financial interests in those firms. Companies that have not received approval from the Food and Drug Administration for their products – projects still in the testing phases – do not have to report payments they make to doctors.
Serving on boards can be lucrative. In 2017, Dr. Baselga received $260,000 in cash and stock awards to sit on Varian’s board of directors, according to the company’s corporate filings.
ProPublica and the Times analyzed Dr. Baselga’s publications in medical journals since 2013, the year he joined Memorial Sloan Kettering. He failed to disclose any industry relationships in more than 100, or about 60% of the time, a figure that has increased with each passing year. Last year, he did not list any potential conflicts in 87% of the articles that he wrote or cowrote.
Dr. Baselga compiled a color-coded list of his articles and offered a different interpretation. Sixty-two of the papers for which he did not disclose any potential conflict represented “conceptual, basic laboratory or translational work,” and did not require one, he said. Questions could be raised about others, he said, but he added that most “had no clinical nor financial implications.” That left the 17 papers he plans to correct.
Early-stage research often carries financial weight because it helps companies decide whether to move ahead with a product. In about two-thirds of Dr. Baselga’s articles that lacked details of his industry ties, one or more of his coauthors listed theirs.
In 2015, Dr. Baselga published an article in the New England Journal about a Roche-sponsored trial of one of the company’s drugs, Zelboraf. Despite his financial ties to Roche, he declared that he had “nothing to disclose.” Fourteen of his coauthors reported ties to Roche.
Dr. Baselga defended the articles, saying that “these are high-quality manuscripts reporting on important clinical trials that led to a better understanding of cancer treatments.”
The guidelines enacted by most major medical journals and professional societies ask authors and presenters to list recent financial relationships that could pose a conflict.
But much of this reporting still relies on the honor system. A study in August in the journal JAMA Oncology found that one-third of authors in a sample of cancer trials did not report all payments from the studies’ sponsors.
“We don’t routinely check because we don’t have those kind of resources,” said Rita F. Redberg, MD, the editor of JAMA Internal Medicine, who has been critical of the influence of industry on medical practice. “We rely on trust and integrity. It’s kind of an assumed part of the professional relationship.”
Jennifer Zeis, a spokeswoman for the New England Journal of Medicine, said in an email that it had now asked Dr. Baselga to amend his disclosures. She said the journal planned to overhaul its tracking of industry relationships.
The AACR said it had begun an “extensive review” of the disclosure forms submitted by Dr. Baselga.
It said that it had never barred an author from publishing, and that “such an action would be necessary only in cases of egregious, consistent violations of the rules.”
Among the most prominent relationships that Dr. Baselga has often failed to disclose is with the Swiss pharmaceutical giant Roche and its United States subsidiary Genentech.
In June 2017, at the annual meeting of the ASCO in Chicago, Dr. Baselga spoke at a Roche-sponsored investor event about study results that the company had been counting on to persuade oncologists to move patients from Herceptin – which was facing competition from cheaper alternatives – to a combination treatment involving Herceptin and a newer, more expensive drug, Perjeta.
The results were so underwhelming that Roche’s stock fell 5 % on the news. One analyst described the results as a “lead balloon,” and an editorial in the New England Journal called it a “disappointment.”
Dr. Baselga, however, told analysts that critiques were “weird” and “strange.”
This June, at the same cancer conference, Dr. Baselga struck an upbeat note about the results of a Roche trial of the drug taselisib, saying in a blog post published on the cancer center website that the results were “incredibly exciting” while conceding the side effects from the drug were high.
That same day, Roche announced it was scrapping plans to develop the drug. The news was another disappointment involving the class of drugs called PI3K inhibitors, which is a major focus of Dr. Baselga’s current research.
In neither case did Dr. Baselga reveal that his ties to Roche and Genentech went beyond serving as a trial investigator. In 2014, Roche acquired Seragon, a cancer research company in which Dr. Baselga had an ownership stake, for $725 million. Dr. Baselga received more than $3 million in 2014 and 2015 for his stake in the company, according to the federal Open Payments database.
From 2013 to 2017, Roche also paid Dr. Baselga more than $50,000 in consulting fees, according to the database.
These details were not included in the conflict-of-interest statements that are required of all presenters at the ASCO conference, although he did disclose ownership interests and consulting relationships with several other companies in the prior two years.
ASCO said it would conduct an internal review of Dr. Baselga’s disclosures and would refer the findings to a panel.
Dr. Baselga said that he played no role in the Seragon acquisition and that he had cut ties with Roche since joining the board of a competitor, Bristol-Myers, in March. As for his presentations at the ASCO meetings in the last 2 years, he said he had also noted shortcomings in the studies.
The combination of Perjeta with Herceptin was later approved by the FDA for certain high-risk patients. As for taselisib, Dr. Baselga stands by his belief that the PI3K class of drugs will be an important target for fighting cancer.
Katie Thomas covers the pharmaceutical industry for the New York Times.
This article was produced in partnership with The New York Times.
One of the world’s top breast cancer doctors failed to disclose millions of dollars in payments from drug and health care companies in recent years, omitting his financial ties from dozens of research articles in prestigious publications like the New England Journal of Medicine and the Lancet.
The researcher, José Baselga, MD, a towering figure in the cancer world, is the chief medical officer at Memorial Sloan Kettering Cancer Center in New York. He has held board memberships or advisory roles with Roche and Bristol-Myers Squibb, among other corporations; has had a stake in start-ups testing cancer therapies; and played a key role in the development of breakthrough drugs that have revolutionized treatments for breast cancer.
According to an analysis by ProPublica and the New York Times, Dr. Baselga did not follow financial disclosure rules set by the American Association for Cancer Research when he was president of the group. He also left out payments he received from companies connected to cancer research in his articles published in the group’s journal, Cancer Discovery. At the same time, he has been one of the journal’s two editors in chief.
At a conference this year and before analysts in 2017, he put a positive spin on the results of two Roche-sponsored clinical trials that many others considered disappointments, without disclosing his relationship to the company. Since 2014, he has received more than $3 million from Roche in consulting fees and for his stake in a company it acquired.
Dr. Baselga did not dispute his relationships with at least a dozen companies. In an interview, he said the disclosure lapses were unintentional.
He stressed that much of his industry work was publicly known although he declined to provide payment figures from his involvement with some biotech start-ups. “I acknowledge that there have been inconsistencies, but that’s what it is,” he said. “It’s not that I do not appreciate the importance.”
Dr. Baselga’s extensive corporate relationships – and his frequent failure to disclose them – illustrate how permeable the boundaries remain between academic research and industry, and how weakly reporting requirements are enforced by the medical journals and professional societies charged with policing them.
A decade ago, a series of scandals involving the secret influence of the pharmaceutical industry on drug research prompted the medical community to beef up its conflict-of-interest disclosure requirements. Ethicists worry that outside entanglements can shape the way studies are designed and medications are prescribed to patients, allowing bias to influence medical practice. Disclosing those connections allows the public, other scientists, and doctors to evaluate the research and weigh potential conflicts.
If leaders don’t follow the rules, then we don’t really have rules,” said Walid Gellad, MD, an of the department of medicine at the University of Pittsburgh and director of its Center for Pharmaceutical Policy and Prescribing. “It says that the rules don’t matter.”
The penalties for such ethical lapses are not severe. The cancer research group, the American Association for Cancer Research, warns authors who fill out disclosure forms for its journals that they face a 3-year ban on publishing if they are found to have financial relationships that they did not disclose. But the ban is not included in the conflict-of-interest policy posted on its website, and the group said no author had ever been barred.
Many journals and professional societies do not check conflicts and simply require authors to correct the record.
Officials at the AACR, the American Society of Clinical Oncology and the New England Journal of Medicine said they were looking into Dr. Baselga’s omissions after inquiries from the Times and ProPublica. The Lancet declined to say whether it would look into the matter.
Christine Hickey, a spokeswoman for Memorial Sloan Kettering, said that Dr. Baselga had properly informed the hospital of his outside industry work and that it was Dr. Baselga’s responsibility to disclose such relationships to entities such as medical journals. The cancer center, she said, “has a rigorous and comprehensive compliance program in place to promote honesty and objectivity in scientific research.”
Asked if he planned to correct his disclosures, Dr. Baselga asked reporters what they would recommend. In a statement several days later, he said he would correct his conflict-of-interest reporting for 17 articles, including in the New England Journal of Medicine, the Lancet, and the publication he edits, Cancer Discovery. He said that he did not believe disclosure was required for dozens of other articles detailing early stages of research.
“I have spent my career caring for cancer patients and bringing new therapies to the clinic with the goal of extending and saving lives,” Dr. Baselga said in the statement. “While I have been inconsistent with disclosures and acknowledge that fact, that is a far cry from compromising my responsibilities as a physician, as a scientist and as a clinical leader.”
The corporate imprint on cancer research
Dr. Baselga, 59, supervises clinical operations at Memorial Sloan Kettering, one of the nation’s top cancer centers and wields influence over the lives of patients and companies wishing to conduct trials there. He was paid more than $1.5 million in compensation by the cancer center in 2016, according to the hospital’s latest available tax disclosures, but that does not include his consulting or board fees from outside companies.
Many top medical researchers have ties to the for-profit health care industry, and some overlap is seen as a good thing – after all, these are the companies charged with developing the drugs, medical devices and diagnostic tests of the future.
Dr. Baselga’s relationship to industry is extensive. In addition to sitting on the board of Bristol-Myers Squibb, he is a director of Varian Medical Systems, which sells radiation equipment and for whom Memorial Sloan Kettering is a client.
In all, Dr. Baselga has served on the boards of at least six companies since 2013, positions that have required him to assume a fiduciary responsibility to protect the interests of those companies, even as he oversees the cancer center’s medical operations.
The hospital and Dr. Baselga said steps had been taken to prevent him from having a say in any business between the cancer center and the companies on whose boards he sits.
The chief executive of Memorial Sloan Kettering, Craig B. Thompson, MD, settled lawsuits several years ago that were filed by the University of Pennsylvania, Philadelphia, and an affiliated research center. They contended that he hid research conducted while he was at Penn to start a new company, Agios Pharmaceuticals, and did not share the earnings. Dr. Thompson disputed the allegations. He now sits on the board of Merck, which manufactures Keytruda, a blockbuster cancer therapy.
Ms. Hickey said the cancer center cannot fulfill its charitable mission without working with industry. “We encourage collaboration and are proud that our work has led to the approval of novel, lifesaving cancer treatments for patients around the world,” she said.
Some disclosures are required; others aren’t
After the scandals a decade ago over lack of disclosure, the federal government began requiring drug and device manufacturers to publicly disclose payments to doctors in 2013.
From August 2013 through 2017, Dr. Baselga received nearly $3.5 million from nine companies, according to the federal Open Payments database, which compiles disclosures filed by drug and device companies.
Dr. Baselga has disclosed in other forums investments and advisory roles in biotech start-ups, but he declined to provide a tally of financial interests in those firms. Companies that have not received approval from the Food and Drug Administration for their products – projects still in the testing phases – do not have to report payments they make to doctors.
Serving on boards can be lucrative. In 2017, Dr. Baselga received $260,000 in cash and stock awards to sit on Varian’s board of directors, according to the company’s corporate filings.
ProPublica and the Times analyzed Dr. Baselga’s publications in medical journals since 2013, the year he joined Memorial Sloan Kettering. He failed to disclose any industry relationships in more than 100, or about 60% of the time, a figure that has increased with each passing year. Last year, he did not list any potential conflicts in 87% of the articles that he wrote or cowrote.
Dr. Baselga compiled a color-coded list of his articles and offered a different interpretation. Sixty-two of the papers for which he did not disclose any potential conflict represented “conceptual, basic laboratory or translational work,” and did not require one, he said. Questions could be raised about others, he said, but he added that most “had no clinical nor financial implications.” That left the 17 papers he plans to correct.
Early-stage research often carries financial weight because it helps companies decide whether to move ahead with a product. In about two-thirds of Dr. Baselga’s articles that lacked details of his industry ties, one or more of his coauthors listed theirs.
