User login
A Rare Case of Primary Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type
CASE REPORT
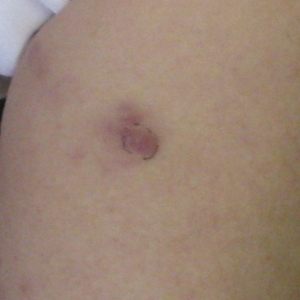
A 74-year-old woman presented with a painful lesion on the left lower leg that was getting larger and more edematous and erythematous over the last 5 months. She experienced numbness and burning of the left lower leg 1 year prior to the development of the lesion. A review of her medical history revealed an otherwise healthy woman with no constitutional symptoms of fever, chills, nausea, vomiting, diarrhea, or chest pain. The patient did not exhibit mucosal, genital, or nail involvement. Physical examination revealed a group of four 1-cm, ill-defined, irregularly bordered, violaceous plaques on the left anterior tibial leg with faint surrounding erythematous to violaceous patches (Figure 1). The plaques were tender to palpation with no bleeding or drainage.
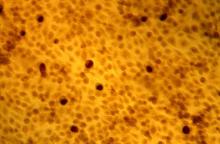
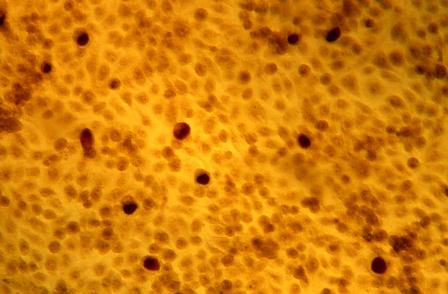
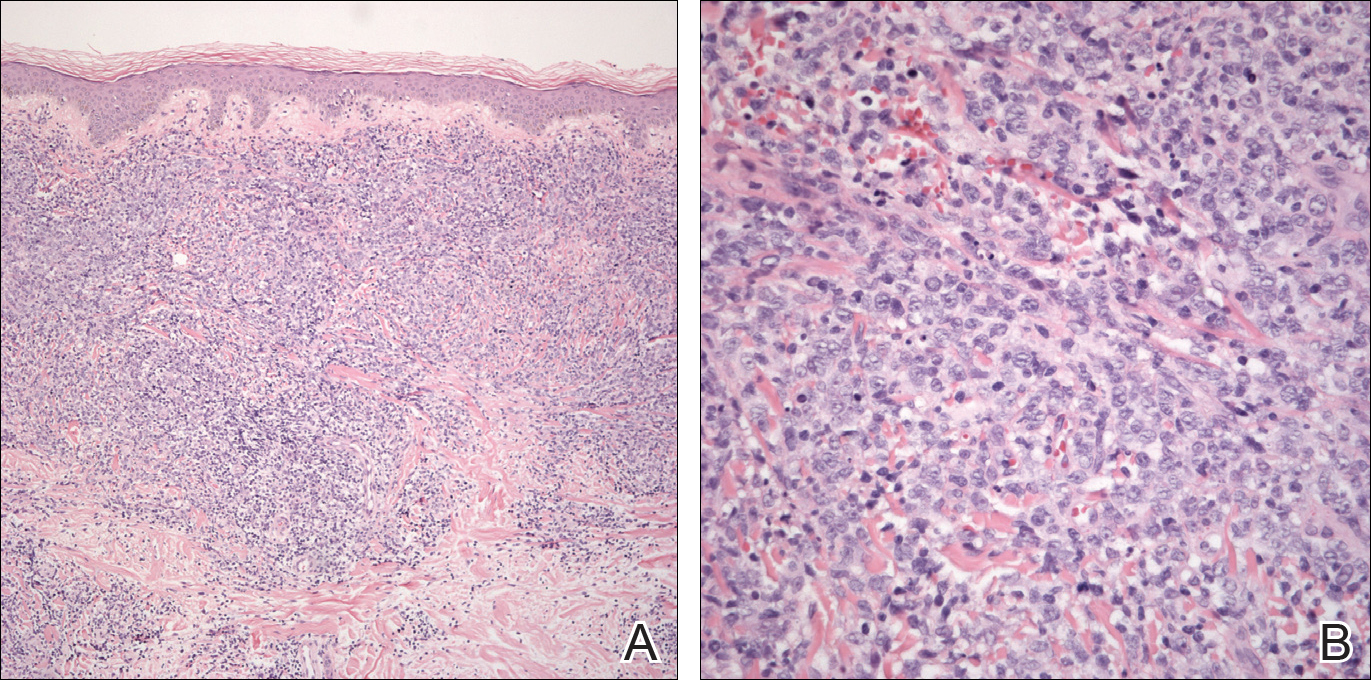
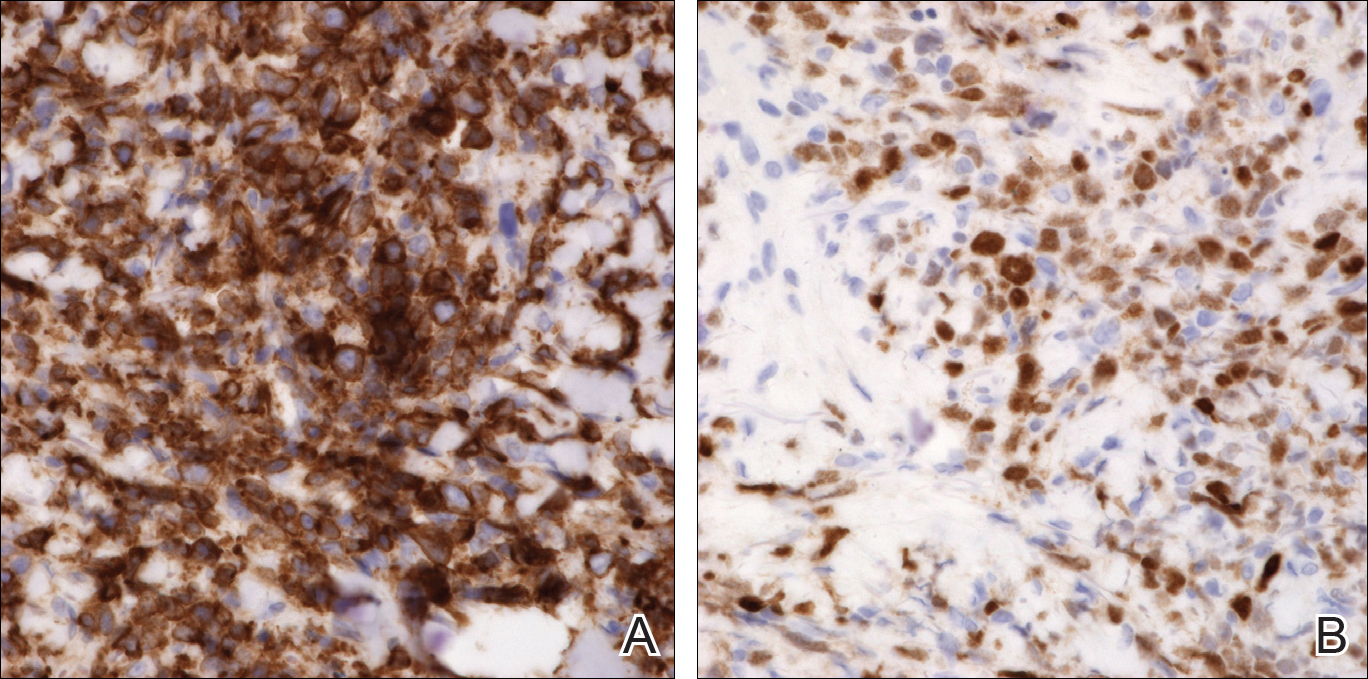
An 8.0-mm punch biopsy of the lesion was obtained. Hematoxylin and eosin staining on low-power magnification demonstrated a diffuse lymphocytic inflammatory infiltrate in the dermis and subcutis. Notable sparing of the subepidermal area (free grenz zone) was present (Figure 2A). On higher power, centroblasts and immunoblasts were visualized alongside extravasated red blood cells (Figure 2B). A diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) was made. Various immunohistochemical stains confirmed the diagnosis, including B-cell lymphoma 2 (BCL-2)(Figure 3A) and multiple myeloma oncogene 1 (MUM-1)(Figure 3B), which were highly positive in our patient. The patient had a negative bone marrow biopsy and positron emission tomography scan. She was started on rituximab infusions and multiple radiation treatments. At 2-year follow-up the lymphoma continued to recur despite radiation therapy.
COMMENT
Incidence and Clinical Characteristics
Primary cutaneous DLBCLLT is an intermediately aggressive form of primary cutaneous B-cell lymphoma (CBCL) that accounts for approximately 10% to 20% of all primary CBCLs and 1% to 3% of all cutaneous lymphomas.1 Diffuse large B-cell lymphoma, leg type primarily affects elderly patients (median age, 70 years). Women are more commonly affected. Clinically, primary cutaneous DLBCLLT presents as red-brown to bluish nodules or tumors on one or both distal legs.
Histopathology
The diagnosis of DLBCLLT is best made histologically. There is a dense inflammatory infiltrate present in the dermis and subcutis that may extend upward into the dermoepidermal junction. Often a subepidermal free grenz zone may be seen, and adnexal structures may be destroyed. This infiltrate is composed of confluent sheets of large round cells including centroblasts and immunoblasts.2 Centroblasts are large cells that have nuclei with several small nucleoli adhering to the membrane, while immunoblasts are large round cells containing nuclei with large central nucleoli. Both centroblasts and immunoblasts stain positively for BCL-2. Centrocytes typically are absent. Staining for BCL-2 can be important in distinguishing DLBCLLT from other forms of CBCL. Diffuse large B-cell lymphoma, leg type also can demonstrate clusters of large atypical cells in the epidermis simulating epidermotropism and Pautrier microabscesses. Neoplastic cells in this condition may express monoclonal surface and cytoplasmic immunoglobulins. Primary cutaneous DLBCLLT typically is positive for B-cell markers CD20 and CD79a. Additionally, MUM-1/IRF4 (interferon regulatory factor 4) and forkhead box protein 1 (FOXP1) are strongly expressed by most patients, which helps distinguish it from other forms of CBCL.
Treatment
Diffuse large B-cell lymphoma, leg type is a relatively aggressive form of CBCL that requires more aggressive treatment than the conservative watchful waiting of some of the more indolent forms of primary CBCL. One regimen involves using cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Local chemotherapy or radiation with rituximab is another treatment option.1,2 In patients with severe comorbidities, rituximab alone may be administered. The prognosis for DLBCLLT is not as favorable as other types of primary CBCL, with an estimated 5-year survival rate of approximately 50%.2
Differential Diagnosis
Lymphomas are malignancies of the lymphocytes that may be subdivided depending on the organ of origin. Both primary nodal lymphomas and primary cutaneous lymphomas exist. Primary nodal lymphomas arise from the lymph nodes and are divided into Hodgkin and non-Hodgkin lymphomas. There are 2 major types of primary cutaneous lymphomas: cutaneous T-cell lymphoma (CTCL) and CBCL. Most primary cutaneous lymphomas are CTCLs, accounting for 75% to 80%.3
Pseudolymphoma
Pseudolymphoma is an inflammatory condition that may histologically mimic cutaneous lymphoma but has a benign clinical course. Pseudolymphoma is not a specific disease but rather is a reactive lymphoproliferative response to a known or unknown stimulus.4 Pseudolymphoma can be broken down into 2 or 3 major categories: cutaneous B-cell pseudolymphoma; cutaneous T-cell pseudolymphoma; and debatably lymphomatoid papulosis, a chronic, self-remitting, papulonecrotic condition that resembles lymphoma histologically but clinically appears benign. It is unknown if lymphomatoid papulosis represents a pseudolymphoma or a true lymphoma. Lymphomatoid papulosis may represent an early indolent form of CTCL.4
Pseudolymphomas can be triggered by a variety of causes. Most cases are idiopathic, and a causative stimulus is never identified. Drugs are known to cause many cases of pseudolymphoma, either by a causing a hypersensitivity reaction or by depressing immunosurveillance.5 Pseudolymphomas may result from exogenous stimuli such as jewelry, tattoo dyes, injectable fillers (eg, silicone), insect bites, vaccines, and trauma.6,7 Lastly, infections in the form of Borrelia, varicella, and molluscum contagiosum can potentially cause pseudolymphomas.4
Clinically, pseudolymphomas may demonstrate a B-cell or T-cell pattern. In cutaneous B-cell pseudolymphomas, asymptomatic solitary erythematous, violaceous, or flesh-colored nodules appear on the face, followed by the chest and arms. Cutaneous T-cell pseudolymphomas present with erythematous patches that are more likely to be symptomatic.4
Histologically, pseudolymphomas also are classified as demonstrating B-cell or T-cell patterns. The nodular inflammatory infiltrate of cutaneous B-cell pseudolymphoma corresponds with its clinically apparent nodules. It can be distinguished from lymphoma in that it is not solely a lymphocytic infiltrate but rather a mixed infiltrate including histiocytes, lymphocytes, eosinophils, and plasma cells. Additionally, cutaneous B-cell pseudolymphoma does not penetrate the dermis as deeply as CBCL.8 Cutaneous T-cell pseudolymphoma is more difficult to distinguish from CTCL because it also demonstrates a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism.9
Treatment must address the underlying cause of pseudolymphoma for resolution. Other treatment options include surgery, cryotherapy, local radiotherapy, topical steroids, and topical immunomodulators. Spontaneous resolution also can occur. The prognosis is better when a known trigger is eliminated, though idiopathic pseudolymphomas may be chronic in nature. It is important to rule out concurrent cutaneous lymphoma or rare transformation into cutaneous lymphoma.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphomas are a diverse group of neoplasms that account for most cutaneous lymphomas seen by dermatologists. In 1806, the first case of CTCL in the form of mycosis fungoides (MF) was described by Jean Louis Alibert. Mycosis fungoides represents the most common form of CTCL, accounting for approximately 50% of all primary cutaneous lymphomas.10 Mycosis fungoides was named after its morphological resemblance to mushrooms. Although not all cases exhibit a classic progression, MF is known for its stepwise progression from patch stage to tumor stage.
Clinically, lesions typically begin as patches that progress to plaques and finally tumors. This progression may not always occur and often can take years to decades to progress. Patches are characterized by erythematous, finely scaling lesions that may be easily confused with eczema or psoriasis. Lesions occur primarily in a swimming trunk distribution.
Mycosis fungoides histologically demonstrates a bandlike lymphocytic infiltrate with epidermotropism, which occurs when lymphocytes infiltrate the epidermis without spongiosis. These lymphocytes are larger, darker, and more angulated than normal lymphocytes. Intraepidermal nests of these atypical lymphocytes creating Pautrier microabscesses may be present. Tumor-stage lesions demonstrate diminished epidermotropism with dense sheets of lymphocytes in the dermis, and fat cells with cerebriform nuclei are present.
