User login
Service, please: Hospital setting matters for pneumonia
the National Center for Health Statistics (NCHS) reported.
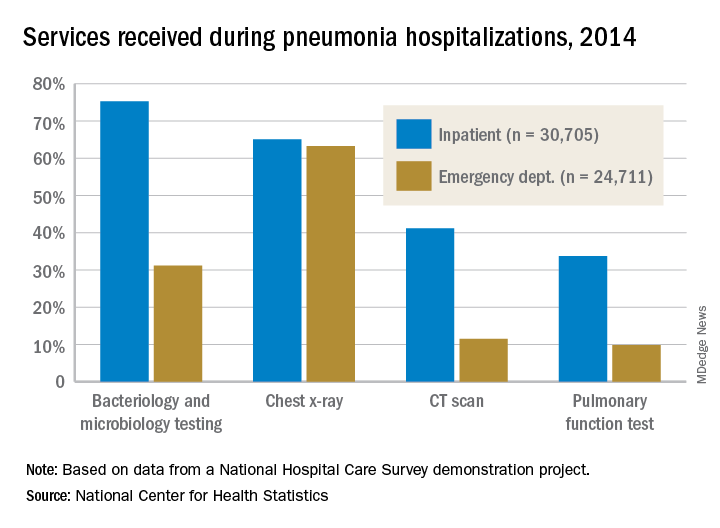
The percentages were not as close, however, for other diagnostic services. Inpatient stays were much more likely than ED encounters to involve bacteriology and microbiology testing (75.3% vs. 31.2%), CT scans (41.2% vs. 11.5%), and pulmonary function tests (33.7% vs. 9.8%), investigators from the NCHS said.
The age distribution of the two patient populations also were quite different, with those aged 65 years and older making up the largest share (46%) of pneumonia inpatients and the 15-and-under group representing the largest proportion (47%) of ED visits. For the inpatient setting, the smallest age group was those aged 15-44 years (10%), and for the ED it was those aged 65 years and older (14%), they reported.
The National Hospital Care Survey “is not yet nationally representative,” the NCHS investigators wrote – the overall sample for 2014 consisted of 581 hospitals – but “the number of encounters and the inclusion of [personally identifiable information] allow an example of analysis that was not previously possible.”
the National Center for Health Statistics (NCHS) reported.

The percentages were not as close, however, for other diagnostic services. Inpatient stays were much more likely than ED encounters to involve bacteriology and microbiology testing (75.3% vs. 31.2%), CT scans (41.2% vs. 11.5%), and pulmonary function tests (33.7% vs. 9.8%), investigators from the NCHS said.
The age distribution of the two patient populations also were quite different, with those aged 65 years and older making up the largest share (46%) of pneumonia inpatients and the 15-and-under group representing the largest proportion (47%) of ED visits. For the inpatient setting, the smallest age group was those aged 15-44 years (10%), and for the ED it was those aged 65 years and older (14%), they reported.
The National Hospital Care Survey “is not yet nationally representative,” the NCHS investigators wrote – the overall sample for 2014 consisted of 581 hospitals – but “the number of encounters and the inclusion of [personally identifiable information] allow an example of analysis that was not previously possible.”
the National Center for Health Statistics (NCHS) reported.

The percentages were not as close, however, for other diagnostic services. Inpatient stays were much more likely than ED encounters to involve bacteriology and microbiology testing (75.3% vs. 31.2%), CT scans (41.2% vs. 11.5%), and pulmonary function tests (33.7% vs. 9.8%), investigators from the NCHS said.
The age distribution of the two patient populations also were quite different, with those aged 65 years and older making up the largest share (46%) of pneumonia inpatients and the 15-and-under group representing the largest proportion (47%) of ED visits. For the inpatient setting, the smallest age group was those aged 15-44 years (10%), and for the ED it was those aged 65 years and older (14%), they reported.
The National Hospital Care Survey “is not yet nationally representative,” the NCHS investigators wrote – the overall sample for 2014 consisted of 581 hospitals – but “the number of encounters and the inclusion of [personally identifiable information] allow an example of analysis that was not previously possible.”
Topical Corticosteroid Tachyphylaxis: Why Don’t Patients Adhere to Treatment?
Pancreatic abnormalities found in one-quarter of patients with high-risk germline mutations
according to a recent study. However, screening individuals under the age of 50 years, even those with risk factors, is a low-yield strategy.
In a retrospective analysis of 86 asymptomatic adult patients with high-risk germline mutations followed at a single center, screening by a variety of imaging modalities showed a pancreatic abnormality in about one-quarter of patients (23 of 86, 26.7%). No cancers were detected on initial screening or during a median 29.8-month follow-up period, though the investigators saw more abnormalities in patients over the age of 60 years (P = .043).
The mutations were detected through genetic screening at the University of Texas MD Anderson Cancer Center, Houston, where the study was conducted.
“At our institution, based on oncology and/or medical genetics assessments, patients with a personal history of breast cancer and/or family history of [pancreatic cancer] are screened for BRCAs and BRCA1 mutations. Patients with other types of cancers in the family are screened for P53, STK11, MSH2, ATM, and APC mutations,” explained first author R. Tomás DaVee, MD, of the division of gastroenterology, hepatology, and nutrition at the University of Texas, Houston, and his collaborators.
Patients were considered to have a family history of pancreatic cancer (PC) if any first-, second-, or third-degree relative had PC; overall, this amounted to 37 patients (43%). For the study, familial PC was defined as having either two first-degree or three or more relatives of any degree diagnosed with PC.
Patients, who were a median age of 48.5 years, were included in the study if they had any of the germline mutations, which are associated with an increase in relative risk of PC ranging from 2.26 for BRCA1 to 132 for STK11/LKB1, the mutation that causes Peutz-Jeghers syndrome. Patients who had a family history alone, without high-risk germline mutations, were excluded from the study. Most participants (79.8%) were women, and most of the participants were BRCA2 or BRCA1 positive (58.1% and 16.3%, respectively).
Of the abnormalities found, almost half (47.8%) were cysts, and almost as many (43.5%) were hyperechoic strands and foci. Two patients (8.7% of abnormalities) had mild pancreatic duct dilation. Patients who had only fatty infiltration or pancreas divisum were classified as having normal variations rather than abnormalities.
The pancreatic abnormalities had been found through endoscopic ultrasound (EUS), CT, or MRI, though the study’s primary aim was to look at outcomes and diagnostic yield for high-risk germline mutation patients receiving EUS. A secondary aim of the study, said the authors, was to compare EUS with both MRI and CT as methods to screen for early PC.
In this regard, Dr. DaVee and his coauthors wrote that “EUS-based screening conferred a higher yield in detecting abnormal pancreatic findings, compared with MRI [P = .007].” For MRI in comparison with EUS as the criterion standard, sensitivity and specificity were 55.6% and 93.8% for detecting pancreatic abnormalities. CT fared a little worse, with sensitivity of 50% and specificity of 89.5% for detecting pancreatic abnormalities.
Overall, the investigators noted that “the frequency of [pancreatic abnormalities] in our study is lower than reported in some studies using alternate criteria.” The relatively young age of the study cohort may be partly responsible for this finding, they said, adding that “our data further support evidence that abnormal pancreatic findings increase with age ... in asymptomatic [high-risk individuals], including the subtle finding of hyperechoic strands and foci.”
To improve survival, “premalignant and early PC identification is key,” wrote Dr. DaVee and his coauthors, pointing out that, of the 10% of patients diagnosed with PC when it is still localized, the 5-year survival rate is about 31.5%, compared with the overall 8.2% of individuals with PC who are still alive 5 years after their diagnosis. The latter figure, they noted, is a small improvement over the 3% 5-year survival figure from 1975.