In 2015, Dr. Baselga published an article in the New England Journal about a Roche-sponsored trial of one of the company’s drugs, Zelboraf. Despite his financial ties to Roche, he declared that he had “nothing to disclose.” Fourteen of his coauthors reported ties to Roche.
Dr. Baselga defended the articles, saying that “these are high-quality manuscripts reporting on important clinical trials that led to a better understanding of cancer treatments.”
The guidelines enacted by most major medical journals and professional societies ask authors and presenters to list recent financial relationships that could pose a conflict.
But much of this reporting still relies on the honor system. A study in August in the journal JAMA Oncology found that one-third of authors in a sample of cancer trials did not report all payments from the studies’ sponsors.
“We don’t routinely check because we don’t have those kind of resources,” said Rita F. Redberg, MD, the editor of JAMA Internal Medicine, who has been critical of the influence of industry on medical practice. “We rely on trust and integrity. It’s kind of an assumed part of the professional relationship.”
Jennifer Zeis, a spokeswoman for the New England Journal of Medicine, said in an email that it had now asked Dr. Baselga to amend his disclosures. She said the journal planned to overhaul its tracking of industry relationships.
The AACR said it had begun an “extensive review” of the disclosure forms submitted by Dr. Baselga.
It said that it had never barred an author from publishing, and that “such an action would be necessary only in cases of egregious, consistent violations of the rules.”
Among the most prominent relationships that Dr. Baselga has often failed to disclose is with the Swiss pharmaceutical giant Roche and its United States subsidiary Genentech.
In June 2017, at the annual meeting of the ASCO in Chicago, Dr. Baselga spoke at a Roche-sponsored investor event about study results that the company had been counting on to persuade oncologists to move patients from Herceptin – which was facing competition from cheaper alternatives – to a combination treatment involving Herceptin and a newer, more expensive drug, Perjeta.
The results were so underwhelming that Roche’s stock fell 5 % on the news. One analyst described the results as a “lead balloon,” and an editorial in the New England Journal called it a “disappointment.”
Dr. Baselga, however, told analysts that critiques were “weird” and “strange.”
This June, at the same cancer conference, Dr. Baselga struck an upbeat note about the results of a Roche trial of the drug taselisib, saying in a blog post published on the cancer center website that the results were “incredibly exciting” while conceding the side effects from the drug were high.
That same day, Roche announced it was scrapping plans to develop the drug. The news was another disappointment involving the class of drugs called PI3K inhibitors, which is a major focus of Dr. Baselga’s current research.
In neither case did Dr. Baselga reveal that his ties to Roche and Genentech went beyond serving as a trial investigator. In 2014, Roche acquired Seragon, a cancer research company in which Dr. Baselga had an ownership stake, for $725 million. Dr. Baselga received more than $3 million in 2014 and 2015 for his stake in the company, according to the federal Open Payments database.
From 2013 to 2017, Roche also paid Dr. Baselga more than $50,000 in consulting fees, according to the database.
These details were not included in the conflict-of-interest statements that are required of all presenters at the ASCO conference, although he did disclose ownership interests and consulting relationships with several other companies in the prior two years.
ASCO said it would conduct an internal review of Dr. Baselga’s disclosures and would refer the findings to a panel.
Dr. Baselga said that he played no role in the Seragon acquisition and that he had cut ties with Roche since joining the board of a competitor, Bristol-Myers, in March. As for his presentations at the ASCO meetings in the last 2 years, he said he had also noted shortcomings in the studies.
The combination of Perjeta with Herceptin was later approved by the FDA for certain high-risk patients. As for taselisib, Dr. Baselga stands by his belief that the PI3K class of drugs will be an important target for fighting cancer.
Katie Thomas covers the pharmaceutical industry for the New York Times.
This article was produced in partnership with The New York Times.
One of the world’s top breast cancer doctors failed to disclose millions of dollars in payments from drug and health care companies in recent years, omitting his financial ties from dozens of research articles in prestigious publications like the New England Journal of Medicine and the Lancet.
The researcher, José Baselga, MD, a towering figure in the cancer world, is the chief medical officer at Memorial Sloan Kettering Cancer Center in New York. He has held board memberships or advisory roles with Roche and Bristol-Myers Squibb, among other corporations; has had a stake in start-ups testing cancer therapies; and played a key role in the development of breakthrough drugs that have revolutionized treatments for breast cancer.
According to an analysis by ProPublica and the New York Times, Dr. Baselga did not follow financial disclosure rules set by the American Association for Cancer Research when he was president of the group. He also left out payments he received from companies connected to cancer research in his articles published in the group’s journal, Cancer Discovery. At the same time, he has been one of the journal’s two editors in chief.
At a conference this year and before analysts in 2017, he put a positive spin on the results of two Roche-sponsored clinical trials that many others considered disappointments, without disclosing his relationship to the company. Since 2014, he has received more than $3 million from Roche in consulting fees and for his stake in a company it acquired.
Dr. Baselga did not dispute his relationships with at least a dozen companies. In an interview, he said the disclosure lapses were unintentional.
He stressed that much of his industry work was publicly known although he declined to provide payment figures from his involvement with some biotech start-ups. “I acknowledge that there have been inconsistencies, but that’s what it is,” he said. “It’s not that I do not appreciate the importance.”
Dr. Baselga’s extensive corporate relationships – and his frequent failure to disclose them – illustrate how permeable the boundaries remain between academic research and industry, and how weakly reporting requirements are enforced by the medical journals and professional societies charged with policing them.
A decade ago, a series of scandals involving the secret influence of the pharmaceutical industry on drug research prompted the medical community to beef up its conflict-of-interest disclosure requirements. Ethicists worry that outside entanglements can shape the way studies are designed and medications are prescribed to patients, allowing bias to influence medical practice. Disclosing those connections allows the public, other scientists, and doctors to evaluate the research and weigh potential conflicts.
If leaders don’t follow the rules, then we don’t really have rules,” said Walid Gellad, MD, an of the department of medicine at the University of Pittsburgh and director of its Center for Pharmaceutical Policy and Prescribing. “It says that the rules don’t matter.”
The penalties for such ethical lapses are not severe. The cancer research group, the American Association for Cancer Research, warns authors who fill out disclosure forms for its journals that they face a 3-year ban on publishing if they are found to have financial relationships that they did not disclose. But the ban is not included in the conflict-of-interest policy posted on its website, and the group said no author had ever been barred.
Many journals and professional societies do not check conflicts and simply require authors to correct the record.
Officials at the AACR, the American Society of Clinical Oncology and the New England Journal of Medicine said they were looking into Dr. Baselga’s omissions after inquiries from the Times and ProPublica. The Lancet declined to say whether it would look into the matter.
Christine Hickey, a spokeswoman for Memorial Sloan Kettering, said that Dr. Baselga had properly informed the hospital of his outside industry work and that it was Dr. Baselga’s responsibility to disclose such relationships to entities such as medical journals. The cancer center, she said, “has a rigorous and comprehensive compliance program in place to promote honesty and objectivity in scientific research.”
Asked if he planned to correct his disclosures, Dr. Baselga asked reporters what they would recommend. In a statement several days later, he said he would correct his conflict-of-interest reporting for 17 articles, including in the New England Journal of Medicine, the Lancet, and the publication he edits, Cancer Discovery. He said that he did not believe disclosure was required for dozens of other articles detailing early stages of research.
“I have spent my career caring for cancer patients and bringing new therapies to the clinic with the goal of extending and saving lives,” Dr. Baselga said in the statement. “While I have been inconsistent with disclosures and acknowledge that fact, that is a far cry from compromising my responsibilities as a physician, as a scientist and as a clinical leader.”
The corporate imprint on cancer research
Dr. Baselga, 59, supervises clinical operations at Memorial Sloan Kettering, one of the nation’s top cancer centers and wields influence over the lives of patients and companies wishing to conduct trials there. He was paid more than $1.5 million in compensation by the cancer center in 2016, according to the hospital’s latest available tax disclosures, but that does not include his consulting or board fees from outside companies.
Many top medical researchers have ties to the for-profit health care industry, and some overlap is seen as a good thing – after all, these are the companies charged with developing the drugs, medical devices and diagnostic tests of the future.
Dr. Baselga’s relationship to industry is extensive. In addition to sitting on the board of Bristol-Myers Squibb, he is a director of Varian Medical Systems, which sells radiation equipment and for whom Memorial Sloan Kettering is a client.
In all, Dr. Baselga has served on the boards of at least six companies since 2013, positions that have required him to assume a fiduciary responsibility to protect the interests of those companies, even as he oversees the cancer center’s medical operations.
The hospital and Dr. Baselga said steps had been taken to prevent him from having a say in any business between the cancer center and the companies on whose boards he sits.
The chief executive of Memorial Sloan Kettering, Craig B. Thompson, MD, settled lawsuits several years ago that were filed by the University of Pennsylvania, Philadelphia, and an affiliated research center. They contended that he hid research conducted while he was at Penn to start a new company, Agios Pharmaceuticals, and did not share the earnings. Dr. Thompson disputed the allegations. He now sits on the board of Merck, which manufactures Keytruda, a blockbuster cancer therapy.
Ms. Hickey said the cancer center cannot fulfill its charitable mission without working with industry. “We encourage collaboration and are proud that our work has led to the approval of novel, lifesaving cancer treatments for patients around the world,” she said.
Some disclosures are required; others aren’t
After the scandals a decade ago over lack of disclosure, the federal government began requiring drug and device manufacturers to publicly disclose payments to doctors in 2013.
From August 2013 through 2017, Dr. Baselga received nearly $3.5 million from nine companies, according to the federal Open Payments database, which compiles disclosures filed by drug and device companies.
Dr. Baselga has disclosed in other forums investments and advisory roles in biotech start-ups, but he declined to provide a tally of financial interests in those firms. Companies that have not received approval from the Food and Drug Administration for their products – projects still in the testing phases – do not have to report payments they make to doctors.
Serving on boards can be lucrative. In 2017, Dr. Baselga received $260,000 in cash and stock awards to sit on Varian’s board of directors, according to the company’s corporate filings.
ProPublica and the Times analyzed Dr. Baselga’s publications in medical journals since 2013, the year he joined Memorial Sloan Kettering. He failed to disclose any industry relationships in more than 100, or about 60% of the time, a figure that has increased with each passing year. Last year, he did not list any potential conflicts in 87% of the articles that he wrote or cowrote.
Dr. Baselga compiled a color-coded list of his articles and offered a different interpretation. Sixty-two of the papers for which he did not disclose any potential conflict represented “conceptual, basic laboratory or translational work,” and did not require one, he said. Questions could be raised about others, he said, but he added that most “had no clinical nor financial implications.” That left the 17 papers he plans to correct.
Early-stage research often carries financial weight because it helps companies decide whether to move ahead with a product. In about two-thirds of Dr. Baselga’s articles that lacked details of his industry ties, one or more of his coauthors listed theirs.
In 2015, Dr. Baselga published an article in the New England Journal about a Roche-sponsored trial of one of the company’s drugs, Zelboraf. Despite his financial ties to Roche, he declared that he had “nothing to disclose.” Fourteen of his coauthors reported ties to Roche.
Dr. Baselga defended the articles, saying that “these are high-quality manuscripts reporting on important clinical trials that led to a better understanding of cancer treatments.”
The guidelines enacted by most major medical journals and professional societies ask authors and presenters to list recent financial relationships that could pose a conflict.
But much of this reporting still relies on the honor system. A study in August in the journal JAMA Oncology found that one-third of authors in a sample of cancer trials did not report all payments from the studies’ sponsors.
“We don’t routinely check because we don’t have those kind of resources,” said Rita F. Redberg, MD, the editor of JAMA Internal Medicine, who has been critical of the influence of industry on medical practice. “We rely on trust and integrity. It’s kind of an assumed part of the professional relationship.”
Jennifer Zeis, a spokeswoman for the New England Journal of Medicine, said in an email that it had now asked Dr. Baselga to amend his disclosures. She said the journal planned to overhaul its tracking of industry relationships.
The AACR said it had begun an “extensive review” of the disclosure forms submitted by Dr. Baselga.