Therapies for MF may control the disease but may not prolong patients’ lives. Topical corticosteroids, phototherapy, and radiotherapy are options for skin-targeting therapies. Systemic chemotherapy and biological response modifiers also are viable treatment options. Prognosis for MF is poor.
There are a few notable variants of MF that are important to consider. Sézary syndrome is an erythrodermic variant of MF characterized by atypical Sézary cells. Clinically, it presents with generalized erythroderma with leonine facies, facial edema, and alopecia with associated symptoms of burning and pruritus. Histologically, Sézary syndrome is similar to MF with an increased CD4:CD8 ratio.10 Sézary syndrome may be treated with methotrexate or photopheresis, but the prognosis remains poor with an average survival of 5 years.
Cutaneous B-Cell Lymphoma
There are 5 types of primary CBCL: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone B-cell lymphoma; primary cutaneous diffuse large B-cell lymphoma, other; precursor B-cell lymphoblastic lymphoma; and primary cutaneous DLBCLLT, which was seen in our patient.11
Primary cutaneous follicle center lymphoma is an indolent neoplastic proliferation in the skin. Clinically, it presents with solitary or grouped pinkish purple papules, plaques, or nodules on the trunk with surrounding patches of erythema.3 Lesions located on the back are referred to as Crosti lymphoma. Histopathology reveals a lymphocytic infiltrate with a diffuse follicular pattern and large round centroblasts, centrocytes, and immunoblasts with epidermal sparing. Tumor cells stain positively for κ or λ light chains, as well as CD20, CD79a, and B-cell lymphoma 6 (BCL-6); however, staining for the protein product of BCL-2 may be negative, which differentiates this form of CBCL from primary nodal B-cell lymphoma. Staining for MUM-1 may be negative, which contrasts with the strong expression seen in DLBCLLT. The follicular pattern of follicle center lymphoma stains positive for CD10, but the diffuse pattern may be CD10 negative. The prognosis for primary cutaneous follicle center lymphoma is favorable, but the recurrence rate is up to 50%.3 Treatment includes local radiotherapy or surgical excision.
Primary cutaneous marginal zone B-cell lymphoma is another indolent primary CBCL subtype that is closely related to mucosa-associated lymphoid tissue lymphomas and arises in areas of acrodermatitis chronica atrophicans and Borrelia infection. Clinically, it presents with recurrent, asymptomatic, red-brown papules, plaques, and nodules of the arms and legs. Histologically, there is a patchy infiltrate in the dermis and subcutis with sparing of the epidermis with pale-staining cells with indented nuclei, along with plasma cells and eosinophils. Primary cutaneous marginal zone B-cell lymphoma typically does not demonstrate epidermotropism. Centrocyte cells stain positively for CD20, CD79a, and BCL-2. The prognosis of primary cutaneous marginal zone B-cell lymphoma is favorable. Treatment is similar to primary cutaneous follicle center lymphoma with surgical excision, radiotherapy, and surveillance being the main modalities.
Primary cutaneous diffuse large B-cell lymphoma, other is an intermediately aggressive form of primary CBCL that is thought to be related to primary cutaneous DLBCLLT. Clinically, it presents with indurated erythematous to violaceous plaques on the trunk and thighs that may resemble a vascular tumor or panniculitis.2,12 Histopathologically, this form of lymphoma presents with a round cell morphology without BCL-2 expression, which distinguishes it from DLBCLLT. If limited to skin, the prognosis is better than the systemic form but is still less favorable than other forms of CBCL.
Precursor B-cell lymphoblastic lymphoma is an extremely rare type of CBCL that potentially can occur in the skin. It primarily affects children and young adults. Clinically, it presents as a solitary large erythematous tumor of the head. Histol
CONCLUSION
We present a rare case of primary cutaneous DLBCLLT. Our case demonstrates the classic presentation of primary cutaneous DLBCLLT in a 74-year-old woman with a tumor on the lower left leg. Histologically, a dense dermal and subcutis infiltrate of centroblasts and immunoblasts with a grenz zone was present. Immunostaining in our patient was consistent with characteristic findings in the literature, staining highly positive for BCL-2 and MUM-1. Primary cutaneous DLBCLLT is an extremely rare and unique form of cutaneous lymphoma that can have potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244.
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:2768-3785.
- Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3:719-734.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244; vii.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Kluger N, Vermeulen C, Moguelet P, et al. Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: a case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-213.
- Burg G, Kerl H, Schmoeckel C. Differentiation between malignant B-cell lymphomas and pseudolymphomas of the skin. J Dermatol Surg Oncol. 1984;10:271-275.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38(6, pt 1):877-895; quiz 896-897.
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sézary syndrome. Blood. 1996;88:2385-2409.
- Kempf W, Ralfkiaer E, Duncan LM, et al. Cutaneous marginal zone B-cell lymphoma. In: LeBoit P, Burg G, Weedon D, et al, eds. Pathology and Genetics of Skin Tumors. Lyon, France: IARC Press; 2006:194-195.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in cutaneous large B-cell lymphomas: a European multicentric study. J Clin Oncol. 2001;19:3602-3610.
- Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385.
CASE REPORT
A 74-year-old woman presented with a painful lesion on the left lower leg that was getting larger and more edematous and erythematous over the last 5 months. She experienced numbness and burning of the left lower leg 1 year prior to the development of the lesion. A review of her medical history revealed an otherwise healthy woman with no constitutional symptoms of fever, chills, nausea, vomiting, diarrhea, or chest pain. The patient did not exhibit mucosal, genital, or nail involvement. Physical examination revealed a group of four 1-cm, ill-defined, irregularly bordered, violaceous plaques on the left anterior tibial leg with faint surrounding erythematous to violaceous patches (Figure 1). The plaques were tender to palpation with no bleeding or drainage.

An 8.0-mm punch biopsy of the lesion was obtained. Hematoxylin and eosin staining on low-power magnification demonstrated a diffuse lymphocytic inflammatory infiltrate in the dermis and subcutis. Notable sparing of the subepidermal area (free grenz zone) was present (Figure 2A). On higher power, centroblasts and immunoblasts were visualized alongside extravasated red blood cells (Figure 2B). A diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) was made. Various immunohistochemical stains confirmed the diagnosis, including B-cell lymphoma 2 (BCL-2)(Figure 3A) and multiple myeloma oncogene 1 (MUM-1)(Figure 3B), which were highly positive in our patient. The patient had a negative bone marrow biopsy and positron emission tomography scan. She was started on rituximab infusions and multiple radiation treatments. At 2-year follow-up the lymphoma continued to recur despite radiation therapy.


COMMENT
Incidence and Clinical Characteristics
Primary cutaneous DLBCLLT is an intermediately aggressive form of primary cutaneous B-cell lymphoma (CBCL) that accounts for approximately 10% to 20% of all primary CBCLs and 1% to 3% of all cutaneous lymphomas.1 Diffuse large B-cell lymphoma, leg type primarily affects elderly patients (median age, 70 years). Women are more commonly affected. Clinically, primary cutaneous DLBCLLT presents as red-brown to bluish nodules or tumors on one or both distal legs.
Histopathology
The diagnosis of DLBCLLT is best made histologically. There is a dense inflammatory infiltrate present in the dermis and subcutis that may extend upward into the dermoepidermal junction. Often a subepidermal free grenz zone may be seen, and adnexal structures may be destroyed. This infiltrate is composed of confluent sheets of large round cells including centroblasts and immunoblasts.2 Centroblasts are large cells that have nuclei with several small nucleoli adhering to the membrane, while immunoblasts are large round cells containing nuclei with large central nucleoli. Both centroblasts and immunoblasts stain positively for BCL-2. Centrocytes typically are absent. Staining for BCL-2 can be important in distinguishing DLBCLLT from other forms of CBCL. Diffuse large B-cell lymphoma, leg type also can demonstrate clusters of large atypical cells in the epidermis simulating epidermotropism and Pautrier microabscesses. Neoplastic cells in this condition may express monoclonal surface and cytoplasmic immunoglobulins. Primary cutaneous DLBCLLT typically is positive for B-cell markers CD20 and CD79a. Additionally, MUM-1/IRF4 (interferon regulatory factor 4) and forkhead box protein 1 (FOXP1) are strongly expressed by most patients, which helps distinguish it from other forms of CBCL.
Treatment
Diffuse large B-cell lymphoma, leg type is a relatively aggressive form of CBCL that requires more aggressive treatment than the conservative watchful waiting of some of the more indolent forms of primary CBCL. One regimen involves using cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Local chemotherapy or radiation with rituximab is another treatment option.1,2 In patients with severe comorbidities, rituximab alone may be administered. The prognosis for DLBCLLT is not as favorable as other types of primary CBCL, with an estimated 5-year survival rate of approximately 50%.2
Differential Diagnosis
Lymphomas are malignancies of the lymphocytes that may be subdivided depending on the organ of origin. Both primary nodal lymphomas and primary cutaneous lymphomas exist. Primary nodal lymphomas arise from the lymph nodes and are divided into Hodgkin and non-Hodgkin lymphomas. There are 2 major types of primary cutaneous lymphomas: cutaneous T-cell lymphoma (CTCL) and CBCL. Most primary cutaneous lymphomas are CTCLs, accounting for 75% to 80%.3
Pseudolymphoma
Pseudolymphoma is an inflammatory condition that may histologically mimic cutaneous lymphoma but has a benign clinical course. Pseudolymphoma is not a specific disease but rather is a reactive lymphoproliferative response to a known or unknown stimulus.4 Pseudolymphoma can be broken down into 2 or 3 major categories: cutaneous B-cell pseudolymphoma; cutaneous T-cell pseudolymphoma; and debatably lymphomatoid papulosis, a chronic, self-remitting, papulonecrotic condition that resembles lymphoma histologically but clinically appears benign. It is unknown if lymphomatoid papulosis represents a pseudolymphoma or a true lymphoma. Lymphomatoid papulosis may represent an early indolent form of CTCL.4
Pseudolymphomas can be triggered by a variety of causes. Most cases are idiopathic, and a causative stimulus is never identified. Drugs are known to cause many cases of pseudolymphoma, either by a causing a hypersensitivity reaction or by depressing immunosurveillance.5 Pseudolymphomas may result from exogenous stimuli such as jewelry, tattoo dyes, injectable fillers (eg, silicone), insect bites, vaccines, and trauma.6,7 Lastly, infections in the form of Borrelia, varicella, and molluscum contagiosum can potentially cause pseudolymphomas.4
Clinically, pseudolymphomas may demonstrate a B-cell or T-cell pattern. In cutaneous B-cell pseudolymphomas, asymptomatic solitary erythematous, violaceous, or flesh-colored nodules appear on the face, followed by the chest and arms. Cutaneous T-cell pseudolymphomas present with erythematous patches that are more likely to be symptomatic.4
Histologically, pseudolymphomas also are classified as demonstrating B-cell or T-cell patterns. The nodular inflammatory infiltrate of cutaneous B-cell pseudolymphoma corresponds with its clinically apparent nodules. It can be distinguished from lymphoma in that it is not solely a lymphocytic infiltrate but rather a mixed infiltrate including histiocytes, lymphocytes, eosinophils, and plasma cells. Additionally, cutaneous B-cell pseudolymphoma does not penetrate the dermis as deeply as CBCL.8 Cutaneous T-cell pseudolymphoma is more difficult to distinguish from CTCL because it also demonstrates a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism.9
Treatment must address the underlying cause of pseudolymphoma for resolution. Other treatment options include surgery, cryotherapy, local radiotherapy, topical steroids, and topical immunomodulators. Spontaneous resolution also can occur. The prognosis is better when a known trigger is eliminated, though idiopathic pseudolymphomas may be chronic in nature. It is important to rule out concurrent cutaneous lymphoma or rare transformation into cutaneous lymphoma.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphomas are a diverse group of neoplasms that account for most cutaneous lymphomas seen by dermatologists. In 1806, the first case of CTCL in the form of mycosis fungoides (MF) was described by Jean Louis Alibert. Mycosis fungoides represents the most common form of CTCL, accounting for approximately 50% of all primary cutaneous lymphomas.10 Mycosis fungoides was named after its morphological resemblance to mushrooms. Although not all cases exhibit a classic progression, MF is known for its stepwise progression from patch stage to tumor stage.
Clinically, lesions typically begin as patches that progress to plaques and finally tumors. This progression may not always occur and often can take years to decades to progress. Patches are characterized by erythematous, finely scaling lesions that may be easily confused with eczema or psoriasis. Lesions occur primarily in a swimming trunk distribution.
Mycosis fungoides histologically demonstrates a bandlike lymphocytic infiltrate with epidermotropism, which occurs when lymphocytes infiltrate the epidermis without spongiosis. These lymphocytes are larger, darker, and more angulated than normal lymphocytes. Intraepidermal nests of these atypical lymphocytes creating Pautrier microabscesses may be present. Tumor-stage lesions demonstrate diminished epidermotropism with dense sheets of lymphocytes in the dermis, and fat cells with cerebriform nuclei are present.
Therapies for MF may control the disease but may not prolong patients’ lives. Topical corticosteroids, phototherapy, and radiotherapy are options for skin-targeting therapies. Systemic chemotherapy and biological response modifiers also are viable treatment options. Prognosis for MF is poor.
There are a few notable variants of MF that are important to consider. Sézary syndrome is an erythrodermic variant of MF characterized by atypical Sézary cells. Clinically, it presents with generalized erythroderma with leonine facies, facial edema, and alopecia with associated symptoms of burning and pruritus. Histologically, Sézary syndrome is similar to MF with an increased CD4:CD8 ratio.10 Sézary syndrome may be treated with methotrexate or photopheresis, but the prognosis remains poor with an average survival of 5 years.
Cutaneous B-Cell Lymphoma
There are 5 types of primary CBCL: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone B-cell lymphoma; primary cutaneous diffuse large B-cell lymphoma, other; precursor B-cell lymphoblastic lymphoma; and primary cutaneous DLBCLLT, which was seen in our patient.11
Primary cutaneous follicle center lymphoma is an indolent neoplastic proliferation in the skin. Clinically, it presents with solitary or grouped pinkish purple papules, plaques, or nodules on the trunk with surrounding patches of erythema.3 Lesions located on the back are referred to as Crosti lymphoma. Histopathology reveals a lymphocytic infiltrate with a diffuse follicular pattern and large round centroblasts, centrocytes, and immunoblasts with epidermal sparing. Tumor cells stain positively for κ or λ light chains, as well as CD20, CD79a, and B-cell lymphoma 6 (BCL-6); however, staining for the protein product of BCL-2 may be negative, which differentiates this form of CBCL from primary nodal B-cell lymphoma. Staining for MUM-1 may be negative, which contrasts with the strong expression seen in DLBCLLT. The follicular pattern of follicle center lymphoma stains positive for CD10, but the diffuse pattern may be CD10 negative. The prognosis for primary cutaneous follicle center lymphoma is favorable, but the recurrence rate is up to 50%.3 Treatment includes local radiotherapy or surgical excision.