Since population-wide screening for PC would not have a favorable cost-benefit profile, the challenge is identifying which individuals might benefit from targeted screening for PC. Dr. DaVee and his colleagues suggested that, as a general rule, high-risk individuals aged younger than 50 years need not be screened, and that EUS be used for the index screening, with MRI used to follow individuals with unremarkable initial screenings.
None of the authors reported relevant conflicts of interest, and no outside source of funding was reported.
SOURCE: DaVee RT et al. Gastrointest Endosc. 2018 Jun; 87(6):1443-50.
according to a recent study. However, screening individuals under the age of 50 years, even those with risk factors, is a low-yield strategy.
In a retrospective analysis of 86 asymptomatic adult patients with high-risk germline mutations followed at a single center, screening by a variety of imaging modalities showed a pancreatic abnormality in about one-quarter of patients (23 of 86, 26.7%). No cancers were detected on initial screening or during a median 29.8-month follow-up period, though the investigators saw more abnormalities in patients over the age of 60 years (P = .043).
The mutations were detected through genetic screening at the University of Texas MD Anderson Cancer Center, Houston, where the study was conducted.
“At our institution, based on oncology and/or medical genetics assessments, patients with a personal history of breast cancer and/or family history of [pancreatic cancer] are screened for BRCAs and BRCA1 mutations. Patients with other types of cancers in the family are screened for P53, STK11, MSH2, ATM, and APC mutations,” explained first author R. Tomás DaVee, MD, of the division of gastroenterology, hepatology, and nutrition at the University of Texas, Houston, and his collaborators.
Patients were considered to have a family history of pancreatic cancer (PC) if any first-, second-, or third-degree relative had PC; overall, this amounted to 37 patients (43%). For the study, familial PC was defined as having either two first-degree or three or more relatives of any degree diagnosed with PC.
Patients, who were a median age of 48.5 years, were included in the study if they had any of the germline mutations, which are associated with an increase in relative risk of PC ranging from 2.26 for BRCA1 to 132 for STK11/LKB1, the mutation that causes Peutz-Jeghers syndrome. Patients who had a family history alone, without high-risk germline mutations, were excluded from the study. Most participants (79.8%) were women, and most of the participants were BRCA2 or BRCA1 positive (58.1% and 16.3%, respectively).
Of the abnormalities found, almost half (47.8%) were cysts, and almost as many (43.5%) were hyperechoic strands and foci. Two patients (8.7% of abnormalities) had mild pancreatic duct dilation. Patients who had only fatty infiltration or pancreas divisum were classified as having normal variations rather than abnormalities.
The pancreatic abnormalities had been found through endoscopic ultrasound (EUS), CT, or MRI, though the study’s primary aim was to look at outcomes and diagnostic yield for high-risk germline mutation patients receiving EUS. A secondary aim of the study, said the authors, was to compare EUS with both MRI and CT as methods to screen for early PC.
In this regard, Dr. DaVee and his coauthors wrote that “EUS-based screening conferred a higher yield in detecting abnormal pancreatic findings, compared with MRI [P = .007].” For MRI in comparison with EUS as the criterion standard, sensitivity and specificity were 55.6% and 93.8% for detecting pancreatic abnormalities. CT fared a little worse, with sensitivity of 50% and specificity of 89.5% for detecting pancreatic abnormalities.
Overall, the investigators noted that “the frequency of [pancreatic abnormalities] in our study is lower than reported in some studies using alternate criteria.” The relatively young age of the study cohort may be partly responsible for this finding, they said, adding that “our data further support evidence that abnormal pancreatic findings increase with age ... in asymptomatic [high-risk individuals], including the subtle finding of hyperechoic strands and foci.”
To improve survival, “premalignant and early PC identification is key,” wrote Dr. DaVee and his coauthors, pointing out that, of the 10% of patients diagnosed with PC when it is still localized, the 5-year survival rate is about 31.5%, compared with the overall 8.2% of individuals with PC who are still alive 5 years after their diagnosis. The latter figure, they noted, is a small improvement over the 3% 5-year survival figure from 1975.
Since population-wide screening for PC would not have a favorable cost-benefit profile, the challenge is identifying which individuals might benefit from targeted screening for PC. Dr. DaVee and his colleagues suggested that, as a general rule, high-risk individuals aged younger than 50 years need not be screened, and that EUS be used for the index screening, with MRI used to follow individuals with unremarkable initial screenings.
None of the authors reported relevant conflicts of interest, and no outside source of funding was reported.
SOURCE: DaVee RT et al. Gastrointest Endosc. 2018 Jun; 87(6):1443-50.
according to a recent study. However, screening individuals under the age of 50 years, even those with risk factors, is a low-yield strategy.
In a retrospective analysis of 86 asymptomatic adult patients with high-risk germline mutations followed at a single center, screening by a variety of imaging modalities showed a pancreatic abnormality in about one-quarter of patients (23 of 86, 26.7%). No cancers were detected on initial screening or during a median 29.8-month follow-up period, though the investigators saw more abnormalities in patients over the age of 60 years (P = .043).
The mutations were detected through genetic screening at the University of Texas MD Anderson Cancer Center, Houston, where the study was conducted.
“At our institution, based on oncology and/or medical genetics assessments, patients with a personal history of breast cancer and/or family history of [pancreatic cancer] are screened for BRCAs and BRCA1 mutations. Patients with other types of cancers in the family are screened for P53, STK11, MSH2, ATM, and APC mutations,” explained first author R. Tomás DaVee, MD, of the division of gastroenterology, hepatology, and nutrition at the University of Texas, Houston, and his collaborators.
Patients were considered to have a family history of pancreatic cancer (PC) if any first-, second-, or third-degree relative had PC; overall, this amounted to 37 patients (43%). For the study, familial PC was defined as having either two first-degree or three or more relatives of any degree diagnosed with PC.
Patients, who were a median age of 48.5 years, were included in the study if they had any of the germline mutations, which are associated with an increase in relative risk of PC ranging from 2.26 for BRCA1 to 132 for STK11/LKB1, the mutation that causes Peutz-Jeghers syndrome. Patients who had a family history alone, without high-risk germline mutations, were excluded from the study. Most participants (79.8%) were women, and most of the participants were BRCA2 or BRCA1 positive (58.1% and 16.3%, respectively).
Of the abnormalities found, almost half (47.8%) were cysts, and almost as many (43.5%) were hyperechoic strands and foci. Two patients (8.7% of abnormalities) had mild pancreatic duct dilation. Patients who had only fatty infiltration or pancreas divisum were classified as having normal variations rather than abnormalities.
The pancreatic abnormalities had been found through endoscopic ultrasound (EUS), CT, or MRI, though the study’s primary aim was to look at outcomes and diagnostic yield for high-risk germline mutation patients receiving EUS. A secondary aim of the study, said the authors, was to compare EUS with both MRI and CT as methods to screen for early PC.
In this regard, Dr. DaVee and his coauthors wrote that “EUS-based screening conferred a higher yield in detecting abnormal pancreatic findings, compared with MRI [P = .007].” For MRI in comparison with EUS as the criterion standard, sensitivity and specificity were 55.6% and 93.8% for detecting pancreatic abnormalities. CT fared a little worse, with sensitivity of 50% and specificity of 89.5% for detecting pancreatic abnormalities.
Overall, the investigators noted that “the frequency of [pancreatic abnormalities] in our study is lower than reported in some studies using alternate criteria.” The relatively young age of the study cohort may be partly responsible for this finding, they said, adding that “our data further support evidence that abnormal pancreatic findings increase with age ... in asymptomatic [high-risk individuals], including the subtle finding of hyperechoic strands and foci.”