It said that it had never barred an author from publishing, and that “such an action would be necessary only in cases of egregious, consistent violations of the rules.”
Among the most prominent relationships that Dr. Baselga has often failed to disclose is with the Swiss pharmaceutical giant Roche and its United States subsidiary Genentech.
In June 2017, at the annual meeting of the ASCO in Chicago, Dr. Baselga spoke at a Roche-sponsored investor event about study results that the company had been counting on to persuade oncologists to move patients from Herceptin – which was facing competition from cheaper alternatives – to a combination treatment involving Herceptin and a newer, more expensive drug, Perjeta.
The results were so underwhelming that Roche’s stock fell 5 % on the news. One analyst described the results as a “lead balloon,” and an editorial in the New England Journal called it a “disappointment.”
Dr. Baselga, however, told analysts that critiques were “weird” and “strange.”
This June, at the same cancer conference, Dr. Baselga struck an upbeat note about the results of a Roche trial of the drug taselisib, saying in a blog post published on the cancer center website that the results were “incredibly exciting” while conceding the side effects from the drug were high.
That same day, Roche announced it was scrapping plans to develop the drug. The news was another disappointment involving the class of drugs called PI3K inhibitors, which is a major focus of Dr. Baselga’s current research.
In neither case did Dr. Baselga reveal that his ties to Roche and Genentech went beyond serving as a trial investigator. In 2014, Roche acquired Seragon, a cancer research company in which Dr. Baselga had an ownership stake, for $725 million. Dr. Baselga received more than $3 million in 2014 and 2015 for his stake in the company, according to the federal Open Payments database.
From 2013 to 2017, Roche also paid Dr. Baselga more than $50,000 in consulting fees, according to the database.
These details were not included in the conflict-of-interest statements that are required of all presenters at the ASCO conference, although he did disclose ownership interests and consulting relationships with several other companies in the prior two years.
ASCO said it would conduct an internal review of Dr. Baselga’s disclosures and would refer the findings to a panel.
Dr. Baselga said that he played no role in the Seragon acquisition and that he had cut ties with Roche since joining the board of a competitor, Bristol-Myers, in March. As for his presentations at the ASCO meetings in the last 2 years, he said he had also noted shortcomings in the studies.
The combination of Perjeta with Herceptin was later approved by the FDA for certain high-risk patients. As for taselisib, Dr. Baselga stands by his belief that the PI3K class of drugs will be an important target for fighting cancer.
Katie Thomas covers the pharmaceutical industry for the New York Times.
Alabama, Oregon, and pediatric asthma
according to estimates from the American Lung Association.
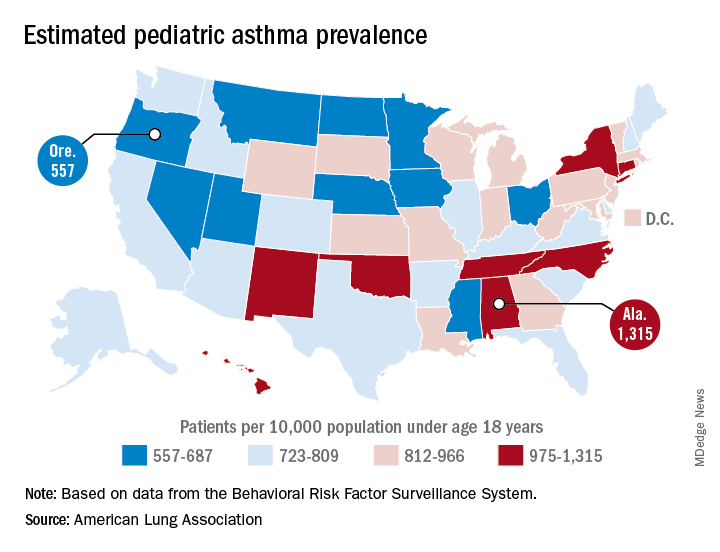
Oregon’s rate comes in at 557 per 10,000 population under the age of 18 years, just ahead of Montana at 574 per 10,000 and Iowa at 577. The prevalence of pediatric asthma in Alabama is 1,315 per 10,000, with North Carolina (1,149), Connecticut (1,107), Hawaii (1,026), and New York (1,005) joining it as members of the over-1,000 club. (MDedge News used the ALA’s estimates for persons under age 18 years with asthma in each state and Census Bureau estimates for population to calculate an unadjusted rate for each state.)
The ALA analysis was based on data from the Behavioral Risk Factor Behavioral System surveys for 2016 (31 states), 2015 (District of Columbia, Louisiana, New Hampshire, Texas), 2014 (Alabama, Maryland, North Carolina, Tennessee, West Virginia), 2012 (North Dakota and Wyoming), and 2011 (Iowa). National data were used for eight states (Alaska, Arkansas, Colorado, Delaware, Idaho, South Carolina, South Dakota, Virginia) that had no data available.
according to estimates from the American Lung Association.

Oregon’s rate comes in at 557 per 10,000 population under the age of 18 years, just ahead of Montana at 574 per 10,000 and Iowa at 577. The prevalence of pediatric asthma in Alabama is 1,315 per 10,000, with North Carolina (1,149), Connecticut (1,107), Hawaii (1,026), and New York (1,005) joining it as members of the over-1,000 club. (MDedge News used the ALA’s estimates for persons under age 18 years with asthma in each state and Census Bureau estimates for population to calculate an unadjusted rate for each state.)
The ALA analysis was based on data from the Behavioral Risk Factor Behavioral System surveys for 2016 (31 states), 2015 (District of Columbia, Louisiana, New Hampshire, Texas), 2014 (Alabama, Maryland, North Carolina, Tennessee, West Virginia), 2012 (North Dakota and Wyoming), and 2011 (Iowa). National data were used for eight states (Alaska, Arkansas, Colorado, Delaware, Idaho, South Carolina, South Dakota, Virginia) that had no data available.
according to estimates from the American Lung Association.

Oregon’s rate comes in at 557 per 10,000 population under the age of 18 years, just ahead of Montana at 574 per 10,000 and Iowa at 577. The prevalence of pediatric asthma in Alabama is 1,315 per 10,000, with North Carolina (1,149), Connecticut (1,107), Hawaii (1,026), and New York (1,005) joining it as members of the over-1,000 club. (MDedge News used the ALA’s estimates for persons under age 18 years with asthma in each state and Census Bureau estimates for population to calculate an unadjusted rate for each state.)
The ALA analysis was based on data from the Behavioral Risk Factor Behavioral System surveys for 2016 (31 states), 2015 (District of Columbia, Louisiana, New Hampshire, Texas), 2014 (Alabama, Maryland, North Carolina, Tennessee, West Virginia), 2012 (North Dakota and Wyoming), and 2011 (Iowa). National data were used for eight states (Alaska, Arkansas, Colorado, Delaware, Idaho, South Carolina, South Dakota, Virginia) that had no data available.
Plan to ‘Learn by Doing’ at the CHEST Annual Meeting 2018
Don’t miss the CHEST Annual Meeting 2018 in San Antonio, Oct 6-10. Watch as CHEST 2018 Program Chair David A. Schulman, MD, MPH, FCCP, walks you through the vision of this year’s meeting. View complete details at chestmeeting.chestnet.org.
Don’t miss the CHEST Annual Meeting 2018 in San Antonio, Oct 6-10. Watch as CHEST 2018 Program Chair David A. Schulman, MD, MPH, FCCP, walks you through the vision of this year’s meeting. View complete details at chestmeeting.chestnet.org.
Don’t miss the CHEST Annual Meeting 2018 in San Antonio, Oct 6-10. Watch as CHEST 2018 Program Chair David A. Schulman, MD, MPH, FCCP, walks you through the vision of this year’s meeting. View complete details at chestmeeting.chestnet.org.
Danish endocarditis strategy halved hospital days
MUNICH – Patients with left-sided endocarditis who are clinically stable after a couple weeks of inpatient intravenous antibiotics may at that point become candidates for discharge on oral antibiotics for the remainder of their treatment course, according to the findings of the randomized, multicenter, Danish POET trial.
“Shifting to oral antibiotic treatment in stabilized patients with endocarditis was as effective and safe as continued intravenous antibiotic treatment and was given during half the antibiotic treatment period. These novel findings may have a significant impact on future clinical practice for the management of patients who are stable,” Henning Bundgård, MD, said at the annual congress of the European Society of Cardiology.
Both ESC and American Heart Association/American College of Cardiology guidelines now recommend treatment of infective endocarditis with intravenous antibiotics for up to 6 weeks. Safely cutting the duration of in-hospital intravenous antibiotics in half is likely to generate major cost savings while improving patient quality of life and avoiding prolonged exposure to the iatrogenic risks inherent to the hospital environment, noted Dr. Bundgård, a cardiologist at Copenhagen University Hospital.
The rationale for the POET (Partial Oral Treatment of Endocarditis) trial was the investigators’ recognition that, even though infectious endocarditis is a feared disease with an in-hospital mortality of 15% or more, the great majority of serious complications occur in the early critical phase of therapy; that is, during the first 10 days or so of inpatient intravenous antibiotic therapy.
“After stabilization, the main reason for staying in the hospital is just to receive IV antibiotics,” Dr. Bundgård noted.
POET included 400 patients with left-sided endocarditis hospitalized at multiple cardiac centers across Denmark, 35% of whom had at least one major comorbid condition. When this reporter observed that this was the smallest study he’d ever seen reported from Denmark, where researchers famously like to utilize interconnected national databases to conduct nationwide observational studies incorporating the country’s entire population, the cardiologist replied, “Denmark is a small country, but we like to make big trials. And this is actually the largest-ever clinical trial in endocarditis, so we are still going big.”
Important to the generalizability of the POET results was the requirement that all 400 participants had to be infected with streptococcus, Staphylococcus aureus, Enterococcus faecalis, or coagulase-negative staphylococci – the major pathogens responsible for three-quarters of all cases of infectious endocarditis.
Once participants were clinically stable after a median of 17 days of intravenous antibiotics, they were randomized to continued in-hospital intravenous antibiotic therapy for a median of another 19 days or to discharge on two oral antibiotics from different classes with different mechanisms of action administered for a median of 17 days, with selection of the oral agents being guided by the results of bacterial susceptibility testing.
The primary outcome was a composite of all-cause mortality, embolic events, unplanned cardiac surgery, and relapse of bacteremia from randomization through 6 months after completion of antibiotic therapy. This occurred in 9.0% of the orally treated group and 12.1% of patients on full-course intravenous therapy for a 28% relative risk reduction, which statistically established the noninferioritiy of the partial oral regimen. The results were similar in patients with native as compared with prosthetic valves, with or without major comorbidities, and in surgically as opposed to conservatively treated patients.
Rates of three of the four components of the composite endpoint were similar in both groups. However, all-cause mortality occurred in 3.5% of the oral therapy group, compared with 6.5% of those on intravenous therapy. Dr. Bundgård said he and his coinvestigators think the disparity in mortality was probably caused by play of chance, although he added that they were struck that four sudden deaths occurred in the intravenous group and none in patients who got oral antibiotics.
Side effects were similarly mild and low frequency in both study arms.
Audience members were eager for details on how the Danish investigators decided patients were clinically stable on intravenous antibiotics and thus ready for randomization, as well as the outpatient follow-up procedures employed in those discharged on oral therapy.
Dr. Bundgård explained that clinical stability required that a patient be afebrile, have C-reactive protein and leukocyte levels less than 35% of their peaks, and needed to have been on intravenous antibiotics for a minimum of 10 days. Moreover, patients who underwent valve surgery during their hospitalization, as did 38% of POET participants, had to wait a minimum of 7 days afterwards before they could be declared clinically stable. Lastly, just prior to randomization all participants underwent transesophageal echocardiography to rule out abscess formation or other valve abnormalities requiring surgery.
Outpatient follow-up required that patients drop in two or three times per week to be checked by a familiar physician or nurse at the hospital ward where they had stayed. Compliance was very good, although it should be noted that only five patients in the POET study were intravenous drug abusers.