Primary cutaneous marginal zone B-cell lymphoma is another indolent primary CBCL subtype that is closely related to mucosa-associated lymphoid tissue lymphomas and arises in areas of acrodermatitis chronica atrophicans and Borrelia infection. Clinically, it presents with recurrent, asymptomatic, red-brown papules, plaques, and nodules of the arms and legs. Histologically, there is a patchy infiltrate in the dermis and subcutis with sparing of the epidermis with pale-staining cells with indented nuclei, along with plasma cells and eosinophils. Primary cutaneous marginal zone B-cell lymphoma typically does not demonstrate epidermotropism. Centrocyte cells stain positively for CD20, CD79a, and BCL-2. The prognosis of primary cutaneous marginal zone B-cell lymphoma is favorable. Treatment is similar to primary cutaneous follicle center lymphoma with surgical excision, radiotherapy, and surveillance being the main modalities.
Primary cutaneous diffuse large B-cell lymphoma, other is an intermediately aggressive form of primary CBCL that is thought to be related to primary cutaneous DLBCLLT. Clinically, it presents with indurated erythematous to violaceous plaques on the trunk and thighs that may resemble a vascular tumor or panniculitis.2,12 Histopathologically, this form of lymphoma presents with a round cell morphology without BCL-2 expression, which distinguishes it from DLBCLLT. If limited to skin, the prognosis is better than the systemic form but is still less favorable than other forms of CBCL.
Precursor B-cell lymphoblastic lymphoma is an extremely rare type of CBCL that potentially can occur in the skin. It primarily affects children and young adults. Clinically, it presents as a solitary large erythematous tumor of the head. Histol
CONCLUSION
We present a rare case of primary cutaneous DLBCLLT. Our case demonstrates the classic presentation of primary cutaneous DLBCLLT in a 74-year-old woman with a tumor on the lower left leg. Histologically, a dense dermal and subcutis infiltrate of centroblasts and immunoblasts with a grenz zone was present. Immunostaining in our patient was consistent with characteristic findings in the literature, staining highly positive for BCL-2 and MUM-1. Primary cutaneous DLBCLLT is an extremely rare and unique form of cutaneous lymphoma that can have potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
CASE REPORT
A 74-year-old woman presented with a painful lesion on the left lower leg that was getting larger and more edematous and erythematous over the last 5 months. She experienced numbness and burning of the left lower leg 1 year prior to the development of the lesion. A review of her medical history revealed an otherwise healthy woman with no constitutional symptoms of fever, chills, nausea, vomiting, diarrhea, or chest pain. The patient did not exhibit mucosal, genital, or nail involvement. Physical examination revealed a group of four 1-cm, ill-defined, irregularly bordered, violaceous plaques on the left anterior tibial leg with faint surrounding erythematous to violaceous patches (Figure 1). The plaques were tender to palpation with no bleeding or drainage.

An 8.0-mm punch biopsy of the lesion was obtained. Hematoxylin and eosin staining on low-power magnification demonstrated a diffuse lymphocytic inflammatory infiltrate in the dermis and subcutis. Notable sparing of the subepidermal area (free grenz zone) was present (Figure 2A). On higher power, centroblasts and immunoblasts were visualized alongside extravasated red blood cells (Figure 2B). A diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) was made. Various immunohistochemical stains confirmed the diagnosis, including B-cell lymphoma 2 (BCL-2)(Figure 3A) and multiple myeloma oncogene 1 (MUM-1)(Figure 3B), which were highly positive in our patient. The patient had a negative bone marrow biopsy and positron emission tomography scan. She was started on rituximab infusions and multiple radiation treatments. At 2-year follow-up the lymphoma continued to recur despite radiation therapy.


COMMENT
Incidence and Clinical Characteristics
Primary cutaneous DLBCLLT is an intermediately aggressive form of primary cutaneous B-cell lymphoma (CBCL) that accounts for approximately 10% to 20% of all primary CBCLs and 1% to 3% of all cutaneous lymphomas.1 Diffuse large B-cell lymphoma, leg type primarily affects elderly patients (median age, 70 years). Women are more commonly affected. Clinically, primary cutaneous DLBCLLT presents as red-brown to bluish nodules or tumors on one or both distal legs.
Histopathology
The diagnosis of DLBCLLT is best made histologically. There is a dense inflammatory infiltrate present in the dermis and subcutis that may extend upward into the dermoepidermal junction. Often a subepidermal free grenz zone may be seen, and adnexal structures may be destroyed. This infiltrate is composed of confluent sheets of large round cells including centroblasts and immunoblasts.2 Centroblasts are large cells that have nuclei with several small nucleoli adhering to the membrane, while immunoblasts are large round cells containing nuclei with large central nucleoli. Both centroblasts and immunoblasts stain positively for BCL-2. Centrocytes typically are absent. Staining for BCL-2 can be important in distinguishing DLBCLLT from other forms of CBCL. Diffuse large B-cell lymphoma, leg type also can demonstrate clusters of large atypical cells in the epidermis simulating epidermotropism and Pautrier microabscesses. Neoplastic cells in this condition may express monoclonal surface and cytoplasmic immunoglobulins. Primary cutaneous DLBCLLT typically is positive for B-cell markers CD20 and CD79a. Additionally, MUM-1/IRF4 (interferon regulatory factor 4) and forkhead box protein 1 (FOXP1) are strongly expressed by most patients, which helps distinguish it from other forms of CBCL.
Treatment
Diffuse large B-cell lymphoma, leg type is a relatively aggressive form of CBCL that requires more aggressive treatment than the conservative watchful waiting of some of the more indolent forms of primary CBCL. One regimen involves using cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Local chemotherapy or radiation with rituximab is another treatment option.1,2 In patients with severe comorbidities, rituximab alone may be administered. The prognosis for DLBCLLT is not as favorable as other types of primary CBCL, with an estimated 5-year survival rate of approximately 50%.2
Differential Diagnosis
Lymphomas are malignancies of the lymphocytes that may be subdivided depending on the organ of origin. Both primary nodal lymphomas and primary cutaneous lymphomas exist. Primary nodal lymphomas arise from the lymph nodes and are divided into Hodgkin and non-Hodgkin lymphomas. There are 2 major types of primary cutaneous lymphomas: cutaneous T-cell lymphoma (CTCL) and CBCL. Most primary cutaneous lymphomas are CTCLs, accounting for 75% to 80%.3
Pseudolymphoma
Pseudolymphoma is an inflammatory condition that may histologically mimic cutaneous lymphoma but has a benign clinical course. Pseudolymphoma is not a specific disease but rather is a reactive lymphoproliferative response to a known or unknown stimulus.4 Pseudolymphoma can be broken down into 2 or 3 major categories: cutaneous B-cell pseudolymphoma; cutaneous T-cell pseudolymphoma; and debatably lymphomatoid papulosis, a chronic, self-remitting, papulonecrotic condition that resembles lymphoma histologically but clinically appears benign. It is unknown if lymphomatoid papulosis represents a pseudolymphoma or a true lymphoma. Lymphomatoid papulosis may represent an early indolent form of CTCL.4
Pseudolymphomas can be triggered by a variety of causes. Most cases are idiopathic, and a causative stimulus is never identified. Drugs are known to cause many cases of pseudolymphoma, either by a causing a hypersensitivity reaction or by depressing immunosurveillance.5 Pseudolymphomas may result from exogenous stimuli such as jewelry, tattoo dyes, injectable fillers (eg, silicone), insect bites, vaccines, and trauma.6,7 Lastly, infections in the form of Borrelia, varicella, and molluscum contagiosum can potentially cause pseudolymphomas.4
Clinically, pseudolymphomas may demonstrate a B-cell or T-cell pattern. In cutaneous B-cell pseudolymphomas, asymptomatic solitary erythematous, violaceous, or flesh-colored nodules appear on the face, followed by the chest and arms. Cutaneous T-cell pseudolymphomas present with erythematous patches that are more likely to be symptomatic.4
Histologically, pseudolymphomas also are classified as demonstrating B-cell or T-cell patterns. The nodular inflammatory infiltrate of cutaneous B-cell pseudolymphoma corresponds with its clinically apparent nodules. It can be distinguished from lymphoma in that it is not solely a lymphocytic infiltrate but rather a mixed infiltrate including histiocytes, lymphocytes, eosinophils, and plasma cells. Additionally, cutaneous B-cell pseudolymphoma does not penetrate the dermis as deeply as CBCL.8 Cutaneous T-cell pseudolymphoma is more difficult to distinguish from CTCL because it also demonstrates a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism.9
Treatment must address the underlying cause of pseudolymphoma for resolution. Other treatment options include surgery, cryotherapy, local radiotherapy, topical steroids, and topical immunomodulators. Spontaneous resolution also can occur. The prognosis is better when a known trigger is eliminated, though idiopathic pseudolymphomas may be chronic in nature. It is important to rule out concurrent cutaneous lymphoma or rare transformation into cutaneous lymphoma.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphomas are a diverse group of neoplasms that account for most cutaneous lymphomas seen by dermatologists. In 1806, the first case of CTCL in the form of mycosis fungoides (MF) was described by Jean Louis Alibert. Mycosis fungoides represents the most common form of CTCL, accounting for approximately 50% of all primary cutaneous lymphomas.10 Mycosis fungoides was named after its morphological resemblance to mushrooms. Although not all cases exhibit a classic progression, MF is known for its stepwise progression from patch stage to tumor stage.
Clinically, lesions typically begin as patches that progress to plaques and finally tumors. This progression may not always occur and often can take years to decades to progress. Patches are characterized by erythematous, finely scaling lesions that may be easily confused with eczema or psoriasis. Lesions occur primarily in a swimming trunk distribution.
Mycosis fungoides histologically demonstrates a bandlike lymphocytic infiltrate with epidermotropism, which occurs when lymphocytes infiltrate the epidermis without spongiosis. These lymphocytes are larger, darker, and more angulated than normal lymphocytes. Intraepidermal nests of these atypical lymphocytes creating Pautrier microabscesses may be present. Tumor-stage lesions demonstrate diminished epidermotropism with dense sheets of lymphocytes in the dermis, and fat cells with cerebriform nuclei are present.
Therapies for MF may control the disease but may not prolong patients’ lives. Topical corticosteroids, phototherapy, and radiotherapy are options for skin-targeting therapies. Systemic chemotherapy and biological response modifiers also are viable treatment options. Prognosis for MF is poor.
There are a few notable variants of MF that are important to consider. Sézary syndrome is an erythrodermic variant of MF characterized by atypical Sézary cells. Clinically, it presents with generalized erythroderma with leonine facies, facial edema, and alopecia with associated symptoms of burning and pruritus. Histologically, Sézary syndrome is similar to MF with an increased CD4:CD8 ratio.10 Sézary syndrome may be treated with methotrexate or photopheresis, but the prognosis remains poor with an average survival of 5 years.
Cutaneous B-Cell Lymphoma
There are 5 types of primary CBCL: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone B-cell lymphoma; primary cutaneous diffuse large B-cell lymphoma, other; precursor B-cell lymphoblastic lymphoma; and primary cutaneous DLBCLLT, which was seen in our patient.11
Primary cutaneous follicle center lymphoma is an indolent neoplastic proliferation in the skin. Clinically, it presents with solitary or grouped pinkish purple papules, plaques, or nodules on the trunk with surrounding patches of erythema.3 Lesions located on the back are referred to as Crosti lymphoma. Histopathology reveals a lymphocytic infiltrate with a diffuse follicular pattern and large round centroblasts, centrocytes, and immunoblasts with epidermal sparing. Tumor cells stain positively for κ or λ light chains, as well as CD20, CD79a, and B-cell lymphoma 6 (BCL-6); however, staining for the protein product of BCL-2 may be negative, which differentiates this form of CBCL from primary nodal B-cell lymphoma. Staining for MUM-1 may be negative, which contrasts with the strong expression seen in DLBCLLT. The follicular pattern of follicle center lymphoma stains positive for CD10, but the diffuse pattern may be CD10 negative. The prognosis for primary cutaneous follicle center lymphoma is favorable, but the recurrence rate is up to 50%.3 Treatment includes local radiotherapy or surgical excision.
Primary cutaneous marginal zone B-cell lymphoma is another indolent primary CBCL subtype that is closely related to mucosa-associated lymphoid tissue lymphomas and arises in areas of acrodermatitis chronica atrophicans and Borrelia infection. Clinically, it presents with recurrent, asymptomatic, red-brown papules, plaques, and nodules of the arms and legs. Histologically, there is a patchy infiltrate in the dermis and subcutis with sparing of the epidermis with pale-staining cells with indented nuclei, along with plasma cells and eosinophils. Primary cutaneous marginal zone B-cell lymphoma typically does not demonstrate epidermotropism. Centrocyte cells stain positively for CD20, CD79a, and BCL-2. The prognosis of primary cutaneous marginal zone B-cell lymphoma is favorable. Treatment is similar to primary cutaneous follicle center lymphoma with surgical excision, radiotherapy, and surveillance being the main modalities.
Primary cutaneous diffuse large B-cell lymphoma, other is an intermediately aggressive form of primary CBCL that is thought to be related to primary cutaneous DLBCLLT. Clinically, it presents with indurated erythematous to violaceous plaques on the trunk and thighs that may resemble a vascular tumor or panniculitis.2,12 Histopathologically, this form of lymphoma presents with a round cell morphology without BCL-2 expression, which distinguishes it from DLBCLLT. If limited to skin, the prognosis is better than the systemic form but is still less favorable than other forms of CBCL.
Precursor B-cell lymphoblastic lymphoma is an extremely rare type of CBCL that potentially can occur in the skin. It primarily affects children and young adults. Clinically, it presents as a solitary large erythematous tumor of the head. Histol
CONCLUSION
We present a rare case of primary cutaneous DLBCLLT. Our case demonstrates the classic presentation of primary cutaneous DLBCLLT in a 74-year-old woman with a tumor on the lower left leg. Histologically, a dense dermal and subcutis infiltrate of centroblasts and immunoblasts with a grenz zone was present. Immunostaining in our patient was consistent with characteristic findings in the literature, staining highly positive for BCL-2 and MUM-1. Primary cutaneous DLBCLLT is an extremely rare and unique form of cutaneous lymphoma that can have potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244.
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:2768-3785.
- Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3:719-734.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244; vii.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Kluger N, Vermeulen C, Moguelet P, et al. Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: a case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-213.
- Burg G, Kerl H, Schmoeckel C. Differentiation between malignant B-cell lymphomas and pseudolymphomas of the skin. J Dermatol Surg Oncol. 1984;10:271-275.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38(6, pt 1):877-895; quiz 896-897.
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sézary syndrome. Blood. 1996;88:2385-2409.
- Kempf W, Ralfkiaer E, Duncan LM, et al. Cutaneous marginal zone B-cell lymphoma. In: LeBoit P, Burg G, Weedon D, et al, eds. Pathology and Genetics of Skin Tumors. Lyon, France: IARC Press; 2006:194-195.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in cutaneous large B-cell lymphomas: a European multicentric study. J Clin Oncol. 2001;19:3602-3610.
- Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244.
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:2768-3785.
- Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3:719-734.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244; vii.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Kluger N, Vermeulen C, Moguelet P, et al. Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: a case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-213.
- Burg G, Kerl H, Schmoeckel C. Differentiation between malignant B-cell lymphomas and pseudolymphomas of the skin. J Dermatol Surg Oncol. 1984;10:271-275.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38(6, pt 1):877-895; quiz 896-897.
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sézary syndrome. Blood. 1996;88:2385-2409.
- Kempf W, Ralfkiaer E, Duncan LM, et al. Cutaneous marginal zone B-cell lymphoma. In: LeBoit P, Burg G, Weedon D, et al, eds. Pathology and Genetics of Skin Tumors. Lyon, France: IARC Press; 2006:194-195.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in cutaneous large B-cell lymphomas: a European multicentric study. J Clin Oncol. 2001;19:3602-3610.
- Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385.
Practice Points
- Primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) is characterized by the presence of large round cells on histopathology.
- There are potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
Malpractice Counsel: Diverticulitis
A 44-year-old woman presented to the ED complaining of crampy lower abdominal pain with nausea and vomiting. The patient described gradual onset 2 days prior, with symptoms worsening over the previous 12 hours. She denied diarrhea, constipation, or blood in her stool. She did admit to frequency of urination, but no dysuria or hematuria. She was gravida 2, para 2, aborta 0, with a last menstrual period 3 weeks prior. She denied vaginal bleeding or discharge. Her past medical history was unremarkable, she was on no medications, and denied alcohol use. She did admit to smoking one pack of cigarettes per day.
On physical examination, the patient’s vital signs were: blood pressure, 132/68 mm Hg; heart rate, 96 beats/min; respiratory rate, 18 breaths/min; and temperature, 99.8°F. Oxygen saturation was 99% on room air.
The head, ears, eyes, nose, and throat (HEENT) examination was completely normal, as was the heart and lung examination. The patient was tender to palpation in the lower abdomen, but without guarding or rebound. Bowel sounds were present and normoactive. A pelvic examination, including a bimanual examination, demonstrated mild left ovarian tenderness but without mass or cervical motion tenderness. The patient did not exhibit any costovertebral angle tenderness bilaterally. No rectal examination was performed.
The emergency physicians (EPs) ordered a complete blood count (CBC), basic metabolic profile (BMP), urinalysis, urine pregnancy test, and a vaginal wet preparation. In addition, the patient was administered 500 cc’s of normal saline intravenously (IV) and ondansetron (Zofran) 4 mg IV.
The urine pregnancy test result came back negative. The urinalysis was remarkable for positive leukocyte esterase, with five to 10 white cells and bacteria present. The CBC showed a mild leukocytosis, but with a normal hemoglobin and hematocrit. The BMP and vaginal wet preparation were completely normal.
The EP was concerned the patient might have something more serious than a simple urinary tract infection (UTI), so she ordered a computed tomography (CT) scan of the abdomen and pelvis with IV contrast.
The radiologist interpreted the CT scan as normal. The patient was discharged home with a prescription for an antibiotic for her UTI, encouraged to drink liquids, and instructed to follow-up with her primary care physician in 1 week.
The patient returned to the same ED approximately 48 hours later with worsening abdominal pain. On this presentation, she was tachycardic (110 beats/min) with a temperature of 101°F. The abdominal examination was remarkable for diffuse tenderness and voluntary guarding. The patient was administered IV fluids, morphine, and ondansetron. A repeat CT scan of the abdomen and pelvis with IV contrast showed a perforated sigmoid colon, with leakage of bowel contents into the peritoneum. The EP immediately started IV fluid resuscitation and administered IV antibiotics. The patient was taken emergently to the operating room by general surgery. The colon was repaired and a colostomy placed. The patient was able to be discharged home on day number 5.
The patient sued the hospital and the treating EP for failure to make the proper diagnosis on the initial ED visit, resulting in the patient having a long and difficult recovery, and the need for a colostomy. At trial, the jury returned a defense verdict.
Discussion
Diverticulitis, and its complications, account for a significant number of ED visits. It is the third most common inpatient gastrointestinal diagnosis in the United States, costing two billion dollars annually.1It is defined as clinically evident microscopic inflammation of a diverticulum or diverticula, and occurs in approximately 4% of patients with diverticulosis.1It is estimated that roughly 15% of these patients will experience a complication, defined as an abscess, perforation, fistula, or colonic obstruction; 15% to 30% will experience a recurrence.
The mean age of patients admitted to the hospital for diverticulitis is 63 years. While considered a disease of older patients, it should be included in the differential diagnosis for younger patients, as approximately 16% of admissions for acute diverticulitis are in patients less than 45 years.2Risk factors include poor diet (ie, low fiber, high fat, red meat), obesity, and smoking. The clinical presentation of diverticulitis has sometimes been referred to as “left-sided appendicitis” because of the similarities between the two entities. Patients will frequently complain of anorexia, change in bowel habits (either diarrhea or constipation), crampy abdominal pain (primarily in the left lower quadrant), low grade fever, and nausea with vomiting. Interestingly, 10% to 15% of patients with acute diverticulitis will complain of dysuria, urgency, or frequency (as in this patient) due to irritation of the bladder from an inflamed sigmoid colon.
Physical examination may reveal a low grade fever and tachycardia, if significant vomiting has been present. The abdomen is tender primarily in the left lower quadrant. The presence of severe tachycardia, hypotension, or a rigid abdomen with guarding and rebound suggests perforation. A pelvic examination should be performed on all women of child-bearing age. The rectal examination may reveal hemoccult positive stool; gross blood is rare.
Laboratory testing should include a CBC, BMP, urinalysis, and a urine pregnancy test (for women of child-bearing age). The CBC will usually reveal a mild leukocytosis. The urinalysis may reveal sterile pyuria for the reason previously described. Additional testing may be indicated by the history and physical examination.
A CT scan of the abdomen and pelvis is considered the gold standard with regards to imaging, with a reported sensitivity of 94% and specificity of 99%.3 Ideally, the CT scan should include both oral and IV contrast; however, IV alone is frequently used. In addition to identifying diverticulitis, CT can also visualize complications, including abscesses, perforation, and bowel obstruction. Ultrasound using high-resolution, graded compassion has a similar sensitivity and specificity as CT, with the advantage of less cost, can be performed at the bedside, and avoids radiation exposure.3 However, it is operator dependent and inferior to CT regarding visualizing complications.
Historically, antibiotics have been considered the treatment of choice for patients with acute uncomplicated diverticulitis, usually as an outpatient. Typically, this involves prescribing ciprofloxacin (or trimethoprim-sulfamethoxazole) plus metronidazole for 7 to 10 days. Monotherapy consisting of either moxifloxacin or amoxicillin/clavulanic acid is also acceptable. However, as our understanding of the important role of inflammation in this disease process, combined with the negative effects associated with antibiotic use, the role of antibiotics in uncomplicated diverticulitis has been called into question. In one recent study of 155 patients with acute uncomplicated diverticulitis, 97% were managed successfully as outpatients without antibiotics, admission, or complications.4 The American Gastroenterological Association (AGA) recommends that antibiotics should be used selectively, rather than routinely, in patients with acute uncomplicated diverticulitis. However, this is considered a “conditional recommendation with a low quality of evidence.”1In other words, this recommendation could easily change based on newer studies. Similarly, other “conditional recommendations” by the AGA include suggesting a fiber-rich diet, or fiber supplementation, and no need to avoid the consumption of nuts and popcorn.1 The majority of these patients begin to feel better in 2 to 3 days and have a good outcome.
Summary
For patients that appear ill, have significant comorbidities, are immunocompromised, or have a complication of acute diverticulitis, admission to the hospital with surgery consultation is recommended. For abscesses, interventional radiology has been used with success for CT-guided percutaneous drainage of diverticular abscesses. Intravenous antibiotics should be initiated; appropriate medications include metronidazole plus a third-generation cephalosporin (such as ceftriaxone or cefotaxime) or a fluoroquinolone (such as ciprofloxacin or levofloxacin). Monotherapy for the moderately ill patient includes piperacillin/tazobactam, ampicillin/sulbactam, ticarcillin/clavulanic acid, and imipenem. In addition, these patients should be placed at bowel rest (ie, nothing by mouth) with IV fluid resuscitation and hydration.
1. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149(7):1944-1949. doi:10.1053/j.gastro.2015.10.003.
2. Nguyen GC, Sam J, Anand N. Epidemiological trends and geographic variation in hospital admissions for diverticulitis in the United States. World J Gastroenterol. 2011;17(12):1600-1605. doi:10.3748/wjg.v17.i12.1600.
3. Laméris W, van Randen A, Bipat S, Bossuyt PM, Boermeester MA, Stoker J. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur Radiol. 2008;18(11):2498-2511. doi:10.1007/s00330-008-1018-6.
4. Isacson D, Thorisson A, Andreasson K, Nikberg M, Smedh K, Chabok A. Outpatient, non-antibiotic management in acute uncomplicated diverticulitis: a prospective study. Int J Colorectal Dis. 2015;30(9):1229-1234. doi:10.1007/s00384-015-2258-y.
A 44-year-old woman presented to the ED complaining of crampy lower abdominal pain with nausea and vomiting. The patient described gradual onset 2 days prior, with symptoms worsening over the previous 12 hours. She denied diarrhea, constipation, or blood in her stool. She did admit to frequency of urination, but no dysuria or hematuria. She was gravida 2, para 2, aborta 0, with a last menstrual period 3 weeks prior. She denied vaginal bleeding or discharge. Her past medical history was unremarkable, she was on no medications, and denied alcohol use. She did admit to smoking one pack of cigarettes per day.
On physical examination, the patient’s vital signs were: blood pressure, 132/68 mm Hg; heart rate, 96 beats/min; respiratory rate, 18 breaths/min; and temperature, 99.8°F. Oxygen saturation was 99% on room air.
The head, ears, eyes, nose, and throat (HEENT) examination was completely normal, as was the heart and lung examination. The patient was tender to palpation in the lower abdomen, but without guarding or rebound. Bowel sounds were present and normoactive. A pelvic examination, including a bimanual examination, demonstrated mild left ovarian tenderness but without mass or cervical motion tenderness. The patient did not exhibit any costovertebral angle tenderness bilaterally. No rectal examination was performed.
The emergency physicians (EPs) ordered a complete blood count (CBC), basic metabolic profile (BMP), urinalysis, urine pregnancy test, and a vaginal wet preparation. In addition, the patient was administered 500 cc’s of normal saline intravenously (IV) and ondansetron (Zofran) 4 mg IV.
The urine pregnancy test result came back negative. The urinalysis was remarkable for positive leukocyte esterase, with five to 10 white cells and bacteria present. The CBC showed a mild leukocytosis, but with a normal hemoglobin and hematocrit. The BMP and vaginal wet preparation were completely normal.
The EP was concerned the patient might have something more serious than a simple urinary tract infection (UTI), so she ordered a computed tomography (CT) scan of the abdomen and pelvis with IV contrast.
The radiologist interpreted the CT scan as normal. The patient was discharged home with a prescription for an antibiotic for her UTI, encouraged to drink liquids, and instructed to follow-up with her primary care physician in 1 week.
The patient returned to the same ED approximately 48 hours later with worsening abdominal pain. On this presentation, she was tachycardic (110 beats/min) with a temperature of 101°F. The abdominal examination was remarkable for diffuse tenderness and voluntary guarding. The patient was administered IV fluids, morphine, and ondansetron. A repeat CT scan of the abdomen and pelvis with IV contrast showed a perforated sigmoid colon, with leakage of bowel contents into the peritoneum. The EP immediately started IV fluid resuscitation and administered IV antibiotics. The patient was taken emergently to the operating room by general surgery. The colon was repaired and a colostomy placed. The patient was able to be discharged home on day number 5.
The patient sued the hospital and the treating EP for failure to make the proper diagnosis on the initial ED visit, resulting in the patient having a long and difficult recovery, and the need for a colostomy. At trial, the jury returned a defense verdict.
Discussion
Diverticulitis, and its complications, account for a significant number of ED visits. It is the third most common inpatient gastrointestinal diagnosis in the United States, costing two billion dollars annually.1It is defined as clinically evident microscopic inflammation of a diverticulum or diverticula, and occurs in approximately 4% of patients with diverticulosis.1It is estimated that roughly 15% of these patients will experience a complication, defined as an abscess, perforation, fistula, or colonic obstruction; 15% to 30% will experience a recurrence.
The mean age of patients admitted to the hospital for diverticulitis is 63 years. While considered a disease of older patients, it should be included in the differential diagnosis for younger patients, as approximately 16% of admissions for acute diverticulitis are in patients less than 45 years.2Risk factors include poor diet (ie, low fiber, high fat, red meat), obesity, and smoking. The clinical presentation of diverticulitis has sometimes been referred to as “left-sided appendicitis” because of the similarities between the two entities. Patients will frequently complain of anorexia, change in bowel habits (either diarrhea or constipation), crampy abdominal pain (primarily in the left lower quadrant), low grade fever, and nausea with vomiting. Interestingly, 10% to 15% of patients with acute diverticulitis will complain of dysuria, urgency, or frequency (as in this patient) due to irritation of the bladder from an inflamed sigmoid colon.
Physical examination may reveal a low grade fever and tachycardia, if significant vomiting has been present. The abdomen is tender primarily in the left lower quadrant. The presence of severe tachycardia, hypotension, or a rigid abdomen with guarding and rebound suggests perforation. A pelvic examination should be performed on all women of child-bearing age. The rectal examination may reveal hemoccult positive stool; gross blood is rare.
Laboratory testing should include a CBC, BMP, urinalysis, and a urine pregnancy test (for women of child-bearing age). The CBC will usually reveal a mild leukocytosis. The urinalysis may reveal sterile pyuria for the reason previously described. Additional testing may be indicated by the history and physical examination.