To improve survival, “premalignant and early PC identification is key,” wrote Dr. DaVee and his coauthors, pointing out that, of the 10% of patients diagnosed with PC when it is still localized, the 5-year survival rate is about 31.5%, compared with the overall 8.2% of individuals with PC who are still alive 5 years after their diagnosis. The latter figure, they noted, is a small improvement over the 3% 5-year survival figure from 1975.
Since population-wide screening for PC would not have a favorable cost-benefit profile, the challenge is identifying which individuals might benefit from targeted screening for PC. Dr. DaVee and his colleagues suggested that, as a general rule, high-risk individuals aged younger than 50 years need not be screened, and that EUS be used for the index screening, with MRI used to follow individuals with unremarkable initial screenings.
None of the authors reported relevant conflicts of interest, and no outside source of funding was reported.
SOURCE: DaVee RT et al. Gastrointest Endosc. 2018 Jun; 87(6):1443-50.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: Pancreatic abnormalities increased with age in high-risk individuals.
Major finding: Imaging found pancreatic abnormalities in 23 of 86 high-risk individuals (26.7%).
Study details: A retrospective, single-center study of 86 patients with germline mutations placing them at high risk for pancreatic cancer.
Disclosures: None of the authors reported relevant conflicts of interest, and no outside source of funding was reported.
Source: DaVee RT et al. Gastrointest Endosc. 2018 Jun;87(6):1443-540.
Physician groups call for CMS to drop E/M proposal
More than 170 physician groups are calling on the Centers for Medicare & Medicaid Services to withdraw a provision in the proposed 2019 physician fee schedule that would flatten evaluation and management payments.
The controversial proposal would set the payment rate for a level 1 evaluation and management (E/M) office visit for a new patient at $44, down from the $45 using the current methodology. Payment for levels 2-5 would be $135. Currently, payments for level 2 new patient visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For E/M office visits with established patients, the proposed rate would be $24 for level 1, up from the current payment of $22. Payment for levels 2-5 would be $93. Under the current methodology, payments for established patient level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
In an Aug. 28 letter to the CMS, led by the American College of Rheumatology, physician groups applauded CMS recognition of the problems with the current E/M documentation guidelines and codes, but urged them to reconsider plans to “cut and consolidate evaluation and management services.” Doing so would “severely reduce Medicare patients’ access to care by cutting payments for complex office visits, adversely affecting the care and treatment of patients with complex conditions, and potentially exacerbate physician workforce shortages.”
A separate letter, led by the American Medical Association, made similar assertions that the current proposal has the potential to “hurt physicians and other health care professionals in specialties that treat the sickest patients, as well as those who provide comprehensive primary care, ultimately jeopardizing patients’ access to care.”
The American Gastroenterological Association sigend on to both letters.
AGA, along with the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, sent out a member alert, asking their members to tell CMS not to move forward with the proposed change because all three societies believe that such a payment system undervalues care provided to their sickest and most vulnerable seniors and other Medicare beneficiaries.
Another concern related to the implementation of this proposal is the financial impact on physicians.
Implementation of the CMS proposal, as currently written, “would be amazingly expensive for private practice [doctors] and really for anyone else because we would have to change our EMRs,” Barbara Levy, MD, cochair of the CPT/RUC Work Group at the AMA.
“We would have to reprogram our billing software. All of that comes with a significant cost,” said Dr. Levy, who also serves as vice president of health policy at the American College of Obstetricians and Gynecologists.
Part of the selling point of the CMS proposal is the reduction in documentation that accompanies the E/M payment changes. The goal, according to the CMS, is to reduce time spent on paperwork and free up physicians to devote more time to patient care. But some physicians are skeptical it would work out that way.
"CMS has clearly heard from physicians about the need to reduce administrative burdens for physicians, and AGA appreciates that they're listening," said Peter S. Margolis, MD, AGAF, AGA Practice Councillor, University Gastroenterology, Providence, Rhode Island. "However, CMS' proposal drastically undervalues the care gastroenterologists and hepatologists provide to complex patients, including but not limited to those with inflammatory bowel disease, motility disorders, and chronic liver disease. Additionally, our experience shows that utilization management methods, such as prior authorization and step therapy appeals, are far more burdensome to physicians and physician practices than current E/M documentation requirements."
Another element of the proposal that is raising concerns among physician groups is a proposed payment reduction when a visit involves more than one service. For example, when a single office visit includes both an E/M code and a procedure code, the proposal calls for the E/M code to be cut in half.
“From the patients’ perspective, the potential threat is that doctors could be incentivized to spend less time with patients or potentially bring patients back for subsequent visits to handle multiple problems,” Angus Worthing, MD, chair of the American College of Rheumatology’s Committee on Government Affairs, said in an interview.
Comments on the proposed update to the 2019 Medicare physician fee schedule are due Sept. 10.
More than 170 physician groups are calling on the Centers for Medicare & Medicaid Services to withdraw a provision in the proposed 2019 physician fee schedule that would flatten evaluation and management payments.
The controversial proposal would set the payment rate for a level 1 evaluation and management (E/M) office visit for a new patient at $44, down from the $45 using the current methodology. Payment for levels 2-5 would be $135. Currently, payments for level 2 new patient visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For E/M office visits with established patients, the proposed rate would be $24 for level 1, up from the current payment of $22. Payment for levels 2-5 would be $93. Under the current methodology, payments for established patient level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
In an Aug. 28 letter to the CMS, led by the American College of Rheumatology, physician groups applauded CMS recognition of the problems with the current E/M documentation guidelines and codes, but urged them to reconsider plans to “cut and consolidate evaluation and management services.” Doing so would “severely reduce Medicare patients’ access to care by cutting payments for complex office visits, adversely affecting the care and treatment of patients with complex conditions, and potentially exacerbate physician workforce shortages.”
A separate letter, led by the American Medical Association, made similar assertions that the current proposal has the potential to “hurt physicians and other health care professionals in specialties that treat the sickest patients, as well as those who provide comprehensive primary care, ultimately jeopardizing patients’ access to care.”
The American Gastroenterological Association sigend on to both letters.
AGA, along with the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, sent out a member alert, asking their members to tell CMS not to move forward with the proposed change because all three societies believe that such a payment system undervalues care provided to their sickest and most vulnerable seniors and other Medicare beneficiaries.
Another concern related to the implementation of this proposal is the financial impact on physicians.
Implementation of the CMS proposal, as currently written, “would be amazingly expensive for private practice [doctors] and really for anyone else because we would have to change our EMRs,” Barbara Levy, MD, cochair of the CPT/RUC Work Group at the AMA.
“We would have to reprogram our billing software. All of that comes with a significant cost,” said Dr. Levy, who also serves as vice president of health policy at the American College of Obstetricians and Gynecologists.
Part of the selling point of the CMS proposal is the reduction in documentation that accompanies the E/M payment changes. The goal, according to the CMS, is to reduce time spent on paperwork and free up physicians to devote more time to patient care. But some physicians are skeptical it would work out that way.
"CMS has clearly heard from physicians about the need to reduce administrative burdens for physicians, and AGA appreciates that they're listening," said Peter S. Margolis, MD, AGAF, AGA Practice Councillor, University Gastroenterology, Providence, Rhode Island. "However, CMS' proposal drastically undervalues the care gastroenterologists and hepatologists provide to complex patients, including but not limited to those with inflammatory bowel disease, motility disorders, and chronic liver disease. Additionally, our experience shows that utilization management methods, such as prior authorization and step therapy appeals, are far more burdensome to physicians and physician practices than current E/M documentation requirements."