Asked why investigators discharged patients on oral therapy rather than on home intravenous antibiotics, Dr. Bundgård explained that home intravenous antibiotic therapy isn’t utilized in Denmark because of the expense and logistic complexity.
Discussant Chris P. Gale, MD, urged care in generalizing the study findings.
“The ‘O’ in POET does not stand for ‘outpatient.’ Outpatients were only selected for oral therapy if they had no heart failure, no emboli, no arrhythmia, no complicating comorbidities, and they were strictly monitored – and frequently. Should we elect to adopt POET into practice, I would recommend strict adherence to the study’s patient selection and monitoring criteria,” said Dr. Gale, a cardiologist at the University of Leeds (England).
The POET results clearly swayed the full-house audience attending the late-breaking Hot Line session in the conference main arena. Immediately before Dr. Bundgård’s presentation, 66% of the audience indicated electronically that they would continue intravenous antibiotics for another 2-4 weeks in a patient with infectious endocarditis who had responded well to 2 weeks of such therapy. After seeing the study results, however, only 19% would still follow that course of action, while 59% of the audience would switch to oral antibiotics and discharge the patient.
Dr. Bundgård reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Simultaneous with Dr. Bundgård’s presentation in Munich, the POET results were published online by the New England Journal of Medicine (2018 Aug 28. doi: 10.1056/NEJMoa1808312).
MUNICH – Patients with left-sided endocarditis who are clinically stable after a couple weeks of inpatient intravenous antibiotics may at that point become candidates for discharge on oral antibiotics for the remainder of their treatment course, according to the findings of the randomized, multicenter, Danish POET trial.
“Shifting to oral antibiotic treatment in stabilized patients with endocarditis was as effective and safe as continued intravenous antibiotic treatment and was given during half the antibiotic treatment period. These novel findings may have a significant impact on future clinical practice for the management of patients who are stable,” Henning Bundgård, MD, said at the annual congress of the European Society of Cardiology.
Both ESC and American Heart Association/American College of Cardiology guidelines now recommend treatment of infective endocarditis with intravenous antibiotics for up to 6 weeks. Safely cutting the duration of in-hospital intravenous antibiotics in half is likely to generate major cost savings while improving patient quality of life and avoiding prolonged exposure to the iatrogenic risks inherent to the hospital environment, noted Dr. Bundgård, a cardiologist at Copenhagen University Hospital.
The rationale for the POET (Partial Oral Treatment of Endocarditis) trial was the investigators’ recognition that, even though infectious endocarditis is a feared disease with an in-hospital mortality of 15% or more, the great majority of serious complications occur in the early critical phase of therapy; that is, during the first 10 days or so of inpatient intravenous antibiotic therapy.
“After stabilization, the main reason for staying in the hospital is just to receive IV antibiotics,” Dr. Bundgård noted.
POET included 400 patients with left-sided endocarditis hospitalized at multiple cardiac centers across Denmark, 35% of whom had at least one major comorbid condition. When this reporter observed that this was the smallest study he’d ever seen reported from Denmark, where researchers famously like to utilize interconnected national databases to conduct nationwide observational studies incorporating the country’s entire population, the cardiologist replied, “Denmark is a small country, but we like to make big trials. And this is actually the largest-ever clinical trial in endocarditis, so we are still going big.”
Important to the generalizability of the POET results was the requirement that all 400 participants had to be infected with streptococcus, Staphylococcus aureus, Enterococcus faecalis, or coagulase-negative staphylococci – the major pathogens responsible for three-quarters of all cases of infectious endocarditis.
Once participants were clinically stable after a median of 17 days of intravenous antibiotics, they were randomized to continued in-hospital intravenous antibiotic therapy for a median of another 19 days or to discharge on two oral antibiotics from different classes with different mechanisms of action administered for a median of 17 days, with selection of the oral agents being guided by the results of bacterial susceptibility testing.
The primary outcome was a composite of all-cause mortality, embolic events, unplanned cardiac surgery, and relapse of bacteremia from randomization through 6 months after completion of antibiotic therapy. This occurred in 9.0% of the orally treated group and 12.1% of patients on full-course intravenous therapy for a 28% relative risk reduction, which statistically established the noninferioritiy of the partial oral regimen. The results were similar in patients with native as compared with prosthetic valves, with or without major comorbidities, and in surgically as opposed to conservatively treated patients.
Rates of three of the four components of the composite endpoint were similar in both groups. However, all-cause mortality occurred in 3.5% of the oral therapy group, compared with 6.5% of those on intravenous therapy. Dr. Bundgård said he and his coinvestigators think the disparity in mortality was probably caused by play of chance, although he added that they were struck that four sudden deaths occurred in the intravenous group and none in patients who got oral antibiotics.
Side effects were similarly mild and low frequency in both study arms.
Audience members were eager for details on how the Danish investigators decided patients were clinically stable on intravenous antibiotics and thus ready for randomization, as well as the outpatient follow-up procedures employed in those discharged on oral therapy.
Dr. Bundgård explained that clinical stability required that a patient be afebrile, have C-reactive protein and leukocyte levels less than 35% of their peaks, and needed to have been on intravenous antibiotics for a minimum of 10 days. Moreover, patients who underwent valve surgery during their hospitalization, as did 38% of POET participants, had to wait a minimum of 7 days afterwards before they could be declared clinically stable. Lastly, just prior to randomization all participants underwent transesophageal echocardiography to rule out abscess formation or other valve abnormalities requiring surgery.
Outpatient follow-up required that patients drop in two or three times per week to be checked by a familiar physician or nurse at the hospital ward where they had stayed. Compliance was very good, although it should be noted that only five patients in the POET study were intravenous drug abusers.
Asked why investigators discharged patients on oral therapy rather than on home intravenous antibiotics, Dr. Bundgård explained that home intravenous antibiotic therapy isn’t utilized in Denmark because of the expense and logistic complexity.
Discussant Chris P. Gale, MD, urged care in generalizing the study findings.
“The ‘O’ in POET does not stand for ‘outpatient.’ Outpatients were only selected for oral therapy if they had no heart failure, no emboli, no arrhythmia, no complicating comorbidities, and they were strictly monitored – and frequently. Should we elect to adopt POET into practice, I would recommend strict adherence to the study’s patient selection and monitoring criteria,” said Dr. Gale, a cardiologist at the University of Leeds (England).
The POET results clearly swayed the full-house audience attending the late-breaking Hot Line session in the conference main arena. Immediately before Dr. Bundgård’s presentation, 66% of the audience indicated electronically that they would continue intravenous antibiotics for another 2-4 weeks in a patient with infectious endocarditis who had responded well to 2 weeks of such therapy. After seeing the study results, however, only 19% would still follow that course of action, while 59% of the audience would switch to oral antibiotics and discharge the patient.
Dr. Bundgård reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Simultaneous with Dr. Bundgård’s presentation in Munich, the POET results were published online by the New England Journal of Medicine (2018 Aug 28. doi: 10.1056/NEJMoa1808312).
MUNICH – Patients with left-sided endocarditis who are clinically stable after a couple weeks of inpatient intravenous antibiotics may at that point become candidates for discharge on oral antibiotics for the remainder of their treatment course, according to the findings of the randomized, multicenter, Danish POET trial.
“Shifting to oral antibiotic treatment in stabilized patients with endocarditis was as effective and safe as continued intravenous antibiotic treatment and was given during half the antibiotic treatment period. These novel findings may have a significant impact on future clinical practice for the management of patients who are stable,” Henning Bundgård, MD, said at the annual congress of the European Society of Cardiology.
Both ESC and American Heart Association/American College of Cardiology guidelines now recommend treatment of infective endocarditis with intravenous antibiotics for up to 6 weeks. Safely cutting the duration of in-hospital intravenous antibiotics in half is likely to generate major cost savings while improving patient quality of life and avoiding prolonged exposure to the iatrogenic risks inherent to the hospital environment, noted Dr. Bundgård, a cardiologist at Copenhagen University Hospital.
The rationale for the POET (Partial Oral Treatment of Endocarditis) trial was the investigators’ recognition that, even though infectious endocarditis is a feared disease with an in-hospital mortality of 15% or more, the great majority of serious complications occur in the early critical phase of therapy; that is, during the first 10 days or so of inpatient intravenous antibiotic therapy.
“After stabilization, the main reason for staying in the hospital is just to receive IV antibiotics,” Dr. Bundgård noted.
POET included 400 patients with left-sided endocarditis hospitalized at multiple cardiac centers across Denmark, 35% of whom had at least one major comorbid condition. When this reporter observed that this was the smallest study he’d ever seen reported from Denmark, where researchers famously like to utilize interconnected national databases to conduct nationwide observational studies incorporating the country’s entire population, the cardiologist replied, “Denmark is a small country, but we like to make big trials. And this is actually the largest-ever clinical trial in endocarditis, so we are still going big.”
Important to the generalizability of the POET results was the requirement that all 400 participants had to be infected with streptococcus, Staphylococcus aureus, Enterococcus faecalis, or coagulase-negative staphylococci – the major pathogens responsible for three-quarters of all cases of infectious endocarditis.
Once participants were clinically stable after a median of 17 days of intravenous antibiotics, they were randomized to continued in-hospital intravenous antibiotic therapy for a median of another 19 days or to discharge on two oral antibiotics from different classes with different mechanisms of action administered for a median of 17 days, with selection of the oral agents being guided by the results of bacterial susceptibility testing.
The primary outcome was a composite of all-cause mortality, embolic events, unplanned cardiac surgery, and relapse of bacteremia from randomization through 6 months after completion of antibiotic therapy. This occurred in 9.0% of the orally treated group and 12.1% of patients on full-course intravenous therapy for a 28% relative risk reduction, which statistically established the noninferioritiy of the partial oral regimen. The results were similar in patients with native as compared with prosthetic valves, with or without major comorbidities, and in surgically as opposed to conservatively treated patients.
Rates of three of the four components of the composite endpoint were similar in both groups. However, all-cause mortality occurred in 3.5% of the oral therapy group, compared with 6.5% of those on intravenous therapy. Dr. Bundgård said he and his coinvestigators think the disparity in mortality was probably caused by play of chance, although he added that they were struck that four sudden deaths occurred in the intravenous group and none in patients who got oral antibiotics.
Side effects were similarly mild and low frequency in both study arms.
Audience members were eager for details on how the Danish investigators decided patients were clinically stable on intravenous antibiotics and thus ready for randomization, as well as the outpatient follow-up procedures employed in those discharged on oral therapy.
Dr. Bundgård explained that clinical stability required that a patient be afebrile, have C-reactive protein and leukocyte levels less than 35% of their peaks, and needed to have been on intravenous antibiotics for a minimum of 10 days. Moreover, patients who underwent valve surgery during their hospitalization, as did 38% of POET participants, had to wait a minimum of 7 days afterwards before they could be declared clinically stable. Lastly, just prior to randomization all participants underwent transesophageal echocardiography to rule out abscess formation or other valve abnormalities requiring surgery.
Outpatient follow-up required that patients drop in two or three times per week to be checked by a familiar physician or nurse at the hospital ward where they had stayed. Compliance was very good, although it should be noted that only five patients in the POET study were intravenous drug abusers.
Asked why investigators discharged patients on oral therapy rather than on home intravenous antibiotics, Dr. Bundgård explained that home intravenous antibiotic therapy isn’t utilized in Denmark because of the expense and logistic complexity.
Discussant Chris P. Gale, MD, urged care in generalizing the study findings.
“The ‘O’ in POET does not stand for ‘outpatient.’ Outpatients were only selected for oral therapy if they had no heart failure, no emboli, no arrhythmia, no complicating comorbidities, and they were strictly monitored – and frequently. Should we elect to adopt POET into practice, I would recommend strict adherence to the study’s patient selection and monitoring criteria,” said Dr. Gale, a cardiologist at the University of Leeds (England).