A CT scan of the abdomen and pelvis is considered the gold standard with regards to imaging, with a reported sensitivity of 94% and specificity of 99%.3 Ideally, the CT scan should include both oral and IV contrast; however, IV alone is frequently used. In addition to identifying diverticulitis, CT can also visualize complications, including abscesses, perforation, and bowel obstruction. Ultrasound using high-resolution, graded compassion has a similar sensitivity and specificity as CT, with the advantage of less cost, can be performed at the bedside, and avoids radiation exposure.3 However, it is operator dependent and inferior to CT regarding visualizing complications.
Historically, antibiotics have been considered the treatment of choice for patients with acute uncomplicated diverticulitis, usually as an outpatient. Typically, this involves prescribing ciprofloxacin (or trimethoprim-sulfamethoxazole) plus metronidazole for 7 to 10 days. Monotherapy consisting of either moxifloxacin or amoxicillin/clavulanic acid is also acceptable. However, as our understanding of the important role of inflammation in this disease process, combined with the negative effects associated with antibiotic use, the role of antibiotics in uncomplicated diverticulitis has been called into question. In one recent study of 155 patients with acute uncomplicated diverticulitis, 97% were managed successfully as outpatients without antibiotics, admission, or complications.4 The American Gastroenterological Association (AGA) recommends that antibiotics should be used selectively, rather than routinely, in patients with acute uncomplicated diverticulitis. However, this is considered a “conditional recommendation with a low quality of evidence.”1In other words, this recommendation could easily change based on newer studies. Similarly, other “conditional recommendations” by the AGA include suggesting a fiber-rich diet, or fiber supplementation, and no need to avoid the consumption of nuts and popcorn.1 The majority of these patients begin to feel better in 2 to 3 days and have a good outcome.
Summary
For patients that appear ill, have significant comorbidities, are immunocompromised, or have a complication of acute diverticulitis, admission to the hospital with surgery consultation is recommended. For abscesses, interventional radiology has been used with success for CT-guided percutaneous drainage of diverticular abscesses. Intravenous antibiotics should be initiated; appropriate medications include metronidazole plus a third-generation cephalosporin (such as ceftriaxone or cefotaxime) or a fluoroquinolone (such as ciprofloxacin or levofloxacin). Monotherapy for the moderately ill patient includes piperacillin/tazobactam, ampicillin/sulbactam, ticarcillin/clavulanic acid, and imipenem. In addition, these patients should be placed at bowel rest (ie, nothing by mouth) with IV fluid resuscitation and hydration.
A 44-year-old woman presented to the ED complaining of crampy lower abdominal pain with nausea and vomiting. The patient described gradual onset 2 days prior, with symptoms worsening over the previous 12 hours. She denied diarrhea, constipation, or blood in her stool. She did admit to frequency of urination, but no dysuria or hematuria. She was gravida 2, para 2, aborta 0, with a last menstrual period 3 weeks prior. She denied vaginal bleeding or discharge. Her past medical history was unremarkable, she was on no medications, and denied alcohol use. She did admit to smoking one pack of cigarettes per day.
On physical examination, the patient’s vital signs were: blood pressure, 132/68 mm Hg; heart rate, 96 beats/min; respiratory rate, 18 breaths/min; and temperature, 99.8°F. Oxygen saturation was 99% on room air.
The head, ears, eyes, nose, and throat (HEENT) examination was completely normal, as was the heart and lung examination. The patient was tender to palpation in the lower abdomen, but without guarding or rebound. Bowel sounds were present and normoactive. A pelvic examination, including a bimanual examination, demonstrated mild left ovarian tenderness but without mass or cervical motion tenderness. The patient did not exhibit any costovertebral angle tenderness bilaterally. No rectal examination was performed.
The emergency physicians (EPs) ordered a complete blood count (CBC), basic metabolic profile (BMP), urinalysis, urine pregnancy test, and a vaginal wet preparation. In addition, the patient was administered 500 cc’s of normal saline intravenously (IV) and ondansetron (Zofran) 4 mg IV.
The urine pregnancy test result came back negative. The urinalysis was remarkable for positive leukocyte esterase, with five to 10 white cells and bacteria present. The CBC showed a mild leukocytosis, but with a normal hemoglobin and hematocrit. The BMP and vaginal wet preparation were completely normal.
The EP was concerned the patient might have something more serious than a simple urinary tract infection (UTI), so she ordered a computed tomography (CT) scan of the abdomen and pelvis with IV contrast.
The radiologist interpreted the CT scan as normal. The patient was discharged home with a prescription for an antibiotic for her UTI, encouraged to drink liquids, and instructed to follow-up with her primary care physician in 1 week.
The patient returned to the same ED approximately 48 hours later with worsening abdominal pain. On this presentation, she was tachycardic (110 beats/min) with a temperature of 101°F. The abdominal examination was remarkable for diffuse tenderness and voluntary guarding. The patient was administered IV fluids, morphine, and ondansetron. A repeat CT scan of the abdomen and pelvis with IV contrast showed a perforated sigmoid colon, with leakage of bowel contents into the peritoneum. The EP immediately started IV fluid resuscitation and administered IV antibiotics. The patient was taken emergently to the operating room by general surgery. The colon was repaired and a colostomy placed. The patient was able to be discharged home on day number 5.
The patient sued the hospital and the treating EP for failure to make the proper diagnosis on the initial ED visit, resulting in the patient having a long and difficult recovery, and the need for a colostomy. At trial, the jury returned a defense verdict.
Discussion
Diverticulitis, and its complications, account for a significant number of ED visits. It is the third most common inpatient gastrointestinal diagnosis in the United States, costing two billion dollars annually.1It is defined as clinically evident microscopic inflammation of a diverticulum or diverticula, and occurs in approximately 4% of patients with diverticulosis.1It is estimated that roughly 15% of these patients will experience a complication, defined as an abscess, perforation, fistula, or colonic obstruction; 15% to 30% will experience a recurrence.
The mean age of patients admitted to the hospital for diverticulitis is 63 years. While considered a disease of older patients, it should be included in the differential diagnosis for younger patients, as approximately 16% of admissions for acute diverticulitis are in patients less than 45 years.2Risk factors include poor diet (ie, low fiber, high fat, red meat), obesity, and smoking. The clinical presentation of diverticulitis has sometimes been referred to as “left-sided appendicitis” because of the similarities between the two entities. Patients will frequently complain of anorexia, change in bowel habits (either diarrhea or constipation), crampy abdominal pain (primarily in the left lower quadrant), low grade fever, and nausea with vomiting. Interestingly, 10% to 15% of patients with acute diverticulitis will complain of dysuria, urgency, or frequency (as in this patient) due to irritation of the bladder from an inflamed sigmoid colon.
Physical examination may reveal a low grade fever and tachycardia, if significant vomiting has been present. The abdomen is tender primarily in the left lower quadrant. The presence of severe tachycardia, hypotension, or a rigid abdomen with guarding and rebound suggests perforation. A pelvic examination should be performed on all women of child-bearing age. The rectal examination may reveal hemoccult positive stool; gross blood is rare.
Laboratory testing should include a CBC, BMP, urinalysis, and a urine pregnancy test (for women of child-bearing age). The CBC will usually reveal a mild leukocytosis. The urinalysis may reveal sterile pyuria for the reason previously described. Additional testing may be indicated by the history and physical examination.
A CT scan of the abdomen and pelvis is considered the gold standard with regards to imaging, with a reported sensitivity of 94% and specificity of 99%.3 Ideally, the CT scan should include both oral and IV contrast; however, IV alone is frequently used. In addition to identifying diverticulitis, CT can also visualize complications, including abscesses, perforation, and bowel obstruction. Ultrasound using high-resolution, graded compassion has a similar sensitivity and specificity as CT, with the advantage of less cost, can be performed at the bedside, and avoids radiation exposure.3 However, it is operator dependent and inferior to CT regarding visualizing complications.
Historically, antibiotics have been considered the treatment of choice for patients with acute uncomplicated diverticulitis, usually as an outpatient. Typically, this involves prescribing ciprofloxacin (or trimethoprim-sulfamethoxazole) plus metronidazole for 7 to 10 days. Monotherapy consisting of either moxifloxacin or amoxicillin/clavulanic acid is also acceptable. However, as our understanding of the important role of inflammation in this disease process, combined with the negative effects associated with antibiotic use, the role of antibiotics in uncomplicated diverticulitis has been called into question. In one recent study of 155 patients with acute uncomplicated diverticulitis, 97% were managed successfully as outpatients without antibiotics, admission, or complications.4 The American Gastroenterological Association (AGA) recommends that antibiotics should be used selectively, rather than routinely, in patients with acute uncomplicated diverticulitis. However, this is considered a “conditional recommendation with a low quality of evidence.”1In other words, this recommendation could easily change based on newer studies. Similarly, other “conditional recommendations” by the AGA include suggesting a fiber-rich diet, or fiber supplementation, and no need to avoid the consumption of nuts and popcorn.1 The majority of these patients begin to feel better in 2 to 3 days and have a good outcome.
Summary
For patients that appear ill, have significant comorbidities, are immunocompromised, or have a complication of acute diverticulitis, admission to the hospital with surgery consultation is recommended. For abscesses, interventional radiology has been used with success for CT-guided percutaneous drainage of diverticular abscesses. Intravenous antibiotics should be initiated; appropriate medications include metronidazole plus a third-generation cephalosporin (such as ceftriaxone or cefotaxime) or a fluoroquinolone (such as ciprofloxacin or levofloxacin). Monotherapy for the moderately ill patient includes piperacillin/tazobactam, ampicillin/sulbactam, ticarcillin/clavulanic acid, and imipenem. In addition, these patients should be placed at bowel rest (ie, nothing by mouth) with IV fluid resuscitation and hydration.
1. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149(7):1944-1949. doi:10.1053/j.gastro.2015.10.003.
2. Nguyen GC, Sam J, Anand N. Epidemiological trends and geographic variation in hospital admissions for diverticulitis in the United States. World J Gastroenterol. 2011;17(12):1600-1605. doi:10.3748/wjg.v17.i12.1600.
3. Laméris W, van Randen A, Bipat S, Bossuyt PM, Boermeester MA, Stoker J. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur Radiol. 2008;18(11):2498-2511. doi:10.1007/s00330-008-1018-6.
4. Isacson D, Thorisson A, Andreasson K, Nikberg M, Smedh K, Chabok A. Outpatient, non-antibiotic management in acute uncomplicated diverticulitis: a prospective study. Int J Colorectal Dis. 2015;30(9):1229-1234. doi:10.1007/s00384-015-2258-y.
1. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149(7):1944-1949. doi:10.1053/j.gastro.2015.10.003.
2. Nguyen GC, Sam J, Anand N. Epidemiological trends and geographic variation in hospital admissions for diverticulitis in the United States. World J Gastroenterol. 2011;17(12):1600-1605. doi:10.3748/wjg.v17.i12.1600.
3. Laméris W, van Randen A, Bipat S, Bossuyt PM, Boermeester MA, Stoker J. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur Radiol. 2008;18(11):2498-2511. doi:10.1007/s00330-008-1018-6.
4. Isacson D, Thorisson A, Andreasson K, Nikberg M, Smedh K, Chabok A. Outpatient, non-antibiotic management in acute uncomplicated diverticulitis: a prospective study. Int J Colorectal Dis. 2015;30(9):1229-1234. doi:10.1007/s00384-015-2258-y.
New MI definition from ESC 2018
Also today, aspirin and fish oil flop in patients with diabetes, CT angiography cuts MI in patients with stable chest pain, and rivaroxaban is no help for heart failure outcomes.
Also today, aspirin and fish oil flop in patients with diabetes, CT angiography cuts MI in patients with stable chest pain, and rivaroxaban is no help for heart failure outcomes.
Also today, aspirin and fish oil flop in patients with diabetes, CT angiography cuts MI in patients with stable chest pain, and rivaroxaban is no help for heart failure outcomes.
Age cutoff suggested for STI screening in HIV patients
WASHINGTON – Current guidelines recommend a minimum of annual screening for gonorrhea and chlamydia in all sexually active individuals with HIV infection. However, this goal, which is frequently not attained in HIV clinics, may be excessive in certain populations with HIV infection.
In particular, women as well as men who have sex exclusively with women (MSW) may best be served by targeted, age-based screening rather than universal screening, according to a presentation by Susan A. Tuddenham, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore.
“Detection and treatment of gonorrhea and chlamydia in HIV-positive patients in the United States is a priority both because of patient morbidity and because of the potential for these infections to enhance transmission of HIV,” said Dr. Tuddenham.
She and her colleagues assessed data from 16,864 gonorrhea and chlamydia tests of all adults in care at three HIV Research Network sites during 2011-2014. She presented the data at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
They assessed the number needed to screen (NNS) in order to identify a single infection across three risk populations of individuals with HIV infection: 1,123 women, 1,236 men who have sex only with women (MSW), and 3,501 men who have sex with men (MSM). NNS was defined as the number of persons tested divided by the number who tested positive and was calculated for the three risk groups for urogenital and extragenital (rectal and pharyngeal) sampling and by age.
Dr. Tuddenham and her colleagues found that NNS based on urogenital screening was similar in all three groups for those individuals aged younger than or equal to 25 years: 15 for women (95% confidence interval, 9-71); 21 for MSW (95% CI, 6-171); and 20 for MSM (95% CI, 12-36). However at ages greater than 25 years, the picture changed, with urogenital NNS increasing to 363 for women (95% CI, 167-1000); 160 for MSW (95% CI, 100-333). For MSM over the age of 25 years, however, the NNS only increased to 46 (95% CI, 38-56).
There were insufficient numbers of extragenital screenings of women and MSW for analysis. But for MSM, rectal NNS was 5 and 10 for those men aged 25 years and younger and those aged over age 25 years, respectively, and pharyngeal NNS was 8 and 20 for the two groups, respectively.
“Our results provide some support for age-based screening cutoffs for women and MSW, with universal screening appropriate for those less than or equal to 25 years of age, and targeted screening for those over 25,” said Dr. Tuddenham. She emphasized the importance of continued universal screening of MSM of all ages for gonorrhea/chlamydia, in particular using extragenital screening as well in order to capture those missed by urogenital screening alone.
Dr. Tuddenham reported that she had no disclosures.
WASHINGTON – Current guidelines recommend a minimum of annual screening for gonorrhea and chlamydia in all sexually active individuals with HIV infection. However, this goal, which is frequently not attained in HIV clinics, may be excessive in certain populations with HIV infection.
In particular, women as well as men who have sex exclusively with women (MSW) may best be served by targeted, age-based screening rather than universal screening, according to a presentation by Susan A. Tuddenham, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore.
“Detection and treatment of gonorrhea and chlamydia in HIV-positive patients in the United States is a priority both because of patient morbidity and because of the potential for these infections to enhance transmission of HIV,” said Dr. Tuddenham.