Another element of the proposal that is raising concerns among physician groups is a proposed payment reduction when a visit involves more than one service. For example, when a single office visit includes both an E/M code and a procedure code, the proposal calls for the E/M code to be cut in half.
“From the patients’ perspective, the potential threat is that doctors could be incentivized to spend less time with patients or potentially bring patients back for subsequent visits to handle multiple problems,” Angus Worthing, MD, chair of the American College of Rheumatology’s Committee on Government Affairs, said in an interview.
Comments on the proposed update to the 2019 Medicare physician fee schedule are due Sept. 10.
More than 170 physician groups are calling on the Centers for Medicare & Medicaid Services to withdraw a provision in the proposed 2019 physician fee schedule that would flatten evaluation and management payments.
The controversial proposal would set the payment rate for a level 1 evaluation and management (E/M) office visit for a new patient at $44, down from the $45 using the current methodology. Payment for levels 2-5 would be $135. Currently, payments for level 2 new patient visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For E/M office visits with established patients, the proposed rate would be $24 for level 1, up from the current payment of $22. Payment for levels 2-5 would be $93. Under the current methodology, payments for established patient level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
In an Aug. 28 letter to the CMS, led by the American College of Rheumatology, physician groups applauded CMS recognition of the problems with the current E/M documentation guidelines and codes, but urged them to reconsider plans to “cut and consolidate evaluation and management services.” Doing so would “severely reduce Medicare patients’ access to care by cutting payments for complex office visits, adversely affecting the care and treatment of patients with complex conditions, and potentially exacerbate physician workforce shortages.”
A separate letter, led by the American Medical Association, made similar assertions that the current proposal has the potential to “hurt physicians and other health care professionals in specialties that treat the sickest patients, as well as those who provide comprehensive primary care, ultimately jeopardizing patients’ access to care.”
The American Gastroenterological Association sigend on to both letters.
AGA, along with the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, sent out a member alert, asking their members to tell CMS not to move forward with the proposed change because all three societies believe that such a payment system undervalues care provided to their sickest and most vulnerable seniors and other Medicare beneficiaries.
Another concern related to the implementation of this proposal is the financial impact on physicians.
Implementation of the CMS proposal, as currently written, “would be amazingly expensive for private practice [doctors] and really for anyone else because we would have to change our EMRs,” Barbara Levy, MD, cochair of the CPT/RUC Work Group at the AMA.
“We would have to reprogram our billing software. All of that comes with a significant cost,” said Dr. Levy, who also serves as vice president of health policy at the American College of Obstetricians and Gynecologists.
Part of the selling point of the CMS proposal is the reduction in documentation that accompanies the E/M payment changes. The goal, according to the CMS, is to reduce time spent on paperwork and free up physicians to devote more time to patient care. But some physicians are skeptical it would work out that way.
"CMS has clearly heard from physicians about the need to reduce administrative burdens for physicians, and AGA appreciates that they're listening," said Peter S. Margolis, MD, AGAF, AGA Practice Councillor, University Gastroenterology, Providence, Rhode Island. "However, CMS' proposal drastically undervalues the care gastroenterologists and hepatologists provide to complex patients, including but not limited to those with inflammatory bowel disease, motility disorders, and chronic liver disease. Additionally, our experience shows that utilization management methods, such as prior authorization and step therapy appeals, are far more burdensome to physicians and physician practices than current E/M documentation requirements."
Another element of the proposal that is raising concerns among physician groups is a proposed payment reduction when a visit involves more than one service. For example, when a single office visit includes both an E/M code and a procedure code, the proposal calls for the E/M code to be cut in half.
“From the patients’ perspective, the potential threat is that doctors could be incentivized to spend less time with patients or potentially bring patients back for subsequent visits to handle multiple problems,” Angus Worthing, MD, chair of the American College of Rheumatology’s Committee on Government Affairs, said in an interview.
Comments on the proposed update to the 2019 Medicare physician fee schedule are due Sept. 10.
Cancer researchers fall short on financial disclosures
New research suggests that investigators involved in oncology trials sometimes fail to disclose payments from the pharmaceutical industry.
Researchers looked at clinical trials associated with cancer drugs recently approved in the United States and assessed whether funding was properly disclosed when the trial results were published in scientific journals.
The data showed that roughly a third of investigators failed to completely disclose payments from trial sponsors.
“We know that pharmaceutical companies sponsor trials of their own drugs. That’s not a surprise,” Cole Wayant, a DO/PhD student at Oklahoma State University in Tulsa, said in a statement. “But what is a surprise, and what warrants concern, is that this funding is often not disclosed in the publication of clinical trials that form the basis of FDA [Food and Drug Administration] approvals and clinical practice guidelines.”
Mr. Wayant and his colleagues conducted this research and reported the findings in a research letter published in JAMA Oncology.
The researchers began by searching the FDA Hematology/Oncology Approvals & Safety Notifications website for oncology drugs approved from Jan. 1, 2016, to Aug. 31, 2017.
The team then identified the published trials supporting these drug approvals and searched the Open Payments Database for industry payment data for each U.S.-based oncologist involved in the trials. Finally, the researchers compared the Open Payments data to the disclosure statements from the publications.
There were 344 authors of clinical trials associated with oncology drugs approved during the period studied. Most authors (76.5%) received at least one industry payment, and the total amount they received exceeded $216 million.
Nearly a third of the authors (32%, n = 110) did not fully disclose payments from a trial sponsor.
In all, the authors received about $6.3 million in general payments, such as speaking fees, and $1.7 million of that was undisclosed.
They received more than $500,000 in research payments, such as fees for study coordination, and more than $200,000 of that was undisclosed.
The authors received close to $210 million in associated research payments, such as grants, and about $78 million of that was undisclosed.
Mr. Wayant and his colleagues said these results suggest financial relationships between the pharmaceutical industry and oncology trial investigators “may be common, expensive, and frequently undisclosed.”
However, the research also suggests that Open Payments data could be used to ensure complete disclosure of industry payments.
The researchers reported having no financial disclosures.
SOURCE: Wayant C et al. JAMA Oncol. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3738.
New research suggests that investigators involved in oncology trials sometimes fail to disclose payments from the pharmaceutical industry.
Researchers looked at clinical trials associated with cancer drugs recently approved in the United States and assessed whether funding was properly disclosed when the trial results were published in scientific journals.
The data showed that roughly a third of investigators failed to completely disclose payments from trial sponsors.
“We know that pharmaceutical companies sponsor trials of their own drugs. That’s not a surprise,” Cole Wayant, a DO/PhD student at Oklahoma State University in Tulsa, said in a statement. “But what is a surprise, and what warrants concern, is that this funding is often not disclosed in the publication of clinical trials that form the basis of FDA [Food and Drug Administration] approvals and clinical practice guidelines.”
Mr. Wayant and his colleagues conducted this research and reported the findings in a research letter published in JAMA Oncology.
The researchers began by searching the FDA Hematology/Oncology Approvals & Safety Notifications website for oncology drugs approved from Jan. 1, 2016, to Aug. 31, 2017.
The team then identified the published trials supporting these drug approvals and searched the Open Payments Database for industry payment data for each U.S.-based oncologist involved in the trials. Finally, the researchers compared the Open Payments data to the disclosure statements from the publications.
There were 344 authors of clinical trials associated with oncology drugs approved during the period studied. Most authors (76.5%) received at least one industry payment, and the total amount they received exceeded $216 million.
Nearly a third of the authors (32%, n = 110) did not fully disclose payments from a trial sponsor.
In all, the authors received about $6.3 million in general payments, such as speaking fees, and $1.7 million of that was undisclosed.