The POET results clearly swayed the full-house audience attending the late-breaking Hot Line session in the conference main arena. Immediately before Dr. Bundgård’s presentation, 66% of the audience indicated electronically that they would continue intravenous antibiotics for another 2-4 weeks in a patient with infectious endocarditis who had responded well to 2 weeks of such therapy. After seeing the study results, however, only 19% would still follow that course of action, while 59% of the audience would switch to oral antibiotics and discharge the patient.
Dr. Bundgård reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Simultaneous with Dr. Bundgård’s presentation in Munich, the POET results were published online by the New England Journal of Medicine (2018 Aug 28. doi: 10.1056/NEJMoa1808312).
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Clinically stable patients with left-sided infectious endocarditis can safely and effectively be discharged on oral antibiotics after completing half of a full course of intravenous antibiotics.
Major finding: Key 6-month outcomes were similar in patients with left-sided infectious endocarditis regardless of whether they were discharged early on carefully selected oral antibiotics or remained in hospital to complete a full course of intravenous antibiotics.
Study details: This prospective, multicenter, Danish randomized trial included 400 patients with left-sided infectious endocarditis.
Disclosures: The presenter reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
When Rodents Attack: A Review of Rabies and Post-Exposure Prophylaxis
In this case report of a 65-year-old woman who presented to the ED for evaluation of an animal bite, the authors review the literature about the treatment of rabies with post-exposure prophylaxis.
Case
A 65-year-old woman presented to the ED with a chief complaint of an animal bite, in Southwest Ohio. The patient reported picking up what she thought to be an injured bird, which in fact turned out to be a chipmunk. When she handled the creature, it jumped up and bit her on the right arm, latching on through the sleeve of her sweater. Her husband struck the chipmunk with a shovel, thus terminating the threat. The patient was brought to the ED by her husband for evaluation, along with the lifeless animal which had been placed in a plastic bag. On examination, the patient had a small superficial abrasion to her right forearm with shallow skin puncture. This was treated with local wound care, including copious irrigation with normal saline and chlorhexidine. Given that she sustained an animal bite wound, she was treated empirically with amoxicillin/clavulanic acid (Augmentin) orally. Her tetanus vaccine was up to date. The critically important question regarding management of the patient was whether she should be treated with post-exposure prophylaxis for rabies after being bitten by the rodent.
Discussion
According to the Centers for Disease Control and Prevention (CDC) website regarding the indications for rabies post-exposure prophylaxis, it was found that small rodents, including chipmunks, have not been known to transmit rabies to humans. While these small animals are rarely infected with rabies, there have been reports of squirrels infected with rabies in the United States.1,2 Therefore, it is recommended that in all cases of rodent bites, the state or local health department should be contacted prior to making a decision regarding post-exposure prophylaxis.1 The state health department was contacted and agreed that post-exposure prophylaxis was not indicated; however, they requested the animal be sent to them for testing, which was arranged. An in-depth literature review found a case report in India of a 7-year-old boy who contracted rabies after being bitten by a squirrel. Per this research, small rodents are rarely infected with rabies, with woodchucks being indicated as local vectors. This particular patient received a tetanus vaccine and wound care initially after the squirrel bite, but presented 2 months later with difficulty with oral feeding, fever, and cough. He succumbed to his illness just 4 hours after admission, and rabies was confirmed through a corneal impression smear.3 Birhane et al4 reported three human deaths in the United States from rabies during 2015. The deaths included one due to a rabid dog bite while abroad, one due to contact with a bat, and one from a mongoose bite. None of these patients had been treated with post-exposure prophylaxis.4
Rabies is an RNA virus in the genus Lyssavirus. Once contracted, the rabies virus initially binds to the nicotinic acetylcholine receptor in muscle, replicates, and ascends along the nerves until it reaches the central nervous system (CNS), then propagates outward via the peripheral nerves. Replication in the CNS occurs in the Negri bodies, which are highly specific for rabies. There are two forms of rabies, a paralytic form and an encephalitic form, with the encephalitic form occurring far more commonly. The encephalitic form presents with hallucinations, and disorientation intermixed with lucid intervals. The paralytic form presents with weakness in the affected limb(s), progressing to quadriparesis and facial weakness, eventually leading to organ failure. The classic hydrophobia that is thought of in connection with rabies is present in only 50% of cases.5Transmission occurs from an infected host via bite most commonly, but can occur through exposure to mucous membranes, aerosol transmission, or exposure in a laboratory setting. The rabies virus can be “shed in the saliva concomitantly with, before or after the development of clinical signs.”6 Lyssaviruses, such as the rabies virus, do not persist in the environment. Once outside the host, the virus is rapidly inactivated, therefore, fomites do not play a role in transmission.6 Rabies hosts vary significantly, and the virus has been found in almost all mammalian orders and on all continents except for Antarctica. The primary reservoir for rabies is the bat, followed by dogs; however, cats, foxes, coyotes, jackals, wolves, mongoose, and raccoons are all vectors of rabies. Animals infected with rabies typically show signs of CNS disturbance. According to Rupprecht,2 “the most reliable signs, regardless of species, are acute behavioral changes and unexplained progressive paralysis.” Notably, wild animals infected with rabies “may lose their fear of people, and nocturnal species may be seen wandering about during the daytime.”2According to Rupprecht et al,6 “Rodents and lagomorphs, although used heavily as laboratory models, are not important in the epidemiology of the disease [rabies], except in the public-health resources devoted to consultation or prophylaxis after routine contact with these ubiquitous small mammals.”
Post-exposure prophylaxis is indicated when someone has been in a room with a bat, even if direct contact with the animal is uncertain. Examples of this would include “a sleeping person [who] awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person.”7 Following a bite requiring post-exposure prophylaxis treatment, it is pertinent to note if the patient has had a previous immunization. Regardless of immunization status, all bite areas must be thoroughly cleansed and irrigated. The CDC recommends using a virucidal agent, such as a povidine-iodine solution, in the cleansing process. If an individual has been previously immunized to rabies, then the rabies immunoglobulin (RIG) should not be administered; rather, the patient should be given the rabies vaccine, such as the human diploid cell culture rabies vaccine (HDCV) or the purified chick embryo cell vaccine (PCECV). The dose is 1 mL intramuscularly on day 0 and day 3. If a patient has not had either pre-exposure or prior post-exposure vaccinations, then RIG is also indicated. The full dose of RIG should be given at the site of the bite; however, if this is not feasible due to the location of the wound, then any remainder should be given at a site distant from the vaccine. The rabies vaccine (HDCV or PCECV) should be administered intramuscularly on days 0, 3, 7, and 14. A fifth dose may be considered on day 28 for immunocompromised patients. In adults, the vaccine should be given in the deltoid region, whereas in children it can also be given in the anterolateral aspect of the thigh. It should never be administered in the gluteal region because it may result in lower antibody titers.8 It is extremely important to administer the RIG in unvaccinated persons. A case report was reviewed from India in which a 45-year-old woman presented with fever, headache, dizziness, and hearing loss 1 month after being bitten by a mongoose on her right leg. She was given 4 doses of a rabies vaccine on days 0, 3, 7, and 28 but was not given RIG. Rabies virus neutralizing antibody titers in the cerebral spinal fluid were initially 2,048 IU/mL and increased after 2 weeks to greater than 16,384 IU/mL confirming the diagnosis of rabies encephalitis. The patient died 1 month after admission.9 The incubation period for rabies is 1 to 3 months in general, but a range from days to years has been reported.6 Post-exposure prophylaxis should typically be initiated as soon as possible after a bite; however, it may be delayed up to 10 days after exposure if the animal has been captured and can be monitored for signs of rabies or euthanized and tested. It is recommended that anyone who presents for evaluation after possible exposure, regardless of timeline, should be treated as if the contact had just occurred.10
Case Conclusion
In this case of the chipmunk bite, in accordance with the state health department, rabies prophylaxis was not indicated. It was recommended that the chipmunk be sent off for a necropsy (noting to leave the chipmunk intact and not to behead it). Following shared decision making with the patient and her husband, the chipmunk was sent off for testing with the results to be sent to the patient. She was discharged from the ED with Augmentin, but without rabies post-exposure prophylaxis. According to review of outpatient medical records, the patient was doing well at primary care appointments after the injury.
1. Centers for Disease Control and Prevention. Rabies: Other Wild Animals. https://www.cdc.gov/rabies/exposure/animals/other.html. Published April 29, 2016. Accessed July 20, 2017.
2. Rupprecht CERE. Overview of Rabies – Nervous System. Merck Veterinary Manual. https://www.merckvetmanual.com/nervous-system/rabies/overview-of-rabies. Accessed June 15, 2018.
3. Kumari PL, Mohanan KR, Kailas L, Chacko KP. A case or rabies after squirrel bite. Indian J Pediatr. 2014;81(2):198. doi:10.1007/s12098-013-0990-2.
4. Birhane MG, Cleaton JM, Monroe BP, et al. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250(10):1117-1130. doi:10.2460/javma.250.10.1117.
5. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016.
6. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327-343.
7. Centers for Disease Control and Prevention. Rabies: Bats. https://www.cdc.gov/rabies/exposure/animals/bats.html. Published July 5, 2017. Accessed June 15, 2018.
8. Centers for Disease Control and Prevention. Rabies: Rabies Vaccine. https://www.cdc.gov/rabies/medical_care/vaccine.html. Published September 24, 2014. Accessed July 20, 2017.
9. Mani RS, Moorkoth AP, Balasubramanian P, Devi KL, Madhusudana SN. Rabies following mongoose bite. Indian J Med Microbiol. 2016;34(2):256-257. doi:10.4103/0255-0857.176848.
10. Petersen B. Rabies: What’s an Exposure? Know When to Vaccinate. Medscape. https://www.medscape.com/viewarticle/877636. Published April 03, 2017. Accessed September 3, 2018.
In this case report of a 65-year-old woman who presented to the ED for evaluation of an animal bite, the authors review the literature about the treatment of rabies with post-exposure prophylaxis.
In this case report of a 65-year-old woman who presented to the ED for evaluation of an animal bite, the authors review the literature about the treatment of rabies with post-exposure prophylaxis.
Case
A 65-year-old woman presented to the ED with a chief complaint of an animal bite, in Southwest Ohio. The patient reported picking up what she thought to be an injured bird, which in fact turned out to be a chipmunk. When she handled the creature, it jumped up and bit her on the right arm, latching on through the sleeve of her sweater. Her husband struck the chipmunk with a shovel, thus terminating the threat. The patient was brought to the ED by her husband for evaluation, along with the lifeless animal which had been placed in a plastic bag. On examination, the patient had a small superficial abrasion to her right forearm with shallow skin puncture. This was treated with local wound care, including copious irrigation with normal saline and chlorhexidine. Given that she sustained an animal bite wound, she was treated empirically with amoxicillin/clavulanic acid (Augmentin) orally. Her tetanus vaccine was up to date. The critically important question regarding management of the patient was whether she should be treated with post-exposure prophylaxis for rabies after being bitten by the rodent.