She and her colleagues assessed data from 16,864 gonorrhea and chlamydia tests of all adults in care at three HIV Research Network sites during 2011-2014. She presented the data at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
They assessed the number needed to screen (NNS) in order to identify a single infection across three risk populations of individuals with HIV infection: 1,123 women, 1,236 men who have sex only with women (MSW), and 3,501 men who have sex with men (MSM). NNS was defined as the number of persons tested divided by the number who tested positive and was calculated for the three risk groups for urogenital and extragenital (rectal and pharyngeal) sampling and by age.
Dr. Tuddenham and her colleagues found that NNS based on urogenital screening was similar in all three groups for those individuals aged younger than or equal to 25 years: 15 for women (95% confidence interval, 9-71); 21 for MSW (95% CI, 6-171); and 20 for MSM (95% CI, 12-36). However at ages greater than 25 years, the picture changed, with urogenital NNS increasing to 363 for women (95% CI, 167-1000); 160 for MSW (95% CI, 100-333). For MSM over the age of 25 years, however, the NNS only increased to 46 (95% CI, 38-56).
There were insufficient numbers of extragenital screenings of women and MSW for analysis. But for MSM, rectal NNS was 5 and 10 for those men aged 25 years and younger and those aged over age 25 years, respectively, and pharyngeal NNS was 8 and 20 for the two groups, respectively.
“Our results provide some support for age-based screening cutoffs for women and MSW, with universal screening appropriate for those less than or equal to 25 years of age, and targeted screening for those over 25,” said Dr. Tuddenham. She emphasized the importance of continued universal screening of MSM of all ages for gonorrhea/chlamydia, in particular using extragenital screening as well in order to capture those missed by urogenital screening alone.
Dr. Tuddenham reported that she had no disclosures.
WASHINGTON – Current guidelines recommend a minimum of annual screening for gonorrhea and chlamydia in all sexually active individuals with HIV infection. However, this goal, which is frequently not attained in HIV clinics, may be excessive in certain populations with HIV infection.
In particular, women as well as men who have sex exclusively with women (MSW) may best be served by targeted, age-based screening rather than universal screening, according to a presentation by Susan A. Tuddenham, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore.
“Detection and treatment of gonorrhea and chlamydia in HIV-positive patients in the United States is a priority both because of patient morbidity and because of the potential for these infections to enhance transmission of HIV,” said Dr. Tuddenham.
She and her colleagues assessed data from 16,864 gonorrhea and chlamydia tests of all adults in care at three HIV Research Network sites during 2011-2014. She presented the data at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
They assessed the number needed to screen (NNS) in order to identify a single infection across three risk populations of individuals with HIV infection: 1,123 women, 1,236 men who have sex only with women (MSW), and 3,501 men who have sex with men (MSM). NNS was defined as the number of persons tested divided by the number who tested positive and was calculated for the three risk groups for urogenital and extragenital (rectal and pharyngeal) sampling and by age.
Dr. Tuddenham and her colleagues found that NNS based on urogenital screening was similar in all three groups for those individuals aged younger than or equal to 25 years: 15 for women (95% confidence interval, 9-71); 21 for MSW (95% CI, 6-171); and 20 for MSM (95% CI, 12-36). However at ages greater than 25 years, the picture changed, with urogenital NNS increasing to 363 for women (95% CI, 167-1000); 160 for MSW (95% CI, 100-333). For MSM over the age of 25 years, however, the NNS only increased to 46 (95% CI, 38-56).
There were insufficient numbers of extragenital screenings of women and MSW for analysis. But for MSM, rectal NNS was 5 and 10 for those men aged 25 years and younger and those aged over age 25 years, respectively, and pharyngeal NNS was 8 and 20 for the two groups, respectively.
“Our results provide some support for age-based screening cutoffs for women and MSW, with universal screening appropriate for those less than or equal to 25 years of age, and targeted screening for those over 25,” said Dr. Tuddenham. She emphasized the importance of continued universal screening of MSM of all ages for gonorrhea/chlamydia, in particular using extragenital screening as well in order to capture those missed by urogenital screening alone.
Dr. Tuddenham reported that she had no disclosures.
REPORTING FROM THE 2018 STD PREVENTION CONFERENCE
Key clinical point: Men who have sex with men with HIV should be regularly screened for STIs regardless of age.
Major finding: The number needed to screen to detect an STI in individuals infected with HIV aged over 25 years were 363 (women); 160 (men who have sex exclusively with women); and 46 (men who have sex with men).
Study details: Gonorrhea/chlamydia tests were assessed from 16,864 individuals infected with HIV and number needed to screen calculated.
Disclosures: Dr. Tuddenham reported that she had no disclosures.
Baseline patient-reported physical function predicts SLE-related mortality
The baseline physical component score of the Medical Outcomes Short Form 36 (SF-36) was an independent predictor of mortality for patients with systemic lupus erythematosus (SLE), according to recent analysis of data from the University of California, San Francisco, (UCSF) Lupus Outcomes Study.
Desiree R. Azizoddin, PsyD, of Stanford Health Care in Palo Alto, Calif., and her colleagues analyzed patient-reported outcomes (PROs) of 728 patients with SLE from the UCSF study, which included measures such as self-rated health, the Center for Epidemiologic Studies Depression scale (CESD), and SF-36 mental and physical component scores between 2007 and 2015. Patients had a mean age of 50.6 years and consisted of mostly whites (68.5%) and women (92.2%), with a mean SLE disease duration of 16.7 years.
“As PROs can be measured easily, quickly, reliably in a systematic manner, without use of many resources (including cost) and experts, PROs can serve as an important tool in community settings that provide care for individuals with SLE,” Dr. Azizoddin and her colleagues wrote in Arthritis Care & Research. “Implementation and integration of PROs may potentially help decrease mortality among patients with SLE in the long run.”
The researchers reported 71 deaths (9.8%) in the study. A univariate analysis showed that self-rated health as fair or poor and all SF-36 subscale scores, except for mental health and role emotional subscales, were predictors of mortality. After the investigators performed a multivariate analysis, they found that baseline SF-36 physical component scores alone were associated with an increased mortality risk (hazard ratio, 0.97; 95% confidence interval, 0.94-0.99; P less than .01), with a 3.5% lower risk for each point-rating increase in the score.
“Insight to predictors of mortality, above and beyond those that require physician resources such as assessments of renal involvement and traditional physician-completed measures of disease activity and damage in this complex disease, affords rheumatologists and SLE providers with additional tools to assess potential patient health trajectories,” Dr. Azizoddin and her colleagues wrote in their study. “Identification of such predictors will expand opportunities for early interventions aimed at the reduction of such risk, including allocation of appropriate medical resources in areas with large numbers of patients with compromised health.”
The researchers noted that bias in self-reporting, a lack of prospective evaluations at each visit, and clinical applicability to only similar cohorts were limitations in the study.
This study was funded by the Robert Wood Johnson Foundation Investigator Awards in Health Policy Research and individually was awarded grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
SOURCE: Azizoddin DR et al. Arthritis Care Res. 2018 Aug 24. doi: 10.1002/acr.23734.
The baseline physical component score of the Medical Outcomes Short Form 36 (SF-36) was an independent predictor of mortality for patients with systemic lupus erythematosus (SLE), according to recent analysis of data from the University of California, San Francisco, (UCSF) Lupus Outcomes Study.
Desiree R. Azizoddin, PsyD, of Stanford Health Care in Palo Alto, Calif., and her colleagues analyzed patient-reported outcomes (PROs) of 728 patients with SLE from the UCSF study, which included measures such as self-rated health, the Center for Epidemiologic Studies Depression scale (CESD), and SF-36 mental and physical component scores between 2007 and 2015. Patients had a mean age of 50.6 years and consisted of mostly whites (68.5%) and women (92.2%), with a mean SLE disease duration of 16.7 years.
“As PROs can be measured easily, quickly, reliably in a systematic manner, without use of many resources (including cost) and experts, PROs can serve as an important tool in community settings that provide care for individuals with SLE,” Dr. Azizoddin and her colleagues wrote in Arthritis Care & Research. “Implementation and integration of PROs may potentially help decrease mortality among patients with SLE in the long run.”
The researchers reported 71 deaths (9.8%) in the study. A univariate analysis showed that self-rated health as fair or poor and all SF-36 subscale scores, except for mental health and role emotional subscales, were predictors of mortality. After the investigators performed a multivariate analysis, they found that baseline SF-36 physical component scores alone were associated with an increased mortality risk (hazard ratio, 0.97; 95% confidence interval, 0.94-0.99; P less than .01), with a 3.5% lower risk for each point-rating increase in the score.
“Insight to predictors of mortality, above and beyond those that require physician resources such as assessments of renal involvement and traditional physician-completed measures of disease activity and damage in this complex disease, affords rheumatologists and SLE providers with additional tools to assess potential patient health trajectories,” Dr. Azizoddin and her colleagues wrote in their study. “Identification of such predictors will expand opportunities for early interventions aimed at the reduction of such risk, including allocation of appropriate medical resources in areas with large numbers of patients with compromised health.”
The researchers noted that bias in self-reporting, a lack of prospective evaluations at each visit, and clinical applicability to only similar cohorts were limitations in the study.
This study was funded by the Robert Wood Johnson Foundation Investigator Awards in Health Policy Research and individually was awarded grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
SOURCE: Azizoddin DR et al. Arthritis Care Res. 2018 Aug 24. doi: 10.1002/acr.23734.
The baseline physical component score of the Medical Outcomes Short Form 36 (SF-36) was an independent predictor of mortality for patients with systemic lupus erythematosus (SLE), according to recent analysis of data from the University of California, San Francisco, (UCSF) Lupus Outcomes Study.
Desiree R. Azizoddin, PsyD, of Stanford Health Care in Palo Alto, Calif., and her colleagues analyzed patient-reported outcomes (PROs) of 728 patients with SLE from the UCSF study, which included measures such as self-rated health, the Center for Epidemiologic Studies Depression scale (CESD), and SF-36 mental and physical component scores between 2007 and 2015. Patients had a mean age of 50.6 years and consisted of mostly whites (68.5%) and women (92.2%), with a mean SLE disease duration of 16.7 years.
“As PROs can be measured easily, quickly, reliably in a systematic manner, without use of many resources (including cost) and experts, PROs can serve as an important tool in community settings that provide care for individuals with SLE,” Dr. Azizoddin and her colleagues wrote in Arthritis Care & Research. “Implementation and integration of PROs may potentially help decrease mortality among patients with SLE in the long run.”
The researchers reported 71 deaths (9.8%) in the study. A univariate analysis showed that self-rated health as fair or poor and all SF-36 subscale scores, except for mental health and role emotional subscales, were predictors of mortality. After the investigators performed a multivariate analysis, they found that baseline SF-36 physical component scores alone were associated with an increased mortality risk (hazard ratio, 0.97; 95% confidence interval, 0.94-0.99; P less than .01), with a 3.5% lower risk for each point-rating increase in the score.
“Insight to predictors of mortality, above and beyond those that require physician resources such as assessments of renal involvement and traditional physician-completed measures of disease activity and damage in this complex disease, affords rheumatologists and SLE providers with additional tools to assess potential patient health trajectories,” Dr. Azizoddin and her colleagues wrote in their study. “Identification of such predictors will expand opportunities for early interventions aimed at the reduction of such risk, including allocation of appropriate medical resources in areas with large numbers of patients with compromised health.”
The researchers noted that bias in self-reporting, a lack of prospective evaluations at each visit, and clinical applicability to only similar cohorts were limitations in the study.
This study was funded by the Robert Wood Johnson Foundation Investigator Awards in Health Policy Research and individually was awarded grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
SOURCE: Azizoddin DR et al. Arthritis Care Res. 2018 Aug 24. doi: 10.1002/acr.23734.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: There was a 3.5% lower risk of mortality per each increased point rating on the SF-36 physical component score.
Study details: An analysis of 728 patients with SLE from the University of California, San Francisco, Lupus Outcomes Study.
Disclosures: This study was funded by the Robert Wood Johnson Foundation Investigator Awards in Health Policy Research and individually was awarded grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Source: Azizoddin DR et al. Arthritis Care Res. 2018 Aug 24. doi: 10.1002/acr.23734.
England green-lights coverage of one CAR T-cell therapy
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
Researchers find drug target in anaplastic large-cell lymphoma
Preclinical research indicates that TYK2 inhibitors could be effective in treating anaplastic large-cell lymphoma (ALCL).
Researchers found evidence to suggest that TYK2 “is highly expressed in all cases of human ALCL.”
The team also discovered that TYK2 inhibition induces apoptosis in human ALCL cells, and it delays tumor onset, and prolongs survival in a mouse model of ALCL.
Olaf Merkel, PhD, of the Medical University of Vienna in Austria, and his colleagues detailed these findings in Leukemia.
The researchers said their analyses suggest TYK2 is expressed in all types of ALCL, regardless of ALK status, and TYK2 mediates the same anti-apoptotic response across ALCLs.
“Therefore, we could consider TYK2 signaling as the Achilles’ heel of ALCL, as, in all patients we have analyzed, the tumor cells relied on this activity to support the essential survival signal,” Dr. Merkel said in a statement.
He and his colleagues found that disrupting TYK2 – either via gene knockdown or with small-molecule TYK2 inhibitors – induced apoptosis in human ALCL cells in vitro.
In a mouse model of NPM-ALK-induced lymphoma, Tyk2 deletion slowed the rate of tumor growth and significantly prolonged survival. The median survival was 53.3 weeks in mice with Tyk2 deletion and 16.0 weeks in control mice (P less than .0001).
Additional experiments in human ALCL cell lines showed that “TYK2 is activated by autocrine production of IL-10 and IL-22 and by interaction with specific receptors expressed by the cells,” the researchers said.
They also found that “activated TYK2 leads to STAT1 and STAT3 phosphorylation, activated expression of MCL1, and aberrant ALCL cell survival.”
Taking these findings together, the researchers concluded that TYK2 inhibitors could be effective for treating ALCL.
“We are looking forward to TYK2 inhibitors becoming available,” said study coauthor Lukas Kenner, MD, of the Medical University of Vienna. “[I]n the more rare lymphomas, we urgently need better therapies.”
The researchers received grant funding from various organizations but reported having no conflicts of interest.
SOURCE: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
Preclinical research indicates that TYK2 inhibitors could be effective in treating anaplastic large-cell lymphoma (ALCL).
Researchers found evidence to suggest that TYK2 “is highly expressed in all cases of human ALCL.”
The team also discovered that TYK2 inhibition induces apoptosis in human ALCL cells, and it delays tumor onset, and prolongs survival in a mouse model of ALCL.