They received more than $500,000 in research payments, such as fees for study coordination, and more than $200,000 of that was undisclosed.
The authors received close to $210 million in associated research payments, such as grants, and about $78 million of that was undisclosed.
Mr. Wayant and his colleagues said these results suggest financial relationships between the pharmaceutical industry and oncology trial investigators “may be common, expensive, and frequently undisclosed.”
However, the research also suggests that Open Payments data could be used to ensure complete disclosure of industry payments.
The researchers reported having no financial disclosures.
SOURCE: Wayant C et al. JAMA Oncol. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3738.
New research suggests that investigators involved in oncology trials sometimes fail to disclose payments from the pharmaceutical industry.
Researchers looked at clinical trials associated with cancer drugs recently approved in the United States and assessed whether funding was properly disclosed when the trial results were published in scientific journals.
The data showed that roughly a third of investigators failed to completely disclose payments from trial sponsors.
“We know that pharmaceutical companies sponsor trials of their own drugs. That’s not a surprise,” Cole Wayant, a DO/PhD student at Oklahoma State University in Tulsa, said in a statement. “But what is a surprise, and what warrants concern, is that this funding is often not disclosed in the publication of clinical trials that form the basis of FDA [Food and Drug Administration] approvals and clinical practice guidelines.”
Mr. Wayant and his colleagues conducted this research and reported the findings in a research letter published in JAMA Oncology.
The researchers began by searching the FDA Hematology/Oncology Approvals & Safety Notifications website for oncology drugs approved from Jan. 1, 2016, to Aug. 31, 2017.
The team then identified the published trials supporting these drug approvals and searched the Open Payments Database for industry payment data for each U.S.-based oncologist involved in the trials. Finally, the researchers compared the Open Payments data to the disclosure statements from the publications.
There were 344 authors of clinical trials associated with oncology drugs approved during the period studied. Most authors (76.5%) received at least one industry payment, and the total amount they received exceeded $216 million.
Nearly a third of the authors (32%, n = 110) did not fully disclose payments from a trial sponsor.
In all, the authors received about $6.3 million in general payments, such as speaking fees, and $1.7 million of that was undisclosed.
They received more than $500,000 in research payments, such as fees for study coordination, and more than $200,000 of that was undisclosed.
The authors received close to $210 million in associated research payments, such as grants, and about $78 million of that was undisclosed.
Mr. Wayant and his colleagues said these results suggest financial relationships between the pharmaceutical industry and oncology trial investigators “may be common, expensive, and frequently undisclosed.”
However, the research also suggests that Open Payments data could be used to ensure complete disclosure of industry payments.
The researchers reported having no financial disclosures.
SOURCE: Wayant C et al. JAMA Oncol. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3738.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Among study authors involved in oncology trials, 32% did not fully disclose payments from trial sponsors.
Study details: A comparison of financial disclosure statements in published studies with information from the Open Payments database. The researchers looked at studies that supported U.S. oncology drug approvals from Jan. 1, 2016, to Aug. 31, 2017.
Disclosures: The researchers reported having no financial disclosures.
Source: Wayant C et al. JAMA Oncol. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3738.
Hydrocortisone plus fludrocortisone for adults with septic shock
Background: Septic shock is a serious and common health problem, associated with a more than 50% mortality rate. It is characterized by a dysregulated patient response to infection, resulting in life-threatening circulatory, cellular, and metabolic abnormalities. The benefit of corticosteroid use in septic shock is still controversial.
Study design: Double-blinded, randomized, placebo-controlled trial.
Setting: The study was conducted in 34 centers in France.
Synopsis: All 1,241 septic shock patients received usual care. Of these patients, 614 patients received hydrocortisone and fludrocortisone (HF), while 627 patients received placebo. HF patients had a lower rate of 90-day mortality (43.0% vs. 49.1%; P = .03), mortality at ICU discharge (35.4% vs. 41.0%; P = .04), mortality at hospital discharge (39.0% vs. 45.3%; P = .02), and mortality at day 180 (46.6% vs. 52.5%; P = .04). There was no significant difference between mortality at day 28 (33.7% in the HF group versus 38.9% in the placebo group; P = .06), ventilator-free days (11 vs. 10 days; P = .07), and rate of serious adverse events (53.1% vs. 58.0%; P = .08). The number of vasopressor-free days to day 28 was significantly higher in the HF group (17 vs. 15 days; P less than .001), as were the number of organ failure–free days (14 vs. 12 days; P = .003). There was more hyperglycemia in the HF group (89.1% vs. 83.1%; P = .002).
Bottom line: Patients with septic shock who received hydrocortisone plus fludrocortisone have a lower rate of 90-day all-cause mortality, compared with placebo.
Citation: Annane D et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018 Mar 1. 378(9):809-18.
Dr. Salih is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Septic shock is a serious and common health problem, associated with a more than 50% mortality rate. It is characterized by a dysregulated patient response to infection, resulting in life-threatening circulatory, cellular, and metabolic abnormalities. The benefit of corticosteroid use in septic shock is still controversial.
Study design: Double-blinded, randomized, placebo-controlled trial.
Setting: The study was conducted in 34 centers in France.
Synopsis: All 1,241 septic shock patients received usual care. Of these patients, 614 patients received hydrocortisone and fludrocortisone (HF), while 627 patients received placebo. HF patients had a lower rate of 90-day mortality (43.0% vs. 49.1%; P = .03), mortality at ICU discharge (35.4% vs. 41.0%; P = .04), mortality at hospital discharge (39.0% vs. 45.3%; P = .02), and mortality at day 180 (46.6% vs. 52.5%; P = .04). There was no significant difference between mortality at day 28 (33.7% in the HF group versus 38.9% in the placebo group; P = .06), ventilator-free days (11 vs. 10 days; P = .07), and rate of serious adverse events (53.1% vs. 58.0%; P = .08). The number of vasopressor-free days to day 28 was significantly higher in the HF group (17 vs. 15 days; P less than .001), as were the number of organ failure–free days (14 vs. 12 days; P = .003). There was more hyperglycemia in the HF group (89.1% vs. 83.1%; P = .002).
Bottom line: Patients with septic shock who received hydrocortisone plus fludrocortisone have a lower rate of 90-day all-cause mortality, compared with placebo.
Citation: Annane D et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018 Mar 1. 378(9):809-18.
Dr. Salih is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Septic shock is a serious and common health problem, associated with a more than 50% mortality rate. It is characterized by a dysregulated patient response to infection, resulting in life-threatening circulatory, cellular, and metabolic abnormalities. The benefit of corticosteroid use in septic shock is still controversial.
Study design: Double-blinded, randomized, placebo-controlled trial.
Setting: The study was conducted in 34 centers in France.
Synopsis: All 1,241 septic shock patients received usual care. Of these patients, 614 patients received hydrocortisone and fludrocortisone (HF), while 627 patients received placebo. HF patients had a lower rate of 90-day mortality (43.0% vs. 49.1%; P = .03), mortality at ICU discharge (35.4% vs. 41.0%; P = .04), mortality at hospital discharge (39.0% vs. 45.3%; P = .02), and mortality at day 180 (46.6% vs. 52.5%; P = .04). There was no significant difference between mortality at day 28 (33.7% in the HF group versus 38.9% in the placebo group; P = .06), ventilator-free days (11 vs. 10 days; P = .07), and rate of serious adverse events (53.1% vs. 58.0%; P = .08). The number of vasopressor-free days to day 28 was significantly higher in the HF group (17 vs. 15 days; P less than .001), as were the number of organ failure–free days (14 vs. 12 days; P = .003). There was more hyperglycemia in the HF group (89.1% vs. 83.1%; P = .002).