Discussion
According to the Centers for Disease Control and Prevention (CDC) website regarding the indications for rabies post-exposure prophylaxis, it was found that small rodents, including chipmunks, have not been known to transmit rabies to humans. While these small animals are rarely infected with rabies, there have been reports of squirrels infected with rabies in the United States.1,2 Therefore, it is recommended that in all cases of rodent bites, the state or local health department should be contacted prior to making a decision regarding post-exposure prophylaxis.1 The state health department was contacted and agreed that post-exposure prophylaxis was not indicated; however, they requested the animal be sent to them for testing, which was arranged. An in-depth literature review found a case report in India of a 7-year-old boy who contracted rabies after being bitten by a squirrel. Per this research, small rodents are rarely infected with rabies, with woodchucks being indicated as local vectors. This particular patient received a tetanus vaccine and wound care initially after the squirrel bite, but presented 2 months later with difficulty with oral feeding, fever, and cough. He succumbed to his illness just 4 hours after admission, and rabies was confirmed through a corneal impression smear.3 Birhane et al4 reported three human deaths in the United States from rabies during 2015. The deaths included one due to a rabid dog bite while abroad, one due to contact with a bat, and one from a mongoose bite. None of these patients had been treated with post-exposure prophylaxis.4
Rabies is an RNA virus in the genus Lyssavirus. Once contracted, the rabies virus initially binds to the nicotinic acetylcholine receptor in muscle, replicates, and ascends along the nerves until it reaches the central nervous system (CNS), then propagates outward via the peripheral nerves. Replication in the CNS occurs in the Negri bodies, which are highly specific for rabies. There are two forms of rabies, a paralytic form and an encephalitic form, with the encephalitic form occurring far more commonly. The encephalitic form presents with hallucinations, and disorientation intermixed with lucid intervals. The paralytic form presents with weakness in the affected limb(s), progressing to quadriparesis and facial weakness, eventually leading to organ failure. The classic hydrophobia that is thought of in connection with rabies is present in only 50% of cases.5Transmission occurs from an infected host via bite most commonly, but can occur through exposure to mucous membranes, aerosol transmission, or exposure in a laboratory setting. The rabies virus can be “shed in the saliva concomitantly with, before or after the development of clinical signs.”6 Lyssaviruses, such as the rabies virus, do not persist in the environment. Once outside the host, the virus is rapidly inactivated, therefore, fomites do not play a role in transmission.6 Rabies hosts vary significantly, and the virus has been found in almost all mammalian orders and on all continents except for Antarctica. The primary reservoir for rabies is the bat, followed by dogs; however, cats, foxes, coyotes, jackals, wolves, mongoose, and raccoons are all vectors of rabies. Animals infected with rabies typically show signs of CNS disturbance. According to Rupprecht,2 “the most reliable signs, regardless of species, are acute behavioral changes and unexplained progressive paralysis.” Notably, wild animals infected with rabies “may lose their fear of people, and nocturnal species may be seen wandering about during the daytime.”2According to Rupprecht et al,6 “Rodents and lagomorphs, although used heavily as laboratory models, are not important in the epidemiology of the disease [rabies], except in the public-health resources devoted to consultation or prophylaxis after routine contact with these ubiquitous small mammals.”
Post-exposure prophylaxis is indicated when someone has been in a room with a bat, even if direct contact with the animal is uncertain. Examples of this would include “a sleeping person [who] awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person.”7 Following a bite requiring post-exposure prophylaxis treatment, it is pertinent to note if the patient has had a previous immunization. Regardless of immunization status, all bite areas must be thoroughly cleansed and irrigated. The CDC recommends using a virucidal agent, such as a povidine-iodine solution, in the cleansing process. If an individual has been previously immunized to rabies, then the rabies immunoglobulin (RIG) should not be administered; rather, the patient should be given the rabies vaccine, such as the human diploid cell culture rabies vaccine (HDCV) or the purified chick embryo cell vaccine (PCECV). The dose is 1 mL intramuscularly on day 0 and day 3. If a patient has not had either pre-exposure or prior post-exposure vaccinations, then RIG is also indicated. The full dose of RIG should be given at the site of the bite; however, if this is not feasible due to the location of the wound, then any remainder should be given at a site distant from the vaccine. The rabies vaccine (HDCV or PCECV) should be administered intramuscularly on days 0, 3, 7, and 14. A fifth dose may be considered on day 28 for immunocompromised patients. In adults, the vaccine should be given in the deltoid region, whereas in children it can also be given in the anterolateral aspect of the thigh. It should never be administered in the gluteal region because it may result in lower antibody titers.8 It is extremely important to administer the RIG in unvaccinated persons. A case report was reviewed from India in which a 45-year-old woman presented with fever, headache, dizziness, and hearing loss 1 month after being bitten by a mongoose on her right leg. She was given 4 doses of a rabies vaccine on days 0, 3, 7, and 28 but was not given RIG. Rabies virus neutralizing antibody titers in the cerebral spinal fluid were initially 2,048 IU/mL and increased after 2 weeks to greater than 16,384 IU/mL confirming the diagnosis of rabies encephalitis. The patient died 1 month after admission.9 The incubation period for rabies is 1 to 3 months in general, but a range from days to years has been reported.6 Post-exposure prophylaxis should typically be initiated as soon as possible after a bite; however, it may be delayed up to 10 days after exposure if the animal has been captured and can be monitored for signs of rabies or euthanized and tested. It is recommended that anyone who presents for evaluation after possible exposure, regardless of timeline, should be treated as if the contact had just occurred.10
Case Conclusion
In this case of the chipmunk bite, in accordance with the state health department, rabies prophylaxis was not indicated. It was recommended that the chipmunk be sent off for a necropsy (noting to leave the chipmunk intact and not to behead it). Following shared decision making with the patient and her husband, the chipmunk was sent off for testing with the results to be sent to the patient. She was discharged from the ED with Augmentin, but without rabies post-exposure prophylaxis. According to review of outpatient medical records, the patient was doing well at primary care appointments after the injury.
Case
A 65-year-old woman presented to the ED with a chief complaint of an animal bite, in Southwest Ohio. The patient reported picking up what she thought to be an injured bird, which in fact turned out to be a chipmunk. When she handled the creature, it jumped up and bit her on the right arm, latching on through the sleeve of her sweater. Her husband struck the chipmunk with a shovel, thus terminating the threat. The patient was brought to the ED by her husband for evaluation, along with the lifeless animal which had been placed in a plastic bag. On examination, the patient had a small superficial abrasion to her right forearm with shallow skin puncture. This was treated with local wound care, including copious irrigation with normal saline and chlorhexidine. Given that she sustained an animal bite wound, she was treated empirically with amoxicillin/clavulanic acid (Augmentin) orally. Her tetanus vaccine was up to date. The critically important question regarding management of the patient was whether she should be treated with post-exposure prophylaxis for rabies after being bitten by the rodent.
Discussion
According to the Centers for Disease Control and Prevention (CDC) website regarding the indications for rabies post-exposure prophylaxis, it was found that small rodents, including chipmunks, have not been known to transmit rabies to humans. While these small animals are rarely infected with rabies, there have been reports of squirrels infected with rabies in the United States.1,2 Therefore, it is recommended that in all cases of rodent bites, the state or local health department should be contacted prior to making a decision regarding post-exposure prophylaxis.1 The state health department was contacted and agreed that post-exposure prophylaxis was not indicated; however, they requested the animal be sent to them for testing, which was arranged. An in-depth literature review found a case report in India of a 7-year-old boy who contracted rabies after being bitten by a squirrel. Per this research, small rodents are rarely infected with rabies, with woodchucks being indicated as local vectors. This particular patient received a tetanus vaccine and wound care initially after the squirrel bite, but presented 2 months later with difficulty with oral feeding, fever, and cough. He succumbed to his illness just 4 hours after admission, and rabies was confirmed through a corneal impression smear.3 Birhane et al4 reported three human deaths in the United States from rabies during 2015. The deaths included one due to a rabid dog bite while abroad, one due to contact with a bat, and one from a mongoose bite. None of these patients had been treated with post-exposure prophylaxis.4
Rabies is an RNA virus in the genus Lyssavirus. Once contracted, the rabies virus initially binds to the nicotinic acetylcholine receptor in muscle, replicates, and ascends along the nerves until it reaches the central nervous system (CNS), then propagates outward via the peripheral nerves. Replication in the CNS occurs in the Negri bodies, which are highly specific for rabies. There are two forms of rabies, a paralytic form and an encephalitic form, with the encephalitic form occurring far more commonly. The encephalitic form presents with hallucinations, and disorientation intermixed with lucid intervals. The paralytic form presents with weakness in the affected limb(s), progressing to quadriparesis and facial weakness, eventually leading to organ failure. The classic hydrophobia that is thought of in connection with rabies is present in only 50% of cases.5Transmission occurs from an infected host via bite most commonly, but can occur through exposure to mucous membranes, aerosol transmission, or exposure in a laboratory setting. The rabies virus can be “shed in the saliva concomitantly with, before or after the development of clinical signs.”6 Lyssaviruses, such as the rabies virus, do not persist in the environment. Once outside the host, the virus is rapidly inactivated, therefore, fomites do not play a role in transmission.6 Rabies hosts vary significantly, and the virus has been found in almost all mammalian orders and on all continents except for Antarctica. The primary reservoir for rabies is the bat, followed by dogs; however, cats, foxes, coyotes, jackals, wolves, mongoose, and raccoons are all vectors of rabies. Animals infected with rabies typically show signs of CNS disturbance. According to Rupprecht,2 “the most reliable signs, regardless of species, are acute behavioral changes and unexplained progressive paralysis.” Notably, wild animals infected with rabies “may lose their fear of people, and nocturnal species may be seen wandering about during the daytime.”2According to Rupprecht et al,6 “Rodents and lagomorphs, although used heavily as laboratory models, are not important in the epidemiology of the disease [rabies], except in the public-health resources devoted to consultation or prophylaxis after routine contact with these ubiquitous small mammals.”
Post-exposure prophylaxis is indicated when someone has been in a room with a bat, even if direct contact with the animal is uncertain. Examples of this would include “a sleeping person [who] awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person.”7 Following a bite requiring post-exposure prophylaxis treatment, it is pertinent to note if the patient has had a previous immunization. Regardless of immunization status, all bite areas must be thoroughly cleansed and irrigated. The CDC recommends using a virucidal agent, such as a povidine-iodine solution, in the cleansing process. If an individual has been previously immunized to rabies, then the rabies immunoglobulin (RIG) should not be administered; rather, the patient should be given the rabies vaccine, such as the human diploid cell culture rabies vaccine (HDCV) or the purified chick embryo cell vaccine (PCECV). The dose is 1 mL intramuscularly on day 0 and day 3. If a patient has not had either pre-exposure or prior post-exposure vaccinations, then RIG is also indicated. The full dose of RIG should be given at the site of the bite; however, if this is not feasible due to the location of the wound, then any remainder should be given at a site distant from the vaccine. The rabies vaccine (HDCV or PCECV) should be administered intramuscularly on days 0, 3, 7, and 14. A fifth dose may be considered on day 28 for immunocompromised patients. In adults, the vaccine should be given in the deltoid region, whereas in children it can also be given in the anterolateral aspect of the thigh. It should never be administered in the gluteal region because it may result in lower antibody titers.8 It is extremely important to administer the RIG in unvaccinated persons. A case report was reviewed from India in which a 45-year-old woman presented with fever, headache, dizziness, and hearing loss 1 month after being bitten by a mongoose on her right leg. She was given 4 doses of a rabies vaccine on days 0, 3, 7, and 28 but was not given RIG. Rabies virus neutralizing antibody titers in the cerebral spinal fluid were initially 2,048 IU/mL and increased after 2 weeks to greater than 16,384 IU/mL confirming the diagnosis of rabies encephalitis. The patient died 1 month after admission.9 The incubation period for rabies is 1 to 3 months in general, but a range from days to years has been reported.6 Post-exposure prophylaxis should typically be initiated as soon as possible after a bite; however, it may be delayed up to 10 days after exposure if the animal has been captured and can be monitored for signs of rabies or euthanized and tested. It is recommended that anyone who presents for evaluation after possible exposure, regardless of timeline, should be treated as if the contact had just occurred.10
Case Conclusion
In this case of the chipmunk bite, in accordance with the state health department, rabies prophylaxis was not indicated. It was recommended that the chipmunk be sent off for a necropsy (noting to leave the chipmunk intact and not to behead it). Following shared decision making with the patient and her husband, the chipmunk was sent off for testing with the results to be sent to the patient. She was discharged from the ED with Augmentin, but without rabies post-exposure prophylaxis. According to review of outpatient medical records, the patient was doing well at primary care appointments after the injury.
1. Centers for Disease Control and Prevention. Rabies: Other Wild Animals. https://www.cdc.gov/rabies/exposure/animals/other.html. Published April 29, 2016. Accessed July 20, 2017.
2. Rupprecht CERE. Overview of Rabies – Nervous System. Merck Veterinary Manual. https://www.merckvetmanual.com/nervous-system/rabies/overview-of-rabies. Accessed June 15, 2018.
3. Kumari PL, Mohanan KR, Kailas L, Chacko KP. A case or rabies after squirrel bite. Indian J Pediatr. 2014;81(2):198. doi:10.1007/s12098-013-0990-2.