Olaf Merkel, PhD, of the Medical University of Vienna in Austria, and his colleagues detailed these findings in Leukemia.
The researchers said their analyses suggest TYK2 is expressed in all types of ALCL, regardless of ALK status, and TYK2 mediates the same anti-apoptotic response across ALCLs.
“Therefore, we could consider TYK2 signaling as the Achilles’ heel of ALCL, as, in all patients we have analyzed, the tumor cells relied on this activity to support the essential survival signal,” Dr. Merkel said in a statement.
He and his colleagues found that disrupting TYK2 – either via gene knockdown or with small-molecule TYK2 inhibitors – induced apoptosis in human ALCL cells in vitro.
In a mouse model of NPM-ALK-induced lymphoma, Tyk2 deletion slowed the rate of tumor growth and significantly prolonged survival. The median survival was 53.3 weeks in mice with Tyk2 deletion and 16.0 weeks in control mice (P less than .0001).
Additional experiments in human ALCL cell lines showed that “TYK2 is activated by autocrine production of IL-10 and IL-22 and by interaction with specific receptors expressed by the cells,” the researchers said.
They also found that “activated TYK2 leads to STAT1 and STAT3 phosphorylation, activated expression of MCL1, and aberrant ALCL cell survival.”
Taking these findings together, the researchers concluded that TYK2 inhibitors could be effective for treating ALCL.
“We are looking forward to TYK2 inhibitors becoming available,” said study coauthor Lukas Kenner, MD, of the Medical University of Vienna. “[I]n the more rare lymphomas, we urgently need better therapies.”
The researchers received grant funding from various organizations but reported having no conflicts of interest.
SOURCE: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
Preclinical research indicates that TYK2 inhibitors could be effective in treating anaplastic large-cell lymphoma (ALCL).
Researchers found evidence to suggest that TYK2 “is highly expressed in all cases of human ALCL.”
The team also discovered that TYK2 inhibition induces apoptosis in human ALCL cells, and it delays tumor onset, and prolongs survival in a mouse model of ALCL.
Olaf Merkel, PhD, of the Medical University of Vienna in Austria, and his colleagues detailed these findings in Leukemia.
The researchers said their analyses suggest TYK2 is expressed in all types of ALCL, regardless of ALK status, and TYK2 mediates the same anti-apoptotic response across ALCLs.
“Therefore, we could consider TYK2 signaling as the Achilles’ heel of ALCL, as, in all patients we have analyzed, the tumor cells relied on this activity to support the essential survival signal,” Dr. Merkel said in a statement.
He and his colleagues found that disrupting TYK2 – either via gene knockdown or with small-molecule TYK2 inhibitors – induced apoptosis in human ALCL cells in vitro.
In a mouse model of NPM-ALK-induced lymphoma, Tyk2 deletion slowed the rate of tumor growth and significantly prolonged survival. The median survival was 53.3 weeks in mice with Tyk2 deletion and 16.0 weeks in control mice (P less than .0001).
Additional experiments in human ALCL cell lines showed that “TYK2 is activated by autocrine production of IL-10 and IL-22 and by interaction with specific receptors expressed by the cells,” the researchers said.
They also found that “activated TYK2 leads to STAT1 and STAT3 phosphorylation, activated expression of MCL1, and aberrant ALCL cell survival.”
Taking these findings together, the researchers concluded that TYK2 inhibitors could be effective for treating ALCL.
“We are looking forward to TYK2 inhibitors becoming available,” said study coauthor Lukas Kenner, MD, of the Medical University of Vienna. “[I]n the more rare lymphomas, we urgently need better therapies.”
The researchers received grant funding from various organizations but reported having no conflicts of interest.
SOURCE: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
FROM LEUKEMIA
Key clinical point:
Major finding: TYK2 was expressed in all types of ALCL studied and mediated the same anti-apoptotic response across ALCLs.
Study details: A preclinical study of mouse and human ALCL cell lines.
Disclosures: The researchers received grant funding from various organizations but reported having no conflicts of interest.
Source: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
ESMO scale offers guidance on cancer targets
The European Society for Medical Oncology (ESMO) has published a proposed scale that would rank molecular targets for various cancers by how well they can be treated with new or emerging drugs.
The ESMO Scale of Clinical Actionability for Molecular Targets is designed to “harmonize and standardize the reporting and interpretation of clinically relevant genomics data,” according to Joaquin Mateo, MD, PhD, from the Vall d’Hebron Institute of Oncology in Barcelona, Spain, and his fellow members of the ESMO Translational Research and Precision Medicine Working Group.
“A major challenge for oncologists in the clinic is to distinguish between findings that represent proven clinical value or potential value based on preliminary clinical or preclinical evidence from hypothetical gene-drug matches and findings that are currently irrelevant for clinical practice,” they wrote in Annals of Oncology.
The scale groups targets into one of six tiers based on levels of evidence ranging from the gold standard of prospective, randomized clinical trials to targets for which there are no evidence and only hypothetical actionability. The primary goal is to help oncologists assign priority to potential targets when they review results of gene-sequencing panels for individual patients, according to the developers.
Briefly, the six tiers are:
Tier I includes targets that are agreed to be suitable for routine use and a recommended specific drug when a specific molecular alteration is detected. Examples include trastuzumab for human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and inhibitors of epidermal growth factor receptor (EGFR) in patients with non–small cell lung cancer positive for EGFR mutations.
Tier II includes “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” This tier includes agents that work in the phosphatidylinostiol 3-kinase pathway.
Tier III is similar to Tier II, in that it includes investigational targets that define a patient population with proven benefit from a targeted therapy, but in this case the target is detected in a different tumor type that has not previously been studied. For example, the targeted agent vemurafenib (Zelboraf), which extends survival of patients with metastatic melanomas carrying the BRAF V600E mutation, has only limited activity against BRAF-mutated colorectal cancers.
Tier IV includes targets with preclinical evidence of actionability.
Tier V includes targets with “evidence of relevant antitumor activity, not resulting in clinical meaningful benefit as single treatment but supporting development of cotargeting approaches.” The authors cite the example of PIK3CA inhibitors in patients with estrogen receptor–positive, HER2-negative breast cancers who also have PIK3CA activating mutations. In clinical trials, this strategy led to objective responses but not change outcomes.
The final tier is not Tier VI, as might be expected, but Tier X, with the X in this case being the unknown – that is, alterations/mutations for which there is neither preclinical nor clinical evidence to support their hypothetical use as a drug target.
“This clinical benefit–centered classification system offers a common language for all the actors involved in clinical cancer drug development. Its implementation in sequencing reports, tumor boards, and scientific communication can enable precise treatment decisions and facilitate discussions with patients about novel therapeutic options,” Dr. Mateo and his associates wrote in their conclusion.
The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
SOURCE: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
The European Society for Medical Oncology (ESMO) has published a proposed scale that would rank molecular targets for various cancers by how well they can be treated with new or emerging drugs.
The ESMO Scale of Clinical Actionability for Molecular Targets is designed to “harmonize and standardize the reporting and interpretation of clinically relevant genomics data,” according to Joaquin Mateo, MD, PhD, from the Vall d’Hebron Institute of Oncology in Barcelona, Spain, and his fellow members of the ESMO Translational Research and Precision Medicine Working Group.
“A major challenge for oncologists in the clinic is to distinguish between findings that represent proven clinical value or potential value based on preliminary clinical or preclinical evidence from hypothetical gene-drug matches and findings that are currently irrelevant for clinical practice,” they wrote in Annals of Oncology.
The scale groups targets into one of six tiers based on levels of evidence ranging from the gold standard of prospective, randomized clinical trials to targets for which there are no evidence and only hypothetical actionability. The primary goal is to help oncologists assign priority to potential targets when they review results of gene-sequencing panels for individual patients, according to the developers.
Briefly, the six tiers are:
Tier I includes targets that are agreed to be suitable for routine use and a recommended specific drug when a specific molecular alteration is detected. Examples include trastuzumab for human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and inhibitors of epidermal growth factor receptor (EGFR) in patients with non–small cell lung cancer positive for EGFR mutations.
Tier II includes “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” This tier includes agents that work in the phosphatidylinostiol 3-kinase pathway.
Tier III is similar to Tier II, in that it includes investigational targets that define a patient population with proven benefit from a targeted therapy, but in this case the target is detected in a different tumor type that has not previously been studied. For example, the targeted agent vemurafenib (Zelboraf), which extends survival of patients with metastatic melanomas carrying the BRAF V600E mutation, has only limited activity against BRAF-mutated colorectal cancers.
Tier IV includes targets with preclinical evidence of actionability.
Tier V includes targets with “evidence of relevant antitumor activity, not resulting in clinical meaningful benefit as single treatment but supporting development of cotargeting approaches.” The authors cite the example of PIK3CA inhibitors in patients with estrogen receptor–positive, HER2-negative breast cancers who also have PIK3CA activating mutations. In clinical trials, this strategy led to objective responses but not change outcomes.
The final tier is not Tier VI, as might be expected, but Tier X, with the X in this case being the unknown – that is, alterations/mutations for which there is neither preclinical nor clinical evidence to support their hypothetical use as a drug target.
“This clinical benefit–centered classification system offers a common language for all the actors involved in clinical cancer drug development. Its implementation in sequencing reports, tumor boards, and scientific communication can enable precise treatment decisions and facilitate discussions with patients about novel therapeutic options,” Dr. Mateo and his associates wrote in their conclusion.
The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
SOURCE: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
The European Society for Medical Oncology (ESMO) has published a proposed scale that would rank molecular targets for various cancers by how well they can be treated with new or emerging drugs.
The ESMO Scale of Clinical Actionability for Molecular Targets is designed to “harmonize and standardize the reporting and interpretation of clinically relevant genomics data,” according to Joaquin Mateo, MD, PhD, from the Vall d’Hebron Institute of Oncology in Barcelona, Spain, and his fellow members of the ESMO Translational Research and Precision Medicine Working Group.
“A major challenge for oncologists in the clinic is to distinguish between findings that represent proven clinical value or potential value based on preliminary clinical or preclinical evidence from hypothetical gene-drug matches and findings that are currently irrelevant for clinical practice,” they wrote in Annals of Oncology.
The scale groups targets into one of six tiers based on levels of evidence ranging from the gold standard of prospective, randomized clinical trials to targets for which there are no evidence and only hypothetical actionability. The primary goal is to help oncologists assign priority to potential targets when they review results of gene-sequencing panels for individual patients, according to the developers.
Briefly, the six tiers are:
Tier I includes targets that are agreed to be suitable for routine use and a recommended specific drug when a specific molecular alteration is detected. Examples include trastuzumab for human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and inhibitors of epidermal growth factor receptor (EGFR) in patients with non–small cell lung cancer positive for EGFR mutations.
Tier II includes “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” This tier includes agents that work in the phosphatidylinostiol 3-kinase pathway.
Tier III is similar to Tier II, in that it includes investigational targets that define a patient population with proven benefit from a targeted therapy, but in this case the target is detected in a different tumor type that has not previously been studied. For example, the targeted agent vemurafenib (Zelboraf), which extends survival of patients with metastatic melanomas carrying the BRAF V600E mutation, has only limited activity against BRAF-mutated colorectal cancers.
Tier IV includes targets with preclinical evidence of actionability.
Tier V includes targets with “evidence of relevant antitumor activity, not resulting in clinical meaningful benefit as single treatment but supporting development of cotargeting approaches.” The authors cite the example of PIK3CA inhibitors in patients with estrogen receptor–positive, HER2-negative breast cancers who also have PIK3CA activating mutations. In clinical trials, this strategy led to objective responses but not change outcomes.
The final tier is not Tier VI, as might be expected, but Tier X, with the X in this case being the unknown – that is, alterations/mutations for which there is neither preclinical nor clinical evidence to support their hypothetical use as a drug target.
“This clinical benefit–centered classification system offers a common language for all the actors involved in clinical cancer drug development. Its implementation in sequencing reports, tumor boards, and scientific communication can enable precise treatment decisions and facilitate discussions with patients about novel therapeutic options,” Dr. Mateo and his associates wrote in their conclusion.
The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
SOURCE: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
FROM ANNALS OF ONCOLOGY
Key clinical point: The scale is intended to standardize reporting and interpretation of cancer gene panel results to help oncologists plan treatment.
Major finding: The scale divides current and future therapeutic targets into tiers based on levels of clinical and preclinical evidence.
Study details: Proposed guiding principles for a classification system developed by the Translational Research and Precision Medicine Working Group of the European Society of Medical Oncology.
Disclosures: The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
Source: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
MRI doubles rate of observation in low-risk prostate cancer
Men who undergo MRI of the prostate around the time of a low-risk prostate cancer diagnosis are nearly twice as likely to be managed with active surveillance as are men who do not get MRI, investigators found.
The findings suggest that MRI at the time of diagnosis can enhance patient and physician confidence in the decision to choose active surveillance (AS) over immediate surgery or radiation therapy in men with low-risk disease, according to Michael S. Leapman, MD, and his colleagues from Yale University, New Haven, Conn.
“Despite initial high costs associated with obtaining and interpreting MRI studies of the prostate, economic modeling studies imply that MRI would be cost effective if it resulted in increased utilization of AS for low- and very-low-risk PCa [prostate cancer]. The association identified in our study between MRI use and initial observation may serve as an informative basis for examining strategies to improve the quality of PCa care with the anticipated growing use of this technology,” they wrote in Urology.
Although active surveillance is increasingly accepted as an initial management strategy for patients with low-risk (Gleason score 6 or less) localized prostate cancer, the majority of patients with low-risk disease still receive definitive treatment.
“Although longitudinal studies support the safety of AS, uncertainty about the possibility of underestimating an indvidual’s risk of harboring aggressive disease remains a strong motivator to treat,” Dr. Leapman and his associates noted.
To see whether MRI of the prostate may have an effect on the use of active surveillance in men with low-risk disease, the investigators reviewed records from the Surveillance, Epidemiology and End Results (SEER) Medicare database to identify men diagnosed with low-risk prostate cancer during 2010-2013.
They looked at the association between MRI and patient management (ascertained by claims) and evaluated clinical and demographic factors associated with the receipt of MRI.
They identified 8,144 patients with low-risk prostate cancer during the study period, of whom 495 (6.1%) had undergone MRI scans. They found that the use of MRI in patients with low-risk cancer increased from 3.4% in 2010 to 10.5% in 2013.
MRI was performed significantly more frequently among 3,060 patients who were managed with observation, with 265 (8.7%) receiving scans, compared with 230 (4.5%) of the 5,084 patients who underwent treatment within a year of diagnosis.
In multivariable analysis that controlled for demographics, factors significantly associated with increased likelihood of undergoing observation versus definitive therapy included MRI, white vs. nonwhite race, later years of diagnosis, higher income status (by ZIP code), unmarried vs. married, treatment region (more common in the West and Midwest versus Northeast or South), and in referral regions with higher population density of urologists.