Bottom line: Patients with septic shock who received hydrocortisone plus fludrocortisone have a lower rate of 90-day all-cause mortality, compared with placebo.
Citation: Annane D et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018 Mar 1. 378(9):809-18.
Dr. Salih is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
September 2018 Issue Highlights
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Click here to view the articles published in September 2018.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Click here to view the articles published in September 2018.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Click here to view the articles published in September 2018.
Escalating methotrexate may improve survival in T-cell ALL
An escalating methotrexate strategy provided superior survival outcomes compared with high-dose methotrexate in a chemotherapy regimen for children and young adults with T-cell acute lymphoblastic leukemia (T-ALL), results of a large, randomized trial show.
There were also fewer relapses reported for escalating versus high-dose methotrexate in the study, which evaluated the effects of these two intensification strategies in patients receiving an augmented Berlin-Frankfurt-Muenster (ABFM) chemotherapy regimen.
These findings come from a report in the Journal of Clinical Oncology on the Children’s Oncology Group (COG) AALL0434 trial, which to the knowledge of the investigators is the largest T-ALL study ever conducted.
The improved survival outcomes in AALL0434 are the “opposite effect” of what was observed in a parallel trial, AALL0232, showing that high-dose methotrexate was superior to the escalating strategy in B-cell acute lymphoblastic leukemia (B-ALL), the authors reported.
The parallel trial design was in fact used because of the known differences between T-ALL and B-ALL in sensitivity to methotrexate and pegaspargase, according to investigator Stuart S. Winter, MD, of Children’s Minnesota Cancer and Blood Disorders Program, Minneapolis, and his coauthors.
“Although treatment intensification has improved survival for children with ALL, the best timing and sequence of key therapeutic interventions, such as asparaginase and methotrexate, which seem to be particularly important for T-ALL, remain unclear,” Dr. Winter and his colleagues said.
In the AALL0434 study, a total of 1,031 T-ALL patients between 1 and 31 years of age without CNS3 disease or testicular leukemia were randomized to postinduction therapy that included either the so-called Capizzi-style escalating intravenous methotrexate or high-dose methotrexate.
The escalating intravenous regimen was superior to high-dose methotrexate, according to investigators. Respectively, the 5-year rate of disease-free survival was 91.5% versus 85.3% (P = .005) and the 5-year rate of overall survival was 93.7% versus 89.4% (P = .036).
Relapses were observed in 32 patients receiving the escalating regimen, versus 59 for patients receiving high-dose methotrexate.
By contrast, the parallel AALL0232 study of B-ALL patients showed that high-dose methotrexate had superior 5-year event-free survival and overall survival, leading Dr. Winter and his colleagues to speculate on how the findings could be reconciled.
Neither trial was a strict comparison of two different methotrexate schedules, due to differences in doses of pegaspargase, 6-MP, and vincristine between arms, as well as differences in the timing of cranial radiation therapy.
Of note, patients randomized to escalated methotrexate had two additional doses of pegaspargase. As a result, enhanced asparagine depletion in that arm may have also prevented relapse events, the investigators said.
Differences in adherence could also have played a role, as the cost and time burden of the escalated approach are “substantially less” than the high-dose approach, they added.
The AALL0434 trial also included a second randomization to an addition of five, 6-day cycles of nelarabine versus no nelarabine. Results of that randomization, reported earlier this year, showed that nelarabine improved disease-free survival, including a 91% 4-year disease-free survival rate for patients receiving both nelarabine and escalating-dose methotrexate.
The study was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. Dr. Winter reported relationships with Amgen and Jazz Pharmaceuticals. Study coauthors reported relationships with Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
SOURCE: Winter SS et al. J Clin Oncol. 2018 Aug 23: doi: 10.1200/JCO.2018.77.7250.
An escalating methotrexate strategy provided superior survival outcomes compared with high-dose methotrexate in a chemotherapy regimen for children and young adults with T-cell acute lymphoblastic leukemia (T-ALL), results of a large, randomized trial show.
There were also fewer relapses reported for escalating versus high-dose methotrexate in the study, which evaluated the effects of these two intensification strategies in patients receiving an augmented Berlin-Frankfurt-Muenster (ABFM) chemotherapy regimen.
These findings come from a report in the Journal of Clinical Oncology on the Children’s Oncology Group (COG) AALL0434 trial, which to the knowledge of the investigators is the largest T-ALL study ever conducted.
The improved survival outcomes in AALL0434 are the “opposite effect” of what was observed in a parallel trial, AALL0232, showing that high-dose methotrexate was superior to the escalating strategy in B-cell acute lymphoblastic leukemia (B-ALL), the authors reported.
The parallel trial design was in fact used because of the known differences between T-ALL and B-ALL in sensitivity to methotrexate and pegaspargase, according to investigator Stuart S. Winter, MD, of Children’s Minnesota Cancer and Blood Disorders Program, Minneapolis, and his coauthors.
“Although treatment intensification has improved survival for children with ALL, the best timing and sequence of key therapeutic interventions, such as asparaginase and methotrexate, which seem to be particularly important for T-ALL, remain unclear,” Dr. Winter and his colleagues said.
In the AALL0434 study, a total of 1,031 T-ALL patients between 1 and 31 years of age without CNS3 disease or testicular leukemia were randomized to postinduction therapy that included either the so-called Capizzi-style escalating intravenous methotrexate or high-dose methotrexate.
The escalating intravenous regimen was superior to high-dose methotrexate, according to investigators. Respectively, the 5-year rate of disease-free survival was 91.5% versus 85.3% (P = .005) and the 5-year rate of overall survival was 93.7% versus 89.4% (P = .036).
Relapses were observed in 32 patients receiving the escalating regimen, versus 59 for patients receiving high-dose methotrexate.
By contrast, the parallel AALL0232 study of B-ALL patients showed that high-dose methotrexate had superior 5-year event-free survival and overall survival, leading Dr. Winter and his colleagues to speculate on how the findings could be reconciled.
Neither trial was a strict comparison of two different methotrexate schedules, due to differences in doses of pegaspargase, 6-MP, and vincristine between arms, as well as differences in the timing of cranial radiation therapy.
Of note, patients randomized to escalated methotrexate had two additional doses of pegaspargase. As a result, enhanced asparagine depletion in that arm may have also prevented relapse events, the investigators said.
Differences in adherence could also have played a role, as the cost and time burden of the escalated approach are “substantially less” than the high-dose approach, they added.
The AALL0434 trial also included a second randomization to an addition of five, 6-day cycles of nelarabine versus no nelarabine. Results of that randomization, reported earlier this year, showed that nelarabine improved disease-free survival, including a 91% 4-year disease-free survival rate for patients receiving both nelarabine and escalating-dose methotrexate.
The study was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. Dr. Winter reported relationships with Amgen and Jazz Pharmaceuticals. Study coauthors reported relationships with Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
SOURCE: Winter SS et al. J Clin Oncol. 2018 Aug 23: doi: 10.1200/JCO.2018.77.7250.
An escalating methotrexate strategy provided superior survival outcomes compared with high-dose methotrexate in a chemotherapy regimen for children and young adults with T-cell acute lymphoblastic leukemia (T-ALL), results of a large, randomized trial show.
There were also fewer relapses reported for escalating versus high-dose methotrexate in the study, which evaluated the effects of these two intensification strategies in patients receiving an augmented Berlin-Frankfurt-Muenster (ABFM) chemotherapy regimen.
These findings come from a report in the Journal of Clinical Oncology on the Children’s Oncology Group (COG) AALL0434 trial, which to the knowledge of the investigators is the largest T-ALL study ever conducted.