4. Birhane MG, Cleaton JM, Monroe BP, et al. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250(10):1117-1130. doi:10.2460/javma.250.10.1117.
5. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016.
6. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327-343.
7. Centers for Disease Control and Prevention. Rabies: Bats. https://www.cdc.gov/rabies/exposure/animals/bats.html. Published July 5, 2017. Accessed June 15, 2018.
8. Centers for Disease Control and Prevention. Rabies: Rabies Vaccine. https://www.cdc.gov/rabies/medical_care/vaccine.html. Published September 24, 2014. Accessed July 20, 2017.
9. Mani RS, Moorkoth AP, Balasubramanian P, Devi KL, Madhusudana SN. Rabies following mongoose bite. Indian J Med Microbiol. 2016;34(2):256-257. doi:10.4103/0255-0857.176848.
10. Petersen B. Rabies: What’s an Exposure? Know When to Vaccinate. Medscape. https://www.medscape.com/viewarticle/877636. Published April 03, 2017. Accessed September 3, 2018.
1. Centers for Disease Control and Prevention. Rabies: Other Wild Animals. https://www.cdc.gov/rabies/exposure/animals/other.html. Published April 29, 2016. Accessed July 20, 2017.
2. Rupprecht CERE. Overview of Rabies – Nervous System. Merck Veterinary Manual. https://www.merckvetmanual.com/nervous-system/rabies/overview-of-rabies. Accessed June 15, 2018.
3. Kumari PL, Mohanan KR, Kailas L, Chacko KP. A case or rabies after squirrel bite. Indian J Pediatr. 2014;81(2):198. doi:10.1007/s12098-013-0990-2.
4. Birhane MG, Cleaton JM, Monroe BP, et al. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250(10):1117-1130. doi:10.2460/javma.250.10.1117.
5. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016.
6. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327-343.
7. Centers for Disease Control and Prevention. Rabies: Bats. https://www.cdc.gov/rabies/exposure/animals/bats.html. Published July 5, 2017. Accessed June 15, 2018.
8. Centers for Disease Control and Prevention. Rabies: Rabies Vaccine. https://www.cdc.gov/rabies/medical_care/vaccine.html. Published September 24, 2014. Accessed July 20, 2017.
9. Mani RS, Moorkoth AP, Balasubramanian P, Devi KL, Madhusudana SN. Rabies following mongoose bite. Indian J Med Microbiol. 2016;34(2):256-257. doi:10.4103/0255-0857.176848.
10. Petersen B. Rabies: What’s an Exposure? Know When to Vaccinate. Medscape. https://www.medscape.com/viewarticle/877636. Published April 03, 2017. Accessed September 3, 2018.
Tai chi tempers risk of falls in older adults
A tai chi exercise program was more effective than multimodal exercise or stretching at reducing falls among older adults in a 6-month study of 670 participants aged 70 years and older.
Although exercise can be a safe way to reduce falls in older adults, “few comparative effectiveness data are available, especially for older adults with high fall risk,” wrote Fuzhong Li, PhD, of the Oregon Research Institute in Eugene, and his colleagues.
In a study published in JAMA Internal Medicine, the researchers randomized participants to a tailored tai chi program, Tai Ji Quan: Moving for Better Balance (TJQMBB), multimodal exercise, or a control intervention of basic stretching. Each intervention consisted of a twice-weekly 60-minute session for 24 weeks.
The study population included 670 community-dwelling adults aged 70 years and older at seven urban and suburban cities in Oregon. All participants had fallen within the past year or had impaired mobility that put them at increased fall risk. The average age of the participants was 78 years, 65% were women, and 92% were white.
A total of 152 falls occurred among 85 individuals in the TJQMBB group during the study period, compared with 218 falls among 112 individuals in the multimodal exercise group and 363 falls among 127 individuals in the stretching group. Overall, the tai chi group had a 58% reduced incidence of falls, compared with stretching, and a 31% reduced risk, compared with multimodal exercise.
The tai chi program used in the study included balance and stability training, described by the researchers as “unilateral weight-bearing and weight-shifting movements, trunk and pelvic rotation, ankle sway, and eye-head-hand movements.” The multimodal exercise program included aerobic conditioning, strength, balance, and flexibility activities, and the stretching intervention included stretching, breathing, and relaxation.
The results were limited by several factors, including the use of self-reports, low representation of African American participants, and collection of data from a single state, the researchers noted.
However, the findings support the potential of tai chi programs to reduce the risk of falls in older adults. “With increasing evidence of community adoption and implementation and information from cost-benefit and cost-effectiveness analyses, the intervention program represents a promising approach to low-cost and easily implementable fall prevention programs,” the researchers explained.
The study was supported in part by the National Institute on Aging. Dr. Li reported that he is the founder and owner of a consulting company, Exercise Alternatives, and that a licensing free for TJQMBB is paid directly to that company.
SOURCE: Li F et al. JAMA Intern Med. 2018 Sep 10. doi: 10.1001/jamainternmed.2018.3915.
A tai chi exercise program was more effective than multimodal exercise or stretching at reducing falls among older adults in a 6-month study of 670 participants aged 70 years and older.
Although exercise can be a safe way to reduce falls in older adults, “few comparative effectiveness data are available, especially for older adults with high fall risk,” wrote Fuzhong Li, PhD, of the Oregon Research Institute in Eugene, and his colleagues.
In a study published in JAMA Internal Medicine, the researchers randomized participants to a tailored tai chi program, Tai Ji Quan: Moving for Better Balance (TJQMBB), multimodal exercise, or a control intervention of basic stretching. Each intervention consisted of a twice-weekly 60-minute session for 24 weeks.
The study population included 670 community-dwelling adults aged 70 years and older at seven urban and suburban cities in Oregon. All participants had fallen within the past year or had impaired mobility that put them at increased fall risk. The average age of the participants was 78 years, 65% were women, and 92% were white.
A total of 152 falls occurred among 85 individuals in the TJQMBB group during the study period, compared with 218 falls among 112 individuals in the multimodal exercise group and 363 falls among 127 individuals in the stretching group. Overall, the tai chi group had a 58% reduced incidence of falls, compared with stretching, and a 31% reduced risk, compared with multimodal exercise.
The tai chi program used in the study included balance and stability training, described by the researchers as “unilateral weight-bearing and weight-shifting movements, trunk and pelvic rotation, ankle sway, and eye-head-hand movements.” The multimodal exercise program included aerobic conditioning, strength, balance, and flexibility activities, and the stretching intervention included stretching, breathing, and relaxation.
The results were limited by several factors, including the use of self-reports, low representation of African American participants, and collection of data from a single state, the researchers noted.
However, the findings support the potential of tai chi programs to reduce the risk of falls in older adults. “With increasing evidence of community adoption and implementation and information from cost-benefit and cost-effectiveness analyses, the intervention program represents a promising approach to low-cost and easily implementable fall prevention programs,” the researchers explained.
The study was supported in part by the National Institute on Aging. Dr. Li reported that he is the founder and owner of a consulting company, Exercise Alternatives, and that a licensing free for TJQMBB is paid directly to that company.
SOURCE: Li F et al. JAMA Intern Med. 2018 Sep 10. doi: 10.1001/jamainternmed.2018.3915.
A tai chi exercise program was more effective than multimodal exercise or stretching at reducing falls among older adults in a 6-month study of 670 participants aged 70 years and older.
Although exercise can be a safe way to reduce falls in older adults, “few comparative effectiveness data are available, especially for older adults with high fall risk,” wrote Fuzhong Li, PhD, of the Oregon Research Institute in Eugene, and his colleagues.
In a study published in JAMA Internal Medicine, the researchers randomized participants to a tailored tai chi program, Tai Ji Quan: Moving for Better Balance (TJQMBB), multimodal exercise, or a control intervention of basic stretching. Each intervention consisted of a twice-weekly 60-minute session for 24 weeks.
The study population included 670 community-dwelling adults aged 70 years and older at seven urban and suburban cities in Oregon. All participants had fallen within the past year or had impaired mobility that put them at increased fall risk. The average age of the participants was 78 years, 65% were women, and 92% were white.
A total of 152 falls occurred among 85 individuals in the TJQMBB group during the study period, compared with 218 falls among 112 individuals in the multimodal exercise group and 363 falls among 127 individuals in the stretching group. Overall, the tai chi group had a 58% reduced incidence of falls, compared with stretching, and a 31% reduced risk, compared with multimodal exercise.
The tai chi program used in the study included balance and stability training, described by the researchers as “unilateral weight-bearing and weight-shifting movements, trunk and pelvic rotation, ankle sway, and eye-head-hand movements.” The multimodal exercise program included aerobic conditioning, strength, balance, and flexibility activities, and the stretching intervention included stretching, breathing, and relaxation.
The results were limited by several factors, including the use of self-reports, low representation of African American participants, and collection of data from a single state, the researchers noted.
However, the findings support the potential of tai chi programs to reduce the risk of falls in older adults. “With increasing evidence of community adoption and implementation and information from cost-benefit and cost-effectiveness analyses, the intervention program represents a promising approach to low-cost and easily implementable fall prevention programs,” the researchers explained.
The study was supported in part by the National Institute on Aging. Dr. Li reported that he is the founder and owner of a consulting company, Exercise Alternatives, and that a licensing free for TJQMBB is paid directly to that company.
SOURCE: Li F et al. JAMA Intern Med. 2018 Sep 10. doi: 10.1001/jamainternmed.2018.3915.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Tai chi reduced the incidence of falls in high-risk older adults more effectively than stretching or conventional exercise.
Major finding: Tai chi participants had a 58% reduced incidence of falls, compared with stretching, and a 31% reduced risk, compared with multimodal exercise.
Study details: The data came from a single-blind, randomized trial of 670 adults aged 70 years and older.
Disclosures: The study was supported in part by the National Institute on Aging. Dr. Li reported that he is the founder and owner of a consulting company, Exercise Alternatives, and that a licensing free for Tai Ji Quan: Moving for Better Balance is paid directly to that company.
Source: Li F et al. JAMA Intern Med. 2018 Sep 10. doi: 10.1001/jamainternmed.2018.3915.
Pertussis vaccine at birth shows immune response, tolerability
compared with a group receiving only the hepatitis B vaccine, a randomized clinical trial from Australia has found.
“These results indicate that a birth dose of aP vaccine is immunogenic in newborns and significantly narrows the immunity gap between birth and 14 days after receipt of DTaP at 6 or 8 weeks of age, marking the critical period when infants are most vulnerable to severe pertussis infection,” reported Nicholas Wood, PhD, of the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases in New South Wales, Australia, and his colleagues.
“Administration of the acellular pertussis vaccine at birth has the potential to reduce severe morbidity from Bordetella pertussis infection in the first 3 months of life, especially for infants of mothers who have not received a pertussis vaccine during pregnancy,” the researchers concluded in JAMA Pediatrics.
The researchers enrolled 417 infants from Sydney, Melbourne, Adelaide, and Perth between June 2010 and March 2013 and randomized them to receive either the hepatitis B vaccine alone (n = 205) or the hepatitis B vaccine with a monovalent acellular pertussis vaccine (n = 212) within the first 5 days after birth. The randomization was stratified for mothers’ receipt of the Tdap before pregnancy.
The Centers for Disease Control and Prevention currently recommends all newborns receive the hepatitis B vaccine shortly after birth and that pregnant women receive the Tdap vaccine during each pregnancy. There is not currently a monovalent acellular pertussis vaccine licensed in the United States.
The study infants then received the hexavalent DTaP-Hib-hep B-polio vaccine and the 10-valent pneumococcal conjugate vaccine at 6 weeks, 4 months, and 6 months.
The primary outcome was detectable levels of IgG antibody to pertussis toxin and pertactin at 10 weeks old.
Of the 206 infants receiving the pertussis vaccine at birth, 93% had detectable antibodies to pertussis toxin and pertactin at 10 weeks, compared with 51% of the 193 infants who received only the hepatitis B shot (P less than .001). Geometric mean concentration for pertussis toxin IgG also was four times higher in infants who received the pertussis vaccine at birth.