In a propensity score–matched analysis designed to smooth out potential confounders, the investigators found that receipt of MRI around the time of diagnosis was associated with a significantly higher likelihood of active surveillance, with an odds ratio of 1.90 (95% confidence interval, 1.56-2.32).
“Efforts to facilitate observational approaches for low-risk PCa are highly valuable to improving the quality of cancer care. Because the use of prostate MRI has grown, and is likely to continue expanding, the cost-effectiveness of MRI-driven pathways are increasingly relevant to the sustainability of the practice,” the authors wrote.
SOURCE: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
Men who undergo MRI of the prostate around the time of a low-risk prostate cancer diagnosis are nearly twice as likely to be managed with active surveillance as are men who do not get MRI, investigators found.
The findings suggest that MRI at the time of diagnosis can enhance patient and physician confidence in the decision to choose active surveillance (AS) over immediate surgery or radiation therapy in men with low-risk disease, according to Michael S. Leapman, MD, and his colleagues from Yale University, New Haven, Conn.
“Despite initial high costs associated with obtaining and interpreting MRI studies of the prostate, economic modeling studies imply that MRI would be cost effective if it resulted in increased utilization of AS for low- and very-low-risk PCa [prostate cancer]. The association identified in our study between MRI use and initial observation may serve as an informative basis for examining strategies to improve the quality of PCa care with the anticipated growing use of this technology,” they wrote in Urology.
Although active surveillance is increasingly accepted as an initial management strategy for patients with low-risk (Gleason score 6 or less) localized prostate cancer, the majority of patients with low-risk disease still receive definitive treatment.
“Although longitudinal studies support the safety of AS, uncertainty about the possibility of underestimating an indvidual’s risk of harboring aggressive disease remains a strong motivator to treat,” Dr. Leapman and his associates noted.
To see whether MRI of the prostate may have an effect on the use of active surveillance in men with low-risk disease, the investigators reviewed records from the Surveillance, Epidemiology and End Results (SEER) Medicare database to identify men diagnosed with low-risk prostate cancer during 2010-2013.
They looked at the association between MRI and patient management (ascertained by claims) and evaluated clinical and demographic factors associated with the receipt of MRI.
They identified 8,144 patients with low-risk prostate cancer during the study period, of whom 495 (6.1%) had undergone MRI scans. They found that the use of MRI in patients with low-risk cancer increased from 3.4% in 2010 to 10.5% in 2013.
MRI was performed significantly more frequently among 3,060 patients who were managed with observation, with 265 (8.7%) receiving scans, compared with 230 (4.5%) of the 5,084 patients who underwent treatment within a year of diagnosis.
In multivariable analysis that controlled for demographics, factors significantly associated with increased likelihood of undergoing observation versus definitive therapy included MRI, white vs. nonwhite race, later years of diagnosis, higher income status (by ZIP code), unmarried vs. married, treatment region (more common in the West and Midwest versus Northeast or South), and in referral regions with higher population density of urologists.
In a propensity score–matched analysis designed to smooth out potential confounders, the investigators found that receipt of MRI around the time of diagnosis was associated with a significantly higher likelihood of active surveillance, with an odds ratio of 1.90 (95% confidence interval, 1.56-2.32).
“Efforts to facilitate observational approaches for low-risk PCa are highly valuable to improving the quality of cancer care. Because the use of prostate MRI has grown, and is likely to continue expanding, the cost-effectiveness of MRI-driven pathways are increasingly relevant to the sustainability of the practice,” the authors wrote.
SOURCE: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
Men who undergo MRI of the prostate around the time of a low-risk prostate cancer diagnosis are nearly twice as likely to be managed with active surveillance as are men who do not get MRI, investigators found.
The findings suggest that MRI at the time of diagnosis can enhance patient and physician confidence in the decision to choose active surveillance (AS) over immediate surgery or radiation therapy in men with low-risk disease, according to Michael S. Leapman, MD, and his colleagues from Yale University, New Haven, Conn.
“Despite initial high costs associated with obtaining and interpreting MRI studies of the prostate, economic modeling studies imply that MRI would be cost effective if it resulted in increased utilization of AS for low- and very-low-risk PCa [prostate cancer]. The association identified in our study between MRI use and initial observation may serve as an informative basis for examining strategies to improve the quality of PCa care with the anticipated growing use of this technology,” they wrote in Urology.
Although active surveillance is increasingly accepted as an initial management strategy for patients with low-risk (Gleason score 6 or less) localized prostate cancer, the majority of patients with low-risk disease still receive definitive treatment.
“Although longitudinal studies support the safety of AS, uncertainty about the possibility of underestimating an indvidual’s risk of harboring aggressive disease remains a strong motivator to treat,” Dr. Leapman and his associates noted.
To see whether MRI of the prostate may have an effect on the use of active surveillance in men with low-risk disease, the investigators reviewed records from the Surveillance, Epidemiology and End Results (SEER) Medicare database to identify men diagnosed with low-risk prostate cancer during 2010-2013.
They looked at the association between MRI and patient management (ascertained by claims) and evaluated clinical and demographic factors associated with the receipt of MRI.
They identified 8,144 patients with low-risk prostate cancer during the study period, of whom 495 (6.1%) had undergone MRI scans. They found that the use of MRI in patients with low-risk cancer increased from 3.4% in 2010 to 10.5% in 2013.
MRI was performed significantly more frequently among 3,060 patients who were managed with observation, with 265 (8.7%) receiving scans, compared with 230 (4.5%) of the 5,084 patients who underwent treatment within a year of diagnosis.
In multivariable analysis that controlled for demographics, factors significantly associated with increased likelihood of undergoing observation versus definitive therapy included MRI, white vs. nonwhite race, later years of diagnosis, higher income status (by ZIP code), unmarried vs. married, treatment region (more common in the West and Midwest versus Northeast or South), and in referral regions with higher population density of urologists.
In a propensity score–matched analysis designed to smooth out potential confounders, the investigators found that receipt of MRI around the time of diagnosis was associated with a significantly higher likelihood of active surveillance, with an odds ratio of 1.90 (95% confidence interval, 1.56-2.32).
“Efforts to facilitate observational approaches for low-risk PCa are highly valuable to improving the quality of cancer care. Because the use of prostate MRI has grown, and is likely to continue expanding, the cost-effectiveness of MRI-driven pathways are increasingly relevant to the sustainability of the practice,” the authors wrote.
SOURCE: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
FROM UROLOGY
Key clinical point: MRI at screening or diagnosis of low-risk prostate cancer is associated with a higher likelihood of observation versus immediate definitive therapy.
Major finding: MRI was associated with a near doubling of the likelihood of observation.
Study details: Review of SEER Medicare data on 8,144 men diagnosed with low-risk prostate cancers during 2010-2013.
Disclosures: The study was supported by the National Cancer Institute, California Department of Public Health, and Centers for Disease Control and Prevention. The authors reported no relevant conflicts of interest.
Source: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
Rituximab/lenalidomide similar to rituximab/chemotherapy for follicular lymphoma
Rituximab plus lenalidomide had efficacy similar to that of rituximab plus chemotherapy in treatment of follicular lymphoma, according to results from a phase 3 trial.
RELEVANCE (NCT01476787) was a multicenter, international, randomized, open-label trial designed to determine the superiority of rituximab/lenalidomide over rituximab/chemotherapy.
This trial randomized 1,030 patients with previously untreated follicular lymphoma to receive either rituximab plus lenalidomide (n = 513) or rituximab plus chemotherapy (n = 517) for 18 cycles; both groups then went on to receive rituximab maintenance therapy for 12 cycles. The total duration of treatment was 120 weeks. The median age of the combined groups was 59 years. The study was published in the New England Journal of Medicine.
One of the coprimary endpoints was complete response (confirmed or unconfirmed) by the end of the treatment period; the other was progression-free survival, which was planned to be assessed through three analyses, including two interim analyses, the first of which was reported in this study.
After a median follow-up of 37.9 months, the rates of coprimary endpoints were similar between the two groups. Complete response (confirmed or unconfirmed) was seen in 48% of the rituximab/lenalidomide group (95% confidence interval [CI], 44-53) and in 53% of the rituximab/chemotherapy group (95% CI, 49-57; P = .13). The hazard ratio for progression or death from any cause was 1.10 (95% CI, 0.85-1.43; P = .48).
In the subgroup analyses, the efficacy of rituximab plus chemotherapy was greater in low-risk patients (based on Follicular Lymphoma International Prognostic Index scores) and in patients whose follicular lymphoma was Ann Arbor stage I or II, whereas efficacy of rituximab/lenalidomide was independent of prognostic factors.
Safety was the biggest area of difference, with some events being more common in one group than in the other. For example, cutaneous reactions, diarrhea, rash, and myalgia were more common with rituximab/lenalidomide treatment, whereas anemia, fatigue, nausea, and febrile neutropenia were more common with rituximab/chemotherapy treatment. Among grade 3 or 4 events, cutaneous reactions were more common with rituximab/lenalidomide, and grade 3 or 4 neutropenia was more common with rituximab/chemotherapy.
“Overall, both treatment groups showed good outcomes, and a median has not yet been reached for either progression-free survival or overall survival,” the study authors wrote.
The RELEVANCE trial was sponsored by Celgene and the Lymphoma Academic Research Organisation. The study authors reported various disclosures, including financial ties to Celgene.
SOURCE: Morschhauser F et al. N Engl J Med. 2018;379:934-47.
Rituximab plus lenalidomide had efficacy similar to that of rituximab plus chemotherapy in treatment of follicular lymphoma, according to results from a phase 3 trial.
RELEVANCE (NCT01476787) was a multicenter, international, randomized, open-label trial designed to determine the superiority of rituximab/lenalidomide over rituximab/chemotherapy.
This trial randomized 1,030 patients with previously untreated follicular lymphoma to receive either rituximab plus lenalidomide (n = 513) or rituximab plus chemotherapy (n = 517) for 18 cycles; both groups then went on to receive rituximab maintenance therapy for 12 cycles. The total duration of treatment was 120 weeks. The median age of the combined groups was 59 years. The study was published in the New England Journal of Medicine.
One of the coprimary endpoints was complete response (confirmed or unconfirmed) by the end of the treatment period; the other was progression-free survival, which was planned to be assessed through three analyses, including two interim analyses, the first of which was reported in this study.
After a median follow-up of 37.9 months, the rates of coprimary endpoints were similar between the two groups. Complete response (confirmed or unconfirmed) was seen in 48% of the rituximab/lenalidomide group (95% confidence interval [CI], 44-53) and in 53% of the rituximab/chemotherapy group (95% CI, 49-57; P = .13). The hazard ratio for progression or death from any cause was 1.10 (95% CI, 0.85-1.43; P = .48).
In the subgroup analyses, the efficacy of rituximab plus chemotherapy was greater in low-risk patients (based on Follicular Lymphoma International Prognostic Index scores) and in patients whose follicular lymphoma was Ann Arbor stage I or II, whereas efficacy of rituximab/lenalidomide was independent of prognostic factors.
Safety was the biggest area of difference, with some events being more common in one group than in the other. For example, cutaneous reactions, diarrhea, rash, and myalgia were more common with rituximab/lenalidomide treatment, whereas anemia, fatigue, nausea, and febrile neutropenia were more common with rituximab/chemotherapy treatment. Among grade 3 or 4 events, cutaneous reactions were more common with rituximab/lenalidomide, and grade 3 or 4 neutropenia was more common with rituximab/chemotherapy.
“Overall, both treatment groups showed good outcomes, and a median has not yet been reached for either progression-free survival or overall survival,” the study authors wrote.
The RELEVANCE trial was sponsored by Celgene and the Lymphoma Academic Research Organisation. The study authors reported various disclosures, including financial ties to Celgene.
SOURCE: Morschhauser F et al. N Engl J Med. 2018;379:934-47.
Rituximab plus lenalidomide had efficacy similar to that of rituximab plus chemotherapy in treatment of follicular lymphoma, according to results from a phase 3 trial.
RELEVANCE (NCT01476787) was a multicenter, international, randomized, open-label trial designed to determine the superiority of rituximab/lenalidomide over rituximab/chemotherapy.
This trial randomized 1,030 patients with previously untreated follicular lymphoma to receive either rituximab plus lenalidomide (n = 513) or rituximab plus chemotherapy (n = 517) for 18 cycles; both groups then went on to receive rituximab maintenance therapy for 12 cycles. The total duration of treatment was 120 weeks. The median age of the combined groups was 59 years. The study was published in the New England Journal of Medicine.
One of the coprimary endpoints was complete response (confirmed or unconfirmed) by the end of the treatment period; the other was progression-free survival, which was planned to be assessed through three analyses, including two interim analyses, the first of which was reported in this study.
After a median follow-up of 37.9 months, the rates of coprimary endpoints were similar between the two groups. Complete response (confirmed or unconfirmed) was seen in 48% of the rituximab/lenalidomide group (95% confidence interval [CI], 44-53) and in 53% of the rituximab/chemotherapy group (95% CI, 49-57; P = .13). The hazard ratio for progression or death from any cause was 1.10 (95% CI, 0.85-1.43; P = .48).
In the subgroup analyses, the efficacy of rituximab plus chemotherapy was greater in low-risk patients (based on Follicular Lymphoma International Prognostic Index scores) and in patients whose follicular lymphoma was Ann Arbor stage I or II, whereas efficacy of rituximab/lenalidomide was independent of prognostic factors.
Safety was the biggest area of difference, with some events being more common in one group than in the other. For example, cutaneous reactions, diarrhea, rash, and myalgia were more common with rituximab/lenalidomide treatment, whereas anemia, fatigue, nausea, and febrile neutropenia were more common with rituximab/chemotherapy treatment. Among grade 3 or 4 events, cutaneous reactions were more common with rituximab/lenalidomide, and grade 3 or 4 neutropenia was more common with rituximab/chemotherapy.
“Overall, both treatment groups showed good outcomes, and a median has not yet been reached for either progression-free survival or overall survival,” the study authors wrote.
The RELEVANCE trial was sponsored by Celgene and the Lymphoma Academic Research Organisation. The study authors reported various disclosures, including financial ties to Celgene.
SOURCE: Morschhauser F et al. N Engl J Med. 2018;379:934-47.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Complete responses were seen in 48% of rituximab/lenalidomide patients versus 53% in the rituximab/chemotherapy patients (P = .13).
Study details: A phase 3 superiority trial of 1,030 patients with previously untreated follicular lymphoma.
Disclosures: Celgene and the Lymphoma Academic Research Organization funded the study. The authors reported various disclosures, including financial ties to Celgene.
Source: Morschhauser F et al. N Engl J Med. 2018;379:934-47.