The improved survival outcomes in AALL0434 are the “opposite effect” of what was observed in a parallel trial, AALL0232, showing that high-dose methotrexate was superior to the escalating strategy in B-cell acute lymphoblastic leukemia (B-ALL), the authors reported.
The parallel trial design was in fact used because of the known differences between T-ALL and B-ALL in sensitivity to methotrexate and pegaspargase, according to investigator Stuart S. Winter, MD, of Children’s Minnesota Cancer and Blood Disorders Program, Minneapolis, and his coauthors.
“Although treatment intensification has improved survival for children with ALL, the best timing and sequence of key therapeutic interventions, such as asparaginase and methotrexate, which seem to be particularly important for T-ALL, remain unclear,” Dr. Winter and his colleagues said.
In the AALL0434 study, a total of 1,031 T-ALL patients between 1 and 31 years of age without CNS3 disease or testicular leukemia were randomized to postinduction therapy that included either the so-called Capizzi-style escalating intravenous methotrexate or high-dose methotrexate.
The escalating intravenous regimen was superior to high-dose methotrexate, according to investigators. Respectively, the 5-year rate of disease-free survival was 91.5% versus 85.3% (P = .005) and the 5-year rate of overall survival was 93.7% versus 89.4% (P = .036).
Relapses were observed in 32 patients receiving the escalating regimen, versus 59 for patients receiving high-dose methotrexate.
By contrast, the parallel AALL0232 study of B-ALL patients showed that high-dose methotrexate had superior 5-year event-free survival and overall survival, leading Dr. Winter and his colleagues to speculate on how the findings could be reconciled.
Neither trial was a strict comparison of two different methotrexate schedules, due to differences in doses of pegaspargase, 6-MP, and vincristine between arms, as well as differences in the timing of cranial radiation therapy.
Of note, patients randomized to escalated methotrexate had two additional doses of pegaspargase. As a result, enhanced asparagine depletion in that arm may have also prevented relapse events, the investigators said.
Differences in adherence could also have played a role, as the cost and time burden of the escalated approach are “substantially less” than the high-dose approach, they added.
The AALL0434 trial also included a second randomization to an addition of five, 6-day cycles of nelarabine versus no nelarabine. Results of that randomization, reported earlier this year, showed that nelarabine improved disease-free survival, including a 91% 4-year disease-free survival rate for patients receiving both nelarabine and escalating-dose methotrexate.
The study was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. Dr. Winter reported relationships with Amgen and Jazz Pharmaceuticals. Study coauthors reported relationships with Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
SOURCE: Winter SS et al. J Clin Oncol. 2018 Aug 23: doi: 10.1200/JCO.2018.77.7250.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: An (T-ALL).
Major finding: The 5-year disease-free survival rate was 91.5% versus 85.3% (P = .005) and overall survival was 93.7% versus 89.4% (P = .036), respectively, for the escalating and high-dose approaches.
Study details: Results after methotrexate randomization in 1,031 T-ALL patients without CNS3 disease or testicular leukemia in the Children’s Oncology Group (COG) AALL0434 trial.
Disclosures: The study was supported by grants from the National Institutes of Health and by St. Baldrick’s Foundation. The authors reported disclosures related to Amgen, Jazz Pharmaceuticals, Novo Nordisk, Tandem, Pfizer, Novartis, and TypeZero Technologies, among others.
Source: Winter SS et al. J Clin Oncol. 2018 Aug 23. doi: 10.1200/JCO.2018.77.7250.
Obstructive sleep apnea may promote gout
Adults with obstructive sleep apnea are approximately twice as likely as are those without it to develop gout, according to data from a large, retrospective study with a median 5-year follow-up.
Data from previous studies have shown an increased risk in developing gout within the first year of an obstructive sleep apnea (OSA) diagnosis, wrote Milica Blagojevic-Bucknall, PhD, of Keele (England) University, and her colleagues.
In a study published in Arthritis & Rheumatology, the researchers compared 15,879 patients with OSA and 63,296 without.
Overall, 4.9% of OSA patients and 2.6% non‐OSA controls developed gout over a median follow‐up period of 5.8 years. The incidence rate for gout per 1,000 person‐years was 7.83 among patients with OSA and 4.03 for controls (adjusted hazard ratio, 1.42). The greatest risk for gout in the OSA patients occurred approximately 1-2 years after their diagnosis.
The researchers also found significant associations between body mass index and gout risk in sleep apnea across all BMI categories, but the strongest association occurred in the normal BMI group (HR 2.02) at 2-5 years after the index date of OSA.
“The novelty of this study lies in assessing both the short- and long-term association of OSA with incident gout in a large primary care-based population,” the researchers said. They proposed that the most likely explanation for the events was that “intermittent hypoxia increases nucleotide turnover which enhances endogenous uric acid production.”
The study findings were limited by several factors including potential misclassification of OSA and the impact of confounding variables such as genetics and diet, they noted.
However, the results support the association between sleep apnea and gout, but also serve to highlight that clinicians should “consider the possibility of gout in patients with sleep apnea regardless of obesity,” the researchers wrote.
The National Institute for Health Research funded the study. The authors have no conflicts of interest to declare.
SOURCE: Blagojevic-Bucknall M et al. Arthritis Rheumatol. 2018 Aug 30. doi: 10.1002/art.40662.
Adults with obstructive sleep apnea are approximately twice as likely as are those without it to develop gout, according to data from a large, retrospective study with a median 5-year follow-up.
Data from previous studies have shown an increased risk in developing gout within the first year of an obstructive sleep apnea (OSA) diagnosis, wrote Milica Blagojevic-Bucknall, PhD, of Keele (England) University, and her colleagues.
In a study published in Arthritis & Rheumatology, the researchers compared 15,879 patients with OSA and 63,296 without.
Overall, 4.9% of OSA patients and 2.6% non‐OSA controls developed gout over a median follow‐up period of 5.8 years. The incidence rate for gout per 1,000 person‐years was 7.83 among patients with OSA and 4.03 for controls (adjusted hazard ratio, 1.42). The greatest risk for gout in the OSA patients occurred approximately 1-2 years after their diagnosis.
The researchers also found significant associations between body mass index and gout risk in sleep apnea across all BMI categories, but the strongest association occurred in the normal BMI group (HR 2.02) at 2-5 years after the index date of OSA.
“The novelty of this study lies in assessing both the short- and long-term association of OSA with incident gout in a large primary care-based population,” the researchers said. They proposed that the most likely explanation for the events was that “intermittent hypoxia increases nucleotide turnover which enhances endogenous uric acid production.”
The study findings were limited by several factors including potential misclassification of OSA and the impact of confounding variables such as genetics and diet, they noted.
However, the results support the association between sleep apnea and gout, but also serve to highlight that clinicians should “consider the possibility of gout in patients with sleep apnea regardless of obesity,” the researchers wrote.
The National Institute for Health Research funded the study. The authors have no conflicts of interest to declare.
SOURCE: Blagojevic-Bucknall M et al. Arthritis Rheumatol. 2018 Aug 30. doi: 10.1002/art.40662.
Adults with obstructive sleep apnea are approximately twice as likely as are those without it to develop gout, according to data from a large, retrospective study with a median 5-year follow-up.
Data from previous studies have shown an increased risk in developing gout within the first year of an obstructive sleep apnea (OSA) diagnosis, wrote Milica Blagojevic-Bucknall, PhD, of Keele (England) University, and her colleagues.
In a study published in Arthritis & Rheumatology, the researchers compared 15,879 patients with OSA and 63,296 without.
Overall, 4.9% of OSA patients and 2.6% non‐OSA controls developed gout over a median follow‐up period of 5.8 years. The incidence rate for gout per 1,000 person‐years was 7.83 among patients with OSA and 4.03 for controls (adjusted hazard ratio, 1.42). The greatest risk for gout in the OSA patients occurred approximately 1-2 years after their diagnosis.