Adverse events were similar in the two groups both at birth and at 32 weeks, demonstrating that the pertussis birth dose is safe and tolerable.
“More important, in this study, the prevalence of fever after receipt of the birth dose, which can mistakenly be associated with potential sepsis and result in additional investigations in the neonatal period, was similar in both the group that received the aP vaccine at birth and the control group,” the authors reported.
A remaining question is the potential impact of maternal antibodies on protection from pertussis.
“The presence of maternal pertussis antibodies at birth can negatively affect postprimary responses to pertussis, diphtheria, and diphtheria-related CRM197 conjugate vaccines with a variety of infant immunization schedules and vaccines,” the authors noted. “The clinical significance of reductions in pertussis antibody related to maternal interference will require ongoing clinical evaluation, because there are no accepted serologic correlates of protection.”
The research was funded by a Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reported having no conflicts of interest.
SOURCE: Wood N et al, JAMA Pediatr. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2349.
Pertussis is most likely to cause morbidity or kill neonates between birth and when they are given their first pertussis vaccine at 6-8 weeks of age. This is well known.
In the current study giving the acellular pertussis (aP) vaccine at birth led to “significantly higher antibody titers to pertussis antigens at 10 weeks of age,” compared with those who did not receive it. Those infants who received the birth dose of aP vaccine also had higher pertussis antibodies at 6 weeks, whether or not their mothers had received Tdap within 5 years prior to delivery.
When this study began in 2009, maternal immunization was not a well accepted concept, but this attitude has changed, in part due to the safe vaccination of pregnant women with the pandemic flu vaccine. Despite this, Centers for Disease Control and Prevention 2016 data showed that only 49% of pregnant women in the United Stated received Tdap. These rates need to increase.
Administering the aP vaccine with the existing hepatitis B vaccine at birth to infants whose mothers who did not receive Tdap during pregnancy would be a practical solution, if the aP vaccine were universally available.
But the aP vaccine currently is not available in the United States and many other countries as a standalone vaccine, and the administration of DTaP as a birth dose has been linked with “significant immune interference.” The aP vaccine could have a place in countries where it is available, and there is no maternal immunization program. Otherwise, boosting maternal immunization appears to be the primary approach for now.
Kathryn M. Edwards, MD, is the Sarah H. Sell and Cornelius Vanderbilt Chair in Pediatrics at Vanderbilt University, Nashville. She specializes in pediatric infectious diseases. These comments are a summary of her editorial accompanying the article by Wood et al. (Pediatrics. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2363). Dr. Edwards said she had no conflicts of interest.
Pertussis is most likely to cause morbidity or kill neonates between birth and when they are given their first pertussis vaccine at 6-8 weeks of age. This is well known.
In the current study giving the acellular pertussis (aP) vaccine at birth led to “significantly higher antibody titers to pertussis antigens at 10 weeks of age,” compared with those who did not receive it. Those infants who received the birth dose of aP vaccine also had higher pertussis antibodies at 6 weeks, whether or not their mothers had received Tdap within 5 years prior to delivery.
When this study began in 2009, maternal immunization was not a well accepted concept, but this attitude has changed, in part due to the safe vaccination of pregnant women with the pandemic flu vaccine. Despite this, Centers for Disease Control and Prevention 2016 data showed that only 49% of pregnant women in the United Stated received Tdap. These rates need to increase.
Administering the aP vaccine with the existing hepatitis B vaccine at birth to infants whose mothers who did not receive Tdap during pregnancy would be a practical solution, if the aP vaccine were universally available.
But the aP vaccine currently is not available in the United States and many other countries as a standalone vaccine, and the administration of DTaP as a birth dose has been linked with “significant immune interference.” The aP vaccine could have a place in countries where it is available, and there is no maternal immunization program. Otherwise, boosting maternal immunization appears to be the primary approach for now.
Kathryn M. Edwards, MD, is the Sarah H. Sell and Cornelius Vanderbilt Chair in Pediatrics at Vanderbilt University, Nashville. She specializes in pediatric infectious diseases. These comments are a summary of her editorial accompanying the article by Wood et al. (Pediatrics. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2363). Dr. Edwards said she had no conflicts of interest.
Pertussis is most likely to cause morbidity or kill neonates between birth and when they are given their first pertussis vaccine at 6-8 weeks of age. This is well known.
In the current study giving the acellular pertussis (aP) vaccine at birth led to “significantly higher antibody titers to pertussis antigens at 10 weeks of age,” compared with those who did not receive it. Those infants who received the birth dose of aP vaccine also had higher pertussis antibodies at 6 weeks, whether or not their mothers had received Tdap within 5 years prior to delivery.
When this study began in 2009, maternal immunization was not a well accepted concept, but this attitude has changed, in part due to the safe vaccination of pregnant women with the pandemic flu vaccine. Despite this, Centers for Disease Control and Prevention 2016 data showed that only 49% of pregnant women in the United Stated received Tdap. These rates need to increase.
Administering the aP vaccine with the existing hepatitis B vaccine at birth to infants whose mothers who did not receive Tdap during pregnancy would be a practical solution, if the aP vaccine were universally available.
But the aP vaccine currently is not available in the United States and many other countries as a standalone vaccine, and the administration of DTaP as a birth dose has been linked with “significant immune interference.” The aP vaccine could have a place in countries where it is available, and there is no maternal immunization program. Otherwise, boosting maternal immunization appears to be the primary approach for now.
Kathryn M. Edwards, MD, is the Sarah H. Sell and Cornelius Vanderbilt Chair in Pediatrics at Vanderbilt University, Nashville. She specializes in pediatric infectious diseases. These comments are a summary of her editorial accompanying the article by Wood et al. (Pediatrics. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2363). Dr. Edwards said she had no conflicts of interest.
compared with a group receiving only the hepatitis B vaccine, a randomized clinical trial from Australia has found.
“These results indicate that a birth dose of aP vaccine is immunogenic in newborns and significantly narrows the immunity gap between birth and 14 days after receipt of DTaP at 6 or 8 weeks of age, marking the critical period when infants are most vulnerable to severe pertussis infection,” reported Nicholas Wood, PhD, of the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases in New South Wales, Australia, and his colleagues.
“Administration of the acellular pertussis vaccine at birth has the potential to reduce severe morbidity from Bordetella pertussis infection in the first 3 months of life, especially for infants of mothers who have not received a pertussis vaccine during pregnancy,” the researchers concluded in JAMA Pediatrics.
The researchers enrolled 417 infants from Sydney, Melbourne, Adelaide, and Perth between June 2010 and March 2013 and randomized them to receive either the hepatitis B vaccine alone (n = 205) or the hepatitis B vaccine with a monovalent acellular pertussis vaccine (n = 212) within the first 5 days after birth. The randomization was stratified for mothers’ receipt of the Tdap before pregnancy.
The Centers for Disease Control and Prevention currently recommends all newborns receive the hepatitis B vaccine shortly after birth and that pregnant women receive the Tdap vaccine during each pregnancy. There is not currently a monovalent acellular pertussis vaccine licensed in the United States.
The study infants then received the hexavalent DTaP-Hib-hep B-polio vaccine and the 10-valent pneumococcal conjugate vaccine at 6 weeks, 4 months, and 6 months.
The primary outcome was detectable levels of IgG antibody to pertussis toxin and pertactin at 10 weeks old.
Of the 206 infants receiving the pertussis vaccine at birth, 93% had detectable antibodies to pertussis toxin and pertactin at 10 weeks, compared with 51% of the 193 infants who received only the hepatitis B shot (P less than .001). Geometric mean concentration for pertussis toxin IgG also was four times higher in infants who received the pertussis vaccine at birth.
Adverse events were similar in the two groups both at birth and at 32 weeks, demonstrating that the pertussis birth dose is safe and tolerable.
“More important, in this study, the prevalence of fever after receipt of the birth dose, which can mistakenly be associated with potential sepsis and result in additional investigations in the neonatal period, was similar in both the group that received the aP vaccine at birth and the control group,” the authors reported.
A remaining question is the potential impact of maternal antibodies on protection from pertussis.
“The presence of maternal pertussis antibodies at birth can negatively affect postprimary responses to pertussis, diphtheria, and diphtheria-related CRM197 conjugate vaccines with a variety of infant immunization schedules and vaccines,” the authors noted. “The clinical significance of reductions in pertussis antibody related to maternal interference will require ongoing clinical evaluation, because there are no accepted serologic correlates of protection.”
The research was funded by a Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reported having no conflicts of interest.
SOURCE: Wood N et al, JAMA Pediatr. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2349.
compared with a group receiving only the hepatitis B vaccine, a randomized clinical trial from Australia has found.
“These results indicate that a birth dose of aP vaccine is immunogenic in newborns and significantly narrows the immunity gap between birth and 14 days after receipt of DTaP at 6 or 8 weeks of age, marking the critical period when infants are most vulnerable to severe pertussis infection,” reported Nicholas Wood, PhD, of the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases in New South Wales, Australia, and his colleagues.
“Administration of the acellular pertussis vaccine at birth has the potential to reduce severe morbidity from Bordetella pertussis infection in the first 3 months of life, especially for infants of mothers who have not received a pertussis vaccine during pregnancy,” the researchers concluded in JAMA Pediatrics.
The researchers enrolled 417 infants from Sydney, Melbourne, Adelaide, and Perth between June 2010 and March 2013 and randomized them to receive either the hepatitis B vaccine alone (n = 205) or the hepatitis B vaccine with a monovalent acellular pertussis vaccine (n = 212) within the first 5 days after birth. The randomization was stratified for mothers’ receipt of the Tdap before pregnancy.
The Centers for Disease Control and Prevention currently recommends all newborns receive the hepatitis B vaccine shortly after birth and that pregnant women receive the Tdap vaccine during each pregnancy. There is not currently a monovalent acellular pertussis vaccine licensed in the United States.
The study infants then received the hexavalent DTaP-Hib-hep B-polio vaccine and the 10-valent pneumococcal conjugate vaccine at 6 weeks, 4 months, and 6 months.
The primary outcome was detectable levels of IgG antibody to pertussis toxin and pertactin at 10 weeks old.
Of the 206 infants receiving the pertussis vaccine at birth, 93% had detectable antibodies to pertussis toxin and pertactin at 10 weeks, compared with 51% of the 193 infants who received only the hepatitis B shot (P less than .001). Geometric mean concentration for pertussis toxin IgG also was four times higher in infants who received the pertussis vaccine at birth.
Adverse events were similar in the two groups both at birth and at 32 weeks, demonstrating that the pertussis birth dose is safe and tolerable.
“More important, in this study, the prevalence of fever after receipt of the birth dose, which can mistakenly be associated with potential sepsis and result in additional investigations in the neonatal period, was similar in both the group that received the aP vaccine at birth and the control group,” the authors reported.
A remaining question is the potential impact of maternal antibodies on protection from pertussis.
“The presence of maternal pertussis antibodies at birth can negatively affect postprimary responses to pertussis, diphtheria, and diphtheria-related CRM197 conjugate vaccines with a variety of infant immunization schedules and vaccines,” the authors noted. “The clinical significance of reductions in pertussis antibody related to maternal interference will require ongoing clinical evaluation, because there are no accepted serologic correlates of protection.”
The research was funded by a Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reported having no conflicts of interest.
SOURCE: Wood N et al, JAMA Pediatr. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2349.
FROM JAMA PEDIATRICS
Key clinical point: A monovalent acellular pertussis vaccine dose at birth appears safe, tolerable, and effective.
Major finding: 93% of 212 newborns receiving an acellular pertussis vaccine at birth showed antibodies against pertussis toxin and pertactin at 10 weeks, compared with 51% of 205 newborns without the birth dose.
Study details: The findings are based on a randomized controlled trial involving 417 healthy term newborns in four Australian cities from June 2010 to March 2013.
Disclosures: The research was funded by an Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reporting having no conflicts of interest.
Source: Wood N et al. JAMA Pediatr. 2018 Sep. 10. doi: 10.1001/jamapediatrics.2018.2349.