The researchers also found significant associations between body mass index and gout risk in sleep apnea across all BMI categories, but the strongest association occurred in the normal BMI group (HR 2.02) at 2-5 years after the index date of OSA.
“The novelty of this study lies in assessing both the short- and long-term association of OSA with incident gout in a large primary care-based population,” the researchers said. They proposed that the most likely explanation for the events was that “intermittent hypoxia increases nucleotide turnover which enhances endogenous uric acid production.”
The study findings were limited by several factors including potential misclassification of OSA and the impact of confounding variables such as genetics and diet, they noted.
However, the results support the association between sleep apnea and gout, but also serve to highlight that clinicians should “consider the possibility of gout in patients with sleep apnea regardless of obesity,” the researchers wrote.
The National Institute for Health Research funded the study. The authors have no conflicts of interest to declare.
SOURCE: Blagojevic-Bucknall M et al. Arthritis Rheumatol. 2018 Aug 30. doi: 10.1002/art.40662.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Adults with obstructive sleep apnea were more likely to develop gout than were those without the condition.
Major finding: Over a median follow‐up of 5.8 years, 4.9% of OSA patients and 2.6% of controls without OSA developed gout.
Study details: A matched retrospective cohort study of 15,879 OSA patients and 63,296 controls.
Disclosures: The National Institute for Health Research funded the study. The researchers had no financial conflicts to disclose.
Source: Blagojevich-Bucknall M et al. Arthritis Rheumatol. 2018 Aug 30. doi: 10.1002/art.40662.
Caplacizumab approved in Europe to treat aTTP
The , a humanized bivalent nanobody that inhibits the interaction between von Willebrand factor and platelets.
Caplacizumab is now approved to treat adults with acquired thrombotic thrombocytopenic purpura (aTTP) in all member countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The drug has been accepted for priority review in the United States and the Food and Drug Administration is expected to make a decision by Feb. 6, 2019.
The European Commission’s approval of caplacizumab is supported by data from the phase 2 TITAN study and the phase 3 HERCULES study.
The TITAN trial included 75 aTTP patients who were randomized to caplacizumab (n = 36) or placebo (n = 39), with all patients receiving the current standard of care – daily plasma exchange and immunosuppressive therapy (N Engl J Med. 2016;374:511-22).
Patients in the caplacizumab arm had a 39% reduction in the median time to response, compared with patients in the placebo arm (P = .005).
The rate of adverse events (AEs) thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. The rate of serious AEs was 37% and 32%, respectively.
There were no deaths in the caplacizumab arm and two in the placebo arm. One death was attributable to severe, refractory TTP, and the other was attributable to cerebral hemorrhage.Results from the HERCULES trial were presented at the 2017 annual meeting of the American Society of Hematology.
The study enrolled patients with an acute episode of aTTP. They were randomized to receive caplacizumab (n = 72) or placebo (n = 73) in addition to standard care – plasma exchange and immunosuppression.
The study’s primary endpoint was the time to platelet count response (normalization). There was a significant reduction in time to platelet count response in the caplacizumab arm, compared with the placebo arm. The platelet normalization rate ratio was 1.55 (P less than .01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least one major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n = 9) in the caplacizumab arm and 49.3% (n = 36) in the placebo arm (P less than .0001).
The incidence of aTTP-related death was 0% (n = 0) in the caplacizumab arm and 4.1% (n = 3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n = 3) in the caplacizumab arm and 38.4% in the placebo arm (n = 28), and the incidence of at least one major thromboembolic event was 8.5% (n = 6) and 8.2% (n = 6), respectively.
The , a humanized bivalent nanobody that inhibits the interaction between von Willebrand factor and platelets.
Caplacizumab is now approved to treat adults with acquired thrombotic thrombocytopenic purpura (aTTP) in all member countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The drug has been accepted for priority review in the United States and the Food and Drug Administration is expected to make a decision by Feb. 6, 2019.
The European Commission’s approval of caplacizumab is supported by data from the phase 2 TITAN study and the phase 3 HERCULES study.
The TITAN trial included 75 aTTP patients who were randomized to caplacizumab (n = 36) or placebo (n = 39), with all patients receiving the current standard of care – daily plasma exchange and immunosuppressive therapy (N Engl J Med. 2016;374:511-22).
Patients in the caplacizumab arm had a 39% reduction in the median time to response, compared with patients in the placebo arm (P = .005).
The rate of adverse events (AEs) thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. The rate of serious AEs was 37% and 32%, respectively.
There were no deaths in the caplacizumab arm and two in the placebo arm. One death was attributable to severe, refractory TTP, and the other was attributable to cerebral hemorrhage.Results from the HERCULES trial were presented at the 2017 annual meeting of the American Society of Hematology.
The study enrolled patients with an acute episode of aTTP. They were randomized to receive caplacizumab (n = 72) or placebo (n = 73) in addition to standard care – plasma exchange and immunosuppression.
The study’s primary endpoint was the time to platelet count response (normalization). There was a significant reduction in time to platelet count response in the caplacizumab arm, compared with the placebo arm. The platelet normalization rate ratio was 1.55 (P less than .01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least one major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n = 9) in the caplacizumab arm and 49.3% (n = 36) in the placebo arm (P less than .0001).
The incidence of aTTP-related death was 0% (n = 0) in the caplacizumab arm and 4.1% (n = 3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n = 3) in the caplacizumab arm and 38.4% in the placebo arm (n = 28), and the incidence of at least one major thromboembolic event was 8.5% (n = 6) and 8.2% (n = 6), respectively.
The , a humanized bivalent nanobody that inhibits the interaction between von Willebrand factor and platelets.
Caplacizumab is now approved to treat adults with acquired thrombotic thrombocytopenic purpura (aTTP) in all member countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The drug has been accepted for priority review in the United States and the Food and Drug Administration is expected to make a decision by Feb. 6, 2019.
The European Commission’s approval of caplacizumab is supported by data from the phase 2 TITAN study and the phase 3 HERCULES study.
The TITAN trial included 75 aTTP patients who were randomized to caplacizumab (n = 36) or placebo (n = 39), with all patients receiving the current standard of care – daily plasma exchange and immunosuppressive therapy (N Engl J Med. 2016;374:511-22).
Patients in the caplacizumab arm had a 39% reduction in the median time to response, compared with patients in the placebo arm (P = .005).
The rate of adverse events (AEs) thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. The rate of serious AEs was 37% and 32%, respectively.
There were no deaths in the caplacizumab arm and two in the placebo arm. One death was attributable to severe, refractory TTP, and the other was attributable to cerebral hemorrhage.Results from the HERCULES trial were presented at the 2017 annual meeting of the American Society of Hematology.
The study enrolled patients with an acute episode of aTTP. They were randomized to receive caplacizumab (n = 72) or placebo (n = 73) in addition to standard care – plasma exchange and immunosuppression.
The study’s primary endpoint was the time to platelet count response (normalization). There was a significant reduction in time to platelet count response in the caplacizumab arm, compared with the placebo arm. The platelet normalization rate ratio was 1.55 (P less than .01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least one major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n = 9) in the caplacizumab arm and 49.3% (n = 36) in the placebo arm (P less than .0001).
The incidence of aTTP-related death was 0% (n = 0) in the caplacizumab arm and 4.1% (n = 3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n = 3) in the caplacizumab arm and 38.4% in the placebo arm (n = 28), and the incidence of at least one major thromboembolic event was 8.5% (n = 6) and 8.2% (n = 6), respectively.











